DOI: https://doi.org/10.1111/nph.19676
PMID: https://pubmed.ncbi.nlm.nih.gov/38482544
تاريخ النشر: 2024-03-14
آليات جذور النباتات وتأثيرها على تراكم الكربون والمغذيات في النظم البيئية الصحراوية تحت تغييرات استخدام الأراضي والمناخ
الملخص
أكاش طارق
آيرس، الأرجنتين؛
نيو فيتولوجيست (2024) 242: 916-934 doi: 10.1111/nph. 19676
الملخص
الملخص تمثل الصحاري خزانات كربون رئيسية، ومع ذلك، فإن تهديد هذه الأنظمة له آثار على التنوع البيولوجي وتغير المناخ. تركز هذه المراجعة على كيفية تأثير هذه التغييرات على النظم البيئية الصحراوية، وخاصة أنظمة جذور النباتات وتأثيرها على مخزونات الكربون والمغذيات المعدنية. تمتلك النباتات الصحراوية هياكل جذرية متنوعة تتشكل من استراتيجيات اكتساب المياه، مما يؤثر على الكتلة الحيوية للنباتات ومخزونات الكربون والمغذيات بشكل عام. يمكن أن disrupt تغير المناخ المجتمعات النباتية الصحراوية، حيث تؤثر الجفاف على كل من النباتات ذات الجذور الضحلة والعميقة مع تقلب مستويات المياه الجوفية. تؤثر ممارسات إدارة الغطاء النباتي، مثل الرعي، بشكل كبير على المجتمعات النباتية، وتكوين التربة، والميكروبات الجذرية، والكتلة الحيوية، ومخزونات المغذيات. النباتات ذات الجذور الضحلة أكثر عرضة لتغير المناخ والتدخل البشري. لحماية النظم البيئية الصحراوية، فإن فهم هيكل الجذر والطبقات العميقة من التربة أمر بالغ الأهمية. يمكن أن تساعد تنفيذ ممارسات الإدارة الاستراتيجية مثل تقليل ضغط الرعي، والحفاظ على مستويات الحصاد المعتدلة، وتبني التسميد المعتدل في الحفاظ على أنظمة النبات والتربة. إن استخدام نهج اجتماعي-بيئي لاستعادة المجتمع يعزز احتفاظ الكربون والمغذيات، ويحد من توسع الصحراء، ويقلل من
المقدمة
وأقارب المحاصيل البرية، غالبًا تهديدات وغالبًا ما يتم تجاهلها (تشانغ وآخرون، 2023). بينما تمتد النظم البيئية الجافة عبر مجموعة من مستويات الجفاف، تمثل الصحاري الطرف المتطرف وغالبًا ما يتم تهميشها من منظور الحفظ. على الرغم من استضافتها لعدد أقل من الأنواع مقارنة بالمناطق الاستوائية الرطبة (سافرييل وزفار، 2005)، تظهر الصحاري تنوعًا وظيفيًا عاليًا وندرة. وبالتالي، فإن فقدان الأنواع في هذه النظم البيئية يمكن أن يكون له تأثير أكثر وضوحًا من المناطق الرطبة والغنية بالأنواع (مايستري وآخرون، 2021). علاوة على ذلك، يتطلب استعادة النظم البيئية الصحراوية المتدهورة استراتيجيات متخصصة بسبب قدرتها المحدودة على التجديد والنمو (برينسيبي وآخرون، 2014). نتيجة لذلك، تحتل النظم البيئية الصحراوية موقعًا فريدًا وتتطلب اهتمامًا كبيرًا بسبب تعرضها المتزايد لتغير المناخ والأنشطة البشرية. علاوة على ذلك، لا يزال التصحر وتوسع الصحاري يُنظر إليهما على أنهما مشكلتان، مما يظلل الآثار الأكثر تعقيدًا لتغير المناخ على النظم البيئية الصحراوية، فضلاً عن الآثار المميزة لمختلف جوانب التغيير البيئي على مجتمعات النباتات المختلفة. تعاني النباتات والميكروبات في الصحاري من ضغوط غير حيوية متعددة، مستمدة أساسًا من فترات طويلة من انخفاض توفر المياه، وملوحة التربة، وانخفاض محتوى المغذيات وحركتها، ودرجات الحرارة القصوى، والإشعاع العالي، والعواصف الرملية والرياح المتكررة (الشارف وآخرون، 2020). تعتمد الكائنات الحية في هذه المناطق بشكل كبير على نبضات المياه المتقطعة التي تحدث في فترات غير منتظمة، مما يجعل هطول الأمطار عاملاً محوريًا يؤثر على نمو النباتات ونشاط الميكروبات في التربة (سول-تشيركاس وستاينبرغر، 2009؛ كولينز وآخرون، 2017؛ رونثيرو-راموس وآخرون، 2022؛ فيكرام وآخرون، 2023). بالإضافة إلى ذلك، تعمل الدورات الجيوكيميائية ببطء في الصحاري بسبب بيئاتها القاسية والمحرومة من المياه، مما يؤدي إلى توفر محدود من المغذيات وحركتها في التربة (طارق وآخرون، 2022أ؛ موريش وآخرون، 2023). نتيجة لذلك، تظهر أنواع نباتات صحراوية متنوعة تعديلات شكلية وفسيولوجية فريدة في أوراقها، وسيقانها، وهياكل جذورها لضمان البقاء. ومع ذلك، بينما تم دراسة الأعضاء فوق الأرض بشكل مكثف بسبب سهولة الوصول إليها، تم تجاهل تعديلات أنظمة الجذور وعواقبها إلى حد كبير (الشارف وآخرون، 2020؛ كيرشنر وآخرون، 2021). يحدث اكتساب المياه في النباتات الصحراوية بشكل أساسي من خلال التربة، مما يجعل هياكل الجذور المميزة حاسمة للبقاء (لينش، 2022). تؤثر هندسة نظام الجذر (RSA) بشكل كبير على الوصول إلى المياه، واكتساب المغذيات، وتخزين الكربون (C)، والوظيفة العامة للنبات (مايخت وآخرون، 2013). وبالتالي، فإن الهيكل الجذري المميز للنباتات الصحراوية، سواء كان عميقًا أو ضحلًا، يؤثر على مخزونات C والمغذيات، حيث يمنح الوصول إلى المياه الجوفية أو مياه الأمطار، مما يؤدي إلى استجابات مختلفة للتغيرات البيئية. مؤخرًا، تم استكشاف دور المياه الجوفية في تخزين C في الصحاري (لي وآخرون، 2016)، ولكن لا يزال هناك فجوة ملحوظة في فهم كيفية تأثير RSA على تراكم المغذيات وC في مجتمعات النباتات الصحراوية.
(كيل، 2011). بالمقابل، تكون الكتلة الحيوية فوق الأرض أكثر عرضة لتغيرات استخدام الأراضي (مثل الحرائق، والرعي، والحصاد) والاضطرابات المناخية، مما يؤدي إلى زيادة إطلاق C في الغلاف الجوي وتفاقم تغير المناخ. بالإضافة إلى ذلك، يمكن أن يُعزى انخفاض كثافة الكتلة الحيوية في الصحاري إلى كل من القيود البيئية (مغذيات التربة، والهطول الموسمي، وتوزيع درجات الحرارة) والاضطرابات البشرية، مما يساهم بشكل أكبر في انخفاض مخزونات المغذيات وC في نظام النبات والتربة (هوتون وآخرون، 2009).
هيكل جذور النباتات في النظم البيئية الصحراوية
مناطق صغيرة. بالمقابل، تتلقى الصحاري الباردة هطولًا في شكل ثلوج، تغطي السطح بالكامل وتوفر مياه سائلة للنباتات خلال موسم النمو الدافئ (فان وآخرون، 2014). الندى هو مصدر إضافي للمياه للنباتات، خاصة في الصحاري الساحلية، حيث يتم امتصاصه بواسطة الأوراق ثم نقله إلى السيقان والجذور الأكثر جفافًا (كيدرون وستارينسكي، 2019). علاوة على ذلك، تتمتع النباتات الصحراوية بالقدرة على سحب المياه من مستوى المياه الجوفية والمسطحات المائية القريبة. ومع ذلك، نظرًا لأن النباتات الصحراوية تكتسب المياه بشكل أساسي من خلال جذورها من التربة الجافة، فإن الهياكل المميزة لأنظمة جذورها حاسمة للبقاء (لينش، 1995).
خلال بضعة أيام بسبب معدل نموها السريع، بسبب مدة هطول الأمطار المحدودة.

أشجار أو شجيرات فريثوفيلية (جذور >5 م عمق)
رافعة هيدروليكية عندما تصل إلى المياه الجوفية
معدل التبخر العالي
تحمل نقص المياه عندما تكون صغيرًا
يستخدم المياه من طبقة المياه الجوفية العميقة
يعتمد على هطول الأمطار عندما يكون صغيرًا، وبعد ذلك يعتمد على مستوى المياه الجوفية
التكيف الأسموزي في الجذور والأوراق عندما تكون صغيرة

أشجار عميقة الجذور، نخل أو شجيرات (جذور < 5 م عمق)
رفع هيدروليكي من الطبقات العميقة إلى الطبقات الضحلة
معدل التبخر العالي
تحمل نقص المياه
يستخدم الماء من التربة العميقة ومن مستوى المياه فقط إذا كان ضحلًا. يعتمد على هطول الأمطار ويمكنه العيش بالقرب من المسطحات المائية السطحية أو حيث يكون مستوى المياه ضحلًا.
التكيف الأسموزي في الأوراق والجذور

عابرات
معدل التمثيل الضوئي ومعدل النتح مرتفع جداً
جذور سطحية صغيرة، أوراق عشبية، إثمار سريع ووفير
الهروب إلى نقص المياه
يستخدم الماء من سطح التربة
يعتمد ذلك على هطول الأمطار أو الفيضانات أو ذوبان الثلوج
التكيف الأسموزي في الأوراق والجذور
ندرة ناتجة عن جفاف مطول (هولتين وآخرون، 2003أ، ب، 2004؛ شولتز وآخرون، 2007، 2008، 2010؛ بارون-غافورد وآخرون، 2017). هذه الاستراتيجية تدعم الاستقرار على المدى الطويل للمجتمع المختلط.
الهياكل في البيئات الجافة وذات العناصر الغذائية القليلة. هذه الهياكل المتباينة لها تداعيات على وظائف الجذور الأخرى، والتي سنناقشها بشكل أعمق في هذا الاستعراض.
مخزونات الكربون والعناصر الغذائية في النظم البيئية الصحراوية
تقليل تآكل التربة وفقدان الكربون (بالاز et al.، 2022). على الرغم من أن الكتلة الحيوية لجذور الصبار والنباتات العصارية منخفضة، إلا أنها تظهر أوقات دوران ممتدة بمجرد تثبيت الكربون من خلال عملية التمثيل الضوئي. تظهر نباتات القيامة، والأعشاب المعمرة، والأعشاب كتلة حيوية قصيرة العمر للأجزاء الهوائية بينما تحافظ على دوران جذري أبطأ بسبب عمر جذورها الأطول. حتى
جدول لتعزيز تحلل المادة العضوية وامتصاص العناصر الغذائية بالقرب من سطح التربة (هولتين وآخرون، 2003أ، ب). يمكن أن تتلقى الأعشاب ذات الجذور الضحلة ما يصل إلى
تظهر الأحياء البيولوجية كميزة رئيسية في النظم البيئية الجافة، حيث تساهم بشكل كبير في تراكم المغذيات والمياه وتعزز التسلسل الإيجابي في النظم البيئية الصحراوية.
أثر تغيير استخدام الأراضي على مجتمعات النباتات الصحراوية واحتياطيات الكربون والعناصر الغذائية
الرعي
يقلل بشكل كبير من الكتلة الحيوية ويقلل من القدرة على التعافي الكامل. بشكل عام، تنخفض مخزونات الكربون والعناصر الغذائية بسبب تقليل الكتلة الحيوية، ويمكن أن تؤدي ممارسات الرعي المفرط على المدى الطويل إلى تغيير هيكل المجتمع.
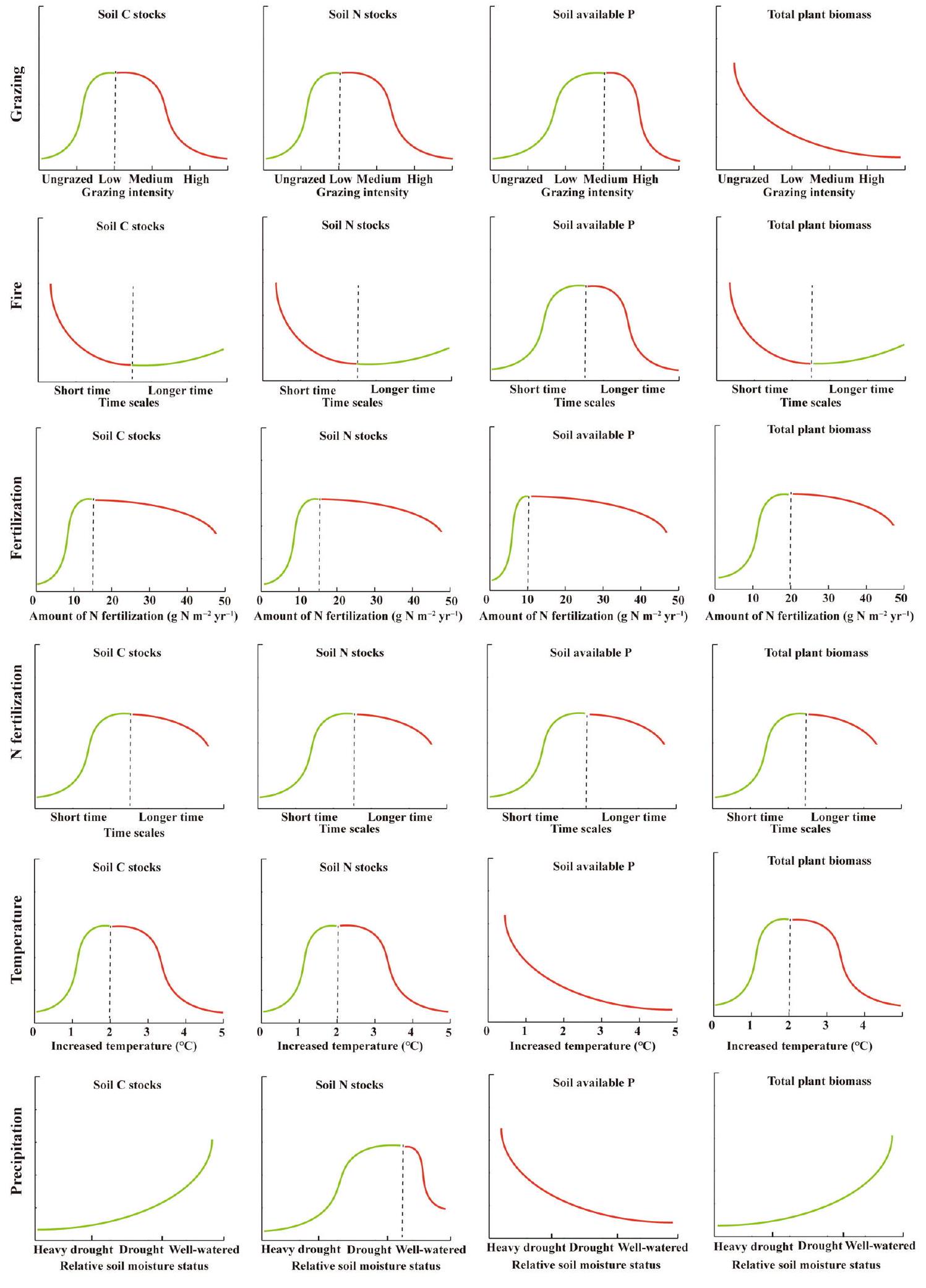
يجب أخذ وفرة وأنماط النمو في ظروف هطول الأمطار المت varyingة، بالإضافة إلى قابلية كل نوع للأكل، في الاعتبار. علاوة على ذلك، يجب مراعاة الديناميات الزمنية لمخزونات الكربون في الكتلة الحيوية فوق الأرض عند تقييم استخدامات الأراضي البشرية.
