DOI: https://doi.org/10.1016/j.ijhydene.2024.03.223
تاريخ النشر: 2024-03-27
التطورات في مواد تخزين الهيدروجين: استغلال التكنولوجيا المبتكرة، من التعلم الآلي إلى الكيمياء الحاسوبية، لحلول تخزين الطاقة
نُشر في:
نسخة الوثيقة:
جامعة كوينز بلفاست – بوابة البحث:
حقوق الناشر
هذه مقالة مفتوحة الوصول نُشرت بموجب ترخيص المشاع الإبداعي للاستخدام مع النسبة.https://creativecommons.org/licenses/by/4.0/الذي يسمح بالاستخدام غير المقيد، والتوزيع، والاستنساخ في أي وسيلة، بشرط ذكر المؤلف والمصدر.
الحقوق العامة
سياسة الإزالة
الوصول المفتوح
التقدم في مواد تخزين الهيدروجين: استغلال التكنولوجيا المبتكرة، من التعلم الآلي إلى الكيمياء الحاسوبية، لحلول تخزين الطاقة
معلومات المقال
الكلمات المفتاحية:
الهيدروجين المضغوط
الهيدروجين المسال
الفحص عالي الإنتاجية
الكيمياء الحاسوبية
تعلم الآلة
الملخص
يتزايد الطلب على حلول الطاقة النظيفة والمستدامة مع نمو السكان العالمي وتطور الاقتصاديات. تساهم الوقود الأحفوري، الذي يهيمن حاليًا على قطاع الطاقة، في انبعاثات غازات الدفيئة وتدهور البيئة. استجابةً لهذه التحديات، ظهرت تقنيات تخزين الهيدروجين كمسار واعد لتحقيق استدامة الطاقة. تقدم هذه المراجعة نظرة عامة على التقدمات الأخيرة في مواد وتقنيات تخزين الهيدروجين، مع التأكيد على أهمية التخزين الفعال لتعظيم إمكانيات الهيدروجين. تبرز المراجعة طرق التخزين الفيزيائية مثل الهيدروجين المضغوط (الذي يصل ضغطه إلى 70 ميغاباسكال) والنهج المعتمدة على المواد التي تستخدم الهيدريدات المعدنية والمواد المحتوية على الكربون. كما تستكشف اعتبارات التصميم، والكيمياء الحاسوبية، والفحص عالي الإنتاجية، وتقنيات التعلم الآلي المستخدمة في تطوير مواد تخزين الهيدروجين الفعالة. تعرض هذه التحليل الشامل إمكانيات تخزين الهيدروجين في تلبية الطلبات الطاقية، وتقليل انبعاثات غازات الدفيئة، ودفع الابتكار في الطاقة النظيفة.
1. المقدمة
والآثار الضارة على صحة الإنسان. لقد ظهرت التوترات الجيوسياسية والمخاوف بشأن أمن الطاقة بسبب التوزيع غير المتساوي لموارد الوقود الأحفوري.
2. تقنيات تخزين الهيدروجين
2.1. تقنيات التخزين الفيزيائي
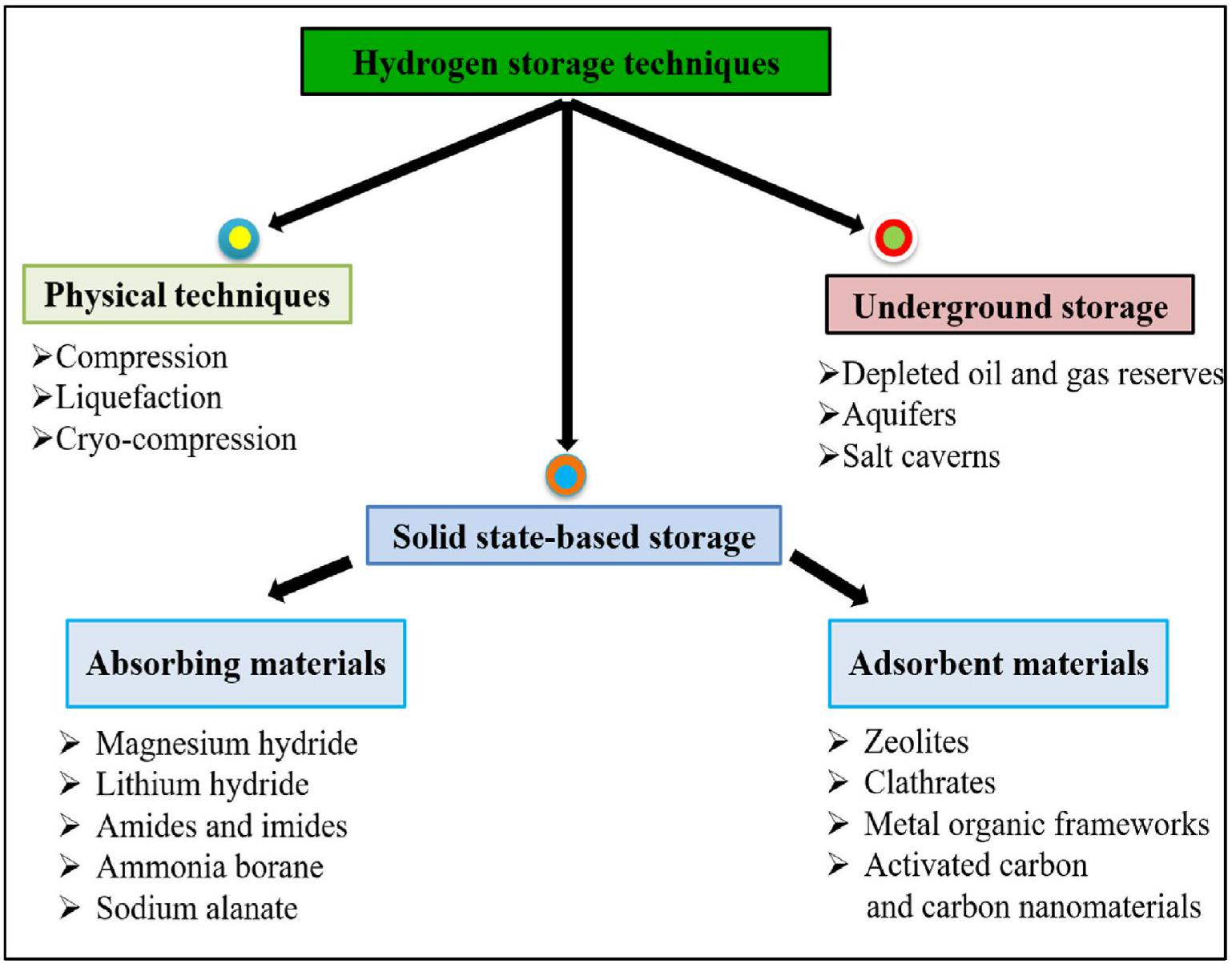
لمعالجة هذه القضية، تم تطوير تقنيات تخزين فيزيائية مختلفة للهيدروجين. تشمل هذه الطرق، التي تتضمن تقنيات الضغط والتسييل، إما بشكل فردي أو مجتمعة، حلولاً مبتكرة لتخزين الهيدروجين في أشكال كثيفة ومستقرة. تلعب هذه التقنيات دورًا محوريًا في التغلب على القيود المرتبطة بخفة وزن الهيدروجين وطبيعته الغازية، مما يمكّن من نقله واستخدامه بكفاءة عبر تطبيقات متنوعة. إنها تمثل بوابة لفتح إمكانيات الهيدروجين كحامل للطاقة النظيفة، مع تطبيقات تمتد إلى مركبات خلايا الوقود والعمليات الصناعية. الشكل 1 يقدم نظرة عامة موجزة عن تقنيات تخزين الهيدروجين المختلفة.
2.1.1. تقنية الهيدروجين المضغوط
2.1.2. تخزين الهيدروجين المسال
يمكن تسهيل نقل الهيدروجين من خلال أنابيب كريوجينية مخصصة أو صهاريج طرق متخصصة مزودة بأنظمة تخزين كريوجينية. علاوة على ذلك، تشمل الاتجاهات العالمية الناشئة النقل البحري للهيدروجين المسال داخل حاويات مخصصة على السفن.
2.1.3. تخزين الهيدروجين المضغوط بالتبريد
تخزين الغاز تحت ضغط عالٍ التقليدي، جنبًا إلى جنب مع الفقدان الحتمي الناتج عن التبخر الذي يتم مواجهته في نموذج تخزين الهيدروجين المبرد. الهدف الرئيسي من هذا النهج هو تحسين حلول تخزين الهيدروجين عبر تطبيقات متنوعة، مع تركيز ملحوظ على قطاع السيارات.
2.2. تخزين الهيدروجين القائم على المواد
تم استكشاف الهيدريدات [43]، المركبات القائمة على الأمونيا [44]، المواد الكربونية، الأطر العضوية المعدنية [45]، الأطر العضوية التساهمية [46]، الكلاترات [47]، وغيرها من المواد الحيوية والمواد المسامية [48] لتخزين الهيدروجين الكيميائي. على سبيل المثال، تشكل الهيدريدات المعدنية روابط قوية بين المعدن والهيدروجين، مما يمكّن من امتصاص الهيدروجين وإطلاقه من خلال التسخين أو التحفيز [49]. من ناحية أخرى، تُعرف الهيدريدات المعقدة بأنها مركبات متعددة العناصر معروفة بسعات التخزين الكبيرة عبر العمليات الكيميائية [50]. كل مادة لها نقاط قوتها وقيودها الفريدة بناءً على عوامل مختلفة [51]. لا يوجد مادة تخزين هيدروجين مثالية عالمياً؛ يعتمد الاختيار على سعة تخزين الهيدروجين، وظروف التشغيل، والديناميكا الحرارية، والحركية، والاستقرار، والعكسية، والتوافر، والتكلفة، والأثر البيئي. ستتناول الأقسام التالية التقدم في المواد المختارة لتخزين الهيدروجين الكيميائي.
2.2.1. الممتزات الكيميائية
2.2.1.1. هيدريدات المعادن. هيدريدات المعادن، وهي فئة من المواد المكونة من المعدن والهيدروجين، قد حظيت باهتمام كبير مؤخرًا بسبب قدرتها الكبيرة على تخزين الهيدروجين، مما يجعلها واعدة لأنظمة الطاقة المعتمدة على الهيدروجين. إن خصائصها، بما في ذلك كثافة الطاقة العالية، والتكلفة المنخفضة نسبيًا، وصداقة البيئة، تجعلها جذابة للتطبيقات التي تشمل الإلكترونيات المحمولة، والمركبات الكهربائية، وأنظمة الطاقة المتجددة. ومع ذلك، فإن تقدمها في تخزين الهيدروجين يواجه عقبات كبيرة، لا سيما تحديد المواد ذات القدرة العالية على تخزين الهيدروجين مع الحفاظ على الاستقرار والسلامة والجدوى الاقتصادية [55].
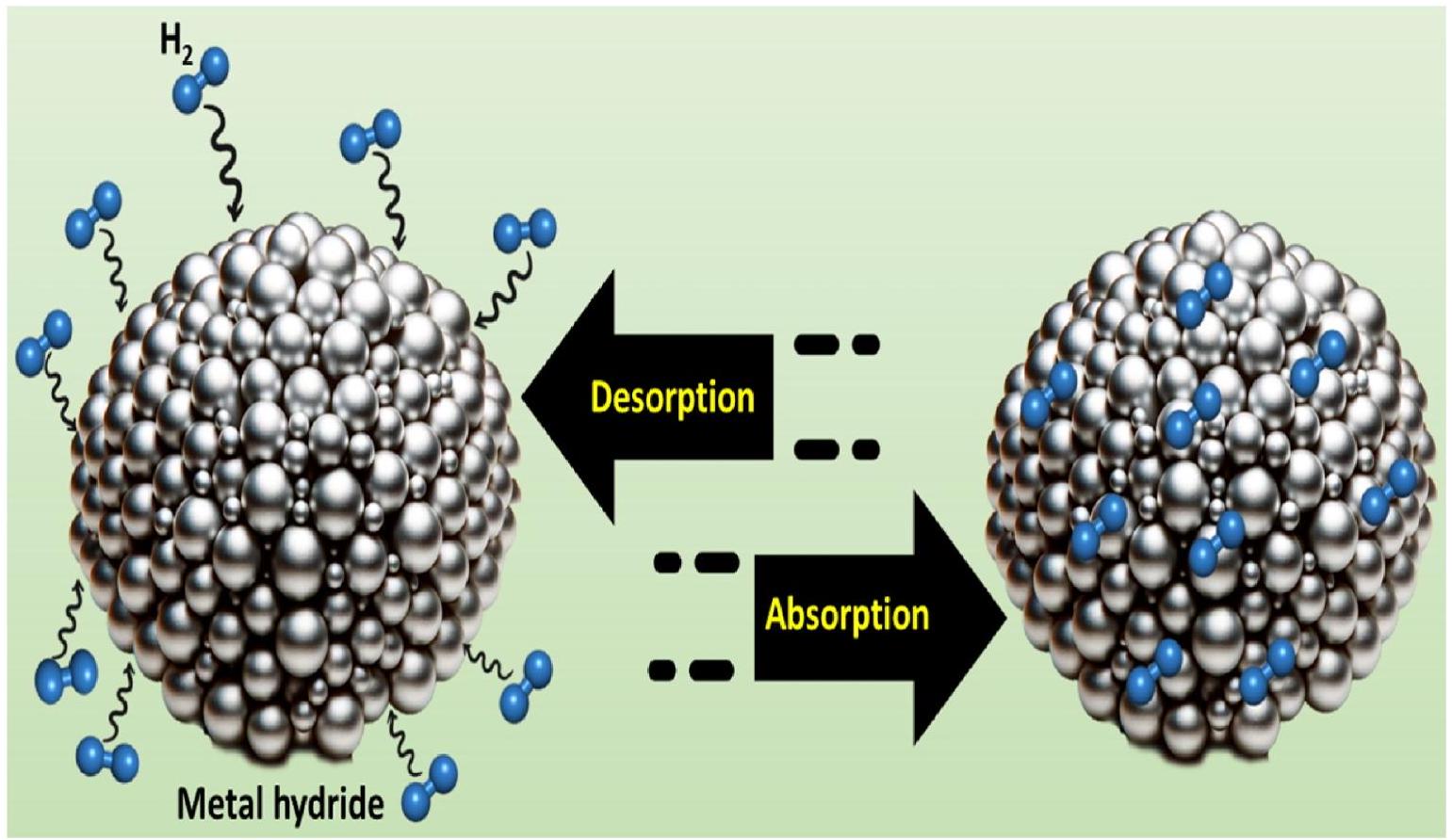
مقيد بتراكم الشوائب داخل الخزانات، التي تسد المساحات التي يتم عادةً تخزين الهيدروجين فيها، مما يقلل من سعة الخزان. كمية الحرارة المنقولة، والهيدروجين الممتص، والهيدروجين المنفصل في هيدريد معدني قابل للعكس يعمل في درجة حرارة الغرفة والضغط الجوي تعتمد عمومًا على سبيكة المعدن المستخدمة لتخزين الهيدروجين. نموذج مبسط يوضح كيفية تخزين الهيدروجين وامتصاصه في هيدريد معدني موضح في الشكل 2.
2.2.1.1.1. هيدريد المغنيسيوم (
التجمع. تم استخدام هذه الاستراتيجية في تحقيق حديث [64]، حيث تم استخدام هيكل نانوي ثلاثي الأبعاد من الكربون المنشط المسامي المعدل بـ Ni و Fe لعملية النقع
2.2.1.1.2. هيدريد الليثيوم (LiH). يُعتبر هيدريد الليثيوم (LiH) واحدًا من أخف هيدريدات المعادن على الإطلاق، وله سعة تخزين هيدروجين جاذبية عالية تصل إلى حوالي
2.2.1.1.3. ألانيت الصوديوم (
2.2.1.1.4. بوران الأمونيوم (
إطلاق الأمونيا من البوران يتضمن التحلل المائي. يظهر البوران الأموني مقاومة ملحوظة للتحلل المائي في المحاليل المائية، مما يتطلب وجود محفز فعال لتحفيز إزالة الهيدروجين التحليلية عند درجات حرارة محيطة. يظهر التحلل المائي المحفز بالمعادن كخيار قابل للتطبيق، مما يوفر إطلاق كمية كبيرة من الهيدروجين.
2.2.1.1.5. نيتريدات المعادن بما في ذلك الأميدات
2.2.2. الممتصات الفيزيائية
نحو تحسين الخصائص الحجمية [88].
2.2.2.1. الكربون المنشط ومواد الكربون النانوية. سعة تخزين الهيدروجين للكربون المنشط عادة ما تكون حوالي
سعة تخزين أنابيب الكربون النانوية.
2.2.2.2. الأطر العضوية المعدنية (MOFs). فئة من المواد المسامية المكونة من أيونات المعادن أو تجمعات المعادن المرتبطة بالأغلفة العضوية. تتمتع هذه المواد بمساحة سطح كبيرة وأقطار مسام قابلة للتعديل. نظرًا لارتفاع مساميتها ومساحتها السطحية الكبيرة، أظهرت MOFs إمكانات قوية لتخزين الهيدروجين من خلال الامتزاز الفيزيائي. أظهرت بعض MOFs سعات هيدروجين تتجاوز
2.2.2.3. الأطر العضوية التساهمية (COFs). الأطر العضوية التساهمية (COFs) هي فئة من المواد التي تشبه الأطر المعدنية العضوية (MOFs) ولكنها لا تحتوي على أيونات المعادن الثقيلة. يتم بناؤها باستخدام كتل بناء عضوية من خلال طرق التكوين التساهمي الديناميكي. تتمتع COFs بهياكل بلورية موسعة مع مساحات سطحية عالية ومنخفضة.
2.2.2.4. البوليمرات العضوية المسامية (POPs). ظهرت البوليمرات العضوية المسامية غير المتبلورة (POPs) كمرشحين واعدين لتخزين الهيدروجين بسبب سهولة معالجتها وخصائصها الميكانيكية القوية. يمكن تصنيف البوليمرات العضوية المسامية إلى أربع فئات رئيسية: (i) الأطر العطرية المسامية (PAFs)، (ii) البوليمرات الدقيقة المترافقة (CMPs)، (iii) البوليمرات المترابطة بشكل مفرط (HCPs) و (iv) البوليمرات ذات المسامية الدقيقة الجوهرية (PIMs) [115].
مواد مسامية أخرى وحتى مقارنة بأفضل القيم المبلغ عنها في MOFs.
أو الكربون المنشط. لاختبار استقرار PIM لتخزين الهيدروجين، قام روشات وآخرون [130] بدراسة الاستقرار على المدى الطويل لـ PIM-1 لتطبيقات تخزين الهيدروجين. على مدى فترة 400 يوم، تم فحص الخصائص الميكانيكية والسطحية لـ PIM-1. أظهرت النتائج أن معظم الخصائص الميكانيكية والسطحية ظلت مستقرة مع مرور الوقت، بما في ذلك القوة الميكانيكية، والمرونة، والمساحة السطحية. ومع ذلك، كان هناك انخفاض صغير ولكنه ذو دلالة إحصائية في سعة تخزين الهيدروجين لـ PIM-1، خاصة في المراحل الأولية من الشيخوخة. وقد تم عزو هذا الانخفاض إلى إعادة ترتيب بطيئة للهيكل البوليمري. بشكل عام، أظهرت الدراسة أن PIM-1 يمتلك الاستقرار اللازم على المدى الطويل لتطبيقات تخزين الهيدروجين الواقعية.
2.2.2.5. الزيوليت. الزيوليت هي سيليكات ألومنيوم بلورية ذات هياكل مسامية محددة جيدًا. نظرًا لطبيعتها الميكروية الفريدة ومساحتها السطحية الكبيرة، تم التحقيق في الزيوليت كمواد محتملة لتخزين الهيدروجين. يخزن الزيوليت الهيدروجين بشكل رئيسي من خلال الامتصاص الفيزيائي، وهو نوع من الامتصاص الفيزيائي الناتج عن قوى فان der Waals الضعيفة بين جزيئات الهيدروجين وسطح الزيوليت. عملية الامتصاص قابلة للعكس، لذا يمكن إطلاق الهيدروجين بسهولة عند الحاجة.
