DOI: https://doi.org/10.1186/s43094-024-00594-5
تاريخ النشر: 2024-02-12
الأهمية الصيدلانية للأسس شيف: نظرة عامة
الملخص
تعتبر قواعد شيف مجموعة متنوعة من المركبات العضوية ذات الأهمية الصيدلانية الكبيرة بسبب وجود روابط مزدوجة بين الكربون والنيتروجين (
الملخص الرسومي
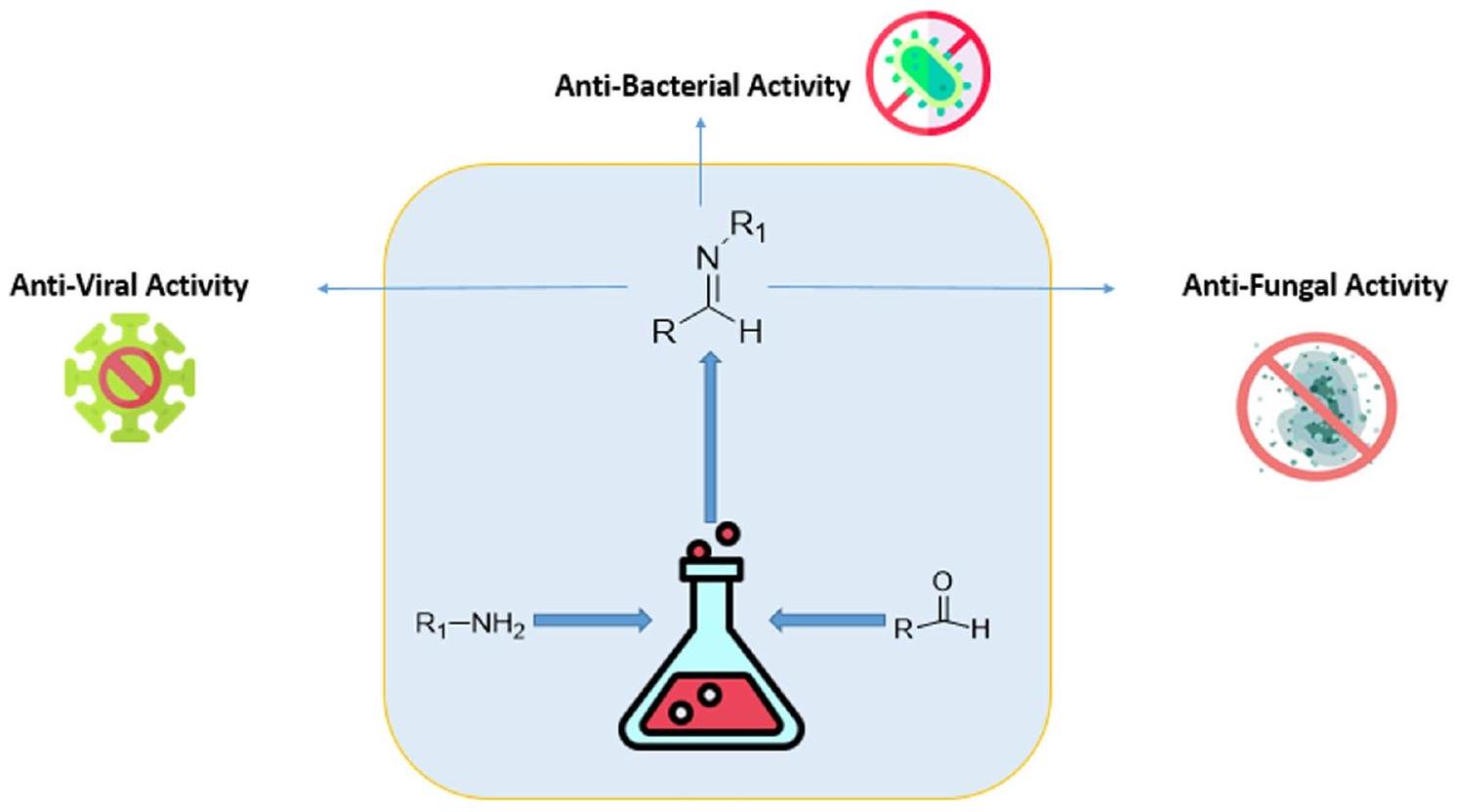
الخلفية
تعود اكتشاف قواعد شيف إلى القرن التاسع عشر حيث قام كيميائي يُدعى “هوجو شيف” بتوثيق تفاعل يظهر التكثيف بين الأمينات ومجموعات الكربونيل الوظيفية [13، 18-21]. في العصر الحديث، شهد هذا المجال من البحث العلمي حول كيمياء تنسيق قواعد شيف نموًا وتوسعًا كبيرين [22، 23]. لقد تم الاعتراف على نطاق واسع بأهمية معقدات قواعد شيف في مجالات
علوم المواد، التطبيقات الطبية الحيوية، الكيمياء الحيوية غير العضوية، عمليات التضمين، الكيمياء فوق الجزيئية، التحفيز، والفصل، وتوليد جزيئات ذات خصائص وهياكل استثنائية تم الاعتراف بها على نطاق واسع وتم فحصها بشكل مكثف في الأدبيات الحالية [24-27]. وثقت الأدبيات استخدام قواعد شيف المشتقة من الساليسيلدهيد كعوامل لتنظيم نمو النباتات، بالإضافة إلى إظهار خصائص مضادة للبكتيريا أو مضادة للفطريات [28-30]. لقد أظهرت قواعد شيف أيضًا استخدامات تحليلية. تساعد أهمية هذه القواعد في تفسير الآليات الصعبة داخل الأنظمة البيولوجية وتعطي معنى صحيحًا لنشاط مجموعة الإيمين. كما توفر أيضًا نشاطًا واسع الطيف ضد عدة أنواع، بما في ذلك Candida و Plasmopora viticola و Trichophyton gypsum و Staphylococcus aureus و Erysiphe graminis و Mycobacterium و Albicans و Bacillus polymyxa و Escherichia coli [31-34].
النص الرئيسي
الأهمية الصيدلانية لقواعد شيف
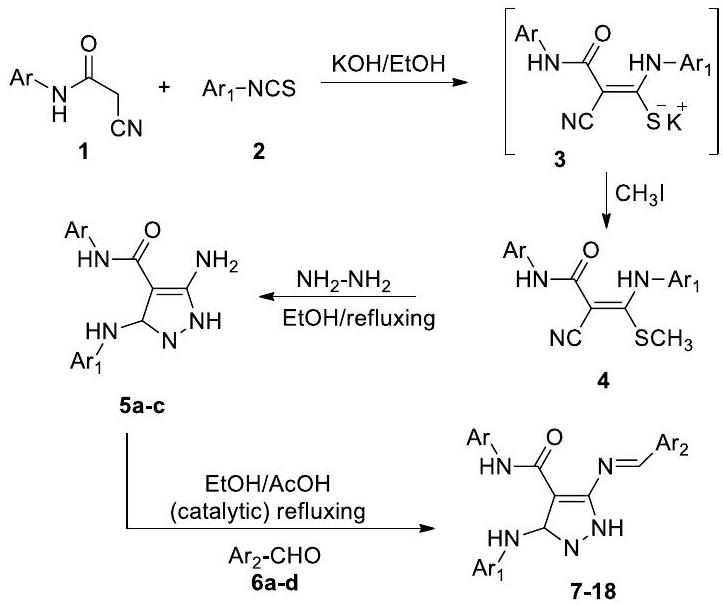
مضاد للبكتيريا
تم إنتاج قواعد شيف المشتقة من السليلوز من خلال تفاعل التكثيف بين p-أمينوفينول ومجموعات الألدهيد وقد أظهرت فعالية مضادة للبكتيريا ضد Staphylococcus aureus و Escherichia coli و Enterococcus faecalis [62].


“تحليل سطح الجهد الكهربائي الجزيئي (MEPS)” و”علاقة الهيكل-النشاط الكمي (QSAR).” تشير نتائج تحليل QSAR، التي شملت نظرية الكثافة الوظيفية (DFT) ووصفًا حجميًا وكارهًا للماء، إلى أن المركبات التي تظهر كارهة للماء أعلى ولحظة ثنائية القطب أقل لها خصائص مضادة للبكتيريا ضد “Klebsiella pneumoniae ATCC700603” [69].
