DOI: https://doi.org/10.1038/s41538-023-00245-8
PMID: https://pubmed.ncbi.nlm.nih.gov/38172179
تاريخ النشر: 2024-01-03
الجوانب الإيجابية والسلبية للبكتريوفاجات ودورها الهائل في سلسلة الغذاء
الملخص
تقوم البكتريوفاجات بالعدوى والتكاثر داخل مضيف بكتيري، كما أنها تعمل كعوامل تحكم حيوية طبيعية. كانت الفاجات تُعتبر في السابق إزعاجات تسبب فشل التخمير في صناعة الجبن وغيرها من العمليات الصناعية، مما يؤدي إلى خسائر اقتصادية، ولكن يتم الآن ملاحظة الفاجات بشكل متزايد كعوامل مضادة للميكروبات واعدة يمكن أن تحارب البكتيريا المسببة للتلف والمرض. الوجبات الخالية من مسببات الأمراض التي تلبي متطلبات الصناعة دون إضافات صناعية دائمًا ما تكون مطلوبة في قطاع الغذاء. تقدم هذه الدراسة للقراء تاريخ ومصادر وبيولوجيا البكتريوفاجات، والتي تشمل نطاقات مضيفيها، وآليات الامتصاص، والملفات الانحلالية، والملفات الاندماجية، وتأثير العوامل الخارجية على نمو الفاجات. ظهرت الفاجات ومشتقاتها كعوامل مضادة للميكروبات، وكاشفات حيوية، ومتحكمات في الأغشية الحيوية، والتي تم مناقشتها بشكل شامل بالإضافة إلى تطبيقاتها المحتملة في الغذاء والجهاز الهضمي، وهي خيار قابل للتطبيق وآمن لمنع أو علاج أو القضاء على الملوثات في مختلف الأطعمة وبيئات معالجة الطعام. علاوة على ذلك، يمكن اعتبار الفاجات والبروتينات الانحلالية المشتقة من الفاجات مضادات ميكروبات محتملة في السياق التقليدي من المزرعة إلى المائدة، والتي تشمل خلطات قائمة على الفاجات ومنتجات فاج متاحة تجاريًا. تختتم هذه الورقة ببعض المخاوف المحتملة المتعلقة بالسلامة التي تحتاج إلى معالجة لتمكين استخدام البكتريوفاجات بشكل فعال.
مقدمة
يجب أن تظل مكون درجة الحرارة للياقة الفاج حاسماً عند النظر في استخدام الفيروسات من أجل إدارة العدوى البكتيرية في الزراعة و/أو البيئة، لأن هذه المنتجات المعتمدة على الفاج قد تكون لها نشاط غير متسق على نفس المرض، وذلك بسبب الاختلافات في الظروف المناخية، مثل درجات الحرارة. يمكن أن تعمل الفاج كحلفاء وأعداء في الأنشطة البشرية، وقد تتطور البكتيريا لمقاومة الفاج من خلال آليات دفاع مختلفة.
تاريخ، مصادر، وبيولوجيا الفاجات
نطاق مضيف الفاجات
آلية الامتصاص
الدورة التحللية
ملف التحلل
الدورة اللسينية
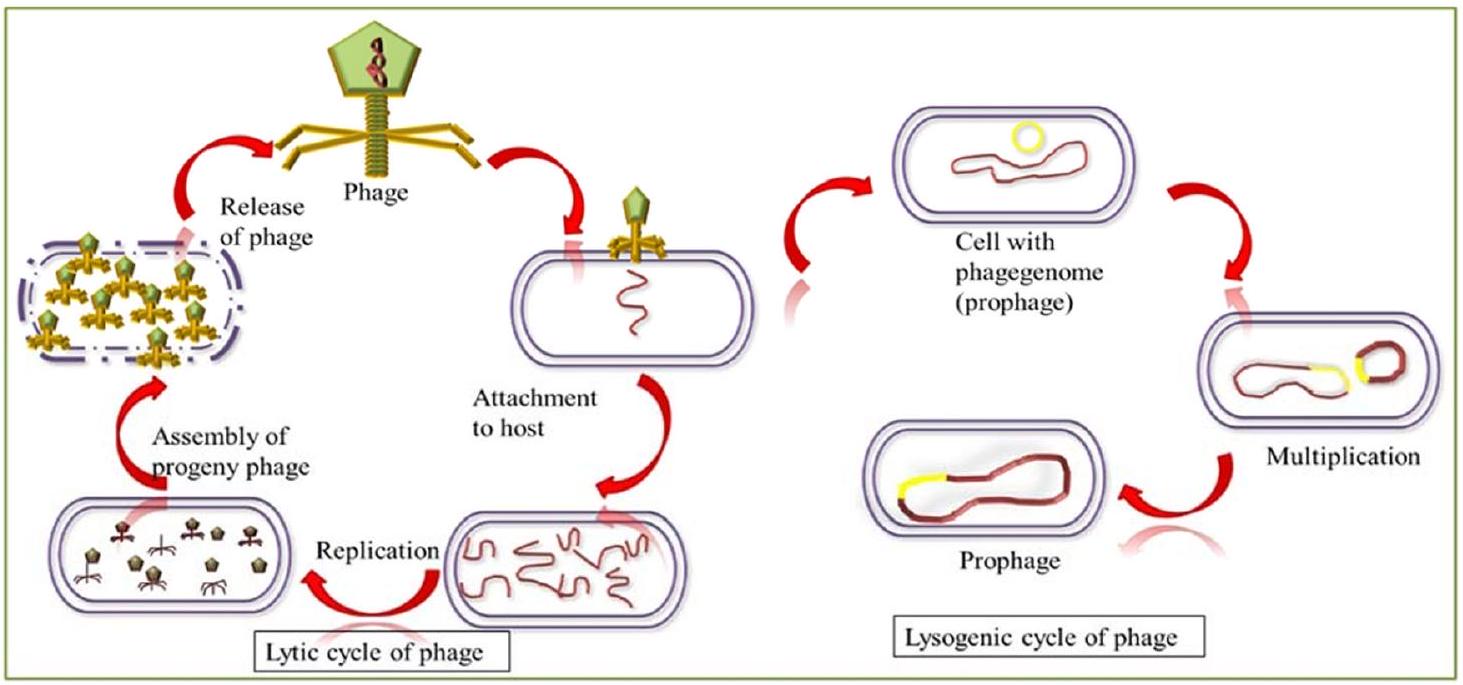
تأثير العوامل الخارجية على الفاجات
درجة الحرارة
تُعطّل الفاجات في الحمأة المجففة والصرف الصحي الخام. تم اكتشاف المقاومة الحرارية للفاجات السوماتية، التي هي فاجات قادرة على إصابة بكتيريا بكتيرويدس فراجيلس، وفاجات RNA الخاصة بـ F. تشير هذه الدراسة إلى أن الفاجات أكثر مقاومة للعلاج الحراري من البكتيريا. المعامل الأكثر أهمية فيما يتعلق بتحديد نشاط الفاجات هو درجة حرارة التخزين. كانت فاجات باكillus cereus CP-51 حساسة لدرجات الحرارة المنخفضة ومستقرة في درجة حرارة الغرفة، على الرغم من أن تخزين الفاجات في درجة حرارة الغرفة غير ممكن. الفاجات ذات الذيل هي الأكثر مقاومة للتخزين ولها أطول عمر. بعض الفاجات، مثل T4 وT5 وT7، كانت قابلة للحياة بعد
درجة حموضة البيئة
كان بالإمكان تغييره عند القيمة الأكبر، ويمكن إعادة توزيع الفاجات عن طريق هزها. وجد الباحثون أن التجلط والترسيب غير القابلين للعكس قد يكونان العامل المحدد لنشاط الفاج. لوحظ أيضًا فقدان طفيف للعدوى بالقرب من pH 7. كان فاج PM2 حساسًا عند pH منخفض، حيث فقد النشاط تمامًا عند pH 5.0. اختفت جزيئات فاج T1 عند pH 3.0، بينما نجت فاج M13 حتى عند pH 2. تظهر هذه التفسيرات أن التغير في pH البيئي قد يحمي نشاط الفاج عند درجة حرارة منخفضة.
الملوحة والأيونات
الجوانب الإيجابية للبكتريوفاجات
عوامل مضادة للميكروبات
وجد ديريل أن الفاجات المدمرة كانت فعالة ضد عصية الديزنتاريا (شيغيلا) في براز المرضى المتعافين في عام 1917، مما يجعله الأول الذي يعتبر الفاجات خيارًا للعلاج البيولوجي.
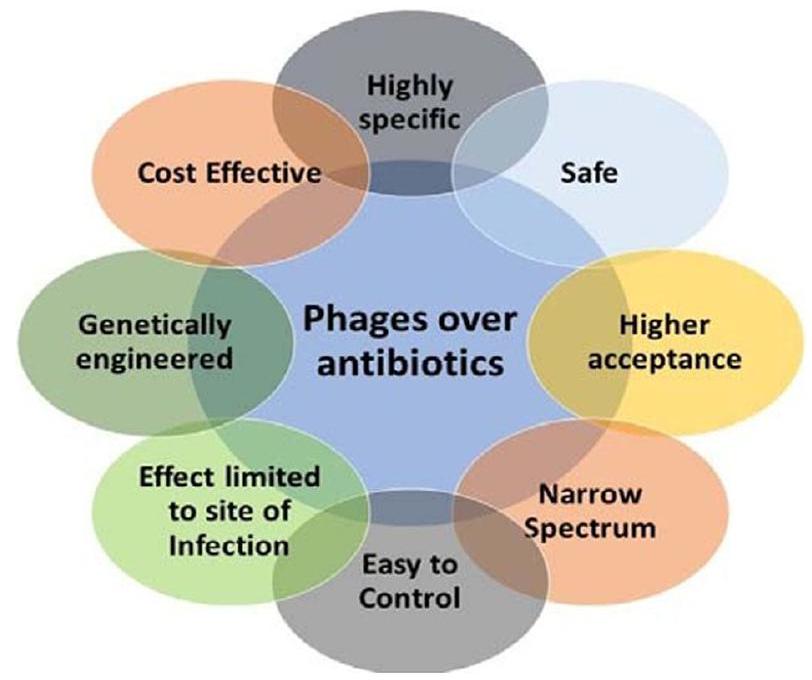
بدائل للمضادات الحيوية
تم ربطه بالمرض. تم إعطاء الدواء ثلاث مرات في اليوم عن طريق استنشاق رذاذ الأنف. لم يُلاحظ أي زيادة في الوزن لمدة عام قبل العلاج، لكن الحالة العامة للطفل تحسنت بشكل ملحوظ بعد ستة أيام من العلاج، وتمت ملاحظة زيادة في الوزن بمقدار 1 كجم بعد عشرين يومًا. لم يكن هناك أي اكتشاف لبكتيريا المكورات العنقودية الذهبية وPseudomonas aeruginosa في البلغم بعد ثلاث جلسات علاج، والتي شملت واحدة مع التتراسيكلين.
التحكم في البكتيريا المسببة للأمراض والبكتيريا المسببة للتلف في الأغذية
إزالة البكتيريا. السالمونيلا هي أكثر مسببات الأمراض المنقولة بالغذاء شيوعًا، وهي واحدة من الأسباب الأربعة الرئيسية عالميًا للأمراض المعوية وفقًا لمنظمة الصحة العالمية. تنتشر العدوى بالسالمونيلا بشكل رئيسي عن طريق اللحوم والدواجن والبيض والحليب الملوث. يمكن أن يضر الاتصال المباشر مع الحيوانات المصابة والدم والبول والبراز بصحة الإنسان. أصبحت المضادات الحيوية تُستخدم بشكل أوسع لعلاج العدوى في الماشية وزيادة إنتاج الغذاء من خلال تسريع انتشار البكتيريا المقاومة للمضادات الحيوية.
