DOI: https://doi.org/10.1038/s41467-024-46388-4
PMID: https://pubmed.ncbi.nlm.nih.gov/38448464
تاريخ النشر: 2024-03-06
جزيئات النحاس النانوية المحاطة بإطار الإيميدازولات الزيوليتية-8 كعامل حفاز مستقر وانتقائي لهدرجة ثاني أكسيد الكربون
تاريخ القبول: 23 فبراير 2024
تاريخ النشر على الإنترنت: 06 مارس 2024
(أ) تحقق من التحديثات
الملخص
لقد جذبت الأطر العضوية المعدنية الانتباه كعوامل حفازة محتملة بفضل كيميائها السطحية الفريدة القابلة للتعديل وسهولة الوصول إليها. ومع ذلك، فإن تطبيقها في التحفيز الحراري كان محدودًا بسبب عدم استقرارها تحت درجات الحرارة والضغوط القاسية، مثل هدرجة
الانتقائية
توزيع جزيئات النحاس وسعة الامتصاص، واختبار النشاط-الاستقرار لـ
النتائج
تخليق أنواع النحاس في ZIF-8
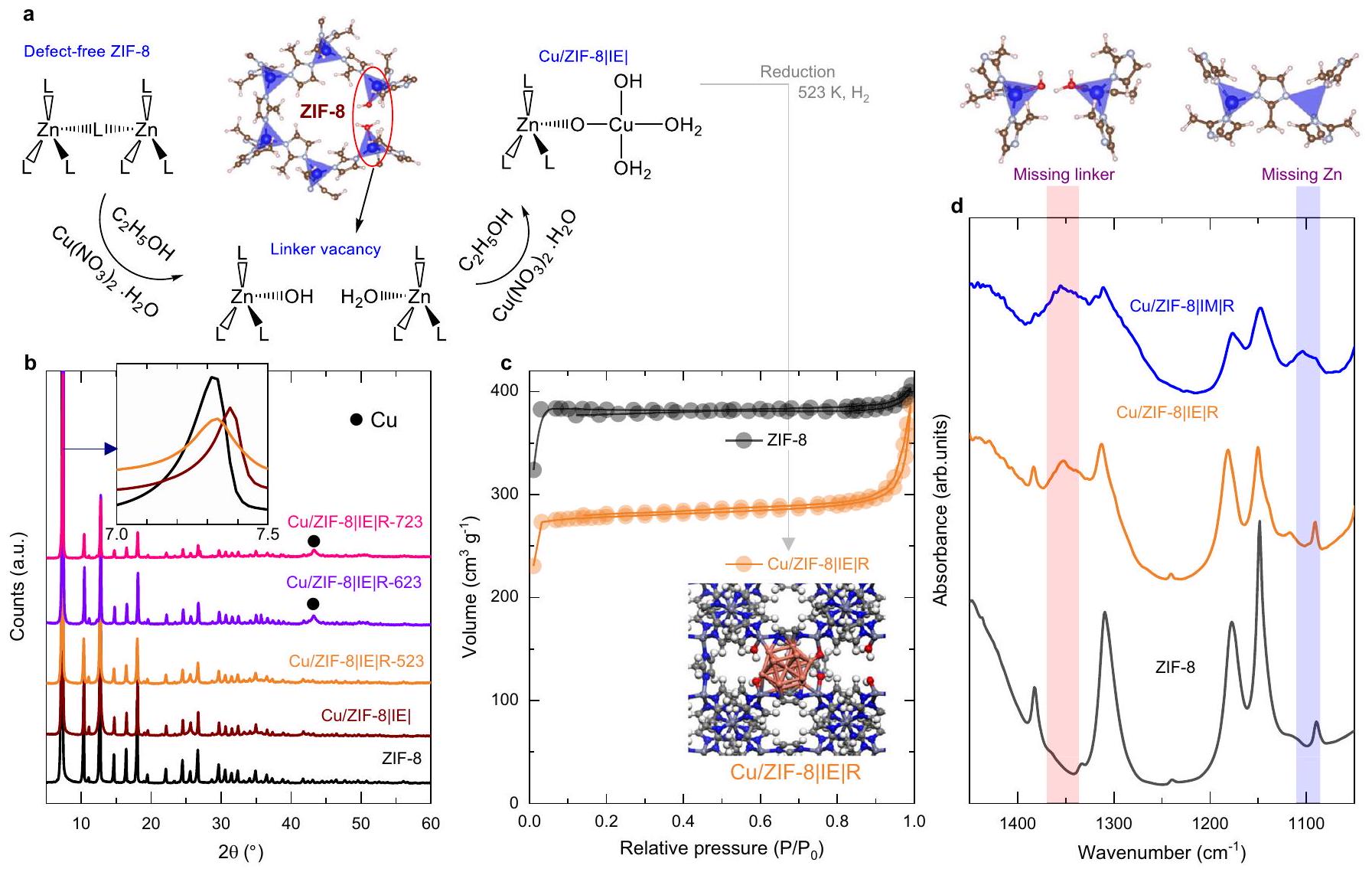
بعد التخفيف عند 523 كلفن إلى 723 كلفن.
