DOI: https://doi.org/10.1038/s41698-024-00522-z
PMID: https://pubmed.ncbi.nlm.nih.gov/38341519
تاريخ النشر: 2024-02-10
استهداف البلعميات المرتبطة بالأورام من نوع M2 هو نهج علاجي محتمل للتغلب على مقاومة الأدوية المضادة للأورام
الملخص
تظهر مقاومة الأدوية في الأورام من تفاعل عاملين حاسمين: تباين الخلايا الورمية وطبيعة البيئة الدقيقة المثبطة للمناعة للأورام. تشكل البلعميات المرتبطة بالورم (TAMs) مكونات أساسية في البيئة الدقيقة للورم. تعتبر البلعميات من النوع M2 ضرورية في تسهيل انتشار الورم بالإضافة إلى تعزيز مقاومة الأورام للأدوية. تلخص هذه المراجعة الآليات التي تستخدمها البلعميات من النوع M2 لتعزيز مقاومة الأدوية في الأورام. كما نصف الاستراتيجيات العلاجية الناشئة التي تستهدف حاليًا البلعميات من النوع M2 بالتزامن مع أدوية مضادة للأورام أخرى، مع وجود بعضها لا يزال قيد التقييم في التجارب السريرية. علاوة على ذلك، نقوم بتلخيص وتحليل مختلف الأساليب الحالية لتطوير أدوية جديدة تستهدف البلعميات من النوع M2 للتغلب على مقاومة الأورام، مع تسليط الضوء على كيفية أن استهداف البلعميات من النوع M2 يمكن أن يوقف فعليًا نمو الورم وانتشاره ويتغلب على مقاومة الأدوية في الأورام.
آليات. كما نقوم بتحليل إمكانيات التطوير المستقبلية لهذه الاستراتيجية العلاجية الجديدة.
الميزة البيولوجية لخلايا المناعة المرتبطة بالورم
أصل TAMs
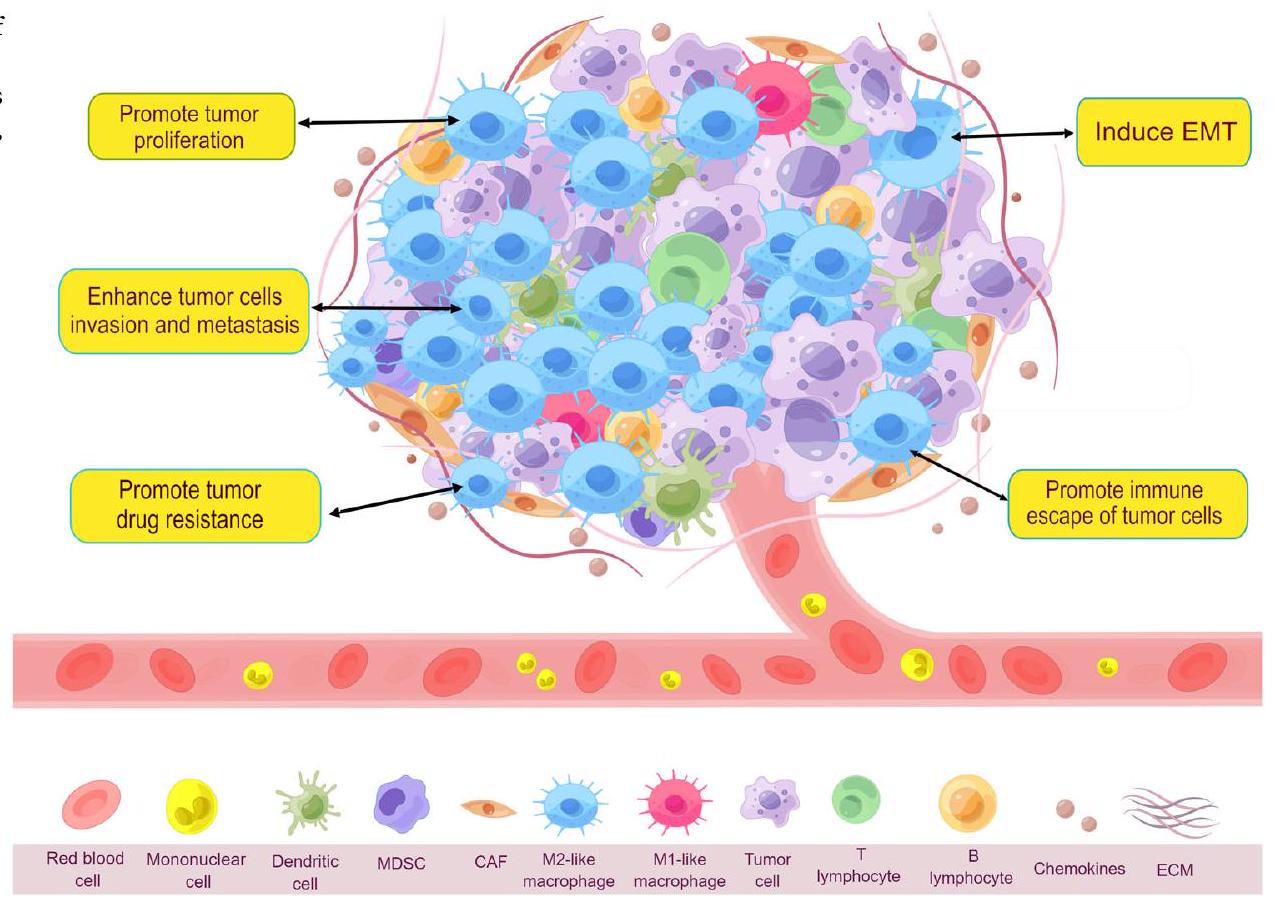
تنظيم اللدونة والاستقطاب للخلايا المناعية المرتبطة بالورم (TAMs) وتسميتها
الأنماط الظاهرية ووظائف TAMs
طرفي TAM الديناميكي المستمر
محور الاستقطاب، موضحًا أدوارها الفريدة في البيئة المجهرية للورم. كل من TAMs الشبيهة بـ M1 وTAMs الشبيهة بـ M2 لها علامات سطح خلوية محددة وعوامل وظيفية. تتولى TAMs الشبيهة بـ M1 في البيئة المجهرية للورم أدوارًا في تعزيز الالتهاب، تثبيط التكاثر، القضاء على مسببات الأمراض، والاستجابات المضادة للورم. بينما تشارك TAMs الشبيهة بـ M2 في الأنشطة المضادة للالتهابات، وتعزيز تكوين الأوعية الدموية، وتأثيرها على تجديد الأنسجة والشفاء، وتعزيز توليد الورم، وتكاثره، وانتشاره، ومقاومته للأدوية. (بواسطة Figdraw).
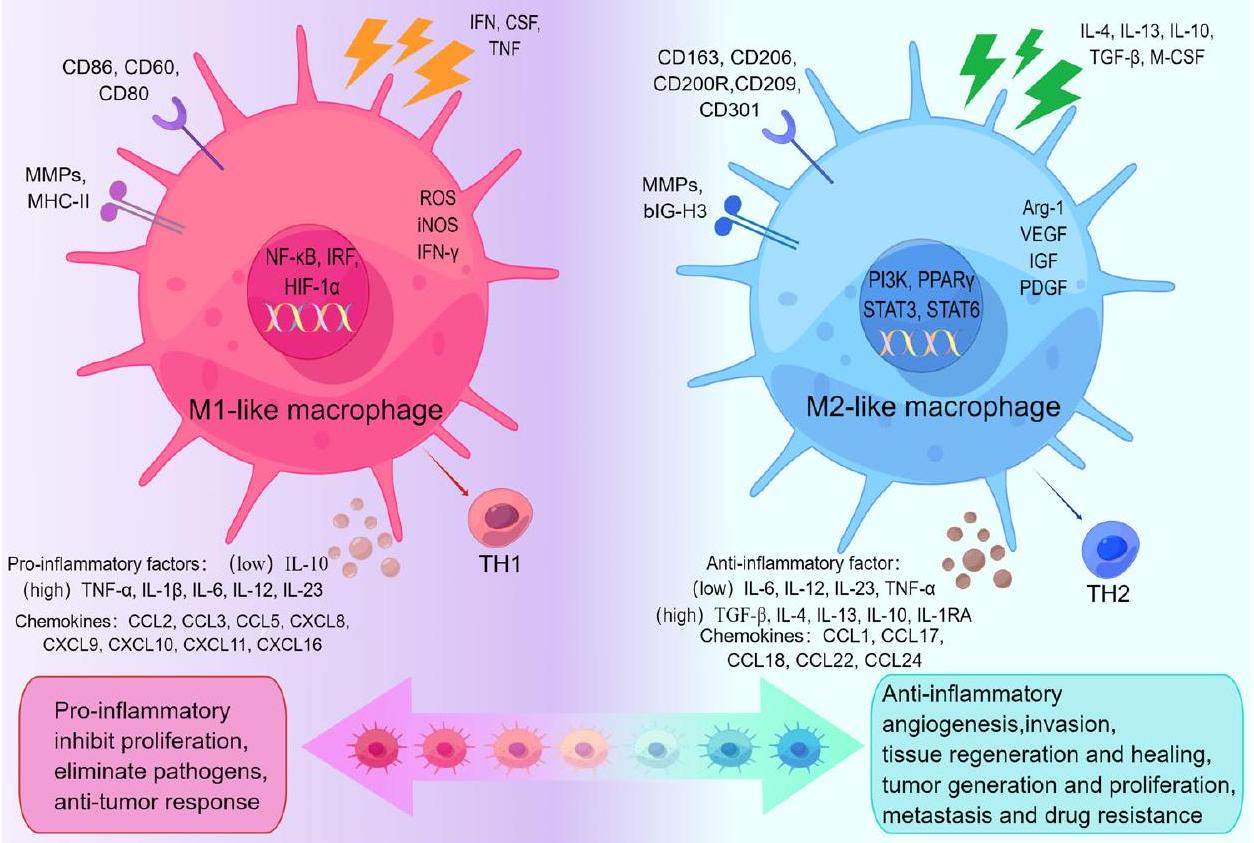
آليات تنظيم استقطاب البلعميات من نوع M1 / M2
| الظواهر | وظائف | عوامل التحفيز | علامات | إخراج | المراجع |
| M1 | مقاومة الورم، مؤيد للالتهابات، تثبيط التكاثر، القضاء على مسببات الأمراض | إنترفيرون-
|
IL-12 (مرتفع)/IL-10 (منخفض)، CD80، CD86، CD60، MMPs، MHCII | CCL2، CCL3، CCL5، CXCL8، CXCL9، IL-1
|
32 |
| M2a | الحساسية، البروفيبروتيك، تكاثر الخلايا، مضاد الالتهاب، شفاء الجروح، تقدم الورم | IL-4، IL-10، IL-13، (PPAR-
|
CD11b، CD45، CD86، CD14، CD206، CD163، CD209، IL-1R، Dectin-1، YM1، RELM
|
TGF-
|
٣٢-٣٥ |
| M2b | تنشيط Th2، تنظيم المناعة، الالتهاب، تقدم الورم | IL-1
|
CD163، CD14، CD86، MHC-II، IL-10 (مرتفع)، IL-12 (منخفض)، IL-6R | TNF-
|
٣٢،٣٥ |
| M2c | تنظيم المناعة، الاستجابة الالتهابية، شفاء الجروح، التليف | IL-6، IL-10، الجلوكوكورتيكويدات، TGF-
|
CD163، CD206، CD14، CD16، CD86، CXCR4، MerTK، TLR-1، TLR-8 | IL-10، TGF-
|
٣٢،٣٥ |
| M2d | تكاثر الورم، الغزو، وتكوين الأوعية الدموية، قمع المناعة | IL-6، TLR، ريجادينوسون، LPS | IL-10 (مرتفع)، IL-12 (منخفض)، VEGF، TNF-
|
IL-10، IL-6، TGF-
|
32 |
تمتلك الخلايا المناعية الشبيهة بـ M2 دورًا في تعزيز مقاومة الأدوية المتعددة في خلايا الورم
تقوم خلايا المناعة الشبيهة بـ M2 بتعديل مسارات الإشارة لتعزيز مقاومة الورم
| هدف | خلية مضيفة | استراتيجية | آلية | الحكام | |
| مسار الإشارة | PI3K/ Akt | MCF7 | تاموكسيفين | تنشيط إشارة PI3K/Akt/mTOR بواسطة CCL2 المفرز من TAM يعزز حلقة التغذية الراجعة لمقاومة الغدد الصماء في بيئة الورم | ٤٥ |
| MCF7 | تاموكسيفين | تنشيط خلايا سرطان الثدي عبر إشارات EGFR/PI3K/Akt من خلال زيادة تنظيم SGLT1 كاستجابة راجعة | ٤٦ | ||
| جاك/ ستات | بي تي 549، تي 47 دي | باكليتاكسيل | تعديل مسار إشارة IL-10/STAT3/bcl-2 | ٤٩ | |
| MKN45 | 5-FU | إفراز CCL8 لتنشيط فسفرة إشارة JAK1/STAT3 | 50 | ||
| جاجد1/نوتش | MCF7 | مثبط الأروماتاز | إعادة برمجة TAMs من خلال التعبير العالي لمسار Jagged1-Notch | ٥٩ | |
| NF-κB | تي إف كيه-1 | جيمسيتابين | مشتقات TAMs من نوع M2 tgf-
|
63 | |
| فرس النهر | جي بي إم | هنا | تعزيز التحفيز على استقطاب M2 بواسطة SOH | 66 | |
| إكسوسوم | miR-21 | MFC، MGC-803 | سيسبلاتين | تعديل نقل إشارة PTEN/PI3K/Akt بين الخلايا المناعية المرتبطة بالورم (TAMs) وخلايا السرطان بواسطة miR-21 المشتق من M2 عبر ApoE المحدد لـ M2 | 81 |
| OVCAR3، HO-8910 | العلاج الكيميائي | المعزز miR-21 الذي تم توصيله بواسطة M2 زاد من مقاومة OCA عبر إشارة PI3K/Akt | 82 | ||
| MSTRG.292666.16 | H1975 | أوسيمرتينيب | المستخلص المستمد من M2 MSTRG.292666.16 عزز مقاومة الأوزيمرتينيب من خلال تنظيم محور miR-6386-5p / MAPK8IP3 | 83 | |
| ميكرو RNA-155-5p | DLD1، HCT-8، HT-29، LoVo | 5-FU | تنشيط إشارة IL-6R/STAT3/miR-204-5p بواسطة miR-155-5p في TAMs من خلال تنظيم C/EBP
|
84 | |
| DLBCL-exo | OCI-LY1. OCI-LY3 | إبيروبيسين | يمكن أن تعزز الإكسوزومات الناتجة عن DLBCL استقطاب M2 من خلال تنشيط مسار الإشارات GP130/STAT3 والتعبير العالي عن IL-10 وCD206 وCD163. | 85 | |
| miR-588 | SGC7901 | سيسبلاتين | تحفيز مسار إشارة NF-kB بواسطة miR-588 من خلال استهداف جزئي لمرض السيليندروماتوز في سرطان المعدة لمنع الموت الخلوي المبرمج | 86 | |
| MCF7-exo | MCF7/S، MCF7/DOC | دوستكسل | توصيل الإكسوزومات وإطلاق P-gp يقومان بتصدير العوامل الكيميائية العلاجية خارج خلايا الورم | ٨٨ | |
| miR-365 | K989 | جيمسيتابين | نقل miR-365 في الخلايا المناعية المرتبطة بالورم أدى إلى مقاومة الجيمسيتابين | 89 | |
| miR-1246 | هيA8، سكوف3يب1، A2780 | باكليتاكسيل | الميكرو RNA-1246 ينشط
|
90 | |
| SOX2-OT | H1975 | مثبطات إنزيمات مستقبلات عامل نمو البشرة | SOX2-OT، كحاجز للميكرو RNA، استهدف نشاط miR-627-3p وزاد من تعبير Smads، مما أعاد برمجة TAMs. | 93 | |
| SNHG7 | H1299، SPC-A1 | دوستكسل | يحفز SNHG7 تقليل مستوى PTEN من خلال استقطاب CUL4A، مما ينشط مسار إشارة PI3K/Akt لتحفيز البلعمة والتوجه نحو M2. | 94 | |
| إنك-تالك | LN229، GL261، HMC3، BV-2 | تموزولوميد | يمكن أن يرتبط TALC الذي يتم توصيله بواسطة GBM بـ ENO1 لتنشيط إشارة p38MAPK، مما يزيد من إفراز C5/C5a لتعزيز استقطاب M2. | 96 | |
| LINC00337 | MCF7، MDA-MB-231 | باكليتاكسيل | تجنيد TAMs شبيهة بـ M2 بواسطة LINC00337 يحفز تطور الورم ومقاومة العلاج الكيميائي | 98 | |
| HCG18 | SW620 | سيتوكسيماب | تعزيز استقطاب M2 بواسطة HCG18 عبر محور miR-365a-3p/FOXO1/CSF-1 | 99 | |
| MIR155HG | كاكو2، إتش تي 29 | أوكساليبلاتين | تسريع تطور CRC بواسطة MIR155HG من خلال تعديل محور miR-650/ANXA2 يعزز مقاومة الأوكساليبلاتين | ١٠١ | |
| CRNDE | MFC، SGC7901 | سيسبلاتين | CRNDE الذي يتم توصيله بواسطة M2 يمنع يوبكويتين PTEN لتقليل قابلية السيكلوفسفاميد. | ١٠١ | |
| سيتوكين | TNF-
|
LM2 | العلاج الكيميائي | TNF-
|
١٠٩ |
| MDAMB231، 4T1، E0771 | بيفاسيزوماب | M2b TAMs تعزز انتشار الورم عبر TNF-
|
١١٠ |
| الجدول 2 (مستمر) | آليات تحفيز مقاومة الأدوية للأورام من خلال TAMs الشبيهة بـ M2 | |||||
| هدف | خلية مضيفة | استراتيجية | آلية | الحكام | |
| SMMC-7721 | دواء مضاد للأورام | تروج TAMs من نوع M2 لعملية التحول الظهاري والسرطانات الجذعية عبر مسار Wnt/
|
111 | ||
| إنترلوكين | هيب جي 2، إس إم إم سي 7721 | أوكساليبلاتين | تنشيط مسار إشارات CMA بواسطة TAMs شبيهة M2 عبر مسار IL-17/IL-17R | ١١٦ | |
| MCF7 | دوكسوروبيسين | تزيد استقطابية TAMs الشبيهة بـ M2 من حلقة IL-6 الجانبية بين TAMs وخلايا الورم | ١١٧ | ||
| M109، H1975، PC-9 | أوسيمرتينيب | نشطت طفرة EGFR T790M-cis-L792F مسار JAK/STAT3 لتعزيز الاستقطاب M2 | ١١٨ | ||
