DOI: https://doi.org/10.1021/acs.biomac.3c01183
PMID: https://pubmed.ncbi.nlm.nih.gov/38319691
تاريخ النشر: 2024-02-06
تعزيز خصائص الالتصاق المخاطي للجيلاتين من خلال التعديل الكيميائي مع الأنهدريدات غير المشبعة
الوصول المفتوح
شاتاباييفا، إ. ORCID:https://orcid.org/0000-0001-91535198كالديبيكوف، د. ب. ORCID:https://orcid.org/0000-0002-7191-5465أولمانوفا، ل.، زهايسانباييفا، ب. أ.، مون، إ. أ.، كينيسوفا، ز. أ. ORCID:https://orcid.org/0000-0003-2768824Xكودايبرغينوف، س. إ. وخوتوريانسكي، ف. ف. ORCID:https://orcid.org/0000-0002-7221-2630 (2024) تعزيز الخصائص اللاصقة للمخاط للجيلاتين من خلال التعديل الكيميائي مع الأنهدريدات غير المشبعة. بيومكرومولكيولز. ISSN 1526-4602 doi: 10.1021/acs.biomac.3c01183 متاح على https://centaur.reading.ac.uk/115238/
الناشر: الجمعية الكيميائية الأمريكية
www.reading.ac.uk/centaur
سنتر
مخرجات أبحاث ريدينغ على الإنترنت
تعزيز الخصائص اللزجة للجيلاتين من خلال التعديل الكيميائي مع الأنهدريدات غير المشبعة
اقرأ على الإنترنت
تم التنزيل عبر جامعة ريدينغ في 23 فبراير 2024 الساعة 14:53:45 (UTC).
انظرhttps://pubs.acs.org/sharingguidelinesلخيارات حول كيفية مشاركة المقالات المنشورة بشكل قانوني.
الملخص
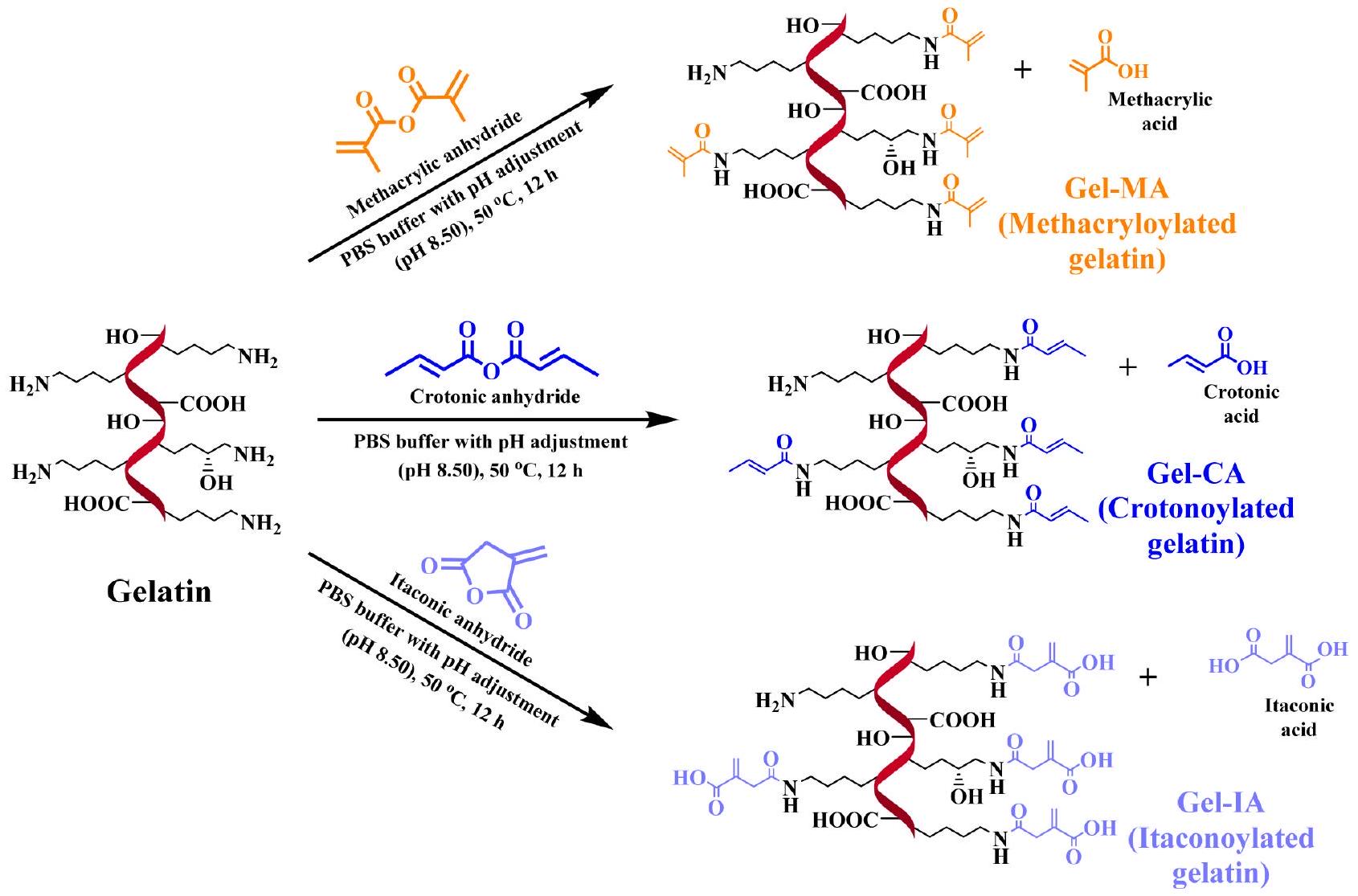
الجيلاتين هو بوليمر طبيعي قابل للذوبان في الماء وله خصائص لاصقة مخاطية ضعيفة. لقد تم استخدامه تقليديًا كمكون رئيسي في العديد من الأدوية، بما في ذلك الكبسولات اللينة والصلبة، والتحاميل، وهندسة الأنسجة، والطب التجديدي. يمكن تحسين الخصائص اللاصقة المخاطية للجيلاتين عن طريق تعديله من خلال الاقتران مع مجموعات لاصقة غير مشبعة محددة. في هذه الدراسة، تم تعديل الجيلاتين من خلال التفاعل مع أنهدريدات الكروتونيك، والإيتاكونيك، والميثاكريليك بنسب مولية متفاوتة لإنتاج جيلاتين كروتونيلات، وإيتاكونيلات، وميثاكريولات (المختصرة كـ Gel-CA، Gel-IA، وGel-MA، على التوالي). تم تأكيد التخليق الناجح باستخدام
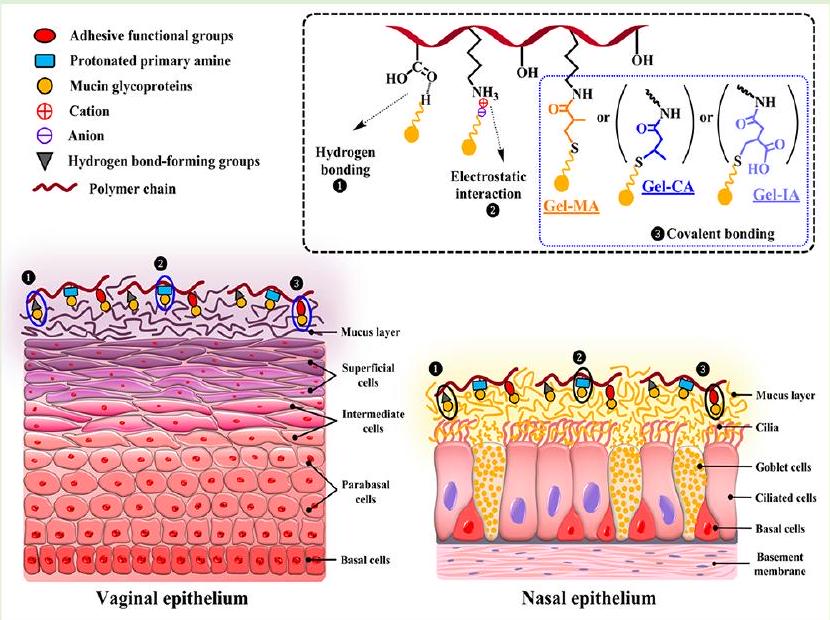 الجيلاتين غير المعدل. تم تقييم سلامة مشتقات الجيلاتين المعدل من خلال اختبار تهيج الغشاء المخاطي للرخويات في الجسم الحي (SMIT) واختبار MTT في المختبر باستخدام خط خلايا الألياف الرئوية البشرية. تم إعداد شكلين مختلفين من الجرعات، مثل الجل الفيزيائي والميكروكريات المجففة بالرش، وتم تقييم خصائصها اللاصقة للمخاط باستخدام تقنية التدفق مع الكشف الفلوري واختبار الشد باستخدام أنسجة المهبل الخنزيرية و الغشاء المخاطي الأنفي للأغنام. أظهرت الجيلاتينات المعدلة بمجموعات غير مشبعة خصائص لاصقة للمخاط متفوقة مقارنة بالجيلاتين الأصلي. إن القدرة المعززة للجيلاتين المعدل بهذه المجموعات الوظيفية غير المشبعة ترجع إلى تكوين روابط تساهمية مع المجالات الفرعية الغنية بالسيستين الموجودة في الميوسين من خلال تفاعلات الإضافة من نوع مايكل بتفاعل ثيول-إين التي تحدث في ظل ظروف ذات صلة فسيولوجيًا.
الجيلاتين غير المعدل. تم تقييم سلامة مشتقات الجيلاتين المعدل من خلال اختبار تهيج الغشاء المخاطي للرخويات في الجسم الحي (SMIT) واختبار MTT في المختبر باستخدام خط خلايا الألياف الرئوية البشرية. تم إعداد شكلين مختلفين من الجرعات، مثل الجل الفيزيائي والميكروكريات المجففة بالرش، وتم تقييم خصائصها اللاصقة للمخاط باستخدام تقنية التدفق مع الكشف الفلوري واختبار الشد باستخدام أنسجة المهبل الخنزيرية و الغشاء المخاطي الأنفي للأغنام. أظهرت الجيلاتينات المعدلة بمجموعات غير مشبعة خصائص لاصقة للمخاط متفوقة مقارنة بالجيلاتين الأصلي. إن القدرة المعززة للجيلاتين المعدل بهذه المجموعات الوظيفية غير المشبعة ترجع إلى تكوين روابط تساهمية مع المجالات الفرعية الغنية بالسيستين الموجودة في الميوسين من خلال تفاعلات الإضافة من نوع مايكل بتفاعل ثيول-إين التي تحدث في ظل ظروف ذات صلة فسيولوجيًا.
1. المقدمة
الهيدروجيلات القابلة للعكس الحراري المرتبطة عند التبريد تحت
خصائص الالتصاق المخاطي. وتشمل تطوير النانو هلام،
2. القسم التجريبي
محلول، بيكربونات الصوديوم، دوديكانول سلفات الصوديوم (SDS)، حمض 2،4،6-ثلاثي نيتروبنزين سلفونيك (TNBSA،
2.2. تخليق مشتقات الجيلاتين. تم تعديل الجيلاتين كيميائيًا باستخدام أنهدريدات غير مشبعة مختلفة وفقًا للإجراءات الموصوفة سابقًا مع بعض التعديلات.
2.3. تحضير الجسيمات الدقيقة المجففة بالرش. تم إذابة عينات الجيلاتين المعدلة كيميائيًا وغير المعدلة (0.5 جرام) في البداية في 100 مل من المحاليل المائية التي تحتوي على ملح فلوريسئين الصوديوم (
2.4. التوصيف. 2.4.1. قياس درجة التFunctionalization. تم تأكيد تعديل الجيلاتين باستخدام
المجموعات الأمينية الحرة المتبقية بعد تحويل الجيلاتين مع تغييرات طفيفة.
2.4.2. مطيافية تحويل فورييه بالأشعة تحت الحمراء (FTIR). تم تسجيل طيف FTIR للجيلاتين غير المعدل والمعدل باستخدام مطياف FTIR Nicolet iS10 (Thermo Scientific، المملكة المتحدة) مع ملحق iTX للانعكاس الكلي المخفف (ATR) المزود بكريستال ماسي. تم جمع الأطياف من متوسط 32 مسحًا بين 4000 و
2.4.3. المجهر الإلكتروني الماسح (SEM). تم فحص شكل وحجم الجسيمات الدقيقة المجففة بالرش المستندة إلى الجيلاتين ومشتقاته المعدلة (Gel-CA و Gel-IA و Gel-MA) باستخدام مجهر إلكتروني ماسح من نوع Zeiss Crossbeam 540 (Carl Zeiss Microscopy GmbH، يينا، ألمانيا) عند جهد تسريع قدره 5 كيلوفولت. تم طلاء العينات بالذهب قبل التصوير. ثم تم تحليل الصور الملتقطة باستخدام برنامج ImageJ (NIH، الولايات المتحدة الأمريكية) لتحديد متوسط قطر الجسيمات الدقيقة.
