DOI: https://doi.org/10.1038/s41598-024-57049-3
PMID: https://pubmed.ncbi.nlm.nih.gov/38509209
تاريخ النشر: 2024-03-20
أثر معالجة البذور بجزيئات السيلينيوم النانوية على إنبات ونمو شتلات الطماطم
الملخص
يمكن أن تؤدي ضعف الإنبات ونمو الشتلات إلى خسائر اقتصادية كبيرة للمزارعين، لذلك هناك حاجة ماسة لاستراتيجيات زراعية مستدامة لتحسين الإنبات والنمو المبكر للمحاصيل. كان الهدف من هذا العمل هو تقييم جزيئات السيلينيوم النانوية (Se NPs) كعوامل تنشيط نانوية لبذور الطماطم (Solanum lycopersicum) التي نمت دون ظروف ضغط في كل من الأطباق وصواني الإنبات. تم تحديد جودة الإنبات، ونمو الشتلات، والتآزر-التضاد بين السيلينيوم وعناصر أخرى، ومصير جزيئات السيلينيوم النانوية، كدالة لتركيزات مختلفة من جزيئات السيلينيوم النانوية (1، 10 و50 جزء في المليون). أشارت النتائج إلى أن معدل الإنبات في الأطباق قد تحسن عند 10 جزء في المليون، بينما قدمت صواني الإنبات أفضل النتائج عند 1 جزء في المليون، بزيادة قدرها 10 و
يتطلب تحقيق الأمن الغذائي العالمي ومنع المزيد من تدهور البيئة تنفيذ ممارسات زراعية مستدامة.
المواد والطرق
مصدر وتوصيف الجسيمات النانوية السيلينيوم
تجارب تنشيط بذور الطماطم
قياس إنبات البذور
مصير جزيئات النانو Se في بذور الطماطم المهيأة
نمو شتلات الطماطم
إجمالي القدرة المضادة للأكسدة (TAC)
محتوى الكلوروفيل
محتوى البرولين
تم قياس الامتصاص في الكروموفور عند 520 نانومتر وتم حساب محتوى البرولين باستخدام المعادلة (8) والتعبير عنه في
تحديد النيتروجين
تحديد المعادن
التحليل الإحصائي
النتائج
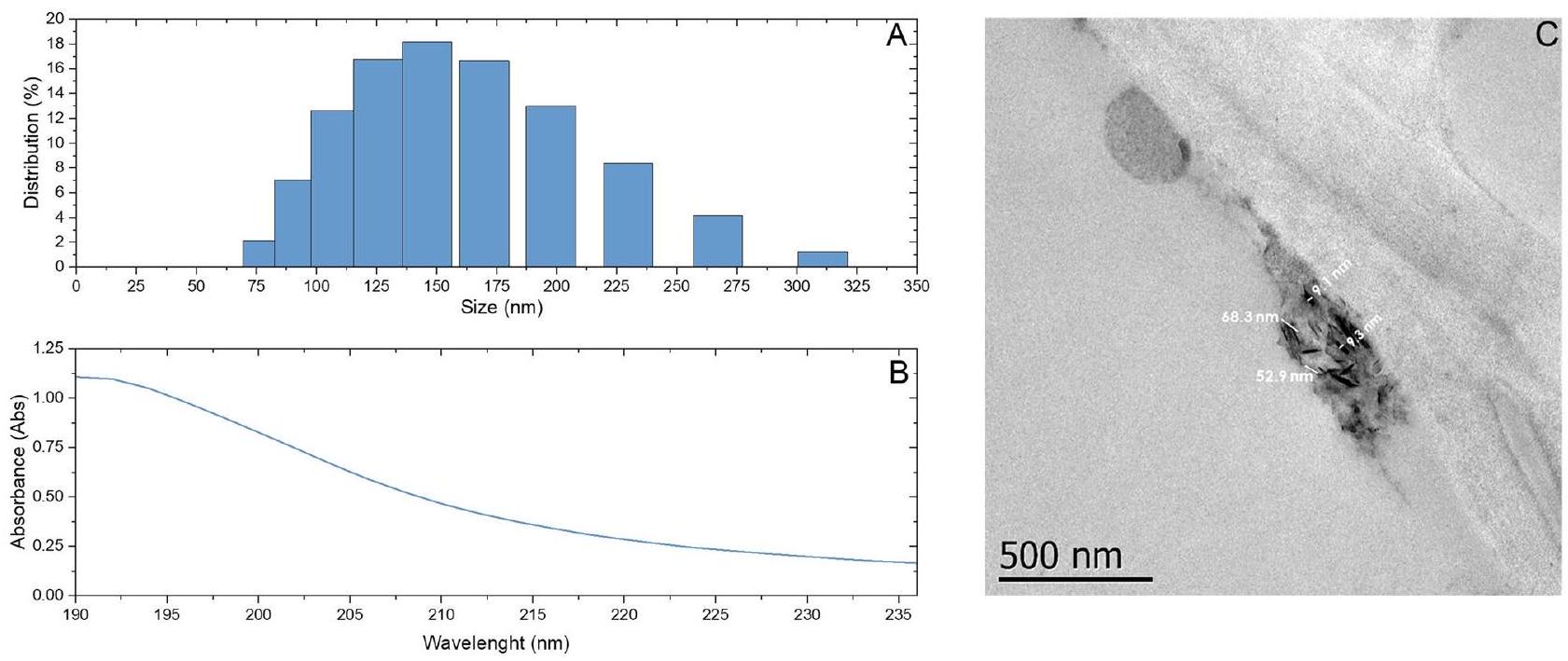
توصيف جزيئات السيلينيوم النانوية
إنبات بذور الطماطم المعالجة بجزيئات السيلينيوم النانوية
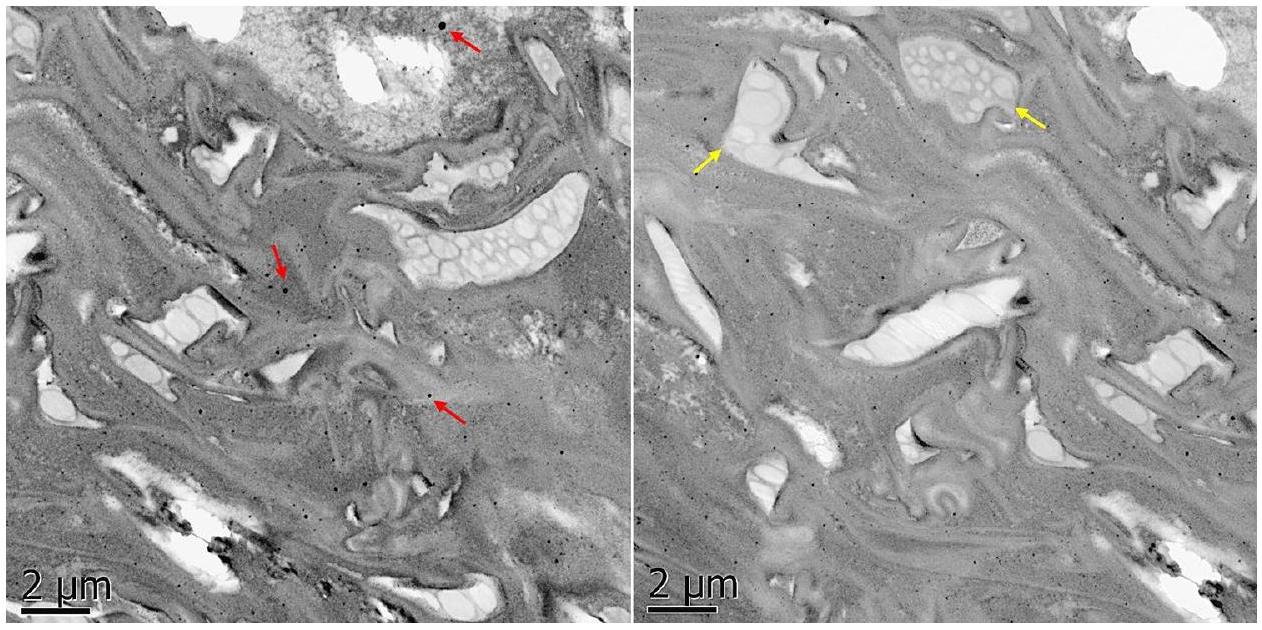
مصير جزيئات النانو Se داخل بذور الطماطم المهيأة
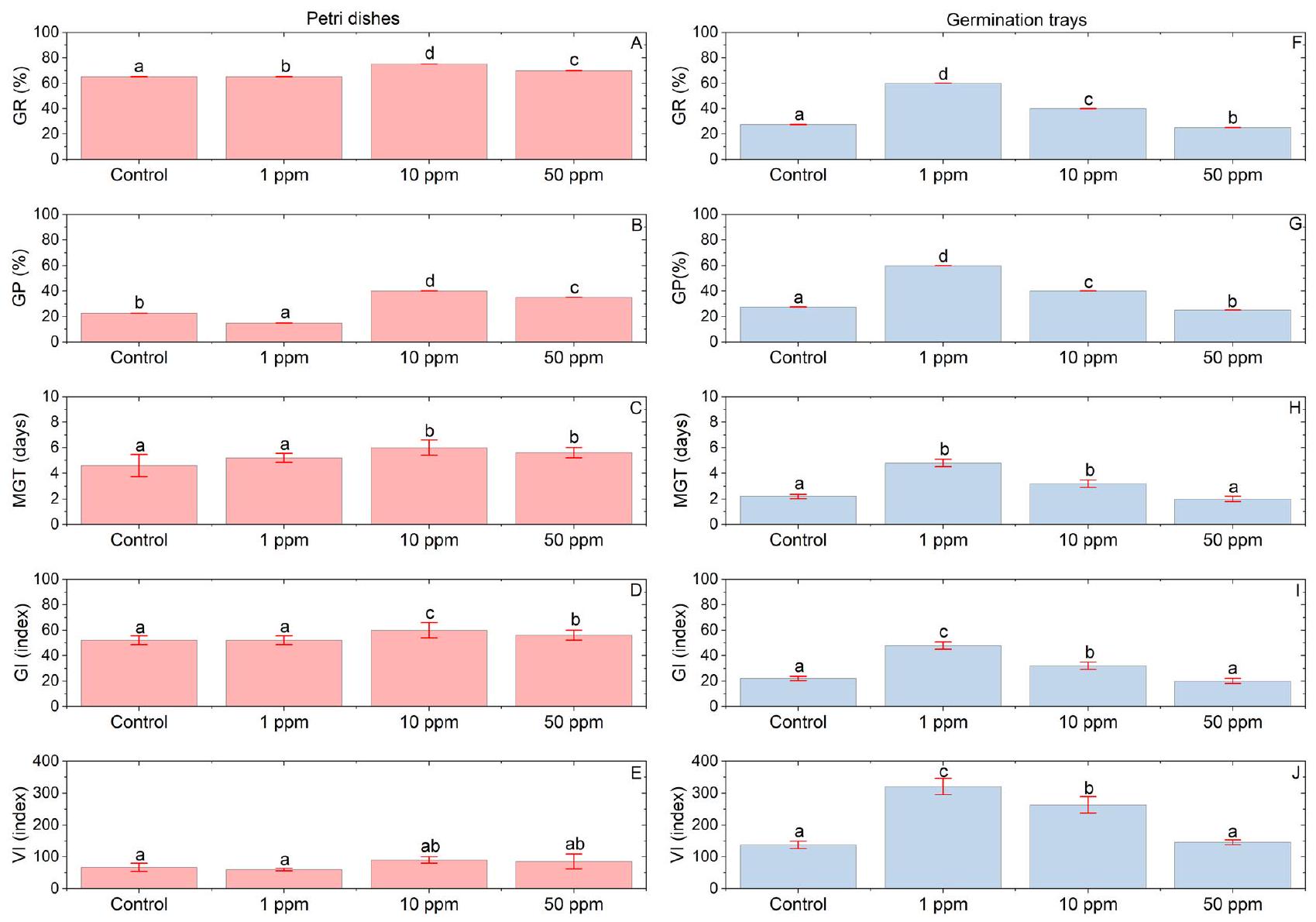
نمو الشتلات

إجمالي القدرة المضادة للأكسدة
محتوى الكلوروفيل
محتوى البرولين
محتوى النيتروجين
محتوى المعدن
| تحكم | 1 جزء في المليون | 10 جزء في المليون | 50 جزء في المليون | |
| سي |
|
|
|
|
| لا |
|
|
|
|
| مغنيسيوم |
|
|
|
|
| ال |
|
|
|
|
| ك |
|
|
|
|
| كا |
|
|
|
|
| تي |
|
|
|
|
| من |
|
|
|
|
| حديد |
|
|
|
|
| نحاس |
|
|
|
|
| زن |
|
|
|
|
| مو |
|
|
|
|
| تحكم | 1 جزء في المليون | 10 جزء في المليون | 50 جزء في المليون | |
| سي |
|
|
|
|
| لا |
|
|
|
|
| مغنيسيوم |
|
|
|
|
| ال |
|
|
|
|
| ك |
|
|
|
|
| كا |
|
|
|
|
| تي |
|
|
|
|
| من |
|
|
|
|
| حديد |
|
|
|
|
| نحاس |
|
|
|
|
| زن |
|
|
|
|
| مو |
|
|
|
|
المناقشة
دورة الغلوتاثيون-حمض الأسكوربيك التي تعمل على الحفاظ على التوازن داخل الخلية ومواجهة الأنواع التفاعلية من الأكسجين (ROS)، من بين أمور أخرى
الاستنتاجات
