DOI: https://doi.org/10.1007/s40820-024-01375-8
PMID: https://pubmed.ncbi.nlm.nih.gov/38512500
تاريخ النشر: 2024-03-21
رسائل النانو والميكرو
(2024) 16:160
تم القبول: 5 فبراير 2024
نُشر على الإنترنت: 21 مارس 2024
© المؤلف(ون) 2024
تعزيز أداء تخزين الهيدروجين لـ MgH2 بواسطة طبقة نانوية غنية بفجوات الأكسجين H-V2O5 كمضخة هيدروجين مثارة
النقاط البارزة
- ثنائي الأبعاد شبيه بالجرافين
تم تصميم الأوراق النانوية الغنية بفراغات الأكسجين كعوامل تحفيز متعددة الوظائف لتصنيع المركبات. - يبدأ إطلاق الهيدروجين من
واحتفاظ السعة مرتفع كما هو بعد 100 دورة عند . - تتميز المركبات بسرعة الديناميكا وقدرة مذهلة على امتصاص الهيدروجين عند درجات حرارة قريبة من درجة حرارة الغرفة.
- يمكن أن تعزز الفجوات الأكسجينية الديناميات بشكل مباشر
بينما يثير بشكل غير مباشر تأثير “مضخة الهيدروجين” .
الملخص
MgH}_{2}$ هو مادة واعدة لتخزين الهيدروجين في الحالة الصلبة بسعة عالية، بينما تعيق تطبيقاته بشكل كبير درجة حرارة الإزالة العالية والحركية البطيئة. هنا، يتم تقديم فراغات الأكسجين الغنية ثنائية الأبعاد المتشابكة.
1 المقدمة
تستخدم معادن الفاناديوم (V) وأكاسيدها المقابلة غالبًا كعوامل حفازة نظرًا لتعدد التكافؤ ونشاطها الحفاز العالي.
أداء تخزين الهيدروجين لـ
قسم التجربة 2
2.1 إعداد العينة
2.1.1 تخليق الهيدروجين المائي
ماء مقطر تحت التحريك. ثم، 1 مل من حمض الهيدروكلوريك المركز (
2.1.2 تخليق
2.2 توصيف وقياسات
تمت عملية الصوتنة، ثم تم إسقاط العينة على شبكة نحاسية ونقلها بسرعة إلى المعدات. كانت ظروف تحضير العينات لتحليلات SEM مشابهة لملاحظات TEM. الاختلاف الوحيد هو أن العينات تم تفريقها في السيكلوهكسان وتم إسقاطها على شريحة السيليكون. تم إجراء مطيافية الأشعة السينية للألكترونات (XPS، Kratos AXIS Ultra DLD) لتحليل حالة التكافؤ وطبيعة الروابط الكيميائية للعناصر المكونة لـ
2.3 الحسابات النظرية
3 النتائج والمناقشة
3.1 توصيف المادة التي تم تصنيعها
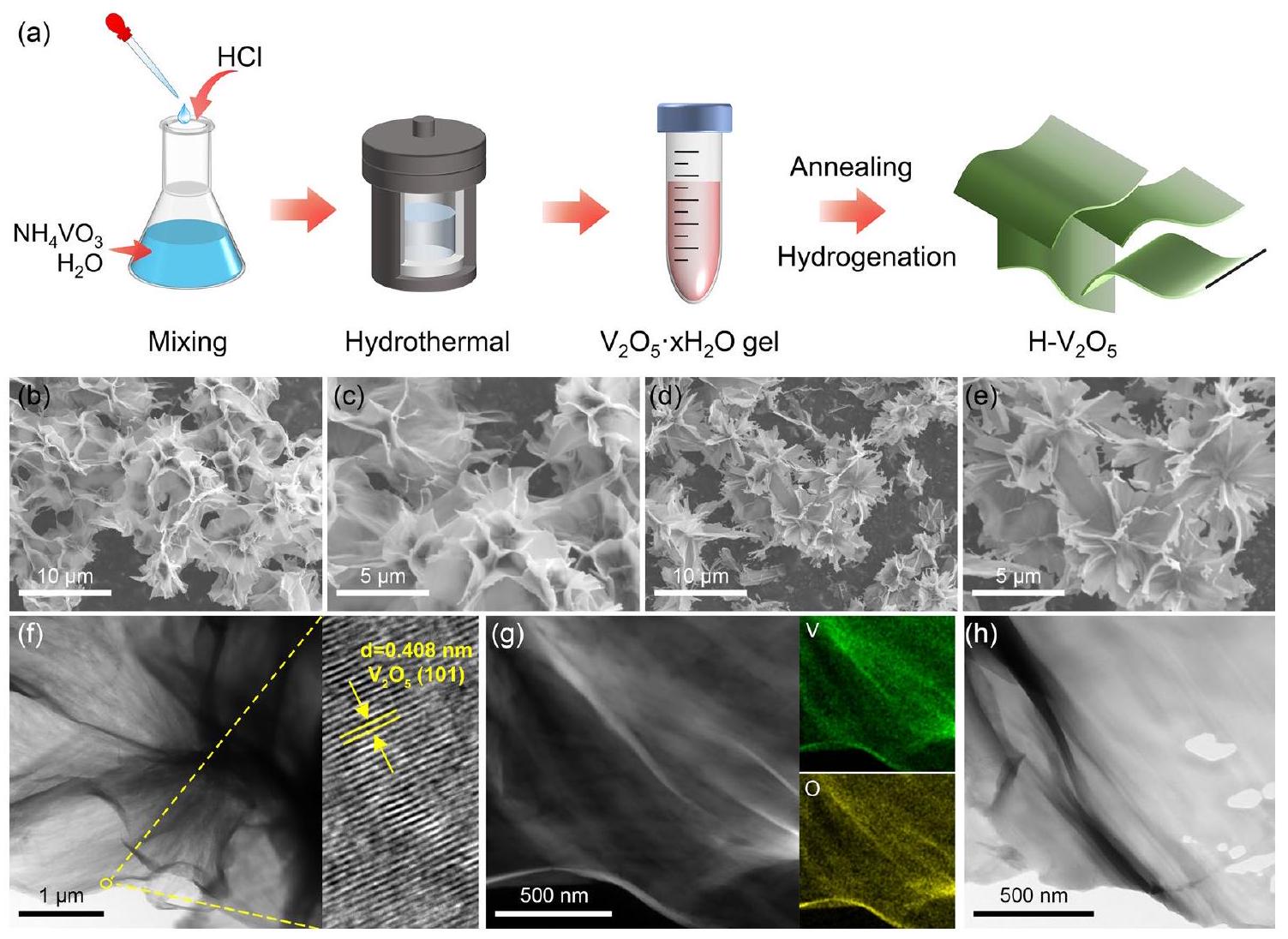
مما يؤدي إلى تحسين أداء تخزين الهيدروجين
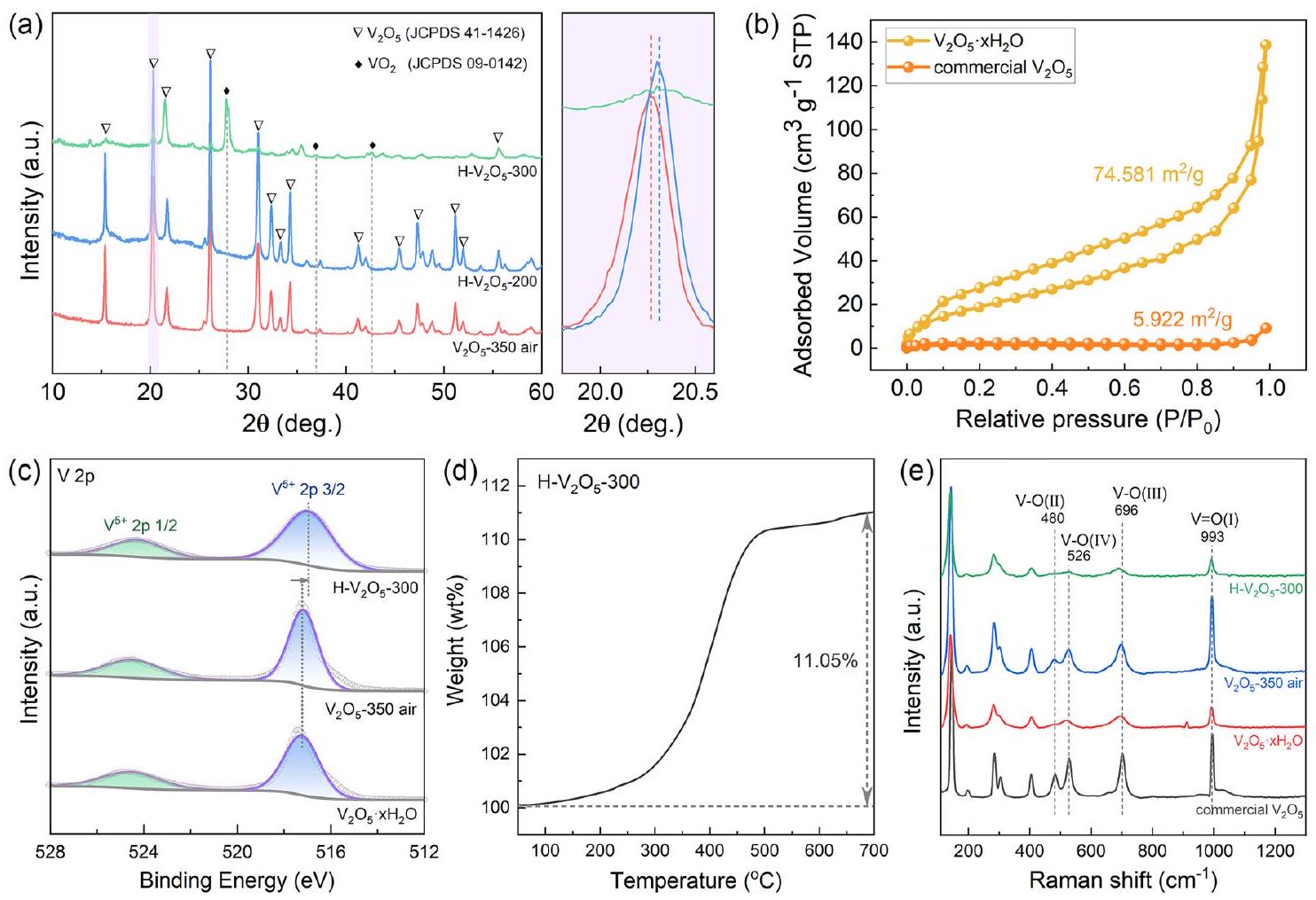
في جو من الأكسجين. بافتراض الصيغة الكيميائية لـ
3.2 التأثير الحفزي لـ
سعة تبلغ حوالي
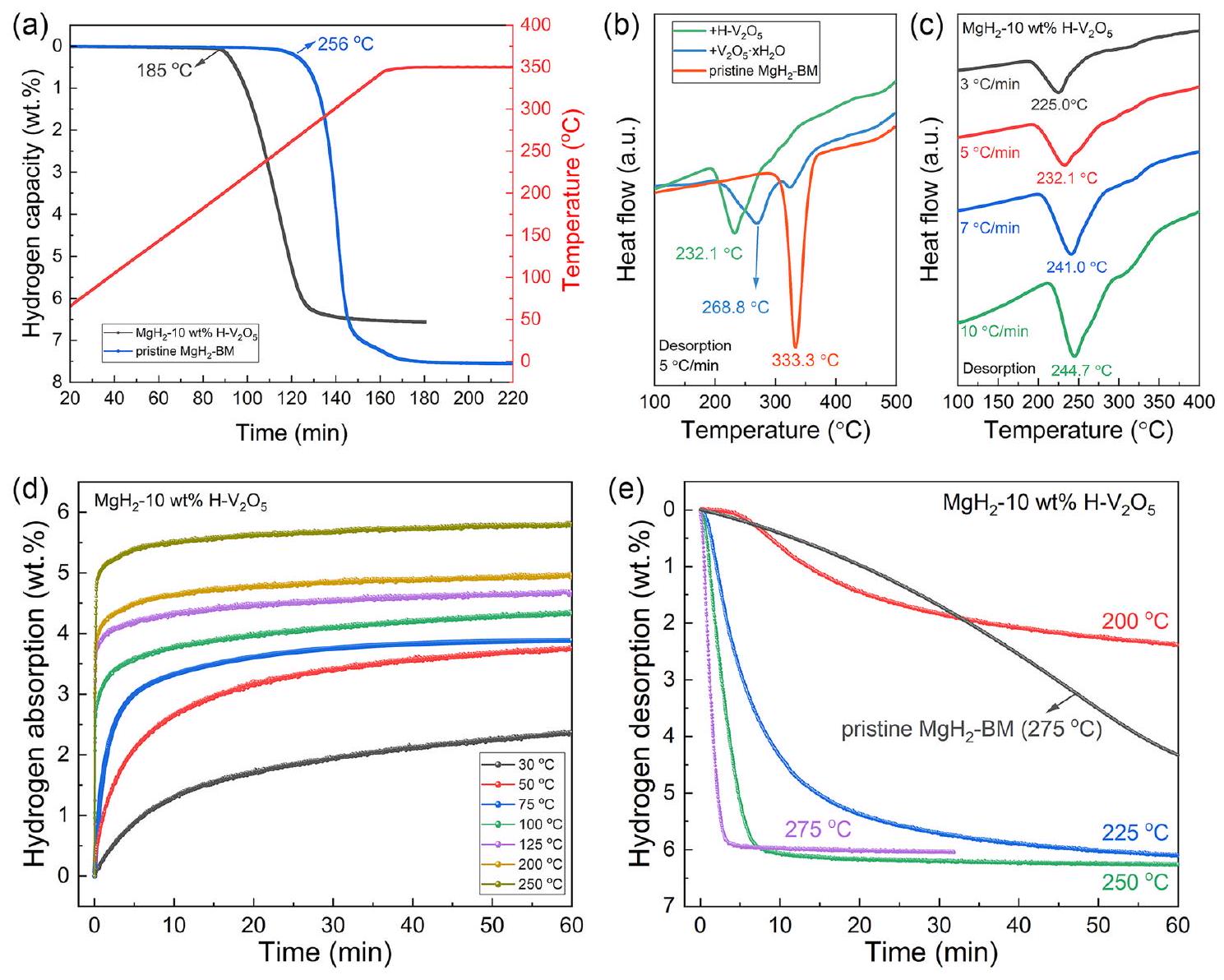
بسعة امتصاص الهيدروجين
طاقة (
الإعلانات التجارية
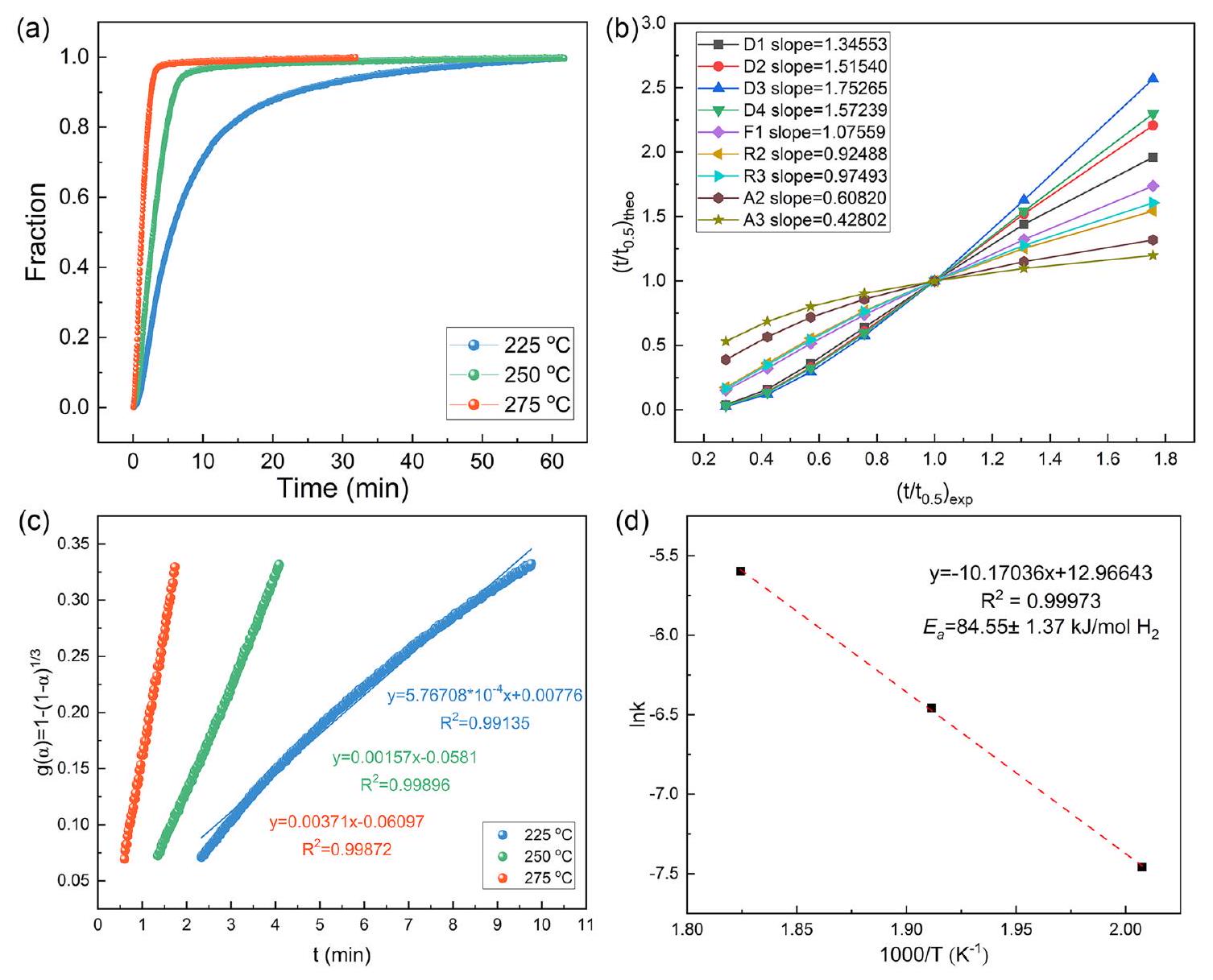
من
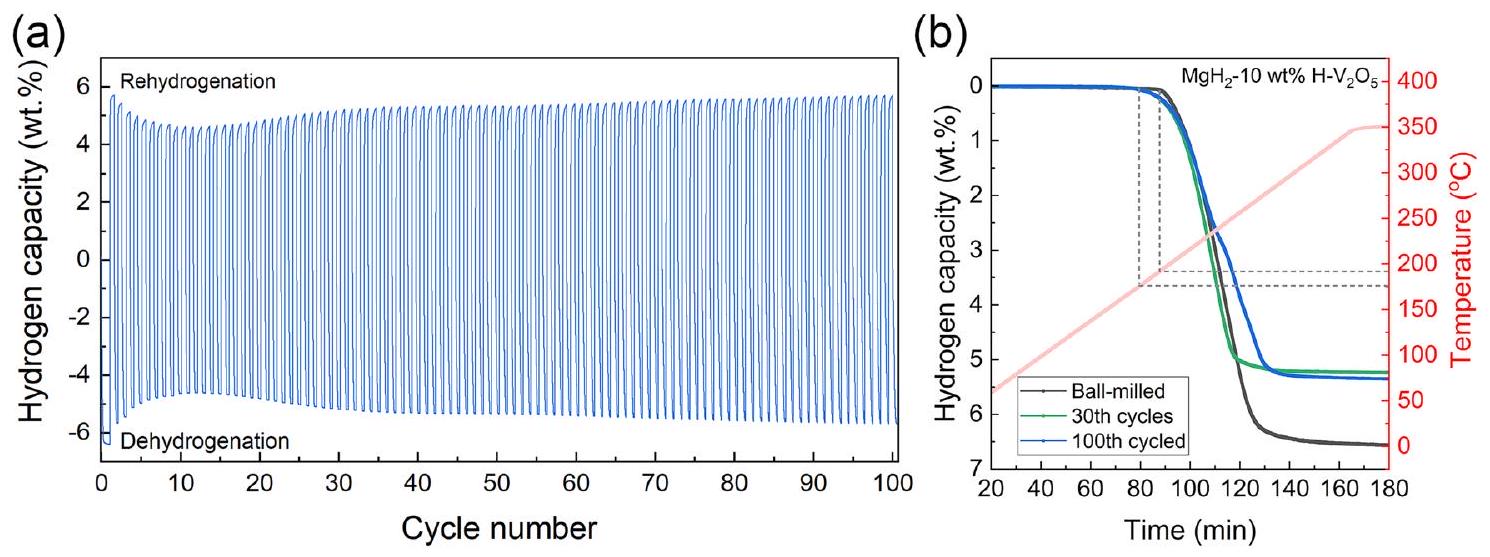
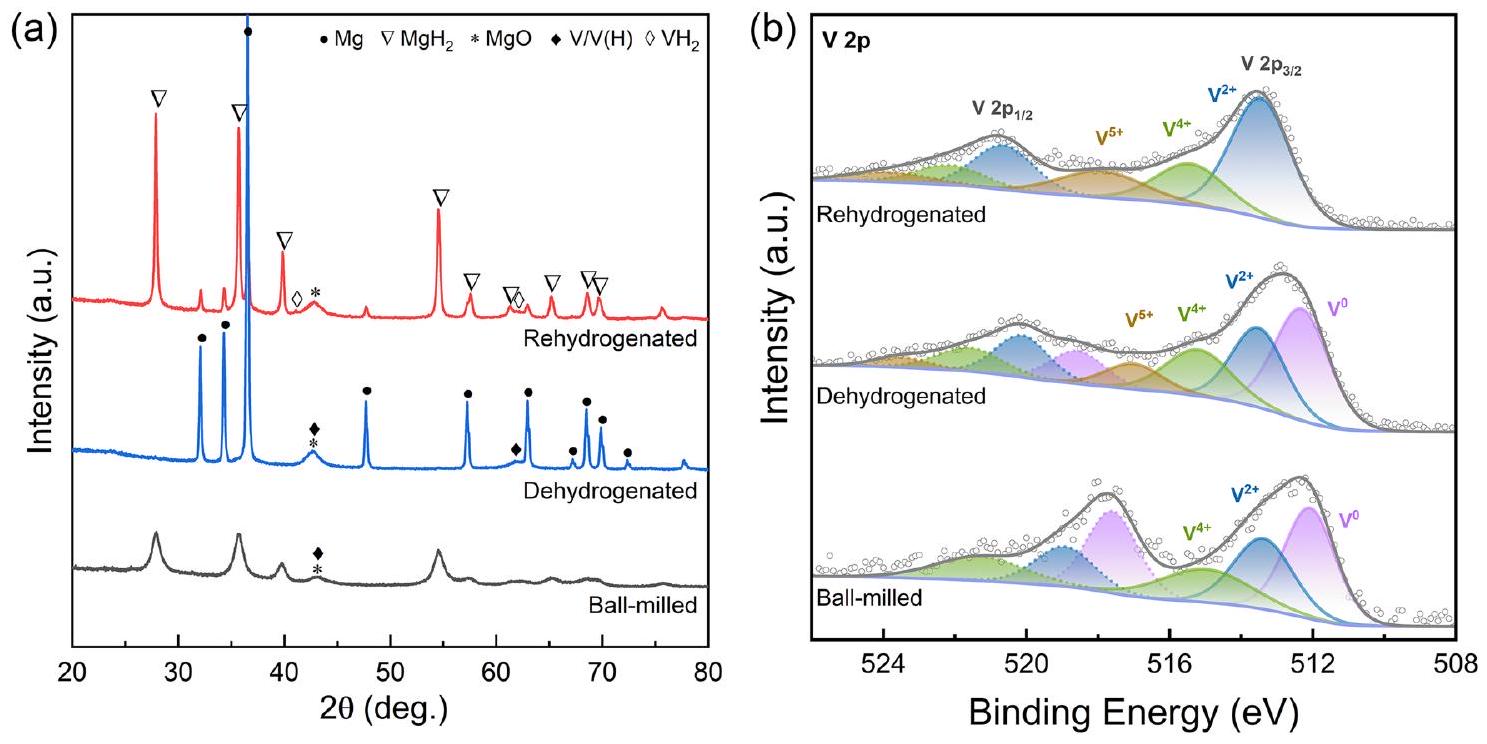
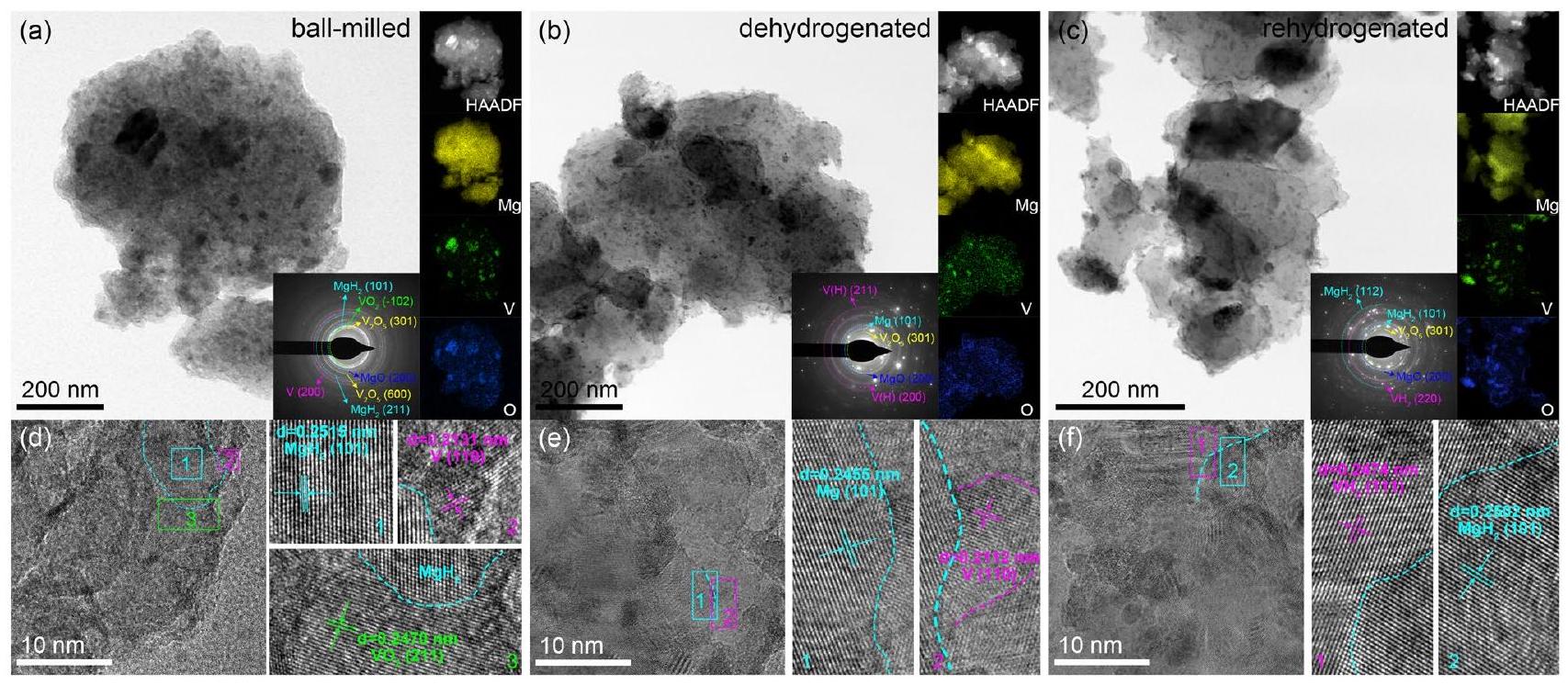
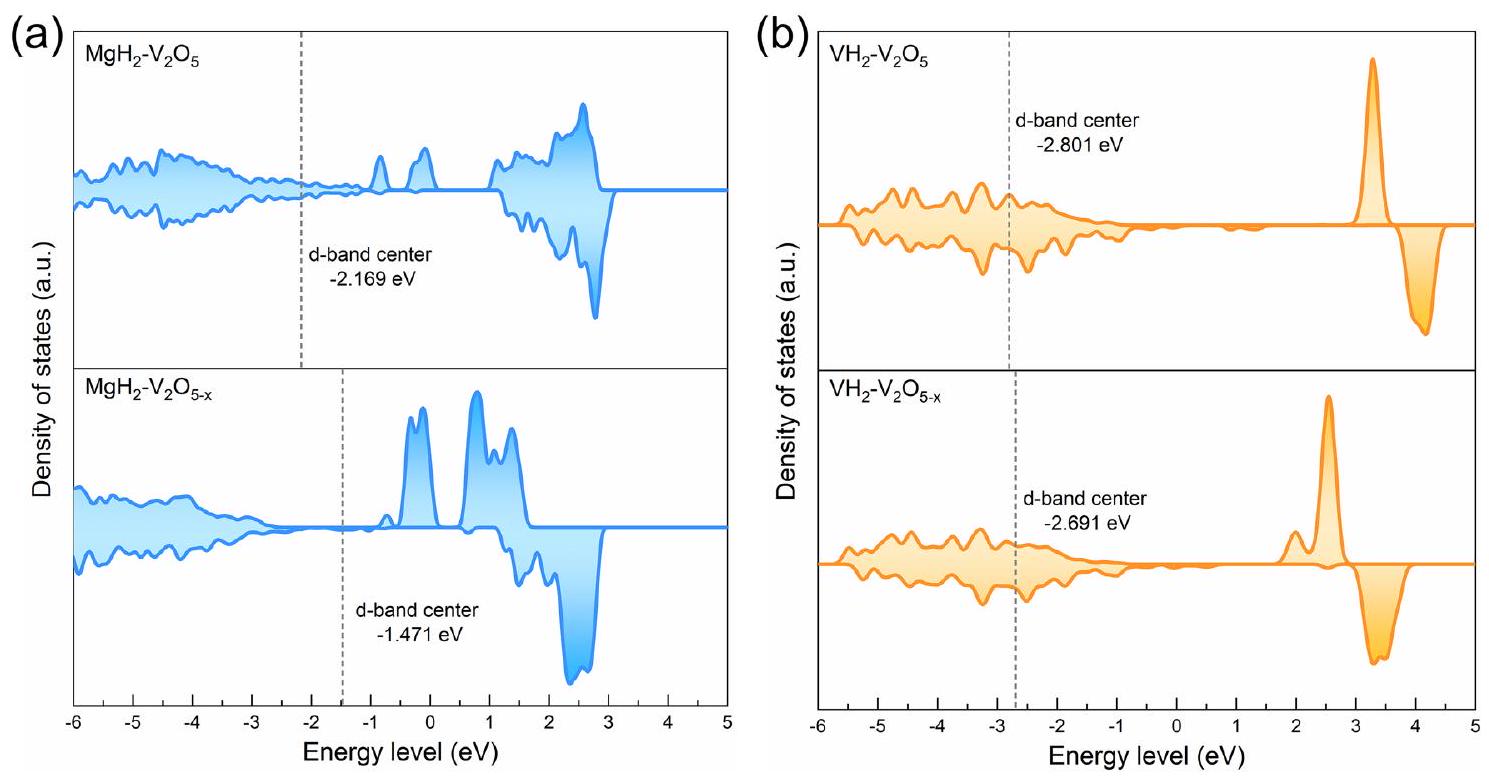
3.3 فهم آلية التحفيز التآزري
تمت بشكل دقيق لدراسة الهياكل والتركيبات المقابلة لـ
تم إزالة الهيدروجين لإنتاج
إطالة الـ
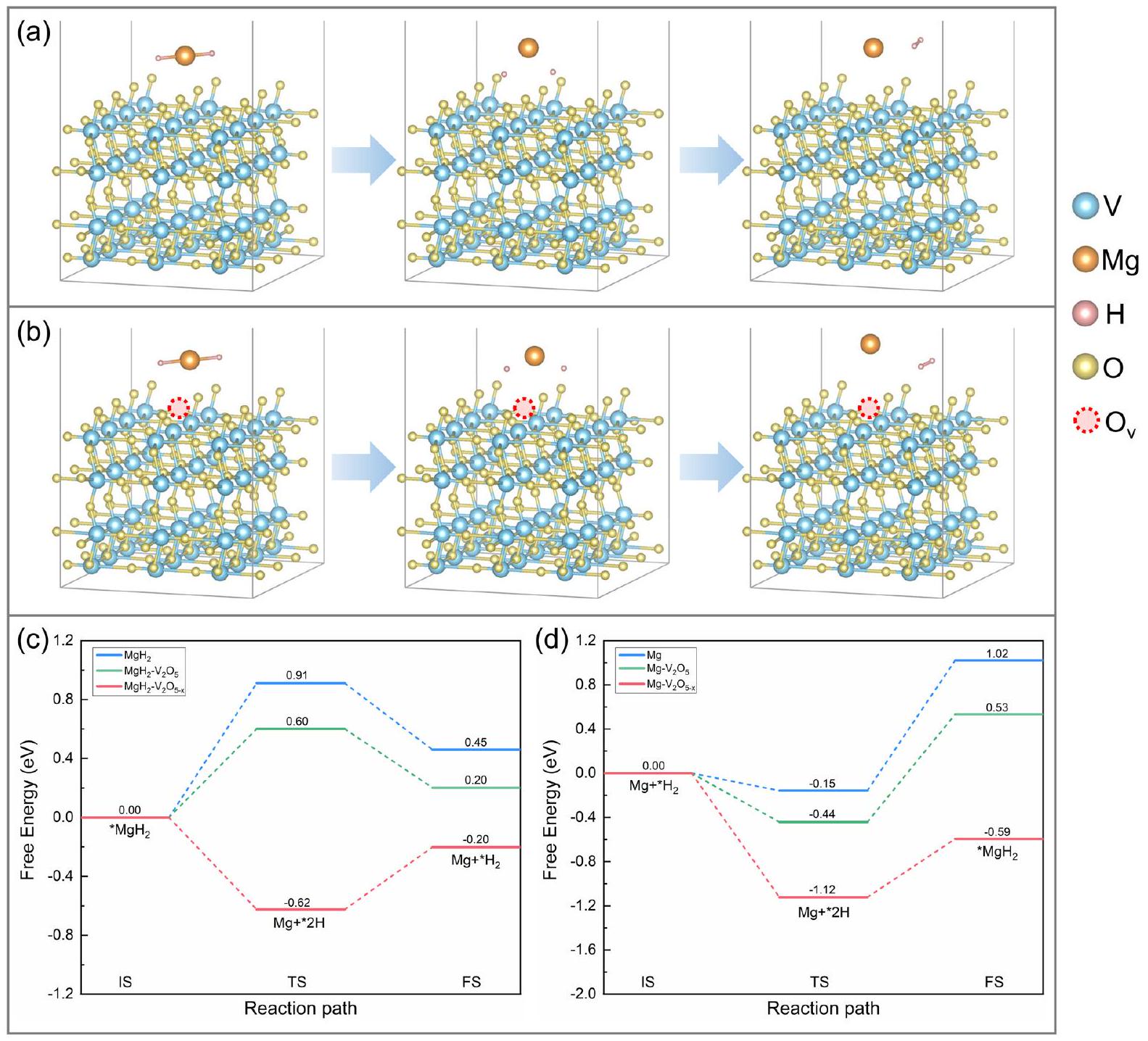

4 الخاتمة
وتشكيل
الإعلانات
References
- Z. Abdin, A. Zafaranloo, A. Rafiee, W. Mérida, W. Lipiński et al., Hydrogen as an energy vector. Renew. Sustain. Energy Rev. 120, 109620 (2020). https://doi.org/10.1016/j.rser. 2019.109620
- I. Staffell, D. Scamman, A.V. Abad, P. Balcombe, P.E. Dodds et al., The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 12, 463-491 (2019). https://doi.org/10.1039/c8ee01157e
