DOI: https://doi.org/10.1038/s41467-024-45457-y
PMID: https://pubmed.ncbi.nlm.nih.gov/38341421
تاريخ النشر: 2024-02-10
الروابط القوية في الأطر العضوية التساهمية النشطة ضوئيًا تمكّن التفاعلات الضوئية الفعالة تحت ظروف قاسية
تاريخ القبول: 25 يناير 2024
تاريخ النشر على الإنترنت: 10 فبراير 2024
(أ) تحقق من التحديثات
الملخص
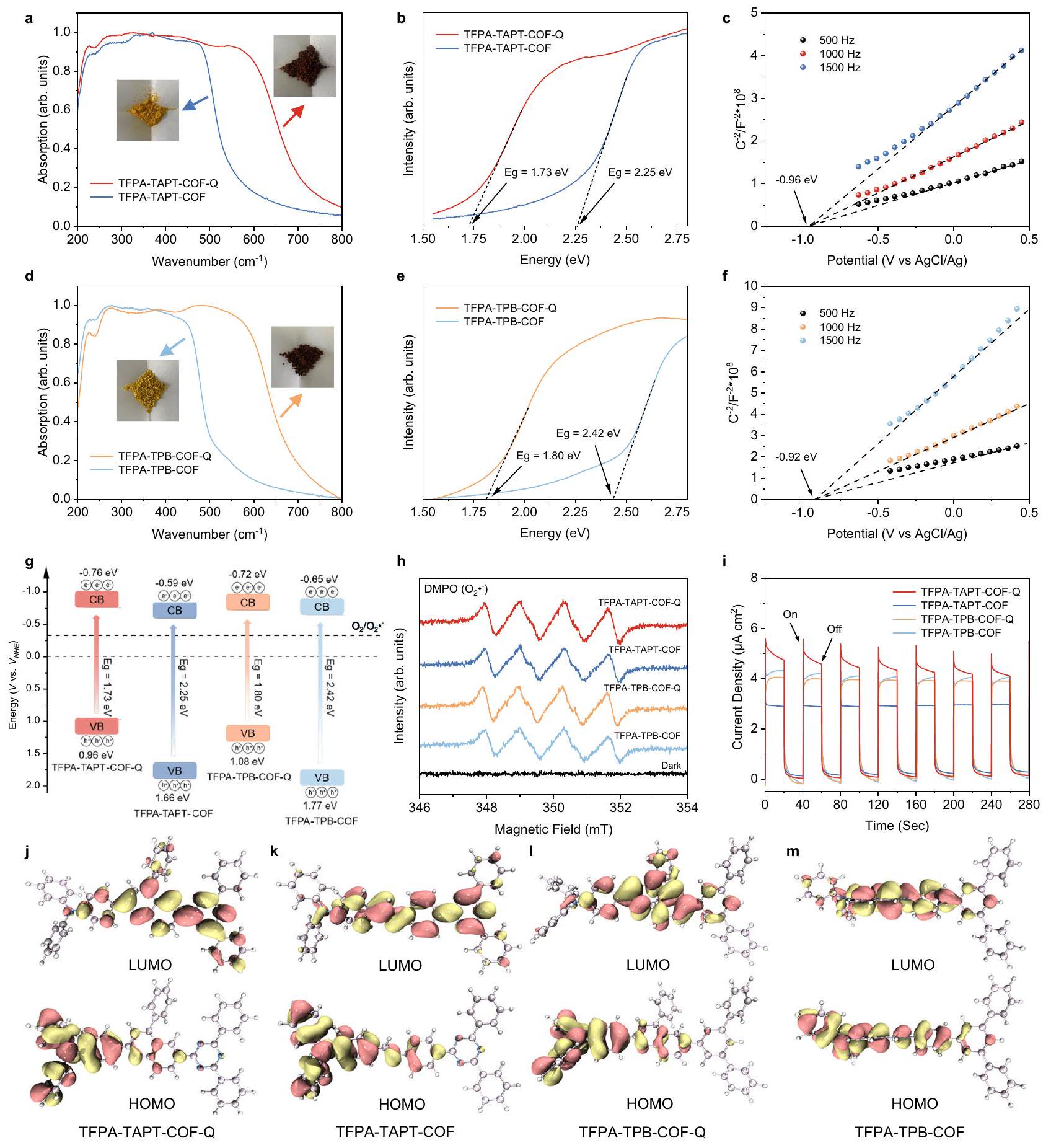
تطوير المحفزات الضوئية غير المتجانسة للتطبيقات في ظروف قاسية هو أمر ذو أهمية عالية ولكنه تحدي. هنا، من خلال تحويل الروابط الأمينية إلى مجموعات كينولين في الأطر العضوية التساهمية (COFs) المدمجة مع ثلاثي الفينيل أمين، تم بناء إطارين ضوئيين، وهما TFPA-TAPT-COF-Q و TFPA-TPB-COF-Q، بنجاح. الأطر المرتبطة بكينولين المعتمدة تظهر استقرارًا محسّنًا ونشاطًا ضوئيًا، مما يجعلها محفزات ضوئية مناسبة للتفاعلات الضوئية تحت ظروف قاسية، كما تم التحقق من ذلك من خلال التفاعلات الضوئية القابلة لإعادة الاستخدام للأحماض العضوية التي تشمل إزالة الكربوكسيل المؤكسد وقاعدة عضوية تشمل اقتران بنزيل أمين. تحت ظروف مؤكسدة قوية، تظهر الأطر المرتبطة بكينولين كفاءة عالية تصل إلى