| كيموكين | HCT-8، HCT-116، SW620، SW480، DLD1 CT26، HT-29 | إيسي | تنشيط إشارة p65/STAT3-CSN5-PD-L1 بواسطة CCL5 المفرز من TAM يثبط استجابات خلايا T CD8 + في خلايا الورم | ١٢٢ | |
| DLD1، HT29 | 5-FU | إفراز CCL22 ينشط برنامج EMT، ومسار PI3K/Akt، والتموت الخلوي المسبب بواسطة الكاسبيز | 123 | ||
| IGF | SUIT2، ميا-باكا-2 | جيمسيتابين | تنشيط إشارات البقاء للأنسولين/IGF1R بواسطة TAMs الشبيهة بـ M2 أو بواسطة IGF، التي يتم تعديلها بواسطة M2، يعزز المقاومة للجيمسيتابين | ١٢٥ | |
| تي إم إي | CCL5 | DU145، PC-3 | باكليتاكسيل، دوكسوروبيسين | تنشيط مسار الإشارة المرتبط بـ STAT3 بواسطة CCL5 المفرز من TAM يزيد من مستوى Nanog | 128 |
| تريغ | CNE1، CNE2، 5-8 ف | هنا | تقوم الخلايا المناعية الشبيهة بـ M2 بتجنيد خلايا Treg الناضجة عن طريق إفراز CCL22 وCCL18 وتعزز تحويل الخلايا التائية الساذجة إلى Treg عن طريق إفراز TGF-
|
١٣٠ | |
| عامل مؤيد لتكوين الأوعية | VEGF | يو87-إم جي | بيفاسيزوماب | نقص VEGF يسبب تقليل MIF ويعزز استقطاب M2 | ١٣٧ |
| LN229، U251 | تموزولوميد | إفراز VEGF بواسطة البلعميات الشبيهة بـ M2 في ظروف نقص الأكسجين من خلال تنشيط مسار PI3K/Akt/Nrf2 | 138 | ||
| VEGF-A | شركة ذات مسؤولية محدودة | سيكلوفوسفاميد، سيسبلاتين | إفراز VEGF-A بواسطة TAMs الشبيهة بـ M2 لتعزيز فسفرة VEGFR2 | ١٣٩ | |
| A549 | دوكسوروبيسين | تعمل البلعميات الشبيهة بـ M2 على تعزيز تعبير VEGF-C و VEGFR3 مما يؤدي إلى تثبيط تعبير p53 و PTEN | ١٤٠ | ||
| مراكز خدمة العملاء | GSC20، GSC267 | جي بي إم | هنا | إفراز GDEs لتحفيز تحول وحيدات النوى إلى ماكروفاجات شبيهة بـ M2 عبر إشارة STAT3 | ١٤٤ |
| CSC | كال27 | فينكريستين | تعمل الخلايا المناعية الشبيهة بـ M2 على تعزيز خلايا OSCC لإنتاج خلايا شبيهة بالخلايا الجذعية وزيادة التعبير عن الجينات المرتبطة بالخصائص الجذعية. | 149 | |
| جي إس سي | جي بي إم | العلاج الكيميائي | تعمل البلعميات الشبيهة بـ M2 على تعزيز خلايا السرطان الجذعية للتعبير عن ميزات الجذعية من خلال تحفيز إشارات باراكرين PTN – PTPRZ1. | 150 | |
الإكسوزومات المشتقة من TME تستهدف TAMs الشبيهة بـ M2 لتعزيز مقاومة الورم
تعدل TAMs الشبيهة بـ M2 السيتوكينات لتعزيز مقاومة الورم
المجموعات التالية بناءً على وظائفها الأساسية العديدة: (1) ILs هي جزيئات حيوية مسؤولة عن نقل المعلومات المناعية بين الكريات البيضاء. من بين السيتوكينات في علم المناعة، تعتبر ILs الأكثر شيوعًا وأهمية؛ (2) CSFs هي سيتوكينات تحفز بشكل انتقائي تكاثر خلايا السلف المكونة للدم في الجسم الحي وكذلك في المختبر، مما يؤدي إلى تمايزها وتشكيل مستعمرات من سلالات خلايا محددة. تُسمى عوامل تحفيز المستعمرات بناءً على نطاق عملها مثل GCSF، M-CSF، GM-CSF؛ (3) هناك حوالي 7 أشكال مختلفة من IFNs: IFN-
تعدل البلعميات الشبيهة بـ M2 البيئة الدقيقة المناعية مما يسهل TME المثبط للمناعة لتعزيز مقاومة الورم
الاستجابات المناعية، ويسهل مقاومة أدوية الورم. علاوة على ذلك، فإن وجود PD-1 في TAMs يثبط نشاطها البلعمي والسمي ضد خلايا الورم، ويؤثر على استقطاب TAMs، مما يؤدي إلى نمط ظاهري M2 المثبط للمناعة، ويعزز آليات هروب المناعة لخلايا الورم، ويؤثر على البيئة الدقيقة للورم
تعدل الخلايا المناعية الشبيهة بـ M2 في الورم تكوين الأوعية الدموية في الورم لتعزيز مقاومة الورم
تعبير p53 و PTEN مع زيادة تعبير VEGF-C و VEGFR3، مما يقلل من موت الخلايا المبرمج في خلايا السرطان ويؤدي إلى مقاومة الدوكسوروبيسين.
تقوم خلايا المناعة الشبيهة بـ M2 وخلايا السرطان الجذعية بتعديل بعضها البعض لتعزيز مقاومة الورم
استهداف الخلايا المناعية الشبيهة بـ M2 للتغلب على مقاومة الورم
تقليل عدد TAMs الشبيهة بـ M2 في TME بشكل مباشر أو غير مباشر
أظهر الباكليتاكسيل في المرضى الذين يعانون من أورام صلبة متقدمة/نقيلية نقصًا في الخلايا المناعية المثبطة من نوع M2 في البيئة المجهرية للورم. ومع ذلك، لم ينتج عن استخدامه بمفرده أو بالاشتراك مع الباكليتاكسيل أي نشاط مضاد للورم ذي صلة سريريًا.
أدوية نانوية مضادة للورم تستهدف الخلايا المناعية الشبيهة بـ M2
| استراتيجية | عامل علاجي | نموذج الورم | آلية علاجية | الحكام |
| نانو مان سور | سورافينيب | نموذج الفأر O.T. لسرطان الكبد | توصيل مشترك لثاني أكسيد المنغنيز المنتج للأكسجين
|
170 |
| ليبوبسوم محمل بـ C6-سيراميد (LipC6) | سيراميد | نموذج الفأر O.T. لسرطان الكبد (HCC) | تنشيط المناعة المضادة للأورام من خلال تحفيز إعادة برمجة الخلايا المناعية المرتبطة بالورم عن طريق تنظيم إشارات ROS. | 171 |
| معقد الجسيمات النانوية/البكتيريا (Ec-PR848) | ريسيكيمود (R848) | نموذج الفأر O.T. 4T1 لسرطان الثدي | إعادة برمجة TAMs من خلال تنشيط TRL7/8 | 172 |
| مجس يستهدف TAM يتكون من جسم مضاد CD206 مرتبط بصبغة الفثالوسيانين القريبة من الأشعة تحت الحمراء (IRD-
|
سورافينيب | نموذج الفأر M.T. 4T1 لسرطان الثدي | نقصان الخلايا المناعية المرتبطة بالورم بواسطة العلاج الضوئي | 173 |
إعادة استقطاب البلعميات من نوع M2 إلى نوع M1
| تدخل | نوع ورم الحالة | حالة | معرف الحكومة | الحكام | |
| تقليل الخلايا المناعية الشبيهة بـ M2 في بيئة الورم | |||||
| مثبط JAK | روكسوليتينيب | مرضى المايلوما المتعددة الذين انتكسوا/مقاومون للعلاج | مكتمل | NCT03311854 | 155 |
| مثبط CSF-1R | بيكسيدارتينيب | الساركومة غير القابلة للاستئصال و MPNST | مكتمل | NCT02584647 | 151 |
| إيمكتوزوماب | الأورام الصلبة المتقدمة/النقيلية | مكتمل | NCT01494688 | ١٥٢ | |
| AMG 820 | الأورام الصلبة المتقدمة | مكتمل | NCT02713529 | 196 | |
| كابيراليزوماب | الميلانوما، سرطان الخلايا الكلوية، أو سرطان الرئة غير صغير الخلايا المقاوم لمضادات PD-1/PD-L1 | مكتمل | NCT03502330 | ١٩٧ | |
| مثبط CCR5 | مارافيروك | سرطان القولون المستقيمي النقيلي المقاوم للعلاج MMRp/MSS | جارٍ | NCT03274804 | 152 |
| مثبط TAM | سيترافاتينيب | سرطان الخلايا الكلوية الصافي المتقدم | مكتمل | NCT03015740 | 198 |
| مثبط VEGFR2 | أنلوتينيب | سرطان الرئة غير صغير الخلايا المتقدم | مكتمل | NCT03628521 | 199 |
| إعادة استقطاب TAMs من نوع M2 إلى نوع M1 | |||||
| مثبط CSF-1R | بيكسيدارتينيب | الأورام الصلبة المتقدمة والمقاومة للعلاج | مكتمل | NCT01525602 | ١٧٥ |
| ARRY-382 | الأورام الصلبة المتقدمة | مكتمل | NCT02880371 | ٢٠٠ | |
| BLZ945 | الأورام الصلبة المتقدمة | جارٍ | NCT02829723 | ١٧٥ | |
| LY3022855 | الأورام الصلبة المتقدمة | مكتمل | NCT02718911 | ٢٠١ | |
| مثبط CSF-1 | لاكوتوزوماب | سرطان الثدي الثلاثي السلبي المتقدم | لم يبدأ التوظيف بعد | NCT02435680 | ٢٠٢ |
| PD-0360324 | الأورام الصلبة المتقدمة محليًا أو المنتشرة | جارٍ | NCT02554812 | ١٧٥ | |
| مثبط BRD4 | AZD5153 | الأورام الصلبة المتكررة/المقاومة أو اللمفوما | مكتمل | NCT03205176 | ٢٠٣ |
| CD47-SIRP
|
إيفورباست | HNSCC | مكتمل | NCT03013218 | 178 |
| منشط CD40 | APX005M | الميلانوما، سرطان الرئة غير صغير الخلايا، أو سرطان الكلى | مكتمل | NCT03502330 | ١٩٧ |
| SEA-CD40 | الأورام الصلبة المتقدمة واللمفوما | مكتمل | NCT02376699. | 181 | |
| PI3K
|
IPI-549 | الأورام الصلبة المتقدمة | مكتمل | NCT02637531 | 204 |
| مثبط Src | داساتينيب | سرطان الرئة غير صغير الخلايا | جارٍ | NCT00826449 | ٢٠٥ |
| مثبطات HDAC | توسيدينوسات | ENKTCL في المراحل المبكرة ذات المخاطر المتوسطة والعالية | مكتمل | NCT04511351 | ٢٠٦ |
| مثبطات STAT 3 | دانفاتيرسن | الأورام الصلبة المتقدمة | مكتمل | NCT03394144 | ٢٠٧ |
| تي جي إف
|
غالونيسيرتيب | سرطان البنكرياس غير القابل للجراحة | مكتمل | NCT02734160 | ٢٠٨ |
| منبه TLR | فيدوتوليمود | الميلانوما النقيلي | مكتمل | NCT03084640 | ٢٠٩ |
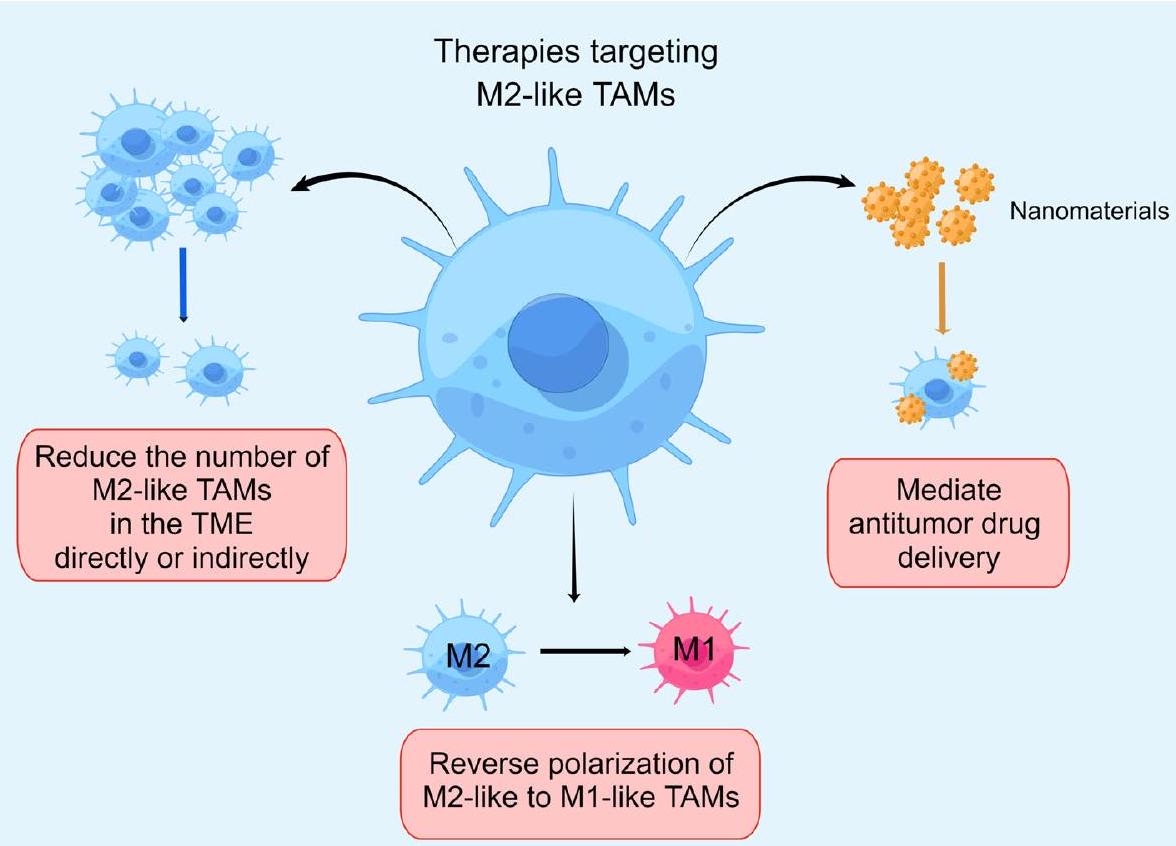
التجارب وأنشأت مجموعة متنوعة من الأدوية ذات الصلة التي تحفز الاستقطاب من ماكروفاجات شبيهة بـ M2 إلى ماكروفاجات شبيهة بـ M1
نقاش
ملخص التقرير
References
- Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 71, 209-249 (2021).
