DOI: https://doi.org/10.1186/s12964-023-01466-w
PMID: https://pubmed.ncbi.nlm.nih.gov/38195584
تاريخ النشر: 2024-01-09
cGAS-STING، الإنفلامازومات والبيوربتوسيس: نظرة عامة على آلية التفاعل لتنشيط وتنظيم
الملخص
الخلفية: تعمل مسارات استشعار الحمض النووي داخل الخلايا cGAS-STING، والانفلامازومات، والبيوربتوسيس كمحاور إشارات مناعية طبيعية حاسمة للعدوى الميكروبية، والالتهاب المزمن، وتقدم السرطان، وانحلال الأعضاء، لكن الآلية وتنظيم شبكة التفاعل لا يزال غير واضح. الجسم الرئيسي للملخص: ي disrupt الإجهاد الخلوي التوازن الميتوكوندري، ويسهل فتح ثقب الانتقال النفاذي للميتوكوندريا وتسرب الحمض النووي الميتوكوندري إلى غشاء الخلية، مما يحفز الاستجابات الالتهابية عن طريق تنشيط إشارة cGAS-STING، ويؤدي بعد ذلك إلى تنشيط الانفلامازومات وبداية البيوربتوسيس. في الوقت نفسه، فإن البروتين المرتبط بالانفلامازوم كاسبيز-1، غازدرمين D، مجال CARD من ASC وقناة البوتاسيوم تشارك في تنظيم مسار cGAS-STING. من المهم أن هذه الشبكة التفاعلية لها تأثير تضخيم متسلسل يزيد من الاستجابة المناعية الالتهابية، مما يؤدي إلى تفاقم العملية المرضية للأمراض الالتهابية والمناعية الذاتية. نظرًا لأهمية هذه الشبكة التفاعلية من cGAS-STING، والانفلامازومات، والبيوربتوسيس في تنظيم المناعة الفطرية، فإنها تبرز كطريق جديد لاستكشاف آليات نشوء الأمراض المتعددة. لذلك، تم أو يتم بذل جهود لتحديد استراتيجيات لتعديل cGAS-STING، والانفلامازومات، والبيوربتوسيس بشكل انتقائي في سياقات مرضية مختلفة. في هذه المراجعة، سنصف كيف أن هذا الفهم الميكانيكي يدفع العلاجات المحتملة التي تستهدف هذه الشبكة التفاعلية، مع التركيز على البروتينات المتفاعلة أو التنظيمية، والمسارات، ومركز ميتوكوندري تنظيمي بين cGAS-STING، والانفلامازومات، والبيوربتوسيس. الخاتمة القصيرة: تهدف هذه المراجعة إلى تقديم رؤى حول الأدوار الحاسمة وآليات التنظيم لشبكة التفاعل بين cGAS-STING، والانفلامازومات، والبيوربتوسيس، وإبراز بعض الاتجاهات الواعدة للبحث والتدخل في المستقبل.
خلفية
تقوم الإنفلامازومات، مثل بروتين 3 المحتوي على مجالات NACHT وLRR وPYD (NLRP3) وغياب في الميلانوما 2 (AIM2)، ببدء إطلاق السيتوكينات المؤيدة للالتهابات عند تلقي إشارات الخطر لتنشيط الاستجابة المناعية الفطرية، وهي ضرورية للتخلص من مسببات الأمراض أو الخلايا التالفة. NLRP3 هو مستشعر داخل الخلايا يتعرف على مجموعة واسعة من الأنماط الميكروبية، وإشارات الخطر الذاتية، والمهيجات البيئية، مما يؤدي إلى تشكيل وتنشيط إنفلامازوم NLRP3. تتطلب عملية من خطوتين من التهيئة والتنشيط لإنفلامازوم NLRP3. في مرحلة التهيئة، يتم تنشيط NF-кВ أولاً بواسطة مستقبلات التعرف مثل مستقبلات التشابه مع البكتيريا (TLRs) التي تتعرف على الأنماط الجزيئية المرتبطة بالمسببات المرضية (PAMPs) أو الأنماط الجزيئية لإشارات الخطر.
(DAMPs)، تليها زيادة تعبير NLRP3 و pro-IL-1
تنظم الإنفلامازومات والبايروبتوس نظام cGAS-STING
تنظم إنفلامازوم AIM2 مسار cGAS-STING
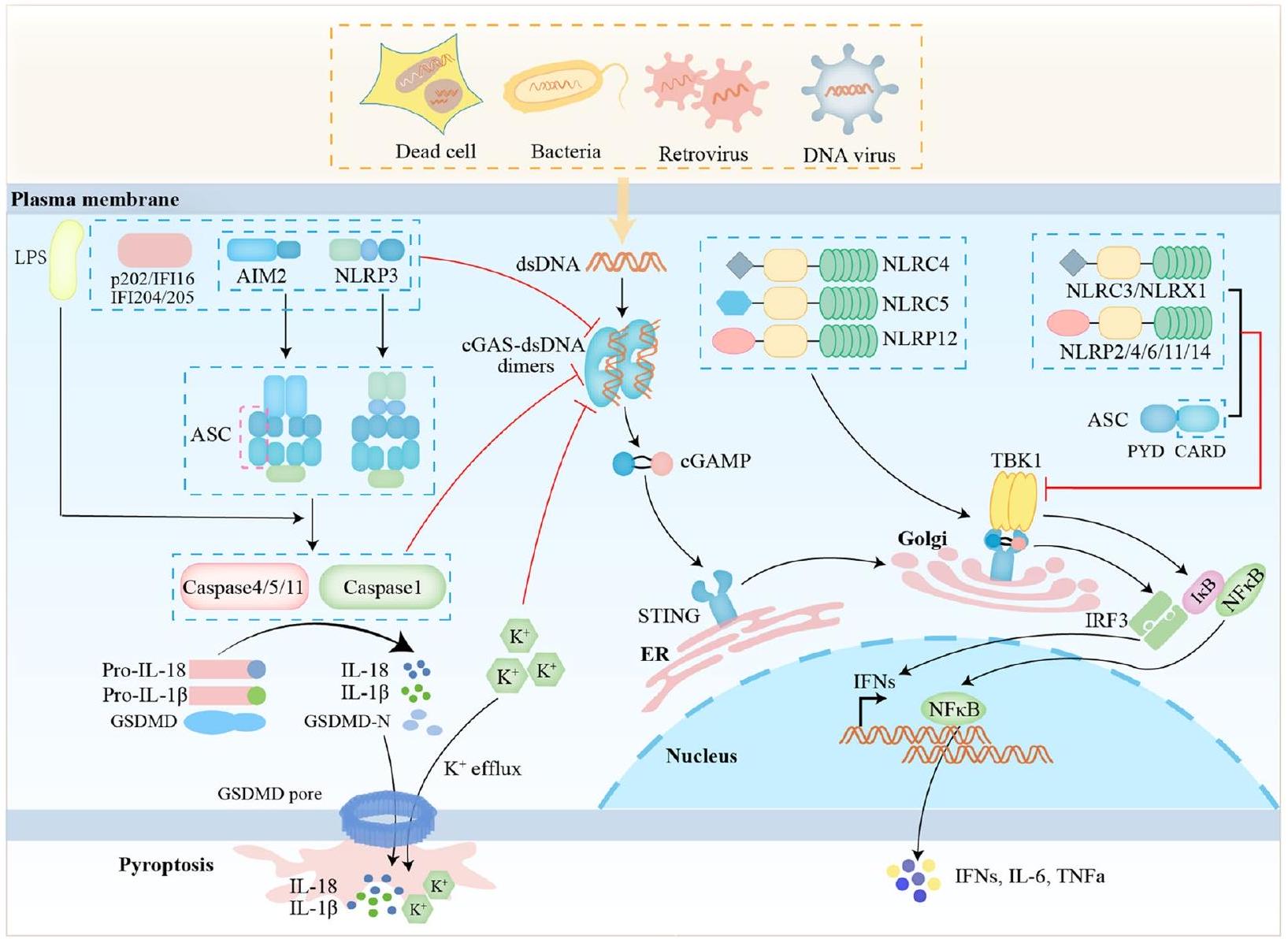
تقوم مستقبلات شبيهة AIM2 (ALRs) بتنظيم cGAS-STING
تم اقتراح IFI16، وهو مستشعر نووي فطري مستقل عن التسلسل، أيضًا لتحفيز مسارات خلوية أخرى عند ارتباطه بالحمض النووي الفيروسي. تؤكد عدة تقارير أن الحمض النووي لفيروسات الهربس مثل فيروس كابوسي ساركوما المرتبط بالهربس (KSHV) وفيروس إبشتاين-بار (EBV) وفيروس الهربس البسيط 1 (HSV-1) أثناء العدوى يشكل هيكلًا أوليغومريًا يحتوي على IFI16، مما يؤدي إلى إنتاج كاسبيز-1 نشط و IL-
نظام الالتهاب NLRP3 ينظم cGAS-STING
في IFN- الذي يتم بوساطة cGAS-STING
تنظم الكاسبازات cGAS-STING
تنظم GSDMD مسار cGAS-STING
مجال CARD من ASC ينظم cGAS-STING
المستقبلات الشبيهة بالعقد (NLRs) تنظم cGAS-STING
cGAS-STING ينظم inflammasomes و pyroptosis cGAS-STING ينظم inflammasome NLRP3 و pyroptosis
تشير الدراسات المتاحة إلى أن تفاعل STING مع NLRP3 استجابةً لتحفيز الحمض النووي السيتوبلازمي يعزز تنشيط inflammasome NLRP3 بعدة طرق. أولاً، قام STING بتجنيد NLRP3 لتعزيز موضعه في الشبكة الإندوبلازمية، مما يعزز تشكيل inflammasome NLRP3. ثانياً، تفاعل TM5 (151-160aa) من STING مع مجال NACHT و LRR في NLRP3 لتقليل تعدد يوبكويتين NLRP3 المرتبط بـ K48 و K63، أي أن STING قام بإزالة يوبكويتين.
cGAS-STING ينظم inflammasome AIM2
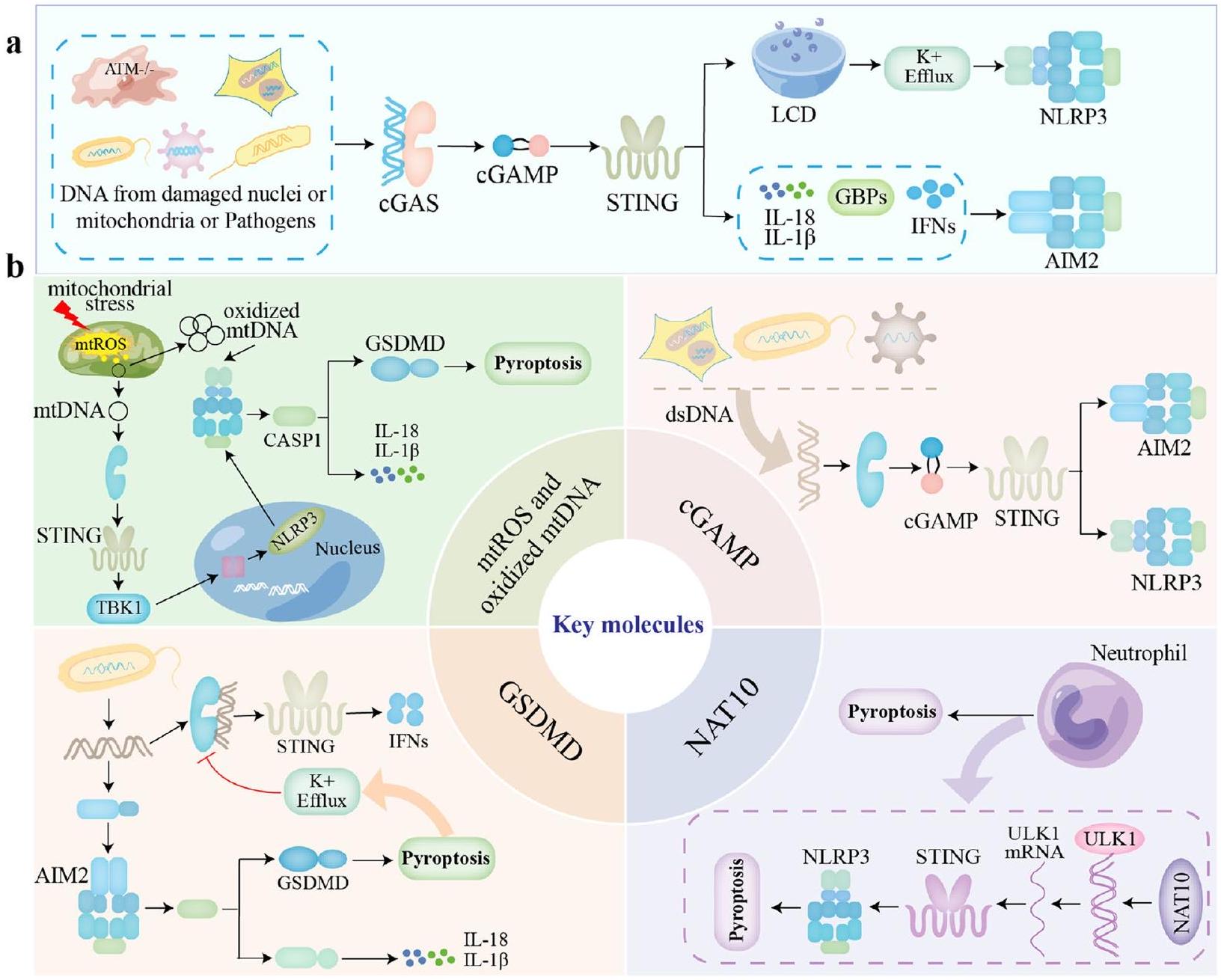
الجزيئات الرئيسية في شبكة التفاعل بين cGAS-STING، والانفلامسومات، والبيوروبتوسيس Ox-mtDNA و mtROS
[116، 117]. بالإضافة إلى ذلك، فإن توليد أكسيد النيتريك سينثاز (iNOS) بواسطة IFN من النوع الأول وNO يمنع تكتل بروتين NLRP3، مما يمنع تجميع inflammasome NLRP3 [118].
GSDMD
نات10 و ULK1
أدى إلى تنشيط inflammasome NLRP3 واحتضار العدلات [125]. من ناحية أخرى، تم إظهار أن ULK1 يشارك في البلعمة الذاتية لـ NLRP3، مما يشير إلى أن ULK1 له تأثير تنظيمي مباشر على inflammasome NLRP3 بالإضافة إلى تثبيط STING (الشكل 2) [126].
cGAMP
الأمراض الناتجة عن شبكة التفاعل بين cGAS-STING، والانفلامازومات، والبيوروبتوسيس
في تنشيط الانفلامازوم [79، 86] والاحتضار الناتج عن GSDMD [135]، والذي يتميز بخلل في وظائف الجهاز المناعي والإفراز الشاذ للسيتوكينات الالتهابية. نتيجة لذلك، فإن التفاعل بين محور cGAS-STING والانفلامازوم والاحتضار يبني مجموعة واسعة من أنظمة المراقبة المهمة استجابةً لتلف الأنسجة وغزو مسببات الأمراض. تسبب الشذوذات في هذا التواصل المتبادل مجموعة متنوعة من الأمراض البشرية، بما في ذلك الأمراض المعدية، والأمراض المناعية الذاتية، والأورام، وتليف الأعضاء، والأمراض التنكسية العصبية [11، 136-138]. نظرًا للدور الحاسم لـ cGAS-STING والانفلامازوم والاحتضار في الاستجابات المناعية والالتهابية، ركزنا بعد ذلك على الأمراض ذات الصلة التي تسببها شبكة التواصل هذه بهدف تقديم أدلة على الوقاية والعلاج (الشكل 3).
خلل القلب
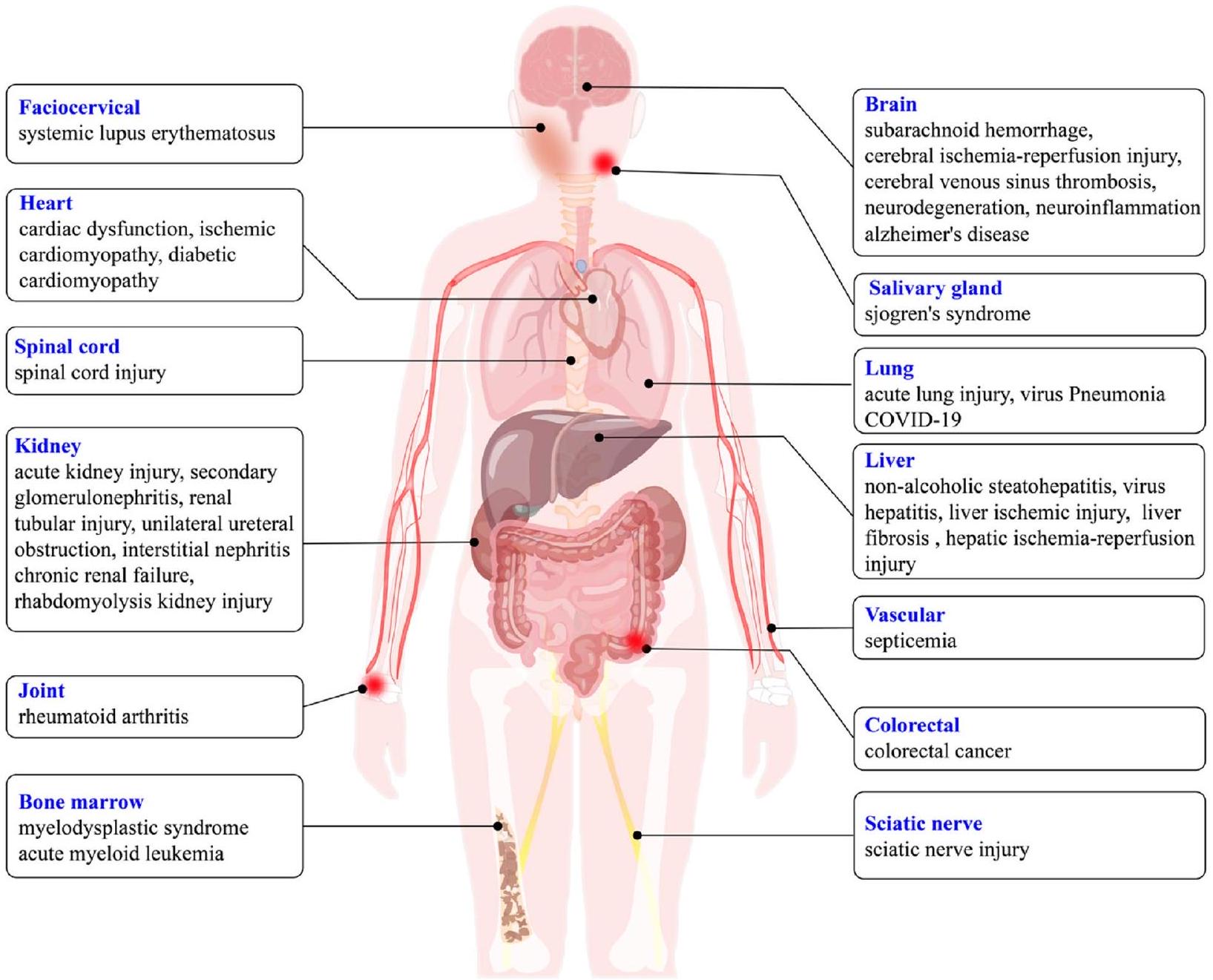
إصابة الرئة الحادة (ALI)
أمراض الكبد
وزيادة تنشيط cGAS-STING في نسيج الكبد، بينما أدى نقص STING إلى تقليل التهاب الكبد وتليفها [153-155]. أظهرت تسلسلات RNA من أكباد الفئران مع
أمراض الكلى
تمت ملاحظته في نماذج الفشل الكلوي الحاد المتعددة في الفئران ومرضى الفشل الكلوي الحاد. أظهرت الفئران التي تم حذف جين STING انخفاضًا في وظيفة الكلى، وتلف الأنابيب، والالتهاب بعد علاج السيكلوفسفاميد. بالإضافة إلى ذلك، كان لـ STING دور في الالتهاب الكلوي الثانوي وإصابة الأنابيب. لعبت مسارات STING وNLRP3 inflammasome أدوارًا مهمة في انسداد الحالب الأحادي، والتهاب الكلى الأنبوبي الناتج عن الأدينين، والفشل الكلوي المزمن. أدى تعبير بروتين الشحم G2 من نوع APOL1 (G2 APOL1) في خلايا كلى الفئران إلى تنشيط cGAS-STING وNLRP3 inflammasome، وكان تعبير APOL1 مرتبطًا بمستويات الكاسبيز-1 وGSDMD. في نموذج RIAKI للفئران، على الرغم من أن نقص AIM2 منع موت الماكروفاجات الكلوية، إلا أنه زاد بشكل مفاجئ من الالتهاب غير الطبيعي كما يتضح من تجمع الماكروفاجات الضخم.
