DOI: https://doi.org/10.1007/s42452-024-05897-z
تاريخ النشر: 2024-05-16
حاملات النانو من الدهون الصلبة لتوصيل الأدوية: ابتكارات التصميم واستراتيجيات التوصيف – مراجعة شاملة
تم النشر على الإنترنت: 16 مايو 2024
© المؤلفون 2024 مفتوح
الملخص
توفر الحاملات الكولودية المعتمدة على الدهون، وخاصة جزيئات الدهون الصلبة (SLNs)، منصة متعددة الاستخدامات لصياغة الأدوية غير القابلة للذوبان في الماء، مما يقدم آثارًا صيدلانية كبيرة عبر مجالات متنوعة. تستكشف هذه الورقة المنهجيات المستخدمة في إنتاج SLN، بدءًا من التجانس عالي الضغط إلى تقنيات المستحلبات الدقيقة، حيث يؤثر كل أسلوب على خصائص وفعالية الجزيئات النانوية الناتجة. توجد طرق إدارة متنوعة لـ SLNs، تستفيد من خصائص المصفوفة الدهنية الواقية لحماية الأدوية المحصورة، مما يقلل من التحلل ويعزز الفعالية العلاجية. علاوة على ذلك، تظهر SLNs خصائص إطلاق مستدام، مما يسهل توصيل الأدوية لفترات طويلة ويقلل من الحاجة إلى الجرعات المتكررة. تساهم أحجامها الصغيرة ومساحتها السطحية الكبيرة في تحسين ذوبان الأدوية، وزيادة التوافر البيولوجي، ومدة الاحتفاظ داخل الجسم. تؤكد وجود براءات اختراع متعددة على الأبحاث الكبيرة التي أجريت في مجال SLNs، مع توفر العديد من التركيبات التجارية على مستوى العالم. في الختام، تسلط هذه العمل الضوء على الطبيعة المعقدة لـ SLNs ودورها المحوري في تقدم تقنيات توصيل الأدوية. تُوجه الجهود المستمرة نحو التغلب على التحديات واستكشاف طرق علاجية جديدة، مما يبرز المشهد الديناميكي والمتطور لأبحاث وتطبيقات SLN.
أبرز المقالات
- تتراوح جزيئات الدهون الصلبة، التي تتراوح عادةً من 50 إلى 1000 نانومتر، من دهون متوافقة حيويًا وقابلة للتحلل، مما يوفر فوائد عديدة مقارنةً بأنظمة توصيل الأدوية التقليدية.
- لقد عززت التقدمات الأخيرة في تقنيات التحضير من قدرات وتطبيقات SLNs، مما يجعلها منصة واعدة لتوصيل مجموعة متنوعة من العوامل العلاجية.
- في المقالة الحالية، نناقش تقنيات التقييم المختلفة المصممة لتقييم جوانب مختلفة من SLNs.
| SLNs | جزيئات الدهون الصلبة |
| NLSs | حاملات الدهون النانوية |
| API | المكون الصيدلاني النشط |
| SEEDS | أنظمة توصيل الأدوية ذاتية الاستحلاب |
| PVP | بولي فينيل بيروليدون |
| PVA | بولي فينيل كحول |
| DPPC | فوسفاتيديل كولين ثنائي بالميتوي |
| DMPG | فوسفاتيديل جليسرول ثنائي الميرستوي |
| SCMC | صوديوم كربوكسي ميثيل السليلوز |
| HPMC | هيدروكسي بروبيل ميثيل السليلوز |
| HPH | التجانس عالي الضغط |
| PCS | طيف تداخل الفوتون |
| LD | تشتت الليزر |
| SLS | تشتت الضوء الثابت |
| SEM | المجهر الإلكتروني الماسح |
| TEM | المجهر الإلكتروني الناقل |
| XRD | تحليل تشتت الأشعة السينية |
| AFM | المجهر الذري |
1 المقدمة
- نظام المستحلب: يتكون من المستحلبات الدقيقة، وأنظمة توصيل الأدوية ذاتية الاستحلاب، والمستحلبات النانوية، والمستحلبات بيكرينغ.
- النظام الحويصلي: يشمل الليبوزومات، النيوسومات، الفارماكوسومات، الفيتوسومات، الترانسفورزومات، الإيثوسومات، الأرتوسومات، الفيسوسومات، الكولوديسومات، والهيربوسومات.
- نظام الجسيمات الدهنية: يشمل الليبوسفيرات، والميكرو جزيئات الدهون الصلبة، وجزيئات الدهون الصلبة، والجسيمات النانوية، وحاملات الدهون، ومركبات الأدوية الدهنية [10].
2 صياغة جزيئات الدهون الصلبة
2.1 مصفوفة الدهون
2.2 المواد الخافضة للتوتر السطحي
2.3 المواد المساعدة السطحية
2.4 المستحلبات
يعززون دوران الجسيمات الدهنية الصلبة عن طريق تثبيط النظام الشبكي البيني وتعزيز توصيل الأدوية إلى الدماغ.
2.5 المواد المساعدة للمستحلبات
2.6 المواد الحافظة للتجميد
2.7 معدلات الشحن
2.8 عوامل تحسين وقت الدوران
2.8.1 مزايا الجسيمات الدهنية الصلبة
- تنظم وتوجه إطلاق الأدوية.
- تمتلك توافقاً استثنائياً مع الكائنات الحية.
- تحسن استقرار الأدوية.
- تشمل تركيزاً مرتفعاً ومكثفاً للأدوية.
- سهلة التعقيم وتمتلك حجمًا أكبر.
- تعزز تنظيم معدل إطلاق المواد المحصورة.
- تحسن قدرة الجسم على امتصاص واستخدام المركبات الحيوية المحصورة.
- تحمي المركبات القابلة للتفكك من خلال الحماية الكيميائية.
- أسهل بكثير في الإنتاج مقارنةً بالجسيمات النانوية البوليمرية.
- لا حاجة لمذيب محدد.
- يمكن استخدام الطرق التقليدية لتصنيع المستحلبات.
- المواد الخام المطلوبة هي نفسها المستخدمة في المستحلبات.
- مستوى الاستقرار على المدى الطويل كبير.
- هناك درجة أكبر من المرونة.
- يمكن استخدام عملية التعقيم التجارية.
- يمكن تحقيق إطلاق دوائي مستدام واستهداف الأدوية.
- تحسن استقرار الأدوية.
- يتم تحقيق حمولة دوائية عالية.
- لا يظهر الحامل سمية حيوية.
- يتم القضاء على الحاجة للمذيبات العضوية.
- يمكن دمج الأدوية المحبة للدهون والمحبة للماء، ويتم تحسين التوافر الحيوي للمركبات الحيوية المحصورة.
- لا توجد مشاكل تتعلق بتصنيع وتعقيم هذا المنتج على نطاق واسع.
- إنه مضاف جديد يستخدم في اللقاحات.
- تُستخدم الجسيمات الدهنية الصلبة على نطاق واسع في علاج السرطان.
2.8.2 عيوب الجسيمات الدهنية الصلبة
- من الممكن حدوث تجمع للجسيمات.
- ميل غير متوقع للتجلي.
- ديناميات غير متوقعة لتغيرات الطور البوليمري.
- إطلاق مفاجئ.
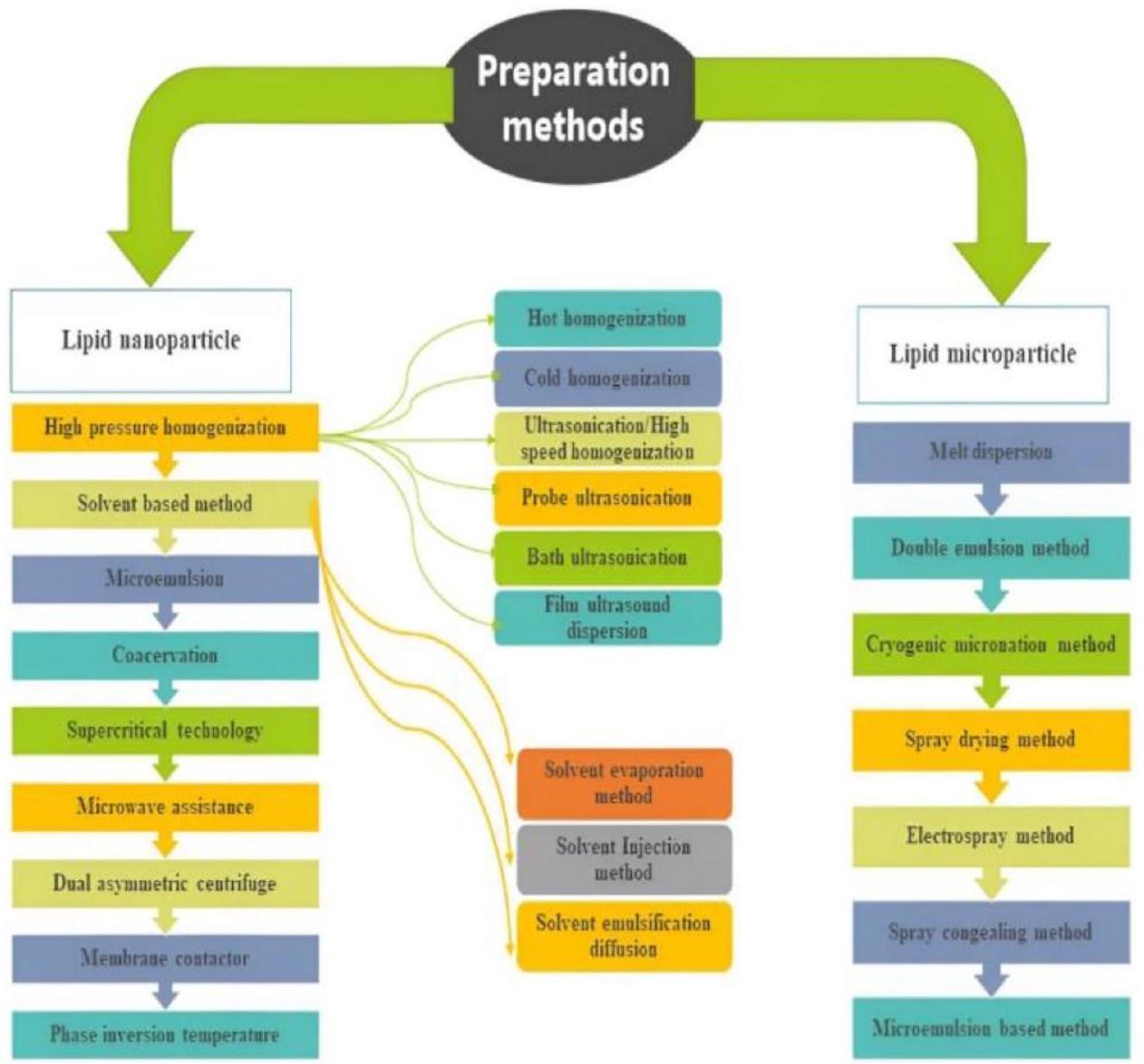
3 تحضير جزيئات الدهون
3.1 التجانس عالي الضغط (HPH)
| رقم التسلسل | اسم الدواء | شركة/اسم | منتج مُسوَّق |
| 1 | زيت بذور الكشمش الأسود وزيت المانوكا | مختبر كيميائي د. كورت ريشتر، CLR-برلين، ألمانيا | استعادة نانو ليبيد CLR |
| 2 | مساعد الإنزيم Q10، بولي ببتيد، مستخلص الكركديه، مستخلص الزنجبيل، كيتوسكر | د. ريمبلر GmbH، ويديمارك، ألمانيا | كريم كوتانوفا نانو ريبير كيو10 |
| ٣ | الأنزيم المساعد Q10، بولي ببتيد، مستخلص المافان | د. ريمبلر GmbH، ويديمارك، ألمانيا | سيروم مكثف نانو ريبير Q10 |
| ٤ | مساعد الإنزيم Q10
|
د. ريمبلر، جيم إتش، ويدمارك، ألمانيا | كريم كوتانوفا نانوفيتال كيو 10 |
| ٥ |
|
بياتي يوهين GmbH، أشهايم، ألمانيا | كريم إصلاح العمق NLC |
| ٦ | مساعد الإنزيم Q10، والأحماض الدهنية غير المشبعة -3 و -6 | شركة أمورباكفيك. سيول، كوريا الجنوبية | منعم إضافي مرطب |
| ٧ | زيت بذور م. تيرنيفوليا، الأفوكادو، اليوريا، زيت بذور الكشمش الأسود | شول، مانهايم، ألمانيا | كريم التجديد المكثف |
| ٨ | زيت جوز الكوكوي، مونوئي تيكي تاهيتي، ببتيد زائف، بروتين القمح المهدرج | لانكراي إنترناشيونال S.A. باريس، فرنسا | كريم سورمر |
| 9 | الأنزيم المساعد Q10، أوليغوسكاريد نشط للغاية | مختبر ACM للأمراض الجلدية | سيروم العين العميق NLC |
| 10 | زيت بذور م. تيرنيفوليا، الأفوكادو، اليوريا، زيت بذور الكشمش الأسود | د. هاوشكا وولا هيلميتيل GmbH | كريم التجديد المكثف |
| 11 | زيت جوز الهند، مستخلص وايلدر، ببتيد زائف، مستخلص الحليب من جوز الهند | ابنة الصيف | سيروم إكسير الجمال نانو فيتاليسانت |
| 12 | Q10، بولي ببتيد، مستخلص الكركديه،
|
الدكتور ريمبلر، جيم إتش | كريم كوتانوفا نانوفيتال وإصلاح Q10 |
| ١٣ | زيت جوز الكوكوي، مونوئي تيكي تاهيتي، ببتيد زائف، بروتين القمح المهدرج | ابنة الصيف | سيروم كريم كونتور العينين نانو ريموديلانت |
| رقم البراءة | عنوان | رقم المرجع |
| O2010112749 | تركيب جلدي لتوصيل المينوكسيديل | [33] |
| SLN المحملة بالتيربينافين المدمجة في جل مع تحسين ترسيب الجلد وزيادة النشاط المضاد للفطريات | [٣٤] | |
| N611/MUM/2011 | تم دمج ميبيروسين SLN في جل قائم على الكاربوبول لتعزيز الفعالية في علاج التهابات الجلد | [35] |
| N3658/MUM/2014 | تم استخدام SLN كحامل في تطوير لاصقات عبر الجلد لاختراق الديلتيازيم | [36] |
| تحضير الأقراص من تعليقات SLN | [37] | |
| N201711046022 | علاج السل والأمراض التي تسببها هيليكوباكتر بيلوري من خلال الإدارة الفموية لشرائح نانوية محملة بالريفابوتين والريفامبيسين | [٣٨] |
| S2011082214 | جزيئات الدهون الصلبة من الفيناسترايد وطريقة التحضير | [39] |
| طريقة تحضير جزيئات الدهون الصلبة من الكاتالاز | [40] | |
| A200900215 | حمل جزيئات الدهون الصلبة من الكركمين والبيبرين وطريقة التحضير | [41] |
| CN101559038B | طريقة تحضير نانوسفير الدهون الصلبة المستهدفة بحمض الفوليك والسليمارين | [42] |
| N101972229B | كرات نانوية دهنية للإعطاء عن طريق الفم | [43] |
| استخدام جزيئات الدهون الصلبة التي تتكون من بروبيونات الكوليسترول و/أو بيوتيرات الكوليسترول | [٤٤] | |
| N103784421B | تكوين ماصات الأشعة فوق البنفسجية من خلال الإدماج في جزيئات الدهون الصلبة | [٤٥] |
| N105708803A | جزيئات الدهون الصلبة | [46] |
| P0167825 | جزيئات نانوية من الدهون الصلبة كحامل للجينات أو الأدوية، التركيبة وطريقة تحضيرها | [47] |
| O06128888 | جزيئات الدهون الصلبة من السيكلوسبورين ذات استقرار فيزيائي جيد أثناء التخزين وطريقة تحضيرها | [٤٨] |
| S7147841 | جزيئات الدهون الصلبة الكلية للفلافونويد من أمبيلوبسيس غروسيدينتاتا وطريقة التحضير | [٤٩] |
| جزيئات الدهون الصلبة من ريسفيراترول وطريقة تحضيرها |
3.1.1 التجانس الساخن
3.1.2 التجانس البارد

