DOI: https://doi.org/10.1021/acsomega.3c07911
PMID: https://pubmed.ncbi.nlm.nih.gov/38405471
تاريخ النشر: 2024-01-29
المحفزات الكهربائية المعدنية لإنتاج الهيدروجين من تحليل الماء
اقرأ على الإنترنت
المقاييس والمزيد
توصيات المقالات
تم التنزيل عبر 86.143.216.125 في 21 مارس 2024 الساعة 15:12:53 (UTC).
انظرhttps://pubs.acs.org/sharingguidelinesللحصول على خيارات حول كيفية مشاركة المقالات المنشورة بشكل قانوني.
الملخص
إن الطلب المتزايد على الوقود الأحفوري والتلوث الناتج عنه قد أثار مخاوف بيئية بشأن إنتاج الطاقة. لا شك أن الهيدروجين هو أفضل مرشح لإنتاج طاقة نظيفة ومستدامة الآن وفي المستقبل. يعتبر تحليل الماء عملية واعدة وفعالة لإنتاج الهيدروجين، حيث تلعب المحفزات دورًا رئيسيًا في تفاعل تطور الهيدروجين (HER). يمكن أن يتم تحفيز HER بشكل جيد بواسطة البلاتين مع جهد زائد منخفض قريب من الصفر وانحدار تافل يبلغ حوالي 30.
 التقدم في تصميم وتطوير المحفزات الكهربائية النانوية للمعادن الثمينة وغير الثمينة في التحفيز الكهربائي لتفاعل الهيدروجين. بشكل عام، فإن الاستراتيجيات بما في ذلك التعديل، والتحكم في التبلور، والهندسة الهيكلية، ومواد الكربون النانوية، وزيادة المواقع النشطة من خلال تغيير الشكل، تساعد في تحسين أداء تفاعل الهيدروجين. أخيرًا، سيتم وصف التحديات وآفاق المستقبل في تصميم محفزات كهربائية وظيفية ومستقرة لتفاعل الهيدروجين في إنتاج الهيدروجين بكفاءة من التحليل الكهربائي للماء.
التقدم في تصميم وتطوير المحفزات الكهربائية النانوية للمعادن الثمينة وغير الثمينة في التحفيز الكهربائي لتفاعل الهيدروجين. بشكل عام، فإن الاستراتيجيات بما في ذلك التعديل، والتحكم في التبلور، والهندسة الهيكلية، ومواد الكربون النانوية، وزيادة المواقع النشطة من خلال تغيير الشكل، تساعد في تحسين أداء تفاعل الهيدروجين. أخيرًا، سيتم وصف التحديات وآفاق المستقبل في تصميم محفزات كهربائية وظيفية ومستقرة لتفاعل الهيدروجين في إنتاج الهيدروجين بكفاءة من التحليل الكهربائي للماء.
1. المقدمة
حالياً حوالي

2. لها
| معايير | مصدر | إنتاج | رد فعل | ||||
|
|
|
|
||||
| تحويل الفحم إلى غاز | فحم، بخار |
|
|
||||
| تحليل الماء بالكهرباء | ماء |
|
|
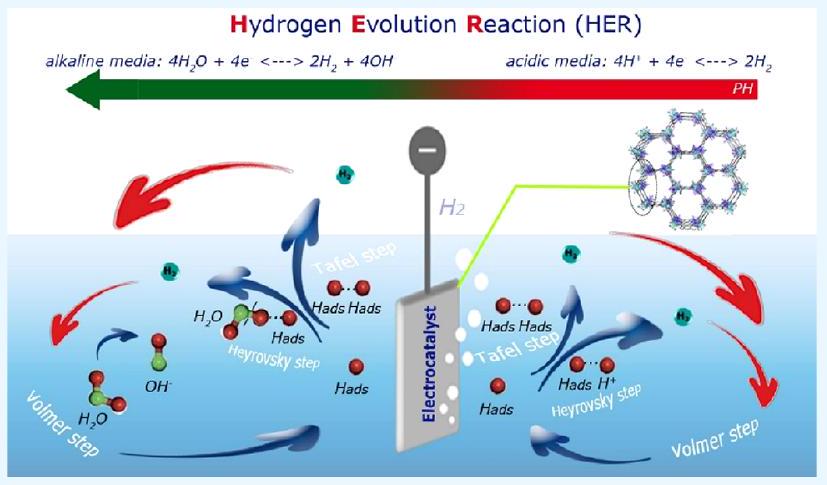
تطبيقات مفيدة. تعتبر الخلايا الكهروكيميائية تقنية واعدة لتوليد وقود الهيدروجين من الماء بشكل متجدد؛ هذه هي عملية تحويل الماء إلى هيدروجين نقي ومستقر تتكون من تفاعلين نصف خلية: تفاعل تطور الأكسجين (OER) وتفاعل تطور الهيدروجين (HER).
2.1. أساسيات تفاعل تقليل الهيدروجين. الماء، على عكس الوقود الأحفوري، هو مورد وفير ومتجدد على الأرض، لذا فإن الهيدروجين
2.2. الوسائط الحمضية والقلوية. يحدث نصف التفاعل الرئيسي لإنتاج الهيدروجين في تفكيك الماء عند القطب السالب، والذي يتضمن نقل إلكترونين معتمدين بشدة في ظروف بيئية. البيئة القلوية هي الآن محور تطوير الهيدروجين من خلال تفاعل تقليل الهيدروجين لاستبدال الوقود النظيف لمختلف أنظمة الطاقة. بسبب عملية تفكيك الماء الإضافية، فإن حركية هذا التفاعل بطيئة وتسبب انخفاضًا كبيرًا في الأداء الكهروكيميائي. لذلك، يمكن أن تؤدي المحفزات الكهروكيميائية الحديثة أداءً جيدًا في البيئات الحمضية.
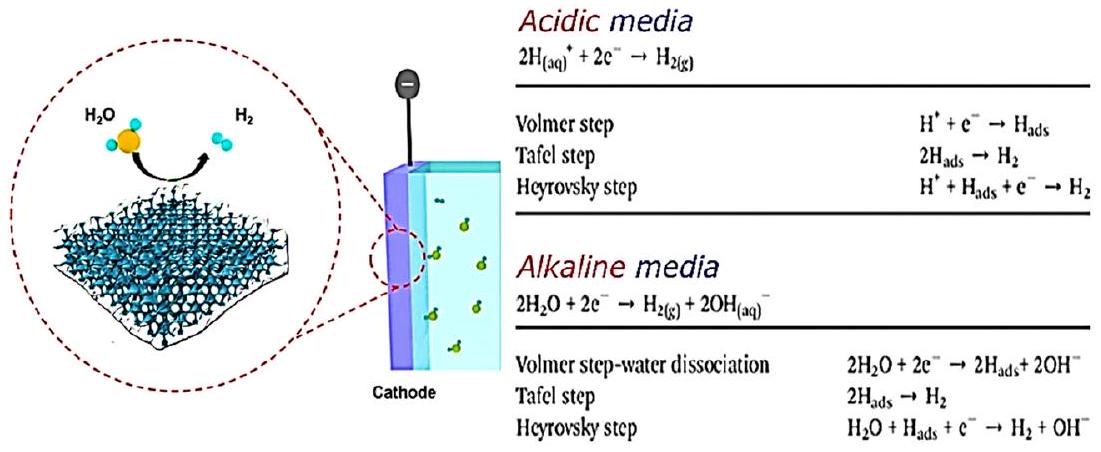

جزيء الماء، المعروف باسم مسار فولمر. ثم يستمر مسار تافل أو هيروفكسي لإنتاج الهيدروجين.
2.3. معدل التفاعل. تم حساب جهد حراري قدره 1.23 فولت عند
ضد التوقعات النظرية مع الكفاءة الفارادية وتردد الدوران.
2.4. السعة الزائفة. في عملية تقليل الهيدروجين، يجب أن يتم امتصاص الهيدروجين على سطح المحفز الكهربائي في الخطوة الأولى. في الخطوة التالية، يجب فصل الهيدروجين الممتص عن سطح المحفز الكهربائي وإعادته بشكل لا رجعة فيه إلى الإلكتروليت. هذه الخاصية الزائفة للمحفز الكهربائي حاسمة لأداء تقليل الهيدروجين. في امتصاص الهيدروجين، تعتبر جميع المحفزات الكهربائية لتقليل الهيدروجين تقريبًا مكثفات زائفة ممتازة. من أجل إزالة كل الهيدروجين الممتص، يجب أن تكون المكثف الزائف فعالًا للغاية. لذلك، فإن دراسة أداء السعة الزائفة قبل جهد تقليل الهيدروجين تقدم معلومات حيوية حول كفاءة المحفز الكهربائي. الأداء الأمثل للسعة الزائفة، الذي يتميز بشكل مستطيل في الفولتمترية الدورية (CV)، مرتبط بشكل كبير بنشاط المحفز الكهربائي لتقليل الهيدروجين.
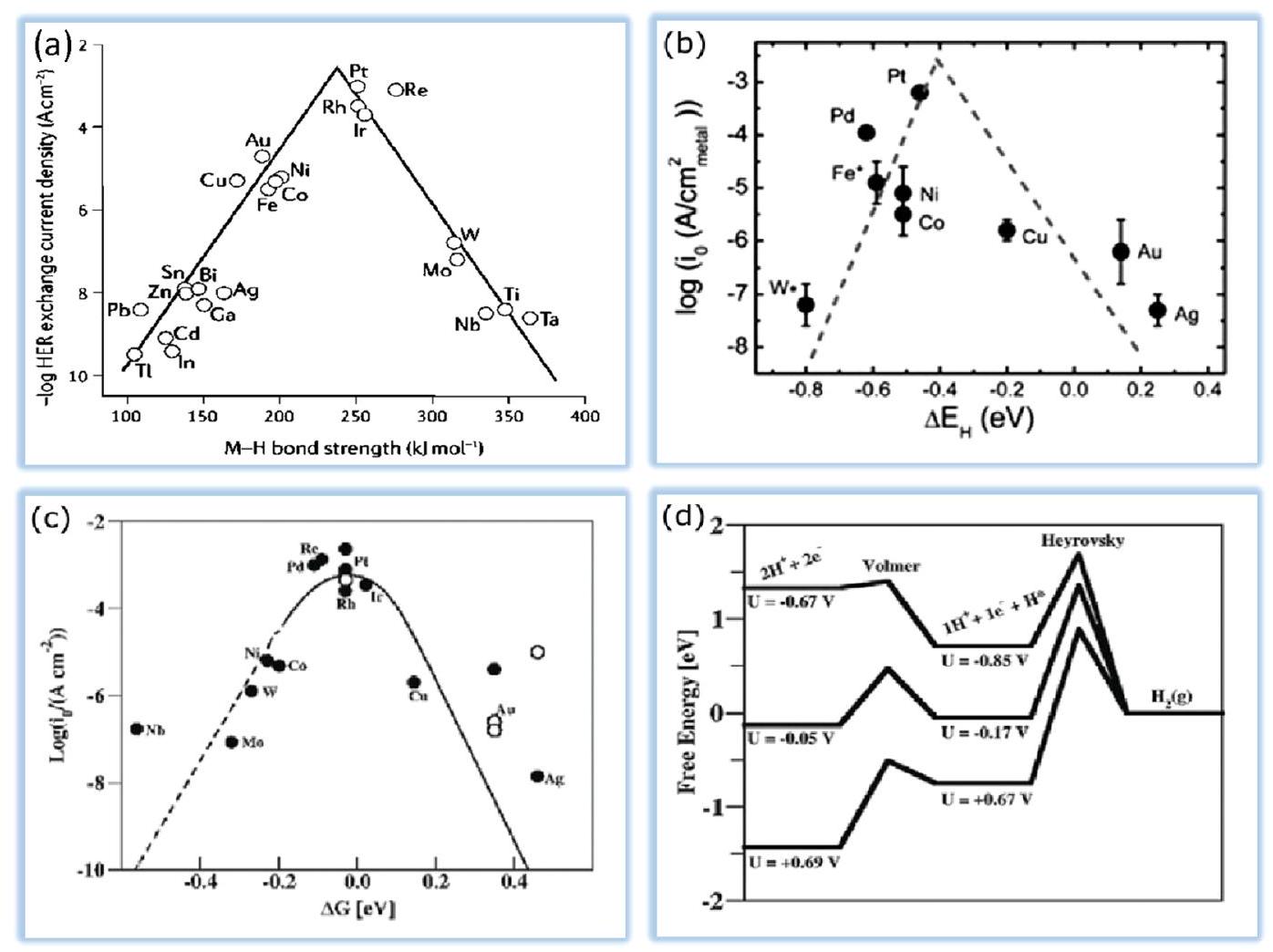
2.5. مخططات البراكين. كما ذُكر في الأقسام السابقة، تثبت المحاكاة النظرية أن نشاط HER مرتبط ارتباطًا وثيقًا
مرتبط بامتصاص الهيدروجين (
2.6. تأثير النظائر الحركي (KIE). طريقة مهمة لدراسة التفاعلات الكيميائية هي تأثير النظائر الحركي (KIE). تستند هذه النظرية إلى الملاحظة أن معدل التفاعل يمكن أن يتغير مع كتلة الذرة. تؤثر كتلة الجسيم على طاقة حالاته الاهتزازية والدورانية، مما يؤثر بدوره على احتمال النفق. النفق هو عملية ميكانيكية كمومية تسمح للجسيمات بالمرور عبر الحواجز المحتملة دون أن تمتلك طاقة كافية لتجاوزها.
يتم حساب التأثيرات باستخدام نسبة ثابتتي معدل تفاعلين مستبدلين نظائريًا حيث لا يكون الفرق في الكتلة بين الذرات المعنية في الخطوة المحددة لمعدل التفاعل ولكن بين الذرات المعنية في ثابت التوازن. يمكن دراسة التفاعلات الكيميائية باستخدام KIE. من الممكن استخدامها لتحديد الخطوة المحددة لمعدل التفاعل، لتحديد طاقة حالة الانتقال، ولدراسة آلية التفاعلات التي يصعب دراستها باستخدام طرق أخرى. يتم نقل إلكترون من القطب إلى أيون هيدروجين في الإلكتروليت كجزء من عملية HER. كإلكتروليت، كلا
(1) يتم نقل إلكترون من القطب إلى أيون الهيدروجين في المحلول الكهربائي، مما يشكل أيون الهيدرونيوم.
(2) أيون الهيدرونيوم ينفصل إلى بروتون وجزيء ماء.
(3) ينفذ البروتون من خلال جزيء الماء ويهاجم سطح القطب، مكونًا ذرة هيدروجين.
(4) يتم تحرير ذرة الهيدروجين من سطح القطب، مكونة غاز الهيدروجين.
تشير KIE إلى أنه يتم نقل إلكترون من القطب إلى أيون الهيدروجين في الإلكتروليت لتحديد معدل التفاعل. تأثير النظير أكبر بكثير لهذه الخطوة مقارنة بأي من الخطوات الأخرى.
3. متطلبات المحفزات الكهربائية لها
3.1. الجهد الزائد، ميل تافل، وكثافة التيار المتبادل. الجهد الزائد (
إن انخفاض الجهد الزائد مرتبط مباشرة بالنشاط الكهروكيميائي العالي. يحدث انقسام الماء عند جهد خلية يبلغ 1.23 فولت (0 فولت لتفاعل الهيدروجين و1.23 فولت لتفاعل الأكسجين). تتطلب كل من عمليات تفاعل الهيدروجين وتفاعل الأكسجين جهدًا إضافيًا، بشكل رئيسي بسبب العقبات التنشيطية الداخلية الموجودة. يجب زيادة الجهد المطبق لحدوث التفاعل.
3.2. المساحة السطحية النشطة كيميائيًا (ECSA). تعتبر المساحة السطحية الكهروكيميائية لمادة القطب (ECSA) واحدة من الظواهر الأساسية في اختيار المحفزات الكهروكيميائية،
تشير إلى مساحة مادة القطب المتاحة للإلكتروليت. لقد ثبت أنه من الصعب قياس السطح النشط كهربائياً لأي مادة كقطب. يتم استخدام هذه السطح لنقل الشحنات و/أو التخزين في الخلايا الكهروكيميائية (الجلفانية/التحليل الكهربائي). يمكن توسيع سطح القطب باستخدام مجموعة متنوعة من التقنيات. تشمل هذه التقنيات استخدام الهياكل النانوية، والفولتمترية الدورية (CV)، والفولتمترية ذات المسح الخطي (LSV).
3.3. الكفاءة فاراداي. الكفاءة فاراداي (المعروفة أيضًا بكفاءة التيار) هي معلمة أخرى تُستخدم لتقييم نشاط المحفز الكهربائي لتفاعل الهيدروجين. بدلاً من التفاعل الجانبي، تحسب الكفاءة فاراداي (FE) كمية الشحنات (الإلكترونات) في التفاعل المرغوب. في عملية HER، تعتبر FE نسبة من تم تحديده تجريبيًا.
3.4. تردد الدوران. ي quantifies تردد الدوران (TOF) لمركز التحفيز نشاطه الخاص من خلال عدد المتفاعلات التي تم تحويلها إلى المنتج المختار لكل وحدة زمنية. يتم حساب مقدار TOF لـ HER و OER بناءً على المعادلات التالية:
3.5. طاقة الروابط الهيدروجينية. في تفاعل تطور الهيدروجين (HER)، وهو عملية كيميائية كهربائية تولد غاز الهيدروجين من انقسام الماء، تلعب طاقة الروابط الهيدروجينية (HBE) دورًا حاسمًا. تصف طاقة الروابط الهيدروجينية قوة تفاعل ذرات الهيدروجين مع الذرات المجاورة، وعادة ما تكون ذرات الأكسجين في الماء أو على سطح المحفز. يؤثر هذا التفاعل على الحركية العامة لتفاعل HER، مما يؤثر على معدل تطور الهيدروجين واستقرار الأنواع الهيدروجينية الممتصة. تسهل الروابط الهيدروجينية القوية بين ذرات الهيدروجين وسطح المحفز امتصاص الوسائط الهيدروجينية على سطح المحفز. إذا كانت HBE قوية جدًا، فقد تمنع جزيئات الهيدروجين من الانفصال، مما يقلل من كفاءة تطور الهيدروجين. من أجل تحقيق HER فعال ومستدام، يجب أن تكون هناك HBE مثالية. تم استخدام العديد من التقنيات لدراسة تأثيرات HBE على نشاط HER، بما في ذلك
(1) طبيعة سطح المحفز: يمكن أن تتأثر طاقة الربط الهيدروجيني بشكل كبير بنوع المعدن أو المادة المستخدمة لسطح المحفز. تميل المحفزات التي تحتوي على كثافة عالية من المجموعات المحتوية على الأكسجين، مثل الهيدروكسيدات والأكاسيد، إلى إظهار طاقة ربط هيدروجيني أقوى من المحفزات التي تحتوي على كثافة أقل.
(2) طرق التحضير: يمكن أن تؤثر طريقة تحضير المحفز أيضًا على طاقته الربط الهيدروجيني. من المرجح أن تحدث تشكيل طاقة الربط الهيدروجيني في المحفزات التي تم تحضيرها باستخدام طرق تُدخل عيوبًا أو خشونة في أسطحها.
(3) تركيبة الإلكتروليت: يمكن أن تتأثر طاقة الربط الهيدروجيني أيضًا بتركيبة الإلكتروليت. تميل الإلكتروليتات ذات قيم pH الأعلى، على سبيل المثال، إلى تشكيل طاقة ربط هيدروجيني أقوى من الإلكتروليتات ذات قيم pH الأقل.
تحسين طاقة الربط الهيدروجيني وتأثيرها على إنتاج الهيدروجين المستدام. يتطلب تطوير محفزات فعالة لتفاعل تطور الهيدروجين تحسين طاقة الربط الهيدروجيني. يمكن للعلماء تصميم محفزات بمعدلات عالية من تفاعل تطور الهيدروجين واستقرار من خلال فهم العوامل التي تؤثر على
3.6. الاستقرار. يعد الاستقرار معلمة مهمة أخرى لاختيار المحفز الكهربائي المناسب لتفاعل تطور الهيدروجين. هناك طريقتان لتقييم عامل الاستقرار. واحدة هي LSV أو CV؛ والأخرى هي التحليل الكهربائي الثابت الجهد أو الثابت التيار من خلال اختبارات الكرونوستاتيكية الطويلة الأمد (CP) أو الكرونوأمبيرومترية (CA). تُستخدم هذه الطريقة الفولتامترية لمقارنة التعديلات في الجهد الزائد، قبل وبعد فترة معينة من الدورات لـ 1000-10000 فولتاموجرام دوري بمعدل مسح مثل
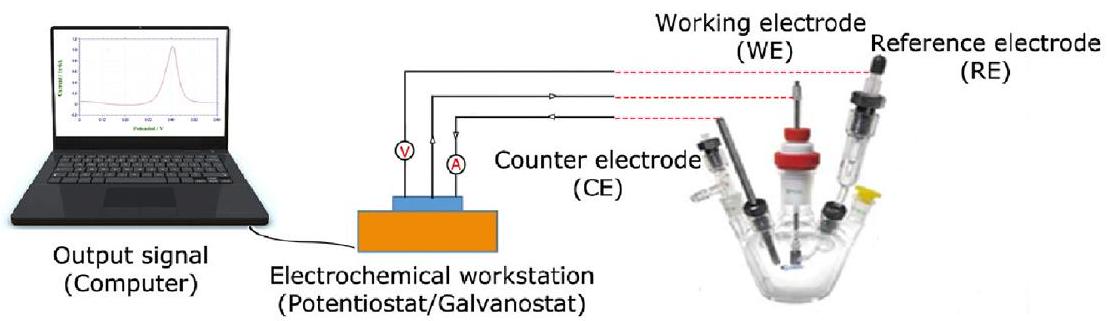
3.7. الطرق الكهروكيميائية (خلية ثلاثية الأقطاب). في عملية تفاعل تطور الهيدروجين، يُقترح استخدام قطب عمل قرصي دوار (RDE) للحصول على بيانات تجريبية دقيقة. يمكن لهذا القطب، بدقة عالية، قياس معدل نقل الكتلة وحركية التفاعل بشكل جيد. يُستخدم RDE كقطب عمل في أنظمة ثلاثية الأقطاب لفولتامترية الدراسات الكهروكيميائية عند فحص آليات التفاعل في كيمياء الأكسدة والاختزال، من بين ظواهر كيميائية أخرى.
محاط بمادة غير موصلة مثل بوليمر أو راتنج خامد. وفقًا للشكل 4، في نظام كهروكيميائي، يُستخدم القطب العامل (WE) غالبًا مع قطب مضاد (CE) وقطب مرجعي (RE) في نظام ثلاثي الأقطاب.
3.8. عوائق القياس وتفسير البيانات في
4. المحفزات الكهروكيميائية لتفاعل تطور الهيدروجين
(1) المعادن النبيلة مع المركبات والسبائك
(2) المواد القائمة على المعادن الانتقالية منخفضة التكلفة بدون معادن ثمينة
(3) المحفزات الكهروكيميائية المستمدة من MOF
4.1. المحفزات الكهروكيميائية القائمة على المعادن النبيلة. إن الأداء الكهروكيميائي للمعادن النبيلة، مثل معادن مجموعة البلاتين (PGMs، بما في ذلك Pt وPd وRh وRu وIr)، جذاب لتفاعل تطور الهيدروجين.
