DOI: https://doi.org/10.1038/s41467-024-45243-w
PMID: https://pubmed.ncbi.nlm.nih.gov/38302469
تاريخ النشر: 2024-02-01
كرات كربونية مسامية متوسطة مع داخل قابل للبرمجة كنانواتفاعلات فعالة لتخليق H2O2 الكهربائي
تم القبول: 16 يناير 2024
نُشر على الإنترنت: 01 فبراير 2024
(أ) التحقق من التحديثات
الملخص
يحتفظ النانو رياكتور بوعود كبيرة حيث يحاكي العمليات الطبيعية للكائنات الحية لتسهيل التفاعلات الكيميائية، مما يوفر إمكانيات هائلة في تحويل الطاقة الحفازة بفضل وظيفته الهيكلية الفريدة. هنا، نقترح استخدام كرات الكربون المصممة بدقة ككتل بناء، مع دمج الميكانيكا الدقيقة والتخليق القابل للتحكم لاستكشاف وظائفها الحفازة في تفاعلات اختزال الأكسجين ذات الإلكترونين. بعد إجراء تجارب ومحاكاة دقيقة، نقدم أدلة مقنعة على تأثيرات نقل الكتلة المعززة وتعديل الميكروبيئة التي تقدمها هذه الكرات الكربونية المجوفة المسامية، خاصة عند امتلاكها لهندسة مجوفة بحجم مناسب. ومن المثير للإعجاب، أن الإنجاز المحوري يكمن في النجاح في فحص محفز قوي، انتقائي، ودائم لتفاعل اختزال الأكسجين ذو الإلكترونين من أجل التخليق المباشر لمطهر بيروكسيد الهيدروجين من الدرجة الطبية. كعرض نموذجي لهندسة النانو رياكتور في فحص المحفزات، يبرز هذا العمل الإمكانيات الهائلة لمجموعة متنوعة من النانو رياكتورات الكربون المصممة بشكل جيد في تطبيقات واسعة.
سلوك التحفيز الجزيئي
تلاعب في هيكل مواد كرات الكربون واستغلال الوظائف التحفيزية، بما في ذلك الانتشار وتعديل البيئة الدقيقة داخل النانو رياكتورات، هناك فرصة واعدة لتجاوز هذه التحديات وتطوير استراتيجيات عملية لـ
النتائج
تحسين المعلمات الهندسية المتعلقة بالانتشار في الشبكات النانوية المعدنية
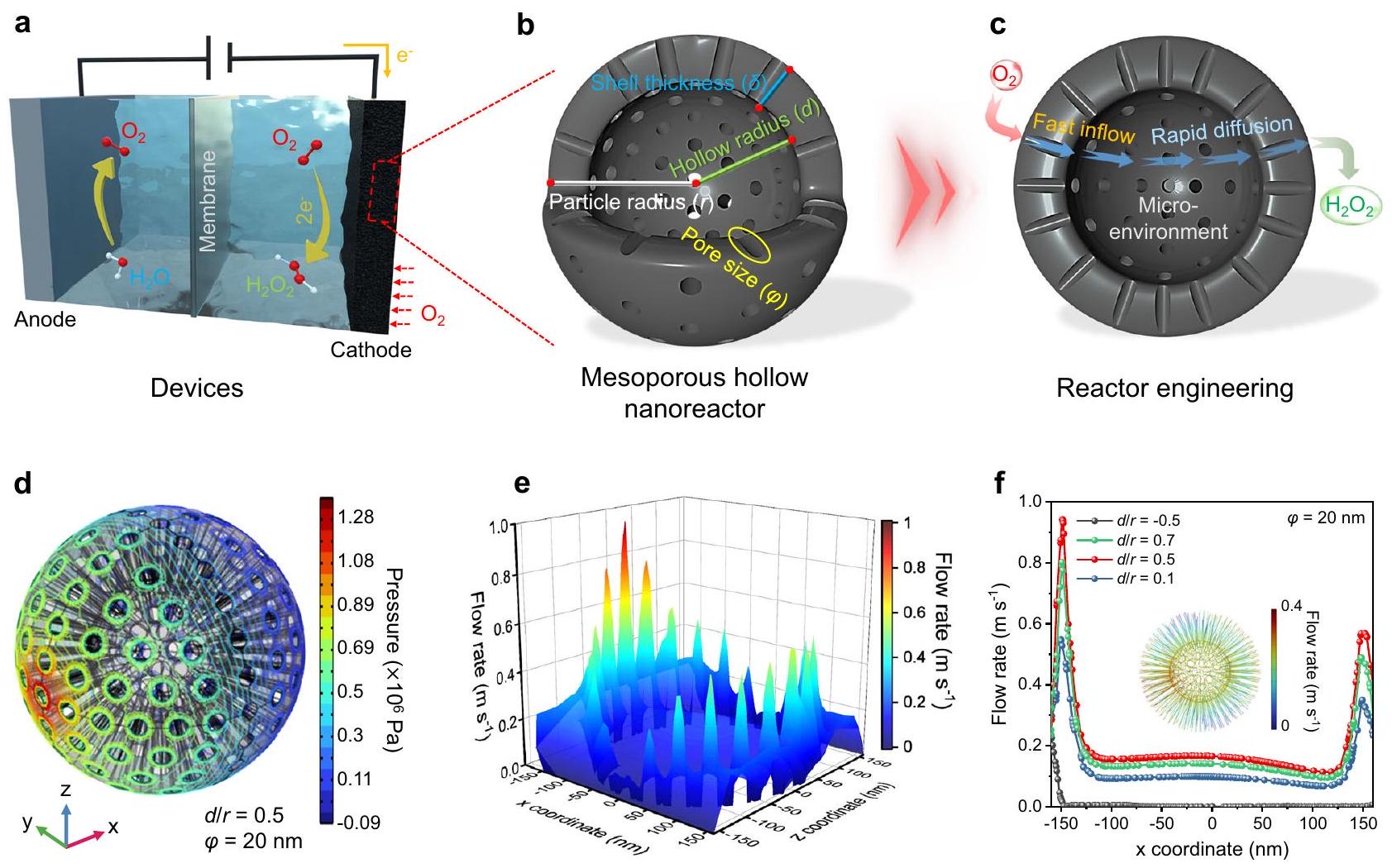
توزيع سرعة التدفق المكاني على المقطع العرضي.
المواقع. قد تؤسس تأثيرات التخفيف السائل في المنطقة المجوفة بيئة ميكروية داخلية مستقرة محتملة. لتلخيص ذلك، تم استخدام محاكاة الديناميات الميكانيكية الدقيقة لتحديد المعلمات الهيكلية المواتية للاختلاط.
