DOI: https://doi.org/10.1038/s41467-024-45678-1
PMID: https://pubmed.ncbi.nlm.nih.gov/38341441
تاريخ النشر: 2024-02-10
الصلابة المزدوجة تنشط الفلورية الفوسفورية العضوية عالية الحرارة طويلة الأمد
تاريخ القبول: 31 يناير 2024
تاريخ النشر على الإنترنت: 10 فبراير 2024
(D) تحقق من التحديثات
الملخص
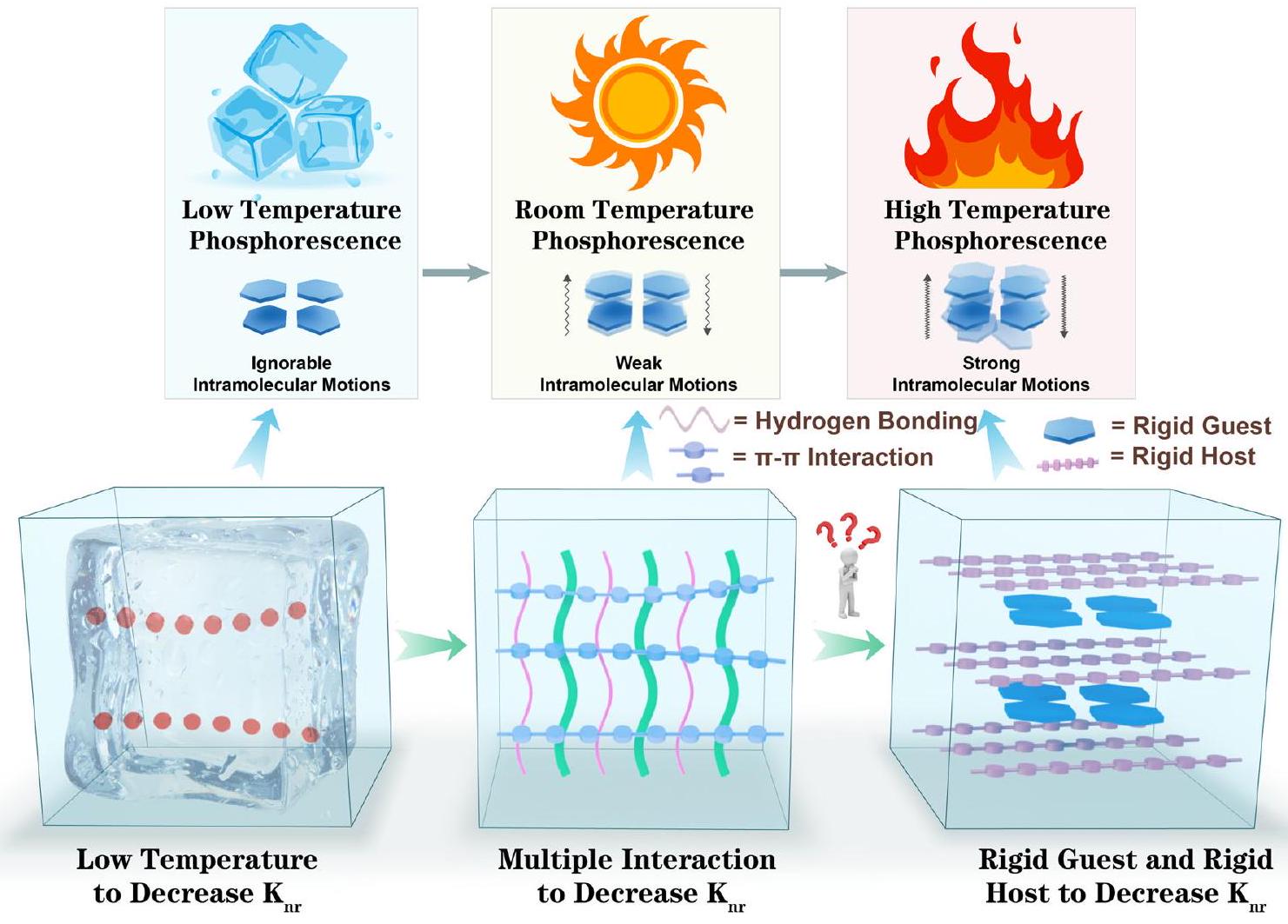
تم ابتكار استراتيجية لتحقيق الفوسفوريسنس عالي الحرارة باستخدام جزيئات صلبة مسطحة كضيوف وبوليمرات صلبة كوسط مضيف. يمكن أن تقاوم التكوين الصلب المسطح اهتزاز الضيف الحراري عند درجات حرارة عالية، وتزيد صلابة الوسط من مقاومة الضيف للحرارة العالية. تظهر المواد المدعومة توهجًا بعد 40 ثانية عند
الإثارات الأحادية) حساسة لدرجة الحرارة وعرضة للتعطيل الحراري، مما يؤدي إلى إنهاء سريع للتوهج عند درجات حرارة عالية
قوية) بمزيد من الاتصال بجزيء BCZ لتوليد خمسة ضيوف تحكم (BCZ-Me، BCZ-nBu، BCZ-Be، BCZ-Ph، وBCZ-TPA). تؤثر المجموعات الألكيلية (مثل الميثيل أو
النتائج
التركيب والخصائص الضوئية
خصائص HTP للنظام المخدر
تأثير الضيوف على HTP
ستة ضيوف عند درجات حرارة مختلفة (DMSO كمذيب،
حلقة البنزين (الشكل التكميلي 11). تشير نتائج هذه الحسابات إلى أن تأثير المجموعات الألكيلية على طاقة الحالة المثارة للضيف يمكن أن يكون شبه مهمل، في حين أن مجموعة البنزيل أظهرت تأثيرًا ضعيفًا، بينما أظهرت مجموعات الفينيل والتري فينيل أمين تأثيرًا قويًا. بعبارة أخرى، يمكن استخدام هاتين المجموعتين الأخيرتين كدوار لاستهلاك طاقة الحالة المثارة.
بواسطة الحركة الحرارية. في المذيب غير القطبي
انخفضت بشكل متناسب (الشكل 3f). أظهرت التجارب الضابطة المذكورة أعلاه أن الفشل في تثبيط الحركة الجزيئية يمكن أن يتسبب في ضرر كبير لخصائص HTP للمواد العضوية، وأكدت أن اختيار جزيئات صلبة مسطحة كضيوف ذات ميل ضعيف للخضوع للحركة الجزيئية هو استراتيجية فعالة لبناء مواد HTP. تم أيضًا اختبار انبعاث الفلورسنت لستة أفلام مخدرة عند درجات حرارة مختلفة. أظهر انبعاث الفلورسنت لـ BCZ/PVP وBCZ-Me/PVP وBCZ-nBu/PVP خصائص مقاومة لدرجات الحرارة العالية تقريبًا متشابهة؛ بينما من BCZ-Be/PVP إلى BCZ-TPA/PVP، انخفضت قدرة مقاومة درجات الحرارة العالية لانبعاث الفلورسنت تدريجيًا (الشكل التكميلي 18). ومع ذلك، بسبب الاستقرار الفائق للحالات المثارة الأحادية مقارنة بالحالات المثارة الثلاثية، كان الانخفاض في شدة الفلورسنت للأفلام المخدرة أقل من ذلك في شدة الفوسفور.