حصاد النباتات
خصوبة وتكوين وكتلة الكائنات الحية في التربة، فضلاً عن الحالة الغذائية للنباتات (الشكل 5). على سبيل المثال، أظهر تجربة طويلة الأمد استمرت 12 عامًا، أجريت في صحراء تكلامكان (غرب الصين)، حيث تم قطع نباتات الألهاجي سبارسيفوليا سنويًا، أن حصاد النباتات أدى إلى تغييرات في التركيب الكيميائي للتربة على طول عمود التربة (طارق وآخرون، 2022أ؛ الجدول S1). كما زادت هذه الممارسة من نشاط الميكروبات في التربة وأدت إلى تقليل التغذية الورقية العامة. وبالمثل، في نظام بيئي آخر من المراعي الجافة في ستيب باتاغونيا (أمريكا الجنوبية)، لم يؤثر الحصاد المكثف للأوراق بشكل ملحوظ على كتلة الجذور أو السيقان، لكنه كان له تأثير ملحوظ على تلقيح الجذور بواسطة الفطريات الميكوريزية، مما أدى إلى تقليل وجودها (توليدو وآخرون، 2022). يمكن أن يؤدي الانخفاض في الارتباط الميكوريزي، بسبب انخفاض كتلة الجذور، إلى إعاقة قدرة النباتات الصحراوية المعمرة على امتصاص الماء والمواد الغذائية. وذلك لأن الرابطة التبادلية بين الفطريات والجذور حيوية لتمديد فترة النمو إلى ما بعد موسم الأمطار. تؤكد هذه الدراسات على الدور الحاسم لتأثيرات إدارة النباتات على بيولوجيا التربة، والتي بدورها تؤثر على التغذية النباتية، مما يؤدي في النهاية إلى تغييرات في دورة الكربون والمواد الغذائية. تستحق هذه العوامل مزيدًا من الاستكشاف في الصحاري، حيث يمكن أن تكون توفر المواد الغذائية محدودًا. وهذا يجعل تأثير استخراج المواد الغذائية من خلال الحصاد أكثر وضوحًا عند مقارنته بأنظمة بيئية أخرى.
حرائق
تغذية النبات. على سبيل المثال، في صحراء تاكلماكان (غرب الصين)، حيث
التسميد
الري، مما يدل على قدرة الشجيرات الأكبر على امتصاص المياه من طبقات التربة الأعمق (درينوسكي وريتشاردز، 2004). في صحراء سونورا (أمريكا الشمالية)، زادت تركيزات النيتروجين في الأوراق مع تخصيب النيتروجين، ولكن في السنوات الأكثر رطوبة، كانت إنتاجية الكتلة الحيوية أعلى، مما أدى إلى انخفاض في تركيز النيتروجين بسبب تخصيص أكبر لإنتاج الكتلة الحيوية للأوراق (هال وآخرون، 2011). وهذا يسمح بتراكم العناصر الغذائية في الأنسجة خلال السنوات الرطبة. وبالمثل، في تجربة تخصيب أجريت في مجتمعات عابرة في صحراء تشيهواهوا، زادت ثلاثة أنواع سنوية شتوية فقط من تغطية السطح عندما تم تخصيبها بالنيتروجين أو الكبريت وتم ريها، بينما لم يُلاحظ أي استجابة ذات مغزى في المواقع المعتمدة على الأمطار (لودفيغ وآخرون، 1989). وهذا يوضح أن الاستجابات لزيادة هطول الأمطار وترسيب العناصر الغذائية أو التخصيب تعتمد بشكل كبير على الأنواع والمواقع، ولا يمكن التنبؤ بسهولة بتكوين وإنتاجية الصحاري. وبالتالي، حيث أن المياه تحد بشكل كبير من حركة المعادن في التربة الجافة وامتصاص النباتات للمياه والعناصر الغذائية في الأراضي الجافة، من الضروري اختبار استجابات الأنواع الصحراوية في بيئاتها المحددة، حيث لا يمكن تطبيق النتائج من البيئات الرطبة بسهولة على الصحاري.
آثار التأثيرات غير المباشرة لتغير المناخ: الفائزون والخاسرون من النباتات والعواقب
عمق مستوى المياه ونضوب المياه الجوفية
تحت الأشجار في واحة ذات محتوى مائي مرتفع (تاريخ وآخرون، 2022ب). الأنواع ذات المرونة الظاهرية العالية والموائل البيئية الواسعة، مثل A. sparsifolia، قد تؤدي بشكل أفضل تحت تغير المناخ مقارنة بالأنواع ذات المتطلبات البيئية الضيقة.
ارتفاع درجات الحرارة
تغير في هطول الأمطار
أنسجة النسيج، وسمك القشرة (للحفاظ على احتباس المياه وكفاءة التمثيل الضوئي)، ومعدل نمو الجذور، والتوصيل الهيدروليكي للخشب، لكن زيادة ضغط الجفاف وعمق المياه الجوفية لم تكن مواتية للتطور (تشوانغ وتشين، 2006؛ الجدول S1).
نباتات الفريثوفيت والنباتات ذات الجذور العميقة زادت من تخصيصها للأجزاء الهوائية، بينما خصصت الأنواع ذات الجذور الضحلة المزيد للجذور، مستفيدة من المياه الإضافية لاستكشاف التربة بشكل أفضل. كما تظهر أنواع أخرى اختلافات في تخصيص الجذور عبر موائل مختلفة، مما يبرز الفجوة بين أنظمة الجذور العميقة والضحلة. على سبيل المثال، تمتلك Prosopis flexuosa التي تنمو في الكثبان الجافة جذورًا أعمق من نفس النوع الذي ينمو في الوديان الأكثر رطوبة (Guevara et al., 2010). وبالمثل، تعتمد تجمعات Argania spinosa من المناطق الساحلية ذات معدلات الأمطار السنوية الأعلى أقل على الجذور العميقة مقارنة بالتجمعات من المواقع الداخلية الأكثر جفافًا (Zunzunegui et al., 2018). مثال مثير للاهتمام هو A. sparsifolia، وهو فريثوفيت يتطور
عواصف رملية
وجهات نظر للبحوث المستقبلية
(1) تتأثر النظم البيئية الصحراوية بتفاعلات متعددة الأوجه بين المناخ والتربة ومجتمعات النباتات. يمكن أن يكون تفاعل هذه العوامل معقدًا وصعبًا لفهمه بالكامل.
(2) الدراسات طويلة الأمد التي تشمل دورات مناخية متعددة ضرورية لفهم مرونة وتكيف مجتمعات النباتات الصحراوية على مر الزمن.
(3) بينما تركز هذه المراجعة على التغيرات المناخية والتغيرات الناتجة عن الأنشطة البشرية، يمكن أن تؤثر عوامل أخرى مثل خصائص التربة والعمليات الجيولوجية أيضًا على دورة الكربون والعناصر الغذائية.
(4) على الرغم من أن التقنيات المتقدمة تحمل وعودًا، إلا أن تطبيقها على النظم البيئية الصحراوية لا يزال يتطور، ولا يزال من غير المعروف إمكاناتها الكاملة في التنبؤ باستجابة النباتات. كانت الدراسات التقليدية لهندسة الجذور تتضمن الحفر والتحليل الدقيق للجذور، ويمكن أن تساعد التقنيات المتقدمة مثل تصوير المقاومة الكهربائية والمحللات ثلاثية الأبعاد في التنبؤ باستجابة النباتات الصحراوية لتغير المناخ. هيكل المجتمع وخصائص النباتات ضرورية لفهم مرونة النظم البيئية الصحراوية تجاه تغير المناخ والإدارة، مما يؤثر على أدوارها في دورة المغذيات.
الاستنتاجات
التوسع، كفاءة استخدام المياه، وتحريك المغذيات. يصبح الرفع الهيدروليكي حاسمًا بمجرد أن تصل جذور النباتات إلى المياه الجوفية، مما يعزز امتصاص المغذيات بالقرب من سطح التربة ويساعد النباتات ذات الجذور الضحلة في الحصول على المياه والمغذيات.
شكر وتقدير
المصالح المتنافسة
مساهمات المؤلفين
الأدب. نظم كل من AT وFZ وCG وJS وACH وJP المعلومات وهيكلوها. قام جميع المؤلفين بمراجعة والمساهمة في نص المخطوطة.
أوركيد
سكندر علي (د)https://orcid.org/0000-0001-9018-2837
يانجو قاو (د)https://orcid.org/0000-0003-1867-1454
كورينا غراسيانو (دhttps://orcid.org/0000-0003-0803-4128
أليس سي. هيوز (دhttps://orcid.org/0000-0002-0675-7552
جوزيب بينويلاس (د)https://orcid.org/0000-0002-7215-0150
جوردي ساردانس (دhttps://orcid.org/0000-0003-2478-0219
أكاش طارق (د)https://orcid.org/0000-0002-5382-9336
عبد الله (دhttps://orcid.org/0000-0003-1570-0176
فانجيانغ زينغ (ديhttps://orcid.org/0000-0003-4209-6971
References
Alon A, Steinberger Y. 1999. Effect of nitrogen amendments on microbial biomass, above-ground biomass and nematode population in the Negev Desert soil. Journal of Arid Environments 41: 429-441.
Alsharif W, Saad MM, Hirt H. 2020. Desert microbes for boosting sustainable agriculture in extreme environments. Frontiers in Microbiology 11: 1666.
An H, Li G. 2015. Effects of grazing on carbon and nitrogen in plants and soils in a semiarid desert grassland, China. Journal of Arid Land 7: 341-349.
Andresen LC, Müller C, de Dato G, Dukes JS, Emmett BA, Estiarte M, Jentsch A, Kröel-Dulay G, Lüscher A, Niu S et al. 2016. Shifting impacts of climate change: long-term patterns of plant response to elevated
Apple ME. 2010. Aspects of mycorrhizae in desert plants. In: Ramawat KG, ed. Desert plants. Berlin, Heidelberg, Germany: Springer Berlin Heidelberg, 121134.
Arndt SK, Kahmen A, Arampatsis C, Popp M, Adams M. 2004b. Nitrogen fixation and metabolism by groundwater-dependent perennial plants in a hyperarid desert. Oecologia 141: 385-394.
Bacilio M, Vazquez P, Bashan Y. 2011. Water versus spacing: a possible growth preference among young individuals of the giant cardon cactus of the Baja California Peninsula. Environmental and Experimental Botany 70: 29-36.
Balazs KR, Munson SM, Butterfield BJ. 2022. Functional composition of plant communities mediates biomass effects on ecosystem service recovery across an experimental dryland restoration network. Functional Ecology 36: 2317-2330.
Barger NN, Weber B, Garcia-Pichel F, Zaady E, Belnap J. 2016. Patterns and controls on nitrogen cycling of biological soil crusts. In: Weber B, Büdel B, Belnap J, eds. Biological soil crusts: an organizing principle in drylands. Ecological studies, vol. 226. Cham, Switzerland: Springer, 273-302.
Barnes PW, Throop HL, Archer SR, Breshears DD, McCulley RL, Tobler MA. 2015. Sunlight and soil-litter mixing: drivers of litter decomposition in drylands. In: Lüttge U, Beyschlag W, eds. Progress in botany. Progress in botany. Cham, Switzerland: Springer International, 273-302.
Barron-Gafford GA, Sanchez-Cañete EP, Minor RL, Hendryx SM, Lee E, Sutter LF, Tran N, Parra E, Colella T, Murphy PC et al. 2017. Impacts of hydraulic redistribution on grass-tree competition vs facilitation in a semi-arid savanna. New Phytologist 215: 1451-1461.
Bates TR, Lynch JP. 1996. Stimulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability. Plant, Cell & Environment 19: 529-538.
Bell LW, Sparling B, Tenuta M, Entz MH. 2012. Soil profile carbon and nutrient stocks under long-term conventional and organic crop and alfalfa-crop rotations and re-established grassland. Agriculture, Ecosystems & Environment 158: 156-163.
Bhanot V, Fadanavis SV, Panwar J. 2021. Revisiting the architecture, biosynthesis and functional aspects of the plant cuticle: there is more scope. Environmental and Experimental Botany 183: 104364.
Biruk LN, Fernández ME, González CV, Guevara A, Rovida-Kojima E, Giordano CV. 2022. High and diverse plastic responses to water availability in four desert woody species of South America. Trees 36: 1881-1894.
Bodí MB, Martin DA, Balfour VN, Santín C, Doerr SH, Pereira P, Cerdà A, Mataix-Solera J. 2014. Wildland fire ash: production, composition and eco-hydro-geomorphic effects. Earth-Science Reviews 130: 103-127.
Bunn RA, Simpson DT, Bullington LS, Lekberg Y, Janos DP. 2019. Revisiting the ‘direct mineral cycling’ hypothesis: arbuscular mycorrhizal fungi colonize leaf litter, but why? The ISME Journal 13: 1891-1898.
Carrera AL, Bertiller MB, Larreguy C. 2008. Leaf litterfall, fine-root production, and decomposition in shrublands with different canopy structure induced by grazing in the Patagonian Monte, Argentina. Plant and Soil 311: 39-50.
