DOI: https://doi.org/10.1016/j.ijpharm.2024.124071
PMID: https://pubmed.ncbi.nlm.nih.gov/38554738
تاريخ النشر: 2024-03-29
بارافيلم® M وسترات-م® كمحاكيات للجلد في اختراق المختبر للرقع الدقيقة القابلة للذوبان المحملة بالبروتينات
نسخة الناشر بصيغة PDF، والمعروفة أيضًا باسم النسخة المسجلة
رابط إلى سجل النشر في بوابة أبحاث جامعة كوينز بلفاست
حقوق الطبع والنشر 2024 المؤلفون.
هذه مقالة مفتوحة الوصول نُشرت بموجب ترخيص المشاع الإبداعي للاستخدام مع الإشارة إلى المؤلفhttps://creativecommons.org/licenses/by/4.0/الذي يسمح بالاستخدام غير المقيد، والتوزيع، والاستنساخ في أي وسيلة، بشرط ذكر المؤلف والمصدر.
حقوق النشر للمطبوعات المتاحة عبر بوابة أبحاث جامعة كوينز بلفاست محفوظة من قبل المؤلفين و/أو مالكي حقوق النشر الآخرين، ومن شروط الوصول إلى هذه المطبوعات أن يعترف المستخدمون ويلتزموا بالمتطلبات القانونية المرتبطة بهذه الحقوق.
بوابة البحث هي المستودع المؤسسي لجامعة كوينز الذي يوفر الوصول إلى مخرجات أبحاث جامعة كوينز. تم بذل كل جهد لضمان أن المحتوى في بوابة البحث لا ينتهك حقوق أي شخص، أو القوانين البريطانية المعمول بها. إذا اكتشفت محتوى في بوابة البحث تعتقد أنه ينتهك حقوق الطبع والنشر أو يخالف أي قانون، يرجى الاتصال بـopenaccess@qub.ac.uk.
بارافيلم
معلومات المقال
الكلمات المفتاحية:
سترات-م®
جلد خنزير مقطع بالدرماتوم
بروتين
دراسات الإدخال
دراسات النفاذية في المختبر
الملخص