التهاب الجهاز العصبي
يمكن أن يُعزى هذا العملية إلى النشاط المحفز للمناعة، وخاصة مسار cGAS-STING. زاد المحفز STING CMA بشكل كبير من تعبير STING في الخلايا الدبقية بعد النزيف تحت العنكبوتية (SAH) وزاد من تفاقم تلف الخلايا العصبية. بالإضافة إلى ذلك، في أدمغة المرضى الذين يعانون من أمراض تنكس عصبي مختلفة، كان مستوى كيناز السيروم/الجلوكوكورتيكويد المرتبط 1 (SGK1) مرتفعًا. يتم الكشف عن تعبير SGK1 على نطاق واسع في الدماغ، ويزداد في الظروف المرضية مثل متلازمة ريت، مرض الزهايمر، التصلب المتعدد، التصلب الجانبي الضموري، والألم العصبي، مما يشير مجتمعة إلى أن SGK1 يلعب أدوارًا مسببة للأمراض في الاضطرابات التنكسية العصبية. إن تثبيط SGK1 الدبقي يصحح الخصائص المؤيدة للالتهاب للخلايا الدبقية من خلال تقليل NF-кB داخل الخلايا، والانفلامازوم NLRP3، ومسارات الالتهاب التي تتوسطها cGAS-STING. أدى تنشيط مسار cGAS-STING في الفئران المصابة بمرض الزهايمر إلى تحفيز تكوين الانفلامازوم NLRP3، وزيادة الشيخوخة الخلوية والاستجابات الالتهابية، وأظهر علاج النياسيناميد ريبوزيد (NR) تأثيرات مفيدة من خلال مسار cGAS-STING. علاوة على ذلك، كانت استجابة الالتهاب التي أدت إلى تنشيط الخلايا الدبقية مرتبطة بالعجز العصبي بعد إصابة الدماغ الرضحية (TBI). على النقيض من ذلك، فإن تنشيط cGAS-STING في الخلايا الدبقية عزز الاستجابات العصبية الالتهابية بعد TBI، جزئيًا من خلال تنشيط الانفلامازوم NLRP3. في الختام، قد يعمل مسار الإشارة cGAS-STING-NLRP3 كهدف علاجي محتمل للخلل العصبي الناتج عن الالتهاب العصبي.
متلازمة خلل التنسج النقوي (MDSs) وإصابة الحبل الشوكي (SCI)
مع مدى تنكس القرص الفقري بواسطة التصوير بالرنين المغناطيسي (MRI) وعلم الأمراض النسيجية. بدأ الإجهاد التأكسدي تفعيل محور cGAS-STING المعتمد على STING والالتهاب الناتج عن NLRP3 في خلايا النواة اللبية البشرية. مجتمعة، تشير هذه البيانات إلى الدور الأساسي لمحور cGAS-STING-NLRP3 والالتهاب الناتج عن NLRP3 في تطور تنكس القرص الفقري وتقدم نهج علاج محتمل لإدارة آلام أسفل الظهر الناتجة عن الأقراص.
الأمراض المناعية الذاتية
الأورام الخبيثة
الانتقال من سرطان القولون والمستقيم [206]. ومع ذلك، يمكن أن يؤدي تنشيط cGASSTING المرتبط بالالتهاب المزمن أيضًا إلى تعزيز انتشار الورم من خلال تحفيز بيئة الورم المثبطة للمناعة [9]. زاد cGAMP المنتج من خلايا السرطان من نمو الورم ومقاومته للعلاج الكيميائي من خلال تنشيط STING في الخلايا النجمية وإنتاج السيتوكينات الالتهابية [207].
كوفيد-19
منظمو شبكة التداخل بين cGAS-STING، والانفلامازوم، والبيوروبتوسيس
المنتجات الطبيعية
حمض 4-أوكتيل (4-OI)، وهو مشتق من المناعة يتراكم خلال تنشيط البلعميات، جذب اهتمامًا واسعًا لخصائصه المضادة للالتهابات ومضادات الأكسدة. أظهرت التجارب في المختبر وفي الجسم الحي أن 4-OI قام بتثبيط تنشيط مسار cGAS-STING-IRF3 من خلال القضاء على إنتاج mtROS وتسرب mtDNA في البلعميات الهوائية تحت الضغط التأكسدي، بينما خفف من الموت الخلوي الناتج عن inflammasome NLRP3 المستحث بواسطة LPS، مما ساهم في تحسين متلازمة الضائقة التنفسية الحادة (ARDS). الإبيغالوكاتشين غالات (EGCG) هو مونومر كاتيكين معزول من الشاي ويعتبر مكونًا رئيسيًا من بوليفينولات الشاي الأخضر. أظهرت دراسة متقدمة في المختبر أن EGCG يمكن أن يمنع تنشيط inflammasome NLRP3 من خلال تقليل تنشيط مسار cGAS-STING-IRF3، وبالتالي كان له تأثيرات وقائية كبيرة ضد
لقد أظهرت عدة دراسات أن التركيز الفسيولوجي لغاز كبريتيد الهيدروجين
الوظائف البيولوجية وصيانة التوازن الداخلي في الجسم [219، 220]. وعلى العكس، فإن نقص المواد الذاتية
مادة صناعية
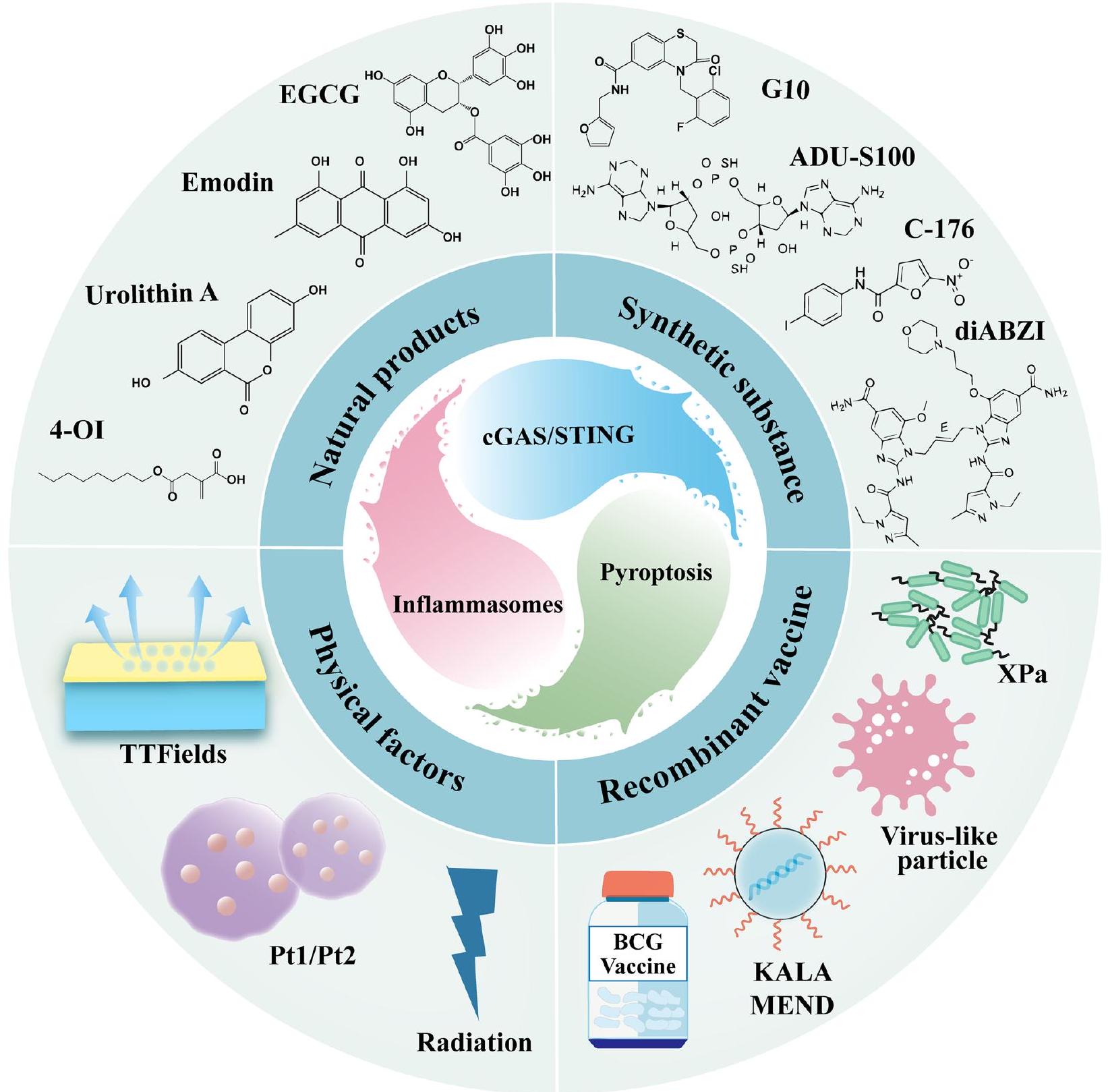
أدى إعطاء مضاد STING C-176 إلى تقليل تنشيط الالتهاب الناتج عن TBI في الميكروغليا وتقليل البيروبتوز [194].
لقاح مؤتلف
أدى إلى محور cGAS-STING-IFNs من النوع الأول ونشط إنزيمات AIM2 وNLRP3، مما أدى إلى نسبة أعلى من
عوامل فيزيائية
المناقشة والاستنتاج
الكبد، الرئة، الكلى، الحبل الشوكي، التهاب الجهاز العصبي، يحفز الأمراض المناعية الذاتية ويعزز تقدم الأورام الخبيثة. بينما يستمر تحسين فهمنا لـ cGAS-STING، والانفلامازوم، والبيوربتوس، فإن استهداف هذه الشبكة التفاعلية كعلاج لعدة أمراض يتقدم بسرعة. لذلك، نحن نلخص مشاركة المنتجات الطبيعية، والمواد الاصطناعية، واللقاحات المؤتلفة، والعوامل الفيزيائية في تنظيم شبكة التفاعل بين مسارات cGAS-STING، والانفلامازومات، والبيوربتوس، مما يوفر مرشحين محتملين لعلاج الأمراض ذات الصلة. باعتبارها تجسيدًا للطب الدقيق في الأمراض الالتهابية، فإن الاستمرار في تصنيف، وتحسين، وإعادة توجيه المعدلات المباشرة والمحددة سيعزز الترجمة السريرية المستقبلية.
باختصار، فإن مسار الإشارات cGAS-STING يولد تأثيرات تضخيم متسلسلة بين inflammasomes و pyroptosis، وينشط الاستجابات الالتهابية المناعية. من ناحية، يمكن أن يؤثر التداخل بين هذه المسارات الإشارية على الأعضاء الحشوية مثل القلب والكبد والرئة والكلى، ويزيد من تفاقم عملية تطور الأمراض الالتهابية؛ بالإضافة إلى ذلك، فإنه مرتبط ارتباطًا وثيقًا بتقدم العديد من الأمراض المناعية الذاتية. لذلك، فإن التحقيقات الإضافية واعدة لكشف آليات تنظيمية جديدة قد توفر فرصًا جديدة للتدخل العلاجي في المجال المثير لشبكة التداخل بين cGAS-STING و inflammasomes ومحور إشارات pyroptosis.
شكر وتقدير
مساهمات المؤلفين
تمويل
توفر البيانات والمواد
الإعلانات
موافقة الأخلاقيات والموافقة على المشاركة
موافقة على النشر
المصالح المتنافسة
تفاصيل المؤلف
مستشفى جامعة تشنغدو للطب الصيني التقليدي، مقاطعة سيتشوان، تشنغدو 610075، الصين.
نُشر على الإنترنت: 09 يناير 2024
References
- Ahn J, Barber GN. STING signaling and host defense against microbial infection. Exp Mol Med. 2019;51(12):1-10.
- Zhang H, You QD, Xu XL. Targeting stimulator of interferon genes (STING): a medicinal chemistry perspective. J Med Chem. 2020;63(8):3785-816.
- Cohen D, Melamed S, Millman A, Shulman G, Oppenheimer-Shaanan Y, Kacen A, et al. Cyclic GMP-AMP signalling protects bacteria against viral infection. Nature. 2019;574(7780):691-5.
- Manes NP, Nita-Lazar A. Molecular mechanisms of the toll-like receptor, STING, MAVS, Inflammasome, and Interferon Pathways. mSystems. 2021;6(3):e0033621.
- Peng Y, Zhuang J, Ying G, Zeng H, Zhou H, Cao Y, et al. Stimulator of IFN genes mediates neuroinflammatory injury by suppressing AMPK signal in experimental subarachnoid hemorrhage. J Neuroinflammation. 2020;17(1):165.
- Chen Q, Sun L, Chen ZJ. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol. 2016;17(10):1142-9.
- Zhang X, Bai XC, Chen ZJ. Structures and mechanisms in the cGASSTING innate immunity pathway. Immunity. 2020;53(1):43-53.
- Jiang M, Chen P, Wang L, Li W, Chen B, Liu Y, et al. cGAS-STING, an important pathway in cancer immunotherapy. J Hematol Oncol. 2020;13(1):81.
- Kwon J, Bakhoum SF. The cytosolic DNA-sensing cGAS-STING pathway in Cancer. Cancer Discov. 2020;10(1):26-39.
- Samson N, Ablasser A. The cGAS-STING pathway and cancer. Nat Cancer. 2022;3(12):1452-63.
- Wang Y, Luo J, Alu A, Han X, Wei Y, Wei X. cGAS-STING pathway in cancer biotherapy. Mol Cancer. 2020;19(1):136.
- Ramanjulu JM, Pesiridis GS, Yang J, Concha N, Singhaus R, Zhang SY, et al. Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature. 2018;564(7736):439-43.
- Van Herck S, Feng B, Tang L. Delivery of STING agonists for adjuvanting subunit vaccines. Adv Drug Deliv Rev. 2021;179:114020.
- Wang J, Li P, Yu Y, Fu Y, Jiang H, Lu M, et al. Pulmonary surfactant-biomimetic nanoparticles potentiate heterosubtypic influenza immunity. Science (New York, NY). 2020;367(6480).
- Luo J, Liu XP, Xiong FF, Gao FX, Yi YL, Zhang M, et al. Enhancing immune response and Heterosubtypic protection ability of inactivated H7N9 vaccine by using STING agonist as a mucosal adjuvant. Front Immunol. 2019;10:2274.
- Motwani M, Pawaria S, Bernier J, Moses S, Henry K, Fang T, et al. Hierarchy of clinical manifestations in SAVI N153S and V154M mouse models. Proc Natl Acad Sci U S A. 2019;116(16):7941-50.
- Taguchi T, Mukai K. Innate immunity signalling and membrane trafficking. Curr Opin Cell Biol. 2019;59:1-7.
- Decout A, Katz JD, Venkatraman S, Ablasser A. The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat Rev Immunol. 2021;21(9):548-69.
- Paul BD, Snyder SH, Bohr VA. Signaling by cGAS-STING in neurodegeneration, Neuroinflammation, and aging. Trends Neurosci. 2021;44(2):83-96.
- Karmakar M, Katsnelson MA, Dubyak GR, Pearlman E. Neutrophil P2X7 receptors mediate NLRP3 inflammasome-dependent IL-1
secretion in response to ATP. Nat Commun. 2016;7:10555. - Paik S, Kim JK, Silwal P, Sasakawa C, Jo EK. An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell Mol Immunol. 2021;18(5):1141-60.
- Molyvdas A, Georgopoulou U, Lazaridis N, Hytiroglou P, Dimitriadis A, Foka P, et al. The role of the NLRP3 inflammasome and the activation of IL-1
in the pathogenesis of chronic viral hepatic inflammation. Cytokine. 2018;110:389-96. - Karki R, Lee E, Sharma BR, Banoth B, Kanneganti TD. IRF8 regulates gram-negative Bacteria-mediated NLRP3 Inflammasome activation and cell death. J Immunol (Baltimore, Md : 1950). 2020;204(9):2514-22.
- Wu Y, Ren J, Zhou B, Ding C, Chen J, Wang G, et al. Gene silencing of non-obese diabetic receptor family (NLRP3) protects against the sepsis-induced hyper-bile acidaemia in a rat model. Clin Exp Immunol. 2015;179(2):277-93.
- Wang Y, Shi P, Chen Q, Huang Z, Zou D, Zhang J, et al. Mitochondrial ROS promote macrophage pyroptosis by inducing GSDMD oxidation. J Mol Cell Biol. 2019;11(12):1069-82.
- Dick MS, Sborgi L, Rühl S, Hiller S, Broz P. Corrigendum: ASC filament formation serves as a signal amplification mechanism for inflammasomes. Nat Commun. 2017;8:15030.
- Cheng Q, Pan J, Zhou ZL, Yin F, Xie HY, Chen PP, et al. Caspase-11/4 and gasdermin D-mediated pyroptosis contributes to podocyte injury in mouse diabetic nephropathy. Acta Pharmacol Sin. 2021;42(6):954-63.
- Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11(12):1136-42.