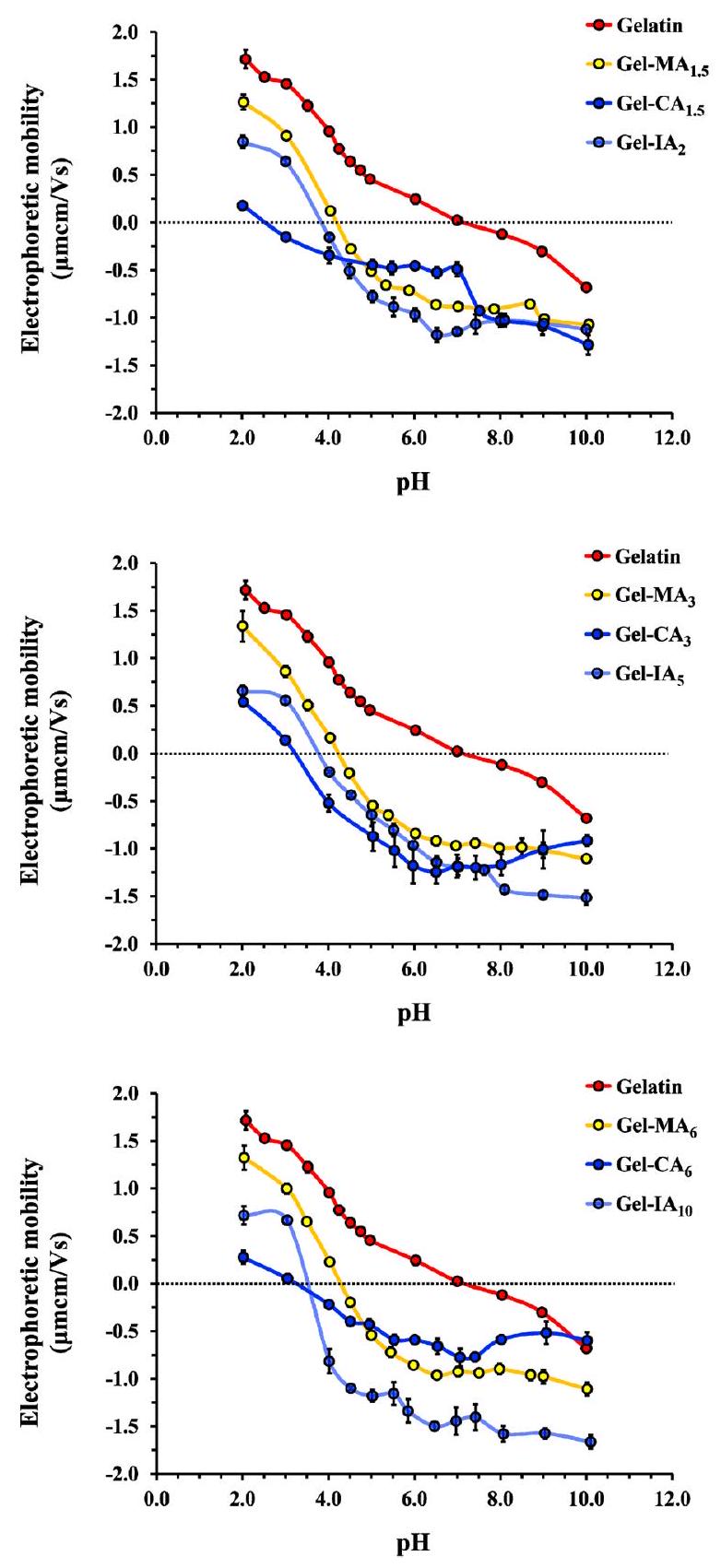
2.4.4. تحديد النقطة المعزولة كهربائياً (IEP). تم تحديد النقاط المعزولة كهربائياً للجيلاتين المعدل وغير المعدل باستخدام تقنية اللزوجة التقليدية.
2.4.5. الدراسات الريولوجية. جهاز قياس اللزوجة TA DHR-1 (TA Instruments، نيو كاسل، ديلاوير، الولايات المتحدة الأمريكية) مزود بلوحة بيلتير ذات درجة حرارة متغيرة وجيومتري مخروط-قرص من الفولاذ المقاوم للصدأ.
من خلال اختبارات سحب درجة الحرارة من 0 إلى
2.5. تقييم السمية. 2.5.1. اختبار حيوية الخلايا في المختبر. تم زراعة خط خلايا الألياف الرئوية البشرية (HPF) في وسط ديلبيكو المعدل (DMEM) المدعوم بـ
2.6. دراسات الاحتفاظ خارج الجسم على الغشاء المخاطي المهبلي للخنازير والغشاء المخاطي الأنفي للأغنام. 2.6.1. تصميم شكل الجرعة النموذجي. 0.1
تم إذابة مشتقات الجيلاتين المعدلة (Gel-MA) بشكل منفصل في 10 مل من المحاليل المائية لـ NaFl. تم تحريك الخلطات لمدة 12 ساعة في درجة حرارة الغرفة حتى تشكلت محاليل متجانسة، وتم تغطيتها بورق الألمنيوم، وتخزينها في الثلاجة للاستخدام لاحقًا. تم استخدام هذه التركيبات المعتمدة على الجيلاتين المعدلة وغير المعدلة المحملة بـ NaFl في دراسات الالتصاق المخاطي في المهبل الخنزيري.
2.6.2. تحضير الأنسجة. تم استلام أنسجة المهبل من الخنازير ورؤوس الأغنام من مسالخ P.C. Turner (فارنبورو، المملكة المتحدة) مباشرة بعد ذبح الحيوانات، وتم تعبئتها ونقلها إلى المختبر في حاويات بلاستيكية باردة، واستخدامها خلال 24 ساعة من جمعها. تم تشريح أنسجة المهبل بعناية (مع تجنب الاتصال بالغشاء المخاطي الداخلي) باستخدام شفرات حادة يمكن التخلص منها للحصول على
2.6.3. تقنية التدفق المستمر. تم إجراء تجارب لتقييم احتفاظ الغشاء المخاطي بالتركيبات المعتمدة على الجيلاتين المعدل وغير المعدل على أنسجة المهبل الخنازير والأنف الأغنام خارج الجسم باستخدام طريقة التدفق المستمر المعروفة مع تعديلات طفيفة تتعلق بالكشف الفلوري.
نفس تقنية التدفق من خلال الزجاج كما هو موصوف أعلاه مع بعض التعديلات. تم استخدام عينات من الجسيمات الدقيقة غير المتشابكة فقط في هذه التجربة. حوالي 100 ملغ من الجسيمات الدقيقة القائمة على الجيلاتين (تتضمن الجيلاتين، Gel-CA، Gel-IA، أو Gel-MA) تحتوي على 1
2.6.4. طريقة الشد (الانفصال). تم استخدام جهاز تحليل القوام TA.XT Plus (Stable Micro Systems Ltd.، ساري، المملكة المتحدة) الذي يعمل في وضع اختبار اللصق لتقييم أداء اللصق المخاطي للجزيئات الدقيقة المجففة بالرش المستندة إلى الجيلاتين ومشتقاته. تم استخدام عينات من الجزيئات الدقيقة المتشابكة وغير المتشابكة في هذه التجربة لتقييم مساهمة انتشار الجزيئات الكبيرة والقدرة على التكوين.
طبقة متداخلة مع المخاط في الالتصاق المخاطي. تم استخدام أنسجة أنف الأغنام المعزولة حديثًا خلال 24 ساعة من الاسترجاع لهذا التجربة. كما تم الإبلاغ عنه سابقًا وتم تعديله ببعض التغييرات،
2.7. التحليل الإحصائي. تم إجراء جميع القياسات في الدراسة الحالية ثلاث مرات على الأقل وتم التعبير عن البيانات كمتوسط
3. النتائج والمناقشة
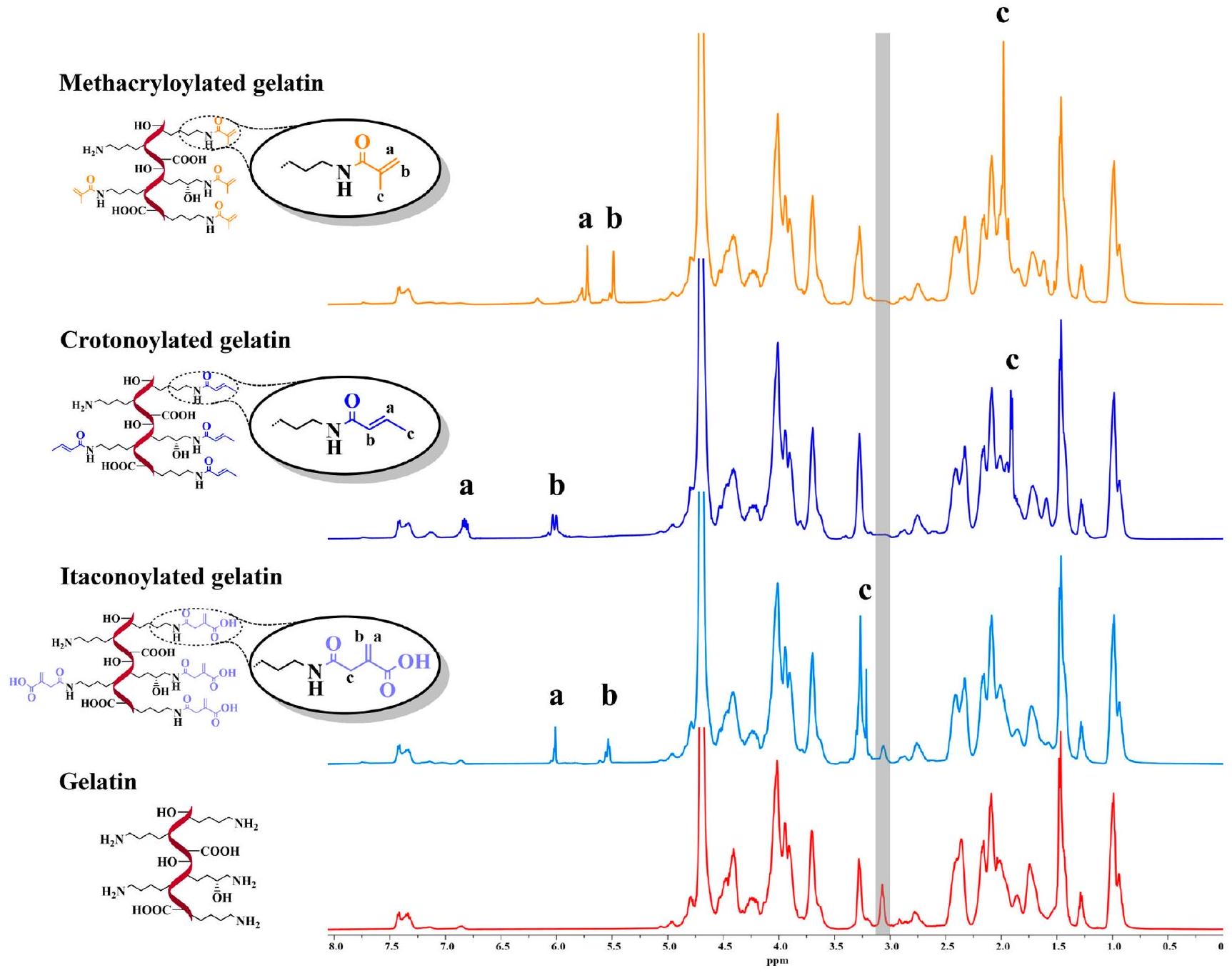
تم تحديد (DoF) من خلال مقارنة تكاملات قمم الهيدروجين المزدوجة المميزة لكل بديل جيلاتين معدل وتكامل المنطقة المقابلة للقمم المجمعة لبروتونات الأروماتية للفينيل ألانين والتيروزين، حيث كانت إشاراتهم بمثابة مرجع. بناءً على
| عينة
|
|
عمق المجال بواسطة
|
DoF بواسطة اختبار TNBSA
|
العائد (%) | برنامج التعليم الفردي
|
|
| جيلاتين |
|
٧.٠ |
|
|||
| جيل-ما
|
1.5 |
|
0.373 |
|
٤.٢ | ٤.٢ |
| جيل-ما
|
٣ |
|
0.380 |
|
٤.٢ | ٤.١ |
| جيل-ما
|
٦ |
|
0.384 |
|
٤.٣ | ٤.٤ |
| جل-CA
|
1.5 |
|
0.355 |
|
2.5 | ND |
| جل-
|
٣ |
|
0.358 |
|
3.2 | ND |
| جل-CA
|
٦ |
|
0.360 |
|
3.2 | ND |
| جيل-IA
|
2 |
|
0.274 |
|
3.8 | ٤.٠ |
| جيل-IA
|
٥ |
|
0.298 |
|
3.8 | ٤.٠ |
| جيل-IA
|
10 |
|
0.298 |
|
٣.٥ | ND |
مع بيانات FTIR عن الجيلاتين المبلغ عنها في الأدبيات.
قياسات الريولوجيا. التغيرات الريولوجية التي تحدث مع
| عينة |
|
|
| جيلاتين |
|
|
| Gel-MA
|
|
|
| Gel-MA
|
|
|
| Gel-MA
|
|
|
| Gel-CA
|
|
|
| Gel-
|
|
|
| Gel-CA
|
|
|
| Gel-IA
|
|
|
| Gel-IA
|
|
|
| Gel-IA
|
|
|
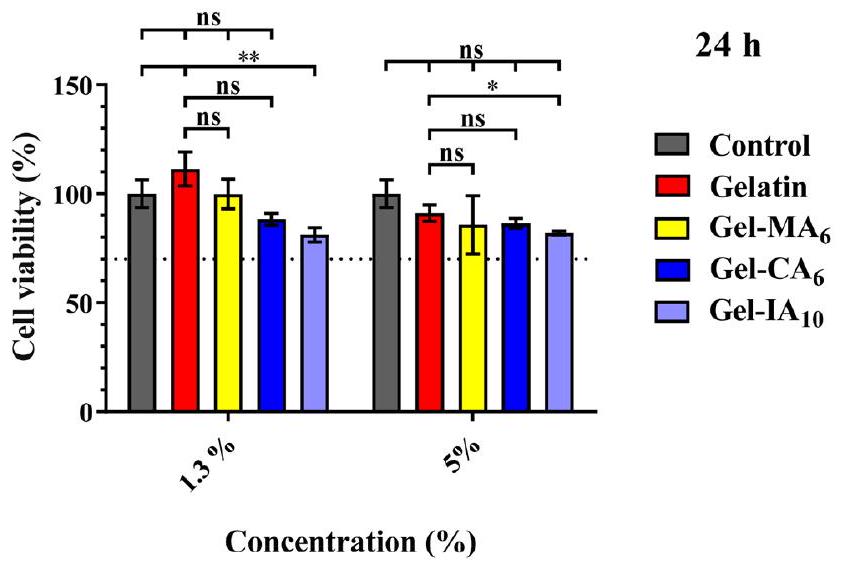
3.2. علم السموم. 3.2.1. حيوية الخلايا. تم دراسة السمية الخلوية في المختبر للجيلاتين ومشتقاته المعدلة (Gel-CA و Gel-IA و Gel-MA) باستخدام اختبار MTT مع خلايا الألياف الرئوية البشرية (HPF). يعتمد الاختبار على قدرة الميتوكوندريا في الخلايا الحية على تقليل 3-(4،5-ثنائي ميثيل ثيازول-2-يل)-2،5-ثنائي فينيل تيترازوليوم بروميد (MTT)، وهي مادة صفراء، إلى بلورات فورمازان غير قابلة للذوبان (لون بنفسجي). تتيح هذه التقنية حساب عدد الخلايا الحية بعد المعالجة بالمادة الاختبارية. تم معالجة خلايا HPF بالجيلاتين، Gel-CA
3.2.2. تهيج الغشاء المخاطي. تم تطوير اختبار تهيج الغشاء المخاطي للرخويات (SMIT) في الأصل بواسطة أدريانس وزملائه
لديها وعي محدود.

