DOI: https://doi.org/10.1007/s11356-023-31729-5
PMID: https://pubmed.ncbi.nlm.nih.gov/38191734
تاريخ النشر: 2024-01-08
دراسات حية ودراسات حاسوبية لتحديد آليات سمية البيرميثرين مع الدور المخفف للسمية للزنجبيل
© المؤلفون 2024
الملخص
في هذه الدراسة، تم التحقيق في التأثيرات السامة للبيرميثرين على Allium cepa L. والدور الوقائي لمستخلص جذور الزنجبيل (Zoex). في هذا السياق، تم تشكيل 6 مجموعات مختلفة. بينما تم معالجة مجموعة التحكم بمياه الصنبور، تم معالجة المجموعتين II و III بـ
إمين يالتشين
emine.yalcin@giresun.edu.tr
داملا هيمتاش
damlahim@giresun.edu.tr
كولتيغين تشافوش أوغلو
kultigin.cavusoglu@giresun.edu.tr
علي عكار
ali.acar@giresun.edu.tr
1 قسم البيولوجيا، معهد العلوم الطبيعية، جامعة جيرسون، 28200 جيرسون، تركيا
2 قسم البيولوجيا، كلية العلوم والفنون، جامعة جيرسون، 28200 جيرسون، تركيا
3 قسم الخدمات الطبية والتقنيات، المدرسة المهنية للخدمات الصحية، جامعة جيرسون، 28200 جيرسون، تركيا
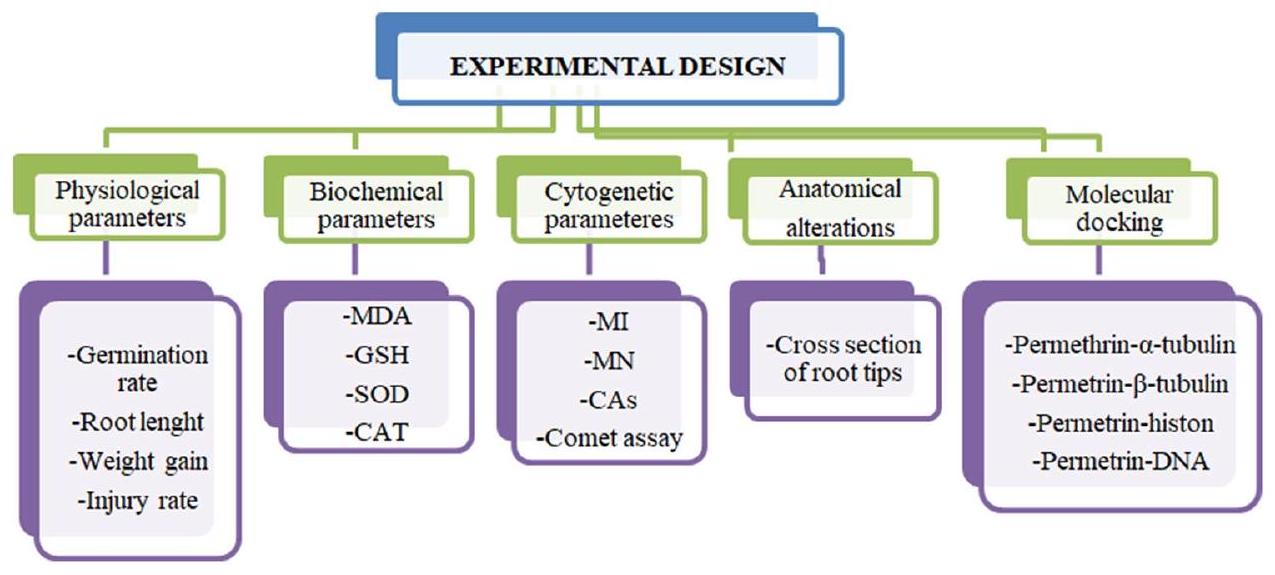
مقدمة
has نشاط مضاد للأكسدة قوي. يظهر Z. officinale نشاطًا مضادًا للأكسدة من خلال آليات مختلفة بسبب المركبات النشطة التي تحتوي عليها ويوفر الحماية ضد العديد من الأمراض الناجمة عن الإجهاد التأكسدي. زيادة تعبير إنزيمات مضادات الأكسدة، منع تكوين الجذور الحرة، منع أكسدة الدهون، وتحفيز تخليق الجلوتاثيون هي بعض من هذه الآليات.
المواد والطرق
استخراج الزنجبيل
المجموعات التجريبية
معايير الإنبات
المعلمات السيتوجينية
اختبار المذنب
دراسة حاسوبية حول تفاعلات البيرميثرين مع الجزيئات الخلوية
التحليل الطيفي لتفاعل بيرميثرين مع الحمض النووي
تمت دراسة طيف الحمض النووي المعزول من الأليوم. تم عزل الحمض النووي من خلايا طرف الجذر لنبات A. cepa وفقًا للطريقة التي طورها شارما وآخرون (2002). تم تقييم تفاعلات الحمض النووي مع البيرميثرين من خلال قياس امتصاصات محلول الحمض النووي عند أطوال موجية مختلفة في وجود وغياب البيرميثرين (حمض نووي/بيرميثرين،
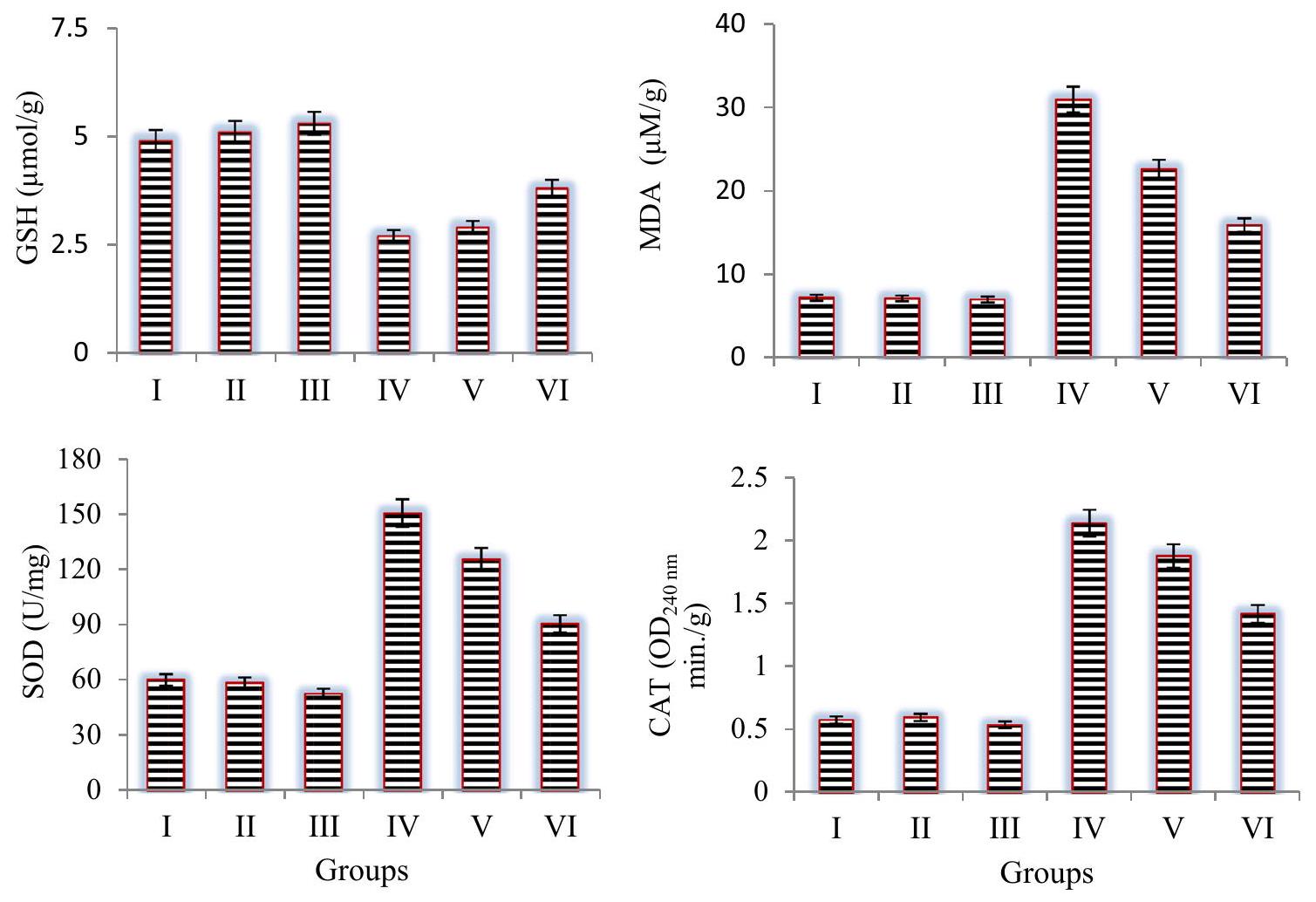
اضطرابات في توازن مضادات الأكسدة والأكسدة
آثار التعافي من زوكس
تغيرات تشريحية
التحليل الإحصائي
النتائج والمناقشة