Chapin FS III, Matson PA, Vitousek P. 2011. Principles of terrestrial ecosystem ecology. New York, NY, USA: Springer.
Chen S, Wang W, Xu W, Wang Y, Wan H, Chen D, Tang Z, Tang X, Zhou G, Xie Z et al. 2018. Plant diversity enhances productivity and soil carbon storage. Proceedings of the National Academy of Sciences, USA 115: 4027-4032.
Chong PF, Li HY, Li Y. 2015. Physiological responses of seedling roots of the desert plant Reaumuria soongorica to drought stress. Acta Prataculturae Sinica 24: 72-80.
Collins SL, Ladwig LM, Petrie MD, Jones SK, Mulhouse JM, Thibault JR, Pockman WT. 2017. Press-pulse interactions: effects of warming, N deposition, altered winter precipitation, and fire on desert grassland community structure and dynamics. Global Change Biology 23: 1095-1108.
Cooper DJ, Sanderson JS, Stannard DI, Groeneveld DP. 2006. Effects of longterm water-table drawdown on evapotranspiration and vegetation in an arid region phreatophyte community. Journal of Hydrology 325: 21-34.
Copeland SM, Bradford JB, Hardegree SP, Schlaepfer DR, Badik KJ. 2023. Management and environmental factors associated with simulated restoration seeding barriers in sagebrush steppe. Restoration Ecology 31: e13722.
Cui H, Fan M, Wang Y, Zhang X, Xu W, Li Y, Song W, Ma JY, Sun W. 2023. Impacts of mowing and N addition on soil organic phosphorus mineralization rates in a semi-natural grassland in Northeast China. Plant and Soil 482: 7-23.
DeFalco LA, Esque TC, Scoles-Sciulla SJ, Rodgers J. 2010. Desert wildfire and severe drought diminish survivorship of the long-lived Joshua tree (Yucca brevifolia; Agavaceae). American Journal of Botany 97: 243-250.
Deng L, Shangguan Z, Bell SM, Soromotin AV, Peng C, An S, Wu X, Xu X, Wang K, Li J et al. 2023. Carbon in Chinese grasslands: meta-analysis and theory of grazing effects. Carbon Research 2: 19.
Drenovsky RE, Richards JH. 2004. Critical N:P values: predicting nutrient deficiencies in desert shrublands. Plant and Soil 259: 59-69.
Dubrovsky JG, Shishkova S. 2013. Developmental adaptations in roots of desert plants with special emphasis on cacti. In: Eshel A, Beeckman T, eds. Plant roots: the hidden half,
Dubrovsky JG, North GB. 2002. Root structure and function. In: Nobel PS, ed. Cacti biology and uses. Oakland, CA, USA: University of California Press, 290.
Elbert W, Weber B, Burrows S, Steinkamp J, Büdel B, Andreae MO, Pöschl U. 2012. Contribution of cryptogamic covers to the global cycles of carbon and nitrogen. Nature Geoscience 5: 459-462.
Escolano JJ, Navarro Pedreño J, Gomez Lucas I, Almendro MB, Zorpas AA. 2018. In: Muñoz MA, Zornoza R, eds. Chapter 1 – Decreased organic carbon associated with land management in Mediterranean environments soil management and climate change: effects of organic carbon, nitrogen dynamics, and greenhouse gas emissions. Berkeley, CA, USA: Elsevier, 1-13.
Esque TC, Medica PA, Shryock DF, DeFalco LA, Webb RH, Hunter RB. 2015. Direct and indirect effects of environmental variability on growth and survivorship of pre-reproductive Joshua trees, Yucca brevifolia Engelm. (Agavaceae). American Journal of Botany 102: 85-91.
Feng S, Fu Q. 2013. Expansion of global drylands under a warming climate. Atmospheric Chemistry and Physics 13: 10081-10094.
Gao Y, Tian J, Pang Y, Liu J. 2017. Soil inorganic carbon sequestration following afforestation is probably induced by pedogenic carbonate formation in NorthwestChina. Frontiers in Plant Sciences 8: 1282.
Gao Y, Zhang Z, Zeng F, Ma X. 2023. Root morphological and physiological traits are committed to the phosphorus acquisition of the desert plants in phosphorusdeficient soils. BMC Plant Biology 23: 188.
Geng DM, Shan LS, Li Y. 2014. Effect of soil water stress on fine root morphology and functional characteristics of Reaumuria Soongorica. Bulletin of Soil and Water Conservation 36: 36-42.
Geng M, Wang X, Liu X, Lv P. 2023. Effects of grazing exclusion on microbial community diversity and soil metabolism in desert grasslands. Sustainability 15: 11263.
Guevara A, Giordano CV, Aranibar J, Quiroga M, Villagra PE. 2010. Phenotypic plasticity of the coarse root system of Prosopis flexuosa, a phreatophyte tree, in the Monte Desert (Argentina). Plant and Soil 330: 447-464.
Gurrero-campo J, Palacio S, Pérez-Rontomé C, Montserrat-Martí G. 2006. Effect of root system morphology on root sprouting and shoot-rooting abilities in 123 plant species from eroded lands in North-east Spain. Annals of Botany 98: 439447.
Harrower JT, Gilbert GS. 2021. Parasitism to mutualism continuum for Joshua trees inoculated with different communities of arbuscular mycorrhizal fungi from a desert elevation gradient. PLoS ONE 16: e0256068.
Hein L, De Ridder N. 2006. Desertification in the Sahel: a reinterpretation. Global Change Biology 12: 751-758.
Holzapfel C. 2008. Deserts. In: Jørgensen SE, Fath BD, eds. Encyclopedia of ecology, vol. 2. Oxford, UK: Elsevier, 879-898.
Horn KJ, Wilkinson J, White S, St. Clair SB. 2015. Desert wildfire impacts on plant community function. Plant Ecology 216: 1623-1634.
Houghton RA, Hall F, Goetz SJ. 2009. Importance of biomass in the global carbon cycle. Journal of Geophysical Research Biogeosciences 114: G00E03.
Huang F, Zhang D, Chen X. 2019. Vegetation response to groundwater variation in arid environments: visualization of research evolution, synthesis of response types, and estimation of groundwater threshold. International Journal of Environmental Research and Public Health 16: 1849.
Hukin D, Cochard H, Dreyer E, Le Thiec D, Bogeat-Triboulot MB. 2005. Cavitation vulnerability in roots and shoots: does Populus euphratica Oliv., a poplar from arid areas of Central Asia, differ from other poplar species? Journal of Experimental Botany 56: 2003-2010.
Hultine KR, Cable WL, Burgess SSO, Williams DG. 2003a. Hydraulic redistribution by deep roots of a Chihuahuan Desert phreatophyte. Tree Physiology 23: 353-360.
Hultine KR, Dettman DL, Williams DG, Puente R, English NB, Butterfield BJ, Búrquez A. 2018. Relationships among climate, stem growth, and biomass
Hultine KR, Scott RL, Cable WL, Goodrich DC, Williams DG. 2004. Hydraulic redistribution by a dominant, warm-desert phreatophyte: seasonal patterns and response to precipitation pulses. Functional Ecology 18: 530-538.
Hultine KR, Williams DG, Burgess SSO, Keefer TO. 2003b. Contrasting patterns of hydraulic redistribution in three desert phreatophytes. Oecologia 135: 167175.
Kell DB. 2011. Breeding crop plants with deep roots: their role in sustainable carbon, nutrient and water sequestration. Annals of Botany 108: 407-418.
Kidron GJ, Starinsky A. 2019. Measurements and ecological implications of nonrainfall water in desert ecosystems-a review. Ecohydrology 12: e2121.
Kim H, Kim K, Lee SJ. 2018. Hydraulic strategy of cactus root-stem junction for effective water transport. Frontiers in Plant Science 9: 799.
Kirschner GK, Xiao TT, Blilou I. 2021. Rooting in the desert: a developmental overview on desert plants. Genes 12: 709.
Ladwig LM, Collins SL, Swann AL, Xia Y, Allen MF, Allen EB. 2012. Above- and belowground responses to nitrogen addition in a Chihuahuan Desert grassland. Oecologia 169: 177-185.
Lasché SN, Schroeder RWR, McIntosh MM, Lucero JE, Spiegal SA, Funk MP, Beck RF, Holechek JL, Faist AM. 2023. Long-term growing season aridity and grazing seasonality effects on perennial grass biomass in a Chihuahuan Desert rangeland. Journal of Arid Environments 209: 104902.
Li B, Wang L, Kaseke KF, Li L, Seely MK. 2016. The impact of rainfall on soil moisture dynamics in a foggy desert. PLoS ONE 11: e0164982.
Li L, Gao X, Gui D, Liu B, Zhang B, Li X. 2017. Stoichiometry in aboveground and fine roots of Seriphidium korovinii in desert grassland in response to artificial nitrogen addition. Journal of Plant Research 130: 689-697.
Liu S, Xu G, Chen T, Wu X, Li Y. 2023. Quantifying the effects of precipitation exclusion and groundwater drawdown on functional traits of Haloxylon ammodendron – how does this xeric shrub survive the drought? Science of the Total Environment 904: 166945.
Liu Z, Liu K, Shi X, Ryan Lock T, Kallenbach RL, Yuan Z. 2022. Changes in grassland phenology and growth rate, rather than diversity, drive biomass production after fire. Agricultural and Forest Meteorology 322: 109028.
Lu J, Feng S, Wang S, Zhang B, Ning Z, Wang R, Chen X, Yu L, Zhao H, Lan D et al. 2023. Patterns and driving mechanism of soil organic carbon, nitrogen, and phosphorus stoichiometry across northern China’s desert-grassland transition zone. Catena 220: 106695.
Lu Y, Liu H, Chen Y, Zhang L, Kudusi K, Song J. 2022. Effects of drought and salt stress on seed germination of ephemeral plants in desert of northwest China. Frontiers in Ecology and Evolution 10: 1026095.
Ludwig JA, Whitford WG, Cornelius JM. 1989. Effects of water, nitrogen and sulfur amendments on cover, density and size of Chihuahuan Desert ephemerals. Journal of Arid Environments 16: 35-42.
Lynch J. 1995. Root architecture and plant productivity. Plant Physiology 109: 7-13.
Lynch J, Marschner P, Rengel Z. 2012. Effect of internal and external factors on root growth and development. In: Marschner’s mineral nutrition of higher plants. London, UK: Academic Press, 331-346.
Lynch JP. 2007. Roots of the Second Green revolution. Australian Journal of Botany 55: 493.
Lynch JP. 2019. Root phenotypes for improved nutrient capture: an underexploited opportunity for global agriculture. New Phytologist 223: 548-564.
Lynch JP. 2022. Harnessing root architecture to address global challenges. The Plant Journal 109: 415-431.
Ma Q, Li Y, Zhu Y, Liu X, Yu H, Li L, Qi M, Sun H, Yin Z, Wang Y et al. 2022. Precipitation variations, rather than N deposition, determine plant ecophysiological traits in a desert steppe in Northern China. Ecological Indicators 141: 109144.
Maeght JL, Rewald B, Pierret A. 2013. How to study deep roots – and why it matters. Frontiers of Plant Science 4: 299.
Maestre FT, Bagousse-Pinguet YL, Delgado-Baquerizo M, Eldrige DJ, Saiz H, Berdugo M, Gozalo B, Ochoa V, Guirado E, Garcia- Gomez M et al. 2022. Grazing and ecosystem service delivery in global drylands. Science 378: 915920.
Manning DAC. 2008. Biological enhancement of soil carbonate precipitation: passive removal of atmospheric
Marks E, Aflakpul GKS, Nkem J, Poch RM, Khouma M, Kokou K, Sagoe R, Sebastià MT. 2008. Coservation of soil organic carbon, biodiversity and the provision of other ecosystems services along climatic gradients in West Africa. Biogeosciences Discussions 5: 4413-4452.
Matos IS, Binks O, Eller CB, Zorger BB, Meir P, Dawson TE, Rosado BHP. 2022. Revisiting plant hydrological niches: the importance of atmospheric resources for ground-rooted plants. Journal of Ecology 110: 1746-1756.
Maurice K, Laurent-Webb L, Dehail A, Bourceret A, Boivin S, Boukcim H, Selosse M-A, Ducousso M. 2023. Fertility islands, keys to the establishment of plant and microbial diversity in a highly alkaline hot desert. Journal of Arid Environments 219: 105074.
McKenna DM, Grams SE, Barasha M, Antoninka AJ, Johnson NC. 2022. Organic and inorganic soil carbon in a semi-arid rangeland is primarily related to abiotic factors and not livestock grazing. Geoderma 419: 115844.
Miao L, Li S, Zhang F, Chen T, Shan Y, Zhang Y. 2020. Future drought in the dry lands of asia under the 1.5 and
Mohanta K, Mohanta YK, Kaushik P, Kumar J. 2023. Physiology, genomics, and evolutionary aspects of desert plants. Journal of Advanced Research, in press. doi: 10.1016/j.jare.2023.04.019.
Nara K. 2006. Ectomycorrhizal networks and seedling establishment during early primary succession. New Phytologist 169: 169-178.
Nevo E. 2012. “Evolution Canyon,” a potential microscale monitor of global warming across life. Proceedings of the National Academy of Sciences, USA 109: 2960-2965.
Nilsen ET, Sharifi MR, Rundel PW. 1984. Comparative water relations of phreatophytes in the Sonoran Desert of California. Ecology 65: 767-778.
Nobel PS, ed. 2002. Cacti biology and uses. Oakland, CA, USA: University of California Press.
Otieno DO, Schmidt MWT, Kinyamario JI, Tenhunen J. 2005. Responses of Acacia tortilis and Acacia xanthophloea to seasonal changes in soil water availability in the savanna region of Kenya. Journal of Arid Environments 62: 377-400.
Paula S, Pausas JG. 2011. Root traits explain different foraging strategies between resprouting life strategies. Physiological Ecology 165: 321-331.
Peek MS, Forseth IN. 2003. Enhancement of photosynthesis and growth of an aridland perennial in response to soil nitrogen pulses generated by mule deer. Environmental and Experimental Botany 49: 169-180.
Pellegrini AFA, Harden J, Georgiou K, Hemes KS, Malhotra A, Nolan CJ, Jackson RB. 2022. Fire effects on the persistence of soil organic matter and long-term carbon storage. Nature Geoscience 15: 5-13.
Pérez ALS, Camargo-Ricalde SL, Montaño NM, García-Oliva F, Alarcón A, Montaño-Arias SA, Esperón-Rodríguez M. 2016. Biocrusts, inside and outside resource islands of Mimosa luisana (Leguminosae), improve soil carbon and nitrogen dynamics in a tropical semiarid ecosystem. European Journal of Soil Biology 74: 93-103.