الكواشف الحيوية المحتملة
مستخدمة في تطوير أنظمة الكشف عن البكتيريا المعتمدة على الفاجات
بروتينات ليتك المشتقة من الفاج
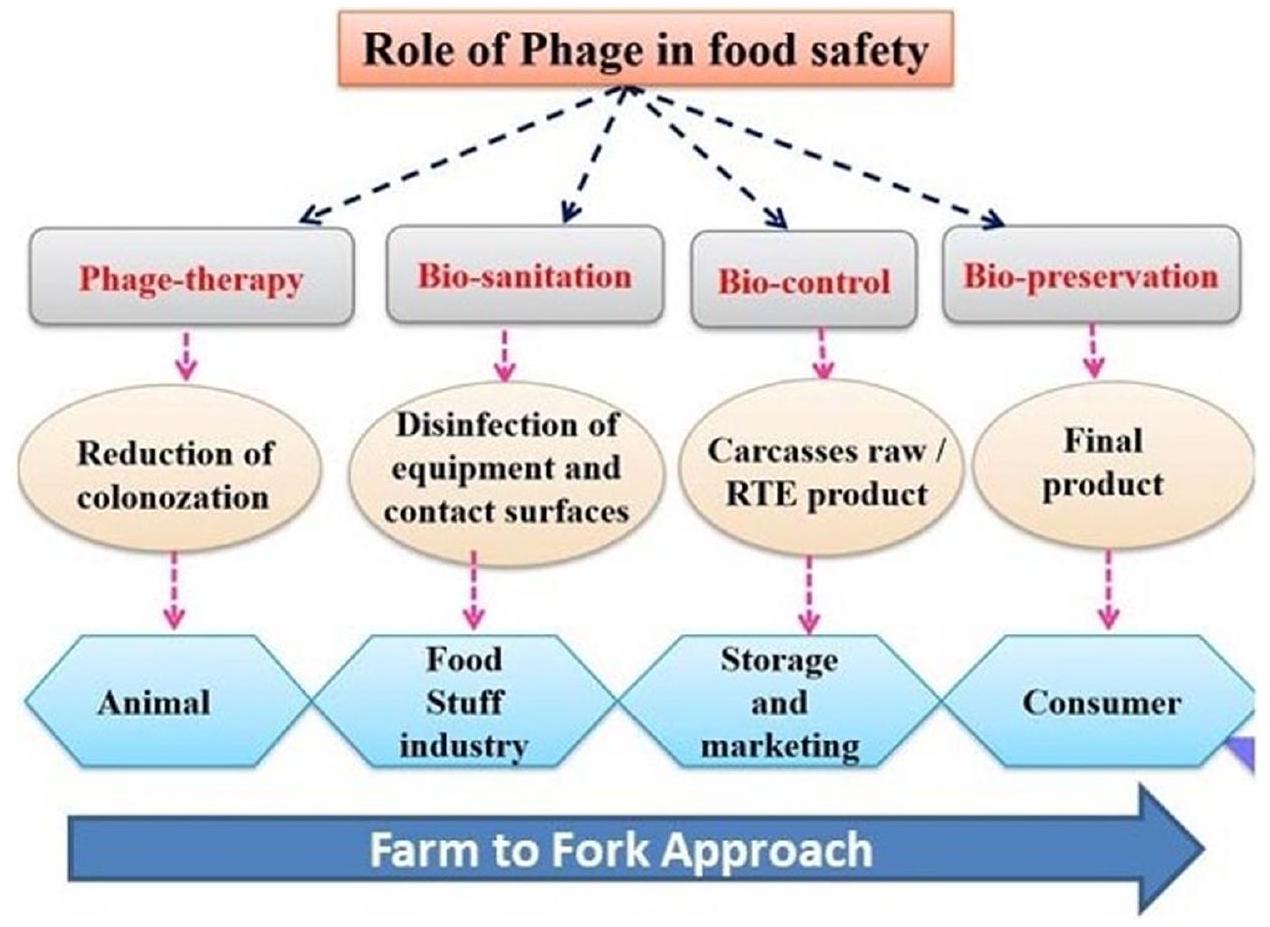
التطبيق في صناعة المواد الغذائية
آلية التحكم في الأغشية الحيوية
تأثير على لون المنتج أو قوامه أو نكهته. البكتيريا الزائفة ذات الأصل الحليبي مقاومة للفيروسات التي تم عزلها من اللحم البقري النيء.
التطبيق في الجهاز الهضمي
| البكتيريا/pathogens المسببة للتلف | خليط الفاجات | تطبيق | كفاءة الفاج | المراجع |
| ب. ليكنيفورميس
|
FBL1 | زجاج | استعادة الأغشية الحيوية
|
١٣٩ |
| E. coli EPEC 920
|
دي تي 1 (
|
تخمر الحليب | 1.1 تقليل في السجل بعد 24 ساعة عند
|
١٤٠ |
| E. كولاي 0157:H7 (
|
BEC8 (خليط الفاج) | الفولاذ المقاوم للصدأ، بلاط السيراميك، والبولي إيثيلين عالي الكثافة | تعداد الخلايا المكونة للأغشية الحيوية لا يمكن تتبعه بعد ساعة واحدة من العلاج عند 12،23، و
|
141 |
| L. monocytogenes (
|
العاثيات LiMN4L (
|
عينة من الفولاذ المقاوم للصدأ | البيوفيلم البكتيري غير مرئي بعد 75 دقيقة | ١٤٢ |
| المكورات العنقودية الذهبية (S. aureus)
|
DRA88 والفيروس
|
بوليسترين | إزالة الكتلة الحيوية بعد 48 ساعة | 99 |
| المكورات العنقودية الذهبية (S. aureus)
|
فيل بلا-سي1 سي والفيروسات فيل بلا-رودي و(
|
بوليسترين | تقليل بواسطة
|
143 |
| المكورات العنقودية الذهبية (S. aureus)
|
سانف (
|
حليب مبستر تجاري |
|
١٤٤ |
| المكورات العنقودية الذهبية Sa9
|
فيروس
|
حليب كامل مبستر | انخفاض كامل، 24 ساعة عند
|
68 |
| المكورات العنقودية الذهبية Sa9
|
بكتريوفاج
|
حليب UHT | انخفاض كامل بعد ساعتين عند
|
100 |
تطبيق في تدمير الأغشية الحيوية
- تتكاثر الفاجات داخل خلايا العائل، مما يزيد من عدد الفاجات المحلية (التكبير). يتم إطلاق الفاجات المعدية وتخترق الغشاء الحيوي.
- تتكاثر الفاجات في جميع أنحاء الأغشية الحيوية وتقتل البكتيريا المنتجة للبوليمرات السكرية الخارجية، مما يزيل الأغشية الحيوية ويقلل من فرصة التجدد.
- يمكن أن تنقل الفاجات أو تعبر عن إنزيمات تفكيك تدمر الإكسيبوليسكاريد من داخل الجينوم المضيف. يمكن للفاجات أن تصيب الخلايا المستمرة حتى لو كانت غير قادرة على التكاثر وتدمر الخلية غير النشطة. تبقى هذه داخل الخلية حتى تصبح نشطة وتشكل خلية نباتية، تبدأ في التكاثر وتدمير الخلية من خلال العمل الليتيك بعد ذلك. إذا كان هناك عدد كبير من الفاجات، يمكنها قتل خلايا المضيف المستهدفة دون التكاثر.
. ومع ذلك، فإن هذه الأنواع من الحالات نادرة، والحصول على أعداد كبيرة مثل هذه في المختبر أمر صعب. يتم استخدام عدد أقل من الفيروسات البكتيرية للتكاثر، وقتل الخلية المضيفة، وتدمير الخلية المضيفة، ثم تكرار الدورة مع عدد أكبر من البكتيريا في دورة التحلل. لا توجد خلايا مضيفة كافية، لذا يتم تعطيل هذه الدورة وقطعها. تعتبر الأغشية الحيوية شائعة جدًا وتحتوي على العديد من البكتيريا، لذا فإن استهداف الفيروس البكتيري الناجح للبكتيريا داخل الأغشية الحيوية يمثل على الأرجح تغييرًا تطوريًا لاستخدام هذا المصدر الوفير. يُعتقد أن آليات القيام بذلك تعتمد على حاجتهم للتعامل مع السكريات المتعددة السكاريدية الكبسولية البكتيرية خلال المسار المعتاد للمرض.
تحتوي العديد من جينومات الفاجات على جينات لإنزيمات تفكيك يمكنها تكسير مصفوفة البيوفيلم. هذه الإنزيمات القابلة للذوبان التي تستهدف البكتيريا عن طريق كسر جدران خلاياها تُطلق من الخلية المضيفة. يمكن لهذه الإنزيمات أيضًا أن تؤثر على وتفكك الإكسيبوليسكاريد في البيوفيلم. يؤدي تدهور الخلية المضيفة إلى إطلاق الحمض النووي، الذي يبقى مرتبطًا بتكوين البيوفيلم. تتطلب الفاجات الذيل داخل الإنزيم للإصابة، وهو نموذج عام للفاجات ذات الذيل. يتم التعرف على السكريات البوليمرية الكبسولية وهضمها بواسطة مكون ذيل الفاج في هذا السيناريو، مما يسمح للذيل بالوصول إلى أغشية الخلايا وحقن الجينوم البكتيري..
الجوانب السلبية للبكتيريوفاجات
المشاكل المتعلقة بالملوثات. أدت تلوث الفاج أثناء التخمر إلى تقديم أول دليل في صناعة الألبان، مما وفر معلومات حاسمة حول وجود الفاجات في صناعة الغذاء. تعتبر هذه البيئات الغذائية موطناً للبكتيريا والفاجات لتتعايش. قد تحدد العديد من العوامل استخدام الفاجات وإنشاء تركيبات علاجية جديدة. علاوة على ذلك، فإن إعداد الفاجات للاستخدام الطبي يمثل تحدياً، ولم يتم حل جميع القضايا التي ترتبط ارتباطاً وثيقاً بعلم الفاجات.
زيادة خطر مقاومة المضادات الحيوية
أثر الفيروسات البكتيرية على صناعة الغذاء
يمكن أن تؤدي تفشي الفيروسات إلى انخفاض جودة المنتجات، وزيادة الفساد، والميكروبات المعدية، أو حتى فقدان الإنتاج بالكامل.
مقاومة البكتيريا ضد الفيروسات البكتيرية
الإطار القانوني للبكتريوفاجات
كمنتج بيولوجي من قبل مكتب أبحاث ومراجعة اللقاحات التابع لإدارة الغذاء والدواء، وبالتالي فهو خاضع للوائح وإنتاج تشمل ممارسات التصنيع الجيدة، والبحوث ما قبل السريرية، وتوثيق التجارب السريرية.