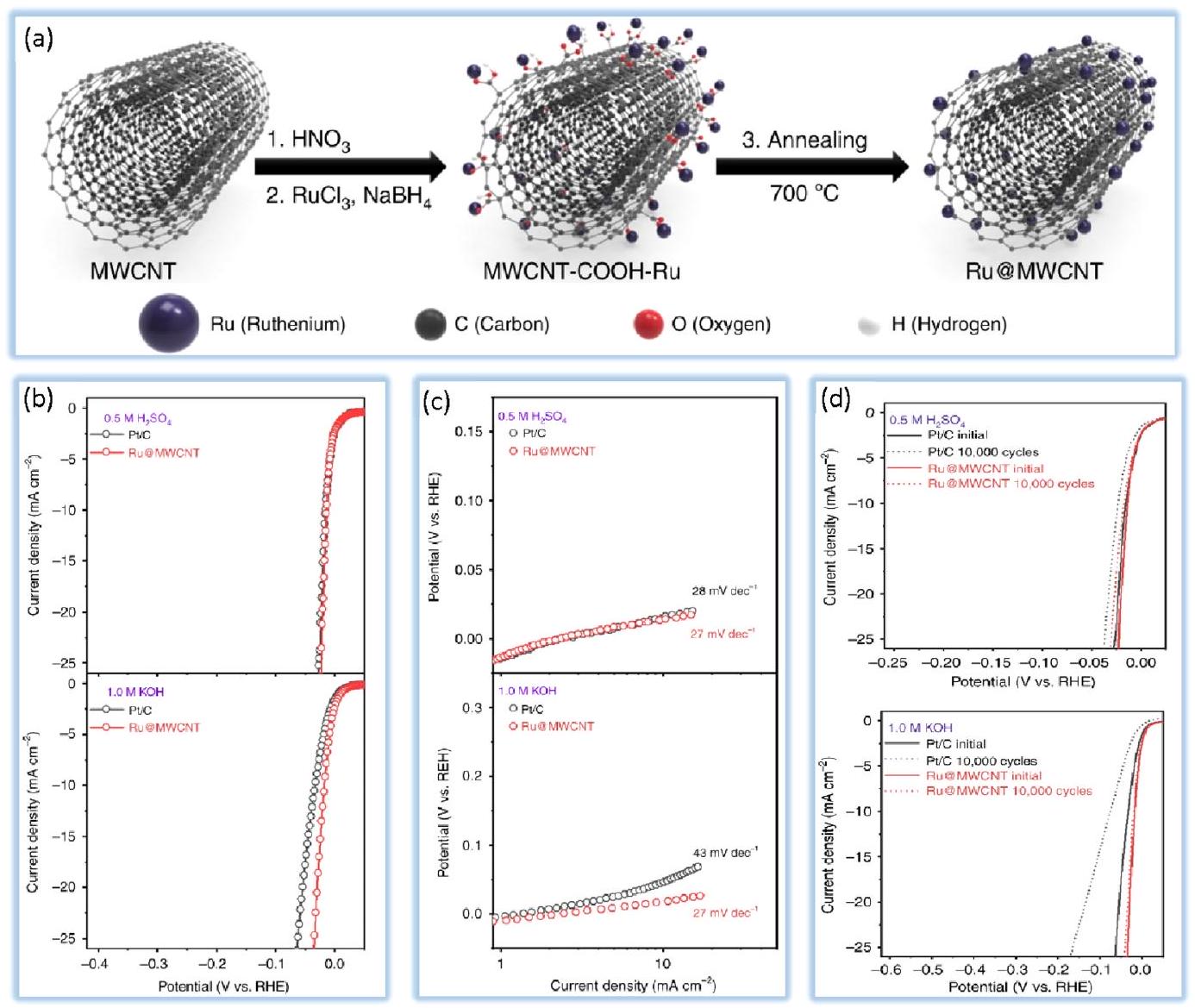
إيجابي على النشاط التحفيزي للمادة. يمكن صنع بعض سبائك البلاتين مع
تم تصميمه باستخدام حمض الروديوم-البالاديوم
4.2. المحفزات الكهروكيميائية القائمة على المعادن غير النفيسة. كما تم تلخيصه في الفصل السابق، فإن المحفزات الكهروكيميائية القائمة على المعادن غير النفيسة هي الخيار الوحيد القابل للتطبيق لتطوير عملية التحليل الكهربائي للمياه على نطاق واسع من أجل تحويل الطاقة.
| مادة | هيكل | ميل الطاولة
|
|
المراجع |
| نيكون | نيتريد | ١٠٥.٢ | 145 | 76 |
|
|
نيتريد | 64 | 31 | 77 |
|
|
سيلينيد | ٤٦.٩ | 249 مللي فولت عند 100 مللي أمبير | 78 |
|
|
سيلينيد | 31.6 | ١٠٨ | 79 |
| NiMoNx/C | نيتريد | ٣٥ | 78 مللي فولت عند 100 مللي أمبير | ٨٠ |
| مو-في-سي بي | سيلينيد | ٥٧.٧ | 86.9 | 81 |
|
|
نيتريد | ٤٧ | ٥٦ | 82 |
|
|
فوسفيد | ٥٥ | 191 | 83 |
|
|
فوسفيد | 60 | 174 | 83 |
|
|
فوسفيد | ١٠١ | ٤٠٦ | 84 |
|
|
نيتريد | ١١٥.٧ | 217 | 85 |
| FeP | فوسفيد | 37 | 50 | 86 |
|
|
كربيد | ٣٤.٥ | ٣٨ | 87 |
| WN/CC | نيتريد | ٥٧.١ | ١٣٠ | ٨٨ |
| مسامي
|
فوسفيد | 67 | ٥٦ | 89 |
| موNx | نيتريد | 114 | 148 | 90 |
| VMoN | نيتريد | 60 | ١٠٨ | 91 |
|
|
سيلينيد | 40 | 87 | 92 |
| CoNiSe/NC | سيلينيد | 66.5 | 100 | 93 |
|
|
كربيد | 52 | 114 | 94 |
| نانو موك@جي إس | كربيد | 43 | ١٢٤ | 95 |
| مو إن | نيتريد | ١٢٠ | ٣٨٩ | 96 |
|
|
نيتريد | 85 | 242 | 97 |
| ثقب NiCoP | فوسفيد | ٥٧ | ٥٨ | 98 |
|
|
فوسفيد | 50 | ٨٨ | 99 |
4.2.1. كربيدات المعادن الانتقالية. تُظهر كربيدات المعادن الانتقالية (TMCs) خصائص مشابهة للبلاتين من حيث النشاط الكهروكيميائي لتفاعل تقليل الهيدروجين (HER) بسبب التحول في مركز نطاق d؛ ولهذا السبب، فإن تطوير هذه المحفزات الكهروكيميائية كمحفزات قائمة على المعادن غير الثمينة يحظى باهتمام كبير.
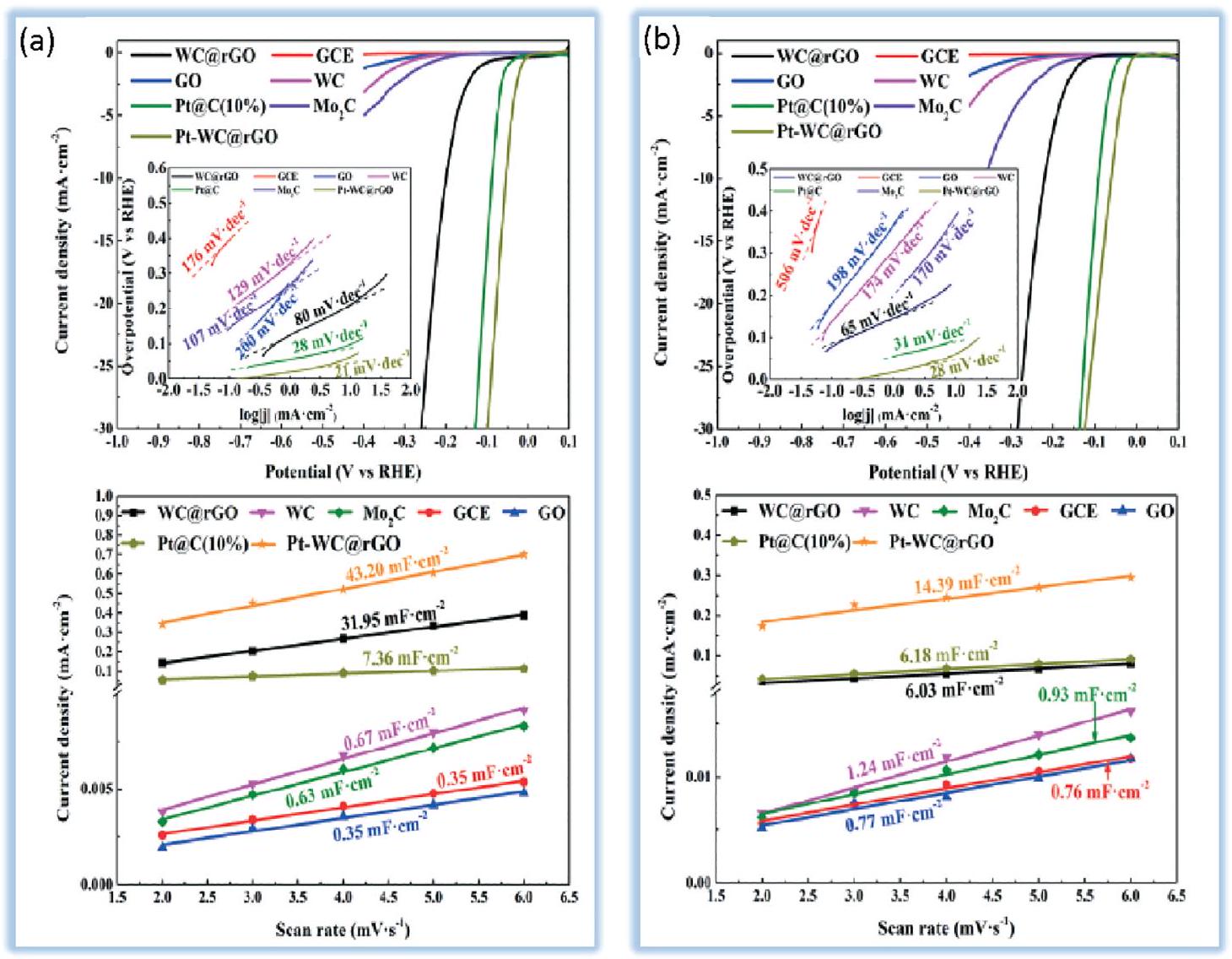
ت disrupt الكربون التنجستن المواقع النشطة في تفاعل المحفز الكهربائي HER. وهذا يتناقض مع الكالكوجينيدات المعدنية الانتقالية التي يمكن أن يحسن وجود الأكسجين أداء HER. في تفاعل المحفز الكهربائي مع الماء، disrupt سطح WC ويعطل تشكيل
تظل العمليات المتقطعة مصدر قلق حاسم حيث إن المواد القائمة على الكربون عرضة للتآكل بشكل طبيعي، حتى في ظل ظروف أكسدة خفيفة. نتيجة لذلك، يُقترح أن تركز الدراسات المستقبلية في هذا المجال على تعزيز الاستقرار تحت الاستقطاب الأنودي الذي يمكن أن يحدث بعد إيقاف تشغيل الإلكتروليزر.
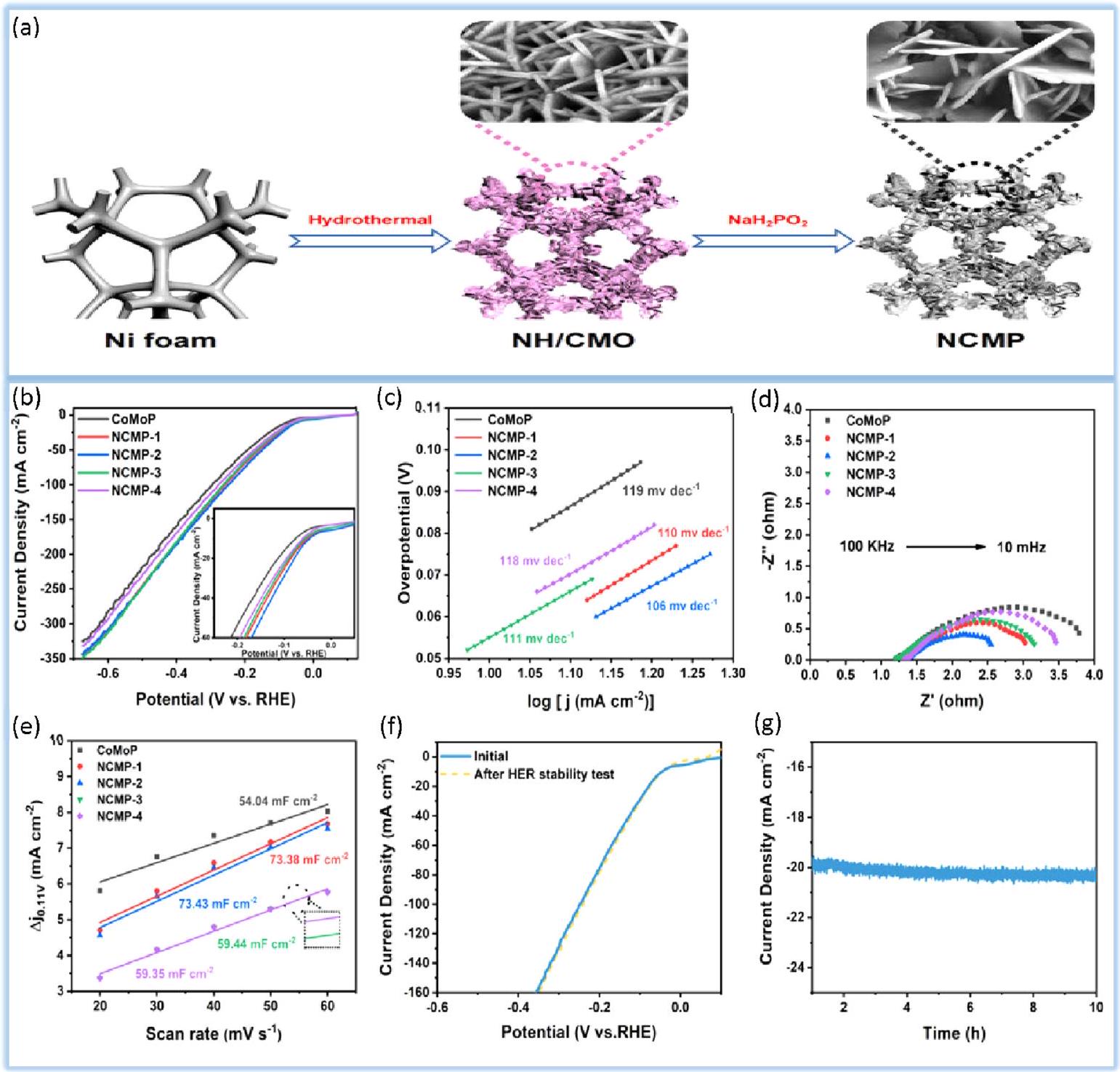
4.2.2. فوسفيدات المعادن الانتقالية. تعتبر فوسفيدات المعادن الانتقالية (TMPs)، نظرًا لنشاطها الفطري واستقرارها العالي في البيئات الحمضية والقلوية، مرشحة محتملة لتحفيز تفاعل تقليل الهيدروجين (HER).
الجرعة هي 0.02 مللي مول، وتظهر أكبر فروق جهد بمقدار 46 مللي فولت عند
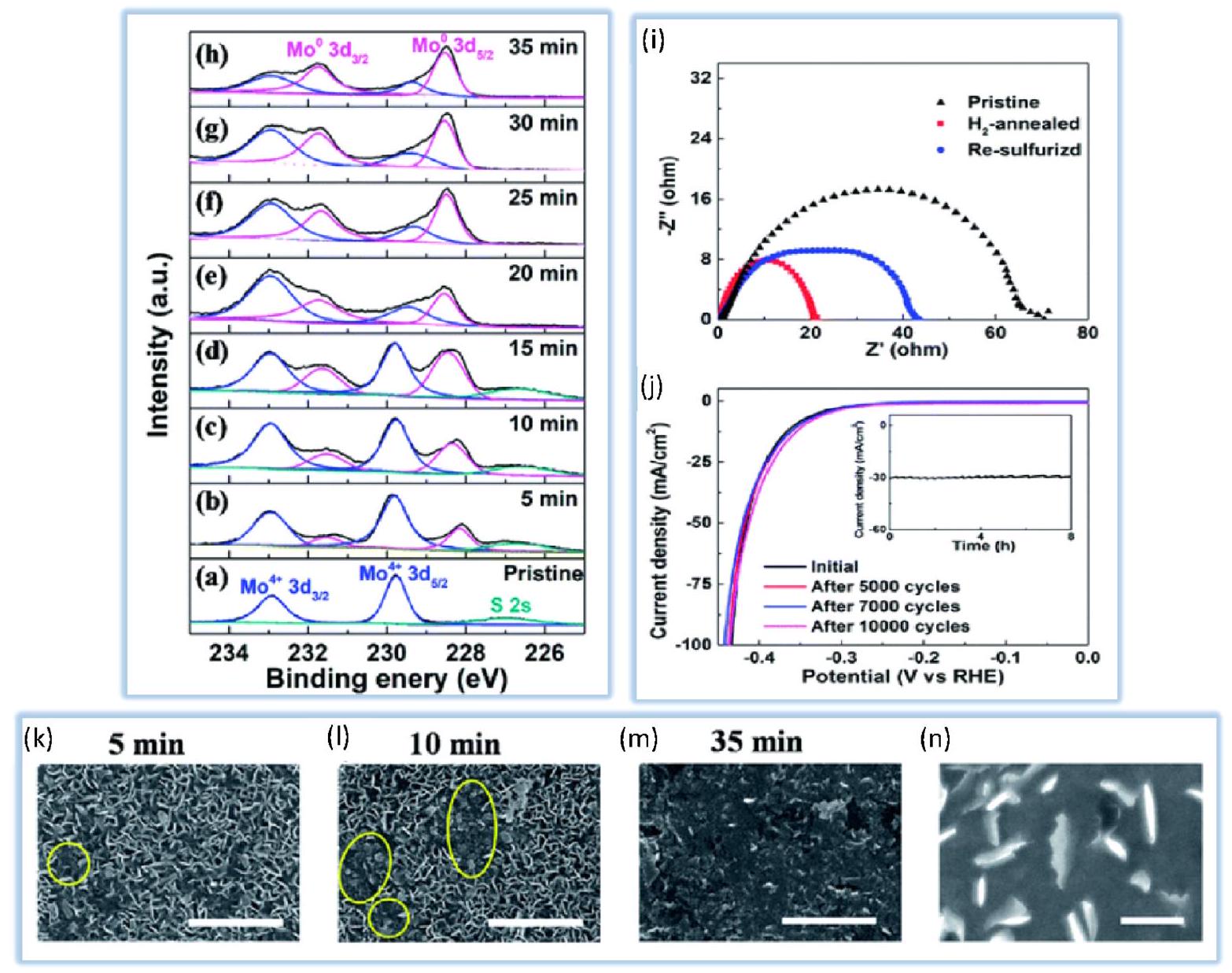
4.2.3. الكالكوجينات من المعادن الانتقالية (الكبريتيدات والسيلينيدات). تعتبر الكالكوجينات من المعادن الانتقالية، التي تعتمد على خصائص مثل كونها غير مكلفة وسهلة التحضير، مرشحة واعدة لاستبدال المعادن النبيلة في تفاعل تقليل الهيدروجين. وفقًا للدراسات الحالية، تظهر السيلينيدات المعدنية نشاطًا تحفيزيًا أعلى من الأعضاء الآخرين في الكالكوجينات.
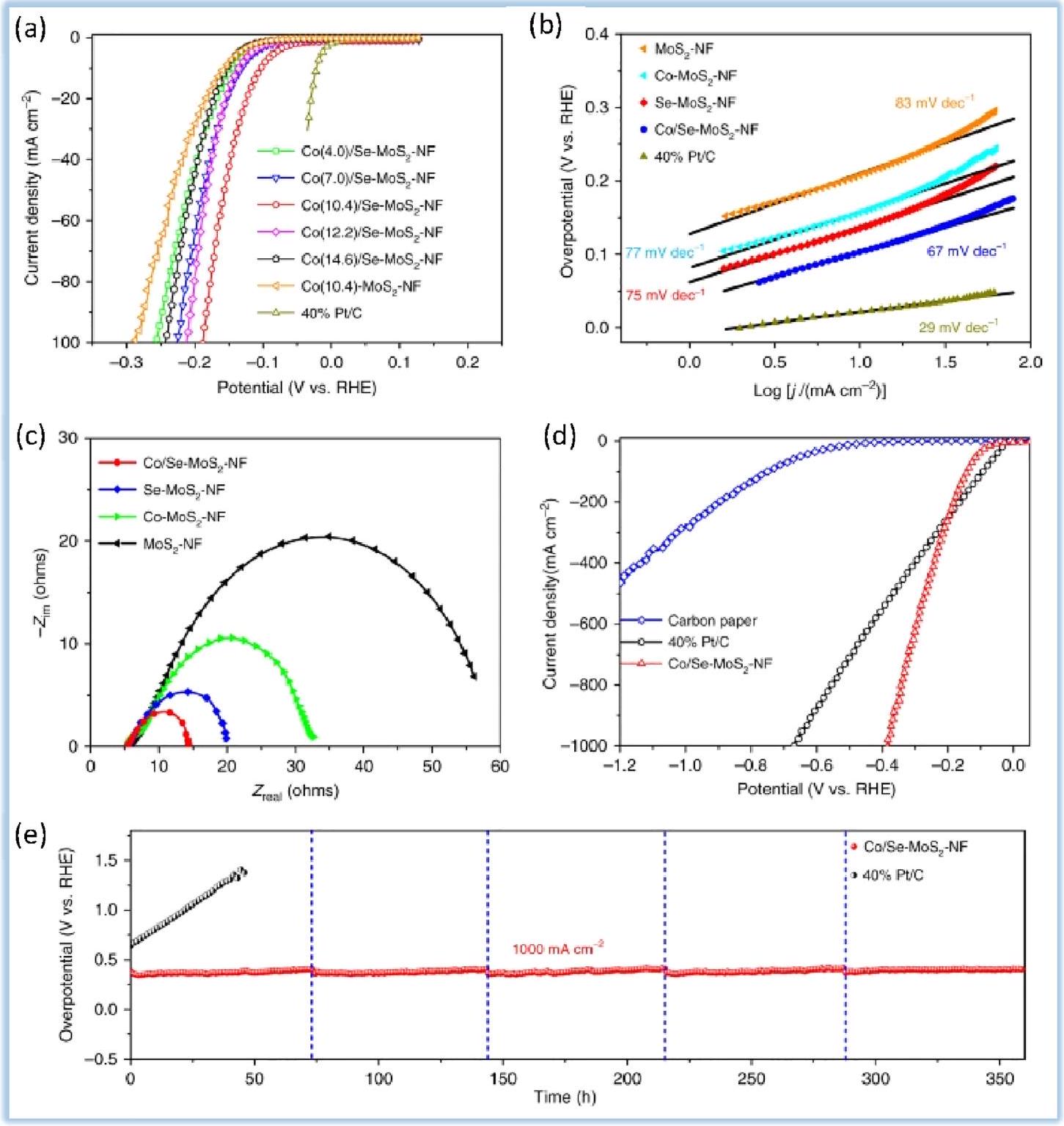
نشاط HER العمودي
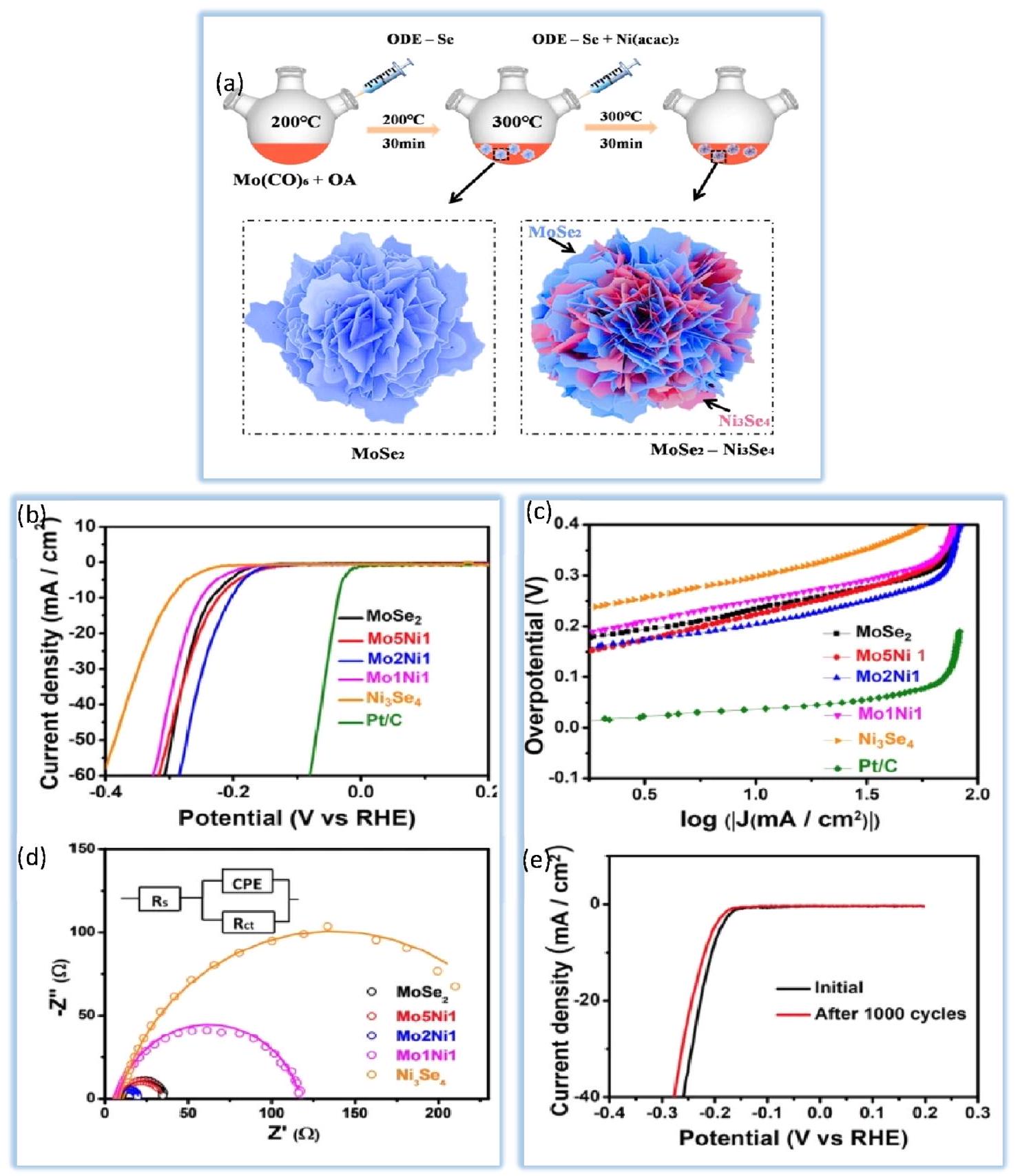
محفزات لتطبيقات إنتاج الهيدروجين الصناعي. في هذه الدراسة، تُظهر الشكل 9a منحنيات استقطاب HER للمواد المضافة بالسيليكون.
نقل الإلكترون في تفاعل HER. يشير ميل تافل الأقل إلى معدل أسرع لنقل الإلكترون، مما يعني وجود محفز أكثر كفاءة.
قياسات الطيفية (EIS) لـ
تم اختبار السيلينيدات المعدنية المتوسطة بنجاح في الأوساط الحمضية.