تلعب دراسات النفاذية في المختبر دورًا حاسمًا في تحسين الصيغ في المراحل المبكرة قبل إجراء تحقيقات واسعة النطاق على نماذج حيوانية. تُستخدم الأغشية البيولوجية عادةً في هذه الدراسات لمحاكاة ظروف جلد الإنسان بدقة. ومع ذلك، عند التركيز على توصيل البروتينات والبيبتيدات عبر الجلد، يمكن أن يؤدي استخدام الأغشية البيولوجية إلى تعقيد عمليات التحليل والتquantification. تهدف هذه الدراسة إلى استكشاف بارافيلم.
1. المقدمة
تم استخدام حقن الأجسام المضادة والببتيدات، مما يسمح بالإدارة الذاتية المحتملة من قبل المرضى في المنزل (Doughty et al., 2016; Bittner et al., 2018). ومع ذلك، يسعى المرضى إلى بدائل في شكل أشكال جرعات يمكن إدارتها ذاتيًا، خالية من الألم، وقليلة التوغل لتقليل زيارات المستشفى وتخفيف العبء على الرعاية الصحية المرتبطة بالعلاج (Anjani et al., 2023a). يظهر توصيل الأدوية عبر الجلد، وخاصة من خلال لاصقات الميكروأري (MAPs)، كحل محتمل. تهدف تقنية MAP، التي تم بحثها بشكل مكثف في الطب الحيوي، إلى تسهيل توصيل الأدوية واللقاحات بشكل فعال (Donnelly and Prausnitz, 2023; Vora et al., 2023). تعمل MAPs عن طريق اختراق الطبقة القرنية (SC) بدون ألم، مما يخلق قنوات دقيقة تساعد في انتشار الدواء إلى طبقات الجلد الأعمق (Anjani et al., 2022a). تتجاوز هذه الطريقة تحديات انتشار الدواء السلبي، مما يسمح بتوصيل مجموعة واسعة من المواد الكيميائية.
2. المواد والأساليب
2.1. المواد
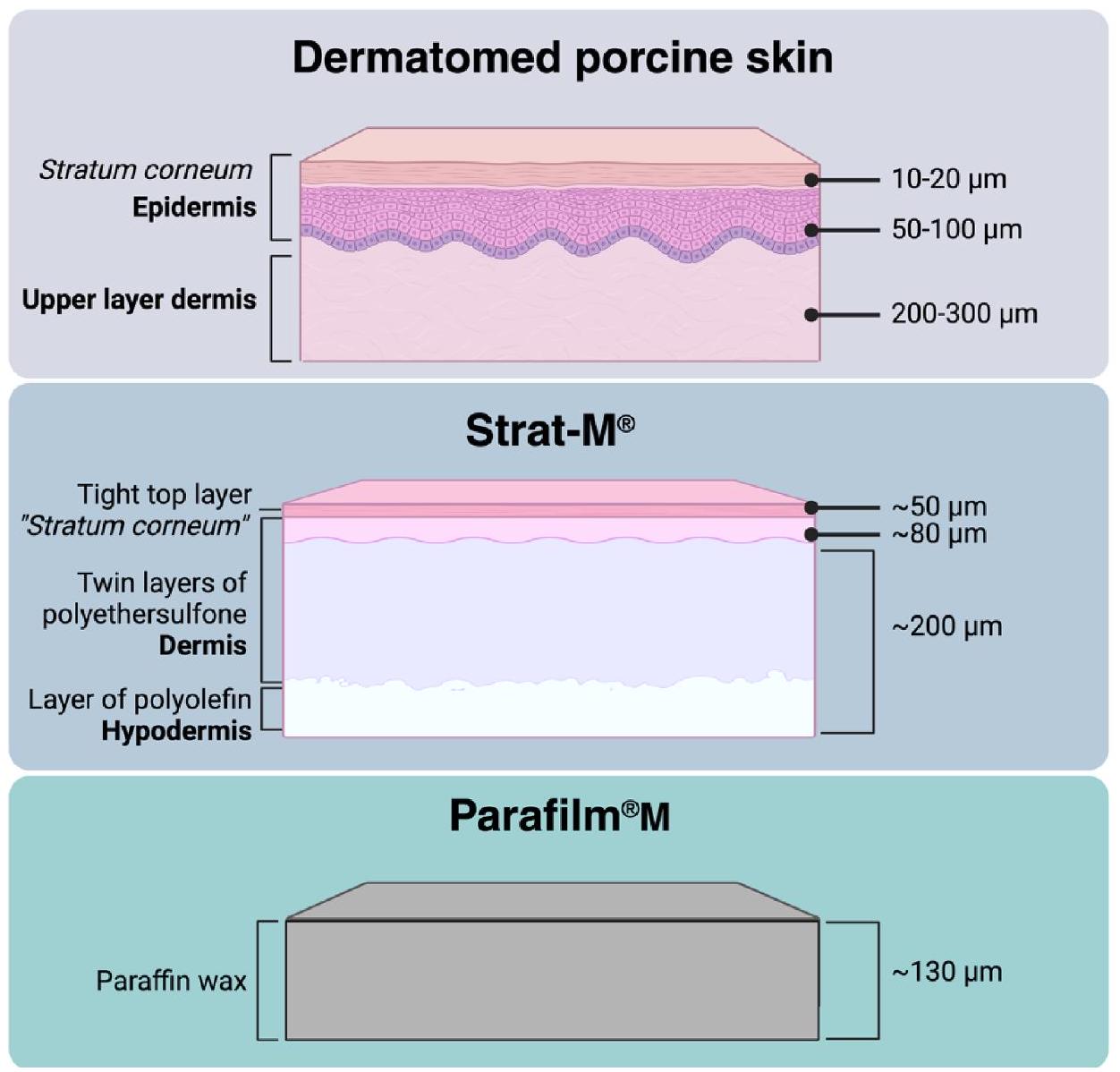
(لوغبره، المملكة المتحدة). تم الحصول على جلد الخنازير حديثي الولادة من خنازير ميتة أقل من 24 ساعة بعد الولادة، وتم شطفه في محلول فوسفات الملح (PBS pH 7.4)، وتم تقطيعه إلى سمك