تم رؤية تحول القمم قبل تقليل العينة، مما يعزز الفكرة بأن النحاس تم إدخاله في الهيكل بعد معالجة التخفيف. لم يتم الإبلاغ عن مراحل النحاس بعد التخفيف عند 523 كلفن، وهو ما يمكن أن يُعزى إلى تشتت جزيئات النحاس بشكل كبير في العامل الحفاز. أظهر التخفيف عند درجات حرارة عالية (623 كلفن و723 كلفن) خطوط حيود نحاسية مخفضة في الغالب بسبب عملية التكتل المحتملة للنحاس عند تعرضه لدرجات حرارة عالية (
هيكل والبيئة الكيميائية لـ
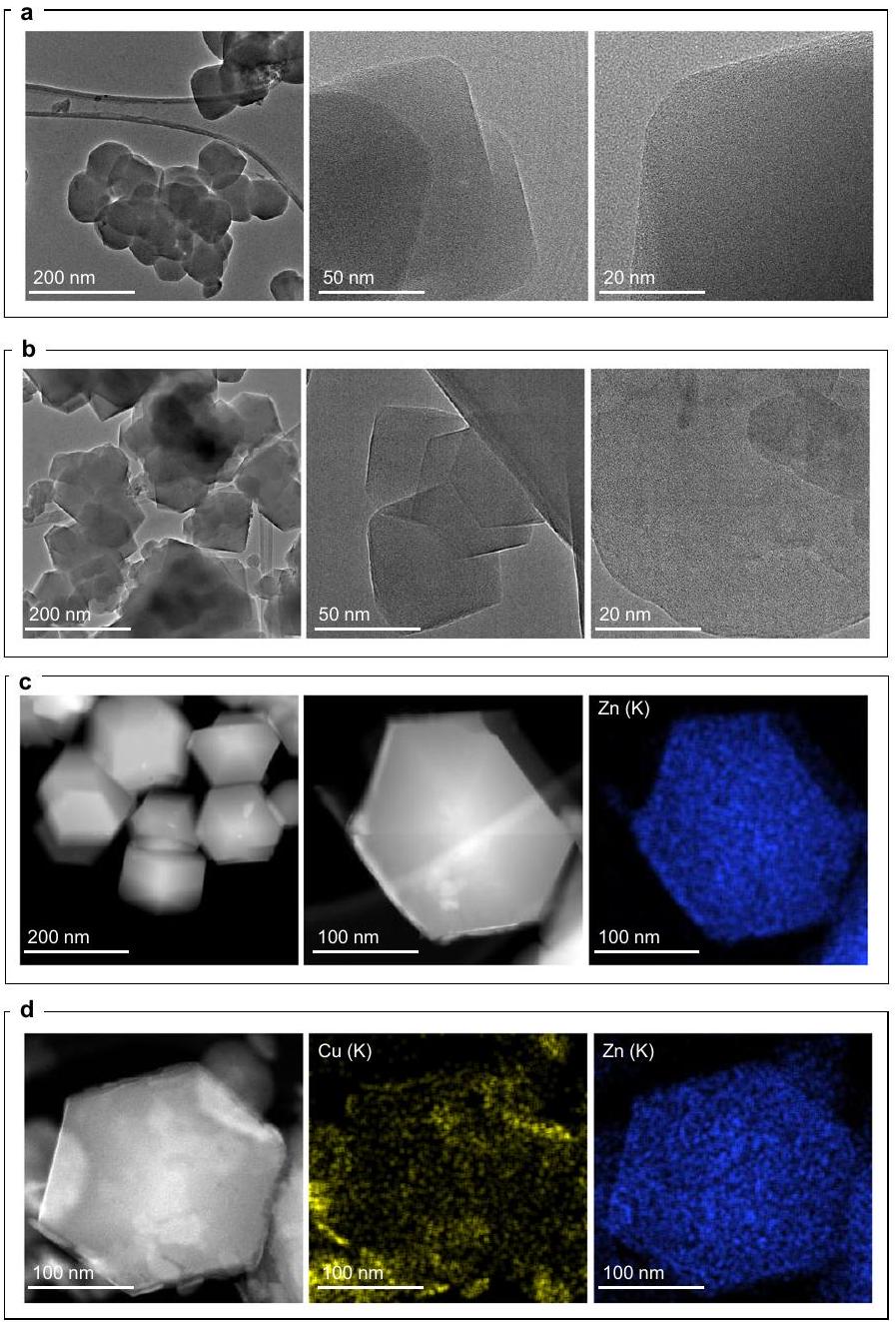
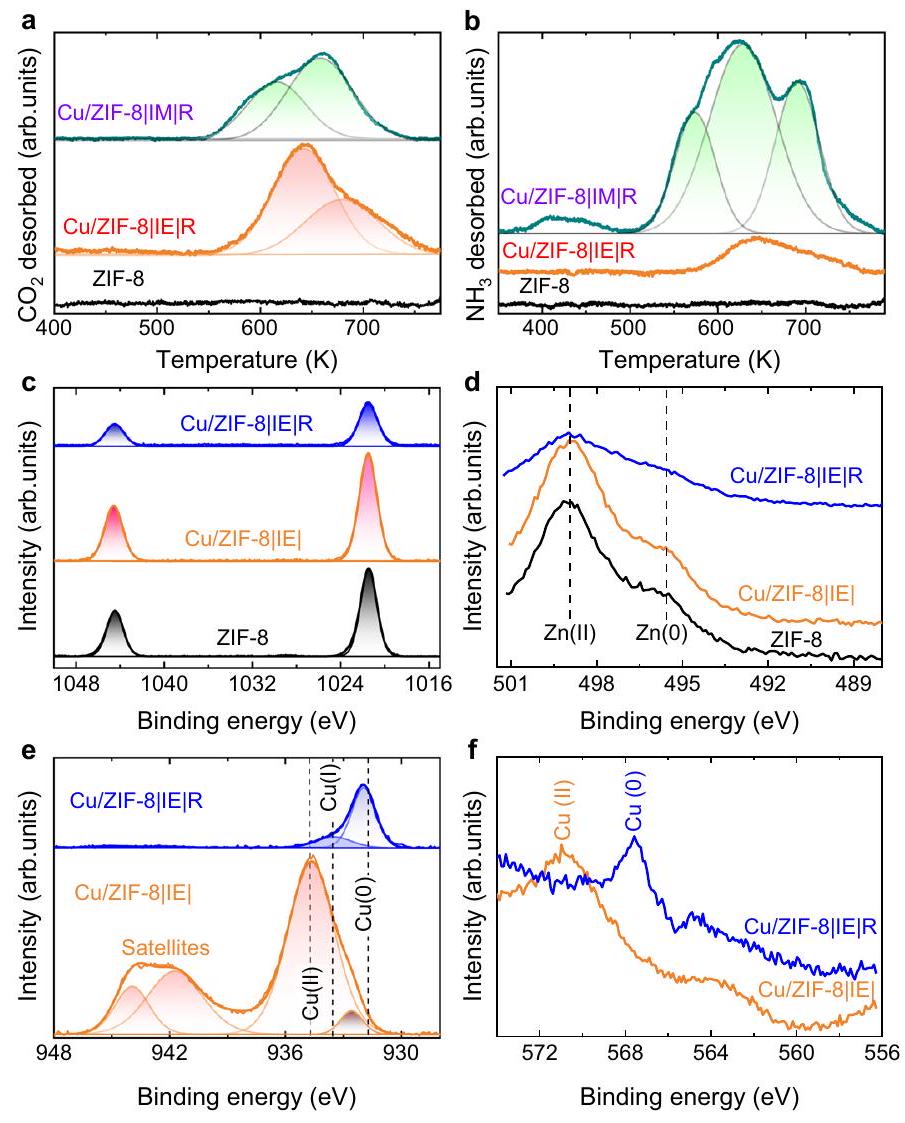

النشاط التحفيزي، الانتقائية، والاستقرار لـ
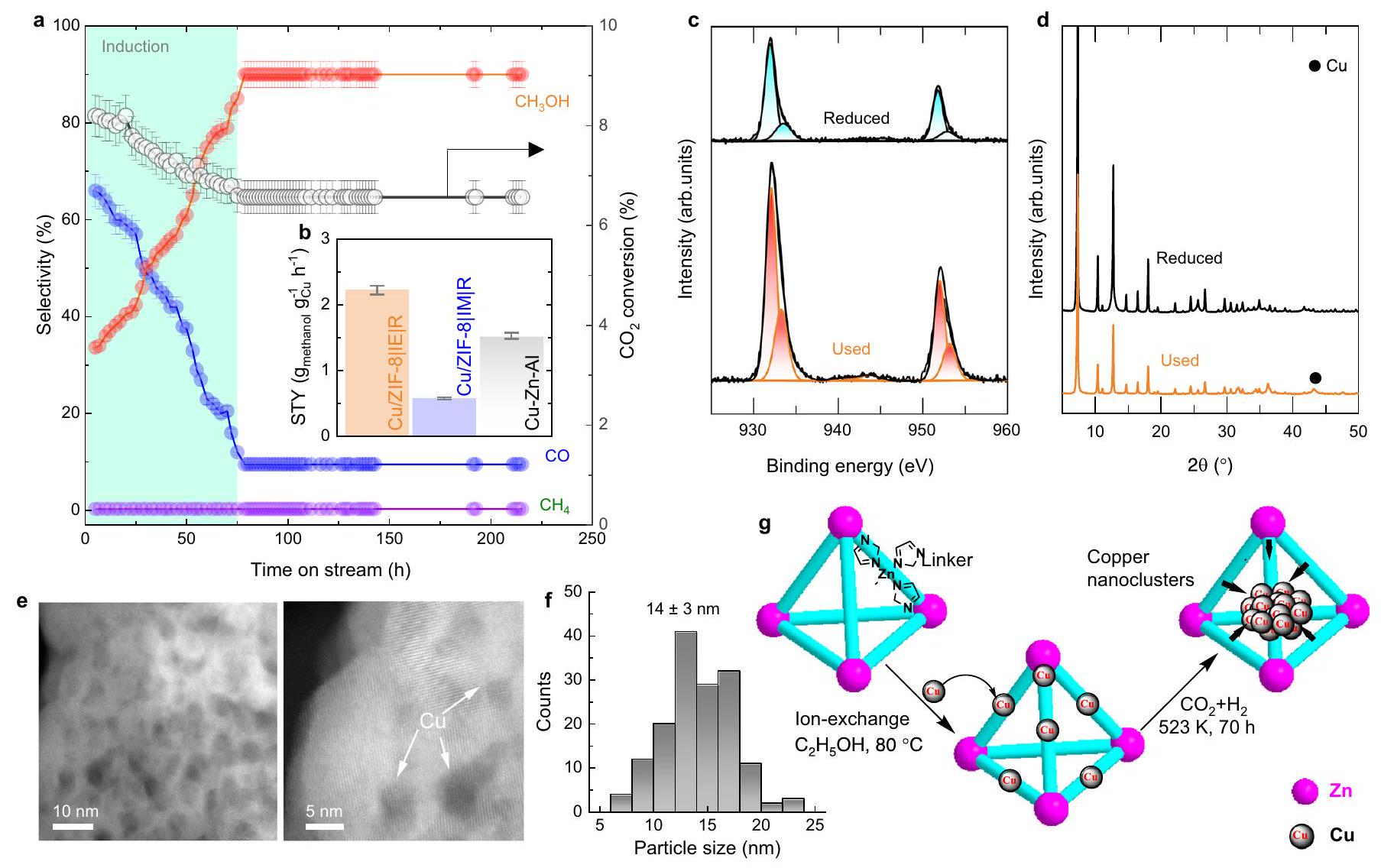
المحفزات. د أنماط حيود الأشعة السينية للبودرة، (هـ) صور TEM عالية الدقة لـ Cu/ZIF-8|IE|R-100 ساعة. ف توزيع حجم الجسيمات لـ Cu كما تم الحصول عليه في صور HR-TEM لـ Cu/ZIF-8 | IE | R-100 ساعة. ج تمثيل تصويري لعمليات معالجة ZIF-8 المختلفة التي تؤدي في النهاية إلى تشكيل جزيئات نانوية من النحاس تحت
ذروة الانكسار عند
الأشعة تحت الحمراء في الموقع لآلية التفاعل
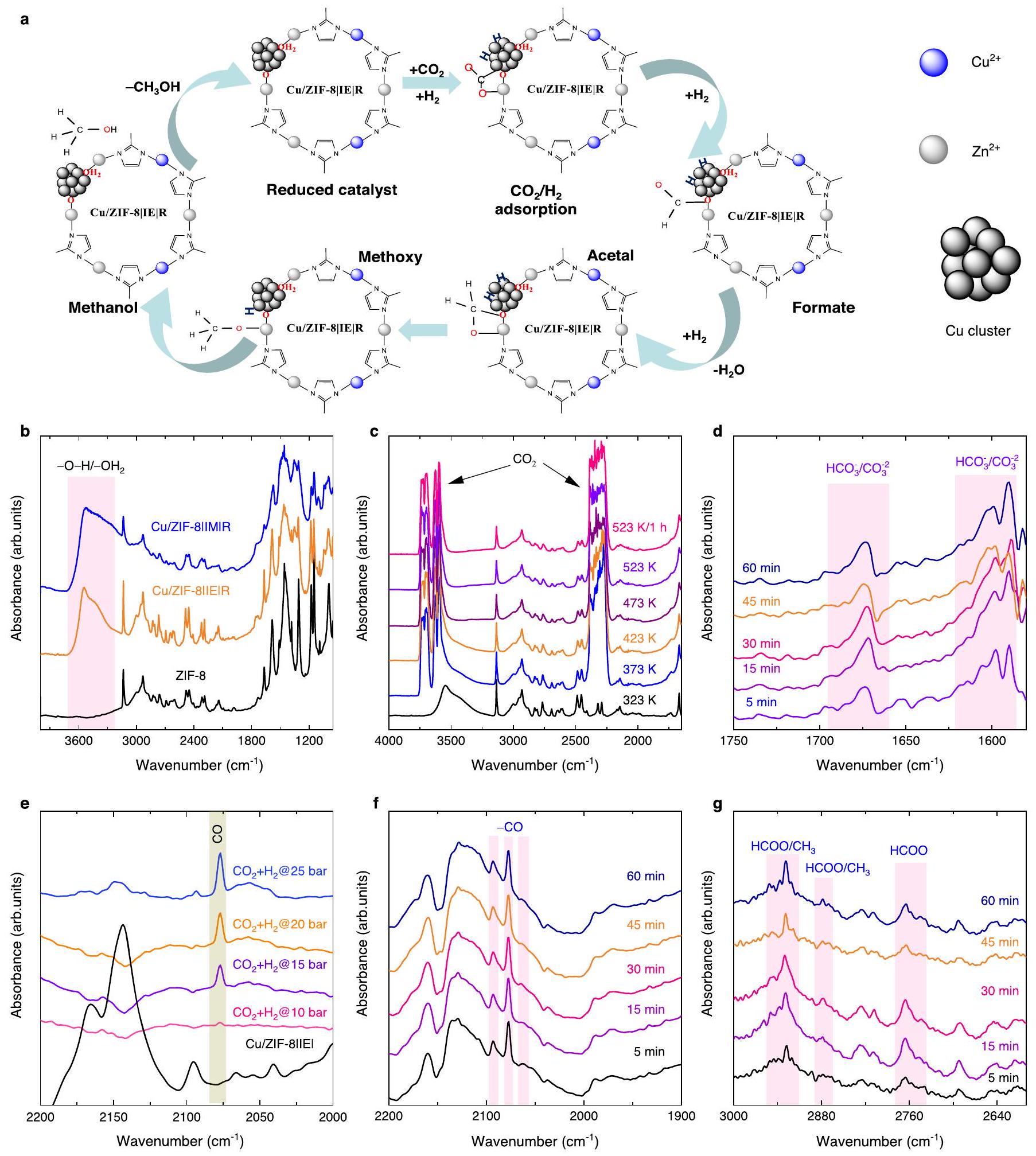
ثلاثة متميزة

نقاش
طرق
تحضير المحفز
تم تشبع الدعم بنترات النحاس باستخدام الإيثانول كمذيب مع التحريك المستمر لمدة ساعتين عند 300 كلفن. تم تبخير المذيب عن طريق التجفيف عند 353 كلفن والنتيجة
توصيف المواد
دراسات الأشعة تحت الحمراء في الموقع
حسابات نظرية الكثافة (DFT)
الزيفيات الموجية المعززة (PAW)
اختبارات التحفيز
توفر البيانات
References
- Zhu, Y. et al. Copper-zirconia interfaces in UiO-66 enable selective catalytic hydrogenation of CO2 to methanol. Nat. Commun. 11, 1-11 (2020).
- Zhu, Y. et al. Inverse iron oxide/metal catalysts from galvanic replacement. Nat. Commun. 11, 1-7 (2020).
