DOI: https://doi.org/10.2147/idr.s456403
PMID: https://pubmed.ncbi.nlm.nih.gov/38444772
تاريخ النشر: 2024-03-01
هيدروجيل قائم على الألجينات والكيتوزان يعزز نشاط العامل المضاد للبكتيريا عند التطبيق الموضعي
الملخص
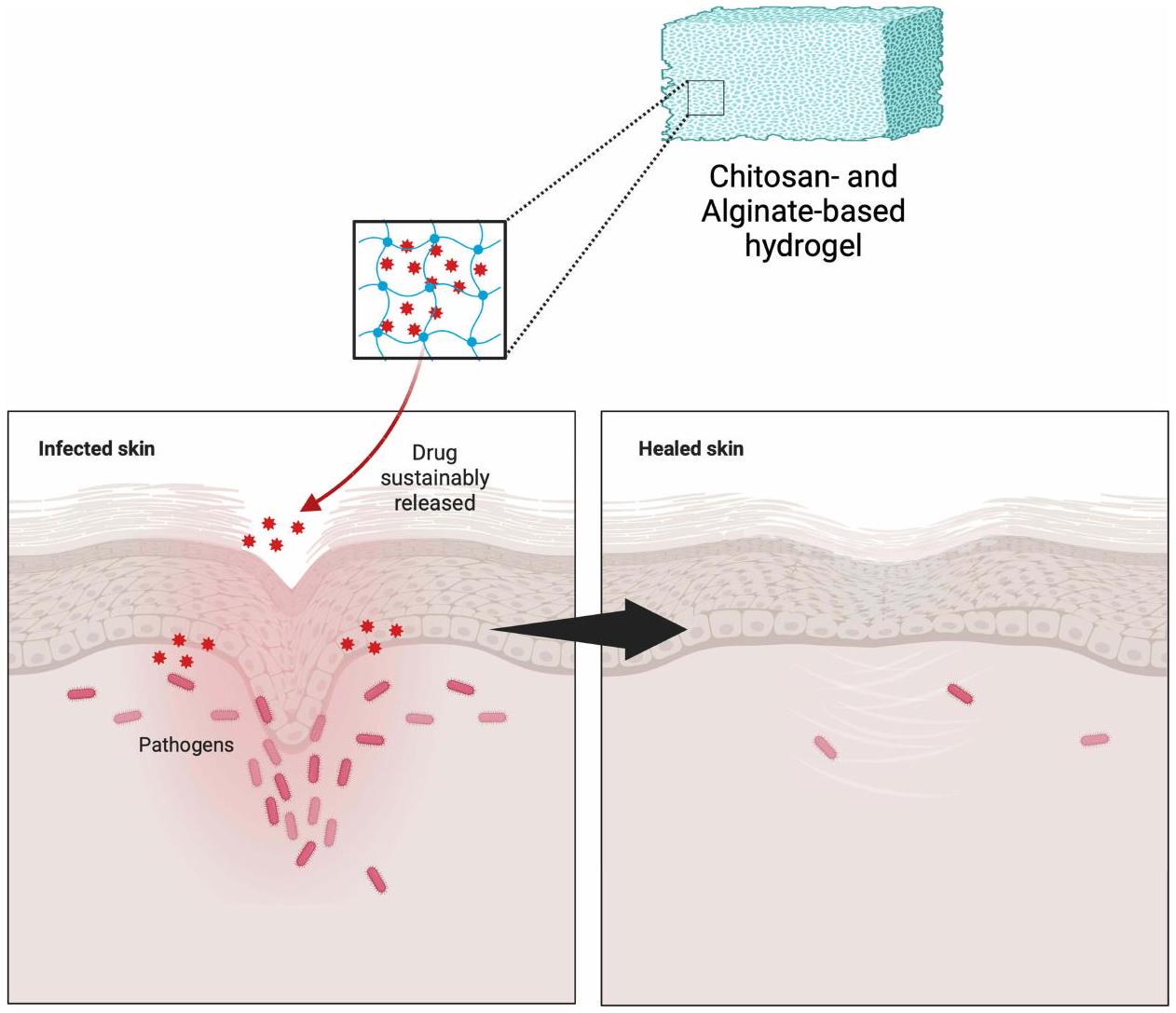
يمكن أن تصبح العدوى الموضعية غير المعالجة مزمنة، مما يسبب مشاكل صحية خطيرة. الالتصاق الجلدي الأمثل أمر حاسم في معالجة مثل هذه العدوى. في هذا السياق، يظهر الكيتوزان والألجينات كمرشحين واعدين للاستخدام كأساس في تطوير الهيدروجيلات الموضعية. الهدف من هذه المراجعة هو فحص الأدبيات حول تركيبات الهيدروجيل الموضعية التي تستخدم الكيتوزان والألجينات كأساس، وخاصة في سياق العوامل المضادة للبكتيريا الموضعية. تتضمن منهجية البحث مراجعة أدبية من خلال فحص المقالات المنشورة في قواعد بيانات مثل PubMed وScopus وScienceDirect وGoogle Scholar. كانت الكلمات الرئيسية المستخدمة خلال البحث هي “الألجينات”، “الكيتوزان”، “الهيدروجيل”، و”المضادات الحيوية”. يعمل الكيتوزان والألجينات كأساس في الهيدروجيلات الموضعية لتوصيل مكونات نشطة متنوعة، وخاصة العوامل المضادة للبكتيريا، كما تشير نتائج البحث. لقد أظهر كلاهما فعالية مضادة للبكتيريا ملحوظة، كما يتضح من انخفاض عدد مستعمرات البكتيريا وزيادة مناطق التثبيط. هذا يدعم بقوة فكرة أن الكيتوزان والألجينات يمكن أن تستخدم معًا لصنع هيدروجيلات موضعية تقتل البكتيريا بشكل فعال. في الختام، تظهر الهيدروجيلات المعتمدة على الكيتوزان والألجينات إمكانات كبيرة في علاج العدوى البكتيرية على سطح الجلد. إن دمج الكيتوزان والألجينات في تركيبات الهيدروجيل يساعد في الاحتفاظ بالعوامل المضادة للبكتيريا، مما يسمح بإطلاقها التدريجي على مدى فترة مثالية. لذلك، فإن الهيدروجيلات المصممة خصيصًا مع الكيتوزان والألجينات لديها القدرة على أن تكون حلاً لمواجهة التحديات في علاج العدوى البكتيرية الموضعية.
مقدمة
لقد أثبتت الأدلة أن استخدام الهيدروجيل له إمكانيات كبيرة في مجال ضمادات الجروح بسبب خصائصه الفيزيائية التي تشبه الأنسجة الحية وخصائصه الممتازة مثل محتوى الماء العالي، ونفاذية الأكسجين، والنعومة.
طريقة
عدوى موضعية
بروبيونيباكتيريوم أكنيس
كوريباكتيريوم
ثم تقوم البكتيريا بحل ألياف الكيراتين في المساحات بين الخلايا وداخل الخلايا، مما ينتج البورفيرينات. العلاج الأول هو الإريثروميسين الموضعي، الكليندامايسين، أو كريم الميكونازول. تشمل بعض الأسماء التجارية الشائعة للكليندامايسين الموضعي Anerocid، Acne Clin، وBenzasil. في الوقت نفسه، تشمل بعض الأسماء التجارية الشائعة لكريم الميكونازول MonistatDerm وMicatin.
ستافيلوكوكوس إpidermidis
ستافيلوكوكوس أوريوس
ستربتوكوكوس بيوجينيس
الهيدروجيل كدواء موضعي واعد
تعريف الهيدروجيل
المواد المساعدة المستخدمة بشكل شائع
طريقة التحضير
تحضير ووزن المواد الخام
الخلط
| مادة مساعدة | الاستخدام |
| وسيلة | الهيدروجيل هو في الأساس مصفوفات قائمة على الماء، والماء هو مكون أساسي يشكل هيكل الهيدروجيل.
|
| عوامل التجلط | تُستخدم عوامل التجلط مثل الكاربومرات، صمغ الجيلان، الأجاروز، أو السليلوز كربوكسي ميثيل الصوديوم (NaCMC) لتوفير الكثافة واللزوجة المناسبة للهيدروجيل.
|
| معدلات الحموضة | تُستخدم مواد مثل هيدروكسيد الصوديوم أو حمض الستريك لضبط درجة حموضة الهيدروجيل لتناسب متطلبات تطبيقه الطبي.
|
| مواد حافظة | تتطلب بعض تركيبات الهيدروجيل مواد حافظة مثل كلوريد البنزالكونيوم أو الفينول لمنع نمو الكائنات الدقيقة.
|
| معدلات القوام | تُستخدم بعض المواد المساعدة مثل الجلايكول البروبيلي أو الإيثانول لتنظيم القوام واللزوجة للهيدروجيل.
|
| معززات التماسك | تُستخدم مواد مساعدة مثل حمض البولي أكريليك لتعزيز تماسك الهيدروجيل.
|
| ملونات وعطور | في بعض الحالات، قد تُضاف ملونات أو عطور لأغراض جمالية أو لامتثال المرضى.