بوليمرات عضوية بلورية مسامية تتكون من مكونات عضوية مرتبطة دوريًا بواسطة روابط تساهمية عضوية، مما منحها قابلية التعديل الكيميائي والوظائف من خلال تزيين إما الوحدات العضوية أو الروابط التساهمية. من خلال أخذ المركبات العضوية ذات الخصائص الحساسة للضوء كوحدات عضوية، يمكن عادةً الحصول على الأطر العضوية النشطة ضوئيًا، والتي تظهر آفاقًا مشرقة في التحفيز الضوئي غير المتجانس
الأطر التي تم تصنيعها يمكن أن تحفز بشكل فعال توليد أيون الجذري الفائق الأكسيد (
النتائج
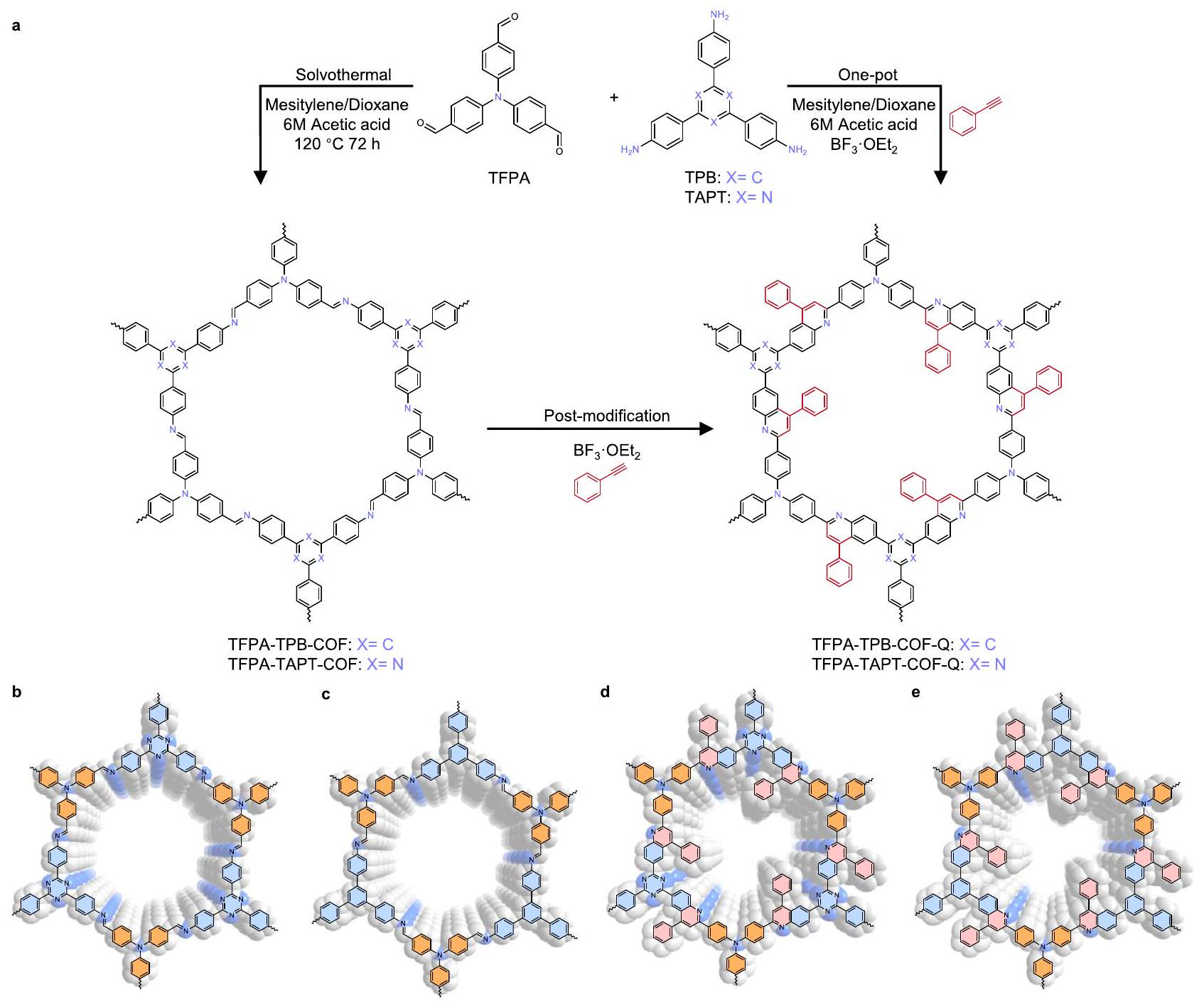
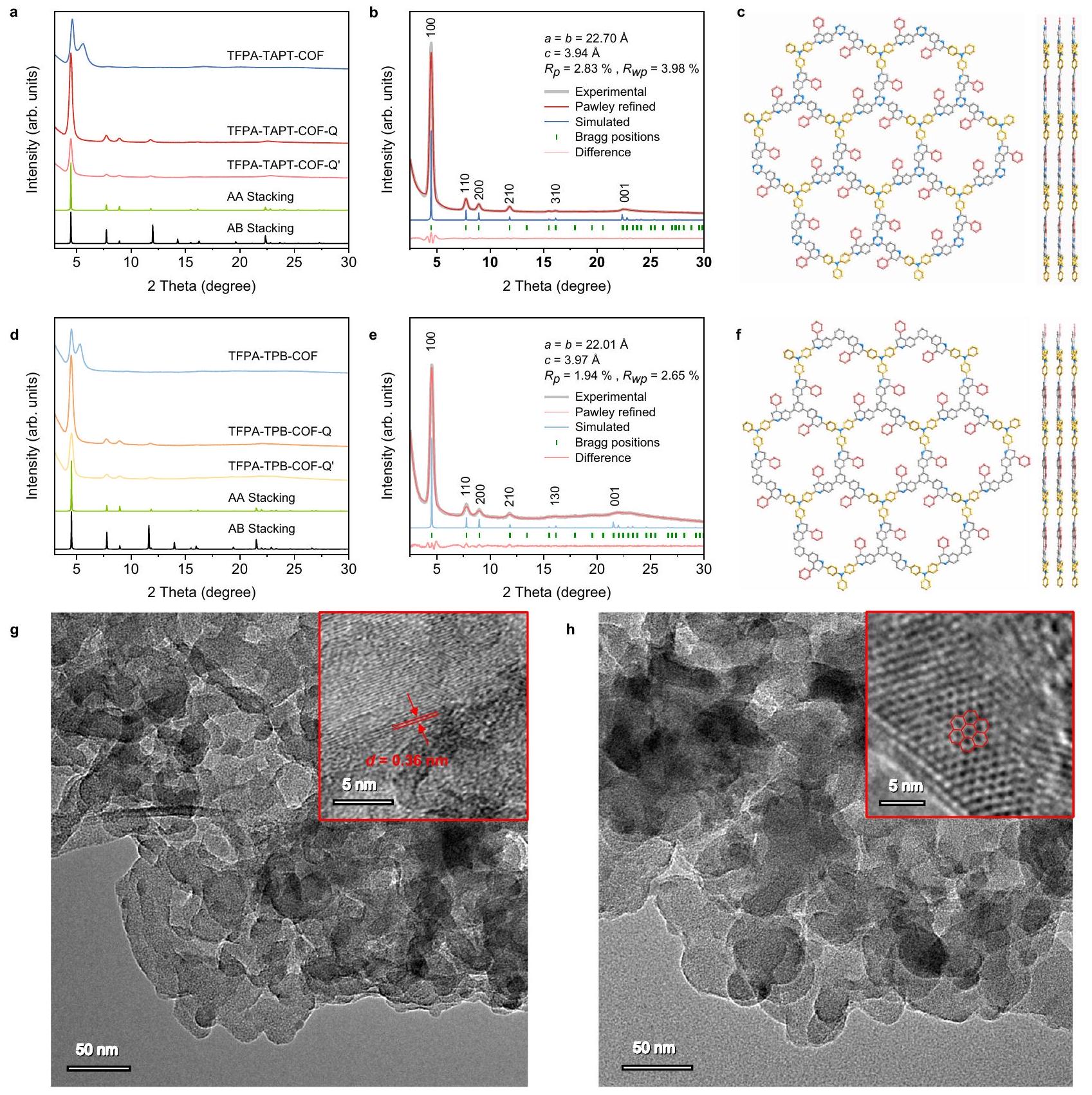
لذلك، يتم استخدام TFPA-TAPT-COF-Q الذي تم تصنيعه بطريقة الوعاء الواحد للتحليلات الهيكلية التالية وتوصيف الأداء التحفيزي. استنادًا إلى هيكل TFPA-TAPT-COF المرتبط بالإيمين، فإن إطارًا ثنائي الأبعاد (2D) مرتبطًا بالكينولين ممكن.
تحويل روابط الإيمين إلى حلقة الكوينولين خلال تفاعلات التعديل
طاقة الربط عند
مرتبط TFPA-TAPT-COF-Q يقلل من طاقة الربط لـ
تم حسابها بواسطة طريقة بروناور-إيميت-تيلر (BET) هي 1058.99 و
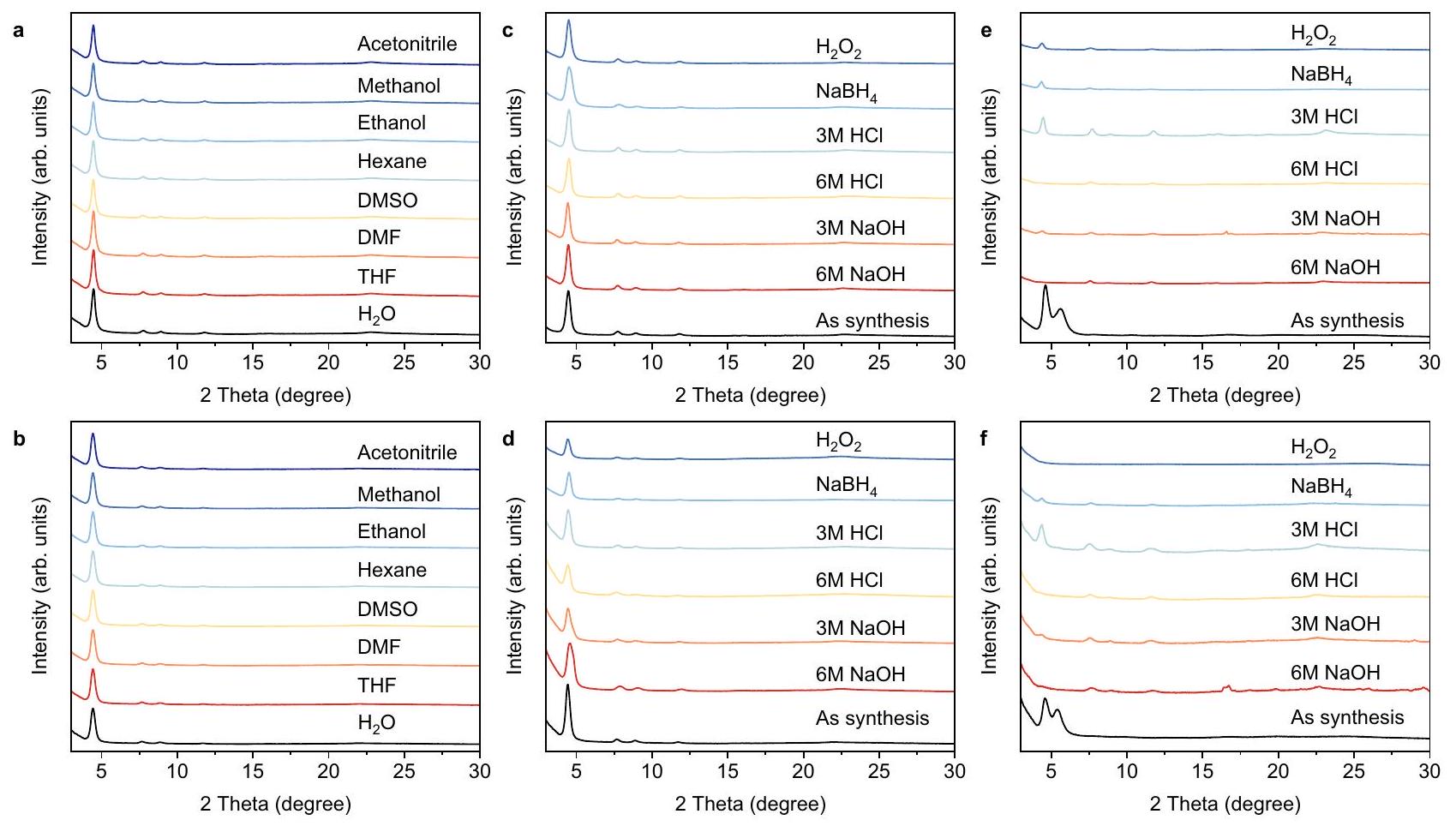
بعد نقعها في تركيزات مختلفة من الأحماض والقواعد،
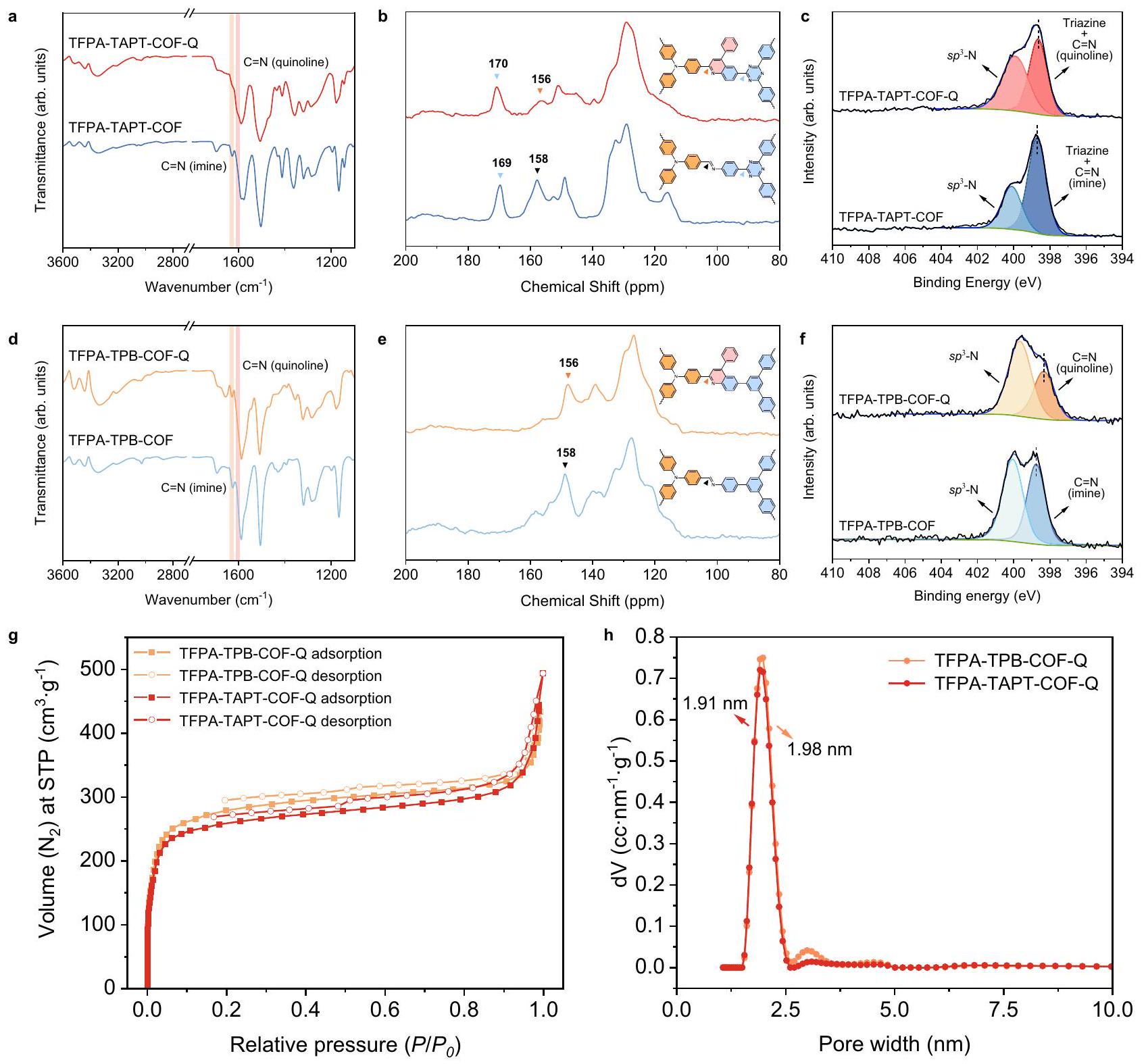
