DOI: https://doi.org/10.1038/s41541-024-00874-4
PMID: https://pubmed.ncbi.nlm.nih.gov/38600250
تاريخ النشر: 2024-04-10
رؤى حول اللقاحات لكبار السن: من تأثيرات الشيخوخة المناعية إلى استراتيجيات التوصيل
الملخص
تزيد الشيخوخة المناعية من خطر وشدة الأمراض لدى كبار السن وتؤدي إلى ضعف المناعة الناتجة عن اللقاحات. مع تقدم العمر في السكان العالميين وزيادة خطر الأوبئة، فإن تطوير المحفزات واللقاحات لكبار السن لتحسين حمايتهم المناعية أمر حيوي للشيخوخة الصحية في جميع أنحاء العالم. يمكن أن يعجل تعميق فهمنا لدور الشيخوخة المناعية في فعالية اللقاحات البحث الذي يركز على تحسين توصيل اللقاحات لكبار السن. في هذه المراجعة، قمنا بتحليل خصائص الشيخوخة المناعية على المستويات الخلوية والجزيئية. تم تلخيص استراتيجيات تحسين فعالية التطعيم لدى كبار السن، بما في ذلك زيادة جرعة المستضد، إعداد لقاحات مستضد متعددة القيم، إضافة محفزات مناسبة، تثبيط الالتهاب المزمن، وتثبيط الشيخوخة المناعية. نأمل أن توفر هذه المراجعة مراجعة للاكتشافات الجديدة فيما يتعلق بتأثيرات الشيخوخة المناعية على الحماية الناتجة عن اللقاحات وتلهم تطوير لقاحات فردية لكبار السن.
مرض، عدوى فيروس الجهاز التنفسي المخلوي، الكزاز، والدفتيريا
أثر الشيخوخة المناعية على فعالية اللقاح
، امتصاص المستضد، تقديم المستضد المتقاطع، وتنظيم خلايا تقديم المستضد (APC) لتحفيز خلايا T
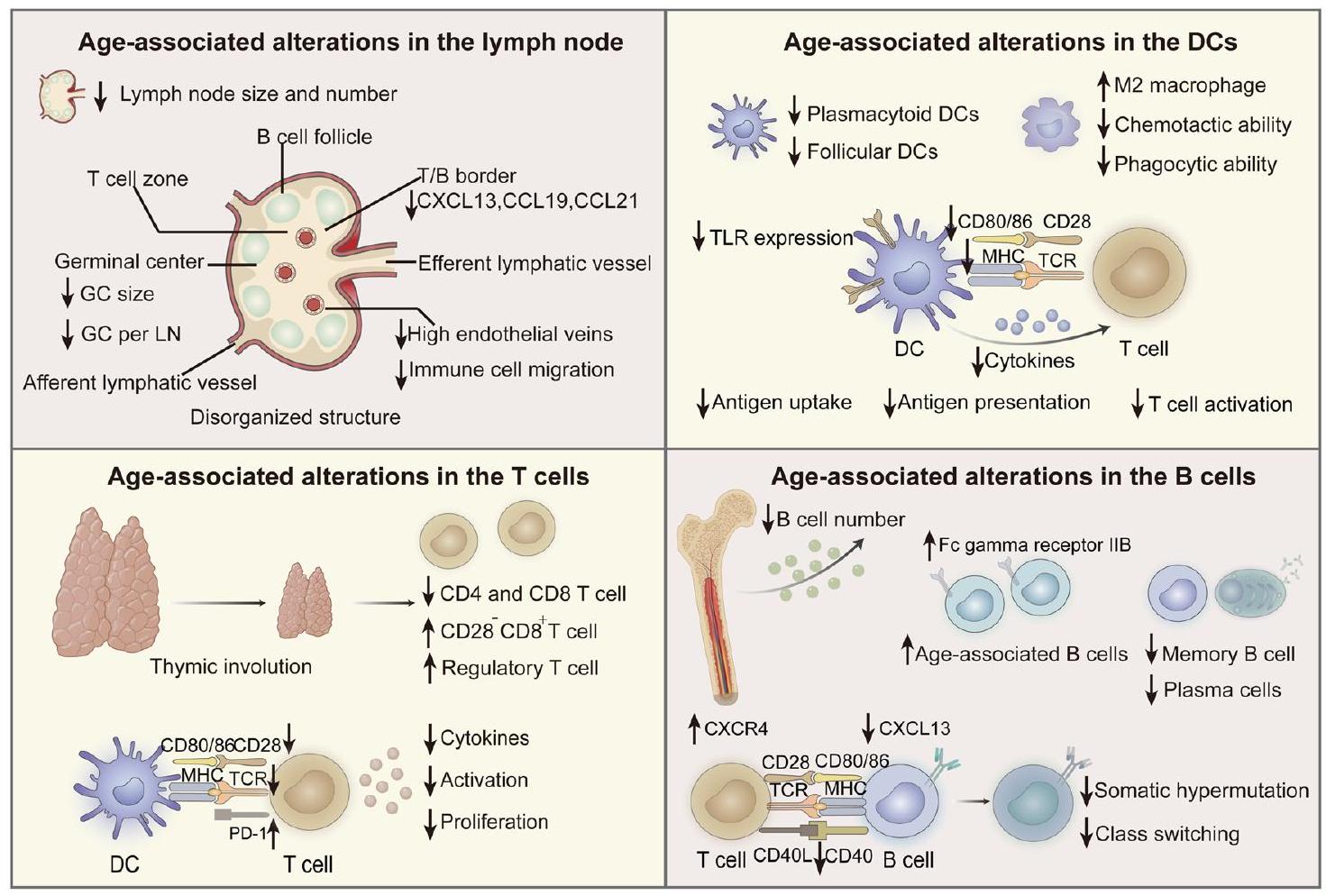
منطقة GC، قللت مباشرة من كميات المستضدات المقدمة لخلايا B، وانخفضت الاستجابات المناعية النوعية. علاوة على ذلك، أظهرت الأبحاث أن تعبير إنزيم دياميناز السيتيدين المحفز بالتحفيز الضروري لنضوج الألفة وتبديل الفئة في خلايا B ينخفض بشكل ملحوظ لدى الأفراد المسنين.
الالتهاب والمناعة
قد يؤدي الميكروبيوم في الأفراد المسنين أيضًا إلى زيادة مستوى السيتوكينات الالتهابية المتداولة.
استجابات المناعة المحددة بمستضد
السمات الجزيئية للشيخوخة والمناعة
ترويجها بواسطة البلعمة الذاتية في خلايا العرض. يمكن أن تؤدي البلعمة الذاتية إلى تشكيل حُجرات تخزين المستضدات في خلايا العرض، وتطيل الوقت المسموح به لتخزين المستضدات، وتسهّل عرض المستضدات وبدء العملية اللاحقة.
استنادًا إلى الحويصلات المحتوية على التيلوميرات. ومع ذلك، كانت الحماية طويلة الأمد لمجموعة الحويصلات المحتوية على التيلوميرات أفضل بشكل ملحوظ من تلك الخاصة بالمجموعة التي لا تحتوي على تيلوميرات. أظهرت هذه النتيجة بشكل أكبر أن الحويصلات المحتوية على التيلوميرات حسنت الذاكرة المناعية والمناعة طويلة الأمد. تشير نتائج هذه الدراسة إلى أن الحويصلات التي تحمل التيلوميرات قد تكون استراتيجية محتملة لتثبيط شيخوخة المناعة في خلايا T وتوفر أفكارًا جديدة لتصميم اللقاحات للأفراد المسنين.
استراتيجيات تصميم اللقاحات للأفراد المسنين
لقاحات الجرعات العالية
اللقاحات متعددة القيم
اللقاحات المعززة
تثبيط الالتهاب المزمن
الخلايا المتقدمة في السن زادت من مستويات العديد من العلامات البيولوجية: المرتبطة بالشيخوخة
تثبيط الشيخوخة المناعية
نظام التسليم ولكن غالبًا ما يتم تجاهل تأثير البيئة الدقيقة المناعية للأفراد المسنين على الاستجابة المناعية. دون تحسين جذري للشيخوخة المناعية لدى الأفراد المسنين، لن تتمكن اللقاحات من إنتاج فعالية مناعية قوية وطويلة الأمد. سيكون تثبيط الشيخوخة المناعية لدى الأفراد المسنين إجراءً حاسمًا لتحسين الاستجابات المناعية للقاحات. إن العثور على مواد مساعدة ومكونات لقاح يمكن أن تثبط الشيخوخة المناعية هو اتجاه جذاب للبحث في المستقبل.
استراتيجيات التصميم الحالية والأمثلة




| استراتيجية التصميم | مُمْرِض | معلومات اللقاح | المراجع | |||
| لقاحات الجرعات العالية | فيروس الإنفلونزا | فلوزون
|
79 | |||
| اللقاحات متعددة القيم | المكورات الرئوية | لقاح PCV13 (لقاح المكورات الرئوية المتقارن 13-valent) | 90 | |||
| المكورات الرئوية | PPSV23 (لقاح بوليسكاريد المكورات الرئوية 23-valent) | 91 | ||||
| اللقاحات المعززة | فيروس الإنفلونزا | فلواد
|
|
|||
| فيروس الإنفلونزا | باندمريكس
|
|
||||
| فيروس الإنفلونزا | يحتوي على معقد السابونين M-1 كمساعد | NCT03293498 | ||||
| فيروس الإنفلونزا | يحتوي على محفز TLR 7/8 ريسيكيمود | NCT01737580 | ||||
| الهربس النطاقي | شينغريكس
|
١٠٤ | ||||
| فيروس الجهاز التنفسي المخلوي | أريكسفي
|
https://www.fda.gov/news-events/press-announcements/ fda-approves-first-respiratory-syncytial-virus-rsv-vaccine | ||||
| سارس-CoV-2 | تتفاعل المحفزات TLR9 مع مادة الهيدروكسيد الألمنيوم المساعدة بشكل متآزر | 114 | ||||
| تثبيط الالتهاب المزمن | / | مثبط p38 MAPK | ١١٥ | |||
| / | مثبط mTOR (الراباميسين) | NCT02874924 | ||||
| / | مثبط COX-2 | ١٢٥ | ||||
| / |
|
١٣٠ | ||||
| تثبيط الشيخوخة المناعية | فيروس SARS-CoV-2 أو فيروس الإنفلونزا | سبيرميدين (مكمل غذائي) لاستعادة الالتهام الذاتي في خلايا T و B | NCT05421546 | |||
| فيروس الإنفلونزا | الميتفورمين لاستعادة البلعمة الذاتية في خلايا T و B | NCT03996538 | ||||
| المكورات الرئوية | الميتفورمين لاستعادة البلعمة الذاتية في خلايا T و B | NCT03713801 | ||||
| لقاحات mRNA | سارس-CoV-2 | بي إن تي 162 بي 2 و مRNA-1273 | 141 | |||
| تعزيز التطعيم | سارس-CoV-2 | جرعة معززة ثالثة | 142 | |||
| التطعيم المتأخر | سارس-CoV-2 | تم تمديد الفاصل بين الجرعتين من 3 أسابيع إلى
|
143 | |||
| حقن داخل الأدمة | فيروس الإنفلونزا | إنتانزا
|
146 |
 من الراباميسين.
