DOI: https://doi.org/10.1007/s11270-023-06782-y
تاريخ النشر: 2024-01-01
الطحالب الزرقاء الخضراء الضارة: الأسباب، التأثيرات، وإدارة المخاطر
© المؤلفون 2024
الملخص
تعتبر الطحالب الزرقاء الخضراء الضارة (cHABs) تهديدًا متزايدًا للحياة المائية، والسياحة البيئية، وبعض الاستثمارات العقارية. إن حدوثها العشوائي والمتقطع جعل تدابير التخفيف مهمة شاقة؛ علاوة على ذلك، فإن الاتجاهات الحالية المتعلقة بالأنشطة البشرية، خاصة في الزراعة والصناعة، تنذر بمزيد من الأحداث غير المرغوب فيها. بخلاف التدهور الجمالي الذي تخلقه في بيئاتها،
1 المقدمة
وأشارت الدراسات إلى زيادة عالمية في الطحالب الزرقاء في النظام البيئي المائي. على سبيل المثال، في عام 2017، توقعت دراسة آثار تغير المناخ على تركيز الطحالب الزرقاء في خزانات المياه الكبيرة في الولايات المتحدة باستخدام إطار نمذجة إحصائية (تشابرا وآخرون، 2017). استخدم الإطار توقعات تغير المناخ من خمسة نماذج حسابية عالمية، ونمو الطحالب الزرقاء، وسيناريوهين لانبعاثات غازات الدفيئة مقترنة بنموذج جودة المياه/الشبكة الهيدرولوجية للولايات المتحدة المتجاورة. توقع النموذج زيادة محتملة في عام 2017 في متوسط عدد أيام cHABs الضارة من 7 أيام في السنة لكل جسم مائي إلى 16-23 يومًا في عام 2050 و18-39 يومًا في عام 2090 بسبب زيادة مستويات المغذيات وكذلك ارتفاع درجة حرارة المياه في البيئة المائية (تشابرا وآخرون، 2017). يمتد انتشار ازدهارات الطحالب الزرقاء في بحيرة تايهو حاليًا عبر تقريبًا
(DSP) ، التسمم بالصدفيات السامة العصبية (NSP) ، والتسمم بالصدفيات المسببة للنسيان (ASP) (غولامي وآخرون ، 2019). تشمل المتلازمات الأخرى المرتبطة بالازدهار الطحلبي الضار التسمم بالباليتوكسين ، التسمم بالأزاسبيراسيد (AZP) والتسمم بالتيتروتوكسين (تسيكوتي وجينيتساريس ، 2021) ، البريفوتوكسينات (BTX) ، حمض الأوكادويك (OA) ، حمض الدومويك (DA) ، والعديد من السموم الأخرى (دوران-فينيت وآخرون ، 2021). يتم نقل هذه السموم إلى البشر من خلال استهلاك الكائنات الحية التي نجت والتي تراكمت فيها هذه السموم من خلال التقدم الغذائي المتسلسل في شبكة الغذاء (مارامبوتي وآخرون ، 2021). وفقًا للبيانات التي تم جمعها من قاعدة بيانات أحداث الطحالب الضارة (HAEDAT) من 1987 إلى 2022 ، حدثت حوالي 82 حالة وفاة بشرية ، و4045 حالة تسمم في المأكولات البحرية و181 تقريرًا عن تغير لون المياه بسبب الازدهارات الطحلبية الضارة.http://haedat.iode.org/). كان مثالاً على تحدي ازدهار السيانوبكتيريا هو أزمة مياه ووشي في مايو 2007، التي أثرت على حوالي 2 مليون نسمة لم يكن لديهم وصول إلى مياه الشرب لأكثر من أسبوع بسبب ازدهار سموم ميكروسيستيس spp. الضخمة (تشين وآخرون، 2010).
(بيرل وأوتن 2016). ومع ذلك، يتم بدء عدة cHABs بـ
(ط) إنتاج المستقلبات السامة، بالإضافة إلى الاستبعاد التنافسي بين السيانوبكتيريا، مجتمع الازدهار الذي يقمع تكاثر الفيتوبلانكتون (شيوه وآخرون، 2018)
(ii) التغذية الانتقائية أو البديلة للزوبلانكتون على الفيتوبلانكتون والسايانو باكتيريا، مما يعزز إما تكوين أو قمع ازدهار السايانو باكتيريا (Zhao et al., 2021).
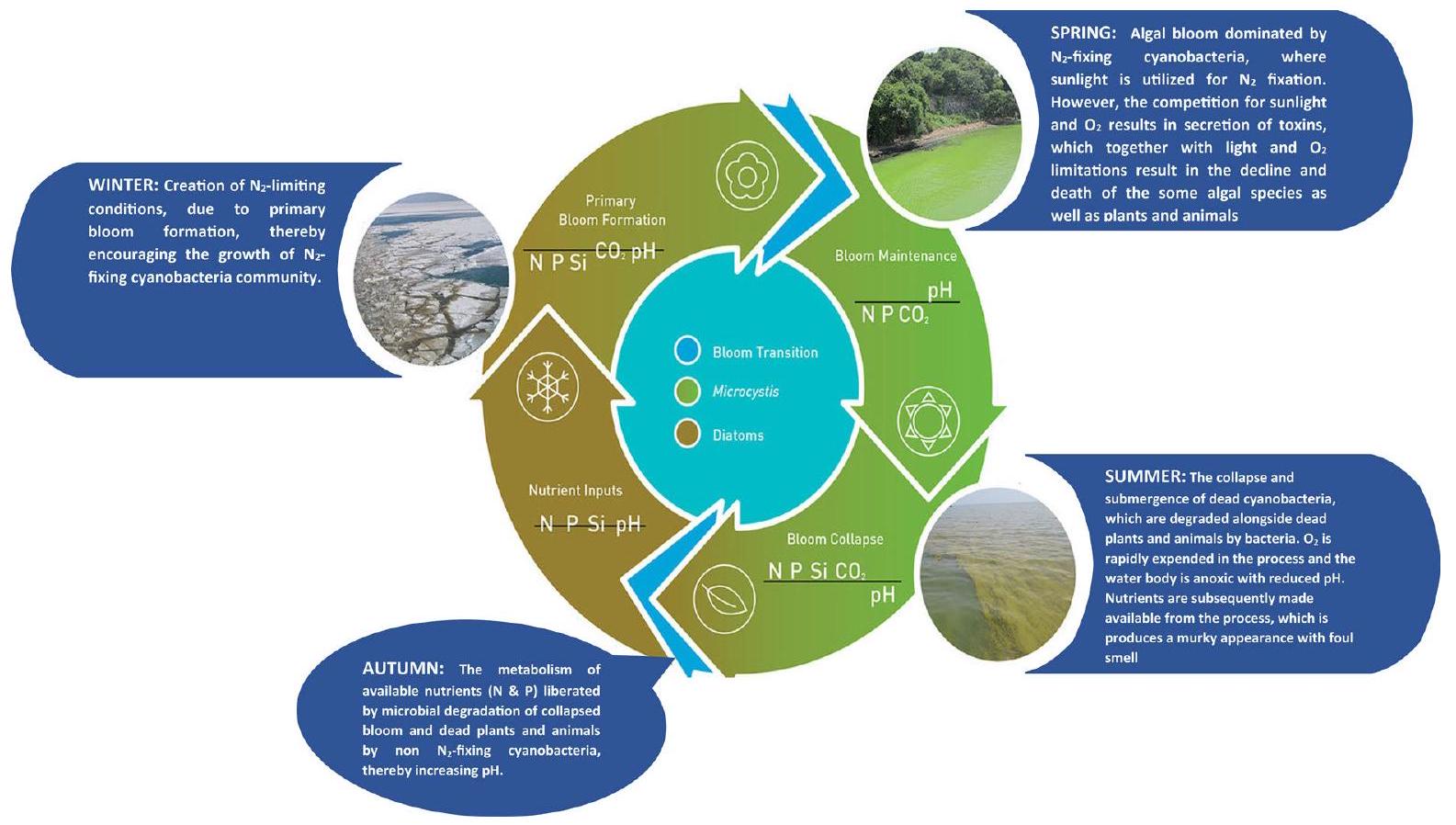
2 عوامل تؤثر على ازدهار الطحالب الضارة
النمو والصيانة. غالبًا ما تحدد تنظيم هذه العوامل ديناميات سكانها وبالتالي تشكيل الكائنات الحية الضارة المتكررة (cHABs) في أي مصفوفة بيئية معينة. علاوة على ذلك، يمكن أن تؤثر هذه العوامل على حالات الكائنات الحية الضارة المتقطعة والعفوية، سواء بشكل فردي أو بشكل متداخل. ومن ثم، يمكن تصنيف المحركات الرئيسية لتشكيل الكائنات الحية الضارة المتكررة (cHABs) وغيرها من الكائنات الحية الضارة إلى ما يلي:
2.1 الإثراء الغذائي
ظروف المياه غير المرغوب فيها، جودة المياه، وتوازن الكائنات الحية في الماء لتشكيل الطحالب (نامساراييف وآخرون، 2020). عادةً ما تزداد التغذية الزائدة والتلوث بسبب هطول الأمطار نتيجة لنقل الملوثات والجريان الغني بالمغذيات عبر البيئات المائية العذبة إلى الاستمرارية البحرية. وقد تم الإبلاغ عنها كسبب رئيسي لزيادة حدوث الطحالب الضارة السامة (cHABs) وهي من العوامل الرئيسية التي تدفع لتشكيل ازدهار الطحالب الزرقاء (تشيريكو وآخرون، 2020). تشمل المسارات الرئيسية للمغذيات التي تؤدي إلى التغذية الزائدة تآكل التربة، تصريف مياه الصرف الصحي، تسرب أو فيضان المجاري المنزلية، تخصيب الأراضي الزراعية، وتصريف الأراضي الزراعية (الشكل 2) (لي وآخرون، 2019). تؤدي التغذية الزائدة إلى نمو الطحالب الزرقاء التي تشكل بعد ذلك ازدهار الطحالب وتصبح واحدة من التحديات الرئيسية.
مرتبط بجودة المياه في المناطق ذات الكثافة السكانية العالية. كشفت أصباغ السيانوبكتيريا في نوى الرواسب من مسح لأكثر من 100 بحيرة في أمريكا الشمالية وأوروبا عن زيادة تقارب
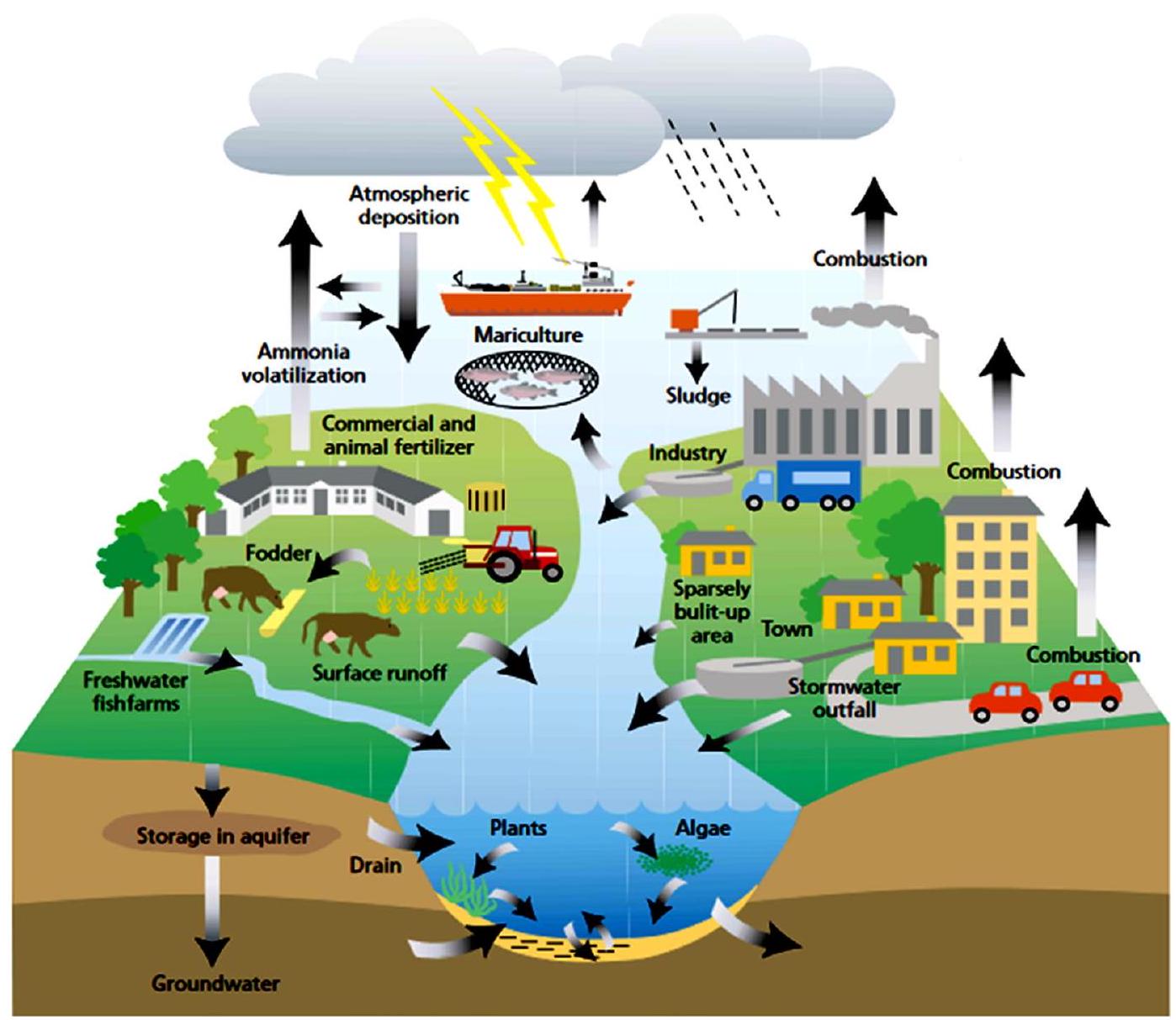
2.2 الأنشطة البشرية
أرضًا مثالية للتبرز في العراء. نظرًا لأن المنظفات وبراز الإنسان غنيان بـ N و P، فإن المسطحات المائية المجاورة قد تكون لديها احتمالية عالية لتدفق المغذيات والتحميل الزائد خلال الفيضانات المفاجئة. تشمل الملوثات الأخرى التي تلوث المسطحات المائية عبر التفريغ العرضي وتخريب الأنابيب بسبب الأنشطة البشرية التي تشجع بشكل كبير على عمليات الازدهار العرضي منتجات البترول مثل الكيروسين، وزيت التشحيم المستعمل، والوقود الممتاز، والديزل، والبنزين، وزيت الغاز للسيارات، والمعادن الثقيلة (Nwankwegu et al., 2019). وقد تم الإبلاغ عن أن الأنشطة البشرية تغير هياكل المجتمع العائم، وأنماط الهجرة، وتوزيع الأنواع التي تؤدي إلى تغييرات في الجغرافيا الحيوية وديناميات HAB، وخاصة cHABs (Müller et al., 2020).
2.3 المتغيرات الهيدروديناميكية
الظروف الهيدروديناميكية وأحمال التلوث (Mao et al., 2015). وبالمثل، حيث أن معظم الكتلة الحية، والجسيمات المعلقة، والملوثات، والغازات المذابة مختلطة ومحمولة بواسطة الحركات المضطربة، قد تتأثر تفاعلات متغيرات جودة المياه المختلفة بشكل كبير بالظروف الهيدروديناميكية المتقلبة لتشكيل الازدهارات (Mao et al., 2015). في النظم البيئية النهرية، تعتبر التغييرات في الظروف الهيدرولوجية، مثل مستوى المياه، والاهتزاز، والشفافية، ذات تأثير كبير على نمو الطحالب، والانتشار، والهجرة، وتراكم الازدهار (Cheng et al., 2019).
2.4 تغير المناخ
الجفاف. يؤدي ذلك إلى زيادة زمن بقاء المغذيات في البيئات التي تخلق ظروفًا مثالية لتكوين ازدهار الكائنات الحية الدقيقة السامة (cHABs) وغيرها من الكائنات الحية السامة (HABs) (ميشالك وآخرون، 2013). ساهم تغير المناخ في الزيادة المستمرة في عدد الكائنات الدقيقة (البكتيريا الزرقاء) في البيئة المائية لتكوين ازدهار الطحالب. يمكن أن تؤدي الطبقة السميكة من تجمعات الكائنات الدقيقة إلى حدوث الكائنات الحية السامة. في هذه الحالة، يتم منع الضوء من اختراق الجسم المائي مما يؤدي إلى موت النباتات المائية التي تحتاج إلى الضوء لعملية التمثيل الضوئي (كاسان وآخرون، 2021). تشمل التأثيرات الأخرى المدفوعة بالمناخ على حدوث وتوزيع مجموعات الفيتوبلانكتون المكونة للزهور مثل البكتيريا الزرقاء الملوحة، والأكسجين المذاب، وشدة الضوء، ودرجة الحرارة، ودرجة الحموضة. ومن المثير للاهتمام أن هذه البيئات قد تم تمييزها بانتظام بوجود أو وجود السميات الزرقاء (كوسوماواتي ومانغكوديهاردجو، 2021). في البيئة المائية، يزيد وجود cHABs
2.5 استراتيجيات التكيف الإيكولوجي الفسيولوجي
عادةً ما كانت تتفوق على الكتل الأكبر في استغلال الضوء.