تغيرات الطاقة والبصريات للضيوف عند درجات حرارة مرتفعة
بشكل كبير مع زيادة درجة الحرارة. من 293 كلفن إلى 443 كلفن، زاد الزاوية ذات أعلى احتمال للحدوث تدريجياً من
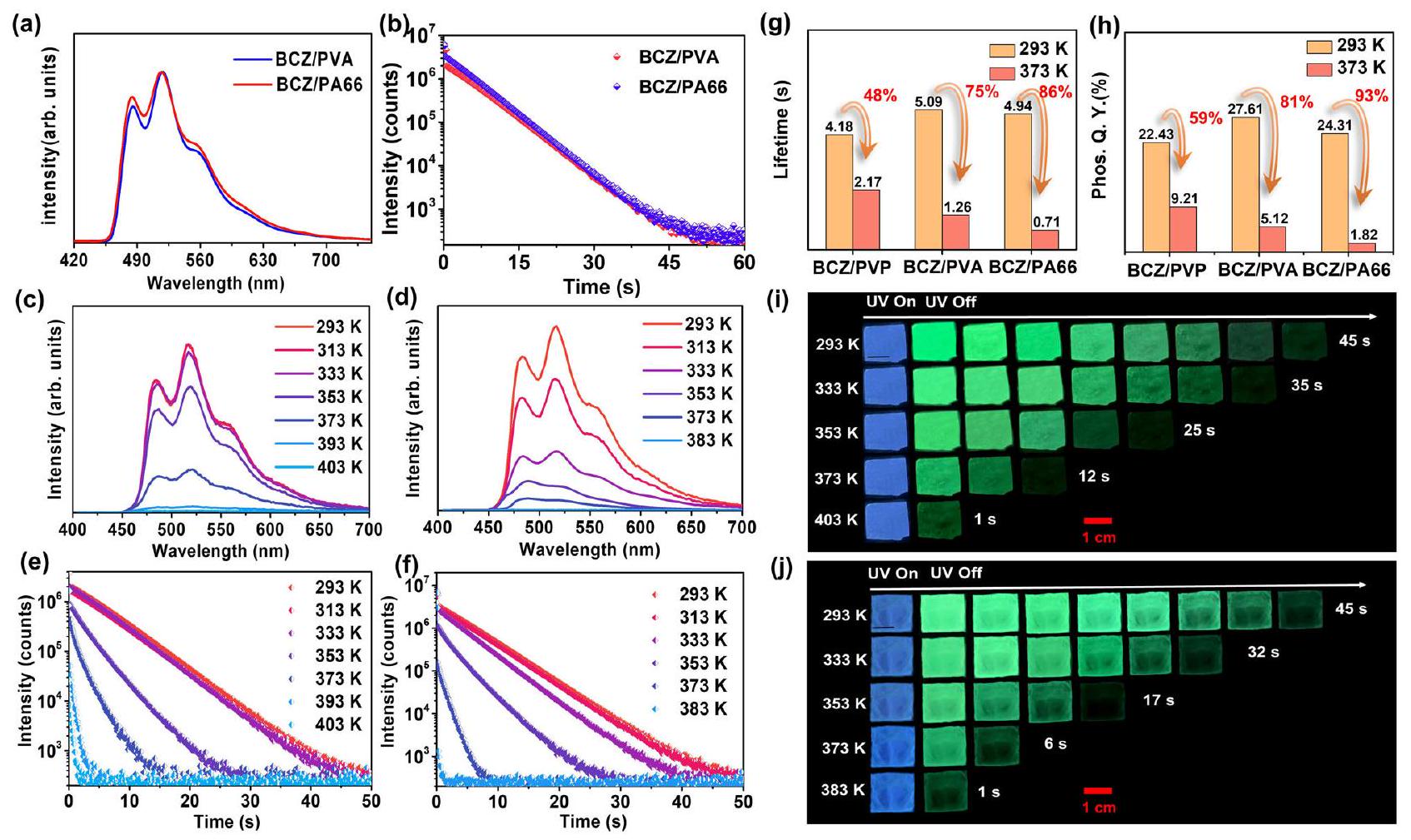
تأثير المضيفين على HTP
كانت أداء الفلورية الفوسفورية لـ BCZ/PVA و BCZ/PA66 عند درجات الحرارة العالية أقل من أداء BCZ/PVP، مما يشير إلى أن البوليمرات الحاضنة ذات المستوى المنخفض
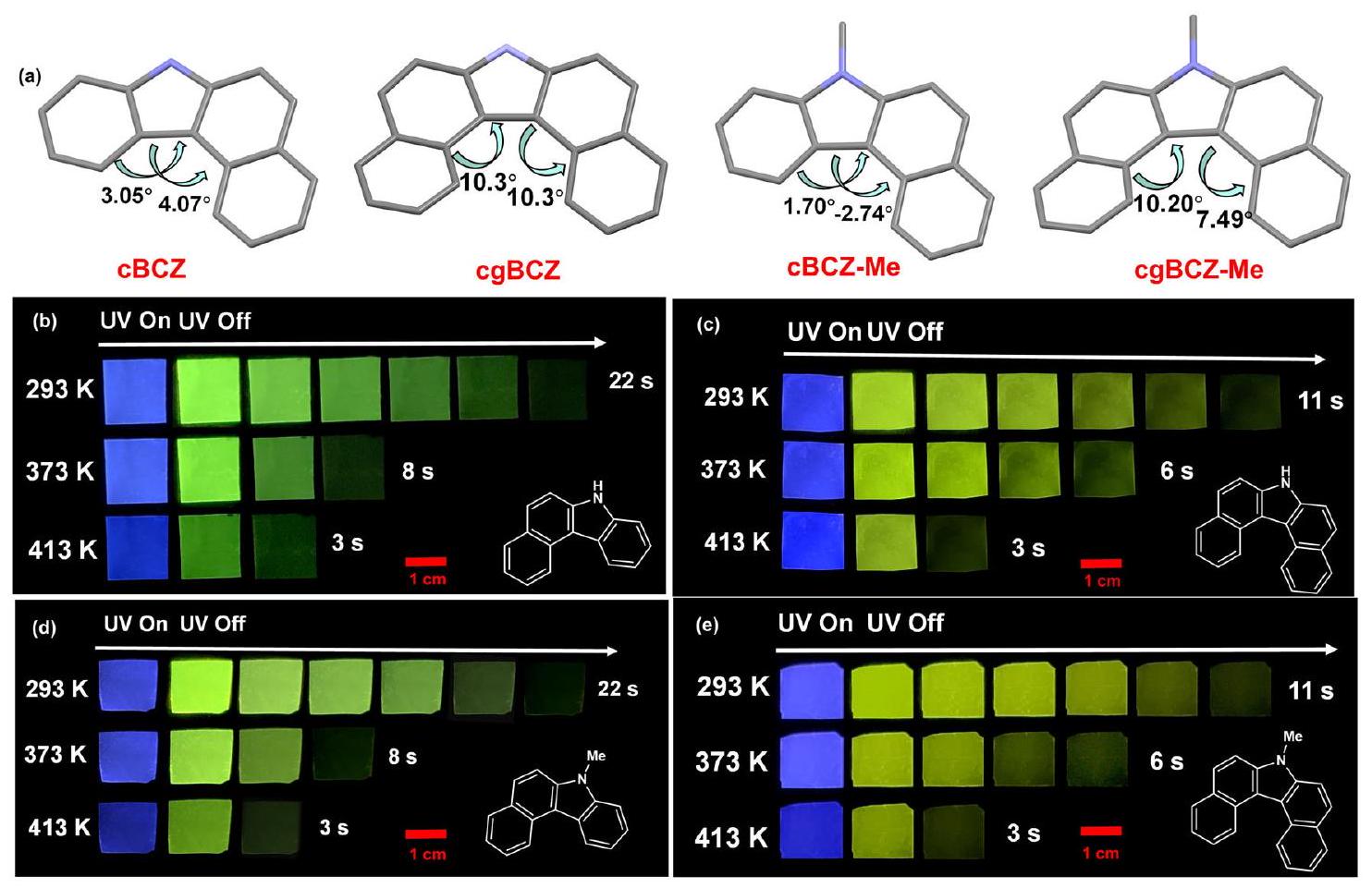
عالمية الاستراتيجية
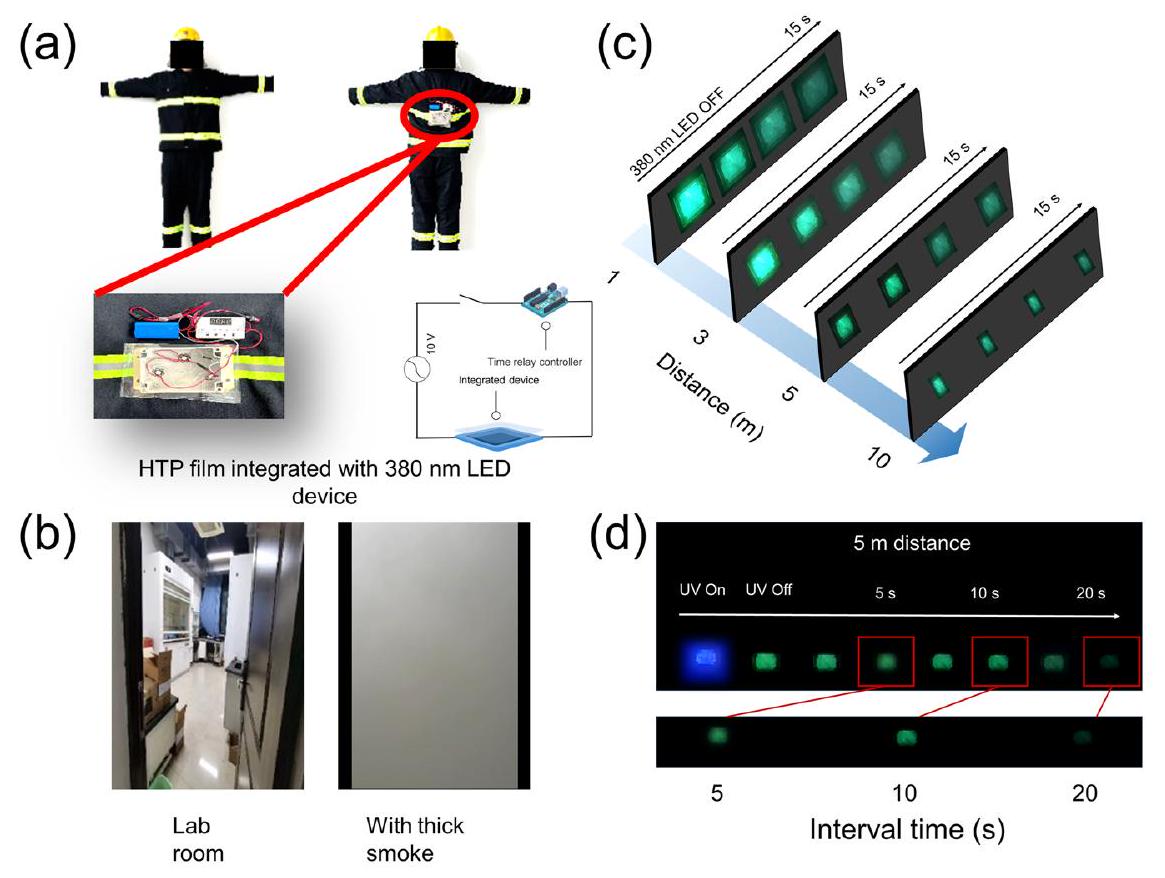
تطبيق مواد HTP في الحماية من الحرائق
وبيئات الدخان الكثيف. قدرة اختراق الدخان لمصادر الإضاءة مثل الفلورية والفوسفورية أقوى من تلك الخاصة بمصادر الإضاءة المتوهجة مثل المصابيح العادية. بالإضافة إلى ذلك، فإن المواد الفوسفورية ذات زمن التوهج الطويل لا تتطلب تحفيزًا مستمرًا، مما لا يوفر الكهرباء فحسب، بل يقلل أيضًا من إشعاع مصدر التحفيز على جسم الإنسان. لذلك، فإن المواد الفوسفورية مناسبة للتعرف الضوئي في عمليات البحث والإنقاذ. ومع ذلك، فإن