مع نانو الجسيمات من طبيعة مختلفة، لكن المعلومات حول تأثير معالجة البذور بنانو الجسيمات السيلينيوم في الطماطم دون ظروف إجهاد مستحثة نادرة. نستنتج أن نانو الجسيمات السيلينيوم يمكن استخدامها كعامل معالجة فعال لزيادة جودة الإنبات ونمو الطماطم، بسبب التأثير الإيجابي الموصوف سابقًا على الإنبات مع 1 جزء في المليون ونمو الشتلات مع 10 جزء في المليون. ومع ذلك، فإن الآليات التي تحفز بها نانو الجسيمات السيلينيوم مثل هذه الزيادات في جودة الإنبات ونمو الشتلات غير معروفة، لذلك، من الضروري توضيح تعقيدات تفاعلات نانو الجسيمات السيلينيوم مع الأنظمة النباتية المختلفة قبل تنفيذ استخدام نانو الجسيمات السيلينيوم في الزراعة المستدامة بشكل صحيح. لتحقيق ذلك، هناك حاجة إلى دراسات كمية مع الميتابولوميات تتناول مسار الجلوتاثيون لنباتات الطماطم المعالجة بنانو الجسيمات السيلينيوم. علاوة على ذلك، يمكن أيضًا استخدام الجينوميات، والترانسكريبتوميات، والبروتيوميات لفهم التفاعلات بين نانو الجسيمات السيلينيوم وأنسجة النبات على نطاق أعمق، بينما يمكن أن تكون دراسات دورة الحياة الكاملة للمحاصيل مفيدة أيضًا لتحديد ما إذا كانت نانو الجسيمات السيلينيوم يمكن أن تؤثر على البلاستيدات الخضراء وغيرها من العضيات داخل أنسجة النبات كوظيفة للوقت.
توفر البيانات
تم النشر عبر الإنترنت: 20 مارس 2024
References
- Muhie, S. H. Novel approaches and practices to sustainable agriculture. J. Agric. Food Res. 10, 100446 (2022).
- Arora, S., Murmu, G., Mukherjee, K., Saha, S. & Maity, D. A comprehensive overview of nanotechnology in sustainable agriculture. J. Biotechnol. 355, 21-41 (2022).
- Altaf, M. A. et al. Melatonin alleviates salt damage in tomato seedling: A root architecture system, photosynthetic capacity, ion homeostasis, and antioxidant enzymes analysis. Sci. Hortic. 285, 110145 (2021).
- Kou, E. et al. Nitrogen and sulfur co-doped carbon dots enhance drought resistance in tomato and mung beans. ACS Appl. Bio Mater. 4, 6093-6102 (2021).
- Quinet, M. et al. Tomato fruit development and metabolism. Front. Plant Sci. https://doi.org/10.3389/fpls.2019.01554 (2019).
- Zulfiqar, F., Navarro, M., Ashraf, M., Akram, N. A. & Munné-Bosch, S. Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant Sci. 289, 110270. https://doi.org/10.1016/j.plantsci.2019.110270 (2019).
- Singh, R. P., Handa, R. & Manchanda, G. Nanoparticles in sustainable agriculture: An emerging opportunity. J. Controll. Release 329,1234-1248. https://doi.org/10.1016/j.jconrel.2020.10.051 (2021).
