DOI: https://doi.org/10.1038/s41413-024-00319-7
PMID: https://pubmed.ncbi.nlm.nih.gov/38424439
تاريخ النشر: 2024-02-29
جزيئات نانوية قائمة على رباعي السطوح DNA لتثبيط الفيروبتوز: توصيل متفوق للكركمين وتخفيف هشاشة العظام السكري
الملخص
هشاشة العظام الناتجة عن السكري (DOP) هي مضاعفة هامة تشكل تهديدًا مستمرًا لصحة العظام لدى مرضى السكري؛ ومع ذلك، لا توجد حاليًا استراتيجيات علاجية فعالة. في مرضى السكري، تؤثر المستويات المرتفعة من الفيروبتوزيس على الالتزام العظمي وتمايز خلايا الساق العظمية (BMSCs)، مما يؤدي إلى تغييرات هيكلية كبيرة. لمعالجة هذه المشكلة، هدفنا إلى استهداف الفيروبتوزيس واقتراح نهج علاجي جديد لعلاج DOP. قمنا بتخليق جزيئات نانوية مثبطة للفيروبتوزيس، والتي يمكن أن توصل الكركمين، وهو مركب طبيعي، إلى نخاع العظام باستخدام الحمض النووي الإطاري الرباعي (tFNA). أظهر نظام التوصيل هذا توافر حيوي ممتاز واستقرار للكركمين، بالإضافة إلى خصائص تآزرية مع tFNA. كشفت التجارب في المختبر وفي الجسم الحي أن الجزيئات النانوية يمكن أن تعزز وظيفة الميتوكوندريا من خلال تنشيط مسار عامل النواة E2 المرتبط بالعامل 2 (NRF2)/بيروكسيداز الجلوتاثيون 4 (GPX4)، مما يثبط الفيروبتوزيس، ويعزز التمايز العظمي لـ BMSCs في البيئة الدقيقة للسكري، ويقلل من فقدان العظام الإسفنجية، ويزيد من تكوين العظام. تشير هذه النتائج إلى أن الجزيئات النانوية المثبطة للفيروبتوزيس المستندة إلى الحمض النووي الرباعي المحتوي على الكركمين لديها إمكانات واعدة لعلاج DOP وأمراض أخرى مرتبطة بالفيروبتوزيس.
مقدمة
نُشر على الإنترنت: 29 فبراير 2024
النتائج
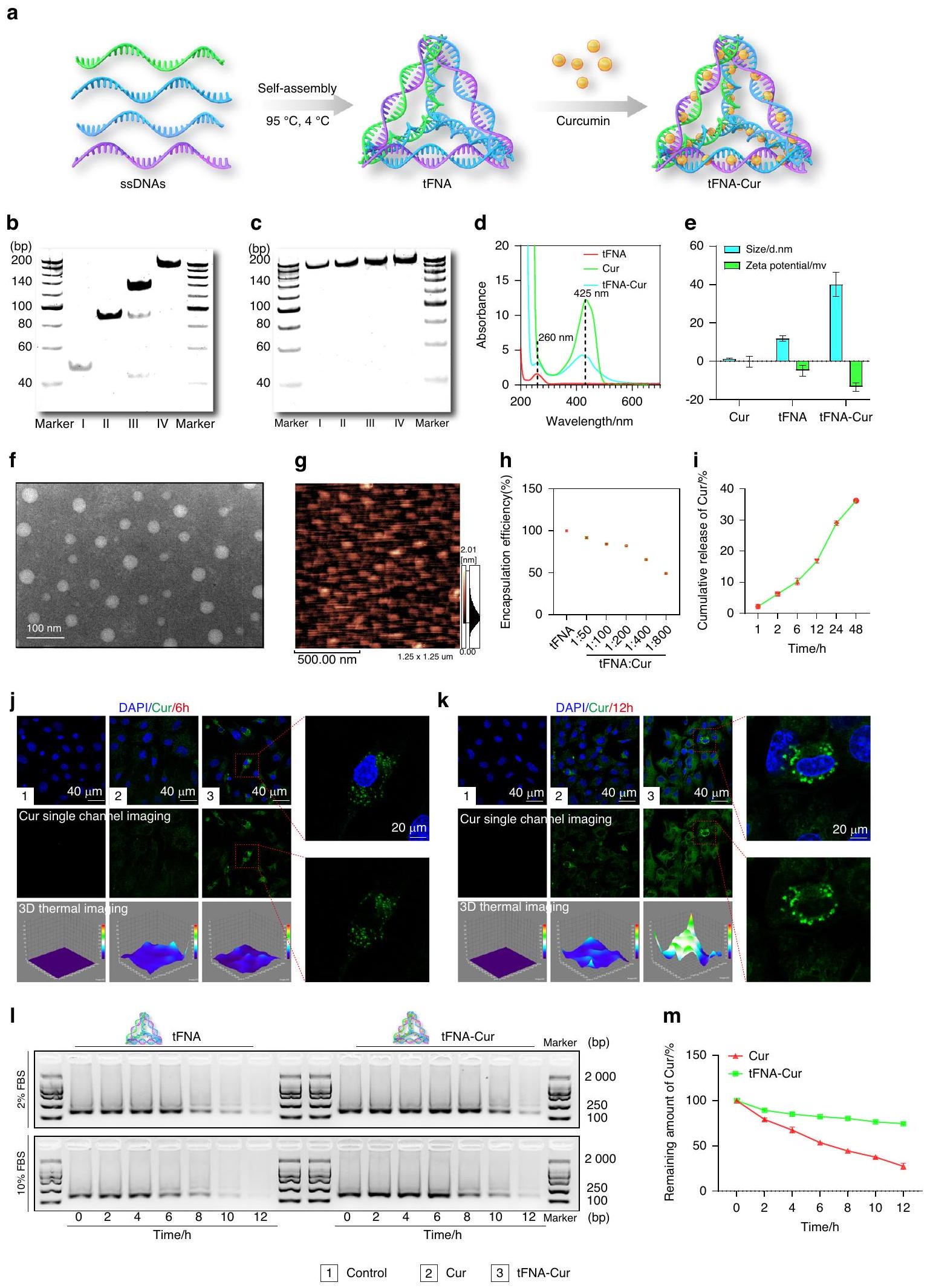
أداء الجسيمات النانوية المثبطة للفيروبتوز