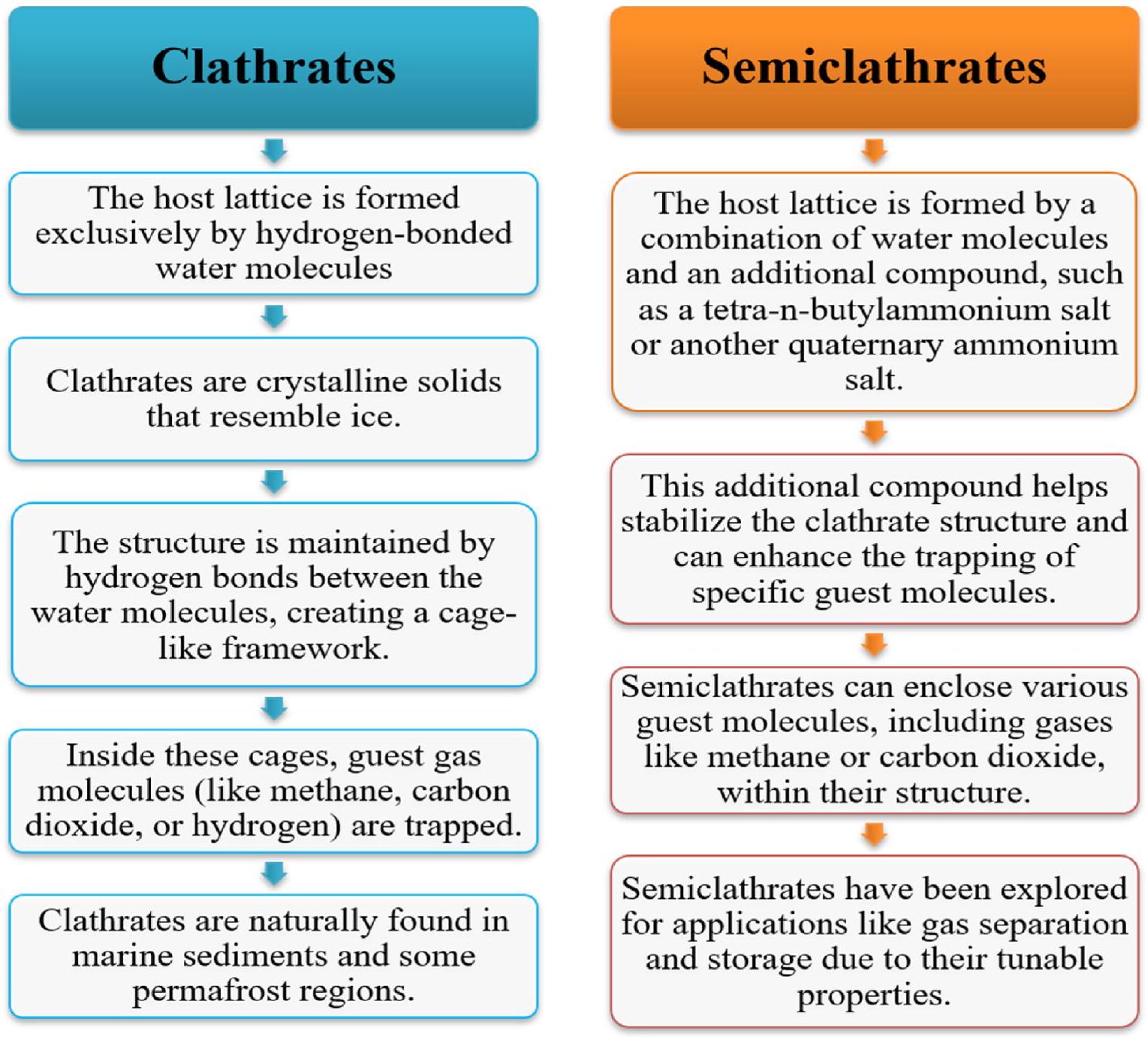
2.2.2.6. الكلاترات. الكلاترات، التي تُعرف علمياً باسم هيدرات الغاز، هي
تشكيلات بلورية معقدة تنشأ من الترتيب الهيكلي لجزيئات الماء. تتخذ هذه التشكيلات بنية تشبه القفص، قادرة على احتواء جزيئات ضيفية دون أي ارتباط كيميائي. عادةً ما تظهر هذه الجزيئات الضيفية كغازات. نظرًا للطلب المتزايد على مواد آمنة وفعالة مناسبة لتخزين الهيدروجين، ظهرت الكلاترات كفئة مشجعة من المواد للاستخدام المحتمل. إن احتواء الهيدروجين داخل أقفاص الماء، كما تسهل الكلاترات، يساعد على تقليل المخاطر المرتبطة بإطلاق الغاز المفاجئ أو الحوادث الانفجارية بشكل كبير.
تصنيف الكلاترات بناءً على الخصائص البلورية.
| الهيكل I (sI) | الهيكل II (sII) | الهيكل H (sH) |
| – هيكل مكعب، و | – هيكل مكعب. | – هيكل سداسي. |
| هو أحد الأكثر | – يتكون من صغير | – إنه صغير |
| أنواع شائعة من | ذو اثني عشر وجهًا وأكبر | اثنا عشري، متوسط- |
| كلاثرات | سداسي عشر الوجوه | مُتَسَاوِي الأبعاد مع شكل سداسي عشر الوجوه |
| – يتكون من صغير | الأقفاص التي تواجه. | وأيكونوساكايوكتاهيدرا الكبير- |
| اثنا عشر وجهاً (دوديكاهيدرال) | – الغازات الأكبر، مثل | أقفاص درال (ذات 24 وجهًا). |
| وأكبر | الإيثان، البروبان، و | – الغازات مثل الكريبتون، |
| رباعي عشر الوجوه | ثاني أكسيد الكربون، الشكل sII | زينون، وبعض الأكبر |
| الأقفاص التي تواجه. | مرطبات. | تشكل الهيدروكربونات sH |
| – الغازات الشائعة التي | – هيدرات sII أكثر | مرطبات. |
| تشمل هيدرات الشكل sI | يتكيف مع | – يمكن للهيدروجين أيضًا أن يشكل |
| الميثان، الإيثيلين، و | الهيدروجين، خاصة في | يتم ترطيب sH تحت ظروف معينة |
| الهيدروجين عادةً | غازات المحفز. لـ | عندما يكون هناك ضيوف أكبر |
| لا يشكل sI مستقر | على سبيل المثال، يمكن للهيدروجين | الجزيئات موجودة لـ |
| مرطبات. | تشكل هيدرات sII في | احتلال الأقفاص الكبيرة. |
| مع غازات أخرى يمكن أن, | وجود | |
| ومع ذلك، استقر على | بعض المواد العضوية الأخرى | |
| تكوين هيدرات السليكون. | جزيئات. |
مستخدمة في تشكيل هيدرات شبه الكلايثرات. تم استكشاف جدوى استخدام كلوريد التترا بوتيل أمونيوم وبروميد التترا بوتيل فوسفونيم القائمين على شبه الكلايثرات لتخزين الهيدروجين من قبل ديشامب و دالمزون [154]. وقد حددوا أن هذه المواد أظهرت سعات تخزين الهيدروجين من
2.3. التخزين تحت الأرض للهيدروجين
تعتبر مناسبة لاستيعاب مصادر الطاقة المتقطعة. تتضمن منشأة تخزين الغاز تحت الأرض التراكم الاصطناعي للغاز في البيئة الطبيعية، عادة على عمق كبير، وغالبًا ما يتجاوز عدة مئات من الأمتار. يتكون الغاز المخزن من مكونين: الغاز العامل، الذي يتم حقنه في التخزين واستخراجه منه، وغاز الوسادة، الذي يبقى داخل المنشأة طوال فترة تشغيلها. الغرض من غاز الوسادة هو مزدوج: الحفاظ على مستوى ضغط أدنى يمنع دخول الماء إلى مساحة التخزين وضمان ظروف مثالية لحقن الغاز.
1. سلامة محسّنة: المنشآت تحت الأرض أقل عرضة للمخاطر مثل الحرائق، الهجمات الإرهابية، أو الأعمال العسكرية مقارنة بخزانات التخزين السطحية.
II. إدارة المساحة بكفاءة: يمكن أن تخزن المنشآت تحت الأرض كميات كبيرة من الغاز بينما تشغل مساحات سطحية صغيرة نسبيًا. بالمقابل، ستحتاج الخزانات السطحية إلى مساحة واسعة لتحقيق نفس سعة التخزين. الطبيعة المدمجة للمنشآت تحت الأرض تسهل التكامل مع المناظر الطبيعية والبنية التحتية القائمة.
III. الجدوى الاقتصادية: بناء منشآت تخزين الغاز تحت الأرض يكون عمومًا أكثر اقتصادية مقارنة بالمنشآت السطحية.
مقارنة شاملة في جدول لمواد تخزين الهيدروجين.
| مواد التخزين | سعة تخزين الهيدروجين الجاذبية (نسبة الوزن%) | درجة الحرارة (ك) | الضغط (ميغاباسكال) | مرجع |
| هيدريد المغنيسيوم المطحون بالكرات | 6.28 | 523 | 1 | [62] |
| هيدريد المغنيسيوم المشبع بهيكل نانوي ثلاثي الأبعاد من الكربون المنشط المسطح المعدل بالنيكل والحديد | 6.63 | ٤٥٣ | 1 | [64] |
| ألانيت الصوديوم مع هيدريد التيتانيوم كأطباق نانوية مدعومة على الجرافين | ٥ | 353 | 10 | [77] |
| إيميد الليثيوم-المغنيسيوم | ٤.١ | 523 | 0 | [85] |
| مركب ليثيوم-مغنيسيوم معزز بنسبة 10% من فاندات الليثيوم@أكسيد الفاندوم الليثيوم | ٤.٧ | |||
| فحم نشط من مادة اللجنين المحترق المستندة إلى رقائق الأوكاليبتوس | 1.8 | 77 | 0.1 | [90] |
| فحم نشط قائم على الكيتوزان | 2.95 | 77 | 0.1 | [91] |
| 5.61 | ٤ | |||
| مادة كربونية مصنوعة من حبوب القهوة | 0.6 | 298 | 12 | [92] |
| ٤ | 77 | |||
| أنابيب الكربون المخدّرة بالنيتروجين مختلطة بأكسيد اللانثانوم | 7.4 | 373 | 1.8 | [97] |
| إطار جامعة أوسلو 66 (UIO-66) | 3.8 | 77 | 10 | [101] |
| إطار جامعة أوسلو 66 (UIO-66) المهدد بالهيدروكسيل | ٤.٦ | |||
| إطار معدني عضوي متساوي الشبكة 1 (IRMOF-1) | 7.1 | 77 | ٤ | [102] |
| 11.5 | 17 | |||
| إطار جامعة أوسلو القائم على 2,2-بيبيريدين-5,5′-ديكربوكسيليت (bpdc) | ٥.٧ | 77 | 2 | [156] |
| هيدريد المغنيسيوم | 6.91 | 648 | 1 | [157] |
| إطار معدني عضوي قائم على المغنيسيوم والكوبالت (II) | 5.19 | |||
| إطار معدني عضوي قائم على المغنيسيوم والحديد (II) | 5.37 | |||
| هيدريد المغنيسيوم المدعوم بإطار معدني عضوي قائم على الفاناديوم | 6.4 | 573 | 3.2 | [158] |
| الهيكل الأول من الكلاترات المدعومة بالميثان | 0.02 | 273 | 70 | [142] |
| الهيكل الأول من الكلاترات المدعومة بالبروبان | 0.17 | ٢٧٠ | 12 | [143] |
| الهيكل الأول من الكلاترات المدعومة بالإيثان | 2.5 | ٢٥٠ | ٣٠٠ | [144] |
| الهيكل الثاني من الكلاترات المدعومة بالتتراهيدروفوران (الهيدرات الثنائية) | 1 | ٢٧٩.٦ | ٥ | [145] |
| الهيكل الثاني من الكلاترات المدعومة بالتتراهيدروفوران (الهيدرات الثنائية) | ٤.٠٣ | ٢٧٠ | 12 | [146] |
| الهيكل الثاني من الكلاترات المدعومة بواسطة التتراهيدروفوران (الهيدرات الثنائية) | 3.4 | 255 | 70 | [147] |
| الهيدرات النقية للهيدروجين | ٤.٤ | ١٤٠ | 0.1 | [148] |
| الهيدرات الثنائية للهيدروجين والتتراهيدروفوران | 1.6-3.8 | |||
| الهيكل الثاني من الكلاترات المدعومة بالميثان (الهيدرات الثنائية) | 2.6 | ٢٥٠ | 70 | [149] |
| الهيكل الثاني من الكلاترات المدعومة بالميثان (الهيدرات الثنائية) | 3.43 | ٢٧٠ | 20 | [150] |
| البنية الثانية من الكلاترات المدعومة بالنيتروجين (الهيدرات الثنائية) | ٤.٤ | 243 | 15 | [151] |
| البنية الثانية من الكلاترات المدعومة بالإيبوكسي سيكلوبنتان (الهيدرات الثنائية) | 0.63 | 262 | 18.2 | [152] |
| الهيدرات الصلبة-هايستور المدعومة بواسطة ميثيل سيكلوهكسان (الهيدرات الثنائية) | 1.4 | ٢٧٤ | ٥٠٠ | [153] |
| المركبات شبه الكلازات القائمة على كلوريد التترا بوتيل أمونيوم | 0.12 | ٢٨٨.٩ | 15 | [154] |
| نصف هيدرات قائم على بروميد تترا بوتيل فوسفونيم | 0.14 | ٢٨٥ | ||
| الهاليدات شبه المائية القائمة على بروميد التترا-ن-بيوتيل أمونيوم | 0.21 | ٢٧٩.٥ | 13.8 | [155] |
| زيوليت X القائم على الصوديوم | 1.79 | 77 | 1.5 | [135] |
| زيوليت X المعتمد على البوتاسيوم | 1.96 | |||
| زيوليت X المعتمد على الريبيديوم | 1.46 | |||
| زيوليت X المعتمد على السيزيوم | 1.32 | |||
| زيوليت X المعتمد على المغنيسيوم | 1.62 | |||
| زيوليت X المعتمد على الكالسيوم | 2.19 | |||
| زيوليت X المعتمد على السترونتيوم | 1.68 | |||
| زيوليت Y فائق الاستقرار | 0.4 | ٣٠٣ | ٥ | [136] |
| زيوليت سوكوني موبايل-5 | 2.89 | 77 | 1.2 | [137] |
IV. وفرة الهياكل الجيولوجية المناسبة: تمتلك العديد من الدول والمناطق الكبيرة تشكيلات جيولوجية مناسبة لتخزين الغاز تحت الأرض. توفر هذه الهياكل ظروفًا ملائمة لإنشاء وتشغيل المنشآت تحت الأرض.
2.3.1. كهف الملح
الكهوف ذات الأبعاد المحددة [166،167]. هذه الكهوف، التي تُبنى عادةً حتى عمق 2000 متر بحجوم تصل إلى 1,000,000 متر مكعب، مناسبة تمامًا لتخزين مواد متنوعة، وخاصة الغازات، تحت ضغوط عالية [167]. يتراوح ضغط التشغيل عادةً بين
عدد المواقع التي تستوعب حاليًا تخزين الهيدروجين داخل كهوف الملح. تشمل الأمثلة البارزة تيسايد في المملكة المتحدة ودوم كليمنس، سبيندلتوب، وموس بلاف في الولايات المتحدة [161،176]. تستخدم منشأة تيسايد، التي تعمل منذ السبعينيات، كهوف ملح ذات شكل بيضاوي تقع على أعماق تتراوح بين 350 إلى 450 م، وتتمتع بحجم إجمالي يبلغ 210,000 متر مكعب. بالمقابل، تحتوي كهوف الملح في دوم كليمنس وموس بلاف، الواقعة على عمق 800 م من قمة الكهف، على سعات أكبر تبلغ حوالي 580,000 متر مكعب لكل منهما. كانت دوم كليمنس تعمل منذ عام 1983، بينما بدأت موس بلاف عملياتها في عام 2007. تعتبر هذه المشاريع الطويلة الأمد دليلًا قويًا على الجدوى التقنية لتخزين الهيدروجين تحت الأرض على مدى فترات طويلة [176،177].
2.3.2. المياه الجوفية
في الضغط. أثناء الحقن، يقوم الغاز بإزاحة الماء، مما يخلق حدودًا ديناميكية للغاز/الماء تتحرك أثناء تشغيل منشأة التخزين. ومع ذلك، فإن أحد العيوب هو أن بعض الغاز يبقى غير قابل للاسترداد في المياه الجوفية [161،169،170].
2.3.3. رواسب النفط والغاز المستنفدة
الميثان وثاني أكسيد الكربون لا يضمنان ملاءمة الهيدروجين بسبب خصائصه المختلفة. يمكن أن تؤثر تفاعلية الهيدروجين مع صخور الخزان على هياكل المسام، مما يؤثر على القدرة على الحقن وسعة التخزين. بينما تكون خسارة الهيدروجين أثناء التخزين ضئيلة، تحدث خسائر كبيرة من خلال الاحتجاز المتبقي، والتفاعلات الجيوكيميائية، وتسرب الصخور القابلة للغطاء. إن فهم هجرة السوائل عبر الخزانات أمر بالغ الأهمية، مع الأخذ في الاعتبار التغيرات المعدنية الناتجة عن تفاعل الهيدروجين.
3. تصميم مواد لتخزين الهيدروجين
3.1. اعتبارات التصميم واستراتيجيات مواد تخزين الهيدروجين
[189].
تحدث درجات الحرارة والضغوط المنخفضة نسبيًا المشابهة لتلك الموجودة في مركبات خلايا الوقود، ويحدث إطلاق الهيدروجين من الهيدريدات المعدنية عبر عملية ماصة للحرارة. يمكن أن يحدث تحرير الهيدروجين من الهيدريدات المعدنية إما من خلال زيادة في درجة الحرارة أو تقليل في الضغط الخارجي.
بحث التخزين [201].
التجميع الذاتي لمجموعات المعادن غير العضوية والروابط العضوية. تقدم هذه العملية الفريدة للتجميع العديد من التباينات في وحدات البناء، مما يساهم في الطيف الواسع من الخصائص التي تظهرها MOFs، لا سيما من حيث المساحة السطحية، التي غالبًا ما تتجاوز تلك الخاصة بالمواد الأخرى. ومع ذلك، بينما تتيح هذه المرونة في التصميم تخصيص خصائص MOF، فإنها تقدم تحديات في تحديد التركيبات المثلى بسبب المساحة الواسعة من المعلمات التي تحتاج إلى استكشاف.
لقد حظيت هذه المواد باهتمام كبير بسبب إمكانياتها في تطبيقات تخزين الهيدروجين، نظرًا لكونها تتمتع بموصلية حرارية عالية. ومن الجدير بالذكر أن الجرافين وأنابيب الكربون النانوية تظهران قدرات مثيرة للإعجاب في امتصاص الهيدروجين، وذلك بفضل مساحاتها السطحية الواسعة والمتاحة وهياكلها المسامية.
3.2. الفحص الحسابي عالي الإنتاجية وتعلم الآلة
الخصائص المستهدفة، تعريف مساحات الفحص، توقع الخصائص، واختيار المواد المرشحة. يُعتبر تحديد الخصائص المستهدفة من بين الخطوات الأكثر أهمية وتحديًا في هذه العمليات. بالنسبة لعلماء المواد، فإن تسمية الخصائص الماكروسكوبية المرغوبة للمواد الوظيفية في أجهزة تحويل أو تخزين الطاقة عمومًا ممكنة.
جهود في الفحص الحسابي عالي الإنتاجية (HTCS) للمواد من أجل
زيادة تقريبية بمقدار الضعف مقارنةً بـ FPGNs غير المنقاة.
بالإضافة إلى ذلك، زاد تشويب الليثيوم من سعة تخزين الهيدروجين الزائد في FPGNs حتى ثلاثة أضعاف عند درجة حرارة الغرفة. تشير هذه النتائج إلى أن Li-FPGNs تحمل إمكانيات كمواد فعالة لتطبيقات تخزين الهيدروجين. من خلال استخدام طرق حسابية مثل محاكاة GCMC، توفر الأبحاث رؤى قيمة حول تحسين أداء Li-FPGNs في امتصاص الهيدروجين. تبرز القيم العددية، مثل سعة الامتصاص الوزني ونسب التحسين، التعزيزات الكبيرة التي تم تحقيقها من خلال تشويب الليثيوم في النانومركبات المدروسة.
لتخزين الهيدروجين. تم اختيار أربعة هيدريدات معدنية عضوية محددة لمزيد من التحقيق والتخليق. أشارت التوقعات النظرية إلى أن أزواج إندوليد الليثيوم وإندوليد أوكتاهيدرو الليثيوم كانت لديها سعة هيدروجين نظرية قدرها
الكميات المتعلقة بمواد تخزين الهيدروجين، مثل الطاقة الحرة لتفاعلات تخزين/إطلاق الهيدروجين وعمليات تحلل المواد. ومع ذلك، فإن تحديد المسارات التفاعلية الأكثر ملاءمة، ومواقع الامتزاز، والهياكل الكيميائية يتطلب التخمين من بين عمليات وتهيئات مرشحة متنوعة، تعتمد بشكل أساسي على الحدس الكيميائي. قد تكون هذه الطريقة غير مكتملة أو عرضة للأخطاء، خاصة عند معالجة المشكلات المعقدة. لمواجهة هذه التحديات “المفتوحة”، الطموح هو وجود أطر نظرية قادرة على التنبؤ تلقائيًا بالمسارات التفاعلية المثلى حراريًا، والحالات، والتهيئات استنادًا فقط إلى التركيب الكيميائي للنظام أو معلومات مشابهة. تقدم تقنيات التعلم الآلي حلاً، مع التركيز على القدرة الحاسوبية والموضوعية بدلاً من الحدس الكيميائي.
درجة الحرارة (303 كلفن) باستخدام نماذج التعلم الآلي. تضمنت الميزات المدخلة للنماذج درجة الحرارة، والضغط، وتسع تركيبات سبائك. تم تقييم دقة التنبؤ من خلال مقارنة القيم المتوقعة بالقيم المقاسة، وأشارت قيم الارتباط الأعلى إلى أداء تنبؤ أفضل. بشكل عام، أظهرت الأبحاث فعالية التعلم الآلي في التنبؤ بمنحنيات PCT لسبائك تخزين الهيدروجين. من خلال استخدام نماذج التعلم الآلي، قدمت الدراسة رؤى حول سلوك
مواد متطورة. تم تحديد MOFs التي تم التعرف عليها من خلال كثافات منخفضة (
4. الخاتمة
إعلان عن تضارب المصالح
شكر وتقدير
References
[2] Zhang L, Jia C, Bai F, Wang W, An S, Zhao K, et al. A comprehensive review of the promising clean energy carrier: hydrogen production, transportation, storage, and utilization (HPTSU) technologies. Fuel 2024;355:129455.
[3] Hutchins DA, Jansson JK, Remais JV, Rich VI, Singh BK, Trivedi P. Climate change microbiology – problems and perspectives. Nat Rev Microbiol 2019;17: 391-6.
[4] Jones MW, Peters GP, Gasser T, Andrew RM, Schwingshackl C, Gütschow J, et al. National contributions to climate change due to historical emissions of carbon dioxide, methane, and nitrous oxide since 1850. Sci Data 2023;10:155.
[5] Kabir M, Habiba UE, Khan W, Shah A, Rahim S, Rios-Escalante PRD, et al. Climate change due to increasing concentration of carbon dioxide and its impacts on environment in 21st century; a mini review. J King Saud Univ Sci 2023;35: 102693.