- Bayik, D. & Lathia, J. D. Cancer stem cell-immune cell crosstalk in tumour progression. Nat. Rev. Cancer 21, 526-536 (2021).
- Rey-Giraud, F., Hafner, M. & Ries, C. H. In vitro generation of monocyte-derived macrophages under serum-free conditions improves their tumor promoting functions. Plos One 7, e42656 (2012).
- Ruffell, B. & Coussens, L. M. Macrophages and therapeutic resistance in cancer. Cancer Cell. 27, 462-472 (2015).
- Altorki, N. K. et al. The lung microenvironment: an important regulator of tumour growth and metastasis. Nat. Rev. Cancer 19, 9-31 (2019).
- Morrison, C. Immuno-oncologists eye up macrophage targets. Nat. Rev. Drug Discov. 15, 373-374 (2016).
- Casanova-Acebes, M. et al. Tissue-resident macrophages provide a pro-tumorigenic niche to early NSCLC cells. Nature 595, 578-584 (2021).
- Yang, Q. et al. The role of tumor-associated macrophages (TAMs) in tumor progression and relevant advance in targeted therapy. Acta Pharm. Sin. B. 10, 2156-2170 (2020).
- Schmid, M. C. et al. Combined blockade of integrin-alpha4beta1 plus cytokines SDF-1alpha or IL-1beta potently inhibits tumor inflammation and growth. Cancer Res. 71, 6965-6975 (2011).
- Christofides, A. et al. The complex role of tumor-infiltrating macrophages. Nat. Immunol. 23, 1148-1156 (2022).
- Chamseddine, A. N., Assi, T., Mir, O. & Chouaib, S. Modulating tumor-associated macrophages to enhance the efficacy of immune checkpoint inhibitors: A TAM-pting approach. Pharmacol. Ther. 231, 107986 (2022).
- Locati, M., Curtale, G. & Mantovani, A. Diversity, mechanisms, and significance of macrophage plasticity. Annu. Rev. Pathol. 15, 123-147 (2020).
- Murray, P. J. et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14-20 (2014).
- Mantovani, A., Sica, A. & Locati, M. Macrophage polarization comes of age. Immunity 23, 344-346 (2005).
- Bleriot, C., Chakarov, S. & Ginhoux, F. Determinants of resident tissue macrophage identity and function. Immunity 52, 957-970 (2020).
- Mulder, K. et al. Cross-tissue single-cell landscape of human monocytes and macrophages in health and disease. Immunity 54, 1883-1900 (2021).
- Aibar, S. et al. SCENIC: single-cell regulatory network inference and clustering. Nat. Methods 14, 1083-1086 (2017).
- Ma, R. Y., Black, A. & Qian, B. Z. Macrophage diversity in cancer revisited in the era of single-cell omics. Trends Immunol. 43, 546-563 (2022).
- Schultze, J. L. & Schmidt, S. V. Molecular features of macrophage activation. Semin. Immunol. 27, 416-423 (2015).
- Jeannin, P., Paolini, L., Adam, C. & Delneste, Y. The roles of CSFs on the functional polarization of tumor-associated macrophages. FEBS J. 285, 680-699 (2018).
- Qian, B. Z. & Pollard, J. W. Macrophage diversity enhances tumor progression and metastasis. Cell 141, 39-51 (2010).
- Lin, Y., Xu, J. & Lan, H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J. Hematol. Oncol. 12, 76 (2019).
- Li, X. et al. Harnessing tumor-associated macrophages as aids for cancer immunotherapy. Mol. Cancer 18, 177 (2019).
- Pan, Y., Yu, Y., Wang, X. & Zhang, T. Tumor-associated macrophages in tumor immunity. Front. Immunol. 11, 583084 (2020).
- Gratchev, A. et al. Alternatively activated macrophages differentially express fibronectin and its splice variants and the extracellular matrix protein betalG-H3. Scand. J. Immunol. 53, 386-392 (2001).
- Hesse, M. et al. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. J. Immunol. 167, 6533-6544 (2001).
- Sunderkotter, C., Steinbrink, K., Goebeler, M., Bhardwaj, R. & Sorg, C. Macrophages and angiogenesis. J. Leukoc. Biol. 55, 410-422 (1994).
- Moeini, P. & Niedzwiedzka-Rystwej, P. Tumor-associated macrophages: Combination of therapies, the approach to improve cancer treatment. Int. J. Mol. Sci. 22, 7239 (2021).
- Yao, Z. et al. Imatinib prevents lung cancer metastasis by inhibiting M2-like polarization of macrophages. Pharmacol. Res. 133, 121-131 (2018).
- Sica, A. & Mantovani, A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787-795 (2012).
- DeNardo, D. G. & Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 19, 369-382 (2019).
- Gharavi, A. T., Hanjani, N. A., Movahed, E. & Doroudian, M. The role of macrophage subtypes and exosomes in immunomodulation. Cell. Mol. Biol. Lett. 27, 83 (2022).
- Zhang, Q. & Sioud, M. Tumor-associated macrophage subsets: Shaping polarization and targeting. Int. J. Mol. Sci. 24, 7493 (2023).
- Wang, Y. et al. Macrophage-derived extracellular vesicles: diverse mediators of pathology and therapeutics in multiple diseases. Cell Death Dis. 11, 924 (2020).
- Wang, L. X., Zhang, S. X., Wu, H. J., Rong, X. L. & Guo, J. M2b tumorassociated macrophage subsets: Shaping polarization and targeting. Dis. J. Leukoc. Biol. 106, 345-358 (2019).
- Hagemann, T. et al. Re-educating” tumor-associated macrophages by targeting NF-kappaB. J. Exp. Med. 205, 1261-1268 (2008).
- Daniel, B. et al. The IL-4/STAT6/PPARgamma signaling axis is driving the expansion of the RXR heterodimer cistrome, providing complex ligand responsiveness in macrophages. Nucleic Acids Res. 46, 4425-4439 (2018).
- Liu, H., Amakye, W. K. & Ren, J. Codonopsis pilosula polysaccharide in synergy with dacarbazine inhibits mouse melanoma by repolarizing M2-like tumor-associated macrophages into M1-like tumor-associated macrophages. Biomed. Pharmacother. 142, 112016 (2021).
- Vergadi, E., leronymaki, E., Lyroni, K., Vaporidi, K. & Tsatsanis, C. Akt signaling pathway in macrophage activation and M1/M2 polarization. J. Immunol. 198, 1006-1014 (2017).
- Zhao, Y. et al. IncRNA-Xist/miR-101-3p/KLF6/C/EBPalpha axis promotes TAM polarization to regulate cancer cell proliferation and migration. Mol. Ther. Nucleic Acids 23, 536-551 (2021).
- Schoumacher, M. & Burbridge, M. Key roles of AXL and MER receptor tyrosine kinases in resistance to multiple anticancer therapies. Curr. Oncol. Rep. 19, 19 (2017).
- Fruman, D. A. et al. The PI3K pathway in human disease. Cell 170, 605-635 (2017).
- Jiang, N. et al. Role of PI3K/AKT pathway in cancer: The framework of malignant behavior. Mol. Biol. Rep. 47, 4587-4629 (2020).
- Su, P. et al. Crosstalk between tumor-associated macrophages and tumor cells promotes chemoresistance via CXCL5/PI3K/AKT/ mTOR pathway in gastric cancer. Cancer Cell Int. 22, 290 (2022).
- Li, D. et al. Tumor-associated macrophages secrete CC-chemokine ligand 2 and induce tamoxifen resistance by activating PI3K/Akt/ mTOR in breast cancer. Cancer Sci. 111, 47-58 (2020).
- Niu, X. et al. Sodium/glucose cotransporter 1-dependent metabolic alterations induce tamoxifen resistance in breast cancer by promoting macrophage M2 polarization. Cell Death Dis. 12, 509 (2021).
- Owen, K. L., Brockwell, N. K. & Parker, B. S. JAK-STAT signaling: A double-edged Sword of immune regulation and cancer progression. Cancers 11, 2002 (2019).
- Hu, X., Li, J., Fu, M., Zhao, X. & Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 6, 402 (2021).
- Yang, C. et al. Increased drug resistance in breast cancer by tumorassociated macrophages through IL-10/STAT3/bcl-2 signaling pathway. Med. Oncol. 32, 352 (2015).
- He, Z. et al. Yes associated protein 1 promotes resistance to 5-fluorouracil in gastric cancer by regulating GLUT3-dependent glycometabolism reprogramming of tumor-associated macrophages. Arch. Biochem. Biophys. 702, 108838 (2021).
- Burotto, M., Chiou, V. L., Lee, J. M. & Kohn, E. C. The MAPK pathway across different malignancies: A new perspective. Cancer 120, 3446-3456 (2014).
- Vogelstein, B. et al. Cancer genome landscapes. Science 339, 1546-1558 (2013).
- Yang, S. H., Sharrocks, A. D. & Whitmarsh, A. J. MAP kinase signalling cascades and transcriptional regulation. Gene 513, 1-13 (2013).
- Zhou, D. et al. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell. Signal. 26, 192-197 (2014).
- Takebe, N., Nguyen, D. & Yang, S. X. Targeting notch signaling pathway in cancer: clinical development advances and challenges. Pharmacol. Ther. 141, 140-149 (2014).
- Palaga, T., Wongchana, W. & Kueanjinda, P. Notch signaling in macrophages in the context of cancer immunity. Front. Immunol. 9, 652 (2018).
- Huang, Y. H. et al. CREBBP/EP300 mutations promoted tumor progression in diffuse large B-cell lymphoma through altering tumor-associated macrophage polarization via FBXW7-NOTCHCCL2/CSF1 axis. Signal Transduct. Target. Ther. 6, 10 (2021).
- Tao, S., Chen, Q., Lin, C. & Dong, H. Linc00514 promotes breast cancer metastasis and M2 polarization of tumor-associated macrophages via Jagged1-mediated notch signaling pathway. J. Exp. Clin. Cancer Res. 39, 191 (2020).
- Liu, H. et al. Jagged1 promotes aromatase inhibitor resistance by modulating tumor-associated macrophage differentiation in breast cancer patients. Breast Cancer Res. Treat. 166, 95-107 (2017).
- Huang, F. et al. miR-148a-3p mediates notch signaling to promote the differentiation and M1 activation of macrophages. Front Immunol. 8, 1327 (2017).
- Rasmi, R. R., Sakthivel, K. M. & Guruvayoorappan, C. NF-kappaB inhibitors in treatment and prevention of lung cancer. Biomed. Pharmacother. 130, 110569 (2020).
- Usman, M. W. et al. Macrophages confer resistance to PI3K inhibitor GDC-0941 in breast cancer through the activation of NF-kappaB signaling. Cell Death Dis. 9, 809 (2018).
- Yang, T. et al. Macrophages-aPKC(i)-CCL5 feedback loop modulates the progression and chemoresistance in cholangiocarcinoma. J. Exp. Clin. Cancer Res. 41, 23 (2022).
- Badouel, C. & McNeill, H. SnapShot: The hippo signaling pathway. Cell 145, 484 (2011).
- Chen, J. et al. Tumor-derived exosomal miR-19b-3p facilitates M2 macrophage polarization and exosomal LINC00273 secretion to promote lung adenocarcinoma metastasis via Hippo pathway. Clin. Transl. Med. 11, e478 (2021).
- Kim, E. H. et al. Silence of hippo pathway associates with protumoral immunosuppression: Potential therapeutic target of glioblastomas. Cells 9, 1761 (2020).
- Urbanelli, L. et al. Signaling pathways in exosomes biogenesis, secretion and fate. Genes 4, 152-170 (2013).
- Kalluri, R. & LeBleu, V. S. The biology, function, and biomedical applications of exosomes. Science 367, eaau6977 (2020).
- Balaji, S., Kim, U., Muthukkaruppan, V. & Vanniarajan, A. Emerging role of tumor microenvironment derived exosomes in therapeutic resistance and metastasis through epithelial-to-mesenchymal transition. Life Sci. 280, 119750 (2021).
- Tan, S. et al. Exosomal miRNAs in tumor microenvironment. J. Exp. Clin. Cancer Res. 39, 67 (2020).
- Mashouri, L. et al. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol. Cancer 18, 75 (2019).
- Baig, M. S. et al. Tumor-derived exosomes in the regulation of macrophage polarization. Inflamm. Res. 69, 435-451 (2020).
- Mori, M. A., Ludwig, R. G., Garcia-Martin, R., Brandao, B. B. & Kahn, C. R. Extracellular miRNAs: From biomarkers to mediators of physiology and disease. Cell Metab. 30, 656-673 (2019).
- Ludwig, N., Yerneni, S. S., Razzo, B. M. & Whiteside, T. L. Exosomes from HNSCC promote angiogenesis through reprogramming of endothelial cells. Mol. Cancer Res. 16, 1798-1808 (2018).
- Cheng, Z. et al. Tumor-derived exosomes induced M2 macrophage polarization and promoted the metastasis of osteosarcoma cells through Tim-3. Arch. Med. Res. 52, 200-210 (2021).
- Mi, X. et al. M2 macrophage-derived exosomal IncRNA AFAP1-AS1 and MicroRNA-26a affect cell migration and metastasis in esophageal cancer. Mol. Ther. Nucleic Acids 22, 779-790 (2020).
- Dong, X. et al. Exosomes and breast cancer drug resistance. Cell Death Dis. 11, 987 (2020).
- Xin, L. et al. Transfer of LncRNA CRNDE in TAM-derived exosomes is linked with cisplatin resistance in gastric cancer. EMBO Rep. 22, e52124 (2021).
- Chen, S. W. et al. Cancer cell-derived exosomal circUSP7 induces CD8(+) T cell dysfunction and anti-PD1 resistance by regulating the miR-934/SHP2 axis in NSCLC. Mol. Cancer 20, 144 (2021).
- Wang, X. et al. Targeting feedback activation of signaling transduction pathways to overcome drug resistance in cancer. Drug Resist. Update 65, 100884 (2022).
- Zheng, P. et al. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J. Exp. Clin. Cancer Res. 36, 53 (2017).
- An, Y. & Yang, Q. MiR-21 modulates the polarization of macrophages and increases the effects of M2 macrophages on promoting the chemoresistance of ovarian cancer. Life Sci. 242, 117162 (2020).
- Wan, X. et al. Exosomes derived from M2 type tumor-associated macrophages promote osimertinib resistance in non-small cell lung cancer through MSTRG.292666.16-miR-6836-5p-MAPK8IP3 axis. Cancer Cell Int. 22, 83 (2022).
- Yin, Y. et al. The immune-microenvironment confers chemoresistance of colorectal cancer through macrophage-derived IL6. Clin. Cancer Res. 23, 7375-7387 (2017).
- Ling, H. Y. et al. Diffuse large B-cell lymphoma-derived exosomes push macrophage polarization toward M2 phenotype via GP130/ STAT3 signaling pathway. Chem. Biol. Interact. 352, 109779 (2022).
- Cui, H. Y. et al. Exosomal microRNA-588 from M2 polarized macrophages contributes to cisplatin resistance of gastric cancer cells. World J. Gastroenterol. 27, 6079-6092 (2021).
- Robey, R. W. et al. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 18, 452-464 (2018).
- Lv, M. M. et al. Exosomes mediate drug resistance transfer in MCF-7 breast cancer cells and a probable mechanism is delivery of P-glycoprotein. Tumour Biol. 35, 10773-10779 (2014).
- Binenbaum, Y. et al. Transfer of miRNA in macrophage-derived exosomes induces drug resistance in pancreatic adenocarcinoma. Cancer Res. 78, 5287-5299 (2018).