من الراباميسين.قد يكون الآلية هي أن الرنا المرسال يمكن التعرف عليه بواسطة TLR3 و 7 و 8 لتحفيز نشاط مشابه للمواد المساعدة وزيادة تعبير المستضد وعرض المستضد.
الاستنتاجات والآفاق
اتجاه بحث جذاب سيكون اكتشاف المناعية المعدلة وتركيبات اللقاحات التي يمكن أن تثبط الشيخوخة المناعية. يمكن أن يؤثر اختيار المحفزات بشكل كبير على نوع وحجم الاستجابة المناعية.
البحث وتطوير اللقاحات؛ قد توفر هذه التعاونات استراتيجيات أكثر ابتكارًا وتثير أفكارًا لتصميم لقاحات مخصصة.
توفر البيانات
References
- Li, X. et al. Inflammation and aging: signaling pathways and intervention therapies. Signal Transduct. Target Ther. 8, 239 (2023).
- United Nations. Department of Economic and Social Affairs, Population Division (2019). World Population Ageing 2019: Highlights (ST/ESA/SER.A/430) (2022).
- Gralinski, L. E. & Menachery, V. D. Return of the Coronavirus: 2019nCoV. Viruses 12, 135 (2020).
- Koff, W. C. et al. Accelerating next-generation vaccine development for global disease prevention. Science 340, 1232910 (2013).
- Mascola, J. R. & Fauci, A. S. Novel vaccine technologies for the 21st century. Nat. Rev. Immunol. 20, 87-88 (2020).
- Hou, Y. et al. Advanced subunit vaccine delivery technologies: from vaccine cascade obstacles to design strategies. Acta Pharm. Sin. B 13, 3321-3338 (2023).
- Cunningham, A. L., McIntyre, P., Subbarao, K., Booy, R. & Levin, M. J. Vaccines for older adults. BMJ 372, n188 (2021).
- Bell, M. R. & Kutzler, M. A. An old problem with new solutions: strategies to improve vaccine efficacy in the elderly. Adv. Drug Deliv. Rev. 183, 114175 (2022).
- Osterholm, M. T. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect. Dis. 12, 36-44 (2012).
- Walford, R. L. The immunologic theory of aging. Gerontologist 4, 195-197 (1964).
- Willyard, C. How anti-ageing drugs could boost COVID vaccines in older people. Nature 586, 352-354 (2020).
- Qin, X., Jian, D. & Yi, C. Role of CD8
T lymphocyte cells: interplay with stromal cells in tumor microenvironment. Acta Pharm. Sin. B 11, 1365-1378 (2021). - Riese, P. et al. Distinct immunological and molecular signatures underpinning influenza vaccine responsiveness in the elderly. Nat. Commun. 13, 6894 (2022).
- Roukens, A. H. et al. Elderly subjects have a delayed antibody response and prolonged viraemia following yellow fever vaccination: a prospective controlled cohort study. PloS One 6, e27753 (2011).
- Schulz, A. R. et al. Low thymic activity and dendritic cell numbers are associated with the immune response to primary viral infection in elderly humans. J. Immunol. 195, 4699-4711 (2015).
- Ding, Y., Li, Z., Jaklenec, A. & Hu, Q. Vaccine delivery systems toward lymph nodes. Adv. Drug Deliv. Rev. 179, 113914 (2021).
- Lefebvre, J. S., Masters, A. R., Hopkins, J. W. & Haynes, L. Agerelated impairment of humoral response to influenza is associated with changes in antigen specific
follicular helper cell responses. Sci. Rep. 6, 25051 (2016). - Chen, J., Deng, J. C. & Goldstein, D. R. How aging impacts vaccine efficacy: known molecular and cellular mechanisms and future directions. Trends Mol. Med. 28, 1100-1111 (2022).
- Hadamitzky, C. et al. Age-dependent histoarchitectural changes in human lymph nodes: an underestimated process with clinical relevance? J. Anat. 216, 556-562 (2010).
- Agrawal, A. et al. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3 -kinase-signaling pathway. J. Immunol. 178, 6912-6922 (2007).
- Yang, Y., Guo, X., Hu, B., He, P. & Feng, M. Generated SecPen_NY-ESO-1_ubiquitin-pulsed dendritic cell cancer vaccine elicits stronger and specific
cell immune responses. Acta Pharm. Sin. B 11, 476-487 (2020). - Eisenbarth, S. C. Dendritic cell subsets in T cell programming: location dictates function. Nat. Rev. Immunol. 19, 89-103 (2019).
- Heath, W. R., Kato, Y., Steiner, T. M. & Caminschi, I. Antigen presentation by dendritic cells for B cell activation. Curr. Opin. Immunol. 58, 44-52 (2019).
- Wang, J., Geiger, H. & Rudolph, K. L. Immunoaging induced by hematopoietic stem cell aging. Curr. Opin. Immunol. 23, 532-536 (2011).
- Panda, A. et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J. Immunol. 184, 2518-2527 (2010).
- Leleux, J., Atalis, A. & Roy, K. Engineering immunity: modulating dendritic cell subsets and lymph node response to direct immunepolarization and vaccine efficacy. J. Control. Release 219, 610-621 (2015).
- Jackaman, C. et al. Targeting macrophages rescues age-related immune deficiencies in C57BL/6J geriatric mice. Aging Cell 12, 345-357 (2013).
- Prieto, L. I. et al. Senescent alveolar macrophages promote earlystage lung tumorigenesis. Cancer Cell 41, 1261-1275.e6 (2023).
- Wang, J., Yang, J. & Kopecek, J. Nanomedicines in B cell-targeting therapies. Acta Biomater. 137, 1-19 (2022).
- Frasca, D. & Blomberg, B. B. Aging affects human B cell responses. J. Clin. Immunol. 31, 430-435 (2011).
- Pritz, T. et al. Plasma cell numbers decrease in bone marrow of old patients. Eur. J. Immunol. 45, 738-746 (2015).
- Cancro, M. P. Age-associated B cells. Annu. Rev. Immunol. 38, 315-340 (2020).
- Yam-Puc, J. C. et al. Age-associated
cells predict impaired humoral immunity after COVID-19 vaccination in patients receiving immune checkpoint blockade. Nat. Commun. 14, 3292 (2023). - Allen, C. D. et al. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat. Immunol. 5, 943-952 (2004).
- Wols, H. A. et al. Migration of immature and mature B cells in the aged microenvironment. Immunology 129, 278-290 (2010).
- Frasca, D., Blomberg, B. B., Garcia, D., Keilich, S. R. & Haynes, L. Age-related factors that affect
cell responses to vaccination in mice and humans. Immunol. Rev. 296, 142-154 (2020). - Lefebvre, J. S. et al. The aged microenvironment contributes to the age-related functional defects of CD4 T cells in mice. Aging Cell 11, 732-740 (2012).
- Silva-Cayetano, A. et al. Spatial dysregulation of T follicular helper cells impairs vaccine responses in aging. Nat. Immunol. 24, 1124-1137 (2023).
- Khurana, S., Frasca, D., Blomberg, B. & Golding, H. AID activity in B cells strongly correlates with polyclonal antibody affinity maturation in-vivo following pandemic 2009-H1N1 vaccination in humans. PLoS Pathog. 8, e1002920 (2012).
- Stiasny, K., Aberle, J. H., Keller, M., Grubeck-Loebenstein, B. & Heinz, F. X. Age affects quantity but not quality of antibody responses after vaccination with an inactivated flavivirus vaccine against tick-borne encephalitis. PLoS One 7, e34145 (2012).
- Goronzy, J. J. & Weyand, C. M. Understanding immunosenescence to improve responses to vaccines. Nat. Immunol. 14, 428-436 (2013).
- Chen, X., Liu, Q. & Xiang, A. P. CD8
CD28 T cells: not only agerelated cells but a subset of regulatory T cells. Cell Mol. Immunol. 15, 734-736 (2018). - Kumagai, S. et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat. Immunol. 21, 1346-1358 (2020).
- Gustafson, C. E., Weyand, C. M. & Goronzy, J. J. T follicular helper cell development and functionality in immune ageing. Clin. Sci. 132, 1925-1935 (2018).
- Herati, R. S. et al. Circulating
response predicts influenza vaccine antibody responses in young adults but not elderly adults. J. Immunol. 193, 3528-3537 (2014). - Franceschi, C. et al. Inflamm aging: an evolutionary perspective on immunosenescence. Ann. N.Y. Acad. Sci. 908, 244-254 (2000).
- Franceschi, C. et al. Inflammaging and ‘Garb-aging. Trends Endocrinol. Metab. 28, 199-212 (2017).
- Gilroy, D. & De Maeyer, R. New insights into the resolution of inflammation. Semin. Immunol. 27, 161-168 (2015).
- Chambers, E. S. & Akbar, A. N. Can blocking inflammation enhance immunity during aging? J. Allergy Clin. Immunol. 145, 1323-1331 (2020).