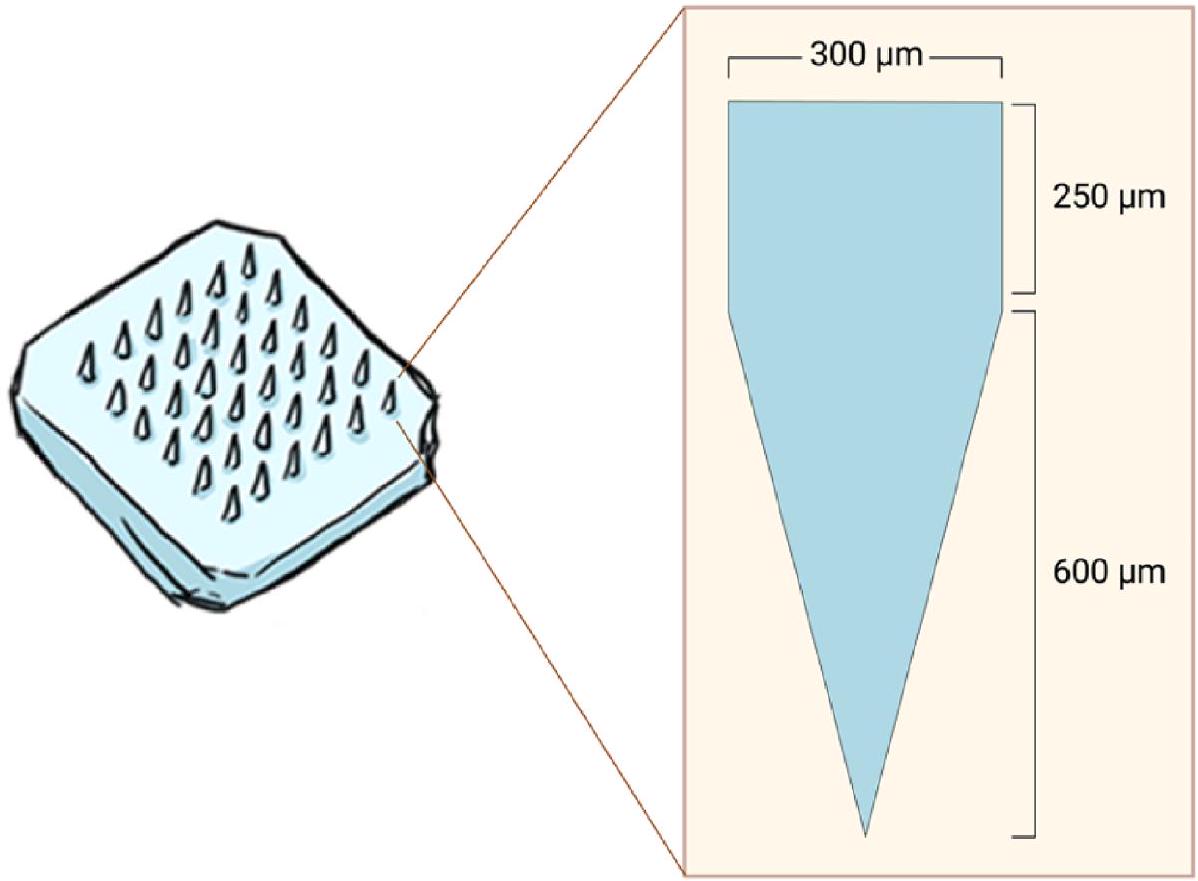
2.2. إعداد MAPs القابلة للذوبان
2.3. تصوير التداخل البصري وتصوير المسام
2.4. تحديد البروتين المفرز من الأغشية
صياغة الطبقة الأولى من MAP القابلة للذوبان.
| تركيب | نوع البروتين | AMSC-MP | |
| ألبومين | جمعية الكشافة الأمريكية | ||
| البروتين (%وزن/وزن) | 10 | 10 | 10 |
| خليط مائي PP2 (%وزن/وزن) | 30 | 30 | 30 |
| ماء منزوع الأيونات (%وزن/وزن) | 60 | 60 | 60 |

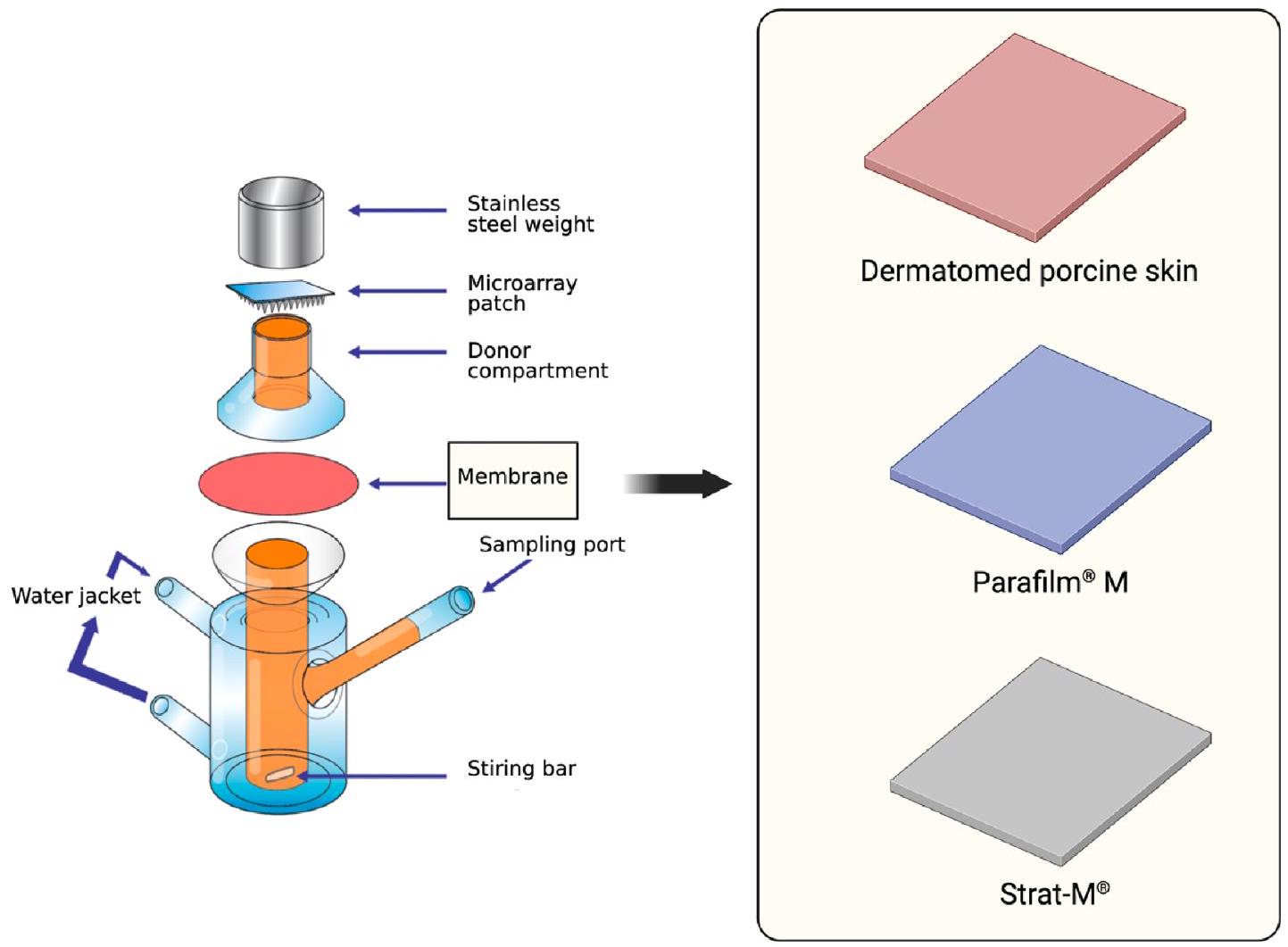
2.5. دراسات النفاذية في المختبر باستخدام خلايا فرانز
2.6. التقدير باستخدام مجموعة اختبار بروتين الميكرو-BCA
2.7. التحليل الإحصائي
3. النتائج والمناقشة
3.1. إعداد MAPs القابلة للذوبان
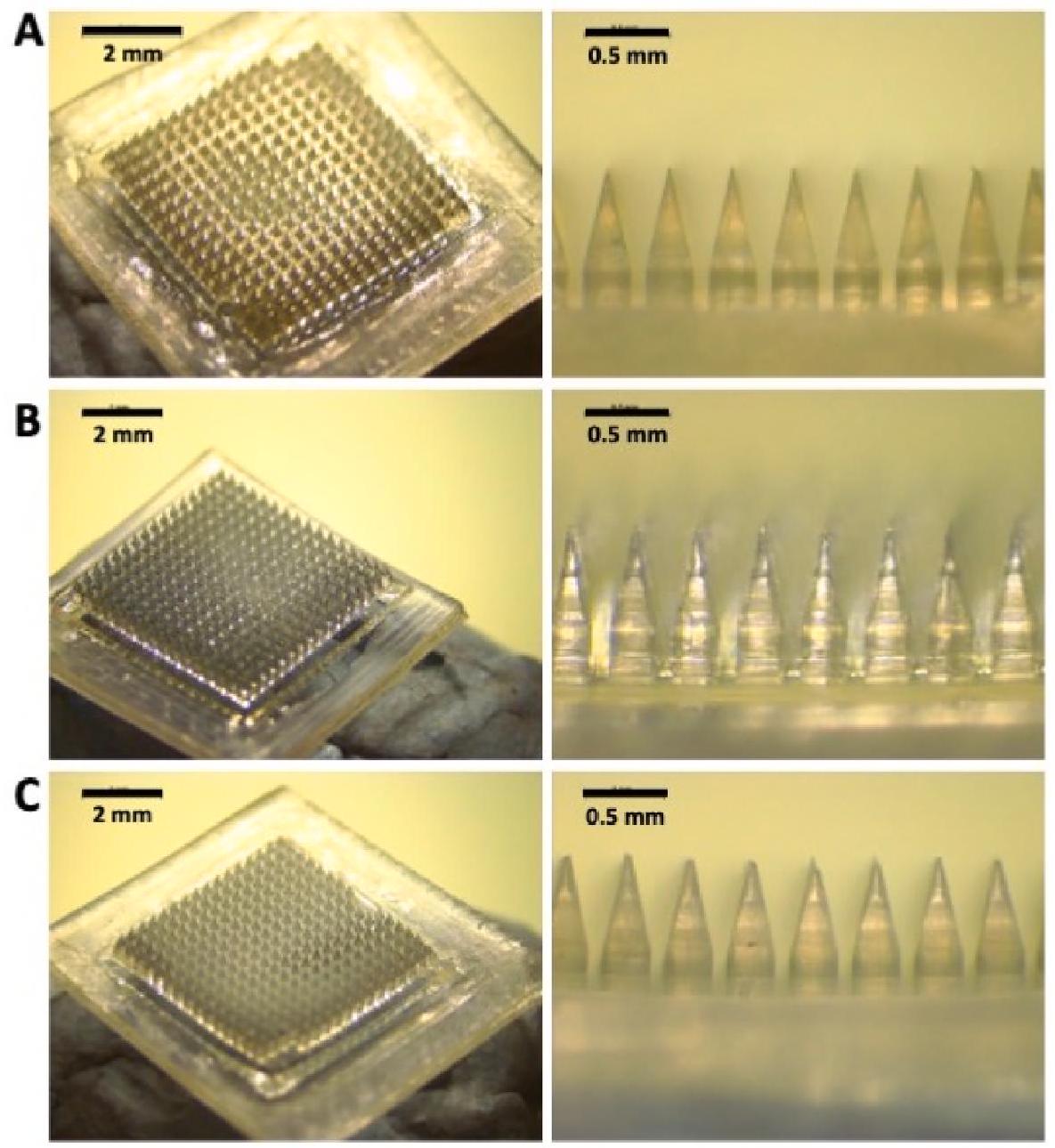
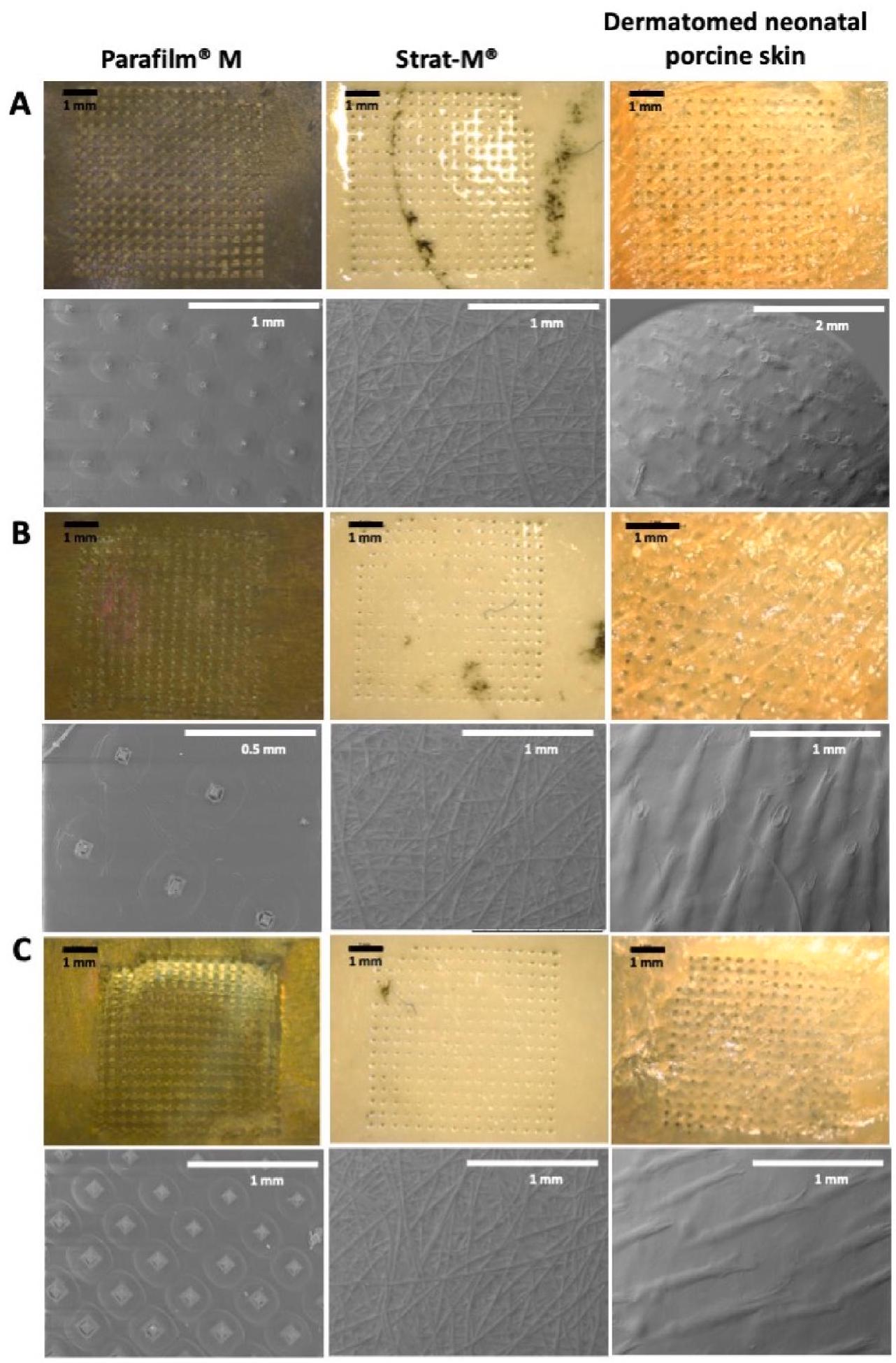
تحتوي التركيبات على بروتينات (أنكاوينت وونغ وآخرون، 2020؛ كوديل وآخرون، 2018). في هذه الدراسة، تم تحميل كل بروتين في رؤوس الإبر بطريقة بسيطة مع ضمان الحد الأدنى من هدر البروتين. لهذا الغرض، تم دمج البروتين مع خليط من PVA و PVP. تعرض الشكل 4 صورًا مجهرية لمادة MAPs القابلة للذوبان الناتجة، مما يوضح تجانسها وبنيتها المحددة جيدًا، التي تتميز بإبر حادة. شكل الإبرة هو مزيج من الشكل المكعب والهرمي، نتيجة لقوالب بولي ديميثيل سيليوكسان المستخدمة في هذه التجربة. تم اختيار تصميم القالب هذا لقدرته على تسهيل الإدخال الفعال وتوصيل الدواء إلى الجلد، والذي أظهر تفوقًا مقارنة بأشكال أخرى (كورديرو وآخرون، 2020). أخيرًا، خضعت هذه MAPs القابلة للذوبان لاختبارات إدخال لاحقة وتقييم اختراق في المختبر.