المواد الفوسفورية العضوية التقليدية حساسة لدرجة الحرارة، ويمكن أن تؤدي درجات الحرارة العالية الناتجة عن الحرائق إلى إخماد انبعاث الفوسفور بشكل كبير. لذلك، من المتوقع أن تساعد مادة الفوسفور ذات العمر الطويل والمقاومة لدرجات الحرارة العالية التي تم تصميمها في هذه الدراسة في تحديد رجال الإطفاء أثناء عمليات الإنقاذ ومكافحة الحرائق. وبناءً عليه، تم إعداد جهاز مريح ينبعث منه الفوسفور عند تحفيز بسيط. يتكون الجهاز من بطارية، ووحدة تحكم، وثلاثة مصابيح تحفيز ضوئي (Ex.
نقاش
طرق
تركيب أو شراء المركبات الضيفية
عند
تمت تنقية المتبقي باستخدام الكروماتوغرافيا السريعة على هلام السيليكا للحصول على المنتج المستهدف BCZ-nBu تحت ضغط منخفض.
تحضير المواد المخدرة باستخدام PVP كالمضيف
تحضير المواد المخدرة باستخدام PVA كالمضيف
طريقة تحضير المواد المخدرة باستخدام PA66 كالمضيف
ملخص التقرير
توفر البيانات
References
- Chen, W. et al. Highly bright and stable single-crystal perovskite light-emitting diodes. Nat. Photon. 17, 401-407 (2023).