21a-27a: $mathrm{X}=mathrm{H}, mathrm{R}^{1}=mathrm{OMe}, mathrm{H}, mathrm{Me} ; mathrm{R}^{2}=mathrm{H}, mathrm{OMe}^{1}$
$mathrm{R}^{3}=mathrm{H}, mathrm{Br}, mathrm{OMe}, mathrm{R}^{4}=mathrm{COOH}, mathrm{Br}, mathrm{H}$
21b-25b: $mathrm{X}=mathrm{Br}, mathrm{R}^{1}=mathrm{OMe}, mathrm{H}, mathrm{Cl}, mathrm{Me}$;
$mathrm{R}^{2}=mathrm{H}, mathrm{OMe}, mathrm{R}^{3}=mathrm{H}, mathrm{R}^{4}=mathrm{COOH}, mathrm{Br}$


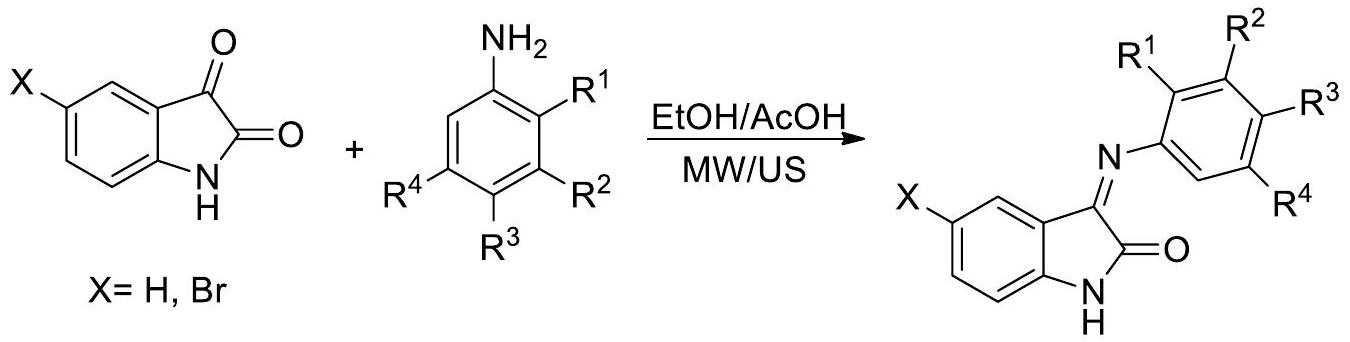
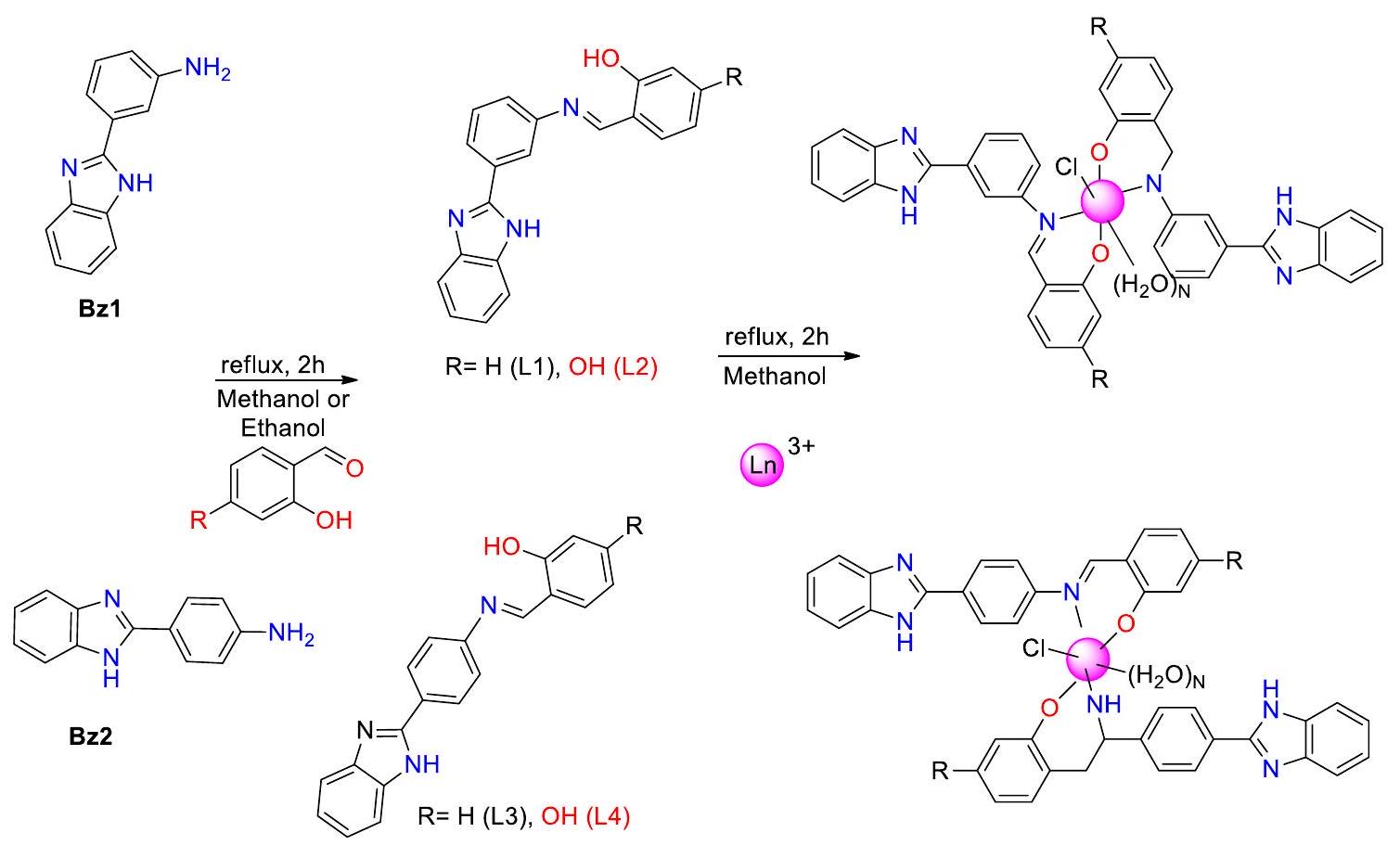
باختصار، تم تصنيع سلسلة من معقدات اللانثانيد الجديدة باستخدام ربيطات قاعدة شيف، جنبًا إلى جنب مع “جزء البنزيميدازول.” تم توصيف هذه المعقدات بدقة باستخدام عدة منهجيات تحليلية، مما يضمن تحديدها وفهمها بشكل لا لبس فيه. تم إجراء تجارب أولية للمركبات لتقييم استخدامات دوائية متنوعة للمواد قيد البحث. تم إجراء هذه التقييمات باستخدام مجموعة من الاختبارات البيولوجية،
بما في ذلك اختبارات النشاط المضاد للتكاثر، والنشاط المضاد للطفيليات، والنشاط المضاد للبكتيريا. تشير النتائج إلى أن الأنشطة البيولوجية للمركبات تتأثر بالتعديلات الهيكلية. على وجه التحديد، أظهرت الرابطة L1 وL2، جنبًا إلى جنب مع معقداتها المعدنية، قيم الحد الأدنى من التركيز المثبط (MIC) مقارنةً بالرابطة L3 وL4 ومعقداتها المعنية. قد يُعزى هذا التباين في قيم MIC إلى الاستبدالات على “حلقة الأمينوفينول.” بنفس الطريقة، تم إظهار أن المركبات كان لها تأثير على سيولة غشاء الخلية من خلال تعديل المنطقة الكارهة للماء داخل ثنائي طبقة الدهون. تشير هذه الملاحظة إلى وجود علاقة محتملة بين هذه التعديلات والاستخدامات العلاجية المحتملة لهذه المركبات. يمكن أن تكون النتيجة المذكورة أعلاه نقطة مرجعية قيمة في المساعي المستقبلية الهادفة إلى تطوير أدوية جديدة. تستكشف التحقيقات الجارية في المختبر الآن تأثير التعديلات على الهيكل الجزيئي.
مضاد للفطريات
تأثرت مشتقات الإينولين بالعديد من المعايير، مثل درجة الاستبدال (DS)، بالإضافة إلى كمية وموقع مجموعات الهيدروكسيل الفينولية. تُظهر المنتجات الموضحة في هذه المخطوطة وعدًا كبيرًا كمواد حيوية تتميز بنشاط حيوي مناسب وتوافق حيوي. يستدعي الأمر مزيدًا من التحقيق في العلاقة بين التركيب والنشاط في جهود البحث المستقبلية [75، 76] (المخطط 7).