المبلغ المتبقي يصل فقط إلى
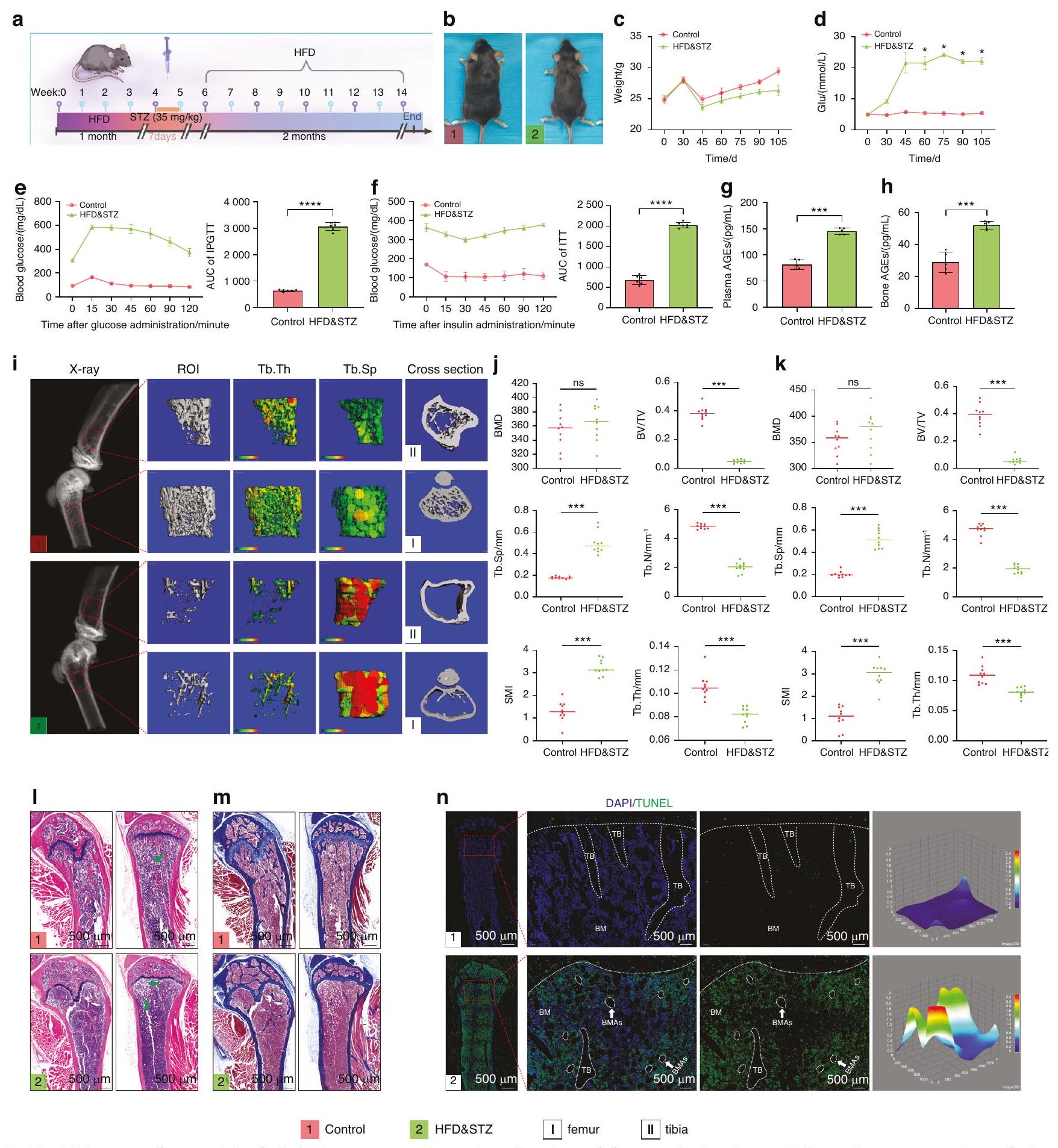
قمنا بإنشاء نموذج للداء السكري من النوع الثاني باستخدام نظام غذائي عالي الدهون (HFD) وجرعة منخفضة من ستربتوزوتوسين (STZ) (الشكل 2أ). تم إجراء تقديرات أسبوعية لوزن الجسم ومستويات الجلوكوز في البلازما، وتم إجراء اختبار تحمل الجلوكوز داخل الصفاق (IPGTT) واختبار تحمل الأنسولين (ITT) في يوم القتل الرحيم. استمرت مستويات الجلوكوز في البلازما في الفئران المعالجة بـ HFD&STZ في الارتفاع، بينما انخفضت أوزانها (الشكل 2ب-د). أظهر IPGTT وITT ضعف تحمل الجلوكوز ومقاومة الأنسولين في الفئران المعالجة بـ HFD&STZ (الشكل 2هـ، و). كما دعمت مقاطع البنكرياس هذه النتيجة (الشكل S3). من المثير للاهتمام، أننا وجدنا أن مستويات المنتجات النهائية المتقدمة للجليكوزيل (AGEs) في البلازما والعظام لدى الفئران المعالجة بـ HFD&STZ قد زادت أيضًا (الشكل 2ز، ح). تُعرف AGEs بأنها علامات على مرض السكري، وتراكمها يؤدي إلى مضاعفات مختلفة.
زيادة القدرة الأوستيوجينية لخلايا النخاع العظمي الجذعية في بيئة ميكروية سكرية بواسطة tFNA-Cur
تم تثبيطها بشكل كبير في مجموعة العلاج ولكن تم عكسها بواسطة معالجة tFNA-Cur (الشكل 3i-I). تم دعم هذه النتائج بشكل أكبر من خلال تحليلات مستوى البروتين (الشكل 3m-q، الشكل S7a-c). ALP (علامة للخلايا العظمية)، OSX (علامة محددة لخلايا سلف الخلايا العظمية) وRUNX2 (علامة للخلايا العظمية الناضجة)
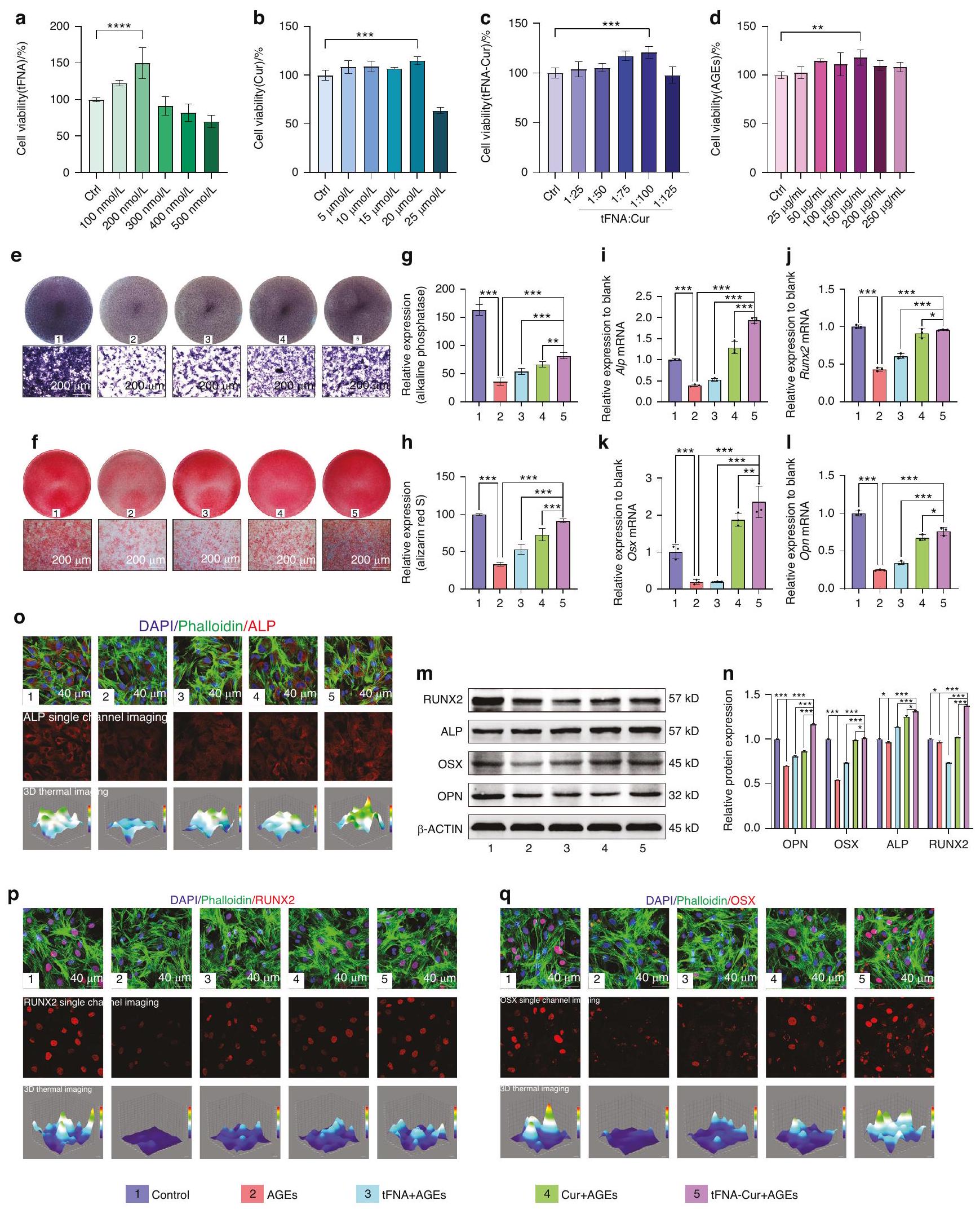
تم تحديدها باستخدام تقنية الوسترن بلوت (WB) والتألق المناعي. أظهرت الأنماط المتسقة أن tFNA-Cur عزز بشكل كبير تكوين العظام في البيئة الدقيقة السكري مقارنةً بـ tFNA أو الكركمين بمفرده.