- Wang, X. et al. Organic phosphors with bright triplet excitons for efficient X-ray-excited luminescence. Nat. Photon. 15, 187-192 (2021).
- Ye, W. et al. Confining isolated chromophores for highly efficient blue phosphorescence. Nat. Mater. 20, 1539-1544 (2021).
- Liang, Y. et al. UV-curing-enhanced organic long-persistent luminescence materials. Adv. Mater. 35, e2304820 (2023).
- Zhang, Y. et al. Ultraviolet irradiation-responsive dynamic ultralong organic phosphorescence in polymeric systems. Nat. Commun. 12, 2297 (2021).
- Wang, Y. et al. High performance of simple organic phosphorescence host-guest materials and their application in time-resolved bioimaging. Adv. Mater. 33, e2007811 (2021).
- Tao, Y. et al. Resonance-induced stimuli-responsive capacity modulation of organic ultralong room temperature phosphorescence. J. Am. Chem. Soc 144, 6946-6953 (2022).
- Zhang, X. et al. Multicolor hyperafterglow from isolated fluorescence chromophores. Nat. Commun. 14, 475 (2023).
- Zhai, Y. et al. Room temperature phosphorescence from natural wood activated by external chloride anion treatment. Nat. Commun. 14, 2614 (2023).
- Yang, Y. et al. Efficient and color-tunable dual-mode afterglow from large-area and flexible polymer-based transparent films for anticounterfeiting and information encryption. Angew. Chem. Int. Ed. 61, e202201820 (2022).
- Wang, H., Li, Q. & Tang, B. Z. High-quality manuscripts complement scientific value and readability. Matter 5, 3072-3075 (2022).
- Dai, W. et al. Halogen bonding: a new platform for achieving multi-stimuli-responsive persistent phosphorescence. Angew. Chem. Int. Ed. 61, e202200236 (2022).
- Xiao, F. et al. Guest-host doped strategy for constructing ultralonglifetime near-infrared organic phosphorescence materials for bioimaging. Nat. Commun. 13, 186 (2022).
- Huang, A. et al. Organic persistent RTP crystals: from brittle to flexible by tunable self-partitioned molecular packing. Adv. Mater. 35, e2209166 (2023).
- Yan, Z. A., Lin, X., Sun, S., Ma, X. & Tian, H. Activating roomtemperature phosphorescence of organic luminophores via external heavy-atom effect and rigidity of ionic polymer matrix. Angew. Chem. Int. Ed. 60, 19735-19739 (2021).
- Zhu, T., Yang, T., Zhang, Q. & Yuan, W. Z. Clustering and halogen effects enabled red/near-infrared room temperature phosphorescence from aliphatic cyclic imides. Nat. Commun. 13, 2658 (2022).
- Wang, J. et al. A facile strategy for realizing room temperature phosphorescence and single molecule white light emission. Nat. Commun. 9, 2963 (2018).
- Zhang, K. et al. Aromatic amides: a smart backbone toward isolated ultralong bright blue-phosphorescence in confined polymeric films. Angew. Chem. Int. Ed. 62, e202300927 (2023).
- Li, J. et al. A direct observation of up-converted room-temperature phosphorescence in an anti-Kasha dopant-matrix system. Nat. Commun. 14, 1987 (2023).
- Wang, T. et al. Thermochromic aggregation-induced dual phosphorescence via temperature-dependent sp(3)-linked donoracceptor electronic coupling. Nat. Commun. 12, 1364 (2021).
- Xia, Y. et al. Host-guest doping in flexible organic crystals for roomtemperature phosphorescence. Angew. Chem. Int. Ed. 62, e202217547 (2023).
- Ding, B., Ma, L., Huang, Z., Ma, X. & Tian, H. Engendering persistent organic room temperature phosphorescence by trace ingredient incorporation. Sci. Adv. 7, eabf9668 (2021).
- Lei, Y. et al. Wide-range color-tunable organic phosphorescence materials for printable and writable security inks. Angew. Chem. Int. Ed. 59, 16054-16060 (2020).
- Ma, H., Peng, Q., An, Z., Huang, W. & Shuai, Z. Efficient and longlived room-temperature organic phosphorescence: theoretical descriptors for molecular designs. J. Am. Chem. Soc. 141, 1010-1015 (2019).
- Zhao, W. et al. Boosting the efficiency of organic persistent roomtemperature phosphorescence by intramolecular triplet-triplet energy transfer. Nat. Commun. 10, 1595 (2019).
- Zhao, W., He, Z. & Tang, B. Z. Room-temperature phosphorescence from organic aggregates. Nat. Rev. Mater. 5, 869-885 (2020).
- Wang, T., Gupta, A. K., Wu, S., Slawin, A. M. Z. & Zysman, C. E. Conjugation-modulated excitonic coupling brightens multiple triplet excited states. J. Am. Chem. Soc. 145, 1945-1954 (2023).
- Shao, W. & Kim, J. Metal-free organic phosphors toward fast and efficient room-temperature phosphorescence. Acc. Chem. Res. 55, 1573-1585 (2022).
- Peng, Q., Ma, H. & Shuai, Z. Theory of long-lived room-temperature phosphorescence in organic aggregates. Acc. Chem. Res. 54, 940-949 (2021).
- Hirata, S. Molecular physics of persistent room temperature phosphorescence and long-lived triplet excitons. Appl. Phys. Rev. 9, 011304 (2022).
- Gao, R., Kodaimati, M. S. & Yan, D. Recent advances in persistent luminescence based on molecular hybrid materials. Chem. Soc. Rev. 50, 5564-5589 (2021).
- Niu, Y. et al. A universal strategy for achieving dual cross-linked networks to obtain ultralong polymeric room temperature phosphorescence. Sci. China Chem. 66, 1161-1168 (2023).
- Zhu, Y. et al. Ultralong polymeric room temperature phosphorescence materials fabricated by multiple hydrogen bondings resistant to temperature and humidity. Adv. Optical. Mater. 9, 2100782 (2021).
- Zhang, J. et al. Highly efficient and robust full-color organic afterglow through 2d superlattices embedment. Adv. Mater. 34, e2206712 (2022).
- Lv, H. et al. Highly stable metal-free long-persistent luminescent copolymer for low flicker AC-LEDs. Angew. Chem. Int. Ed. 61, e202204209 (2022).
- Cai, S. et al. Enabling long-lived organic room temperature phosphorescence in polymers by subunit interlocking. Nat. Commun. 10, 4247 (2019).
- Wei, J. et al. Conformation-dependent dynamic organic phosphorescence through thermal energy driven molecular rotations. Nat. Commun. 14, 627 (2023).
- Liu, X., Liao, Q., Yang, J., Li, Z. & Li, Q. Unveiling one-to-one correspondence between excited triplet states and determinate interactions by temperature-controllable blue-green-yellow afterglow. Angew. Chem. Int. Ed. 62, e202302792 (2023).
- Guo, S. et al. Recent progress in pure organic room temperature phosphorescence of small molecular host-guest systems. ACS Mater. Lett. 3, 379-397 (2021).
- Yan, X. et al. Recent advances on host-guest material systems toward organic room temperature phosphorescence. Small 18, e2104073 (2022).