تنقيح وتوصيف الشبكات النانوية الكربونية
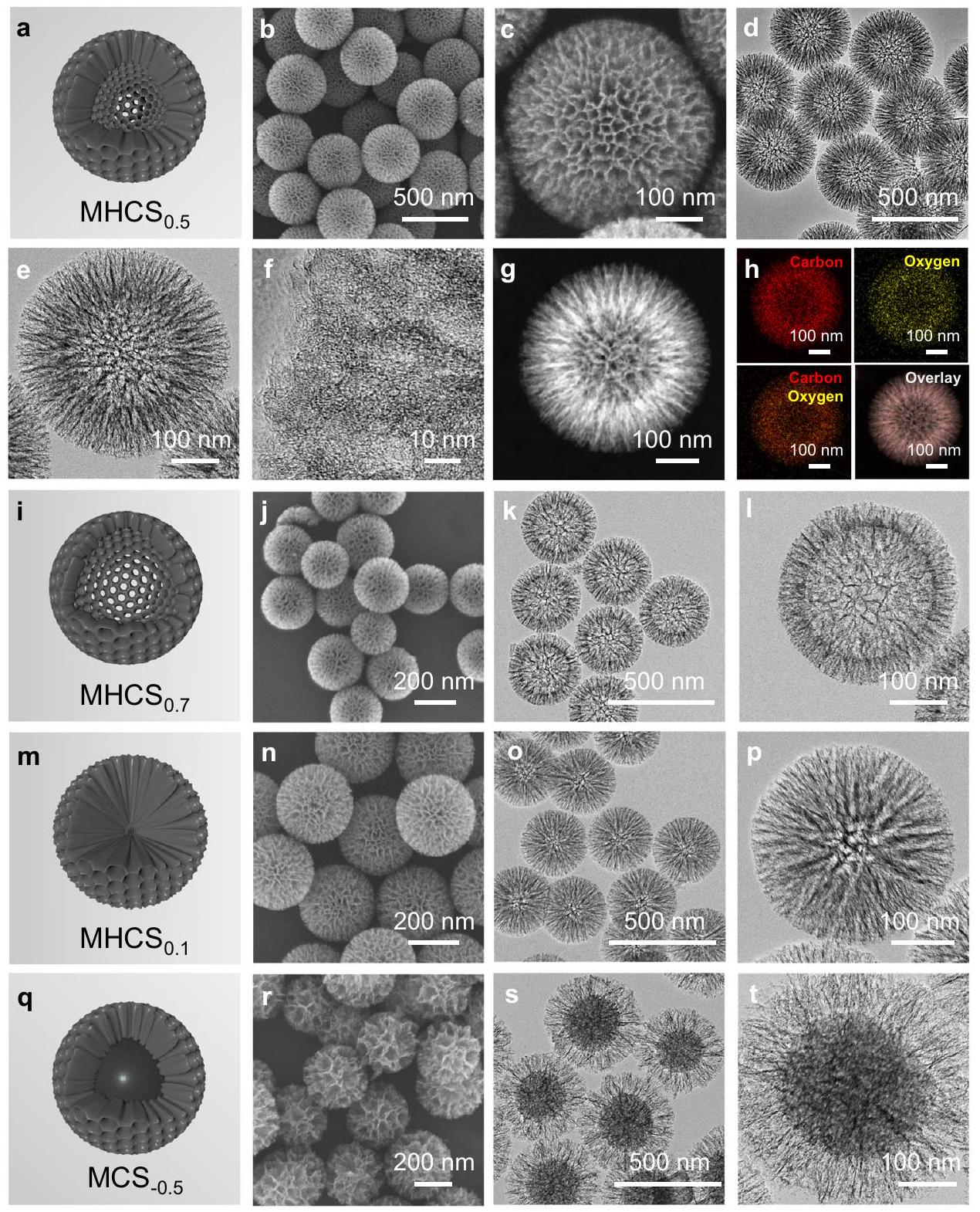
متقدم، مما يؤدي إلى تشكيل أكبر
الأصداف المسامية المتوسطة، مما يجعلها نماذج مثالية لاستكشاف فعالية MHNs في التفاعلات الحفزية.
أداء التحفيز الكهربائي
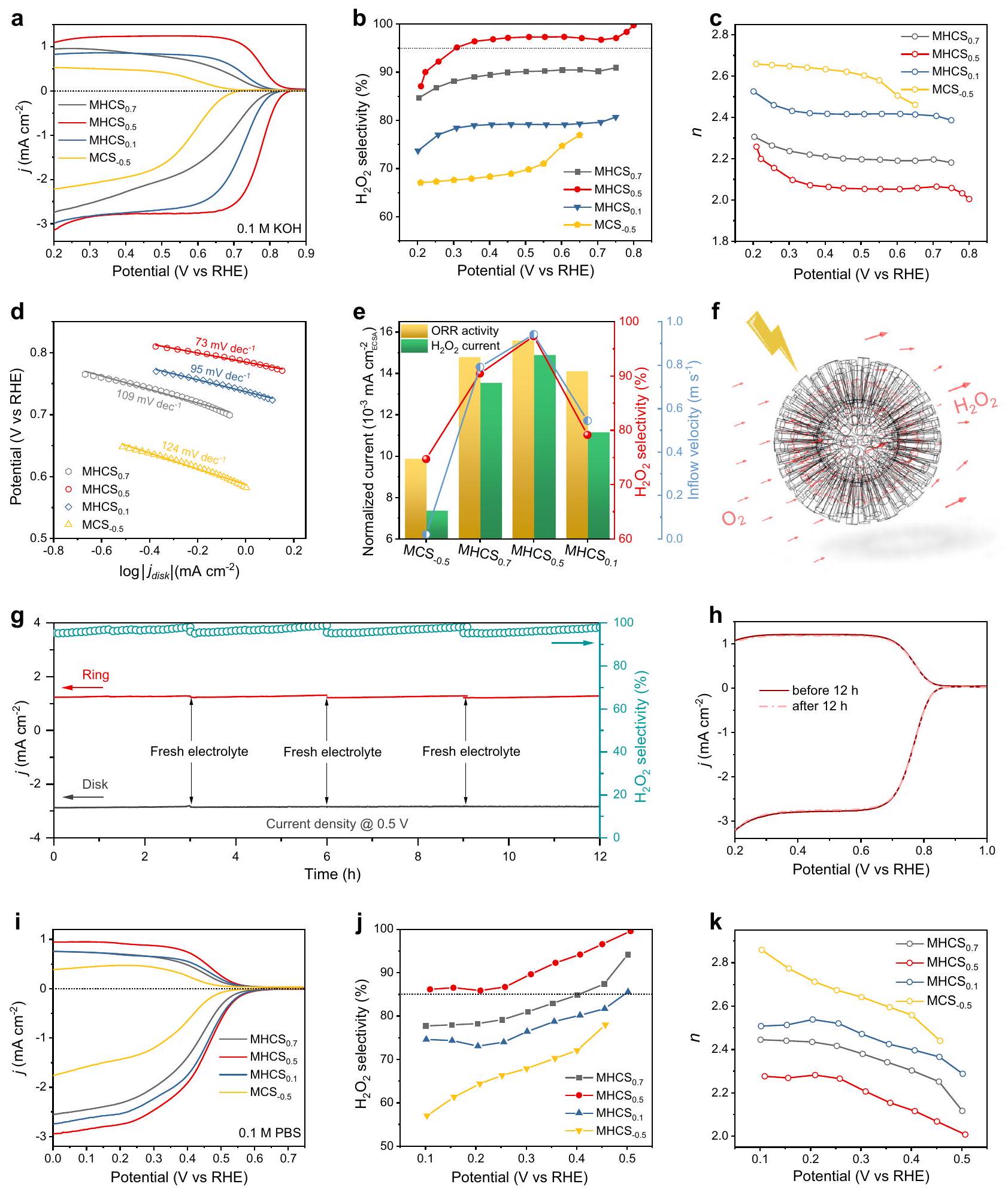
الأكسجين وعملية اختزال الأكسجين على
في عملية الاختزال الكهروكيميائي لتفاعل الأكسدة الاختزالية للأكسجين. لقد قمنا بتقليل
الغالبية العظمى من المحفزات المعتمدة على الكربون المبلغ عنها في الأدبيات (الشكل 4i-k والجدول التكميلي 4). الاتجاهات الملحوظة في نشاط ORR وانتقائيته لـ
فعلي
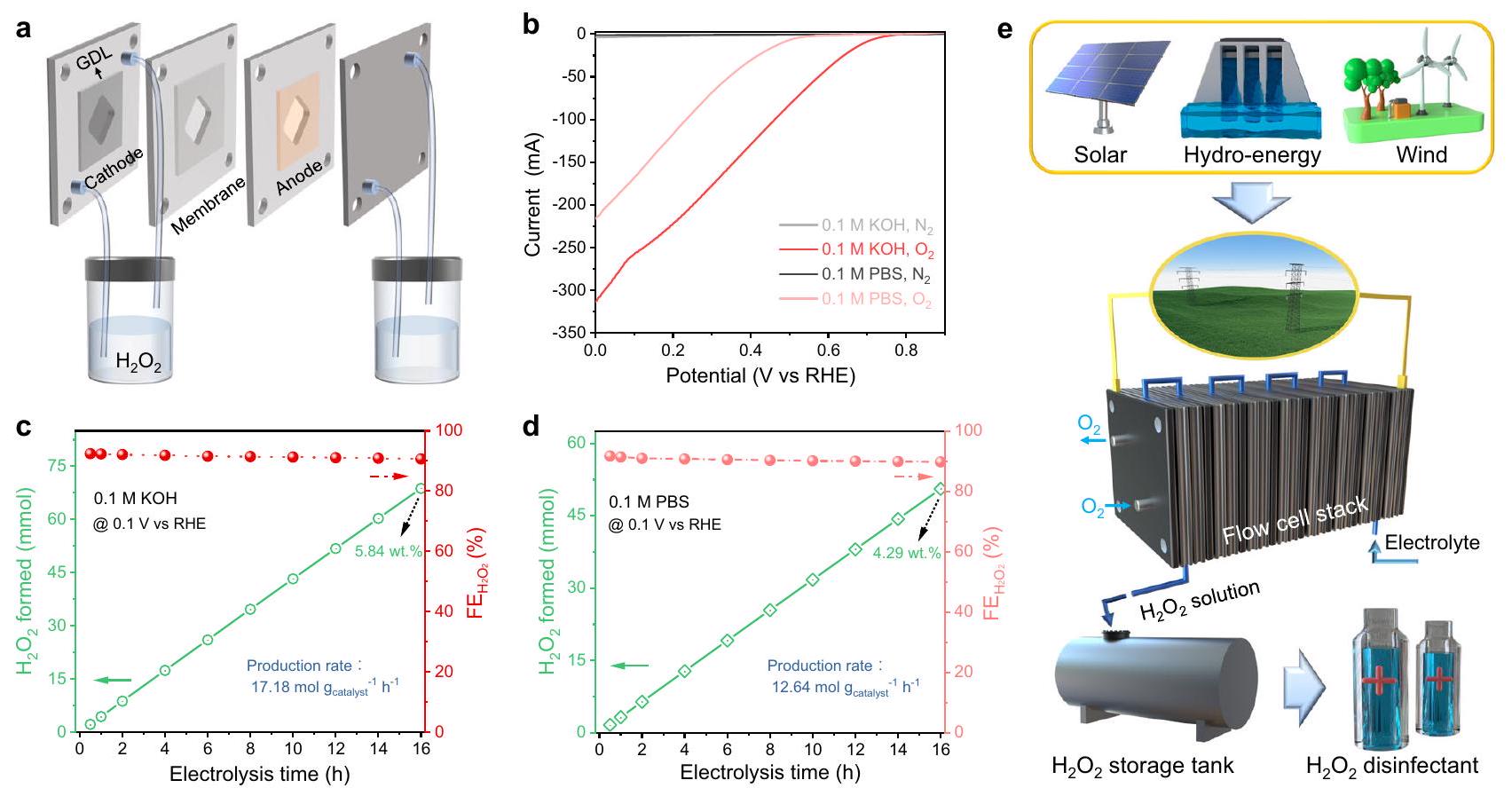
اعتمادًا على الوقت
تؤكد التمييز في الأداء الكهروكيميائي بشكل أكبر على فعالية الهياكل النانوية القائمة على كرات الكربون المصممة.
تأثير تعديل الميكروبيئة للنانوركترات
تسارع كبير في تدفق السائل في منطقة المسام المتوسطة، بينما كانت سرعة التدفق داخل المنطقة المجوفة منخفضة نسبيًا (الشكل التوضيحي 46). يمكن أن تزيد معدلات التدفق العالية من انخراط