[6] Halawy SA, Osman AI, Nasr M, Rooney DW. Mg-O-F nanocomposite catalysts defend against global warming via the efficient, dynamic, and rapid capture of CO(2) at different temperatures under ambient pressure. ACS Omega 2022;7: 38856-68.
[7] Asghar U, Rafiq S, Anwar A, Iqbal T, Ahmed A, Jamil F, et al. Review on the progress in emission control technologies for the abatement of
[8] Obaideen K, Olabi AG, Al Swailmeen Y, Shehata N, Abdelkareem MA, Alami AH, et al. Solar energy: applications, trends analysis, bibliometric analysis and research contribution to sustainable development goals (SDGs). Sustainability 2023;15:1418.
[9] Zhang Z, Liu X, Zhao D, Post S, Chen J. Overview of the development and application of wind energy in New Zealand. Energy and Built Environment 2023; 4:725-42.
[10] Xiaosan Z, Qingquan J, Shoukat Iqbal K, Manzoor A, Zia Ur R. Achieving sustainability and energy efficiency goals: assessing the impact of hydroelectric and renewable electricity generation on carbon dioxide emission in China. Energy Pol 2021;155:112332.
[11] Nasr M, Abdelkader A, El-Nahas S, Osman AI, Abdelhaleem A, El Nazer HA, et al. Utilizing undissolved portion (UNP) of cement kiln dust as a versatile multicomponent catalyst for bioethylene production from bioethanol: an innovative approach to address the energy crisis. ACS Omega 2023.
[12] Pandit K, Jeffrey C, Keogh J, Tiwari MS, Artioli N, Manyar HG. Techno-economic assessment and sensitivity analysis of glycerol valorization to biofuel additives via esterification. Ind Eng Chem Res 2023;62:9201-10.
[13] Osman AI, Mehta N, Elgarahy AM, Hefny M, Al-Hinai A, Al-Muhtaseb AH, et al. Hydrogen production, storage, utilisation and environmental impacts: a review (vol 20, pg 153, 2022). Environ Chem Lett 2022;20:2213.
[14] Hassan Q, Sameen AZ, Salman HM, Jaszczur M, Al-Jiboory AK. Hydrogen energy future: advancements in storage technologies and implications for sustainability. J Energy Storage 2023;72:108404.
[15] Jahanbakhsh A, Louis Potapov-Crighton A, Mosallanezhad A, Tohidi Kaloorazi N, Maroto-Valer MM. Underground hydrogen storage: a UK perspective. Renew Sustain Energy Rev 2024;189:114001.
[16] Mondal K, Malode SJ, Shetti NP, Alqarni SA, Pandiaraj S, Alodhayb A. Porous nanostructures for hydrogen generation and storage. J Energy Storage 2024;76: 109719.
[17] Hassan IA, Ramadan HS, Saleh MA, Hissel D. Hydrogen storage technologies for stationary and mobile applications: review, analysis and perspectives. Renew Sustain Energy Rev 2021;149:111311.
[18] Navlani-García M, Mori K, Kuwahara Y, Yamashita H. Recent strategies targeting efficient hydrogen production from chemical hydrogen storage materials over carbon-supported catalysts. NPG Asia Mater 2018;10:277-92.
[19] Eberle U, Felderhoff M, Schuth F. Chemical and physical solutions for hydrogen storage. Angew Chem Int Ed Engl 2009;48:6608-30.
[20] Dalebrook AF, Gan W, Grasemann M, Moret S, Laurenczy G. Hydrogen storage: beyond conventional methods. Chem Commun 2013;49:8735-51.
[21] Lu Z. Computational discovery of energy materials in the era of big data and machine learning: a critical review. Materials Reports: Energy 2021;1:100047.
[22] Tashie-Lewis BC, Nnabuife SG. Hydrogen production, distribution, storage and power conversion in a hydrogen economy – a technology review. Chemical Engineering Journal Advances 2021;8:100172.
[23] Zhang L, Jia CQ, Bai FQ, Wang WS, An SY, Zhao KY, et al. A comprehensive review of the promising clean energy carrier: hydrogen production, transportation, storage, and utilization (HPTSU) technologies. Fuel 2024;355: 129455.
[24] Du ZM, Liu CM, Zhai JX, Guo XY, Xiong YL, Su W, et al. A review of hydrogen purification technologies for fuel cell vehicles. Catalysts 2021;11:393.
[25] Qazi UY. Future of hydrogen as an alternative fuel for next-generation industrial applications; challenges and expected opportunities. Energies 2022;15:4741.
[26] Usman MR. Hydrogen storage methods: review and current status. Renew Sustain Energy Rev 2022;167:112743.
[27] Demirocak DE. Hydrogen storage technologies. Nanostructured Materials for Next-Generation Energy Storage and Conversion: Hydrogen Production, Storage, and Utilization 2017:117-42.
[28] Greene DL, Ogden JM, Lin ZH. Challenges in the designing, planning and deployment of hydrogen refueling infrastructure for fuel cell electric vehicles. Etransportation 2020;6:100086.
[29] Barthélémy H, Weber M, Barbier F. Hydrogen storage: recent improvements and industrial perspectives. Int J Hydrogen Energy 2017;42:7254-62.
[30] Moradi R, Groth KM. Hydrogen storage and delivery: review of the state of the art technologies and risk and reliability analysis. Int J Hydrogen Energy 2019;44: 12254-69.
[31] Durbin D, Malardier-Jugroot C. Review of hydrogen storage techniques for on board vehicle applications. Int J Hydrogen Energy 2013;38:14595-617.
[32] AlZohbi G, Almoaikel A, AlShuhail L. An overview on the technologies used to store hydrogen. Energy Rep 2023;9:28-34.
[33] Abe JO, Popoola A, Ajenifuja E, Popoola OM. Hydrogen energy, economy and storage: review and recommendation. Int J Hydrogen Energy 2019;44:15072-86.
[34] Ball M, Wietschel M. The future of hydrogen-opportunities and challenges. Int J Hydrogen Energy 2009;34:615-27.
[35] Ahluwalia RK, Hua T, Peng J. On-board and Off-board performance of hydrogen storage options for light-duty vehicles. Int J Hydrogen Energy 2012;37:2891-910.
[36] Schlapbach L, Züttel A. Hydrogen-storage materials for mobile applications. Nature 2001;414:353-8.
[37] Bossel U. Does a hydrogen economy make sense? Proc IEEE 2006;94:1826-37.
[38] Wang M, Wang G, Sun Z, Zhang Y, Xu D. Review of renewable energy-based hydrogen production processes for sustainable energy innovation. Global Energy Interconnection 2019;2:436-43.
[39] Yanxing Z, Maoqiong G, Yuan Z, Xueqiang D, Jun S. Thermodynamics analysis of hydrogen storage based on compressed gaseous hydrogen, liquid hydrogen and cryo-compressed hydrogen. Int J Hydrogen Energy 2019;44:16833-40.
[40] Sui Y, Yuan Z, Zhou D, Zhai T, Li X, Feng D, et al. Recent progress of nanotechnology in enhancing hydrogen storage performance of magnesiumbased materials: a review. Int J Hydrogen Energy 2022;47:30546-66.
[41] Bellosta von Colbe J, Ares J-R, Barale J, Baricco M, Buckley C, Capurso G, et al. Application of hydrides in hydrogen storage and compression: achievements, outlook and perspectives. Int J Hydrogen Energy 2019;44:7780-808.
[42] Sun Y, Shen C, Lai Q, Liu W, Wang D-W, Aguey-Zinsou K-F. Tailoring magnesium based materials for hydrogen storage through synthesis: current state of the art. Energy Storage Mater 2018;10:168-98.
[43] Ley MB, Jepsen LH, Lee Y-S, Cho YW, Von Colbe JMB, Dornheim M, et al. Complex hydrides for hydrogen storage-new perspectives. Mater Today 2014;17: 122-8.
[44] Demirci UB. Ammonia borane, a material with exceptional properties for chemical hydrogen storage. Int J Hydrogen Energy 2017;42:9978-10013.
[45] Xu J, Liu J, Li Z, Wang X, Xu Y, Chen S, et al. Optimized synthesis of Zr (iv) metal organic frameworks (MOFs-808) for efficient hydrogen storage. New J Chem 2019;43:4092-9.
[46] Bian L-Y, Li X-D, Huang X-Y, Yang P-h, Wang Y-D, Liu X-Y, et al. Molecular simulation on hydrogen storage properties of five novel covalent organic frameworks with the higher valency. Int J Hydrogen Energy 2022;47:29390-8.
[47] Gupta A, Baron GV, Perreault P, Lenaerts S, Ciocarlan R-G, Cool P, et al. Hydrogen clathrates: next generation hydrogen storage materials. Energy Storage Mater 2021;41:69-107.
[48] Ariharan A, Ramesh K, Vinayagamoorthi R, Rani MS, Viswanathan B, Ramaprabhu S, et al. Biomass derived phosphorous containing porous carbon material for hydrogen storage and high-performance supercapacitor applications. J Energy Storage 2021;35:102185.
[49] Von Colbe JB, Ares J-R, Barale J, Baricco M, Buckley C, Capurso G, et al. Application of hydrides in hydrogen storage and compression: achievements, outlook and perspectives. Int J Hydrogen Energy 2019;44:7780-808.
[50] Lai Q, Pratthana C, Yang Y, Rawal A, Aguey-Zinsou K-F. Nanoconfinement of complex borohydrides for hydrogen storage. ACS Appl Nano Mater 2021;4: 973-8.
[51] Rusman NAA, Dahari M. A review on the current progress of metal hydrides material for solid-state hydrogen storage applications. Int J Hydrogen Energy 2016;41:12108-26.
[52] Preuster P, Papp C, Wasserscheid P. Liquid organic hydrogen carriers (LOHCs): toward a hydrogen-free hydrogen economy. Acc Chem Res 2017;50:74-85.
[53] Sathe RY, Kumar TJD, Ahuja R. Furtherance of the material-based hydrogen storage based on theory and experiments. Int J Hydrogen Energy 2023;48: 12767-95.
[54] Qureshi F, Yusuf M, Khan MA, Ibrahim H, Ekeoma BC, Kamyab H, et al. A State-of-The-Art Review on the Latest trends in Hydrogen production, storage, and transportation techniques. Fuel 2023;340:127574.
[55] Kukkapalli VK, Kim S, Thomas SA. Thermal management techniques in metal hydrides for hydrogen storage applications: a review. Energies 2023;16:3444.
[56] Lototskyy MV, Tolj I, Pickering L, Sita C, Barbir F, Yartys V. The use of metal hydrides in fuel cell applications. Prog Nat Sci: Mater Int 2017;27:3-20.
[57] Manoharan K, Sundaram R, Raman K. Expeditious re-hydrogenation kinetics of ball-milled magnesium hydride (
[58] Bogdanović B, Ritter A, Spliethoff B. Active MgH 2 Mg systems for reversible chemical energy storage. Angew Chem Int Ed Engl 2003;29:223-34.
[59] Wang L, Zhang L, Lu X, Wu F, Sun X, Zhao H, et al. Surprising cocktail effect in high entropy alloys on catalyzing magnesium hydride for solid-state hydrogen storage. Chem Eng J 2023;465:142766.
[60] Schneemann A, White JL, Kang S, Jeong S, Wan LF, Cho ES, et al. Nanostructured metal hydrides for hydrogen storage. Chem Rev 2018;118:10775-839.
[61] Yang H, Ding Z, Li Y-T, Li S-Y, Wu P-K, Hou Q-H, et al. Recent advances in kinetic and thermodynamic regulation of magnesium hydride for hydrogen storage. Rare Met 2023:1-22.
[62] El-Eskandarany MS, AlMatrouk H, Shaban E, Al-Duweesh A. Effect of the nanocatalysts on the thermal stability and hydrogenation/dehydrogenation kinetics of MgH 2 nanocrystalline powders. Mater Today Proc 2016;3:2608-16.
[63] Zhou C, Peng Y, Zhang Q. Growth kinetics of MgH2 nanocrystallites prepared by ball milling. J Mater Sci Technol 2020;50:178-83.
[64] Shinde S, Kim D-H, Yu J-Y, Lee J-H. Self-assembled air-stable magnesium hydride embedded in 3-D activated carbon for reversible hydrogen storage. Nanoscale 2017;9:7094-103.
[65] Meng Y, Ju S, Chen W, Chen X, Xia G, Sun D, et al. Design of bifunctional Nb/V interfaces for improving reversible hydrogen storage performance of MgH 2 . Small Structures 2022;3:2200119.
[66] Bramwell PL, Ngene P, de Jongh PE. Carbon supported lithium hydride nanoparticles: impact of preparation conditions on particle size and hydrogen sorption. Int J Hydrogen Energy 2017;42:5188-98.
[67] Milanese C, Jensen TR, Hauback BC, Pistidda C, Dornheim M, Yang H, et al. Complex hydrides for energy storage. Int J Hydrogen Energy 2019;44:7860-74.
[68] Bahou S, Labrim H, Lakhal M, Ez-Zahraouy H. Improving the hydrogen storage properties of lithium hydride ( LiH ) by lithium vacancy defects: ab initio calculations. Solid State Commun 2023;371:115167.
[69] Vajo JJ, Mertens F, Ahn CC, Bowman RC, Fultz B. Altering hydrogen storage properties by hydride destabilization through alloy formation:: LiH and MgH destabilized with Si. J Phys Chem B 2004;108:13977-83.
[70] Abbas MA, Grant DM, Brunelli M, Hansen TC, Walker GS. Reducing the dehydrogenation temperature of lithium hydride through alloying with germanium. Phys Chem Chem Phys 2013;15:12139-46.
[71] Jain A, Kawasako E, Miyaoka H, Ma T, Isobe S, Ichikawa T, et al. Destabilization of LiH by Li insertion into Ge . J Phys Chem C 2013;117:5650-7.
[72] Wang L, Quadir MZ, Aguey-Zinsou KF. Ni coated LiH nanoparticles for reversible hydrogen storage. Int J Hydrogen Energy 2016;41:6376-86.
[73] Pluengphon P, Tsuppayakorn-aek P, Inceesungvorn B, Ahuja R, Bovornratanaraks T. Formation of lightweight ternary polyhydrides and their hydrogen storage mechanism. J Phys Chem C 2021;125:1723-30.
[74] Miyaoka H, Ishida W, Ichikawa T, Kojima Y. Synthesis and characterization of lithium-carbon compounds for hydrogen storage. J Alloys Compd 2011;509: 719-23.
[75] Schneemann A, White JL, Kang S, Jeong S, Wan LF, Cho ES, et al. Nanostructured metal hydrides for hydrogen storage. Chem Rev 2018;118:10775-839.
[76] Beatrice CAG, Moreira BR, de Oliveira AD, Passador FR, Neto GRD, Leiva DR, et al. Development of polymer nanocomposites with sodium alanate for hydrogen storage. Int J Hydrogen Energy 2020;45:5337-46.
[77] Ren Z, Zhang X, Li H-W, Huang Z, Hu J, Gao M, et al. Titanium hydride nanoplates enable
[78] Li YT, Fang F, Fu HL, Qiu JM, Song Y, Li YS, et al. Carbon nanomaterial-assisted morphological tuning for thermodynamic and kinetic destabilization in sodium alanates. J Mater Chem A 2013;1:5238-46.
[79] Hudson MSL, Raghubanshi H, Pukazhselvan D, Srivastava ON. Carbon nanostructures as catalyst for improving the hydrogen storage behavior of sodium aluminum hydride. Int J Hydrogen Energy 2012;37:2750-5.
[80] Li H, Yao Z, Wang X, Zhu Y, Chen Y. Review on hydrogen production from catalytic ammonia borane methanolysis: advances and perspectives. Energy Fuels 2022;36:11745-59.
[81] Tang Z, Li S, Yang Z, Yu X. Ammonia borane nanofibers supported by poly (vinyl pyrrolidone) for dehydrogenation. J Mater Chem 2011;21:14616-21.
[82] Wu H, Cheng Y, Fan Y, Lu X, Li L, Liu B, et al. Metal-catalyzed hydrolysis of ammonia borane: mechanism, catalysts, and challenges. Int J Hydrogen Energy 2020;45:30325-40.
[83] Akbayrak S, Ozkar S. Ammonia borane as hydrogen storage materials. Int J Hydrogen Energy 2018;43:18592-606.
[84] Sakintuna B, Lamari-Darkrim F, Hirscher M. Metal hydride materials for solid hydrogen storage: a review. Int J Hydrogen Energy 2007;32:1121-40.
[85] Che H, Wu Y, Wang X, Liu H, Yan M. Improved hydrogen storage properties of Li-Mg-N-H system by lithium vanadium oxides. J Alloys Compd 2023;931:167603.
[86] Costanzo F, Silvestrelli PL, Ancilotto F. Physisorption, diffusion, and chemisorption pathways of H2 molecule on graphene and on
[87] Nazir H, Muthuswamy N, Louis C, Jose S, Prakash J, Buan ME, et al. Is the H2 economy realizable in the foreseeable future? Part II: H2 storage, transportation, and distribution. Int J Hydrogen Energy 2020;45:20693-708.
[88] Samantaray SS, Putnam ST, Stadie NP. Volumetrics of hydrogen storage by physical adsorption. Inorganics 2021;9:45.
[89] Shindo K, Kondo T, Arakawa M, Sakurai Y. Hydrogen adsorption/desorption properties of mechanically milled activated carbon. J Alloys Compd 2003;359: 267-71.
[90] Rowlandson JL, Edler KJ, Tian M, Ting VP. Toward process-resilient ligninderived activated carbons for hydrogen storage applications. ACS Sustainable Chem Eng 2020;8:2186-95.
[91] Wróbel-Iwaniec I, Díez N, Gryglewicz G. Chitosan-based highly activated carbons for hydrogen storage. Int J Hydrogen Energy 2015;40:5788-96.
[92] Akasaka H, Takahata T, Toda I, Ono H, Ohshio S, Himeno S, et al. Hydrogen storage ability of porous carbon material fabricated from coffee bean wastes. Int J Hydrogen Energy 2011;36:580-5.
[93] Dillon AC, Jones K, Bekkedahl T, Kiang C, Bethune D, Heben M. Storage of hydrogen in single-walled carbon nanotubes. Nature 1997;386:377-9.
[94] Su Rather. Preparation, characterization and hydrogen storage studies of carbon nanotubes and their composites: a review. Int J Hydrogen Energy 2020;45: 4653-72.
[95] Akbarzadeh R, Ghaedi M, Kokhdan SN, Vashaee D. Remarkably improved electrochemical hydrogen storage by multi-walled carbon nanotubes decorated with nanoporous bimetallic
[96] Kadri A, Jia Y, Chen Z, Yao X. Catalytically enhanced hydrogen sorption in MgMgH 2 by coupling vanadium-based catalyst and carbon nanotubes. Materials 2015;8:3491-507.
[97] Liang H, Du X, Li J, Sun L, Song M, Li W. Manipulating active sites on carbon nanotube materials for highly efficient hydrogen storage. Appl Surf Sci 2023;619: 156740.
[98] Zhao D, Wang X, Yue L, He Y, Chen B. Porous metal-organic frameworks for hydrogen storage. Chem Commun 2022;58:11059-78.
[99] Zhou H, Zhang J, Zhang J, Yan XF, Shen XP, Yuan AH. Spillover enhanced hydrogen storage in Pt-doped MOF/graphene oxide composite produced via an impregnation method. Inorg Chem Commun 2015;54:54-6.
[100] Guo JH, Li SJ, Su Y, Chen G. Theoretical study of hydrogen storage by spillover on porous carbon materials. Int J Hydrogen Energy 2020;45:25900-11.
[101] Bambalaza SE, Langmi HW, Mokaya R, Musyoka NM, Khotseng LE. Experimental demonstration of dynamic temperature-dependent behavior of UiO-66 metal-organic framework: compaction of hydroxylated and dehydroxylated forms of UiO-66 for high-pressure hydrogen storage. ACS Appl Mater Interfaces 2020;12:24883-94.
[102] Kaye SS, Dailly A, Yaghi OM, Long JR. Impact of preparation and handling on the hydrogen storage properties of Zn40 (1, 4-benzenedicarboxylate) 3 (MOF-5). J Am Chem Soc 2007;129:14176-7.
[103] Zunkovič E, Mazaj M, Mali G, Rangus M, Devic T, Serre C, et al. Structural study of Ni-or Mg-based complexes incorporated within UiO-66-NH2 framework and their impact on hydrogen sorption properties. J Solid State Chem 2015;225: 209-15.
[104] Orcajo G, Montes-Andrés H, Villajos JA, Martos C, Botas JA, Calleja G. Li-Crown ether complex inclusion in MOF materials for enhanced H2 volumetric storage capacity at room temperature. Int J Hydrogen Energy 2019;44:19285-93.
[105] Chafiq M, Chaouiki A, Ko YG. Advances in COFs for energy storage devices: harnessing the potential of covalent organic framework materials. Energy Storage Mater 2023;63:103014.
[106] Cote AP, Benin AI, Ockwig NW, O’Keeffe M, Matzger AJ, Yaghi OM. Porous, crystalline, covalent organic frameworks. Science 2005;310:1166-70.
[107] El-Kaderi HM, Hunt JR, Mendoza-Cortes JL, Cote AP, Taylor RE, O’Keeffe M, et al. Designed synthesis of 3D covalent organic frameworks. Science 2007;316: 268-72.
[108] Han SS, Furukawa H, Yaghi OM, Goddard WA. Covalent organic frameworks as exceptional hydrogen storage materials. J Am Chem Soc 2008;130:11580-+.
[109] Furukawa H, Yaghi OM. Storage of hydrogen, methane, and carbon dioxide in highly porous covalent organic frameworks for clean energy applications. J Am Chem Soc 2009;131:8875-83.
[110] Ghosh S, Singh JK. Hydrogen adsorption in pyridine bridged porphyrin-covalent organic framework. Int J Hydrogen Energy 2019;44:1782-96.