أنماط COFs المرتبطة بالإيمين بعد الغمر في محاليل الكحول التي تحتوي على عوامل مؤكسدة وعوامل مختزلة (الشكل 4e، f). كشفت التحقيقات في الاستقرار أنه بعد تحويل الروابط الإيمينية إلى روابط كينولين، يمكن لـ TFPA-TAPT-COF-Q و TFPA-TPB-COF-Q الحفاظ على استقرارها الكيميائي بشكل جيد في المحاليل الحمضية والقاعدية القاسية، وكذلك في محاليل العوامل المؤكسدة والمختزلة.
قوة للاختزال الضوئي المساعد لـ
تؤدي إلى خصائص ضوئية كيميائية، مما يوضح أن COFs المرتبطة بالكينولين التي تم تصنيعها يجب أن تكون محفزات ضوئية فعالة للأكسدة الهوائية. نظرًا لأن COFs المرتبطة بالكينولين تمتلك خصائص استجابة ضوئية أعلى، تم بعد ذلك دراسة أكسدة الأكسجين الهوائية المحفزة ضوئيًا. الأكسدة الضوئية للكبريتيد كعملية نموذجية من خلال توليد…
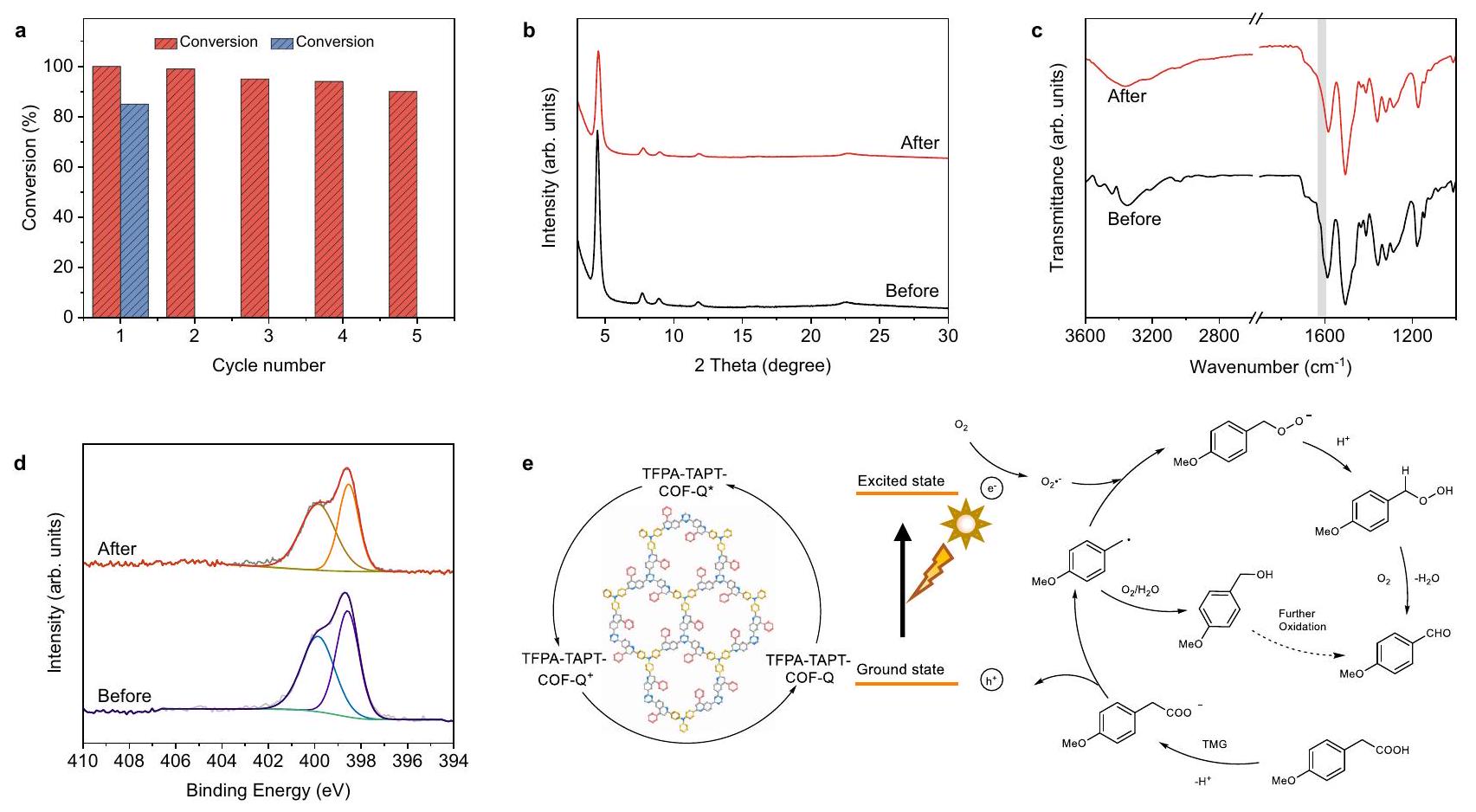
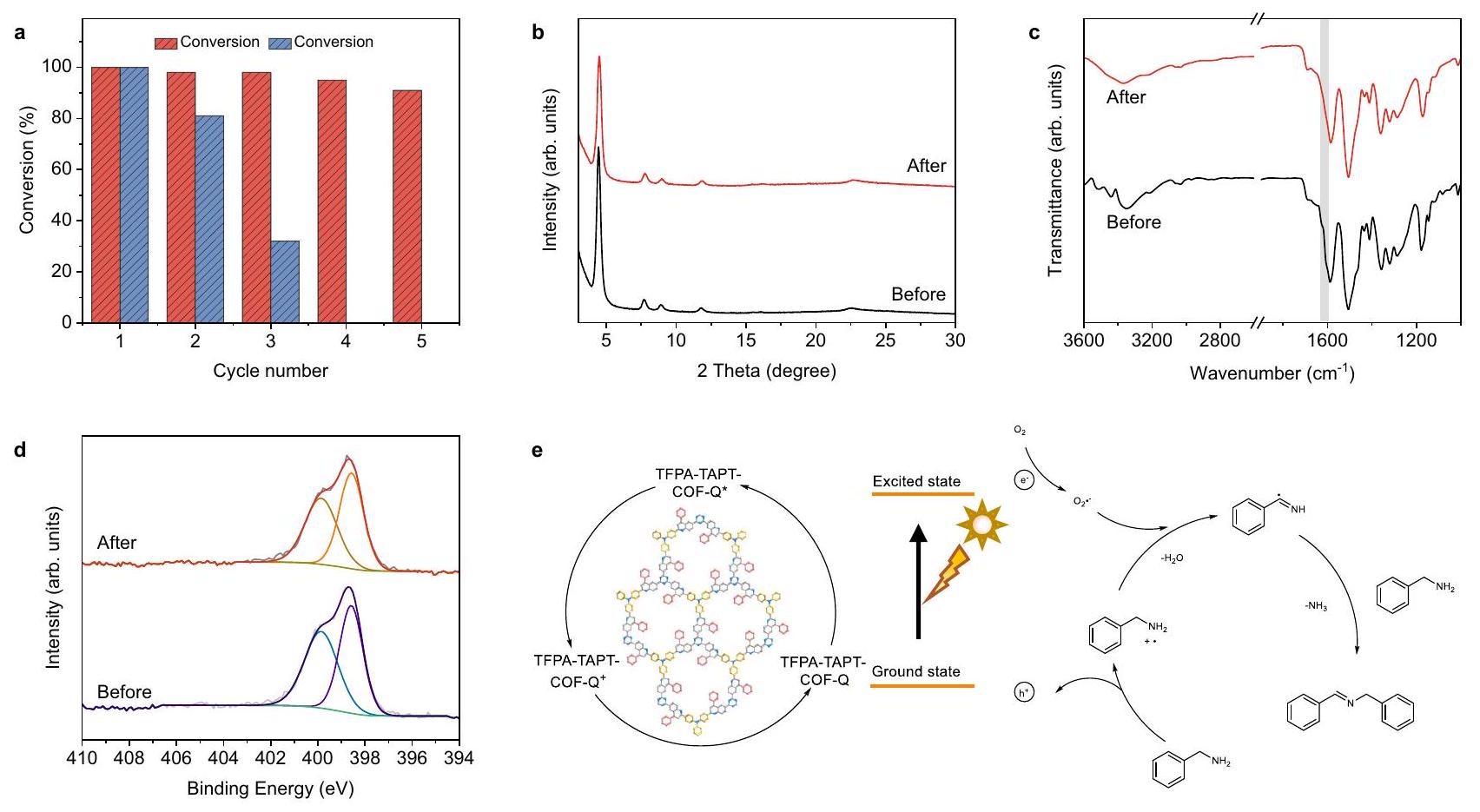
تمت ملاحظة نفس النتائج كما في الدورة الأولى من التفاعل في جميع خمس جولات إعادة التدوير على الرغم من أن نشاطها الضوئي ليس مرتفعًا مثل نشاط TFPA-TAPT-COF-Q (الأشكال التكميلية 46-49). أظهرت هذه النتائج أن COFs المرتبطة بالكينولين، TFPA-TAPT-COF-Q و TFPA-TPB-COF-Q، يمكن استخدامها كعوامل تحفيز ضوئي غير متجانسة قابلة لإعادة التدوير بشكل فعال في ظروف الأحماض العضوية القاسية نسبيًا.
