DOI: https://doi.org/10.1007/s00204-024-03735-0
PMID: https://pubmed.ncbi.nlm.nih.gov/38582802
تاريخ النشر: 2024-04-06
الأيض البشري لأربعة أفيونات بنزيميدازول الاصطناعية: إيزوتونيتازين، ميتونيتازين، إيتوديسنيتازين، وميتوديسنيتازين
© المؤلف(ون) 2024
الملخص
بعد جدولة الإيزوتونيتازين في عام 2019، زادت توفر بدائل الأفيونيات من نوع 2-بنزيل بنزيميدازول (نيتازينات) في سوق المخدرات العالمي، مما أدى إلى العديد من الوفيات في جميع أنحاء العالم. تعتبر النيتازينات قوية.
مقدمة
تم تقييم تفعيل مستقبلات المورفين في تجارب في المختبر (Vandeputte et al. 2021). تم التعرف مؤخرًا على المستقلبات الرئيسية من المرحلة الأولى لإيتوديسنيتازين في الجرذان (Grigoryev et al. 2023). لم يتم تقييم مصير الأيض لمواد أفيونية أخرى من عائلة البنزيميدازول بعد.
المواد والطرق
المواد الكيميائية والمواد الكيماوية
توقع المستقلبات باستخدام الحاسوب
تم ضرب المستقلبات من الجيل الثاني في درجة المستقلب من الجيل الأول المقابل لحساب “الدرجة المعدلة”.
حضانات خلايا الكبد البشرية
عينات ما بعد الوفاة
تحضير العينة
تفقيس
عينات من البول والدم
إعدادات كروماتوغرافيا السائل-مطياف الكتلة عالي الدقة
الكروماتوغرافيا السائلة
العودة إلى الظروف الأولية خلال 0.1 دقيقة؛ كان وقت إعادة التوازن 4.9 دقيقة.
مطيافية الكتلة الثنائية عالية الدقة
تحديد المستقلبات
4′-هيدروكسي-نيتازين
النتائج
توقع المستقلبات باستخدام الحاسوب
