DOI: https://doi.org/10.1038/s41392-023-01679-y
PMID: https://pubmed.ncbi.nlm.nih.gov/38169461
تاريخ النشر: 2024-01-03
الأيض الخلوي للزنك وإشارات الزنك: من الوظائف البيولوجية إلى الأمراض والأهداف العلاجية
الملخص
يعد أيض الزنك على المستوى الخلوي أمرًا حيويًا للعديد من العمليات البيولوجية في الجسم. من الملاحظات الرئيسية هو اضطراب التوازن الخلوي، الذي غالبًا ما يتزامن مع تقدم المرض. باعتباره عاملًا أساسيًا في الحفاظ على التوازن الخلوي، أصبح الزنك الخلوي محط اهتمام متزايد في سياق تطور الأمراض. تشير الأبحاث المكثفة إلى تورط الزنك في تعزيز الخباثة والغزو في خلايا السرطان، على الرغم من تركيزه المنخفض في الأنسجة. وقد أدى ذلك إلى تزايد الأدبيات التي تحقق في أيض الزنك الخلوي، لا سيما وظائف ناقلات الزنك وآليات التخزين خلال تقدم السرطان. يخضع نقل الزنك لسيطرة عائلتين رئيسيتين من الناقلات: SLC30 (ZnT) لإفراز الزنك وSLC39 (ZIP) لامتصاص الزنك. بالإضافة إلى ذلك، يتم تخزين هذا العنصر الأساسي بشكل رئيسي بواسطة الميتالوثيونينات (MTs). تجمع هذه المراجعة المعرفة حول الوظائف الحيوية لإشارات الزنك الخلوية وتبرز المسارات الجزيئية المحتملة التي تربط أيض الزنك بتقدم المرض، مع تركيز خاص على السرطان. كما نلخص التجارب السريرية التي تشمل أيونات الزنك. نظرًا للتموضع الرئيسي لناقلات الزنك على غشاء الخلية، فإن الإمكانيات للعلاجات المستهدفة، بما في ذلك الجزيئات الصغيرة والأجسام المضادة وحيدة النسيلة، تقدم آفاقًا واعدة للاستكشاف المستقبلي.
مقدمة
(ZnT) وSLC39 (بروتينات شبيهة بـ Zrt وIrt/ZIP)، بالإضافة إلى البروتينات المرتبطة بالزنك (MTs).
تنظيم إشارات الزنك الخلوية
توزيع الزنك
الإشارات الخلوية للزنك
الذي يساعد في الحفاظ على تركيز الزنك في نطاق البيكو مول في السيتوسول.
الإشارات الزنك خارج الخلية
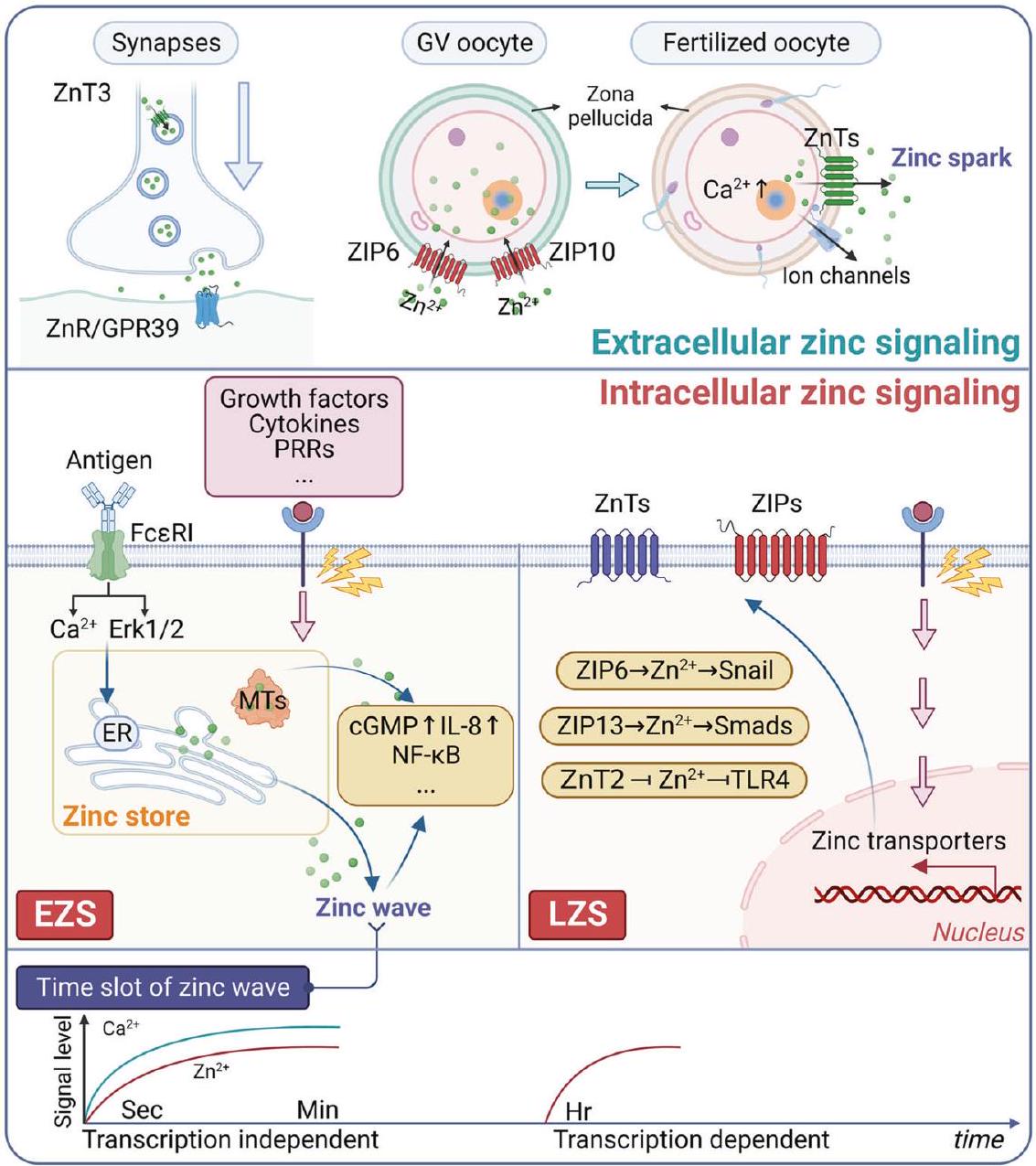
الإشارات الزنك وتكوّن الأورام
تجسيد الأنشطة الحالية للخلايا، مثل الوظيفة، والنمو، والتكاثر. تفسر عدة آليات الوظيفة المضادة للأورام للزنك، وتشمل تلف الحمض النووي، وإصلاح الحمض النووي، ووظيفة الجهاز المناعي، والإجهاد التأكسدي، والالتهاب.
المسار في سرطان البلعوم الأنفي (NPC).
تنظيم أيض الزنك الخلوي
مُجمّعات ZIP. تتألف عائلة SLC39 من أربع مجموعات متميزة بناءً على تشابه تسلسل الأحماض الأمينية: العائلة الفرعية الأولى (ZIP9)؛ العائلة الفرعية الثانية (ZIP1، 2، و3)؛ العائلة الفرعية LIV-1 (ZIP4، 5، 6، 7، 8، 10، 12، 13، و14)؛ والعائلة الفرعية gufA التي تحتوي على ZIP11.
امتصاص أيونات المعادن إلى داخل الخلايا. يقع ZIP7 في جهاز جولجي والشبكة الإندوبلازمية، بينما ZIP13، الأقرب تطوريًا إلى ZIP7، يتموضع في جهاز جولجي والحويصلات السيتوبلازمية.

في تخزين الأنسولين، ZnT 4 في إفراز البروستاتا، وZnT 2 في الرضاعة.
عضو في بروتينات ناقل الزنك ZnT في الثدييات.
المتالثيونينات الثديية هي عائلة فائقة من الببتيدات غير الإنزيمية التي تتكون عادة من 61-68 حمضًا أمينيًا.
دور أيض الزنك الخلوي تحت الظروف الفسيولوجية
دعم وظيفة الجهاز المناعي. تعتبر الخلايا التائية مكونًا حيويًا في الجهاز المناعي.
متمركزة بشكل رئيسي في طوافات الدهون المشاركة في تكوين المشبك المناعي (IS) بعد تحفيز مستقبلات الخلايا التائية (TCR).

يتم التوسط بواسطة ZIP7، حيث أن ZIP7 يتواجد بشكل رئيسي في الشبكة الإندوبلازمية، ومنع إسكات ZIP باستخدام siRNA حدوث موجة الزنك.

وتشوهات في العظام والأنسجة الضامة، تعكس الأنماط الظاهرية التي لوحظت في مرضى متلازمة إهلرز-دانلوس المرتبطة بمرض الخلايا المنجلية.
المشاركة في تكاثر الخلايا، التمايز، والموت المبرمج. أظهرت العديد من الدراسات أن البروتينات المعدنية (MTs) تنظم الزنك، لا سيما فيما يتعلق بتنظيم دورة الخلية وتكاثر الخلايا.
التفريق هو وظيفة غير مباشرة، تتضمن كبت PPAR
إزالة السموم من المعادن الثقيلة، وخاصة الكادميوم والزرنيخ.
كما ذُكر سابقًا، هناك علاقة بين تغيرات مستويات الزنك وتقدم السرطان. ومع ذلك، من الضروري الاعتراف بأن طبيعة هذه العلاقة قد تختلف بين أنواع السرطان المختلفة. تؤكد التأثيرات المتعددة الأوجه للزنك في تعزيز أو تثبيط نمو الأورام على هذه التعقيدات، مع وجود آليات مميزة تعمل في أنواع السرطان المختلفة. تتراكم الأدلة الحديثة التي تشير إلى وجود صلة بين نقص الزنك وتطور السرطانات. تشارك العديد من العمليات في النشاط المضاد للأورام للزنك، بما في ذلك تلف وإصلاح الحمض النووي، التأكسج، المناعة، وعملية الالتهاب.
يُلاحظ ارتفاع تنظيمه في سرطانات الثدي الإيجابية لمستقبلات الإستروجين ويظهر ارتباطًا إيجابيًا مع حالة مستقبلات الإستروجين. خلال التكوّن الجنيني في سمك الزبرا، يتم تنشيط zip6 بواسطة STAT3. يؤدي التعبير المرتفع لـ zip6 إلى احتجاز نووي لـ Snail، المعروف أيضًا بأنه عامل نسخ يحتوي على إصبع زنك، والذي يقوم بعد ذلك بكبت تعبير E-cadherin، مما يؤدي إلى هجرة الخلايا.

تم العثور على نسيج الدماغ بمعدل ضعف ما هو موجود في خط خلايا سرطان الثدي الثلاثي السلبية MDA-MB231. بالإضافة إلى ذلك، تم إثبات زيادة التعبير عن ZIP8 وZIP9 وZIP13 في خلايا BrM2. يُفترض وجود علاقة بين تركيز الزنك داخل الخلايا وإمكانات انتقال خلايا سرطان الثدي.

لوحظ انخفاض التنظيم في تضخم البروستاتا الحميد (BPH)، وخلايا PC-3، والأنسجة الخبيثة للبروستاتا البشرية. يتم تعزيز تعبير MT1/2 بشكل ملحوظ بواسطة علاج الزنك في كل من خلايا PC-3 وBPH، بالتزامن مع استعادة تركيزات الزنك داخل الخلايا. على وجه التحديد، في خلايا BPH، تم تحديد MT3، الذي يعمل كعامل مثبط للنمو، وكانت مستوياته مرتفعة بفعل الزنك. علاوة على ذلك، يُعد تعبير MT3 سمة مميزة توجد حصريًا في خلايا BPH.

الأنماط الظاهرية والنتائج السريرية السيئة لدى مرضى سرطان القولون والمستقيم.
الأيض. ومن المثير للاهتمام أن هذه البروتينات الغنية بالميثيونين غالبًا ما تعمل كجينات كابحة للأورام في سرطان القولون والمستقيم. تم تحديد علاقة ملحوظة بين انخفاض تعبير MT1B أو MT1H أو MT1L وزيادة خطر النتائج السلبية.
أن MT1M لديها القدرة على تقليل الخباثة وخصائص الخلايا الجذعية لسرطان المعدة عن طريق تثبيط GLI1، وهو مكون من مكونات مسار إشارات Hedgehog، المعروف بعدد كبير من مجالات الزنك الإصبعي.
تشن وآخرون