| دخول | ركيزة |
|
TFPA-TAPT-COF اختيار التحويل (%) | ||||
| 1 | 100 | 86 | 85 | 76 | |||
| 2 | 78 | 81 | 72 | 78 | |||
| ٣ | 77 | ٨٠ | ٤٤ | 77 | |||
| ٤ | 64 | 84 | ٥٦ | 75 | |||
| ٥ | 83 | 92 | ٣٦ | 77 | |||
| ٦ | 85 | 92 | 70 | 82 | |||
| ٧ | 100 | 84 | 100 | 84 | |||
النشاط الضوئي التحفيزي خلال الدورات الخمس من التفاعلات (الشكل 7أ)، بينما تم الكشف عن فقدان كبير في الكتلة لمسحوق TFPA-TAPT-COF المستعاد بعد كل دورة، والذي لا يمكن استعادته بعد الدورة الثالثة من التفاعل (الشكل التكميلي 53). أظهرت قياسات PXRD على العينة المستعادة من TFPA-TAPT-COF-Q أن القمم الرئيسية لا تزال محفوظة بعد خمس دورات من التفاعلات (الشكل 7ب).
تم تحضير COFs المرتبطة بالكينولين تحت ظروف أكسدة قوية لإنتاج ضوئي لـ
 |
||||
| مدخل | ||||
| ركيزة |
|
TFPA-TAPT-COF تحويل (%) | ||
| 1 | 100 | 100 | ||
| 2 | 98 | 96 | ||
| ٣ | 100 | 100 | ||
| ٤ | 65 | 53 | ||
| ٥ | 81 | 70 | ||
| ٦ | 96 | 81 | ||
| ٧ | 85 | 81 | ||
مع TFPA-TAPT-COF-Q و TFPA-TPB-COF-Q كعوامل تحفيز ضوئي يمكن أن تزيد بشكل مستمر حتى
نقاش
طرق
تركيب TAPA-TAPT-COF-Q
تركيب TAPA-TAPT-COF-Q’
تفاعل في
تحضير TAPA-TAPT-COF-Q على مقياس جرام
تركيب TFPA-TAPT-COF
تركيب TAPA-TPB-COF-Q
تركيب TAPA-TPB-COF-Q’
تركيب TFPA-TPB-COF
توفر البيانات
References
- Balaraman, E., Khaskin, E., Leitus, G. & Milstein, D. Catalytic transformation of alcohols to carboxylic acid salts and
using water as the oxygen atom source. Nat. Chem. 5, 122-125 (2013). - Huang, Z. et al. Chemical recycling of polystyrene to valuable chemicals via selective acid-catalyzed aerobic oxidation under visible light. J. Am. Chem. Soc. 144, 6532-6542 (2022).
- Dai, Z. et al. Crystal defect engineering of aurivillius
by Ce doping for increased reactive species production in photocatalysis. ACS Catal. 6, 3180-3192 (2016). - Pan, L. et al. Manipulating spin polarization of titanium dioxide for efficient photocatalysis. Nat. Commun. 11, 418 (2020).
- Duan, Y., Waerenborgh, J. C., Clemente-Juan, J. M., Gimenez-Saiz, C. & Coronado, E. Light-induced decarboxylation in a photoresponsive iron-containing complex based on polyoxometalate and oxalato ligands. Chem. Sci. 8, 305-315 (2017).
- Yu, B., Zhang, S. & Wang, X. Helical microporous nanorods assembled by polyoxometalate clusters for the photocatalytic oxidation of toluene. Angew. Chem. Int. Ed. 60, 17404-17409 (2021).
- Bagal, D. B. et al. Trifluoromethylchlorosulfonylation of alkenes: Evidence for an inner-sphere mechanism by a copper phenanthroline photoredox catalyst. Angew. Chem. Int. Ed. 54, 6999-7002 (2015).
- Wenger, O. S. Photoactive complexes with earth-abundant metals. J. Am. Chem. Soc. 140, 13522-13533 (2018).
- Shi, D. et al. Merging of the photocatalysis and copper catalysis in metal-organic frameworks for oxidative
bond formation. Chem. Sci. 6, 1035-1042 (2015). - Côté, A. P. et al. Porous, crystalline, covalent organic frameworks. J. Am. Chem. Soc. 310, 1166-1170 (2005).
- Wang, S. et al. Ultrathin ionic COF membrane via polyelectrolytemediated assembly for efficient
separation. Adv. Funct. Mater. 33, 2300386 (2023). - Li, H., Zhang, D., Cheng, K., Li, Z. & Li, P.-Z. Effective iodine adsorption by nitrogen-rich nanoporous covalent organic frameworks. ACS Appl. Nano Mater. 6, 1295-1302 (2023).
- Cheng, K., Li, H., Wang, J.-R., Li, P.-Z. & Zhao, Y. From supramolecular organic cages to porous covalent organic frameworks for enhancing iodine adsorption capability by fully exposed nitrogenrich sites. Small 19, 2301998 (2023).
- Cheng, K., Li, H., Li, Z., Li, P.-Z. & Zhao, Y. Linking nitrogen-rich organic cages into isoreticular covalent organic frameworks for enhancing iodine adsorption capability. ACS Mater. Lett. 5, 1546-1555 (2023).
- Wang, X. et al. Homochiral 2D porous covalent organic frameworks for heterogeneous asymmetric catalysis. J. Am. Chem. Soc. 138, 12332-12335 (2016).
- Sun, J. et al. Pyrene-based covalent organic frameworks for photocatalytic hydrogen peroxide production. Angew. Chem. Int. Ed. 62, e202216719 (2023).
- Wang, S. et al. Programming covalent organic frameworks for photocatalysis: Investigation of chemical and structural variations. Matter 2, 416-427 (2020).
- Zhi, Q. et al. Piperazine-linked metalphthalocyanine frameworks for highly efficient visible-light-driven
photosynthesis. J. Am. Chem. Soc. 144, 21328-21336 (2022). - Zou, X.-N. et al. Incorporating photochromic triphenylamine into a zirconium-organic framework for highly effective photocatalytic aerobic oxidation of sulfides. ACS Appl. Mater. Interfaces 13, 20137-20144 (2021).
- Zhang, D. et al. Highly effective photocatalytic radical reactions triggered by a photoactive metal-organic framework. ACS Appl. Mater. Interfaces 14, 23518-23526 (2022).
- Li, Y. et al. Effective photocatalytic initiation of reactive oxygen species by a photoactive covalent organic framework for oxidation reactions. ACS Mater. Lett. 4, 1160-1167 (2022).
- Luan, T.-X. et al. Highly enhancing
photoreduction by metallization of an imidazole-linked robust covalent organic framework. Small 19, 2303324 (2023). - Yu, S.-Y. et al. Direct synthesis of a covalent triazine-based framework from aromatic amides. Angew. Chem. Int. Ed. 57, 8438-8442 (2018).
- Lin, Y. et al. A covalent organic framework as a long-life and highrate anode suitable for both aqueous acidic and alkaline batteries. Angew. Chem. Int. Ed. 62, e202218745 (2023).
- Luan, T.-X. et al. Highly effective generation of singlet oxygen by an imidazole-linked robust photosensitizing covalent organic framework. ACS Nano 16, 21565-21575 (2022).