خليط الفاجات والمنتجات المتاحة تجارياً
تعتبر البكتيريوفاجات كعوامل علاجية مجالًا مثيرًا للدراسة في السعي لإيجاد علاجات جديدة وفعالة ضد العدوى البكتيرية، خاصة تلك التي تسببها سلالات مقاومة للمضادات الحيوية. تم استكشاف واستخدام البكتيريوفاجات، أو الفاجات اختصارًا، ككاشفات حيوية فعالة لمراقبة واكتشاف مسببات الأمراض الميكروبية غير المرغوب فيها في بيئات مختلفة، بما في ذلك الأغذية والأدوية. بشكل عام، تمثل الكاشفات الحيوية المعتمدة على الفاجات نهجًا قيمًا لمراقبة وضمان سلامة العديد من المنتجات، بما في ذلك الأغذية والأدوية. من المهم ملاحظة أن تصميم خلطات الفاجات للاستخدام العلاجي يتطلب اعتبارات دقيقة، بما في ذلك فهم الخصائص المحددة للبكتيريا المستهدفة، وديناميات العدوى، والتفاعلات المحتملة بين الفاجات المختلفة. تستمر الأبحاث في علاج الفاجات في استكشاف استراتيجيات مثلى لتصميم خلطات فاجات فعالة لمكافحة العدوى البكتيرية. لقد كانت تجارية المنتجات المعتمدة على الفاجات تطورًا ملحوظًا في مجال الميكروبيولوجيا والتكنولوجيا الحيوية. تم استكشاف وتطوير العديد من المنتجات المعتمدة على الفاجات، وفي بعض الحالات، تم تسويقها بنجاح كعلاجات أو أدوية. تستفيد هذه المنتجات من الخصائص الفريدة للبكتيريوفاجات لعلاج العدوى البكتيرية. في الختام، يعد التصدي لتحدي مقاومة الفاجات أمرًا محوريًا لنجاح واستدامة التطبيقات المعتمدة على الفاجات على المدى الطويل. تعتبر الأبحاث المستمرة، والابتكار التكنولوجي، وفهم شامل للديناميات بين الفاجات والبكتيريا ضرورية لتطوير استراتيجيات فعالة للتغلب على المقاومة وتقليلها في التطبيقات المستقبلية.
ملخص التقرير
توفر البيانات
REFERENCES
- Summers, W. C. Félix Hubert d’Herelle (1873-1949): history of a scientific mind. Bacteriophage 6, e1270090 (2016).
- Czajkowski, R., Jackson, R. W. & Lindow, S. E. Environmental bacteriophages: from biological control applications to directed bacterial evolution. Front. Microbiol. 10, 1830 (2019).
- Karthik et al. Bacteriophages: effective alternative to antibiotics. Adv. Anim. Vet. Sci. 2, 1-7 (2014).
- Pinto, A. M., Cerqueira, M. A., Bañobre-Lópes, M., Pastrana, L. M. & Sillankorva, S. Bacteriophages for chronic wound treatment: From traditional to novel delivery systems. Viruses 12, 235 (2020).
- Moye, Z. D., Woolston, J. & Sulakvelidze, A. Bacteriophage applications for food production and processing. Viruses 10, 205 (2018).
- Wu et al. Phages in fermented foods: interactions and applications. Fermentation 9, 201 (2023).
- Gamachu, S. B. & Debalo, M. Review of bacteriophage and its applications. Int. J. Vet. Sci. Res. 8, 133-147 (2022).
- Fruciano, D. E. & Bourne, S. Phage as an antimicrobial agent: d’Herelle’s heretical theories and their role in the decline of phage prophylaxis in the West. Can. J. Infect. Dis. Med. Microbiol. 18, 19-26 (2007).
- Sulakvelidze, A. Bacteriophages: Biology and Application 381-436 (CRC Press, 2005).
- Ackermann, H. W. 5500 Phages examined in the electron microscope. Arch. Virol. 152, 227-243 (2007).
- Principi, N., Silvestri, E. & Esposito, S. Advantages and limitations of bacteriophages for the treatment of bacterial infections. Front. pharmacol. 10, 513 (2019).
- Deresinski, S. Bacteriophage therapy: exploiting smaller fleas. Clin. Infect. Dis. 48, 1096-1101 (2009).
- Hyman, P. Phages for phage therapy: isolation, characterization, and host range breadth. Pharmaceuticals 12, 35 (2019).
- Atterbury, R. J., & Barrow, P. A. in Bacteriophages: Biology, Technology, Therapy (eds Harper, D. R., Abedon, S. T., Burrowes, B. H. & McConville, M. L.) 953-987 (Springer, 2021).
- Samson, J. E. & Moineau, S. Bacteriophages in food fermentations: new frontiers in a continuous arms race. Annu. Rev. Food Sci. Technol. 4, 347-368 (2013).
- Kumar, R., Aneja, K. R., Punia, A. K. & Roy, P. Changing pattern of biotypes, phage types and drug resistance of Salmonella typhi in Ludhiana during 1980-1999. Indian J. Med. Res 113, 175 (2001).
- Labrie, S. J., Samson, J. E. & Moineau, S. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 8, 317-327 (2010).
- Laanto, E., Hoikkala, V., Ravantti, J. & Sundberg, L. R. Long-term genomic coevolution of host-parasite interaction in the natural environment. Nat. Commun. 8, 1-8 (2017).
- Ross, A., Ward, S. & Hyman, P. More is better: selecting for broad host range bacteriophages. Front. Microbiol 7, 1352 (2016).
- Anand, T. et al. Isolation and characterization of a bacteriophage with broad host range, displaying potential in preventing bovine diarrhoea. Virus genes 51, 315-321 (2015).
- Harada, L. K. et al. Biotechnological applications of bacteriophages: State of the art. Microbiol. Res. 212, 38-58 (2018).
- Tan, C. W. et al. Isolation and characterization of six Vibrio parahaemolyticus lytic bacteriophages from seafood samples. Front. Microbiol. 12, 616548 (2021).
- Sharma, M. Lytic bacteriophages: potential interventions against enteric bacterial pathogens on produce. Bacteriophage 3, e25518 (2013).
- Abdelsattar, A. et al. Bacteriophages: from isolation to application. Curr. Pharm. Biotechnol. 23, 337-360 (2022).
- Ly-Chatain, M. H. The factors affecting effectiveness of treatment in phages therapy. Front. Microbiol. 5, 51 (2014).
- Olson, M. R., Axler, R. P. & Hicks, R. E. Effects of freezing and storage temperature on MS2 viability. J. Virol. Methods 122, 147-152 (2004).
- Tey, B. T. et al. Production of fusion m 13 phage bearing the di-sulphide constrained peptide sequence (C-WSFFSNI-C) that interacts with hepatitis B core antigen. Afr. J. Biotechnol. 8, 268-273 (2009).
- Breitbart, M., Wegley, L., Leeds, S., Schoenfeld, T. & Rohwer, F. Phage community dynamics in hot springs. Appl. Environ. Microbiol. 70, 1633-1640 (2004).
- Mocé-Llivina, L., Muniesa, M., Pimenta-Vale, H., Lucena, F. & Jofre, J. Survival of bacterial indicator species and bacteriophages after thermal treatment of sludge and sewage. Appl. Environ. Microbiol 69, 1452-1456 (2003).
- Hatch, M. T. & Warren, J. C. Enhanced recovery of airborne T3 coliphage and Pasteurella pestis bacteriophage by means of a presampling humidification technique. J. Appl. Microbiol. 17, 685-689 (1969).
- Lu et al. Bacteriophage ecology in commercial sauerkraut fermentations. Appl. Environ. Microbiol. 69, 3192-3202 (2003).
- Kerby, G. P. et al. Purification, pH stability and sedimentation properties of the T7 bacteriophage of Escherichia coli. J. Immunol. 63, 93-107 (1994).
- Wick, C. H. et al. Mass spectrometry and integrated virus detection system characterization of MS2 bacteriophage. Toxicol. Mech. Methods 17, 241-254 (2007).
- Höglund, C., Ashbolt, N., Stenström, T. A. & Svensson, L. Viral persistence in source-separated human urine. Adv. Environ. Res. 6, 265-275 (2002).
- Vinner, G. K., Vladisavljević, G. T., Clokie, M. R. & Malik, D. J. Microencapsulation of Clostridium difficile specific bacteriophages using microfluidic glass capillary devices for colon delivery using pH triggered release. PLOS ONE 12, e0186239 (2017).
- Vinner, G. K., Richards, K., Leppanen, M., Sagona, A. P. & Malik, D. J. Microencapsulation of enteric bacteriophages in a pH-responsive solid oral dosage formulation using a scalable membrane emulsification process. Pharmaceutics 11, 475 (2019).
- Chandra, M. et al. Combined effect of disinfectant and phage on the survivality of S. Typhimurium and its biofilm phenotype. Internet J. Food Saf. 17, 25-31 (2015).
- Chen, X. et al. Characterization and adsorption of a Lactobacillus plantarum virulent phage. J. Dairy Sci. 102, 3879-3886 (2019).
- Whitman, P. A. & Marshall, R. T. Characterization of two psychrophilic Pseudomonas bacteriophages isolated from ground beef. J. Appl. Microbiol 22, 463-468 (1971).
- Wichels, A. et al. Bacteriophage diversity in the North Sea. Appl. Environ. Microbiol. 64, 4128-4133 (1998).
- Hidaka, T. “On the stability of marine bacteriophages.”. Bull. Jpn. Soc. Sci. Fish. 38, 517-523 (1972).
- Seaman, P. F. & Day, M. J. Isolation and characterization of a bacteriophage with an unusually large genome from the Great Salt Plains National Wildlife Refuge, Oklahoma, USA. FEMS Microbiol. Ecol. 60, 1-13 (2007).
- Greer, G. G. Bacteriophage control of foodborne bacteria. J. Food Prot. 68, 1102-1111 (2005).
- Anand, T. et al. Phage therapy for treatment of virulent Klebsiella pneumoniae infection in a mouse model. J. Glob. Antimicrob. Resist 21, 34-41 (2020).
- Fernández, L. et al. Bacteriophages in the dairy environment: From enemies to allies. Antibiotics 6, 27 (2017).
- Kaur, R., & Sethi, N. in Emerging Modalities in Mitigation of Antimicrobial Resistance (eds Akhtar, N., Singh, K. S., Prerna & Goyal, D.) 357-74 (Springer International Publishing, 2022).
- Pecetta, S. & Rappuoli, R. Bacteriophages, a multi-tool to fight infectious disease. Med 2, 209-210 (2021).
- Azam, A. H., Tan, X. E., Veeranarayanan, S., Kiga, K. & Cui, L. Bacteriophage technology and modern medicine. Antibiotics 10, 999 (2021).
- Lin, H., Paff, M. L., Molineux, I. J. & Bull, J. J. Therapeutic application of phage capsule depolymerases against K1, K5, and K30 capsulated E. coli in mice. Front. Microbiol. 8, 2257 (2017).
- Li, J. et al. Challenges for the application of bacteriophages as effective antibacterial agents in the food industry. J. Sci. Food Agric. 102, 461-471 (2022).
- Ghosh, C., Sarkar, P., Issa, R. & Haldar, J. Alternatives to conventional antibiotics in the era of antimicrobial resistance. Trends Microbiol 27, 323-338 (2019).
- Dery, K. J., Górski, A., Miedzybrodzki, R., Farmer, D. G. & Kupiec-Weglinski, J. W. Therapeutic perspectives and mechanistic insights of phage therapy in allotransplantation. Transplantation 105, 1449-1458 (2021).
- Dabrowska, K. et al. Immunogenicity studies of proteins forming the T4 phage head surface. J. Virol. 88, 12551-12557 (2014).
- Anand, T. et al. Phage therapy in tackling AMR: potential and prospects. Indian J. Comp. Microbiol Immunol. Infect. Dis. 43, 50-57 (2022).
- Rios, A. C. et al. Structural and functional stabilization of bacteriophage particles within the aqueous core of a W/O/W multiple emulsion: a potential biotherapeutic system for the inhalational treatment of bacterial pneumonia. Process Biochem. 64, 177-192 (2018).
- Cao, Y. et al. Characterization and application of a novel Aeromonas bacteriophage as treatment for pathogenic Aeromonas hydrophila infection in rainbow trout. Aquaculture 523, 735193 (2020).