- Hadrup, S. R. et al. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J. Immunol. 176, 2645-2653 (2006).
- Frasca, D., Blomberg, B. B. & Paganelli, R. Aging, obesity, and inflammatory age-related diseases. Front. Immunol. 8, 1745 (2017).
- Kim, K. A., Jeong, J. J., Yoo, S. Y. & Kim, D. H. Gut microbiota lipopolysaccharide accelerates inflamm-aging in mice. BMC Microbiol 16, 9 (2016).
- Claesson, M. J. et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 488, 178-184 (2012).
- De Maeyer, R. P. H. et al. Blocking elevated p38 MAPK restores efferocytosis and inflammatory resolution in the elderly. Nat. Immunol. 21, 615-625 (2020).
- Lasry, A. & Ben-Neriah, Y. Senescence-associated inflammatory responses: aging and cancer perspectives. Trends Immunol. 36, 217-228 (2015).
- Gulen, M. F. et al. cGAS-STING drives ageing-related inflammation and neurodegeneration. Nature 620, 374-380 (2023).
- Gritsenko, A., Green, J. P., Brough, D. & Lopez-Castejon, G. Mechanisms of NLRP3 priming in inflammaging and age-related diseases. Cytokine Growth Factor Rev. 55, 15-25 (2020).
- Lim, S.O. et al. Deubiquitination and stabilization of PD-L1 by CSN5. Cancer Cell, 925-939 (2016).
- Hamilton, J. A. G. et al. Interleukin-37 improves T-cell-mediated immunity and chimeric antigen receptor T-cell therapy in aged backgrounds. Aging Cell 20, e13309 (2021).
- Chen, X., Baumel, M., Männel, D. N., Howard, O. M. Z. & Oppenheim, J. J. Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse
T regulatory cells. J. Immunol. 179, 154-161 (2007). - Watanabe, R., Shirai, T., Hong, N., Zhang, H. & Weyand, C. M. Pyruvate controls the checkpoint inhibitor PD-L1 and suppresses T cell immunity. J. Clin. Investig. 127, 2725-2738 (2017).
- Chen, J. H. et al. Prostaglandin E2 and programmed cell death 1 signaling coordinately impair CTL function and survival during chronic viral infection. Nat. Med. 21, 327-334 (2015).
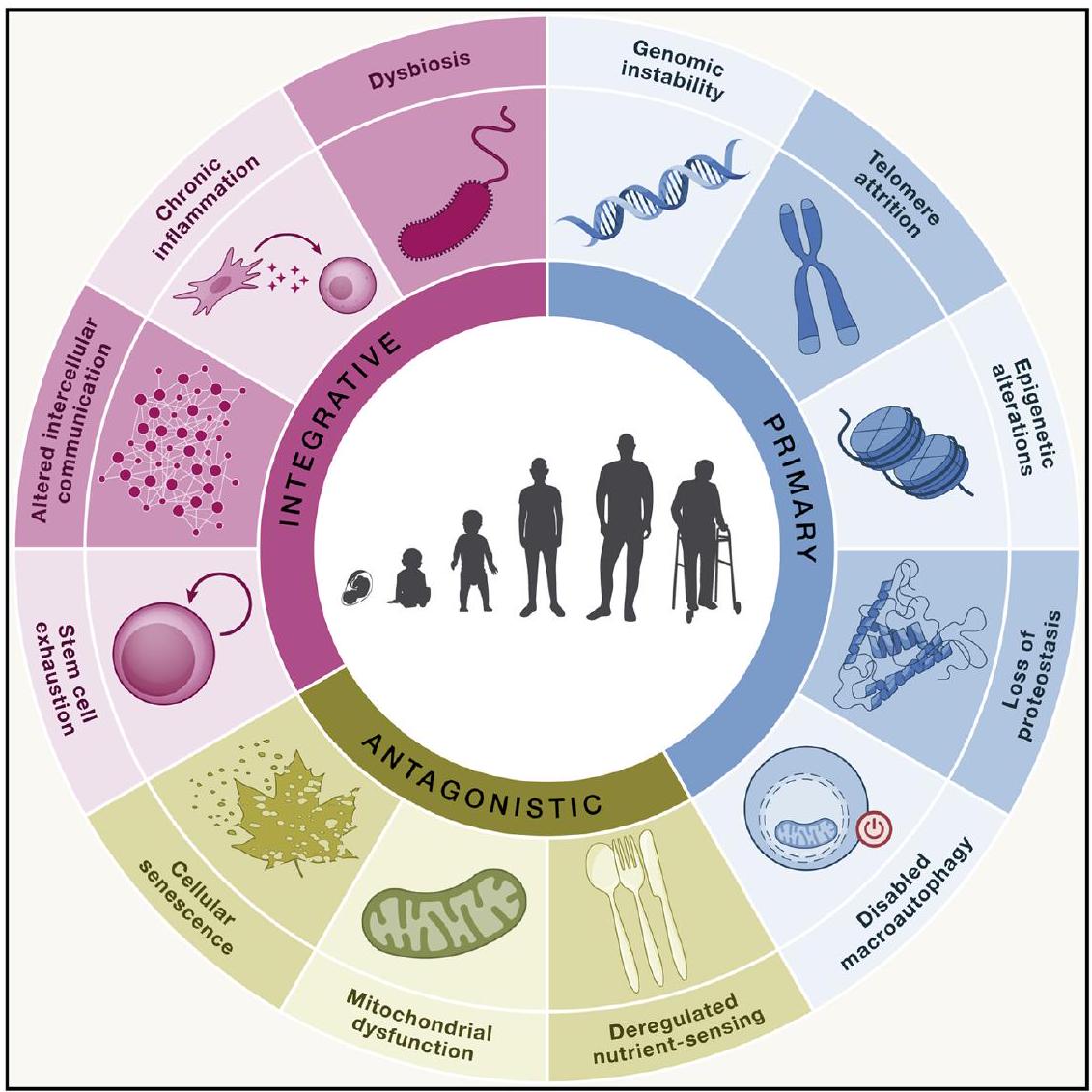
- López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: an expanding universe. Cell 186, 243-278 (2023).
- Guo, J. et al. Aging and aging-related diseases: from molecular mechanisms to interventions and treatments. Signal Transduct. Target Ther. 7, 391 (2022).
- Hansen, M., Rubinsztein, D. C. & Walker, D. W. Autophagy as a promoter of longevity: insights from model organisms. Nat. Rev. Mol. Cell Biol. 19, 579-593 (2018).
- Zhang, H. et al. Polyamines control eIF5A hypusination, TFEB translation, and autophagy to reverse B cell senescence. Mol. Cell. 76, 110-125.e9 (2019).
- Aman, Y. et al. Autophagy in healthy aging and disease. Nat. Aging 1, 634-650 (2021).
- Kim, J., Kundu, M., Viollet, B. & Guan, K. L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 13, 132-141 (2011).
- Cirone, M. Perturbation of bulk and selective macroautophagy, abnormal UPR activation and their interplay pave the way to immune dysfunction, cancerogenesis and neurodegeneration in ageing. Ageing Res. Rev. 58, 101026 (2020).
- Wang, Y. et al. In situ manipulation of dendritic cells by an autophagy-regulative nanoactivator enables effective cancer immunotherapy. ACS Nano 13, 7568-7577 (2019).
- Wang, S. et al. Exploration of antigen-induced
nanoparticles for therapeutic vaccine. Small 14, e1704272 (2018). - Hubbard, V. M. et al. Macroautophagy regulates energy metabolism during effector T cell activation. J. Immunol. 185, 7349-7357 (2010).
- Fan, J., Feng, Z. & Chen, N. Spermidine as a target for cancer therapy. Pharmacol. Res. 159, 104943 (2020).
- De Risi, M. et al. Mechanisms by which autophagy regulates memory capacity in ageing. Aging Cell 19, e13189 (2020).
- Ma, T. et al. Low-dose metformin targets the lysosomal AMPK pathway through PEN2. Nature 603, 159-165 (2022).
- Kroemer, G. & Zitvogel, L. CD4
T cells at the center of inflammaging. Cell Metab. 32, 4-5 (2020). - Chakravarti, D., LaBella, K. A. & DePinho, R. A. Telomeres: history, health, and hallmarks of aging. Cell 184, 306-322 (2021).
- Lanna, A. et al. An intercellular transfer of telomeres rescues T cells from senescence and promotes long-term immunological memory. Nat. Cell Biol. 24, 1461-1474 (2022).
- Gravenstein, S. et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccination on numbers of US nursing home residents admitted to hospital: a cluster-randomised trial. Lancet Respir. Med. 5, 738-746 (2017).
- Couch, R. B. et al. Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects. Vaccine 25, 7656-7663 (2007).
- Wilkinson, K. et al. Efficacy and safety of high-dose influenza vaccine in elderly adults: a systematic review and meta-analysis. Vaccine 35, 2775-2780 (2017).
- Goronzy, J. J., Fang, F., Cavanagh, M. M., Qi, Q. & Weyand, C. M. Naive T cell maintenance and function in human aging. J. Immunol. 194, 4073-4080 (2015).
- Sun, Y. et al. Metal-organic framework nanocarriers for drug delivery in biomedical applications. Nanomicro Lett. 12, 103 (2020).
- Hong, X. et al. The pore size of mesoporous silica nanoparticles regulates their antigen delivery efficiency. Sci. Adv. 6, eaaz4462 (2020).
- Xia, Y. et al. Exploiting the pliability and lateral mobility of Pickering emulsion for enhanced vaccination. Nat. Mater. 17, 187-194 (2018).
- Liu, K. et al. A novel multifunctional vaccine platform with dendritic cell-targeting and pH -responsive for cancer immunotherapy: antigen-directed biomimetic fabrication of a cabbage-like mannatide-zinc-antigen hybrid microparticles. Chem. Eng. J. 426, 130867 (2021).
- Zhao, J. et al. A minimalist binary vaccine carrier for personalized postoperative cancer vaccine therapy. Adv. Mater. 34, e2109254 (2022).
- Pereira, B., Xu, X. N. & Akbar, A. N. Targeting inflammation and immunosenescence to improve vaccine responses in the rlderly. Front. Immunol. 11, 583019 (2020).