معايير متعلقة بالإنبات
| مجموعات | الهامش الإجمالي (%) | طول الجذر (سم) | الوزن الابتدائي (غ) | الوزن النهائي (غ) | زيادة الوزن (غ) | معدل الإصابة النسبي |
| أنا | 100 |
|
|
|
|
ND |
| الثاني | 100 |
|
|
|
|
ND |
| ثالثاً | 100 |
|
|
|
|
ND |
| الرابع | 65 |
|
|
|
|
0.53 |
| ف | 71 |
|
|
|
|
0.29 |
| السادس | 79 |
|
|
|
|
0.21 |
ديناميكية مضادات الأكسدة/الأكسدة
آثار سامة للخلايا
| تحكم |
|
|
|
|
|
|||||
| MN |
|
|
|
|
|
|
||||
| FRG |
|
|
|
|
|
|
||||
| SC |
|
|
|
|
|
|
||||
| VN |
|
|
|
|
|
|
||||
| UDC |
|
|
|
|
|
|
||||
| ب |
|
|
|
|
|
|
||||
| قبل الميلاد |
|
|
|
|
|
|
||||
| RP |
|
|
|
|
|
|
||||
|
ج | د | ||||||||
 |
ف | ج | ح | |||||||
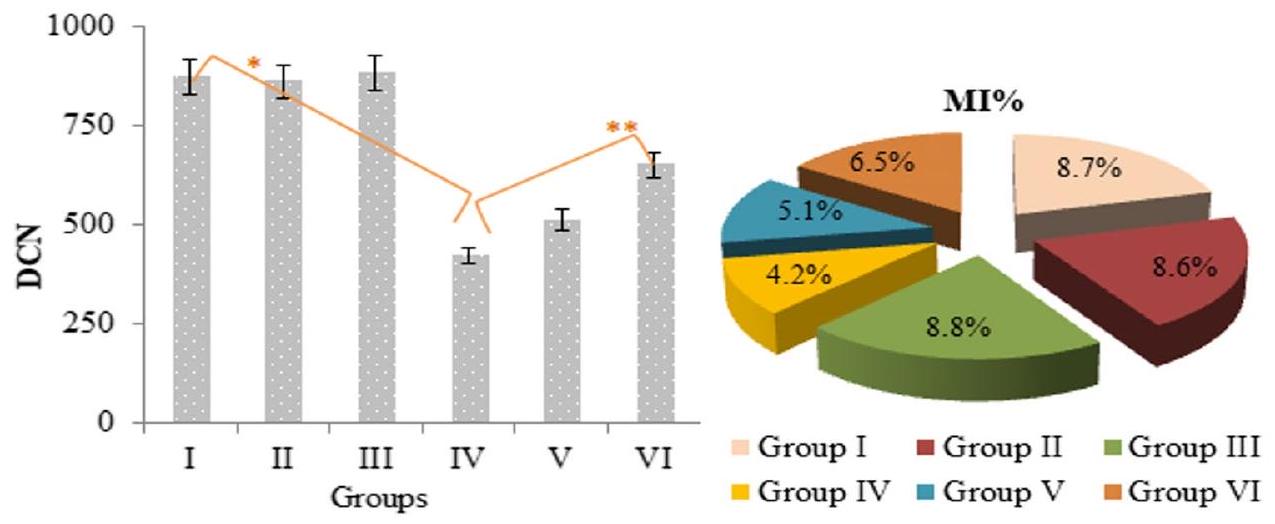
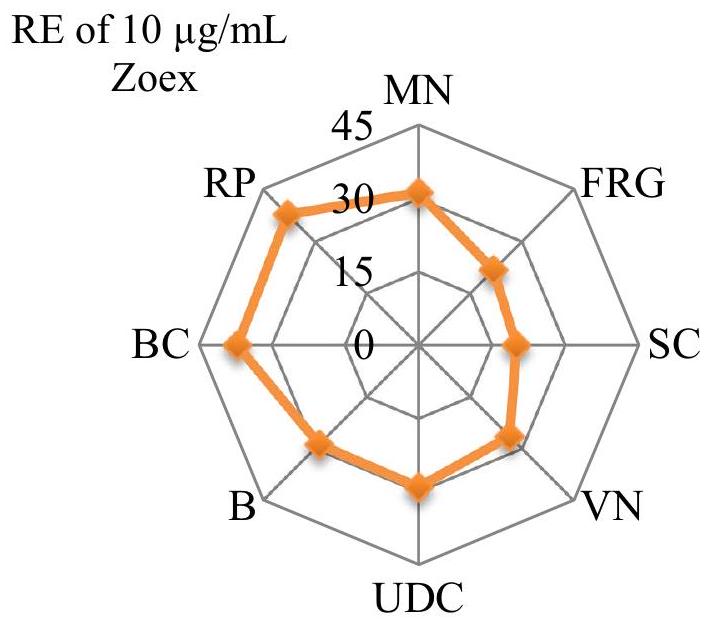
تمت ملاحظته أيضًا، جنبًا إلى جنب مع انخفاض في معدل MI. تم اكتشاف تشكيل MN في إجمالي 72.84 خلية في المجموعة المعالجة بالبرميثرين (الجدول 2). تدعم التردد العالي لـ MN، الذي تم اكتشافه في مجموعة البرميثرين، أيضًا الانخفاض في معدل MI. يمكن أن تؤدي الشذوذات المغزلية، التي تؤدي إلى انخفاض في معدل MI، أيضًا إلى تحفيز تشكيل MN (فينش ونيفيل 1992). وجد دليك (1998) أن إعطاء البرميثرين بتركيزات من
دورة الانقسام الميتوزي وتكوين MN. يحمي زوكس الخلايا من مثل هذه الأكسدة ويضمن استمرار دورة الانقسام الميتوزي الطبيعية. المركبات الفينولية مثل الزنجرول والشوجول في زوكس لها تأثير في تقليل الإجهاد التأكسدي وزيادة مستويات مضادات الأكسدة مثل GSH. هذا التأثير أيضًا يعادل الضرر التأكسدي في الخلايا ويحمي البروتينات مثل المغزل وغيرها من الجزيئات الكبيرة من الضرر (كوهاد وآخرون 2006؛ دوغاساني وآخرون 2020). أفاد أوكيسولا وآخرون (2019) أن Z. officianale قلل من تكرار تكوين MN وحسن معدلات MI، وأفادوا أن هذه الخاصية الواقية كانت بسبب المكونات النشطة مثل الفينولات والصابونين والقلويدات في الزنجبيل. نتيجة لذلك، أظهر البيرميثرين تأثيرًا جينيًا عن طريق التسبب في انخفاض معدلات MI وزيادة تكرار MN، بينما قدم تطبيق زوكس الحماية ضد السمية الخلوية، وكان يُعتقد أن هذه الحماية مرتبطة بالمركبات النشطة في زوكس.