Predick KI, Archer SR, Aguillon SM, Keller DA, Throop HL, Barnes PW. 2018. UV-B radiation and shrub canopy effects on surface litter decomposition in a shrub-invaded dry grassland. Journal of Arid Environments 157: 13-21.
Principe A, Nunes A, Pinho P, do Rosário L, Correira O, Branquinho C. 2014. Modeling the long-term natural regeneration potential of woodlands in semi-arid regions to guide restoration efforts. European Journal of Forest Research 133: 757767.
Rodriguez-Caballero E, Belnap J, Buedel B, Crutzen PJ, Andreae MO, Pöschl U, Weber B. 2018. Dryland photoautotrophic soil surface communities endangered by global change. Nature Geoscience 11: 185-189.
Roncero-Ramos B, Román JR, Acién G, Cantón Y. 2022. Towards large scale biocrust restoration: Producing an efficient and low-cost inoculum of N-fixing cyanobacteria. Science of the Total Environment 848: 157704.
Rüger L, Ganther M, Freudenthal J, Jansa J, Heintz-Buschart A, Tarkka MT, Bonkowski M. 2023. Root cap is an important determinant of rhizosphere microbiome assembly. New Phytologist 239: 1434-1448.
Salo LF, McPherson GR, Williams DG. 2005. Sonoran desert winter annuals affected by density of red brome and soil nitrogen. The American Midland Naturalist 153: 95-109.
Šantrůček J. 2022. The why and how of sunken stomata: does the behaviour of encrypted stomata and the leaf cuticle matter? Annals of Botany 130: 285-300.
Sardans J, Peñuelas J. 2012. The role of plants in the effects of global change on nutrient availability and stoichiometry in the plant-soil system. Plant Physiology 160: 1741-1761.
Saul-Tcherkas V, Steinberger Y. 2009. Temporal and shrub adaptation effect on soil microbial functional diversity in a desert system. European Journal of Soil Science 60: 871-882.
Schlesinger WH. 2017. An evaluation of abiotic carbon sinks in deserts. Global Change Biology 23: 25-27.
Scholz FG, Bucci SJ, Goldstein G, Meinzer FC, Franco AC, Miralles-Wilhelm F. 2007. Removal of nutrient limitations by long-term fertilization decreases nocturnal water loss in savanna trees. Tree Physiology 27: 551-559.
Scholz FG, Bucci SJ, Hoffmann WA, Meinzer FC, Goldstein G. 2010. Hydraulic lift in a Neotropical savanna: experimental manipulation and model simulations. Agricultural and Forest Meteorology 150: 629-639.
Scholz FG, Moreira MZ, Franco AC, Miralles-Wilhelm F. 2008. Biophysical and life-history determinants of hydraulic lift in Neotropical savanna trees. Functional Ecology 22: 773-786.
Shadwell E, February E. 2017. Effects of groundwater abstraction on two keystone tree species in an arid savanna national park. Peer 5: e2923.
Shishkova S, Dubrovsky JG. 2005. Developmental programmed cell death in primary roots of Sonoran desert cactaceae. American Journal of Botany 92: 15901594.
Singh G, Shukla S. 2011. Effects of Azadirachta indica canopy manipulation and nitrogen fertilization on diversity and productivity of herbaceous vegetation in an Arid Environment of India. Arid Land Research and Management 25: 128-149.
Snyman HA. 2006. Root distribution with changes in distance and depth of two-year-old cactus pears Opuntia ficus-indica and O. robusta plants. South African Journal of Botany 72: 434-441.
Spinoni J, Barbosa P, Cherlet M, Forzieri G, McCormick N, Naumann G, Vogt JV, Dosio A. 2021. How will the progressive global increase of arid areas affect population and land-use in the 21st century? Global and Planetary Change 205: 103597.
Su YG, Chen YW, Padilla FM, Zhang YM, Huang G. 2020. The influence of biocrusts on the spatial pattern of soil bacterial communities: a case study at landscape and slope scales. Soil Biology & Biochemistry 142: 107721.
Sweet LC, Green T, Heintz JGC, Frakes N, Graver N, Rangitsch JS, Rodgers JE, Heacox S, Barrows CW. 2019. Congruence between future distribution models and empirical data for an iconic species at Joshua Tree National Park. Ecosphere 10: e02763.
Tao Y, Shang T, Yan J, Hu Y, Zhao Y, Liu Y. 2022. Effects of sand burial depth on Xanthium spinosum seed germination and seedling growth. BMC Plant Biology 22: 43.
Tariq A, Ullah A, Sardans J, Zeng F, Graciano C, Li X, Wang W, Ahmed Z, Ali S, Zhang Z et al. 2022b. Alhagi sparsifolia: an ideal phreatophyte for combating desertification and land degradation. Science of the Total Environment 844 : 157228.
Toledo S, Fontenla SB, Peri PL. 2022. Effect of defoliation frequency on Rytidosperma Virescens plants and arbuscular mycorrhizal fungi colonization. Rangeland Ecology & Management 84: 1-9.
Ullah A, Tariq A, Zeng F, Sardans J, Graciano C, Ullah S, Chai X, Zhang Z, Keyimu M, Asghar MA et al. 2022. Phosphorous supplementation alleviates droughtinduced physio-biochemical damages in Calligonum mongolicum. Plants 11: 3054.
Valone TJ. 2003. Examination of interaction effects of multiple disturbances on an arid plant community. The Southwestern Naturalis 48: 481-490.
Vasar M, Davison J, Sepp S-K, Öpik M, Moora M, Koorem K, Meng Y, Oja J, Akhmetzhanova AA, Al-Quraishy S et al. 2021. Arbuscular mycorrhizal fungal communities in the soils of desert habitats. Microorganisms 9: 229.
Vikram S, Ramond J-B, Ortiz M, Maggs-Kölling G, Pelser K, Cowan DA. 2023. Soil fungal diversity and assembly along a xeric stress gradient in the central Namib Desert. Fungal Biology 127: 997-1003.
Wang L, Zhao C, Li J, Liu Z, Wang J. 2015. Root plasticity of Populus euphratica seedlings in response to different water-table depths and contrasting sediment types. PLoS ONE 10: e0118691.
Wang Q, Zhang Q, Han Y, Zhang D, Zhang CC, Hu C. 2022. Carbon cycle in the microbial ecosystems of biological soil crusts. Soil Biology and Biochemistry 171: 108729.
Wang Z, Jiang S, Struik PC, Wang H, Jin K, Wu R, Na R, Mu H, Ta N. 2023. Plant and soil responses to grazing intensity drive changes in the soil microbiome in a desert steppe. Plant and Soil 491: 219-237.
Wang Z, Zhao M, Yan Z, Yang Y, Niklas KJ, Huang H, Mipam TD, He X, Hu X, Wright SJ. 2022. Global patterns and predictors of soil microbial biomass carbon, nitrogen, and phosphorus in terrestrial ecosystems. Catena 211: 106037.
Xu H, Li Y, Xu G, Zou T. 2007. Ecophysiological response and morphological adjustment of two Central Asian desert shrubs towards variation in summer precipitation. Plant, Cell & Environment 30: 399-409.
Yang Y, Li T, Pokharel P, Liu L, Qiao J, Wang Y, An S, Chang SX. 2022. Global effects on soil respiration and its temperature sensitivity depend on nitrogen addition rate. Soil Biology and Biochemistry 174: 108814.
Yao J, Liu H, Huang J, Gao Z, Wang G, Li D, Yu H, Chen X. 2020. Accelerated dryland expansion regulates future variability in dryland gross primary production. Nature Communications 11: 1665.
Yin H, Tariq A, Zhang B, Lv G, Zeng F, Graciano C, Santos M, Zhang Z, Wang P, Mu S. 2021a. Coupling relationship of leaf economic and hydraulic traits of Alhagi sparsifolia Shap. in a hyper-arid desert ecosystem. Plants 10: 1867.
Yin H, Zheng H, Zhang B, Tariq A, Lv G, Zeng F, Graciano C. 2021b. Stoichiometry of
York LM, Nord EA, Lynch JP. 2013. Integration of root phenes for soil resource acquisition Integration of root phenes for soil resource acquisition. Frontiers in Plant Science 4: e355.
Young KE, Ferrenberg S, Reibold R, Reed SC, Swenson T, Northen T, DarrouzetNardi A. 2022. Vertical movement of soluble carbon and nutrients from biocrusts to subsurface mineral soils. Geoderma 405: 115495.
Yuan S, Tang H, Yan Y. 2009. Photosynthetic characteristics of spring ephemerals in the desert ecosystem of Dzungaria Basin, northwest China. Environmental Earth Sciences 59: 501-510.
Zeng F, Song C, Guo H, Liu B, Luo W, Gui D, Arndt S, Guo D. 2013. Responses of root growth of Alhagi sparsifolia Shap. (Fabaceae) to different simulated groundwater depths in the southern fringe of the Taklimakan Desert, China. Journal of Arid Land 5: 220-232.
Zhang B, Gao X, Li L, Lu Y, Shareef M, Huang C, Liu G, Gui D, Zeng F. 2018. Groundwater depth affects phosphorus but not carbon and nitrogen concentrations of a desert phreatophyte in northwest China. Frontiers in Plant Science 9: 1-10.
Zhang Y, Tariq A, Hughes AC, Hong D, Wei F, Sun H, Sardans J, Peñuelas J, Perry G, Qiao J et al. 2023. Challenges and solutions to biodiversity conservation in arid lands. Science of the Total Environment 857: 159695.
Zhang Z, Chai X, Tariq A, Zeng F, Graciano C, Li X, Gao Y, Ullah A. 2021a. Coordinated patterns in the allocation, composition, and variability of multiple elements among organs of two desert shrubs under nitrogen addition and drought. Journal of Soil Science and Plant Nutrition 22: 47-58.
Zhang Z, Shan L, Li Y. 2018. Prolonged dry periods between rainfall events shorten the growth period of the resurrection plant Reaumuria soongorica. Ecology and Evolution 8: 920-927.
Zhang Z, Tariq A, Fanjiang Z, Graciano C, Bo Z. 2020. Nitrogen application mitigates drought-induced metabolic changes in Alhagi sparsifolia seedlings by regulating nutrient and biomass allocation patterns. Plant Physiology and Biochemistry 155: 828-841.
Zhang Z, Tariq A, Zeng F, Chai X, Graciano C. 2021b. Involvement of soluble proteins in growth and metabolic adjustments of drought stressed Calligonum mongolicum seedlings under nitrogen addition. Plant Biology 23: 32-43.
Zhou L, Zhou X, He Y, Fu Y, Du Z, Lu M, Sun X, Li C, Lu C, Liu R et al. 2022. Global systematic review with meta-analysis shows that warming effects on terrestrial plant biomass allocation are influenced by precipitation and mycorrhizal association. Nature Communications 13: 4914.
Zhou X, Tao Y, Yin BF, Tucker C, Zhang YM. 2020a. Nitrogen pools in soil covered by biological soil crusts of different successional stages in a temperate desert in Central Asia. Geoderma 366: 114166.
Zhou X, Yue P, Cui X, Tao Y, Zhang Y, Liu X. 2020b. Impacts of nitrogen deposition on China’s desert ecosystems. In: Liu X, Du E, eds. Atmospheric reactive nitrogen in China. Singapore City, Singapore: Springer Singapore, 245-261.
Zhuang L, Chen Y. 2006. Physiological response of Tamarix ramosissima under water stress along the lower reaches of Tarim River. Chinese Science Bulletin 51: 1123-1129.
Zoccatelli D, Marra F, Armon M, Rinat Y, Smith JA, Morin E. 2019. Contrasting rainfall-runoff characteristics of floods in desert and Mediterranean basins. Hydrology and Earth System Sciences 23: 2665-2678.
المعلومات الداعمة
طرق S1 المنهجية.
ملاحظات حول مخزونات الكربون في النظم البيئية الصحراوية.
ملاحظات S3 الكائنات الحية في التربة المرتبطة بالجذور في الصحاري.
ملاحظات S4 التأثيرات غير الخطية وتأثيرات العتبة.
ملاحظات S5 استراتيجيات الشكلية للنباتات الصحراوية للتكيف مع البيئات الجافة.