[111] Zhao H, Guan YR, Guo HL, Du RJ, Yan CX. Hydrogen storage capacity on Lidecorated covalent organic framework-1: a first-principles study. Mater Res Express 2020;7:035506.
[112] Shangguan W, Zhao H, Dai JQ, Cai J, Yan C. First-principles study of hydrogen storage of Sc-modified semiconductor covalent organic framework-1. ACS Omega 2021;6:21985-93.
[113] Choi YJ, Lee JW, Choi JH, Kang JK. Ideal metal-decorated three dimensional covalent organic frameworks for reversible hydrogen storage. Appl Phys Lett 2008;92.
[114] Kalidindi SB, Oh H, Hirscher M, Esken D, Wiktor C, Turner S, et al. Metal@COFs: covalent organic frameworks as templates for Pd nanoparticles and hydrogen storage properties of Pd@COF-102 hybrid material. Chemistry 2012;18: 10848-56.
[115] Chen ZJ, Kirlikovali KO, Idrees KB, Wasson MC, Farha OK. Porous materials for hydrogen storage. Chem-Us 2022;8:693-716.
[116] Peng YY, Yu JS, Liu XH, Meng TM, Zhao XH, Li LA. Enhanced pore structure regulation in porous aromatic framework materials via montmorillonite templating. Colloid Surface 2023;678:132468.
[117] Ben T, Ren H, Ma S, Cao D, Lan J, Jing X, et al. Targeted synthesis of a porous aromatic framework with high stability and exceptionally high surface area. Angew Chem Int Ed Engl 2009;48:9457-60.
[118] Yuan D, Lu W, Zhao D, Zhou HC. Highly stable porous polymer networks with exceptionally high gas-uptake capacities. Adv Mater 2011;23:3723-5.
[119] Hayat A, Sohail M, El Jery A, Al-Zaydi KM, Raza S, Ali H, et al. Recent advances in ground-breaking conjugated microporous polymers-based materials, their synthesis, modification and potential applications. Mater Today 2023;64: 180-208.
[120] Qiao S, Du Z, Yang R. Design and synthesis of novel carbazole-spacer-carbazole type conjugated microporous networks for gas storage and separation. J Mater Chem A 2014;2:1877-85.
[121] Zeng W, Zhang Y, Zhao XB, Qin ML, Li XY, Jin WS, et al. One-pot synthesis of conjugated microporous polymers based on extended molecular graphenes for hydrogen storage. Polymer 2019;174:96-100.
[122] Tsyurupa MP, Davankov VA. Porous structure of hypercrosslinked polystyrene: state-of-the-art mini-review. React Funct Polym 2006;66:768-79.
[123] Raza S, Nazeer S, Abid A, Kanwal A. Recent research progress in the synthesis, characterization and applications of hyper cross-linked polymer. J Polym Res 2023;30:415.
[124] Lee JY, Wood CD, Bradshaw D, Rosseinsky MJ, Cooper AI. Hydrogen adsorption in microporous hypercrosslinked polymers. Chem Commun 2006:2670-2.
[125] Wood CD, Tan B, Trewin A, Niu HJ, Bradshaw D, Rosseinsky MJ, et al. Hydrogen storage in microporous hypercrosslinked organic polymer networks. Chem Mater 2007;19:2034-48.
[126] Tian M, Rochatz S, Fawcett H, Burrows AD, Bowen CR, Mays TJ. Chemical modification of the polymer of intrinsic microporosity PIM-1 for enhanced hydrogen storage. Adsorption 2020;26:1083-91.
[127] Rochat S, Polak-Krasna K, Tian M, Holyfield LT, Mays TJ, Bowen CR, et al. Hydrogen storage in polymer-based processable microporous composites. J Mater Chem A 2017;5:18752-61.
[128] Tian M, Rochat S, Polak-Krasna K, Holyfield LT, Burrows AD, Bowen CR, et al. Nanoporous polymer-based composites for enhanced hydrogen storage. Adsorption 2019;25:889-901.
[129] Budd PM, Elabas ES, Ghanem BS, Makhseed S, McKeown NB, Msayib KJ, et al. Solution-processed, organophilic membrane derived from a polymer of intrinsic microporosity. Adv Mater 2004;16:456-9.
[130] Rochat S, Polak-Krasna K, Tian M, Mays TJ, Bowen CR, Burrows AD. Assessment of the long-term stability of the polymer of intrinsic microporosity PIM-1 for hydrogen storage applications. Int J Hydrogen Energy 2019;44:332-7.
[131] Bhattacharyya R, Mohan S. Solid state storage of hydrogen and its isotopes: an engineering overview. Renew Sustain Energy Rev 2015;41:872-83.
[132] Li J, Gao ZR, Lin Q-F, Liu C, Gao F, Lin C, et al. A 3D extra-large-pore zeolite enabled by 1D-to-3D topotactic condensation of a chain silicate. Science 2023; 379:283-7.
[133] Li M, Yin W, Pan J, Zhu Y, Sun N, Zhang X, et al. Hydrogen spillover as a promising strategy for boosting heterogeneous catalysis and hydrogen storage. Chem Eng J 2023;471:144691.
[134] Allendorf MD, Hulvey Z, Gennett T, Ahmed A, Autrey T, Camp J, et al. An assessment of strategies for the development of solid-state adsorbents for vehicular hydrogen storage. Energy Environ Sci 2018;11:2784-812.
[135] Langmi HW, Book D, Walton A, Johnson SR, Al-Mamouri MM, Speight JD, et al. Hydrogen storage in ion-exchanged zeolites. J Alloys Compd 2005;404-406: 637-42.
[136] Chung K-H. High-pressure hydrogen storage on microporous zeolites with varying pore properties. Energy 2010;35:2235-41.
[137] Fatriansyah JF, Dhaneswara D, Suhariadi I, Widyantoro MI, Ramadhan BA, Rahmatullah MZ, et al. Simple molecular dynamics simulation of hydrogen adsorption on ZSM 5, graphite nanofiber, graphene oxide framework, and reduced graphene oxide. Heliyon 2021;7:08528.
[138] Zhang Y, Bhattacharjee G, Kumar R, Linga P. Solidified hydrogen storage (SolidHyStore) via clathrate hydrates. Chem Eng J 2022;431:133702.
[139] Gupta A, Baron GV, Perreault P, Lenaerts S, Ciocarlan R-G, Cool P, et al. Hydrogen clathrates: next generation hydrogen storage materials. Energy Storage Mater 2021;41:69-107.
[140] Veluswamy HP, Kumar R, Linga P. Hydrogen storage in clathrate hydrates: current state of the art and future directions. Appl Energy 2014;122:112-32.
[141] Ogata K, Hashimoto S, Sugahara T, Moritoki M, Sato H, Ohgaki K. Storage capacity of hydrogen in tetrahydrofuran hydrate. Chem Eng Sci 2008;63:5714-8.
[142] Matsumoto Y, Grim RG, Khan NM, Sugahara T, Ohgaki K, Sloan ED, et al. Investigating the thermodynamic stabilities of hydrogen and methane binary gas hydrates. J Phys Chem C 2014;118:3783-8.
[143] Park J, Lee H. Spectroscopic evidences of the double hydrogen hydrates stabilized with ethane and propane. Kor J Chem Eng 2007;24:624-7.
[144] Belosludov R, Zhdanov RK, Subbotin O, Mizuseki H, Souissi M, Kawazoe Y, et al. Theoretical modelling of the phase diagrams of clathrate hydrates for hydrogen storage applications. Mol Simulat 2012;38:773-80.
[145] Florusse LJ, Peters CJ, Schoonman J, Hester KC, Koh CA, Dec SF, et al. Stable lowpressure hydrogen clusters stored in a binary clathrate hydrate. Science 2004; 306:469-71.
[146] Lee H, Lee J-w, Kim DY, Park J, Seo Y-T, Zeng H, et al. Tuning clathrate hydrates for hydrogen storage. Nature 2005;434:743-6.
[147] Sugahara T, Haag JC, Prasad PS, Warntjes AA, Sloan ED, Sum AK, et al. Increasing hydrogen storage capacity using tetrahydrofuran. J Am Chem Soc 2009;131: 14616-7.
[148] Liu J, Hou J, Xu J, Liu H, Chen G, Zhang J. Ab initio study of the molecular hydrogen occupancy in pure H2 and binary H2-THF clathrate hydrates. Int J Hydrogen Energy 2017;42:17136-43.
[149] Belosludov R, Zhdanov R, Subbotin O, Mizuseki H, Kawazoe Y, Belosludov V. Theoretical study of hydrogen storage in binary hydrogen-methane clathrate hydrates. J Renew Sustain Energy 2014;6.
[150] Liu S, Zhang W, Wu H, Wang J, Yuan Y, Wang S, et al. Molecular hydrogen storage in binary H2-CH4 clathrate hydrates. J Mol Liq 2023;376:121496.
[151] Liu J, Hou J, Xu J, Liu H, Li S, Chen G, et al. Theoretical investigation of exchange of N2 and H2 in sII clathrate hydrates. Chem Phys Lett 2016;660:266-71.
[152] Chen S, Wang Y, Lang X, Fan S, Li G. Rapid and high hydrogen storage in epoxycyclopentane hydrate at moderate pressure. Energy 2023;268:126638.
[153] Papadimitriou N, Tsimpanogiannis I, Peters C, Papaioannou AT, Stubos A. Hydrogen storage in sH hydrates: a Monte Carlo study. J Phys Chem B 2008;112: 14206-11.
[154] Deschamps J, Dalmazzone D. Hydrogen storage in semiclathrate hydrates of tetrabutyl ammonium chloride and tetrabutyl phosphonium bromide. J Chem Eng Data 2010;55:3395-9.
[155] Strobel TA, Koh CA, Sloan ED. Hydrogen storage properties of clathrate hydrate materials. Fluid Phase Equil 2007;261:382-9.
[156] Li L, Tang S, Wang C, Lv X, Jiang M, Wu H, et al. High gas storage capacities and stepwise adsorption in a UiO type metal-organic framework incorporating Lewis basic bipyridyl sites. Chem Commun 2014;50:2304-7.
[157] Ma Z, Zou J, Khan D, Zhu W, Hu C, Zeng X, et al. Preparation and hydrogen storage properties of MgH 2 -trimesic acid-TM MOF (
[158] Lu Z, He J, Song M, Zhang Y, Wu F, Zheng J, et al. Bullet-like vanadium-based MOFs as a highly active catalyst for promoting the hydrogen storage property in MgH 2 . Int J Miner Metall Mater 2023;30:44-53.
[159] Muhammed NS, Haq B, Al Shehri D, Al-Ahmed A, Rahman MM, Zaman E. A review on underground hydrogen storage: insight into geological sites, influencing factors and future outlook. Energy Rep 2022;8:461-99.
[160] Tarkowski R, Uliasz-Misiak B. Towards underground hydrogen storage: a review of barriers. Renew Sustain Energy Rev 2022;162:112451.
[161] Tarkowski R. Underground hydrogen storage: characteristics and prospects. Renew Sustain Energy Rev 2019;105:86-94.
[162] Bünger U, Michalski J, Crotogino F, Kruck O. Large-scale underground storage of hydrogen for the grid integration of renewable energy and other applications. Compendium of Hydrogen Energy: Elsevier 2016:133-63.
[163] Lord AS, Kobos PH, Borns DJ. Geologic storage of hydrogen: scaling up to meet city transportation demands. Int J Hydrogen Energy 2014;39:15570-82.
[164] Raad SMJ, Leonenko Y, Hassanzadeh H. Hydrogen storage in saline aquifers: opportunities and challenges. Renew Sustain Energy Rev 2022;168:112846.
[165] Osman AI, Mehta N, Elgarahy AM, Hefny M, Al-Hinai A, Al-Muhtaseb AaH, et al. Hydrogen production, storage, utilisation and environmental impacts: a review. Environ Chem Lett 2022:1-36.
[166] Lemieux A, Sharp K, Shkarupin A. Preliminary assessment of underground hydrogen storage sites in Ontario, Canada. Int J Hydrogen Energy 2019;44: 15193-204.
[167] Michalski J, Bünger U, Crotogino F, Donadei S, Schneider GS, Pregger T, et al. Hydrogen generation by electrolysis and storage in salt caverns: potentials, economics and systems aspects with regard to the German energy transition. Int J Hydrogen Energy 2017;42:13427-43.
[168] Crotogino F, Donadei S, Bünger U, Landinger H. Large-scale hydrogen underground storage for securing future energy supplies. In: 18th World hydrogen energy conference; 2010. p. 37-45.
[169] Sainz-Garcia A, Abarca E, Rubi V, Grandia F. Assessment of feasible strategies for seasonal underground hydrogen storage in a saline aquifer. Int J Hydrogen Energy 2017;42:16657-66.
[170] Zivar D, Kumar S, Foroozesh J. Underground hydrogen storage: a comprehensive review. Int J Hydrogen Energy 2021;46:23436-62.
[171] Tarkowski R, Czapowski G. Salt domes in Poland – potential sites for hydrogen storage in caverns. Int J Hydrogen Energy 2018;43:21414-27.
[172] Lord AS. Overview of geologic storage of natural gas with an emphasis on assessing the feasibility of storing hydrogen. Albuquerque, NM, and Livermore, CA: Sandia National Laboratories (SNL); 2009.
[173] Tackie-Otoo BN, Haq MB. A comprehensive review on geo-storage of H2 in salt caverns: prospect and research advances. Fuel 2024;356:129609.
[174] Raad SMJ, Leonenko Y, Hassanzadeh H. Hydrogen storage in saline aquifers: opportunities and challenges. Renew Sustain Energy Rev 2022;168:112846.
[175] Cyran K. Insight into a shape of salt storage caverns. Arch Min Sci 2020;65: 363-98.
[176] Landinger H, Crotogino F. The role of large-scale hydrogen storage for future renewable energy utilisation. In: Second international renewable energy storage conference (IRES II); 2007.
[177] Caglayan DG, Weber N, Heinrichs HU, Linssen J, Robinius M, Kukla PA, et al. Technical potential of salt caverns for hydrogen storage in Europe. Int J Hydrogen Energy 2020;45:6793-805.
[178] Dias W, Roehl D, Mejia C, Sotomayor P. Cavern integrity for underground hydrogen storage in the Brazilian pre-salt fields. Int J Hydrogen Energy 2023;48: 26853-69.
[179] Liu W, Zhang ZX, Chen J, Jiang DY, Wei F, Fan JY, et al. Feasibility evaluation of large-scale underground hydrogen storage in bedded salt rocks of China: a case study in Jiangsu province. Energy 2020;198:117348.
[180] Panfilov M, Gravier G, Fillacier S. Underground storage of H2 and H2-CO2-CH4 mixtures. In: ECMOR X-10th European conference on the mathematics of oil recovery. European Association of Geoscientists & Engineers; 2006. 23-00003.
[181] Kruck O, Crotogino F, Prelicz R, Tjkutg Rudolph. Assessment of the potential, the actors and relevant business cases for large scale and seasonal storage of renewable electricity by hydrogen underground storage in Europe. 2013. p. 1-32.
[182] Navaid HB, Emadi H, Watson M. A comprehensive literature review on the challenges associated with underground hydrogen storage. Int J Hydrogen Energy 2023;48:10603-35.
[183] Bo ZK, Zeng LP, Chen YQ, Xie Q. Geochemical reactions-induced hydrogen loss during underground hydrogen storage in sandstone reservoirs. Int J Hydrogen Energy 2021;46:19998-20009.
[184] Perera MSA. A review of underground hydrogen storage in depleted gas reservoirs: insights into various rock-fluid interaction mechanisms and their impact on the process integrity. Fuel 2023;334:126677.
[185] Moser A. Bioprocess technology: kinetics and reactors. Springer Science & Business Media; 2012.
[186] Al-Yaseri A, Esteban L, Giwelli A, Sarout J, Lebedev M, Sarmadivaleh M. Initial and residual trapping of hydrogen and nitrogen in Fontainebleau sandstone using nuclear magnetic resonance core flooding. Int J Hydrogen Energy 2022;47: 22482-94.
[187] Sekar LK, Kiran R, Okoroafor ER, Wood DA. Review of reservoir challenges associated with subsurface hydrogen storage and recovery in depleted oil and gas reservoirs. J Energy Storage 2023;72:108605.
[188] Matos CR, Carneiro JF, Silva PP. Overview of large-scale underground energy storage technologies for integration of renewable energies and criteria for reservoir identification. J Energy Storage 2019;21:241-58.
[189] Lai Q, Sun Y, Wang T, Modi P, Cazorla C, Demirci UB, et al. How to design hydrogen storage materials? Fundamentals, synthesis, and storage tanks. Advanced Sustainable Systems 2019;3:1900043.
[190] Zhang F, Zhao PC, Niu M, Maddy J. The survey of key technologies in hydrogen energy storage. Int J Hydrogen Energy 2016;41:14535-52.
[191] Ramimoghadam D, Gray EM, Webb CJ. Review of polymers of intrinsic microporosity for hydrogen storage applications. Int J Hydrogen Energy 2016;41: 16944-65.
[192] Kaur M, Pal K. Review on hydrogen storage materials and methods from an electrochemical viewpoint. J Energy Storage 2019;23:234-49.
[193] Blackman JM, Patrick JW, Arenillas A, Shi W, Snape CE. Activation of carbon nanofibres for hydrogen storage. Carbon 2006;44:1376-85.
[194] Aceves SM, Berry GD, Martinez-Frias J, Espinosa-Loza F. Vehicular storage of hydrogen in insulated pressure vessels. Int J Hydrogen Energy 2006;31:2274-83.
[195] Wang Y, Adroher XC, Chen JX, Yang XG, Miller T. Three-dimensional modeling of hydrogen sorption in metal hydride hydrogen storage beds. J Power Sources 2009;194:997-1006.
[196] Klopčič N, Grimmer I, Winkler F, Sartory M, Trattner A. A review on metal hydride materials for hydrogen storage. J Energy Storage 2023;72:108456.
[197] Lototskyy MV, Yartys VA, Pollet BG, Bowman RC. Metal hydride hydrogen compressors: a review. Int J Hydrogen Energy 2014;39:5818-51.
[198] Tarasov BP, Fursikov PV, Volodin AA, Bocharnikov MS, Shimkus YY, Kashin AM, et al. Metal hydride hydrogen storage and compression systems for energy storage technologies. Int J Hydrogen Energy 2021;46:13647-57.
[199] Modi P, Aguey-Zinsou KF. Room temperature metal hydrides for stationary and heat storage applications: a review. Front Energy Res 2021;9:616115.
[200] Salman MS, Lai QW, Luo XX, Pratthana C, Rambhujun N, Costalin M, et al. The power of multifunctional metal hydrides: a key enabler beyond hydrogen storage. J Alloys Compd 2022;920:165936.
[201] He T, Cao H, Chen P. Complex hydrides for energy storage, conversion, and utilization. Adv Mater 2019;31:e1902757.
[202] Orimo S, Nakamori Y, Eliseo JR, Zuttel A, Jensen CM. Complex hydrides for hydrogen storage. Chem Rev 2007;107:4111-32.
[203] de Jongh PE, Adelhelm P. Nanosizing and nanoconfinement: new strategies towards meeting hydrogen storage goals. ChemSusChem 2010;3:1332-48.
[204] Pang Y, Liu Y, Gao M, Ouyang L, Liu J, Wang H, et al. A mechanical-force-driven physical vapour deposition approach to fabricating complex hydride nanostructures. Nat Commun 2014;5:3519.
[205] Balde CP, Hereijgers BP, Bitter JH, de Jong KP. Sodium alanate nanoparticleslinking size to hydrogen storage properties. J Am Chem Soc 2008;130:6761-5.
[206] Pratthana C, Yang YW, Rawal A, Aguey-Zinsou KF. Nanoconfinement of lithium alanate for hydrogen storage. J Alloys Compd 2022;926:166834.
[207] Gutowska A, Li L, Shin Y, Wang CM, Li XS, Linehan JC, et al. Nanoscaffold mediates hydrogen release and the reactivity of ammonia borane. Angew Chem Int Ed Engl 2005;44:3578-82.
[208] Wahab MA, Zhao HJ, Yao XD. Nano-confined ammonia borane for chemical hydrogen storage. Front Chem Sci Eng 2012;6:27-33.
[209] Valero-Pedraza MJ, Cot D, Petit E, Aguey-Zinsou KF, Alauzun JG, Demirci UB. Ammonia borane nanospheres for hydrogen storage. ACS Appl Nano Mater 2019; 2:1129-38.
[210] Lai QW, Pratthana C, Yang YW, Rawal A, Aguey-Zinsou KF. Nanoconfinement of complex borohydrides for hydrogen storage. ACS Appl Nano Mater 2021;4:973-8.
[211] Ngene P, Adelhelm P, Beale AM, de Jong KP, de Jongh PE. LiBH/SBA-15 nanocomposites prepared by melt infiltration under hydrogen pressure: synthesis and hydrogen sorption properties. J Phys Chem C 2010;114:6163-8.
[212] Le TT, Pistidda C, Nguyen VH, Singh P, Raizada P, Klassen T, et al.
Nanoconfinement effects on hydrogen storage properties of MgH and LiBH . Int J Hydrogen Energy 2021;46:23723-36.
[213] Zhang QY, Huang YK, Ma TC, Li K, Ye F, Wang XC, et al. Facile synthesis of small MgH nanoparticles confined in different carbon materials for hydrogen storage. J Alloys Compd 2020;825:153953.
[214] Ma Z, Zhang Q, Panda S, Zhu W, Sun F, Khan D, et al. In situ catalyzed and nanoconfined magnesium hydride nanocrystals in a Ni-MOF scaffold for hydrogen storage. Sustain Energy Fuels 2020;4:4694-703.