- Jorgensen I, Zhang Y, Krantz BA, Miao EA. Pyroptosis triggers poreinduced intracellular traps (PITs) that capture bacteria and lead to their clearance by efferocytosis. J Exp Med. 2016;213(10):2113-28.
- Bai B, Yang Y, Wang Q, Li M, Tian C, Liu Y, et al. NLRP3 inflammasome in endothelial dysfunction. Cell Death Dis. 2020;11(9):776.
- Aachoui Y, Leaf IA, Hagar JA, Fontana MF, Campos CG, Zak DE, et al. Caspase-11 protects against bacteria that escape the vacuole. Science (New York, NY). 2013;339(6122):975-8.
- Corrales L, Woo SR, Williams JB, McWhirter SM, Dubensky TW Jr, Gajewski TF. Antagonism of the STING pathway via activation of the AIM2 Inflammasome by intracellular DNA. J Immunol (Baltimore, Md : 1950). 2016;196(7):3191-8.
- Baatarjav C, Komada T, Karasawa T, Yamada N, Sampilvanjil A, Matsumura T, et al. dsDNA-induced AIM2 pyroptosis halts aberrant inflammation during rhabdomyolysis-induced acute kidney injury. Cell Death Differ. 2022;29(12):2487-502.
- Yan S, Shen H, Lian Q, Jin W, Zhang R, Lin X, et al. Deficiency of the AIM2-ASC signal uncovers the STING-driven Overreactive response of type I IFN and reciprocal depression of protective IFN-
immunity in mycobacterial infection. J Immunol. 2018;200(3):1016-26. - Gray EE, Winship D, Snyder JM, Child SJ, Geballe AP, Stetson DB. The AIM2-like receptors are dispensable for the interferon response to intracellular DNA. Immunity. 2016;45(2):255-66.
- Jiang H, Swacha P, Gekara NO. Nuclear AIM2-like receptors drive genotoxic tissue injury by inhibiting DNA repair. Adv Sci (Weinh). 2021;8(22):e2102534.
- Ratsimandresy RA, Dorfleutner A, Stehlik C. An update on PYRIN domain-containing pattern recognition receptors: from immunity to pathology. Front Immunol. 2013;4:440.
- Brunette RL, Young JM, Whitley DG, Brodsky IE, Malik HS, Stetson DB. Extensive evolutionary and functional diversity among mammalian AlM2-like receptors. J Exp Med. 2012;209(11):1969-83.
- Kumar V. The trinity of cGAS, TLR9, and ALRs guardians of the cellular galaxy against host-derived self-DNA. Front Immunol. 2020;11:624597.
- Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma S, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11(11):997-1004.
- Schattgen SA, Fitzgerald KA. The PYHIN protein family as mediators of host defenses. Immunol Rev. 2011;243(1):109-18.
- Nakaya Y, Lilue J, Stavrou S, Moran EA, Ross SR. AIM2-like receptors positively and negatively regulate the interferon response induced by cytosolic DNA. mBio. 2017;8(4).
- Panchanathan
, Duan , Shen , Rathinam VA, Erickson LD, Fitzgerald KA, et al. Aim2 deficiency stimulates the expression of IFN-inducible Ifi202, a lupus susceptibility murine gene within the Nba2 autoimmune susceptibility locus. J Immunol (Baltimore, Md : 1950). 2010;185(12):7385-93. - Storek KM, Gertsvolf NA, Ohlson MB, Monack DM. cGAS and Ifi204 cooperate to produce type I IFNs in response to Francisella infection. J Immunol (Baltimore, Md : 1950). 2015;194(7):3236-45.
- Bühler M, Li D, Li L, Runft S, Waltl I, Pavlou A, et al. IFNAR signaling of neuroectodermal cells is essential for the survival of C57BL/6 mice infected with Theiler’s murine encephalomyelitis virus. J Neuroinflammation. 2023;20(1):58.
- Almine JF, O’Hare CA, Dunphy G, Haga IR, Naik RJ, Atrih A, et al. IFI16 and cGAS cooperate in the activation of STING during DNA sensing in human keratinocytes. Nat Commun. 2017;8:14392.
- Lee MN, Roy M, Ong SE, Mertins P, Villani AC, Li W, et al. Identification of regulators of the innate immune response to cytosolic DNA and retroviral infection by an integrative approach. Nat Immunol. 2013;14(2):179-85.
- Cao L, Ji Y, Zeng L, Liu Q, Zhang Z, Guo S, et al. P200 family protein IFI204 negatively regulates type I interferon responses by targeting IRF7 in nucleus. PLoS Pathog. 2019;15(10):e1008079.
- Chunfa L, Xin S, Qiang L, Sreevatsan S, Yang L, Zhao D, et al. The central role of IFI204 in IFN-
release and autophagy activation during Mycobacterium bovis infection. Front Cell Infect Microbiol. 2017;7:169. - Chen W, Yu SX, Zhou FH, Zhang XJ, Gao WY, Li KY, et al. DNA sensor IFI204 contributes to host defense against Staphylococcus aureus infection in mice. Front Immunol. 2019;10:474.
- Ansari MA, Dutta S, Veettil MV, Dutta D, Iqbal J, Kumar B, et al. Herpesvirus genome recognition induced acetylation of nuclear IFI16 is essential for its cytoplasmic translocation, Inflammasome and IFN-
responses. PLoS Pathog. 2015;11(7):e1005019. - Pisano G, Roy A, Ahmed Ansari M, Kumar B, Chikoti L, Chandran B. Interferon-
-inducible protein 16 (IFI16) is required for the maintenance of Epstein-Barr virus latency. Virol J. 2017;14(1):221. - Diner BA, Lum KK, Toettcher JE, Cristea IM. Viral DNA sensors IFI16 and cyclic GMP-AMP synthase possess distinct functions in regulating viral gene expression, immune defenses, and apoptotic responses during herpesvirus infection. mBio. 2016;7(6).
- Hansen K, Prabakaran T, Laustsen A, Jørgensen SE, Rahbæk SH, Jensen SB, et al. Listeria monocytogenes induces IFN
expression through an IFI16-, cGAS- and STING-dependent pathway. EMBO J. 2014;33(15):1654-66. - Wu T, Gao J, Liu W, Cui J, Yang M, Guo W, et al. NLRP3 protects mice from radiation-induced colon and skin damage via attenuating cGAS-STING signaling. Toxicol Appl Pharmacol. 2021;418:115495.
- Zheng Y, Liu Q, Wu Y, Ma L, Zhang Z, Liu T, et al. Zika virus elicits inflammation to evade antiviral response by cleaving cGAS via NS1-caspase-1 axis. EMBO J. 2018;37(18).
- Wang Y, Ning X, Gao P, Wu S, Sha M, Lv M, et al. Inflammasome activation triggers Caspase-1-mediated cleavage of cGAS to regulate responses to DNA virus infection. Immunity. 2017;46(3):393-404.
- Aglietti RA, Estevez A, Gupta A, Ramirez MG, Liu PS, Kayagaki N, et al. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc Natl Acad Sci U S A. 2016;113(28):7858-63.
- Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535(7610):111-6.
- Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, et al. Inflamma-some-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535(7610):153-8.
- Sborgi L, Rühl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H, et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016;35(16):1766-78.
- Banerjee I, Behl B, Mendonca M, Shrivastava G, Russo AJ, Menoret A, et al. Gasdermin D restrains type I interferon response to cytosolic DNA by disrupting ionic homeostasis. Immunity. 2018;49(3):413-26. e5.
- Eren E, Berber M, Özören N. NLRC3 protein inhibits inflammation by disrupting NALP3 inflammasome assembly via competition with the adaptor protein ASC for pro-caspase-1 binding. J Biol Chem. 2017;292(30):12691-701.
- Sandstrom A, Mitchell PS, Goers L, Mu EW, Lesser CF, Vance RE. Functional degradation: a mechanism of NLRP1 inflammasome activation by diverse pathogen enzymes. Science (New York, NY). 2019;364(6435).
- Chui AJ, Okondo MC, Rao SD, Gai K, Griswold AR, Johnson DC, et al. N-terminal degradation activates the NLRP1B inflammasome. Science (New York, NY). 2019;364(6435):82-5.
- Zheng C. The emerging roles of NOD-like receptors in antiviral innate immune signaling pathways. Int J Biol Macromol. 2021;169:407-13.
- Li X, Deng M, Petrucelli AS, Zhu C, Mo J, Zhang L, et al. Viral DNA binding to NLRC3, an inhibitory nucleic acid sensor, unleashes STING, a cyclic dinucleotide receptor that activates type I interferon. Immunity. 2019;50(3):591-9.e6.
- Zhang L, Mo J, Swanson KV, Wen H, Petrucelli A, Gregory SM, et al. NLRC3, a member of the NLR family of proteins, is a negative regulator of innate immune signaling induced by the DNA sensor STING. Immunity. 2014;40(3):329-41.
- Guo H, König R, Deng M, Riess M, Mo J, Zhang L, et al. NLRX1 sequesters STING to negatively regulate the interferon response, thereby facilitating the replication of HIV-1 and DNA viruses. Cell Host Microbe. 2016;19(4):515-28.
- Yang Y, Lang X, Sun S, Gao C, Hu J, Ding S, et al. NLRP2 negatively regulates antiviral immunity by interacting with TBK1. Eur J Immunol. 2018;48(11):1817-25.
- Cui J, Li Y, Zhu L, Liu D, Songyang Z, Wang HY, et al. NLRP4 negatively regulates type I interferon signaling by targeting the kinase TBK1 for degradation via the ubiquitin ligase DTX4. Nat Immunol. 2012;13(4):387-95.
- Ellwanger K, Becker E, Kienes I, Sowa A, Postma Y, Cardona Gloria Y, et al. The NLR family pyrin domain-containing 11 protein contributes to the regulation of inflammatory signaling. J Biol Chem. 2018;293(8):2701-10.
- Abe T, Lee A, Sitharam R, Kesner J, Rabadan R, Shapira SD. Germ-cellspecific Inflammasome component NLRP14 negatively regulates cytosolic nucleic acid sensing to promote fertilization. Immunity. 2017;46(4):621-34.
- Wang P, Zhu S, Yang L, Cui S, Pan W, Jackson R, et al. NIrp6 regulates intestinal antiviral innate immunity. Science (New York, NY). 2015;350(6262):826-30.
- Zhang R, Yang W, Zhu H, Zhai J, Xue M, Zheng C. NLRC4 promotes the cGAS-STING signaling pathway by facilitating CBL-mediated K63-linked polyubiquitination of TBK1. J Med Virol. 2023;95(8):e29013.
- Sundaram B, Kanneganti TD. Advances in understanding activation and function of the NLRC4 Inflammasome. Int J Mol Sci. 2021;22(3).
- Kuenzel S, Till A, Winkler M, Häsler R, Lipinski S, Jung S, et al. The nucleo-tide-binding oligomerization domain-like receptor NLRC5 is involved in IFN-dependent antiviral immune responses. J Immunol (Baltimore, Md : 1950). 2010;184(4):1990-2000.
- Neerincx A, Lautz K, Menning M, Kremmer E, Zigrino P, Hösel M, et al. A role for the human nucleotide-binding domain, leucine-rich repeatcontaining family member NLRC5 in antiviral responses. J Biol Chem. 2010;285(34):26223-32.
- Gaidt MM, Ebert TS, Chauhan D, Ramshorn K, Pinci F, Zuber S, et al. The DNA Inflammasome in human myeloid cells is initiated by a STING-cell death program upstream of NLRP3. Cell. 2017;171(5):1110-24.e18.
- Webster SJ, Brode S, Ellis L, Fitzmaurice TJ, Elder MJ, Gekara NO, et al. Detection of a microbial metabolite by STING regulates inflammasome activation in response to chlamydia trachomatis infection. PLoS Pathog. 2017;13(6):e1006383.
- Zhong W, Rao Z, Rao J, Han G, Wang P, Jiang T, et al. Aging aggravated liver ischemia and reperfusion injury by promoting STING-mediated NLRP3 activation in macrophages. Aging Cell. 2020;19(8):e13186.
- Li N, Zhou H, Wu H, Wu Q, Duan M, Deng W, et al. STING-IRF3 contributes to lipopolysaccharide-induced cardiac dysfunction, inflammation, apoptosis and pyroptosis by activating NLRP3. Redox Biol. 2019;24:101215.
- Zhang H, Chen Z, Zhou J, Gu J, Wu H, Jiang Y, et al. NAT10 regulates neutrophil pyroptosis in sepsis via acetylating ULK1 RNA and activating STING pathway. Commun Biol. 2022;5(1):916.
- McLemore AF, Hou HA, Meyer BS, Lam NB, Ward GA, Aldrich AL, et al. Somatic gene mutations expose cytoplasmic DNA to co-opt the cGAS/ STING/NLRP3 axis in myelodysplastic syndromes. JCI Insight. 2022;7(15).
- Aits S, Jäättelä M. Lysosomal cell death at a glance. J Cell Sci. 2013;126(Pt 9):1905-12.
- Wang W, Hu D, Wu C, Feng Y, Li A, Liu W, et al. STING promotes NLRP3 localization in ER and facilitates NLRP3 deubiquitination to
activate the inflammasome upon HSV-1 infection. PLoS Pathog. 2020;16(3):e1008335. - Xiao Y, Zhao C, Tai Y, Li B, Lan T, Lai E, et al. STING mediates hepatocyte pyroptosis in liver fibrosis by epigenetically activating the NLRP3 inflammasome. Redox Biol. 2023;62:102691.
- Cridland JA, Curley EZ, Wykes MN, Schroder K, Sweet MJ, Roberts TL, et al. The mammalian PYHIN gene family: phylogeny, evolution and expression. BMC Evol Biol. 2012;12:140.
- Man SM, Karki R, Kanneganti TD. AIM2 inflammasome in infection, cancer, and autoimmunity: role in DNA sensing, inflammation, and innate immunity. Eur J Immunol. 2016;46(2):269-80.
- Liu F, Niu Q, Fan X, Liu C, Zhang J, Wei Z, et al. Priming and activation of Inflammasome by canarypox virus vector ALVAC via the cGAS/IFI16-STING-type I IFN pathway and AIM2 sensor. J Immunol. 2017;199(9):3293-305.
- Song X, Ma F, Herrup K. Accumulation of cytoplasmic DNA due to ATM deficiency activates the microglial viral response system with neurotoxic consequences. J Neurosci. 2019;39(32):6378-94.
- Man SM, Karki R, Malireddi RK, Neale G, Vogel P, Yamamoto M, et al. The transcription factor IRF1 and guanylate-binding proteins target activation of the AIM2 inflammasome by Francisella infection. Nat Immunol. 2015;16(5):467-75.
- Costa Franco MM, Marim F, Guimarães ES, Assis NRG, Cerqueira DM, Alves-Silva J, et al. Brucella abortus triggers a cGAS-independent STING pathway to induce host protection that involves guanylate-binding proteins and Inflammasome activation. J Immunol. 2018;200(2):607-22.
- Brault M, Olsen TM, Martinez J, Stetson DB, Oberst A. Intracellular nucleic acid sensing triggers necroptosis through synergistic type I IFN and TNF signaling. J Immunol (Baltimore, Md : 1950). 2018;200(8):2748-56.
- Chen D, Tong J, Yang L, Wei L, Stolz DB, Yu J, et al. PUMA amplifies necroptosis signaling by activating cytosolic DNA sensors. Proc Natl Acad Sci U S A. 2018;115(15):3930-5.
- Cui Y, Zhao D, Sreevatsan S, Liu C, Yang W, Song Z, et al. Mycobacterium bovis induces endoplasmic reticulum stress mediated-apoptosis by activating IRF3 in a murine macrophage cell line. Front Cell Infect Microbiol. 2016;6:182.
- Petrasek J, Iracheta-Vellve A, Csak T, Satishchandran A, Kodys K, KurtJones EA, et al. STING-IRF3 pathway links endoplasmic reticulum stress with hepatocyte apoptosis in early alcoholic liver disease. Proc Natl Acad Sci U S A. 2013;110(41):16544-9.
- Wu J, Chen YJ, Dobbs N, Sakai T, Liou J, Miner JJ, et al. STING-mediated disruption of calcium homeostasis chronically activates ER stress and primes T cell death. J Exp Med. 2019;216(4):867-83.
- Gurung P, Lukens JR, Kanneganti TD. Mitochondria: diversity in the regulation of the NLRP3 inflammasome. Trends Mol Med. 2015;21(3):193-201.
- West AP, Khoury-Hanold W, Staron M, Tal MC, Pineda CM, Lang SM, et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature. 2015;520(7548):553-7.
- Hopfner KP, Hornung V. Molecular mechanisms and cellular functions of cGAS-STING signalling. Nat Rev Mol Cell Biol. 2020;21(9):501-21.
- Lawrence G, Holley CL, Schroder K. Come on mtDNA, light my fire. Immunity. 2022;55(8):1331-3.
- Zhong Z, Umemura A, Sanchez-Lopez E, Liang S, Shalapour S, Wong J, et al. NF-kB restricts Inflammasome activation via elimination of damaged mitochondria. Cell. 2016;164(5):896-910.
- Xian H, Watari K, Sanchez-Lopez E, Offenberger J, Onyuru J, Sampath H, et al. Oxidized DNA fragments exit mitochondria via mPTP- and VDACdependent channels to activate NLRP3 inflammasome and interferon signaling. Immunity. 2022;55(8):1370-85.e8.
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84(1):277-359.
- Fenzl A, Kiefer FW. Brown adipose tissue and thermogenesis. Horm Mol Biol Clin Investig. 2014;19(1):25-37.
- Montanari T, Pošćić N, Colitti M. Factors involved in white-to-brown adipose tissue conversion and in thermogenesis: a review. Obes Rev. 2017;18(5):495-513.
- Lee JH, Park A, Oh KJ, Lee SC, Kim WK, Bae KH. The role of adipose tissue mitochondria: regulation of mitochondrial function for the treatment of metabolic diseases. Int J Mol Sci. 2019;20(19).
- Rosina M, Ceci V, Turchi R, Chuan L, Borcherding N, Sciarretta F, et al. Ejection of damaged mitochondria and their removal by macrophages ensure efficient thermogenesis in brown adipose tissue. Cell Metab. 2022;34(4):533-48.e12.
- Wang G, Meyer JG, Cai W, Softic S, Li ME, Verdin E, et al. Regulation of UCP1 and mitochondrial metabolism in Brown adipose tissue by reversible Succinylation. Mol Cell. 2019;74(4):844-57.e7.
- Huang Y, Zhou JH, Zhang H, Canfran-Duque A, Singh AK, Perry RJ, et al. Brown adipose TRX2 deficiency activates mtDNA-NLRP3 to impair thermogenesis and protect against diet-induced insulin resistance. J Clin Invest. 2022;132(9).
- Liu Z, Wang M, Wang X, Bu Q, Wang Q, Su W, et al. XBP1 deficiency promotes hepatocyte pyroptosis by impairing mitophagy to activate mtDNA-cGAS-STING signaling in macrophages during acute liver injury. Redox Biol. 2022;52:102305.
