DOI: https://doi.org/10.1038/s41563-024-01819-x
PMID: https://pubmed.ncbi.nlm.nih.gov/38366155
تاريخ النشر: 2024-02-16
توليد طلاءات أكسيد هجينة محفزات نحاسية مستقرة للاختزال الكهربائي لثاني أكسيد الكربون
تم القبول: 25 يناير 2024
نُشر على الإنترنت: 16 فبراير 2024
الملخص
لقد ساهمت المواد الهجينة العضوية/غير العضوية في حل تحديات مهمة في مجالات مختلفة من العلوم. واحدة من أكبر التحديات من أجل مجتمع أكثر استدامة هي الحصول على محفزات نشطة ومستقرة تمكّن الانتقال من الوقود الأحفوري إلى المواد الأولية المتجددة، وتقليل استهلاك الطاقة وتقليل الأثر البيئي. هنا نقوم بتخليق مواد هجينة جديدة حيث يحيط طلاء أكسيدي غير متبلور مع روابط عضوية مدفونة بلورات نانوية معدنية. نحن نثبت أن الطلاء الهجين هو وسيلة قوية لإنشاء محفزات كهربائية مستقرة ضد إعادة البناء الهيكلي خلال
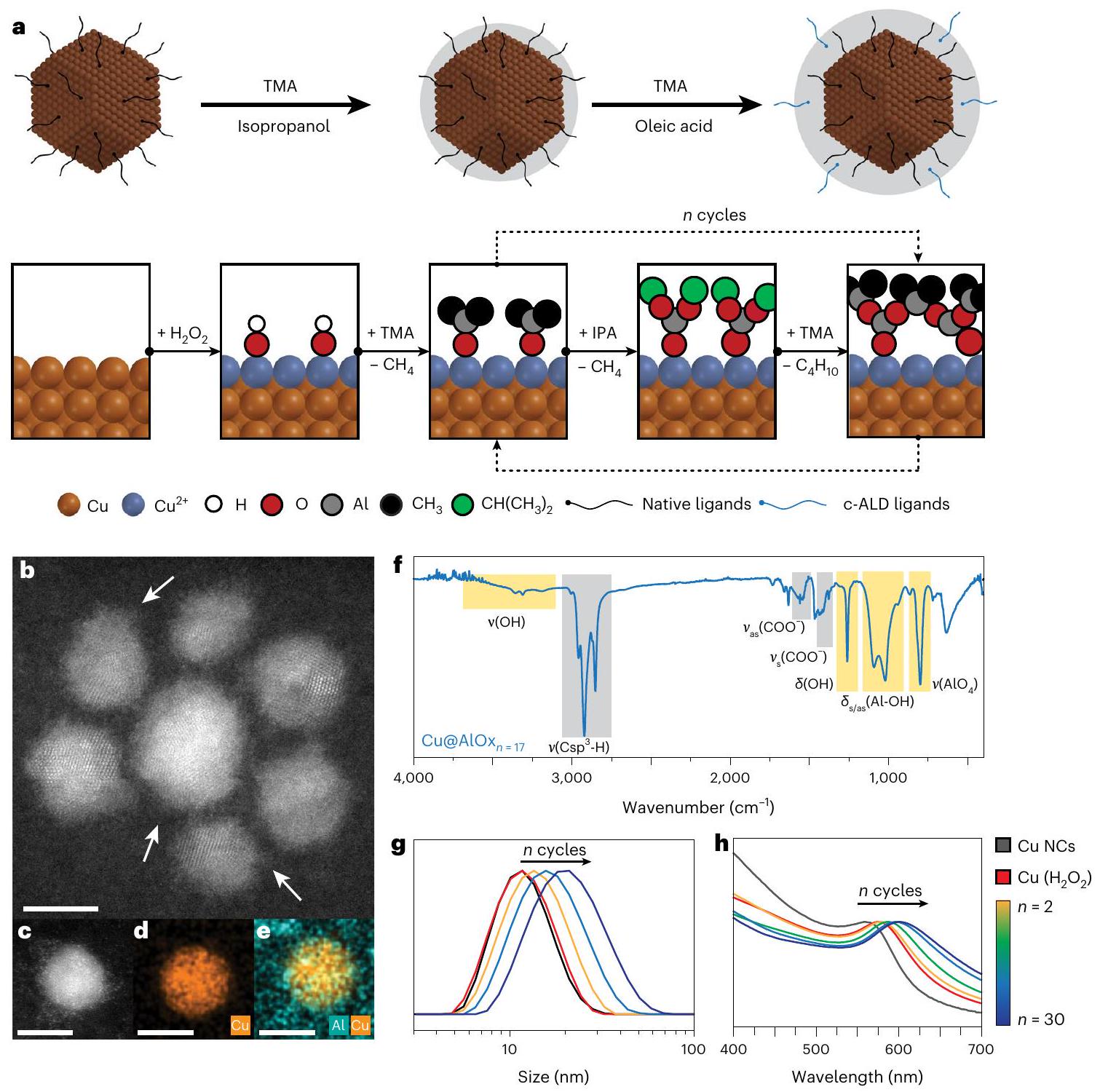
أ، مخطط البروتوكول الاصطناعي. ب، صورة تمثيلية بتقنية HAADF-STEM لـ
مساهمة غلاف AlOx غير العضوي، المميز باللون الأصفر، ومساهمة الروابط العضوية (وهي TOA وTDPA وOLAC)، المميزة باللون الرمادي. ج، ح، طيف DLS وUV-Vis التمثيلي لـ Cu@AlOx لمختلف
قذائف
الذي يمكّن من فهم عميق للآلية وراء الاستقرار الهيكلي المحقق.
