DOI: https://doi.org/10.1038/s41467-024-46815-6
PMID: https://pubmed.ncbi.nlm.nih.gov/38509091
تاريخ النشر: 2024-03-20
تأمين الأكسجين الشبكي في RuO2 لاستقرار مواقع الروثينيوم النشطة للغاية في أكسدة الماء الحمضية
تم القبول: 4 مارس 2024
نُشر على الإنترنت: 20 مارس 2024
(أ) التحقق من التحديثات
الملخص
ثاني أكسيد الروثينيوم هو حاليًا أكثر المحفزات نشاطًا لتفاعل تطور الأكسجين (OER) في الوسط الحمضي، ولكنه يعاني من ذوبان الروثينيوم الشديد الناتج عن التساهمية العالية لروابط الروثينيوم-أكسجين مما يؤدي إلى أكسدة الأكسجين الشبكي. هنا، نبلغ عن استراتيجية تخديم السيليكون بين الفجوات لاستقرار المواقع النشطة للغاية للروثينيوم.
تفعيل مسار LOM، مما يؤدي إلى تحسين النشاط ولكن بضعف الاستقرار. تم الإبلاغ عن عدد قليل جداً من العناصر واستخدامها لتقليل تساهمية رابطة RuO وتحسين استقرار أكاسيد الروثينيوم. مجموعة زانغ
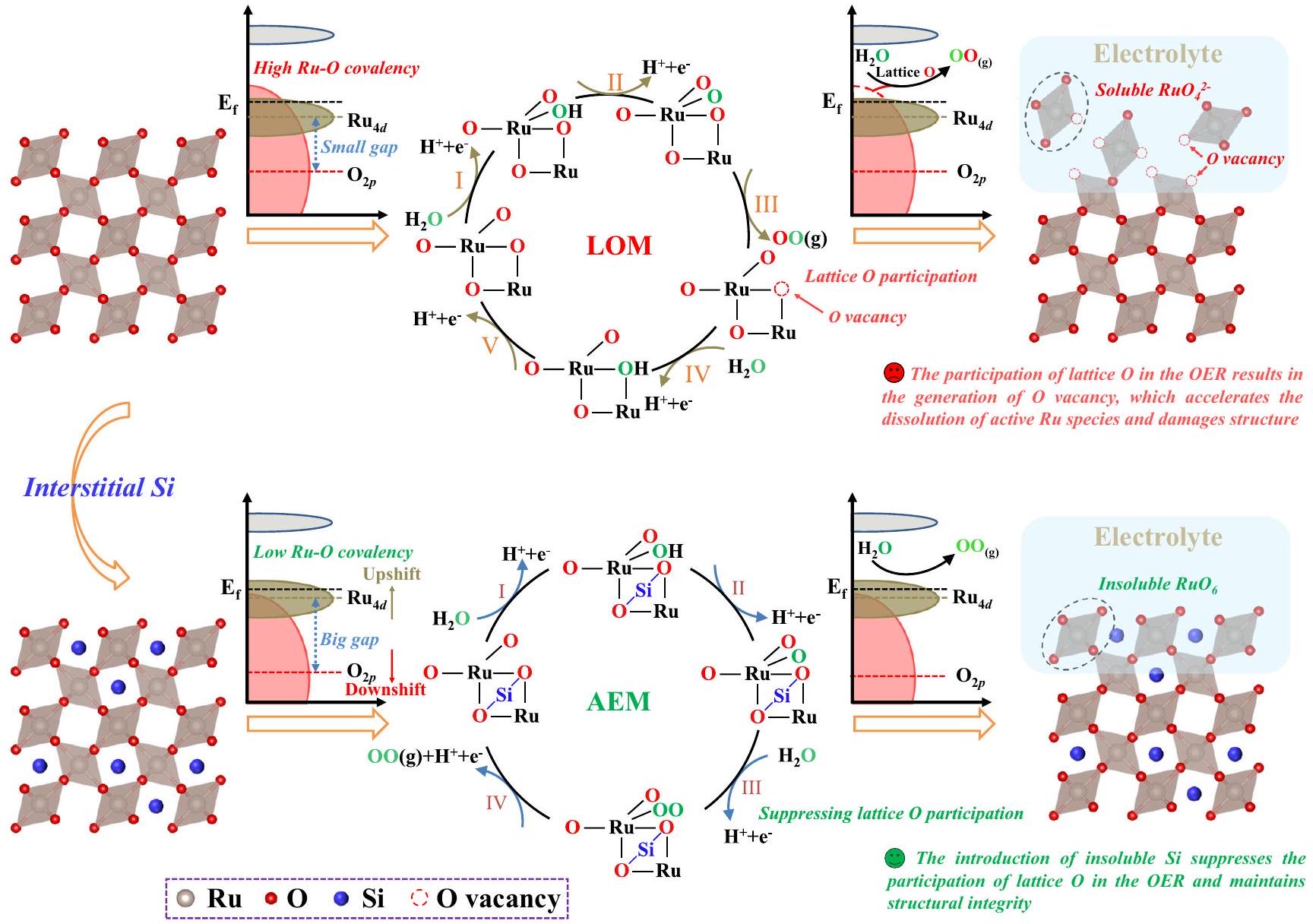
مع ذرات الدوبينغ، التي تحد من كمية هياكل Ru-O في المحفز، وليس لها فائدة في تحسين نشاط أكاسيد الروثينيوم. لذلك، فإن استكشاف استراتيجيات دوبينغ فعالة لتقليل تساهمية رابطة Ru-O دون فقدان كمية هيكل Ru-O هو أمر مرغوب فيه للغاية ولكنه يمثل تحديًا.