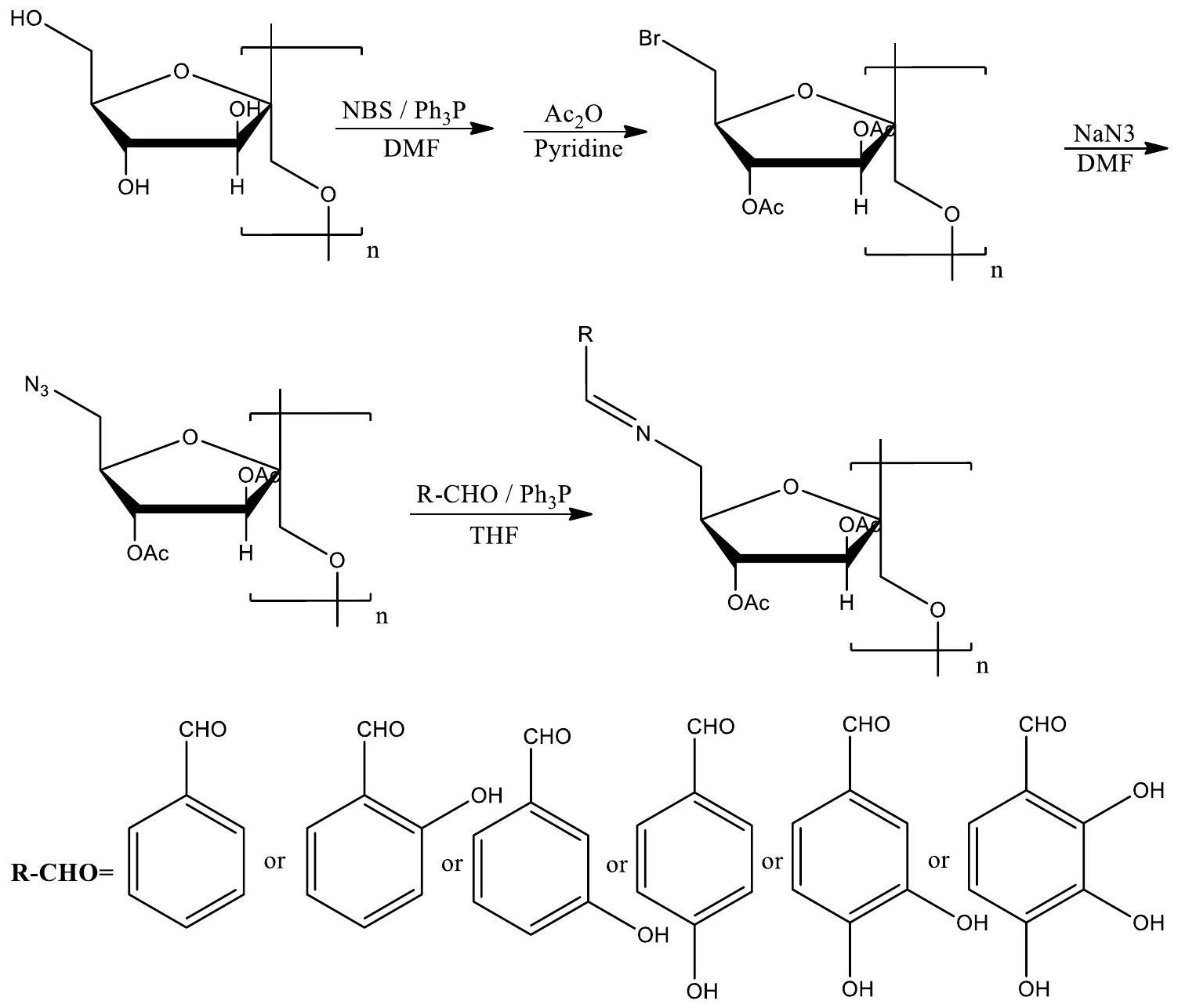

تم إجراء الدراسة لتقييم الفعالية المضادة للفطريات ضد ثلاثة فطريات نباتية مرضية شائعة، وهي Botrytis cinerea وFusarium oxysporum f. sp. cucumerinum وFusarium oxysporum f. sp. niveum، من خلال قياسات الهيفات في المختبر. تشير نتائج الدراسة إلى أن النشاط المضاد للفطريات لقاعدتي شيف المزدوجتين من الكيتوزان
مضاد الفيروسات
و
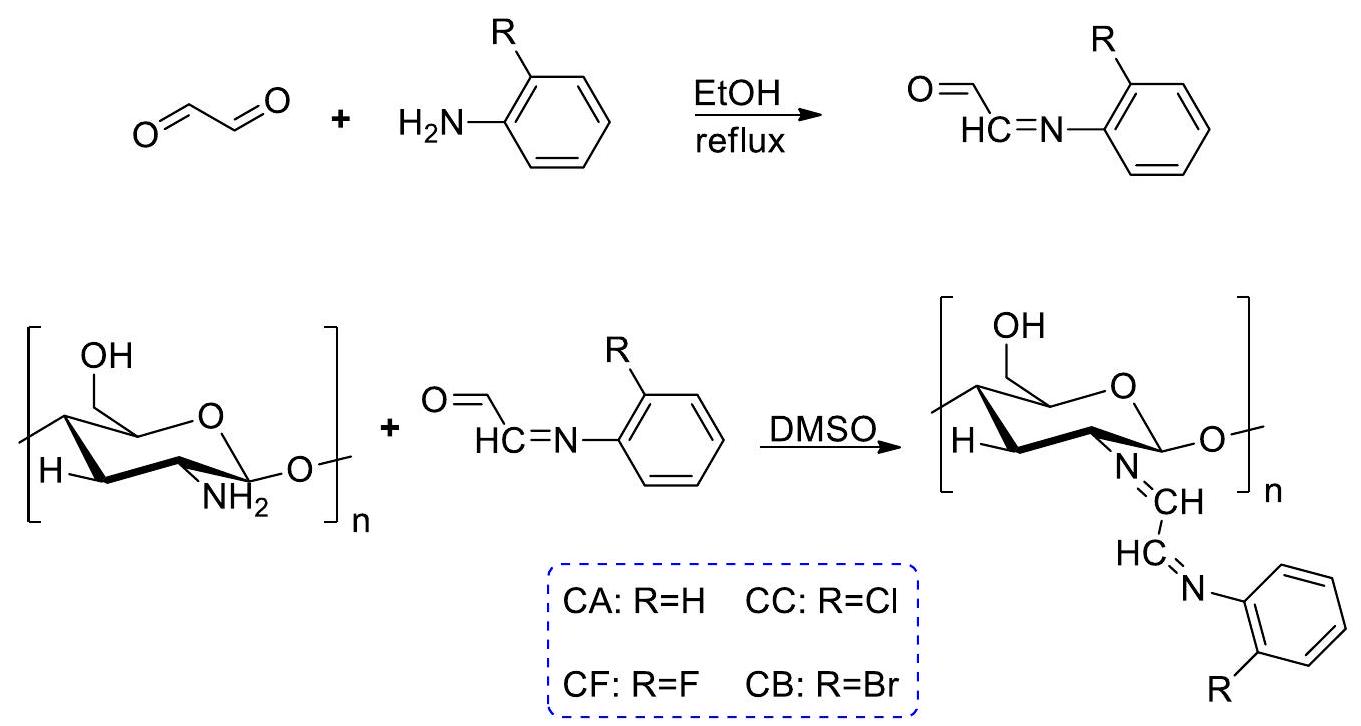
تم تطوير سلسلة روايات تتكون من مركبات 3-(بنزيليدين أمين). شملت تخليق 2-فينيل كوينازولين-4(3H)-ونات إنتاج قواعد شيف من مركبات 3-أمين. تم تفاعل -2-فينيل كوينازولين-4(3)H-ون مع عدة مركبات كربونيلية تم استبدالها. تم تحديد الهياكل الكيميائية للمركبات باستخدام تحليل الطيف. تم تقييم السمية الخلوية والنشاط المضاد للفيروسات للمركبات المختبرة ضد مجموعة من الفيروسات، بما في ذلك فيروس الإنفلونزا B، فيروس الإنفلونزا A من النمط الفرعي H3N2، فيروس الإنفلونزا A من النمط الفرعي H1N1، فيروس اللقاح، فيروس الهربس القططي، فيروس الهربس البسيط-1 TK-KOS ACVr، فيروس بونتا تورو، فيروس الهربس البسيط-2 (G)، فيروس بارا إنفلونزا-3، فيروس ريو، فيروس سندبيس، فيروس كوكساكي B4، فيروس التهاب الفم الحويصلي، فيروس الجهاز التنفسي المخلوي، فيروس الهربس البسيط-1 (KOS)، وفيروس كورونا القططي (FIPV). أظهر المركب المتكون فعالية مضادة للفيروسات تفوق جميع السلالات الفيروسية التي تم تقييمها.