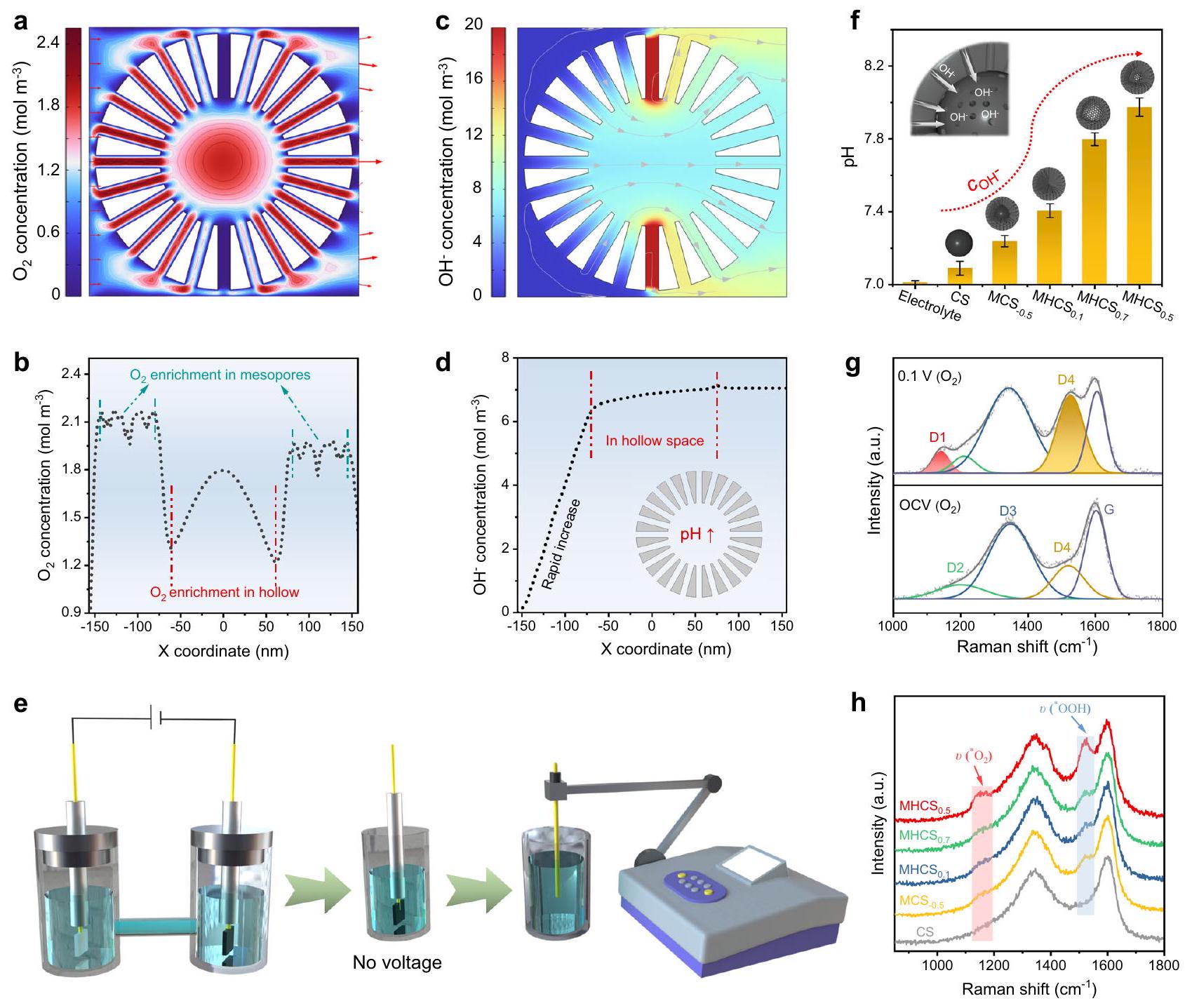
نموذج كرة الكربون المسامية المتوسطة على
مقارنة لطيف رامان في الموقع على
إنتاج
تشير قمة D4 الأكثر حدة المقابلة إلى تكوين أكبر لمتوسط *OOH
تُعزز التكوينات المجوفة ارتفاع درجة الحموضة المحلية، مما يوفر بيئة ميكروية مناسبة لتفاعل اختزال الأكسجين ثنائي الإلكترون (2e ORR). تؤكد نتائج التجارب، وقياسات رامان في الموقع، هذه الوظائف للنانوهياكل المجوفة المستندة إلى كرات الكربون المهندسة، والتي يمكن مقارنتها ببعض الوظائف الهيكلية للخلايا: حيث إن الغلاف المسامي للنانوهياكل المجوفة يشبه غشاء الخلية ويسيطر بشكل فعال على دخول وخروج الركائز والمنتجات؛ بينما يوفر الجزء الداخلي المجوف للنانوهياكل، الذي يشبه السيتوبلازم داخل الخلايا، بيئة داخلية ملائمة للتفاعل. على الرغم من أن مثالنا قد يفتقر إلى الوحدات الوظيفية المعقدة والمتطورة الموجودة في البيولوجيا، إلا أنه لا يزال مصدر إلهام لبناء النانو رياكتورات من خلال تقليد الطبيعة.