- T. Capurso, M. Stefanizzi, M. Torresi, S.M. Camporeale, Perspective of the role of hydrogen in the 21st century energy transition. Energy Convers. Manag. 251, 114898 (2022). https://doi.org/10.1016/j.enconman.2021.114898
- N. Mac Dowell, N. Sunny, N. Brandon, H. Herzog, A.Y. Ku et al., The hydrogen economy: a pragmatic path forward. Joule 5, 2524-2529 (2021). https://doi.org/10.1016/j.joule. 2021.09.014
- O. Faye, J. Szpunar, U. Eduok, A critical review on the current technologies for the generation, storage, and transportation of hydrogen. Int. J. Hydrog. Energy 47, 13771-13802 (2022). https://doi.org/10.1016/j.ijhydene.2022.02.112
- M. van der Spek, C. Banet, C. Bauer, P. Gabrielli, W. Goldthorpe et al., Perspective on the hydrogen economy as a pathway to reach net-zero
emissions in Europe. Energy Environ. Sci. 15, 1034-1077 (2022). https://doi.org/10.1039/ D1EE02118D - A.I. Osman, N. Mehta, A.M. Elgarahy, M. Hefny, A. Al-Hinai et al., Hydrogen production, storage, utilisation and environmental impacts: a review. Environ. Chem. Lett. 20, 153-188 (2022). https://doi.org/10.1007/ s10311-021-01322-8
- G. Nazir, A. Rehman, S. Hussain, S. Aftab, K. Heo et al., Recent advances and reliable assessment of solid-state materials for hydrogen storage: a step forward toward a sustainable
economy. Adv. Sustain. Syst. 6, 2200276 (2022). https:// doi.org/10.1002/adsu. 202200276 - L. Ren, Y. Li, N. Zhang, Z. Li, X. Lin et al., Nanostructuring of Mg-based hydrogen storage materials: recent advances for promoting key applications. Nano-Micro Lett. 15, 93 (2023). https://doi.org/10.1007/s40820-023-01041-5
- V.A. Yartys, M.V. Lototskyy, E. Akiba, R. Albert, V.E. Antonov et al., Magnesium based materials for hydrogen based energy storage: past, present and future. Int. J. Hydrog. Energy 44, 7809-7859 (2019). https://doi.org/10.1016/j.ijhyd ene.2018.12.212
- M. Song, L. Zhang, F. Wu, H. Zhang, H. Zhao et al., Recent advances of magnesium hydride as an energy storage material. J. Mater. Sci. Technol. 149, 99-111 (2023). https://doi.org/10. 1016/j.jmst.2022.11.032
- L. Ren, Y. Li, X. Lin, W. Ding, J. Zou, Promoting hydrogen industry with high-capacity Mg -based solid-state hydrogen storage materials and systems. Front. Energy 17, 320-323 (2023). https://doi.org/10.1007/s11708-023-0889-1
- Z.-Y. Li, Y.-J. Sun, C.-C. Zhang, S. Wei, L. Zhao et al., Optimizing hydrogen ad/desorption of Mg -based hydrides
for energy-storage applications. J. Mater. Sci. Technol. 141, 221-235 (2023). https://doi.org/10.1016/j.jmst.2022.08.047 - Y. Luo, Q. Wang, J. Li, F. Xu, L. Sun et al., Enhanced hydrogen storage/sensing of metal hydrides by nanomodification. Mater. Today Nano 9, 100071 (2020). https://doi.org/10. 1016/j.mtnano.2019.100071
- M. Rueda, L.M. Sanz-Moral, Á. Martín, Innovative methods to enhance the properties of solid hydrogen storage materials based on hydrides through nanoconfinement: a review. J. Supercrit. Fluids 141, 198-217 (2018). https://doi.org/10. 1016/j.supflu.2018.02.010