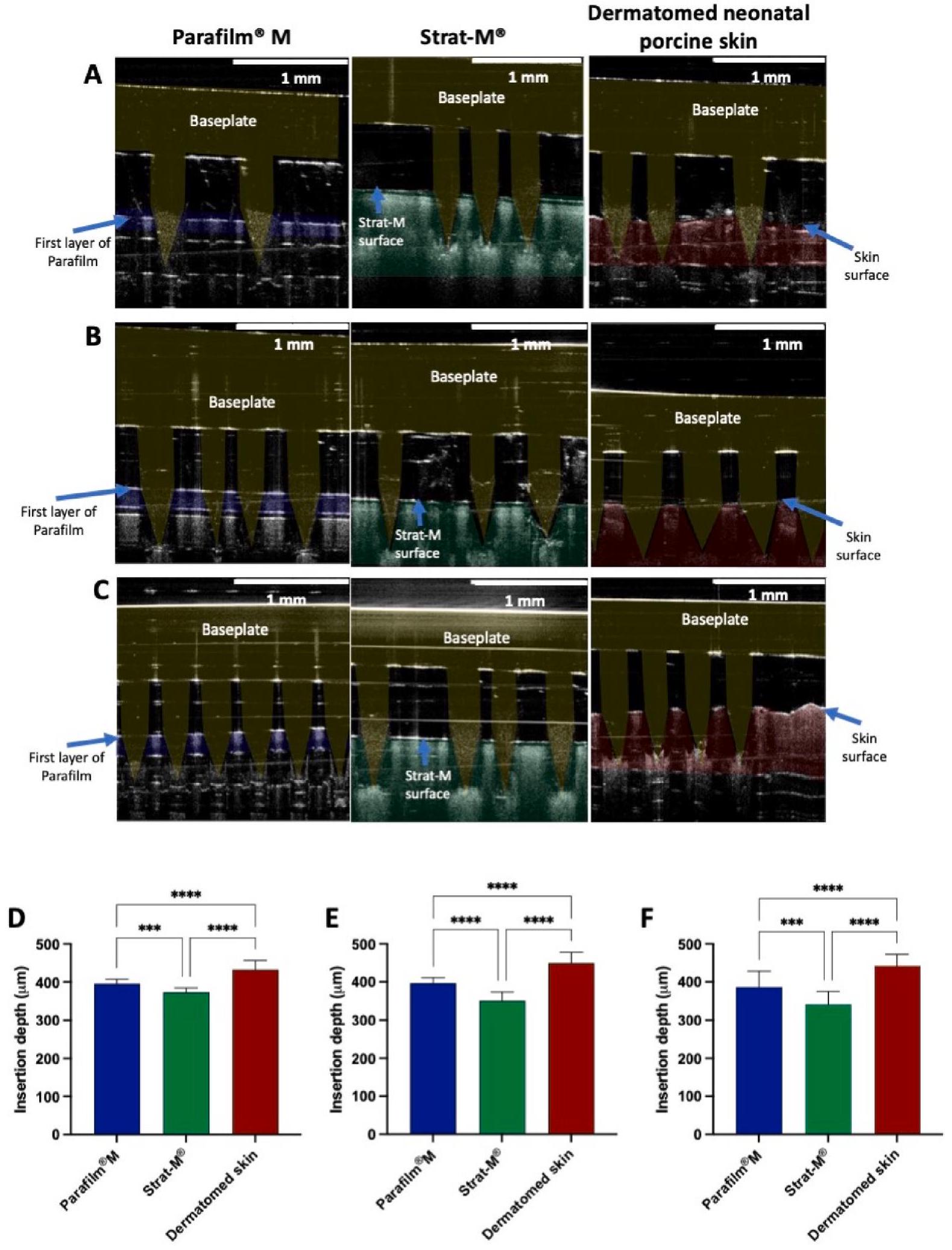
3.2. التصوير المقطعي البصري وتصور المسام
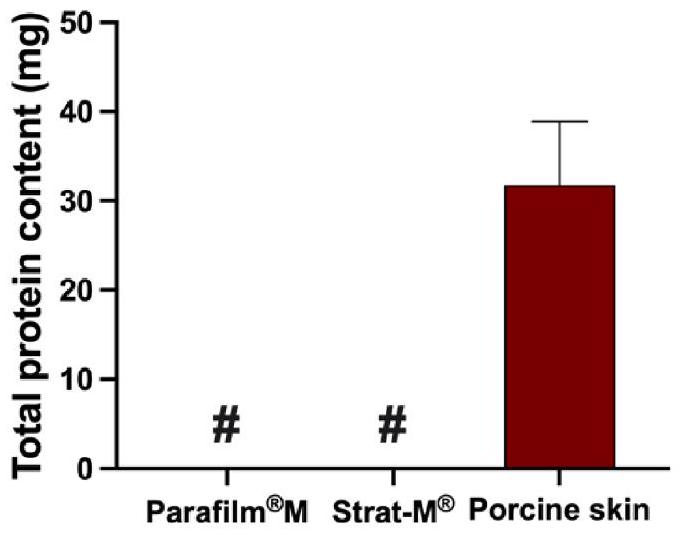
3.3. تحديد البروتين المفرز من الأغشية
3.4. دراسات النفاذية في المختبر باستخدام خلايا فرانز

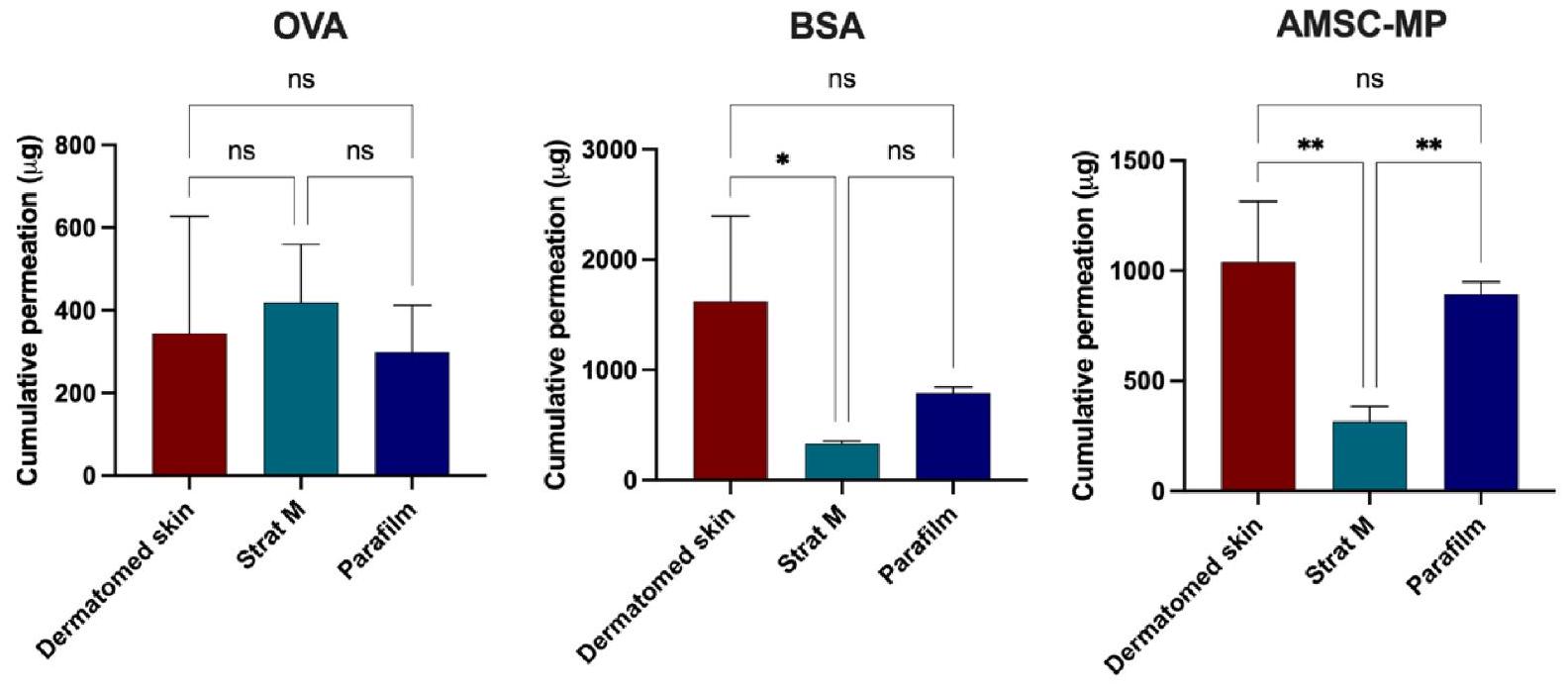
مخصص للتوصيل عبر الجلد. بالإضافة إلى ذلك، تشمل الخطط المستقبلية لهذه الدراسة إنشاء عامل تشابه. التركيز الحالي هو على الاستخدام المحتمل لبارافيلم.
4. الخاتمة
بيان مساهمة مؤلفي CRediT
إعلان عن تضارب المصالح
توفر البيانات
References
Anjani, Q.K., Bin Sabri, A.H., Donnelly, R., 2021a. Development and validation of simple and sensitive HPLC-UV method for ethambutol hydrochloride detection following transdermal application. Anal. Methods. https://doi.org/10.1039/D1AY01414E.
Anjani, Q.K., Permana, A.D., Cárcamo-Martínez, Á., Domínguez-Robles, J., Tekko, I.A., Larrañeta, E., Vora, L.K., Ramadon, D., Donnelly, R.F., 2021b. Versatility of hydrogel-forming microneedles in in vitro transdermal delivery of tuberculosis drugs. Eur. J. Pharm. Biopharm. 294-312, 294-312. https://doi.org/10.1016/j. ejpb.2020.12.003.
Anjani, Q.K., Hidayat, A., Sabri, B., Moreno-Castellanos, N., Utomo, E., CárcamoMartínez, Á., Domínguez-Robles, J., Ahmadi, L., Wardoyo, H., Donnelly, R.F., 2022a. Soluplus
Anjani, Q.K., Bin Sabri, A.H., Utomo, E., Domínguez-Robles, J., Donnelly, R.F., 2022b. Elucidating the impact of surfactants on the performance of dissolving microneedle array patches. Mol. Pharm. 19, 1191-1208, 10.1021/ACS. MOLPHARMACEUT.1C00988/ASSET/IMAGES/LARGE/MP1C00988_0014.JPEG.
Anjani, Q.K., Bin Sabri, A.H., McGuckin, M.B., Li, H., Hamid, K.A., Donnelly, R.F., 2022c. In vitro permeation studies on carvedilol containing dissolving microarray patches quantified using a rapid and simple HPLC-UV analytical method. AAPS PharmSciTech 23, 1-13. https://doi.org/10.1208/S12249-022-02422-6/FIGURES/ 4.
Anjani, Q.K., Bin Sabri, A.H., Hutton, A.J., Cárcamo-Martínez, Á., Wardoyo, L.A.H., Mansoor, A.Z., Donnelly, R.F., 2023a. Microarray patches for managing infections at a global scale. J. Control. Release 359, 97-115. https://doi.org/10.1016/j. jconrel.2023.05.038.