خاصة خلال موسم الجفاف (الذي يتميز عادة بالجفاف الشديد)، تشكل بعض أنواع السيانوبكتيريا (أنابينا، نودولاريا، سيليندروسبرموبسيس وجلوترشيا) الأكينيتات أو الخلايا الساكنة ذات الجدران السميكة، والتي تكون أكثر كثافة من الخلايا النباتية وبالتالي تترسب. في هذه المرحلة، يسمح الطلب القليل أو المعدوم للخلايا للسيانوبكتيريا بالبقاء على قيد الحياة في ظروف الرواسب القاعية غير المواتية لفترات طويلة. ومن المثير للاهتمام أن الخلايا الغارقة يمكن أن تتحمل ظروف بيئية قاسية، مثل درجات الحرارة العالية، وتبدأ في الإنبات عندما يتم استعادة الظروف الملائمة.
3 السيانوبكتيريا والسموم الخاصة بها
| فئة السموم السيانينية | الأجناس السمية | المراجع | |||
| السموم العصبية | |||||
| ساكسي توكسين (>60) | سيليندروسبرموبسيس، أنابينا، لينغبيا، أفيزومينون وبلانكتوثريكس | لاجو وآخرون، 2015 | |||
| أناتوكسين-أ | أنابينا، سيليندروبرم، رافيديوبيس، أفيزومينون، أوسيلاطوريا، وبلاكتوثريكس |
|
|||
| بيتا-N-ميثيل أمينو-L-ألانين (BMAA) | أنابينا، سيليندروسبرموبسين، ميكروسيستيس، نوستوك، وبلانكتوثريكس (أوسيلاطوريا) | تشونغ وآخرون، 2013 | |||
| أناتوكسي-أ | دوليكوسبرموم | هويزمان وآخرون، 2018 | |||
| ساكسي توكسين | أفانيزومينون فلوس-أكوي، دوليكوسبرموم (سابقًا أنابينا) سيرسينا ليس، لينغبيا ووللي، بلانكتوثريكس spp.، وعزلة برازيلية من رافيديوبيس راسيبورسكي | وكالة حماية البيئة الأمريكية، 2022ب | |||
| السموم الداخلية | |||||
| ليبوبوليسكاريد | جميع السيانوبكتيريا | بلاها وآخرون، 2009 | |||
| السميات الخلوية | |||||
| سيليندروسبرموبسين (3) | أنواع Cylindrospermopsis و Aphanizomenon و Anabaena و Raphidiopsis و Umezakia و Oscillatoria |
|
|||
| سموم كبدية | |||||
| نودولارين (10) | نودولاريا | لوبيكس وآخرون 2008؛ | |||
| الميكروسيستينات (> 100) | أنابينا، نوستوك، أناباينوبسيس، أوسيلاطوريا، أفانوكابس، أرتروسبيرا، هابالوسيفون، ميكروسيستيس، بلانكتوثريكس، سنويلا، سينكوكوستيس، وورونيشينيا | كارمايكل وبوير، 2016 | |||
| السموم الجلدية | |||||
| سموم الأبلسيا | لينغبيا، شيزوتريكس، وأوسيلاطوريا | هان وآخرون، 2018 | |||
| سموم لينغبي (>8) | لينغبيا، شيزوتريكس، وأوسيلاطوريا | جيانغ وآخرون 2014 | |||
| غير محدد | |||||
| الأيروجينوسينات (>15) | ميكروسيستيس، أوسيلاطوريا، نوستوك، وبلانكتوثريكس | مانينغ ونوبلز، 2017 | |||
| أمبيغول (3) | فيشرلا | مانينغ ونوبلز، 2017 | |||
(ii) السموم العصبية (أناتوكسي-أ، ساكسي توكسين، أناتوكسي
(iii) السموم الكبدية (النودولارين والميكروسيستينات): هذه الفئة من السموم تشارك في تثبيط بروتينات الفوسفات 1 A و 2 A، مما يؤدي إلى تعزيز السرطان، تشوه الخلايا الكبدية، تلف الكبد، وزيادة الفسفرة في خيوط الهيكل الخلوي (كاثرين وآخرون، 2017).
(رابعاً) السموم الجلدية (لينغبياتوكسين، ديبروموأبليزياتوكسين وأبليزياتوكسينات)، و
المواد السامة المزعجة (السموم الداخلية من الليبوبوليسكاريد): هذه هي المسؤولة عن التهاب الغاز-
تهيج الجهاز الهضمي والجلد (ميليوني وآخرون، 2021).
تشمل الأجناس من السيانوبكتيريا ضمن فصيلة السيانوفيسي التي يمكن أن تشكل تجمعات ضارة بالأسماك (cHABs) كل من Microcystis وNodularia وCylindrospermopsis وDolichospermum وPlanktothrix (Huisman et al.، 2018). في المياه الاستوائية، يُعرف كل من Microcystis وRaphidiopsis كأجناس السيانوبكتيريا التي عادةً ما تشكل ازدهارات مرتبطة بمجموعة متنوعة من السموم التي ت degrade جودة المياه. غالبًا ما يتم إفراز السموم الناتجة عن بعض أنواع السيانوبكتيريا داخل الخلايا، ثم يتم إطلاقها في البيئة عندما تنفجر الخلايا أو تموت خلال فترات الملوحة الشديدة. على العكس، قد يتم إفراز السموم أو إطلاقها في نهاية دورة حياة ازدهار السيانوبكتيريا. يمكن أن تسبب السموم العصبية، والسموم الكبدية، وسموم الجلد الناتجة عن أنواع السيانوبكتيريا آثارًا صحية حادة ومزمنة على الإنسان (Smucker et al.، 2021).
4 طرق التعرض للسموم السيانينية وتأثيراتها على الصحة العامة
شلل في عضلات البطن وعضلات الصدر بالإضافة إلى الوفاة (مارامبوتي وآخرون، 2021). متلازمة أخرى مرتبطة بسموم الطحالب الضارة تُعرف بتسمم المحار المسبب للنسيان (ASP) يمكن أن تسبب أيضًا صداعًا، دوارًا، ارتباكًا، نقصًا حركيًا، فقدان الذاكرة القصيرة الأمد، وارتباكًا (كوديلا وآخرون، 2015).
وفقًا للتشريع البلغاري، لا يوجد حد مقبول للسموم الزرقاء في المياه (إيلييفا وآخرون، 2019). ومع ذلك، أوصت وكالة حماية البيئة الأمريكية (US EPA) بـ
تم الإبلاغ عن ذلك بعد التعرض الترفيهي لتركيزات عالية من MCs (فيدال وآخرون، 2017)
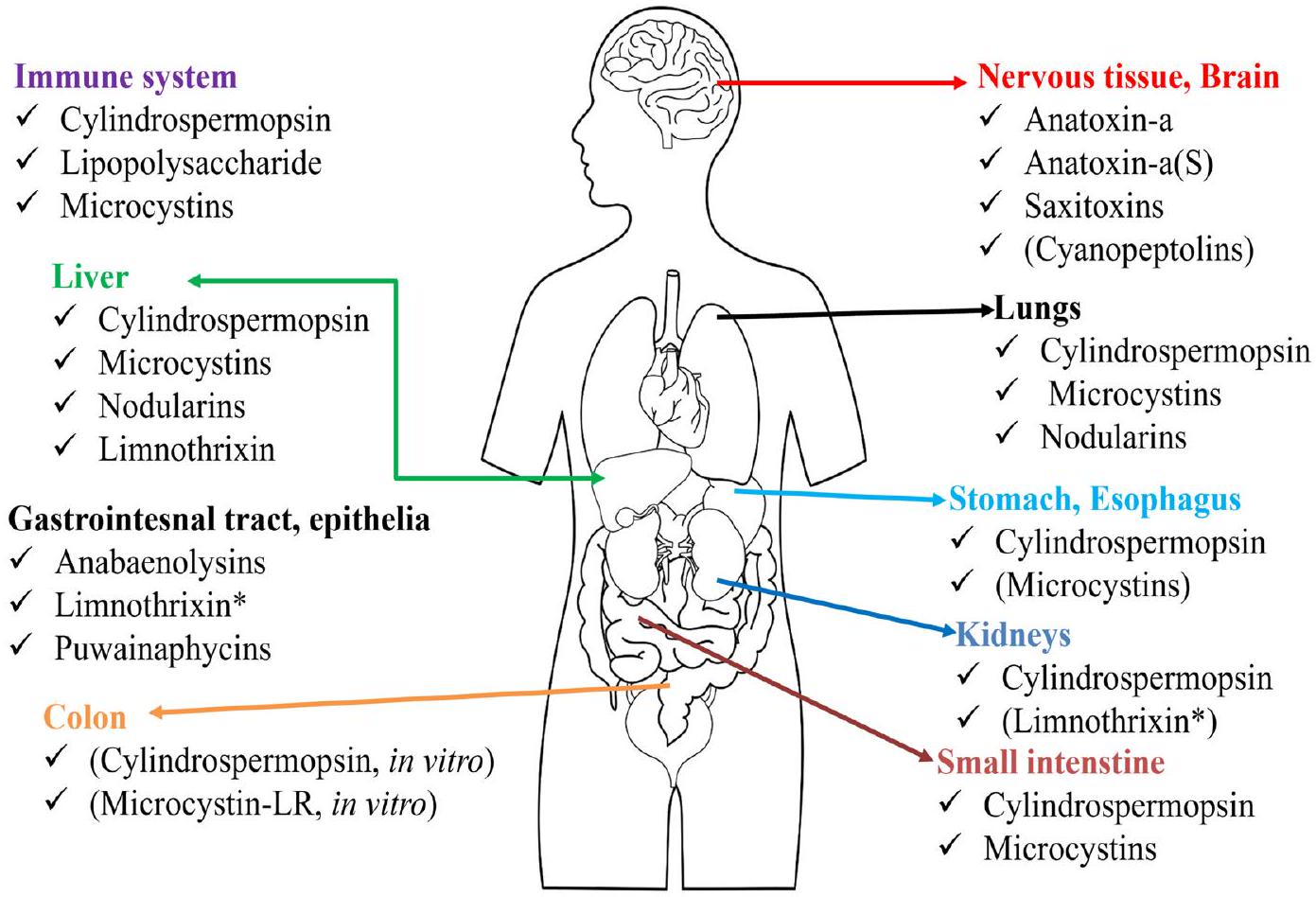
تظهر السموم الناتجة عن السيانوبكتيريا كما هو موضح في الشكل 3 (كوبكوفا وآخرون، 2019)، بينما تحتوي الجدول 2 على بعض المعلومات حول السموم المختارة وتأثيراتها الصحية المحتملة على المدى القصير والطويل.
5 تأثيرات للسموم الزرقاء على الأسماك والحيوانات الأليفة والماشية
| لا | أجناس السيانوبكتيريا | سم | آثار صحية قصيرة الأجل | آثار صحية طويلة الأمد | مرجع |
| 1 | أنابينا، أفيزومينون، أوسيلاطوريا، وبلانكتوثريكس، كريسوسبوروم (أفيزومينون) أوفالسپوروم، كوسبيدوثريكس، رافيديوبيس، سيليندروسبرموم، دوليشوسبرموم، ميكروسيستيس، فورميديوم، دوليشوسبرموم فلوس-أكواي، أ. ليمرمانيي، رافيديوبيس ميديترانيانا (سلالة من رافيديوبيس راسيبورسكي)، تيكونيما وورونيشينيا | السموم المناعية | تنميل، خدر، حرقان، كلام غير متماسك، نعاس، سيلان اللعاب، نعاس، تنميل، حرقان خدر، كلام غير متماسك، شلل تنفسي، ووفاة | اضطراب نظم القلب الذي يؤدي إلى الوفاة | لوبيكس وآخرون. 2008؛ وكالة حماية البيئة الأمريكية، 2022ب |
| 2 | أنابينا، سيليندروسبرموبسين، ميكروسيستيس، نوستوك، وبلانكتوثريكس | BMAA | غير معروف | ضمور العضلات، فقدان التنسيق، والمساهمات المحتملة في الأمراض التنكسية العصبية | هويزمان وآخرون، 2018 |
| ٣ | لينغبيا | سموم لينغبي | التهاب الجلد | أورام الجلد | فوجيكي وآخرون، 1990 |
| ٤ | أفانيزومون، وأوسيلاطور | ليبوبوليسكاريد | الجهاز الهضمي والتهاب الجلد | لوبيكس وآخرون 2008 | |
| ٥ | أنابينا، أفيزومينون، سيليندروسبرموبسيس ولينغبيا | ساكسي توكسين | تنميل، حرق، وخز، نعاس، كلام غير متماسك، شلل تنفسي، ووفاة | غير معروف | لوبيكس وآخرون 2008 |
| ٦ | أفانوكابس، ميكروسيستيس، أنابينا، نوستوك، أوسيلاطوريا، هابالوسفون، وبلانكتوثريكس، فيشرلا، غلوترشيا | الميكروسيستينات | الالتهاب الرئوي، التهاب الكبد، مشاكل الجهاز الهضمي، التهاب الجلد، النزيف، فشل الكبد والموت، التهاب الحلق، ألم في البطن، صداع، سعال، غثيان، تقرحات حول الفم، والالتهاب الرئوي | سمية تناسلية، تعزيز الأورام، تقليل إصلاح الحمض النووي، تلف الكبد والكلى | هويزمان وآخرون، 2018؛ وكالة حماية البيئة الأمريكية، 2022 |
| ٧ | أفانيزومينون، سيليندروسبرموبسيس، وأوميزاكيا، أفانيزومينون فلوس-أكوي، أفانيزومينون غراسيلي، دوليشوسبرموم بيرغي، دوليشوسبرموم لابونيكا، دوليشوسبرموم بلانكتونيكا، لينغبيا ووللي، رافيديوبيس كورفاتا، ورافيديوبيس ميديتراني | سيليندروسبرموبسين | التهاب الرئة، الجهاز الهضمي، نزيف، كبد، حمى، صداع، قيء، إسهال دموي، التهاب، والتهاب الجلد | فقدان الشهية، الشعور بالضيق، وفشل الكبد مما يؤدي إلى الوفاة | لوبيكس وآخرون 2008؛ وكالة حماية البيئة الأمريكية، 2022ب |
| ٨ | نودولاريا سبوميجينا | نودولارين | نفس التأثيرات مثل MCs | نفس التأثيرات مثل MCs | هايسمان وآخرون، 2018؛ |
المواشي، وخنازير غينيا بسبب استهلاك النودولارين (Chen et al., 2021b). في أفريقيا، كانت السموم السيانوبكتيرية غالبًا السبب الرئيسي المشتبه به في حالات الوفاة الجماعية للثدييات الأرضية الكبيرة والمتوسطة الحجم مثل الماشية (الأغنام والأبقار) وكذلك الثدييات غير القابلة للغوص (الحمار الوحشي، الإيمبالا، الويلدبيس الأزرق، الزرافات، ووحيد القرن الأبيض) (Wang et al., 2021b). في بوتسوانا، بين مايو ويونيو 2020، كانت حالات الوفاة الجماعية لأكثر من 330 فيلًا أفريقيًا يُعتقد أنها مرتبطة بالسيانوبكتيريا (السموم البيولوجية) (Wang et al., 2021b). كما كشف تقرير Backer et al. (2013) أن القطط والكلاب وثدييات أخرى قد ماتت أيضًا بسبب تسمم الأناتوكسيين-أ والميكروسيستين بعد التعرض لمياه ملوثة. بالإضافة إلى الآثار الضارة للسيانوبكتيريا المنتجة للسموم على الكائنات المائية أو الحيوانات، يمكن أن تسبب الطحالب غير السامة أيضًا آثارًا سلبية في البيئات المائية من خلال تقليل الأكسجين المذاب، واختناق الكائنات الحية القاعية والنباتات، وعرقلة خياشيم الأسماك مما يؤدي إلى موت الأسماك (Davidson 2014).
6 الآثار الاجتماعية والاقتصادية للازدهارات الضارة للطحالب الزرقاء
لقد تم الإبلاغ عنها بأنها تسبب آثارًا اجتماعية خطيرة مثل تعطيل الممارسات الاجتماعية والثقافية، والخسائر الاقتصادية (مور وآخرون، 2020)، والآثار الصحية السلبية (باكر ومور، 2012)، والخسائر لكل من الرفاهية الخاصة والعامة (ويليس وآخرون، 2018).
“لا تشرب” لسكان شمال غرب أوهايو في الولايات المتحدة الأمريكية في عام 2014 (جيتو وآخرون، 2015).
7 أساليب تقييم وإدارة المخاطر للزهور الطحلبية الضارة
يجب على المديرين اتخاذ الإجراءات المناسبة عندما تهدد هذه الأحداث مصادر المياه (USEPA، 2015b). ومن ثم، تم اعتماد استراتيجيات متنوعة من قبل العديد من الدول والشركات التجارية لإدارة ومراقبة الكائنات الحية الضارة المتزايدة (cHABs) وغيرها من الكائنات الحية الضارة في المياه الساحلية (أندرسون 2012). وفقًا لكوركوران وهنت (2021)، يمكن تقسيم أساليب الإدارة للكائنات الحية الضارة المتزايدة وغيرها من الكائنات الحية الضارة إلى ثلاث فئات مختلفة تشمل الوقاية، والسيطرة، والتخفيف.
(ط) الوقاية: في أساليب الإدارة المتعلقة بالزهور الضارة بالطحالب (cHABs)، تشير الوقاية إلى الإجراءات المتخذة لتقليل حدوث وشدة الزهور أو لمنع حدوث cHABs أو التأثير المباشر على بعض الموارد مثل إضافة المبيدات الكيميائية للطحالب، وتقليل الأحمال الغذائية، وإدارة المياه، والمنافسة البيولوجية، وتنظيم الديناميكا المائية (Zhu et al., 2021). تشمل الأساليب الفعالة الأخرى لمنع الزهور الضارة بالطحالب بما في ذلك cHABs تقليل الأحمال الغذائية في مياه الصرف الصحي ومنع التحميل الزائد للأسمدة في التربة الزراعية، والتحكم في المياه الجوفية وجريان مياه الأمطار من خلال حلول قائمة على الطبيعة مثل الترسيب، وأنظمة الترشيح الحيوي، ومناطق الإيكوتون، وحواجز إزالة النيتروجين (Morón-López, 2021; Paerl & Barnard, 2020).