- Kanlikilicer, P. et al. Corrigendum to ‘Exosomal miRNA confers chemo resistance via targeting Cav1/p-gp/M2-type macrophage axis in ovarian cancer’ [EBioMedicine 38 (2018) 100-112]. Ebiomedicine 52, 102630 (2020).
- Akhade, V. S., Pal, D. & Kanduri, C. Long noncoding RNA: Genome organization and mechanism of action. Adv. Exp. Med. Biol. 1008, 47-74 (2017).
- Chen, F. et al. The functional roles of exosomes-derived long noncoding RNA in human cancer. Cancer Biol. Ther. 20, 583-592 (2019).
- Zhou, D. et al. Exosomal long non-coding RNA SOX2 overlapping transcript enhances the resistance to EGFR-TKIs in non-small cell lung cancer cell line H1975. Hum. Cell. 34, 1478-1489 (2021).
- Zhang, K. et al. Exosome-mediated transfer of SNHG7 enhances docetaxel resistance in lung adenocarcinoma. Cancer Lett. 526, 142-154 (2022).
- Meng, X. et al. DNA damage repair alterations modulate M2 polarization of microglia to remodel the tumor microenvironment via the p53-mediated MDK expression in glioma. Ebiomedicine 41, 185-199 (2019).
- Li, Z. et al. Glioblastoma cell-derived IncRNA-containing exosomes induce microglia to produce complement C5, promoting chemotherapy resistance. Cancer Immunol. Res. 9, 1383-1399 (2021).
- Yousefi, H. et al. Long noncoding RNAs and exosomal IncRNAs: classification, and mechanisms in breast cancer metastasis and drug resistance. Oncogene 39, 953-974 (2020).
- Xing, Z. et al. LINC00337 induces tumor development and chemoresistance to paclitaxel of breast cancer by recruiting M2 tumor-associated macrophages. Mol. Immunol. 138, 1-9 (2021).
- Gao, C., Hu, W., Zhao, J., Ni, X. & Xu, Y. LncRNA HCG18 promotes M2 macrophage polarization to accelerate cetuximab resistance in colorectal cancer through regulating miR-365a-3p/FOXO1/CSF-1 axis. Pathol. Res. Pract. 240, 154227 (2022).
- Wu, W. et al. The miR155HG/miR-185/ANXA2 loop contributes to glioblastoma growth and progression. J. Exp. Clin. Cancer Res. 38, 133 (2019).
- Zhou, L., Li, J., Liao, M., Zhang, Q. & Yang, M. LncRNA MIR155HG induces M2 macrophage polarization and drug resistance of colorectal cancer cells by regulating ANXA2. Cancer Immunol. Immunother. 71, 1075-1091 (2022).
- Ramani, T. et al. Cytokines: The good, the bad, and the deadly. Int. J. Toxicol. 34, 355-365 (2015).
- Kelso, A. Cytokines and their receptors: An overview. Ther. Drug Monit. 22, 40-43 (2000).
- Asadullah, K., Sterry, W. & Trefzer, U. Cytokines: Interleukin and interferon therapy in dermatology. Clin. Exp. Dermatol. 27, 578-584 (2002).
- Yang, S., Wang, J., Brand, D. D. & Zheng, S. G. Role of TNF-TNF receptor 2 signal in regulatory T cells and its therapeutic implications. Front. Immunol. 9, 784 (2018).
- Tan, W. et al. TNF-alpha is a potential therapeutic target to overcome sorafenib resistance in hepatocellular carcinoma. Ebiomedicine 40, 446-456 (2019).
- Laha, D., Grant, R., Mishra, P. & Nilubol, N. The role of tumor necrosis factor in manipulating the immunological response of tumor microenvironment. Front. Immunol. 12, 656908 (2021).
- Cruceriu, D., Baldasici, O., Balacescu, O. & Berindan-Neagoe, I. The dual role of tumor necrosis factor-alpha (TNF-alpha) in breast cancer: molecular insights and therapeutic approaches. Cell. Oncol. 43, 1-18 (2020).
- Acharyya, S. et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell 150, 165-178 (2012).
- Liu, Y. et al. Tumor necrosis factor alpha inhibition overcomes immunosuppressive M2b macrophage-induced bevacizumab resistance in triple-negative breast cancer. Cell Death Dis. 11, 993 (2020).
- Chen, Y. et al. TNF-alpha derived from M2 tumor-associated macrophages promotes epithelial-mesenchymal transition and cancer stemness through the Wnt/beta-catenin pathway in SMMC7721 hepatocellular carcinoma cells. Exp. Cell Res. 378, 41-50 (2019).
- Catalan-Dibene, J., McIntyre, L. L. & Zlotnik, A. Interleukin 30 to Interleukin 40. J. Interferon Cytokine Res. 38, 423-439 (2018).
- Gelfo, V. et al. Roles of IL-1 in cancer: From tumor progression to resistance to targeted therapies. Int. J. Mol. Sci. 21, 6009 (2020).
- Kumari, N., Dwarakanath, B. S., Das, A. & Bhatt, A. N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 37, 11553-11572 (2016).
- Zhang, Y. et al. Interleukin-17-induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. J. Exp. Med. 217, e20190354 (2020).
- Bin, G. Mechanism of M2-TAMs Induced Chemotherapy resistant in Hepatocellular Carcinoma Cells Through IL-17/IL-17R-CMA Pathway. North China University of Science and Technology, 2018.
- Li, J., He, K., Liu, P. & Xu, L. X. Iron participated in breast cancer chemoresistance by reinforcing IL-6 paracrine loop. Biochem. Biophys. Res. Commun. 475, 154-160 (2016).
- Sun, Y. et al. Blockade of STAT3/IL-4 overcomes EGFR T790M-cis-L792F-induced resistance to osimertinib via suppressing M2 macrophages polarization. Ebiomedicine 83, 104200 (2022).
- Hughes, C. E. & Nibbs, R. A guide to chemokines and their receptors. FEBS J. 285, 2944-2971 (2018).
- Lopez-Cotarelo, P., Gomez-Moreira, C., Criado-Garcia, O., Sanchez, L. & Rodriguez-Fernandez, J. L. Beyond chemoattraction: Multifunctionality of chemokine receptors in leukocytes. Trends Immunol. 38, 927-941 (2017).
- Susek, K. H., Karvouni, M., Alici, E. & Lundqvist, A. The role of CXC chemokine receptors 1-4 on immune cells in the tumor microenvironment. Front. Immunol. 9, 2159 (2018).
- Liu, C. et al. Macrophage-derived CCL5 facilitates immune escape of colorectal cancer cells via the p65/STAT3-CSN5-PD-L1 pathway. Cell Death Differ. 27, 1765-1781 (2020).
- Wei, C. et al. M2 macrophages confer resistance to 5-fluorouracil in colorectal cancer through the activation of CCL22/PI3K/AKT signaling. Oncotargets Ther. 12, 3051-3063 (2019).
- Nwabo, K. A. et al. Insulin-like growth factor-1 signaling in the tumor microenvironment: Carcinogenesis, cancer drug resistance, and therapeutic potential. Front. Endocrinol. 13, 927390 (2022).
- Ireland, L. et al. Chemoresistance in pancreatic cancer is driven by stroma-derived insulin-like growth factors. Cancer Res. 76, 6851-6863 (2016).
- Gordon, S. R. et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 545, 495-499 (2017).
- Li, W. et al. Correlation between PD-1/PD-L1 expression and polarization in tumor-associated macrophages: A key player in tumor immunotherapy. Cytokine Growth Factor Rev. 67, 49-57 (2022).
- Ma, J. et al. Tumor-associated macrophage-derived CCL5 promotes chemotherapy resistance and metastasis in prostatic cancer. Cell Biol. Int. 45, 2054-2062 (2021).
- Noy, R. & Pollard, J. W. Tumor-associated macrophages: from mechanisms to therapy. Immunity 41, 49-61 (2014).
- Wang, J. et al. Tumor cells induced-M2 macrophage favors accumulation of Treg in nasopharyngeal carcinoma. Int. J. Clin. Exp. Pathol. 10, 8389-8401 (2017).
- Fu, L. Q. et al. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell. Immunol. 353, 104119 (2020).
- Ferrara, N., Gerber, H. P. & LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 9, 669-676 (2003).
- Itatani, Y., Kawada, K., Yamamoto, T. & Sakai, Y. Resistance to antiangiogenic therapy in cancer-alterations to Anti-VEGF pathway. Int. J. Mol. Sci. 19, 1232 (2018).
- Siveen, K. S. et al. Vascular endothelial growth factor (VEGF) signaling in tumour vascularization: Potential and challenges. Curr. Vasc. Pharmacol. 15, 339-351 (2017).
- Ma, F. et al. Hypoxic macrophage-derived VEGF promotes proliferation and invasion of gastric cancer cells. Dig. Dis. Sci. 64, 3154-3163 (2019).
- Wheeler, K. C. et al. VEGF may contribute to macrophage recruitment and M2 polarization in the decidua. Plos One 13, e191040 (2018).
- Castro, B. A. et al. Macrophage migration inhibitory factor downregulation: a novel mechanism of resistance to anti-angiogenic therapy. Oncogene 36, 3749-3759 (2017).
- Zhang, G., Tao, X., Ji, B. & Gong, J. Hypoxia-driven M2-polarized macrophages facilitate cancer aggressiveness and temozolomide resistance in glioblastoma. Oxid. Med. Cell. Longev. 2022, 1614336 (2022).
- Stockmann, C. et al. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature 456, 814-818 (2008).
- Li, Y. et al. VEGFR3 inhibition chemosensitizes lung adenocarcinoma A549 cells in the tumor-associated macrophage microenvironment through upregulation of p53 and PTEN. Oncol. Rep. 38, 2761-2773 (2017).
- Wang, T. et al. JAK/STAT3-regulated fatty acid beta-oxidation is critical for breast cancer stem cell self-renewal and chemoresistance. Cell Metab. 27, 136-150 (2018).
- Wang, Q. et al. Cancer stem-like cells-oriented surface selfassembly to conquer radioresistance. Adv. Mater. 35, e2302916 (2023).
- Najafi, M., Mortezaee, K. & Majidpoor, J. Cancer stem cell (CSC) resistance drivers. Life Sci. 234, 116781 (2019).
- Gabrusiewicz, K. et al. Glioblastoma stem cell-derived exosomes induce M2 macrophages and PD-L1 expression on human monocytes. Oncoimmunology 7, e1412909 (2018).
- Vahidian, F. et al. Interactions between cancer stem cells, immune system and some environmental components: Friends or foes? Immunol. Lett. 208, 19-29 (2019).
- Li, L. et al. CD105: tumor diagnosis, prognostic marker and future tumor therapeutic target. Clin. Transl. Oncol. 24, 1447-1458 (2022).
- Hassn, M. M., Syafruddin, S. E., Mohtar, M. A. & Syahir, A. CD44: A multifunctional mediator of cancer progression. Biomolecules 11, 1850 (2021).
- Yaghobi, Z. et al. The role of CD44 in cancer chemoresistance: A concise review. Eur. J. Pharmacol. 903, 174147 (2021).
- Li, X. et al. CXCL12/CXCR4 pathway orchestrates CSC-like properties by CAF recruited tumor associated macrophage in OSCC. Exp. Cell Res. 378, 131-138 (2019).
- Shi, Y. et al. Tumour-associated macrophages secrete pleiotrophin to promote PTPRZ1 signalling in glioblastoma stem cells for tumour growth. Nat. Commun. 8, 15080 (2017).
- Manji, G. A. et al. A phase I study of the combination of pexidartinib and sirolimus to target tumor-associated macrophages in unresectable sarcoma and malignant peripheral nerve sheath tumors. Clin. Cancer Res. 27, 5519-5527 (2021).
- Gomez-Roca, C. A. et al. Phase I study of emactuzumab single agent or in combination with paclitaxel in patients with advanced/ metastatic solid tumors reveals depletion of immunosuppressive M2-like macrophages. Ann. Oncol. 30, 1381-1392 (2019).
- Guan, W. et al. Tumor-associated macrophage promotes the survival of cancer cells upon docetaxel chemotherapy via the CSF1/ CSF1R-CXCL12/CXCR4 axis in castration-resistant prostate cancer. Genes 12, 773 (2021).
- Yang, Y. I., Wang, Y. Y., Ahn, J. H., Kim, B. H. & Choi, J. H. CCL2 overexpression is associated with paclitaxel resistance in ovarian cancer cells via autocrine signaling and macrophage recruitment. Biomed. Pharmacother. 153, 113474 (2022).
- Berenson, J. R. et al. A phase 1 study of ruxolitinib, steroids and lenalidomide for relapsed/refractory multiple myeloma patients. Hematol. Oncol. 40, 906-913 (2022).
- Chen, H. et al. JAK1/2 pathway inhibition suppresses M2 polarization and overcomes resistance of myeloma to lenalidomide by reducing TRIB1, MUC1, CD44, CXCL12, and CXCR4 expression. Br. J. Haematol. 188, 283-294 (2020).
- Nie, Y. et al. Breast phyllodes tumors recruit and repolarize tumorassociated macrophages via secreting CCL5 to promote malignant progression, which can be inhibited by CCR5 inhibition therapy. Clin. Cancer Res. 25, 3873-3886 (2019).
- Halama, N. et al. Tumoral immune cell exploitation in colorectal cancer metastases can be targeted effectively by anti-CCR5 therapy in cancer patients. Cancer Cell. 29, 587-601 (2016).
- Huang, H. et al. The CCR5 antagonist maraviroc causes remission of pancreatic cancer liver metastasis in nude rats based on cell cycle inhibition and apoptosis induction. Cancer Lett. 474, 82-93 (2020).
- Yue, L. Chang Weiqing enhances chemosensitivity of colon cancer oxaliplatin by inhibiting M2 macrophages., Shanghai University of Traditional Chinese Medicine, 2019.
- Zhang, Z., Deng, Q., Xiao, C., Li, Z. & Yang, X. Rational design of nanotherapeutics based on the five features principle for potent elimination of cancer stem cells. Acc. Chem. Res. 55, 526-536 (2022).
- Li, Z. et al. Influence of nanomedicine mechanical properties on tumor targeting delivery. Chem. Soc. Rev. 49, 2273-2290 (2020).
- Ramesh, A., Brouillard, A. & Kulkarni, A. Supramolecular nanotherapeutics for macrophage immunotherapy. Acs Appl. Bio Mater. 4, 4653-4666 (2021).
- Choi, M. R. et al. A cellular Trojan Horse for delivery of therapeutic nanoparticles into tumors. Nano Lett. 7, 3759-3765 (2007).
- Kennedy, L. C. et al. A new era for cancer treatment: gold-nanoparticle-mediated thermal therapies. Small 7, 169-183 (2011).
- Cheng, Y. et al. Tumor associated macrophages and TAMs-based anti-tumor nanomedicines. Adv. Heathc. Mater 10, e2100590 (2021).
- Wang, Y. et al. Engineering endogenous tumor-associated macrophage-targeted biomimetic nano-rbc to reprogram tumor immunosuppressive microenvironment for enhanced chemoimmunotherapy. Adv. Mater. 33, e2103497 (2021).
- Wu, L. et al. Multiwalled carbon nanotubes prevent tumor metastasis through switching M2-polarized macrophages to M1 via TLR4. Activat. J. Biomed. Nanotechnol. 15, 138-150 (2019).
- Wei, Z., Zhang, X. & Zhang, Z. Engineered Iron-Based nanoplatform amplifies repolarization of M2-like tumor-associated macrophages for enhanced cancer immunotherapy. Chem. Eng. J. 433, 133847 (2022).
- Chang, C. C. et al. Nanoparticle delivery of
and antiangiogenic therapy to overcome hypoxia-driven tumor escape and suppress hepatocellular carcinoma. Acs Appl. Mater. Interfaces 12, 44407-44419 (2020). - Li, G. et al. Nanoliposome C6-ceramide increases the anti-tumor immune response and slows growth of liver tumors in mice. Gastroenterology 154, 1024-1036 (2018).
- Wei, B. et al. Polarization of tumor-associated macrophages by nanoparticle-loaded escherichia coli combined with immunogenic cell death for cancer immunotherapy. Nano Lett. 21, 4231-4240 (2021).
- Zhang, C. et al. Inhibition of tumor growth and metastasis by photoimmunotherapy targeting tumor-associated macrophage in a sorafenib-resistant tumor model. Biomaterials 84, 1-12 (2016).
- Zhang, S. Y. et al. Tumor-associated macrophages: A promising target for a cancer immunotherapeutic strategy. Pharmacol. Res. 161, 105111 (2020).