[215] Pohlmann C, Röntzsch L, Hu J, Weißgärber T, Kieback B, Fichtner M. Tailored heat transfer characteristics of pelletized
[216] Milanese C, Garroni S, Gennari F, Marini A, Klassen T, Dornheim M, et al. Solid state hydrogen storage in alanates and alanate-based compounds: a review. Metals 2018;8:567.
[217] Gao Q, Xia G, Yu X. Confined NaAlH(4) nanoparticles inside CeO(2) hollow nanotubes towards enhanced hydrogen storage. Nanoscale 2017;9:14612-9.
[218] Broom DP, Webb CJ, Fanourgakis GS, Froudakis GE, Trikalitis PN, Hirscher M. Concepts for improving hydrogen storage in nanoporous materials. Int J Hydrogen Energy 2019;44:7768-79.
[219] Shet SP, Priya SS, Sudhakar K, Tahir M. A review on current trends in potential use of metal-organic framework for hydrogen storage. Int J Hydrogen Energy 2021;46:11782-803.
[220] Yuan S, Zou L, Qin JS, Li J, Huang L, Feng L, et al. Construction of hierarchically porous metal-organic frameworks through linker labilization. Nat Commun 2017; 8:15356.
[221] Ahmed A, Seth S, Purewal J, Wong-Foy AG, Veenstra M, Matzger AJ, et al. Exceptional hydrogen storage achieved by screening nearly half a million metalorganic frameworks. Nat Commun 2019;10:1568.
[222] Yang SL, Karve VV, Justin A, Kochetygov I, Espín J, Asgari M, et al. Enhancing MOF performance through the introduction of polymer guests. Coord Chem Rev 2021;427:213525.
[223] Zhang X, Zhang S, Tang Y, Huang X, Pang H. Recent advances and challenges of metal-organic framework/graphene-based composites. Compos B Eng 2022;230: 109532.
[224] Kang PC, Ou YS, Li GL, Chang JK, Wang CY. Room-temperature hydrogen adsorption via spillover in Pt nanoparticle-decorated UiO-66 nanoparticles: implications for hydrogen storage. ACS Appl Nano Mater 2021;4:11269-80.
[225] Chen L, Zhang X, Cheng X, Xie Z, Kuang Q, Zheng L. The function of metalorganic frameworks in the application of MOF-based composites. Nanoscale Adv 2020;2:2628-47.
[226] Ren J, Huang Y, Zhu H, Zhang B, Zhu H, Shen S, et al. Recent progress on MOFderived carbon materials for energy storage. Carbon Energy 2020;2:176-202.
[227] Li X, Yang X, Xue H, Pang H, Xu Q. Metal-organic frameworks as a platform for clean energy applications. Inside Energy 2020;2:100027.
[228] Niu S, Wang Z, Zhou T, Yu M, Yu M, Qiu J. A polymetallic metal-organic framework-derived strategy toward synergistically multidoped metal oxide electrodes with ultralong cycle life and high volumetric capacity. Adv Funct Mater 2016;27:1605332.
[229] Shi W, Ye C, Xu X, Liu X, Ding M, Liu W, et al. High-performance membrane capacitive deionization based on metal-organic framework-derived hierarchical carbon structures. ACS Omega 2018;3:8506-13.
[230] Bao W, Yu J, Chen F, Du H, Zhang W, Yan S, et al. Controllability construction and structural regulation of metal-organic frameworks for hydrogen storage at ambient condition: a review. Int J Hydrogen Energy 2023.
[231] Chavan S, Vitillo JG, Gianolio D, Zavorotynska O, Civalleri B, Jakobsen S, et al. H 2 storage in isostructural UiO-67 and UiO-66 MOFs. Phys Chem Chem Phys 2012;14:1614-26.
[232] Xia YD, Yang ZX, Zhu YQ. Porous carbon-based materials for hydrogen storage: advancement and challenges. J Mater Chem A 2013;1:9365-81.
[233] Xu H, Shang H, Wang C, Du Y. Low-dimensional metallic nanomaterials for advanced electrocatalysis. Adv Funct Mater 2020;30:2006317.
[234] Van der Ven A, Deng Z, Banerjee S, Ong SP. Rechargeable alkali-ion battery materials: theory and computation. Chem Rev 2020;120:6977-7019.
[235] Wang ZF, Jin KH, Liu F. Computational design of two-dimensional topological materials. WIREs Computational Molecular Science 2017;7:e1304.
[236] Mroz AM, Posligua V, Tarzia A, Wolpert EH, Jelfs KE. Into the unknown: how computation can help explore uncharted material space. J Am Chem Soc 2022; 144:18730-43.
[237] Miracle DB, Li M, Zhang ZH, Mishra R, Flores KM. Emerging capabilities for the high-throughput characterization of structural materials. Annu Rev Mater Res 2021;51(2021):131-64. 51.
[238] Xu D, Zhang Q, Huo X, Wang Y, Yang M. Advances in data-assisted highthroughput computations for material design. Materials Genome Engineering Advances 2023;1:e11.
[239] Cazorla C. The role of density functional theory methods in the prediction of nanostructured gas-adsorbent materials. Coord Chem Rev 2015;300:142-63.
[240] Altintas C, Keskin S. On the shoulders of high-throughput computational screening and machine learning: design and discovery of MOFs for H2 storage and purification. Mater Today Energy 2023;38:101426.
[241] Ren E, Guilbaud P, Coudert FX. High-throughput computational screening of nanoporous materials in targeted applications. Digital Discovery 2022;1:355-74.
[242] Colón YJ, Fairen-Jimenez D, Wilmer CE, Snurr RQ. High-throughput screening of porous crystalline materials for hydrogen storage capacity near room temperature. J Phys Chem C 2014;118:5383-9.
[243] Ahmed A, Liu YY, Purewal J, Tran LD, Wong-Foy AG, Veenstra M, et al. Balancing gravimetric and volumetric hydrogen density in MOFs. Energy Environ Sci 2017; 10:2459-71.
[244] Deniz CU, Mert H, Baykasoglu C. Li-doped fullerene pillared graphene nanocomposites for enhancing hydrogen storage: a computational study. Comput Mater Sci 2021;186:110023.
[245] Bian LY, Li XD, Huang XY, Yang PH, Wang YD, Liu XY, et al. Molecular simulation on hydrogen storage properties of five novel covalent organic frameworks with the higher valency. Int J Hydrogen Energy 2022;47:29390-8.
[246] Li QY, Qiu SY, Wu CZ, Lau T, Sun CH, Jia BH. Computational investigation of
[247] Rana S, Monder DS, Chatterjee A. Thermodynamic calculations using reverse Monte Carlo: a computational workflow for accelerated construction of phase diagrams for metal hydrides. Comput Mater Sci 2024;233:112727.
[248] Jing ZJ, Yuan QQ, Yu Y, Kong XT, Tan KC, Wang JT, et al. Developing ideal metalorganic hydrides for hydrogen storage: from theoretical prediction to rational fabrication. ACS Mater Lett 2021;3:1417-25.
[249] Liu H, Xu C, Liang J. Dependency distance: a new perspective on syntactic patterns in natural languages. Phys Life Rev 2017;21:171-93.
[250] Montejo-Ráez A, Jiménez-Zafra SM. Current approaches and applications in natural language processing. Appl Sci 2022;12:4859.
[251] Mahadevkar SV, Khemani B, Patil S, Kotecha K, Vora DR, Abraham A, et al. A review on machine learning styles in computer vision-techniques and future directions. IEEE Access 2022;10:107293-329.
[252] Shetty SK, Siddiqa A. Deep learning algorithms and applications in computer vision. Int J Comput Sci Eng 2019;7:195-201.
[253] Jovel J, Greiner R. An introduction to machine learning approaches for biomedical research. Front Med 2021;8:771607.
[254] Athanasopoulou K, Daneva GN, Adamopoulos PG, Scorilas A. Artificial intelligence: the milestone in modern biomedical research. BioMedInformatics 2022;2:727-44.
[255] Cebollada S, Paya L, Flores M, Peidró A, Reinoso O. A state-of-the-art review on mobile robotics tasks using artificial intelligence and visual data. Expert Syst Appl 2021;167:114195.
[256] Cully A, Clune J, Tarapore D, Mouret JB. Robots that can adapt like animals. Nature 2015;521. 503-U476.
[257] Tsai C-W, Lai C-F, Chiang M-C, Yang LT. Data mining for internet of things: a survey. IEEE Communications Surveys & Tutorials 2014;16:77-97.
[258] Wang T, Pan R, Martins ML, Cui J, Huang Z, Thapaliya BP, et al. Machine-learning-assisted material discovery of oxygen-rich highly porous carbon active materials for aqueous supercapacitors. Nat Commun 2023;14:4607.
[259] Borboudakis G, Stergiannakos T, Frysali M, Klontzas E, Tsamardinos I, Froudakis GE. Chemically intuited, large-scale screening of MOFs by machine learning techniques. npj Comput Mater 2017;3:40.
[260] Lv C, Zhou X, Zhong L, Yan C, Srinivasan M, Seh ZW, et al. Machine learning: an advanced platform for materials development and state prediction in lithium-ion batteries. Adv Mater 2022;34:e2101474.
[261] Kim J, Kang D, Kim S, Jang HW. Catalyze materials science with machine learning. ACS Mater Lett 2021;3:1151-71.
[262] Shi Z, Yang W, Deng X, Cai C, Yan Y, Liang H, et al. Machine-learning-assisted high-throughput computational screening of high performance metal-organic frameworks. Molecular Systems Design & Engineering 2020;5:725-42.
[263] Jäger MO, Morooka EV, Federici Canova F, Himanen L, Foster AS. Machine learning hydrogen adsorption on nanoclusters through structural descriptors, 4; 2018. p. 37.
[264] Hattrick-Simpers JR, Choudhary K, Corgnale C. A simple constrained machine learning model for predicting high-pressure-hydrogen-compressor materials. Molecular Systems Design & Engineering 2018;3:509-17.
[265] Nations S, Nandi T, Ramazani A, Wang SN, Duan YH. Metal hydride compositionderived parameters as machine learning features for material design and H2 storage. J Energy Storage 2023;70:107980.
[266] Kim JM, Ha T, Lee J, Lee Y-S, Shim J-H. Prediction of pressure-compositiontemperature curves of AB2-type hydrogen storage alloys by machine learning. Met Mater Int 2022;29:861-9.
[267] Thanh HV, Taremsari SE, Ranjbar B, Mashhadimoslem H, Rahimi E, Rahimi M, et al. Hydrogen storage on porous carbon adsorbents: rediscovery by naturederived algorithms in random forest machine learning model. Energies 2023;16: 2348.
[268] Shekhar S, Chowdhury C. Prediction of hydrogen storage in metal-organic frameworks: a neural network based approach. Results in Surfaces and Interfaces 2024;14:100166.
[269] Ahmed A, Siegel DJ. Predicting hydrogen storage in MOFs via machine learning. Patterns (N Y) 2021;2:100291.
[270] Salehi K, Rahmani M, Atashrouz S. Machine learning assisted predictions for hydrogen storage in metal-organic frameworks. Int J Hydrogen Energy 2023;48: 33260-75.
[271] Borja NK, Fabros CJE, Doma BT. Prediction of hydrogen adsorption and moduli of metal-organic frameworks (MOFs) using machine learning strategies. Energies 2024;17:927.
- Corresponding author. School of Chemistry and Chemical Engineering, Queen’s University Belfast, David Keir Building, Stranmillis Road, Belfast, BT9 5AG, Northern Ireland, United Kingdom.
E-mail address: aosmanahmed01@qub.ac.uk (A.I. Osman).
DOI: https://doi.org/10.1016/j.ijhydene.2024.03.223
Publication Date: 2024-03-27
Advances in hydrogen storage materials: harnessing innovative technology, from machine learning to computational chemistry, for energy storage solutions
Published in:
Document Version:
Queen’s University Belfast – Research Portal:
Publisher rights
This is an open access article published under a Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the author and source are cited.
General rights
Take down policy
Open Access
Advances in hydrogen storage materials: harnessing innovative technology, from machine learning to computational chemistry, for energy storage solutions
ARTICLE INFO
Keywords:
Compressed hydrogen
Liquified hydrogen
High-throughput screening
Computational chemistry
Machine learning
Abstract
The demand for clean and sustainable energy solutions is escalating as the global population grows and economies develop. Fossil fuels, which currently dominate the energy sector, contribute to greenhouse gas emissions and environmental degradation. In response to these challenges, hydrogen storage technologies have emerged as a promising avenue for achieving energy sustainability. This review provides an overview of recent advancements in hydrogen storage materials and technologies, emphasizing the importance of efficient storage for maximizing hydrogen’s potential. The review highlights physical storage methods such as compressed hydrogen (reaching pressures of up to 70 MPa ) and material-based approaches utilizing metal hydrides and carboncontaining substances. It also explores design considerations, computational chemistry, high-throughput screening, and machine-learning techniques employed in developing efficient hydrogen storage materials. This comprehensive analysis showcases the potential of hydrogen storage in addressing energy demands, reducing greenhouse gas emissions, and driving clean energy innovation.
1. Introduction
and detrimental impacts on human health. Geopolitical tensions and concerns about energy security have arisen due to the uneven distribution of fossil fuel resources [7].
2. Hydrogen storage technologies
2.1. Physical storage technologies
tackle this issue, various physical storage technologies for hydrogen have been developed. These methods, which include compression and liquefaction technologies, either individually or in combination, offer innovative solutions for storing hydrogen in dense and stable forms. Such technologies play a pivotal role in overcoming the limitations associated with hydrogen’s lightweight and gaseous nature, enabling its efficient transportation and utilization across various applications [22]. They serve as a gateway to unlock hydrogen’s potential as a clean energy carrier [23], with applications spanning fuel cell vehicles [24] and industrial processes [25]. Fig. 1 Provides a concise overview of various hydrogen storage technologies.
2.1.1. Compressed hydrogen technique

2.1.2. Liquefied hydrogen storage
hydrogen can be facilitated through dedicated cryogenic pipelines or specialized road tankers equipped with cryogenic storage systems. Moreover, an emerging global trend encompasses the maritime transportation of liquefied hydrogen within dedicated containers on ships [34].
2.1.3. Cryo-compressed hydrogen storage
conventional high-pressure gas storage, along with the inescapable boiloff losses encountered in the cryogenic hydrogen storage paradigm [32]. The focal aim of this approach is to optimize hydrogen storage solutions across diverse applications, with a pronounced emphasis on the automotive sector.
2.2. Material-based hydrogen storage
hydrides [43], ammonia-based compounds [44], carbon materials, metal-organic frameworks [45], covalent organic frameworks [46], clathrates [47], and other bio-waste and porous materials [48], has been explored for chemical hydrogen storage. For instance, metal hydrides form robust metal-hydrogen bonds, enabling hydrogen absorption and release through heating or catalysis [49]. Complex hydrides, on the other hand, are multi-element compounds known for their substantial storage capacities via chemical processes [50]. Each material has its unique strengths and limitations based on various factors [51]. There is no universally ideal hydrogen storage material; the choice depends on hydrogen storage capacity, operating conditions, thermodynamics, kinetics, stability, reversibility, availability, cost, and environmental impact. Subsequent sections will delve into advancements in selected materials for chemical hydrogen storage.
2.2.1. Chemical sorbents
2.2.1.1. Metal hydrides. Metal hydrides, a category of materials composed of metal and hydrogen, have garnered considerable attention recently due to their substantial hydrogen-storage capacities, rendering them promising for hydrogen-based energy systems. Their attributes, including high energy density, relatively low cost, and environmental friendliness, render them attractive for applications spanning portable electronics, electric vehicles, and renewable energy systems. However, their advancement for hydrogen storage encounters significant hurdles, notably the identification of materials with high hydrogen-storage capacity while maintaining stability, safety, and economic viability [55].
limited by the accumulation of impurities within the tanks, which clog the spaces where hydrogen would typically be stored, reducing the tank’s capacity. The amount of heat transferred, hydrogen absorbed, and hydrogen desorbed in a reversible metal hydride operating at room temperature and atmospheric pressure is generally dependent on the metal alloy used for hydrogen storage [22]. A simplified model illustrating how hydrogen is stored and absorbed in a metal hydride is depicted in Fig. 2.
2.2.1.1.1. Magnesium hydride (
agglomeration. This strategy was employed in a recent investigation [64], in which a porous three-dimensional activated carbon nanostructure modified with Ni and Fe was used to impregnate
2.2.1.1.2. Lithium hydride (LiH). Lithium hydride (LiH), which is considered one of the lightest metal hydrides ever, has a high gravimetric hydrogen storage capacity of up to around

2.2.1.1.3. Sodium alanate (
2.2.1.1.4. Ammonia borane (
release from ammonia borane involves hydrolysis. Ammonia borane exhibits a notable resistance to hydrolysis in aqueous solutions, thus demanding the presence of an efficient catalyst to induce hydrolytic dehydrogenation at ambient temperatures. Metal-catalyzed hydrolysis emerges as a viable choice, affording the release of a substantial quantity (
2.2.1.1.5. Metal nitrides including amides (
2.2.2. Physical sorbents
towards optimizing volumetric properties [88].
2.2.2.1. Activated carbon and carbon nanomaterials. The hydrogen storage capacity of activated carbon is typically around
capacity of carbon nanotubes.
2.2.2.2. Metal-organic frameworks (MOFs). A class of porous materials made up of metal ions or metal clusters coupled to organic ligands. These materials have a large surface area and adjustable pore diameters. Due to their high porosity and substantial surface area, MOFs have demonstrated a strong potential for storing hydrogen through physisorption. Some MOFs have demonstrated hydrogen capacities exceeding
2.2.2.3. Covalent organic frameworks (COFs). Covalent organic frameworks (COFs) are a class of materials that resemble MOFs but do not contain heavy-metal ions. They are constructed using organic building blocks through dynamic covalent formation methods. COFs have extended crystalline structures with high surface areas and low

2.2.2.4. Porous organic polymers (POPs). Amorphous porous organic polymers (POPs) have emerged as promising contenders for hydrogen storage due to their convenient processability and robust mechanical properties. POPs can be classified into four main categories: (i) porous aromatic frameworks (PAFs), (ii) conjugated microporous polymers (CMPs), (iii) hyper-cross-linked polymers (HCPs) and (iv) polymers of intrinsic microporosity (PIMs) [115].
other porous materials and even comparable to the best values reported in MOFs.
or activated carbon. For testing the stability of PIM for hydrogen storage, Rochat et al. [130] investigated the long-term stability of the PIM-1 for hydrogen storage applications. Over a period of 400 days, the mechanical and surface properties of PIM-1 were examined. The results showed that most mechanical and surface properties remained stable over time, including mechanical strength, elasticity, and surface area. However, there was a small but statistically significant decrease in the hydrogen storage capacity of PIM-1, particularly in the initial stages of aging. This decrease was attributed to the slow rearrangement of the polymer scaffold. Overall, the study demonstrated that PIM-1 possesses the necessary long-term stability for realistic hydrogen storage applications.
2.2.2.5. Zeolites. Zeolites are crystalline aluminosilicates with welldefined porous structures. Due to their unique microporous nature and large surface area, zeolites have been investigated as potential materials for hydrogen storage [131]. Zeolites store hydrogen mainly through physisorption, which is a type of physical adsorption resulting from weak Van der Waals forces between the hydrogen molecules and the surface of the zeolite. The adsorption process is reversible, so hydrogen can easily be released when needed.
2.2.2.6. Clathrates. Clathrates, scientifically termed gas hydrates, are
intricate crystalline formations arising from the structural arrangement of water molecules. These formations assume a cage-like architecture, adept at encapsulating guest molecules devoid of any chemical bonding. Typically, these guest molecules manifest as gases. Given the heightened demand for safe and efficient materials conducive to hydrogen storage, clathrates have emerged as an encouraging category of substances for potential application. The containment of hydrogen within water cages, as facilitated by clathrates, serves to substantially reduce the associated hazards related to sudden gas release or explosive occurrences [138].
Classification of clathrates based on crystallographic attributes.
| Structure I (sI) | Structure II (sII) | Structure H (sH) |
| – A cubic structure, and | – A cubic structure. | – A hexagonal structure. |
| is one of the most | – It consists of small | – It has small |
| common types of | dodecahedral and larger | dodecahedral, medium- |
| clathrates. | hexakaidecahedral (16- | sized hexakaidecahedral, |
| – It consists of small | faced) cages. | and large icosakaioctahe- |
| dodecahedral (12-faced) | – Larger gases, like | dral (24-faced) cages. |
| and larger | ethane, propane, and | – Gases like krypton, |
| tetrakaidecahedral (14- | carbon dioxide, form sII | xenon, and some larger |
| faced) cages. | hydrates. | hydrocarbons form sH |
| – Common gases that | – sII hydrates are more | hydrates. |
| form sI hydrates include | accommodating to | – Hydrogen can also form |
| methane, ethylene, and | hydrogen, especially in | sH hydrates under certain |
| hydrogen typically | promoter gases. For | when other larger guest |
| doesn’t form stable sI | instance, hydrogen can | molecules are present to |
| hydrates. | form sII hydrates in the | occupy the large cages. |
| with other gases can, | presence of | |
| however, stabilize the | certain other organic | |
| formation of sI hydrates. | molecules. |
used in the formation of semiclathrates hydrates. The feasibility of utilizing tetrabutylammonium chloride and tetrabutylphosphonium bromide-based semi-clathrates for hydrogen storage was explored by Deschamps and Dalmazzone [154]. They determined that these materials exhibited hydrogen storage capacities of
2.3. Underground storage of hydrogen
them suitable for accommodating intermittent energy sources [160]. An underground gas storage facility involves the artificial accumulation of gas in the natural environment, typically at a considerable depth, often exceeding several hundred meters. The stored gas consists of two components: the working gas, which is injected into and extracted from the storage, and the cushion gas, which remains within the facility throughout its operational lifespan. The purpose of the cushion gas is twofold: to maintain a minimum pressure level that prevents the ingress of water into the storage space and to ensure optimal conditions for gas injection [161,162].