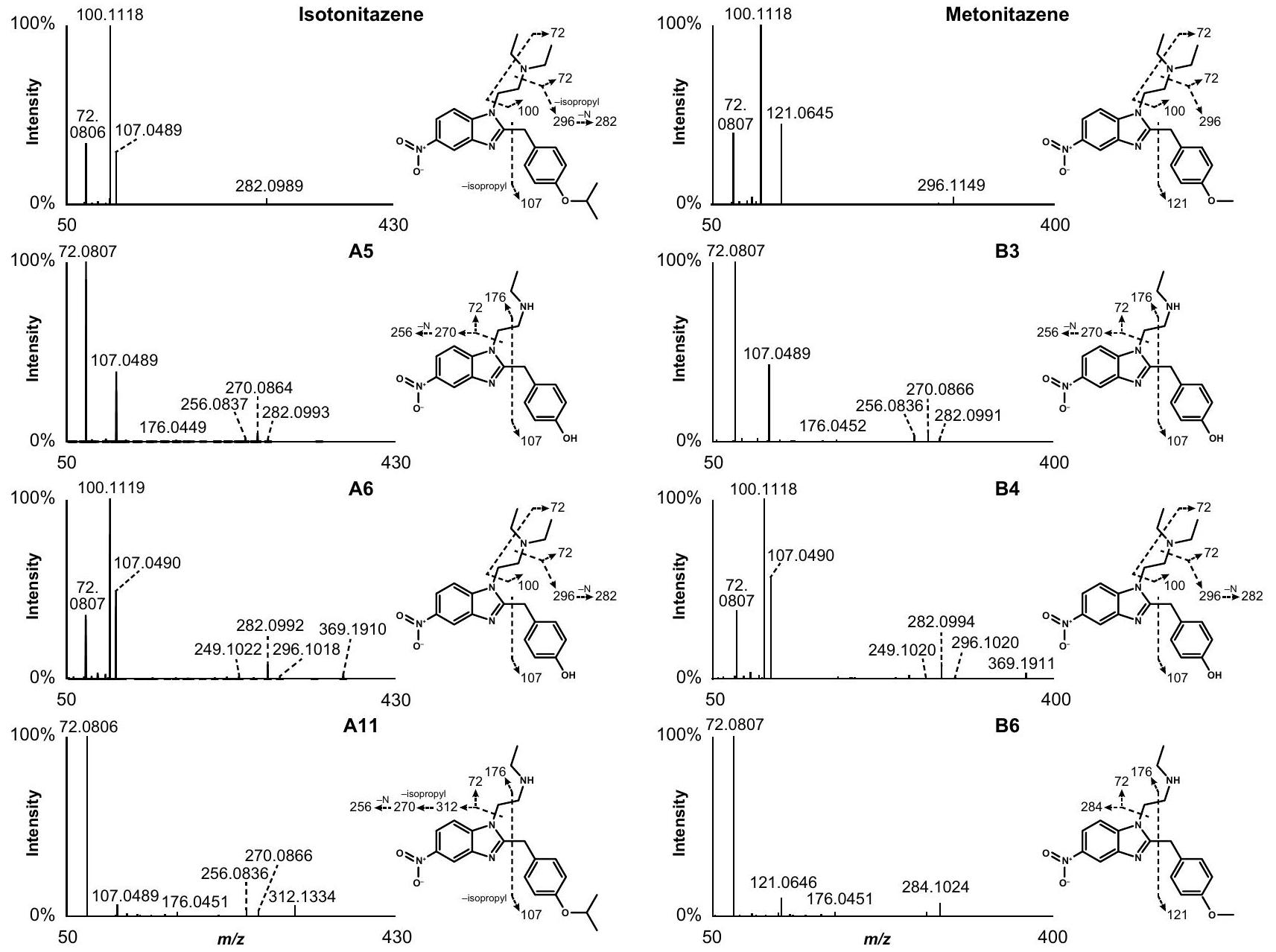
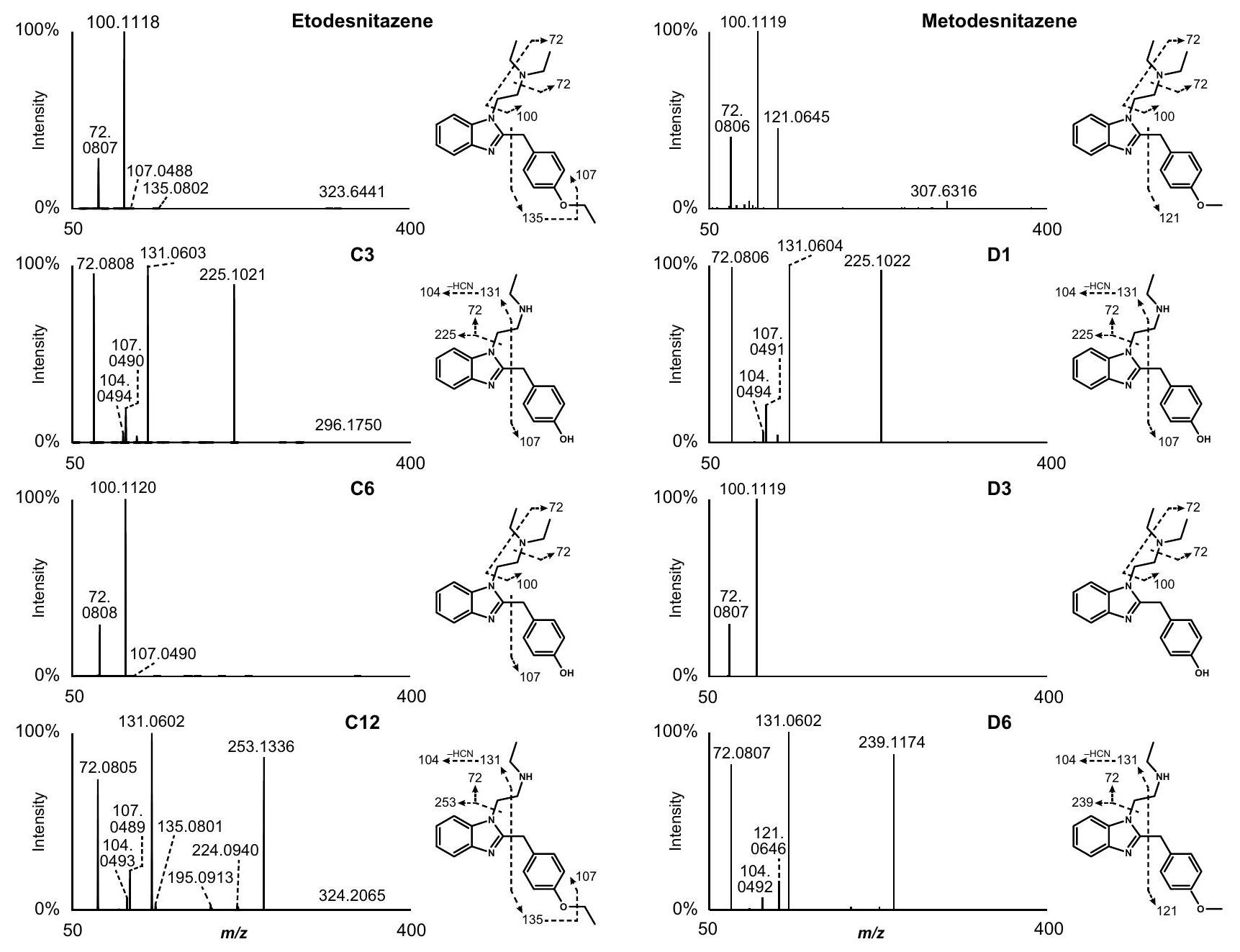
نمط تفتت HRMS/MS للإيزوتونيتازين، الميتونيتازين، الإيتوديسنيتازين، والميتوديسنيتازين
تحديد المستقلبات في حضانات الكبد البشري
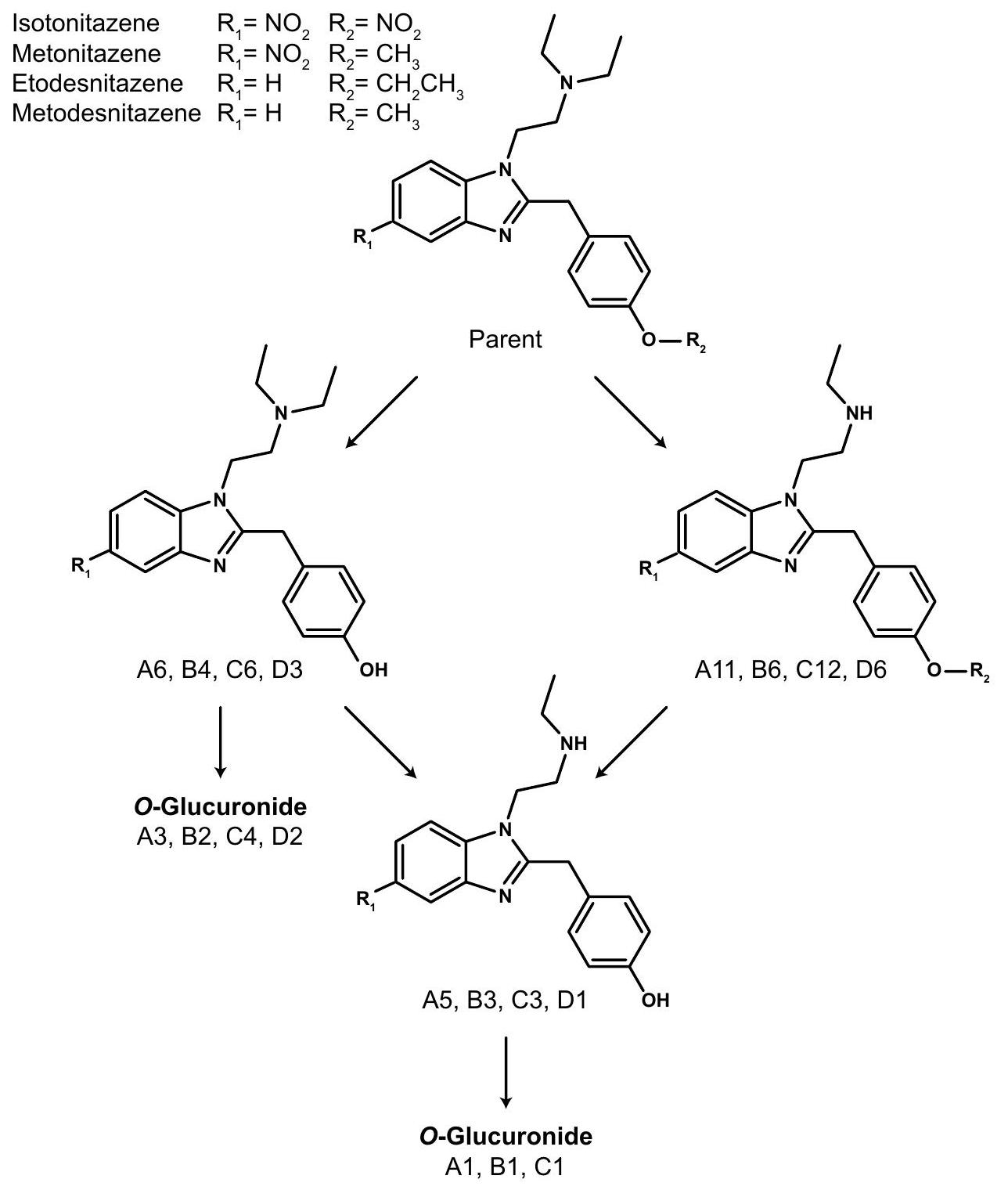
إزالة الألكيل من الأكسجين وعمليات الغلوكورونيدation الإضافية
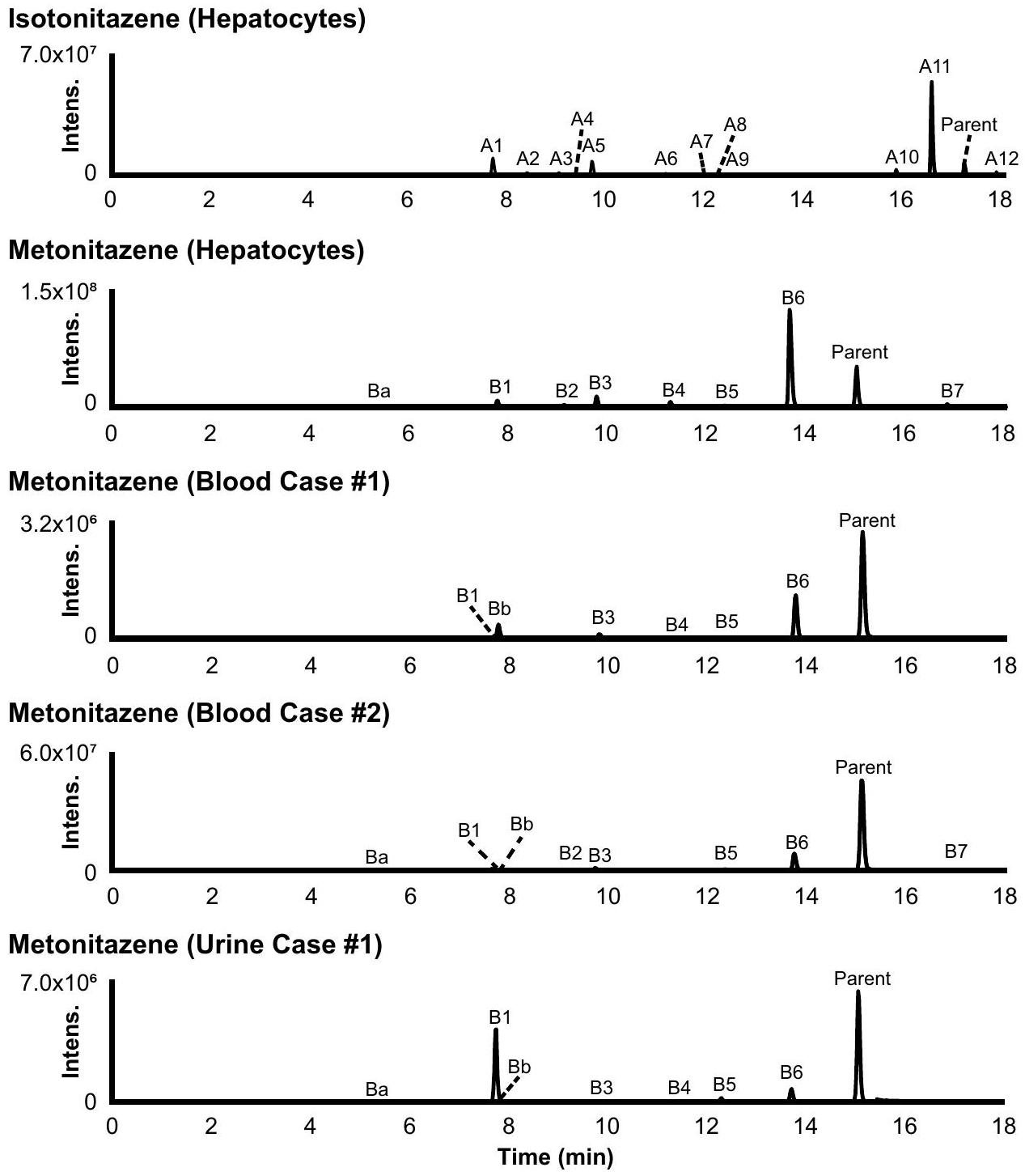
تم تأكيد (4′-هيدروكسي-نيتازين) من خلال حقن المعيار المرجعي المتاح تجارياً.
دمج
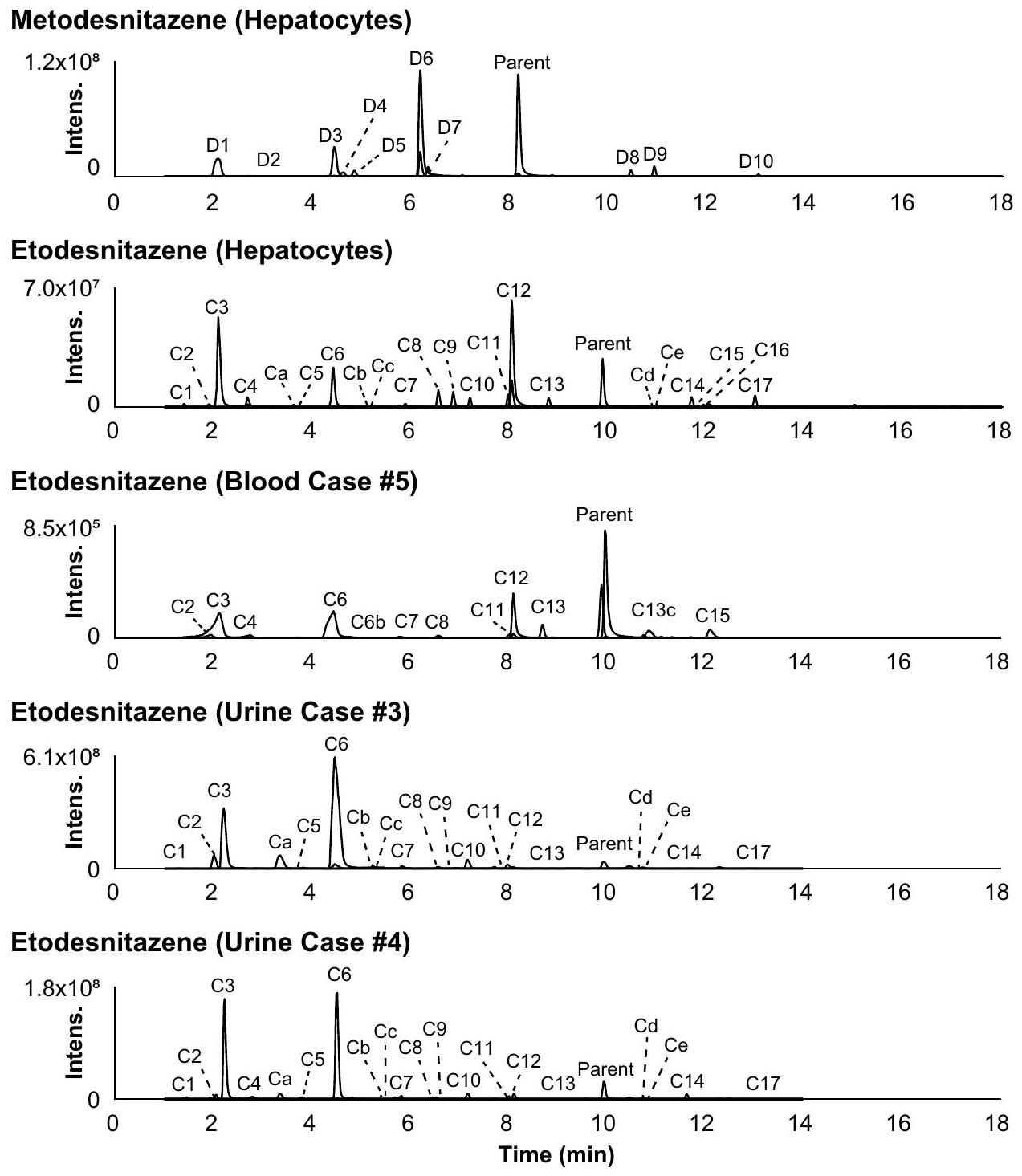
على التوالي. ومن المRemarkably، تم فصل C 1 في بداية تدرج LC، مما يوضح أهمية تحسين إعدادات LC لدراسات تحديد المستقلبات لتجنب فقدان المستقلبات الأكثر قطبية.