- Wassermann R, Gulen MF, Sala C, Perin SG, Lou Y, Rybniker J, et al. Mycobacterium tuberculosis differentially activates cGAS- and Inflammas-ome-dependent intracellular immune responses through ESX-1. Cell Host Microbe. 2015;17(6):799-810.
- Wiens KE, Ernst JD. The mechanism for type I interferon induction by mycobacterium tuberculosis is bacterial strain-dependent. PLoS Pathog. 2016;12(8):e1005809.
- Kim BR, Kim BJ, Kook YH, Kim BJ. Mycobacterium abscessus infection leads to enhanced production of type 1 interferon and NLRP3 inflammasome activation in murine macrophages via mitochondrial oxidative stress. PLoS Pathog. 2020;16(3):e1008294.
- Guarda G, Braun M, Staehli F, Tardivel A, Mattmann C, Förster I, et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 2011;34(2):213-23.
- Mayer-Barber KD, Yan B. Clash of the cytokine titans: counter-regulation of interleukin-1 and type I interferon-mediated inflammatory responses. Cell Mol Immunol. 2017;14(1):22-35.
- Kopitar-Jerala N. The role of interferons in inflammation and Inflammasome activation. Front Immunol. 2017;8:873.
- Seo GJ, Kim C, Shin WJ, Sklan EH, Eoh H, Jung JU. TRIM56-mediated monoubiquitination of cGAS for cytosolic DNA sensing. Nat Commun. 2018;9(1):613.
- Wang C, Guan Y, Lv M, Zhang R, Guo Z, Wei X, et al. Manganese increases the sensitivity of the cGAS-STING pathway for doublestranded DNA and is required for the host defense against DNA viruses. Immunity. 2018;48(4):675-87.e7.
- Zi J, Han Q, Gu S, McGrath M, Kane S, Song C, et al. Targeting NAT10 induces apoptosis associated with enhancing endoplasmic reticulum stress in acute myeloid leukemia cells. Front Oncol. 2020;10:598107.
- Liu X, Cai S, Zhang C, Liu Z, Luo J, Xing B, et al. Deacetylation of NAT10 by Sirt1 promotes the transition from rRNA biogenesis to autophagy upon energy stress. Nucleic Acids Res. 2018;46(18):9601-16.
- Denning NL, Aziz M, Gurien SD, Wang P. DAMPs and NETs in Sepsis. Front Immunol. 2019;10:2536.
- Qiao H, Chiu Y, Liang X, Xia S, Ayrapetyan M, Liu S, et al. Microglia innate immune response contributes to the antiviral defense and blood-CSF barrier function in human choroid plexus organoids during HSV-1 infection. J Med Virol. 2023;95(2):e28472.
- Konno H, Konno K, Barber GN. Cyclic dinucleotides trigger ULK1 (ATG1) phosphorylation of STING to prevent sustained innate immune signaling. Cell. 2013;155(3):688-98.
- Kimura T, Jain A, Choi SW, Mandell MA, Schroder K, Johansen T, et al. TRIM-mediated precision autophagy targets cytoplasmic regulators of innate immunity. J Cell Biol. 2015;210(6):973-89.
- Swanson KV, Junkins RD, Kurkjian CJ, Holley-Guthrie E, Pendse AA, El Morabiti R, et al. A noncanonical function of cGAMP in inflammasome priming and activation. J Exp Med. 2017;214(12):3611-26.
- Denk D, Greten FR. Inflammation: the incubator of the tumor microenvironment. Trends Cancer. 2022;8(11):901-14.
- Afify SM, Hassan G, Seno A, Seno M. Cancer-inducing niche: the force of chronic inflammation. Br J Cancer. 2022;127(2):193-201.
- Greten FR, Grivennikov SI. Inflammation and Cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27-41.
- Chen Z, Zhou L, Liu L, Hou Y, Xiong M, Yang Y, et al. Singlecell RNA sequencing highlights the role of inflammatory
cancer-associated fibroblasts in bladder urothelial carcinoma. Nat Commun. 2020;11(1):5077. - Fleming TP, Watkins AJ, Velazquez MA, Mathers JC, Prentice AM, Stephenson J, et al. Origins of lifetime health around the time of conception: causes and consequences. Lancet. 2018;391(10132):1842-52.
- Renz H, Holt PG, Inouye M, Logan AC, Prescott SL, Sly PD. An exposome perspective: early-life events and immune development in a changing world. J Allergy Clin Immunol. 2017;140(1):24-40.
- Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25(12):1822-32.
- Li Q, Cao Y, Dang C, Han B, Han R, Ma H, et al. Inhibition of doublestrand DNA-sensing cGAS ameliorates brain injury after ischemic stroke. EMBO Mol Med. 2020;12(4):e11002.
- Du Y, Hu Z, Luo Y, Wang HY, Yu X, Wang RF. Function and regulation of cGAS-STING signaling in infectious diseases. Front Immunol. 2023;14:1130423.
- Zhang D, Liu Y, Zhu Y, Zhang Q, Guan H, Liu S, et al. A non-canonical cGAS-STING-PERK pathway facilitates the translational program critical for senescence and organ fibrosis. Nat Cell Biol. 2022;24(5):766-82.
- Gulen MF, Samson N, Keller A, Schwabenland M, Liu C, Glück S, et al. cGAS-STING drives ageing-related inflammation and neurodegeneration. Nature. 2023;620(7973):374-80.
- Yan M, Li Y, Luo Q, Zeng W, Shao X, Li L, et al. Mitochondrial damage and activation of the cytosolic DNA sensor cGAS-STING pathway lead to cardiac pyroptosis and hypertrophy in diabetic cardiomyopathy mice. Cell Death Discov. 2022;8(1):258.
- Han J, Dai S, Zhong L, Shi X, Fan X, Zhong X, et al. GSDMD (Gasdermin D) Mediates Pathological Cardiac Hypertrophy and Generates a Feed-Forward Amplification Cascade via Mitochondria-STING (Stimulator of Interferon Genes) Axis. Hypertension (Dallas, Tex : 1979). 2022;79(11):2505-18.
- Lv N, Zhao Y, Liu X, Ye L, Liang Z, Kang Y, et al. Dysfunctional telomeres through mitostress-induced cGAS/STING activation to aggravate immune senescence and viral pneumonia. Aging Cell. 2022;21(4):e13594.
- Ning L, Wei W, Wenyang J, Rui X, Qing G. Cytosolic DNA-STING-NLRP3 axis is involved in murine acute lung injury induced by lipopolysaccharide. Clin Transl Med. 2020;10(7):e228.
- Long G, Gong R, Wang Q, Zhang D, Huang C. Role of released mitochondrial DNA in acute lung injury. Front Immunol. 2022;13:973089.
- Messaoud-Nacer Y, Culerier E, Rose S, Maillet I, Rouxel N, Briault S, et al. STING agonist diABZI induces PANoptosis and DNA mediated acute respiratory distress syndrome (ARDS). Cell Death Dis. 2022;13(3):269.
- Zhang Y, Li Z, Hong W, Hsu S, Wang B, Zeng Z, et al. STING-dependent sensing of self-DNA driving pyroptosis contributes to radiation-induced lung injury. Int J Radiat Oncol Biol Phys. 2023;117.
- Xu D, Tian Y, Xia Q, Ke B. The cGAS-STING pathway: novel perspectives in liver diseases. Front Immunol. 2021;12:682736.
- Wang Z, Chen N, Li Z, Xu G, Zhan X, Tang J, et al. The cytosolic DNAsensing cGAS-STING pathway in liver diseases. Front Cell Dev Biol. 2021;9:717610.
- de Carvalho RM, Szabo G. Role of the Inflammasome in liver disease. Annu Rev Pathol. 2022;17:345-65.
- Szabo G, Csak T. Inflammasomes in liver diseases. J Hepatol. 2012;57(3):642-54.
- Yu Y, Liu Y, An W, Song J, Zhang Y, Zhao X. STING-mediated inflammation in Kupffer cells contributes to progression of nonalcoholic steatohepatitis. J Clin Invest. 2019;129(2):546-55.
- Luo X, Li H, Ma L, Zhou J, Guo X, Woo SL, et al. Expression of STING is increased in liver tissues from patients with NAFLD and promotes macrophage-mediated hepatic inflammation and fibrosis in mice. Gastroenterology. 2018;155(6):1971-84.e4.
- Xian H, Liu Y, Rundberg Nilsson A, Gatchalian R, Crother TR, Tourtellotte WG, et al. Metformin inhibition of mitochondrial ATP and DNA synthesis abrogates NLRP3 inflammasome activation and pulmonary inflammation. Immunity. 2021;54(7):1463-77.e11.
- Iracheta-Vellve A, Petrasek J, Gyongyosi B, Satishchandran A, Lowe P, Kodys K, et al. Endoplasmic reticulum stress-induced hepatocellular death pathways mediate liver injury and fibrosis via stimulator of interferon genes. J Biol Chem. 2016;291(52):26794-805
- Yong H, Wang S, Song F. Activation of cGAS/STING pathway upon TDP-43-mediated mitochondrial injury may be involved in the pathogenesis of liver fibrosis. Liver Int : Off J Int Assoc Study Liver. 2021;41(8):1969-71.
- Li Y, He M, Wang Z, Duan Z, Guo Z, Wang Z, et al. STING signaling activation inhibits HBV replication and attenuates the severity of liver injury and HBV-induced fibrosis. Cell Mol Immunol. 2022;19(1):92-107.
- Gautheron J, Gores GJ, Rodrigues CMP. Lytic cell death in metabolic liver disease. J Hepatol. 2020;73(2):394-408.
- Wu J, Lin S, Wan B, Velani B, Zhu Y. Pyroptosis in liver disease: new insights into disease mechanisms. Aging Dis. 2019;10(5):1094-108.
- Wree A, Eguchi A, McGeough MD, Pena CA, Johnson CD, Canbay A, et al. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology (Baltimore, Md). 2014;59(3):898-910.
- Gaul S, Leszczynska A, Alegre F, Kaufmann B, Johnson CD, Adams LA, et al. Hepatocyte pyroptosis and release of inflammasome particles induce stellate cell activation and liver fibrosis. J Hepatol. 2021;74(1):156-67.
- Wu XY, Chen YJ, Liu CA, Gong JH, Xu XS. STING induces liver ischemiareperfusion injury by promoting calcium-dependent caspase 1-GSDMD processing in macrophages. Oxidative Med Cell Longev. 2022;2022:8123157.
- Li HY, Chien Y, Chen YJ, Chen SF, Chang YL, Chiang CH, et al. Reprogramming induced pluripotent stem cells in the absence of c-Myc for differentiation into hepatocyte-like cells. Biomaterials. 2011;32(26):5994-6005.
- Dat NQ, Thuy LTT, Hieu VN, Hai H, Hoang DV, Thi Thanh Hai N, et al. Hexa Histidine-Tagged Recombinant Human Cytoglobin Deactivates Hepatic Stellate Cells and Inhibits Liver Fibrosis by Scavenging Reactive Oxygen Species. Hepatology (Baltimore, Md). 2021;73(6):2527-45.
- Evavold CL, Hafner-Bratkovič I, Devant P, D’Andrea JM, Ngwa EM, Boršić E, et al. Control of gasdermin D oligomerization and pyroptosis by the Ragulator-rag-mTORC1 pathway. Cell. 2021;184(17):4495-511.e19.
- Jia D, Gong L, Li Y, Cao S, Zhao W, Hao L, et al. {BiW(8) O(30) } exerts antitumor effect by triggering pyroptosis and upregulating reactive oxygen species. Angewandte Chemie (International ed in English). 2021;60(39):21449-56.
- Lameire NH, Bagga A, Cruz D, De Maeseneer J, Endre Z, Kellum JA, et al. Acute kidney injury: an increasing global concern. Lancet (London, England). 2013;382(9887):170-9.
- Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, et al. Acute kidney disease and renal recovery: consensus report of the acute disease quality initiative (ADQI) 16 workgroup. Nat Rev Nephrol. 2017;13(4):241-57.
- Tsuji N, Tsuji T, Ohashi N, Kato A, Fujigaki Y, Yasuda H. Role of mitochondrial DNA in septic AKI via toll-like receptor 9. J Am Soc Nephrol : JASN. 2016;27(7):2009-20.
- Maekawa H, Inoue T, Ouchi H, Jao TM, Inoue R, Nishi H, et al. Mitochondrial damage causes inflammation via cGAS-STING signaling in acute kidney injury. Cell Rep. 2019;29(5):1261-73.e6.
- Homolová J, Janovičová L’, Konečná B, VIková B, Celec P, Tóthová L’, et al. Plasma concentrations of extracellular DNA in acute kidney injury. Diagnostics (Basel, Switzerland). 2020;10(3).
- Inoue T, Abe C, Sung SS, Moscalu S, Jankowski J, Huang L, et al. Vagus nerve stimulation mediates protection from kidney ischemiareperfusion injury through a7nAChR+ splenocytes. J Clin Invest. 2016;126(5):1939-52.
- Kojima I, Tanaka T, Inagi R, Kato H, Yamashita T, Sakiyama A, et al. Protective role of hypoxia-inducible factor-2alpha against ischemic damage and oxidative stress in the kidney. J Am Soc Nephrol : JASN. 2007;18(4):1218-26.
- Correa-Costa M, Braga TT, Semedo P, Hayashida CY, Bechara LR, Elias RM, et al. Pivotal role of toll-like receptors 2 and 4, its adaptor molecule MyD88, and inflammasome complex in experimental tubule-interstitial nephritis. PLoS One. 2011;6(12):e29004.
- Vilaysane A, Chun J, Seamone ME, Wang W, Chin R, Hirota S, et al. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J Am Soc Nephrol : JASN. 2010;21(10):1732-44.
- Gong W, Mao S, Yu J, Song J, Jia Z, Huang S, et al. NLRP3 deletion protects against renal fibrosis and attenuates mitochondrial
abnormality in mouse withnephrectomy. Am J Physiol Renal Physiol. 2016;310(10):F1081-8. - Wu J, Raman A, Coffey NJ, Sheng X, Wahba J, Seasock MJ, et al. The key role of NLRP3 and STING in APOL1-associated podocytopathy. J Clin Invest. 2021;131(20).
- Eldahshan W, Fagan SC, Ergul A. Inflammation within the neurovascular unit: focus on microglia for stroke injury and recovery. Pharmacol Res. 2019;147:104349.
- Han B, Jiang W, Cui P, Zheng K, Dang C, Wang J, et al. Microglial PGC-1 a protects against ischemic brain injury by suppressing neuroinflammation. Genome Med. 2021;13(1):47.
- Xu P, Hong Y, Xie Y, Yuan K, Li J, Sun R, et al. TREM-1 exacerbates Neuroinflammatory injury via NLRP3 Inflammasome-mediated Pyroptosis in experimental subarachnoid hemorrhage. Transl Stroke Res. 2021;12(4):643-59.
- Ran Y, Su W, Gao F, Ding Z, Yang S, Ye L, et al. Curcumin ameliorates white matter injury after ischemic stroke by inhibiting microglia/macrophage Pyroptosis through NF-kB suppression and NLRP3 Inflammasome inhibition. Oxidative Med Cell Longev. 2021;2021:1552127.
- Xu P, Zhang X, Liu Q, Xie Y, Shi X, Chen J, et al. Microglial TREM-1 receptor mediates neuroinflammatory injury via interaction with SYK in experimental ischemic stroke. Cell Death Dis. 2019;10(8):555.
- Xu S, Wang J, Zhong J, Shao M, Jiang J, Song J, et al. CD73 alleviates GSDMD-mediated microglia pyroptosis in spinal cord injury through PI3K/AKT/Foxo1 signaling. Clin Transl Med. 2021;11(1):e269.
- Ding R, Li H, Liu Y, Ou W, Zhang X, Chai H, et al. Activating cGASSTING axis contributes to neuroinflammation in CVST mouse model and induces inflammasome activation and microglia pyroptosis. J Neuroinflammation. 2022;19(1):137.
- Liu J, Zhang X, Wang H. The cGAS-STING-mediated NLRP3 inflammasome is involved in the neurotoxicity induced by manganese exposure. Biomed Pharmacother = Biomed Pharmacother. 2022;154:113680.
- Wang D, Zhang J, Jiang W, Cao Z, Zhao F, Cai T, et al. The role of NLRP3CASP1 in inflammasome-mediated neuroinflammation and autophagy dysfunction in manganese-induced, hippocampal-dependent impairment of learning and memory ability. Autophagy. 2017;13(5):914-27.
- Sarkar S, Rokad D, Malovic E, Luo J, Harischandra DS, Jin H, et al. Manganese activates NLRP3 inflammasome signaling and propagates exosomal release of ASC in microglial cells. Sci Signal. 2019;12(563).
- Nuber UA, Kriaucionis S, Roloff TC, Guy J, Selfridge J, Steinhoff C, et al. Up-regulation of glucocorticoid-regulated genes in a mouse model of Rett syndrome. Hum Mol Genet. 2005;14(15):2247-56.
- Lang F, Strutz-Seebohm N, Seebohm G, Lang UE. Significance of SGK1 in the regulation of neuronal function. J Physiol. 2010;588(Pt 18):3349-54.
- Zhang Z, Li XG, Wang ZH, Song M, Yu SP, Kang SS, et al. ઈ-secretasecleaved tau stimulates
production via upregulating STAT1-BACE1 signaling in Alzheimer’s disease. Mol Psychiatry. 2021;26(2):586-603. - Wang L, Li B, Quan MY, Li L, Chen Y, Tan GJ, et al. Mechanism of oxidative stress p38MAPK-SGK1 signaling axis in experimental autoimmune encephalomyelitis (EAE). Oncotarget. 2017;8(26):42808-16.
- Schoenebeck B, Bader V, Zhu XR, Schmitz B, Lübbert H, Stichel CC. Sgk1, a cell survival response in neurodegenerative diseases. Mol Cell Neurosci. 2005;30(2):249-64.
- Peng HY, Chen GD, Lai CY, Hsieh MC, Lin TB. Spinal serum-inducible and glucocorticoid-inducible kinase 1 mediates neuropathic pain via kalirin and downstream PSD-95-dependent NR2B phosphorylation in rats. J Neurosci. 2013;33(12):5227-40.
- Kwon OC, Song JJ, Yang Y, Kim SH, Kim JY, Seok MJ, et al. SGK1 inhibition in glia ameliorates pathologies and symptoms in Parkinson disease animal models. EMBO Mol Med. 2021;13(4):e13076.
- Hou Y, Wei Y, Lautrup S, Yang B, Wang Y, Cordonnier S, et al. NAD(+) supplementation reduces neuroinflammation and cell senescence in a transgenic mouse model of Alzheimer’s disease via cGAS-STING. Proc Natl Acad Sci U S A. 2021;118(37).
- Zhang LM, Xin Y, Wu ZY, Song RX, Miao HT, Zheng WC, et al. STING mediates neuroinflammatory response by activating NLRP3related pyroptosis in severe traumatic brain injury. J Neurochem. 2022;162(5):444-62.