النتائج والمناقشات
تركيب وتوصيف
(المشار إليه بـ
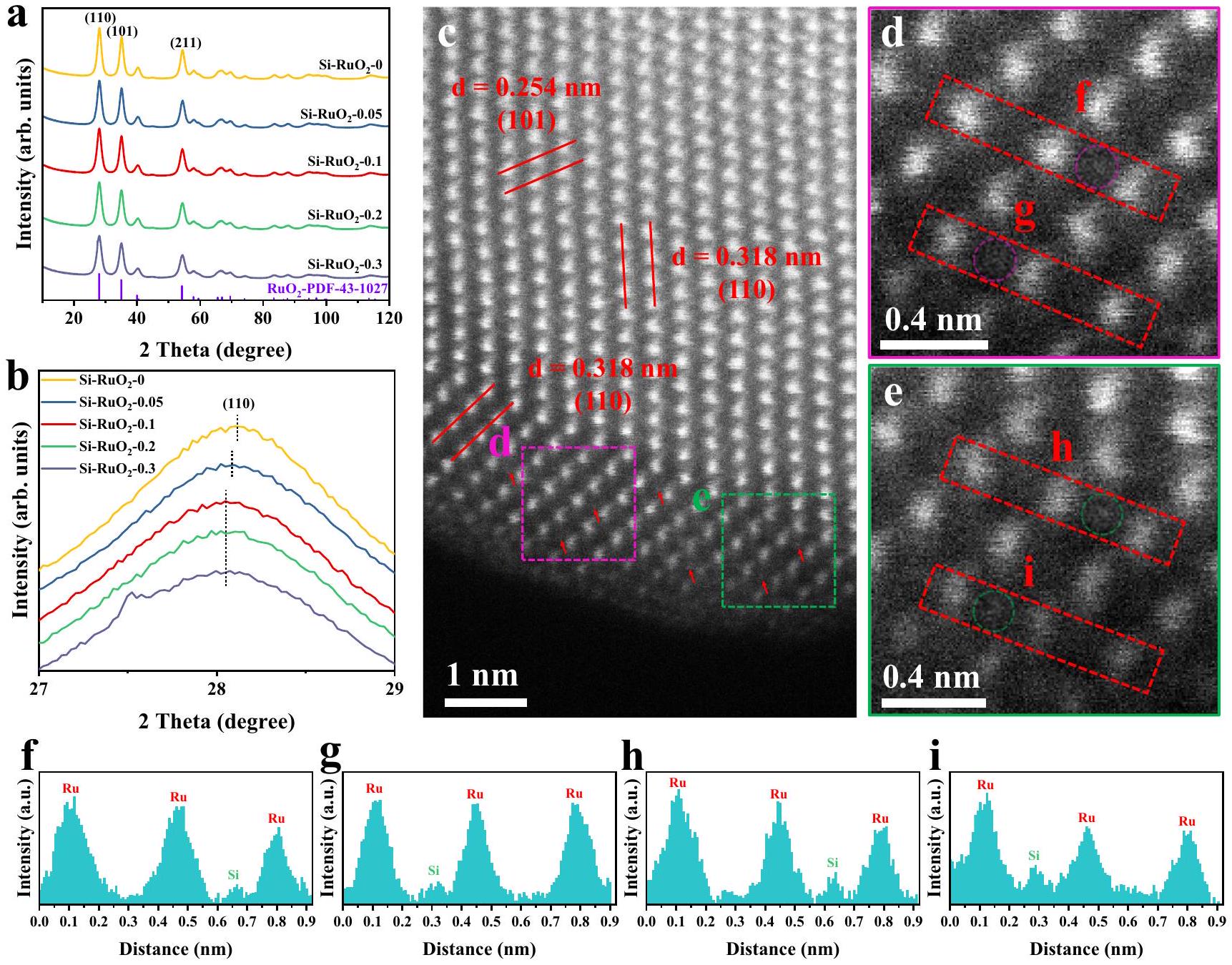
تخطيط العناصر. كما هو موضح في الأشكال التكميلية 1a، b-5a، b، صور TEM لجميع
الذي تم نسبه إلى رابطة Si-O المرتبطة بالسيليكون البيني. عندما زاد محتوى السيليكون إلى 0.2 و 0.3، ظهر ذروة إضافية عند 104.1 إلكترون فولت تتماشى مع
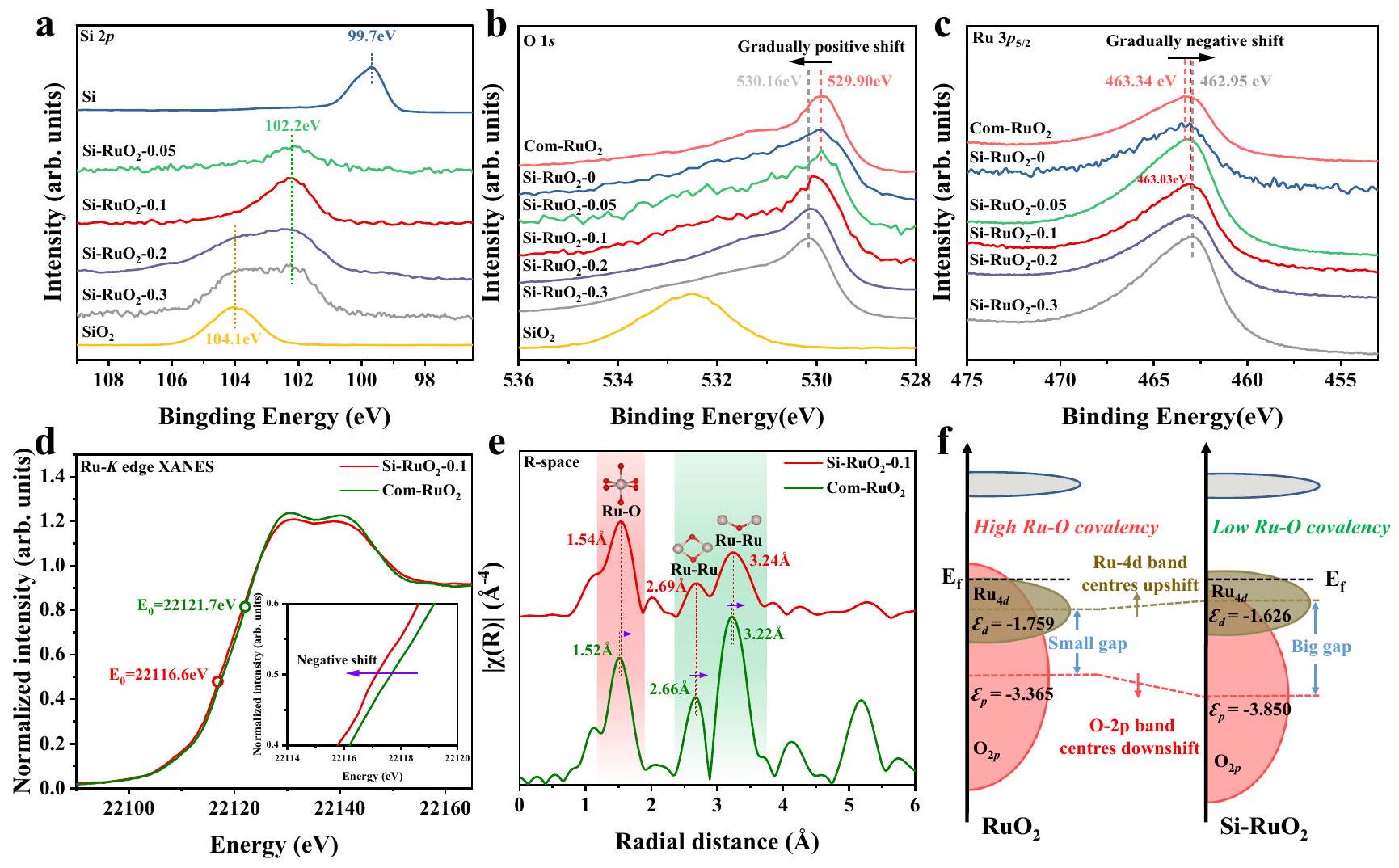
مخطط تخطيطي لهياكل النطاقات لـ
طيف الامتصاص بالأشعة السينية الدقيقة الممتدة (FT-EXAFS) لسي-
قياس أداء OER وأصل النشاط المعزز
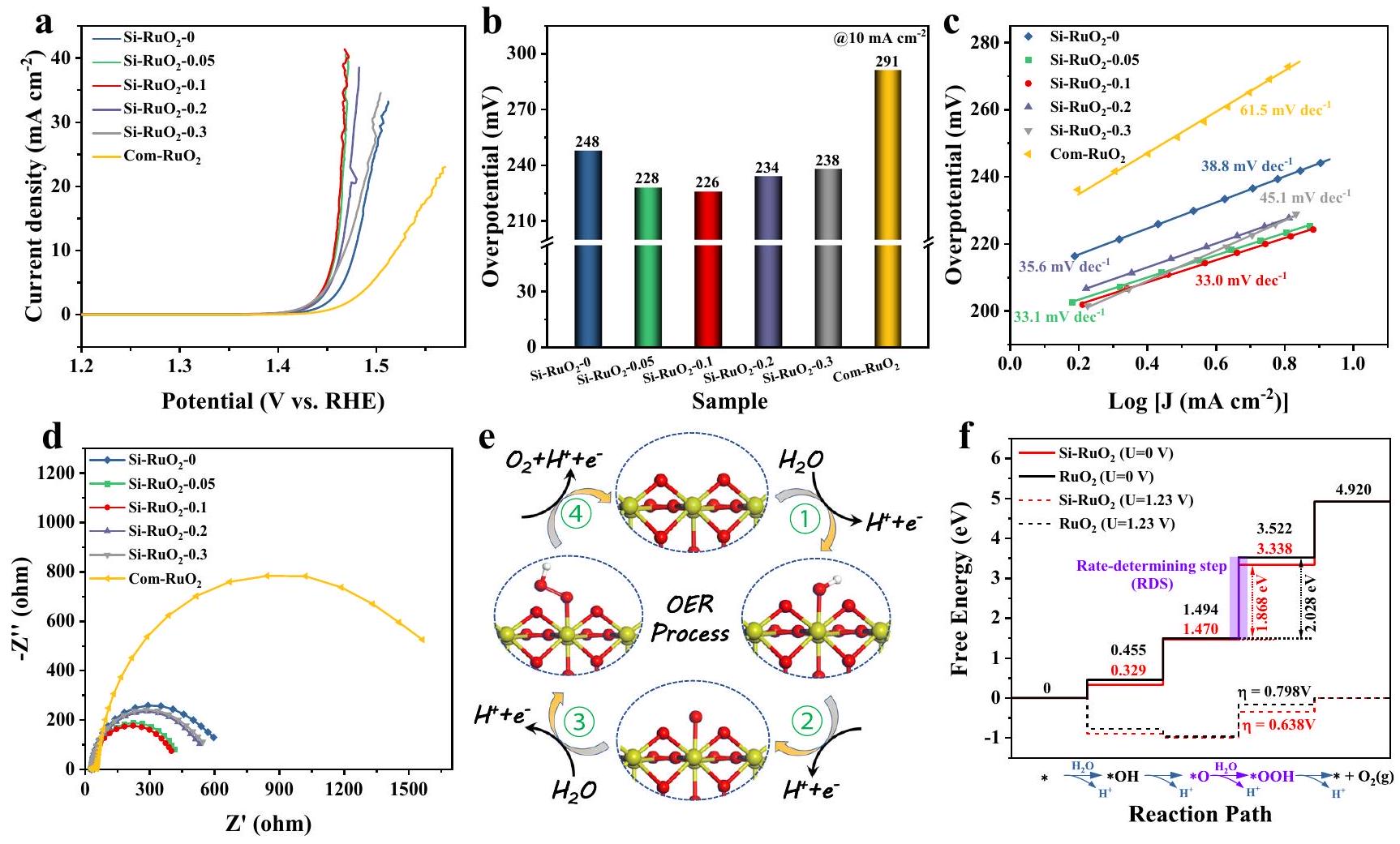
تقييم استقرار التحفيز
توصيف
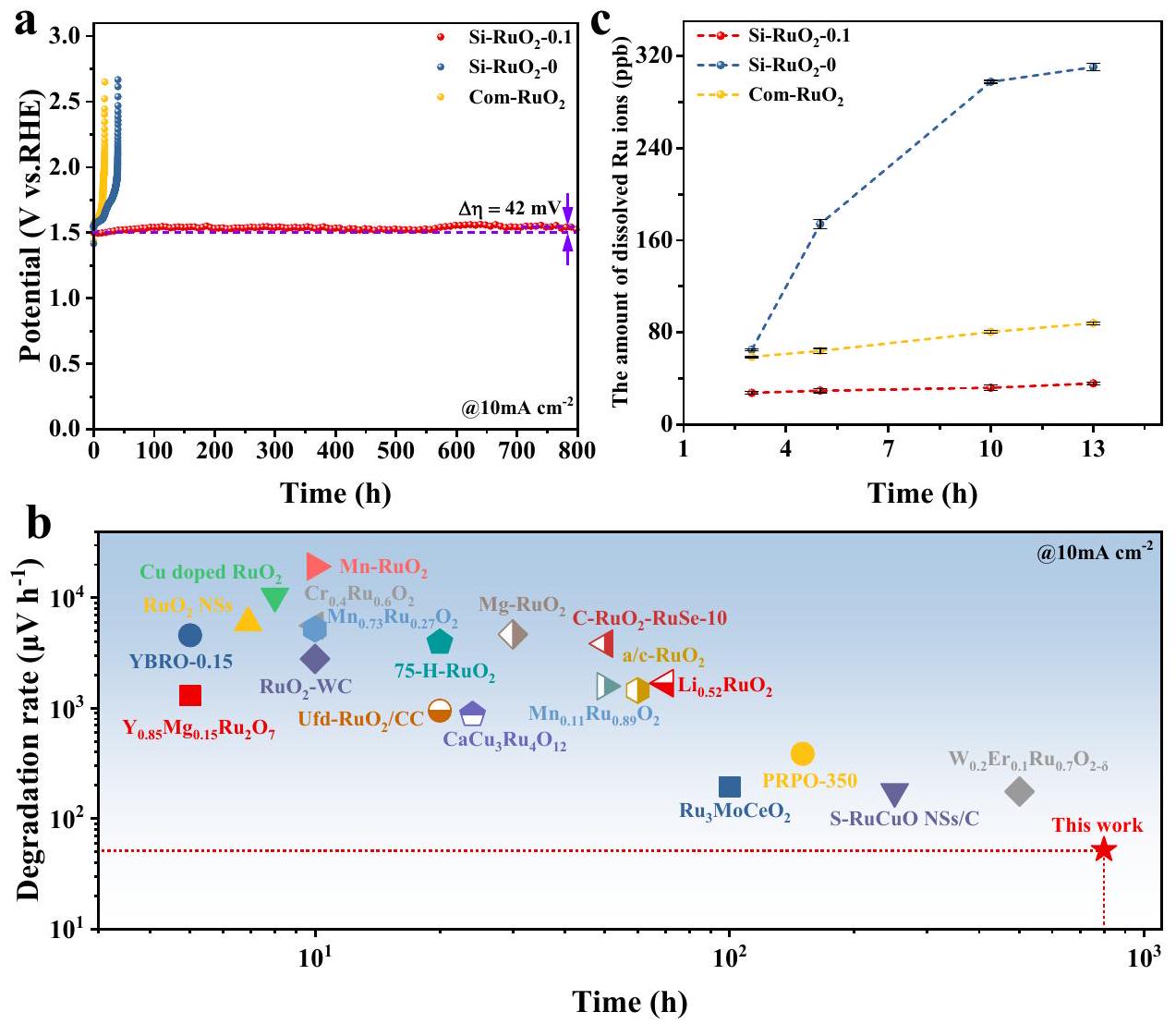
منحنيات استقرار الكرونوبوتومترية لـ
استقرار ومعدل التحلل لمحفز أكسيد الروثينيوم
و كوم-
قياس DEMS
طرق
المواد الكيميائية والمواد
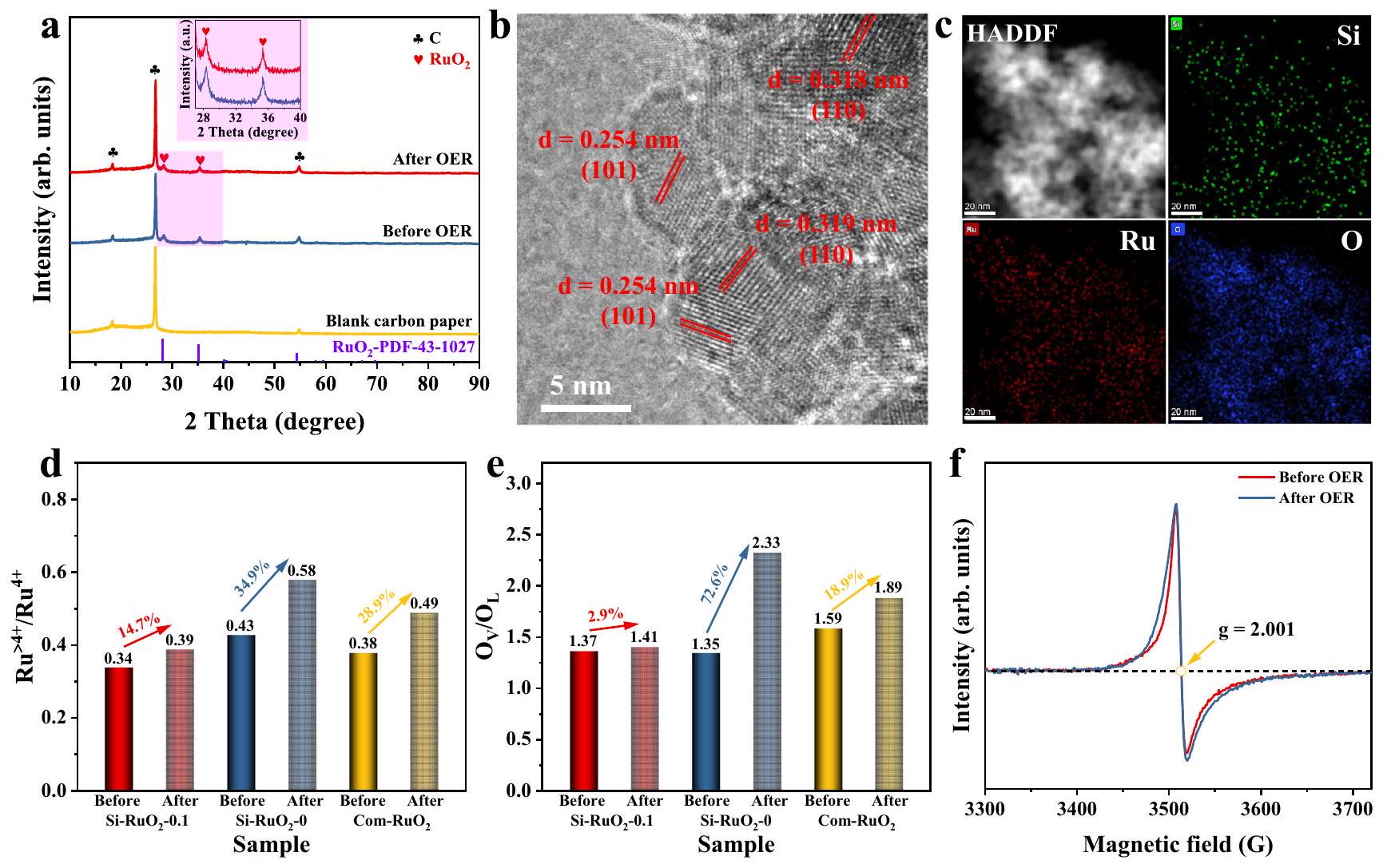
نسب لـ
توصيف المواد
تم الحصول على طيف الرنين (EPR) على مطياف Bruker EMXPLUS بتردد ميكروويف قدره 9.84 GHz.
القياسات الكهروكيميائية
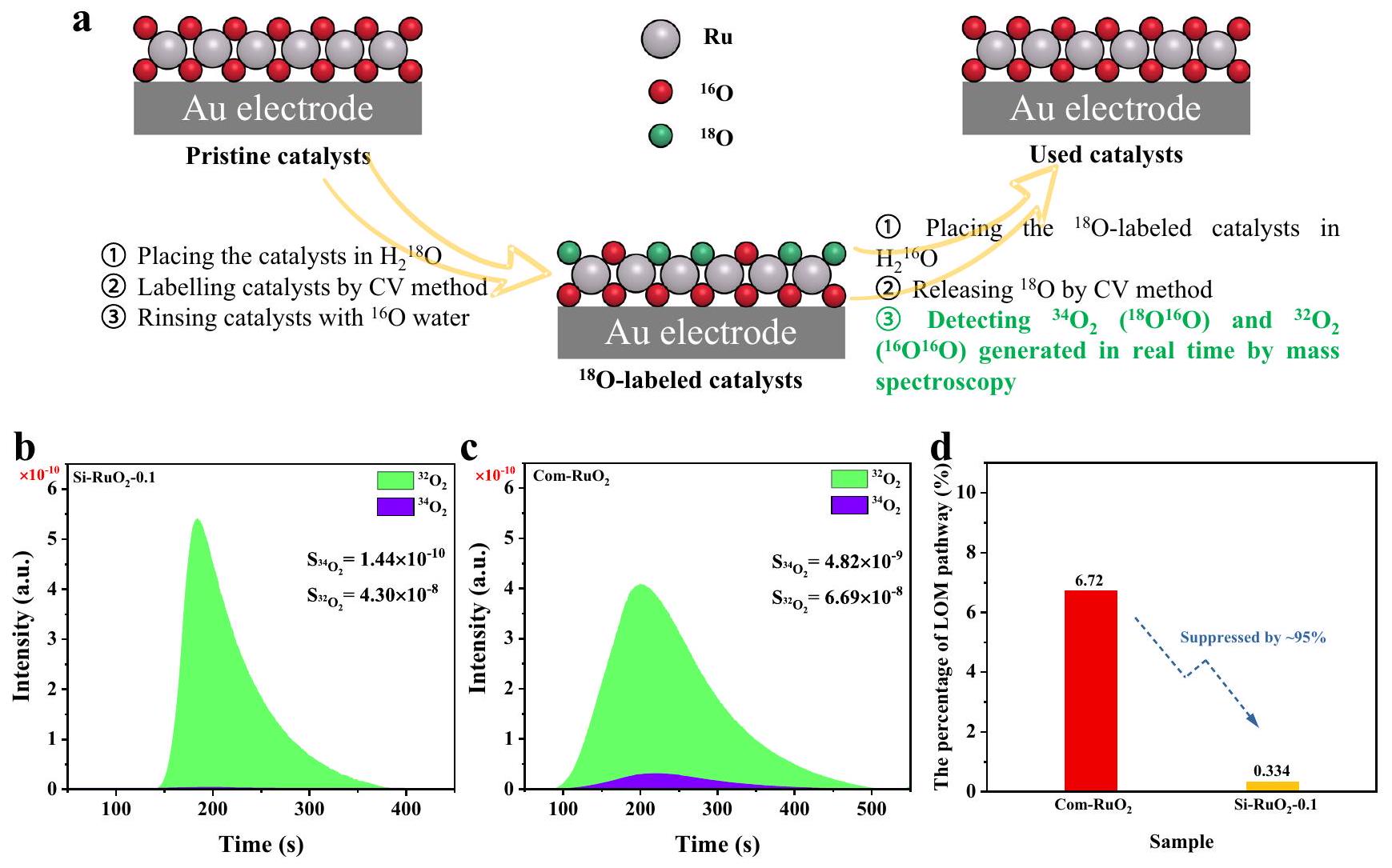
تحليل الطيف الكتلي للبلز المتصل بالتحليل (ICP-MS) لذوبان أيونات Ru
التحليل الطيفي الكهروكيميائي التفاضلي (DEMS)
الحسابات النظرية
نموذج استنادًا إلى محتوى Si المتوقع في النص الرئيسي. خلال عملية تحسين الهيكل، تم إجراء تكامل منطقة Brillouin مع
توفر البيانات
References
- Yeo, K.-R., Lee, K.-S., Kim, H., Lee, J. & Kim, S.-K. A highly active and stable 3D dandelion spore-structured self-supporting Ir-based electrocatalyst for proton exchange membrane water electrolysis fabricated using structural reconstruction. Energy Environ. Sci. 15, 3449-3461 (2022).