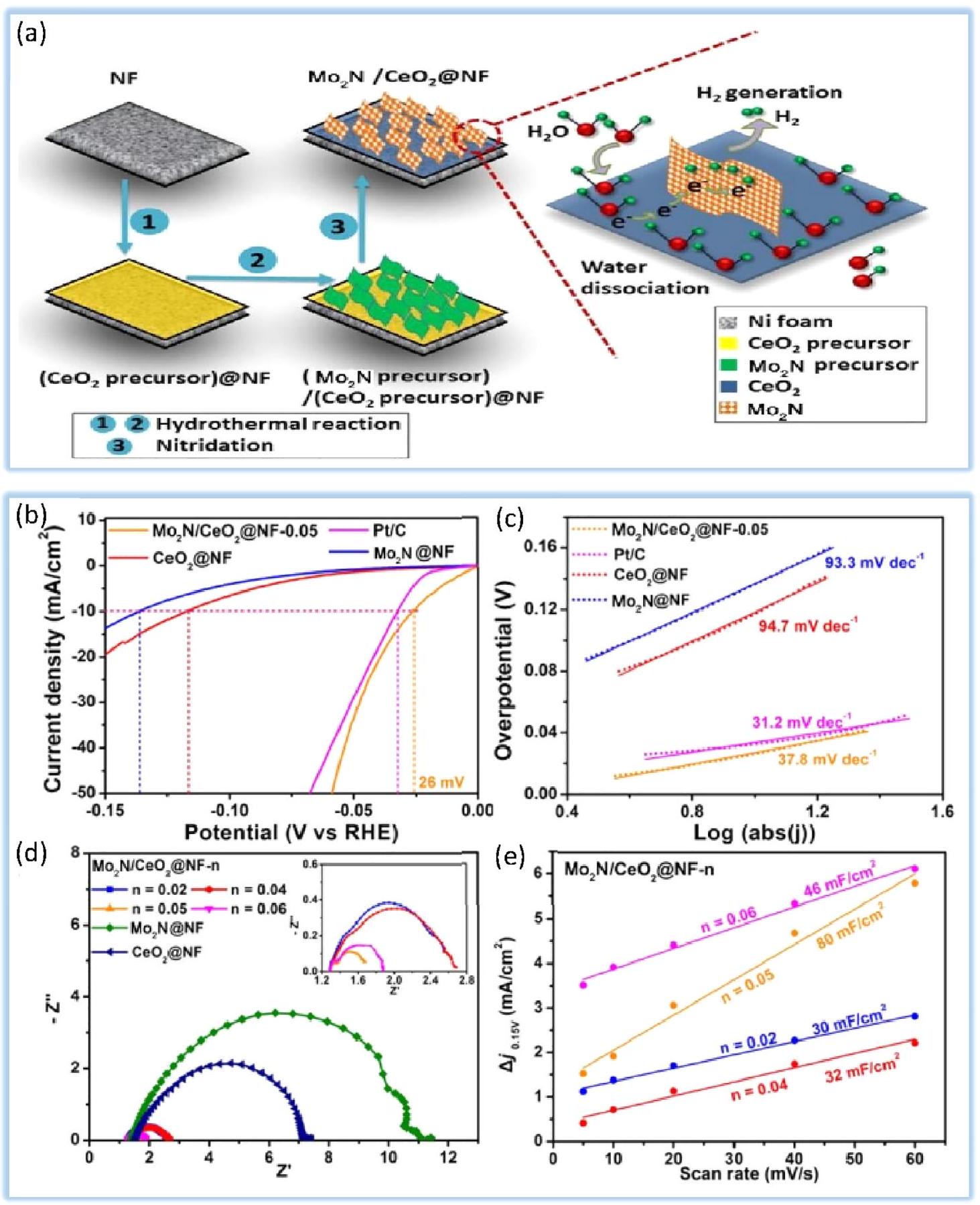
4.2.4. نيتريدات المعادن الانتقالية. تم اقتراح نيتريدات المعادن الانتقالية (TMNs)، المعروفة باسم سبائك الانتقال، مؤخرًا كعوامل تحفيز فعالة لتفاعل تقليل الهيدروجين (HER) كبدائل لعوامل التحفيز المعدنية النبيلة بسبب توصيلها الكهربائي الاستثنائي، ومقاومتها للتآكل، وقوتها الميكانيكية، واستقرارها الكهروكيميائي العالي.
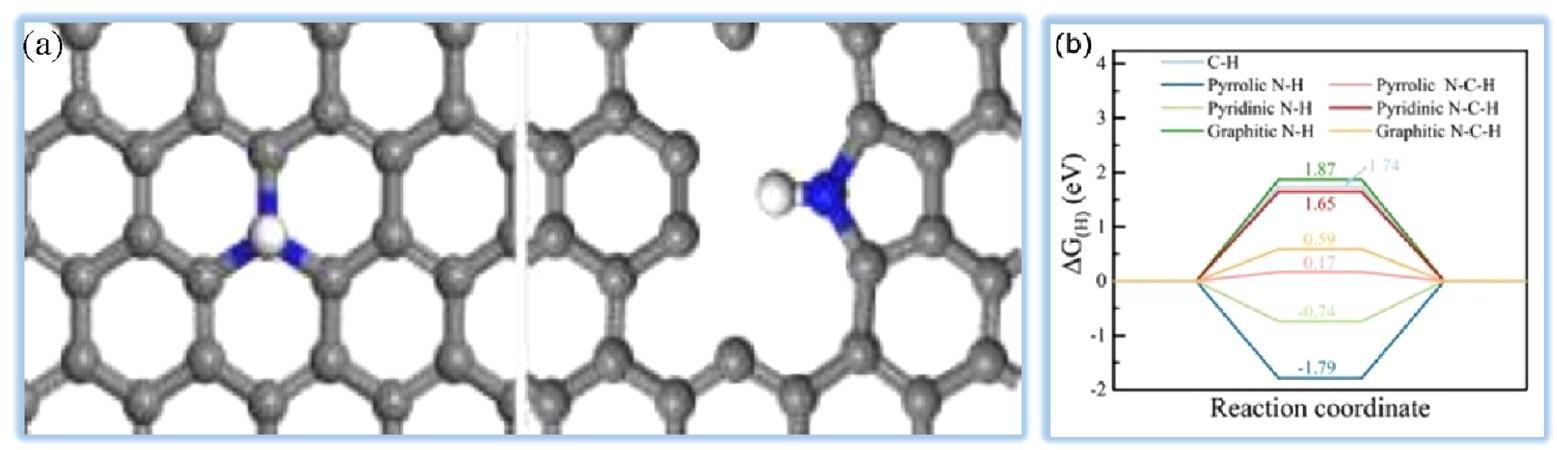
4.3. المحفزات الكهروكيميائية القائمة على الإطارات العضوية المعدنية. إن استخدام الإطارات العضوية المعدنية (MOFs) في العمليات الكهروكيميائية، والضوئية، والكيميائية يجعلها خيارًا قويًا لتحقيق فعالية عالية في تفاعل تقليل الهيدروجين (HER).
تعتبر الجسيمات النانوية وترابط الأنواع السطحية الوظيفية مفيدة في زيادة نشاط المحفزات.
الجرافين. زين وآخرون.
5. الملخص ووجهات النظر
(أ) يمكن أن يكون دمج المعادن الانتقالية في تصميم المحفز الكهربائي مفيدًا. على الرغم من أن هذه التأثيرات المفيدة قابلة للتعديل، إلا أن التأثيرات التآزرية المثلى بين المعادن المختلفة تعزز القدرات الكهروكيميائية.
(ب) تصميم النواة/الصدفة للمحفز هو استراتيجية واقعية وعملية لتعزيز المواقع النشطة لتفاعل الهيدروجين الكهربائي، مع جعل المادة قابلة للتطوير. إنه فعال وناجح بشكل خاص في زيادة المواقع النشطة حول حدود الهياكل ثنائية الأبعاد الطبقية. ومع ذلك، على الرغم من أن تصميم النواة/الصدفة يعزز الأداء التحفيزي، قد تكون البنية العامة غير كافية للتشغيل الصناعي.
(ج) يتطلب دعمًا عالي المستوى من الكربون. لتحقيق أداء كافٍ، حتى مع البلاتين، يجب استخدام دعم كربوني كافٍ (وليس فقط انخفاض الجهد الزائد). ومع ذلك، فإن الهيكل الفيزيائي والكيميائي للكربون مهم بلا شك في فعالية التحفيز الكهربائي.
(د) المواد الكيميائية المعتمدة على الانتقال مثل الكبريتيدات والسيلينيدات والفوسفيدات والكربيدات هي الخيارات الأكثر وعدًا.
(هـ) يمكن أن يكون استخدام المعادن الانتقالية في بنية التحفيز الكهربائي، مشابهًا للتطعيم، مفيدًا.
(ز) تلعب سطح الركيزة دورًا مهمًا، خاصةً بالنسبة للأحادية الكهربائية أو الأفلام الرقيقة جدًا. يُلاحظ هذا التأثير على كل من السطح الأملس وتغليف الجسيمات.
(H) يمكن أن تساعد تشوهات السطح الهندسية وهندسة الإجهاد في تنشيط المواقع المحتملة للتفاعل الكهروكيميائي على الأسطح الأساسية.
(ط) تُظهر المواد المعتمدة على MOF نشاطًا كبيرًا في تفاعل تقليل الهيدروجين (HER) بسبب مساميتها العالية، والمسامية المتحكم بها، والبنية المناسبة. تُستخدم هذه المواد كعوامل تحفيز كهربائية لتفاعل HER لعدة أسباب. أولاً، توفر فرصة لتعزيز واستبدال العوامل التحفيزية القائمة على المعادن الثمينة باهظة الثمن بمعادن أكثر تكلفة. ثانيًا، تساعد في تقليل الجهد الزائد اللازم لتفاعل HER، مما يحسن الكفاءة العامة. أخيرًا، تسهم المواد المعتمدة على MOF في تحسين حركية التفاعل، مما يمكّن من عمليات HER أكثر كفاءة. بشكل عام، تجعل الخصائص الفريدة لـ MOFs منها مرشحين واعدين لتحفيز HER.
على الرغم من التقدم الكبير في فهم العمليات الكهروكيميائية وتصميم الأقطاب المناسبة لتفاعل تقليل الهيدروجين، إلا أن هناك العديد من التحديات أمام الإنتاج الفعال من حيث التكلفة للهيدروجين على نطاق واسع من خلال التحليل الكهربائي للماء. لضمان التقدم الناجح للبحث، من الضروري دمج بروتوكول الاختبار من أجل القدرة على مقارنة المواد المختلفة. بالإضافة إلى ذلك، عند تصميم محفزات كاثودية جديدة في مرحلة البحث، يجب مراعاة سهولة التحضير وإمكانية التوسع للتطبيقات الصناعية. ومع ذلك، من المتوقع أن يؤدي الاهتمام الأخير بنهج مختلف لمصادر الطاقة، وأهمية حماية البيئة في المستقبل، والقضايا الاقتصادية إلى تحقيق تقدم جديد في تصميم محفزات HER نشطة ومستدامة ومنخفضة التكلفة للتسويق الجماعي لإنتاج الهيدروجين القائم على الماء.
معلومات المؤلف
المؤلفون المراسلون
زاري طهراني – معهد أبحاث التصنيع المستقبلي، كلية العلوم والهندسة، جامعة سوانسي، SA1 8EN سوانسي، المملكة المتحدة؛ © orcid.org/0000-0002-5069-7921; البريد الإلكتروني: z.tehrani@swansea.ac.uk
المؤلف
معلومات الاتصال الكاملة متاحة على:
https://pubs.acs.org/10.1021/acsomega.3c07911
ملاحظات
الشكر والتقدير
REFERENCES
(2) Dou, Y.; Wang, A.; Zhao, L.; Yang, X.; Wang, Q.; Sudi, M. S.; Zhu, W.; Shang, D. Boosted hydrogen evolution reaction for a nitrogen-rich azo-bridged metallated porphyrin network. J. Colloid Interface Sci. 2023, 650, 943-950.
(3) Vijayapradeep, S.; Logeshwaran, N.; Ramakrishnan, S.; Kim, A. R.; Sampath, P.; Kim, D. H.; Yoo, D. J. Novel Pt-carbon core-shell decorated hierarchical CoMo2S4 as efficient electrocatalysts for alkaline/seawater hydrogen evolution reaction. Chem. Eng. J. 2023, 473, 145348.
(4) Ramachandran, E.; Krishnaiah, R.; Venkatesan, E. P.; Shaik, S.; Saleel, C. A.; Hussain, F. Investigation into the Ideal Concoction for Performance and Emissions Enhancement of Jatropha Biodiesel-Diesel with CuO Nanoparticles Using Response Surface Methodology. ACS omega 2023, 8 (42), 39067-39079.
(5) Riaz, A.; Qyyum, M. A.; Hussain, A.; Lee, M. Tapping the energy and exergy benefits of channeling liquid air energy system in the hydrogen liquefaction process. Journal of Energy Storage 2023, 72, 108193.
(6) Hoang, A. T.; Pandey, A.; De Osés, F. J. M.; Chen, W.-H.; Said, Z.; Ng, K. H.; Ağbulut, D. C.; Tarełko, W.; Olçer, A. I.; Nguyen, X. P. Technological solutions for boosting hydrogen role in decarbonization strategies and net-zero goals of world shipping: Challenges and perspectives. Renewable Sustainable Energy Rev. 2023, 188, 113790.
(7) Longchamps, R. S.; Yang, X.-G.; Wang, C.-Y. Fundamental Insights into Battery Thermal Management and Safety. ACS Energy Lett. 2022, 7 (3), 1103-1111.
(8) Li, Y.; Zhou, Q.; Weng, S.; Ding, F.; Qi, X.; Lu, J.; Li, Y.; Zhang, X.; Rong, X.; Lu, Y.; et al. Interfacial engineering to achieve an energy density of over 200 Wh kg- 1 in sodium batteries. Nat. Energy 2022, 7 (6), 511-519. Kumar, A.; Bui, V. Q.; Lee, J.; Jadhav, A. R.; Hwang, Y.; Kim, M. G.; Kawazoe, Y.; Lee, H. Modulating interfacial charge density of NiP2-FeP2 via coupling with metallic Cu for accelerating alkaline hydrogen evolution. ACS Energy Lett. 2021, 6 (2), 354-363.
(9) Biggins, F.; Kataria, M.; Roberts, D.; Brown, S. Green hydrogen investments: Investigating the option to wait. Energy 2022, 241, 122842. Fei, B.; Chen, Z.; Liu, J.; Xu, H.; Yan, X.; Qing, H.; Chen, M.;
(10) Thirumal, V.; Yuvakkumar, R.; Saravanakumar, B.; Ravi, G.; Isacfranklin, M.; Shobana, M.; Al-Sehemi, A. G.; Velauthapillai, D. Carbonization and optimization of biomass waste for HER application. Fuel 2022, 324, 124466.
(11) Fakayode, O. A.; Yusuf, B. A.; Zhou, C.; Xu, Y.; Ji, Q.; Xie, J.; Ma, H. Simplistic two-step fabrication of porous carbon-based biomassderived electrocatalyst for efficient hydrogen evolution reaction. Energy Conversion and Management 2021, 227, 113628.
(12) Huner, B.; Demir, N.; Kaya, M. F. Hydrogen Evolution Reaction Performance of Ni-Co-Coated Graphene-Based 3D Printed Electrodes. ACS omega 2023, 8 (6), 5958-5974.
(13) McCrum, I. T.; Koper, M. The role of adsorbed hydroxide in hydrogen evolution reaction kinetics on modified platinum. Nature Energy 2020, 5 (11), 891-899.
(14) Hao, J.; Zhuang, Z.; Cao, K.; Gao, G.; Wang, C.; Lai, F.; Lu, S.; Ma, P.; Dong, W.; Liu, T.; et al. Unraveling the electronegativitydominated intermediate adsorption on high-entropy alloy electrocatalysts. Nat. Commun. 2022, 13 (1), 1-13.
(15) Tang, Z.; Wei, S.; Wang, Y.; Dai, L. Three-dimensional reduced graphene oxide decorated with cobalt metaphosphate as high costefficiency electrocatalysts for the hydrogen evolution reaction. RSC Adv. 2022, 12 (17), 10522-10533.
(16) Wang, T.; Tao, L.; Zhu, X.; Chen, C.; Chen, W.; Du, S.; Zhou, Y.; Zhou, B.; Wang, D.; Xie, C.; et al. Combined anodic and cathodic hydrogen production from aldehyde oxidation and hydrogen evolution reaction. Nat. Catal. 2022, 5 (1), 66-73.
(17) Song, D.; Sun, J.; Sun, L.; Zhai, S.; Ho, G. W.; Wu, H.; Deng, W. Q. Acidic media regulated hierarchical cobalt compounds with phosphorous doping as water splitting electrocatalysts. Adv. Energy Mater. 2021, 11 (22), 2100358.
(18) Voitic, G.; Hacker, V. Recent advancements in chemical looping water splitting for the production of hydrogen. Rsc Advances 2016, 6 (100), 98267-98296.
(19) Hermesmann, M.; Müller, T. Green, Turquoise, Blue, or Grey? Environmentally friendly Hydrogen Production in Transforming Energy Systems. Prog. Energy Combust. Sci. 2022, 90, 100996. Haverkort, J.; Rajaei, H. Voltage losses in zero-gap alkaline water electrolysis. J. Power Sources 2021, 497, 229864.
(20) Li, Y.; Shi, X.; Phoumin, H. A strategic roadmap for large-scale green hydrogen demonstration and commercialisation in China: A review and survey analysis. Int. J. Hydrogen Energy 2022, 47, 2459224609.
(21) Miller, H. A.; Bouzek, K.; Hnat, J.; Loos, S.; Bernäcker, C. I.; Weißgärber, T.; Röntzsch, L.; Meier-Haack, J. Green hydrogen from anion exchange membrane water electrolysis: a review of recent developments in critical materials and operating conditions. Sustainable Energy & Fuels 2020, 4 (5), 2114-2133.
(22) Zhang, P.; Cheng, H.; Gu, F.; Hong, S.; Dong, H.; Li, C. Progress on iron-series metal-organic frameworks materials towards electrocatalytic hydrogen evolution reaction. Surfaces and Interfaces 2023, 42, 103368.
(23) Wang, J.; Gao, Y.; Kong, H.; Kim, J.; Choi, S.; Ciucci, F.; Hao, Y.; Yang, S.; Shao, Z.; Lim, J. Non-precious-metal catalysts for alkaline water electrolysis: operando characterizations, theoretical calculations, and recent advances. Chem. Soc. Rev. 2020, 49 (24), 9154-9196.
(24) Dinh, K. N.; Sun, Y.; Pei, Z.; Yuan, Z.; Suwardi, A.; Huang, Q.; Liao, X.; Wang, Z.; Chen, Y.; Yan, Q. Electronic modulation of nickel disulfide toward efficient water electrolysis. Small 2020, 16 (17), 1905885. Grigoriev, S.; Fateev, V.; Bessarabov, D.; Millet, P. Current status, research trends, and challenges in water electrolysis science and technology. Int. J. Hydrogen Energy 2020, 45 (49), 26036-26058.
(25) Wan, L.; Xu, Z.; Wang, P.; Lin, Y.; Wang, B. H2SO4-doped polybenzimidazole membranes for hydrogen production with acidalkaline amphoteric water electrolysis. J. Membr. Sci. 2021, 618, 118642. Cheikh, J. A.; Zakari, R.; Bhosale, A. C.; Villagra, A.; Leclerc, N.; Floquet, S.; Ghosh, P. C.; Ranjbari, A.; Cadot, E.; Millet, P.; et al. Electrocatalytic properties of {Mo 3 S 4}-based complexes with regard to the hydrogen evolution reaction and application to PEM water electrolysis. Mater. Adv. 2020, 1 (3), 430-440.
(26) Huang, W.-H.; Li, X.-M.; Yang, X.-F.; Zhang, H.-B.; Wang, F.; Zhang, J. Highly efficient electrocatalysts for overall water splitting: mesoporous
(27) Lin, L.; Sherrell, P.; Liu, Y.; Lei, W.; Zhang, S.; Zhang, H.; Wallace, G. G.; Chen, J. Engineered 2D transition metal dichalcoge-nides-a vision of viable hydrogen evolution reaction catalysis. Adv. Energy Mater. 2020, 10 (16), 1903870.
(28) Wang, X.; Zheng, Y.; Sheng, W.; Xu, Z. J.; Jaroniec, M.; Qiao, S.Z . Strategies for design of electrocatalysts for hydrogen evolution under alkaline conditions. Mater. Today 2020, 36, 125-138. Pu, Z.; Amiinu, I. S.; Cheng, R.; Wang, P.; Zhang, C.; Mu, S.; Zhao, W.; Su, F.; Zhang, G.; Liao, S.; et al. Single-atom catalysts for electrochemical hydrogen evolution reaction: recent advances and future perspectives. NanoMicro Lett. 2020, 12 (1), 1-29. Shang, P.; Ye, Z.; Ding, Y.; Zhu, Z.; Peng, X.; Ma, G.; Li, D. Nanosponge-like solid solution of NiMo with a high hydrogen evolution reaction performance over a wide range of current densities. ACS Sustainable Chem. Eng. 2020, 8 (29), 1066410672.
(29) Liu, Y.; Yong, X.; Liu, Z.; Chen, Z.; Kang, Z.; Lu, S. Unified catalyst for efficient and stable hydrogen production by both the electrolysis of water and the hydrolysis of ammonia borane. Adv. Sustainable Syst. 2019, 3 (5), 1800161. dos Santos, K. G.; Eckert, C. T.; De Rossi, E.; Bariccatti, R. A.; Frigo, E. P.; Lindino, C. A.; Alves, H. J.
(30) Wang, Y.; Sun, D.; Wang, M.; Feng, Z.; Hall, A. S. Oxygen Reduction Electrocatalysis on Ordered Intermetallic Pd-Bi Electrodes Is Enhanced by a Low Coverage of Spectator Species. J. Phys. Chem. C 2020, 124 (9), 5220-5224. Wang, J.; Qiu, T.; Chen, X.; Lu, Y.; Yang, W. N-doped carbon@ Ni-Al2O3 nanosheet array@ graphene oxide composite as an electrocatalyst for hydrogen evolution reaction in alkaline medium. J. Power Sources 2015, 293, 178-186. Schmidt, T. J.; Stamenkovic, V.; Ross, P. N., Jr.; Markovic, N. M. Temperature dependent surface electrochemistry on Pt single crystals in alkaline electrolyte Part 3. The oxygen reduction reaction. Phys. Chem. Chem. Phys. 2003, 5 (2), 400-406.
(31) Cai, J.; Ding, J.; Wei, D.; Xie, X.; Li, B.; Lu, S.; Zhang, J.; Liu, Y.; Cai, Q.; Zang, S. Coupling of Ru and O-Vacancy on 2D Mo-Based Electrocatalyst Via a Solid-Phase Interface Reaction Strategy for Hydrogen Evolution Reaction. Adv. Energy Mater. 2021, 11 (26), 2100141. Kou, T.; Chen, M.; Wu, F.; Smart, T. J.; Wang, S.; Wu, Y.; Zhang, Y.; Li, S.; Lall, S.; Zhang, Z. J. N. c.; et al. Carbon doping switching on the hydrogen adsorption activity of NiO for hydrogen evolution reaction. Nat. Commun. 2020, 11 (1), 1-10. Li, W.; Liu, G.; Li, J.; Wang, Y.; Ricardez-Sandoval, L.; Zhang, Y.; Zhang, Z. J. A. S. S. Hydrogen evolution reaction mechanism on 2H-MoS2 electrocatalyst. Appl. Surf. Sci. 2019, 498, 143869. Liu, Z.; Yang, X.; Hu, G.; Feng, L. Ru nanoclusters coupled on
(32) Yu, X.; Xu, S.; Wang, Z.; Cheng, X.; Du, Y.; Chen, G.; Sun, X.; Wu, Q. An Mn -doped NiCoP flower-like structure as a highly efficient electrocatalyst for hydrogen evolution reaction in acidic and alkaline solutions with long duration. Nanoscale 2021, 13 (25), 11069-11076.
(33) He, H.-z.; Zhang, Y.; Li, Y.; Wang, P. Recent innovations of silkderived electrocatalysts for hydrogen evolution reaction, oxygen evolution reaction and oxygen reduction reaction. Int. J. Hydrogen Energy 2021, 46 (11), 7848-7865.
(34) Wen, Q.; Zhao, Y.; Liu, Y.; Li, H.; Zhai, T. Ultrahigh-CurrentDensity and Long-Term-Durability Electrocatalysts for Water Splitting. Small 2022, 18 (4), 2104513. Hao, J.; Wei, F.; Zhang, X.; Li, L.; Zhang, C.; Liang, D.; Ma, X.; Lu, P. Defect and doping engineered pentagraphene for catalysis of hydrogen evolution reaction. Nanoscale Res. Lett. 2021, 16 (1), 1-9.
(35) Wang, S.; Xu, B.; Huo, W.; Feng, H.; Zhou, X.; Fang, F.; Xie, Z.; Shang, J. K.; Jiang, J. Efficient FeCoNiCuPd thin-film electrocatalyst for alkaline oxygen and hydrogen evolution reactions. Appl. Catal. B: Environmental 2022, 313, 121472.
(36) Tymoczko, J.; Calle-Vallejo, F.; Schuhmann, W.; Bandarenka, A. S. Making the hydrogen evolution reaction in polymer electrolyte membrane electrolysers even faster. Nat. Commun. 2016, 7 (1), 1-6. Lačnjevac, U.; Vasilić, R.; Dobrota, A.; Đurđić, S.; Tomanec, O.; Zbořil, R.; Mohajernia, S.; Nguyen, N. T.; Skorodumova, N.; Manojlović, D.; et al. High-performance hydrogen evolution electrocatalysis using proton-intercalated TiO 2 nanotube arrays as interactive supports for Ir nanoparticles. J. Mater. Chem. A 2020, 8 (43), 22773-22790. Zhang, W.; Huang, B.; Wang, K.; Yang, W.; Lv, F.; Li, N.; Chao, Y.; Zhou, P.; Yang, Y.; Li, Y.; et al. WOx-Surface Decorated PtNi@ Pt Dendritic Nanowires as Efficient pH-Universal Hydrogen Evolution Electrocatalysts. Adv. Energy Mater. 2021, 11 (3), 2003192.
(37) Wendt, H.; Spinacé, E. V.; Oliveira Neto, A.; Linardi, M. J. Q. N. Electrocatalysis and electrocatalysts for low temperature fuel cells: fundamentals, state of the art, research and development. Quimica Nova 2005, 28 (6), 1066-1075. Koper, M. T. Thermodynamic theory of multi-electron transfer reactions: Implications for electrocatalysis.
(38) Sheng, W.; Myint, M.; Chen, J. G.; Yan, Y. J. E. Correlating the hydrogen evolution reaction activity in alkaline electrolytes with the
hydrogen binding energy on monometallic surfaces. Energy Environ. Sci. 2013, 6 (5), 1509-1512.
(39) Zheng, Y.; Jiao, Y.; Vasileff, A.; Qiao, S. Z. The hydrogen evolution reaction in alkaline solution: from theory, single crystal models, to practical electrocatalysts. Angew. Chem., Int. Ed. 2018, 57 (26), 7568-7579.
(40) Kim, J.; Jung, H.; Jung, S.-M.; Hwang, J.; Kim, D. Y.; Lee, N.; Kim, K.-S.; Kwon, H.; Kim, Y.-T.; Han, J. W.; et al. Tailoring binding abilities by incorporating oxophilic transition metals on 3D nanostructured Ni arrays for accelerated alkaline hydrogen evolution reaction. J. Am. Chem. Soc. 2021, 143 (3), 1399-1408. Ugwu, L. I.; Morgan, Y.; Ibrahim, H. Application of density functional theory and machine learning in heterogenous-based catalytic reactions for hydrogen production. Int. J. Hydrogen Energy 2022, 47 (4), 2245-2267.
