DOI: https://doi.org/10.1016/j.plipres.2024.101275
PMID: https://pubmed.ncbi.nlm.nih.gov/38280491
تاريخ النشر: 2024-01-26
أكسدة الدهون في المستحلبات: رؤى جديدة من العقدين الماضيين
للاستشهاد بهذه النسخة:
أكسدة الدهون في المستحلبات: رؤى جديدة من العقدين الماضيين
معلومات المقال
الكلمات المفتاحية:
المستحلبات
قطرات الدهون
واجهة
مضادات الأكسدة
المستحلبات
التأكسد المشترك
الملخص
تشكل أكسدة الدهون المصدر الرئيسي لتدهور الأطعمة الغنية بالدهون، بما في ذلك المستحلبات الغذائية. إن تعقيد التفاعلات المعنية، بالإضافة إلى الطلب المتزايد من المستهلكين على الأطعمة الأقل معالجة والأكثر طبيعية، يؤدي إلى تحديات إضافية في السيطرة على هذه الظاهرة. تقدم هذه المراجعة لمحة عامة عن الرؤى التي تم اكتسابها على مدى العقدين الماضيين حول فهم أكسدة الدهون في الزيت في الماء.
1. المقدمة
للتحكم في هذه الظاهرة، لا سيما في المنتجات التي تحتوي على كمية عالية من الأحماض الدهنية (البولية) غير المشبعة التي تكون حساسة بشكل خاص للأكسدة. يمكن أن تحدث أكسدة الدهون من خلال ثلاثة مسارات رئيسية: الأكسدة الإنزيمية عبر عمل إنزيمات مثل
- المؤلف المراسل.
** المؤلف المراسل في: INRAE، UR BIA، نانت 44300، فرنسا.
عنوان البريد الإلكتروني: مارie.hennebelle@wur.nl (م. هينيبيل).
الليبواوكسيجيناز، الأكسدة الضوئية من خلال تنشيط مادة حساسة بواسطة الضوء والأكسدة الذاتية. بينما يتم عادة التحكم في الأولين بشكل جيد في المنتجات الغذائية من خلال المعالجة الحرارية والتعبئة، تظل الأكسدة الذاتية تحديًا، لا سيما لتلبية الطلب المتزايد من المستهلكين على منتجات أكثر استدامة وطبيعية (أي، ملصق نظيف). تحتاج المنهجيات الحالية لمنع أكسدة الدهون (أي، تخزين سلسلة التبريد، التعبئة في فراغ أو جو محكم، إضافة مضادات أكسدة صناعية) إلى إعادة النظر، وتظهر اتجاهات جديدة، بما في ذلك استخدام مضادات أكسدة أكثر طبيعية من مستخلصات النباتات، وتطوير مكونات معاد تدويرها من الجوانب الجانبية لصناعة المواد الغذائية، وإدخال مكونات منخفضة أو معالجة بشكل خفيف، أو زيادة استخدام المستحلبات النباتية.
الحليب، تركيبات الرضع، المنتجات القائمة على الألبان، المايونيز، الصلصات، بالإضافة إلى الأدوية، مستحضرات التجميل، ومنتجات العناية الشخصية. تتكون هذه المستحلبات من قطرات زيتية موزعة داخل مرحلة مائية مستمرة ومستقرة بواسطة جزيئات نشطة سطحياً، والتي تمتص عند واجهة الزيت والماء. من المعروف أن واجهة الزيت والماء تلعب دوراً حاسماً كونها الموقع الذي تتلامس فيه الدهون والأكسجين والعوامل المؤكسدة (للمراجعات، انظر [3،4]). المساحة الكبيرة للواجهة التي توجد عادة في المستحلبات تعزز تعرض الدهون للعوامل المؤكسدة، وبالتالي تسرع من أكسدة الدهون مقارنة بالزيوت السائبة. العديد من العوامل، مثل حجم القطرات، التركيب الواجهوي، نوع المستحلب، التقسيم والتفاعل للعوامل المؤكسدة والمضادة للأكسدة يمكن أن تؤثر على عملية أكسدة الدهون في الأطعمة المستحلبة، مما يجعل من الصعب فهم السيطرة على التفاعلات بالكامل، وبالتالي، تبرير استراتيجيات التركيب.
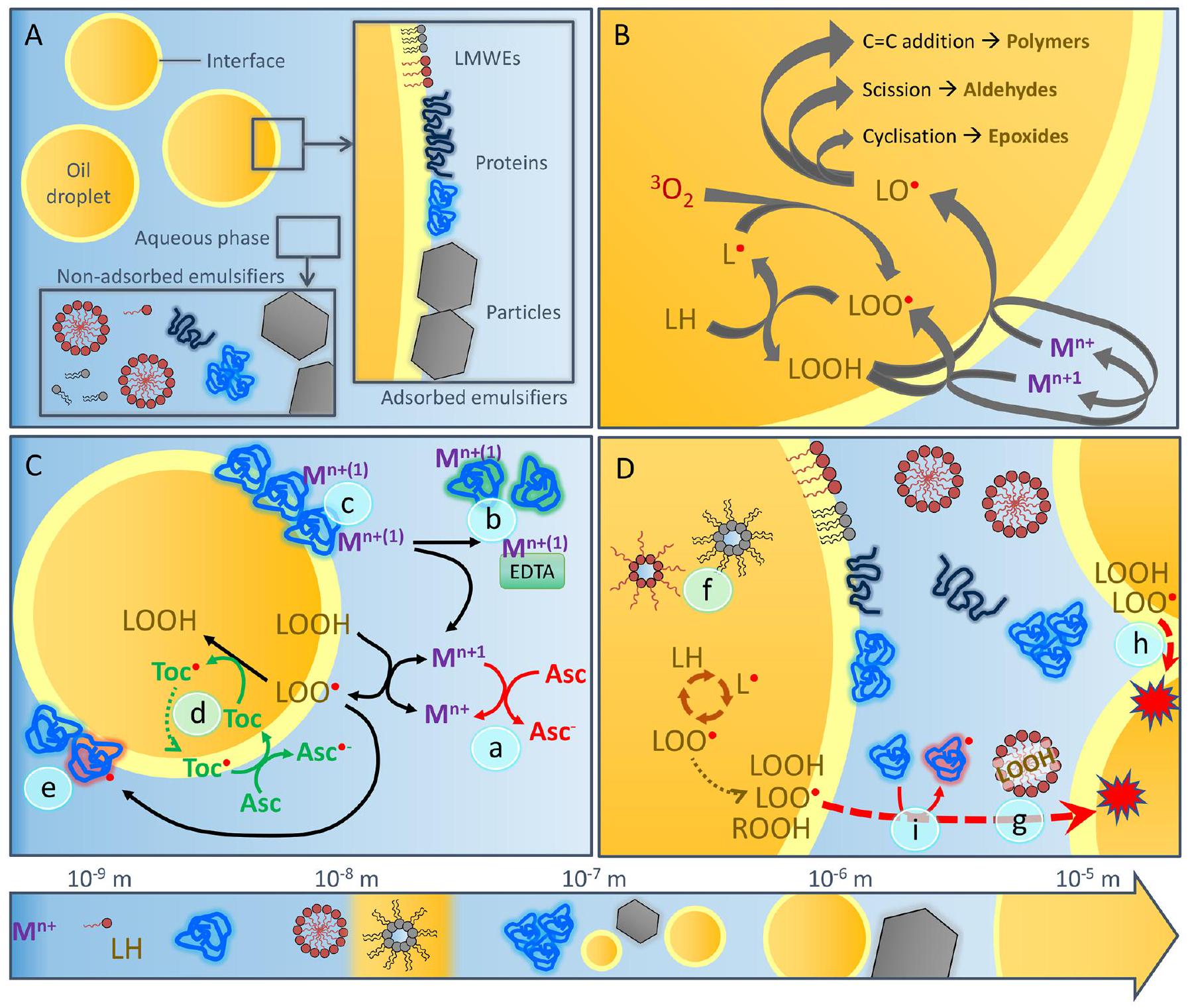
2. آليات أكسدة الدهون في المستحلبات الزيتية في الماء
2.1. الخصائص العامة للمستحلبات وواجهات الزيت والماء
كثافة القطرات، مما قد يؤدي إلى ترسيب القطرات بدلاً من التكوين. تنشأ قوى شعرية جانبية جذابة بين الجسيمات المحبوسة داخل الغشاء السطحي من تشوه واجهة السائل حول الجسيمات، مما يمنح الطبقة السطحية استقرارًا ميكانيكيًا عاليًا وصلابة. هذا، جنبًا إلى جنب مع الطاقة العالية للامتزاز للجسيمات بمجرد امتصاصها، يمنح هذه المستحلبات استقرارًا فيزيائيًا عاليًا، خاصة فيما يتعلق بمقاومة التكتل [15،23].
2.2. نظرة عامة عالمية على مسارات أكسدة الدهون
دورة انتشار أكسدة الدهون. يتم تعديل دورة الأكسدة والاختزال المعدنية في الشكل 1B بشكل كبير من خلال وجود عوامل اختزال قابلة للذوبان في الماء، مثل حمض الأسكوربيك، التي تساعد في تجديد المعادن الانتقالية ثنائية التكافؤ (الآلية (أ) في الشكل 1C) وبالتالي تظهر تأثيرًا مؤكسدًا [39]، أو خافضات المعادن (الآلية (ب) في الشكل 1C)، وبالتالي لها تأثير مضاد للأكسدة في الحالة الأخيرة.
2.3. ما وراء المسار الكلاسيكي لأكسدة الدهون
منتجات غذائية. ومع ذلك، كما سيتم تسليط الضوء عليه بمزيد من التفصيل في الأقسام التالية، فإن واجهة الزيت والماء في
2.4. الدقة الزمانية والمكانية لأكسدة الدهون في المستحلبات
2.4.1. الدور المركزي للواجهة بين النفط والماء
2.4.2. تعديل حركيات الأكسدة من خلال تشكيل الهياكل الغروية داخل المرحلة الزيتية
2.4.3. النقل عبر المرحلة المائية المستمرة
أوكتادكان [26،28،98،99]، ومنتجات أكسدة الدهون الثانوية المحددة التي تحمل مجموعة هيدروبيروكسيد (مثل 4-هيدروبيروكسي 2-نونينال)، والأحماض الدهنية الحرة (مثل حمض اللينوليك)، بالإضافة إلى مركبات الأكسدة الثانوية (مثل 2،4-ديكادينال)، يمكن أن تنتشر عبر نظام المستحلب وقد تنشر تفاعلات أكسدة إلى قطرات الدهون المجاورة [27،99-101]. أشارت التجارب التي أجريت باستخدام قياس التدفق الخلوي إلى أن النقل المساعد بالميكل هو آلية نقل نشطة في المستحلبات النموذجية O/W [100]. أكدت الدراسات التي استخدمت مستحلبات نموذجية معدة بعناية أن هذه الآليات النقل محدودة بمنتجات أكسدة الدهون المحددة؛ على سبيل المثال، الهيدروبيروكسيدات المرتبطة بعمود TAG ليست عرضة للنقل، ربما لأن إدخالها في القلب الكاره للماء للميكل السطحي ليس ممكنًا من الناحية الاستيريو، بينما الجزيئات الصغيرة القابلة للذوبان في الماء هي [27].
2.4.4. تفاعلات الأكسدة داخل المرحلة المائية المستمرة
2.5. نهج النمذجة
بين العينات ذات التغيرات المحددة (مثل، تركيزات مختلفة من مضادات الأكسدة) وقد تم استخدامها حتى للتنبؤ بعمر التخزين للمواد المستحلبة، مثل المايونيز. من ناحية أخرى، يمكن أن تكون تفسير معلماتها معقدًا لأنها لا ترتبط مباشرة بمعلمات النظام نفسه (مثل، تركيبة العينات أو ظروف الحضانة) ولا تأخذ في الاعتبار سلسلة التفاعلات الكاملة المعنية. مؤخرًا، تم اقتراح نموذج حركي آلي أكثر شمولاً في الزيوت السائبة، مع الأخذ في الاعتبار التفاعلات المتعددة المعنية في أكسدة الدهون، ونقل الكتلة للأكسجين، بالإضافة إلى التركيبة الأولية للزيت. يقدم هذا النموذج ميزة تمكين التنبؤ بعمر التخزين لزيوت نباتية مختلفة مخزنة تحت ظروف أكسجين مختلفة، لكنه يظل محدودًا بالزيوت السائبة. في المستحلبات O/W، تم إظهار نموذج حركي يدمج حركية التفاعل ونقل الكتلة لوصف عملية الأكسدة بشكل جيد في المستحلبات المدعمة بخمسة مستحلبات مختلفة.
3. تأثير تركيبة المستحلب وبنيته على أكسدة الدهون
3.1. تأثير معالجة المستحلب والخصائص الهيكلية على أكسدة الدهون
الفرق بين العناصر الدوارة والثابتة لتحفيز القص من أجل تفكك القطرات، ولكنها عادة ما تتمتع بكثافة طاقة منخفضة، مما يجعل من الصعب إنشاء قطرات صغيرة. توجد طرق أخرى للتجانس، مثل تجانس الأغشية، لكنها غير عملية حاليًا لمعظم التطبيقات التجارية [124]. أشار كوستا وآخرون [125] إلى أن التجانس، خاصة عند استخدام قوى ميكانيكية شديدة، يمكن أن يؤثر على جزيئات المستحلب أو الهياكل فوق الجزيئية. غالبًا ما يتم تجاهل التأثير المشترك لدرجة الحرارة والقص على المستحلبات في أبحاث المستحلبات. على سبيل المثال، يمكن أن تؤدي الضغوط العالية إلى تغيير التركيب الثلاثي للبروتينات الكروية، مما يؤدي إلى تفكك البروتين حتى عندما تظل درجة حرارة المنتج أقل من درجة حرارة تغيير البروتين، مما قد يؤدي إلى تجمع البروتين. تعتبر ظروف التجانس، بما في ذلك نوع المعدات ومدخلات الطاقة، مهمة أيضًا لأنها تحدد توزيع حجم القطرات المحدد، والذي يؤثر مباشرة على قدرة المستحلب على مقاومة الأكسدة بسبب التغيرات في المساحة السطحية الكلية [126،127]. من حيث المبدأ، يجب أن تعزز القطرات الأصغر، وبالتالي مساحة الواجهة بين الزيت والماء الأكبر، أكسدة الدهون. بينما تم ملاحظة هذا التأثير غالبًا تجريبيًا، كانت هناك تقارير متضاربة، ربما ناتجة عن التحديات المتعلقة بتغيير حجم القطرات دون التأثير على خصائص المستحلب الأخرى [4]. أظهرت عدة دراسات، وخاصة من مجموعة C. Jacobsen، أن ظروف التجانس يمكن أن تؤثر على أكسدة الدهون في أنواع مختلفة من المستحلبات التي تحتوي على زيت السمك [128-130]. قام هورن وآخرون [128] بدراسة تأثير ضغوط ودرجات حرارة التجانس المختلفة على السمك.
(حلقات البوليمر الحيوي، المسام)، مما يثير الشكوك حول ما إذا كانت هذه المعلمة تلعب دورًا حاسمًا في أكسدة المستحلبات.
3.2. اتجاهات جديدة في مستحلبات الطعام
المستحلبات (الشكل 2). قبل عشرين عامًا، كانت هذه النسب حوالي
3.2.1. المستحلبات القائمة على بروتينات النباتات
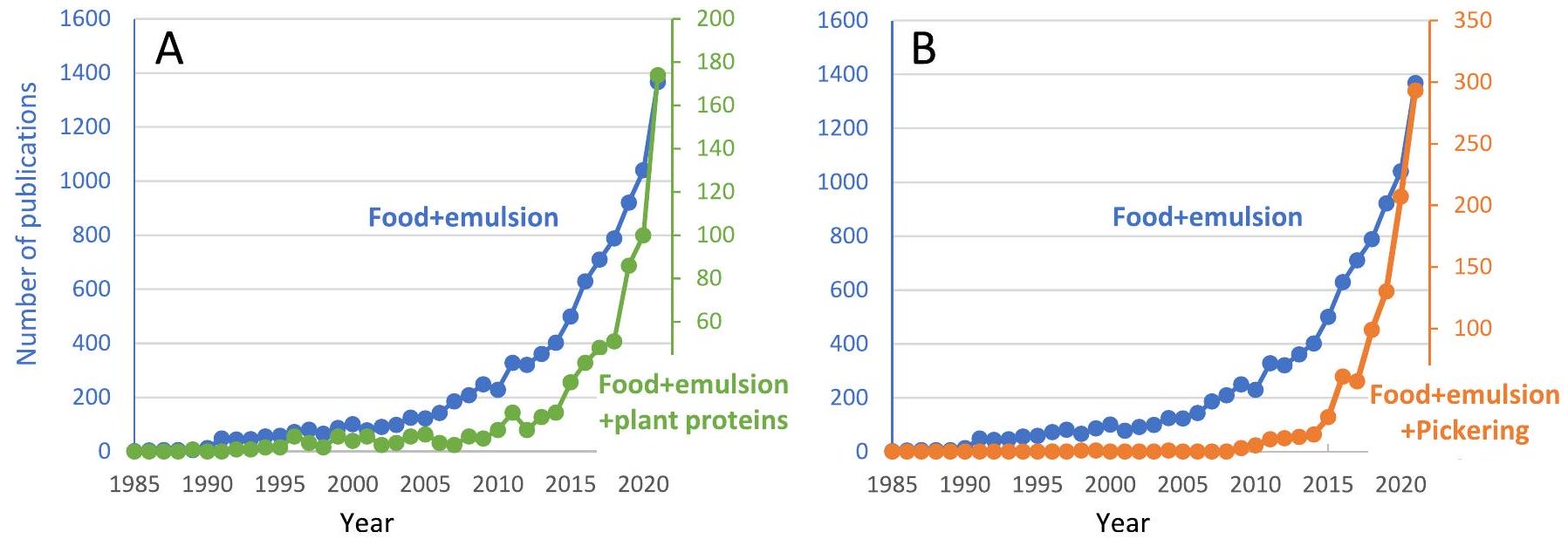
3.2.2. مستحلبات بيكيرينغ
هيكل مختلف تمامًا، يفتقر إلى الجزيئات السطحية. أشارت النتائج إلى أن جزيئات بيكرينغ لم تمنع، بحد ذاتها، أكسدة الدهون مقارنةً بالمستحلبات المدعومة بالكازينيت، مما يشير إلى أن هذه الجزيئات لم تحفز تأثير حاجز فيزيائي عند السطح. ومع ذلك، أظهر المستحلب بيكرينغ استقرارًا أكسديًا أعلى مقارنةً بمستحلب التحكم الذي يحتوي على دهون ذات نقطة انصهار عالية في نواة القطيرات. تم نسب ذلك إلى تبلور الدهون داخل القطيرات في النظام الأخير، مما طرد الأحماض الدهنية المتعددة غير المشبعة القابلة للتغيير من داخل القطيرات إلى السطح، مما يسهل اتصالها بالعوامل المؤكسدة في المرحلة المائية. استكشف باحثون آخرون أيضًا استخدام جزيئات مختلفة قائمة على الدهون لتثبيت مستحلبات بيكرينغ [165]. وأبرزوا أهمية نوع الدهون المستخدمة لتشكيل الجزيئات في التأثير على أكسدة نواة قطيرات الزيت. على سبيل المثال، أظهرت المستحلبات بيكرينغ المصنوعة من جزيئات التريميستين أو زيت الزيتون مقاومة أكبر لأكسدة الدهون مقارنةً بمستحلب مدعوم بالبروتين التقليدي، بينما لم تظهر تلك المصنوعة من جزيئات زيت النخيل ميزة واضحة. من المثير للاهتمام، أن التأثير الوقائي للجزيئات السابقة لوحظ فقط عندما كانت موجودة أثناء تجانس المستحلب، وليس عند إضافتها بعد الاستحلاب، وهو تحكم مهم لنسب التأثير الوقائي للجزيئات إلى موضعها السطحي. علاوة على ذلك، لم يبدو أن الحالة الفيزيائية (بلورية مقابل سائلة) لمادة الدهون داخل الجزيئات كانت عاملًا مهيمنًا يؤثر على التأثير الوقائي لطبقة الجزيئات [165].
3.3. التوازن بين المستحلبات الممتصة/غير الممتصة
النشاط المضاد للأكسدة في المستحلبات الغذائية.
طريقة إضافة المضاد للأكسدة أثناء تحضير المستحلبات الغذائية مهمة أيضًا [167-169]. على سبيل المثال، فإن إضافة
تشارك في تسريع أكسدة القطرات المحيطة خلال خطوة الانتشار. على العكس، في حالة الدهون المحبة للدهون جدًا (مثل TAG-OOH، إلخ)، قد يشك المرء في أن انتشارها المحتمل سيكون مقيدًا، مما سيحد من تأثير المستحلبات غير الممتصة ونشاط المحفزات للأكسدة بين القطرات في المرحلة المبكرة من الأكسدة [27،98]. أخيرًا، تعتبر الهيدروبيروكسيدات الناتجة عن الأحماض الدهنية الحرة (FFA-OOH) أكثر نشاطًا سطحيًا من نظائرها التي تتكون على الدهون ذات الوزن الجزيئي الأعلى (TAGs، ثنائي الجليسريدات، إلخ) وأكثر استقرارًا من جذورها (FFA-OO•)، التي لها عمر نصف محدود، مما يجعلها مرشحًا جادًا في نشاط المحفزات للأكسدة بين القطرات [100]. ومع ذلك، ليس من الواضح ما إذا كان يمكن تحقيق مثل هذه الميكلية بواسطة FFAOOH وحدها أو ما إذا كان سيكون من الضروري وجود فائض من المستحلبات غير الممتصة (تكوين ميكلي مشترك).
زادت جزيئات مضادات الأكسدة مثل التوكوفيرولات في المنطقة الواجهة بسرعة مع زيادة تركيز السطحي [181،182]. تسلط هذه النتائج، إلى جانب العديد من النتائج الأخرى [101،173،183،184]، الضوء على دور الميسيلات في فعالية مضادات الأكسدة. وفقًا للأدبيات، بالنسبة لأكثر مضادات الأكسدة المحبة للدهون، فإن تكوين الميسيلات من شأنه تعزيز فعاليتها من خلال تحسين انتشارها و/أو تركيزها في المنطقة الواجهة، مما يعني أيضًا أن الشكل المؤكسد عند واجهة قطرات الدهون يمكن أن يتم استبداله بسرعة أكبر. بالنسبة لمضادات الأكسدة ذات القطبية المتوسطة والطبيعة الأمفيفيلية، فإن تكوين الميسيلات سيلجأ إلى تقليل تأثيرها، على الأرجح من خلال تقليل تركيزها عند واجهة الزيت والماء (“تأثير التخفيف”). بالنسبة لمضادات الأكسدة القابلة للذوبان في الماء بشكل كبير، لا يبدو أن هناك أي اتجاه محدد، على الأرجح لأن التأثير على توزيع مضادات الأكسدة سيكون أقل أهمية من التأثيرات المؤكسدة بين القطرات الناتجة عن الدهون والمعادن.
4. حساسية المستحلبات نفسها للأكسدة
4.1. مواد خافضة للتوتر السطحي ذات الوزن الجزيئي المنخفض
الكميات (
4.2. الفوسفوليبيدات
المستحلبات حسّنت من الاستقرار الأكسيدي لـ
تشمل المنتجات الوسيطة التي تتشكل في مسار التفاعل هذا استرات دهنية كيتونية إبوكسي، وإبوكسي ألكينال، وهيدروكسي ألكينال [235،239]. تم توثيق أنماط وسرعة أكسدة الفوسفوليبيدات بشكل كبير عند التعامل مع أنظمة ليبوسوم للغذاء أو التطبيقات الصيدلانية أو التجميلية. يتأثر الاستقرار الأكسيدي لليبوسومات بشكل كبير بحجمها وتركيبها الجزيئي بالإضافة إلى التركيب الجزيئي للوسط المحيط بها [240]. على سبيل المثال، بالنسبة للفوسفوليبيدات البحرية، فإن هذا التحول سريع جدًا لدرجة أن التكوين المعتاد لهيدروبيروكسايد الدهون والألدهيدات الذي يتم تقييمه من خلال قياس قيمة البيروكسيد (PV)، قيمة الأنيزيدين (AV) أو المواد الثيوباربيتورية (TBARS) لا يمكن اكتشافه، مما قد يؤدي إلى استنتاج خاطئ بعدم حدوث أكسدة للدهون [241]. فيما يتعلق بالمستحلبات التي يتم تثبيتها بواسطة الليسيثينات، على حد علمنا، لا توجد أبحاث حيث تم مقارنة حركيات أكسدة الفوسفوليبيدات مقابل تلك الخاصة بـ TAGs. ومع ذلك، في مثل هذه المستحلبات، حيث تقع الفوسفوليبيدات إما في المنطقة الواجهة أو كتركيبات غروانية في المرحلة المستمرة، يمكن افتراض أنه، مقارنة بقطرات الدهون، ستكون الفوسفوليبيدات أكثر اتصالًا بالمؤكسدات ويمكن أن تتأكسد بشكل أسرع. بالمثل، يمكن الإشارة إلى الدراسات المختلفة التي أجريت على دراسة الاستقرار الأكسيدي في نظام اللحم النموذجي. قام إجن [242] بتقييم تأثير TAGs والفوسفوليبيدات على تطور الرائحة باستخدام ألياف عضلية خالية من الدهون بالاشتراك مع TAGs أو فوسفوليبيدات مضافة. أظهرت النتائج أن كل من TAGs والفوسفوليبيدات تساهم في تطور الرائحة، على الرغم من أن الفوسفوليبيدات كانت الأولى في الأكسدة. لاحقًا، أظهرت نفس مجموعة البحث أن PE و PC كانا حساسين بشكل خاص للأكسدة في اللحم المجمد [243]. أكد آخرون هذه الملاحظة ووجدوا أن
4.3. البروتينات
كشفت عن توزيع غير متجانس لأكسدة البروتين عند الواجهة مما يشير إلى حبيبات البروتين الدهني التي توجد عادة في صفار البيض. أخيرًا، في غياب التوكوفيرولات (زيت مُنزع)، تم تعزيز أكسدة البروتين سواء عند الواجهة أو في المرحلة المستمرة. وقد تم عزو هذه الزيادة في أكسدة البروتين عند إزالة التوكوفيرول إلى نقل الجذور الحرة الدهنية من قطرات الزيت إلى الواجهة والمرحلة المستمرة. كما قام المؤلفون بتقييم تأثير حمض الأسكوربيك على أكسدة الدهون والبروتين. في وجود التوكوفيرولات، أظهر حمض الأسكوربيك تأثيرًا مضادًا للأكسدة تجاه الدهون، بينما في غياب التوكوفيرولات، تصرف كعامل مؤكسد. ومع ذلك، أظهرت دراسات أخرى أن حمض الأسكوربيك يعمل كعامل محفز قوي لأكسدة الدهون في المايونيز المُعد بمزيج من زيت اللفت وزيت السمك، الذي يحتوي على التوكوفيرولات. فيما يتعلق بأكسدة البروتين، عمل حمض الأسكوربيك كعامل مؤكسد للبروتينات الممتصة عند الواجهة وكذلك لتلك الموجودة في المرحلة المستمرة. ومن المثير للاهتمام، تم الإبلاغ عن سلوكيات متناقضة مع مضادات الأكسدة الأخرى فيما يتعلق بأكسدة الدهون أو البروتين.
5. مضادات أكسدة جديدة من الأنظمة النموذجية إلى تطبيقات الطعام الحقيقية
مما دفع إلى إعادة تقييم المفارقة القطبية وإدخال مفاهيم ونظريات جديدة [278]. على سبيل المثال، في الزيت السائب، كما تم شرحه سابقًا (القسم 2.4.2)، تم إثبات أن الموقع الحرج للأكسدة ليس هو واجهة الهواء والزيت، كما تم اقتراحه سابقًا في المفارقة القطبية. بدلاً من ذلك، تحدث الأكسدة في الكولودات المرتبطة التي تتكون من آثار من الماء وجزيئات نشطة سطحياً مثل الفوسفوليبيدات. في نفس الفترة، تم إثبات أنه في المستحلبات O/W، ينطبق علاقة غير خطية بين الكارهة للماء وقدرة مضادات الأكسدة [279]. تم دراسة هذا التأثير، المعروف باسم تأثير القطع، بشكل موسع وتم تأكيده، كما هو موضح في ما يلي (القسم 5.1).
5.1. الفينوليبيدات وتأثير القطع
أو الهيدروكسيل الأولي مع الأحماض الكربوكسيلية الأليفاتية على التوالي. وبالتالي، من خلال الحفاظ على الهيدروكسيل الفينولي، احتفظت الجزيئات الناتجة، التي تُسمى “الفينوليبيدات”، بجميع تفاعليتها مع الجذور الحرة. تعتبر زيادة المحبة للدهون عن طريق الاسترification، التي تُجرى عادةً في ظروف قاسية مع محفزات حمضية قوية (حمض الهيدروكلوريك، حمض الكبريتيك، حمض بارا-تولوين السلفوني، راتنجات الأحماض السلفونية)، فعالة جدًا وكميّة، ويمكن دفعها إلى الاكتمال من خلال التجفيف المستمر للوسط عندما يتم إنتاج الماء. على العكس من ذلك، تسمح الإنزيمات بتخليق الفينوليبيدات في ظروف أكثر اعتدالًا مع اختيار أفضل وأقل تفاعلات جانبية. ومع ذلك، فإن التحفيز الإنزيمي غالبًا ما يكون أبطأ بكثير، وقد يكون عرضة لظواهر التثبيط، وكما تم وصفه مؤخرًا في عدة مراجعات، يتطلب ضبطًا دقيقًا للعديد من المعلمات ليكون فعالًا [283-285].
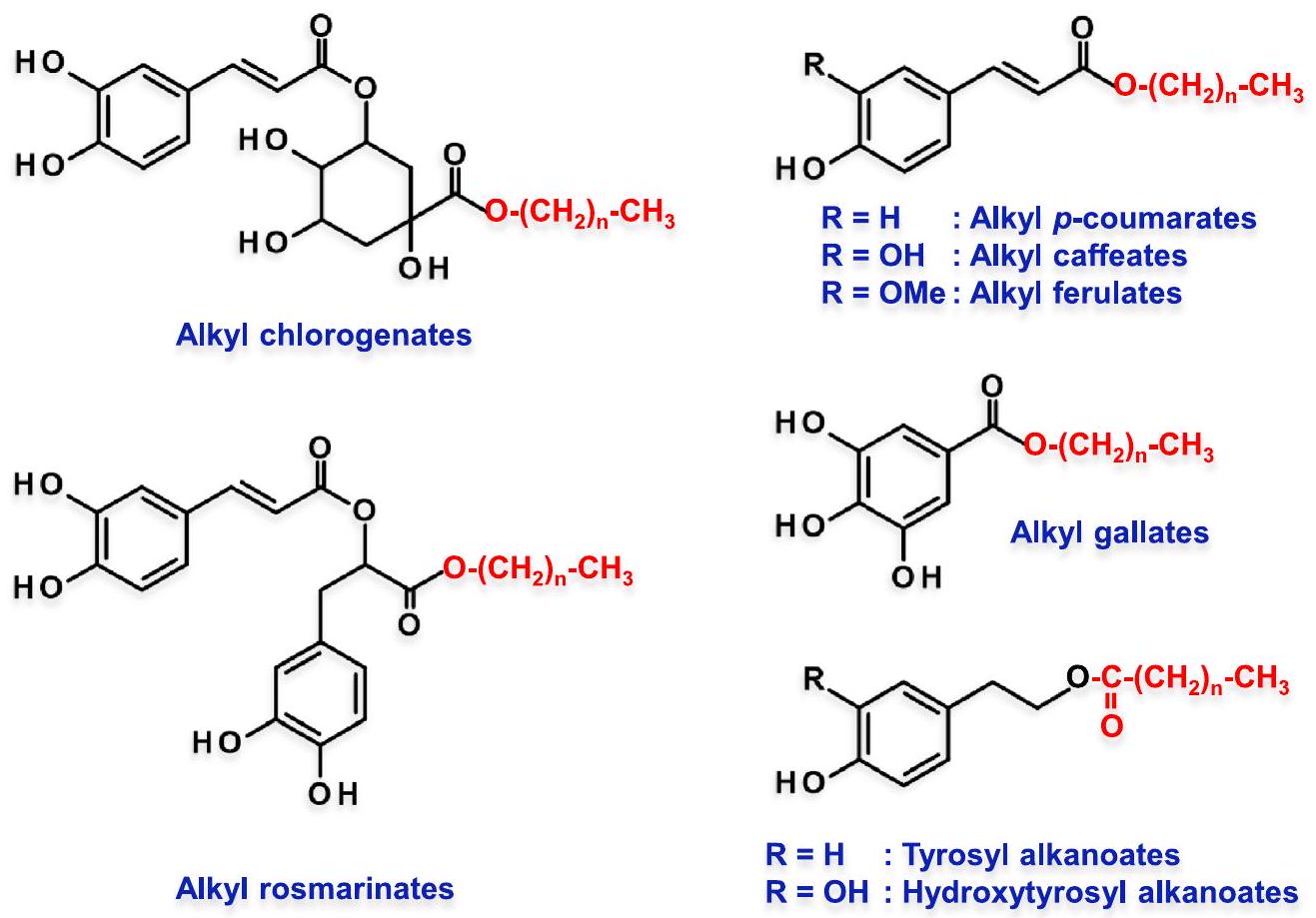
بروبيل؛ C8، أوكتيل؛ C12، أوكتادسيل) (الشكل 3) في مستحلبات زيت الزيتون أو زيت الذرة في الماء بنسب مختلفة من الزيت: الماء (
أطوال السلسلة الحرجة (CCL) ضمن سلسلة الفينوليد.
| فينوليبدات | سلاسل الألكيل المختبرة
|
نظام الدهون | مستحلب | CCL
|
ملاحظات | مرجع |
| ألكانات هيدروكسي تيروسيل | 0، 2، 4، 8، 12 | مستحلبات السمك O/W | ليسيثين | ٨ | [292] | |
| روسمارينات الألكيل |
|
مستحلب تونغ O/W | بريج 35 | ٨ | [293] | |
| روسمارينات الألكيل | 0، 4، 8، 12، 18، 20 | مستحلب فول الصويا O/W | توين 20 | ٤ | [180] | |
| كومارات الألكيل | 1، 4، 8، 12، 16، 18، 20 | مستحلب تونغ O/W | بريج 35 | 12 | نشاط مضاد للأكسدة ضعيف جداً لجميع المركبات الفينولية | [294] |
| فوريلات الألكيل |
|
مستحلب تونغ O/W | بريج 35 | ٤-١٢ | [294] | |
|
|
حليب مدعم بزيت السمك | – | 1 | أثر مؤكسد لـ C12 و C8؛ C16 و C20 شبه غير نشط | [295] | |
|
|
مستحلب تونغ O/W | بريج 35 | ٨ | [294] | ||
| كافئات الألكيل | 0، 1، 4، 8، 12، 18 | مايونيز مدعم بزيت السمك | – | ٤-١٢ | مايونيز قائم على زيت اللفت | |
|
|
حليب مدعم بزيت السمك | – | 1-4 | [296] | ||
| بروتوكاتشوات الألكيل | 1، 2، 4، 6، 8، 10، 12، 14، 16، 18 | مستحلب تونغ O/W | بريج 35 | 2-4 | عملت C2-C6 ككاسرات سلسلة، بينما عملت C12-C20 كمؤخرات. | [297] |
| الألكيل ريسورسينولات من نخالة الجاودار الطبيعية | 17، 19، 21، 23، 25 | مستحلب الطحالب O/W | بريج L23 | 21 | كانت جميع الألكيل ريسورسينولات أكثر فعالية من الأورسينول | [298] |
| غالات الألكيل | 0، 3، 8، 12، 16 | مستحلب مجفف بالرش (بروتين مصل اللبن + كازينات + زيوت عباد الشمس والأسماك) | – | ٨ | تأثير قطع طفيف جداً. جالات الهيدروفوبية (
|
[299] |
5.2. الببتيدات (على سبيل المثال، المشتقة من المنتجات الثانوية)
5.2.1. النهج من الأعلى إلى الأسفل للحصول على ببتيدات مضادة للأكسدة
5.2.2. النهج التصاعدي للحصول على ببتيدات مضادة للأكسدة
أظهرت الدراسة نقص تقنيات الفحص الموثوقة لاختبار خلب المعادن للببتيدات الاصطناعية والحاجة إلى تقنيات متقدمة جديدة. من ناحية أخرى، تم اعتبار معلمات أخرى مهمة لنشاط خلب المعادن مثل النقطة المعزولة والشحنة الصافية عند pH 7.0 لاختيار الببتيدات الخالبة للمعادن. أظهرت النتائج أن جميع الببتيدات قدمت استقرارًا أكسديًا أفضل أو مشابهًا مقارنةً بالإيمولسيون الضابطة بدون مضادات الأكسدة وبالتالي منعت أكسدة الدهون في نظام إيمولسيون نموذجي. بشكل خاص، كانت الببتيدات المشحونة تؤدي بشكل أفضل من الببتيدات المحايدة، وكان طول الببتيدات النشطة يتراوح بين 6 و 14 حمضًا أمينيًا. أظهرت هذه الدراسة إمكانيات المعلوماتية الحيوية والبروتيوميات في تحديد الببتيدات الطبيعية المستدامة الغنية بمضادات الأكسدة الموجودة في البروتينات الأم. تتيح المنهجية استهداف الببتيدات بدقة من خلال التحلل المائي الإنزيمي المصمم، باستخدام خصوصية البروتياز وتحليل تسلسل المعلوماتية الحيوية.