نقاش
طرق
تركيب كرات الكربون المسامية النانوية ذات الهياكل الداخلية المختلفة
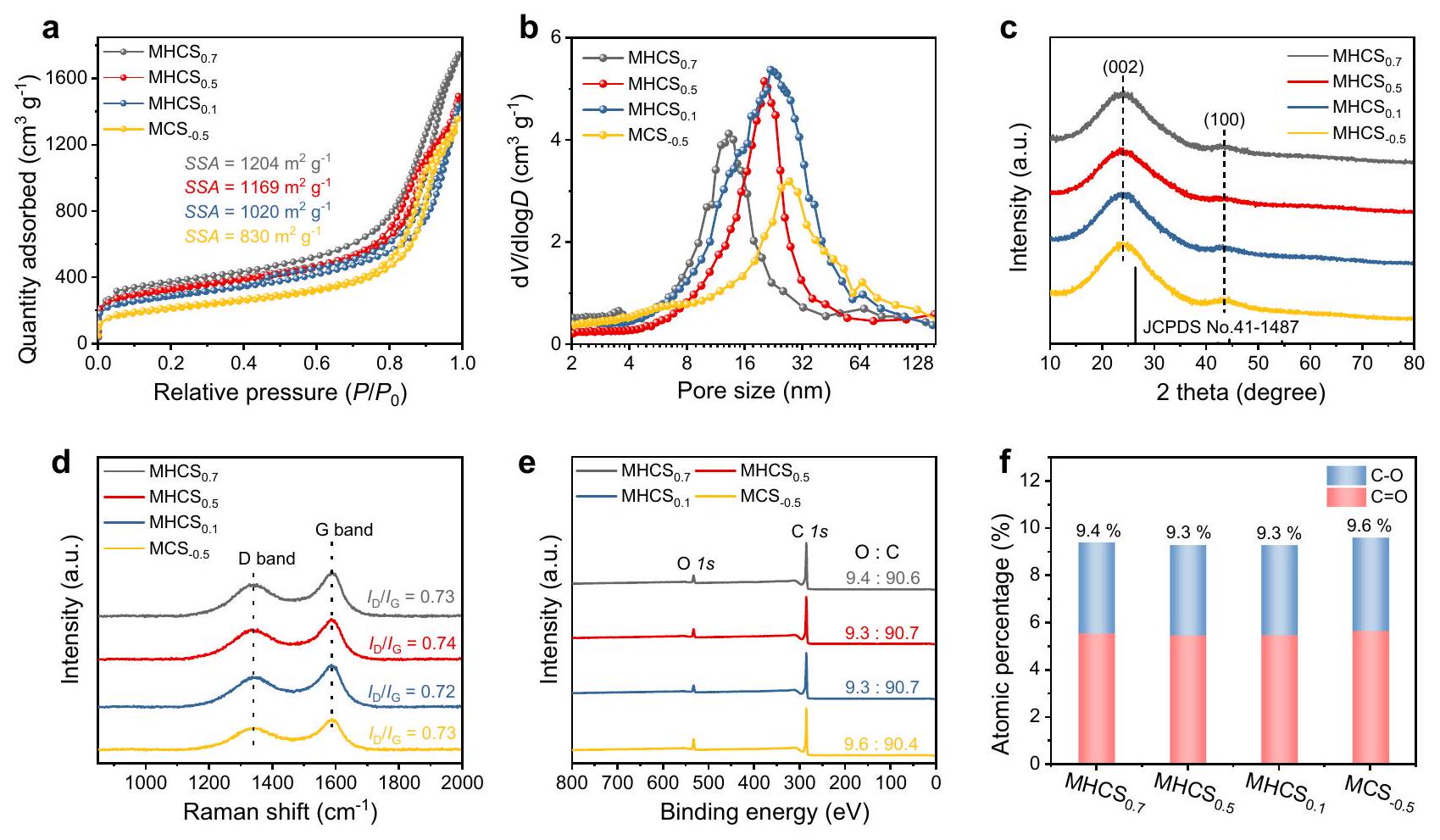
خصائص المواد
تم تسجيل خطوط العزل عند 77 كلفن (Tristar 3020، الولايات المتحدة الأمريكية). تم جمع بيانات طيف الإلكترون الضوئي (XPS) باستخدام مصدر أحادي اللون من الألمنيوم (KRATOS، Axis Ultra). تم إجراء قياسات الرنين المغناطيسي الإلكتروني (EPR) باستخدام مطياف EPR من نوع Bruker ECSEMX في درجة حرارة الغرفة.
القياسات الكهروكيميائية
قياس المساحة السطحية النشطة كيميائيًا
التحليل الكهربائي بالجملة
أداء
الكشف المباشر عن التغيرات المحلية في درجة الحموضة على القطب
محاكاة العناصر المحدودة
توفر البيانات
References
- Vriezema, D. M. et al. Self-assembled nanoreactors. Chem. Rev. 105, 1445-1490 (2005).
- Yu, Z. et al. Kinetics driven by hollow nanoreactors: an opportunity for controllable catalysis. Angew. Chem. Int. Ed. 62, e202213612 (2023).
- Petrosko, S. H., Johnson, R., White, H. & Mirkin, C. A. Nanoreactors: small spaces, big implications in chemistry. J. Am. Chem. Soc. 138, 7443-7445 (2016).
- Marguet, M., Bonduelle, C. & Lecommandoux, S. Multicompartmentalized polymeric systems: towards biomimetic cellular structure and function. Chem. Soc. Rev. 42, 512-529 (2013).
- Weiss, M. et al. Sequential bottom-up assembly of mechanically stabilized synthetic cells by microfluidics. Nat. Mater. 17, 89-96 (2018).
- Wang, G.-H. et al. Platinum-cobalt bimetallic nanoparticles in hollow carbon nanospheres for hydrogenolysis of 5-hydroxymethylfurfural. Nat. Mater. 13, 293-300 (2014).
- Zhu, W. et al. Functionalization of hollow nanomaterials for catalytic applications: nanoreactor construction. Adv. Mater. 31, 1800426 (2019).
- Ding, S. et al. Formation of
hollow nanospheres inside mesoporous silica nanoreactors. J. Am. Chem. Soc. 133, 21-23 (2011). - Tian, H. et al. Construction of hollow mesoporous silica nanoreactors for enhanced photo-oxidations over Au-Pt catalysts. Nat. Sci. Rev. 7, 1647-1655 (2020).
- Yu, Z. et al. Ruthenium-nanoparticle-loaded hollow carbon spheres as nanoreactors for hydrogenation of levulinic acid: explicitly recognizing the void-confinement effect. Angew. Chem. Int. Ed. 60, 20786-20794 (2021).
- Dong, C. et al. Hollow carbon sphere nanoreactors loaded with PdCu nanoparticles: void-confinement effects in liquid-phase hydrogenations. Angew. Chem. Int. Ed. 59, 18374-18379 (2020).
- Yu, Q. et al. Space-confined carbon-doped Pd nanoparticles as a highly efficient catalyst for selective phenol hydrogenation. ACS Catal. 13, 3925-3933 (2023).
- Tian, Q. et al. Exceptional photocatalytic hydrogen peroxide production from sandwich-structured graphene interlayered phenolic resins nanosheets with mesoporous channels. Adv. Funct. Mater. 33, 2213173 (2023).
- Tang,
. et al. Carbon nanocage with maximum utilization of atomically dispersed iron as efficient oxygen electroreduction nanoreactor. Adv. Mater. 35, 2208942 (2023). - Hung, C.-T. et al. Gradient hierarchically porous structure for rapid capillary-assisted catalysis. J. Am. Chem. Soc. 144, 6091-6099 (2022).
- Yu, Z. et al. Metal-loaded hollow carbon nanostructures as nanoreactors: microenvironment effects and prospects for biomass hydrogenation applications. ACS Sustain. Chem. Engin. 9, 2990-3010 (2021).
- Xie, G., Zhang, J. & Ma, X. Compartmentalization of multiple catalysts into outer and inner shells of hollow mesoporous nanospheres for heterogeneous multi-catalyzed/multi-component asymmetric organocascade. ACS Catal. 9, 9081-9086 (2019).
