DOI: https://doi.org/10.1186/s12906-024-04381-w
PMID: https://pubmed.ncbi.nlm.nih.gov/38350963
تاريخ النشر: 2024-02-13
جزيئات الفضة النانوية المُركبة بيئيًا من مستخلص الزنجبيل: الإمكانيات المضادة للأكسدة، التوافق الحيوي، خصائص مضادة للـ LOX، والتحليل الحاسوبي
الملخص
مقدمة لقد برز مستخلص الزنجبيل (Zingiber officinale) كمرشح قوي للتخليق الأخضر للجسيمات النانوية، مما يوفر تطبيقات متنوعة في مجالات الطب ومستحضرات التجميل والتغذية. تتناول هذه الدراسة التحقيق في سمية الجسيمات النانوية المستخلصة من الزنجبيل (GE-AgNPs) في المختبر وتستكشف الفائدة الطبية للجسيمات النانوية التي تم تخليقها بطريقة خضراء. الطرق استخدمنا بروتوكولات معتمدة لتقييم الجوانب المختبرية مثل القدرة المضادة للأكسدة، والقدرة المضادة للالتهابات، والتوافق الحيوي لـ GE-AgNPs. بالإضافة إلى ذلك، تم استخدام الربط الجزيئي لتقييم نشاطها المضاد للـ lipoxygenase (anti-LOX). النتائج تسلط نتائجنا الضوء على أن استخراج مستخلص الزنجبيل عند درجة حموضة 6، باستخدام مزيج من المذيبات من الإيثانول والأسيتات الإيثيلية بنسبة 1:1، ينتج قدرة مضادة للأكسدة مرتفعة تعزى إلى محتواه الغني من المركبات الفينولية والفلافونويد. في سياق تخليق الجسيمات النانوية الفضية، ينتج استخراج درجة حموضة 6 أعلى كمية من الجسيمات النانوية، والتي تتميز بحجم متوسط من
مقدمة
تشكل تقنية إزالة الليزر [9]، والإشعاع الجاما [10]، واستخدام المواد الكيميائية [11] كعوامل مختزلة ومغلفة مجرد جزء من المنهجيات المتنوعة المتاحة لتخليق جزيئات الفضة النانوية (AgNPs). إن التكاليف الباهظة المرتبطة بهذه الأساليب، إلى جانب استخدام المواد الكيميائية الخطرة، تمثل عوائق كبيرة بسبب آثارها السلبية على صحة الإنسان والبيئة [12]. في مجال تكنولوجيا النانو، ظهرت تقنية التخليق الأخضر.
لقد حظيت باهتمام كبير كبديل قابل للتطبيق لتصنيع الجسيمات النانوية. لقد أظهرت الجسيمات النانوية الناتجة عن التخليق الأخضر فعالية ملحوظة ضد الأغشية الحيوية الأولية. ومن الجدير بالذكر أن استخدام النباتات كمصدر لتخليق الجسيمات النانوية يوفر مزايا مميزة مقارنة بالطرق الفيزيائية والكيميائية التقليدية. في السنوات الأخيرة، اكتسب استخدام مستخلصات النباتات لتخليق الجسيمات النانوية زخمًا كبيرًا نظرًا لتوافرها الواسع، وخصائصها المستدامة بيئيًا، وسهولة تنفيذها، وتنوع مجموعة المستقلبات الثانوية التي تحتويها، والتي يمكن استغلالها كعوامل مختزلة قوية.
الأمراض الالتهابية. تمارس هذه المركبات النشطة حيوياً تأثيراتها العلاجية بشكل أساسي من خلال تثبيط إنتاج البروستاجلاندينات عبر مسارات السيكلوأوكسيجيناز (COX) والليبوأوكسيجيناز (LOX) [20]. لقد أدى الاستخدام التاريخي لمستخلصات الزنجبيل في إدارة حالات مثل الروماتيزم والتهاب المفاصل إلى إجراء أبحاث واسعة حول الآليات المضادة للالتهابات لمستقلبات النبات الثانوية. وفقًا لعدة باحثين [21،22]، يمكن أن تُعزى الخصائص المضادة للالتهابات لـ 6-جينجيرول إلى قدرتها على تقليل مستويات السيتوكينات المؤيدة للالتهابات وإعاقة تقديم المستضدات بواسطة البلعميات المنشطة بواسطة الليببوليسكاريد (LPS). أجرى ليانغ وآخرون [23] دراسة تُظهر أن الشوجولز وجميع الجينجيرولات تُظهر تقليلاً يعتمد على الجرعة في إنتاج أكسيد النيتريك (NO) في خلايا RAW 264.7 المعالجة بـ LPS. وبالتالي، فإن دمج مستخلص الزنجبيل كعامل مختزل في تخليق جزيئات الفضة النانوية (AgNPs) يخدم غرضين: تعزيز العمل المضاد للالتهابات وتقليل المتطلبات المادية الإجمالية لتطوير المنتجات المضادة للالتهابات. على الرغم من إمكانية استخدام الزنجبيل لتخليق AgNPs، إلا أن الأبحاث التي تستكشف مستخلصات الزنجبيل ذات التركيزات المرتفعة من المكونات الفينولية والفلافونويدية تفتقر حاليًا. تنشأ هذه الفجوة البحثية من الاعتراف بأن استخراج المكونات الكيميائية النشطة بيولوجيًا من فئات الفينولات والفلافونويدات هو أمر بالغ الأهمية لتحقيق خصائص استثنائية كعوامل مختزلة [24]. علاوة على ذلك، فإن المركبات النشطة الموجودة في الزنجبيل، التي تُظهر محتوى كبير من الفينولات والفلافونويدات، تحمل وعدًا لتعزيز تثبيط الالتهاب [25]. يمكن أن تلعب الدراسات المعتمدة على الكمبيوتر دورًا محوريًا في تقليل الأخطاء المتأصلة في الإجراءات التجريبية وزيادة احتمالية النتائج الناجحة، وبالتالي تجنب الحاجة إلى التجربة والخطأ الواسعة. وبالتالي، فإن التحقيق في فعالية المركبات الرئيسية في الزنجبيل في تثبيط الإنتاج الالتهابي، الذي يتم تحقيقه من خلال استخدام عمليات الربط الجزيئي لإعاقة نشاط إنزيم LOX، يساهم في فهم أعمق لفعالية الزنجبيل.
التحليل. إن إجراء فحص شامل أمر حيوي للحصول على فهم معقد لتكوين وتركيز العناصر النشطة بيولوجيًا الموجودة في مستخلص الزنجبيل. إن دراسة خصائص المركبات التي تم التحقيق فيها فيما يتعلق بقدرتها على تثبيط إنزيم LOX هي في غاية الأهمية. يمكن أن تسهل استخدام منهجيات حسابية متقدمة، مثل الربط الجزيئي، تحقيق هذا الهدف. توفر هذه المنهجية تحليلًا شاملاً للتفاعل بين المواد الكيميائية الموجودة في مستخلص الزنجبيل وإنزيم LOX، مما يقدم رؤى قيمة حول آثارها المثبطة المحتملة على تكوين الالتهاب. من خلال تنفيذ تحقيقات دقيقة، يمكن تحقيق فهم شامل للتكوين الكيميائي لمستخلص الزنجبيل وقدرته كمثبط لإنتاج الالتهابات. إن اكتساب هذه المعرفة يعزز الفهم العلمي ضمن هذا المجال ويمكّن من صياغة استراتيجيات فعالة لاستغلال مستخلص الزنجبيل كعامل مخفض، مما يظهر تطبيقات محتملة عبر عدة مجالات. بالإضافة إلى ذلك، من الضروري تقييم القدرة المضادة للأكسدة لمستخلص الزنجبيل فيما يتعلق بإزالة الجذور الحرة 2،2-diphenyl-1-picrylhydrazyl (DPPH) و2،2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS). تم إخضاع AgNPs التي تم تصنيعها لتوصيف دقيق باستخدام تشتت الضوء الديناميكي (DLS) وميكروسكوب الإلكترون الناقل (TEM) وطيف الأشعة فوق البنفسجية-المرئية (UV-Vis) وطيف الأشعة تحت الحمراء بتحويل فورييه (FTIR). بالإضافة إلى ذلك، تم إجراء تقييمات شاملة للتوافق الحيوي تشمل السمية الخلوية باستخدام خلايا L929 لضمان سلامة وملاءمة AgNPs.
المواد والأساليب
المواد
تم استخدام مواد زراعة خلايا شاملة لدعم هذه الدراسات، وفقًا للبروتوكولات المخبرية المعتمدة. تم شراء هذه المواد من شركة ميرك (دارمشتات، ألمانيا). تم الحصول على إنزيم ليبوكسجيناز (LOX) وحمض اللينوليك من شركة سيغما-ألدريتش (ميسوري، الولايات المتحدة الأمريكية).
المجموعة 3، التي تضمنت دراسات وتحليلات من خلال أنظمة الكمبيوتر، استخدمت البرامج التالية: AutoDock 1.5.6، Python 3.8.2، MGLTools 1.5.4، Discovery Studio-2017، ArgusLab 4.0.1، وAvogadro. تم استخدام هذه البرامج تحت سيطرة نظام كمبيوتر بالمواصفات التالية مع المعالج: Intel Xeon-E5-2678v3 12C/24 T CPU @
مجموعة من النباتات
استخراج النبات استخراج الماء
استخراج
تحضير مستخلص الزنجبيل لتحليل العائد
كمية المركبات الفينولية
تم تحديد محتوى الفينولات من حيث مكافئات حمض الجاليك (GAE) المعبر عنها بالملغ لكل غرام من مستخلص النبات المجفف.