5.2.3. فحص الببتيدات الخالبة للمعادن
(IMAC). تعتبر تقنية SPR تقنية بصرية لتحديد ثوابت التفكك (
5.3. جزيئات بيكرينغ المحملة بمضادات الأكسدة: جزيئات مصممة عمدًا (من الأسفل إلى الأعلى) مقابل الهياكل الطبيعية (من الأعلى إلى الأسفل)
(DMSNs)، التي تعمل كحاملات نانوية لمضادات الأكسدة المحبة للماء أو الكارهة للماء (تم إثبات ذلك بالنسبة للإيبيغالوكتشين غالاتي والريسفيراترول) وكمنظمات بيكرينغ [335]. على الرغم من أن هذا النظام تم اختباره فقط لتثبيت زيت النكهة (وليس زيت غني بالأحماض الدهنية المتعددة غير المشبعة)، إلا أن الجسيمات احتفظت بشكل فعال بمضاد الأكسدة المستهدف داخل هيكلها الداخلي وحمت بشكل كبير السيترال من الأكسدة. لقد أثبتت هذه التصاميم المتقدمة الهرمية للجسيمات البيكرينغ ثنائية الوظيفة مفهوم الخزانات المضادة للأكسدة على الواجهة وتقدم وعدًا لتعزيز فعالية مضادات الأكسدة الطبيعية. ومع ذلك، فإن أحد العيوب الرئيسية التي قد تحد من تطبيقها على نطاق واسع هو التعقيد والتكلفة لمثل هذه الاستراتيجية، والتي قد لا تتماشى مع الاتجاه الحالي نحو أنظمة الغذاء الطبيعية، ذات الملصقات النظيفة، والمعالجة بشكل ضئيل. قد تتضمن نهجًا بديلاً، مع الاستمرار في استخدام مفهوم الجسيمات البيكرينغ المحملة بمضادات الأكسدة، الاستفادة من المحتوى الطبيعي لمضادات الأكسدة في الجسيمات الموجودة بشكل طبيعي (أي، اعتماد نهج “من الأعلى إلى الأسفل” بدلاً من “من الأسفل إلى الأعلى”، كما في الأمثلة السابقة)، مثل الجوانب الجانبية للمنتجات الغذائية والمنتجات المستندة إلى البيولوجيا. بينما سيكون مستوى التحكم في التركيب والبنية لهذه الجسيمات بالضرورة أقل من تلك الجسيمات المركبة المصممة خصيصًا، فإن لديها إمكانات كبيرة من حيث الاستدامة والطبيعية والاحتفاظ بالمضادات الأكسدة على الواجهة [159]. أحد الأمثلة على هذه الاستراتيجية تتضمن المستحلبات البيكرينغ المستقرة بجسيمات الأرز الأحمر المطحون التي تحتوي على الأنثوسيانين [336]. أظهرت هذه الدراسة أن هذه الجسيمات الغنية بالبوليفينول قدمت حماية أفضل لقطرات الزيت المستحلب ضد الأكسدة مقارنة بالمستحلبات المستقرة بالنشا من الأرز الأبيض والزيت السائب. أظهرت دراسة حديثة أخرى تركزت على تحضير المستحلبات البيكرينغ باستخدام جسيمات نباتية متنوعة، أن المستحلبات المستقرة بجسيمات شاي الماتشا وأوراق السبانخ كانت مستقرة للغاية ضد أكسدة الدهون، على عكس المستحلبات المرجعية المستقرة بواسطة المستحلبات التقليدية [337]. من المحتمل أن يُعزى هذا التأثير الوقائي إلى وجود مضادات الأكسدة الذاتية في هذه الفئات، مثل الفينولات التي تلتقط الجذور الحرة والأحماض العضوية المخلبية، على التوالي. يبدو أن استخدام مثل هذه الجسيمات الطبيعية هو نهج واعد لتثبيت المستحلبات ذات الملصقات النظيفة بشكل مادي وأكسدي. نظرًا للوجود الواسع والتنوع لمضادات الأكسدة الفينولية في المواد النباتية المتوافقة مع الغذاء، فلا شك أن هناك العديد من المصادر المحتملة لجسيمات البيكرينغ لاستكشافها.
5.4. نحو مكونات نظيفة و متعددة الوظائف
نظرة عامة على المكونات (المحتملة) ذات العلامة النظيفة التي تمتلك نشاطًا مضادًا للأكسدة في مستحلبات الطعام O/W.
| مستحلب الطعام | مكون حيوي | المكونات/ الآليات المقترحة لمضادات الأكسدة | مرجع |
| مايونيز | بقايا التفاح | الفينولات | [342] |
| مسحوق الزنجبيل | غير محدد | [343] | |
| مستخلص بذور العنب | التخلص من الجذور الحرة بواسطة الفينولات | ||
| خل العنب والتفاح | الفينولات | [345] | |
| خل البلسميك | ميلانويدينات | [346] | |
| مستخلص قشر التفاح | الفينولات | [347] | |
| مستخلصات الطحالب البنية | الفينولات | [348] | |
| جذر الشمندر | الفينولات، البيتالين | [349] | |
| قشر الشمندر | الفينولات، البيتالين | [٣٥٠] | |
| منتج ثانوي من الطماطم | كاروتينات | [351] | |
| مستخلصات الذرة الأرجوانية | الأنثوسيانين | [352] | |
| مستخلصات بذور الخيار | الفينولات | [353] | |
| مايونيز نباتي | نفايات معصرة الزيتون | الفينولات | [354] |
| دقيق الفاكهة | الفينولات | [355] | |
| خل الزيتون | الفينولات | [356] | |
| مستخلص كعكة الكمون | الفينولات، التخلص من الجذور الحرة | [357] | |
| نموذج مستحلب O/W | نسب القهوة المحمصة | تقسيم MRPs | [358] |
| خل ورق الزيتون | الفينولات، أوليوروبين | [359] | |
| مستخلصات نخالة الأرز | غير محدد | [360] |
المصدر من النفايات أو الجوانب الجانبية. بالنسبة لمعظم المكونات في الجدول 2، تم نسب نشاطها المضاد للأكسدة إلى وجود الفينولات في المرحلة المائية من مستحلبات الطعام، ولكن دون إقامة رابط آلي سببي واضح.
ضمان سلامة الغذاء، بما في ذلك المنتجات الثانوية المستخدمة كمكونات غذائية. ومع ذلك، عندما تعمل هذه المنتجات الثانوية كإضافات طبيعية، يصبح الامتثال لائحة الأغذية الجديدة (اللائحة الأوروبية رقم 2015/2283) إلزاميًا. تحدد هذه اللائحة فئات الأغذية الجديدة التي تنشأ من النباتات والحيوانات والميكروبات وتقنيات الإنتاج الجديدة. تتطلب هذه الأغذية الحصول على إذن وإدراجها في قائمة الأغذية الجديدة المصرح بها التي تديرها الهيئة الأوروبية لسلامة الغذاء (EFSA). يجب الإشارة إلى أن عدم وجود تعريف واضح لنفايات الطعام والمنتجات الثانوية يشكل تحديًا، مما يعيق التقييم الدقيق ويعوق التدابير لمعالجة فقدان الطعام والنفايات. لذلك، يبدو أن التعريفات الواضحة لهذه المصطلحات ضرورية للبحوث المستقبلية وبرامج الأعمال لتعزيز الابتكار في نظام الاقتصاد الدائري. توحيد السياسات، وتقليل الأعباء الإدارية، وإرساء تنظيمات مستقرة هي أمور حيوية لتعزيز الاستثمارات وتسهيل تحويل النفايات إلى موارد قيمة. علاوة على ذلك، يعد الكودكس أليمنتاريوس، وهو مبادرة من منظمة الأغذية والزراعة (FAO) ومنظمة الصحة العالمية (WHO)، مرجعًا عالميًا لسلامة الغذاء، حيث يضع معايير وإرشادات دولية. غالبًا ما تتضمن هذه الإرشادات حدودًا مسموح بها للملوثات والإضافات والمغذيات، والتي تعتبر ضرورية لتطوير تنظيمات سلامة غذاء سليمة. تحدد الهيئات التنظيمية مثل إدارة الغذاء والدواء (FDA) في الولايات المتحدة والهيئة الأوروبية لسلامة الغذاء (EFSA) مستويات الاستهلاك اليومي المقبول (ADI) للإضافات لحماية المستهلكين من المواد الضارة. ومع ذلك، لا تزال التحديات قائمة في تقييم ملاءمة وسلامة المنتجات الثانوية بسبب غياب حدود قانونية محددة. لذلك، غالبًا ما تعتمد القيم المرجعية لتقييم المنتجات الثانوية في هذا الصدد على الحدود المحددة للمواد الأصلية أو المصفوفات المماثلة، مما يؤدي إلى تعقيد تفسير النتائج وضمان سلامة المستهلك.
6. الاستنتاجات والرؤية المستقبلية
التقدمات المنهجية الرائدة التي هي قيد التطوير النشط، سيكون إنشاء ممارسات موحدة محورياً في معالجة تعقيد تفاعلات أكسدة الدهون. هذا التوافق في المنهجيات عبر الجهود البحثية لن يعزز فقط القابلية للمقارنة ولكن سيسهل أيضاً فهمًا أكثر شمولية لآليات الأكسدة. في الوقت نفسه، فإن تطور تقنيات النمذجة يظهر كحدود حاسمة. سيكون تحسين وتطوير هذه النماذج ضروريًا في التنبؤ ومحاكاة سلوك أكسدة الدهون داخل هذه الأنظمة، مما يساعد في تصميم التجارب وكشف العلاقات المعقدة بين العوامل المعنية.
مصادر التمويل
بيان مساهمة المؤلفين وفقاً لنظام CRediT
إعلان عن تضارب المصالح
References
[2] Cosgrove JP, Church DF, Pryor WA. The kinetics of the autoxidation of polyunsaturated fatty acids. Lipids. 1987;22(5):299-304. https://doi.org/ 10.1007/BF02533996.
[3] McClements DJ, Decker EA. Lipid oxidation in oil-in-water emulsions: impact of molecular environment on chemical reactions in heterogeneous food systems.
[4] Berton-Carabin CC, Ropers MH, Genot C. Lipid oxidation in oil-in-water emulsions: involvement of the interfacial layer. Comprehen Rev Food Sci Food Safet 2014;13(5):945-77. https://doi.org/10.1111/1541-4337.12097.
[5] ten Klooster S, Boerkamp V, Lazaridi E, Yang S, Takeuchi M, Berton-Carabin C, et al. Lipid oxidation in food emulsions: Analytical challenges and recent developments. In: Bravo-Diaz C, editor. Lipid oxidation in food and biological systems: A physical chemistry perspective. Cham: Springer International Publishing; 2022. p. 3-29. https://doi.org/10.1007/978-3-030-87222-9_1.
[6] Barriuso B, Astiasarán I, Ansorena D. A review of analytical methods measuring lipid oxidation status in foods: a challenging task. Eur Food Res Technol 2013; 236(1):1-15. https://doi.org/10.1007/s00217-012-1866-9.
[7] Laguerre M, Lecomte J, Villeneuve P. Evaluation of the ability of antioxidants to counteract lipid oxidation: existing methods, new trends and challenges. Prog Lipid Res 2007;46(5):244-82. https://doi.org/10.1016/j.plipres.2007.05.002.
[8] Xia W, Budge SM. Techniques for the analysis of minor lipid oxidation products derived from Triacylglycerols: epoxides, alcohols, and ketones. Compr Rev Food Sci Food Saf 2017;16(4):735-58. https://doi.org/10.1111/1541-4337.12276.
[9] Jacobsen C, García-Moreno PJ, Yesiltas B, Sørensen A-DM. Chapter 9 – lipid oxidation and traditional methods for evaluation. In: García-Moreno PJ, et al., editors. Omega-3 delivery systems. Academic Press; 2021. p. 183-200. https:// doi.org/10.1016/B978-0-12-821391-9.00009-0.
[10] McClements DJ. Food emulsions: Principles, practices, and techniques. 3rd edition ed. Boca Raton: CRC Press; 2015. https://doi.org/10.1201/b18868.
[11] Food emulsifiers and their applications. 3rd edition ed. Springer Cham; 2019. p. 522. https://doi.org/10.1007/978-3-030-29187-7.
[12] Drusch S, Klost M, Kieserling H. Current knowledge on the interfacial behaviour limits our understanding of plant protein functionality in emulsions. Curr Opin Colloid Interface Sci 2021;56:101503. https://doi.org/10.1016/j. cocis.2021.101503.
[13] Dickinson E. Proteins at interfaces and in emulsions stability, rheology and interactions. J Chem Soc Faraday Trans 1998;94(12):1657-69. https://doi.org/ 10.1039/A801167B.
[14] Dickinson E. Use of nanoparticles and microparticles in the formation and stabilization of food emulsions. Trends Food Sci Technol 2012;24(1):4-12. https://doi.org/10.1016/j.tifs.2011.09.006.
[15] Berton-Carabin CC, Schroen K. Pickering emulsions for food applications: background, trends, and challenges. Annu Rev Food Sci Technol 2015;6:263-97. https://doi.org/10.1146/annurev-food-081114-110822.
[16] Murray BS. Pickering emulsions for food and drinks. Curr Opin Food Sci 2019;27: 57-63. https://doi.org/10.1016/j.cofs.2019.05.004.
[17] Pickering SU. CXCVI.-emulsions. J Chem Soc Trans 1907;91(0):2001-21. https://doi.org/10.1039/CT9079102001.
[18] Tcholakova S, Denkov N, Lips A. Comparison of solid particles, globular proteins and surfactants as emulsifiers. Phys Chem Chem Phys 2008;10(12):1608-27. https://doi.org/10.1039/B715933C.
[19] Berton-Carabin CC, Sagis L, Schroen K. Formation, structure, and functionality of interfacial layers in food emulsions. Annu Rev Food Sci Technol 2018;9:551-87. https://doi.org/10.1146/annurev-food-030117-012405.
[20] Bos MA, van Vliet T. Interfacial rheological properties of adsorbed protein layers and surfactants: a review. Adv Colloid Interface Sci 2001;91(3):437-71. https:// doi.org/10.1016/S0001-8686(00)00077-4.
[21] Murray BS. Rheological properties of protein films. Curr Opin Colloid Interface Sci 2011;16(1):27-35. https://doi.org/10.1016/j.cocis.2010.06.005.
[22] Hinderink EB, Sagis L, Schroën K, Berton-Carabin CC. Behavior of plant-dairy protein blends at air-water and oil-water interfaces. Colloids Surf B Biointerfaces 2020;192:111015. https://doi.org/10.1016/j.colsurfb.2020.111015.
[23] Chevalier Y, Bolzinger M-A. Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloids Surf A Physicochem Eng Asp 2013;439:23-34. https://doi.org/10.1016/j.colsurfa.2013.02.054.
[24] ten Klooster S, Takeuchi M, Schroën K, Tuinier R, Joosten R, Friedrich H, et al. Tiny, yet impactful: detection and oxidative stability of very small oil droplets in surfactant-stabilized emulsions. J Colloid Interface Sci 2023. https://doi.org/ 10.1016/j.jcis.2023.09.005.
[25] Guzey D, McClements DJ. Formation, stability and properties of multilayer emulsions for application in the food industry. Adv Colloid Interface Sci 2006; 128-130:227-48. https://doi.org/10.1016/j.cis.2006.11.021.
[26] Samtlebe M, Yucel U, Weiss J, Coupland JN. Stability of solid lipid nanoparticles in the presence of liquid oil emulsions. J Am Oil Chem Soc 2012;89(4):609-17. https://doi.org/10.1007/s11746-011-1944-3.
[27] Klooster ST, Schroën K, Berton-Carabin C. Lipid oxidation products in model food emulsions: do they stay in or leave droplets, that’s the question. Food Chem 2023; 405:134992. https://doi.org/10.1016/j.foodchem.2022.134992.
[28] McClements D, Dungan S, German J, Kinsella J. Oil exchange between oil-inwater emulsion droplets stabilised with a non-ionic surfactant. Food Hydrocoll 1992;6(5):415-22. https://doi.org/10.1016/S0268-005X(09)80027-1.
[29] Schaich KM. Lipid oxidation: theoretical aspects. In: Bailey’s Industrial Oil and Fat Products; 2005. https://doi.org/10.1002/047167849X.bio067.
[30] Frankel EN. Chapter 1 – free radical oxidation. In: Frankel EN, editor. Lipid oxidation. 2nd ed. Woodhead Publishing; 2012. p. 15-24. https://doi.org/ 10.1533/9780857097927.15.
[31] Frankel EN. Lipid Oxidation. Elsevier; 2014.
[32] Kerr JA. Bond dissociation energies by kinetic methods. Chem Rev 1966;66(5): 465-500. https://doi.org/10.1021/cr60243a001.
[33] Frankel EN. Volatile lipid oxidation products. Prog Lipid Res 1983;22(1):1-33. https://doi.org/10.1016/0163-7827(83)90002-4.
[34] Schaich KM. Metals and lipid oxidation. Contemporary issues. Lipids. 1992;27(3): 209-18. https://doi.org/10.1007/BF02536181.
[35] Minotti G, Aust SD. Redox cycling of iron and lipid peroxidation. Lipids. 1992;27 (3):219-26. https://doi.org/10.1007/BF02536182.
[36] Mozuraityte R, Kristinova V, Rustad T, Storrø I. The role of iron in peroxidation of PUFA: effect of pH and chelators. Eur J Lipid Sci Technol 2016;118(4):658-68. https://doi.org/10.1002/ejlt.201400590.
[37] Mozuraityte R, Rustad T, Storrø I. The role of iron in peroxidation of polyunsaturated fatty acids in liposomes. J Agric Food Chem 2008;56(2):537-43. https://doi.org/10.1021/jf0716073.
[38] Ingold KU. Inhibition of the autoxidation of organic substances in the liquid phase. Chem Rev 1961;61(6):563-89. https://doi.org/10.1021/cr60214a002.
[39] Jacobsen C, Timm M, Meyer AS. Oxidation in fish oil enriched mayonnaise: ascorbic acid and low pH increase oxidative deterioration. J Agric Food Chem 2001;49(8):3947-56. https://doi.org/10.1021/jf001253e.
[40] Jacobsen C, Let MB, Nielsen NS, Meyer AS. Antioxidant strategies for preventing oxidative flavour deterioration of foods enriched with n-3 polyunsaturated lipids: a comparative evaluation. Trends Food Sci Technol 2008;19(2):76-93. https:// doi.org/10.1016/j.tifs.2007.08.001.
[41] Shahidi F, Ambigaipalan P. Phenolics and polyphenolics in foods, beverages and spices: antioxidant activity and health effects-A review. J Funct Foods 2015;18: 820-97. https://doi.org/10.1016/j.jff.2015.06.018.
[42] Quideau S, Deffieux D, Douat-Casassus C, Pouységu L. Plant polyphenols: chemical properties, biological activities, and synthesis. Angew Chem Int Ed 2011;50(3):586-621. https://doi.org/10.1002/anie.201000044.
[43] Jacobsen C, Hartvigsen K, Thomsen MK, Hansen LF, Lund P, Skibsted LH, et al. Lipid oxidation in fish oil enriched mayonnaise: calcium disodium ethylenediaminetetraacetate, but not gallic acid, strongly inhibited oxidative deterioration. J Agric Food Chem 2001;49(2):1009-19. https://doi.org/10.1021/ jf000729r.
[44] Gumus CE, Decker EA, McClements DJ. Impact of legume protein type and location on lipid oxidation in fish oil-in-water emulsions: lentil, pea, and faba bean proteins. Food Res Int 2017;100(Pt 2):175-85. https://doi.org/10.1016/j. foodres.2017.08.029.
[45] Berton C, M.H.L.N. Ropers, Guibert D, Solé VR, Genot C. Modifications of interfacial proteins in oil-in-water emulsions prior to and during lipid oxidation. J Agric Food Chem 2012;60(35):8659-71. https://doi.org/10.1021/jf300490w.
[46] Schaich KM. Lipid oxidation: new perspectives on an old reaction. In: Bailey’s Industrial Oil and Fat Products; 2020. p. 1-72. https://doi.org/10.1002/ 047167849X.bio067.pub2.
[47] Mubiru E, Shrestha K, Papastergiadis A, De Meulenaer B. Improved gas chromatography-flame ionization detector analytical method for the analysis of epoxy fatty acids. J Chromatogr A 2013;1318:217-25. https://doi.org/10.1016/j. chroma.2013.10.025.
[48] Marmesat S, Velasco J, Dobarganes MC. Quantitative determination of epoxy acids, keto acids and hydroxy acids formed in fats and oils at frying temperatures. J Chromatogr A 2008;1211(1):129-34. https://doi.org/10.1016/j. chroma.2008.09.077.
[49] Velasco J, Berdeaux O, Márquez-Ruiz G, Dobarganes MC. Sensitive and accurate quantitation of monoepoxy fatty acids in thermoxidized oils by gas-liquid chromatography. J Chromatogr A 2002;982(1):145-52. https://doi.org/ 10.1016/S0021-9673(02)01481-4.
[50] Velasco J, Marmesat S, Bordeaux O, Márquez-Ruiz G, Dobarganes C. Formation and evolution of Monoepoxy fatty acids in Thermoxidized olive and sunflower oils and quantitation in used frying oils from restaurants and fried-food outlets. J Agric Food Chem 2004;52(14):4438-43. https://doi.org/10.1021/jf030753f.
[51] Mubiru E, Shrestha K, Papastergiadis A, De Meulenaer B. Development and validation of a gas chromatography-flame ionization detection method for the determination of epoxy fatty acids in food matrices. J Agric Food Chem 2014;62 (13):2982-8. https://doi.org/10.1021/jf405664c.
[52] Grüneis V, Fruehwirth S, Zehl M, Ortner J, Schamann A, J.r. König, and M. Pignitter.. Simultaneous analysis of epoxidized and hydroperoxidized triacylglycerols in canola oil and margarine by LC-MS. J Agric Food Chem 2019; 67(36):10174-84. https://doi.org/10.1021/acs.jafc.9b03601.
[53] Fruehwirth S, Egger S, Flecker T, Ressler M, Firat N, Pignitter M. Acetone as indicator of lipid oxidation in stored margarine. Antioxidants. 2021;10(1):59. https://doi.org/10.3390/antiox10010059.
[54] Boerkamp VJ, Merkx DW, Wang J, Vincken J-P, Hennebelle M, van Duynhoven JP. Quantitative assessment of epoxide formation in oil and mayonnaise by 1H-13C HSQC NMR spectroscopy. Food Chem 2022;390:133145. https://doi.org/10.1016/j.foodchem.2022.133145.
[55] Steenhorst-Slikkerveer L, Louter A, Janssen H-G, Bauer-Plank C. Analysis of nonvolatile lipid oxidation products in vegetable oils by normal-phase highperformance liquid chromatography with mass spectrometric detection. J Am Oil Chem Soc 2000;77(8):837. https://doi.org/10.1007/s11746-000-0134-1.
[56] Morales A, Marmesat S, Ruiz-Méndez MV, Márquez-Ruiz G, Velasco J. Formation of oxidation products in edible vegetable oils analyzed as FAME derivatives by HPLC-UV-ELSD. Food Res Int 2014;62:1080-6. https://doi.org/10.1016/j. foodres.2014.05.063.
[57] Morales A, Dobarganes C, Márquez-Ruiz G, Velasco J. Quantitation of Hydroperoxy-, keto- and Hydroxy-dienes during oxidation of FAMEs from highlinoleic and high-oleic sunflower oils. J Am Oil Chem Soc 2010;87(11):1271-9. https://doi.org/10.1007/s11746-010-1624-8.
[58] Morales A, Marmesat S, Dobarganes MC, Márquez-Ruiz G, Velasco J. Quantitative analysis of hydroperoxy-, keto- and hydroxy-dienes in refined vegetable oils. J Chromatogr A 2012;1229:190-7. https://doi.org/10.1016/j. chroma.2012.01.039.
[59] Morales A, Marmesat S, Dobarganes MC, Márquez-Ruiz G, Velasco J. Formation of Hydroperoxy-, keto- and Hydroxy-dienes in FAME from oils: influence of temperature and addition of
[60] Emami S, Zhang Z, Taha AY. Quantitation of Oxylipins in fish and algae oil supplements using optimized hydrolysis procedures and ultra-high performance liquid chromatography coupled to tandem mass-spectrometry. J Agric Food Chem 2020;68(35):9329-44. https://doi.org/10.1021/acs.jafc.0c02461.
[61] Richardson CE, Hennebelle M, Otoki Y, Zamora D, Yang J, Hammock BD, et al. Lipidomic analysis of oxidized fatty acids in plant and algae oils. J Agric Food Chem 2017;65(9):1941-51. https://doi.org/10.1021/acs.jafc.6b05559.
[62] Zhou L, Zhao M, Bindler F, Marchioni E. Identification of oxidation compounds of 1-Stearoyl-2-linoleoyl-sn-glycero-3-phosphoethanolamine during thermal oxidation. J Agric Food Chem 2015;63(43):9615-20. https://doi.org/10.1021/ acs.jafc.5b03753.
[63] Le Grandois J, Marchioni E, Ennahar S, Giuffrida F, Bindler F. Identification and kinetics of oxidized compounds from phosphatidylcholine molecular species. Food Chem 2010;119(3):1233-8. https://doi.org/10.1016/j. foodchem.2009.08.042.
[64] Parchem K, Kusznierewicz B, Chmiel T, Maciołek P, Bartoszek A. Profiling and qualitative assessment of enzymatically and thermally oxidized egg yolk phospholipids using a two-step high-performance liquid chromatography protocol. J Am Oil Chem Soc 2019;96(6):693-706. https://doi.org/10.1002/ aocs. 12218.
[65] Kato S, Iseki T, Hanzawa Y, Otoki Y, Ito J, Kimura F, et al. Evaluation of the mechanisms of mayonnaise phospholipid oxidation. J Oleo Sci 2017;66(4): 369-74. https://doi.org/10.5650/jos.ess16187.
[66] Laguerre M, Tenon M, Bily A, Birtić S. Toward a spatiotemporal model of oxidation in lipid dispersions: A hypothesis-driven review. Eur J Lipid Sci Technol 2020;122(3):1900209. https://doi.org/10.1002/ejlt.201900209.
[67] Ghelichi S, Hajfathalian M, Yesiltas B, Sørensen ADM, García-Moreno PJ, Jacobsen C. Oxidation and oxidative stability in emulsions. Comprehen Rev Food Sci Food Safet 2023;22(3):1864-901. https://doi.org/10.1111/15414337.13134.
[68] Mei L, McClements DJ, Wu J, Decker EA. Iron-catalyzed lipid oxidation in emulsion as affected by surfactant, pH and NaCl . Food Chem 1998;61(3):307-12. https://doi.org/10.1016/S0308-8146(97)00058-7.
[69] Waraho T, McClements DJ, Decker EA. Mechanisms of lipid oxidation in food dispersions. Trends Food Sci Technol 2011;22(1):3-13. https://doi.org/10.1016/ j.tifs.2010.11.003.
[70] Rampon V, Lethuaut L, Mouhous-Riou N, Genot C. Interface characterization and aging of bovine serum albumin stabilized oil-in-water emulsions as revealed by front-surface fluorescence. J Agric Food Chem 2001;49(8):4046-51. https://doi. org/10.1021/jf001170y.
[71] Chen J, Li X, Cao C, Kong B, Wang H, Zhang H, et al. Effects of different pH conditions on interfacial composition and protein-lipid co-oxidation of whey protein isolate-stabilised O/W emulsions. Food Hydrocoll 2022;131:107752. https://doi.org/10.1016/j.foodhyd.2022.107752.
[72] Raudsepp P, Bruggemann DA, Andersen ML. Detection of radicals in single droplets of oil-in-water emulsions with the lipophilic fluorescent probe BODIPY (665/676) and confocal laser scanning microscopy. Free Radic Biol Med 2014;70: 233-40. https://doi.org/10.1016/j.freeradbiomed.2014.02.026.
[73] Yang S, Takeuchi M, Friedrich H, van Duynhoven JP, Hohlbein J. Unravelling mechanisms of protein and lipid oxidation in mayonnaise at multiple length scales. Food Chem 2023;402:134417. https://doi.org/10.1016/j. foodchem.2022.134417.
[74] Yang S, Verhoeff AA, Merkx DWH, van Duynhoven JPM, Hohlbein J. Quantitative spatiotemporal mapping of lipid and protein oxidation in mayonnaise. Antioxidants (Basel) 2020;9(12). https://doi.org/10.3390/antiox9121278.
[75] Berton-Carabin C, Genot C, Gaillard C, Guibert D, Ropers M. Design of interfacial films to control lipid oxidation in oil-in-water emulsions. Food Hydrocoll 2013;33 (1):99-105. https://doi.org/10.1016/j.foodhyd.2013.02.021.
[76] Berton C, Genot C, Guibert D, Ropers M-H. Effect of lateral heterogeneity in mixed surfactant-stabilized interfaces on the oxidation of unsaturated lipids in oil-in-water emulsions. J Colloid Interface Sci 2012;377(1):244-50. https://doi. org/10.1016/j.jcis.2012.03.084.
[77] Hohlbein J. Single-molecule localization microscopy as an emerging tool to probe multiscale food structures. Food Struct 2021;30:100236. https://doi.org/ 10.1016/j.foostr.2021.100236.
[78] Jabermoradi A, Yang S, Gobes MI, Van Duynhoven JPM, Hohlbein J. Enabling single-molecule localization microscopy in turbid food emulsions. Philos Trans R Soc A Math Phys Eng Sci 2022;380(2220). https://doi.org/10.1098/ rsta.2020.0164.
[79] Villeneuve P, Bourlieu-Lacanal C, Durand E, Lecomte J, McClements DJ, Decker EA. Lipid oxidation in emulsions and bulk oils: a review of the importance of micelles. Crit Rev Food Sci Nutr 2023;63(20):4687-727. https://doi.org/ 10.1080/10408398.2021.2006138.
[80] Budilarto ES, Kamal-Eldin A. The supramolecular chemistry of lipid oxidation and antioxidation in bulk oils. Eur J Lipid Sci Technol 2015;117(8):1095-137. https://doi.org/10.1002/ejlt.201400200.
[81] Chaiyasit W, Elias RJ, McClements DJ, Decker EA. Role of physical structures in bulk oils on lipid oxidation. Crit Rev Food Sci Nutr 2007;47(3):299-317. https:// doi.org/10.1080/10408390600754248.
[82] Chen B, Han A, McClements DJ, Decker EA. Physical structures in soybean oil and their impact on lipid oxidation. J Agric Food Chem 2010;58(22):11993-9. https://doi.org/10.1021/jf102763p.
[83] Cui L, McClements DJ, Decker EA. Impact of phosphatidylethanolamine on the antioxidant activity of alpha-tocopherol and trolox in bulk oil. J Agric Food Chem 2015;63(12):3288-94. https://doi.org/10.1021/acs.jafc.5b00243.
[84] Koga T, Nagao A, Terao J, Sawada K, Mukai K. Synthesis of a phosphatidyl derivative of vitamin E and its antioxidant activity in phospholipid bilayers. Lipids. 1994;29(2):83-9. https://doi.org/10.1007/BF02537147.
[85] Koga T, Terao J. Phospholipids increase radical-scavenging activity of vitamin E in a bulk oil model system. J Agric Food Chem 1995;43(6):1450-4. https://doi. org/10.1021/jf00054a007.
[86] Yoo K, Kim S, Kim M-J, Oh W, Lee J. Effects of association colloidal structures on the oxygen solubility in oil-in-water emulsion matrix. Food Sci Biotechnol 2023: 1-9. https://doi.org/10.1007/s10068-023-01338-6.