I. Enhanced safety: Underground facilities are less vulnerable to risks such as fires, terrorist attacks, or military actions compared to surface storage tanks.
II. Efficient space management: Underground facilities can store significant amounts of gas while occupying relatively small surface areas. In contrast, surface tanks would require extensive space to achieve the same storage capacity. The compact nature of underground facilities allows for easier integration with the landscape and existing infrastructure.
III. Cost-effectiveness: Constructing underground gas storage facilities is generally more economical compared to surface facilities

Comprehensive tabulated comparison of hydrogen storage materials.
| Storage material | Gravimetric hydrogen storage capacity (wt%) | Temperature (K) | Pressure (MPa) | Reference |
| Ball-milled magnesium hydride | 6.28 | 523 | 1 | [62] |
| Magnesium hydride impregnated with a porous 3D activated carbon nanostructure modified with nickel and iron | 6.63 | 453 | 1 | [64] |
| Sodium alanate with titanium hydride as nanoplates supported on graphene | 5 | 353 | 10 | [77] |
| Lithium-magnesium imide | 4.1 | 523 | 0 | [85] |
| Lithium-magnesium imide enhanced by 10% lithium vanadate@lithium vanadium oxide | 4.7 | |||
| Activated carbon from pyrolyzed lignin material based-eucalyptus chips | 1.8 | 77 | 0.1 | [90] |
| Chitosan-based activated carbon | 2.95 | 77 | 0.1 | [91] |
| 5.61 | 4 | |||
| Coffee bean-based carbon material | 0.6 | 298 | 12 | [92] |
| 4 | 77 | |||
| Nitrogen-doped carbon nanotubes mixed with lanthanum oxide | 7.4 | 373 | 1.8 | [97] |
| University of Oslo Framework 66 (UIO-66) | 3.8 | 77 | 10 | [101] |
| Hydroxylated University of Oslo Framework 66 (UIO-66) | 4.6 | |||
| Isoreticular metal-organic framework 1 (IRMOF-1) | 7.1 | 77 | 4 | [102] |
| 11.5 | 17 | |||
| 2,2-bipyridine-5,5′-dicarboxylate (bpdc)-based University of Oslo framework | 5.7 | 77 | 2 | [156] |
| Magnesium hydride | 6.91 | 648 | 1 | [157] |
| Magnesium@cobalt (II)-based metal-organic framework | 5.19 | |||
| Magnesium@iron (II)-based metal-organic framework | 5.37 | |||
| Magnesium hydride promoted by vanadium-based metal-organic framework | 6.4 | 573 | 3.2 | [158] |
| Structure I of clathrates promoted by methane | 0.02 | 273 | 70 | [142] |
| Structure I of clathrates promoted by propane | 0.17 | 270 | 12 | [143] |
| Structure I of clathrates promoted by ethane | 2.5 | 250 | 300 | [144] |
| Structure II of clathrates promoted by tetrahydrofuran (binary hydrates) | 1 | 279.6 | 5 | [145] |
| Structure II of clathrates promoted by tetrahydrofuran (binary hydrates) | 4.03 | 270 | 12 | [146] |
| Structure II of clathrates promoted by tetrahydrofuran (binary hydrates) | 3.4 | 255 | 70 | [147] |
| Pure hydrogen hydrates | 4.4 | 140 | 0.1 | [148] |
| Binary hydrates of hydrogen and tetrahydrofuran | 1.6-3.8 | |||
| Structure II of clathrates promoted by methane (binary hydrates) | 2.6 | 250 | 70 | [149] |
| Structure II of clathrates promoted by methane (binary hydrates) | 3.43 | 270 | 20 | [150] |
| Structure II of clathrates promoted by nitrogen (binary hydrates) | 4.4 | 243 | 15 | [151] |
| Structure II of clathrates promoted by epoxycyclopentane (binary hydrates) | 0.63 | 262 | 18.2 | [152] |
| Solid-HyStore hydrate promoted by methylcyclohexane (binary hydrates) | 1.4 | 274 | 500 | [153] |
| Tetrabutylammonium chloride-based semiclathrates | 0.12 | 288.9 | 15 | [154] |
| Tetrabutylphosphonium bromide-based semiclathrates | 0.14 | 285 | ||
| Tetra-n-butylammonium bromide-based semiclathrates | 0.21 | 279.5 | 13.8 | [155] |
| Sodium based-zeolite X | 1.79 | 77 | 1.5 | [135] |
| Potassium based-zeolite X | 1.96 | |||
| Rubidium based-zeolite X | 1.46 | |||
| Cesium based-zeolite X | 1.32 | |||
| Magnesium based-zeolite X | 1.62 | |||
| Calcium based-zeolite X | 2.19 | |||
| Strontium based-zeolite X | 1.68 | |||
| Ultra-stable Y zeolite | 0.4 | 303 | 5 | [136] |
| Zeolite socony mobil-5 | 2.89 | 77 | 1.2 | [137] |
IV. Abundance of suitable geological structures: Many countries and large areas possess suitable geological formations for underground gas storage. These structures provide favorable conditions for the establishment and operation of underground facilities.
2.3.1. Salt cavern
caverns with specific dimensions [166,167]. These caverns, typically built up to 2000 m deep with volumes reaching 1,000,000 cubic meters, are well-suited for storing various substances, particularly gases, under high pressures [167]. The pressure range during operation usually falls between
number of sites currently accommodate hydrogen storage within salt caverns. Notable examples include Teesside in the UK and Clemens Dome, Spindletop, and Moss Bluff in the US [161,176]. The Teesside facility, operational since the 1970s, utilizes elliptically shaped salt caverns situated at depths ranging from 350 to 450 m , boasting a collective volume of 210,000 cubic meters. Conversely, the salt caverns at Clemens Dome and Moss Bluff, located at a depth of 800 m from the cavern’s top, have larger capacities of approximately 580,000 cubic meters each. Clemens Dome has been in operation since 1983, while Moss Bluff commenced operations in 2007. These longstanding projects serve as compelling evidence of the technical viability of underground hydrogen storage over extended periods [176,177].
2.3.2. Aquifers
in pressure. During injection, gas displaces water, creating a dynamic gas/water boundary that shifts during the storage facility’s operation. However, a drawback is that some gas remains unrecoverable in the aquifer [161,169,170].
2.3.3. Depleted oil and gas reservoirs
methane and carbon dioxide does not guarantee suitability for hydrogen due to its different characteristics. Hydrogen’s reactivity with reservoir rocks can alter pore structures, affecting injectivity and storage capacity. While hydrogen loss during storage is minimal, significant losses occur through residual trapping, geochemical reactions, and caprock leakage. Understanding fluid migration through reservoirs is crucial, considering mineralogical changes induced by hydrogen interaction [184].
3. Designing materials for hydrogen storage
3.1. Design considerations and strategies for hydrogen storage material
[189].
relatively low temperatures and pressures akin to those found in fuel cell vehicles, and the release of hydrogen from metal hydrides occurs via an endothermic process. Hydrogen liberation from metal hydrides can occur either through an increase in temperature or a reduction in external pressure [51].
storage research [201].
self-assembly of inorganic metal clusters and organic linkers. This unique assembly process offers numerous variations in building blocks, contributing to the vast spectrum of properties exhibited by MOFs, particularly in terms of surface area, which often surpasses that of other materials. However, while this design flexibility enables the customization of MOF properties, it presents challenges in identifying optimal compositions due to the extensive parameter space that needs exploration [220,221].
thermal conductivity, these materials have garnered attention for their potential in hydrogen storage applications. Notably, graphene and carbon nanotubes demonstrate impressive hydrogen adsorption capacities attributed to their expansive, accessible surface areas and porous architectures.
3.2. High-throughput computational screening and machine learning
target properties, definition of screening spaces, property prediction, and selection of candidate materials. Identifying target properties stands out as one of the most crucial and challenging steps in these processes. For materials scientists, naming the desired macroscopic properties of functional materials in energy conversion or storage devices is generally feasible [21].
efforts in high-throughput computational screening (HTCS) of materials for
approximately a two-fold increase compared to undoped FPGNs.
Additionally, Li doping enhanced the excess hydrogen storage capacity of FPGNs up to three times at ambient temperature. These findings suggest that Li-FPGNs hold potential as effective materials for hydrogen storage applications. By utilizing computational methods such as GCMC simulations, the research provides valuable insights into the optimization of Li-FPGNs’ performance for hydrogen adsorption. Numerical values, such as the gravimetric adsorption capacity and the improvement ratios, highlight the significant enhancements achieved through Li doping in the studied nanocomposites.
for hydrogen storage. Four specific metalorganic hydrides were selected for further investigation and synthesis. Theoretical predictions indicated that lithium indolide and lithium octahydroindolide pairs had a theoretical hydrogen capacity of
quantities relevant to hydrogen storage materials, such as the free energy of hydrogen storage/release reactions and materials decomposition processes. Yet, determining the most favorable reaction pathways, adsorption sites, and chemical structures necessitates guessing from various candidate processes and configurations, primarily reliant on chemical intuition. This approach might be incomplete or prone to errors, especially when addressing intricate problems. To tackle these “open” challenges, the aspiration is for theoretical frameworks capable of automatically predicting the thermodynamically optimal reaction paths, states, and configurations based solely on the system’s chemical composition or similar information. ML techniques offer a solution, focusing on computational affordability and objectiveness over chemical intuition [189].
temperature ( 303 K ) using machine learning models. The input features for the models included temperature, pressure, and nine alloy compositions. The prediction accuracy was assessed by comparing the predicted values with the measured values, and higher correlation values indicated better prediction performance. Overall, the research demonstrated the effectiveness of machine learning in predicting PCT curves of hydrogen storage alloys. By utilizing machine learning models, the study provided insights into the behavior of
state-of-the-art materials. The identified MOFs were characterized by low densities (
4. Conclusion
Declaration of competing interest
Acknowledgement
References
[2] Zhang L, Jia C, Bai F, Wang W, An S, Zhao K, et al. A comprehensive review of the promising clean energy carrier: hydrogen production, transportation, storage, and utilization (HPTSU) technologies. Fuel 2024;355:129455.
[3] Hutchins DA, Jansson JK, Remais JV, Rich VI, Singh BK, Trivedi P. Climate change microbiology – problems and perspectives. Nat Rev Microbiol 2019;17: 391-6.
[4] Jones MW, Peters GP, Gasser T, Andrew RM, Schwingshackl C, Gütschow J, et al. National contributions to climate change due to historical emissions of carbon dioxide, methane, and nitrous oxide since 1850. Sci Data 2023;10:155.
[5] Kabir M, Habiba UE, Khan W, Shah A, Rahim S, Rios-Escalante PRD, et al. Climate change due to increasing concentration of carbon dioxide and its impacts on environment in 21st century; a mini review. J King Saud Univ Sci 2023;35: 102693.
[6] Halawy SA, Osman AI, Nasr M, Rooney DW. Mg-O-F nanocomposite catalysts defend against global warming via the efficient, dynamic, and rapid capture of CO(2) at different temperatures under ambient pressure. ACS Omega 2022;7: 38856-68.
[7] Asghar U, Rafiq S, Anwar A, Iqbal T, Ahmed A, Jamil F, et al. Review on the progress in emission control technologies for the abatement of
[8] Obaideen K, Olabi AG, Al Swailmeen Y, Shehata N, Abdelkareem MA, Alami AH, et al. Solar energy: applications, trends analysis, bibliometric analysis and research contribution to sustainable development goals (SDGs). Sustainability 2023;15:1418.
[9] Zhang Z, Liu X, Zhao D, Post S, Chen J. Overview of the development and application of wind energy in New Zealand. Energy and Built Environment 2023; 4:725-42.
[10] Xiaosan Z, Qingquan J, Shoukat Iqbal K, Manzoor A, Zia Ur R. Achieving sustainability and energy efficiency goals: assessing the impact of hydroelectric and renewable electricity generation on carbon dioxide emission in China. Energy Pol 2021;155:112332.
[11] Nasr M, Abdelkader A, El-Nahas S, Osman AI, Abdelhaleem A, El Nazer HA, et al. Utilizing undissolved portion (UNP) of cement kiln dust as a versatile multicomponent catalyst for bioethylene production from bioethanol: an innovative approach to address the energy crisis. ACS Omega 2023.
[12] Pandit K, Jeffrey C, Keogh J, Tiwari MS, Artioli N, Manyar HG. Techno-economic assessment and sensitivity analysis of glycerol valorization to biofuel additives via esterification. Ind Eng Chem Res 2023;62:9201-10.
[13] Osman AI, Mehta N, Elgarahy AM, Hefny M, Al-Hinai A, Al-Muhtaseb AH, et al. Hydrogen production, storage, utilisation and environmental impacts: a review (vol 20, pg 153, 2022). Environ Chem Lett 2022;20:2213.
[14] Hassan Q, Sameen AZ, Salman HM, Jaszczur M, Al-Jiboory AK. Hydrogen energy future: advancements in storage technologies and implications for sustainability. J Energy Storage 2023;72:108404.
[15] Jahanbakhsh A, Louis Potapov-Crighton A, Mosallanezhad A, Tohidi Kaloorazi N, Maroto-Valer MM. Underground hydrogen storage: a UK perspective. Renew Sustain Energy Rev 2024;189:114001.
[16] Mondal K, Malode SJ, Shetti NP, Alqarni SA, Pandiaraj S, Alodhayb A. Porous nanostructures for hydrogen generation and storage. J Energy Storage 2024;76: 109719.
[17] Hassan IA, Ramadan HS, Saleh MA, Hissel D. Hydrogen storage technologies for stationary and mobile applications: review, analysis and perspectives. Renew Sustain Energy Rev 2021;149:111311.
[18] Navlani-García M, Mori K, Kuwahara Y, Yamashita H. Recent strategies targeting efficient hydrogen production from chemical hydrogen storage materials over carbon-supported catalysts. NPG Asia Mater 2018;10:277-92.
[19] Eberle U, Felderhoff M, Schuth F. Chemical and physical solutions for hydrogen storage. Angew Chem Int Ed Engl 2009;48:6608-30.
[20] Dalebrook AF, Gan W, Grasemann M, Moret S, Laurenczy G. Hydrogen storage: beyond conventional methods. Chem Commun 2013;49:8735-51.
[21] Lu Z. Computational discovery of energy materials in the era of big data and machine learning: a critical review. Materials Reports: Energy 2021;1:100047.
[22] Tashie-Lewis BC, Nnabuife SG. Hydrogen production, distribution, storage and power conversion in a hydrogen economy – a technology review. Chemical Engineering Journal Advances 2021;8:100172.
[23] Zhang L, Jia CQ, Bai FQ, Wang WS, An SY, Zhao KY, et al. A comprehensive review of the promising clean energy carrier: hydrogen production, transportation, storage, and utilization (HPTSU) technologies. Fuel 2024;355: 129455.
[24] Du ZM, Liu CM, Zhai JX, Guo XY, Xiong YL, Su W, et al. A review of hydrogen purification technologies for fuel cell vehicles. Catalysts 2021;11:393.
[25] Qazi UY. Future of hydrogen as an alternative fuel for next-generation industrial applications; challenges and expected opportunities. Energies 2022;15:4741.
[26] Usman MR. Hydrogen storage methods: review and current status. Renew Sustain Energy Rev 2022;167:112743.
[27] Demirocak DE. Hydrogen storage technologies. Nanostructured Materials for Next-Generation Energy Storage and Conversion: Hydrogen Production, Storage, and Utilization 2017:117-42.
[28] Greene DL, Ogden JM, Lin ZH. Challenges in the designing, planning and deployment of hydrogen refueling infrastructure for fuel cell electric vehicles. Etransportation 2020;6:100086.
[29] Barthélémy H, Weber M, Barbier F. Hydrogen storage: recent improvements and industrial perspectives. Int J Hydrogen Energy 2017;42:7254-62.
[30] Moradi R, Groth KM. Hydrogen storage and delivery: review of the state of the art technologies and risk and reliability analysis. Int J Hydrogen Energy 2019;44: 12254-69.
[31] Durbin D, Malardier-Jugroot C. Review of hydrogen storage techniques for on board vehicle applications. Int J Hydrogen Energy 2013;38:14595-617.
[32] AlZohbi G, Almoaikel A, AlShuhail L. An overview on the technologies used to store hydrogen. Energy Rep 2023;9:28-34.
[33] Abe JO, Popoola A, Ajenifuja E, Popoola OM. Hydrogen energy, economy and storage: review and recommendation. Int J Hydrogen Energy 2019;44:15072-86.
[34] Ball M, Wietschel M. The future of hydrogen-opportunities and challenges. Int J Hydrogen Energy 2009;34:615-27.
[35] Ahluwalia RK, Hua T, Peng J. On-board and Off-board performance of hydrogen storage options for light-duty vehicles. Int J Hydrogen Energy 2012;37:2891-910.
[36] Schlapbach L, Züttel A. Hydrogen-storage materials for mobile applications. Nature 2001;414:353-8.
[37] Bossel U. Does a hydrogen economy make sense? Proc IEEE 2006;94:1826-37.
[38] Wang M, Wang G, Sun Z, Zhang Y, Xu D. Review of renewable energy-based hydrogen production processes for sustainable energy innovation. Global Energy Interconnection 2019;2:436-43.
[39] Yanxing Z, Maoqiong G, Yuan Z, Xueqiang D, Jun S. Thermodynamics analysis of hydrogen storage based on compressed gaseous hydrogen, liquid hydrogen and cryo-compressed hydrogen. Int J Hydrogen Energy 2019;44:16833-40.
[40] Sui Y, Yuan Z, Zhou D, Zhai T, Li X, Feng D, et al. Recent progress of nanotechnology in enhancing hydrogen storage performance of magnesiumbased materials: a review. Int J Hydrogen Energy 2022;47:30546-66.
[41] Bellosta von Colbe J, Ares J-R, Barale J, Baricco M, Buckley C, Capurso G, et al. Application of hydrides in hydrogen storage and compression: achievements, outlook and perspectives. Int J Hydrogen Energy 2019;44:7780-808.
[42] Sun Y, Shen C, Lai Q, Liu W, Wang D-W, Aguey-Zinsou K-F. Tailoring magnesium based materials for hydrogen storage through synthesis: current state of the art. Energy Storage Mater 2018;10:168-98.
[43] Ley MB, Jepsen LH, Lee Y-S, Cho YW, Von Colbe JMB, Dornheim M, et al. Complex hydrides for hydrogen storage-new perspectives. Mater Today 2014;17: 122-8.
[44] Demirci UB. Ammonia borane, a material with exceptional properties for chemical hydrogen storage. Int J Hydrogen Energy 2017;42:9978-10013.
[45] Xu J, Liu J, Li Z, Wang X, Xu Y, Chen S, et al. Optimized synthesis of Zr (iv) metal organic frameworks (MOFs-808) for efficient hydrogen storage. New J Chem 2019;43:4092-9.
[46] Bian L-Y, Li X-D, Huang X-Y, Yang P-h, Wang Y-D, Liu X-Y, et al. Molecular simulation on hydrogen storage properties of five novel covalent organic frameworks with the higher valency. Int J Hydrogen Energy 2022;47:29390-8.
[47] Gupta A, Baron GV, Perreault P, Lenaerts S, Ciocarlan R-G, Cool P, et al. Hydrogen clathrates: next generation hydrogen storage materials. Energy Storage Mater 2021;41:69-107.
[48] Ariharan A, Ramesh K, Vinayagamoorthi R, Rani MS, Viswanathan B, Ramaprabhu S, et al. Biomass derived phosphorous containing porous carbon material for hydrogen storage and high-performance supercapacitor applications. J Energy Storage 2021;35:102185.
[49] Von Colbe JB, Ares J-R, Barale J, Baricco M, Buckley C, Capurso G, et al. Application of hydrides in hydrogen storage and compression: achievements, outlook and perspectives. Int J Hydrogen Energy 2019;44:7780-808.
[50] Lai Q, Pratthana C, Yang Y, Rawal A, Aguey-Zinsou K-F. Nanoconfinement of complex borohydrides for hydrogen storage. ACS Appl Nano Mater 2021;4: 973-8.
[51] Rusman NAA, Dahari M. A review on the current progress of metal hydrides material for solid-state hydrogen storage applications. Int J Hydrogen Energy 2016;41:12108-26.
[52] Preuster P, Papp C, Wasserscheid P. Liquid organic hydrogen carriers (LOHCs): toward a hydrogen-free hydrogen economy. Acc Chem Res 2017;50:74-85.
[53] Sathe RY, Kumar TJD, Ahuja R. Furtherance of the material-based hydrogen storage based on theory and experiments. Int J Hydrogen Energy 2023;48: 12767-95.
[54] Qureshi F, Yusuf M, Khan MA, Ibrahim H, Ekeoma BC, Kamyab H, et al. A State-of-The-Art Review on the Latest trends in Hydrogen production, storage, and transportation techniques. Fuel 2023;340:127574.
[55] Kukkapalli VK, Kim S, Thomas SA. Thermal management techniques in metal hydrides for hydrogen storage applications: a review. Energies 2023;16:3444.
[56] Lototskyy MV, Tolj I, Pickering L, Sita C, Barbir F, Yartys V. The use of metal hydrides in fuel cell applications. Prog Nat Sci: Mater Int 2017;27:3-20.
[57] Manoharan K, Sundaram R, Raman K. Expeditious re-hydrogenation kinetics of ball-milled magnesium hydride (
[58] Bogdanović B, Ritter A, Spliethoff B. Active MgH 2 Mg systems for reversible chemical energy storage. Angew Chem Int Ed Engl 2003;29:223-34.
[59] Wang L, Zhang L, Lu X, Wu F, Sun X, Zhao H, et al. Surprising cocktail effect in high entropy alloys on catalyzing magnesium hydride for solid-state hydrogen storage. Chem Eng J 2023;465:142766.
[60] Schneemann A, White JL, Kang S, Jeong S, Wan LF, Cho ES, et al. Nanostructured metal hydrides for hydrogen storage. Chem Rev 2018;118:10775-839.