- Wobma H, Shin DS, Chou J, Dedeoд
lu F. Dysregulation of the cGAS-STING pathway in monogenic autoinflammation and lupus. Front Immunol. 2022;13:905109. - Basiorka AA, McGraw KL, Eksioglu EA, Chen X, Johnson J, Zhang L, et al. The NLRP3 inflammasome functions as a driver of the myelodysplastic syndrome phenotype. Blood. 2016;128(25):2960-75.
- Zhang W, Li G, Luo R, Lei J, Song Y, Wang B, et al. Cytosolic escape of mitochondrial DNA triggers cGAS-STING-NLRP3 axis-dependent nucleus pulposus cell pyroptosis. Exp Mol Med. 2022;54(2):129-42.
- Barrera MJ, Aguilera S, Castro I, Carvajal P, Jara D, Molina C, et al. Dysfunctional mitochondria as critical players in the inflammation of autoimmune diseases: potential role in Sjögren’s syndrome. Autoimmun Rev. 2021;20(8):102867.
- Lin B, Goldbach-Mansky R. Pathogenic insights from genetic causes of autoinflammatory inflammasomopathies and interferonopathies. J Allergy Clin Immunol. 2022;149(3):819-32.
- Inokuchi S, Mitoma H, Kawano S, Ayano M, Kimoto Y, Akahoshi M, et al. Activation of caspase-1 is mediated by stimulation of interferon genes and NLR family pyrin domain containing 3 in monocytes of active systemic lupus erythematosus. Clin Exp Rheumatol. 2022;40(3):522-31.
- Kogan AA, Topper MJ, Dellomo AJ, Stojanovic L, McLaughlin LJ, Creed TM, et al. Activating STING1-dependent immune signaling in TP53 mutant and wild-type acute myeloid leukemia. Proc Natl Acad Sci U S A. 2022;119(27):e2123227119.
- Gu L, Sun Y, WuT, Chen G, Tang X, Zhao L, et al. A novel mechanism for macrophage pyroptosis in rheumatoid arthritis induced by pol
deficiency. Cell Death Dis. 2022;13(7):583. - Hou J, Karin M, Sun B. Targeting cancer-promoting inflammation – have anti-inflammatory therapies come of age? Nat Rev Clin Oncol. 2021;18(5):261-79.
- Li A, Yi M, Qin S, Song Y, Chu Q, Wu K. Activating cGAS-STING pathway for the optimal effect of cancer immunotherapy. J Hematol Oncol. 2019;12(1):35.
- Sun Y, Hu H, Liu Z, Xu J, Gao Y, Zhan X, et al. Macrophage STING signaling promotes NK cell to suppress colorectal cancer liver metastasis via 4-1BBL/4-1BB co-stimulation. J Immunother Cancer. 2023;11 (3).
- Dupaul-Chicoine J, Arabzadeh A, Dagenais M, Douglas T, Champagne C, Morizot A, et al. The NIrp3 Inflammasome suppresses colorectal Cancer metastatic growth in the liver by promoting natural killer cell Tumoricidal activity. Immunity. 2015;43(4):751-63.
- Chen Q, Boire A, Jin X, Valiente M, Er EE, Lopez-Soto A, et al. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature. 2016;533(7604):493-8.
- Sefik E, Qu R, Junqueira C, Kaffe E, Mirza H, Zhao J, et al. Inflammasome activation in infected macrophages drives COVID-19 pathology. Nature. 2022;606(7914):585-93.
- Zhao N, Di B, Xu LL. The NLRP3 inflammasome and COVID-19: activation, pathogenesis and therapeutic strategies. Cytokine Growth Factor Rev. 2021;61:2-15.
- Vora SM, Lieberman J,Wu H. Inflammasome activation at the crux of severe COVID-19. Nat Rev Immunol. 2021;21(11):694-703.
- Potere N, Del Buono MG, Caricchio R, Cremer PC, Vecchie A, Porreca E, et al. Interleukin-1 and the NLRP3 inflammasome in COVID-19: Pathogenetic and therapeutic implications. EBioMedicine. 2022;85:104299.
- Domizio JD, Gulen MF, Saidoune F, Thacker VV, Yatim A, Sharma K, et al. The cGAS-STING pathway drives type I IFN immunopathology in COVID-19. Nature. 2022;603(7899):145-51.
- Xiao R, Zhang A. Involvement of the STING signaling in COVID-19. Front Immunol. 2022;13:1006395.
- Li M, Ferretti M, Ying B, Descamps H, Lee E, Dittmar M, et al. Pharmacological activation of STING blocks SARS-CoV-2 infection. Sci Immunol. 2021;6(59).
- Zhang Y, Yan J, Hou X, Wang C, Kang DD, Xue Y, et al. STING agonist-derived LNP-mRNA vaccine enhances protective immunity against SARS-CoV-2. Nano Lett. 2023;23(7):2593-600.
- Wu Y, Zhang M, Yuan C, Ma Z, Li W, Zhang Y, et al. Progress of cGASSTING signaling in response to SARS-CoV-2 infection. Front Immunol. 2022;13:1010911.
- Wu YT, Xu WT, Zheng L, Wang S, Wei J, Liu MY, et al. 4-octyl itaconate ameliorates alveolar macrophage pyroptosis against ARDS via rescuing mitochondrial dysfunction and suppressing the cGAS/STING pathway. Int Immunopharmacol. 2023;118:110104.
- Tian Y, Bao Z, JiY, Mei X, Yang H. Epigallocatechin-3-Gallate protects H(2)O(2)induced nucleus pulposus cell apoptosis and inflammation by inhibiting cGAS/Sting/NLRP3 activation. Drug Des Devel Ther. 2020;14:2113-22.
- Meng G, Zhao S, Xie L, Han Y, Ji Y. Protein S-sulfhydration by hydrogen sulfide in cardiovascular system. Br J Pharmacol. 2018;175(8):1146-56.
- Yuan S, Shen X, Kevil CG. Beyond a Gasotransmitter: hydrogen sulfide and polysulfide in cardiovascular health and immune response. Antioxid Redox Signal. 2017;27(10):634-53.
- Mani S, Li H, Untereiner A, Wu L, Yang G, Austin RC, et al. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation. 2013;127(25):2523-34.
- Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science (New York, NY). 2008;322(5901):587-90.
- LaPenna KB, Polhemus DJ, Doiron JE, Hidalgo HA, Li Z, Lefer DJ. Hydrogen sulfide as a potential therapy for heart failure-past, present, and future. Antioxidants (Basel, Switzerland). 2021;10(3).
- Bai L, Dai J, Xia Y, He K, Xue H, Guo Q, et al. Hydrogen sulfide ameliorated high choline-induced cardiac dysfunction by inhibiting cGAS-STING-NLRP3 Inflammasome pathway. Oxidative Med Cell Longev. 2022;2022:1392896.
- Shen P, Han L, Chen G, Cheng Z, Liu Q. Emodin attenuates acetaminopheninduced hepatotoxicity via the cGAS-STING pathway. Inflammation. 2022;45(1):74-87.
- Zhang C, Song Y, Chen L, Chen P, Yuan M, Meng Y, et al. Urolithin a attenuates Hyperuricemic nephropathy in fructose-fed mice by impairing STINGNLRP3 Axis-mediated inflammatory response via restoration of Parkindependent Mitophagy. Front Pharmacol. 2022;13:907209.
- Ma Z, Ni G, Damania B. Innate sensing of DNA virus genomes. Annu RevVirol. 2018;5(1):341-62.
- SuT, Zhang Y, Valerie K, Wang XY, Lin S, Zhu G. STING activation in cancer immunotherapy. Theranostics. 2019;9(25):7759-71.
- Ming SL, Zeng L, Guo YK, Zhang S, Li GL, Ma YX, et al. The human-specific STING agonist G10 activates type I interferon and the NLRP3 Inflammasome in porcine cells. Front Immunol. 2020;11:575818.
- Gröschel MI, Sayes F, Shin SJ, Frigui W, Pawlik A, Orgeur M, et al. Recombinant BCG expressing ESX-1 of Mycobacterium marinum combines low virulence with cytosolic immune signaling and improved TB protection. Cell Rep. 2017;18(11):2752-65.
- Ma C, Ma X, Jiang B, Pan H, Liao X, Zhang L, et al. A novel inactivated wholecell Pseudomonas aeruginosa vaccine that acts through the cGAS-STING pathway. Signal Transduct Target Ther. 2021;6(1):353.
- Miura N, Shaheen SM, Akita H, Nakamura T, Harashima H. A KALA-modified lipid nanoparticle containing CpG-free plasmid DNA as a potential DNA vaccine carrier for antigen presentation and as an immune-stimulative adjuvant. Nucleic Acids Res. 2015;43(3):1317-31.
- Xu X, Fan H, Yang Y, Yao S, Yu W, Guo Z, et al. Virus-Like Particle-Induced cGAS-STING Activation and AIM2 Inflammasome-Mediated Pyroptosis for Robust Cancer Immunotherapy. Angewandte Chemie (International ed in English). 2023;135:e202303010.
- Chen D, Le SB, Hutchinson TE, Calinescu AA, Sebastian M, Jin D, et al. Tumor treating fields dually activate STING and AIM2 inflammasomes to induce adjuvant immunity in glioblastoma. J Clin Invest. 2022;132(8).
- Ling YY, Xia XY, Hao L, Wang WJ, Zhang H, Liu LY, et al. Simultaneous Photoactivation of cGAS-STING pathway and Pyroptosis by platinum(II) Triphenylamine complexes for Cancer immunotherapy. Angewandte Chemie (International ed in English). 2022;61(43):e202210988.
- Liu YG, Chen JK, Zhang ZT, Ma XJ, Chen YC, Du XM, et al. NLRP3 inflammasome activation mediates radiation-induced pyroptosis in bone marrowderived macrophages. Cell Death Dis. 2017;8(2):e2579.
- Kabiljo J, Harpain F, Carotta S, Bergmann M. Radiotherapy as a backbone for novel concepts in Cancer immunotherapy. Cancers. 2019;12(1).
- Barber GN. STING: infection, inflammation and cancer. Nat Rev Immunol. 2015;15(12):760-70.
ملاحظة الناشر
جينغوين ليو، جينغ زو ويو لينغ لوان هم المؤلفون الرئيسيون المشاركون.
*المراسلة:
جيانيون تانغ
tangjy@cdutcm.edu.cn
زهيلي وانغ
wangzl1993@outlook.com
قائمة كاملة بمعلومات المؤلف متاحة في نهاية المقال
DOI: https://doi.org/10.1186/s12964-023-01466-w
PMID: https://pubmed.ncbi.nlm.nih.gov/38195584
Publication Date: 2024-01-09
cGAS-STING, inflammasomes and pyroptosis: an overview of crosstalk mechanism of activation and regulation
Abstract
Background Intracellular DNA-sensing pathway cGAS-STING, inflammasomes and pyroptosis act as critical natural immune signaling axes for microbial infection, chronic inflammation, cancer progression and organ degeneration, but the mechanism and regulation of the crosstalk network remain unclear. Main body of the abstract Cellular stress disrupts mitochondrial homeostasis, facilitates the opening of mitochondrial permeability transition pore and the leakage of mitochondrial DNA to cell membrane, triggers inflammatory responses by activating cGAS-STING signaling, and subsequently induces inflammasomes activation and the onset of pyroptosis. Meanwhile, the inflammasome-associated protein caspase-1, Gasdermin D, the CARD domain of ASC and the potassium channel are involved in regulating cGAS-STING pathway. Importantly, this crosstalk network has a cascade amplification effect that exacerbates the immuno-inflammatory response, worsening the pathological process of inflammatory and autoimmune diseases. Given the importance of this crosstalk network of cGAS-STING, inflammasomes and pyroptosis in the regulation of innate immunity, it is emerging as a new avenue to explore the mechanisms of multiple disease pathogenesis. Therefore, efforts to define strategies to selectively modulate cGAS-STING, inflammasomes and pyroptosis in different disease settings have been or are ongoing. In this review, we will describe how this mechanistic understanding is driving possible therapeutics targeting this crosstalk network, focusing on the interacting or regulatory proteins, pathways, and a regulatory mitochondrial hub between cGASSTING, inflammasomes, and pyroptosis. Short conclusion This review aims to provide insight into the critical roles and regulatory mechanisms of the crosstalk network of cGAS-STING, inflammasomes and pyroptosis, and to highlight some promising directions for future research and intervention.
Background
Inflammasomes, such as NACHT, LRR, and PYD domains-containing protein 3 (NLRP3) and absent in melanoma 2 (AIM2), initiate the release of pro-inflammatory cytokines upon receipt of danger signals to activate the innate immune response and are essential for the clearance of pathogens or damaged cells. NLRP3 is an intracellular sensor that recognizes a wide variety of microbial motifs, endogenous danger signals and environmental irritants, triggering the formation and activation of the NLRP3 inflammasome. A two-step process of priming and activation is required for NLRP3 inflammasome [20]. In the priming stage, NF-кВ is first activated by recognition receptors such as Toll-like receptors (TLRs) that recognize pathogen-associated molecular patterns (PAMPs) or danger signaling molecular patterns
(DAMPs), followed by upregulation of NLRP3 and pro-IL-1
Inflammasomes and pyroptosis regulate cGAS-STING
AIM2 inflammasome regulates cGAS-STING

AIM2-like receptors (ALRs) regulate cGAS-STING
IFI16, a sequence-independent nuclear innate sensor ALR, was also proposed to stimulate other cellular pathways upon its binding to viral DNA [40]. Several reports assert that DNA of herpesviruses Kaposi’s sar-coma-associated herpesvirus (KSHV), Epstein-Barr virus (EBV), and herpes simplex virus 1 (HSV-1) during infection assembles an IFI16-containing oligomeric structure, leading to the production of active caspase- 1 and IL-
NLRP3 inflammasome regulates cGAS-STING
in cGAS-STING-mediated IFN-
Caspases regulate cGAS-STING
GSDMD regulates cGAS-STING
The CARD domain of ASC regulates cGAS-STING
Nod-like acceptors (NLRs) regulate cGAS-STING
cGAS-STING regulates inflammasomes and pyroptosis cGAS-STING regulates NLRP3 inflammasome and pyroptosis

Available studies indicate that STING interaction with NLRP3 in response to cytoplasmic DNA stimulation promotes NLRP3 inflammasome activation in several ways. Firstly, STING recruited NLRP3 to promote its localization in the ER, thereby promoting the formation of NLRP3 inflammasome [86]. Secondly, TM5 (151-160aa) of STING interacted with NACHT and LRR domain in NLRP3 to attenuate NLRP3 polyubiquitination associated with K48 and K63, i.e., STING deubiquitinated
cGAS-STING regulates AIM2 inflammasome
Key molecules in the crosstalk network of cGAS-STING, inflammasomes, and pyroptosis Ox-mtDNA and mtROS
[116, 117]. In addition, type I IFN-mediated generation of nitric oxide synthase (iNOS) and NO inhibited NLRP3 protein oligomerization, thereby preventing the assembly of NLRP3 inflammasome [118].
GSDMD
NAT10 and ULK1
triggered NLRP3 inflammasome activation and neutrophil pyroptosis [125]. On the other hand, ULK1 has been shown to be involved in NLRP3 autophagy, suggesting that ULK1 has a direct regulatory effect on the NLRP3 inflammasome in addition to inhibiting STING (Fig. 2) [126].
cGAMP
Diseases induced by the crosstalk network of cGAS-STING, inflammasomes, and pyroptosis
in inflammasome activation [79, 86] and GSDMDtriggered pyroptosis [135], which is characterized by the dysfunctions of the immune system and the aberrant secretion of inflammatory cytokine. As a result, the interplay among the cGAS-STING axis, inflammasome, and pyroptosis builds a wide range of important monitoring systems in response to tissue damage and pathogen invasion. Abnormalities of this crosstalk cause a variety of human diseases, including infectious diseases, autoimmune diseases, tumors, organ fibrosis and neurodegenerative diseases [11, 136-138]. In view of the critical role of cGAS-STING, inflammasomes and pyroptosis in immune and inflammatory responses, we then focused on the related diseases induced by this crosstalk network with the aim of providing clues for their prevention and treatment (Fig. 3).
Cardiac dysfunction

Acute lung injury (ALI)
Liver diseases
and enhanced cGAS-STING activation in liver tissue, while STING deficiency attenuated liver inflammation and fibrosis [153-155]. RNA sequencing of livers from mice with
Kidney diseases
was observed in multiple AKI mouse models and AKI patients [168, 170, 171]. STING knockout mice exhibited reduced renal function, tubular damage and inflammation after cisplatin treatment [168]. In addition, STING mediated secondary renal inflammation and tubular injury. STING and NLRP3 inflammasome pathways played important roles in unilateral ureteral obstruction, adenine-induced tubulointerstitial nephritis and chronic renal failure [172-174]. Expression of G2-type apolipoprotein APOL1 (G2 APOL1) in mouse kidney cells led to activation of cGAS-STING and NLRP3 inflammasome, and APOL1 expression correlated with caspase-1 and GSDMD levels [175]. In a RIAKI mouse model, although AIM2 deficiency inhibited renal macrophage pyroptosis, it surprisingly accentuated abnormal inflammation as evidenced by massive macrophage aggregation (CXCR3
Nervous system inflammation
process may be attributed to the immune stimulating activity, especially the cGAS-STING pathway [183-185]. The STING agonist CMA significant increased STING expression in microglia after subarachnoid hemorrhage (SAH) and exacerbation of neuronal damage [5]. In addition, in the brains of patients with different neurodegenerative diseases, serum/glucocorticoid-related kinase 1 (SGK1) was elevated. SGK1 expression is widely detected in the brain, and it is increased in pathologic conditions such as Rett syndrome [186], Alzheimer disease (AD) [187, 188], multiple sclerosis [189], amyotrophic lateral sclerosis [190], and neuropathic pain [191], collectively suggesting that SGK1 plays pathogenic roles in neurodegenerative disorders. Inhibition of glial SGK1 corrects the pro-inflammatory characteristics of glia by reducing intracellular NF-кB, NLRP3 inflammasome and cGASSTING mediated inflammatory pathways [192]. Activation of the cGAS-STING pathway in AD mice triggered the formation of NLRP3 inflammasome, exacerbated cellular senescence and inflammatory responses, and nicotinamide riboside (NR) treatment exerted beneficial effects through the cGAS-STING pathway [193]. Furthermore, inflammatory response-induced microglia activation was associated with neurological deficits after traumatic brain injury (TBI). In contrast, microglia cGAS-STING activation promoted neuroinflammatory responses after TBI, in part through activation of the NLRP3 inflammasome [194]. In conclusion, the cGAS-STING-NLRP3 signaling pathway may serve as a potential therapeutic target for neuroinflammation-induced neurological dysfunction.
Myelodysplastic syndrome (MDSs) and spinal injury (SCI)
with the extent of intervertebral disc degeneration by magnetic resonance imaging (MRI) and histopathology. Oxidative stress initiated the STING-dependent activation of the cGAS-STING axis and NLRP3-inflammas-ome-mediated pyroptosis in human nucleus pulposus cells [197]. Taken together, these data implicate the essential role of the cGAS-STING-NLRP3 axis and pyroptosis in the development of IVD degeneration and offer a potential treatment approach for the management of discogenic low back pain.