|
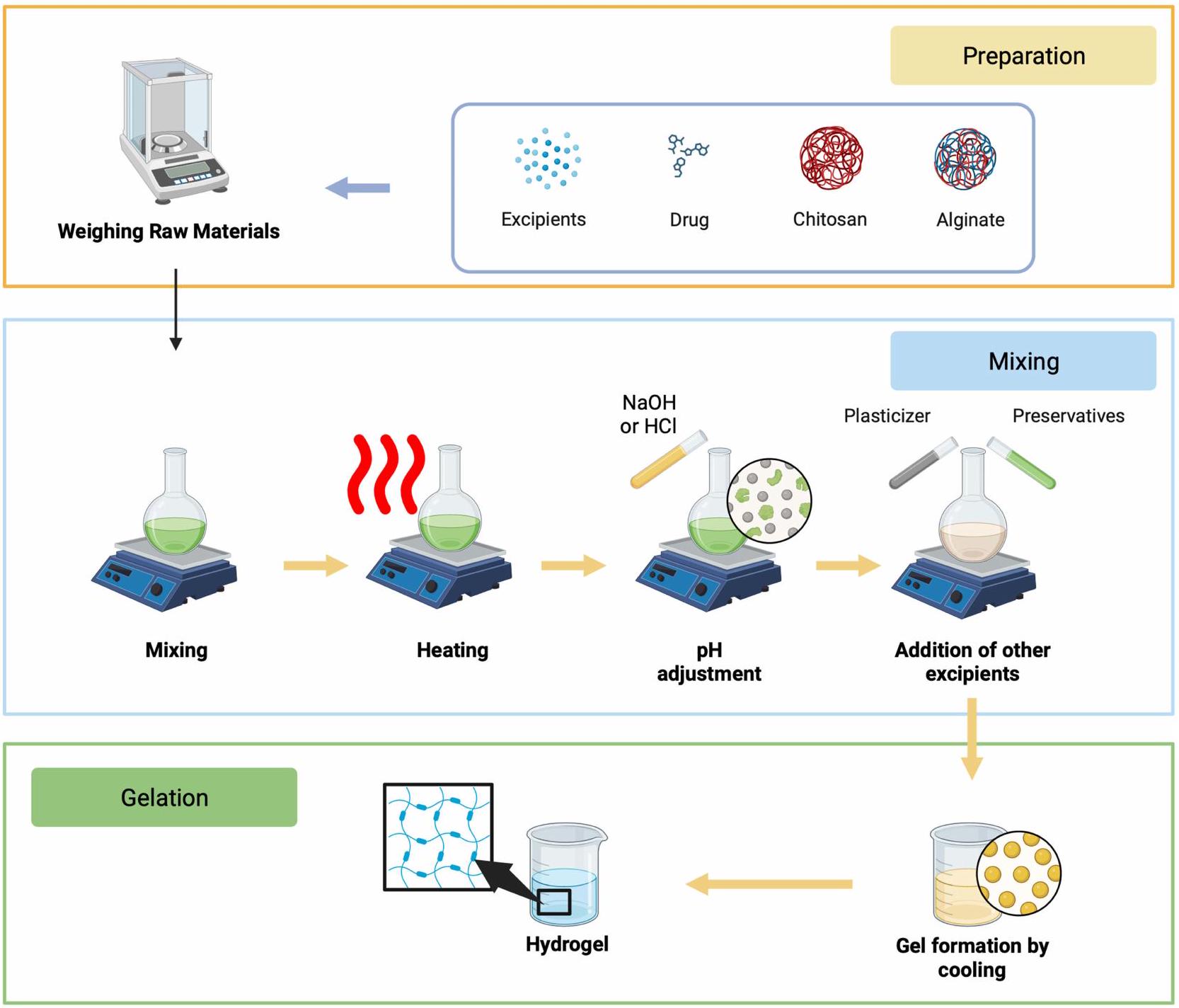
التسخين
ضبط الحموضة
إضافة المواد الحافظة والمواد المساعدة الأخرى
التجلي
الترشيح والتعقيم
التعبئة والتغليف
توصيف شكل جرعة الهيدروجيل
فعالية الكيتوزان والألجينات كبوليمرات طبيعية قابلة للتحلل الحيوي قائمة على الهيدروجيل
الألجينات
كوسائط توصيل للجزيئات التي تستهدف الأنسجة بدقة. يمكن أن يؤدي استخدام الألجينات إلى تغيير الخصائص الفيزيائية والكيميائية للأدوية، مما يعزز فعاليتها وسلامتها في أنظمة توصيل الأدوية.
الكيتوزان
تطبيق هيدروجيل قائم على الألجينات والكيتوزان لعلاج البكتيريا الموضعي
هيدروجيل قائم على الألجينات والكيتوزان
| قاعدة الهيدروجيل | المادة الفعالة | تركيز الدواء | بكتيريا | فعالية مضادة للبكتيريا | مرجع |
| الألجينات-الكيتوزان | بيربرين |
|
المكورات العنقودية الذهبية |
|
[6I] |
| كارفاكرول |
|
المكورات العنقودية الذهبية والإشريكية القولونية |
|
[62] | |
| سيبروفلوكساسين، أموكسيسيلين وفانكومايسين |
|
المكورات العنقودية الذهبية |
|
[63] | |
| الكركمين-
|
10% (وزن/وزن) | إي. كولاي و س. أوريس |
|
[64] | |
| هيسبيريدين | 10% (وزن/حجم) | المكورات العنقودية الذهبية و الزائفة الزنجارية |
|
[65] | |
| هيسبيريدين | 10% (وزن/وزن) | المكورات العنقودية الذهبية و الزائفة الزنجارية |
|
[66] | |
| ميترونيدازول | 1% (وزن/حجم) | المكورات العنقودية الذهبية والإشريكية القولونية |
|
[67] | |
| ميترونيدازول |
|
المكورات العنقودية الذهبية والإشريكية القولونية |
|
[68] | |
| ميترونيدازول | 4% (وزن/حجم) | المكورات العنقودية الذهبية وجرثومة غاردنريلا المهبلية |
|
[69] | |
| أكسيد النيتريك | 2% (وزن/وزن) | المكورات العنقودية الذهبية والإشريكية القولونية |
|
[70] | |
| مستخلص أوراق السولانوم نيجروم L. |
|
المكورات العنقودية الذهبية، الزائفة الزنجارية وباسيلاس سوبتيليس |
|
[71] | |
| حمض التانيك | 10% (وزن/حجم) | إشريشيا كولاي و ستافيلوكوكوس أوريوس |
|
[72] | |
| هيدروكلوريد التتراسيكلين |
|
إشريشيا كولاي و ستافيلوكوكوس أوريوس |
|
[73] | |
| تيلميكوسين |
|
المكورات العنقودية الذهبية |
|
[74] | |
| توبرايسين | غير متوفر | إشريشيا كولاي و ستافيلوكوكوس أوريوس |
|
[75] | |
| توبرايسين |
|
المكورات العنقودية الذهبية والإشريكية القولونية | قتل حوالي 95% من المكورات العنقودية الذهبية عند تركيز
|
[76] | |
| فانكومايسين | 0.1% (وزن/حجم) | المكورات العنقودية الذهبية |
|
[77] | |
| أكسيد الزنك |
|
المكورات العنقودية الذهبية (S. aureus)، الإشريكية القولونية (E. coli)، العصوية الرقيقة (B. subtilis) |
|
[78] |
| جل الألجينات-الكيتوزان-الجيلاتين | الدوبامين | 0.2% (وزن/حجم) | المكورات العنقودية الذهبية والإشريكية القولونية |
|
[79] |
| ليفوفلوكساسين |
|
إي. كولاي و س. أوريس |
|
[80] | |
| ألجينات-كيتوزان-جيلاتين-ناCMC | مستخلص المشيمة البشرية | 10% (حجم/حجم) | المكورات العنقودية الذهبية والإشريكية القولونية |
|
[8I] |
| ألجينات-كيتوزان-بكتين | دوكسيسيكلين | 2% (وزن/وزن) | المكورات العنقودية الذهبية |
|
[82] |
| ألجينات-كيتوزان PVA | 5-فلورويوراسيل | 2% (وزن/حجم) | إي. كولاي و س. أوريس |
|
[83] |
| مستخلص زهرة القطيفة الطبية | 20% (وزن/حجم) | أسيتيتوباكتر باومانني، ستافيلوكوكوس إيبيديرميديس، بروتيوس ميرابيلس، وستافيلوكوكوس أوريوس. |
|
[84] | |
| ألجينات-كيتوزان صوديوم غليسيروفوسفات | هيدروكلوريد الفانكومايسين |
|
المكورات العنقودية الذهبية والإشريكية القولونية |
|
[85] |
| مركب الكيتوزان-بلورونيك | الكركمين النانوي | 15% (وزن/حجم) | إي. كولاي، س. تايفيموريوم، ب. أيروجينوزا، وس. أوريوس |
|
[86] |
| كيتوزان-ناCMC | ألانطوين | 0.5% (وزن/حجم) | المكورات العنقودية الذهبية والإشريكية القولونية |
|
[87] |
المواد. يمكن تطبيقه على كل من المستقلبات الثانوية والمركبات الكيميائية الاصطناعية.
الألجينات-الكيتوزان المدمج مع بوليمرات أخرى
التحديات والقيود
وجهة نظر مستقبلية
الخاتمة
بوليمر بلورونيك، وفوسفات الجلسرين الصوديوم. يمكن أن يؤدي استخدام هيدروجيل الكيتوزان-الألجينات إلى تمديد مدة إطلاق العوامل المضادة للبكتيريا وقد أظهر فعالية في تقليل عدد مستعمرات البكتيريا وتثبيط مناطق النمو في اختبارات أقراص اللوحة.