- Wesolowski, R. et al. Phase lb study of the combination of pexidartinib (PLX3397), a CSF-1R inhibitor, and paclitaxel in patients with advanced solid tumors. Ther. Adv. Med. Oncol. 11, 432498018 (2019).
- Omstead, A. N. et al. CSF-1R inhibitor, pexidartinib, sensitizes esophageal adenocarcinoma to PD-1 immune checkpoint blockade in a rat model. Carcinogenesis 43, 842-850 (2022).
- Li, X. et al. BRD4 inhibition by AZD5153 promotes antitumor immunity via depolarizing M2 macrophages. Front. Immunol. 11, 89 (2020).
- Kauder, S. E. et al. ALX148 blocks CD47 and enhances innate and adaptive antitumor immunity with a favorable safety profile. Plos One 13, e201832 (2018).
- Djureinovic, D., Wang, M. & Kluger, H. M. Agonistic CD40 antibodies in cancer treatment. Cancers 13, 1302 (2021).
- Kaneda, M. M. et al. PI3Kgamma is a molecular switch that controls immune suppression. Nature 539, 437-442 (2016).
- Coveler, A. L. et al. Phase 1 dose-escalation study of SEA-CD40: a non-fucosylated CD40 agonist, in advanced solid tumors and Iymphomas. J. Immunother. Cancer 11, e005584 (2023).
- Huang, W. C., Kuo, K. T., Wang, C. H., Yeh, C. T. & Wang, Y. Cisplatin resistant lung cancer cells promoted M2 polarization of tumorassociated macrophages via the Src/CD155/MIF. Funct. Pathw. J. Exp. Clin. Cancer Res. 38, 180 (2019).
- Zhang, P. et al. Optimized dose selective HDAC inhibitor tucidinostat overcomes anti-PD-L1 antibody resistance in experimental solid tumors. BMC Med. 20, 435 (2022).
- Christofides, A. et al. SHP-2 and PD-1-SHP-2 signaling regulate myeloid cell differentiation and antitumor responses. Nat. Immunol. 24, 55-68 (2023).
- Gao, J. et al. Allosteric inhibition reveals SHP2-mediated tumor immunosuppression in colon cancer by single-cell transcriptomics. Acta Pharm. Sin. B. 12, 149-166 (2022).
- Proia, T. A. et al. STAT3 antisense oligonucleotide remodels the suppressive tumor microenvironment to enhance immune activation in combination with Anti-PD-L1. Clin. Cancer Res. 26, 6335-6349 (2020).
- Yue, Y. et al. Novel myeloma patient-derived xenograft models unveil the potency of anlotinib to overcome bortezomib resistance. Front. Oncol. 12, 894279 (2022).
- Cao, H., Wang, D., Gao, R., Feng, Y. & Chen, L. Qi Ling decreases paclitaxel resistance in the human prostate cancer by reversing tumor-associated macrophages function. Aging (Albany Ny.). 14, 1812-1821 (2022).
- Xu, B., Zhang, H., Wang, Y., Huang, S. & Xu, L. Mechanism of Xu Li’s experiential prescription for the treatment of EGFR-positive NSCLC. Evid. -Based Complement Altern. Med. 2020, 8787153 (2020).
- Lai, Z. et al. Hedyotis diffusa Willd suppresses metastasis in 5-fluorouracil-resistant colorectal cancer cells by regulating the TGF-beta signaling pathway. Mol. Med. Rep. 16, 7752-7758 (2017).
- J, F. Zhi Zhen Fomula Regulating Tumor-associated Macrophages to Inhibit Drug Resistance of Colorectal Cancer., Shanghai University of Traditional Chinese Medicine, 2020.
- H, H. Study on the mechanism of triptolide inhibition on Cisplatin resistant epithelial ovarian cancer based on the polarization of TAMs., Nanchang University, 2020.
- Haider, T., Pandey, V., Banjare, N., Gupta, P. N. & Soni, V. Drug resistance in cancer: mechanisms and tackling strategies. Pharmacol. Rep. 72, 1125-1151 (2020).
- Zhong, L. et al. Small molecules in targeted cancer therapy: advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 6, 201 (2021).
- Bagchi, S., Yuan, R. & Engleman, E. G. Immune checkpoint inhibitors for the treatment of cancer: Clinical impact and mechanisms of response and resistance. Annu. Rev. Pathol. 16, 223-249 (2021).
- Razak, A. R. et al. Safety and efficacy of AMG 820, an anti-colonystimulating factor 1 receptor antibody, in combination with pembrolizumab in adults with advanced solid tumors. J. Immunother. Cancer 8, e001006 (2020).
- Weiss, S. A. et al. A Phase I Study of APX005M and Cabiralizumab with or without Nivolumab in Patients with Melanoma, Kidney Cancer, or Non-Small Cell Lung Cancer Resistant to Anti-PD-1/PDL1. Clin. Cancer Res. 27, 4757-4767 (2021).
- Msaouel, P. et al. A phase 1-2 trial of sitravatinib and nivolumab in clear cell renal cell carcinoma following progression on antiangiogenic therapy. Sci. Transl. Med. 14, eabm6420 (2022).
- Chu, T. et al. Phase 1b study of sintilimab plus anlotinib as first-line therapy in patients with advanced NSCLC. J. Thorac. Oncol. 16, 643-652 (2021).
- Johnson, M. et al. ARRY-382 in combination with pembrolizumab in patients with advanced solid tumors: Results from a phase 1b/ 2 study. Clin. Cancer Res. 28, 2517-2526 (2022).
- Falchook, G. S. et al. A phase 1a/1b trial of CSF-1R inhibitor LY3022855 in combination with durvalumab or tremelimumab in patients with advanced solid tumors. Invest. New. Drugs 39, 1284-1297 (2021).
- Kuemmel, S. et al. A randomized phase II sudy of anti-CSF1 monoclonal antibody lacnotuzumab (MCS110) combined with gemcitabine and carboplatin in advanced triple-negative breast cancer. Clin. Cancer Res. 28, 106-115 (2022).
- Hamilton, E. P. et al. First-in-human study of AZD5153, a small molecule inhibitor of bromodomain protein 4, in patients with relapsed/refractory malignant solid tumors and lymphoma. Mol. Cancer Ther. 22, 1154-1165 (2023).
- Tolcher, A. et al. Abstract CT089: IPI-549-01 – A Phase 1/1b, first-inhuman study of IPI-549, a PI3K-
inhibitor, as monotherapy and in combination with nivolumab in patients with advanced solid tumors. Cancer Res. 13, CT89 (2017). - Gold, K. A. et al. A phase I/II study combining erlotinib and dasatinib for non-small cell lung cancer. Oncologist 19, 1040-1041 (2014).
- Chai, Y. et al. First-line chemoradiation with or without chidamide (tucidinostat) in patients with intermediate- and high-risk early-stage extranodal nasal-type natural killer/T-cell lymphoma: A randomized phase 2 study in China. Int. J. Radiat. Oncol. Biol. Phys. 113, 833-844 (2022).
- Nishina, T. et al. Safety, tolerability, pharmacokinetics and preliminary antitumour activity of an antisense oligonucleotide targeting STAT3 (danvatirsen) as monotherapy and in combination with durvalumab in Japanese patients with advanced solid malignancies: A phase 1 study. BMJ Open. 12, e55718 (2022).
- Melisi, D. et al. Galunisertib plus gemcitabine vs. gemcitabine for first-line treatment of patients with unresectable pancreatic cancer. Br. J. Cancer 119, 1208-1214 (2018).
- Ribas, A. et al. Overcoming PD-1 blockade resistance with CpG-A toll-like receptor 9 agonist vidutolimod in patients with metastatic melanoma. Cancer Discov. 11, 2998-3007 (2021).
شكر وتقدير
مساهمات المؤلفين
المصالح المتنافسة
معلومات إضافية
المواد التكميلية متاحة على
https://doi.org/10.1038/s41698-024-00522-z.
http://www.nature.com/reprints
ملاحظة الناشر: تظل شركة سبرينغر ناتشر محايدة فيما يتعلق بالمطالبات القضائية في الخرائط المنشورة والانتماءات المؤسسية.
© المؤلف(ون) 2024
أول كلية طبية سريرية لجامعة غوانغتشو للطب الصيني، غوانغتشو، الصين. المستشفى الأول التابع لجامعة قوانغتشو للطب الصيني، قوانغتشو، الصين. أكاديمية قوانغدونغ للبحوث السريرية في الطب الصيني، قوانغتشو، الصين. مركز لينغنان للبحوث الطبية، جامعة الطب الصيني في قوانغتشو، قوانغتشو، الصين. البريد الإلكتروني: gzucmlinlz@163.com معهد هورميل
جامعة مينيسوتا
DOI: https://doi.org/10.1038/s41698-024-00522-z
PMID: https://pubmed.ncbi.nlm.nih.gov/38341519
Publication Date: 2024-02-10
Targeting M2-like tumor-associated macrophages is a potential therapeutic approach to overcome antitumor drug resistance
Abstract
Tumor drug resistance emerges from the interaction of two critical factors: tumor cellular heterogeneity and the immunosuppressive nature of the tumor microenvironment (TME). Tumorassociated macrophages (TAMs) constitute essential components of the TME. M2-like TAMs are essential in facilitating tumor metastasis as well as augmenting the drug resistance of tumors. This review encapsulates the mechanisms that M2-like TAMs use to promote tumor drug resistance. We also describe the emerging therapeutic strategies that are currently targeting M2-like TAMs in combination with other antitumor drugs, with some still undergoing clinical trial evaluation. Furthermore, we summarize and analyze various existing approaches for developing novel drugs that target M2-like TAMs to overcome tumor resistance, highlighting how targeting M2-like TAMs can effectively stop tumor growth, metastasis, and overcome tumor drug resistance.
mechanisms. Also, we analyze the future development potential of this novel therapeutic strategy.
Biological feature of TAMs
The origin of TAMs

The plasticity and polarization regulation of TAMs and their nomenclature
The phenotypes and functions of TAMs
opposite ends of a continuous dynamic TAM
polarization axis, illustrating their unique roles in the TME. Both M1-like TAMs and M2-like TAMs have specific cell surface markers and functional factors. M1-like TAMs in the TME undertake roles in promoting inflammation, inhibiting proliferation, eliminating pathogens, and anti-tumor responses. M2-like TAMs in the TME are involved in anti-inflammatory activities, promoting angiogenesis, influencing tissue regeneration and healing, and fostering tumor generation, proliferation, metastasis, and drug resistance. (By Figdraw).

The regulation mechanisms of M1-like /M2-like macrophages polarization
| Phenotypes | Functions | Stimulator factors | Markers | Excretion | References |
| M1 | Tumor resistance, pro-inflammatory, inhibit proliferation, eliminate pathogens | IFN-
|
IL-12 (high)/IL-10(low), CD80, CD86, CD60, MMPs, MHCII | CCL2, CCL3, CCL5, CXCL8, CXCL9, IL-1
|
32 |
| M2a | Allergy, profibrotic, cell proliferation, anti-inflammatory, wound healing, tumor progression | IL-4, IL-10, IL-13, (PPAR-
|
CD11b, CD45, CD86, CD14, CD206, CD163, CD209, IL-1R, Dectin-1, YM1, RELM
|
TGF-
|
32-35 |
| M2b | Th2 activation, immunoregulation, inflammation, tumor progression | IL-1
|
CD163, CD14, CD86, MHC-II, IL-10 (high), IL-12 (low), IL-6R | TNF-
|
32,35 |
| M2c | Immunoregulation, inflammatory response, wound healing, fibrosis | IL-6, IL-10, glucocorticoids, TGF-
|
CD163, CD206, CD14, CD16, CD86, CXCR4, MerTK, TLR-1、TLR-8 | IL-10, TGF-
|
32,35 |
| M2d | Tumor proliferation, invasion, and angiogenesis, immune suppression | IL-6, TLR, Regadenoson, LPS | IL-10 (high), IL-12 (low), VEGF, TNF-
|
IL-10, IL-6, TGF-
|
32 |
M2-like TAMs have involvement in promoting multidrug resistance in tumor cells
M2-like TAMs modulate signaling pathways to enhance tumor resistance
| Target | Host cell | Strategy | Mechanism | Refs | |
| Signaling pathway | PI3K/ Akt | MCF7 | Tamoxifen | Activation of PI3K/Akt/mTOR signaling by TAM-secreted CCL2 promotes the TME endocrine resistance feedback loop | 45 |
| MCF7 | Tamoxifen | Activation of breast cancer cells via EGFR/PI3K/Akt signaling by feedback upregulation of SGLT1 | 46 | ||
| JAK/ STAT | BT549, T47D | Paclitaxel | Modulation of IL-10/STAT3/bcl-2 signaling pathway | 49 | |
| MKN45 | 5-FU | Secretion of CCL8 to activate JAK1/STAT3 signaling phosphorylation | 50 | ||
| Jagged1/Notch | MCF7 | Aromatase inhibitor | Reprogramming TAMs through high expression of the Jagged1-Notch pathway | 59 | |
| NF-кВ | TFK-1 | Gemcitabine | M2-like TAMs-derived tgf-
|
63 | |
| Hippo | GBM | ICI | Promotion of promotes M2 polarization by SOH | 66 | |
| Exosome | miR-21 | MFC, MGC-803 | Cisplatin | Modulation of the transfer of PTEN/PI3K/Akt signaling between TAMs and cancer cells by M2derived miR-21 via the M2-specific ApoE | 81 |
| OVCAR3, HO-8910 | Chemotherapy | M2-delivered miR-21 enhanced OCA resistance via PI3K/Akt signaling | 82 | ||
| MSTRG.292666.16 | H1975 | Osimertinib | M2-derived MSTRG.292666.16 promoted osimertinib resistance by regulating the miR-6386-5p/ MAPK8IP3 axis | 83 | |
| miR-155-5p | DLD1, HCT-8, HT-29, LoVo | 5-FU | Activation of the IL-6R/STAT3/miR-204-5p signaling by miR-155-5p in TAMs through regulating C/EBP
|
84 | |
| DLBCL-exo | OCI-LY1. OCI-LY3 | Epirubicin | DLBCL-generated exosomes may promote M2 polarization through activating the GP130/STAT3 signaling pathway and highly expressing IL-10, CD206 and CD163 expression | 85 | |
| miR-588 | SGC7901 | Cisplatin | Stimulation of the NF-kB signaling pathway by miR-588 through partially targeting cylindromatosis in GC to prevent apoptosis | 86 | |
| MCF7-exo | MCF7/S, MCF7/DOC | Docetaxel | Exosomal delivery and the release of P-gp export the chemotherapeutic agents outside tumor cells | 88 | |
| miR-365 | K989 | Gemcitabine | Adoptive transfer of miR-365 in TAMs induced gemcitabine resistance | 89 | |
| miR-1246 | HeyA8, Skov3ip1, A2780 | Paclitaxel | miR-1246 actives
|
90 | |
| SOX2-OT | H1975 | EGFR-TKIs | SOX2-OT, as a miRNA sponge, targeted miR-627-3p activity and upregulated Smads expression, thereby reprogramming TAMs | 93 | |
| SNHG7 | H1299, SPC-A1 | Docetaxel | SNHG7 induces PTEN downregulation iva recruiting CUL4A, thus stimulates the PI3K/Akt signaling pathway to induce autophagy and M2 polarization. | 94 | |
| Inc-TALC | LN229, GL261, HMC3, BV-2 | Temozolomide | GBM-delivered Inc-TALC can bound to ENO1 to activate p38MAPK signaling, thus increasing C5/ C5a secretion to promote M2 polarization | 96 | |
| LINC00337 | MCF7,MDA-MB-231 | Paclitaxel | Recruitment of M2-like TAMs by LINC00337 induces tumor development and chemoresistance | 98 | |
| HCG18 | SW620 | Cetuximab | Promotion of M2 polarization by HCG18 via the miR-365a-3p/FOXO1/CSF-1 axis | 99 | |
| MIR155HG | Caco2, HT29 | Oxaliplatin | Acceleration of the CRC evolution by MIR155HG through modulating the miR-650/ANXA2 axis enhances oxaliplatin resistance | 101 | |
| CRNDE | MFC, SGC7901 | Cisplatin | M2-delivered CRNDE inhibits PTEN ubiquitination to reduce the susceptibility of cisplatin | 101 | |
| Cytokine | TNF-
|
LM2 | Chemotherapy | TNF-
|
109 |
| MDAMB231, 4T1, E0771 | Bevacizumab | M2b TAMs promote tumor metastasis via TNF-
|
110 |
| Table 2 (continued) | Mechanisms of inducing tumor drug resistance through M2-like TAMs | |||||