كمية مركبات الفلافونويد
نشاط التقاط الجذور الحرة لثنائي الفينيل-1-بيكريل هيدرازيل (DPPH)
تحليل GC-MS/MS
ABTS
تبع الملف الشخصي دورة مدتها 69 دقيقة تم التخطيط لها بعناية، تبدأ عند درجة حرارة أولية من
مقارنة مع المكتبات الكيميائية المتاحة في برنامج Agilent MassHunter للتحليل الكمي (الإصدار B.09.00). تطابق مع درجة
استخدام مستخلصات الزنجبيل المائية والمذيبات المساعدة في إنتاج جزيئات الفضة النانوية
توصيف
تم إجراء (FTIR) على المسحوق المجفف بالتجميد المستمد من محلول AgNP. تم إجراء التحليل باستخدام جهاز Bruker Tensor 27، مع استخدام كريات KBr كوسيلة عينة، وشمل نطاق الطول الموجي من 400 إلى
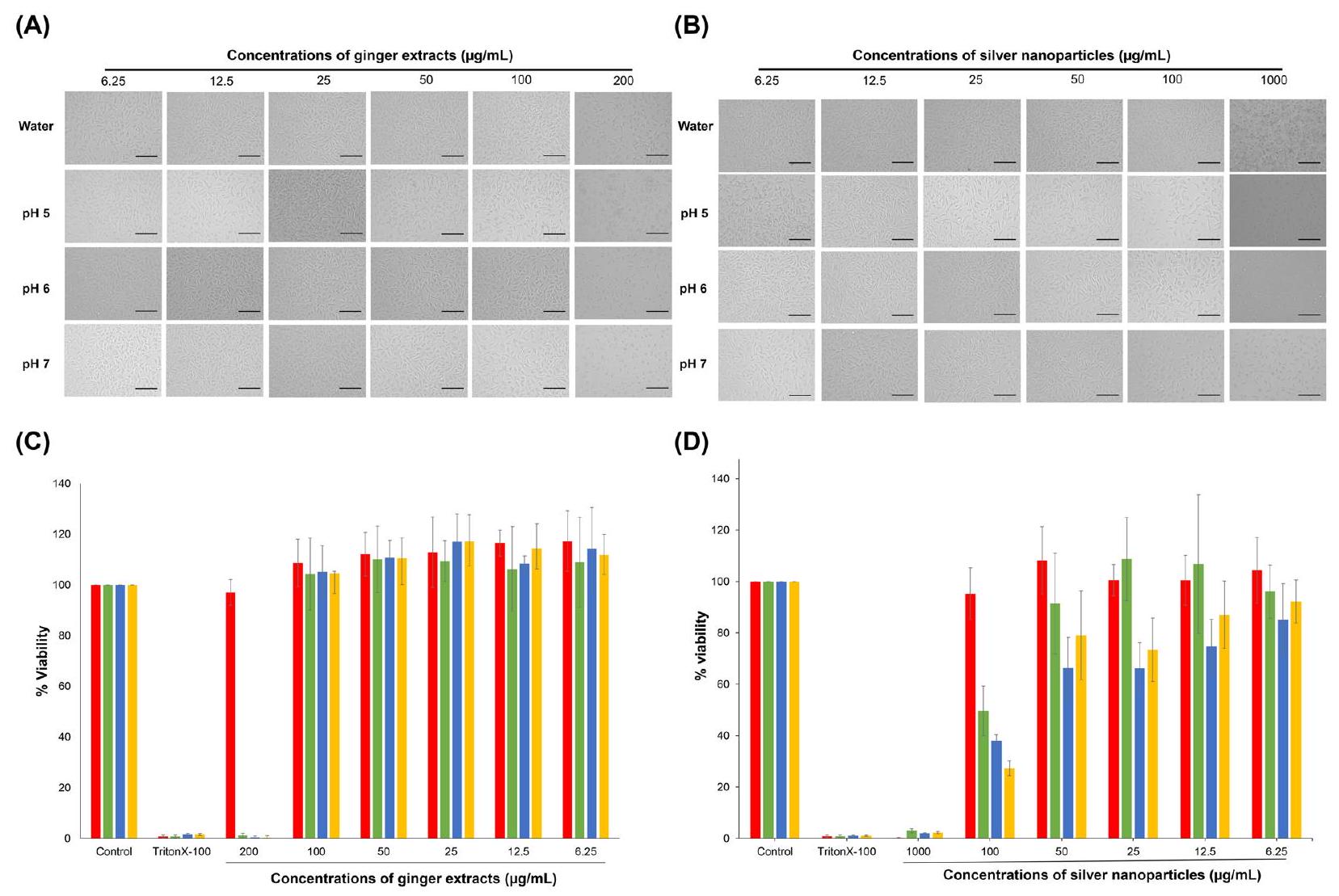
زراعة خلايا ليفوبلاست L929
السُمية الخلوية في المختبر
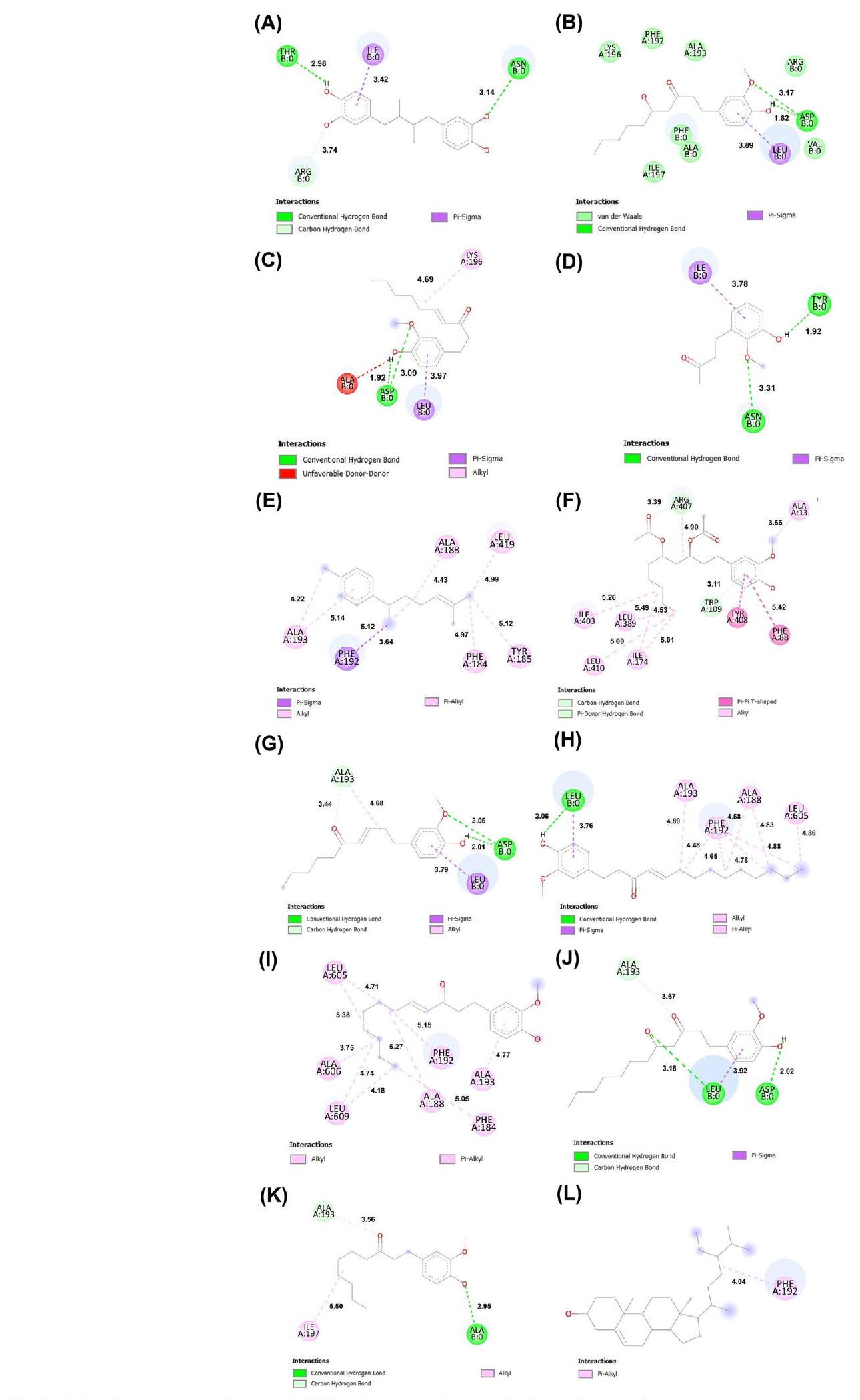
التثبيت الجزيئي لتقييم تثبيط LOX
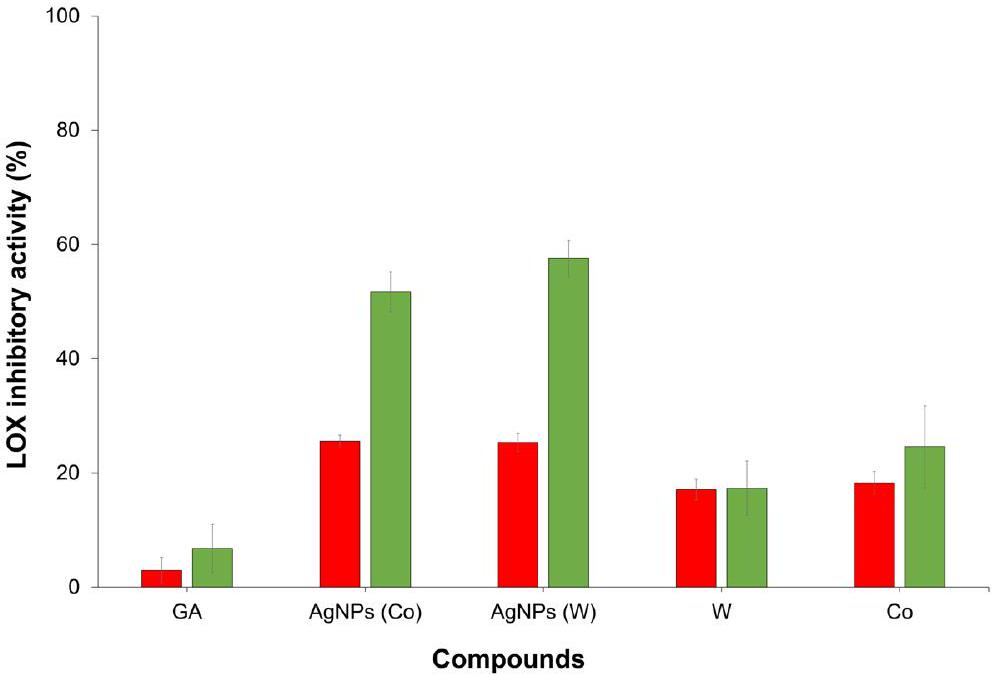
نشاط مثبط لليبوكسجيناز في المختبر
| استخراج | عائد |
| ماء |
|
| رقم الحموضة 5 (إيثانول + إيثيل أسيتات) |
|
| pH 6 (إيثانول + إيثيل أسيتات) |
|
| رقم الهيدروجين 7 (إيثانول + إيثيل أسيتات) |
|
التحليل الإحصائي
النتائج والمناقشة
تحليل مستخلص الزنجبيل
استخراج المواد الكيميائية الفينولية من المواد النباتية. وبالتالي، من أجل استخراج الفينولات والفلافونويدات بشكل مثالي من مستخلص الزنجبيل، يُوصى باستخدام استخراج الإيثانول: الأسيتات الإيثيلية عند درجة حموضة 6. الدراسات البحثية العديدة [48، 49] تدعم الفكرة القائلة بأن الإيثانول أو الأسيتات الإيثيلية يمكن استخدامها بفعالية لاستخراج المركبات النشطة بيولوجيًا، بما في ذلك الفينولات والفلافونويدات.