الشكر
التمويل
الإفصاح
References
- Okamoto S, Ogai K, Mukai K, Sugama J. Association of skin microbiome with the onset and recurrence of pressure injury in bedridden elderly people. Microorganisms. 2021;9(8). doi:10.3390/microorganisms9081603
- Gould L, Li WW. Defining complete wound closure: closing the gap in clinical trials and practice. Wound Repair Regener. 2019;27(3). doi:10.1111/wrr.12707
- Buzzá HH, Alves F, Tomé AJB, et al. Porphyrin nanoemulsion for antimicrobial photodynamic therapy: effective delivery to inactivate biofilm-related infections. Proc Natl Acad Sci U S A. 2022;119(46). doi:10.1073/pnas. 2216239119
- Punjataewakupt A, Napavichayanun S, Aramwit P. The downside of antimicrobial agents for wound healing. Eur J Clin Microbiol Infect Dis. 2019;38(1). doi:10.1007/s10096-018-3393-5
- Landeck L, John SM, Geier J. Topical ophthalmic agents as allergens in periorbital dermatitis. Br J Ophthalmol. 2014;98(2). doi:10.1136/ bjophthalmol-2013-304197
- Bandyopadhyay D. Topical antibacterials in dermatology. Indian J Dermatol. 2021;66(2). doi:10.4103/ijd.IJD_99_18
- Neri I, Miraglia Del Giudice M, Novelli A, Ruggiero G, Pappagallo G, Galli L. Ideal features of topical antibiotic therapy for the treatment of impetigo: an Italian expert consensus report. Curr Ther Res Clin Exp. 2023;98. doi:10.1016/j.curtheres.2022.100690
- Moeini A, Pedram P, Makvandi P, Malinconico M, Ayala G. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: a review. Carbohydr Polym. 2020;233. doi:10.1016/j.carbpol.2020.115839
- Siavash M, Noursina A. The ideal wound dressing. Burns. 2023. doi:10.1016/j.burns.2023.04.007
- Rezvani Ghomi E, Khalili S, Nouri Khorasani S, Esmaeely Neisiany R, Ramakrishna S. Wound dressings: current advances and future directions. J Appl Polym Sci. 2019;136(27). doi:10.1002/app. 47738
- Williamson DA, Carter GP, Howden BP. Current and emerging topical antibacterials and antiseptics: agents, action, and resistance patterns. Clin Microbiol Rev. 2017;30(3). doi:10.1128/CMR.00112-16
- Alsaab HO, Alharbi FD, Alhibs AS, et al. PLGA-based nanomedicine: history of advancement and development in clinical applications of multiple diseases. Pharmaceutics. 2022;14(12). doi:10.3390/pharmaceutics14122728
- Yanat M, Schroën K. Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packaging. React Funct Polym. 2021;161. doi:10.1016/j.reactfunctpolym.2021.104849
- Schmieg B, Döbber J, Kirschhöfer F, Pohl M, Franzreb M. Advantages of hydrogel-based 3D-printed enzyme reactors and their limitations for biocatalysis. Front Bioeng Biotechnol. 2019;6. doi:10.3389/fbioe.2018.00211
- Aswathy SH, Narendrakumar U, Manjubala I. Commercial hydrogels for biomedical applications. Heliyon. 2020;6(4). doi:10.1016/j.heliyon.
- Kibungu C, Kondiah PPD, Kumar P, Choonara YE. This review recent advances in chitosan and alginate-based hydrogels for wound healing application. Front Mater. 2021;8. doi:10.3389/fmats.2021.681960
- Ghobashy MM. The application of natural polymer-based hydrogels for agriculture. In: Hydrogels Based on Natural Polymers. Elsevier; 2019. doi10.1016/B978-0-12-816421-1.00013-6
- Sanchez-Salvador JL, Balea A, Monte MC, Negro C, Blanco A. Chitosan grafted/cross-linked with biodegradable polymers: a review. Int J Biol Macromol. 2021;178. doi:10.1016/j.ijbiomac.2021.02.200
- Yilmaz Atay H. Antibacterial activity of chitosan-based systems. Function Chitosan. 2020;457-489. doi:10.1007/978-981-15-0263-7_15
- Long S, Xie C, Lu X. Natural polymer-based adhesive hydrogel for biomedical applications. Biosurf Biotribol. 2022;8(2). doi:10.1049/bsb2.12036
- Bao Z, Xian C, Yuan Q, Liu G, Wu J. Natural polymer-based hydrogels with enhanced mechanical performances: preparation, structure, and property. Adv Healthc Mater. 2019;8(17). doi:10.1002/adhm. 201900670
- Abdel Maksoud MIA, Ghobashy MM, Kodous AS, et al. Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications. Nanotechnol Rev. 2022;11(1). doi:10.1515/ntrev-2022-0027
- Motelica L, Ficai D, Oprea O, et al. Biodegradable alginate films with ZnO nanoparticles and citronella essential oil-A novel antimicrobial structure. Pharmaceutics. 2021;13(7). doi:10.3390/pharmaceutics13071020
- Tomić SL, Babić Radić MM, Vuković JS, Filipović V, Nikodinovic-Runic J, Vukomanović M. Alginate-based hydrogels and scaffolds for biomedical applications. Mar Drugs. 2023;21(3). doi:10.3390/md21030177
- Suhandi C, Mohammed AFA, Wilar G, El-Rayyes A, Wathoni N. Effectiveness of mesenchymal stem cell secretome on wound healing: a systematic review and meta-analysis. Tissue Eng Regen Med. 2023. doi:10.1007/s13770-023-00570-9
- Ma Y, Zhang N, Wu S, Huang H, Cao Y. Antimicrobial activity of topical agents against Propionibacterium acnes: an in vitro study of clinical isolates from a hospital in Shanghai, China. Front Med. 2016;10(4). doi:10.1007/s11684-016-0480-9
- Salemi SZ, Memar MY, Kafil HS, et al. The prevalence and antibiotics susceptibility patterns of Corynebacterium minutissimum isolates from skin lesions of patients with suspected erythrasma from Tabriz, Iran. Can J Infect Dis Med Microbiol. 2022;2022. doi:10.1155/2022/4016173
- Pinheiro L, Brito CI, Pereira VC, de Oliveira A, Camargo CH. Reduced susceptibility to vancomycin and biofilm formation in methicillin-resistant Staphylococcus epidermidis isolated from blood cultures. Mem Inst Oswaldo Cruz. 2014;109(7). doi:10.1590/0074-0276140120
- Guo Y, Song G, Sun M, Wang J, Wang Y. Prevalence and therapies of antibiotic-resistance in Staphylococcus aureus. Front Cell Infect Microbiol. 2020;10. doi:10.3389/fcimb.2020.00107
- Tayeb-Fligelman E, Tabachnikov O, Moshe A, et al. The cytotoxic Staphylococcus aureus PSM
reveals a cross- amyloid-like fibril. Science. 2017;355(6327). doi:10.1126/science.aaf4901 - Nong Y, Taiaroa G, Pasricha S, et al. Clinical relevance of topical antibiotic use in coselecting for multidrug-resistant staphylococcus aureus: insights from in vitro and ex vivo models. Antimicrob Agents Chemother. 2021;65(5). doi:10.1128/AAC.02048-20
- Jespersen MG, Lacey JA, Tong SYC, Davies MR. Global genomic epidemiology of Streptococcus pyogenes. Infect Genet Evol. 2020;86. doi:10.1016/j.meegid.2020.104609
- Avire NJ, Whiley H, Ross K. A review of streptococcus pyogenes: public health risk factors, prevention and control. Pathogens. 2021;10(2). doi:10.3390/pathogens10020248
- Chowdhury S, Khakzad H, Bergdahl GE, et al. Streptococcus pyogenes forms serotype- and local environment-dependent interspecies protein complexes. mSystems. 2021;6(5). doi:10.1128/msystems.00271-21
- Stevens DL, Bryant AE. Impetigo, Erysipelas and Cellulitis. Search life-sciences literature; 2016.
- Thakur S, Thakur VK, Arotiba OA. History, Classification, Properties and Application of Hydrogels: An Overview. Springer; 2018. doi:10.1007/ 978-981-10-6077-9_2
- Elsayed MM. Hydrogel preparation technologies: relevance kinetics, thermodynamics and scaling up aspects. J Polym Environ. 2019;27(4). doi:10.1007/s10924-019-01376-4
- Jiang P, Yan C, Ji Z, et al. Drawing high-definition and reversible hydrogel paintings with grayscale exposure. ACS Appl Mater Interfaces. 2019;11 (45). doi:10.1021/acsami.9b14342
- Ickenstein LM, Garidel P. Hydrogel formulations for biologicals: current spotlight from a commercial perspective. Ther Deliv. 2018;9(3). doi:10.4155/tde-2017-0085
- Li J, Mooney DJ. Designing hydrogels for controlled drug delivery. Nat Rev Mater. 2016;1(12). doi:10.1038/natrevmats. 2016.71
- Almoshari YH. Novel hydrogels for topical applications: an updated comprehensive review based on source. Gels. 2022;8(3). doi:10.3390/ gels8030174
- Thang NH, Chien TB, Cuong DX. Polymer-based hydrogels applied in drug delivery: an overview. Gels. 2023;9(7). doi:10.3390/gels9070523
- Ciolacu DE, Nicu R, Ciolacu F. Cellulose-based hydrogels as sustained drug-delivery systems. Materials. 2020;13(22). doi:10.3390/ma13225270
- Yang J, Chen Y, Zhao L, et al. Preparation of a chitosan/carboxymethyl chitosan/AgNPs polyelectrolyte composite physical hydrogel with self-healing ability, antibacterial properties, and good biosafety simultaneously, and its application as a wound dressing. Compos B Eng. 2020:197. doi:10.1016/j.compositesb.2020.108139
- Ahmed EM. Hydrogel: preparation, characterization, and applications: a review.