تحديد المستقلبات في العينات بعد الوفاة
مساحة قمة الطيف الكتلي للإيزوتونيتازين والمواد الأيضية في وضع التأين الإيجابي بعد 3 ساعات من الحضانة مع خلايا الكبد البشرية
| هوية | التحول الحيوي | التركيب العنصري | RT، دقيقة | نسبة الكتلة إلى الشحنة (m/z)
|
منطقة الذروة في الحضانة لمدة 3 ساعات | |||
| أ1 |
|
|
7.69 | 517.1923 (-1.13) |
|
|||
| A2 |
|
|
8.39 | 313.1292 (-0.88) |
|
|||
| A3 |
|
|
9.03 | 545.2243 (0.17) |
|
|||
| A4 |
|
|
9.37 | 561.2188 (-0.66) |
|
|||
| A5 |
|
|
9.69 | ٣٤١.١٦٠٣ (-١.٤٠) |
|
|||
| A6 |
|
|
11.19 | 369.1920 (-0.34) |
|
|||
| أ7 |
|
|
12.02 | ٣٩٩.٢٠٢٣ (-٠.٩٦) |
|
|||
| أ8 |
|
|
12.27 | 314.1134 (-0.52) |
|
|||
| أ9 |
|
|
12.41 | 355.1400 (-0.29) |
|
|||
| A10 |
|
|
15.84 | 355.1760 (-1.23) |
|
|||
| أ11 |
|
|
١٦.٥٦ | 383.2072 (- 1.45) |
|
|||
| إيزوتونيتازين (الأصل) |
|
17.21 | 411.2386 (- 1.09) |
|
||||
| أ12 |
|
|
17.86 | 603.2296 (- 0.22) |
|
تم اكتشافها في جميع العينات، على الرغم من أنها لم تكن مستقلبات رئيسية. تم اكتشاف Ba و Bb بأثر رجعي في الحاضنات، ولكن مع شدة إشارة أقل من العتبة المحددة.
(C3) ، و etodesnitazene كانت سائدة في الدم؛ و
نقاش
في المختبر مقابل العينات الأصلية والمقارنة بين النظائر
مساحة قمة الطيف الكتلي للميتونيتيزين والمواد الأيضية في وضع التأين الإيجابي بعد 3 ساعات من الحضانة مع خلايا الكبد البشرية وفي العينات بعد الوفاة
| هوية | التركيب العنصري | RT، دقيقة |
|
منطقة الذروة في الحاضنات لمدة 3 ساعات | قضية الدم #1 | قضية الدم #2 | منطقة الذروة في البول الحالة رقم 1 | |
| بدون التحلل المائي | مع التحلل المائي | |||||||
| با |
|
٥.٥٢ | 353.2336 (0.03) |
|
ND |
|
|
|
| ب1 |
|
7.76 | 517.1926 (0.58) |
|
|
|
|
ND |
| بي بي |
|
٧.٧ | 395.2442 (0.12) |
|
|
|
|
|
| ب2 |
|
9.11 | 545.2241 (0.16) |
|
ND | ND | ND | ND |
| ب3 |
|
9.77 | ٣٤١.١٦٠٦ (٠.٦٣) |
|
|
|
ND |
|
| ب4 |
|
11.27 | ٣٦٩.١٩١٩ (٠.٥٨) |
|
|
ND |
|
|
| ب5 |
|
12.36 | 314.1134 (0.42) |
|
|
|
|
|
| ب6 |
|
١٣.٦٦ | 355.1759 (1.59) |
|
|
|
|
|
| ميتونيتيزين (الأصل) |
|
15.02 | ٣٨٣.٢٠٧٣ (١.٢٩) |
|
|
|
|
|
| ب7 |
|
16.84 | ٣٦٩.١٥٦١ (٠.٩٩) |
|
ND |
|
ND | ND |
تم العثور عليها بأثر رجعي في الحاضنات بعد التعرف عليها في عينات أصلية
حالات العمل (Krotulski وآخرون 2020، 2021). ومع ذلك،
الهيدروكسيل
| هوية | التحول الحيوي | التركيب العنصري | RT، دقيقة |
|
منطقة الذروة في الحاضنات لمدة 3 ساعات | حالة الدم رقم 5 | منطقة الذروة في البول | |||
| حالة البول رقم 3 | حالة البول رقم 4 | |||||||||
| بدون التحلل المائي | مع التحلل المائي | بدون التحلل المائي | مع التحلل المائي | |||||||
| C1 |
|
|
1.39 | 472.2080 (0.33) |
|
ND |
|
|
|
|
| C2 |
|
|
1.90 | 340.2019 (-0.07) |
|
|
|
|
|
|
| C3 |
|
|
2.10 | 296.1758 (0.24) |
|
|
|
|
|
|
| C4 |
|
|
2.69 | 500.2395 (0.81) |
|
|
|
|
|
|
| كا |
|
|
3.21 | 340.2019 (-0.05) |
|
ND |
|
|
|
|
| C5 |
|
|
3.63 | 340.2020 (0.05) |
|
ND |
|
|
|
|
| C6 |
|
|
٤.٤٣ | ٣٢٤.٢٠٧١ (٠.٢٥) |
|
|
|
|
|
|
| سي بي |
|
|
5.39 | 445.1605 (-0.09) |
|
ND |
|
ND |
|
|
| نسخة إلى | هيدروكسيليشن (O-إيثيل) |
|
٥.٧٦ | 368.2331 (-0.14) |
|
|
|
|
|
|
| C7 |
|
|
5.89 | ٢٨٣.١٠٧٥ (-٠.٨٤) |
|
|
|
|
|
|
| C8 |
|
|
6.56 | 340.2016 (-0.95) |
|
|
|
|
|
|
| سي9 |
|
|
6.86 | 296.1756 (-0.47) |
|
ND |
|
|
|
|
| C10 |
|
|
7.19 | 269.1283 (-0.46) |
|
ND |
|
|
|
|
| هوية | التحول الحيوي | التركيب العنصري | RT، دقيقة | نسبة الكتلة إلى الشحنة (m/z)
|
منطقة الذروة في
|
حالة الدم رقم 5 | منطقة الذروة في البول | |||
| حالة البول رقم 3 | حالة البول رقم 4 | |||||||||
| بدون التحلل المائي | مع التحلل المائي | بدون التحلل المائي | مع التحلل المائي | |||||||
| سي11 | الهيدروكسيل (البنزيميدازول أو
|
|
7.97 | 368.2330 (-0.61) |
|
|
|
|
|
|
| C12 |
|
|
8.05 | ٣٢٤.٢٠٦٦ (-١.٣٥) |
|
|
|
|
|
|
| سي 13 |
|
|
٨.٨٠ | ٣٢٢.١٩١١ (٠.٩٠) |
|
|
|
|
|
|
| إيتوديسنيتازين (الأصل) |
|
9.89 | 352.2379 (-1.39) |
|
|
|
|
|
|
|
| سي دي |
|
|
10.46 | ٣٣٨.١٨٦٣ (٠.٠٠) |
|
ND |
|
|
|
|
| هذا | الهيدروكسيل (البنزيميدازول) |
|
10.69 | 368.2332 (-0.05) |
|
|
|
|
|
|
| C14 | إزالة الأمين التأكسدية
|
|
11.69 | 311.1389 (-0.42) |
|
ND |
|
|
|
|
| C15 | إزالة الأمين التأكسدية + الهيدروكسلة
|
|
12.04 | 313.1544 (0.99) |
|
|
ND | ND | ND | ND |
| سي16 |
|
|
12.42 | ٣٣٨.١٨٦٠ (-٠.٩٠) |
|
ND | ND | ND | ND | ND |
| C17 | إزالة الأمين التأكسدية |
|
12.98 | 297.1597 (-0.18) |
|
ND |
|
|
|
|
تم العثور عليها بأثر رجعي في الحاضنات بعد التعرف عليها في عينات أصلية
مساحة قمة الطيف الكتلي لمادة الميتوديسنيتازين والمواد الأيضية في وضع التأين الإيجابي بعد 3 ساعات من الحضانة مع خلايا الكبد البشرية
| هوية | التحول الحيوي | التركيب العنصري | RT، دقيقة |
|
منطقة الذروة في الحاضنات لمدة 3 ساعات |
| D1 |
|
|
2.09 | 296.1756 (1.35) |
|
| D2 |
|
|
2.47 | 500.2392 (0.15) |
|
| D3 |
|
|
٤.٤٤ | ٣٢٤.٢٠٧١ (٠.١٨) |
|
| D4 |
|
|
٤.٦٣ | 326.1863 (-0.01) |
|
| D5 |
|
|
٤.٨٥ | 282.1601 (0.04) |
|
| D6 |
|
|
6.18 | 310.1911 (-0.9) |
|
| D7 | الهيدروكسيل (إيميدازول) |
|
6.35 | ٣٥٤.٢١٧٥ (-٠.٣) |
|
| ميتوديسنيتازين |
|
8.17 | ٣٣٨.٢٢٢٢ (١.٤٥) |
|
|
| D8 |
|
|
10.46 | ٣٢٤.١٧٠٥ (-٠.٥) |
|
| D9 | إزالة الأمين التأكسدية |
|
10.93 | 283.1440 (-0.4) |
|
| D10 |
|
|
13.04 | 326.1863 (-0.01) |
|
تم الكشف عن المستقلبات بشدة أقل بكثير في عينات الميتونيتازين مقارنةً بالعينات الأصلية، مما قد يشير إلى الأيض خارج الكبد. على عكس ملاحظاتنا، وجد كروتولسكي وآخرون أن 5-أمينوميتونيتازين كان المستقلب الرئيسي في دم جميع الحالات الأربعة عشر التي تم فيها الكشف عن مستقلبات الميتونيتازين (كروتولسكي وآخرون 2021). أعد المؤلفون العينات باستخدام استخراج سائل-سائل، مما قد يكون أثر على الاسترداد النسبي للمستقلبات، ولكن، على العكس، قد يكون ترسيب البروتين في الدراسة الحالية قد أثر على التأثير النسبي للمصفوفة للمستقلبات. قد تكون هناك أسباب محتملة أخرى لهذه الفجوة بسبب عدم استقرار المستقلب، أو إعادة توزيع ما بعد الوفاة المختلفة، أو التباينات الجينية بين الأفراد. هناك حاجة إلى مزيد من التحقيق باستخدام معيار مرجعي نقي للمستقلب لفهم أهميته بشكل أفضل.