نشاط مضادات الأكسدة
| المعلمات | المبلغ | |||
| ماء | رقم الهيدروجين 5 | رقم الهيدروجين 6 | رقم الهيدروجين 7 | |
| إجمالي الفينولات (ملغ GAE/غ) |
|
|
|
|
| إجمالي الفلافونويد (ملغ QE/غ) |
|
|
|
|
| DPPH (ملغ TE/غ) |
|
|
|
|
| DPPH (%/ملغ) |
|
|
|
|
| IC
|
|
|
|
|
| ABTS (ملغ TE/غ) |
|
|
|
|
| ABTS (%/ملغ) |
|
|
|
|
| IC
|
|
|
|
|
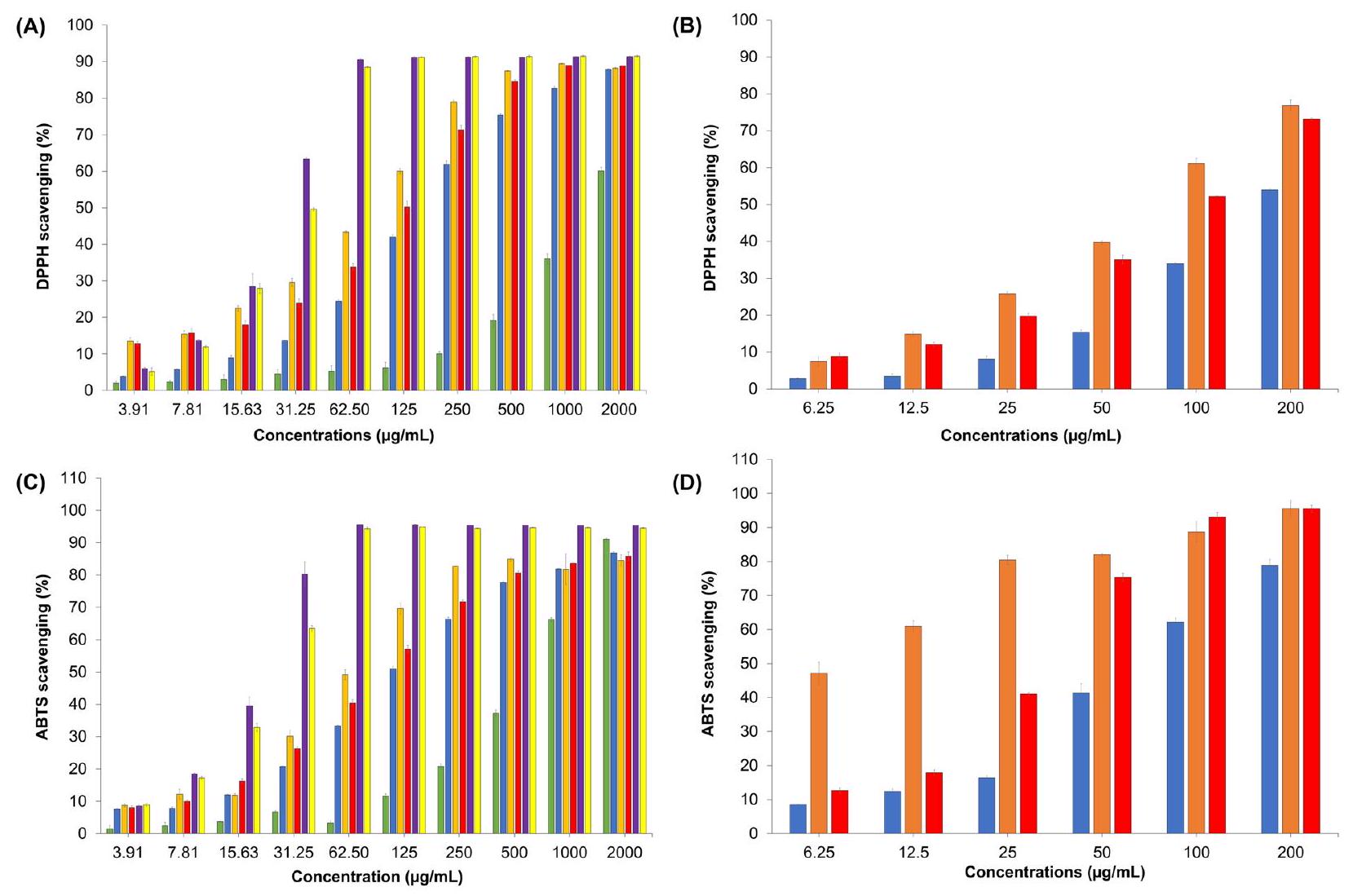
تمت من خلال استخدام اختبار 1,1-ثنائي الفينيل-2-بيكريل هيدرازيل (DPPH)، كما هو موضح في الشكل 1. النتائج التي تم الحصول عليها من تقييم قدرة التخلص من الجذور الحرة باستخدام ملح ثنائي الأمونيوم 2,2′-أزينو-بيس(3-إيثيل بنزوثيازولين-6-سلفونيك) (ABTS+)
| المركبات | DPPH (
|
ABTS (
|
| حمضي |
|
|
| تروكس |
|
|
| GE ( الرقم الهيدروجيني 5 ) |
|
|
| GE-AgNPs (pH5) |
|
|
| GE ( الرقم الهيدروجيني 6 ) |
|
|
| GE-AgNPs (pH6) |
|
|
| GE ( pH 7 ) |
|
|
| GE-AgNPs (pH7) |
|
|
تحليل GC-MS/MS
تم التعرف على المكونات الموجودة في مستخلص الزنجبيل المائي، والتي كانت 5-هيدروكسي-1-(4-هيدروكسي-3-ميثوكسي فينيل) دكان-3-ون، والمعروفة أيضًا باسم 6-جينجيرول (27.69%)، مما يبرز أهميتها ووجودها في تركيبة المستخلص.
توصيف جزيئات الفضة النانوية باستخدام مطيافية الأشعة فوق البنفسجية والمرئية
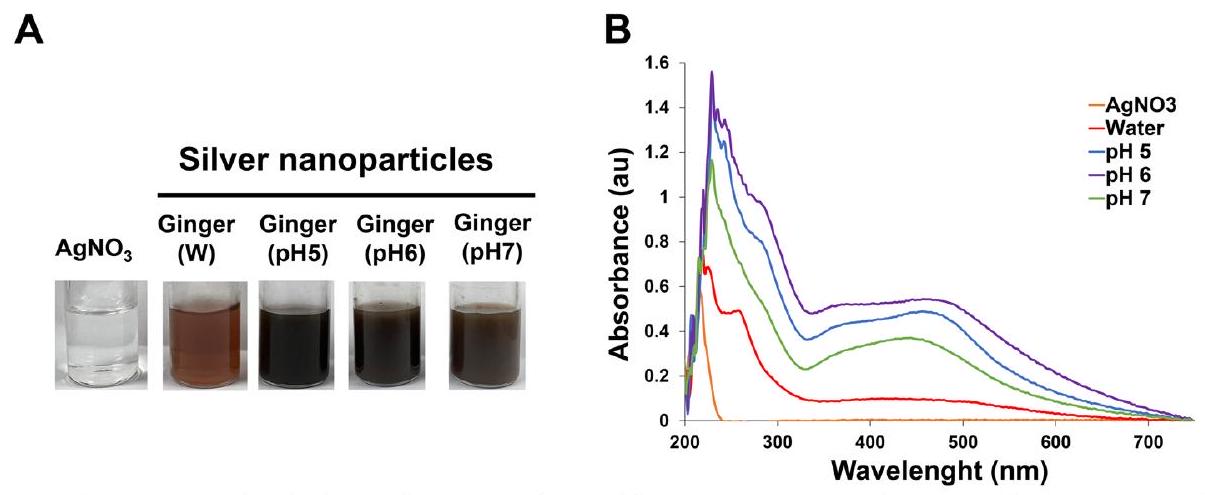
توضح الشكل 2B أن طول موجة مستخلص الزنجبيل يصل إلى أقصى قيمة له عند 457 نانومتر عندما يتم تحضير pH لمستخلص الزنجبيل إلى 6. تظهر قيم الامتصاص تحولات طفيفة في pH أخرى، مما يشير إلى تغييرات في حجم الجسيمات [53]. علاوة على ذلك، تؤكد النتائج التجريبية أن امتصاص الضوء في نطاق الطول الموجي من 400 إلى 500 نانومتر يكون أكثر وضوحًا عند pH 6. يمكن أن يُعزى هذا الظاهرة إلى التركيز المرتفع من المكونات الفينولية [54] والفلافونويدات الموجودة في مستخلص الزنجبيل عند pH 6. نتيجة لذلك، يتم تحضير مستخلصات الزنجبيل باستخدام الإيثانول والإيثيل.
الأسيتات عند pH 6 تحمل قيمة كبيرة كعوامل مختزلة في تخليق GE-AgNPs.
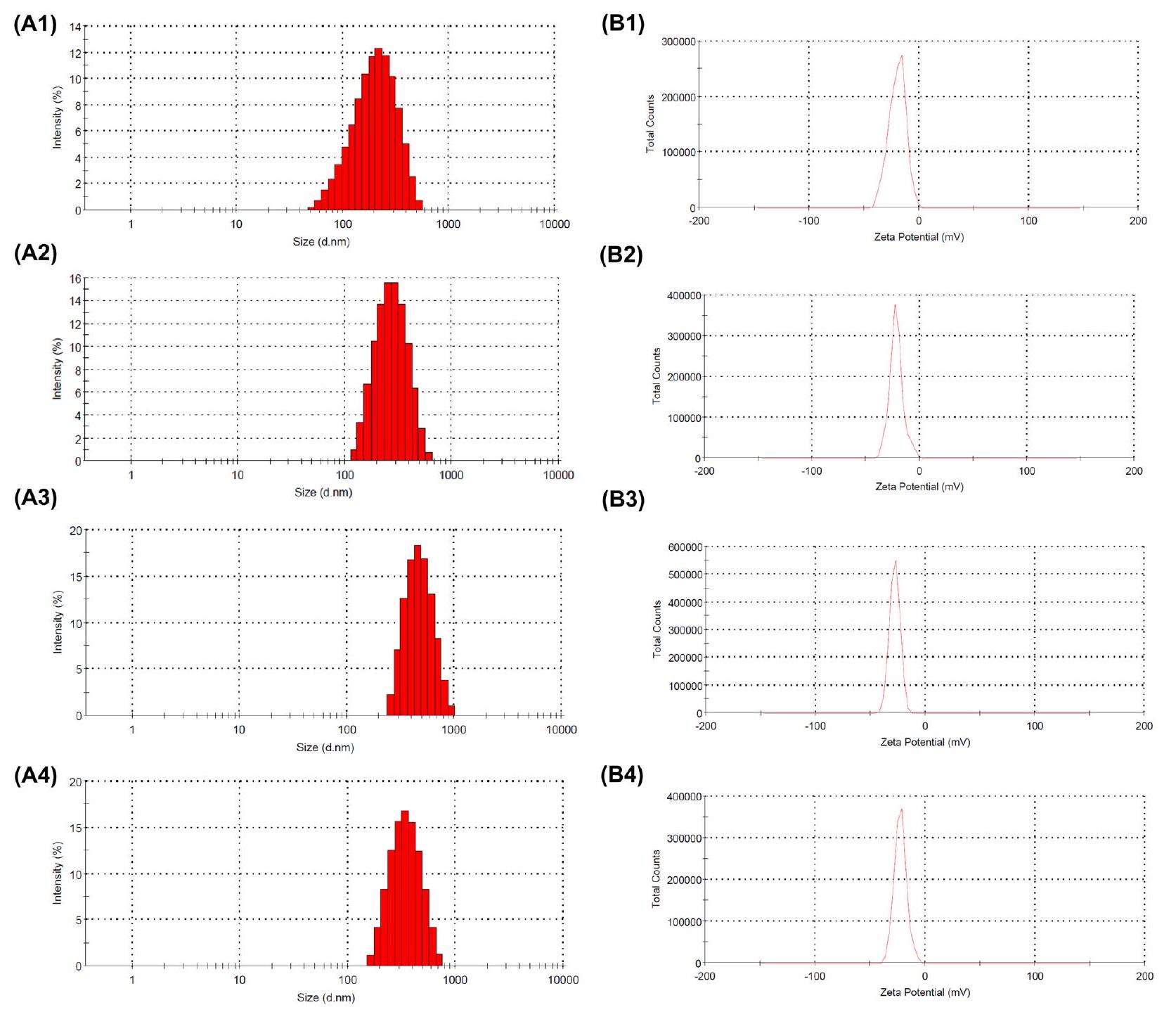
الحجم والشحنة
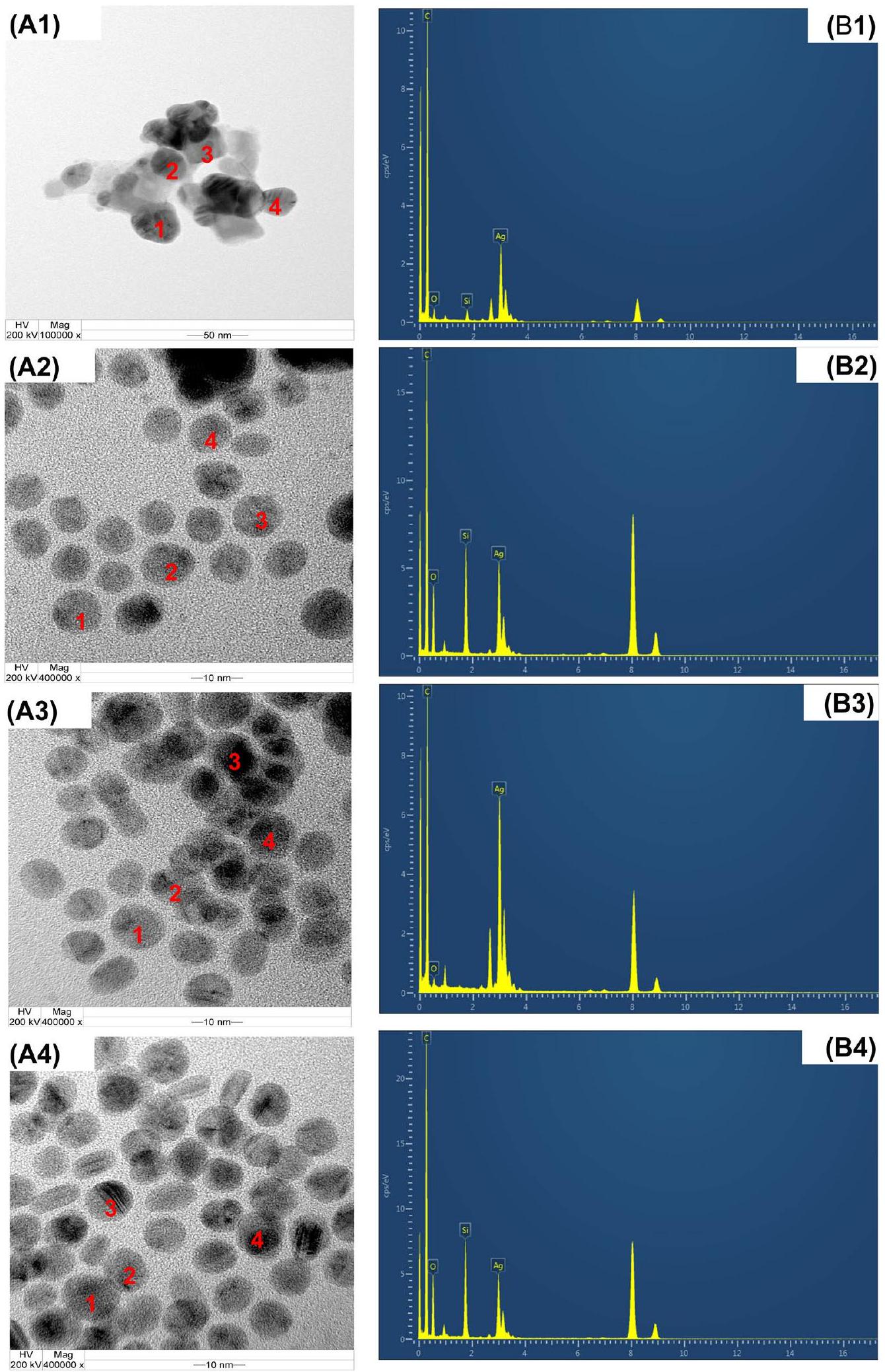
المجهر الإلكتروني الناقل (TEM) وطيف الأشعة السينية المشتتة بالطاقة (EDS)
تم استخراجها في كل من أنظمة الماء والمذيب المساعد. كشفت فحوصات TEM أن جزيئات الفضة النانوية المنتجة في هذه الظروف أظهرت شكلًا كرويًا بشكل أساسي. تم تحديد الحجم المتوسط لجزيئات الفضة النانوية ليكون بين 28 و 105 نانومتر، كما يتضح من صور TEM (الشكل 4A).
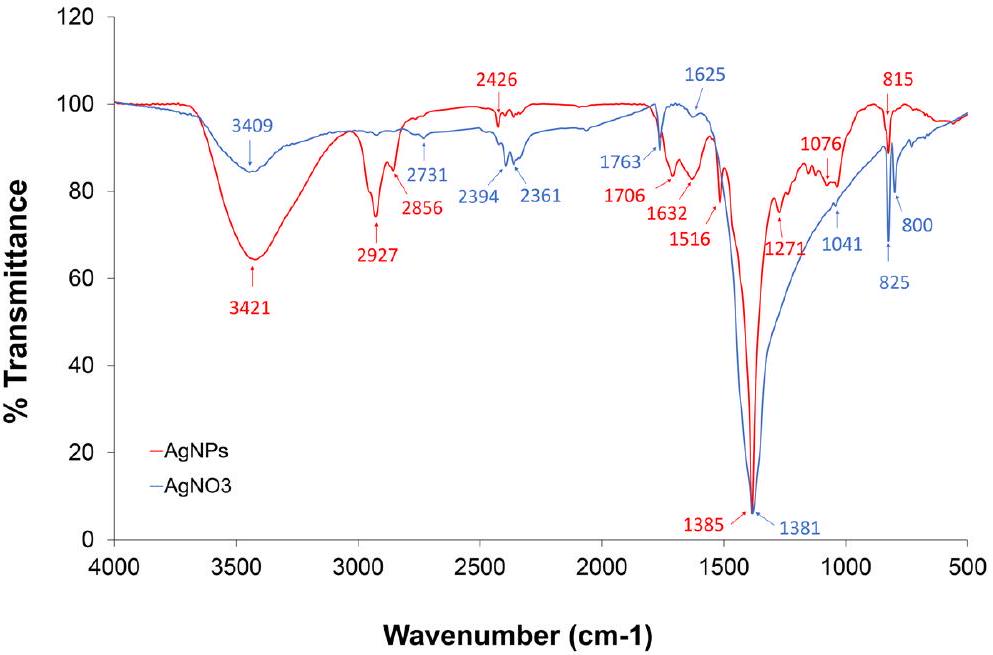
طيف الأشعة تحت الحمراء بواسطة تحويل فورييه (FTIR)
الجسيمات النانوية والمكونات البروتينية النباتية السائدة الموجودة في الزنجبيل. تصل إلى ذروتها عند
السُمية الخلوية في المختبر
الربط الجزيئي
فصل مكاني لـ
نشاط مثبط لليبوكسجيناز في المختبر
| المركبات | المحتويات (%) | الارتباط الجزيئي
|
|||
| ماء | مذيب مشترك | أرجوس لاب | فينا | أوتودوك | |
| حمض النورديدروغوايايريتيك | -11.5195 | -8.0 | -5.12 | ||
| 6-جينجرول | ٢٧.٦٩ | 9.83 | -11.1077 | -6.4 | -4.11 |
| 6-شوجاول | 11.56 | 9.11 | -12.2919 | -6.3 | -4.51 |
| بيوتان-2-ون، 4-(3-هيدروكسي-2-ميثوكسي فينيل)- | 9.26 | ٤.٤٣ | -9.0293 | -6.2 | -4.08 |
| ألفا-زنجبيرين | 6.29 | 7.35 | -12.0969 | -7.9 | -5.83 |