- Zhuang, X. et al. A two-dimensional conjugated polymer framework with fully
-bonded carbon skeleton. Polym. Chem. 7, 4176-4181 (2016). - Shi, J.-L., Chen, R., Hao, H., Wang, C. & Lang, X. 2D sp² carbonconjugated porphyrin covalent organic framework for cooperative photocatalysis with TEMPO. Angew. Chem. Int. Ed. 59, 9088-9093 (2020).
- Haase, F. et al. Topochemical conversion of an imine- into a thiazole-linked covalent organic framework enabling real structure analysis. Nat. Commun. 9, 2600 (2018).
- Yang, S. et al. Transformation of covalent organic frameworks from N -acylhydrazone to oxadiazole linkages for smooth electron transfer in photocatalysis. Angew. Chem. Int. Ed. 61, e202115655 (2022).
- Qian, C. et al. Imine and imine-derived linkages in twodimensional covalent organic frameworks. Nat. Rev. Chem. 6, 881-898 (2022).
- Tran, L. D. et al. Divergent properties in structural isomers of triphenylamine-based covalent organic frameworks. Chem. Mater. 34, 529-536 (2022).
- Xie, Y. et al. Efficient and simultaneous capture of iodine and methyl iodide achieved by a covalent organic framework. Nat. Commun. 13, 2878 (2022).
- Uribe-Romo et al. A crystalline imine-linked 3-D porous covalent organic framework. J. Am. Chem. Soc. 131, 4570-4571 (2009).
- Ding, S.-Y. et al. Construction of covalent organic framework for catalysis: Pd/COF-LZU1 in Suzuki-Miyaura coupling reaction. J. Am. Chem. Soc. 133, 19816-19822 (2011).
- Zhou, Z.-B. et al. A facile, efficient, and general synthetic method to amide-linked covalent organic frameworks. J. Am. Chem. Soc. 144, 1138-1143 (2022).
- Liu, H. et al. Covalent organic frameworks linked by amine bonding for concerted electrochemical reduction of
. Chem. 4, 1696-1709 (2018). - Lyu, H., Li, H., Hanikel, N., Wang, K. & Yaghi, O. M. Covalent organic frameworks for carbon dioxide capture from air. J. Am. Chem. Soc. 144, 12989-12995 (2022).
- Li, X. et al. Facile transformation of imine covalent organic frameworks into ultrastable crystalline porous aromatic frameworks. Nat. Commun. 9, 2998 (2018).
- Li, X.-T. et al. Construction of covalent organic frameworks via three-component one-pot Strecker and Povarov reactions. J. Am. Chem. Soc. 142, 6521-6526 (2020).
- Zhao, Y. et al. A trifluoromethyl-grafted ultra-stable fluorescent covalent organic framework for adsorption and detection of pesticides. J. Mater. Chem. A 8, 25156-25164 (2020).
- Zhao, Y. et al. Pyrimidine-functionalized covalent organic framework and its cobalt complex as an efficient electrocatalyst for oxygen evolution reaction. ChemSusChem. 14, 4556-4562 (2021).
- Ding, L.-G. et al. Metalloporphyrin and ionic liquid-functionalized covalent organic frameworks for catalytic
cycloaddition via visible-light-induced photothermal conversion. Inorg. Chem. 60, 12591-12601 (2021). - Chen, R. et al. Post-synthetic fully п-conjugated three-dimensional covalent organic frameworks for high-performance lithium storage. ACS Appl. Mater. Interfaces 15, 830-837 (2022).
- Ren, X.-R. et al. Constructing stable chromenoquinoline-based covalent organic frameworks via intramolecular Povarov reaction. J. Am. Chem. Soc. 144, 2488-2494 (2022).
- Xiao, Z. et al. Boric acid functional fluorescent covalent-organic framework for sensitive and selective visualization of
. ACS Appl. Mater. Interfaces 15, 9524-9532 (2023). - Yang, Y. et al. Constructing chemical stable 4-carboxyl-quinoline linked covalent organic frameworks via Doebner reaction for nanofiltration. Nat. Commun. 13, 2615 (2022).
- Li, X.-T. et al. Construction of acid-base bifunctional covalent organic frameworks via Doebner reaction for catalysing cascade reaction. Chem. Commun. 58, 2508-2511 (2022).
- Das, P. et al. Integrating bifunctionality and chemical stability in covalent organic frameworks via one-pot multicomponent reactions for solar-driven
production. J. Am. Chem. Soc. 145, 2975-2984 (2023). - Zhao, X. et al. Construction of ultrastable nonsubstituted quinolinebridged covalent organic frameworks via rhodium-catalyzed dehydrogenative annulation. Angew. Chem. Int. Ed. 61, e202208833 (2022).
- Pang, H., Huang, D., Zhu, Y., Zhao, X. & Xiang, Y. One-pot cascade construction of nonsubstituted quinoline-bridged covalent organic frameworks. Chem. Sci. 14, 1543-1550 (2023).
- Das, P., Roeser, J. & Thomas, A. Solar light driven
production and selective oxidations using a covalent organic framework photocatalyst prepared by a multicomponent reaction. Angew. Chem. Int. Ed. 62, e202304349 (2023). - Liu, M. et al. Crystalline covalent triazine frameworks by in situ oxidation of alcohols to aldehyde monomers. Angew. Chem. Int. Ed. 57, 11968-11972 (2018).
- Zhai, L., Huang, N., Xu, H., Chen, Q. & Jiang, D. A backbone design principle for covalent organic frameworks: The impact of weakly
interacting units onadsorption. Chem. Commun. 53, 4242-4245 (2017). - Luo, R. et al. Intrareticular charge transfer regulated electrochemiluminescence of donor-acceptor covalent organic frameworks. Nat. Commun. 12, 6808 (2021).
- Kandambeth, S., Dey, K. & Banerjee, R. Covalent organic frameworks: Chemistry beyond the structure. J. Am. Chem. Soc. 141, 1807-1822 (2019).
- Haase, F. et al. Tuning the stacking behaviour of a 2D covalent organic framework through non-covalent interactions. Mater. Chem. Front. 1, 1354-1361 (2017).
- Keller, N. et al. Enforcing extended porphyrin J-aggregate stacking in covalent organic frameworks. J. Am. Chem. Soc. 140, 16544-16552 (2018).
- Yang, J. et al. Constitutional isomerism of the linkages in donoracceptor covalent organic frameworks and its impact on photocatalysis. Nat. Commun. 13, 6317 (2022).
- Zhu, D. & Verduzco, R. Ultralow surface tension solvents enable facile COF activation with reduced pore collapse. ACS Appl. Mater. Interfaces 12, 33121-33127 (2020).
- Yang, J. et al. Protonated imine-linked covalent organic frameworks for photocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 60, 19797-19803 (2021).
- Sing, K. S. W. et al. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 57, 603-619 (1985).
- Woods-Robinson, R. et al. Wide band gap chalcogenide semiconductors. Chem. Rev. 120, 4007-4055 (2020).
- Zhang, M. et al. Semiconductor/covalent-organic-framework Z-scheme heterojunctions for artificial photosynthesis. Angew. Chem. Int. Ed. 59, 6500-6506 (2020).
- Wang, X., Dong, M.-J. & Wu, C.-D. Tuning the pore structures and photocatalytic properties of a 2D covalent organic framework with multi-branched photoactive moieties. Nanoscale 12, 16136-16142 (2020).
- Chen, X. et al. Recent progress in the development of fluorescent, luminescent and colorimetric probes for detection of reactive oxygen and nitrogen species. Chem. Soc. Rev. 45, 2976-3016 (2016).
- Nosaka, Y. & Nosaka, A. Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 117, 11302-11336 (2017).
- Koppenol, W. H., Stanbury, D. M. & Bounds, P. L. Electrode potentials of partially reduced oxygen species, from dioxygen to water. Free Radic. Biol. Med. 49, 317-322 (2010).
- Karabacak, M., Bilgili, S., Mavis, T., Eskici, M. & Atac, A. Molecular structure, spectroscopic characterization (FT-IR, FT-Raman, UV and NMR), HOMO and LUMO analysis of 3-ethynylthiophene with DFT quantum chemical calculations. Spectrochim. Acta A Mol. Biomol. Spectrosc. 115, 709-718 (2013).
- Wen, W. et al. N-Acylamino saccharin as an emerging cysteinedirected covalent warhead and its application in the identification of novel FBPase inhibitors toward glucose reduction. J. Med. Chem. 65, 9126-9143 (2022).
- Lu, T. & Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580-592 (2012).
- Zhang, W.-Q. et al. Highly efficient and selective photooxidation of sulfur mustard simulant by a triazolobenzothiadiazole-moietyfunctionalized metal-organic framework in air. Inorg. Chem. 57, 4230-4233 (2018).
- Wei, L.-Q. & Ye, B.-H. Cyclometalated Ir-Zr metal-organic frameworks as recyclable visible-light photocatalysts for sulfide oxidation into sulfoxide in water. ACS Appl. Mater. Interfaces 11, 41448-41457 (2019).
- He, S. et al. Visible-light-promoted oxidative decarboxylation of arylacetic acids in air: Metal-free synthesis of aldehydes and ketones at room temperature. Chin. Chem. Lett. 31, 1863-1867 (2020).
- Shirase, S. et al. Cerium(IV) carboxylate photocatalyst for catalytic radical formation from carboxylic acids: decarboxylative oxygenation of aliphatic carboxylic acids and lactonization of aromatic carboxylic acids. J. Am. Chem. Soc. 142, 5668-5675 (2020).
- Reichle, A. et al. Copper(II)-photocatalyzed decarboxylative oxygenation of carboxylic acids. Chem. Commun. 58, 4456-4459 (2022).
- Goossen, L. J., Rodriguez, N. & Goossen, K. Carboxylic acids as substrates in homogeneous catalysis. Angew. Chem. Int. Ed. 47, 3100-3120 (2008).