(41) Ibn Shamsah, S. M. Earth-abundant electrocatalysts for water splitting: current and future directions. Catalysts 2021, 11 (4), 429.
(42) Krishtalik, L. I. Kinetic isotope effect in the hydrogen evolution reaction. Electrochimica acta 2001, 46 (19), 2949-2960. Qi, Q.; Shao, D.; Zhou, Y.; Wang, Q.; Yu, X.-Y. Plasma-induced implanting of active species in metal-organic frameworks for efficient hydrogen evolution reaction. Journal of Materials Chemistry A 2023, 11 (29), 15663-15669.
(43) Wu, H.; Feng, C.; Zhang, L.; Zhang, J.; Wilkinson, D. P. Nonnoble metal electrocatalysts for the hydrogen evolution reaction in water electrolysis. Electrochemical Energy Reviews 2021, 4(3), 473-507.
(44) Zhou, F.; Zhou, Y.; Liu, G.-G.; Wang, C.-T.; Wang, J. Recent advances in nanostructured electrocatalysts for hydrogen evolution reaction. Rare Metals 2021, 40 (12), 3375-3405. Chen, L.-W.; Liang, H.-W. Ir-based bifunctional electrocatalysts for overall water splitting. Catal. Sci. Technol. 2021, 11, 4673-4689.
(45) Zhang, X.-Y.; Xie, J.-Y.; Ma, Y.; Dong, B.; Liu, C.-G.; Chai, Y.-M. An overview of the active sites in transition metal electrocatalysts and their practical activity for hydrogen evolution reaction. Chem. Eng. J. 2022, 430, 132312.
(46) Theerthagiri, J.; Lee, S. J.; Murthy, A. P.; Madhavan, J.; Choi, M. Y. Fundamental aspects and recent advances in transition metal nitrides as electrocatalysts for hydrogen evolution reaction: A review. Curr. Opin. Solid State Mater. Sci. 2020, 24 (1), 100805.
(47) Ge, Z.; Fu, B.; Zhao, J.; Li, X.; Ma, B.; Chen, Y. A review of the electrocatalysts on hydrogen evolution reaction with an emphasis on Fe, Co and Ni-based phosphides. J. Mater. Sci. 2020, 55 (29), 1408114104.
(48) Anantharaj, S.; Kundu, S.; Noda, S. Progress in nickel chalcogenide electrocatalyzed hydrogen evolution reaction. Journal of Materials Chemistry A 2020, 8 (8), 4174-4192.
(49) Li, T.; Hu, T.; Dai, L.; Li, C. M. Metal-free photo-and electrocatalysts for hydrogen evolution reaction. Journal of Materials Chemistry A 2020, 8 (45), 23674-23698.
(50) Luo, W.; Wang, Y.; Cheng, C. Ru-based electrocatalysts for hydrogen evolution reaction: recent research advances and perspectives. Materials Today Physics 2020, 15, 100274. Ogundipe, T. O.; Shen, L.; Lu, Z.; Yan, C.; Yan, C. Recent Advances on Bimetallic Transition Metal Phosphides for Enhanced Hydrogen Evolution Reaction. ChemistrySelect 2022, 7 (23), No. e202200291.
(51) Chen, Q.; Yu, Y.; Li, J.; Nan, H.; Luo, S.; Jia, C.; Deng, P.; Zhong, S.; Tian, X. Recent Progress in Layered Double Hydroxide-Based Electrocatalyst for Hydrogen Evolution Reaction. ChemElectroChem. 2022, 9 (9), No. e202101387. Zhou, D.; Li, P.; Lin, X.; McKinley, A.; Kuang, Y.; Liu, W.; Lin, W.-F.; Sun, X.; Duan, X. Layered double hydroxide-based electrocatalysts for the oxygen evolution reaction: identification and tailoring of active sites, and superaerophobic nanoarray electrode assembly. Chem. Soc. Rev. 2021, 50 (15), 87908817.
(52) Chen, J.; Cheng, H.; Ding, L.-X.; Wang, H. Competing hydrogen evolution reaction: a challenge in electrocatalytic nitrogen fixation. Materials Chemistry Frontiers 2021, 5 (16), 5954-5969. Zhu, J.; Yang, R.; Zhang, G. Atomically thin transition metal dichalcogenides for the hydrogen evolution reaction. ChemPhysMater. 2022, 1, 102.
(53) Hansen, J. N.; Prats, H.; Toudahl, K. K.; Mørch Secher, N.; Chan, K.; Kibsgaard, J.; Chorkendorff, I. Is there anything better than Pt for
(54) Aggarwal, P.; Sarkar, D.; Awasthi, K.; Menezes, P. W. Functional role of single-atom catalysts in electrocatalytic hydrogen evolution: Current developments and future challenges. Coord. Chem. Rev. 2022, 452, 214289.
(55) Zahra, R.; Pervaiz, E.; Yang, M.; Rabi, O.; Saleem, Z.; Ali, M.; Farrukh, S. A review on nickel cobalt sulphide and their hybrids: Earth abundant, pH stable electro-catalyst for hydrogen evolution reaction. Int. J. Hydrogen Energy 2020, 45 (46), 24518-24543.
(56) Yang, W.; Chen, S. Recent progress in electrode fabrication for electrocatalytic hydrogen evolution reaction: A mini review. Chem. Eng. J. 2020, 393, 124726.
(57) Chen, S.; Pan, Y. Influence of group III and IV elements on the hydrogen evolution reaction of MoS2 disulfide. J. Phys. Chem. C 2021, 125 (22), 11848-11856.
(58) Feng, W.; Pang, W.; Xu, Y.; Guo, A.; Gao, X.; Qiu, X.; Chen, W. Transition metal selenides for electrocatalytic hydrogen evolution reaction. ChemElectroChem. 2020, 7(1), 31-54.
(59) Drosou, M.; Kamatsos, F.; Mitsopoulou, C. A. Recent advances in the mechanisms of the hydrogen evolution reaction by non-innocent sulfur-coordinating metal complexes. Inorganic Chemistry Frontiers 2020, 7 (1), 37-71.
(60) Liu, Y.; Wang, Q.; Zhang, J.; Ding, J.; Cheng, Y.; Wang, T.; Li, J.; Hu, F.; Yang, H. B.; Liu, B. Recent Advances in Carbon-Supported Noble-Metal Electrocatalysts for Hydrogen Evolution Reaction: Syntheses, Structures, and Properties. Adv. Energy Mater. 2022, 12, 2200928.
(61) Zheng, X.; Peng, L.; Li, L.; Yang, N.; Yang, Y.; Li, J.; Wang, J.; Wei, Z. J. C. s. Role of non-metallic atoms in enhancing the catalytic activity of nickel-based compounds for hydrogen evolution reaction. Chemical science 2018, 9 (7), 1822-1830.
(62) Kozhushner, A.; Zion, N.; Elbaz, L. J. C. O. i. E. Methods for assessment and measurement of the active site density in platinum group metal-free oxygen reduction reaction catalysts. Current Opinion in Electrochemistry 2021, 25, 100620. Carmo, M.; Fritz, D. L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38 (12), 4901-4934. Safizadeh, F.; Ghali, E.; Houlachi, G. Electrocatalysis developments for hydrogen evolution reaction in alkaline solutions-A review. Int. J. Hydrogen Energy 2015, 40 (1), 256-274.
(63) Markovića, N. M.; Sarraf, S. T.; Gasteiger, H. A.; Ross, P. N. Faraday Transactions. Hydrogen electrochemistry on platinum lowindex single-crystal surfaces in alkaline solution. J. Chem. Soc., Faraday Trans. 1996, 92 (20), 3719-3725. Gottesfeld, S. Fuel cell science: Theory, fundamentals, and biocatalysis; John Wiley & Sons: New York, 2011. Kobayashi, S.; Tryk, D.; Uchida, H. J. E. C. Enhancement of hydrogen evolution activity on Pt-skin/Pt3Co [(111),(100), and (110)] single crystal electrodes. Electrochem. Commun. 2020, 110, 106615.
(64) Lee, W.-J.; Wan, Z.; Kim, C.-M.; Oh, I.-K.; Harada, R.; Suzuki, K.; Choi, E.-A.; Kwon, S.-H. J. C. o. M. Atomic layer deposition of Pt thin films using dimethyl (
(65) Fu, H. Q.; Zhou, M.; Liu, P. F.; Liu, P.; Yin, H.; Sun, K. Z.; Yang, H. G.; Al-Mamun, M.; Hu, P.; Wang, H.-F.; et al. Hydrogen spilloverbridged Volmer/Tafel processes enabling ampere-level current density alkaline hydrogen evolution reaction under low overpotential. J. Am. Chem. Soc. 2022, 144 (13), 6028-6039. Lao, M.; Li, P.; Jiang, Y.; Pan, H.; Dou, S. X.; Sun, W. From Fundamentals and Theories to Heterostructured Electrocatalyst Design: An In-depth Understanding of Alkaline Hydrogen Evolution Reaction. Nano Energy 2022, 98, 107231.
(66) Zhai, L.; She, X.; Zhuang, L.; Li, Y.; Ding, R.; Guo, X.; Zhang, Y.; Zhu, Y.; Xu, K.; Fan, H. J.; et al. Modulating Built-In Electric Field via Variable Oxygen Affinity for Robust Hydrogen Evolution Reaction in Neutral Media. Angew. Chem., Int. Ed. 2022, 61 (14), No. e202116057. Chen, Y.; Ding, R.; Li, J.; Liu, J. Highly active atomically dispersed platinum-based electrocatalyst for hydrogen evolution reaction achieved by defect anchoring strategy. Appl. Catal. B: Environmental 2022, 301, 120830.
(67) Ghamami, S.; Kazemi Korayem, A.; Baqeri, N. Production and purification of titanium dioxide with titanium tetrachloride nanoparticles from eliminate concentrate of Kahnooj mine in Kerman. J. Appl. Chem. 2020, 15 (55), 189-206. Ren, B.; Jin, Q.; Li, Y.; Li, Y.; Cui, H.; Wang, C. Activating titanium dioxide as a new efficient electrocatalyst: from theory to experiment. ACS Appl. Mater. Interfaces 2020, 12 (10), 11607-11615.
(68) Kweon, D. H.; Okyay, M. S.; Kim, S.-J.; Jeon, J.-P.; Noh, H.-J.; Park, N.; Mahmood, J.; Baek, J.-B. Ruthenium anchored on carbon nanotube electrocatalyst for hydrogen production with enhanced Faradaic efficiency. Nat. Commun. 2020, 11 (1), 1-10.
(69) Bao, X.; Chen, Y.; Mao, S.; Wang, Y.; Yang, Y.; Gong, Y. Boosting the performance gain of
(70) Malik, B.; Anantharaj, S.; Karthick, K.; Pattanayak, D. K.; Kundu, S. J. C. S. Technology. Magnetic CoPt nanoparticle-decorated ultrathin
(71) Scofield, M. E.; Zhou, Y.; Yue, S.; Wang, L.; Su, D.; Tong, X.; Vukmirovic, M. B.; Adzic, R. R.; Wong, S. S. Role of chemical composition in the enhanced catalytic activity of Pt-based alloyed ultrathin nanowires for the hydrogen oxidation reaction under alkaline conditions. ACS Catal. 2016, 6 (6), 3895-3908.
(72) Santos, D.; Sequeira, C.; Macciò, D.; Saccone, A.; Figueiredo, J. Platinum-rare earth electrodes for hydrogen evolution in alkaline water electrolysis. International journal of hydrogen energy 2013, 38 (8), 31373145. Stojić, D. L.; Grozdić, T. D.; Kaninski, M. M.; Maksić, A. D.; Simić, N. D. Intermetallics as advanced cathode materials in hydrogen production via electrolysis. International journal of hydrogen energy 2006, 31 (7), 841-846.
(73) Naveen, M. H.; Huang, Y.; Bisalere Kantharajappa, S.; Seo, K.-D.; Park, D.-S.; Shim, Y.-B. Enhanced electrocatalytic activities of in situ produced
(74) Zhang, L.; Xiao, W.; Zhang, Y.; Han, F.; Yang, X. Nanocarbon encapsulating Ni-doped MoP/graphene composites for highly improved electrocatalytic hydrogen evolution reaction. Compos. Соттии. 2021, 26, 100792.
(75) Pu, M.; Wang, D.; Zhang, Z.; Guo, Y.; Guo, W. Flexoelectricity enhanced water splitting and hydrogen evolution reaction on grain boundaries of monolayer transition metal dichalcogenides. Nano Research 2022, 15 (2), 978-984.
(76) Lai, J.; Huang, B.; Chao, Y.; Chen, X.; Guo, S. J. A. M. Strongly Coupled Nickel-Cobalt Nitrides/Carbon Hybrid Nanocages with PtLike Activity for Hydrogen Evolution Catalysis. Adv. Mater. 2019, 31 (2), 1805541.
(77) Kuttiyiel, K. A.; Sasaki, K.; Chen, W.-F.; Su, D.; Adzic, R. R. Coreshell, hollow-structured iridium-nickel nitride nanoparticles for the hydrogen evolution reaction. J. Mater. Chem. A 2014, 2 (3), 591-594.
(78) Zhang, L.; Wang, T.; Sun, L.; Sun, Y.; Hu, T.; Xu, K.; Ma, F. Hydrothermal synthesis of 3D hierarchical MoSe 2/NiSe 2 composite nanowires on carbon fiber paper and their enhanced electrocatalytic
activity for the hydrogen evolution reaction. J. Mater. Chem. A 2017, 5 (37), 19752-19759.
(79) Wang, X.; Zheng, Y.; Yuan, J.; Shen, J.; Hu, J.; Wang, A.-j.; Wu, L.; Niu, L. J. E. A. Porous NiCo diselenide nanosheets arrayed on carbon cloth as promising advanced catalysts used in water splitting. Electrochim. Acta 2017, 225, 503-513.
(80) Chen, Q.; Wang, R.; Yu, M.; Zeng, Y.; Lu, F.; Kuang, X.; Lu, X. J. E. A. Bifunctional iron-nickel nitride nanoparticles as flexible and robust electrode for overall water splitting. Electrochim. Acta 2017, 247, 666— 673.
(81) Chen, Y.; Zhang, J.; Guo, P.; Liu, H.; Wang, Z.; Liu, M.; Zhang, T.; Wang, S.; Zhou, Y.; Lu, X. J. A. a. m.; et al. interfaces. Coupled heterostructure of
(82) Zhang, N.; Cao, L.; Feng, L.; Huang, J.; Kajiyoshi, K.; Li, C.; Liu, Q.; Yang, D.; He, J. J. N. Co, N-Codoped porous vanadium nitride nanoplates as superior bifunctional electrocatalysts for hydrogen evolution and oxygen reduction reactions. Nanoscale 2019, 11 (24), 11542-11549.
(83) Read, C. G.; Callejas, J. F.; Holder, C. F.; Schaak, R. E. J. A. a. m. interfaces. General strategy for the synthesis of transition metal phosphide films for electrocatalytic hydrogen and oxygen evolution. ACS applied materials 2016, 8 (20), 12798-12803.
(84) Pan, Y.; Lin, Y.; Chen, Y.; Liu, Y.; Liu, C. Cobalt phosphide-based electrocatalysts: synthesis and phase catalytic activity comparison for hydrogen evolution. J. Mater. Chem. A 2016, 4 (13), 4745-4754.
(85) Wang, M.-Q.; Tang, C.; Ye, C.; Duan, J.; Li, C.; Chen, Y.; Bao, S.J.; Xu, M. J. J. o. M. C. A. Engineering the nanostructure of molybdenum nitride nanodot embedded N -doped porous hollow carbon nanochains for rapid all pH hydrogen evolution. J. Mater. Chem. A 2018, 6 (30), 14734-14741.
(86) Callejas, J. F.; McEnaney, J. M.; Read, C. G.; Crompton, J. C.; Biacchi, A. J.; Popczun, E. J.; Gordon, T. R.; Lewis, N. S.; Schaak, R. E. J. A. n. Electrocatalytic and photocatalytic hydrogen production from acidic and neutral-pH aqueous solutions using iron phosphide nanoparticles. ACS Nano 2014, 8 (11), 11101-11107.
(87) Liu, Y.; Kelly, T. G.; Chen, J. G.; Mustain, W. E. J. A. C. Metal carbides as alternative electrocatalyst supports. ACS Catal. 2013, 3 (6), 1184-1194.
(88) Ramesh, R.; Nandi, D. K.; Kim, T. H.; Cheon, T.; Oh, J.; Kim, S.H. J. A. a. m. xsinterfaces. Atomic-layer-deposited MoN x thin films on three-dimensional Ni foam as efficient catalysts for the electrochemical hydrogen evolution reaction. ACS applied materials 2019, 11 (19), 17321-17332.
(89) Zhou, Y.; Yang, Y.; Wang, R.; Wang, X.; Zhang, X.; Qiang, L.; Wang, W.; Wang, Q.; Hu, Z. Rhombic porous CoP 2 nanowire arrays synthesized by alkaline etching as highly active hydrogen-evolutionreaction electrocatalysts. J. Mater. Chem. A 2018, 6 (39), 19038-19046.
(90) Jin, H.; Liu, X.; Vasileff, A.; Jiao, Y.; Zhao, Y.; Zheng, Y.; Qiao, S.Z. J. A. N. Single-crystal nitrogen-rich two-dimensional Mo5N6 nanosheets for efficient and stable seawater splitting. ACS Nano 2018, 12 (12), 12761-12769.
(91) Yu, M.; Zhao, S.; Feng, H.; Hu, L.; Zhang, X.; Zeng, Y.; Tong, Y.; Lu, X. J. A. E. L. Engineering thin MoS2 nanosheets on TiN nanorods: advanced electrochemical capacitor electrode and hydrogen evolution electrocatalyst. ACS Energy Lett. 2017, 2 (8), 1862-1868.
(92) Chen, T.; Tan, Y. J. N. R. Hierarchical CoNiSe2 nanoarchitecture as a high-performance electrocatalyst for water splitting. Nano Research 2018, 11 (3), 1331-1344.
(93) Jiao, C.; Bo, X.; Zhou, M. J. J. o. E. C. Electrocatalytic water splitting at nitrogen-doped carbon layers-encapsulated nickel cobalt selenide. J. Energy Chem. 2019, 34, 161-170.
(94) Liang, C.; Ying, P.; Li, C. J. C. o. m. Nanostructured
(95) Shi, Z.; Wang, Y.; Lin, H.; Zhang, H.; Shen, M.; Xie, S.; Zhang, Y.; Gao, Q.; Tang, Y. Porous nanoMoC@ graphite shell derived from a
(96) Cao, B.; Veith, G. M.; Neuefeind, J. C.; Adzic, R. R.; Khalifah, P. G . Mixed close-packed cobalt molybdenum nitrides as non-noble metal electrocatalysts for the hydrogen evolution reaction. J. Am. Chem. Soc. 2013, 135 (51), 19186-19192.
(97) Chen, W. F.; Schneider, J. M.; Sasaki, K.; Wang, C. H.; Schneider, J.; Iyer, S.; Iyer, S.; Zhu, Y.; Muckerman, J. T.; Fujita, E. J. C. Tungsten carbide-nitride on graphene nanoplatelets as a durable hydrogen evolution electrocatalyst. ChemSusChem 2014, 7 (9), 2414-2418.
(98) Fang, Z.; Peng, L.; Qian, Y.; Zhang, X.; Xie, Y.; Cha, J. J.; Yu, G. Dual tuning of Ni-Co-A (
(99) Wang, J.; Yang, W.; Liu, J. CoP 2 nanoparticles on reduced graphene oxide sheets as a super-efficient bifunctional electrocatalyst for full water splitting. J. Mater. Chem. A 2016, 4 (13), 4686-4690.
(100) Majhi, K. C.; Yadav, M. Fuels. Sphere-Shaped Bimetallic Sulphoselenide: An Efficient Electrocatalyst for Hydrogen Evolution Reaction. Energy 2021, 35 (15), 12473-12481. Li, D.; Liao, L.; Zhou, H.; Zhao, Y.; Cai, F.; Zeng, J.; Liu, F.; Wu, H.; Tang, D.; Yu, F. J. M. T. P. Highly active non-noble electrocatalyst from
(101) Lin, F.; Dong, Z.; Yao, Y.; Yang, L.; Fang, F.; Jiao, L. Electrocatalytic hydrogen evolution of ultrathin Co-Mo5N6 heterojunction with interfacial electron redistribution. Adv. Energy Mater. 2020, 10 (42), 2002176.
(102) Zhang, F.; Shan, W.; Hu, Q.; Jiang, W.; Li, D.; Zhang, B. The Effect of Mo Addition on Electrocatalytic Activity and Stability of Fe-Co-PC Metallic Glasses for Hydrogen Evolution. J. Electrochem. Soc. 2021, 168 (7), 076510. Pentland, N.; Bockris, J. M.; Sheldon, E. Hydrogen evolution reaction on copper, gold, molybdenum, palladium, rhodium, and iron: mechanism and measurement technique under high purity conditions. J. Electrochem. Soc. 1957, 104 (3), 182.
(103) Hu, X.; Tian, X.; Lin, Y.-W.; Wang, Z. J. R. a. Nickel foam and stainless steel mesh as electrocatalysts for hydrogen evolution reaction, oxygen evolution reaction and overall water splitting in alkaline media. RSC Adv. 2019, 9 (54), 31563-31571. Lin, Y.; Sun, K.; Chen, X.; Chen, C.; Pan, Y.; Li, X.; Zhang, J.. High-precision regulation synthesis of Fedoped Co 2 P nanorod bundles as efficient electrocatalysts for hydrogen evolution in all-pH range and seawater. J. Energy Chem. 2021, 55, 92101.
(104) Mahmood, N.; Yao, Y.; Zhang, J. W.; Pan, L.; Zhang, X.; Zou, J. J. Electrocatalysts for hydrogen evolution in alkaline electrolytes: mechanisms, challenges, and prospective solutions. Adv. Sci. 2018, 5 (2), 1700464.
(105) Ali Shah, S.; Xu, L.; Sayyar, R.; Bian, T.; Liu, Z.; Yuan, A.; Shen, X.; Khan, I.; Ali Tahir, A.; Ullah, H. J. C. E. J. Growth of MoS2 nanosheets on
(106) Müller, C. I.; Rauscher, T.; Schmidt, A.; Schubert, T.; Weißgärber, T.; Kieback, B.; Röntzsch, L. Electrochemical investigations on amorphous Fe-base alloys for alkaline water electrolysis. Int. J. Hydrogen Energy 2014, 39 (17), 8926-8937. Schäfer, H.; Chatenet, M. J. A. E. L. Steel: the resurrection of a forgotten watersplitting catalyst. ACS Energy Lett. 2018, 3 (3), 574-591.
(107) Ekspong, J.; Wågberg, T. J. M. Stainless steel as a bi-functional electrocatalyst-A top-down approach. Materials 2019, 12 (13), 2128. Benavente Llorente, V.; Diaz, L. A.; Lacconi, G. I.; Abuin, G. C.;
(108) Kim, M.; Ha, J.; Shin, N.; Kim, Y.-T.; Choi, J. J. E. A. Selfactivated anodic nanoporous stainless steel electrocatalysts with high durability for the hydrogen evolution reaction. Electrochim. Acta 2020, 364, 137315.
(109) Liu, X.; You, B.; Sun, Y. J. A. S. C. Engineering. Facile surface modification of ubiquitous stainless steel led to competent electrocatalysts for overall water splitting. ACS Sustainable Chemistry 2017, 5 (6), 4778-4784. Zayat, B.; Mitra, D.; Narayanan, S. R. Inexpensive and efficient alkaline water electrolyzer with robust steel-based electrodes. J. Electrochem. Soc. 2020, 167 (11), 114513. Gao, Y.; Xiong, T.; Li, Y.; Huang, Y.; Li, Y.; Balogun, M.-S. J. T. A simple and scalable approach to remarkably boost the overall water splitting activity of stainless steel electrocatalysts. ACS Omega 2019, 4 (14), 16130-16138.
(110) Yang, C.; Zhao, R.; Xiang, H.; Wu, J.; Zhong, W.; Li, W.; Zhang, Q.; Yang, N.; Li, X. Ni-activated transition metal carbides for efficient hydrogen evolution in acidic and alkaline solutions. Adv. Energy Mater. 2020, 10 (37), 2002260. Jun, H.; Kim, S.; Lee, J. Development strategies in transition metal carbide for hydrogen evolution reaction: A review. Korean Journal of Chemical Engineering 2020, 37 (8), 13171330. Peng, O.; Hu, Q.; Zhou, X.; Zhang, R.; Du, Y.; Li, M.; Ma, L.; Xi, S.; Fu, W.; Xu, Z.-X.; et al. Swinging hydrogen evolution to nitrate reduction activity in molybdenum carbide by ruthenium doping. ACS Catal. 2022, 12 (24), 15045-15055.
(111) Chen, P.; Ye, J.; Wang, H.; Ouyang, L.; Zhu, M. Recent progress of transition metal carbides/nitrides for electrocatalytic water splitting. J. Alloys Compd. 2021, 883, 160833. He, C.; Tao, J. Transition metal carbides coupled with nitrogen-doped carbon as efficient and stable
(112) Zhang, X.; Zhu, Z.; Liang, X.; Ma, F.-X.; Zhang, J.; Tan, Y.; Pan, Z.; Bo, Y.; Wu, C.-M. L. Encapsulating dual-phased Mo2C-WC nanocrystals into ultrathin carbon nanosheet assemblies for efficient electrocatalytic hydrogen evolution. Chem. Eng. J. 2021, 408, 127270. Yan, P.; Wu, Y.; Wei, X.; Zhu, X.; Su, W. Preparation of robust hydrogen evolution reaction electrocatalyst WC/C by molten salt. Nanomaterials 2020, 10 (9), 1621. Hu, Z.; Chen, J.; Pan, P.; Liu, C.; Zeng, J.; Ou, Y.; Qi, X.; Liang, T. Porous N-doped Mo2C@ C nanoparticles for highperformance hydrogen evolution reaction. Int. J. Hydrogen Energy 2022, 47 (7), 4641-4652.