| Target | Host cell | Strategy | Mechanism | Refs | |
| SMMC-7721 | Anti-tumor drug | M2-like TAMs promote EMT and CSCs via the Wnt/
|
111 | ||
| Interleukin | HepG2, SMMC7721, | Oxaliplatin | Activation of CMA signaling pathway by M2-like TAMs via IL-17/IL-17R pathway | 116 | |
| MCF7 | Doxorubicin | The polarization of M2-like TAMs enhances the IL-6 paracrine loop between TAMs and tumor cells | 117 | ||
| M109, H1975, PC-9 | Osimertinib | EGFR T790M-cis-L792F activated the JAK/STAT3 pathway to promote the M2 polarization | 118 | ||
| Chemokine | HCT-8, HCT-116, SW620, SW480, DLD1 CT26, HT-29 | ICI | Activation of p65/STAT3-CSN5-PD-L1 signaling by TAM-secreted CCL5 inhibits CD8 + T cell responses in tumor cells | 122 | |
| DLD1, HT29 | 5-FU | Secretion of CCL22 activates the EMT program, PI3K/Akt pathway and Caspase-mediated apoptosis | 123 | ||
| IGF | SUIT2, MIA-PaCa-2 | Gemcitabine | Activation of insulin/IGF1R survival signaling by M2-like TAMs or by IGF, which is modulated by M2, enhances the resistance to gemcitabine | 125 | |
| TME | CCL5 | DU145, PC-3 | Paclitaxel, Doxorubicin | Activation of STAT3-related signaling pathway by TAM-secreted CCL5 upregulates Nanog | 128 |
| Treg | CNE1, CNE2, 5-8 F | ICI | M2-like TAMs recruit mature Treg by secreting CCL22, CCL18 and promote the conversion of naive T cells to Treg by secreting TGF-
|
130 | |
| Proangiogenic factor | VEGF | U87-MG | Bevacizumab | Depletion of VEGF causes MIF downregulation and promotes M2 polarization | 137 |
| LN229, U251 | Temozolomide | Secretion of VEGF by hypoxic M2-like macrophages through activating the PI3K/Akt/Nrf2 pathway | 138 | ||
| VEGF-A | LLC | Cyclophosphamide, Cisplatin | Secretion of VEGF-A by M2-like TAMs to promote VEGFR2 phosphorylation | 139 | |
| A549 | Doxorubicin | M2-like macrophages promote VEGF-C and VEGFR3 expression thereby inhibiting p53 and PTEN expression | 140 | ||
| CSCs | GSC20,GSC267 | GBM | ICI | secretion of GDEs to induce monocyte polarize into M2-like macrophages via STAT3 signaling | 144 |
| CSC | Cal27 | Vincristine | M2-like TAMs promote OSCC cells to produce csc-like cells and overexpress stemnessrelated genes | 149 | |
| GSC | GBM | Chemotherapy | M2-like macrophages promote CSCs to express stemness features by mediating PTN – PTPRZ1 paracrine signaling induction | 150 | |
TME-derived exosomes target M2-like TAMs to enhance tumor resistance
M2-like TAMs modulate cytokines to enhance tumor resistance
following groups based on their many primary functions: (1) ILs are biomolecules responsible for transmitting immunomodulatory information between leukocytes. Among cytokines in immunology, ILs are the most prevalent and significant; (2) CSFs are cytokines that selectively stimulate the hematopoietic progenitor cell proliferation in vivo as well as in vitro, causing them to differentiate and form colonies of specific cell lineages. The colony-stimulating factors are named based on their range of action as GCSF, M-CSF, GM-CSF; (3) There are approximately 7 different forms of IFNs: IFN-
M2-like TAMs modulate the immunological microenvironment facilitating immunosuppressive TME to enhance tumor resistance
immune responses, and facilitating tumor drug resistance. Furthermore, the presence of PD-1 in TAMs suppresses their phagocytic and cytotoxic activity against tumor cells, affects the polarization of TAMs, leading to an immunosuppressive M2 phenotype, enhances the immune escape mechanisms of tumor cells, and affects the tumor microenvironment
M2-like TAMs modulate tumor angiogenesis to enhance tumor resistance
expression of p53 and PTEN while upregulating the expression of VEGF-C and VEGFR3, thereby attenuating apoptosis in cancer cells and inducing DOX resistance
M2-like TAMs and CSCs modulate each other to enhance tumor resistance
Targeting M2-like TAMs to overcome tumor resistance
Reduce the number of M2-like TAMs in the TME directly or indirectly
paclitaxel in patients with advanced/metastatic solid tumors, demonstrated immunosuppressive M2-like TAMs depletion in the TME. However, neither in isolation nor in combination with paclitaxel did it result in clinically relevant antitumor activity
Antitumor nanodrugs targeting M2-like TAMs
| Strategy | Therapeutic agent | Tumor model | Therapeutic mechanism | Refs |
| NanoMnSor | sorafenib | O.T. mouse model of HCC | Co-deliver of oxygen-producing manganese dioxide
|
170 |
| a nanoliposome-loaded C6-ceremide (LipC6) | ceramide | O.T. mouse model of HCC | Activation of antitumor immunity by inducing TAMs reprogramming through regulating ROS signaling. | 171 |
| nanoparticles/bacteria complex (Ec-PR848) | Resiquimod (R848) | O.T. 4T1 mouse model of breast cancer | Reprogramming of TAMs by activating TRL7/8 | 172 |
| a TAM-targeting probe consisting of CD206 antibody coupled to nearinfrared phthalocyanine dye (IRD-
|
sorafenib | M.T. 4T1 mouse model of breast cancer | Depletion of TAMs by phototherapy | 173 |
Repolarize M2-like to M1-like macrophages
| Intervention | Statustumor type | Status | gov identifier | Refs | |
| Reduce M2-like TAMs in TME | |||||
| JAK inhibitor | Ruxolitinib | Relapsed/refractory multiple myeloma patients | Completed | NCT03311854 | 155 |
| CSF-1R inhibitor | Pexidartinib | Unresectable sarcoma and MPNST | Completed | NCT02584647 | 151 |
| Emactuzumab | Advanced/metastatic solid tumors | Completed | NCT01494688 | 152 | |
| AMG 820 | Advanced solid tumors | Completed | NCT02713529 | 196 | |
| Cabiralizumab | Melanoma, RCC, or NSCLC resistant to anti-PD-1/PD-L1 | Completed | NCT03502330 | 197 | |
| CCR5 inhibitor | Maraviroc | refractory MMRp/MSS metastatic CRC | Ongoing | NCT03274804 | 152 |
| TAM inhibitor | Sitravatinib | Advanced clear cell renal cell carcinoma | Completed | NCT03015740 | 198 |
| VEGFR2 inhibitor | Anlotinib | Advanced NSCLC | Completed | NCT03628521 | 199 |
| Repolarize M2-like to M1-like TAMs | |||||
| CSF-1R inhibitor | Pexidartinib | Advanced, treatment refractory solid tumors | Completed | NCT01525602 | 175 |
| ARRY-382 | Advanced solid tumors | Completed | NCT02880371 | 200 | |
| BLZ945 | Advanced solid tumors | Ongoing | NCT02829723 | 175 | |
| LY3022855 | Advanced solid tumors | Completed | NCT02718911 | 201 | |
| CSF-1 inhibitor | Lacnotuzumab | Advanced triple-negative breast cancer | Not yet recruiting | NCT02435680 | 202 |
| PD-0360324 | Locally advanced or metastatic solid tumors | Ongoing | NCT02554812 | 175 | |
| BRD4 inhibitor | AZD5153 | relapsed/refractory solid tumors or lymphoma | Completed | NCT03205176 | 203 |
| CD47-SIRP
|
Evorpacept | HNSCC | Completed | NCT03013218 | 178 |
| CD40 agonist | APX005M | Melanoma, NSCLC, or RCC | Completed | NCT03502330 | 197 |
| SEA-CD40 | Advanced solid tumors and lymphomas | Completed | NCT02376699. | 181 | |
| PI3K
|
IPI-549 | Advanced solid tumors | Completed | NCT02637531 | 204 |
| Src inhibitor | Dasatinib | NSCLC | Ongoing | NCT00826449 | 205 |
| HDAC inhibitor | Tucidinostat | intermediate- and high-risk early-stage ENKTCL | Completed | NCT04511351 | 206 |
| STAT 3 inhibitors | Danvatirsen | Advanced solid tumors | Completed | NCT03394144 | 207 |
| TGF
|
Galunisertib | Unresectable pancreatic cancer | Completed | NCT02734160 | 208 |
| TLR agonist | Vidutolimod | Metastatic melanoma | Completed | NCT03084640 | 209 |

experiments and created a variety of related drugs that induce the polarization from M2-like to M1-like macrophages
Discussion
Reporting summary
References
- Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 71, 209-249 (2021).
- Bayik, D. & Lathia, J. D. Cancer stem cell-immune cell crosstalk in tumour progression. Nat. Rev. Cancer 21, 526-536 (2021).
- Rey-Giraud, F., Hafner, M. & Ries, C. H. In vitro generation of monocyte-derived macrophages under serum-free conditions improves their tumor promoting functions. Plos One 7, e42656 (2012).
- Ruffell, B. & Coussens, L. M. Macrophages and therapeutic resistance in cancer. Cancer Cell. 27, 462-472 (2015).
- Altorki, N. K. et al. The lung microenvironment: an important regulator of tumour growth and metastasis. Nat. Rev. Cancer 19, 9-31 (2019).
- Morrison, C. Immuno-oncologists eye up macrophage targets. Nat. Rev. Drug Discov. 15, 373-374 (2016).
- Casanova-Acebes, M. et al. Tissue-resident macrophages provide a pro-tumorigenic niche to early NSCLC cells. Nature 595, 578-584 (2021).
- Yang, Q. et al. The role of tumor-associated macrophages (TAMs) in tumor progression and relevant advance in targeted therapy. Acta Pharm. Sin. B. 10, 2156-2170 (2020).
- Schmid, M. C. et al. Combined blockade of integrin-alpha4beta1 plus cytokines SDF-1alpha or IL-1beta potently inhibits tumor inflammation and growth. Cancer Res. 71, 6965-6975 (2011).
- Christofides, A. et al. The complex role of tumor-infiltrating macrophages. Nat. Immunol. 23, 1148-1156 (2022).
- Chamseddine, A. N., Assi, T., Mir, O. & Chouaib, S. Modulating tumor-associated macrophages to enhance the efficacy of immune checkpoint inhibitors: A TAM-pting approach. Pharmacol. Ther. 231, 107986 (2022).
- Locati, M., Curtale, G. & Mantovani, A. Diversity, mechanisms, and significance of macrophage plasticity. Annu. Rev. Pathol. 15, 123-147 (2020).
- Murray, P. J. et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14-20 (2014).
- Mantovani, A., Sica, A. & Locati, M. Macrophage polarization comes of age. Immunity 23, 344-346 (2005).
- Bleriot, C., Chakarov, S. & Ginhoux, F. Determinants of resident tissue macrophage identity and function. Immunity 52, 957-970 (2020).
- Mulder, K. et al. Cross-tissue single-cell landscape of human monocytes and macrophages in health and disease. Immunity 54, 1883-1900 (2021).
- Aibar, S. et al. SCENIC: single-cell regulatory network inference and clustering. Nat. Methods 14, 1083-1086 (2017).
- Ma, R. Y., Black, A. & Qian, B. Z. Macrophage diversity in cancer revisited in the era of single-cell omics. Trends Immunol. 43, 546-563 (2022).
- Schultze, J. L. & Schmidt, S. V. Molecular features of macrophage activation. Semin. Immunol. 27, 416-423 (2015).
- Jeannin, P., Paolini, L., Adam, C. & Delneste, Y. The roles of CSFs on the functional polarization of tumor-associated macrophages. FEBS J. 285, 680-699 (2018).
- Qian, B. Z. & Pollard, J. W. Macrophage diversity enhances tumor progression and metastasis. Cell 141, 39-51 (2010).
- Lin, Y., Xu, J. & Lan, H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J. Hematol. Oncol. 12, 76 (2019).
- Li, X. et al. Harnessing tumor-associated macrophages as aids for cancer immunotherapy. Mol. Cancer 18, 177 (2019).
- Pan, Y., Yu, Y., Wang, X. & Zhang, T. Tumor-associated macrophages in tumor immunity. Front. Immunol. 11, 583084 (2020).
- Gratchev, A. et al. Alternatively activated macrophages differentially express fibronectin and its splice variants and the extracellular matrix protein betalG-H3. Scand. J. Immunol. 53, 386-392 (2001).
- Hesse, M. et al. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. J. Immunol. 167, 6533-6544 (2001).
- Sunderkotter, C., Steinbrink, K., Goebeler, M., Bhardwaj, R. & Sorg, C. Macrophages and angiogenesis. J. Leukoc. Biol. 55, 410-422 (1994).
- Moeini, P. & Niedzwiedzka-Rystwej, P. Tumor-associated macrophages: Combination of therapies, the approach to improve cancer treatment. Int. J. Mol. Sci. 22, 7239 (2021).
- Yao, Z. et al. Imatinib prevents lung cancer metastasis by inhibiting M2-like polarization of macrophages. Pharmacol. Res. 133, 121-131 (2018).
- Sica, A. & Mantovani, A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787-795 (2012).
- DeNardo, D. G. & Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 19, 369-382 (2019).
- Gharavi, A. T., Hanjani, N. A., Movahed, E. & Doroudian, M. The role of macrophage subtypes and exosomes in immunomodulation. Cell. Mol. Biol. Lett. 27, 83 (2022).
- Zhang, Q. & Sioud, M. Tumor-associated macrophage subsets: Shaping polarization and targeting. Int. J. Mol. Sci. 24, 7493 (2023).
- Wang, Y. et al. Macrophage-derived extracellular vesicles: diverse mediators of pathology and therapeutics in multiple diseases. Cell Death Dis. 11, 924 (2020).
- Wang, L. X., Zhang, S. X., Wu, H. J., Rong, X. L. & Guo, J. M2b tumorassociated macrophage subsets: Shaping polarization and targeting. Dis. J. Leukoc. Biol. 106, 345-358 (2019).
- Hagemann, T. et al. Re-educating” tumor-associated macrophages by targeting NF-kappaB. J. Exp. Med. 205, 1261-1268 (2008).
- Daniel, B. et al. The IL-4/STAT6/PPARgamma signaling axis is driving the expansion of the RXR heterodimer cistrome, providing complex ligand responsiveness in macrophages. Nucleic Acids Res. 46, 4425-4439 (2018).
- Liu, H., Amakye, W. K. & Ren, J. Codonopsis pilosula polysaccharide in synergy with dacarbazine inhibits mouse melanoma by repolarizing M2-like tumor-associated macrophages into M1-like tumor-associated macrophages. Biomed. Pharmacother. 142, 112016 (2021).
- Vergadi, E., leronymaki, E., Lyroni, K., Vaporidi, K. & Tsatsanis, C. Akt signaling pathway in macrophage activation and M1/M2 polarization. J. Immunol. 198, 1006-1014 (2017).
- Zhao, Y. et al. IncRNA-Xist/miR-101-3p/KLF6/C/EBPalpha axis promotes TAM polarization to regulate cancer cell proliferation and migration. Mol. Ther. Nucleic Acids 23, 536-551 (2021).
- Schoumacher, M. & Burbridge, M. Key roles of AXL and MER receptor tyrosine kinases in resistance to multiple anticancer therapies. Curr. Oncol. Rep. 19, 19 (2017).
- Fruman, D. A. et al. The PI3K pathway in human disease. Cell 170, 605-635 (2017).
- Jiang, N. et al. Role of PI3K/AKT pathway in cancer: The framework of malignant behavior. Mol. Biol. Rep. 47, 4587-4629 (2020).
- Su, P. et al. Crosstalk between tumor-associated macrophages and tumor cells promotes chemoresistance via CXCL5/PI3K/AKT/ mTOR pathway in gastric cancer. Cancer Cell Int. 22, 290 (2022).
- Li, D. et al. Tumor-associated macrophages secrete CC-chemokine ligand 2 and induce tamoxifen resistance by activating PI3K/Akt/ mTOR in breast cancer. Cancer Sci. 111, 47-58 (2020).