| ألفا-كيركومين | 6.26 | ٣.٥٧ | -13.141 | -7.6 | -5.45 |
| سيكويبيلاندرين | 3.73 | ٤.٢٢ | -12.1881 | -6.7 | -5.98 |
| بيتا-بيسابولين | ٣.٦٠ | ٤.٢٤ | -12.8127 | -7.7 | -5.56 |
| دياسيتوكسي-6-زنجبيرديول | 1.92 | 2.05 | -10.2678 | -7.0 | -2.97 |
| 6-إيزوشوجول | 0.93 | 9.11 | 12.7547 | -6.8 | -5.46 |
| 3-ديكانون، 1-(4-هيدروكسي-3-ميثوكسي فينيل)- | 0.63 | 1.11 | -11.7984 | -6.1 | -4.68 |
| 8-شوجاول | 1.35 | ٢.٥٧ | -12.7358 | -7.0 | -5.33 |
| (S)-8-جنجرول | 1.28 | 1.31 | -12.2436 | -6.5 | -4.25 |
| 1-(4-هيدروكسي-3-ميثوكسي فينيل) تيترا دك-4-إن-3-ون | 1.08 | 2.87 | -11.2954 | -6.9 | -4.95 |
| كليوناستيرول | 0.83 | 1.37 | -14.1923 | -8.9 | -8.61 |
الخاتمة
معلومات إضافية
شكر وتقدير
مساهمات المؤلفين
تمويل
توفر البيانات والمواد
الإعلانات
موافقة الأخلاقيات والموافقة على المشاركة
موافقة على النشر
المصالح المتنافسة
تفاصيل المؤلف
تم النشر على الإنترنت: 13 فبراير 2024
References
- Hernández-Díaz JA, Garza-García JJO, Zamudio-Ojeda A, León-Morales JM, López-Velázquez JC, García-Morales S. Plant-mediated synthesis of nanoparticles and their antimicrobial activity against phytopathogens. J Sci Food Agric. 2021;101(4):1270-87.
- Thipe VC, Karikachery AR, Çakılkaya P, Farooq U, Genedy HH, Kaeokhamloed N, Phan D-H, Rezwan R, Tezcan G, Roger E, et al. Green nanotech-nology-An innovative pathway towards biocompatible and medically relevant gold nanoparticles. J Drug Delivery Sci Technol. 2022;70:103256.
- Mohammadinejad R, Karimi S, Iravani S, Varma RS. Plant-derived nanostructures: types and applications. J Green Chemistry. 2016;18(1):20-52.
- Zhang M, Hu W, Cai C, Wu Y, Li J, Dong S. Advanced application of stimuliresponsive drug delivery system for inflammatory arthritis treatment. Materials Today Bio. 2022;14:100223.
- Ziegler D. Painful diabetic neuropathy: treatment and future aspects. Diabetes Metab Res Rev. 2008;24(S1):S52-7.
- Harris J-D. Management of expected and unexpected opioid-related side effects. Clin J Pain. 2008;24:58-13.
- Beg S, Swain S, Hasan H, Barkat MA, Hussain MS. Systematic review of herbals as potential anti-inflammatory agents: recent advances, current clinical status and future perspectives. Pharmacogn Rev. 2011;5(10):120-37.
- Skulachev VP. Cationic antioxidants as a powerful tool against mitochondrial oxidative stress. Biochem Biophys Res Commun. 2013;441(2):275-9.
- Rhim J-W, Wang L-F, Lee Y, Hong S-I. Preparation and characterization of bio-nanocomposite films of agar and silver nanoparticles: laser ablation method. Carbohyd Polym. 2014;103:456-65.
- Yue Y, Zhou B, Shi J, Chen C, Li N, Xu Z, Liu L, Kuang L, Ma M, Fu H.
-Irradiation assisted synthesis of graphene oxide sheets supported Ag nanoparticles with single crystalline structure and parabolic distribution from interlamellar limitation. Appl Surf Sci. 2017;403:282-93. - Shyam A, Chandran SS, George B. E S: Plant mediated synthesis of AgNPs and its applications: an overview. Inorganic Nano-Metal Chemist. 2021;51(12):1646-62.