- Li, X. et al. Amorphous NiFe oxide-based nanoreactors for efficient electrocatalytic water oxidation. Angew. Chem. Int. Ed. 62, e202300478 (2023).
- Wang, X. et al. Balancing mass transfer and active sites to improve electrocatalytic oxygen reduction by B, N Codoped C nanoreactors. Nano Lett. 23, 4699-4707 (2023).
- Boyjoo, Y. et al. Engineering nanoreactors for metal-chalcogen batteries. Energy Environ. Sci. 14, 540-575 (2021).
- Swisher, J. H., Jibril, L., Petrosko, S. H. & Mirkin, C. A. Nanoreactors for particle synthesis. Nat. Rev. Mater. 7, 428-448 (2022).
- Tian, H., Liang, J. & Liu, J. Nanoengineering carbon spheres as nanoreactors for sustainable energy applications. Adv. Mater. 31, 1903886 (2019).
- Liu, J., Wickramaratne, N. P., Qiao, S. Z. & Jaroniec, M. Molecularbased design and emerging applications of nanoporous carbon spheres. Nat. Mater. 14, 763-774 (2015).
- Wang, T., Okejiri, F., Qiao, Z. A. & Dai, S. Tailoring polymer colloids derived porous carbon spheres based on specific chemical reactions. Adv. Mater. 32, 2002475 (2020).
- Chen, S. et al. Chemical identification of catalytically active sites on oxygen-doped carbon nanosheet to decipher the high activity for electro-synthesis hydrogen peroxide. Angew. Chem. Int. Ed. 133, 16743-16750 (2021).
- Lim, J. S. et al. Designing highly active nanoporous carbon
production electrocatalysts through active site identification. Chemistry 7, 3114-3130 (2021). - Wang, Z. et al. Hydrogen peroxide generation with
faradaic efficiency on metal-free carbon black. ACS Catal. 11, 2454-2459 (2021). - Wang, W., Zheng, Y., Hu, Y., Liu, Y. & Chen, S. Intrinsic carbon defects for the electrosynthesis of
. J. Phys. Chem. Lett. 13, 8914-8920 (2022). - San Roman, D. et al. Engineering three-dimensional (3D) out-ofplane graphene edge sites for highly selective two-electron oxygen reduction electrocatalysis. ACS Catal. 10, 1993-2008 (2020).
- Sa, Y. J., Kim, J. H. & Joo, S. H. Active edge-site-rich carbon nanocatalysts with enhanced electron transfer for efficient electrochemical hydrogen peroxide production. Angew. Chem. Int. Ed. 58, 1100-1105 (2019).
- Ding, Y. & Qiao, Z. A. Carbon surface chemistry: new insight into the old story. Adv. Mater. 34, 2206025 (2022).
- Li, Z., Li, B., Yu, C., Wang, H. & Li, Q. Recent progress of hollow carbon nanocages: general design fundamentals and diversified electrochemical applications. Adv. Sci. 10, 2206605 (2023).
- Xia, C., Xia, Y., Zhu, P., Fan, L. & Wang, H. T. Direct electrosynthesis of pure aqueous
solutions up to by weight using a solid electrolyte. Science 366, 226-231 (2019). - Jiang, Y. et al. Selective electrochemical
production through two-electron oxygen electrochemistry. Adv. Energy Mater. 8, 1801909 (2018). - Zhou, Y., Chen, G. & Zhang, J. A review of advanced metal-free carbon catalysts for oxygen reduction reactions towards the selective generation of hydrogen peroxide. J. Mater. Chem. A 8, 20849-20869 (2020).
- Sun, Y., Han, L. & Strasser, P. A comparative perspective of electrochemical and photochemical approaches for catalytic
production. Chem. Soc. Rev. 49, 6605-6631 (2020). - Tian, Z. et al. Constructing interfacial boron-nitrogen moieties in turbostratic carbon for electrochemical hydrogen peroxide production. Angew. Chem. Int. Ed. 134, e202206915 (2022).
- Hu, C., Paul, R., Dai, Q. & Dai, L. Carbon-based metal-free electrocatalysts: from oxygen reduction to multifunctional electrocatalysis. Chem. Soc. Rev. 50, 11785-11843 (2021).
- Bu, Y. et al. Carbon-based electrocatalysts for efficient hydrogen peroxide production. Adv. Mater. 33, 2103266 (2021).
- Jiang, K., Zhao, J. & Wang, H. Catalyst design for electrochemical oxygen reduction toward hydrogen peroxide. Adv. Funct. Mater. 30, 2003321 (2020).
- Li, L. et al. Tailoring selectivity of electrochemical hydrogen peroxide generation by tunable pyrrolic-nitrogen-carbon. Adv. Energy Mater. 10, 2000789 (2020).
- Perry, S. C. et al. Electrochemical synthesis of hydrogen peroxide from water and oxygen. Nat. Rev. Chem. 3, 442-458 (2019).
- Jing, L. et al. Mesoscale diffusion enhancement of carbon-bowl-shaped nanoreactor toward high-performance electrochemical
production. ACS Appl. Mater. Inter. 13, 39763-39771 (2021). - Deng, Z., Gong, M., Gong, Z. & Wang, X. Mesoscale mass transport enhancement on well-defined porous carbon platform for electrochemical
synthesis. Nano Lett. 22, 9551-9558 (2022). - Park, J., Nabae, Y., Hayakawa, T. & Kakimoto, M. A. Highly selective two-electron oxygen reduction catalyzed by mesoporous nitrogendoped carbon. ACS Catal. 4, 3749-3754 (2014).
- Grunberg, L. & Nissan, A. H. Mixture law for viscosity. Nature 164, 799-800 (1949).
- Zhang, H. et al. Surfactant-free assembly of mesoporous carbon hollow spheres with large tunable pore sizes. ACS Nano 10, 4579-4586 (2016).
- Mei, S. C. et al. Sequential assembly tailored interior of porous carbon spheres for boosted water decontamination through peroxymonosulfate activation. Adv. Funct. Mater. 32, 2111184 (2022).
- Xie, L. et al. Kinetics-controlled super-assembly of asymmetric porous and hollow carbon nanoparticles as light-sensitive smart nanovehicles. J. Am. Chem. Soc. 144, 1634-1646 (2022).
- Tian, Q. et al. Micelle-templating interfacial self-assembly of twodimensional mesoporous nanosheets for sustainable
electrosynthesis. Sustain. Mater. Technol. 32, e00398 (2022). - Schwan, J., Ulrich, S., Batori, V., Ehrhardt, H. & Silva, S. Raman spectroscopy on amorphous carbon films. J. Appl. Phys. 80, 440-447 (1996).
- Kim, H. W. et al. Efficient hydrogen peroxide generation using reduced graphene oxide-based oxygen reduction electrocatalysts. Nat. Catal. 1, 282-290 (2018).
- Wu, K.-H. et al. Highly selective hydrogen peroxide electrosynthesis on carbon: in situ interface engineering with surfactants. Chemistry 6, 1443-1458 (2020).
- Chen, S. et al. Defective carbon-based materials for the electrochemical synthesis of hydrogen peroxide. ACS Sustain. Chem. Engin. 6, 311-317 (2018).
- Tang, C. et al. Coordination tunes selectivity: two-electron oxygen reduction on high-loading molybdenum single-atom catalysts. Angew. Chem. Int. Ed. 132, 9256-9261 (2020).
- Han, G.-F. et al. Building and identifying highly active oxygenated groups in carbon materials for oxygen reduction to
. Nat. Commun. 11, 2209 (2020). - Chang, Q. et al. Promoting
production via 2-electron oxygen reduction by coordinating partially oxidized Pd with defect carbon. Nat. Commun. 11, 2178 (2020). - Peng, L. et al. Versatile nanoemulsion assembly approach to synthesize functional mesoporous carbon nanospheres with tunable pore sizes and architectures. J. Am. Chem. Soc. 141, 7073-7080 (2019).
- Lu, Z. Y. et al. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials. Nat. Catal. 1, 156-162 (2018).
- Zhao, K. M. et al. Insight into the mechanism of axial ligands regulating the catalytic activity of Fe-N4 sites for oxygen reduction reaction. Adv. Energy Mater. 12, 2103588 (2022).