- Wang, X., Shi, H. & Szanyi, J. Controlling selectivities in CO2 reduction through mechanistic understanding. Nat. Commun. 8, 1-6 (2017).
- Graciani, J. et al. Highly active copper-ceria and copper-ceria-titania catalysts for methanol synthesis from
. Science (80-). 345, 546-CO550 (2014). - Sehested, J. Industrial and scientific directions of methanol catalyst development. J. Catal. 371, 368-375 (2019).
- Bonura, G., Cordaro, M., Cannilla, C., Arena, F. & Frusteri, F. The changing nature of the active site of
catalysts for the CO 2 hydrogenation reaction to methanol. Appl. Catal. B Environ. 152-153, 152-161 (2014). - Kuld, S. et al. Quantifying the promotion of Cu catalysts by ZnO for methanol synthesis. Science (80-.). 352, 969-974 (2016).
- Martin, O. et al. Operando synchrotron X-ray powder diffraction and modulated-excitation infrared spectroscopy elucidate the CO2 promotion on a commercial methanol synthesis catalyst. Angew. Chemie Int. Ed. 55, 11031-11036 (2016).
- Beck, A. et al. Following the structure of copper-zinc-alumina across the pressure gap in carbon dioxide hydrogenation. Nat. Catal. 202146 4, 488-497 (2021).
- Prieto, G., Zečević, J., Friedrich, H., De Jong, K. P. & De Jongh, P. E. Towards stable catalysts by controlling collective properties of supported metal nanoparticles. Nat. Mater. 2012121 12, 34-39 (2012).
- Martin, O. & Pérez-Ramírez, J. New and revisited insights into the promotion of methanol synthesis catalysts by