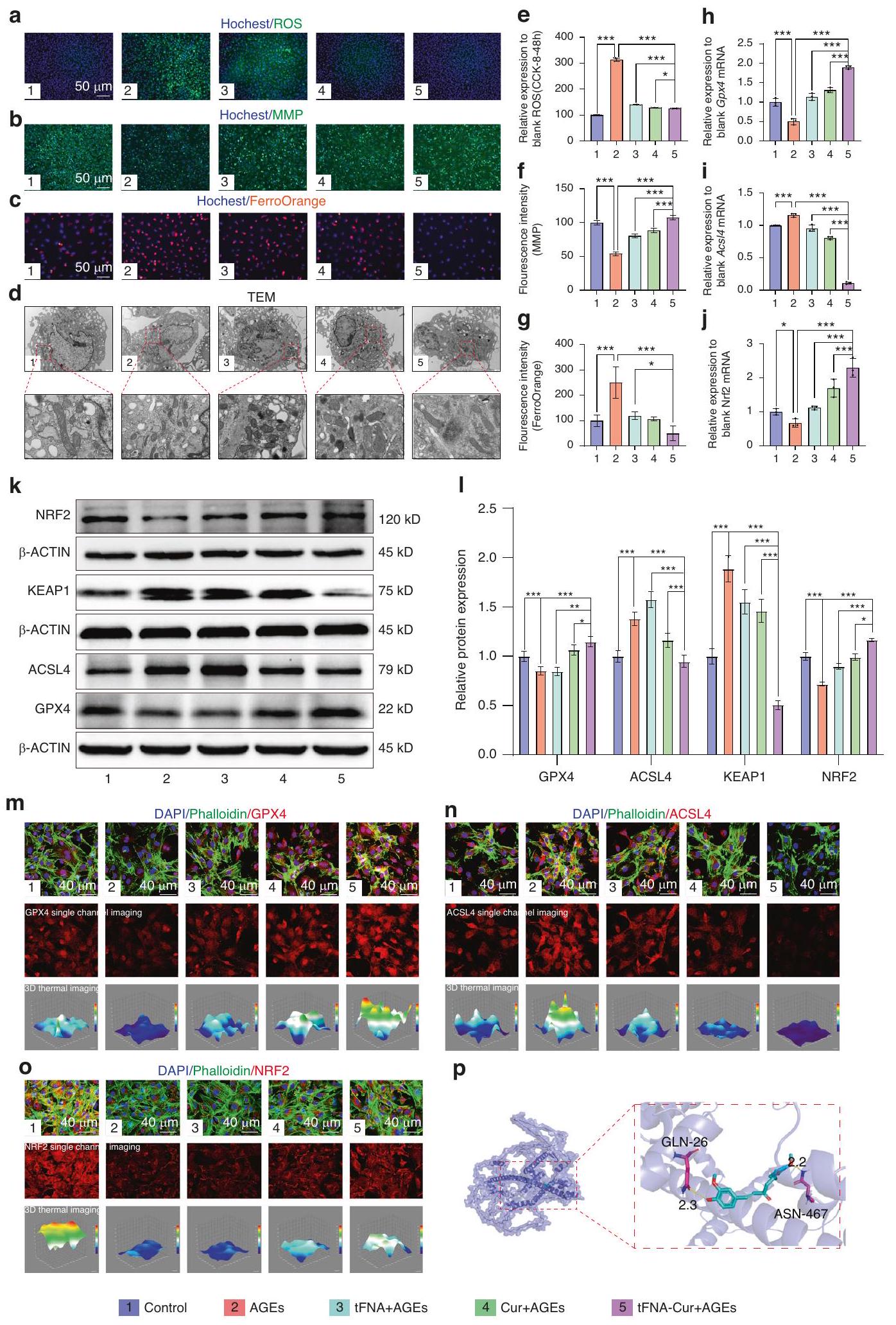
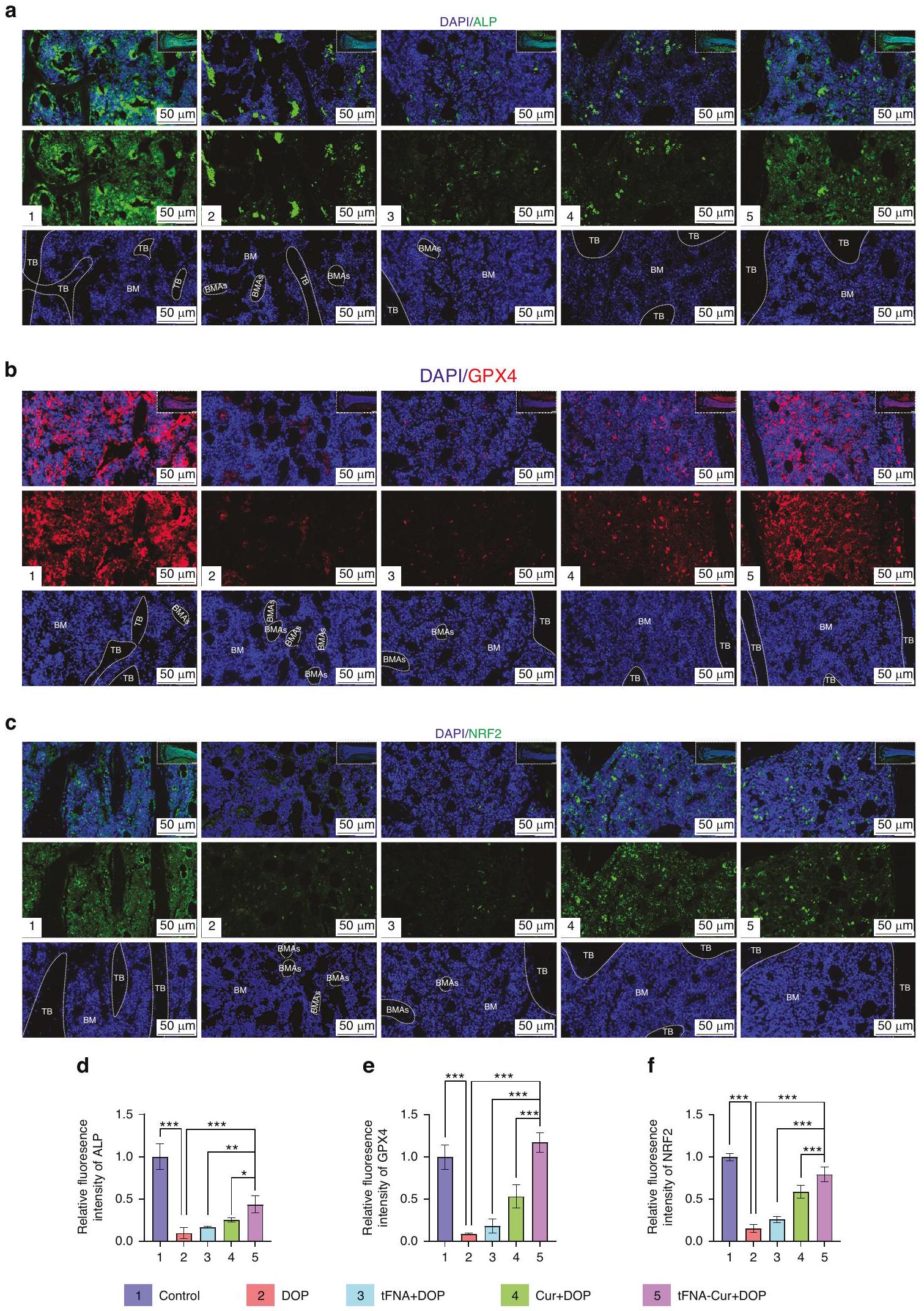
تثبط FNA-Cur الموت الخلوي الناتج عن الحديد بسبب AGEs عبر مسار NRF2/GPX4
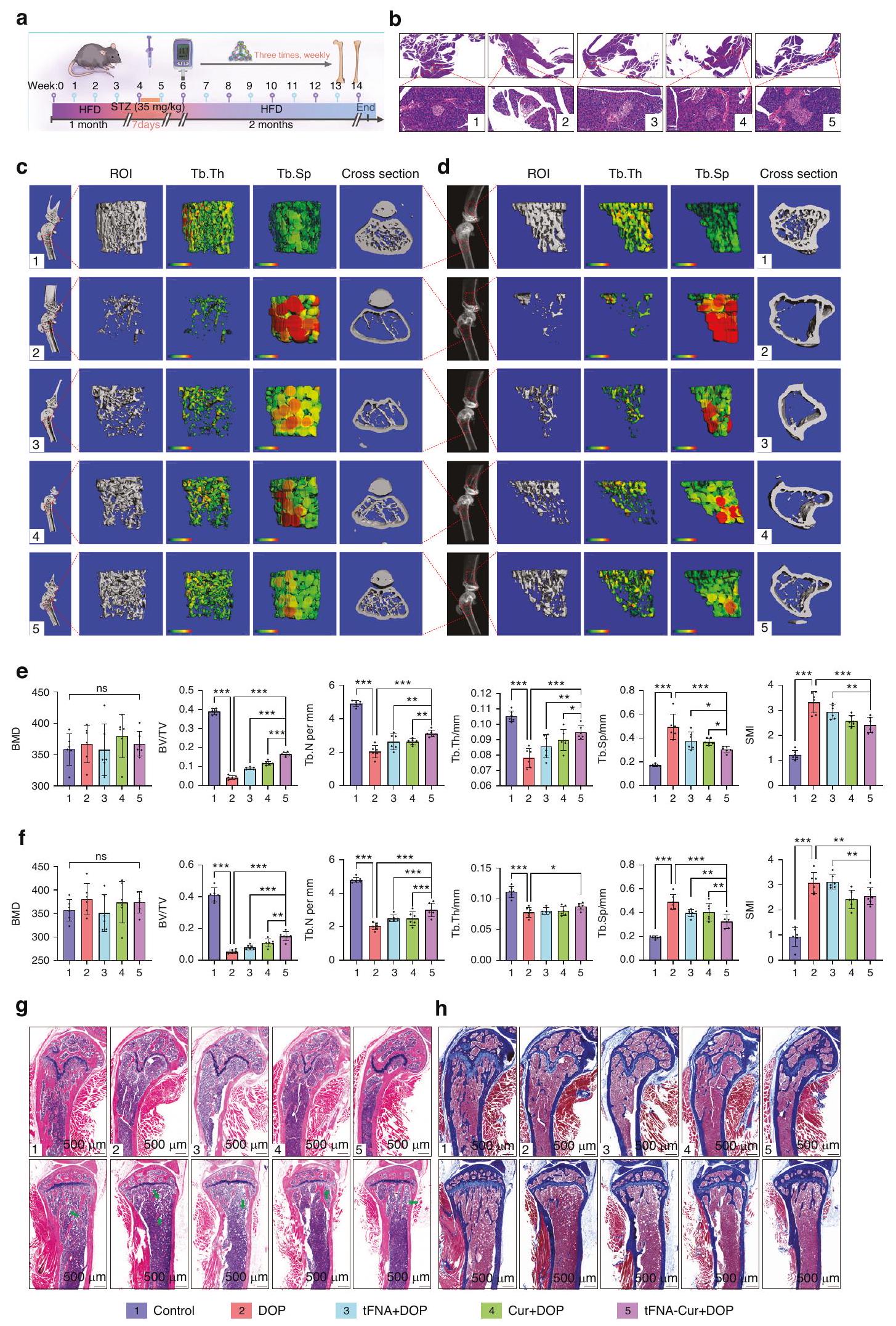
استهداف الفيروبتوزيس يخفف من DOP باستخدام tFNA-Cur
سلامة التربيق وتقليل فقدان العظام بشكل فعال عند مقارنتها بمجموعات العلاج الأخرى. وقد دعمت هذه النتائج المزيد من خلال صبغات H&E وماسون، مما كشف عن زيادة كبيرة في وفرة التربيقات وألياف الكولاجين (الشكل 5g، h). ومن