Adv Res. 2015;6(2). doi:10.1016/j.jare.2013.07.006 - Gopala Kumari SV, Manikandan NA, Pakshirajan K, Pugazhenthi G. Sustained drug release and bactericidal activity of a novel, highly biocompatible and biodegradable polymer nanocomposite loaded with norfloxacin for potential use in antibacterial therapy. J Drug Deliv Sci Technol. 2020;59. doi:10.1016/j.jddst.2020.101900
- Li S, Shi X, Xu B, et al. In vitro drug release and antibacterial activity evaluation of silk fibroin coated vancomycin hydrochloride loaded poly (lactic-co-glycolic acid) (PLGA) sustained release microspheres. J Biomater Appl. 2022;36(9). doi:10.1177/08853282211064098
- Saliani M, Jalal R, Goharshadi EK. Effects of pH and temperature on antibacterial activity of zinc oxide nanofluid against Escherichia coli O157: H7 and staphylococcus aureus. Jundishapur J Microbiol. 2015;8(2). doi:10.5812/jjm. 17115
- Zakharova L, Pashirova T, Kashapov R, Gabdrakhmanov D, Sinyashin O. Drug delivery mediated by confined nanosystems: structure-activity relations and factors responsible for the efficacy of formulations. In: Nanostructures for Drug Delivery. Elsevier; 2017. doi10.1016/B978-0-323-46143-6.00024-5
- Sosnik A. Alginate particles as platform for drug delivery by the oral route: state-of-the-art. ISRN Pharm. 2014;2014. doi:10.1155/2014/926157
- Yang J, Han S, Zheng H, Dong H, Liu J. Preparation and application of micro/nanoparticles based on natural polysaccharides. Carbohydr Polym. 2015;123. doi:10.1016/j.carbpol.2015.01.029
- Silva SS, Fernandes EM, Pina S, et al. 2.11 Polymers of biological origin. In: Comprehensive Biomaterials II. Elsevier; 2017. doi:10.1016/B978-0-12-803581-8.10134-1
- Hassabo AG, Mohamed AL. Extraction, structural properties, and applications of alginic acid. In: Natural Gums. Elsevier; 2023. doi10.1016/b978-0-323-99468-2.00023-1
- Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37(1). doi:10.1016/j.progpolymsci.2011.06.003
- Jarrah R, Sammak S, Onyedimma C, et al. The role of alginate hydrogels as a potential treatment modality for spinal cord injury: a comprehensive review of the literature. Neurospine. 2022;19(2). doi:10.14245/ns.2244186.093
- Milanda T, Cindana Mo FR, Mohammed AFA, et al. Alginate/chitosan-based hydrogel film containing
-mangostin for recurrent aphthous stomatitis therapy in rats. Pharmaceutics. 2022;14(8). doi:10.3390/pharmaceutics14081709 - Chen H, Cheng J, Ran L, et al. An injectable self-healing hydrogel with adhesive and antibacterial properties effectively promotes wound healing. Carbohydr Polym. 2018:201. doi:10.1016/j.carbpol.2018.08.090
- Duceac IA, Coseri S. Biopolymers and their derivatives: key components of advanced biomedical technologies. Biotechnol Adv. 2022;61. doi:10.1016/j.biotechadv.2022.108056
- Baysal K, Aroguz AZ, Adiguzel Z, Baysal BM. Chitosan/alginate crosslinked hydrogels: preparation, characterization and application for cell growth purposes. Int J Biol Macromol. 2013;59. doi:10.1016/j.ijbiomac.2013.04.073
- Istiqomah A, Utami MR, Firdaus M, Suryanti V, Kusumaningsih T. Antibacterial chitosan-Dioscorea alata starch film enriched with essential oils optimally prepared by following response surface methodology. Food Biosci. 2022;46. doi:10.1016/j.fbio.2022.101603
- Hu H, Zhong D, Li W, et al. Microalgae-based bioactive hydrogel loaded with quorum sensing inhibitor promotes infected wound healing. Nano Today. 2022:42. doi:10.1016/j.nantod.2021.101368
- Cheng M, Cui Y, Guo Y, et al. Design of carboxymethyl chitosan-reinforced pH -responsive hydrogels for on-demand release of carvacrol and simulation of release kinetics. Food Chem. 2023:405. doi:10.1016/j.foodchem.2022.134856
- Khan YA, Ozaltin K, Bernal-Ballen A, Di Martino A. Chitosan-alginate hydrogels for simultaneous and sustained releases of ciprofloxacin, amoxicillin and vancomycin for combination therapy. J Drug Deliv Sci Technol. 2021;61. doi:10.1016/j.jddst.2020.102126
- Kiti K, Suwantong O. Bilayer wound dressing based on sodium alginate incorporated with curcumin-
-cyclodextrin inclusion complex/chitosan hydrogel. Int J Biol Macromol. 2020;164. doi:10.1016/j.jjbiomac.2020.09.013 - Bagher Z, Ehterami A, Nasrolahi M, Azimi M, Salehi M. Hesperidin promotes peripheral nerve regeneration based on tissue engineering strategy using alginate/chitosan hydrogel: in vitro and in vivo study. Int J Polym Mater Polym Biomater. 2021;70(5). doi:10.1080/00914037.2020.1713781
- Bagher Z, Ehterami A, Safdel MH, et al. Wound healing with alginate/chitosan hydrogel containing hesperidin in rat model. J Drug Deliv Sci Technol. 2020:55. doi:10.1016/j.jddst.2019.101379
- Permana AD, Asri RM, Amir MN, et al. Development of thermoresponsive hydrogels with mucoadhesion properties loaded with metronidazole gel-flakes for improved bacterial vaginosis treatment. Pharmaceutics. 2023;15(5). doi:10.3390/pharmaceutics15051529
- Feyissa Z, Edossa GD, Gupta NK, Negera D. Development of double crosslinked sodium alginate/chitosan based hydrogels for controlled release of metronidazole and its antibacterial activity. Heliyon. 2023;9(9). doi:10.1016/j.heliyon.2023.e20144
- Tentor F, Siccardi G, Sacco P, et al. Long lasting mucoadhesive membrane based on alginate and chitosan for intravaginal drug delivery.