. Catal. Sci. Technol. 3, 3343-3352 (2013). - Bavykina, A. et al. Turning a methanation Co catalyst into an In-Co methanol producer. ACS Catal. 9, 6910-6918 (2019).
- Frei, M. S. et al. Nanostructure of nickel-promoted indium oxide catalysts drives selectivity in CO2 hydrogenation. Nat. Commun. 2021121 12, 1-9 (2021).
- Rungtaweevoranit, B. et al. Copper nanocrystals encapsulated in Zr-based metal-organic frameworks for highly selective CO2 hydrogenation to methanol. Nano Lett. 16, 7645-7649 (2016).
- Jiang, H. L. et al. Au@ZIF-8: CO oxidation over gold nanoparticles deposited to metal-organic framework. J. Am. Chem. Soc. 131, 11302-11303 (2009).
- Jiang, H. L., Akita, T., Ishida, T., Haruta, M. & Xu, Q. Synergistic catalysis of Au@Ag core-shell nanoparticles stabilized on metalorganic framework. J. Am. Chem. Soc. 133, 1304-1306 (2011).
- Zhang, J. et al. Neighboring
Sites in a Metal-Organic Framework for CO2Hydrogenation. J. Am. Chem. Soc. 143, 8829-8837 (2021). - An, B. et al. Confinement of ultrasmall
nanoparticles in metal-organic frameworks for selective methanol synthesis from catalytic hydrogenation of . J. Am. Chem. Soc. 139, 3834-3840 (2017). - Sholl, D. S. & Lively, R. P. Defects in metal-organic frameworks: challenge or opportunity? J. Phys. Chem. Lett. 6, 3437-3444 (2015).
- Zhang, C., Han, C., Sholl, D. S. & Schmidt, J. R. Computational characterization of defects in metal-organic frameworks: spontaneous and water-induced point defects in ZIF-8. J. Phys. Chem. Lett. 7, 459-464 (2016).