3.1.3 الموجات فوق الصوتية/التجانس عالي السرعة
3.1.4 الموجات فوق الصوتية في الحمام

3.1.5 السونكشن باستخدام المجس
3.1.6 تشتت الفيلم بالموجات فوق الصوتية
3.2 الطريقة المعتمدة على المذيب
3.2.1 طريقة تبخر المذيب
3.2.2 طريقة حقن المذيب
3.2.3 طريقة استحلاب المذيب-الانتشار
3.3 الطريقة المعتمدة على الميكروإيمولشن


3.4 التكتل
3.5 التكنولوجيا الفائقة
الأدوية، بما في ذلك الكيتوبروفين، والإندوميثاسين، والكامبتوثيسين. المادة الناتجة هي مسحوق مجفف مع نطاق محدود من أحجام الجزيئات، وقدرة محسنة على التدفق، وكمية مخفضة من المذيب العضوي المتبقي. يعزز ذلك إنشاء تركيبات دوائية سائلة أو صلبة محسنة. الطريقة مستدامة بيئيًا ولديها القدرة على التوسع [64]. الخطوات المعنية في التحضير بواسطة التكنولوجيا الفائقة موضحة في الشكل 6.
3.6 المساعدة بالموجات الدقيقة
3.7 الطرد المركزي غير المتماثل المزدوج
3.8 مقاول الغشاء



3.9 درجة حرارة انقلاب الطور

4 طرق تحضير الجسيمات الدقيقة الدهنية
4.1 تشتت الانصهار
4.2 طريقة المستحلب المزدوج
4.3 طريقة التجميد المجهري

4.4 طريقة التجفيف بالرش
4.5 طريقة الرش الكهربائي
4.6 طريقة التصلب بالرش

تعتبر هذه التقنيات فعالة للغاية ومثبتة في الصناعة (الشكل 12)، مما يجعلها خيارًا موثوقًا لمصنعي الأدوية [72].
4.7 طريقة قائمة على المستحلبات الدقيقة
5 معلمات التوصيف
5.1 التوصيف الفيزيائي الكيميائي لجزيئات الدهون الصلبة النانوية
(1) الحجم المرئي والتوزيع
i. مطيافية تداخل الفوتون
ii. تشتت الليزر
iii. تشتت الضوء الديناميكي
iv. مطيافية الليزر الثابتة
(2) الجهد السطحي الكهربائي
i. جهد زتا
ii. مسبار حساس لدرجة الحموضة [76]
(3) الشكل والهيكل السطحي
i. مجهر إلكتروني نافذ
ii. مجهر إلكتروني ماسح
iii. مجهر القوة الذرية
iv. المجهر الضوئي [77]
(4) البلورية
i. حيود الأشعة السينية
(5) الكارهية السطحية
i. تقسيم ذو مرحلتين
ii. قياس زاوية الاتصال
(6) الكثافة
i. بيكنومتر الغاز
(7) اللزوجة
i. مقياس اللزوجة [78]
(8) الوزن الجزيئي
i. كروماتوغرافيا نفاذ الهلام
ii. إطلاق الدواء في المختبر
- أنابيب الغسيل
- غسيل عكسي
- خلية الانتشار العابر
(9) كفاءة الاحتجاز [79]
6 توصيف جزيئات الدهون الصلبة النانوية
6.1 توزيع الحجم المرئي
6.1.1 مطيافية تداخل الفوتون (PCS)
6.1.2 تشتت الليزر (LD)
6.1.3 تشتت الضوء الديناميكي
6.1.4 تشتت الضوء الثابت (SLS)
6.2 الجهد السطحي الكهربائي ودرجة الحموضة
6.2.1 جهد زتا
6.2.2 المجسات الحساسة لدرجة الحموضة
6.3 الشكل والهيكل السطحي
6.3.1 مجهر إلكتروني نافذ



6.3.2 مجهر إلكتروني ماسح
6.3.3 مجهر القوة الذرية
6.3.4 المجهر الضوئي
6.4 البلورية
6.4.1 حيود الأشعة السينية
6.5 الكارهية السطحية
6.5.1 تقسيم ذو مرحلتين