التأثيرات الجينية
تم الكشف عن تشكيلات في المجموعة الرابعة، التي تم إعطاؤها البيرميثرين فقط. بينما تحدث الشظايا بأعلى تكرار بين الشذوذ الكروموسومي، فإن الكروموسومات اللزجة، النواة المتجوفة، التوزيع غير المتساوي للكروماتين، الجسر، الخلايا ثنائية النواة، والاستقطاب العكسي هي الشذوذ الكروموسومي الأخرى. إن حقيقة أن البيرميثرين يسبب الشظايا بمعدل مرتفع تظهر أنه يحفز الانكسارات في الحمض النووي. في المراحل اللاحقة من انقسام الخلايا، تصبح هذه الشظايا MN. الشذوذ الآخر الذي يسببه البيرميثرين يعرف باسم الكروموسوم اللزج، الذي يتطور نتيجة لزيادة تفكك الحمض النووي، وتكثيف الكروموسومات، والانحلال الجزئي للبروتينات النووية. الكروموسومات اللزجة هي علامة على عواقب ضارة للغاية لأنها غالبًا ما تكون غير قابلة للعكس وقد تسبب موت الخلايا. الكروموسومات المتشردة، التي يتم اكتشافها بتكرار عالٍ نتيجة لتطبيق البيرميثرين، تعمل بشكل منفصل عن مجموعة الكروموسومات التي تُسحب إلى الأقطاب، مما يتسبب في فصل غير متساوٍ لعدد الكروموسومات في خلايا الابنة (خانا وشارما 2013). إن حقيقة أن البيرميثرين يحفز أنواعًا مختلفة من الشذوذ الكروموسومي وأن كل شذوذ يحدث بآليات مختلفة تشير إلى أن البيرميثرين لا يظهر تأثيرًا جينيًا محددًا ولكنه يحفز تشكيلات الشذوذ الكروموسومي بآليات متعددة. أفاد فالتشوني وآخرون (2010) أن
آلية السمية الخلوية والجينية المدعومة بالتجميع الجزيئي

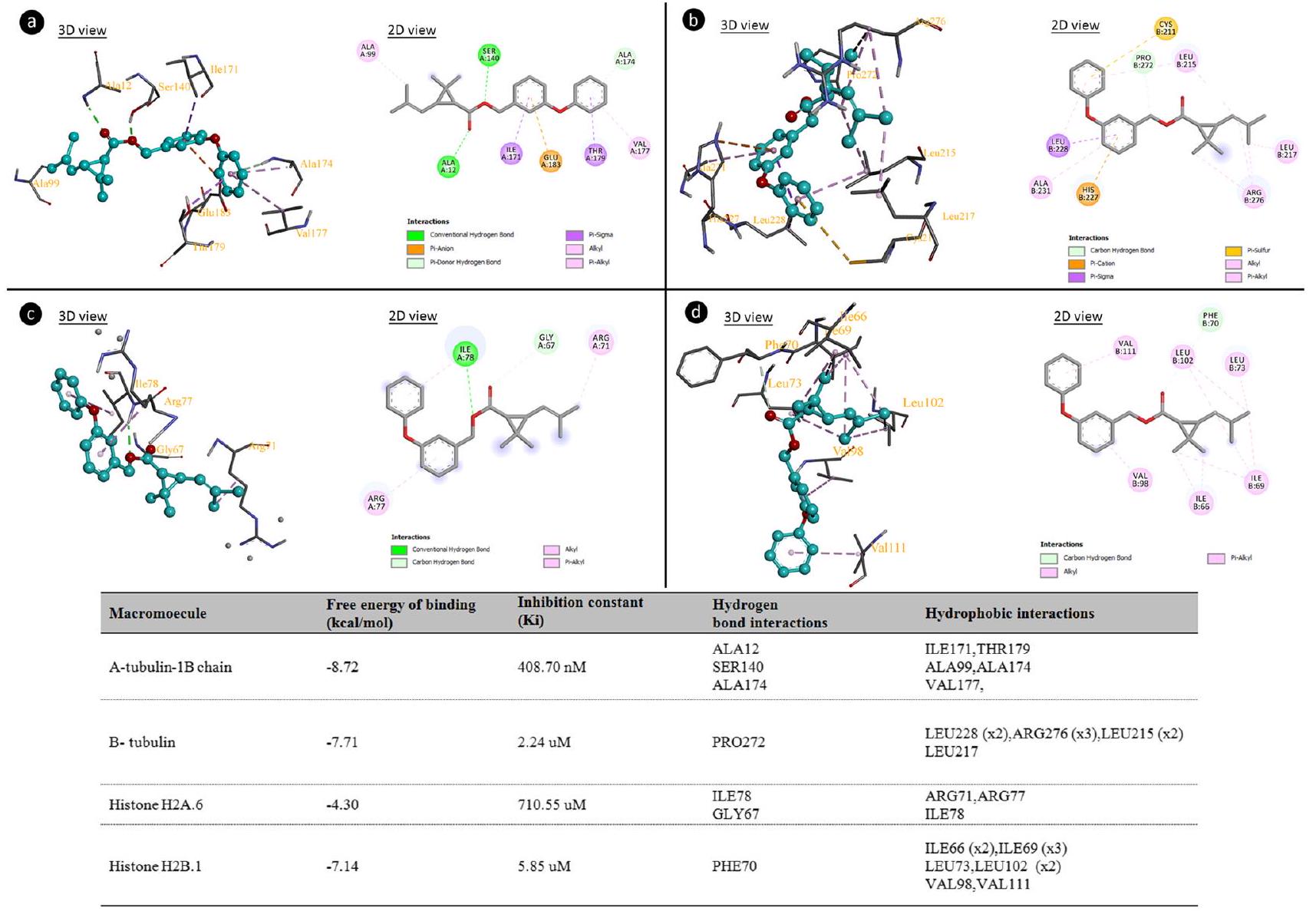
البروتينات أيضًا إلى تعطيل تجميع الأنابيب الدقيقة. إن التفاعل المحتمل للبيرميثرين مع بروتينات التوبولين يؤدي إلى شذوذ في بنية البوليببتيد. نتيجة لهذا الشذوذ، يتم منع تجميع الأنابيب الدقيقة ويتم تقييد حركة الكروموسومات نحو الأقطاب، مما يؤدي إلى تعطيل المراحل الميتوزية والشذوذ الكروموسومي. تعتبر تفاعلات البيرميثرين-التوبولين أساس التأثير السمي للبيرميثرين، والذي يتجلى من خلال انخفاض معدل MI في A. cepa. قد يحدث التأثير الجيني للبيرميثرين نتيجة لتفاعلات البيرميثرين-الهيستون (الشكل 5) وتفاعلات البيرميثرين-DNA (الشكل 6). يتمتع البيرميثرين بالطاقة الربطية -4.30 و
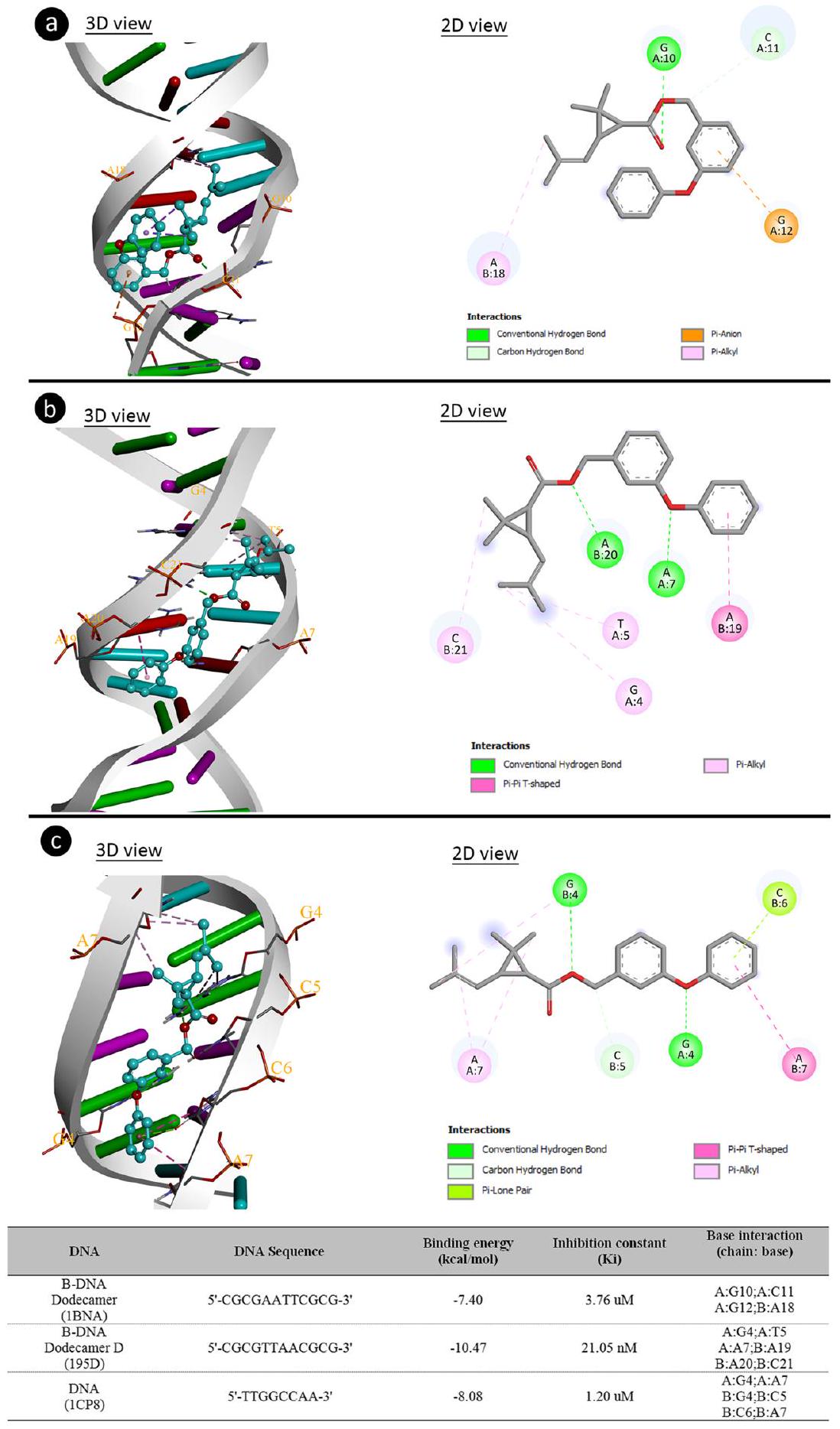
غير الحمض النووي. من بين هذه التأثيرات تشمل تثبيط تخليق الحمض النووي أو الحمض النووي الريبي، طفرات الإزاحة، وانكسارات الحمض النووي المرتبطة بالبروتين. وُجد أن البيرميثرين له تأثير جيني سام من خلال الارتباط بأنواع مختلفة من الحمض النووي ومن خلال عمله كعامل إدخال. المركبات القادرة على الإدخال في الحمض النووي تؤدي إلى زيادة في التأثيرات الكلاستوجينية. نتيجة لهذه التأثيرات، قد تحدث شذوذات في تخليق الحمض النووي، انكسارات في الخيوط، إدراج، حذف، أو إعادة ترتيب الكروموسومات (فيرغسون وديني 2007).