(113) Lv, Z.; Liu, D.; Tian, W.; Dang, J. Designed synthesis of WCbased nanocomposites as low-cost, efficient and stable electrocatalysts for the hydrogen evolution reaction. CrystEngComm 2020, 22 (27), 4580-4590.
(114) Wang, F.; Wu, Y.; Dong, B.; Lv, K.; Shi, Y.; Ke, N.; Hao, L.; Yin, L.; Bai, Y.; Xu, X.; et al. Robust Porous WC-Based Self-Supported Ceramic Electrodes for High Current Density Hydrogen Evolution Reaction. Adv. Sci. 2022, 9, 2106029 . Huang, J.; Jian, C.; Cai, Q.; Hong, W.; Liu, W. A large scale self-supported WP-W 2 C nanoporous network for efficient hydrogen evolution reaction in alkaline media.
(115) Morishita, M.; Nozaki, A.; Yamamoto, H.; Fukumuro, N.; Mori, M.; Araki, K.; Sakamoto, F.; Nakamura, A.; Yanagita, H. Catalytic activity of Co-nanocrystal-doped tungsten carbide arising from an internal magnetic field. RSC Adv. 2021, 11 (23), 14063-14070. Tian, D.; Denny, S. R.; Li, K.; Wang, H.; Kattel, S.; Chen, J. G. Density functional theory studies of transition metal carbides and nitrides as electrocatalysts. Chem. Soc. Rev. 2021, 50, 12338.
(116) Ma, L.; Ting, L. R. L.; Molinari, V.; Giordano, C.; Yeo, B. S. Efficient hydrogen evolution reaction catalyzed by molybdenum carbide and molybdenum nitride nanocatalysts synthesized via the
urea glass route. Journal of Materials Chemistry A 2015, 3 (16), 83618368.
(117) Liu, W.; Wang, X.; Wang, F.; Du, K.; Zhang, Z.; Guo, Y.; Yin, H.; Wang, D. A durable and pH -universal self-standing MoC-Mo2C heterojunction electrode for efficient hydrogen evolution reaction. Nat. Commun. 2021, 12 (1), 1-10.
(118) Ji, X.; Wang, K.; Zhang, Y.; Sun, H.; Zhang, Y.; Ma, T.; Ma, Z.; Hu, P.; Qiu, Y. MoC based Mott-Schottky electrocatalyst for boosting the hydrogen evolution reaction performance. Sustainable Energy & Fuels 2020, 4 (1), 407-416.
(119) Hu, W.; Zheng, M.; Xu, B.; Wei, Y.; Zhu, W.; Li, Q.; Pang, H. Design of hollow carbon-based materials derived from metal-organic frameworks for electrocatalysis and electrochemical energy storage. Journal of Materials Chemistry A 2021, 9 (7), 3880-3917.
(120) Chen, Y.; Li, T.; Zhao, Q.; Liu, D.; Li, C. M. The in situ preparation of iron phosphide using ionic liquids as iron and phosphorus sources for efficient hydrogen evolution reactions. RSC Adv. 2020, 10 (55), 33026-33032. Zhou, Q.; Wang, D. 3D nanoporous NiCoP as a highly efficient electrocatalyst for the hydrogen evolution reaction in alkaline electrolyte. New J. Chem. 2022, 46 (16), 74907496.
(121) Kumari, A.; Kaushal, S.; Singh, P. P. Bimetallic metal organic frameworks heterogeneous catalysts: Design, construction, and applications. Materials Today Energy 2021, 20, 100667. Sirisomboonchai, S.; Kitiphatpiboon, N.; Chen, M.; Li, S.; Li, X.; Kongparakul, S.; Samart, C.; Zhang, L.; Abudula, A.; Guan, G. MultiHierarchical Porous Mn-Doped CoP Catalyst on Nickel Phosphide Foam for Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2022, 5 (1), 149-158. Park, Y.; Kang, H.; Hong, Y.-k.; Cho, G.; Choi, M.; Cho, J.; Ha, D.-H. Influence of the phosphorus source on iron phosphide nanoparticle synthesis for hydrogen evolution reaction catalysis. Int. J. Hydrogen Energy 2020, 45 (57), 32780-32788.
(122) Zhang, C.; Li, D.; Xu, Y. Mn-doped NiP: Facile synthesis and enhanced electrocatalytic activity for hydrogen evolution. J. Mater. Res. 2022, 37 (3), 807-817.
(123) Wang, Y.; Wang, Y.; Bai, J.; Lau, W.-M. Trace Amount of NiP2 Cooperative CoMoP Nanosheets Inducing Efficient Hydrogen Evolution. ACS omega 2021, 6 (48), 33057-33066.
(124) Ge, L.; Yuan, H.; Min, Y.; Li, L.; Chen, S.; Xu, L.; Goddard III, W. A. Predicted optimal bifunctional electrocatalysts for the hydrogen evolution reaction and the oxygen evolution reaction using chalcogenide heterostructures based on machine learning analysis of in silico quantum mechanics based high throughput screening. J. Phys. Chem. Lett. 2020, 11 (3), 869-876.
(125) Tseng, C.-A.; Lee, C.-P. Transition Metal Chalcogenides for the Electrocatalysis of Water. In Advanced Functional Materials; IntechOpen: London, 2020. Iffelsberger, C.; Ng, S.; Pumera, M. Catalyst coating of 3D printed structures via electrochemical deposition: Case of the transition metal chalcogenide MoSx for hydrogen evolution reaction. Appl. Mater. Today 2020, 20, 100654. Moschkowitsch, W.; Lori, O.; Elbaz, L. Recent Progress and Viability of PGM-Free Catalysts for Hydrogen Evolution Reaction and Hydrogen Oxidation Reaction. ACS Catal. 2022, 12 (2), 1082-1089.
(126) Ali, T.; Qiao, W.; Zhang, D.; Liu, W.; Sajjad, S.; Yan, C.; Su, R. Surface sulfur vacancy engineering of metal sulfides promoted desorption of hydrogen atoms for enhanced electrocatalytic hydrogen evolution. J. Phys. Chem. C 2021, 125 (23), 12707-12712. Duraisamy, S.; Ganguly, A.; Sharma, P. K.; Benson, J.; Davis, J.; Papakonstantinou, P. One-step hydrothermal synthesis of phase-engineered
(127) Mei, X.; Li, C.; Lam, F. L.-Y.; Hu, X. Nanosheet-like ternary metal sulfide as a pH -universal catalyst for the hydrogen evolution reaction. ACS Appl. Energy Mater. 2020, 3 (7), 6172-6179. Aslan, E.; Yanalak, G.; Hatay Patir, I. Enhanced Hydrogen Evolution Reaction Catalysis at Template-Free Liquid/Liquid Interfaces by In Situ Electrodeposited Amorphous Molybdenum Sulfide on Carbon Nanotubes. ACS Appl. Energy Mater. 2021, 4 (8), 8330-8339.
(128) Wang, S.; Huang, B.; Dai, Y.; Wei, W. Origin of the Enhanced Hydrogen Evolution Reaction Activity of Grain Boundaries in MoS2Monolayers. J. Phys. Chem. C 2022, 126 (14), 6215-6222. Zhou, W.; Dong, L.; Tan, L.; Tang, Q. First-principles study of sulfur vacancy concentration effect on the electronic structures and hydrogen evolution reaction of MoS2. Nanotechnology 2021, 32 (14), 145718.
(129) He, M.; Kong, F.; Yin, G.; Lv, Z.; Sun, X.; Shi, H.; Gao, B. Enhanced hydrogen evolution reaction activity of hydrogen-annealed vertical MoS 2 nanosheets. RSC Adv. 2018, 8 (26), 14369-14376.
(130) Zhu, J.; Tu, Y.; Cai, L.; Ma, H.; Chai, Y.; Zhang, L.; Zhang, W. Defect-Assisted Anchoring of Pt Single Atoms on MoS2 Nanosheets Produces High-Performance Catalyst for Industrial Hydrogen Evolution Reaction. Small 2022, 18 (4), 2104824. Zheng, J.; Lu, L.; Lebedev, K.; Wu, S.; Zhao, P.; McPherson, I. J.; Wu, T.-S.; Kato, R.; Li, Y.; Ho, P.-L.; et al. Fe on molecular-layer MoS2 as inorganic Fe-S2-Mo motifs for light-driven nitrogen fixation to ammonia at elevated temperatures. Chem. Catalysis 2021, 1 (1), 162-182.
(131) Zheng, Z.; Yu, L.; Gao, M.; Chen, X.; Zhou, W.; Ma, C.; Wu, L.; Zhu, J.; Meng, X.; Hu, J.; et al. Boosting hydrogen evolution on MoS2 via co-confining selenium in surface and cobalt in inner layer. Nat. Commun. 2020, 11 (1), 1-10.
(132) Kazemi Korayem, A.; Ghamami, S.; Bahrami, Z. Fractal properties and morphological investigation of nano-amiodarone using image processing. Signal Image Video Process. 2019, 13 (2), 281-287. Guo, B.; Yu, K.; Li, H.; Qi, R.; Zhang, Y.; Song, H.; Tang, Z.; Zhu, Z.; Chen, M. Coral-shaped MoS2 decorated with graphene quantum dots performing as a highly active electrocatalyst for hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2017, 9 (4), 3653-3660. Park, T.; Bae, C.; Lee, H.; Leem, M.; Kim, H.; Ahn, W.; Kim, J.; Lee, E.; Shin, H.; Kim, H. Non-equilibrium fractal growth of MoS 2 for electrocatalytic hydrogen evolution. CrystEngComm 2019, 21 (3), 478-486. Kazemi Korayem, A.; Ghamami, S.; Bahrami, Z. Fractal properties and morphological investigation of Nano hydrochlorothiazide is used to treat hypertension. BMC Pharmacol. Toxicol. 2018, 19, 1-9.
(133) Ge, Y.; Shi, Z.; Tan, C.; Chen, Y.; Cheng, H.; He, Q.; Zhang, H. Two-dimensional nanomaterials with unconventional phases. Chem. 2020, 6 (6), 1237-1253. Ma, X.; Yang, J.; Xu, X.; Yang, H.; Peng, C.
(134) Deng, W.; Xie, W.; Li, D.; Gai, Y.; Chen, Z.; Yu, J.; Yang, R.; Bao, X.; Jiang, F. Controllable tuning of polymetallic Co-Ni-Ru-S-Se ultrathin nanosheets to boost electrocatalytic oxygen evolution. NPG Asia Mater. 2022, 14 (1), 1-13.
(135) Wang, K.; Lin, Z.; Tang, Y.; Tang, Z.; Tao, C.-L.; Qin, D.-D.; Tian, Y. Selenide/sulfide heterostructured NiCo2Se4/NiCoS4 for oxygen evolution reaction, hydrogen evolution reaction, water splitting and Zn -air batteries. Electrochim. Acta 2021, 368, 137584. Pang, Y.; Zhu, S.; Cui, Z.; Liang, Y.; Li, Z.; Wu, S. Self-supported amorphous nanoporous nickel-cobalt phosphide catalyst for hydrogen evolution reaction. Progress in Natural Science: Materials International 2021, 31 (2), 201-206.
(136) Xiao, D.; Bao, D.-L.; Liang, X.; Wang, Y.; Shen, J.; Cheng, C.; Chu, P. K. Experimental and theoretical investigation of the control and balance of active sites on oxygen plasma-functionalized MoSe2 nanosheets for efficient hydrogen evolution reaction. Appl. Catal. B: Environmental 2021, 288, 119983.
(137) Chang, Y.; Chen, C.; Ho, C.; Cheng, C.; Chen, H.; Fu, T.; Huang, Y.; Ke, S.; Du, H.; Lee, K.; et al. Surface electron accumulation and enhanced hydrogen evolution reaction in MoSe2 basal planes. Nano Energy 2021, 84, 105922.
(138) Yang, X.; Liu, W.; Han, C.; Zhao, C.; Tang, H.; Liu, Q.; Xu, J. Mechanistic insights into charge carrier dynamics in MoSe2/CdS heterojunctions for boosted photocatalytic hydrogen evolution. Materials Today Physics 2020, 15, 100261. Ruqia, B.; Kabiraz, M. K.; Hong, J. W.; Choi, S.-I. Catalyst activation: Surface doping effects of group VI transition metal dichalcogenides towards hydrogen evolution reaction in acidic media. J. Energy Chem. 2022, 72, 217.
(139) Wu, P.; Sun, G.; Chen, Y.; Xu, W.; Zheng, H.; Xu, J.; Wang, L.; Peng, D.-L. MoSe2-Ni3Se4 hybrid nanoelectrocatalysts and their enhanced electrocatalytic activity for hydrogen evolution reaction. Nanoscale Res. Lett. 2020, 15 (1), 1-10.
(140) Hu, W.; Shi, Q.; Chen, Z.; Yin, H.; Zhong, H.; Wang, P. Co2N/ Co 2 Mo 3 O 8 heterostructure as a highly active electrocatalyst for an alkaline hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2021, 13 (7), 8337-8343. Dan, Z.; Liang, W.; Gong, X.; Lin, X.; Zhang, W.; Le, Z.; Xie, F.; Chen, J.; Yang, M.; Wang, N.; et al. Substitutional Doping Engineering toward W2N Nanorod for Hydrogen Evolution Reaction at High Current Density. ACS Mater. Lett. 2022, 4 (7), 13741380.
(141) Tong, R.; Xu, M.; Huang, H.; Zhang, C.; Ma, Y.; Wang, X.; Hu, X.; Qu, Y.; Wang, S.; Pan, H. Co2N0. 67/MoO2 Heterostructure as High-Efficiency Electrocatalysts for the Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2022, 5 (1), 440-448. Xing, Z.; Li, Q.; Wang, D.; Yang, X.; Sun, X. Self-supported nickel nitride as an efficient highperformance three-dimensional cathode for the alkaline hydrogen evolution reaction. Electrochim. Acta 2016, 191, 841-845.
(142) Zhou, W.; Huang, S.; Sun, C. Ni3Mo3N coupled with nitrogenrich carbon microspheres as an efficient hydrogen evolution reaction catalyst and electrochemical sensor for H 2 O 2 detection. Int. J. Hydrogen Energy 2022, 47 (33), 14906-14915.
(143) Park, S. H.; Kang, S. H.; Youn, D. H. Direct One-Step Growth of Bimetallic Ni2Mo3N on Ni Foam as an Efficient Oxygen Evolution Electrocatalyst. Materials 2021, 14 (16), 4768.
(144) Wang, C.; Lv, X.; Zhou, P.; Liang, X.; Wang, Z.; Liu, Y.; Wang, P.; Zheng, Z.; Dai, Y.; Li, Y. Molybdenum nitride electrocatalysts for hydrogen evolution more efficient than platinum/carbon: Mo2N/ CeO2@ nickel foam. ACS Appl. Mater. Interfaces 2020, 12 (26), 29153-29161.
(145) Balaji, D.; Madhavan, J.; AlSalhi, M. S.; Aljaafreh, M. J.; Prasad, S.; Show, P. L. Carbon supported
(146) Zhang, J.; Zhang, L.; Du, L.; Xin, H. L.; Goodenough, J. B.; Cui, Z. Composition-Tunable Antiperovskite CuxIn1- xNNi3 as Superior Electrocatalysts for the Hydrogen Evolution Reaction. Angew. Chem., Int. Ed. 2020, 59 (40), 17488-17493.
(147) Dastafkan, K.; Shen, X.; Hocking, R. K.; Meyer, Q.; Zhao, C. Monometallic interphasic synergy via nano-hetero-interfacing for hydrogen evolution in alkaline electrolytes. Nat. Commun. 2023, 14 (1), 547.
(148) Wang, H.-F.; Chen, L.; Pang, H.; Kaskel, S.; Xu, Q. MOFderived electrocatalysts for oxygen reduction, oxygen evolution and hydrogen evolution reactions. Chem. Soc. Rev. 2020, 49 (5), 14141448. Sahu, N.; Das, J. K.; Behera, J. NiSe2 Nanoparticles Encapsulated in N-Doped Carbon Matrix Derived from a One-Dimensional NiMOF: An Efficient and Sustained Electrocatalyst for Hydrogen Evolution Reaction. Inorg. Chem. 2022, 61 (6), 2835-2845. Aleksandrzak, M.; Sielicki, K.; Mijowska, E. Enhancement of photocatalytic hydrogen evolution with catalysts based on carbonized MOF-5 and gC 3 N 4. RSC Adv. 2020, 10 (7), 4032-4039.
(149) Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J. M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nature materials 2009, 8 (1), 76-80. Sprick, R. S.; Bonillo, B.; Clowes, R.; Guiglion, P.; Brownbill, N. J.; Slater, B. J.; Blanc, F.; Zwijnenburg, M. A.; Adams, D. J.; Cooper, A. I. Visible-light-driven hydrogen evolution using planarized conjugated polymer photocatalysts. Angew. Chem., Int. Ed. 2016, 55 (5), 1792-1796. Rahman, M. Z.; Davey, K.; Qiao, S.-Z. Carbon, nitrogen and phosphorus containing metal-free photocatalysts for hydrogen production: progress and challenges. J. Mater. Chem. A 2018, 6 (4), 1305-1322. Gopi, S.; Panda, A.; Ramu, A.; Theerthagiri, J.; Kim, H.; Yun, K. J. Bifunctional electrocatalysts for water splitting from a bimetallic (V doped-NixFey) Metal-Organic framework MOF@ Graphene oxide composite. Int. J. Hydrogen Energy 2022, 47, 42122. Liu, Y.; Cheng, H.; Cheng, M.; Liu, Z.; Huang, D.; Zhang, G.; Shao, B.; Liang, Q.; Luo, S.; Wu, T. J. C. E. J.; et al. The application of Zeolitic
imidazolate frameworks (ZIFs) and their derivatives based materials for photocatalytic hydrogen evolution and pollutants treatment. Chem. Eng. J. 2021, 417, 127914.
(150) Zhu, B.; Qu, C.; Gao, S.; Liang, Z.; Zhang, H.; Zou, R. J. C. Ultralow Loading Ruthenium Nanoparticles on Nitrogen-Doped Graphene Aerogel for Trifunctional Electrocatalysis. ChemCatChem. 2018, 10 (5), 1113-1121. Zhu, B.; Wen, D.; Liang, Z.; Zou, R. J. C. C. R. Conductive metal-organic frameworks for electrochemical energy conversion and storage. Coord. Chem. Rev. 2021, 446, 214119.
(151) Gao, H.; Shen, H.; Wu, H.; Jing, H.; Sun, Y.; Liu, B.; Chen, Z.; Song, J.; Lu, L.; Wu, Z. J. E.; et al. Review of Pristine Metal-Organic Frameworks for Supercapacitors: Recent Progress and Perspectives. Energy Fuels 2021, 35 (16), 12884-12901. Qiu, T.; Gao, S.; Liang, Z.; Wang, D. G.; Tabassum, H.; Zhong, R.; Zou, R. J. A. C. I. E. Pristine Hollow Metal-Organic Frameworks: Design, Synthesis and Application. Angew. Chem., Int. Ed. 2021, 60, 17314.
(152) Sonowal, K.; Saikia, L. Metal-organic frameworks and their composites for fuel and chemical production via CO 2 conversion and water splitting. RSC Adv. 2022, 12 (19), 11686-11707. Li, P.; Zheng, D.; Gao, M.; Zuo, X.; Sun, L.; Zhou, Q.; Lin, J. Bimetallic MOFTemplated Fabrication of Porous
(153) Ao, K.; Wei, Q.; Daoud, W. A. MOF-derived sulfide-based electrocatalyst and scaffold for boosted hydrogen production. ACS Appl. Mater. Interfaces 2020, 12 (30), 33595-33602. Qi, W.; Wang, C.; Yu, J.; Adimi, S.; Thomas, T.; Guo, H.; Liu, S.; Yang, M. MOF-Derived Porous Ternary Nickel Iron Nitride Nanocube as a Functional Catalyst toward Water Splitting Hydrogen Evolution for Solar to Chemical Energy Conversion. ACS Appl. Energy Mater. 2022, 5, 6155.
(154) Kazemi, A.; Moghadaskhou, F.; Pordsari, M. A.; Manteghi, F.; Tadjarodi, A.; Ghaemi, A. Enhanced CO2 capture potential of UiO-66NH2 synthesized by sonochemical method: experimental findings and performance evaluation. Sci. Rep. 2023, 13 (1), 19891. Ramezanalizadeh, H.; Manteghi, F. Mixed cobalt/nickel metal-organic framework, an efficient catalyst for one-pot synthesis of substituted imidazoles. Monatshefte für Chemie-Chemical Monthly 2017, 148, 347355. Ramezanalizadeh, H.; Manteghi, F. Synthesis of a novel MOF/ CuWO4 heterostructure for efficient photocatalytic degradation and removal of water pollutants. Journal of Cleaner Production 2018, 172, 2655-2666. Abhari, P. S.; Manteghi, F.; Tehrani, Z. Adsorption of lead ions by a green AC/HKUST-1 nanocomposite. Nanomaterials 2020, 10 (9), 1647.
(155) Li, Y.-W.; Wu, Q.; Ma, R.-C.; Sun, X.-Q.; Li, D.-D.; Du, H.-M.; Ma, H.-Y.; Li, D.-C.; Wang, S.-N.; Dou, J.-M. A Co-MOF-derived Co 9 S 8@ NS-C electrocatalyst for efficient hydrogen evolution reaction. RSC Adv. 2021, 11 (11), 5947-5957. Sun, Y.; Xue, Z.; Liu, Q.; Jia, Y.; Li, Y.; Liu, K.; Lin, Y.; Liu, M.; Li, G.; Su, C.-Y. Modulating electronic structure of metal-organic frameworks by introducing atomically dispersed Ru for efficient hydrogen evolution. Nat. Commun. 2021, 12 (1), 1369.
(156) Yan, L.; Cao, L.; Dai, P.; Gu, X.; Liu, D.; Li, L.; Wang, Y.; Zhao, X. J. A. F. M. Metal-organic frameworks derived nanotube of nickelcobalt bimetal phosphides as highly efficient electrocatalysts for overall water splitting. Adv. Funct. Mater. 2017, 27 (40), 1703455.
(157) Hao, X.; Jin, Z.; Yang, H.; Lu, G.; Bi, Y. J. A. C. B. E. Peculiar synergetic effect of MoS2 quantum dots and graphene on MetalOrganic Frameworks for photocatalytic hydrogen evolution. Appl. Catal. B.: Environ. 2017, 210, 45-56.
(158) Zhen, W.; Ma, J.; Lu, G. Small-sized Ni (lll) particles in metal-organic frameworks with low over-potential for visible photocatalytic hydrogen generation. Appl. Catal. B: Environmental 2016, 190, 12-25.
(159) Li, Y.; Ai, C.; Deng, S.; Wang, Y.; Tong, X.; Wang, X.; Xia, X.; Tu, J. Nitrogen doped vertical graphene as metal-free electrocatalyst for hydrogen evolution reaction. Mater. Res. Bull. 2021, 134, 111094.
- Received: October 10, 2023
Revised: December 28, 2023
Accepted: December 29, 2023
Published: January 29, 2024
DOI: https://doi.org/10.1021/acsomega.3c07911
PMID: https://pubmed.ncbi.nlm.nih.gov/38405471
Publication Date: 2024-01-29
Metal Electrocatalysts for Hydrogen Production in Water Splitting
Read Online
Metrics & More
Article Recommendations
Downloaded via 86.143.216.125 on March 21, 2024 at 15:12:53 (UTC).
See https://pubs.acs.org/sharingguidelines for options on how to legitimately share published articles.
Abstract
The rising demand for fossil fuels and the resulting pollution have raised environmental concerns about energy production. Undoubtedly, hydrogen is the best candidate for producing clean and sustainable energy now and in the future. Water splitting is a promising and efficient process for hydrogen production, where catalysts play a key role in the hydrogen evolution reaction (HER). HER electrocatalysis can be well performed by Pt with a low overpotential close to zero and a Tafel slope of about 30
 advances in the design and development of nanostructured electrocatalysts for noble and non-noble metals in HER electrocatalysis. In general, strategies including doping, crystallization control, structural engineering, carbon nanomaterials, and increasing active sites by changing morphology are helpful to improve HER performance. Finally, the challenges and future perspectives in designing functional and stable electrocatalysts for HER in efficient hydrogen production from water-splitting electrolysis will be described.
advances in the design and development of nanostructured electrocatalysts for noble and non-noble metals in HER electrocatalysis. In general, strategies including doping, crystallization control, structural engineering, carbon nanomaterials, and increasing active sites by changing morphology are helpful to improve HER performance. Finally, the challenges and future perspectives in designing functional and stable electrocatalysts for HER in efficient hydrogen production from water-splitting electrolysis will be described.
1. INTRODUCTION
currently around

2. HER
| criteria | source | production | reaction | ||||
|
|
|
|
||||
| coal gasification | coal, steam |
|
|
||||
| water electrolysis | water |
|
|
useful applications. Electrochemical WE is a promising technology to generate hydrogen fuel from water renewably; this is the process of converting water to pure and stable hydrogen that is made up of two half-cell reactions: the oxygen evolution (OER) and the hydrogen evolution (HER) reaction processes.
2.1. Fundamentals of the HER. Water, unlike fossil fuels, is an abundant and renewable resource on Earth, so the hydrogen
2.2. Acidic and Alkaline Media. The key half-reaction for hydrogen production in water splitting occurs at the cathode, which involves the transfer of two highly dependent electrons in environmental conditions. The alkaline environment is now the focus of hydrogen development through the HER to substitute clean fuel for different energy systems. Due to an extra water dissociation process, the kinetics of this reaction are sluggish and cause a significant reduction in electrocatalytic performance. Therefore, modern electrocatalysts can perform well in acidic environments.


water molecule, called the Volmer pathway. Then, the Tafel or Hirovsky path continues to produce hydrogen.

2.3. Rate of Reaction. A thermodynamic potential of 1.23 V at
results against theoretical predictions with faradaic efficiency and turnover frequency.