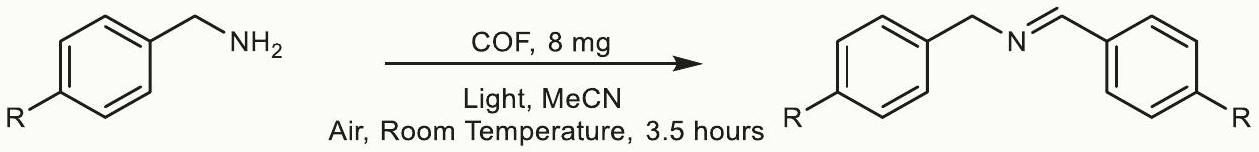
- Che, G. et al. Efficient photocatalytic oxidative coupling of benzylamine over uranyl-organic frameworks. Inorg. Chem. 61, 12301-12307 (2022).
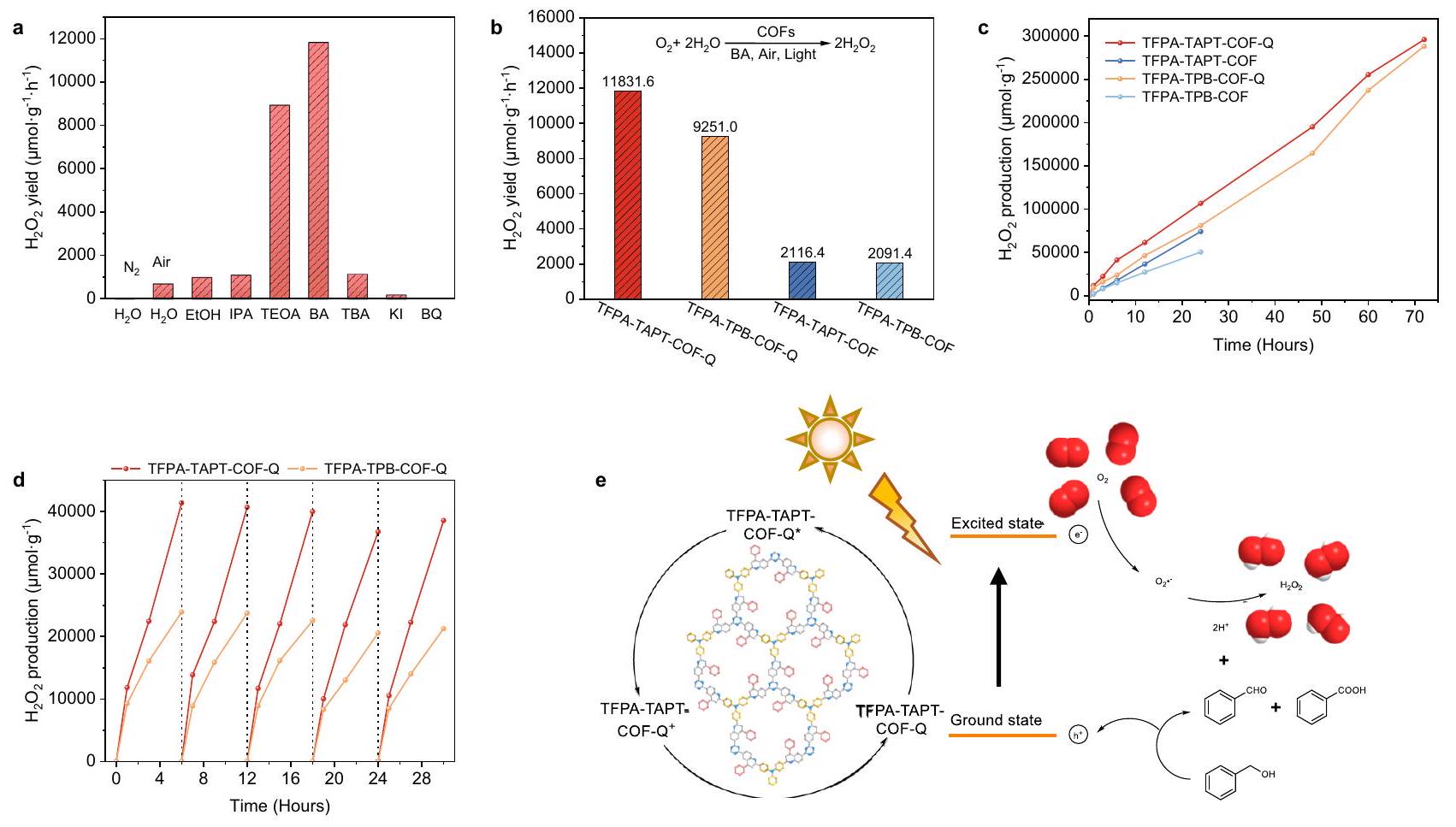
- Hou, H., Zeng, X. & Zhang, X. Production of hydrogen peroxide by photocatalytic processes. Angew. Chem. Int. Ed. 59, 17356-17376 (2020).
- Isaka, Y., Kawase, Y., Kuwahara, Y., Mori, K. & Yamashita, H. Twophase system utilizing hydrophobic metal-organic frameworks (MOFs) for photocatalytic synthesis of hydrogen peroxide. Angew. Chem. Int. Ed. 58, 5402-5406 (2019).
- Ye, Y.-X. et al. Highly efficient photosynthesis of hydrogen peroxide in ambient conditions. Proc. Natl Acad. Sci. USA 118, e2103964118 (2021).
- Zhao, W. et al. Accelerated synthesis and discovery of covalent organic framework photocatalysts for hydrogen peroxide production. J. Am. Chem. Soc. 144, 9902-9909 (2022).
- Kou, M. et al. Molecularly engineered covalent organic frameworks for hydrogen peroxide photosynthesis. Angew. Chem. Int. Ed. 61, e202200413 (2022).
- Tan, F. et al. Aqueous synthesis of covalent organic frameworks as photocatalysts for hydrogen peroxide production. CCS Chem. 4, 3751-3761 (2022).
- Mo, Y. et al. Linkage microenvironment of azoles-related covalent organic frameworks precisely regulates photocatalytic generation of hydrogen peroxide. Angew. Chem. Int. Ed. 62, e202309480 (2023).
الشكر والتقدير
مساهمات المؤلفين
المصالح المتنافسة
معلومات إضافية
المواد التكميلية المتاحة على
https://doi.org/10.1038/s41467-024-45457-y.
http://www.nature.com/reprints
© المؤلفون 2024
مدرسة الكيمياء والهندسة الكيميائية، مختبر شاندونغ الرئيسي لعلوم إنشاء المواد وتحويل الطاقة، مركز العلوم لإنشاء المواد وتحويل الطاقة، جامعة شاندونغ، رقم 27 طريق شندا الجنوبي، جينان 250100، جمهورية الصين الشعبية. مدرسة الكيمياء والهندسة الكيميائية والتكنولوجيا الحيوية، جامعة نانيانغ التكنولوجية، 21 رابط نانيانغ، 637371 سنغافورة، سنغافورة. البريد الإلكتروني:pzli@sdu.edu.cn; zhaoyanli@ntu.edu.sg
DOI: https://doi.org/10.1038/s41467-024-45457-y
PMID: https://pubmed.ncbi.nlm.nih.gov/38341421
Publication Date: 2024-02-10
Robust links in photoactive covalent organic frameworks enable effective photocatalytic reactions under harsh conditions
Accepted: 25 January 2024
Published online: 10 February 2024
(A) Check for updates
Abstract
Developing heterogeneous photocatalysts for the applications in harsh conditions is of high importance but challenging. Herein, by converting the imine linkages into quinoline groups of triphenylamine incorporated covalent organic frameworks (COFs), two photosensitive COFs, namely TFPA-TAPT-COF-Q and TFPA-TPB-COF-Q, are successfully constructed. The obtained quinoline-linked COFs display improved stability and photocatalytic activity, making them suitable photocatalysts for photocatalytic reactions under harsh conditions, as verified by the recyclable photocatalytic reactions of organic acid involving oxidative decarboxylation and organic base involving benzylamine coupling. Under strong oxidative condition, the quinoline-linked COFs show a high efficiency up to
crystalline porous organic polymers composed of organic components periodically linked by organic covalent bonds, which endowed them with chemical tunability and functionality by decorating either the organic moieties or the covalent linkages. By taking organic compounds with photosensitive properties as organic modules, photoactive COFs usually can be obtained, which exhibit a bright prospect in heterogeneous photocatalysis
synthesized COFs can effectively catalyze the generation of superoxide radical anion (
Results

“Method” section). Therefore, TFPA-TAPT-COF-Q synthesized by the one-pot method is used for the following structural analyses and catalytic performance characterizations. Based on the structure of iminelinked TFPA-TAPT-COF, a possible quinoline-linked two-dimensional (2D) framework

conversion of imine linkages into quinoline-ring during the modification reactions
binding energy at

linked TFPA-TAPT-COF-Q lowering the binding energy of
calculated by the Brunauer-Emmett-Teller (BET) method are 1058.99 and

after socking in different concentrations of acids, bases,
patterns of imine-linked COFs after immersing in alcohol solutions containing oxidizing and reducing agents (Fig. 4e, f). The stability investigations revealed that after converting the imine linkages into quinoline linkages, TFPA-TAPT-COF-Q and TFPA-TPB-COF-Q can maintain their chemical stability well in harsh acid, base and oxidizing, and reducing agent solutions.