Mater Sci Mater Med. 2020;31(3). doi:10.1007/s10856-020-6359-y - Zhang M, Fan Z, Zhang J, et al. Multifunctional chitosan/alginate hydrogel incorporated with bioactive glass nanocomposites enabling photothermal and nitric oxide release activities for bacteria-infected wound healing. Int J Biol Macromol. 2023:232. doi:10.1016/j.ijbiomac.2023.123445
- Najafpour F, Arabzadeh S, Kalalinia F, et al. Evaluation of wound healing effect of Solanum nigrum L. leaf extract-loaded sodium alginate nanoparticles embedded in chitosan hydrogel, In vivo study. Nanomed J. 2022;9(1). doi:10.22038/NMJ.2022.62218.1644
- Jafari H, Ghaffari-bohlouli P, Podstawczyk D, Nie L, Shavandi A. Tannic acid post-treatment of enzymatically crosslinked chitosan-alginate hydrogels for biomedical applications. Carbohydr Polym. 2022;295. doi:10.1016/j.carbpol.2022.119844
- Chen H, Xing X, Tan H, et al. Covalently antibacterial alginate-chitosan hydrogel dressing integrated gelatin microspheres containing tetracycline hydrochloride for wound healing. Mater Sci Eng C. 2017;70(Part 2). doi:10.1016/j.msec.2016.08.086
- Zhou K, Wang X, Chen D, et al. Enhanced treatment effects of tilmicosin against staphylococcus aureus cow mastitis by self-assembly sodium alginate-chitosan nanogel. Pharmaceutics. 2019;11(10). doi:10.3390/pharmaceutics11100524
- Xiao W, Gu S, Yuan W. Injectable nanocomposite hydrogels with the NIR-triggered low-temperature photothermal effect and low-dose antibiotic release. ACS Appl Polym Mater. 2023;5(7). doi:10.1021/acsapm.3c00676
- Shi M, Xu Y, Li S, Wang L, Gu J, Zhang YX. The development of a polysaccharide-based hydrogel encapsulating tobramycin-loaded gelatine microspheres as an antibacterial system. Gels. 2023;9(3). doi:10.3390/gels9030219
- Pawar V, Topkar H, Srivastava R. Chitosan nanoparticles and povidone iodine containing alginate gel for prevention and treatment of orthopedic implant associated infections. Int
Biol Macromol. 2018;115. doi:10.1016/j.ijbiomac.2018.04.166 - Zhang M, Qiao X, Han W, Jiang T, Liu F, Zhao X. Alginate-chitosan oligosaccharide-ZnO composite hydrogel for accelerating wound healing. Carbohydr Polym. 2021;266. doi:10.1016/j.carbpol.2021.118100
- Lu Y, Xu J, Su Y, et al. A biocompatible double-crosslinked gelatin/ sodium alginate/dopamine/quaterniazed chitosan hydrogel for wound dressings based on 3D bioprinting technology. Int J Bioprint. 2022;9(2). doi:10.18063/IJB. 689
- Cao J, Xiao L, Shi X. Injectable drug-loaded polysaccharide hybrid hydrogels for hemostasis. RSC Adv. 2019;9(63). doi:10.1039/c9ra07116d
- Seifi S, Shamloo A, Tavoosi SN, Almasi-Jaf A, Shaygani H, Sayah MR. A novel multifunctional chitosan-gelatin/carboxymethyl cellulose-alginate bilayer hydrogel containing human placenta extract for accelerating full-thickness wound healing. Int J Biol Macromol. 2023. doi:10.1016/j. ijbiomac.2023.126929
- Amante C, Esposito T, Del Gaudio P, et al. A novel three-polysaccharide blend in situ gelling powder for wound healing applications. Pharmaceutics. 2021;13(10). doi:10.3390/pharmaceutics13101680
- Chen M, Zhai X, Pan Y, Tan H. Covalent and environment-responsive biopolymer hydrogel for drug delivery and wound healing. J Macromol Sci Part A. 2021;58(11). doi:10.1080/10601325.2021.1929316
- Ghasemi AH, Farazin A, Mohammadimehr M, Naeimi H. Fabrication and characterization of biopolymers with antibacterial nanoparticles and Calendula officinalis flower extract as an active ingredient for modern hydrogel wound dressings. Mater Today Commun. 2022;31. doi:10.1016/j. mtcomm.2022.103513
- Sun M, Cheng L, Xu Z, et al. Preparation and characterization of vancomycin hydrochloride-loaded mesoporous silica composite hydrogels. Front Bioeng Biotechnol. 2022:10. doi:10.3389/fbioe.2022.826971
- Dang LH, Nguyen TH, Tran HLB, Doan VN, Tran NQ. Injectable nanocurcumin-formulated chitosan-g-pluronic hydrogel exhibiting a great potential for burn treatment. J Healthc Eng. 2018;2018. doi:10.1155/2018/5754890
- Haki M, Shamloo A, Eslami SS, Mir-Mohammad-Sadeghi F, Maleki S, Hajizadeh A. Fabrication and characterization of an antibacterial chitosan-coated allantoin-loaded NaCMC/SA skin scaffold for wound healing applications. Int J Biol Macromol. 2023;253. doi:10.1016/j. ijbiomac.2023.127051
- Suhandi C, Alfathonah SS, Hasanah AN. Potency of xanthone derivatives from garcinia mangostana L. for COVID-19 treatment through angiotensin-converting enzyme 2 and main protease blockade: a computational study. Molecules. 2023;28(13). doi:10.3390/molecules28135187
- Suhandi C, Wilar G, Lesmana R, et al. Propolis-based nanostructured lipid carriers for
-mangostin delivery: formulation, characterization, and in vitro antioxidant activity evaluation. Molecules. 2023;28(16). doi:10.3390/molecules28166057 - Suharyani I, Suhandi C, Rizkiyan Y, et al. Molecular docking in prediction of
-mangostin/cyclodextrin inclusion complex formation. In: AIP Conference Proceedings. Vol. 2706; 2023. doi:10.1063/5.0120782. - Andreazza R, Morales A, Pieniz S, Labidi J. Gelatin-based hydrogels: potential biomaterials for remediation. Polymers. 2023;15(4). doi:10.3390/ polym15041026
- Oliveira RN, McGuinness GB. Blended Gels of Sodium Carboxymethyl Cellulose Incorporating Antimicrobials for Absorbance and Wound Healing Applications. Springer; 2018. doi:10.1007/978-3-319-76573-0_39-1
- Chen W, Yuan S, Shen J, Chen Y, Xiao Y. A composite hydrogel based on pectin/cellulose via chemical cross-linking for hemorrhage. Front Bioeng Biotechnol. 2021;8. doi:10.3389/fbioe.2020.627351
- Deng A, Kang X, Zhang J, Yang Y, Yang S. Enhanced gelation of chitosan/
-sodium glycerophosphate thermosensitive hydrogel with sodium bicarbonate and biocompatibility evaluated. Mater Sci Eng C. 2017;78. doi:10.1016/j.msec.2017.04.109 - Aslam M, Barkat K, Malik NS, et al. pH sensitive pluronic acid/agarose-hydrogels as controlled drug delivery carriers: design, characterization and toxicity evaluation. Pharmaceutics. 2022;14(6). doi:10.3390/pharmaceutics14061218
- Ghobashy MM, Elbarbary AM, Hegazy DE, Maziad NA. Radiation synthesis of pH -sensitive 2-(dimethylamino)ethyl methacrylate/ polyethylene oxide/ZnS nanocomposite hydrogel membrane for wound dressing application. J Drug Deliv Sci Technol. 2022;73. doi:10.1016/j.jddst.2022.103399
- El-banna FS, Mahfouz ME, Leporatti S, El-Kemary M, Hanafy NAN. Chitosan as a natural copolymer with unique properties for the development of hydrogels. Appl Sci. 2019;9(11). doi:10.3390/app9112193
Dovepress
انشر عملك في هذه المجلة
DOI: https://doi.org/10.2147/idr.s456403
PMID: https://pubmed.ncbi.nlm.nih.gov/38444772
Publication Date: 2024-03-01
Alginate and Chitosan-Based Hydrogel Enhance Antibacterial Agent Activity on Topical Application
Abstract
Untreated topical infections can become chronic, posing serious health issues. Optimal skin adherence is crucial in addressing such infections. In this context, chitosan and alginate emerge as promising candidates for use as a foundation in the development of topical hydrogels. The aim of this review is to examine the literature on topical hydrogel formulations that use chitosan and alginate as foundations, specifically in the context of topical antibacterial agents. The research methodology involves a literature review by examining articles published in databases such as PubMed, Scopus, ScienceDirect, and Google Scholar. The keywords employed during the research were “Alginate”, “Chitosan”, “Hydrogel”, and “Antibacterial”. Chitosan and alginate serve as bases in topical hydrogels to deliver various active ingredients, particularly antibacterial agents, as indicated by the search results. Both have demonstrated significant antibacterial effectiveness, as evidenced by a reduction in bacterial colony counts and an increase in inhibition zones. This strongly supports the idea that chitosan and alginate could be used together to make topical hydrogels that kill bacteria that work well. In conclusion, chitosan and alginate-based hydrogels show great potential in treating bacterial infections on the skin surface. The incorporation of chitosan and alginate into hydrogel formulations aids in retaining antibacterial agents, allowing for their gradual release over an optimal period. Therefore, hydrogels specifically formulated with chitosan and alginate have the potential to serve as a solution to address challenges in the treatment of topical bacterial infections.
Introduction
evidence, the application of hydrogels has proven to have great potential in the field of wound dressings due to their physical properties closely resembling living tissues and excellent characteristics such as high-water content, oxygen permeability, and softness.
Method
Topical Infection
Propionibacterium Acnes
Corynebacterium
bacteria then dissolve keratin fibrils in intercellular spaces and within cells, producing porphyrins. The first-line therapy is topical erythromycin, clindamycin, or miconazole cream. Some common brand names for topical clindamycin include Anerocid, Acne Clin, and Benzasil. Meanwhile, some common brand names for miconazole cream include MonistatDerm and Micatin.
Staphylococcus Epidermidis
Staphylococcus Aureus
Streptococcus Pyogenes
Hydrogel as Promising Topical Drug Delivery
Definition of Hydrogel
Commonly Used Excipients
Preparation method
Preparing and Weighing of Raw Materials
Mixing
| Excipient | Usage |
| Vehicle | Hydrogels are essentially water-based matrices, and water is a primary component that forms the hydrogel structure.
|
| Gelling Agents | Gelling agents such as carbomers, gellan gum, agarose, or sodium carboxymethyl cellulose (NaCMC) are used to provide the hydrogel with appropriate density and viscosity.
|
| pH Adjusters | Substances like sodium hydroxide or citric acid are employed to adjust the pH of the hydrogel to suit the requirements of its medicinal application.
|
| Preservatives | Certain hydrogel formulations require preservatives such as benzalkonium chloride or phenol to prevent the growth of microorganisms.
|
| Texture Modifiers | Some excipients like propylene glycol or ethanol are used to regulate the texture and viscosity of the hydrogel.
|
| Consistency Enhancers | Excipients like polyacrylic acid glycol are employed to enhance the cohesiveness of the hydrogel.
|
| Colorants and Fragrances | In some cases, colorants or fragrances may be added for aesthetic purposes or patient compliance.
|

Heating
pH Adjustment
Preservatives and Other Excipients Addition
Gelation
Filtration and Sterilization
Filling and Packaging
Characterization of Hydrogel Dosage Form
Potency of Chitosan and Alginate as Natural Biodegradable Polymers-Based Hydrogel
Alginate
can function as delivery vectors for molecules precisely targeting tissues. The use of alginate can alter the physicochemical characteristics of drugs, enhancing their effectiveness and safety in drug delivery systems.