- Ramos-Fernandez, E. V., Redondo-Murcia, A., Grau-Atienza, A., Sepúlveda-Escribano, A. & Narciso, J. Clean production of Zeolitic Imidazolate Framework 8 using Zamak residues as metal precursor and substrate. J. Clean. Prod. 260, 121081 (2020).
- Li, M. et al. Thermal stability of size-selected copper nanoparticles: Effect of size, support and CO2 hydrogenation atmosphere. Appl. Surf. Sci. 510, 145439 (2020).
- Thommes, M. et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 87, 1051-1069 (2015).
- Ta, D. N. et al. Preparation of nano-ZIF-8 in methanol with high yield. Can. J. Chem. Eng. 96, 1518-1531 (2018).
- Nordin, N. A. H. M., Ismail, A. F., Mustafa, A., Murali, R. S. & Matsuura, T. The impact of ZIF-8 particle size and heat treatment on
CH4 separation using asymmetric mixed matrix membrane. RSC Adv. 4, 52530-52541 (2014). - Jamil, N. et al. Green one-pot synthesis and characterisation of hybrid reduced graphene oxide/zeolitic imidazole framework-8 (rGO/ZIF-8.J. Iran. Chem. Soc. 18, 363-373 (2021).
- Yue, B., Li, Q., Iwai, H., Kako, T. & Ye, J. Hydrogen production using zinc-doped carbon nitride catalyst irradiated with visible light. Sci. Technol. Adv. Mater. 12, 34401-34408 (2011).
- Ramos-Fernández, E. V., Ferreira, A. F. P., Sepúlveda-Escribano, A., Kapteijn, F. & Rodríguez-Reinoso, F. Enhancing the catalytic performance of
in the selective hydrogenation of cinnamaldehyde by Cr addition to the support. J. Catal. 258, 52-60 (2008). - Zhen, S. Y. et al. Metal-organic framework derived hollow porous
dodecahedrons as a cathode catalyst for batteries. RSC Adv. 9, 16288-16295 (2019). - La Rosa-Toro, A. et al. Preparation and characterization of copperdoped cobalt oxide electrodes. J. Phys. Chem. B 110, 24021-24029 (2006).
- Wang, W., Qu, Z., Song, L. & Fu, Q. CO2 hydrogenation to methanol over
and catalysts: Tuning methanol selectivity via metal-support interaction. J. Energy Chem. 40, 22-30 (2020). - Liao, F. et al. Morphology-dependent interactions of ZnO with Cu nanoparticles at the materials’ interface in selective hydrogenation of CO 2 to CH 3 OH . Angew. Chemie Int. Ed. 50, 2162-2165 (2011).
- Li, D., Wang, G., Cheng, L., Wang, C. & Mei, X. Engineering the selfassembly induced emission of copper nanoclusters as 3D nanomaterials with mesoporous sphere structures by the crosslinking of Ce3. ACS Omega 3, 14755-14765 (2018).
- Frei, E. et al. Cu-Zn alloy formation as unfavored state for efficient methanol catalysts. ChemCatChem 12, 4029-4033 (2020).
- Newton, M. A. et al. On the mechanism underlying the direct conversion of methane to methanol by copper hosted in zeolites; braiding Cu K-edge XANES and reactivity studies. J. Am. Chem. Soc. 140, 10090-10093 (2018).
- Nian, J. N., Chen, S. A., Tsai, C. C. & Teng, H. Structural feature and catalytic performance of Cu species distributed over TiO 2 nanotubes. J. Phys. Chem. B 110, 25817-25824 (2006).
- Yuan, L. et al. Dynamic evolution of atomically dispersed Cu species for CO2 photoreduction to solar fuels. ACS Catal. 9, 4824-4833 (2019).
- Ma, Q., Buchholz, D. B. & Chang, R. P. H. Local structures of copperdoped ZnO films. Phys. Rev. B – Condens. Matter Mater. Phys. 78, 214429 (2008).
- Boada, R. et al. Unraveling the molecular details of the ‘gate opening’ phenomenon in ZIF-8 with X-ray absorption spectroscopy. J. Phys. Chem. C 126, 5935-5943 (2022).
- Dalebout, R. et al. Insight into the nature of the ZnOx promoter during methanol synthesis. ACS Catal. 12, 6628-6639 (2022).
- Wang, Y. et al. Exploring the ternary interactions in
catalysts for efficient CO2 hydrogenation to methanol. Nat. Commun. 2019101 10, 1-10 (2019). - Graaf, G. H., Sijtsema, P. J. J. M., Stamhuis, E. J. & Joosten, G. E. H. Chemical equilibria in methanol synthesis. Chem. Eng. Sci. 41, 2883-2890 (1986).
- Hu, B. et al. Cu@ZIF-8 derived inverse
catalyst with sub-5 nm ZnO for efficient CO2 hydrogenation to methanol. Catal. Sci. Technol. 9, 2673-2681 (2019). - Beebe, T. P., Crowell, J. E. & Yates, J. T. Reaction of methyl chloride with alumina surfaces: a study of the methoxy surface species by transmission infrared spectroscopy. J. Phys. Chem. 92, 1296-1301 (2002).
- Fujita, S. I., Usui, M., Ohara, E. & Takezawa, N. Methanol synthesis from carbon dioxide at atmospheric pressure over
catalyst. Role of methoxide species formed on ZnO support. Catal. Lett. 1992 134 13, 349-358 (1992). - Wang, L. L. & Johnson, D. D. Density functional study of structural trends for late-transition-metal 13-atom clusters. Phys. Rev. B Condens. Matter Mater. Phys. 75, 235405 (2007).
- Fan, Q. Y., Sun, J. J., Wang, F. & Cheng, J. Adsorption-induced liquid-to-solid phase transition of Cu clusters in catalytic dissociation of
. J. Phys. Chem. Lett. 11, 7954-7959 (2020). - Natesakhawat, S. et al. Active sites and structure-activity relationships of copper-based catalysts for carbon dioxide hydrogenation to methanol. ACS Catal. 2, 1667-1676 (2012).
- Tang, W., Sanville, E. & Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter 21, 084204 (2009).
- Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169-11186 (1996).
- Lewis, D. W. et al. Zeolitic imidazole frameworks: structural and energetics trends compared with their zeolite analogues. CrystEngComm 11, 2272-2276 (2009).
- Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953 (1994).
- Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15-50 (1996).
- Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
- Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
- Pack, J. D ., & Monkhorst, H. J. Special points for Brillonin-zone integrations*. Phys. Rev. B 13, 5188-5192 (1976).
- Zhang, W. & Xiao, Y. Mechanism of electrocatalytically active precious metal (Ni, Pd, Pt, and Ru) complexes in the graphene basal plane for ORR applications in novel fuel cells. Energy Fuels 34, 2425-2434 (2020).
- Ahmad, R. & Singh, A. K. Synergistic core-shell interactions enable ultra-low overpotentials for enhanced CO 2 electro-reduction activity. J. Mater. Chem. A 6, 21120-21130 (2018).
شكر وتقدير
مساهمات المؤلفين
المصالح المتنافسة
معلومات إضافية
المواد التكميلية متاحة على
https://doi.org/10.1038/s41467-024-46388-4.
http://www.nature.com/reprints
© المؤلف(ون) 2024
هندسة التفاعل متعددة المقاييس، مركز كاوست للتحفيز (KCC)، جامعة الملك عبدالله للعلوم والتقنية (KAUST)، ثول 23955-6900، المملكة العربية السعودية. مركز كاوست للتحفيز (KCC)، جامعة الملك عبدالله للعلوم والتقنية (KAUST)، ثول 23955-6900، المملكة العربية السعودية. جامعة الملك عبدالله للعلوم والتقنية (كاوست)، قسم العلوم الفيزيائية والهندسة، مركز الأغشية المتقدمة والمواد المسامية (AMPM)، ثول 23955-6900، المملكة العربية السعودية. مرفق إشعاع السنكروترون في بكين، معهد الفيزياء عالية الطاقة، الأكاديمية الصينية للعلوم، بكين 100049، الصين. مختبر المواد المتقدمة، قسم الكيمياء غير العضوية – المعهد الجامعي للمواد في أليكانتي، جامعة أليكانتي، صندوق بريد 99، E-03080 أليكانتي، إسبانيا. المواد الحفازة المتقدمة (ACM)، مركز التحفيز بجامعة الملك عبد الله للعلوم والتقنية (KCC)، جامعة الملك عبد الله للعلوم والتقنية، ثول، المملكة العربية السعودية. برنامج الهندسة الكيميائية، قسم العلوم الفيزيائية والهندسة (PSE)، جامعة الملك عبد الله للعلوم والتقنية، ثول، المملكة العربية السعودية. البريد الإلكتروني: pedro.castano@kaust.edu.sa
DOI: https://doi.org/10.1038/s41467-024-46388-4
PMID: https://pubmed.ncbi.nlm.nih.gov/38448464
Publication Date: 2024-03-06
Copper nanoparticles encapsulated in zeolitic imidazolate framework- 8 as a stable and selective
Accepted: 23 February 2024
Published online: 06 March 2024
(A) Check for updates
Abstract
Metal-organic frameworks have drawn attention as potential catalysts owing to their unique tunable surface chemistry and accessibility. However, their application in thermal catalysis has been limited because of their instability under harsh temperatures and pressures, such as the hydrogenation of
selectivity
disposition of the Cu nanoparticles and adsorption capacity, and activity-stability test of
Results
Synthesis of Cu species in ZIF-8

after reduction at 523 K to 723 K .
shift of the peaks is seen before reducing the sample, thus reinforcing the idea that Cu is incorporated into the structure after reduction treatment. Cu phases were not reported after reduction at 523 K , which could be attributed to Cu particles being highly dispersed in the catalyst. Reduction at high temperatures ( 623 K and 723 K ) showed reduced copper diffraction lines mostly due to the sintering process possible for copper when subject to high temperatures (
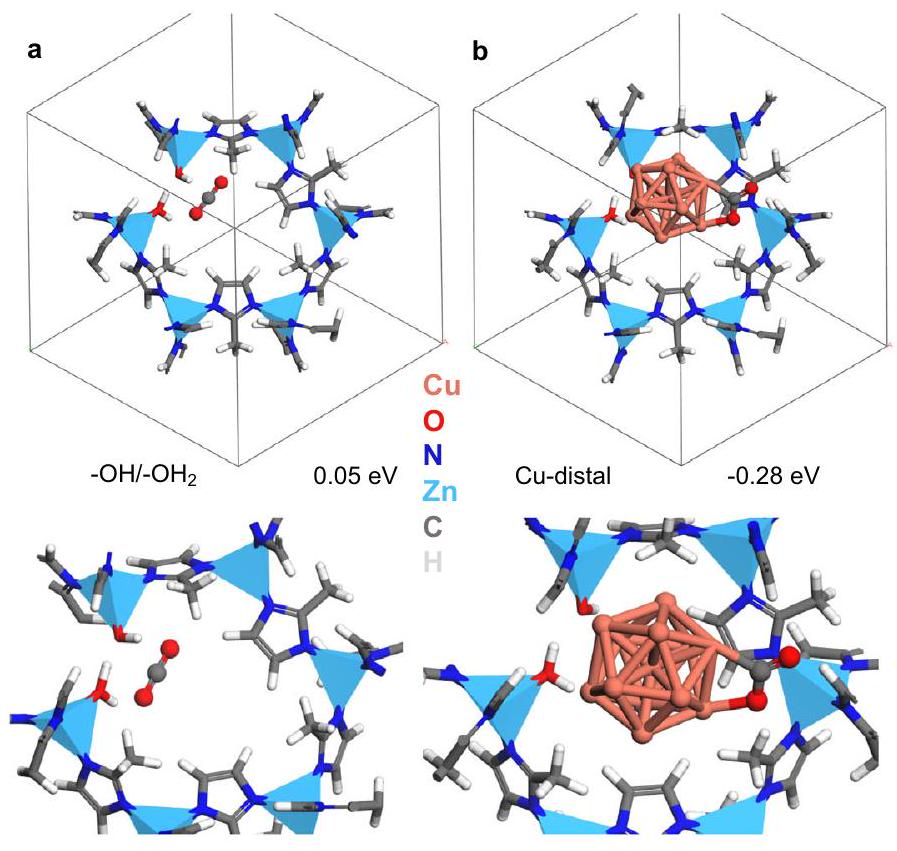
Structure and chemical environment of



Catalytic activity, selectivity, and stability for

catalysts. d Powder XRD patterns, (e) HR-TEM images of Cu/ZIF-8|IE|R-100 h. f Particle size distribution of the Cu particles as obtained in the HR-TEM images for the Cu/ZIF-8 | IE | R-100 h. g Pictorial representation of different ZIF-8 treatment processes ultimately leading to Cu nanoparticle formation under
diffraction peak at
In situ IR for reaction mechanism

three distinct


Discussion
Methods
Catalyst synthesis
support was impregnated with Cu nitrate using ethanol as a solvent with constant stirring for 2 h at 300 K . The solvent was evaporated by drying at 353 K and the resulting
Material characterization
In situ IR studies
Density functional theory (DFT) calculations
augmented wave (PAW) pseudopotentials
Catalytic tests
Data availability
References
- Zhu, Y. et al. Copper-zirconia interfaces in UiO-66 enable selective catalytic hydrogenation of CO2 to methanol. Nat. Commun. 11, 1-11 (2020).