force for the photocatalytic reduction of
results in photochemical properties, demonstrating that the synthesized quinoline-linked COFs should be effective photocatalysts for aerobic oxidations. As the quinoline-linked COFs possess higher photo-responsive properties, their photocatalytic aerobic oxidations were then investigated. Photocatalytic oxidation of sulfide as a typical reaction with the process by photocatalytically generating

out for TFPA-TPB-COF-Q, and the same outcomes as the first cycle of reaction were detected in all five recycling runs although its photoactivity is not as high as that of TFPA-TAPT-COF-Q (Supplementary Figs. 46-49). These results demonstrated that the quinoline-linked COFs, TFPA-TAPT-COF-Q and TFPA-TPB-COF-Q, can be used as effective recyclable heterogeneous photocatalysts in relatively harsh organic acid conditions.

| Entry | Substrate |
|
TFPA-TAPT-COF Conv. Sel. (%) | ||||
| 1 | 100 | 86 | 85 | 76 | |||
| 2 | 78 | 81 | 72 | 78 | |||
| 3 | 77 | 80 | 44 | 77 | |||
| 4 | 64 | 84 | 56 | 75 | |||
| 5 | 83 | 92 | 36 | 77 | |||
| 6 | 85 | 92 | 70 | 82 | |||
| 7 | 100 | 84 | 100 | 84 | |||
photocatalytic activity within the five cycles of reactions (Fig. 7a), while a great loss in mass for the recovered powder of TFPA-TAPT-COF after each cycle was detected, which cannot be recollected after the third cycle of reaction (Supplementary Fig. 53). The PXRD measurements on the recollected sample of TFPA-TAPT-COF-Q revealed that the main peaks were still maintained after five runs of reactions (Fig. 7b).

prepared quinoline-linked COFs under such a strong oxidation condition for photocatalytic production of
 |
||||
| Entry | ||||
| Substrate |
|
TFPA-TAPT-COF Conv. (%) | ||
| 1 | 100 | 100 | ||
| 2 | 98 | 96 | ||
| 3 | 100 | 100 | ||
| 4 | 65 | 53 | ||
| 5 | 81 | 70 | ||
| 6 | 96 | 81 | ||
| 7 | 85 | 81 | ||
with TFPA-TAPT-COF-Q and TFPA-TPB-COF-Q as photocatalysts can increase steadily up to

Discussion
Methods
Synthesis of TAPA-TAPT-COF-Q
Synthesis of TAPA-TAPT-COF-Q’
reacted at
Gram scale synthesis of TAPA-TAPT-COF-Q
Synthesis of TFPA-TAPT-COF
Synthesis of TAPA-TPB-COF-Q
Synthesis of TAPA-TPB-COF-Q’
Synthesis of TFPA-TPB-COF
Data availability
References
- Balaraman, E., Khaskin, E., Leitus, G. & Milstein, D. Catalytic transformation of alcohols to carboxylic acid salts and
using water as the oxygen atom source. Nat. Chem. 5, 122-125 (2013). - Huang, Z. et al. Chemical recycling of polystyrene to valuable chemicals via selective acid-catalyzed aerobic oxidation under visible light. J. Am. Chem. Soc. 144, 6532-6542 (2022).
- Dai, Z. et al. Crystal defect engineering of aurivillius
by Ce doping for increased reactive species production in photocatalysis. ACS Catal. 6, 3180-3192 (2016). - Pan, L. et al. Manipulating spin polarization of titanium dioxide for efficient photocatalysis. Nat. Commun. 11, 418 (2020).
- Duan, Y., Waerenborgh, J. C., Clemente-Juan, J. M., Gimenez-Saiz, C. & Coronado, E. Light-induced decarboxylation in a photoresponsive iron-containing complex based on polyoxometalate and oxalato ligands. Chem. Sci. 8, 305-315 (2017).
- Yu, B., Zhang, S. & Wang, X. Helical microporous nanorods assembled by polyoxometalate clusters for the photocatalytic oxidation of toluene. Angew. Chem. Int. Ed. 60, 17404-17409 (2021).
- Bagal, D. B. et al. Trifluoromethylchlorosulfonylation of alkenes: Evidence for an inner-sphere mechanism by a copper phenanthroline photoredox catalyst. Angew. Chem. Int. Ed. 54, 6999-7002 (2015).
- Wenger, O. S. Photoactive complexes with earth-abundant metals. J. Am. Chem. Soc. 140, 13522-13533 (2018).
- Shi, D. et al. Merging of the photocatalysis and copper catalysis in metal-organic frameworks for oxidative
bond formation. Chem. Sci. 6, 1035-1042 (2015). - Côté, A. P. et al. Porous, crystalline, covalent organic frameworks. J. Am. Chem. Soc. 310, 1166-1170 (2005).
- Wang, S. et al. Ultrathin ionic COF membrane via polyelectrolytemediated assembly for efficient
separation. Adv. Funct. Mater. 33, 2300386 (2023). - Li, H., Zhang, D., Cheng, K., Li, Z. & Li, P.-Z. Effective iodine adsorption by nitrogen-rich nanoporous covalent organic frameworks. ACS Appl. Nano Mater. 6, 1295-1302 (2023).
- Cheng, K., Li, H., Wang, J.-R., Li, P.-Z. & Zhao, Y. From supramolecular organic cages to porous covalent organic frameworks for enhancing iodine adsorption capability by fully exposed nitrogenrich sites. Small 19, 2301998 (2023).
- Cheng, K., Li, H., Li, Z., Li, P.-Z. & Zhao, Y. Linking nitrogen-rich organic cages into isoreticular covalent organic frameworks for enhancing iodine adsorption capability. ACS Mater. Lett. 5, 1546-1555 (2023).
- Wang, X. et al. Homochiral 2D porous covalent organic frameworks for heterogeneous asymmetric catalysis. J. Am. Chem. Soc. 138, 12332-12335 (2016).
- Sun, J. et al. Pyrene-based covalent organic frameworks for photocatalytic hydrogen peroxide production. Angew. Chem. Int. Ed. 62, e202216719 (2023).
- Wang, S. et al. Programming covalent organic frameworks for photocatalysis: Investigation of chemical and structural variations. Matter 2, 416-427 (2020).
- Zhi, Q. et al. Piperazine-linked metalphthalocyanine frameworks for highly efficient visible-light-driven
photosynthesis. J. Am. Chem. Soc. 144, 21328-21336 (2022). - Zou, X.-N. et al. Incorporating photochromic triphenylamine into a zirconium-organic framework for highly effective photocatalytic aerobic oxidation of sulfides. ACS Appl. Mater. Interfaces 13, 20137-20144 (2021).
- Zhang, D. et al. Highly effective photocatalytic radical reactions triggered by a photoactive metal-organic framework. ACS Appl. Mater. Interfaces 14, 23518-23526 (2022).
- Li, Y. et al. Effective photocatalytic initiation of reactive oxygen species by a photoactive covalent organic framework for oxidation reactions. ACS Mater. Lett. 4, 1160-1167 (2022).
- Luan, T.-X. et al. Highly enhancing
photoreduction by metallization of an imidazole-linked robust covalent organic framework. Small 19, 2303324 (2023). - Yu, S.-Y. et al. Direct synthesis of a covalent triazine-based framework from aromatic amides. Angew. Chem. Int. Ed. 57, 8438-8442 (2018).
- Lin, Y. et al. A covalent organic framework as a long-life and highrate anode suitable for both aqueous acidic and alkaline batteries. Angew. Chem. Int. Ed. 62, e202218745 (2023).
- Luan, T.-X. et al. Highly effective generation of singlet oxygen by an imidazole-linked robust photosensitizing covalent organic framework. ACS Nano 16, 21565-21575 (2022).
- Zhuang, X. et al. A two-dimensional conjugated polymer framework with fully
-bonded carbon skeleton. Polym. Chem. 7, 4176-4181 (2016). - Shi, J.-L., Chen, R., Hao, H., Wang, C. & Lang, X. 2D sp² carbonconjugated porphyrin covalent organic framework for cooperative photocatalysis with TEMPO. Angew. Chem. Int. Ed. 59, 9088-9093 (2020).
- Haase, F. et al. Topochemical conversion of an imine- into a thiazole-linked covalent organic framework enabling real structure analysis. Nat. Commun. 9, 2600 (2018).
- Yang, S. et al. Transformation of covalent organic frameworks from N -acylhydrazone to oxadiazole linkages for smooth electron transfer in photocatalysis. Angew. Chem. Int. Ed. 61, e202115655 (2022).
- Qian, C. et al. Imine and imine-derived linkages in twodimensional covalent organic frameworks. Nat. Rev. Chem. 6, 881-898 (2022).
- Tran, L. D. et al. Divergent properties in structural isomers of triphenylamine-based covalent organic frameworks. Chem. Mater. 34, 529-536 (2022).
- Xie, Y. et al. Efficient and simultaneous capture of iodine and methyl iodide achieved by a covalent organic framework. Nat. Commun. 13, 2878 (2022).
- Uribe-Romo et al. A crystalline imine-linked 3-D porous covalent organic framework. J. Am. Chem. Soc. 131, 4570-4571 (2009).
- Ding, S.-Y. et al. Construction of covalent organic framework for catalysis: Pd/COF-LZU1 in Suzuki-Miyaura coupling reaction. J. Am. Chem. Soc. 133, 19816-19822 (2011).
- Zhou, Z.-B. et al. A facile, efficient, and general synthetic method to amide-linked covalent organic frameworks. J. Am. Chem. Soc. 144, 1138-1143 (2022).
- Liu, H. et al. Covalent organic frameworks linked by amine bonding for concerted electrochemical reduction of
. Chem. 4, 1696-1709 (2018). - Lyu, H., Li, H., Hanikel, N., Wang, K. & Yaghi, O. M. Covalent organic frameworks for carbon dioxide capture from air. J. Am. Chem. Soc. 144, 12989-12995 (2022).
- Li, X. et al. Facile transformation of imine covalent organic frameworks into ultrastable crystalline porous aromatic frameworks. Nat. Commun. 9, 2998 (2018).