DOI: https://doi.org/10.1111/nph.19676
PMID: https://pubmed.ncbi.nlm.nih.gov/38482544
Publication Date: 2024-03-14
Plant root mechanisms and their effects on carbon and nutrient accumulation in desert ecosystems under changes in land use and climate
Abstract
Akash Tariq
Aires, Argentina;
New Phytologist (2024) 242: 916-934 doi: 10.1111/nph. 19676
Abstract
Summary Deserts represent key carbon reservoirs, yet as these systems are threatened this has implications for biodiversity and climate change. This review focuses on how these changes affect desert ecosystems, particularly plant root systems and their impact on carbon and mineral nutrient stocks. Desert plants have diverse root architectures shaped by water acquisition strategies, affecting plant biomass and overall carbon and nutrient stocks. Climate change can disrupt desert plant communities, with droughts impacting both shallow and deep-rooted plants as groundwater levels fluctuate. Vegetation management practices, like grazing, significantly influence plant communities, soil composition, root microorganisms, biomass, and nutrient stocks. Shallow-rooted plants are particularly susceptible to climate change and human interference. To safeguard desert ecosystems, understanding root architecture and deep soil layers is crucial. Implementing strategic management practices such as reducing grazing pressure, maintaining moderate harvesting levels, and adopting moderate fertilization can help preserve plant-soil systems. Employing socio-ecological approaches for community restoration enhances carbon and nutrient retention, limits desert expansion, and reduces
Introduction
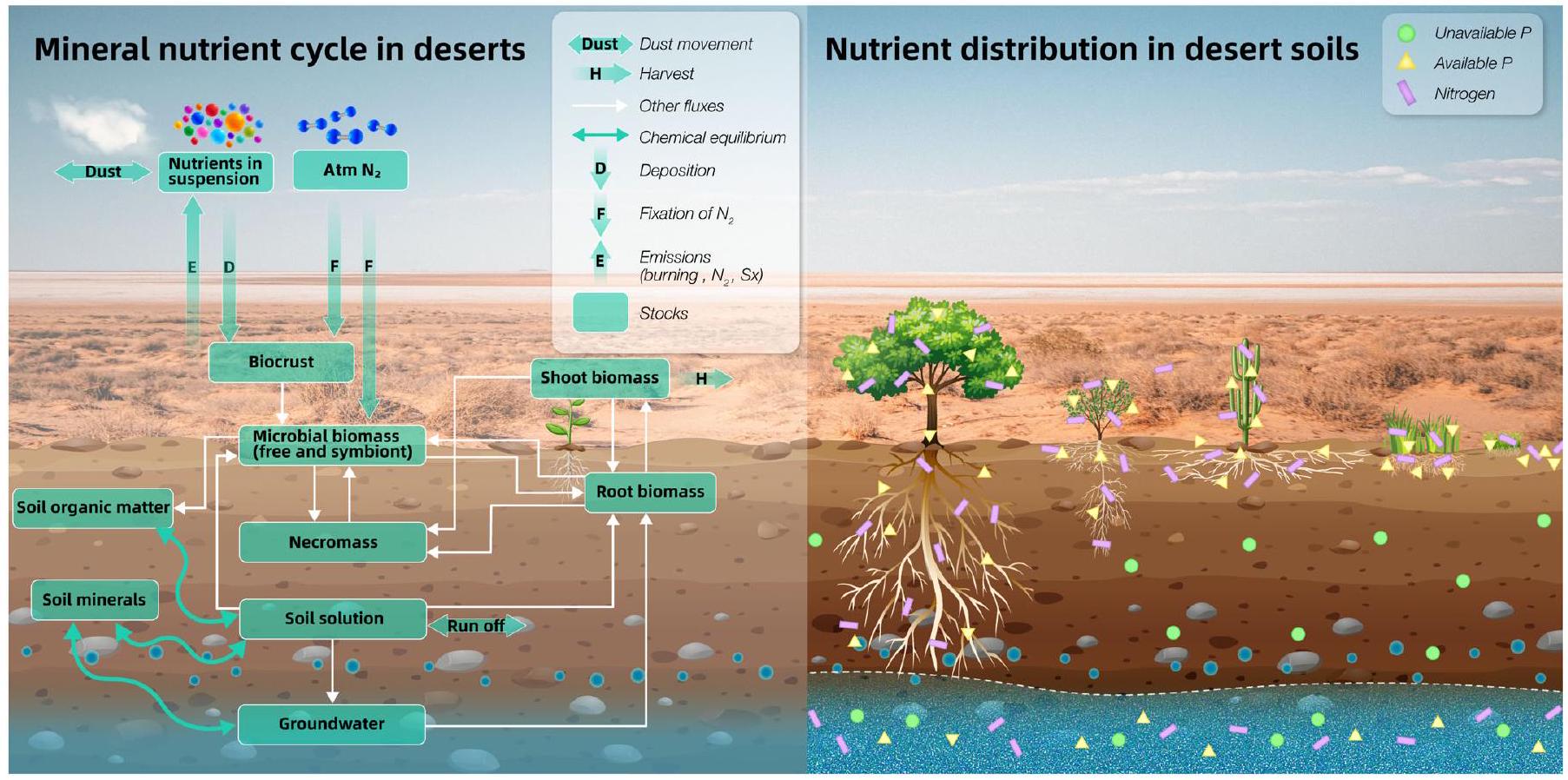
and crop wild relatives, often face threats and are frequently overlooked (Zhang et al., 2023). While arid ecosystems span a range of aridity levels, deserts represent the extreme end and are frequently marginalized from a conservation standpoint. Despite hosting fewer species than humid tropics (Safriel & Zafar, 2005), deserts exhibit high functional diversity and endemism. Consequently, the loss of species in these ecosystems can have a more pronounced impact than in wetter and species-rich regions (Maestre et al., 2021). Furthermore, restoring degraded desert ecosystems requires specialized strategies due to their limited capacity for regeneration and growth (Principe et al., 2014). As a result, desert ecosystems hold a unique position and necessitate significant attention due to their heightened vulnerability to climate change and human activities. Furthermore, desertification and the expansion of deserts continue to be viewed as problems, overshadowing the much more nuanced impacts of climate change on desert ecosystems, as well as the distinct impacts of various facets of environmental change on different plant communities. Plants and microorganisms in deserts suffer multiple abiotic stresses, mainly derived from long lapses of low water availability, soil salinity, low nutrient content and mobility, extreme temperatures, high irradiance, and frequent wind and sand storms (Alsharif et al., 2020). The biota in these regions heavily rely on intermittent water pulses that occur at irregular intervals, making rainfall a pivotal factor influencing plant growth and soil microorganismal activity (Saul-Tcherkas & Steinberger, 2009; Collins et al., 2017; Roncero-Ramos et al., 2022; Vikram et al., 2023). Additionally, geochemical cycles operate sluggishly in deserts due to their harsh and water-deprived environments, resulting in limited nutrient availability and mobility in the soil (Tariq et al., 2022a; Maurice et al., 2023). Consequently, diverse desert plant species exhibit unique morpho-physiological adaptations in their leaf, stem, and root architectures to ensure survival. However, while aboveground organs have been extensively studied due to their accessibility, adaptations of root systems and their consequences have largely been neglected (Alsharif et al., 2020; Kirschner et al., 2021). Water acquisition in desert plants primarily occurs through the soil, making distinct root architectures crucial for survival (Lynch, 2022). Root-system architecture (RSA) significantly influences water access, nutrient acquisition, carbon (C) sequestration, and overall plant function (Maeght et al., 2013). Thus, the characteristic root structure of desert plants, whether deep or shallow, impacts C and nutrient stocks, as they give access to groundwater or rainwater, respectively, resulting in differential responses to environmental changes. Recently, the role of groundwater in C storage in deserts has been explored (Li et al., 2016), but there remains a notable gap in understanding how RSA impacts nutrient and C accumulation in desert plant communities.
components (Kell, 2011). By contrast, aboveground biomass is more susceptible to land-use changes (such as fire, grazing, harvesting) and climate perturbations, resulting in increased C release into the atmosphere and exacerbating climate change. Additionally, reduced biomass density in deserts can be attributed to both environmental limitations (soil nutrients, seasonal precipitation and temperature distribution) and anthropogenic disturbances, further contributing to lower nutrient and C stocks in the plant-soil system (Houghton et al., 2009).
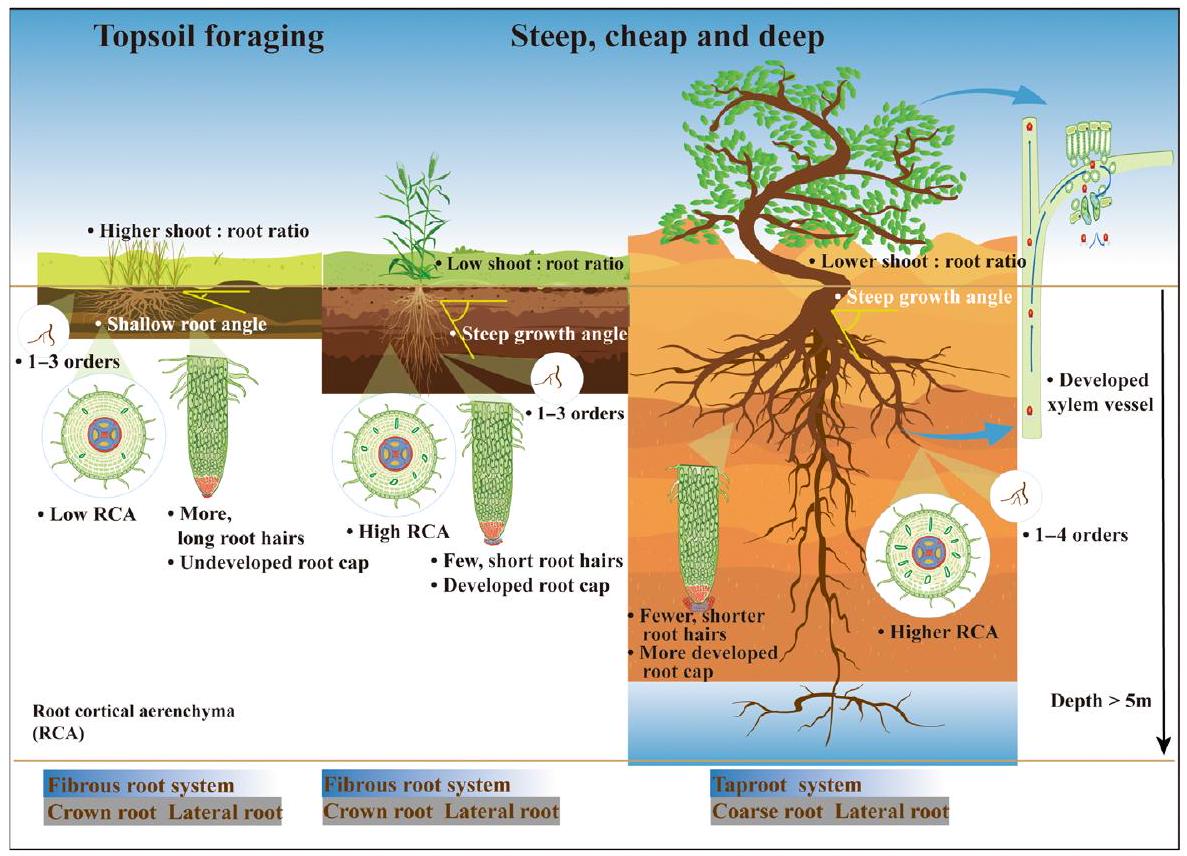
Plant root architecture in desert ecosystems
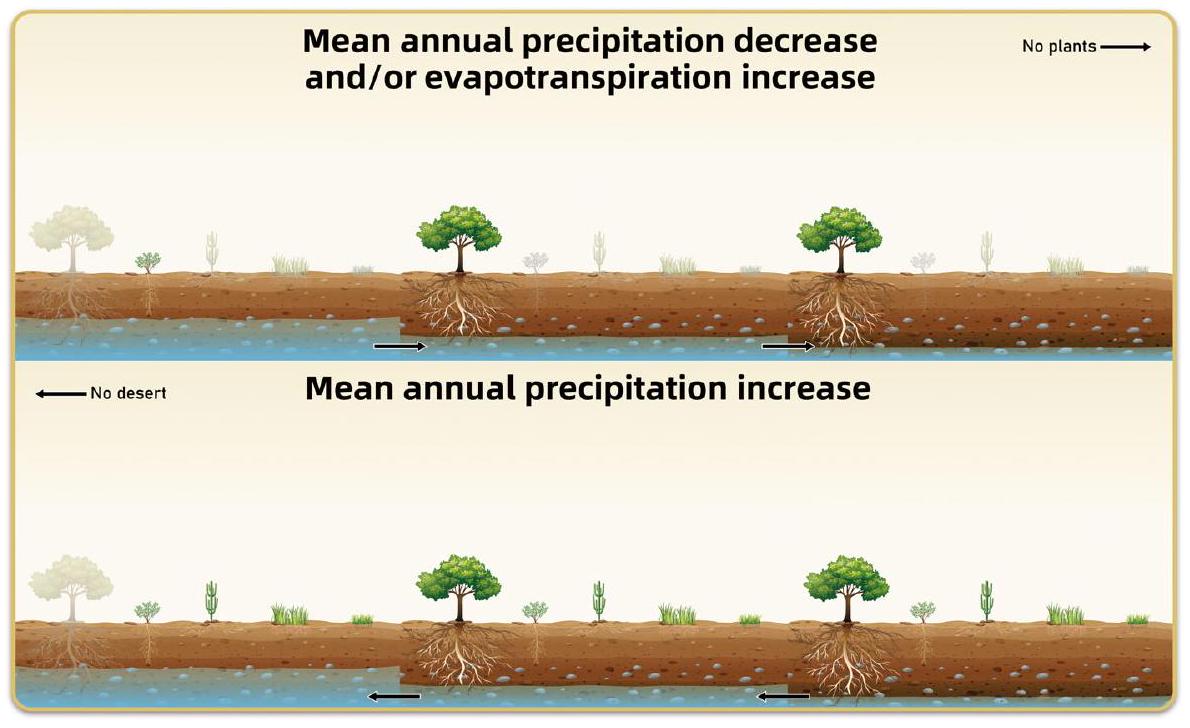
small regions. By contrast, cold deserts receive precipitation in the form of snow, blanketing the entire surface and providing liquid water to plants during the warm growth season (Fan et al., 2014). Dew is an additional water source for plants, particularly in coastal deserts, where it is absorbed by leaves and subsequently transported to drier stems and roots (Kidron & Starinsky, 2019). Moreover, desert plants have the capacity to draw water from the water table and nearby surface water bodies. Yet, given that desert plants predominantly acquire water through their roots from dry soils, the distinctive architectures of their root systems are critical for survival (Lynch, 1995).
within a few days due to their rapid growth rate, due to limited duration of rainfalls.

Phreatophytic trees or shrubs (roots >5 m depth)
Hydraulic lift when it reaches the groundwater
High transpiration rate
Endure water deficit when young
Uses water from deep water table
Depends on rainfall when young, thereafter it depends on water table
Osmotic adjustment in roots and leaves when young

Deep rooted trees, palms or shrubs (roots < 5 m depth)
Hydraulic lift from deep to shallow layers
High transpiration rate
Endure water deficit
Uses water from deep soil and from water table only if it is shallow Depends on rainfall and it can live near superficial water bodies or where the water table is shallow
Osmotic adjustment in leaves and roots

Ephemerals
Very high photosynthetic rate and transpiration rate
Superficial tiny roots, herbaceous leaves, fast and abundant fructification
Escape to water deficit
Uses water from the soil surface
It depends on rainfall, floods or snowmelt
Osmotic adjustment in leaves and roots
scarcity caused by prolonged drought (Hultine et al., 2003a,b, 2004; Scholz et al., 2007, 2008, 2010; Barron-Gafford et al., 2017). This strategy sustains the long-term stability of mixed community
structures in arid and nutrient-deficient environments. These contrasting architectures have implications for other root functions, which we will discuss further in this review.