- Lei, Y. et al. Stimulus-responsive organic phosphorescence materials based on small molecular host-guest doped systems. J. Phys. Chem. Lett. 14, 1794-1807 (2023).
- Zhang, T. et al. Molecular engineering for metal-free amorphous materials with room-temperature phosphorescence. Angew. Chem. Int. Ed. 59, 11206-11216 (2020).
- Dai, W. et al. Red-emissive organic room-temperature phosphorescence material for time-resolved luminescence bioimaging. CCS Chem. 4, 2550-2559 (2022).
- Lei, Y. et al. The dual-state luminescent mechanism of 2,3,4,5-tet-raphenyl-1H-pyrrole. Chem. Eur. J. 24, 14269-14274 (2018).
- Zhao, Y. & Truhlar, D. G. The MO6 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 120, 215-241 (2007).
- Dunning, T. H. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 90, 1007-1023 (1989).
- Riplinger, C. & Neese, F. An efficient and near linear scaling pair natural orbital based local coupled cluster method. J. Chem. Phys 38, 034106 (2013).
- Neese, F., Wennmohs, F., Becker, U. & Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 52, 224108 (2020).
- Lu, T. & Chen, Q. Shermo: A general code for calculating molecular thermochemistry properties. Comput. Theor. Chem. 1200, 113249 (2021).
- Grimme, S. Supramolecular binding thermodynamics by dispersion-corrected density functional theory. Chem. Eur. J. 18, 9955-9964 (2012).
- Becke, A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 8, 5648-5652 (1993).
- Brandenburg, J. G., Bannwarth, C., Hansen, A. & Grimme, S. B97-3c: A revised low-cost variant of the B97-D density functional method. J. Chem. Phys. 48, 064104 (2018).
- Bussi, G., Donadio, D. & Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 26, 014101 (2007).
الشكر والتقدير
مساهمات المؤلفين
المصالح المتنافسة
معلومات إضافية
المواد التكميلية المتاحة على
https://doi.org/10.1038/s41467-024-45678-1.
http://www.nature.com/reprints
© المؤلفون 2024
مدرسة الكيمياء وهندسة المواد، جامعة ونتشو، ونتشو 325035، جمهورية الصين الشعبية. مدرسة علوم المواد والهندسة، معهد بكين للتكنولوجيا، 10081 بكين، جمهورية الصين الشعبية. المختبر الرئيسي للمواد المتقدمة ومركز أبحاث مشترك لعالم نوبل في فيرينغا، مركز العلوم الأمامية لعلم المواد والكيماويات الديناميكية، مدرسة الكيمياء والهندسة الجزيئية، جامعة شرق الصين للعلوم والتكنولوجيا، طريق ميلونغ 130، شنغهاي 200237، جمهورية الصين الشعبية. ساهم هؤلاء المؤلفون بالتساوي: كايجون تشين، يونغفينغ تشانغ. - البريد الإلكتروني: yunxianglei@wzu.edu.cn; xiaobhuang@wzu.edu.cn; maxiang@ecust.edu.cn
DOI: https://doi.org/10.1038/s41467-024-45678-1
PMID: https://pubmed.ncbi.nlm.nih.gov/38341441