- Li, X.-T. et al. Construction of covalent organic frameworks via three-component one-pot Strecker and Povarov reactions. J. Am. Chem. Soc. 142, 6521-6526 (2020).
- Zhao, Y. et al. A trifluoromethyl-grafted ultra-stable fluorescent covalent organic framework for adsorption and detection of pesticides. J. Mater. Chem. A 8, 25156-25164 (2020).
- Zhao, Y. et al. Pyrimidine-functionalized covalent organic framework and its cobalt complex as an efficient electrocatalyst for oxygen evolution reaction. ChemSusChem. 14, 4556-4562 (2021).
- Ding, L.-G. et al. Metalloporphyrin and ionic liquid-functionalized covalent organic frameworks for catalytic
cycloaddition via visible-light-induced photothermal conversion. Inorg. Chem. 60, 12591-12601 (2021). - Chen, R. et al. Post-synthetic fully п-conjugated three-dimensional covalent organic frameworks for high-performance lithium storage. ACS Appl. Mater. Interfaces 15, 830-837 (2022).
- Ren, X.-R. et al. Constructing stable chromenoquinoline-based covalent organic frameworks via intramolecular Povarov reaction. J. Am. Chem. Soc. 144, 2488-2494 (2022).
- Xiao, Z. et al. Boric acid functional fluorescent covalent-organic framework for sensitive and selective visualization of
. ACS Appl. Mater. Interfaces 15, 9524-9532 (2023). - Yang, Y. et al. Constructing chemical stable 4-carboxyl-quinoline linked covalent organic frameworks via Doebner reaction for nanofiltration. Nat. Commun. 13, 2615 (2022).
- Li, X.-T. et al. Construction of acid-base bifunctional covalent organic frameworks via Doebner reaction for catalysing cascade reaction. Chem. Commun. 58, 2508-2511 (2022).
- Das, P. et al. Integrating bifunctionality and chemical stability in covalent organic frameworks via one-pot multicomponent reactions for solar-driven
production. J. Am. Chem. Soc. 145, 2975-2984 (2023). - Zhao, X. et al. Construction of ultrastable nonsubstituted quinolinebridged covalent organic frameworks via rhodium-catalyzed dehydrogenative annulation. Angew. Chem. Int. Ed. 61, e202208833 (2022).
- Pang, H., Huang, D., Zhu, Y., Zhao, X. & Xiang, Y. One-pot cascade construction of nonsubstituted quinoline-bridged covalent organic frameworks. Chem. Sci. 14, 1543-1550 (2023).
- Das, P., Roeser, J. & Thomas, A. Solar light driven
production and selective oxidations using a covalent organic framework photocatalyst prepared by a multicomponent reaction. Angew. Chem. Int. Ed. 62, e202304349 (2023). - Liu, M. et al. Crystalline covalent triazine frameworks by in situ oxidation of alcohols to aldehyde monomers. Angew. Chem. Int. Ed. 57, 11968-11972 (2018).
- Zhai, L., Huang, N., Xu, H., Chen, Q. & Jiang, D. A backbone design principle for covalent organic frameworks: The impact of weakly
interacting units onadsorption. Chem. Commun. 53, 4242-4245 (2017). - Luo, R. et al. Intrareticular charge transfer regulated electrochemiluminescence of donor-acceptor covalent organic frameworks. Nat. Commun. 12, 6808 (2021).
- Kandambeth, S., Dey, K. & Banerjee, R. Covalent organic frameworks: Chemistry beyond the structure. J. Am. Chem. Soc. 141, 1807-1822 (2019).
- Haase, F. et al. Tuning the stacking behaviour of a 2D covalent organic framework through non-covalent interactions. Mater. Chem. Front. 1, 1354-1361 (2017).
- Keller, N. et al. Enforcing extended porphyrin J-aggregate stacking in covalent organic frameworks. J. Am. Chem. Soc. 140, 16544-16552 (2018).
- Yang, J. et al. Constitutional isomerism of the linkages in donoracceptor covalent organic frameworks and its impact on photocatalysis. Nat. Commun. 13, 6317 (2022).
- Zhu, D. & Verduzco, R. Ultralow surface tension solvents enable facile COF activation with reduced pore collapse. ACS Appl. Mater. Interfaces 12, 33121-33127 (2020).
- Yang, J. et al. Protonated imine-linked covalent organic frameworks for photocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 60, 19797-19803 (2021).
- Sing, K. S. W. et al. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 57, 603-619 (1985).
- Woods-Robinson, R. et al. Wide band gap chalcogenide semiconductors. Chem. Rev. 120, 4007-4055 (2020).
- Zhang, M. et al. Semiconductor/covalent-organic-framework Z-scheme heterojunctions for artificial photosynthesis. Angew. Chem. Int. Ed. 59, 6500-6506 (2020).
- Wang, X., Dong, M.-J. & Wu, C.-D. Tuning the pore structures and photocatalytic properties of a 2D covalent organic framework with multi-branched photoactive moieties. Nanoscale 12, 16136-16142 (2020).
- Chen, X. et al. Recent progress in the development of fluorescent, luminescent and colorimetric probes for detection of reactive oxygen and nitrogen species. Chem. Soc. Rev. 45, 2976-3016 (2016).
- Nosaka, Y. & Nosaka, A. Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 117, 11302-11336 (2017).
- Koppenol, W. H., Stanbury, D. M. & Bounds, P. L. Electrode potentials of partially reduced oxygen species, from dioxygen to water. Free Radic. Biol. Med. 49, 317-322 (2010).
- Karabacak, M., Bilgili, S., Mavis, T., Eskici, M. & Atac, A. Molecular structure, spectroscopic characterization (FT-IR, FT-Raman, UV and NMR), HOMO and LUMO analysis of 3-ethynylthiophene with DFT quantum chemical calculations. Spectrochim. Acta A Mol. Biomol. Spectrosc. 115, 709-718 (2013).
- Wen, W. et al. N-Acylamino saccharin as an emerging cysteinedirected covalent warhead and its application in the identification of novel FBPase inhibitors toward glucose reduction. J. Med. Chem. 65, 9126-9143 (2022).
- Lu, T. & Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580-592 (2012).
- Zhang, W.-Q. et al. Highly efficient and selective photooxidation of sulfur mustard simulant by a triazolobenzothiadiazole-moietyfunctionalized metal-organic framework in air. Inorg. Chem. 57, 4230-4233 (2018).
- Wei, L.-Q. & Ye, B.-H. Cyclometalated Ir-Zr metal-organic frameworks as recyclable visible-light photocatalysts for sulfide oxidation into sulfoxide in water. ACS Appl. Mater. Interfaces 11, 41448-41457 (2019).
- He, S. et al. Visible-light-promoted oxidative decarboxylation of arylacetic acids in air: Metal-free synthesis of aldehydes and ketones at room temperature. Chin. Chem. Lett. 31, 1863-1867 (2020).
- Shirase, S. et al. Cerium(IV) carboxylate photocatalyst for catalytic radical formation from carboxylic acids: decarboxylative oxygenation of aliphatic carboxylic acids and lactonization of aromatic carboxylic acids. J. Am. Chem. Soc. 142, 5668-5675 (2020).
- Reichle, A. et al. Copper(II)-photocatalyzed decarboxylative oxygenation of carboxylic acids. Chem. Commun. 58, 4456-4459 (2022).
- Goossen, L. J., Rodriguez, N. & Goossen, K. Carboxylic acids as substrates in homogeneous catalysis. Angew. Chem. Int. Ed. 47, 3100-3120 (2008).
- Che, G. et al. Efficient photocatalytic oxidative coupling of benzylamine over uranyl-organic frameworks. Inorg. Chem. 61, 12301-12307 (2022).
- Hou, H., Zeng, X. & Zhang, X. Production of hydrogen peroxide by photocatalytic processes. Angew. Chem. Int. Ed. 59, 17356-17376 (2020).
- Isaka, Y., Kawase, Y., Kuwahara, Y., Mori, K. & Yamashita, H. Twophase system utilizing hydrophobic metal-organic frameworks (MOFs) for photocatalytic synthesis of hydrogen peroxide. Angew. Chem. Int. Ed. 58, 5402-5406 (2019).
- Ye, Y.-X. et al. Highly efficient photosynthesis of hydrogen peroxide in ambient conditions. Proc. Natl Acad. Sci. USA 118, e2103964118 (2021).
- Zhao, W. et al. Accelerated synthesis and discovery of covalent organic framework photocatalysts for hydrogen peroxide production. J. Am. Chem. Soc. 144, 9902-9909 (2022).
- Kou, M. et al. Molecularly engineered covalent organic frameworks for hydrogen peroxide photosynthesis. Angew. Chem. Int. Ed. 61, e202200413 (2022).
- Tan, F. et al. Aqueous synthesis of covalent organic frameworks as photocatalysts for hydrogen peroxide production. CCS Chem. 4, 3751-3761 (2022).
- Mo, Y. et al. Linkage microenvironment of azoles-related covalent organic frameworks precisely regulates photocatalytic generation of hydrogen peroxide. Angew. Chem. Int. Ed. 62, e202309480 (2023).
Acknowledgements
Author contributions
Competing interests
Additional information
supplementary material available at
https://doi.org/10.1038/s41467-024-45457-y.
http://www.nature.com/reprints
© The Author(s) 2024
School of Chemistry and Chemical Engineering, Shandong Provincial Key Laboratory for Science of Material Creation and Energy Conversion, Science Center for Material Creation and Energy Conversion, Shandong University, No. 27 Shanda South Road, Ji’nan 250100, PR China. School of Chemistry, Chemical Engineering and Biotechnology, Nanyang Technological University, 21 Nanyang Link, 637371 Singapore, Singapore. e-mail: pzli@sdu.edu.cn; zhaoyanli@ntu.edu.sg