تركيب وتوصيف نانوكريات الهجين Cu@AlOx
مجموعات الهيدروكسيل، التي تثبت غلاف الألومينا على السطح أثناء التكوين (الشكل التكميلي 2). ثم تسمح الحقن المتتالية من ثلاثي ميثيل الألمنيوم (TMA) والإيزوبروبانول (IPA) بنمو غلاف الألومينا حول نانو كريستالات النحاس (الشكل التكميلي 3 و4). عدد دورات ALD (
صور المجهر الإلكتروني الناقل في مجال الضوء (BF-TEM) (الشكل 1ب، ج والشكل التكميلي 5 و6) تثبت أن جسيمات النحاس النانوية (Cu NCs) هي كرات بلورية بقطر 7 نانومتر، كل منها محاطة بقشرة غير متبلورة بعد عملية الترسيب الكيميائي بالطبقات (c-ALD). تؤكد مطيافية الأشعة السينية المشتتة بالطاقة (EDX) وجود الألومينا حول جسيمات النحاس النانوية (الشكل 1د، هـ والشكل التكميلي 6). تشير مطيافية الأشعة تحت الحمراء بتحويل فورييه (FT-IR) (الشكل 1و والشكل التكميلي 7) إلى وجود نطاقات مميزة مرتبطة بهيكل ألومينا غير متبلور/شبيه بالبوهيميت.