(ii) السيطرة: من منظور إداري، تشير السيطرة إلى التدابير المتخذة لقتل أو تدمير الكائنات الحية الضارة (cHABs) وغيرها من الكائنات الحية الضارة (HABs) لوقف تكوين الزهور بسرعة (أندرسون، 2009). في السيطرة على الكائنات الحية الضارة، يتم استخدام خمس استراتيجيات شائعة لمكافحة الأنواع الضارة أو الخبيثة، وتشمل هذه السيطرة الكيميائية، الميكانيكية، الوراثية، البيولوجية، والبيئية (كيو وآخرون، 2021). تشمل طرق السيطرة الحصاد، التكتل والترسيب، الخلط، الشطف، استخدام المعالجة الكيميائية، البكتيريا القاتلة للطحالب، الكائنات الحية التي تتغذى على الفلاتر، الفيروسات الزرقاء، تقنية تفريغ البلازما، نظام الأشعة فوق البنفسجية/فينتون، والهندسة الوراثية (بهات وآخرون، 2023؛ كوركوران وهنت، 2021؛ الشيخ وآخرون، 2023؛ لي وآخرون، 2023).
(iii) التخفيف: يشير التخفيف إلى التدابير المتخذة لتقليل الآثار السلبية للطحالب الضارة المتزايدة (cHABs) وغيرها من الطحالب الضارة على النظام البيئي وصحة الإنسان والاقتصاد. تشمل أمثلة إجراءات التخفيف برامج المراقبة، ومعالجة مياه الشرب، وإغلاق مناطق الصيد للرخويات.
مناطق الحصاد، حركة منتجات الأسماك أو القشريات بعيدًا عن مناطق الازدهار، إغلاق الشواطئ والبحيرات، واستخدام النماذج التنبؤية (كوركوران وهنت، 2021).
7.1 التطبيق المحتمل للذكاء الاصطناعي في تقييم وإدارة مخاطر ازدهار الطحالب الضارة
الرطوبة، والهطول. هذه التكنولوجيا، إذا تم استغلالها بشكل جيد ونشرها بشكل صحيح، يمكن أن تقدم حلاً أكثر موثوقية وتوجيهًا في اتخاذ القرارات.
8 تحديات تواجه إدارة ازدهار الطحالب الضارة
9 آفاق المستقبل
في منع آثار الكائنات الحية الدقيقة السامة، يجب اعتماد الاستراتيجيات التالية.
لإنشاء مراكز ترفيهية أخرى للحفاظ على السياحة، وهذا سيساعد الأعمال المحلية خلال فترات الازدهار.
(ii) لتطوير برامج خاصة أو عامة ستوفر الدعم الاجتماعي والاقتصادي للعمال العاطلين عن العمل مؤقتًا مثل الصيادين.
(iii) لتطوير برامج تعليمية ستساعد السكان المتنوعين عرقياً على تجنب التعرض.
تم التحقيق فيه. أولاً، لوحظ أن معالجة المستخلصات قيدت نمو Microcystis aeruginosa ولكن كان لها تأثير ضئيل على الطحالب الخضراء (Scenedesmus obliqus). أيضًا، يمكن أن تعزز الأعمدة المثبتة صناعيًا أو غابات الخيزران على ضفاف الأنهار نمو المتنافسين، خاصة الدياتومات التي يمكن أن تغزو مستعمرات السيانوبكتيريا (Hao et al.، 2022). وبالمثل، فإن توحيد إجراءات تحليل السموم الزرقاء وكذلك توفير مواد مرجعية موحدة لتحديد كميتها أمر حيوي لمراقبة المياه المنتظمة أو الروتينية (Welker et al.، 2021). بعيدًا عن أجهزة الاستشعار الكهروكيميائية المذكورة سابقًا، يمكن أن يساعد تطوير أجهزة استشعار قوية في الموقع قادرة على تحديد كميات خلايا HABs والسموم في المسطحات المائية أيضًا في منع تفشي HABs والنشر المناسب للذكاء الاصطناعي. علاوة على ذلك، فإن تجنب المخاطر بشكل استباقي وكذلك خلق الوعي العام من خلال النشر ووسائل الإعلام الإخبارية ووضع علامات تحذيرية واستشارية حيث تم الإبلاغ عن CHABs وغيرها من HABs سيكون له أهمية كبيرة في منع مخاطر التسمم البشري والحيواني المحلي من HABs.
10 الخاتمة
باستخدام الذكاء الاصطناعي وإجراءات العلاج لحماية المواطنين من هذه التهديدات الصحية المحتملة.
الإعلانات
References
Ærtebjerg, G., Anderson, J. H., Hanson, O. S. (Eds.) (2003). Nutrients and eutrophication in danish marine waters. A challenge for science and management. Danish Environmental Protection Agency & National Environmental Research Institute. pp 126.
Anderson, D. M., Cembella, A. D., & Hallegraeff, G. M. (2012). Progress in understanding harmful algal blooms: Paradigm shifts and new technologies for research, monitoring, and management. Annual Review of Marine Science, 4, 143-176.
Annadotter, H., Cronberg, G., Lawton, L., Hansson, H.-B., Göthe, U., & Skulberg, O. (2001). An extensive outbreak of gastroenteritis associated with the toxic cyanobacterium Planktothrix agardhii (Oscillatoriales, Cyanophyceae) in Scania, south Sweden. In I. Chorus (Ed.), Cyanotoxins: Occurrence, causes, consequences (pp. 200-208). Springer-Verlag.
Azevedo, S. M., Carmichael, W., Jochimsen, E. M., Rinehart, K. L., Lau, S., Shaw, G. R., & Eaglesham, G. (2001). Human intoxication by microcystins during renal dialysis treatment in Caruaru-Brazil. Toxicology, 164(1-3), 32-32.
Backer, L. C., & Moore, S. K. (2012). Harmful algal blooms: Future threats in a warmer world. In A. E. Nemr (Ed.), Environmental pollution and its relation to climate change (pp. 485-512). Nova Science Publishers.
Backer, L. C., McNeel, S. V., Barber, T., Kirkpatrick, B., Williams, C., Irvin, M., Zhou, Y., Johnson, T. B., Nierenberg, K., Aubel, M., et al. (2010). Recreational exposure to microcystins during algal blooms in two California lakes. Toxicol, 55, 909-921.
Backer, L. C., Landsberg, J. H., Miller, M., Keel, K., & Taylor, T. K. (2013). Canine cyanotoxin poisonings in the United States (1920s-2012): Review of suspected and confirmed cases from three data sources. Toxins, 5(9), 1597-1628.
Barsanti, L., & Gualtieri, P. (2014). Algae: Anatomy, biochemistry, and biotechnology. CRC Press.
Beaulieu, J. J., DelSontro, T., & Downing, J. A. (2019). Eutrophication will increase methane emissions from lakes and impoundments during the 21st century. Nature Communications, 10, 1375.
Bechard, A. (2021). Gone with the wind: Declines in property values as harmful blooms are blown towards the shore. The Journal of Real Estate Finance and Economics, 62, 242-257.
Benayache, N. Y., Afri-Mehennaoui, F. Z., Kherief-Nacereddine, S., Vo-Quoc, B., Hushchyna, K., Nguyen-Quang, T., & Bouaïcha, N. (2022). Massive fish death associated with the toxic cyanobacterial Planktothrix sp. bloom in the Béni-Haroun Reservoir (Algeria). Environmental Science and Pollution Research, 29(53), pp 80849-80859.
Bhatt, P., Engel, B.A., Reuhs, M. and Simsek, H. (2023). Cyanophage technology in removal of cyanobacteria mediated harmful algal blooms: A novel and eco-friendly method. Chemosphere, p. 137769
Bláha, L., Babica, P., & Maršálek, B. (2009). Toxins produced in cyanobacterial water blooms-Toxicity and risks. Interdisciplinary Toxicology, 2(2), 36.
Bouaïcha, N., Miles, C. O., Beach, D. G., Labidi, Z., Djabri, A., Benayache, N. Y., & Nguyen-Quang, T. (2019). Structural diversity, characterization and toxicology of microcystins. Toxins, 11(12), 714.
Cao, H., Han, L., & Li, L. (2022). A deep learning method for cyanobacterial harmful algae blooms prediction in Taihu Lake China. Harmful Algae, 113, 102189.
Carmichael, W. W., & Boyer, G. L. (2016). Health impacts from cyanobacteria harmful algae blooms: Implications for the North American Great Lakes. Harmful Algae, 54, 194-212.
Catherine, A., Bernard, C., Spoof, L., & Bruno, M. (2017). Microcystins and nodularins. Handbook Cyanobacterial Monitoring Cyanotoxin Analysis, 1, 107-126.
Chapra, S. C., Boehlert, B., Fant, C., Bierman, V. J., Jr., Henderson, J., Mills, D., Mas, D. M., Rennels, L., Jantarasami, L., Martinich, J., & Strzepek, K. M. (2017). Climate change impacts on harmful algal blooms in US freshwaters: A screening-level assessment. Environmental Science and Technology, 51(16), 8933-8943.
Chatziefthimiou, A. D., Banack, S. A., & Cox, P. A. (2021). Biocrust-produced cyanotoxins are found vertically in the desert soil profile. Neurotoxicity Research, 39(1), 42-48.
Chen, L., Chen, J., Zhang, X., & Xie, P. (2016). A review of reproductive toxicity of microcystins. Journal of Hazardous Materials, 301, 381-399.
Chen, L., Giesy, J. P., Adamovsky, O., Svirčev, Z., Meriluoto, J., Codd, G. A., Mijovic, B., Shi, T., Tuo, X., Li, S. C., & Pan, B. Z. (2021). Challenges of using blooms of Microcystis spp in animal feeds: A comprehensive review of nutritional, toxicological and microbial health evaluation. Science of The Total Environment, 764, 142319.
Chen, M.-Y., Teng, W.-K., Zhao, L., Hu, C.-X., Zhou, Y.-K., Han, B.-P., Song, L.-R., & Shu, W.-S. (2021b). Comparative genomics reveals insights into cyanobacterial evolution and habitat adaptation. ISME Journal, 15, 211-227.
Chen, G., Wang, L., Wang, M., & Hu, T. (2021c). Comprehensive insights into the occurrence and toxicological issues of nodularins. Marine Pollution Bulletin, 162, 111884.
Cheng, B., Xia, R., Zhang, Y., Yang, Z., Hu, S., Guo, F., & Ma, S. (2019). Characterization and causes analysis for algae blooms in large river system. Sustainable Cities and Society, 51, 101707.
Cheung, M. Y., Liang, S., & Lee, J. (2013). Toxin-producing cyanobacteria in freshwater: A review of the problems, impact on drinking water safety, and efforts for protecting public health. Journal of Microbiology, 51(1), 1-10.
Chirico, N., António, D. C., Pozzoli, L., Marinov, D., Malagó, A., Sanseverino, I., Beghi, A., Genoni, P., Dobricic, S., & Lettieri, T. (2020). Cyanobacterial blooms in Lake Varese: Analysis and characterization over ten years of observations. Water, 12(3), 675.
Christensen, V. G., & Khan, E. (2020). Freshwater neurotoxins and concerns for human, animal, and ecosystem health: A review of anatoxin-a and saxitoxin. Science of the Total Environment, 736, 139515.
Codd, G. A., Testai, E., Funari, E., Svirčev, Z. (2020). Cyanobacteria, cyanotoxins, and human health. In: Water
treatment for purification from cyanobacteria and cyanotoxins, Anastasia E. Hiskia, Theodoros M. Triantis, Maria G. Antoniou, Triantafyllos Kaludis, Dionysios D. DIonysios, Editors, pp 37-68. John Wiley & Sons
Corcoran, A. A., & Hunt, R. W. (2021). Capitalizing on harmful algal blooms: From problems to products. Algal Research, 55, 102265.
Davis, D. A., Cox, P. A., Banack, S. A., Lecusay, P. D., Garamszegi, S. P., Hagan, M. J., Powell, J. T., Metcalf, J. S., Palmour, R. M., Beierschmitt, A., & Bradley, W. G. (2020). L-serine reduces spinal cord pathology in a vervet model of preclinical ALS/MND. Journal of Neuropathology & Experimental Neurology, 79(4), 393-406.
Dev, P. J., Sukenik, A., Mishra, D. R., & Ostrovsky, I. (2022). Cyanobacterial pigment concentrations in inland waters: Novel semi-analytical algorithms for multi-and hyperspectral remote sensing data. Science of the Total Environment, 805, 150423.
Dittmann, E., Fewer, D. P., & Neilan, B. A. (2013). Cyanobacterial toxins: Biosynthetic routes and evolutionary roots. FEMS Microbiology Reviews, 37(1), 23-43.
Drobac, B. D., Tokodi, N., Marinović, Z., Lujić, J., Dulić, T., Simić, S. B., Đorđević, N. B., Kitanović, N., Šćekić, I., Urbányi, B., & Meriluoto, J. (2021). Cyanobacteria, cyanotoxins, and their histopathological effects on fish tissues in Fehérvárcsurgó reservoir, Hungary. Environmental Monitoring and Assessment, 193(9), 1-14.
Durán-Vinet, B., Araya-Castro, K., Chao, T. C., Wood, S. A., Gallardo, V., Godoy, K., & Abanto, M. (2021). Potential applications of CRISPR/Cas for next-generation biomonitoring of harmful algae blooms: A review. Harmful Algae, 103, 102027.
El-Sheekh, M. M., Abd Al-Halim, M. A., Mohammed, S. A., (2023). Algae processing by plasma discharge technology: A review. Algal Research, 70, 102983.
Farrer, D., Counter, M., Hillwig, R., & Cude, C. (2015). Health-based cyanotoxin guideline values allow for cyanotoxin-based monitoring and efficient public health response to cyanobacterial blooms. Toxins, 7, 457-477.
Fujiki, H., Suganuma, M., Suguri, H., Yoshizawa, S., Takagi, K., Nakayasu, M., Ojika, M., Yamada, K., Yasumoto, T., Moore, R. E., & Sugimura, T. (1990). New tumor promoters from marine natural products. In S. Hall & G. Strichartz (Eds.), Marine toxins: Origin, structure and molecular pharmacology (pp. 232-240). American Chemical Society.
Funari, E., & Testai, E. (2008). Human health risk assessment related to cyanotoxins exposure. Critical Reviews in Toxicology, 38(2), 97-125.
Ger, K. A., Hansson, L. A., & Lürling, M. (2014). Understanding cyanobacteria-zooplankton interactions in a more eutrophic world. Freshwater Biology, 59(9), 1783-1798.
Ghaffar, S., Stevenson, R. J., & Khan, Z. (2016). Cyanobacteria dominance in lakes and evaluation of its predictors: A study of Southern Appalachians Ecoregion, USA. Matec Web Conf, 60, 02001.
Gholami, Z., Mortazavi, M. S., & Karbassi, A. (2019). Environmental risk assessment of harmful algal blooms case study: Persian Gulf and Oman Sea located at Hormozgan Province, Iran. Human and Ecological Risk Assessment, 25(1-2), 271-296.
Griffiths, D. J., & Saker, M. L. (2003). The Palm Island mystery disease 20 years on: A review of research on the cyanotoxin cylindrospermopsin. Environmental Toxicology: An International Journal, 18(2), 78-93.
Guo, L. (2007). Doing battle with the green monster of Taihu Lake. Science, 317(5842), 1166-1166.
Guzmán-Guillén, R., Puerto, M., Gutiérrez-Praena, D., Prieto, A. I., Pichardo, S., Jos, Á., Campos, A., Vasconcelos, V., & Cameán, A. M. (2017). Potential use of chemoprotectants against the toxic effects of cyanotoxins: A review. Toxins, 9(6), 175.
Han, B. N., Liang, T. T., Keen, L. J., Fan, T. T., Zhang, X. D., Xu, L., Zhao, Q., Wang, S. P., & Lin, H. W. (2018). Two marine cyanobacterial aplysiatoxins polyketides, neo-debromoaplysiatoxin A and B, with K+ channel inhibition activity. Organic Letters, 20, 578-581.
Hao, A., Su, M., Kobayashi, S., Zhao, M., & Iseri, Y. (2022). Multiple roles of bamboo as a regulator of cyanobacterial bloom in aquatic systems. Scientific Reports, 12(1), 1-12.
Hilborn, E. D., Roberts, V. A., Backer, L., DeConno, E., Egan, J. S., Hyde, J. B., Nicholas, D. C., Wiegert, E. J., Billing, L. M., DiOrio, M., & Mohr, M. C. (2014). Algal bloom-associated disease outbreaks among users of freshwater lakes-United States, 2009-2010. Morbidity and Mortality Weekly Report, 63(1), 11.
Hofbauer, W. K. (2021). Toxic or otherwise harmful algae and the built environment. Toxins, 13(7), 465.
Huang, J. D., & Zheng, H. (2017). Current trend of metagenomic data analytics for cyanobacteria blooms. Journal of Geoscience and Environment Protection, 5(06), 198-213.
Huang, Y. L., Huang, G. H., Liu, D. F., Zhu, H., & Sun, W. (2012). Simulation-based inexact chance-constrained nonlinear programming for eutrophication management in the Xiangxi Bay of Three Gorges Reservoir. Journal of Environmental Management, 108, 54-65.
Huisman, J., Codd, G. A., Paerl, H. W., Ibelings, B. W., Verspagen, J. M., & Visser, P. M. (2018). Cyanobacterial blooms. Nature Reviews Microbiology, 16(8), 471-483.