- Saravanan A, Kumar PS, Hemavathy RV, Jeevanantham S, Harikumar P, Priyanka G, Devakirubai DRA. A comprehensive review on sources, analysis and toxicity of environmental pollutants and its removal methods from water environment. Sci Total Environ. 2022;812:152456.
- Shafey AME. Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: a review. Green Processing Synthesis. 2020;9(1):304-39.
- Rudrappa M, Kumar RS, Nagaraja SK, Hiremath H, Gunagambhire PV, Almansour Al, Perumal K, Nayaka S. Myco-nanofabrication of silver nanoparticles by penicillium brasilianum and their antimicrobial, photoprotective and anticancer effect on breast cancer cell line. Antibiotics. 2023;12(3):567.
- Rudrappa M, Rudayni HA, Assiri RA, Bepari A, Basavarajappa DS, Nagaraja SK, Chakraborty B, Swamy PS, Agadi SN, Niazi SK, et al. Plumeria albamediated green synthesis of silver nanoparticles exhibits antimicrobial effect and anti-oncogenic activity against glioblastoma U118 MG cancer cell line. Nanomaterials. 2022;12(3):493.
- Ling JK, Hadinoto K: Deep Eutectic Solvent as Green Solvent in Extraction of Biological Macromolecules: A Review. In: International Journal of Molecular Sciences. 2022;23.
- Liao DW, Cheng C, Liu JP, Zhao LY, Huang DC, Chen GT. Characterization and antitumor activities of polysaccharides obtained from ginger by different extraction methods. Int J Biol Macromol. 2020;152:894-903.
- Zhang M, Zhao R, Wang D, Wang L, Zhang Q, Wei S, Lu F, Peng W, Wu C. Ginger and its bioactive components are potential resources for health beneficial agents. Phytother Res. 2021;35(2):711-42.
- Wang Q, Kuang H, Su Y, Sun Y, Feng J, Guo R, Chan K. Naturally derived anti-inflammatory compounds from Chinese medicinal plants. J Ethnopharmacol. 2013;146(1):9-39.
- Sudlna GF, Pushkareva MA, Shephard P, Klein T. Cyclooxygenase (COX) and 5-lipoxygenase (5-LOX) selectivity of COX inhibitors. Prostaglandins, Leukotrienes Essential Fatty Acids. 2008;78(2):99-108.
- Lee T-Y, Lee K-C, Chen S-Y, Chang H-H. 6-Gingerol inhibits ROS and iNOS through the suppression of PKC-a and NF-kB pathways in lipopolysac-charide-stimulated mouse macrophages. Biochem Biophys Res Commun. 2009;382(1):134-9.
- Zhang F-L, Zhou B-W, Yan Z-Z, Zhao J, Zhao B-C, Liu W-F, Li C, Liu K-X. 6-Gingerol attenuates macrophages pyroptosis via the inhibition of MAPK signaling pathways and predicts a good prognosis in sepsis. Cytokine. 2020;125:154854.
- Liang N, Sang Y, Liu W, Yu W, Wang X. Anti-inflammatory effects of gingerol on lipopolysaccharide-stimulated RAW 264.7 cells by inhibiting NF-kB signaling pathway. Inflammation. 2018;41(3):835-45.
- Devadiga A, Shetty KV, Saidutta MB. Timber industry waste-teak leaf extract mediated synthesis of antibacterial silver nanoparticles. Int Nano Letters. 2015;5(4):205-14.
- Zhang L, Ravipati AS, Koyyalamudi SR, Jeong SC, Reddy N, Smith PT, Bartlett J, Shanmugam K, Münch G, Wu MJ. Antioxidant and anti-inflammatory activities of selected medicinal plants containing phenolic and flavonoid compounds. J Agric Food Chem. 2011;59(23):12361-7.
- Ongtanasup T, Prommee N, Jampa O, Limcharoen T, Wanmasae S, Nissapatorn V, Paul AK, Pereira MD, Wilairatana P, Nasongkla N et al: The Cholesterol-Modulating Effect of the New Herbal Medicinal Recipe from Yellow Vine (Coscinium fenestratum (Goetgh.)), Ginger (Zingiber officinale Roscoe.), and Safflower (Carthamus tinctorius L.) on Suppressing PCSK9 Expression to Upregulate LDLR Expression in HepG2 Cells. In: Plants. vol. 11; 2022.
- Magalhães LM, Santos F, Segundo MA, Reis S, Lima JLFC. Rapid microplate high-throughput methodology for assessment of Folin-Ciocalteu reducing capacity. Talanta. 2010;83(2):441-7.
- Mammen D, Daniel M. A critical evaluation on the reliability of two aluminum chloride chelation methods for quantification of flavonoids. Food Chem. 2012;135(3):1365-8.
- Moragot C, Pitaksit S, Patcharaporn P, Chantanapa C, Sutida W, Jiraphat N, Jitbanjong T, Jitbanjong T: Antioxidant and Tyrosinase Inhibitory Properties of an Aqueous Extract of Garcinia atroviridis Griff. ex. T. Anderson Fruit Pericarps. Pharmacognosy Journal 2020, 12(1).
- Chakraborty B, Kumar RS, Almansour Al, Kotresha D, Rudrappa M, Pallavi SS, Hiremath H, Perumal K, Nayaka S. Evaluation of antioxidant, antimicrobial and antiproliferative activity of silver nanoparticles derived from Galphimia glauca leaf extract. J King Saud University – Sci. 2021;33(8):101660.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med. 1999;26(9):1231-7.
- Lu J, Zeng X, Feng Y, Li S, Wang Y, Liu Y, Chen F, Guan Z, Chen T, Wei F. Inhibitory effects of Jasminum grandiflorum L. essential oil on lipopolysaccharide-induced microglia activation-integrated characteristic analysis of volatile compounds, network pharmacology, and BV-2 cell. Front Pharmacol. 2023;14:1180618.
- Satpathy S, Patra A, Ahirwar B, Delwar Hussain M. Antioxidant and anticancer activities of green synthesized silver nanoparticles using aqueous extract of tubers of Pueraria tuberosa. Artificial Cells Nanomedicine Biotechnol. 2018;46(sup3):71-85.
- Anandalakshmi K, Venugobal J, Ramasamy V. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl Nanosci. 2016;6(3):399-408.
- Nasongkla N, Tuchinda P, Munyoo B, Eawsakul K. Preparation and Characterization of MUC-30-loaded polymeric micelles against MCF-7 cell lines using molecular docking methods and in vitro study. Evidence-Based Complement Alternat Med. 2021;2021:5597681.
- Pooprommin P, Manaspon C, Dwivedi A, Mazumder A, Sangkaew S, Wanmasae S, Tangpong J, Ongtanasup T, Eawsakul K. Alginate/pectin dressing with niosomal mangosteen extract for enhanced wound healing: evaluating skin irritation by structure-activity relationship. Heliyon. 2022;8(12):e12032.
- Jongwannasiri C, Krasaesin A, Pinijsuwan S, Udomsom S, Boonprakong L, Eawsakul K, Osathanon T, Manaspon C. Diamond-like carbon (DLC)coated titanium surface inhibits bacterial growth and modulates human alveolar bone cell responses in vitro. Diam Relat Mater. 2023;136:110022.
- Ongtanasup T, Mazumder A, Dwivedi A, Eawsakul K: Homology Modeling, Molecular Docking, Molecular Dynamic Simulation, and Drug-Likeness of the Modified Alpha-Mangostin against the β-Tubulin Protein of Acanthamoeba Keratitis. In: Molecules. vol. 27; 2022.
- Eawsakul K, Ongtanasup T, Ngamdokmai N, Bunluepuech K. Alpha-glucosidase inhibitory activities of astilbin contained in Bauhinia strychnifolia Craib. stems: an investigation by in silico and in vitro studies. BMC Complement Med Ther. 2023;23(1):25.
- Eawsakul K, Panichayupakaranant P, Ongtanasup T, Warinhomhoun S, Noonong K, Bunluepuech K. Computational study and in vitro alphaglucosidase inhibitory effects of medicinal plants from a Thai folk remedy. Heliyon. 2021;7(9):e08078.
- Ongtanasup T, Wanmasae S, Srisang S, Manaspon C, Net-anong S, Eawsakul K. In silico investigation of ACE2 and the main protease of SARS-CoV-2 with phytochemicals from Myristica fragrans (Houtt.) for the discovery of a novel COVID-19 drug. Saudi J Biol Sci. 2022;29(9):103389.
- Goodsell DS, Morris GM, Olson AJ. Automated docking of flexible ligands: applications of autodock. J Mol Recognit. 1996;9(1):1-5.
- Trott O, Olson AJ. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31(2):455-61.
- Bitencourt-Ferreira G, de Azevedo WF: Molecular Docking Simulations with ArgusLab. In: Docking Screens for Drug Discovery. edn. Edited by de Azevedo Jr WF. New York, NY: Springer New York; 2019: 203-220.
- Li M, Kamdenlek P, Kuntanawat P, Eawsakul K, Porntaveetus T, Osathanon T, Manaspon CJCMUJNS. In vitro preparation and evaluation of chitosan/ pluronic hydrogel as a local delivery of crude extract of phycocyanin for treating gingivitis. Chiang Mai Univ J Nat Sci. 2022;21:e2022052.
- Marathe SJ, Hamzi W, Bashein AM, Deska J, Seppänen-Laakso T, Singhal RS, Shamekh S. Anti-Angiogenic effect of cantharellus cibarius extracts, its correlation with lipoxygenase inhibition, and role of the bioactives therein. Nutr Cancer. 2022;74(2):724-34.
- Singh PP, Saldaña MDA. Subcritical water extraction of phenolic compounds from potato peel. Food Res Int. 2011;44(8):2452-8.
- Zhu H, Zhang J, Li C, Liu S, Wang L. Morinda citrifolia L. leaves extracts obtained by traditional and eco-friendly extraction solvents: Relation between phenolic compositions and biological properties by multivariate analysis. Industrial Crops Prod. 2020;153:112586.
- Mohd Hazli UHA, Abdul-Aziz A, Mat-Junit S, Chee CF, Kong KW. Solidliquid extraction of bioactive compounds with antioxidant potential from Alternanthera sesillis (red) and identification of the polyphenols using UHPLC-QqQ-MS/MS. Food Res Int. 2019;115:241-50.
- Altaf MM, Ahmad Khan MS, Ahmad I: Chapter 2 – Diversity of Bioactive Compounds and Their Therapeutic Potential. In: New Look to Phytomedicine. edn. Edited by Ahmad Khan MS, Ahmad I, Chattopadhyay D: Academic Press; 2019: 15-34.
- Atanasov AG, Waltenberger B, Pferschy-Wenzig E-M, Linder T, Wawrosch C, Uhrin P, Temml V, Wang L, Schwaiger S, Heiss EH, et al. Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol Adv. 2015;33(8):1582-614.
- Govorov AO, Fan Z, Hernandez P, Slocik JM, Naik RR. Theory of circular dichroism of nanomaterials comprising chiral molecules and nanocrystals: plasmon enhancement, dipole interactions, and dielectric effects. Nano Lett. 2010;10(4):1374-82.
- de Aragão AP, de Oliveira TM, Quelemes PV, Perfeito MLG, Araújo MC, Santiago JDAS, Cardoso VS, Quaresma P, de Souza de Almeida Leite JR, da Silva DA. Green synthesis of silver nanoparticles using the seaweed Gracilaria birdiae and their antibacterial activity. Arabian J Chemist. 2019;12(8):4182-8.