المثير للاهتمام أن فئران DOP أظهرت زيادة في تراكم الخلايا الدهنية في نخاع العظام (BMAs)، وهو ما يتماشى مع الأبحاث السابقة (الشكل S9a-c).
نقاش
تم استخدام FNA، كمواد نانوية متعددة الوظائف، على نطاق واسع في مجالات العلوم الطبية الحيوية المختلفة لتوصيل الحمض النووي DNA، والحمض النووي الريبي RNA، والببتيدات، والمركبات الصغيرة.
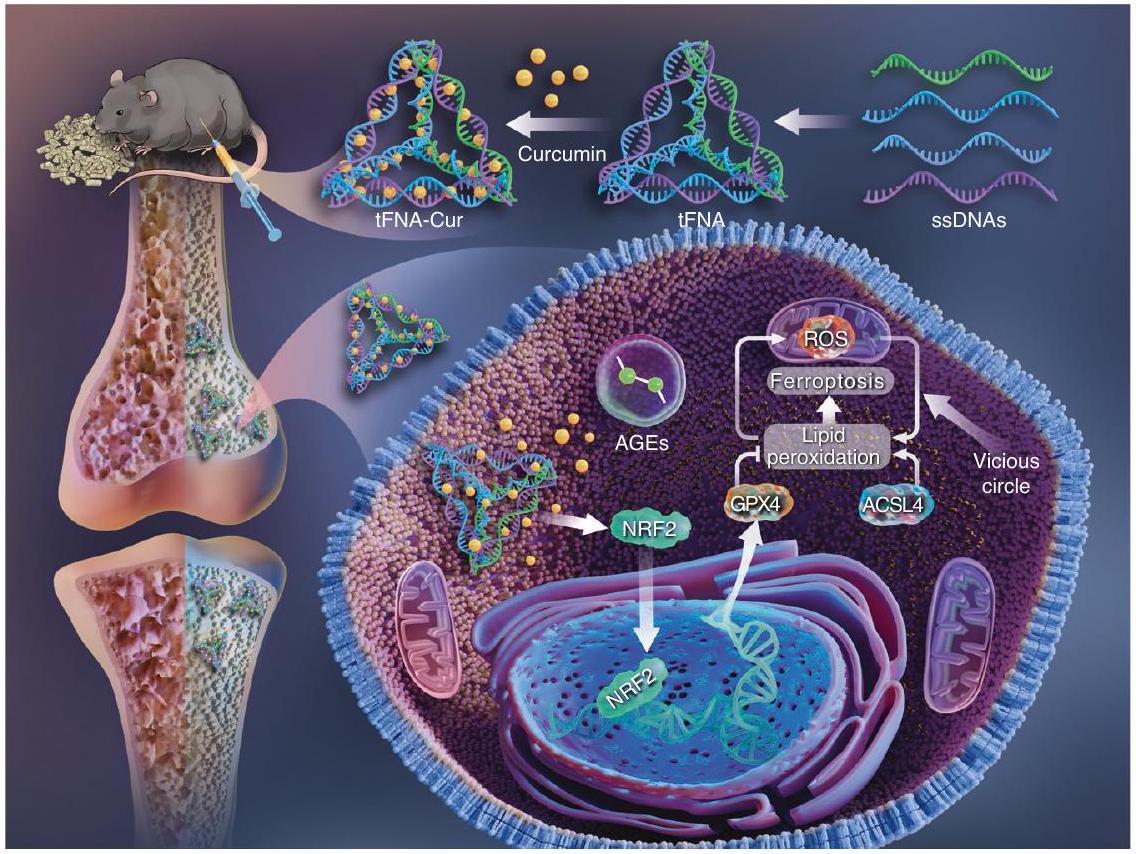
تغليف غشاء خلايا جذعية من النخاع العظمي على الجسيمات النانوية المثبطة للفيروبتوز.