قد يفسر هذا التباين،
مؤشرات الاستهلاك
سلسلة جانبية. وبالمثل، إيتوديسنيتازين وميتوديسنيتازين
الاستنتاجات
الإعلانات
References
Barrios R, Lawrence SV, Rosen LW (2024) China primer: illicit fentanyl and China’s role. https://crsreports.congress.gov/product/pdf/ IF/IF10890. Accessed 2 Feb 2024
Berardinelli D, Taoussi O, Carlier J et al (2024) In vitro, in vivo metabolism and quantification of the novel synthetic opioid N-piperidinyl etonitazene (etonitazepipne). Clin Chem Lab Med. https://doi. org/10.1515/cclm-2023-1360
Brunetti P, Pirani F, Carlier J et al (2021) A 2017-2019 update on acute intoxications and fatalities from illicit fentanyl and analogs. J Anal Toxicol 45:537-554. https://doi.org/10.1093/JAT/BKAA115
Brunetti P, Lo Faro AF, Di Trana A et al (2023)
De Bruyn KC, Šícho M, Mazzolari A, Kirchmair J (2021) GLORYx: Prediction of the metabolites resulting from phase 1 and phase 2 biotransformations of xenobiotics. Chem Res Toxicol 34:286299. https://doi.org/10.1021/acs.chemrestox.0c00224
Carlier J, Diao X, Huestis MA (2018) Synthetic cannabinoid BB-22 (QUCHIC): human hepatocytes metabolism with liquid chroma-tography-high resolution mass spectrometry detection. J Pharm Biomed Anal 157:27-35. https://doi.org/10.1016/J.JPBA.2018. 05.007
analysis and targeted/untargeted data mining. J Chromatogr B Analyt Technol Biomed Life Sci 1193:123162. https://doi.org/ 10.1016/j.jchromb.2022.123162
Di Trana A, Brunetti P, Giorgetti R et al (2021) In silico prediction, LC-HRMS/MS analysis, and targeted/untargeted data-mining workflow for the profiling of phenylfentanyl in vitro metabolites. Talanta 235:122740. https://doi.org/10.1016/J.TALANTA.2021. 122740
Di Trana A, La Maida N, Froldi R et al (2023) The new synthetic benzimidazole opioid etonitazepipne: an emerging fatal harm and a challenge for laboratory medicine. Clin Chem Lab Med 61:E200E202. https://doi.org/10.1515/CCLM-2023-0186
Drug Enforcement Administration (DEA) (2018) Schedules of controlled substances: temporary placement of fentanyl-related substances in schedule I. Temporary amendment; temporary scheduling order. Fed Regist 83:5188-5192
Drug Enforcement Administration (DEA) Diversion control division (2023) isotonitazene. https://www.deadiversion.usdoj.gov/drug_ chem_info/isotonitazene.pdf. Accessed 2 February 2024
European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) (2022) European drug report 2022: trends and developments. https://www.emcdda.europa.eu/publications/edr/trendsdevelopments/2022_en. accessed 2 Feb 2024
European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) (2023a) The drug situation in Europe up to 2023 – an overview and assessment of emerging threats and new developments (european drug report 2023). https://www.emcdda.europa. eu/publications/european-drug-report/2023/drug-situation-in-europe-up-to-2023_en. accessed 2 Feb 2024
European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) (2023b) New psychoactive substances – the current situation in europe (european drug report 2023). https://www. emcdda.europa.eu/publications/european-drug-report/2023/new-psychoactive-substances_en. accessed 2 Feb 2024
Grigoryev A, Kavanagh P, Dowling G, Rodin I (2023) Tentative identification of etazene (etodesnitazene) metabolites in rat serum and urine by gas chromatography-mass spectrometry and accurate mass liquid chromatography-mass spectrometry. J Anal Toxicol 46:1032-1037. https://doi.org/10.1093/JAT/BKAC001
Hunger A, Kebrle J, Rossi A, Hoffmann K (1957) Synthesis of analgesically active benzimidazole derivatives with basic substitutions. Experientia 13:400-401. https://doi.org/10.1007/BF02161116
Hunger A, Kebrle J, Rossi A, Hoffmann K (1960) Benzimidazol-derivate und verwandte heterocyclen VI. Synthese von phenyl-[1-ami-noalkyl-benzimidazolyl-(2)]-essigsäure-estern und -amiden. Helv Chim Acta 43:1727-1733. https://doi.org/10.1002/hlca. 19600 430634
Keller BO, Sui J, Young AB, Whittal RM (2008) Interferences and contaminants encountered in modern mass spectrometry. Anal Chim Acta 627:71-81. https://doi.org/10.1016/J.ACA.2008.04.043
Krotulski AJ, Papsun DM, Kacinko SL, Logan BK (2020) Isotonitazene quantitation and metabolite discovery in authentic forensic casework. J Anal Toxicol 44:521-530. https://doi.org/10.1093/ JAT/BKAA016
Krotulski AJ, Papsun DM, Walton SE, Logan BK (2021) Metonitazene in the united states-forensic toxicology assessment of a
potent new synthetic opioid using liquid chromatography mass spectrometry. Drug Test Anal 13:1697-1711. https://doi.org/10. 1002/dta. 3115
Mattson CL, Tanz LJ, Quinn K et al (2021) Trends and geographic patterns in drug and synthetic opioid overdose deaths-United States, 2013-2019. MMWR Morb Mortal Wkly Rep 70(6):202207. https://doi.org/10.15585/MMWR.MM7006A4
Papsun DM, Krotulski AJ, Logan BK (2022) Proliferation of novel synthetic opioids in postmortem investigations after core-structure scheduling for fentanyl-related substances. Am J Forensic Med Pathol 43:315-327. https://doi.org/10.1097/PAF. 0000000000 000787
Prekupec MP, Mansky PA, Baumann MH (2017) Misuse of novel synthetic opioids: a deadly new trend. J Addict Med 11:256-265. https://doi.org/10.1097/ADM. 0000000000000324