- Niu, X. et al. Sodium/glucose cotransporter 1-dependent metabolic alterations induce tamoxifen resistance in breast cancer by promoting macrophage M2 polarization. Cell Death Dis. 12, 509 (2021).
- Owen, K. L., Brockwell, N. K. & Parker, B. S. JAK-STAT signaling: A double-edged Sword of immune regulation and cancer progression. Cancers 11, 2002 (2019).
- Hu, X., Li, J., Fu, M., Zhao, X. & Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 6, 402 (2021).
- Yang, C. et al. Increased drug resistance in breast cancer by tumorassociated macrophages through IL-10/STAT3/bcl-2 signaling pathway. Med. Oncol. 32, 352 (2015).
- He, Z. et al. Yes associated protein 1 promotes resistance to 5-fluorouracil in gastric cancer by regulating GLUT3-dependent glycometabolism reprogramming of tumor-associated macrophages. Arch. Biochem. Biophys. 702, 108838 (2021).
- Burotto, M., Chiou, V. L., Lee, J. M. & Kohn, E. C. The MAPK pathway across different malignancies: A new perspective. Cancer 120, 3446-3456 (2014).
- Vogelstein, B. et al. Cancer genome landscapes. Science 339, 1546-1558 (2013).
- Yang, S. H., Sharrocks, A. D. & Whitmarsh, A. J. MAP kinase signalling cascades and transcriptional regulation. Gene 513, 1-13 (2013).
- Zhou, D. et al. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell. Signal. 26, 192-197 (2014).
- Takebe, N., Nguyen, D. & Yang, S. X. Targeting notch signaling pathway in cancer: clinical development advances and challenges. Pharmacol. Ther. 141, 140-149 (2014).
- Palaga, T., Wongchana, W. & Kueanjinda, P. Notch signaling in macrophages in the context of cancer immunity. Front. Immunol. 9, 652 (2018).
- Huang, Y. H. et al. CREBBP/EP300 mutations promoted tumor progression in diffuse large B-cell lymphoma through altering tumor-associated macrophage polarization via FBXW7-NOTCHCCL2/CSF1 axis. Signal Transduct. Target. Ther. 6, 10 (2021).
- Tao, S., Chen, Q., Lin, C. & Dong, H. Linc00514 promotes breast cancer metastasis and M2 polarization of tumor-associated macrophages via Jagged1-mediated notch signaling pathway. J. Exp. Clin. Cancer Res. 39, 191 (2020).
- Liu, H. et al. Jagged1 promotes aromatase inhibitor resistance by modulating tumor-associated macrophage differentiation in breast cancer patients. Breast Cancer Res. Treat. 166, 95-107 (2017).
- Huang, F. et al. miR-148a-3p mediates notch signaling to promote the differentiation and M1 activation of macrophages. Front Immunol. 8, 1327 (2017).
- Rasmi, R. R., Sakthivel, K. M. & Guruvayoorappan, C. NF-kappaB inhibitors in treatment and prevention of lung cancer. Biomed. Pharmacother. 130, 110569 (2020).
- Usman, M. W. et al. Macrophages confer resistance to PI3K inhibitor GDC-0941 in breast cancer through the activation of NF-kappaB signaling. Cell Death Dis. 9, 809 (2018).
- Yang, T. et al. Macrophages-aPKC(i)-CCL5 feedback loop modulates the progression and chemoresistance in cholangiocarcinoma. J. Exp. Clin. Cancer Res. 41, 23 (2022).
- Badouel, C. & McNeill, H. SnapShot: The hippo signaling pathway. Cell 145, 484 (2011).
- Chen, J. et al. Tumor-derived exosomal miR-19b-3p facilitates M2 macrophage polarization and exosomal LINC00273 secretion to promote lung adenocarcinoma metastasis via Hippo pathway. Clin. Transl. Med. 11, e478 (2021).
- Kim, E. H. et al. Silence of hippo pathway associates with protumoral immunosuppression: Potential therapeutic target of glioblastomas. Cells 9, 1761 (2020).
- Urbanelli, L. et al. Signaling pathways in exosomes biogenesis, secretion and fate. Genes 4, 152-170 (2013).
- Kalluri, R. & LeBleu, V. S. The biology, function, and biomedical applications of exosomes. Science 367, eaau6977 (2020).
- Balaji, S., Kim, U., Muthukkaruppan, V. & Vanniarajan, A. Emerging role of tumor microenvironment derived exosomes in therapeutic resistance and metastasis through epithelial-to-mesenchymal transition. Life Sci. 280, 119750 (2021).
- Tan, S. et al. Exosomal miRNAs in tumor microenvironment. J. Exp. Clin. Cancer Res. 39, 67 (2020).
- Mashouri, L. et al. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol. Cancer 18, 75 (2019).
- Baig, M. S. et al. Tumor-derived exosomes in the regulation of macrophage polarization. Inflamm. Res. 69, 435-451 (2020).
- Mori, M. A., Ludwig, R. G., Garcia-Martin, R., Brandao, B. B. & Kahn, C. R. Extracellular miRNAs: From biomarkers to mediators of physiology and disease. Cell Metab. 30, 656-673 (2019).
- Ludwig, N., Yerneni, S. S., Razzo, B. M. & Whiteside, T. L. Exosomes from HNSCC promote angiogenesis through reprogramming of endothelial cells. Mol. Cancer Res. 16, 1798-1808 (2018).
- Cheng, Z. et al. Tumor-derived exosomes induced M2 macrophage polarization and promoted the metastasis of osteosarcoma cells through Tim-3. Arch. Med. Res. 52, 200-210 (2021).
- Mi, X. et al. M2 macrophage-derived exosomal IncRNA AFAP1-AS1 and MicroRNA-26a affect cell migration and metastasis in esophageal cancer. Mol. Ther. Nucleic Acids 22, 779-790 (2020).
- Dong, X. et al. Exosomes and breast cancer drug resistance. Cell Death Dis. 11, 987 (2020).
- Xin, L. et al. Transfer of LncRNA CRNDE in TAM-derived exosomes is linked with cisplatin resistance in gastric cancer. EMBO Rep. 22, e52124 (2021).
- Chen, S. W. et al. Cancer cell-derived exosomal circUSP7 induces CD8(+) T cell dysfunction and anti-PD1 resistance by regulating the miR-934/SHP2 axis in NSCLC. Mol. Cancer 20, 144 (2021).
- Wang, X. et al. Targeting feedback activation of signaling transduction pathways to overcome drug resistance in cancer. Drug Resist. Update 65, 100884 (2022).
- Zheng, P. et al. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J. Exp. Clin. Cancer Res. 36, 53 (2017).
- An, Y. & Yang, Q. MiR-21 modulates the polarization of macrophages and increases the effects of M2 macrophages on promoting the chemoresistance of ovarian cancer. Life Sci. 242, 117162 (2020).
- Wan, X. et al. Exosomes derived from M2 type tumor-associated macrophages promote osimertinib resistance in non-small cell lung cancer through MSTRG.292666.16-miR-6836-5p-MAPK8IP3 axis. Cancer Cell Int. 22, 83 (2022).
- Yin, Y. et al. The immune-microenvironment confers chemoresistance of colorectal cancer through macrophage-derived IL6. Clin. Cancer Res. 23, 7375-7387 (2017).
- Ling, H. Y. et al. Diffuse large B-cell lymphoma-derived exosomes push macrophage polarization toward M2 phenotype via GP130/ STAT3 signaling pathway. Chem. Biol. Interact. 352, 109779 (2022).
- Cui, H. Y. et al. Exosomal microRNA-588 from M2 polarized macrophages contributes to cisplatin resistance of gastric cancer cells. World J. Gastroenterol. 27, 6079-6092 (2021).
- Robey, R. W. et al. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 18, 452-464 (2018).
- Lv, M. M. et al. Exosomes mediate drug resistance transfer in MCF-7 breast cancer cells and a probable mechanism is delivery of P-glycoprotein. Tumour Biol. 35, 10773-10779 (2014).
- Binenbaum, Y. et al. Transfer of miRNA in macrophage-derived exosomes induces drug resistance in pancreatic adenocarcinoma. Cancer Res. 78, 5287-5299 (2018).
- Kanlikilicer, P. et al. Corrigendum to ‘Exosomal miRNA confers chemo resistance via targeting Cav1/p-gp/M2-type macrophage axis in ovarian cancer’ [EBioMedicine 38 (2018) 100-112]. Ebiomedicine 52, 102630 (2020).
- Akhade, V. S., Pal, D. & Kanduri, C. Long noncoding RNA: Genome organization and mechanism of action. Adv. Exp. Med. Biol. 1008, 47-74 (2017).
- Chen, F. et al. The functional roles of exosomes-derived long noncoding RNA in human cancer. Cancer Biol. Ther. 20, 583-592 (2019).
- Zhou, D. et al. Exosomal long non-coding RNA SOX2 overlapping transcript enhances the resistance to EGFR-TKIs in non-small cell lung cancer cell line H1975. Hum. Cell. 34, 1478-1489 (2021).
- Zhang, K. et al. Exosome-mediated transfer of SNHG7 enhances docetaxel resistance in lung adenocarcinoma. Cancer Lett. 526, 142-154 (2022).
- Meng, X. et al. DNA damage repair alterations modulate M2 polarization of microglia to remodel the tumor microenvironment via the p53-mediated MDK expression in glioma. Ebiomedicine 41, 185-199 (2019).
- Li, Z. et al. Glioblastoma cell-derived IncRNA-containing exosomes induce microglia to produce complement C5, promoting chemotherapy resistance. Cancer Immunol. Res. 9, 1383-1399 (2021).
- Yousefi, H. et al. Long noncoding RNAs and exosomal IncRNAs: classification, and mechanisms in breast cancer metastasis and drug resistance. Oncogene 39, 953-974 (2020).
- Xing, Z. et al. LINC00337 induces tumor development and chemoresistance to paclitaxel of breast cancer by recruiting M2 tumor-associated macrophages. Mol. Immunol. 138, 1-9 (2021).
- Gao, C., Hu, W., Zhao, J., Ni, X. & Xu, Y. LncRNA HCG18 promotes M2 macrophage polarization to accelerate cetuximab resistance in colorectal cancer through regulating miR-365a-3p/FOXO1/CSF-1 axis. Pathol. Res. Pract. 240, 154227 (2022).
- Wu, W. et al. The miR155HG/miR-185/ANXA2 loop contributes to glioblastoma growth and progression. J. Exp. Clin. Cancer Res. 38, 133 (2019).
- Zhou, L., Li, J., Liao, M., Zhang, Q. & Yang, M. LncRNA MIR155HG induces M2 macrophage polarization and drug resistance of colorectal cancer cells by regulating ANXA2. Cancer Immunol. Immunother. 71, 1075-1091 (2022).
- Ramani, T. et al. Cytokines: The good, the bad, and the deadly. Int. J. Toxicol. 34, 355-365 (2015).
- Kelso, A. Cytokines and their receptors: An overview. Ther. Drug Monit. 22, 40-43 (2000).
- Asadullah, K., Sterry, W. & Trefzer, U. Cytokines: Interleukin and interferon therapy in dermatology. Clin. Exp. Dermatol. 27, 578-584 (2002).
- Yang, S., Wang, J., Brand, D. D. & Zheng, S. G. Role of TNF-TNF receptor 2 signal in regulatory T cells and its therapeutic implications. Front. Immunol. 9, 784 (2018).
- Tan, W. et al. TNF-alpha is a potential therapeutic target to overcome sorafenib resistance in hepatocellular carcinoma. Ebiomedicine 40, 446-456 (2019).
- Laha, D., Grant, R., Mishra, P. & Nilubol, N. The role of tumor necrosis factor in manipulating the immunological response of tumor microenvironment. Front. Immunol. 12, 656908 (2021).
- Cruceriu, D., Baldasici, O., Balacescu, O. & Berindan-Neagoe, I. The dual role of tumor necrosis factor-alpha (TNF-alpha) in breast cancer: molecular insights and therapeutic approaches. Cell. Oncol. 43, 1-18 (2020).
- Acharyya, S. et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell 150, 165-178 (2012).
- Liu, Y. et al. Tumor necrosis factor alpha inhibition overcomes immunosuppressive M2b macrophage-induced bevacizumab resistance in triple-negative breast cancer. Cell Death Dis. 11, 993 (2020).
- Chen, Y. et al. TNF-alpha derived from M2 tumor-associated macrophages promotes epithelial-mesenchymal transition and cancer stemness through the Wnt/beta-catenin pathway in SMMC7721 hepatocellular carcinoma cells. Exp. Cell Res. 378, 41-50 (2019).
- Catalan-Dibene, J., McIntyre, L. L. & Zlotnik, A. Interleukin 30 to Interleukin 40. J. Interferon Cytokine Res. 38, 423-439 (2018).
- Gelfo, V. et al. Roles of IL-1 in cancer: From tumor progression to resistance to targeted therapies. Int. J. Mol. Sci. 21, 6009 (2020).
- Kumari, N., Dwarakanath, B. S., Das, A. & Bhatt, A. N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 37, 11553-11572 (2016).
- Zhang, Y. et al. Interleukin-17-induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. J. Exp. Med. 217, e20190354 (2020).
- Bin, G. Mechanism of M2-TAMs Induced Chemotherapy resistant in Hepatocellular Carcinoma Cells Through IL-17/IL-17R-CMA Pathway. North China University of Science and Technology, 2018.
- Li, J., He, K., Liu, P. & Xu, L. X. Iron participated in breast cancer chemoresistance by reinforcing IL-6 paracrine loop. Biochem. Biophys. Res. Commun. 475, 154-160 (2016).
- Sun, Y. et al. Blockade of STAT3/IL-4 overcomes EGFR T790M-cis-L792F-induced resistance to osimertinib via suppressing M2 macrophages polarization. Ebiomedicine 83, 104200 (2022).
- Hughes, C. E. & Nibbs, R. A guide to chemokines and their receptors. FEBS J. 285, 2944-2971 (2018).
- Lopez-Cotarelo, P., Gomez-Moreira, C., Criado-Garcia, O., Sanchez, L. & Rodriguez-Fernandez, J. L. Beyond chemoattraction: Multifunctionality of chemokine receptors in leukocytes. Trends Immunol. 38, 927-941 (2017).
- Susek, K. H., Karvouni, M., Alici, E. & Lundqvist, A. The role of CXC chemokine receptors 1-4 on immune cells in the tumor microenvironment. Front. Immunol. 9, 2159 (2018).
- Liu, C. et al. Macrophage-derived CCL5 facilitates immune escape of colorectal cancer cells via the p65/STAT3-CSN5-PD-L1 pathway. Cell Death Differ. 27, 1765-1781 (2020).
- Wei, C. et al. M2 macrophages confer resistance to 5-fluorouracil in colorectal cancer through the activation of CCL22/PI3K/AKT signaling. Oncotargets Ther. 12, 3051-3063 (2019).
- Nwabo, K. A. et al. Insulin-like growth factor-1 signaling in the tumor microenvironment: Carcinogenesis, cancer drug resistance, and therapeutic potential. Front. Endocrinol. 13, 927390 (2022).
- Ireland, L. et al. Chemoresistance in pancreatic cancer is driven by stroma-derived insulin-like growth factors. Cancer Res. 76, 6851-6863 (2016).
- Gordon, S. R. et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 545, 495-499 (2017).
- Li, W. et al. Correlation between PD-1/PD-L1 expression and polarization in tumor-associated macrophages: A key player in tumor immunotherapy. Cytokine Growth Factor Rev. 67, 49-57 (2022).
- Ma, J. et al. Tumor-associated macrophage-derived CCL5 promotes chemotherapy resistance and metastasis in prostatic cancer. Cell Biol. Int. 45, 2054-2062 (2021).
- Noy, R. & Pollard, J. W. Tumor-associated macrophages: from mechanisms to therapy. Immunity 41, 49-61 (2014).
- Wang, J. et al. Tumor cells induced-M2 macrophage favors accumulation of Treg in nasopharyngeal carcinoma. Int. J. Clin. Exp. Pathol. 10, 8389-8401 (2017).
- Fu, L. Q. et al. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell. Immunol. 353, 104119 (2020).
- Ferrara, N., Gerber, H. P. & LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 9, 669-676 (2003).
- Itatani, Y., Kawada, K., Yamamoto, T. & Sakai, Y. Resistance to antiangiogenic therapy in cancer-alterations to Anti-VEGF pathway. Int. J. Mol. Sci. 19, 1232 (2018).