2.4. Pseudocapacitance. In the HER process, hydrogen must be adsorbed on the surface of the electrocatalyst in the first step. In the next step, the absorbed hydrogen must be separated from the electrocatalyst surface and returned irreversibly to the electrolyte. This electrocatalyst pseudocapacitive characteristic is critical to HER performance. In hydrogen adsorption, practically all HER electrocatalysts are excellent pseudocapacitors. In order to desorb all of the hydrogen absorbed, a good pseudocapacitor must be highly efficient. Therefore, studying pseudocapacitive performance before the HER potential offers vital information on electrocatalyst efficiency. The optimal pseudocapacitive performance, which is typified by a rectangular shape in cyclic voltammetry (CV), is significantly more closely related to HER electrocatalytic activity.
2.5. Volcano Plots. As stated in the previous sections, theoretical simulations prove that HER activity is closely
connected to hydrogen adsorption (
2.6. Kinetic Isotope Effect (KIE). An important method for studying chemical reactions is the kinetic isotope effect (KIE). This theory is based on the observation that the rate of a reaction can vary with atom mass. A particle’s mass affects the energy of its vibrational and rotational states, which, in turn, affects the probability of tunneling. Tunneling is a quantum mechanical process that allows particles to pass through potential barriers without having enough energy to overcome them.
effects are calculated using the ratio of the rate constants of two isotopically substituted reactions in which the difference in mass is not between atoms involved in the rate-determining step but between atoms involved in the equilibrium constant. Chemical reactions can be studied using a KIE. It is possible to use them to identify the rate-determining step, to determine the energy of the transition state, and to study the mechanism of reactions that are difficult to study using other methods. An electron is transferred from the electrode to a hydrogen ion in the electrolyte as part of the HER process. As an electrolyte, both
(1) An electron is transferred from the electrode to a hydrogen ion in the electrolyte, forming a hydronium ion.
(2) The hydronium ion dissociates into a proton and water molecule.
(3) The proton tunnels through the water molecule and attacks the surface of the electrode, forming a hydrogen atom.
(4) The hydrogen atom desorbs from the surface of the electrode, forming hydrogen gas.
A KIE indicates that an electron is transferred from the electrode to a hydrogen ion in the electrolyte to determine the rate of the reaction. The isotope effect is much larger for this step than for any of the other steps.
3. HER ELECTROCATALYSTS REQUIREMENTS
3.1. Overpotential, Tafel Slope, and Exchange Current Density. The overpotential (
of low overpotential is directly related to the high electrocatalytic activity. Water splitting occurs at a cell potential of 1.23 V ( 0 V for HER and 1.23 V for the OER). Both HER and OER processes need additional potential, mainly from the intrinsic activation obstacles present. The applied potential must be increased for the reaction to occur.
3.2. Electrochemically Active Surface Area (ECSA). The electrode material’s electrochemical surface area (ECSA) is one of the fundamental phenomena in selecting electrocatalysts,
indicating the area of the electrode material available to the electrolyte. It has been proven that it is challenging to measure the electrochemically active surface of any material as an electrode. This surface is used for charge transfer and/or storage in electrochemical cells (galvanic/electrolytic). The electrode surface may be expanded using a variety of techniques. These include using nanostructures, cyclic voltammetry (CV), and linear sweep voltammetry (LSV).
3.3. Faradaic Efficiency. Faraday efficiency (also known as current efficiency) is another parameter used to assess the activity of the HER electrocatalyst. In place of the side reaction, faradaic efficiency (FE) calculates the amount of charges (electrons) in the desired reaction. In the HER process, FE is the ratio of experimentally identified
3.4. Turnover Frequency. The turnover frequency (TOF) of a catalytic center quantifies its particular activity by the number of reactants transformed to the chosen product per unit time. The amount of TOF for HER and OER is calculated based on the following equations:
3.5. Hydrogen-Bonding Energy. In the hydrogen evolution reaction (HER), an electrochemical process that generates hydrogen gas from water splitting, hydrogen-bonding energy (HBE) plays a crucial role. Hydrogen bond energy describes the strength of hydrogen atoms’ interaction with neighboring atoms, usually oxygen atoms in water or on the catalyst surface. The interaction affects the overall kinetics of the HER, affecting the rate of hydrogen evolution and the stability of adsorbed hydrogen species. Adsorption of hydrogen intermediates on the catalyst surface is made easier by a strong hydrogen bond between hydrogen atoms and the catalyst surface. If the HBE is too strong, it can prevent hydrogen molecules from desorbing, reducing hydrogen evolution efficiency. In order to achieve efficient and sustainable HER, an optimal HBE must be in place. Numerous techniques have been used to study the effects of HBE on HER activity, including

(1) Nature of the catalyst surface: HBE can be significantly affected by the type of metal or material used for the catalyst surface. Catalysts containing a high density of oxygen-containing groups, such as hydroxides and oxides, tend to exhibit stronger HBE than catalysts containing a lower density.
(2) Preparation methods: A catalyst’s preparation method can also affect its HBE. The formation of HBE is more likely to occur in catalysts prepared using methods that introduce defects or roughness into their surfaces.
(3) Electrolyte composition: HBE can also be affected by the electrolyte composition. Electrolytes with higher pH values, for example, tend to form stronger HBE than electrolytes with lower pH values.
Optimizing HBE and Impact on Sustainable Hydrogen Production. Developing efficient HER catalysts requires optimizing HBE. Scientists can design catalysts with high HER rates and stability by understanding the factors that influence
3.6. Stability. Another important parameter for choosing the suitable HER electrocatalyst is stability. There are two approaches to assess the stability factor. One is LSV or CV; the other is potentiostatic or galvanostatic electrolysis by longterm chronopotentiometric (CP) or chronoamperometric (CA) tests. This voltammetric method is utilized to compare the overpotential modifications, before and after a certain period of cycles for 1000-10000 cyclic voltammograms at a scan rate such as
3.7. Electrochemical Methods (Three-Electrode Cell). In the HER process, a rotating disk work electrode (RDE) is suggested to obtain accurate experimental data. With high accuracy, this electrode can quantify the mass transfer rate and reaction kinetics well. The RDE is a working electrode used in three-electrode systems for voltammetry of electrochemical studies when examining the reaction mechanisms of redox chemistry, among other chemical phenomena.
surrounded by a nonconductive material such as a polymer or an inert resin. According to Figure 4, in an electrochemical system, the working electrode (WE) is often used in conjunction with a counter electrode (CE) and a reference electrode (RE) in a three-electrode system.
3.8. Measurement Pitfalls and Data Interpretation in
4. HER ELECTROCATALYSTS
(1) Noble metals with compounds and alloys
(2) Low-cost transition metal-based materials without precious metals
(3) MOF-derived material-based electrocatalysts
4.1. Noble Metal-Based Electrocatalysts. The electrocatalytic performance of noble metals, such as Pt group metals (PGMs, including Pt, Pd, Rh, Ru, and Ir), is appealing for HER.

favorable influence on the material’s catalytic activity. Some Ptbased alloys can be made with
is designed by using a palladium-rubeanic acid (
4.2. Non-Noble Metal-Based Electrocatalysts. As summarized in the previous chapter, non-noble metal-based electrocatalysts are the only viable option for the future development of large-scale water splitting for energy conversion.
| material | structure | Tafel slope (
|
|
refs |
| NiCoN | nitride | 105.2 | 145 | 76 |
|
|
nitride | 64 | 31 | 77 |
|
|
selenide | 46.9 | 249 mV at 100 mA | 78 |
|
|
selenide | 31.6 | 108 | 79 |
| NiMoNx/C | nitride | 35 | 78 mV at 100 mA | 80 |
| Mo-Fe-SeCP | selenide | 57.7 | 86.9 | 81 |
|
|
nitride | 47 | 56 | 82 |
|
|
phosphide | 55 | 191 | 83 |
|
|
phosphide | 60 | 174 | 83 |
|
|
phosphide | 101 | 406 | 84 |
|
|
nitride | 115.7 | 217 | 85 |
| FeP | phosphide | 37 | 50 | 86 |
|
|
carbide | 34.5 | 38 | 87 |
| WN/CC | nitride | 57.1 | 130 | 88 |
| porous
|
phosphide | 67 | 56 | 89 |
| MoNx | nitride | 114 | 148 | 90 |
| VMoN | nitride | 60 | 108 | 91 |
|
|
selenide | 40 | 87 | 92 |
| CoNiSe/NC | selenide | 66.5 | 100 | 93 |
|
|
carbide | 52 | 114 | 94 |
| nanoMoC@GS | carbide | 43 | 124 | 95 |
| MoN | nitride | 120 | 389 | 96 |
|
|
nitride | 85 | 242 | 97 |
| NiCoP holey | phosphide | 57 | 58 | 98 |
|
|
phosphide | 50 | 88 | 99 |
4.2.1. Transition Metal Carbides. Transition metal carbides (TMCs) demonstrate Pt-like properties for HER electrocatalytic activity due to the shift in the d-band center; for this reason, the development of these electrocatalysts as non-noble metal-based electrocatalysts is of great interest.

tungsten carbon disrupt the active sites in the HER electrocatalyst reaction. This is in contrast to transition metal chalcogenides in which the presence of oxygen can improve HER performance. In the interaction of the electrocatalyst with water, the WC surface disrupts and inactivates the formation of a

intermittent operation remains a critical concern since carbonbased materials are naturally prone to corrosion, even under mild oxidative circumstances. As a result, it is proposed that future study in this field concentrates on strengthening the stability under anodic polarization that can occur after the electrolyzer is turned off.
4.2.2. Transition Metal Phosphides. Transition metal phosphides (TMPs), due to their inherent activity and high stability in both acidic and alkaline environments, have presented themselves as potential candidates for HER electrocatalysis.

dose is 0.02 mM , it shows the most prominent overpotentials of 46 mV at
4.2.3. Transition Metal Chalcogenides (Sulfides and Selenides). Transition metal chalcogenides, relying on properties such as being inexpensive and ease of preparation, are promising candidates to replace noble metals for HER. According to the existing studies, metal selenides show higher catalytic activity than other members of the chalcogenides.

the HER activity of vertical

catalysts for industrial hydrogen production applications. In this study, Figure 9a shows the HER polarization curves of the Sedoped

electron transfer in the HER reaction. A lower Tafel slope indicates a faster rate of electron transfer, which means a more efficient catalyst. The
spectroscopy (EIS) measurements of the
medium, metal selenides have been successfully tested in acidic media.
4.2.4. Transition Metal Nitrides. Transition metal nitrides (TMNs), known as transition alloys, have recently been proposed as efficient HER electrocatalysts as alternatives to noble metal electrocatalysts due to their exceptional electrical conductivity, corrosion resistance, mechanical robustness, and high electrochemical stability.

4.3. MOF-Based Electrocatalysts. The use of metalorganic frameworks (MOFs) in electrocatalytic, photocatalytic, and chemocatalytic processes makes them a strong choice for extremely effective HER.
nanoparticles and the bonding of functional surface species, are useful in increasing the activity of the catalysts.
graphene. Zhen et al.
5. SUMMARY AND PERSPECTIVES
(a) The incorporation of transition metals into the electrocatalyst design can be advantageous. Although these beneficial effects are tunable, optimal synergistic effects between various metals boost electrocatalytic capabilities.
(b) The catalyst core/shell design is a realistic and practical strategy to enhance the active sites for HER electrocatalysis while making the material feasible for development. It is particularly effective and successful in increasing active sites around the borders of twodimensional layered structures. However, although the core/shell design enhances catalytic performance, the overall structure may be inadequate for industrial operation.
(c) A high-level carbon catalyst is required for support. To obtain adequate performance, even with Pt, a sufficient carbon catalyst support must be utilized (and not just low overpotential). However, the physical and chemical structure of carbon is undeniably important in electrocatalytic efficacy.
(d) Transition-based chemicals such as sulfides, selenides, phosphides, and carbides are the most promising options.
(e) The employment of transition metals in electrocatalytic architecture, similar to doping, can be advantageous.
(g) The substrate surface plays an important role, especially for electroactive monolayers or ultrathin films. This effect is seen on both the smooth surface and the particle coating.
(H) Planar deformation and strain engineering can assist activate putative electrocatalytic sites on base surfaces.
(i) MOF-based materials demonstrate significant activity in the HER due to their high porosity, controlled porosity, and suitable structure. These materials are utilized as electrocatalysts for HER for several reasons. First, they offer an opportunity to enhance and replace expensive noble metal-based catalysts with more affordable metals. Second, they aid in reducing the necessary overpotential for HER, thereby improving the overall efficiency. Lastly, MOF-based materials contribute to improved reaction kinetics, enabling more efficient HER processes. Overall, the unique characteristics of MOFs make them promising candidates for HER electrocatalysis.
Despite significant advances in understanding electrocatalytic processes and designing suitable electrodes for the HER, there are several challenges to the cost-effective production of largescale hydrogen by split water electrolysis. To ensure the successful progress of the research, it is necessary to integrate the test protocol in order to be able to compare different materials. In addition, when designing novel cathodic catalysts in the research phase, the ease of preparation and potential scalability for industrial applications should be considered. However, recent interest in a different approach to energy sources, the importance of environmental protection in the future, and economic issues are expected to lead to new advances in the design of active, sustainable, and low-cost HER electrocatalysts for mass commercialization of water-based hydrogen production.
AUTHOR INFORMATION
Corresponding Authors
Zari Tehrani – The Future Manufacturing Research Institute, Faculty of Science and Engineering, Swansea University, SA1 8EN Swansea, United Kingdom; © orcid.org/0000-0002-5069-7921; Email: z.tehrani@swansea.ac.uk
Author
Complete contact information is available at:
https://pubs.acs.org/10.1021/acsomega.3c07911
Notes
ACKNOWLEDGMENTS
REFERENCES
(2) Dou, Y.; Wang, A.; Zhao, L.; Yang, X.; Wang, Q.; Sudi, M. S.; Zhu, W.; Shang, D. Boosted hydrogen evolution reaction for a nitrogen-rich azo-bridged metallated porphyrin network. J. Colloid Interface Sci. 2023, 650, 943-950.
(3) Vijayapradeep, S.; Logeshwaran, N.; Ramakrishnan, S.; Kim, A. R.; Sampath, P.; Kim, D. H.; Yoo, D. J. Novel Pt-carbon core-shell decorated hierarchical CoMo2S4 as efficient electrocatalysts for alkaline/seawater hydrogen evolution reaction. Chem. Eng. J. 2023, 473, 145348.
(4) Ramachandran, E.; Krishnaiah, R.; Venkatesan, E. P.; Shaik, S.; Saleel, C. A.; Hussain, F. Investigation into the Ideal Concoction for Performance and Emissions Enhancement of Jatropha Biodiesel-Diesel with CuO Nanoparticles Using Response Surface Methodology. ACS omega 2023, 8 (42), 39067-39079.
(5) Riaz, A.; Qyyum, M. A.; Hussain, A.; Lee, M. Tapping the energy and exergy benefits of channeling liquid air energy system in the hydrogen liquefaction process. Journal of Energy Storage 2023, 72, 108193.
(6) Hoang, A. T.; Pandey, A.; De Osés, F. J. M.; Chen, W.-H.; Said, Z.; Ng, K. H.; Ağbulut, D. C.; Tarełko, W.; Olçer, A. I.; Nguyen, X. P. Technological solutions for boosting hydrogen role in decarbonization strategies and net-zero goals of world shipping: Challenges and perspectives. Renewable Sustainable Energy Rev. 2023, 188, 113790.
(7) Longchamps, R. S.; Yang, X.-G.; Wang, C.-Y. Fundamental Insights into Battery Thermal Management and Safety. ACS Energy Lett. 2022, 7 (3), 1103-1111.
(8) Li, Y.; Zhou, Q.; Weng, S.; Ding, F.; Qi, X.; Lu, J.; Li, Y.; Zhang, X.; Rong, X.; Lu, Y.; et al. Interfacial engineering to achieve an energy density of over 200 Wh kg- 1 in sodium batteries. Nat. Energy 2022, 7 (6), 511-519. Kumar, A.; Bui, V. Q.; Lee, J.; Jadhav, A. R.; Hwang, Y.; Kim, M. G.; Kawazoe, Y.; Lee, H. Modulating interfacial charge density of NiP2-FeP2 via coupling with metallic Cu for accelerating alkaline hydrogen evolution. ACS Energy Lett. 2021, 6 (2), 354-363.
(9) Biggins, F.; Kataria, M.; Roberts, D.; Brown, S. Green hydrogen investments: Investigating the option to wait. Energy 2022, 241, 122842. Fei, B.; Chen, Z.; Liu, J.; Xu, H.; Yan, X.; Qing, H.; Chen, M.;
(10) Thirumal, V.; Yuvakkumar, R.; Saravanakumar, B.; Ravi, G.; Isacfranklin, M.; Shobana, M.; Al-Sehemi, A. G.; Velauthapillai, D. Carbonization and optimization of biomass waste for HER application. Fuel 2022, 324, 124466.
(11) Fakayode, O. A.; Yusuf, B. A.; Zhou, C.; Xu, Y.; Ji, Q.; Xie, J.; Ma, H. Simplistic two-step fabrication of porous carbon-based biomassderived electrocatalyst for efficient hydrogen evolution reaction. Energy Conversion and Management 2021, 227, 113628.
(12) Huner, B.; Demir, N.; Kaya, M. F. Hydrogen Evolution Reaction Performance of Ni-Co-Coated Graphene-Based 3D Printed Electrodes. ACS omega 2023, 8 (6), 5958-5974.
(13) McCrum, I. T.; Koper, M. The role of adsorbed hydroxide in hydrogen evolution reaction kinetics on modified platinum. Nature Energy 2020, 5 (11), 891-899.
(14) Hao, J.; Zhuang, Z.; Cao, K.; Gao, G.; Wang, C.; Lai, F.; Lu, S.; Ma, P.; Dong, W.; Liu, T.; et al. Unraveling the electronegativitydominated intermediate adsorption on high-entropy alloy electrocatalysts. Nat. Commun. 2022, 13 (1), 1-13.
(15) Tang, Z.; Wei, S.; Wang, Y.; Dai, L. Three-dimensional reduced graphene oxide decorated with cobalt metaphosphate as high costefficiency electrocatalysts for the hydrogen evolution reaction. RSC Adv. 2022, 12 (17), 10522-10533.
(16) Wang, T.; Tao, L.; Zhu, X.; Chen, C.; Chen, W.; Du, S.; Zhou, Y.; Zhou, B.; Wang, D.; Xie, C.; et al. Combined anodic and cathodic hydrogen production from aldehyde oxidation and hydrogen evolution reaction. Nat. Catal. 2022, 5 (1), 66-73.
(17) Song, D.; Sun, J.; Sun, L.; Zhai, S.; Ho, G. W.; Wu, H.; Deng, W. Q. Acidic media regulated hierarchical cobalt compounds with phosphorous doping as water splitting electrocatalysts. Adv. Energy Mater. 2021, 11 (22), 2100358.
(18) Voitic, G.; Hacker, V. Recent advancements in chemical looping water splitting for the production of hydrogen. Rsc Advances 2016, 6 (100), 98267-98296.
(19) Hermesmann, M.; Müller, T. Green, Turquoise, Blue, or Grey? Environmentally friendly Hydrogen Production in Transforming Energy Systems. Prog. Energy Combust. Sci. 2022, 90, 100996. Haverkort, J.; Rajaei, H. Voltage losses in zero-gap alkaline water electrolysis. J. Power Sources 2021, 497, 229864.
(20) Li, Y.; Shi, X.; Phoumin, H. A strategic roadmap for large-scale green hydrogen demonstration and commercialisation in China: A review and survey analysis. Int. J. Hydrogen Energy 2022, 47, 2459224609.
(21) Miller, H. A.; Bouzek, K.; Hnat, J.; Loos, S.; Bernäcker, C. I.; Weißgärber, T.; Röntzsch, L.; Meier-Haack, J. Green hydrogen from anion exchange membrane water electrolysis: a review of recent developments in critical materials and operating conditions. Sustainable Energy & Fuels 2020, 4 (5), 2114-2133.
(22) Zhang, P.; Cheng, H.; Gu, F.; Hong, S.; Dong, H.; Li, C. Progress on iron-series metal-organic frameworks materials towards electrocatalytic hydrogen evolution reaction. Surfaces and Interfaces 2023, 42, 103368.
(23) Wang, J.; Gao, Y.; Kong, H.; Kim, J.; Choi, S.; Ciucci, F.; Hao, Y.; Yang, S.; Shao, Z.; Lim, J. Non-precious-metal catalysts for alkaline water electrolysis: operando characterizations, theoretical calculations, and recent advances. Chem. Soc. Rev. 2020, 49 (24), 9154-9196.
(24) Dinh, K. N.; Sun, Y.; Pei, Z.; Yuan, Z.; Suwardi, A.; Huang, Q.; Liao, X.; Wang, Z.; Chen, Y.; Yan, Q. Electronic modulation of nickel disulfide toward efficient water electrolysis. Small 2020, 16 (17), 1905885. Grigoriev, S.; Fateev, V.; Bessarabov, D.; Millet, P. Current status, research trends, and challenges in water electrolysis science and technology. Int. J. Hydrogen Energy 2020, 45 (49), 26036-26058.
(25) Wan, L.; Xu, Z.; Wang, P.; Lin, Y.; Wang, B. H2SO4-doped polybenzimidazole membranes for hydrogen production with acidalkaline amphoteric water electrolysis. J. Membr. Sci. 2021, 618, 118642. Cheikh, J. A.; Zakari, R.; Bhosale, A. C.; Villagra, A.; Leclerc, N.; Floquet, S.; Ghosh, P. C.; Ranjbari, A.; Cadot, E.; Millet, P.; et al. Electrocatalytic properties of {Mo 3 S 4}-based complexes with regard to the hydrogen evolution reaction and application to PEM water electrolysis. Mater. Adv. 2020, 1 (3), 430-440.
(26) Huang, W.-H.; Li, X.-M.; Yang, X.-F.; Zhang, H.-B.; Wang, F.; Zhang, J. Highly efficient electrocatalysts for overall water splitting: mesoporous
(27) Lin, L.; Sherrell, P.; Liu, Y.; Lei, W.; Zhang, S.; Zhang, H.; Wallace, G. G.; Chen, J. Engineered 2D transition metal dichalcoge-nides-a vision of viable hydrogen evolution reaction catalysis. Adv. Energy Mater. 2020, 10 (16), 1903870.
(28) Wang, X.; Zheng, Y.; Sheng, W.; Xu, Z. J.; Jaroniec, M.; Qiao, S.Z . Strategies for design of electrocatalysts for hydrogen evolution under alkaline conditions. Mater. Today 2020, 36, 125-138. Pu, Z.; Amiinu, I. S.; Cheng, R.; Wang, P.; Zhang, C.; Mu, S.; Zhao, W.; Su, F.; Zhang, G.; Liao, S.; et al. Single-atom catalysts for electrochemical hydrogen evolution reaction: recent advances and future perspectives. NanoMicro Lett. 2020, 12 (1), 1-29. Shang, P.; Ye, Z.; Ding, Y.; Zhu, Z.; Peng, X.; Ma, G.; Li, D. Nanosponge-like solid solution of NiMo with a high hydrogen evolution reaction performance over a wide range of current densities. ACS Sustainable Chem. Eng. 2020, 8 (29), 1066410672.
(29) Liu, Y.; Yong, X.; Liu, Z.; Chen, Z.; Kang, Z.; Lu, S. Unified catalyst for efficient and stable hydrogen production by both the electrolysis of water and the hydrolysis of ammonia borane. Adv. Sustainable Syst. 2019, 3 (5), 1800161. dos Santos, K. G.; Eckert, C. T.; De Rossi, E.; Bariccatti, R. A.; Frigo, E. P.; Lindino, C. A.; Alves, H. J.
(30) Wang, Y.; Sun, D.; Wang, M.; Feng, Z.; Hall, A. S. Oxygen Reduction Electrocatalysis on Ordered Intermetallic Pd-Bi Electrodes Is Enhanced by a Low Coverage of Spectator Species. J. Phys. Chem. C 2020, 124 (9), 5220-5224. Wang, J.; Qiu, T.; Chen, X.; Lu, Y.; Yang, W. N-doped carbon@ Ni-Al2O3 nanosheet array@ graphene oxide composite as an electrocatalyst for hydrogen evolution reaction in alkaline medium. J. Power Sources 2015, 293, 178-186. Schmidt, T. J.; Stamenkovic, V.; Ross, P. N., Jr.; Markovic, N. M. Temperature dependent surface electrochemistry on Pt single crystals in alkaline electrolyte Part 3. The oxygen reduction reaction. Phys. Chem. Chem. Phys. 2003, 5 (2), 400-406.
(31) Cai, J.; Ding, J.; Wei, D.; Xie, X.; Li, B.; Lu, S.; Zhang, J.; Liu, Y.; Cai, Q.; Zang, S. Coupling of Ru and O-Vacancy on 2D Mo-Based Electrocatalyst Via a Solid-Phase Interface Reaction Strategy for Hydrogen Evolution Reaction. Adv. Energy Mater. 2021, 11 (26), 2100141. Kou, T.; Chen, M.; Wu, F.; Smart, T. J.; Wang, S.; Wu, Y.; Zhang, Y.; Li, S.; Lall, S.; Zhang, Z. J. N. c.; et al. Carbon doping switching on the hydrogen adsorption activity of NiO for hydrogen evolution reaction. Nat. Commun. 2020, 11 (1), 1-10. Li, W.; Liu, G.; Li, J.; Wang, Y.; Ricardez-Sandoval, L.; Zhang, Y.; Zhang, Z. J. A. S. S. Hydrogen evolution reaction mechanism on 2H-MoS2 electrocatalyst. Appl. Surf. Sci. 2019, 498, 143869. Liu, Z.; Yang, X.; Hu, G.; Feng, L. Ru nanoclusters coupled on
(32) Yu, X.; Xu, S.; Wang, Z.; Cheng, X.; Du, Y.; Chen, G.; Sun, X.; Wu, Q. An Mn -doped NiCoP flower-like structure as a highly efficient electrocatalyst for hydrogen evolution reaction in acidic and alkaline solutions with long duration. Nanoscale 2021, 13 (25), 11069-11076.
(33) He, H.-z.; Zhang, Y.; Li, Y.; Wang, P. Recent innovations of silkderived electrocatalysts for hydrogen evolution reaction, oxygen evolution reaction and oxygen reduction reaction. Int. J. Hydrogen Energy 2021, 46 (11), 7848-7865.