- Lauer, K. B., Borrow, R. & Blancharda, T. J. Multivalent and multipathogen viral vector vaccines. Clin. Vaccin. Immunol. 24, e00298-16 (2017).
- Lewnard, J. A. et al. Effectiveness of 13-Valent pneumococcal conjugate vaccine against medically attended lower respiratory tract
infection and pneumonia among older adults. Clin. Infect. Dis. 75, 832-841 (2022). - Lawrence, H. et al. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against vaccine serotype pneumococcal pneumonia in adults: a case-control test-negative design study. PLoS Med. 17, e1003326 (2020).
- Hernandez-Davies, J. E. et al. Administration of multivalent influenza virus recombinant hemagglutinin vaccine in combination-adjuvant elicits broad reactivity beyond the vaccine components. Front. Immunol. 12, 692151 (2021).
- Reed, S. G., Orr, M. T. & Fox, C. B. Key roles of adjuvants in modern vaccines. Nat. Med. 19, 1597-1608 (2013).
- Pulendran, B., Arunachalam, P. S. & O’Hagan, D. T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 20, 454-475 (2021).
- Peletta, A., Lemoine, C., Courant, T., Collin, N. & Borchard, G. Meeting vaccine formulation challenges in an emergency setting: towards the development of accessible vaccines. Pharmacol. Res. 189, 106699 (2023).
- Nanishi, E. et al. Precision vaccine adjuvants for older adults: a scoping review. Clin. Infect. Dis. 75, S72-S80 (2022).
- Ciabattini, A. et al. Vaccination in the elderly: the challenge of immune changes with aging. Semin. Immunol. 40, 83-94 (2018).
- Nicolay, U., Heijnen, E., Nacci, P., Patriarca, P. A. & Leav, B. Immunogenicity of alIV3, MF59-adjuvanted seasonal trivalent influenza vaccine, in older adults
years of age: meta-analysis of cumulative clinical experience. Int. J. Infect. Dis. 85S, S1-S9 (2019). - Isakova Sivak, I. & Rudenko, L. Cross-protective potential of a MF59-adjuvanted quadrivalent influenza vaccine in older adults. Lancet Infect. Dis. 21, 900-901 (2021).
- Morel, S. et al. Adjuvant System AS03 containing
-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine 29, 2461-2473 (2011). - Yam, K. K. et al. AS03-adjuvanted, very-low-dose influenza vaccines induce distinctive immune responses compared to unadjuvanted high-dose vaccines in BALB/c mice. Front. Immunol. 6, 207 (2015).
- Dendouga, N., Fochesato, M., Lockman, L., Mossman, S. & Giannini, S. L. Cell-mediated immune responses to a varicella-zoster virus glycoprotein E vaccine using both a TLR agonist and QS21 in mice. Vaccine 30, 3126-3135 (2012).
- Nam, H. J. et al. An adjuvanted zoster vaccine elicits potent cellular immune responses in mice without QS21. NPJ Vaccines 7, 45 (2022).
- Lal, H. et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N. Engl. J. Med. 372, 2087-2096 (2015).
- Renshaw, M. et al. Cutting Edge: impaired toll-like receptor expression and function in aging. J. Immunol. 169, 4697-4701 (2002).
- Metcalf, T. U. et al. Global analyses revealed age-related alterations in innate immune responses after stimulation of pathogen recognition receptors. Aging Cell 14, 421-432 (2015).
- Janssen, J. M., Jackson, S., Heyward, W. L. & Janssen, R. S. Immunogenicity of an investigational hepatitis B vaccine with a tolllike receptor 9 agonist adjuvant (HBsAg-1018) compared with a licensed hepatitis B vaccine in subpopulations of healthy adults 1870 years of age. Vaccine 33, 3614-3618 (2015).
- Lim, J. S. et al. Flagellin-dependent TLR5/caveolin-1 as a promising immune activator in immunosenescence. Aging Cell 14, 907-915 (2015).
- Denton, A. E. et al. Targeting TLR4 during vaccination boosts MAdCAM-1+lymphoid stromal cell activation and promotes the aged germinal center response. Sci. Immunol. 7, eabk0018 (2022).
- Zareian, N. et al. Triggering of toll-like receptors in old individuals. Relevance for vaccination. Curr. Pharm. Des. 25, 4163-4167 (2019).
- Wu, T. Y. et al. Rational design of small molecules as vaccine adjuvants. Sci. Transl. Med. 6, 263ra160 (2014).
- Ross, K. A. et al. Novel nanoadjuvants balance immune activation with modest inflammation: implications for older adult vaccines. Immun. Ageing 20, 28 (2023).
- Ananya, A. et al. Just right” combinations of adjuvants with nanoscale carriers activate aged dendritic cells without overt inflammation. Immun. Ageing 20, 10 (2023).
- Nanishi, E., Borriello, F., O’Meara, T. R., Mcgrath, M. E. & Dowling, D. J. An aluminum hydroxide:CpG adjuvant enhances protection elicited by a SARS-CoV-2 receptor-binding domain vaccine in aged mice. Sci. Transl. Med. 14, eabj5305 (2021).
- Lanna, A. et al. A sestrin-dependent Erk-Jnk-p38 MAPK activation complex inhibits immunity during aging. Nat. Immunol. 18, 354-363 (2017).
- Kennedy, R. B. et al. Immunosenescence-related transcriptomic and immunologic changes in older individuals following influenza vaccination. Front. Immunol. 7, 450 (2016).
- Mannick, J. B. et al. mTOR inhibition improves immune function in the elderly. Sci. Transl. Med. 6, 268 ra179 (2014).
- Song, S., Lam, E. W. F., Tchkonia, T., Kirkland, J. L. & Sun, Y. Senescent cells: emerging targets for human aging and age-related diseases. Trends Biochem. Sci. 45, 578-592 (2020).
- Wissler Gerdes, E. O., Misra, A., Netto, J. M. E., Tchkonia, T. & Kirkland, J. L. Strategies for late phase preclinical and early clinical trials of senolytics. Mech. Ageing Dev. 200, 111591 (2021).
- Kirkland, J. L. & Tchkonia, T. Senolytic drugs: from discovery to translation. J. Intern. Med. 288, 518-536 (2020).
- Zhang, Q., Li, S., Chen, F., Zeng, R. & Tong, R. Targeted delivery strategy: a beneficial partner for emerging senotherapy. Biomed. Pharmacother. 155, 113737 (2022).
- Chen, S. et al. Metformin in aging and aging-related diseases: clinical applications and relevant mechanisms. Theranostics 12, 2722-2740 (2022).
- Bharath, L. P. et al. Metformin enhances autophagy and normalizes mitochondrial function to alleviate aging-associated inflammation. Cell Metab. 32, 44-55.e6 (2020).
- Hung, I. F. et al. Immunogenicity of intradermal trivalent influenza vaccine with topical imiquimod: a double blind randomized controlled trial. Clin. Infect. Dis. 59, 1246-1255 (2014).
- Pettersen, F. O. et al. An exploratory trial of cyclooxygenase type 2 inhibitor in HIV-1 infection: downregulated immune activation and improved T cell-dependent vaccine responses. J. Virol. 85, 6557-6566 (2011).
- Madeo, F., Eisenberg, T., Pietrocola, F. & Kroemer, G. Spermidine in health and disease. Science 359, eaan2788 (2018).
- Nakamura, A. et al. Symbiotic polyamine metabolism regulates epithelial proliferation and macrophage differentiation in the colon. Nat. Commun. 12, 2105 (2021).
- Aggarwal, V. et al. Molecular mechanisms of action of epigallocatechin gallate in cancer: recent trends and advancement. Semin. Cancer Biol. 80, 256-275 (2022).
- Yuan, H. et al. The phytochemical epigallocatechin gallate prolongs the lifespan by improving lipid metabolism, reducing inflammation and oxidative stress in high-fat diet-fed obese rats. Aging Cell 19, e13199 (2020).
- Tavenier, J. et al. Alterations of monocyte NF-kappaB p65/RelA signaling in a cohort of older medical patients, agematched controls, and healthy young adults. Immun. Ageing 17, 25 (2020).
- Cheong, Y. et al. Epigallocatechin-3-Gallate as a novel vaccine adjuvant. Front. Immunol. 12, 769088 (2021).
- Herati, R. S. et al. Vaccine-induced
circulating Tfh are sensitive biosensors of age-related changes in inflammatory pathways. Cell Rep. Med. 2, 100262 (2021). - Carrasco, E. et al. The role of T cells in age-related diseases. Nat. Rev. Immunol. 22, 97-111 (2022).
- Heitmann, J. S. et al. A COVID-19 peptide vaccine for the induction of SARS-CoV-2 T cell immunity. Nature 601, 617-622 (2022).
- Hu, Y. et al. Synergistic tumor immunological strategy by combining tumor nanovaccine with gene-mediated extracellular matrix scavenger. Biomaterials 252, 120114 (2020).
- Mittelbrunn, M. & Kroemer, G. Hallmarks of T cell aging. Nat. Immunol. 22, 687-698 (2021).
- Fear, V. S. et al. Tumour draining lymph node-generated CD8 T cells play a role in controlling lung metastases after a primary tumour is removed but not when adjuvant immunotherapy is used. Cancer Immunol. Immunother. 70, 3259 (2021).
- Liu, X., Hoft, D. F. & Peng, G. Senescent T cells within suppressive tumor microenvironments: emerging target for tumor immunotherapy. J. Clin. Investig. 130, 1073-1083 (2020).
- Liu, C. et al. A nanovaccine for antigen self-presentation and immunosuppression reversal as a personalized cancer immunotherapy strategy. Nat. Nanotechnol. 17, 531-540 (2022).
- Antonangeli, F., Zingoni, A., Soriani, A. & Santoni, A. Senescent cells: living or dying is a matter of NK cells. J. Leukoc. Biol. 105, 1275-1283 (2019).
- Chaudhary, N., Weissman, D. & Whitehead, K. A. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat. Rev. Drug Discov. 20, 817-838 (2021).