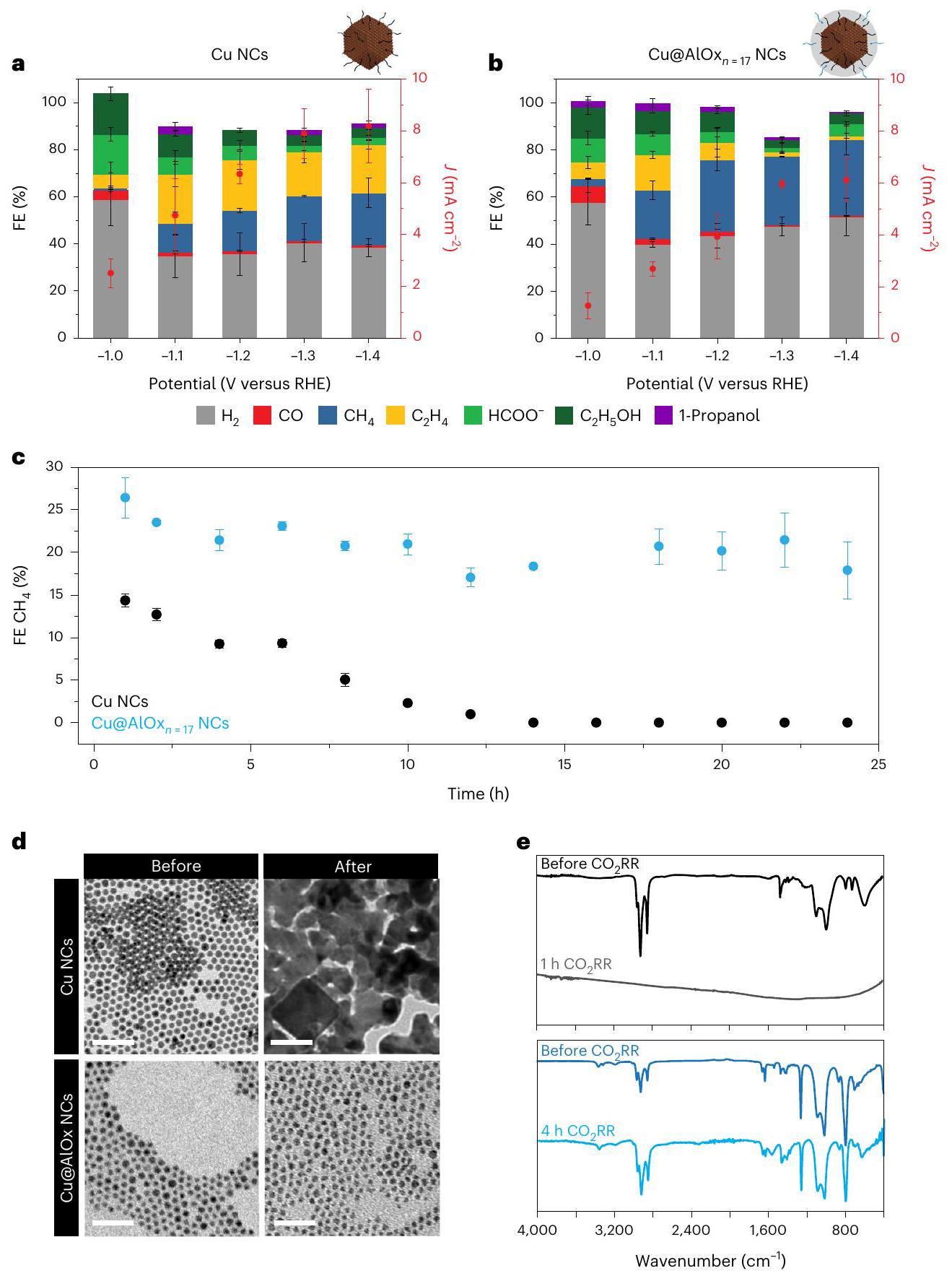
الأداء التحفيزي والاستقرار الهيكلي خلال
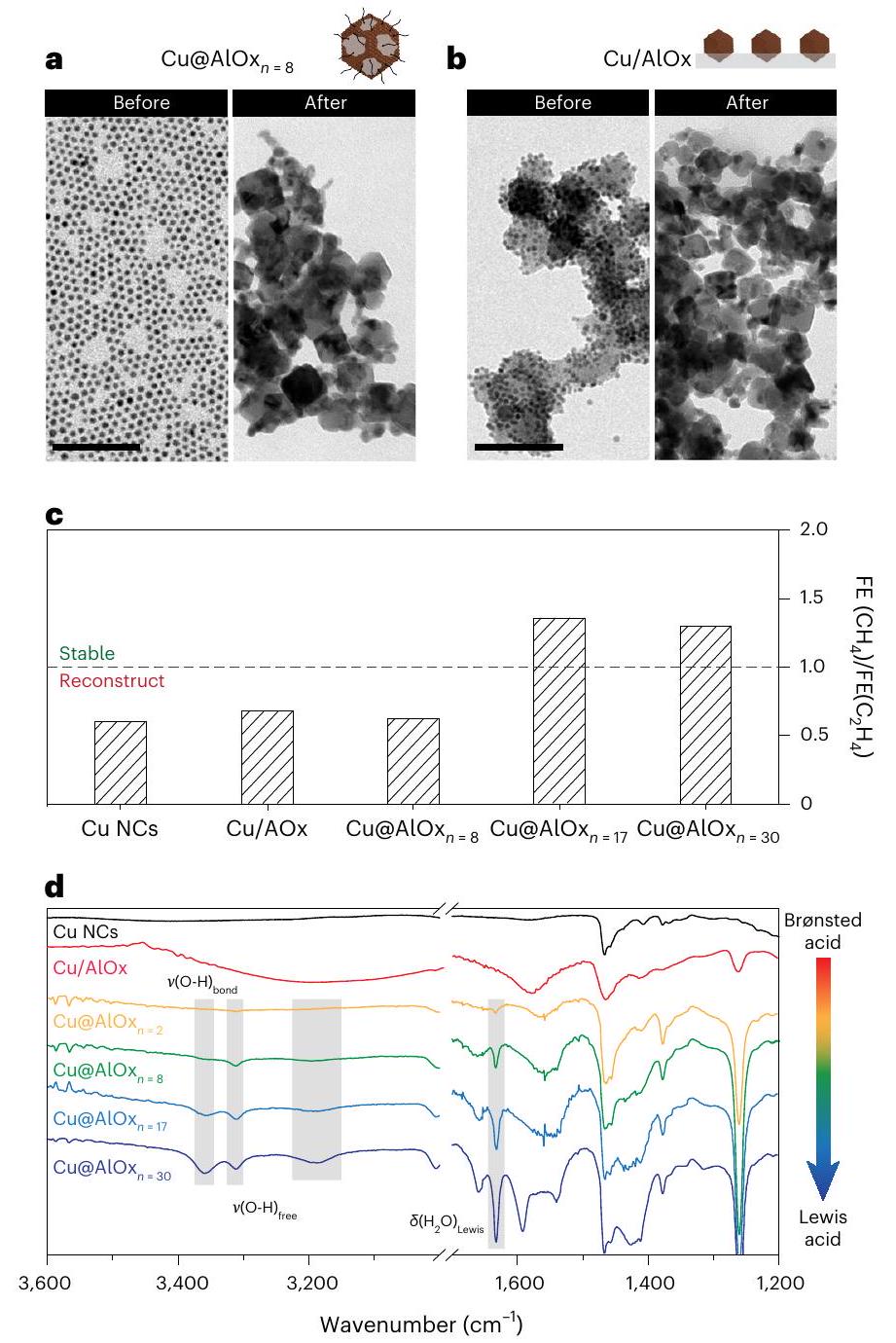
آلية الاستقرار الهيكلي أثناء
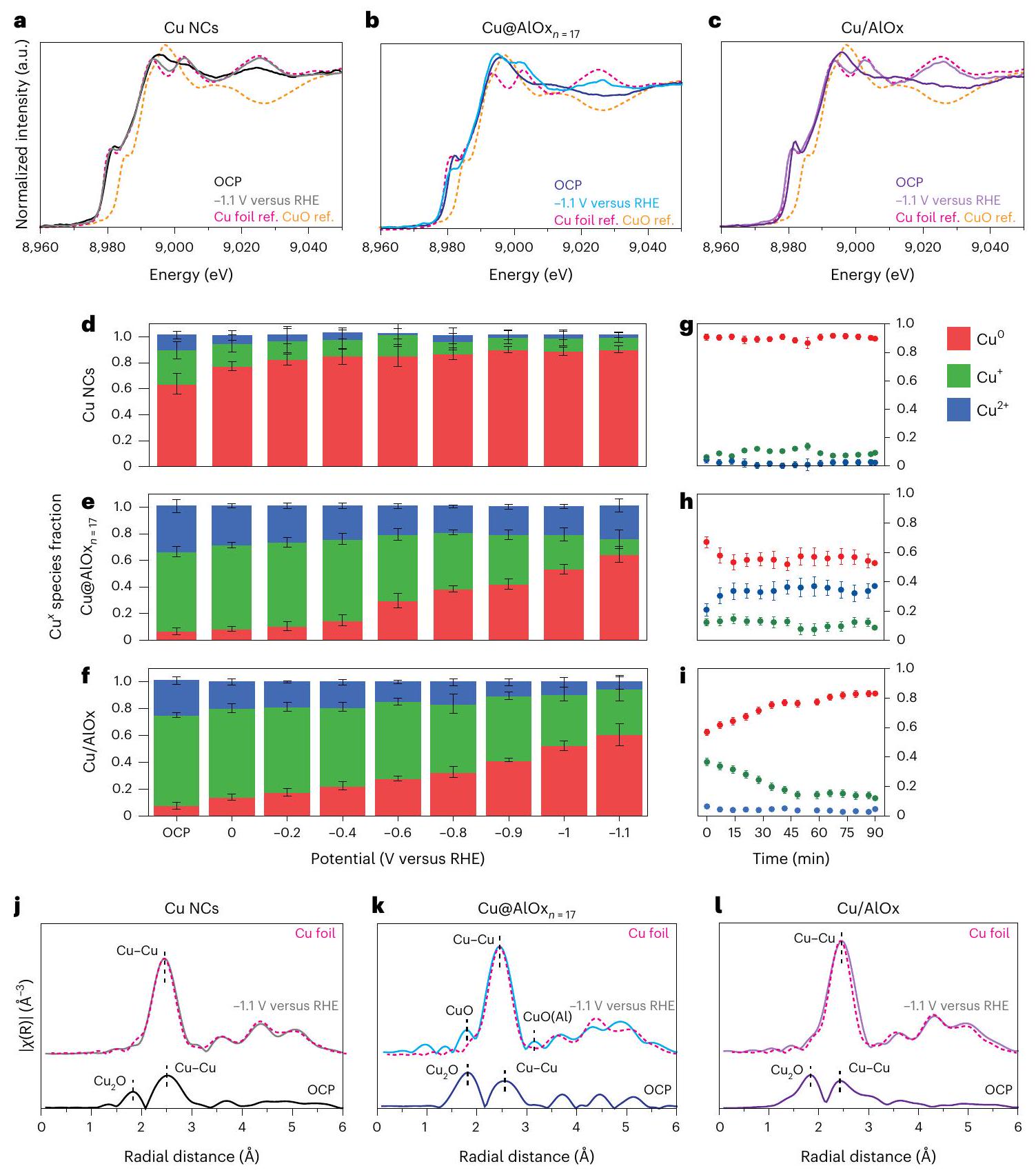
XAS. أ-ج، طيف XANES عند حافة Cu K عند جهد الدائرة المفتوحة (لون داكن) وعند -1.1 فولت مقابل RHE (لون فاتح) لـ
تؤكد القشور الأكثر سمكًا بشكل أكبر من خلال زيادة شدة وضع الانحناء لـ
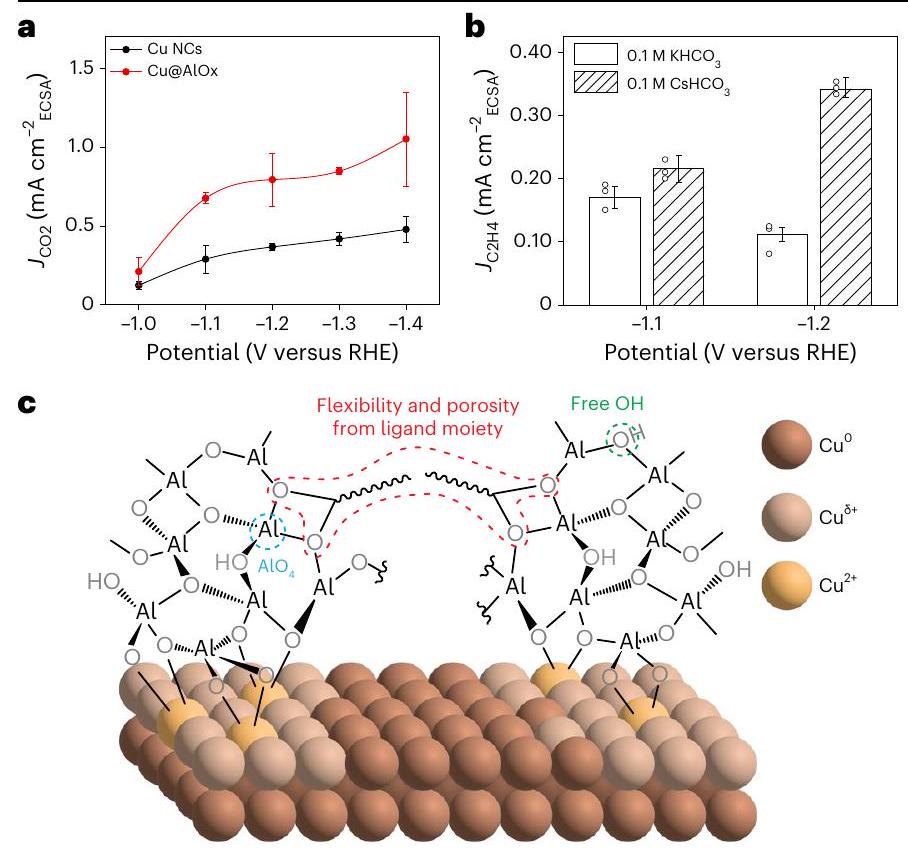
كثافة التيار الجزئي لـ
على التوالي
النشاط الجوهري والتلاعب بالانتقائية
تظهر معالجة الميكروبيئة كاستراتيجية مستقبلية لتعديل وتوجيه انتقائية محفزات Cu@AlOx نحو منتجات مختلفة عن الميثان. في الواقع، تغيير الإلكتروليت من
المحتوى عبر الإنترنت
References
- Sanchez, C., Belleville, P., Popall, M. & Nicole, L. Applications of advanced hybrid organic-inorganic nanomaterials: from laboratory to market. Chem. Soc. Rev. 40, 696-753 (2011).
- Li, W. et al. Chemically diverse and multifunctional hybrid organic-inorganic perovskites. Nat. Rev. Mater. 2, 1-18 (2017).
- Trickett, C. A. et al. The chemistry of metal-organic frameworks for
capture, regeneration and conversion. Nat. Rev. Mater. 2, 1-16 (2017). - Mitchell, S., Qin, R., Zheng, N. & Pérez-Ramírez, J. Nanoscale engineering of catalytic materials for sustainable technologies. Nat. Nanotechnol. 16, 129-139 (2021).
- Cheng, K. et al. Maximizing noble metal utilization in solid catalysts by control of nanoparticle location. Science 377, 204-208 (2022).
- Birdja, Y. Y. et al. Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels. Nat. Energy 4, 732-745 (2019).