6.5.2 قياس زاوية الاتصال
6.6 الكثافة
6.6.1 بيكنومتر الغاز
6.7 اللزوجة
6.7.1 جهاز قياس اللزوجة
6.8 الوزن الجزيئي
6.8.1 كروماتوغرافيا نفاذية الجل
6.8.2 الإفراج في المختبر
6.8.2.1 أنبوب الغسيل يمكن تحقيق الإفراج عن الدواء في بيئة مختبرية (في المختبر) من خلال استخدام أنبوب الغسيل. يتم احتواء تشتت الجسيمات النانوية الدهنية الصلبة داخل أنبوب غسيل تم شطفه مسبقًا، والذي يمكن إغلاقه بإحكام. بعد ذلك، يتم إخضاع كيس الغسيل للغسيل ضد وسط ذوبان مناسب عند درجة حرارة الغرفة. في فترات مناسبة، يتم استخراج عينات من وسط الذوبان، وتخضع للطرد المركزي، وتحلل لمحتوى الدواء باستخدام تقنية تحليل مناسبة [101].
6.8.2.2 الغسيل العكسي تتضمن هذه التقنية وضع عدة أكياس غسيل صغيرة، كل منها يحتوي على 1 مل من وسط الذوبان، في تشتت SLN. بعد ذلك، يتم نقل SLNs إلى المحلول المحيط [102].
6.8.2.3 خلية انتشار فرانز من أجل تحديد مدى قدرة الأدوية على اختراق بيئة صناعية، تعتبر خلايا فرانز طريقة موثوقة للغاية. واحدة من فوائد هذه الاختبارات هي أنها تتطلب فقط كمية صغيرة من الدواء للتحليل، وتلاعب محدود بالأنسجة، وعدم الحاجة لجمع عينات مستمرة. بالإضافة إلى ذلك، لا يوجد متطلبات لجمع عينات مستمرة. أحد مكونات نظام FDC هو حجرة استقبال تحتوي على خمسة ملليلترات من PBS. بعد مرور المركب عبر بديل الجلد، يتم إطلاقه في هذه الحجرة. من الممكن إجراء تحليل شامل لديناميات الاختراق بمرور الوقت من خلال تطبيق شرط التجربة ذو الجرعة اللانهائية مباشرة على هذا البديل أثناء وجوده في حجرة المانح. يمكن العثور على محرك مغناطيسي وحمام مائي يتم التحكم فيه بواسطة ترموستات في نظام خلية انتشار فرانز. يمكن لهذا الحمام المائي الحفاظ على درجة حرارة بدقة
6.9 كفاءة الاحتجاز
تساهم إعداد وتوصيف الجسيمات النانوية الدهنية الصلبة في فعاليتها ومرونتها وملاءمتها لمجموعة واسعة من التطبيقات في توصيل الأدوية، مستحضرات التجميل، التصوير، وغيرها من المجالات. تمكن هذه المساهمات من تطوير تركيبات متقدمة مع أداء محسّن وفوائد علاجية.
7 الخاتمة
استهداف أنسجة أو خلايا معينة مباشرة لتوصيل الأدوية، وهو ما لا يمكن تحقيقه مع أنظمة توصيل الأدوية الأخرى. لنقل SLNs بفعالية من المختبر إلى الاستخدام السريري، من الضروري معالجة القضايا المتعلقة بالتحجيم، والاستقرار، وإمكانية التكرار، على الرغم من وجود تقدم ملحوظ في هذا المجال. تعتبر الأبحاث والتطوير المستمرة ضرورية للتغلب على هذه التحديات واستغلال الإمكانات الكاملة لـ SLNs في توصيل الأدوية.
8 آفاق المستقبل
توفر البيانات مجموعات البيانات / المعلومات المستخدمة في هذه الدراسة متاحة عند الطلب المعقول.
الإعلانات
الموافقة على النشر قرأ جميع المؤلفين ووافقوا على النسخة النهائية من النتائج كما هو موجود في المخطوطة.
المصالح المتنافسة جميع المؤلفين يذكرون أنه لم يكن هناك أي مصلحة متنافسة في هذا العمل.
References
- Ahmad J. Lipid nanoparticles based cosmetics with potential application in alleviating skin disorders. Cosmetics. 2021;8:84. https://doi. org/10.3390/cosmetics8030084.
- Shrestha H, Bala R, Arora S. Lipid-based drug delivery systems. J Pharm (Cairo). 2014;2014:801820. https://doi.org/10.1155/2014/801820.
- Williams HD, Trevaskis NL, Yeap YY, Anby MU, Pouton CW, Porter CJH. Lipid-based formulations and drug supersaturation: harnessing the unique benefits of the lipid digestion/absorption pathway. Pharm Res. 2013;30:2976-92. https://doi.org/10.1007/s11095-013-1126-0.
- Ravichandran R. Nanotechnology-based drug delivery systems. NanoBiotechnology. 2009;5:17-33. https://doi.org/10.1007/ s12030-009-9028-2.
- Jawahar N, Meyyanathan SN, Reddy G, Sood S. ChemInform abstract: solid lipid nanoparticles for oral delivery of poorly soluble drugs. ChemInform. 2013. https://doi.org/10.1002/chin.201327225.
- Park K. Facing the truth about nanotechnology in drug delivery. ACS Nano. 2013;7:7442-7. https://doi.org/10.1021/nn404501g.
- Sultana K, et al. Review of solid lipid nano particle. Int J Res Trend Innov. 2020;5(5):1 (ISSN: 2456-3315).
- Prabhakaran E, et al. Solid lipid nanoparticles. Sci Revs Chem Commun. 2012;2(1):80-102 (ISSN 2277-2669).
- Ramadon D. Solid lipid nanoparticles (SLN): formulation and fabrication. Pharm Sci Res. 2023. https://doi.org/10.7454/psr.v10i2.1313.
- Surender V, Deepika M. Solid lipid nanoparticles: a comprehensive review. J Chem Pharm Res. 2016;8(8):102-14.
- Manjunath K, Reddy JS, Venkateswarlu V. Solid lipid nanoparticles as drug delivery systems. Methods Find Exp Clin Pharmacol. 2005;27:127-44. https://doi.org/10.1358/mf.2005.27.2.876286.
- Yadav N, Khatak S, Sara U. Solid lipid nanoparticles-a review. Int J Appl Pharm. 2013;5:8-18.
- Luo WC, Lu X. Solid lipid nanoparticles for drug delivery. Methods Mol Biol. 2023;2622:139-46. https://doi.org/10.1007/978-1-0716-2954-3_12.
- Shirure PD, Pathan MA, Surwase PR, Kareppa MS. Review on solid lipid nanoparticles: as a promising approach for targeted drug delivery system. World J Pharm. 2019;8(3):433-50. https://doi.org/10.20959/wjpps20193-13273.
- Nazarova A, Yakimova L, Filimonova D, Stoikov I. Surfactant effect on the physicochemical characteristics of solid lipid nanoparticles based on Pillar[5]arenes. Int J Mol Sci. 2022;23:779. https://doi.org/10.3390/ijms23020779.
- Khatak S, Dureja H. Recent techniques and patents on solid lipid nanoparticles as novel carrier for drug delivery. Recent Pat Nanotechnol. 2015;9:150-77. https://doi.org/10.2174/1872210510999151126105754.
- Sarangi MK, Padhi S. Solid lipid nanoparticles—a review. J Crit Rev. 2016. https://doi.org/10.31838/jcr.03.01.02.
- Das S, Chaudhury A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech. 2011;12:62-76. https://doi.org/10.1208/s12249-010-9563-0.
- Alsaad A, Hussien A, Gareeb M. Solid lipid nanoparticles (SLN) as a novel drug delivery system: a theoretical review. Syst Rev Pharm. 2020;11(5):259-73.https://doi.org/10.31838/srp.2020.5.39.
- Qushawy M, Nasr A. Solid lipid nanoparticles (SLNs) as nano drug delivery carriers: preparation, characterization and application. Int J App Pharm. 2019. https://doi.org/10.22159/ijap.2020v12i1.35312.
- Ghasemiyeh P, Mohammadi-Samani S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages. Res Pharm Sci. 2018;13:288-303. https://doi.org/10.4103/1735-5362.235156.
- El-Housiny S, Shams Eldeen MA, El-Attar YA, Salem HA, Attia D, Bendas ER, El-Nabarawi MA. Fluconazole-loaded solid lipid nanoparticles topical gel for treatment of pityriasis versicolor: formulation and clinical study. Drug Deliv. 2018;25:78-90. https://doi.org/10.1080/10717 544.2017.1413444.
- Mukherjee S, Ray S, Thakur RS. Solid lipid nanoparticles: a modern formulation approach in drug delivery system. Indian J Pharm Sci. 2009;71:349-58. https://doi.org/10.4103/0250-474X.57282.
- Singh VK, et al. Formulation and evaluation of topical gel of acelofenac containing piparine. Indo Am J Pharm Res. 2013. https://doi.org/ 10.1044/1980-iajpr.00404.
- Sanna V, Gavini E, Cossu M, Rassu G, Giunchedi P. Solid lipid nanoparticles (SLN) as carriers for the topical delivery of econazole nitrate: in-vitro characterization, ex-vivo and in-vivo studies. J Pharm Pharmacol. 2007;59:1057-64. https://doi.org/10.1211/jpp.59.8.0002.
- Khan AS, Shah KU, Mohaini MA, Alsalman AJ, Hawaj MAA, Alhashem YN, Ghazanfar S, Khan KA, Niazi ZR, Farid A. Tacrolimus-loaded solid lipid nanoparticle gel: formulation development and in vitro assessment for topical applications. Gels. 2022;8(2):129. https://doi.org/ 10.3390/gels8020129.
- Chandrakala V, Mamatha HS, Usha A, Priya B. Formulation and evaluation of solid lipid nanoparticles-based gel containing miconazole nitrate (an antifungal agent). Adv Concepts Pharm Res. 2024;5:108-19. https://doi.org/10.9734/bpi/acpr/v5/6727C.
- Arumugarajan AK, et al. Preparation and in vitro evaluation of etodolac extended release tablets prepared by wet granulation method employing kollidon
SR. Indo Am J Pharm Sci. 2015;2(7):1133. - Rostamkalaei SS, Akbari J, Saeedi M, Morteza-Semnani K, Nokhodchi A. Topical gel of Metformin solid lipid nanoparticles: a hopeful promise as a dermal delivery system. Colloids Surf B Biointerfaces. 2019;175:150-7. https://doi.org/10.1016/j.colsurfb.2018.11.072.
- Garse H, Jagtap P, Kadam V. Solid lipid nanoparticles based gel for topicals delivary of antifungal agents. Int J Pharm Sci Res. 2015;6(8):3571. https://doi.org/10.13040/IJPSR.0975-8232.6(8).3571-79.
- Dolatabadi JE, Valizadeh H, Hamishehkar H. Solid lipid nanoparticles as efficient drug and gene delivery systems: recent breakthroughs. Adv Pharm Bull. 2015;5:151-9. https://doi.org/10.15171/apb.2015.022.
- Purohit DK. Nano-lipid carriers for topical application: current scenario. Asian J Pharm (AJP). 2016. https://doi.org/10.22377/ajp.v10i1. 544.
- Padois K, Pirot F, Falson F. Solid lipid nanoparticles encapsulating minoxidil and aqueous suspension containing same. Patent WO2010112749. 2010.
- Vavia PR, Wavikar PR. Solid lipid nanoparticles based formulation of antifungal agent and preparation method thereof. Patent IN611/ MUM/2011. 2011.
- Mandal PA. Topical gel containing solid lipid nanoparticles. Patent IN3658/MUM/2014. 2014.
- Gupta GD. Development of transdermal matrix type patch of diltiazem hydrochloride using solid lipid nanoparticles for arrhythmia. Patent IN201711046022. 2017.
- Faure A, Voorspoels JF, Mertens RJ, Kiekens FR, inventors; Gruenenthal GmbH, assignee. Process for the preparation of a solid dosage form, in particular a tablet, for pharmaceutical use and process for the preparation of a precursor for a solid dosage form, in particular a tablet. Patent US2011082214, 2011.
- Babii VVE, Ignatiev AV, Gelperina SE, Maksi menko OO, Vachugova LV, Shipulo EV. Pharmaceu- tical composition for treatment tuberculosis and diseases caused by Helicobacter pylori based on solid lipid nano- particles and method for tuberculosis treatment. Patent EA200900215. 2009.
- Jun T, Dangguo W. Haitao G, et al. Solid lipid nanoparticles of finasteride and preparation method thereof. CN101559038B. 2011.
- Qi C, Chen Y, Jing Q-Z, Wang X-G. Preparation and characterization of catalase-loaded solid lipid nanoparticles protecting enzyme against proteolysis. Int J Mol Sci. 2011;12:4282-93. https://doi.org/10.3390/ijms12074282.
- Jingling T, Jinmei R, Linhua W, Hongyu J, Mengting L, Chao C. Curcumin and piperine carried solid lipid nanoparticles and preparation method thereof. CN103784421A. 2014.
- Liantian Y, Zhao L, Yang Y, Jin CS, Weitong S, Tong DYZ. Folic acid targeting silymarin solid lipid nanosphere preparation method. CN105708803A. 2014.
- Speiser P. Lipid nano pellets as drug carriers for oral administration. EP0167825A3. 1985.
- Gasco MR. Use of solid lipid nanoparticles comprising cholesteryl propionate and/or cholesteryl butyrate. WO2006128888A1. 2006.
- Herzog B. Formulation of UV absorbers by incorporation in solid lipid nanoparticles. EP1378231A1. 2003.
- Weiss J, Schweiggert C, Leuenberger B, Novotny M, Tedeschi C, Kessler A. Solid lipid nanoparticles. US9616001B2. 2014.
- V Shastri, E Sussman, A Jayagopal. Functionalized solid lipid nanoparticles and methods of making and using same. US20060083781A1. 2005.
- Penkler LJ, Müller RH, Runge SA, Ravelli V. Pharmaceutical cyclosporin formulation with improved biopharmaceutical properties, improved physical quality and greater stability, and method for producing said formulation. EP1073426B1. 1999.
- Zhicheng W, Kexin Z, Bing W, Feng C, Jianlin R, Tong Z, Qi Z, Shiyu Z. Resveratrol solid lipid nano-particles and preparation method thereof. CN104688715A. 2015.
- Schwarz J, Weisspapir M. Colloidal solid lipid vehicle for pharmaceutical use. US20060222716A1. 2006.