تم التضاعف المشترك لجين SLC30A8 وSLC39A4 في جميع مرضى السرطان تقريبًا. ومن المثير للاهتمام أن الحالات التي تظهر حذفًا لجين SLC39A14 تبدو أكثر من تلك التي تظهر تضاعفًا (الشكل 8). على الرغم من أن بروتينات ZIP تُعتبر عادةً جينات مسرطنة في السرطان، إلا أن سرطان البروستاتا يشكل استثناءً. كما أشارت الدراسات إلى أن وظيفة ناقلات الزنك قد تكون متناقضة بين أنواع السرطان المختلفة. أثناء تعمقنا في التغيرات الجينية في MTs، يجذب انتباهنا التباين المذهل والمتسق الذي لوحظ بين جميع أعضاء MTs (الشكل 8). ومن الجدير بالذكر أن مجموعة البيانات القوية من مرضى الأورام النموذجيين تعرض اتجاهات متجانسة بشكل ملحوظ في التغيرات الجينية بين جميع أعضاء MTs. وتتمثل هذه التغيرات بشكل رئيسي في…
تشمل التوسعات والتسلسلات العميقة، مما يشير إلى أدوار محورية للبروتينات المعدنية في سياق السرطانات. على الرغم من الاتجاهات المماثلة في تغيرات الجينات، لوحظت ملفات تعبير mRNA مختلفة لأعضاء البروتينات المعدنية المختلفة. تشير هذه الملاحظة المثيرة إلى تورط آليات تنظيم نسخ معقدة تتحكم في جينات البروتينات المعدنية. قد تنشأ التنوع في مستويات تعبير mRNA بسبب عوامل عديدة، قد تكون مرتبطة بالسياق الخلوي، خصوصية الأنسجة، وحتى أنواع السرطان. لذلك، لا يزال البحث في ناقلات الزنك والبروتينات المعدنية في تكوين الأورام في بداياته.
الأيض الخلوي للزنك في أمراض القلب والأوعية الدموية
لقد تم اقتراح أن تقليل إنتاج أكسيد النيتريك في المناطق المعرضة للتصلب العصيدي، إلى جانب زيادة تعبير ZnT 1 وMT، قد يؤدي إلى انخفاض الزنك الحر داخل الخلايا.
الصمامات من مرضى تضيق الصمام التاجي التكلسي (CAVD). التأثير المضاد للتكلس للزنك على تكلس خلايا بينية الصمام البشري (hVIC) يتم، على الأقل جزئياً، من خلال تثبيط الاستماتة والتمايز العظمي عبر مسار الإشارة ERK1/2 المعتمد على GPR39. بالإضافة إلى ذلك، يلعب كل من ZIP13 وZIP14 أدوارًا مهمة في تكلس hVIC والتمايز العظمي في المختبر.
يلعب الزنك أدوارًا مختلفة في الأمراض المناعية الذاتية، بما في ذلك دوره كعامل مؤثر في الجهاز المناعي والالتهاب والتمثيل الغذائي. كما ذُكر سابقًا، تعمل عائلة ZIP وعائلة ZnT والبروتينات المعدنية (MTs) كمنظمات حاسمة لمستويات الزنك وتشارك في تطور أمراض مناعية ذاتية مختلفة، مثل إنتاج الأجسام المضادة الذاتية والاستجابات الالتهابية.
يعكس الفقدان المستمر لـ
الأيض الخلوي للزنك في الأمراض المعدية
استعادة حساسية الكاربابينيم في الأكنيتوباكتر بوماني وتحسن البقاء على قيد الحياة في الفئران المصابة بالأسبيرجيلوس فوميغاتوس عندما تم تجويع الممرضات بمُخلبات الزنك.
الحويصلات البلعومية عبر بروتينات ZIP.
تم اقتراح أن التغيرات في توازن الزنك مرتبطة ارتباطًا وثيقًا بتطور بعض الأمراض التنكسية العصبية.
الأهداف العلاجية لأيض الزنك الخلوي
ناقلات الزنك
| الجدول 1. مستويات التعبير، الارتباطات السريرية المرضية، والجزيئات الصغيرة المحتملة لناقلات الزنك في التسرطن | ||||||
| عضو | نوع السرطان | تعبير | علامة تشخيصية | علامة تنبؤية | جزيئات صغيرة | المراجع |
| زيب4 | سرطان الخلايا الكبدية | مرتفع التعبير | – |
|
– | ٣٩٧ |
| الأورام الدبقية | مرتفع التعبير |
|
|
– | ٦٧٩ | |
| قيادة العمليات الخاصة التابعة للجيش الأمريكي | مرتفع التعبير |
|
– | – | ٥٨٥ | |
| الحاسوب الشخصي | مرتفع التعبير |
|
|
– | ٣٩١، ٣٩٣، ٣٩٤، ٣٩٦، ٣٩٩ | |
| شخصية غير قابلة للعب | مرتفع التعبير |
|
|
– | ٧٧ | |
| سرطان الرئة غير صغير الخلايا | مرتفع التعبير | – |
|
– | ٥٨٣ | |
| ZIP5 | ESCC | مرتفع التعبير | – | – | مي آر-193ب | ٥٩٦ |
| زيب6 | ESCC | مرتفع التعبير | – |
|
– | ٤٤٧ |
| قبل الميلاد | مرتفع التعبير |
|
|
SGN-LIV1A/LV (NCT01969643، NCT03310957، NCT03424005، NCT01042379، NCT04032704، NCT02093858) | ١٥٦٬٦٠١٬٦٨٠ | |
| جسم مضاد ZIP6-Y | ١٥٦ | |||||
| فاسلوديكس، 4-هيدروكسي تاموكسيفين | ١٥٧ | |||||
| M1S9 | ٦٠٢ | |||||
| زيب7 | قبل الميلاد | مرتفع التعبير |
|
|
دي إم إيه تي، تي بي بي | ١٦٨ |
| اللمفوما التائية الحادة | مرتفع التعبير | – |
|
NVS-ZP7-4 | ٦٠٣ | |
| زيب9 | سرطان الخلايا الكبدية | مرتفع التعبير | – |
|
– | ٦٨١ |
| سرطان المثانة | مرتفع التعبير | – |
|
دوتاستيريد | ٦٠٤٬٦٠٥ | |
| الميلانوما | مرتفع التعبير | بيكالوتاميد | ٤٦١ | |||
| زيب10 | سرطان العظم | مرتفع التعبير | – |
|
666-15، GSK690693 | ٥٨٩ |
| قبل الميلاد | مرتفع التعبير | – |
|
جسم مضاد ZIP10B | ١٥٦٬٣٣٤ | |
| GC | مرتفع التعبير | – |
|
إكس واي إيه-2 | ٦٨٢ | |
| زيب13 | سرطان المبيض | مرتفع التعبير | – |
|
– | ٧٣ |
| زيب14 | CRC تعني “رمز تصحيح الخطأ الدوري” | مرتفع التعبير | – |
|
– | ٤١٧٬٦٨٣ |
البيئة الدقيقة، تساعد خلايا السرطان على توليد مقاومة كيميائية من خلال تنظيم تركيز الزنك.
الانتشار.
| الجدول 2. إمكانية استهداف استقلاب الزنك في عدة أمراض | |||||||
| بروتين | مرض | تعبير | القيمة الحالية أو المحتملة للاستهداف | ||||
| ZIP5 | داء السكري | منخفض التعبير | الهدف العلاجي المحتمل للأمراض المرتبطة بالسكري.
|
||||
| زيب10 | مرض الدم النخاعي | منخفض التعبير | استهداف ZIP10 قد يكون استراتيجية علاجية جديدة ضد فقر الدم الجنيني المبكر.
|
||||
| زيب14 | العضلات التنكسية | مرتفع التعبير | يؤكد على أهمية تنظيم توازن الزنك في ضمور العضلات الناجم عن السرطان النقيلي ويقترح مسار علاج جديد من خلال استهداف ZIP14.
|
||||
| تليف الكبد | مرتفع التعبير | طريق علاجي جديد محتمل لمنع تليف الكبد الناجم عن موت الحديد.
|
|||||
| ZnT8 | السكري | منخفض النشاط |
|
||||
| MT1/2 | ميلادي | – | تعديل تعبير MT-I/II هو هدف علاجي محتمل لعلاج بداية وتطور ضعف الإدراك.
|
||||
| تكوّن أوعية دموية جديدة في العين | – | MT1/2 هو هدف علاجي جديد محتمل للأمراض التي تنطوي على تكوّن الأوعية الدموية في العين.
|
|||||
الإمكانات العلاجية للبروتينات الغنية بالميثيونين
العلاجات القائمة على الزنك والقياس
تشن وآخرون
| مرض | الجرعة وأنواع الزنك | التأثير/التعليقات | رقم تسجيل التجربة | المراجع |
| التطبيقات السريرية لمكملات الزنك | ||||
| مرحلة ما قبل السكري | 30 ملغ جلوكونات الزنك/يوم، 90 يومًا. | أدى تناول مكملات الزنك إلى انخفاض كبير في مؤشر كتلة الجسم وتحسن في مستوى الجلوكوز الصائم، والجلوكوز بعد ساعتين من الأكل، والهيموغلوبين السكري، والأنسولين، وحساسية الأنسولين، ومقاومة الأنسولين. | – | ٦٨٤ |
| داء السكري من النوع الثاني | 30 ملغ كبريتات الزنك/يوم، 6 أشهر. | تحسين مكملات الزنك لتركيز الجلوكوز الصائم ومؤشر HOMA. كما أظهرت وظيفة خلايا بيتا، وحساسية الأنسولين، ومقاومة الأنسولين تحسناً ملحوظاً. | – | ٦٨٥ |
| 40 ملغ كبريتات الزنك/يوم، 12 أسبوعًا. | لم يُلاحظ تأثير لمكملات الزنك على تركيزات مؤشرات الالتهاب أو التغير النسبي في تعبير جينات ناقل الزنك وجينات البروتين المرتبط بالميتالوثيونين. | NCT01505803 | 686 | |
| 50 ملغ جلوكونات الزنك/يوم، 8 أسابيع. | تم رفع السعة الكلية لمضادات الأكسدة بشكل ملحوظ (
|
IRCT2015083102 | ٦٨٧ | |
| السكري مع الثلاسيميا | 25 ملغ كبريتات الزنك/يوم، 3 أشهر. | مكملات الزنك تحسن توازن الجلوكوز في الثلاسيميا. | NCT01772680 | ٦٨٨ |
| كما | 45 ملغ من جلوكونات الزنك/يوم، لمدة 6 أشهر. | أدى تناول مكملات الزنك إلى خفض مستويات بروتين سي التفاعلي (CRP) والإنترلوكين-6 (IL-6) في البلازما لدى الرجال والنساء. قد يكون للزنك تأثير وقائي على تصلب الشرايين بسبب وظائفه المضادة للالتهابات والمضادة للأكسدة. | – | ٦٨٩ |
| كوفيد-19 | 25 ملغ من الزنك العنصري على شكل كبسولة/يوم، لمدة 15 يومًا. | يمكن أن يقلل الزنك الفموي من معدل الوفاة خلال 30 يومًا، ومعدل دخول وحدة العناية المركزة، ويمكن أن يختصر مدة الأعراض. | NCT05212480. | ٦٩٠ |
| كوفيد-19 | 15 ملغ من الزنك في منتج نشط/يوم، 30 يومًا. | إدارة منتج نشط (ABB C1
|
NCT04798677 | ٦٩١ |
| مرض بهجت | 30 ملغ جلوكونات الزنك/يوم، 12 أسبوعًا. | يمكن اعتبار مكملات جلوكونات الزنك كعلاج مساعد في تخفيف الالتهاب والقرح التناسلية لدى مرضى داء بهجت. | – | ٦٩٢ |
| 30 ملغ جلوكونات الزنك/يوم، 12 أسبوعًا. | أدى تناول مكملات الزنك إلى تحسن كبير في درجة مرض بهجت غير العيني وتعبير TLR-2. | NCT05098678 | ٦٩٣ | |
| فيروس نقص المناعة البشرية-1 | 10 ملغ كبريتات الزنك/يوم، 6 أشهر. | لا يؤدي تناول مكملات الزنك إلى زيادة في الحمل الفيروسي لفيروس HIV-1 في البلازما وقد يقلل من المراضة الناجمة عن الإسهال. | – | ٦٩٤ |
| الكوليرا | 30 ملغ أسيتات الزنك/يوم، حتى زوال الإسهال أو لمدة تصل إلى سبعة أيام. | أدى تناول مكملات الزنك إلى تقليل مدة الإسهال وكمية البراز بشكل كبير لدى الأطفال المصابين بالكوليرا. | NCT00226616 | ٦٩٥ |
| الملاريا | 10 ملغ جلوكونات الزنك/يوم، متوسط فترة المتابعة: 331 يومًا | لم يؤثر الزنك ولا المغذيات المتعددة على معدلات الملاريا | NCT00623857 | ٦٩٦ |
| الثلاسيميا الكبرى | 25 ملغ كبريتات الزنك/يوم، 18 شهرًا. | أدى تناول مكملات الزنك إلى زيادة أكبر في كتلة العظام الكلية للجسم لدى المرضى الشباب المصابين بمرض الثلاسيميا الكبرى. | NCT00459732 | ٦٩٧ |
| الغسيل الكلوي | 78 ملغ من جلوكونات الزنك/يوم، لمدة شهرين. | تكملة الزنك تحسن من تركيزات الألفا-1 البلازمية المرتفعة بشكل غير طبيعي والإجهاد التأكسدي وتحسن حالة السيلينيوم لدى مرضى الغسيل الكلوي طويل الأمد. | – | ٦٩٨ |
| 34 ملغ غسيل دموي/يوم، 12 شهرًا. | يقلل تناول مكملات الزنك من مؤشر استجابة الإريثروبويتين لدى المرضى الذين يخضعون لغسيل الكلى وقد يكون استراتيجية علاجية جديدة للمرضى الذين يعانون من فقر الدم الكلوي وانخفاض مستويات الزنك في الدم. | – | ٦٩٩ | |
| سرطانات الرأس والعنق | 25 ملغ برو-زينك (مسحوق مستخلص من غدة البروستاتا البقرية ثم مرتبط بالزنك) / يوم، لمدة شهرين. | يمكن أن يؤخر تناول مكملات الزنك مع العلاج الإشعاعي تطور التهاب الغشاء المخاطي والتهاب الجلد الشديد لدى المرضى المصابين بسرطانات الرأس والعنق. | – | ٧٠٠ |
| سرطان القولون والمستقيم | 308 ملغ كبريتات الزنك/يوم، 108 أيام. | أدى تناول مكملات الزنك خلال دورات العلاج الكيميائي إلى زيادة نشاط إنزيم SOD والحفاظ على تركيزات فيتامين E، مما يشير إلى إنتاج جذور حرة مستقرة، والتي قد يكون لها تأثير إيجابي على علاج السرطان. | NCT02106806 | ٧٠١ |
| 70 ملغ كبريتات الزنك/يوم، 16 أسبوعًا | مكملات الزنك على مؤشرات الإجهاد التأكسدي في سرطان القولون والمستقيم بعد الجراحة خلال دورات العلاج الكيميائي. | NCT02106806 | – | |
| غلوكونات الزنك، جرعة غير معروفة، 8 أسابيع. | مكمل الزنك في مريض سرطان القولون والمستقيم النقيلي المعالج بريجورافينيب (ZnCORRECT). | NCT03898102 | – | |
| 70 ملغ كبريتات الزنك/يوم، 4 أشهر. | تعديل الاستجابة المناعية عن طريق مكملات الزنك الفموية في العلاج الكيميائي لسرطان القولون والمستقيم. | NCT01261962 | – | |
| ESCC و GC | 22.5 ملغ أكسيد الزنك/يوم، 15.25 سنة. | كان تناول مكملات الزنك مرتبطًا بزيادة في إجمالي الوفيات ووفيات السكتة الدماغية. | – | ٧٠٢ |
| سرطان الجهاز الهضمي | كبريتات الزنك، الجرعة غير معروفة | تأثيرات مكملات الزنك على جودة الحياة لدى مرضى سرطان الجهاز الهضمي. | NCT03819088 | – |
| التطبيق السريري لمُخلِّبات الزنك | ||||
| الصرع | أسبوعان
|
لفحص النشاط المضاد للنوبات المحتمل لكليوكينول في مجموعة صغيرة من المراهقين المصابين بالصرع المقاوم للأدوية | NCT05727943 | – |
| السرطان الدموي | 800 ملغ كليوكينول/يوم، 28 يومًا. | لتقييم السمية المحددة للجرعة، والجرعة القصوى المحتملة التحمل، والجرعة الموصى بها للمرحلة الثانية من عقار الكليوكينول في المرضى الذين يعانون من أمراض دموية خبيثة متكررة أو مقاومة للعلاج. | NCT00963495 | – |
| الفئة | الاسم | كد | العُضيّات المستهدفة | المراجع |
| فريت | زيف |
|
– | ٧٠٣ |
| زاب سي واي 1 |
|
جولجي، الشبكة الإندوبلازمية، الميتوكوندريا | ٦٧٢٬٦٧٨ | |
| eCALWY-4 |
|
قسم الطوارئ، الميتوكوندريا | ٦٧٥ | |
| eZinCh-2 |
|
الطوارئ، الميتوكوندريا | ٦٧٦ | |
| GZnP1 |
|
– | ٧٠٤ | |
| بريت | BLZinCh-1 |
|
الطوارئ، الميتوكوندريا | ٦٧١ |
| BLZinCh-2 |
|
الطوارئ، الميتوكوندريا | ٦٧١ | |
| BLZinCh-3 |
|
قسم الطوارئ، الميتوكوندريا | ٦٧١ | |
| LMW | فلوازين-3-إيه إم |
|
الطوارئ، الميتوكوندريا | ٧٠٥ |
| زينباير (ZP) |
|
جولجي، الميتوكوندريا | ٧٠٦ | |
| ZnAF |
|
– | ٧٠٧ | |
| رودزين-353 | – | الميتوكوندريا | ٧٠٨،٧٠٩ | |
| زيرف |
|
– | ٧١٠ | |
| TSQ | – | السيتوبلازم | ٧١١ |
يخفف من التوتر المعدني والأكسدي في أنسجة الكلى لدى الفئران المصابة بالسكري الناتج عن الستربتوزوتوسين، مما يمنع تطور اعتلال الكلية السكري.
نحاس.
وبروتينات الفلورسنت المشفرة وراثيًا.
الخاتمة والاتجاه المستقبلي
الميثلة في سرطان القولون والمستقيم. التعبير الشاذ أو فرط تنشيط ناقلات الزنك قد يساهم أيضًا في مقاومة الورم، مما قد يكون عاملًا سيئًا في توقعات المرضى المصابين بالسرطان. لذلك، من المتوقع أن يؤدي استهداف ناقلات الزنك إلى تحسين فعالية علاجات الأورام. في الوقت نفسه، نظرًا لأن بروتينات ناقلات الزنك موزعة بشكل رئيسي على أغشية الخلايا، فإن تطوير جزيئات صغيرة أو أجسام مضادة وحيدة النسيلة للاستهداف المحدد أمر ممكن.
الشكر والتقدير
مجموعات البيانات والتحليل. تم إنشاء جزء من الصور بواسطة BioRender (https:// biorender.com/) و GEPIA2 (http://gepia2.cancer-pku.cn/#isoform). نحن نقدر أيضًا الدعم الفني من قسم المرافق الأساسية لجينوميات السرطان والبيولوجيا المرضية في قسم علم الأمراض التشريحي والخلوي، الجامعة الصينية في هونغ كونغ.
مساهمات المؤلف
معلومات إضافية
REFERENCES
- Huang, L. & Tepaamorndech, S. The SLC30 family of zinc transporters – a review of current understanding of their biological and pathophysiological roles. Mol. Asp. Med. 34, 548-560 (2013).
- Kambe, T., Tsuji, T., Hashimoto, A. & Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 95, 749-784 (2015).
- Kimura, T. & Kambe, T. The Functions of Metallothionein and ZIP and ZnT Transporters: An Overview and Perspective. Int J. Mol. Sci. 17, 336 (2016).
- Hu, H. et al. New anti-cancer explorations based on metal ions. J. Nanobiotechnol. 20, 457 (2022).
- Stockwell, B. R., Jiang, X. & Gu, W. Emerging mechanisms and disease relevance of ferroptosis. Trends Cell Biol. 30, 478-490 (2020).
- Andreini, C., Bertini, I. & Rosato, A. Metalloproteomes: a bioinformatic approach. Acc. Chem. Res. 42, 1471-1479 (2009).
- Angus-Hill, M. L. et al. A Rsc3/Rsc30 zinc cluster dimer reveals novel roles for the chromatin remodeler RSC in gene expression and cell cycle control. Mol. Cell. 7, 741-751 (2001).
- Kim, A. M. et al. Zinc sparks are triggered by fertilization and facilitate cell cycle resumption in mammalian eggs. ACS Chem. Biol. 6, 716-723 (2011).
- Lo, M. N. et al. Single cell analysis reveals multiple requirements for zinc in the mammalian cell cycle. Elife 9, e51107 (2020).
- Haase, H. & Rink, L. Multiple impacts of zinc on immune function. Metallomics 6, 1175-1180 (2014).
- Que, E. L. et al. Quantitative mapping of zinc fluxes in the mammalian egg reveals the origin of fertilization-induced zinc sparks. Nat. Chem. 7, 130-139 (2015).
- Maret, W. Analyzing free zinc(II) ion concentrations in cell biology with fluorescent chelating molecules. Metallomics 7, 202-211 (2015).
- Hennigar, S. R., Kelley, A. M. & McClung, J. P. Metallothionein and zinc transporter expression in circulating human blood cells as biomarkers of zinc status: a systematic review. Adv. Nutr. 7, 735-746 (2016).
- Bafaro, E., Liu, Y., Xu, Y. & Dempski, R. E. The emerging role of zinc transporters in cellular homeostasis and cancer. Signal Transduct. Target Ther. 2, 17029- (2017).
- Calesnick, B. & Dinan, A. M. Zinc deficiency and zinc toxicity. Am. Fam. Physician 37, 267-270 (1988).
- Stefanidou, M., Maravelias, C., Dona, A. & Spiliopoulou, C. Zinc: a multipurpose trace element. Arch. Toxicol. 80, 1-9 (2006).
- Gilbert, R., Peto, T., Lengyel, I. & Emri, E. Zinc nutrition and inflammation in the aging retina. Mol. Nutr. Food Res. 63, e1801049 (2019).
- Pfeiffer, C. C. & Braverman, E. R. Zinc, the brain and behavior. Biol. Psychiatry 17, 513-532 (1982).
- Tapiero, H. & Tew, K. D. Trace elements in human physiology and pathology: zinc and metallothioneins. Biomed. Pharmacother. 57, 399-411 (2003).
- Costello, L. C., Fenselau, C. C. & Franklin, R. B. Evidence for operation of the direct zinc ligand exchange mechanism for trafficking, transport, and reactivity of zinc in mammalian cells. J. Inorg. Biochem. 105, 589-599 (2011).
- Maret, W. Zinc coordination environments in proteins as redox sensors and signal transducers. Antioxid. Redox Signal. 8, 1419-1441 (2006).
- Turan, B. & Tuncay, E. Impact of labile zinc on heart function: from physiology to pathophysiology. Int J. Mol. Sci. 18, 2395 (2017).
- Coyle, P., Philcox, J. C., Carey, L. C. & Rofe, A. M. Metallothionein: the multipurpose protein. Cell Mol. Life Sci. 59, 627-647 (2002).
- Outten, C. E. & O’Halloran, T. V. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 292, 2488-2492 (2001).
30
25. Blindauer, C. A. & Leszczyszyn, O. I. Metallothioneins: unparalleled diversity in structures and functions for metal ion homeostasis and more. Nat. Prod. Rep. 27, 720-741 (2010).
26. Wang, X. L., Schnoor, M. & Yin, L. M. Metallothionein-2: an emerging target in inflammatory diseases and cancers. Pharm. Ther. 244, 108374 (2023).
27. Amagai, Y. et al. Zinc homeostasis governed by Golgi-resident ZnT family members regulates ERp44-mediated proteostasis at the ER-Golgi interface. Nat. Commun. 14, 2683 (2023).
28. Fang, H. et al. Simultaneous
29. Frederickson, C. J., Koh, J. Y. & Bush, A. I. The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 6, 449-462 (2005).
30. Eide, D. J. The SLC39 family of metal ion transporters. Pflug. Arch. 447, 796-800 (2004).
31. Bin, B. H. et al. Molecular pathogenesis of spondylocheirodysplastic EhlersDanlos syndrome caused by mutant ZIP13 proteins. EMBO Mol. Med. 6, 1028-1042 (2014).
32. Wang, Z., Tymianski, M., Jones, O. T. & Nedergaard, M. Impact of cytoplasmic calcium buffering on the spatial and temporal characteristics of intercellular calcium signals in astrocytes. J. Neurosci. 17, 7359-7371 (1997).
33. Krezel, A. & Maret, W. Zinc-buffering capacity of a eukaryotic cell at physiological pZn. J. Biol. Inorg. Chem. 11, 1049-1062 (2006).
34. Atrián-Blasco, E. et al. Chemistry of mammalian metallothioneins and their interaction with amyloidogenic peptides and proteins. Chem. Soc. Rev. 46, 7683-7693 (2017).
35. Krezel, A. & Maret, W. Dual nanomolar and picomolar
36. Colvin, R. A., Holmes, W. R., Fontaine, C. P. & Maret, W. Cytosolic zinc buffering and muffling: their role in intracellular zinc homeostasis. Metallomics 2, 306-317 (2010).
37. Ueda, S. et al. Early secretory pathway-resident Zn transporter proteins contribute to cellular sphingolipid metabolism through activation of sphingomyelin phosphodiesterase 1. Am. J. Physiol. Cell Physiol. 322, C948-c959 (2022).
38. Wagatsuma, T. et al. Pigmentation and TYRP1 expression are mediated by zinc through the early secretory pathway-resident ZNT proteins. Commun. Biol. 6, 403 (2023).
39. Chandler, P. et al. Subtype-specific accumulation of intracellular zinc pools is associated with the malignant phenotype in breast cancer. Mol. Cancer 15, 2 (2016).
40. Beyer, N. et al. ZnT 3 mRNA levels are reduced in Alzheimer’s disease postmortem brain. Mol. Neurodegener. 4, 53 (2009).
41. Chimienti, F., Devergnas, S., Favier, A. & Seve, M. Identification and cloning of a beta-cell-specific zinc transporter,
42. Maret, W. Redox biochemistry of mammalian metallothioneins. J. Biol. Inorg. Chem. 16, 1079-1086 (2011).
43. Hirano, T. et al. Roles of zinc and zinc signaling in immunity: zinc as an intracellular signaling molecule. Adv. Immunol. 97, 149-176 (2008).
44. Yamasaki, S. et al. Zinc is a novel intracellular second messenger. J. Cell Biol. 177, 637-645 (2007).
45. Bonaventura, P., Benedetti, G., Albarède, F. & Miossec, P. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 14, 277-285 (2015).
46. Liu, W. et al. Lactate regulates cell cycle by remodelling the anaphase promoting complex. Nature 616, 790-797 (2023).
47. Wang, L. et al. Co-implantation of magnesium and zinc ions into titanium regulates the behaviors of human gingival fibroblasts. Bioact. Mater. 6, 64-74 (2021).
48. Xiao, W. et al. Therapeutic targeting of the USP2-E2F4 axis inhibits autophagic machinery essential for zinc homeostasis in cancer progression. Autophagy 18, 2615-2635 (2022).
49. Supasai, S. et al. Zinc deficiency affects the STAT1/3 signaling pathways in part through redox-mediated mechanisms. Redox Biol. 11, 469-481 (2017).
50. He, X. et al. The zinc transporter SLC39A10 plays an essential role in embryonic hematopoiesis. Adv. Sci. 10, e2205345 (2023).
51. Feske, S., Wulff, H. & Skolnik, E. Y. Ion channels in innate and adaptive immunity. Annu Rev. Immunol. 33, 291-353 (2015).
52. Chaigne-Delalande, B. & Lenardo, M. J. Divalent cation signaling in immune cells. Trends Immunol. 35, 332-344 (2014).
53. Ma, T. et al. A pair of transporters controls mitochondrial
54. Chen, H. C. et al. Sub-acute restraint stress progressively increases oxidative/ nitrosative stress and inflammatory markers while transiently upregulating antioxidant gene expression in the rat hippocampus. Free Radic. Biol. Med. 130, 446-457 (2019).
55. Si, M. & Lang, J. The roles of metallothioneins in carcinogenesis. J. Hematol. Oncol. 11, 107 (2018).
56. Aras, M. A. & Aizenman, E. Redox regulation of intracellular zinc: molecular signaling in the life and death of neurons. Antioxid. Redox Signal. 15, 2249-2263 (2011).
57. McCord, M. C. & Aizenman, E. Convergent Ca2+ and
58. Millward, D. J. Nutrition, infection and stunting: the roles of deficiencies of individual nutrients and foods, and of inflammation, as determinants of reduced linear growth of children. Nutr. Res Rev. 30, 50-72 (2017).
59. Ren, M. et al. Associations between hair levels of trace elements and the risk of preterm birth among pregnant Wwomen: a prospective nested case-control study in Beijing Birth Cohort (BBC), China. Environ. Int. 158, 106965 (2022).
60. Chorin, E. et al. Upregulation of KCC2 activity by zinc-mediated neurotransmission via the mZnR/GPR39 receptor. J. Neurosci. 31, 12916-12926 (2011).
61. Anderson, C. T. et al. Modulation of extrasynaptic NMDA receptors by synaptic and tonic zinc. Proc. Natl Acad. Sci. USA. 112, E2705-E2714 (2015).
62. Medvedeva, Y. V., Ji, S. G., Yin, H. Z. & Weiss, J. H. Differential vulnerability of CA1 versus CA3 pyramidal neurons after ischemia: possible relationship to sources of
63. Michelotti, F. C. et al. PET/MRI enables simultaneous in vivo quantification of
64. Carver, C. M., Chuang, S. H. & Reddy, D. S. Zinc selectively blocks neurosteroidsensitive extrasynaptic
65. Dostalova, Z. et al. Human
66. Sensi, S. L., Paoletti, P., Bush, A. I. & Sekler, I. Zinc in the physiology and pathology of the CNS. Nat. Rev. Neurosci. 10, 780-791 (2009).
67. Olesen, R. H. et al. Obesity and age-related alterations in the gene expression of zinc-transporter proteins in the human brain. Transl. Psychiatry 6, e838 (2016).
68. Ren, L. et al. Amperometric measurements and dynamic models reveal a mechanism for how zinc alters neurotransmitter release. Angew. Chem. Int Ed. Engl. 59, 3083-3087 (2020).
69. Hershfinkel, M. The zinc sensing receptor, ZnR/GPR39, in health and disease. Int J. Mol. Sci. 19, 439 (2018).
70. Ho, E. & Ames, B. N. Low intracellular zinc induces oxidative DNA damage, disrupts p53, NFkappa B, and AP1 DNA binding, and affects DNA repair in a rat glioma cell line. Proc. Natl Acad. Sci. USA. 99, 16770-16775 (2002).
71. Nuñez, N. N. et al. The zinc linchpin motif in the DNA repair glycosylase MUTYH: identifying the
72. Lecane, P. S. et al. Motexafin gadolinium and zinc induce oxidative stress responses and apoptosis in B-cell lymphoma lines. Cancer Res. 65, 11676-11688 (2005).
73. Cheng, X. et al. Zinc transporter SLC39A13/ZIP13 facilitates the metastasis of human ovarian cancer cells via activating Src/FAK signaling pathway. J. Exp. Clin. Cancer Res. 40, 199 (2021).
74. Liu, M. et al. Zinc-dependent regulation of ZEB1 and YAP1 coactivation promotes epithelial-mesenchymal transition plasticity and metastasis in pancreatic cancer. Gastroenterology 160, 1771-1783.e1771 (2021).
75. Yang, J. et al. ZIP4 promotes muscle wasting and cachexia in mice with orthotopic pancreatic tumors by stimulating RAB27B-regulated release of extracellular vesicles from cancer cells. Gastroenterology 156, 722-734.e726 (2019).
76. Wagner, E. F. & Nebreda, A. R. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 9, 537-549 (2009).
77. Zeng, Q. et al. Inhibition of ZIP4 reverses epithelial-to-mesenchymal transition and enhances the radiosensitivity in human nasopharyngeal carcinoma cells. Cell Death Dis. 10, 588 (2019).
78. Qi, J. et al. MCOLN1/TRPML1 finely controls oncogenic autophagy in cancer by mediating zinc influx. Autophagy 17, 4401-4422 (2021).
79. Su, X. et al. Disruption of zinc homeostasis by a novel platinum(IV)-terthiophene complex for antitumor immunity. Angew. Chem. Int Ed. Engl. 62, e202216917 (2023).
80. Jeong, J. & Eide, D. J. The SLC39 family of zinc transporters. Mol. Asp. Med. 34, 612-619 (2013).
81. Zhang, T., Sui, D. & Hu, J. Structural insights of ZIP4 extracellular domain critical for optimal zinc transport. Nat. Commun. 7, 11979 (2016).
82. Zhang, T. et al. Crystal structures of a ZIP zinc transporter reveal a binuclear metal center in the transport pathway. Sci. Adv. 3, e1700344 (2017).
83. Pang, C. et al. Structural mechanism of intracellular autoregulation of zinc uptake in ZIP transporters. Nat. Commun. 14, 3404 (2023).
84. Bogdan, A. R., Miyazawa, M., Hashimoto, K. & Tsuji, Y. Regulators of iron homeostasis: new players in metabolism, cell death, and disease. Trends Biochem Sci. 41, 274-286 (2016).
85. Jeong, J. et al. Promotion of vesicular zinc efflux by ZIP13 and its implications for spondylocheiro dysplastic Ehlers-Danlos syndrome. Proc. Natl Acad. Sci. USA. 109, E3530-E3538 (2012).
86. Bin, B. H. et al. Biochemical characterization of human ZIP13 protein: a homodimerized zinc transporter involved in the spondylocheiro dysplastic EhlersDanlos syndrome. J. Biol. Chem. 286, 40255-40265 (2011).
87. Lichten, L. A. et al. MTF-1-mediated repression of the zinc transporter Zip10 is alleviated by zinc restriction. PLoS One 6, e21526 (2011).
88. Ryu, M. S., Lichten, L. A., Liuzzi, J. P. & Cousins, R. J. Zinc transporters ZnT1 (Slc30a1), Zip8 (SIc39a8), and Zip10 (SIc39a10) in mouse red blood cells are differentially regulated during erythroid development and by dietary zinc deficiency. J. Nutr. 138, 2076-2083 (2008).
89. Liuzzi, J. P. et al. Responsive transporter genes within the murine intestinalpancreatic axis form a basis of zinc homeostasis. Proc. Natl Acad. Sci. USA. 101, 14355-14360 (2004).
90. Taylor, K. M. & Nicholson, R. I. The LZT proteins; the LIV-1 subfamily of zinc transporters. Biochim. Biophys. Acta 1611, 16-30 (2003).
91. Xin, Y. et al. Manganese transporter Slc39a14 deficiency revealed its key role in maintaining manganese homeostasis in mice. Cell Discov. 3, 17025 (2017).
92. Polesel, M. et al. Functional characterization of SLC39 family members ZIP5 and ZIP10 in overexpressing HEK293 cells reveals selective copper transport activity. Biometals 36, 227-237 (2023).
93. Boycott, K. M. et al. Autosomal-recessive intellectual disability with cerebellar atrophy syndrome caused by mutation of the manganese and zinc transporter gene SLC39A8. Am. J. Hum. Genet. 97, 886-893 (2015).
94. Jorge-Nebert, L. F. et al. Comparing gene expression during cadmium uptake and distribution: untreated versus oral Cd-treated wild-type and ZIP14 knockout mice. Toxicol. Sci. 143, 26-35 (2015).
95. Himeno, S., Yanagiya, T. & Fujishiro, H. The role of zinc transporters in cadmium and manganese transport in mammalian cells. Biochimie 91, 1218-1222 (2009).
96. Nebert, D. W. & Liu, Z. SLC39A8 gene encoding a metal ion transporter: discovery and bench to bedside. Hum. Genomics. 13, 51 (2019).
97. Liu, Z. et al. Cd2+ versus Zn2+ uptake by the ZIP8 HCO3-dependent symporter: kinetics, electrogenicity and trafficking. Biochem. Biophys. Res Commun. 365, 814-820 (2008).
98. Napolitano, J. R. et al. Cadmium-mediated toxicity of lung epithelia is enhanced through NF-кB-mediated transcriptional activation of the human zinc transporter ZIP8. Am. J. Physiol. Lung Cell Mol. Physiol. 302, L909-L918 (2012).
99. Girijashanker, K. et al. Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: similarities to the ZIP8 transporter. Mol. Pharmacol. 73, 1413-1423 (2008).
100. Pinilla-Tenas, J. J. et al. Zip14 is a complex broad-scope metal-ion transporter whose functional properties support roles in the cellular uptake of zinc and nontransferrin-bound iron. Am. J. Physiol. Cell Physiol. 301, C862-C871 (2011).
101. Liuzzi, J. P. et al. Zip14 (SIc39a14) mediates non-transferrin-bound iron uptake into cells. Proc. Natl Acad. Sci. USA. 103, 13612-13617 (2006).
102. Wang, C. Y. et al. ZIP8 is an iron and zinc transporter whose cell-surface expression is up-regulated by cellular iron loading. J. Biol. Chem. 287, 34032-34043 (2012).
103. Jenkitkasemwong, S. et al. SLC39A14 is required for the development of hepatocellular iron overload in murine models of hereditary hemochromatosis. Cell Metab. 22, 138-150 (2015).
104. Kambe, T., Matsunaga, M. & Takeda, T. A. Understanding the contribution of zinc transporters in the function of the early secretory pathway. Int J. Mol. Sci. 18, 2179 (2017).
105. Davidson, H. W., Wenzlau, J. M. & O’Brien, R. M. Zinc transporter 8 (ZnT8) and beta cell function. Trends Endocrinol. Metab. 25, 415-424 (2014).
106. Suzuki, T. et al. Zinc transporters, ZnT5 and ZnT7, are required for the activation of alkaline phosphatases, zinc-requiring enzymes that are glycosylphosphatidylinositol-anchored to the cytoplasmic membrane. J. Biol. Chem. 280, 637-643 (2005).
107. Nishito, Y. & Kambe, T. Zinc transporter 1 (ZNT1) expression on the cell surface is elaborately controlled by cellular zinc levels. J. Biol. Chem. 294, 15686-15697 (2019).
108. Lichten, L. A. & Cousins, R. J. Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev. Nutr. 29, 153-176 (2009).
109. Wang, Y. et al. Zinc application alleviates the adverse renal effects of arsenic stress in a protein quality control way in common carp. Environ. Res. 191, 110063 (2020).
110. Dwivedi, O. P. et al. Loss of ZnT 8 function protects against diabetes by enhanced insulin secretion. Nat. Genet. 51, 1596-1606 (2019).
111. Henshall, S. M. et al. Expression of the zinc transporter ZnT 4 is decreased in the progression from early prostate disease to invasive prostate cancer. Oncogene 22, 6005-6012 (2003).
112. Sanchez, V. B., Ali, S., Escobar, A. & Cuajungco, M. P. Transmembrane 163 (TMEM163) protein effluxes zinc. Arch. Biochem. Biophys. 677, 108166 (2019).
113. Styrpejko, D. J. & Cuajungco, M. P. Transmembrane 163 (TMEM163) protein: a new member of the zinc efflux transporter family. Biomedicines 9, 220 (2021).
114. do Rosario, M. C. et al. Variants in the zinc transporter TMEM163 cause a hypomyelinating leukodystrophy. Brain 145, 4202-4209 (2022).
115. Kia, D. A. et al. Identification of candidate Parkinson disease genes by integrating genome-wide association study, expression, and epigenetic data sets. JAMA Neurol. 78, 464-472 (2021).
116. Yuan, Y. et al. A zinc transporter, transmembrane protein 163, is critical for the biogenesis of platelet dense granules. Blood 137, 1804-1817 (2021).
117. Braun, W. et al. Comparison of the NMR solution structure and the x-ray crystal structure of rat metallothionein-2. Proc. Natl Acad. Sci. USA. 89, 10124-10128 (1992).
118. Krężel, A. & Maret, W. The bioinorganic chemistry of mammalian metallothioneins. Chem. Rev. 121, 14594-14648 (2021).
119. Merlos Rodrigo, M. A. et al. Metallothionein isoforms as double agents – their roles in carcinogenesis, cancer progression and chemoresistance. Drug Resist. Updat. 52, 100691 (2020).
120. Go, Y. M., Chandler, J. D. & Jones, D. P. The cysteine proteome. Free Radic. Biol. Med. 84, 227-245 (2015).
121. Marreiro, D. D. et al. Zinc and oxidative stress: current mechanisms. Antioxidants. 6, 24 (2017).
122. Guo, L. et al. STAT5-glucocorticoid receptor interaction and MTF-1 regulate the expression of ZnT2 (Slc30a2) in pancreatic acinar cells. Proc. Natl Acad. Sci. USA. 107, 2818-2823 (2010).
123. Lu, Y. J. et al. Coordinative modulation of human zinc transporter 2 gene expression through active and suppressive regulators. J. Nutr. Biochem. 26, 351-359 (2015).
124. Mocchegiani, E., Giacconi, R. & Malavolta, M. Zinc signalling and subcellular distribution: emerging targets in type 2 diabetes. Trends Mol. Med. 14, 419-428 (2008).
125. O’Donnell, J. S., Teng, M. W. L. & Smyth, M. J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 16, 151-167 (2019).
126. Kim, B., Kim, H. Y. & Lee, W. W. Zap70 regulates TCR-mediated Zip6 activation at the immunological synapse. Front. Immunol. 12, 687367 (2021).
127. Lee, W. W. et al. Age-dependent signature of metallothionein expression in primary CD4 T cell responses is due to sustained zinc signaling. Rejuvenation Res. 11, 1001-1011 (2008).
128. Pommier, A. et al. Inflammatory monocytes are potent antitumor effectors controlled by regulatory CD4+ T cells. Proc. Natl Acad. Sci. USA. 110, 13085-13090 (2013).
129. Aydemir, T. B., Liuzzi, J. P., McClellan, S. & Cousins, R. J. Zinc transporter ZIP8 (SLC39A8) and zinc influence IFN-gamma expression in activated human T cells. J. Leukoc. Biol. 86, 337-348 (2009).
130. Liu, M. J. et al. ZIP8 regulates host defense through zinc-mediated inhibition of NF-kappaB. Cell Rep. 3, 386-400 (2013).
131. Begum, N. A. et al. Mycobacterium bovis BCG cell wall and lipopolysaccharide induce a novel gene, BIGM103, encoding a 7-TM protein: identification of a new protein family having Zn-transporter and Zn-metalloprotease signatures. Genomics 80, 630-645 (2002).
132. Kim, B. et al. Cytoplasmic zinc promotes IL-1 beta production by monocytes and macrophages through mTORC1-induced glycolysis in rheumatoid arthritis. Sci. Signal. 15, eabi7400 (2022).