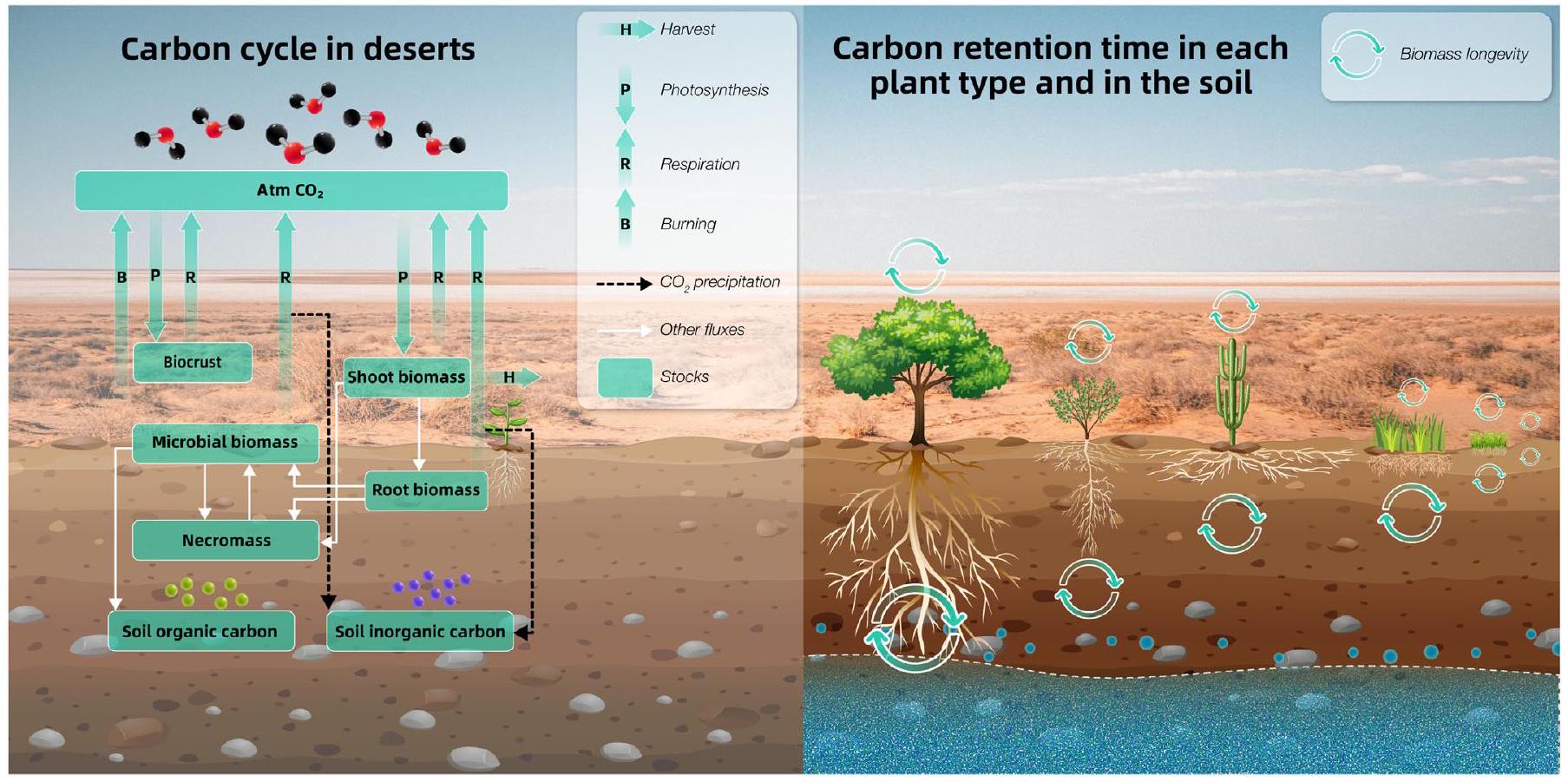
Carbon and nutrient stocks in desert ecosystems
reducing soil erosion and C loss (Balazs et al., 2022). Although the root biomass of cacti and succulents is low, they display extended turnover times once C is fixed through photosynthesis. Resurrection plants, perennial grasses, and herbs exhibit short-lived shoot biomass while maintaining slower root turnover due to their longer root lifespan. Up to

table to enhance organic matter decomposition and nutrient uptake near the soil surface (Hultine et al., 2003a,b). Shallowrooted grasses can receive up to

short-lived plants in desert ecosystems (Zhang et al., 2006). Thus, biocrusts emerge as a key feature of arid ecosystems, contributing significantly to nutrient and water accumulation and promoting positive succession in desert ecosystems.
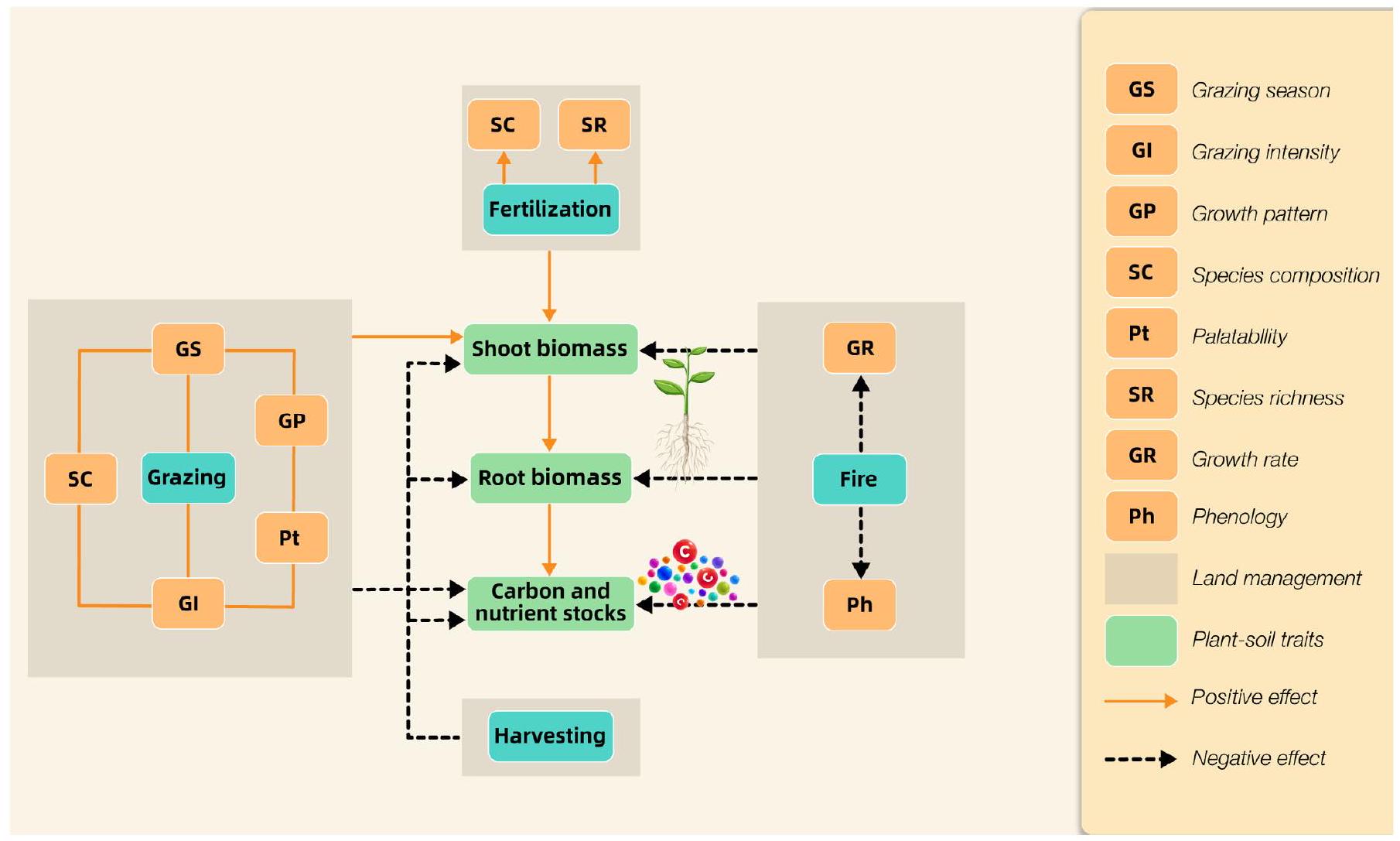
Impact of land-use change on desert plant communities and carbon and nutrient stocks
Grazing

strongly reduces biomass reduces capacity for complete recovery. Overall, C and nutrient stocks decrease due to biomass reduction, and long-term overgrazing practices can even alter the community structure.

abundance and growth patterns in varying rainfall conditions, along with the palatability of each species, should be considered. Furthermore, the temporal dynamics of C stocks in aboveground biomass should be considered when assessing anthropogenic land uses.
Plant harvesting
fertility, composition and biomass of soil biota, as well as the nutritional status of plants (Fig. 5). For example, a long-term experiment spanning 12 yr , conducted in the Taklamakan desert (West China), where Alhagi sparsifolia plants were annually cut, demonstrated that plant harvesting induced alterations in soil chemical composition throughout the soil profile (Tariq et al., 2022a; Table S1). This practice also heightened soil microbial activity and led to a reduction in overall foliar nutrition. Similarly, within another arid grassland ecosystem in the Patagonia steppe (South America), intensive foliar harvesting did not markedly alter root or shoot biomass but did have a notable effect on root inoculation by arbuscular mycorrhiza, resulting in a reduction of its presence (Toledo et al., 2022). The decline in mycorrhizal association, due to diminished root biomass, can impede the capacity of perennial desert plants to take up water and nutrients. This is because the symbiotic bond between fungi and roots is pivotal for extending the growing period beyond the wet season. These studies underscore the crucial role of plant management effects on soil biology, which in turn impacts, and is influenced by plant nutrition, ultimately leading to shifts in C and nutrient cycling. These factors merit further exploration in deserts, where nutrient availability can be constrained. This makes the impact of nutrient extraction through harvesting more pronounced when compared to other ecosystems.
Fires
plant nutrition. For instance, in the Taklamakan desert (West China), where
Fertilization
irrigation, indicating the greater water uptake capacity of shrubs from deeper soil layers (Drenovsky & Richards, 2004). In the Sonora desert (North America), N concentration in leaves increased with N fertilization, but in wetter years, biomass production was higher, leading to a decrease in N concentration due to greater allocation to more leaf’s biomass production (Hall et al., 2011). This allows accumulation of nutrients in tissues during wetter years. Similarly, in a fertilization trial conducted in ephemeral communities in the Chihuahua desert, only three winter annual species increased canopy cover when fertilized with N or S and irrigated, while no meaningful response was observed in rainfed plots (Ludwig et al., 1989). This demonstrates that responses to increased rainfall and nutrient deposition or fertilization are highly species and site-specific, and the composition and productivity of deserts cannot be easily predicted. Thus, as water dramatically limits mineral movement in arid soil and plant water and nutrient uptake in arid lands, it is necessary to test the responses of desert species in their specific environments, as results from mesic environments cannot be readily applied to deserts.
Impacts of indirect effects of climate change: Plant winners and losers and the consequences
Water table depth and depleting aquifers
under trees in an oasis with high SWC (Tariq et al., 2022b). Species with high phenotypic plasticity and wide ecological niches, like A. sparsifolia, could potentially perform better under climate change compared to species with narrower ecological requirements.
Increasing temperatures
Variation in precipitation

palisade tissues, cortical thickness (to maintain water retention and photosynthetic efficiency), root growth rate, and hydraulic conductance of xylem, but increasing drought stress and groundwater depth were not conducive to development (Zhuang & Chen, 2006; Table S1).
phreatophyte and a deep-rooted plant increased allocation to shoots, while a shallow-rooted species allocated more to roots, utilizing the additional water for enhanced soil exploration. Other species also demonstrate variations in root allocation across different habitats, underscoring the dichotomy between deep and shallow root systems. For instance, Prosopis flexuosa growing in arid dunes possesses deeper roots than the same species growing in more humid valleys (Guevara et al., 2010). Similarly, Argania spinosa populations from coastal regions with higher MAP rely less on deep rooting than populations from drier inland sites (Zunzunegui et al., 2018). An interesting example is A. sparsifolia, a phreatophyte that develops
Sandstorms
Perspectives for future research
(1) Desert ecosystems are influenced by multifaceted interactions between climate, soil, and plant communities. The interplay of these factors can be complex and challenging to fully unravel.
(2) Long-term studies encompassing multiple climatic cycles are essential to understand the resilience and adaptability of desert plant communities over time.
(3) While this review focuses on climatic and human-induced changes, other factors like soil characteristics and geological processes can also impact C and nutrient cycling.
(4) Though advanced technologies hold promises, their application to desert ecosystems is still evolving, and their full potential in predicting plant responses remains to be seen. Traditional studies of root architecture have involved excavation and careful analysis of roots, advanced technologies like electrical resistance imaging and 3D analysers can aid in predicting desert plant responses to climate change. The structure of the community and plant traits are crucial for understanding the resilience of desert ecosystems to changing climate and management, affecting their roles in nutrient cycling.
Conclusions
expansion, water-use efficiency, and nutrient mobilization. Hydraulic lift becomes crucial once plant roots reach the groundwater, boosting nutrient uptake near the soil surface and assisting shallow-rooted plants in acquiring water and nutrients.
Acknowledgements
Competing interests
Author contributions
literature. AT, FZ, CG, JS, ACH and JP organized and structured the information. All authors reviewed and contributed to the text of the manuscript.
ORCID
Sikandar Ali (D) https://orcid.org/0000-0001-9018-2837
Yanju Gao (D) https://orcid.org/0000-0003-1867-1454
Corina Graciano (D https://orcid.org/0000-0003-0803-4128
Alice C. Hughes (D https://orcid.org/0000-0002-0675-7552
Josep Peñuelas (D) https://orcid.org/0000-0002-7215-0150
Jordi Sardans (D https://orcid.org/0000-0003-2478-0219
Akash Tariq (D) https://orcid.org/0000-0002-5382-9336
Abd Ullah (D https://orcid.org/0000-0003-1570-0176
Fanjiang Zeng (D https://orcid.org/0000-0003-4209-6971
References
Alon A, Steinberger Y. 1999. Effect of nitrogen amendments on microbial biomass, above-ground biomass and nematode population in the Negev Desert soil. Journal of Arid Environments 41: 429-441.
Alsharif W, Saad MM, Hirt H. 2020. Desert microbes for boosting sustainable agriculture in extreme environments. Frontiers in Microbiology 11: 1666.
An H, Li G. 2015. Effects of grazing on carbon and nitrogen in plants and soils in a semiarid desert grassland, China. Journal of Arid Land 7: 341-349.
Andresen LC, Müller C, de Dato G, Dukes JS, Emmett BA, Estiarte M, Jentsch A, Kröel-Dulay G, Lüscher A, Niu S et al. 2016. Shifting impacts of climate change: long-term patterns of plant response to elevated
Apple ME. 2010. Aspects of mycorrhizae in desert plants. In: Ramawat KG, ed. Desert plants. Berlin, Heidelberg, Germany: Springer Berlin Heidelberg, 121134.
Arndt SK, Kahmen A, Arampatsis C, Popp M, Adams M. 2004b. Nitrogen fixation and metabolism by groundwater-dependent perennial plants in a hyperarid desert. Oecologia 141: 385-394.
Bacilio M, Vazquez P, Bashan Y. 2011. Water versus spacing: a possible growth preference among young individuals of the giant cardon cactus of the Baja California Peninsula. Environmental and Experimental Botany 70: 29-36.