- Tang, C. et al. Tailoring acidic oxygen reduction selectivity on single-atom catalysts via modification of first and second coordination spheres. J. Am. Chem. Soc. 143, 7819-7827 (2021).
- Bonakdarpour, A., Dahn, T. R., Atanasoski, R., Debe, M. K. & Dahn, J.
release during oxygen reduction reaction on Pt nanoparticles. Electrochem. Solid-State Lett. 11, B2O8 (2008). - Yao, W. et al. Hierarchically ordered macro-mesoporous electrocatalyst with hydrophilic surface for efficient oxygen reduction reaction. Adv. Mater. 35, 2301894 (2023).
- Ding, J. et al. Tunable periodically ordered mesoporosity in palladium membranes enables exceptional enhancement of intrinsic electrocatalytic activity for formic acid oxidation. Angew. Chem. Int. Ed. 132, 5130-5139 (2020).
- Wu, Z.-Y. et al. A general synthesis of single atom catalysts with controllable atomic and mesoporous structures. Nature Synth. 1, 658-667 (2022).
- Jung, E., Shin, H., Hooch Antink, W., Sung, Y.-E. & Hyeon, T. Recent advances in electrochemical oxygen reduction to
: catalyst and cell design. ACS Energy Lett. 5, 1881-1892 (2020). - Wen, Y. et al. Electrochemical reactors for continuous decentralized
production. Angew. Chem. Int. Ed. 134, e202205972 (2022). - Li, Y. et al. Single-atom iron catalyst with biomimetic active center to accelerate proton spillover for medical-level electrosynthesis of
disinfectant. Angew. Chem. Int. Ed. 62, e202306491 (2023). - Wei, X. et al. Synergistically enhanced single-atomic site Fe by
for boosted oxygen reduction in neutral electrolyte. Nano Energy 84, 105840 (2021). - Jiang, K. et al. Highly selective oxygen reduction to hydrogen peroxide on transition metal single atom coordination. Nat. Commun. 10, 3997 (2019).
- Zhang, L., Han, L., Liu, H., Liu, X. & Luo, J. Potential-cycling synthesis of single platinum atoms for efficient hydrogen evolution in neutral media. Angew. Chem. Int. Ed. 129, 13882-13886 (2017).
- Chu, S. & Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 488, 294-303 (2012).
- Gong, F. et al. Modulation of Mo-Fe-C sites over mesoscale dif-fusion-enhanced hollow sub-micro reactors toward boosted electrochemical water oxidation. Adv. Funct. Mater. 32, 2202141 (2022).
- Yang, C. et al. Interfacial
accumulation affects microenvironment in carbon-based electrocatalysts for production. ACS Energy Lett. 7, 4398-4407 (2022). - Melchionna, M., Fornasiero, P. & Prato, M. The rise of hydrogen peroxide as the main product by metal-free catalysis in oxygen reductions. Adv. Mater. 31, 1802920 (2019).
- Dong, K. et al. Honeycomb carbon nanofibers: a superhydrophilic
-entrapping electrocatalyst enables ultrahigh mass activity for the two-electron oxygen reduction reaction. Angew. Chem. Int. Ed. 60, 10583-10587 (2021). - Wei, J. et al. Probing the oxygen reduction reaction intermediates and dynamic active site structures of molecular and pyrolyzed Fe-N-C electrocatalysts by in situ Raman spectroscopy. ACS Catal. 12, 7811-7820 (2022).
- Tse, E. C. et al. Proton transfer dynamics control the mechanism of
reduction by a non-precious metal electrocatalyst. Nat. Mater. 15, 754-759 (2016).
شكر وتقدير
مساهمات المؤلفين
المصالح المتنافسة
معلومات إضافية
المواد التكميلية متاحة على
https://doi.org/10.1038/s41467-024-45243-w.
http://www.nature.com/reprints
© المؤلف(ون) 2024
مختبر شنتشن الرئيسي لمواد التحفيز الكهربائي للطاقة، مركز غوانغدونغ للبحث في الهندسة السطحية للمواد الوظيفية، كلية علوم وهندسة المواد، جامعة شنتشن، شنتشن، الصين. كلية الفيزياء والهندسة البصرية والإلكترونية، جامعة شنتشن، شنتشن، الصين. كلية الكيمياء والهندسة البيئية، جامعة شنتشن، شنتشن، الصين. المختبر الوطني الرئيسي للتفاعل الكيميائي، معهد داليان للفيزياء الكيميائية، الأكاديمية الصينية للعلوم، داليان، الصين. المعهد العالمي لتكنولوجيا المستقبل، جامعة جياوتونغ بشنغهاي، شنغهاي، الصين. البريد الإلكتروني: lingyan.jing@foxmail.com; yangjl18@szu.edu.cn
DOI: https://doi.org/10.1038/s41467-024-45243-w
PMID: https://pubmed.ncbi.nlm.nih.gov/38302469
Publication Date: 2024-02-01
Mesoporous carbon spheres with programmable interiors as efficient nanoreactors for
Accepted: 16 January 2024
Published online: 01 February 2024
(A) Check for updates
Abstract
The nanoreactor holds great promise as it emulates the natural processes of living organisms to facilitate chemical reactions, offering immense potential in catalytic energy conversion owing to its unique structural functionality. Here, we propose the utilization of precisely engineered carbon spheres as building blocks, integrating micromechanics and controllable synthesis to explore their catalytic functionalities in two-electron oxygen reduction reactions. After conducting rigorous experiments and simulations, we present compelling evidence for the enhanced mass transfer and microenvironment modulation effects offered by these mesoporous hollow carbon spheres, particularly when possessing a suitably sized hollow architecture. Impressively, the pivotal achievement lies in the successful screening of a potent, selective, and durable two-electron oxygen reduction reaction catalyst for the direct synthesis of medical-grade hydrogen peroxide disinfectant. Serving as an exemplary demonstration of nanoreactor engineering in catalyst screening, this work highlights the immense potential of various well-designed carbon-based nanoreactors in extensive applications.
molecular catalytic behavior
manipulate the structure of carbon sphere materials and harnessing the catalytic functions, including diffusion and microenvironmental modulation within nanoreactors, there is a promising opportunity to overcome these challenges and advance practical strategies for
Results
Optimizing diffusion-related geometrical parameters of MHNs

color mapping of the spatial flow velocity distribution on the cross-section.
sites. The fluid buffering effects in the hollow region may establish a potentially stable internal microenvironment. To summarize, micromechanical dynamics simulations were employed to identify diffusionfavorable structural parameters (
Refinement and characterization of carbon-based MHNs

is advanced,leading to the formation of larger

mesoporous shells, making them ideal models for exploring the efficacy of MHNs for catalytic reactions.
Electrocatalytic performance

oxygen and the oxygen reduction process on the
in electrocatalytic ORR. We reduced the
majority of carbon-based catalysts reported in the literature (Fig. 4i-k and Supplementary Table. 4). The trends observed in the ORR activity and selectivity of
Actual

time-dependent
distinction in electrochemical performance further underscores the effectiveness of the engineered carbon sphere-based MHNs.
Microenvironmental modulation effect of nanoreactors
significant acceleration of the fluid flow in the mesopore region, while the flow velocity within the hollow region was relatively low (Supplementary Fig. 46). High flow rates can increase the entrainment of

mesoporous carbon sphere model on
h Comparison of in-situ Raman spectra on
production of
corresponding sharper D4 peak indicates a greater formation of *OOH intermediate
hollow configuration promote localized pH elevation, providing a suitable microenvironment for the 2e ORR. FES, experimental results, and in-situ Raman spectroscopy verify these functions of the MHNs based on engineered carbon spheres, which can be compared to certain structural functions of cells: the mesoporous shell of the MHNs is analogous to the cell membrane and effectively controls the entry and exit of substrates and products; while the hollow interior of the MHNs, akin to the cytoplasm within cells, furnishes a conducive internal microenvironment for the reaction. Whilst our example may lack the intricate and sophisticated functional units found in biology, it nonetheless serves as a source of inspiration for the construction of nanoreactors through biomimicry.
