DOI: https://doi.org/10.1038/s41467-024-46513-3
PMID: https://pubmed.ncbi.nlm.nih.gov/38461315
تاريخ النشر: 2024-03-09
محفز Ce-CuZn مع مواقع نشطة وفيرة من Cu/Zn-OV-Ce لهيدروجين CO2 إلى الميثانول
تم القبول: 29 فبراير 2024
نُشر على الإنترنت: 09 مارس 2024
(أ) التحقق من التحديثات
الملخص
تنفيذ
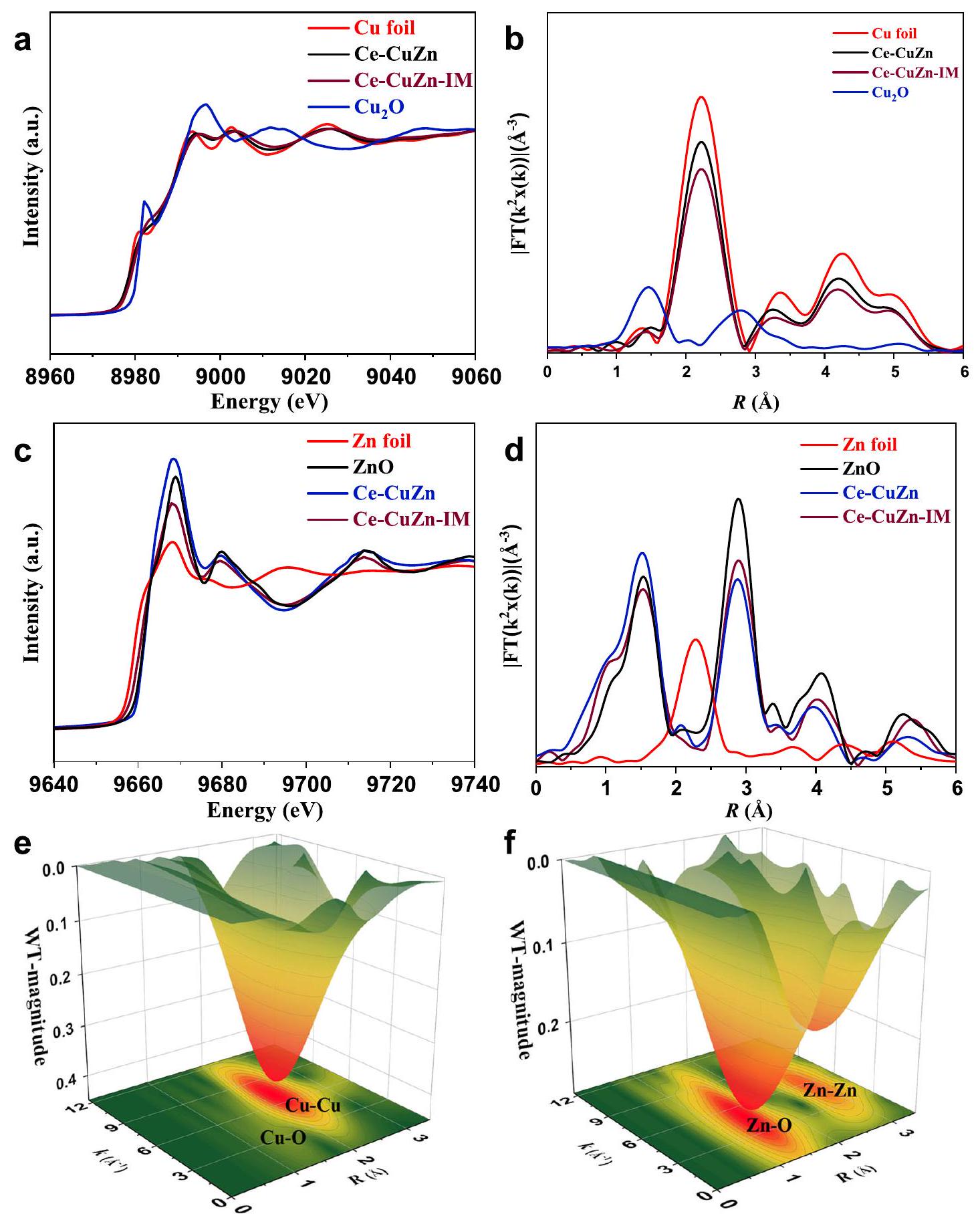
الاتصال بين مراحل الزنك والنحاس
النتائج
الطريق الاصطناعي
الخصائص الشكلية والنسيجية للعوامل المساعدة
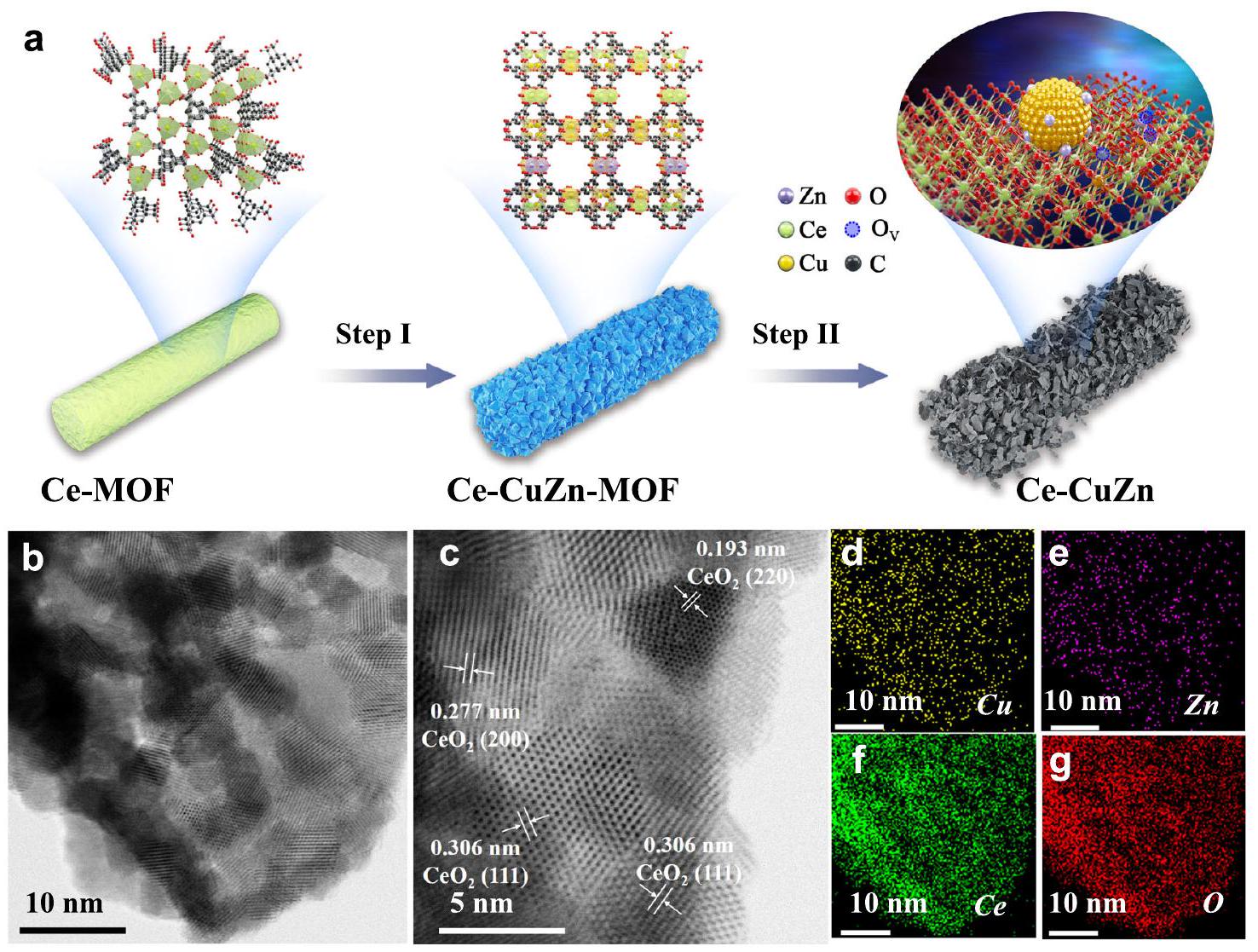
صور المجهر الإلكتروني النافذ (TEM) والمجهر الإلكتروني الماسح (SEM) لـ
على وجه الخصوص، التكتل الجزئي لـ
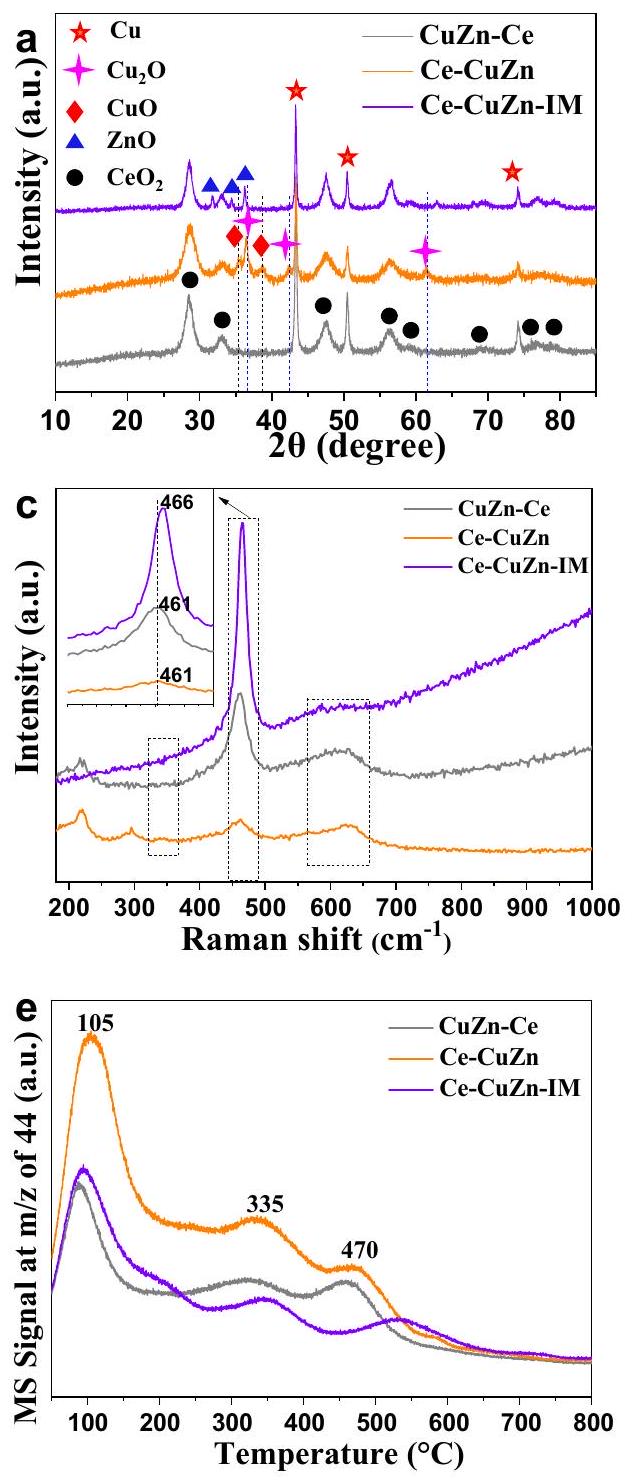
تطور الطور البلوري وخصائص السطح
| العوامل المساعدة | كو
|
|
|
|
حجم المسام (نانومتر) | حجم المسام
|
|
د
|
|
| CuZn | 80.65 | ٣.٥٥ | – | 2.9 | ٢٦.٩ | 0.01 | 3.7 | 0.7 | – |