- Newman, J. et al. Neutralizing antibody activity against 21 SARS-CoV-2 variants in older adults vaccinated with BNT162b2. Nat. Microbiol. 7, 1180-1188 (2022).
- Parry, H. et al. Extended interval BNT162b2 vaccination enhances peak antibody generation. NPJ Vaccines 7, 14 (2022).
- Thompson, W. W. et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289, 179-186 (2003).
- Di Pasquale, A., Preiss, S., Tavares Da Silva, F. & Garcon, N. Vaccine adjuvants: from 1920 to 2015 and beyond. Vaccines 3, 320-343 (2015).
- Tsang, P. et al. Immunogenicity and safety of Fluzone
intradermal and high-dose influenza vaccines in older adults years of age: a randomized, controlled, phase II trial. Vaccine 32, 2507-2517 (2014). - Muszkat, M. et al. Local and systemic immune response in nursing-home elderly following intranasal or intramuscular immunization with inactivated influenza vaccine. Vaccine 21, 1180-1186 (2003).
- Mosafer, J., Sabbaghi, A. H., Badiee, A., Dehghan, S. & Tafaghodi, M. Preparation, characterization and in vivo evaluation of alginatecoated chitosan and trimethylchitosan nanoparticles loaded with PR8 influenza virus for nasal immunization. Asian J. Pharm. Sci. 14, 216-221 (2019).
- Andrew, M. K. et al. The importance of frailty in the assessment of influenza vaccine effectiveness against influenza-related hospitalization in elderly people. J. Infect. Dis. 216, 405-414 (2017).
- Huang, C. Q., Vishwanath, S., Carnell, G. W., Chan, A. C. Y. & Heeney, J. L. Immune imprinting and next-generation coronavirus vaccines. Nat. Microbiol. 8, 1971-1985 (2023).
- Jirru, E. et al. Impact of influenza on pneumococcal vaccine effectiveness during Streptococcus pneumoniae infection in aged murine lung. Vaccines 8, 298 (2020).
- Wang, J. et al. Broadly reactive IgG responses to heterologous H5 prime-boost influenza vaccination are shaped by antigenic relatedness to priming strains. mBio 12, e0044921 (2021).
- Kim, J. H., Davis, W. G., Sambhara, S. & Jacob, J. Strategies to alleviate original antigenic sin responses to influenza viruses. Proc. Natl Acad. Sci. USA 109, 13751-13756 (2012).
الشكر والتقدير
مساهمات المؤلفين
المصالح المتنافسة
معلومات إضافية
http://www.nature.com/reprints
© المؤلفون 2024
قسم الصيدلة، أكاديمية سيتشوان للعلوم الطبية ومستشفى الشعب الإقليمي في سيتشوان، كلية الطب، جامعة العلوم والتكنولوجيا الإلكترونية في الصين، تشنغدو 610072، الصين. مختبر الأدوية الشخصية الرئيسي في مقاطعة سيتشوان، كلية الطب، جامعة العلوم والتكنولوجيا الإلكترونية في الصين، تشنغدو 610072، الصين. ساهم هؤلاء المؤلفون بالتساوي: ينغ ينغ هو، مين تشين، يوان بيان. البريد الإلكتروني: zhl7235301@163.com; zhuyuxuan6688@163.com; tongrs@126.comعلوم اللقاحات
DOI: https://doi.org/10.1038/s41541-024-00874-4
PMID: https://pubmed.ncbi.nlm.nih.gov/38600250
Publication Date: 2024-04-10
Insights into vaccines for elderly individuals: from the impacts of immunosenescence to delivery strategies
Abstract
Immunosenescence increases the risk and severity of diseases in elderly individuals and leads to impaired vaccine-induced immunity. With aging of the global population and the emerging risk of epidemics, developing adjuvants and vaccines for elderly individuals to improve their immune protection is pivotal for healthy aging worldwide. Deepening our understanding of the role of immunosenescence in vaccine efficacy could accelerate research focused on optimizing vaccine delivery for elderly individuals. In this review, we analyzed the characteristics of immunosenescence at the cellular and molecular levels. Strategies to improve vaccination potency in elderly individuals are summarized, including increasing the antigen dose, preparing multivalent antigen vaccines, adding appropriate adjuvants, inhibiting chronic inflammation, and inhibiting immunosenescence. We hope that this review can provide a review of new findings with regards to the impacts of immunosenescence on vaccine-mediated protection and inspire the development of individualized vaccines for elderly individuals.
disease, respiratory syncytial virus infection, tetanus, and diphtheria
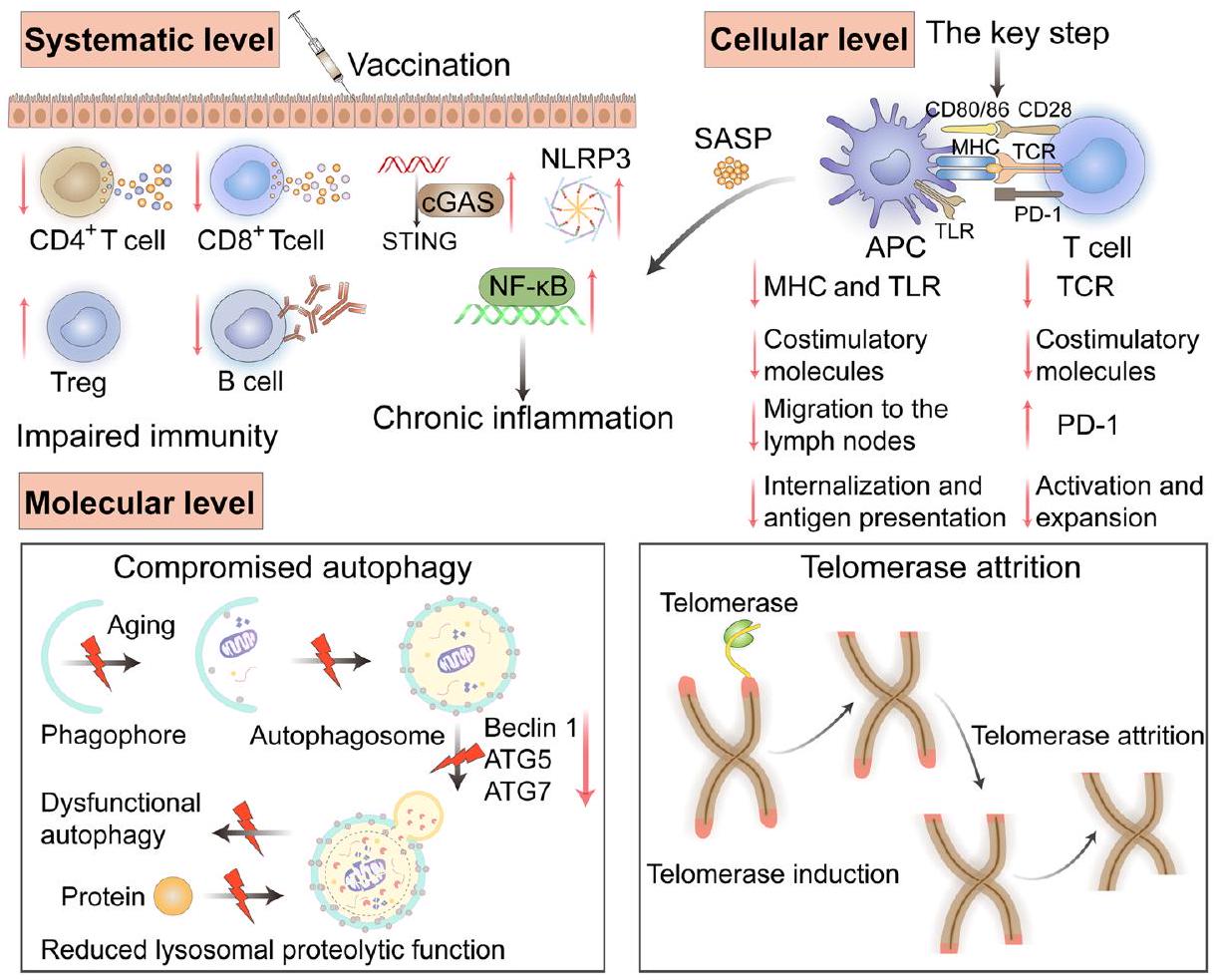
Impact of immunosenescence on vaccine efficacy
cell modulation, antigen uptake, antigen cross-presentation, and antigenpresenting cell (APC) regulation to stimulate T cells

zone of GC, directly decreased the amounts of antigens presented to B cells, and decreased specific antibody responses. Moreover, research has shown that the expression of activation-induced cytidine deaminase necessary for affinity maturation and class switching in B cells is significantly reduced in elderly individuals
Inflammation and immunity
microbiome in elderly individuals may also elevate the level of circulating inflammatory cytokines
antigen-specific immune responses
Molecular hallmarks of aging and immunity


promoted by autophagy in DCs. Autophagy can lead to the formation of antigen storage compartments in APCs, prolong the allowed storage time of antigens, and facilitate antigen presentation and subsequent initiation of
based on telomere EVs. However, the long-term protection of the telomere EV group was dramatically better than that of the group without telomere EVs. This finding further showed that telomere-containing EVs improved immune memory and long-lasting immunity. The results of this study suggest that vesicles carrying telomeres may be a potential strategy for inhibiting T cell immunosenescence and provide new ideas for the design of vaccines for elderly individuals.
Design strategies for vaccines for elderly individuals
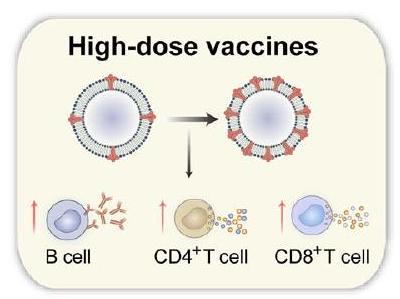
High-dose vaccines
Multivalent vaccines
Adjuvanted vaccines
Inhibiting chronic inflammation
senescent cells upregulated many biomarkers: Senescence-associated
Inhibiting immunosenescence
delivery system but often ignore the influence of the immune microenvironment of older individuals on the immune response. Without fundamentally improving the immunosenescence of elderly individuals, vaccines will not be able to produce strong and long-lasting immune efficacy. Inhibiting immunosenescence in elderly individuals will be a critical measure to improve immune responses to vaccines. Finding adjuvants and vaccine components that can inhibit immunosenescence is an attractive direction for future research.