Anjani, Q.K., Bin Sabri, A.H., Hamid, K.A., Moreno-Castellanos, N., Li, H., Donnelly, R.F., 2023b. Tip loaded cyclodextrin-carvedilol complexes microarray patches. Carbohydr. Polym. 320, 121194 https://doi.org/10.1016/j.carbpol.2023.121194.
Anjani, Q.K., Cárcamo-Martínez, Á., Wardoyo, L.A.H., Moreno-Castellanos, N., Bin Sabri, A.H., Larrañeta, E., Donnelly, R.F., 2023d. MAP-box: a novel, low-cost and easy-to-fabricate 3D-printed box for the storage and transportation of dissolving microneedle array patches. Drug Deliv. Transl. Res. https://doi.org/10.1007/ s13346-023-01393-w.
Anjani, Q.K., Bin Sabri, A.H., Hamid, K.A., Moreno-Castellanos, N., Li, H., Donnelly, R.F., 2023e. Tip loaded cyclodextrin-carvedilol complexes microarray patches. Carbohydr. Polym. 320, 121194.
Anjani, Q.K., Volpe-Zanutto, F., Hamid, K.A., Bin Sabri, A.H., Moreno-Castellano, N., Gaitán, X.A., Calit, J., Bargieri, D.Y., Donnelly, R.F., 2023f. Primaquine and chloroquine nano-sized solid dispersion-loaded dissolving microarray patches for the improved treatment of malaria caused by Plasmodium vivax. J. Controll. Release 361, 385-401. https://doi.org/10.1016/J.JCONREL.2023.08.009.
Antosova, Z., Mackova, M., Kral, V., Macek, T., 2009. Therapeutic application of peptides and proteins: parenteral forever? Trends Biotechnol. 27, 628-635. https://doi.org/ 10.1016/J.TIBTECH.2009.07.009.
Bin Sabri, A.H., Anjani, Q.K., Donnelly, R.F., Hidayat Bin Sabri, A., Kurnia Anjani, Q., Donnelly, R.F., 2021. Synthesis and characterization of sorbitol laced hydrogelforming microneedles for therapeutic drug monitoring. Int. J. Pharm. 607, 121049.
Bin Sabri, A.H., Anjani, Q.K., Utomo, E., Ripolin, A., Donnelly, R.F., 2022. Development and characterization of a dry reservoir-hydrogel-forming microneedles composite for minimally invasive delivery of cefazolin. Int. J. Pharm. 617, 121593 https://doi.org/ 10.1016/J.IJPHARM.2022.121593.