- Zoecklein BW, Fugelsang KC, Gump BH, Nury FS: Phenolic Compounds and Wine Color. In: Production Wine Analysis. edn. Edited by Zoecklein BW, Fugelsang KC, Gump BH, Nury FS. Boston, MA: Springer US; 1990: 129-168.
- Capek I: Noble Metal Nanoparticles. In: Noble Metal Nanoparticles: Preparation, Composite Nanostructures, Biodecoration and Collective Properties. edn. Edited by Capek I. Tokyo: Springer Japan; 2017: 125-210.
- Cumberland SA, Lead JR. Particle size distributions of silver nanoparticles at environmentally relevant conditions. J Chromatogr A. 2009;1216(52):9099-105.
- Fernando I, Zhou Y. Impact of pH on the stability, dissolution and aggregation kinetics of silver nanoparticles. Chemosphere. 2019;216:297-305.
- Vijayaraghavan K, Nalini SPK, Prakash NU, Madhankumar D. Biomimetic synthesis of silver nanoparticles by aqueous extract of Syzygium aromaticum. Mater Lett. 2012;75:33-5.
- Sreelekha E, George B, Shyam A, Sajina N, Mathew B. A comparative study on the synthesis, characterization, and antioxidant activity of green and chemically synthesized silver nanoparticles. BioNanoScience. 2021;11(2):489-96.
- Kumar Panda M, Kumar Dhal N, Kumar M, Manjari Mishra P, Kumar Behera R. Green synthesis of silver nanoparticles and its potential effect on phytopathogens. Materials Today: Proceedings. 2021;35:233-8.
- Iyer RI, Panda T. Biosynthesis of gold and silver nanoparticles with antimicrobial activity by callus cultures of Michelia champaca L. J Nanosci Nanotechnol. 2016;16(7):7345-57.
- Sathiyaseelan A, Shajahan A, Kalaichelvan PT, Kaviyarasan V. Fungal chitosan based nanocomposites sponges-an alternative medicine for wound dressing. Int J Biol Macromol. 2017;104:1905-15.
- Shankar SS, Ahmad A, Sastry M. Geranium leaf assisted biosynthesis of silver nanoparticles. Biotechnol Prog. 2003;19(6):1627-31.
- Chandran Priyadarshni K, Krishnamoorthi R, Mumtha C, Ulagan Mahalingam P. Biochemical analysis of cultivated mushroom, Pleurotus florida and synthesis of silver nanoparticles for enhanced antimicrobial effects on clinically important human pathogens. Inorg Chem Commun. 2022;142:109673.
- Saikia J, Washmin N, Borah T, Sarmah P, Konwar P, Siga A, Haldar S, Banik D. Physicochemical properties, chemical composition and sensory attributes of Alpinia nigra (Gaertn.) B.L. Burtt rhizome: an underutilized spice source. European Food Res Technol. 2023;249(4):1097-112.
- Panigrahi S, Praharaj S, Basu S, Ghosh SK, Jana S, Pande S, Vo-Dinh T, Jiang H, Pal T. Self-assembly of silver nanoparticles: synthesis, stabilization, optical properties, and application in surface-enhanced raman scattering. J Phys Chem B. 2006;110(27):13436-44.
- Wallin RF, Arscott E. A practical guide to ISO 10993-5: Cytotoxicity. J Med Device Diagnostic Indust. 1998;20:96-8.
- Pournaderi PS, Yaghmaei P, Khodaei H, Noormohammadi Z, Hejazi SH. The effects of 6-Gingerol on reproductive improvement, liver functioning and Cyclooxygenase-2 gene expression in estradiol valerate – Induced polycystic ovary syndrome in Wistar rats. Biochem Biophys Res Commun. 2017;484(2):461-6.
- Rudrappa M, Nayaka S, Kumar RS. In silico molecular docking approach of melanin against melanoma causing MITF proteins and anticancer, oxida-tion-reduction, photoprotection, and drug-binding affinity properties of extracted melanin from streptomyces sp. strain MR28. Appl Biochemist Biotechnol. 2023;195(7):4368-86.
ملاحظة الناشر
DOI: https://doi.org/10.1186/s12906-024-04381-w
PMID: https://pubmed.ncbi.nlm.nih.gov/38350963
Publication Date: 2024-02-13
Green-synthesized silver nanoparticles
Check for updates from Zingiber officinale extract: antioxidant potential, biocompatibility, anti-LOX properties, and in silico analysis
Abstract
Introduction Zingiber officinale extract has emerged as a compelling candidate for green synthesis of nanoparticles, offering diverse applications across medicine, cosmetics, and nutrition. This study delves into the investigation of in vitro toxicity and explores the biomedical utility of green-synthesized silver nanoparticles derived from ginger extract (GE-AgNPs). Methods We employed established protocols to evaluate in vitro aspects such as antioxidant capacity, anti-inflammatory potential, and biocompatibility of GE-AgNPs. Additionally, molecular docking was employed to assess their anti-lipoxygenase (anti-LOX) activity. Results Our findings highlight that the extraction of ginger extract at a pH of 6, utilizing a cosolvent blend of ethanol and ethyl acetate in a 1:1 ratio, yields heightened antioxidant capacity attributed to its rich phenolic and flavonoid content. In the context of silver nanoparticle synthesis, pH 6 extraction yields the highest quantity of nanoparticles, characterized by an average size of
Introduction
Laser ablation [9], gamma irradiation [10], and the employment of chemical agents [11] as reducing and capping agents represent only a subset of the diverse methodologies available for the synthesis of AgNPs. The exorbitant costs associated with these approaches, coupled with the use of hazardous chemical agents, present significant impediments due to their adverse impacts on human health and the environment [12]. Within the field of nanotechnology, the emergence of green synthesis
has garnered considerable attention as a viable alternative for NP fabrication. Green synthesis NPs have demonstrated remarkable efficacy against primary biofilms. Notably, the utilization of plants as a source for NP synthesis offers distinct advantages over conventional physical and chemical methods [13]. In recent years, the utilization of plant extracts for NP synthesis [14, 15] has gained considerable traction owing to their widespread availability, environmentally sustainable characteristics, ease of implementation, and the diverse array of secondary metabolites they harbor, which can be harnessed as potent reducing agents [16].
inflammatory ailments. These bioactive compounds exert their therapeutic actions primarily by inhibiting the production of prostaglandins via the cyclooxygenase (COX) and lipoxygenase (LOX) pathways [20]. The historical utilization of ginger infusions for the management of conditions such as rheumatism and arthritis has prompted extensive research into the anti-inflammatory mechanisms of the plant’s secondary metabolites. According to several researchers [21,22], the anti-inflammatory properties of 6-gingerol can be attributed to its capacity to diminish pro-inflammatory cytokine levels and impede antigen presentation by macrophages stimulated by lipopolysaccharides (LPS). Liang et al. [23] conducted a study demonstrating that shogaols and all gingerols exhibit a dose-dependent reduction in the production of nitric oxide (NO) in RAW 264.7 cells treated with LPS. Thus, the integration of ginger extract as a reducing agent in the synthesis of AgNPs serves a dual purpose of enhancing anti-inflammatory action and minimizing the overall material requirements for anti-inflammatory product development. Notwithstanding the feasibility of utilizing ginger for AgNPs synthesis, investigations exploring ginger extracts with elevated concentrations of phenolic and flavonoid constituents are currently lacking. This research gap arises from the recognition that the extraction of biologically active chemical constituents from the phenolic and flavonoid classes is of paramount importance in attaining exceptional properties as reducing agents [24]. Furthermore, the active compounds present in ginger, which exhibit significant phenolic and flavonoid content, hold promise for enhancing the inhibition of inflammation [25]. The utilization of computerbased studies can play a pivotal role in reducing errors inherent in experimental procedures and increasing the likelihood of successful outcomes, thereby circumventing the need for extensive trial and error. Consequently, an investigation into the efficacy of ginger’s main compounds in inhibiting inflammatory production, achieved through the use of molecular docking processes to impede the activity of the LOX enzyme, contributes to a deeper understanding of the effectiveness of ginger.
analysis. Conducting a thorough examination is vital to acquire an intricate comprehension of the composition and concentration of the bioactive elements included in the ginger extract. The examination of the characteristics of the investigated compounds in relation to their capacity to hinder the LOX enzyme is of utmost importance. The utilization of sophisticated computational methodologies, such as molecular docking, can facilitate the attainment of this objective. This methodology provides a comprehensive analysis of the interaction between the chemicals present in ginger extract and the LOX enzyme, thereby offering valuable insights into their possible inhibitory effects on the formation of inflammation. Through the implementation of meticulous investigations, a thorough comprehension of the chemical makeup of ginger extract and its capacity as an inhibitor of inflammatory production can be attained. The acquisition of this knowledge enhances scientific comprehension within the discipline and enables the formulation of efficient approaches for harnessing ginger extract as a reducing agent, displaying potential applications across several fields. In addition, it is crucial to evaluate the antioxidant potential of the ginger extract in relation to the scavenging of 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radicals. The synthesized AgNPs were subjected to meticulous characterization using dynamic light scattering (DLS), transmission electron microscopy (TEM), ultraviolet-visible spectroscopy (UV-Vis), and Fouriertransform infrared spectroscopy (FTIR). Additionally, comprehensive biocompatibility assessments encompassing cytotoxicity were performed using L929 cells to ensure the safety and viability of the AgNPs.
Materials and methods
Materials
comprehensive cell culture materials were utilized to support these studies, in accordance with established laboratory protocols. These materials were purchased from Merck (Darmstadt, Germany). Lipoxygenase (LOX) enzyme and linoleic acid were obtained from Sigma-Aldrich (Missouri, USA).
Group 3, which involved studies and analysis through computer systems, utilized the following software programs: AutoDock 1.5.6, Python 3.8.2, MGLTools 1.5.4, Discovery Studio-2017, ArgusLab 4.0.1, and Avogadro. These software programs were used under the control of a computer system with the following specifications with processor: Intel Xeon-E5-2678v3 12C/24 T CPU @
Collection of plant
Plant extraction Water extraction
The extraction of
Preparation of ginger extract for yield analysis
Quantity of phenolic compounds
phenolic content was quantified in terms of gallic acid equivalents (GAE) expressed in milligrams per gram of the dried plant extract.
Quantity of flavonoid compounds
2,2-Diphennyl-1-picrylhydrazyl (DPPH) radical scavenging activity
GC-MS/MS analysis
ABTS
profile adhered to a carefully planned 69 min cycle, beginning at an initial temperature of
compared against the chemical libraries available in the Agilent MassHunter Quantitative Analysis software (version B.09.00). A match with a score of
The use of Zingiber officinale aqueous and cosolvent extracts in the production of silver nanoparticles
Characterization
(FTIR) was performed on the freeze-dried powder derived from the AgNP solution. The analysis was conducted utilizing a Bruker Tensor 27 apparatus, utilising KBr pellets as the sample medium, and including a wavelength range spanning from 400 to
Cell culture of L929 fibroblasts
In vitro cytotoxicity
Molecular docking to evaluate the inhibition of LOX
In-vitro lipoxygenase inhibitory activity
| Extraction | Yield |
| Water |
|
| pH 5 (ethanol + Ethyl acetate) |
|
| pH 6 (ethanol + Ethyl acetate) |
|
| pH 7 (ethanol + Ethyl acetate) |
|
Statistical analysis
Results and discussion
The analysis of ginger extract
the extraction of phenolic chemicals from plant materials. Thus, for the optimal extraction of phenolics and flavonoids from the ginger extract, it is recommended to employ ethanol: ethyl acetate extraction at a pH of 6 . The several research studies [48, 49] corroborate the notion that ethanol or ethyl acetate can effectively be utilized for the extraction of bioactive compounds, including phenolics and flavonoids.