- Singh, B. K., Trivedi, P., Egidi, E., Macdonald, C. A. & Delgado-Baquerizo, M. Crop microbiome and sustainable agriculture. Nat. Rev. Microbiol. 18, 601-602. https://doi.org/10.1038/s41579-020-00446-y (2020).
- Talebian, S. et al. Facts and figures on materials science and nanotechnology progress and investment. ACS Nano 15, 15940-15952 (2021).
- Cervantes-Avilés, P., Huang, X. & Keller, A. A. Dissolution and aggregation of metal oxide nanoparticles in root exudates and soil leachate: Implications for nanoagrochemical application. Environ. Sci. Technol. 55, 13443-13451 (2021).
- Biswas, S., Seal, P., Majumder, B. & Biswas, A. K. Efficacy of seed priming strategies for enhancing salinity tolerance in plants: An overview of the progress and achievements. Plant Stress 9, 100186. https://doi.org/10.1016/j.stress.2023.100186 (2023).
- Eevera, T. et al. Unleashing the potential of nanoparticles on seed treatment and enhancement for sustainable farming. Environ. Res. 236, 116849. https://doi.org/10.1016/j.envres.2023.116849 (2023).
- Mazhar, M. W. et al. Seed priming with iron oxide nanoparticles improves yield and antioxidant status of garden pea (Pisum sativum L.) grown under drought stress. South Afr. J. Bot. 162, 577-587 (2023).
- Mazhar, M. W., Ishtiaq, M., Maqbool, M. & Akram, R. Seed priming with Calcium oxide nanoparticles improves germination, biomass, antioxidant defence and yield traits of canola plants under drought stress. South Afr. J. Bot. 151, 889-899 (2022).
- Zhao, L. et al. Nanobiotechnology-based strategies for enhanced crop stress resilience. Nat. Food 3, 829-836 (2022).
- Dangi, K. & Verma, A. K. Efficient & eco-friendly smart nano-pesticides: Emerging prospects for agriculture. Mater. Today Proc. 45, 3819-3824 (2020).
- Elsherbiny, A. S., Galal, A., Ghoneem, K. M. & Salahuddin, N. A. Novel chitosan-based nanocomposites as ecofriendly pesticide carriers: Synthesis, root rot inhibition and growth management of tomato plants. Carbohydr. Polym. 282, 119111 (2022).
- Ozcan, A. et al. Copper-fixed quat: A hybrid nanoparticle for application as a locally systemic pesticide (LSP) to manage bacterial spot disease of tomato. Nanoscale Adv. 3, 1473-1483 (2021).
- Keller, A. A. et al. Comparative environmental fate and toxicity of copper nanomaterials. NanoImpact 7, 28-40 (2017).
- Younes, N. A., Hassan, H. S., Elkady, M. F., Hamed, A. M. & Dawood, M. F. A. Impact of synthesized metal oxide nanomaterials on seedlings production of three Solanaceae crops. Heliyon 6, e03188 (2020).