المواد والأساليب
تصنيع tFNA-Cur
توصيف tFNA-Cur
استنادًا إلى الأبحاث السابقة،
تم استخدامه لتحليل نتائج الاستقرار. أخيرًا، تم التقاط صور لـ tFNA و tFNA-Cur باستخدام جهاز التعرض للأشعة فوق البنفسجية (Bio-Rad، هيركوليس، الولايات المتحدة الأمريكية).
عزل وزراعة خلايا جذعية من نخاع العظم
امتصاص خلايا جذعية من النخاع العظمي للكركمين و tFNA-Cur
صبغة ALP والأليزارين الأحمر
اختبار الكشف عن مستوى ROS و MMP
صبغة فيروأورانج
تم
تحليل RT-PCR الكمي
تحليل البقعة الغربية
تلطيخ IF
تجارب الحيوانات
تم حقن المجموعة داخل الصفاق بـ STZ (
تحليلات الميكرو-سي تي
فحص AGEs في العظام والمصل
اختبار TUNEL
التحليل النسيجي
النمذجة الجزيئية ثلاثية الأبعاد والتثبيت للكرسيتين إلى NRF2
التحليل الإحصائي
شكر وتقدير
مساهمات المؤلفين
معلومات إضافية
REFERENCES
- Shanbhogue, V., Mitchell, D., Rosen, C. & Bouxsein, M. Type 2 diabetes and the skeleton: new insights into sweet bones. Lancet. Diabetes Endocrinol. 4, 159-173 (2016).
- Napoli, N. et al. Mechanisms of diabetes mellitus-induced bone fragility. Nat. Rev. Endocrinol. 13, 208-219 (2017).
- Shanbhogue, V. V., Hansen, S., Frost, M., Brixen, K. & Hermann, A. P. Bone disease in diabetes: another manifestation of microvascular disease? Lancet Diabetes Endocrinol. 5, 827-838 (2017).
- Devlin, M. J. & Rosen, C. J. The bone-fat interface: basic and clinical implications of marrow adiposity. Lancet Diabetes Endocrinol. 3, 141-147 (2015).
- Greenhill, C. Shared variants for osteoporosis and T2DM. Nat. Rev. Endocrinol. 14, 627 (2018).
- Sheu, A., Greenfield, J., White, C. & Center, J. Assessment and treatment of osteoporosis and fractures in type 2 diabetes. Trends Endocrinol. Metab. 33, 333-344 (2022).
- Khosla, S., Samakkarnthai, P., Monroe, D. G. & Farr, J. N. Update on the pathogenesis and treatment of skeletal fragility in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 17, 685-697 (2021).
- Hofbauer, L. et al. Bone fragility in diabetes: novel concepts and clinical implications. Lancet Diabetes Endocrinol. 10, 207-220 (2022).
- Ru, Q. et al. Fighting age-related orthopedic diseases: focusing on ferroptosis. Bone Res. 11, 12 (2023).
- Balogh, E. et al. Iron overload inhibits osteogenic commitment and differentiation of mesenchymal stem cells via the induction of ferritin. Biochim. Biophys. Acta Mol. Basis Disease 1862, 1640-1649 (2016).
- Yang, Y. et al. Targeting ferroptosis suppresses osteocyte glucolipotoxicity and alleviates diabetic osteoporosis. Bone Res. 10, 26 (2022).
- Tong, L. et al. Current understanding of osteoarthritis pathogenesis and relevant new approaches. Bone Res. 10, 60 (2022).
- Xue, Y. et al. Alkaline “nanoswords” coordinate ferroptosis-like bacterial death for antibiosis and osseointegration. ACS Nano 17, 2711-2724 (2023).
- Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060-1072 (2012).
- Tonnus, W. et al. The role of regulated necrosis in endocrine diseases. Nat. Rev. Endocrinol. 17, 497-510 (2021).
- Jiang, X., Stockwell, B. R. & Conrad, M. Ferroptosis: mechanisms, biology, and role in disease. Nat. Rev. Mol. Cell Biol. 22, 266-282 (2021).
- Mishima, E. et al. A non-canonical vitamin K cycle is a potent ferroptosis suppressor. Nature 608, 778-783 (2022).
- Barayeu, U. et al. Hydropersulfides inhibit lipid peroxidation and ferroptosis by scavenging radicals. Nat. Chem. Biol. 19, 28-37 (2022).
- Stockwell, B. R. Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications. Cell 185, 2401-2421 (2022).
- Tang, D., Chen, X., Kang, R. & Kroemer, G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 31, 107-125 (2021).
- Mao, C. et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 593, 586-590 (2021).
- Cui, S. et al. Autophagosomes defeat ferroptosis by decreasing generation and increasing discharge of free
in skin repair cells to accelerate diabetic wound healing. Adv. Sci. 10, e2300414 (2023). - Liang, D. et al. Ferroptosis surveillance independent of GPX4 and differentially regulated by sex hormones. Cell 186, 2748-2764.e2722 (2023).
- Hu, X. et al. Optineurin regulates NRF2-mediated antioxidant response in a mouse model of Paget’s disease of bone. Sci. Adv. 9, eade6998 (2023).
- Yue, L. et al. Chemotaxis-guided self-propelled macrophage motor for targeted treatment of acute pneumonia. Adv. Mater. 35, e2211626 (2023).
- Wang, D. et al. Cell Membrane vesicles with enriched CXCR4 display enhances their targeted delivery as drug carriers to inflammatory sites. Adv. Sci. 8, e2101562 (2021).
- Rashwan, A. K. et al. An updated and comprehensive review on the potential health effects of curcumin-encapsulated micro/nanoparticles. Crit. Rev. Food Sci. Nutr. 63, 9731-9751 (2023).
- Chen, Z. et al. Multifaceted role of phyto-derived polyphenols in nanodrug delivery systems. Adv. Drug Deliv. Rev. 176, 113870 (2021).
- Tian, T. et al. A dynamic DNA tetrahedron framework for active targeting. Nat. Protoc. 18, 1028-1055 (2023).
- Zhang, T. et al. Design, fabrication and applications of tetrahedral DNA nanostructure-based multifunctional complexes in drug delivery and biomedical treatment. Nat. Protoc. 15, 2728-2757 (2020).
- Li, J. et al. Modulation of the crosstalk between schwann cells and macrophages for nerve regeneration: a therapeutic strategy based on multifunctional tetrahedral framework nucleic acids system. Adv. Mater. 34, e2202513 (2022).
- Ma, W. et al. Biomimetic nanoerythrosome-coated aptamer-DNA tetrahedron/ maytansine conjugates: pH-responsive and targeted cytotoxicity for HER2positive breast cancer. Adv. Mater. 34, e2109609 (2022).