- Lin C-H, Chen C-H, Lin Z-C, Fang J-Y. Recent advances in oral delivery of drugs and bioactive natural products using solid lipid nanoparticles as the carriers. J Food Drug Anal. 2017;25:219-34. https://doi.org/10.1016/j.jfda.2017.02.001.
- Yadav N, Khatak S, Sara US. Solid Lipid Nanoparticles- A Review. International Journal of Applied Pharmaceutics. 2013;5(2):8-18.
- Battaglia L, Ugazio E. Lipid nano- and microparticles: an overview of patent-related research. J Nanomater. 2019. https://doi.org/ 10.1155/2019/2834941.
- Mirchandani Y, Patravale VB, Brijesh S. Solid lipid nanoparticles for hydrophilic drugs. J Controll Release. 2021;335:457-64. https:// doi.org/10.1016/j.jconrel.2021.05.032.
- Duan Y, Dhar A, Patel C, Khimani M, Neogi S, Sharma P, Siva Kumar N, Vekariya RL. A brief review on solid lipid nanoparticles: part and parcel of contemporary drug delivery systems. RSC Adv. 2020;10:26777-91. https://doi.org/10.1039/d0ra03491f.
- Shirodkar RK, Kumar L, Mutalik S, Lewis S. Solid lipid nanoparticles and nanostructured lipid carriers: emerging lipid based drug delivery systems. Pharm Chem J. 2019;53:440-53. https://doi.org/10.1007/s11094-019-02017-9.
- Uner M. Preparation, characterization and physico-chemical properties of solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC): their benefits as colloidal drug carrier systems. Pharmazie. 2006;61:375-86.
- Sun S-B, Liu P, Shao F-M, Miao Q-L. Formulation and evaluation of PLGA nanoparticles loaded capecitabine for prostate cancer. Int J Clin Exp Med. 2015;8:19670-81.
- Duong V-A, Nguyen T-T-L, Maeng H-J. Preparation of solid lipid nanoparticles and nanostructured lipid carriers for drug delivery and the effects of preparation parameters of solvent injection method. Molecules. 2020;25:4781. https://doi.org/10.3390/molecules2 5204781.
- Amoabediny G, Haghiralsadat F, Naderinezhad S, Helder MN, Akhoundi Kharanaghi E, Mohammadnejad Arough J, Zandieh-Doulabi B. Overview of preparation methods of polymeric and lipid-based (niosome, solid lipid, liposome) nanoparticles: a comprehensive review. Int J Polym Mater Polym Biomater. 2018;67:383-400. https://doi.org/10.1080/00914037.2017.1332623.
- Harde H, Das M, Jain S. Solid lipid nanoparticles: an oral bioavailability enhancer vehicle. Expert Opin Drug Deliv. 2011;8:1407-24. https:// doi.org/10.1517/17425247.2011.604311.
- Geszke-Moritz M, Moritz M. Solid lipid nanoparticles as attractive drug vehicles: composition, properties and therapeutic strategies. Mater Sci Eng C Mater Biol Appl. 2016;68:982-94. https://doi.org/10.1016/j.msec.2016.05.119.
- Hao J, Wang F, Wang X, Zhang D, Bi Y, Gao Y, Zhao X, Zhang Q. Development and optimization of baicalin-loaded solid lipid nanoparticles prepared by coacervation method using central composite design. Eur J Pharm Sci. 2012;47:497-505. https://doi.org/10.1016/j.ejps. 2012.07.006.
- Andrade LN, Oliveira DML, Chaud MV, Alves TFR, Nery M, da Silva CF, Gonsalves JKC, Nunes RS, Corrêa CB, Amaral RG, Sanchez-Lopez E, Souto EB, Severino P. Praziquantel-solid lipid nanoparticles produced by supercritical carbon dioxide extraction: physicochemical characterization, release profile, and cytotoxicity. Molecules. 2019;24:3881. https://doi.org/10.3390/molecules24213881.
- Dunn SS, Beckford Vera DR, Benhabbour SR, Parrott MC. Rapid microwave-assisted synthesis of sub- 30 nm lipid nanoparticles. J Colloid Interface Sci. 2017;488:240-5. https://doi.org/10.1016/j.jcis.2016.10.093.
- Koehler JK, Schmager S, Bender V, Steiner D, Massing U. Preparation of nanosized pharmaceutical formulations by dual centrifugation. Pharmaceuticals. 2023;16:1519. https://doi.org/10.3390/ph16111519.
- Bagul US, Pisal VV, Solanki NV, Karnavat A. Current status of solid lipid nanoparticles: a review. Mod Appl Bioequivalence Bioavailab. 2018;3(4):1-10.
- Ram D, Debnath S, Babu M, Nath T, Thejeswi B. A review on solid lipid nanoparticles. Res J Pharm Technol. 2012;5:1359-68.
- Khadka P, Ro J, Kim H, Kim I, Kim JT, Kim H, Cho JM, Yun G, Lee J. Pharmaceutical particle technologies: an approach to improve drug solubility, dissolution and bioavailability. Asian J Pharm Sci. 2014;9:304-16. https://doi.org/10.1016/j.ajps.2014.05.005.
- Sawant KK, Dodiya SS. Recent advances and patents on solid lipid nanoparticles. Recent Pat Drug Deliv Formul. 2008;2:120-35. https:// doi.org/10.2174/187221108784534081.
- Shanaghi E, Aghajani M, Esmaeli F, Faramarzi MA, Jahandar H, Amani A. Application of electrospray in preparing solid lipid nanoparticles and optimization of nanoparticles using artificial neural networks. Avicenna J Med Biotechnol. 2020;12:251-4.
- Candiani A, Milanesi A, Foglio Bonda A, Diana G, Bari E, Segale L, Torre ML, Giovannelli L. Solid lipid microparticles by spray congealing of water/oil emulsion: an effective/versatile loading strategy for a highly soluble drug. Pharmaceutics. 2022;14:2805. https://doi.org/ 10.3390/pharmaceutics14122805.
- Khairnar SV, Pagare P, Thakre A, Nambiar AR, Junnuthula V, Abraham MC, Kolimi P, Nyavanandi D, Dyawanapelly S. Review on the scaleup methods for the preparation of solid lipid nanoparticles. Pharmaceutics. 2022;14:1886. https://doi.org/10.3390/pharmaceutics14 091886.
- Bondì ML, Craparo EF. Solid lipid nanoparticles for applications in gene therapy: a review of the state of the art. Expert Opin Drug Deliv. 2010;7:7-18. https://doi.org/10.1517/17425240903362410.
- Shahgaldian P, Da Silva E, Coleman AW, Rather B, Zaworotko MJ. Para-acyl-calix-arene based solid lipid nanoparticles (SLNs): a detailed study of preparation and stability parameters. Int J Pharm. 2003;253:23-38. https://doi.org/10.1016/s0378-5173(02)00639-7.
- Schubert MA, Müller-Goymann CC. Solvent injection as a new approach for manufacturing lipid nanoparticles-evaluation of the method and process parameters. Eur J Pharm Biopharm. 2003;55:125-31. https://doi.org/10.1016/s0939-6411(02)00130-3.
- Bose S, Michniak-Kohn B. Preparation and characterization of lipid based nanosystems for topical delivery of quercetin. Eur J Pharm Sci. 2013;48:442-52. https://doi.org/10.1016/j.ejps.2012.12.005.
- Sastri T, Gadela R, Pidikiti S, Vajjhala P. Solid lipid nanoparticles: Preparation techniques, their characterization, and an update on recent studies. J Appl Pharm Sci. 2020;10:126-41. https://doi.org/10.7324/JAPS.2020.10617.
- Balka SR, Sundari PT. Formulation and evaluation of solid lipid nanoparticles of etoricoxib by employing glyceryl monostearate and gelucire. 48/16. 2019) https://doi.org/10.5281/ZENODO.2583501.
- Montasser I, Shahgaldian P, Perret F, Coleman AW. Solid lipid nanoparticle-based calix[n]arenes and calix-resorcinarenes as building blocks: synthesis, formulation and characterization. Int J Mol Sci. 2013;14:21899-942. https://doi.org/10.3390/ijms141121899.
- Kumar R, Singh A, Sharma K, Dhasmana D, Garg N, Siril PF. Preparation, characterization and in vitro cytotoxicity of fenofibrate and nabumetone loaded solid lipid nanoparticles. Mater Sci Eng C Mater Biol Appl. 2020;106:110184. https://doi.org/10.1016/j.msec.2019. 110184.
- Van de Ven H, Vermeersch M, Shunmugaperumal T, Vandervoort J, Maes L, Ludwig A. Solid lipid nanoparticle (SLN) formulations as a potential tool for the reduction of cytotoxicity of saponins. Pharmazie. 2009;64:172-6.
- Garud A, Singh D, Garud N. Solid lipid nanoparticles (SLN): method, characterization and applications. Int Curr Pharm J. 2012;1:384-93. https://doi.org/10.3329/icpj.v1i11.12065.
- Mishra DK, Dhote V, Bhatnagar P, Mishra PK. Engineering solid lipid nanoparticles for improved drug delivery: promises and challenges of translational research. Drug Deliv Transl Res. 2012;2:238-53. https://doi.org/10.1007/s13346-012-0088-9.
- Plajnšek KT, Pajk S, Govedarica B, Pečar S, Srčič S, Kristl J. A novel fluorescent probe for more effective monitoring of nanosized drug delivery systems within the cells. Int J Pharm. 2011;416:384-93. https://doi.org/10.1016/j.ijpharm.2011.06.046.
- Jores K, Mehnert W, Drechsler M, Bunjes H, Johann C, Mäder K. Investigations on the structure of solid lipid nanoparticles (SLN) and oil-loaded solid lipid nanoparticles by photon correlation spectroscopy, field-flow fractionation and transmission electron microscopy. J Control Release. 2004;95:217-27. https://doi.org/10.1016/j.jconrel.2003.11.012.
- Daghighpoor Z. Back pain and herniated disc linked to depression. Am J Ethnomed. 2017. https://doi.org/10.21767/2348-9502-C1-003.
- Carrillo C, Sánchez-Hernández N, García-Montoya E, Pérez-Lozano P, Suñé-Negre JM, Ticó JR, Suñé C, Miñarro M. DNA delivery via cationic solid lipid nanoparticles (SLNs). Eur J Pharm Sci. 2013;49:157-65. https://doi.org/10.1016/j.ejps.2013.02.011.
- Sharifi M, Attar F, Saboury AA, Akhtari K, Hooshmand N, Hasan A, El-Sayed MA, Falahati M. Plasmonic gold nanoparticles: optical manipulation, imaging, drug delivery and therapy. J Control Release. 2019;311-312:170-89. https://doi.org/10.1016/j.jconrel.2019.08.032.
- Noack A, Hause G, Mäder K. Physicochemical characterization of curcuminoid-loaded solid lipid nanoparticles. Int J Pharm. 2012;423:44051. https://doi.org/10.1016/j.ijpharm.2011.12.011.
- Paumelle R, Blanquart C, Briand O, Barbier O, Duhem C, Woerly G, Percevault F, Fruchart JC, Dombrowicz D, Glineur C, Staels B. Acute antiinflammatory properties of statins involve peroxisome proliferator-activated receptor-alpha via inhibition of the protein kinase C signaling pathway. Circ Res. 2006;98(3):361-9. https://doi.org/10.1161/01.RES.0000202706.70992.95.
- Milsmann J, Oehlke K, Greiner R, Steffen-Heins A. Fate of edible solid lipid nanoparticles (SLN) in surfactant stabilized o/w emulsions. Part 2: Release and partitioning behavior of lipophilic probes from SLN into different phases of o/w emulsions. Colloids Surf A Physicochem Eng Asp. 2018;558:623-31. https://doi.org/10.1016/j.colsurfa.2017.05.050.
- Tang K, Lv X, Wu S, Xuan S, Huang X, Bai C. Measurement for contact angle of iron ore particles and water. ISIJ Int. 2018;58:379-400. https://doi.org/10.2355/isijinternational.ISIJINT-2017-424.
- Poumellec M-A, Dejode M, Figl A, Darcourt J, Haudebourg J, Sabah Y, Voury A, Martaens A, Barranger E. Sentinel node detection using optonuclear probe (gamma and fluorescence) after green indocyanine and radio-isotope injections. Gynecol Obstet Fertil. 2016;44:20710. https://doi.org/10.1016/j.gyobfe.2016.02.012.
- Patravale VB, Mirani AG. Preparation and characterization of solid lipid nanoparticles-based gel for topical delivery. Methods Mol Biol. 2019;2000:293-302. https://doi.org/10.1007/978-1-4939-9516-5_20.
- Abdelbary G, Fahmy RH. Diazepam-loaded solid lipid nanoparticles: design and characterization. AAPS PharmSciTech. 2009;10:211-9. https://doi.org/10.1208/s12249-009-9197-2.
- Ramteke KH, Joshi SA, Dhole SN. Solid lipid nanoparticle: a review. IOSR J Pharm. 2012;2:34-44. https://doi.org/10.9790/3013-26103444.
- Narala A, Veerabrahma K. Preparation, characterization and evaluation of quetiapine fumarate solid lipid nanoparticles to improve the oral bioavailability. J Pharm (Cairo). 2013;2013:265741. https://doi.org/10.1155/2013/265741.
- Zeng J, Pang X, Zhang L, Medina A, Rozelle S. Gender inequality in education in China: a meta-regression analysis. Contemp Econ Policy. 2014;32(2):474-91. https://doi.org/10.1111/coep.12006.