| CuZn-Ce | ٥٢.٣٣ | 0.03 | ٢٨.٧١ | ٤١.٥ | ٦.٧ | 0.07 | 31.1 | 9.2 | 6.0 |
| سي-نحاس-زنك | 52.82 | 1.12 | 31.74 | ٤٠.٢ | 12.1 | 0.12 | ٢٣.١ | ٦.٧ | 18.1 |
| سي-كوبالت-زنك-إيم | ٢٢.٣٦ | 11.47 | ٤٨.٨٠ | 41.4 | 7.2 | 0.08 | 22.0 | 15.2 | 2.9 |

يُنسب أيضًا إلى
واصلت تشغيل اختبار XRD في الموقع تحت
(الشكل التوضيحي 10). وهذا يشير إلى أن أنواع النحاس كانت مختلفة في عينات سلسلة CuZnCe. بالإضافة إلى ذلك،
الشبكة. لذلك، فإن فراغات الأكسجين تظهر عند 531.2 إلكترون فولت في
النشاط التحفيزي والاستقرار
قدمت أداءً تحفيزيًا ضعيفًا. لذلك، من الضروري إعداد النظام الثلاثي. بالنسبة لترتيب تقديم الـ
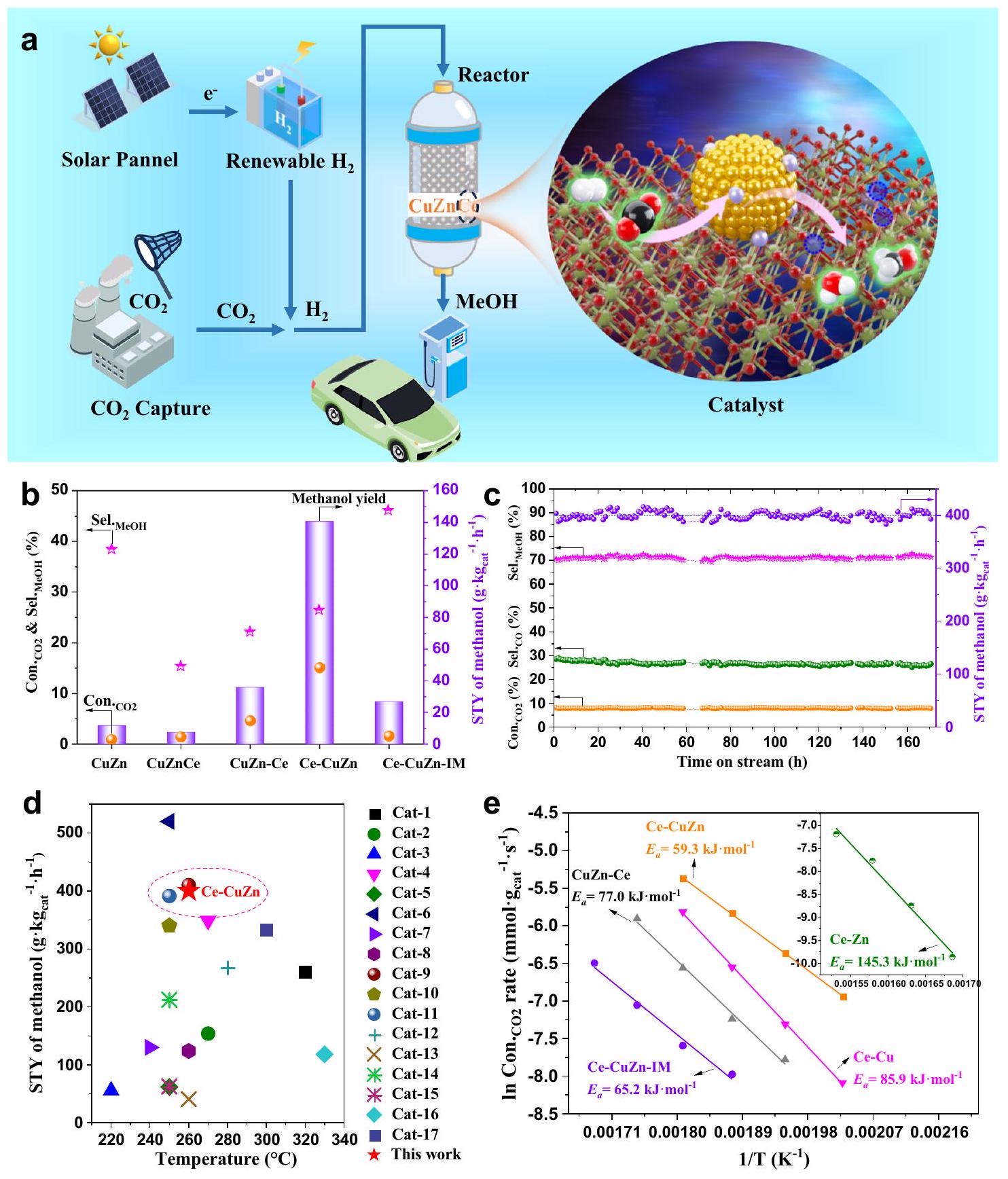
أولاً، الـ
إجراءات التحضير تتكون من ثلاث خطوات رئيسية. وقد تم إظهار أن
المحسّن
يظهر نمط STY من الميثانول قابل للمقارنة تحت ظروف تفاعل مماثلة (الشكل 4d والجدول التكميلي 6).