[87] Kittipongpittaya K, Panya A, McClements DJ, Decker EA. Impact of free fatty acids and phospholipids on reverse micelles formation and lipid oxidation in bulk oil. J Am Oil Chem Soc 2014;91:453-62. https://doi.org/10.1007/s11746-013-2388-8.
[88] Farhoosh R. Reliable determination of the induction period and critical reverse micelle concentration of lipid hydroperoxides exploiting a model composed of pseudo-first and -second order reaction kinetics. LWT. 2018;98:406-10. https:// doi.org/10.1016/j.lwt.2018.09.003.
[89] Merkx DW, Swager A, van Velzen EJ, van Duynhoven JP, Hennebelle M. Quantitative and predictive modelling of lipid oxidation in mayonnaise. Antioxidants. 2021;10(2):287. https://doi.org/10.3390/antiox10020287.
[90] Laguerre M, Bily A, Roller M, Birtić S. Mass transport phenomena in lipid oxidation and antioxidation. Annu Rev Food Sci Technol 2017;8:391-411. https://doi.org/10.1146/annurev-food-030216-025812.
[91] Raudsepp P, Brüggemann DA, Andersen ML. Evidence for Transfer of Radicals between Oil-in-Water Emulsion Droplets as Detected by the Probe (E,E)-3,5-Bis(4-phenyl-1,3-butadienyl)-4,4-difluoro-4-bora-3a,4a-diaza-s-indacene, BODIPY665/ 676. J Agric Food Chem 2014;62(51):12428-35. https://doi.org/10.1021/ jf504404a.
[92] Raudsepp P, Brüggemann DA, Knudsen JC, Andersen ML. Localized lipid autoxidation initiated by two-photon irradiation within single oil droplets in oil-in-water emulsions. Food Chem 2016;199:760-7. https://doi.org/10.1016/j. foodchem.2015.12.070.
[93] Banerjee C, Breitenbach T, Ogilby PR. Spatially resolved experiments to monitor the singlet oxygen initiated oxidation of lipid droplets in emulsions. ChemPhotoChem. 2018;2(7):586-95. https://doi.org/10.1002/cptc.201800005.
[94] Banerjee C, Westberg M, Breitenbach T, Bregnhøj M, Ogilby PR. Monitoring interfacial lipid oxidation in oil-in-water emulsions using spatially resolved optical techniques. Anal Chem 2017;89(11):6239-47. https://doi.org/10.1021/ acs.analchem.7b01228.
[95] Yesiltas B, García-Moreno PJ, Sørensen A-DM, Banerjee C, Anankanbil S, Guo Z, et al. The use of soy and egg phosphatidylcholines modified with Caffeic acid enhances the oxidative stability of high-fat (70%) fish oil-in-water emulsions. Coll Interf 2023;7(3):60. https://doi.org/10.3390/colloids7030060.
[96] Pryor WA. Oxy-radicals and related species: their formation, lifetimes, and reactions. Annu Rev Physiol 1986;48:657-67. https://doi.org/10.1146/annurev. ph.48.030186.003301.
[97] Termini J. Peroxyl and alkoxyl radical mediated DNA damage. In: Cutler RG, Rodriguez H, editors. Critical Reviews of Oxidative Stress and Aging. Advances in Basic Science, Diagnostics and Intervention. World Scientific Publishing Co. Pte. Ltd.; 2003. p. 39-53. https://doi.org/10.1142/9789812775733_0003.
[98] Coupland JN, Zhu Z, Wan H, McClements DJ, Nawar WW, Chinachoti P. Droplet composition affects the rate of oxidation of emulsified ethyl linoleate. J Am Oil Chem Soc 1996;73(6):795-901. https://doi.org/10.1007/BF02517957.
[99] Malassagne-Bulgarelli N, McGrath KM. Emulsion ageing: effect on the dynamics of oil exchange in oil-in-water emulsions. Soft Matter 2013;9(1):48-59. https:// doi.org/10.1039/C2SM26229K.
[100] Li P, McClements DJ, Decker EA. Application of flow cytometry as novel technology in studying lipid oxidation and mass transport phenomena in oil-inwater emulsions. Food Chem 2020;315:126225. https://doi.org/10.1016/j. foodchem.2020.126225.
[101] Nuchi CD, Hernandez P, McClements DJ, Decker EA. Ability of lipid hydroperoxides to partition into surfactant micelles and alter lipid oxidation rates in emulsions. J Agric Food Chem 2002;50(19):5445-9. https://doi.org/10.1021/ jf020095j.
[102] Verleyen T, Kamal-Eldin A, Dobarganes C, Verhé R, Dewettinck K, Huyghebaert A. Modeling of
[103] Barretto ACS, Ida EI, Silva RSF, Torres EAFS, Shimokomaki M. Empirical models for describing poultry meat lipid oxidation inhibition by natural antioxidants. J Food Compos Anal 2003;16(5):587-94. https://doi.org/10.1016/S0889-1575 (03)00023-1.
[104] Høy M, Mozuraityte R, Segtnan V, Storrø I, Bjørn Helge M, Rustad T, et al. The use of experimental design methodology for investigating a lipid oxidation rate assay. Chemom Intel Lab Syst 2008;91(2):164-72. https://doi.org/10.1016/j. chemolab.2007.11.001.
[105] Kerrihard AL, Nagy K, Craft BD, Beggio M, Pegg RB. Oxidative stability of commodity fats and oils: modeling based on fatty acid composition. J Am Oil Chem Soc 2015;92(8):1153-63. https://doi.org/10.1007/s11746-015-2686-4.
[106] Pinchuk I, Lichtenberg D. Analysis of the kinetics of lipid peroxidation in terms of characteristic time-points. Chem Phys Lipids 2014;178:63-76. https://doi.org/ 10.1016/j.chemphyslip.2013.12.001.
[107] Murado MA, Vázquez JA. Mathematical model for the characterization and objective comparison of antioxidant activities. J Agric Food Chem 2010;58(3): 1622-9. https://doi.org/10.1021/jf903709z.
[108] Farhoosh R. A reconsidered approach providing kinetic parameters and rate constants to analyze the oxidative stability of bulk lipid systems. Food Chem 2020;327:127088. https://doi.org/10.1016/j.foodchem.2020.127088.
[109] Farhoosh R. New insights into the kinetic and thermodynamic evaluations of lipid peroxidation. Food Chem 2022;375:131659. https://doi.org/10.1016/j. foodchem.2021.131659.
[110] Li X, Wu G, Huang J, Zhang H, Jin Q, Wang X. Kinetic models to understand the coexistence of formation and decomposition of hydroperoxide during lipid oxidation. Food Res Int 2020;136:109314. https://doi.org/10.1016/j. foodres.2020.109314.
[111] Aragao GMF, Corradini MG, Peleg M. A phenomenological model of the peroxide Value’s rise and fall during lipid oxidation. J Am Oil Chem Soc 2008;85(12):1143. https://doi.org/10.1007/s11746-008-1305-z.
[112] Ozilgen S, Ozilgen M. Kinetic model of lipid oxidation in foods. J Food Sci 1990; 55(2):498-501. https://doi.org/10.1111/j.1365-2621.1990.tb06795.x.
[113] McPherson PAC, Bole A, Cruz KA, Young IS, McEneny J. A curvilinear approach to the kinetic analysis of linoleate peroxidation in aqueous liposomes by 2,2’azobis (2-amidoinopropane) dihydrochloride. Chem Phys Lipids 2012;165(6):682-8. https://doi.org/10.1016/j.chemphyslip.2012.07.004.
[114] Merkx DW, Hong GS, Ermacora A, Van Duynhoven JP. Rapid quantitative profiling of lipid oxidation products in a food emulsion by 1H NMR. Anal Chem 2018;90(7):4863-70. https://doi.org/10.1021/acs.analchem.8b00380.
[115] Nguyen KA, Hennebelle M, van Duynhoven JPM, Dubbelboer A, Boerkamp VJP, Wierenga PA. Mechanistic kinetic modelling of lipid oxidation in vegetable oils to estimate shelf-life. Food Chem 2024;433:137266. https://doi.org/10.1016/j. foodchem.2023.137266.
[116] Schroën K, Berton-Carabin CC. A unifying approach to lipid oxidation in emulsions: modelling and experimental validation. Food Res Int 2022;160: 111621. https://doi.org/10.1016/j.foodres.2022.111621.
[117] Tan CP, Che Man YB, Selamat J, Yusoff MSA. Application of arrhenius kinetics to evaluate oxidative stability in vegetable oils by isothermal differential scanning calorimetry. J Am Oil Chem Soc 2001;78(11):1133-8. https://doi.org/10.1007/ s11746-001-0401-1.
[118] Farhoosh R. Critical kinetic parameters and rate constants representing lipid peroxidation as affected by temperature. Food Chem 2021;340:128137. https:// doi.org/10.1016/j.foodchem.2020.128137.
[119] Sullivan JC, Budge SM, St-Onge M. Modeling the primary oxidation in commercial fish oil preparations. Lipids. 2011;46(1):87-93. https://doi.org/
[120] Shim SD, Lee SJ. Shelf-life prediction of perilla oil by considering the induction period of lipid oxidation. Eur J Lipid Sci Technol 2011;113(7):904-9. https://doi. org/10.1002/ejlt.201000325.
[121] Bravo-Diaz C. Advances in the control of lipid peroxidation in oil-in-water emulsions: kinetic approaches(dagger). Crit Rev Food Sci Nutr 2022:1-33. https://doi.org/10.1080/10408398.2022.2029827.
[122] Romsted LS, Bravo-Díaz C. Modeling chemical reactivity in emulsions. Curr Opin Colloid Interface Sci 2013;18(1):3-14. https://doi.org/10.1016/j. cocis.2012.11.001.
[123] Genot C. Distributions of phenolic acid antioxidants between the interfacial and aqueous regions of corn oil emulsions-a commentary. Eur J Lipid Sci Technol 2015;117(11):1684-6. https://doi.org/10.1002/ejlt.201500210.
[124] Nazir A, Schroën K, Boom R. Premix emulsification: A review. J Membr Sci 2010; 362(1):1-11. https://doi.org/10.1016/j.memsci.2010.06.044.
[125] Costa ALR, Gomes A, Andrade CCPD, Cunha RL. Emulsifier functionality and process engineering: Progress and challenges. Food Hydrocoll 2017;68:69-80. https://doi.org/10.1016/j.foodhyd.2016.10.012.
[126] Neves MA, Wang Z, Kobayashi I, Nakajima M. Assessment of oxidative stability in fish oil-in-water emulsions: effect of emulsification process, droplet size and storage temperature. J Food Process Eng 2017;40(1):e12316. https://doi.org/ 10.1111/jfpe.12316.
[127] Walker R, Decker EA, McClements DJ. Development of food-grade nanoemulsions and emulsions for delivery of omega-3 fatty acids: opportunities and obstacles in the food industry. Food Funct 2015;6(1):42-55. https://doi.org/10.1039/ c4fo00723a.
[128] Horn AF, Barouh N, Nielsen NS, Baron CP, Jacobsen C. Homogenization pressure and temperature affect protein partitioning and oxidative stability of emulsions. J Am Oil Chem Soc 2013;90(10):1541-50. https://doi.org/10.1007/s11746-013-2292-2.
[129] Let MB, Jacobsen C, Sorensen AD, Meyer AS. Homogenization conditions affect the oxidative stability of fish oil enriched milk emulsions: lipid oxidation. J Agric Food Chem 2007;55(5):1773-80. https://doi.org/10.1021/jf062391s.
[130] Sorensen AD, Baron CP, Let MB, Bruggemann DA, Pedersen LR, Jacobsen C. Homogenization conditions affect the oxidative stability of fish oil enriched milk emulsions: oxidation linked to changes in protein composition at the oil-water interface. J Agric Food Chem 2007;55(5):1781-9. https://doi.org/10.1021/ jf0623900.
[131] Hu M, McClements DJ, Decker EA. Lipid oxidation in corn oil-in-water emulsions stabilized by casein, whey protein isolate, and soy protein isolate. J Agric Food Chem 2003;51(6):1696-700. https://doi.org/10.1021/jf020952j.
[132] Shao Y, Tang C-H. Characteristics and oxidative stability of soy protein-stabilized oil-in-water emulsions: influence of ionic strength and heat pretreatment. Food Hydrocoll 2014;37:149-58. https://doi.org/10.1016/j.foodhyd.2013.10.030.
[133] Kellerby SS, Gu YS, McClements DJ, Decker EA. Lipid oxidation in a menhaden oil-in-water emulsion stabilized by sodium Caseinate cross-linked with transglutaminase. J Agric Food Chem 2006;54(26):10222-7. https://doi.org/ 10.1021/jf062143w.
[134] Lesmes U, Sandra S, Decker EA, McClements DJ. Impact of surface deposition of lactoferrin on physical and chemical stability of omega-3 rich lipid droplets stabilised by caseinate. Food Chem 2010;123(1):99-106. https://doi.org/ 10.1016/j.foodchem.2010.04.007.
[135] Chen B, Li H, Ding Y, Rao J. Improvement of physicochemical stabilities of emulsions containing oil droplets coated by non-globular protein-beet pectin complex membranes. Food Res Int 2011;44(5):1468-75. https://doi.org/ 10.1016/j.foodres.2011.03.024.
[136] Gudipati V, Sandra S, McClements DJ, Decker EA. Oxidative stability and in vitro digestibility of fish oil-in-water emulsions containing multilayered membranes. J Agric Food Chem 2010;58(13):8093-9. https://doi.org/10.1021/jf101348c.
[137] McClements DJ, Decker E. Interfacial antioxidants: A review of natural and synthetic emulsifiers and Coemulsifiers that can inhibit lipid oxidation. J Agric Food Chem 2018;66(1):20-35. https://doi.org/10.1021/acs.jafc.7b05066.
[138] Dickinson E. Mixed biopolymers at interfaces: competitive adsorption and multilayer structures. Food Hydrocoll 2011;25(8):1966-83. https://doi.org/ 10.1016/j.foodhyd.2010.12.001.
[139] García-Moreno PJ, Horn AF, Jacobsen C. Influence of casein-phospholipid combinations as emulsifier on the physical and oxidative stability of fish oil-inwater emulsions. J Agric Food Chem 2014;62(5):1142-52. https://doi.org/ 10.1021/jf405073x.
[140] Yesiltas B, Soria Caindec AM, García-Moreno PJ, Echers SG, Olsen TH, Jones NC, et al. Physical and oxidative stability of fish oil-in-water emulsions stabilized with emulsifier peptides derived from seaweed, methanotrophic bacteria and potato proteins. Colloids Surf A Physicochem Eng Asp 2023;663:131069. https://doi. org/10.1016/j.colsurfa.2023.131069.
[141] Yesiltas B, Torkkeli M, Almásy L, Dudás Z, García-Moreno PJ, Sørensen A-DM, et al. Small-angle neutron scattering study of high fat fish oil-in-water emulsion stabilized with sodium Caseinate and phosphatidylcholine. Langmuir. 2020;36 (9):2300-6. https://doi.org/10.1021/acs.langmuir.9b03269.
[142] Ozturk B, McClements DJ. Progress in natural emulsifiers for utilization in food emulsions. Curr Opin Food Sci 2016;7:1-6. https://doi.org/10.1016/j. cofs.2015.07.008.
[143] Sharif HR, Williams PA, Sharif MK, Abbas S, Majeed H, Masamba KG, et al. Current progress in the utilization of native and modified legume proteins as emulsifiers and encapsulants-A review. Food Hydrocoll 2018;76:2-16. https:// doi.org/10.1016/j.foodhyd.2017.01.002.
[144] Burger TG, Zhang Y. Recent progress in the utilization of pea protein as an emulsifier for food applications. Trends Food Sci Technol 2019;86:25-33. https:// doi.org/10.1016/j.tifs.2019.02.007.
[145] Lam RSH, Nickerson MT. Food proteins: A review on their emulsifying properties using a structure-function approach. Food Chem 2013;141(2):975-84. https:// doi.org/10.1016/j.foodchem.2013.04.038.
[146] Schutyser M, Van der Goot A. The potential of dry fractionation processes for sustainable plant protein production. Trends Food Sci Technol 2011;22(4): 154-64. https://doi.org/10.1016/j.tifs.2010.11.006.
[147] Faraji H, McClements DJ, Decker EA. Role of continuous phase protein on the oxidative stability of fish oil-in-water emulsions. J Agric Food Chem 2004;52(14): 4558-64. https://doi.org/10.1021/jf035346i.
[148] Feng J, Schroën K, Fogliano V, Berton-Carabin C. Antioxidant potential of nonmodified and glycated soy proteins in the continuous phase of oil-in-water emulsions. Food Hydrocoll 2021;114:106564. https://doi.org/10.1016/j. foodhyd.2020.106564.
[149] Hinderink EBA, Schröder A, Sagis L, Schroën K, Berton-Carabin CC. Physical and oxidative stability of food emulsions prepared with pea protein fractions. LWT. 2021;146:111424. https://doi.org/10.1016/j.lwt.2021.111424.
[150] Liu C, Bhattarai M, Mikkonen KS, Heinonen M. Effects of enzymatic hydrolysis of fava bean protein isolate by Alcalase on the physical and oxidative stability of oil-in-water emulsions. J Agric Food Chem 2019;67(23):6625-32. https://doi.org/ 10.1021/acs.jafc.9b00914.
[151] Keuleyan E, Gélébart P, Beaumal V, Kermarrec A, Ribourg-Birault L, Le Gall S, et al. Pea and lupin protein ingredients: new insights into endogenous lipids and the key effect of high-pressure homogenization on their aqueous suspensions. Food Hydrocoll 2023;141:108671. https://doi.org/10.1016/j. foodhyd.2023.108671.
[152] Pei Y, Deng Q, McClements DJ, Li J, Li B. Impact of Phytic acid on the physical and oxidative stability of protein-stabilized oil-in-water emulsions. Food Biophys 2020;15(4):433-41. https://doi.org/10.1007/s11483-020-09641-z.
[153] Dickinson E. Advances in food emulsions and foams: reflections on research in the neo-Pickering era. Curr Opin Food Sci 2020;33:52-60. https://doi.org/10.1016/j. cofs.2019.12.009.
[154] Calabrese V, Courtenay JC, Edler KJ, Scott JL. Pickering emulsions stabilized by naturally derived or biodegradable particles. Curr Opin Green Sustain Chem 2018;12:83-90. https://doi.org/10.1016/j.cogsc.2018.07.002.
[155] Dickinson E. Biopolymer-based particles as stabilizing agents for emulsions and foams. Food Hydrocoll 2017;68:219-31. https://doi.org/10.1016/j. foodhyd.2016.06.024.
[156] Linke C, Drusch S. Pickering emulsions in foods – opportunities and limitations. Crit Rev Food Sci Nutr 2018;58(12):1971-85. https://doi.org/10.1080/ 10408398.2017 .1290578.
[157] Rayner M. Current status on novel ways for stabilizing food dispersions by oleosins, particles and microgels. Curr Opin Food Sci 2015;3:94-109. https://doi. org/10.1016/j.cofs.2015.05.006.
[158] Tavernier I, Wijaya W, Van der Meeren P, Dewettinck K, Patel AR. Food-grade particles for emulsion stabilization. Trends Food Sci Technol 2016;50:159-74. https://doi.org/10.1016/j.tifs.2016.01.023.
[159] Berton-Carabin C, Schröder A, Schroën K, Laguerre M. Chapter 14 – Lipid oxidation in Pickering emulsions. In: García-Moreno PJ, editor. Omega-3 Delivery Systems. Academic Press; 2021. p. 275-93. https://doi.org/10.1016/B978-0-12-821391-9.00011-9.
[160] Kargar M, Fayazmanesh K, Alavi M, Spyropoulos F, Norton IT. Investigation into the potential ability of Pickering emulsions (food-grade particles) to enhance the oxidative stability of oil-in-water emulsions. J Colloid Interface Sci 2012;366(1): 209-15. https://doi.org/10.1016/j.jcis.2011.09.073.
[161] Kargar M, Spyropoulos F, Norton IT. The effect of interfacial microstructure on the lipid oxidation stability of oil-in-water emulsions. J Colloid Interface Sci 2011; 357(2):527-33. https://doi.org/10.1016/j.jcis.2011.02.019.
[162] Xiao J, Li C, Huang Q. Kafirin nanoparticle-stabilized Pickering emulsions as Oral delivery vehicles: physicochemical stability and in vitro digestion profile. J Agric Food Chem 2015;63(47):10263-70. https://doi.org/10.1021/acs.jafc.5b04385.
[163] Huang X-N, Zhou F-Z, Yang T, Yin S-W, Tang C-H, Yang X-Q. Fabrication and characterization of Pickering high internal phase emulsions (HIPEs) stabilized by chitosan-caseinophosphopeptides nanocomplexes as oral delivery vehicles. Food Hydrocoll 2019;93:34-45. https://doi.org/10.1016/j.foodhyd.2019.02.005.
[164] Schröder A, Sprakel J, Boerkamp W, Schroën K, Berton-Carabin CC. Can we prevent lipid oxidation in emulsions by using fat-based Pickering particles? Food Res Int 2019;120:352-63. https://doi.org/10.1016/j.foodres.2019.03.004.
[165] Okubanjo SS, Loveday SM, Ye A, Wilde PJ, Singh H. Droplet-stabilized oil-inwater emulsions protect unsaturated lipids from oxidation. J Agric Food Chem 2019;67(9):2626-36. https://doi.org/10.1021/acs.jafc.8b02871.
[166] Timgren A, Rayner M, Dejmek P, Marku D, Sjoo M. Emulsion stabilizing capacity of intact starch granules modified by heat treatment or octenyl succinic anhydride. Food Sci Nutr 2013;1(2):157-71. https://doi.org/10.1002/fsn3.17.
[167] Barden L, Barouh N, Villeneuve P, Decker E. Impact of hydrophobicity on antioxidant efficacy in low-moisture food. J Agric Food Chem 2015;63(24): 5821-7. https://doi.org/10.1021/acs.jafc.5b01085.
[168] Barden L, Decker EA. Lipid oxidation in low-moisture food: A review. Crit Rev Food Sci Nutr 2016;56(15):2467-82. https://doi.org/10.1080/ 10408398.2013.848833.
[169] Ferreira da Silveira TF, Laguerre M, Bourlieu-Lacanal C, Lecomte J, Durand E, Figueroa-Espinoza MC, et al. Impact of surfactant concentration and antioxidant mode of incorporation on the oxidative stability of oil-in-water nanoemulsions. LWT. 2021;141:110892. https://doi.org/10.1016/j.lwt.2021.110892.
[170] Inchingolo R, Bayram I, Uluata S, Kiralan SS, Rodriguez-Estrada MT, McClements DJ, et al. Ability of sodium dodecyl sulfate (SDS) micelles to increase the antioxidant activity of alpha-tocopherol. J Agric Food Chem 2021;69(20): 5702-8. https://doi.org/10.1021/acs.jafc.1c01199.
[171] McClements DJ, Dungan SR. Factors that affect the rate of oil exchange between oil-in-water emulsion droplets stabilized by a nonionic surfactant: droplet size, surfactant concentration, and ionic strength. J Phys Chem 1993;97(28):7304-8. https://doi.org/10.1021/j100130a030.
[172] Raudsepp P, Brüggemann DA, Lenferink A, Otto C, Andersen ML. Oxidative stabilization of mixed mayonnaises made with linseed oil and saturated mediumchain triglyceride oil. Food Chem 2014;152:378-85. https://doi.org/10.1016/j. foodchem.2013.11.141.
[173] Cho YJ, McClements DJ, Decker EA. Ability of surfactant micelles to alter the physical location and reactivity of iron in oil-in-water emulsion. J Agric Food Chem 2002;50(20):5704-10. https://doi.org/10.1021/jf020433g.
[174] Anton M. Egg yolk: structures, functionalities and processes. J Sci Food Agric 2013;93(12):2871-80. https://doi.org/10.1002/jsfa.6247.
[175] Samaraweera H, Zhang WG, Lee EJ, Ahn DU. Egg yolk phosvitin and functional phosphopeptides. J Food Sci 2011;76(7):R143-50. https://doi.org/10.1111/ j.1750-3841.2011.02291.x.
[176] Hu M, McClements DJ, Decker EA. Impact of whey protein emulsifiers on the oxidative stability of Salmon oil-in-water emulsions. J Agric Food Chem 2003;51 (5):1435-9. https://doi.org/10.1021/jf0203794.
[177] Sugiarto M, Ye A, Taylor MW, Singh H. Milk protein-iron complexes: inhibition of lipid oxidation in an emulsion. Dairy Sci Technol 2010;90(1):87-98. https://doi. org/10.1051/dst/2009053.
[178] Villiere A, Viau M, Bronnec I, Moreau N, Genot C. Oxidative stability of bovine serum albumin- and sodium Caseinate-stabilized emulsions depends on metal availability. J Agric Food Chem 2005;53(5):1514-20. https://doi.org/10.1021/ jf0486951.
[179] Elias RJ, Kellerby SS, Decker EA. Antioxidant activity of proteins and peptides. Crit Rev Food Sci Nutr 2008;48(5):430-41. https://doi.org/10.1080/ 10408390701425615.
[180] Panya A, Laguerre M, Bayrasy C, Lecomte J, Villeneuve P, McClements DJ, et al. An investigation of the versatile antioxidant mechanisms of action of rosmarinate alkyl esters in oil-in-water emulsions. J Agric Food Chem 2012;60(10):2692-700. https://doi.org/10.1021/jf204848b.
[181] Martinez-Senra T, Losada-Barreiro S, Bravo-Diaz C. Efficiency of delta-tocopherol in inhibiting lipid oxidation in emulsions: effects of surfactant charge and of surfactant concentration. Antioxidants (Basel) 2023;12(6). https://doi.org/ 10.3390/antiox12061158.
[182] Losada-Barreiro S, Paiva-Martins F, Bravo-Diaz C. Partitioning of antioxidants in edible oil-water binary systems and in oil-in-water emulsions. Antioxidants (Basel) 2023;12(4). https://doi.org/10.3390/antiox12040828.
[183] Kiralan SS, Doğu-Baykut E, Kittipongpittaya K, McClements DJ, Decker EA. Increased antioxidant efficacy of tocopherols by surfactant Solubilization in oil-in-water emulsions. J Agric Food Chem 2014;62(43):10561-6. https://doi.org/ 10.1021/jf503115j.
[184] Richards MP, Chaiyasit W, McClements DJ, Decker EA. Ability of surfactant micelles to alter the partitioning of phenolic antioxidants in oil-in-water emulsions. J Agric Food Chem 2002;50(5):1254-9. https://doi.org/10.1021/ jf011324p.
[185] Dai T, Chen J, McClements DJ, Hu P, Ye X, Liu C, et al. Protein-polyphenol interactions enhance the antioxidant capacity of phenolics: analysis of rice glutelin-procyanidin dimer interactions. Food Funct 2019;10(2):765-74. https:// doi.org/10.1039/c8fo02246a.
[186] Schild K, Sonnichsen FD, Martin D, Garamus VM, Van der Goot AJ, Schwarz K, et al. Unraveling the effects of low protein-phenol binding affinity on the structural properties of beta-lactoglobulin. Food Chem 2023;426:136496. https://doi.org/10.1016/j.foodchem.2023.136496.
[187] Tang L, Cao M, Liao C, Liu R, Chang M, Wang X. Migration of tocopherols from the oil phase to the oil-water interface using phospholipids improved the oxidative stability of O/W emulsions. Food Chem 2023;414:135719. https://doi. org/10.1016/j.foodchem.2023.135719.
[188] Chen J, Li X, Kong B, Chen Q, Liu Q. Comparative study of protein-lipid cooxidation in whey protein isolate-stabilised oil-in-water emulsions prepared by different homogenisation methods. Colloids Surf A Physicochem Eng Asp 2022; 633:127916. https://doi.org/10.1016/j.colsurfa.2021.127916.
[189] Chen J, Zhao J, Kong B, Chen Q, Liu Q, Liu C. Comparative study of oxidative structural modifications of Unadsorbed and adsorbed proteins in whey protein isolate-stabilized oil-in-water emulsions under the stress of primary and secondary lipid oxidation products. Foods. 2021;10(3). https://doi.org/10.3390/ foods10030593.
[190] Donnelly JL, Decker EA, McClements DJ. Iron-catalyzed oxidation of menhaden oil as affected by emulsifiers. J Food Sci 1998;63(6):997-1000. https://doi.org/ 10.1111/j.1365-2621.1998.tb15841.x.
[191] Ma KK, Greis M, Lu J, Nolden AA, McClements DJ, Kinchla AJ. Functional performance of plant proteins. Foods 2022;11:4. https://doi.org/10.3390/ foods11040594.
[192] Donbrow M, Azaz E, Pillersdorf A. Autoxidation of polysorbates. J Pharm Sci 1978;67(12):1676-81. https://doi.org/10.1002/jps.2600671211.
[193] Hamburger R, Azaz E, Donbrow M. Autoxidation of polyoxyethylenic non-ionic surfactants and of polyethylene glycols. Pharm Acta Helv 1975;50(1-2):10-7.
[194] Lever M. Peroxides in detergents as interfering factors in biochemical analysis. Anal Biochem 1977;83(1):274-84. https://doi.org/10.1016/0003-2697(77) 90536-X.
[195] Jaeger J, Sorensen K, Wolff SP. Peroxide accumulation in detergents. J Biochem Biophys Methods 1994;29(1):77-81. https://doi.org/10.1016/0165-022x(94) 90058-2.
[196] Mancuso JR, McClements DJ, Decker EA. The effects of surfactant type, pH , and chelators on the oxidation of salmon oil-in-water emulsions. J Agric Food Chem 1999;47(10):4112-6. https://doi.org/10.1021/jf990203a.
[197] Ha E, Wang W, Wang YJ. Peroxide formation in polysorbate 80 and protein stability. J Pharm Sci 2002;91(10):2252-64. https://doi.org/10.1002/jps.10216.
[198] Segal R, Azaz E, Donbrow M. Peroxide removal from non-ionic surfactants. J Pharm Pharmacol 1979;31(1):39-40. https://doi.org/10.1111/j.20427158.1979.tb13418.x.
[199] Donbrow M, Hamburger R, Azaz E, Pillersdorf A. Development of acidity in nonionic surfactants: formic and acetic acid. Analyst. 1978;103(1225):400-2. https://doi.org/10.1039/AN9780300400.
[200] Kerwin BA. Polysorbates 20 and 80 used in the formulation of protein biotherapeutics: structure and degradation pathways. J Pharm Sci 2008;97(8): 2924-35. https://doi.org/10.1002/jps. 21190.
[201] Yao J, Dokuru DK, Noestheden M, Park SS, Kerwin BA, Jona J, et al. A quantitative kinetic study of polysorbate autoxidation: the role of unsaturated fatty acid ester substituents. Pharm Res 2009;26(10):2303-13. https://doi.org/ 10.1007/s11095-009-9946-7.
[202] Kishore RS, Pappenberger A, Dauphin IB, Ross A, Buergi B, Staempfli A, et al. Degradation of polysorbates 20 and 80: studies on thermal autoxidation and hydrolysis. J Pharm Sci 2011;100(2):721-31. https://doi.org/10.1002/ jps. 22290.
[203] Hvattum E, Yip WL, Grace D, Dyrstad K. Characterization of polysorbate 80 with liquid chromatography mass spectrometry and nuclear magnetic resonance spectroscopy: specific determination of oxidation products of thermally oxidized polysorbate 80. J Pharm Biomed Anal 2012;62:7-16. https://doi.org/10.1016/j. jpba.2011.12.009.
[204] Li X, Wang Z, Zheng B, Wang Y, Zhang J. Novel strategy to rapidly profile and identify oxidized species of Polysorbate 80 using ultra-high-performance liquid chromatography coupled with high-resolution mass spectrometry. Anal Chem 2023;95(24):9156-63. https://doi.org/10.1021/acs.analchem.2c04956.
[205] Bai L, Zhang Y, Zhang C, Lu Y, Li Z, Huang G, et al. Investigation of excipients impact on polysorbate 80 degradation in biopharmaceutical formulation buffers.
[206] Mittag JJ, Trutschel ML, Kruschwitz H, Mader K, Buske J, Garidel P. Characterization of radicals in polysorbate 80 using electron paramagnetic resonance (EPR) spectroscopy and spin trapping. Int J Pharm X 2022;4:100123. https://doi.org/10.1016/j.ijpx.2022.100123.
[207] Larson NR, Wei Y, Prajapati I, Chakraborty A, Peters B, Kalonia C, et al. Comparison of Polysorbate 80 hydrolysis and oxidation on the aggregation of a monoclonal antibody. J Pharm Sci 2020;109(1):633-9. https://doi.org/10.1016/ j.xphs.2019.10.069.
[208] Peters BH, Wei Y, Middaugh CR, Schoneich C. Intra-micellar and extra-micellar oxidation in phosphate and histidine buffers containing Polysorbate 80. J Pharm Sci 2022;111(9):2435-44. https://doi.org/10.1016/j.xphs.2022.06.011.
[209] Nuchi CD, McClements DJ, Decker EA. Impact of tween 20 hydroperoxides and iron on the oxidation of methyl linoleate and salmon oil dispersions. J Agric Food Chem 2001;49(10):4912-6. https://doi.org/10.1021/jf010370m.
[210] Schwarz K, Huang SW, German JB, Tiersch B, Hartmann J, Frankel EN. Activities of antioxidants are affected by colloidal properties of oil-in-water and water-in-oil emulsions and bulk oils. J Agric Food Chem 2000;48(10):4874-82. https://doi. org/10.1021/jf991289a.
[211] Mabrouk AF, Dugan Jr L. Kinetic investigation into glucose-, fructose-, and sucrose-activated autoxidation of methyl linoleate emulsion. J Am Oil Chem Soc 1961;38(12):692-5. https://doi.org/10.1007/BF02633057.
[212] Haahr AM, Jacobsen C. Emulsifier type, metal chelation and pH affect oxidative stability of n-3-enriched emulsions. Eur J Lipid Sci Technol 2008;110(10): 949-61. https://doi.org/10.1002/ejlt.200800035.
[213] Kasaikina O, Golyavin A, Krugovov D, Kartasheva Z, Pisarenko L. Micellar catalysis in the oxidation of lipids. Mosc Univ Chem Bull 2010;65:206-9. https:// doi.org/10.3103/S0027131410030193.
[214] Uluata S, McClements DJ, Decker EA. Physical stability, autoxidation, and photosensitized oxidation of omega-3 oils in Nanoemulsions prepared with natural and synthetic surfactants. J Agric Food Chem 2015;63(42):9333-40. https://doi.org/10.1021/acs.jafc.5b03572.