- F. Guo, T. Zhang, L. Shi, L. Song, Hydrogen absorption/desorption cycling performance of Mg-based alloys with in situ formed
and nanocrystallines. J. Magnes. Alloys 11, 1180-1192 (2023). https://doi.org/10.1016/j.jma. 2021.06.013 - X. Ding, R. Chen, X. Chen, H. Fang, Q. Wang et al., A novel method towards improving the hydrogen storage properties of hypoeutectic Mg -Ni alloy via ultrasonic treatment. J. Magnes. Alloys 11, 903-915 (2023). https://doi.org/10.1016/j.jma. 2021.06.003
- S.T. Kelly, S.L. Van Atta, J.J. Vajo, G.L. Olson, B.M. Clemens, Kinetic limitations of the
system for reversible hydrogen storage. Nanotechnology 20, 204017 (2009). https:// doi.org/10.1088/0957-4484/20/20/204017 - W.J. Botta, G. Zepon, T.T. Ishikawa, D.R. Leiva, Metallurgical processing of Mg alloys and
for hydrogen storage. J. Alloys Compd. 897, 162798 (2022). https://doi.org/10.1016/j. jallcom.2021.162798 - Z. Ding, Y. Li, H. Yang, Y. Lu, J. Tan et al., Tailoring
for hydrogen storage through nanoengineering and catalysis. J. Magnes. Alloys 10, 2946-2967 (2022). https://doi.org/10. 1016/j.jma.2022.09.028 - X. Ding, R. Chen, J. Zhang, W. Cao, Y. Su et al., Recent progress on enhancing the hydrogen storage properties of Mg -based materials via fabricating nanostructures: a critical review. J. Alloys Compd. 897, 163137 (2022). https://doi.org/ 10.1016/j.jallcom.2021.163137
- X. Xie, M. Chen, M. Hu, B. Wang, R. Yu et al., Recent advances in magnesium-based hydrogen storage materials with multiple catalysts. Int. J. Hydrog. Energy 44, 1069410712 (2019). https://doi.org/10.1016/j.ijhydene.2019.02.237
- Y. Meng, J. Zhang, S. Ju, Y. Yang, Z. Li et al., Understanding and unlocking the role of V in boosting the reversible hydrogen storage performance of
. J. Mater. Chem. A 11, 9762-9771 (2023). https://doi.org/10.1039/D3TA01029E - Y. Meng, S. Ju, W. Chen, X. Chen, G. Xia et al., Design of bifunctional
interfaces for improving reversible hydrogen storage performance of . Small Struct. 3, 2270030 (2022). https://doi.org/10.1002/sstr. 202270030 - Z. Lan, X. Wen, L. Zeng, Z. Luo, H. Liang et al., In situ incorporation of highly dispersed nickel and vanadium trioxide nanoparticles in nanoporous carbon for the hydrogen storage performance enhancement of magnesium hydride. Chem. Eng. J. 446, 137261 (2022). https://doi.org/10.1016/j. cej.2022.137261
- J. Zang, S. Wang, R. Hu, H. Man, J. Zhang et al., Ni, beyond thermodynamic tuning, maintains the catalytic activity of V species in
doped . J. Mater. Chem. A 9, 8341-8349 (2021). https://doi.org/10.1039/d0ta12079k - J. Cui, J. Liu, H. Wang, L. Ouyang, D. Sun et al., Mg-TM (TM: Ti, Nb, V Co, Mo or Ni) core-shell like nanostructures: synthesis, hydrogen storage performance and catalytic mechanism. J. Mater. Chem. A 2, 9645-9655 (2014). https://doi.org/ 10.1039/c4ta00221k