Ilieva, V., Kondeva-Burdina, M., Georgieva, T., & Pavlova, V. (2019). Toxicity of cyanobacteria Organotropy of cyanotoxins and toxicodynamics of cyanotoxins by species. Pharmacia, 66, 91.
Jetoo, S., Grover, V. I., & Krantzberg, G. (2015). The Toledo drinking water advisory: Suggested application of the water safety planning approach. Sustainability, 7(8), 9787-9808.
Ji, X., Verspagen, J. M., Van de Waal, D. B., Rost, B., & Huisman, J. (2020). Phenotypic plasticity of carbon fixation stimulates cyanobacterial blooms at elevated CO2. Science Advances, 6(8), eaax2926.
Jia-Fong, H., Ouddane, B., Hwang, J. S., & Dahms, H. U. (2021). In silico assessment of human health risks caused by cyanotoxins from cyanobacteria. Biocell, 45(1), 65.
Kankaanpää, H. T., Sipia, V. O., Kuparinen, J. S., Ott, J. L., & Carmichael, W. W. (2001). Nodularin analyses and toxicity of a Nodulariaspumigena (Nostocales, Cyanobacteria) water-bloom in the western Gulf of Finland, Baltic Sea, in August 1999. Phyciologia, 40, 268-274.
Karlson, B., Andersen, P., Arneborg, L., Cembella, A., Eikrem, W., John, U., West, J. J., Klemm, K., Kobos, J., Lehtinen, S., & Lundholm, N. (2021). Harmful algal blooms and their effects in coastal seas of Northern Europe. Harmful Algae, 102, 101989.
Kasan, N. A., Yusof, S. Z. M., Manan, H., Khairul, W. M., & Zakeri, H. A. (2021). Inhibitory effect of thiourea derivatives on the growth of blue-green algae. Journal of Environmental Management, 294, 113008.
Keliri, E., Paraskeva, C., Sofokleous, A., Sukenik, A., Dziga, D., Chernova, E., Brient, L., & Antoniou, M. G. (2021). Occurrence of a single-species cyanobacterial bloom in a lake in Cyprus: Monitoring and treatment with hydrogen peroxide-releasing granules. Environmental Sciences Europe, 33(1), 1-14.
Kim, J., Jonoski, A., & Solomatine, D. P. (2022). A classifi-cation-based machine learning approach to the prediction of cyanobacterial blooms in Chilgok Weir South Korea. Water, 14(4), 542.
Krienitz, L., Ballot, A., Kotut, K., Wiegand, C., Pütz, S., Metcalf, J. S., Codd, G. A., & Stephan, P. (2003). Contribution of hot spring cyanobacteria to the mysterious deaths of Lesser Flamingos at Lake Bogoria, Kenya. FEMS Microbiology Ecology, 43(2), 141-148.
Kubickova, B., Babica, P., Hilscherová, K., & Šindlerová, L. (2019). Effects of cyanobacterial toxins on the human gastrointestinal tract and the mucosal innate immune system. Environmental Sciences Europe, 31(1), 1-27.
Kudela, R., Berdalet, E., Urban, E. (2015) Harmful algal blooms: A scientific summary for policy makers. IOC/ UNESCO, Paris (IOC/INF-1320)
Kusumawati, D. I., & Mangkoedihardjo, S. (2021). Problems and use of cyanobacteria for environmental improve-ment-A. Journal of Aridland Agriculture, 7, 9-14.
Lad, A., Breidenbach, J. D., Su, R. C., Murray, J., Kuang, R., Mascarenhas, A., Najjar, J., Patel, S., Hegde, P., Youssef, M., & Breuler, J. (2022). As we drink and breathe: Adverse health effects of microcystins and other harmful algal bloom toxins in the liver, gut, lungs and beyond. Life, 12(3), 418.
Lago, J., Rodríguez, P. L., Blanco, L., Vieites, J. M., & Cabado, A. G. (2015). Tetrodotoxin, an extremely potent marine neurotoxin: Distribution, toxicity, origin and therapeutical uses. Marine Drugs, 13, 6384-6406.
Landsberg, J. H., Hendrickson, J., Tabuchi, M., Kiryu, Y., Williams, B. J., & Tomlinson, M. C. (2020). A largescale sustained fish kill in the St. Johns River, Florida: A complex consequence of cyanobacteria blooms. Harmful Algae, 92, 101771.
Li, J., Li, R., & Li, J. (2017). Current research scenario for microcystins biodegradation-a review on fundamental knowledge, application prospects and challenges. Science of the Total Environment, 595(Suppl C), 615-632.
Li, S. M., Liu, J. P., Song, K. S., Liang, C., & Gao, J. (2019). Analysis of temporal and spatial variation characteristics and driving factors of blue alga blooms in Chaohu Lake based on Landsat images. Resources and Environment in the Yangtze Basin, 28, 205-213.
Li, X., Liu, B., Wang, Y., Yang, Y., Liang, R., Peng, F., Xue, S., Zhu, Z., & Li, K. (2020). Hydrodynamic and environmental characteristics of a tributary bay influenced by backwater jacking and intrusions from a main reservoir. Hydrology and Earth System Sciences, 24(11), 5057-5076.
Li, H., Gu, X., Chen, H., Mao, Z., Zeng, Q., Yang, H., & Kan, K. (2021). Comparative toxicological effects of planktonic Microcystis and benthic Oscillatoria on zebrafish embryonic development: Implications for cyanobacteria risk assessment. Environmental Pollution, 274, 115852.
Li, L., Zhang, H., Mubashar, M., Chen, L., Cheng, S., & Zhang, X. (2023). Parallel filtration for solid-liquid separation: A case study of highly-efficient algal removal under parallel configuration driven by magnetic force. Separation and Purification Technology, 310, 123098.
Lim, M. H., Tay, H. S. M., Devotta, D. A., Mowe, M. A., & Mitrovic, S. M. (2020). Risk management of cyanotoxins in Singapore. Journal of Water Resource and Protection, 12(6), 512-525.
Lin, Q., Wu, Z., Singh, V. P., Sadeghi, S. H. R., He, H., & Lu, G. (2017). Correlation between hydrological drought, climatic factors, reservoir operation, and vegetation cover in the Xijiang Basin, South China. Journal of Hydrology, 549, 512-524.
Liu, W., Qiao, Q., Chen, Y., Wu, K., & Zhang, X. (2014). Microcystin-LR exposure to adult zebrafish (Danio rerio) leads to growth inhibition and immune dysfunction in F1 offspring, a parental transmission effect of toxicity. Aquatic Toxicology, 155, 360-367.
Lopez, C. B., Jewett, E. B., Dortch, Q., Walton, B. T., Barsanti, L., & Gualtieri, P. (2005). Algae: Anatomy, biochemistry, and biotechnology. CRC Press.
Lopez, C. B., Jewett, E. B., Dortch, Q., Walton, B. T., Hudnell, H. K. (2008). Scientific assessment of freshwater harmful algal blooms. Interagency Working Group on Harmful Algal Blooms, Hypoxia, and Human Health of the Joint Subcommittee on Ocean Science and Technology. Washington, D.C., USA
Lopez-Rodas, V., Maneiro, E., Lanzarot, M. P., Perdigones, N., & Costas, E. (2008). Mass wildlife mortality due to cyanobacteria in the Doñana National Park, Spain. Veterinary Record, 162(10), 317.
Lu, J., Zhu, B., Struewing, I., Xu, N., & Duan, S. (2019). Nitro-gen-phosphorus-associated metabolic activities during the development of a cyanobacterial bloom revealed by metatranscriptomics. Science and Reports, 9(1), 1-11.
Ma, M., Wang, X., Veroustraete, F., & Dong, L. (2007). Change in area of Ebinur Lake during the 1998-2005 period. International Journal of Remote Sensing, 28, 5523-5533.
Maity, S., Guchhait, R., Chatterjee, A., & Pramanick, K. (2021). Co-occurrence of co-contaminants: Cyanotoxins and microplastics, in soil system and their health impacts on plant-A comprehensive review. Science of the Total Environment, 794, 148752.
Manning, S. R., & Nobles, D. R. (2017). Impact of global warming on water toxicity: Cyanotoxins. Current Opinion in Food Science, 18, 14-20.
Mao, J., Jiang, D., & Dai, H. (2015). Spatial-temporal hydrodynamic and algal bloom modelling analysis of a reservoir tributary embayment. Journal of Hydro-Environment Research, 9(2), 200-215.
Marampouti, C., Buma, A. G., & de Boer, M. K. (2021). Mediterranean alien harmful algal blooms: Origins and impacts. Environmental Science and Pollution Research, 28(4), 3837-3851.
Massey, I. Y., Al Osman, M., Yang, F. (2020). An overview on cyanobacterial blooms and toxins production: Their occurrence and influencing factors. Toxin Reviews, 41(1), 326-346.
Mccarthy, F. M. G., Riddick, N. L., Volik, O., Danesh, D. C., & Krueger, A. M. (2018). Algal palynomorphs as proxies of human impact on freshwater resources in the Great Lakes region. Anthropocene, 21, 16-31.
Mehinto, A. C., Smith, J., Wenger, E., Stanton, B., Linville, R., Brooks, B. W., Sutula, M. A., & Howard, M. D. (2021). Synthesis of ecotoxicological studies on cyanotoxins in freshwater habitats-Evaluating the basis for developing thresholds protective of aquatic life in the United States. Science of the Total Environment, 795, 148864.
Michalak, A. M., Anderson, E. J., Beletsky, D., Boland, S., Bosch, N. S., Bridgeman, T. B., DePinto, J. V., Evans, M. A., Fahnenstiel, G. L., & He, L. (2013). Recordsetting algal bloom in Lake Erie caused by agricultural and meteorological trends consistent with expected future conditions. Proceedings of the National Academy of Sciences of the United States of America, 110(16), 6448-6452.
Miglione, A., Napoletano, M., & Cinti, S. (2021). Electrochemical biosensors for tracing cyanotoxins in food and environmental matrices. Biosensors, 11(9), 315.
Misiou, O., Koutsoumanis, K. (2022). Climate change and its implications for food safety and spoilage. Trends in Food Science and Technology, 126, 142-152.
Moestrup, Ø. (1996). Toxic blue-green algae (cyanobacteria) in 1833. Phycologia, 35(sup6), 5-5.
Moore, S. K., Dreyer, S. J., Ekstrom, J. A., Moore, K., Norman, K., Klinger, T., Allison, E. H., & Jardine, S. L. (2020). Harmful algal blooms and coastal communities:
Moreira, C., Azevedo, J., Antunes, A., & Vasconcelos, V. (2013). Cylindrospermopsin: Occurrence, methods of detection and toxicology. Journal of Applied Microbiology, 114(3), 605-620.
Moreira, C., Ramos, V., Azevedo, J., & Vasconcelos, V. (2014). Methods to detect cyanobacteria and their toxins in the environment. Applied Microbiology and Biotechnology, 98(19), 8073-8082.
Morón-López, J. (2021). A holistic water monitoring approach for effective ecosystem management. Ecohydrology and Hydrobiology, 21(3), 549-554.
Müller, M. N., Mardones, J. I., & Dorantes-Aranda, J. J. (2020). Harmful algal blooms (HABs) in Latin America. Frontiers in Marine Science, 7, 34.
Namsaraev, Z., Melnikova, A., Komova, A., Ivanov, V., Rudenko, A., & Ivanov, E. (2020). Algal bloom occurrence and effects in Russia. Water, 12(1), 285.
Nguyen, T. A. D., Nguyen, L. T., Enright, A., Pham, L. T., Tran, H. Y. T., Tran, T. T., Nguyen, V. H. T., & Tran, D. N. (2021). Health risk assessment related to cyanotoxins exposure of a community living near Tri An Reservoir, Vietnam. Environ Sci Poll Res, 28(40), 56079-56091.
Ni, J., Liu, R., Li, Y., Tang, G., & Shi, P. (2022). An improved transfer learning model for cyanobacterial bloom concentration prediction. Water, 14(8), 1300.
Nwankwegu, A. S., Li, Y., Huang, Y., Wei, J., Norgbey, E., Sarpong, L., Lai, Q., & Wang, K. (2019). Harmful algal blooms under changing climate and constantly increasing anthropogenic actions: the review of management implications. Biotech 3, 9(12), 1-19.
Ogungbile, A. O., Ashur, I., Icin, I., Shapiro, O. H., & Vernick, S. (2021). Rapid detection and quantification of microcystins in surface water by an impedimetric immunosensor. Sensors and Actuators b: Chem, 348, 130687.
Ohio State University. (2017). Algal blooms cost Ohio homeowner
Paerl, H. W., & Barnard, M. A. (2020). Mitigating the global expansion of harmful cyanobacterial blooms: Moving targets in a human-and climatically altered world. Harmful Algae, 96, 101845.
Paerl, H. W., & Otten, T. G. (2013). Harmful cyanobacterial blooms: Causes, consequences, and controls. Microbial Ecology, 65, 995-1010.
Paerl, H. W., & Otten, T. G. (2016). Duelling ‘CyanoHABs’: Unravelling the environmental drivers controlling dominance and succession among diazotrophic and non-N2-fixing harmful cyanobacteria. Environmental Microbiology, 18(2), 316-324.
Paerl, H. W., Xu, H., Hall, N. S., Rossignol, K. L., Joyner, R., Zhu, G., & Qin, B. (2015). Nutrient limitation dynamics examined on a multi-annual scale in Lake Taihu, China:
Paerl, H. W., Gardner, W. S., Havens, K. E., Joyner, A. R., McCarthy, M. J., Newell, S. E., Qin, B., & Scott, J. T. (2016). Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae, 54, 213-222.
Pal, M., Yesankar, P. J., Dwivedi, A., & Qureshi, A. (2021). Biotic control of harmful algal blooms (HABs): A brief review. Journal of Environmental Management, 268, 110687.
Petterson, L. H., Pozdnyakov, D. (2013). Quantification, species variety, and consequences of harmful algal blooms (HABs). Chapter 1 in : Monitoring of harmful algal blooms. Springer-Praxis, Berlin, pp 1-24.
Pham, T. L., & Utsumi, M. (2018). An overview of the accumulation of microcystins in aquatic ecosystems. Journal of Environmental Management, 213, 520-529.
Pitcher, G. C., & Louw, D. C. (2021). Harmful algal blooms of the Benguela Eastern Boundary upwelling system. Harmful Algae, 102, 101898.
Poniedziałek, B., Rzymski, P., & Kokociński, M. (2012). Cylindrospermopsin: Water-linked potential threat to human health in Europe. Environmental Toxicology and Pharmacology, 34(3), 651-660.
Pouria, S., de Andrade, A., Barbosa, J., Cavalcanti, R. L., Barreto, V. T., Ward, C. J., & Codd, G. A. (1998). Fatal microcystin intoxication in haemodialysis unit in Caruaru Brazil. Lancet, 352(9121), 21-26.
Prabha, R., Singh, D. P., Somvanshi, P., & Rai, A. (2016). Functional profiling of cyanobacterial genomes and its role in ecological adaptations. Genomics Data, 9, 89-94.
Qiao, X., Saha., B. (2021). Quantifying the socio-economic impacts of harmful algal blooms in South-West Florida in 2018. Food and resource economics department, University of Florida institute of food and agricultural sciences. Accessed 16 Mar 2022.
Qin, B., Zhu, G., Gao, G., Zhang, Y., Li, W., Paerl, H. W., & Carmichael, W. W. (2010). A drinking water crisis in Lake Taihu, China: Linkage to climatic variability and lake management. Environmental Management, 45(1), 105-112.
Qu, M., Anderson, S., Lyu, P., Malang, Y., Lai, J., Liu, J., Jiang, B., Xie, F., Liu, H. H., Lefebvre, D. D., & Wang, Y. S. (2019). Effective aerial monitoring of cyanobacterial harmful algal blooms is dependent on understanding cellular migration. Harmful Algae, 87, 101620.
Rashidi, H., Baulch, H., Gill, A., Bharadwaj, L., & Bradford, L. (2021). Monitoring, managing, and communicating risk of harmful algal blooms (HABs) in recreational resources across Canada. Environmental Health Insights, 15, 11786302211014400.
Rodger, H. D., Turnbull, T., Edwards, C., & Codd, G. A. (1994). Cyanobacterial (blue-green-algal) bloom associated pathology in brown trout, Salmo trutta L., in
Sakamoto, S., Lim, W. A., Lu, D., Dai, X., Orlova, T., & Iwataki, M. (2021). Harmful algal blooms and associated fisheries damage in East Asia: Current status and trends in China, Japan Korea and Russia. Harmful Algae, 102, 101787.
Schaefer, A. M., Yrastorza, L., Stockley, N., Harvey, K., Harris, N., Grady, R., Sullivan, J., McFarland, M., & Reif, J. S. (2020). Exposure to microcystin among coastal residents during cyanobacteria bloom in Florida. Harmful Algae, 92, 101769.
Schmale, D. G., Ault, A. P., Saad, W., Scott, D. T., & Westrick, J. A. (2019). Perspectives on harmful algal blooms (HABs) and the cyberbiosecurity of freshwater systems. Front Bioeng Biotechnol, 7, 128.
Serrà, A., Philippe, L., Perreault, F., & Garcia-Segura, S. (2021). Photocatalytic treatment of natural waters. Reality or hype? The case of cyanotoxins remediation. Water Research, 188, 116543.
Shahmohamadloo, R. S., Poirier, D. G., Almirall, X. O., Bhavsar, S. P., & Sibley, P. K. (2020). Assessing the toxicity of cell-bound microcystins on freshwater pelagic and benthic invertebrates. Ecotoxicology and Environmental Safety, 188, 109945.