133. Kang, J. A. et al. ZIP8 exacerbates collagen-induced arthritis by increasing pathogenic T cell responses. Exp. Mol. Med. 53, 560-571 (2021).
134. Abd El-Rehim, D. M. et al. High-throughput protein expression analysis using tissue microarray technology of a large well-characterised series identifies biologically distinct classes of breast cancer confirming recent cDNA expression analyses. Int J. Cancer 116, 340-350 (2005).
135. Lee, D. S. W., Rojas, O. L. & Gommerman, J. L. B cell depletion therapies in autoimmune disease: advances and mechanistic insights. Nat. Rev. Drug Discov. 20, 179-199 (2021).
136. Taniguchi, M. et al. Essential role of the zinc transporter ZIP9/SLC39A9 in regulating the activations of Akt and Erk in B-cell receptor signaling pathway in DT40 cells. PLoS One 8, e58022 (2013).
137. Miyai, T. et al. Zinc transporter SLC39A10/ZIP10 facilitates antiapoptotic signaling during early B-cell development. Proc. Natl Acad. Sci. USA. 111, 11780-11785 (2014).
138. Hojyo, S. et al. Zinc transporter SLC39A10/ZIP10 controls humoral immunity by modulating B-cell receptor signal strength. Proc. Natl Acad. Sci. USA. 111, 11786-11791 (2014).
139. Ma, Z. et al. SLC39A10 upregulation predicts poor prognosis, promotes proliferation and migration, and correlates with immune infiltration in hepatocellular carcinoma. J. Hepatocell. Carcinoma 8, 899-912 (2021).
140. Stafford, S. L. et al. Metal ions in macrophage antimicrobial pathways: emerging roles for zinc and copper. Biosci. Rep. 33, e00049 (2013).
141. Locati, M., Curtale, G. & Mantovani, A. Diversity, mechanisms, and significance of macrophage plasticity. Annu. Rev. Pathol. 15, 123-147 (2020).
142. Gao, H. et al. Metal transporter Slc39a10 regulates susceptibility to inflammatory stimuli by controlling macrophage survival. Proc. Natl Acad. Sci. USA. 114, 12940-12945 (2017).
143. Sriskandan, S. & Altmann, D. M. The immunology of sepsis. J. Pathol. 214, 211-223 (2008).
144. Wong, H. R. et al. Genome-level expression profiles in pediatric septic shock indicate a role for altered zinc homeostasis in poor outcome. Physiol. Genomics. 30, 146-155 (2007).
145. Besecker, B. et al. The human zinc transporter SLC39A8 (Zip8) is critical in zincmediated cytoprotection in lung epithelia. Am. J. Physiol. Lung Cell Mol. Physiol. 294, L1127-L1136 (2008).
146. Besecker, B. Y. et al. A comparison of zinc metabolism, inflammation, and disease severity in critically ill infected and noninfected adults early after intensive care unit admission. Am. J. Clin. Nutr. 93, 1356-1364 (2011).
147. Wessels, I. & Cousins, R. J. Zinc dyshomeostasis during polymicrobial sepsis in mice involves zinc transporter Zip14 and can be overcome by zinc supplementation. Am. J. Physiol. Gastrointest. Liver Physiol. 309, G768-G778 (2015).
148. Hogstrand, C., Kille, P., Nicholson, R. I. & Taylor, K. M. Zinc transporters and cancer: a potential role for ZIP7 as a hub for tyrosine kinase activation. Trends Mol. Med. 15, 101-111 (2009).
149. Adulcikas, J. et al. The zinc transporter SLC39A7 (ZIP7) harbours a highlyconserved histidine-rich N-terminal region that potentially contributes to zinc homeostasis in the endoplasmic reticulum. Comput Biol. Med. 100, 196-202 (2018).
150. Uchida, R. et al. L-type calcium channel-mediated zinc wave is involved in the regulation of IL-6 by stimulating non-IgE with LPS and IL-33 in mast cells and dendritic cells. Biol. Pharm. Bull. 42, 87-93 (2019).
151. Levy, S. et al. Molecular basis for zinc transporter 1 action as an endogenous inhibitor of L-type calcium channels. J. Biol. Chem. 284, 32434-32443 (2009).
152. Maret, W. Zinc in cellular regulation: the nature and significance of “zinc signals”. Int J. Mol. Sci. 18, 2285 (2017).
153. Kim, A. M., Vogt, S., O’Halloran, T. V. & Woodruff, T. K. Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nat. Chem. Biol. 6, 674-681 (2010).
154. Taylor, K. M. et al. Zinc transporter ZIP10 forms a heteromer with ZIP6 which regulates embryonic development and cell migration. Biochem J. 473, 2531-2544 (2016).
155. Kong, B. Y. et al. Maternally-derived zinc transporters ZIP6 and ZIP10 drive the mammalian oocyte-to-egg transition. Mol. Hum. Reprod. 20, 1077-1089 (2014).
156. Nimmanon, T. et al. The ZIP6/ZIP10 heteromer is essential for the zinc-mediated trigger of mitosis. Cell Mol. Life Sci. 78, 1781-1798 (2021).
157. Hogstrand, C. et al. A mechanism for epithelial-mesenchymal transition and anoikis resistance in breast cancer triggered by zinc channel ZIP6 and STAT3 (signal transducer and activator of transcription 3). Biochem. J. 455, 229-237 (2013).
158. Mulay, I. L. et al. Trace-metal analysis of cancerous and noncancerous human tissues. J. Natl Cancer Inst. 47, 1-13 (1971).
159. Chen, P. H. et al. Zinc transporter ZIP7 is a novel determinant of ferroptosis. Cell Death Dis. 12, 198 (2021).
160. Makhov, P. et al. Zinc chelation induces rapid depletion of the X-linked inhibitor of apoptosis and sensitizes prostate cancer cells to TRAIL-mediated apoptosis. Cell Death Differ. 15, 1745-1751 (2008).
161. Zhang, R. et al. Zinc regulates primary ovarian tumor growth and metastasis through the epithelial to mesenchymal transition. Free Radic. Biol. Med. 160, 775-783 (2020).
162. Hernandez-Camacho, J. D., Vicente-Garcia, C., Parsons, D. S. & Navas-Enamorado, I. Zinc at the crossroads of exercise and proteostasis. Redox Biol. 35, 101529 (2020).
163. Ohashi, K. et al. Zinc promotes proliferation and activation of myogenic cells via the PI3K/Akt and ERK signaling cascade. Exp. Cell Res. 333, 228-237 (2015).
164. Lee, H. Y. et al. Deletion of Jazf1 gene causes early growth retardation and insulin resistance in mice. Proc. Natl Acad. Sci. USA. 119, e2213628119 (2022).
165. Jinno, N., Nagata, M. & Takahashi, T. Marginal zinc deficiency negatively affects recovery from muscle injury in mice. Biol. Trace Elem. Res. 158, 65-72 (2014).
166. Lin, P. H. et al. Zinc in wound healing modulation. Nutrients 10, 16 (2017).
167. Postigo, A. A. & Dean, D. C. Differential expression and function of members of the zfh-1 family of zinc finger/homeodomain repressors. Proc. Natl Acad. Sci. USA. 97, 6391-6396 (2000).
168. Taylor, K. M. et al. Protein kinase CK2 triggers cytosolic zinc signaling pathways by phosphorylation of zinc channel ZIP7. Sci. Signal. 5, ra11 (2012).
169. Mnatsakanyan, H., Serra, R. S. I., Rico, P. & Salmeron-Sanchez, M. Zinc uptake promotes myoblast differentiation via Zip7 transporter and activation of Akt signalling transduction pathway. Sci. Rep. 8, 13642 (2018).
170. Nimmanon, T. et al. Phosphorylation of zinc channel ZIP7 drives MAPK, PI3K and mTOR growth and proliferation signalling. Metallomics 9, 471-481 (2017).
171. Mapley, J. I., Wagner, P., Officer, D. L. & Gordon, K. C. Computational and spectroscopic analysis of beta-indandione modified zinc porphyrins. J. Phys. Chem. A. 122, 4448-4456 (2018).
172. Giunta, C. et al. Spondylocheiro dysplastic form of the Ehlers-Danlos syndrome-an autosomal-recessive entity caused by mutations in the zinc transporter gene SLC39A13. Am. J. Hum. Genet. 82, 1290-1305 (2008).
173. Fukada, T. et al. The zinc transporter SLC39A13/ZIP13 is required for connective tissue development; its involvement in BMP/TGF-beta signaling pathways. PLoS One 3, e3642 (2008).
174. Shusterman, E. et al. Zinc transport and the inhibition of the L-type calcium channel are two separable functions of ZnT-1. Metallomics 9, 228-238 (2017).
175. Hennigar, S. R. & McClung, J. P. Zinc transport in the mammalian intestine. Compr. Physiol. 9, 59-74 (2018).
176. Geiser, J., Venken, K. J., De Lisle, R. C. & Andrews, G. K. A mouse model of acrodermatitis enteropathica: loss of intestine zinc transporter ZIP4 (SIc39a4) disrupts the stem cell niche and intestine integrity. PLoS Genet. 8, e1002766 (2012).
177. Dufner-Beattie, J., Kuo, Y. M., Gitschier, J. & Andrews, G. K. The adaptive response to dietary zinc in mice involves the differential cellular localization and zinc regulation of the zinc transporters ZIP4 and ZIP5. J. Biol. Chem. 279, 49082-49090 (2004).
178. Dufner-Beattie, J. et al. The acrodermatitis enteropathica gene ZIP4 encodes a tissue-specific, zinc-regulated zinc transporter in mice. J. Biol. Chem. 278, 33474-33481 (2003).
179. Kury, S. et al. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat. Genet. 31, 239-240 (2002).
180. Wang, K. et al. A novel member of a zinc transporter family is defective in acrodermatitis enteropathica. Am. J. Hum. Genet. 71, 66-73 (2002).
181. Weaver, B. P., Dufner-Beattie, J., Kambe, T. & Andrews, G. K. Novel zincresponsive post-transcriptional mechanisms reciprocally regulate expression of the mouse Slc39a4 and Slc39a5 zinc transporters (Zip4 and Zip5). Biol. Chem. 388, 1301-1312 (2007).
182. Yu, Y. Y., Kirschke, C. P. & Huang, L. Immunohistochemical analysis of ZnT1, 4, 5, 6, and 7 in the mouse gastrointestinal tract. J. Histochem Cytochem. 55, 223-234 (2007).
183. McMahon, R. J. & Cousins, R. J. Regulation of the zinc transporter
184. Wu, J., Ma, N., Johnston, L. J. & Ma, X. Dietary nutrients mediate intestinal host defense peptide expression. Adv. Nutr. 11, 92-102 (2020).
185. Podany, A. B. et al. ZnT2-mediated zinc import into paneth cell granules is necessary for coordinated secretion and paneth cell function in mice. Cell Mol. Gastroenterol. Hepatol. 2, 369-383 (2016).
186. Hennigar, S. R. & Kelleher, S. L. TNFalpha post-translationally targets ZnT2 to accumulate zinc in lysosomes. J. Cell Physiol. 230, 2345-2350 (2015).
187. Ohashi, W. et al. Zinc transporter SLC39A7/ZIP7 promotes intestinal epithelial self-renewal by resolving ER stress. PLoS Genet. 12, e1006349 (2016).
188. Turner, J. R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799-809 (2009).
189. Higashimura, Y. et al. Zinc deficiency activates the IL-23/Th17 axis to aggravate experimental colitis in mice. J. Crohns Colitis 14, 856-866 (2020).
190. Hering, N. A., Fromm, M. & Schulzke, J. D. Determinants of colonic barrier function in inflammatory bowel disease and potential therapeutics. J. Physiol. 590, 1035-1044 (2012).
191. Guthrie, G. J. et al. Influence of ZIP14 (slc39A14) on intestinal zinc processing and barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 308, G171-G178 (2015).
192. Kim, J. et al. Deletion of metal transporter Zip14 (SIc39a14) produces skeletal muscle wasting, endotoxemia, Mef2c activation and induction of miR-675 and Hspb7. Sci. Rep. 10, 4050 (2020).
193. Aydemir, T. B. & Cousins, R. J. The multiple faces of the metal transporter ZIP14 (SLC39A14). J. Nutr. 148, 174-184 (2018).
194. McGourty, K. et al. ZnT2 is critical for TLR4-mediated cytokine expression in colonocytes and modulates mucosal inflammation in mice. Int J. Mol. Sci. 23, 11467 (2022).
195. Hennigar, S. R. et al. ZnT 2 is a critical mediator of lysosomal-mediated cell death during early mammary gland involution. Sci. Rep. 5, 8033 (2015).
196. Liu, M. J. et al. ZIP8 regulates host defense through zinc-mediated inhibition of NF-кВ. Cell Rep. 3, 386-400 (2013).
197. Li, D. et al. A pleiotropic missense variant in SLC39A8 is associated with Crohn’s disease and human gut microbiome composition. Gastroenterology 151, 724-732 (2016).
198. Vergnano, A. M. et al. Zinc dynamics and action at excitatory synapses. Neuron 82, 1101-1114 (2014).
199. Kalappa, B. I. et al. AMPA receptor inhibition by synaptically released zinc. Proc. Natl Acad. Sci. USA. 112, 15749-15754 (2015).
200. Huang, Y. Z., Pan, E., Xiong, Z. Q. & McNamara, J. O. Zinc-mediated transactivation of TrkB potentiates the hippocampal mossy fiber-CA3 pyramid synapse. Neuron 57, 546-558 (2008).
201. Pan, E. et al. Vesicular zinc promotes presynaptic and inhibits postsynaptic longterm potentiation of mossy fiber-CA3 synapse. Neuron 71, 1116-1126 (2011).
202. Eom, K. et al. Intracellular
203. Anderson, C. T., Kumar, M., Xiong, S. & Tzounopoulos, T. Cell-specific gain modulation by synaptically released zinc in cortical circuits of audition. Elife 6, e29893 (2017).
204. Kumar, M., Xiong, S., Tzounopoulos, T. & Anderson, C. T. Fine control of sound frequency tuning and frequency discrimination acuity by synaptic zinc signaling in mouse auditory cortex. J. Neurosci. 39, 854-865 (2019).
205. Besser, L. et al. Synaptically released zinc triggers metabotropic signaling via a zinc-sensing receptor in the hippocampus. J. Neurosci. 29, 2890-2901 (2009).
206. Palmiter, R. D., Cole, T. B., Quaife, C. J. & Findley, S. D. ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc. Natl Acad. Sci. USA. 93, 14934-14939 (1996).
207. Sikora, J., Kieffer, B. L., Paoletti, P. & Ouagazzal, A. M. Synaptic zinc contributes to motor and cognitive deficits in 6-hydroxydopamine mouse models of Parkinson’s disease. Neurobiol. Dis. 134, 104681 (2020).
208. Upmanyu, N. et al. Colocalization of different neurotransmitter transporters on synaptic vesicles is sparse except for VGLUT1 and ZnT3. Neuron 110, 1483-1497.e1487 (2022).
209. McAllister, B. B. & Dyck, R. H. Zinc transporter 3 (ZnT3) and vesicular zinc in central nervous system function. Neurosci. Biobehav. Rev. 80, 329-350 (2017).
210. Perez-Rosello, T. et al. Tonic zinc inhibits spontaneous firing in dorsal cochlear nucleus principal neurons by enhancing glycinergic neurotransmission. Neurobiol. Dis. 81, 14-19 (2015).
211. Sindreu, C., Palmiter, R. D. & Storm, D. R. Zinc transporter ZnT-3 regulates presynaptic Erk1/2 signaling and hippocampus-dependent memory. Proc. Natl Acad. Sci. USA. 108, 3366-3370 (2011).
212. Mellone, M. et al. Zinc transporter-1: a novel NMDA receptor-binding protein at the postsynaptic density. J. Neurochem. 132, 159-168 (2015).
213. Krall, R. F. et al. Synaptic zinc inhibition of NMDA receptors depends on the association of GluN2A with the zinc transporter ZnT1. Sci. Adv. 6, eabb1515 (2020).
214. Chowanadisai, W. et al. Neurulation and neurite extension require the zinc transporter ZIP12 (slc39a12). Proc. Natl Acad. Sci. USA. 110, 9903-9908 (2013).
215. Kambe, T., Yamaguchi-Iwai, Y., Sasaki, R. & Nagao, M. Overview of mammalian zinc transporters. Cell Mol. Life Sci. 61, 49-68 (2004).
216. Scarr, E. et al. Increased cortical expression of the zinc transporter SLC39A12 suggests a breakdown in zinc cellular homeostasis as part of the pathophysiology of schizophrenia. NPJ Schizophr. 2, 16002 (2016).
217. Bogdanovic, M. et al. The ZIP3 zinc transporter is localized to mossy fiber terminals and is required for kainate-induced degeneration of CA3 neurons. J. Neurosci. 42, 2824-2834 (2022).
218. De Benedictis, C. A. et al. Expression analysis of zinc transporters in nervous tissue cells reveals neuronal and synaptic localization of ZIP4. Int J. Mol. Sci. 22, 4511 (2021).
219. Pickrell, J. K. et al. Detection and interpretation of shared genetic influences on 42 human traits. Nat. Genet. 48, 709-717 (2016).
220. Park, J. H. et al. SLC39A8 deficiency: a disorder of manganese transport and glycosylation. Am. J. Hum. Genet. 97, 894-903 (2015).
221. Müller, N. Inflammation and the glutamate system in schizophrenia: implications for therapeutic targets and drug development. Expert Opin. Ther. Targets 12, 1497-1507 (2008).
222. Tseng, W. C. et al. Schizophrenia-associated SLC39A8 polymorphism is a loss-offunction allele altering glutamate receptor and innate immune signaling. Transl. Psychiatry 11, 136 (2021).
223. Derewenda, U. et al. Phenol stabilizes more helix in a new symmetrical zinc insulin hexamer. Nature 338, 594-596 (1989).
224. Barman, S. & Srinivasan, K. Diabetes and zinc dyshomeostasis: can zinc supplementation mitigate diabetic complications? Crit. Rev. Food Sci. Nutr. 62, 1046-1061 (2022).
225. Davidson, H. W., Wenzlau, J. M. & O’Brien, R. M. Zinc transporter 8 (ZnT8) and
226. Rutter, G. A. & Chimienti, F. SLC30A8 mutations in type 2 diabetes. Diabetologia 58, 31-36 (2015).
227. Tamaki, M. et al. The diabetes-susceptible gene SLC30A8/ZnT8 regulates hepatic insulin clearance. J. Clin. Invest. 123, 4513-4524 (2013).
228. Sladek, R. et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445, 881-885 (2007).
229. Fukunaka, A. & Fujitani, Y. Role of zinc homeostasis in the pathogenesis of diabetes and obesity. Int J. Mol. Sci. 19, 476 (2018).
230. Ma, Q. et al. ZnT8 loss-of-function accelerates functional maturation of hESCderived
231. Regnell, S. E. & Lernmark, Å. Early prediction of autoimmune (type 1) diabetes. Diabetologia 60, 1370-1381 (2017).
232. Lemaire, K. et al. Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. Proc. Natl Acad. Sci. USA. 106, 14872-14877 (2009).
233. Wenzlau, J. M. et al. The cation efflux transporter
234. Smidt, K. et al. SLC30A3 responds to glucose- and zinc variations in beta-cells and is critical for insulin production and in vivo glucose-metabolism during beta-cell stress. PLoS One 4, e5684 (2009).
235. Petersen, A. B. et al. siRNA-mediated knock-down of ZnT 3 and ZnT 8 affects production and secretion of insulin and apoptosis in INS-1E cells. Apmis 119, 93-102 (2011).
236. Hardy, A. B. et al. Zip4 mediated zinc influx stimulates insulin secretion in pancreatic beta cells. PLoS One 10, e0119136 (2015).
237. Liu, Y. et al. Characterization of zinc influx transporters (ZIPs) in pancreatic
238. Gyulkhandanyan, A. V. et al. Investigation of transport mechanisms and regulation of intracellular
239. Solomou, A. et al. Over-expression of Slc30a8/ZnT8 selectively in the mouse a cell impairs glucagon release and responses to hypoglycemia. Nutr. Metab. 13, 46 (2016).
240. Balaz, M. et al. Subcutaneous adipose tissue zinc-a2-glycoprotein is associated with adipose tissue and whole-body insulin sensitivity. Obesity 22, 1821-1829 (2014).
241. Wang, W. & Seale, P. Control of brown and beige fat development. Nat. Rev. Mol. Cell Biol. 17, 691-702 (2016).
242. Fukunaka, A. et al. Zinc transporter ZIP13 suppresses beige adipocyte biogenesis and energy expenditure by regulating C/EBP-
243. Hay, N. Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nat. Rev. Cancer 16, 635-649 (2016).
244. Luo, X. et al. Emerging roles of lipid metabolism in cancer metastasis. Mol. Cancer 16, 76 (2017).
245. Gumulec, J. et al. Insight to physiology and pathology of zinc(II) ions and their actions in breast and prostate carcinoma. Curr. Med. Chem. 18, 5041-5051 (2011).
246. Takahashi, Y., Ogra, Y. & Suzuki, K. T. Nuclear trafficking of metallothionein requires oxidation of a cytosolic partner. J. Cell Physiol. 202, 563-569 (2005).
247. Nagel, W. W. & Vallee, B. L. Cell cycle regulation of metallothionein in human colonic cancer cells. Proc. Natl Acad. Sci. USA. 92, 579-583 (1995).
248. Formigari, A., Santon, A. & Irato, P. Efficacy of zinc treatment against ironinduced toxicity in rat hepatoma cell line H4-II-E-C3. Liver Int. 27, 120-127 (2007).
249. Chen, W. Y. et al. Expression of metallothionein gene during embryonic and early larval development in zebrafish. Aquat. Toxicol. 69, 215-227 (2004).
250. Chen, W. Y., John, J. A., Lin, C. H. & Chang, C. Y. Expression pattern of metallothionein, MTF-1 nuclear translocation, and its dna-binding activity in zebrafish (Danio rerio) induced by zinc and cadmium. Environ. Toxicol. Chem. 26, 110-117 (2007).
251. Xia, N., Liu, L., Yi, X. & Wang, J. Studies of interaction of tumor suppressor p53 with apo-MT using surface plasmon resonance. Anal. Bioanal. Chem. 395, 2569-2575 (2009).
252. Rana, U. et al. Zinc binding ligands and cellular zinc trafficking: apo-metallothionein, glutathione, TPEN, proteomic zinc, and Zn-Sp1. J. Inorg. Biochem. 102, 489-499 (2008).
253. Huang, M., Shaw, I. C. & Petering, D. H. Interprotein metal exchange between transcription factor Illa and apo-metallothionein. J. Inorg. Biochem. 98, 639-648 (2004).
254. Parreno, V., Martinez, A. M. & Cavalli, G. Mechanisms of Polycomb group protein function in cancer. Cell Res. 32, 231-253 (2022).
34
255. Di Foggia, V. et al. Bmi1 enhances skeletal muscle regeneration through MT1mediated oxidative stress protection in a mouse model of dystrophinopathy. J. Exp. Med. 211, 2617-2633 (2014).
256. Dünkelberg, S. et al. The interaction of sodium and zinc in the priming of T cell subpopulations regarding Th17 and treg cells. Mol. Nutr. Food Res. 64, e1900245 (2020).
257. Spiering, R. et al. Membrane-bound metallothionein 1 of murine dendritic cells promotes the expansion of regulatory T cells in vitro. Toxicol. Sci. 138, 69-75 (2014).
258. Li, S. et al. Metallothionein 3 promotes osteoblast differentiation in C2C12 cells via reduction of oxidative stress. Int J. Mol. Sci. 22, 4312 (2021).
259. Shin, C. H. et al. Identification of XAF1-MT2A mutual antagonism as a molecular switch in cell-fate decisions under stressful conditions. Proc. Natl Acad. Sci. USA. 114, 5683-5688 (2017).
260. Korkola, N. C. & Stillman, M. J. Structural role of cadmium and zinc in metallothionein oxidation by hydrogen peroxide: the resilience of metal-thiolate clusters. J. Am. Chem. Soc. 145, 6383-6397 (2023).
261. Ma, H. et al. HMBOX1 interacts with MT2A to regulate autophagy and apoptosis in vascular endothelial cells. Sci. Rep. 5, 15121 (2015).
262. Murphy, M. P. et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 4, 651-662 (2022).
263. Song, Q. X. et al. Potential role of oxidative stress in the pathogenesis of diabetic bladder dysfunction. Nat. Rev. Urol. 19, 581-596 (2022).
264. Vatner, S. F. et al. Healthful aging mediated by inhibition of oxidative stress. Ageing Res. Rev. 64, 101194 (2020).
265. Niu, B. et al. Application of glutathione depletion in cancer therapy: enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials 277, 121110 (2021).
266. Otterbein, L. E., Foresti, R. & Motterlini, R. Heme oxygenase-1 and carbon monoxide in the heart: the balancing act between danger signaling and prosurvival. Circ. Res. 118, 1940-1959 (2016).
267. Maret, W. & Li, Y. Coordination dynamics of zinc in proteins. Chem. Rev. 109, 4682-4707 (2009).
268. Pluth, M. D., Tomat, E. & Lippard, S. J. Biochemistry of mobile zinc and nitric oxide revealed by fluorescent sensors. Annu Rev. Biochem. 80, 333-355 (2011).
269. Rowsell, S. et al. Crystal structure of human MMP9 in complex with a reverse hydroxamate inhibitor. J. Mol. Biol. 319, 173-181 (2002).
270. Choi, S., Liu, X. & Pan, Z. Zinc deficiency and cellular oxidative stress: prognostic implications in cardiovascular diseases. Acta Pharm. Sin. 39, 1120-1132 (2018).
271. D’Amico, E., Factor-Litvak, P., Santella, R. M. & Mitsumoto, H. Clinical perspective on oxidative stress in sporadic amyotrophic lateral sclerosis. Free Radic. Biol. Med. 65, 509-527 (2013).
272. Wu, W., Bromberg, P. A. & Samet, J. M. Zinc ions as effectors of environmental oxidative lung injury. Free Radic. Biol. Med. 65, 57-69 (2013).
273. Roel, M. et al. Crambescin C1 exerts a cytoprotective effect on HepG2 cells through metallothionein induction. Mar. Drugs 13, 4633-4653 (2015).
274. Cavalca, E. et al. Metallothioneins are neuroprotective agents in lysosomal storage disorders. Ann. Neurol. 83, 418-432 (2018).
275. Yang, M. & Chitambar, C. R. Role of oxidative stress in the induction of metallothionein-2A and heme oxygenase-1 gene expression by the antineoplastic agent gallium nitrate in human lymphoma cells. Free Radic. Biol. Med. 45, 763-772 (2008).
276. Qu, W., Pi, J. & Waalkes, M. P. Metallothionein blocks oxidative DNA damage in vitro. Arch. Toxicol. 87, 311-321 (2013).
277. Koh, J. Y. & Lee, S. J. Metallothionein-3 as a multifunctional player in the control of cellular processes and diseases. Mol. Brain. 13, 116 (2020).
278. Álvarez-Barrios, A. et al. Antioxidant defenses in the human eye: a focus on metallothioneins. Antioxidants 10, 89 (2021).
279. Maret, W. The redox biology of redox-inert zinc ions. Free Radic. Biol. Med. 134, 311-326 (2019).
280. Oteiza, P. I. Zinc and the modulation of redox homeostasis. Free Radic. Biol. Med. 53, 1748-1759 (2012).
281. Hübner, C. & Haase, H. Interactions of zinc- and redox-signaling pathways. Redox Biol. 41, 101916 (2021).
282. Kim, H. G. et al. The epigenetic regulator SIRT6 protects the liver from alcoholinduced tissue injury by reducing oxidative stress in mice. J. Hepatol. 71, 960-969 (2019).
283. Hwang, S. et al. Interleukin-22 ameliorates neutrophil-driven nonalcoholic steatohepatitis through multiple targets. Hepatology 72, 412-429 (2020).
284. Wang, B. et al. D609 protects retinal pigmented epithelium as a potential therapy for age-related macular degeneration. Signal Transduct. Target Ther. 5, 20 (2020).
285. Phillippi, J. A. et al. Basal and oxidative stress-induced expression of metallothionein is decreased in ascending aortic aneurysms of bicuspid aortic valve patients. Circulation 119, 2498-2506 (2009).
286. Bahadorani, S., Mukai, S., Egli, D. & Hilliker, A. J. Overexpression of metalresponsive transcription factor (MTF-1) in Drosophila melanogaster ameliorates life-span reductions associated with oxidative stress and metal toxicity. Neurobiol. Aging 31, 1215-1226 (2010).
287. Esposito, K. et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation 106, 2067-2072 (2002).
288. Stankovic, R. K., Chung, R. S. & Penkowa, M. Metallothioneins I and II: neuroprotective significance during CNS pathology. Int J. Biochem. Cell Biol. 39, 484-489 (2007).
289. Inoue, K., Takano, H. & Satoh, M. Protective role of metallothionein in coagulatory disturbance accompanied by acute liver injury induced by LPS/D-GalN. Thromb. Haemost. 99, 980-983 (2008).
290. Inoue, K. et al. Role of metallothionein in coagulatory disturbance and systemic inflammation induced by lipopolysaccharide in mice. Faseb J. 20, 533-535 (2006).
291. Takano, H. et al. Protective role of metallothionein in acute lung injury induced by bacterial endotoxin. Thorax 59, 1057-1062 (2004).
292. Subramanian Vignesh, K. et al. Granulocyte macrophage-colony stimulating factor induced Zn sequestration enhances macrophage superoxide and limits intracellular pathogen survival. Immunity 39, 697-710 (2013).
293. Liu, Y. et al. EOLA1 protects lipopolysaccharide induced IL-6 production and apoptosis by regulation of MT2A in human umbilical vein endothelial cells. Mol. Cell Biochem. 395, 45-51 (2014).
294. Wu, H. et al. Metallothionein deletion exacerbates intermittent hypoxia-induced renal injury in mice. Toxicol. Lett. 232, 340-348 (2015).
295. Vasto, S. et al. Zinc and inflammatory/immune response in aging. Ann. N. Y. Acad. Sci. 1100, 111-122 (2007).
296. Majumder, S. et al. Loss of metallothionein predisposes mice to diethylnitrosamine-induced hepatocarcinogenesis by activating NF-kappaB target genes. Cancer Res. 70, 10265-10276 (2010).
297. Butcher, H. L. et al. Metallothionein mediates the level and activity of nuclear factor kappa B in murine fibroblasts. J. Pharm. Exp. Ther. 310, 589-598 (2004).
298. Pan, Y. et al. Metallothionein 2 A inhibits
299. Toh, P. P. et al. Modulation of metallothionein isoforms is associated with collagen deposition in proliferating keloid fibroblasts in vitro. Exp. Dermatol. 19, 987-993 (2010).
300. Cong, W. et al. Metallothionein prevents age-associated cardiomyopathy via inhibiting NF-kB pathway activation and associated nitrative damage to 2-OGD. Antioxid. Redox Signal. 25, 936-952 (2016).
301. Read, S. A. et al. Zinc is a potent and specific inhibitor of IFN-
302. Chen, Q. Y., DesMarais, T. & Costa, M. Metals and mechanisms of carcinogenesis. Annu. Rev. Pharm. Toxicol. 59, 537-554 (2019).
303. Ganger, R. et al. Protective effects of zinc against acute arsenic toxicity by regulating antioxidant defense system and cumulative metallothionein expression. Biol. Trace Elem. Res. 169, 218-229 (2016).
304. Polykretis, P. et al. Cadmium effects on superoxide dismutase 1 in human cells revealed by NMR. Redox Biol. 21, 101102 (2019).
305. Petering, D. H., Loftsgaarden, J., Schneider, J. & Fowler, B. Metabolism of cadmium, zinc and copper in the rat kidney: the role of metallothionein and other binding sites. Environ. Health Perspect. 54, 73-81 (1984).
306. Chen, X. et al. The association between renal tubular dysfunction and zinc level in a Chinese population environmentally exposed to cadmium. Biol. Trace Elem. Res. 186, 114-121 (2018).
307. Hu, Y. et al. The role of reactive oxygen species in arsenic toxicity. Biomolecules 10, 240 (2020).
308. Rahman, M. T. & De Ley, M. Arsenic induction of metallothionein and metallothionein induction against arsenic cytotoxicity. Rev. Environ. Contam Toxicol. 240, 151-168 (2017).
309. Ho, E. Zinc deficiency, DNA damage and cancer risk. J. Nutr. Biochem. 15, 572-578 (2004).
310. Song, Y. et al. Marginal zinc deficiency increases oxidative DNA damage in the prostate after chronic exercise. Free Radic. Biol. Med. 48, 82-88 (2010).
311. Stepien, M. et al. Circulating copper and zinc levels and risk of hepatobiliary cancers in Europeans. Br. J. Cancer 116, 688-696 (2017).
312. Jayaraman, A. K. & Jayaraman, S. Increased level of exogenous zinc induces cytotoxicity and up-regulates the expression of the
313. Wu, X., Tang, J. & Xie, M. Serum and hair zinc levels in breast cancer: a metaanalysis. Sci. Rep. 5, 12249 (2015).
314. Seeler, J. F. et al. Metal ion fluxes controlling amphibian fertilization. Nat. Chem. 13, 683-691 (2021).
315. Kambe, T., Hashimoto, A. & Fujimoto, S. Current understanding of ZIP and ZnT zinc transporters in human health and diseases. Cell Mol. Life Sci. 71, 3281-3295 (2014).
316. Margalioth, E. J., Schenker, J. G. & Chevion, M. Copper and zinc levels in normal and malignant tissues. Cancer 52, 868-872 (1983).
317. Gammoh, N. Z. & Rink, L. Zinc in infection and inflammation. Nutrients 9, 624 (2017).
318. Cui, Y. et al. Levels of zinc, selenium, calcium, and iron in benign breast tissue and risk of subsequent breast cancer. Cancer Epidemiol. Biomark. Prev. 16, 1682-1685 (2007).
319. Santoliquido, P. M., Southwick, H. W. & Olwin, J. H. Trace metal levels in cancer of the breast. Surg. Gynecol. Obstet. 142, 65-70 (1976).
320. Taylor, K. M. et al. The emerging role of the LIV-1 subfamily of zinc transporters in breast cancer. Mol. Med. 13, 396-406 (2007).
321. Kasper, G. et al. Expression levels of the putative zinc transporter LIV-1 are associated with a better outcome of breast cancer patients. Int J. Cancer 117, 961-973 (2005).
322. Yamashita, S. et al. Zinc transporter LIVI controls epithelial-mesenchymal transition in zebrafish gastrula organizer. Nature 429, 298-302 (2004).
323. Kowalski, P. J., Rubin, M. A. & Kleer, C. G. E-cadherin expression in primary carcinomas of the breast and its distant metastases. Breast Cancer Res. 5, R217-R222 (2003).
324. Oka, H. et al. Expression of E-cadherin cell adhesion molecules in human breast cancer tissues and its relationship to metastasis. Cancer Res. 53, 1696-1701 (1993).
325. Lopez, V. & Kelleher, S. L. Zip6-attenuation promotes epithelial-to-mesenchymal transition in ductal breast tumor (T47D) cells. Exp. Cell Res. 316, 366-375 (2010).
326. Shen, H., Qin, H. & Guo, J. Concordant correlation of LIV-1 and E-cadherin expression in human breast cancer cell MCF-7. Mol. Biol. Rep. 36, 653-659 (2009).
327. Matsui, C. et al. Zinc and its transporter ZIP6 are key mediators of breast cancer cell survival under high glucose conditions. FEBS Lett. 591, 3348-3359 (2017).
328. Gao, T. et al. The mechanism between epithelial mesenchymal transition in breast cancer and hypoxia microenvironment. Biomed. Pharmacother. 80, 393-405 (2016).
329. Dave, B., Mittal, V., Tan, N. M. & Chang, J. C. Epithelial-mesenchymal transition, cancer stem cells and treatment resistance. Breast Cancer Res. 14, 202 (2012).
330. Chung, C. H., Bernard, P. S. & Perou, C. M. Molecular portraits and the family tree of cancer. Nat. Genet. 32, 533-540 (2002).
331. Tozlu, S. et al. Identification of novel genes that co-cluster with estrogen receptor alpha in breast tumor biopsy specimens, using a large-scale real-time reverse transcription-PCR approach. Endocr. Relat. Cancer 13, 1109-1120 (2006).
332. Althobiti, M. et al. Oestrogen-regulated protein SLC39A6: a biomarker of good prognosis in luminal breast cancer. Breast Cancer Res Treat. 189, 621-630 (2021).
333. Kambe, T. [Overview of and update on the physiological functions of mammalian zinc transporters]. Nihon Eiseigaku Zasshi. 68, 92-102 (2013).