Chitosan
Alginate and Chitosan-Based Hydrogel Application for Topical Bacterial Treatment
Alginate-Chitosan-Based Hydrogel
| Hydrogel Base | Active Pharmacological Ingredient | Drug Concentration | Bacteria | Antibacterial Effectivity | Reference |
| Alginate-Chitosan | Berberine |
|
S. aureus |
|
[6I] |
| Carvacrol |
|
S. aureus and E. coli |
|
[62] | |
| Ciprofloxacin, amoxicillin and vancomycin |
|
S. aureus |
|
[63] | |
| Curcumin-
|
10% (w/w) | E. coli and S. aureus |
|
[64] | |
| Hesperidin | 10% (w/v) | Staphylococcus aureus dan Pseudomonas aeruginosa |
|
[65] | |
| Hesperidin | 10% (w/w) | S. aureus and P. aeruginosa |
|
[66] | |
| Metronidazole | 1% (w/v) | S. aureus dan E. coli |
|
[67] | |
| Metronidazole |
|
S. aureus and E. coli |
|
[68] | |
| Metronidazole | 4% (w/v) | Staphylococcus aureus and Gardnerella vaginalis |
|
[69] | |
| Nitric oxide | 2% (w/w) | S. aureus and E. coli |
|
[70] | |
| Solanum nigrum L. leave extract |
|
Staphylococcus aureus, Pseudomonas aeruginosa and Bacillus subtilis |
|
[71] | |
| Tannic acid | 10% (w/v) | Escherichia coli and Staphylococcus aureus |
|
[72] | |
| Tetracycline hydrochloride |
|
Escherichia coli and Staphylococcus aureus |
|
[73] | |
| Tilmicosin |
|
S. aureus |
|
[74] | |
| Tobramycin | N/A | Escherichia coli and Staphylococcus aureus |
|
[75] | |
| Tobramycin |
|
S. aureus and E. coli | Kill almost 95% S. aureus at concentration of
|
[76] | |
| Vancomycin | 0.1% (w/v) | S. aureus |
|
[77] | |
| Zinc oxide |
|
Staphylococcus aureus (S. aureus), Escherichia coli (E. coli), bacillus subtilis (B. subtilis) |
|
[78] |
| Alginate-ChitosanGelatin | Dopamine | 0.2% (w/v) | S. aureus and E. coli |
|
[79] |
| Levofloxacin |
|
E. coli and S. aureus |
|
[80] | |
| Alginate-Chitosan-Gelatin-NaCMC | Human placenta extract | 10% (v/v) | S. aureus and E. coli |
|
[8I] |
| Alginate-ChitosanPectin | Doxycycline | 2% (w/w) | Staphylococcus aureus |
|
[82] |
| Alginate-ChitosanPVA | 5-fluorouracil | 2% (w/v) | E. coli and S. aureus |
|
[83] |
| Calendula officinalis flower extract | 20% (w/v) | Acinetobacter baumannii, Staphylococcus epidermidis, Proteus mirabilis, and Staphylococcus aureus. |
|
[84] | |
| Alginate-ChitosanSodium Glycerophosphate | Vancomycin hydrochloride |
|
S. aureus and E. coli |
|
[85] |
| Chitosan-pluronic copolymer | Nano-curcumin | 15% (w/v) | E.coli, S. typhimurium, P. aeruginosa, and S. aureus |
|
[86] |
| Chitosan-NaCMC | Allantoin | 0.5% (w/v) | S. aureus and E. coli |
|
[87] |
substances. It can be applied to both secondary metabolites and synthetic chemical compounds.

Alginate-Chitosan Combined with Other Polymers
Challenges and Limitations
Future Perspective
Conclusion
pluronic copolymer, and sodium glycerophosphate. The utilization of chitosan-alginate hydrogels can extend the release duration of antibacterial agents and has demonstrated effectiveness in reducing bacterial colony counts and inhibiting growth zones in plate disc tests.
Acknowledgments
Funding
Disclosure
References
- Okamoto S, Ogai K, Mukai K, Sugama J. Association of skin microbiome with the onset and recurrence of pressure injury in bedridden elderly people. Microorganisms. 2021;9(8). doi:10.3390/microorganisms9081603
- Gould L, Li WW. Defining complete wound closure: closing the gap in clinical trials and practice. Wound Repair Regener. 2019;27(3). doi:10.1111/wrr.12707
- Buzzá HH, Alves F, Tomé AJB, et al. Porphyrin nanoemulsion for antimicrobial photodynamic therapy: effective delivery to inactivate biofilm-related infections. Proc Natl Acad Sci U S A. 2022;119(46). doi:10.1073/pnas. 2216239119
- Punjataewakupt A, Napavichayanun S, Aramwit P. The downside of antimicrobial agents for wound healing. Eur J Clin Microbiol Infect Dis. 2019;38(1). doi:10.1007/s10096-018-3393-5
- Landeck L, John SM, Geier J. Topical ophthalmic agents as allergens in periorbital dermatitis. Br J Ophthalmol. 2014;98(2). doi:10.1136/ bjophthalmol-2013-304197
- Bandyopadhyay D. Topical antibacterials in dermatology. Indian J Dermatol. 2021;66(2). doi:10.4103/ijd.IJD_99_18
- Neri I, Miraglia Del Giudice M, Novelli A, Ruggiero G, Pappagallo G, Galli L. Ideal features of topical antibiotic therapy for the treatment of impetigo: an Italian expert consensus report. Curr Ther Res Clin Exp. 2023;98. doi:10.1016/j.curtheres.2022.100690
- Moeini A, Pedram P, Makvandi P, Malinconico M, Ayala G. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: a review. Carbohydr Polym. 2020;233. doi:10.1016/j.carbpol.2020.115839
- Siavash M, Noursina A. The ideal wound dressing. Burns. 2023. doi:10.1016/j.burns.2023.04.007
- Rezvani Ghomi E, Khalili S, Nouri Khorasani S, Esmaeely Neisiany R, Ramakrishna S. Wound dressings: current advances and future directions. J Appl Polym Sci. 2019;136(27). doi:10.1002/app. 47738
- Williamson DA, Carter GP, Howden BP. Current and emerging topical antibacterials and antiseptics: agents, action, and resistance patterns. Clin Microbiol Rev. 2017;30(3). doi:10.1128/CMR.00112-16
- Alsaab HO, Alharbi FD, Alhibs AS, et al. PLGA-based nanomedicine: history of advancement and development in clinical applications of multiple diseases. Pharmaceutics. 2022;14(12). doi:10.3390/pharmaceutics14122728
- Yanat M, Schroën K. Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packaging. React Funct Polym. 2021;161. doi:10.1016/j.reactfunctpolym.2021.104849
- Schmieg B, Döbber J, Kirschhöfer F, Pohl M, Franzreb M. Advantages of hydrogel-based 3D-printed enzyme reactors and their limitations for biocatalysis. Front Bioeng Biotechnol. 2019;6. doi:10.3389/fbioe.2018.00211
- Aswathy SH, Narendrakumar U, Manjubala I. Commercial hydrogels for biomedical applications. Heliyon. 2020;6(4). doi:10.1016/j.heliyon.