- Wang, Y. et al. Tetrahedral framework nucleic acids can alleviate taurocholateinduced severe acute pancreatitis and its subsequent multiorgan injury in mice. Nano Lett. 22, 1759-1768 (2022).
- Zhang, Y. et al. Multi-targeted antisense oligonucleotide delivery by a framework nucleic acid for inhibiting biofilm formation and virulence. Nanomicro Lett. 12, 74 (2020).
- Gao, Y. et al. A LYsosome-activated tetrahedral nanobox for encapsulated sirna delivery. Adv. Mater. 34, e2201731 (2022).
- Li, S. et al. A tetrahedral framework DNA-based bioswitchable mirna inhibitor delivery system: application to skin anti-aging. Adv. Mater. 34, e2204287 (2022).
- Zhang, T. et al. Myelosuppression alleviation and hematopoietic regeneration by tetrahedral-framework nucleic-acid nanostructures functionalized with osteogenic growth peptide. Adv. Sci. 9, e2202058 (2022).
- Liu, Z. et al. Suppression of lipopolysaccharide-induced sepsis by tetrahedral framework nucleic acid loaded with quercetin. Adv. Funct. Mater. 32, 2204587 (2022).
- Yan, R. et al. Typhaneoside-tetrahedral framework nucleic acids system: mitochondrial recovery and antioxidation for acute kidney injury treatment. ACS Nano 17, 8767-8781 (2023).
- Zhao, Y. et al. Effects of puerarin-loaded tetrahedral framework nucleic acids on osteonecrosis of the femoral head. Small 19, e2302326 (2023).
- Gao, S. et al. Tetrahedral framework nucleic acids induce immune tolerance and prevent the onset of type 1 diabetes. Nano Lett. 21, 4437-4446 (2021).
- Li, Y. et al. Tetrahedral framework nucleic acid-based delivery of resveratrol alleviates insulin resistance: from innate to adaptive immunity. Nanomicro Lett. 13, 86 (2021).
- Li, Y. et al. Tetrahedral framework nucleic acids ameliorate insulin resistance in type 2 diabetes mellitus via the PI3K/Akt Pathway. ACS Appl. Mater. Interfaces 13, 40354-40364 (2021).
- Wu, T. et al. METTL3-m(6) A methylase regulates the osteogenic potential of bone marrow mesenchymal stem cells in osteoporotic rats via the Wnt signalling pathway. Cell Prolif. 55, e13234 (2022).
- Li, Y. et al. Advanced glycation end products inhibit the osteogenic differentiation potential of adipose-derived stem cells by modulating Wnt/
-catenin signalling pathway via DNA methylation. Cell Prolif. 53, e12834 (2020). - Peng, S. et al. LncRNA-AK137033 inhibits the osteogenic potential of adiposederived stem cells in diabetic osteoporosis by regulating Wnt signaling pathway via DNA methylation. Cell Prolif. 55, e13174 (2022).
- Salhotra, A., Shah, H. N., Levi, B. & Longaker, M. T. Mechanisms of bone development and repair. Nat. Rev. Mol. Cell Biol. 21, 696-711 (2020).
- Li, S. et al. Bioswitchable delivery of microRNA by framework nucleic acids: application to bone regeneration. Small 17, e2104359 (2021).
- Eberhardt, J., Santos-Martins, D., Tillack, A. F. & Forli, S. AutoDock Vina 1.2.0: new docking methods, expanded force field, and python bindings. J. Chem. Inf. Model 61, 3891-3898 (2021).
- Drake, Z. C., Seffernick, J. T. & Lindert, S. Protein complex prediction using Rosetta, AlphaFold, and mass spectrometry covalent labeling. Nat. Commun. 13, 7846 (2022).
- Gao, M., Nakajima, An,D., Parks, J. M. & Skolnick, J. AF2Complex predicts direct physical interactions in multimeric proteins with deep learning. Nat. Commun. 13, 1744 (2022).
- Tevlin, R. et al. Pharmacological rescue of diabetic skeletal stem cell niches. Sci. Transl. Med. 9, eaag2809 (2017).
- Zhang, Y. et al. Co-delivery of doxorubicin and curcumin via cRGD-peptide modified PEG-PLA self-assembly nanomicelles for lung cancer therapy. Chin. Chem. Lett. 33, 2507-2511 (2022).
- Zhang, C. et al. Oral colon-targeted mucoadhesive micelles with enzymeresponsive controlled release of curcumin for ulcerative colitis therapy. Chin. Chem. Lett. 33, 4924-4929 (2022).
- Zhang, T., Tian, T. & Lin, Y. Functionalizing framework nucleic-acid-based nanostructures for biomedical application. Adv. Mater. 34, e2107820 (2022).
- Shi, S. et al. Amelioration of osteoarthritis via tetrahedral framework nucleic acids delivering microrna-124 for cartilage regeneration. Adv. Funct. Mater. 33, 202305558 (2023).
- Zhang, T., et al Nanomaterials targeting toll-like receptor 4 prevent bispho-sphonate-related osteonecrosis of the jaw via regulating mitochondrial homeostasis in macrophages. Adv. Funct. Mater. 33, 202213401 (2023).
- Zhou, B. O., Yue, R., Murphy, M. M., Peyer, J. G. & Morrison, S. J. Leptin-receptorexpressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 15, 154-168 (2014).
- Sun, Y. et al. The long noncoding RNA Inc-ob1 facilitates bone formation by upregulating Osterix in osteoblasts. Nat. Metab. 1, 485-496 (2019).
- Lutz, T. A. Mammalian models of diabetes mellitus, with a focus on type 2 diabetes mellitus. Nat. Rev. Endocrinol. 19, 350-360 (2023).
- Kallai, I. et al. Microcomputed tomography-based structural analysis of various bone tissue regeneration models. Nat. Protoc. 6, 105-110 (2011).
© The Author(s) 2024
State Key Laboratory of Oral Diseases & National Center for Stomatology & National Clinical Research Center for Oral Diseases, West China Hospital of Stomatology, Sichuan University, Chengdu, Sichuan 610041, PR China; ²Department of Oral and Maxillofacial Surgery, Affiliated Stomatological Hospital, Southwest Medical University, Luzhou, Sichuan 646000, PR China and Sichuan Provincial Engineering Research Center of Oral Biomaterials, Chengdu, Sichuan 610041, China
Correspondence: En Luo (luoen521125@sina.com) or Yunfeng Lin (yunfenglin@scu.edu.cn)
DOI: https://doi.org/10.1038/s41413-024-00319-7
PMID: https://pubmed.ncbi.nlm.nih.gov/38424439
Publication Date: 2024-02-29
A DNA tetrahedron-based ferroptosis-suppressing nanoparticle: superior delivery of curcumin and alleviation of diabetic osteoporosis
Abstract
Diabetic osteoporosis (DOP) is a significant complication that poses continuous threat to the bone health of patients with diabetes; however, currently, there are no effective treatment strategies. In patients with diabetes, the increased levels of ferroptosis affect the osteogenic commitment and differentiation of bone mesenchymal stem cells (BMSCs), leading to significant skeletal changes. To address this issue, we aimed to target ferroptosis and propose a novel therapeutic approach for the treatment of DOP. We synthesized ferroptosis-suppressing nanoparticles, which could deliver curcumin, a natural compound, to the bone marrow using tetrahedral framework nucleic acid (tFNA). This delivery system demonstrated excellent curcumin bioavailability and stability, as well as synergistic properties with tFNA. Both in vitro and in vivo experiments revealed that nanoparticles could enhance mitochondrial function by activating the nuclear factor E2-related factor 2 (NRF2)/glutathione peroxidase 4 (GPX4) pathway, inhibiting ferroptosis, promoting the osteogenic differentiation of BMSCs in the diabetic microenvironment, reducing trabecular loss, and increasing bone formation. These findings suggest that curcumin-containing DNA tetrahedron-based ferroptosissuppressing nanoparticles have a promising potential for the treatment of DOP and other ferroptosis-related diseases.