- Zhu, Y. et al. Inverse iron oxide/metal catalysts from galvanic replacement. Nat. Commun. 11, 1-7 (2020).
- Wang, X., Shi, H. & Szanyi, J. Controlling selectivities in CO2 reduction through mechanistic understanding. Nat. Commun. 8, 1-6 (2017).
- Graciani, J. et al. Highly active copper-ceria and copper-ceria-titania catalysts for methanol synthesis from
. Science (80-). 345, 546-CO550 (2014). - Sehested, J. Industrial and scientific directions of methanol catalyst development. J. Catal. 371, 368-375 (2019).
- Bonura, G., Cordaro, M., Cannilla, C., Arena, F. & Frusteri, F. The changing nature of the active site of
catalysts for the CO 2 hydrogenation reaction to methanol. Appl. Catal. B Environ. 152-153, 152-161 (2014). - Kuld, S. et al. Quantifying the promotion of Cu catalysts by ZnO for methanol synthesis. Science (80-.). 352, 969-974 (2016).
- Martin, O. et al. Operando synchrotron X-ray powder diffraction and modulated-excitation infrared spectroscopy elucidate the CO2 promotion on a commercial methanol synthesis catalyst. Angew. Chemie Int. Ed. 55, 11031-11036 (2016).
- Beck, A. et al. Following the structure of copper-zinc-alumina across the pressure gap in carbon dioxide hydrogenation. Nat. Catal. 202146 4, 488-497 (2021).
- Prieto, G., Zečević, J., Friedrich, H., De Jong, K. P. & De Jongh, P. E. Towards stable catalysts by controlling collective properties of supported metal nanoparticles. Nat. Mater. 2012121 12, 34-39 (2012).
- Martin, O. & Pérez-Ramírez, J. New and revisited insights into the promotion of methanol synthesis catalysts by
. Catal. Sci. Technol. 3, 3343-3352 (2013). - Bavykina, A. et al. Turning a methanation Co catalyst into an In-Co methanol producer. ACS Catal. 9, 6910-6918 (2019).
- Frei, M. S. et al. Nanostructure of nickel-promoted indium oxide catalysts drives selectivity in CO2 hydrogenation. Nat. Commun. 2021121 12, 1-9 (2021).
- Rungtaweevoranit, B. et al. Copper nanocrystals encapsulated in Zr-based metal-organic frameworks for highly selective CO2 hydrogenation to methanol. Nano Lett. 16, 7645-7649 (2016).
- Jiang, H. L. et al. Au@ZIF-8: CO oxidation over gold nanoparticles deposited to metal-organic framework. J. Am. Chem. Soc. 131, 11302-11303 (2009).
- Jiang, H. L., Akita, T., Ishida, T., Haruta, M. & Xu, Q. Synergistic catalysis of Au@Ag core-shell nanoparticles stabilized on metalorganic framework. J. Am. Chem. Soc. 133, 1304-1306 (2011).
- Zhang, J. et al. Neighboring
Sites in a Metal-Organic Framework for CO2Hydrogenation. J. Am. Chem. Soc. 143, 8829-8837 (2021). - An, B. et al. Confinement of ultrasmall
nanoparticles in metal-organic frameworks for selective methanol synthesis from catalytic hydrogenation of . J. Am. Chem. Soc. 139, 3834-3840 (2017). - Sholl, D. S. & Lively, R. P. Defects in metal-organic frameworks: challenge or opportunity? J. Phys. Chem. Lett. 6, 3437-3444 (2015).
- Zhang, C., Han, C., Sholl, D. S. & Schmidt, J. R. Computational characterization of defects in metal-organic frameworks: spontaneous and water-induced point defects in ZIF-8. J. Phys. Chem. Lett. 7, 459-464 (2016).
- Ramos-Fernandez, E. V., Redondo-Murcia, A., Grau-Atienza, A., Sepúlveda-Escribano, A. & Narciso, J. Clean production of Zeolitic Imidazolate Framework 8 using Zamak residues as metal precursor and substrate. J. Clean. Prod. 260, 121081 (2020).
- Li, M. et al. Thermal stability of size-selected copper nanoparticles: Effect of size, support and CO2 hydrogenation atmosphere. Appl. Surf. Sci. 510, 145439 (2020).
- Thommes, M. et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 87, 1051-1069 (2015).
- Ta, D. N. et al. Preparation of nano-ZIF-8 in methanol with high yield. Can. J. Chem. Eng. 96, 1518-1531 (2018).
- Nordin, N. A. H. M., Ismail, A. F., Mustafa, A., Murali, R. S. & Matsuura, T. The impact of ZIF-8 particle size and heat treatment on
CH4 separation using asymmetric mixed matrix membrane. RSC Adv. 4, 52530-52541 (2014). - Jamil, N. et al. Green one-pot synthesis and characterisation of hybrid reduced graphene oxide/zeolitic imidazole framework-8 (rGO/ZIF-8.J. Iran. Chem. Soc. 18, 363-373 (2021).
- Yue, B., Li, Q., Iwai, H., Kako, T. & Ye, J. Hydrogen production using zinc-doped carbon nitride catalyst irradiated with visible light. Sci. Technol. Adv. Mater. 12, 34401-34408 (2011).
- Ramos-Fernández, E. V., Ferreira, A. F. P., Sepúlveda-Escribano, A., Kapteijn, F. & Rodríguez-Reinoso, F. Enhancing the catalytic performance of
in the selective hydrogenation of cinnamaldehyde by Cr addition to the support. J. Catal. 258, 52-60 (2008). - Zhen, S. Y. et al. Metal-organic framework derived hollow porous
dodecahedrons as a cathode catalyst for batteries. RSC Adv. 9, 16288-16295 (2019). - La Rosa-Toro, A. et al. Preparation and characterization of copperdoped cobalt oxide electrodes. J. Phys. Chem. B 110, 24021-24029 (2006).