- Liao, Y. Y. et al. Magnesium oxide nanomaterial, an alternative for commercial copper bactericides: Field-scale tomato bacterial spot disease management and total and bioavailable metal accumulation in soil. Environ. Sci. Technol. 55, 13561-13570 (2021).
- Carvalho, R., Duman, K., Jones, J. B. & Paret, M. L. Bactericidal activity of copper-zinc hybrid nanoparticles on copper-tolerant Xanthomonas perforans. Sci. Rep. https://doi.org/10.1038/s41598-019-56419-6 (2019).
- Singh, A., Singh, N. B., Hussain, I. & Singh, H. Effect of biologically synthesized copper oxide nanoparticles on metabolism and antioxidant activity to the crop plants Solanum lycopersicum and Brassica oleracea var. botrytis. J. Biotechnol. 262, 11-27 (2017).
- Malandrakis, A. A. et al. Metal nanoparticles: Phytotoxicity on tomato and effect on symbiosis with the Fusarium solani FsK strain. Sci. Total Environ. 787, 147606 (2021).
- Ahmed, B., Rizvi, A., Zaidi, A., Khan, M. S. & Musarrat, J. Understanding the phyto-interaction of heavy metal oxide bulk and nanoparticles: Evaluation of seed germination, growth, bioaccumulation, and metallothionein production. RSC Adv. 9, 4210-4225 (2019).
- El-Badri, A. M. et al. Selenium and zinc oxide nanoparticles modulate the molecular and morpho-physiological processes during seed germination of Brassica napus under salt stress. Ecotoxicol. Environ. Saf. 225, 112695 (2021).
- Shalaby, T. A. et al. Nano-selenium, silicon and H 2 O 2 boost growth and productivity of cucumber under combined salinity and heat stress. Ecotoxicol. Environ. Saf. 212, 111962 (2021).
- Mateus, M. P. B. et al. Selenium biofortification enhances ROS scavenge system increasing yield of coffee plants. Ecotoxicol. Environ. Saf. 209, 111772 (2021).
- Abid, S. et al. Synthesis and characterization of glycol chitosan coated selenium nanoparticles acts synergistically to alleviate oxidative stress and increase ginsenoside content in Panax ginseng. Carbohydr. Polym. 267, 118195 (2021).
- Gudkov, S. V. et al. Production and use of selenium nanoparticles as fertilizers. ACS Omega 5, 17767-17774 (2020).
- Joshi, S. M., De Britto, S. & Jogaiah, S. Myco-engineered selenium nanoparticles elicit resistance against tomato late blight disease by regulating differential expression of cellular, biochemical and defense responsive genes. J. Biotechnol. 325, 196-206 (2021).
- Liu, J. et al. Defense and inhibition integrated mesoporous nanoselenium delivery system against tomato gray mold. Environ. Sci. Nano 7, 210-227 (2020).
- Huang, X., Cervantes-Avilés, P., Li, W. & Keller, A. A. Drilling into the metabolomics to enhance insight on corn and wheat responses to molybdenum trioxide nanoparticles. Environ. Sci. Technol. 55, 13452-13464 (2021).
- Itroutwar, P. D., Kasivelu, G., Raguraman, V., Malaichamy, K. & Sevathapandian, S. K. Effects of biogenic zinc oxide nanoparticles on seed germination and seedling vigor of maize (Zea mays). Biocatal. Agric. Biotechnol. 29, 101778 (2020).
- Gong, C. et al. Responses of seed germination and shoot metabolic profiles of maize (: Zea mays L.) to Y2O3 nanoparticle stress. RSC Adv. 9, 27720-27731 (2019).
- Wang, Z. et al. Xylem- and phloem-based transport of CuO nanoparticles in maize (Zea mays L.). Environ. Sci. Technol. 46, 4434-4441 (2012).
- Cervantes-Avilés, P., Díaz Barriga-Castro, E., Palma-Tirado, L. & Cuevas-Rodríguez, G. Interactions and effects of metal oxide nanoparticles on microorganisms involved in biological wastewater treatment. Microsc. Res. Tech. 80, 1103-1112 (2017).
- Adhikary, S. et al. Seed priming with selenium and zinc nanoparticles modifies germination, growth, and yield of direct-seeded rice (Oryza sativa L.). Sci. Rep. https://doi.org/10.1038/s41598-022-11307-4 (2022).
- Pisoschi, A. M., Pop, A., Cimpeanu, C. & Predoi, G. Antioxidant capacity determination in plants and plant-derived products: A review. Oxidat. Med. Cell. Longev. 2016, 1-36. https://doi.org/10.1155/2016/9130976 (2016).
- Sigma-Aldrich Co. LLC. USA. Total Antioxidant Capacity Assay Kit. (2018).
- Wintermans, J. F. G. M. & De Mots, A. Spectrophotometric characteristics of chlorophylls a and b and their Pheophytins in Ethanol. Biochimica et Biophysica Acta (BBA) Biophys. Incl. Photosynth. https://doi.org/10.1016/0926-6585(65)90170-6 (1965).
- Bates, L. S., Waldren, R. P. & Teare, I. D. Rapid determination of free proline for water-stress studies. Plant Soil https://doi.org/10. 1007/BF00018060 (1973).
- Hach Lange GmbH. USA. Total Nitrogen, Persulfate Digestion Method. (2015).
- Zhao, L., Hu, Q., Huang, Y. & Keller, A. A. Response at genetic, metabolic, and physiological levels of maize (Zea mays) exposed to a
- Rohal, C. B., Adams, C. R., Martin, C. W., Tevlin, S. & Reynolds, L. K. Seed bank and germination ecology of sub-tropical Vallisneria americana. Aquat. Bot. 190, 103721 (2024).
- Nagdalian, A. A. et al. Effect of selenium nanoparticles on biological and morphofunctional parameters of barley seeds (Hordéum vulgáre L.). Sci. Rep. https://doi.org/10.1038/s41598-023-33581-6 (2023).
- Zhan, T. et al. Chitin combined with selenium reduced nitrogen loss in soil and improved nitrogen uptake efficiency in Guanxi pomelo orchard. Sci. Total Environ. 799, 149414 (2021).
- Abouelhamd, N., Gharib, F. A. E. L., Amin, A. A. & Ahmed, E. Z. Impact of foliar spray with Se, nano-Se and sodium sulfate on growth, yield and metabolic activities of red kidney bean. Sci. Rep. https://doi.org/10.1038/s41598-023-43677-8 (2023).
- Ghanbari, F., Bag-Nazari, M. & Azizi, A. Exogenous application of selenium and nano-selenium alleviates salt stress and improves secondary metabolites in lemon verbena under salinity stress. Sci. Rep. https://doi.org/10.1038/s41598-023-32436-4 (2023).