Balazs KR, Munson SM, Butterfield BJ. 2022. Functional composition of plant communities mediates biomass effects on ecosystem service recovery across an experimental dryland restoration network. Functional Ecology 36: 2317-2330.
Barger NN, Weber B, Garcia-Pichel F, Zaady E, Belnap J. 2016. Patterns and controls on nitrogen cycling of biological soil crusts. In: Weber B, Büdel B, Belnap J, eds. Biological soil crusts: an organizing principle in drylands. Ecological studies, vol. 226. Cham, Switzerland: Springer, 273-302.
Barnes PW, Throop HL, Archer SR, Breshears DD, McCulley RL, Tobler MA. 2015. Sunlight and soil-litter mixing: drivers of litter decomposition in drylands. In: Lüttge U, Beyschlag W, eds. Progress in botany. Progress in botany. Cham, Switzerland: Springer International, 273-302.
Barron-Gafford GA, Sanchez-Cañete EP, Minor RL, Hendryx SM, Lee E, Sutter LF, Tran N, Parra E, Colella T, Murphy PC et al. 2017. Impacts of hydraulic redistribution on grass-tree competition vs facilitation in a semi-arid savanna. New Phytologist 215: 1451-1461.
Bates TR, Lynch JP. 1996. Stimulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability. Plant, Cell & Environment 19: 529-538.
Bell LW, Sparling B, Tenuta M, Entz MH. 2012. Soil profile carbon and nutrient stocks under long-term conventional and organic crop and alfalfa-crop rotations and re-established grassland. Agriculture, Ecosystems & Environment 158: 156-163.
Bhanot V, Fadanavis SV, Panwar J. 2021. Revisiting the architecture, biosynthesis and functional aspects of the plant cuticle: there is more scope. Environmental and Experimental Botany 183: 104364.
Biruk LN, Fernández ME, González CV, Guevara A, Rovida-Kojima E, Giordano CV. 2022. High and diverse plastic responses to water availability in four desert woody species of South America. Trees 36: 1881-1894.
Bodí MB, Martin DA, Balfour VN, Santín C, Doerr SH, Pereira P, Cerdà A, Mataix-Solera J. 2014. Wildland fire ash: production, composition and eco-hydro-geomorphic effects. Earth-Science Reviews 130: 103-127.
Bunn RA, Simpson DT, Bullington LS, Lekberg Y, Janos DP. 2019. Revisiting the ‘direct mineral cycling’ hypothesis: arbuscular mycorrhizal fungi colonize leaf litter, but why? The ISME Journal 13: 1891-1898.
Carrera AL, Bertiller MB, Larreguy C. 2008. Leaf litterfall, fine-root production, and decomposition in shrublands with different canopy structure induced by grazing in the Patagonian Monte, Argentina. Plant and Soil 311: 39-50.
Chapin FS III, Matson PA, Vitousek P. 2011. Principles of terrestrial ecosystem ecology. New York, NY, USA: Springer.
Chen S, Wang W, Xu W, Wang Y, Wan H, Chen D, Tang Z, Tang X, Zhou G, Xie Z et al. 2018. Plant diversity enhances productivity and soil carbon storage. Proceedings of the National Academy of Sciences, USA 115: 4027-4032.
Chong PF, Li HY, Li Y. 2015. Physiological responses of seedling roots of the desert plant Reaumuria soongorica to drought stress. Acta Prataculturae Sinica 24: 72-80.
Collins SL, Ladwig LM, Petrie MD, Jones SK, Mulhouse JM, Thibault JR, Pockman WT. 2017. Press-pulse interactions: effects of warming, N deposition, altered winter precipitation, and fire on desert grassland community structure and dynamics. Global Change Biology 23: 1095-1108.
Cooper DJ, Sanderson JS, Stannard DI, Groeneveld DP. 2006. Effects of longterm water-table drawdown on evapotranspiration and vegetation in an arid region phreatophyte community. Journal of Hydrology 325: 21-34.
Copeland SM, Bradford JB, Hardegree SP, Schlaepfer DR, Badik KJ. 2023. Management and environmental factors associated with simulated restoration seeding barriers in sagebrush steppe. Restoration Ecology 31: e13722.
Cui H, Fan M, Wang Y, Zhang X, Xu W, Li Y, Song W, Ma JY, Sun W. 2023. Impacts of mowing and N addition on soil organic phosphorus mineralization rates in a semi-natural grassland in Northeast China. Plant and Soil 482: 7-23.
DeFalco LA, Esque TC, Scoles-Sciulla SJ, Rodgers J. 2010. Desert wildfire and severe drought diminish survivorship of the long-lived Joshua tree (Yucca brevifolia; Agavaceae). American Journal of Botany 97: 243-250.
Deng L, Shangguan Z, Bell SM, Soromotin AV, Peng C, An S, Wu X, Xu X, Wang K, Li J et al. 2023. Carbon in Chinese grasslands: meta-analysis and theory of grazing effects. Carbon Research 2: 19.
Drenovsky RE, Richards JH. 2004. Critical N:P values: predicting nutrient deficiencies in desert shrublands. Plant and Soil 259: 59-69.
Dubrovsky JG, Shishkova S. 2013. Developmental adaptations in roots of desert plants with special emphasis on cacti. In: Eshel A, Beeckman T, eds. Plant roots: the hidden half,
Dubrovsky JG, North GB. 2002. Root structure and function. In: Nobel PS, ed. Cacti biology and uses. Oakland, CA, USA: University of California Press, 290.
Elbert W, Weber B, Burrows S, Steinkamp J, Büdel B, Andreae MO, Pöschl U. 2012. Contribution of cryptogamic covers to the global cycles of carbon and nitrogen. Nature Geoscience 5: 459-462.
Escolano JJ, Navarro Pedreño J, Gomez Lucas I, Almendro MB, Zorpas AA. 2018. In: Muñoz MA, Zornoza R, eds. Chapter 1 – Decreased organic carbon associated with land management in Mediterranean environments soil management and climate change: effects of organic carbon, nitrogen dynamics, and greenhouse gas emissions. Berkeley, CA, USA: Elsevier, 1-13.
Esque TC, Medica PA, Shryock DF, DeFalco LA, Webb RH, Hunter RB. 2015. Direct and indirect effects of environmental variability on growth and survivorship of pre-reproductive Joshua trees, Yucca brevifolia Engelm. (Agavaceae). American Journal of Botany 102: 85-91.
Feng S, Fu Q. 2013. Expansion of global drylands under a warming climate. Atmospheric Chemistry and Physics 13: 10081-10094.
Gao Y, Tian J, Pang Y, Liu J. 2017. Soil inorganic carbon sequestration following afforestation is probably induced by pedogenic carbonate formation in NorthwestChina. Frontiers in Plant Sciences 8: 1282.
Gao Y, Zhang Z, Zeng F, Ma X. 2023. Root morphological and physiological traits are committed to the phosphorus acquisition of the desert plants in phosphorusdeficient soils. BMC Plant Biology 23: 188.
Geng DM, Shan LS, Li Y. 2014. Effect of soil water stress on fine root morphology and functional characteristics of Reaumuria Soongorica. Bulletin of Soil and Water Conservation 36: 36-42.
Geng M, Wang X, Liu X, Lv P. 2023. Effects of grazing exclusion on microbial community diversity and soil metabolism in desert grasslands. Sustainability 15: 11263.
Guevara A, Giordano CV, Aranibar J, Quiroga M, Villagra PE. 2010. Phenotypic plasticity of the coarse root system of Prosopis flexuosa, a phreatophyte tree, in the Monte Desert (Argentina). Plant and Soil 330: 447-464.
Gurrero-campo J, Palacio S, Pérez-Rontomé C, Montserrat-Martí G. 2006. Effect of root system morphology on root sprouting and shoot-rooting abilities in 123 plant species from eroded lands in North-east Spain. Annals of Botany 98: 439447.
Harrower JT, Gilbert GS. 2021. Parasitism to mutualism continuum for Joshua trees inoculated with different communities of arbuscular mycorrhizal fungi from a desert elevation gradient. PLoS ONE 16: e0256068.
Hein L, De Ridder N. 2006. Desertification in the Sahel: a reinterpretation. Global Change Biology 12: 751-758.
Holzapfel C. 2008. Deserts. In: Jørgensen SE, Fath BD, eds. Encyclopedia of ecology, vol. 2. Oxford, UK: Elsevier, 879-898.
Horn KJ, Wilkinson J, White S, St. Clair SB. 2015. Desert wildfire impacts on plant community function. Plant Ecology 216: 1623-1634.
Houghton RA, Hall F, Goetz SJ. 2009. Importance of biomass in the global carbon cycle. Journal of Geophysical Research Biogeosciences 114: G00E03.
Huang F, Zhang D, Chen X. 2019. Vegetation response to groundwater variation in arid environments: visualization of research evolution, synthesis of response types, and estimation of groundwater threshold. International Journal of Environmental Research and Public Health 16: 1849.
Hukin D, Cochard H, Dreyer E, Le Thiec D, Bogeat-Triboulot MB. 2005. Cavitation vulnerability in roots and shoots: does Populus euphratica Oliv., a poplar from arid areas of Central Asia, differ from other poplar species? Journal of Experimental Botany 56: 2003-2010.
Hultine KR, Cable WL, Burgess SSO, Williams DG. 2003a. Hydraulic redistribution by deep roots of a Chihuahuan Desert phreatophyte. Tree Physiology 23: 353-360.
Hultine KR, Dettman DL, Williams DG, Puente R, English NB, Butterfield BJ, Búrquez A. 2018. Relationships among climate, stem growth, and biomass
Hultine KR, Scott RL, Cable WL, Goodrich DC, Williams DG. 2004. Hydraulic redistribution by a dominant, warm-desert phreatophyte: seasonal patterns and response to precipitation pulses. Functional Ecology 18: 530-538.
Hultine KR, Williams DG, Burgess SSO, Keefer TO. 2003b. Contrasting patterns of hydraulic redistribution in three desert phreatophytes. Oecologia 135: 167175.
Kell DB. 2011. Breeding crop plants with deep roots: their role in sustainable carbon, nutrient and water sequestration. Annals of Botany 108: 407-418.
Kidron GJ, Starinsky A. 2019. Measurements and ecological implications of nonrainfall water in desert ecosystems-a review. Ecohydrology 12: e2121.
Kim H, Kim K, Lee SJ. 2018. Hydraulic strategy of cactus root-stem junction for effective water transport. Frontiers in Plant Science 9: 799.
Kirschner GK, Xiao TT, Blilou I. 2021. Rooting in the desert: a developmental overview on desert plants. Genes 12: 709.
Ladwig LM, Collins SL, Swann AL, Xia Y, Allen MF, Allen EB. 2012. Above- and belowground responses to nitrogen addition in a Chihuahuan Desert grassland. Oecologia 169: 177-185.
Lasché SN, Schroeder RWR, McIntosh MM, Lucero JE, Spiegal SA, Funk MP, Beck RF, Holechek JL, Faist AM. 2023. Long-term growing season aridity and grazing seasonality effects on perennial grass biomass in a Chihuahuan Desert rangeland. Journal of Arid Environments 209: 104902.
Li B, Wang L, Kaseke KF, Li L, Seely MK. 2016. The impact of rainfall on soil moisture dynamics in a foggy desert. PLoS ONE 11: e0164982.
Li L, Gao X, Gui D, Liu B, Zhang B, Li X. 2017. Stoichiometry in aboveground and fine roots of Seriphidium korovinii in desert grassland in response to artificial nitrogen addition. Journal of Plant Research 130: 689-697.
Liu S, Xu G, Chen T, Wu X, Li Y. 2023. Quantifying the effects of precipitation exclusion and groundwater drawdown on functional traits of Haloxylon ammodendron – how does this xeric shrub survive the drought? Science of the Total Environment 904: 166945.
Liu Z, Liu K, Shi X, Ryan Lock T, Kallenbach RL, Yuan Z. 2022. Changes in grassland phenology and growth rate, rather than diversity, drive biomass production after fire. Agricultural and Forest Meteorology 322: 109028.
Lu J, Feng S, Wang S, Zhang B, Ning Z, Wang R, Chen X, Yu L, Zhao H, Lan D et al. 2023. Patterns and driving mechanism of soil organic carbon, nitrogen, and phosphorus stoichiometry across northern China’s desert-grassland transition zone. Catena 220: 106695.
Lu Y, Liu H, Chen Y, Zhang L, Kudusi K, Song J. 2022. Effects of drought and salt stress on seed germination of ephemeral plants in desert of northwest China. Frontiers in Ecology and Evolution 10: 1026095.
Ludwig JA, Whitford WG, Cornelius JM. 1989. Effects of water, nitrogen and sulfur amendments on cover, density and size of Chihuahuan Desert ephemerals. Journal of Arid Environments 16: 35-42.
Lynch J. 1995. Root architecture and plant productivity. Plant Physiology 109: 7-13.
Lynch J, Marschner P, Rengel Z. 2012. Effect of internal and external factors on root growth and development. In: Marschner’s mineral nutrition of higher plants. London, UK: Academic Press, 331-346.
Lynch JP. 2007. Roots of the Second Green revolution. Australian Journal of Botany 55: 493.
Lynch JP. 2019. Root phenotypes for improved nutrient capture: an underexploited opportunity for global agriculture. New Phytologist 223: 548-564.
Lynch JP. 2022. Harnessing root architecture to address global challenges. The Plant Journal 109: 415-431.
Ma Q, Li Y, Zhu Y, Liu X, Yu H, Li L, Qi M, Sun H, Yin Z, Wang Y et al. 2022. Precipitation variations, rather than N deposition, determine plant ecophysiological traits in a desert steppe in Northern China. Ecological Indicators 141: 109144.
Maeght JL, Rewald B, Pierret A. 2013. How to study deep roots – and why it matters. Frontiers of Plant Science 4: 299.
Maestre FT, Bagousse-Pinguet YL, Delgado-Baquerizo M, Eldrige DJ, Saiz H, Berdugo M, Gozalo B, Ochoa V, Guirado E, Garcia- Gomez M et al. 2022. Grazing and ecosystem service delivery in global drylands. Science 378: 915920.
Manning DAC. 2008. Biological enhancement of soil carbonate precipitation: passive removal of atmospheric
Marks E, Aflakpul GKS, Nkem J, Poch RM, Khouma M, Kokou K, Sagoe R, Sebastià MT. 2008. Coservation of soil organic carbon, biodiversity and the provision of other ecosystems services along climatic gradients in West Africa. Biogeosciences Discussions 5: 4413-4452.
Matos IS, Binks O, Eller CB, Zorger BB, Meir P, Dawson TE, Rosado BHP. 2022. Revisiting plant hydrological niches: the importance of atmospheric resources for ground-rooted plants. Journal of Ecology 110: 1746-1756.