- Mehnert W, Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2001;47:165-96. https://doi.org/10.1016/s0169-409x(01)00105-3.
- Aljaeid B, Hosny KM. Miconazole-loaded solid lipid nanoparticles: formulation and evaluation of a novel formula with high bioavailability and antifungal activity. IJN. 2016. https://doi.org/10.2147/IJN.S100625.
- Venishetty VK, Chede R, Komuravelli R, Adepu L, Sistla R, Diwan PV. Design and evaluation of polymer coated carvedilol loaded solid lipid nanoparticles to improve the oral bioavailability: a novel strategy to avoid intraduodenal administration. Colloids Surf B Biointerfaces. 2012;95:1-9. https://doi.org/10.1016/j.colsurfb.2012.01.001.
- Wissing SA, Müller RH, Manthei L, Mayer C. Structural characterization of Q10-loaded solid lipid nanoparticles by NMR spectroscopy. Pharm Res. 2004;21:400-5. https://doi.org/10.1023/B:PHAM.0000019291.36636.c1.
- Güney G, Kutlu HM, Genç L. Preparation and characterization of ascorbic acid loaded solid lipid nanoparticles and investigation of their apoptotic effects. Colloids Surf B Biointerfaces. 2014;121:270-80. https://doi.org/10.1016/j.colsurfb.2014.05.008.
- Pulsoni I, Lubda M, Aiello M, Fedi A, Marzagalli M, Von Hagen J, Scaglione S. Comparison between franz diffusion cell and a novel microphysiological system for in vitro penetration assay using different skin models. SLAS Technol. 2022;27:161-71. https://doi.org/10.1016/j. slast.2021.12.006.
Sarad Pawar Naik Bukke, drsaradpawar@kiu.ac.ug; Chandrakala Venkatesh, chandrakala.epcp@eastpoint.ac.in Department of Pharmaceutics and Pharmaceutical Technology, Kampala International University, Western Campus, P.O. Box 71, Ishaka-Bushenyi, Uganda. Department of Pharmaceutics, East Paint College of Pharmacy, Bidarahalli, Bangalore 560049, Karnataka, India. Department of Pharmaceutics, SRM College of Pharmacy, SRM Institute of Science and Technology, Kattankulathur, Tamilnadu 603203, India. Department of Pharmaceutical Chemistry, School of Pharmaceutical Sciences, Delhi Pharmaceutical Sciences and Research University, New Delhi, India. Department of Clinical Pharmacy and Pharmacy Practice, Kampala International University, Western Campus, P.O. Box 71, Ishaka-Bushenyi, Uganda.
DOI: https://doi.org/10.1007/s42452-024-05897-z
Publication Date: 2024-05-16
Solid lipid nanocarriers for drug delivery: design innovations and characterization strategies-a comprehensive review
Published online: 16 May 2024
© The Author(s) 2024 OPEN
Abstract
Lipid-based colloidal carriers, particularly Solid Lipid Nanoparticles (SLNs), offer a versatile platform for formulating hydrophobic drugs, presenting significant pharmaceutical implications across diverse fields. This paper explores methodologies utilized in SLN production, ranging from high-pressure homogenization to microemulsion techniques, with each method influencing the characteristics and efficacy of the resultant nanoparticles. Various administration routes for SLNs exist, leveraging the lipid matrix’s protective properties to shield encapsulated drugs, thus minimizing degradation and enhancing therapeutic efficacy. Furthermore, SLNs exhibit sustained release properties, facilitating prolonged drug delivery and reducing the need for frequent dosing. Their small size and high surface area contribute to improved drug dissolution, enhanced bioavailability, and extended retention within the body. The existence of multiple patents underscores the substantial research conducted in the domain of SLNs, with numerous commercial formulations available globally. In conclusion, this work highlights the intricate nature of SLNs and their pivotal role in advancing drug delivery techniques. Ongoing efforts are directed toward overcoming challenges and exploring novel therapeutic avenues, highlighting the dynamic and evolving landscape of SLN research and application.
Article Highlights
- Solid Lipid particles, typically ranging from 50 to 1000 nm , are composed of biocompatible and biodegradable lipids, offering numerous benefits over conventional drug delivery systems.
- Recent advancements in preparation techniques have further enhanced the capabilities and applications of SLNs, making them a promising platform for delivering various therapeutic agents.
- In the present article, we discuss various evaluation techniques tailored to assess different aspects of SLNs.
| SLNs | Solid lipid nanoparticles |
| NLSs | Nanostructured lipid carriers |
| API | Active pharmaceutical ingredient |
| SEEDS | Self-emulsifying drug delivery systems |
| PVP | Polyvinyl pyrrolidone |
| PVA | Polyvinyl alcohol |
| DPPC | Dipalmitoyl phosphatidyl choline |
| DMPG | Dimyristoyl phophatidyl glycerol |
| SCMC | Sodium carboxy methyl cellulose |
| HPMC | Hydroxyl propyl methyl cellulose |
| HPH | High pressure homogenization |
| PCS | Photon correlation spectroscopy |
| LD | Laser diffraction |
| SLS | Static light scattering |
| SEM | Scanning electron microscopy |
| TEM | Transmission Electron Microscopy |
| XRD | X-Ray diffraction analysis |
| AFM | Atomic force microscopy |
1 Introduction
- The emulsion system: This comprises microemulsions, Self-nanoemulsifying Drug Delivery Systems nanoemulsions, and pickering emulsions.
- The vesicular system: It includes liposomes, niosomes, pharmacosomes, phytosomes, transferosomes, ethosomes, arthosomes, vesosomes, colloidosomes, and herbosomes.
- The lipid particulate system: It includes lipospheres, solid lipid microparticles, solid lipid nanoparticles, nanoparticles, lipid carriers, and lipid drug conjugates [10].
2 Formulation of solid lipid nanoparticles
2.1 Lipid matrix
2.2 Surfactants
2.3 Co-surfactants
2.4 Emulsifiers
enhance the circulation of SLNs by inhibiting the Reticuloendothelial System and enhancing the delivery of drugs to the brain [15].
2.5 Co-emulsifiers
2.6 Cryoprotectants
2.7 Charge modifiers
2.8 Agents for improving circulation time
2.8.1 Advantages of SLNs [20]
- Regulate and direct the release of drugs.
- Have exceptional compatibility with living organisms.
- Improve the stability of pharmaceuticals.
- Involve elevated and intensified drug concentration.
- Simple to sterilize and possess increased size.
- Enhance the regulation of the rate at which enclosed substances are released.
- Improve the body’s ability to absorb and use trapped bioactive compounds.
- Safeguard labile-incorporated compounds through chemical protection.
- Significantly more straightforward to produce compared to biopolymeric nanoparticles.
- No requirement for a specific solvent.
- Traditional methods for manufacturing emulsions can be used.
- The raw materials needed are identical to those used in emulsions.
- The level of long-term stability is significant.
- There is a greater degree of versatility.
- The process of commercial sterilization can be utilized.
- Controlled drug release and drug targeting are possible.
- Drug stability is enhanced.
- A high drug payload is achieved.
- The carrier does not exhibit bio-toxicity.
- The need for organic solvents is eliminated.
- Both lipophilic and hydrophilic drugs can be incorporated, and the bioavailability of entrapped bioactive compounds is enhanced [22].
- There are no issues related to the manufacturing and sterilization of this product on a large scale.
- It is a novel additive used in vaccines.
- SLNs are extensively employed in cancer treatment.
2.8.2 Disadvantages of SLNs [21]
- Particle agglomeration is possible.
- Unforeseeable propensity for gelation.
- Unforeseen kinetics of polymeric phase changes.
- Burst release
3 Lipid nanoparticles preparation
3.1 High-pressure homogenization (HPH)
| S. No | Drug name | Company/Name | Marketed product |
| 1 | Black currant seed oil and manuka oil | Chemisches Laboratium Dr. Kurt Richter, CLR-Berlin, Germany | NanoLipid Restore CLR |
| 2 | Coenzyme Q10, polypeptide, hibiscus extract, ginger extract, ketosugar | Dr. Rimpler GmbH, Wedemark, Germany | Cutanova Cream Nanorepair Q10 |
| 3 | Coenzyme Q10, polypeptide, mafane extract | Dr. Rimpler GmbH, Wedemark, Germany | Intensive Serum Nanorepair Q10 |
| 4 | Coenzyme Q10,
|
Dr. Rimpler, GimH, Wedemark, Germany | Cutanova Cream Nanovital Q10 |
| 5 |
|
Beate Johnen GmbH, Aschheim, Germany | NLC Deep Effect Repair Cream |
| 6 | Coenzyme Q10, -3 und -6 unsaturated fatty acids | Amorepacific Corp. Seoul, South Korea | Extra Moist Softener |
| 7 | M. Ternifolia seed oil, avocado, urea, black currant seed oil | Scholl, Mannheium, Germany | Regeneration crème intensive |
| 8 | Kukui nut oil, Monoi Tiki Tahiti, pseudopeptide, hydrolyzed wheat protein | Lancray International S.A. Paris, France | SURMER Crème |
| 9 | Coenzyme Q10, highly active oligosaccharides | ACM Laboratoire Dermatologique | NLC Deep Effect Eye Serum |
| 10 | M. Ternifolia seed oil, avocado, urea, black currant seed oil | Dr. Hauschka WALA Heilmittei GmbH | Regenerations crème intensive |
| 11 | Coconut oil, wilder extract, pseudo peptide, milk extract from coconut | Summer’s Daughter | Surmer Elixir de Beatue Nano vitalisant |
| 12 | Q10, polypetide, hibiscus extract,
|
Dr. Rimpler, GimH | Cutanova Cream Nanovital and Repair Q10 |
| 13 | Kukui nut oil, Monoi Tiki Tahiti, pseudo peptide, hydolysed wheat protein | Summer’s Daughter | Surmer Crème Contour Des Yeux Nano Remodelante |
| Patent number | Title | Ref. no |
| O2010112749 | Dermal composition for minoxidil delivery | [33] |
| Terbinafine-loaded SLN incorporated into a gel with enhanced skin deposition and improved antifungal activity | [34] | |
| N611/MUM/2011 | Mupirocin SLN incorporated into a Carbopol-based gel to enhance efficacy in skin infection treatment | [35] |
| N3658/MUM/2014 | SLN used as a carrier in the development of transdermal patches for diltiazem permeation | [36] |
| Preparation of tablets from SLN suspensions | [37] | |
| N201711046022 | Treatment of tuberculosis and diseases mediated by Helicobacter pylori via the peroral administration of SLN loaded with rifabutin and rifampicin | [38] |
| S2011082214 | Solid lipid nanoparticles of finasteride and preparation method | [39] |
| Preparation method of catalase solid lipid nanoparticles preparation | [40] | |
| A200900215 | Carrying solid lipid nanoparticles of curcumin and piperine and the preparation method | [41] |
| CN101559038B | Folic acid targeting silymarin solid lipid nanosphere preparation method | [42] |
| N101972229B | Lipid nano pellets for oral administration | [43] |
| Use of solid lipid nanoparticles comprising cholesteryl propionate and/or cholesteryl butyrate | [44] | |
| N103784421B | Formulation of UV absorbers by incorporation in solid lipid nanoparticles | [45] |
| N105708803A | Solid lipid nanoparticles | [46] |
| P0167825 | Solid lipid nanoparticle as a gene or drug carrier, formulation and method for preparing the same | [47] |
| O06128888 | Cyclosporin solid lipid nanoparticles with good storage physical stability and preparation method thereof | [48] |
| S7147841 | Ampelopsis grossedentata total flavonoid solid lipid nanoparticles and preparation method | [49] |
| Resveratrol solid lipid nanoparticles and preparation method thereof |