- Pan, S. et al. Efficient and stable noble-metal-free catalyst for acidic water oxidation. Nat. Commun. 13, 2294 (2022).
- An, L. et al. Recent development of oxygen evolution electrocatalysts in acidic environment. Adv. Mater. 33, 2006328 (2021).
- Spöri, C., Kwan, J. T. H., Bonakdarpour, A., Wilkinson, D. P. & Strasser, P . The stability challenges of oxygen evolving catalysts: towards a common fundamental understanding and mitigation of catalyst degradation. Angew. Chem. Int. Ed. 56, 5994-6021 (2017).
- Zheng, Y.-R. et al. Monitoring oxygen production on mass-selected iridium-tantalum oxide electrocatalysts. Nat. Energy 7, 55-64 (2022).
- He, J., Zhou, X., Xu, P. & Sun, J. Regulating electron redistribution of intermetallic iridium oxide by incorporating Ru for efficient acidic water oxidation. Adv. Energy Mater. 11, 2102883 (2021).
- Wang, H. et al. Significantly enhanced overall water splitting performance by partial oxidation of Ir through Au modification in core-shell alloy structure. J. Am. Chem. Soc. 143, 4639-4645 (2021).
- Chen, Y. et al. Exceptionally active iridium evolved from a pseudocubic perovskite for oxygen evolution in acid. Nat. Commun. 10, 572 (2019).
- Zhang, J. et al. Core-shell nanostructured Ru@lr-O electrocatalysts for superb oxygen evolution in acid. Small 18, 2108031 (2022).