334. Kagara, N., Tanaka, N., Noguchi, S. & Hirano, T. Zinc and its transporter ZIP10 are involved in invasive behavior of breast cancer cells. Cancer Sci. 98, 692-697 (2007).
335. Pal, D., Sharma, U., Singh, S. K. & Prasad, R. Association between ZIP10 gene expression and tumor aggressiveness in renal cell carcinoma. Gene 552, 195-198 (2014).
336. Pawlus, M. R., Wang, L. & Hu, C. J. STAT3 and HIF1alpha cooperatively activate HIF1 target genes in MDA-MB-231 and RCC4 cells. Oncogene 33, 1670-1679 (2014).
337. Armanious, H. et al. STAT3 upregulates the protein expression and transcriptional activity of beta-catenin in breast cancer. Int J. Clin. Exp. Pathol. 3, 654-664 (2010).
338. Chung, S. S., Giehl, N., Wu, Y. & Vadgama, J. V. STAT3 activation in HER2overexpressing breast cancer promotes epithelial-mesenchymal transition and cancer stem cell traits. Int J. Oncol. 44, 403-411 (2014).
339. Taylor, K. M. et al. ZIP7-mediated intracellular zinc transport contributes to aberrant growth factor signaling in antihormone-resistant breast cancer Cells. Endocrinology 149, 4912-4920 (2008).
340. Ziliotto, S. et al. Activated zinc transporter ZIP7 as an indicator of anti-hormone resistance in breast cancer. Metallomics 11, 1579-1592 (2019).
341. Huang, L., Kirschke, C. P., Zhang, Y. & Yu, Y. Y. The ZIP7 gene (SIc39a7) encodes a zinc transporter involved in zinc homeostasis of the Golgi apparatus. J. Biol. Chem. 280, 15456-15463 (2005).
342. de Nonneville, A. et al. Prognostic and predictive value of LIV1 expression in early breast cancer and by molecular subtype. Pharmaceutics 15, 938 (2023).
343. Vogel-Gonzalez, M., Musa-Afaneh, D., Rivera Gil, P. & Vicente, R. Zinc favors triple-negative breast cancer’s microenvironment modulation and cell plasticity. Int J. Mol. Sci. 22, 9188 (2021).
344. Yap, X. et al. Over-expression of metallothionein predicts chemoresistance in breast cancer. J. Pathol. 217, 563-570 (2009).
345. Jadhav, R. R. et al. Genome-wide DNA methylation analysis reveals estrogenmediated epigenetic repression of metallothionein-1 gene cluster in breast cancer. Clin. Epigenetics. 7, 13 (2015).
346. Lopez, V., Foolad, F. & Kelleher, S. L. ZnT2-overexpression represses the cytotoxic effects of zinc hyper-accumulation in malignant metallothionein-null T47D breast tumor cells. Cancer Lett. 304, 41-51 (2011).
347. Lim, D., Jocelyn, K. M., Yip, G. W. & Bay, B. H. Silencing the Metallothionein-2A gene inhibits cell cycle progression from G1- to S-phase involving ATM and cdc25A signaling in breast cancer cells. Cancer Lett. 276, 109-117 (2009).
348. Sun, L. et al. Zinc regulates the ability of Cdc25C to activate MPF/cdk1. J. Cell Physiol. 213, 98-104 (2007).
349. Banin, S. et al. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 281, 1674-1677 (1998).
350. Deng, C. et al. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 82, 675-684 (1995).
351. Li, D., Stovall, D. B., Wang, W. & Sui, G. Advances of zinc signaling studies in prostate cancer. Int J. Mol. Sci. 21, 667 (2020).
352. Zhao, J. et al. Comparative study of serum zinc concentrations in benign and malignant prostate disease: a systematic review and meta-analysis. Sci. Rep. 6, 25778 (2016).
353. McNeal, J. E. Normal histology of the prostate. Am. J. Surg. Pathol. 12, 619-633 (1988).
354. Costello, L. C. & Franklin, R. B. A comprehensive review of the role of zinc in normal prostate function and metabolism; and its implications in prostate cancer. Arch. Biochem. Biophys. 611, 100-112 (2016).
355. Vartsky, D. et al. Prostatic zinc and prostate specific antigen: an experimental evaluation of their combined diagnostic value. J. Urol. 170, 2258-2262 (2003).
356. Dakubo, G. D. et al. Altered metabolism and mitochondrial genome in prostate cancer. J. Clin. Pathol. 59, 10-16 (2006).
357. Feng, P. et al. The involvement of Bax in zinc-induced mitochondrial apoptogenesis in malignant prostate cells. Mol. Cancer 7, 25 (2008).
358. Nardinocchi, L. et al. Zinc downregulates HIF-1alpha and inhibits its activity in tumor cells in vitro and in vivo. PLoS One 5, e15048 (2010).
359. Uzzo, R. G. et al. Zinc inhibits nuclear factor-kappa B activation and sensitizes prostate cancer cells to cytotoxic agents. Clin. Cancer Res. 8, 3579-3583 (2002).
360. Ishii, K. et al. Evidence that the prostate-specific antigen (PSA)/Zn2+ axis may play a role in human prostate cancer cell invasion. Cancer Lett. 207, 79-87 (2004).
361. Uzzo, R. G. et al. Diverse effects of zinc on NF-kappaB and AP-1 transcription factors: implications for prostate cancer progression. Carcinogenesis 27, 1980-1990 (2006).
362. Ishii, K. et al. Inhibition of aminopeptidase N (AP-N) and urokinase-type plasminogen activator (uPA) by zinc suppresses the invasion activity in human urological cancer cells. Biol. Pharm. Bull. 24, 226-230 (2001).
363. Singh, K. K., Desouki, M. M., Franklin, R. B. & Costello, L. C. Mitochondrial aconitase and citrate metabolism in malignant and nonmalignant human prostate tissues. Mol. Cancer 5, 14 (2006).
364. Fontana, F., Anselmi, M. & Limonta, P. Unraveling the peculiar features of mitochondrial metabolism and dynamics in prostate cancer. Cancers. 15, 1192 (2023).
365. Costello, L. C. et al. Human prostate cancer ZIP1/zinc/citrate genetic/metabolic relationship in the TRAMP prostate cancer animal model. Cancer Biol. Ther. 12, 1078-1084 (2011).
366. Costello, L. C. & Franklin, R. B. The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: connecting the dots. Mol. Cancer 5, 17 (2006).
367. Franklin, R. B. et al. hZIP1 zinc uptake transporter down regulation and zinc depletion in prostate cancer. Mol. Cancer 4, 32 (2005).
368. An, Y. et al. A novel tetrapeptide fluorescence sensor for early diagnosis of prostate cancer based on imaging
369. Fong, L. Y. et al. Human-like hyperplastic prostate with low ZIP1 induced solely by Zn deficiency in rats. Proc. Natl Acad. Sci. USA. 115, E11091-e11100 (2018).
370. Costello, L. C., Franklin, R. B., Zou, J. & Naslund, M. J. Evidence that human prostate cancer is a ZIP1-deficient malignancy that could be effectively treated with a zinc ionophore (Clioquinol) approach. Chemotherapy 4, 152 (2015).
371. Huang, L., Kirschke, C. P. & Zhang, Y. Decreased intracellular zinc in human tumorigenic prostate epithelial cells: a possible role in prostate cancer progression. Cancer Cell Int. 6, 10 (2006).
372. Makhov, P. et al. Transcriptional regulation of the major zinc uptake protein hZip1 in prostate cancer cells. Gene 431, 39-46 (2009).
373. Thiagalingam, A. et al. RREB-1, a novel zinc finger protein, is involved in the differentiation response to Ras in human medullary thyroid carcinomas. Mol. Cell Biol. 16, 5335-5345 (1996).
374. Zhang, S. et al. p16 INK4a gene promoter variation and differential binding of a repressor, the ras-responsive zinc-finger transcription factor, RREB. Oncogene 22, 2285-2295 (2003).
36
375. Gioeli, D. Signal transduction in prostate cancer progression. Clin. Sci. 108, 293-308 (2005).
376. Milon, B. C. et al. Ras responsive element binding protein-1 (RREB-1) downregulates hZIP1 expression in prostate cancer cells. Prostate 70, 288-296 (2010).
377. Aguirre-Portoles, C. et al. ZIP9 is a druggable determinant of sex differences in melanoma. Cancer Res. 81, 5991-6003 (2021).
378. Berg, A. H. et al. Identification and characterization of membrane androgen receptors in the ZIP9 zinc transporter subfamily: I. Discovery in female atlantic croaker and evidence ZIP9 mediates testosterone-induced apoptosis of ovarian follicle cells. Endocrinology 155, 4237-4249 (2014).
379. Thomas, P., Pang, Y., Dong, J. & Berg, A. H. Identification and characterization of membrane androgen receptors in the ZIP9 zinc transporter subfamily: II. Role of human ZIP9 in testosterone-induced prostate and breast cancer cell apoptosis. Endocrinology 155, 4250-4265 (2014).
380. Desouki, M. M. et al. hZip2 and hZip3 zinc transporters are down regulated in human prostate adenocarcinomatous glands. Mol. Cancer 6, 37 (2007).
381. Kelleher, S. L., McCormick, N. H., Velasquez, V. & Lopez, V. Zinc in specialized secretory tissues: roles in the pancreas, prostate, and mammary gland. Adv. Nutr. 2, 101-111 (2011).
382. Franklin, R. B. et al. Human ZIP1 is a major zinc uptake transporter for the accumulation of zinc in prostate cells. J. Inorg. Biochem. 96, 435-442 (2003).
383. Prasad, R. R. et al. Stage-specific differential expression of zinc transporter SLC30A and SLC39A family proteins during prostate tumorigenesis. Mol. Carcinog. 61, 454-471 (2022).
384. Kim, Y. R. et al. HOXB13 downregulates intracellular zinc and increases NFkappaB signaling to promote prostate cancer metastasis. Oncogene 33, 4558-4567 (2014).
385. Beck, F. W. et al. Differential expression of
386. Inoue, K. et al. Osteopenia and male-specific sudden cardiac death in mice lacking a zinc transporter gene, Znt5. Hum. Mol. Genet. 11, 1775-1784 (2002).
387. Wei, H. et al. Differential expression of metallothioneins (MTs) 1,2 , and 3 in response to zinc treatment in human prostate normal and malignant cells and tissues. Mol. Cancer 7, 7 (2008).
388. Han, Y. C. et al. Metallothionein 1 h tumour suppressor activity in prostate cancer is mediated by euchromatin methyltransferase 1. J. Pathol. 230, 184-193 (2013).
389. Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 73, 17-48 (2023).
390. Costello, L. C. et al. Decreased zinc and downregulation of ZIP3 zinc uptake transporter in the development of pancreatic adenocarcinoma. Cancer Biol. Ther. 12, 297-303 (2011).
391. Li, M. et al. Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. Proc. Natl Acad. Sci. USA. 104, 18636-18641 (2007).
392. Shakri, A. R. et al. Upregulation of ZIP14 and altered zinc homeostasis in muscles in pancreatic cancer cachexia. Cancers. 12, 3 (2019).
393. Li, M. et al. Down-regulation of ZIP4 by RNA interference inhibits pancreatic cancer growth and increases the survival of nude mice with pancreatic cancer xenografts. Clin. Cancer Res. 15, 5993-6001 (2009).
394. Liu, M. et al. ZIP4 promotes pancreatic cancer progression by repressing ZO-1 and Claudin-1 through a ZEB1-dependent transcriptional mechanism. Clin. Cancer Res. 24, 3186-3196 (2018).
395. Liu, M. et al. ZIP4 increases expression of transcription factor ZEB1 to promote Integrin
396. Shi, X. et al. Circular RNA ANAPC7 inhibits tumor growth and muscle wasting via PHLPP2-AKT-TGF-
397. Xu, X. et al. ZIP4, a novel determinant of tumor invasion in hepatocellular carcinoma, contributes to tumor recurrence after liver transplantation. Int J. Biol. Sci. 10, 245-256 (2014).
398. Zhang, Y. et al. ZIP4 regulates pancreatic cancer cell growth by activating IL-6/ STAT3 pathway through zinc finger transcription factor CREB. Clin. Cancer Res. 16, 1423-1430 (2010).
399. Zhang, Y. et al. A novel epigenetic CREB-miR-373 axis mediates ZIP4-induced pancreatic cancer growth. EMBO Mol. Med. 5, 1322-1334 (2013).
400. Shi, X. et al. Circular RNA ANAPC7 inhibits tumor growth and muscle wasting via PHLPP2-AKT-TGF-beta signaling axis in pancreatic cancer. Gastroenterology 162, 2004-2017.e2002 (2022).
401. Krebs, A. M. et al. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat. Cell Biol. 19, 518-529 (2017).
402. Franklin, R. B., Zou, J. & Costello, L. C. The cytotoxic role of RREB1, ZIP3 zinc transporter, and zinc in human pancreatic adenocarcinoma. Cancer Biol. Ther. 15, 1431-1437 (2014).
403. Li, K. et al. Metallothionein-1G suppresses pancreatic cancer cell stemness by limiting activin A secretion via NF-KB inhibition. Theranostics 11, 3196-3212 (2021).
404. Li, P. et al. Association between zinc intake and risk of digestive tract cancers: a systematic review and meta-analysis. Clin. Nutr. 33, 415-420 (2014).
405. Jaiswal, A. S. & Narayan, S. Zinc stabilizes adenomatous polyposis coli (APC) protein levels and induces cell cycle arrest in colon cancer cells. J. Cell Biochem. 93, 345-357 (2004).
406. Shangkuan, W. C. et al. Risk analysis of colorectal cancer incidence by gene expression analysis. PeerJ 5, e3003 (2017).
407. Yagi, K. et al. Three DNA methylation epigenotypes in human colorectal cancer. Clin. Cancer Res. 16, 21-33 (2010).
408. Hou, L., Liu, P. & Zhu, T. Long noncoding RNA SLC30A10 promotes colorectal tumor proliferation and migration via miR-21c/APC axis. Eur. Rev. Med Pharm. Sci. 24, 6682-6691 (2020).
409. Yao, H. et al. KCTD9 inhibits the Wnt/
410. Chen, Y. H. et al. Role of GAC63 in transcriptional activation mediated by betacatenin. Nucleic Acids Res. 35, 2084-2092 (2007).
411. Zhao, H. et al. Wnt signaling in colorectal cancer: pathogenic role and therapeutic target. Mol. Cancer 21, 144 (2022).
412. Barresi, V. et al. Transcriptome analysis reveals an altered expression profile of zinc transporters in colorectal cancer. J. Cell Biochem. 119, 9707-9719 (2018).
413. Sheng, N. et al. Knockdown of SLC39A7 inhibits cell growth and induces apoptosis in human colorectal cancer cells. Acta Biochim. Biophys. Sin. (Shanghai). 49, 926-934 (2017).
414. Jbara, A. et al. RBFOX2 modulates a metastatic signature of alternative splicing in pancreatic cancer. Nature 617, 147-153 (2023).
415. Marasco, L. E. & Kornblihtt, A. R. The physiology of alternative splicing. Nat. Rev. Mol. Cell Biol. 24, 242-254 (2023).
416. Wan, L. et al. Splicing factor SRSF1 promotes pancreatitis and KRASG12Dmediated pancreatic cancer. Cancer Discov. 13, 1678-1695 (2023).
417. Thorsen, K. et al. Alternative splicing of SLC39A14 in colorectal cancer is regulated by the Wnt pathway. Mol. Cell Proteom. 10, M110 002998 (2011).
418. Cao, X. et al. Cadmium induced BEAS-2B cells apoptosis and mitochondria damage via MAPK signaling pathway. Chemosphere 263, 128346 (2021).
419. Jin, Y. H. et al. Cadmium is a mutagen that acts by inhibiting mismatch repair. Nat. Genet. 34, 326-329 (2003).
420. Hung, K. C. et al. The expression profile and prognostic significance of metallothionein genes in colorectal cancer. Int J. Mol. Sci. 20, 3849 (2019).
421. Arriaga, J. M., Greco, A., Mordoh, J. & Bianchini, M. Metallothionein 1 G and zinc sensitize human colorectal cancer cells to chemotherapy. Mol. Cancer Ther. 13, 1369-1381 (2014).
422. Liu, X. et al. Metallothionein 2 A (MT2A) controls cell proliferation and liver metastasis by controlling the MST1/LATS2/YAP1 signaling pathway in colorectal cancer. Cancer Cell Int. 22, 205 (2022).
423. Arriaga, J. M. et al. Metallothionein expression in colorectal cancer: relevance of different isoforms for tumor progression and patient survival. Hum. Pathol. 43, 197-208 (2012).
424. Chen, H. et al. Nutrient intakes and adenocarcinoma of the esophagus and distal stomach. Nutr. Cancer 42, 33-40 (2002).
425. Rogers, M. A. et al. A case-control study of element levels and cancer of the upper aerodigestive tract. Cancer Epidemiol. Biomark. Prev. 2, 305-312 (1993).
426. Pakseresht, M. et al. Dietary habits and gastric cancer risk in north-west Iran. Cancer Causes Control. 22, 725-736 (2011).
427. He, Y. et al. Cancer incidence and mortality in Hebei province, 2013. Medicine 96, e7293 (2017).
428. Li, D. et al. Cancer survival in Cixian of China, 2003-2013: a population-based study. Cancer Med. 7, 1537-1545 (2018).
429. Liang, D. et al. Gastric cancer burden of last 40 years in North China (Hebei Province): a population-based study. Medicine 96, e5887 (2017).
430. Guo, Y. & He, Y. Comprehensive analysis of the expression of SLC30A family genes and prognosis in human gastric cancer. Sci. Rep. 10, 18352 (2020).
431. Guan, X. et al. Dual inhibition of MYC and SLC39A10 by a novel natural product STAT3 inhibitor derived from Chaetomium globosum suppresses tumor growth and metastasis in gastric cancer. Pharm. Res. 189, 106703 (2023).
432. Zhang, Y. et al. SLC39A7, regulated by miR-139-5p, induces cell proliferation, migration and inhibits apoptosis in gastric cancer via Akt/mTOR signaling pathway. Biosci. Rep. 40, BSR20200041 (2020).
433. Janssen, A. M. et al. Metallothionein in human gastrointestinal cancer. J. Pathol. 192, 293-300 (2000).
434. Lin, S. et al. Transcription factor myeloid zinc-finger 1 suppresses human gastric carcinogenesis by interacting with metallothionein 2 A. Clin. Cancer Res. 25, 1050-1062 (2019).
435. Cho, Y. H. et al. A role of metallothionein-3 in radiation-induced autophagy in glioma cells. Sci. Rep. 10, 2015 (2020).
436. Li, K. et al. MT1M regulates gastric cancer progression and stemness by modulating the Hedgehog pathway protein GLI1. Biochem. Biophys. Res. Commun. 670, 63-72 (2023).
437. Fiches, G. N. et al. Profiling of immune related genes silenced in EBV-positive gastric carcinoma identified novel restriction factors of human gammaherpesviruses. PLoS Pathog. 16, e1008778 (2020).
438. Takahashi, S. Molecular functions of metallothionein and its role in hematological malignancies. J. Hematol. Oncol. 5, 41 (2012).
439. Pan, Y. et al. Epigenetic upregulation of metallothionein 2 A by diallyl trisulfide enhances chemosensitivity of human gastric cancer cells to docetaxel through attenuating NF-кB activation. Antioxid. Redox Signal. 24, 839-854 (2016).
440. Habel, N. et al. Zinc chelation: a metallothionein 2 A ‘s mechanism of action involved in osteosarcoma cell death and chemotherapy resistance. Cell Death Dis. 4, e874 (2013).
441. Zalewska, M., Trefon, J. & Milnerowicz, H. The role of metallothionein interactions with other proteins. Proteomics 14, 1343-1356 (2014).
442. Kolenko, V., Teper, E., Kutikov, A. & Uzzo, R. Zinc and zinc transporters in prostate carcinogenesis. Nat. Rev. Urol. 10, 219-226 (2013).
443. Kim, C. H., Kim, J. H., Lee, J. & Ahn, Y. S. Zinc-induced NF-kappaB inhibition can be modulated by changes in the intracellular metallothionein level. Toxicol. Appl Pharmacol. 190, 189-196 (2003).
444. Fong, L. Y. & Magee, P. N. Dietary zinc deficiency enhances esophageal cell proliferation and N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumor incidence in C57BL/6 mouse. Cancer Lett. 143, 63-69 (1999).
445. Abnet, C. C. et al. Zinc concentration in esophageal biopsy specimens measured by x-ray fluorescence and esophageal cancer risk. J. Natl Cancer Inst. 97, 301-306 (2005).
446. Fong, L. Y., Nguyen, V. T. & Farber, J. L. Esophageal cancer prevention in zincdeficient rats: rapid induction of apoptosis by replenishing zinc. J. Natl Cancer Inst. 93, 1525-1533 (2001).
447. Wu, C. et al. Genome-wide association study identifies common variants in SLC39A6 associated with length of survival in esophageal squamous-cell carcinoma. Nat. Genet. 45, 632-638 (2013).
448. Cui, X. B. et al. SLC39A6: a potential target for diagnosis and therapy of esophageal carcinoma. J. Transl. Med. 13, 321 (2015).
449. Cheng, X. et al. Solute carrier family 39 member 6 gene promotes aggressiveness of esophageal carcinoma cells by increasing intracellular levels of zinc, activating phosphatidylinositol 3 -kinase signaling, and up-regulating genes that regulate metastasis. Gastroenterology 152, 1985-1997.e1912 (2017).
450. Jin, J. et al. Knockdown of zinc transporter ZIP5 (SLC39A5) expression significantly inhibits human esophageal cancer progression. Oncol. Rep. 34, 1431-1439 (2015).
451. Kumar, A., Chatopadhyay, T., Raziuddin, M. & Ralhan, R. Discovery of deregulation of zinc homeostasis and its associated genes in esophageal squamous cell carcinoma using cDNA microarray. Int J. Cancer 120, 230-242 (2007).
452. Li, Q. et al. Knockdown of zinc transporter ZIP5 by RNA interference inhibits esophageal cancer growth in vivo. Oncol. Res. 24, 205-214 (2016).
453. Huang, J. X. et al. Relationship between COX-2 and cell cycle-regulatory proteins in patients with esophageal squamous cell carcinoma. World J. Gastroenterol. 16, 5975-5981 (2010).
454. Shimizu, M. et al. Metallothionein 2 A expression in cancer-associated fibroblasts and cancer cells promotes esophageal squamous cell carcinoma progression. Cancers. 13, 4552 (2021).
455. Wong, T. S., Gao, W. & Chan, J. Y. Transcription regulation of E-cadherin by zinc finger E -box binding homeobox proteins in solid tumors. Biomed. Res Int. 2014, 921564 (2014).
456. ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Consortium. Pan-cancer analysis of whole genomes. Nature 578, 82-93 (2020).
457. Agrawal, A. et al. Zinc-binding groups modulate selective inhibition of MMPs. ChemMedChem 3, 812-820 (2008).
458. Puerta, D. T. & Cohen, S. M. Examination of novel zinc-binding groups for use in matrix metalloproteinase inhibitors. Inorg. Chem. 42, 3423-3430 (2003).
459. Lheureux, S., Braunstein, M. & Oza, A. M. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J. Clin. 69, 280-304 (2019).
460. Wei, T. et al. ZnT7 RNAi favors Raf(GOF)scrib(-/-)-induced tumor growth and invasion in Drosophila through JNK signaling pathway. Oncogene 40, 2217-2229 (2021).
461. Aguirre-Portolés, C. et al. ZIP9 is a druggable determinant of sex differences in melanoma. Cancer Res. 81, 5991-6003 (2021).
462. Jaiswal, S. & Libby, P. Clonal haematopoiesis: connecting ageing and inflammation in cardiovascular disease. Nat. Rev. Cardiol. 17, 137-144 (2020).
463. Bekele, T. H. et al. Dietary recommendations for ethiopians on the basis of priority diet-related diseases and causes of death in ethiopia: an umbrella review. Adv. Nutr. 14, 895-913 (2023).
464. Mohammadifard, N. et al. Trace minerals intake: Risks and benefits for cardiovascular health. Crit. Rev. Food Sci. Nutr. 59, 1334-1346 (2019).
465. Libby, P. The changing landscape of atherosclerosis. Nature 592, 524-533 (2021).
466. Förstermann, U., Xia, N. & Li, H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res. 120, 713-735 (2017).
467. Conway, D. E. et al. Endothelial metallothionein expression and intracellular free zinc levels are regulated by shear stress. Am. J. Physiol. Cell Physiol. 299, C1461-C1467 (2010).
468. Hara, T. et al. Role of ScI39a13/ZIP13 in cardiovascular homeostasis. PLoS One 17, e0276452 (2022).
469. Allen-Redpath, K. et al. Marginal dietary zinc deficiency in vivo induces vascular smooth muscle cell apoptosis in large arteries. Cardiovasc Res. 99, 525-534 (2013).
470. Alcantara, E. H. et al. Long-term zinc deprivation accelerates rat vascular smooth muscle cell proliferation involving the down-regulation of JNK1/2 expression in MAPK signaling. Atherosclerosis 228, 46-52 (2013).
471. Patrushev, N., Seidel-Rogol, B. & Salazar, G. Angiotensin II requires zinc and downregulation of the zinc transporters ZnT 3 and ZnT 10 to induce senescence of vascular smooth muscle cells. PLoS One 7, e33211 (2012).
472. min, L. J., Mogi, M., Iwai, M. & Horiuchi, M. Signaling mechanisms of angiotensin II in regulating vascular senescence. Ageing Res Rev. 8, 113-121 (2009).
473. Reed, G. W., Rossi, J. E. & Cannon, C. P. Acute myocardial infarction. Lancet 389, 197-210 (2017).
474. McIntosh, R. et al. The critical role of intracellular zinc in adenosine A(2) receptor activation induced cardioprotection against reperfusion injury. J. Mol. Cell Cardiol. 49, 41-47 (2010).
475. Du, L. et al. The critical role of the zinc transporter Zip2 (SLC39A2) in ischemia/reperfusion injury in mouse hearts. J. Mol. Cell Cardiol. 132, 136-145 (2019).
476. Zhao, H. et al. Endoplasmic reticulum stress/Ca(2+)-calmodulin-dependent protein kinase/signal transducer and activator of transcription 3 pathway plays a role in the regulation of cellular zinc deficiency in myocardial ischemia/reperfusion injury. Front. Physiol. 12, 736920 (2021).
477. Zhang, H. et al. The zinc transporter ZIP7 (SIc39a7) controls myocardial reperfusion injury by regulating mitophagy. Basic Res. Cardiol. 116, 54 (2021).
478. Beharier, O. et al. ZnT-1 protects HL-1 cells from simulated ischemia-reperfusion through activation of Ras-ERK signaling. J. Mol. Med. 90, 127-138 (2012).
479. Bruinsma, J. J., Jirakulaporn, T., Muslin, A. J. & Kornfeld, K. Zinc ions and cation diffusion facilitator proteins regulate Ras-mediated signaling. Dev. Cell. 2, 567-578 (2002).
480. Lazarczyk, M. et al. Regulation of cellular zinc balance as a potential mechanism of EVER-mediated protection against pathogenesis by cutaneous oncogenic human papillomaviruses. J. Exp. Med. 205, 35-42 (2008).
481. Murphy, E. & Steenbergen, C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol. Rev. 88, 581-609 (2008).
482. Smith, M. J. et al. Redox and metal profiles in human coronary endothelial and smooth muscle cells under hyperoxia, physiological normoxia and hypoxia: Effects of NRF2 signaling on intracellular zinc. Redox Biol. 62, 102712 (2023).
483. Cai, L. et al. Attenuation by metallothionein of early cardiac cell death via suppression of mitochondrial oxidative stress results in a prevention of diabetic cardiomyopathy. J. Am. Coll. Cardiol. 48, 1688-1697 (2006).
484. Wang, Y. et al. Inactivation of GSK-3beta by metallothionein prevents diabetesrelated changes in cardiac energy metabolism, inflammation, nitrosative damage, and remodeling. Diabetes 58, 1391-1402 (2009).
485. Dong, F. et al. Metallothionein prevents high-fat diet induced cardiac contractile dysfunction: role of peroxisome proliferator activated receptor gamma coactivator 1alpha and mitochondrial biogenesis. Diabetes 56, 2201-2212 (2007).
486. Wang, J. et al. Cardiac metallothionein induction plays the major role in the prevention of diabetic cardiomyopathy by zinc supplementation. Circulation 113, 544-554 (2006).
487. Hu, N. et al. Cardiac-specific overexpression of metallothionein rescues nicotineinduced cardiac contractile dysfunction and interstitial fibrosis. Toxicol. Lett. 202, 8-14 (2011).
488. Zhou, G. et al. Metallothionein suppresses angiotensin II-induced nicotinamide adenine dinucleotide phosphate oxidase activation, nitrosative stress, apoptosis, and pathological remodeling in the diabetic heart. J. Am. Coll. Cardiol. 52, 655-666 (2008).
489. Zhang, Y. et al. Cardiac overexpression of metallothionein rescues cold exposure-induced myocardial contractile dysfunction through attenuation of cardiac fibrosis despite cardiomyocyte mechanical anomalies. Free Radic. Biol. Med. 53, 194-207 (2012).
38
490. Cai, L. et al. Inhibition of superoxide generation and associated nitrosative damage is involved in metallothionein prevention of diabetic cardiomyopathy. Diabetes 54, 1829-1837 (2005).
491. Gu, J. et al. Metallothionein preserves Akt2 activity and cardiac function via inhibiting TRB3 in diabetic hearts. Diabetes 67, 507-517 (2018).
492. Dabravolski, S. A. et al. Interplay between
493. Woodier, J., Rainbow, R. D., Stewart, A. J. & Pitt, S. J. Intracellular zinc modulates cardiac ryanodine receptor-mediated calcium release. J. Biol. Chem. 290, 17599-17610 (2015).
494. Gaburjakova, J. & Gaburjakova, M. The cardiac ryanodine receptor provides a suitable pathway for the rapid transport of zinc (
495. Mor, M. et al. ZnT-1 enhances the activity and surface expression of T-type calcium channels through activation of Ras-ERK signaling. Am. J. Physiol. Cell Physiol. 303, C192-C203 (2012).
496. Liu, B., Cai, Z. Q. & Zhou, Y. M. Deficient zinc levels and myocardial infarction : association between deficient zinc levels and myocardial infarction: a metaanalysis. Biol. Trace Elem. Res. 165, 41-50 (2015).
497. Wang, J. et al. Downregulation of the zinc transporter SLC39A13 (ZIP13) is responsible for the activation of CaMKII at reperfusion and leads to myocardial ischemia/reperfusion injury in mouse hearts. J. Mol. Cell Cardiol. 152, 69-79 (2021).
498. Chen, Z. et al. Zinc ameliorates human aortic valve calcification through GPR39 mediated ERK1/2 signalling pathway. Cardiovasc. Res. 117, 820-835 (2021).
499. Fang, Y. et al. Slc39a2-mediated zinc homeostasis modulates innate immune signaling in phenylephrine-induced cardiomyocyte hypertrophy. Front. Cardiovasc. Med. 8, 736911 (2021).
500. Jiang, D. S. et al. IRF8 suppresses pathological cardiac remodelling by inhibiting calcineurin signalling. Nat. Commun. 5, 3303 (2014).
501. Jiang, D. S. et al. Interferon regulatory factor 9 protects against cardiac hypertrophy by targeting myocardin. Hypertension 63, 119-127 (2014).
502. Jiang, D. S. et al. Interferon regulatory factor 7 functions as a novel negative regulator of pathological cardiac hypertrophy. Hypertension 63, 713-722 (2014).
503. Lin, W. et al. Zinc transporter Slc39a8 is essential for cardiac ventricular compaction. J. Clin. Invest. 128, 826-833 (2018).
504. Lehuen, A., Diana, J., Zaccone, P. & Cooke, A. Immune cell crosstalk in type 1 diabetes. Nat. Rev. Immunol. 10, 501-513 (2010).
505. Baekkeskov, S. et al. Identification of the 64 K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature 347, 151-156 (1990).
506. Vehik, K. et al. Hierarchical order of distinct autoantibody spreading and progression to type 1 diabetes in the TEDDY study. Diabetes Care. 43, 2066-2073 (2020).
507. Palmer, J. P. et al. Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science 222, 1337-1339 (1983).
508. Achenbach, P. et al. Autoantibodies to zinc transporter 8 and SLC30A8 genotype stratify type 1 diabetes risk. Diabetologia 52, 1881-1888 (2009).
509. Kawasaki, E. et al. Differences in the humoral autoreactivity to zinc transporter 8 between childhood- and adult-onset type 1 diabetes in Japanese patients. Clin. Immunol. 138, 146-153 (2011).
510. Vermeulen, I. et al. Contribution of antibodies against IA-2
511. Wenzlau, J. M. et al. Kinetics of the post-onset decline in zinc transporter 8 autoantibodies in type 1 diabetic human subjects. J. Clin. Endocrinol. Metab. 95, 4712-4719 (2010).
512. Long, A. E. et al. Humoral responses to islet antigen-2 and zinc transporter 8 are attenuated in patients carrying HLA-A24 alleles at the onset of type 1 diabetes. Diabetes 62, 2067-2071 (2013).
513. Ye, J. et al. Attenuated humoral responses in HLA-A24-positive individuals at risk of type 1 diabetes. Diabetologia 58, 2284-2287 (2015).
514. Énée, É. et al.
515. Scotto, M. et al. Zinc transporter (ZnT)8(186-194) is an immunodominant CD8+ T cell epitope in HLA-A2 + type 1 diabetic patients. Diabetologia 55, 2026-2031 (2012).
516. Culina, S. et al. Islet-reactive CD8(+) T cell frequencies in the pancreas, but not in blood, distinguish type 1 diabetic patients from healthy donors. Sci. Immunol. 3, eaao4013 (2018).
517. Lampasona, V. & Liberati, D. Islet autoantibodies. Curr. Diab. Rep. 16, 53 (2016).
518. Wenzlau, J. M. et al. A common nonsynonymous single nucleotide polymorphism in the SLC30A8 gene determines ZnT8 autoantibody specificity in type 1 diabetes. Diabetes 57, 2693-2697 (2008).
519. Kawasaki, E. et al. Association between anti-ZnT8 autoantibody specificities and SLC30A8 Arg325Trp variant in Japanese patients with type 1 diabetes. Diabetologia 51, 2299-2302 (2008).
520. Shruthi, S., Mohan, V., Maradana, M. R. & Aravindhan, V. In silico identification and wet lab validation of novel cryptic
521. Hanna, S. J. et al. Slow progressors to type 1 diabetes lose islet autoantibodies over time, have few islet antigen-specific CD8(+) T cells and exhibit a distinct CD95(hi) B cell phenotype. Diabetologia 63, 1174-1185 (2020).
522. Wenzlau, J. M. et al. Changes in zinc transporter 8 autoantibodies following type 1 diabetes onset: the type 1 diabetes genetics consortium autoantibody workshop. Diabetes Care. 38, S14-S20 (2015).
523. Flannick, J. et al. Loss-of-function mutations in SLC30A8 protect against type 2 diabetes. Nat. Genet. 46, 357-363 (2014).
524. Choi, B. Y. et al. Zinc transporter 3 (ZnT3) gene deletion reduces spinal cord white matter damage and motor deficits in a murine MOG-induced multiple sclerosis model. Neurobiol. Dis. 94, 205-212 (2016).
525. Penkowa, M. & Hidalgo, J. Metallothionein I+II expression and their role in experimental autoimmune encephalomyelitis. Glia 32, 247-263 (2000).
526. Kim, B. et al. Cytoplasmic zinc promotes IL-1
527. Yoon, B. R. et al. Preferential induction of the T cell auxiliary signaling molecule B7-H3 on synovial monocytes in rheumatoid arthritis. J. Biol. Chem. 291, 4048-4057 (2016).
528. Cassat, J. E. & Skaar, E. P. Metal ion acquisition in Staphylococcus aureus: overcoming nutritional immunity. Semin. Immunopathol. 34, 215-235 (2012).
529. Baum, M. K. et al. Randomized, controlled clinical trial of zinc supplementation to prevent immunological failure in HIV-infected adults. Clin. Infect. Dis. 50, 1653-1660 (2010).
530. Kehl-Fie, T. E. & Skaar, E. P. Nutritional immunity beyond iron: a role for manganese and zinc. Curr. Opin. Chem. Biol. 14, 218-224 (2010).