(34) Wen, Q.; Zhao, Y.; Liu, Y.; Li, H.; Zhai, T. Ultrahigh-CurrentDensity and Long-Term-Durability Electrocatalysts for Water Splitting. Small 2022, 18 (4), 2104513. Hao, J.; Wei, F.; Zhang, X.; Li, L.; Zhang, C.; Liang, D.; Ma, X.; Lu, P. Defect and doping engineered pentagraphene for catalysis of hydrogen evolution reaction. Nanoscale Res. Lett. 2021, 16 (1), 1-9.
(35) Wang, S.; Xu, B.; Huo, W.; Feng, H.; Zhou, X.; Fang, F.; Xie, Z.; Shang, J. K.; Jiang, J. Efficient FeCoNiCuPd thin-film electrocatalyst for alkaline oxygen and hydrogen evolution reactions. Appl. Catal. B: Environmental 2022, 313, 121472.
(36) Tymoczko, J.; Calle-Vallejo, F.; Schuhmann, W.; Bandarenka, A. S. Making the hydrogen evolution reaction in polymer electrolyte membrane electrolysers even faster. Nat. Commun. 2016, 7 (1), 1-6. Lačnjevac, U.; Vasilić, R.; Dobrota, A.; Đurđić, S.; Tomanec, O.; Zbořil, R.; Mohajernia, S.; Nguyen, N. T.; Skorodumova, N.; Manojlović, D.; et al. High-performance hydrogen evolution electrocatalysis using proton-intercalated TiO 2 nanotube arrays as interactive supports for Ir nanoparticles. J. Mater. Chem. A 2020, 8 (43), 22773-22790. Zhang, W.; Huang, B.; Wang, K.; Yang, W.; Lv, F.; Li, N.; Chao, Y.; Zhou, P.; Yang, Y.; Li, Y.; et al. WOx-Surface Decorated PtNi@ Pt Dendritic Nanowires as Efficient pH-Universal Hydrogen Evolution Electrocatalysts. Adv. Energy Mater. 2021, 11 (3), 2003192.
(37) Wendt, H.; Spinacé, E. V.; Oliveira Neto, A.; Linardi, M. J. Q. N. Electrocatalysis and electrocatalysts for low temperature fuel cells: fundamentals, state of the art, research and development. Quimica Nova 2005, 28 (6), 1066-1075. Koper, M. T. Thermodynamic theory of multi-electron transfer reactions: Implications for electrocatalysis.
(38) Sheng, W.; Myint, M.; Chen, J. G.; Yan, Y. J. E. Correlating the hydrogen evolution reaction activity in alkaline electrolytes with the
hydrogen binding energy on monometallic surfaces. Energy Environ. Sci. 2013, 6 (5), 1509-1512.
(39) Zheng, Y.; Jiao, Y.; Vasileff, A.; Qiao, S. Z. The hydrogen evolution reaction in alkaline solution: from theory, single crystal models, to practical electrocatalysts. Angew. Chem., Int. Ed. 2018, 57 (26), 7568-7579.
(40) Kim, J.; Jung, H.; Jung, S.-M.; Hwang, J.; Kim, D. Y.; Lee, N.; Kim, K.-S.; Kwon, H.; Kim, Y.-T.; Han, J. W.; et al. Tailoring binding abilities by incorporating oxophilic transition metals on 3D nanostructured Ni arrays for accelerated alkaline hydrogen evolution reaction. J. Am. Chem. Soc. 2021, 143 (3), 1399-1408. Ugwu, L. I.; Morgan, Y.; Ibrahim, H. Application of density functional theory and machine learning in heterogenous-based catalytic reactions for hydrogen production. Int. J. Hydrogen Energy 2022, 47 (4), 2245-2267.
(41) Ibn Shamsah, S. M. Earth-abundant electrocatalysts for water splitting: current and future directions. Catalysts 2021, 11 (4), 429.
(42) Krishtalik, L. I. Kinetic isotope effect in the hydrogen evolution reaction. Electrochimica acta 2001, 46 (19), 2949-2960. Qi, Q.; Shao, D.; Zhou, Y.; Wang, Q.; Yu, X.-Y. Plasma-induced implanting of active species in metal-organic frameworks for efficient hydrogen evolution reaction. Journal of Materials Chemistry A 2023, 11 (29), 15663-15669.
(43) Wu, H.; Feng, C.; Zhang, L.; Zhang, J.; Wilkinson, D. P. Nonnoble metal electrocatalysts for the hydrogen evolution reaction in water electrolysis. Electrochemical Energy Reviews 2021, 4(3), 473-507.
(44) Zhou, F.; Zhou, Y.; Liu, G.-G.; Wang, C.-T.; Wang, J. Recent advances in nanostructured electrocatalysts for hydrogen evolution reaction. Rare Metals 2021, 40 (12), 3375-3405. Chen, L.-W.; Liang, H.-W. Ir-based bifunctional electrocatalysts for overall water splitting. Catal. Sci. Technol. 2021, 11, 4673-4689.
(45) Zhang, X.-Y.; Xie, J.-Y.; Ma, Y.; Dong, B.; Liu, C.-G.; Chai, Y.-M. An overview of the active sites in transition metal electrocatalysts and their practical activity for hydrogen evolution reaction. Chem. Eng. J. 2022, 430, 132312.
(46) Theerthagiri, J.; Lee, S. J.; Murthy, A. P.; Madhavan, J.; Choi, M. Y. Fundamental aspects and recent advances in transition metal nitrides as electrocatalysts for hydrogen evolution reaction: A review. Curr. Opin. Solid State Mater. Sci. 2020, 24 (1), 100805.
(47) Ge, Z.; Fu, B.; Zhao, J.; Li, X.; Ma, B.; Chen, Y. A review of the electrocatalysts on hydrogen evolution reaction with an emphasis on Fe, Co and Ni-based phosphides. J. Mater. Sci. 2020, 55 (29), 1408114104.
(48) Anantharaj, S.; Kundu, S.; Noda, S. Progress in nickel chalcogenide electrocatalyzed hydrogen evolution reaction. Journal of Materials Chemistry A 2020, 8 (8), 4174-4192.
(49) Li, T.; Hu, T.; Dai, L.; Li, C. M. Metal-free photo-and electrocatalysts for hydrogen evolution reaction. Journal of Materials Chemistry A 2020, 8 (45), 23674-23698.
(50) Luo, W.; Wang, Y.; Cheng, C. Ru-based electrocatalysts for hydrogen evolution reaction: recent research advances and perspectives. Materials Today Physics 2020, 15, 100274. Ogundipe, T. O.; Shen, L.; Lu, Z.; Yan, C.; Yan, C. Recent Advances on Bimetallic Transition Metal Phosphides for Enhanced Hydrogen Evolution Reaction. ChemistrySelect 2022, 7 (23), No. e202200291.
(51) Chen, Q.; Yu, Y.; Li, J.; Nan, H.; Luo, S.; Jia, C.; Deng, P.; Zhong, S.; Tian, X. Recent Progress in Layered Double Hydroxide-Based Electrocatalyst for Hydrogen Evolution Reaction. ChemElectroChem. 2022, 9 (9), No. e202101387. Zhou, D.; Li, P.; Lin, X.; McKinley, A.; Kuang, Y.; Liu, W.; Lin, W.-F.; Sun, X.; Duan, X. Layered double hydroxide-based electrocatalysts for the oxygen evolution reaction: identification and tailoring of active sites, and superaerophobic nanoarray electrode assembly. Chem. Soc. Rev. 2021, 50 (15), 87908817.
(52) Chen, J.; Cheng, H.; Ding, L.-X.; Wang, H. Competing hydrogen evolution reaction: a challenge in electrocatalytic nitrogen fixation. Materials Chemistry Frontiers 2021, 5 (16), 5954-5969. Zhu, J.; Yang, R.; Zhang, G. Atomically thin transition metal dichalcogenides for the hydrogen evolution reaction. ChemPhysMater. 2022, 1, 102.
(53) Hansen, J. N.; Prats, H.; Toudahl, K. K.; Mørch Secher, N.; Chan, K.; Kibsgaard, J.; Chorkendorff, I. Is there anything better than Pt for
(54) Aggarwal, P.; Sarkar, D.; Awasthi, K.; Menezes, P. W. Functional role of single-atom catalysts in electrocatalytic hydrogen evolution: Current developments and future challenges. Coord. Chem. Rev. 2022, 452, 214289.
(55) Zahra, R.; Pervaiz, E.; Yang, M.; Rabi, O.; Saleem, Z.; Ali, M.; Farrukh, S. A review on nickel cobalt sulphide and their hybrids: Earth abundant, pH stable electro-catalyst for hydrogen evolution reaction. Int. J. Hydrogen Energy 2020, 45 (46), 24518-24543.
(56) Yang, W.; Chen, S. Recent progress in electrode fabrication for electrocatalytic hydrogen evolution reaction: A mini review. Chem. Eng. J. 2020, 393, 124726.
(57) Chen, S.; Pan, Y. Influence of group III and IV elements on the hydrogen evolution reaction of MoS2 disulfide. J. Phys. Chem. C 2021, 125 (22), 11848-11856.
(58) Feng, W.; Pang, W.; Xu, Y.; Guo, A.; Gao, X.; Qiu, X.; Chen, W. Transition metal selenides for electrocatalytic hydrogen evolution reaction. ChemElectroChem. 2020, 7(1), 31-54.
(59) Drosou, M.; Kamatsos, F.; Mitsopoulou, C. A. Recent advances in the mechanisms of the hydrogen evolution reaction by non-innocent sulfur-coordinating metal complexes. Inorganic Chemistry Frontiers 2020, 7 (1), 37-71.
(60) Liu, Y.; Wang, Q.; Zhang, J.; Ding, J.; Cheng, Y.; Wang, T.; Li, J.; Hu, F.; Yang, H. B.; Liu, B. Recent Advances in Carbon-Supported Noble-Metal Electrocatalysts for Hydrogen Evolution Reaction: Syntheses, Structures, and Properties. Adv. Energy Mater. 2022, 12, 2200928.
(61) Zheng, X.; Peng, L.; Li, L.; Yang, N.; Yang, Y.; Li, J.; Wang, J.; Wei, Z. J. C. s. Role of non-metallic atoms in enhancing the catalytic activity of nickel-based compounds for hydrogen evolution reaction. Chemical science 2018, 9 (7), 1822-1830.
(62) Kozhushner, A.; Zion, N.; Elbaz, L. J. C. O. i. E. Methods for assessment and measurement of the active site density in platinum group metal-free oxygen reduction reaction catalysts. Current Opinion in Electrochemistry 2021, 25, 100620. Carmo, M.; Fritz, D. L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38 (12), 4901-4934. Safizadeh, F.; Ghali, E.; Houlachi, G. Electrocatalysis developments for hydrogen evolution reaction in alkaline solutions-A review. Int. J. Hydrogen Energy 2015, 40 (1), 256-274.
(63) Markovića, N. M.; Sarraf, S. T.; Gasteiger, H. A.; Ross, P. N. Faraday Transactions. Hydrogen electrochemistry on platinum lowindex single-crystal surfaces in alkaline solution. J. Chem. Soc., Faraday Trans. 1996, 92 (20), 3719-3725. Gottesfeld, S. Fuel cell science: Theory, fundamentals, and biocatalysis; John Wiley & Sons: New York, 2011. Kobayashi, S.; Tryk, D.; Uchida, H. J. E. C. Enhancement of hydrogen evolution activity on Pt-skin/Pt3Co [(111),(100), and (110)] single crystal electrodes. Electrochem. Commun. 2020, 110, 106615.
(64) Lee, W.-J.; Wan, Z.; Kim, C.-M.; Oh, I.-K.; Harada, R.; Suzuki, K.; Choi, E.-A.; Kwon, S.-H. J. C. o. M. Atomic layer deposition of Pt thin films using dimethyl (
(65) Fu, H. Q.; Zhou, M.; Liu, P. F.; Liu, P.; Yin, H.; Sun, K. Z.; Yang, H. G.; Al-Mamun, M.; Hu, P.; Wang, H.-F.; et al. Hydrogen spilloverbridged Volmer/Tafel processes enabling ampere-level current density alkaline hydrogen evolution reaction under low overpotential. J. Am. Chem. Soc. 2022, 144 (13), 6028-6039. Lao, M.; Li, P.; Jiang, Y.; Pan, H.; Dou, S. X.; Sun, W. From Fundamentals and Theories to Heterostructured Electrocatalyst Design: An In-depth Understanding of Alkaline Hydrogen Evolution Reaction. Nano Energy 2022, 98, 107231.
(66) Zhai, L.; She, X.; Zhuang, L.; Li, Y.; Ding, R.; Guo, X.; Zhang, Y.; Zhu, Y.; Xu, K.; Fan, H. J.; et al. Modulating Built-In Electric Field via Variable Oxygen Affinity for Robust Hydrogen Evolution Reaction in Neutral Media. Angew. Chem., Int. Ed. 2022, 61 (14), No. e202116057. Chen, Y.; Ding, R.; Li, J.; Liu, J. Highly active atomically dispersed platinum-based electrocatalyst for hydrogen evolution reaction achieved by defect anchoring strategy. Appl. Catal. B: Environmental 2022, 301, 120830.
(67) Ghamami, S.; Kazemi Korayem, A.; Baqeri, N. Production and purification of titanium dioxide with titanium tetrachloride nanoparticles from eliminate concentrate of Kahnooj mine in Kerman. J. Appl. Chem. 2020, 15 (55), 189-206. Ren, B.; Jin, Q.; Li, Y.; Li, Y.; Cui, H.; Wang, C. Activating titanium dioxide as a new efficient electrocatalyst: from theory to experiment. ACS Appl. Mater. Interfaces 2020, 12 (10), 11607-11615.
(68) Kweon, D. H.; Okyay, M. S.; Kim, S.-J.; Jeon, J.-P.; Noh, H.-J.; Park, N.; Mahmood, J.; Baek, J.-B. Ruthenium anchored on carbon nanotube electrocatalyst for hydrogen production with enhanced Faradaic efficiency. Nat. Commun. 2020, 11 (1), 1-10.
(69) Bao, X.; Chen, Y.; Mao, S.; Wang, Y.; Yang, Y.; Gong, Y. Boosting the performance gain of
(70) Malik, B.; Anantharaj, S.; Karthick, K.; Pattanayak, D. K.; Kundu, S. J. C. S. Technology. Magnetic CoPt nanoparticle-decorated ultrathin
(71) Scofield, M. E.; Zhou, Y.; Yue, S.; Wang, L.; Su, D.; Tong, X.; Vukmirovic, M. B.; Adzic, R. R.; Wong, S. S. Role of chemical composition in the enhanced catalytic activity of Pt-based alloyed ultrathin nanowires for the hydrogen oxidation reaction under alkaline conditions. ACS Catal. 2016, 6 (6), 3895-3908.
(72) Santos, D.; Sequeira, C.; Macciò, D.; Saccone, A.; Figueiredo, J. Platinum-rare earth electrodes for hydrogen evolution in alkaline water electrolysis. International journal of hydrogen energy 2013, 38 (8), 31373145. Stojić, D. L.; Grozdić, T. D.; Kaninski, M. M.; Maksić, A. D.; Simić, N. D. Intermetallics as advanced cathode materials in hydrogen production via electrolysis. International journal of hydrogen energy 2006, 31 (7), 841-846.
(73) Naveen, M. H.; Huang, Y.; Bisalere Kantharajappa, S.; Seo, K.-D.; Park, D.-S.; Shim, Y.-B. Enhanced electrocatalytic activities of in situ produced
(74) Zhang, L.; Xiao, W.; Zhang, Y.; Han, F.; Yang, X. Nanocarbon encapsulating Ni-doped MoP/graphene composites for highly improved electrocatalytic hydrogen evolution reaction. Compos. Соттии. 2021, 26, 100792.
(75) Pu, M.; Wang, D.; Zhang, Z.; Guo, Y.; Guo, W. Flexoelectricity enhanced water splitting and hydrogen evolution reaction on grain boundaries of monolayer transition metal dichalcogenides. Nano Research 2022, 15 (2), 978-984.
(76) Lai, J.; Huang, B.; Chao, Y.; Chen, X.; Guo, S. J. A. M. Strongly Coupled Nickel-Cobalt Nitrides/Carbon Hybrid Nanocages with PtLike Activity for Hydrogen Evolution Catalysis. Adv. Mater. 2019, 31 (2), 1805541.
(77) Kuttiyiel, K. A.; Sasaki, K.; Chen, W.-F.; Su, D.; Adzic, R. R. Coreshell, hollow-structured iridium-nickel nitride nanoparticles for the hydrogen evolution reaction. J. Mater. Chem. A 2014, 2 (3), 591-594.
(78) Zhang, L.; Wang, T.; Sun, L.; Sun, Y.; Hu, T.; Xu, K.; Ma, F. Hydrothermal synthesis of 3D hierarchical MoSe 2/NiSe 2 composite nanowires on carbon fiber paper and their enhanced electrocatalytic
activity for the hydrogen evolution reaction. J. Mater. Chem. A 2017, 5 (37), 19752-19759.
(79) Wang, X.; Zheng, Y.; Yuan, J.; Shen, J.; Hu, J.; Wang, A.-j.; Wu, L.; Niu, L. J. E. A. Porous NiCo diselenide nanosheets arrayed on carbon cloth as promising advanced catalysts used in water splitting. Electrochim. Acta 2017, 225, 503-513.
(80) Chen, Q.; Wang, R.; Yu, M.; Zeng, Y.; Lu, F.; Kuang, X.; Lu, X. J. E. A. Bifunctional iron-nickel nitride nanoparticles as flexible and robust electrode for overall water splitting. Electrochim. Acta 2017, 247, 666— 673.
(81) Chen, Y.; Zhang, J.; Guo, P.; Liu, H.; Wang, Z.; Liu, M.; Zhang, T.; Wang, S.; Zhou, Y.; Lu, X. J. A. a. m.; et al. interfaces. Coupled heterostructure of
(82) Zhang, N.; Cao, L.; Feng, L.; Huang, J.; Kajiyoshi, K.; Li, C.; Liu, Q.; Yang, D.; He, J. J. N. Co, N-Codoped porous vanadium nitride nanoplates as superior bifunctional electrocatalysts for hydrogen evolution and oxygen reduction reactions. Nanoscale 2019, 11 (24), 11542-11549.
(83) Read, C. G.; Callejas, J. F.; Holder, C. F.; Schaak, R. E. J. A. a. m. interfaces. General strategy for the synthesis of transition metal phosphide films for electrocatalytic hydrogen and oxygen evolution. ACS applied materials 2016, 8 (20), 12798-12803.
(84) Pan, Y.; Lin, Y.; Chen, Y.; Liu, Y.; Liu, C. Cobalt phosphide-based electrocatalysts: synthesis and phase catalytic activity comparison for hydrogen evolution. J. Mater. Chem. A 2016, 4 (13), 4745-4754.
(85) Wang, M.-Q.; Tang, C.; Ye, C.; Duan, J.; Li, C.; Chen, Y.; Bao, S.J.; Xu, M. J. J. o. M. C. A. Engineering the nanostructure of molybdenum nitride nanodot embedded N -doped porous hollow carbon nanochains for rapid all pH hydrogen evolution. J. Mater. Chem. A 2018, 6 (30), 14734-14741.
(86) Callejas, J. F.; McEnaney, J. M.; Read, C. G.; Crompton, J. C.; Biacchi, A. J.; Popczun, E. J.; Gordon, T. R.; Lewis, N. S.; Schaak, R. E. J. A. n. Electrocatalytic and photocatalytic hydrogen production from acidic and neutral-pH aqueous solutions using iron phosphide nanoparticles. ACS Nano 2014, 8 (11), 11101-11107.
(87) Liu, Y.; Kelly, T. G.; Chen, J. G.; Mustain, W. E. J. A. C. Metal carbides as alternative electrocatalyst supports. ACS Catal. 2013, 3 (6), 1184-1194.
(88) Ramesh, R.; Nandi, D. K.; Kim, T. H.; Cheon, T.; Oh, J.; Kim, S.H. J. A. a. m. xsinterfaces. Atomic-layer-deposited MoN x thin films on three-dimensional Ni foam as efficient catalysts for the electrochemical hydrogen evolution reaction. ACS applied materials 2019, 11 (19), 17321-17332.
(89) Zhou, Y.; Yang, Y.; Wang, R.; Wang, X.; Zhang, X.; Qiang, L.; Wang, W.; Wang, Q.; Hu, Z. Rhombic porous CoP 2 nanowire arrays synthesized by alkaline etching as highly active hydrogen-evolutionreaction electrocatalysts. J. Mater. Chem. A 2018, 6 (39), 19038-19046.
(90) Jin, H.; Liu, X.; Vasileff, A.; Jiao, Y.; Zhao, Y.; Zheng, Y.; Qiao, S.Z. J. A. N. Single-crystal nitrogen-rich two-dimensional Mo5N6 nanosheets for efficient and stable seawater splitting. ACS Nano 2018, 12 (12), 12761-12769.
(91) Yu, M.; Zhao, S.; Feng, H.; Hu, L.; Zhang, X.; Zeng, Y.; Tong, Y.; Lu, X. J. A. E. L. Engineering thin MoS2 nanosheets on TiN nanorods: advanced electrochemical capacitor electrode and hydrogen evolution electrocatalyst. ACS Energy Lett. 2017, 2 (8), 1862-1868.
(92) Chen, T.; Tan, Y. J. N. R. Hierarchical CoNiSe2 nanoarchitecture as a high-performance electrocatalyst for water splitting. Nano Research 2018, 11 (3), 1331-1344.
(93) Jiao, C.; Bo, X.; Zhou, M. J. J. o. E. C. Electrocatalytic water splitting at nitrogen-doped carbon layers-encapsulated nickel cobalt selenide. J. Energy Chem. 2019, 34, 161-170.
(94) Liang, C.; Ying, P.; Li, C. J. C. o. m. Nanostructured
(95) Shi, Z.; Wang, Y.; Lin, H.; Zhang, H.; Shen, M.; Xie, S.; Zhang, Y.; Gao, Q.; Tang, Y. Porous nanoMoC@ graphite shell derived from a
(96) Cao, B.; Veith, G. M.; Neuefeind, J. C.; Adzic, R. R.; Khalifah, P. G . Mixed close-packed cobalt molybdenum nitrides as non-noble metal electrocatalysts for the hydrogen evolution reaction. J. Am. Chem. Soc. 2013, 135 (51), 19186-19192.
(97) Chen, W. F.; Schneider, J. M.; Sasaki, K.; Wang, C. H.; Schneider, J.; Iyer, S.; Iyer, S.; Zhu, Y.; Muckerman, J. T.; Fujita, E. J. C. Tungsten carbide-nitride on graphene nanoplatelets as a durable hydrogen evolution electrocatalyst. ChemSusChem 2014, 7 (9), 2414-2418.
(98) Fang, Z.; Peng, L.; Qian, Y.; Zhang, X.; Xie, Y.; Cha, J. J.; Yu, G. Dual tuning of Ni-Co-A (
(99) Wang, J.; Yang, W.; Liu, J. CoP 2 nanoparticles on reduced graphene oxide sheets as a super-efficient bifunctional electrocatalyst for full water splitting. J. Mater. Chem. A 2016, 4 (13), 4686-4690.
(100) Majhi, K. C.; Yadav, M. Fuels. Sphere-Shaped Bimetallic Sulphoselenide: An Efficient Electrocatalyst for Hydrogen Evolution Reaction. Energy 2021, 35 (15), 12473-12481. Li, D.; Liao, L.; Zhou, H.; Zhao, Y.; Cai, F.; Zeng, J.; Liu, F.; Wu, H.; Tang, D.; Yu, F. J. M. T. P. Highly active non-noble electrocatalyst from
(101) Lin, F.; Dong, Z.; Yao, Y.; Yang, L.; Fang, F.; Jiao, L. Electrocatalytic hydrogen evolution of ultrathin Co-Mo5N6 heterojunction with interfacial electron redistribution. Adv. Energy Mater. 2020, 10 (42), 2002176.
(102) Zhang, F.; Shan, W.; Hu, Q.; Jiang, W.; Li, D.; Zhang, B. The Effect of Mo Addition on Electrocatalytic Activity and Stability of Fe-Co-PC Metallic Glasses for Hydrogen Evolution. J. Electrochem. Soc. 2021, 168 (7), 076510. Pentland, N.; Bockris, J. M.; Sheldon, E. Hydrogen evolution reaction on copper, gold, molybdenum, palladium, rhodium, and iron: mechanism and measurement technique under high purity conditions. J. Electrochem. Soc. 1957, 104 (3), 182.
(103) Hu, X.; Tian, X.; Lin, Y.-W.; Wang, Z. J. R. a. Nickel foam and stainless steel mesh as electrocatalysts for hydrogen evolution reaction, oxygen evolution reaction and overall water splitting in alkaline media. RSC Adv. 2019, 9 (54), 31563-31571. Lin, Y.; Sun, K.; Chen, X.; Chen, C.; Pan, Y.; Li, X.; Zhang, J.. High-precision regulation synthesis of Fedoped Co 2 P nanorod bundles as efficient electrocatalysts for hydrogen evolution in all-pH range and seawater. J. Energy Chem. 2021, 55, 92101.
(104) Mahmood, N.; Yao, Y.; Zhang, J. W.; Pan, L.; Zhang, X.; Zou, J. J. Electrocatalysts for hydrogen evolution in alkaline electrolytes: mechanisms, challenges, and prospective solutions. Adv. Sci. 2018, 5 (2), 1700464.
(105) Ali Shah, S.; Xu, L.; Sayyar, R.; Bian, T.; Liu, Z.; Yuan, A.; Shen, X.; Khan, I.; Ali Tahir, A.; Ullah, H. J. C. E. J. Growth of MoS2 nanosheets on
(106) Müller, C. I.; Rauscher, T.; Schmidt, A.; Schubert, T.; Weißgärber, T.; Kieback, B.; Röntzsch, L. Electrochemical investigations on amorphous Fe-base alloys for alkaline water electrolysis. Int. J. Hydrogen Energy 2014, 39 (17), 8926-8937. Schäfer, H.; Chatenet, M. J. A. E. L. Steel: the resurrection of a forgotten watersplitting catalyst. ACS Energy Lett. 2018, 3 (3), 574-591.