-
. et al. In-situ reconstructed Ru atom array on with enhanced performance for acidic water oxidation. Nat. Catal. 4, 1012-1023 (2021). - Hao, S. et al. Torsion strained iridium oxide for efficient acidic water oxidation in proton exchange membrane electrolyzers. Nat. Nanotechnol. 16, 1371-1377 (2021).
- Seitz, L. C. et al. A highly active and stable IrO
catalyst for the oxygen evolution reaction. Science 353, 1011-1014 (2016). - An, L. et al. A functionally stable RuMn electrocatalyst for oxygen evolution eraction in acid. Adv. Funct. Mater. 32, 2200131 (2022).
- He, J., Li, W., Xu, P. & Sun, J. Tuning electron correlations of
by co-doping of Mo and Ce for boosting electrocatalytic water oxidation in acidic media. Appl. Catal. B Environ. 298, 120528 (2021). - Wang, K. et al. Highly active ruthenium sites stabilized by modulating electron-feeding for sustainable acidic oxygen-evolution electrocatalysis. Energy Environ. Sci. 15, 2356-2365 (2022).
- Yao, Q. et al. S incorporated
-based nanorings for active and stable water oxidation in acid. Nano Res. 15, 3964-3970 (2022). - Grimaud, A. et al. Activating lattice oxygen redox reactions in metal oxides to catalyse oxygen evolution. Nat. Chem. 9, 457-465 (2017).
- Hao, S. et al. Dopants fixation of ruthenium for boosting acidic oxygen evolution stability and activity. Nat. Commun. 11, 5368 (2020).