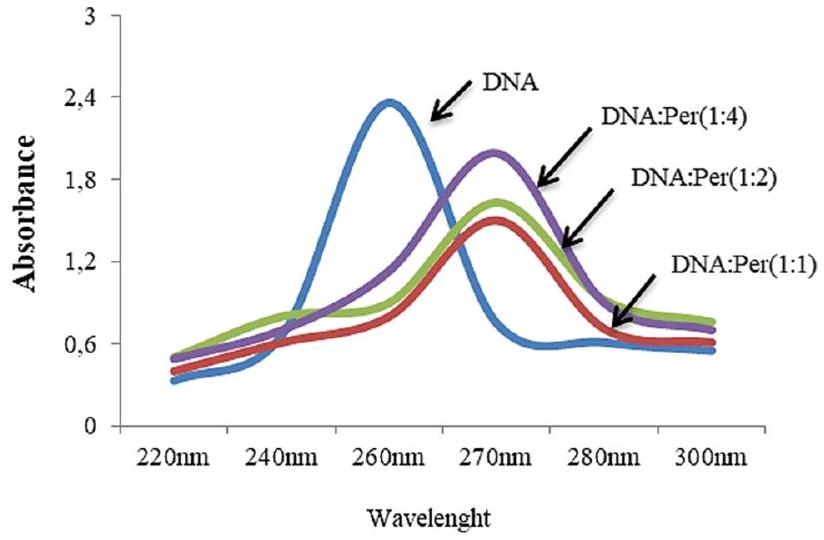
تفاعل DNA مع البيرميثرين تم تأكيده من خلال التحول الطيفي
اختبار المذنب

تغيرات تشريحية
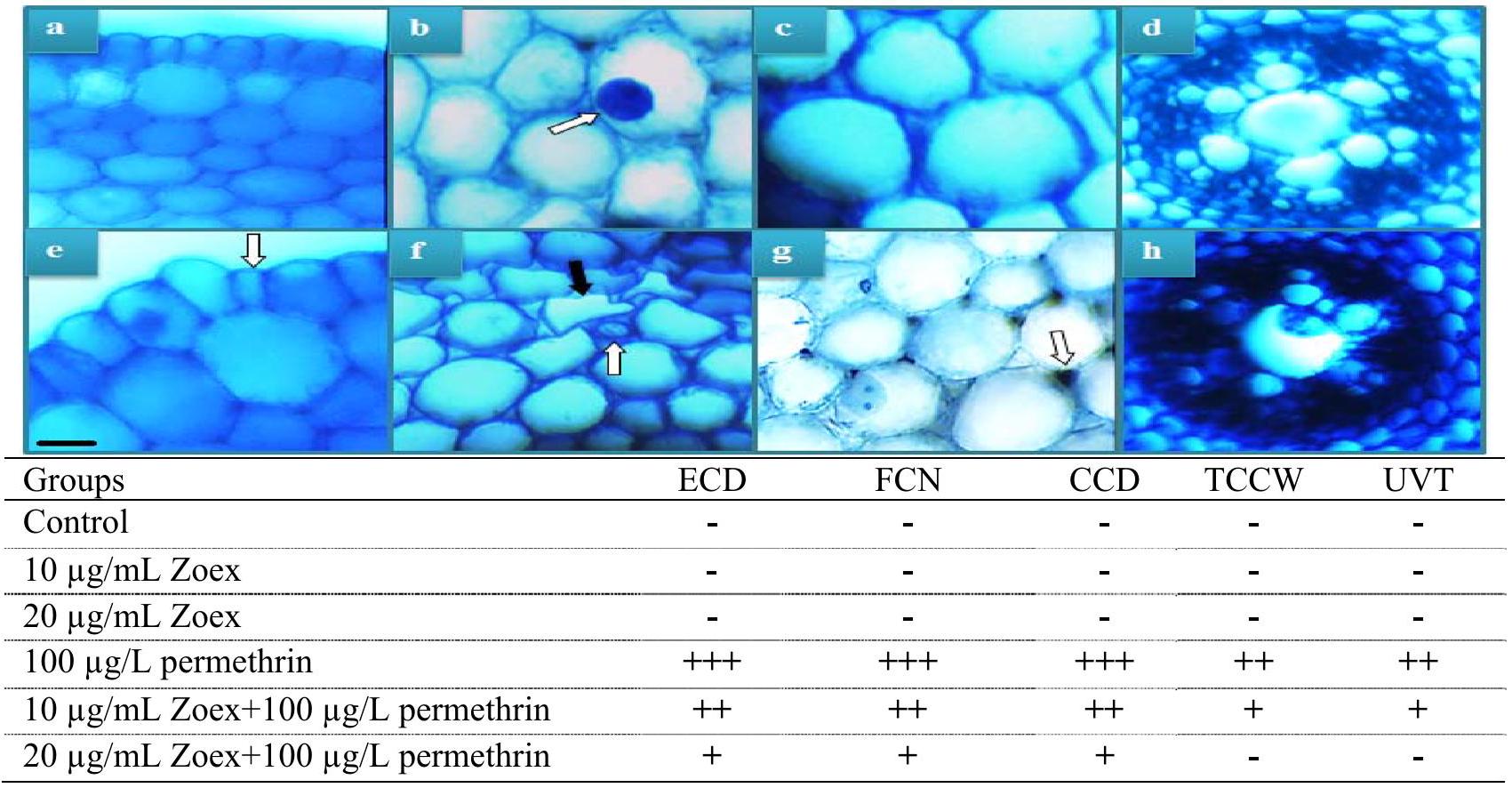
هذا التغيير يعمل كحاجز ضد المواد الكيميائية الضارة ويمنع المركبات السامة من دخول الأنسجة الوعائية. تغيير شكل النواة هو شذوذ آخر، وقد تكون التغيرات الجينية والبيوكيميائية التي تسببها البيرميثرين هي سبب تسطح النواة. قد تنتج التغيرات في شكل النواة عن تدهور حجم النواة، وتركيز البروتين، وسلامة الحمض النووي، والكثافة (دال وآخرون 2008؛ داور وورمان 2009). وبالمثل، وجد يالتشين وآخرون (2019) أن العديد من التغيرات الشكلية، مثل تشوه خلايا البشرة وتثخين جدران الخلايا، لوحظت في تشريح الجذر تحت ضغط كيميائي. تشمل الشذوذات الأخرى التي تسببها البيرميثرين الأنسجة الوعائية غير المنظمة والأضرار الخلوية للبشرة. قد يكون سبب كل هذه الشذوذات هو الإجهاد التأكسدي الناتج عن البيرميثرين، وتأثيراته السامة للخلايا والجينات. تمنع هذه الشذوذات التشريحية النبات من امتصاص العناصر الغذائية ونقلها إلى أنسجة أخرى وقد تؤخر أيضًا النمو. أدى تطبيق زوكس مع البيرميثرين إلى انخفاض في حدوث الأضرار التشريحية. تطبيق
الخاتمة
إعلانات
موافقة للمشاركة غير قابلة للتطبيق.