- M. Chen, M. Hu, X. Xie, T. Liu, High loading nanoconfinement of V-decorated Mg with 1 nm carbon shells: hydrogen storage properties and catalytic mechanism. Nanoscale 11, 10045-10055 (2019). https://doi.org/10.1039/c8nr09909j
- X. Zhang, X. Zhang, L. Zhang, Z. Huang, F. Fang et al., Remarkable low-temperature hydrogen cycling kinetics of Mg enabled by VH nanoparticles. J. Mater. Sci. Technol. 144, 168-177 (2023). https://doi.org/10.1016/j.jmst.2022.10.029
- Q. Li, M. Yan, Y. Xu, X.L. Zhang, K.T. Lau et al., Computational investigation of
for hydrogen storage. J. Phys. Chem. C 125, 8862-8868 (2021). https://doi.org/10. 1021/acs.jpcc.1c01554 - L. Ren, W. Zhu, Y. Li, X. Lin, H. Xu et al., Oxygen vacancyrich 2D
nanosheets: a bridge toward high stability and rapid hydrogen storage kinetics of nano-confined . Nano-Micro Lett. 14, 144 (2022). https://doi.org/10.1007/ s40820-022-00891-9 - H. Zhang, Q. Kong, S. Hu, D. Zhang, H. Chen et al., Engineering the oxygen vacancies in
for boosting its catalytic performance in hydrogen storage. ACS Sustain. Chem. Eng. 10, 363-371 (2022). https://doi.org/10.1021/acssuschem eng.1c06444 - P. Liu, H. Chen, H. Yu, X. Liu, R. Jiang et al., Oxygen vacancy in magnesium/cerium composite from ball milling for hydrogen storage improvement. Int. J. Hydrog. Energy 44, 1360613612 (2019). https://doi.org/10.1016/j.ijhydene.2019.03.258
- Z. Han, H. Chen, S. Zhou, Dissociation and diffusion of hydrogen on defect-free and vacancy defective Mg (0001) surfaces: a density functional theory study. Appl. Surf. Sci. 394, 371377 (2017). https://doi.org/10.1016/j.apsusc.2016.10.101
- C. Duan, M. Wu, X. Wang, D. Fu, Y. Zhang et al., The effect of vacancy defective Mg (0001) surface on hydrogenation of Ni-Mg-CNTs: a mechanistic investigation. Fuel 341, 127730 (2023). https://doi.org/10.1016/j.fuel.2023.127730
- G. Kresse, J. Furthmüller, Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15-50 (1996). https://doi.org/ 10.1016/0927-0256(96)00008-0
- G. Kresse, J. Furthmüller, Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter 54, 11169-11186 (1996). https://doi.org/10.1103/physrevb.54.11169
- J.P. Perdew, K. Burke, M. Ernzerhof, Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865-3868 (1996). https://doi.org/10.1103/PhysRevLett.77.3865
- P.E. Blöchl, Projector augmented-wave method. Phys. Rev. B Condens. Matter 50, 17953-17979 (1994). https://doi.org/10. 1103/physrevb.50.17953
- G. Kresse, D. Joubert, From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 17581775 (1999). https://doi.org/10.1103/physrevb.59.1758
- A. Badreldin, M.D. Imam, Y. Wubulikasimu, K. Elsaid, A.E. Abusrafa et al., Surface microenvironment engineering of black
nanostructures for visible light photodegradation of methylene blue. J. Alloys Compd. 871, 159615 (2021). https://doi.org/10.1016/j.jallcom.2021.159615 - R. Zou, J. Li, W. Zhang, G. Lei, Z. Li et al., Enhancing the cycling stability of