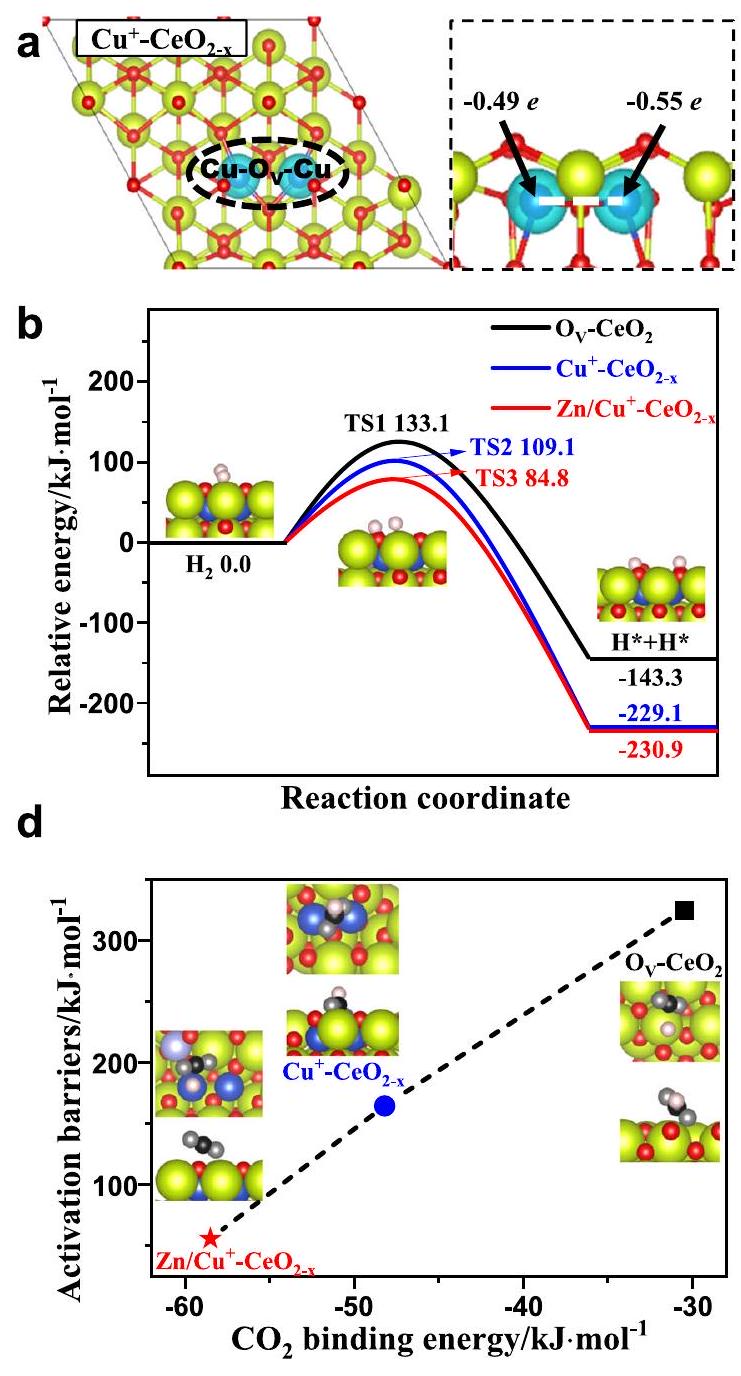
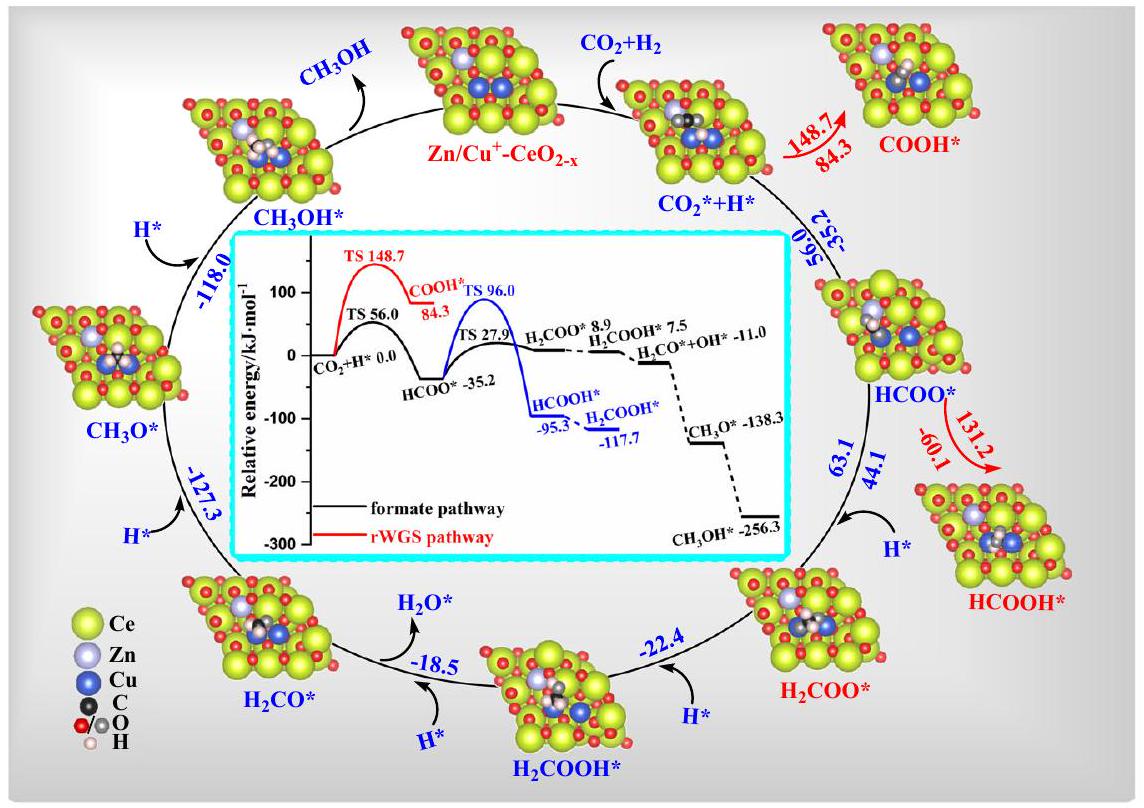
آلية التفاعل وحسابات DFT
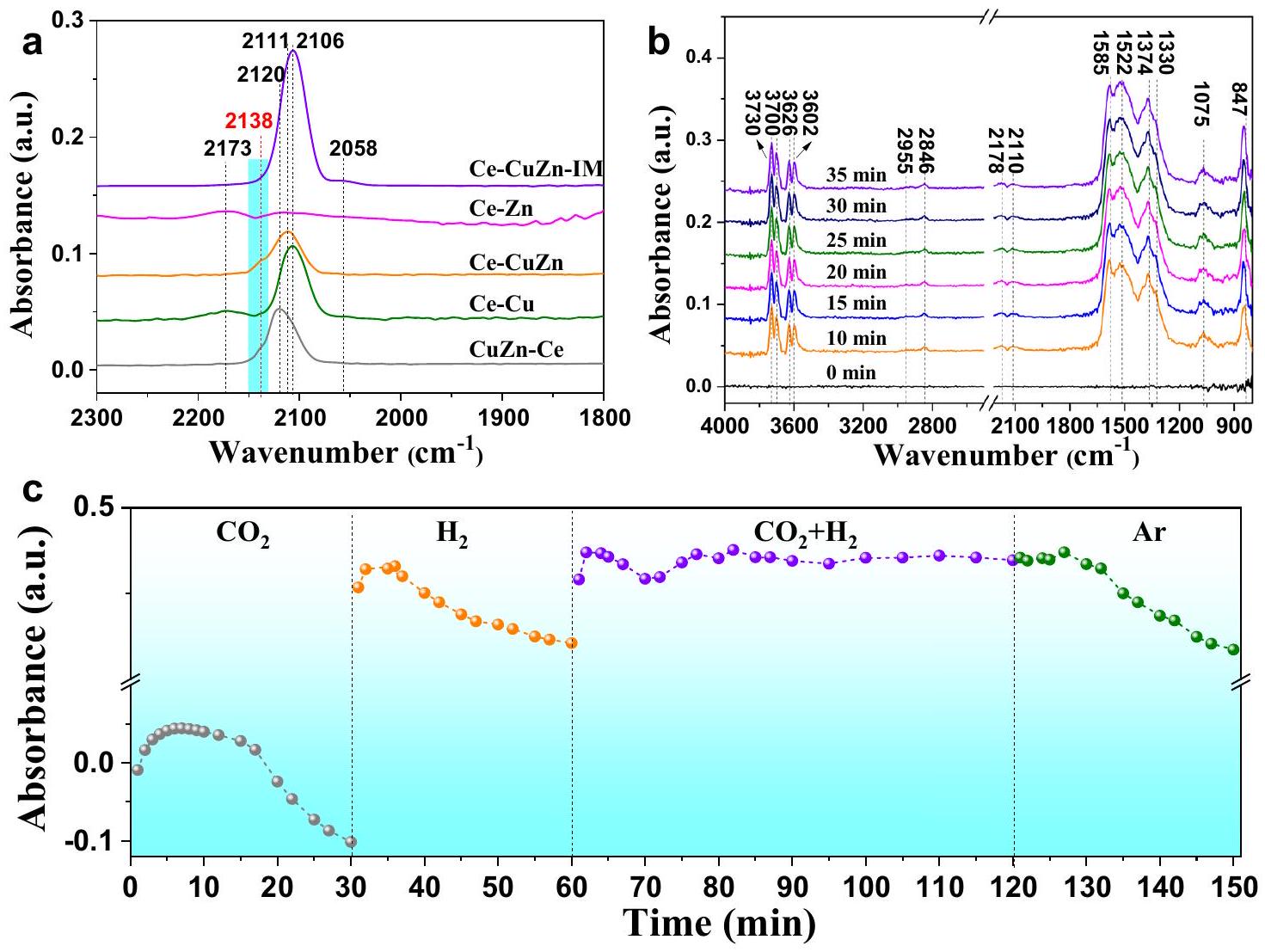
تجارب على
سطح


امتصاص
تطعيم النحاس
نقاش
المحفز الناتج عن استبدال النحاس والزنك على المستوى الذري في سلف Ce-MOF أنتج العديد من الأنشطة الفعالة
طرق
المواد الكيميائية
تحضير المحفز
توصيف المحفز
حسابات DFT
تقييم المحفز
توفر البيانات
References
- Ye, R. P. et al.
hydrogenation to high-value products via heterogeneous catalysis. Nat. Commun. 10, 5698 (2019). - Yang, M. et al. Probing the nature of zinc in copper-zinc-zirconium catalysts by operando spectroscopies for
hydrogenation to methanol. Angew. Chem. Int. Ed. 62, e202216803 (2023). - Pinheiro Araujo, T. et al. Flame-made ternary
catalyst with enhanced oxygen vacancy generation for hydrogenation to methanol. Nat. Commun. 13, 5610 (2022). - Monai, M. et al. Restructuring of titanium oxide overlayers over nickel nanoparticles during catalysis. Science 380, 644-651 (2023).
- Kumar, A., Daw, P. & Milstein, D. Homogeneous catalysis for sustainable energy: Hydrogen and methanol economies, fuels from biomass, and related topics. Chem. Rev. 122, 385-441 (2021).
- Zhong, J. et al. State of the art and perspectives in heterogeneous catalysis of
hydrogenation to methanol. Chem. Soc. Rev. 49, 1385-1413 (2020). - Tian, P., Wei, Y., Ye, M. & Liu, Z. Methanol to olefins (MTO): From fundamentals to commercialization. ACS Catal. 5, 1922-1938 (2015).
- Jiang, X., Nie, X., Guo, X., Song, C. & Chen, J. G. Recent Advances in Carbon Dioxide Hydrogenation to Methanol via Heterogeneous Catalysis. Chem. Rev. 120, 7984-8034 (2020).