- Pereira, A. E. S., Oliveira, H. C. & Fraceto, L. F. Polymeric nanoparticles as an alternative for application of gibberellic acid in sustainable agriculture: A field study. Sci. Rep. https://doi.org/10.1038/s41598-019-43494-y (2019).
- Hubbard, J. D., Lui, A. & Landry, M. P. Multiscale and multidisciplinary approach to understanding nanoparticle transport in plants: Multiscale and multidisciplinary approach to understanding nanoparticle transport in plants. Curr. Opin. Chem. Eng. 30, 135-143. https://doi.org/10.1016/j.coche.2020.100659 (2020).
- Zhao, L., Huang, Y., Adeleye, A. S. & Keller, A. A. Metabolomics reveals
- Tanaka, A. & Tanaka, R. Chlorophyll metabolism. Curr. Opin. Plant Biol. 9, 248-255 (2006).
- Dumanović, J., Nepovimova, E., Natić, M., Kuča, K. & Jaćević, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. https://doi.org/10.3389/fpls.2020.552969 (2021).
- Verbruggen, N. & Hermans, C. Proline accumulation in plants: A review. Amino Acids 35, 753-759. https://doi.org/10.1007/ s00726-008-0061-6 (2008).
- Mattioli, R., Costantino, P. & Trovato, M. Proline accumulation in plants: Not only stress. Plant Signal. Behav. 4, 1016-1018. https:// doi.org/10.4161/psb.4.11.9797 (2009).
- Aaseth, J., Gerhardsson, L., Skaug, M. A. & Alexander, J. General chemistry of metal toxicity and basis for metal complexation. In Chelation Therapy in the Treatment of Metal Intoxication (ed. Aaseth, J.) 1-33 (Elsevier Inc., 2016). https://doi.org/10.1016/ B978-0-12-803072-1.00001-8.
- Noctor, G. et al. Glutathione in plants: An integrated overview. Plant Cell Environ. 35, 454-484 (2012).
- Salbitani, G., Vona, V., Bottone, C., Petriccione, M. & Carfagna, S. Sulphur deprivation results in oxidative perturbation in chlorella Sorokiniana (211/8k). Plant Cell Physiol. https://doi.org/10.1093/pcp/pcv015 (2015).
- Schiavon, M. et al. Selenium fertilization alters the chemical composition and antioxidant constituents of tomato (Solanum lycopersicon L.). J. Agric. Food Chem. 61, 10542-10554 (2013).
- Hasanuzzaman, M., Nahar, K., Anee, T. I. & Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 23, 249-268. https://doi.org/10.1007/s12298-017-0422-2 (2017).
- Müller-Schüssele, S. J. et al. Chloroplasts require glutathione reductase to balance reactive oxygen species and maintain efficient photosynthesis. Plant J. 103, 1140-1154 (2020).
- Pu, Z. et al. Selenium and anthocyanins share the same transcription factors R2R3MYB and bHLH in wheat. Food Chem. 356, 129699 (2021).
- Csiszár, J. et al. Glutathione transferase supergene family in tomato: Salt stress-regulated expression of representative genes from distinct GST classes in plants primed with salicylic acid. Plant Physiol. Biochem. 78, 15-26 (2014).
- Romero, I., Sanchez-Ballesta, M. T., Escribano, M. I. & Merodio, C. Individual anthocyanins and their contribution to total antioxidant capacity in response to low temperature and high
- Hayat, S. et al. Role of proline under changing environments: A review. Plant Signal. Behav. 7, 1456-1466. https://doi.org/10.4161/ psb. 21949 (2012).
- Verslues, P. E. & Sharma, S. Proline metabolism and its implications for plant-environment interaction. Arabidopsis Book 8, e0140 (2010).
- White, P. J., Bowen, H. C., Marshall, B. & Broadley, M. R. Extraordinarily high leaf selenium to sulfur ratios define ‘Se-accumulator’ plants. Ann. Bot. 100, 111-118 (2007).
- White, P. J., Broadley, M. R., Bowen, H. C. & Johnson, S. E. Chapter 1010 Selenium and its Relationship with Sulfur (Springer, 2007).
- White, P. J. Selenium in soils and crops. In Molecular and Integrative Toxicology (ed. Michalke, B.) 29-50 (Springer Science + Business Media B.V., 2018). https://doi.org/10.1007/978-3-319-95390-8_2.
- White, P. J. Selenium metabolism in plants. Biochimica et Biophysica Acta Gen. Subj. 1862, 2333-2342. https://doi.org/10.1016/j. bbagen.2018.05.006 (2018).
- Tolu, J. et al. Understanding soil selenium accumulation and bioavailability through size resolved and elemental characterization of soil extracts. Nat. Commun. https://doi.org/10.1038/s41467-022-34731-6 (2022).
- Shrivastava, M. et al. Monitoring of engineered nanoparticles in soil-plant system: A review. Environ. Nanotechnol. Monit. Manag. 11, 100218. https://doi.org/10.1016/j.enmm.2019.100218 (2019).
- Majumdar, S. & Keller, A. A. Omics to address the opportunities and challenges of nanotechnology in agriculture. Crit. Rev. Environ. Sci. Technol. 51, 2595-2636 (2021).
- Kumari, M. et al. Omics-based mechanistic insight into the role of bioengineered nanoparticles for biotic stress amelioration by modulating plant metabolic pathways. Front. Bioeng. Biotechnol. https://doi.org/10.3389/fbioe.2020.00242 (2020).
الشكر والتقدير
مساهمات المؤلفين
التمويل
المصالح المتنافسة
معلومات إضافية
معلومات إعادة الطبع والتصاريح متاحة على www.nature.com/reprints.
ملاحظة الناشر تظل Springer Nature محايدة فيما يتعلق بالمطالبات القضائية في الخرائط المنشورة والانتماءات المؤسسية.
© المؤلفون 2024
- مدرسة الهندسة والعلوم، تقنية مونتيري، احتياطي إقليمي أتلتيكايول، الرمز البريدي 72453 بويبلا، بويب، المكسيك.