Caudill, C.L., Perry, J.L., Tian, S., Luft, J.C., DeSimone, J.M., 2018. Spatially controlled coating of continuous liquid interface production microneedles for transdermal protein delivery. J. Control. Release 284, 122-132. https://doi.org/10.1016/J. JCONREL. 2018.05.042.
Cordeiro, A.S., Tekko, I.A., Jomaa, M.H., Vora, L., McAlister, E., Volpe-Zanutto, F., Nethery, M., Baine, P.T., Mitchell, N., McNeill, D.W., Donnelly, R.F., 2020. Twophoton polymerisation 3D printing of microneedle array templates with versatile designs: application in the development of polymeric drug delivery systems. Pharm. Res. 37, 1-15. https://doi.org/10.1007/S11095-020-02887-9/FIGURES/9.
11. Correlation and regression, (n.d.). https://www.bmj.com/about-bmj/resources-readers/publications/statistics-square-one/11-correlation-and-regression (Accessed December 2, 2023).
Demir, Y.K., Akan, Z., Kerimoglu, O., 2013. Sodium alginate microneedle arrays mediate the transdermal delivery of bovine serum albumin. PLoS One 8, e63819.
Donnelly, R.F., Prausnitz, M.R., 2023. The promise of microneedle technologies for drug delivery. Drug Deliv. Transl. Res. 1-8. https://doi.org/10.1007/S13346-023-014308/FIGURES/2.
Donnelly, R.F., Garland, M.J., Morrow, D.I.J., Migalska, K., Singh, T.R.R., Majithiya, R., Woolfson, A.D., 2010. Optical coherence tomography is a valuable tool in the study of the effects of microneedle geometry on skin penetration characteristics and in-skin dissolution. J. Control. Release 147, 333-341. https://doi.org/10.1016/J. JCONREL. 2010.08.008.
Donnelly, R.F., Majithiya, R., Singh, T.R.R., Morrow, D.I.J., Garland, M.J., Demir, Y.K., Migalska, K., Ryan, E., Gillen, D., Scott, C.J., Woolfson, A.D., 2011. Design, optimization and characterisation of polymeric microneedle arrays prepared by a novel laser-based micromoulding technique. Pharm. Res. 28, 41-57. https://doi.org/ 10.1007/S11095-010-0169-8/FIGURES/12.
Garland, M.J., Migalska, K., Tuan-Mahmood, T.M., Raghu Raj Singh, T., Majithija, R., Caffarel-Salvador, E., McCrudden, C.M., McCarthy, H.O., David Woolfson, A., Donnelly, R.F., 2012. Influence of skin model on in vitro performance of drug-loaded soluble microneedle arrays. Int. J. Pharm. 434, 80-89. https://doi.org/10.1016/J. IJPHARM.2012.05.069.
Haq, A., Goodyear, B., Ameen, D., Joshi, V., Michniak-Kohn, B., 2018. Strat-M® synthetic membrane: permeability comparison to human cadaver skin. Int. J. Pharm. 547, 432-437. https://doi.org/10.1016/J.IJPHARM.2018.06.012.
Jiskoot, W., Randolph, T.W., Volkin, D.B., Middaugh, C.R., Schöneich, C., Winter, G., Friess, W., Crommelin, D.J.A., Carpenter, J.F., 2012. Protein instability and immunogenicity: roadblocks to clinical application of injectable protein delivery systems for sustained release. J. Pharm. Sci. 101, 946-954. https://doi.org/10.1002/ JPS. 23018.
Kirkby, M., Hutton, A.R.J., Donnelly, R.F., 2020. Microneedle mediated transdermal delivery of protein, peptide and antibody based therapeutics: current status and future considerations. Pharm. Res. 37, 1-18. https://doi.org/10.1007/S11095-020-02844-6/TABLES/2.
E. Larrañeta, M.T.C. McCrudden, A.J. Courtenay, R.F. Donnelly, Microneedles: a new frontier in nanomedicine delivery, Pharmaceut. Res. 33 (2016) 1055-1073. doi: 10.1007/S11095-016-1885-5.
R. Neupane, S.H.S. Boddu, J. Renukuntla, R.J. Babu, A.K. Tiwari, Alternatives to biological skin in permeation studies: current trends and possibilities, Pharmaceutics 12 (2020) 152. doi: 10.3390/PHARMACEUTICS12020152.
Oberli, M.A., Schoellhammer, C.M., Langer, R., Blankschtein, D., 2014. Ultrasoundenhanced transdermal delivery: recent advances and future challenges. Ther. Deliv. 5, 843-857. https://doi.org/10.4155/tde.14.32.
Pulsoni, I., Lubda, M., Aiello, M., Fedi, A., Marzagalli, M., von Hagen, J., Scaglione, S., 2022. Comparison between franz diffusion cell and a novel micro-physiological system for in vitro penetration assay using different skin models. SLAS Technol. 27, 161-171. https://doi.org/10.1016/J.SLAST.2021.12.006.
Van Der Maaden, K., Varypataki, E.M., Romeijn, S., Ossendorp, F., Jiskoot, W., Bouwstra, J., 2014. Ovalbumin-coated pH -sensitive microneedle arrays effectively induce ovalbumin-specific antibody and T-cell responses in mice. Eur. J. Pharm. Biopharm. 88, 310-315. https://doi.org/10.1016/J.EJPB.2014.05.003.
Vora, L.K., Sabri, A.H., Naser, Y., Himawan, A., Hutton, A.R.J., Anjani, Q.K., VolpeZanutto, F., Mishra, D., Li, M., Rodgers, A.M., Paredes, A.J., Larrañeta, E., Thakur, R. R.S., Donnelly, R.F., 2023. Long-acting microneedle formulations. Adv. Drug Deliv. Rev. 201, 115055 https://doi.org/10.1016/J.ADDR.2023.115055.
- Open Access
This research has been made openly available by Queen’s academics and its Open Research team. We would love to hear how access to this research benefits you. – Share your feedback with us: http://go.qub.ac.uk/oa-feedback - Corresponding author at: Chair in Pharmaceutical Technology, School of Pharmacy, Queen’s University Belfast, Medical Biology Centre, 97 Lisburn Road, Belfast BT9 7BL, Northern Ireland, UK.
E-mail address: r.donnelly@qub.ac.uk (R.F. Donnelly).
DOI: https://doi.org/10.1016/j.ijpharm.2024.124071
PMID: https://pubmed.ncbi.nlm.nih.gov/38554738
Publication Date: 2024-03-29
Parafilm
Publisher’s PDF, also known as Version of record
Link to publication record in Queen’s University Belfast Research Portal
Copyright 2024 The Authors.
This is an open access article published under a Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the author and source are cited.
Copyright for the publications made accessible via the Queen’s University Belfast Research Portal is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights.
The Research Portal is Queen’s institutional repository that provides access to Queen’s research output. Every effort has been made to ensure that content in the Research Portal does not infringe any person’s rights, or applicable UK laws. If you discover content in the Research Portal that you believe breaches copyright or violates any law, please contact openaccess@qub.ac.uk.