using nitrogen modified titanate. J. Mater. Chem. A 11, 11748-11754 (2023). https://doi.org/10. 1039/d3ta01557b - H. Zhang, Y. Bu, W. Xiong, K. He, T. Yu et al., Effect of bimetallic nitride NiCoN on the hydrogen absorption and desorption properties of
and the catalytic effect of in situ formed and phases. J. Alloys Compd. 965, 171431 (2023). https://doi.org/10.1016/j.jallcom.2023.171431 - X. Xiao, X. Peng, H. Jin, T. Li, C. Zhang et al., Freestanding mesoporous VN/CNT hybrid electrodes for flexible all-solidstate supercapacitors. Adv. Mater. 25, 5091-5097 (2013). https://doi.org/10.1002/adma. 201301465
- X. Peng, X. Zhang, L. Wang, L. Hu, S.H.-S. Cheng et al., Hydrogenated
nanosheets for superior lithium storage properties. Adv. Funct. Mater. 26, 784-791 (2016). https:// doi.org/10.1002/adfm. 201503859 - X. Xiao, Z. Peng, C. Chen, C. Zhang, M. Beidaghi et al., Freestanding
-nanobelt/carbon nanotube films for Li ion intercalation pseudocapacitors. Nano Energy 9, 355-363 (2014). https://doi.org/10.1016/j.nanoen.2014.08.001 - X.-J. Wang, R. Nesper, C. Villevieille, P. Novák, Ammonolyzed
nanobelts as novel cathode material of rechargeable Li-ion batteries. Adv. Energy Mater. 3, 606-614 (2013). https://doi.org/10.1002/aenm.201200692 - Y. Wei, C.-W. Ryu, K.-B. Kim, Improvement in electrochemical performance of
by Cu doping. J. Power. Sources 165, 386-392 (2007). https://doi.org/10.1016/j.jpowsour.2006.12. 016 - B. Yan, L. Liao, Y. You, X. Xu, Z. Zheng et al., Single-crystalline
ultralong nanoribbon waveguides. Adv. Mater. 21, 2436-2440 (2009). https://doi.org/10.1002/adma. 200803684 - R. Baddour-Hadjean, J.P. Pereira-Ramos, C. Navone, M. Smirnov, Raman microspectrometry study of electrochemical lithium intercalation into sputtered crystalline
thin films. Chem. Mater. 20, 1916-1923 (2008). https://doi.org/10. 1021/cm702979k - R. Baddour-Hadjean, M.B. Smirnov, K.S. Smirnov, V.Y. Kazimirov, J.M. Gallardo-Amores et al., Lattice dynamics of
: Raman spectroscopic insight into the atomistic structure of a high-pressure vanadium pentoxide polymorph. Inorg. Chem. 51, 3194-3201 (2012). https://doi.org/10.1021/ic202 651b - V. Eyert, K.-H. Höck, Electronic structure of
: role of octahedral deformations. Phys. Rev. B 57, 12727-12737 (1998). https://doi.org/10.1103/physrevb.57.12727 - H.E. Kissinger, Reaction kinetics in differential thermal analysis. Anal. Chem. 29, 1702-1706 (1957). https://doi.org/10. 1021/ac60131a045
- L. Ren, W. Zhu, Q. Zhang, C. Lu, F. Sun et al.,
confinement in MOF-derived N -doped porous carbon nanofibers for enhanced hydrogen storage. Chem. Eng. J. 434, 134701 (2022). https://doi.org/10.1016/j.cej.2022.134701 - J.H. Sharp, G.W. Brindley, B.N. Narahari Achar, Numerical data for some commonly used solid state reaction equations. J. Am. Ceram. Soc. 49, 379-382 (1966). https://doi.org/10. 1111/j.1151-2916.1966.tb13289.x