531. Bao, B. et al. Zinc supplementation decreases oxidative stress, incidence of infection, and generation of inflammatory cytokines in sickle cell disease patients. Transl. Res. 152, 67-80 (2008).
532. Laskaris, P. et al. Administration of zinc chelators improves survival of mice infected with aspergillus fumigatus both in monotherapy and in combination with caspofungin. Antimicrob. Agents Chemother. 60, 5631-5639 (2016).
533. Corbin, B. D. et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 319, 962-965 (2008).
534. Hantke, K. Bacterial zinc uptake and regulators. Curr. Opin. Microbiol. 8, 196-202 (2005).
535. Lappann, M. et al. In vitro resistance mechanisms of Neisseria meningitidis against neutrophil extracellular traps. Mol. Microbiol. 89, 433-449 (2013).
536. Botella, H. et al. Metallobiology of host-pathogen interactions: an intoxicating new insight. Trends Microbiol. 20, 106-112 (2012).
537. Branch, A. H., Stoudenmire, J. L., Seib, K. L. & Cornelissen, C. N. Acclimation to nutritional immunity and metal intoxication requires zinc, manganese, and copper homeostasis in the pathogenic neisseriae. Front Cell Infect. Microbiol. 12, 909888 (2022).
538. Ishida, T. J. A. J. B. S. R. Review on the role of
539. Alamir, O. F., Oladele, R. O. & Ibe, C. Nutritional immunity: targeting fungal zinc homeostasis. Heliyon 7, e07805 (2021).
540. Subramanian Vignesh, K. & Deepe, G. S. Jr. Immunological orchestration of zinc homeostasis: the battle between host mechanisms and pathogen defenses. Arch. Biochem. Biophys. 611, 66-78 (2016).
541. Wagner, D. et al. Elemental analysis of Mycobacterium avium-, Mycobacterium tuberculosis-, and Mycobacterium smegmatis-containing phagosomes indicates pathogen-induced microenvironments within the host cell’s endosomal system. J. Immunol. 174, 1491-1500 (2005).
542. Botella, H. et al. Mycobacterial p(1)-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host Microbe 10, 248-259 (2011).
543. Neyrolles, O., Wolschendorf, F., Mitra, A. & Niederweis, M. Mycobacteria, metals, and the macrophage. Immunol. Rev. 264, 249-263 (2015).
544. Neyrolles, O., Mintz, E. & Catty, P. Zinc and copper toxicity in host defense against pathogens: mycobacterium tuberculosis as a model example of an emerging paradigm. Front. Cell Infect. Microbiol. 3, 89 (2013).
545. Sayadi, A., Nguyen, A. T., Bard, F. A. & Bard-Chapeau, E. A. Zip14 expression induced by lipopolysaccharides in macrophages attenuates inflammatory response. Inflamm. Res. 62, 133-143 (2013).
546. Stocks, C. J. et al. Uropathogenic Escherichia coli employs both evasion and resistance to subvert innate immune-mediated zinc toxicity for dissemination. Proc. Natl Acad. Sci. USA. 116, 6341-6350 (2019).
547. Padilla-Benavides, T . et al. A novel
548. Chandrangsu, P., Rensing, C. & Helmann, J. D. Metal homeostasis and resistance in bacteria. Nat. Rev. Microbiol. 15, 338-350 (2017).
549. Sensi, S. L. et al. The neurophysiology and pathology of brain zinc. J. Neurosci. 31, 16076-16085 (2011).
550. Szewczyk, B. Zinc homeostasis and neurodegenerative disorders. Front Aging Neurosci. 5, 33 (2013).
551. Walsh, D. M. et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416, 535-539 (2002).
552. Adlard, P. A. et al. Metal chaperones prevent zinc-mediated cognitive decline. Neurobiol. Dis. 81, 196-202 (2015).
553. Bjorklund, N. L. et al. Absence of amyloid
554. Bush, A. I. The metallobiology of Alzheimer’s disease. Trends Neurosci. 26, 207-214 (2003).
555. Whitfield, D. R. et al. Depression and synaptic zinc regulation in Alzheimer disease, dementia with lewy bodies, and Parkinson disease dementia. Am. J. Geriatr. Psychiatry 23, 141-148 (2015).
556. Adlard, P. A., Parncutt, J. M., Finkelstein, D. I. & Bush, A. I. Cognitive loss in zinc transporter-3 knock-out mice: a phenocopy for the synaptic and memory deficits of Alzheimer’s disease? J. Neurosci. 30, 1631-1636 (2010).
557. Adlard, P. A. et al. A novel approach to rapidly prevent age-related cognitive decline. Aging Cell. 13, 351-359 (2014).
558. Lang, M. et al. Genetic inhibition of solute-linked carrier 39 family transporter 1 ameliorates a
559. Meloni, G. et al. Metal swap between Zn 7 -metallothionein-3 and amyloid-betaCu protects against amyloid-beta toxicity. Nat. Chem. Biol. 4, 366-372 (2008).
560. Lyubartseva, G., Smith, J. L., Markesbery, W. R. & Lovell, M. A. Alterations of zinc transporter proteins
561. Bosomworth, H. J., Adlard, P. A., Ford, D. & Valentine, R. A. Altered expression of ZnT10 in Alzheimer’s disease brain. PLoS One 8, e65475 (2013).
562. Song, L. et al. ZIP9 mediates the effects of DHT on learning, memory and hippocampal synaptic plasticity of male Tfm and APP/PS1 mice. Front Endocrinol. 14, 1139874 (2023).
563. Sikora, J. & Ouagazzal, A. M. Synaptic zinc: an emerging player in Parkinson’s disease. Int J. Mol. Sci. 22, 4724 (2021).
564. Valiente-Gabioud, A. A. et al. Structural basis behind the interaction of
565. Sepers, M. D. & Raymond, L. A. Mechanisms of synaptic dysfunction and excitotoxicity in Huntington’s disease. Drug Discov. Today 19, 990-996 (2014).
566. Fourie, C. et al. Dietary zinc supplementation prevents autism related behaviors and striatal synaptic dysfunction in Shank3 Exon 13-16 mutant mice. Front. Cell Neurosci. 12, 374 (2018).
567. Lee, K. et al. Dietary zinc supplementation rescues fear-based learning and synaptic function in the Tbr1(+/-) mouse model of autism spectrum disorders. Mol. Autism 13, 13 (2022).
568. Squadrone, S., Brizio, P., Abete, M. C. & Brusco, A. Trace elements profile in the blood of Huntington’ disease patients. J. Trace Elem. Med. Biol. 57, 18-20 (2020).
569. Niu, L. et al. Disruption of zinc transporter ZnT 3 transcriptional activity and synaptic vesicular zinc in the brain of Huntington’s disease transgenic mouse. Cell Biosci. 10, 106 (2020).
570. Ayton, S. et al. Brain zinc deficiency exacerbates cognitive decline in the
571. Kaneko, M. et al. Zinc transporters ZnT3 and ZnT6 are downregulated in the spinal cords of patients with sporadic amyotrophic lateral sclerosis. J. Neurosci. Res. 93, 370-379 (2015).
572. Huang, J. et al. Structural basis of the zinc-induced cytoplasmic aggregation of the RNA-binding protein SFPQ. Nucleic Acids Res. 48, 3356-3365 (2020).
573. Gordon, P. M., Hamid, F., Makeyev, E. V. & Houart, C. A conserved role for the ALS-linked splicing factor SFPQ in repression of pathogenic cryptic last exons. Nat. Commun. 12, 1918 (2021).
574. Younas, N. et al. SFPQ and Tau: critical factors contributing to rapid progression of Alzheimer’s disease. Acta Neuropathol. 140, 317-339 (2020).
575. Bayik, D. & Lathia, J. D. Cancer stem cell-immune cell crosstalk in tumour progression. Nat. Rev. Cancer 21, 526-536 (2021).
576. Polyak, K. & Weinberg, R. A. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat. Rev. Cancer 9, 265-273 (2009).
577. Medema, J. P. Cancer stem cells: the challenges ahead. Nat. Cell Biol. 15, 338-344 (2013).
578. Singh, A. & Settleman, J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 29, 4741-4751 (2010).
579. Holohan, C. et al. Cancer drug resistance: an evolving paradigm. Nat. Rev. Cancer 13, 714-726 (2013).
580. Nabhan, C. et al. Caspase activation is required for gemcitabine activity in multiple myeloma cell lines. Mol. Cancer Ther. 1, 1221-1227 (2002).
581. Cui, X. et al. ZIP4 confers resistance to zinc deficiency-induced apoptosis in pancreatic cancer. Cell Cycle 13, 1180-1186 (2014).
582. Hessmann, E., Johnsen, S. A., Siveke, J. T. & Ellenrieder, V. Epigenetic treatment of pancreatic cancer: is there a therapeutic perspective on the horizon? Gut 66, 168-179 (2017).
583. Jiang, Y. et al. ZIP4 promotes non-small cell lung cancer metastasis by activating snail-N-cadherin signaling axis. Cancer Lett. 521, 71-81 (2021).
584. Wu, D. M. et al. SLC39A4 expression is associated with enhanced cell migration, cisplatin resistance, and poor survival in non-small cell lung cancer. Sci. Rep. 7, 7211 (2017).
585. Fan, Q., Zhang, W., Emerson, R. E. & Xu, Y. ZIP4 is a novel cancer stem cell marker in high-grade serous ovarian cancer. Cancers 12, 3692 (2020).
586. Ivan, C. et al. Epigenetic analysis of the Notch superfamily in high-grade serous ovarian cancer. Gynecol. Oncol. 128, 506-511 (2013).
587. Geles, K. G. et al. NOTCH3-targeted antibody drug conjugates regress tumors by inducing apoptosis in receptor cells and through transendocytosis into ligand cells. Cell Rep. Med. 2, 100279 (2021).
588. Farra, R. et al. Strategies for delivery of siRNAs to ovarian cancer cells. Pharmaceutics 11, 547 (2019).
589. Li, H. et al. ZIP10 drives osteosarcoma proliferation and chemoresistance through ITGA10-mediated activation of the PI3K/AKT pathway. J. Exp. Clin. Cancer Res. 40, 340 (2021).
590. Chen, Q. et al. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature 533, 493-498 (2016).
591. Maynard, A. et al. Therapy-induced evolution of human lung cancer revealed by single-cell RNA sequencing. Cell 182, 1232-1251.e1222 (2020).
592. Ni, C. et al. ZIP1(+) fibroblasts protect lung cancer against chemotherapy via connexin-43 mediated intercellular
593. Jia, C., Guo, Y. & Wu, F. G. Chemodynamic therapy via fenton and fenton-like nanomaterials: strategies and recent advances. Small 18, e2103868 (2022).
594. Ho, E., Wong, C. P. & King, J. C. Impact of zinc on DNA integrity and age-related inflammation. Free Radic. Biol. Med. 178, 391-397 (2022).
595. He, Y. et al. Evaluation of miR-21 and miR-375 as prognostic biomarkers in oesophageal cancer in high-risk areas in China. Clin. Exp. Metastasis. 34, 73-84 (2017).
596. Jin, J. et al. Methylation-associated silencing of miR-193b improves the radiotherapy sensitivity of esophageal cancer cells by targeting cyclin D1 in areas with zinc deficiency. Radiother. Oncol. 150, 104-113 (2020).
597. Kang, Y. et al. Advances in targeted therapy mainly based on signal pathways for nasopharyngeal carcinoma. Signal Transduct. Target Ther. 5, 245 (2020).
598. Criscitiello, C., Morganti, S. & Curigliano, G. Antibody-drug conjugates in solid tumors: a look into novel targets. J. Hematol. Oncol. 14, 20 (2021).
599. Nagayama, A., Vidula, N., Ellisen, L. & Bardia, A. Novel antibody-drug conjugates for triple negative breast cancer. Ther. Adv. Med. Oncol. 12, 1758835920915980 (2020).
600. Trail, P. A., Dubowchik, G. M. & Lowinger, T. B. Antibody drug conjugates for treatment of breast cancer: novel targets and diverse approaches in ADC design. Pharm. Ther. 181, 126-142 (2018).
601. Barroso-Sousa, R. & Tolaney, S. M. Clinical development of new antibody-drug conjugates in breast cancer: to infinity and beyond. BioDrugs 35, 159-174 (2021).
602. Lim, W. F., Mohamad Yusof, M. I., Teh, L. K. & Salleh, M. Z. Significant decreased expressions of CaN, VEGF, SLC39A6 and SFRP1 in MDA-MB-231 xenograft breast tumor mice treated with moringa oleifera leaves and seed residue (MOLSr) extracts. Nutrients 12, 2993 (2020).
603. Nolin, E. et al. Discovery of a ZIP7 inhibitor from a Notch pathway screen. Nat. Chem. Biol. 15, 179-188 (2019).
604. Chen, J. et al. Androgen dihydrotestosterone (DHT) promotes the bladder cancer nuclear AR-negative cell invasion via a newly identified membrane androgen receptor (mAR-SLC39A9)-mediated Gai protein/MAPK/MMP9 intracellular signaling. Oncogene 39, 574-586 (2020).
605. Seok, J. et al. Anti-oncogenic effects of dutasteride, a dual 5-alpha reductase inhibitor and a drug for benign prostate hyperplasia, in bladder cancer. J. Transl. Med. 21, 129 (2023).
606. Ashrafizadeh, M. et al. Noncoding RNAs as regulators of STAT3 pathway in gastrointestinal cancers: Roles in cancer progression and therapeutic response. Med. Res. Rev., 43, 1263-1321 (2023).
607. Yang, J. et al. Gene profile identifies zinc transporters differentially expressed in normal human organs and human pancreatic cancer. Curr. Mol. Med. 13, 401-409 (2013).
608. Ferrari, G., Thrasher, A. J. & Aiuti, A. Gene therapy using haematopoietic stem and progenitor cells. Nat. Rev. Genet. 22, 216-234 (2021).
609. Pramanik, S. K. et al. Nanoparticles for super-resolution microscopy: intracellular delivery and molecular targeting. Chem. Soc. Rev. 51, 9882-9916 (2022).
610. Wandt, V. K. et al. Ageing-associated effects of a long-term dietary modulation of four trace elements in mice. Redox Biol. 46, 102083 (2021).
611. Vrieling, F. & Stienstra, R. Obesity and dysregulated innate immune responses: impact of micronutrient deficiencies. Trends Immunol. 44, 217-230 (2023).
612. Wang, X. et al. The zinc transporter Slc39a5 controls glucose sensing and insulin secretion in pancreatic
613. Wang, G. et al. Metastatic cancers promote cachexia through ZIP14 upregulation in skeletal muscle. Nat. Med. 24, 770-781 (2018).
614. Yu, Y. et al. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood 136, 726-739 (2020).
615. Carvalho, C. S. et al. Blood cell responses and metallothionein in the liver, kidney and muscles of bullfrog tadpoles, Lithobates catesbeianus, following exposure to different metals. Environ. Pollut. 221, 445-452 (2017).
616. Chen, G. H. et al. Functional analysis of MTF-1 and MT promoters and their transcriptional response to zinc ( Zn ) and copper ( Cu ) in yellow catfish Pelteobagrus fulvidraco. Chemosphere 246, 125792 (2020).
617. Santoro, A. et al. The glutathione/metallothionein system challenges the design of efficient
618. Zaręba, N. & Kepinska, M. The function of transthyretin complexes with metallothionein in Alzheimer’s disease. Int J. Mol. Sci. 21, 9003 (2020).
619. Manso, Y. et al. Characterization of the role of metallothionein-3 in an animal model of Alzheimer’s disease. Cell Mol. Life Sci. 69, 3683-3700 (2012).
620. Kang, Y. C. et al. Cell-penetrating artificial mitochondria-targeting peptideconjugated metallothionein 1 A alleviates mitochondrial damage in Parkinson’s disease models. Exp. Mol. Med. 50, 1-13 (2018).
621. Carrasco, J. et al. Metallothionein-I and -III expression in animal models of Alzheimer disease. Neuroscience 143, 911-922 (2006).
622. Manso, Y. et al. Characterization of the role of the antioxidant proteins metallothioneins 1 and 2 in an animal model of Alzheimer’s disease. Cell Mol. Life Sci. 69, 3665-3681 (2012).
623. Nakamura, S. et al. Role of metallothioneins 1 and 2 in ocular neovascularization. Invest Ophthalmol. Vis. Sci. 55, 6851-6860 (2014).
624. Tiwari, R. et al. SPINK1 promotes colorectal cancer progression by downregulating Metallothioneins expression. Oncogenesis 4, e162 (2015).
625. Na, H. et al. Novel roles of DC-SIGNR in colon cancer cell adhesion, migration, invasion, and liver metastasis. J. Hematol. Oncol. 10, 28 (2017).
626. Mendes Garrido Abregú, F., Caniffi, C., Arranz, C. T. & Tomat, A. L. Impact of zinc deficiency during prenatal and/or postnatal life on cardiovascular and metabolic diseases: experimental and clinical evidence. Adv. Nutr. 13, 833-845 (2022).
627. Read, S. A., Obeid, S., Ahlenstiel, C. & Ahlenstiel, G. The role of zinc in antiviral immunity. Adv. Nutr. 10, 696-710 (2019).
628. Gomes, M. J. C., Martino, H. S. D. & Tako, E. Zinc-biofortified staple food crops to improve zinc status in humans: a systematic review. Crit. Rev. Food Sci. Nutr. 63, 4966-4978 (2023).
629. Gibson, R. S., King, J. C. & Lowe, N. A review of dietary zinc recommendations. Food Nutr. Bull. 37, 443-460 (2016).
630. Fairweather-Tait, S. J. & de Sesmaisons, A. Approaches used to estimate bioavailability when deriving dietary reference values for iron and zinc in adults. Proc. Nutr. Soc. 78, 1-7 (2018).
631. Duan, M. et al. Zinc nutrition and dietary zinc supplements. Crit. Rev. Food Sci. Nutr. 63, 1277-1292 (2023).
632. Brown, K. H. et al. International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr. Bull. 25, S99-S203 (2004).
633. Tran, C. D. et al. Zinc absorption as a function of the dose of zinc sulfate in aqueous solution. Am. J. Clin. Nutr. 80, 1570-1573 (2004).
634. Sapota, A. et al. The bioavailability of different zinc compounds used as human dietary supplements in rat prostate: a comparative study. Biometals 27, 495-505 (2014).
635. Chukwuma, C. I. et al. A comprehensive review on zinc(II) complexes as antidiabetic agents: The advances, scientific gaps and prospects. Pharm. Res. 155, 104744 (2020).
636. Jansen, J., Karges, W. & Rink, L. Zinc and diabetes-clinical links and molecular mechanisms. J. Nutr. Biochem. 20, 399-417 (2009).
637. Tang, Y. et al. Zinc supplementation partially prevents renal pathological changes in diabetic rats. J. Nutr. Biochem. 21, 237-246 (2010).
638. Jayawardena, R. et al. Effects of zinc supplementation on diabetes mellitus: a systematic review and meta-analysis. Diabetol. Metab. Syndr. 4, 13 (2012).
639. Ranasinghe, P. et al. Effects of Zinc supplementation on serum lipids: a systematic review and meta-analysis. Nutr. Metab. 12, 26 (2015).
640. Pompano, L. M. & Boy, E. Effects of dose and duration of zinc interventions on risk factors for type 2 diabetes and cardiovascular disease: a systematic review and meta-analysis. Adv. Nutr. 12, 141-160 (2021).
641. Özcelik, D. et al. Zinc supplementation attenuates metallothionein and oxidative stress changes in kidney of streptozotocin-induced diabetic rats. Biol. Trace Elem. Res. 150, 342-349 (2012).
642. Barman, S., Pradeep, S. R. & Srinivasan, K. Zinc supplementation alleviates the progression of diabetic nephropathy by inhibiting the overexpression of oxidative-stress-mediated molecular markers in streptozotocin-induced experimental rats. J. Nutr. Biochem. 54, 113-129 (2018).
643. Liu, F. et al. Zinc supplementation alleviates diabetic peripheral neuropathy by inhibiting oxidative stress and upregulating metallothionein in peripheral nerves of diabetic rats. Biol. Trace Elem. Res. 158, 211-218 (2014).
644. Foster, M., Chu, A., Petocz, P. & Samman, S. Zinc transporter gene expression and glycemic control in post-menopausal women with Type 2 diabetes mellitus. J. Trace Elem. Med Biol. 28, 448-452 (2014).
645. Sakurai, H., Yoshikawa, Y. & Yasui, H. Current state for the development of metallopharmaceutics and anti-diabetic metal complexes. Chem. Soc. Rev. 37, 2383-2392 (2008).
646. Tang, K. S. The current and future perspectives of zinc oxide nanoparticles in the treatment of diabetes mellitus. Life Sci. 239, 117011 (2019).
647. Patel, A. et al. Therapeutic value of zinc supplementation in acute and persistent diarrhea: a systematic review. PLoS One 5, e10386 (2010).
648. Chang, M. N. et al. Effects of different types of zinc supplement on the growth, incidence of diarrhea, immune function, and rectal microbiota of newborn dairy calves. J. Dairy Sci. 103, 6100-6113 (2020).
649. Bhandari, N. et al. Substantial reduction in severe diarrheal morbidity by daily zinc supplementation in young north Indian children. Pediatrics 109, e86 (2002).
650. Brooks, W. A. et al. Effect of weekly zinc supplements on incidence of pneumonia and diarrhoea in children younger than 2 years in an urban, low-income population in Bangladesh: randomised controlled trial. Lancet 366, 999-1004 (2005).
651. Dong, J., Li, H. & Min, W. Preparation, characterization and bioactivities of Athelia rolfsii exopolysaccharide-zinc complex (AEPS-zinc). Int J. Biol. Macromol. 113, 20-28 (2018).
652. Martinelli, D. et al. MEDNIK syndrome: a novel defect of copper metabolism treatable by zinc acetate therapy. Brain 136, 872-881 (2013).
653. Camarata, M. A., Ala, A. & Schilsky, M. L. Zinc maintenance therapy for wilson disease: a comparison between zinc acetate and alternative zinc preparations. Hepatol. Commun. 3, 1151-1158 (2019).
654. Duncan, A., Yacoubian, C., Watson, N. & Morrison, I. The risk of copper deficiency in patients prescribed zinc supplements. J. Clin. Pathol. 68, 723-725 (2015).
655. Guo, C. H. & Wang, C. L. Effects of zinc supplementation on plasma copper/zinc ratios, oxidative stress, and immunological status in hemodialysis patients. Int J. Med. Sci. 10, 79-89 (2013).
656. Hemilä, H. Zinc lozenges and the common cold: a meta-analysis comparing zinc acetate and zinc gluconate, and the role of zinc dosage. JRSM Open. 8, 2054270417694291 (2017).
657. Granum, B. Opinion of the Scientific Committee on Consumer safety (SCCS) Final opinion on water-soluble zinc salts used in oral hygiene products. Regul. Toxicol. Pharmacol. 99, 249-250 (2018).
658. Franklin, R. B. & Costello, L. C. The important role of the apoptotic effects of zinc in the development of cancers. J. Cell Biochem. 106, 750-757 (2009).
659. Hashemi, M. et al. Cytotoxic effects of intra and extracellular zinc chelation on human breast cancer cells. Eur. J. Pharmacol. 557, 9-19 (2007).
660. Richter, M. et al. Zinc chelators inhibit eotaxin, RANTES, and MCP-1 production in stimulated human airway epithelium and fibroblasts. Am. J. Physiol. Lung Cell Mol. Physiol. 285, L719-L729 (2003).
661. Albulescu, L. O. et al. Preclinical validation of a repurposed metal chelator as an early-intervention therapeutic for hemotoxic snakebite. Sci. Transl. Med. 12, eaay8314 (2020).
662. Nyborg, J. K. & Peersen, O. B. That zincing feeling: the effects of EDTA on the behaviour of zinc-binding transcriptional regulators. Biochem J. 381, e3-e4 (2004).
663. Hellmich, H. L. et al. Protective effects of zinc chelation in traumatic brain injury correlate with upregulation of neuroprotective genes in rat brain. Neurosci. Lett. 355, 221-225 (2004).
664. Bareggi, S. R. & Cornelli, U. Clioquinol: review of its mechanisms of action and clinical uses in neurodegenerative disorders. CNS Neurosci. Ther. 18, 41-46 (2012).
665. Doraiswamy, P. M. & Finefrock, A. E. Metals in our minds: therapeutic implications for neurodegenerative disorders. Lancet Neurol. 3, 431-434 (2004).
666. Labbé, R. F., Vreman, H. J. & Stevenson, D. K. Zinc protoporphyrin: a metabolite with a mission. Clin. Chem. 45, 2060-2072 (1999).
667. Faller, P. & Hureau, C. Bioinorganic chemistry of copper and zinc ions coordinated to amyloid-beta peptide. Dalton Trans. 7, 1080-1094 (2009).
668. Jackson, K. W. & Mahmood, T. M. Atomic absorption, atomic emission, and flame emission spectrometry. Anal. Chem. 66, 252r-279r (1994).
669. Carter, K. P., Young, A. M. & Palmer, A. E. Fluorescent sensors for measuring metal ions in living systems. Chem. Rev. 114, 4564-4601 (2014).
670. Denk, C. et al. Design, synthesis, and evaluation of a low-molecular-weight (11) C-labeled tetrazine for pretargeted PET imaging applying bioorthogonal in vivo click chemistry. Bioconjug. Chem. 27, 1707-1712 (2016).
671. Aper, S. J., Dierickx, P. & Merkx, M. Dual Readout BRET/FRET Sensors for Measuring Intracellular Zinc. ACS Chem. Biol. 11, 2854-2864 (2016).
672. Wei, T. et al. Directed evolution of the genetically encoded zinc(II) FRET sensor ZapCY1. Biochim Biophys. Acta Gen. Subj. 1866, 130201 (2022).
673. Bacart, J. et al. The BRET technology and its application to screening assays. Biotechnol. J. 3, 311-324 (2008).
674. Qin, Y. et al. Measuring steady-state and dynamic endoplasmic reticulum and Golgi Zn2+ with genetically encoded sensors. Proc. Natl Acad. Sci. Usa. 108, 7351-7356 (2011).
675. Chabosseau, P. et al. Mitochondrial and ER-targeted eCALWY probes reveal high levels of free
676. Hessels, A. M. et al. eZinCh-2: a versatile, genetically encoded FRET sensor for cytosolic and intraorganelle
677. Hessels, A. M., Taylor, K. M. & Merkx, M. Monitoring cytosolic and ER Zn(2+) in stimulated breast cancer cells using genetically encoded FRET sensors. Metallomics 8, 211-217 (2016).
678. Park, J. G., Qin, Y., Galati, D. F. & Palmer, A. E. New sensors for quantitative measurement of mitochondrial
679. Lin, Y. et al. ZIP4 is a novel molecular marker for glioma. Neuro Oncol. 15, 1008-1016 (2013).
680. Saravanan, R. et al. Zinc transporter LIV1: a promising cell surface target for triple negative breast cancer. J. Cell Physiol. 237, 4132-4156 (2022).
681. Gou, Y. et al. The transcription of ZIP9 is associated with the macrophage polarization and the pathogenesis of hepatocellular carcinoma. Front Immunol. 13, 725595 (2022).
682. Changizzadeh, P. N., Mukkamalla, S. K. R. & Armenio, V. A. Combined checkpoint inhibitor therapy causing diabetic ketoacidosis in metastatic melanoma. J. Immunother. Cancer 5, 97 (2017).
683. Sveen, A. et al. The exon-level biomarker SLC39A14 has organ-confined cancerspecificity in colorectal cancer. Int J. Cancer 131, 1479-1485 (2012).
684. Karandish, M. et al. The effect of curcumin and zinc co-supplementation on glycemic parameters in overweight or obese prediabetic subjects: a phase 2 randomized, placebo-controlled trial with a multi-arm, parallel-group design. Phytother. Res. 35, 4377-4387 (2021).
685. Islam, M. R. et al. Zinc supplementation for improving glucose handling in prediabetes: a double blind randomized placebo controlled pilot study. Diabetes Res Clin. Pract. 115, 39-46 (2016).
686. Foster, M., Petocz, P. & Samman, S. Inflammation markers predict zinc transporter gene expression in women with type 2 diabetes mellitus. J. Nutr. Biochem. 24, 1655-1661 (2013).
687. Nazem, M. R. et al. Zinc supplementation ameliorates type 2 diabetes markers through the enhancement of total antioxidant capacity in overweight patients. Postgrad. Med. J. 99, 862-867 (2023).
688. Fung, E. B. et al. Zinc supplementation improves markers of glucose homeostasis in thalassaemia. Br. J. Haematol. 190, e162-e166 (2020).
689. Bao, B. et al. Zinc decreases C-reactive protein, lipid peroxidation, and inflammatory cytokines in elderly subjects: a potential implication of zinc as an atheroprotective agent. Am. J. Clin. Nutr. 91, 1634-1641 (2010).
690. Ben Abdallah, S. et al. Twice-Daily Oral Zinc in the Treatment of Patients With Coronavirus Disease 2019: A Randomized Double-Blind Controlled Trial. Clin. Infect. Dis. 76, 185-191 (2023).
691. Rodriguez, J. A. M. et al. Effect and tolerability of a nutritional supplement based on a synergistic combination of
692. Faghfouri, A. H. et al. Regulation of NLRP3 inflammasome by zinc supplementation in Behçet’s disease patients: a double-blind, randomized placebocontrolled clinical trial. Int Immunopharmacol. 109, 108825 (2022).
693. Faghfouri, A. H. et al. Immunomodulatory and clinical responses to zinc gluconate supplementation in patients with Behçet’s disease: a doubleblind, randomized placebo-controlled clinical trial. Clin. Nutr. 41, 1083-1092 (2022).
694. Bobat, R. et al. Safety and efficacy of zinc supplementation for children with HIV1 infection in South Africa: a randomised double-blind placebo-controlled trial. Lancet 366, 1862-1867 (2005).
695. Roy, S. K. et al. Zinc supplementation in children with cholera in Bangladesh: randomised controlled trial. BMJ 336, 266-268 (2008).
696. Veenemans, J. et al. Effect of supplementation with zinc and other micronutrients on malaria in Tanzanian children: a randomised trial. PLoS Med. 8, e1001125 (2011).
697. Fung, E. B. et al. Zinc supplementation improves bone density in patients with thalassemia: a double-blind, randomized, placebo-controlled trial. Am. J. Clin. Nutr. 98, 960-971 (2013).
698. Guo, C. H., Chen, P. C., Hsu, G. S. & Wang, C. L. Zinc supplementation alters plasma aluminum and selenium status of patients undergoing dialysis: a pilot study. Nutrients 5, 1456-1470 (2013).
699. Kobayashi, H. et al. Oral zinc supplementation reduces the erythropoietin responsiveness index in patients on hemodialysis. Nutrients 7, 3783-3795 (2015).
700. Lin, L. C., Que, J., Lin, L. K. & Lin, F. C. Zinc supplementation to improve mucositis and dermatitis in patients after radiotherapy for head-and-neck cancers: a double-blind, randomized study. Int J. Radiat. Oncol. Biol. Phys. 65, 745-750 (2006).
701. Ribeiro, S. M. et al. Effect of zinc supplementation on antioxidant defenses and oxidative stress markers in patients undergoing chemotherapy for colorectal cancer: a placebo-controlled, prospective randomized trial. Biol. Trace Elem. Res. 169, 8-16 (2016).
702. Qiao, Y. L. et al. Total and cancer mortality after supplementation with vitamins and minerals: follow-up of the Linxian General Population Nutrition Intervention Trial. J. Natl Cancer Inst. 101, 507-518 (2009).
703. Ye, W. et al. A sensitive FRET biosensor based on carbon dots-modified nanoporous membrane for 8-hydroxy-2′-Deoxyguanosine (8-OHdG) detection with Au@ZIF-8 nanoparticles as signal quenchers. Nanomaterials 10, 2044 (2020).
704. Qin, Y. et al. Development of an optical
705. Han, Y., Goldberg, J. M., Lippard, S. J. & Palmer, A. E. Superiority of SpiroZin2 Versus FluoZin-3 for monitoring vesicular
706. Nolan, E. M. & Lippard, S. J. Small-molecule fluorescent sensors for investigating zinc metalloneurochemistry. Acc. Chem. Res. 42, 193-203 (2009).
707. Ueno, S. et al. Mossy fiber Zn2+ spillover modulates heterosynaptic N-methyl-D-aspartate receptor activity in hippocampal CA3 circuits. J. Cell Biol. 158, 215-220 (2002).
708. Kao, Y. Y. et al. Zinc oxide nanoparticles interfere with zinc ion homeostasis to cause cytotoxicity. Toxicol. Sci. 125, 462-472 (2012).
709. Sensi, S. L. et al. A new mitochondrial fluorescent zinc sensor. Cell Calcium 34, 281-284 (2003).
710. You, Y. et al. Phosphorescent sensor for biological mobile zinc. J. Am. Chem. Soc. 133, 18328-18342 (2011).
711. Meeusen, J. W., Tomasiewicz, H., Nowakowski, A. & Petering, D. H. TSQ (6-methoxy-8-p-toluenesulfonamido-quinoline), a common fluorescent sensor for cellular zinc, images zinc proteins. Inorg. Chem. 50, 7563-7573 (2011).
© The Author(s) 2023
Department of Anatomical and Cellular Pathology, State Key Laboratory of Translational Oncology, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong, China; State Key Laboratory of Digestive Disease, Institute of Digestive Disease, The Chinese University of Hong Kong, Hong Kong, China; CUHK-Shenzhen Research Institute, The Chinese University of Hong Kong, Shenzhen, China; Department of Pathology, Nanfang Hospital and Basic Medical College, Southern Medical University, Guangdong Province Key Laboratory of Molecular Tumor Pathology, Guangzhou, China; Institute of Biomedical Research, Taihe Hospital, Hubei University of Medicine, Shiyan, China; Department of Pediatrics, The Chinese University of Hong Kong, Hong Kong, China and Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Hong Kong, China
Correspondence: Wei Kang (weikang@cuhk.edu.hk) or Ka Fai To (kfto@cuhk.edu.hk)
These authors contributed equally: Bonan Chen, Peiyao Yu
DOI: https://doi.org/10.1038/s41392-023-01679-y
PMID: https://pubmed.ncbi.nlm.nih.gov/38169461
Publication Date: 2024-01-03
Cellular zinc metabolism and zinc signaling: from biological functions to diseases and therapeutic targets
Abstract
Zinc metabolism at the cellular level is critical for many biological processes in the body. A key observation is the disruption of cellular homeostasis, often coinciding with disease progression. As an essential factor in maintaining cellular equilibrium, cellular zinc has been increasingly spotlighted in the context of disease development. Extensive research suggests zinc’s involvement in promoting malignancy and invasion in cancer cells, despite its low tissue concentration. This has led to a growing body of literature investigating zinc’s cellular metabolism, particularly the functions of zinc transporters and storage mechanisms during cancer progression. Zinc transportation is under the control of two major transporter families: SLC30 (ZnT) for the excretion of zinc and SLC39 (ZIP) for the zinc intake. Additionally, the storage of this essential element is predominantly mediated by metallothioneins (MTs). This review consolidates knowledge on the critical functions of cellular zinc signaling and underscores potential molecular pathways linking zinc metabolism to disease progression, with a special focus on cancer. We also compile a summary of clinical trials involving zinc ions. Given the main localization of zinc transporters at the cell membrane, the potential for targeted therapies, including small molecules and monoclonal antibodies, offers promising avenues for future exploration.
INTRODUCTION
( ZnT ) and SLC39 (Zrt- and Irt-like proteins/ZIP), as well as the zincbinding (MTs).
REGULATION OF CELLULAR ZINC SIGNALING
Zinc distribution
Intracellular zinc signaling
which helps maintain zinc concentration at the pM range in the cytosol.
Extracellular zinc signaling