- Wang, W., Qu, Z., Song, L. & Fu, Q. CO2 hydrogenation to methanol over
and catalysts: Tuning methanol selectivity via metal-support interaction. J. Energy Chem. 40, 22-30 (2020). - Liao, F. et al. Morphology-dependent interactions of ZnO with Cu nanoparticles at the materials’ interface in selective hydrogenation of CO 2 to CH 3 OH . Angew. Chemie Int. Ed. 50, 2162-2165 (2011).
- Li, D., Wang, G., Cheng, L., Wang, C. & Mei, X. Engineering the selfassembly induced emission of copper nanoclusters as 3D nanomaterials with mesoporous sphere structures by the crosslinking of Ce3. ACS Omega 3, 14755-14765 (2018).
- Frei, E. et al. Cu-Zn alloy formation as unfavored state for efficient methanol catalysts. ChemCatChem 12, 4029-4033 (2020).
- Newton, M. A. et al. On the mechanism underlying the direct conversion of methane to methanol by copper hosted in zeolites; braiding Cu K-edge XANES and reactivity studies. J. Am. Chem. Soc. 140, 10090-10093 (2018).
- Nian, J. N., Chen, S. A., Tsai, C. C. & Teng, H. Structural feature and catalytic performance of Cu species distributed over TiO 2 nanotubes. J. Phys. Chem. B 110, 25817-25824 (2006).
- Yuan, L. et al. Dynamic evolution of atomically dispersed Cu species for CO2 photoreduction to solar fuels. ACS Catal. 9, 4824-4833 (2019).
- Ma, Q., Buchholz, D. B. & Chang, R. P. H. Local structures of copperdoped ZnO films. Phys. Rev. B – Condens. Matter Mater. Phys. 78, 214429 (2008).
- Boada, R. et al. Unraveling the molecular details of the ‘gate opening’ phenomenon in ZIF-8 with X-ray absorption spectroscopy. J. Phys. Chem. C 126, 5935-5943 (2022).
- Dalebout, R. et al. Insight into the nature of the ZnOx promoter during methanol synthesis. ACS Catal. 12, 6628-6639 (2022).
- Wang, Y. et al. Exploring the ternary interactions in
catalysts for efficient CO2 hydrogenation to methanol. Nat. Commun. 2019101 10, 1-10 (2019). - Graaf, G. H., Sijtsema, P. J. J. M., Stamhuis, E. J. & Joosten, G. E. H. Chemical equilibria in methanol synthesis. Chem. Eng. Sci. 41, 2883-2890 (1986).
- Hu, B. et al. Cu@ZIF-8 derived inverse
catalyst with sub-5 nm ZnO for efficient CO2 hydrogenation to methanol. Catal. Sci. Technol. 9, 2673-2681 (2019). - Beebe, T. P., Crowell, J. E. & Yates, J. T. Reaction of methyl chloride with alumina surfaces: a study of the methoxy surface species by transmission infrared spectroscopy. J. Phys. Chem. 92, 1296-1301 (2002).
- Fujita, S. I., Usui, M., Ohara, E. & Takezawa, N. Methanol synthesis from carbon dioxide at atmospheric pressure over
catalyst. Role of methoxide species formed on ZnO support. Catal. Lett. 1992 134 13, 349-358 (1992). - Wang, L. L. & Johnson, D. D. Density functional study of structural trends for late-transition-metal 13-atom clusters. Phys. Rev. B Condens. Matter Mater. Phys. 75, 235405 (2007).
- Fan, Q. Y., Sun, J. J., Wang, F. & Cheng, J. Adsorption-induced liquid-to-solid phase transition of Cu clusters in catalytic dissociation of
. J. Phys. Chem. Lett. 11, 7954-7959 (2020). - Natesakhawat, S. et al. Active sites and structure-activity relationships of copper-based catalysts for carbon dioxide hydrogenation to methanol. ACS Catal. 2, 1667-1676 (2012).
- Tang, W., Sanville, E. & Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter 21, 084204 (2009).
- Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169-11186 (1996).
- Lewis, D. W. et al. Zeolitic imidazole frameworks: structural and energetics trends compared with their zeolite analogues. CrystEngComm 11, 2272-2276 (2009).
- Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953 (1994).
- Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15-50 (1996).
- Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
- Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
- Pack, J. D ., & Monkhorst, H. J. Special points for Brillonin-zone integrations*. Phys. Rev. B 13, 5188-5192 (1976).
- Zhang, W. & Xiao, Y. Mechanism of electrocatalytically active precious metal (Ni, Pd, Pt, and Ru) complexes in the graphene basal plane for ORR applications in novel fuel cells. Energy Fuels 34, 2425-2434 (2020).
- Ahmad, R. & Singh, A. K. Synergistic core-shell interactions enable ultra-low overpotentials for enhanced CO 2 electro-reduction activity. J. Mater. Chem. A 6, 21120-21130 (2018).
Acknowledgements
Author contributions
Competing interests
Additional information
Supplementary Material available at
https://doi.org/10.1038/s41467-024-46388-4.
http://www.nature.com/reprints
© The Author(s) 2024
Multiscale Reaction Engineering, KAUST Catalysis Center (KCC), King Abdullah University of Science and Technology (KAUST), Thuwal 23955-6900, Saudi Arabia. KAUST Catalysis Center (KCC), King Abdullah University of Science and Technology (KAUST), Thuwal 23955-6900, Saudi Arabia. King Abdullah University of Science and Technology (KAUST), Physical Sciences and Engineering Division, Advanced Membranes and Porous Materials (AMPM) Center, Thuwal 23955-6900, Saudi Arabia. Beijing Synchrotron Radiation Facility, Institute of High Energy Physics, Chinese Academy of Sciences, Beijing 100049, China. Laboratorio de Materiales Avanzados, Departamento de Química Inorgánica – Instituto Universitario de Materiales de Alicante, Universidad de Alicante, Apartado 99, E-03080 Alicante, Spain. Advanced Catalytic Materials (ACM), KAUST Catalysis Center (KCC), KAUST, Thuwal, Saudi Arabia. Chemical Engineering Program, Physical Science and Engineering (PSE) Division, KAUST, Thuwal, Saudi Arabia. e-mail: pedro.castano@kaust.edu.sa