- Z. Ma, S. Panda, Q. Zhang, F. Sun, D. Khan et al., Improving hydrogen sorption performances of
through nanoconfinement in a mesoporous CoS nano-boxes scaffold. Chem. Eng. J. 406, 126790 (2021). https://doi.org/10.1016/j.cej.2020. 126790 - Z. Lan, H. Fu, R. Zhao, H. Liu, W. Zhou et al., Roles of in situ-formed NbN and
from N -doped MXene in regulating the re/hydrogenation and cycling performance of magnesium hydride. Chem. Eng. J. 431, 133985 (2022). https://doi.org/10.1016/j.cej.2021.133985 - H. Fu, J. Hu, Y. Lu, X. Li, Y.-A. Chen et al., Synergistic effect of a facilely synthesized
catalyst on improving the low-temperature kinetic properties of . ACS Appl.
59. S. Kumar, A. Jain, T. Ichikawa, Y. Kojima, G.K. Dey, Development of vanadium based hydrogen storage material: a review. Renew. Sustain. Energy Rev. 72, 791-800 (2017). https://doi. org/10.1016/j.rser.2017.01.063
60. P. Khajondetchairit, L. Ngamwongwan, P. Hirunsit, S. Suthirakun, Synergistic effects of V and Ni catalysts on hydrogen sorption kinetics of Mg -based hydrogen storage materials: a computational study. J. Phys. Chem. C 126, 5483-5492 (2022). https://doi.org/10.1021/acs.jpcc.1c10535
61. D. Zhou, P. Peng, J. Liu, First-principles calculation of dehydrogenating properties of
62. A.J. Du, S.C. Smith, X.D. Yao, C.H. Sun, L. Li et al., The role of
- Jianxin Zou, zoujx@sjtu.edu.cn
National Engineering Research Center of Light Alloys Net Forming & State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University, Shanghai 200240, People’s Republic of China
Shanghai Engineering Research Center of Mg Materials and Applications & School of Materials Science and Engineering, Shanghai Jiao Tong University, Shanghai 200240, People’s Republic of China
Center of Hydrogen Science, Shanghai Jiao Tong University, Shanghai 200240, People’s Republic of China
Instrumental Analysis Center of SJTU, Shanghai Jiao Tong University, Shanghai 200240, People’s Republic of China - Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/ s40820-024-01375-8.
DOI: https://doi.org/10.1007/s40820-024-01375-8
PMID: https://pubmed.ncbi.nlm.nih.gov/38512500
Publication Date: 2024-03-21
Nano-Micro Lett.
(2024) 16:160
Accepted: 5 February 2024
Published online: 21 March 2024
© The Author(s) 2024
Boosting Hydrogen Storage Performance of
HIGHLIGHTS
- Graphene-like 2D
nanosheets rich in oxygen vacancies are designed as multi-functional catalysts to fabricate composites. - Hydrogen release starts from
and capacity retention is as high as after 100 cycles at . - The composites present rapid kinetics and impressive hydrogen absorption capability at near room temperature.
- The oxygen vacancies could directly enhance kinetics of
while indirectly exciting “hydrogen pump” effect of .