Zinc signaling and tumorigenesis
manifesting the cells’ current activities, e.g., function, growth, and proliferation. Several mechanisms explain the antitumor function of zinc, encompassing DNA damage, DNA repair, immune function, oxidative stress, and inflammation.
pathway in nasopharyngeal carcinoma (NPC).
REGULATION OF CELLULAR ZINC METABOLISM
ZIPs. The SLC39 family comprises four distinct groups based on amino acid sequence similarities: subfamily I (ZIP9); subfamily II (ZIP1, 2, and 3); the LIV-1 subfamily (ZIP4, 5, 6, 7, 8, 10, 12, 13, and 14); and the gufA subfamily containing ZIP11.
metal ion uptake into cells. ZIP7 is situated in the Golgi apparatus and ER, while ZIP13, evolutionarily closest to ZIP7, is localized in the Golgi apparatus and cytoplasmic vesicles.

in insulin storage, ZnT 4 in prostate secretion, and ZnT 2 in lactation.
member of the mammalian ZnT transporter proteins.
Mammalian MTs are a superfamily of nonenzymatic polypeptides that typically consist of 61-68 amino acids.
ROLE OF CELLULAR ZINC METABOLISM UNDER PHYSIOLOGICAL CONDITIONS
Supporting immune function. T cells are a critical component of the immune system.
predominantly localized to lipid rafts involved in the immune synapse (IS) formation following T cell receptor (TCR) stimulation.