- Kordus, D. et al. Shape-dependent
hydrogenation to methanol over nanocubes supported on ZnO . J. Am. Chem. Soc. 145, 3016-3030 (2023). - Shao, Y., Kosari, M., Xi, S. & Zeng, H. C. Single solid precursorderived three-dimensional nanowire networks of CuZn-silicate for
hydrogenation to methanol. ACS Catal. 12, 5750-5765 (2022). - Wang, Y. et al. Strong evidence of the role of
in affecting methanol selectivity from hydrogenation over . Chem 6, 419-430 (2020). - Wang, J. et al. A highly selective and stable
solid solution catalyst for hydrogenation to methanol. Sci. Adv. 3, e1701290 (2017). - Gao, Y. et al. Recent advances in intensified ethylene production-a review. ACS Catal. 9, 8592-8621 (2019).
- Prašnikar, A., Pavlišič, A., Ruiz-Zepeda, F., Kovač, J. & Likozar, B. Mechanisms of copper-based catalyst deactivation during
reduction to methanol. Ind. Eng. Chem. Res. 58, 13021-13029 (2019). - Liang, B. et al. Investigation on deactivation of
catalyst for hydrogenation to methanol. Ind. Eng. Chem. Res. 58, 9030-9037 (2019). - Graciani, J. et al. Highly active copper-ceria and copper-ceria-titania catalysts for methanol synthesis from
. Science 345, 546-550 (2014). - Rasteiro, L. F. et al. Insights into the alloy-support synergistic effects for the
hydrogenation towards methanol on oxide-supported catalysts: An experimental and DFT study. Appl. Catal. B: Environ. 302, 120842 (2022). - Divins, N. J. et al. Operando high-pressure investigation of sizecontrolled CuZn catalysts for the methanol synthesis reaction. Nat. Commun. 12, 1435 (2021).