Discussion
Methods
Synthesis of mesoporous carbon sphere nanoreactors with different internal structures
Material characterizations
isotherms were recorded at 77 K (Tristar 3020, USA). X-ray photoelectron spectroscopy (XPS) data were collected with a monochromatic Al source (KRATOS, Axis Ultra). Electron paramagnetic resonance (EPR) measurements were carried out using a Bruker ECSEMX X-band EPR spectrometer at room temperature.
Electrochemical measurements
Measurement of electrochemically active surface area
Bulk electrolysis
performance of
Direct detection of the local pH changes on electrode
Finite element simulation
Data availability
References
- Vriezema, D. M. et al. Self-assembled nanoreactors. Chem. Rev. 105, 1445-1490 (2005).
- Yu, Z. et al. Kinetics driven by hollow nanoreactors: an opportunity for controllable catalysis. Angew. Chem. Int. Ed. 62, e202213612 (2023).
- Petrosko, S. H., Johnson, R., White, H. & Mirkin, C. A. Nanoreactors: small spaces, big implications in chemistry. J. Am. Chem. Soc. 138, 7443-7445 (2016).
- Marguet, M., Bonduelle, C. & Lecommandoux, S. Multicompartmentalized polymeric systems: towards biomimetic cellular structure and function. Chem. Soc. Rev. 42, 512-529 (2013).
- Weiss, M. et al. Sequential bottom-up assembly of mechanically stabilized synthetic cells by microfluidics. Nat. Mater. 17, 89-96 (2018).
- Wang, G.-H. et al. Platinum-cobalt bimetallic nanoparticles in hollow carbon nanospheres for hydrogenolysis of 5-hydroxymethylfurfural. Nat. Mater. 13, 293-300 (2014).
- Zhu, W. et al. Functionalization of hollow nanomaterials for catalytic applications: nanoreactor construction. Adv. Mater. 31, 1800426 (2019).
- Ding, S. et al. Formation of
hollow nanospheres inside mesoporous silica nanoreactors. J. Am. Chem. Soc. 133, 21-23 (2011). - Tian, H. et al. Construction of hollow mesoporous silica nanoreactors for enhanced photo-oxidations over Au-Pt catalysts. Nat. Sci. Rev. 7, 1647-1655 (2020).
- Yu, Z. et al. Ruthenium-nanoparticle-loaded hollow carbon spheres as nanoreactors for hydrogenation of levulinic acid: explicitly recognizing the void-confinement effect. Angew. Chem. Int. Ed. 60, 20786-20794 (2021).
- Dong, C. et al. Hollow carbon sphere nanoreactors loaded with PdCu nanoparticles: void-confinement effects in liquid-phase hydrogenations. Angew. Chem. Int. Ed. 59, 18374-18379 (2020).
- Yu, Q. et al. Space-confined carbon-doped Pd nanoparticles as a highly efficient catalyst for selective phenol hydrogenation. ACS Catal. 13, 3925-3933 (2023).
- Tian, Q. et al. Exceptional photocatalytic hydrogen peroxide production from sandwich-structured graphene interlayered phenolic resins nanosheets with mesoporous channels. Adv. Funct. Mater. 33, 2213173 (2023).
- Tang,
. et al. Carbon nanocage with maximum utilization of atomically dispersed iron as efficient oxygen electroreduction nanoreactor. Adv. Mater. 35, 2208942 (2023). - Hung, C.-T. et al. Gradient hierarchically porous structure for rapid capillary-assisted catalysis. J. Am. Chem. Soc. 144, 6091-6099 (2022).
- Yu, Z. et al. Metal-loaded hollow carbon nanostructures as nanoreactors: microenvironment effects and prospects for biomass hydrogenation applications. ACS Sustain. Chem. Engin. 9, 2990-3010 (2021).
- Xie, G., Zhang, J. & Ma, X. Compartmentalization of multiple catalysts into outer and inner shells of hollow mesoporous nanospheres for heterogeneous multi-catalyzed/multi-component asymmetric organocascade. ACS Catal. 9, 9081-9086 (2019).
- Li, X. et al. Amorphous NiFe oxide-based nanoreactors for efficient electrocatalytic water oxidation. Angew. Chem. Int. Ed. 62, e202300478 (2023).
- Wang, X. et al. Balancing mass transfer and active sites to improve electrocatalytic oxygen reduction by B, N Codoped C nanoreactors. Nano Lett. 23, 4699-4707 (2023).
- Boyjoo, Y. et al. Engineering nanoreactors for metal-chalcogen batteries. Energy Environ. Sci. 14, 540-575 (2021).
- Swisher, J. H., Jibril, L., Petrosko, S. H. & Mirkin, C. A. Nanoreactors for particle synthesis. Nat. Rev. Mater. 7, 428-448 (2022).
- Tian, H., Liang, J. & Liu, J. Nanoengineering carbon spheres as nanoreactors for sustainable energy applications. Adv. Mater. 31, 1903886 (2019).
- Liu, J., Wickramaratne, N. P., Qiao, S. Z. & Jaroniec, M. Molecularbased design and emerging applications of nanoporous carbon spheres. Nat. Mater. 14, 763-774 (2015).
- Wang, T., Okejiri, F., Qiao, Z. A. & Dai, S. Tailoring polymer colloids derived porous carbon spheres based on specific chemical reactions. Adv. Mater. 32, 2002475 (2020).
- Chen, S. et al. Chemical identification of catalytically active sites on oxygen-doped carbon nanosheet to decipher the high activity for electro-synthesis hydrogen peroxide. Angew. Chem. Int. Ed. 133, 16743-16750 (2021).
- Lim, J. S. et al. Designing highly active nanoporous carbon
production electrocatalysts through active site identification. Chemistry 7, 3114-3130 (2021). - Wang, Z. et al. Hydrogen peroxide generation with
faradaic efficiency on metal-free carbon black. ACS Catal. 11, 2454-2459 (2021). - Wang, W., Zheng, Y., Hu, Y., Liu, Y. & Chen, S. Intrinsic carbon defects for the electrosynthesis of
. J. Phys. Chem. Lett. 13, 8914-8920 (2022). - San Roman, D. et al. Engineering three-dimensional (3D) out-ofplane graphene edge sites for highly selective two-electron oxygen reduction electrocatalysis. ACS Catal. 10, 1993-2008 (2020).
- Sa, Y. J., Kim, J. H. & Joo, S. H. Active edge-site-rich carbon nanocatalysts with enhanced electron transfer for efficient electrochemical hydrogen peroxide production. Angew. Chem. Int. Ed. 58, 1100-1105 (2019).
- Ding, Y. & Qiao, Z. A. Carbon surface chemistry: new insight into the old story. Adv. Mater. 34, 2206025 (2022).
- Li, Z., Li, B., Yu, C., Wang, H. & Li, Q. Recent progress of hollow carbon nanocages: general design fundamentals and diversified electrochemical applications. Adv. Sci. 10, 2206605 (2023).
- Xia, C., Xia, Y., Zhu, P., Fan, L. & Wang, H. T. Direct electrosynthesis of pure aqueous
solutions up to by weight using a solid electrolyte. Science 366, 226-231 (2019). - Jiang, Y. et al. Selective electrochemical
production through two-electron oxygen electrochemistry. Adv. Energy Mater. 8, 1801909 (2018). - Zhou, Y., Chen, G. & Zhang, J. A review of advanced metal-free carbon catalysts for oxygen reduction reactions towards the selective generation of hydrogen peroxide. J. Mater. Chem. A 8, 20849-20869 (2020).
- Sun, Y., Han, L. & Strasser, P. A comparative perspective of electrochemical and photochemical approaches for catalytic
production. Chem. Soc. Rev. 49, 6605-6631 (2020). - Tian, Z. et al. Constructing interfacial boron-nitrogen moieties in turbostratic carbon for electrochemical hydrogen peroxide production. Angew. Chem. Int. Ed. 134, e202206915 (2022).
- Hu, C., Paul, R., Dai, Q. & Dai, L. Carbon-based metal-free electrocatalysts: from oxygen reduction to multifunctional electrocatalysis. Chem. Soc. Rev. 50, 11785-11843 (2021).
- Bu, Y. et al. Carbon-based electrocatalysts for efficient hydrogen peroxide production. Adv. Mater. 33, 2103266 (2021).