Autoimmune diseases
Malignant tumors
metastasis from colorectal cancer [206]. However, cGASSTING activation-mediated chronic inflammation can also promote tumor metastasis through the induction of immunosuppressive TME [9]. Cancer cell-produced cGAMP enhanced tumor growth and chemoresistance through activation of astrocyte STING and production of inflammatory cytokines [207].
COVID-19
Regulators of the crosstalk network of cGAS-STING, inflammasome, and pyroptosis
Natural products
4-Octylic acid (4-OI), an immunomodulatory derivative accumulated during macrophage activation, has attracted widespread attention for its anti-inflammatory and antioxidant properties. In vitro and in vivo experiments have shown that 4-OI inhibited the activation of the cGAS-STING-IRF3 pathway by eliminating mtROS production and mtDNA leakage in alveolar macrophages under oxidative stress, while alleviated LPS-induced NLRP3 inflammasome-mediated pyroptosis, which in turn ameliorated acute respiratory distress syndrome (ARDS) [217]. Epigallocatechin gallate (EGCG) is a catechin monomer isolated from tea and is a major component of green tea polyphenols. Advanced in vitro study that EGCG could block the activation of NLRP3 inflammasome through down-regulation of cGAS-STINGIRF3 pathway, and thus had significant protective effects against
Several studies have shown that the physiologic concentration of hydrogen sulfide
biological functions and the maintenance of homeostasis in the body [219, 220]. Conversely, the lack of endogenous
Synthetic substance

administration of the STING antagonist C-176 attenuated TBI-induced inflammatory activation of microglia and reduced pyroptosis [194].
Recombinant vaccine
induced the cGAS-STING-type I IFNs axis and activated the AIM2 and NLRP3 inflammasomes, resulting in a higher proportion of
Physical factors
Discussion and conclusion
liver, lung, kidney, spinal cord, nervous system inflammation, induces autoimmune disease and promotes the progression of malignant tumors. While refinement of our understanding of cGAS-STING, inflammasome and pyroptosis continues, targeting of this crosstalk network as a therapeutic for multiple diseases is rapidly progressing. We therefore summarize the involvement of natural products, synthetic substances, recombinant vaccines, and physical factors in regulating the cGAS-STING, inflammasomes and pyroptosis pathways crosstalk network, providing potential candidates for the treatment of related diseases. As the epitome of precision medicine in inflammatory diseases, the continued profiling, refinement and re-purposing of direct and specific modulators will drive future clinical translation.
In summary, the cGAS-STING signaling pathway generates cascade amplification effects between inflammasomes, and pyroptosis, and activates immune inflammatory responses. On the one hand, the crosstalk of these signaling pathways can affect parenchymal organs such as heart, liver, lung, and kidney, and aggravate the development process of inflammatory diseases; in addition, it is also closely related to the progression of several autoimmune diseases. Therefore, further investigations are promising to uncover novel regulatory mechanisms that may provide new opportunities for therapeutic intervention in the exciting field of the crosstalk network of cGAS-STING, inflammasomes and the pyroptosis signaling axis.
Acknowledgements
Authors’ contributions
Funding
Availability of data and materials
Declarations
Ethics approval and consent to participate
Consent for publication
Competing interests
Author details
of Sichuan Province, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu 610075, China.
Published online: 09 January 2024
References
- Ahn J, Barber GN. STING signaling and host defense against microbial infection. Exp Mol Med. 2019;51(12):1-10.
- Zhang H, You QD, Xu XL. Targeting stimulator of interferon genes (STING): a medicinal chemistry perspective. J Med Chem. 2020;63(8):3785-816.
- Cohen D, Melamed S, Millman A, Shulman G, Oppenheimer-Shaanan Y, Kacen A, et al. Cyclic GMP-AMP signalling protects bacteria against viral infection. Nature. 2019;574(7780):691-5.
- Manes NP, Nita-Lazar A. Molecular mechanisms of the toll-like receptor, STING, MAVS, Inflammasome, and Interferon Pathways. mSystems. 2021;6(3):e0033621.
- Peng Y, Zhuang J, Ying G, Zeng H, Zhou H, Cao Y, et al. Stimulator of IFN genes mediates neuroinflammatory injury by suppressing AMPK signal in experimental subarachnoid hemorrhage. J Neuroinflammation. 2020;17(1):165.
- Chen Q, Sun L, Chen ZJ. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol. 2016;17(10):1142-9.
- Zhang X, Bai XC, Chen ZJ. Structures and mechanisms in the cGASSTING innate immunity pathway. Immunity. 2020;53(1):43-53.
- Jiang M, Chen P, Wang L, Li W, Chen B, Liu Y, et al. cGAS-STING, an important pathway in cancer immunotherapy. J Hematol Oncol. 2020;13(1):81.
- Kwon J, Bakhoum SF. The cytosolic DNA-sensing cGAS-STING pathway in Cancer. Cancer Discov. 2020;10(1):26-39.
- Samson N, Ablasser A. The cGAS-STING pathway and cancer. Nat Cancer. 2022;3(12):1452-63.
- Wang Y, Luo J, Alu A, Han X, Wei Y, Wei X. cGAS-STING pathway in cancer biotherapy. Mol Cancer. 2020;19(1):136.
- Ramanjulu JM, Pesiridis GS, Yang J, Concha N, Singhaus R, Zhang SY, et al. Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature. 2018;564(7736):439-43.
- Van Herck S, Feng B, Tang L. Delivery of STING agonists for adjuvanting subunit vaccines. Adv Drug Deliv Rev. 2021;179:114020.
- Wang J, Li P, Yu Y, Fu Y, Jiang H, Lu M, et al. Pulmonary surfactant-biomimetic nanoparticles potentiate heterosubtypic influenza immunity. Science (New York, NY). 2020;367(6480).
- Luo J, Liu XP, Xiong FF, Gao FX, Yi YL, Zhang M, et al. Enhancing immune response and Heterosubtypic protection ability of inactivated H7N9 vaccine by using STING agonist as a mucosal adjuvant. Front Immunol. 2019;10:2274.
- Motwani M, Pawaria S, Bernier J, Moses S, Henry K, Fang T, et al. Hierarchy of clinical manifestations in SAVI N153S and V154M mouse models. Proc Natl Acad Sci U S A. 2019;116(16):7941-50.
- Taguchi T, Mukai K. Innate immunity signalling and membrane trafficking. Curr Opin Cell Biol. 2019;59:1-7.
- Decout A, Katz JD, Venkatraman S, Ablasser A. The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat Rev Immunol. 2021;21(9):548-69.
- Paul BD, Snyder SH, Bohr VA. Signaling by cGAS-STING in neurodegeneration, Neuroinflammation, and aging. Trends Neurosci. 2021;44(2):83-96.
- Karmakar M, Katsnelson MA, Dubyak GR, Pearlman E. Neutrophil P2X7 receptors mediate NLRP3 inflammasome-dependent IL-1
secretion in response to ATP. Nat Commun. 2016;7:10555. - Paik S, Kim JK, Silwal P, Sasakawa C, Jo EK. An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell Mol Immunol. 2021;18(5):1141-60.
- Molyvdas A, Georgopoulou U, Lazaridis N, Hytiroglou P, Dimitriadis A, Foka P, et al. The role of the NLRP3 inflammasome and the activation of IL-1
in the pathogenesis of chronic viral hepatic inflammation. Cytokine. 2018;110:389-96. - Karki R, Lee E, Sharma BR, Banoth B, Kanneganti TD. IRF8 regulates gram-negative Bacteria-mediated NLRP3 Inflammasome activation and cell death. J Immunol (Baltimore, Md : 1950). 2020;204(9):2514-22.
- Wu Y, Ren J, Zhou B, Ding C, Chen J, Wang G, et al. Gene silencing of non-obese diabetic receptor family (NLRP3) protects against the sepsis-induced hyper-bile acidaemia in a rat model. Clin Exp Immunol. 2015;179(2):277-93.
- Wang Y, Shi P, Chen Q, Huang Z, Zou D, Zhang J, et al. Mitochondrial ROS promote macrophage pyroptosis by inducing GSDMD oxidation. J Mol Cell Biol. 2019;11(12):1069-82.
- Dick MS, Sborgi L, Rühl S, Hiller S, Broz P. Corrigendum: ASC filament formation serves as a signal amplification mechanism for inflammasomes. Nat Commun. 2017;8:15030.
- Cheng Q, Pan J, Zhou ZL, Yin F, Xie HY, Chen PP, et al. Caspase-11/4 and gasdermin D-mediated pyroptosis contributes to podocyte injury in mouse diabetic nephropathy. Acta Pharmacol Sin. 2021;42(6):954-63.
- Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11(12):1136-42.
- Jorgensen I, Zhang Y, Krantz BA, Miao EA. Pyroptosis triggers poreinduced intracellular traps (PITs) that capture bacteria and lead to their clearance by efferocytosis. J Exp Med. 2016;213(10):2113-28.
- Bai B, Yang Y, Wang Q, Li M, Tian C, Liu Y, et al. NLRP3 inflammasome in endothelial dysfunction. Cell Death Dis. 2020;11(9):776.
- Aachoui Y, Leaf IA, Hagar JA, Fontana MF, Campos CG, Zak DE, et al. Caspase-11 protects against bacteria that escape the vacuole. Science (New York, NY). 2013;339(6122):975-8.
- Corrales L, Woo SR, Williams JB, McWhirter SM, Dubensky TW Jr, Gajewski TF. Antagonism of the STING pathway via activation of the AIM2 Inflammasome by intracellular DNA. J Immunol (Baltimore, Md : 1950). 2016;196(7):3191-8.
- Baatarjav C, Komada T, Karasawa T, Yamada N, Sampilvanjil A, Matsumura T, et al. dsDNA-induced AIM2 pyroptosis halts aberrant inflammation during rhabdomyolysis-induced acute kidney injury. Cell Death Differ. 2022;29(12):2487-502.
- Yan S, Shen H, Lian Q, Jin W, Zhang R, Lin X, et al. Deficiency of the AIM2-ASC signal uncovers the STING-driven Overreactive response of type I IFN and reciprocal depression of protective IFN-
immunity in mycobacterial infection. J Immunol. 2018;200(3):1016-26. - Gray EE, Winship D, Snyder JM, Child SJ, Geballe AP, Stetson DB. The AIM2-like receptors are dispensable for the interferon response to intracellular DNA. Immunity. 2016;45(2):255-66.
- Jiang H, Swacha P, Gekara NO. Nuclear AIM2-like receptors drive genotoxic tissue injury by inhibiting DNA repair. Adv Sci (Weinh). 2021;8(22):e2102534.
- Ratsimandresy RA, Dorfleutner A, Stehlik C. An update on PYRIN domain-containing pattern recognition receptors: from immunity to pathology. Front Immunol. 2013;4:440.
- Brunette RL, Young JM, Whitley DG, Brodsky IE, Malik HS, Stetson DB. Extensive evolutionary and functional diversity among mammalian AlM2-like receptors. J Exp Med. 2012;209(11):1969-83.
- Kumar V. The trinity of cGAS, TLR9, and ALRs guardians of the cellular galaxy against host-derived self-DNA. Front Immunol. 2020;11:624597.
- Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma S, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11(11):997-1004.
- Schattgen SA, Fitzgerald KA. The PYHIN protein family as mediators of host defenses. Immunol Rev. 2011;243(1):109-18.
- Nakaya Y, Lilue J, Stavrou S, Moran EA, Ross SR. AIM2-like receptors positively and negatively regulate the interferon response induced by cytosolic DNA. mBio. 2017;8(4).
- Panchanathan
, Duan , Shen , Rathinam VA, Erickson LD, Fitzgerald KA, et al. Aim2 deficiency stimulates the expression of IFN-inducible Ifi202, a lupus susceptibility murine gene within the Nba2 autoimmune susceptibility locus. J Immunol (Baltimore, Md : 1950). 2010;185(12):7385-93. - Storek KM, Gertsvolf NA, Ohlson MB, Monack DM. cGAS and Ifi204 cooperate to produce type I IFNs in response to Francisella infection. J Immunol (Baltimore, Md : 1950). 2015;194(7):3236-45.
- Bühler M, Li D, Li L, Runft S, Waltl I, Pavlou A, et al. IFNAR signaling of neuroectodermal cells is essential for the survival of C57BL/6 mice infected with Theiler’s murine encephalomyelitis virus. J Neuroinflammation. 2023;20(1):58.
- Almine JF, O’Hare CA, Dunphy G, Haga IR, Naik RJ, Atrih A, et al. IFI16 and cGAS cooperate in the activation of STING during DNA sensing in human keratinocytes. Nat Commun. 2017;8:14392.
- Lee MN, Roy M, Ong SE, Mertins P, Villani AC, Li W, et al. Identification of regulators of the innate immune response to cytosolic DNA and retroviral infection by an integrative approach. Nat Immunol. 2013;14(2):179-85.
- Cao L, Ji Y, Zeng L, Liu Q, Zhang Z, Guo S, et al. P200 family protein IFI204 negatively regulates type I interferon responses by targeting IRF7 in nucleus. PLoS Pathog. 2019;15(10):e1008079.
- Chunfa L, Xin S, Qiang L, Sreevatsan S, Yang L, Zhao D, et al. The central role of IFI204 in IFN-
release and autophagy activation during Mycobacterium bovis infection. Front Cell Infect Microbiol. 2017;7:169. - Chen W, Yu SX, Zhou FH, Zhang XJ, Gao WY, Li KY, et al. DNA sensor IFI204 contributes to host defense against Staphylococcus aureus infection in mice. Front Immunol. 2019;10:474.
- Ansari MA, Dutta S, Veettil MV, Dutta D, Iqbal J, Kumar B, et al. Herpesvirus genome recognition induced acetylation of nuclear IFI16 is essential for its cytoplasmic translocation, Inflammasome and IFN-
responses. PLoS Pathog. 2015;11(7):e1005019. - Pisano G, Roy A, Ahmed Ansari M, Kumar B, Chikoti L, Chandran B. Interferon-
-inducible protein 16 (IFI16) is required for the maintenance of Epstein-Barr virus latency. Virol J. 2017;14(1):221. - Diner BA, Lum KK, Toettcher JE, Cristea IM. Viral DNA sensors IFI16 and cyclic GMP-AMP synthase possess distinct functions in regulating viral gene expression, immune defenses, and apoptotic responses during herpesvirus infection. mBio. 2016;7(6).
- Hansen K, Prabakaran T, Laustsen A, Jørgensen SE, Rahbæk SH, Jensen SB, et al. Listeria monocytogenes induces IFN
expression through an IFI16-, cGAS- and STING-dependent pathway. EMBO J. 2014;33(15):1654-66. - Wu T, Gao J, Liu W, Cui J, Yang M, Guo W, et al. NLRP3 protects mice from radiation-induced colon and skin damage via attenuating cGAS-STING signaling. Toxicol Appl Pharmacol. 2021;418:115495.
- Zheng Y, Liu Q, Wu Y, Ma L, Zhang Z, Liu T, et al. Zika virus elicits inflammation to evade antiviral response by cleaving cGAS via NS1-caspase-1 axis. EMBO J. 2018;37(18).
- Wang Y, Ning X, Gao P, Wu S, Sha M, Lv M, et al. Inflammasome activation triggers Caspase-1-mediated cleavage of cGAS to regulate responses to DNA virus infection. Immunity. 2017;46(3):393-404.
- Aglietti RA, Estevez A, Gupta A, Ramirez MG, Liu PS, Kayagaki N, et al. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc Natl Acad Sci U S A. 2016;113(28):7858-63.
- Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535(7610):111-6.
- Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, et al. Inflamma-some-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535(7610):153-8.
- Sborgi L, Rühl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H, et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016;35(16):1766-78.
- Banerjee I, Behl B, Mendonca M, Shrivastava G, Russo AJ, Menoret A, et al. Gasdermin D restrains type I interferon response to cytosolic DNA by disrupting ionic homeostasis. Immunity. 2018;49(3):413-26. e5.
- Eren E, Berber M, Özören N. NLRC3 protein inhibits inflammation by disrupting NALP3 inflammasome assembly via competition with the adaptor protein ASC for pro-caspase-1 binding. J Biol Chem. 2017;292(30):12691-701.
- Sandstrom A, Mitchell PS, Goers L, Mu EW, Lesser CF, Vance RE. Functional degradation: a mechanism of NLRP1 inflammasome activation by diverse pathogen enzymes. Science (New York, NY). 2019;364(6435).
- Chui AJ, Okondo MC, Rao SD, Gai K, Griswold AR, Johnson DC, et al. N-terminal degradation activates the NLRP1B inflammasome. Science (New York, NY). 2019;364(6435):82-5.
- Zheng C. The emerging roles of NOD-like receptors in antiviral innate immune signaling pathways. Int J Biol Macromol. 2021;169:407-13.
- Li X, Deng M, Petrucelli AS, Zhu C, Mo J, Zhang L, et al. Viral DNA binding to NLRC3, an inhibitory nucleic acid sensor, unleashes STING, a cyclic dinucleotide receptor that activates type I interferon. Immunity. 2019;50(3):591-9.e6.
- Zhang L, Mo J, Swanson KV, Wen H, Petrucelli A, Gregory SM, et al. NLRC3, a member of the NLR family of proteins, is a negative regulator of innate immune signaling induced by the DNA sensor STING. Immunity. 2014;40(3):329-41.
- Guo H, König R, Deng M, Riess M, Mo J, Zhang L, et al. NLRX1 sequesters STING to negatively regulate the interferon response, thereby facilitating the replication of HIV-1 and DNA viruses. Cell Host Microbe. 2016;19(4):515-28.
- Yang Y, Lang X, Sun S, Gao C, Hu J, Ding S, et al. NLRP2 negatively regulates antiviral immunity by interacting with TBK1. Eur J Immunol. 2018;48(11):1817-25.
- Cui J, Li Y, Zhu L, Liu D, Songyang Z, Wang HY, et al. NLRP4 negatively regulates type I interferon signaling by targeting the kinase TBK1 for degradation via the ubiquitin ligase DTX4. Nat Immunol. 2012;13(4):387-95.
- Ellwanger K, Becker E, Kienes I, Sowa A, Postma Y, Cardona Gloria Y, et al. The NLR family pyrin domain-containing 11 protein contributes to the regulation of inflammatory signaling. J Biol Chem. 2018;293(8):2701-10.
- Abe T, Lee A, Sitharam R, Kesner J, Rabadan R, Shapira SD. Germ-cellspecific Inflammasome component NLRP14 negatively regulates cytosolic nucleic acid sensing to promote fertilization. Immunity. 2017;46(4):621-34.
- Wang P, Zhu S, Yang L, Cui S, Pan W, Jackson R, et al. NIrp6 regulates intestinal antiviral innate immunity. Science (New York, NY). 2015;350(6262):826-30.