موافقة للنشر غير قابلة للتطبيق.
المصالح المتنافسة يعلن المؤلفون عدم وجود مصالح متنافسة.
References
Ahmad P, Jaleel CA, Salem MA, Nabi G, Sharma S (2010) Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit Rev Biotechnol 30:161-175. https://doi.org/10.3109/ 07388550903524243
Akgeyik AU, Yalçın E, Çavuşoğlu K (2023) Phytochemical fingerprint and biological activity of raw and heat-treated Ornithogalum umbellatum. Sci Rep 13(1):13733. https://doi.org/10.1038/ s41598-023-41057-w
Akgündüz MÇ, Çavuşoğlu K, Yalçın E (2020) The potential risk assessment of phenoxyethanol with a versatile model system. Sci Rep 10(1):1-10. https://doi.org/10.1038/s41598-020-58170-9
Al-Amoudi WM (2018) Toxic effects of lambda-cyhalothrin, on the rat thyroid: involvement of oxidative stress and ameliorative effect of ginger extract. Toxicol Rep 5:728-736. https://doi.org/10.1016/j. toxrep.2018.06.005
Al-Nahain A, Jahan R, Rahmatullah M (2014) Zingiber officinale: a potential plant against rheumatoid arthritis. Arthritis 2014. https:// doi.org/10.1155/2014/159089
Aydin D, Yalçın E, Çavuşoğlu K (2022) Metal chelating and anti-radical activity of Salvia officinalis in the ameliorative effects against uranium toxicity. Sci Rep 12(1):15845. https://doi.org/10.1038/ s41598-022-20115-9
Balendiran K, Rao ST, Sekharudu CY, Zon G, Sundaralingam M (1995) X-ray structures of the B-DNA dodecamer d (CGCGTT AACGCG) with an inverted central tetranucleotide and its netropsin complex. Acta Crystallogr D Biol Crystallogr 51:190-198. https://doi.org/10.1107/S0907444994010759
Banerjee S, Mullick HI, Banerjee J, Ghosh A (2011) Zingiber officinale: ‘a natural gold.’ Int J Pharmaceutical Bio-Sci 2:283-294
Borowik A, Wyszkowska J, Zaborowska M, Kucharski J (2023) The impact of permethrin and cypermethrin on plants, soil enzyme
activity, and microbial communities. Int J Mol Sci 24(3):2892. https://doi.org/10.3390/ijms24032892
Çakir F, Kutluer F, Yalçın E, Çavuşoğlu K, Acar A (2023) Deep neural network and molecular docking supported toxicity profile of prometryn. Chemosphere 139962. https://doi.org/10.1016/j.chemo sphere.2023.139962
Çavuşoğlu K, Yalçın E (2023) Spectral shift supported epichlorohydrin toxicity and the protective role of sage. Environ Sci Pollut Res 30(1):1374-1385. https://doi.org/10.1007/s11356-022-22288-2
Çavuşoğlu K, Gür B, Yalçın E, Demirtaş G, Çiçek F (2014) The effect of lambda-cyhalothrin on root tip cytology, pigment contents and antioxidant defense system of Allium cepa. Cytologia 79(1):95101. https://doi.org/10.1508/cytologia.79.95
Chakraborty R, Mukherjee AK, Mukherjee A (2009) Evaluation of genotoxicity of coal fly ash in Allium cepa root cells by combining comet assay with the Allium test. Environ Monit Assess 153:351-357. https://doi.org/10.1007/s10661-008-0361-z
Collins AR (2004) The comet assay for DNA damage and repair. Mol Biotechnol 26(3):249-261. https://doi.org/10.1385/MB:26:3:249
Dahl KN, Ribeiro AJ, Lammerding J (2008) Nuclear shape, mechanics, and mechanotransduction. Circ Res 102:1307-1318. https://doi. org/10.1161/CIRCRESAHA.108.173989
Dauer WT, Worman HJ (2009) The nuclear envelope as a signaling node in development and disease. Dev Cell 17(5):626-638. https://doi.org/10.1016/j.devcel.2009.10.016
Davoodi R, Gholamreza ABDİ (2012) Comparative study on the acute toxicity of synthetic pesticides, permethrin
Delic N (1998) Effect of permethrin on mitotic activity of cultured human lymphocytes. Pesticides 13:233-238
Demirtaş G, Çavuşoğlu K, Yalçın E (2020) Aneugenic, clastogenic, and multi-toxic effects of diethyl phthalate exposure. Environ Sci Pollut Res 27:5503-5510. https://doi.org/10.1007/ s11356-019-07339-5
Drew HR, Wing RM, Takano T, Broka C, Tanaka S, Itakura K, Dickerson RE (1981) Structure of a B-DNA dodecamer: conformation and dynamics. Proc Natl Acad Sci 78(4):2179-2183. https://doi. org/10.1073/pnas.78.4.2179
Dubus IG, Hollis JM, Brown CD (2000) Pesticide in rainfall in Europe. Environ Pollut 110:331-344. https://doi.org/10.1016/S0269-7491(99)00295-X
Dugasani S, Pichika MR, Nadarajah VD, Balijepalli MK, Tandra S, Korlakunta JN (2020) Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J Ethnopharmacol 127(2):515-520. https://doi.org/ 10.1016/j.jep.2009.10.004
Fenech M, Neville S (1992) Conversion of excision-repairable DNA lesions to micronuclei within one cell cycle in human lymphocytes. Environ Mol Mut 19:27-36. https://doi.org/10.1002/em. 2850190106
Ferguson LR, Denny WA (2007) Genotoxicity of non-covalent interactions: DNA intercalators. Mutat Res Fundam Mol Mech Mutagen 623(1-2):14-23. https://doi.org/10.1016/j.mrfmmm.2007.03.014
Gabbianelli R, Palan M, Flis DJ, Fedeli D, Nasuti C, Skarydova L, Ziolkowski W (2013) Imbalance in redox system of rat liver following permethrin treatment in adolescence and neonatal age. Xenobiotica 43:1103-1110. https://doi.org/10.3109/00498254. 2013.796427
Guex N, Peitsch M (2005) CSWISS-MODEL and the Swiss-Pdb Viewer: an environment for comparative protein modeling. Electrophoresis 18:2714-2723. https://doi.org/10.1002/elps. 11501 81505
Hausladen A, Alscher RG (1993) Glutathione. In: Alscher RG, Hess JL (eds) Antioxidants in higher plants. CRC Press, Boca Raton, pp 1-30
Jeena K, Liju VB, Ramanath V, Kuttan R (2016) Protection against whole body gamma-irradiation induced oxidative stress and clastogenic damage in mice by ginger essential oil. APJCP 17(3):1325-1332. https://doi.org/10.7314/APJCP.2016.17.3. 1325
Junquera P (2021) Permethrin: safety summary for veterinary use in dogs, cats, horses, cattle, sheep, goats, swine and poultry. Poisoning, intoxication, overdose, antidote. https://parasitipedia. net/index.php?option=com_content&view=article&id=2676& Itemid=. Accessed 27 Apr 2023
Katahira R, Katahira M, Yamashita Y, Ogawa H, Kyogoku Y, Yoshida M (1998) Solution structure of the novel antitumor drug UCH9 complexed with d (TTGGCCAA) 2 as determined by NMR. Nucleic Acids Res 26:744-755. https://doi.org/10. 1093/nar/26.3.744
Kerksick C, Willoughby D (2005) The antioxidant role of glutathione and N -acetyl-cysteine supplements and exercise-induced oxidative stress. J Internat Soc Sports Nutr 2(2):1-7. https://doi.org/ 10.1186/1550-2783-2-2-38
Kim YS, Hong CS, Lee SW, Nam JH, Kim BJ (2017) Effects of ginger and its pungent constituents on transient receptor potential channels. Biophys J 112(3):250
Końca K, Lankoff A, Banasik A, Lisowska H, Kuszewski T, Góźdź S, Koza Z, Wojcik A (2003) A cross-platform public domain PC image-analysis program for the comet assay. Mutat Res 534:1520. https://doi.org/10.1016/s1383-5718(02)00251-6
Kurt D, Yalçin E, Çavuşoğlu K (2023) GC-MS and HPLC supported phytochemical analysis of watercress and the protective role against paraben toxicity. Environ Sci Pollut Res 30(3):60336046. https://doi.org/10.1007/s11356-022-22380-7
Lacey SE, He S, Scheres SH, Carter AP (2019) Cryo-EM of dynein microtubule-binding domains shows how an axonemal dynein distorts the microtubule. Elife 8:e47145. https://doi.org/10. 7554/eLife. 47145
Luo Q, Wang B, Wu Z, Jiang W, Wang Y, Du K, Zhou N, Zheng L, Gan J, Shen WH, Ma J, Dong A (2020) NAP1-Related Protein 1 (NRP1) has multiple interaction modes for chaperoning histones H2A-H2B. Proc Natl Acad Sci USA 117(48):30391-30399. https://doi.org/10.1073/pnas. 2011089117
Macar O, Kalefetoğlu Macar T, Çavuşoğlu K, Yalçın E (2020) Protective effects of anthocyanin-rich bilberry (Vaccinium myrtillus L.) extract against copper (II) chloride toxicity.