Schumann JL, Syrjanen R, Alford K et al (2023) Intoxications in an Australian emergency department involving “nitazene” benzylbenzimidazole synthetic opioids (etodesnitazene, butonitazene and protonitazene). J Anal Toxicol 47:E6-E9. https://doi.org/10.1093/ JAT/BKAC062
Ujváry I, Christie R, Evans-Brown M et al (2021) DARK classics in chemical neuroscience: etonitazene and related benzimidazoles. ACS Chem Neurosci 12:1072-1092. https://doi.org/10.1021/ ACSCHEMNEURO.1C00037
United Nations Office on Drugs and Crime (UNODC) (2020) The growing complexity of the opioid crisis-Global SMART update, Volume 24. https://www.unodc.org/documents/scientific/Global_ SMART_Update_2020-Vol.24-Eng.pdf Accessed 2 Feb 2024
Vandeputte MM, Van Uytfanghe K, Layle NK (2021) Synthesis, chemical characterization, and-opioid receptor activity assessment of the emerging group of nitazene new synthetic opioids. ACS Chem Neurosci 12:1241-1251. https://doi.org/10.1021/acschemneuro. 1c00064
Vandeputte MM, Krotulski AJ, Walther D et al (2022a) Pharmacological evaluation and forensic case series of N -pyrrolidino etonitazene (etonitazepyne), a newly emerging 2-benzylbenzimidazole “nitazene” synthetic opioid. Arch Toxicol 96:1845-1863. https:// doi.org/10.1007/S00204-022-03276-4
Vandeputte MM, Verougstraete N, Walther D et al (2022b) First identification, chemical analysis and pharmacological characterization of N-piperidinyl etonitazene (etonitazepipne), a recent addition to the 2-benzylbenzimidazole opioid subclass. Arch Toxicol 96:1865-1880. https://doi.org/10.1007/S00204-022-03294-2
Verougstraete N, Verhaeghe A, Germonpré J et al (2023) Identification of etazene (etodesnitazene) metabolites in human urine by LC-HRMS. Drug Test Anal 15:235-239. https://doi.org/10.1002/ DTA. 3377
- Francesco P. Busardò
fra.busardo@libero.it
Unit of Forensic Toxicology, Section of Legal Medicine, Department of Biomedical Sciences and Public Health, Marche Polytechnic University, Via Tronto 10/a, 60126 Ancona AN, Italy
2 Department of Anatomical, Histological, Forensic and Orthopaedic Sciences, Sapienza University of Rome, Rome, Italy
Department of Trauma Surgery, IRCCS Galeazzi Orthopedic Institute, Milan, Italy
4 Department of Forensic Genetics and Forensic Toxicology, National Board of Forensic Medicine, Linköping, Sweden
5 Institute of Forensic Medicine, Forensic Toxicology, Medical Center, University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany
DOI: https://doi.org/10.1007/s00204-024-03735-0
PMID: https://pubmed.ncbi.nlm.nih.gov/38582802
Publication Date: 2024-04-06
Human metabolism of four synthetic benzimidazole opioids: isotonitazene, metonitazene, etodesnitazene, and metodesnitazene
© The Author(s) 2024
Abstract
Following isotonitazene scheduling in 2019, the availability of alternative 2-benzylbenzimidazole opioids (nitazenes) on the global drug market increased, resulting in many fatalities worldwide. Nitazenes are potent
Introduction
parent in in vitro experiments evaluating MOR activation (Vandeputte et al. 2021). Etodesnitazene major phase I metabolites were recently identified in rats (Grigoryev et al. 2023). The metabolic fate of other benzimidazole opioids was not yet assessed.
Materials and methods
Chemicals and reagents
In silico metabolite prediction
second-generation metabolites was multiplied to the score of the corresponding first-generation metabolite to calculate an “adjusted score”.
Human hepatocyte incubations
Postmortem samples
Sample preparation
Incubates
Urine and blood samples
Liquid chromatography-high-resolution tandem mass spectrometry settings
Liquid chromatography
returning to initial conditions within 0.1 min ; re-equilibration time was 4.9 min .
High-resolution tandem mass spectrometry
Metabolite identification
4′-Hydroxyl-nitazene,
Results
In silico metabolite prediction
HRMS/MS fragmentation pattern of isotonitazene, metonitazene, etodesnitazene, and metodesnitazene

Metabolite identification in human hepatocyte incubations

O-dealkylation and further O-glucuronidation

(4′-hydroxyl-nitazene) was confirmed through the injection of the commercially available reference standard.
Combination of

respectively. Remarkably, C 1 eluted at the very beginning of the LC gradient, demonstrating the importance of LC setting optimization for metabolite identification studies to avoid missing more polar metabolites.
Metabolite identification in postmortem samples
spectrometry peak area of isotonitazene and metabolites in positiveionization mode after 3-h Incubation with human hepatocytes
| ID | Biotransformation | Elemental composition | RT, min | m/z (
|
Peak area in 3-h incubate | |||
| A1 |
|
|
7.69 | 517.1923 (-1.13) |
|
|||
| A2 |
|
|
8.39 | 313.1292 (-0.88) |
|
|||
| A3 |
|
|
9.03 | 545.2243 (0.17) |
|
|||
| A4 |
|
|
9.37 | 561.2188 (-0.66) |
|
|||
| A5 |
|
|
9.69 | 341.1603 (-1.40) |
|
|||
| A6 |
|
|
11.19 | 369.1920 (-0.34) |
|
|||
| A7 |
|
|
12.02 | 399.2023 (-0.96) |
|
|||
| A8 |
|
|
12.27 | 314.1134 (-0.52) |
|
|||
| A9 |
|
|
12.41 | 355.1400 (-0.29) |
|
|||
| A10 |
|
|
15.84 | 355.1760 (-1.23) |
|
|||
| A11 |
|
|
16.56 | 383.2072 (- 1.45) |
|
|||
| Isotonitazene (parent) |
|
17.21 | 411.2386 (- 1.09) |
|
||||
| A12 |
|
|
17.86 | 603.2296 (- 0.22) |
|
were detected in all samples, although they were not major metabolites. Ba and Bb were retrospectively detected in incubates, but with a signal intensity below the established threshold.