دواء، مع زيادة بمقدار 32 مرة في النشاط كما يتضح من قيمة EC50 الخاصة به البالغة 1.6 ميكرومول. عند درجة حموضة 7.4 ودرجة حرارة
الخاتمة
الاختصارات
| MDRB | البكتيريا المقاومة لمتعدد الأدوية |
| DMSO | ثنائي ميثيل سلفوكسيد |
| MW | ميكروويف |
| IR | الأشعة تحت الحمراء |
| NMR | الرنين المغناطيسي النووي |
| MEPS | سطح الجهد الكهربائي الجزيئي |
| QSAR | علاقة الهيكل-النشاط الكمية |
| DmChDp | 2,2′-(5,5-ثنائي ميثيل سيكلوهكسان-1,3-دييلدين)بيس(أزان-1-يل-1يلدين)دايفينول |
EC50 التركيز الفعال نصف الأقصى
CT-DNA الحمض النووي الورمي المتداول
الشكر والتقدير
مساهمات المؤلفين
التمويل
توفر البيانات والمواد
الإعلانات
موافقة الأخلاقيات والموافقة على المشاركة
الموافقة على النشر
المصالح المتنافسة
تفاصيل المؤلف
تاريخ الاستلام: 18 سبتمبر 2023 تاريخ القبول: 6 فبراير 2024
References
- Berhanu AL, Mohiuddin I, Malik AK, Aulakh JS, Kumar V, Kim KH (2019) A review of the applications of Schiff bases as optical chemical sensors. TrAC Trends Anal Chem 116:74-91
- Abu-Dief AM, Mohamed IM (2015) A review of versatile applications of transition metal complexes incorporating Schiff bases. Beni-suef Univ J Basic Appl Sci 4(2):119-133
- Juyal VK, Pathak A, Panwar M, Thakuri SC, Prakash O, Agrwal A, Nand V (2023) Schiff base metal complexes as a versatile catalyst: a review. J Organomet Chem 2023:122825
- Boulechfar C, Ferkous H, Delimi A, Djedouani A, Kahlouche A, Boublia A, Benguerba Y (2023) Schiff bases and their metal complexes: a review on the history, synthesis, and applications. Inorg Chem Commun 150:110451
- Ashraf T, Ali B, Qayyum H, Haroone MS, Shabbir G (2023) Pharmacological aspects of Schiff base metal complexes: a critical review. Inorg Chem Commun 150:110449
- Alam MZ, Alimuddin, Khan SA (2023) A review on Schiff base as a versatile fluorescent chemo-sensors tool for detection of
and metal ion. J Fluoresc 33:1-32 - Hameed A, Al-Rashida M, Uroos M, Abid Ali S, Khan KM (2017) Schiff bases in medicinal chemistry: a patent review (2010-2015). Expert Opin Ther Pat 27(1):63-79
- Manvatkar VD, Patle RY, Meshram PH, Dongre RS (2023) Azomethinefunctionalized organic-inorganic framework: an overview. Chem Pap 77:1-22
- Uddin MN, Ahmed SS, Alam SR (2020) Biomedical applications of Schiff base metal complexes. J Coord Chem 73(23):3109-3149
- Li M, Cheng Z, Sun J, Tian Y, He J, Chen Y, Liu Z (2023) Nitrogen-doped porous carbon nanosheets based on a Schiff base reaction for highperformance lithium-ion batteries anode. Energies 16(4):1733
- Antony R, Arun T, Manickam STD (2019) A review on applications of chitosan-based Schiff bases. Int J Biol Macromol 129:615-633
- Yadav M, Yadav D, Singh DP, Kapoor JK (2023) Pharmaceutical properties of macrocyclic Schiff base transition metal complexes: urgent need in today’s world. Inorg Chim Acta 546:121300
- Zhang J, Xu L, Wong WY (2018) Energy materials based on metal Schiff base complexes. Coord Chem Rev 355:180-198
- Jia Y, Li J (2015) Molecular assembly of Schiff base interactions: construction and application. Chem Rev 115(3):1597-1621
- Al Zoubi W, Al-Hamdani AAS, Kaseem M (2016) Synthesis and antioxidant activities of Schiff bases and their complexes: a review. Appl Organomet Chem 30(10):810-817
- El-Sonbati AZ, Mahmoud WH, Mohamed GG, Diab MA, Morgan SM, Abbas SY (2019) Synthesis, characterization of Schiff base metal complexes and their biological investigation. Appl Organomet Chem 33(9):e5048
- Kakkassery JT, Raphael VP, Johnson R (2021) In vitro antibacterial and in silico docking studies of two Schiff bases on Staphylococcus aureus and its target proteins. Future J Pharm Sci 7(1):1-9
- lacopetta D, Ceramella J, Catalano A, Saturnino C, Bonomo MG, Franchini C, Sinicropi MS (2021) Schiff bases: interesting scaffolds with promising antitumoral properties. Appl Sci 11(4):1877
- Qin W, Long S, Panunzio M, Biondi S (2013) Schiff bases: a short survey on an evergreen chemistry tool. Molecules 18(10):12264-12289
- Chaturvedi D, Kamboj M (2016) Role of Schiff base in drug discovery research. Chem Sci J 7(2):7-8
- Parveen S (2020) Recent advances in anticancer ruthenium Schiff base complexes. Appl Organomet Chem 34(8):e5687
- Udhayakumari D, Inbaraj V (2020) A review on Schiff base fluorescent chemosensors for cell imaging applications. J Fluoresc 30:1203-1223
- Collinson SR, Fenton DE (1996) Metal complexes of bibracchial Schiff base macrocycles. Coord Chem Rev 148:19-40
- Xu J, Liu Y, Hsu SH (2019) Hydrogels based on Schiff base linkages for biomedical applications. Molecules 24(16):3005
- Ghanghas P, Choudhary A, Kumar D, Poonia K (2021) Coordination metal complexes with Schiff bases: useful pharmacophores
with comprehensive biological applications. Inorg Chem Commun 130:108710 - Verma C, Quraishi MA (2021) Recent progresses in Schiff bases as aqueous phase corrosion inhibitors: design and applications. Coord Chem Rev 446:214105
- Matar SA, Talib WH, Mustafa MS, Mubarak MS, AIDamen MA (2015) Synthesis, characterization, and antimicrobial activity of Schiff bases derived from benzaldehydes and 3,3′-diaminodipropylamine. Arab J Chem 8(6):850-857
- Liu X, Manzur C, Novoa N, Celedón S, Carrillo D, Hamon JR (2018) Multidentate unsymmetrically-substituted Schiff bases and their metal complexes: synthesis, functional materials properties, and applications to catalysis. Coord Chem Rev 357:144-172
- Shakir M, Hanif S, Sherwani MA, Mohammad O, Al-Resayes SI (2015) Pharmacologically significant complexes of Mn (II), Co (II), Ni (II), Cu (II) and Zn (II) of novel Schiff base ligand,(E)-N-(furan-2-yl methylene) quinolin-8-amine: Synthesis, spectral, XRD, SEM, antimicrobial, antioxidant and in vitro cytotoxic studies. J Mol Struct 1092:143-159
- Balakrishnan B, Joshi N, Banerjee R (2013) Borate aided Schiff’s base formation yields in situ gelling hydrogels for cartilage regeneration. J Mater Chem B 1(41):5564-5577
- Yousif E, Majeed A, Al-Sammarrae K, Salih N, Salimon J, Abdullah B (2017) Metal complexes of Schiff base: preparation, characterization and antibacterial activity. Arab J Chem 10:S1639-S1644
- Liu X, Hamon JR (2019) Recent developments in penta-, hexa-and heptadentate Schiff base ligands and their metal complexes. Coord Chem Rev 389:94-118
- More MS, Joshi PG, Mishra YK, Khanna PK (2019) Metal complexes driven from Schiff bases and semicarbazones for biomedical and allied applications: a review. Mater Today Chem 14:100195
- El-Gammal OA, Mohamed FS, Rezk GN, El-Bindary AA (2021) Structural characterization and biological activity of a new metal complexes based of Schiff base. J Mol Liq 330:115522
- Shellaiah M, Rajan YC, Balu P, Murugan A (2015) A pyrene based Schiff base probe for selective fluorescence turn-on detection of
ions with live cell application. New J Chem 39(4):2523-2531 - Keshavarzian E, Asadi Z, Poupon M, Dusek M, Rastegari B (2022) Heterodinuclear Cu-Gd (3d-4f) complex with di-compartmental Schiff base ligand in biological activity: synthesis, crystal structure, catecholase activity and DNA & BSA-binding studies. J Mol Liq 345:117785
- Bitu MNA, Hossain MS, Zahid AASM, Zakaria CM, Kudrat-E-Zahan M (2019) Anti-pathogenic activity of cu (II) complexes incorporating Schiff bases: a short review. Am J Heterocyclic Chem 5(1):11-23
- Spinu C, Kriza A (2000) Co (II), Ni (II) and Cu (II) complexes of bidentate Schiff bases. Acta Chim Slov 47(2):179-186