Antioxidant activity
| Parameters | Amount | |||
| Water | pH 5 | pH 6 | pH 7 | |
| Total phenolic (mg GAE/g) |
|
|
|
|
| Total flavonoid (mg QE/g) |
|
|
|
|
| DPPH (mg TE/g) |
|
|
|
|
| DPPH (%/mg) |
|
|
|
|
| IC
|
|
|
|
|
| ABTS (mg TE/g) |
|
|
|
|
| ABTS (%/mg) |
|
|
|
|
| IC
|
|
|
|
|
conducted through the utilization of the 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay, as represented in Fig. 1. The results obtained from the evaluation of radical scavenging capacity employing the 2,2′-azino-bis(3-ethylben-zothiazoline-6-sulfonic acid) diammonium salt (ABTS+)

| Compounds | DPPH (
|
ABTS (
|
| Ascorbic |
|
|
| Trolox |
|
|
| GE ( pH 5 ) |
|
|
| GE-AgNPs (pH5) |
|
|
| GE ( pH 6 ) |
|
|
| GE-AgNPs (pH6) |
|
|
| GE ( pH 7 ) |
|
|
| GE-AgNPs (pH7) |
|
|
GC-MS/MS analysis
constituents identified in the aqueous ginger extract were found to be 5-hydroxy-1-(4-hydroxy-3-methoxyphenyl) decan-3-one as known as 6-gingerol (27.69%), exemplifying their prominence and significance in the composition of the extract.
Silver nanoparticle characterization UV-visible (vis) spectroscopy
Figure 2B illustrates that the wavelength of the ginger extract reaches its maximum value at 457 nm when the pH of the ginger extract is prepared to 6 . The absorbance values exhibit slight shifts in other pHs , indicating changes in particle size [53]. Furthermore, the experimental findings establish that the absorption of light in the wavelength range of 400 to 500 nm is most pronounced at pH 6 . This phenomenon can be attributed to the elevated concentration of phenolic components [54] and flavonoids present in the ginger extract at pH 6 . As a result, ginger extracts prepared using ethanol and ethyl
acetate at pH 6 hold significant value as reducing agents in the synthesis of GE-AgNPs.
Size and charge


Transmission electron microscopy (TEM) and energy-dispersive X-ray spectroscopy (EDS)
extract in both water and cosolvent systems. The TEM examination unveiled that the AgNPs produced under these conditions displayed a predominantly spherical shape. The average size of the AgNPs s was determined to range between 28 and 105 nm , as evidenced by the TEM images (Fig. 4A).

Fourier transform infrared spectroscopy (FTIR)
nanoparticles and the predominant proteinaceous phytoconstituents present in ginger. Peaking at

In vitro cytotoxicity

Molecular docking
a spatial separation of
In-vitro lipoxygenase inhibitory activity
| Compounds | Contents (%) | Binding affinity (
|
|||
| Water | Cosolvent | Arguslab | Vina | Autodock | |
| Nordihydroguaiaretic acid | -11.5195 | -8.0 | -5.12 | ||
| 6-gingerol | 27.69 | 9.83 | -11.1077 | -6.4 | -4.11 |
| 6-shogaol | 11.56 | 9.11 | -12.2919 | -6.3 | -4.51 |
| Butan-2-one, 4-(3-hydroxy-2-methoxyphenyl)- | 9.26 | 4.43 | -9.0293 | -6.2 | -4.08 |
| Alpha-zingiberene | 6.29 | 7.35 | -12.0969 | -7.9 | -5.83 |
| Alpha-curcumene | 6.26 | 3.57 | -13.141 | -7.6 | -5.45 |
| Sesquiphellandrene | 3.73 | 4.22 | -12.1881 | -6.7 | -5.98 |
| Beta-bisabolene | 3.60 | 4.24 | -12.8127 | -7.7 | -5.56 |
| Diacetoxy-6-gingerdiol | 1.92 | 2.05 | -10.2678 | -7.0 | -2.97 |
| 6-isoshogaol | 0.93 | 9.11 | 12.7547 | -6.8 | -5.46 |
| 3-decanone,1-(4-hydroxy-3-methoxyphenyl)- | 0.63 | 1.11 | -11.7984 | -6.1 | -4.68 |
| 8-shogaol | 1.35 | 2.57 | -12.7358 | -7.0 | -5.33 |
| (S)-8-gingerol | 1.28 | 1.31 | -12.2436 | -6.5 | -4.25 |
| 1-(4-hydroxy-3-methoxyphenyl)tetradec-4-en-3-one | 1.08 | 2.87 | -11.2954 | -6.9 | -4.95 |
| Clionasterol | 0.83 | 1.37 | -14.1923 | -8.9 | -8.61 |

Conclusion

Supplementary Information
Acknowledgements
Authors’ contributions
Funding
Availability of data and materials
Declarations
Ethics approval and consent to participate
Consent for publication
Competing interests
Author details
Published online: 13 February 2024
References
- Hernández-Díaz JA, Garza-García JJO, Zamudio-Ojeda A, León-Morales JM, López-Velázquez JC, García-Morales S. Plant-mediated synthesis of nanoparticles and their antimicrobial activity against phytopathogens. J Sci Food Agric. 2021;101(4):1270-87.
- Thipe VC, Karikachery AR, Çakılkaya P, Farooq U, Genedy HH, Kaeokhamloed N, Phan D-H, Rezwan R, Tezcan G, Roger E, et al. Green nanotech-nology-An innovative pathway towards biocompatible and medically relevant gold nanoparticles. J Drug Delivery Sci Technol. 2022;70:103256.
- Mohammadinejad R, Karimi S, Iravani S, Varma RS. Plant-derived nanostructures: types and applications. J Green Chemistry. 2016;18(1):20-52.
- Zhang M, Hu W, Cai C, Wu Y, Li J, Dong S. Advanced application of stimuliresponsive drug delivery system for inflammatory arthritis treatment. Materials Today Bio. 2022;14:100223.
- Ziegler D. Painful diabetic neuropathy: treatment and future aspects. Diabetes Metab Res Rev. 2008;24(S1):S52-7.
- Harris J-D. Management of expected and unexpected opioid-related side effects. Clin J Pain. 2008;24:58-13.
- Beg S, Swain S, Hasan H, Barkat MA, Hussain MS. Systematic review of herbals as potential anti-inflammatory agents: recent advances, current clinical status and future perspectives. Pharmacogn Rev. 2011;5(10):120-37.
- Skulachev VP. Cationic antioxidants as a powerful tool against mitochondrial oxidative stress. Biochem Biophys Res Commun. 2013;441(2):275-9.
- Rhim J-W, Wang L-F, Lee Y, Hong S-I. Preparation and characterization of bio-nanocomposite films of agar and silver nanoparticles: laser ablation method. Carbohyd Polym. 2014;103:456-65.
- Yue Y, Zhou B, Shi J, Chen C, Li N, Xu Z, Liu L, Kuang L, Ma M, Fu H.
-Irradiation assisted synthesis of graphene oxide sheets supported Ag nanoparticles with single crystalline structure and parabolic distribution from interlamellar limitation. Appl Surf Sci. 2017;403:282-93. - Shyam A, Chandran SS, George B. E S: Plant mediated synthesis of AgNPs and its applications: an overview. Inorganic Nano-Metal Chemist. 2021;51(12):1646-62.
- Saravanan A, Kumar PS, Hemavathy RV, Jeevanantham S, Harikumar P, Priyanka G, Devakirubai DRA. A comprehensive review on sources, analysis and toxicity of environmental pollutants and its removal methods from water environment. Sci Total Environ. 2022;812:152456.
- Shafey AME. Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: a review. Green Processing Synthesis. 2020;9(1):304-39.
- Rudrappa M, Kumar RS, Nagaraja SK, Hiremath H, Gunagambhire PV, Almansour Al, Perumal K, Nayaka S. Myco-nanofabrication of silver nanoparticles by penicillium brasilianum and their antimicrobial, photoprotective and anticancer effect on breast cancer cell line. Antibiotics. 2023;12(3):567.
- Rudrappa M, Rudayni HA, Assiri RA, Bepari A, Basavarajappa DS, Nagaraja SK, Chakraborty B, Swamy PS, Agadi SN, Niazi SK, et al. Plumeria albamediated green synthesis of silver nanoparticles exhibits antimicrobial effect and anti-oncogenic activity against glioblastoma U118 MG cancer cell line. Nanomaterials. 2022;12(3):493.
- Ling JK, Hadinoto K: Deep Eutectic Solvent as Green Solvent in Extraction of Biological Macromolecules: A Review. In: International Journal of Molecular Sciences. 2022;23.
- Liao DW, Cheng C, Liu JP, Zhao LY, Huang DC, Chen GT. Characterization and antitumor activities of polysaccharides obtained from ginger by different extraction methods. Int J Biol Macromol. 2020;152:894-903.
- Zhang M, Zhao R, Wang D, Wang L, Zhang Q, Wei S, Lu F, Peng W, Wu C. Ginger and its bioactive components are potential resources for health beneficial agents. Phytother Res. 2021;35(2):711-42.
- Wang Q, Kuang H, Su Y, Sun Y, Feng J, Guo R, Chan K. Naturally derived anti-inflammatory compounds from Chinese medicinal plants. J Ethnopharmacol. 2013;146(1):9-39.
- Sudlna GF, Pushkareva MA, Shephard P, Klein T. Cyclooxygenase (COX) and 5-lipoxygenase (5-LOX) selectivity of COX inhibitors. Prostaglandins, Leukotrienes Essential Fatty Acids. 2008;78(2):99-108.
- Lee T-Y, Lee K-C, Chen S-Y, Chang H-H. 6-Gingerol inhibits ROS and iNOS through the suppression of PKC-a and NF-kB pathways in lipopolysac-charide-stimulated mouse macrophages. Biochem Biophys Res Commun. 2009;382(1):134-9.
- Zhang F-L, Zhou B-W, Yan Z-Z, Zhao J, Zhao B-C, Liu W-F, Li C, Liu K-X. 6-Gingerol attenuates macrophages pyroptosis via the inhibition of MAPK signaling pathways and predicts a good prognosis in sepsis. Cytokine. 2020;125:154854.
- Liang N, Sang Y, Liu W, Yu W, Wang X. Anti-inflammatory effects of gingerol on lipopolysaccharide-stimulated RAW 264.7 cells by inhibiting NF-kB signaling pathway. Inflammation. 2018;41(3):835-45.
- Devadiga A, Shetty KV, Saidutta MB. Timber industry waste-teak leaf extract mediated synthesis of antibacterial silver nanoparticles. Int Nano Letters. 2015;5(4):205-14.
- Zhang L, Ravipati AS, Koyyalamudi SR, Jeong SC, Reddy N, Smith PT, Bartlett J, Shanmugam K, Münch G, Wu MJ. Antioxidant and anti-inflammatory activities of selected medicinal plants containing phenolic and flavonoid compounds. J Agric Food Chem. 2011;59(23):12361-7.
- Ongtanasup T, Prommee N, Jampa O, Limcharoen T, Wanmasae S, Nissapatorn V, Paul AK, Pereira MD, Wilairatana P, Nasongkla N et al: The Cholesterol-Modulating Effect of the New Herbal Medicinal Recipe from Yellow Vine (Coscinium fenestratum (Goetgh.)), Ginger (Zingiber officinale Roscoe.), and Safflower (Carthamus tinctorius L.) on Suppressing PCSK9 Expression to Upregulate LDLR Expression in HepG2 Cells. In: Plants. vol. 11; 2022.