[61] Yang H, Ding Z, Li Y-T, Li S-Y, Wu P-K, Hou Q-H, et al. Recent advances in kinetic and thermodynamic regulation of magnesium hydride for hydrogen storage. Rare Met 2023:1-22.
[62] El-Eskandarany MS, AlMatrouk H, Shaban E, Al-Duweesh A. Effect of the nanocatalysts on the thermal stability and hydrogenation/dehydrogenation kinetics of MgH 2 nanocrystalline powders. Mater Today Proc 2016;3:2608-16.
[63] Zhou C, Peng Y, Zhang Q. Growth kinetics of MgH2 nanocrystallites prepared by ball milling. J Mater Sci Technol 2020;50:178-83.
[64] Shinde S, Kim D-H, Yu J-Y, Lee J-H. Self-assembled air-stable magnesium hydride embedded in 3-D activated carbon for reversible hydrogen storage. Nanoscale 2017;9:7094-103.
[65] Meng Y, Ju S, Chen W, Chen X, Xia G, Sun D, et al. Design of bifunctional Nb/V interfaces for improving reversible hydrogen storage performance of MgH 2 . Small Structures 2022;3:2200119.
[66] Bramwell PL, Ngene P, de Jongh PE. Carbon supported lithium hydride nanoparticles: impact of preparation conditions on particle size and hydrogen sorption. Int J Hydrogen Energy 2017;42:5188-98.
[67] Milanese C, Jensen TR, Hauback BC, Pistidda C, Dornheim M, Yang H, et al. Complex hydrides for energy storage. Int J Hydrogen Energy 2019;44:7860-74.
[68] Bahou S, Labrim H, Lakhal M, Ez-Zahraouy H. Improving the hydrogen storage properties of lithium hydride ( LiH ) by lithium vacancy defects: ab initio calculations. Solid State Commun 2023;371:115167.
[69] Vajo JJ, Mertens F, Ahn CC, Bowman RC, Fultz B. Altering hydrogen storage properties by hydride destabilization through alloy formation:: LiH and MgH destabilized with Si. J Phys Chem B 2004;108:13977-83.
[70] Abbas MA, Grant DM, Brunelli M, Hansen TC, Walker GS. Reducing the dehydrogenation temperature of lithium hydride through alloying with germanium. Phys Chem Chem Phys 2013;15:12139-46.
[71] Jain A, Kawasako E, Miyaoka H, Ma T, Isobe S, Ichikawa T, et al. Destabilization of LiH by Li insertion into Ge . J Phys Chem C 2013;117:5650-7.
[72] Wang L, Quadir MZ, Aguey-Zinsou KF. Ni coated LiH nanoparticles for reversible hydrogen storage. Int J Hydrogen Energy 2016;41:6376-86.
[73] Pluengphon P, Tsuppayakorn-aek P, Inceesungvorn B, Ahuja R, Bovornratanaraks T. Formation of lightweight ternary polyhydrides and their hydrogen storage mechanism. J Phys Chem C 2021;125:1723-30.
[74] Miyaoka H, Ishida W, Ichikawa T, Kojima Y. Synthesis and characterization of lithium-carbon compounds for hydrogen storage. J Alloys Compd 2011;509: 719-23.
[75] Schneemann A, White JL, Kang S, Jeong S, Wan LF, Cho ES, et al. Nanostructured metal hydrides for hydrogen storage. Chem Rev 2018;118:10775-839.
[76] Beatrice CAG, Moreira BR, de Oliveira AD, Passador FR, Neto GRD, Leiva DR, et al. Development of polymer nanocomposites with sodium alanate for hydrogen storage. Int J Hydrogen Energy 2020;45:5337-46.
[77] Ren Z, Zhang X, Li H-W, Huang Z, Hu J, Gao M, et al. Titanium hydride nanoplates enable
[78] Li YT, Fang F, Fu HL, Qiu JM, Song Y, Li YS, et al. Carbon nanomaterial-assisted morphological tuning for thermodynamic and kinetic destabilization in sodium alanates. J Mater Chem A 2013;1:5238-46.
[79] Hudson MSL, Raghubanshi H, Pukazhselvan D, Srivastava ON. Carbon nanostructures as catalyst for improving the hydrogen storage behavior of sodium aluminum hydride. Int J Hydrogen Energy 2012;37:2750-5.
[80] Li H, Yao Z, Wang X, Zhu Y, Chen Y. Review on hydrogen production from catalytic ammonia borane methanolysis: advances and perspectives. Energy Fuels 2022;36:11745-59.
[81] Tang Z, Li S, Yang Z, Yu X. Ammonia borane nanofibers supported by poly (vinyl pyrrolidone) for dehydrogenation. J Mater Chem 2011;21:14616-21.
[82] Wu H, Cheng Y, Fan Y, Lu X, Li L, Liu B, et al. Metal-catalyzed hydrolysis of ammonia borane: mechanism, catalysts, and challenges. Int J Hydrogen Energy 2020;45:30325-40.
[83] Akbayrak S, Ozkar S. Ammonia borane as hydrogen storage materials. Int J Hydrogen Energy 2018;43:18592-606.
[84] Sakintuna B, Lamari-Darkrim F, Hirscher M. Metal hydride materials for solid hydrogen storage: a review. Int J Hydrogen Energy 2007;32:1121-40.
[85] Che H, Wu Y, Wang X, Liu H, Yan M. Improved hydrogen storage properties of Li-Mg-N-H system by lithium vanadium oxides. J Alloys Compd 2023;931:167603.
[86] Costanzo F, Silvestrelli PL, Ancilotto F. Physisorption, diffusion, and chemisorption pathways of H2 molecule on graphene and on
[87] Nazir H, Muthuswamy N, Louis C, Jose S, Prakash J, Buan ME, et al. Is the H2 economy realizable in the foreseeable future? Part II: H2 storage, transportation, and distribution. Int J Hydrogen Energy 2020;45:20693-708.
[88] Samantaray SS, Putnam ST, Stadie NP. Volumetrics of hydrogen storage by physical adsorption. Inorganics 2021;9:45.
[89] Shindo K, Kondo T, Arakawa M, Sakurai Y. Hydrogen adsorption/desorption properties of mechanically milled activated carbon. J Alloys Compd 2003;359: 267-71.
[90] Rowlandson JL, Edler KJ, Tian M, Ting VP. Toward process-resilient ligninderived activated carbons for hydrogen storage applications. ACS Sustainable Chem Eng 2020;8:2186-95.
[91] Wróbel-Iwaniec I, Díez N, Gryglewicz G. Chitosan-based highly activated carbons for hydrogen storage. Int J Hydrogen Energy 2015;40:5788-96.
[92] Akasaka H, Takahata T, Toda I, Ono H, Ohshio S, Himeno S, et al. Hydrogen storage ability of porous carbon material fabricated from coffee bean wastes. Int J Hydrogen Energy 2011;36:580-5.
[93] Dillon AC, Jones K, Bekkedahl T, Kiang C, Bethune D, Heben M. Storage of hydrogen in single-walled carbon nanotubes. Nature 1997;386:377-9.
[94] Su Rather. Preparation, characterization and hydrogen storage studies of carbon nanotubes and their composites: a review. Int J Hydrogen Energy 2020;45: 4653-72.
[95] Akbarzadeh R, Ghaedi M, Kokhdan SN, Vashaee D. Remarkably improved electrochemical hydrogen storage by multi-walled carbon nanotubes decorated with nanoporous bimetallic
[96] Kadri A, Jia Y, Chen Z, Yao X. Catalytically enhanced hydrogen sorption in MgMgH 2 by coupling vanadium-based catalyst and carbon nanotubes. Materials 2015;8:3491-507.
[97] Liang H, Du X, Li J, Sun L, Song M, Li W. Manipulating active sites on carbon nanotube materials for highly efficient hydrogen storage. Appl Surf Sci 2023;619: 156740.
[98] Zhao D, Wang X, Yue L, He Y, Chen B. Porous metal-organic frameworks for hydrogen storage. Chem Commun 2022;58:11059-78.
[99] Zhou H, Zhang J, Zhang J, Yan XF, Shen XP, Yuan AH. Spillover enhanced hydrogen storage in Pt-doped MOF/graphene oxide composite produced via an impregnation method. Inorg Chem Commun 2015;54:54-6.
[100] Guo JH, Li SJ, Su Y, Chen G. Theoretical study of hydrogen storage by spillover on porous carbon materials. Int J Hydrogen Energy 2020;45:25900-11.
[101] Bambalaza SE, Langmi HW, Mokaya R, Musyoka NM, Khotseng LE. Experimental demonstration of dynamic temperature-dependent behavior of UiO-66 metal-organic framework: compaction of hydroxylated and dehydroxylated forms of UiO-66 for high-pressure hydrogen storage. ACS Appl Mater Interfaces 2020;12:24883-94.
[102] Kaye SS, Dailly A, Yaghi OM, Long JR. Impact of preparation and handling on the hydrogen storage properties of Zn40 (1, 4-benzenedicarboxylate) 3 (MOF-5). J Am Chem Soc 2007;129:14176-7.
[103] Zunkovič E, Mazaj M, Mali G, Rangus M, Devic T, Serre C, et al. Structural study of Ni-or Mg-based complexes incorporated within UiO-66-NH2 framework and their impact on hydrogen sorption properties. J Solid State Chem 2015;225: 209-15.
[104] Orcajo G, Montes-Andrés H, Villajos JA, Martos C, Botas JA, Calleja G. Li-Crown ether complex inclusion in MOF materials for enhanced H2 volumetric storage capacity at room temperature. Int J Hydrogen Energy 2019;44:19285-93.
[105] Chafiq M, Chaouiki A, Ko YG. Advances in COFs for energy storage devices: harnessing the potential of covalent organic framework materials. Energy Storage Mater 2023;63:103014.
[106] Cote AP, Benin AI, Ockwig NW, O’Keeffe M, Matzger AJ, Yaghi OM. Porous, crystalline, covalent organic frameworks. Science 2005;310:1166-70.
[107] El-Kaderi HM, Hunt JR, Mendoza-Cortes JL, Cote AP, Taylor RE, O’Keeffe M, et al. Designed synthesis of 3D covalent organic frameworks. Science 2007;316: 268-72.
[108] Han SS, Furukawa H, Yaghi OM, Goddard WA. Covalent organic frameworks as exceptional hydrogen storage materials. J Am Chem Soc 2008;130:11580-+.
[109] Furukawa H, Yaghi OM. Storage of hydrogen, methane, and carbon dioxide in highly porous covalent organic frameworks for clean energy applications. J Am Chem Soc 2009;131:8875-83.
[110] Ghosh S, Singh JK. Hydrogen adsorption in pyridine bridged porphyrin-covalent organic framework. Int J Hydrogen Energy 2019;44:1782-96.
[111] Zhao H, Guan YR, Guo HL, Du RJ, Yan CX. Hydrogen storage capacity on Lidecorated covalent organic framework-1: a first-principles study. Mater Res Express 2020;7:035506.
[112] Shangguan W, Zhao H, Dai JQ, Cai J, Yan C. First-principles study of hydrogen storage of Sc-modified semiconductor covalent organic framework-1. ACS Omega 2021;6:21985-93.
[113] Choi YJ, Lee JW, Choi JH, Kang JK. Ideal metal-decorated three dimensional covalent organic frameworks for reversible hydrogen storage. Appl Phys Lett 2008;92.
[114] Kalidindi SB, Oh H, Hirscher M, Esken D, Wiktor C, Turner S, et al. Metal@COFs: covalent organic frameworks as templates for Pd nanoparticles and hydrogen storage properties of Pd@COF-102 hybrid material. Chemistry 2012;18: 10848-56.
[115] Chen ZJ, Kirlikovali KO, Idrees KB, Wasson MC, Farha OK. Porous materials for hydrogen storage. Chem-Us 2022;8:693-716.
[116] Peng YY, Yu JS, Liu XH, Meng TM, Zhao XH, Li LA. Enhanced pore structure regulation in porous aromatic framework materials via montmorillonite templating. Colloid Surface 2023;678:132468.
[117] Ben T, Ren H, Ma S, Cao D, Lan J, Jing X, et al. Targeted synthesis of a porous aromatic framework with high stability and exceptionally high surface area. Angew Chem Int Ed Engl 2009;48:9457-60.
[118] Yuan D, Lu W, Zhao D, Zhou HC. Highly stable porous polymer networks with exceptionally high gas-uptake capacities. Adv Mater 2011;23:3723-5.
[119] Hayat A, Sohail M, El Jery A, Al-Zaydi KM, Raza S, Ali H, et al. Recent advances in ground-breaking conjugated microporous polymers-based materials, their synthesis, modification and potential applications. Mater Today 2023;64: 180-208.
[120] Qiao S, Du Z, Yang R. Design and synthesis of novel carbazole-spacer-carbazole type conjugated microporous networks for gas storage and separation. J Mater Chem A 2014;2:1877-85.
[121] Zeng W, Zhang Y, Zhao XB, Qin ML, Li XY, Jin WS, et al. One-pot synthesis of conjugated microporous polymers based on extended molecular graphenes for hydrogen storage. Polymer 2019;174:96-100.
[122] Tsyurupa MP, Davankov VA. Porous structure of hypercrosslinked polystyrene: state-of-the-art mini-review. React Funct Polym 2006;66:768-79.
[123] Raza S, Nazeer S, Abid A, Kanwal A. Recent research progress in the synthesis, characterization and applications of hyper cross-linked polymer. J Polym Res 2023;30:415.
[124] Lee JY, Wood CD, Bradshaw D, Rosseinsky MJ, Cooper AI. Hydrogen adsorption in microporous hypercrosslinked polymers. Chem Commun 2006:2670-2.
[125] Wood CD, Tan B, Trewin A, Niu HJ, Bradshaw D, Rosseinsky MJ, et al. Hydrogen storage in microporous hypercrosslinked organic polymer networks. Chem Mater 2007;19:2034-48.
[126] Tian M, Rochatz S, Fawcett H, Burrows AD, Bowen CR, Mays TJ. Chemical modification of the polymer of intrinsic microporosity PIM-1 for enhanced hydrogen storage. Adsorption 2020;26:1083-91.
[127] Rochat S, Polak-Krasna K, Tian M, Holyfield LT, Mays TJ, Bowen CR, et al. Hydrogen storage in polymer-based processable microporous composites. J Mater Chem A 2017;5:18752-61.
[128] Tian M, Rochat S, Polak-Krasna K, Holyfield LT, Burrows AD, Bowen CR, et al. Nanoporous polymer-based composites for enhanced hydrogen storage. Adsorption 2019;25:889-901.
[129] Budd PM, Elabas ES, Ghanem BS, Makhseed S, McKeown NB, Msayib KJ, et al. Solution-processed, organophilic membrane derived from a polymer of intrinsic microporosity. Adv Mater 2004;16:456-9.
[130] Rochat S, Polak-Krasna K, Tian M, Mays TJ, Bowen CR, Burrows AD. Assessment of the long-term stability of the polymer of intrinsic microporosity PIM-1 for hydrogen storage applications. Int J Hydrogen Energy 2019;44:332-7.
[131] Bhattacharyya R, Mohan S. Solid state storage of hydrogen and its isotopes: an engineering overview. Renew Sustain Energy Rev 2015;41:872-83.
[132] Li J, Gao ZR, Lin Q-F, Liu C, Gao F, Lin C, et al. A 3D extra-large-pore zeolite enabled by 1D-to-3D topotactic condensation of a chain silicate. Science 2023; 379:283-7.
[133] Li M, Yin W, Pan J, Zhu Y, Sun N, Zhang X, et al. Hydrogen spillover as a promising strategy for boosting heterogeneous catalysis and hydrogen storage. Chem Eng J 2023;471:144691.
[134] Allendorf MD, Hulvey Z, Gennett T, Ahmed A, Autrey T, Camp J, et al. An assessment of strategies for the development of solid-state adsorbents for vehicular hydrogen storage. Energy Environ Sci 2018;11:2784-812.
[135] Langmi HW, Book D, Walton A, Johnson SR, Al-Mamouri MM, Speight JD, et al. Hydrogen storage in ion-exchanged zeolites. J Alloys Compd 2005;404-406: 637-42.
[136] Chung K-H. High-pressure hydrogen storage on microporous zeolites with varying pore properties. Energy 2010;35:2235-41.
[137] Fatriansyah JF, Dhaneswara D, Suhariadi I, Widyantoro MI, Ramadhan BA, Rahmatullah MZ, et al. Simple molecular dynamics simulation of hydrogen adsorption on ZSM 5, graphite nanofiber, graphene oxide framework, and reduced graphene oxide. Heliyon 2021;7:08528.
[138] Zhang Y, Bhattacharjee G, Kumar R, Linga P. Solidified hydrogen storage (SolidHyStore) via clathrate hydrates. Chem Eng J 2022;431:133702.
[139] Gupta A, Baron GV, Perreault P, Lenaerts S, Ciocarlan R-G, Cool P, et al. Hydrogen clathrates: next generation hydrogen storage materials. Energy Storage Mater 2021;41:69-107.
[140] Veluswamy HP, Kumar R, Linga P. Hydrogen storage in clathrate hydrates: current state of the art and future directions. Appl Energy 2014;122:112-32.
[141] Ogata K, Hashimoto S, Sugahara T, Moritoki M, Sato H, Ohgaki K. Storage capacity of hydrogen in tetrahydrofuran hydrate. Chem Eng Sci 2008;63:5714-8.
[142] Matsumoto Y, Grim RG, Khan NM, Sugahara T, Ohgaki K, Sloan ED, et al. Investigating the thermodynamic stabilities of hydrogen and methane binary gas hydrates. J Phys Chem C 2014;118:3783-8.
[143] Park J, Lee H. Spectroscopic evidences of the double hydrogen hydrates stabilized with ethane and propane. Kor J Chem Eng 2007;24:624-7.
[144] Belosludov R, Zhdanov RK, Subbotin O, Mizuseki H, Souissi M, Kawazoe Y, et al. Theoretical modelling of the phase diagrams of clathrate hydrates for hydrogen storage applications. Mol Simulat 2012;38:773-80.
[145] Florusse LJ, Peters CJ, Schoonman J, Hester KC, Koh CA, Dec SF, et al. Stable lowpressure hydrogen clusters stored in a binary clathrate hydrate. Science 2004; 306:469-71.
[146] Lee H, Lee J-w, Kim DY, Park J, Seo Y-T, Zeng H, et al. Tuning clathrate hydrates for hydrogen storage. Nature 2005;434:743-6.
[147] Sugahara T, Haag JC, Prasad PS, Warntjes AA, Sloan ED, Sum AK, et al. Increasing hydrogen storage capacity using tetrahydrofuran. J Am Chem Soc 2009;131: 14616-7.
[148] Liu J, Hou J, Xu J, Liu H, Chen G, Zhang J. Ab initio study of the molecular hydrogen occupancy in pure H2 and binary H2-THF clathrate hydrates. Int J Hydrogen Energy 2017;42:17136-43.
[149] Belosludov R, Zhdanov R, Subbotin O, Mizuseki H, Kawazoe Y, Belosludov V. Theoretical study of hydrogen storage in binary hydrogen-methane clathrate hydrates. J Renew Sustain Energy 2014;6.
[150] Liu S, Zhang W, Wu H, Wang J, Yuan Y, Wang S, et al. Molecular hydrogen storage in binary H2-CH4 clathrate hydrates. J Mol Liq 2023;376:121496.
[151] Liu J, Hou J, Xu J, Liu H, Li S, Chen G, et al. Theoretical investigation of exchange of N2 and H2 in sII clathrate hydrates. Chem Phys Lett 2016;660:266-71.
[152] Chen S, Wang Y, Lang X, Fan S, Li G. Rapid and high hydrogen storage in epoxycyclopentane hydrate at moderate pressure. Energy 2023;268:126638.
[153] Papadimitriou N, Tsimpanogiannis I, Peters C, Papaioannou AT, Stubos A. Hydrogen storage in sH hydrates: a Monte Carlo study. J Phys Chem B 2008;112: 14206-11.
[154] Deschamps J, Dalmazzone D. Hydrogen storage in semiclathrate hydrates of tetrabutyl ammonium chloride and tetrabutyl phosphonium bromide. J Chem Eng Data 2010;55:3395-9.
[155] Strobel TA, Koh CA, Sloan ED. Hydrogen storage properties of clathrate hydrate materials. Fluid Phase Equil 2007;261:382-9.
[156] Li L, Tang S, Wang C, Lv X, Jiang M, Wu H, et al. High gas storage capacities and stepwise adsorption in a UiO type metal-organic framework incorporating Lewis basic bipyridyl sites. Chem Commun 2014;50:2304-7.
[157] Ma Z, Zou J, Khan D, Zhu W, Hu C, Zeng X, et al. Preparation and hydrogen storage properties of MgH 2 -trimesic acid-TM MOF (
[158] Lu Z, He J, Song M, Zhang Y, Wu F, Zheng J, et al. Bullet-like vanadium-based MOFs as a highly active catalyst for promoting the hydrogen storage property in MgH 2 . Int J Miner Metall Mater 2023;30:44-53.
[159] Muhammed NS, Haq B, Al Shehri D, Al-Ahmed A, Rahman MM, Zaman E. A review on underground hydrogen storage: insight into geological sites, influencing factors and future outlook. Energy Rep 2022;8:461-99.
[160] Tarkowski R, Uliasz-Misiak B. Towards underground hydrogen storage: a review of barriers. Renew Sustain Energy Rev 2022;162:112451.
[161] Tarkowski R. Underground hydrogen storage: characteristics and prospects. Renew Sustain Energy Rev 2019;105:86-94.
[162] Bünger U, Michalski J, Crotogino F, Kruck O. Large-scale underground storage of hydrogen for the grid integration of renewable energy and other applications. Compendium of Hydrogen Energy: Elsevier 2016:133-63.
[163] Lord AS, Kobos PH, Borns DJ. Geologic storage of hydrogen: scaling up to meet city transportation demands. Int J Hydrogen Energy 2014;39:15570-82.