[215] Gutiérrez-Méndez N, Chavez-Garay DR, Leal-Ramos MY. Lecithins: A comprehensive review of their properties and their use in formulating microemulsions. J Food Biochem 2022;46(7):e14157. https://doi.org/10.1111/ jfbc. 14157.
[216] Caparosa MH, Hartel RW. Characterizing lecithin interactions in chocolate using interfacial properties and rheology. J Am Oil Chem Soc 2020;97(12):1309-17. https://doi.org/10.1002/aocs.12419.
[217] Alhajj MJ, Montero N, Yarce CJ, Salamanca CH. Lecithins from vegetable, land, and marine animal sources and their potential applications for cosmetic, food, and pharmaceutical sectors. Cosmetics. 2020;7(4):87. https://doi.org/10.3390/ cosmetics7040087.
[218] Robert C, Couëdelo L, Vaysse C, Michalski M-C. Vegetable lecithins: A review of their compositional diversity, impact on lipid metabolism and potential in cardiometabolic disease prevention. Biochimie. 2020;169:121-32. https://doi. org/10.1016/j.biochi.2019.11.017.
[219] Dacaranhe CD, Terao J. A unique antioxidant activity of phosphatidylserine on iron-induced lipid peroxidation of phospholipid bilayers. Lipids. 2001;36(10): 1105-10. https://doi.org/10.1007/s11745-001-0820-7.
[220] Cardenia V, Waraho T, Rodriguez-Estrada MT, Julian McClements D, Decker EA. Antioxidant and Prooxidant activity behavior of phospholipids in stripped soybean oil-in-water emulsions. J Am Oil Chem Soc 2011;88(9):1409-16. https:// doi.org/10.1007/s11746-011-1807-y.
[221] Judde A, Villeneuve P, Rossignol-Castera A, Le Guillou A. Antioxidant effect of soy lecithins on vegetable oil stability and their synergism with tocopherols. J Am Oil Chem Soc 2003;80(12):1209-15. https://doi.org/10.1007/s11746-003-08444.
[222] Hildebrand D, Terao J, Kito M. Phospholipids plus tocopherols increase soybean oil stability. J Am Oil Chem Soc 1984;61(3):552-5. https://doi.org/10.1007/ BF02677029.
[223] Khan MA, Shahidi F. Tocopherols and phospholipids enhance the oxidative stability of borage and evening primrose triacylglycerols. J Food Lipids 2000;7 (3):143-50. https://doi.org/10.1111/j.1745-4522.2000.tb00167.x.
[224] Kashima M, Cha G-S, Isoda Y, Hirano J, Miyazawa T. The antioxidant effects of phospholipids on perilla oil. J Am Oil Chem Soc 1991;68:119-22. https://doi. org/10.1007/BF02662331.
[225] Lee J, Choe E. Effects of phospholipids on the antioxidant activity of alphatocopherol in the singlet oxygen oxidation of canola oil. N Biotechnol 2011;28(6): 691-7. https://doi.org/10.1016/j.nbt.2011.04.013.
[226] Weng X, Gordon MH. Antioxidant synergy between phosphatidyl ethanolamine and
[227] Doert M, Jaworska K, Moersel J-T, Kroh LW. Synergistic effect of lecithins for tocopherols: lecithin-based regeneration of
[228] Takenaka A, Hosokawa M, Miyashita K. Unsaturated phosphatidylethanolamine as effective synergist in combination with
[229] Shimajiri J, Shiota M, Hosokawa M, Miyashita K. Synergistic antioxidant activity of milk sphingomyeline and its sphingoid base with
[230] Hidalgo FJ, Nogales F, Zamora R. Changes produced in the antioxidative activity of phospholipids as a consequence of their oxidation. J Agric Food Chem 2005;53 (3):659-62. https://doi.org/10.1021/jf0483220.
[231] Bandarra NM, Campos RM, Batista I, Nunes ML, Empis JM. Antioxidant synergy of
[232] Lu FS, Nielsen NS, Baron CP, Diehl BW, Jacobsen C. Oxidative stability of dispersions prepared from purified marine phospholipid and the role of alphatocopherol. J Agric Food Chem 2012;60(50):12388-96. https://doi.org/10.1021/ jf303560f.
[233] Bacot S, Bernoud-Hubac N, Chantegrel B, Deshayes C, Doutheau A, Ponsin G, et al. Evidence for in situ ethanolamine phospholipid adducts with hydroxyalkenals. J Lipid Res 2007;48(4):816-25. https://doi.org/10.1194/jlr.M600340JLR200.
[234] van Nieuwenhuyzen W, Tomás MC. Update on vegetable lecithin and phospholipid technologies. Eur J Lipid Sci Technol 2008;110(5):472-86. https:// doi.org/10.1002/ejlt.200800041.
[235] Lu F, Nielsen NS, Baron CP, Jacobsen C. Marine phospholipids: the current understanding of their oxidation mechanisms and potential uses for food fortification. Crit Rev Food Sci Nutr 2017;57(10):2057-70. https://doi.org/ 10.1080/10408398.2014.925422.
[236] Dong Y, Yong VW. Oxidized phospholipids as novel mediators of neurodegeneration. Trends Neurosci 2022. https://doi.org/10.1016/j. tins.2022.03.002.
[237] Dushianthan A, Postle A. Methodology to detect oxidised phospholipids and their relevance in disease. Biochem Soc Trans 2021;49(3):1241-50. https://doi.org/ 10.1042/BST20200852.
[238] Nie J, Yang J, Wei Y, Wei X. The role of oxidized phospholipids in the development of disease. Mol Aspects Med 2020;76:100909. https://doi.org/ 10.1016/j.mam.2020.100909.
[239] Reis A, Spickett CM. Chemistry of phospholipid oxidation. Biochim Biophys Acta 2012;1818(10):2374-87. https://doi.org/10.1016/j.bbamem.2012.02.002.
[240] Henna Lu FS, Nielsen NS, Timm-Heinrich M, Jacobsen C. Oxidative stability of marine phospholipids in the liposomal form and their applications. Lipids. 2011; 46(1):3-23. https://doi.org/10.1007/s11745-010-3496-y.
[241] Thomsen BR, Haugsgjerd BO, Griinari M, Lu HFS, Bruheim I, Vogt G, et al. Investigation of oxidative degradation and non-enzymatic browning reactions in krill and fish oils. Eur J Lipid Sci Technol 2013;115(12):1357-66. https://doi. org/10.1002/ejlt.201300141.
[242] Igene J, Pearson A, Dugan Jr L, Price J. Role of triglycerides and phospholipids on development of rancidity in model meat systems during frozen storage. Food Chem 1980;5(4):263-76. https://doi.org/10.1016/0308-8146(80)90048-5.
[243] Igene J, Pearson A, Gray J. Effects of length of frozen storage, cooking and holding temperatures upon component phospholipids and the fatty acid composition of meat triglycerides and phospholipids. Food Chem 1981;7(4): 289-303. https://doi.org/10.1016/0308-8146(81)90034-0.
[244] Pikul J, Kummerow FA. Relative role of individual phospholipids on thiobarbituric acid reactive substances formation in chicken meat, skin and swine aorta. J Food Sci 1990;55(5):1243-8. https://doi.org/10.1111/j.13652621.1990.tb03907.x.
[245] Pan Y, Tikekar RV, Nitin N. Effect of antioxidant properties of lecithin emulsifier on oxidative stability of encapsulated bioactive compounds. Int J Pharm 2013; 450(1-2):129-37. https://doi.org/10.1016/j.ijpharm.2013.04.038.
[246] Burgos-Díaz C, Wandersleben T, Marqués AM, Rubilar M. Multilayer emulsions stabilized by vegetable proteins and polysaccharides. Curr Opin Colloid Interface Sci 2016;25:51-7. https://doi.org/10.1016/j.cocis.2016.06.014.
[247] McClements DJ. Protein-stabilized emulsions. Curr Opin Colloid Interface Sci 2004;9(5):305-13. https://doi.org/10.1016/j.cocis.2004.09.003.
[248] Zhang M, Fan L, Liu Y, Huang S, Li J. Effects of proteins on emulsion stability: the role of proteins at the oil-water interface. Food Chem 2022;397:133726. https:// doi.org/10.1016/j.foodchem.2022.133726.
[249] Decker EA. Strategies for manipulating the prooxidative/antioxidative balance of foods to maximize oxidative stability. Trends Food Sci Technol 1998;9(6):241-8. https://doi.org/10.1016/S0924-2244(98)00045-4.
[250] Singh P, Singh TP, Gandhi N. Prevention of lipid oxidation in muscle foods by milk proteins and peptides: A review. Food Rev Intl 2018;34(3):226-47. https:// doi.org/10.1080/87559129.2016.1261297.
[251] Berton-Carabin C, Schroën K. Towards new food emulsions: designing the interface and beyond. Curr Opin Food Sci 2019;27:74-81. https://doi.org/ 10.1016/j.cofs.2019.06.006.
[252] Cheng W, Zheng X, Yang M. Hydrogen peroxide induced protein oxidation during storage and Lyophilization process. J Pharm Sci 2016;105(6):1837-42. https:// doi.org/10.1016/j.xphs.2016.03.034.
[253] Duque-Estrada P, Kyriakopoulou K, de Groot W, van der Goot AJ, BertonCarabin CC. Oxidative stability of soy proteins: from ground soybeans to structured products. Food Chem 2020;318:126499. https://doi.org/10.1016/j. foodchem.2020.126499.
[254] Tan Y, Wang J, Chen F, Niu S, Yu J. Effect of protein oxidation on kinetics of droplets stability probed by microrheology in O/W and W/O emulsions of whey protein concentrate. Food Res Int 2016;85:259-65. https://doi.org/10.1016/j. foodres.2016.05.005.
[255] Li H, Cai Y, Li F, Zhang B, Wu X, Wu W. Rancidity-induced protein oxidation affects the interfacial dynamic properties and the emulsion rheological behavior of rice bran protein. Food Hydrocoll 2022;131:107794. https://doi.org/10.1016/ j.foodhyd.2022.107794.
[256] Zhou Q, Li H, Li F, Zhang B, Wu X, Wu W. Strategy and mechanism of Rice bran protein emulsion stability based on rancidity-induced protein oxidation: an ultrasonic case study. Foods. 2022;11(23). https://doi.org/10.3390/ foods11233896.
[257] Wang J, Tan Y, Xu H, Niu S, Yu J. Effect of 2,2-azobis (2-amidinopropane) dihydrochloride oxidized casein on the microstructure and microrheology properties of emulsions. Food Sci Biotechnol 2016;25(5):1283-90. https://doi. org/10.1007/s10068-016-0202-8.
[258] Hinderink EB, Kaade W, Sagis L, Schroën K, Berton-Carabin CC. Microfluidic investigation of the coalescence susceptibility of pea protein-stabilised emulsions: effect of protein oxidation level. Food Hydrocoll 2020;102:105610. https://doi. org/10.1016/j.foodhyd.2019.105610.
[259] Obando M, Papastergiadis A, Li S, De Meulenaer B. Impact of lipid and protein cooxidation on digestibility of dairy proteins in oil-in-water (
[260] Zhou Q, Wang J, Li H, Wu X, Wu W. Effect of protein oxidation on the emulsion carrier prepared by rice bran protein for improving stability and bioavailability of
[261] Zdanowska-Sąsiadek Z, Damaziak K, Marchewka J, Horbańczuk OK, Jóźwiak A, Wójcik W, et al. Lipid-and protein oxidation during storage and in vitro gastrointestinal digestion of ostrich, beef and chicken jerky snacks. Anim Sci Pap Rep 2022;40:305-16.
[262] Schaich KM. Chapter 8: Co-oxidations of oxidizing lipids: Reactions with proteins. In: Kamal-Eldin A, Min D, editors. Lipid oxidation pathways. AOCS Publishing; 2008. p. 183-274.
[263] Wu W, Hou L, Zhang C, Kong X, Hua Y. Structural modification of soy protein by 13-hydroperoxyoctadecadienoic acid. Eur Food Res Technol 2009;229:771-8. https://doi.org/10.1007/s00217-009-1113-1.
[264] Gürbüz G, Heinonen M. LC-MS investigations on interactions between isolated
[265] Yang J, Xiong YL. Comparative time-course of lipid and myofibrillar protein oxidation in different biphasic systems under hydroxyl radical stress. Food Chem 2018;243:231-8. https://doi.org/10.1016/j.foodchem.2017.09.146.
[266] Baron CP, Kjaersgard IV, Jessen F, Jacobsen C. Protein and lipid oxidation during frozen storage of rainbow trout (Oncorhynchus mykiss). J Agric Food Chem 2007; 55(20):8118-25. https://doi.org/10.1021/jf070686f.
[267] Yan S, Yao Y, Xie X, Zhang S, Huang Y, Zhu H, et al. Comparison of the physical stabilities and oxidation of lipids and proteins in natural and polyphenol-modified soybean protein isolate-stabilized emulsions. Food Res Int 2022;162(Pt B): 112066. https://doi.org/10.1016/j.foodres.2022.112066.
[268] Yi J, Ning J, Zhu Z, Cui L, Decker EA, McClements DJ. Impact of interfacial composition on co-oxidation of lipids and proteins in oil-in-water emulsions: competitive displacement of casein by surfactants. Food Hydrocoll 2019;87:20-8. https://doi.org/10.1016/j.foodhyd.2018.07.025.
[269] Zhu Z, Zhao C, Yi J, Liu N, Cao Y, Decker EA, et al. Impact of interfacial composition on lipid and protein co-oxidation in oil-in-water emulsions containing mixed Emulisifers. J Agric Food Chem 2018;66(17):4458-68. https:// doi.org/10.1021/acs.jafc.8b00590.
[270] Tian L, Zhang S, Yi J, Zhu Z, Decker EA, McClements DJ. The impact of konjac glucomannan on the physical and chemical stability of walnut oil-in-water emulsions coated by whey proteins. J Sci Food Agric 2022;102(10):4003-11. https://doi.org/10.1002/jsfa. 11748.
[271] Sørensen ADM, Nielsen NS, Hyldig G, Jacobsen C. Influence of emulsifier type on lipid oxidation in fish oil-enriched light mayonnaise. Eur J Lipid Sci Technol 2010;112(9):1012-23. https://doi.org/10.1002/ejlt.200900288.
[272] Jacobsen C, Adler-Nissen J, Meyer AS. Effect of ascorbic acid on iron release from the emulsifier interface and on the oxidative flavor deterioration in fish oil enriched mayonnaise. J Agric Food Chem 1999;47(12):4917-26. https://doi.org/ 10.1021/jf990241u.
[273] Raes K, Doolaege EH, Deman S, Vossen E, De Smet S. Effect of carnosic acid, quercetin and alpha-tocopherol on lipid and protein oxidation in an in vitro simulated gastric digestion model. Int J Food Sci Nutr 2015;66(2):216-21. https://doi.org/10.3109/09637486.2014.959900.
[274] Tian L, Kejing Y, Zhang S, Yi J, Zhu Z, Decker EA, et al. Impact of tea polyphenols on the stability of oil-in-water emulsions coated by whey proteins. Food Chem 2021;343:128448. https://doi.org/10.1016/j.foodchem.2020.128448.
[275] Perez-Gregorio MR, Simal-Gandara J. A critical review of the characterization of polyphenol-protein interactions and of their potential use for improving food quality. Curr Pharm Des 2017;23(19):2742-53. https://doi.org/10.2174/ 1381612823666170202112530.
[276] Quan TH, Benjakul S, Sae-leaw T, Balange AK, Maqsood S. Protein-polyphenol conjugates: antioxidant property, functionalities and their applications. Trends Food Sci Technol 2019;91:507-17. https://doi.org/10.1016/j.tifs.2019.07.049.
[277] Yan S, Xu J, Zhang S, Zhu H, Qi B, Li Y. Effect of interfacial composition on the physical stability and co-oxidation of proteins and lipids in a soy protein isolate-(-)-epigallocatechin gallate conjugate emulsion. Food Hydrocoll 2022;130: 107720. https://doi.org/10.1016/j.foodhyd.2022.107720.
[278] Laguerre M, Bayrasy C, Panya A, Weiss J, McClements DJ, Lecomte J, et al. What makes good antioxidants in lipid-based systems? The next theories beyond the polar paradox. Crit Rev Food Sci Nutr 2015;55(2):183-201. https://doi.org/ 10.1080/10408398.2011.650335.
[279] Laguerre M, Giraldo LJ, Lecomte J, Figueroa-Espinoza MC, Barea B, Weiss J, et al. Chain length affects antioxidant properties of chlorogenate esters in emulsion: the
cutoff theory behind the polar paradox. J Agric Food Chem 2009;57(23): 11335-42. https://doi.org/10.1021/jf9026266.
[280] Figueroa-Espinoza MC, Laguerre M, Villeneuve P, Lecomte J. From phenolics to phenolipids: optimizing antioxidants in lipid dispersions. Lipid Technol 2013;25 (6):131-4. https://doi.org/10.1002/lite.201300277.
[281] Figueroa-Espinoza MC, Villeneuve P. Phenolic acids enzymatic lipophilization. J Agric Food Chem 2005;53(8):2779-87. https://doi.org/10.1021/jf0484273.
[282] Grasso S, Siracusa L, Spatafora C, Renis M, Tringali C. Hydroxytyrosol lipophilic analogues: enzymatic synthesis, radical scavenging activity and DNA oxidative damage protection. Bioorg Chem 2007;35(2):137-52. https://doi.org/10.1016/j. bioorg.2006.09.003.
[283] Farooq S, Abdullah H Zhang, Weiss J. A comprehensive review on polarity, partitioning, and interactions of phenolic antioxidants at oil-water interface of food emulsions. Comprehen Rev Food Sci Food Safet 2021;20(5):4250-77. https://doi.org/10.1111/1541-4337.12792.
[284] Figueroa-Espinoza MC, Bourlieu C, Durand E, Lecomte J, Villeneuve P. Lipophilized antioxidants. In: Melton L, Shahidi F, Varelis P, editors. Encyclopedia of food chemistry. Oxford: Academic Press; 2019. p. 193-201. https://doi.org/10.1016/B978-0-08-100596-5.21673-9.
[285] Kahveci D, Laguerre M, Villeneuve P. 7 – Phenolipids as new antioxidants: Production. In: Ahmad MU, Xu X, editors. Activity, and potential applications, in polar lipids. Elsevier; 2015. p. 185-214. https://doi.org/10.1016/B978-1-63067-044-3.50011-X.
[286] Medina S, Auñón D, Lehoux J, Durand T, Crauste C, Gil-Izquierdo Á. Hydroxytyrosol fatty acid esters as new candidate markers for detecting olive oil inadequate storage conditions by UHPLC-QqQ-MS/MS. Microchem J 2022;181: 107656. https://doi.org/10.1016/j.microc.2022.107656.
[287] Caillol S. Cardanol: A promising building block for biobased polymers and additives. Curr Opin Green Sustain Chem 2018;14:26-32. https://doi.org/ 10.1016/j.cogsc.2018.05.002.
[288] Sonar VP, Corona A, Distinto S, Maccioni E, Meleddu R, Fois B, et al. Natural product-inspired esters and amides of ferulic and caffeic acid as dual inhibitors of HIV-1 reverse transcriptase. Eur J Med Chem 2017;130:248-60. https://doi.org/ 10.1016/j.ejmech.2017.02.054.
[289] Crauste C, Rosell M, Durand T, Vercauteren J. Omega-3 polyunsaturated lipophenols, how and why? Biochimie. 2016;120:62-74. https://doi.org/ 10.1016/j.biochi.2015.07.018.
[290] Choudhary MI, Begum A, Abbaskhan A, Ajaz A, Shafique ur R, Atta ur R. Phenyl polypropanoids from Lindelofia stylosa. Chem Pharm Bull(Tokyo) 2005;53(11): 1469-71. https://doi.org/10.1248/cpb.53.1469.
[291] Saha MM, Mallik UK, Mallik AK. A chromenoflavanone and two caffeic esters fromPongamia glabra. Phytochemistry. 1991;30(11):3834-6. https://doi.org/ 10.1016/0031-9422(91)80130-S.
[292] Medina I, Lois S, Alcantara D, Lucas R, Morales JC. Effect of lipophilization of hydroxytyrosol on its antioxidant activity in fish oils and fish oil-in-water emulsions. J Agric Food Chem 2009;57(20):9773-9. https://doi.org/10.1021/ jf9023867.
[293] Laguerre M, López Giraldo LJ, Lecomte J, Figueroa-Espinoza M-C, Baréa B, Weiss J, et al. Relationship between hydrophobicity and antioxidant ability of “Phenolipids” in emulsion: A parabolic effect of the chain length of Rosmarinate esters. J Agric Food Chem 2010;58(5):2869-76. https://doi.org/10.1021/ jf904119v.
[294] Sørensen A-DM, Durand E, Laguerre M, Bayrasy C, Lecomte J, Villeneuve P, et al. Antioxidant properties and efficacies of synthesized alkyl Caffeates, Ferulates, and Coumarates. J Agric Food Chem 2014;62(52):12553-62. https://doi.org/ 10.1021/jf500588s.
[295] Sørensen A-DM, Lyneborg KS, Villeneuve P, Jacobsen C. Alkyl chain length impacts the antioxidative effect of lipophilized ferulic acid in fish oil enriched milk. J Funct Foods 2015;18:959-67. https://doi.org/10.1016/j.jff.2014.04.008.
[296] Alemán M, Bou R, Guardiola F, Durand E, Villeneuve P, Jacobsen C, et al. Antioxidative effect of lipophilized caffeic acid in fish oil enriched mayonnaise and milk. Food Chem 2015;167:236-44. https://doi.org/10.1016/j. foodchem.2014.06.083.
[297] Grajeda-Iglesias C, Salas E, Barouh N, Baréa B, Panya A, Figueroa-Espinoza MC. Antioxidant activity of protocatechuates evaluated by DPPH, ORAC, and CAT methods. Food Chem 2016;194:749-57. https://doi.org/10.1016/j. foodchem.2015.07.119.
[298] Elder AS, Coupland JN, Elias RJ. Effect of alkyl chain length on the antioxidant activity of alkylresorcinol homologues in bulk oils and oil-in-water emulsions. Food Chem 2021;346:128885. https://doi.org/10.1016/j. foodchem.2020.128885.
[299] ten Klooster S, Villeneuve P, Bourlieu-Lacanal C, Durand E, Schroën K, BertonCarabin C. Alkyl chain length modulates antioxidant activity of gallic acid esters in spray-dried emulsions. Food Chem 2022;387:132880. https://doi.org/ 10.1016/j.foodchem.2022.132880.
[300] Lucas R, Comelles F, Alcantara D, Maldonado OS, Curcuroze M, Parra JL, et al. Surface-active properties of lipophilic antioxidants tyrosol and hydroxytyrosol fatty acid esters: a potential explanation for the nonlinear hypothesis of the antioxidant activity in oil-in-water emulsions. J Agric Food Chem 2010;58(13): 8021-6. https://doi.org/10.1021/jf1009928.
[301] Losada Barreiro S, Bravo-Diaz C, Paiva-Martins F, Romsted LS. Maxima in antioxidant distributions and efficiencies with increasing hydrophobicity of gallic acid and its alkyl esters. The pseudophase model interpretation of the “cutoff effect”. J Agric Food Chem 2013;61(26):6533-43. https://doi.org/10.1021/ jf400981x.
[302] Costa M, Losada-Barreiro S, Bravo-Diaz C, Vicente AA, Monteiro LS, PaivaMartins F. Influence of AO chain length, droplet size and oil to water ratio on the distribution and on the activity of gallates in fish oil-in-water emulsified systems: emulsion and nanoemulsion comparison. Food Chem 2020;310:125716. https:// doi.org/10.1016/j.foodchem.2019.125716.
[303] Mitrus O, Zuraw M, Losada-Barreiro S, Bravo-Diaz C, Paiva-Martins F. Targeting antioxidants to interfaces: control of the oxidative stability of lipid-based emulsions. J Agric Food Chem 2019;67(11):3266-74. https://doi.org/10.1021/ acs.jafc.8b06545.
[304] Freiria-Gandara J, Losada-Barreiro S, Paiva-Martins F, Bravo-Diaz C. Enhancement of the antioxidant efficiency of gallic acid derivatives in intact fish oil-in-water emulsions through optimization of their interfacial concentrations. Food Funct 2018;9(8):4429-42. https://doi.org/10.1039/c8fo00977e.
[305] Costa M, Losada-Barreiro S, Paiva-Martins F, Bravo-Díaz C. Physical evidence that the variations in the efficiency of homologous series of antioxidants in emulsions are a result of differences in their distribution. J Sci Food Agric 2017;97(2): 564-71. https://doi.org/10.1002/jsfa. 7765.
[306] Costa M, Losada-Barreiro S, Paiva-Martins F, Bravo-Díaz C, Romsted LS. A direct correlation between the antioxidant efficiencies of caffeic acid and its alkyl esters and their concentrations in the interfacial region of olive oil emulsions. The pseudophase model interpretation of the “cut-off” effect. Food Chem 2015;175: 233-42. https://doi.org/10.1016/j.foodchem.2014.10.016.
[307] Almeida J, Losada-Barreiro S, Costa M, Paiva-Martins F, Bravo-Diaz C, Romsted LS. Interfacial concentrations of Hydroxytyrosol and its lipophilic esters in intact olive oil-in-water emulsions: effects of antioxidant hydrophobicity, surfactant concentration, and the oil-to-water ratio on the oxidative stability of the emulsions. J Agric Food Chem 2016;64(25):5274-83. https://doi.org/ 10.1021/acs.jafc.6b01468.
[308] Stöckmann H, Schwarz K, Huynh-Ba T. The influence of various emulsifiers on the partitioning and antioxidant activity of hydroxybenzoic acids and their derivatives in oil-in-water emulsions. J Am Oil Chem Soc 2000;77(5):535-42. https://doi.org/10.1007/s11746-000-0085-6.
[309] Gonzalez MJ, Medina I, Maldonado OS, Lucas R, Morales JC. Antioxidant activity of alkyl gallates and glycosyl alkyl gallates in fish oil in water emulsions: relevance of their surface active properties and of the type of emulsifier. Food Chem 2015;183:190-6. https://doi.org/10.1016/j.foodchem.2015.03.035.
[310] Sørensen A-DM, Villeneuve P, Jacobsen C. Alkyl caffeates as antioxidants in O/W emulsions: impact of emulsifier type and endogenous tocopherols. Eur J Lipid Sci Technol 2017;119(6):1600276. https://doi.org/10.1002/ejlt.201600276.
[311] Hajfathalian M, Ghelichi S, Garcia-Moreno PJ, Moltke Sorensen AD, Jacobsen C. Peptides: production, bioactivity, functionality, and applications. Crit Rev Food Sci Nutr 2018;58(18):3097-129. https://doi.org/10.1080/ 10408398.2017.1352564.
[312] Lorenzo JM, Munekata PES, Gómez B, Barba FJ, Mora L, Pérez-Santaescolástica C, et al. Bioactive peptides as natural antioxidants in food products – A review. Trends Food Sci Technol 2018;79:136-47. https://doi.org/10.1016/j. tifs.2018.07.003.
[313] Zou TB, He TP, Li HB, Tang HW, Xia EQ. The structure-activity relationship of the antioxidant peptides from natural proteins. Molecules. 2016;21(1):72. https:// doi.org/10.3390/molecules21010072.
[314] Sun JZ, Kaur H, Halliwell B, Li XY, Bolli R. Use of aromatic hydroxylation of phenylalanine to measure production of hydroxyl radicals after myocardial ischemia in vivo. Direct evidence for a pathogenetic role of the hydroxyl radical in myocardial stunning. Circ Res 1993;73(3):534-49. https://doi.org/10.1161/01. res.73.3.534.
[315] Ospina-Quiroga JL, García-Moreno PJ, Guadix A, Guadix EM, AlmécijaRodríguez MDC, Pérez-Gálvez R. Evaluation of plant protein hydrolysates as natural antioxidants in fish oil-in-water emulsions. Antioxidants. 2022;11(8): 1612. https://doi.org/10.3390/antiox11081612.
[316] Liu Y, Qi Y, Wang Q, Yin F, Zhan H, Wang H, et al. Antioxidative effect of chlorella Pyrenoidosa protein hydrolysates and their application in krill oil-inwater emulsions. Mar Drugs 2022;20(6):345. https://doi.org/10.3390/ md20060345.
[317] Ghelichi S, Sørensen A-DM, García-Moreno PJ, Hajfathalian M, Jacobsen C. Physical and oxidative stability of fish oil-in-water emulsions fortified with enzymatic hydrolysates from common carp (Cyprinus carpio) roe. Food Chem 2017;237:1048-57. https://doi.org/10.1016/j.foodchem.2017.06.048.
[318] Olsen TH, Yesiltas B, Marin FI, Pertseva M, García-Moreno PJ, Gregersen S, et al. AnOxPePred: using deep learning for the prediction of antioxidative properties of peptides. Sci Rep 2020;10(1):21471. https://doi.org/10.1038/s41598-020-78319-w.
[319] Yesiltas B, García-Moreno PJ, Gregersen S, Olsen TH, Jones NC, Hoffmann SV, et al. Antioxidant peptides derived from potato, seaweed, microbial and spinach proteins: oxidative stability of 5% fish oil-in-water emulsions. Food Chem 2022; 385:132699. https://doi.org/10.1016/j.foodchem.2022.132699.
[320] Varona E, García-Moreno PJ, Gregersen Echers S, Olsen TH, Marcatili P, Guardiola F, et al. Antioxidant peptides from alternative sources reduce lipid oxidation in 5% fish oil-in-water emulsions ( pH 4 ) and fish oil-enriched mayonnaise. Food Chem 2023;426:136498. https://doi.org/10.1016/j. foodchem.2023.136498.
[321] García-Moreno PJ, Yesiltas B, Gregersen Echers S, Marcatili P, Overgaard MT, Hansen EB, et al. Recent advances in the production of emulsifying peptides with the aid of proteomics and bioinformatics. Curr Opin Food Sci 2023;51:101039. https://doi.org/10.1016/j.cofs.2023.101039.
[322] Pearson RG. Hard and soft acids and bases. J Am Chem Soc 1963;85(22):3533-9. https://doi.org/10.1021/ja00905a001.
[323] Irankunda R, Camaño Echavarría JA, Paris C, Stefan L, Desobry S, Selmeczi K, et al. Metal-chelating peptides separation using immobilized metal ion affinity chromatography: Experimental methodology and simulation. Separations. 2022;9 (11):370. https://doi.org/10.3390/separations9110370.
[324] Canabady-Rochelle LLS, Selmeczi K, Collin S, Pasc A, Muhr L, Boschi-Muller S. SPR screening of metal chelating peptides in a hydrolysate for their antioxidant properties. Food Chem 2018;239:478-85. https://doi.org/10.1016/j. foodchem. 2017.06.116.
[325] El Hajj S, Irankunda R, Camaño Echavarría JA, Arnoux P, Paris C, Stefan L, et al. Metal-chelating activity of soy and pea protein hydrolysates obtained after different enzymatic treatments from protein isolates. Food Chem 2023;405: 134788. https://doi.org/10.1016/j.foodchem.2022.134788.
[326] Guo L, Harnedy PA, O’Keeffe MB, Zhang L, Li B, Hou H, et al. Fractionation and identification of Alaska Pollock skin collagen-derived mineral chelating peptides. Food Chem 2015;173:536-42. https://doi.org/10.1016/j. foodchem. 2014.10.055.
[327] Chen D, Liu Z, Huang W, Zhao Y, Dong S, Zeng M. Purification and characterisation of a zinc-binding peptide from oyster protein hydrolysate. J Funct Foods 2013;5(2):689-97. https://doi.org/10.1016/j.jff.2013.01.012.
[328] Xie N, Huang J, Li B, Cheng J, Wang Z, Yin J, et al. Affinity purification and characterisation of zinc chelating peptides from rapeseed protein hydrolysates: possible contribution of characteristic amino acid residues. Food Chem 2015;173: 210-7. https://doi.org/10.1016/j.foodchem.2014.10.030.
[329] Schröder A, Laguerre M, Sprakel J, Schroën K, Berton-Carabin CC. Pickering particles as interfacial reservoirs of antioxidants. J Colloid Interface Sci 2020;575: 489-98. https://doi.org/10.1016/j.jcis.2020.04.069.
[330] Laguerre M, Sprakel JHB, Birtic S, Schroen CGPH, Berton-Carabin C, Schröder A. Emulsion comprising antioxidant particles. Naturex SA: Wageningen University; 2019.
[331] Zhou B, Gao S, Li X, Liang H, Li S. Antioxidant Pickering emulsions stabilised by zein/tannic acid colloidal particles with low concentration. Int J Food Sci Technol 2020;55(5):1924-34. https://doi.org/10.1111/ijfs.14419.
[332] Zou Y, Zhong J, Pan R, Wan Z, Guo J, Wang J, et al. Zein/tannic acid complex nanoparticles-stabilised emulsion as a novel delivery system for controlled release of curcumin. Int J Food Sci Technol 2017;52(5):1221-8. https://doi.org/ 10.1111/ijfs. 13380.
[333] Zhou FZ, Yan L, Yin SW, Tang CH, Yang XQ. Development of Pickering emulsions stabilized by gliadin/Proanthocyanidins hybrid particles (GPHPs) and the fate of lipid oxidation and digestion. J Agric Food Chem 2018;66(6):1461-71. https:// doi.org/10.1021/acs.jafc.7b05261.
[334] Peng LP, Tang CH. Outstanding antioxidant Pickering high internal phase emulsions by co-assembled polyphenol-soy beta-conglycinin nanoparticles. Food Res Int 2020;136:109509. https://doi.org/10.1016/j.foodres.2020.109509.
[335] Hu J, Du P, Xu R, Deng W. Supersmall dendritic mesoporous silica Nanospheres as antioxidant Nanocarriers for Pickering emulsifiers. J Agric Food Chem 2021;69 (49):14893-905. https://doi.org/10.1021/acs.jafc.1c03016.
[336] Lu X, Huang Q. Nano/submicrometer milled red Rice particles-stabilized Pickering emulsions and their Antioxidative properties. J Agric Food Chem 2020; 68(1):292-300. https://doi.org/10.1021/acs.jafc.9b04827.
[337] Schröder A, Laguerre M, Tenon M, Schroën K, Berton-Carabin CC. Natural particles can armor emulsions against lipid oxidation and coalescence. Food Chem 2021;347:129003. https://doi.org/10.1016/j.foodchem.2021.129003.