Mao QQ, Xu XY, Cao SY, Gan RY, Corke H, Beta T, Li HB (2019) Bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe). Foods 8(6):185. https://doi.org/10.3390/foods80601 85
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30:2785-2791. https://doi.org/10.1002/jcc. 21256
O’Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR (2011) Open Babel: an open chemical toolbox. J Cheminform 3:33. https://doi.org/10.1186/1758-2946-3-33
Okesola MA, Ajiboye BO, Oyinloye BE, Ojo OA (2019) Effect of Zingiber officinale on some biochemical parameters and cytogenic analysis in lead-induced toxicity in experimental rats. Tox Mech Meth 29(4):255-262
Ozluer C, Satana Kara HE (2014) In vitro DNA binding studies of anticancer drug idarubicin using spectroscopic techniques. J Photochem Photobiol B 138:36-42. https://doi.org/10.1016/j.jphot obiol.2014.05.015
Policegoudra RS, Rehna K, Rao LJ, Aradhya SM (2010) Antimicrobial, antioxidant, cytotoxicity and platelet aggregation inhibitory activity of a novel molecule isolated and characterized from mango ginger rhizome. J Biosci 35(2):231-240. https://doi.org/10.1007/ s12038-010-0027-1
Roma GC, De Oliveira PR, Araujo AM, Bechara GH, Mathias MIC (2012) Genotoxic and mutagenic effects of permethrin in mice: micronuclei analysis in peripheral blood erythrocytes. Mic Res Tech 75(12):1732-1736. https://doi.org/10.1002/jemt. 22124
Saha C, Kumar R, Das A (2017) Understanding nucleosomal histone and DNA interactions: a biophysical study. J Biomol Struct Dyn 35(12):2531-2538. https://doi.org/10.1080/07391102.2016.1225603
Sellami B, Louati H, Dellali M, Aissa P, Mahmoudi E, Coelho AV, Sheehan
Sharma AD, Gill PK, Singh P (2002) DNA isolation from dry and fresh samples of polysaccharide-rich plants. Plant Mol Biol Rep 20:415. https://doi.org/10.1007/BF02772129
Singh D, Pal M, Singh R, Singh CK, Chaturvedi AK (2015) Physiological and biochemical characteristics of Vigna species for Al stress tolerance. Acta Physiol Plant 37:1-13
Sun YJ, Liang YJ, Yang L, Long DX, Wang HP, Wu YJ (2022) Longterm low-dose exposure of permethrin induces liver and kidney damage in rats. BMC Pharmacol Toxicol 23:46. https://doi.org/ 10.1186/s40360-022-00586-2
Tiryaki O, Canhilal R, Horuz S (2010) The use of pesticides and their risks. Erciyes Univ J Inst Sci Technol 26(2):154-169
Tütüncü E, Yalçin E, Acar A, Yapar K, Çavuşoğlu K (2019) Investigation of the toxic effects of a carbamate insecticide methiocarb in Allium cepa L. Cytologia 84(2):113-117. https://doi.org/10.1508/ cytologia.84.113
Weidinger A, Kozlov AV (2015) Biological activities of reactive oxygen and nitrogen species: oxidative stress versus signal transduction. Biomolecules 5(2):472-548. https://doi.org/10.3390/biom5 020472
Yalçın E, Uzun A, Çavuşoğlu K (2019) In vivo epiclorohidrine toxicity: cytogenetic, biochemical, physiological, and anatomical evidences. Environ Sci Pollut Res 26:22400-22406. https://doi.org/ 10.1007/s11356-019-05518-y
Yalçin E, Çavuşoğlu K (2022b) Spectroscopic contribution to glyphosate toxicity profile and the remedial effects of Momordica charantia. Sci Rep 12(1):20020. https://doi.org/10.1038/ s41598-022-24692-7
- Values shown with different letters in the same column are statistically significant
not determined - ECD, epidermis cell damage; CCD, cortex cell damage; TCCW, thickening of cortex cell walls; UVT, unclear vascular tissue; FCN, flattened cell nucleus. (-) no damage, (+) minor damage, () medium damage, (+) severe damage. Bar
DOI: https://doi.org/10.1007/s11356-023-31729-5
PMID: https://pubmed.ncbi.nlm.nih.gov/38191734
Publication Date: 2024-01-08
In-vivo and in-silico studies to identify toxicity mechanisms of permethrin with the toxicity-reducing role of ginger
© The Author(s) 2024
Abstract
In this study, the toxic effects of permethrin on Allium cepa L. and the protective role of Zingiber officinale rhizome extract (Zoex) were investigated. In this context, 6 different groups were formed. While the control group was treated with tap water, the groups II and III were treated with
Emine Yalçin
emine.yalcin@giresun.edu.tr
Damla Himtaş
damlahim@giresun.edu.tr
Kültiğin Çavuşoğlu
kultigin.cavusoglu@giresun.edu.tr
Ali Acar
ali.acar@giresun.edu.tr
1 Department of Biology, Institute of Natural Sciences, University of Giresun, 28200 Giresun, Turkey
2 Department of Biology, Faculty of Science and Art, University of Giresun, 28200 Giresun, Turkey
3 Department of Medical Services and Techniques, Vocational School of Health Services, University of Giresun, 28200 Giresun, Turkey
Introduction
has potent antioxidant activity. Z. officinale exhibits antioxidant activity through various mechanisms due to the active compounds they contain and provide protection against numerous diseases caused by oxidative stress. Increasing the expression of antioxidant enzymes, preventing the formation of free radicals, preventing lipid peroxidation, and stimulating glutathione synthesis are some of these mechanisms.
Material and methods
Zingiber officinale extraction
Experimental groups
Germination related parameters
Cytogenetic parameters
Comet test

In silico study on permethrin interactions with cellular molecules
Spectral analysis of permethrin-DNA interaction
spectrum of DNA isolated from Allium were investigated. DNA was isolated from root tip cells of A. cepa according to the method developed by Sharma et al. (2002). DNApermethrin interactions were evaluated by measuring the absorbances of the DNA solution at different wavelengths in the presence and absence of permethrin (DNA/permethrin,
Disruptions in antioxidant-oxidant balance
Recovery effects of Zoex
Anatomical alterations
Statistical analysis
Results and discussion
Germination-related parameters
| Groups | GP (%) | Root length (cm) | Initial weight (g) | Final weight (g) | Weight gain (g) | Relative injury rate |
| I | 100 |
|
|
|
|
ND |
| II | 100 |
|
|
|
|
ND |
| III | 100 |
|
|
|
|
ND |
| IV | 65 |
|
|
|
|
0.53 |
| V | 71 |
|
|
|
|
0.29 |
| VI | 79 |
|
|
|
|
0.21 |
Antioxidant/oxidant dynamic

Cytotoxic effects

| Control |
|
|
|
|
|
|||||
| MN |
|
|
|
|
|
|
||||
| FRG |
|
|
|
|
|
|
||||
| SC |
|
|
|
|
|
|
||||
| VN |
|
|
|
|
|
|
||||
| UDC |
|
|
|
|
|
|
||||
| B |
|
|
|
|
|
|
||||
| BC |
|
|
|
|
|
|
||||
| RP |
|
|
|
|
|
|
||||
|
c | d | ||||||||
 |
f | g | h | |||||||
also observed, along with a decrease in the MI rate. The formation of MN was detected in a total of 72.84 cells in the group treated with permethrin (Table 2). The high frequency of MN, detected in the permethrin group, also supports the decrease in the MI rate. Spindle abnormalities, which lead to a decrease in the MI rate, can also trigger the formation of MN (Fenech and Neville 1992). Delic (1998) found that the administration of permethrin at concentrations of
the mitotic cycle, and MN formation. Zoex protects cells against such oxidations and ensures the continuation of the normal mitotic cycle. Phenolic compounds such as gingerol and shogaol in Zoex have the effect of reducing oxidative stress and increasing the levels of antioxidants such as GSH. This effect also neutralizes oxidative damage in cells and protects proteins such as spindle and other macromolecules from damage (Kuhad et al. 2006; Dugasani et al. 2020). Okesola et al. (2019) reported that Z. officianale reduced the frequency of formation of MN and improved the rates of MI, and reported that this protective property was due to active components such as phenols, saponins, and alkaloids in ginger. As a result, permethrin showed a genotoxic effect by causing a decrease in MI rates and an increase in MN frequency, while the Zoex application provided protection against cytotoxicity, and this protection was thought to be related to the active compounds in Zoex.