[164] Raad SMJ, Leonenko Y, Hassanzadeh H. Hydrogen storage in saline aquifers: opportunities and challenges. Renew Sustain Energy Rev 2022;168:112846.
[165] Osman AI, Mehta N, Elgarahy AM, Hefny M, Al-Hinai A, Al-Muhtaseb AaH, et al. Hydrogen production, storage, utilisation and environmental impacts: a review. Environ Chem Lett 2022:1-36.
[166] Lemieux A, Sharp K, Shkarupin A. Preliminary assessment of underground hydrogen storage sites in Ontario, Canada. Int J Hydrogen Energy 2019;44: 15193-204.
[167] Michalski J, Bünger U, Crotogino F, Donadei S, Schneider GS, Pregger T, et al. Hydrogen generation by electrolysis and storage in salt caverns: potentials, economics and systems aspects with regard to the German energy transition. Int J Hydrogen Energy 2017;42:13427-43.
[168] Crotogino F, Donadei S, Bünger U, Landinger H. Large-scale hydrogen underground storage for securing future energy supplies. In: 18th World hydrogen energy conference; 2010. p. 37-45.
[169] Sainz-Garcia A, Abarca E, Rubi V, Grandia F. Assessment of feasible strategies for seasonal underground hydrogen storage in a saline aquifer. Int J Hydrogen Energy 2017;42:16657-66.
[170] Zivar D, Kumar S, Foroozesh J. Underground hydrogen storage: a comprehensive review. Int J Hydrogen Energy 2021;46:23436-62.
[171] Tarkowski R, Czapowski G. Salt domes in Poland – potential sites for hydrogen storage in caverns. Int J Hydrogen Energy 2018;43:21414-27.
[172] Lord AS. Overview of geologic storage of natural gas with an emphasis on assessing the feasibility of storing hydrogen. Albuquerque, NM, and Livermore, CA: Sandia National Laboratories (SNL); 2009.
[173] Tackie-Otoo BN, Haq MB. A comprehensive review on geo-storage of H2 in salt caverns: prospect and research advances. Fuel 2024;356:129609.
[174] Raad SMJ, Leonenko Y, Hassanzadeh H. Hydrogen storage in saline aquifers: opportunities and challenges. Renew Sustain Energy Rev 2022;168:112846.
[175] Cyran K. Insight into a shape of salt storage caverns. Arch Min Sci 2020;65: 363-98.
[176] Landinger H, Crotogino F. The role of large-scale hydrogen storage for future renewable energy utilisation. In: Second international renewable energy storage conference (IRES II); 2007.
[177] Caglayan DG, Weber N, Heinrichs HU, Linssen J, Robinius M, Kukla PA, et al. Technical potential of salt caverns for hydrogen storage in Europe. Int J Hydrogen Energy 2020;45:6793-805.
[178] Dias W, Roehl D, Mejia C, Sotomayor P. Cavern integrity for underground hydrogen storage in the Brazilian pre-salt fields. Int J Hydrogen Energy 2023;48: 26853-69.
[179] Liu W, Zhang ZX, Chen J, Jiang DY, Wei F, Fan JY, et al. Feasibility evaluation of large-scale underground hydrogen storage in bedded salt rocks of China: a case study in Jiangsu province. Energy 2020;198:117348.
[180] Panfilov M, Gravier G, Fillacier S. Underground storage of H2 and H2-CO2-CH4 mixtures. In: ECMOR X-10th European conference on the mathematics of oil recovery. European Association of Geoscientists & Engineers; 2006. 23-00003.
[181] Kruck O, Crotogino F, Prelicz R, Tjkutg Rudolph. Assessment of the potential, the actors and relevant business cases for large scale and seasonal storage of renewable electricity by hydrogen underground storage in Europe. 2013. p. 1-32.
[182] Navaid HB, Emadi H, Watson M. A comprehensive literature review on the challenges associated with underground hydrogen storage. Int J Hydrogen Energy 2023;48:10603-35.
[183] Bo ZK, Zeng LP, Chen YQ, Xie Q. Geochemical reactions-induced hydrogen loss during underground hydrogen storage in sandstone reservoirs. Int J Hydrogen Energy 2021;46:19998-20009.
[184] Perera MSA. A review of underground hydrogen storage in depleted gas reservoirs: insights into various rock-fluid interaction mechanisms and their impact on the process integrity. Fuel 2023;334:126677.
[185] Moser A. Bioprocess technology: kinetics and reactors. Springer Science & Business Media; 2012.
[186] Al-Yaseri A, Esteban L, Giwelli A, Sarout J, Lebedev M, Sarmadivaleh M. Initial and residual trapping of hydrogen and nitrogen in Fontainebleau sandstone using nuclear magnetic resonance core flooding. Int J Hydrogen Energy 2022;47: 22482-94.
[187] Sekar LK, Kiran R, Okoroafor ER, Wood DA. Review of reservoir challenges associated with subsurface hydrogen storage and recovery in depleted oil and gas reservoirs. J Energy Storage 2023;72:108605.
[188] Matos CR, Carneiro JF, Silva PP. Overview of large-scale underground energy storage technologies for integration of renewable energies and criteria for reservoir identification. J Energy Storage 2019;21:241-58.
[189] Lai Q, Sun Y, Wang T, Modi P, Cazorla C, Demirci UB, et al. How to design hydrogen storage materials? Fundamentals, synthesis, and storage tanks. Advanced Sustainable Systems 2019;3:1900043.
[190] Zhang F, Zhao PC, Niu M, Maddy J. The survey of key technologies in hydrogen energy storage. Int J Hydrogen Energy 2016;41:14535-52.
[191] Ramimoghadam D, Gray EM, Webb CJ. Review of polymers of intrinsic microporosity for hydrogen storage applications. Int J Hydrogen Energy 2016;41: 16944-65.
[192] Kaur M, Pal K. Review on hydrogen storage materials and methods from an electrochemical viewpoint. J Energy Storage 2019;23:234-49.
[193] Blackman JM, Patrick JW, Arenillas A, Shi W, Snape CE. Activation of carbon nanofibres for hydrogen storage. Carbon 2006;44:1376-85.
[194] Aceves SM, Berry GD, Martinez-Frias J, Espinosa-Loza F. Vehicular storage of hydrogen in insulated pressure vessels. Int J Hydrogen Energy 2006;31:2274-83.
[195] Wang Y, Adroher XC, Chen JX, Yang XG, Miller T. Three-dimensional modeling of hydrogen sorption in metal hydride hydrogen storage beds. J Power Sources 2009;194:997-1006.
[196] Klopčič N, Grimmer I, Winkler F, Sartory M, Trattner A. A review on metal hydride materials for hydrogen storage. J Energy Storage 2023;72:108456.
[197] Lototskyy MV, Yartys VA, Pollet BG, Bowman RC. Metal hydride hydrogen compressors: a review. Int J Hydrogen Energy 2014;39:5818-51.
[198] Tarasov BP, Fursikov PV, Volodin AA, Bocharnikov MS, Shimkus YY, Kashin AM, et al. Metal hydride hydrogen storage and compression systems for energy storage technologies. Int J Hydrogen Energy 2021;46:13647-57.
[199] Modi P, Aguey-Zinsou KF. Room temperature metal hydrides for stationary and heat storage applications: a review. Front Energy Res 2021;9:616115.
[200] Salman MS, Lai QW, Luo XX, Pratthana C, Rambhujun N, Costalin M, et al. The power of multifunctional metal hydrides: a key enabler beyond hydrogen storage. J Alloys Compd 2022;920:165936.
[201] He T, Cao H, Chen P. Complex hydrides for energy storage, conversion, and utilization. Adv Mater 2019;31:e1902757.
[202] Orimo S, Nakamori Y, Eliseo JR, Zuttel A, Jensen CM. Complex hydrides for hydrogen storage. Chem Rev 2007;107:4111-32.
[203] de Jongh PE, Adelhelm P. Nanosizing and nanoconfinement: new strategies towards meeting hydrogen storage goals. ChemSusChem 2010;3:1332-48.
[204] Pang Y, Liu Y, Gao M, Ouyang L, Liu J, Wang H, et al. A mechanical-force-driven physical vapour deposition approach to fabricating complex hydride nanostructures. Nat Commun 2014;5:3519.
[205] Balde CP, Hereijgers BP, Bitter JH, de Jong KP. Sodium alanate nanoparticleslinking size to hydrogen storage properties. J Am Chem Soc 2008;130:6761-5.
[206] Pratthana C, Yang YW, Rawal A, Aguey-Zinsou KF. Nanoconfinement of lithium alanate for hydrogen storage. J Alloys Compd 2022;926:166834.
[207] Gutowska A, Li L, Shin Y, Wang CM, Li XS, Linehan JC, et al. Nanoscaffold mediates hydrogen release and the reactivity of ammonia borane. Angew Chem Int Ed Engl 2005;44:3578-82.
[208] Wahab MA, Zhao HJ, Yao XD. Nano-confined ammonia borane for chemical hydrogen storage. Front Chem Sci Eng 2012;6:27-33.
[209] Valero-Pedraza MJ, Cot D, Petit E, Aguey-Zinsou KF, Alauzun JG, Demirci UB. Ammonia borane nanospheres for hydrogen storage. ACS Appl Nano Mater 2019; 2:1129-38.
[210] Lai QW, Pratthana C, Yang YW, Rawal A, Aguey-Zinsou KF. Nanoconfinement of complex borohydrides for hydrogen storage. ACS Appl Nano Mater 2021;4:973-8.
[211] Ngene P, Adelhelm P, Beale AM, de Jong KP, de Jongh PE. LiBH/SBA-15 nanocomposites prepared by melt infiltration under hydrogen pressure: synthesis and hydrogen sorption properties. J Phys Chem C 2010;114:6163-8.
[212] Le TT, Pistidda C, Nguyen VH, Singh P, Raizada P, Klassen T, et al.
Nanoconfinement effects on hydrogen storage properties of MgH and LiBH . Int J Hydrogen Energy 2021;46:23723-36.
[213] Zhang QY, Huang YK, Ma TC, Li K, Ye F, Wang XC, et al. Facile synthesis of small MgH nanoparticles confined in different carbon materials for hydrogen storage. J Alloys Compd 2020;825:153953.
[214] Ma Z, Zhang Q, Panda S, Zhu W, Sun F, Khan D, et al. In situ catalyzed and nanoconfined magnesium hydride nanocrystals in a Ni-MOF scaffold for hydrogen storage. Sustain Energy Fuels 2020;4:4694-703.
[215] Pohlmann C, Röntzsch L, Hu J, Weißgärber T, Kieback B, Fichtner M. Tailored heat transfer characteristics of pelletized
[216] Milanese C, Garroni S, Gennari F, Marini A, Klassen T, Dornheim M, et al. Solid state hydrogen storage in alanates and alanate-based compounds: a review. Metals 2018;8:567.
[217] Gao Q, Xia G, Yu X. Confined NaAlH(4) nanoparticles inside CeO(2) hollow nanotubes towards enhanced hydrogen storage. Nanoscale 2017;9:14612-9.
[218] Broom DP, Webb CJ, Fanourgakis GS, Froudakis GE, Trikalitis PN, Hirscher M. Concepts for improving hydrogen storage in nanoporous materials. Int J Hydrogen Energy 2019;44:7768-79.
[219] Shet SP, Priya SS, Sudhakar K, Tahir M. A review on current trends in potential use of metal-organic framework for hydrogen storage. Int J Hydrogen Energy 2021;46:11782-803.
[220] Yuan S, Zou L, Qin JS, Li J, Huang L, Feng L, et al. Construction of hierarchically porous metal-organic frameworks through linker labilization. Nat Commun 2017; 8:15356.
[221] Ahmed A, Seth S, Purewal J, Wong-Foy AG, Veenstra M, Matzger AJ, et al. Exceptional hydrogen storage achieved by screening nearly half a million metalorganic frameworks. Nat Commun 2019;10:1568.
[222] Yang SL, Karve VV, Justin A, Kochetygov I, Espín J, Asgari M, et al. Enhancing MOF performance through the introduction of polymer guests. Coord Chem Rev 2021;427:213525.
[223] Zhang X, Zhang S, Tang Y, Huang X, Pang H. Recent advances and challenges of metal-organic framework/graphene-based composites. Compos B Eng 2022;230: 109532.
[224] Kang PC, Ou YS, Li GL, Chang JK, Wang CY. Room-temperature hydrogen adsorption via spillover in Pt nanoparticle-decorated UiO-66 nanoparticles: implications for hydrogen storage. ACS Appl Nano Mater 2021;4:11269-80.
[225] Chen L, Zhang X, Cheng X, Xie Z, Kuang Q, Zheng L. The function of metalorganic frameworks in the application of MOF-based composites. Nanoscale Adv 2020;2:2628-47.
[226] Ren J, Huang Y, Zhu H, Zhang B, Zhu H, Shen S, et al. Recent progress on MOFderived carbon materials for energy storage. Carbon Energy 2020;2:176-202.
[227] Li X, Yang X, Xue H, Pang H, Xu Q. Metal-organic frameworks as a platform for clean energy applications. Inside Energy 2020;2:100027.
[228] Niu S, Wang Z, Zhou T, Yu M, Yu M, Qiu J. A polymetallic metal-organic framework-derived strategy toward synergistically multidoped metal oxide electrodes with ultralong cycle life and high volumetric capacity. Adv Funct Mater 2016;27:1605332.
[229] Shi W, Ye C, Xu X, Liu X, Ding M, Liu W, et al. High-performance membrane capacitive deionization based on metal-organic framework-derived hierarchical carbon structures. ACS Omega 2018;3:8506-13.
[230] Bao W, Yu J, Chen F, Du H, Zhang W, Yan S, et al. Controllability construction and structural regulation of metal-organic frameworks for hydrogen storage at ambient condition: a review. Int J Hydrogen Energy 2023.
[231] Chavan S, Vitillo JG, Gianolio D, Zavorotynska O, Civalleri B, Jakobsen S, et al. H 2 storage in isostructural UiO-67 and UiO-66 MOFs. Phys Chem Chem Phys 2012;14:1614-26.
[232] Xia YD, Yang ZX, Zhu YQ. Porous carbon-based materials for hydrogen storage: advancement and challenges. J Mater Chem A 2013;1:9365-81.
[233] Xu H, Shang H, Wang C, Du Y. Low-dimensional metallic nanomaterials for advanced electrocatalysis. Adv Funct Mater 2020;30:2006317.
[234] Van der Ven A, Deng Z, Banerjee S, Ong SP. Rechargeable alkali-ion battery materials: theory and computation. Chem Rev 2020;120:6977-7019.
[235] Wang ZF, Jin KH, Liu F. Computational design of two-dimensional topological materials. WIREs Computational Molecular Science 2017;7:e1304.
[236] Mroz AM, Posligua V, Tarzia A, Wolpert EH, Jelfs KE. Into the unknown: how computation can help explore uncharted material space. J Am Chem Soc 2022; 144:18730-43.
[237] Miracle DB, Li M, Zhang ZH, Mishra R, Flores KM. Emerging capabilities for the high-throughput characterization of structural materials. Annu Rev Mater Res 2021;51(2021):131-64. 51.
[238] Xu D, Zhang Q, Huo X, Wang Y, Yang M. Advances in data-assisted highthroughput computations for material design. Materials Genome Engineering Advances 2023;1:e11.
[239] Cazorla C. The role of density functional theory methods in the prediction of nanostructured gas-adsorbent materials. Coord Chem Rev 2015;300:142-63.
[240] Altintas C, Keskin S. On the shoulders of high-throughput computational screening and machine learning: design and discovery of MOFs for H2 storage and purification. Mater Today Energy 2023;38:101426.
[241] Ren E, Guilbaud P, Coudert FX. High-throughput computational screening of nanoporous materials in targeted applications. Digital Discovery 2022;1:355-74.
[242] Colón YJ, Fairen-Jimenez D, Wilmer CE, Snurr RQ. High-throughput screening of porous crystalline materials for hydrogen storage capacity near room temperature. J Phys Chem C 2014;118:5383-9.
[243] Ahmed A, Liu YY, Purewal J, Tran LD, Wong-Foy AG, Veenstra M, et al. Balancing gravimetric and volumetric hydrogen density in MOFs. Energy Environ Sci 2017; 10:2459-71.
[244] Deniz CU, Mert H, Baykasoglu C. Li-doped fullerene pillared graphene nanocomposites for enhancing hydrogen storage: a computational study. Comput Mater Sci 2021;186:110023.
[245] Bian LY, Li XD, Huang XY, Yang PH, Wang YD, Liu XY, et al. Molecular simulation on hydrogen storage properties of five novel covalent organic frameworks with the higher valency. Int J Hydrogen Energy 2022;47:29390-8.
[246] Li QY, Qiu SY, Wu CZ, Lau T, Sun CH, Jia BH. Computational investigation of
[247] Rana S, Monder DS, Chatterjee A. Thermodynamic calculations using reverse Monte Carlo: a computational workflow for accelerated construction of phase diagrams for metal hydrides. Comput Mater Sci 2024;233:112727.
[248] Jing ZJ, Yuan QQ, Yu Y, Kong XT, Tan KC, Wang JT, et al. Developing ideal metalorganic hydrides for hydrogen storage: from theoretical prediction to rational fabrication. ACS Mater Lett 2021;3:1417-25.
[249] Liu H, Xu C, Liang J. Dependency distance: a new perspective on syntactic patterns in natural languages. Phys Life Rev 2017;21:171-93.
[250] Montejo-Ráez A, Jiménez-Zafra SM. Current approaches and applications in natural language processing. Appl Sci 2022;12:4859.
[251] Mahadevkar SV, Khemani B, Patil S, Kotecha K, Vora DR, Abraham A, et al. A review on machine learning styles in computer vision-techniques and future directions. IEEE Access 2022;10:107293-329.
[252] Shetty SK, Siddiqa A. Deep learning algorithms and applications in computer vision. Int J Comput Sci Eng 2019;7:195-201.
[253] Jovel J, Greiner R. An introduction to machine learning approaches for biomedical research. Front Med 2021;8:771607.
[254] Athanasopoulou K, Daneva GN, Adamopoulos PG, Scorilas A. Artificial intelligence: the milestone in modern biomedical research. BioMedInformatics 2022;2:727-44.
[255] Cebollada S, Paya L, Flores M, Peidró A, Reinoso O. A state-of-the-art review on mobile robotics tasks using artificial intelligence and visual data. Expert Syst Appl 2021;167:114195.
[256] Cully A, Clune J, Tarapore D, Mouret JB. Robots that can adapt like animals. Nature 2015;521. 503-U476.
[257] Tsai C-W, Lai C-F, Chiang M-C, Yang LT. Data mining for internet of things: a survey. IEEE Communications Surveys & Tutorials 2014;16:77-97.
[258] Wang T, Pan R, Martins ML, Cui J, Huang Z, Thapaliya BP, et al. Machine-learning-assisted material discovery of oxygen-rich highly porous carbon active materials for aqueous supercapacitors. Nat Commun 2023;14:4607.
[259] Borboudakis G, Stergiannakos T, Frysali M, Klontzas E, Tsamardinos I, Froudakis GE. Chemically intuited, large-scale screening of MOFs by machine learning techniques. npj Comput Mater 2017;3:40.
[260] Lv C, Zhou X, Zhong L, Yan C, Srinivasan M, Seh ZW, et al. Machine learning: an advanced platform for materials development and state prediction in lithium-ion batteries. Adv Mater 2022;34:e2101474.
[261] Kim J, Kang D, Kim S, Jang HW. Catalyze materials science with machine learning. ACS Mater Lett 2021;3:1151-71.
[262] Shi Z, Yang W, Deng X, Cai C, Yan Y, Liang H, et al. Machine-learning-assisted high-throughput computational screening of high performance metal-organic frameworks. Molecular Systems Design & Engineering 2020;5:725-42.
[263] Jäger MO, Morooka EV, Federici Canova F, Himanen L, Foster AS. Machine learning hydrogen adsorption on nanoclusters through structural descriptors, 4; 2018. p. 37.
[264] Hattrick-Simpers JR, Choudhary K, Corgnale C. A simple constrained machine learning model for predicting high-pressure-hydrogen-compressor materials. Molecular Systems Design & Engineering 2018;3:509-17.
[265] Nations S, Nandi T, Ramazani A, Wang SN, Duan YH. Metal hydride compositionderived parameters as machine learning features for material design and H2 storage. J Energy Storage 2023;70:107980.
[266] Kim JM, Ha T, Lee J, Lee Y-S, Shim J-H. Prediction of pressure-compositiontemperature curves of AB2-type hydrogen storage alloys by machine learning. Met Mater Int 2022;29:861-9.
[267] Thanh HV, Taremsari SE, Ranjbar B, Mashhadimoslem H, Rahimi E, Rahimi M, et al. Hydrogen storage on porous carbon adsorbents: rediscovery by naturederived algorithms in random forest machine learning model. Energies 2023;16: 2348.
[268] Shekhar S, Chowdhury C. Prediction of hydrogen storage in metal-organic frameworks: a neural network based approach. Results in Surfaces and Interfaces 2024;14:100166.
[269] Ahmed A, Siegel DJ. Predicting hydrogen storage in MOFs via machine learning. Patterns (N Y) 2021;2:100291.
[270] Salehi K, Rahmani M, Atashrouz S. Machine learning assisted predictions for hydrogen storage in metal-organic frameworks. Int J Hydrogen Energy 2023;48: 33260-75.
[271] Borja NK, Fabros CJE, Doma BT. Prediction of hydrogen adsorption and moduli of metal-organic frameworks (MOFs) using machine learning strategies. Energies 2024;17:927.
- Corresponding author. School of Chemistry and Chemical Engineering, Queen’s University Belfast, David Keir Building, Stranmillis Road, Belfast, BT9 5AG, Northern Ireland, United Kingdom.
E-mail address: aosmanahmed01@qub.ac.uk (A.I. Osman).