Abstract
MgH}_{2}$ is a promising high-capacity solid-state hydrogen storage material, while its application is greatly hindered by the high desorption temperature and sluggish kinetics. Herein, intertwined 2D oxygen vacancy-rich
1 Introduction
multi-valance and high catalytic activity, vanadium (V) metal and its corresponding oxide counterparts are often used as catalysts for
the hydrogen storage performances of
2 Experiment Section
2.1 Sample Preparation
2.1.1 Synthesis of Hydrogenated
distilled water under stirring. Then, 1 mL of concentrated HCl (
2.1.2 Synthesis of
2.2 Characterization and Measurements
sonicated, dropped cast on a copper grid and rapidly transferred to the equipment. The preparation conditions of samples for SEM analyses were similar to the TEM observations. The only difference was that the samples were dispersed in cyclohexane and dropped to the silicon wafer. X-ray photoelectron spectroscopy (XPS, Kratos AXIS Ultra DLD) was conducted to analyze the valence state and chemical bonding nature of constituent elements of
2.3 Theoretical Calculations
3 Results and Discussion
3.1 Characterization of the As-Synthesized

giving rise to an improved hydrogen storage performance of

in an oxygen atmosphere. Assuming the chemical formula of
3.2 Catalytic Effect of
capacity of about

with a hydrogen desorption capacity of
energy (
the commercial

of


3.3 Understanding the Synergistic Catalytic Mechanism
carefully performed to study the corresponding structures and compositions of the
dehydrogenated to produce

elongation of the



4 Conclusion
and the formation of
Declarations
References
- Z. Abdin, A. Zafaranloo, A. Rafiee, W. Mérida, W. Lipiński et al., Hydrogen as an energy vector. Renew. Sustain. Energy Rev. 120, 109620 (2020). https://doi.org/10.1016/j.rser. 2019.109620
- I. Staffell, D. Scamman, A.V. Abad, P. Balcombe, P.E. Dodds et al., The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 12, 463-491 (2019). https://doi.org/10.1039/c8ee01157e
- T. Capurso, M. Stefanizzi, M. Torresi, S.M. Camporeale, Perspective of the role of hydrogen in the 21st century energy transition. Energy Convers. Manag. 251, 114898 (2022). https://doi.org/10.1016/j.enconman.2021.114898
- N. Mac Dowell, N. Sunny, N. Brandon, H. Herzog, A.Y. Ku et al., The hydrogen economy: a pragmatic path forward. Joule 5, 2524-2529 (2021). https://doi.org/10.1016/j.joule. 2021.09.014
- O. Faye, J. Szpunar, U. Eduok, A critical review on the current technologies for the generation, storage, and transportation of hydrogen. Int. J. Hydrog. Energy 47, 13771-13802 (2022). https://doi.org/10.1016/j.ijhydene.2022.02.112
- M. van der Spek, C. Banet, C. Bauer, P. Gabrielli, W. Goldthorpe et al., Perspective on the hydrogen economy as a pathway to reach net-zero
emissions in Europe. Energy Environ. Sci. 15, 1034-1077 (2022). https://doi.org/10.1039/ D1EE02118D - A.I. Osman, N. Mehta, A.M. Elgarahy, M. Hefny, A. Al-Hinai et al., Hydrogen production, storage, utilisation and environmental impacts: a review. Environ. Chem. Lett. 20, 153-188 (2022). https://doi.org/10.1007/ s10311-021-01322-8
- G. Nazir, A. Rehman, S. Hussain, S. Aftab, K. Heo et al., Recent advances and reliable assessment of solid-state materials for hydrogen storage: a step forward toward a sustainable
economy. Adv. Sustain. Syst. 6, 2200276 (2022). https:// doi.org/10.1002/adsu. 202200276 - L. Ren, Y. Li, N. Zhang, Z. Li, X. Lin et al., Nanostructuring of Mg-based hydrogen storage materials: recent advances for promoting key applications. Nano-Micro Lett. 15, 93 (2023). https://doi.org/10.1007/s40820-023-01041-5
- V.A. Yartys, M.V. Lototskyy, E. Akiba, R. Albert, V.E. Antonov et al., Magnesium based materials for hydrogen based energy storage: past, present and future. Int. J. Hydrog. Energy 44, 7809-7859 (2019). https://doi.org/10.1016/j.ijhyd ene.2018.12.212
- M. Song, L. Zhang, F. Wu, H. Zhang, H. Zhao et al., Recent advances of magnesium hydride as an energy storage material. J. Mater. Sci. Technol. 149, 99-111 (2023). https://doi.org/10. 1016/j.jmst.2022.11.032
- L. Ren, Y. Li, X. Lin, W. Ding, J. Zou, Promoting hydrogen industry with high-capacity Mg -based solid-state hydrogen storage materials and systems. Front. Energy 17, 320-323 (2023). https://doi.org/10.1007/s11708-023-0889-1
- Z.-Y. Li, Y.-J. Sun, C.-C. Zhang, S. Wei, L. Zhao et al., Optimizing hydrogen ad/desorption of Mg -based hydrides
for energy-storage applications. J. Mater. Sci. Technol. 141, 221-235 (2023). https://doi.org/10.1016/j.jmst.2022.08.047 - Y. Luo, Q. Wang, J. Li, F. Xu, L. Sun et al., Enhanced hydrogen storage/sensing of metal hydrides by nanomodification. Mater. Today Nano 9, 100071 (2020). https://doi.org/10. 1016/j.mtnano.2019.100071
- M. Rueda, L.M. Sanz-Moral, Á. Martín, Innovative methods to enhance the properties of solid hydrogen storage materials based on hydrides through nanoconfinement: a review. J. Supercrit. Fluids 141, 198-217 (2018). https://doi.org/10. 1016/j.supflu.2018.02.010
- F. Guo, T. Zhang, L. Shi, L. Song, Hydrogen absorption/desorption cycling performance of Mg-based alloys with in situ formed
and nanocrystallines. J. Magnes. Alloys 11, 1180-1192 (2023). https://doi.org/10.1016/j.jma. 2021.06.013 - X. Ding, R. Chen, X. Chen, H. Fang, Q. Wang et al., A novel method towards improving the hydrogen storage properties of hypoeutectic Mg -Ni alloy via ultrasonic treatment. J. Magnes. Alloys 11, 903-915 (2023). https://doi.org/10.1016/j.jma. 2021.06.003
- S.T. Kelly, S.L. Van Atta, J.J. Vajo, G.L. Olson, B.M. Clemens, Kinetic limitations of the
system for reversible hydrogen storage. Nanotechnology 20, 204017 (2009). https:// doi.org/10.1088/0957-4484/20/20/204017 - W.J. Botta, G. Zepon, T.T. Ishikawa, D.R. Leiva, Metallurgical processing of Mg alloys and
for hydrogen storage. J. Alloys Compd. 897, 162798 (2022). https://doi.org/10.1016/j. jallcom.2021.162798 - Z. Ding, Y. Li, H. Yang, Y. Lu, J. Tan et al., Tailoring
for hydrogen storage through nanoengineering and catalysis. J. Magnes. Alloys 10, 2946-2967 (2022). https://doi.org/10. 1016/j.jma.2022.09.028 - X. Ding, R. Chen, J. Zhang, W. Cao, Y. Su et al., Recent progress on enhancing the hydrogen storage properties of Mg -based materials via fabricating nanostructures: a critical review. J. Alloys Compd. 897, 163137 (2022). https://doi.org/ 10.1016/j.jallcom.2021.163137
- X. Xie, M. Chen, M. Hu, B. Wang, R. Yu et al., Recent advances in magnesium-based hydrogen storage materials with multiple catalysts. Int. J. Hydrog. Energy 44, 1069410712 (2019). https://doi.org/10.1016/j.ijhydene.2019.02.237
- Y. Meng, J. Zhang, S. Ju, Y. Yang, Z. Li et al., Understanding and unlocking the role of V in boosting the reversible hydrogen storage performance of
. J. Mater. Chem. A 11, 9762-9771 (2023). https://doi.org/10.1039/D3TA01029E - Y. Meng, S. Ju, W. Chen, X. Chen, G. Xia et al., Design of bifunctional
interfaces for improving reversible hydrogen storage performance of . Small Struct. 3, 2270030 (2022). https://doi.org/10.1002/sstr. 202270030 - Z. Lan, X. Wen, L. Zeng, Z. Luo, H. Liang et al., In situ incorporation of highly dispersed nickel and vanadium trioxide nanoparticles in nanoporous carbon for the hydrogen storage performance enhancement of magnesium hydride. Chem. Eng. J. 446, 137261 (2022). https://doi.org/10.1016/j. cej.2022.137261
- J. Zang, S. Wang, R. Hu, H. Man, J. Zhang et al., Ni, beyond thermodynamic tuning, maintains the catalytic activity of V species in
doped . J. Mater. Chem. A 9, 8341-8349 (2021). https://doi.org/10.1039/d0ta12079k - J. Cui, J. Liu, H. Wang, L. Ouyang, D. Sun et al., Mg-TM (TM: Ti, Nb, V Co, Mo or Ni) core-shell like nanostructures: synthesis, hydrogen storage performance and catalytic mechanism. J. Mater. Chem. A 2, 9645-9655 (2014). https://doi.org/ 10.1039/c4ta00221k