- Cui, X. et al. Robust interface Ru centers for high-performance acidic oxygen evolution. Adv. Mater. 32, 1908126 (2020).
- Miao, X. et al. Quadruple perovskite ruthenate as a highly efficient catalyst for acidic water oxidation. Nat. Commun. 10, 3809 (2019).
- Cao, L. et al. Dynamic oxygen adsorption on single-atomic ruthenium catalyst with high performance for acidic oxygen evolution reaction. Nat. Commun. 10, 4849 (2019).
- Yao, Y. et al. Engineering the electronic structure of single atom Ru sites via compressive strain boosts acidic water oxidation electrocatalysis. Nat. Catal. 2, 304-313 (2019).
- Zhang, Y., Zhu, X., Zhang, G., Shi, P. & Wang, A.-L. Rational catalyst design for oxygen evolution under acidic conditions: strategies toward enhanced electrocatalytic performance. J. Mater. Chem. A 9, 5890-5914 (2021).
- Tian, Y. et al. A Co-doped nanorod-like
electrocatalyst with abundant oxygen vacancies for acidic water oxidation. iScience 23, 100756 (2020). - Zhang, L. et al. Sodium-decorated amorphous/crystalline
with rich oxygen vacancies: a robust pH -universal oxygen evolution electrocatalyst. Angew. Chem. Int. Ed. 60, 18821-18829 (2021). - Lin, Y. et al. Chromium-ruthenium oxide solid solution electrocatalyst for highly efficient oxygen evolution reaction in acidic media. Nat. Commun. 10, 162 (2019).