- Pan, F. Flghting Antimicrobial Resistant (Amr) Bacteria: from Bacteriophage-based Specific Capture to Controlled Killing Doctoral Dissertation, ETH Zurich (2022).
- Kutateladze, Á. & Adamia, R. Phage therapy experience at the Eliava Institute. Médecine Maladies Infectieuses 38, 426-430 (2008).
- Fiscarelli, E. V. et al. In vitro newly isolated environmental phage activity against biofilms preformed by Pseudomonas aeruginosa from patients with cystic fibrosis. Microorganisms 9, 478 (2021).
- Morello, E. et al. Pulmonary bacteriophage therapy on Pseudomonas aeruginosa cystic fibrosis strains: first steps towards treatment and prevention. PLoS ONE 6, e16963 (2011).
- Roach, D. R. et al. Synergy between the host immune system and bacteriophage is essential for successful phage therapy against an acute respiratory pathogen. Cell Host Microbe 22, 38-47 (2017).
- Semler, D. D., Goudie, A. D., Finlay, W. H. & Dennis, J. J. Aerosol phage therapy efficacy in Burkholderia cepacia complex respiratory infections. Antimicrob. Agents Chemother. 58, 4005-4013 (2014).
- Kwiatek, M., Parasion, S. & Nakonieczna, A. Therapeutic bacteriophages as a rescue treatment for drug-resistant infections-an in vivo studies overview. J. Appl. Microbiol. 128, 985-1002 (2020).
- Oliver, K. M., Degnan, P. H., Hunter, M. S. & Moran, N. A. Bacteriophages encode factors required for protection in a symbiotic mutualism. Science 325, 992-994 (2009).
- CDC. Antimicrobial Resistance Threat Report. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Washington, DC (2013).
- Abdeltawab, A. A., El-Nahas, E. M., Askora, A. A. & Abdelaziz, H. S. Bacteriological characterization of Salmonella species isolated from laying ducks. Benha Med. J. 34, 404-412 (2018).
- Esmael, A. et al. Isolation and characterization of two lytic bacteriophages infecting a multi-drug resistant Salmonella Typhimurium and their efficacy to combat salmonellosis in ready-to-use foods. Microorganisms 9, 423 (2021).
- Garcia, R. et al. Bacteriophage production models: an overview. Front. Microbiol. 10, 1187 (2019).
- Bhandare, S., & Goodridge, L. in Bacteriophages: Biology, Technology, Therapy (eds Harper, D. R., Abedon, S. T., Burrowes, B. H. & McConville, M. L.) 769-788 (Springer, 2021).
- Rodríguez-Rubio, L. et al. Listeriaphages and coagulin C23 act synergistically to kill Listeria monocytogenes in milk under refrigeration conditions. Int. J. Food Microbiol. 205, 68-72 (2015).
- López-Cuevas, O., Medrano-Félix, J. A., Castro-Del Campo, N. & Chaidez, C. Bacteriophage applications for fresh produce food safety. Int. J. Environ. Health Res. 31, 687-702 (2021).
- Kuek, M., McLean, S. K. & Palombo, E. A. Application of bacteriophages in food production and their potential as biocontrol agents in the organic farming industry. Biol. Control 165, 104817 (2022).
- Sillankorva, S. M., Oliveira, H., & Azeredo, J. Bacteriophages and their role in food safety. Int. J. Microbiol. 2012, 863945 (2012).
- Jun, J. W. et al. Bacteriophage application to control the contaminated water with Shigella. Sci. Rep. 6, 1-7 (2016).
- Prashantha, S. T., Yadav, J., Sunilkumar, V. P., & HP, N. P. The Variability and Mechanisms of Infection by Gram-Positive, Plant Associated Bacteria. International Year of Millets 2023, 51 (2023).
- Kaptchouang Tchatchouang, C. D. et al. Listeriosis outbreak in South Africa: a comparative analysis with previously reported cases worldwide. Microorganisms 8, 135 (2020).
- Kawacka, I., Olejnik-Schmidt, A., Schmidt, M. & Sip, A. Effectiveness of phagebased inhibition of Listeria monocytogenes in food products and food processing environments. Microorganisms 8, 1764 (2020).
- Leverentz, B., Conway, W. S., Janisiewicz, W. & Camp, M. J. Optimizing concentration and timing of a phage spray application to reduce Listeria monocytogenes on honeydew melon tissue. J. Food Prot. 67, 1682-1686 (2004).
- Komora, N. et al. Non-thermal approach to Listeria monocytogenes inactivation in milk: the combined effect of high pressure, pediocin PA-1 and bacteriophage P100. Food Microbiol. 86, 103315 (2020).
- Komora, N. et al. Innovative hurdle system towards Listeria monocytogenes inactivation in a fermented meat sausage model-high pressure processing assisted by bacteriophage P100 and bacteriocinogenic Pediococcus acidilactici. Food Res. Int. 148, 110628 (2021).
- Stefani, E. et al. Bacteriophage-mediated control of phytopathogenic xanthomonads: A promising green solution for the future. Microorganisms 9, 1056 (2021).
- Schwarczinger, I. et al. Characterization of Myoviridae and Podoviridae family bacteriophages of Erwinia amylovora from Hungary-potential of application in Biol. Control of fire blight. Eur. J. Plant Pathol. 149, 639-652 (2017).
- Kizheva, Y. et al. Broad host range bacteriophages found in rhizosphere soil of a healthy tomato plant in Bulgaria. Heliyon 7, e07084 (2021).
- Clavijo, V. et al. Phage cocktail SalmoFREE
reduces Salmonella on a commercial broiler farm. Poult. Sci. J. 98, 5054-5063 (2019). - Firlieyanti, A. S., Connerton, P. L. & Connerton, I. F. Campylobacters and their bacteriophages from chicken liver: the prospect for phage biocontrol. Int. J. Food Microbiol. 237, 121-127 (2016).
- Leverentz, B. et al. Examination of bacteriophage as a biocontrol method for Salmonella on fresh-cut fruit: a model study. J. Food Prot. 64, 1116-1121 (2001).
- Patel, D., Zhou, Y. & Ramasamy, R. P. A bacteriophage-based electrochemical biosensor for detection of methicillin-resistant Staphylococcus aureus. J. Electrochem. Soc. 168, 057523 (2021).
- Janczuk, M. et al. Bacteriophage-based bioconjugates as a flow cytometry probe for fast bacteria detection. Bioconjug. Chem. 28, 419-425 (2017).
- Paczesny, J., Wdowiak, M., & Ochirbat, E. in Nanotechnology for Infectious Diseases 439-473 (Springer, 2022)
- Pierce, C. L., Rees, J. C., & Barr, J. R. in Bacteriophages: Biology, Technology, Therapy (eds Harper, D. R., Abedon, S. T., Burrowes, B. H. & McConville, M. L.) 645-656 (2021).
- Srivastava, K. R., Awasthi, S., Mishra, P. K., & Srivastava, P. K. in Waterborne Pathogens (eds Prasad, M. N. V. & Grobelak, A.) 237-277 (Banaras Hindu University, 2020)
- Farooq, U., Yang, Q., Ullah, M. W. & Wang, S. Bacterial biosensing: recent advances in phage-based bioassays and biosensors. Biosens. Bioelectron. 118, 204-216 (2018).
- Anand, T. et al. Phage display technique as a tool for diagnosis and antibody selection for coronaviruses. Curr. Microbiol. 78, 1124-1134 (2021).
- Nachimuthu, R., Royam, M. M., Manohar, P. & Leptihn, S. Application of bacteriophages and endolysins in aquaculture as a biocontrol measure. Biol. Control 160, 104678 (2021).
- Lai, W. C. B., Chen, X., Ho, M. K. Y., Xia, J. & Leung, S. S. Y. Bacteriophage-derived endolysins to target gram-negative bacteria. Int. J. Pharm. 589, 119833 (2020).
- Huang, Z. et al. Phages and their lysins: Toolkits in the battle against foodborne pathogens in the post-antibiotic era. Compr. Rev. Food Sci. 20, 3319-3343 (2021).
- Schmelcher, M. & Loessner, M. J. Bacteriophage endolysins: applications for food safety. Curr. Opin. Biotechnol. 37, 76-87 (2016).
- Bruttin, A. & Brussow, H. Human volunteers receiving Escherichia coli phage T4 orally: a safety test of phage therapy. Antimicrob. Agents Chemother. 49, 2874-2878 (2005).
- Alves, D. R. Combined use of bacteriophage K and a novel bacteriophage to reduce Staphylococcus aureus biofilm formation. Appl. Environ. Microbiol. 80, 6694-6703 (2014).
- Viazis, S., Akhtar, M., Feirtag, J. & Diez-Gonzalez, F. Reduction of Escherichia coli O157: H7 viability on leafy green vegetables by treatment with a bacteriophage mixture and trans-cinnamaldehyde. Food microbiol 28, 149-157 (2011).
- Soni, K. A. & Nannapaneni, R. Removal of Listeria monocytogenes biofilms with bacteriophage P100. J. Food Prot. 73, 1519-1524 (2010).
- Patel, T. R. & Jackman, D. M. Susceptibility of psychrotrophic pseudomonads of milk origin to psychrotrophic bacteriophages. Appl. Environ. Microbiol. 51, 446-448 (1986).
- Goode, D., Allen, V. M. & Barrow, P. A. Reduction of experimental Salmonella and Campylobacter contamination of chicken skin by application of lytic bacteriophages. Appl. Environ. Microbiol. 69, 5032-5036 (2003).
- Fernández, L., Duarte, A. C., Rodríguez, A. & García, P. The relationship between the phageome and human health: are bacteriophages beneficial or harmful microbes? Benef. Microbes 12, 107-120 (2021).
- Dalmasso, M., Hill, C. & Ross, R. P. Exploiting gut bacteriophages for human health. Trends Microbiol 22, 399-405 (2014).
- Mirzaei, M. K. & Maurice, C. F. Ménage à trois in the human gut: interactions between host, bacteria and phages. Nat. Rev. Microbiol. 15, 397-408 (2017).
- Kutter, E. et al. Phage therapy in clinical practice: treatment of human infections. Curr. Pharm. Biotechnol. 11, 69-86 (2010).
- Merabishvili, M. et al. Selection and characterization of a candidate therapeutic bacteriophage that lyses the Escherichia coli O104: H4 strain from the 2011 outbreak in Germany. PLoS ONE 7, e52709 (2012).
- Siringan, P., Connerton, P. L., Payne, R. J. & Connerton, I. F. Bacteriophagemediated dispersal of Campylobacter jejuni biofilms. Appl. Environ. Microbiol. 77, 3320-3326 (2011).
- Łusiak-Szelachowska, M., Weber-Dabrowska, B. & Górski, A. Bacteriophages and lysins in biofilm control. Virol. Sin. 35, 125-133 (2020).
- Stanford, K. et al. Oral delivery systems for encapsulated bacteriophages targeted at Escherichia coli O157: H7 in feedlot cattle. J. Food Prot. 73, 1304-1312 (2010).
- Liu, M. et al. Comparative genomics of Acinetobacter baumannii and therapeutic bacteriophages from a patient undergoing phage therapy. Nat. Commun. 13, 3776 (2022).
- Abedon, S. T., García, P., Mullany, P. & Aminov, R. Phage therapy: past, present and future. Front. Microbiol. 8, 981 (2017).
- Pires, D. P., Oliveira, H., Melo, L. D., Sillankorva, S. & Azeredo, J. Bacteriophageencoded depolymerases: their diversity and biotechnological applications. Appl. Microbiol. Biotechnol. 100, 2141-2151 (2016).
- Ferriol-González, C. & Domingo-Calap, P. Phages for biofilm removal. Antibiotics 9, 268 (2020).