mediated by ZIP7, as ZIP7 predominantly resides in the ER, and silencing ZIP using siRNA prevented the occurrence of the zinc wave.

and skeletal and connective tissue abnormalities, mirroring the phenotypes observed in SCD-EDS patients.
Involvement in cell proliferation, differentiation, and apoptosis. Numerous studies have demonstrated that MTs regulate zinc, notably in relation to cell cycle regulation and cell proliferation.
differentiation is an indirect function, involving the suppression of PPAR
heavy metal detoxification, particularly for cadmium and arsenic.
As previously mentioned, there exists a correlation between changes in zinc levels and cancer progression. However, it is essential to acknowledge that the nature of this correlation may vary among various kinds of cancer. Multifaceted effects of zinc in promoting or inhibiting tumor growth underscores this complexity, with distinct mechanisms operating in various cancer types. Recent evidence has been accumulating, suggesting a link between ZD and the development of cancers. Numerous processes are involved in zinc’s anti-tumor activity, encompassing DNA damage and repair, oxygenation, immunity, and the inflammatory process.
is observed to be upregulated in estrogen receptor-positive breast cancers and shows a positive correlation with estrogen receptor status. During gastrulation in zebrafish, zip6 is transactivated by STAT3. Elevated expression of zip6 results in nuclear retention of Snail, which is also known to be a zinc-finger transcription factor, which subsequently represses the expression of E-cadherin, resulting in cell migration