- M. Chen, M. Hu, X. Xie, T. Liu, High loading nanoconfinement of V-decorated Mg with 1 nm carbon shells: hydrogen storage properties and catalytic mechanism. Nanoscale 11, 10045-10055 (2019). https://doi.org/10.1039/c8nr09909j
- X. Zhang, X. Zhang, L. Zhang, Z. Huang, F. Fang et al., Remarkable low-temperature hydrogen cycling kinetics of Mg enabled by VH nanoparticles. J. Mater. Sci. Technol. 144, 168-177 (2023). https://doi.org/10.1016/j.jmst.2022.10.029
- Q. Li, M. Yan, Y. Xu, X.L. Zhang, K.T. Lau et al., Computational investigation of
for hydrogen storage. J. Phys. Chem. C 125, 8862-8868 (2021). https://doi.org/10. 1021/acs.jpcc.1c01554 - L. Ren, W. Zhu, Y. Li, X. Lin, H. Xu et al., Oxygen vacancyrich 2D
nanosheets: a bridge toward high stability and rapid hydrogen storage kinetics of nano-confined . Nano-Micro Lett. 14, 144 (2022). https://doi.org/10.1007/ s40820-022-00891-9 - H. Zhang, Q. Kong, S. Hu, D. Zhang, H. Chen et al., Engineering the oxygen vacancies in
for boosting its catalytic performance in hydrogen storage. ACS Sustain. Chem. Eng. 10, 363-371 (2022). https://doi.org/10.1021/acssuschem eng.1c06444 - P. Liu, H. Chen, H. Yu, X. Liu, R. Jiang et al., Oxygen vacancy in magnesium/cerium composite from ball milling for hydrogen storage improvement. Int. J. Hydrog. Energy 44, 1360613612 (2019). https://doi.org/10.1016/j.ijhydene.2019.03.258
- Z. Han, H. Chen, S. Zhou, Dissociation and diffusion of hydrogen on defect-free and vacancy defective Mg (0001) surfaces: a density functional theory study. Appl. Surf. Sci. 394, 371377 (2017). https://doi.org/10.1016/j.apsusc.2016.10.101
- C. Duan, M. Wu, X. Wang, D. Fu, Y. Zhang et al., The effect of vacancy defective Mg (0001) surface on hydrogenation of Ni-Mg-CNTs: a mechanistic investigation. Fuel 341, 127730 (2023). https://doi.org/10.1016/j.fuel.2023.127730
- G. Kresse, J. Furthmüller, Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15-50 (1996). https://doi.org/ 10.1016/0927-0256(96)00008-0
- G. Kresse, J. Furthmüller, Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter 54, 11169-11186 (1996). https://doi.org/10.1103/physrevb.54.11169
- J.P. Perdew, K. Burke, M. Ernzerhof, Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865-3868 (1996). https://doi.org/10.1103/PhysRevLett.77.3865
- P.E. Blöchl, Projector augmented-wave method. Phys. Rev. B Condens. Matter 50, 17953-17979 (1994). https://doi.org/10. 1103/physrevb.50.17953
- G. Kresse, D. Joubert, From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 17581775 (1999). https://doi.org/10.1103/physrevb.59.1758
- A. Badreldin, M.D. Imam, Y. Wubulikasimu, K. Elsaid, A.E. Abusrafa et al., Surface microenvironment engineering of black
nanostructures for visible light photodegradation of methylene blue. J. Alloys Compd. 871, 159615 (2021). https://doi.org/10.1016/j.jallcom.2021.159615 - R. Zou, J. Li, W. Zhang, G. Lei, Z. Li et al., Enhancing the cycling stability of
using nitrogen modified titanate. J. Mater. Chem. A 11, 11748-11754 (2023). https://doi.org/10. 1039/d3ta01557b - H. Zhang, Y. Bu, W. Xiong, K. He, T. Yu et al., Effect of bimetallic nitride NiCoN on the hydrogen absorption and desorption properties of
and the catalytic effect of in situ formed and phases. J. Alloys Compd. 965, 171431 (2023). https://doi.org/10.1016/j.jallcom.2023.171431 - X. Xiao, X. Peng, H. Jin, T. Li, C. Zhang et al., Freestanding mesoporous VN/CNT hybrid electrodes for flexible all-solidstate supercapacitors. Adv. Mater. 25, 5091-5097 (2013). https://doi.org/10.1002/adma. 201301465
- X. Peng, X. Zhang, L. Wang, L. Hu, S.H.-S. Cheng et al., Hydrogenated
nanosheets for superior lithium storage properties. Adv. Funct. Mater. 26, 784-791 (2016). https:// doi.org/10.1002/adfm. 201503859 - X. Xiao, Z. Peng, C. Chen, C. Zhang, M. Beidaghi et al., Freestanding
-nanobelt/carbon nanotube films for Li ion intercalation pseudocapacitors. Nano Energy 9, 355-363 (2014). https://doi.org/10.1016/j.nanoen.2014.08.001 - X.-J. Wang, R. Nesper, C. Villevieille, P. Novák, Ammonolyzed
nanobelts as novel cathode material of rechargeable Li-ion batteries. Adv. Energy Mater. 3, 606-614 (2013). https://doi.org/10.1002/aenm.201200692 - Y. Wei, C.-W. Ryu, K.-B. Kim, Improvement in electrochemical performance of
by Cu doping. J. Power. Sources 165, 386-392 (2007). https://doi.org/10.1016/j.jpowsour.2006.12. 016 - B. Yan, L. Liao, Y. You, X. Xu, Z. Zheng et al., Single-crystalline
ultralong nanoribbon waveguides. Adv. Mater. 21, 2436-2440 (2009). https://doi.org/10.1002/adma. 200803684 - R. Baddour-Hadjean, J.P. Pereira-Ramos, C. Navone, M. Smirnov, Raman microspectrometry study of electrochemical lithium intercalation into sputtered crystalline
thin films. Chem. Mater. 20, 1916-1923 (2008). https://doi.org/10. 1021/cm702979k - R. Baddour-Hadjean, M.B. Smirnov, K.S. Smirnov, V.Y. Kazimirov, J.M. Gallardo-Amores et al., Lattice dynamics of
: Raman spectroscopic insight into the atomistic structure of a high-pressure vanadium pentoxide polymorph. Inorg. Chem. 51, 3194-3201 (2012). https://doi.org/10.1021/ic202 651b - V. Eyert, K.-H. Höck, Electronic structure of
: role of octahedral deformations. Phys. Rev. B 57, 12727-12737 (1998). https://doi.org/10.1103/physrevb.57.12727 - H.E. Kissinger, Reaction kinetics in differential thermal analysis. Anal. Chem. 29, 1702-1706 (1957). https://doi.org/10. 1021/ac60131a045
- L. Ren, W. Zhu, Q. Zhang, C. Lu, F. Sun et al.,
confinement in MOF-derived N -doped porous carbon nanofibers for enhanced hydrogen storage. Chem. Eng. J. 434, 134701 (2022). https://doi.org/10.1016/j.cej.2022.134701 - J.H. Sharp, G.W. Brindley, B.N. Narahari Achar, Numerical data for some commonly used solid state reaction equations. J. Am. Ceram. Soc. 49, 379-382 (1966). https://doi.org/10. 1111/j.1151-2916.1966.tb13289.x
- Z. Ma, S. Panda, Q. Zhang, F. Sun, D. Khan et al., Improving hydrogen sorption performances of
through nanoconfinement in a mesoporous CoS nano-boxes scaffold. Chem. Eng. J. 406, 126790 (2021). https://doi.org/10.1016/j.cej.2020. 126790 - Z. Lan, H. Fu, R. Zhao, H. Liu, W. Zhou et al., Roles of in situ-formed NbN and
from N -doped MXene in regulating the re/hydrogenation and cycling performance of magnesium hydride. Chem. Eng. J. 431, 133985 (2022). https://doi.org/10.1016/j.cej.2021.133985 - H. Fu, J. Hu, Y. Lu, X. Li, Y.-A. Chen et al., Synergistic effect of a facilely synthesized
catalyst on improving the low-temperature kinetic properties of . ACS Appl.
59. S. Kumar, A. Jain, T. Ichikawa, Y. Kojima, G.K. Dey, Development of vanadium based hydrogen storage material: a review. Renew. Sustain. Energy Rev. 72, 791-800 (2017). https://doi. org/10.1016/j.rser.2017.01.063
60. P. Khajondetchairit, L. Ngamwongwan, P. Hirunsit, S. Suthirakun, Synergistic effects of V and Ni catalysts on hydrogen sorption kinetics of Mg -based hydrogen storage materials: a computational study. J. Phys. Chem. C 126, 5483-5492 (2022). https://doi.org/10.1021/acs.jpcc.1c10535
61. D. Zhou, P. Peng, J. Liu, First-principles calculation of dehydrogenating properties of
62. A.J. Du, S.C. Smith, X.D. Yao, C.H. Sun, L. Li et al., The role of
- Jianxin Zou, zoujx@sjtu.edu.cn
National Engineering Research Center of Light Alloys Net Forming & State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University, Shanghai 200240, People’s Republic of China
Shanghai Engineering Research Center of Mg Materials and Applications & School of Materials Science and Engineering, Shanghai Jiao Tong University, Shanghai 200240, People’s Republic of China
Center of Hydrogen Science, Shanghai Jiao Tong University, Shanghai 200240, People’s Republic of China
Instrumental Analysis Center of SJTU, Shanghai Jiao Tong University, Shanghai 200240, People’s Republic of China - Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/ s40820-024-01375-8.