[338] Ghorbani Gorji S, Smyth HE, Sharma M, Fitzgerald M. Lipid oxidation in mayonnaise and the role of natural antioxidants: A review. Trends Food Sci Technol 2016;56:88-102. https://doi.org/10.1016/j.tifs.2016.08.002.
[339] Fadda A, Sanna D, Sakar EH, Gharby S, Mulas M, Medda S, et al. Innovative and sustainable technologies to enhance the oxidative stability of vegetable oils. Sustainability. 2022;14(2):849. https://doi.org/10.3390/su14020849.
[340] Savani P, Puthiyedath A, George S, Prasad PS, Annapure US. Evaluation of the sensory properties and antioxidant activity of clean rosemary extracts for an effective replacement of EDTA in mayonnaise. Appl Food Res 2023;3(1):100302. https://doi.org/10.1016/j.afres.2023.100302.
[341] Faustino M, Veiga M, Sousa P, Costa EM, Silva S, Pintado M. Agro-food byproducts as a new source of natural food additives. Molecules. 2019;24(6): 1056. https://doi.org/10.3390/molecules24061056.
[342] Mangiapelo L, Ianni F, Pagano C, Grispoldi L, Blasi F, Cenci-Goga B, et al. Role of apple pomace in the formulation of a novel healthy mayonnaise. Eur Food Res Technol 2023. https://doi.org/10.1007/s00217-023-04331-9.
[343] Kishk YFM, Elsheshetawy HE. Effect of ginger powder on the mayonnaise oxidative stability, rheological measurements, and sensory characteristics. Ann Agricult Sci 2013;58(2):213-20. https://doi.org/10.1016/j.aoas.2013.07.016.
[344] Altunkaya A, Hedegaard RV, Harholt J, Brimer L, Gökmen V, Skibsted LH. Oxidative stability and chemical safety of mayonnaise enriched with grape seed extract. Food Funct 2013;4(11):1647-53. https://doi.org/10.1039/C3FO60204D.
[345] Alnokkari A. Effect of vinegar on the oxidative stability of mayonnaise during its storage. J Chromatogr Sci 2023. https://doi.org/10.1093/chromsci/bmad036.
[346] Acharya P, Martins SIDFS, Der Maaten V, Vreeker R. EDTA-free mayonnaise and method for the production thereof. US 2015/0181914 A1.Google. Patents 2015.
[347] Khalid MU, Shabbir MA, Mustafa S, Hina S, Quddoos MY, Mahmood S, et al. Effect of apple peel as an antioxidant on the quality characteristics and oxidative stability of mayonnaise. Appl Food Res 2021;1(2):100023. https://doi.org/ 10.1016/j.afres.2021.100023.
[348] Honold PJ, Jacobsen C, Jónsdóttir R, Kristinsson HG, Hermund DB. Potential seaweed-based food ingredients to inhibit lipid oxidation in fish-oil-enriched mayonnaise. Eur Food Res Technol 2016;242:571-84. https://doi.org/10.1007/ s00217-015-2567-y.
[349] Raikos V, McDonagh A, Ranawana V, Duthie G. Processed beetroot (Beta vulgaris L.) as a natural antioxidant in mayonnaise: effects on physical stability, texture and sensory attributes. Food Sci Human Wellness 2016;5(4):191-8. https://doi. org/10.1016/j.fshw.2016.10.002.
[350] Lazăr S, Constantin OE, Horincar G, Andronoiu DG, Stănciuc N, Muresan C, et al. Beetroot by-product as a functional ingredient for obtaining value-added mayonnaise. Processes. 2022;10(2):227. https://doi.org/10.3390/pr10020227.
[351] Blejan AM, Nour V. Physico-chemical characteristics, sensory attributes and oxidative stability of soy Milk mayonnaise enriched in carotenoids from tomato by-products. Appl Sci 2023;13(12):7101. https://doi.org/10.3390/app13127101.
[352] Li C-Y, Kim H-W, Li H, Lee D-C, Rhee H-I. Antioxidative effect of purple corn extracts during storage of mayonnaise. Food Chem 2014;152:592-6. https://doi. org/10.1016/j.foodchem.2013.11.152.
[353] Azhagu Saravana Babu P, Aafrin B Vajiha, Archana G, Sabina K, Sudharsan K, Krishnan K Radha, et al. Polyphenolic and phytochemical content of Cucumis sativus seeds and study on mechanism of preservation of nutritional and quality outcomes in enriched mayonnaise. Int J Food Sci Technol 2016;51(6):1417-24. https://doi.org/10.1111/ijfs.13109.
[354] De Bruno A, Romeo R, Gattuso A, Piscopo A, Poiana M. Functionalization of a vegan mayonnaise with high value ingredient derived from the agro-industrial sector. Foods. 2021;10(11):2684. https://doi.org/10.3390/foods10112684.
[355] Vieira MR, Simões S, Carrera-Sánchez C, Raymundo A. Development of a clean label mayonnaise using fruit flour. Foods. 2023;12(11):2111. https://doi.org/ 10.3390/foods12112111.
[356] De Leonardis A, Macciola V, Iftikhar A, Lopez F. Antioxidant effect of traditional and new vinegars on functional oil/vinegar dressing-based formulations. Eur Food Res Technol 2022;248(6):1573-82. https://doi.org/10.1007/s00217-022-03986-0.
[357] Włodarczyk K, Tymczewska A, Rabiej-Kozioł D, Szydłowska-Czerniak A. Effect of black cumin cake extract, Octyl Caffeate, and active packaging on antioxidant properties of egg-free mayonnaise during storage. Appl Sci 2023;13(10):6245. https://doi.org/10.3390/app13106245.
[358] Feng J, Schroën K, Guyot S, Gacel A, Fogliano V, Berton-Carabin CC. Physical and oxidative stabilization of oil-in-water emulsions by roasted coffee fractions: Interface-and continuous phase-related effects. J Agric Food Chem 2023;71(11): 4717-28. https://doi.org/10.1021/acs.jafc.2c07365.
[359] De Leonardis A, Macciola V, Iftikhar A, Lopez F. Characterization, sensory and oxidative stability analysis of vegetable mayonnaise formulated with olive leaf vinegar as an active ingredient. Foods. 2022;11(24):4006. https://doi.org/ 10.3390/foods11244006.
[360] Martillanes S, Rocha-Pimienta J, Gil MV, Ayuso-Yuste MC, Delgado-Adámez J. Antioxidant and antimicrobial evaluation of rice bran (Oryza sativa L.) extracts in a mayonnaise-type emulsion. Food Chem 2020;308:125633. https://doi.org/ 10.1016/j.foodchem.2019.125633.
[361] Habinshuti I, Chen X, Yu J, Mukeshimana O, Duhoranimana E, Karangwa E, et al. Antimicrobial, antioxidant and sensory properties of Maillard reaction products (MRPs) derived from sunflower, soybean and corn meal hydrolysates. LWT. 2019; 101:694-702. https://doi.org/10.1016/j.lwt.2018.11.083.
[362] Feng J, Berton-Carabin CC, Fogliano V, Schroën K. Maillard reaction products as functional components in oil-in-water emulsions: A review highlighting interfacial and antioxidant properties. Trends Food Sci Technol 2022;121: 129-41. https://doi.org/10.1016/j.tifs.2022.02.008.
[363] Feng J, Berton-Carabin CC, Guyot S, Gacel A, Fogliano V, Schroën K. Coffee melanoidins as emulsion stabilizers. Food Hydrocoll 2023;139:108522. https:// doi.org/10.1016/j.foodhyd.2023.108522.
[364] Verzelloni E, Tagliazucchi D, Conte A. Relationship between the antioxidant properties and the phenolic and flavonoid content in traditional balsamic vinegar. Food Chem 2007;105(2):564-71. https://doi.org/10.1016/j. foodchem.2007.04.014.
[365] Tagliazucchi D, Verzelloni E, Conte A. Contribution of melanoidins to the antioxidant activity of traditional balsamic vinegar during aging. J Food Biochem 2010;34(5):1061-78. https://doi.org/10.1111/j.1745-4514.2010.00349.x.
[366] Verzelloni E, Tagliazucchi D, Conte A. Changes in major antioxidant compounds during aging of traditional balsamic vinegar. J Food Biochem 2010;34(1):152-71. https://doi.org/10.1111/j.1745-4514.2009.00271.x.
[367] Verzelloni E, Tagliazucchi D, Conte A. From balsamic to healthy: traditional balsamic vinegar melanoidins inhibit lipid peroxidation during simulated gastric digestion of meat. Food Chem Toxicol 2010;48(8):2097-102. https://doi.org/ 10.1016/j.fct.2010.05.010.
[368] Smetana S, Pleissner D, Zuin Zeidler V. Waste to Food: Returning Nutrients to the Food Chain. Leiden, The Netherlands: Wageningen Academic; 2023. https://doi. org/10.3920/978-90-8686-929-9.
[369] Ribeiro TB, Voss GB, Coelho MC, Pintado ME. Chapter 33 – food waste and byproduct valorization as an integrated approach with zero waste: Future challenges. In: Bhat R, editor. Future foods. Academic Press; 2022. p. 569-96. https://doi.org/10.1016/B978-0-323-91001-9.00017-7.
[370] Socas-Rodríguez B, Álvarez-Rivera G, Valdés A, Ibáñez E, Cifuentes A. Food byproducts and food wastes: are they safe enough for their valorization? Trends Food Sci Technol 2021;114:133-47. https://doi.org/10.1016/j.tifs.2021.05.002.
[371] Ahmed Z, Chen J, Tufail T, Latif A, Arif M, Ullah R, et al. Fundamental opportunities and challenges of nutraceutical noodles enriched with Agri-food byproducts. Trends Food Sci Technol 2024;143:104299. https://doi.org/10.1016/j. tifs.2023.104299.
[372] Amft J, Meissner PM, Steffen-Heins A, Hasler M, Stöckmann H, Meynier A, et al. Interlaboratory study on lipid oxidation during accelerated storage trials with rapeseed and sunflower oil analyzed by conjugated dienes as primary oxidation products. Eur J Lipid Sci Technol 2023;125(10):2300067. https://doi.org/ 10.1002/ejlt. 202300067.
- Abbreviations: A•, antioxidant radical; ABTS, 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonate); AFM, atomic force microscopy; AH, antioxidant; AMVN, 2,2’azobis (2,4-dimethylvaleronitrile); AV, anidisine value; BHT, butyl hydroxytoluene; CCL, critical chain length; CLSM, confocal laser scanning microscopy; CLP, colloidal solid lipid particles; CMC, critical micelle concentration; cryo-TEM, cryo-transmission electron microscopy; DLPC, dilauroylphosphatidylcholine; DMSN, dendritic mesoporous silica nanospheres; DPPH, 2,2-Diphenyl-1-picrylhydrazyl; EDTA, ethylenediaminetetraacetic acid; FFA-OOH, free fatty acid hydroperoxide; FID, flame ionization detector; GA, gallic acid; GC, gas chromatography; HLB, hydrophilic-lipophilic balance; HSAB theory, hard and soft acid and base theory; IMAC, immobilized metal ion affinity chromatography; L•, lipid alkyl radical; LC, liquid chromatography; LDL, low-density lipoprotein; LH, unsaturated fatty acid; LMWE, low molecular weight emulsifier; LO•, lipid alkoxyl radical; LOO•, lipid peroxyl radical; LOOH, lipid hydroperoxide; MCP, metal chelating peptide; MRP, Maillard reaction product; MS, mass spectrometry; NMR, nuclear magnetic resonance;
, hydroxyl radical; OSA, octenyl succinic anhydride; O/W emulsions, oil-inwater emulsions; PA, phosphatidic acid; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PG, phosphatidylglycerol; PI, phosphatidylinositol; PS, phosphatidylserine; PV, peroxide value; R•, alkyl radical; ROO•, peroxyl radical; SDS, sodium dodecyl sulfate; SPI, soy protein isolate; SPME, solid phase microextraction; SPR, surface plasmon resonance; switchSENSE®, electrically switchable nanolever technology; TAG, triacylglycerol; TBARS, thiobarbituric acid reactive substances; WPI, whey protein isolate.. Number of carbon atoms in the alkyl chain.
Critical chain length: number of carbon atoms in the alkyl chain at the maximum antioxidant capacity.
DOI: https://doi.org/10.1016/j.plipres.2024.101275
PMID: https://pubmed.ncbi.nlm.nih.gov/38280491
Publication Date: 2024-01-26
Lipid oxidation in emulsions: New insights from the past two decades
To cite this version:
Lipid oxidation in emulsions: New insights from the past two decades
A R T I C L E I N F O
Keywords:
Emulsions
Lipid droplets
Interface
Antioxidants
Colloids
Co-oxidation
Abstract
Lipid oxidation constitutes the main source of degradation of lipid-rich foods, including food emulsions. The complexity of the reactions at play combined with the increased demand from consumers for less processed and more natural foods result in additional challenges in controlling this phenomenon. This review provides an overview of the insights acquired over the past two decades on the understanding of lipid oxidation in oil-inwater (
1. Introduction
to control this phenomenon, in particular in products containing a high amount of (poly)unsaturated fatty acids that are especially sensitive to oxidation [1,2]. Lipid oxidation can occur through three major pathways: enzymatic oxidation via the action of enzymes such as
- Corresponding author.
** Corresponding author at: INRAE, UR BIA, Nantes 44300, France.
E-mail address: marie.hennebelle@wur.nl (M. Hennebelle).
lipoxygenases, photooxidation via the activation of a sensitizer by light and autooxidation. While the first two are usually well controlled in food products through heat treatment and packaging, autooxidation remains a challenge, in particular to answer the increased demand by the consumers for more sustainable and natural products (i.e., clean label). Current methodologies to prevent lipid oxidation (i.e., cold chain storage, vacuum- or controlled atmosphere packaging, addition of synthetic antioxidants) need to be reconsidered and new trends are appearing, including the use of more natural antioxidants from plant extracts, the development of upcycled ingredients from food industry side streams, the introduction of low or mildly processed ingredients, or the increased use of plant-based emulsifiers.
milk, infant formulas, dairy-based goods, mayonnaises, dressings, as well as pharmaceuticals, cosmetics, and personal care products. Such emulsions consist of oil droplets dispersed within a continuous aqueous phase and stabilized by surface-active molecules, which adsorb at the oil-water interface. The oil-water interface is known to play a crucial role by being the location where lipids, oxygen, and pro-oxidants enter into contact (for reviews, see [3,4]). The large interfacial area typically found in emulsions promotes the exposure of lipids to pro-oxidant agents, and thus accelerates lipid oxidation compared to bulk oils. Many factors, such as the droplet size, interfacial composition, emulsifier type, partitioning and reactivity of pro- and antioxidants can impact the lipid oxidation process in emulsified foods, making it a challenge to fully understand and control the reactions, and therefore, to rationalize formulation strategies.

2. Lipid oxidation mechanisms in oil-in-water emulsions
2.1. General properties of emulsions and oil-water interfaces
can modify droplet density, potentially leading to droplet sedimentation rather than creaming. Attractive lateral capillary forces between trapped particles within the interfacial film stem from the deformation of the fluid interface around particles, granting the interfacial layer high mechanical stability and rigidity. This, combined with the high desorption energy of the particles once adsorbed, confers such emulsions with a high physical stability, in particular regarding resistance to coalescence [15,23].
2.2. Global overview of lipid oxidation pathways
propagation cycle of lipid oxidation. The metal redox cycle in Fig. 1B is also strongly modulated by the presence of water-soluble reducing agents, such as ascorbic acid, that aid regenerating bivalent transition metals (mechanism (a) in Fig. 1C) and thus display a pro-oxidant effect [39], or metal chelators (mechanism (b) in Fig. 1C), thus having an antioxidant action in the latter case.
2.3. Beyond the classical pathway of lipid oxidation
food products. However, as will be highlighted in further detail in the following sections, the oil-water interface in
2.4. Spatiotemporal resolution of lipid oxidation in emulsions
2.4.1. The central role of the interface between oil and water
2.4.2. Modulation of oxidation kinetics by the formation of colloidal structures within the oil phase
2.4.3. Transport through the continuous aqueous phase
octadecane [26,28,98,99], specific secondary lipid oxidation products bearing a hydroperoxide group (e.g., 4-hydroperoxy 2-nonenal), free fatty acids (e.g., linoleic acid), as well as secondary oxidation compounds (e.g., 2,4-decadienal), can diffuse through the emulsion system and may propagate oxidative reactions to neighboring lipid droplets [27,99-101]. Experiments performed using flow cytometry suggested that micelle-facilitated transport is an active transport mechanism in model O/W emulsions [100]. Studies using carefully prepared model emulsions confirmed that these transport mechanisms are limited to specific lipid oxidation products; for instance, hydroperoxides bound to a TAG backbone are not prone to transport, probably because their insertion into the hydrophobic core of surfactant micelles is not sterically possible, whereas smaller hydrophilic molecules are [27].
2.4.4. Oxidation reactions within the continuous aqueous phase
2.5. Modelling approaches
between samples with specific variations (e.g., different concentrations of antioxidant) and have even been used to predict the shelf-life of emulsions, such as mayonnaise [114]. On the other hand, the interpretation of their parameters can be complex as they do not relate directly to parameters from the system itself (e.g., composition of the samples or conditions of incubation) and they do not consider the full cascade of reactions involved. More recently, a more extensive mechanistic kinetic model has been proposed in bulk oils, taking into consideration the multiple reactions involved in lipid oxidation, the oxygen mass transfer, as well as the initial composition of the oil [115]. This model presents the advantage of enabling the shelf-life prediction of different vegetable oils stored under different oxygen conditions, but remains limited to bulk oils. In O/W emulsions, a kinetic model incorporating reaction kinetics and mass transfer was shown to well describe the oxidation process in emulsions stabilized with five different emulsifiers [116].
3. Impact of emulsion formulation and structure on lipid oxidation
3.1. Effects of emulsion processing and structural properties on lipid oxidation
difference between rotating and stationary elements to induce shear for droplet breakup, but they typically have low energy density, making it difficult to create small droplets. Other emulsification methods, such as membrane emulsification, exist but are currently impractical for most commercial applications [124]. Costa et al. [125] highlighted that homogenization, particularly when involving intense mechanical forces, can impact emulsifier molecules or supramolecular structures. The combined influence of temperature and shear on emulsifiers is often overlooked in emulsion research. For example, high pressures can alter the tertiary structure of globular proteins, leading to protein unfolding even when the product temperature remains below the protein denaturation temperature, potentially resulting in protein aggregation. The conditions of emulsification, including the type of equipment and energy input, are also important as they determine the specific droplet size distribution, which directly influences the emulsion’s ability to resist oxidation due to variations in total surface area [126,127]. In principle, smaller droplets, and thus a larger oil-water interfacial area, should promote lipid oxidation. While this effect has often been observed experimentally, there have been conflicting reports, possibly stemming from the challenges of altering droplet size without affecting other emulsion properties [4]. Several studies, in particular by C. Jacobsen’s group, have demonstrated that the homogenization conditions can impact lipid oxidation in various types of emulsions containing fish oil [128-130]. Horn et al. [128] investigated the effect of different homogenization pressures and temperatures on fish
(biopolymer loops, pores), raising doubts about whether this parameter plays a decisive role in the oxidation of emulsions.
3.2. New trends in food emulsifiers
emulsions (Fig. 2). Twenty years ago, these percentages were only around
3.2.1. Plant protein-based emulsions

3.2.2. Pickering emulsions
a completely different structure, lacking interfacial particles. The findings indicated that the Pickering particles did not, per se, prevent lipid oxidation compared to caseinate-stabilized emulsions, suggesting that these particles did not induce a physical barrier effect at the interface. However, the Pickering emulsion exhibited higher oxidative stability compared to the control emulsion with high melting point fat in the droplet core. This was attributed to intra-droplet lipid crystallization in the latter system, which expelled labile polyunsaturated fatty acids from inside the droplets to the surface, facilitating their contact with aqueous phase pro-oxidants. Other researchers also explored the use of various lipid-based particles to stabilize Pickering emulsions [165]. They highlighted the importance of the lipid type used to form the particles in influencing the oxidation of the oil droplet core. For example, Pickering emulsions made with trimyristin or olive oil particles showed greater resistance to lipid oxidation compared to a conventional proteinstabilized emulsion, whereas those made with palm olein particles did not exhibit a clear advantage. Interestingly, the protective effect of the former particles was only observed when they were present during emulsion homogenization, not when added post-emulsification, which is an important control to ascribe the protective effect of the particles to their interfacial localization. Furthermore, the physical state (crystalline vs. liquid) of the lipid material within the particles did not appear to be a dominant factor affecting the protective effect of the particle layer [165].
3.3. Trade-off between adsorbed/non-adsorbed emulsifiers
antioxidant activity in food emulsions.
The mode of addition of the antioxidant during the preparation of food emulsions is also important [167-169]. For example, the addition of
participate in accelerating the oxidation of surrounding droplets during the propagation step. Conversely, in the case of very lipophilic lipids (e. g., TAG-OOH, etc.), one may suspect that their potential diffusion will be restricted, which would limit the influence of non-adsorbed emulsifiers and inter-droplet pro-oxidant activity in the early stage of oxidation [27,98]. Finally, hydroperoxides from free fatty acids (FFA-OOH) are more surface-active than their homologues formed on higher molecular weight lipids (TAGs, diacylglycerols, etc.) and more stable than their radicals (FFA-OO•), which have a limited half-life time, making them a serious candidate in the inter-droplet pro-oxidant activity [100]. However, it is unclear whether such a micellization can be achieved by FFAOOH alone or whether an excess of non-adsorbed emulsifiers would be necessary (co-micellization).
antioxidant molecules such as tocopherols in the interfacial region increased rapidly with increasing surfactant concentration [181,182]. These results, along with a multitude of others [101,173,183,184], highlight the role of micelles on the antioxidant efficacy. According to the literature, for the most lipophilic antioxidants, micellization would promote their effectiveness by improving their diffusion and/or concentration in interfacial region meaning also that the oxidized form at the interface of the lipid droplets could be more quickly replaced. For antioxidants of intermediate polarity and amphiphilic nature, micellization will tend to reduce their action, likely by reducing their concentration at the oil-water interface (“dilution effect”). For highly watersoluble antioxidants, there does not seem to be any definite trend, likely because the effect on antioxidant distribution will be less important than the inter-droplet pro-oxidant effects of lipids and metals.
4. Sensitivity of the emulsifiers themselves to oxidation
4.1. Low molecular weight surfactants
quantities (
4.2. Phospholipids
emulsifiers improved the oxidative stability of
aldehydes. Intermediate products formed in this reaction pathway include epoxy keto fatty esters, epoxyalkenals and hydroxyalkenals [235,239]. The patterns and kinetics of phospholipid oxidation are largely documented when dealing with liposomal systems for food, pharmaceutical or cosmetic applications. The oxidative stability of liposomes is greatly influenced by their size and molecular composition as well as the molecular composition of their surrounding medium [240]. For example, for marine phospholipids, this transformation is so fast that the usual formation of lipid hydroperoxides and aldehydes evaluated by measurement of peroxide value (PV), anisidine value (AV) or thiobarbituric subtances (TBARS) cannot be detected, which can lead to the erroneous conclusion that no lipid oxidation is taking place [241]. Concerning emulsions that are stabilized by lecithins, to the best of our knowledge, no research work exists where the oxidation kinetics of phospholipids versus the ones of TAGs were compared. Still, in such emulsions, as phospholipids locate either at the interfacial area or as colloidal structures in the continuous phase, one can assume that, compared to the lipid droplets, phospholipids would be more in contact with pro-oxidants and could be then oxidized faster. By analogy, one can cite the various studies made on the study of oxidative stability in model meat system. Igene et al. [242] evaluated the effect of TAGs and phospholipids on development of rancidity using lipid-free muscle fibers in combination with added TAGs or phospholipids. Results showed that both TAGs and phospholipids contribute to development of rancidity, although phospholipids were the first to oxidize. Later, the same research group showed that PE and PC were particularly sensitive to oxidation in frozen meat [243]. Others confirmed this observation and found that
4.3. Proteins
revealed a heterogeneous distribution of protein oxidation at the interface pointing towards lipoprotein granules that are typically present in egg yolk. Finally, in the absence of tocopherols (stripped oil), protein oxidation was enhanced both at the interface and in the continuous phase. This enhanced protein oxidation upon removal of tocopherol was attributed to the transport of lipid radicals from the oil droplets to the interface and continuous phase. The authors also evaluated the effect of ascorbic acid on lipid and protein oxidation. In the presence of tocopherols, ascorbic acid showed an antioxidant effect towards lipids, whereas in the absence of tocopherols, it behaved as a pro-oxidant. However, other studies have shown that ascorbic acid acts as a strong catalyst of lipid oxidation in mayonnaise prepared with a mixture of rapeseed oil and fish oil, which contained tocopherols [272]. Concerning protein oxidation, ascorbic acid acted as a pro-oxidant for adsorbed proteins at the interface as well as for the ones in the continuous phase. Interestingly, contrasting behaviors with other antioxidants regarding lipid or protein oxidation were also reported. For instance, Raes et al. [273] showed that
5. Novel antioxidants from model systems to real food applications
prompting a revaluation of the polar paradox and the introduction of new concepts and hypotheses [278]. For instance, in bulk oil, as explained earlier (section 2.4.2), it was demonstrated that the critical site of oxidation is not the air-oil interface, as previously proposed in the polar paradox. Instead, oxidation occurs at association colloids formed by traces of water and surface-active molecules such as phospholipids. At the same period, it was demonstrated that in O/W emulsions, a nonlinear relationship between hydrophobicity and antioxidant capacity applies [279]. This effect, referred to as the cut-off effect, was then amply further studied and confirmed, as detailed in the following (section 5.1).
5.1. Phenolipids and the cut-off effect
alcohols or the primary hydroxyl with aliphatic carboxylic acids respectively. Thus, by preserving the phenolic hydroxyl, the resulting molecules, called “phenolipids,” retained all their reactivity with free radicals. Chemical lipophilization by esterification, usually carried out under harsh conditions with strong acid catalysts (hydrochloric acid, sulfuric acid, paratoluene sulfonic acid, sulfonic acid resins), is quite efficient and quantitative, and can be driven to completion by continuous drying of the medium when water is produced. Conversely, enzymes allow the synthesis of phenolipids under milder conditions with better selectivity and fewer side reactions. However, enzymatic catalysis is often much slower, potentially subject to inhibition phenomena, and, as recently described in several reviews, requires the fine-tuning of numerous parameters to be effective [283-285].

propyl; C8, octyl; C12, octadecyl) (Fig. 3) in stripped olive or corn oil-inwater emulsions of different oil:water (
Critical chain lengths (CCL) within phenolipid series.
| Phenolipids | Alkyl chains tested
|
Lipid system | Emulsifier | CCL
|
Remarks | Reference |
| Hydroxytyrosyl alkanoates | 0, 2, 4, 8, 12 | Fish O/W emulsions | Lecithin | 8 | [292] | |
| Alkyl rosmarinates |
|
Tung O/W emulsion | Brij 35 | 8 | [293] | |
| Alkyl rosmarinates | 0, 4, 8, 12, 18, 20 | Soybean O/W emulsion | Tween 20 | 4 | [180] | |
| Alkyl coumarates | 1, 4, 8, 12, 16, 18, 20 | Tung O/W emulsion | Brij 35 | 12 | Very weak antioxidant activity of all phenolics | [294] |
| Alkyl ferulates |
|
Tung O/W emulsion | Brij 35 | 4-12 | [294] | |
|
|
Fish oil-enriched milk | – | 1 | Pro-oxidant effect of C12 and C8; C16 and C20 almost inactive | [295] | |
|
|
Tung O/W emulsion | Brij 35 | 8 | [294] | ||
| Alkyl caffeates | 0, 1, 4, 8, 12, 18 | Fish oil-enriched mayonnaise | – | 4-12 | Rapeseed oil-based mayonnaise | |
|
|
Fish oil-enriched milk | – | 1-4 | [296] | ||
| Alkyl protocatechuates | 1, 2, 4, 6, 8, 10, 12, 14, 16, 18 | Tung O/W emulsion | Brij 35 | 2-4 | C2-C6 acted as chain-breakers, while C12-C20 as retarders | [297] |
| Natural rye bran alkylresorcinols | 17, 19, 21, 23, 25 | Algae O/W emulsion | Brij L23 | 21 | All alkyl resorcinols were more effective than orcinol | [298] |
| Alkyl gallates | 0,3,8,12,16 | Spray-dried emulsion (WPI + caseinate + sunflower and fish oils) | – | 8 | Very slight cut-off effect. Hydrophobic gallates (
|
[299] |
5.2. Peptides (e.g., derived from by-products)
5.2.1. Top-down approach to obtain antioxidant peptides
5.2.2. Bottom-up approach to obtain antioxidant peptides
study highlighted the lack of reliable screening techniques for testing the metal chelation of synthetic peptides and the need for new advanced techniques. On the other hand, other important parameters for metal chelating activity were considered such as isoelectric point and net charge at pH 7.0 for the selection of metal chelating peptides. Results showed that all the peptides provided better or similar oxidative stability compared to the control emulsion without antioxidants and thus prevented lipid oxidation in a model emulsion system. Particularly, charged peptides performed better than neutral ones, and active peptides’ lengths ranged between 6 and 14 amino acids. This study demonstrated the potential of bioinformatics and proteomics for identifying natural, sustainable antioxidant peptides abundant in parent proteins. The methodology enables precise peptide targeting through designed enzymatic hydrolysis, using protease specificity and bioinformatics sequence analysis.
5.2.3. Screening of metal chelating peptides
chromatography (IMAC). The SPR technology is an optical technique for the determination of dissociation constants (
5.3. Antioxidant-loaded Pickering particles: purposely designed particles (bottom-up) vs natural structures (top-down)
(DMSNs), which function both as nanocarriers for hydrophilic or hydrophobic antioxidants (demonstrated for epigallocatechin gallate and resveratrol) and as Pickering stabilizers [335]. Although this system was only tested for stabilizing flavor oil (not PUFA-rich oil), the particles effectively retained the targeted antioxidant within their internal structure and significantly protected citral against oxidation. These advanced hierarchical designs for bi-functional Pickering particles have established the proof of concept for interfacial antioxidant reservoirs and offer promise for enhancing the effectiveness of natural antioxidants. However, a major drawback that may limit their widespread application is the complexity and cost of such a strategy, which may not align with the current trend towards natural, clean-label, and minimally processed food systems. An alternative approach, while still utilizing the concept of antioxidant-loaded Pickering particles, could involve leveraging the endogenous antioxidant content of naturally occurring particles (i.e., deploying a “top-down” approach rather than “bottomup”, as in the previous examples), such as food and biobased product side-streams. While the level of control over the composition and structure of such particles will be necessarily lower than that of tailormade composite particles, they hold significant potential in terms of sustainability, naturalness, and interfacial retention of antioxidants [159]. One example of such a strategy involves Pickering emulsions stabilized with milled red rice particles containing anthocyanins [336]. This study showed that these polyphenol-rich particles provided better protection for emulsified oil droplets against oxidation compared to white rice starch-stabilized emulsions and bulk oil. Another recent study focusing on preparing Pickering emulsions using various plant-based particles, demonstrated that emulsions stabilized by matcha tea and spinach leaf particles were highly stable against lipid oxidation, in contrast to reference emulsions stabilized by conventional emulsifiers [337]. This protective effect is likely attributed to the presence of endogenous antioxidants in these fractions, such as free radicalscavenging phenolics and chelating organic acids, respectively. Using such natural particles appears to be a promising approach for physically and oxidatively stabilizing clean-label emulsions. Given the widespread presence and diversity of phenolic antioxidants in food-compatible plant materials, there are undoubtedly numerous prospective sources of Pickering particles to explore.
5.4. Towards clean-label and multifunctional ingredients
Overview of (potential) clean-label ingredients with antioxidant activity in O/W food emulsions.
| Food emulsion | Biobased ingredient | Proposed antioxidant components/ mechanisms | Reference |
| Mayonnaise | Apple pomace | Phenolics | [342] |
| Ginger powder | Not defined | [343] | |
| Grape seed extract | Radical scavenging by phenolics | ||
| Grape, apple vinegar | Phenolics | [345] | |
| Balsamic vinegar | Melanoidins | [346] | |
| Apple peel extract | Phenolics | [347] | |
| Brown seaweed extracts | Phenolics | [348] | |
| Beet root | Phenolics, betalain | [349] | |
| Beet root peel | Phenolics, betalain | [350] | |
| Tomato byproduct | Carotenoids | [351] | |
| Purple corn extracts | Anthocyanins | [352] | |
| Cucumis seed extracts | Phenolics | [353] | |
| Vegan mayonnaise | Olive mill waste | Phenolics | [354] |
| Fruit flour | Phenolics | [355] | |
| Olive vinegar | Phenolics | [356] | |
| Cumin cake extract | Phenolics, radical scavenging | [357] | |
| Model O/W emulsion | Roasted coffee fractions | Partitioning of MRPs | [358] |
| Olive leaf vinegar | Phenolics, oleuropin | [359] | |
| Rice bran extracts | Not specified | [360] |
originating from waste- or side-streams. For most of the ingredients in Table 2, their antioxidant activity was attributed to presence of phenolics in the water phase of food emulsions, yet without establishing a clear causal mechanistic link.
ensuring food safety, encompassing by-products used as food ingredients. However, when these by-products function as natural additives, compliance with the Novel Food Regulation (EU Regulation No. 2015/2283) becomes mandatory. This regulation delineates novel food categories originating from plants, animals, microorganisms, and novel production technologies. These foods require authorization and indexing on the list of authorized novel foods managed by the European Food Safety Authority (EFSA) [368]. It should be pointed out that the lack of a clear definition of food waste and by-products poses a challenge, hampering accurate assessment and impeding measures to address food loss and waste. Therefore, clear definitions of these terms seem crucial for future research and business programs to promote innovation in a circular economy scheme [368,370]. Harmonizing policies, reducing administrative burdens, and establishing stable regulations are pivotal to foster investments and facilitate the transformation of waste into valuable resources. Furthermore, the Codex Alimentarius, an initiative by the Food and Agriculture Organization (FAO) and the World Health Organization (WHO), serves as a global reference for food safety, establishing international standards and guidelines [371]. These guidelines often include permissible limits for contaminants, additives, and nutrients, which are crucial to develop sound food safety regulations. Regulatory bodies such as the FDA (US) and EFSA determine acceptable daily intake (ADI) levels for additives to safeguard consumers from harmful substances. However, challenges persist in assessing byproducts’ suitability and safety due to the absence of specific legal limits. Therefore, reference values for evaluating by-products in that respect often rely on limits set for original materials or similar matrices, leading to complexity in interpreting results and ensuring consumer safety [370].