- Strathdee, S. A., Hatfull, G. F., Mutalik, V. K. & Schooley, R. T. Phage therapy: from biological mechanisms to future directions. Cell 186, 17-31 (2023).
- Drulis-Kawa, Z., Majkowska-Skrobek, G. & Maciejewska, B. Bacteriophages and phage-derived proteins-application approaches. Curr. Medicinal Chem. 22, 1757-1773 (2015).
- Monjezi, R., Tey, B. T., Sieo, C. C. & Tan, W. S. Purification of bacteriophage M13 by anion exchange chromatography. J. Chromatogr. B 878, 1855-1859 (2010).
- Henein, A. What are the limitations on the wider therapeutic use of phage? Bacteriophage 3, e24872 (2013).
- Marcó, M. B., Moineau, S. & Quiberoni, A. Bacteriophages and dairy fermentations. Bacteriophage 2, 149-158 (2012).
- Maiques, E. et al. Role of staphylococcal phage and SaPI integrase in intra-and interspecies SaPI transfer. J. Bacteriol. 189, 5608-5616 (2007).
- Anand, T. et al. Abundance of antibiotic resistance genes in environmental bacteriophages. J. G. Virol. 97, 3458-3466 (2016).
- Hemme, T., Uddin, M. M. & Ndambi, O. A. Benchmarking cost of milk production in 46 countries. Glob. Econ. Rev. 3, 254-270 (2014).
- Seed, K. D. Battling phages: how bacteria defend against viral attack. PLoS Pathog. 11, e1004847 (2015).
- Drulis-Kawa, Z., Majkowska-Skrobek, G., Maciejewska, B., Delattre, A. S. & Lavigne, R. Learning from bacteriophages-advantages and limitations of phage and phage-encoded protein applications. Curr. Protein Pept. Sci. 13, 699-722 (2012).
- Seed, K. D., Lazinski, D. W., Calderwood, S. B. & Camilli, A. A bacteriophage encodes its own CRISPR/Cas adaptive response to evade host innate immunity. Nature 494, 489-491 (2013).
- Verbeken, G. et al. Optimizing the European regulatory framework for sustainable bacteriophage therapy in human medicine. Arch. Immunol. Therapiae Experimentalis 60, 161-172 (2012).
- Fontaine, N., & Reynders, D. Directive 2001/83/EC of the European Parliament and of the Council of 6 November. on the Community code relating to medicinal products for human use. Official J. Eur. Communities L 311, 67-128 (2001).
- Międzybrodzki, R. et al. in Bacteriophages: Biology, Technology, Therapy (eds Harper, D. R., Abedon, S. T., Burrowes, B. H. & McConville, M. L.) 921-951 (2021).
- Żaczek, M. et al. A thorough synthesis of phage therapy unit activity in Polandits history, milestones and international recognition. Viruses 14, 1170 (2022).
- Hartmann, M. & Hartmann-Vareilles, F. The clinical trials directive: how is it affecting Europe’s noncommercial research. PLoS Clin. trials 1, e13 (2006).
- Naureen, Z. et al. Comparison between American and European legislation in the therapeutical and alimentary bacteriophage usage. Acta Bio Medica: Atenei Parmensis 91 (2020).
- Jones, J. D., Trippett, C., Suleman, M., Clokie, M. R. & Clark, J. R. The future of clinical phage therapy in the United Kingdom. Viruses 15, 721 (2023).
- Lin, R. C., Fabijan, A. P., Attwood, L., & Iredell, J. State of the regulatory affair: Regulation of phage therapy in Australia (2019). Available at: https:// phage.directory/capsid/phage-therapy-regulation-australia.
- Furfaro, L. L., Payne, M. S. & Chang, B. J. Bacteriophage therapy: clinical trials and regulatory hurdles. Front. Cell. Infect. Microbiol. 8, 376 (2018).
- Johri, P. Antimicrobial resistance and phage therapy in India. The Microbiologist (2023). Available at: https://www.the-microbiologist.com/features/antimicrobial-resistance-and-phage-therapy-in-india/1386.article.
- eCFR, 71 FR 47731, Aug. 18, 2006, as amended at 81 FR v5591, Feb. 3, 2016; 88 FR 17720, Mar. 24, 2023 Available at: https://www.ecfr.gov/current/title-21/ chapter-I/subchapter-B/part-172/subpart-H/section-172.785.
- Wang, Z. & Zhao, X. The application and research progress of bacteriophages in food safety. J. Appl. Microbiol. 133, 2137-2147 (2022).
- Prada-Peñaranda, C. Phage preparation FBL1 prevents Bacillus licheniformis biofilm, bacterium responsible for the mortality of the Pacific White Shrimp Litopenaeus vannamei. Aquaculture 484, 160-167 (2018).
- Tomat, D., Casabonne, C., Aquili, V., Balagué, C. & Quiberoni, A. Evaluation of a novel cocktail of six lytic bacteriophages against Shiga toxin-producing Escherichia coli in broth, milk and meat. Food Microbiol. 76, 434-442 (2018).
- Wang, L. et al. Use of bacteriophages to control Escherichia coli O157: H7 in domestic ruminants, meat products, and fruits and vegetables. Foodborne Pathog. Dis. 14, 483-493 (2017).
- Arachchi, G. G. et al. Characteristics of three listeriaphages isolated from New Zealand seafood environments. J. Appl. Microbiol. 115, 1427-1438 (2013).
- Gutiérrez, D., Rodríguez-Rubio, L., Martínez, B., Rodríguez, A. & García, P. Bacteriophages as weapons against bacterial biofilms in the food industry. Front. Microbiol. 7, 825 (2016).
- Tahir, A., Asif, M., Abbas, Z. & Rehman, S. U. Three bacteriophages SA, SA2 and SNAF can control growth of milk isolated Staphylococcal species. Pak. J. Zool. 49, 425-759 (2017).
شكر وتقدير
مساهمات المؤلفين
المصالح المتنافسة
الموافقة الأخلاقية
معلومات إضافية
© المؤلفون 2024، نشر مصحح 2024
قسم ميكروبيولوجيا الألبان، المعهد الوطني للبحوث الزراعية للألبان، كارنا 132001، الهند. قسم التغذية السريرية، كلية العلوم الطبية التطبيقية، جامعة جازان، جازان 45142، المملكة العربية السعودية. قسم بيولوجيا التغذية، كلية العلوم التطبيقية والمتعددة التخصصات، الجامعة المركزية في هاريانا، مهيندرغار 123031، الهند. المركز الوطني للبحوث على الخيول – ICAR، طريق سيرسا، هيزار 125001، الهند. المعهد الجامعي للتكنولوجيا الحيوية، جامعة تشانديغار، ساهيبزادا أجيط سينغ ناغار 140413، الهند. قسم الميكروبيولوجيا، معهد VCSG الحكومي للعلوم الطبية والبحث، جانجانالي سريكوت، سريناغار باوري غاروال 246174، الهند. مركز الصحة الواحدة، كلية العلوم البيطرية، جامعة جورو أنغاد ديف للعلوم البيطرية والحيوانية، لوديانا، الهند. مركز بول هيبرت لتشفير الحمض النووي ودراسات التنوع البيولوجي، جامعة د. بابا صاحب أمبيدكار ماراثوادا، أورانجاباد، الهند. CBIOS (مركز الأبحاث لعلوم الحياة والتقنيات الصحية)، جامعة لوسوفونا للعلوم الإنسانية والتكنولوجيا، كامبو غراندي 376، 1749-024 لشبونة، البرتغال. كلية إدارة الضيافة والسياحة، جامعة سيجونغ، 98 غونجا-دونغ، منطقة غوانجين، سيول 143-747، جمهورية كوريا. البريد الإلكتروني:antonio.raposo@ulusofona.pt; heesup.han@gmail.com; akpuniya@gmail.com; Anil.Puniya@icar.gov.in
DOI: https://doi.org/10.1038/s41538-023-00245-8
PMID: https://pubmed.ncbi.nlm.nih.gov/38172179
Publication Date: 2024-01-03
Positive and negative aspects of bacteriophages and their immense role in the food chain
Abstract
Bacteriophages infect and replicate inside a bacterial host as well as serve as natural bio-control agents. Phages were once viewed as nuisances that caused fermentation failures with cheese-making and other industrial processes, which lead to economic losses, but phages are now increasingly being observed as being promising antimicrobials that can fight against spoilage and pathogenic bacteria. Pathogen-free meals that fulfil industry requirements without synthetic additives are always in demand in the food sector. This study introduces the readers to the history, sources, and biology of bacteriophages, which include their host ranges, absorption mechanisms, lytic profiles, lysogenic profiles, and the influence of external factors on the growth of phages. Phages and their derivatives have emerged as antimicrobial agents, biodetectors, and biofilm controllers, which have been comprehensively discussed in addition to their potential applications in the food and gastrointestinal tract, and they are a feasible and safe option for preventing, treating, and/or eradicating contaminants in various foods and food processing environments. Furthermore, phages and phage-derived lytic proteins can be considered potential antimicrobials in the traditional farm-to-fork context, which include phage-based mixtures and commercially available phage products. This paper concludes with some potential safety concerns that need to be addressed to enable bacteriophage use efficiently.
INTRODUCTION
temperature component of phage fitness should remain critical when considering the use of viruses in order to manage bacterial infections in agriculture and/ or the environment because these phage-based products may have inconsistent activity on the same disease, which is due to differences in climatic conditions, such as temperatures. Phages can act as allies and enemies in human activities, and bacteria may evolve phage resistance via different defense mechanisms
HISTORY, SOURCES, AND BIOLOGY OF PHAGES
Host range of phages
Absorption mechanism
Lytic cycle
Lysis profile
Lysogenic cycle

INFLUENCE OF EXTERNAL FACTORS ON PHAGES
Temperature
inactivates phages in dewatered sludge and raw sewage. The thermal resistance of somatic coliphages, which are phages capable of infecting Bacteriodes fragilis, and F-specific RNA phages was discovered. This study suggests that phages are more resistant to thermal treatment than bacteria. The most significant parameter in regards to determining phage activity is the storage temperature. Bacillus cereus CP-51 phages were sensitive to low temperatures and stable at room temperature, even though phage storage at room temperature is impossible. Tailed phages are the most resistant to storage and have the most extended longevity. Some phages, such as T4, T5, and T7 were viable after
pH of the environment
was alterable at the greater value, and the phages could be redisposed by shaking them. The researchers found that irreversible coagulation and precipitation might be the limiting factor of the phage activity. A little loss of infectivity nearby at pH 7 was also observed. The PM2 phage was sensitive at a low pH , completely losing activity at pH 5.0. Particles of the T1 phage vanished at pH 3.0 , and the M13 phage survived even at pH 2. These interpretations show that the alteration in environmental pH may shelter the phage activity at a low temperature.