- Kibungu C, Kondiah PPD, Kumar P, Choonara YE. This review recent advances in chitosan and alginate-based hydrogels for wound healing application. Front Mater. 2021;8. doi:10.3389/fmats.2021.681960
- Ghobashy MM. The application of natural polymer-based hydrogels for agriculture. In: Hydrogels Based on Natural Polymers. Elsevier; 2019. doi10.1016/B978-0-12-816421-1.00013-6
- Sanchez-Salvador JL, Balea A, Monte MC, Negro C, Blanco A. Chitosan grafted/cross-linked with biodegradable polymers: a review. Int J Biol Macromol. 2021;178. doi:10.1016/j.ijbiomac.2021.02.200
- Yilmaz Atay H. Antibacterial activity of chitosan-based systems. Function Chitosan. 2020;457-489. doi:10.1007/978-981-15-0263-7_15
- Long S, Xie C, Lu X. Natural polymer-based adhesive hydrogel for biomedical applications. Biosurf Biotribol. 2022;8(2). doi:10.1049/bsb2.12036
- Bao Z, Xian C, Yuan Q, Liu G, Wu J. Natural polymer-based hydrogels with enhanced mechanical performances: preparation, structure, and property. Adv Healthc Mater. 2019;8(17). doi:10.1002/adhm. 201900670
- Abdel Maksoud MIA, Ghobashy MM, Kodous AS, et al. Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications. Nanotechnol Rev. 2022;11(1). doi:10.1515/ntrev-2022-0027
- Motelica L, Ficai D, Oprea O, et al. Biodegradable alginate films with ZnO nanoparticles and citronella essential oil-A novel antimicrobial structure. Pharmaceutics. 2021;13(7). doi:10.3390/pharmaceutics13071020
- Tomić SL, Babić Radić MM, Vuković JS, Filipović V, Nikodinovic-Runic J, Vukomanović M. Alginate-based hydrogels and scaffolds for biomedical applications. Mar Drugs. 2023;21(3). doi:10.3390/md21030177
- Suhandi C, Mohammed AFA, Wilar G, El-Rayyes A, Wathoni N. Effectiveness of mesenchymal stem cell secretome on wound healing: a systematic review and meta-analysis. Tissue Eng Regen Med. 2023. doi:10.1007/s13770-023-00570-9
- Ma Y, Zhang N, Wu S, Huang H, Cao Y. Antimicrobial activity of topical agents against Propionibacterium acnes: an in vitro study of clinical isolates from a hospital in Shanghai, China. Front Med. 2016;10(4). doi:10.1007/s11684-016-0480-9
- Salemi SZ, Memar MY, Kafil HS, et al. The prevalence and antibiotics susceptibility patterns of Corynebacterium minutissimum isolates from skin lesions of patients with suspected erythrasma from Tabriz, Iran. Can J Infect Dis Med Microbiol. 2022;2022. doi:10.1155/2022/4016173
- Pinheiro L, Brito CI, Pereira VC, de Oliveira A, Camargo CH. Reduced susceptibility to vancomycin and biofilm formation in methicillin-resistant Staphylococcus epidermidis isolated from blood cultures. Mem Inst Oswaldo Cruz. 2014;109(7). doi:10.1590/0074-0276140120
- Guo Y, Song G, Sun M, Wang J, Wang Y. Prevalence and therapies of antibiotic-resistance in Staphylococcus aureus. Front Cell Infect Microbiol. 2020;10. doi:10.3389/fcimb.2020.00107
- Tayeb-Fligelman E, Tabachnikov O, Moshe A, et al. The cytotoxic Staphylococcus aureus PSM
reveals a cross- amyloid-like fibril. Science. 2017;355(6327). doi:10.1126/science.aaf4901 - Nong Y, Taiaroa G, Pasricha S, et al. Clinical relevance of topical antibiotic use in coselecting for multidrug-resistant staphylococcus aureus: insights from in vitro and ex vivo models. Antimicrob Agents Chemother. 2021;65(5). doi:10.1128/AAC.02048-20
- Jespersen MG, Lacey JA, Tong SYC, Davies MR. Global genomic epidemiology of Streptococcus pyogenes. Infect Genet Evol. 2020;86. doi:10.1016/j.meegid.2020.104609
- Avire NJ, Whiley H, Ross K. A review of streptococcus pyogenes: public health risk factors, prevention and control. Pathogens. 2021;10(2). doi:10.3390/pathogens10020248
- Chowdhury S, Khakzad H, Bergdahl GE, et al. Streptococcus pyogenes forms serotype- and local environment-dependent interspecies protein complexes. mSystems. 2021;6(5). doi:10.1128/msystems.00271-21
- Stevens DL, Bryant AE. Impetigo, Erysipelas and Cellulitis. Search life-sciences literature; 2016.
- Thakur S, Thakur VK, Arotiba OA. History, Classification, Properties and Application of Hydrogels: An Overview. Springer; 2018. doi:10.1007/ 978-981-10-6077-9_2
- Elsayed MM. Hydrogel preparation technologies: relevance kinetics, thermodynamics and scaling up aspects. J Polym Environ. 2019;27(4). doi:10.1007/s10924-019-01376-4
- Jiang P, Yan C, Ji Z, et al. Drawing high-definition and reversible hydrogel paintings with grayscale exposure. ACS Appl Mater Interfaces. 2019;11 (45). doi:10.1021/acsami.9b14342
- Ickenstein LM, Garidel P. Hydrogel formulations for biologicals: current spotlight from a commercial perspective. Ther Deliv. 2018;9(3). doi:10.4155/tde-2017-0085
- Li J, Mooney DJ. Designing hydrogels for controlled drug delivery. Nat Rev Mater. 2016;1(12). doi:10.1038/natrevmats. 2016.71
- Almoshari YH. Novel hydrogels for topical applications: an updated comprehensive review based on source. Gels. 2022;8(3). doi:10.3390/ gels8030174
- Thang NH, Chien TB, Cuong DX. Polymer-based hydrogels applied in drug delivery: an overview. Gels. 2023;9(7). doi:10.3390/gels9070523
- Ciolacu DE, Nicu R, Ciolacu F. Cellulose-based hydrogels as sustained drug-delivery systems. Materials. 2020;13(22). doi:10.3390/ma13225270
- Yang J, Chen Y, Zhao L, et al. Preparation of a chitosan/carboxymethyl chitosan/AgNPs polyelectrolyte composite physical hydrogel with self-healing ability, antibacterial properties, and good biosafety simultaneously, and its application as a wound dressing. Compos B Eng. 2020:197. doi:10.1016/j.compositesb.2020.108139
- Ahmed EM. Hydrogel: preparation, characterization, and applications: a review.
Adv Res. 2015;6(2). doi:10.1016/j.jare.2013.07.006 - Gopala Kumari SV, Manikandan NA, Pakshirajan K, Pugazhenthi G. Sustained drug release and bactericidal activity of a novel, highly biocompatible and biodegradable polymer nanocomposite loaded with norfloxacin for potential use in antibacterial therapy. J Drug Deliv Sci Technol. 2020;59. doi:10.1016/j.jddst.2020.101900
- Li S, Shi X, Xu B, et al. In vitro drug release and antibacterial activity evaluation of silk fibroin coated vancomycin hydrochloride loaded poly (lactic-co-glycolic acid) (PLGA) sustained release microspheres. J Biomater Appl. 2022;36(9). doi:10.1177/08853282211064098
- Saliani M, Jalal R, Goharshadi EK. Effects of pH and temperature on antibacterial activity of zinc oxide nanofluid against Escherichia coli O157: H7 and staphylococcus aureus. Jundishapur J Microbiol. 2015;8(2). doi:10.5812/jjm. 17115
- Zakharova L, Pashirova T, Kashapov R, Gabdrakhmanov D, Sinyashin O. Drug delivery mediated by confined nanosystems: structure-activity relations and factors responsible for the efficacy of formulations. In: Nanostructures for Drug Delivery. Elsevier; 2017. doi10.1016/B978-0-323-46143-6.00024-5
- Sosnik A. Alginate particles as platform for drug delivery by the oral route: state-of-the-art. ISRN Pharm. 2014;2014. doi:10.1155/2014/926157
- Yang J, Han S, Zheng H, Dong H, Liu J. Preparation and application of micro/nanoparticles based on natural polysaccharides. Carbohydr Polym. 2015;123. doi:10.1016/j.carbpol.2015.01.029
- Silva SS, Fernandes EM, Pina S, et al. 2.11 Polymers of biological origin. In: Comprehensive Biomaterials II. Elsevier; 2017. doi:10.1016/B978-0-12-803581-8.10134-1
- Hassabo AG, Mohamed AL. Extraction, structural properties, and applications of alginic acid. In: Natural Gums. Elsevier; 2023. doi10.1016/b978-0-323-99468-2.00023-1
- Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37(1). doi:10.1016/j.progpolymsci.2011.06.003
- Jarrah R, Sammak S, Onyedimma C, et al. The role of alginate hydrogels as a potential treatment modality for spinal cord injury: a comprehensive review of the literature. Neurospine. 2022;19(2). doi:10.14245/ns.2244186.093
- Milanda T, Cindana Mo FR, Mohammed AFA, et al. Alginate/chitosan-based hydrogel film containing
-mangostin for recurrent aphthous stomatitis therapy in rats. Pharmaceutics. 2022;14(8). doi:10.3390/pharmaceutics14081709 - Chen H, Cheng J, Ran L, et al. An injectable self-healing hydrogel with adhesive and antibacterial properties effectively promotes wound healing. Carbohydr Polym. 2018:201. doi:10.1016/j.carbpol.2018.08.090
- Duceac IA, Coseri S. Biopolymers and their derivatives: key components of advanced biomedical technologies. Biotechnol Adv. 2022;61. doi:10.1016/j.biotechadv.2022.108056
- Baysal K, Aroguz AZ, Adiguzel Z, Baysal BM. Chitosan/alginate crosslinked hydrogels: preparation, characterization and application for cell growth purposes. Int J Biol Macromol. 2013;59. doi:10.1016/j.ijbiomac.2013.04.073
- Istiqomah A, Utami MR, Firdaus M, Suryanti V, Kusumaningsih T. Antibacterial chitosan-Dioscorea alata starch film enriched with essential oils optimally prepared by following response surface methodology. Food Biosci. 2022;46. doi:10.1016/j.fbio.2022.101603
- Hu H, Zhong D, Li W, et al. Microalgae-based bioactive hydrogel loaded with quorum sensing inhibitor promotes infected wound healing. Nano Today. 2022:42. doi:10.1016/j.nantod.2021.101368
- Cheng M, Cui Y, Guo Y, et al. Design of carboxymethyl chitosan-reinforced pH -responsive hydrogels for on-demand release of carvacrol and simulation of release kinetics. Food Chem. 2023:405. doi:10.1016/j.foodchem.2022.134856
- Khan YA, Ozaltin K, Bernal-Ballen A, Di Martino A. Chitosan-alginate hydrogels for simultaneous and sustained releases of ciprofloxacin, amoxicillin and vancomycin for combination therapy. J Drug Deliv Sci Technol. 2021;61. doi:10.1016/j.jddst.2020.102126
- Kiti K, Suwantong O. Bilayer wound dressing based on sodium alginate incorporated with curcumin-
-cyclodextrin inclusion complex/chitosan hydrogel. Int J Biol Macromol. 2020;164. doi:10.1016/j.jjbiomac.2020.09.013 - Bagher Z, Ehterami A, Nasrolahi M, Azimi M, Salehi M. Hesperidin promotes peripheral nerve regeneration based on tissue engineering strategy using alginate/chitosan hydrogel: in vitro and in vivo study. Int J Polym Mater Polym Biomater. 2021;70(5). doi:10.1080/00914037.2020.1713781
- Bagher Z, Ehterami A, Safdel MH, et al. Wound healing with alginate/chitosan hydrogel containing hesperidin in rat model. J Drug Deliv Sci Technol. 2020:55. doi:10.1016/j.jddst.2019.101379
- Permana AD, Asri RM, Amir MN, et al. Development of thermoresponsive hydrogels with mucoadhesion properties loaded with metronidazole gel-flakes for improved bacterial vaginosis treatment. Pharmaceutics. 2023;15(5). doi:10.3390/pharmaceutics15051529
- Feyissa Z, Edossa GD, Gupta NK, Negera D. Development of double crosslinked sodium alginate/chitosan based hydrogels for controlled release of metronidazole and its antibacterial activity. Heliyon. 2023;9(9). doi:10.1016/j.heliyon.2023.e20144
- Tentor F, Siccardi G, Sacco P, et al. Long lasting mucoadhesive membrane based on alginate and chitosan for intravaginal drug delivery.