Current design strategies and examples





| Design strategy | Pathogen | Vaccine information | References | |||
| High-dose vaccines | Influenza virus | Fluzone
|
79 | |||
| Multivalent vaccines | Streptococcus pneumoniae | PCV13 (13-valent pneumococcal conjugate vaccine) | 90 | |||
| Streptococcus pneumoniae | PPSV23 (23-valent pneumococcal polysaccharide vaccine) | 91 | ||||
| Adjuvanted vaccines | Influenza virus | Fluad
|
|
|||
| Influenza virus | Pandemrix
|
|
||||
| Influenza virus | Containing Matrix M-1 saponin complex adjuvant | NCT03293498 | ||||
| Influenza virus | Containing TLR 7/8 agonist resiquimod | NCT01737580 | ||||
| Herpes zoster | Shingrix
|
104 | ||||
| Respiratory syncytial virus | Arexvy
|
https://www.fda.gov/news-events/press-announcements/ fda-approves-first-respiratory-syncytial-virus-rsv-vaccine | ||||
| SARS-CoV-2 | TLR9 agonists synergizes with aluminum hydroxide adjuvant | 114 | ||||
| Inhibiting chronic inflammation | / | p38 MAPK inhibitor | 115 | |||
| / | mTOR inhibitor (rapamycin) | NCT02874924 | ||||
| / | COX-2 inhibitor | 125 | ||||
| / |
|
130 | ||||
| Inhibiting immunosenescence | SARS-CoV-2 or influenza virus | Spermidine (Dietary Supplement) to restore autophagy in T and B cells | NCT05421546 | |||
| Influenza virus | Metformin to restore autophagy in T and B cells | NCT03996538 | ||||
| Streptococcus pneumoniae | Metformin to restore autophagy in T and B cells | NCT03713801 | ||||
| mRNA vaccines | SARS-CoV-2 | BNT162b2 and mRNA-1273 | 141 | |||
| Boost vaccination | SARS-CoV-2 | A third booster dose | 142 | |||
| Delayed interval vaccination | SARS-CoV-2 | The interval between the two doses was extended from 3 weeks to
|
143 | |||
| Intradermal injection | Influenza virus | Intanza
|
146 |
 of rapamycin.
of rapamycin.mechanism may be that mRNA can be recognized by TLR3, 7, and 8 to induce adjuvant-like activity and enhanced antigen expression and antigen presentation
Conclusions and perspectives
efficacy against various antigens can be improved. An attractive research direction will be discovering immunomodulators and vaccine formulations that can inhibit immunosenescence. The selection of adjuvants can greatly impact the type and magnitude of the immune response
research and development of vaccines; this collaboration could provide more innovative strategies and spark ideas for personalized vaccine design.
Data availability
References
- Li, X. et al. Inflammation and aging: signaling pathways and intervention therapies. Signal Transduct. Target Ther. 8, 239 (2023).
- United Nations. Department of Economic and Social Affairs, Population Division (2019). World Population Ageing 2019: Highlights (ST/ESA/SER.A/430) (2022).
- Gralinski, L. E. & Menachery, V. D. Return of the Coronavirus: 2019nCoV. Viruses 12, 135 (2020).
- Koff, W. C. et al. Accelerating next-generation vaccine development for global disease prevention. Science 340, 1232910 (2013).
- Mascola, J. R. & Fauci, A. S. Novel vaccine technologies for the 21st century. Nat. Rev. Immunol. 20, 87-88 (2020).
- Hou, Y. et al. Advanced subunit vaccine delivery technologies: from vaccine cascade obstacles to design strategies. Acta Pharm. Sin. B 13, 3321-3338 (2023).
- Cunningham, A. L., McIntyre, P., Subbarao, K., Booy, R. & Levin, M. J. Vaccines for older adults. BMJ 372, n188 (2021).
- Bell, M. R. & Kutzler, M. A. An old problem with new solutions: strategies to improve vaccine efficacy in the elderly. Adv. Drug Deliv. Rev. 183, 114175 (2022).
- Osterholm, M. T. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect. Dis. 12, 36-44 (2012).
- Walford, R. L. The immunologic theory of aging. Gerontologist 4, 195-197 (1964).
- Willyard, C. How anti-ageing drugs could boost COVID vaccines in older people. Nature 586, 352-354 (2020).
- Qin, X., Jian, D. & Yi, C. Role of CD8
T lymphocyte cells: interplay with stromal cells in tumor microenvironment. Acta Pharm. Sin. B 11, 1365-1378 (2021). - Riese, P. et al. Distinct immunological and molecular signatures underpinning influenza vaccine responsiveness in the elderly. Nat. Commun. 13, 6894 (2022).
- Roukens, A. H. et al. Elderly subjects have a delayed antibody response and prolonged viraemia following yellow fever vaccination: a prospective controlled cohort study. PloS One 6, e27753 (2011).
- Schulz, A. R. et al. Low thymic activity and dendritic cell numbers are associated with the immune response to primary viral infection in elderly humans. J. Immunol. 195, 4699-4711 (2015).
- Ding, Y., Li, Z., Jaklenec, A. & Hu, Q. Vaccine delivery systems toward lymph nodes. Adv. Drug Deliv. Rev. 179, 113914 (2021).
- Lefebvre, J. S., Masters, A. R., Hopkins, J. W. & Haynes, L. Agerelated impairment of humoral response to influenza is associated with changes in antigen specific
follicular helper cell responses. Sci. Rep. 6, 25051 (2016). - Chen, J., Deng, J. C. & Goldstein, D. R. How aging impacts vaccine efficacy: known molecular and cellular mechanisms and future directions. Trends Mol. Med. 28, 1100-1111 (2022).
- Hadamitzky, C. et al. Age-dependent histoarchitectural changes in human lymph nodes: an underestimated process with clinical relevance? J. Anat. 216, 556-562 (2010).
- Agrawal, A. et al. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3 -kinase-signaling pathway. J. Immunol. 178, 6912-6922 (2007).
- Yang, Y., Guo, X., Hu, B., He, P. & Feng, M. Generated SecPen_NY-ESO-1_ubiquitin-pulsed dendritic cell cancer vaccine elicits stronger and specific
cell immune responses. Acta Pharm. Sin. B 11, 476-487 (2020). - Eisenbarth, S. C. Dendritic cell subsets in T cell programming: location dictates function. Nat. Rev. Immunol. 19, 89-103 (2019).
- Heath, W. R., Kato, Y., Steiner, T. M. & Caminschi, I. Antigen presentation by dendritic cells for B cell activation. Curr. Opin. Immunol. 58, 44-52 (2019).
- Wang, J., Geiger, H. & Rudolph, K. L. Immunoaging induced by hematopoietic stem cell aging. Curr. Opin. Immunol. 23, 532-536 (2011).
- Panda, A. et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J. Immunol. 184, 2518-2527 (2010).
- Leleux, J., Atalis, A. & Roy, K. Engineering immunity: modulating dendritic cell subsets and lymph node response to direct immunepolarization and vaccine efficacy. J. Control. Release 219, 610-621 (2015).
- Jackaman, C. et al. Targeting macrophages rescues age-related immune deficiencies in C57BL/6J geriatric mice. Aging Cell 12, 345-357 (2013).
- Prieto, L. I. et al. Senescent alveolar macrophages promote earlystage lung tumorigenesis. Cancer Cell 41, 1261-1275.e6 (2023).
- Wang, J., Yang, J. & Kopecek, J. Nanomedicines in B cell-targeting therapies. Acta Biomater. 137, 1-19 (2022).
- Frasca, D. & Blomberg, B. B. Aging affects human B cell responses. J. Clin. Immunol. 31, 430-435 (2011).
- Pritz, T. et al. Plasma cell numbers decrease in bone marrow of old patients. Eur. J. Immunol. 45, 738-746 (2015).
- Cancro, M. P. Age-associated B cells. Annu. Rev. Immunol. 38, 315-340 (2020).
- Yam-Puc, J. C. et al. Age-associated
cells predict impaired humoral immunity after COVID-19 vaccination in patients receiving immune checkpoint blockade. Nat. Commun. 14, 3292 (2023). - Allen, C. D. et al. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat. Immunol. 5, 943-952 (2004).
- Wols, H. A. et al. Migration of immature and mature B cells in the aged microenvironment. Immunology 129, 278-290 (2010).
- Frasca, D., Blomberg, B. B., Garcia, D., Keilich, S. R. & Haynes, L. Age-related factors that affect
cell responses to vaccination in mice and humans. Immunol. Rev. 296, 142-154 (2020). - Lefebvre, J. S. et al. The aged microenvironment contributes to the age-related functional defects of CD4 T cells in mice. Aging Cell 11, 732-740 (2012).
- Silva-Cayetano, A. et al. Spatial dysregulation of T follicular helper cells impairs vaccine responses in aging. Nat. Immunol. 24, 1124-1137 (2023).
- Khurana, S., Frasca, D., Blomberg, B. & Golding, H. AID activity in B cells strongly correlates with polyclonal antibody affinity maturation in-vivo following pandemic 2009-H1N1 vaccination in humans. PLoS Pathog. 8, e1002920 (2012).
- Stiasny, K., Aberle, J. H., Keller, M., Grubeck-Loebenstein, B. & Heinz, F. X. Age affects quantity but not quality of antibody responses after vaccination with an inactivated flavivirus vaccine against tick-borne encephalitis. PLoS One 7, e34145 (2012).
- Goronzy, J. J. & Weyand, C. M. Understanding immunosenescence to improve responses to vaccines. Nat. Immunol. 14, 428-436 (2013).
- Chen, X., Liu, Q. & Xiang, A. P. CD8
CD28 T cells: not only agerelated cells but a subset of regulatory T cells. Cell Mol. Immunol. 15, 734-736 (2018). - Kumagai, S. et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat. Immunol. 21, 1346-1358 (2020).