- Sorochinsky AE, Aceña JL, Moriwaki H, Sato T, Soloshonok VA (2013) Asymmetric synthesis of a-amino acids via homologation of Ni (II) complexes of glycine Schiff bases; Part 1: alkyl halide alkylations. Amino Acids 45:691-718
- Liu J, Wu BW, Zhang B, Liu Y (2006) Synthesis and characterization of metal complexes of Cu (II), Ni (II), Zn (II), Co (II), Mn (II) and Cd (II) with tetradentate Schiff bases. Turk J Chem 30(1):41-48
- Ma L, Li W, Zhu S, Wang L, Guan S (2021) Corrosion inhibition of Schiff bases for
alloy in normal saline: experimental and theoretical investigations. Corros Sci 184:109268 - Mohanty P, Behura R, Bhardwaj V, Dash PP, Sahoo SK, Jali BR (2022) Recent advancement on chromo-fluorogenic sensing of aluminum (III) with Schiff bases. Trends Environ Anal Chem 34:e00166
- Hachem K, Jasim SA, Al-Gazally ME, Riadi Y, Yasin G, TurkiJalil A, DehnoKhalaji A (2022) Retracted: adsorption of Pb (II) and Cd (II) by magnetic chitosan-salicylaldehyde Schiff base: synthesis, characterization, thermal study and antibacterial activity. J Chin Chem Soc 69(3):512-521
- Abdel-Rahman LH, Abu-Dief AM, Atlam FM, Abdel-Mawgoud AH, Alothman AA, Alsalme AM, Nafady A (2020) Chemical, physical, and biological properties of Pd (II), V (IV) O, and Ag (I) complexes of N3 tridentate pyridine-based Schiff base ligand. J Coord Chem 73(23):3150-3173
- Kaczmarek MT, Zabiszak M, Nowak M, Jastrzab R (2018) Lanthanides: Schiff base complexes, applications in cancer diagnosis, therapy, and antibacterial activity. Coord Chem Rev 370:42-54
- Malik MA, Dar OA, Gull P, Wani MY, Hashmi AA (2018) Heterocyclic Schiff base transition metal complexes in antimicrobial and anticancer chemotherapy. MedChemComm 9(3):409-436
- de Fátima Â, de Paula Pereira C, Olímpio CRSDG, de Freitas Oliveira BG, Franco LL, da Silva PHC (2018) Schiff bases and their metal complexes as urease inhibitors-a brief review. J Adv Res 13:113-126
- Almashal FA, Mohammed MQ, Hassan QMA, Emshary CA, Sultan HA, Dhumad AM (2020) Spectroscopic and thermal nonlinearity study of a Schiff base compound. Opt Mater 100:109703
- AI Zoubi W, Ko YG (2016) Organometallic complexes of Schiff bases: Recent progress in oxidation catalysis. J Organomet Chem 822:173-188
- Salama HE, Saad GR, Sabaa MW (2015) Synthesis, characterization and biological activity of Schiff bases based on chitosan and arylpyrazole moiety. Int J Biol Macromol 79:996-1003
- Anush SM, Vishalakshi B, Kalluraya B, Manju N (2018) Synthesis of pyrazole-based Schiff bases of chitosan: evaluation of antimicrobial activity. Int J Biol Macromol 119:446-452
- Das P, Linert W (2016) Schiff base-derived homogeneous and heterogeneous palladium catalysts for the Suzuki-Miyaura reaction. Coord Chem Rev 311:1-23
- Divya K, Pinto GM, Pinto AF (2017) Application of metal complexes of Schiff bases as an antimicrobial drug: a review of recent works. Int J Curr Pharm Res 9(3):27-30
- Gupta KC, Sutar AK (2008) Catalytic activities of Schiff base transition metal complexes. Coord Chem Rev 252(12-14):1420-1450
- Rana K, Pandurangan A, Singh N, Tiwari AK (2012) A systemic review of Schiff bases as an analgesic, anti-inflammatory. Int J Curr Pharm Res 4(2):5-11
- Da Silva CM, da Silva DL, Modolo LV, Alves RB, de Resende MA, Martins CV, de Fátima  (2011) Schiff bases: a short review of their antimicrobial activities. J Adv Res 2(1):1-8
- Vigato PA, Tamburini S (2004) The challenge of cyclic and acyclic Schiff bases and related derivatives. Coord Chem Rev 248(17-20):1717-2128
- Kajal A, Bala S, Kamboj S, Sharma N, Saini V (2013) Schiff bases: a versatile pharmacophore. J Catal 2013:3512
- Balouiri M, Sadiki M, Ibnsouda SK (2016) Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal 6(2):71-79
- Verma M, Pandeya SN, Singh KN, Stables JP (2004) Anticonvulsant activity of Schiff bases of isatin derivatives. Acta Pharm 54(1):49-56
- Azizian J, Mohammadi MK, Firuzi O, Razzaghi-asI N, Miri R (2012) Synthesis, biological activity and docking study of some new isatin Schiff base derivatives. Med Chem Res 21:3730-3740
- Xu Y, Shi Y, Lei F, Dai L (2020) A novel and green cellulose-based Schiff base-Cu (II) complex and its excellent antibacterial activity. Carbohydr Polym 230:115671
- Hassan AS, Askar AA, Nossier ES, Naglah AM, Moustafa GO, AI-Omar MA (2019) Antibacterial evaluation, in silico characters and molecular docking of Schiff bases derived from 5-aminopyrazoles. Molecules 24(17):3130
- Ahmed A, Mushtaq I, Chinnam S (2023) Suzuki-Miyaura cross-couplings for alkyl boron reagent: recent developments—a review. Future J Pharm Sci 9(1):67
- GÜmÜŞ A, OkumuŞ V, GÜmÜŞ S (2020) Synthesis, biological evaluation of antioxidant-antibacterial activities and computational studies of novel anthracene- and pyrene-based Schiff base derivatives. Turk J Chem 44(4):1200-1215
- Jesmin M, Ali MM, Khanam JA (2010) Antitumour activities of some Schiff bases derived from benzoin, salicylaldehyde, amino phenol and 2,4 dinitrophenyl hydrazine. Thai J Pharm Sci 34(1):20-31
- Makawana JA, Sangani CB, Lin L, Zhu HL (2014) Schiff’s base derivatives bearing nitroimidazole and quinoline nuclei: new class of anticancer agents and potential EGFR tyrosine kinase inhibitors. Bioorg Med Chem Lett 24(7):1734-1736
- Zhang K, Wang P, Xuan LN, Fu XY, Jing F, Li S, Chen BQ (2014) Synthesis and antitumor activities of novel hybrid molecules containing 1,3,4-oxadiazole and 1,3,4-thiadiazole bearing Schiff base moiety. Bioorg Med Chem Lett 24(22):5154-5156
- Chemchem M, Menacer R, Merabet N, Bouridane H, Yahiaoui S, Moussaoui S, Belkhiri L (2020) Green synthesis, antibacterial evaluation and QSAR analysis of some isatin Schiff bases. J Mol Struct 1208:127853
- Salihović M, Pazalja M, Halilović SŠ, Veljović E, Mahmutović-Dizdarević I, Roca S, Trifunović S (2021) Synthesis, characterization, antimicrobial activity and DFT study of some novel Schiff bases. J Mol Struct 1241:130670
- Mushtaq I, Ahmed A (2023) Synthesis of biologically active sulfonamidebased indole analogs: a review. Future J Pharm Sci 9(1):1-13
- Aragón-Muriel A, Liscano Y, Upegui Y, Robledo SM, Ramírez-Apan MT, Morales-Morales D, Polo-Cerón D (2021) In vitro evaluation of the potential pharmacological activity and molecular targets of new benzimida-zole-based Schiff base metal complexes. Antibiotics 10(6):728
- Chen Y, Mi Y, Li Q, Dong F, Guo Z (2020) Synthesis of Schiff bases modified inulin derivatives for potential antifungal and antioxidant applications. Int J Biol Macromol 143:714-723
- Cheng LX, Tang JJ, Luo H, Jin XL, Dai F, Yang J, Zhou B (2010) Antioxidant and antiproliferative activities of hydroxyl-substituted Schiff bases. Bioorg Med Chem Lett 20(8):2417-2420
- Devi P, Singh K, Kubavat B (2023) Synthesis, spectroscopic, quantum, thermal and kinetics, antibacterial and antifungal studies: Novel Schiff base 5-methyl-3-((5-bromosalicylidene) amino)-pyrazole and its transition metal complexes. Results Chem. 5:100813
- Ejidike IP (2018) Cu (II) Complexes of 4-[(1 E)-N-{2-[(Z)-Benzylideneamino] ethyl3 ethanimidoyl] benzene-1, 3-diol Schiff base: synthesis, spectroscopic, in-vitro antioxidant, antifungal and antibacterial studies. Molecules 23(7):1581
- Hamad A, Chen Y, Khan MA, Jamshidi S, Saeed N, Clifford M, Rahman KM (2021) Schiff bases of sulphonamides as a new class of antifungal agent against multidrug-resistant Candida auris. MicrobiologyOpen 10(4):e1218
- Wei L, Zhang J, Tan W, Wang G, Li Q, Dong F, Guo Z (2021) Antifungal activity of double Schiff bases of chitosan derivatives bearing active halogeno-benzenes. Int J Biol Macromol 179:292-298
- Al-Masoudi NA, Aziz NM, Mohammed AT (2009) Synthesis and In vitro anti-HIV activity of some new Schiff base ligands derived from 5-Amino-4-phenyl-4 H-1, 2, 4-triazole-3-thiol and their metal complexes. Phosphorus Sulfur Silicon 184(11):2891-2901
- Kumar KS, Ganguly S, Veerasamy R, De Clercq E (2010) Synthesis, antiviral activity and cytotoxicity evaluation of Schiff bases of some 2-phenyl quinazoline-4 (3) H-ones. Eur J Med Chem 45(11):5474-5479
- Sriram D, Yogeeswari P, Myneedu NS, Saraswat V (2006) Abacavir prodrugs: microwave-assisted synthesis and their evaluation of anti-HIV activities. Bioorg Med Chem Lett 16(8):2127-2129
ملاحظة الناشر
- *المراسلة:
إيرفان مشتاك
irfanmushtaq820@gmail.com
القائمة الكاملة لمعلومات المؤلف متاحة في نهاية المقال
DOI: https://doi.org/10.1186/s43094-024-00594-5
Publication Date: 2024-02-12
Pharmaceutical significance of Schiff bases: an overview
Abstract
Schiff bases are a diverse group of organic compounds with great pharmaceutical importance due to the presence of carbon-nitrogen double bonds (
Graphical abstract

Background
The discovery of Schiff bases dates back to the nineteenth century during which a chemist named “Hugo Schiff” first documented a reaction showing condensation between amines and carbonyl functional groups [13, 18-21]. In contemporary times, this domain of scientific inquiry about Schiff base coordination chemistry has seen significant growth and expansion [22, 23]. The significance of Schiff base complexes in the fields
of material science, biomedical applications, bioinorganic chemistry, encapsulation processes, supramolecular chemistry, catalysis, and separation, and the generation of molecules with exceptional characteristics and structures has been widely acknowledged and extensively examined in the existing literature [24-27]. The literature has documented the use of Schiff bases derived from salicylaldehydes as agents for regulating plant development, as well as exhibiting antibacterial or antimycotic properties [28-30]. Schiff bases have been shown to possess analytical uses as well. The significance of these bases helps explain the difficult mechanisms inside biological systems and gives proper meaning to the imine group activity. These also provide broad-spectrum activity against several species, including Candida, Plasmopora viticola, Trichophyton gypsum, Staphylococcus aureus, Erysiphe graminis, Mycobacterium, Albicans, Bacillus polymyxa, and Escherichia coli [31-34].
Main text
Pharmaceutical significance of Schiff bases
Antibacterial
bases derived from cellulose are produced by the condensation reaction between p-aminophenol and aldehyde moieties and have shown antibacterial efficacy against, Staphylococcus aureus, Escherichia coli, and Enterococcus faecalis [62].



“Molecular Electrostatic Potential Surface (MEPS) analysis” and “Quantitative Structure-Activity Relationship (QSAR).” The findings of the QSAR analysis, which included density functional theory (DFT)-based, steric, and hydrophobic descriptors, indicate that compounds exhibiting higher hydrophobicity and lower dipole moment have antibacterial properties against “Klebsiella pneumoniae ATCC700603” [69].

21a-27a: $mathrm{X}=mathrm{H}, mathrm{R}^{1}=mathrm{OMe}, mathrm{H}, mathrm{Me} ; mathrm{R}^{2}=mathrm{H}, mathrm{OMe}^{1}$
$mathrm{R}^{3}=mathrm{H}, mathrm{Br}, mathrm{OMe}, mathrm{R}^{4}=mathrm{COOH}, mathrm{Br}, mathrm{H}$
21b-25b: $mathrm{X}=mathrm{Br}, mathrm{R}^{1}=mathrm{OMe}, mathrm{H}, mathrm{Cl}, mathrm{Me}$;
$mathrm{R}^{2}=mathrm{H}, mathrm{OMe}, mathrm{R}^{3}=mathrm{H}, mathrm{R}^{4}=mathrm{COOH}, mathrm{Br}$


In brief, a series of new lanthanide complexes were synthesized by the use of Schiff base ligands, together with a “benzimidazole moiety.” These complexes were thoroughly characterized by utilizing several analytical methodologies, ensuring their unambiguous identification and understanding. Initial experiments for the compounds were performed to assess various pharmacological uses of the substances under investigation. These assessments were undertaken using a range of bioassays,
including tests for antiproliferative activity, antiparasitic activity, and antibacterial activity. The findings indicate that the biological activities of the compounds are influenced by structural modifications. Specifically, ligands L1 and L2, along with their metal complexes, demonstrated minimum inhibitory concentration (MIC) values in comparison with ligands L3 and L4 and their respective complexes. This disparity in MIC values may be attributed to substitutions on the “aminophenol ring.” In a similar vein, it was shown that the compounds had an impact on the fluidity of the cell membrane by modifying the hydrophobic region inside the lipid bilayer. This observation suggests a possible correlation between such alterations and the potential therapeutic uses of these compounds. The aforementioned result has the potential to serve as a valuable point of reference in future endeavors aimed at the advancement of novel pharmacological medicines. Ongoing investigations in the laboratory are now exploring the impact of the alterations on the molecular architecture.

Antifungal
of the inulin derivatives was influenced by many parameters, such as the degree of substitution (DS), as well as the quantity and location of phenolic hydroxyl groups. The products elucidated in this manuscript show significant promise as biomaterials characterized by favorable bioactivity and biocompatibility. Further investigation of the structure-activity link is warranted in future research endeavors [75, 76] (Scheme 7).


was conducted to assess the antifungal efficacy against three prevalent plant pathogenic fungi, namely Botrytis cinerea, Fusarium oxysporum f. sp. cucumerinum, and Fusarium oxysporum f. sp. niveum, by in vitro hyphal measurements. The findings of the study indicate that the antifungal activity of double Schiff bases of chitosan

Antiviral
and
A novel series comprising 3-(benzylideneamino) compounds has been developed. The synthesis of 2-phenylquinazoline-4(3H)-ones included the production of Schiff bases from 3 -amino compounds. The reaction of -2-phenyl quinazoline-4(3)H-one with several carbonyl compounds that have been replaced. The chemical structures of the compounds were determined by the use of spectrum analysis. The cytotoxicity and antiviral activity of the tested compounds were assessed against a range of viruses, including influenza B virus, influenza A H3N2 subtype, influenza A H1N1 subtype, vaccinia virus, feline herpes virus, herpes simplex virus-1 TK-KOS ACVr, Punta Toro virus, herpes simplex virus-2 ( G ), para influenza-3 virus, reovirus-1, Sindbis virus, Coxsackie virus B4, vesicular stomatitis virus, respiratory syncytial virus herpes simplex virus-1 (KOS), and feline coronavirus (FIPV). A compound formed exhibited superior antiviral efficacy against all evaluated viral strains [80] (Scheme 11).


medication, with a 32 -fold increase in activity as shown by its EC50 value of 1.6 lM . At a pH of 7.4 and a temperature of

Conclusion
Abbreviations
| MDRB | Multi-drug resistant bacteria |
| DMSO | Dimethyl sulfoxide |
| MW | Microwave |
| IR | Infrared |
| NMR | Nuclear magnetic resonance |
| MEPS | Molecular electrostatic potential surface |
| QSAR | Quantitative structure-activity relationship |
| DmChDp | 2,2′-(5,5-Dimethylcyclohexane-1,3-diylidene)bis(azan-1-yl-1ylidene)diphenol |
EC50 Half maximal effective concentration
CT-DNA Circulating tumor DNA
Acknowledgements
Author contributions
Funding
Availability of data and materials
Declarations
Ethics approval and consent to participate
Consent for publication
Competing interests
Author details
Received: 18 September 2023 Accepted: 6 February 2024
References
- Berhanu AL, Mohiuddin I, Malik AK, Aulakh JS, Kumar V, Kim KH (2019) A review of the applications of Schiff bases as optical chemical sensors. TrAC Trends Anal Chem 116:74-91
- Abu-Dief AM, Mohamed IM (2015) A review of versatile applications of transition metal complexes incorporating Schiff bases. Beni-suef Univ J Basic Appl Sci 4(2):119-133
- Juyal VK, Pathak A, Panwar M, Thakuri SC, Prakash O, Agrwal A, Nand V (2023) Schiff base metal complexes as a versatile catalyst: a review. J Organomet Chem 2023:122825
- Boulechfar C, Ferkous H, Delimi A, Djedouani A, Kahlouche A, Boublia A, Benguerba Y (2023) Schiff bases and their metal complexes: a review on the history, synthesis, and applications. Inorg Chem Commun 150:110451
- Ashraf T, Ali B, Qayyum H, Haroone MS, Shabbir G (2023) Pharmacological aspects of Schiff base metal complexes: a critical review. Inorg Chem Commun 150:110449
- Alam MZ, Alimuddin, Khan SA (2023) A review on Schiff base as a versatile fluorescent chemo-sensors tool for detection of
and metal ion. J Fluoresc 33:1-32 - Hameed A, Al-Rashida M, Uroos M, Abid Ali S, Khan KM (2017) Schiff bases in medicinal chemistry: a patent review (2010-2015). Expert Opin Ther Pat 27(1):63-79
- Manvatkar VD, Patle RY, Meshram PH, Dongre RS (2023) Azomethinefunctionalized organic-inorganic framework: an overview. Chem Pap 77:1-22
- Uddin MN, Ahmed SS, Alam SR (2020) Biomedical applications of Schiff base metal complexes. J Coord Chem 73(23):3109-3149
- Li M, Cheng Z, Sun J, Tian Y, He J, Chen Y, Liu Z (2023) Nitrogen-doped porous carbon nanosheets based on a Schiff base reaction for highperformance lithium-ion batteries anode. Energies 16(4):1733
- Antony R, Arun T, Manickam STD (2019) A review on applications of chitosan-based Schiff bases. Int J Biol Macromol 129:615-633
- Yadav M, Yadav D, Singh DP, Kapoor JK (2023) Pharmaceutical properties of macrocyclic Schiff base transition metal complexes: urgent need in today’s world. Inorg Chim Acta 546:121300
- Zhang J, Xu L, Wong WY (2018) Energy materials based on metal Schiff base complexes. Coord Chem Rev 355:180-198
- Jia Y, Li J (2015) Molecular assembly of Schiff base interactions: construction and application. Chem Rev 115(3):1597-1621
- Al Zoubi W, Al-Hamdani AAS, Kaseem M (2016) Synthesis and antioxidant activities of Schiff bases and their complexes: a review. Appl Organomet Chem 30(10):810-817
- El-Sonbati AZ, Mahmoud WH, Mohamed GG, Diab MA, Morgan SM, Abbas SY (2019) Synthesis, characterization of Schiff base metal complexes and their biological investigation. Appl Organomet Chem 33(9):e5048
- Kakkassery JT, Raphael VP, Johnson R (2021) In vitro antibacterial and in silico docking studies of two Schiff bases on Staphylococcus aureus and its target proteins. Future J Pharm Sci 7(1):1-9
- lacopetta D, Ceramella J, Catalano A, Saturnino C, Bonomo MG, Franchini C, Sinicropi MS (2021) Schiff bases: interesting scaffolds with promising antitumoral properties. Appl Sci 11(4):1877
- Qin W, Long S, Panunzio M, Biondi S (2013) Schiff bases: a short survey on an evergreen chemistry tool. Molecules 18(10):12264-12289
- Chaturvedi D, Kamboj M (2016) Role of Schiff base in drug discovery research. Chem Sci J 7(2):7-8
- Parveen S (2020) Recent advances in anticancer ruthenium Schiff base complexes. Appl Organomet Chem 34(8):e5687
- Udhayakumari D, Inbaraj V (2020) A review on Schiff base fluorescent chemosensors for cell imaging applications. J Fluoresc 30:1203-1223
- Collinson SR, Fenton DE (1996) Metal complexes of bibracchial Schiff base macrocycles. Coord Chem Rev 148:19-40
- Xu J, Liu Y, Hsu SH (2019) Hydrogels based on Schiff base linkages for biomedical applications. Molecules 24(16):3005
- Ghanghas P, Choudhary A, Kumar D, Poonia K (2021) Coordination metal complexes with Schiff bases: useful pharmacophores
with comprehensive biological applications. Inorg Chem Commun 130:108710 - Verma C, Quraishi MA (2021) Recent progresses in Schiff bases as aqueous phase corrosion inhibitors: design and applications. Coord Chem Rev 446:214105
- Matar SA, Talib WH, Mustafa MS, Mubarak MS, AIDamen MA (2015) Synthesis, characterization, and antimicrobial activity of Schiff bases derived from benzaldehydes and 3,3′-diaminodipropylamine. Arab J Chem 8(6):850-857
- Liu X, Manzur C, Novoa N, Celedón S, Carrillo D, Hamon JR (2018) Multidentate unsymmetrically-substituted Schiff bases and their metal complexes: synthesis, functional materials properties, and applications to catalysis. Coord Chem Rev 357:144-172
- Shakir M, Hanif S, Sherwani MA, Mohammad O, Al-Resayes SI (2015) Pharmacologically significant complexes of Mn (II), Co (II), Ni (II), Cu (II) and Zn (II) of novel Schiff base ligand,(E)-N-(furan-2-yl methylene) quinolin-8-amine: Synthesis, spectral, XRD, SEM, antimicrobial, antioxidant and in vitro cytotoxic studies. J Mol Struct 1092:143-159
- Balakrishnan B, Joshi N, Banerjee R (2013) Borate aided Schiff’s base formation yields in situ gelling hydrogels for cartilage regeneration. J Mater Chem B 1(41):5564-5577
- Yousif E, Majeed A, Al-Sammarrae K, Salih N, Salimon J, Abdullah B (2017) Metal complexes of Schiff base: preparation, characterization and antibacterial activity. Arab J Chem 10:S1639-S1644
- Liu X, Hamon JR (2019) Recent developments in penta-, hexa-and heptadentate Schiff base ligands and their metal complexes. Coord Chem Rev 389:94-118
- More MS, Joshi PG, Mishra YK, Khanna PK (2019) Metal complexes driven from Schiff bases and semicarbazones for biomedical and allied applications: a review. Mater Today Chem 14:100195
- El-Gammal OA, Mohamed FS, Rezk GN, El-Bindary AA (2021) Structural characterization and biological activity of a new metal complexes based of Schiff base. J Mol Liq 330:115522
- Shellaiah M, Rajan YC, Balu P, Murugan A (2015) A pyrene based Schiff base probe for selective fluorescence turn-on detection of
ions with live cell application. New J Chem 39(4):2523-2531 - Keshavarzian E, Asadi Z, Poupon M, Dusek M, Rastegari B (2022) Heterodinuclear Cu-Gd (3d-4f) complex with di-compartmental Schiff base ligand in biological activity: synthesis, crystal structure, catecholase activity and DNA & BSA-binding studies. J Mol Liq 345:117785
- Bitu MNA, Hossain MS, Zahid AASM, Zakaria CM, Kudrat-E-Zahan M (2019) Anti-pathogenic activity of cu (II) complexes incorporating Schiff bases: a short review. Am J Heterocyclic Chem 5(1):11-23
- Spinu C, Kriza A (2000) Co (II), Ni (II) and Cu (II) complexes of bidentate Schiff bases. Acta Chim Slov 47(2):179-186
- Sorochinsky AE, Aceña JL, Moriwaki H, Sato T, Soloshonok VA (2013) Asymmetric synthesis of a-amino acids via homologation of Ni (II) complexes of glycine Schiff bases; Part 1: alkyl halide alkylations. Amino Acids 45:691-718
- Liu J, Wu BW, Zhang B, Liu Y (2006) Synthesis and characterization of metal complexes of Cu (II), Ni (II), Zn (II), Co (II), Mn (II) and Cd (II) with tetradentate Schiff bases. Turk J Chem 30(1):41-48
- Ma L, Li W, Zhu S, Wang L, Guan S (2021) Corrosion inhibition of Schiff bases for