- Popović, S. et al. Stability and degradation mechanisms of copper-based catalysts for electrochemical
reduction. Angew. Chem. Int. Ed. 59, 14736-14746 (2020). - Ross, M. B. et al. Designing materials for electrochemical carbon dioxide recycling. Nat. Catal. 2, 648-658 (2019).
- Nitopi, S. et al. Progress and perspectives of electrochemical
reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610-7672 (2019). - Mistry, H., Varela, A. S., Kühl, S., Strasser, P. & Cuenya, B. R. Nanostructured electrocatalysts with tunable activity and selectivity. Nat. Rev. Mater. 1, 1-14 (2016).
- Loiudice, A. et al. Tailoring copper nanocrystals towards
products in electrochemical reduction. Angew. Chem. Int. Ed. 55, 5789-5792 (2016). - De Gregorio, G. L. et al. Facet-dependent selectivity of Cu catalysts in electrochemical
reduction at commercially viable current densities. ACS Catal. 10, 4854-4862 (2020). - Huang, J. et al. Potential-induced nanoclustering of metallic catalysts during electrochemical
reduction. Nat. Commun. 9, 3117 (2018). - Grosse, P. et al. Dynamic transformation of cubic copper catalysts during
electroreduction and its impact on catalytic selectivity. Nat. Commun. 12, 6736 (2021). - Osowiecki, W. T. et al. Factors and dynamics of Cu nanocrystal reconstruction under
reduction. ACS Appl. Energy Mater. 2, 7744-7749 (2019). - Kim, D., Kley, C. S., Li, Y. & Yang, P. Copper nanoparticle ensembles for selective electroreduction of
to products. Proc. Natl Acad. Sci. USA 114, 10560-10565 (2017). - Li, Y. et al. Electrochemically scrambled nanocrystals are catalytically active for
-to-multicarbons. Proc. Natl Acad. Sci. USA 117, 9194-9201 (2020). - Yang, Y. et al. Operando studies reveal active Cu nanograins for
electroreduction. Nature 614, 262-269 (2023). - Vavra, J., Shen, T. H., Stoian, D., Tileli, V. & Buonsanti, R. Real-time monitoring reveals dissolution/redeposition mechanism in copper nanocatalysts during the initial stages of the
reduction reaction. Angew. Chem. Int. Ed. 60, 1347-1354 (2021). - Hochfilzer, D., Chorkendorff, I. & Kibsgaard, J. Catalyst stability considerations for electrochemical energy conversion with non-noble metals: do we measure on what we synthesized? ACS Energy Lett. 8, 1607-1612 (2023).
- Okatenko, V. et al. Alloying as a strategy to boost the stability of copper nanocatalysts during the electrochemical
reduction reaction. J. Am. Chem. Soc. 145, 37 (2022). - Lu, X. K., Lu, B., Li, H., Lim, K. & Seitz, L. C. Stabilization of undercoordinated Cu sites in strontium copper oxides for enhanced formation of
products in electrochemical reduction. ACS Catal. 12, 6663-6671 (2022). - Li, Y. et al. Structure-sensitive
electroreduction to hydrocarbons on ultrathin 5-fold twinned copper nanowires. Nano Lett. 17, 1312-1317 (2017). - Martín, A. J., Mitchell, S., Mondelli, C., Jaydev, S. & Pérez-Ramírez, J. Unifying views on catalyst deactivation. Nat. Catal. 5, 854-866 (2022).
- Otor, H. O., Steiner, J. B., García-Sancho, C. & Alba-Rubio, A. C. Encapsulation methods for control of catalyst deactivation: a review. ACS Catal. 10, 7630-7656 (2020).
- Das, S. et al. Core-shell structured catalysts for thermocatalytic, photocatalytic, and electrocatalytic conversion of
. Chem. Soc. Rev. 49, 2937-3004 (2020). - Munnik, P., De Jongh, P. E. & De Jong, K. P. Control and impact of the nanoscale distribution of supported cobalt particles used in fischer-tropsch catalysis. J. Am. Chem. Soc. 136, 7333-7340 (2014).
- van Deelen, T. W., Hernández Mejía, C. & de Jong, K. P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2, 955-970 (2019).
- Aitbekova, A. et al. Templated encapsulation of platinum-based catalysts promotes high-temperature stability to
. Nat. Mater. 21, 1290-1297 (2022). - Loiudice, A., Strach, M., Saris, S., Chernyshov, D. & Buonsanti, R. Universal oxide shell growth enables in situ structural studies of perovskite nanocrystals during the anion exchange reaction. J. Am. Chem. Soc. 141, 8254-8263 (2019).
- Loiudice, A., Segura Lecina, O., Bornet, A., Luther, J. M. & Buonsanti, R. Ligand locking on quantum dot surfaces via a mild reactive surface treatment. J. Am. Chem. Soc. 143, 13418-13427 (2021).
- Segura Lecina, O. et al. Colloidal-ALD-grown hybrid shells nucleate via a ligand-precursor complex. J. Am. Chem. Soc. 144, 3998-4008 (2022).
- Green, P. B., Lecina, O. S., Albertini, P. P., Loiudice, A. & Buonsanti, R. Colloidal-ALD-grown metal oxide shells enable the synthesis of photoactive ligand/nanocrystal composite materials. J. Am. Chem. Soc. 145, 8189-8197 (2023).
- Morterra, C., Emanuel, C., Cerrato, G. & Magnacca, G. Infrared study of some surface properties of boehmite
. J. Chem. Soc. Faraday Trans. 88, 339-348 (1992). - Pankhurst, J. R., Iyengar, P., Loiudice, A., Mensi, M. & Buonsanti, R. Metal-ligand bond strength determines the fate of organic ligands on the catalyst surface during the electrochemical
reduction reaction. Chem. Sci. 11, 9296-9302 (2020). - Kaushik, M. et al. Atomic-scale structure and its impact on chemical properties of aluminum oxide layers prepared by atomic layer deposition on silica. Chem. Mater. 33, 3335-3348 (2021).