- Su, J. et al. Assembling ultrasmall copper-doped ruthenium oxide nanocrystals into hollow porous polyhedra: highly robust electrocatalysts for oxygen evolution in acidic media. Adv. Mater. 30, 1801351 (2018).
- Macounova, K., Makarova, M. & Krtil, P. Oxygen evolution on nanocrystalline
and electrodes-DEMS approach to reaction mechanism determination. Electrochem. Commun. 11, 1865-1868 (2009). - Shi, Z. et al. Customized reaction route for ruthenium oxide towards stabilized water oxidation in high-performance PEM electrolyzers. Nat. Commun. 14, 843 (2023).
- Liu, Y. et al. Iridium-containing water-oxidation catalysts in acidic electrolyte. Chin. J. Catal. 42, 1054-1077 (2021).
- Chen, F.-Y., Wu, Z.-Y., Adler, Z. & Wang, H. Stability challenges of electrocatalytic oxygen evolution reaction: from mechanistic understanding to reactor design. Joule 5, 1704-1731 (2021).
- Kasian, O., Grote, J.-P., Geiger, S., Cherevko, S. & Mayrhofer, K. J. J. The common intermediates of oxygen evolution and dissolution reactions during water electrolysis on iridium. Angew. Chem. Int. Ed. 57, 2488-2491 (2018).
- Dean, J. A. Lange’s Handbook of Chemistry 15th edn, Vol. 1424, 4.30-4.50 (McGraw-Hill Professional, 1999).
- Pan, Y. et al. Direct evidence of boosted oxygen evolution over perovskite by enhanced lattice oxygen participation. Nat. Commun. 11, 2002 (2020).
- Kim, D. et al. Blue-silica by Eu
-activator occupied in interstitial sites. RSC Adv. 5, 74790-74801 (2015). - Gu, Y. et al. Crystal splintering of
induced by interstitial Ru doping toward reversible oxygen conversion. Chem. Mater. 33, 4135-4145 (2021). - Liu, C., Jiang, Y., Wang, T., Li, Q. & Liu, Y. Nano Si-doped ruthenium oxide particles from caged precursors for high-performance acidic oxygen evolution. Adv. Sci. 10, 2207429 (2023).
- Ping, X. et al. Tailoring B-site of lead-ruthenate pyrochlore for boosting acidic water oxidation activity and stability. Appl. Catal. B Environ. 318, 121884 (2022).
- Zhu, Y. et al. Self-assembled ruddlesden-popper/perovskite hybrid with lattice-oxygen activation as a superior oxygen evolution electrocatalyst. Small 16, 2001204 (2020).
- Shen, Z. et al. Increased activity in the oxygen evolution reaction by
-induced hole states in perovskite . J. Mater. Chem. A 8, 4407-4415 (2020). - Huang, Z.-F. et al. Chemical and structural origin of lattice oxygen oxidation in Co-Zn oxyhydroxide oxygen evolution electrocatalysts. Nat. Energy 4, 329-338 (2019).
- Qin, Y. et al. RuO2 electronic structure and lattice strain dual engineering for enhanced acidic oxygen evolution reaction performance. Nat. Commun. 13, 3784 (2022).
- Kim, J. et al. A porous pyrochlore
electrocatalyst for enhanced performance towards the oxygen evolution reaction in acidic media. Angew. Chem. Int. Ed. 57, 13877-13881 (2018). - Wang, J. et al. Exceptionally active and stable
with interstitial carbon for water oxidation in acid. Chem 8, 1673-1687 (2022). - Wang, X., Zhong, H., Xi, S., Lee, W. S. V. & Xue, J. Understanding of oxygen redox in the oxygen evolution reaction. Adv. Mater. 34, 2107956 (2022).
- Hwang, J. et al. Perovskites in catalysis and electrocatalysis. Science 358, 751-756 (2017).
- Liang, X. et al. Perovskite-type solid solution nano-electrocatalysts enable simultaneously enhanced activity and stability for oxygen evolution. Adv. Mater. 32, 2001430 (2020).
- Shin, J. F., Apperley, D. C. & Slater, P. R. Silicon doping in
: example of a beneficial effect of silicon incorporation on oxide ion/ proton conductivity. Chem. Mater. 22, 5945-5948 (2010). - Appel, C. C. & Bonanos, N. Structural and electrical characterisation of silica-containing yttria-stabilised zirconia. J. Eur. Ceram. Soc. 19, 847-851 (1999).
- Ge, R. et al. Ultrafine defective
electrocatayst integrated on carbon cloth for robust water oxidation in acidic media. Adv. Energy Mater. 9, 1901313 (2019). - Chen, S. et al. Mn-doped
nanocrystals as highly active electrocatalysts for enhanced oxygen evolution in acidic media. ACS Catal. 10, 1152-1160 (2020). - Wang, Q. et al. Ultrahigh-loading of Ir single atoms on NiO matrix to dramatically enhance oxygen evolution reaction. J. Am. Chem. Soc. 142, 7425-7433 (2020).
- Shi, Z. et al. Confined Ir single sites with triggered lattice oxygen redox: toward boosted and sustained water oxidation catalysis. Joule 5, 2164-2176 (2021).
- Geiger, S. et al. The stability number as a metric for electrocatalyst stability benchmarking. Nat. Catal. 1, 508-515 (2018).
- Li, R. et al. IrW nanochannel support enabling ultrastable electrocatalytic oxygen evolution at
in acidic media. Nat. Commun. 12, 3540 (2021). - Xue, Y. et al. Sulfate-functionalized RuFeOx as highly efficient oxygen evolution reaction electrocatalyst in acid. Adv. Funct. Mater. 31, 2101405 (2021).
- Shi, Z. et al. Enhanced acidic water oxidation by dynamic migration of oxygen species at the
catalyst/support interfaces. Angew. Chem. Int. Ed. 61, e202212341 (2022). - Liang, H. et al. Tungsten blue oxide as a reusable electrocatalyst for acidic water oxidation by plasma-induced vacancy engineering. CCS Chem. 3, 1553-1561 (2020).
- Yu, B. et al. Densely populated tiny
crystallites supported by hierarchically porous carbon for full acidic water splitting. Mater. Horiz. 10, 4589-4596 (2023). - Wen, Y. et al. Stabilizing highly active Ru sites by suppressing lattice oxygen participation in acidic water oxidation. J. Am. Chem. Soc. 143, 6482-6490 (2021).