Parafilm
ARTICLE INFO
Keywords:
Strat-M®
Dermatomed porcine skin
Protein
Insertion studies
In vitro permeation studies
Abstract
In vitro permeation studies play a crucial role in early formulation optimisation before extensive animal model investigations. Biological membranes are typically used in these studies to mimic human skin conditions accurately. However, when focusing on protein and peptide transdermal delivery, utilising biological membranes can complicate analysis and quantification processes. This study aims to explore Parafilm
1. Introduction
injection of antibodies and peptides has been employed, allowing for potential self-administration by patients at home (Doughty et al., 2016; Bittner et al., 2018). However, patients seek alternatives in the form of self-administrable, painless, and minimally invasive dosage forms to reduce hospital visits and alleviate the healthcare burden associated with therapy (Anjani et al., 2023a). Transdermal drug delivery, notably via microarray patches (MAPs), emerges as a potential solution. MAP technology, intensively researched in biomedicine, aims to facilitate effective drug and vaccine delivery (Donnelly and Prausnitz, 2023; Vora et al., 2023). MAPs function by painlessly breaching the stratum corneum (SC), creating microchannels to aid drug diffusion into deeper dermal layers (Anjani et al., 2022a). This approach circumvents passive drug diffusion challenges, allowing delivery of a wide range of chemical
2. Materials and methods
2.1. Materials
(Loughborough, UK). Neonatal porcine skin was obtained from stillborn piglets less than 24 h after birth, rinsed in phosphate buffer saline (PBS pH 7.4 ), dermatomed to a thickness of
2.2. Preparation of dissolving MAPs
2.3. Optical coherence tomography and pore visualisation
2.4. Determination of protein released from membranes
Formulation of first layer dissolving MAP.
| Composition | Protein Type | AMSC-MP | |
| Albumin | BSA | ||
| Protein (%w/w) | 10 | 10 | 10 |
| Aqueous mixture PP2 (%w/w) | 30 | 30 | 30 |
| Deionised water (%w/w) | 60 | 60 | 60 |


2.5. In vitro permeation studies using Franz cells

2.6. Quantification using micro-BCA protein assay kit
2.7. Statistical analysis
3. Results and discussion
3.1. Preparation of dissolving MAPs
arrays with formulations containing proteins (Angkawinitwong et al., 2020; Caudill et al., 2018). In this study, each protein was loaded into the needle tips in a simple way and ensuring minimum waste of the protein cargo. For this purpose, the protein was combined with a mixture of PVA and PVP. Fig. 4 displays microscopic images of the resulting dissolving MAPs, demonstrating their homogeneity and welldefined structure, characterised by sharp needles. The needle shape is a combination of cuboidal and pyramidal, a result of the polydimethylsiloxane moulds used in this experiment. This mould design was chosen for its ability to facilitate effective insertion and drug delivery into the skin, which has shown superiority compared to other shapes (Cordeiro et al., 2020). Finally, these dissolving MAPs underwent subsequent insertion testing and in vitro permeation profiling.
3.2. Optical coherence tomography and pore visualisation

3.3. Determination of protein released from membranes

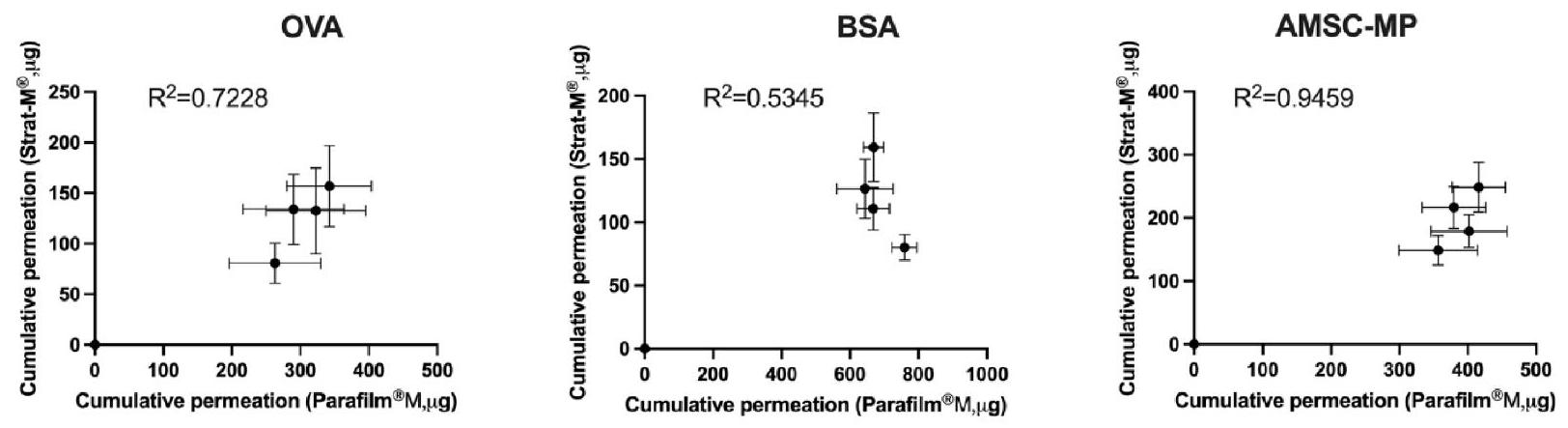
3.4. In vitro permeation studies using Franz cells





intended for transdermal delivery. Additionally, future plans for this study include establishing a similarity factor. The current focus is on the potential use of Parafilm
4. Conclusion

CRediT authorship contribution statement
Declaration of competing interest
Data availability
References
Anjani, Q.K., Bin Sabri, A.H., Donnelly, R., 2021a. Development and validation of simple and sensitive HPLC-UV method for ethambutol hydrochloride detection following transdermal application. Anal. Methods. https://doi.org/10.1039/D1AY01414E.
Anjani, Q.K., Permana, A.D., Cárcamo-Martínez, Á., Domínguez-Robles, J., Tekko, I.A., Larrañeta, E., Vora, L.K., Ramadon, D., Donnelly, R.F., 2021b. Versatility of hydrogel-forming microneedles in in vitro transdermal delivery of tuberculosis drugs. Eur. J. Pharm. Biopharm. 294-312, 294-312. https://doi.org/10.1016/j. ejpb.2020.12.003.
Anjani, Q.K., Hidayat, A., Sabri, B., Moreno-Castellanos, N., Utomo, E., CárcamoMartínez, Á., Domínguez-Robles, J., Ahmadi, L., Wardoyo, H., Donnelly, R.F., 2022a. Soluplus
Anjani, Q.K., Bin Sabri, A.H., Utomo, E., Domínguez-Robles, J., Donnelly, R.F., 2022b. Elucidating the impact of surfactants on the performance of dissolving microneedle array patches. Mol. Pharm. 19, 1191-1208, 10.1021/ACS. MOLPHARMACEUT.1C00988/ASSET/IMAGES/LARGE/MP1C00988_0014.JPEG.
Anjani, Q.K., Bin Sabri, A.H., McGuckin, M.B., Li, H., Hamid, K.A., Donnelly, R.F., 2022c. In vitro permeation studies on carvedilol containing dissolving microarray patches quantified using a rapid and simple HPLC-UV analytical method. AAPS PharmSciTech 23, 1-13. https://doi.org/10.1208/S12249-022-02422-6/FIGURES/ 4.
Anjani, Q.K., Bin Sabri, A.H., Hutton, A.J., Cárcamo-Martínez, Á., Wardoyo, L.A.H., Mansoor, A.Z., Donnelly, R.F., 2023a. Microarray patches for managing infections at a global scale. J. Control. Release 359, 97-115. https://doi.org/10.1016/j. jconrel.2023.05.038.
Anjani, Q.K., Bin Sabri, A.H., Hamid, K.A., Moreno-Castellanos, N., Li, H., Donnelly, R.F., 2023b. Tip loaded cyclodextrin-carvedilol complexes microarray patches. Carbohydr. Polym. 320, 121194 https://doi.org/10.1016/j.carbpol.2023.121194.
Anjani, Q.K., Cárcamo-Martínez, Á., Wardoyo, L.A.H., Moreno-Castellanos, N., Bin Sabri, A.H., Larrañeta, E., Donnelly, R.F., 2023d. MAP-box: a novel, low-cost and easy-to-fabricate 3D-printed box for the storage and transportation of dissolving microneedle array patches. Drug Deliv. Transl. Res. https://doi.org/10.1007/ s13346-023-01393-w.
Anjani, Q.K., Bin Sabri, A.H., Hamid, K.A., Moreno-Castellanos, N., Li, H., Donnelly, R.F., 2023e. Tip loaded cyclodextrin-carvedilol complexes microarray patches. Carbohydr. Polym. 320, 121194.