(C3), and etodesnitazene were preponderant in blood; and
Discussion
In vitro versus authentic samples and comparison between analogues
spectrometry peak area of metonitazene and metabolites in positiveionization mode after 3-h Incubation with human hepatocytes and in postmortem samples
| ID | Elemental composition | RT, min |
|
Peak area in 3-h incubates | Blood Case #1 | Blood Case #2 | Peak area in urine Case #1 | |
| Without hydrolysis | With hydrolysis | |||||||
| Ba |
|
5.52 | 353.2336 (0.03) |
|
ND |
|
|
|
| B1 |
|
7.76 | 517.1926 (0.58) |
|
|
|
|
ND |
| Bb |
|
7.7 | 395.2442 (0.12) |
|
|
|
|
|
| B2 |
|
9.11 | 545.2241 (0.16) |
|
ND | ND | ND | ND |
| B3 |
|
9.77 | 341.1606 (0.63) |
|
|
|
ND |
|
| B4 |
|
11.27 | 369.1919 (0.58) |
|
|
ND |
|
|
| B5 |
|
12.36 | 314.1134 (0.42) |
|
|
|
|
|
| B6 |
|
13.66 | 355.1759 (1.59) |
|
|
|
|
|
| Metonitazene (parent) |
|
15.02 | 383.2073 (1.29) |
|
|
|
|
|
| B7 |
|
16.84 | 369.1561 (0.99) |
|
ND |
|
ND | ND |
*Found retrospectively in incubates after identification in authentic samples
casework (Krotulski et al. 2020, 2021). However,
hydroxylation and
| ID | Biotransformation | Elemental composition | RT, min |
|
Peak area in 3-h incubates | Blood case #5 | Peak area in urine | |||
| Urine case #3 | Urine case #4 | |||||||||
| Without hydrolysis | With hydrolysis | Without hydrolysis | With hydrolysis | |||||||
| C1 |
|
|
1.39 | 472.2080 (0.33) |
|
ND |
|
|
|
|
| C2 |
|
|
1.90 | 340.2019 (-0.07) |
|
|
|
|
|
|
| C3 |
|
|
2.10 | 296.1758 (0.24) |
|
|
|
|
|
|
| C4 |
|
|
2.69 | 500.2395 (0.81) |
|
|
|
|
|
|
| Ca |
|
|
3.21 | 340.2019 (-0.05) |
|
ND |
|
|
|
|
| C5 |
|
|
3.63 | 340.2020 (0.05) |
|
ND |
|
|
|
|
| C6 |
|
|
4.43 | 324.2071 (0.25) |
|
|
|
|
|
|
| Cb |
|
|
5.39 | 445.1605 (-0.09) |
|
ND |
|
ND |
|
|
| Cc | Hydroxylation (O-ethyl) |
|
5.76 | 368.2331 (-0.14) |
|
|
|
|
|
|
| C7 |
|
|
5.89 | 283.1075 (-0.84) |
|
|
|
|
|
|
| C8 |
|
|
6.56 | 340.2016 (-0.95) |
|
|
|
|
|
|
| C9 |
|
|
6.86 | 296.1756 (-0.47) |
|
ND |
|
|
|
|
| C10 |
|
|
7.19 | 269.1283 (-0.46) |
|
ND |
|
|
|
|
| ID | Biotransformation | Elemental composition | RT, min | m/z (
|
Peak area in
|
Blood case #5 | Peak area in urine | |||
| Urine case #3 | Urine case #4 | |||||||||
| Without hydrolysis | With hydrolysis | Without hydrolysis | With hydrolysis | |||||||
| C11 | Hydroxylation (benzimidazole or
|
|
7.97 | 368.2330 (-0.61) |
|
|
|
|
|
|
| C12 |
|
|
8.05 | 324.2066 (-1.35) |
|
|
|
|
|
|
| C13 |
|
|
8.80 | 322.1911 (0.90) |
|
|
|
|
|
|
| Etodesnitazene (parent) |
|
9.89 | 352.2379 (-1.39) |
|
|
|
|
|
|
|
| Cd |
|
|
10.46 | 338.1863 (0.00) |
|
ND |
|
|
|
|
| Ce | Hydroxylation (benzimidazole) |
|
10.69 | 368.2332 (-0.05) |
|
|
|
|
|
|
| C14 | Oxidative deamination
|
|
11.69 | 311.1389 (-0.42) |
|
ND |
|
|
|
|
| C15 | Oxidative deamination + Hydroxylation (
|
|
12.04 | 313.1544 (0.99) |
|
|
ND | ND | ND | ND |
| C16 |
|
|
12.42 | 338.1860 (-0.90) |
|
ND | ND | ND | ND | ND |
| C17 | Oxidative deamination |
|
12.98 | 297.1597 (-0.18) |
|
ND |
|
|
|
|
*Found retrospectively in incubates after identification in authentic samples
trometry peak area of metodesnitazene and metabolites in positiveionization mode after 3-h Incubation with human hepatocytes
| ID | Biotransformation | Elemental composition | RT, min |
|
Peak area in 3-h incubates |
| D1 |
|
|
2.09 | 296.1756 (1.35) |
|
| D2 |
|
|
2.47 | 500.2392 (0.15) |
|
| D3 |
|
|
4.44 | 324.2071 (0.18) |
|
| D4 |
|
|
4.63 | 326.1863 (-0.01) |
|
| D5 |
|
|
4.85 | 282.1601 (0.04) |
|
| D6 |
|
|
6.18 | 310.1911 (-0.9) |
|
| D7 | Hydroxylation (imidazole) |
|
6.35 | 354.2175 (-0.3) |
|
| Metodesnitazene |
|
8.17 | 338.2222 (1.45) |
|
|
| D8 |
|
|
10.46 | 324.1705 (-0.5) |
|
| D9 | Oxidative deamination |
|
10.93 | 283.1440 (-0.4) |
|
| D10 |
|
|
13.04 | 326.1863 (-0.01) |
|
metabolites were detected with a much lower intensity in metonitazene incubates compared to authentic samples, potentially indicating extra-hepatic metabolism. Contrary to our observations, Krotulski et al. found that 5 -aminometonitazene was the main metabolite in the blood of all 14 cases in which metonitazene metabolites were detected (Krotulski et al. 2021). The authors prepared the samples with a liquid-liquid extraction, which might have affected the relative recovery of the metabolites, but, conversely, the protein precipitation of the present study might have affected the relative matrix effect of the metabolites. Other potential reasons to this discrepancy might be due to the instability of the metabolite, a different postmortem redistribution, or interindividual genetic variations. Further investigation with a purified reference standard of the metabolite is necessary to further understand its significance.