DOI: https://doi.org/10.1038/s41598-024-57049-3
PMID: https://pubmed.ncbi.nlm.nih.gov/38509209
Publication Date: 2024-03-20
Impact of seed priming with Selenium nanoparticles on germination and seedlings growth of tomato
Abstract
Poor germination and seedlings growth can lead to significant economic losses for farmers, therefore, sustainable agricultural strategies to improve germination and early growth of crops are urgently needed. The objective of this work was to evaluate selenium nanoparticles (Se NPs) as nanopriming agents for tomato (Solanum lycopersicum) seeds germinated without stress conditions in both trays and Petri dishes. Germination quality, seedlings growth, synergism-antagonism of Se with other elements, and fate of Se NPs, were determined as function of different Se NPs concentrations (1, 10 and 50 ppm ). Results indicated that the germination rate in Petri dishes improved with 10 ppm , while germination trays presented the best results at 1 ppm, increasing by 10 and
Achieving global food security and preventing further environmental degradation requires the implementation of sustainable agricultural practices
Materials and methods
Se NPs source and characterization
Tomato seed priming experiments
Measurement of seed germination
Fate of Se NPs in tomato primed seeds
Tomato seedlings growth
Total antioxidant capacity (TAC)
Chlorophyll content
Proline content
the absorbance in the chromophore was measured at 520 nm and the proline content was calculated using the Eq. (8) and expressed in
Nitrogen determination
Metals determination
Statistical analysis
Results
Characterization of Se NPs

Germination of tomato seeds primed with Se NPs
Fate of Se NPs within tomato primed seeds


Seedlings growth

Total antioxidant capacity
Chlorophyll content
Proline content
Nitrogen content
Metal content
| Control | 1 ppm | 10 ppm | 50 ppm | |
| Se |
|
|
|
|
| Na |
|
|
|
|
| Mg |
|
|
|
|
| Al |
|
|
|
|
| K |
|
|
|
|
| Ca |
|
|
|
|
| Ti |
|
|
|
|
| Mn |
|
|
|
|
| Fe |
|
|
|
|
| Cu |
|
|
|
|
| Zn |
|
|
|
|
| Mo |
|
|
|
|
| Control | 1 ppm | 10 ppm | 50 ppm | |
| Se |
|
|
|
|
| Na |
|
|
|
|
| Mg |
|
|
|
|
| Al |
|
|
|
|
| K |
|
|
|
|
| Ca |
|
|
|
|
| Ti |
|
|
|
|
| Mn |
|
|
|
|
| Fe |
|
|
|
|
| Cu |
|
|
|
|
| Zn |
|
|
|
|
| Mo |
|
|
|
|
Discussion
the glutathione-ascorbate cycle which serve to maintain homeostasis within the cell and counteract reactive oxygen species (ROS), among others
Conclusions
with NPs of different nature, but information of the impact of seed priming with Se NPs in tomato without induced stress conditions is scarce. We conclude that Se NPs can be used as an effective nanopriming agent to increase the germination quality and growth of tomato, because of the positive impact previously described on the germination with 1 ppm and the seedlings growth with 10 ppm . However, the mechanisms in which Se NPs prompt such increases in the germination quality and seedling growth are unknown, therefore, it is essential to elucidate the intricacies of Se NPs interactions with the different plant systems before properly implementing the use of Se NPs in sustainable agriculture. To achieve this, quantitative studies with metabolomics addressing the glutathione pathway of tomato plants treated with Se NPs are needed. Furthermore, genomics, transcriptomics and proteomics can also be utilized to understand the interactions between Se NPs and plant tissues on a deeper scale, while full life cycle studies of crops can also be useful to determine if the Se NPs can impact chloroplasts and other organelles within plant tissue as function of time.
Data availability
Published online: 20 March 2024
References
- Muhie, S. H. Novel approaches and practices to sustainable agriculture. J. Agric. Food Res. 10, 100446 (2022).
- Arora, S., Murmu, G., Mukherjee, K., Saha, S. & Maity, D. A comprehensive overview of nanotechnology in sustainable agriculture. J. Biotechnol. 355, 21-41 (2022).
- Altaf, M. A. et al. Melatonin alleviates salt damage in tomato seedling: A root architecture system, photosynthetic capacity, ion homeostasis, and antioxidant enzymes analysis. Sci. Hortic. 285, 110145 (2021).
- Kou, E. et al. Nitrogen and sulfur co-doped carbon dots enhance drought resistance in tomato and mung beans. ACS Appl. Bio Mater. 4, 6093-6102 (2021).
- Quinet, M. et al. Tomato fruit development and metabolism. Front. Plant Sci. https://doi.org/10.3389/fpls.2019.01554 (2019).
- Zulfiqar, F., Navarro, M., Ashraf, M., Akram, N. A. & Munné-Bosch, S. Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant Sci. 289, 110270. https://doi.org/10.1016/j.plantsci.2019.110270 (2019).
- Singh, R. P., Handa, R. & Manchanda, G. Nanoparticles in sustainable agriculture: An emerging opportunity. J. Controll. Release 329,1234-1248. https://doi.org/10.1016/j.jconrel.2020.10.051 (2021).
- Singh, B. K., Trivedi, P., Egidi, E., Macdonald, C. A. & Delgado-Baquerizo, M. Crop microbiome and sustainable agriculture. Nat. Rev. Microbiol. 18, 601-602. https://doi.org/10.1038/s41579-020-00446-y (2020).
- Talebian, S. et al. Facts and figures on materials science and nanotechnology progress and investment. ACS Nano 15, 15940-15952 (2021).
- Cervantes-Avilés, P., Huang, X. & Keller, A. A. Dissolution and aggregation of metal oxide nanoparticles in root exudates and soil leachate: Implications for nanoagrochemical application. Environ. Sci. Technol. 55, 13443-13451 (2021).
- Biswas, S., Seal, P., Majumder, B. & Biswas, A. K. Efficacy of seed priming strategies for enhancing salinity tolerance in plants: An overview of the progress and achievements. Plant Stress 9, 100186. https://doi.org/10.1016/j.stress.2023.100186 (2023).
- Eevera, T. et al. Unleashing the potential of nanoparticles on seed treatment and enhancement for sustainable farming. Environ. Res. 236, 116849. https://doi.org/10.1016/j.envres.2023.116849 (2023).
- Mazhar, M. W. et al. Seed priming with iron oxide nanoparticles improves yield and antioxidant status of garden pea (Pisum sativum L.) grown under drought stress. South Afr. J. Bot. 162, 577-587 (2023).
- Mazhar, M. W., Ishtiaq, M., Maqbool, M. & Akram, R. Seed priming with Calcium oxide nanoparticles improves germination, biomass, antioxidant defence and yield traits of canola plants under drought stress. South Afr. J. Bot. 151, 889-899 (2022).
- Zhao, L. et al. Nanobiotechnology-based strategies for enhanced crop stress resilience. Nat. Food 3, 829-836 (2022).
- Dangi, K. & Verma, A. K. Efficient & eco-friendly smart nano-pesticides: Emerging prospects for agriculture. Mater. Today Proc. 45, 3819-3824 (2020).
- Elsherbiny, A. S., Galal, A., Ghoneem, K. M. & Salahuddin, N. A. Novel chitosan-based nanocomposites as ecofriendly pesticide carriers: Synthesis, root rot inhibition and growth management of tomato plants. Carbohydr. Polym. 282, 119111 (2022).
- Ozcan, A. et al. Copper-fixed quat: A hybrid nanoparticle for application as a locally systemic pesticide (LSP) to manage bacterial spot disease of tomato. Nanoscale Adv. 3, 1473-1483 (2021).
- Keller, A. A. et al. Comparative environmental fate and toxicity of copper nanomaterials. NanoImpact 7, 28-40 (2017).
- Younes, N. A., Hassan, H. S., Elkady, M. F., Hamed, A. M. & Dawood, M. F. A. Impact of synthesized metal oxide nanomaterials on seedlings production of three Solanaceae crops. Heliyon 6, e03188 (2020).
- Liao, Y. Y. et al. Magnesium oxide nanomaterial, an alternative for commercial copper bactericides: Field-scale tomato bacterial spot disease management and total and bioavailable metal accumulation in soil. Environ. Sci. Technol. 55, 13561-13570 (2021).
- Carvalho, R., Duman, K., Jones, J. B. & Paret, M. L. Bactericidal activity of copper-zinc hybrid nanoparticles on copper-tolerant Xanthomonas perforans. Sci. Rep. https://doi.org/10.1038/s41598-019-56419-6 (2019).
- Singh, A., Singh, N. B., Hussain, I. & Singh, H. Effect of biologically synthesized copper oxide nanoparticles on metabolism and antioxidant activity to the crop plants Solanum lycopersicum and Brassica oleracea var. botrytis. J. Biotechnol. 262, 11-27 (2017).
- Malandrakis, A. A. et al. Metal nanoparticles: Phytotoxicity on tomato and effect on symbiosis with the Fusarium solani FsK strain. Sci. Total Environ. 787, 147606 (2021).
- Ahmed, B., Rizvi, A., Zaidi, A., Khan, M. S. & Musarrat, J. Understanding the phyto-interaction of heavy metal oxide bulk and nanoparticles: Evaluation of seed germination, growth, bioaccumulation, and metallothionein production. RSC Adv. 9, 4210-4225 (2019).
- El-Badri, A. M. et al. Selenium and zinc oxide nanoparticles modulate the molecular and morpho-physiological processes during seed germination of Brassica napus under salt stress. Ecotoxicol. Environ. Saf. 225, 112695 (2021).
- Shalaby, T. A. et al. Nano-selenium, silicon and H 2 O 2 boost growth and productivity of cucumber under combined salinity and heat stress. Ecotoxicol. Environ. Saf. 212, 111962 (2021).
- Mateus, M. P. B. et al. Selenium biofortification enhances ROS scavenge system increasing yield of coffee plants. Ecotoxicol. Environ. Saf. 209, 111772 (2021).
- Abid, S. et al. Synthesis and characterization of glycol chitosan coated selenium nanoparticles acts synergistically to alleviate oxidative stress and increase ginsenoside content in Panax ginseng. Carbohydr. Polym. 267, 118195 (2021).
- Gudkov, S. V. et al. Production and use of selenium nanoparticles as fertilizers. ACS Omega 5, 17767-17774 (2020).
- Joshi, S. M., De Britto, S. & Jogaiah, S. Myco-engineered selenium nanoparticles elicit resistance against tomato late blight disease by regulating differential expression of cellular, biochemical and defense responsive genes. J. Biotechnol. 325, 196-206 (2021).
- Liu, J. et al. Defense and inhibition integrated mesoporous nanoselenium delivery system against tomato gray mold. Environ. Sci. Nano 7, 210-227 (2020).
- Huang, X., Cervantes-Avilés, P., Li, W. & Keller, A. A. Drilling into the metabolomics to enhance insight on corn and wheat responses to molybdenum trioxide nanoparticles. Environ. Sci. Technol. 55, 13452-13464 (2021).
- Itroutwar, P. D., Kasivelu, G., Raguraman, V., Malaichamy, K. & Sevathapandian, S. K. Effects of biogenic zinc oxide nanoparticles on seed germination and seedling vigor of maize (Zea mays). Biocatal. Agric. Biotechnol. 29, 101778 (2020).
- Gong, C. et al. Responses of seed germination and shoot metabolic profiles of maize (: Zea mays L.) to Y2O3 nanoparticle stress. RSC Adv. 9, 27720-27731 (2019).
- Wang, Z. et al. Xylem- and phloem-based transport of CuO nanoparticles in maize (Zea mays L.). Environ. Sci. Technol. 46, 4434-4441 (2012).
- Cervantes-Avilés, P., Díaz Barriga-Castro, E., Palma-Tirado, L. & Cuevas-Rodríguez, G. Interactions and effects of metal oxide nanoparticles on microorganisms involved in biological wastewater treatment. Microsc. Res. Tech. 80, 1103-1112 (2017).
- Adhikary, S. et al. Seed priming with selenium and zinc nanoparticles modifies germination, growth, and yield of direct-seeded rice (Oryza sativa L.). Sci. Rep. https://doi.org/10.1038/s41598-022-11307-4 (2022).
- Pisoschi, A. M., Pop, A., Cimpeanu, C. & Predoi, G. Antioxidant capacity determination in plants and plant-derived products: A review. Oxidat. Med. Cell. Longev. 2016, 1-36. https://doi.org/10.1155/2016/9130976 (2016).
- Sigma-Aldrich Co. LLC. USA. Total Antioxidant Capacity Assay Kit. (2018).
- Wintermans, J. F. G. M. & De Mots, A. Spectrophotometric characteristics of chlorophylls a and b and their Pheophytins in Ethanol. Biochimica et Biophysica Acta (BBA) Biophys. Incl. Photosynth. https://doi.org/10.1016/0926-6585(65)90170-6 (1965).
- Bates, L. S., Waldren, R. P. & Teare, I. D. Rapid determination of free proline for water-stress studies. Plant Soil https://doi.org/10. 1007/BF00018060 (1973).
- Hach Lange GmbH. USA. Total Nitrogen, Persulfate Digestion Method. (2015).
- Zhao, L., Hu, Q., Huang, Y. & Keller, A. A. Response at genetic, metabolic, and physiological levels of maize (Zea mays) exposed to a
- Rohal, C. B., Adams, C. R., Martin, C. W., Tevlin, S. & Reynolds, L. K. Seed bank and germination ecology of sub-tropical Vallisneria americana. Aquat. Bot. 190, 103721 (2024).
- Nagdalian, A. A. et al. Effect of selenium nanoparticles on biological and morphofunctional parameters of barley seeds (Hordéum vulgáre L.). Sci. Rep. https://doi.org/10.1038/s41598-023-33581-6 (2023).
- Zhan, T. et al. Chitin combined with selenium reduced nitrogen loss in soil and improved nitrogen uptake efficiency in Guanxi pomelo orchard. Sci. Total Environ. 799, 149414 (2021).
- Abouelhamd, N., Gharib, F. A. E. L., Amin, A. A. & Ahmed, E. Z. Impact of foliar spray with Se, nano-Se and sodium sulfate on growth, yield and metabolic activities of red kidney bean. Sci. Rep. https://doi.org/10.1038/s41598-023-43677-8 (2023).
- Ghanbari, F., Bag-Nazari, M. & Azizi, A. Exogenous application of selenium and nano-selenium alleviates salt stress and improves secondary metabolites in lemon verbena under salinity stress. Sci. Rep. https://doi.org/10.1038/s41598-023-32436-4 (2023).
- Pereira, A. E. S., Oliveira, H. C. & Fraceto, L. F. Polymeric nanoparticles as an alternative for application of gibberellic acid in sustainable agriculture: A field study. Sci. Rep. https://doi.org/10.1038/s41598-019-43494-y (2019).
- Hubbard, J. D., Lui, A. & Landry, M. P. Multiscale and multidisciplinary approach to understanding nanoparticle transport in plants: Multiscale and multidisciplinary approach to understanding nanoparticle transport in plants. Curr. Opin. Chem. Eng. 30, 135-143. https://doi.org/10.1016/j.coche.2020.100659 (2020).
- Zhao, L., Huang, Y., Adeleye, A. S. & Keller, A. A. Metabolomics reveals
- Tanaka, A. & Tanaka, R. Chlorophyll metabolism. Curr. Opin. Plant Biol. 9, 248-255 (2006).
- Dumanović, J., Nepovimova, E., Natić, M., Kuča, K. & Jaćević, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. https://doi.org/10.3389/fpls.2020.552969 (2021).
- Verbruggen, N. & Hermans, C. Proline accumulation in plants: A review. Amino Acids 35, 753-759. https://doi.org/10.1007/ s00726-008-0061-6 (2008).
- Mattioli, R., Costantino, P. & Trovato, M. Proline accumulation in plants: Not only stress. Plant Signal. Behav. 4, 1016-1018. https:// doi.org/10.4161/psb.4.11.9797 (2009).
- Aaseth, J., Gerhardsson, L., Skaug, M. A. & Alexander, J. General chemistry of metal toxicity and basis for metal complexation. In Chelation Therapy in the Treatment of Metal Intoxication (ed. Aaseth, J.) 1-33 (Elsevier Inc., 2016). https://doi.org/10.1016/ B978-0-12-803072-1.00001-8.
- Noctor, G. et al. Glutathione in plants: An integrated overview. Plant Cell Environ. 35, 454-484 (2012).
- Salbitani, G., Vona, V., Bottone, C., Petriccione, M. & Carfagna, S. Sulphur deprivation results in oxidative perturbation in chlorella Sorokiniana (211/8k). Plant Cell Physiol. https://doi.org/10.1093/pcp/pcv015 (2015).
- Schiavon, M. et al. Selenium fertilization alters the chemical composition and antioxidant constituents of tomato (Solanum lycopersicon L.). J. Agric. Food Chem. 61, 10542-10554 (2013).
- Hasanuzzaman, M., Nahar, K., Anee, T. I. & Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 23, 249-268. https://doi.org/10.1007/s12298-017-0422-2 (2017).
- Müller-Schüssele, S. J. et al. Chloroplasts require glutathione reductase to balance reactive oxygen species and maintain efficient photosynthesis. Plant J. 103, 1140-1154 (2020).
- Pu, Z. et al. Selenium and anthocyanins share the same transcription factors R2R3MYB and bHLH in wheat. Food Chem. 356, 129699 (2021).
- Csiszár, J. et al. Glutathione transferase supergene family in tomato: Salt stress-regulated expression of representative genes from distinct GST classes in plants primed with salicylic acid. Plant Physiol. Biochem. 78, 15-26 (2014).
- Romero, I., Sanchez-Ballesta, M. T., Escribano, M. I. & Merodio, C. Individual anthocyanins and their contribution to total antioxidant capacity in response to low temperature and high
- Hayat, S. et al. Role of proline under changing environments: A review. Plant Signal. Behav. 7, 1456-1466. https://doi.org/10.4161/ psb. 21949 (2012).
- Verslues, P. E. & Sharma, S. Proline metabolism and its implications for plant-environment interaction. Arabidopsis Book 8, e0140 (2010).
- White, P. J., Bowen, H. C., Marshall, B. & Broadley, M. R. Extraordinarily high leaf selenium to sulfur ratios define ‘Se-accumulator’ plants. Ann. Bot. 100, 111-118 (2007).
- White, P. J., Broadley, M. R., Bowen, H. C. & Johnson, S. E. Chapter 1010 Selenium and its Relationship with Sulfur (Springer, 2007).
- White, P. J. Selenium in soils and crops. In Molecular and Integrative Toxicology (ed. Michalke, B.) 29-50 (Springer Science + Business Media B.V., 2018). https://doi.org/10.1007/978-3-319-95390-8_2.
- White, P. J. Selenium metabolism in plants. Biochimica et Biophysica Acta Gen. Subj. 1862, 2333-2342. https://doi.org/10.1016/j. bbagen.2018.05.006 (2018).
- Tolu, J. et al. Understanding soil selenium accumulation and bioavailability through size resolved and elemental characterization of soil extracts. Nat. Commun. https://doi.org/10.1038/s41467-022-34731-6 (2022).
- Shrivastava, M. et al. Monitoring of engineered nanoparticles in soil-plant system: A review. Environ. Nanotechnol. Monit. Manag. 11, 100218. https://doi.org/10.1016/j.enmm.2019.100218 (2019).
- Majumdar, S. & Keller, A. A. Omics to address the opportunities and challenges of nanotechnology in agriculture. Crit. Rev. Environ. Sci. Technol. 51, 2595-2636 (2021).
- Kumari, M. et al. Omics-based mechanistic insight into the role of bioengineered nanoparticles for biotic stress amelioration by modulating plant metabolic pathways. Front. Bioeng. Biotechnol. https://doi.org/10.3389/fbioe.2020.00242 (2020).
Acknowledgements
Author contributions
Funding
Competing interests
Additional information
Reprints and permissions information is available at www.nature.com/reprints.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
© The Author(s) 2024
- Escuela de Ingeniería y Ciencias, Tecnologico de Monterrey, Reserva Territorial Atlixcáyotl, CP 72453 Puebla, Pue, México.