- Beck, A. et al. Following the structure of copper-zinc-alumina across the pressure gap in carbon dioxide hydrogenation. Nat. Catal. 4, 488-497 (2021).
- Li, M. M. J. et al.
hydrogenation to methanol over catalysts derived from single cationic layer CuZnGa LDH precursors. ACS Catal. 8, 4390-4401 (2018). - Zhu, J. et al. Flame synthesis of
catalysts: Synergistic metal-dupport interactions promote selectivity in hydrogenation. ACS Catal. 11, 4880-4892 (2021). - Hou, X. X. et al. Improved methanol synthesis from
hydrogenation over CuZnAlZr catalysts with precursor pre-activation by formaldehyde. J. Catal. 379, 147-153 (2019). - Kattel, S., Ramírez, P. J., Chen, J. G., Rodriguez, J. A. & Liu, P. Active sites for
hydrogenation to methanol on catalysts. Science 355, 1296-1299 (2017). - Stangeland, K., Li, H. & Yu, Z.
hydrogenation to methanol: the structure-activity relationships of different catalyst systems. Energ. Ecol. Environ. 5, 272-285 (2020). - Kosari, M. et al. Revamping
spheres by core-shell porosity endowment to construct a mazelike nanoreactor for enhanced catalysis in hydrogenation to methanol. Adv. Funct. Mater. 31, 2102896 (2021). - Behrens, M. et al. The active site of methanol synthesis over
industrial catalysts. Science 336, 893-897 (2012). - Amann, P. et al. The state of zinc in methanol synthesis over a
model catalyst. Science 376, 603-608 (2022). - Zabilskiy, M. et al. The unique interplay between copper and zinc during catalytic carbon dioxide hydrogenation to methanol. Nat. Commun. 11, 2409 (2020).
- Xia, W., Mahmood, A., Zou, R. & Xu, Q. Metal-organic frameworks and their derived nanostructures for electrochemical energy storage and conversion. Energy Environ. Sci. 8, 1837-1866 (2015).
- Ye, R. P. et al. Synthesis of robust MOF-derived
catalyst with low copper loading via sol-gel method for the dimethyl oxalate hydrogenation reaction. ACS Catal. 8, 3382-3394 (2018). - Zhang, B. et al. Designing MOF nanoarchitectures for electrochemical water splitting. Adv. Mater. 33, e2006042 (2021).
- Bai, X. J., Zhai, X., Zhang, L. Y., Fu, Y. & Wei, Q. Site-directed reduction engineering within bimetal-organic frameworks for efficient size-selective catalysis. Matter 4, 2919-2935 (2021).
- Wang, F. et al. A Photoactivated
Catalyst with Active Species Designed through MOF Crystal Engineering. Angew. Chem. Int. Ed. 59, 8203-8209 (2020). - Yang, B., Deng, W., Guo, L. & Ishihara, T. Copper-ceria solid solution with improved catalytic activity for hydrogenation of
to . Chin. J. Catal. 41, 1348-1359 (2020). - Liu, C., Shi, J. W., Gao, C. & Niu, C. M. Manganese oxide-based catalysts for low-temperature selective catalytic reduction of
with : A review. Appl. Catal. A: Gen. 522, 54-69 (2016). - Niu, J. et al. Process and mechanism of toluene oxidation using
sepiolite prepared by the co-precipitation method. J. Hazard. Mater. 357, 332-340 (2018). - Yu, J. et al. Sulfur poisoning resistant mesoporous Mn-base catalyst for low-temperature SCR of NO with
. Appl. Catal. B Environ. 95, 160-168 (2010). - Alvarez, A. et al. Challenges in the greener production of formates/ formic acid, methanol, and DME by heterogeneously catalyzed
hydrogenation processes. Chem. Rev. 117, 9804-9838 (2017). - Zhang, Y. et al. Highly efficient
-hollow nanospheres catalyst for the reverse water-gas shift reaction: Investigation on the role of oxygen vacancies through in situ UV-Raman and DRIFTS. Appl. Surf. Sci. 516, 146035 (2020). - Zhu, J. et al. Mechanism and nature of active sites for methanol synthesis from
on . ACS Catal. 10, 11532-11544 (2020). - Liu, S. et al. Producing ultrastable Ni-ZrO2 nanoshell catalysts for dry reforming of methane by flame synthesis and Ni exsolution. Chem Catal. 2, 2262-2274 (2022).
- Cheng, X. et al. NO reduction by CO over copper catalyst supported on mixed
and : Catalyst design and activity test. Appl. Catal. B: Environ. 239, 485-501 (2018). - Jiang, J., Yang, H., Jiang, H., Hu, Y. & Li, C. Boosting catalytic activity of Cu-Ce solid solution catalysts by flame spray pyrolysis with high
concentration and oxygen vacancies. Chem. Eng. J. 471, 144439 (2023). - Chen, J. et al. Characterization and catalytic performance of
and catalysts for NO reduction by CO . Appl. Catal. A: Gen. 363, 208-215 (2009). - Chen, A. et al. Structure of the catalytically active copper-ceria interfacial perimeter. Nat. Catal. 2, 334-341 (2019).
- Mosrati, J. et al. Tiny species with big impact: High activity of Cu single atoms on
deciphered by operando spectroscopy. ACS Catal. 11, 10933-10949 (2021). - Abdel-Mageed, A. M. et al. Highly active and stable single-atom Cu catalysts supported by a metal-organic framework. J. Am. Chem. Soc. 141, 5201-5210 (2019).
- Szanyi, J., Kwak, J. H., Zhu, H. & Peden, C. H. Characterization of Cu-SSZ-13
SCR catalysts: an in situ FTIR study. Phys. Chem. Chem. Phys. 15, 2368-2380 (2013). - Guo, Y. et al. Low-temperature
methanation over -supported Ru single atoms, nanoclusters, and nanoparticles competitively tuned by strong metal-support interactions and H -spillover effect. ACS Catal. 8, 6203-6215 (2018). - Chen, S., Zou, H., Liu, Z. & Lin, W. DRIFTS study of different gas adsorption for CO selective oxidation on
catalysts. Appl. Surf. Sci. 255, 6963-6967 (2009). - Polster, C. S., Nair, H. & Baertsch, C. D. Study of active sites and mechanism responsible for highly selective CO oxidation in
rich atmospheres on a mixed Cu and Ce oxide catalyst. J. Catal. 266, 308-319 (2009). - Wang, W., Qu, Z., Song, L. & Fu, Q.
hydrogenation to methanol over and catalysts: Tuning methanol selectivity via metal-support interaction. J. Energy Chem. 40, 22-30 (2020). - Ye, R. P. et al. High-performance of nanostructured Ni/CeO2 catalyst on
methanation. Appl. Catal. B: Environ. 268, 118474 (2020). - Liu, X. et al. In situ spectroscopic characterization and theoretical calculations identify partially reduced
interfaces for methanol synthesis from . Angew. Chem. Int. Ed. 134, e202202330 (2022). - Kumari, N., Haider, M. A., Agarwal, M., Sinha, N. & Basu, S. Role of reduced
surface for reduction to CO methanol. J. Phys. Chem. C. 120, 16626-16635 (2016). - Chen, J. et al. The synergetic mechanism between copper species and ceria in NO abatement over
catalysts. Appl. Catal. A: Gen. 377, 121-127 (2010). - Wang, Z. Q. et al. High-performance and long-lived
nanocatalyst for hydrogenation. ACS Catal. 5, 4255-4259 (2015). - Yang, H. Y. et al. Core-shell structured
and catalysts for methanol synthesis from hydrogenation. Catal. Commun. 84, 56-60 (2016). - Jiang, Y. et al. Slurry methanol synthesis from
hydrogenation over micro-spherical support catalysts. J. Util. 26, 642-651 (2018). - Toyir, J., de la Piscina, P. R., Fierro, J. L. G. & Homs, N. Highly effective conversion of
to methanol over supported and promoted copper-based catalysts: influence of support and promoter. Appl. Catal. B: Environ. 29, 207-215 (2001). - Liu, X. M., Lu, G. Q. & Yan, Z. F. Nanocrystalline zirconia as catalyst support in methanol synthesis. Appl. Catal. A: Gen. 279, 241-245 (2005).
- Gao, P. et al. Influence of modifier (
and Y ) on the performance of catalysts via hydrotalcite-like precursors for hydrogenation to methanol. Appl. Catal. A: Gen. 468, 442-452 (2013). - Lei, H., Hou, Z. & Xie, J. Hydrogenation of
to over catalysts prepared via a solvent-free routine. Fuel 164, 191-198 (2016). - Tan, Q., Shi, Z. & Wu, D.
hydrogenation to methanol over a highly active -nanotube natalyst. Ind. Eng. Chem. Res. 57, 10148-10158 (2018). - Hu, J. et al. Sulfur vacancy-rich
as a catalyst for the hydrogenation of to methanol. Nat. Catal. 4, 242-250 (2021). - An, B. et al. Confinement of ultrasmall
nanoparticles in metal-organic frameworks for selective methanol synthesis from
catalytic hydrogenation of. J. Am. Chem. Soc. 139, 3834-3840 (2017). - Choi, E. J., Lee, Y. H., Lee, D.-W., Moon, D.-J. & Lee, K.-Y. Hydrogenation of
to methanol over catalysts. Mol. Catal. 434, 146-153 (2017). - Zhang, C., Liao, P., Wang, H., Sun, J. & Gao, P. Preparation of novel bimetallic CuZn-BTC coordination polymer nanorod for methanol synthesis from
hydrogenation. Mater. Chem. Phys. 215, 211-220 (2018). - Sun, K. H. et al. Hydrogenation of
to methanol over . Catal. J. Util. 12, 1-6 (2015). - Dang, S. et al. Rationally designed indium oxide catalysts for
hydrogenation to methanol with high activity and selectivity. Sci. Adv. 6, eaaz2060 (2020).
شكر وتقدير
مساهمات المؤلفين
تفسير بيانات EXAFS وTEM وXPS. R.Y. وL.M. وJ.M. كتبوا المسودة وقام المؤلفون الآخرون بمراجعة المخطوطة.
المصالح المتنافسة
معلومات إضافية
المواد التكميلية المتاحة على
https://doi.org/10.1038/s41467-024-46513-3.
يجب توجيه المراسلات وطلبات المواد إلى Riguang Zhang وMelis Seher Duyar وZheng Jiang أو Jian Liu.
http://www.nature.com/reprints
ملاحظة الناشر تظل Springer Nature محايدة فيما يتعلق بالمطالبات القضائية في الخرائط المنشورة والانتماءات المؤسسية.
© المؤلفون 2024
المختبر الرئيسي لمقاطعة جيانغشي لتحفيز البيئة والطاقة، معهد الكيمياء التطبيقية، كلية الكيمياء والهندسة الكيميائية، جامعة نانتشانغ، نانتشانغ 330031، جمهورية الصين الشعبية. المختبر الرئيسي للدولة للاستخدام النظيف والفعال للفحم، كلية الهندسة الكيميائية والتكنولوجيا، جامعة تاييوان للتكنولوجيا، تاييوان 030024 شانشي، جمهورية الصين الشعبية. معهد شنغهاي للفيزياء التطبيقية، الأكاديمية الصينية للعلوم، شنغهاي 201204، جمهورية الصين الشعبية. مركز علوم المواد والهندسة البصرية، جامعة الأكاديمية الصينية للعلوم، بكين 100049، جمهورية الصين الشعبية. المختبر الرئيسي للدولة للتحفيز، معهد داليان للفيزياء الكيميائية، الأكاديمية الصينية للعلوم، داليان 116023 لياونينغ، جمهورية الصين الشعبية. مركز SUNCAT لعلوم الواجهة والتحفيز، مختبر SLAC الوطني للتسريع، 2575 طريق ساندهيل، مينلو بارك، كاليفورنيا 94025، الولايات المتحدة الأمريكية. كلية الكيمياء والهندسة الكيميائية، جامعة منغوليا الداخلية، هولونغو 010021، جمهورية الصين الشعبية. مركز DICP-Surrey المشترك للمواد المستقبلية، ومعهد التكنولوجيا المتقدمة، جامعة ساري، غيلفورد، ساري GU2 7XH، المملكة المتحدة. كلية الكيمياء والهندسة الكيميائية، جامعة ساري، غيلفورد، ساري GU2 7XH، المملكة المتحدة. المختبر الوطني للأشعة السينية، جامعة العلوم والتكنولوجيا الصينية، هيفي 230029، جمهورية الصين الشعبية.
هؤلاء المؤلفون ساهموا بالتساوي: Runping Ye وLixuan Ma وJianing Mao. البريد الإلكتروني: zhangriguang@tyut.edu.cn; m.duyar@surrey.ac.uk; jiangz@ustc.edu.cn; jian.liu@surrey.ac.uk
DOI: https://doi.org/10.1038/s41467-024-46513-3
PMID: https://pubmed.ncbi.nlm.nih.gov/38461315
Publication Date: 2024-03-09
A
Accepted: 29 February 2024
Published online: 09 March 2024
(A) Check for updates
Abstract
implementation of
contact between the zinc and copper phases
Results
Synthetic route
Morphological and textural properties of the catalysts

microscopy (TEM) and scanning electron microscopy (SEM) images of
particular, the partial agglomeration of
Evolution of crystalline phase and surface properties
| Catalysts | Cu
|
|
|
|
Pore size (nm) | Pore volume (
|
|
D
|
|
| CuZn | 80.65 | 3.55 | – | 2.9 | 26.9 | 0.01 | 3.7 | 0.7 | – |
| CuZn-Ce | 52.33 | 0.03 | 28.71 | 41.5 | 6.7 | 0.07 | 31.1 | 9.2 | 6.0 |
| Ce-CuZn | 52.82 | 1.12 | 31.74 | 40.2 | 12.1 | 0.12 | 23.1 | 6.7 | 18.1 |
| Ce-CuZn-IM | 22.36 | 11.47 | 48.80 | 41.4 | 7.2 | 0.08 | 22.0 | 15.2 | 2.9 |


is also ascribed to
further operated the in-situ XRD test under the
(Supplementary Fig. 10). This suggests that the copper species were different over CuZnCe series samples. In addition, the
lattice. Therefore, the oxygen vacancies results at 531.2 eV in
Catalytic activity and stability

presented poor catalytic performance. Thus, it is necessary to prepare the ternary system. For the order of introducing the

first, the
preparation procedures with three main steps. It was shown that the
the optimized
exhibits comparable STY of methanol under similar reaction conditions (Fig. 4d and Supplementary Table 6).