might explain this discrepancy,

Biomarkers of consumption
side chain. Similarly, etodesnitazene and metodesnitazene
Conclusions
Declarations
References
Barrios R, Lawrence SV, Rosen LW (2024) China primer: illicit fentanyl and China’s role. https://crsreports.congress.gov/product/pdf/ IF/IF10890. Accessed 2 Feb 2024
Berardinelli D, Taoussi O, Carlier J et al (2024) In vitro, in vivo metabolism and quantification of the novel synthetic opioid N-piperidinyl etonitazene (etonitazepipne). Clin Chem Lab Med. https://doi. org/10.1515/cclm-2023-1360
Brunetti P, Pirani F, Carlier J et al (2021) A 2017-2019 update on acute intoxications and fatalities from illicit fentanyl and analogs. J Anal Toxicol 45:537-554. https://doi.org/10.1093/JAT/BKAA115
Brunetti P, Lo Faro AF, Di Trana A et al (2023)
De Bruyn KC, Šícho M, Mazzolari A, Kirchmair J (2021) GLORYx: Prediction of the metabolites resulting from phase 1 and phase 2 biotransformations of xenobiotics. Chem Res Toxicol 34:286299. https://doi.org/10.1021/acs.chemrestox.0c00224
Carlier J, Diao X, Huestis MA (2018) Synthetic cannabinoid BB-22 (QUCHIC): human hepatocytes metabolism with liquid chroma-tography-high resolution mass spectrometry detection. J Pharm Biomed Anal 157:27-35. https://doi.org/10.1016/J.JPBA.2018. 05.007
analysis and targeted/untargeted data mining. J Chromatogr B Analyt Technol Biomed Life Sci 1193:123162. https://doi.org/ 10.1016/j.jchromb.2022.123162
Di Trana A, Brunetti P, Giorgetti R et al (2021) In silico prediction, LC-HRMS/MS analysis, and targeted/untargeted data-mining workflow for the profiling of phenylfentanyl in vitro metabolites. Talanta 235:122740. https://doi.org/10.1016/J.TALANTA.2021. 122740
Di Trana A, La Maida N, Froldi R et al (2023) The new synthetic benzimidazole opioid etonitazepipne: an emerging fatal harm and a challenge for laboratory medicine. Clin Chem Lab Med 61:E200E202. https://doi.org/10.1515/CCLM-2023-0186
Drug Enforcement Administration (DEA) (2018) Schedules of controlled substances: temporary placement of fentanyl-related substances in schedule I. Temporary amendment; temporary scheduling order. Fed Regist 83:5188-5192
Drug Enforcement Administration (DEA) Diversion control division (2023) isotonitazene. https://www.deadiversion.usdoj.gov/drug_ chem_info/isotonitazene.pdf. Accessed 2 February 2024
European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) (2022) European drug report 2022: trends and developments. https://www.emcdda.europa.eu/publications/edr/trendsdevelopments/2022_en. accessed 2 Feb 2024
European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) (2023a) The drug situation in Europe up to 2023 – an overview and assessment of emerging threats and new developments (european drug report 2023). https://www.emcdda.europa. eu/publications/european-drug-report/2023/drug-situation-in-europe-up-to-2023_en. accessed 2 Feb 2024
European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) (2023b) New psychoactive substances – the current situation in europe (european drug report 2023). https://www. emcdda.europa.eu/publications/european-drug-report/2023/new-psychoactive-substances_en. accessed 2 Feb 2024
Grigoryev A, Kavanagh P, Dowling G, Rodin I (2023) Tentative identification of etazene (etodesnitazene) metabolites in rat serum and urine by gas chromatography-mass spectrometry and accurate mass liquid chromatography-mass spectrometry. J Anal Toxicol 46:1032-1037. https://doi.org/10.1093/JAT/BKAC001
Hunger A, Kebrle J, Rossi A, Hoffmann K (1957) Synthesis of analgesically active benzimidazole derivatives with basic substitutions. Experientia 13:400-401. https://doi.org/10.1007/BF02161116
Hunger A, Kebrle J, Rossi A, Hoffmann K (1960) Benzimidazol-derivate und verwandte heterocyclen VI. Synthese von phenyl-[1-ami-noalkyl-benzimidazolyl-(2)]-essigsäure-estern und -amiden. Helv Chim Acta 43:1727-1733. https://doi.org/10.1002/hlca. 19600 430634
Keller BO, Sui J, Young AB, Whittal RM (2008) Interferences and contaminants encountered in modern mass spectrometry. Anal Chim Acta 627:71-81. https://doi.org/10.1016/J.ACA.2008.04.043
Krotulski AJ, Papsun DM, Kacinko SL, Logan BK (2020) Isotonitazene quantitation and metabolite discovery in authentic forensic casework. J Anal Toxicol 44:521-530. https://doi.org/10.1093/ JAT/BKAA016
Krotulski AJ, Papsun DM, Walton SE, Logan BK (2021) Metonitazene in the united states-forensic toxicology assessment of a
potent new synthetic opioid using liquid chromatography mass spectrometry. Drug Test Anal 13:1697-1711. https://doi.org/10. 1002/dta. 3115
Mattson CL, Tanz LJ, Quinn K et al (2021) Trends and geographic patterns in drug and synthetic opioid overdose deaths-United States, 2013-2019. MMWR Morb Mortal Wkly Rep 70(6):202207. https://doi.org/10.15585/MMWR.MM7006A4
Papsun DM, Krotulski AJ, Logan BK (2022) Proliferation of novel synthetic opioids in postmortem investigations after core-structure scheduling for fentanyl-related substances. Am J Forensic Med Pathol 43:315-327. https://doi.org/10.1097/PAF. 0000000000 000787
Prekupec MP, Mansky PA, Baumann MH (2017) Misuse of novel synthetic opioids: a deadly new trend. J Addict Med 11:256-265. https://doi.org/10.1097/ADM. 0000000000000324
Schumann JL, Syrjanen R, Alford K et al (2023) Intoxications in an Australian emergency department involving “nitazene” benzylbenzimidazole synthetic opioids (etodesnitazene, butonitazene and protonitazene). J Anal Toxicol 47:E6-E9. https://doi.org/10.1093/ JAT/BKAC062
Ujváry I, Christie R, Evans-Brown M et al (2021) DARK classics in chemical neuroscience: etonitazene and related benzimidazoles. ACS Chem Neurosci 12:1072-1092. https://doi.org/10.1021/ ACSCHEMNEURO.1C00037
United Nations Office on Drugs and Crime (UNODC) (2020) The growing complexity of the opioid crisis-Global SMART update, Volume 24. https://www.unodc.org/documents/scientific/Global_ SMART_Update_2020-Vol.24-Eng.pdf Accessed 2 Feb 2024
Vandeputte MM, Van Uytfanghe K, Layle NK (2021) Synthesis, chemical characterization, and-opioid receptor activity assessment of the emerging group of nitazene new synthetic opioids. ACS Chem Neurosci 12:1241-1251. https://doi.org/10.1021/acschemneuro. 1c00064
Vandeputte MM, Krotulski AJ, Walther D et al (2022a) Pharmacological evaluation and forensic case series of N -pyrrolidino etonitazene (etonitazepyne), a newly emerging 2-benzylbenzimidazole “nitazene” synthetic opioid. Arch Toxicol 96:1845-1863. https:// doi.org/10.1007/S00204-022-03276-4
Vandeputte MM, Verougstraete N, Walther D et al (2022b) First identification, chemical analysis and pharmacological characterization of N-piperidinyl etonitazene (etonitazepipne), a recent addition to the 2-benzylbenzimidazole opioid subclass. Arch Toxicol 96:1865-1880. https://doi.org/10.1007/S00204-022-03294-2
Verougstraete N, Verhaeghe A, Germonpré J et al (2023) Identification of etazene (etodesnitazene) metabolites in human urine by LC-HRMS. Drug Test Anal 15:235-239. https://doi.org/10.1002/ DTA. 3377
- Francesco P. Busardò
fra.busardo@libero.it
Unit of Forensic Toxicology, Section of Legal Medicine, Department of Biomedical Sciences and Public Health, Marche Polytechnic University, Via Tronto 10/a, 60126 Ancona AN, Italy
2 Department of Anatomical, Histological, Forensic and Orthopaedic Sciences, Sapienza University of Rome, Rome, Italy
Department of Trauma Surgery, IRCCS Galeazzi Orthopedic Institute, Milan, Italy
4 Department of Forensic Genetics and Forensic Toxicology, National Board of Forensic Medicine, Linköping, Sweden
5 Institute of Forensic Medicine, Forensic Toxicology, Medical Center, University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany