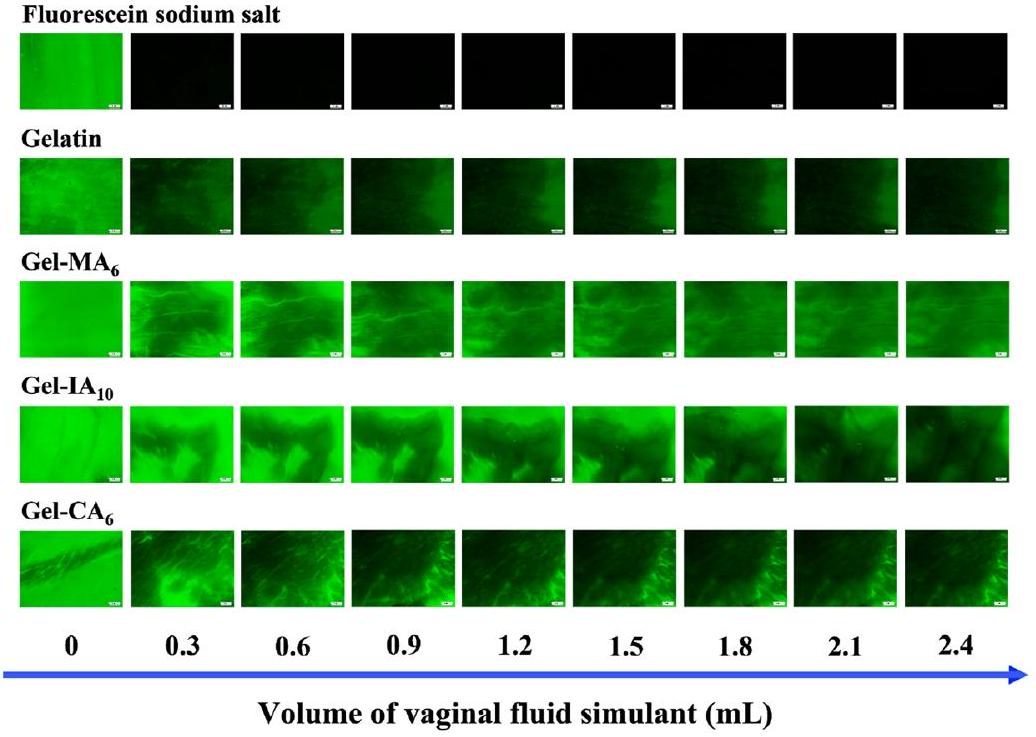
3.3. دراسات الالتصاق المخاطي. 3.3.1. الاحتفاظ في ظروف خارج الجسم
تم إعداد التركيبات باستخدام فلوريسئين الصوديوم (NaFl)، وهو علامة فلورية تسهل الكشف والقياس السهل لمستويات الاحتفاظ بالغشاء المخاطي. لقد تم استخدام هذه الطريقة على نطاق واسع لدراسة احتفاظ التركيبات المختلفة على أسطح مخاطية مختلفة، بما في ذلك الأنسجة المهبلية.
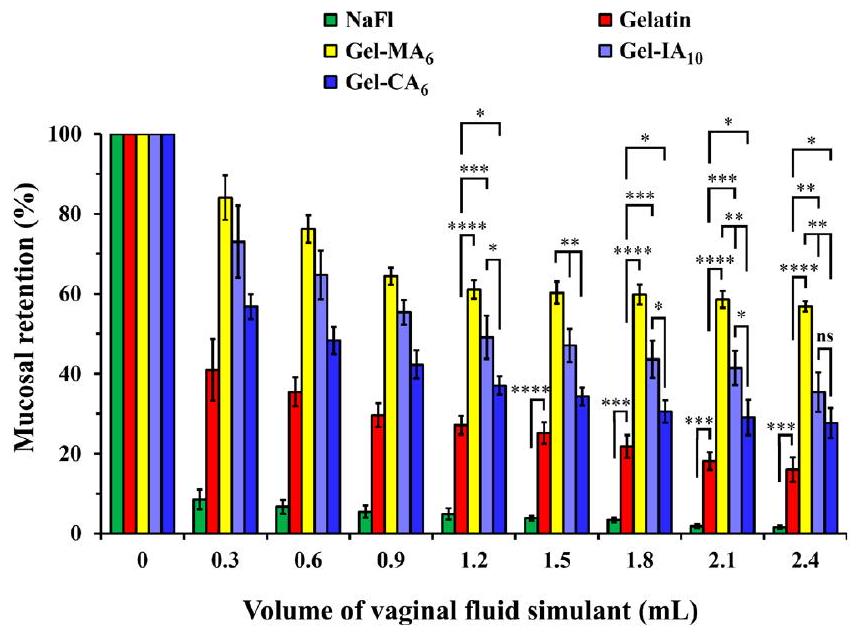
كما هو متوقع، ينخفض احتفاظ جميع التركيبات على مدار عملية الغسيل، ومع ذلك، يتم ملاحظة الاتجاه التالي: Gel-

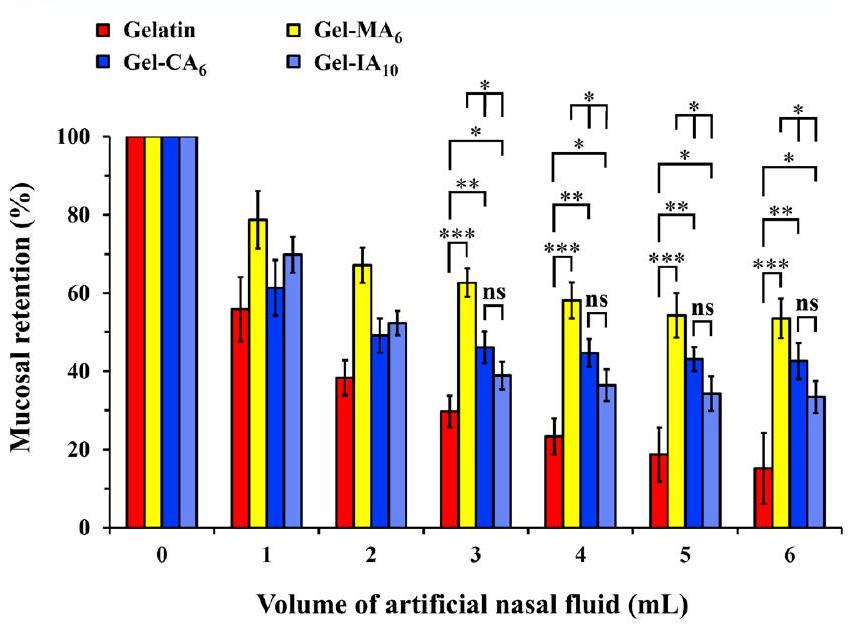
3.3.2. الاحتفاظ على أنسجة أنف الأغنام الحية. يوفر الإعطاء داخل الأنف وسيلة غير جراحية لتوصيل الأدوية. تعمل العوامل العلاجية التي يتم توصيلها إلى تجويف الأنف محليًا وتوفر هدفًا مباشرًا للجهاز العصبي المركزي. يتراوح الإنتاج اليومي المتوقع للمخاط الأنفي بين 0.1 و
ملمس سطح مجعد، خالية من البلورات، المسام، والشقوق (انظر الأشكال S14 وS15 في المعلومات الداعمة). أدت جميع التركيبات إلى جزيئات دقيقة ذات مورفولوجيات مشابهة وكان متوسط أقطار الجزيئات
4. الاستنتاجات
– المحتوى المرتبط
(س) المعلومات الداعمة
(SEM) للجسيمات الدقيقة المجففة بالرش المستندة إلى عينات الجيلاتين المعدلة وغير المعدلة؛ منحنيات اللزوجة المحددة مقابل منحنيات درجة الحموضة لتحديد نقاط التكافؤ؛ منحنيات تخزين وفقدان المودول المستخدمة لتحديد نقاط الجل القابلة للعكس حراريًا؛ توضيح تخطيطي لاختبار تهيج الغشاء المخاطي (SMIT) باستخدام نوع Arion lusitanicus؛ صور نموذجية لإنتاج المخاط بواسطة رخويات Arion lusitanicus عند الاتصال بالمواد الاختبارية؛ ملفات انفصال نموذجية للمواد الاختبارية (PDF)
– معلومات المؤلف
المؤلفون المتجاوبون
داولت ب. كالديبيكوف – مدرسة ريدينغ للصيدلة، جامعة ريدينغ، وايتكنيتس RG6 6DX ريدينغ، المملكة المتحدة؛ قسم الكيمياء والتكنولوجيا الكيميائية، جامعة الفارابي كازاخستان الوطنية، 050040 ألماتي، كازاخستان؛ معهد مواد البوليمر والتكنولوجيا، 050019 ألماتي، كازاخستان؛ © orcid.org/ 0000-0002-7191-5465; البريد الإلكتروني: dauletchem@gmail.com, d.kaldybekov@reading.ac.uk
المؤلفون
ليلى أولمانوفا – مدرسة العلوم والإنسانية، جامعة نازارباييف، 010000 أستانا، كازاخستان
بالنور أ. زهايسنباييفا – مدرسة الهندسة والعلوم الرقمية، جامعة نازارباييف، 010000 أستانا، كازاخستان
إلينا أ. مون – مدرسة العلوم والإنسانية، جامعة نازارباييف، 010000 أستانا، كازاخستان
زارينا أ. كينيسوفا – قسم الكيمياء والتكنولوجيا الكيميائية، جامعة الفارابي كازاخستان الوطنية، 050040 ألماتي، كازاخستان؛ © orcid.org/0000-0003-2768-824X
سركيت إ. كودايبرغينوف – معهد مواد البوليمر والتكنولوجيا، 050019 ألماتي، كازاخستان
معلومات الاتصال الكاملة متاحة على:
https://pubs.acs.org/10.1021/acs.biomac.3c01183
مساهمات المؤلفين
ملاحظات
– الشكر والتقدير
توفير أنسجة المهبل الخنزير وأنسجة أنف الأغنام لتجارب الالتصاق المخاطي. V.V.K. يشكر الجمعية الملكية على زمالته الصناعية (IF
– الاختصارات
– REFERENCES
(2) Schrieber, R.; Gareis, H. Gelatine Handbook: Theory and Industrial Practice; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, 2007.
(3) Alipal, J.; Mohd Pu’ad, N. A. S.; Lee, T. C.; Nayan, N. H. M.; Sahari, N.; Basri, H.; Idris, M. I.; Abdullah, H. Z. A Review of Gelatin: Properties, Sources, Process, Applications, and Commercialisation. Mater. Today: Proc. 2021, 42, 240-250.
(4) Gullapalli, R. P.; Mazzitelli, C. L. Gelatin and Non-Gelatin Capsule Dosage Forms. J. Pharm. Sci. 2017, 106 (6), 1453-1465.
(5) Osmałek, T.; Froelich, A.; Jadach, B.; Tatarek, A.; Gadzinski, P.; Falana, A.; Gralinska, K.; Ekert, M.; Puri, V.; Wrotynska-Barczynska, J.; Michniak-Kohn, B. Recent Advances in Polymer-Based Vaginal Drug Delivery Systems. Pharmaceutics 2021, 13 (6), 884.
(6) Bhagat, V.; Becker, M. L. Degradable Adhesives for Surgery and Tissue Engineering. Biomacromolecules 2017, 18 (10), 3009-3039.
(7) De Clercq, K.; Schelfhout, C.; Bracke, M.; De Wever, O.; Van Bockstal, M.; Ceelen, W.; Remon, J. P.; Vervaet, C. GenipinCrosslinked Gelatin Microspheres as a Strategy to Prevent Postsurgical Peritoneal Adhesions: In Vitro and in Vivo Characterization. Biomaterials 2016, 96, 33-46.
(8) Yuk, H.; Varela, C. E.; Nabzdyk, C. S.; Mao, X.; Padera, R. F.; Roche, E. T.; Zhao, X. Dry Double-Sided Tape for Adhesion of Wet Tissues and Devices. Nature 2019, 575, 169-174.
(9) Li, C.; Marton, I.; Harari, D.; Shemesh, M.; Kalchenko, V.; Pardo, M.; Schreiber, G.; Rudich, Y. Gelatin Stabilizes Nebulized Proteins in Pulmonary Drug Delivery against COVID-19. ACS Biomater. Sci. Eng. 2022, 8 (6), 2553-2563.
(10) Khutoryanskiy, V. V. Advances in Mucoadhesion and Mucoadhesive Polymers. Macromol. Biosci. 2011, 11 (6), 748-764.
(11) Serra, L.; Doménech, J.; Peppas, N. A. Engineering Design and Molecular Dynamics of Mucoadhesive Drug Delivery Systems as Targeting Agents. Eur. J. Pharm. Biopharm. 2009, 71 (3), 519-528.
(12) Bernkop-Schnürch, A. Thiomers: A New Generation of Mucoadhesive Polymers. Adv. Drug Delivery Rev. 2005, 57 (11), 1569-1582.
(13) Laffleur, F.; Bernkop-Schnürch, A. Thiomers: Promising Platform for Macromolecular Drug Delivery. Future Med. Chem. 2012, 4, 2205-2216.
(14) Laffleur, F.; Hörmann, N.; Gust, R.; Ganner, A. Synthesis, Characterization and Evaluation of Hyaluronic Acid-Based Polymers for Nasal Delivery. Int. J. Pharm. 2023, 631, No. 122496.
(15) Davidovich-Pinhas, M.; Bianco-Peled, H. Novel Mucoadhesive System Based on Sulfhydryl-Acrylate Interactions. J. Mater. Sci.: Mater. Med. 2010, 21 (7), 2027-2034.
(16) Kolawole, O. M.; Lau, W. M.; Khutoryanskiy, V. V. Methacrylated Chitosan as a Polymer with Enhanced Mucoadhesive Properties for Transmucosal Drug Delivery. Int. J. Pharm. 2018, 550 (1-2), 123-129.
(17) Tonglairoum, P.; Brannigan, R. P.; Opanasopit, P.; Khutoryanskiy, V. V. Maleimide-Bearing Nanogels as Novel Mucoadhesive Materials for Drug Delivery. J. Mater. Chem. B 2016, 4 (40), 6581-6587.
(18) Kim, K.; Kim, K.; Ryu, J. H.; Lee, H. Chitosan-Catechol: A Polymer with Long-Lasting Mucoadhesive Properties. Biomaterials 2015, 52 (1), 161-170.
(19) Prosperi-Porta, G.; Kedzior, S.; Muirhead, B.; Sheardown, H. Phenylboronic-Acid-Based Polymeric Micelles for Mucoadhesive Anterior Segment Ocular Drug Delivery. Biomacromolecules 2016, 17 (4), 1449-1457.
(20) Menzel, C.; Hauser, M.; Frey, A.; Jelkmann, M.; Laffleur, F.; Götzfried, S. K.; Gust, R.; Bernkop-Schnürch, A. Covalently Binding Mucoadhesive Polymers: N-Hydroxysuccinimide Grafted Polyacrylates. Eur. J. Pharm. Biopharm. 2019, 139, 161-167.
(21) Eshel-Green, T.; Bianco-Peled, H. Mucoadhesive Acrylated Block Copolymers Micelles for the Delivery of Hydrophobic Drugs. Colloids Surf., B 2016, 139, 42-51.
(22) Kaldybekov, D. B.; Tonglairoum, P.; Opanasopit, P.; Khutoryanskiy, V. V. Mucoadhesive Maleimide-Functionalised Liposomes for Drug Delivery to Urinary Bladder. Eur. J. Pharm. Sci. 2018, 111, 83-90.
(23) Kaldybekov, D. B.; Filippov, S. K.; Radulescu, A.; Khutoryanskiy, V. V. Maleimide-Functionalised PLGA-PEG Nanoparticles as Mucoadhesive Carriers for Intravesical Drug Delivery. Eur. J. Pharm. Biopharm. 2019, 143, 24-34.
(24) Shan, X.; Aspinall, S.; Kaldybekov, D. B.; Buang, F.; Williams, A. C.; Khutoryanskiy, V. V. Synthesis and Evaluation of Methacrylated Poly(2-Ethyl-2-Oxazoline) as a Mucoadhesive Polymer for Nasal Drug Delivery. ACS Appl. Polym. Mater. 2021, 3 (11), 5882-5892.
(25) Agibayeva, L. E.; Kaldybekov, D. B.; Porfiryeva, N. N.; Garipova, V. R.; Mangazbayeva, R. A.; Moustafine, R. I.; Semina, I. I.; Mun, G. A.; Kudaibergenov, S. E.; Khutoryanskiy, V. V. Gellan Gum and Its Methacrylated Derivatives as in Situ Gelling Mucoadhesive Formulations of Pilocarpine: In Vitro and in Vivo Studies. Int. J. Pharm. 2020, 577, No. 119093.
(26) Buang, F.; Fu, M.; Chatzifragkou, A.; Amin, M. C. I. M.; Khutoryanskiy, V. V. Hydroxyethyl Cellulose Functionalised with Maleimide Groups as a New Excipient with Enhanced Mucoadhesive Properties. Int. J. Pharm. 2023, 642, No. 123113.
(27) Brotherton, E. E.; Neal, T. J.; Kaldybekov, D. B.; Smallridge, M. J.; Khutoryanskiy, V. V.; Armes, S. P. Aldehyde-Functional Thermoresponsive Diblock Copolymer Worm Gels Exhibit Strong Mucoadhesion. Chem. Sci. 2022, 13, 6888-6898.
(28) Hunter, S. J.; Abu Elella, M. H.; Johnson, E. C.; Taramova, L.; Brotherton, E. E.; Armes, S. P.; Khutoryanskiy, V. V.; Smallridge, M. J. Mucoadhesive Pickering Nanoemulsions via Dynamic Covalent Chemistry. J. Colloid Interface Sci. 2023, 651, 334-345.
(29) Bonferoni, M. C.; Chetoni, P.; Giunchedi, P.; Rossi, S.; Ferrari, F.; Burgalassi, S.; Caramella, C. Carrageenan-Gelatin Mucoadhesive Systems for Ion-Exchange Based Ophthalmic Delivery: In Vitro and Preliminary in Vivo Studies. Eur. J. Pharm. Biopharm. 2004, 57 (3), 465-472.
(30) Padhi, J. R.; Nayak, D.; Nanda, A.; Rauta, P. R.; Ashe, S.; Nayak, B. Development of Highly Biocompatible Gelatin & ICarrageenan Based Composite Hydrogels: In Depth Physiochemical Analysis for Biomedical Applications. Carbohydr. Polym. 2016, 153, 292-301.
(31) Liu, Y.; Cheong NG, S.; Yu, J.; Tsai, W. B. Modification and Crosslinking of Gelatin-Based Biomaterials as Tissue Adhesives. Colloids Surf., B 2019, 174, 316-323.
(32) Jovanović, M.; Tomić, N.; Cvijić, S.; Stojanović, D.; Ibrić, S.; Uskoković, P. Mucoadhesive Gelatin Buccal Films with Propranolol Hydrochloride: Evaluation of Mechanical, Mucoadhesive, and Biopharmaceutical Properties. Pharmaceutics 2021, 13 (2), 273.
(33) Abruzzo, A.; Bigucci, F.; Cerchiara, T.; Cruciani, F.; Vitali, B.; Luppi, B. Mucoadhesive Chitosan/Gelatin Films for Buccal Delivery of Propranolol Hydrochloride. Carbohydr. Polym. 2012, 87 (1), 581588.
(34) Abruzzo, A.; Cerchiara, T.; Bigucci, F.; Gallucci, M. C.; Luppi, B. Mucoadhesive Buccal Tablets Based on Chitosan/Gelatin Microparticles for Delivery of Propranolol Hydrochloride. J. Pharm. Sci. 2015, 104 (12), 4365-4372.
(35) Trastullo, R.; Abruzzo, A.; Saladini, B.; Gallucci, M. C.; Cerchiara, T.; Luppi, B.; Bigucci, F. Design and Evaluation of Buccal Films as Paediatric Dosage Form for Transmucosal Delivery of Ondansetron. Eur. J. Pharm. Biopharm. 2016, 105, 115-121.
(36) Mahaling, B.; Katti, D. S. Understanding the Influence of Surface Properties of Nanoparticles and Penetration Enhancers for Improving Bioavailability in Eye Tissues In Vivo. Int. J. Pharm. 2016, 501, 1-9.
(37) Giordani, B.; Abruzzo, A.; Prata, C.; Nicoletta, F. P.; Dalena, F.; Cerchiara, T.; Luppi, B.; Bigucci, F. Ondansetron Buccal Administration for Paediatric Use: A Comparison between Films and Wafers. Int. J. Pharm. 2020, 580, No. 119228.
(38) Kudaibergenov, S. E. Synthetic and Natural Polyampholytes: Structural and Behavioral Similarity. Polym. Adv. Technol. 2021, 32 (3), 906-918.
(39) Ahmady, A.; Abu Samah, N. H. A Review: Gelatine as a Bioadhesive Material for Medical and Pharmaceutical Applications. Int. J. Pharm. 2021, 608, No. 121037.
(40) Van Den Bulcke, A. I.; Bogdanov, B.; De Rooze, N.; Schacht, E. H.; Cornelissen, M.; Berghmans, H. Structural and Rheological Properties of Methacrylamide Modified Gelatin Hydrogels. Biomacromolecules 2000, 1(1), 31-38.
(41) Claaßen, C.; Claaßen, M. H.; Truffault, V.; Sewald, L.; Tovar, G. E. M.; Borchers, K.; Southan, A. Quantification of Substitution of Gelatin Methacryloyl: Best Practice and Current Pitfalls. Biomacromolecules 2018, 19 (1), 42-52.
(42) Brinkman, W. T.; Nagapudi, K.; Thomas, B. S.; Chaikof, E. L. Photo-Cross-Linking of Type I Collagen Gels in the Presence of Smooth Muscle Cells: Mechanical Properties, Cell Viability, and Function. Biomacromolecules 2003, 4 (4), 890-895.
(43) Nichol, J. W.; Koshy, S. T.; Bae, H.; Hwang, C. M.; Yamanlar, S.; Khademhosseini, A. Cell-Laden Microengineered Gelatin Methacrylate Hydrogels. Biomaterials 2010, 31 (21), 5536-5544.
(44) Norris, S. C. P.; Delgado, S. M.; Kasko, A. M. Mechanically Robust Photodegradable Gelatin Hydrogels for 3D Cell Culture and in Situ Mechanical Modification. Polym. Chem. 2019, 10 (23), 31803193.
(45) Habeeb, A. F. S. A. Determination of Free Amino Groups in Proteins by Trinitrobenzenesulfonic Acid. Anal. Biochem. 1966, 14 (3), 328-336.
(46) Lee, B. H.; Shirahama, H.; Cho, N. J.; Tan, L. P. Efficient and Controllable Synthesis of Highly Substituted Gelatin Methacrylamide for Mechanically Stiff Hydrogels. RSC Adv. 2015, 5 (128), 106094106097.
(47) Zhu, M.; Wang, Y.; Ferracci, G.; Zheng, J.; Cho, N. J.; Lee, B. H. Gelatin Methacryloyl and Its Hydrogels with an Exceptional Degree of Controllability and Batch-to-Batch Consistency. Sci. Rep. 2019, 9, 6863.
(48) Benson, J. E. Viscometric Determination of the Isoelectric Point of a Protein. J. Chem. Educ. 1963, 40 (9), 468-469.
(49) Khutoryanskaya, O. V.; Morrison, P. W. J.; Seilkhanov, S. K.; Mussin, M. N.; Ozhmukhametova, E. K.; Rakhypbekov, T. K.; Khutoryanskiy, V. V. Hydrogen-Bonded Complexes and Blends of Poly(Acrylic Acid) and Methylcellulose: Nanoparticles and Mucoadhesive Films for Ocular Delivery of Riboflavin. Macromol. Biosci. 2014, 14 (2), 225-234.
(50) Irmukhametova, G. S.; Mun, G. A.; Khutoryanskiy, V. V. Thiolated Mucoadhesive and PEGylated Nonmucoadhesive Organosilica Nanoparticles from 3-Mercaptopropyltrimethoxysilane. Langmuir 2011, 27 (15), 9551-9556.
(51) Mun, E. A.; Williams, A. C.; Khutoryanskiy, V. V. Adhesion of Thiolated Silica Nanoparticles to Urinary Bladder Mucosa: Effects of PEGylation, Thiol Content and Particle Size. Int. J. Pharm. 2016, 512 (1), 32-38.
(52) Porfiryeva, N. N.; Nasibullin, S. F.; Abdullina, S. G.; Tukhbatullina, I. K.; Moustafine, R. I.; Khutoryanskiy, V. V. Acrylated Eudragit® E PO as a Novel Polymeric Excipient with Enhanced Mucoadhesive Properties for Application in Nasal Drug Delivery. Int. J. Pharm. 2019, 562, 241-248.
(53) Haddow, P. J.; da Silva, M. A.; Kaldybekov, D. B.; Dreiss, C. A.; Hoffman, E.; Hutter, V.; Khutoryanskiy, V. V.; Kirton, S. B.; Mahmoudi, N.; McAuley, W. J.; Cook, M. T. Polymer Architecture Effects on Poly(
(54) Kolawole, O. M.; Lau, W. M.; Khutoryanskiy, V. V. Synthesis and Evaluation of Boronated Chitosan as a Mucoadhesive Polymer for Intravesical Drug Delivery. J. Pharm. Sci. 2019, 108 (9), 3046-3053.
(55) Hoch, E.; Schuh, C.; Hirth, T.; Tovar, G. E. M.; Borchers, K. Stiff Gelatin Hydrogels Can Be Photo-Chemically Synthesized from Low Viscous Gelatin Solutions Using Molecularly Functionalized Gelatin with a High Degree of Methacrylation. J. Mater. Sci. Mater. Med. 2012, 23 (11), 2607-2617.
(56) Kim, S.; Nimni, M. E.; Yang, Z.; Han, B. Chitosan/GelatinBased Films Crosslinked by Proanthocyanidin. J. Biomed. Mater. Res. 2005, 75B (2), 442-450.
(57) Barth, A. Infrared Spectroscopy of Proteins. Biochim. Biophys. Acta – Bioenerg. 2007, 1767 (9), 1073-1101.
(58) Hashim, D. M.; Man, Y. B. C.; Norakasha, R.; Shuhaimi, M.; Salmah, Y.; Syahariza, Z. A. Potential Use of Fourier Transform Infrared Spectroscopy for Differentiation of Bovine and Porcine Gelatins. Food Chem. 2010, 118 (3), 856-860.
(59) Cebi, N.; Durak, M. Z.; Toker, O. S.; Sagdic, O.; Arici, M. An Evaluation of Fourier Transforms Infrared Spectroscopy Method for the Classification and Discrimination of Bovine, Porcine and Fish Gelatins. Food Chem. 2016, 190, 1109-1115.
(60) Rahali, K.; Ben Messaoud, G.; Kahn, C. J. F.; SanchezGonzalez, L.; Kaci, M.; Cleymand, F.; Fleutot, S.; Linder, M.; Desobry, S.; Arab-Tehrany, E. Synthesis and Characterization of Nanofunctionalized Gelatin Methacrylate Hydrogels. Int. J. Mol. Sci. 2017, 18 (12), 2675.
(61) Sheppard, S. E.; Houck, R. C. The Structure of Gelatin Sols and Gels. III. J. Phys. Chem. 1930, 34 (10), 2187-2201.
(62) Johlin, J. M. The Isoelectric Point of Gelatin and Its Relation to the Minimum Physical Properties of Gelatin. J. Biol. Chem. 1930, 86 (1), 231-243.
(63) Lowe, A. B.; McCormick, C. L. Synthesis and Solution Properties of Zwitterionic Polymers. Chem. Rev. 2002, 102 (11), 4177-4190.
(64) Gudmundsson, M. Rheological Properties of Fish Gelatins. J. Food Sci. 2002, 67 (6), 2172-2176.
(65) Goudoulas, T. B.; Germann, N. Phase Transition Kinetics and Rheology of Gelatin-Alginate Mixtures. Food Hydrocolloids 2017, 66, 49-60.
(66) Boran, G.; Mulvaney, S. J.; Regenstein, J. M. Rheological Properties of Gelatin from Silver Carp Skin Compared to Commercially Available Gelatins from Different Sources. J. Food Sci. 2010, 75 (8), E565-E571.
(67) ISO 10993-5:2009(en), Biological evaluation of medical devices – Part 5: Tests for in vitro cytotoxicity. https://www.iso.org/ obp/ui/#iso:std:iso:10993:-5:ed-3:v1:en (accessed 2022-09-03).
(68) Elzoghby, A. O.; Samy, W. M.; Elgindy, N. A. Protein-Based Nanocarriers as Promising Drug and Gene Delivery Systems. J. Controlled Release 2012, 161 (1), 38-49.
(69) Suresh, D.; Suresh, A.; Kannan, R. Engineering Biomolecular Systems: Controlling the Self-Assembly of Gelatin to Form UltraSmall Bioactive Nanomaterials. Bioact. Mater. 2022, 18, 321-336.
(70) Ulubayram, K.; Aksu, E.; Gurhan, S. I. D.; Serbetci, K.; Hasirci, N. Cytotoxicity Evaluation of Gelatin Sponges Prepared with Different Cross-Linking Agents. J. Biomater. Sci. Polym. Ed. 2002, 13 (11), 1203-1219.
(71) Won, Y.-W.; Kim, Y.-H. Preparation and Cytotoxicity Comparison of Type a Gelatin Nanoparticles with Recombinant Human Gelatin Nanoparticles. Macromol. Res. 2009, 17 (7), 464468.
(72) Shin, H.; Olsen, B. D.; Khademhosseini, A. The Mechanical Properties and Cytotoxicity of Cell-Laden Double-Network Hydrogels Based on Photocrosslinkable Gelatin and Gellan Gum Biomacromolecules. Biomaterials 2012, 33 (11), 3143-3152.
(73) Adriaens, E.; Remon, J. P. Gastropods as an Evaluation Tool for Screening the Irritating Potency of Absorption Enhancers and Drugs. Pharm. Res. 1999, 16 (8), 1240-1244.
(74) Adriaens, E.; Dierckens, K.; Bauters, T. G. M.; Nelis, H. J.; Van Goethem, F.; Vanparys, P.; Remon, J. P. The Mucosal Toxicity of Different Benzalkonium Chloride Analogues Evaluated with an Alternative Test Using Slugs. Pharm. Res. 2001, 18 (7), 937-942.
(75) Callens, C.; Adriaens, E.; Dierckens, K.; Remon, J. P. Toxicological Evaluation of a Bioadhesive Nasal Powder Containing a Starch and Carbopol® 974 P on Rabbit Nasal Mucosa and Slug Mucosa. J. Controlled Release 2001, 76 (1-2), 81-91.
(76) Ceulemans, J.; Vermeire, A.; Adriaens, E.; Remon, J. P.; Ludwig, A. Evaluation of a Mucoadhesive Tablet for Ocular Use. J. Controlled Release 2001, 77 (3), 333-344.
(77) Adriaens, E.; Ameye, D.; Dhondt, M. M. M.; Foreman, P.; Remon, J. P. Evaluation of the Mucosal Irritation Potency of Co-Spray Dried Amioca®/Poly(Acrylic Acid) and Amioca®/Carbopol® 974P Mixtures. J. Controlled Release 2003, 88 (3), 393-399.
(78) Dhondt, M.; Adriaens, E.; Roey, J.; Remon, J. The Evaluation of the Local Tolerance of Vaginal Formulations Containing Dapivirine Using the Slug Mucosal Irritation Test and the Rabbit Vaginal Irritation Test. Eur. J. Pharm. Biopharm. 2005, 60 (3), 419425.
(79) Adriaens, E.; Remon, J. P. Mucosal Irritation Potential of Personal Lubricants Relates to Product Osmolality as Detected by the Slug Mucosal Irritation Assay. Sex. Transm. Dis. 2008, 35 (5), 512516.
(80) Adriaens, E.; Bytheway, H.; De Wever, B.; Eschrich, D.; Guest, R.; Hansen, E.; Vanparys, P.; Schoeters, G.; Warren, N.; Weltens, R.; Whittingham, A.; Remon, J. P. Successful Prevalidation of the Slug Mucosal Irritation Test to Assess the Eye Irritation Potency of Chemicals. Toxicol. In Vitro 2008, 22 (5), 1285-1296.
(81) Lenoir, J.; Adriaens, E.; Remon, J. P. New Aspects of the Slug Mucosal Irritation Assay: Predicting Nasal Stinging, Itching and Burning Sensations. J. Appl. Toxicol. 2011, 31 (7), 640-648.
(82) Lenoir, J.; Bachert, C.; Remon, J. P.; Adriaens, E. The Slug Mucosal Irritation (SMI) Assay: A Tool for the Evaluation of Nasal Discomfort. Toxicol. In Vitro 2013, 27 (6), 1954-1961.
(83) Petit, J. Y.; Doré, V.; Marignac, G.; Perrot, S. Assessment of Ocular Discomfort Caused by 5 Shampoos Using the Slug Mucosal Irritation Test. Toxicol. In Vitro 2017, 40, 243-247.
(84) Balls, M.; Goldberg, A. M.; Fentem, J. H.; Broadhead, C. L.; Burch, R. L.; Festing, M. F. W.; Frazier, J. M.; Hendriksen, C. F. M.; Jennings, M.; van der Kamp, M. D. O.; Morton, D. B.; Rowan, A. N.; Russell, C.; Russell, W. M. S.; Spielmann, H.; Stephens, M. L.; Stokes, W. S.; Straughan, D. W.; Yager, J. D.; Zurlo, J.; van Zutphen, B. F. M. The Three Rs: The Way Forward: The Report and Recommendations of ECVAM Workshop 11. Altern. to Lab. Anim. 1995, 23 (6), 838866.
(85) Moiseev, R. V.; Steele, F.; Khutoryanskiy, V. V. Polyaphron Formulations Stabilised with Different Water-Soluble Polymers for Ocular Drug Delivery. Pharmaceutics 2022, 14 (5), 926.
(86) Hussain, A.; Ahsan, F. The Vagina as a Route for Systemic Drug Delivery. J. Controlled Release 2005, 103 (2), 301-313.
(87) Krogstad, E. A.; Rathbone, M. J.; Woodrow, K. A. Vaginal Drug Delivery. In Focal Controlled Drug Delivery. Advances in Delivery Science
and Technology; Domb, A. J., Khan, W., Eds.; Springer: Boston, MA, 2014; pp 607-651.
(88) Shapiro, R. L.; DeLong, K.; Zulfiqar, F.; Carter, D.; Better, M.; Ensign, L. M. In Vitro and Ex Vivo Models for Evaluating Vaginal Drug Delivery Systems. Adv. Drug Delivery Rev. 2022, 191, No. 114543.
(89) Davidovich-Pinhas, M.; Bianco-Peled, H. Methods to Study Mucoadhesive Dosage Forms. In Mucoadhesive Materials and Drug Delivery Systems; Khutoryanskiy, V. V., Ed.; John Wiley & Sons, Ltd: Chichester, U.K., 2014; pp 175-196.
(90) Eschenbach, D. A.; Thwin, S. S.; Patton, D. L.; Hooton, T. M.; Stapleton, A. E.; Agnew, K.; Winter, C.; Meier, A.; Stamm, W. E. Influence of the Normal Menstrual Cycle on Vaginal Tissue, Discharge, and Microflora. Clin. Infect. Dis. 2000, 30 (6), 901-907.
(91) Sogias, I. A.; Williams, A. C.; Khutoryanskiy, V. V. Why Is Chitosan Mucoadhesive? Biomacromolecules 2008, 9 (7), 1837-1842.
(92) Fu, M.; Filippov, S. K.; Williams, A. C.; Khutoryanskiy, V. V. On the Mucoadhesive Properties of Synthetic and Natural Polyampholytes. J. Colloid Interface Sci. 2024, 659, 849-858.
(93) Lale, A. M.; Mason, J. D. T.; Jones, N. S. Mucociliary Transport and Its Assessment: A Review. Clin. Otolaryngol. Allied Sci. 1998, 23 (5), 388-396.
(94) Sahin-Yilmaz, A.; Naclerio, R. M. Anatomy and Physiology of the Upper Airway. Proc. Am. Thorac. Soc. 2011, 8 (1), 31-39.
(95) Beule, A. G. Physiology and Pathophysiology of Respiratory Mucosa of the Nose and the Paranasal Sinuses. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2010, 9, Doc07.
(96) Chavda, V. P.; Jogi, G.; Shah, N.; Athalye, M. N.; Bamaniya, N.; K Vora, L.; Cláudia Paiva-Santos, A. Advanced Particulate CarrierMediated Technologies for Nasal Drug Delivery. J. Drug Delivery Sci. Technol. 2022, 74, No. 103569.
(97) Favaro-Trindade, C. S.; Santana, A. S.; Monterrey-Quintero, E. S.; Trindade, M. A.; Netto, F. M. The Use of Spray Drying Technology to Reduce Bitter Taste of Casein Hydrolysate. Food Hydrocolloids 2010, 24 (4), 336-340.
(98) Osman, R.; Kan, P. L.; Awad, G.; Mortada, N.; El-Shamy, A. E.; Alpar, O. Spray Dried Inhalable Ciprofloxacin Powder with Improved Aerosolisation and Antimicrobial Activity. Int. J. Pharm. 2013, 449 (12), 44-58.
- All outputs in CentAUR are protected by Intellectual Property Rights law, including copyright law. Copyright and IPR is retained by the creators or other copyright holders. Terms and conditions for use of this material are defined in
- Received: October 31, 2023
Revised: January 13, 2024
Accepted: January 16, 2024
DOI: https://doi.org/10.1021/acs.biomac.3c01183
PMID: https://pubmed.ncbi.nlm.nih.gov/38319691
Publication Date: 2024-02-06
Enhancing mucoadhesive properties of gelatin through chemical modification with unsaturated anhydrides
Open Access
Shatabayeva, E. ORCID: https://orcid.org/0000-0001-91535198, Kaldybekov, D. B. ORCID: https://orcid.org/0000-0002-7191-5465, Ulmanova, L., Zhaisanbayeva, B. A., Mun, E. A., Kenessova, Z. A. ORCID: https://orcid.org/0000-0003-2768824X, Kudaibergenov, S. E. and Khutoryanskiy, V. V. ORCID: https://orcid.org/0000-0002-7221-2630 (2024) Enhancing mucoadhesive properties of gelatin through chemical modification with unsaturated anhydrides. Biomacromolecules. ISSN 1526-4602 doi: 10.1021/acs.biomac.3c01183 Available at https://centaur.reading.ac.uk/115238/
Publisher: American Chemical Society
www.reading.ac.uk/centaur
CentAUR
Reading’s research outputs online
Enhancing Mucoadhesive Properties of Gelatin through Chemical Modification with Unsaturated Anhydrides
Read Online
Downloaded via UNIV OF READING on February 23, 2024 at 14:53:45 (UTC).
See https://pubs.acs.org/sharingguidelines for options on how to legitimately share published articles.
Abstract
Gelatin is a water-soluble natural polyampholyte with poor mucoadhesive properties. It has traditionally been used as a major ingredient in many pharmaceuticals, including soft and hard capsules, suppositories, tissue engineering, and regenerative medicine. The mucoadhesive properties of gelatin can be improved by modifying it through conjugation with specific adhesive unsaturated groups. In this study, gelatin was modified by reacting with crotonic, itaconic, and methacrylic anhydrides in varying molar ratios to yield crotonoylated-, itaconoylated-, and methacryloylated gelatins (abbreviated as Gel-CA, Gel-IA, and Gel-MA, respectively). The successful synthesis was confirmed using
 unmodified gelatins. The safety of modified gelatin derivatives was assessed with an in vivo slug mucosal irritation test (SMIT) and an in vitro MTT assay utilizing human pulmonary fibroblasts cell line. Two different model dosage forms, such as physical gels and spray-dried microparticles, were prepared and their mucoadhesive properties were evaluated using a flow-through technique with fluorescent detection and a tensile test with ex vivo porcine vaginal tissues and sheep nasal mucosa. Gelatins modified with unsaturated groups exhibited superior mucoadhesive properties compared to native gelatin. The enhanced ability of gelatin modified with these unsaturated functional groups is due to the formation of covalent bonds with cysteine-rich subdomains present in the mucin via thiol-ene click Michael-type addition reactions occurring under physiologically relevant conditions.
unmodified gelatins. The safety of modified gelatin derivatives was assessed with an in vivo slug mucosal irritation test (SMIT) and an in vitro MTT assay utilizing human pulmonary fibroblasts cell line. Two different model dosage forms, such as physical gels and spray-dried microparticles, were prepared and their mucoadhesive properties were evaluated using a flow-through technique with fluorescent detection and a tensile test with ex vivo porcine vaginal tissues and sheep nasal mucosa. Gelatins modified with unsaturated groups exhibited superior mucoadhesive properties compared to native gelatin. The enhanced ability of gelatin modified with these unsaturated functional groups is due to the formation of covalent bonds with cysteine-rich subdomains present in the mucin via thiol-ene click Michael-type addition reactions occurring under physiologically relevant conditions.
1. INTRODUCTION
linked thermoreversible hydrogels upon cooling below
mucoadhesive properties. These include the development of nanogels,
2. EXPERIMENTAL SECTION
solution, sodium bicarbonate, sodium dodecyl sulfate (SDS), 2,4,6trinitrobenzenesulfonic acid (TNBSA,
2.2. Synthesis of Gelatin Derivatives. Gelatin was chemically functionalized with different unsaturated anhydrides using previously described procedures with some modifications.
2.3. Preparation of Spray-Dried Microparticles. Both chemically modified and unmodified gelatin samples ( 0.5 g ) were initially dissolved in 100 mL of aqueous solutions containing fluorescein sodium salt (
2.4. Characterization. 2.4.1. Quantification of the Degree of Functionalization. The modification of gelatin was confirmed using
remaining free amino groups after gelatin derivatization with minor changes.
2.4.2. Fourier Transform Infrared (FTIR) Spectroscopy. FTIR spectra of unmodified and modified gelatins were recorded using a Nicolet iS10 FTIR spectrophotometer (Thermo Scientific, U.K.) with an iTX attenuated total reflectance (ATR) accessory equipped with a diamond crystal. The spectra were collected from an average of 32 scans between 4000 and
2.4.3. Scanning Electron Microscopy (SEM). The morphology and size of spray-dried microparticles based on gelatin and its modified (Gel-CA, Gel-IA, and Gel-MA) derivatives were examined using a Zeiss Crossbeam 540 scanning electron microscope (Carl Zeiss Microscopy GmbH, Jena, Germany) at an accelerating voltage of 5 kV . The samples were sputter-coated with gold prior to imaging. The acquired images were then analyzed with ImageJ software (NIH, U.S.A.) to determine the average mean diameter of the microparticles.
2.4.4. Determination of the Isoelectric Point (IEP). The isoelectric points of modified and unmodified gelatin were determined using a conventional viscometric technique
2.4.5. Rheological Studies. A TA DHR-1 rheometer (TA Instruments, New Castle, DE, U.S.A.) equipped with a variable temperature Peltier plate and a stainless steel cone-plate geometry (
by temperature sweep tests from 0 to
2.5. Toxicity Assessment. 2.5.1. In Vitro Cell Viability Assay. Human pulmonary fibroblasts (HPF) cell line was cultured in Dulbecco’s modified eagle medium (DMEM) fortified with
2.6. Ex Vivo Retention Studies on Porcine Vaginal and Sheep Nasal Mucosae. 2.6.1. Model Dosage Form Design. A 0.1
lated (Gel-MA) derivatives were separately dissolved in 10 mL of aqueous solutions of NaFl . The mixtures were stirred for 12 h at room temperature until homogeneous solutions formed, covered with aluminum foil, and stored in a fridge for further use. These NaFlloaded modified and unmodified gelatin-based formulations were employed in porcine vaginal mucoadhesion studies.
2.6.2. Tissue Preparation. Porcine vaginal tissues and sheep heads were received from P.C. Turner Abattoirs (Farnborough, U.K.) immediately after animal slaughter, packed, transported to the laboratory in cold plastic containers, and used within 24 h of collection. The vaginal tissues were carefully dissected (avoiding contact with the internal mucosa) using disposable sharp blades to yield
2.6.3. Flow-through Technique. Experiments to evaluate the mucosal retention of modified and unmodified gelatin-based formulations on ex vivo porcine vaginal and sheep nasal tissues were conducted using a well-established flow-through method involving fluorescent detection with minor modifications.

same in vitro flow-through technique as described above with some modifications. Only non-cross-linked microparticle samples were used in this experiment. Approximately 100 mg of gelatin-based microparticles (included gelatin, Gel-CA, Gel-IA, or Gel-MA) containing 1
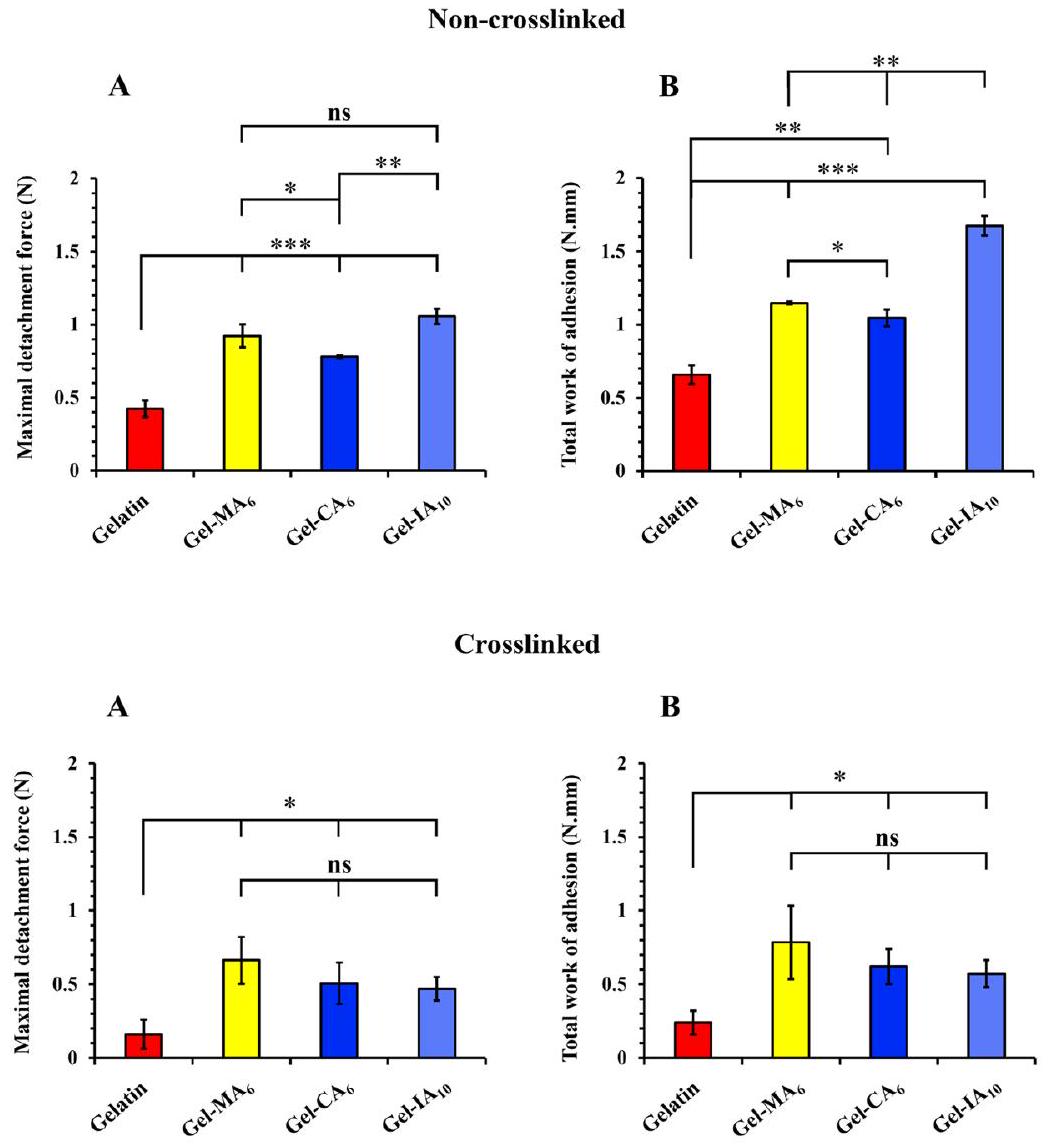
2.6.4. Tensile (Detachment) Method. A TA.XT Plus Texture Analyzer (Stable Micro Systems Ltd., Surrey, U.K.) operated in its adhesive test mode was used to evaluate the mucoadhesive performance of spray-dried microparticles based on gelatin and its derivatives. Both cross-linked and non-cross-linked microparticle samples were employed in this experiment to evaluate the contribution of macromolecules diffusion and ability to form
interpenetrating layer with mucus in mucoadhesion. Freshly isolated sheep nasal tissues were used within 24 h of retrieval for this experiment. As previously reported and adapted with some changes,
2.7. Statistical Analysis. All measurements in the present study were conducted at a minimum of three times and data were expressed as mean

3. RESULTS AND DISCUSSION
(DoF) was determined by comparing the integrals of the characteristic double bond hydrogen peaks of each modified gelatin substituent and the integration of the area corresponding to the combined peaks of the aromatic protons of phenylalanine and tyrosine, where their signals were served as a reference. Based on
| sample
|
|
DoF by
|
DoF by TNBSA assay (
|
yield (%) | IEP
|
|
| gelatin |
|
7.0 |
|
|||
| Gel-MA
|
1.5 |
|
0.373 |
|
4.2 | 4.2 |
| Gel-MA
|
3 |
|
0.380 |
|
4.2 | 4.1 |
| Gel-MA
|
6 |
|
0.384 |
|
4.3 | 4.4 |
| Gel-CA
|
1.5 |
|
0.355 |
|
2.5 | ND |
| Gel-
|
3 |
|
0.358 |
|
3.2 | ND |
| Gel-CA
|
6 |
|
0.360 |
|
3.2 | ND |
| Gel-IA
|
2 |
|
0.274 |
|
3.8 | 4.0 |
| Gel-IA
|
5 |
|
0.298 |
|
3.8 | 4.0 |
| Gel-IA
|
10 |
|
0.298 |
|
3.5 | ND |
with the FTIR data on gelatin reported in the literature.

rheological measurements. The rheological changes happening with
| sample |
|
|
| gelatin |
|
|
| Gel-MA
|
|
|
| Gel-MA
|
|
|
| Gel-MA
|
|
|
| Gel-CA
|
|
|
| Gel-
|
|
|
| Gel-CA
|
|
|
| Gel-IA
|
|
|
| Gel-IA
|
|
|
| Gel-IA
|
|
|
3.2. Toxicology. 3.2.1. Cell Viability. In vitro cytotoxicity of gelatin and its modified derivatives (Gel-CA, Gel-IA, and Gel-MA) was studied using MTT assay with human pulmonary fibroblasts (HPF) cells. The assay is based on the ability of mitochondria of live cells to reduce 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT reagent), a yellow substance, to insoluble formazan crystals (violet color). This technique allows to calculate the number of viable cells after treatment with the test material. HPF cells were treated with gelatin, Gel-CA

3.2.2. Mucosal Irritancy. The in vivo slug mucosal irritation test (SMIT) was originally developed by Adriaens and coworkers
considered to have limited sentience.


3.3. Mucoadhesion Studies. 3.3.1. Retention on Ex Vivo
formulations were prepared with fluorescein sodium ( NaFl ), which is a fluorescent marker that facilitates easy detection and measurement of mucosal retention levels. This method has been widely employed to study the retention of various formulations on different mucosal surfaces, including vaginal tissues.

As expected, retention of all formulations declines over the course of the washing, yet the following trend is observed: Gel-

3.3.2. Retention on Ex Vivo Sheep Nasal Tissues. Intranasal administration offers a noninvasive route of drug delivery. Therapeutic agents delivered to the nasal cavity act locally and provide a direct target to the central nervous system. Estimated daily production of nasal mucus varies between 0.1 and
wrinkled surface texture, free of crystals, pores, and cracks (see Figures S14 and S15 in the Supporting Information). All the formulations led to microparticles with similar morphologies and the mean diameters of the particles were


4. CONCLUSIONS
– ASSOCIATED CONTENT
(s) Supporting Information
(SEM) images of spray-dried microparticles based on modified and unmodified gelatin samples; Specific viscosity versus pH curves to determine isoelectric points; Storage and loss moduli curves used to determine thermo-reversible gelation points; Schematic illustration of slug mucosal irritation test (SMIT) procedure using Arion lusitanicus species; Exemplar photographs of mucus production by Arion lusitanicus slugs in contact with test materials; Exemplar detachment profiles of test materials (PDF)
– AUTHOR INFORMATION
Corresponding Authors
Daulet B. Kaldybekov – Reading School of Pharmacy, University of Reading, Whiteknights RG6 6DX Reading, United Kingdom; Department of Chemistry and Chemical Technology, Al-Farabi Kazakh National University, 050040 Almaty, Kazakhstan; Institute of Polymer Materials and Technology, 050019 Almaty, Kazakhstan; © orcid.org/ 0000-0002-7191-5465; Email: dauletchem@gmail.com, d.kaldybekov@reading.ac.uk
Authors
Leila Ulmanova – School of Sciences and Humanities, Nazarbayev University, 010000 Astana, Kazakhstan
Balnur A. Zhaisanbayeva – School of Engineering and Digital Sciences, Nazarbayev University, 010000 Astana, Kazakhstan
Ellina A. Mun – School of Sciences and Humanities, Nazarbayev University, 010000 Astana, Kazakhstan
Zarina A. Kenessova – Department of Chemistry and Chemical Technology, Al-Farabi Kazakh National University, 050040 Almaty, Kazakhstan; © orcid.org/0000-0003-2768-824X
Sarkyt E. Kudaibergenov – Institute of Polymer Materials and Technology, 050019 Almaty, Kazakhstan
Complete contact information is available at:
https://pubs.acs.org/10.1021/acs.biomac.3c01183
Author Contributions
Notes
– ACKNOWLEDGMENTS
supplying porcine vaginal and sheep nasal tissues for mucoadhesion experiments. V.V.K. acknowledges the Royal Society for his industry fellowship (IF
– ABBREVIATIONS
– REFERENCES
(2) Schrieber, R.; Gareis, H. Gelatine Handbook: Theory and Industrial Practice; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, 2007.
(3) Alipal, J.; Mohd Pu’ad, N. A. S.; Lee, T. C.; Nayan, N. H. M.; Sahari, N.; Basri, H.; Idris, M. I.; Abdullah, H. Z. A Review of Gelatin: Properties, Sources, Process, Applications, and Commercialisation. Mater. Today: Proc. 2021, 42, 240-250.
(4) Gullapalli, R. P.; Mazzitelli, C. L. Gelatin and Non-Gelatin Capsule Dosage Forms. J. Pharm. Sci. 2017, 106 (6), 1453-1465.
(5) Osmałek, T.; Froelich, A.; Jadach, B.; Tatarek, A.; Gadzinski, P.; Falana, A.; Gralinska, K.; Ekert, M.; Puri, V.; Wrotynska-Barczynska, J.; Michniak-Kohn, B. Recent Advances in Polymer-Based Vaginal Drug Delivery Systems. Pharmaceutics 2021, 13 (6), 884.
(6) Bhagat, V.; Becker, M. L. Degradable Adhesives for Surgery and Tissue Engineering. Biomacromolecules 2017, 18 (10), 3009-3039.
(7) De Clercq, K.; Schelfhout, C.; Bracke, M.; De Wever, O.; Van Bockstal, M.; Ceelen, W.; Remon, J. P.; Vervaet, C. GenipinCrosslinked Gelatin Microspheres as a Strategy to Prevent Postsurgical Peritoneal Adhesions: In Vitro and in Vivo Characterization. Biomaterials 2016, 96, 33-46.
(8) Yuk, H.; Varela, C. E.; Nabzdyk, C. S.; Mao, X.; Padera, R. F.; Roche, E. T.; Zhao, X. Dry Double-Sided Tape for Adhesion of Wet Tissues and Devices. Nature 2019, 575, 169-174.
(9) Li, C.; Marton, I.; Harari, D.; Shemesh, M.; Kalchenko, V.; Pardo, M.; Schreiber, G.; Rudich, Y. Gelatin Stabilizes Nebulized Proteins in Pulmonary Drug Delivery against COVID-19. ACS Biomater. Sci. Eng. 2022, 8 (6), 2553-2563.
(10) Khutoryanskiy, V. V. Advances in Mucoadhesion and Mucoadhesive Polymers. Macromol. Biosci. 2011, 11 (6), 748-764.
(11) Serra, L.; Doménech, J.; Peppas, N. A. Engineering Design and Molecular Dynamics of Mucoadhesive Drug Delivery Systems as Targeting Agents. Eur. J. Pharm. Biopharm. 2009, 71 (3), 519-528.
(12) Bernkop-Schnürch, A. Thiomers: A New Generation of Mucoadhesive Polymers. Adv. Drug Delivery Rev. 2005, 57 (11), 1569-1582.
(13) Laffleur, F.; Bernkop-Schnürch, A. Thiomers: Promising Platform for Macromolecular Drug Delivery. Future Med. Chem. 2012, 4, 2205-2216.
(14) Laffleur, F.; Hörmann, N.; Gust, R.; Ganner, A. Synthesis, Characterization and Evaluation of Hyaluronic Acid-Based Polymers for Nasal Delivery. Int. J. Pharm. 2023, 631, No. 122496.
(15) Davidovich-Pinhas, M.; Bianco-Peled, H. Novel Mucoadhesive System Based on Sulfhydryl-Acrylate Interactions. J. Mater. Sci.: Mater. Med. 2010, 21 (7), 2027-2034.
(16) Kolawole, O. M.; Lau, W. M.; Khutoryanskiy, V. V. Methacrylated Chitosan as a Polymer with Enhanced Mucoadhesive Properties for Transmucosal Drug Delivery. Int. J. Pharm. 2018, 550 (1-2), 123-129.
(17) Tonglairoum, P.; Brannigan, R. P.; Opanasopit, P.; Khutoryanskiy, V. V. Maleimide-Bearing Nanogels as Novel Mucoadhesive Materials for Drug Delivery. J. Mater. Chem. B 2016, 4 (40), 6581-6587.
(18) Kim, K.; Kim, K.; Ryu, J. H.; Lee, H. Chitosan-Catechol: A Polymer with Long-Lasting Mucoadhesive Properties. Biomaterials 2015, 52 (1), 161-170.
(19) Prosperi-Porta, G.; Kedzior, S.; Muirhead, B.; Sheardown, H. Phenylboronic-Acid-Based Polymeric Micelles for Mucoadhesive Anterior Segment Ocular Drug Delivery. Biomacromolecules 2016, 17 (4), 1449-1457.
(20) Menzel, C.; Hauser, M.; Frey, A.; Jelkmann, M.; Laffleur, F.; Götzfried, S. K.; Gust, R.; Bernkop-Schnürch, A. Covalently Binding Mucoadhesive Polymers: N-Hydroxysuccinimide Grafted Polyacrylates. Eur. J. Pharm. Biopharm. 2019, 139, 161-167.
(21) Eshel-Green, T.; Bianco-Peled, H. Mucoadhesive Acrylated Block Copolymers Micelles for the Delivery of Hydrophobic Drugs. Colloids Surf., B 2016, 139, 42-51.
(22) Kaldybekov, D. B.; Tonglairoum, P.; Opanasopit, P.; Khutoryanskiy, V. V. Mucoadhesive Maleimide-Functionalised Liposomes for Drug Delivery to Urinary Bladder. Eur. J. Pharm. Sci. 2018, 111, 83-90.
(23) Kaldybekov, D. B.; Filippov, S. K.; Radulescu, A.; Khutoryanskiy, V. V. Maleimide-Functionalised PLGA-PEG Nanoparticles as Mucoadhesive Carriers for Intravesical Drug Delivery. Eur. J. Pharm. Biopharm. 2019, 143, 24-34.
(24) Shan, X.; Aspinall, S.; Kaldybekov, D. B.; Buang, F.; Williams, A. C.; Khutoryanskiy, V. V. Synthesis and Evaluation of Methacrylated Poly(2-Ethyl-2-Oxazoline) as a Mucoadhesive Polymer for Nasal Drug Delivery. ACS Appl. Polym. Mater. 2021, 3 (11), 5882-5892.
(25) Agibayeva, L. E.; Kaldybekov, D. B.; Porfiryeva, N. N.; Garipova, V. R.; Mangazbayeva, R. A.; Moustafine, R. I.; Semina, I. I.; Mun, G. A.; Kudaibergenov, S. E.; Khutoryanskiy, V. V. Gellan Gum and Its Methacrylated Derivatives as in Situ Gelling Mucoadhesive Formulations of Pilocarpine: In Vitro and in Vivo Studies. Int. J. Pharm. 2020, 577, No. 119093.
(26) Buang, F.; Fu, M.; Chatzifragkou, A.; Amin, M. C. I. M.; Khutoryanskiy, V. V. Hydroxyethyl Cellulose Functionalised with Maleimide Groups as a New Excipient with Enhanced Mucoadhesive Properties. Int. J. Pharm. 2023, 642, No. 123113.
(27) Brotherton, E. E.; Neal, T. J.; Kaldybekov, D. B.; Smallridge, M. J.; Khutoryanskiy, V. V.; Armes, S. P. Aldehyde-Functional Thermoresponsive Diblock Copolymer Worm Gels Exhibit Strong Mucoadhesion. Chem. Sci. 2022, 13, 6888-6898.
(28) Hunter, S. J.; Abu Elella, M. H.; Johnson, E. C.; Taramova, L.; Brotherton, E. E.; Armes, S. P.; Khutoryanskiy, V. V.; Smallridge, M. J. Mucoadhesive Pickering Nanoemulsions via Dynamic Covalent Chemistry. J. Colloid Interface Sci. 2023, 651, 334-345.
(29) Bonferoni, M. C.; Chetoni, P.; Giunchedi, P.; Rossi, S.; Ferrari, F.; Burgalassi, S.; Caramella, C. Carrageenan-Gelatin Mucoadhesive Systems for Ion-Exchange Based Ophthalmic Delivery: In Vitro and Preliminary in Vivo Studies. Eur. J. Pharm. Biopharm. 2004, 57 (3), 465-472.
(30) Padhi, J. R.; Nayak, D.; Nanda, A.; Rauta, P. R.; Ashe, S.; Nayak, B. Development of Highly Biocompatible Gelatin & ICarrageenan Based Composite Hydrogels: In Depth Physiochemical Analysis for Biomedical Applications. Carbohydr. Polym. 2016, 153, 292-301.
(31) Liu, Y.; Cheong NG, S.; Yu, J.; Tsai, W. B. Modification and Crosslinking of Gelatin-Based Biomaterials as Tissue Adhesives. Colloids Surf., B 2019, 174, 316-323.
(32) Jovanović, M.; Tomić, N.; Cvijić, S.; Stojanović, D.; Ibrić, S.; Uskoković, P. Mucoadhesive Gelatin Buccal Films with Propranolol Hydrochloride: Evaluation of Mechanical, Mucoadhesive, and Biopharmaceutical Properties. Pharmaceutics 2021, 13 (2), 273.
(33) Abruzzo, A.; Bigucci, F.; Cerchiara, T.; Cruciani, F.; Vitali, B.; Luppi, B. Mucoadhesive Chitosan/Gelatin Films for Buccal Delivery of Propranolol Hydrochloride. Carbohydr. Polym. 2012, 87 (1), 581588.
(34) Abruzzo, A.; Cerchiara, T.; Bigucci, F.; Gallucci, M. C.; Luppi, B. Mucoadhesive Buccal Tablets Based on Chitosan/Gelatin Microparticles for Delivery of Propranolol Hydrochloride. J. Pharm. Sci. 2015, 104 (12), 4365-4372.
(35) Trastullo, R.; Abruzzo, A.; Saladini, B.; Gallucci, M. C.; Cerchiara, T.; Luppi, B.; Bigucci, F. Design and Evaluation of Buccal Films as Paediatric Dosage Form for Transmucosal Delivery of Ondansetron. Eur. J. Pharm. Biopharm. 2016, 105, 115-121.
(36) Mahaling, B.; Katti, D. S. Understanding the Influence of Surface Properties of Nanoparticles and Penetration Enhancers for Improving Bioavailability in Eye Tissues In Vivo. Int. J. Pharm. 2016, 501, 1-9.
(37) Giordani, B.; Abruzzo, A.; Prata, C.; Nicoletta, F. P.; Dalena, F.; Cerchiara, T.; Luppi, B.; Bigucci, F. Ondansetron Buccal Administration for Paediatric Use: A Comparison between Films and Wafers. Int. J. Pharm. 2020, 580, No. 119228.
(38) Kudaibergenov, S. E. Synthetic and Natural Polyampholytes: Structural and Behavioral Similarity. Polym. Adv. Technol. 2021, 32 (3), 906-918.
(39) Ahmady, A.; Abu Samah, N. H. A Review: Gelatine as a Bioadhesive Material for Medical and Pharmaceutical Applications. Int. J. Pharm. 2021, 608, No. 121037.
(40) Van Den Bulcke, A. I.; Bogdanov, B.; De Rooze, N.; Schacht, E. H.; Cornelissen, M.; Berghmans, H. Structural and Rheological Properties of Methacrylamide Modified Gelatin Hydrogels. Biomacromolecules 2000, 1(1), 31-38.
(41) Claaßen, C.; Claaßen, M. H.; Truffault, V.; Sewald, L.; Tovar, G. E. M.; Borchers, K.; Southan, A. Quantification of Substitution of Gelatin Methacryloyl: Best Practice and Current Pitfalls. Biomacromolecules 2018, 19 (1), 42-52.
(42) Brinkman, W. T.; Nagapudi, K.; Thomas, B. S.; Chaikof, E. L. Photo-Cross-Linking of Type I Collagen Gels in the Presence of Smooth Muscle Cells: Mechanical Properties, Cell Viability, and Function. Biomacromolecules 2003, 4 (4), 890-895.
(43) Nichol, J. W.; Koshy, S. T.; Bae, H.; Hwang, C. M.; Yamanlar, S.; Khademhosseini, A. Cell-Laden Microengineered Gelatin Methacrylate Hydrogels. Biomaterials 2010, 31 (21), 5536-5544.
(44) Norris, S. C. P.; Delgado, S. M.; Kasko, A. M. Mechanically Robust Photodegradable Gelatin Hydrogels for 3D Cell Culture and in Situ Mechanical Modification. Polym. Chem. 2019, 10 (23), 31803193.
(45) Habeeb, A. F. S. A. Determination of Free Amino Groups in Proteins by Trinitrobenzenesulfonic Acid. Anal. Biochem. 1966, 14 (3), 328-336.
(46) Lee, B. H.; Shirahama, H.; Cho, N. J.; Tan, L. P. Efficient and Controllable Synthesis of Highly Substituted Gelatin Methacrylamide for Mechanically Stiff Hydrogels. RSC Adv. 2015, 5 (128), 106094106097.
(47) Zhu, M.; Wang, Y.; Ferracci, G.; Zheng, J.; Cho, N. J.; Lee, B. H. Gelatin Methacryloyl and Its Hydrogels with an Exceptional Degree of Controllability and Batch-to-Batch Consistency. Sci. Rep. 2019, 9, 6863.
(48) Benson, J. E. Viscometric Determination of the Isoelectric Point of a Protein. J. Chem. Educ. 1963, 40 (9), 468-469.
(49) Khutoryanskaya, O. V.; Morrison, P. W. J.; Seilkhanov, S. K.; Mussin, M. N.; Ozhmukhametova, E. K.; Rakhypbekov, T. K.; Khutoryanskiy, V. V. Hydrogen-Bonded Complexes and Blends of Poly(Acrylic Acid) and Methylcellulose: Nanoparticles and Mucoadhesive Films for Ocular Delivery of Riboflavin. Macromol. Biosci. 2014, 14 (2), 225-234.