- Magalhães LM, Santos F, Segundo MA, Reis S, Lima JLFC. Rapid microplate high-throughput methodology for assessment of Folin-Ciocalteu reducing capacity. Talanta. 2010;83(2):441-7.
- Mammen D, Daniel M. A critical evaluation on the reliability of two aluminum chloride chelation methods for quantification of flavonoids. Food Chem. 2012;135(3):1365-8.
- Moragot C, Pitaksit S, Patcharaporn P, Chantanapa C, Sutida W, Jiraphat N, Jitbanjong T, Jitbanjong T: Antioxidant and Tyrosinase Inhibitory Properties of an Aqueous Extract of Garcinia atroviridis Griff. ex. T. Anderson Fruit Pericarps. Pharmacognosy Journal 2020, 12(1).
- Chakraborty B, Kumar RS, Almansour Al, Kotresha D, Rudrappa M, Pallavi SS, Hiremath H, Perumal K, Nayaka S. Evaluation of antioxidant, antimicrobial and antiproliferative activity of silver nanoparticles derived from Galphimia glauca leaf extract. J King Saud University – Sci. 2021;33(8):101660.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med. 1999;26(9):1231-7.
- Lu J, Zeng X, Feng Y, Li S, Wang Y, Liu Y, Chen F, Guan Z, Chen T, Wei F. Inhibitory effects of Jasminum grandiflorum L. essential oil on lipopolysaccharide-induced microglia activation-integrated characteristic analysis of volatile compounds, network pharmacology, and BV-2 cell. Front Pharmacol. 2023;14:1180618.
- Satpathy S, Patra A, Ahirwar B, Delwar Hussain M. Antioxidant and anticancer activities of green synthesized silver nanoparticles using aqueous extract of tubers of Pueraria tuberosa. Artificial Cells Nanomedicine Biotechnol. 2018;46(sup3):71-85.
- Anandalakshmi K, Venugobal J, Ramasamy V. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl Nanosci. 2016;6(3):399-408.
- Nasongkla N, Tuchinda P, Munyoo B, Eawsakul K. Preparation and Characterization of MUC-30-loaded polymeric micelles against MCF-7 cell lines using molecular docking methods and in vitro study. Evidence-Based Complement Alternat Med. 2021;2021:5597681.
- Pooprommin P, Manaspon C, Dwivedi A, Mazumder A, Sangkaew S, Wanmasae S, Tangpong J, Ongtanasup T, Eawsakul K. Alginate/pectin dressing with niosomal mangosteen extract for enhanced wound healing: evaluating skin irritation by structure-activity relationship. Heliyon. 2022;8(12):e12032.
- Jongwannasiri C, Krasaesin A, Pinijsuwan S, Udomsom S, Boonprakong L, Eawsakul K, Osathanon T, Manaspon C. Diamond-like carbon (DLC)coated titanium surface inhibits bacterial growth and modulates human alveolar bone cell responses in vitro. Diam Relat Mater. 2023;136:110022.
- Ongtanasup T, Mazumder A, Dwivedi A, Eawsakul K: Homology Modeling, Molecular Docking, Molecular Dynamic Simulation, and Drug-Likeness of the Modified Alpha-Mangostin against the β-Tubulin Protein of Acanthamoeba Keratitis. In: Molecules. vol. 27; 2022.
- Eawsakul K, Ongtanasup T, Ngamdokmai N, Bunluepuech K. Alpha-glucosidase inhibitory activities of astilbin contained in Bauhinia strychnifolia Craib. stems: an investigation by in silico and in vitro studies. BMC Complement Med Ther. 2023;23(1):25.
- Eawsakul K, Panichayupakaranant P, Ongtanasup T, Warinhomhoun S, Noonong K, Bunluepuech K. Computational study and in vitro alphaglucosidase inhibitory effects of medicinal plants from a Thai folk remedy. Heliyon. 2021;7(9):e08078.
- Ongtanasup T, Wanmasae S, Srisang S, Manaspon C, Net-anong S, Eawsakul K. In silico investigation of ACE2 and the main protease of SARS-CoV-2 with phytochemicals from Myristica fragrans (Houtt.) for the discovery of a novel COVID-19 drug. Saudi J Biol Sci. 2022;29(9):103389.
- Goodsell DS, Morris GM, Olson AJ. Automated docking of flexible ligands: applications of autodock. J Mol Recognit. 1996;9(1):1-5.
- Trott O, Olson AJ. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31(2):455-61.
- Bitencourt-Ferreira G, de Azevedo WF: Molecular Docking Simulations with ArgusLab. In: Docking Screens for Drug Discovery. edn. Edited by de Azevedo Jr WF. New York, NY: Springer New York; 2019: 203-220.
- Li M, Kamdenlek P, Kuntanawat P, Eawsakul K, Porntaveetus T, Osathanon T, Manaspon CJCMUJNS. In vitro preparation and evaluation of chitosan/ pluronic hydrogel as a local delivery of crude extract of phycocyanin for treating gingivitis. Chiang Mai Univ J Nat Sci. 2022;21:e2022052.
- Marathe SJ, Hamzi W, Bashein AM, Deska J, Seppänen-Laakso T, Singhal RS, Shamekh S. Anti-Angiogenic effect of cantharellus cibarius extracts, its correlation with lipoxygenase inhibition, and role of the bioactives therein. Nutr Cancer. 2022;74(2):724-34.
- Singh PP, Saldaña MDA. Subcritical water extraction of phenolic compounds from potato peel. Food Res Int. 2011;44(8):2452-8.
- Zhu H, Zhang J, Li C, Liu S, Wang L. Morinda citrifolia L. leaves extracts obtained by traditional and eco-friendly extraction solvents: Relation between phenolic compositions and biological properties by multivariate analysis. Industrial Crops Prod. 2020;153:112586.
- Mohd Hazli UHA, Abdul-Aziz A, Mat-Junit S, Chee CF, Kong KW. Solidliquid extraction of bioactive compounds with antioxidant potential from Alternanthera sesillis (red) and identification of the polyphenols using UHPLC-QqQ-MS/MS. Food Res Int. 2019;115:241-50.
- Altaf MM, Ahmad Khan MS, Ahmad I: Chapter 2 – Diversity of Bioactive Compounds and Their Therapeutic Potential. In: New Look to Phytomedicine. edn. Edited by Ahmad Khan MS, Ahmad I, Chattopadhyay D: Academic Press; 2019: 15-34.
- Atanasov AG, Waltenberger B, Pferschy-Wenzig E-M, Linder T, Wawrosch C, Uhrin P, Temml V, Wang L, Schwaiger S, Heiss EH, et al. Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol Adv. 2015;33(8):1582-614.
- Govorov AO, Fan Z, Hernandez P, Slocik JM, Naik RR. Theory of circular dichroism of nanomaterials comprising chiral molecules and nanocrystals: plasmon enhancement, dipole interactions, and dielectric effects. Nano Lett. 2010;10(4):1374-82.
- de Aragão AP, de Oliveira TM, Quelemes PV, Perfeito MLG, Araújo MC, Santiago JDAS, Cardoso VS, Quaresma P, de Souza de Almeida Leite JR, da Silva DA. Green synthesis of silver nanoparticles using the seaweed Gracilaria birdiae and their antibacterial activity. Arabian J Chemist. 2019;12(8):4182-8.
- Zoecklein BW, Fugelsang KC, Gump BH, Nury FS: Phenolic Compounds and Wine Color. In: Production Wine Analysis. edn. Edited by Zoecklein BW, Fugelsang KC, Gump BH, Nury FS. Boston, MA: Springer US; 1990: 129-168.
- Capek I: Noble Metal Nanoparticles. In: Noble Metal Nanoparticles: Preparation, Composite Nanostructures, Biodecoration and Collective Properties. edn. Edited by Capek I. Tokyo: Springer Japan; 2017: 125-210.
- Cumberland SA, Lead JR. Particle size distributions of silver nanoparticles at environmentally relevant conditions. J Chromatogr A. 2009;1216(52):9099-105.
- Fernando I, Zhou Y. Impact of pH on the stability, dissolution and aggregation kinetics of silver nanoparticles. Chemosphere. 2019;216:297-305.
- Vijayaraghavan K, Nalini SPK, Prakash NU, Madhankumar D. Biomimetic synthesis of silver nanoparticles by aqueous extract of Syzygium aromaticum. Mater Lett. 2012;75:33-5.
- Sreelekha E, George B, Shyam A, Sajina N, Mathew B. A comparative study on the synthesis, characterization, and antioxidant activity of green and chemically synthesized silver nanoparticles. BioNanoScience. 2021;11(2):489-96.
- Kumar Panda M, Kumar Dhal N, Kumar M, Manjari Mishra P, Kumar Behera R. Green synthesis of silver nanoparticles and its potential effect on phytopathogens. Materials Today: Proceedings. 2021;35:233-8.
- Iyer RI, Panda T. Biosynthesis of gold and silver nanoparticles with antimicrobial activity by callus cultures of Michelia champaca L. J Nanosci Nanotechnol. 2016;16(7):7345-57.
- Sathiyaseelan A, Shajahan A, Kalaichelvan PT, Kaviyarasan V. Fungal chitosan based nanocomposites sponges-an alternative medicine for wound dressing. Int J Biol Macromol. 2017;104:1905-15.
- Shankar SS, Ahmad A, Sastry M. Geranium leaf assisted biosynthesis of silver nanoparticles. Biotechnol Prog. 2003;19(6):1627-31.
- Chandran Priyadarshni K, Krishnamoorthi R, Mumtha C, Ulagan Mahalingam P. Biochemical analysis of cultivated mushroom, Pleurotus florida and synthesis of silver nanoparticles for enhanced antimicrobial effects on clinically important human pathogens. Inorg Chem Commun. 2022;142:109673.
- Saikia J, Washmin N, Borah T, Sarmah P, Konwar P, Siga A, Haldar S, Banik D. Physicochemical properties, chemical composition and sensory attributes of Alpinia nigra (Gaertn.) B.L. Burtt rhizome: an underutilized spice source. European Food Res Technol. 2023;249(4):1097-112.
- Panigrahi S, Praharaj S, Basu S, Ghosh SK, Jana S, Pande S, Vo-Dinh T, Jiang H, Pal T. Self-assembly of silver nanoparticles: synthesis, stabilization, optical properties, and application in surface-enhanced raman scattering. J Phys Chem B. 2006;110(27):13436-44.
- Wallin RF, Arscott E. A practical guide to ISO 10993-5: Cytotoxicity. J Med Device Diagnostic Indust. 1998;20:96-8.
- Pournaderi PS, Yaghmaei P, Khodaei H, Noormohammadi Z, Hejazi SH. The effects of 6-Gingerol on reproductive improvement, liver functioning and Cyclooxygenase-2 gene expression in estradiol valerate – Induced polycystic ovary syndrome in Wistar rats. Biochem Biophys Res Commun. 2017;484(2):461-6.
- Rudrappa M, Nayaka S, Kumar RS. In silico molecular docking approach of melanin against melanoma causing MITF proteins and anticancer, oxida-tion-reduction, photoprotection, and drug-binding affinity properties of extracted melanin from streptomyces sp. strain MR28. Appl Biochemist Biotechnol. 2023;195(7):4368-86.
Publisher’s Note
- *Correspondence:
Komgrit Eawsakul
komgrit.ea@wu.ac.th
Full list of author information is available at the end of the article