Mater Sci Mater Med. 2020;31(3). doi:10.1007/s10856-020-6359-y - Zhang M, Fan Z, Zhang J, et al. Multifunctional chitosan/alginate hydrogel incorporated with bioactive glass nanocomposites enabling photothermal and nitric oxide release activities for bacteria-infected wound healing. Int J Biol Macromol. 2023:232. doi:10.1016/j.ijbiomac.2023.123445
- Najafpour F, Arabzadeh S, Kalalinia F, et al. Evaluation of wound healing effect of Solanum nigrum L. leaf extract-loaded sodium alginate nanoparticles embedded in chitosan hydrogel, In vivo study. Nanomed J. 2022;9(1). doi:10.22038/NMJ.2022.62218.1644
- Jafari H, Ghaffari-bohlouli P, Podstawczyk D, Nie L, Shavandi A. Tannic acid post-treatment of enzymatically crosslinked chitosan-alginate hydrogels for biomedical applications. Carbohydr Polym. 2022;295. doi:10.1016/j.carbpol.2022.119844
- Chen H, Xing X, Tan H, et al. Covalently antibacterial alginate-chitosan hydrogel dressing integrated gelatin microspheres containing tetracycline hydrochloride for wound healing. Mater Sci Eng C. 2017;70(Part 2). doi:10.1016/j.msec.2016.08.086
- Zhou K, Wang X, Chen D, et al. Enhanced treatment effects of tilmicosin against staphylococcus aureus cow mastitis by self-assembly sodium alginate-chitosan nanogel. Pharmaceutics. 2019;11(10). doi:10.3390/pharmaceutics11100524
- Xiao W, Gu S, Yuan W. Injectable nanocomposite hydrogels with the NIR-triggered low-temperature photothermal effect and low-dose antibiotic release. ACS Appl Polym Mater. 2023;5(7). doi:10.1021/acsapm.3c00676
- Shi M, Xu Y, Li S, Wang L, Gu J, Zhang YX. The development of a polysaccharide-based hydrogel encapsulating tobramycin-loaded gelatine microspheres as an antibacterial system. Gels. 2023;9(3). doi:10.3390/gels9030219
- Pawar V, Topkar H, Srivastava R. Chitosan nanoparticles and povidone iodine containing alginate gel for prevention and treatment of orthopedic implant associated infections. Int
Biol Macromol. 2018;115. doi:10.1016/j.ijbiomac.2018.04.166 - Zhang M, Qiao X, Han W, Jiang T, Liu F, Zhao X. Alginate-chitosan oligosaccharide-ZnO composite hydrogel for accelerating wound healing. Carbohydr Polym. 2021;266. doi:10.1016/j.carbpol.2021.118100
- Lu Y, Xu J, Su Y, et al. A biocompatible double-crosslinked gelatin/ sodium alginate/dopamine/quaterniazed chitosan hydrogel for wound dressings based on 3D bioprinting technology. Int J Bioprint. 2022;9(2). doi:10.18063/IJB. 689
- Cao J, Xiao L, Shi X. Injectable drug-loaded polysaccharide hybrid hydrogels for hemostasis. RSC Adv. 2019;9(63). doi:10.1039/c9ra07116d
- Seifi S, Shamloo A, Tavoosi SN, Almasi-Jaf A, Shaygani H, Sayah MR. A novel multifunctional chitosan-gelatin/carboxymethyl cellulose-alginate bilayer hydrogel containing human placenta extract for accelerating full-thickness wound healing. Int J Biol Macromol. 2023. doi:10.1016/j. ijbiomac.2023.126929
- Amante C, Esposito T, Del Gaudio P, et al. A novel three-polysaccharide blend in situ gelling powder for wound healing applications. Pharmaceutics. 2021;13(10). doi:10.3390/pharmaceutics13101680
- Chen M, Zhai X, Pan Y, Tan H. Covalent and environment-responsive biopolymer hydrogel for drug delivery and wound healing. J Macromol Sci Part A. 2021;58(11). doi:10.1080/10601325.2021.1929316
- Ghasemi AH, Farazin A, Mohammadimehr M, Naeimi H. Fabrication and characterization of biopolymers with antibacterial nanoparticles and Calendula officinalis flower extract as an active ingredient for modern hydrogel wound dressings. Mater Today Commun. 2022;31. doi:10.1016/j. mtcomm.2022.103513
- Sun M, Cheng L, Xu Z, et al. Preparation and characterization of vancomycin hydrochloride-loaded mesoporous silica composite hydrogels. Front Bioeng Biotechnol. 2022:10. doi:10.3389/fbioe.2022.826971
- Dang LH, Nguyen TH, Tran HLB, Doan VN, Tran NQ. Injectable nanocurcumin-formulated chitosan-g-pluronic hydrogel exhibiting a great potential for burn treatment. J Healthc Eng. 2018;2018. doi:10.1155/2018/5754890
- Haki M, Shamloo A, Eslami SS, Mir-Mohammad-Sadeghi F, Maleki S, Hajizadeh A. Fabrication and characterization of an antibacterial chitosan-coated allantoin-loaded NaCMC/SA skin scaffold for wound healing applications. Int J Biol Macromol. 2023;253. doi:10.1016/j. ijbiomac.2023.127051
- Suhandi C, Alfathonah SS, Hasanah AN. Potency of xanthone derivatives from garcinia mangostana L. for COVID-19 treatment through angiotensin-converting enzyme 2 and main protease blockade: a computational study. Molecules. 2023;28(13). doi:10.3390/molecules28135187
- Suhandi C, Wilar G, Lesmana R, et al. Propolis-based nanostructured lipid carriers for
-mangostin delivery: formulation, characterization, and in vitro antioxidant activity evaluation. Molecules. 2023;28(16). doi:10.3390/molecules28166057 - Suharyani I, Suhandi C, Rizkiyan Y, et al. Molecular docking in prediction of
-mangostin/cyclodextrin inclusion complex formation. In: AIP Conference Proceedings. Vol. 2706; 2023. doi:10.1063/5.0120782. - Andreazza R, Morales A, Pieniz S, Labidi J. Gelatin-based hydrogels: potential biomaterials for remediation. Polymers. 2023;15(4). doi:10.3390/ polym15041026
- Oliveira RN, McGuinness GB. Blended Gels of Sodium Carboxymethyl Cellulose Incorporating Antimicrobials for Absorbance and Wound Healing Applications. Springer; 2018. doi:10.1007/978-3-319-76573-0_39-1
- Chen W, Yuan S, Shen J, Chen Y, Xiao Y. A composite hydrogel based on pectin/cellulose via chemical cross-linking for hemorrhage. Front Bioeng Biotechnol. 2021;8. doi:10.3389/fbioe.2020.627351
- Deng A, Kang X, Zhang J, Yang Y, Yang S. Enhanced gelation of chitosan/
-sodium glycerophosphate thermosensitive hydrogel with sodium bicarbonate and biocompatibility evaluated. Mater Sci Eng C. 2017;78. doi:10.1016/j.msec.2017.04.109 - Aslam M, Barkat K, Malik NS, et al. pH sensitive pluronic acid/agarose-hydrogels as controlled drug delivery carriers: design, characterization and toxicity evaluation. Pharmaceutics. 2022;14(6). doi:10.3390/pharmaceutics14061218
- Ghobashy MM, Elbarbary AM, Hegazy DE, Maziad NA. Radiation synthesis of pH -sensitive 2-(dimethylamino)ethyl methacrylate/ polyethylene oxide/ZnS nanocomposite hydrogel membrane for wound dressing application. J Drug Deliv Sci Technol. 2022;73. doi:10.1016/j.jddst.2022.103399
- El-banna FS, Mahfouz ME, Leporatti S, El-Kemary M, Hanafy NAN. Chitosan as a natural copolymer with unique properties for the development of hydrogels. Appl Sci. 2019;9(11). doi:10.3390/app9112193
Dovepress