- Siveen, K. S. et al. Vascular endothelial growth factor (VEGF) signaling in tumour vascularization: Potential and challenges. Curr. Vasc. Pharmacol. 15, 339-351 (2017).
- Ma, F. et al. Hypoxic macrophage-derived VEGF promotes proliferation and invasion of gastric cancer cells. Dig. Dis. Sci. 64, 3154-3163 (2019).
- Wheeler, K. C. et al. VEGF may contribute to macrophage recruitment and M2 polarization in the decidua. Plos One 13, e191040 (2018).
- Castro, B. A. et al. Macrophage migration inhibitory factor downregulation: a novel mechanism of resistance to anti-angiogenic therapy. Oncogene 36, 3749-3759 (2017).
- Zhang, G., Tao, X., Ji, B. & Gong, J. Hypoxia-driven M2-polarized macrophages facilitate cancer aggressiveness and temozolomide resistance in glioblastoma. Oxid. Med. Cell. Longev. 2022, 1614336 (2022).
- Stockmann, C. et al. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature 456, 814-818 (2008).
- Li, Y. et al. VEGFR3 inhibition chemosensitizes lung adenocarcinoma A549 cells in the tumor-associated macrophage microenvironment through upregulation of p53 and PTEN. Oncol. Rep. 38, 2761-2773 (2017).
- Wang, T. et al. JAK/STAT3-regulated fatty acid beta-oxidation is critical for breast cancer stem cell self-renewal and chemoresistance. Cell Metab. 27, 136-150 (2018).
- Wang, Q. et al. Cancer stem-like cells-oriented surface selfassembly to conquer radioresistance. Adv. Mater. 35, e2302916 (2023).
- Najafi, M., Mortezaee, K. & Majidpoor, J. Cancer stem cell (CSC) resistance drivers. Life Sci. 234, 116781 (2019).
- Gabrusiewicz, K. et al. Glioblastoma stem cell-derived exosomes induce M2 macrophages and PD-L1 expression on human monocytes. Oncoimmunology 7, e1412909 (2018).
- Vahidian, F. et al. Interactions between cancer stem cells, immune system and some environmental components: Friends or foes? Immunol. Lett. 208, 19-29 (2019).
- Li, L. et al. CD105: tumor diagnosis, prognostic marker and future tumor therapeutic target. Clin. Transl. Oncol. 24, 1447-1458 (2022).
- Hassn, M. M., Syafruddin, S. E., Mohtar, M. A. & Syahir, A. CD44: A multifunctional mediator of cancer progression. Biomolecules 11, 1850 (2021).
- Yaghobi, Z. et al. The role of CD44 in cancer chemoresistance: A concise review. Eur. J. Pharmacol. 903, 174147 (2021).
- Li, X. et al. CXCL12/CXCR4 pathway orchestrates CSC-like properties by CAF recruited tumor associated macrophage in OSCC. Exp. Cell Res. 378, 131-138 (2019).
- Shi, Y. et al. Tumour-associated macrophages secrete pleiotrophin to promote PTPRZ1 signalling in glioblastoma stem cells for tumour growth. Nat. Commun. 8, 15080 (2017).
- Manji, G. A. et al. A phase I study of the combination of pexidartinib and sirolimus to target tumor-associated macrophages in unresectable sarcoma and malignant peripheral nerve sheath tumors. Clin. Cancer Res. 27, 5519-5527 (2021).
- Gomez-Roca, C. A. et al. Phase I study of emactuzumab single agent or in combination with paclitaxel in patients with advanced/ metastatic solid tumors reveals depletion of immunosuppressive M2-like macrophages. Ann. Oncol. 30, 1381-1392 (2019).
- Guan, W. et al. Tumor-associated macrophage promotes the survival of cancer cells upon docetaxel chemotherapy via the CSF1/ CSF1R-CXCL12/CXCR4 axis in castration-resistant prostate cancer. Genes 12, 773 (2021).
- Yang, Y. I., Wang, Y. Y., Ahn, J. H., Kim, B. H. & Choi, J. H. CCL2 overexpression is associated with paclitaxel resistance in ovarian cancer cells via autocrine signaling and macrophage recruitment. Biomed. Pharmacother. 153, 113474 (2022).
- Berenson, J. R. et al. A phase 1 study of ruxolitinib, steroids and lenalidomide for relapsed/refractory multiple myeloma patients. Hematol. Oncol. 40, 906-913 (2022).
- Chen, H. et al. JAK1/2 pathway inhibition suppresses M2 polarization and overcomes resistance of myeloma to lenalidomide by reducing TRIB1, MUC1, CD44, CXCL12, and CXCR4 expression. Br. J. Haematol. 188, 283-294 (2020).
- Nie, Y. et al. Breast phyllodes tumors recruit and repolarize tumorassociated macrophages via secreting CCL5 to promote malignant progression, which can be inhibited by CCR5 inhibition therapy. Clin. Cancer Res. 25, 3873-3886 (2019).
- Halama, N. et al. Tumoral immune cell exploitation in colorectal cancer metastases can be targeted effectively by anti-CCR5 therapy in cancer patients. Cancer Cell. 29, 587-601 (2016).
- Huang, H. et al. The CCR5 antagonist maraviroc causes remission of pancreatic cancer liver metastasis in nude rats based on cell cycle inhibition and apoptosis induction. Cancer Lett. 474, 82-93 (2020).
- Yue, L. Chang Weiqing enhances chemosensitivity of colon cancer oxaliplatin by inhibiting M2 macrophages., Shanghai University of Traditional Chinese Medicine, 2019.
- Zhang, Z., Deng, Q., Xiao, C., Li, Z. & Yang, X. Rational design of nanotherapeutics based on the five features principle for potent elimination of cancer stem cells. Acc. Chem. Res. 55, 526-536 (2022).
- Li, Z. et al. Influence of nanomedicine mechanical properties on tumor targeting delivery. Chem. Soc. Rev. 49, 2273-2290 (2020).
- Ramesh, A., Brouillard, A. & Kulkarni, A. Supramolecular nanotherapeutics for macrophage immunotherapy. Acs Appl. Bio Mater. 4, 4653-4666 (2021).
- Choi, M. R. et al. A cellular Trojan Horse for delivery of therapeutic nanoparticles into tumors. Nano Lett. 7, 3759-3765 (2007).
- Kennedy, L. C. et al. A new era for cancer treatment: gold-nanoparticle-mediated thermal therapies. Small 7, 169-183 (2011).
- Cheng, Y. et al. Tumor associated macrophages and TAMs-based anti-tumor nanomedicines. Adv. Heathc. Mater 10, e2100590 (2021).
- Wang, Y. et al. Engineering endogenous tumor-associated macrophage-targeted biomimetic nano-rbc to reprogram tumor immunosuppressive microenvironment for enhanced chemoimmunotherapy. Adv. Mater. 33, e2103497 (2021).
- Wu, L. et al. Multiwalled carbon nanotubes prevent tumor metastasis through switching M2-polarized macrophages to M1 via TLR4. Activat. J. Biomed. Nanotechnol. 15, 138-150 (2019).
- Wei, Z., Zhang, X. & Zhang, Z. Engineered Iron-Based nanoplatform amplifies repolarization of M2-like tumor-associated macrophages for enhanced cancer immunotherapy. Chem. Eng. J. 433, 133847 (2022).
- Chang, C. C. et al. Nanoparticle delivery of
and antiangiogenic therapy to overcome hypoxia-driven tumor escape and suppress hepatocellular carcinoma. Acs Appl. Mater. Interfaces 12, 44407-44419 (2020). - Li, G. et al. Nanoliposome C6-ceramide increases the anti-tumor immune response and slows growth of liver tumors in mice. Gastroenterology 154, 1024-1036 (2018).
- Wei, B. et al. Polarization of tumor-associated macrophages by nanoparticle-loaded escherichia coli combined with immunogenic cell death for cancer immunotherapy. Nano Lett. 21, 4231-4240 (2021).
- Zhang, C. et al. Inhibition of tumor growth and metastasis by photoimmunotherapy targeting tumor-associated macrophage in a sorafenib-resistant tumor model. Biomaterials 84, 1-12 (2016).
- Zhang, S. Y. et al. Tumor-associated macrophages: A promising target for a cancer immunotherapeutic strategy. Pharmacol. Res. 161, 105111 (2020).
- Wesolowski, R. et al. Phase lb study of the combination of pexidartinib (PLX3397), a CSF-1R inhibitor, and paclitaxel in patients with advanced solid tumors. Ther. Adv. Med. Oncol. 11, 432498018 (2019).
- Omstead, A. N. et al. CSF-1R inhibitor, pexidartinib, sensitizes esophageal adenocarcinoma to PD-1 immune checkpoint blockade in a rat model. Carcinogenesis 43, 842-850 (2022).
- Li, X. et al. BRD4 inhibition by AZD5153 promotes antitumor immunity via depolarizing M2 macrophages. Front. Immunol. 11, 89 (2020).
- Kauder, S. E. et al. ALX148 blocks CD47 and enhances innate and adaptive antitumor immunity with a favorable safety profile. Plos One 13, e201832 (2018).
- Djureinovic, D., Wang, M. & Kluger, H. M. Agonistic CD40 antibodies in cancer treatment. Cancers 13, 1302 (2021).
- Kaneda, M. M. et al. PI3Kgamma is a molecular switch that controls immune suppression. Nature 539, 437-442 (2016).
- Coveler, A. L. et al. Phase 1 dose-escalation study of SEA-CD40: a non-fucosylated CD40 agonist, in advanced solid tumors and Iymphomas. J. Immunother. Cancer 11, e005584 (2023).
- Huang, W. C., Kuo, K. T., Wang, C. H., Yeh, C. T. & Wang, Y. Cisplatin resistant lung cancer cells promoted M2 polarization of tumorassociated macrophages via the Src/CD155/MIF. Funct. Pathw. J. Exp. Clin. Cancer Res. 38, 180 (2019).
- Zhang, P. et al. Optimized dose selective HDAC inhibitor tucidinostat overcomes anti-PD-L1 antibody resistance in experimental solid tumors. BMC Med. 20, 435 (2022).
- Christofides, A. et al. SHP-2 and PD-1-SHP-2 signaling regulate myeloid cell differentiation and antitumor responses. Nat. Immunol. 24, 55-68 (2023).
- Gao, J. et al. Allosteric inhibition reveals SHP2-mediated tumor immunosuppression in colon cancer by single-cell transcriptomics. Acta Pharm. Sin. B. 12, 149-166 (2022).
- Proia, T. A. et al. STAT3 antisense oligonucleotide remodels the suppressive tumor microenvironment to enhance immune activation in combination with Anti-PD-L1. Clin. Cancer Res. 26, 6335-6349 (2020).
- Yue, Y. et al. Novel myeloma patient-derived xenograft models unveil the potency of anlotinib to overcome bortezomib resistance. Front. Oncol. 12, 894279 (2022).
- Cao, H., Wang, D., Gao, R., Feng, Y. & Chen, L. Qi Ling decreases paclitaxel resistance in the human prostate cancer by reversing tumor-associated macrophages function. Aging (Albany Ny.). 14, 1812-1821 (2022).
- Xu, B., Zhang, H., Wang, Y., Huang, S. & Xu, L. Mechanism of Xu Li’s experiential prescription for the treatment of EGFR-positive NSCLC. Evid. -Based Complement Altern. Med. 2020, 8787153 (2020).
- Lai, Z. et al. Hedyotis diffusa Willd suppresses metastasis in 5-fluorouracil-resistant colorectal cancer cells by regulating the TGF-beta signaling pathway. Mol. Med. Rep. 16, 7752-7758 (2017).
- J, F. Zhi Zhen Fomula Regulating Tumor-associated Macrophages to Inhibit Drug Resistance of Colorectal Cancer., Shanghai University of Traditional Chinese Medicine, 2020.
- H, H. Study on the mechanism of triptolide inhibition on Cisplatin resistant epithelial ovarian cancer based on the polarization of TAMs., Nanchang University, 2020.
- Haider, T., Pandey, V., Banjare, N., Gupta, P. N. & Soni, V. Drug resistance in cancer: mechanisms and tackling strategies. Pharmacol. Rep. 72, 1125-1151 (2020).
- Zhong, L. et al. Small molecules in targeted cancer therapy: advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 6, 201 (2021).
- Bagchi, S., Yuan, R. & Engleman, E. G. Immune checkpoint inhibitors for the treatment of cancer: Clinical impact and mechanisms of response and resistance. Annu. Rev. Pathol. 16, 223-249 (2021).
- Razak, A. R. et al. Safety and efficacy of AMG 820, an anti-colonystimulating factor 1 receptor antibody, in combination with pembrolizumab in adults with advanced solid tumors. J. Immunother. Cancer 8, e001006 (2020).
- Weiss, S. A. et al. A Phase I Study of APX005M and Cabiralizumab with or without Nivolumab in Patients with Melanoma, Kidney Cancer, or Non-Small Cell Lung Cancer Resistant to Anti-PD-1/PDL1. Clin. Cancer Res. 27, 4757-4767 (2021).
- Msaouel, P. et al. A phase 1-2 trial of sitravatinib and nivolumab in clear cell renal cell carcinoma following progression on antiangiogenic therapy. Sci. Transl. Med. 14, eabm6420 (2022).
- Chu, T. et al. Phase 1b study of sintilimab plus anlotinib as first-line therapy in patients with advanced NSCLC. J. Thorac. Oncol. 16, 643-652 (2021).
- Johnson, M. et al. ARRY-382 in combination with pembrolizumab in patients with advanced solid tumors: Results from a phase 1b/ 2 study. Clin. Cancer Res. 28, 2517-2526 (2022).
- Falchook, G. S. et al. A phase 1a/1b trial of CSF-1R inhibitor LY3022855 in combination with durvalumab or tremelimumab in patients with advanced solid tumors. Invest. New. Drugs 39, 1284-1297 (2021).
- Kuemmel, S. et al. A randomized phase II sudy of anti-CSF1 monoclonal antibody lacnotuzumab (MCS110) combined with gemcitabine and carboplatin in advanced triple-negative breast cancer. Clin. Cancer Res. 28, 106-115 (2022).
- Hamilton, E. P. et al. First-in-human study of AZD5153, a small molecule inhibitor of bromodomain protein 4, in patients with relapsed/refractory malignant solid tumors and lymphoma. Mol. Cancer Ther. 22, 1154-1165 (2023).
- Tolcher, A. et al. Abstract CT089: IPI-549-01 – A Phase 1/1b, first-inhuman study of IPI-549, a PI3K-
inhibitor, as monotherapy and in combination with nivolumab in patients with advanced solid tumors. Cancer Res. 13, CT89 (2017). - Gold, K. A. et al. A phase I/II study combining erlotinib and dasatinib for non-small cell lung cancer. Oncologist 19, 1040-1041 (2014).
- Chai, Y. et al. First-line chemoradiation with or without chidamide (tucidinostat) in patients with intermediate- and high-risk early-stage extranodal nasal-type natural killer/T-cell lymphoma: A randomized phase 2 study in China. Int. J. Radiat. Oncol. Biol. Phys. 113, 833-844 (2022).
- Nishina, T. et al. Safety, tolerability, pharmacokinetics and preliminary antitumour activity of an antisense oligonucleotide targeting STAT3 (danvatirsen) as monotherapy and in combination with durvalumab in Japanese patients with advanced solid malignancies: A phase 1 study. BMJ Open. 12, e55718 (2022).
- Melisi, D. et al. Galunisertib plus gemcitabine vs. gemcitabine for first-line treatment of patients with unresectable pancreatic cancer. Br. J. Cancer 119, 1208-1214 (2018).
- Ribas, A. et al. Overcoming PD-1 blockade resistance with CpG-A toll-like receptor 9 agonist vidutolimod in patients with metastatic melanoma. Cancer Discov. 11, 2998-3007 (2021).
Acknowledgements
Author contributions
Competing interests
Additional information
supplementary material available at
https://doi.org/10.1038/s41698-024-00522-z.
http://www.nature.com/reprints
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
© The Author(s) 2024
The First Clinical Medical School of Guangzhou University of Chinese Medicine, Guangzhou, China. The First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China. Guangdong Clinical Research Academy of Chinese Medicine, Guangzhou, China. Lingnan Medical Research Center, Guangzhou University of Chinese Medicine, Guangzhou, China. e-mail: gzucmlinlz@163.com The Hormel Institute
University of Minnesota