alloy in normal saline: experimental and theoretical investigations. Corros Sci 184:109268 - Mohanty P, Behura R, Bhardwaj V, Dash PP, Sahoo SK, Jali BR (2022) Recent advancement on chromo-fluorogenic sensing of aluminum (III) with Schiff bases. Trends Environ Anal Chem 34:e00166
- Hachem K, Jasim SA, Al-Gazally ME, Riadi Y, Yasin G, TurkiJalil A, DehnoKhalaji A (2022) Retracted: adsorption of Pb (II) and Cd (II) by magnetic chitosan-salicylaldehyde Schiff base: synthesis, characterization, thermal study and antibacterial activity. J Chin Chem Soc 69(3):512-521
- Abdel-Rahman LH, Abu-Dief AM, Atlam FM, Abdel-Mawgoud AH, Alothman AA, Alsalme AM, Nafady A (2020) Chemical, physical, and biological properties of Pd (II), V (IV) O, and Ag (I) complexes of N3 tridentate pyridine-based Schiff base ligand. J Coord Chem 73(23):3150-3173
- Kaczmarek MT, Zabiszak M, Nowak M, Jastrzab R (2018) Lanthanides: Schiff base complexes, applications in cancer diagnosis, therapy, and antibacterial activity. Coord Chem Rev 370:42-54
- Malik MA, Dar OA, Gull P, Wani MY, Hashmi AA (2018) Heterocyclic Schiff base transition metal complexes in antimicrobial and anticancer chemotherapy. MedChemComm 9(3):409-436
- de Fátima Â, de Paula Pereira C, Olímpio CRSDG, de Freitas Oliveira BG, Franco LL, da Silva PHC (2018) Schiff bases and their metal complexes as urease inhibitors-a brief review. J Adv Res 13:113-126
- Almashal FA, Mohammed MQ, Hassan QMA, Emshary CA, Sultan HA, Dhumad AM (2020) Spectroscopic and thermal nonlinearity study of a Schiff base compound. Opt Mater 100:109703
- AI Zoubi W, Ko YG (2016) Organometallic complexes of Schiff bases: Recent progress in oxidation catalysis. J Organomet Chem 822:173-188
- Salama HE, Saad GR, Sabaa MW (2015) Synthesis, characterization and biological activity of Schiff bases based on chitosan and arylpyrazole moiety. Int J Biol Macromol 79:996-1003
- Anush SM, Vishalakshi B, Kalluraya B, Manju N (2018) Synthesis of pyrazole-based Schiff bases of chitosan: evaluation of antimicrobial activity. Int J Biol Macromol 119:446-452
- Das P, Linert W (2016) Schiff base-derived homogeneous and heterogeneous palladium catalysts for the Suzuki-Miyaura reaction. Coord Chem Rev 311:1-23
- Divya K, Pinto GM, Pinto AF (2017) Application of metal complexes of Schiff bases as an antimicrobial drug: a review of recent works. Int J Curr Pharm Res 9(3):27-30
- Gupta KC, Sutar AK (2008) Catalytic activities of Schiff base transition metal complexes. Coord Chem Rev 252(12-14):1420-1450
- Rana K, Pandurangan A, Singh N, Tiwari AK (2012) A systemic review of Schiff bases as an analgesic, anti-inflammatory. Int J Curr Pharm Res 4(2):5-11
- Da Silva CM, da Silva DL, Modolo LV, Alves RB, de Resende MA, Martins CV, de Fátima  (2011) Schiff bases: a short review of their antimicrobial activities. J Adv Res 2(1):1-8
- Vigato PA, Tamburini S (2004) The challenge of cyclic and acyclic Schiff bases and related derivatives. Coord Chem Rev 248(17-20):1717-2128
- Kajal A, Bala S, Kamboj S, Sharma N, Saini V (2013) Schiff bases: a versatile pharmacophore. J Catal 2013:3512
- Balouiri M, Sadiki M, Ibnsouda SK (2016) Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal 6(2):71-79
- Verma M, Pandeya SN, Singh KN, Stables JP (2004) Anticonvulsant activity of Schiff bases of isatin derivatives. Acta Pharm 54(1):49-56
- Azizian J, Mohammadi MK, Firuzi O, Razzaghi-asI N, Miri R (2012) Synthesis, biological activity and docking study of some new isatin Schiff base derivatives. Med Chem Res 21:3730-3740
- Xu Y, Shi Y, Lei F, Dai L (2020) A novel and green cellulose-based Schiff base-Cu (II) complex and its excellent antibacterial activity. Carbohydr Polym 230:115671
- Hassan AS, Askar AA, Nossier ES, Naglah AM, Moustafa GO, AI-Omar MA (2019) Antibacterial evaluation, in silico characters and molecular docking of Schiff bases derived from 5-aminopyrazoles. Molecules 24(17):3130
- Ahmed A, Mushtaq I, Chinnam S (2023) Suzuki-Miyaura cross-couplings for alkyl boron reagent: recent developments—a review. Future J Pharm Sci 9(1):67
- GÜmÜŞ A, OkumuŞ V, GÜmÜŞ S (2020) Synthesis, biological evaluation of antioxidant-antibacterial activities and computational studies of novel anthracene- and pyrene-based Schiff base derivatives. Turk J Chem 44(4):1200-1215
- Jesmin M, Ali MM, Khanam JA (2010) Antitumour activities of some Schiff bases derived from benzoin, salicylaldehyde, amino phenol and 2,4 dinitrophenyl hydrazine. Thai J Pharm Sci 34(1):20-31
- Makawana JA, Sangani CB, Lin L, Zhu HL (2014) Schiff’s base derivatives bearing nitroimidazole and quinoline nuclei: new class of anticancer agents and potential EGFR tyrosine kinase inhibitors. Bioorg Med Chem Lett 24(7):1734-1736
- Zhang K, Wang P, Xuan LN, Fu XY, Jing F, Li S, Chen BQ (2014) Synthesis and antitumor activities of novel hybrid molecules containing 1,3,4-oxadiazole and 1,3,4-thiadiazole bearing Schiff base moiety. Bioorg Med Chem Lett 24(22):5154-5156
- Chemchem M, Menacer R, Merabet N, Bouridane H, Yahiaoui S, Moussaoui S, Belkhiri L (2020) Green synthesis, antibacterial evaluation and QSAR analysis of some isatin Schiff bases. J Mol Struct 1208:127853
- Salihović M, Pazalja M, Halilović SŠ, Veljović E, Mahmutović-Dizdarević I, Roca S, Trifunović S (2021) Synthesis, characterization, antimicrobial activity and DFT study of some novel Schiff bases. J Mol Struct 1241:130670
- Mushtaq I, Ahmed A (2023) Synthesis of biologically active sulfonamidebased indole analogs: a review. Future J Pharm Sci 9(1):1-13
- Aragón-Muriel A, Liscano Y, Upegui Y, Robledo SM, Ramírez-Apan MT, Morales-Morales D, Polo-Cerón D (2021) In vitro evaluation of the potential pharmacological activity and molecular targets of new benzimida-zole-based Schiff base metal complexes. Antibiotics 10(6):728
- Chen Y, Mi Y, Li Q, Dong F, Guo Z (2020) Synthesis of Schiff bases modified inulin derivatives for potential antifungal and antioxidant applications. Int J Biol Macromol 143:714-723
- Cheng LX, Tang JJ, Luo H, Jin XL, Dai F, Yang J, Zhou B (2010) Antioxidant and antiproliferative activities of hydroxyl-substituted Schiff bases. Bioorg Med Chem Lett 20(8):2417-2420
- Devi P, Singh K, Kubavat B (2023) Synthesis, spectroscopic, quantum, thermal and kinetics, antibacterial and antifungal studies: Novel Schiff base 5-methyl-3-((5-bromosalicylidene) amino)-pyrazole and its transition metal complexes. Results Chem. 5:100813
- Ejidike IP (2018) Cu (II) Complexes of 4-[(1 E)-N-{2-[(Z)-Benzylideneamino] ethyl3 ethanimidoyl] benzene-1, 3-diol Schiff base: synthesis, spectroscopic, in-vitro antioxidant, antifungal and antibacterial studies. Molecules 23(7):1581
- Hamad A, Chen Y, Khan MA, Jamshidi S, Saeed N, Clifford M, Rahman KM (2021) Schiff bases of sulphonamides as a new class of antifungal agent against multidrug-resistant Candida auris. MicrobiologyOpen 10(4):e1218
- Wei L, Zhang J, Tan W, Wang G, Li Q, Dong F, Guo Z (2021) Antifungal activity of double Schiff bases of chitosan derivatives bearing active halogeno-benzenes. Int J Biol Macromol 179:292-298
- Al-Masoudi NA, Aziz NM, Mohammed AT (2009) Synthesis and In vitro anti-HIV activity of some new Schiff base ligands derived from 5-Amino-4-phenyl-4 H-1, 2, 4-triazole-3-thiol and their metal complexes. Phosphorus Sulfur Silicon 184(11):2891-2901
- Kumar KS, Ganguly S, Veerasamy R, De Clercq E (2010) Synthesis, antiviral activity and cytotoxicity evaluation of Schiff bases of some 2-phenyl quinazoline-4 (3) H-ones. Eur J Med Chem 45(11):5474-5479
- Sriram D, Yogeeswari P, Myneedu NS, Saraswat V (2006) Abacavir prodrugs: microwave-assisted synthesis and their evaluation of anti-HIV activities. Bioorg Med Chem Lett 16(8):2127-2129
Publisher’s Note
- *Correspondence:
Irfan Mushtaq
irfanmushtaq820@gmail.com
Full list of author information is available at the end of the article