INTRODUCTION
Published online: 29 February 2024
RESULTS
Performance of ferroptosis-suppressing nanoparticle
residual amount reaching only
We established a model of DOP using a high-fat diet (HFD) and low-dose streptozotocin (STZ) (Fig. 2a). Biweekly estimations of body weight and plasma glucose levels were conducted, and inraperitoneal glucose tolerance test (IPGTT) and insulin tolerance test (ITT) were performed on the day of euthanasia. The plasma glucose levels in the HFD&STZ mice continued to rise, while their weights decreased (Fig. 2b-d). IPGTT and ITT demonstrated impaired glucose tolerance and insulin resistance in the HFD&STZ mice (Fig. 2e, f). Pancreatic sections also supported this result (Fig. S3). Interestingly, we found the levels of advanced glycation end products (AGEs) in the plasma and bone of HFD&STZ mice also increased (Fig. 2g, h). AGEs are known markers of diabetes, and their accumulation leads to various complications.
Increased osteogenic potential of BMSCs in diabetic microenvironment by tFNA-Cur


were substantially inhibited in the treatment group but reversed by tFNA-Cur pretreatment (Fig. 3i-I). These findings were further supported by protein level analyses (Fig. 3m-q, Fig. S7a-c). ALP (a marker for osteoblasts), OSX (a specific marker for osteoblast progenitor cells) and RUNX2 (a marker for mature osteoblasts)

were determined using western blot (WB) and immunofluorescence. Consistent patterns revealed that tFNA-Cur significantly promoted osteogenesis in the diabetic microenvironment compared to tFNA or curcumin alone.
tFNA-Cur suppresses AGEs-induced ferroptosis via NRF2/GPX4 pathway
Targeting ferroptosis alleviates DOP using tFNA-Cur
trabecular integrity and effectively reduced bone loss when compared to the other treatment groups. These findings were further supported by H&E and masson staining, revealing a substantial increase in the abundance of trabeculae and collagen fibers (Fig. 5g, h). Interestingly, DOP mice exhibited increased accumulation of bone marrow adipocytes (BMAs), consistent with prior research (Fig. S9a-c).
DISCUSSION
tFNA, as a multifunctional nanomaterial, has been widely applied in various fields of biomedical science to deliver DNA, RNA, peptides, and small-molecule compounds.




encapsulating the BMSCs membrane on the ferroptosissuppressing nanoparticle.
MATERIALS AND METHODS
Fabrication of tFNA-Cur
Characterization of tFNA-Cur
Based on previous research,
employed to analyze the stability results. Finally, images of tFNA and tFNA-Cur were captured using an ultraviolet exposure apparatus (Bio-Rad, Hercules, USA).
Isolation and culture of BMSCs
BMSCs uptake of curcumin and tFNA-Cur
ALP and alizarin red staining
ROS, MMP level detection assay
FerroOrange staining
TEM
Quantitative RT-PCR analysis
Western blot analysis
IF staining
Animal experiments
group were injected intraperitoneally with STZ (
Micro-CT analyses
AGEs examination in bone and serum
TUNEL assay
Histological analysis
3D molecular modeling and docking of curcumin to NRF2
Statistical analysis
ACKNOWLEDGEMENTS
AUTHOR CONTRIBUTIONS
ADDITIONAL INFORMATION
REFERENCES
- Shanbhogue, V., Mitchell, D., Rosen, C. & Bouxsein, M. Type 2 diabetes and the skeleton: new insights into sweet bones. Lancet. Diabetes Endocrinol. 4, 159-173 (2016).
- Napoli, N. et al. Mechanisms of diabetes mellitus-induced bone fragility. Nat. Rev. Endocrinol. 13, 208-219 (2017).
- Shanbhogue, V. V., Hansen, S., Frost, M., Brixen, K. & Hermann, A. P. Bone disease in diabetes: another manifestation of microvascular disease? Lancet Diabetes Endocrinol. 5, 827-838 (2017).
- Devlin, M. J. & Rosen, C. J. The bone-fat interface: basic and clinical implications of marrow adiposity. Lancet Diabetes Endocrinol. 3, 141-147 (2015).
- Greenhill, C. Shared variants for osteoporosis and T2DM. Nat. Rev. Endocrinol. 14, 627 (2018).
- Sheu, A., Greenfield, J., White, C. & Center, J. Assessment and treatment of osteoporosis and fractures in type 2 diabetes. Trends Endocrinol. Metab. 33, 333-344 (2022).
- Khosla, S., Samakkarnthai, P., Monroe, D. G. & Farr, J. N. Update on the pathogenesis and treatment of skeletal fragility in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 17, 685-697 (2021).
- Hofbauer, L. et al. Bone fragility in diabetes: novel concepts and clinical implications. Lancet Diabetes Endocrinol. 10, 207-220 (2022).
- Ru, Q. et al. Fighting age-related orthopedic diseases: focusing on ferroptosis. Bone Res. 11, 12 (2023).
- Balogh, E. et al. Iron overload inhibits osteogenic commitment and differentiation of mesenchymal stem cells via the induction of ferritin. Biochim. Biophys. Acta Mol. Basis Disease 1862, 1640-1649 (2016).
- Yang, Y. et al. Targeting ferroptosis suppresses osteocyte glucolipotoxicity and alleviates diabetic osteoporosis. Bone Res. 10, 26 (2022).
- Tong, L. et al. Current understanding of osteoarthritis pathogenesis and relevant new approaches. Bone Res. 10, 60 (2022).
- Xue, Y. et al. Alkaline “nanoswords” coordinate ferroptosis-like bacterial death for antibiosis and osseointegration. ACS Nano 17, 2711-2724 (2023).
- Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060-1072 (2012).
- Tonnus, W. et al. The role of regulated necrosis in endocrine diseases. Nat. Rev. Endocrinol. 17, 497-510 (2021).
- Jiang, X., Stockwell, B. R. & Conrad, M. Ferroptosis: mechanisms, biology, and role in disease. Nat. Rev. Mol. Cell Biol. 22, 266-282 (2021).
- Mishima, E. et al. A non-canonical vitamin K cycle is a potent ferroptosis suppressor. Nature 608, 778-783 (2022).
- Barayeu, U. et al. Hydropersulfides inhibit lipid peroxidation and ferroptosis by scavenging radicals. Nat. Chem. Biol. 19, 28-37 (2022).
- Stockwell, B. R. Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications. Cell 185, 2401-2421 (2022).
- Tang, D., Chen, X., Kang, R. & Kroemer, G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 31, 107-125 (2021).
- Mao, C. et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 593, 586-590 (2021).
- Cui, S. et al. Autophagosomes defeat ferroptosis by decreasing generation and increasing discharge of free
in skin repair cells to accelerate diabetic wound healing. Adv. Sci. 10, e2300414 (2023). - Liang, D. et al. Ferroptosis surveillance independent of GPX4 and differentially regulated by sex hormones. Cell 186, 2748-2764.e2722 (2023).
- Hu, X. et al. Optineurin regulates NRF2-mediated antioxidant response in a mouse model of Paget’s disease of bone. Sci. Adv. 9, eade6998 (2023).
- Yue, L. et al. Chemotaxis-guided self-propelled macrophage motor for targeted treatment of acute pneumonia. Adv. Mater. 35, e2211626 (2023).