Shen, L., Xu, H., & Guo, X. (2012). Satellite remote sensing of harmful algal blooms (HABs) and a potential synthesized framework. Sensors (basel), 12, 7778-7803.
Shi, K., Zhang, Y., Zhou, Y., Liu, X., Zhu, G., Qin, B., & Gao, G. (2017). Long-term MODIS observations of cyanobacterial dynamics in Lake Taihu: Responses to nutrient enrichment and meteorological factors. Scientific Reports, 7(1), 1-16.
Skafi, M., Duy, S. V., Munoz, G., Dinh, Q. T., Simon, D. F., Juneau, P., & Sauvé, S. (2021). Occurrence of microcystins, anabaenopeptins and other cyanotoxins in fish from a freshwater wildlife reserve impacted by harmful cyanobacterial blooms. Toxicon, 194, 44-52.
Smith, R. B., Bass, B., Sawyer, D., Depew, D., & Watson, S. B. (2019). Estimating the economic costs of algal blooms in the Canadian Lake Erie Basin. Harmful Algae, 87, 101624.
Starling, F., Lazzaro, X., Cavalcanti, C., & Moreira, R. (2002). Contribution of omnivorous tilapia to eutrophication of a shallow tropical reservoir: Evidence from a fish kill. Freshwater Biology, 47, 2443-2452.
Sukenik, A., & Kaplan, A. (2021). Cyanobacterial harmful algal blooms in aquatic ecosystems: A comprehensive outlook on current and emerging mitigation and control approaches. Microorganisms, 9(7), 1472.
Svirčev, Z., Lalić, D., Bojadžija Savić, G., Tokodi, N., Drobac Backović, D., Chen, L., Meriluoto, J., & Codd, G. A. (2019). Global geographical and historical overview of cyanotoxin distribution and cyanobacterial poisonings. Archives of Toxicology, 93, 2429-2481.
Tarafdar, L., Mohapatra, M., Muduli, P. R., Kumar, A., Mishra, D. R., & Rastogi, G. (2023). Co-occurrence patterns and environmental factors associated with rapid onset of Microcystis aeruginosa bloom in a tropical coastal lagoon. Journal of Environmental Management, 325, 116580.
Trevino-Garrison, I., DeMent, J., Ahmed, F. S., Haines-Lieber, P., Langer, T., Ménager, H., Neff, J., Van der Merwe, D., & Carney, E. (2015). Human illnesses and animal deaths associated with freshwater harmful algal blooms-Kansas. Toxins, 7(2), 353-366.
Trinchet, I., Cadel-Six, S., Djediat, C., Marie, B., Bernard, C., Puiseux-Dao, S., Krys, S., & Edery, M. (2013). Toxicity of harmful cyanobacterial blooms to bream and roach. Toxicon, 71, 121-127.
Tsikoti, C., & Genitsaris, S. (2021). Review of harmful algal blooms in the Coastal Mediterranean Sea, with a focus on Greek waters. Diversity, 13(8), 396.
Turner, A. D., Lewis, A. M., Bradley, K., & Maskrey, B. H. (2021). Marine invertebrate interactions with harmful algal blooms-Implications for one health. Journal of Invertebrate Pathology, 186, 107555.
USEPA, (2015a). Drinking water health advisories for two cyanobacterial toxins. Office of Water, 820F15003.
USEPA. (2015b). Recommendations for public water systems to manage cyanotoxins in drinking water. Office of Water (4606M), EPA 815-R-15-010.
USEPA Harmful Algal Blooms (2017): www.epa.gov/nutri entpollution/harmful-algal-blooms
USEPA. (2019). Cyanobacteria and cyanotoxins: Information for drinking water systems. United State Environmental Protection Agency. Office of Water, EPA-810F11001. https://www.epa.gov/sites/default/files/201907/docum ents/cyanobacteria_and_cyanotoxins_fact_sheet_for_ pws_final_06282019.pdf.pdf
USEPA. (2022a). United States environmental protection agency. Learn about Cyanobacteria and Cyanotoxins. https://www.epa.gov/cyanohabs/learn-about-cyanobacte ria-and-cyanotoxins
USEPA. (2022b). United States environmental protection agency. Health effects from Cyanotoxins. https://www. epa.gov/cyanohabs/health-effects-cyanotoxins
Veerman, J., Kumar, A., & Mishra, D. R. (2022). Exceptional landscape-wide cyanobacteria bloom in Okavango Delta, Botswana in 2020 coincided with a mass elephant die-off event. Harmful Algae, 111, 102145.
Verspagen, J. M., Van de Waal, D. B., Finke, J. F., Visser, P. M., Van Donk, E., & Huisman, J. (2014). Rising CO2 levels will intensify phytoplankton blooms in eutrophic and hypertrophic lakes. Plos One, 9(8), e104325.
Vidal, F., Sedan, D., D’Agostino, D., Cavalieri, M. L., Mullen, E., Parot Varela, M. M., Flores, C., Caixach, J., & Andrinolo, D. (2017). Recreational exposure during algal bloom in Carrasco Beach, Uruguay: A liver failure case report. Toxins, 9(9), 267.
Vieira-Lanero, R., Barca, S., Cobo, M. C., & Cobo, F. (2022). Occurrence of freshwater cyanobacteria and bloom records in Spanish reservoirs (1981-2017). Hydrobiology, 1(1), 122-136.
Vogiazi, V., de la Cruz, A. A., Varughese, E. A., Heineman, W. R., White, R. J., & Dionysiou, D. D. (2021). Sensitive electrochemical detection of microcystin-LR in water samples via target-induced displacement of aptamer associated [Ru (NH3) 6] 3+. ACS ES&T Engineering, 1(11), 1597-1605.
Wan, X., Steinman, A. D., Gu, Y., Zhu, G., Shu, X., Xue, Q., Zou, W., & Xie, L. (2020). Occurrence and risk assessment of microcystin and its relationship with environmental factors in lakes of the eastern plain ecoregion China. Environmental Science and Pollution Research, 27(36), 45095-45107.
Wang, C., Feng, T., Wang, P., Hou, J., & Qian, J. (2017). Understanding the transport feature of bloom-forming Microcystis in a large shallow lake: A new combined hydrodynamic and spatially explicit agent-based modelling approach. Ecological Engineering, 343, 25-38.
Wang, H., Li, H., Sun, K., Huang, H., Zhu, P., & Lu, Z. (2020). Impact of exogenous nitrogen on the cyanobacterial abundance and community in oil-contaminated sediment: A microcosm study. Science of the Total Environment, 710, 136296.
Wang, K., Mou, X., Cao, H., Struewing, I., Allen, J., & Lu, J. (2021a). Co-occurring microorganisms regulate the succession of cyanobacterial harmful algal blooms. Environmental Pollution, 288, 117682.
Wang, H., Xu, C., Liu, Y., Jeppesen, E., Svenning, J. C., Wu, J., Zhang, W., Zhou, T., Wang, P., Nangombe, S., & Ma, J. (2021b). From unusual suspect to serial killer: Cyanotoxins boosted by climate change may jeopardize African megafauna. The Innovation, 2(2), 100092.
Welker, M., Chorus, I., Scheffer, B. and Urquhard E. (2021). Planning monitoring programmes for cyanobacteria and cyanotoxins. In: Toxic cyanobacteria in water, pp 641668 CRC Press.
Whitman, P., Schaeffer, B., Salls, W., Coffer, M., Mishra, S., Seegers, B., Loftin, K., Stumpf, R., & Werdell, P. J. (2022). A validation of satellite derived cyanobacteria detections with state reported events and recreation advisories across US lakes. Harmful Algae, 115, 102191.
WHO. (2010). IARC Monographs on the evaluation of carcinogenic risks to humans, ingested nitrate and nitrite, and cyanobacterial peptide toxins (Vol. 94, p. 450). World Health Organization.
Wood, R. (2016). Acute animal and human poisonings from cyanotoxin exposure-A review of the literature. Environment International, 91, 276-282.
World Health Organization (2021). WHO guidelines on recreational water quality: Volume 1: Coastal and fresh waters. World Health Organization (WHO).
Wu, T., Qin, B., Zhu, G., Luo, L., Ding, Y., & Bian, G. (2013). Dynamics of cyanobacterial bloom formation during short-term hydrodynamic fluctuation in a large shallow, eutrophic, and wind-exposed Lake Taihu China. Environmental Science and Pollution Research, 20(12), 8546-8556.
Wu, J., Hilborn, E. D., Schaeffer, B. A., Urquhart, E., Coffer, M. M., Lin, C. J., & Egorov, A. I. (2021). Acute health effects associated with satellite-determined cyanobacterial blooms in a drinking water source in Massachusetts. Environmental Health, 20(1), 1-13.
Xie, H., Fischer, A. M., & Strutton, P. G. (2021). Generalized linear models to assess environmental drivers of paralytic shellfish toxin blooms (Southeast Tasmania, Australia). Continental Shelf Research, 223, 104439.
Xu, H., Long, L., Yan, M., Ma, J., Ji, D., Liu, D., & Yang, Z. (2020). Modelling the effects of hydrodynamics on thermal stratification and algal blooms in the Xiangxi Bay of Three Gorges Reservoir. Frontiers in Ecology and Evolution, 8, 453.
Xue, Y., Chen, H., Yang, J. R., Liu, M., Huang, B., & Yang, J. (2018). Distinct patterns and processes of abundant and rare eukaryotic plankton communities following a reservoir cyanobacterial bloom. ISME Journal, 12, 2263-2277.
You, L., Tong, X., Te, S. H., Tran, N. H., Sukarji, N. H., He, Y., & Gin, K. Y. H. (2022). Multi-class secondary metabolites in cyanobacterial blooms from a tropical water body: Distribution patterns and real-time prediction. Water Research, 212, 118129.
Zhang, Y., Husk, B. R., Duy, S. V., Dinh, Q. T., Sanchez, J. S., Sauvé, S., & Whalen, J. K. (2021). Quantitative screening for cyanotoxins in soil and groundwater of agricultural watersheds in Quebec Canada. Chemosphere, 274, 129781.
Zhao, K., Wang, L., You, Q., Pan, Y., Liu, T., Zhou, Y., Zhang, J., Pang, W., & Wang, Q. (2021). Influence of cyanobacterial blooms and environmental variation on zooplankton and eukaryotic phytoplankton in a large, shallow, eutrophic lake in China. Science of the Total Environment, 773, 145421.
Zhou, Y., Wang, L., Zhou, Y., & Mao, X.-Z. (2020). Eutrophication control strategies for highly anthropogenic influenced coastal waters. Science of the Total Environment, 705, 135760.
Zhu, X., Dao, G., Tao, Y., Zhan, X., & Hu, H. (2021). A review on control of harmful algal blooms by plant-derived allelochemicals. Journal of Hazardous Materials, 401, 123403.
DOI: https://doi.org/10.1007/s11270-023-06782-y
Publication Date: 2024-01-01
Cyanobacteria Harmful Algae Blooms: Causes, Impacts, and Risk Management
© The Author(s) 2024
Abstract
Cyanobacteria harmful algal blooms (cHABs) are increasingly becoming an emerging threat to aquatic life, ecotourism, and certain real estate investments. Their spontaneous yet sporadic occurrence has made mitigation measures a cumbersome task; moreover, current trends regarding anthropogenic activities, especially in agriculture and industry portend further undesirable events. Apart from the aesthetic degeneration they create in their respective
1 Introduction
and the studies indicated a global increase of cyanobacteria in the aquatic ecosystem. For instance, in 2017, a study predicted the effects of climate change on the concentration of cyanobacteria in large reservoirs in the USA using statistical modelling framework (Chapra et al., 2017). The framework utilized a forecast of climate change from five global calculation models, two cyanobacteria growth and two greenhouse gas emission scenarios coupled with a water quality/hydrologic network model of the contiguous USA. The model forecasted a likely increase in the year 2017 in the mean number of days of harmful cHABs from 7 days per year per water body to 16-23 days in 2050 and 18-39 days in 2090 due to the increase in nutrient levels as well as the rise in water temperature in the aquatic milieu (Chapra et al., 2017). The expansion of cyanobacteria blooms in Taihu Lake is currently extending across almost approximately
(DSP), neurotoxic shellfish poisoning (NSP), and amnesic shellfish poisoning (ASP) (Gholami et al., 2019). Other HABs-related syndromes include palytoxin poisoning, azaspiracid poisoning (AZP) and tetrodotoxin poisoning (Tsikoti & Genitsaris, 2021), brevetoxins (BTX), okadaic acid (OA), domoic acid (DA), and numerous other toxins (Durán-Vinet et al., 2021). These toxins are transferred to humans through the consumption of surviving organisms that have bioaccumulated these toxins through sequential trophic advancement in the food web (Marampouti et al. Marampouti et al., 2021). According to the data captured by the harmful algal event database (HAEDAT) from 1987 to 2022, about 82 human mortalities, 4045 cases of toxins in seafood and 181 reports of water discoloration because of HABs (http://haedat.iode.org/). An example of the challenge of cyanobacteria blooms was the Wuxi water crisis in May 2007 which affected about 2 million inhabitants who had no access to drinking water for over a week due to massive Microcystis spp. toxin blooms (Qin et al., 2010).
(Pearl and Otten 2016). Nevertheless, several cHABs are initiated with
(i) Production of toxic metabolites, as well as competitive exclusion amongst cyanobacteria, bloom community that repress phytoplankton proliferation (Xue et al., 2018)
(ii) Selective or alternative feeding of zooplankton on phytoplankton and cyanobacteria, thereby promoting either the formation or repression of cyanobacteria blooms (Zhao et al., 2021).

2 Factors that Influence Harmful Algae Blooms
growth and maintenance. The regulation of these factors often dictates their population dynamics and subsequently cHABs formation in any given environmental matrix. Moreover, these factors can influence sporadic and spontaneous HABs situations, either individually or interdependently. Hence, the major drivers for the formation of cHABs and other HABs can be grouped into the following:
2.1 Eutrophication
undesirable water conditions, water quality, and the balance of organisms in the water to form blooms (Namsaraev et al., 2020). Eutrophication and pollution are usually increased by rainfall due to the transport of contaminants and nutrient-rich run-off across freshwater environments to the marine continuum. It has been reported as a major cause of increasing cHABs occurrence and is among the major driving factor for the formation of cyanobacterial bloom (Chirico et al., 2020). The main nutrient pathways leading to eutrophication include soil erosion, wastewater discharge, leaking or overflowing domestic sewers, agricultural fertilization, and farmland drainage (Fig. 2) (Li et al., 2019). Eutrophication induces cyanobacterial growth that subsequently forms algal bloom and is becoming one of the leading challenges
associated with water quality in densely populated areas. Cyanobacteria pigments in sediment cores from a survey of over 100 lakes in North America and Europe revealed an increase of almost

2.2 Anthropogenic Actions
deemed a perfect ground for open defecation. Since detergents and human feces are rich in N and P , adjacent water bodies might therefore have a high likelihood of nutrient infusion and overload during flash floods. Other contaminants that pollute waterbodies via accidental discharge and pipeline vandalism due to human activities that significantly encourage the processes of episodic blooms include petroleum products such as kerosene, spent lubricating oil, premium motor spirit, diesel, gasoline, automobile gas oil, and heavy metals (Nwankwegu et al., 2019). Anthropogenic activities have been reported to alter planktonic community structures, migration patterns, and species distribution that result in changes in the biogeography and HAB dynamics, especially cHABs (Müller et al., 2020).
2.3 Hydrodynamic Variables
hydrodynamic conditions and pollution loads (Mao et al., 2015). Likewise, as the majority of living biomass, suspended particles, pollutants, and dissolved gases are mixed and carried by turbulent motions, the interactions of different water quality variables may be greatly affected by fluctuating hydrodynamic conditions to form blooms (Mao et al., 2015). In riverine ecosystems, changes in hydrological conditions, such as water level, agitation, and transparency, are highly influential on algal growth, diffusion, migration, and bloom accumulation (Cheng et al., 2019).
2.4 Climate Change
drought. This results in an increase in the residence time of nutrients in the environments that create ideal situations for blooms formation of cHABs and other HABs (Michalak et al., 2013). Climate change has contributed to the continuous upsurge of microalgae population (cyanobacteria) in aquatic milieu to form algal bloom. The thick layer of microalgae clusters can lead to HABs. In this condition, light is prevented from penetrating the waterbody and this cause death of aquatic plant that needs light for the photosynthetic process (Kasan et al., 2021). Other climate-driven impacts on the occurrence and distribution of bloomforming phytoplankton groups such as cyanobacteria are salinity, dissolved oxygen, light intensity, temperature, and pH . Interestingly, these environments have been regularly characterized by the occurrence or presence of cyanotoxins (Kusumawati & Mangkoedihardjo, 2021). In the aquatic milieu, the presence of cHABs increases
2.5 Eco-physiological Adaptation Strategies
usually outcompeted larger masses at harnessing light.
especially during the dry season (usually characterized by intense desiccation), some cyanobacteria taxa (Anabaena, Nodularia, Cylindrospermopsis and Gloeotrichia) form akinetes or thick-walled resting cells, which are denser than the vegetative cells and therefore sediment. At this stage, the nonor meagre requirement of the cells permits cyanobacteria to survive the unavailing conditions of bottom sediments for long periods. Interestingly, the sunken cells can tolerate harsh environmental conditions, such as high temperatures, and begin to germinate when favorable conditions are restored.