Maurice K, Laurent-Webb L, Dehail A, Bourceret A, Boivin S, Boukcim H, Selosse M-A, Ducousso M. 2023. Fertility islands, keys to the establishment of plant and microbial diversity in a highly alkaline hot desert. Journal of Arid Environments 219: 105074.
McKenna DM, Grams SE, Barasha M, Antoninka AJ, Johnson NC. 2022. Organic and inorganic soil carbon in a semi-arid rangeland is primarily related to abiotic factors and not livestock grazing. Geoderma 419: 115844.
Miao L, Li S, Zhang F, Chen T, Shan Y, Zhang Y. 2020. Future drought in the dry lands of asia under the 1.5 and
Mohanta K, Mohanta YK, Kaushik P, Kumar J. 2023. Physiology, genomics, and evolutionary aspects of desert plants. Journal of Advanced Research, in press. doi: 10.1016/j.jare.2023.04.019.
Nara K. 2006. Ectomycorrhizal networks and seedling establishment during early primary succession. New Phytologist 169: 169-178.
Nevo E. 2012. “Evolution Canyon,” a potential microscale monitor of global warming across life. Proceedings of the National Academy of Sciences, USA 109: 2960-2965.
Nilsen ET, Sharifi MR, Rundel PW. 1984. Comparative water relations of phreatophytes in the Sonoran Desert of California. Ecology 65: 767-778.
Nobel PS, ed. 2002. Cacti biology and uses. Oakland, CA, USA: University of California Press.
Otieno DO, Schmidt MWT, Kinyamario JI, Tenhunen J. 2005. Responses of Acacia tortilis and Acacia xanthophloea to seasonal changes in soil water availability in the savanna region of Kenya. Journal of Arid Environments 62: 377-400.
Paula S, Pausas JG. 2011. Root traits explain different foraging strategies between resprouting life strategies. Physiological Ecology 165: 321-331.
Peek MS, Forseth IN. 2003. Enhancement of photosynthesis and growth of an aridland perennial in response to soil nitrogen pulses generated by mule deer. Environmental and Experimental Botany 49: 169-180.
Pellegrini AFA, Harden J, Georgiou K, Hemes KS, Malhotra A, Nolan CJ, Jackson RB. 2022. Fire effects on the persistence of soil organic matter and long-term carbon storage. Nature Geoscience 15: 5-13.
Pérez ALS, Camargo-Ricalde SL, Montaño NM, García-Oliva F, Alarcón A, Montaño-Arias SA, Esperón-Rodríguez M. 2016. Biocrusts, inside and outside resource islands of Mimosa luisana (Leguminosae), improve soil carbon and nitrogen dynamics in a tropical semiarid ecosystem. European Journal of Soil Biology 74: 93-103.
Predick KI, Archer SR, Aguillon SM, Keller DA, Throop HL, Barnes PW. 2018. UV-B radiation and shrub canopy effects on surface litter decomposition in a shrub-invaded dry grassland. Journal of Arid Environments 157: 13-21.
Principe A, Nunes A, Pinho P, do Rosário L, Correira O, Branquinho C. 2014. Modeling the long-term natural regeneration potential of woodlands in semi-arid regions to guide restoration efforts. European Journal of Forest Research 133: 757767.
Rodriguez-Caballero E, Belnap J, Buedel B, Crutzen PJ, Andreae MO, Pöschl U, Weber B. 2018. Dryland photoautotrophic soil surface communities endangered by global change. Nature Geoscience 11: 185-189.
Roncero-Ramos B, Román JR, Acién G, Cantón Y. 2022. Towards large scale biocrust restoration: Producing an efficient and low-cost inoculum of N-fixing cyanobacteria. Science of the Total Environment 848: 157704.
Rüger L, Ganther M, Freudenthal J, Jansa J, Heintz-Buschart A, Tarkka MT, Bonkowski M. 2023. Root cap is an important determinant of rhizosphere microbiome assembly. New Phytologist 239: 1434-1448.
Salo LF, McPherson GR, Williams DG. 2005. Sonoran desert winter annuals affected by density of red brome and soil nitrogen. The American Midland Naturalist 153: 95-109.
Šantrůček J. 2022. The why and how of sunken stomata: does the behaviour of encrypted stomata and the leaf cuticle matter? Annals of Botany 130: 285-300.
Sardans J, Peñuelas J. 2012. The role of plants in the effects of global change on nutrient availability and stoichiometry in the plant-soil system. Plant Physiology 160: 1741-1761.
Saul-Tcherkas V, Steinberger Y. 2009. Temporal and shrub adaptation effect on soil microbial functional diversity in a desert system. European Journal of Soil Science 60: 871-882.
Schlesinger WH. 2017. An evaluation of abiotic carbon sinks in deserts. Global Change Biology 23: 25-27.
Scholz FG, Bucci SJ, Goldstein G, Meinzer FC, Franco AC, Miralles-Wilhelm F. 2007. Removal of nutrient limitations by long-term fertilization decreases nocturnal water loss in savanna trees. Tree Physiology 27: 551-559.
Scholz FG, Bucci SJ, Hoffmann WA, Meinzer FC, Goldstein G. 2010. Hydraulic lift in a Neotropical savanna: experimental manipulation and model simulations. Agricultural and Forest Meteorology 150: 629-639.
Scholz FG, Moreira MZ, Franco AC, Miralles-Wilhelm F. 2008. Biophysical and life-history determinants of hydraulic lift in Neotropical savanna trees. Functional Ecology 22: 773-786.
Shadwell E, February E. 2017. Effects of groundwater abstraction on two keystone tree species in an arid savanna national park. Peer 5: e2923.
Shishkova S, Dubrovsky JG. 2005. Developmental programmed cell death in primary roots of Sonoran desert cactaceae. American Journal of Botany 92: 15901594.
Singh G, Shukla S. 2011. Effects of Azadirachta indica canopy manipulation and nitrogen fertilization on diversity and productivity of herbaceous vegetation in an Arid Environment of India. Arid Land Research and Management 25: 128-149.
Snyman HA. 2006. Root distribution with changes in distance and depth of two-year-old cactus pears Opuntia ficus-indica and O. robusta plants. South African Journal of Botany 72: 434-441.
Spinoni J, Barbosa P, Cherlet M, Forzieri G, McCormick N, Naumann G, Vogt JV, Dosio A. 2021. How will the progressive global increase of arid areas affect population and land-use in the 21st century? Global and Planetary Change 205: 103597.
Su YG, Chen YW, Padilla FM, Zhang YM, Huang G. 2020. The influence of biocrusts on the spatial pattern of soil bacterial communities: a case study at landscape and slope scales. Soil Biology & Biochemistry 142: 107721.
Sweet LC, Green T, Heintz JGC, Frakes N, Graver N, Rangitsch JS, Rodgers JE, Heacox S, Barrows CW. 2019. Congruence between future distribution models and empirical data for an iconic species at Joshua Tree National Park. Ecosphere 10: e02763.
Tao Y, Shang T, Yan J, Hu Y, Zhao Y, Liu Y. 2022. Effects of sand burial depth on Xanthium spinosum seed germination and seedling growth. BMC Plant Biology 22: 43.
Tariq A, Ullah A, Sardans J, Zeng F, Graciano C, Li X, Wang W, Ahmed Z, Ali S, Zhang Z et al. 2022b. Alhagi sparsifolia: an ideal phreatophyte for combating desertification and land degradation. Science of the Total Environment 844 : 157228.
Toledo S, Fontenla SB, Peri PL. 2022. Effect of defoliation frequency on Rytidosperma Virescens plants and arbuscular mycorrhizal fungi colonization. Rangeland Ecology & Management 84: 1-9.
Ullah A, Tariq A, Zeng F, Sardans J, Graciano C, Ullah S, Chai X, Zhang Z, Keyimu M, Asghar MA et al. 2022. Phosphorous supplementation alleviates droughtinduced physio-biochemical damages in Calligonum mongolicum. Plants 11: 3054.
Valone TJ. 2003. Examination of interaction effects of multiple disturbances on an arid plant community. The Southwestern Naturalis 48: 481-490.
Vasar M, Davison J, Sepp S-K, Öpik M, Moora M, Koorem K, Meng Y, Oja J, Akhmetzhanova AA, Al-Quraishy S et al. 2021. Arbuscular mycorrhizal fungal communities in the soils of desert habitats. Microorganisms 9: 229.
Vikram S, Ramond J-B, Ortiz M, Maggs-Kölling G, Pelser K, Cowan DA. 2023. Soil fungal diversity and assembly along a xeric stress gradient in the central Namib Desert. Fungal Biology 127: 997-1003.
Wang L, Zhao C, Li J, Liu Z, Wang J. 2015. Root plasticity of Populus euphratica seedlings in response to different water-table depths and contrasting sediment types. PLoS ONE 10: e0118691.
Wang Q, Zhang Q, Han Y, Zhang D, Zhang CC, Hu C. 2022. Carbon cycle in the microbial ecosystems of biological soil crusts. Soil Biology and Biochemistry 171: 108729.
Wang Z, Jiang S, Struik PC, Wang H, Jin K, Wu R, Na R, Mu H, Ta N. 2023. Plant and soil responses to grazing intensity drive changes in the soil microbiome in a desert steppe. Plant and Soil 491: 219-237.
Wang Z, Zhao M, Yan Z, Yang Y, Niklas KJ, Huang H, Mipam TD, He X, Hu X, Wright SJ. 2022. Global patterns and predictors of soil microbial biomass carbon, nitrogen, and phosphorus in terrestrial ecosystems. Catena 211: 106037.
Xu H, Li Y, Xu G, Zou T. 2007. Ecophysiological response and morphological adjustment of two Central Asian desert shrubs towards variation in summer precipitation. Plant, Cell & Environment 30: 399-409.
Yang Y, Li T, Pokharel P, Liu L, Qiao J, Wang Y, An S, Chang SX. 2022. Global effects on soil respiration and its temperature sensitivity depend on nitrogen addition rate. Soil Biology and Biochemistry 174: 108814.
Yao J, Liu H, Huang J, Gao Z, Wang G, Li D, Yu H, Chen X. 2020. Accelerated dryland expansion regulates future variability in dryland gross primary production. Nature Communications 11: 1665.
Yin H, Tariq A, Zhang B, Lv G, Zeng F, Graciano C, Santos M, Zhang Z, Wang P, Mu S. 2021a. Coupling relationship of leaf economic and hydraulic traits of Alhagi sparsifolia Shap. in a hyper-arid desert ecosystem. Plants 10: 1867.
Yin H, Zheng H, Zhang B, Tariq A, Lv G, Zeng F, Graciano C. 2021b. Stoichiometry of
York LM, Nord EA, Lynch JP. 2013. Integration of root phenes for soil resource acquisition Integration of root phenes for soil resource acquisition. Frontiers in Plant Science 4: e355.
Young KE, Ferrenberg S, Reibold R, Reed SC, Swenson T, Northen T, DarrouzetNardi A. 2022. Vertical movement of soluble carbon and nutrients from biocrusts to subsurface mineral soils. Geoderma 405: 115495.
Yuan S, Tang H, Yan Y. 2009. Photosynthetic characteristics of spring ephemerals in the desert ecosystem of Dzungaria Basin, northwest China. Environmental Earth Sciences 59: 501-510.
Zeng F, Song C, Guo H, Liu B, Luo W, Gui D, Arndt S, Guo D. 2013. Responses of root growth of Alhagi sparsifolia Shap. (Fabaceae) to different simulated groundwater depths in the southern fringe of the Taklimakan Desert, China. Journal of Arid Land 5: 220-232.
Zhang B, Gao X, Li L, Lu Y, Shareef M, Huang C, Liu G, Gui D, Zeng F. 2018. Groundwater depth affects phosphorus but not carbon and nitrogen concentrations of a desert phreatophyte in northwest China. Frontiers in Plant Science 9: 1-10.
Zhang Y, Tariq A, Hughes AC, Hong D, Wei F, Sun H, Sardans J, Peñuelas J, Perry G, Qiao J et al. 2023. Challenges and solutions to biodiversity conservation in arid lands. Science of the Total Environment 857: 159695.
Zhang Z, Chai X, Tariq A, Zeng F, Graciano C, Li X, Gao Y, Ullah A. 2021a. Coordinated patterns in the allocation, composition, and variability of multiple elements among organs of two desert shrubs under nitrogen addition and drought. Journal of Soil Science and Plant Nutrition 22: 47-58.
Zhang Z, Shan L, Li Y. 2018. Prolonged dry periods between rainfall events shorten the growth period of the resurrection plant Reaumuria soongorica. Ecology and Evolution 8: 920-927.
Zhang Z, Tariq A, Fanjiang Z, Graciano C, Bo Z. 2020. Nitrogen application mitigates drought-induced metabolic changes in Alhagi sparsifolia seedlings by regulating nutrient and biomass allocation patterns. Plant Physiology and Biochemistry 155: 828-841.
Zhang Z, Tariq A, Zeng F, Chai X, Graciano C. 2021b. Involvement of soluble proteins in growth and metabolic adjustments of drought stressed Calligonum mongolicum seedlings under nitrogen addition. Plant Biology 23: 32-43.
Zhou L, Zhou X, He Y, Fu Y, Du Z, Lu M, Sun X, Li C, Lu C, Liu R et al. 2022. Global systematic review with meta-analysis shows that warming effects on terrestrial plant biomass allocation are influenced by precipitation and mycorrhizal association. Nature Communications 13: 4914.
Zhou X, Tao Y, Yin BF, Tucker C, Zhang YM. 2020a. Nitrogen pools in soil covered by biological soil crusts of different successional stages in a temperate desert in Central Asia. Geoderma 366: 114166.
Zhou X, Yue P, Cui X, Tao Y, Zhang Y, Liu X. 2020b. Impacts of nitrogen deposition on China’s desert ecosystems. In: Liu X, Du E, eds. Atmospheric reactive nitrogen in China. Singapore City, Singapore: Springer Singapore, 245-261.
Zhuang L, Chen Y. 2006. Physiological response of Tamarix ramosissima under water stress along the lower reaches of Tarim River. Chinese Science Bulletin 51: 1123-1129.
Zoccatelli D, Marra F, Armon M, Rinat Y, Smith JA, Morin E. 2019. Contrasting rainfall-runoff characteristics of floods in desert and Mediterranean basins. Hydrology and Earth System Sciences 23: 2665-2678.
Supporting Information
Methods S1 Methodology.
Notes S2 Carbon stocks in desert ecosystems.
Notes S3 Soil biota associated with roots in deserts.
Notes S4 Nonlinear and threshold effects.
Notes S5 Morphological strategies of desert plants to cope with dry environments.