- Jiang, K., Zhao, J. & Wang, H. Catalyst design for electrochemical oxygen reduction toward hydrogen peroxide. Adv. Funct. Mater. 30, 2003321 (2020).
- Li, L. et al. Tailoring selectivity of electrochemical hydrogen peroxide generation by tunable pyrrolic-nitrogen-carbon. Adv. Energy Mater. 10, 2000789 (2020).
- Perry, S. C. et al. Electrochemical synthesis of hydrogen peroxide from water and oxygen. Nat. Rev. Chem. 3, 442-458 (2019).
- Jing, L. et al. Mesoscale diffusion enhancement of carbon-bowl-shaped nanoreactor toward high-performance electrochemical
production. ACS Appl. Mater. Inter. 13, 39763-39771 (2021). - Deng, Z., Gong, M., Gong, Z. & Wang, X. Mesoscale mass transport enhancement on well-defined porous carbon platform for electrochemical
synthesis. Nano Lett. 22, 9551-9558 (2022). - Park, J., Nabae, Y., Hayakawa, T. & Kakimoto, M. A. Highly selective two-electron oxygen reduction catalyzed by mesoporous nitrogendoped carbon. ACS Catal. 4, 3749-3754 (2014).
- Grunberg, L. & Nissan, A. H. Mixture law for viscosity. Nature 164, 799-800 (1949).
- Zhang, H. et al. Surfactant-free assembly of mesoporous carbon hollow spheres with large tunable pore sizes. ACS Nano 10, 4579-4586 (2016).
- Mei, S. C. et al. Sequential assembly tailored interior of porous carbon spheres for boosted water decontamination through peroxymonosulfate activation. Adv. Funct. Mater. 32, 2111184 (2022).
- Xie, L. et al. Kinetics-controlled super-assembly of asymmetric porous and hollow carbon nanoparticles as light-sensitive smart nanovehicles. J. Am. Chem. Soc. 144, 1634-1646 (2022).
- Tian, Q. et al. Micelle-templating interfacial self-assembly of twodimensional mesoporous nanosheets for sustainable
electrosynthesis. Sustain. Mater. Technol. 32, e00398 (2022). - Schwan, J., Ulrich, S., Batori, V., Ehrhardt, H. & Silva, S. Raman spectroscopy on amorphous carbon films. J. Appl. Phys. 80, 440-447 (1996).
- Kim, H. W. et al. Efficient hydrogen peroxide generation using reduced graphene oxide-based oxygen reduction electrocatalysts. Nat. Catal. 1, 282-290 (2018).
- Wu, K.-H. et al. Highly selective hydrogen peroxide electrosynthesis on carbon: in situ interface engineering with surfactants. Chemistry 6, 1443-1458 (2020).
- Chen, S. et al. Defective carbon-based materials for the electrochemical synthesis of hydrogen peroxide. ACS Sustain. Chem. Engin. 6, 311-317 (2018).
- Tang, C. et al. Coordination tunes selectivity: two-electron oxygen reduction on high-loading molybdenum single-atom catalysts. Angew. Chem. Int. Ed. 132, 9256-9261 (2020).
- Han, G.-F. et al. Building and identifying highly active oxygenated groups in carbon materials for oxygen reduction to
. Nat. Commun. 11, 2209 (2020). - Chang, Q. et al. Promoting
production via 2-electron oxygen reduction by coordinating partially oxidized Pd with defect carbon. Nat. Commun. 11, 2178 (2020). - Peng, L. et al. Versatile nanoemulsion assembly approach to synthesize functional mesoporous carbon nanospheres with tunable pore sizes and architectures. J. Am. Chem. Soc. 141, 7073-7080 (2019).
- Lu, Z. Y. et al. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials. Nat. Catal. 1, 156-162 (2018).
- Zhao, K. M. et al. Insight into the mechanism of axial ligands regulating the catalytic activity of Fe-N4 sites for oxygen reduction reaction. Adv. Energy Mater. 12, 2103588 (2022).
- Tang, C. et al. Tailoring acidic oxygen reduction selectivity on single-atom catalysts via modification of first and second coordination spheres. J. Am. Chem. Soc. 143, 7819-7827 (2021).
- Bonakdarpour, A., Dahn, T. R., Atanasoski, R., Debe, M. K. & Dahn, J.
release during oxygen reduction reaction on Pt nanoparticles. Electrochem. Solid-State Lett. 11, B2O8 (2008). - Yao, W. et al. Hierarchically ordered macro-mesoporous electrocatalyst with hydrophilic surface for efficient oxygen reduction reaction. Adv. Mater. 35, 2301894 (2023).
- Ding, J. et al. Tunable periodically ordered mesoporosity in palladium membranes enables exceptional enhancement of intrinsic electrocatalytic activity for formic acid oxidation. Angew. Chem. Int. Ed. 132, 5130-5139 (2020).
- Wu, Z.-Y. et al. A general synthesis of single atom catalysts with controllable atomic and mesoporous structures. Nature Synth. 1, 658-667 (2022).
- Jung, E., Shin, H., Hooch Antink, W., Sung, Y.-E. & Hyeon, T. Recent advances in electrochemical oxygen reduction to
: catalyst and cell design. ACS Energy Lett. 5, 1881-1892 (2020). - Wen, Y. et al. Electrochemical reactors for continuous decentralized
production. Angew. Chem. Int. Ed. 134, e202205972 (2022). - Li, Y. et al. Single-atom iron catalyst with biomimetic active center to accelerate proton spillover for medical-level electrosynthesis of
disinfectant. Angew. Chem. Int. Ed. 62, e202306491 (2023). - Wei, X. et al. Synergistically enhanced single-atomic site Fe by
for boosted oxygen reduction in neutral electrolyte. Nano Energy 84, 105840 (2021). - Jiang, K. et al. Highly selective oxygen reduction to hydrogen peroxide on transition metal single atom coordination. Nat. Commun. 10, 3997 (2019).
- Zhang, L., Han, L., Liu, H., Liu, X. & Luo, J. Potential-cycling synthesis of single platinum atoms for efficient hydrogen evolution in neutral media. Angew. Chem. Int. Ed. 129, 13882-13886 (2017).
- Chu, S. & Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 488, 294-303 (2012).
- Gong, F. et al. Modulation of Mo-Fe-C sites over mesoscale dif-fusion-enhanced hollow sub-micro reactors toward boosted electrochemical water oxidation. Adv. Funct. Mater. 32, 2202141 (2022).
- Yang, C. et al. Interfacial
accumulation affects microenvironment in carbon-based electrocatalysts for production. ACS Energy Lett. 7, 4398-4407 (2022). - Melchionna, M., Fornasiero, P. & Prato, M. The rise of hydrogen peroxide as the main product by metal-free catalysis in oxygen reductions. Adv. Mater. 31, 1802920 (2019).
- Dong, K. et al. Honeycomb carbon nanofibers: a superhydrophilic
-entrapping electrocatalyst enables ultrahigh mass activity for the two-electron oxygen reduction reaction. Angew. Chem. Int. Ed. 60, 10583-10587 (2021). - Wei, J. et al. Probing the oxygen reduction reaction intermediates and dynamic active site structures of molecular and pyrolyzed Fe-N-C electrocatalysts by in situ Raman spectroscopy. ACS Catal. 12, 7811-7820 (2022).
- Tse, E. C. et al. Proton transfer dynamics control the mechanism of
reduction by a non-precious metal electrocatalyst. Nat. Mater. 15, 754-759 (2016).
Acknowledgements
Author contributions
Competing interests
Additional information
supplementary material available at
https://doi.org/10.1038/s41467-024-45243-w.
http://www.nature.com/reprints
© The Author(s) 2024
Shenzhen Key Laboratory of Energy Electrocatalytic Materials, Guangdong Research Center for Interfacial Engineering of Functional Materials, College of Materials Science and Engineering, Shenzhen University, Shenzhen, China. College of Physics and Optoelectronic Engineering, Shenzhen University, Shenzhen, China. College of Chemistry and Environmental Engineering, Shenzhen University, Shenzhen, China. State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, China. Global Institute of Future Technology, Shanghai Jiaotong University, Shanghai, China. e-mail: lingyan.jing@foxmail.com; yangjl18@szu.edu.cn