6. Conclusions and perspective
the-art methodological advancements that are currently under active development, the establishment of standardized practices will be pivotal in addressing the complexity of lipid oxidation reactions [372]. This alignment in methodologies across research endeavors will not only enhance comparability but also facilitate a more holistic understanding of oxidation mechanisms. Simultaneously, the evolution of modelling techniques is emerging as a critical frontier. Refinement and development of these models will be indispensable in predicting and simulating the behavior of lipid oxidation within such systems, aiding in experimental design and unravelling complex interrelationships between the involved factors.
Funding sources
CRediT authorship contribution statement
Declaration of competing interest
References
[2] Cosgrove JP, Church DF, Pryor WA. The kinetics of the autoxidation of polyunsaturated fatty acids. Lipids. 1987;22(5):299-304. https://doi.org/ 10.1007/BF02533996.
[3] McClements DJ, Decker EA. Lipid oxidation in oil-in-water emulsions: impact of molecular environment on chemical reactions in heterogeneous food systems.
[4] Berton-Carabin CC, Ropers MH, Genot C. Lipid oxidation in oil-in-water emulsions: involvement of the interfacial layer. Comprehen Rev Food Sci Food Safet 2014;13(5):945-77. https://doi.org/10.1111/1541-4337.12097.
[5] ten Klooster S, Boerkamp V, Lazaridi E, Yang S, Takeuchi M, Berton-Carabin C, et al. Lipid oxidation in food emulsions: Analytical challenges and recent developments. In: Bravo-Diaz C, editor. Lipid oxidation in food and biological systems: A physical chemistry perspective. Cham: Springer International Publishing; 2022. p. 3-29. https://doi.org/10.1007/978-3-030-87222-9_1.
[6] Barriuso B, Astiasarán I, Ansorena D. A review of analytical methods measuring lipid oxidation status in foods: a challenging task. Eur Food Res Technol 2013; 236(1):1-15. https://doi.org/10.1007/s00217-012-1866-9.
[7] Laguerre M, Lecomte J, Villeneuve P. Evaluation of the ability of antioxidants to counteract lipid oxidation: existing methods, new trends and challenges. Prog Lipid Res 2007;46(5):244-82. https://doi.org/10.1016/j.plipres.2007.05.002.
[8] Xia W, Budge SM. Techniques for the analysis of minor lipid oxidation products derived from Triacylglycerols: epoxides, alcohols, and ketones. Compr Rev Food Sci Food Saf 2017;16(4):735-58. https://doi.org/10.1111/1541-4337.12276.
[9] Jacobsen C, García-Moreno PJ, Yesiltas B, Sørensen A-DM. Chapter 9 – lipid oxidation and traditional methods for evaluation. In: García-Moreno PJ, et al., editors. Omega-3 delivery systems. Academic Press; 2021. p. 183-200. https:// doi.org/10.1016/B978-0-12-821391-9.00009-0.
[10] McClements DJ. Food emulsions: Principles, practices, and techniques. 3rd edition ed. Boca Raton: CRC Press; 2015. https://doi.org/10.1201/b18868.
[11] Food emulsifiers and their applications. 3rd edition ed. Springer Cham; 2019. p. 522. https://doi.org/10.1007/978-3-030-29187-7.
[12] Drusch S, Klost M, Kieserling H. Current knowledge on the interfacial behaviour limits our understanding of plant protein functionality in emulsions. Curr Opin Colloid Interface Sci 2021;56:101503. https://doi.org/10.1016/j. cocis.2021.101503.
[13] Dickinson E. Proteins at interfaces and in emulsions stability, rheology and interactions. J Chem Soc Faraday Trans 1998;94(12):1657-69. https://doi.org/ 10.1039/A801167B.
[14] Dickinson E. Use of nanoparticles and microparticles in the formation and stabilization of food emulsions. Trends Food Sci Technol 2012;24(1):4-12. https://doi.org/10.1016/j.tifs.2011.09.006.
[15] Berton-Carabin CC, Schroen K. Pickering emulsions for food applications: background, trends, and challenges. Annu Rev Food Sci Technol 2015;6:263-97. https://doi.org/10.1146/annurev-food-081114-110822.
[16] Murray BS. Pickering emulsions for food and drinks. Curr Opin Food Sci 2019;27: 57-63. https://doi.org/10.1016/j.cofs.2019.05.004.
[17] Pickering SU. CXCVI.-emulsions. J Chem Soc Trans 1907;91(0):2001-21. https://doi.org/10.1039/CT9079102001.
[18] Tcholakova S, Denkov N, Lips A. Comparison of solid particles, globular proteins and surfactants as emulsifiers. Phys Chem Chem Phys 2008;10(12):1608-27. https://doi.org/10.1039/B715933C.
[19] Berton-Carabin CC, Sagis L, Schroen K. Formation, structure, and functionality of interfacial layers in food emulsions. Annu Rev Food Sci Technol 2018;9:551-87. https://doi.org/10.1146/annurev-food-030117-012405.
[20] Bos MA, van Vliet T. Interfacial rheological properties of adsorbed protein layers and surfactants: a review. Adv Colloid Interface Sci 2001;91(3):437-71. https:// doi.org/10.1016/S0001-8686(00)00077-4.
[21] Murray BS. Rheological properties of protein films. Curr Opin Colloid Interface Sci 2011;16(1):27-35. https://doi.org/10.1016/j.cocis.2010.06.005.
[22] Hinderink EB, Sagis L, Schroën K, Berton-Carabin CC. Behavior of plant-dairy protein blends at air-water and oil-water interfaces. Colloids Surf B Biointerfaces 2020;192:111015. https://doi.org/10.1016/j.colsurfb.2020.111015.
[23] Chevalier Y, Bolzinger M-A. Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloids Surf A Physicochem Eng Asp 2013;439:23-34. https://doi.org/10.1016/j.colsurfa.2013.02.054.
[24] ten Klooster S, Takeuchi M, Schroën K, Tuinier R, Joosten R, Friedrich H, et al. Tiny, yet impactful: detection and oxidative stability of very small oil droplets in surfactant-stabilized emulsions. J Colloid Interface Sci 2023. https://doi.org/ 10.1016/j.jcis.2023.09.005.
[25] Guzey D, McClements DJ. Formation, stability and properties of multilayer emulsions for application in the food industry. Adv Colloid Interface Sci 2006; 128-130:227-48. https://doi.org/10.1016/j.cis.2006.11.021.
[26] Samtlebe M, Yucel U, Weiss J, Coupland JN. Stability of solid lipid nanoparticles in the presence of liquid oil emulsions. J Am Oil Chem Soc 2012;89(4):609-17. https://doi.org/10.1007/s11746-011-1944-3.
[27] Klooster ST, Schroën K, Berton-Carabin C. Lipid oxidation products in model food emulsions: do they stay in or leave droplets, that’s the question. Food Chem 2023; 405:134992. https://doi.org/10.1016/j.foodchem.2022.134992.
[28] McClements D, Dungan S, German J, Kinsella J. Oil exchange between oil-inwater emulsion droplets stabilised with a non-ionic surfactant. Food Hydrocoll 1992;6(5):415-22. https://doi.org/10.1016/S0268-005X(09)80027-1.
[29] Schaich KM. Lipid oxidation: theoretical aspects. In: Bailey’s Industrial Oil and Fat Products; 2005. https://doi.org/10.1002/047167849X.bio067.
[30] Frankel EN. Chapter 1 – free radical oxidation. In: Frankel EN, editor. Lipid oxidation. 2nd ed. Woodhead Publishing; 2012. p. 15-24. https://doi.org/ 10.1533/9780857097927.15.
[31] Frankel EN. Lipid Oxidation. Elsevier; 2014.
[32] Kerr JA. Bond dissociation energies by kinetic methods. Chem Rev 1966;66(5): 465-500. https://doi.org/10.1021/cr60243a001.
[33] Frankel EN. Volatile lipid oxidation products. Prog Lipid Res 1983;22(1):1-33. https://doi.org/10.1016/0163-7827(83)90002-4.
[34] Schaich KM. Metals and lipid oxidation. Contemporary issues. Lipids. 1992;27(3): 209-18. https://doi.org/10.1007/BF02536181.
[35] Minotti G, Aust SD. Redox cycling of iron and lipid peroxidation. Lipids. 1992;27 (3):219-26. https://doi.org/10.1007/BF02536182.
[36] Mozuraityte R, Kristinova V, Rustad T, Storrø I. The role of iron in peroxidation of PUFA: effect of pH and chelators. Eur J Lipid Sci Technol 2016;118(4):658-68. https://doi.org/10.1002/ejlt.201400590.
[37] Mozuraityte R, Rustad T, Storrø I. The role of iron in peroxidation of polyunsaturated fatty acids in liposomes. J Agric Food Chem 2008;56(2):537-43. https://doi.org/10.1021/jf0716073.
[38] Ingold KU. Inhibition of the autoxidation of organic substances in the liquid phase. Chem Rev 1961;61(6):563-89. https://doi.org/10.1021/cr60214a002.
[39] Jacobsen C, Timm M, Meyer AS. Oxidation in fish oil enriched mayonnaise: ascorbic acid and low pH increase oxidative deterioration. J Agric Food Chem 2001;49(8):3947-56. https://doi.org/10.1021/jf001253e.
[40] Jacobsen C, Let MB, Nielsen NS, Meyer AS. Antioxidant strategies for preventing oxidative flavour deterioration of foods enriched with n-3 polyunsaturated lipids: a comparative evaluation. Trends Food Sci Technol 2008;19(2):76-93. https:// doi.org/10.1016/j.tifs.2007.08.001.
[41] Shahidi F, Ambigaipalan P. Phenolics and polyphenolics in foods, beverages and spices: antioxidant activity and health effects-A review. J Funct Foods 2015;18: 820-97. https://doi.org/10.1016/j.jff.2015.06.018.
[42] Quideau S, Deffieux D, Douat-Casassus C, Pouységu L. Plant polyphenols: chemical properties, biological activities, and synthesis. Angew Chem Int Ed 2011;50(3):586-621. https://doi.org/10.1002/anie.201000044.
[43] Jacobsen C, Hartvigsen K, Thomsen MK, Hansen LF, Lund P, Skibsted LH, et al. Lipid oxidation in fish oil enriched mayonnaise: calcium disodium ethylenediaminetetraacetate, but not gallic acid, strongly inhibited oxidative deterioration. J Agric Food Chem 2001;49(2):1009-19. https://doi.org/10.1021/ jf000729r.
[44] Gumus CE, Decker EA, McClements DJ. Impact of legume protein type and location on lipid oxidation in fish oil-in-water emulsions: lentil, pea, and faba bean proteins. Food Res Int 2017;100(Pt 2):175-85. https://doi.org/10.1016/j. foodres.2017.08.029.
[45] Berton C, M.H.L.N. Ropers, Guibert D, Solé VR, Genot C. Modifications of interfacial proteins in oil-in-water emulsions prior to and during lipid oxidation. J Agric Food Chem 2012;60(35):8659-71. https://doi.org/10.1021/jf300490w.
[46] Schaich KM. Lipid oxidation: new perspectives on an old reaction. In: Bailey’s Industrial Oil and Fat Products; 2020. p. 1-72. https://doi.org/10.1002/ 047167849X.bio067.pub2.
[47] Mubiru E, Shrestha K, Papastergiadis A, De Meulenaer B. Improved gas chromatography-flame ionization detector analytical method for the analysis of epoxy fatty acids. J Chromatogr A 2013;1318:217-25. https://doi.org/10.1016/j. chroma.2013.10.025.
[48] Marmesat S, Velasco J, Dobarganes MC. Quantitative determination of epoxy acids, keto acids and hydroxy acids formed in fats and oils at frying temperatures. J Chromatogr A 2008;1211(1):129-34. https://doi.org/10.1016/j. chroma.2008.09.077.
[49] Velasco J, Berdeaux O, Márquez-Ruiz G, Dobarganes MC. Sensitive and accurate quantitation of monoepoxy fatty acids in thermoxidized oils by gas-liquid chromatography. J Chromatogr A 2002;982(1):145-52. https://doi.org/ 10.1016/S0021-9673(02)01481-4.
[50] Velasco J, Marmesat S, Bordeaux O, Márquez-Ruiz G, Dobarganes C. Formation and evolution of Monoepoxy fatty acids in Thermoxidized olive and sunflower oils and quantitation in used frying oils from restaurants and fried-food outlets. J Agric Food Chem 2004;52(14):4438-43. https://doi.org/10.1021/jf030753f.
[51] Mubiru E, Shrestha K, Papastergiadis A, De Meulenaer B. Development and validation of a gas chromatography-flame ionization detection method for the determination of epoxy fatty acids in food matrices. J Agric Food Chem 2014;62 (13):2982-8. https://doi.org/10.1021/jf405664c.
[52] Grüneis V, Fruehwirth S, Zehl M, Ortner J, Schamann A, J.r. König, and M. Pignitter.. Simultaneous analysis of epoxidized and hydroperoxidized triacylglycerols in canola oil and margarine by LC-MS. J Agric Food Chem 2019; 67(36):10174-84. https://doi.org/10.1021/acs.jafc.9b03601.
[53] Fruehwirth S, Egger S, Flecker T, Ressler M, Firat N, Pignitter M. Acetone as indicator of lipid oxidation in stored margarine. Antioxidants. 2021;10(1):59. https://doi.org/10.3390/antiox10010059.
[54] Boerkamp VJ, Merkx DW, Wang J, Vincken J-P, Hennebelle M, van Duynhoven JP. Quantitative assessment of epoxide formation in oil and mayonnaise by 1H-13C HSQC NMR spectroscopy. Food Chem 2022;390:133145. https://doi.org/10.1016/j.foodchem.2022.133145.
[55] Steenhorst-Slikkerveer L, Louter A, Janssen H-G, Bauer-Plank C. Analysis of nonvolatile lipid oxidation products in vegetable oils by normal-phase highperformance liquid chromatography with mass spectrometric detection. J Am Oil Chem Soc 2000;77(8):837. https://doi.org/10.1007/s11746-000-0134-1.
[56] Morales A, Marmesat S, Ruiz-Méndez MV, Márquez-Ruiz G, Velasco J. Formation of oxidation products in edible vegetable oils analyzed as FAME derivatives by HPLC-UV-ELSD. Food Res Int 2014;62:1080-6. https://doi.org/10.1016/j. foodres.2014.05.063.
[57] Morales A, Dobarganes C, Márquez-Ruiz G, Velasco J. Quantitation of Hydroperoxy-, keto- and Hydroxy-dienes during oxidation of FAMEs from highlinoleic and high-oleic sunflower oils. J Am Oil Chem Soc 2010;87(11):1271-9. https://doi.org/10.1007/s11746-010-1624-8.
[58] Morales A, Marmesat S, Dobarganes MC, Márquez-Ruiz G, Velasco J. Quantitative analysis of hydroperoxy-, keto- and hydroxy-dienes in refined vegetable oils. J Chromatogr A 2012;1229:190-7. https://doi.org/10.1016/j. chroma.2012.01.039.
[59] Morales A, Marmesat S, Dobarganes MC, Márquez-Ruiz G, Velasco J. Formation of Hydroperoxy-, keto- and Hydroxy-dienes in FAME from oils: influence of temperature and addition of
[60] Emami S, Zhang Z, Taha AY. Quantitation of Oxylipins in fish and algae oil supplements using optimized hydrolysis procedures and ultra-high performance liquid chromatography coupled to tandem mass-spectrometry. J Agric Food Chem 2020;68(35):9329-44. https://doi.org/10.1021/acs.jafc.0c02461.
[61] Richardson CE, Hennebelle M, Otoki Y, Zamora D, Yang J, Hammock BD, et al. Lipidomic analysis of oxidized fatty acids in plant and algae oils. J Agric Food Chem 2017;65(9):1941-51. https://doi.org/10.1021/acs.jafc.6b05559.
[62] Zhou L, Zhao M, Bindler F, Marchioni E. Identification of oxidation compounds of 1-Stearoyl-2-linoleoyl-sn-glycero-3-phosphoethanolamine during thermal oxidation. J Agric Food Chem 2015;63(43):9615-20. https://doi.org/10.1021/ acs.jafc.5b03753.
[63] Le Grandois J, Marchioni E, Ennahar S, Giuffrida F, Bindler F. Identification and kinetics of oxidized compounds from phosphatidylcholine molecular species. Food Chem 2010;119(3):1233-8. https://doi.org/10.1016/j. foodchem.2009.08.042.
[64] Parchem K, Kusznierewicz B, Chmiel T, Maciołek P, Bartoszek A. Profiling and qualitative assessment of enzymatically and thermally oxidized egg yolk phospholipids using a two-step high-performance liquid chromatography protocol. J Am Oil Chem Soc 2019;96(6):693-706. https://doi.org/10.1002/ aocs. 12218.
[65] Kato S, Iseki T, Hanzawa Y, Otoki Y, Ito J, Kimura F, et al. Evaluation of the mechanisms of mayonnaise phospholipid oxidation. J Oleo Sci 2017;66(4): 369-74. https://doi.org/10.5650/jos.ess16187.
[66] Laguerre M, Tenon M, Bily A, Birtić S. Toward a spatiotemporal model of oxidation in lipid dispersions: A hypothesis-driven review. Eur J Lipid Sci Technol 2020;122(3):1900209. https://doi.org/10.1002/ejlt.201900209.
[67] Ghelichi S, Hajfathalian M, Yesiltas B, Sørensen ADM, García-Moreno PJ, Jacobsen C. Oxidation and oxidative stability in emulsions. Comprehen Rev Food Sci Food Safet 2023;22(3):1864-901. https://doi.org/10.1111/15414337.13134.
[68] Mei L, McClements DJ, Wu J, Decker EA. Iron-catalyzed lipid oxidation in emulsion as affected by surfactant, pH and NaCl . Food Chem 1998;61(3):307-12. https://doi.org/10.1016/S0308-8146(97)00058-7.
[69] Waraho T, McClements DJ, Decker EA. Mechanisms of lipid oxidation in food dispersions. Trends Food Sci Technol 2011;22(1):3-13. https://doi.org/10.1016/ j.tifs.2010.11.003.
[70] Rampon V, Lethuaut L, Mouhous-Riou N, Genot C. Interface characterization and aging of bovine serum albumin stabilized oil-in-water emulsions as revealed by front-surface fluorescence. J Agric Food Chem 2001;49(8):4046-51. https://doi. org/10.1021/jf001170y.
[71] Chen J, Li X, Cao C, Kong B, Wang H, Zhang H, et al. Effects of different pH conditions on interfacial composition and protein-lipid co-oxidation of whey protein isolate-stabilised O/W emulsions. Food Hydrocoll 2022;131:107752. https://doi.org/10.1016/j.foodhyd.2022.107752.
[72] Raudsepp P, Bruggemann DA, Andersen ML. Detection of radicals in single droplets of oil-in-water emulsions with the lipophilic fluorescent probe BODIPY (665/676) and confocal laser scanning microscopy. Free Radic Biol Med 2014;70: 233-40. https://doi.org/10.1016/j.freeradbiomed.2014.02.026.
[73] Yang S, Takeuchi M, Friedrich H, van Duynhoven JP, Hohlbein J. Unravelling mechanisms of protein and lipid oxidation in mayonnaise at multiple length scales. Food Chem 2023;402:134417. https://doi.org/10.1016/j. foodchem.2022.134417.
[74] Yang S, Verhoeff AA, Merkx DWH, van Duynhoven JPM, Hohlbein J. Quantitative spatiotemporal mapping of lipid and protein oxidation in mayonnaise. Antioxidants (Basel) 2020;9(12). https://doi.org/10.3390/antiox9121278.
[75] Berton-Carabin C, Genot C, Gaillard C, Guibert D, Ropers M. Design of interfacial films to control lipid oxidation in oil-in-water emulsions. Food Hydrocoll 2013;33 (1):99-105. https://doi.org/10.1016/j.foodhyd.2013.02.021.
[76] Berton C, Genot C, Guibert D, Ropers M-H. Effect of lateral heterogeneity in mixed surfactant-stabilized interfaces on the oxidation of unsaturated lipids in oil-in-water emulsions. J Colloid Interface Sci 2012;377(1):244-50. https://doi. org/10.1016/j.jcis.2012.03.084.
[77] Hohlbein J. Single-molecule localization microscopy as an emerging tool to probe multiscale food structures. Food Struct 2021;30:100236. https://doi.org/ 10.1016/j.foostr.2021.100236.
[78] Jabermoradi A, Yang S, Gobes MI, Van Duynhoven JPM, Hohlbein J. Enabling single-molecule localization microscopy in turbid food emulsions. Philos Trans R Soc A Math Phys Eng Sci 2022;380(2220). https://doi.org/10.1098/ rsta.2020.0164.
[79] Villeneuve P, Bourlieu-Lacanal C, Durand E, Lecomte J, McClements DJ, Decker EA. Lipid oxidation in emulsions and bulk oils: a review of the importance of micelles. Crit Rev Food Sci Nutr 2023;63(20):4687-727. https://doi.org/ 10.1080/10408398.2021.2006138.
[80] Budilarto ES, Kamal-Eldin A. The supramolecular chemistry of lipid oxidation and antioxidation in bulk oils. Eur J Lipid Sci Technol 2015;117(8):1095-137. https://doi.org/10.1002/ejlt.201400200.
[81] Chaiyasit W, Elias RJ, McClements DJ, Decker EA. Role of physical structures in bulk oils on lipid oxidation. Crit Rev Food Sci Nutr 2007;47(3):299-317. https:// doi.org/10.1080/10408390600754248.
[82] Chen B, Han A, McClements DJ, Decker EA. Physical structures in soybean oil and their impact on lipid oxidation. J Agric Food Chem 2010;58(22):11993-9. https://doi.org/10.1021/jf102763p.
[83] Cui L, McClements DJ, Decker EA. Impact of phosphatidylethanolamine on the antioxidant activity of alpha-tocopherol and trolox in bulk oil. J Agric Food Chem 2015;63(12):3288-94. https://doi.org/10.1021/acs.jafc.5b00243.
[84] Koga T, Nagao A, Terao J, Sawada K, Mukai K. Synthesis of a phosphatidyl derivative of vitamin E and its antioxidant activity in phospholipid bilayers. Lipids. 1994;29(2):83-9. https://doi.org/10.1007/BF02537147.
[85] Koga T, Terao J. Phospholipids increase radical-scavenging activity of vitamin E in a bulk oil model system. J Agric Food Chem 1995;43(6):1450-4. https://doi. org/10.1021/jf00054a007.
[86] Yoo K, Kim S, Kim M-J, Oh W, Lee J. Effects of association colloidal structures on the oxygen solubility in oil-in-water emulsion matrix. Food Sci Biotechnol 2023: 1-9. https://doi.org/10.1007/s10068-023-01338-6.
[87] Kittipongpittaya K, Panya A, McClements DJ, Decker EA. Impact of free fatty acids and phospholipids on reverse micelles formation and lipid oxidation in bulk oil. J Am Oil Chem Soc 2014;91:453-62. https://doi.org/10.1007/s11746-013-2388-8.
[88] Farhoosh R. Reliable determination of the induction period and critical reverse micelle concentration of lipid hydroperoxides exploiting a model composed of pseudo-first and -second order reaction kinetics. LWT. 2018;98:406-10. https:// doi.org/10.1016/j.lwt.2018.09.003.
[89] Merkx DW, Swager A, van Velzen EJ, van Duynhoven JP, Hennebelle M. Quantitative and predictive modelling of lipid oxidation in mayonnaise. Antioxidants. 2021;10(2):287. https://doi.org/10.3390/antiox10020287.
[90] Laguerre M, Bily A, Roller M, Birtić S. Mass transport phenomena in lipid oxidation and antioxidation. Annu Rev Food Sci Technol 2017;8:391-411. https://doi.org/10.1146/annurev-food-030216-025812.
[91] Raudsepp P, Brüggemann DA, Andersen ML. Evidence for Transfer of Radicals between Oil-in-Water Emulsion Droplets as Detected by the Probe (E,E)-3,5-Bis(4-phenyl-1,3-butadienyl)-4,4-difluoro-4-bora-3a,4a-diaza-s-indacene, BODIPY665/ 676. J Agric Food Chem 2014;62(51):12428-35. https://doi.org/10.1021/ jf504404a.
[92] Raudsepp P, Brüggemann DA, Knudsen JC, Andersen ML. Localized lipid autoxidation initiated by two-photon irradiation within single oil droplets in oil-in-water emulsions. Food Chem 2016;199:760-7. https://doi.org/10.1016/j. foodchem.2015.12.070.
[93] Banerjee C, Breitenbach T, Ogilby PR. Spatially resolved experiments to monitor the singlet oxygen initiated oxidation of lipid droplets in emulsions. ChemPhotoChem. 2018;2(7):586-95. https://doi.org/10.1002/cptc.201800005.
[94] Banerjee C, Westberg M, Breitenbach T, Bregnhøj M, Ogilby PR. Monitoring interfacial lipid oxidation in oil-in-water emulsions using spatially resolved optical techniques. Anal Chem 2017;89(11):6239-47. https://doi.org/10.1021/ acs.analchem.7b01228.
[95] Yesiltas B, García-Moreno PJ, Sørensen A-DM, Banerjee C, Anankanbil S, Guo Z, et al. The use of soy and egg phosphatidylcholines modified with Caffeic acid enhances the oxidative stability of high-fat (70%) fish oil-in-water emulsions. Coll Interf 2023;7(3):60. https://doi.org/10.3390/colloids7030060.
[96] Pryor WA. Oxy-radicals and related species: their formation, lifetimes, and reactions. Annu Rev Physiol 1986;48:657-67. https://doi.org/10.1146/annurev. ph.48.030186.003301.
[97] Termini J. Peroxyl and alkoxyl radical mediated DNA damage. In: Cutler RG, Rodriguez H, editors. Critical Reviews of Oxidative Stress and Aging. Advances in Basic Science, Diagnostics and Intervention. World Scientific Publishing Co. Pte. Ltd.; 2003. p. 39-53. https://doi.org/10.1142/9789812775733_0003.
[98] Coupland JN, Zhu Z, Wan H, McClements DJ, Nawar WW, Chinachoti P. Droplet composition affects the rate of oxidation of emulsified ethyl linoleate. J Am Oil Chem Soc 1996;73(6):795-901. https://doi.org/10.1007/BF02517957.
[99] Malassagne-Bulgarelli N, McGrath KM. Emulsion ageing: effect on the dynamics of oil exchange in oil-in-water emulsions. Soft Matter 2013;9(1):48-59. https:// doi.org/10.1039/C2SM26229K.
[100] Li P, McClements DJ, Decker EA. Application of flow cytometry as novel technology in studying lipid oxidation and mass transport phenomena in oil-inwater emulsions. Food Chem 2020;315:126225. https://doi.org/10.1016/j. foodchem.2020.126225.
[101] Nuchi CD, Hernandez P, McClements DJ, Decker EA. Ability of lipid hydroperoxides to partition into surfactant micelles and alter lipid oxidation rates in emulsions. J Agric Food Chem 2002;50(19):5445-9. https://doi.org/10.1021/ jf020095j.
[102] Verleyen T, Kamal-Eldin A, Dobarganes C, Verhé R, Dewettinck K, Huyghebaert A. Modeling of
[103] Barretto ACS, Ida EI, Silva RSF, Torres EAFS, Shimokomaki M. Empirical models for describing poultry meat lipid oxidation inhibition by natural antioxidants. J Food Compos Anal 2003;16(5):587-94. https://doi.org/10.1016/S0889-1575 (03)00023-1.
[104] Høy M, Mozuraityte R, Segtnan V, Storrø I, Bjørn Helge M, Rustad T, et al. The use of experimental design methodology for investigating a lipid oxidation rate assay. Chemom Intel Lab Syst 2008;91(2):164-72. https://doi.org/10.1016/j. chemolab.2007.11.001.
[105] Kerrihard AL, Nagy K, Craft BD, Beggio M, Pegg RB. Oxidative stability of commodity fats and oils: modeling based on fatty acid composition. J Am Oil Chem Soc 2015;92(8):1153-63. https://doi.org/10.1007/s11746-015-2686-4.
[106] Pinchuk I, Lichtenberg D. Analysis of the kinetics of lipid peroxidation in terms of characteristic time-points. Chem Phys Lipids 2014;178:63-76. https://doi.org/ 10.1016/j.chemphyslip.2013.12.001.
[107] Murado MA, Vázquez JA. Mathematical model for the characterization and objective comparison of antioxidant activities. J Agric Food Chem 2010;58(3): 1622-9. https://doi.org/10.1021/jf903709z.
[108] Farhoosh R. A reconsidered approach providing kinetic parameters and rate constants to analyze the oxidative stability of bulk lipid systems. Food Chem 2020;327:127088. https://doi.org/10.1016/j.foodchem.2020.127088.
[109] Farhoosh R. New insights into the kinetic and thermodynamic evaluations of lipid peroxidation. Food Chem 2022;375:131659. https://doi.org/10.1016/j. foodchem.2021.131659.
[110] Li X, Wu G, Huang J, Zhang H, Jin Q, Wang X. Kinetic models to understand the coexistence of formation and decomposition of hydroperoxide during lipid oxidation. Food Res Int 2020;136:109314. https://doi.org/10.1016/j. foodres.2020.109314.
[111] Aragao GMF, Corradini MG, Peleg M. A phenomenological model of the peroxide Value’s rise and fall during lipid oxidation. J Am Oil Chem Soc 2008;85(12):1143. https://doi.org/10.1007/s11746-008-1305-z.
[112] Ozilgen S, Ozilgen M. Kinetic model of lipid oxidation in foods. J Food Sci 1990; 55(2):498-501. https://doi.org/10.1111/j.1365-2621.1990.tb06795.x.
[113] McPherson PAC, Bole A, Cruz KA, Young IS, McEneny J. A curvilinear approach to the kinetic analysis of linoleate peroxidation in aqueous liposomes by 2,2’azobis (2-amidoinopropane) dihydrochloride. Chem Phys Lipids 2012;165(6):682-8. https://doi.org/10.1016/j.chemphyslip.2012.07.004.
[114] Merkx DW, Hong GS, Ermacora A, Van Duynhoven JP. Rapid quantitative profiling of lipid oxidation products in a food emulsion by 1H NMR. Anal Chem 2018;90(7):4863-70. https://doi.org/10.1021/acs.analchem.8b00380.
[115] Nguyen KA, Hennebelle M, van Duynhoven JPM, Dubbelboer A, Boerkamp VJP, Wierenga PA. Mechanistic kinetic modelling of lipid oxidation in vegetable oils to estimate shelf-life. Food Chem 2024;433:137266. https://doi.org/10.1016/j. foodchem.2023.137266.
[116] Schroën K, Berton-Carabin CC. A unifying approach to lipid oxidation in emulsions: modelling and experimental validation. Food Res Int 2022;160: 111621. https://doi.org/10.1016/j.foodres.2022.111621.
[117] Tan CP, Che Man YB, Selamat J, Yusoff MSA. Application of arrhenius kinetics to evaluate oxidative stability in vegetable oils by isothermal differential scanning calorimetry. J Am Oil Chem Soc 2001;78(11):1133-8. https://doi.org/10.1007/ s11746-001-0401-1.
[118] Farhoosh R. Critical kinetic parameters and rate constants representing lipid peroxidation as affected by temperature. Food Chem 2021;340:128137. https:// doi.org/10.1016/j.foodchem.2020.128137.
[119] Sullivan JC, Budge SM, St-Onge M. Modeling the primary oxidation in commercial fish oil preparations. Lipids. 2011;46(1):87-93. https://doi.org/
[120] Shim SD, Lee SJ. Shelf-life prediction of perilla oil by considering the induction period of lipid oxidation. Eur J Lipid Sci Technol 2011;113(7):904-9. https://doi. org/10.1002/ejlt.201000325.
[121] Bravo-Diaz C. Advances in the control of lipid peroxidation in oil-in-water emulsions: kinetic approaches(dagger). Crit Rev Food Sci Nutr 2022:1-33. https://doi.org/10.1080/10408398.2022.2029827.
[122] Romsted LS, Bravo-Díaz C. Modeling chemical reactivity in emulsions. Curr Opin Colloid Interface Sci 2013;18(1):3-14. https://doi.org/10.1016/j. cocis.2012.11.001.
[123] Genot C. Distributions of phenolic acid antioxidants between the interfacial and aqueous regions of corn oil emulsions-a commentary. Eur J Lipid Sci Technol 2015;117(11):1684-6. https://doi.org/10.1002/ejlt.201500210.
[124] Nazir A, Schroën K, Boom R. Premix emulsification: A review. J Membr Sci 2010; 362(1):1-11. https://doi.org/10.1016/j.memsci.2010.06.044.
[125] Costa ALR, Gomes A, Andrade CCPD, Cunha RL. Emulsifier functionality and process engineering: Progress and challenges. Food Hydrocoll 2017;68:69-80. https://doi.org/10.1016/j.foodhyd.2016.10.012.
[126] Neves MA, Wang Z, Kobayashi I, Nakajima M. Assessment of oxidative stability in fish oil-in-water emulsions: effect of emulsification process, droplet size and storage temperature. J Food Process Eng 2017;40(1):e12316. https://doi.org/ 10.1111/jfpe.12316.
[127] Walker R, Decker EA, McClements DJ. Development of food-grade nanoemulsions and emulsions for delivery of omega-3 fatty acids: opportunities and obstacles in the food industry. Food Funct 2015;6(1):42-55. https://doi.org/10.1039/ c4fo00723a.
[128] Horn AF, Barouh N, Nielsen NS, Baron CP, Jacobsen C. Homogenization pressure and temperature affect protein partitioning and oxidative stability of emulsions. J Am Oil Chem Soc 2013;90(10):1541-50. https://doi.org/10.1007/s11746-013-2292-2.
[129] Let MB, Jacobsen C, Sorensen AD, Meyer AS. Homogenization conditions affect the oxidative stability of fish oil enriched milk emulsions: lipid oxidation. J Agric Food Chem 2007;55(5):1773-80. https://doi.org/10.1021/jf062391s.
[130] Sorensen AD, Baron CP, Let MB, Bruggemann DA, Pedersen LR, Jacobsen C. Homogenization conditions affect the oxidative stability of fish oil enriched milk emulsions: oxidation linked to changes in protein composition at the oil-water interface. J Agric Food Chem 2007;55(5):1781-9. https://doi.org/10.1021/ jf0623900.
[131] Hu M, McClements DJ, Decker EA. Lipid oxidation in corn oil-in-water emulsions stabilized by casein, whey protein isolate, and soy protein isolate. J Agric Food Chem 2003;51(6):1696-700. https://doi.org/10.1021/jf020952j.