3 Cyanobacteria and Their Toxins
| Cyanotoxin class | Toxigenic genera | References | |||
| Neurotoxins | |||||
| Saxitoxins (>60) | Cylindrospermopsis, Anabaena, Lyngbya, Aphanizomenon and Planktothrix | Lago et al., 2015 | |||
| Anatoxins-a | Anabaena, Cylindropermum, Raphidiopsis, Aphanizomenon, Oscillatoria, and Placktothrix, |
|
|||
| Beta-N-methylamino-Lalanine (BMAA) | Anabaena, Cylindrospermopsin, Microcystis, Nostoc, and Planktothrix (Oscillatoria) | Cheung et al., 2013 | |||
| Anatoxin-a(s) | Dolichospermum | Huisman et al., 2018 | |||
| Saxitoxins | Aphanizomenon flos-aquae, Dolichospermum (previously Anabaena) circinalis, Lyngbya wollei, Planktothrix spp., and a Brazilian isolate of Raphidiopsis raciborskii | USEPA, 2022b | |||
| Endotoxins | |||||
| Lipopolysaccharides | All cyanobacteria | Bláha et al., 2009 | |||
| Cytotoxins | |||||
| Cylindrospermopsins (3) | Cylindrospermopsis, Aphanizomenon, Anabaena, Raphidiopsis, Umezakia, and Oscillatoria species |
|
|||
| Hepatotoxins | |||||
| Nodularins (10) | Nodularia | Lopex et al. 2008; | |||
| Microcystins (> 100) | Anabaena, Nostoc, Anabaenopsis, Oscillatoria, Aphanocapsa, Arthrospira, Hapalosiphon, Microcystis, Planktothrix, Snowella, Synechocystis, and Woronichinia | Carmichael & Boyer, 2016 | |||
| Dermatotoxins | |||||
| Aplysiatoxins | Lyngbya, Schizotrix, and Oscillatoria | Han et al., 2018 | |||
| Lyngbyatoxins (>8) | Lyngbya, Schizotrix, and Oscillatoria | Jiang et al. 2014 | |||
| Not identified | |||||
| Aeruginosins (>15) | Microcystis, Oscillatoria, Nostoc, and Planktothrix | Manning & Nobles, 2017 | |||
| Ambigol (3) | Fischerella | Manning & Nobles, 2017 | |||
(ii) Neurotoxins (anatoxin-a, saxitoxins, anatoxin
(iii) Hepatotoxins (nodularins and microcystins): this class of toxins are involved in the inhibition of phosphate proteins 1 A and 2 A , which cause cancer promotion, deformation of hepatocytes, liver damage and hyperphosphorylation of cytoskeletal filaments (Catherine et al., 2017).
(iv) Dermatoxins (lyngbyatoxin, debromoaplysiatoxin and aplysiatoxins), and
(v) Irritating toxins (lipopolysaccharide endotoxins): these are responsible for inflammation of the gas-
trointestinal tract and skin irritation (Miglione et al., 2021).
cyanobacteria genera within the Cyanophyceae that can form cHABs include Microcystis, Nodularia, Cylindrospermopsis, Dolichospermum, and Planktothrix (Huisman et al., 2018). In tropical waters, Microcystis and Raphidiopsis are the two known cyanobacteria genera that usually form blooms associated with a variety of toxins that degrade water quality. Oftentimes, CTs produced by some cyanobacteria species are secreted intracellularly and are later released into the environment when the cells rupture or die during periods of extreme salinity. Conversely, toxins might be secreted or released at the end of the cyanobacteria bloom’s lifecycle. Neurotoxins, hepatotoxins, and dermatoxins produced by cyanobacteria species can cause acute and chronic human health effects (Smucker et al., 2021).
4 Cyanotoxins Exposure Routes and Public Health Impacts
paralysis of the abdominal muscles and chest muscles as well as death (Marampouti et al., 2021). Another HAB toxin-associated syndrome called amnesic shellfish poisoning (ASP) can also cause headaches, dizziness, disorientation, motor deficiency, short-term memory loss, and confusion (Kudela et al. 2015).
et al., 2015). According to the Bulgarian legislature, there is no acceptable limit on cyanotoxins in water (Ilieva et al., 2019). However, the United States Environmental protection agency (US EPA) recommended
reported following recreational exposure to high concentrations of MCs (Vidal et al., 2017
by toxins produced by cyanobacteria are as shown in Fig. 3 (Kubickova et al., 2019), while Table 2 contains some information on selected toxins and their potential short- and long-term health impacts.
5 Impacts of Cyanotoxins on Fish, Pets, and Livestock

| No | Cyanobacteria genera | Toxin | Short-term health impacts | Long-term health impacts | Reference |
| 1 | Anabaena, Aphanizomenon, Oscillatoria, and Planktothrix, Chrysosporum (Aphanizomenon) ovalisporum, Cuspidothrix, Raphidiopsis, Cylindrospermum, Dolichospermum, Microcystis, Phormidium, Dolichospermum flos-aquae, A. lemmermannii Raphidiopsis mediterranea (strain of Raphidiopsis raciborskii), Tychonema and Woronichinia | Anatoxins | Tingling, numbness, burning, incoherent speech, drowsiness, salivation, drowsiness, tingling, burning numbness, incoherent speech, respiratory paralysis, and death | Cardiac arrhythmia leading to death | Lopex et al. 2008; USEPA, 2022b |
| 2 | Anabaena, Cylindrospermopsin, Microcystis, Nostoc, and Planktothrix | BMAA | Unknown | Muscle atrophy, loss of coordination, and possible contributions to neurodegenerative diseases | Huisman et al., 2018 |
| 3 | Lyngbya | Lyngbyatoxins | Dermatitis | Skin tumors | Fujiki et al., 1990 |
| 4 | Aphanizomenon, and Oscillatori | Lipopolysaccharide | Gastrointestinal and dermatitis | Lopex et al. 2008 | |
| 5 | Anabaena, Aphanizomenon, Cylindrospermopsis and Lyngbya | Saxitoxins | Numbness, burning, tingling, drowsiness, incoherent speech, respiratory paralysis, and death | Unknown | Lopex et al. 2008 |
| 6 | Aphanocapsa, Microcystis, Anabaena, Nostoc, Oscillatoria, Hapalosphon, and Planktothrix, Fischerella, Gloeotrichia | Microcystins | Pneumonia, liver inflammation, gastrointestinal, dermatitis, hemorrhage, liver failure and death, sore throat, abdominal pain, headache, cough, nausea, blistering around the mouth, and pneumonia | Reproductive toxicity, tumor promotion, reduced DNA repair, liver and kidney damage, | Huisman et al., 2018; USEPA, 2022 |
| 7 | Aphanizomenon, Cylindrospermopsis, and Umezakia, Aphanizomenon flos-aquae, Aphanizomenon gracile, Dolichospermum bergii, Dolichospermum lapponica, Dolichospermum planctonica, Lyngbya wollei, Rhaphidiopsis curvata, and Rhaphidiopsis mediterranea | Cylindrospermopsin | Pneumonia, gastrointestinal, hemorrhage, liver, fever, headache, vomiting, bloody diarrhea, inflammation, and dermatitis | Anorexia, malaise, and liver failure leading to death | Lopex et al. 2008; USEPA, 2022b |
| 8 | Nodularia spumigena | Nodularins | Same impacts as MCs | Same impacts as MCs | Huisman et al., 2018; |
cattle, and guinea pigs due to the consumption of nodularin (Chen et al., 2021b). In Africa, CTs have often been the main suspected cause of mass deaths of both large-sized and medium terrestrial mammals such as livestock (sheep and cattle) as well as nonwading mammals (zebra, impalas, blue wildebeests, giraffes, and white rhinoceros) (Wang et al., 2021b). In Botswana, between May and June 2020, the mass deaths of over 330 African elephants were supposed to be cyanobacteria related (biotoxins) (Wang et al., 2021b). The report of Backer et al. (2013) has also revealed that cats, dogs, and other mammals have also died from anatoxin-a and microcystin poisoning after exposure to contaminated water. Besides the harmful impacts of cyanobacteria producing toxins on aquatic organisms or animals, non-toxic algae can also cause adverse effects in the aquatic environments by reducing dissolved oxygen, suffocating benthic fauna and flora and blocking fish gills leading to fish death (Davidson 2014).
6 Socio-economic Impacts of Cyanobacterial Harmful Algal Blooms
have been reported to cause severe societal impacts such as disruption to social and cultural practices, economic loss (Moore et al., 2020), adverse health effects (Backer & Moore, 2012), and losses to both private and public wellbeing (Willis et al., 2018).
“Do not drink” for the residents of Northwest Ohio in the USA in 2014 (Jetoo et al., 2015).
7 Risk Assessment and Management Approaches for Harmful Algae Blooms
managers to take proper actions when these events threaten the water sources (USEPA, 2015b). Hence, various strategies have been adopted by many countries and commercial enterprises to manage and monitor cHABs and other HABs in coastal waters (Anderson 2012). According to Corcoran and Hunt (2021), management approaches for cHABs and other HABs can be divided into three different categories including prevention, control, and mitigation.
(i) Prevention: in management approaches for cHABs, prevention refers to the actions taken to reduce the occurrence and severity of blooms or to keep cHABs from occurring or directly impacting certain resources such as chemicalalgaecides addition, nutrient-loads reduction, water management, biological competition, and hydrodynamics regulation (Zhu et al., 2021). Other effective approaches to prevent HABs including cHABs are to reduce nutrient loads in sewage effluents and prevent fertilizer overload in agricultural soils, groundwater control and stormwater runoff by nature-based solutions such as sedimentation, biofiltration systems, ecotone zones, and denitrification barriers, (Morón-López, 2021; Paerl & Barnard, 2020).
(ii) Control: from a management perspective, control refers to the measures taken to kill or destroy cHABs and other HABs to quickly interrupt bloom formation (Anderson, 2009). In controlling ABs, five common strategies are employed to combat insidious or harmful species, and these include chemical, mechanical, genetic, biological, and environmental control (Qu et al. 2021). Control methods include harvest, flocculation and settling, mixing, flushing, the use of chemical treatment, algicidal bacteria, filter-feeding organisms, cyanophage, plasma discharge technology, UV/Fenton system, and genetic engineering (Bhatt et al. 2023Corcoran and Hunt 2021; El-Sheekh et al, 2023; Li et al., 2023).
(iii) Mitigation: mitigation refers to the measures taken to reduce the negative effects of cHABs and other HABs on the ecosystem, human health, and the economy. Examples of mitigation procedures include monitoring programs, drinking water treatment, closures of shellfish
harvest areas, movement of fish or shellfish product away from blooms areas, closures of beaches, lakes, and the use of predictive models (Corcoran & Hunt, 2021).
7.1 Potential Application of Artificial Intelligence in the Risk Assessment and Management of Harmful Algae Blooms
humidity, and precipitation. This technology, if well harnessed and properly deployed, could offer a more reliable solution and guide in decision-making.
8 Challenges Facing the Management of Harmful Algal Blooms
9 Future Perspectives
in preventing the impacts of cHABs , the following strategies should be adopted.
(i) To create other recreational centers to keep tourism and this will help local businesses during bloom incidence.
(ii) To develop private or public programs that will provide social and economic support to temporarily unemployed workers such as fishermen.
(iii) To develop educational programs that will help ethnically diverse residents to avoid exposure.
investigated. First, it was observed that extract treatment suppressed Microcystis aeruginosa growth but had little effect on green alga (Scenedesmus obliqus). Also, artificially installed poles or bamboo forest stands along the banks can enhance competitors’ growth especially diatoms that can invade cyanobacteria colonies (Hao et al., 2022). Similarly, standardization of procedures for cyanotoxins analysis as well as the provision of standardized reference material for their quantification is vital for regular or routine water monitoring (Welker et al., 2021). Apart from the earlier mentioned electrochemical sensors, the development of strong in situ sensors capable of quantifying and detecting HABs cells and toxins in waterbodies could also help prevent the outbreak of HABs and appropriate deployment of artificial intelligence. Furthermore, proactive risk avoidance as well as creating public awareness via publication, news media, and placement of warning and advisory signs where CHABs and other HABs have been reported will be significant to preventing human and domestic animal poisoning risk of HABs.
10 Conclusion
using artificial intelligence and treatment procedures to protect citizens from this potential health threat.
Declarations
References
Ærtebjerg, G., Anderson, J. H., Hanson, O. S. (Eds.) (2003). Nutrients and eutrophication in danish marine waters. A challenge for science and management. Danish Environmental Protection Agency & National Environmental Research Institute. pp 126.
Anderson, D. M., Cembella, A. D., & Hallegraeff, G. M. (2012). Progress in understanding harmful algal blooms: Paradigm shifts and new technologies for research, monitoring, and management. Annual Review of Marine Science, 4, 143-176.
Annadotter, H., Cronberg, G., Lawton, L., Hansson, H.-B., Göthe, U., & Skulberg, O. (2001). An extensive outbreak of gastroenteritis associated with the toxic cyanobacterium Planktothrix agardhii (Oscillatoriales, Cyanophyceae) in Scania, south Sweden. In I. Chorus (Ed.), Cyanotoxins: Occurrence, causes, consequences (pp. 200-208). Springer-Verlag.
Azevedo, S. M., Carmichael, W., Jochimsen, E. M., Rinehart, K. L., Lau, S., Shaw, G. R., & Eaglesham, G. (2001). Human intoxication by microcystins during renal dialysis treatment in Caruaru-Brazil. Toxicology, 164(1-3), 32-32.
Backer, L. C., & Moore, S. K. (2012). Harmful algal blooms: Future threats in a warmer world. In A. E. Nemr (Ed.), Environmental pollution and its relation to climate change (pp. 485-512). Nova Science Publishers.
Backer, L. C., McNeel, S. V., Barber, T., Kirkpatrick, B., Williams, C., Irvin, M., Zhou, Y., Johnson, T. B., Nierenberg, K., Aubel, M., et al. (2010). Recreational exposure to microcystins during algal blooms in two California lakes. Toxicol, 55, 909-921.
Backer, L. C., Landsberg, J. H., Miller, M., Keel, K., & Taylor, T. K. (2013). Canine cyanotoxin poisonings in the United States (1920s-2012): Review of suspected and confirmed cases from three data sources. Toxins, 5(9), 1597-1628.
Barsanti, L., & Gualtieri, P. (2014). Algae: Anatomy, biochemistry, and biotechnology. CRC Press.
Beaulieu, J. J., DelSontro, T., & Downing, J. A. (2019). Eutrophication will increase methane emissions from lakes and impoundments during the 21st century. Nature Communications, 10, 1375.
Bechard, A. (2021). Gone with the wind: Declines in property values as harmful blooms are blown towards the shore. The Journal of Real Estate Finance and Economics, 62, 242-257.
Benayache, N. Y., Afri-Mehennaoui, F. Z., Kherief-Nacereddine, S., Vo-Quoc, B., Hushchyna, K., Nguyen-Quang, T., & Bouaïcha, N. (2022). Massive fish death associated with the toxic cyanobacterial Planktothrix sp. bloom in the Béni-Haroun Reservoir (Algeria). Environmental Science and Pollution Research, 29(53), pp 80849-80859.
Bhatt, P., Engel, B.A., Reuhs, M. and Simsek, H. (2023). Cyanophage technology in removal of cyanobacteria mediated harmful algal blooms: A novel and eco-friendly method. Chemosphere, p. 137769
Bláha, L., Babica, P., & Maršálek, B. (2009). Toxins produced in cyanobacterial water blooms-Toxicity and risks. Interdisciplinary Toxicology, 2(2), 36.
Bouaïcha, N., Miles, C. O., Beach, D. G., Labidi, Z., Djabri, A., Benayache, N. Y., & Nguyen-Quang, T. (2019). Structural diversity, characterization and toxicology of microcystins. Toxins, 11(12), 714.
Cao, H., Han, L., & Li, L. (2022). A deep learning method for cyanobacterial harmful algae blooms prediction in Taihu Lake China. Harmful Algae, 113, 102189.
Carmichael, W. W., & Boyer, G. L. (2016). Health impacts from cyanobacteria harmful algae blooms: Implications for the North American Great Lakes. Harmful Algae, 54, 194-212.
Catherine, A., Bernard, C., Spoof, L., & Bruno, M. (2017). Microcystins and nodularins. Handbook Cyanobacterial Monitoring Cyanotoxin Analysis, 1, 107-126.
Chapra, S. C., Boehlert, B., Fant, C., Bierman, V. J., Jr., Henderson, J., Mills, D., Mas, D. M., Rennels, L., Jantarasami, L., Martinich, J., & Strzepek, K. M. (2017). Climate change impacts on harmful algal blooms in US freshwaters: A screening-level assessment. Environmental Science and Technology, 51(16), 8933-8943.
Chatziefthimiou, A. D., Banack, S. A., & Cox, P. A. (2021). Biocrust-produced cyanotoxins are found vertically in the desert soil profile. Neurotoxicity Research, 39(1), 42-48.
Chen, L., Chen, J., Zhang, X., & Xie, P. (2016). A review of reproductive toxicity of microcystins. Journal of Hazardous Materials, 301, 381-399.
Chen, L., Giesy, J. P., Adamovsky, O., Svirčev, Z., Meriluoto, J., Codd, G. A., Mijovic, B., Shi, T., Tuo, X., Li, S. C., & Pan, B. Z. (2021). Challenges of using blooms of Microcystis spp in animal feeds: A comprehensive review of nutritional, toxicological and microbial health evaluation. Science of The Total Environment, 764, 142319.
Chen, M.-Y., Teng, W.-K., Zhao, L., Hu, C.-X., Zhou, Y.-K., Han, B.-P., Song, L.-R., & Shu, W.-S. (2021b). Comparative genomics reveals insights into cyanobacterial evolution and habitat adaptation. ISME Journal, 15, 211-227.
Chen, G., Wang, L., Wang, M., & Hu, T. (2021c). Comprehensive insights into the occurrence and toxicological issues of nodularins. Marine Pollution Bulletin, 162, 111884.