3.1.1 Hot homogenization
3.1.2 Cold homogenization

3.1.3 Ultrasonication/high-speed homogenization
3.1.4 Bath ultrasonication

3.1.5 Probe ultrasonication
3.1.6 Film ultrasound dispersion
3.2 Solvent-based method
3.2.1 Solvent evaporation method
3.2.2 Solvent injection method
3.2.3 Solvent emulsification-diffusion method
3.3 Microemulsion-based method


3.4 Coacervation
3.5 Supercritical technology
of medications, including ketoprofen, indomethacin, and camptothecin. The resulting substance is a desiccated powder with a limited range of particle sizes, improved ability to flow, and a reduced amount of remaining organic solvent. This enhances the creation of enhanced liquid or solid drug formulations. The method is ecologically sustainable and has the capacity to be expanded [64]. Steps involved in the preparation by supercritical technology in shown in Fig. 6.
3.6 Microwave assistance
3.7 Dual asymmetric centrifuge
3.8 Membrane contractor



3.9 Phase inversion temperature

4 Lipid microparticles preparation methods
4.1 Melt dispersion
4.2 Double emulsion method
4.3 Cryogenic micronization method

4.4 Spray drying method
4.5 Electrospray method
4.6 Spray congealing method

techniques are highly efficient and well-established in the industry (Fig. 12), making them a reliable choice for pharmaceutical manufacturers [72].
4.7 Microemulsion-based method
5 Characterization parameters
5.1 Physicochemical characterization of solid lipid nanoparticles
(1) Visual size and distribution
i. Photon correlation spectroscopy
ii. Laser diffraction
iii. Dynamic light scattering
iv. Static laser spectroscopy
(2) Electrical surface potential
i. Zeta potential
ii. pH -sensitive probe [76]
(3) Shape and surface morphology
i. Transmission electron microscopy
ii. Scanning electron microscopy
iii. Atomic force microscopy
iv. Optical microscopy [77]
(4) Crystallinity
i. X-ray diffraction
(5) Surface hydrophobicity
i. Two-phase partition
ii. Contact angle measurement
(6) Density
i. Gas pycnometer
(7) Viscosity
i. Viscometer [78]
(8) Molecular weight
i. Gel permeation chromatography
ii. In vitro drug release
- Dialysis tubing
- Reverse dialysis
- Trans diffusion cell
(9) Entrapment efficiency [79]
6 Characterization of solid lipid nanoparticles
6.1 Visual size distribution
6.1.1 Photon correlation spectroscopy (PCS)
6.1.2 Laser diffraction (LD)
6.1.3 Dynamic light scattering
6.1.4 Static light scattering (SLS)
6.2 Electrical surface potential and pH
6.2.1 Zeta potential
6.2.2 pH-sensitive probes
6.3 Shape and surface morphology
6.3.1 Transmission electron microscopy



6.3.2 Scanning electron microscopy
6.3.3 Atomic force microscopy
6.3.4 Optical microscopy
6.4 Crystallinity
6.4.1 X-ray diffraction
6.5 Surface hydrophobicity
6.5.1 Two-phase partition