Publication Date: 2024-02-10
Twofold rigidity activates ultralong organic high-temperature phosphorescence
Accepted: 31 January 2024
Published online: 10 February 2024
(D) Check for updates
Abstract
A strategy is pioneered for achieving high-temperature phosphorescence using planar rigid molecules as guests and rigid polymers as host matrix. The planar rigid configuration can resist the thermal vibration of the guest at high temperatures, and the rigidity of the matrix further enhances the hightemperature resistance of the guest. The doped materials exhibit an afterglow of 40 s at
singlet excitons) are sensitive to temperature and prone to thermal deactivation, resulting in the rapid termination of the afterglow at high temperature
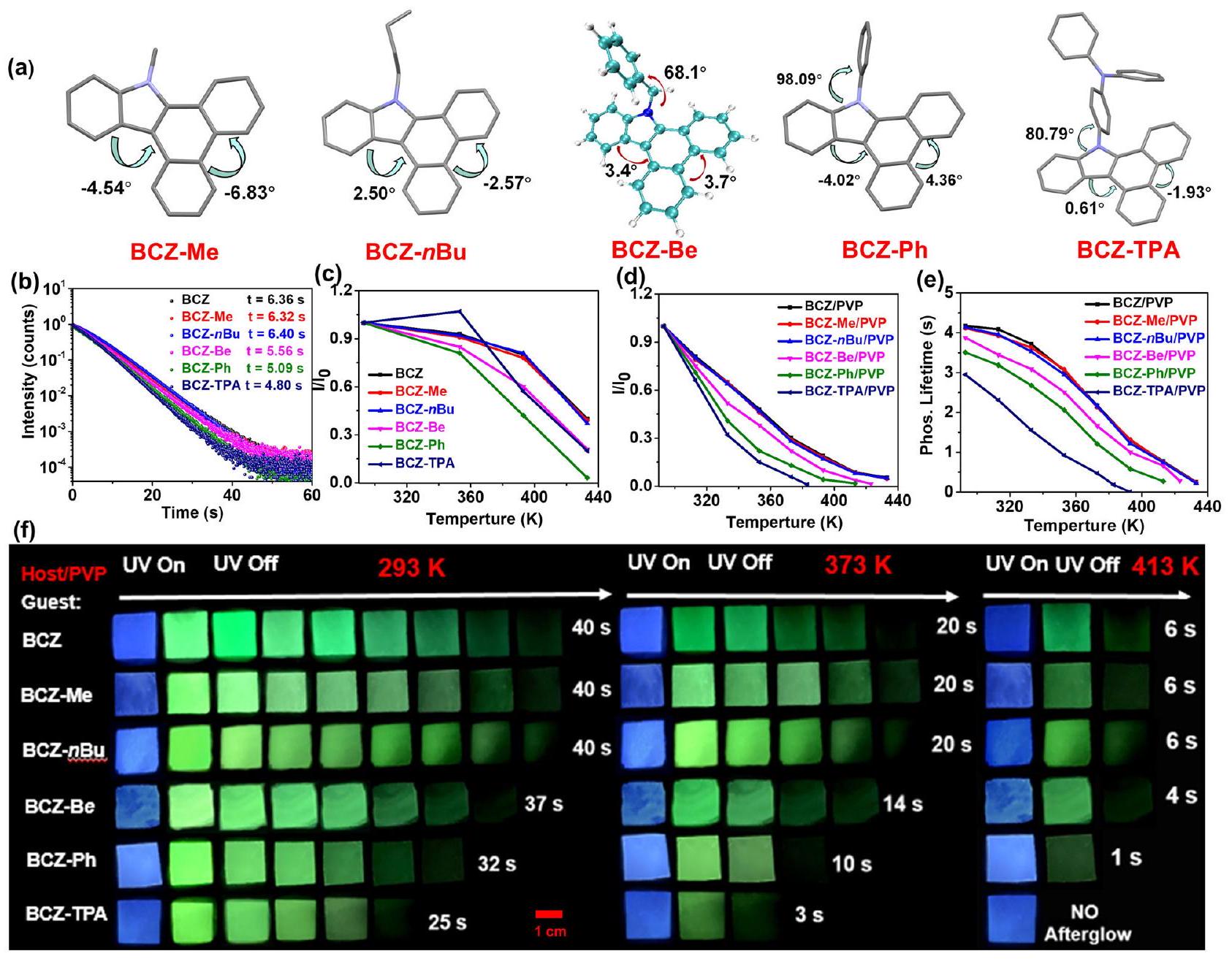
strong rotational ability) were further connected to the BCZ molecule to generate five control guests (BCZ-Me, BCZ-nBu, BCZ-Be, BCZ-Ph, and BCZ-TPA). The alkyl groups (such as methyl or
Results
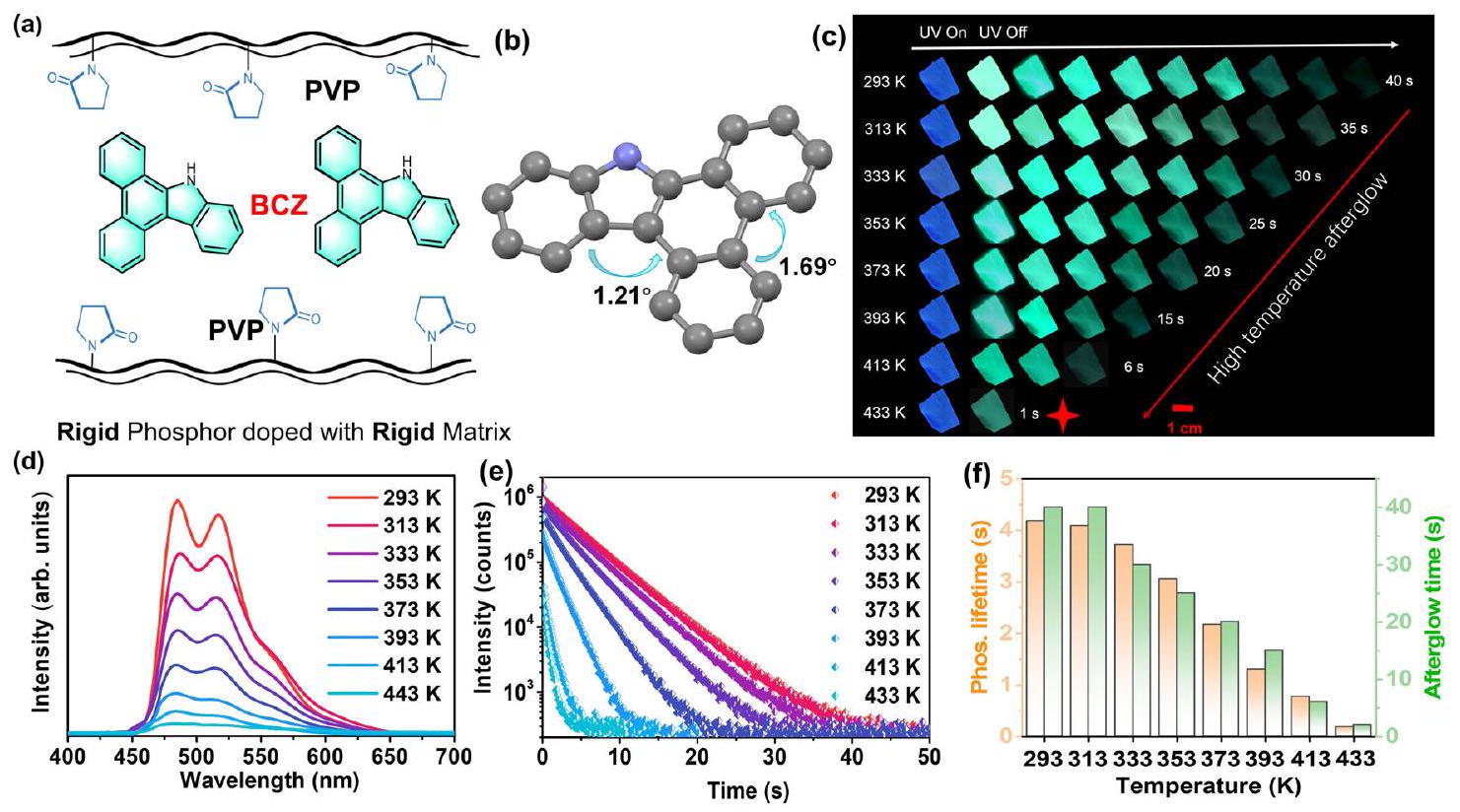
Synthesis and photophysical properties


HTP properties of the doped system
Influence of guests on the HTP

of six guests at various temperatures (DMSO as solvent,
benzene ring (Supplementary Fig. 11). These calculation results indicate that the influence of alkyl substituents on the excited-state energy of the guest can be almost negligible, and the benzyl group exhibited a weak influence, whereas phenyl and triphenylamine groups exhibited a strong influence. In other words, these latter two groups can be used as a rotor to consume the energy of the excited state
by the thermal motion. In the nonpolar solvent
correspondingly decreased (Fig. 3f). The aforementioned control experiments demonstrated that failing to inhibit the molecular motion can substantially damage the HTP properties of organic materials, and confirmed that selecting planar rigid molecules as guests with a weak propensity to undergo molecular motion is an effective strategy for constructing HTP materials. The fluorescence emission of six doped films at various temperatures was also tested. The fluorescence emission of BCZ/PVP, BCZ-Me/PVP, and BCZ-nBu/PVP exhibited almost the same high-temperature resistance properties; whereas from BCZ-Be/PVP to BCZ-TPA/PVP, the high-temperature resistance ability of the fluorescence emission gradually decreased (Supplementary Fig. 18). However, because of the superior stability of singlet excitons compared with triplet excitons, the decrease in the fluorescence intensity of the doped films was less than that of the phosphorescence intensity.