- Gustafson, C. E., Weyand, C. M. & Goronzy, J. J. T follicular helper cell development and functionality in immune ageing. Clin. Sci. 132, 1925-1935 (2018).
- Herati, R. S. et al. Circulating
response predicts influenza vaccine antibody responses in young adults but not elderly adults. J. Immunol. 193, 3528-3537 (2014). - Franceschi, C. et al. Inflamm aging: an evolutionary perspective on immunosenescence. Ann. N.Y. Acad. Sci. 908, 244-254 (2000).
- Franceschi, C. et al. Inflammaging and ‘Garb-aging. Trends Endocrinol. Metab. 28, 199-212 (2017).
- Gilroy, D. & De Maeyer, R. New insights into the resolution of inflammation. Semin. Immunol. 27, 161-168 (2015).
- Chambers, E. S. & Akbar, A. N. Can blocking inflammation enhance immunity during aging? J. Allergy Clin. Immunol. 145, 1323-1331 (2020).
- Hadrup, S. R. et al. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J. Immunol. 176, 2645-2653 (2006).
- Frasca, D., Blomberg, B. B. & Paganelli, R. Aging, obesity, and inflammatory age-related diseases. Front. Immunol. 8, 1745 (2017).
- Kim, K. A., Jeong, J. J., Yoo, S. Y. & Kim, D. H. Gut microbiota lipopolysaccharide accelerates inflamm-aging in mice. BMC Microbiol 16, 9 (2016).
- Claesson, M. J. et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 488, 178-184 (2012).
- De Maeyer, R. P. H. et al. Blocking elevated p38 MAPK restores efferocytosis and inflammatory resolution in the elderly. Nat. Immunol. 21, 615-625 (2020).
- Lasry, A. & Ben-Neriah, Y. Senescence-associated inflammatory responses: aging and cancer perspectives. Trends Immunol. 36, 217-228 (2015).
- Gulen, M. F. et al. cGAS-STING drives ageing-related inflammation and neurodegeneration. Nature 620, 374-380 (2023).
- Gritsenko, A., Green, J. P., Brough, D. & Lopez-Castejon, G. Mechanisms of NLRP3 priming in inflammaging and age-related diseases. Cytokine Growth Factor Rev. 55, 15-25 (2020).
- Lim, S.O. et al. Deubiquitination and stabilization of PD-L1 by CSN5. Cancer Cell, 925-939 (2016).
- Hamilton, J. A. G. et al. Interleukin-37 improves T-cell-mediated immunity and chimeric antigen receptor T-cell therapy in aged backgrounds. Aging Cell 20, e13309 (2021).
- Chen, X., Baumel, M., Männel, D. N., Howard, O. M. Z. & Oppenheim, J. J. Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse
T regulatory cells. J. Immunol. 179, 154-161 (2007). - Watanabe, R., Shirai, T., Hong, N., Zhang, H. & Weyand, C. M. Pyruvate controls the checkpoint inhibitor PD-L1 and suppresses T cell immunity. J. Clin. Investig. 127, 2725-2738 (2017).
- Chen, J. H. et al. Prostaglandin E2 and programmed cell death 1 signaling coordinately impair CTL function and survival during chronic viral infection. Nat. Med. 21, 327-334 (2015).
- López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: an expanding universe. Cell 186, 243-278 (2023).
- Guo, J. et al. Aging and aging-related diseases: from molecular mechanisms to interventions and treatments. Signal Transduct. Target Ther. 7, 391 (2022).
- Hansen, M., Rubinsztein, D. C. & Walker, D. W. Autophagy as a promoter of longevity: insights from model organisms. Nat. Rev. Mol. Cell Biol. 19, 579-593 (2018).
- Zhang, H. et al. Polyamines control eIF5A hypusination, TFEB translation, and autophagy to reverse B cell senescence. Mol. Cell. 76, 110-125.e9 (2019).
- Aman, Y. et al. Autophagy in healthy aging and disease. Nat. Aging 1, 634-650 (2021).
- Kim, J., Kundu, M., Viollet, B. & Guan, K. L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 13, 132-141 (2011).
- Cirone, M. Perturbation of bulk and selective macroautophagy, abnormal UPR activation and their interplay pave the way to immune dysfunction, cancerogenesis and neurodegeneration in ageing. Ageing Res. Rev. 58, 101026 (2020).
- Wang, Y. et al. In situ manipulation of dendritic cells by an autophagy-regulative nanoactivator enables effective cancer immunotherapy. ACS Nano 13, 7568-7577 (2019).
- Wang, S. et al. Exploration of antigen-induced
nanoparticles for therapeutic vaccine. Small 14, e1704272 (2018). - Hubbard, V. M. et al. Macroautophagy regulates energy metabolism during effector T cell activation. J. Immunol. 185, 7349-7357 (2010).
- Fan, J., Feng, Z. & Chen, N. Spermidine as a target for cancer therapy. Pharmacol. Res. 159, 104943 (2020).
- De Risi, M. et al. Mechanisms by which autophagy regulates memory capacity in ageing. Aging Cell 19, e13189 (2020).
- Ma, T. et al. Low-dose metformin targets the lysosomal AMPK pathway through PEN2. Nature 603, 159-165 (2022).
- Kroemer, G. & Zitvogel, L. CD4
T cells at the center of inflammaging. Cell Metab. 32, 4-5 (2020). - Chakravarti, D., LaBella, K. A. & DePinho, R. A. Telomeres: history, health, and hallmarks of aging. Cell 184, 306-322 (2021).
- Lanna, A. et al. An intercellular transfer of telomeres rescues T cells from senescence and promotes long-term immunological memory. Nat. Cell Biol. 24, 1461-1474 (2022).
- Gravenstein, S. et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccination on numbers of US nursing home residents admitted to hospital: a cluster-randomised trial. Lancet Respir. Med. 5, 738-746 (2017).
- Couch, R. B. et al. Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects. Vaccine 25, 7656-7663 (2007).
- Wilkinson, K. et al. Efficacy and safety of high-dose influenza vaccine in elderly adults: a systematic review and meta-analysis. Vaccine 35, 2775-2780 (2017).
- Goronzy, J. J., Fang, F., Cavanagh, M. M., Qi, Q. & Weyand, C. M. Naive T cell maintenance and function in human aging. J. Immunol. 194, 4073-4080 (2015).
- Sun, Y. et al. Metal-organic framework nanocarriers for drug delivery in biomedical applications. Nanomicro Lett. 12, 103 (2020).
- Hong, X. et al. The pore size of mesoporous silica nanoparticles regulates their antigen delivery efficiency. Sci. Adv. 6, eaaz4462 (2020).
- Xia, Y. et al. Exploiting the pliability and lateral mobility of Pickering emulsion for enhanced vaccination. Nat. Mater. 17, 187-194 (2018).
- Liu, K. et al. A novel multifunctional vaccine platform with dendritic cell-targeting and pH -responsive for cancer immunotherapy: antigen-directed biomimetic fabrication of a cabbage-like mannatide-zinc-antigen hybrid microparticles. Chem. Eng. J. 426, 130867 (2021).
- Zhao, J. et al. A minimalist binary vaccine carrier for personalized postoperative cancer vaccine therapy. Adv. Mater. 34, e2109254 (2022).
- Pereira, B., Xu, X. N. & Akbar, A. N. Targeting inflammation and immunosenescence to improve vaccine responses in the rlderly. Front. Immunol. 11, 583019 (2020).
- Lauer, K. B., Borrow, R. & Blancharda, T. J. Multivalent and multipathogen viral vector vaccines. Clin. Vaccin. Immunol. 24, e00298-16 (2017).
- Lewnard, J. A. et al. Effectiveness of 13-Valent pneumococcal conjugate vaccine against medically attended lower respiratory tract
infection and pneumonia among older adults. Clin. Infect. Dis. 75, 832-841 (2022). - Lawrence, H. et al. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against vaccine serotype pneumococcal pneumonia in adults: a case-control test-negative design study. PLoS Med. 17, e1003326 (2020).
- Hernandez-Davies, J. E. et al. Administration of multivalent influenza virus recombinant hemagglutinin vaccine in combination-adjuvant elicits broad reactivity beyond the vaccine components. Front. Immunol. 12, 692151 (2021).
- Reed, S. G., Orr, M. T. & Fox, C. B. Key roles of adjuvants in modern vaccines. Nat. Med. 19, 1597-1608 (2013).
- Pulendran, B., Arunachalam, P. S. & O’Hagan, D. T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 20, 454-475 (2021).
- Peletta, A., Lemoine, C., Courant, T., Collin, N. & Borchard, G. Meeting vaccine formulation challenges in an emergency setting: towards the development of accessible vaccines. Pharmacol. Res. 189, 106699 (2023).
- Nanishi, E. et al. Precision vaccine adjuvants for older adults: a scoping review. Clin. Infect. Dis. 75, S72-S80 (2022).
- Ciabattini, A. et al. Vaccination in the elderly: the challenge of immune changes with aging. Semin. Immunol. 40, 83-94 (2018).
- Nicolay, U., Heijnen, E., Nacci, P., Patriarca, P. A. & Leav, B. Immunogenicity of alIV3, MF59-adjuvanted seasonal trivalent influenza vaccine, in older adults
years of age: meta-analysis of cumulative clinical experience. Int. J. Infect. Dis. 85S, S1-S9 (2019). - Isakova Sivak, I. & Rudenko, L. Cross-protective potential of a MF59-adjuvanted quadrivalent influenza vaccine in older adults. Lancet Infect. Dis. 21, 900-901 (2021).
- Morel, S. et al. Adjuvant System AS03 containing
-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine 29, 2461-2473 (2011). - Yam, K. K. et al. AS03-adjuvanted, very-low-dose influenza vaccines induce distinctive immune responses compared to unadjuvanted high-dose vaccines in BALB/c mice. Front. Immunol. 6, 207 (2015).
- Dendouga, N., Fochesato, M., Lockman, L., Mossman, S. & Giannini, S. L. Cell-mediated immune responses to a varicella-zoster virus glycoprotein E vaccine using both a TLR agonist and QS21 in mice. Vaccine 30, 3126-3135 (2012).