- Ravi, M., Sushkevich, V. L. & van Bokhoven, J. A. Towards a better understanding of Lewis acidic aluminium in zeolites. Nat. Mater. 19, 1047-1056 (2020).
- Raaijman, S. J., Arulmozhi, N. & Koper, M. T. M. Morphological stability of copper surfaces under reducing conditions. ACS Appl. Mater. Interfaces 13, 48730-48744 (2021).
- Campbell, C. T. & Mao, Z. Chemical potential of metal atoms in supported nanoparticles: dependence upon particle size and support. ACS Catal. 7, 8460-8466 (2017).
- Yoo, J. M., Shin, H., Chung, D. Y. & Sung, Y. E. Carbon shell on active nanocatalyst for stable electrocatalysis. Acc. Chem. Res. 55, 1278-1289 (2022).
- Ji, S. G., Kwon, H. C., Kim, T. H., Sim, U. & Choi, C. H. Does the encapsulation strategy of Pt nanoparticles with carbon layers really ensure both highly active and durable electrocatalysis in fuel cells? ACS Catal. 12, 7317-7325 (2022).
- Bhardwaj, A. A. et al. Ultrathin silicon oxide overlayers enable selective oxygen evolution from acidic and unbuffered pH -neutral seawater. ACS Catal. 11, 1316-1330 (2021).
- Li, Q. et al. Tuning Sn-catalysis for electrochemical reduction of
to CO via the core/shell structure. J. Am. Chem. Soc. 139, 4290-4293 (2017). - Xie, H. et al. Boosting tunable syngas formation via electrochemical
reduction on core/shell nanoparticles. ACS Appl. Mater. Interfaces 10, 36996-37004 (2018). - Ye, K. et al. In situ reconstruction of a hierarchical
core/shell catalyst for high-performance electroreduction. Angew. Chem. Int. Ed. 59, 4814-4821 (2020). - Varandili, S. B. et al. Synthesis of
nanocrystalline heterodimers with interfacial active sites to promote electroreduction. ACS Catal. 9, 5035-5046 (2019). - Xu, A. et al. Copper/alkaline earth metal oxide interfaces for electrochemical
-to-alcohol conversion by selective hydrogenation. Nat. Catal. 5, 1081-1088 (2022). - Arán-Ais, R. M., Scholten, F., Kunze, S., Rizo, R. & Roldan Cuenya, B . The role of in situ generated morphological motifs and
species in product selectivity during pulsed electroreduction. Nat. Energy 5, 317-325 (2020). - Zhou, Y. et al. Long-chain hydrocarbons by
electroreduction using polarized nickel catalysts. Nat. Catal. 5, 545-554 (2022). - Edri, E., Cooper, J. K., Sharp, I. D., Guldi, D. M. & Frei, H. Ultrafast charge transfer between light absorber and
water oxidation catalyst across molecular wires embedded in silica membrane. J. Am. Chem. Soc. 139, 5458-5466 (2017).
(c) The Author(s) 2024
طريقة
تركيب نانو كرات النحاس
تخليق Cu@AlOx NCs
تخليق Cu/AlOx
القياسات الكهروكيميائية
توفر البيانات
الشكر والتقدير
مساهمات المؤلفين
التمويل
المصالح المتنافسة
معلومات إضافية
مختبر النانو كيمياء للطاقة، معهد العلوم والهندسة الكيميائية، المدرسة الفيدرالية العليا للعلوم التطبيقية في لوزان، سيون، سويسرا. خطوط الأشعة السويسرية النرويجية، منشأة الإشعاع السنكروتروني الأوروبية، غرونوبل، فرنسا. المركز متعدد التخصصات لميكروسكوب الإلكترون، المدرسة الفيدرالية العليا للعلوم التطبيقية في لوزان، لوزان، سويسرا. البريد الإلكتروني:raffaella.buonsanti@epfl.ch
DOI: https://doi.org/10.1038/s41563-024-01819-x
PMID: https://pubmed.ncbi.nlm.nih.gov/38366155
Publication Date: 2024-02-16
Hybrid oxide coatings generate stable Cucatalysts for
Accepted: 25 January 2024
Published online: 16 February 2024
Abstract
Hybrid organic/inorganic materials have contributed to solve important challenges in different areas of science. One of the biggest challenges for a more sustainable society is to have active and stable catalysts that enable the transition from fossil fuel to renewable feedstocks, reduce energy consumption and minimize the environmental footprint. Here we synthesize novel hybrid materials where an amorphous oxide coating with embedded organic ligands surrounds metallic nanocrystals. We demonstrate that the hybrid coating is a powerful means to create electrocatalysts stable against structural reconstruction during the

a, Schematic of the synthetic protocol. b, Representative HAADF-STEM image of
the contribution of the inorganic AlOx shell, highlighted in yellow, and the contribution of the organic ligands (that is TOA, TDPA and OLAC), highlighted in grey. g,h, Representative DLS and UV-Vis spectra of Cu@AlOx for different
shells
that enables an in-depth understanding of the mechanism behind the achieved structural stability.