Genotoxic effects
formations were detected in group IV, which was administered only permethrin. While fragments occur with the highest frequency among chromosomal aberrations, sticky chromosomes, vacuolated nucleus, unequal distribution of chromatin, bridge, binuclear cells, and reverse polarization are the other chromosomal aberrations. The fact that permethrin causes fragment at a high rate shows that it induces breaks in DNA. In the later stages of cell division, these fragments become MN. Another aberration induced by permethrin is known as the sticky chromosome, which develops as a result of increased depolymerization of DNA, chromosomal condensation, and partial dissolution of nucleoproteins. Sticky chromosomes are a sign of extremely harmful consequences because they are frequently irreversible and may cause cell death. Vagrant chromosomes, which are detected with high frequency as a result of permethrin application, act separately from the chromosome group that is pulled to the poles, causing unequal separation of the chromosome number in daughter cells (Khanna and Sharma 2013). The fact that permethrin induces different types of chromosomal aberrations and that each abnormality occurs with different mechanisms indicates that permethrin does not exhibit a specific genotoxic effect but triggers chromosomal aberration formations with multiple mechanisms. Falcioni et al. (2010) reported that

Molecular docking-supported cytotoxicity and genotoxicity mechanism


proteins can also lead to disruption of microtubule polymerization. The possible interaction of permethrin with tubulin proteins leads to abnormalities in the polypeptide structure. As a result of this abnormality, microtubule polymerization is prevented and chromosome movement to the poles is restricted, leading to disruption of mitotic stages and chromosome abnormalities. Permethrin-tubulin interactions are the basis for the cytotoxic impact of permethrin, which is demonstrated by a reduction in the rate of MI in A. cepa. The genotoxic effect of permethrin may occur as a result of permethrin-histone (Fig. 5) and permethrin-DNA interactions (Fig. 6). Permethrin has binding energies of -4.30 and

on DNA. Among these impacts include inhibition of DNA or RNA synthesis, frameshift mutations, and protein-associated DNA breaks. Permethrin was found to have a genotoxic effect both by binding to different DNA and by functioning as an intercalator agent. Compounds that are able to insert into DNA lead to an increase in clastogenic effects. As a result of these effects, abnormalities in DNA synthesis, strand breaks, insertion, deletion, or rearrangement of chromosomes may occur (Ferguson and Denny 2007).
DNA-permethrin interaction confirmed by spectral shift

Comet assay

Anatomical alterations
this change acts as a barrier against harmful chemicals and prevents toxic compounds from entering the vascular tissue. Nuclear shape change is another abnormality, and genotoxic and biochemical changes induced by permethrin may be the cause of nuclear flattening. Changes in nuclear shape may result from deterioration of nuclear volume, protein concentration, DNA integrity, and density (Dahl et al. 2008; Dauer and Worman 2009). Similarly, Yalçın et al. (2019) found that numerous morphological changes, such as deformation of epidermal cells and thickening of cell walls, were observed in root anatomy under chemicalinduced stress. Other abnormalities caused by permethrin include disorganized vascular tissue and cellular damage to the epidermis. The cause of all these abnormalities could be permethrin-induced oxidative stress, cytotoxic, and genotoxic effects. Such anatomical abnormalities prevent the plant from taking up nutrients and transporting them to other tissues and may also retard growth. Zoex application with permethrin resulted in a decrease in the incidence of anatomical damage. The application of

Conclusion
Declarations
Consent to participate Not applicable.
Consent for publication Not applicable.
Competing interests The authors declare no competing interests.
References
Ahmad P, Jaleel CA, Salem MA, Nabi G, Sharma S (2010) Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit Rev Biotechnol 30:161-175. https://doi.org/10.3109/ 07388550903524243
Akgeyik AU, Yalçın E, Çavuşoğlu K (2023) Phytochemical fingerprint and biological activity of raw and heat-treated Ornithogalum umbellatum. Sci Rep 13(1):13733. https://doi.org/10.1038/ s41598-023-41057-w
Akgündüz MÇ, Çavuşoğlu K, Yalçın E (2020) The potential risk assessment of phenoxyethanol with a versatile model system. Sci Rep 10(1):1-10. https://doi.org/10.1038/s41598-020-58170-9
Al-Amoudi WM (2018) Toxic effects of lambda-cyhalothrin, on the rat thyroid: involvement of oxidative stress and ameliorative effect of ginger extract. Toxicol Rep 5:728-736. https://doi.org/10.1016/j. toxrep.2018.06.005
Al-Nahain A, Jahan R, Rahmatullah M (2014) Zingiber officinale: a potential plant against rheumatoid arthritis. Arthritis 2014. https:// doi.org/10.1155/2014/159089
Aydin D, Yalçın E, Çavuşoğlu K (2022) Metal chelating and anti-radical activity of Salvia officinalis in the ameliorative effects against uranium toxicity. Sci Rep 12(1):15845. https://doi.org/10.1038/ s41598-022-20115-9
Balendiran K, Rao ST, Sekharudu CY, Zon G, Sundaralingam M (1995) X-ray structures of the B-DNA dodecamer d (CGCGTT AACGCG) with an inverted central tetranucleotide and its netropsin complex. Acta Crystallogr D Biol Crystallogr 51:190-198. https://doi.org/10.1107/S0907444994010759
Banerjee S, Mullick HI, Banerjee J, Ghosh A (2011) Zingiber officinale: ‘a natural gold.’ Int J Pharmaceutical Bio-Sci 2:283-294
Borowik A, Wyszkowska J, Zaborowska M, Kucharski J (2023) The impact of permethrin and cypermethrin on plants, soil enzyme
activity, and microbial communities. Int J Mol Sci 24(3):2892. https://doi.org/10.3390/ijms24032892
Çakir F, Kutluer F, Yalçın E, Çavuşoğlu K, Acar A (2023) Deep neural network and molecular docking supported toxicity profile of prometryn. Chemosphere 139962. https://doi.org/10.1016/j.chemo sphere.2023.139962
Çavuşoğlu K, Yalçın E (2023) Spectral shift supported epichlorohydrin toxicity and the protective role of sage. Environ Sci Pollut Res 30(1):1374-1385. https://doi.org/10.1007/s11356-022-22288-2
Çavuşoğlu K, Gür B, Yalçın E, Demirtaş G, Çiçek F (2014) The effect of lambda-cyhalothrin on root tip cytology, pigment contents and antioxidant defense system of Allium cepa. Cytologia 79(1):95101. https://doi.org/10.1508/cytologia.79.95
Chakraborty R, Mukherjee AK, Mukherjee A (2009) Evaluation of genotoxicity of coal fly ash in Allium cepa root cells by combining comet assay with the Allium test. Environ Monit Assess 153:351-357. https://doi.org/10.1007/s10661-008-0361-z
Collins AR (2004) The comet assay for DNA damage and repair. Mol Biotechnol 26(3):249-261. https://doi.org/10.1385/MB:26:3:249
Dahl KN, Ribeiro AJ, Lammerding J (2008) Nuclear shape, mechanics, and mechanotransduction. Circ Res 102:1307-1318. https://doi. org/10.1161/CIRCRESAHA.108.173989
Dauer WT, Worman HJ (2009) The nuclear envelope as a signaling node in development and disease. Dev Cell 17(5):626-638. https://doi.org/10.1016/j.devcel.2009.10.016
Davoodi R, Gholamreza ABDİ (2012) Comparative study on the acute toxicity of synthetic pesticides, permethrin
Delic N (1998) Effect of permethrin on mitotic activity of cultured human lymphocytes. Pesticides 13:233-238