- Nam, H. J. et al. An adjuvanted zoster vaccine elicits potent cellular immune responses in mice without QS21. NPJ Vaccines 7, 45 (2022).
- Lal, H. et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N. Engl. J. Med. 372, 2087-2096 (2015).
- Renshaw, M. et al. Cutting Edge: impaired toll-like receptor expression and function in aging. J. Immunol. 169, 4697-4701 (2002).
- Metcalf, T. U. et al. Global analyses revealed age-related alterations in innate immune responses after stimulation of pathogen recognition receptors. Aging Cell 14, 421-432 (2015).
- Janssen, J. M., Jackson, S., Heyward, W. L. & Janssen, R. S. Immunogenicity of an investigational hepatitis B vaccine with a tolllike receptor 9 agonist adjuvant (HBsAg-1018) compared with a licensed hepatitis B vaccine in subpopulations of healthy adults 1870 years of age. Vaccine 33, 3614-3618 (2015).
- Lim, J. S. et al. Flagellin-dependent TLR5/caveolin-1 as a promising immune activator in immunosenescence. Aging Cell 14, 907-915 (2015).
- Denton, A. E. et al. Targeting TLR4 during vaccination boosts MAdCAM-1+lymphoid stromal cell activation and promotes the aged germinal center response. Sci. Immunol. 7, eabk0018 (2022).
- Zareian, N. et al. Triggering of toll-like receptors in old individuals. Relevance for vaccination. Curr. Pharm. Des. 25, 4163-4167 (2019).
- Wu, T. Y. et al. Rational design of small molecules as vaccine adjuvants. Sci. Transl. Med. 6, 263ra160 (2014).
- Ross, K. A. et al. Novel nanoadjuvants balance immune activation with modest inflammation: implications for older adult vaccines. Immun. Ageing 20, 28 (2023).
- Ananya, A. et al. Just right” combinations of adjuvants with nanoscale carriers activate aged dendritic cells without overt inflammation. Immun. Ageing 20, 10 (2023).
- Nanishi, E., Borriello, F., O’Meara, T. R., Mcgrath, M. E. & Dowling, D. J. An aluminum hydroxide:CpG adjuvant enhances protection elicited by a SARS-CoV-2 receptor-binding domain vaccine in aged mice. Sci. Transl. Med. 14, eabj5305 (2021).
- Lanna, A. et al. A sestrin-dependent Erk-Jnk-p38 MAPK activation complex inhibits immunity during aging. Nat. Immunol. 18, 354-363 (2017).
- Kennedy, R. B. et al. Immunosenescence-related transcriptomic and immunologic changes in older individuals following influenza vaccination. Front. Immunol. 7, 450 (2016).
- Mannick, J. B. et al. mTOR inhibition improves immune function in the elderly. Sci. Transl. Med. 6, 268 ra179 (2014).
- Song, S., Lam, E. W. F., Tchkonia, T., Kirkland, J. L. & Sun, Y. Senescent cells: emerging targets for human aging and age-related diseases. Trends Biochem. Sci. 45, 578-592 (2020).
- Wissler Gerdes, E. O., Misra, A., Netto, J. M. E., Tchkonia, T. & Kirkland, J. L. Strategies for late phase preclinical and early clinical trials of senolytics. Mech. Ageing Dev. 200, 111591 (2021).
- Kirkland, J. L. & Tchkonia, T. Senolytic drugs: from discovery to translation. J. Intern. Med. 288, 518-536 (2020).
- Zhang, Q., Li, S., Chen, F., Zeng, R. & Tong, R. Targeted delivery strategy: a beneficial partner for emerging senotherapy. Biomed. Pharmacother. 155, 113737 (2022).
- Chen, S. et al. Metformin in aging and aging-related diseases: clinical applications and relevant mechanisms. Theranostics 12, 2722-2740 (2022).
- Bharath, L. P. et al. Metformin enhances autophagy and normalizes mitochondrial function to alleviate aging-associated inflammation. Cell Metab. 32, 44-55.e6 (2020).
- Hung, I. F. et al. Immunogenicity of intradermal trivalent influenza vaccine with topical imiquimod: a double blind randomized controlled trial. Clin. Infect. Dis. 59, 1246-1255 (2014).
- Pettersen, F. O. et al. An exploratory trial of cyclooxygenase type 2 inhibitor in HIV-1 infection: downregulated immune activation and improved T cell-dependent vaccine responses. J. Virol. 85, 6557-6566 (2011).
- Madeo, F., Eisenberg, T., Pietrocola, F. & Kroemer, G. Spermidine in health and disease. Science 359, eaan2788 (2018).
- Nakamura, A. et al. Symbiotic polyamine metabolism regulates epithelial proliferation and macrophage differentiation in the colon. Nat. Commun. 12, 2105 (2021).
- Aggarwal, V. et al. Molecular mechanisms of action of epigallocatechin gallate in cancer: recent trends and advancement. Semin. Cancer Biol. 80, 256-275 (2022).
- Yuan, H. et al. The phytochemical epigallocatechin gallate prolongs the lifespan by improving lipid metabolism, reducing inflammation and oxidative stress in high-fat diet-fed obese rats. Aging Cell 19, e13199 (2020).
- Tavenier, J. et al. Alterations of monocyte NF-kappaB p65/RelA signaling in a cohort of older medical patients, agematched controls, and healthy young adults. Immun. Ageing 17, 25 (2020).
- Cheong, Y. et al. Epigallocatechin-3-Gallate as a novel vaccine adjuvant. Front. Immunol. 12, 769088 (2021).
- Herati, R. S. et al. Vaccine-induced
circulating Tfh are sensitive biosensors of age-related changes in inflammatory pathways. Cell Rep. Med. 2, 100262 (2021). - Carrasco, E. et al. The role of T cells in age-related diseases. Nat. Rev. Immunol. 22, 97-111 (2022).
- Heitmann, J. S. et al. A COVID-19 peptide vaccine for the induction of SARS-CoV-2 T cell immunity. Nature 601, 617-622 (2022).
- Hu, Y. et al. Synergistic tumor immunological strategy by combining tumor nanovaccine with gene-mediated extracellular matrix scavenger. Biomaterials 252, 120114 (2020).
- Mittelbrunn, M. & Kroemer, G. Hallmarks of T cell aging. Nat. Immunol. 22, 687-698 (2021).
- Fear, V. S. et al. Tumour draining lymph node-generated CD8 T cells play a role in controlling lung metastases after a primary tumour is removed but not when adjuvant immunotherapy is used. Cancer Immunol. Immunother. 70, 3259 (2021).
- Liu, X., Hoft, D. F. & Peng, G. Senescent T cells within suppressive tumor microenvironments: emerging target for tumor immunotherapy. J. Clin. Investig. 130, 1073-1083 (2020).
- Liu, C. et al. A nanovaccine for antigen self-presentation and immunosuppression reversal as a personalized cancer immunotherapy strategy. Nat. Nanotechnol. 17, 531-540 (2022).
- Antonangeli, F., Zingoni, A., Soriani, A. & Santoni, A. Senescent cells: living or dying is a matter of NK cells. J. Leukoc. Biol. 105, 1275-1283 (2019).
- Chaudhary, N., Weissman, D. & Whitehead, K. A. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat. Rev. Drug Discov. 20, 817-838 (2021).
- Newman, J. et al. Neutralizing antibody activity against 21 SARS-CoV-2 variants in older adults vaccinated with BNT162b2. Nat. Microbiol. 7, 1180-1188 (2022).
- Parry, H. et al. Extended interval BNT162b2 vaccination enhances peak antibody generation. NPJ Vaccines 7, 14 (2022).
- Thompson, W. W. et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289, 179-186 (2003).
- Di Pasquale, A., Preiss, S., Tavares Da Silva, F. & Garcon, N. Vaccine adjuvants: from 1920 to 2015 and beyond. Vaccines 3, 320-343 (2015).
- Tsang, P. et al. Immunogenicity and safety of Fluzone
intradermal and high-dose influenza vaccines in older adults years of age: a randomized, controlled, phase II trial. Vaccine 32, 2507-2517 (2014). - Muszkat, M. et al. Local and systemic immune response in nursing-home elderly following intranasal or intramuscular immunization with inactivated influenza vaccine. Vaccine 21, 1180-1186 (2003).
- Mosafer, J., Sabbaghi, A. H., Badiee, A., Dehghan, S. & Tafaghodi, M. Preparation, characterization and in vivo evaluation of alginatecoated chitosan and trimethylchitosan nanoparticles loaded with PR8 influenza virus for nasal immunization. Asian J. Pharm. Sci. 14, 216-221 (2019).
- Andrew, M. K. et al. The importance of frailty in the assessment of influenza vaccine effectiveness against influenza-related hospitalization in elderly people. J. Infect. Dis. 216, 405-414 (2017).
- Huang, C. Q., Vishwanath, S., Carnell, G. W., Chan, A. C. Y. & Heeney, J. L. Immune imprinting and next-generation coronavirus vaccines. Nat. Microbiol. 8, 1971-1985 (2023).
- Jirru, E. et al. Impact of influenza on pneumococcal vaccine effectiveness during Streptococcus pneumoniae infection in aged murine lung. Vaccines 8, 298 (2020).
- Wang, J. et al. Broadly reactive IgG responses to heterologous H5 prime-boost influenza vaccination are shaped by antigenic relatedness to priming strains. mBio 12, e0044921 (2021).
- Kim, J. H., Davis, W. G., Sambhara, S. & Jacob, J. Strategies to alleviate original antigenic sin responses to influenza viruses. Proc. Natl Acad. Sci. USA 109, 13751-13756 (2012).
Acknowledgements
Author contributions
Competing interests
Additional information
http://www.nature.com/reprints
© The Author(s) 2024
Department of Pharmacy, Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu 610072, China. Personalized Drug Therapy Key Laboratory of Sichuan Province, School of Medicine, University of Electronic Science and Technology of China, Chengdu 610072, China. These authors contributed equally: Yingying Hou, Min Chen, Yuan Bian. e-mail: zhl7235301@163.com; zhuyuxuan6688@163.com; tongrs@126.com Vaccine Sciences