Synthesis and characterization of the hybrid Cu@AlOx NCs

hydroxyl groups, which anchor the alumina shell on the surface during nucleation (Supplementary Fig. 2). Sequential injections of tri-methyl aluminium (TMA) and isopropanol (IPA) then allow the growth of the alumina shell around the Cu NCs (Supplementary Figs. 3 and 4). The number of ALD cycles (
bright-field transmission electron microscopy (BF-TEM) images (Fig. 1b,c and Supplementary Figs. 5 and 6) evidence that the Cu NCs are 7 nm crystalline spheres, each surrounded by an amorphous shell after c-ALD. Energy-dispersive X-ray (EDX) spectroscopy confirms the presence of alumina around the Cu NCs (Fig. 1d, e and Supplementary Fig. 6). Fourier transform infrared (FT-IR) spectroscopy (Fig. 1f and Supplementary Fig. 7) indicates characteristic bands associated to an amorphous/boehmite-like alumina structure

Catalytic performance and structural stability during
Mechanism behind the structural stability during

XAS. a-c, Cu K-edge XANES spectra at OCP (dark colour) and at -1.1 V versus RHE (bright colour) for
the thicker shells is further confirmed by the increased intensity of the bending mode of

a, Partial current density of
respectively
Intrinsic activity and manipulation of selectivity
effects, microenvironment manipulation emerges as a future strategy to modulate and steer the selectivity of the Cu@AlOx catalysts towards products different than methane. In fact, changing the electrolyte from
Online content
References
- Sanchez, C., Belleville, P., Popall, M. & Nicole, L. Applications of advanced hybrid organic-inorganic nanomaterials: from laboratory to market. Chem. Soc. Rev. 40, 696-753 (2011).
- Li, W. et al. Chemically diverse and multifunctional hybrid organic-inorganic perovskites. Nat. Rev. Mater. 2, 1-18 (2017).
- Trickett, C. A. et al. The chemistry of metal-organic frameworks for
capture, regeneration and conversion. Nat. Rev. Mater. 2, 1-16 (2017). - Mitchell, S., Qin, R., Zheng, N. & Pérez-Ramírez, J. Nanoscale engineering of catalytic materials for sustainable technologies. Nat. Nanotechnol. 16, 129-139 (2021).
- Cheng, K. et al. Maximizing noble metal utilization in solid catalysts by control of nanoparticle location. Science 377, 204-208 (2022).
- Birdja, Y. Y. et al. Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels. Nat. Energy 4, 732-745 (2019).
- Popović, S. et al. Stability and degradation mechanisms of copper-based catalysts for electrochemical
reduction. Angew. Chem. Int. Ed. 59, 14736-14746 (2020). - Ross, M. B. et al. Designing materials for electrochemical carbon dioxide recycling. Nat. Catal. 2, 648-658 (2019).
- Nitopi, S. et al. Progress and perspectives of electrochemical
reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610-7672 (2019). - Mistry, H., Varela, A. S., Kühl, S., Strasser, P. & Cuenya, B. R. Nanostructured electrocatalysts with tunable activity and selectivity. Nat. Rev. Mater. 1, 1-14 (2016).
- Loiudice, A. et al. Tailoring copper nanocrystals towards
products in electrochemical reduction. Angew. Chem. Int. Ed. 55, 5789-5792 (2016). - De Gregorio, G. L. et al. Facet-dependent selectivity of Cu catalysts in electrochemical
reduction at commercially viable current densities. ACS Catal. 10, 4854-4862 (2020). - Huang, J. et al. Potential-induced nanoclustering of metallic catalysts during electrochemical
reduction. Nat. Commun. 9, 3117 (2018). - Grosse, P. et al. Dynamic transformation of cubic copper catalysts during
electroreduction and its impact on catalytic selectivity. Nat. Commun. 12, 6736 (2021). - Osowiecki, W. T. et al. Factors and dynamics of Cu nanocrystal reconstruction under
reduction. ACS Appl. Energy Mater. 2, 7744-7749 (2019). - Kim, D., Kley, C. S., Li, Y. & Yang, P. Copper nanoparticle ensembles for selective electroreduction of
to products. Proc. Natl Acad. Sci. USA 114, 10560-10565 (2017). - Li, Y. et al. Electrochemically scrambled nanocrystals are catalytically active for
-to-multicarbons. Proc. Natl Acad. Sci. USA 117, 9194-9201 (2020). - Yang, Y. et al. Operando studies reveal active Cu nanograins for
electroreduction. Nature 614, 262-269 (2023). - Vavra, J., Shen, T. H., Stoian, D., Tileli, V. & Buonsanti, R. Real-time monitoring reveals dissolution/redeposition mechanism in copper nanocatalysts during the initial stages of the
reduction reaction. Angew. Chem. Int. Ed. 60, 1347-1354 (2021). - Hochfilzer, D., Chorkendorff, I. & Kibsgaard, J. Catalyst stability considerations for electrochemical energy conversion with non-noble metals: do we measure on what we synthesized? ACS Energy Lett. 8, 1607-1612 (2023).
- Okatenko, V. et al. Alloying as a strategy to boost the stability of copper nanocatalysts during the electrochemical
reduction reaction. J. Am. Chem. Soc. 145, 37 (2022). - Lu, X. K., Lu, B., Li, H., Lim, K. & Seitz, L. C. Stabilization of undercoordinated Cu sites in strontium copper oxides for enhanced formation of
products in electrochemical reduction. ACS Catal. 12, 6663-6671 (2022). - Li, Y. et al. Structure-sensitive
electroreduction to hydrocarbons on ultrathin 5-fold twinned copper nanowires. Nano Lett. 17, 1312-1317 (2017). - Martín, A. J., Mitchell, S., Mondelli, C., Jaydev, S. & Pérez-Ramírez, J. Unifying views on catalyst deactivation. Nat. Catal. 5, 854-866 (2022).
- Otor, H. O., Steiner, J. B., García-Sancho, C. & Alba-Rubio, A. C. Encapsulation methods for control of catalyst deactivation: a review. ACS Catal. 10, 7630-7656 (2020).