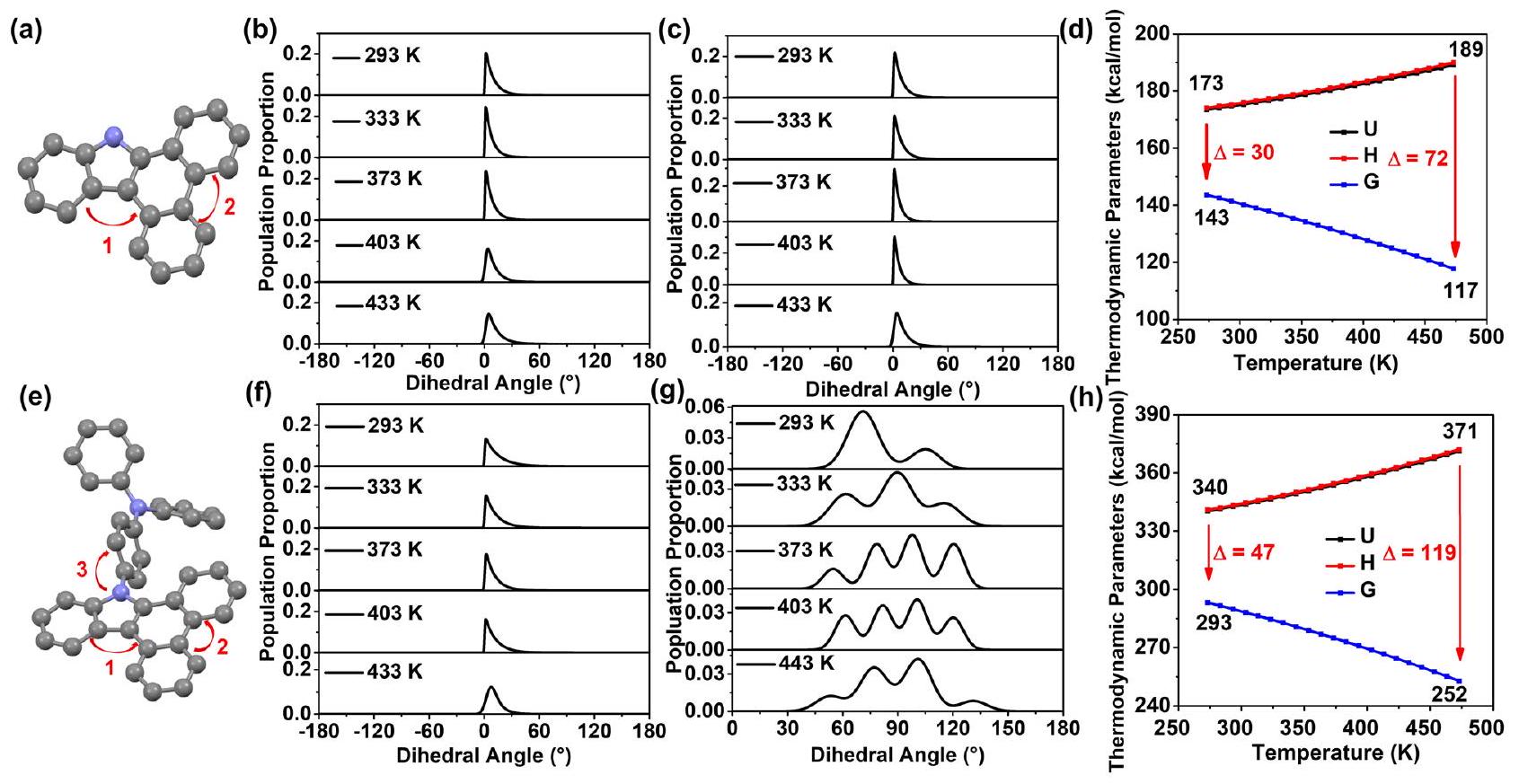
Energy and optical changes of guests at high temperatures
substantially with increasing temperature. From 293 K to 443 K , the angle with the highest probability of occurrence gradually increased from


Influence of hosts on the HTP
phosphorescence performance of BCZ/PVA and BCZ/PA66 at high temperatures was inferior to that of BCZ/PVP, indicating that host polymers with a low
Universality of strategy

Application of HTP materials to fire protection
and thick-smoke environments. The smoke penetration ability of luminescence sources such as fluorescence and phosphorescence are stronger than that of incandescence sources such as common lighting lamps. In addition, phosphorescence materials with a long afterglow time do not require constant excitation, which not only saves electricity but also reduces the radiation of the excitation source to the human body. Therefore, phosphorescence materials are suitable for luminescent recognition in search-and-rescue operations. However, conventional organic phosphorescence materials are sensitive to temperature, and the high temperature generated by fires can substantially quench their phosphorescence emission. Therefore, the long-lifetime and high-temperature resistant phosphorescence material that was designed in this study is expected to help identify firefighters during rescue and firefighting operations. Accordingly, a convenient device was prepared that emits phosphorescence upon straightforward excitation. The device consists of a battery, a controller, three excitation light bulbs (Ex.

Discussion
Methods
Synthesis or purchase of guest compounds
at
solvent under reduced pressure, the residue was purified by flash chromatography on silica gel to afford the target product BCZ-nBu.
Preparation of doped materials with PVP as the host
Preparation of doped materials with PVA as the host
Preparation method of doped materials with PA66 as the host
Reporting summary
Data availability
References
- Chen, W. et al. Highly bright and stable single-crystal perovskite light-emitting diodes. Nat. Photon. 17, 401-407 (2023).
- Wang, X. et al. Organic phosphors with bright triplet excitons for efficient X-ray-excited luminescence. Nat. Photon. 15, 187-192 (2021).
- Ye, W. et al. Confining isolated chromophores for highly efficient blue phosphorescence. Nat. Mater. 20, 1539-1544 (2021).
- Liang, Y. et al. UV-curing-enhanced organic long-persistent luminescence materials. Adv. Mater. 35, e2304820 (2023).
- Zhang, Y. et al. Ultraviolet irradiation-responsive dynamic ultralong organic phosphorescence in polymeric systems. Nat. Commun. 12, 2297 (2021).
- Wang, Y. et al. High performance of simple organic phosphorescence host-guest materials and their application in time-resolved bioimaging. Adv. Mater. 33, e2007811 (2021).
- Tao, Y. et al. Resonance-induced stimuli-responsive capacity modulation of organic ultralong room temperature phosphorescence. J. Am. Chem. Soc 144, 6946-6953 (2022).
- Zhang, X. et al. Multicolor hyperafterglow from isolated fluorescence chromophores. Nat. Commun. 14, 475 (2023).
- Zhai, Y. et al. Room temperature phosphorescence from natural wood activated by external chloride anion treatment. Nat. Commun. 14, 2614 (2023).
- Yang, Y. et al. Efficient and color-tunable dual-mode afterglow from large-area and flexible polymer-based transparent films for anticounterfeiting and information encryption. Angew. Chem. Int. Ed. 61, e202201820 (2022).
- Wang, H., Li, Q. & Tang, B. Z. High-quality manuscripts complement scientific value and readability. Matter 5, 3072-3075 (2022).
- Dai, W. et al. Halogen bonding: a new platform for achieving multi-stimuli-responsive persistent phosphorescence. Angew. Chem. Int. Ed. 61, e202200236 (2022).
- Xiao, F. et al. Guest-host doped strategy for constructing ultralonglifetime near-infrared organic phosphorescence materials for bioimaging. Nat. Commun. 13, 186 (2022).
- Huang, A. et al. Organic persistent RTP crystals: from brittle to flexible by tunable self-partitioned molecular packing. Adv. Mater. 35, e2209166 (2023).
- Yan, Z. A., Lin, X., Sun, S., Ma, X. & Tian, H. Activating roomtemperature phosphorescence of organic luminophores via external heavy-atom effect and rigidity of ionic polymer matrix. Angew. Chem. Int. Ed. 60, 19735-19739 (2021).
- Zhu, T., Yang, T., Zhang, Q. & Yuan, W. Z. Clustering and halogen effects enabled red/near-infrared room temperature phosphorescence from aliphatic cyclic imides. Nat. Commun. 13, 2658 (2022).