- Zhang R, Yang W, Zhu H, Zhai J, Xue M, Zheng C. NLRC4 promotes the cGAS-STING signaling pathway by facilitating CBL-mediated K63-linked polyubiquitination of TBK1. J Med Virol. 2023;95(8):e29013.
- Sundaram B, Kanneganti TD. Advances in understanding activation and function of the NLRC4 Inflammasome. Int J Mol Sci. 2021;22(3).
- Kuenzel S, Till A, Winkler M, Häsler R, Lipinski S, Jung S, et al. The nucleo-tide-binding oligomerization domain-like receptor NLRC5 is involved in IFN-dependent antiviral immune responses. J Immunol (Baltimore, Md : 1950). 2010;184(4):1990-2000.
- Neerincx A, Lautz K, Menning M, Kremmer E, Zigrino P, Hösel M, et al. A role for the human nucleotide-binding domain, leucine-rich repeatcontaining family member NLRC5 in antiviral responses. J Biol Chem. 2010;285(34):26223-32.
- Gaidt MM, Ebert TS, Chauhan D, Ramshorn K, Pinci F, Zuber S, et al. The DNA Inflammasome in human myeloid cells is initiated by a STING-cell death program upstream of NLRP3. Cell. 2017;171(5):1110-24.e18.
- Webster SJ, Brode S, Ellis L, Fitzmaurice TJ, Elder MJ, Gekara NO, et al. Detection of a microbial metabolite by STING regulates inflammasome activation in response to chlamydia trachomatis infection. PLoS Pathog. 2017;13(6):e1006383.
- Zhong W, Rao Z, Rao J, Han G, Wang P, Jiang T, et al. Aging aggravated liver ischemia and reperfusion injury by promoting STING-mediated NLRP3 activation in macrophages. Aging Cell. 2020;19(8):e13186.
- Li N, Zhou H, Wu H, Wu Q, Duan M, Deng W, et al. STING-IRF3 contributes to lipopolysaccharide-induced cardiac dysfunction, inflammation, apoptosis and pyroptosis by activating NLRP3. Redox Biol. 2019;24:101215.
- Zhang H, Chen Z, Zhou J, Gu J, Wu H, Jiang Y, et al. NAT10 regulates neutrophil pyroptosis in sepsis via acetylating ULK1 RNA and activating STING pathway. Commun Biol. 2022;5(1):916.
- McLemore AF, Hou HA, Meyer BS, Lam NB, Ward GA, Aldrich AL, et al. Somatic gene mutations expose cytoplasmic DNA to co-opt the cGAS/ STING/NLRP3 axis in myelodysplastic syndromes. JCI Insight. 2022;7(15).
- Aits S, Jäättelä M. Lysosomal cell death at a glance. J Cell Sci. 2013;126(Pt 9):1905-12.
- Wang W, Hu D, Wu C, Feng Y, Li A, Liu W, et al. STING promotes NLRP3 localization in ER and facilitates NLRP3 deubiquitination to
activate the inflammasome upon HSV-1 infection. PLoS Pathog. 2020;16(3):e1008335. - Xiao Y, Zhao C, Tai Y, Li B, Lan T, Lai E, et al. STING mediates hepatocyte pyroptosis in liver fibrosis by epigenetically activating the NLRP3 inflammasome. Redox Biol. 2023;62:102691.
- Cridland JA, Curley EZ, Wykes MN, Schroder K, Sweet MJ, Roberts TL, et al. The mammalian PYHIN gene family: phylogeny, evolution and expression. BMC Evol Biol. 2012;12:140.
- Man SM, Karki R, Kanneganti TD. AIM2 inflammasome in infection, cancer, and autoimmunity: role in DNA sensing, inflammation, and innate immunity. Eur J Immunol. 2016;46(2):269-80.
- Liu F, Niu Q, Fan X, Liu C, Zhang J, Wei Z, et al. Priming and activation of Inflammasome by canarypox virus vector ALVAC via the cGAS/IFI16-STING-type I IFN pathway and AIM2 sensor. J Immunol. 2017;199(9):3293-305.
- Song X, Ma F, Herrup K. Accumulation of cytoplasmic DNA due to ATM deficiency activates the microglial viral response system with neurotoxic consequences. J Neurosci. 2019;39(32):6378-94.
- Man SM, Karki R, Malireddi RK, Neale G, Vogel P, Yamamoto M, et al. The transcription factor IRF1 and guanylate-binding proteins target activation of the AIM2 inflammasome by Francisella infection. Nat Immunol. 2015;16(5):467-75.
- Costa Franco MM, Marim F, Guimarães ES, Assis NRG, Cerqueira DM, Alves-Silva J, et al. Brucella abortus triggers a cGAS-independent STING pathway to induce host protection that involves guanylate-binding proteins and Inflammasome activation. J Immunol. 2018;200(2):607-22.
- Brault M, Olsen TM, Martinez J, Stetson DB, Oberst A. Intracellular nucleic acid sensing triggers necroptosis through synergistic type I IFN and TNF signaling. J Immunol (Baltimore, Md : 1950). 2018;200(8):2748-56.
- Chen D, Tong J, Yang L, Wei L, Stolz DB, Yu J, et al. PUMA amplifies necroptosis signaling by activating cytosolic DNA sensors. Proc Natl Acad Sci U S A. 2018;115(15):3930-5.
- Cui Y, Zhao D, Sreevatsan S, Liu C, Yang W, Song Z, et al. Mycobacterium bovis induces endoplasmic reticulum stress mediated-apoptosis by activating IRF3 in a murine macrophage cell line. Front Cell Infect Microbiol. 2016;6:182.
- Petrasek J, Iracheta-Vellve A, Csak T, Satishchandran A, Kodys K, KurtJones EA, et al. STING-IRF3 pathway links endoplasmic reticulum stress with hepatocyte apoptosis in early alcoholic liver disease. Proc Natl Acad Sci U S A. 2013;110(41):16544-9.
- Wu J, Chen YJ, Dobbs N, Sakai T, Liou J, Miner JJ, et al. STING-mediated disruption of calcium homeostasis chronically activates ER stress and primes T cell death. J Exp Med. 2019;216(4):867-83.
- Gurung P, Lukens JR, Kanneganti TD. Mitochondria: diversity in the regulation of the NLRP3 inflammasome. Trends Mol Med. 2015;21(3):193-201.
- West AP, Khoury-Hanold W, Staron M, Tal MC, Pineda CM, Lang SM, et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature. 2015;520(7548):553-7.
- Hopfner KP, Hornung V. Molecular mechanisms and cellular functions of cGAS-STING signalling. Nat Rev Mol Cell Biol. 2020;21(9):501-21.
- Lawrence G, Holley CL, Schroder K. Come on mtDNA, light my fire. Immunity. 2022;55(8):1331-3.
- Zhong Z, Umemura A, Sanchez-Lopez E, Liang S, Shalapour S, Wong J, et al. NF-kB restricts Inflammasome activation via elimination of damaged mitochondria. Cell. 2016;164(5):896-910.
- Xian H, Watari K, Sanchez-Lopez E, Offenberger J, Onyuru J, Sampath H, et al. Oxidized DNA fragments exit mitochondria via mPTP- and VDACdependent channels to activate NLRP3 inflammasome and interferon signaling. Immunity. 2022;55(8):1370-85.e8.
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84(1):277-359.
- Fenzl A, Kiefer FW. Brown adipose tissue and thermogenesis. Horm Mol Biol Clin Investig. 2014;19(1):25-37.
- Montanari T, Pošćić N, Colitti M. Factors involved in white-to-brown adipose tissue conversion and in thermogenesis: a review. Obes Rev. 2017;18(5):495-513.
- Lee JH, Park A, Oh KJ, Lee SC, Kim WK, Bae KH. The role of adipose tissue mitochondria: regulation of mitochondrial function for the treatment of metabolic diseases. Int J Mol Sci. 2019;20(19).
- Rosina M, Ceci V, Turchi R, Chuan L, Borcherding N, Sciarretta F, et al. Ejection of damaged mitochondria and their removal by macrophages ensure efficient thermogenesis in brown adipose tissue. Cell Metab. 2022;34(4):533-48.e12.
- Wang G, Meyer JG, Cai W, Softic S, Li ME, Verdin E, et al. Regulation of UCP1 and mitochondrial metabolism in Brown adipose tissue by reversible Succinylation. Mol Cell. 2019;74(4):844-57.e7.
- Huang Y, Zhou JH, Zhang H, Canfran-Duque A, Singh AK, Perry RJ, et al. Brown adipose TRX2 deficiency activates mtDNA-NLRP3 to impair thermogenesis and protect against diet-induced insulin resistance. J Clin Invest. 2022;132(9).
- Liu Z, Wang M, Wang X, Bu Q, Wang Q, Su W, et al. XBP1 deficiency promotes hepatocyte pyroptosis by impairing mitophagy to activate mtDNA-cGAS-STING signaling in macrophages during acute liver injury. Redox Biol. 2022;52:102305.
- Wassermann R, Gulen MF, Sala C, Perin SG, Lou Y, Rybniker J, et al. Mycobacterium tuberculosis differentially activates cGAS- and Inflammas-ome-dependent intracellular immune responses through ESX-1. Cell Host Microbe. 2015;17(6):799-810.
- Wiens KE, Ernst JD. The mechanism for type I interferon induction by mycobacterium tuberculosis is bacterial strain-dependent. PLoS Pathog. 2016;12(8):e1005809.
- Kim BR, Kim BJ, Kook YH, Kim BJ. Mycobacterium abscessus infection leads to enhanced production of type 1 interferon and NLRP3 inflammasome activation in murine macrophages via mitochondrial oxidative stress. PLoS Pathog. 2020;16(3):e1008294.
- Guarda G, Braun M, Staehli F, Tardivel A, Mattmann C, Förster I, et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 2011;34(2):213-23.
- Mayer-Barber KD, Yan B. Clash of the cytokine titans: counter-regulation of interleukin-1 and type I interferon-mediated inflammatory responses. Cell Mol Immunol. 2017;14(1):22-35.
- Kopitar-Jerala N. The role of interferons in inflammation and Inflammasome activation. Front Immunol. 2017;8:873.
- Seo GJ, Kim C, Shin WJ, Sklan EH, Eoh H, Jung JU. TRIM56-mediated monoubiquitination of cGAS for cytosolic DNA sensing. Nat Commun. 2018;9(1):613.
- Wang C, Guan Y, Lv M, Zhang R, Guo Z, Wei X, et al. Manganese increases the sensitivity of the cGAS-STING pathway for doublestranded DNA and is required for the host defense against DNA viruses. Immunity. 2018;48(4):675-87.e7.
- Zi J, Han Q, Gu S, McGrath M, Kane S, Song C, et al. Targeting NAT10 induces apoptosis associated with enhancing endoplasmic reticulum stress in acute myeloid leukemia cells. Front Oncol. 2020;10:598107.
- Liu X, Cai S, Zhang C, Liu Z, Luo J, Xing B, et al. Deacetylation of NAT10 by Sirt1 promotes the transition from rRNA biogenesis to autophagy upon energy stress. Nucleic Acids Res. 2018;46(18):9601-16.
- Denning NL, Aziz M, Gurien SD, Wang P. DAMPs and NETs in Sepsis. Front Immunol. 2019;10:2536.
- Qiao H, Chiu Y, Liang X, Xia S, Ayrapetyan M, Liu S, et al. Microglia innate immune response contributes to the antiviral defense and blood-CSF barrier function in human choroid plexus organoids during HSV-1 infection. J Med Virol. 2023;95(2):e28472.
- Konno H, Konno K, Barber GN. Cyclic dinucleotides trigger ULK1 (ATG1) phosphorylation of STING to prevent sustained innate immune signaling. Cell. 2013;155(3):688-98.
- Kimura T, Jain A, Choi SW, Mandell MA, Schroder K, Johansen T, et al. TRIM-mediated precision autophagy targets cytoplasmic regulators of innate immunity. J Cell Biol. 2015;210(6):973-89.
- Swanson KV, Junkins RD, Kurkjian CJ, Holley-Guthrie E, Pendse AA, El Morabiti R, et al. A noncanonical function of cGAMP in inflammasome priming and activation. J Exp Med. 2017;214(12):3611-26.
- Denk D, Greten FR. Inflammation: the incubator of the tumor microenvironment. Trends Cancer. 2022;8(11):901-14.
- Afify SM, Hassan G, Seno A, Seno M. Cancer-inducing niche: the force of chronic inflammation. Br J Cancer. 2022;127(2):193-201.
- Greten FR, Grivennikov SI. Inflammation and Cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27-41.
- Chen Z, Zhou L, Liu L, Hou Y, Xiong M, Yang Y, et al. Singlecell RNA sequencing highlights the role of inflammatory
cancer-associated fibroblasts in bladder urothelial carcinoma. Nat Commun. 2020;11(1):5077. - Fleming TP, Watkins AJ, Velazquez MA, Mathers JC, Prentice AM, Stephenson J, et al. Origins of lifetime health around the time of conception: causes and consequences. Lancet. 2018;391(10132):1842-52.
- Renz H, Holt PG, Inouye M, Logan AC, Prescott SL, Sly PD. An exposome perspective: early-life events and immune development in a changing world. J Allergy Clin Immunol. 2017;140(1):24-40.
- Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25(12):1822-32.
- Li Q, Cao Y, Dang C, Han B, Han R, Ma H, et al. Inhibition of doublestrand DNA-sensing cGAS ameliorates brain injury after ischemic stroke. EMBO Mol Med. 2020;12(4):e11002.
- Du Y, Hu Z, Luo Y, Wang HY, Yu X, Wang RF. Function and regulation of cGAS-STING signaling in infectious diseases. Front Immunol. 2023;14:1130423.
- Zhang D, Liu Y, Zhu Y, Zhang Q, Guan H, Liu S, et al. A non-canonical cGAS-STING-PERK pathway facilitates the translational program critical for senescence and organ fibrosis. Nat Cell Biol. 2022;24(5):766-82.
- Gulen MF, Samson N, Keller A, Schwabenland M, Liu C, Glück S, et al. cGAS-STING drives ageing-related inflammation and neurodegeneration. Nature. 2023;620(7973):374-80.
- Yan M, Li Y, Luo Q, Zeng W, Shao X, Li L, et al. Mitochondrial damage and activation of the cytosolic DNA sensor cGAS-STING pathway lead to cardiac pyroptosis and hypertrophy in diabetic cardiomyopathy mice. Cell Death Discov. 2022;8(1):258.
- Han J, Dai S, Zhong L, Shi X, Fan X, Zhong X, et al. GSDMD (Gasdermin D) Mediates Pathological Cardiac Hypertrophy and Generates a Feed-Forward Amplification Cascade via Mitochondria-STING (Stimulator of Interferon Genes) Axis. Hypertension (Dallas, Tex : 1979). 2022;79(11):2505-18.
- Lv N, Zhao Y, Liu X, Ye L, Liang Z, Kang Y, et al. Dysfunctional telomeres through mitostress-induced cGAS/STING activation to aggravate immune senescence and viral pneumonia. Aging Cell. 2022;21(4):e13594.
- Ning L, Wei W, Wenyang J, Rui X, Qing G. Cytosolic DNA-STING-NLRP3 axis is involved in murine acute lung injury induced by lipopolysaccharide. Clin Transl Med. 2020;10(7):e228.
- Long G, Gong R, Wang Q, Zhang D, Huang C. Role of released mitochondrial DNA in acute lung injury. Front Immunol. 2022;13:973089.
- Messaoud-Nacer Y, Culerier E, Rose S, Maillet I, Rouxel N, Briault S, et al. STING agonist diABZI induces PANoptosis and DNA mediated acute respiratory distress syndrome (ARDS). Cell Death Dis. 2022;13(3):269.
- Zhang Y, Li Z, Hong W, Hsu S, Wang B, Zeng Z, et al. STING-dependent sensing of self-DNA driving pyroptosis contributes to radiation-induced lung injury. Int J Radiat Oncol Biol Phys. 2023;117.
- Xu D, Tian Y, Xia Q, Ke B. The cGAS-STING pathway: novel perspectives in liver diseases. Front Immunol. 2021;12:682736.
- Wang Z, Chen N, Li Z, Xu G, Zhan X, Tang J, et al. The cytosolic DNAsensing cGAS-STING pathway in liver diseases. Front Cell Dev Biol. 2021;9:717610.
- de Carvalho RM, Szabo G. Role of the Inflammasome in liver disease. Annu Rev Pathol. 2022;17:345-65.
- Szabo G, Csak T. Inflammasomes in liver diseases. J Hepatol. 2012;57(3):642-54.
- Yu Y, Liu Y, An W, Song J, Zhang Y, Zhao X. STING-mediated inflammation in Kupffer cells contributes to progression of nonalcoholic steatohepatitis. J Clin Invest. 2019;129(2):546-55.
- Luo X, Li H, Ma L, Zhou J, Guo X, Woo SL, et al. Expression of STING is increased in liver tissues from patients with NAFLD and promotes macrophage-mediated hepatic inflammation and fibrosis in mice. Gastroenterology. 2018;155(6):1971-84.e4.
- Xian H, Liu Y, Rundberg Nilsson A, Gatchalian R, Crother TR, Tourtellotte WG, et al. Metformin inhibition of mitochondrial ATP and DNA synthesis abrogates NLRP3 inflammasome activation and pulmonary inflammation. Immunity. 2021;54(7):1463-77.e11.
- Iracheta-Vellve A, Petrasek J, Gyongyosi B, Satishchandran A, Lowe P, Kodys K, et al. Endoplasmic reticulum stress-induced hepatocellular death pathways mediate liver injury and fibrosis via stimulator of interferon genes. J Biol Chem. 2016;291(52):26794-805
- Yong H, Wang S, Song F. Activation of cGAS/STING pathway upon TDP-43-mediated mitochondrial injury may be involved in the pathogenesis of liver fibrosis. Liver Int : Off J Int Assoc Study Liver. 2021;41(8):1969-71.
- Li Y, He M, Wang Z, Duan Z, Guo Z, Wang Z, et al. STING signaling activation inhibits HBV replication and attenuates the severity of liver injury and HBV-induced fibrosis. Cell Mol Immunol. 2022;19(1):92-107.
- Gautheron J, Gores GJ, Rodrigues CMP. Lytic cell death in metabolic liver disease. J Hepatol. 2020;73(2):394-408.
- Wu J, Lin S, Wan B, Velani B, Zhu Y. Pyroptosis in liver disease: new insights into disease mechanisms. Aging Dis. 2019;10(5):1094-108.
- Wree A, Eguchi A, McGeough MD, Pena CA, Johnson CD, Canbay A, et al. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology (Baltimore, Md). 2014;59(3):898-910.
- Gaul S, Leszczynska A, Alegre F, Kaufmann B, Johnson CD, Adams LA, et al. Hepatocyte pyroptosis and release of inflammasome particles induce stellate cell activation and liver fibrosis. J Hepatol. 2021;74(1):156-67.
- Wu XY, Chen YJ, Liu CA, Gong JH, Xu XS. STING induces liver ischemiareperfusion injury by promoting calcium-dependent caspase 1-GSDMD processing in macrophages. Oxidative Med Cell Longev. 2022;2022:8123157.