- Das, S. et al. Core-shell structured catalysts for thermocatalytic, photocatalytic, and electrocatalytic conversion of
. Chem. Soc. Rev. 49, 2937-3004 (2020). - Munnik, P., De Jongh, P. E. & De Jong, K. P. Control and impact of the nanoscale distribution of supported cobalt particles used in fischer-tropsch catalysis. J. Am. Chem. Soc. 136, 7333-7340 (2014).
- van Deelen, T. W., Hernández Mejía, C. & de Jong, K. P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2, 955-970 (2019).
- Aitbekova, A. et al. Templated encapsulation of platinum-based catalysts promotes high-temperature stability to
. Nat. Mater. 21, 1290-1297 (2022). - Loiudice, A., Strach, M., Saris, S., Chernyshov, D. & Buonsanti, R. Universal oxide shell growth enables in situ structural studies of perovskite nanocrystals during the anion exchange reaction. J. Am. Chem. Soc. 141, 8254-8263 (2019).
- Loiudice, A., Segura Lecina, O., Bornet, A., Luther, J. M. & Buonsanti, R. Ligand locking on quantum dot surfaces via a mild reactive surface treatment. J. Am. Chem. Soc. 143, 13418-13427 (2021).
- Segura Lecina, O. et al. Colloidal-ALD-grown hybrid shells nucleate via a ligand-precursor complex. J. Am. Chem. Soc. 144, 3998-4008 (2022).
- Green, P. B., Lecina, O. S., Albertini, P. P., Loiudice, A. & Buonsanti, R. Colloidal-ALD-grown metal oxide shells enable the synthesis of photoactive ligand/nanocrystal composite materials. J. Am. Chem. Soc. 145, 8189-8197 (2023).
- Morterra, C., Emanuel, C., Cerrato, G. & Magnacca, G. Infrared study of some surface properties of boehmite
. J. Chem. Soc. Faraday Trans. 88, 339-348 (1992). - Pankhurst, J. R., Iyengar, P., Loiudice, A., Mensi, M. & Buonsanti, R. Metal-ligand bond strength determines the fate of organic ligands on the catalyst surface during the electrochemical
reduction reaction. Chem. Sci. 11, 9296-9302 (2020). - Kaushik, M. et al. Atomic-scale structure and its impact on chemical properties of aluminum oxide layers prepared by atomic layer deposition on silica. Chem. Mater. 33, 3335-3348 (2021).
- Ravi, M., Sushkevich, V. L. & van Bokhoven, J. A. Towards a better understanding of Lewis acidic aluminium in zeolites. Nat. Mater. 19, 1047-1056 (2020).
- Raaijman, S. J., Arulmozhi, N. & Koper, M. T. M. Morphological stability of copper surfaces under reducing conditions. ACS Appl. Mater. Interfaces 13, 48730-48744 (2021).
- Campbell, C. T. & Mao, Z. Chemical potential of metal atoms in supported nanoparticles: dependence upon particle size and support. ACS Catal. 7, 8460-8466 (2017).
- Yoo, J. M., Shin, H., Chung, D. Y. & Sung, Y. E. Carbon shell on active nanocatalyst for stable electrocatalysis. Acc. Chem. Res. 55, 1278-1289 (2022).
- Ji, S. G., Kwon, H. C., Kim, T. H., Sim, U. & Choi, C. H. Does the encapsulation strategy of Pt nanoparticles with carbon layers really ensure both highly active and durable electrocatalysis in fuel cells? ACS Catal. 12, 7317-7325 (2022).
- Bhardwaj, A. A. et al. Ultrathin silicon oxide overlayers enable selective oxygen evolution from acidic and unbuffered pH -neutral seawater. ACS Catal. 11, 1316-1330 (2021).
- Li, Q. et al. Tuning Sn-catalysis for electrochemical reduction of
to CO via the core/shell structure. J. Am. Chem. Soc. 139, 4290-4293 (2017). - Xie, H. et al. Boosting tunable syngas formation via electrochemical
reduction on core/shell nanoparticles. ACS Appl. Mater. Interfaces 10, 36996-37004 (2018). - Ye, K. et al. In situ reconstruction of a hierarchical
core/shell catalyst for high-performance electroreduction. Angew. Chem. Int. Ed. 59, 4814-4821 (2020). - Varandili, S. B. et al. Synthesis of
nanocrystalline heterodimers with interfacial active sites to promote electroreduction. ACS Catal. 9, 5035-5046 (2019). - Xu, A. et al. Copper/alkaline earth metal oxide interfaces for electrochemical
-to-alcohol conversion by selective hydrogenation. Nat. Catal. 5, 1081-1088 (2022). - Arán-Ais, R. M., Scholten, F., Kunze, S., Rizo, R. & Roldan Cuenya, B . The role of in situ generated morphological motifs and
species in product selectivity during pulsed electroreduction. Nat. Energy 5, 317-325 (2020). - Zhou, Y. et al. Long-chain hydrocarbons by
electroreduction using polarized nickel catalysts. Nat. Catal. 5, 545-554 (2022). - Edri, E., Cooper, J. K., Sharp, I. D., Guldi, D. M. & Frei, H. Ultrafast charge transfer between light absorber and
water oxidation catalyst across molecular wires embedded in silica membrane. J. Am. Chem. Soc. 139, 5458-5466 (2017).
(c) The Author(s) 2024
Method
Synthesis of Cu NCs and
Synthesis of Cu@AlOx NCs
Synthesis of Cu/AlOx
Electrochemical measurements
Data availability
Acknowledgements
Author contributions
Funding
Competing interests
Additional information
Laboratory of Nanochemistry for Energy, Institute of Chemical Sciences and Engineering, École Polytechnique Fédérale de Lausanne, Sion, Switzerland. Swiss-Norwegian Beamlines, European Synchrotron Radiation Facility, Grenoble, France. Interdisciplinary Center for Electron Microscopy, Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland. e-mail: raffaella.buonsanti@epfl.ch