- Wang, J. et al. A facile strategy for realizing room temperature phosphorescence and single molecule white light emission. Nat. Commun. 9, 2963 (2018).
- Zhang, K. et al. Aromatic amides: a smart backbone toward isolated ultralong bright blue-phosphorescence in confined polymeric films. Angew. Chem. Int. Ed. 62, e202300927 (2023).
- Li, J. et al. A direct observation of up-converted room-temperature phosphorescence in an anti-Kasha dopant-matrix system. Nat. Commun. 14, 1987 (2023).
- Wang, T. et al. Thermochromic aggregation-induced dual phosphorescence via temperature-dependent sp(3)-linked donoracceptor electronic coupling. Nat. Commun. 12, 1364 (2021).
- Xia, Y. et al. Host-guest doping in flexible organic crystals for roomtemperature phosphorescence. Angew. Chem. Int. Ed. 62, e202217547 (2023).
- Ding, B., Ma, L., Huang, Z., Ma, X. & Tian, H. Engendering persistent organic room temperature phosphorescence by trace ingredient incorporation. Sci. Adv. 7, eabf9668 (2021).
- Lei, Y. et al. Wide-range color-tunable organic phosphorescence materials for printable and writable security inks. Angew. Chem. Int. Ed. 59, 16054-16060 (2020).
- Ma, H., Peng, Q., An, Z., Huang, W. & Shuai, Z. Efficient and longlived room-temperature organic phosphorescence: theoretical descriptors for molecular designs. J. Am. Chem. Soc. 141, 1010-1015 (2019).
- Zhao, W. et al. Boosting the efficiency of organic persistent roomtemperature phosphorescence by intramolecular triplet-triplet energy transfer. Nat. Commun. 10, 1595 (2019).
- Zhao, W., He, Z. & Tang, B. Z. Room-temperature phosphorescence from organic aggregates. Nat. Rev. Mater. 5, 869-885 (2020).
- Wang, T., Gupta, A. K., Wu, S., Slawin, A. M. Z. & Zysman, C. E. Conjugation-modulated excitonic coupling brightens multiple triplet excited states. J. Am. Chem. Soc. 145, 1945-1954 (2023).
- Shao, W. & Kim, J. Metal-free organic phosphors toward fast and efficient room-temperature phosphorescence. Acc. Chem. Res. 55, 1573-1585 (2022).
- Peng, Q., Ma, H. & Shuai, Z. Theory of long-lived room-temperature phosphorescence in organic aggregates. Acc. Chem. Res. 54, 940-949 (2021).
- Hirata, S. Molecular physics of persistent room temperature phosphorescence and long-lived triplet excitons. Appl. Phys. Rev. 9, 011304 (2022).
- Gao, R., Kodaimati, M. S. & Yan, D. Recent advances in persistent luminescence based on molecular hybrid materials. Chem. Soc. Rev. 50, 5564-5589 (2021).
- Niu, Y. et al. A universal strategy for achieving dual cross-linked networks to obtain ultralong polymeric room temperature phosphorescence. Sci. China Chem. 66, 1161-1168 (2023).
- Zhu, Y. et al. Ultralong polymeric room temperature phosphorescence materials fabricated by multiple hydrogen bondings resistant to temperature and humidity. Adv. Optical. Mater. 9, 2100782 (2021).
- Zhang, J. et al. Highly efficient and robust full-color organic afterglow through 2d superlattices embedment. Adv. Mater. 34, e2206712 (2022).
- Lv, H. et al. Highly stable metal-free long-persistent luminescent copolymer for low flicker AC-LEDs. Angew. Chem. Int. Ed. 61, e202204209 (2022).
- Cai, S. et al. Enabling long-lived organic room temperature phosphorescence in polymers by subunit interlocking. Nat. Commun. 10, 4247 (2019).
- Wei, J. et al. Conformation-dependent dynamic organic phosphorescence through thermal energy driven molecular rotations. Nat. Commun. 14, 627 (2023).
- Liu, X., Liao, Q., Yang, J., Li, Z. & Li, Q. Unveiling one-to-one correspondence between excited triplet states and determinate interactions by temperature-controllable blue-green-yellow afterglow. Angew. Chem. Int. Ed. 62, e202302792 (2023).
- Guo, S. et al. Recent progress in pure organic room temperature phosphorescence of small molecular host-guest systems. ACS Mater. Lett. 3, 379-397 (2021).
- Yan, X. et al. Recent advances on host-guest material systems toward organic room temperature phosphorescence. Small 18, e2104073 (2022).
- Lei, Y. et al. Stimulus-responsive organic phosphorescence materials based on small molecular host-guest doped systems. J. Phys. Chem. Lett. 14, 1794-1807 (2023).
- Zhang, T. et al. Molecular engineering for metal-free amorphous materials with room-temperature phosphorescence. Angew. Chem. Int. Ed. 59, 11206-11216 (2020).
- Dai, W. et al. Red-emissive organic room-temperature phosphorescence material for time-resolved luminescence bioimaging. CCS Chem. 4, 2550-2559 (2022).
- Lei, Y. et al. The dual-state luminescent mechanism of 2,3,4,5-tet-raphenyl-1H-pyrrole. Chem. Eur. J. 24, 14269-14274 (2018).
- Zhao, Y. & Truhlar, D. G. The MO6 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 120, 215-241 (2007).
- Dunning, T. H. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 90, 1007-1023 (1989).
- Riplinger, C. & Neese, F. An efficient and near linear scaling pair natural orbital based local coupled cluster method. J. Chem. Phys 38, 034106 (2013).
- Neese, F., Wennmohs, F., Becker, U. & Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 52, 224108 (2020).
- Lu, T. & Chen, Q. Shermo: A general code for calculating molecular thermochemistry properties. Comput. Theor. Chem. 1200, 113249 (2021).
- Grimme, S. Supramolecular binding thermodynamics by dispersion-corrected density functional theory. Chem. Eur. J. 18, 9955-9964 (2012).
- Becke, A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 8, 5648-5652 (1993).
- Brandenburg, J. G., Bannwarth, C., Hansen, A. & Grimme, S. B97-3c: A revised low-cost variant of the B97-D density functional method. J. Chem. Phys. 48, 064104 (2018).
- Bussi, G., Donadio, D. & Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 26, 014101 (2007).
Acknowledgements
Author contributions
Competing interests
Additional information
supplementary material available at
https://doi.org/10.1038/s41467-024-45678-1.
http://www.nature.com/reprints
© The Author(s) 2024
School of Chemistry and Materials Engineering, Wenzhou University, Wenzhou 325035, PR China. School of Materials Science & Engineering, Beijing Institute of Technology, 10081 Beijing, PR China. Key Laboratory for Advanced Materials and Feringa Nobel Prize Scientist Joint Research Center, Frontiers Science Center for Materiobiology and Dynamic Chemistry, School of Chemistry and Molecular Engineering, East China University of Science and Technology, Meilong Road 130, Shanghai 200237, PR China. These authors contributed equally: Kaijun Chen, Yongfeng Zhang.