Anjani, Q.K., Volpe-Zanutto, F., Hamid, K.A., Bin Sabri, A.H., Moreno-Castellano, N., Gaitán, X.A., Calit, J., Bargieri, D.Y., Donnelly, R.F., 2023f. Primaquine and chloroquine nano-sized solid dispersion-loaded dissolving microarray patches for the improved treatment of malaria caused by Plasmodium vivax. J. Controll. Release 361, 385-401. https://doi.org/10.1016/J.JCONREL.2023.08.009.
Antosova, Z., Mackova, M., Kral, V., Macek, T., 2009. Therapeutic application of peptides and proteins: parenteral forever? Trends Biotechnol. 27, 628-635. https://doi.org/ 10.1016/J.TIBTECH.2009.07.009.
Bin Sabri, A.H., Anjani, Q.K., Donnelly, R.F., Hidayat Bin Sabri, A., Kurnia Anjani, Q., Donnelly, R.F., 2021. Synthesis and characterization of sorbitol laced hydrogelforming microneedles for therapeutic drug monitoring. Int. J. Pharm. 607, 121049.
Bin Sabri, A.H., Anjani, Q.K., Utomo, E., Ripolin, A., Donnelly, R.F., 2022. Development and characterization of a dry reservoir-hydrogel-forming microneedles composite for minimally invasive delivery of cefazolin. Int. J. Pharm. 617, 121593 https://doi.org/ 10.1016/J.IJPHARM.2022.121593.
Caudill, C.L., Perry, J.L., Tian, S., Luft, J.C., DeSimone, J.M., 2018. Spatially controlled coating of continuous liquid interface production microneedles for transdermal protein delivery. J. Control. Release 284, 122-132. https://doi.org/10.1016/J. JCONREL. 2018.05.042.
Cordeiro, A.S., Tekko, I.A., Jomaa, M.H., Vora, L., McAlister, E., Volpe-Zanutto, F., Nethery, M., Baine, P.T., Mitchell, N., McNeill, D.W., Donnelly, R.F., 2020. Twophoton polymerisation 3D printing of microneedle array templates with versatile designs: application in the development of polymeric drug delivery systems. Pharm. Res. 37, 1-15. https://doi.org/10.1007/S11095-020-02887-9/FIGURES/9.
11. Correlation and regression, (n.d.). https://www.bmj.com/about-bmj/resources-readers/publications/statistics-square-one/11-correlation-and-regression (Accessed December 2, 2023).
Demir, Y.K., Akan, Z., Kerimoglu, O., 2013. Sodium alginate microneedle arrays mediate the transdermal delivery of bovine serum albumin. PLoS One 8, e63819.
Donnelly, R.F., Prausnitz, M.R., 2023. The promise of microneedle technologies for drug delivery. Drug Deliv. Transl. Res. 1-8. https://doi.org/10.1007/S13346-023-014308/FIGURES/2.
Donnelly, R.F., Garland, M.J., Morrow, D.I.J., Migalska, K., Singh, T.R.R., Majithiya, R., Woolfson, A.D., 2010. Optical coherence tomography is a valuable tool in the study of the effects of microneedle geometry on skin penetration characteristics and in-skin dissolution. J. Control. Release 147, 333-341. https://doi.org/10.1016/J. JCONREL. 2010.08.008.
Donnelly, R.F., Majithiya, R., Singh, T.R.R., Morrow, D.I.J., Garland, M.J., Demir, Y.K., Migalska, K., Ryan, E., Gillen, D., Scott, C.J., Woolfson, A.D., 2011. Design, optimization and characterisation of polymeric microneedle arrays prepared by a novel laser-based micromoulding technique. Pharm. Res. 28, 41-57. https://doi.org/ 10.1007/S11095-010-0169-8/FIGURES/12.
Garland, M.J., Migalska, K., Tuan-Mahmood, T.M., Raghu Raj Singh, T., Majithija, R., Caffarel-Salvador, E., McCrudden, C.M., McCarthy, H.O., David Woolfson, A., Donnelly, R.F., 2012. Influence of skin model on in vitro performance of drug-loaded soluble microneedle arrays. Int. J. Pharm. 434, 80-89. https://doi.org/10.1016/J. IJPHARM.2012.05.069.
Haq, A., Goodyear, B., Ameen, D., Joshi, V., Michniak-Kohn, B., 2018. Strat-M® synthetic membrane: permeability comparison to human cadaver skin. Int. J. Pharm. 547, 432-437. https://doi.org/10.1016/J.IJPHARM.2018.06.012.
Jiskoot, W., Randolph, T.W., Volkin, D.B., Middaugh, C.R., Schöneich, C., Winter, G., Friess, W., Crommelin, D.J.A., Carpenter, J.F., 2012. Protein instability and immunogenicity: roadblocks to clinical application of injectable protein delivery systems for sustained release. J. Pharm. Sci. 101, 946-954. https://doi.org/10.1002/ JPS. 23018.
Kirkby, M., Hutton, A.R.J., Donnelly, R.F., 2020. Microneedle mediated transdermal delivery of protein, peptide and antibody based therapeutics: current status and future considerations. Pharm. Res. 37, 1-18. https://doi.org/10.1007/S11095-020-02844-6/TABLES/2.
E. Larrañeta, M.T.C. McCrudden, A.J. Courtenay, R.F. Donnelly, Microneedles: a new frontier in nanomedicine delivery, Pharmaceut. Res. 33 (2016) 1055-1073. doi: 10.1007/S11095-016-1885-5.
R. Neupane, S.H.S. Boddu, J. Renukuntla, R.J. Babu, A.K. Tiwari, Alternatives to biological skin in permeation studies: current trends and possibilities, Pharmaceutics 12 (2020) 152. doi: 10.3390/PHARMACEUTICS12020152.
Oberli, M.A., Schoellhammer, C.M., Langer, R., Blankschtein, D., 2014. Ultrasoundenhanced transdermal delivery: recent advances and future challenges. Ther. Deliv. 5, 843-857. https://doi.org/10.4155/tde.14.32.
Pulsoni, I., Lubda, M., Aiello, M., Fedi, A., Marzagalli, M., von Hagen, J., Scaglione, S., 2022. Comparison between franz diffusion cell and a novel micro-physiological system for in vitro penetration assay using different skin models. SLAS Technol. 27, 161-171. https://doi.org/10.1016/J.SLAST.2021.12.006.
Van Der Maaden, K., Varypataki, E.M., Romeijn, S., Ossendorp, F., Jiskoot, W., Bouwstra, J., 2014. Ovalbumin-coated pH -sensitive microneedle arrays effectively induce ovalbumin-specific antibody and T-cell responses in mice. Eur. J. Pharm. Biopharm. 88, 310-315. https://doi.org/10.1016/J.EJPB.2014.05.003.
Vora, L.K., Sabri, A.H., Naser, Y., Himawan, A., Hutton, A.R.J., Anjani, Q.K., VolpeZanutto, F., Mishra, D., Li, M., Rodgers, A.M., Paredes, A.J., Larrañeta, E., Thakur, R. R.S., Donnelly, R.F., 2023. Long-acting microneedle formulations. Adv. Drug Deliv. Rev. 201, 115055 https://doi.org/10.1016/J.ADDR.2023.115055.
- Open Access
This research has been made openly available by Queen’s academics and its Open Research team. We would love to hear how access to this research benefits you. – Share your feedback with us: http://go.qub.ac.uk/oa-feedback - Corresponding author at: Chair in Pharmaceutical Technology, School of Pharmacy, Queen’s University Belfast, Medical Biology Centre, 97 Lisburn Road, Belfast BT9 7BL, Northern Ireland, UK.
E-mail address: r.donnelly@qub.ac.uk (R.F. Donnelly).