Demirtaş G, Çavuşoğlu K, Yalçın E (2020) Aneugenic, clastogenic, and multi-toxic effects of diethyl phthalate exposure. Environ Sci Pollut Res 27:5503-5510. https://doi.org/10.1007/ s11356-019-07339-5
Drew HR, Wing RM, Takano T, Broka C, Tanaka S, Itakura K, Dickerson RE (1981) Structure of a B-DNA dodecamer: conformation and dynamics. Proc Natl Acad Sci 78(4):2179-2183. https://doi. org/10.1073/pnas.78.4.2179
Dubus IG, Hollis JM, Brown CD (2000) Pesticide in rainfall in Europe. Environ Pollut 110:331-344. https://doi.org/10.1016/S0269-7491(99)00295-X
Dugasani S, Pichika MR, Nadarajah VD, Balijepalli MK, Tandra S, Korlakunta JN (2020) Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J Ethnopharmacol 127(2):515-520. https://doi.org/ 10.1016/j.jep.2009.10.004
Fenech M, Neville S (1992) Conversion of excision-repairable DNA lesions to micronuclei within one cell cycle in human lymphocytes. Environ Mol Mut 19:27-36. https://doi.org/10.1002/em. 2850190106
Ferguson LR, Denny WA (2007) Genotoxicity of non-covalent interactions: DNA intercalators. Mutat Res Fundam Mol Mech Mutagen 623(1-2):14-23. https://doi.org/10.1016/j.mrfmmm.2007.03.014
Gabbianelli R, Palan M, Flis DJ, Fedeli D, Nasuti C, Skarydova L, Ziolkowski W (2013) Imbalance in redox system of rat liver following permethrin treatment in adolescence and neonatal age. Xenobiotica 43:1103-1110. https://doi.org/10.3109/00498254. 2013.796427
Guex N, Peitsch M (2005) CSWISS-MODEL and the Swiss-Pdb Viewer: an environment for comparative protein modeling. Electrophoresis 18:2714-2723. https://doi.org/10.1002/elps. 11501 81505
Hausladen A, Alscher RG (1993) Glutathione. In: Alscher RG, Hess JL (eds) Antioxidants in higher plants. CRC Press, Boca Raton, pp 1-30
Jeena K, Liju VB, Ramanath V, Kuttan R (2016) Protection against whole body gamma-irradiation induced oxidative stress and clastogenic damage in mice by ginger essential oil. APJCP 17(3):1325-1332. https://doi.org/10.7314/APJCP.2016.17.3. 1325
Junquera P (2021) Permethrin: safety summary for veterinary use in dogs, cats, horses, cattle, sheep, goats, swine and poultry. Poisoning, intoxication, overdose, antidote. https://parasitipedia. net/index.php?option=com_content&view=article&id=2676& Itemid=. Accessed 27 Apr 2023
Katahira R, Katahira M, Yamashita Y, Ogawa H, Kyogoku Y, Yoshida M (1998) Solution structure of the novel antitumor drug UCH9 complexed with d (TTGGCCAA) 2 as determined by NMR. Nucleic Acids Res 26:744-755. https://doi.org/10. 1093/nar/26.3.744
Kerksick C, Willoughby D (2005) The antioxidant role of glutathione and N -acetyl-cysteine supplements and exercise-induced oxidative stress. J Internat Soc Sports Nutr 2(2):1-7. https://doi.org/ 10.1186/1550-2783-2-2-38
Kim YS, Hong CS, Lee SW, Nam JH, Kim BJ (2017) Effects of ginger and its pungent constituents on transient receptor potential channels. Biophys J 112(3):250
Końca K, Lankoff A, Banasik A, Lisowska H, Kuszewski T, Góźdź S, Koza Z, Wojcik A (2003) A cross-platform public domain PC image-analysis program for the comet assay. Mutat Res 534:1520. https://doi.org/10.1016/s1383-5718(02)00251-6
Kurt D, Yalçin E, Çavuşoğlu K (2023) GC-MS and HPLC supported phytochemical analysis of watercress and the protective role against paraben toxicity. Environ Sci Pollut Res 30(3):60336046. https://doi.org/10.1007/s11356-022-22380-7
Lacey SE, He S, Scheres SH, Carter AP (2019) Cryo-EM of dynein microtubule-binding domains shows how an axonemal dynein distorts the microtubule. Elife 8:e47145. https://doi.org/10. 7554/eLife. 47145
Luo Q, Wang B, Wu Z, Jiang W, Wang Y, Du K, Zhou N, Zheng L, Gan J, Shen WH, Ma J, Dong A (2020) NAP1-Related Protein 1 (NRP1) has multiple interaction modes for chaperoning histones H2A-H2B. Proc Natl Acad Sci USA 117(48):30391-30399. https://doi.org/10.1073/pnas. 2011089117
Macar O, Kalefetoğlu Macar T, Çavuşoğlu K, Yalçın E (2020) Protective effects of anthocyanin-rich bilberry (Vaccinium myrtillus L.) extract against copper (II) chloride toxicity.
Mao QQ, Xu XY, Cao SY, Gan RY, Corke H, Beta T, Li HB (2019) Bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe). Foods 8(6):185. https://doi.org/10.3390/foods80601 85
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30:2785-2791. https://doi.org/10.1002/jcc. 21256
O’Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR (2011) Open Babel: an open chemical toolbox. J Cheminform 3:33. https://doi.org/10.1186/1758-2946-3-33
Okesola MA, Ajiboye BO, Oyinloye BE, Ojo OA (2019) Effect of Zingiber officinale on some biochemical parameters and cytogenic analysis in lead-induced toxicity in experimental rats. Tox Mech Meth 29(4):255-262
Ozluer C, Satana Kara HE (2014) In vitro DNA binding studies of anticancer drug idarubicin using spectroscopic techniques. J Photochem Photobiol B 138:36-42. https://doi.org/10.1016/j.jphot obiol.2014.05.015
Policegoudra RS, Rehna K, Rao LJ, Aradhya SM (2010) Antimicrobial, antioxidant, cytotoxicity and platelet aggregation inhibitory activity of a novel molecule isolated and characterized from mango ginger rhizome. J Biosci 35(2):231-240. https://doi.org/10.1007/ s12038-010-0027-1
Roma GC, De Oliveira PR, Araujo AM, Bechara GH, Mathias MIC (2012) Genotoxic and mutagenic effects of permethrin in mice: micronuclei analysis in peripheral blood erythrocytes. Mic Res Tech 75(12):1732-1736. https://doi.org/10.1002/jemt. 22124
Saha C, Kumar R, Das A (2017) Understanding nucleosomal histone and DNA interactions: a biophysical study. J Biomol Struct Dyn 35(12):2531-2538. https://doi.org/10.1080/07391102.2016.1225603
Sellami B, Louati H, Dellali M, Aissa P, Mahmoudi E, Coelho AV, Sheehan
Sharma AD, Gill PK, Singh P (2002) DNA isolation from dry and fresh samples of polysaccharide-rich plants. Plant Mol Biol Rep 20:415. https://doi.org/10.1007/BF02772129
Singh D, Pal M, Singh R, Singh CK, Chaturvedi AK (2015) Physiological and biochemical characteristics of Vigna species for Al stress tolerance. Acta Physiol Plant 37:1-13
Sun YJ, Liang YJ, Yang L, Long DX, Wang HP, Wu YJ (2022) Longterm low-dose exposure of permethrin induces liver and kidney damage in rats. BMC Pharmacol Toxicol 23:46. https://doi.org/ 10.1186/s40360-022-00586-2
Tiryaki O, Canhilal R, Horuz S (2010) The use of pesticides and their risks. Erciyes Univ J Inst Sci Technol 26(2):154-169
Tütüncü E, Yalçin E, Acar A, Yapar K, Çavuşoğlu K (2019) Investigation of the toxic effects of a carbamate insecticide methiocarb in Allium cepa L. Cytologia 84(2):113-117. https://doi.org/10.1508/ cytologia.84.113
Weidinger A, Kozlov AV (2015) Biological activities of reactive oxygen and nitrogen species: oxidative stress versus signal transduction. Biomolecules 5(2):472-548. https://doi.org/10.3390/biom5 020472
Yalçın E, Uzun A, Çavuşoğlu K (2019) In vivo epiclorohidrine toxicity: cytogenetic, biochemical, physiological, and anatomical evidences. Environ Sci Pollut Res 26:22400-22406. https://doi.org/ 10.1007/s11356-019-05518-y
Yalçin E, Çavuşoğlu K (2022b) Spectroscopic contribution to glyphosate toxicity profile and the remedial effects of Momordica charantia. Sci Rep 12(1):20020. https://doi.org/10.1038/ s41598-022-24692-7
- Values shown with different letters in the same column are statistically significant
not determined - ECD, epidermis cell damage; CCD, cortex cell damage; TCCW, thickening of cortex cell walls; UVT, unclear vascular tissue; FCN, flattened cell nucleus. (-) no damage, (+) minor damage, () medium damage, (+) severe damage. Bar