Reaction mechanism and DFT calculations

experiments on
the surface of



adsorption
doping of Cu and

Discussion
catalyst from atomic-level substitution of Cu and Zn into Ce-MOF precursor produced many active
Methods
Chemicals
Catalyst preparation
Catalyst characterization
DFT calculations
Catalyst evaluation
Data availability
References
- Ye, R. P. et al.
hydrogenation to high-value products via heterogeneous catalysis. Nat. Commun. 10, 5698 (2019). - Yang, M. et al. Probing the nature of zinc in copper-zinc-zirconium catalysts by operando spectroscopies for
hydrogenation to methanol. Angew. Chem. Int. Ed. 62, e202216803 (2023). - Pinheiro Araujo, T. et al. Flame-made ternary
catalyst with enhanced oxygen vacancy generation for hydrogenation to methanol. Nat. Commun. 13, 5610 (2022). - Monai, M. et al. Restructuring of titanium oxide overlayers over nickel nanoparticles during catalysis. Science 380, 644-651 (2023).
- Kumar, A., Daw, P. & Milstein, D. Homogeneous catalysis for sustainable energy: Hydrogen and methanol economies, fuels from biomass, and related topics. Chem. Rev. 122, 385-441 (2021).
- Zhong, J. et al. State of the art and perspectives in heterogeneous catalysis of
hydrogenation to methanol. Chem. Soc. Rev. 49, 1385-1413 (2020). - Tian, P., Wei, Y., Ye, M. & Liu, Z. Methanol to olefins (MTO): From fundamentals to commercialization. ACS Catal. 5, 1922-1938 (2015).
- Jiang, X., Nie, X., Guo, X., Song, C. & Chen, J. G. Recent Advances in Carbon Dioxide Hydrogenation to Methanol via Heterogeneous Catalysis. Chem. Rev. 120, 7984-8034 (2020).
- Kordus, D. et al. Shape-dependent
hydrogenation to methanol over nanocubes supported on ZnO . J. Am. Chem. Soc. 145, 3016-3030 (2023). - Shao, Y., Kosari, M., Xi, S. & Zeng, H. C. Single solid precursorderived three-dimensional nanowire networks of CuZn-silicate for
hydrogenation to methanol. ACS Catal. 12, 5750-5765 (2022). - Wang, Y. et al. Strong evidence of the role of
in affecting methanol selectivity from hydrogenation over . Chem 6, 419-430 (2020). - Wang, J. et al. A highly selective and stable
solid solution catalyst for hydrogenation to methanol. Sci. Adv. 3, e1701290 (2017). - Gao, Y. et al. Recent advances in intensified ethylene production-a review. ACS Catal. 9, 8592-8621 (2019).
- Prašnikar, A., Pavlišič, A., Ruiz-Zepeda, F., Kovač, J. & Likozar, B. Mechanisms of copper-based catalyst deactivation during
reduction to methanol. Ind. Eng. Chem. Res. 58, 13021-13029 (2019). - Liang, B. et al. Investigation on deactivation of
catalyst for hydrogenation to methanol. Ind. Eng. Chem. Res. 58, 9030-9037 (2019). - Graciani, J. et al. Highly active copper-ceria and copper-ceria-titania catalysts for methanol synthesis from
. Science 345, 546-550 (2014). - Rasteiro, L. F. et al. Insights into the alloy-support synergistic effects for the
hydrogenation towards methanol on oxide-supported catalysts: An experimental and DFT study. Appl. Catal. B: Environ. 302, 120842 (2022). - Divins, N. J. et al. Operando high-pressure investigation of sizecontrolled CuZn catalysts for the methanol synthesis reaction. Nat. Commun. 12, 1435 (2021).
- Beck, A. et al. Following the structure of copper-zinc-alumina across the pressure gap in carbon dioxide hydrogenation. Nat. Catal. 4, 488-497 (2021).
- Li, M. M. J. et al.
hydrogenation to methanol over catalysts derived from single cationic layer CuZnGa LDH precursors. ACS Catal. 8, 4390-4401 (2018). - Zhu, J. et al. Flame synthesis of
catalysts: Synergistic metal-dupport interactions promote selectivity in hydrogenation. ACS Catal. 11, 4880-4892 (2021). - Hou, X. X. et al. Improved methanol synthesis from
hydrogenation over CuZnAlZr catalysts with precursor pre-activation by formaldehyde. J. Catal. 379, 147-153 (2019). - Kattel, S., Ramírez, P. J., Chen, J. G., Rodriguez, J. A. & Liu, P. Active sites for
hydrogenation to methanol on catalysts. Science 355, 1296-1299 (2017). - Stangeland, K., Li, H. & Yu, Z.
hydrogenation to methanol: the structure-activity relationships of different catalyst systems. Energ. Ecol. Environ. 5, 272-285 (2020). - Kosari, M. et al. Revamping
spheres by core-shell porosity endowment to construct a mazelike nanoreactor for enhanced catalysis in hydrogenation to methanol. Adv. Funct. Mater. 31, 2102896 (2021). - Behrens, M. et al. The active site of methanol synthesis over
industrial catalysts. Science 336, 893-897 (2012). - Amann, P. et al. The state of zinc in methanol synthesis over a
model catalyst. Science 376, 603-608 (2022). - Zabilskiy, M. et al. The unique interplay between copper and zinc during catalytic carbon dioxide hydrogenation to methanol. Nat. Commun. 11, 2409 (2020).
- Xia, W., Mahmood, A., Zou, R. & Xu, Q. Metal-organic frameworks and their derived nanostructures for electrochemical energy storage and conversion. Energy Environ. Sci. 8, 1837-1866 (2015).
- Ye, R. P. et al. Synthesis of robust MOF-derived
catalyst with low copper loading via sol-gel method for the dimethyl oxalate hydrogenation reaction. ACS Catal. 8, 3382-3394 (2018). - Zhang, B. et al. Designing MOF nanoarchitectures for electrochemical water splitting. Adv. Mater. 33, e2006042 (2021).
- Bai, X. J., Zhai, X., Zhang, L. Y., Fu, Y. & Wei, Q. Site-directed reduction engineering within bimetal-organic frameworks for efficient size-selective catalysis. Matter 4, 2919-2935 (2021).
- Wang, F. et al. A Photoactivated
Catalyst with Active Species Designed through MOF Crystal Engineering. Angew. Chem. Int. Ed. 59, 8203-8209 (2020). - Yang, B., Deng, W., Guo, L. & Ishihara, T. Copper-ceria solid solution with improved catalytic activity for hydrogenation of
to . Chin. J. Catal. 41, 1348-1359 (2020). - Liu, C., Shi, J. W., Gao, C. & Niu, C. M. Manganese oxide-based catalysts for low-temperature selective catalytic reduction of
with : A review. Appl. Catal. A: Gen. 522, 54-69 (2016). - Niu, J. et al. Process and mechanism of toluene oxidation using
sepiolite prepared by the co-precipitation method. J. Hazard. Mater. 357, 332-340 (2018). - Yu, J. et al. Sulfur poisoning resistant mesoporous Mn-base catalyst for low-temperature SCR of NO with
. Appl. Catal. B Environ. 95, 160-168 (2010). - Alvarez, A. et al. Challenges in the greener production of formates/ formic acid, methanol, and DME by heterogeneously catalyzed
hydrogenation processes. Chem. Rev. 117, 9804-9838 (2017). - Zhang, Y. et al. Highly efficient
-hollow nanospheres catalyst for the reverse water-gas shift reaction: Investigation on the role of oxygen vacancies through in situ UV-Raman and DRIFTS. Appl. Surf. Sci. 516, 146035 (2020). - Zhu, J. et al. Mechanism and nature of active sites for methanol synthesis from
on . ACS Catal. 10, 11532-11544 (2020). - Liu, S. et al. Producing ultrastable Ni-ZrO2 nanoshell catalysts for dry reforming of methane by flame synthesis and Ni exsolution. Chem Catal. 2, 2262-2274 (2022).