Salinity and ions
POSITIVE ASPECTS OF BACTERIOPHAGES
Antimicrobial agents
d’Herelle found that lytic phages were effective against the dysentery bacillus (Shigella) in the faeces of convalescing patients in 1917, which makes him the first to consider phages as a biotherapeutic option
Alternatives to antibiotics
been associated with the illness. The drug was delivered three times a day via nasal phage nebulization. No weight gain was observed for a year before the treatment, but the child’s general condition significantly improved after six days of therapy, and a weight gain of 1 kg was observed after twenty days. S. aureus and P. aeruginosa were undetectable in the sputum after three therapy sessions, which included one with tetracycline
Control of pathogenic and spoilage bacteria in foods


eliminate the bacteria. Salmonella is the most common foodborne pathogen, and it is one of the four leading global causes of diarrheal diseases according to the World Health Organization. Infections with Salmonella are spread mostly by infected meat, poultry, eggs, and milk. Direct contact with infected animals, blood, urine, and excreta can harm human health. Antibiotics have become more widely used in order to treat infections in livestock and increase food production by accelerating the spread of antimicrobial-resistant bacteria
Potential biodetectors
used in developing phage-based bacterial detection systems
Phage-derived lytic proteins
Application in the food industry
Biofilm control mechanism
influence on the product’s color, texture, or flavor. Pseudomonads of milk origin is resistant to phages that are isolated from raw beef
Application in the gastrointestinal tract
| Spoilage causing bacteria/ pathogens | Phage(s) mixture | Application | Phage efficiency | References |
| B. licheniformis (
|
FBL1 | Glass | Biofilm recovery
|
139 |
| E. coli EPEC 920 (
|
DT1 (
|
Milk fermentation | 1.1 log reduction after 24 h at
|
140 |
| E. coli 0157:H7 (
|
BEC8 (Phage mixture) | Stainless steel, ceramic tile, and highdensity polyethylene | Biofilm-forming cell counts are untraceable after 1 h of treatment at 12,23 , and
|
141 |
| L. monocytogenes (
|
Phages LiMN4L (
|
Stainless steel coupon | Bacterial Biofilm invisible after 75 min | 142 |
| S. aureus (
|
DRA88 and phage
|
Polystyrene | Removal of the biomass after 48 h | 99 |
| S. aureus (
|
PhilPLA-C1C and phages philPLA-RODI and (
|
Polystyrene | Reduction by
|
143 |
| S. aureus (
|
SANF (
|
Commercial pasteurized Milk |
|
144 |
| S. aureus Sa9 (
|
Phage (
|
Pasteurized whole milk | Complete decline, 24 h at
|
68 |
| S. aureus Sa9 (
|
Phage (
|
UHT milk | Complete decline after 2 h at
|
100 |
Application in biofilm destruction
- Phages replicate within their host cells, which increases the localized phage population (amplification). Infectious phages are released and penetrate the biofilm.
- Phages propagate throughout the biofilm and kill exopolysaccharide-producing bacteria, which remove the biofilm and reduce the chance of regeneration.
- Phages may transport or express depolymerizing enzymes that destroy the exopolysaccharide from within the host genome.Phages can infect the persister cell even if they are unable to reproduce and destroy the inactive cell. These remain inside the cell until they become reactive and form a vegetative cell, which begins to multiply and destroy the cell via lytic action afterward. If a large number of phages are present, they can kill their target host cells without replicating
. However, these types of cases are uncommon, and obtaining large numbers like this in the lab is difficult. A smaller number of phages are utilized to replicate, kill the host cell, destroy the host cell, and then repeat the cycle with a larger number of bacteria in the lysis cycle. There are not enough host cells, so this cycle is disrupted and interrupted. Biofilms are quite frequent and contain many bacteria, so the phage’s successful targeting of bacteria within biofilms likely represents an evolutionary change to use this abundant source. Their mechanisms for doing this are thought to be based on their need to deal with bacterial capsular polysaccharides throughout the usual course of an illness.
Many phage genomes contain genes for depolymerizing enzymes that can break down the biofilm matrix. These soluble enzymes that target bacteria by breaking their cell walls are released from the host cell. These enzymes also can affect and degrade the exopolysaccharide in the biofilm. The host cell degradation releases the DNA, which remains attached to the biofilm formation. Phages require the tail within the enzyme for infection, which is a general model of tail phages. Capsular polysaccharides are recognized and digested by a phage tail component in this scenario, which allows the tail to access cell membranes and inject the bacterial genome.
NEGATIVE ASPECTS OF BACTERIOPHAGES
problems that are related to contaminants. Phage contamination during fermentation gave the first evidence in the dairy industry, which provided crucial information on the presence of phages in the food industry. These dietary settings serve as a host for bacteria and phages to coexist. Many factors may limit the use of phages and the creation of new therapeutic formulations. Furthermore, preparing phages for medicinal application is challenging, and not all of the issues that are strictly interrelated with phage biology have been resolved
Increased risk of antibiotic resistance
Impact of phages on the food industry
of components, lower quality products, the growth of spoilage, and infectious microbes, or even total production loss, can all result from phage outbreaks
Bacterial resistance against phages
LEGAL FRAMEWORK FOR BACTERIOPHAGES
as a biological product by the FDA’s Office of Vaccines Research and Review in the Center for Biologics Evaluation and Research, and as such, it is subject to regulations and production that include GMP, preclinical research, and clinical trial documentation
PHAGE MIXTURES AND COMMERCIALLY AVAILABLE PRODUCTS
enzybiotics as therapeutic agents is an exciting area of study in the quest for new and effective treatments against bacterial infections, especially those caused by antibiotic-resistant strains. Bacteriophages, or phages for short, have been explored and utilized as efficient biodetectors to monitor and detect undesired microbial pathogens in various settings, including foods and medicines. Overall, phage-based biodetection represents a valuable approach for monitoring and ensuring the safety of multiple products, including foods and medicines. It’s important to note that the design of phage mixtures for therapeutic use requires careful consideration, including understanding the target bacteria’s specific characteristics, the infection’s dynamics, and the potential interactions between different phages. Research in phage therapy continues to explore optimal strategies for designing effective phage mixtures to combat bacterial infections. The commercialization of phage-based products has been a notable development in the field of microbiology and biotechnology. Several phagebased products have been explored, developed, and, in some cases, successfully marketed as therapies or medicines. These products leverage the unique properties of bacteriophages to treat bacterial infections. In conclusion, addressing the challenge of phage resistance is pivotal for the long-term success and sustainability of phage-based applications. Ongoing research, technological innovation, and a holistic understanding of the dynamics between phages and bacteria are essential for developing effective strategies to overcome and minimize resistance in future applications.
Reporting summary
DATA AVAILABILITY
REFERENCES
- Summers, W. C. Félix Hubert d’Herelle (1873-1949): history of a scientific mind. Bacteriophage 6, e1270090 (2016).
- Czajkowski, R., Jackson, R. W. & Lindow, S. E. Environmental bacteriophages: from biological control applications to directed bacterial evolution. Front. Microbiol. 10, 1830 (2019).
- Karthik et al. Bacteriophages: effective alternative to antibiotics. Adv. Anim. Vet. Sci. 2, 1-7 (2014).
- Pinto, A. M., Cerqueira, M. A., Bañobre-Lópes, M., Pastrana, L. M. & Sillankorva, S. Bacteriophages for chronic wound treatment: From traditional to novel delivery systems. Viruses 12, 235 (2020).
- Moye, Z. D., Woolston, J. & Sulakvelidze, A. Bacteriophage applications for food production and processing. Viruses 10, 205 (2018).
- Wu et al. Phages in fermented foods: interactions and applications. Fermentation 9, 201 (2023).
- Gamachu, S. B. & Debalo, M. Review of bacteriophage and its applications. Int. J. Vet. Sci. Res. 8, 133-147 (2022).
- Fruciano, D. E. & Bourne, S. Phage as an antimicrobial agent: d’Herelle’s heretical theories and their role in the decline of phage prophylaxis in the West. Can. J. Infect. Dis. Med. Microbiol. 18, 19-26 (2007).
- Sulakvelidze, A. Bacteriophages: Biology and Application 381-436 (CRC Press, 2005).
- Ackermann, H. W. 5500 Phages examined in the electron microscope. Arch. Virol. 152, 227-243 (2007).
- Principi, N., Silvestri, E. & Esposito, S. Advantages and limitations of bacteriophages for the treatment of bacterial infections. Front. pharmacol. 10, 513 (2019).
- Deresinski, S. Bacteriophage therapy: exploiting smaller fleas. Clin. Infect. Dis. 48, 1096-1101 (2009).
- Hyman, P. Phages for phage therapy: isolation, characterization, and host range breadth. Pharmaceuticals 12, 35 (2019).
- Atterbury, R. J., & Barrow, P. A. in Bacteriophages: Biology, Technology, Therapy (eds Harper, D. R., Abedon, S. T., Burrowes, B. H. & McConville, M. L.) 953-987 (Springer, 2021).
- Samson, J. E. & Moineau, S. Bacteriophages in food fermentations: new frontiers in a continuous arms race. Annu. Rev. Food Sci. Technol. 4, 347-368 (2013).
- Kumar, R., Aneja, K. R., Punia, A. K. & Roy, P. Changing pattern of biotypes, phage types and drug resistance of Salmonella typhi in Ludhiana during 1980-1999. Indian J. Med. Res 113, 175 (2001).
- Labrie, S. J., Samson, J. E. & Moineau, S. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 8, 317-327 (2010).
- Laanto, E., Hoikkala, V., Ravantti, J. & Sundberg, L. R. Long-term genomic coevolution of host-parasite interaction in the natural environment. Nat. Commun. 8, 1-8 (2017).
- Ross, A., Ward, S. & Hyman, P. More is better: selecting for broad host range bacteriophages. Front. Microbiol 7, 1352 (2016).
- Anand, T. et al. Isolation and characterization of a bacteriophage with broad host range, displaying potential in preventing bovine diarrhoea. Virus genes 51, 315-321 (2015).
- Harada, L. K. et al. Biotechnological applications of bacteriophages: State of the art. Microbiol. Res. 212, 38-58 (2018).
- Tan, C. W. et al. Isolation and characterization of six Vibrio parahaemolyticus lytic bacteriophages from seafood samples. Front. Microbiol. 12, 616548 (2021).
- Sharma, M. Lytic bacteriophages: potential interventions against enteric bacterial pathogens on produce. Bacteriophage 3, e25518 (2013).
- Abdelsattar, A. et al. Bacteriophages: from isolation to application. Curr. Pharm. Biotechnol. 23, 337-360 (2022).
- Ly-Chatain, M. H. The factors affecting effectiveness of treatment in phages therapy. Front. Microbiol. 5, 51 (2014).
- Olson, M. R., Axler, R. P. & Hicks, R. E. Effects of freezing and storage temperature on MS2 viability. J. Virol. Methods 122, 147-152 (2004).
- Tey, B. T. et al. Production of fusion m 13 phage bearing the di-sulphide constrained peptide sequence (C-WSFFSNI-C) that interacts with hepatitis B core antigen. Afr. J. Biotechnol. 8, 268-273 (2009).
- Breitbart, M., Wegley, L., Leeds, S., Schoenfeld, T. & Rohwer, F. Phage community dynamics in hot springs. Appl. Environ. Microbiol. 70, 1633-1640 (2004).
- Mocé-Llivina, L., Muniesa, M., Pimenta-Vale, H., Lucena, F. & Jofre, J. Survival of bacterial indicator species and bacteriophages after thermal treatment of sludge and sewage. Appl. Environ. Microbiol 69, 1452-1456 (2003).
- Hatch, M. T. & Warren, J. C. Enhanced recovery of airborne T3 coliphage and Pasteurella pestis bacteriophage by means of a presampling humidification technique. J. Appl. Microbiol. 17, 685-689 (1969).
- Lu et al. Bacteriophage ecology in commercial sauerkraut fermentations. Appl. Environ. Microbiol. 69, 3192-3202 (2003).
- Kerby, G. P. et al. Purification, pH stability and sedimentation properties of the T7 bacteriophage of Escherichia coli. J. Immunol. 63, 93-107 (1994).
- Wick, C. H. et al. Mass spectrometry and integrated virus detection system characterization of MS2 bacteriophage. Toxicol. Mech. Methods 17, 241-254 (2007).
- Höglund, C., Ashbolt, N., Stenström, T. A. & Svensson, L. Viral persistence in source-separated human urine. Adv. Environ. Res. 6, 265-275 (2002).