6.5.2 Contact angle measurement
6.6 Density
6.6.1 Gas pycnometer
6.7 Viscosity
6.7.1 Viscometer
6.8 Molecular weight
6.8.1 Gel permeation chromatography
6.8.2 In vitro release
6.8.2.1 Dialysis tubing Drug release in a laboratory setting (in vitro) can be accomplished by employing dialysis tubing. The solid lipid nanoparticle dispersion is enclosed within pre-rinsed dialysis tubing, which can be tightly sealed. Subsequently, the dialysis sac is subjected to dialysis against an appropriate dissolution medium at ambient temperature. At appropriate intervals, samples are extracted from the dissolution medium, subjected to centrifugation, and analyzed for drug content using a suitable analytical technique [101].
6.8.2.2 Reverse dialysis This technique involves the placement of multiple small dialysis sacs, each containing 1 ml of dissolution medium, into an SLN dispersion. Subsequently, the SLNs are transferred into the surrounding solution [102].
6.8.2.3 Franz diffusion cell For the purpose of determining the extent to which drugs are able to penetrate an artificial environment, Franz cells are an extremely reliable method. One of the benefits of these tests is that they require only a small amount of drug for analysis, limited tissue manipulation, and no ongoing sample collection. Additionally, there is no requirement for continuous sample collection. One of the components of the FDC system is a receiver compartment that holds five milliliters of PBS. Following the passage of the compound through the skin surrogate, it is then released into this compartment. It is possible to conduct a comprehensive analysis of the penetration kinetics over time by applying the infinite-dose experimental condition directly onto this surrogate while it is located in the donor compartment. A magnetic stirrer and a water bath that is controlled by a thermostat can be found in the Franz diffusion cell system. This water bath is capable of maintaining a temperature of precisely
6.9 Entrapment efficiency
Preparation and characterization of solid lipid nanoparticles contribute to their efficacy, versatility, and suitability for a wide range of applications in drug delivery, cosmetics, imaging, and other fields. These contributions enable the development of advanced formulations with enhanced performance and therapeutic benefits.
7 Conclusion
directly target specific tissues or cells for drug delivery, which is not possible with other drug delivery systems. To effectively move SLNs from the laboratory to clinical use, it is essential to address issues concerning scalability, stability, and reproducibility, even though there have been notable advancements in this area. Continual research and development are essential for overcoming these challenges and fully harnessing the potential of SLNs for drug delivery.
8 Future prospective
Data availability The datasets/information used for this study is available on reasonable request.
Declarations
Consent for publication All the authors have read and agreed to the final copy of the finding as contained in the manuscript.
Competing interests All authors report that there was no competing interest in this work.
References
- Ahmad J. Lipid nanoparticles based cosmetics with potential application in alleviating skin disorders. Cosmetics. 2021;8:84. https://doi. org/10.3390/cosmetics8030084.
- Shrestha H, Bala R, Arora S. Lipid-based drug delivery systems. J Pharm (Cairo). 2014;2014:801820. https://doi.org/10.1155/2014/801820.
- Williams HD, Trevaskis NL, Yeap YY, Anby MU, Pouton CW, Porter CJH. Lipid-based formulations and drug supersaturation: harnessing the unique benefits of the lipid digestion/absorption pathway. Pharm Res. 2013;30:2976-92. https://doi.org/10.1007/s11095-013-1126-0.
- Ravichandran R. Nanotechnology-based drug delivery systems. NanoBiotechnology. 2009;5:17-33. https://doi.org/10.1007/ s12030-009-9028-2.
- Jawahar N, Meyyanathan SN, Reddy G, Sood S. ChemInform abstract: solid lipid nanoparticles for oral delivery of poorly soluble drugs. ChemInform. 2013. https://doi.org/10.1002/chin.201327225.
- Park K. Facing the truth about nanotechnology in drug delivery. ACS Nano. 2013;7:7442-7. https://doi.org/10.1021/nn404501g.
- Sultana K, et al. Review of solid lipid nano particle. Int J Res Trend Innov. 2020;5(5):1 (ISSN: 2456-3315).
- Prabhakaran E, et al. Solid lipid nanoparticles. Sci Revs Chem Commun. 2012;2(1):80-102 (ISSN 2277-2669).
- Ramadon D. Solid lipid nanoparticles (SLN): formulation and fabrication. Pharm Sci Res. 2023. https://doi.org/10.7454/psr.v10i2.1313.
- Surender V, Deepika M. Solid lipid nanoparticles: a comprehensive review. J Chem Pharm Res. 2016;8(8):102-14.
- Manjunath K, Reddy JS, Venkateswarlu V. Solid lipid nanoparticles as drug delivery systems. Methods Find Exp Clin Pharmacol. 2005;27:127-44. https://doi.org/10.1358/mf.2005.27.2.876286.
- Yadav N, Khatak S, Sara U. Solid lipid nanoparticles-a review. Int J Appl Pharm. 2013;5:8-18.
- Luo WC, Lu X. Solid lipid nanoparticles for drug delivery. Methods Mol Biol. 2023;2622:139-46. https://doi.org/10.1007/978-1-0716-2954-3_12.
- Shirure PD, Pathan MA, Surwase PR, Kareppa MS. Review on solid lipid nanoparticles: as a promising approach for targeted drug delivery system. World J Pharm. 2019;8(3):433-50. https://doi.org/10.20959/wjpps20193-13273.
- Nazarova A, Yakimova L, Filimonova D, Stoikov I. Surfactant effect on the physicochemical characteristics of solid lipid nanoparticles based on Pillar[5]arenes. Int J Mol Sci. 2022;23:779. https://doi.org/10.3390/ijms23020779.
- Khatak S, Dureja H. Recent techniques and patents on solid lipid nanoparticles as novel carrier for drug delivery. Recent Pat Nanotechnol. 2015;9:150-77. https://doi.org/10.2174/1872210510999151126105754.
- Sarangi MK, Padhi S. Solid lipid nanoparticles—a review. J Crit Rev. 2016. https://doi.org/10.31838/jcr.03.01.02.
- Das S, Chaudhury A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech. 2011;12:62-76. https://doi.org/10.1208/s12249-010-9563-0.
- Alsaad A, Hussien A, Gareeb M. Solid lipid nanoparticles (SLN) as a novel drug delivery system: a theoretical review. Syst Rev Pharm. 2020;11(5):259-73.https://doi.org/10.31838/srp.2020.5.39.
- Qushawy M, Nasr A. Solid lipid nanoparticles (SLNs) as nano drug delivery carriers: preparation, characterization and application. Int J App Pharm. 2019. https://doi.org/10.22159/ijap.2020v12i1.35312.
- Ghasemiyeh P, Mohammadi-Samani S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages. Res Pharm Sci. 2018;13:288-303. https://doi.org/10.4103/1735-5362.235156.
- El-Housiny S, Shams Eldeen MA, El-Attar YA, Salem HA, Attia D, Bendas ER, El-Nabarawi MA. Fluconazole-loaded solid lipid nanoparticles topical gel for treatment of pityriasis versicolor: formulation and clinical study. Drug Deliv. 2018;25:78-90. https://doi.org/10.1080/10717 544.2017.1413444.
- Mukherjee S, Ray S, Thakur RS. Solid lipid nanoparticles: a modern formulation approach in drug delivery system. Indian J Pharm Sci. 2009;71:349-58. https://doi.org/10.4103/0250-474X.57282.
- Singh VK, et al. Formulation and evaluation of topical gel of acelofenac containing piparine. Indo Am J Pharm Res. 2013. https://doi.org/ 10.1044/1980-iajpr.00404.
- Sanna V, Gavini E, Cossu M, Rassu G, Giunchedi P. Solid lipid nanoparticles (SLN) as carriers for the topical delivery of econazole nitrate: in-vitro characterization, ex-vivo and in-vivo studies. J Pharm Pharmacol. 2007;59:1057-64. https://doi.org/10.1211/jpp.59.8.0002.
- Khan AS, Shah KU, Mohaini MA, Alsalman AJ, Hawaj MAA, Alhashem YN, Ghazanfar S, Khan KA, Niazi ZR, Farid A. Tacrolimus-loaded solid lipid nanoparticle gel: formulation development and in vitro assessment for topical applications. Gels. 2022;8(2):129. https://doi.org/ 10.3390/gels8020129.
- Chandrakala V, Mamatha HS, Usha A, Priya B. Formulation and evaluation of solid lipid nanoparticles-based gel containing miconazole nitrate (an antifungal agent). Adv Concepts Pharm Res. 2024;5:108-19. https://doi.org/10.9734/bpi/acpr/v5/6727C.
- Arumugarajan AK, et al. Preparation and in vitro evaluation of etodolac extended release tablets prepared by wet granulation method employing kollidon
SR. Indo Am J Pharm Sci. 2015;2(7):1133. - Rostamkalaei SS, Akbari J, Saeedi M, Morteza-Semnani K, Nokhodchi A. Topical gel of Metformin solid lipid nanoparticles: a hopeful promise as a dermal delivery system. Colloids Surf B Biointerfaces. 2019;175:150-7. https://doi.org/10.1016/j.colsurfb.2018.11.072.
- Garse H, Jagtap P, Kadam V. Solid lipid nanoparticles based gel for topicals delivary of antifungal agents. Int J Pharm Sci Res. 2015;6(8):3571. https://doi.org/10.13040/IJPSR.0975-8232.6(8).3571-79.
- Dolatabadi JE, Valizadeh H, Hamishehkar H. Solid lipid nanoparticles as efficient drug and gene delivery systems: recent breakthroughs. Adv Pharm Bull. 2015;5:151-9. https://doi.org/10.15171/apb.2015.022.
- Purohit DK. Nano-lipid carriers for topical application: current scenario. Asian J Pharm (AJP). 2016. https://doi.org/10.22377/ajp.v10i1. 544.
- Padois K, Pirot F, Falson F. Solid lipid nanoparticles encapsulating minoxidil and aqueous suspension containing same. Patent WO2010112749. 2010.
- Vavia PR, Wavikar PR. Solid lipid nanoparticles based formulation of antifungal agent and preparation method thereof. Patent IN611/ MUM/2011. 2011.
- Mandal PA. Topical gel containing solid lipid nanoparticles. Patent IN3658/MUM/2014. 2014.
- Gupta GD. Development of transdermal matrix type patch of diltiazem hydrochloride using solid lipid nanoparticles for arrhythmia. Patent IN201711046022. 2017.
- Faure A, Voorspoels JF, Mertens RJ, Kiekens FR, inventors; Gruenenthal GmbH, assignee. Process for the preparation of a solid dosage form, in particular a tablet, for pharmaceutical use and process for the preparation of a precursor for a solid dosage form, in particular a tablet. Patent US2011082214, 2011.
- Babii VVE, Ignatiev AV, Gelperina SE, Maksi menko OO, Vachugova LV, Shipulo EV. Pharmaceu- tical composition for treatment tuberculosis and diseases caused by Helicobacter pylori based on solid lipid nano- particles and method for tuberculosis treatment. Patent EA200900215. 2009.
- Jun T, Dangguo W. Haitao G, et al. Solid lipid nanoparticles of finasteride and preparation method thereof. CN101559038B. 2011.
- Qi C, Chen Y, Jing Q-Z, Wang X-G. Preparation and characterization of catalase-loaded solid lipid nanoparticles protecting enzyme against proteolysis. Int J Mol Sci. 2011;12:4282-93. https://doi.org/10.3390/ijms12074282.
- Jingling T, Jinmei R, Linhua W, Hongyu J, Mengting L, Chao C. Curcumin and piperine carried solid lipid nanoparticles and preparation method thereof. CN103784421A. 2014.
- Liantian Y, Zhao L, Yang Y, Jin CS, Weitong S, Tong DYZ. Folic acid targeting silymarin solid lipid nanosphere preparation method. CN105708803A. 2014.
- Speiser P. Lipid nano pellets as drug carriers for oral administration. EP0167825A3. 1985.
- Gasco MR. Use of solid lipid nanoparticles comprising cholesteryl propionate and/or cholesteryl butyrate. WO2006128888A1. 2006.
- Herzog B. Formulation of UV absorbers by incorporation in solid lipid nanoparticles. EP1378231A1. 2003.
- Weiss J, Schweiggert C, Leuenberger B, Novotny M, Tedeschi C, Kessler A. Solid lipid nanoparticles. US9616001B2. 2014.
- V Shastri, E Sussman, A Jayagopal. Functionalized solid lipid nanoparticles and methods of making and using same. US20060083781A1. 2005.
- Penkler LJ, Müller RH, Runge SA, Ravelli V. Pharmaceutical cyclosporin formulation with improved biopharmaceutical properties, improved physical quality and greater stability, and method for producing said formulation. EP1073426B1. 1999.
- Zhicheng W, Kexin Z, Bing W, Feng C, Jianlin R, Tong Z, Qi Z, Shiyu Z. Resveratrol solid lipid nano-particles and preparation method thereof. CN104688715A. 2015.
- Schwarz J, Weisspapir M. Colloidal solid lipid vehicle for pharmaceutical use. US20060222716A1. 2006.
- Lin C-H, Chen C-H, Lin Z-C, Fang J-Y. Recent advances in oral delivery of drugs and bioactive natural products using solid lipid nanoparticles as the carriers. J Food Drug Anal. 2017;25:219-34. https://doi.org/10.1016/j.jfda.2017.02.001.
- Yadav N, Khatak S, Sara US. Solid Lipid Nanoparticles- A Review. International Journal of Applied Pharmaceutics. 2013;5(2):8-18.
- Battaglia L, Ugazio E. Lipid nano- and microparticles: an overview of patent-related research. J Nanomater. 2019. https://doi.org/ 10.1155/2019/2834941.
- Mirchandani Y, Patravale VB, Brijesh S. Solid lipid nanoparticles for hydrophilic drugs. J Controll Release. 2021;335:457-64. https:// doi.org/10.1016/j.jconrel.2021.05.032.