Cheng, B., Xia, R., Zhang, Y., Yang, Z., Hu, S., Guo, F., & Ma, S. (2019). Characterization and causes analysis for algae blooms in large river system. Sustainable Cities and Society, 51, 101707.
Cheung, M. Y., Liang, S., & Lee, J. (2013). Toxin-producing cyanobacteria in freshwater: A review of the problems, impact on drinking water safety, and efforts for protecting public health. Journal of Microbiology, 51(1), 1-10.
Chirico, N., António, D. C., Pozzoli, L., Marinov, D., Malagó, A., Sanseverino, I., Beghi, A., Genoni, P., Dobricic, S., & Lettieri, T. (2020). Cyanobacterial blooms in Lake Varese: Analysis and characterization over ten years of observations. Water, 12(3), 675.
Christensen, V. G., & Khan, E. (2020). Freshwater neurotoxins and concerns for human, animal, and ecosystem health: A review of anatoxin-a and saxitoxin. Science of the Total Environment, 736, 139515.
Codd, G. A., Testai, E., Funari, E., Svirčev, Z. (2020). Cyanobacteria, cyanotoxins, and human health. In: Water
treatment for purification from cyanobacteria and cyanotoxins, Anastasia E. Hiskia, Theodoros M. Triantis, Maria G. Antoniou, Triantafyllos Kaludis, Dionysios D. DIonysios, Editors, pp 37-68. John Wiley & Sons
Corcoran, A. A., & Hunt, R. W. (2021). Capitalizing on harmful algal blooms: From problems to products. Algal Research, 55, 102265.
Davis, D. A., Cox, P. A., Banack, S. A., Lecusay, P. D., Garamszegi, S. P., Hagan, M. J., Powell, J. T., Metcalf, J. S., Palmour, R. M., Beierschmitt, A., & Bradley, W. G. (2020). L-serine reduces spinal cord pathology in a vervet model of preclinical ALS/MND. Journal of Neuropathology & Experimental Neurology, 79(4), 393-406.
Dev, P. J., Sukenik, A., Mishra, D. R., & Ostrovsky, I. (2022). Cyanobacterial pigment concentrations in inland waters: Novel semi-analytical algorithms for multi-and hyperspectral remote sensing data. Science of the Total Environment, 805, 150423.
Dittmann, E., Fewer, D. P., & Neilan, B. A. (2013). Cyanobacterial toxins: Biosynthetic routes and evolutionary roots. FEMS Microbiology Reviews, 37(1), 23-43.
Drobac, B. D., Tokodi, N., Marinović, Z., Lujić, J., Dulić, T., Simić, S. B., Đorđević, N. B., Kitanović, N., Šćekić, I., Urbányi, B., & Meriluoto, J. (2021). Cyanobacteria, cyanotoxins, and their histopathological effects on fish tissues in Fehérvárcsurgó reservoir, Hungary. Environmental Monitoring and Assessment, 193(9), 1-14.
Durán-Vinet, B., Araya-Castro, K., Chao, T. C., Wood, S. A., Gallardo, V., Godoy, K., & Abanto, M. (2021). Potential applications of CRISPR/Cas for next-generation biomonitoring of harmful algae blooms: A review. Harmful Algae, 103, 102027.
El-Sheekh, M. M., Abd Al-Halim, M. A., Mohammed, S. A., (2023). Algae processing by plasma discharge technology: A review. Algal Research, 70, 102983.
Farrer, D., Counter, M., Hillwig, R., & Cude, C. (2015). Health-based cyanotoxin guideline values allow for cyanotoxin-based monitoring and efficient public health response to cyanobacterial blooms. Toxins, 7, 457-477.
Fujiki, H., Suganuma, M., Suguri, H., Yoshizawa, S., Takagi, K., Nakayasu, M., Ojika, M., Yamada, K., Yasumoto, T., Moore, R. E., & Sugimura, T. (1990). New tumor promoters from marine natural products. In S. Hall & G. Strichartz (Eds.), Marine toxins: Origin, structure and molecular pharmacology (pp. 232-240). American Chemical Society.
Funari, E., & Testai, E. (2008). Human health risk assessment related to cyanotoxins exposure. Critical Reviews in Toxicology, 38(2), 97-125.
Ger, K. A., Hansson, L. A., & Lürling, M. (2014). Understanding cyanobacteria-zooplankton interactions in a more eutrophic world. Freshwater Biology, 59(9), 1783-1798.
Ghaffar, S., Stevenson, R. J., & Khan, Z. (2016). Cyanobacteria dominance in lakes and evaluation of its predictors: A study of Southern Appalachians Ecoregion, USA. Matec Web Conf, 60, 02001.
Gholami, Z., Mortazavi, M. S., & Karbassi, A. (2019). Environmental risk assessment of harmful algal blooms case study: Persian Gulf and Oman Sea located at Hormozgan Province, Iran. Human and Ecological Risk Assessment, 25(1-2), 271-296.
Griffiths, D. J., & Saker, M. L. (2003). The Palm Island mystery disease 20 years on: A review of research on the cyanotoxin cylindrospermopsin. Environmental Toxicology: An International Journal, 18(2), 78-93.
Guo, L. (2007). Doing battle with the green monster of Taihu Lake. Science, 317(5842), 1166-1166.
Guzmán-Guillén, R., Puerto, M., Gutiérrez-Praena, D., Prieto, A. I., Pichardo, S., Jos, Á., Campos, A., Vasconcelos, V., & Cameán, A. M. (2017). Potential use of chemoprotectants against the toxic effects of cyanotoxins: A review. Toxins, 9(6), 175.
Han, B. N., Liang, T. T., Keen, L. J., Fan, T. T., Zhang, X. D., Xu, L., Zhao, Q., Wang, S. P., & Lin, H. W. (2018). Two marine cyanobacterial aplysiatoxins polyketides, neo-debromoaplysiatoxin A and B, with K+ channel inhibition activity. Organic Letters, 20, 578-581.
Hao, A., Su, M., Kobayashi, S., Zhao, M., & Iseri, Y. (2022). Multiple roles of bamboo as a regulator of cyanobacterial bloom in aquatic systems. Scientific Reports, 12(1), 1-12.
Hilborn, E. D., Roberts, V. A., Backer, L., DeConno, E., Egan, J. S., Hyde, J. B., Nicholas, D. C., Wiegert, E. J., Billing, L. M., DiOrio, M., & Mohr, M. C. (2014). Algal bloom-associated disease outbreaks among users of freshwater lakes-United States, 2009-2010. Morbidity and Mortality Weekly Report, 63(1), 11.
Hofbauer, W. K. (2021). Toxic or otherwise harmful algae and the built environment. Toxins, 13(7), 465.
Huang, J. D., & Zheng, H. (2017). Current trend of metagenomic data analytics for cyanobacteria blooms. Journal of Geoscience and Environment Protection, 5(06), 198-213.
Huang, Y. L., Huang, G. H., Liu, D. F., Zhu, H., & Sun, W. (2012). Simulation-based inexact chance-constrained nonlinear programming for eutrophication management in the Xiangxi Bay of Three Gorges Reservoir. Journal of Environmental Management, 108, 54-65.
Huisman, J., Codd, G. A., Paerl, H. W., Ibelings, B. W., Verspagen, J. M., & Visser, P. M. (2018). Cyanobacterial blooms. Nature Reviews Microbiology, 16(8), 471-483.
Ilieva, V., Kondeva-Burdina, M., Georgieva, T., & Pavlova, V. (2019). Toxicity of cyanobacteria Organotropy of cyanotoxins and toxicodynamics of cyanotoxins by species. Pharmacia, 66, 91.
Jetoo, S., Grover, V. I., & Krantzberg, G. (2015). The Toledo drinking water advisory: Suggested application of the water safety planning approach. Sustainability, 7(8), 9787-9808.
Ji, X., Verspagen, J. M., Van de Waal, D. B., Rost, B., & Huisman, J. (2020). Phenotypic plasticity of carbon fixation stimulates cyanobacterial blooms at elevated CO2. Science Advances, 6(8), eaax2926.
Jia-Fong, H., Ouddane, B., Hwang, J. S., & Dahms, H. U. (2021). In silico assessment of human health risks caused by cyanotoxins from cyanobacteria. Biocell, 45(1), 65.
Kankaanpää, H. T., Sipia, V. O., Kuparinen, J. S., Ott, J. L., & Carmichael, W. W. (2001). Nodularin analyses and toxicity of a Nodulariaspumigena (Nostocales, Cyanobacteria) water-bloom in the western Gulf of Finland, Baltic Sea, in August 1999. Phyciologia, 40, 268-274.
Karlson, B., Andersen, P., Arneborg, L., Cembella, A., Eikrem, W., John, U., West, J. J., Klemm, K., Kobos, J., Lehtinen, S., & Lundholm, N. (2021). Harmful algal blooms and their effects in coastal seas of Northern Europe. Harmful Algae, 102, 101989.
Kasan, N. A., Yusof, S. Z. M., Manan, H., Khairul, W. M., & Zakeri, H. A. (2021). Inhibitory effect of thiourea derivatives on the growth of blue-green algae. Journal of Environmental Management, 294, 113008.
Keliri, E., Paraskeva, C., Sofokleous, A., Sukenik, A., Dziga, D., Chernova, E., Brient, L., & Antoniou, M. G. (2021). Occurrence of a single-species cyanobacterial bloom in a lake in Cyprus: Monitoring and treatment with hydrogen peroxide-releasing granules. Environmental Sciences Europe, 33(1), 1-14.
Kim, J., Jonoski, A., & Solomatine, D. P. (2022). A classifi-cation-based machine learning approach to the prediction of cyanobacterial blooms in Chilgok Weir South Korea. Water, 14(4), 542.
Krienitz, L., Ballot, A., Kotut, K., Wiegand, C., Pütz, S., Metcalf, J. S., Codd, G. A., & Stephan, P. (2003). Contribution of hot spring cyanobacteria to the mysterious deaths of Lesser Flamingos at Lake Bogoria, Kenya. FEMS Microbiology Ecology, 43(2), 141-148.
Kubickova, B., Babica, P., Hilscherová, K., & Šindlerová, L. (2019). Effects of cyanobacterial toxins on the human gastrointestinal tract and the mucosal innate immune system. Environmental Sciences Europe, 31(1), 1-27.
Kudela, R., Berdalet, E., Urban, E. (2015) Harmful algal blooms: A scientific summary for policy makers. IOC/ UNESCO, Paris (IOC/INF-1320)
Kusumawati, D. I., & Mangkoedihardjo, S. (2021). Problems and use of cyanobacteria for environmental improve-ment-A. Journal of Aridland Agriculture, 7, 9-14.
Lad, A., Breidenbach, J. D., Su, R. C., Murray, J., Kuang, R., Mascarenhas, A., Najjar, J., Patel, S., Hegde, P., Youssef, M., & Breuler, J. (2022). As we drink and breathe: Adverse health effects of microcystins and other harmful algal bloom toxins in the liver, gut, lungs and beyond. Life, 12(3), 418.
Lago, J., Rodríguez, P. L., Blanco, L., Vieites, J. M., & Cabado, A. G. (2015). Tetrodotoxin, an extremely potent marine neurotoxin: Distribution, toxicity, origin and therapeutical uses. Marine Drugs, 13, 6384-6406.
Landsberg, J. H., Hendrickson, J., Tabuchi, M., Kiryu, Y., Williams, B. J., & Tomlinson, M. C. (2020). A largescale sustained fish kill in the St. Johns River, Florida: A complex consequence of cyanobacteria blooms. Harmful Algae, 92, 101771.
Li, J., Li, R., & Li, J. (2017). Current research scenario for microcystins biodegradation-a review on fundamental knowledge, application prospects and challenges. Science of the Total Environment, 595(Suppl C), 615-632.
Li, S. M., Liu, J. P., Song, K. S., Liang, C., & Gao, J. (2019). Analysis of temporal and spatial variation characteristics and driving factors of blue alga blooms in Chaohu Lake based on Landsat images. Resources and Environment in the Yangtze Basin, 28, 205-213.
Li, X., Liu, B., Wang, Y., Yang, Y., Liang, R., Peng, F., Xue, S., Zhu, Z., & Li, K. (2020). Hydrodynamic and environmental characteristics of a tributary bay influenced by backwater jacking and intrusions from a main reservoir. Hydrology and Earth System Sciences, 24(11), 5057-5076.
Li, H., Gu, X., Chen, H., Mao, Z., Zeng, Q., Yang, H., & Kan, K. (2021). Comparative toxicological effects of planktonic Microcystis and benthic Oscillatoria on zebrafish embryonic development: Implications for cyanobacteria risk assessment. Environmental Pollution, 274, 115852.
Li, L., Zhang, H., Mubashar, M., Chen, L., Cheng, S., & Zhang, X. (2023). Parallel filtration for solid-liquid separation: A case study of highly-efficient algal removal under parallel configuration driven by magnetic force. Separation and Purification Technology, 310, 123098.
Lim, M. H., Tay, H. S. M., Devotta, D. A., Mowe, M. A., & Mitrovic, S. M. (2020). Risk management of cyanotoxins in Singapore. Journal of Water Resource and Protection, 12(6), 512-525.
Lin, Q., Wu, Z., Singh, V. P., Sadeghi, S. H. R., He, H., & Lu, G. (2017). Correlation between hydrological drought, climatic factors, reservoir operation, and vegetation cover in the Xijiang Basin, South China. Journal of Hydrology, 549, 512-524.
Liu, W., Qiao, Q., Chen, Y., Wu, K., & Zhang, X. (2014). Microcystin-LR exposure to adult zebrafish (Danio rerio) leads to growth inhibition and immune dysfunction in F1 offspring, a parental transmission effect of toxicity. Aquatic Toxicology, 155, 360-367.
Lopez, C. B., Jewett, E. B., Dortch, Q., Walton, B. T., Barsanti, L., & Gualtieri, P. (2005). Algae: Anatomy, biochemistry, and biotechnology. CRC Press.
Lopez, C. B., Jewett, E. B., Dortch, Q., Walton, B. T., Hudnell, H. K. (2008). Scientific assessment of freshwater harmful algal blooms. Interagency Working Group on Harmful Algal Blooms, Hypoxia, and Human Health of the Joint Subcommittee on Ocean Science and Technology. Washington, D.C., USA
Lopez-Rodas, V., Maneiro, E., Lanzarot, M. P., Perdigones, N., & Costas, E. (2008). Mass wildlife mortality due to cyanobacteria in the Doñana National Park, Spain. Veterinary Record, 162(10), 317.
Lu, J., Zhu, B., Struewing, I., Xu, N., & Duan, S. (2019). Nitro-gen-phosphorus-associated metabolic activities during the development of a cyanobacterial bloom revealed by metatranscriptomics. Science and Reports, 9(1), 1-11.
Ma, M., Wang, X., Veroustraete, F., & Dong, L. (2007). Change in area of Ebinur Lake during the 1998-2005 period. International Journal of Remote Sensing, 28, 5523-5533.
Maity, S., Guchhait, R., Chatterjee, A., & Pramanick, K. (2021). Co-occurrence of co-contaminants: Cyanotoxins and microplastics, in soil system and their health impacts on plant-A comprehensive review. Science of the Total Environment, 794, 148752.
Manning, S. R., & Nobles, D. R. (2017). Impact of global warming on water toxicity: Cyanotoxins. Current Opinion in Food Science, 18, 14-20.
Mao, J., Jiang, D., & Dai, H. (2015). Spatial-temporal hydrodynamic and algal bloom modelling analysis of a reservoir tributary embayment. Journal of Hydro-Environment Research, 9(2), 200-215.
Marampouti, C., Buma, A. G., & de Boer, M. K. (2021). Mediterranean alien harmful algal blooms: Origins and impacts. Environmental Science and Pollution Research, 28(4), 3837-3851.
Massey, I. Y., Al Osman, M., Yang, F. (2020). An overview on cyanobacterial blooms and toxins production: Their occurrence and influencing factors. Toxin Reviews, 41(1), 326-346.
Mccarthy, F. M. G., Riddick, N. L., Volik, O., Danesh, D. C., & Krueger, A. M. (2018). Algal palynomorphs as proxies of human impact on freshwater resources in the Great Lakes region. Anthropocene, 21, 16-31.
Mehinto, A. C., Smith, J., Wenger, E., Stanton, B., Linville, R., Brooks, B. W., Sutula, M. A., & Howard, M. D. (2021). Synthesis of ecotoxicological studies on cyanotoxins in freshwater habitats-Evaluating the basis for developing thresholds protective of aquatic life in the United States. Science of the Total Environment, 795, 148864.
Michalak, A. M., Anderson, E. J., Beletsky, D., Boland, S., Bosch, N. S., Bridgeman, T. B., DePinto, J. V., Evans, M. A., Fahnenstiel, G. L., & He, L. (2013). Recordsetting algal bloom in Lake Erie caused by agricultural and meteorological trends consistent with expected future conditions. Proceedings of the National Academy of Sciences of the United States of America, 110(16), 6448-6452.
Miglione, A., Napoletano, M., & Cinti, S. (2021). Electrochemical biosensors for tracing cyanotoxins in food and environmental matrices. Biosensors, 11(9), 315.
Misiou, O., Koutsoumanis, K. (2022). Climate change and its implications for food safety and spoilage. Trends in Food Science and Technology, 126, 142-152.
Moestrup, Ø. (1996). Toxic blue-green algae (cyanobacteria) in 1833. Phycologia, 35(sup6), 5-5.
Moore, S. K., Dreyer, S. J., Ekstrom, J. A., Moore, K., Norman, K., Klinger, T., Allison, E. H., & Jardine, S. L. (2020). Harmful algal blooms and coastal communities:
Moreira, C., Azevedo, J., Antunes, A., & Vasconcelos, V. (2013). Cylindrospermopsin: Occurrence, methods of detection and toxicology. Journal of Applied Microbiology, 114(3), 605-620.