الشكر والتقدير
مساهمات المؤلفين
المصالح المتنافسة
معلومات إضافية
(ج) المؤلفون 2024
كلية الكيمياء والهندسة الكيميائية، المختبر الوطني الرئيسي لمصادر الطاقة الكيميائية المتقدمة (SKL-ACPS)، جامعة تشونغتشينغ، تشونغتشينغ، الصين. المختبر الوطني الرئيسي لمواد التحفيز وهندسة التفاعلات، معهد أبحاث معالجة النفط SINOPEC، بكين، الصين. ساهم هؤلاء المؤلفون بالتساوي: شينيو بينغ، يونغدو ليو. -البريد: csg810519@126.com
DOI: https://doi.org/10.1038/s41467-024-46815-6
PMID: https://pubmed.ncbi.nlm.nih.gov/38509091
Publication Date: 2024-03-20
Locking the lattice oxygen in
Accepted: 4 March 2024
Published online: 20 March 2024
(A) Check for updates
Abstract
Ruthenium dioxide is presently the most active catalyst for the oxygen evolution reaction (OER) in acidic media but suffers from severe Ru dissolution resulting from the high covalency of Ru-O bonds triggering lattice oxygen oxidation. Here, we report an interstitial silicon-doping strategy to stabilize the highly active Ru sites of
activating the LOM pathway, resulting in improved activity but poor stability. Very few elements have been reported and used to weaken RuO bond covalency and improve the stability of Ru-based oxides. Zhang’s group

atoms with doping atoms, which, however, limits the quantity of Ru-O structures in the catalyst and is not beneficial for further improving the activity of Ru-based oxides. Therefore, exploring effective doping strategies for weakening the Ru-O bond covalency without losing the quantity of Ru-O structure is extraordinarily desirable but challenging.
Results and discussions
Synthesis and characterization of
(denoted

elemental mapping. As displayed in Supplementary Figs. 1a, b-5a, b, the TEM images of all
which was attributed to the Si-O bond associated with interstitial Si. When the Si content increased to 0.2 and 0.3 , an additional peak at 104.1 eV consistent with the

f Schematic diagram of the band structures of
extended X-ray absorption fine structure (FT-EXAFS) spectra of Si-
OER performance measurement and the origin of the enhanced activity

Catalytic stability evaluation
Characterization of

a Chronopotentiometry stability curves of
the stability and degradation rate of the Ru-based oxide catalyst at
and Com-
DEMS measurement
Methods
Chemicals and materials

ratios for
Material characterization
resonance (EPR) spectra were obtained on a Bruker EMXPLUS spectrometer with a microwave frequency of 9.84 GHz . The
Electrochemical measurements

Inductively coupled plasma-mass spectrometry (ICP-MS) analysis of Ru ion dissolution
Differential electrochemical mass spectrometry (DEMS)
Theoretical calculations
model based on the predicted Si content in the main text. During the structural optimization process, Brillouin zone integration was performed with
Data availability
References
- Yeo, K.-R., Lee, K.-S., Kim, H., Lee, J. & Kim, S.-K. A highly active and stable 3D dandelion spore-structured self-supporting Ir-based electrocatalyst for proton exchange membrane water electrolysis fabricated using structural reconstruction. Energy Environ. Sci. 15, 3449-3461 (2022).
- Pan, S. et al. Efficient and stable noble-metal-free catalyst for acidic water oxidation. Nat. Commun. 13, 2294 (2022).
- An, L. et al. Recent development of oxygen evolution electrocatalysts in acidic environment. Adv. Mater. 33, 2006328 (2021).
- Spöri, C., Kwan, J. T. H., Bonakdarpour, A., Wilkinson, D. P. & Strasser, P . The stability challenges of oxygen evolving catalysts: towards a common fundamental understanding and mitigation of catalyst degradation. Angew. Chem. Int. Ed. 56, 5994-6021 (2017).
- Zheng, Y.-R. et al. Monitoring oxygen production on mass-selected iridium-tantalum oxide electrocatalysts. Nat. Energy 7, 55-64 (2022).
- He, J., Zhou, X., Xu, P. & Sun, J. Regulating electron redistribution of intermetallic iridium oxide by incorporating Ru for efficient acidic water oxidation. Adv. Energy Mater. 11, 2102883 (2021).
- Wang, H. et al. Significantly enhanced overall water splitting performance by partial oxidation of Ir through Au modification in core-shell alloy structure. J. Am. Chem. Soc. 143, 4639-4645 (2021).
- Chen, Y. et al. Exceptionally active iridium evolved from a pseudocubic perovskite for oxygen evolution in acid. Nat. Commun. 10, 572 (2019).
- Zhang, J. et al. Core-shell nanostructured Ru@lr-O electrocatalysts for superb oxygen evolution in acid. Small 18, 2108031 (2022).
-
. et al. In-situ reconstructed Ru atom array on with enhanced performance for acidic water oxidation. Nat. Catal. 4, 1012-1023 (2021). - Hao, S. et al. Torsion strained iridium oxide for efficient acidic water oxidation in proton exchange membrane electrolyzers. Nat. Nanotechnol. 16, 1371-1377 (2021).
- Seitz, L. C. et al. A highly active and stable IrO
catalyst for the oxygen evolution reaction. Science 353, 1011-1014 (2016). - An, L. et al. A functionally stable RuMn electrocatalyst for oxygen evolution eraction in acid. Adv. Funct. Mater. 32, 2200131 (2022).
- He, J., Li, W., Xu, P. & Sun, J. Tuning electron correlations of
by co-doping of Mo and Ce for boosting electrocatalytic water oxidation in acidic media. Appl. Catal. B Environ. 298, 120528 (2021). - Wang, K. et al. Highly active ruthenium sites stabilized by modulating electron-feeding for sustainable acidic oxygen-evolution electrocatalysis. Energy Environ. Sci. 15, 2356-2365 (2022).
- Yao, Q. et al. S incorporated
-based nanorings for active and stable water oxidation in acid. Nano Res. 15, 3964-3970 (2022). - Grimaud, A. et al. Activating lattice oxygen redox reactions in metal oxides to catalyse oxygen evolution. Nat. Chem. 9, 457-465 (2017).
- Hao, S. et al. Dopants fixation of ruthenium for boosting acidic oxygen evolution stability and activity. Nat. Commun. 11, 5368 (2020).
- Cui, X. et al. Robust interface Ru centers for high-performance acidic oxygen evolution. Adv. Mater. 32, 1908126 (2020).
- Miao, X. et al. Quadruple perovskite ruthenate as a highly efficient catalyst for acidic water oxidation. Nat. Commun. 10, 3809 (2019).
- Cao, L. et al. Dynamic oxygen adsorption on single-atomic ruthenium catalyst with high performance for acidic oxygen evolution reaction. Nat. Commun. 10, 4849 (2019).
- Yao, Y. et al. Engineering the electronic structure of single atom Ru sites via compressive strain boosts acidic water oxidation electrocatalysis. Nat. Catal. 2, 304-313 (2019).
- Zhang, Y., Zhu, X., Zhang, G., Shi, P. & Wang, A.-L. Rational catalyst design for oxygen evolution under acidic conditions: strategies toward enhanced electrocatalytic performance. J. Mater. Chem. A 9, 5890-5914 (2021).
- Tian, Y. et al. A Co-doped nanorod-like
electrocatalyst with abundant oxygen vacancies for acidic water oxidation. iScience 23, 100756 (2020). - Zhang, L. et al. Sodium-decorated amorphous/crystalline
with rich oxygen vacancies: a robust pH -universal oxygen evolution electrocatalyst. Angew. Chem. Int. Ed. 60, 18821-18829 (2021). - Lin, Y. et al. Chromium-ruthenium oxide solid solution electrocatalyst for highly efficient oxygen evolution reaction in acidic media. Nat. Commun. 10, 162 (2019).