- Cheng, X. et al. NO reduction by CO over copper catalyst supported on mixed
and : Catalyst design and activity test. Appl. Catal. B: Environ. 239, 485-501 (2018). - Jiang, J., Yang, H., Jiang, H., Hu, Y. & Li, C. Boosting catalytic activity of Cu-Ce solid solution catalysts by flame spray pyrolysis with high
concentration and oxygen vacancies. Chem. Eng. J. 471, 144439 (2023). - Chen, J. et al. Characterization and catalytic performance of
and catalysts for NO reduction by CO . Appl. Catal. A: Gen. 363, 208-215 (2009). - Chen, A. et al. Structure of the catalytically active copper-ceria interfacial perimeter. Nat. Catal. 2, 334-341 (2019).
- Mosrati, J. et al. Tiny species with big impact: High activity of Cu single atoms on
deciphered by operando spectroscopy. ACS Catal. 11, 10933-10949 (2021). - Abdel-Mageed, A. M. et al. Highly active and stable single-atom Cu catalysts supported by a metal-organic framework. J. Am. Chem. Soc. 141, 5201-5210 (2019).
- Szanyi, J., Kwak, J. H., Zhu, H. & Peden, C. H. Characterization of Cu-SSZ-13
SCR catalysts: an in situ FTIR study. Phys. Chem. Chem. Phys. 15, 2368-2380 (2013). - Guo, Y. et al. Low-temperature
methanation over -supported Ru single atoms, nanoclusters, and nanoparticles competitively tuned by strong metal-support interactions and H -spillover effect. ACS Catal. 8, 6203-6215 (2018). - Chen, S., Zou, H., Liu, Z. & Lin, W. DRIFTS study of different gas adsorption for CO selective oxidation on
catalysts. Appl. Surf. Sci. 255, 6963-6967 (2009). - Polster, C. S., Nair, H. & Baertsch, C. D. Study of active sites and mechanism responsible for highly selective CO oxidation in
rich atmospheres on a mixed Cu and Ce oxide catalyst. J. Catal. 266, 308-319 (2009). - Wang, W., Qu, Z., Song, L. & Fu, Q.
hydrogenation to methanol over and catalysts: Tuning methanol selectivity via metal-support interaction. J. Energy Chem. 40, 22-30 (2020). - Ye, R. P. et al. High-performance of nanostructured Ni/CeO2 catalyst on
methanation. Appl. Catal. B: Environ. 268, 118474 (2020). - Liu, X. et al. In situ spectroscopic characterization and theoretical calculations identify partially reduced
interfaces for methanol synthesis from . Angew. Chem. Int. Ed. 134, e202202330 (2022). - Kumari, N., Haider, M. A., Agarwal, M., Sinha, N. & Basu, S. Role of reduced
surface for reduction to CO methanol. J. Phys. Chem. C. 120, 16626-16635 (2016). - Chen, J. et al. The synergetic mechanism between copper species and ceria in NO abatement over
catalysts. Appl. Catal. A: Gen. 377, 121-127 (2010). - Wang, Z. Q. et al. High-performance and long-lived
nanocatalyst for hydrogenation. ACS Catal. 5, 4255-4259 (2015). - Yang, H. Y. et al. Core-shell structured
and catalysts for methanol synthesis from hydrogenation. Catal. Commun. 84, 56-60 (2016). - Jiang, Y. et al. Slurry methanol synthesis from
hydrogenation over micro-spherical support catalysts. J. Util. 26, 642-651 (2018). - Toyir, J., de la Piscina, P. R., Fierro, J. L. G. & Homs, N. Highly effective conversion of
to methanol over supported and promoted copper-based catalysts: influence of support and promoter. Appl. Catal. B: Environ. 29, 207-215 (2001). - Liu, X. M., Lu, G. Q. & Yan, Z. F. Nanocrystalline zirconia as catalyst support in methanol synthesis. Appl. Catal. A: Gen. 279, 241-245 (2005).
- Gao, P. et al. Influence of modifier (
and Y ) on the performance of catalysts via hydrotalcite-like precursors for hydrogenation to methanol. Appl. Catal. A: Gen. 468, 442-452 (2013). - Lei, H., Hou, Z. & Xie, J. Hydrogenation of
to over catalysts prepared via a solvent-free routine. Fuel 164, 191-198 (2016). - Tan, Q., Shi, Z. & Wu, D.
hydrogenation to methanol over a highly active -nanotube natalyst. Ind. Eng. Chem. Res. 57, 10148-10158 (2018). - Hu, J. et al. Sulfur vacancy-rich
as a catalyst for the hydrogenation of to methanol. Nat. Catal. 4, 242-250 (2021). - An, B. et al. Confinement of ultrasmall
nanoparticles in metal-organic frameworks for selective methanol synthesis from
catalytic hydrogenation of. J. Am. Chem. Soc. 139, 3834-3840 (2017). - Choi, E. J., Lee, Y. H., Lee, D.-W., Moon, D.-J. & Lee, K.-Y. Hydrogenation of
to methanol over catalysts. Mol. Catal. 434, 146-153 (2017). - Zhang, C., Liao, P., Wang, H., Sun, J. & Gao, P. Preparation of novel bimetallic CuZn-BTC coordination polymer nanorod for methanol synthesis from
hydrogenation. Mater. Chem. Phys. 215, 211-220 (2018). - Sun, K. H. et al. Hydrogenation of
to methanol over . Catal. J. Util. 12, 1-6 (2015). - Dang, S. et al. Rationally designed indium oxide catalysts for
hydrogenation to methanol with high activity and selectivity. Sci. Adv. 6, eaaz2060 (2020).
Acknowledgements
Author contributions
interpret the EXAFS, TEM and XPS data. R.Y., L.M. and J.M. wrote the draft and the other authors revised the manuscript.
Competing interests
Additional information
supplementary material available at
https://doi.org/10.1038/s41467-024-46513-3.
Correspondence and requests for materials should be addressed to Riguang Zhang, Melis Seher Duyar, Zheng Jiang or Jian Liu.
http://www.nature.com/reprints
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
© The Author(s) 2024
Key Laboratory of Jiangxi Province for Environment and Energy Catalysis, Institute of Applied Chemistry, School of Chemistry and Chemical Engineering, Nanchang University, Nanchang 330031, PR China. State Key Laboratory of Clean and Efficient Coal Utilization, College of Chemical Engineering and Technology, Taiyuan University of Technology, Taiyuan 030024 Shanxi, PR China. Shanghai Institute of Applied Physics, Chinese Academy of Sciences, Shanghai 201204, PR China. Center of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences, Beijing 100049, PR China. State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023 Liaoning, PR China. SUNCAT Center for Interface Science and Catalysis, SLAC National Accelerator Laboratory, 2575 Sand Hill Road, Menlo Park, CA 94025, USA. College of Chemistry and Chemical Engineering, Inner Mongolia University, Hohhot 010021, PR China. DICP-Surrey Joint Centre for Future Materials, and Advanced Technology Institute, University of Surrey, Guilford, Surrey GU2 7XH, United Kingdom. School of Chemistry and Chemical Engineering, University of Surrey, Guildford, Surrey GU2 7XH, United Kingdom. National Synchrotron Radiation Laboratory, University of Science and Technology of China, Hefei 230029, PR China.
These authors contributed equally: Runping Ye, Lixuan Ma, Jianing Mao. e-mail: zhangriguang@tyut.edu.cn; m.duyar@surrey.ac.uk; jiangz@ustc.edu.cn; jian.liu@surrey.ac.uk