(50) Irmukhametova, G. S.; Mun, G. A.; Khutoryanskiy, V. V. Thiolated Mucoadhesive and PEGylated Nonmucoadhesive Organosilica Nanoparticles from 3-Mercaptopropyltrimethoxysilane. Langmuir 2011, 27 (15), 9551-9556.
(51) Mun, E. A.; Williams, A. C.; Khutoryanskiy, V. V. Adhesion of Thiolated Silica Nanoparticles to Urinary Bladder Mucosa: Effects of PEGylation, Thiol Content and Particle Size. Int. J. Pharm. 2016, 512 (1), 32-38.
(52) Porfiryeva, N. N.; Nasibullin, S. F.; Abdullina, S. G.; Tukhbatullina, I. K.; Moustafine, R. I.; Khutoryanskiy, V. V. Acrylated Eudragit® E PO as a Novel Polymeric Excipient with Enhanced Mucoadhesive Properties for Application in Nasal Drug Delivery. Int. J. Pharm. 2019, 562, 241-248.
(53) Haddow, P. J.; da Silva, M. A.; Kaldybekov, D. B.; Dreiss, C. A.; Hoffman, E.; Hutter, V.; Khutoryanskiy, V. V.; Kirton, S. B.; Mahmoudi, N.; McAuley, W. J.; Cook, M. T. Polymer Architecture Effects on Poly(
(54) Kolawole, O. M.; Lau, W. M.; Khutoryanskiy, V. V. Synthesis and Evaluation of Boronated Chitosan as a Mucoadhesive Polymer for Intravesical Drug Delivery. J. Pharm. Sci. 2019, 108 (9), 3046-3053.
(55) Hoch, E.; Schuh, C.; Hirth, T.; Tovar, G. E. M.; Borchers, K. Stiff Gelatin Hydrogels Can Be Photo-Chemically Synthesized from Low Viscous Gelatin Solutions Using Molecularly Functionalized Gelatin with a High Degree of Methacrylation. J. Mater. Sci. Mater. Med. 2012, 23 (11), 2607-2617.
(56) Kim, S.; Nimni, M. E.; Yang, Z.; Han, B. Chitosan/GelatinBased Films Crosslinked by Proanthocyanidin. J. Biomed. Mater. Res. 2005, 75B (2), 442-450.
(57) Barth, A. Infrared Spectroscopy of Proteins. Biochim. Biophys. Acta – Bioenerg. 2007, 1767 (9), 1073-1101.
(58) Hashim, D. M.; Man, Y. B. C.; Norakasha, R.; Shuhaimi, M.; Salmah, Y.; Syahariza, Z. A. Potential Use of Fourier Transform Infrared Spectroscopy for Differentiation of Bovine and Porcine Gelatins. Food Chem. 2010, 118 (3), 856-860.
(59) Cebi, N.; Durak, M. Z.; Toker, O. S.; Sagdic, O.; Arici, M. An Evaluation of Fourier Transforms Infrared Spectroscopy Method for the Classification and Discrimination of Bovine, Porcine and Fish Gelatins. Food Chem. 2016, 190, 1109-1115.
(60) Rahali, K.; Ben Messaoud, G.; Kahn, C. J. F.; SanchezGonzalez, L.; Kaci, M.; Cleymand, F.; Fleutot, S.; Linder, M.; Desobry, S.; Arab-Tehrany, E. Synthesis and Characterization of Nanofunctionalized Gelatin Methacrylate Hydrogels. Int. J. Mol. Sci. 2017, 18 (12), 2675.
(61) Sheppard, S. E.; Houck, R. C. The Structure of Gelatin Sols and Gels. III. J. Phys. Chem. 1930, 34 (10), 2187-2201.
(62) Johlin, J. M. The Isoelectric Point of Gelatin and Its Relation to the Minimum Physical Properties of Gelatin. J. Biol. Chem. 1930, 86 (1), 231-243.
(63) Lowe, A. B.; McCormick, C. L. Synthesis and Solution Properties of Zwitterionic Polymers. Chem. Rev. 2002, 102 (11), 4177-4190.
(64) Gudmundsson, M. Rheological Properties of Fish Gelatins. J. Food Sci. 2002, 67 (6), 2172-2176.
(65) Goudoulas, T. B.; Germann, N. Phase Transition Kinetics and Rheology of Gelatin-Alginate Mixtures. Food Hydrocolloids 2017, 66, 49-60.
(66) Boran, G.; Mulvaney, S. J.; Regenstein, J. M. Rheological Properties of Gelatin from Silver Carp Skin Compared to Commercially Available Gelatins from Different Sources. J. Food Sci. 2010, 75 (8), E565-E571.
(67) ISO 10993-5:2009(en), Biological evaluation of medical devices – Part 5: Tests for in vitro cytotoxicity. https://www.iso.org/ obp/ui/#iso:std:iso:10993:-5:ed-3:v1:en (accessed 2022-09-03).
(68) Elzoghby, A. O.; Samy, W. M.; Elgindy, N. A. Protein-Based Nanocarriers as Promising Drug and Gene Delivery Systems. J. Controlled Release 2012, 161 (1), 38-49.
(69) Suresh, D.; Suresh, A.; Kannan, R. Engineering Biomolecular Systems: Controlling the Self-Assembly of Gelatin to Form UltraSmall Bioactive Nanomaterials. Bioact. Mater. 2022, 18, 321-336.
(70) Ulubayram, K.; Aksu, E.; Gurhan, S. I. D.; Serbetci, K.; Hasirci, N. Cytotoxicity Evaluation of Gelatin Sponges Prepared with Different Cross-Linking Agents. J. Biomater. Sci. Polym. Ed. 2002, 13 (11), 1203-1219.
(71) Won, Y.-W.; Kim, Y.-H. Preparation and Cytotoxicity Comparison of Type a Gelatin Nanoparticles with Recombinant Human Gelatin Nanoparticles. Macromol. Res. 2009, 17 (7), 464468.
(72) Shin, H.; Olsen, B. D.; Khademhosseini, A. The Mechanical Properties and Cytotoxicity of Cell-Laden Double-Network Hydrogels Based on Photocrosslinkable Gelatin and Gellan Gum Biomacromolecules. Biomaterials 2012, 33 (11), 3143-3152.
(73) Adriaens, E.; Remon, J. P. Gastropods as an Evaluation Tool for Screening the Irritating Potency of Absorption Enhancers and Drugs. Pharm. Res. 1999, 16 (8), 1240-1244.
(74) Adriaens, E.; Dierckens, K.; Bauters, T. G. M.; Nelis, H. J.; Van Goethem, F.; Vanparys, P.; Remon, J. P. The Mucosal Toxicity of Different Benzalkonium Chloride Analogues Evaluated with an Alternative Test Using Slugs. Pharm. Res. 2001, 18 (7), 937-942.
(75) Callens, C.; Adriaens, E.; Dierckens, K.; Remon, J. P. Toxicological Evaluation of a Bioadhesive Nasal Powder Containing a Starch and Carbopol® 974 P on Rabbit Nasal Mucosa and Slug Mucosa. J. Controlled Release 2001, 76 (1-2), 81-91.
(76) Ceulemans, J.; Vermeire, A.; Adriaens, E.; Remon, J. P.; Ludwig, A. Evaluation of a Mucoadhesive Tablet for Ocular Use. J. Controlled Release 2001, 77 (3), 333-344.
(77) Adriaens, E.; Ameye, D.; Dhondt, M. M. M.; Foreman, P.; Remon, J. P. Evaluation of the Mucosal Irritation Potency of Co-Spray Dried Amioca®/Poly(Acrylic Acid) and Amioca®/Carbopol® 974P Mixtures. J. Controlled Release 2003, 88 (3), 393-399.
(78) Dhondt, M.; Adriaens, E.; Roey, J.; Remon, J. The Evaluation of the Local Tolerance of Vaginal Formulations Containing Dapivirine Using the Slug Mucosal Irritation Test and the Rabbit Vaginal Irritation Test. Eur. J. Pharm. Biopharm. 2005, 60 (3), 419425.
(79) Adriaens, E.; Remon, J. P. Mucosal Irritation Potential of Personal Lubricants Relates to Product Osmolality as Detected by the Slug Mucosal Irritation Assay. Sex. Transm. Dis. 2008, 35 (5), 512516.
(80) Adriaens, E.; Bytheway, H.; De Wever, B.; Eschrich, D.; Guest, R.; Hansen, E.; Vanparys, P.; Schoeters, G.; Warren, N.; Weltens, R.; Whittingham, A.; Remon, J. P. Successful Prevalidation of the Slug Mucosal Irritation Test to Assess the Eye Irritation Potency of Chemicals. Toxicol. In Vitro 2008, 22 (5), 1285-1296.
(81) Lenoir, J.; Adriaens, E.; Remon, J. P. New Aspects of the Slug Mucosal Irritation Assay: Predicting Nasal Stinging, Itching and Burning Sensations. J. Appl. Toxicol. 2011, 31 (7), 640-648.
(82) Lenoir, J.; Bachert, C.; Remon, J. P.; Adriaens, E. The Slug Mucosal Irritation (SMI) Assay: A Tool for the Evaluation of Nasal Discomfort. Toxicol. In Vitro 2013, 27 (6), 1954-1961.
(83) Petit, J. Y.; Doré, V.; Marignac, G.; Perrot, S. Assessment of Ocular Discomfort Caused by 5 Shampoos Using the Slug Mucosal Irritation Test. Toxicol. In Vitro 2017, 40, 243-247.
(84) Balls, M.; Goldberg, A. M.; Fentem, J. H.; Broadhead, C. L.; Burch, R. L.; Festing, M. F. W.; Frazier, J. M.; Hendriksen, C. F. M.; Jennings, M.; van der Kamp, M. D. O.; Morton, D. B.; Rowan, A. N.; Russell, C.; Russell, W. M. S.; Spielmann, H.; Stephens, M. L.; Stokes, W. S.; Straughan, D. W.; Yager, J. D.; Zurlo, J.; van Zutphen, B. F. M. The Three Rs: The Way Forward: The Report and Recommendations of ECVAM Workshop 11. Altern. to Lab. Anim. 1995, 23 (6), 838866.
(85) Moiseev, R. V.; Steele, F.; Khutoryanskiy, V. V. Polyaphron Formulations Stabilised with Different Water-Soluble Polymers for Ocular Drug Delivery. Pharmaceutics 2022, 14 (5), 926.
(86) Hussain, A.; Ahsan, F. The Vagina as a Route for Systemic Drug Delivery. J. Controlled Release 2005, 103 (2), 301-313.
(87) Krogstad, E. A.; Rathbone, M. J.; Woodrow, K. A. Vaginal Drug Delivery. In Focal Controlled Drug Delivery. Advances in Delivery Science
and Technology; Domb, A. J., Khan, W., Eds.; Springer: Boston, MA, 2014; pp 607-651.
(88) Shapiro, R. L.; DeLong, K.; Zulfiqar, F.; Carter, D.; Better, M.; Ensign, L. M. In Vitro and Ex Vivo Models for Evaluating Vaginal Drug Delivery Systems. Adv. Drug Delivery Rev. 2022, 191, No. 114543.
(89) Davidovich-Pinhas, M.; Bianco-Peled, H. Methods to Study Mucoadhesive Dosage Forms. In Mucoadhesive Materials and Drug Delivery Systems; Khutoryanskiy, V. V., Ed.; John Wiley & Sons, Ltd: Chichester, U.K., 2014; pp 175-196.
(90) Eschenbach, D. A.; Thwin, S. S.; Patton, D. L.; Hooton, T. M.; Stapleton, A. E.; Agnew, K.; Winter, C.; Meier, A.; Stamm, W. E. Influence of the Normal Menstrual Cycle on Vaginal Tissue, Discharge, and Microflora. Clin. Infect. Dis. 2000, 30 (6), 901-907.
(91) Sogias, I. A.; Williams, A. C.; Khutoryanskiy, V. V. Why Is Chitosan Mucoadhesive? Biomacromolecules 2008, 9 (7), 1837-1842.
(92) Fu, M.; Filippov, S. K.; Williams, A. C.; Khutoryanskiy, V. V. On the Mucoadhesive Properties of Synthetic and Natural Polyampholytes. J. Colloid Interface Sci. 2024, 659, 849-858.
(93) Lale, A. M.; Mason, J. D. T.; Jones, N. S. Mucociliary Transport and Its Assessment: A Review. Clin. Otolaryngol. Allied Sci. 1998, 23 (5), 388-396.
(94) Sahin-Yilmaz, A.; Naclerio, R. M. Anatomy and Physiology of the Upper Airway. Proc. Am. Thorac. Soc. 2011, 8 (1), 31-39.
(95) Beule, A. G. Physiology and Pathophysiology of Respiratory Mucosa of the Nose and the Paranasal Sinuses. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2010, 9, Doc07.
(96) Chavda, V. P.; Jogi, G.; Shah, N.; Athalye, M. N.; Bamaniya, N.; K Vora, L.; Cláudia Paiva-Santos, A. Advanced Particulate CarrierMediated Technologies for Nasal Drug Delivery. J. Drug Delivery Sci. Technol. 2022, 74, No. 103569.
(97) Favaro-Trindade, C. S.; Santana, A. S.; Monterrey-Quintero, E. S.; Trindade, M. A.; Netto, F. M. The Use of Spray Drying Technology to Reduce Bitter Taste of Casein Hydrolysate. Food Hydrocolloids 2010, 24 (4), 336-340.
(98) Osman, R.; Kan, P. L.; Awad, G.; Mortada, N.; El-Shamy, A. E.; Alpar, O. Spray Dried Inhalable Ciprofloxacin Powder with Improved Aerosolisation and Antimicrobial Activity. Int. J. Pharm. 2013, 449 (12), 44-58.
- All outputs in CentAUR are protected by Intellectual Property Rights law, including copyright law. Copyright and IPR is retained by the creators or other copyright holders. Terms and conditions for use of this material are defined in
- Received: October 31, 2023
Revised: January 13, 2024
Accepted: January 16, 2024