- Wang, D. et al. Cell Membrane vesicles with enriched CXCR4 display enhances their targeted delivery as drug carriers to inflammatory sites. Adv. Sci. 8, e2101562 (2021).
- Rashwan, A. K. et al. An updated and comprehensive review on the potential health effects of curcumin-encapsulated micro/nanoparticles. Crit. Rev. Food Sci. Nutr. 63, 9731-9751 (2023).
- Chen, Z. et al. Multifaceted role of phyto-derived polyphenols in nanodrug delivery systems. Adv. Drug Deliv. Rev. 176, 113870 (2021).
- Tian, T. et al. A dynamic DNA tetrahedron framework for active targeting. Nat. Protoc. 18, 1028-1055 (2023).
- Zhang, T. et al. Design, fabrication and applications of tetrahedral DNA nanostructure-based multifunctional complexes in drug delivery and biomedical treatment. Nat. Protoc. 15, 2728-2757 (2020).
- Li, J. et al. Modulation of the crosstalk between schwann cells and macrophages for nerve regeneration: a therapeutic strategy based on multifunctional tetrahedral framework nucleic acids system. Adv. Mater. 34, e2202513 (2022).
- Ma, W. et al. Biomimetic nanoerythrosome-coated aptamer-DNA tetrahedron/ maytansine conjugates: pH-responsive and targeted cytotoxicity for HER2positive breast cancer. Adv. Mater. 34, e2109609 (2022).
- Wang, Y. et al. Tetrahedral framework nucleic acids can alleviate taurocholateinduced severe acute pancreatitis and its subsequent multiorgan injury in mice. Nano Lett. 22, 1759-1768 (2022).
- Zhang, Y. et al. Multi-targeted antisense oligonucleotide delivery by a framework nucleic acid for inhibiting biofilm formation and virulence. Nanomicro Lett. 12, 74 (2020).
- Gao, Y. et al. A LYsosome-activated tetrahedral nanobox for encapsulated sirna delivery. Adv. Mater. 34, e2201731 (2022).
- Li, S. et al. A tetrahedral framework DNA-based bioswitchable mirna inhibitor delivery system: application to skin anti-aging. Adv. Mater. 34, e2204287 (2022).
- Zhang, T. et al. Myelosuppression alleviation and hematopoietic regeneration by tetrahedral-framework nucleic-acid nanostructures functionalized with osteogenic growth peptide. Adv. Sci. 9, e2202058 (2022).
- Liu, Z. et al. Suppression of lipopolysaccharide-induced sepsis by tetrahedral framework nucleic acid loaded with quercetin. Adv. Funct. Mater. 32, 2204587 (2022).
- Yan, R. et al. Typhaneoside-tetrahedral framework nucleic acids system: mitochondrial recovery and antioxidation for acute kidney injury treatment. ACS Nano 17, 8767-8781 (2023).
- Zhao, Y. et al. Effects of puerarin-loaded tetrahedral framework nucleic acids on osteonecrosis of the femoral head. Small 19, e2302326 (2023).
- Gao, S. et al. Tetrahedral framework nucleic acids induce immune tolerance and prevent the onset of type 1 diabetes. Nano Lett. 21, 4437-4446 (2021).
- Li, Y. et al. Tetrahedral framework nucleic acid-based delivery of resveratrol alleviates insulin resistance: from innate to adaptive immunity. Nanomicro Lett. 13, 86 (2021).
- Li, Y. et al. Tetrahedral framework nucleic acids ameliorate insulin resistance in type 2 diabetes mellitus via the PI3K/Akt Pathway. ACS Appl. Mater. Interfaces 13, 40354-40364 (2021).
- Wu, T. et al. METTL3-m(6) A methylase regulates the osteogenic potential of bone marrow mesenchymal stem cells in osteoporotic rats via the Wnt signalling pathway. Cell Prolif. 55, e13234 (2022).
- Li, Y. et al. Advanced glycation end products inhibit the osteogenic differentiation potential of adipose-derived stem cells by modulating Wnt/
-catenin signalling pathway via DNA methylation. Cell Prolif. 53, e12834 (2020). - Peng, S. et al. LncRNA-AK137033 inhibits the osteogenic potential of adiposederived stem cells in diabetic osteoporosis by regulating Wnt signaling pathway via DNA methylation. Cell Prolif. 55, e13174 (2022).
- Salhotra, A., Shah, H. N., Levi, B. & Longaker, M. T. Mechanisms of bone development and repair. Nat. Rev. Mol. Cell Biol. 21, 696-711 (2020).
- Li, S. et al. Bioswitchable delivery of microRNA by framework nucleic acids: application to bone regeneration. Small 17, e2104359 (2021).
- Eberhardt, J., Santos-Martins, D., Tillack, A. F. & Forli, S. AutoDock Vina 1.2.0: new docking methods, expanded force field, and python bindings. J. Chem. Inf. Model 61, 3891-3898 (2021).
- Drake, Z. C., Seffernick, J. T. & Lindert, S. Protein complex prediction using Rosetta, AlphaFold, and mass spectrometry covalent labeling. Nat. Commun. 13, 7846 (2022).
- Gao, M., Nakajima, An,D., Parks, J. M. & Skolnick, J. AF2Complex predicts direct physical interactions in multimeric proteins with deep learning. Nat. Commun. 13, 1744 (2022).
- Tevlin, R. et al. Pharmacological rescue of diabetic skeletal stem cell niches. Sci. Transl. Med. 9, eaag2809 (2017).
- Zhang, Y. et al. Co-delivery of doxorubicin and curcumin via cRGD-peptide modified PEG-PLA self-assembly nanomicelles for lung cancer therapy. Chin. Chem. Lett. 33, 2507-2511 (2022).
- Zhang, C. et al. Oral colon-targeted mucoadhesive micelles with enzymeresponsive controlled release of curcumin for ulcerative colitis therapy. Chin. Chem. Lett. 33, 4924-4929 (2022).
- Zhang, T., Tian, T. & Lin, Y. Functionalizing framework nucleic-acid-based nanostructures for biomedical application. Adv. Mater. 34, e2107820 (2022).
- Shi, S. et al. Amelioration of osteoarthritis via tetrahedral framework nucleic acids delivering microrna-124 for cartilage regeneration. Adv. Funct. Mater. 33, 202305558 (2023).
- Zhang, T., et al Nanomaterials targeting toll-like receptor 4 prevent bispho-sphonate-related osteonecrosis of the jaw via regulating mitochondrial homeostasis in macrophages. Adv. Funct. Mater. 33, 202213401 (2023).
- Zhou, B. O., Yue, R., Murphy, M. M., Peyer, J. G. & Morrison, S. J. Leptin-receptorexpressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 15, 154-168 (2014).
- Sun, Y. et al. The long noncoding RNA Inc-ob1 facilitates bone formation by upregulating Osterix in osteoblasts. Nat. Metab. 1, 485-496 (2019).
- Lutz, T. A. Mammalian models of diabetes mellitus, with a focus on type 2 diabetes mellitus. Nat. Rev. Endocrinol. 19, 350-360 (2023).
- Kallai, I. et al. Microcomputed tomography-based structural analysis of various bone tissue regeneration models. Nat. Protoc. 6, 105-110 (2011).
© The Author(s) 2024
State Key Laboratory of Oral Diseases & National Center for Stomatology & National Clinical Research Center for Oral Diseases, West China Hospital of Stomatology, Sichuan University, Chengdu, Sichuan 610041, PR China; ²Department of Oral and Maxillofacial Surgery, Affiliated Stomatological Hospital, Southwest Medical University, Luzhou, Sichuan 646000, PR China and Sichuan Provincial Engineering Research Center of Oral Biomaterials, Chengdu, Sichuan 610041, China
Correspondence: En Luo (luoen521125@sina.com) or Yunfeng Lin (yunfenglin@scu.edu.cn)