- Duan Y, Dhar A, Patel C, Khimani M, Neogi S, Sharma P, Siva Kumar N, Vekariya RL. A brief review on solid lipid nanoparticles: part and parcel of contemporary drug delivery systems. RSC Adv. 2020;10:26777-91. https://doi.org/10.1039/d0ra03491f.
- Shirodkar RK, Kumar L, Mutalik S, Lewis S. Solid lipid nanoparticles and nanostructured lipid carriers: emerging lipid based drug delivery systems. Pharm Chem J. 2019;53:440-53. https://doi.org/10.1007/s11094-019-02017-9.
- Uner M. Preparation, characterization and physico-chemical properties of solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC): their benefits as colloidal drug carrier systems. Pharmazie. 2006;61:375-86.
- Sun S-B, Liu P, Shao F-M, Miao Q-L. Formulation and evaluation of PLGA nanoparticles loaded capecitabine for prostate cancer. Int J Clin Exp Med. 2015;8:19670-81.
- Duong V-A, Nguyen T-T-L, Maeng H-J. Preparation of solid lipid nanoparticles and nanostructured lipid carriers for drug delivery and the effects of preparation parameters of solvent injection method. Molecules. 2020;25:4781. https://doi.org/10.3390/molecules2 5204781.
- Amoabediny G, Haghiralsadat F, Naderinezhad S, Helder MN, Akhoundi Kharanaghi E, Mohammadnejad Arough J, Zandieh-Doulabi B. Overview of preparation methods of polymeric and lipid-based (niosome, solid lipid, liposome) nanoparticles: a comprehensive review. Int J Polym Mater Polym Biomater. 2018;67:383-400. https://doi.org/10.1080/00914037.2017.1332623.
- Harde H, Das M, Jain S. Solid lipid nanoparticles: an oral bioavailability enhancer vehicle. Expert Opin Drug Deliv. 2011;8:1407-24. https:// doi.org/10.1517/17425247.2011.604311.
- Geszke-Moritz M, Moritz M. Solid lipid nanoparticles as attractive drug vehicles: composition, properties and therapeutic strategies. Mater Sci Eng C Mater Biol Appl. 2016;68:982-94. https://doi.org/10.1016/j.msec.2016.05.119.
- Hao J, Wang F, Wang X, Zhang D, Bi Y, Gao Y, Zhao X, Zhang Q. Development and optimization of baicalin-loaded solid lipid nanoparticles prepared by coacervation method using central composite design. Eur J Pharm Sci. 2012;47:497-505. https://doi.org/10.1016/j.ejps. 2012.07.006.
- Andrade LN, Oliveira DML, Chaud MV, Alves TFR, Nery M, da Silva CF, Gonsalves JKC, Nunes RS, Corrêa CB, Amaral RG, Sanchez-Lopez E, Souto EB, Severino P. Praziquantel-solid lipid nanoparticles produced by supercritical carbon dioxide extraction: physicochemical characterization, release profile, and cytotoxicity. Molecules. 2019;24:3881. https://doi.org/10.3390/molecules24213881.
- Dunn SS, Beckford Vera DR, Benhabbour SR, Parrott MC. Rapid microwave-assisted synthesis of sub- 30 nm lipid nanoparticles. J Colloid Interface Sci. 2017;488:240-5. https://doi.org/10.1016/j.jcis.2016.10.093.
- Koehler JK, Schmager S, Bender V, Steiner D, Massing U. Preparation of nanosized pharmaceutical formulations by dual centrifugation. Pharmaceuticals. 2023;16:1519. https://doi.org/10.3390/ph16111519.
- Bagul US, Pisal VV, Solanki NV, Karnavat A. Current status of solid lipid nanoparticles: a review. Mod Appl Bioequivalence Bioavailab. 2018;3(4):1-10.
- Ram D, Debnath S, Babu M, Nath T, Thejeswi B. A review on solid lipid nanoparticles. Res J Pharm Technol. 2012;5:1359-68.
- Khadka P, Ro J, Kim H, Kim I, Kim JT, Kim H, Cho JM, Yun G, Lee J. Pharmaceutical particle technologies: an approach to improve drug solubility, dissolution and bioavailability. Asian J Pharm Sci. 2014;9:304-16. https://doi.org/10.1016/j.ajps.2014.05.005.
- Sawant KK, Dodiya SS. Recent advances and patents on solid lipid nanoparticles. Recent Pat Drug Deliv Formul. 2008;2:120-35. https:// doi.org/10.2174/187221108784534081.
- Shanaghi E, Aghajani M, Esmaeli F, Faramarzi MA, Jahandar H, Amani A. Application of electrospray in preparing solid lipid nanoparticles and optimization of nanoparticles using artificial neural networks. Avicenna J Med Biotechnol. 2020;12:251-4.
- Candiani A, Milanesi A, Foglio Bonda A, Diana G, Bari E, Segale L, Torre ML, Giovannelli L. Solid lipid microparticles by spray congealing of water/oil emulsion: an effective/versatile loading strategy for a highly soluble drug. Pharmaceutics. 2022;14:2805. https://doi.org/ 10.3390/pharmaceutics14122805.
- Khairnar SV, Pagare P, Thakre A, Nambiar AR, Junnuthula V, Abraham MC, Kolimi P, Nyavanandi D, Dyawanapelly S. Review on the scaleup methods for the preparation of solid lipid nanoparticles. Pharmaceutics. 2022;14:1886. https://doi.org/10.3390/pharmaceutics14 091886.
- Bondì ML, Craparo EF. Solid lipid nanoparticles for applications in gene therapy: a review of the state of the art. Expert Opin Drug Deliv. 2010;7:7-18. https://doi.org/10.1517/17425240903362410.
- Shahgaldian P, Da Silva E, Coleman AW, Rather B, Zaworotko MJ. Para-acyl-calix-arene based solid lipid nanoparticles (SLNs): a detailed study of preparation and stability parameters. Int J Pharm. 2003;253:23-38. https://doi.org/10.1016/s0378-5173(02)00639-7.
- Schubert MA, Müller-Goymann CC. Solvent injection as a new approach for manufacturing lipid nanoparticles-evaluation of the method and process parameters. Eur J Pharm Biopharm. 2003;55:125-31. https://doi.org/10.1016/s0939-6411(02)00130-3.
- Bose S, Michniak-Kohn B. Preparation and characterization of lipid based nanosystems for topical delivery of quercetin. Eur J Pharm Sci. 2013;48:442-52. https://doi.org/10.1016/j.ejps.2012.12.005.
- Sastri T, Gadela R, Pidikiti S, Vajjhala P. Solid lipid nanoparticles: Preparation techniques, their characterization, and an update on recent studies. J Appl Pharm Sci. 2020;10:126-41. https://doi.org/10.7324/JAPS.2020.10617.
- Balka SR, Sundari PT. Formulation and evaluation of solid lipid nanoparticles of etoricoxib by employing glyceryl monostearate and gelucire. 48/16. 2019) https://doi.org/10.5281/ZENODO.2583501.
- Montasser I, Shahgaldian P, Perret F, Coleman AW. Solid lipid nanoparticle-based calix[n]arenes and calix-resorcinarenes as building blocks: synthesis, formulation and characterization. Int J Mol Sci. 2013;14:21899-942. https://doi.org/10.3390/ijms141121899.
- Kumar R, Singh A, Sharma K, Dhasmana D, Garg N, Siril PF. Preparation, characterization and in vitro cytotoxicity of fenofibrate and nabumetone loaded solid lipid nanoparticles. Mater Sci Eng C Mater Biol Appl. 2020;106:110184. https://doi.org/10.1016/j.msec.2019. 110184.
- Van de Ven H, Vermeersch M, Shunmugaperumal T, Vandervoort J, Maes L, Ludwig A. Solid lipid nanoparticle (SLN) formulations as a potential tool for the reduction of cytotoxicity of saponins. Pharmazie. 2009;64:172-6.
- Garud A, Singh D, Garud N. Solid lipid nanoparticles (SLN): method, characterization and applications. Int Curr Pharm J. 2012;1:384-93. https://doi.org/10.3329/icpj.v1i11.12065.
- Mishra DK, Dhote V, Bhatnagar P, Mishra PK. Engineering solid lipid nanoparticles for improved drug delivery: promises and challenges of translational research. Drug Deliv Transl Res. 2012;2:238-53. https://doi.org/10.1007/s13346-012-0088-9.
- Plajnšek KT, Pajk S, Govedarica B, Pečar S, Srčič S, Kristl J. A novel fluorescent probe for more effective monitoring of nanosized drug delivery systems within the cells. Int J Pharm. 2011;416:384-93. https://doi.org/10.1016/j.ijpharm.2011.06.046.
- Jores K, Mehnert W, Drechsler M, Bunjes H, Johann C, Mäder K. Investigations on the structure of solid lipid nanoparticles (SLN) and oil-loaded solid lipid nanoparticles by photon correlation spectroscopy, field-flow fractionation and transmission electron microscopy. J Control Release. 2004;95:217-27. https://doi.org/10.1016/j.jconrel.2003.11.012.
- Daghighpoor Z. Back pain and herniated disc linked to depression. Am J Ethnomed. 2017. https://doi.org/10.21767/2348-9502-C1-003.
- Carrillo C, Sánchez-Hernández N, García-Montoya E, Pérez-Lozano P, Suñé-Negre JM, Ticó JR, Suñé C, Miñarro M. DNA delivery via cationic solid lipid nanoparticles (SLNs). Eur J Pharm Sci. 2013;49:157-65. https://doi.org/10.1016/j.ejps.2013.02.011.
- Sharifi M, Attar F, Saboury AA, Akhtari K, Hooshmand N, Hasan A, El-Sayed MA, Falahati M. Plasmonic gold nanoparticles: optical manipulation, imaging, drug delivery and therapy. J Control Release. 2019;311-312:170-89. https://doi.org/10.1016/j.jconrel.2019.08.032.
- Noack A, Hause G, Mäder K. Physicochemical characterization of curcuminoid-loaded solid lipid nanoparticles. Int J Pharm. 2012;423:44051. https://doi.org/10.1016/j.ijpharm.2011.12.011.
- Paumelle R, Blanquart C, Briand O, Barbier O, Duhem C, Woerly G, Percevault F, Fruchart JC, Dombrowicz D, Glineur C, Staels B. Acute antiinflammatory properties of statins involve peroxisome proliferator-activated receptor-alpha via inhibition of the protein kinase C signaling pathway. Circ Res. 2006;98(3):361-9. https://doi.org/10.1161/01.RES.0000202706.70992.95.
- Milsmann J, Oehlke K, Greiner R, Steffen-Heins A. Fate of edible solid lipid nanoparticles (SLN) in surfactant stabilized o/w emulsions. Part 2: Release and partitioning behavior of lipophilic probes from SLN into different phases of o/w emulsions. Colloids Surf A Physicochem Eng Asp. 2018;558:623-31. https://doi.org/10.1016/j.colsurfa.2017.05.050.
- Tang K, Lv X, Wu S, Xuan S, Huang X, Bai C. Measurement for contact angle of iron ore particles and water. ISIJ Int. 2018;58:379-400. https://doi.org/10.2355/isijinternational.ISIJINT-2017-424.
- Poumellec M-A, Dejode M, Figl A, Darcourt J, Haudebourg J, Sabah Y, Voury A, Martaens A, Barranger E. Sentinel node detection using optonuclear probe (gamma and fluorescence) after green indocyanine and radio-isotope injections. Gynecol Obstet Fertil. 2016;44:20710. https://doi.org/10.1016/j.gyobfe.2016.02.012.
- Patravale VB, Mirani AG. Preparation and characterization of solid lipid nanoparticles-based gel for topical delivery. Methods Mol Biol. 2019;2000:293-302. https://doi.org/10.1007/978-1-4939-9516-5_20.
- Abdelbary G, Fahmy RH. Diazepam-loaded solid lipid nanoparticles: design and characterization. AAPS PharmSciTech. 2009;10:211-9. https://doi.org/10.1208/s12249-009-9197-2.
- Ramteke KH, Joshi SA, Dhole SN. Solid lipid nanoparticle: a review. IOSR J Pharm. 2012;2:34-44. https://doi.org/10.9790/3013-26103444.
- Narala A, Veerabrahma K. Preparation, characterization and evaluation of quetiapine fumarate solid lipid nanoparticles to improve the oral bioavailability. J Pharm (Cairo). 2013;2013:265741. https://doi.org/10.1155/2013/265741.
- Zeng J, Pang X, Zhang L, Medina A, Rozelle S. Gender inequality in education in China: a meta-regression analysis. Contemp Econ Policy. 2014;32(2):474-91. https://doi.org/10.1111/coep.12006.
- Mehnert W, Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2001;47:165-96. https://doi.org/10.1016/s0169-409x(01)00105-3.
- Aljaeid B, Hosny KM. Miconazole-loaded solid lipid nanoparticles: formulation and evaluation of a novel formula with high bioavailability and antifungal activity. IJN. 2016. https://doi.org/10.2147/IJN.S100625.
- Venishetty VK, Chede R, Komuravelli R, Adepu L, Sistla R, Diwan PV. Design and evaluation of polymer coated carvedilol loaded solid lipid nanoparticles to improve the oral bioavailability: a novel strategy to avoid intraduodenal administration. Colloids Surf B Biointerfaces. 2012;95:1-9. https://doi.org/10.1016/j.colsurfb.2012.01.001.
- Wissing SA, Müller RH, Manthei L, Mayer C. Structural characterization of Q10-loaded solid lipid nanoparticles by NMR spectroscopy. Pharm Res. 2004;21:400-5. https://doi.org/10.1023/B:PHAM.0000019291.36636.c1.
- Güney G, Kutlu HM, Genç L. Preparation and characterization of ascorbic acid loaded solid lipid nanoparticles and investigation of their apoptotic effects. Colloids Surf B Biointerfaces. 2014;121:270-80. https://doi.org/10.1016/j.colsurfb.2014.05.008.
- Pulsoni I, Lubda M, Aiello M, Fedi A, Marzagalli M, Von Hagen J, Scaglione S. Comparison between franz diffusion cell and a novel microphysiological system for in vitro penetration assay using different skin models. SLAS Technol. 2022;27:161-71. https://doi.org/10.1016/j. slast.2021.12.006.
Sarad Pawar Naik Bukke, drsaradpawar@kiu.ac.ug; Chandrakala Venkatesh, chandrakala.epcp@eastpoint.ac.in Department of Pharmaceutics and Pharmaceutical Technology, Kampala International University, Western Campus, P.O. Box 71, Ishaka-Bushenyi, Uganda. Department of Pharmaceutics, East Paint College of Pharmacy, Bidarahalli, Bangalore 560049, Karnataka, India. Department of Pharmaceutics, SRM College of Pharmacy, SRM Institute of Science and Technology, Kattankulathur, Tamilnadu 603203, India. Department of Pharmaceutical Chemistry, School of Pharmaceutical Sciences, Delhi Pharmaceutical Sciences and Research University, New Delhi, India. Department of Clinical Pharmacy and Pharmacy Practice, Kampala International University, Western Campus, P.O. Box 71, Ishaka-Bushenyi, Uganda.