- Su, J. et al. Assembling ultrasmall copper-doped ruthenium oxide nanocrystals into hollow porous polyhedra: highly robust electrocatalysts for oxygen evolution in acidic media. Adv. Mater. 30, 1801351 (2018).
- Macounova, K., Makarova, M. & Krtil, P. Oxygen evolution on nanocrystalline
and electrodes-DEMS approach to reaction mechanism determination. Electrochem. Commun. 11, 1865-1868 (2009). - Shi, Z. et al. Customized reaction route for ruthenium oxide towards stabilized water oxidation in high-performance PEM electrolyzers. Nat. Commun. 14, 843 (2023).
- Liu, Y. et al. Iridium-containing water-oxidation catalysts in acidic electrolyte. Chin. J. Catal. 42, 1054-1077 (2021).
- Chen, F.-Y., Wu, Z.-Y., Adler, Z. & Wang, H. Stability challenges of electrocatalytic oxygen evolution reaction: from mechanistic understanding to reactor design. Joule 5, 1704-1731 (2021).
- Kasian, O., Grote, J.-P., Geiger, S., Cherevko, S. & Mayrhofer, K. J. J. The common intermediates of oxygen evolution and dissolution reactions during water electrolysis on iridium. Angew. Chem. Int. Ed. 57, 2488-2491 (2018).
- Dean, J. A. Lange’s Handbook of Chemistry 15th edn, Vol. 1424, 4.30-4.50 (McGraw-Hill Professional, 1999).
- Pan, Y. et al. Direct evidence of boosted oxygen evolution over perovskite by enhanced lattice oxygen participation. Nat. Commun. 11, 2002 (2020).
- Kim, D. et al. Blue-silica by Eu
-activator occupied in interstitial sites. RSC Adv. 5, 74790-74801 (2015). - Gu, Y. et al. Crystal splintering of
induced by interstitial Ru doping toward reversible oxygen conversion. Chem. Mater. 33, 4135-4145 (2021). - Liu, C., Jiang, Y., Wang, T., Li, Q. & Liu, Y. Nano Si-doped ruthenium oxide particles from caged precursors for high-performance acidic oxygen evolution. Adv. Sci. 10, 2207429 (2023).
- Ping, X. et al. Tailoring B-site of lead-ruthenate pyrochlore for boosting acidic water oxidation activity and stability. Appl. Catal. B Environ. 318, 121884 (2022).
- Zhu, Y. et al. Self-assembled ruddlesden-popper/perovskite hybrid with lattice-oxygen activation as a superior oxygen evolution electrocatalyst. Small 16, 2001204 (2020).
- Shen, Z. et al. Increased activity in the oxygen evolution reaction by
-induced hole states in perovskite . J. Mater. Chem. A 8, 4407-4415 (2020). - Huang, Z.-F. et al. Chemical and structural origin of lattice oxygen oxidation in Co-Zn oxyhydroxide oxygen evolution electrocatalysts. Nat. Energy 4, 329-338 (2019).
- Qin, Y. et al. RuO2 electronic structure and lattice strain dual engineering for enhanced acidic oxygen evolution reaction performance. Nat. Commun. 13, 3784 (2022).
- Kim, J. et al. A porous pyrochlore
electrocatalyst for enhanced performance towards the oxygen evolution reaction in acidic media. Angew. Chem. Int. Ed. 57, 13877-13881 (2018). - Wang, J. et al. Exceptionally active and stable
with interstitial carbon for water oxidation in acid. Chem 8, 1673-1687 (2022). - Wang, X., Zhong, H., Xi, S., Lee, W. S. V. & Xue, J. Understanding of oxygen redox in the oxygen evolution reaction. Adv. Mater. 34, 2107956 (2022).
- Hwang, J. et al. Perovskites in catalysis and electrocatalysis. Science 358, 751-756 (2017).
- Liang, X. et al. Perovskite-type solid solution nano-electrocatalysts enable simultaneously enhanced activity and stability for oxygen evolution. Adv. Mater. 32, 2001430 (2020).
- Shin, J. F., Apperley, D. C. & Slater, P. R. Silicon doping in
: example of a beneficial effect of silicon incorporation on oxide ion/ proton conductivity. Chem. Mater. 22, 5945-5948 (2010). - Appel, C. C. & Bonanos, N. Structural and electrical characterisation of silica-containing yttria-stabilised zirconia. J. Eur. Ceram. Soc. 19, 847-851 (1999).
- Ge, R. et al. Ultrafine defective
electrocatayst integrated on carbon cloth for robust water oxidation in acidic media. Adv. Energy Mater. 9, 1901313 (2019). - Chen, S. et al. Mn-doped
nanocrystals as highly active electrocatalysts for enhanced oxygen evolution in acidic media. ACS Catal. 10, 1152-1160 (2020). - Wang, Q. et al. Ultrahigh-loading of Ir single atoms on NiO matrix to dramatically enhance oxygen evolution reaction. J. Am. Chem. Soc. 142, 7425-7433 (2020).
- Shi, Z. et al. Confined Ir single sites with triggered lattice oxygen redox: toward boosted and sustained water oxidation catalysis. Joule 5, 2164-2176 (2021).
- Geiger, S. et al. The stability number as a metric for electrocatalyst stability benchmarking. Nat. Catal. 1, 508-515 (2018).
- Li, R. et al. IrW nanochannel support enabling ultrastable electrocatalytic oxygen evolution at
in acidic media. Nat. Commun. 12, 3540 (2021). - Xue, Y. et al. Sulfate-functionalized RuFeOx as highly efficient oxygen evolution reaction electrocatalyst in acid. Adv. Funct. Mater. 31, 2101405 (2021).
- Shi, Z. et al. Enhanced acidic water oxidation by dynamic migration of oxygen species at the
catalyst/support interfaces. Angew. Chem. Int. Ed. 61, e202212341 (2022). - Liang, H. et al. Tungsten blue oxide as a reusable electrocatalyst for acidic water oxidation by plasma-induced vacancy engineering. CCS Chem. 3, 1553-1561 (2020).
- Yu, B. et al. Densely populated tiny
crystallites supported by hierarchically porous carbon for full acidic water splitting. Mater. Horiz. 10, 4589-4596 (2023). - Wen, Y. et al. Stabilizing highly active Ru sites by suppressing lattice oxygen participation in acidic water oxidation. J. Am. Chem. Soc. 143, 6482-6490 (2021).
Acknowledgements
Author contributions
Competing interests
Additional information
(c) The Author(s) 2024
College of Chemistry and Chemical Engineering, State Key Laboratory of Advanced Chemical Power Sources (SKL-ACPS), Chongqing University, Chongqing, China. State Key Laboratory of Catalytic Materials and Reaction Engineering, SINOPEC Research Institute of Petroleum Processing Co., Ltd., Beijing, China. These authors contributed equally: Xinyu Ping, Yongduo Liu. -mail: csg810519@126.com