(107) Ekspong, J.; Wågberg, T. J. M. Stainless steel as a bi-functional electrocatalyst-A top-down approach. Materials 2019, 12 (13), 2128. Benavente Llorente, V.; Diaz, L. A.; Lacconi, G. I.; Abuin, G. C.;
(108) Kim, M.; Ha, J.; Shin, N.; Kim, Y.-T.; Choi, J. J. E. A. Selfactivated anodic nanoporous stainless steel electrocatalysts with high durability for the hydrogen evolution reaction. Electrochim. Acta 2020, 364, 137315.
(109) Liu, X.; You, B.; Sun, Y. J. A. S. C. Engineering. Facile surface modification of ubiquitous stainless steel led to competent electrocatalysts for overall water splitting. ACS Sustainable Chemistry 2017, 5 (6), 4778-4784. Zayat, B.; Mitra, D.; Narayanan, S. R. Inexpensive and efficient alkaline water electrolyzer with robust steel-based electrodes. J. Electrochem. Soc. 2020, 167 (11), 114513. Gao, Y.; Xiong, T.; Li, Y.; Huang, Y.; Li, Y.; Balogun, M.-S. J. T. A simple and scalable approach to remarkably boost the overall water splitting activity of stainless steel electrocatalysts. ACS Omega 2019, 4 (14), 16130-16138.
(110) Yang, C.; Zhao, R.; Xiang, H.; Wu, J.; Zhong, W.; Li, W.; Zhang, Q.; Yang, N.; Li, X. Ni-activated transition metal carbides for efficient hydrogen evolution in acidic and alkaline solutions. Adv. Energy Mater. 2020, 10 (37), 2002260. Jun, H.; Kim, S.; Lee, J. Development strategies in transition metal carbide for hydrogen evolution reaction: A review. Korean Journal of Chemical Engineering 2020, 37 (8), 13171330. Peng, O.; Hu, Q.; Zhou, X.; Zhang, R.; Du, Y.; Li, M.; Ma, L.; Xi, S.; Fu, W.; Xu, Z.-X.; et al. Swinging hydrogen evolution to nitrate reduction activity in molybdenum carbide by ruthenium doping. ACS Catal. 2022, 12 (24), 15045-15055.
(111) Chen, P.; Ye, J.; Wang, H.; Ouyang, L.; Zhu, M. Recent progress of transition metal carbides/nitrides for electrocatalytic water splitting. J. Alloys Compd. 2021, 883, 160833. He, C.; Tao, J. Transition metal carbides coupled with nitrogen-doped carbon as efficient and stable
(112) Zhang, X.; Zhu, Z.; Liang, X.; Ma, F.-X.; Zhang, J.; Tan, Y.; Pan, Z.; Bo, Y.; Wu, C.-M. L. Encapsulating dual-phased Mo2C-WC nanocrystals into ultrathin carbon nanosheet assemblies for efficient electrocatalytic hydrogen evolution. Chem. Eng. J. 2021, 408, 127270. Yan, P.; Wu, Y.; Wei, X.; Zhu, X.; Su, W. Preparation of robust hydrogen evolution reaction electrocatalyst WC/C by molten salt. Nanomaterials 2020, 10 (9), 1621. Hu, Z.; Chen, J.; Pan, P.; Liu, C.; Zeng, J.; Ou, Y.; Qi, X.; Liang, T. Porous N-doped Mo2C@ C nanoparticles for highperformance hydrogen evolution reaction. Int. J. Hydrogen Energy 2022, 47 (7), 4641-4652.
(113) Lv, Z.; Liu, D.; Tian, W.; Dang, J. Designed synthesis of WCbased nanocomposites as low-cost, efficient and stable electrocatalysts for the hydrogen evolution reaction. CrystEngComm 2020, 22 (27), 4580-4590.
(114) Wang, F.; Wu, Y.; Dong, B.; Lv, K.; Shi, Y.; Ke, N.; Hao, L.; Yin, L.; Bai, Y.; Xu, X.; et al. Robust Porous WC-Based Self-Supported Ceramic Electrodes for High Current Density Hydrogen Evolution Reaction. Adv. Sci. 2022, 9, 2106029 . Huang, J.; Jian, C.; Cai, Q.; Hong, W.; Liu, W. A large scale self-supported WP-W 2 C nanoporous network for efficient hydrogen evolution reaction in alkaline media.
(115) Morishita, M.; Nozaki, A.; Yamamoto, H.; Fukumuro, N.; Mori, M.; Araki, K.; Sakamoto, F.; Nakamura, A.; Yanagita, H. Catalytic activity of Co-nanocrystal-doped tungsten carbide arising from an internal magnetic field. RSC Adv. 2021, 11 (23), 14063-14070. Tian, D.; Denny, S. R.; Li, K.; Wang, H.; Kattel, S.; Chen, J. G. Density functional theory studies of transition metal carbides and nitrides as electrocatalysts. Chem. Soc. Rev. 2021, 50, 12338.
(116) Ma, L.; Ting, L. R. L.; Molinari, V.; Giordano, C.; Yeo, B. S. Efficient hydrogen evolution reaction catalyzed by molybdenum carbide and molybdenum nitride nanocatalysts synthesized via the
urea glass route. Journal of Materials Chemistry A 2015, 3 (16), 83618368.
(117) Liu, W.; Wang, X.; Wang, F.; Du, K.; Zhang, Z.; Guo, Y.; Yin, H.; Wang, D. A durable and pH -universal self-standing MoC-Mo2C heterojunction electrode for efficient hydrogen evolution reaction. Nat. Commun. 2021, 12 (1), 1-10.
(118) Ji, X.; Wang, K.; Zhang, Y.; Sun, H.; Zhang, Y.; Ma, T.; Ma, Z.; Hu, P.; Qiu, Y. MoC based Mott-Schottky electrocatalyst for boosting the hydrogen evolution reaction performance. Sustainable Energy & Fuels 2020, 4 (1), 407-416.
(119) Hu, W.; Zheng, M.; Xu, B.; Wei, Y.; Zhu, W.; Li, Q.; Pang, H. Design of hollow carbon-based materials derived from metal-organic frameworks for electrocatalysis and electrochemical energy storage. Journal of Materials Chemistry A 2021, 9 (7), 3880-3917.
(120) Chen, Y.; Li, T.; Zhao, Q.; Liu, D.; Li, C. M. The in situ preparation of iron phosphide using ionic liquids as iron and phosphorus sources for efficient hydrogen evolution reactions. RSC Adv. 2020, 10 (55), 33026-33032. Zhou, Q.; Wang, D. 3D nanoporous NiCoP as a highly efficient electrocatalyst for the hydrogen evolution reaction in alkaline electrolyte. New J. Chem. 2022, 46 (16), 74907496.
(121) Kumari, A.; Kaushal, S.; Singh, P. P. Bimetallic metal organic frameworks heterogeneous catalysts: Design, construction, and applications. Materials Today Energy 2021, 20, 100667. Sirisomboonchai, S.; Kitiphatpiboon, N.; Chen, M.; Li, S.; Li, X.; Kongparakul, S.; Samart, C.; Zhang, L.; Abudula, A.; Guan, G. MultiHierarchical Porous Mn-Doped CoP Catalyst on Nickel Phosphide Foam for Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2022, 5 (1), 149-158. Park, Y.; Kang, H.; Hong, Y.-k.; Cho, G.; Choi, M.; Cho, J.; Ha, D.-H. Influence of the phosphorus source on iron phosphide nanoparticle synthesis for hydrogen evolution reaction catalysis. Int. J. Hydrogen Energy 2020, 45 (57), 32780-32788.
(122) Zhang, C.; Li, D.; Xu, Y. Mn-doped NiP: Facile synthesis and enhanced electrocatalytic activity for hydrogen evolution. J. Mater. Res. 2022, 37 (3), 807-817.
(123) Wang, Y.; Wang, Y.; Bai, J.; Lau, W.-M. Trace Amount of NiP2 Cooperative CoMoP Nanosheets Inducing Efficient Hydrogen Evolution. ACS omega 2021, 6 (48), 33057-33066.
(124) Ge, L.; Yuan, H.; Min, Y.; Li, L.; Chen, S.; Xu, L.; Goddard III, W. A. Predicted optimal bifunctional electrocatalysts for the hydrogen evolution reaction and the oxygen evolution reaction using chalcogenide heterostructures based on machine learning analysis of in silico quantum mechanics based high throughput screening. J. Phys. Chem. Lett. 2020, 11 (3), 869-876.
(125) Tseng, C.-A.; Lee, C.-P. Transition Metal Chalcogenides for the Electrocatalysis of Water. In Advanced Functional Materials; IntechOpen: London, 2020. Iffelsberger, C.; Ng, S.; Pumera, M. Catalyst coating of 3D printed structures via electrochemical deposition: Case of the transition metal chalcogenide MoSx for hydrogen evolution reaction. Appl. Mater. Today 2020, 20, 100654. Moschkowitsch, W.; Lori, O.; Elbaz, L. Recent Progress and Viability of PGM-Free Catalysts for Hydrogen Evolution Reaction and Hydrogen Oxidation Reaction. ACS Catal. 2022, 12 (2), 1082-1089.
(126) Ali, T.; Qiao, W.; Zhang, D.; Liu, W.; Sajjad, S.; Yan, C.; Su, R. Surface sulfur vacancy engineering of metal sulfides promoted desorption of hydrogen atoms for enhanced electrocatalytic hydrogen evolution. J. Phys. Chem. C 2021, 125 (23), 12707-12712. Duraisamy, S.; Ganguly, A.; Sharma, P. K.; Benson, J.; Davis, J.; Papakonstantinou, P. One-step hydrothermal synthesis of phase-engineered
(127) Mei, X.; Li, C.; Lam, F. L.-Y.; Hu, X. Nanosheet-like ternary metal sulfide as a pH -universal catalyst for the hydrogen evolution reaction. ACS Appl. Energy Mater. 2020, 3 (7), 6172-6179. Aslan, E.; Yanalak, G.; Hatay Patir, I. Enhanced Hydrogen Evolution Reaction Catalysis at Template-Free Liquid/Liquid Interfaces by In Situ Electrodeposited Amorphous Molybdenum Sulfide on Carbon Nanotubes. ACS Appl. Energy Mater. 2021, 4 (8), 8330-8339.
(128) Wang, S.; Huang, B.; Dai, Y.; Wei, W. Origin of the Enhanced Hydrogen Evolution Reaction Activity of Grain Boundaries in MoS2Monolayers. J. Phys. Chem. C 2022, 126 (14), 6215-6222. Zhou, W.; Dong, L.; Tan, L.; Tang, Q. First-principles study of sulfur vacancy concentration effect on the electronic structures and hydrogen evolution reaction of MoS2. Nanotechnology 2021, 32 (14), 145718.
(129) He, M.; Kong, F.; Yin, G.; Lv, Z.; Sun, X.; Shi, H.; Gao, B. Enhanced hydrogen evolution reaction activity of hydrogen-annealed vertical MoS 2 nanosheets. RSC Adv. 2018, 8 (26), 14369-14376.
(130) Zhu, J.; Tu, Y.; Cai, L.; Ma, H.; Chai, Y.; Zhang, L.; Zhang, W. Defect-Assisted Anchoring of Pt Single Atoms on MoS2 Nanosheets Produces High-Performance Catalyst for Industrial Hydrogen Evolution Reaction. Small 2022, 18 (4), 2104824. Zheng, J.; Lu, L.; Lebedev, K.; Wu, S.; Zhao, P.; McPherson, I. J.; Wu, T.-S.; Kato, R.; Li, Y.; Ho, P.-L.; et al. Fe on molecular-layer MoS2 as inorganic Fe-S2-Mo motifs for light-driven nitrogen fixation to ammonia at elevated temperatures. Chem. Catalysis 2021, 1 (1), 162-182.
(131) Zheng, Z.; Yu, L.; Gao, M.; Chen, X.; Zhou, W.; Ma, C.; Wu, L.; Zhu, J.; Meng, X.; Hu, J.; et al. Boosting hydrogen evolution on MoS2 via co-confining selenium in surface and cobalt in inner layer. Nat. Commun. 2020, 11 (1), 1-10.
(132) Kazemi Korayem, A.; Ghamami, S.; Bahrami, Z. Fractal properties and morphological investigation of nano-amiodarone using image processing. Signal Image Video Process. 2019, 13 (2), 281-287. Guo, B.; Yu, K.; Li, H.; Qi, R.; Zhang, Y.; Song, H.; Tang, Z.; Zhu, Z.; Chen, M. Coral-shaped MoS2 decorated with graphene quantum dots performing as a highly active electrocatalyst for hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2017, 9 (4), 3653-3660. Park, T.; Bae, C.; Lee, H.; Leem, M.; Kim, H.; Ahn, W.; Kim, J.; Lee, E.; Shin, H.; Kim, H. Non-equilibrium fractal growth of MoS 2 for electrocatalytic hydrogen evolution. CrystEngComm 2019, 21 (3), 478-486. Kazemi Korayem, A.; Ghamami, S.; Bahrami, Z. Fractal properties and morphological investigation of Nano hydrochlorothiazide is used to treat hypertension. BMC Pharmacol. Toxicol. 2018, 19, 1-9.
(133) Ge, Y.; Shi, Z.; Tan, C.; Chen, Y.; Cheng, H.; He, Q.; Zhang, H. Two-dimensional nanomaterials with unconventional phases. Chem. 2020, 6 (6), 1237-1253. Ma, X.; Yang, J.; Xu, X.; Yang, H.; Peng, C.
(134) Deng, W.; Xie, W.; Li, D.; Gai, Y.; Chen, Z.; Yu, J.; Yang, R.; Bao, X.; Jiang, F. Controllable tuning of polymetallic Co-Ni-Ru-S-Se ultrathin nanosheets to boost electrocatalytic oxygen evolution. NPG Asia Mater. 2022, 14 (1), 1-13.
(135) Wang, K.; Lin, Z.; Tang, Y.; Tang, Z.; Tao, C.-L.; Qin, D.-D.; Tian, Y. Selenide/sulfide heterostructured NiCo2Se4/NiCoS4 for oxygen evolution reaction, hydrogen evolution reaction, water splitting and Zn -air batteries. Electrochim. Acta 2021, 368, 137584. Pang, Y.; Zhu, S.; Cui, Z.; Liang, Y.; Li, Z.; Wu, S. Self-supported amorphous nanoporous nickel-cobalt phosphide catalyst for hydrogen evolution reaction. Progress in Natural Science: Materials International 2021, 31 (2), 201-206.
(136) Xiao, D.; Bao, D.-L.; Liang, X.; Wang, Y.; Shen, J.; Cheng, C.; Chu, P. K. Experimental and theoretical investigation of the control and balance of active sites on oxygen plasma-functionalized MoSe2 nanosheets for efficient hydrogen evolution reaction. Appl. Catal. B: Environmental 2021, 288, 119983.
(137) Chang, Y.; Chen, C.; Ho, C.; Cheng, C.; Chen, H.; Fu, T.; Huang, Y.; Ke, S.; Du, H.; Lee, K.; et al. Surface electron accumulation and enhanced hydrogen evolution reaction in MoSe2 basal planes. Nano Energy 2021, 84, 105922.
(138) Yang, X.; Liu, W.; Han, C.; Zhao, C.; Tang, H.; Liu, Q.; Xu, J. Mechanistic insights into charge carrier dynamics in MoSe2/CdS heterojunctions for boosted photocatalytic hydrogen evolution. Materials Today Physics 2020, 15, 100261. Ruqia, B.; Kabiraz, M. K.; Hong, J. W.; Choi, S.-I. Catalyst activation: Surface doping effects of group VI transition metal dichalcogenides towards hydrogen evolution reaction in acidic media. J. Energy Chem. 2022, 72, 217.
(139) Wu, P.; Sun, G.; Chen, Y.; Xu, W.; Zheng, H.; Xu, J.; Wang, L.; Peng, D.-L. MoSe2-Ni3Se4 hybrid nanoelectrocatalysts and their enhanced electrocatalytic activity for hydrogen evolution reaction. Nanoscale Res. Lett. 2020, 15 (1), 1-10.
(140) Hu, W.; Shi, Q.; Chen, Z.; Yin, H.; Zhong, H.; Wang, P. Co2N/ Co 2 Mo 3 O 8 heterostructure as a highly active electrocatalyst for an alkaline hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2021, 13 (7), 8337-8343. Dan, Z.; Liang, W.; Gong, X.; Lin, X.; Zhang, W.; Le, Z.; Xie, F.; Chen, J.; Yang, M.; Wang, N.; et al. Substitutional Doping Engineering toward W2N Nanorod for Hydrogen Evolution Reaction at High Current Density. ACS Mater. Lett. 2022, 4 (7), 13741380.
(141) Tong, R.; Xu, M.; Huang, H.; Zhang, C.; Ma, Y.; Wang, X.; Hu, X.; Qu, Y.; Wang, S.; Pan, H. Co2N0. 67/MoO2 Heterostructure as High-Efficiency Electrocatalysts for the Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2022, 5 (1), 440-448. Xing, Z.; Li, Q.; Wang, D.; Yang, X.; Sun, X. Self-supported nickel nitride as an efficient highperformance three-dimensional cathode for the alkaline hydrogen evolution reaction. Electrochim. Acta 2016, 191, 841-845.
(142) Zhou, W.; Huang, S.; Sun, C. Ni3Mo3N coupled with nitrogenrich carbon microspheres as an efficient hydrogen evolution reaction catalyst and electrochemical sensor for H 2 O 2 detection. Int. J. Hydrogen Energy 2022, 47 (33), 14906-14915.
(143) Park, S. H.; Kang, S. H.; Youn, D. H. Direct One-Step Growth of Bimetallic Ni2Mo3N on Ni Foam as an Efficient Oxygen Evolution Electrocatalyst. Materials 2021, 14 (16), 4768.
(144) Wang, C.; Lv, X.; Zhou, P.; Liang, X.; Wang, Z.; Liu, Y.; Wang, P.; Zheng, Z.; Dai, Y.; Li, Y. Molybdenum nitride electrocatalysts for hydrogen evolution more efficient than platinum/carbon: Mo2N/ CeO2@ nickel foam. ACS Appl. Mater. Interfaces 2020, 12 (26), 29153-29161.
(145) Balaji, D.; Madhavan, J.; AlSalhi, M. S.; Aljaafreh, M. J.; Prasad, S.; Show, P. L. Carbon supported
(146) Zhang, J.; Zhang, L.; Du, L.; Xin, H. L.; Goodenough, J. B.; Cui, Z. Composition-Tunable Antiperovskite CuxIn1- xNNi3 as Superior Electrocatalysts for the Hydrogen Evolution Reaction. Angew. Chem., Int. Ed. 2020, 59 (40), 17488-17493.
(147) Dastafkan, K.; Shen, X.; Hocking, R. K.; Meyer, Q.; Zhao, C. Monometallic interphasic synergy via nano-hetero-interfacing for hydrogen evolution in alkaline electrolytes. Nat. Commun. 2023, 14 (1), 547.
(148) Wang, H.-F.; Chen, L.; Pang, H.; Kaskel, S.; Xu, Q. MOFderived electrocatalysts for oxygen reduction, oxygen evolution and hydrogen evolution reactions. Chem. Soc. Rev. 2020, 49 (5), 14141448. Sahu, N.; Das, J. K.; Behera, J. NiSe2 Nanoparticles Encapsulated in N-Doped Carbon Matrix Derived from a One-Dimensional NiMOF: An Efficient and Sustained Electrocatalyst for Hydrogen Evolution Reaction. Inorg. Chem. 2022, 61 (6), 2835-2845. Aleksandrzak, M.; Sielicki, K.; Mijowska, E. Enhancement of photocatalytic hydrogen evolution with catalysts based on carbonized MOF-5 and gC 3 N 4. RSC Adv. 2020, 10 (7), 4032-4039.
(149) Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J. M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nature materials 2009, 8 (1), 76-80. Sprick, R. S.; Bonillo, B.; Clowes, R.; Guiglion, P.; Brownbill, N. J.; Slater, B. J.; Blanc, F.; Zwijnenburg, M. A.; Adams, D. J.; Cooper, A. I. Visible-light-driven hydrogen evolution using planarized conjugated polymer photocatalysts. Angew. Chem., Int. Ed. 2016, 55 (5), 1792-1796. Rahman, M. Z.; Davey, K.; Qiao, S.-Z. Carbon, nitrogen and phosphorus containing metal-free photocatalysts for hydrogen production: progress and challenges. J. Mater. Chem. A 2018, 6 (4), 1305-1322. Gopi, S.; Panda, A.; Ramu, A.; Theerthagiri, J.; Kim, H.; Yun, K. J. Bifunctional electrocatalysts for water splitting from a bimetallic (V doped-NixFey) Metal-Organic framework MOF@ Graphene oxide composite. Int. J. Hydrogen Energy 2022, 47, 42122. Liu, Y.; Cheng, H.; Cheng, M.; Liu, Z.; Huang, D.; Zhang, G.; Shao, B.; Liang, Q.; Luo, S.; Wu, T. J. C. E. J.; et al. The application of Zeolitic
imidazolate frameworks (ZIFs) and their derivatives based materials for photocatalytic hydrogen evolution and pollutants treatment. Chem. Eng. J. 2021, 417, 127914.
(150) Zhu, B.; Qu, C.; Gao, S.; Liang, Z.; Zhang, H.; Zou, R. J. C. Ultralow Loading Ruthenium Nanoparticles on Nitrogen-Doped Graphene Aerogel for Trifunctional Electrocatalysis. ChemCatChem. 2018, 10 (5), 1113-1121. Zhu, B.; Wen, D.; Liang, Z.; Zou, R. J. C. C. R. Conductive metal-organic frameworks for electrochemical energy conversion and storage. Coord. Chem. Rev. 2021, 446, 214119.
(151) Gao, H.; Shen, H.; Wu, H.; Jing, H.; Sun, Y.; Liu, B.; Chen, Z.; Song, J.; Lu, L.; Wu, Z. J. E.; et al. Review of Pristine Metal-Organic Frameworks for Supercapacitors: Recent Progress and Perspectives. Energy Fuels 2021, 35 (16), 12884-12901. Qiu, T.; Gao, S.; Liang, Z.; Wang, D. G.; Tabassum, H.; Zhong, R.; Zou, R. J. A. C. I. E. Pristine Hollow Metal-Organic Frameworks: Design, Synthesis and Application. Angew. Chem., Int. Ed. 2021, 60, 17314.
(152) Sonowal, K.; Saikia, L. Metal-organic frameworks and their composites for fuel and chemical production via CO 2 conversion and water splitting. RSC Adv. 2022, 12 (19), 11686-11707. Li, P.; Zheng, D.; Gao, M.; Zuo, X.; Sun, L.; Zhou, Q.; Lin, J. Bimetallic MOFTemplated Fabrication of Porous
(153) Ao, K.; Wei, Q.; Daoud, W. A. MOF-derived sulfide-based electrocatalyst and scaffold for boosted hydrogen production. ACS Appl. Mater. Interfaces 2020, 12 (30), 33595-33602. Qi, W.; Wang, C.; Yu, J.; Adimi, S.; Thomas, T.; Guo, H.; Liu, S.; Yang, M. MOF-Derived Porous Ternary Nickel Iron Nitride Nanocube as a Functional Catalyst toward Water Splitting Hydrogen Evolution for Solar to Chemical Energy Conversion. ACS Appl. Energy Mater. 2022, 5, 6155.
(154) Kazemi, A.; Moghadaskhou, F.; Pordsari, M. A.; Manteghi, F.; Tadjarodi, A.; Ghaemi, A. Enhanced CO2 capture potential of UiO-66NH2 synthesized by sonochemical method: experimental findings and performance evaluation. Sci. Rep. 2023, 13 (1), 19891. Ramezanalizadeh, H.; Manteghi, F. Mixed cobalt/nickel metal-organic framework, an efficient catalyst for one-pot synthesis of substituted imidazoles. Monatshefte für Chemie-Chemical Monthly 2017, 148, 347355. Ramezanalizadeh, H.; Manteghi, F. Synthesis of a novel MOF/ CuWO4 heterostructure for efficient photocatalytic degradation and removal of water pollutants. Journal of Cleaner Production 2018, 172, 2655-2666. Abhari, P. S.; Manteghi, F.; Tehrani, Z. Adsorption of lead ions by a green AC/HKUST-1 nanocomposite. Nanomaterials 2020, 10 (9), 1647.
(155) Li, Y.-W.; Wu, Q.; Ma, R.-C.; Sun, X.-Q.; Li, D.-D.; Du, H.-M.; Ma, H.-Y.; Li, D.-C.; Wang, S.-N.; Dou, J.-M. A Co-MOF-derived Co 9 S 8@ NS-C electrocatalyst for efficient hydrogen evolution reaction. RSC Adv. 2021, 11 (11), 5947-5957. Sun, Y.; Xue, Z.; Liu, Q.; Jia, Y.; Li, Y.; Liu, K.; Lin, Y.; Liu, M.; Li, G.; Su, C.-Y. Modulating electronic structure of metal-organic frameworks by introducing atomically dispersed Ru for efficient hydrogen evolution. Nat. Commun. 2021, 12 (1), 1369.
(156) Yan, L.; Cao, L.; Dai, P.; Gu, X.; Liu, D.; Li, L.; Wang, Y.; Zhao, X. J. A. F. M. Metal-organic frameworks derived nanotube of nickelcobalt bimetal phosphides as highly efficient electrocatalysts for overall water splitting. Adv. Funct. Mater. 2017, 27 (40), 1703455.
(157) Hao, X.; Jin, Z.; Yang, H.; Lu, G.; Bi, Y. J. A. C. B. E. Peculiar synergetic effect of MoS2 quantum dots and graphene on MetalOrganic Frameworks for photocatalytic hydrogen evolution. Appl. Catal. B.: Environ. 2017, 210, 45-56.
(158) Zhen, W.; Ma, J.; Lu, G. Small-sized Ni (lll) particles in metal-organic frameworks with low over-potential for visible photocatalytic hydrogen generation. Appl. Catal. B: Environmental 2016, 190, 12-25.
(159) Li, Y.; Ai, C.; Deng, S.; Wang, Y.; Tong, X.; Wang, X.; Xia, X.; Tu, J. Nitrogen doped vertical graphene as metal-free electrocatalyst for hydrogen evolution reaction. Mater. Res. Bull. 2021, 134, 111094.
- Received: October 10, 2023
Revised: December 28, 2023
Accepted: December 29, 2023
Published: January 29, 2024