- Vinner, G. K., Vladisavljević, G. T., Clokie, M. R. & Malik, D. J. Microencapsulation of Clostridium difficile specific bacteriophages using microfluidic glass capillary devices for colon delivery using pH triggered release. PLOS ONE 12, e0186239 (2017).
- Vinner, G. K., Richards, K., Leppanen, M., Sagona, A. P. & Malik, D. J. Microencapsulation of enteric bacteriophages in a pH-responsive solid oral dosage formulation using a scalable membrane emulsification process. Pharmaceutics 11, 475 (2019).
- Chandra, M. et al. Combined effect of disinfectant and phage on the survivality of S. Typhimurium and its biofilm phenotype. Internet J. Food Saf. 17, 25-31 (2015).
- Chen, X. et al. Characterization and adsorption of a Lactobacillus plantarum virulent phage. J. Dairy Sci. 102, 3879-3886 (2019).
- Whitman, P. A. & Marshall, R. T. Characterization of two psychrophilic Pseudomonas bacteriophages isolated from ground beef. J. Appl. Microbiol 22, 463-468 (1971).
- Wichels, A. et al. Bacteriophage diversity in the North Sea. Appl. Environ. Microbiol. 64, 4128-4133 (1998).
- Hidaka, T. “On the stability of marine bacteriophages.”. Bull. Jpn. Soc. Sci. Fish. 38, 517-523 (1972).
- Seaman, P. F. & Day, M. J. Isolation and characterization of a bacteriophage with an unusually large genome from the Great Salt Plains National Wildlife Refuge, Oklahoma, USA. FEMS Microbiol. Ecol. 60, 1-13 (2007).
- Greer, G. G. Bacteriophage control of foodborne bacteria. J. Food Prot. 68, 1102-1111 (2005).
- Anand, T. et al. Phage therapy for treatment of virulent Klebsiella pneumoniae infection in a mouse model. J. Glob. Antimicrob. Resist 21, 34-41 (2020).
- Fernández, L. et al. Bacteriophages in the dairy environment: From enemies to allies. Antibiotics 6, 27 (2017).
- Kaur, R., & Sethi, N. in Emerging Modalities in Mitigation of Antimicrobial Resistance (eds Akhtar, N., Singh, K. S., Prerna & Goyal, D.) 357-74 (Springer International Publishing, 2022).
- Pecetta, S. & Rappuoli, R. Bacteriophages, a multi-tool to fight infectious disease. Med 2, 209-210 (2021).
- Azam, A. H., Tan, X. E., Veeranarayanan, S., Kiga, K. & Cui, L. Bacteriophage technology and modern medicine. Antibiotics 10, 999 (2021).
- Lin, H., Paff, M. L., Molineux, I. J. & Bull, J. J. Therapeutic application of phage capsule depolymerases against K1, K5, and K30 capsulated E. coli in mice. Front. Microbiol. 8, 2257 (2017).
- Li, J. et al. Challenges for the application of bacteriophages as effective antibacterial agents in the food industry. J. Sci. Food Agric. 102, 461-471 (2022).
- Ghosh, C., Sarkar, P., Issa, R. & Haldar, J. Alternatives to conventional antibiotics in the era of antimicrobial resistance. Trends Microbiol 27, 323-338 (2019).
- Dery, K. J., Górski, A., Miedzybrodzki, R., Farmer, D. G. & Kupiec-Weglinski, J. W. Therapeutic perspectives and mechanistic insights of phage therapy in allotransplantation. Transplantation 105, 1449-1458 (2021).
- Dabrowska, K. et al. Immunogenicity studies of proteins forming the T4 phage head surface. J. Virol. 88, 12551-12557 (2014).
- Anand, T. et al. Phage therapy in tackling AMR: potential and prospects. Indian J. Comp. Microbiol Immunol. Infect. Dis. 43, 50-57 (2022).
- Rios, A. C. et al. Structural and functional stabilization of bacteriophage particles within the aqueous core of a W/O/W multiple emulsion: a potential biotherapeutic system for the inhalational treatment of bacterial pneumonia. Process Biochem. 64, 177-192 (2018).
- Cao, Y. et al. Characterization and application of a novel Aeromonas bacteriophage as treatment for pathogenic Aeromonas hydrophila infection in rainbow trout. Aquaculture 523, 735193 (2020).
- Pan, F. Flghting Antimicrobial Resistant (Amr) Bacteria: from Bacteriophage-based Specific Capture to Controlled Killing Doctoral Dissertation, ETH Zurich (2022).
- Kutateladze, Á. & Adamia, R. Phage therapy experience at the Eliava Institute. Médecine Maladies Infectieuses 38, 426-430 (2008).
- Fiscarelli, E. V. et al. In vitro newly isolated environmental phage activity against biofilms preformed by Pseudomonas aeruginosa from patients with cystic fibrosis. Microorganisms 9, 478 (2021).
- Morello, E. et al. Pulmonary bacteriophage therapy on Pseudomonas aeruginosa cystic fibrosis strains: first steps towards treatment and prevention. PLoS ONE 6, e16963 (2011).
- Roach, D. R. et al. Synergy between the host immune system and bacteriophage is essential for successful phage therapy against an acute respiratory pathogen. Cell Host Microbe 22, 38-47 (2017).
- Semler, D. D., Goudie, A. D., Finlay, W. H. & Dennis, J. J. Aerosol phage therapy efficacy in Burkholderia cepacia complex respiratory infections. Antimicrob. Agents Chemother. 58, 4005-4013 (2014).
- Kwiatek, M., Parasion, S. & Nakonieczna, A. Therapeutic bacteriophages as a rescue treatment for drug-resistant infections-an in vivo studies overview. J. Appl. Microbiol. 128, 985-1002 (2020).
- Oliver, K. M., Degnan, P. H., Hunter, M. S. & Moran, N. A. Bacteriophages encode factors required for protection in a symbiotic mutualism. Science 325, 992-994 (2009).
- CDC. Antimicrobial Resistance Threat Report. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Washington, DC (2013).
- Abdeltawab, A. A., El-Nahas, E. M., Askora, A. A. & Abdelaziz, H. S. Bacteriological characterization of Salmonella species isolated from laying ducks. Benha Med. J. 34, 404-412 (2018).
- Esmael, A. et al. Isolation and characterization of two lytic bacteriophages infecting a multi-drug resistant Salmonella Typhimurium and their efficacy to combat salmonellosis in ready-to-use foods. Microorganisms 9, 423 (2021).
- Garcia, R. et al. Bacteriophage production models: an overview. Front. Microbiol. 10, 1187 (2019).
- Bhandare, S., & Goodridge, L. in Bacteriophages: Biology, Technology, Therapy (eds Harper, D. R., Abedon, S. T., Burrowes, B. H. & McConville, M. L.) 769-788 (Springer, 2021).
- Rodríguez-Rubio, L. et al. Listeriaphages and coagulin C23 act synergistically to kill Listeria monocytogenes in milk under refrigeration conditions. Int. J. Food Microbiol. 205, 68-72 (2015).
- López-Cuevas, O., Medrano-Félix, J. A., Castro-Del Campo, N. & Chaidez, C. Bacteriophage applications for fresh produce food safety. Int. J. Environ. Health Res. 31, 687-702 (2021).
- Kuek, M., McLean, S. K. & Palombo, E. A. Application of bacteriophages in food production and their potential as biocontrol agents in the organic farming industry. Biol. Control 165, 104817 (2022).
- Sillankorva, S. M., Oliveira, H., & Azeredo, J. Bacteriophages and their role in food safety. Int. J. Microbiol. 2012, 863945 (2012).
- Jun, J. W. et al. Bacteriophage application to control the contaminated water with Shigella. Sci. Rep. 6, 1-7 (2016).
- Prashantha, S. T., Yadav, J., Sunilkumar, V. P., & HP, N. P. The Variability and Mechanisms of Infection by Gram-Positive, Plant Associated Bacteria. International Year of Millets 2023, 51 (2023).
- Kaptchouang Tchatchouang, C. D. et al. Listeriosis outbreak in South Africa: a comparative analysis with previously reported cases worldwide. Microorganisms 8, 135 (2020).
- Kawacka, I., Olejnik-Schmidt, A., Schmidt, M. & Sip, A. Effectiveness of phagebased inhibition of Listeria monocytogenes in food products and food processing environments. Microorganisms 8, 1764 (2020).
- Leverentz, B., Conway, W. S., Janisiewicz, W. & Camp, M. J. Optimizing concentration and timing of a phage spray application to reduce Listeria monocytogenes on honeydew melon tissue. J. Food Prot. 67, 1682-1686 (2004).
- Komora, N. et al. Non-thermal approach to Listeria monocytogenes inactivation in milk: the combined effect of high pressure, pediocin PA-1 and bacteriophage P100. Food Microbiol. 86, 103315 (2020).
- Komora, N. et al. Innovative hurdle system towards Listeria monocytogenes inactivation in a fermented meat sausage model-high pressure processing assisted by bacteriophage P100 and bacteriocinogenic Pediococcus acidilactici. Food Res. Int. 148, 110628 (2021).
- Stefani, E. et al. Bacteriophage-mediated control of phytopathogenic xanthomonads: A promising green solution for the future. Microorganisms 9, 1056 (2021).
- Schwarczinger, I. et al. Characterization of Myoviridae and Podoviridae family bacteriophages of Erwinia amylovora from Hungary-potential of application in Biol. Control of fire blight. Eur. J. Plant Pathol. 149, 639-652 (2017).
- Kizheva, Y. et al. Broad host range bacteriophages found in rhizosphere soil of a healthy tomato plant in Bulgaria. Heliyon 7, e07084 (2021).
- Clavijo, V. et al. Phage cocktail SalmoFREE
reduces Salmonella on a commercial broiler farm. Poult. Sci. J. 98, 5054-5063 (2019). - Firlieyanti, A. S., Connerton, P. L. & Connerton, I. F. Campylobacters and their bacteriophages from chicken liver: the prospect for phage biocontrol. Int. J. Food Microbiol. 237, 121-127 (2016).
- Leverentz, B. et al. Examination of bacteriophage as a biocontrol method for Salmonella on fresh-cut fruit: a model study. J. Food Prot. 64, 1116-1121 (2001).
- Patel, D., Zhou, Y. & Ramasamy, R. P. A bacteriophage-based electrochemical biosensor for detection of methicillin-resistant Staphylococcus aureus. J. Electrochem. Soc. 168, 057523 (2021).
- Janczuk, M. et al. Bacteriophage-based bioconjugates as a flow cytometry probe for fast bacteria detection. Bioconjug. Chem. 28, 419-425 (2017).
- Paczesny, J., Wdowiak, M., & Ochirbat, E. in Nanotechnology for Infectious Diseases 439-473 (Springer, 2022)
- Pierce, C. L., Rees, J. C., & Barr, J. R. in Bacteriophages: Biology, Technology, Therapy (eds Harper, D. R., Abedon, S. T., Burrowes, B. H. & McConville, M. L.) 645-656 (2021).
- Srivastava, K. R., Awasthi, S., Mishra, P. K., & Srivastava, P. K. in Waterborne Pathogens (eds Prasad, M. N. V. & Grobelak, A.) 237-277 (Banaras Hindu University, 2020)
- Farooq, U., Yang, Q., Ullah, M. W. & Wang, S. Bacterial biosensing: recent advances in phage-based bioassays and biosensors. Biosens. Bioelectron. 118, 204-216 (2018).
- Anand, T. et al. Phage display technique as a tool for diagnosis and antibody selection for coronaviruses. Curr. Microbiol. 78, 1124-1134 (2021).