[132] Shao Y, Tang C-H. Characteristics and oxidative stability of soy protein-stabilized oil-in-water emulsions: influence of ionic strength and heat pretreatment. Food Hydrocoll 2014;37:149-58. https://doi.org/10.1016/j.foodhyd.2013.10.030.
[133] Kellerby SS, Gu YS, McClements DJ, Decker EA. Lipid oxidation in a menhaden oil-in-water emulsion stabilized by sodium Caseinate cross-linked with transglutaminase. J Agric Food Chem 2006;54(26):10222-7. https://doi.org/ 10.1021/jf062143w.
[134] Lesmes U, Sandra S, Decker EA, McClements DJ. Impact of surface deposition of lactoferrin on physical and chemical stability of omega-3 rich lipid droplets stabilised by caseinate. Food Chem 2010;123(1):99-106. https://doi.org/ 10.1016/j.foodchem.2010.04.007.
[135] Chen B, Li H, Ding Y, Rao J. Improvement of physicochemical stabilities of emulsions containing oil droplets coated by non-globular protein-beet pectin complex membranes. Food Res Int 2011;44(5):1468-75. https://doi.org/ 10.1016/j.foodres.2011.03.024.
[136] Gudipati V, Sandra S, McClements DJ, Decker EA. Oxidative stability and in vitro digestibility of fish oil-in-water emulsions containing multilayered membranes. J Agric Food Chem 2010;58(13):8093-9. https://doi.org/10.1021/jf101348c.
[137] McClements DJ, Decker E. Interfacial antioxidants: A review of natural and synthetic emulsifiers and Coemulsifiers that can inhibit lipid oxidation. J Agric Food Chem 2018;66(1):20-35. https://doi.org/10.1021/acs.jafc.7b05066.
[138] Dickinson E. Mixed biopolymers at interfaces: competitive adsorption and multilayer structures. Food Hydrocoll 2011;25(8):1966-83. https://doi.org/ 10.1016/j.foodhyd.2010.12.001.
[139] García-Moreno PJ, Horn AF, Jacobsen C. Influence of casein-phospholipid combinations as emulsifier on the physical and oxidative stability of fish oil-inwater emulsions. J Agric Food Chem 2014;62(5):1142-52. https://doi.org/ 10.1021/jf405073x.
[140] Yesiltas B, Soria Caindec AM, García-Moreno PJ, Echers SG, Olsen TH, Jones NC, et al. Physical and oxidative stability of fish oil-in-water emulsions stabilized with emulsifier peptides derived from seaweed, methanotrophic bacteria and potato proteins. Colloids Surf A Physicochem Eng Asp 2023;663:131069. https://doi. org/10.1016/j.colsurfa.2023.131069.
[141] Yesiltas B, Torkkeli M, Almásy L, Dudás Z, García-Moreno PJ, Sørensen A-DM, et al. Small-angle neutron scattering study of high fat fish oil-in-water emulsion stabilized with sodium Caseinate and phosphatidylcholine. Langmuir. 2020;36 (9):2300-6. https://doi.org/10.1021/acs.langmuir.9b03269.
[142] Ozturk B, McClements DJ. Progress in natural emulsifiers for utilization in food emulsions. Curr Opin Food Sci 2016;7:1-6. https://doi.org/10.1016/j. cofs.2015.07.008.
[143] Sharif HR, Williams PA, Sharif MK, Abbas S, Majeed H, Masamba KG, et al. Current progress in the utilization of native and modified legume proteins as emulsifiers and encapsulants-A review. Food Hydrocoll 2018;76:2-16. https:// doi.org/10.1016/j.foodhyd.2017.01.002.
[144] Burger TG, Zhang Y. Recent progress in the utilization of pea protein as an emulsifier for food applications. Trends Food Sci Technol 2019;86:25-33. https:// doi.org/10.1016/j.tifs.2019.02.007.
[145] Lam RSH, Nickerson MT. Food proteins: A review on their emulsifying properties using a structure-function approach. Food Chem 2013;141(2):975-84. https:// doi.org/10.1016/j.foodchem.2013.04.038.
[146] Schutyser M, Van der Goot A. The potential of dry fractionation processes for sustainable plant protein production. Trends Food Sci Technol 2011;22(4): 154-64. https://doi.org/10.1016/j.tifs.2010.11.006.
[147] Faraji H, McClements DJ, Decker EA. Role of continuous phase protein on the oxidative stability of fish oil-in-water emulsions. J Agric Food Chem 2004;52(14): 4558-64. https://doi.org/10.1021/jf035346i.
[148] Feng J, Schroën K, Fogliano V, Berton-Carabin C. Antioxidant potential of nonmodified and glycated soy proteins in the continuous phase of oil-in-water emulsions. Food Hydrocoll 2021;114:106564. https://doi.org/10.1016/j. foodhyd.2020.106564.
[149] Hinderink EBA, Schröder A, Sagis L, Schroën K, Berton-Carabin CC. Physical and oxidative stability of food emulsions prepared with pea protein fractions. LWT. 2021;146:111424. https://doi.org/10.1016/j.lwt.2021.111424.
[150] Liu C, Bhattarai M, Mikkonen KS, Heinonen M. Effects of enzymatic hydrolysis of fava bean protein isolate by Alcalase on the physical and oxidative stability of oil-in-water emulsions. J Agric Food Chem 2019;67(23):6625-32. https://doi.org/ 10.1021/acs.jafc.9b00914.
[151] Keuleyan E, Gélébart P, Beaumal V, Kermarrec A, Ribourg-Birault L, Le Gall S, et al. Pea and lupin protein ingredients: new insights into endogenous lipids and the key effect of high-pressure homogenization on their aqueous suspensions. Food Hydrocoll 2023;141:108671. https://doi.org/10.1016/j. foodhyd.2023.108671.
[152] Pei Y, Deng Q, McClements DJ, Li J, Li B. Impact of Phytic acid on the physical and oxidative stability of protein-stabilized oil-in-water emulsions. Food Biophys 2020;15(4):433-41. https://doi.org/10.1007/s11483-020-09641-z.
[153] Dickinson E. Advances in food emulsions and foams: reflections on research in the neo-Pickering era. Curr Opin Food Sci 2020;33:52-60. https://doi.org/10.1016/j. cofs.2019.12.009.
[154] Calabrese V, Courtenay JC, Edler KJ, Scott JL. Pickering emulsions stabilized by naturally derived or biodegradable particles. Curr Opin Green Sustain Chem 2018;12:83-90. https://doi.org/10.1016/j.cogsc.2018.07.002.
[155] Dickinson E. Biopolymer-based particles as stabilizing agents for emulsions and foams. Food Hydrocoll 2017;68:219-31. https://doi.org/10.1016/j. foodhyd.2016.06.024.
[156] Linke C, Drusch S. Pickering emulsions in foods – opportunities and limitations. Crit Rev Food Sci Nutr 2018;58(12):1971-85. https://doi.org/10.1080/ 10408398.2017 .1290578.
[157] Rayner M. Current status on novel ways for stabilizing food dispersions by oleosins, particles and microgels. Curr Opin Food Sci 2015;3:94-109. https://doi. org/10.1016/j.cofs.2015.05.006.
[158] Tavernier I, Wijaya W, Van der Meeren P, Dewettinck K, Patel AR. Food-grade particles for emulsion stabilization. Trends Food Sci Technol 2016;50:159-74. https://doi.org/10.1016/j.tifs.2016.01.023.
[159] Berton-Carabin C, Schröder A, Schroën K, Laguerre M. Chapter 14 – Lipid oxidation in Pickering emulsions. In: García-Moreno PJ, editor. Omega-3 Delivery Systems. Academic Press; 2021. p. 275-93. https://doi.org/10.1016/B978-0-12-821391-9.00011-9.
[160] Kargar M, Fayazmanesh K, Alavi M, Spyropoulos F, Norton IT. Investigation into the potential ability of Pickering emulsions (food-grade particles) to enhance the oxidative stability of oil-in-water emulsions. J Colloid Interface Sci 2012;366(1): 209-15. https://doi.org/10.1016/j.jcis.2011.09.073.
[161] Kargar M, Spyropoulos F, Norton IT. The effect of interfacial microstructure on the lipid oxidation stability of oil-in-water emulsions. J Colloid Interface Sci 2011; 357(2):527-33. https://doi.org/10.1016/j.jcis.2011.02.019.
[162] Xiao J, Li C, Huang Q. Kafirin nanoparticle-stabilized Pickering emulsions as Oral delivery vehicles: physicochemical stability and in vitro digestion profile. J Agric Food Chem 2015;63(47):10263-70. https://doi.org/10.1021/acs.jafc.5b04385.
[163] Huang X-N, Zhou F-Z, Yang T, Yin S-W, Tang C-H, Yang X-Q. Fabrication and characterization of Pickering high internal phase emulsions (HIPEs) stabilized by chitosan-caseinophosphopeptides nanocomplexes as oral delivery vehicles. Food Hydrocoll 2019;93:34-45. https://doi.org/10.1016/j.foodhyd.2019.02.005.
[164] Schröder A, Sprakel J, Boerkamp W, Schroën K, Berton-Carabin CC. Can we prevent lipid oxidation in emulsions by using fat-based Pickering particles? Food Res Int 2019;120:352-63. https://doi.org/10.1016/j.foodres.2019.03.004.
[165] Okubanjo SS, Loveday SM, Ye A, Wilde PJ, Singh H. Droplet-stabilized oil-inwater emulsions protect unsaturated lipids from oxidation. J Agric Food Chem 2019;67(9):2626-36. https://doi.org/10.1021/acs.jafc.8b02871.
[166] Timgren A, Rayner M, Dejmek P, Marku D, Sjoo M. Emulsion stabilizing capacity of intact starch granules modified by heat treatment or octenyl succinic anhydride. Food Sci Nutr 2013;1(2):157-71. https://doi.org/10.1002/fsn3.17.
[167] Barden L, Barouh N, Villeneuve P, Decker E. Impact of hydrophobicity on antioxidant efficacy in low-moisture food. J Agric Food Chem 2015;63(24): 5821-7. https://doi.org/10.1021/acs.jafc.5b01085.
[168] Barden L, Decker EA. Lipid oxidation in low-moisture food: A review. Crit Rev Food Sci Nutr 2016;56(15):2467-82. https://doi.org/10.1080/ 10408398.2013.848833.
[169] Ferreira da Silveira TF, Laguerre M, Bourlieu-Lacanal C, Lecomte J, Durand E, Figueroa-Espinoza MC, et al. Impact of surfactant concentration and antioxidant mode of incorporation on the oxidative stability of oil-in-water nanoemulsions. LWT. 2021;141:110892. https://doi.org/10.1016/j.lwt.2021.110892.
[170] Inchingolo R, Bayram I, Uluata S, Kiralan SS, Rodriguez-Estrada MT, McClements DJ, et al. Ability of sodium dodecyl sulfate (SDS) micelles to increase the antioxidant activity of alpha-tocopherol. J Agric Food Chem 2021;69(20): 5702-8. https://doi.org/10.1021/acs.jafc.1c01199.
[171] McClements DJ, Dungan SR. Factors that affect the rate of oil exchange between oil-in-water emulsion droplets stabilized by a nonionic surfactant: droplet size, surfactant concentration, and ionic strength. J Phys Chem 1993;97(28):7304-8. https://doi.org/10.1021/j100130a030.
[172] Raudsepp P, Brüggemann DA, Lenferink A, Otto C, Andersen ML. Oxidative stabilization of mixed mayonnaises made with linseed oil and saturated mediumchain triglyceride oil. Food Chem 2014;152:378-85. https://doi.org/10.1016/j. foodchem.2013.11.141.
[173] Cho YJ, McClements DJ, Decker EA. Ability of surfactant micelles to alter the physical location and reactivity of iron in oil-in-water emulsion. J Agric Food Chem 2002;50(20):5704-10. https://doi.org/10.1021/jf020433g.
[174] Anton M. Egg yolk: structures, functionalities and processes. J Sci Food Agric 2013;93(12):2871-80. https://doi.org/10.1002/jsfa.6247.
[175] Samaraweera H, Zhang WG, Lee EJ, Ahn DU. Egg yolk phosvitin and functional phosphopeptides. J Food Sci 2011;76(7):R143-50. https://doi.org/10.1111/ j.1750-3841.2011.02291.x.
[176] Hu M, McClements DJ, Decker EA. Impact of whey protein emulsifiers on the oxidative stability of Salmon oil-in-water emulsions. J Agric Food Chem 2003;51 (5):1435-9. https://doi.org/10.1021/jf0203794.
[177] Sugiarto M, Ye A, Taylor MW, Singh H. Milk protein-iron complexes: inhibition of lipid oxidation in an emulsion. Dairy Sci Technol 2010;90(1):87-98. https://doi. org/10.1051/dst/2009053.
[178] Villiere A, Viau M, Bronnec I, Moreau N, Genot C. Oxidative stability of bovine serum albumin- and sodium Caseinate-stabilized emulsions depends on metal availability. J Agric Food Chem 2005;53(5):1514-20. https://doi.org/10.1021/ jf0486951.
[179] Elias RJ, Kellerby SS, Decker EA. Antioxidant activity of proteins and peptides. Crit Rev Food Sci Nutr 2008;48(5):430-41. https://doi.org/10.1080/ 10408390701425615.
[180] Panya A, Laguerre M, Bayrasy C, Lecomte J, Villeneuve P, McClements DJ, et al. An investigation of the versatile antioxidant mechanisms of action of rosmarinate alkyl esters in oil-in-water emulsions. J Agric Food Chem 2012;60(10):2692-700. https://doi.org/10.1021/jf204848b.
[181] Martinez-Senra T, Losada-Barreiro S, Bravo-Diaz C. Efficiency of delta-tocopherol in inhibiting lipid oxidation in emulsions: effects of surfactant charge and of surfactant concentration. Antioxidants (Basel) 2023;12(6). https://doi.org/ 10.3390/antiox12061158.
[182] Losada-Barreiro S, Paiva-Martins F, Bravo-Diaz C. Partitioning of antioxidants in edible oil-water binary systems and in oil-in-water emulsions. Antioxidants (Basel) 2023;12(4). https://doi.org/10.3390/antiox12040828.
[183] Kiralan SS, Doğu-Baykut E, Kittipongpittaya K, McClements DJ, Decker EA. Increased antioxidant efficacy of tocopherols by surfactant Solubilization in oil-in-water emulsions. J Agric Food Chem 2014;62(43):10561-6. https://doi.org/ 10.1021/jf503115j.
[184] Richards MP, Chaiyasit W, McClements DJ, Decker EA. Ability of surfactant micelles to alter the partitioning of phenolic antioxidants in oil-in-water emulsions. J Agric Food Chem 2002;50(5):1254-9. https://doi.org/10.1021/ jf011324p.
[185] Dai T, Chen J, McClements DJ, Hu P, Ye X, Liu C, et al. Protein-polyphenol interactions enhance the antioxidant capacity of phenolics: analysis of rice glutelin-procyanidin dimer interactions. Food Funct 2019;10(2):765-74. https:// doi.org/10.1039/c8fo02246a.
[186] Schild K, Sonnichsen FD, Martin D, Garamus VM, Van der Goot AJ, Schwarz K, et al. Unraveling the effects of low protein-phenol binding affinity on the structural properties of beta-lactoglobulin. Food Chem 2023;426:136496. https://doi.org/10.1016/j.foodchem.2023.136496.
[187] Tang L, Cao M, Liao C, Liu R, Chang M, Wang X. Migration of tocopherols from the oil phase to the oil-water interface using phospholipids improved the oxidative stability of O/W emulsions. Food Chem 2023;414:135719. https://doi. org/10.1016/j.foodchem.2023.135719.
[188] Chen J, Li X, Kong B, Chen Q, Liu Q. Comparative study of protein-lipid cooxidation in whey protein isolate-stabilised oil-in-water emulsions prepared by different homogenisation methods. Colloids Surf A Physicochem Eng Asp 2022; 633:127916. https://doi.org/10.1016/j.colsurfa.2021.127916.
[189] Chen J, Zhao J, Kong B, Chen Q, Liu Q, Liu C. Comparative study of oxidative structural modifications of Unadsorbed and adsorbed proteins in whey protein isolate-stabilized oil-in-water emulsions under the stress of primary and secondary lipid oxidation products. Foods. 2021;10(3). https://doi.org/10.3390/ foods10030593.
[190] Donnelly JL, Decker EA, McClements DJ. Iron-catalyzed oxidation of menhaden oil as affected by emulsifiers. J Food Sci 1998;63(6):997-1000. https://doi.org/ 10.1111/j.1365-2621.1998.tb15841.x.
[191] Ma KK, Greis M, Lu J, Nolden AA, McClements DJ, Kinchla AJ. Functional performance of plant proteins. Foods 2022;11:4. https://doi.org/10.3390/ foods11040594.
[192] Donbrow M, Azaz E, Pillersdorf A. Autoxidation of polysorbates. J Pharm Sci 1978;67(12):1676-81. https://doi.org/10.1002/jps.2600671211.
[193] Hamburger R, Azaz E, Donbrow M. Autoxidation of polyoxyethylenic non-ionic surfactants and of polyethylene glycols. Pharm Acta Helv 1975;50(1-2):10-7.
[194] Lever M. Peroxides in detergents as interfering factors in biochemical analysis. Anal Biochem 1977;83(1):274-84. https://doi.org/10.1016/0003-2697(77) 90536-X.
[195] Jaeger J, Sorensen K, Wolff SP. Peroxide accumulation in detergents. J Biochem Biophys Methods 1994;29(1):77-81. https://doi.org/10.1016/0165-022x(94) 90058-2.
[196] Mancuso JR, McClements DJ, Decker EA. The effects of surfactant type, pH , and chelators on the oxidation of salmon oil-in-water emulsions. J Agric Food Chem 1999;47(10):4112-6. https://doi.org/10.1021/jf990203a.
[197] Ha E, Wang W, Wang YJ. Peroxide formation in polysorbate 80 and protein stability. J Pharm Sci 2002;91(10):2252-64. https://doi.org/10.1002/jps.10216.
[198] Segal R, Azaz E, Donbrow M. Peroxide removal from non-ionic surfactants. J Pharm Pharmacol 1979;31(1):39-40. https://doi.org/10.1111/j.20427158.1979.tb13418.x.
[199] Donbrow M, Hamburger R, Azaz E, Pillersdorf A. Development of acidity in nonionic surfactants: formic and acetic acid. Analyst. 1978;103(1225):400-2. https://doi.org/10.1039/AN9780300400.
[200] Kerwin BA. Polysorbates 20 and 80 used in the formulation of protein biotherapeutics: structure and degradation pathways. J Pharm Sci 2008;97(8): 2924-35. https://doi.org/10.1002/jps. 21190.
[201] Yao J, Dokuru DK, Noestheden M, Park SS, Kerwin BA, Jona J, et al. A quantitative kinetic study of polysorbate autoxidation: the role of unsaturated fatty acid ester substituents. Pharm Res 2009;26(10):2303-13. https://doi.org/ 10.1007/s11095-009-9946-7.
[202] Kishore RS, Pappenberger A, Dauphin IB, Ross A, Buergi B, Staempfli A, et al. Degradation of polysorbates 20 and 80: studies on thermal autoxidation and hydrolysis. J Pharm Sci 2011;100(2):721-31. https://doi.org/10.1002/ jps. 22290.
[203] Hvattum E, Yip WL, Grace D, Dyrstad K. Characterization of polysorbate 80 with liquid chromatography mass spectrometry and nuclear magnetic resonance spectroscopy: specific determination of oxidation products of thermally oxidized polysorbate 80. J Pharm Biomed Anal 2012;62:7-16. https://doi.org/10.1016/j. jpba.2011.12.009.
[204] Li X, Wang Z, Zheng B, Wang Y, Zhang J. Novel strategy to rapidly profile and identify oxidized species of Polysorbate 80 using ultra-high-performance liquid chromatography coupled with high-resolution mass spectrometry. Anal Chem 2023;95(24):9156-63. https://doi.org/10.1021/acs.analchem.2c04956.
[205] Bai L, Zhang Y, Zhang C, Lu Y, Li Z, Huang G, et al. Investigation of excipients impact on polysorbate 80 degradation in biopharmaceutical formulation buffers.
[206] Mittag JJ, Trutschel ML, Kruschwitz H, Mader K, Buske J, Garidel P. Characterization of radicals in polysorbate 80 using electron paramagnetic resonance (EPR) spectroscopy and spin trapping. Int J Pharm X 2022;4:100123. https://doi.org/10.1016/j.ijpx.2022.100123.
[207] Larson NR, Wei Y, Prajapati I, Chakraborty A, Peters B, Kalonia C, et al. Comparison of Polysorbate 80 hydrolysis and oxidation on the aggregation of a monoclonal antibody. J Pharm Sci 2020;109(1):633-9. https://doi.org/10.1016/ j.xphs.2019.10.069.
[208] Peters BH, Wei Y, Middaugh CR, Schoneich C. Intra-micellar and extra-micellar oxidation in phosphate and histidine buffers containing Polysorbate 80. J Pharm Sci 2022;111(9):2435-44. https://doi.org/10.1016/j.xphs.2022.06.011.
[209] Nuchi CD, McClements DJ, Decker EA. Impact of tween 20 hydroperoxides and iron on the oxidation of methyl linoleate and salmon oil dispersions. J Agric Food Chem 2001;49(10):4912-6. https://doi.org/10.1021/jf010370m.
[210] Schwarz K, Huang SW, German JB, Tiersch B, Hartmann J, Frankel EN. Activities of antioxidants are affected by colloidal properties of oil-in-water and water-in-oil emulsions and bulk oils. J Agric Food Chem 2000;48(10):4874-82. https://doi. org/10.1021/jf991289a.
[211] Mabrouk AF, Dugan Jr L. Kinetic investigation into glucose-, fructose-, and sucrose-activated autoxidation of methyl linoleate emulsion. J Am Oil Chem Soc 1961;38(12):692-5. https://doi.org/10.1007/BF02633057.
[212] Haahr AM, Jacobsen C. Emulsifier type, metal chelation and pH affect oxidative stability of n-3-enriched emulsions. Eur J Lipid Sci Technol 2008;110(10): 949-61. https://doi.org/10.1002/ejlt.200800035.
[213] Kasaikina O, Golyavin A, Krugovov D, Kartasheva Z, Pisarenko L. Micellar catalysis in the oxidation of lipids. Mosc Univ Chem Bull 2010;65:206-9. https:// doi.org/10.3103/S0027131410030193.
[214] Uluata S, McClements DJ, Decker EA. Physical stability, autoxidation, and photosensitized oxidation of omega-3 oils in Nanoemulsions prepared with natural and synthetic surfactants. J Agric Food Chem 2015;63(42):9333-40. https://doi.org/10.1021/acs.jafc.5b03572.
[215] Gutiérrez-Méndez N, Chavez-Garay DR, Leal-Ramos MY. Lecithins: A comprehensive review of their properties and their use in formulating microemulsions. J Food Biochem 2022;46(7):e14157. https://doi.org/10.1111/ jfbc. 14157.
[216] Caparosa MH, Hartel RW. Characterizing lecithin interactions in chocolate using interfacial properties and rheology. J Am Oil Chem Soc 2020;97(12):1309-17. https://doi.org/10.1002/aocs.12419.
[217] Alhajj MJ, Montero N, Yarce CJ, Salamanca CH. Lecithins from vegetable, land, and marine animal sources and their potential applications for cosmetic, food, and pharmaceutical sectors. Cosmetics. 2020;7(4):87. https://doi.org/10.3390/ cosmetics7040087.
[218] Robert C, Couëdelo L, Vaysse C, Michalski M-C. Vegetable lecithins: A review of their compositional diversity, impact on lipid metabolism and potential in cardiometabolic disease prevention. Biochimie. 2020;169:121-32. https://doi. org/10.1016/j.biochi.2019.11.017.
[219] Dacaranhe CD, Terao J. A unique antioxidant activity of phosphatidylserine on iron-induced lipid peroxidation of phospholipid bilayers. Lipids. 2001;36(10): 1105-10. https://doi.org/10.1007/s11745-001-0820-7.
[220] Cardenia V, Waraho T, Rodriguez-Estrada MT, Julian McClements D, Decker EA. Antioxidant and Prooxidant activity behavior of phospholipids in stripped soybean oil-in-water emulsions. J Am Oil Chem Soc 2011;88(9):1409-16. https:// doi.org/10.1007/s11746-011-1807-y.
[221] Judde A, Villeneuve P, Rossignol-Castera A, Le Guillou A. Antioxidant effect of soy lecithins on vegetable oil stability and their synergism with tocopherols. J Am Oil Chem Soc 2003;80(12):1209-15. https://doi.org/10.1007/s11746-003-08444.
[222] Hildebrand D, Terao J, Kito M. Phospholipids plus tocopherols increase soybean oil stability. J Am Oil Chem Soc 1984;61(3):552-5. https://doi.org/10.1007/ BF02677029.
[223] Khan MA, Shahidi F. Tocopherols and phospholipids enhance the oxidative stability of borage and evening primrose triacylglycerols. J Food Lipids 2000;7 (3):143-50. https://doi.org/10.1111/j.1745-4522.2000.tb00167.x.
[224] Kashima M, Cha G-S, Isoda Y, Hirano J, Miyazawa T. The antioxidant effects of phospholipids on perilla oil. J Am Oil Chem Soc 1991;68:119-22. https://doi. org/10.1007/BF02662331.
[225] Lee J, Choe E. Effects of phospholipids on the antioxidant activity of alphatocopherol in the singlet oxygen oxidation of canola oil. N Biotechnol 2011;28(6): 691-7. https://doi.org/10.1016/j.nbt.2011.04.013.
[226] Weng X, Gordon MH. Antioxidant synergy between phosphatidyl ethanolamine and
[227] Doert M, Jaworska K, Moersel J-T, Kroh LW. Synergistic effect of lecithins for tocopherols: lecithin-based regeneration of
[228] Takenaka A, Hosokawa M, Miyashita K. Unsaturated phosphatidylethanolamine as effective synergist in combination with
[229] Shimajiri J, Shiota M, Hosokawa M, Miyashita K. Synergistic antioxidant activity of milk sphingomyeline and its sphingoid base with
[230] Hidalgo FJ, Nogales F, Zamora R. Changes produced in the antioxidative activity of phospholipids as a consequence of their oxidation. J Agric Food Chem 2005;53 (3):659-62. https://doi.org/10.1021/jf0483220.
[231] Bandarra NM, Campos RM, Batista I, Nunes ML, Empis JM. Antioxidant synergy of
[232] Lu FS, Nielsen NS, Baron CP, Diehl BW, Jacobsen C. Oxidative stability of dispersions prepared from purified marine phospholipid and the role of alphatocopherol. J Agric Food Chem 2012;60(50):12388-96. https://doi.org/10.1021/ jf303560f.
[233] Bacot S, Bernoud-Hubac N, Chantegrel B, Deshayes C, Doutheau A, Ponsin G, et al. Evidence for in situ ethanolamine phospholipid adducts with hydroxyalkenals. J Lipid Res 2007;48(4):816-25. https://doi.org/10.1194/jlr.M600340JLR200.
[234] van Nieuwenhuyzen W, Tomás MC. Update on vegetable lecithin and phospholipid technologies. Eur J Lipid Sci Technol 2008;110(5):472-86. https:// doi.org/10.1002/ejlt.200800041.
[235] Lu F, Nielsen NS, Baron CP, Jacobsen C. Marine phospholipids: the current understanding of their oxidation mechanisms and potential uses for food fortification. Crit Rev Food Sci Nutr 2017;57(10):2057-70. https://doi.org/ 10.1080/10408398.2014.925422.
[236] Dong Y, Yong VW. Oxidized phospholipids as novel mediators of neurodegeneration. Trends Neurosci 2022. https://doi.org/10.1016/j. tins.2022.03.002.
[237] Dushianthan A, Postle A. Methodology to detect oxidised phospholipids and their relevance in disease. Biochem Soc Trans 2021;49(3):1241-50. https://doi.org/ 10.1042/BST20200852.
[238] Nie J, Yang J, Wei Y, Wei X. The role of oxidized phospholipids in the development of disease. Mol Aspects Med 2020;76:100909. https://doi.org/ 10.1016/j.mam.2020.100909.
[239] Reis A, Spickett CM. Chemistry of phospholipid oxidation. Biochim Biophys Acta 2012;1818(10):2374-87. https://doi.org/10.1016/j.bbamem.2012.02.002.
[240] Henna Lu FS, Nielsen NS, Timm-Heinrich M, Jacobsen C. Oxidative stability of marine phospholipids in the liposomal form and their applications. Lipids. 2011; 46(1):3-23. https://doi.org/10.1007/s11745-010-3496-y.
[241] Thomsen BR, Haugsgjerd BO, Griinari M, Lu HFS, Bruheim I, Vogt G, et al. Investigation of oxidative degradation and non-enzymatic browning reactions in krill and fish oils. Eur J Lipid Sci Technol 2013;115(12):1357-66. https://doi. org/10.1002/ejlt.201300141.
[242] Igene J, Pearson A, Dugan Jr L, Price J. Role of triglycerides and phospholipids on development of rancidity in model meat systems during frozen storage. Food Chem 1980;5(4):263-76. https://doi.org/10.1016/0308-8146(80)90048-5.
[243] Igene J, Pearson A, Gray J. Effects of length of frozen storage, cooking and holding temperatures upon component phospholipids and the fatty acid composition of meat triglycerides and phospholipids. Food Chem 1981;7(4): 289-303. https://doi.org/10.1016/0308-8146(81)90034-0.
[244] Pikul J, Kummerow FA. Relative role of individual phospholipids on thiobarbituric acid reactive substances formation in chicken meat, skin and swine aorta. J Food Sci 1990;55(5):1243-8. https://doi.org/10.1111/j.13652621.1990.tb03907.x.
[245] Pan Y, Tikekar RV, Nitin N. Effect of antioxidant properties of lecithin emulsifier on oxidative stability of encapsulated bioactive compounds. Int J Pharm 2013; 450(1-2):129-37. https://doi.org/10.1016/j.ijpharm.2013.04.038.
[246] Burgos-Díaz C, Wandersleben T, Marqués AM, Rubilar M. Multilayer emulsions stabilized by vegetable proteins and polysaccharides. Curr Opin Colloid Interface Sci 2016;25:51-7. https://doi.org/10.1016/j.cocis.2016.06.014.
[247] McClements DJ. Protein-stabilized emulsions. Curr Opin Colloid Interface Sci 2004;9(5):305-13. https://doi.org/10.1016/j.cocis.2004.09.003.
[248] Zhang M, Fan L, Liu Y, Huang S, Li J. Effects of proteins on emulsion stability: the role of proteins at the oil-water interface. Food Chem 2022;397:133726. https:// doi.org/10.1016/j.foodchem.2022.133726.
[249] Decker EA. Strategies for manipulating the prooxidative/antioxidative balance of foods to maximize oxidative stability. Trends Food Sci Technol 1998;9(6):241-8. https://doi.org/10.1016/S0924-2244(98)00045-4.
[250] Singh P, Singh TP, Gandhi N. Prevention of lipid oxidation in muscle foods by milk proteins and peptides: A review. Food Rev Intl 2018;34(3):226-47. https:// doi.org/10.1080/87559129.2016.1261297.
[251] Berton-Carabin C, Schroën K. Towards new food emulsions: designing the interface and beyond. Curr Opin Food Sci 2019;27:74-81. https://doi.org/ 10.1016/j.cofs.2019.06.006.
[252] Cheng W, Zheng X, Yang M. Hydrogen peroxide induced protein oxidation during storage and Lyophilization process. J Pharm Sci 2016;105(6):1837-42. https:// doi.org/10.1016/j.xphs.2016.03.034.
[253] Duque-Estrada P, Kyriakopoulou K, de Groot W, van der Goot AJ, BertonCarabin CC. Oxidative stability of soy proteins: from ground soybeans to structured products. Food Chem 2020;318:126499. https://doi.org/10.1016/j. foodchem.2020.126499.
[254] Tan Y, Wang J, Chen F, Niu S, Yu J. Effect of protein oxidation on kinetics of droplets stability probed by microrheology in O/W and W/O emulsions of whey protein concentrate. Food Res Int 2016;85:259-65. https://doi.org/10.1016/j. foodres.2016.05.005.
[255] Li H, Cai Y, Li F, Zhang B, Wu X, Wu W. Rancidity-induced protein oxidation affects the interfacial dynamic properties and the emulsion rheological behavior of rice bran protein. Food Hydrocoll 2022;131:107794. https://doi.org/10.1016/ j.foodhyd.2022.107794.
[256] Zhou Q, Li H, Li F, Zhang B, Wu X, Wu W. Strategy and mechanism of Rice bran protein emulsion stability based on rancidity-induced protein oxidation: an ultrasonic case study. Foods. 2022;11(23). https://doi.org/10.3390/ foods11233896.
[257] Wang J, Tan Y, Xu H, Niu S, Yu J. Effect of 2,2-azobis (2-amidinopropane) dihydrochloride oxidized casein on the microstructure and microrheology properties of emulsions. Food Sci Biotechnol 2016;25(5):1283-90. https://doi. org/10.1007/s10068-016-0202-8.
[258] Hinderink EB, Kaade W, Sagis L, Schroën K, Berton-Carabin CC. Microfluidic investigation of the coalescence susceptibility of pea protein-stabilised emulsions: effect of protein oxidation level. Food Hydrocoll 2020;102:105610. https://doi. org/10.1016/j.foodhyd.2019.105610.
[259] Obando M, Papastergiadis A, Li S, De Meulenaer B. Impact of lipid and protein cooxidation on digestibility of dairy proteins in oil-in-water (
[260] Zhou Q, Wang J, Li H, Wu X, Wu W. Effect of protein oxidation on the emulsion carrier prepared by rice bran protein for improving stability and bioavailability of
[261] Zdanowska-Sąsiadek Z, Damaziak K, Marchewka J, Horbańczuk OK, Jóźwiak A, Wójcik W, et al. Lipid-and protein oxidation during storage and in vitro gastrointestinal digestion of ostrich, beef and chicken jerky snacks. Anim Sci Pap Rep 2022;40:305-16.
[262] Schaich KM. Chapter 8: Co-oxidations of oxidizing lipids: Reactions with proteins. In: Kamal-Eldin A, Min D, editors. Lipid oxidation pathways. AOCS Publishing; 2008. p. 183-274.
[263] Wu W, Hou L, Zhang C, Kong X, Hua Y. Structural modification of soy protein by 13-hydroperoxyoctadecadienoic acid. Eur Food Res Technol 2009;229:771-8. https://doi.org/10.1007/s00217-009-1113-1.