- Li HY, Chien Y, Chen YJ, Chen SF, Chang YL, Chiang CH, et al. Reprogramming induced pluripotent stem cells in the absence of c-Myc for differentiation into hepatocyte-like cells. Biomaterials. 2011;32(26):5994-6005.
- Dat NQ, Thuy LTT, Hieu VN, Hai H, Hoang DV, Thi Thanh Hai N, et al. Hexa Histidine-Tagged Recombinant Human Cytoglobin Deactivates Hepatic Stellate Cells and Inhibits Liver Fibrosis by Scavenging Reactive Oxygen Species. Hepatology (Baltimore, Md). 2021;73(6):2527-45.
- Evavold CL, Hafner-Bratkovič I, Devant P, D’Andrea JM, Ngwa EM, Boršić E, et al. Control of gasdermin D oligomerization and pyroptosis by the Ragulator-rag-mTORC1 pathway. Cell. 2021;184(17):4495-511.e19.
- Jia D, Gong L, Li Y, Cao S, Zhao W, Hao L, et al. {BiW(8) O(30) } exerts antitumor effect by triggering pyroptosis and upregulating reactive oxygen species. Angewandte Chemie (International ed in English). 2021;60(39):21449-56.
- Lameire NH, Bagga A, Cruz D, De Maeseneer J, Endre Z, Kellum JA, et al. Acute kidney injury: an increasing global concern. Lancet (London, England). 2013;382(9887):170-9.
- Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, et al. Acute kidney disease and renal recovery: consensus report of the acute disease quality initiative (ADQI) 16 workgroup. Nat Rev Nephrol. 2017;13(4):241-57.
- Tsuji N, Tsuji T, Ohashi N, Kato A, Fujigaki Y, Yasuda H. Role of mitochondrial DNA in septic AKI via toll-like receptor 9. J Am Soc Nephrol : JASN. 2016;27(7):2009-20.
- Maekawa H, Inoue T, Ouchi H, Jao TM, Inoue R, Nishi H, et al. Mitochondrial damage causes inflammation via cGAS-STING signaling in acute kidney injury. Cell Rep. 2019;29(5):1261-73.e6.
- Homolová J, Janovičová L’, Konečná B, VIková B, Celec P, Tóthová L’, et al. Plasma concentrations of extracellular DNA in acute kidney injury. Diagnostics (Basel, Switzerland). 2020;10(3).
- Inoue T, Abe C, Sung SS, Moscalu S, Jankowski J, Huang L, et al. Vagus nerve stimulation mediates protection from kidney ischemiareperfusion injury through a7nAChR+ splenocytes. J Clin Invest. 2016;126(5):1939-52.
- Kojima I, Tanaka T, Inagi R, Kato H, Yamashita T, Sakiyama A, et al. Protective role of hypoxia-inducible factor-2alpha against ischemic damage and oxidative stress in the kidney. J Am Soc Nephrol : JASN. 2007;18(4):1218-26.
- Correa-Costa M, Braga TT, Semedo P, Hayashida CY, Bechara LR, Elias RM, et al. Pivotal role of toll-like receptors 2 and 4, its adaptor molecule MyD88, and inflammasome complex in experimental tubule-interstitial nephritis. PLoS One. 2011;6(12):e29004.
- Vilaysane A, Chun J, Seamone ME, Wang W, Chin R, Hirota S, et al. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J Am Soc Nephrol : JASN. 2010;21(10):1732-44.
- Gong W, Mao S, Yu J, Song J, Jia Z, Huang S, et al. NLRP3 deletion protects against renal fibrosis and attenuates mitochondrial
abnormality in mouse withnephrectomy. Am J Physiol Renal Physiol. 2016;310(10):F1081-8. - Wu J, Raman A, Coffey NJ, Sheng X, Wahba J, Seasock MJ, et al. The key role of NLRP3 and STING in APOL1-associated podocytopathy. J Clin Invest. 2021;131(20).
- Eldahshan W, Fagan SC, Ergul A. Inflammation within the neurovascular unit: focus on microglia for stroke injury and recovery. Pharmacol Res. 2019;147:104349.
- Han B, Jiang W, Cui P, Zheng K, Dang C, Wang J, et al. Microglial PGC-1 a protects against ischemic brain injury by suppressing neuroinflammation. Genome Med. 2021;13(1):47.
- Xu P, Hong Y, Xie Y, Yuan K, Li J, Sun R, et al. TREM-1 exacerbates Neuroinflammatory injury via NLRP3 Inflammasome-mediated Pyroptosis in experimental subarachnoid hemorrhage. Transl Stroke Res. 2021;12(4):643-59.
- Ran Y, Su W, Gao F, Ding Z, Yang S, Ye L, et al. Curcumin ameliorates white matter injury after ischemic stroke by inhibiting microglia/macrophage Pyroptosis through NF-kB suppression and NLRP3 Inflammasome inhibition. Oxidative Med Cell Longev. 2021;2021:1552127.
- Xu P, Zhang X, Liu Q, Xie Y, Shi X, Chen J, et al. Microglial TREM-1 receptor mediates neuroinflammatory injury via interaction with SYK in experimental ischemic stroke. Cell Death Dis. 2019;10(8):555.
- Xu S, Wang J, Zhong J, Shao M, Jiang J, Song J, et al. CD73 alleviates GSDMD-mediated microglia pyroptosis in spinal cord injury through PI3K/AKT/Foxo1 signaling. Clin Transl Med. 2021;11(1):e269.
- Ding R, Li H, Liu Y, Ou W, Zhang X, Chai H, et al. Activating cGASSTING axis contributes to neuroinflammation in CVST mouse model and induces inflammasome activation and microglia pyroptosis. J Neuroinflammation. 2022;19(1):137.
- Liu J, Zhang X, Wang H. The cGAS-STING-mediated NLRP3 inflammasome is involved in the neurotoxicity induced by manganese exposure. Biomed Pharmacother = Biomed Pharmacother. 2022;154:113680.
- Wang D, Zhang J, Jiang W, Cao Z, Zhao F, Cai T, et al. The role of NLRP3CASP1 in inflammasome-mediated neuroinflammation and autophagy dysfunction in manganese-induced, hippocampal-dependent impairment of learning and memory ability. Autophagy. 2017;13(5):914-27.
- Sarkar S, Rokad D, Malovic E, Luo J, Harischandra DS, Jin H, et al. Manganese activates NLRP3 inflammasome signaling and propagates exosomal release of ASC in microglial cells. Sci Signal. 2019;12(563).
- Nuber UA, Kriaucionis S, Roloff TC, Guy J, Selfridge J, Steinhoff C, et al. Up-regulation of glucocorticoid-regulated genes in a mouse model of Rett syndrome. Hum Mol Genet. 2005;14(15):2247-56.
- Lang F, Strutz-Seebohm N, Seebohm G, Lang UE. Significance of SGK1 in the regulation of neuronal function. J Physiol. 2010;588(Pt 18):3349-54.
- Zhang Z, Li XG, Wang ZH, Song M, Yu SP, Kang SS, et al. ઈ-secretasecleaved tau stimulates
production via upregulating STAT1-BACE1 signaling in Alzheimer’s disease. Mol Psychiatry. 2021;26(2):586-603. - Wang L, Li B, Quan MY, Li L, Chen Y, Tan GJ, et al. Mechanism of oxidative stress p38MAPK-SGK1 signaling axis in experimental autoimmune encephalomyelitis (EAE). Oncotarget. 2017;8(26):42808-16.
- Schoenebeck B, Bader V, Zhu XR, Schmitz B, Lübbert H, Stichel CC. Sgk1, a cell survival response in neurodegenerative diseases. Mol Cell Neurosci. 2005;30(2):249-64.
- Peng HY, Chen GD, Lai CY, Hsieh MC, Lin TB. Spinal serum-inducible and glucocorticoid-inducible kinase 1 mediates neuropathic pain via kalirin and downstream PSD-95-dependent NR2B phosphorylation in rats. J Neurosci. 2013;33(12):5227-40.
- Kwon OC, Song JJ, Yang Y, Kim SH, Kim JY, Seok MJ, et al. SGK1 inhibition in glia ameliorates pathologies and symptoms in Parkinson disease animal models. EMBO Mol Med. 2021;13(4):e13076.
- Hou Y, Wei Y, Lautrup S, Yang B, Wang Y, Cordonnier S, et al. NAD(+) supplementation reduces neuroinflammation and cell senescence in a transgenic mouse model of Alzheimer’s disease via cGAS-STING. Proc Natl Acad Sci U S A. 2021;118(37).
- Zhang LM, Xin Y, Wu ZY, Song RX, Miao HT, Zheng WC, et al. STING mediates neuroinflammatory response by activating NLRP3related pyroptosis in severe traumatic brain injury. J Neurochem. 2022;162(5):444-62.
- Wobma H, Shin DS, Chou J, Dedeoд
lu F. Dysregulation of the cGAS-STING pathway in monogenic autoinflammation and lupus. Front Immunol. 2022;13:905109. - Basiorka AA, McGraw KL, Eksioglu EA, Chen X, Johnson J, Zhang L, et al. The NLRP3 inflammasome functions as a driver of the myelodysplastic syndrome phenotype. Blood. 2016;128(25):2960-75.
- Zhang W, Li G, Luo R, Lei J, Song Y, Wang B, et al. Cytosolic escape of mitochondrial DNA triggers cGAS-STING-NLRP3 axis-dependent nucleus pulposus cell pyroptosis. Exp Mol Med. 2022;54(2):129-42.
- Barrera MJ, Aguilera S, Castro I, Carvajal P, Jara D, Molina C, et al. Dysfunctional mitochondria as critical players in the inflammation of autoimmune diseases: potential role in Sjögren’s syndrome. Autoimmun Rev. 2021;20(8):102867.
- Lin B, Goldbach-Mansky R. Pathogenic insights from genetic causes of autoinflammatory inflammasomopathies and interferonopathies. J Allergy Clin Immunol. 2022;149(3):819-32.
- Inokuchi S, Mitoma H, Kawano S, Ayano M, Kimoto Y, Akahoshi M, et al. Activation of caspase-1 is mediated by stimulation of interferon genes and NLR family pyrin domain containing 3 in monocytes of active systemic lupus erythematosus. Clin Exp Rheumatol. 2022;40(3):522-31.
- Kogan AA, Topper MJ, Dellomo AJ, Stojanovic L, McLaughlin LJ, Creed TM, et al. Activating STING1-dependent immune signaling in TP53 mutant and wild-type acute myeloid leukemia. Proc Natl Acad Sci U S A. 2022;119(27):e2123227119.
- Gu L, Sun Y, WuT, Chen G, Tang X, Zhao L, et al. A novel mechanism for macrophage pyroptosis in rheumatoid arthritis induced by pol
deficiency. Cell Death Dis. 2022;13(7):583. - Hou J, Karin M, Sun B. Targeting cancer-promoting inflammation – have anti-inflammatory therapies come of age? Nat Rev Clin Oncol. 2021;18(5):261-79.
- Li A, Yi M, Qin S, Song Y, Chu Q, Wu K. Activating cGAS-STING pathway for the optimal effect of cancer immunotherapy. J Hematol Oncol. 2019;12(1):35.
- Sun Y, Hu H, Liu Z, Xu J, Gao Y, Zhan X, et al. Macrophage STING signaling promotes NK cell to suppress colorectal cancer liver metastasis via 4-1BBL/4-1BB co-stimulation. J Immunother Cancer. 2023;11 (3).
- Dupaul-Chicoine J, Arabzadeh A, Dagenais M, Douglas T, Champagne C, Morizot A, et al. The NIrp3 Inflammasome suppresses colorectal Cancer metastatic growth in the liver by promoting natural killer cell Tumoricidal activity. Immunity. 2015;43(4):751-63.
- Chen Q, Boire A, Jin X, Valiente M, Er EE, Lopez-Soto A, et al. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature. 2016;533(7604):493-8.
- Sefik E, Qu R, Junqueira C, Kaffe E, Mirza H, Zhao J, et al. Inflammasome activation in infected macrophages drives COVID-19 pathology. Nature. 2022;606(7914):585-93.
- Zhao N, Di B, Xu LL. The NLRP3 inflammasome and COVID-19: activation, pathogenesis and therapeutic strategies. Cytokine Growth Factor Rev. 2021;61:2-15.
- Vora SM, Lieberman J,Wu H. Inflammasome activation at the crux of severe COVID-19. Nat Rev Immunol. 2021;21(11):694-703.
- Potere N, Del Buono MG, Caricchio R, Cremer PC, Vecchie A, Porreca E, et al. Interleukin-1 and the NLRP3 inflammasome in COVID-19: Pathogenetic and therapeutic implications. EBioMedicine. 2022;85:104299.
- Domizio JD, Gulen MF, Saidoune F, Thacker VV, Yatim A, Sharma K, et al. The cGAS-STING pathway drives type I IFN immunopathology in COVID-19. Nature. 2022;603(7899):145-51.
- Xiao R, Zhang A. Involvement of the STING signaling in COVID-19. Front Immunol. 2022;13:1006395.
- Li M, Ferretti M, Ying B, Descamps H, Lee E, Dittmar M, et al. Pharmacological activation of STING blocks SARS-CoV-2 infection. Sci Immunol. 2021;6(59).
- Zhang Y, Yan J, Hou X, Wang C, Kang DD, Xue Y, et al. STING agonist-derived LNP-mRNA vaccine enhances protective immunity against SARS-CoV-2. Nano Lett. 2023;23(7):2593-600.
- Wu Y, Zhang M, Yuan C, Ma Z, Li W, Zhang Y, et al. Progress of cGASSTING signaling in response to SARS-CoV-2 infection. Front Immunol. 2022;13:1010911.
- Wu YT, Xu WT, Zheng L, Wang S, Wei J, Liu MY, et al. 4-octyl itaconate ameliorates alveolar macrophage pyroptosis against ARDS via rescuing mitochondrial dysfunction and suppressing the cGAS/STING pathway. Int Immunopharmacol. 2023;118:110104.
- Tian Y, Bao Z, JiY, Mei X, Yang H. Epigallocatechin-3-Gallate protects H(2)O(2)induced nucleus pulposus cell apoptosis and inflammation by inhibiting cGAS/Sting/NLRP3 activation. Drug Des Devel Ther. 2020;14:2113-22.
- Meng G, Zhao S, Xie L, Han Y, Ji Y. Protein S-sulfhydration by hydrogen sulfide in cardiovascular system. Br J Pharmacol. 2018;175(8):1146-56.
- Yuan S, Shen X, Kevil CG. Beyond a Gasotransmitter: hydrogen sulfide and polysulfide in cardiovascular health and immune response. Antioxid Redox Signal. 2017;27(10):634-53.
- Mani S, Li H, Untereiner A, Wu L, Yang G, Austin RC, et al. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation. 2013;127(25):2523-34.
- Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science (New York, NY). 2008;322(5901):587-90.
- LaPenna KB, Polhemus DJ, Doiron JE, Hidalgo HA, Li Z, Lefer DJ. Hydrogen sulfide as a potential therapy for heart failure-past, present, and future. Antioxidants (Basel, Switzerland). 2021;10(3).
- Bai L, Dai J, Xia Y, He K, Xue H, Guo Q, et al. Hydrogen sulfide ameliorated high choline-induced cardiac dysfunction by inhibiting cGAS-STING-NLRP3 Inflammasome pathway. Oxidative Med Cell Longev. 2022;2022:1392896.
- Shen P, Han L, Chen G, Cheng Z, Liu Q. Emodin attenuates acetaminopheninduced hepatotoxicity via the cGAS-STING pathway. Inflammation. 2022;45(1):74-87.
- Zhang C, Song Y, Chen L, Chen P, Yuan M, Meng Y, et al. Urolithin a attenuates Hyperuricemic nephropathy in fructose-fed mice by impairing STINGNLRP3 Axis-mediated inflammatory response via restoration of Parkindependent Mitophagy. Front Pharmacol. 2022;13:907209.
- Ma Z, Ni G, Damania B. Innate sensing of DNA virus genomes. Annu RevVirol. 2018;5(1):341-62.
- SuT, Zhang Y, Valerie K, Wang XY, Lin S, Zhu G. STING activation in cancer immunotherapy. Theranostics. 2019;9(25):7759-71.
- Ming SL, Zeng L, Guo YK, Zhang S, Li GL, Ma YX, et al. The human-specific STING agonist G10 activates type I interferon and the NLRP3 Inflammasome in porcine cells. Front Immunol. 2020;11:575818.
- Gröschel MI, Sayes F, Shin SJ, Frigui W, Pawlik A, Orgeur M, et al. Recombinant BCG expressing ESX-1 of Mycobacterium marinum combines low virulence with cytosolic immune signaling and improved TB protection. Cell Rep. 2017;18(11):2752-65.
- Ma C, Ma X, Jiang B, Pan H, Liao X, Zhang L, et al. A novel inactivated wholecell Pseudomonas aeruginosa vaccine that acts through the cGAS-STING pathway. Signal Transduct Target Ther. 2021;6(1):353.
- Miura N, Shaheen SM, Akita H, Nakamura T, Harashima H. A KALA-modified lipid nanoparticle containing CpG-free plasmid DNA as a potential DNA vaccine carrier for antigen presentation and as an immune-stimulative adjuvant. Nucleic Acids Res. 2015;43(3):1317-31.
- Xu X, Fan H, Yang Y, Yao S, Yu W, Guo Z, et al. Virus-Like Particle-Induced cGAS-STING Activation and AIM2 Inflammasome-Mediated Pyroptosis for Robust Cancer Immunotherapy. Angewandte Chemie (International ed in English). 2023;135:e202303010.
- Chen D, Le SB, Hutchinson TE, Calinescu AA, Sebastian M, Jin D, et al. Tumor treating fields dually activate STING and AIM2 inflammasomes to induce adjuvant immunity in glioblastoma. J Clin Invest. 2022;132(8).
- Ling YY, Xia XY, Hao L, Wang WJ, Zhang H, Liu LY, et al. Simultaneous Photoactivation of cGAS-STING pathway and Pyroptosis by platinum(II) Triphenylamine complexes for Cancer immunotherapy. Angewandte Chemie (International ed in English). 2022;61(43):e202210988.
- Liu YG, Chen JK, Zhang ZT, Ma XJ, Chen YC, Du XM, et al. NLRP3 inflammasome activation mediates radiation-induced pyroptosis in bone marrowderived macrophages. Cell Death Dis. 2017;8(2):e2579.
- Kabiljo J, Harpain F, Carotta S, Bergmann M. Radiotherapy as a backbone for novel concepts in Cancer immunotherapy. Cancers. 2019;12(1).
- Barber GN. STING: infection, inflammation and cancer. Nat Rev Immunol. 2015;15(12):760-70.
Publisher’s Note
Jingwen Liu, Jing Zhou and Yuling Luan are co-first author.
*Correspondence:
Jianyuan Tang
tangjy@cdutcm.edu.cn
Zheilei Wang
wangzl1993@outlook.com
Full list of author information is available at the end of the article