- Nachimuthu, R., Royam, M. M., Manohar, P. & Leptihn, S. Application of bacteriophages and endolysins in aquaculture as a biocontrol measure. Biol. Control 160, 104678 (2021).
- Lai, W. C. B., Chen, X., Ho, M. K. Y., Xia, J. & Leung, S. S. Y. Bacteriophage-derived endolysins to target gram-negative bacteria. Int. J. Pharm. 589, 119833 (2020).
- Huang, Z. et al. Phages and their lysins: Toolkits in the battle against foodborne pathogens in the post-antibiotic era. Compr. Rev. Food Sci. 20, 3319-3343 (2021).
- Schmelcher, M. & Loessner, M. J. Bacteriophage endolysins: applications for food safety. Curr. Opin. Biotechnol. 37, 76-87 (2016).
- Bruttin, A. & Brussow, H. Human volunteers receiving Escherichia coli phage T4 orally: a safety test of phage therapy. Antimicrob. Agents Chemother. 49, 2874-2878 (2005).
- Alves, D. R. Combined use of bacteriophage K and a novel bacteriophage to reduce Staphylococcus aureus biofilm formation. Appl. Environ. Microbiol. 80, 6694-6703 (2014).
- Viazis, S., Akhtar, M., Feirtag, J. & Diez-Gonzalez, F. Reduction of Escherichia coli O157: H7 viability on leafy green vegetables by treatment with a bacteriophage mixture and trans-cinnamaldehyde. Food microbiol 28, 149-157 (2011).
- Soni, K. A. & Nannapaneni, R. Removal of Listeria monocytogenes biofilms with bacteriophage P100. J. Food Prot. 73, 1519-1524 (2010).
- Patel, T. R. & Jackman, D. M. Susceptibility of psychrotrophic pseudomonads of milk origin to psychrotrophic bacteriophages. Appl. Environ. Microbiol. 51, 446-448 (1986).
- Goode, D., Allen, V. M. & Barrow, P. A. Reduction of experimental Salmonella and Campylobacter contamination of chicken skin by application of lytic bacteriophages. Appl. Environ. Microbiol. 69, 5032-5036 (2003).
- Fernández, L., Duarte, A. C., Rodríguez, A. & García, P. The relationship between the phageome and human health: are bacteriophages beneficial or harmful microbes? Benef. Microbes 12, 107-120 (2021).
- Dalmasso, M., Hill, C. & Ross, R. P. Exploiting gut bacteriophages for human health. Trends Microbiol 22, 399-405 (2014).
- Mirzaei, M. K. & Maurice, C. F. Ménage à trois in the human gut: interactions between host, bacteria and phages. Nat. Rev. Microbiol. 15, 397-408 (2017).
- Kutter, E. et al. Phage therapy in clinical practice: treatment of human infections. Curr. Pharm. Biotechnol. 11, 69-86 (2010).
- Merabishvili, M. et al. Selection and characterization of a candidate therapeutic bacteriophage that lyses the Escherichia coli O104: H4 strain from the 2011 outbreak in Germany. PLoS ONE 7, e52709 (2012).
- Siringan, P., Connerton, P. L., Payne, R. J. & Connerton, I. F. Bacteriophagemediated dispersal of Campylobacter jejuni biofilms. Appl. Environ. Microbiol. 77, 3320-3326 (2011).
- Łusiak-Szelachowska, M., Weber-Dabrowska, B. & Górski, A. Bacteriophages and lysins in biofilm control. Virol. Sin. 35, 125-133 (2020).
- Stanford, K. et al. Oral delivery systems for encapsulated bacteriophages targeted at Escherichia coli O157: H7 in feedlot cattle. J. Food Prot. 73, 1304-1312 (2010).
- Liu, M. et al. Comparative genomics of Acinetobacter baumannii and therapeutic bacteriophages from a patient undergoing phage therapy. Nat. Commun. 13, 3776 (2022).
- Abedon, S. T., García, P., Mullany, P. & Aminov, R. Phage therapy: past, present and future. Front. Microbiol. 8, 981 (2017).
- Pires, D. P., Oliveira, H., Melo, L. D., Sillankorva, S. & Azeredo, J. Bacteriophageencoded depolymerases: their diversity and biotechnological applications. Appl. Microbiol. Biotechnol. 100, 2141-2151 (2016).
- Ferriol-González, C. & Domingo-Calap, P. Phages for biofilm removal. Antibiotics 9, 268 (2020).
- Strathdee, S. A., Hatfull, G. F., Mutalik, V. K. & Schooley, R. T. Phage therapy: from biological mechanisms to future directions. Cell 186, 17-31 (2023).
- Drulis-Kawa, Z., Majkowska-Skrobek, G. & Maciejewska, B. Bacteriophages and phage-derived proteins-application approaches. Curr. Medicinal Chem. 22, 1757-1773 (2015).
- Monjezi, R., Tey, B. T., Sieo, C. C. & Tan, W. S. Purification of bacteriophage M13 by anion exchange chromatography. J. Chromatogr. B 878, 1855-1859 (2010).
- Henein, A. What are the limitations on the wider therapeutic use of phage? Bacteriophage 3, e24872 (2013).
- Marcó, M. B., Moineau, S. & Quiberoni, A. Bacteriophages and dairy fermentations. Bacteriophage 2, 149-158 (2012).
- Maiques, E. et al. Role of staphylococcal phage and SaPI integrase in intra-and interspecies SaPI transfer. J. Bacteriol. 189, 5608-5616 (2007).
- Anand, T. et al. Abundance of antibiotic resistance genes in environmental bacteriophages. J. G. Virol. 97, 3458-3466 (2016).
- Hemme, T., Uddin, M. M. & Ndambi, O. A. Benchmarking cost of milk production in 46 countries. Glob. Econ. Rev. 3, 254-270 (2014).
- Seed, K. D. Battling phages: how bacteria defend against viral attack. PLoS Pathog. 11, e1004847 (2015).
- Drulis-Kawa, Z., Majkowska-Skrobek, G., Maciejewska, B., Delattre, A. S. & Lavigne, R. Learning from bacteriophages-advantages and limitations of phage and phage-encoded protein applications. Curr. Protein Pept. Sci. 13, 699-722 (2012).
- Seed, K. D., Lazinski, D. W., Calderwood, S. B. & Camilli, A. A bacteriophage encodes its own CRISPR/Cas adaptive response to evade host innate immunity. Nature 494, 489-491 (2013).
- Verbeken, G. et al. Optimizing the European regulatory framework for sustainable bacteriophage therapy in human medicine. Arch. Immunol. Therapiae Experimentalis 60, 161-172 (2012).
- Fontaine, N., & Reynders, D. Directive 2001/83/EC of the European Parliament and of the Council of 6 November. on the Community code relating to medicinal products for human use. Official J. Eur. Communities L 311, 67-128 (2001).
- Międzybrodzki, R. et al. in Bacteriophages: Biology, Technology, Therapy (eds Harper, D. R., Abedon, S. T., Burrowes, B. H. & McConville, M. L.) 921-951 (2021).
- Żaczek, M. et al. A thorough synthesis of phage therapy unit activity in Polandits history, milestones and international recognition. Viruses 14, 1170 (2022).
- Hartmann, M. & Hartmann-Vareilles, F. The clinical trials directive: how is it affecting Europe’s noncommercial research. PLoS Clin. trials 1, e13 (2006).
- Naureen, Z. et al. Comparison between American and European legislation in the therapeutical and alimentary bacteriophage usage. Acta Bio Medica: Atenei Parmensis 91 (2020).
- Jones, J. D., Trippett, C., Suleman, M., Clokie, M. R. & Clark, J. R. The future of clinical phage therapy in the United Kingdom. Viruses 15, 721 (2023).
- Lin, R. C., Fabijan, A. P., Attwood, L., & Iredell, J. State of the regulatory affair: Regulation of phage therapy in Australia (2019). Available at: https:// phage.directory/capsid/phage-therapy-regulation-australia.
- Furfaro, L. L., Payne, M. S. & Chang, B. J. Bacteriophage therapy: clinical trials and regulatory hurdles. Front. Cell. Infect. Microbiol. 8, 376 (2018).
- Johri, P. Antimicrobial resistance and phage therapy in India. The Microbiologist (2023). Available at: https://www.the-microbiologist.com/features/antimicrobial-resistance-and-phage-therapy-in-india/1386.article.
- eCFR, 71 FR 47731, Aug. 18, 2006, as amended at 81 FR v5591, Feb. 3, 2016; 88 FR 17720, Mar. 24, 2023 Available at: https://www.ecfr.gov/current/title-21/ chapter-I/subchapter-B/part-172/subpart-H/section-172.785.
- Wang, Z. & Zhao, X. The application and research progress of bacteriophages in food safety. J. Appl. Microbiol. 133, 2137-2147 (2022).
- Prada-Peñaranda, C. Phage preparation FBL1 prevents Bacillus licheniformis biofilm, bacterium responsible for the mortality of the Pacific White Shrimp Litopenaeus vannamei. Aquaculture 484, 160-167 (2018).
- Tomat, D., Casabonne, C., Aquili, V., Balagué, C. & Quiberoni, A. Evaluation of a novel cocktail of six lytic bacteriophages against Shiga toxin-producing Escherichia coli in broth, milk and meat. Food Microbiol. 76, 434-442 (2018).
- Wang, L. et al. Use of bacteriophages to control Escherichia coli O157: H7 in domestic ruminants, meat products, and fruits and vegetables. Foodborne Pathog. Dis. 14, 483-493 (2017).
- Arachchi, G. G. et al. Characteristics of three listeriaphages isolated from New Zealand seafood environments. J. Appl. Microbiol. 115, 1427-1438 (2013).
- Gutiérrez, D., Rodríguez-Rubio, L., Martínez, B., Rodríguez, A. & García, P. Bacteriophages as weapons against bacterial biofilms in the food industry. Front. Microbiol. 7, 825 (2016).
- Tahir, A., Asif, M., Abbas, Z. & Rehman, S. U. Three bacteriophages SA, SA2 and SNAF can control growth of milk isolated Staphylococcal species. Pak. J. Zool. 49, 425-759 (2017).
ACKNOWLEDGEMENTS
AUTHOR CONTRIBUTIONS
COMPETING INTERESTS
ETHICAL APPROVAL
ADDITIONAL INFORMATION
© The Author(s) 2024, corrected publication 2024
Dairy Microbiology Division, ICAR-National Dairy Research Institute, Karnal 132001, India. Department of Clinical Nutrition, College of Applied Medical Science, Jazan University, Jazan 45142, Saudi Arabia. Department of Nutrition Biology, School of Interdisciplinary and Applied Sciences, Central University of Haryana, Mahendergarh 123031, India. ICAR-National Research Centre on Equines, Sirsa Road, Hisar 125001, India. University Institute of Biotechnology, Chandigarh University, Sahibzada Ajit Singh Nagar 140413, India. Microbiology Department, VCSG Government Institute of Medical Science and Research, Ganganali Srikot, Srinagar Pauri Garhwal 246174, India. Centre of One Health, College of Veterinary Science, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana, India. Paul Hebert Centre for DNA Barcoding and Biodiversity Studies, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, India. CBIOS (Research Center for Biosciences and Health Technologies), Universidade Lusófona de Humanidades e Tecnologias, Campo Grande 376, 1749-024 Lisboa, Portugal. College of Hospitality and Tourism Management, Sejong University, 98 Gunja-Dong, Gwanjin-gu, Seoul 143-747, Republic of Korea. email: antonio.raposo@ulusofona.pt; heesup.han@gmail.com; akpuniya@gmail.com; Anil.Puniya@icar.gov.in