Moreira, C., Ramos, V., Azevedo, J., & Vasconcelos, V. (2014). Methods to detect cyanobacteria and their toxins in the environment. Applied Microbiology and Biotechnology, 98(19), 8073-8082.
Morón-López, J. (2021). A holistic water monitoring approach for effective ecosystem management. Ecohydrology and Hydrobiology, 21(3), 549-554.
Müller, M. N., Mardones, J. I., & Dorantes-Aranda, J. J. (2020). Harmful algal blooms (HABs) in Latin America. Frontiers in Marine Science, 7, 34.
Namsaraev, Z., Melnikova, A., Komova, A., Ivanov, V., Rudenko, A., & Ivanov, E. (2020). Algal bloom occurrence and effects in Russia. Water, 12(1), 285.
Nguyen, T. A. D., Nguyen, L. T., Enright, A., Pham, L. T., Tran, H. Y. T., Tran, T. T., Nguyen, V. H. T., & Tran, D. N. (2021). Health risk assessment related to cyanotoxins exposure of a community living near Tri An Reservoir, Vietnam. Environ Sci Poll Res, 28(40), 56079-56091.
Ni, J., Liu, R., Li, Y., Tang, G., & Shi, P. (2022). An improved transfer learning model for cyanobacterial bloom concentration prediction. Water, 14(8), 1300.
Nwankwegu, A. S., Li, Y., Huang, Y., Wei, J., Norgbey, E., Sarpong, L., Lai, Q., & Wang, K. (2019). Harmful algal blooms under changing climate and constantly increasing anthropogenic actions: the review of management implications. Biotech 3, 9(12), 1-19.
Ogungbile, A. O., Ashur, I., Icin, I., Shapiro, O. H., & Vernick, S. (2021). Rapid detection and quantification of microcystins in surface water by an impedimetric immunosensor. Sensors and Actuators b: Chem, 348, 130687.
Ohio State University. (2017). Algal blooms cost Ohio homeowner
Paerl, H. W., & Barnard, M. A. (2020). Mitigating the global expansion of harmful cyanobacterial blooms: Moving targets in a human-and climatically altered world. Harmful Algae, 96, 101845.
Paerl, H. W., & Otten, T. G. (2013). Harmful cyanobacterial blooms: Causes, consequences, and controls. Microbial Ecology, 65, 995-1010.
Paerl, H. W., & Otten, T. G. (2016). Duelling ‘CyanoHABs’: Unravelling the environmental drivers controlling dominance and succession among diazotrophic and non-N2-fixing harmful cyanobacteria. Environmental Microbiology, 18(2), 316-324.
Paerl, H. W., Xu, H., Hall, N. S., Rossignol, K. L., Joyner, R., Zhu, G., & Qin, B. (2015). Nutrient limitation dynamics examined on a multi-annual scale in Lake Taihu, China:
Paerl, H. W., Gardner, W. S., Havens, K. E., Joyner, A. R., McCarthy, M. J., Newell, S. E., Qin, B., & Scott, J. T. (2016). Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae, 54, 213-222.
Pal, M., Yesankar, P. J., Dwivedi, A., & Qureshi, A. (2021). Biotic control of harmful algal blooms (HABs): A brief review. Journal of Environmental Management, 268, 110687.
Petterson, L. H., Pozdnyakov, D. (2013). Quantification, species variety, and consequences of harmful algal blooms (HABs). Chapter 1 in : Monitoring of harmful algal blooms. Springer-Praxis, Berlin, pp 1-24.
Pham, T. L., & Utsumi, M. (2018). An overview of the accumulation of microcystins in aquatic ecosystems. Journal of Environmental Management, 213, 520-529.
Pitcher, G. C., & Louw, D. C. (2021). Harmful algal blooms of the Benguela Eastern Boundary upwelling system. Harmful Algae, 102, 101898.
Poniedziałek, B., Rzymski, P., & Kokociński, M. (2012). Cylindrospermopsin: Water-linked potential threat to human health in Europe. Environmental Toxicology and Pharmacology, 34(3), 651-660.
Pouria, S., de Andrade, A., Barbosa, J., Cavalcanti, R. L., Barreto, V. T., Ward, C. J., & Codd, G. A. (1998). Fatal microcystin intoxication in haemodialysis unit in Caruaru Brazil. Lancet, 352(9121), 21-26.
Prabha, R., Singh, D. P., Somvanshi, P., & Rai, A. (2016). Functional profiling of cyanobacterial genomes and its role in ecological adaptations. Genomics Data, 9, 89-94.
Qiao, X., Saha., B. (2021). Quantifying the socio-economic impacts of harmful algal blooms in South-West Florida in 2018. Food and resource economics department, University of Florida institute of food and agricultural sciences. Accessed 16 Mar 2022.
Qin, B., Zhu, G., Gao, G., Zhang, Y., Li, W., Paerl, H. W., & Carmichael, W. W. (2010). A drinking water crisis in Lake Taihu, China: Linkage to climatic variability and lake management. Environmental Management, 45(1), 105-112.
Qu, M., Anderson, S., Lyu, P., Malang, Y., Lai, J., Liu, J., Jiang, B., Xie, F., Liu, H. H., Lefebvre, D. D., & Wang, Y. S. (2019). Effective aerial monitoring of cyanobacterial harmful algal blooms is dependent on understanding cellular migration. Harmful Algae, 87, 101620.
Rashidi, H., Baulch, H., Gill, A., Bharadwaj, L., & Bradford, L. (2021). Monitoring, managing, and communicating risk of harmful algal blooms (HABs) in recreational resources across Canada. Environmental Health Insights, 15, 11786302211014400.
Rodger, H. D., Turnbull, T., Edwards, C., & Codd, G. A. (1994). Cyanobacterial (blue-green-algal) bloom associated pathology in brown trout, Salmo trutta L., in
Sakamoto, S., Lim, W. A., Lu, D., Dai, X., Orlova, T., & Iwataki, M. (2021). Harmful algal blooms and associated fisheries damage in East Asia: Current status and trends in China, Japan Korea and Russia. Harmful Algae, 102, 101787.
Schaefer, A. M., Yrastorza, L., Stockley, N., Harvey, K., Harris, N., Grady, R., Sullivan, J., McFarland, M., & Reif, J. S. (2020). Exposure to microcystin among coastal residents during cyanobacteria bloom in Florida. Harmful Algae, 92, 101769.
Schmale, D. G., Ault, A. P., Saad, W., Scott, D. T., & Westrick, J. A. (2019). Perspectives on harmful algal blooms (HABs) and the cyberbiosecurity of freshwater systems. Front Bioeng Biotechnol, 7, 128.
Serrà, A., Philippe, L., Perreault, F., & Garcia-Segura, S. (2021). Photocatalytic treatment of natural waters. Reality or hype? The case of cyanotoxins remediation. Water Research, 188, 116543.
Shahmohamadloo, R. S., Poirier, D. G., Almirall, X. O., Bhavsar, S. P., & Sibley, P. K. (2020). Assessing the toxicity of cell-bound microcystins on freshwater pelagic and benthic invertebrates. Ecotoxicology and Environmental Safety, 188, 109945.
Shen, L., Xu, H., & Guo, X. (2012). Satellite remote sensing of harmful algal blooms (HABs) and a potential synthesized framework. Sensors (basel), 12, 7778-7803.
Shi, K., Zhang, Y., Zhou, Y., Liu, X., Zhu, G., Qin, B., & Gao, G. (2017). Long-term MODIS observations of cyanobacterial dynamics in Lake Taihu: Responses to nutrient enrichment and meteorological factors. Scientific Reports, 7(1), 1-16.
Skafi, M., Duy, S. V., Munoz, G., Dinh, Q. T., Simon, D. F., Juneau, P., & Sauvé, S. (2021). Occurrence of microcystins, anabaenopeptins and other cyanotoxins in fish from a freshwater wildlife reserve impacted by harmful cyanobacterial blooms. Toxicon, 194, 44-52.
Smith, R. B., Bass, B., Sawyer, D., Depew, D., & Watson, S. B. (2019). Estimating the economic costs of algal blooms in the Canadian Lake Erie Basin. Harmful Algae, 87, 101624.
Starling, F., Lazzaro, X., Cavalcanti, C., & Moreira, R. (2002). Contribution of omnivorous tilapia to eutrophication of a shallow tropical reservoir: Evidence from a fish kill. Freshwater Biology, 47, 2443-2452.
Sukenik, A., & Kaplan, A. (2021). Cyanobacterial harmful algal blooms in aquatic ecosystems: A comprehensive outlook on current and emerging mitigation and control approaches. Microorganisms, 9(7), 1472.
Svirčev, Z., Lalić, D., Bojadžija Savić, G., Tokodi, N., Drobac Backović, D., Chen, L., Meriluoto, J., & Codd, G. A. (2019). Global geographical and historical overview of cyanotoxin distribution and cyanobacterial poisonings. Archives of Toxicology, 93, 2429-2481.
Tarafdar, L., Mohapatra, M., Muduli, P. R., Kumar, A., Mishra, D. R., & Rastogi, G. (2023). Co-occurrence patterns and environmental factors associated with rapid onset of Microcystis aeruginosa bloom in a tropical coastal lagoon. Journal of Environmental Management, 325, 116580.
Trevino-Garrison, I., DeMent, J., Ahmed, F. S., Haines-Lieber, P., Langer, T., Ménager, H., Neff, J., Van der Merwe, D., & Carney, E. (2015). Human illnesses and animal deaths associated with freshwater harmful algal blooms-Kansas. Toxins, 7(2), 353-366.
Trinchet, I., Cadel-Six, S., Djediat, C., Marie, B., Bernard, C., Puiseux-Dao, S., Krys, S., & Edery, M. (2013). Toxicity of harmful cyanobacterial blooms to bream and roach. Toxicon, 71, 121-127.
Tsikoti, C., & Genitsaris, S. (2021). Review of harmful algal blooms in the Coastal Mediterranean Sea, with a focus on Greek waters. Diversity, 13(8), 396.
Turner, A. D., Lewis, A. M., Bradley, K., & Maskrey, B. H. (2021). Marine invertebrate interactions with harmful algal blooms-Implications for one health. Journal of Invertebrate Pathology, 186, 107555.
USEPA, (2015a). Drinking water health advisories for two cyanobacterial toxins. Office of Water, 820F15003.
USEPA. (2015b). Recommendations for public water systems to manage cyanotoxins in drinking water. Office of Water (4606M), EPA 815-R-15-010.
USEPA Harmful Algal Blooms (2017): www.epa.gov/nutri entpollution/harmful-algal-blooms
USEPA. (2019). Cyanobacteria and cyanotoxins: Information for drinking water systems. United State Environmental Protection Agency. Office of Water, EPA-810F11001. https://www.epa.gov/sites/default/files/201907/docum ents/cyanobacteria_and_cyanotoxins_fact_sheet_for_ pws_final_06282019.pdf.pdf
USEPA. (2022a). United States environmental protection agency. Learn about Cyanobacteria and Cyanotoxins. https://www.epa.gov/cyanohabs/learn-about-cyanobacte ria-and-cyanotoxins
USEPA. (2022b). United States environmental protection agency. Health effects from Cyanotoxins. https://www. epa.gov/cyanohabs/health-effects-cyanotoxins
Veerman, J., Kumar, A., & Mishra, D. R. (2022). Exceptional landscape-wide cyanobacteria bloom in Okavango Delta, Botswana in 2020 coincided with a mass elephant die-off event. Harmful Algae, 111, 102145.
Verspagen, J. M., Van de Waal, D. B., Finke, J. F., Visser, P. M., Van Donk, E., & Huisman, J. (2014). Rising CO2 levels will intensify phytoplankton blooms in eutrophic and hypertrophic lakes. Plos One, 9(8), e104325.
Vidal, F., Sedan, D., D’Agostino, D., Cavalieri, M. L., Mullen, E., Parot Varela, M. M., Flores, C., Caixach, J., & Andrinolo, D. (2017). Recreational exposure during algal bloom in Carrasco Beach, Uruguay: A liver failure case report. Toxins, 9(9), 267.
Vieira-Lanero, R., Barca, S., Cobo, M. C., & Cobo, F. (2022). Occurrence of freshwater cyanobacteria and bloom records in Spanish reservoirs (1981-2017). Hydrobiology, 1(1), 122-136.
Vogiazi, V., de la Cruz, A. A., Varughese, E. A., Heineman, W. R., White, R. J., & Dionysiou, D. D. (2021). Sensitive electrochemical detection of microcystin-LR in water samples via target-induced displacement of aptamer associated [Ru (NH3) 6] 3+. ACS ES&T Engineering, 1(11), 1597-1605.
Wan, X., Steinman, A. D., Gu, Y., Zhu, G., Shu, X., Xue, Q., Zou, W., & Xie, L. (2020). Occurrence and risk assessment of microcystin and its relationship with environmental factors in lakes of the eastern plain ecoregion China. Environmental Science and Pollution Research, 27(36), 45095-45107.
Wang, C., Feng, T., Wang, P., Hou, J., & Qian, J. (2017). Understanding the transport feature of bloom-forming Microcystis in a large shallow lake: A new combined hydrodynamic and spatially explicit agent-based modelling approach. Ecological Engineering, 343, 25-38.
Wang, H., Li, H., Sun, K., Huang, H., Zhu, P., & Lu, Z. (2020). Impact of exogenous nitrogen on the cyanobacterial abundance and community in oil-contaminated sediment: A microcosm study. Science of the Total Environment, 710, 136296.
Wang, K., Mou, X., Cao, H., Struewing, I., Allen, J., & Lu, J. (2021a). Co-occurring microorganisms regulate the succession of cyanobacterial harmful algal blooms. Environmental Pollution, 288, 117682.
Wang, H., Xu, C., Liu, Y., Jeppesen, E., Svenning, J. C., Wu, J., Zhang, W., Zhou, T., Wang, P., Nangombe, S., & Ma, J. (2021b). From unusual suspect to serial killer: Cyanotoxins boosted by climate change may jeopardize African megafauna. The Innovation, 2(2), 100092.
Welker, M., Chorus, I., Scheffer, B. and Urquhard E. (2021). Planning monitoring programmes for cyanobacteria and cyanotoxins. In: Toxic cyanobacteria in water, pp 641668 CRC Press.
Whitman, P., Schaeffer, B., Salls, W., Coffer, M., Mishra, S., Seegers, B., Loftin, K., Stumpf, R., & Werdell, P. J. (2022). A validation of satellite derived cyanobacteria detections with state reported events and recreation advisories across US lakes. Harmful Algae, 115, 102191.
WHO. (2010). IARC Monographs on the evaluation of carcinogenic risks to humans, ingested nitrate and nitrite, and cyanobacterial peptide toxins (Vol. 94, p. 450). World Health Organization.
Wood, R. (2016). Acute animal and human poisonings from cyanotoxin exposure-A review of the literature. Environment International, 91, 276-282.
World Health Organization (2021). WHO guidelines on recreational water quality: Volume 1: Coastal and fresh waters. World Health Organization (WHO).
Wu, T., Qin, B., Zhu, G., Luo, L., Ding, Y., & Bian, G. (2013). Dynamics of cyanobacterial bloom formation during short-term hydrodynamic fluctuation in a large shallow, eutrophic, and wind-exposed Lake Taihu China. Environmental Science and Pollution Research, 20(12), 8546-8556.
Wu, J., Hilborn, E. D., Schaeffer, B. A., Urquhart, E., Coffer, M. M., Lin, C. J., & Egorov, A. I. (2021). Acute health effects associated with satellite-determined cyanobacterial blooms in a drinking water source in Massachusetts. Environmental Health, 20(1), 1-13.
Xie, H., Fischer, A. M., & Strutton, P. G. (2021). Generalized linear models to assess environmental drivers of paralytic shellfish toxin blooms (Southeast Tasmania, Australia). Continental Shelf Research, 223, 104439.
Xu, H., Long, L., Yan, M., Ma, J., Ji, D., Liu, D., & Yang, Z. (2020). Modelling the effects of hydrodynamics on thermal stratification and algal blooms in the Xiangxi Bay of Three Gorges Reservoir. Frontiers in Ecology and Evolution, 8, 453.
Xue, Y., Chen, H., Yang, J. R., Liu, M., Huang, B., & Yang, J. (2018). Distinct patterns and processes of abundant and rare eukaryotic plankton communities following a reservoir cyanobacterial bloom. ISME Journal, 12, 2263-2277.
You, L., Tong, X., Te, S. H., Tran, N. H., Sukarji, N. H., He, Y., & Gin, K. Y. H. (2022). Multi-class secondary metabolites in cyanobacterial blooms from a tropical water body: Distribution patterns and real-time prediction. Water Research, 212, 118129.
Zhang, Y., Husk, B. R., Duy, S. V., Dinh, Q. T., Sanchez, J. S., Sauvé, S., & Whalen, J. K. (2021). Quantitative screening for cyanotoxins in soil and groundwater of agricultural watersheds in Quebec Canada. Chemosphere, 274, 129781.
Zhao, K., Wang, L., You, Q., Pan, Y., Liu, T., Zhou, Y., Zhang, J., Pang, W., & Wang, Q. (2021). Influence of cyanobacterial blooms and environmental variation on zooplankton and eukaryotic phytoplankton in a large, shallow, eutrophic lake in China. Science of the Total Environment, 773, 145421.
Zhou, Y., Wang, L., Zhou, Y., & Mao, X.-Z. (2020). Eutrophication control strategies for highly anthropogenic influenced coastal waters. Science of the Total Environment, 705, 135760.
Zhu, X., Dao, G., Tao, Y., Zhan, X., & Hu, H. (2021). A review on control of harmful algal blooms by plant-derived allelochemicals. Journal of Hazardous Materials, 401, 123403.