[264] Gürbüz G, Heinonen M. LC-MS investigations on interactions between isolated
[265] Yang J, Xiong YL. Comparative time-course of lipid and myofibrillar protein oxidation in different biphasic systems under hydroxyl radical stress. Food Chem 2018;243:231-8. https://doi.org/10.1016/j.foodchem.2017.09.146.
[266] Baron CP, Kjaersgard IV, Jessen F, Jacobsen C. Protein and lipid oxidation during frozen storage of rainbow trout (Oncorhynchus mykiss). J Agric Food Chem 2007; 55(20):8118-25. https://doi.org/10.1021/jf070686f.
[267] Yan S, Yao Y, Xie X, Zhang S, Huang Y, Zhu H, et al. Comparison of the physical stabilities and oxidation of lipids and proteins in natural and polyphenol-modified soybean protein isolate-stabilized emulsions. Food Res Int 2022;162(Pt B): 112066. https://doi.org/10.1016/j.foodres.2022.112066.
[268] Yi J, Ning J, Zhu Z, Cui L, Decker EA, McClements DJ. Impact of interfacial composition on co-oxidation of lipids and proteins in oil-in-water emulsions: competitive displacement of casein by surfactants. Food Hydrocoll 2019;87:20-8. https://doi.org/10.1016/j.foodhyd.2018.07.025.
[269] Zhu Z, Zhao C, Yi J, Liu N, Cao Y, Decker EA, et al. Impact of interfacial composition on lipid and protein co-oxidation in oil-in-water emulsions containing mixed Emulisifers. J Agric Food Chem 2018;66(17):4458-68. https:// doi.org/10.1021/acs.jafc.8b00590.
[270] Tian L, Zhang S, Yi J, Zhu Z, Decker EA, McClements DJ. The impact of konjac glucomannan on the physical and chemical stability of walnut oil-in-water emulsions coated by whey proteins. J Sci Food Agric 2022;102(10):4003-11. https://doi.org/10.1002/jsfa. 11748.
[271] Sørensen ADM, Nielsen NS, Hyldig G, Jacobsen C. Influence of emulsifier type on lipid oxidation in fish oil-enriched light mayonnaise. Eur J Lipid Sci Technol 2010;112(9):1012-23. https://doi.org/10.1002/ejlt.200900288.
[272] Jacobsen C, Adler-Nissen J, Meyer AS. Effect of ascorbic acid on iron release from the emulsifier interface and on the oxidative flavor deterioration in fish oil enriched mayonnaise. J Agric Food Chem 1999;47(12):4917-26. https://doi.org/ 10.1021/jf990241u.
[273] Raes K, Doolaege EH, Deman S, Vossen E, De Smet S. Effect of carnosic acid, quercetin and alpha-tocopherol on lipid and protein oxidation in an in vitro simulated gastric digestion model. Int J Food Sci Nutr 2015;66(2):216-21. https://doi.org/10.3109/09637486.2014.959900.
[274] Tian L, Kejing Y, Zhang S, Yi J, Zhu Z, Decker EA, et al. Impact of tea polyphenols on the stability of oil-in-water emulsions coated by whey proteins. Food Chem 2021;343:128448. https://doi.org/10.1016/j.foodchem.2020.128448.
[275] Perez-Gregorio MR, Simal-Gandara J. A critical review of the characterization of polyphenol-protein interactions and of their potential use for improving food quality. Curr Pharm Des 2017;23(19):2742-53. https://doi.org/10.2174/ 1381612823666170202112530.
[276] Quan TH, Benjakul S, Sae-leaw T, Balange AK, Maqsood S. Protein-polyphenol conjugates: antioxidant property, functionalities and their applications. Trends Food Sci Technol 2019;91:507-17. https://doi.org/10.1016/j.tifs.2019.07.049.
[277] Yan S, Xu J, Zhang S, Zhu H, Qi B, Li Y. Effect of interfacial composition on the physical stability and co-oxidation of proteins and lipids in a soy protein isolate-(-)-epigallocatechin gallate conjugate emulsion. Food Hydrocoll 2022;130: 107720. https://doi.org/10.1016/j.foodhyd.2022.107720.
[278] Laguerre M, Bayrasy C, Panya A, Weiss J, McClements DJ, Lecomte J, et al. What makes good antioxidants in lipid-based systems? The next theories beyond the polar paradox. Crit Rev Food Sci Nutr 2015;55(2):183-201. https://doi.org/ 10.1080/10408398.2011.650335.
[279] Laguerre M, Giraldo LJ, Lecomte J, Figueroa-Espinoza MC, Barea B, Weiss J, et al. Chain length affects antioxidant properties of chlorogenate esters in emulsion: the
cutoff theory behind the polar paradox. J Agric Food Chem 2009;57(23): 11335-42. https://doi.org/10.1021/jf9026266.
[280] Figueroa-Espinoza MC, Laguerre M, Villeneuve P, Lecomte J. From phenolics to phenolipids: optimizing antioxidants in lipid dispersions. Lipid Technol 2013;25 (6):131-4. https://doi.org/10.1002/lite.201300277.
[281] Figueroa-Espinoza MC, Villeneuve P. Phenolic acids enzymatic lipophilization. J Agric Food Chem 2005;53(8):2779-87. https://doi.org/10.1021/jf0484273.
[282] Grasso S, Siracusa L, Spatafora C, Renis M, Tringali C. Hydroxytyrosol lipophilic analogues: enzymatic synthesis, radical scavenging activity and DNA oxidative damage protection. Bioorg Chem 2007;35(2):137-52. https://doi.org/10.1016/j. bioorg.2006.09.003.
[283] Farooq S, Abdullah H Zhang, Weiss J. A comprehensive review on polarity, partitioning, and interactions of phenolic antioxidants at oil-water interface of food emulsions. Comprehen Rev Food Sci Food Safet 2021;20(5):4250-77. https://doi.org/10.1111/1541-4337.12792.
[284] Figueroa-Espinoza MC, Bourlieu C, Durand E, Lecomte J, Villeneuve P. Lipophilized antioxidants. In: Melton L, Shahidi F, Varelis P, editors. Encyclopedia of food chemistry. Oxford: Academic Press; 2019. p. 193-201. https://doi.org/10.1016/B978-0-08-100596-5.21673-9.
[285] Kahveci D, Laguerre M, Villeneuve P. 7 – Phenolipids as new antioxidants: Production. In: Ahmad MU, Xu X, editors. Activity, and potential applications, in polar lipids. Elsevier; 2015. p. 185-214. https://doi.org/10.1016/B978-1-63067-044-3.50011-X.
[286] Medina S, Auñón D, Lehoux J, Durand T, Crauste C, Gil-Izquierdo Á. Hydroxytyrosol fatty acid esters as new candidate markers for detecting olive oil inadequate storage conditions by UHPLC-QqQ-MS/MS. Microchem J 2022;181: 107656. https://doi.org/10.1016/j.microc.2022.107656.
[287] Caillol S. Cardanol: A promising building block for biobased polymers and additives. Curr Opin Green Sustain Chem 2018;14:26-32. https://doi.org/ 10.1016/j.cogsc.2018.05.002.
[288] Sonar VP, Corona A, Distinto S, Maccioni E, Meleddu R, Fois B, et al. Natural product-inspired esters and amides of ferulic and caffeic acid as dual inhibitors of HIV-1 reverse transcriptase. Eur J Med Chem 2017;130:248-60. https://doi.org/ 10.1016/j.ejmech.2017.02.054.
[289] Crauste C, Rosell M, Durand T, Vercauteren J. Omega-3 polyunsaturated lipophenols, how and why? Biochimie. 2016;120:62-74. https://doi.org/ 10.1016/j.biochi.2015.07.018.
[290] Choudhary MI, Begum A, Abbaskhan A, Ajaz A, Shafique ur R, Atta ur R. Phenyl polypropanoids from Lindelofia stylosa. Chem Pharm Bull(Tokyo) 2005;53(11): 1469-71. https://doi.org/10.1248/cpb.53.1469.
[291] Saha MM, Mallik UK, Mallik AK. A chromenoflavanone and two caffeic esters fromPongamia glabra. Phytochemistry. 1991;30(11):3834-6. https://doi.org/ 10.1016/0031-9422(91)80130-S.
[292] Medina I, Lois S, Alcantara D, Lucas R, Morales JC. Effect of lipophilization of hydroxytyrosol on its antioxidant activity in fish oils and fish oil-in-water emulsions. J Agric Food Chem 2009;57(20):9773-9. https://doi.org/10.1021/ jf9023867.
[293] Laguerre M, López Giraldo LJ, Lecomte J, Figueroa-Espinoza M-C, Baréa B, Weiss J, et al. Relationship between hydrophobicity and antioxidant ability of “Phenolipids” in emulsion: A parabolic effect of the chain length of Rosmarinate esters. J Agric Food Chem 2010;58(5):2869-76. https://doi.org/10.1021/ jf904119v.
[294] Sørensen A-DM, Durand E, Laguerre M, Bayrasy C, Lecomte J, Villeneuve P, et al. Antioxidant properties and efficacies of synthesized alkyl Caffeates, Ferulates, and Coumarates. J Agric Food Chem 2014;62(52):12553-62. https://doi.org/ 10.1021/jf500588s.
[295] Sørensen A-DM, Lyneborg KS, Villeneuve P, Jacobsen C. Alkyl chain length impacts the antioxidative effect of lipophilized ferulic acid in fish oil enriched milk. J Funct Foods 2015;18:959-67. https://doi.org/10.1016/j.jff.2014.04.008.
[296] Alemán M, Bou R, Guardiola F, Durand E, Villeneuve P, Jacobsen C, et al. Antioxidative effect of lipophilized caffeic acid in fish oil enriched mayonnaise and milk. Food Chem 2015;167:236-44. https://doi.org/10.1016/j. foodchem.2014.06.083.
[297] Grajeda-Iglesias C, Salas E, Barouh N, Baréa B, Panya A, Figueroa-Espinoza MC. Antioxidant activity of protocatechuates evaluated by DPPH, ORAC, and CAT methods. Food Chem 2016;194:749-57. https://doi.org/10.1016/j. foodchem.2015.07.119.
[298] Elder AS, Coupland JN, Elias RJ. Effect of alkyl chain length on the antioxidant activity of alkylresorcinol homologues in bulk oils and oil-in-water emulsions. Food Chem 2021;346:128885. https://doi.org/10.1016/j. foodchem.2020.128885.
[299] ten Klooster S, Villeneuve P, Bourlieu-Lacanal C, Durand E, Schroën K, BertonCarabin C. Alkyl chain length modulates antioxidant activity of gallic acid esters in spray-dried emulsions. Food Chem 2022;387:132880. https://doi.org/ 10.1016/j.foodchem.2022.132880.
[300] Lucas R, Comelles F, Alcantara D, Maldonado OS, Curcuroze M, Parra JL, et al. Surface-active properties of lipophilic antioxidants tyrosol and hydroxytyrosol fatty acid esters: a potential explanation for the nonlinear hypothesis of the antioxidant activity in oil-in-water emulsions. J Agric Food Chem 2010;58(13): 8021-6. https://doi.org/10.1021/jf1009928.
[301] Losada Barreiro S, Bravo-Diaz C, Paiva-Martins F, Romsted LS. Maxima in antioxidant distributions and efficiencies with increasing hydrophobicity of gallic acid and its alkyl esters. The pseudophase model interpretation of the “cutoff effect”. J Agric Food Chem 2013;61(26):6533-43. https://doi.org/10.1021/ jf400981x.
[302] Costa M, Losada-Barreiro S, Bravo-Diaz C, Vicente AA, Monteiro LS, PaivaMartins F. Influence of AO chain length, droplet size and oil to water ratio on the distribution and on the activity of gallates in fish oil-in-water emulsified systems: emulsion and nanoemulsion comparison. Food Chem 2020;310:125716. https:// doi.org/10.1016/j.foodchem.2019.125716.
[303] Mitrus O, Zuraw M, Losada-Barreiro S, Bravo-Diaz C, Paiva-Martins F. Targeting antioxidants to interfaces: control of the oxidative stability of lipid-based emulsions. J Agric Food Chem 2019;67(11):3266-74. https://doi.org/10.1021/ acs.jafc.8b06545.
[304] Freiria-Gandara J, Losada-Barreiro S, Paiva-Martins F, Bravo-Diaz C. Enhancement of the antioxidant efficiency of gallic acid derivatives in intact fish oil-in-water emulsions through optimization of their interfacial concentrations. Food Funct 2018;9(8):4429-42. https://doi.org/10.1039/c8fo00977e.
[305] Costa M, Losada-Barreiro S, Paiva-Martins F, Bravo-Díaz C. Physical evidence that the variations in the efficiency of homologous series of antioxidants in emulsions are a result of differences in their distribution. J Sci Food Agric 2017;97(2): 564-71. https://doi.org/10.1002/jsfa. 7765.
[306] Costa M, Losada-Barreiro S, Paiva-Martins F, Bravo-Díaz C, Romsted LS. A direct correlation between the antioxidant efficiencies of caffeic acid and its alkyl esters and their concentrations in the interfacial region of olive oil emulsions. The pseudophase model interpretation of the “cut-off” effect. Food Chem 2015;175: 233-42. https://doi.org/10.1016/j.foodchem.2014.10.016.
[307] Almeida J, Losada-Barreiro S, Costa M, Paiva-Martins F, Bravo-Diaz C, Romsted LS. Interfacial concentrations of Hydroxytyrosol and its lipophilic esters in intact olive oil-in-water emulsions: effects of antioxidant hydrophobicity, surfactant concentration, and the oil-to-water ratio on the oxidative stability of the emulsions. J Agric Food Chem 2016;64(25):5274-83. https://doi.org/ 10.1021/acs.jafc.6b01468.
[308] Stöckmann H, Schwarz K, Huynh-Ba T. The influence of various emulsifiers on the partitioning and antioxidant activity of hydroxybenzoic acids and their derivatives in oil-in-water emulsions. J Am Oil Chem Soc 2000;77(5):535-42. https://doi.org/10.1007/s11746-000-0085-6.
[309] Gonzalez MJ, Medina I, Maldonado OS, Lucas R, Morales JC. Antioxidant activity of alkyl gallates and glycosyl alkyl gallates in fish oil in water emulsions: relevance of their surface active properties and of the type of emulsifier. Food Chem 2015;183:190-6. https://doi.org/10.1016/j.foodchem.2015.03.035.
[310] Sørensen A-DM, Villeneuve P, Jacobsen C. Alkyl caffeates as antioxidants in O/W emulsions: impact of emulsifier type and endogenous tocopherols. Eur J Lipid Sci Technol 2017;119(6):1600276. https://doi.org/10.1002/ejlt.201600276.
[311] Hajfathalian M, Ghelichi S, Garcia-Moreno PJ, Moltke Sorensen AD, Jacobsen C. Peptides: production, bioactivity, functionality, and applications. Crit Rev Food Sci Nutr 2018;58(18):3097-129. https://doi.org/10.1080/ 10408398.2017.1352564.
[312] Lorenzo JM, Munekata PES, Gómez B, Barba FJ, Mora L, Pérez-Santaescolástica C, et al. Bioactive peptides as natural antioxidants in food products – A review. Trends Food Sci Technol 2018;79:136-47. https://doi.org/10.1016/j. tifs.2018.07.003.
[313] Zou TB, He TP, Li HB, Tang HW, Xia EQ. The structure-activity relationship of the antioxidant peptides from natural proteins. Molecules. 2016;21(1):72. https:// doi.org/10.3390/molecules21010072.
[314] Sun JZ, Kaur H, Halliwell B, Li XY, Bolli R. Use of aromatic hydroxylation of phenylalanine to measure production of hydroxyl radicals after myocardial ischemia in vivo. Direct evidence for a pathogenetic role of the hydroxyl radical in myocardial stunning. Circ Res 1993;73(3):534-49. https://doi.org/10.1161/01. res.73.3.534.
[315] Ospina-Quiroga JL, García-Moreno PJ, Guadix A, Guadix EM, AlmécijaRodríguez MDC, Pérez-Gálvez R. Evaluation of plant protein hydrolysates as natural antioxidants in fish oil-in-water emulsions. Antioxidants. 2022;11(8): 1612. https://doi.org/10.3390/antiox11081612.
[316] Liu Y, Qi Y, Wang Q, Yin F, Zhan H, Wang H, et al. Antioxidative effect of chlorella Pyrenoidosa protein hydrolysates and their application in krill oil-inwater emulsions. Mar Drugs 2022;20(6):345. https://doi.org/10.3390/ md20060345.
[317] Ghelichi S, Sørensen A-DM, García-Moreno PJ, Hajfathalian M, Jacobsen C. Physical and oxidative stability of fish oil-in-water emulsions fortified with enzymatic hydrolysates from common carp (Cyprinus carpio) roe. Food Chem 2017;237:1048-57. https://doi.org/10.1016/j.foodchem.2017.06.048.
[318] Olsen TH, Yesiltas B, Marin FI, Pertseva M, García-Moreno PJ, Gregersen S, et al. AnOxPePred: using deep learning for the prediction of antioxidative properties of peptides. Sci Rep 2020;10(1):21471. https://doi.org/10.1038/s41598-020-78319-w.
[319] Yesiltas B, García-Moreno PJ, Gregersen S, Olsen TH, Jones NC, Hoffmann SV, et al. Antioxidant peptides derived from potato, seaweed, microbial and spinach proteins: oxidative stability of 5% fish oil-in-water emulsions. Food Chem 2022; 385:132699. https://doi.org/10.1016/j.foodchem.2022.132699.
[320] Varona E, García-Moreno PJ, Gregersen Echers S, Olsen TH, Marcatili P, Guardiola F, et al. Antioxidant peptides from alternative sources reduce lipid oxidation in 5% fish oil-in-water emulsions ( pH 4 ) and fish oil-enriched mayonnaise. Food Chem 2023;426:136498. https://doi.org/10.1016/j. foodchem.2023.136498.
[321] García-Moreno PJ, Yesiltas B, Gregersen Echers S, Marcatili P, Overgaard MT, Hansen EB, et al. Recent advances in the production of emulsifying peptides with the aid of proteomics and bioinformatics. Curr Opin Food Sci 2023;51:101039. https://doi.org/10.1016/j.cofs.2023.101039.
[322] Pearson RG. Hard and soft acids and bases. J Am Chem Soc 1963;85(22):3533-9. https://doi.org/10.1021/ja00905a001.
[323] Irankunda R, Camaño Echavarría JA, Paris C, Stefan L, Desobry S, Selmeczi K, et al. Metal-chelating peptides separation using immobilized metal ion affinity chromatography: Experimental methodology and simulation. Separations. 2022;9 (11):370. https://doi.org/10.3390/separations9110370.
[324] Canabady-Rochelle LLS, Selmeczi K, Collin S, Pasc A, Muhr L, Boschi-Muller S. SPR screening of metal chelating peptides in a hydrolysate for their antioxidant properties. Food Chem 2018;239:478-85. https://doi.org/10.1016/j. foodchem. 2017.06.116.
[325] El Hajj S, Irankunda R, Camaño Echavarría JA, Arnoux P, Paris C, Stefan L, et al. Metal-chelating activity of soy and pea protein hydrolysates obtained after different enzymatic treatments from protein isolates. Food Chem 2023;405: 134788. https://doi.org/10.1016/j.foodchem.2022.134788.
[326] Guo L, Harnedy PA, O’Keeffe MB, Zhang L, Li B, Hou H, et al. Fractionation and identification of Alaska Pollock skin collagen-derived mineral chelating peptides. Food Chem 2015;173:536-42. https://doi.org/10.1016/j. foodchem. 2014.10.055.
[327] Chen D, Liu Z, Huang W, Zhao Y, Dong S, Zeng M. Purification and characterisation of a zinc-binding peptide from oyster protein hydrolysate. J Funct Foods 2013;5(2):689-97. https://doi.org/10.1016/j.jff.2013.01.012.
[328] Xie N, Huang J, Li B, Cheng J, Wang Z, Yin J, et al. Affinity purification and characterisation of zinc chelating peptides from rapeseed protein hydrolysates: possible contribution of characteristic amino acid residues. Food Chem 2015;173: 210-7. https://doi.org/10.1016/j.foodchem.2014.10.030.
[329] Schröder A, Laguerre M, Sprakel J, Schroën K, Berton-Carabin CC. Pickering particles as interfacial reservoirs of antioxidants. J Colloid Interface Sci 2020;575: 489-98. https://doi.org/10.1016/j.jcis.2020.04.069.
[330] Laguerre M, Sprakel JHB, Birtic S, Schroen CGPH, Berton-Carabin C, Schröder A. Emulsion comprising antioxidant particles. Naturex SA: Wageningen University; 2019.
[331] Zhou B, Gao S, Li X, Liang H, Li S. Antioxidant Pickering emulsions stabilised by zein/tannic acid colloidal particles with low concentration. Int J Food Sci Technol 2020;55(5):1924-34. https://doi.org/10.1111/ijfs.14419.
[332] Zou Y, Zhong J, Pan R, Wan Z, Guo J, Wang J, et al. Zein/tannic acid complex nanoparticles-stabilised emulsion as a novel delivery system for controlled release of curcumin. Int J Food Sci Technol 2017;52(5):1221-8. https://doi.org/ 10.1111/ijfs. 13380.
[333] Zhou FZ, Yan L, Yin SW, Tang CH, Yang XQ. Development of Pickering emulsions stabilized by gliadin/Proanthocyanidins hybrid particles (GPHPs) and the fate of lipid oxidation and digestion. J Agric Food Chem 2018;66(6):1461-71. https:// doi.org/10.1021/acs.jafc.7b05261.
[334] Peng LP, Tang CH. Outstanding antioxidant Pickering high internal phase emulsions by co-assembled polyphenol-soy beta-conglycinin nanoparticles. Food Res Int 2020;136:109509. https://doi.org/10.1016/j.foodres.2020.109509.
[335] Hu J, Du P, Xu R, Deng W. Supersmall dendritic mesoporous silica Nanospheres as antioxidant Nanocarriers for Pickering emulsifiers. J Agric Food Chem 2021;69 (49):14893-905. https://doi.org/10.1021/acs.jafc.1c03016.
[336] Lu X, Huang Q. Nano/submicrometer milled red Rice particles-stabilized Pickering emulsions and their Antioxidative properties. J Agric Food Chem 2020; 68(1):292-300. https://doi.org/10.1021/acs.jafc.9b04827.
[337] Schröder A, Laguerre M, Tenon M, Schroën K, Berton-Carabin CC. Natural particles can armor emulsions against lipid oxidation and coalescence. Food Chem 2021;347:129003. https://doi.org/10.1016/j.foodchem.2021.129003.
[338] Ghorbani Gorji S, Smyth HE, Sharma M, Fitzgerald M. Lipid oxidation in mayonnaise and the role of natural antioxidants: A review. Trends Food Sci Technol 2016;56:88-102. https://doi.org/10.1016/j.tifs.2016.08.002.
[339] Fadda A, Sanna D, Sakar EH, Gharby S, Mulas M, Medda S, et al. Innovative and sustainable technologies to enhance the oxidative stability of vegetable oils. Sustainability. 2022;14(2):849. https://doi.org/10.3390/su14020849.
[340] Savani P, Puthiyedath A, George S, Prasad PS, Annapure US. Evaluation of the sensory properties and antioxidant activity of clean rosemary extracts for an effective replacement of EDTA in mayonnaise. Appl Food Res 2023;3(1):100302. https://doi.org/10.1016/j.afres.2023.100302.
[341] Faustino M, Veiga M, Sousa P, Costa EM, Silva S, Pintado M. Agro-food byproducts as a new source of natural food additives. Molecules. 2019;24(6): 1056. https://doi.org/10.3390/molecules24061056.
[342] Mangiapelo L, Ianni F, Pagano C, Grispoldi L, Blasi F, Cenci-Goga B, et al. Role of apple pomace in the formulation of a novel healthy mayonnaise. Eur Food Res Technol 2023. https://doi.org/10.1007/s00217-023-04331-9.
[343] Kishk YFM, Elsheshetawy HE. Effect of ginger powder on the mayonnaise oxidative stability, rheological measurements, and sensory characteristics. Ann Agricult Sci 2013;58(2):213-20. https://doi.org/10.1016/j.aoas.2013.07.016.
[344] Altunkaya A, Hedegaard RV, Harholt J, Brimer L, Gökmen V, Skibsted LH. Oxidative stability and chemical safety of mayonnaise enriched with grape seed extract. Food Funct 2013;4(11):1647-53. https://doi.org/10.1039/C3FO60204D.
[345] Alnokkari A. Effect of vinegar on the oxidative stability of mayonnaise during its storage. J Chromatogr Sci 2023. https://doi.org/10.1093/chromsci/bmad036.
[346] Acharya P, Martins SIDFS, Der Maaten V, Vreeker R. EDTA-free mayonnaise and method for the production thereof. US 2015/0181914 A1.Google. Patents 2015.
[347] Khalid MU, Shabbir MA, Mustafa S, Hina S, Quddoos MY, Mahmood S, et al. Effect of apple peel as an antioxidant on the quality characteristics and oxidative stability of mayonnaise. Appl Food Res 2021;1(2):100023. https://doi.org/ 10.1016/j.afres.2021.100023.
[348] Honold PJ, Jacobsen C, Jónsdóttir R, Kristinsson HG, Hermund DB. Potential seaweed-based food ingredients to inhibit lipid oxidation in fish-oil-enriched mayonnaise. Eur Food Res Technol 2016;242:571-84. https://doi.org/10.1007/ s00217-015-2567-y.
[349] Raikos V, McDonagh A, Ranawana V, Duthie G. Processed beetroot (Beta vulgaris L.) as a natural antioxidant in mayonnaise: effects on physical stability, texture and sensory attributes. Food Sci Human Wellness 2016;5(4):191-8. https://doi. org/10.1016/j.fshw.2016.10.002.
[350] Lazăr S, Constantin OE, Horincar G, Andronoiu DG, Stănciuc N, Muresan C, et al. Beetroot by-product as a functional ingredient for obtaining value-added mayonnaise. Processes. 2022;10(2):227. https://doi.org/10.3390/pr10020227.
[351] Blejan AM, Nour V. Physico-chemical characteristics, sensory attributes and oxidative stability of soy Milk mayonnaise enriched in carotenoids from tomato by-products. Appl Sci 2023;13(12):7101. https://doi.org/10.3390/app13127101.
[352] Li C-Y, Kim H-W, Li H, Lee D-C, Rhee H-I. Antioxidative effect of purple corn extracts during storage of mayonnaise. Food Chem 2014;152:592-6. https://doi. org/10.1016/j.foodchem.2013.11.152.
[353] Azhagu Saravana Babu P, Aafrin B Vajiha, Archana G, Sabina K, Sudharsan K, Krishnan K Radha, et al. Polyphenolic and phytochemical content of Cucumis sativus seeds and study on mechanism of preservation of nutritional and quality outcomes in enriched mayonnaise. Int J Food Sci Technol 2016;51(6):1417-24. https://doi.org/10.1111/ijfs.13109.
[354] De Bruno A, Romeo R, Gattuso A, Piscopo A, Poiana M. Functionalization of a vegan mayonnaise with high value ingredient derived from the agro-industrial sector. Foods. 2021;10(11):2684. https://doi.org/10.3390/foods10112684.
[355] Vieira MR, Simões S, Carrera-Sánchez C, Raymundo A. Development of a clean label mayonnaise using fruit flour. Foods. 2023;12(11):2111. https://doi.org/ 10.3390/foods12112111.
[356] De Leonardis A, Macciola V, Iftikhar A, Lopez F. Antioxidant effect of traditional and new vinegars on functional oil/vinegar dressing-based formulations. Eur Food Res Technol 2022;248(6):1573-82. https://doi.org/10.1007/s00217-022-03986-0.
[357] Włodarczyk K, Tymczewska A, Rabiej-Kozioł D, Szydłowska-Czerniak A. Effect of black cumin cake extract, Octyl Caffeate, and active packaging on antioxidant properties of egg-free mayonnaise during storage. Appl Sci 2023;13(10):6245. https://doi.org/10.3390/app13106245.
[358] Feng J, Schroën K, Guyot S, Gacel A, Fogliano V, Berton-Carabin CC. Physical and oxidative stabilization of oil-in-water emulsions by roasted coffee fractions: Interface-and continuous phase-related effects. J Agric Food Chem 2023;71(11): 4717-28. https://doi.org/10.1021/acs.jafc.2c07365.
[359] De Leonardis A, Macciola V, Iftikhar A, Lopez F. Characterization, sensory and oxidative stability analysis of vegetable mayonnaise formulated with olive leaf vinegar as an active ingredient. Foods. 2022;11(24):4006. https://doi.org/ 10.3390/foods11244006.
[360] Martillanes S, Rocha-Pimienta J, Gil MV, Ayuso-Yuste MC, Delgado-Adámez J. Antioxidant and antimicrobial evaluation of rice bran (Oryza sativa L.) extracts in a mayonnaise-type emulsion. Food Chem 2020;308:125633. https://doi.org/ 10.1016/j.foodchem.2019.125633.
[361] Habinshuti I, Chen X, Yu J, Mukeshimana O, Duhoranimana E, Karangwa E, et al. Antimicrobial, antioxidant and sensory properties of Maillard reaction products (MRPs) derived from sunflower, soybean and corn meal hydrolysates. LWT. 2019; 101:694-702. https://doi.org/10.1016/j.lwt.2018.11.083.
[362] Feng J, Berton-Carabin CC, Fogliano V, Schroën K. Maillard reaction products as functional components in oil-in-water emulsions: A review highlighting interfacial and antioxidant properties. Trends Food Sci Technol 2022;121: 129-41. https://doi.org/10.1016/j.tifs.2022.02.008.
[363] Feng J, Berton-Carabin CC, Guyot S, Gacel A, Fogliano V, Schroën K. Coffee melanoidins as emulsion stabilizers. Food Hydrocoll 2023;139:108522. https:// doi.org/10.1016/j.foodhyd.2023.108522.
[364] Verzelloni E, Tagliazucchi D, Conte A. Relationship between the antioxidant properties and the phenolic and flavonoid content in traditional balsamic vinegar. Food Chem 2007;105(2):564-71. https://doi.org/10.1016/j. foodchem.2007.04.014.
[365] Tagliazucchi D, Verzelloni E, Conte A. Contribution of melanoidins to the antioxidant activity of traditional balsamic vinegar during aging. J Food Biochem 2010;34(5):1061-78. https://doi.org/10.1111/j.1745-4514.2010.00349.x.
[366] Verzelloni E, Tagliazucchi D, Conte A. Changes in major antioxidant compounds during aging of traditional balsamic vinegar. J Food Biochem 2010;34(1):152-71. https://doi.org/10.1111/j.1745-4514.2009.00271.x.
[367] Verzelloni E, Tagliazucchi D, Conte A. From balsamic to healthy: traditional balsamic vinegar melanoidins inhibit lipid peroxidation during simulated gastric digestion of meat. Food Chem Toxicol 2010;48(8):2097-102. https://doi.org/ 10.1016/j.fct.2010.05.010.
[368] Smetana S, Pleissner D, Zuin Zeidler V. Waste to Food: Returning Nutrients to the Food Chain. Leiden, The Netherlands: Wageningen Academic; 2023. https://doi. org/10.3920/978-90-8686-929-9.
[369] Ribeiro TB, Voss GB, Coelho MC, Pintado ME. Chapter 33 – food waste and byproduct valorization as an integrated approach with zero waste: Future challenges. In: Bhat R, editor. Future foods. Academic Press; 2022. p. 569-96. https://doi.org/10.1016/B978-0-323-91001-9.00017-7.
[370] Socas-Rodríguez B, Álvarez-Rivera G, Valdés A, Ibáñez E, Cifuentes A. Food byproducts and food wastes: are they safe enough for their valorization? Trends Food Sci Technol 2021;114:133-47. https://doi.org/10.1016/j.tifs.2021.05.002.
[371] Ahmed Z, Chen J, Tufail T, Latif A, Arif M, Ullah R, et al. Fundamental opportunities and challenges of nutraceutical noodles enriched with Agri-food byproducts. Trends Food Sci Technol 2024;143:104299. https://doi.org/10.1016/j. tifs.2023.104299.
[372] Amft J, Meissner PM, Steffen-Heins A, Hasler M, Stöckmann H, Meynier A, et al. Interlaboratory study on lipid oxidation during accelerated storage trials with rapeseed and sunflower oil analyzed by conjugated dienes as primary oxidation products. Eur J Lipid Sci Technol 2023;125(10):2300067. https://doi.org/ 10.1002/ejlt. 202300067.
- Abbreviations: A•, antioxidant radical; ABTS, 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonate); AFM, atomic force microscopy; AH, antioxidant; AMVN, 2,2’azobis (2,4-dimethylvaleronitrile); AV, anidisine value; BHT, butyl hydroxytoluene; CCL, critical chain length; CLSM, confocal laser scanning microscopy; CLP, colloidal solid lipid particles; CMC, critical micelle concentration; cryo-TEM, cryo-transmission electron microscopy; DLPC, dilauroylphosphatidylcholine; DMSN, dendritic mesoporous silica nanospheres; DPPH, 2,2-Diphenyl-1-picrylhydrazyl; EDTA, ethylenediaminetetraacetic acid; FFA-OOH, free fatty acid hydroperoxide; FID, flame ionization detector; GA, gallic acid; GC, gas chromatography; HLB, hydrophilic-lipophilic balance; HSAB theory, hard and soft acid and base theory; IMAC, immobilized metal ion affinity chromatography; L•, lipid alkyl radical; LC, liquid chromatography; LDL, low-density lipoprotein; LH, unsaturated fatty acid; LMWE, low molecular weight emulsifier; LO•, lipid alkoxyl radical; LOO•, lipid peroxyl radical; LOOH, lipid hydroperoxide; MCP, metal chelating peptide; MRP, Maillard reaction product; MS, mass spectrometry; NMR, nuclear magnetic resonance;
, hydroxyl radical; OSA, octenyl succinic anhydride; O/W emulsions, oil-inwater emulsions; PA, phosphatidic acid; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PG, phosphatidylglycerol; PI, phosphatidylinositol; PS, phosphatidylserine; PV, peroxide value; R•, alkyl radical; ROO•, peroxyl radical; SDS, sodium dodecyl sulfate; SPI, soy protein isolate; SPME, solid phase microextraction; SPR, surface plasmon resonance; switchSENSE®, electrically switchable nanolever technology; TAG, triacylglycerol; TBARS, thiobarbituric acid reactive substances; WPI, whey protein isolate.. Number of carbon atoms in the alkyl chain.
Critical chain length: number of carbon atoms in the alkyl chain at the maximum antioxidant capacity.