brain tissue, was found to be twice as high as that in the TNBC cell line MDA-MB231. Additionally, ZIP8, ZIP9, and ZIP13 have been demonstrated to be upregulated in BrM2 cells. The correlation between intracellular zinc concentration and BC cell metastatic potential is implied.

downregulation has been observed in benign prostatic hyperplasia (BPH), PC-3 cells, and malignant tissues of the human prostate. MT1/2 expression is notably enhanced by zinc therapy in both PC-3 and BPH cells, coincident with the restoration of intracellular zinc concentrations. Specifically, in BPH cells, MT3, acting as a growth inhibitory agent, was identified, and its levels were elevated by zinc. Furthermore, the expression of MT3 serves as a distinctive feature exclusively found in BPH cells.

phenotypes and poor patient outcomes in CRC.
metabolism. Intriguingly, these MTs often act as tumor suppressor genes in CRC. A notable correlation between low MT1B, MT1H, or MT1L expression and an increased risk of adverse outcomes was identified.
that MT1M has the ability to dampen the malignancy and stem cell-like characteristics of GC by inhibiting GLI1, a component of the Hedgehog signaling pathway, known for its numerous zinc finger domains.
Chen et al.

gene for SLC30A8 and SLC39A4 amplification was co-occurring in almost all cancer patients. Interestingly, the cases with SLC39A14 deletion appear to be more than those with amplification (Fig. 8). Although ZIPs are more commonly regarded as oncogenes in cancer, prostate cancer is an exception. Studies also suggested that the function of the zinc transporters may be contradictory among different cancer types. As we delve into the gene alterations in MTs, our attention is captured by the astonishingly consistent variations observed among all MTs members (Fig. 8). Notably, the compelling set of data from representative tumor patients showcases the remarkably homogeneous trends in gene alterations among all MTs members. Such changes predominantly
encompass amplifications and deep deletions, implying pivotal roles for MTs in the context of cancers. Despite the similar gene alteration trends, disparate mRNA expression profiles are observed for different MTs members. This intriguing observation suggests the involvement of intricate transcriptional regulatory mechanisms governing MTs genes. The diversity in mRNA expression levels might arise due to a myriad of factors, potentially linked to cellular context, tissue specificity, and even cancer types. Thus, research on zinc transporters and MTs in tumorigenesis is still a long way to go.
Cellular zinc metabolism in cardiovascular disease
been suggested that reduced NO generation in atheroprone regions, combined with increased ZnT 1 and MT expression, may lead to decreased intracellular free zinc.
valves from patients with CAVD. The anti-calcific effect of zinc on human valve interstitial cells (hVIC) calcification is, at least in part, mediated through the inhibition of apoptosis and osteogenic differentiation via the GPR39-dependent ERK1/2 signaling pathway. Additionally, ZIP13 and ZIP14 play important roles in hVIC in vitro calcification and osteogenic differentiation.
Zinc plays various roles in autoimmune diseases, including its function as an effector of the immune system, inflammation, and metabolism. As mentioned previously, the ZIP family, ZnT family, and MTs act as crucial regulators of zinc levels and are involved in developing different autoimmune diseases, such as the production of autoantibodies and inflammatory responses.
reflecting the continuous loss of
Cellular zinc metabolism in infectious diseases
the restoration of carbapenem susceptibility in Acinetobacter baumannii and improved survival in mice infected with Aspergillus fumigatus when pathogens were starved with zinc chelators.
phagocytic vesicles through ZIPs.
Zinc homeostasis alterations have been suggested to be closely associated with the development of certain neurodegenerative diseases.
THERAPEUTIC TARGETS FOR CELLULAR ZINC METABOLISM
Zinc transporters
| Table 1. The expression levels, clinicopathological correlation, and potential small molecules for zinc transporters in carcinogenesis | ||||||
| Member | Cancer type | Expression | Diagnostic marker | Prognostic marker | Small molecules | References |
| ZIP4 | HCC | Upregulated | – |
|
– | 397 |
| Gliomas | Upregulated |
|
|
– | 679 | |
| HGSOC | Upregulated |
|
– | – | 585 | |
| PC | Upregulated |
|
|
– | 391,393,394,396,399 | |
| NPC | Upregulated |
|
|
– | 77 | |
| NSCLC | Upregulated | – |
|
– | 583 | |
| ZIP5 | ESCC | Upregulated | – | – | miR-193b | 596 |
| ZIP6 | ESCC | Upregulated | – |
|
– | 447 |
| BC | Upregulated |
|
|
SGN-LIV1A/LV (NCT01969643, NCT03310957, NCT03424005, NCT01042379, NCT04032704, NCT02093858) | 156,601,680 | |
| ZIP6-Y antibody | 156 | |||||
| Faslodex, 4-hydroxytamoxifen | 157 | |||||
| M1S9 | 602 | |||||
| ZIP7 | BC | Upregulated |
|
|
DMAT, TBB | 168 |
| T-ALL | Upregulated | – |
|
NVS-ZP7-4 | 603 | |
| ZIP9 | HCC | Upregulated | – |
|
– | 681 |
| Bladder cancer | Upregulated | – |
|
Dutasteride | 604,605 | |
| Melanoma | Upregulated | Bicalutamide | 461 | |||
| ZIP10 | Osteosarcoma | Upregulated | – |
|
666-15, GSK690693 | 589 |
| BC | Upregulated | – |
|
ZIP10B antibody | 156,334 | |
| GC | Upregulated | – |
|
XYA-2 | 682 | |
| ZIP13 | Ovarian cancer | Upregulated | – |
|
– | 73 |
| ZIP14 | CRC | Upregulated | – |
|
– | 417,683 |
microenvironment, helping cancer cells to generate chemoresistance by regulating zinc concentration.
proliferation.
| Table 2. Possibility for targeting zinc metabolism in multiple diseases | |||||||
| Protein | Disease | Expression | Current or potential targeting value | ||||
| ZIP5 | Diabetes | Downregulated | The potential therapeutic target for diabetes-related diseases.
|
||||
| ZIP10 | Hematopoietic disease | Downregulated | Targeting ZIP10 may be a new therapeutic strategy against early fetal anemia.
|
||||
| ZIP14 | Dystrophic muscles | Upregulated | Underscores the importance of regulated zinc homeostasis in metastatic cancer-induced muscle dystrophy and suggests a novel treatment avenue by targeting ZIP14.
|
||||
| Liver cirrhosis | Upregulated | A new potential therapeutic avenue for preventing iron-death-induced liver fibrosis.
|
|||||
| ZnT8 | Diabetes | Downregulated |
|
||||
| MT1/2 | AD | – | Modulation of MT-I/II expression is a potential therapeutic target to treat the onset and progression of cognitive impairment.
|
||||
| Ocular neovascularization | – | MT1/2 is a potential novel therapeutic target for diseases involving ocular angiogenesis.
|
|||||
Therapeutic potential of MTs
Zinc-based therapeutics and measurement
Chen et al.
| Disease | Dosage and species of zinc | Effect/Comments | Trial registration number | References |
| Clinical applications of zinc supplements | ||||
| Prediabetes | 30 mg zinc gluconate/day, 90 days. | Zinc supplementation significantly decreased BMI and improved FPG, 2hpp, HbA1C, insulin, IS, and IR. | – | 684 |
| Type-2 diabetes | 30 mg zinc sulfate/day, 6 months. | Zinc supplementation improved FBG and HOMA concentration. Beta cell function, insulin sensitivity and insulin resistance showed significant improvement as well. | – | 685 |
| 40 mg zinc sulfate/day, 12 weeks. | Zinc supplementation was observed on inflammatory marker concentrations or fold change in zinc transporter and MT gene expression. | NCT01505803 | 686 | |
| 50 mg zinc gluconate/day, 8 weeks. | The total antioxidant capacity was significantly elevated (
|
IRCT2015083102 | 687 | |
| Diabetes with thalassemia | 25 mg zinc sulfate/day, 3 months. | Zinc supplementation improves glucose homeostasis in thalassemia. | NCT01772680 | 688 |
| AS | 45 mg zinc gluconate/day, 6 months. | Zinc supplementation reduced plasma CRP and IL-6 levels in men and women. Zinc may have a protective effect on AS because of its anti-inflammatory and antioxidant functions. | – | 689 |
| COVID-19 | 25 mg of elemental zinc as capsule/day, 15 days. | Oral zinc can decrease 30-day death, ICU admission rate and can shorten symptom duration. | NCT05212480. | 690 |
| COVID-19 | 15 mg zinc in an active product/day, 30 days. | The administration of an active product (ABB C1
|
NCT04798677 | 691 |
| Behcet’s disease | 30 mg zinc gluconate/day, 12 weeks. | Zinc gluconate supplementation can be considered as an adjuvant therapy in alleviating inflammation and genital ulcer among Behcet’s disease patients. | – | 692 |
| 30 mg zinc gluconate/day, 12 weeks. | Zinc supplementation significantly improved non-ocular Behcet’s disease score and TLR-2 expression. | NCT05098678 | 693 | |
| HIV-1 | 10 mg zinc sulfate/day, 6 months. | Zinc supplementation does not result in an increase in plasma HIV-1 viral load and could reduce morbidity caused by diarrhea. | – | 694 |
| Cholera | 30 mg zinc acetate/day, until resolution of diarrhea or for up to seven days. | Zinc supplementation significantly reduced the duration of diarrhea and stool output in children with cholera. | NCT00226616 | 695 |
| Malaria | 10 mg zinc gluconate/day, median follow-up: 331 days | Neither zinc nor multi-nutrients influenced malaria rates | NCT00623857 | 696 |
| Thalassemia major | 25 mg zinc sulfate/day, 18 months. | Zinc supplementation resulted in greater gains in total-body bone mass in young patients with thalassemia major. | NCT00459732 | 697 |
| Hemodialysis | 78 mg zinc gluconate/day, 2 months. | Zinc supplementation ameliorates abnormally high plasma AI concentrations and oxidative stress and improves selenium status in long-term dialysis patients. | – | 698 |
| 34 mg hemodialysis/day, 12 months. | Zinc supplementation reduces the erythropoietin responsiveness index in patients undergoing hemodialysis and may be a novel therapeutic strategy for patients with renal anemia and low serum zinc levels. | – | 699 | |
| Head and neck cancers | 25 mg Pro-zinc (a powder extracted from bovine prostate then chelated to zinc)/ day, 2 months. | Zinc supplementation used in conjunction with radiotherapy could postpone the development of severe mucositis and dermatitis in patients with cancers of the head and neck. | – | 700 |
| Colorectal cancer | 308 mg zinc sulfate/day, 108 days. | Zinc supplementation during chemotherapy cycles increased SOD activity and maintained vitamin E concentrations, indicating production of stable free radicals, which may have a positive effect on cancer treatment. | NCT02106806 | 701 |
| 70 mg zinc sulfate/day, 16 weeks | Zinc supplementation on markers of oxidative stress in postoperative colorectal cancer during chemotherapy cycles. | NCT02106806 | – | |
| Zinc gluconate, unknown dosage, 8 weeks. | Zinc supplement in regorafenib treated metastatic CRC patient (ZnCORRECT). | NCT03898102 | – | |
| 70 mg zinc sulfate/day, 4 months. | Modulation of immune response by oral zinc supplementation in chemotherapy for CRC. | NCT01261962 | – | |
| ESCC and GC | 22.5 mg zinc oxide/day, 15.25 years. | Zinc supplementation was associated with increased total and stroke mortality. | – | 702 |
| GI cancer | Zinc sulfate, unknown dosage | Effects on quality of life with zinc supplementation in patients with Gl cancer. | NCT03819088 | – |
| Clinical application of zinc chelators | ||||
| Epilepsy | 2 weeks
|
To examine the potential anti-seizure activity of clioquinol in a small cohort of adolescents with drug-resistant epilepsy | NCT05727943 | – |
| Hematological malignancy | 800 mg clioquinol/day, 28 days. | To evaluate the dose-limiting toxicity, maximum tolerated dose, and recommended phase II dose of clioquinol in patients with relapsed or refractory hematologic malignancies. | NCT00963495 | – |
| Category | Name | Kd | Targeted organelles | References |
| FRET | Zif |
|
– | 703 |
| ZapCY1 |
|
Golgi, ER, mitochondria | 672,678 | |
| eCALWY-4 |
|
ER, mitochondria | 675 | |
| eZinCh-2 |
|
ER, mitochondria | 676 | |
| GZnP1 |
|
– | 704 | |
| BRET | BLZinCh-1 |
|
ER, mitochondria | 671 |
| BLZinCh-2 |
|
ER, mitochondria | 671 | |
| BLZinCh-3 |
|
ER, mitochondria | 671 | |
| LMW | FluoZin-3-AM |
|
ER, mitochondria | 705 |
| Zinpyr (ZP) |
|
Golgi, mitochondria | 706 | |
| ZnAF |
|
– | 707 | |
| RhodZin-353 | – | Mitochondria | 708,709 | |
| ZIrF |
|
– | 710 | |
| TSQ | – | Cytoplasm | 711 |
alleviates MT and oxidative stress in renal tissues of streptozotocin-induced diabetic rats, thereby preventing the development of diabetic nephropathy.
copper.
and genetically encoded fluorescent proteins.
CONCLUSION AND FUTURE DIRECTION
methylation in CRC. Aberrant expression or hyperactivation of zinc transporters would also contribute to tumor resistance, which could be a malprognostic factor for cancer patients. Therefore, aiming at zinc transporters is expected to improve the efficacy of tumor therapies. Meanwhile, since zinc transporter proteins are predominantly distributed on cell membranes, developing small molecules or monoclonal antibodies for specific targeting is feasible.
ACKNOWLEDGEMENTS
datasets and analysis. Part of the images was generated by BioRender (https:// biorender.com/) and GEPIA2 (http://gepia2.cancer-pku.cn/#isoform). We also appreciate the technical support from Core Utilities of Cancer Genomics and Pathobiology of the Department of Anatomical and Cellular Pathology, The Chinese University of Hong Kong.
AUTHOR CONTRIBUTIONS
ADDITIONAL INFORMATION
REFERENCES
- Huang, L. & Tepaamorndech, S. The SLC30 family of zinc transporters – a review of current understanding of their biological and pathophysiological roles. Mol. Asp. Med. 34, 548-560 (2013).
- Kambe, T., Tsuji, T., Hashimoto, A. & Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 95, 749-784 (2015).
- Kimura, T. & Kambe, T. The Functions of Metallothionein and ZIP and ZnT Transporters: An Overview and Perspective. Int J. Mol. Sci. 17, 336 (2016).
- Hu, H. et al. New anti-cancer explorations based on metal ions. J. Nanobiotechnol. 20, 457 (2022).
- Stockwell, B. R., Jiang, X. & Gu, W. Emerging mechanisms and disease relevance of ferroptosis. Trends Cell Biol. 30, 478-490 (2020).
- Andreini, C., Bertini, I. & Rosato, A. Metalloproteomes: a bioinformatic approach. Acc. Chem. Res. 42, 1471-1479 (2009).
- Angus-Hill, M. L. et al. A Rsc3/Rsc30 zinc cluster dimer reveals novel roles for the chromatin remodeler RSC in gene expression and cell cycle control. Mol. Cell. 7, 741-751 (2001).
- Kim, A. M. et al. Zinc sparks are triggered by fertilization and facilitate cell cycle resumption in mammalian eggs. ACS Chem. Biol. 6, 716-723 (2011).
- Lo, M. N. et al. Single cell analysis reveals multiple requirements for zinc in the mammalian cell cycle. Elife 9, e51107 (2020).
- Haase, H. & Rink, L. Multiple impacts of zinc on immune function. Metallomics 6, 1175-1180 (2014).
- Que, E. L. et al. Quantitative mapping of zinc fluxes in the mammalian egg reveals the origin of fertilization-induced zinc sparks. Nat. Chem. 7, 130-139 (2015).
- Maret, W. Analyzing free zinc(II) ion concentrations in cell biology with fluorescent chelating molecules. Metallomics 7, 202-211 (2015).
- Hennigar, S. R., Kelley, A. M. & McClung, J. P. Metallothionein and zinc transporter expression in circulating human blood cells as biomarkers of zinc status: a systematic review. Adv. Nutr. 7, 735-746 (2016).
- Bafaro, E., Liu, Y., Xu, Y. & Dempski, R. E. The emerging role of zinc transporters in cellular homeostasis and cancer. Signal Transduct. Target Ther. 2, 17029- (2017).
- Calesnick, B. & Dinan, A. M. Zinc deficiency and zinc toxicity. Am. Fam. Physician 37, 267-270 (1988).
- Stefanidou, M., Maravelias, C., Dona, A. & Spiliopoulou, C. Zinc: a multipurpose trace element. Arch. Toxicol. 80, 1-9 (2006).
- Gilbert, R., Peto, T., Lengyel, I. & Emri, E. Zinc nutrition and inflammation in the aging retina. Mol. Nutr. Food Res. 63, e1801049 (2019).
- Pfeiffer, C. C. & Braverman, E. R. Zinc, the brain and behavior. Biol. Psychiatry 17, 513-532 (1982).
- Tapiero, H. & Tew, K. D. Trace elements in human physiology and pathology: zinc and metallothioneins. Biomed. Pharmacother. 57, 399-411 (2003).
- Costello, L. C., Fenselau, C. C. & Franklin, R. B. Evidence for operation of the direct zinc ligand exchange mechanism for trafficking, transport, and reactivity of zinc in mammalian cells. J. Inorg. Biochem. 105, 589-599 (2011).
- Maret, W. Zinc coordination environments in proteins as redox sensors and signal transducers. Antioxid. Redox Signal. 8, 1419-1441 (2006).
- Turan, B. & Tuncay, E. Impact of labile zinc on heart function: from physiology to pathophysiology. Int J. Mol. Sci. 18, 2395 (2017).
- Coyle, P., Philcox, J. C., Carey, L. C. & Rofe, A. M. Metallothionein: the multipurpose protein. Cell Mol. Life Sci. 59, 627-647 (2002).
- Outten, C. E. & O’Halloran, T. V. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 292, 2488-2492 (2001).
30
25. Blindauer, C. A. & Leszczyszyn, O. I. Metallothioneins: unparalleled diversity in structures and functions for metal ion homeostasis and more. Nat. Prod. Rep. 27, 720-741 (2010).
26. Wang, X. L., Schnoor, M. & Yin, L. M. Metallothionein-2: an emerging target in inflammatory diseases and cancers. Pharm. Ther. 244, 108374 (2023).
27. Amagai, Y. et al. Zinc homeostasis governed by Golgi-resident ZnT family members regulates ERp44-mediated proteostasis at the ER-Golgi interface. Nat. Commun. 14, 2683 (2023).
28. Fang, H. et al. Simultaneous
29. Frederickson, C. J., Koh, J. Y. & Bush, A. I. The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 6, 449-462 (2005).
30. Eide, D. J. The SLC39 family of metal ion transporters. Pflug. Arch. 447, 796-800 (2004).
31. Bin, B. H. et al. Molecular pathogenesis of spondylocheirodysplastic EhlersDanlos syndrome caused by mutant ZIP13 proteins. EMBO Mol. Med. 6, 1028-1042 (2014).
32. Wang, Z., Tymianski, M., Jones, O. T. & Nedergaard, M. Impact of cytoplasmic calcium buffering on the spatial and temporal characteristics of intercellular calcium signals in astrocytes. J. Neurosci. 17, 7359-7371 (1997).
33. Krezel, A. & Maret, W. Zinc-buffering capacity of a eukaryotic cell at physiological pZn. J. Biol. Inorg. Chem. 11, 1049-1062 (2006).
34. Atrián-Blasco, E. et al. Chemistry of mammalian metallothioneins and their interaction with amyloidogenic peptides and proteins. Chem. Soc. Rev. 46, 7683-7693 (2017).
35. Krezel, A. & Maret, W. Dual nanomolar and picomolar
36. Colvin, R. A., Holmes, W. R., Fontaine, C. P. & Maret, W. Cytosolic zinc buffering and muffling: their role in intracellular zinc homeostasis. Metallomics 2, 306-317 (2010).
37. Ueda, S. et al. Early secretory pathway-resident Zn transporter proteins contribute to cellular sphingolipid metabolism through activation of sphingomyelin phosphodiesterase 1. Am. J. Physiol. Cell Physiol. 322, C948-c959 (2022).
38. Wagatsuma, T. et al. Pigmentation and TYRP1 expression are mediated by zinc through the early secretory pathway-resident ZNT proteins. Commun. Biol. 6, 403 (2023).
39. Chandler, P. et al. Subtype-specific accumulation of intracellular zinc pools is associated with the malignant phenotype in breast cancer. Mol. Cancer 15, 2 (2016).
40. Beyer, N. et al. ZnT 3 mRNA levels are reduced in Alzheimer’s disease postmortem brain. Mol. Neurodegener. 4, 53 (2009).
41. Chimienti, F., Devergnas, S., Favier, A. & Seve, M. Identification and cloning of a beta-cell-specific zinc transporter,
42. Maret, W. Redox biochemistry of mammalian metallothioneins. J. Biol. Inorg. Chem. 16, 1079-1086 (2011).
43. Hirano, T. et al. Roles of zinc and zinc signaling in immunity: zinc as an intracellular signaling molecule. Adv. Immunol. 97, 149-176 (2008).
44. Yamasaki, S. et al. Zinc is a novel intracellular second messenger. J. Cell Biol. 177, 637-645 (2007).
45. Bonaventura, P., Benedetti, G., Albarède, F. & Miossec, P. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 14, 277-285 (2015).
46. Liu, W. et al. Lactate regulates cell cycle by remodelling the anaphase promoting complex. Nature 616, 790-797 (2023).
47. Wang, L. et al. Co-implantation of magnesium and zinc ions into titanium regulates the behaviors of human gingival fibroblasts. Bioact. Mater. 6, 64-74 (2021).
48. Xiao, W. et al. Therapeutic targeting of the USP2-E2F4 axis inhibits autophagic machinery essential for zinc homeostasis in cancer progression. Autophagy 18, 2615-2635 (2022).
49. Supasai, S. et al. Zinc deficiency affects the STAT1/3 signaling pathways in part through redox-mediated mechanisms. Redox Biol. 11, 469-481 (2017).
50. He, X. et al. The zinc transporter SLC39A10 plays an essential role in embryonic hematopoiesis. Adv. Sci. 10, e2205345 (2023).
51. Feske, S., Wulff, H. & Skolnik, E. Y. Ion channels in innate and adaptive immunity. Annu Rev. Immunol. 33, 291-353 (2015).
52. Chaigne-Delalande, B. & Lenardo, M. J. Divalent cation signaling in immune cells. Trends Immunol. 35, 332-344 (2014).
53. Ma, T. et al. A pair of transporters controls mitochondrial
54. Chen, H. C. et al. Sub-acute restraint stress progressively increases oxidative/ nitrosative stress and inflammatory markers while transiently upregulating antioxidant gene expression in the rat hippocampus. Free Radic. Biol. Med. 130, 446-457 (2019).
55. Si, M. & Lang, J. The roles of metallothioneins in carcinogenesis. J. Hematol. Oncol. 11, 107 (2018).
56. Aras, M. A. & Aizenman, E. Redox regulation of intracellular zinc: molecular signaling in the life and death of neurons. Antioxid. Redox Signal. 15, 2249-2263 (2011).
57. McCord, M. C. & Aizenman, E. Convergent Ca2+ and
58. Millward, D. J. Nutrition, infection and stunting: the roles of deficiencies of individual nutrients and foods, and of inflammation, as determinants of reduced linear growth of children. Nutr. Res Rev. 30, 50-72 (2017).
59. Ren, M. et al. Associations between hair levels of trace elements and the risk of preterm birth among pregnant Wwomen: a prospective nested case-control study in Beijing Birth Cohort (BBC), China. Environ. Int. 158, 106965 (2022).
60. Chorin, E. et al. Upregulation of KCC2 activity by zinc-mediated neurotransmission via the mZnR/GPR39 receptor. J. Neurosci. 31, 12916-12926 (2011).
61. Anderson, C. T. et al. Modulation of extrasynaptic NMDA receptors by synaptic and tonic zinc. Proc. Natl Acad. Sci. USA. 112, E2705-E2714 (2015).
62. Medvedeva, Y. V., Ji, S. G., Yin, H. Z. & Weiss, J. H. Differential vulnerability of CA1 versus CA3 pyramidal neurons after ischemia: possible relationship to sources of
63. Michelotti, F. C. et al. PET/MRI enables simultaneous in vivo quantification of
64. Carver, C. M., Chuang, S. H. & Reddy, D. S. Zinc selectively blocks neurosteroidsensitive extrasynaptic
65. Dostalova, Z. et al. Human
66. Sensi, S. L., Paoletti, P., Bush, A. I. & Sekler, I. Zinc in the physiology and pathology of the CNS. Nat. Rev. Neurosci. 10, 780-791 (2009).
67. Olesen, R. H. et al. Obesity and age-related alterations in the gene expression of zinc-transporter proteins in the human brain. Transl. Psychiatry 6, e838 (2016).
68. Ren, L. et al. Amperometric measurements and dynamic models reveal a mechanism for how zinc alters neurotransmitter release. Angew. Chem. Int Ed. Engl. 59, 3083-3087 (2020).
69. Hershfinkel, M. The zinc sensing receptor, ZnR/GPR39, in health and disease. Int J. Mol. Sci. 19, 439 (2018).
70. Ho, E. & Ames, B. N. Low intracellular zinc induces oxidative DNA damage, disrupts p53, NFkappa B, and AP1 DNA binding, and affects DNA repair in a rat glioma cell line. Proc. Natl Acad. Sci. USA. 99, 16770-16775 (2002).
71. Nuñez, N. N. et al. The zinc linchpin motif in the DNA repair glycosylase MUTYH: identifying the
72. Lecane, P. S. et al. Motexafin gadolinium and zinc induce oxidative stress responses and apoptosis in B-cell lymphoma lines. Cancer Res. 65, 11676-11688 (2005).
73. Cheng, X. et al. Zinc transporter SLC39A13/ZIP13 facilitates the metastasis of human ovarian cancer cells via activating Src/FAK signaling pathway. J. Exp. Clin. Cancer Res. 40, 199 (2021).
74. Liu, M. et al. Zinc-dependent regulation of ZEB1 and YAP1 coactivation promotes epithelial-mesenchymal transition plasticity and metastasis in pancreatic cancer. Gastroenterology 160, 1771-1783.e1771 (2021).
75. Yang, J. et al. ZIP4 promotes muscle wasting and cachexia in mice with orthotopic pancreatic tumors by stimulating RAB27B-regulated release of extracellular vesicles from cancer cells. Gastroenterology 156, 722-734.e726 (2019).
76. Wagner, E. F. & Nebreda, A. R. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 9, 537-549 (2009).
77. Zeng, Q. et al. Inhibition of ZIP4 reverses epithelial-to-mesenchymal transition and enhances the radiosensitivity in human nasopharyngeal carcinoma cells. Cell Death Dis. 10, 588 (2019).
78. Qi, J. et al. MCOLN1/TRPML1 finely controls oncogenic autophagy in cancer by mediating zinc influx. Autophagy 17, 4401-4422 (2021).
79. Su, X. et al. Disruption of zinc homeostasis by a novel platinum(IV)-terthiophene complex for antitumor immunity. Angew. Chem. Int Ed. Engl. 62, e202216917 (2023).
80. Jeong, J. & Eide, D. J. The SLC39 family of zinc transporters. Mol. Asp. Med. 34, 612-619 (2013).
81. Zhang, T., Sui, D. & Hu, J. Structural insights of ZIP4 extracellular domain critical for optimal zinc transport. Nat. Commun. 7, 11979 (2016).
82. Zhang, T. et al. Crystal structures of a ZIP zinc transporter reveal a binuclear metal center in the transport pathway. Sci. Adv. 3, e1700344 (2017).
83. Pang, C. et al. Structural mechanism of intracellular autoregulation of zinc uptake in ZIP transporters. Nat. Commun. 14, 3404 (2023).
84. Bogdan, A. R., Miyazawa, M., Hashimoto, K. & Tsuji, Y. Regulators of iron homeostasis: new players in metabolism, cell death, and disease. Trends Biochem Sci. 41, 274-286 (2016).
85. Jeong, J. et al. Promotion of vesicular zinc efflux by ZIP13 and its implications for spondylocheiro dysplastic Ehlers-Danlos syndrome. Proc. Natl Acad. Sci. USA. 109, E3530-E3538 (2012).
86. Bin, B. H. et al. Biochemical characterization of human ZIP13 protein: a homodimerized zinc transporter involved in the spondylocheiro dysplastic EhlersDanlos syndrome. J. Biol. Chem. 286, 40255-40265 (2011).
87. Lichten, L. A. et al. MTF-1-mediated repression of the zinc transporter Zip10 is alleviated by zinc restriction. PLoS One 6, e21526 (2011).
88. Ryu, M. S., Lichten, L. A., Liuzzi, J. P. & Cousins, R. J. Zinc transporters ZnT1 (Slc30a1), Zip8 (SIc39a8), and Zip10 (SIc39a10) in mouse red blood cells are differentially regulated during erythroid development and by dietary zinc deficiency. J. Nutr. 138, 2076-2083 (2008).
89. Liuzzi, J. P. et al. Responsive transporter genes within the murine intestinalpancreatic axis form a basis of zinc homeostasis. Proc. Natl Acad. Sci. USA. 101, 14355-14360 (2004).
90. Taylor, K. M. & Nicholson, R. I. The LZT proteins; the LIV-1 subfamily of zinc transporters. Biochim. Biophys. Acta 1611, 16-30 (2003).
91. Xin, Y. et al. Manganese transporter Slc39a14 deficiency revealed its key role in maintaining manganese homeostasis in mice. Cell Discov. 3, 17025 (2017).
92. Polesel, M. et al. Functional characterization of SLC39 family members ZIP5 and ZIP10 in overexpressing HEK293 cells reveals selective copper transport activity. Biometals 36, 227-237 (2023).
93. Boycott, K. M. et al. Autosomal-recessive intellectual disability with cerebellar atrophy syndrome caused by mutation of the manganese and zinc transporter gene SLC39A8. Am. J. Hum. Genet. 97, 886-893 (2015).
94. Jorge-Nebert, L. F. et al. Comparing gene expression during cadmium uptake and distribution: untreated versus oral Cd-treated wild-type and ZIP14 knockout mice. Toxicol. Sci. 143, 26-35 (2015).
95. Himeno, S., Yanagiya, T. & Fujishiro, H. The role of zinc transporters in cadmium and manganese transport in mammalian cells. Biochimie 91, 1218-1222 (2009).
96. Nebert, D. W. & Liu, Z. SLC39A8 gene encoding a metal ion transporter: discovery and bench to bedside. Hum. Genomics. 13, 51 (2019).
97. Liu, Z. et al. Cd2+ versus Zn2+ uptake by the ZIP8 HCO3-dependent symporter: kinetics, electrogenicity and trafficking. Biochem. Biophys. Res Commun. 365, 814-820 (2008).
98. Napolitano, J. R. et al. Cadmium-mediated toxicity of lung epithelia is enhanced through NF-кB-mediated transcriptional activation of the human zinc transporter ZIP8. Am. J. Physiol. Lung Cell Mol. Physiol. 302, L909-L918 (2012).
99. Girijashanker, K. et al. Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: similarities to the ZIP8 transporter. Mol. Pharmacol. 73, 1413-1423 (2008).
100. Pinilla-Tenas, J. J. et al. Zip14 is a complex broad-scope metal-ion transporter whose functional properties support roles in the cellular uptake of zinc and nontransferrin-bound iron. Am. J. Physiol. Cell Physiol. 301, C862-C871 (2011).
101. Liuzzi, J. P. et al. Zip14 (SIc39a14) mediates non-transferrin-bound iron uptake into cells. Proc. Natl Acad. Sci. USA. 103, 13612-13617 (2006).
102. Wang, C. Y. et al. ZIP8 is an iron and zinc transporter whose cell-surface expression is up-regulated by cellular iron loading. J. Biol. Chem. 287, 34032-34043 (2012).
103. Jenkitkasemwong, S. et al. SLC39A14 is required for the development of hepatocellular iron overload in murine models of hereditary hemochromatosis. Cell Metab. 22, 138-150 (2015).
104. Kambe, T., Matsunaga, M. & Takeda, T. A. Understanding the contribution of zinc transporters in the function of the early secretory pathway. Int J. Mol. Sci. 18, 2179 (2017).
105. Davidson, H. W., Wenzlau, J. M. & O’Brien, R. M. Zinc transporter 8 (ZnT8) and beta cell function. Trends Endocrinol. Metab. 25, 415-424 (2014).
106. Suzuki, T. et al. Zinc transporters, ZnT5 and ZnT7, are required for the activation of alkaline phosphatases, zinc-requiring enzymes that are glycosylphosphatidylinositol-anchored to the cytoplasmic membrane. J. Biol. Chem. 280, 637-643 (2005).
107. Nishito, Y. & Kambe, T. Zinc transporter 1 (ZNT1) expression on the cell surface is elaborately controlled by cellular zinc levels. J. Biol. Chem. 294, 15686-15697 (2019).
108. Lichten, L. A. & Cousins, R. J. Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev. Nutr. 29, 153-176 (2009).
109. Wang, Y. et al. Zinc application alleviates the adverse renal effects of arsenic stress in a protein quality control way in common carp. Environ. Res. 191, 110063 (2020).
110. Dwivedi, O. P. et al. Loss of ZnT 8 function protects against diabetes by enhanced insulin secretion. Nat. Genet. 51, 1596-1606 (2019).
111. Henshall, S. M. et al. Expression of the zinc transporter ZnT 4 is decreased in the progression from early prostate disease to invasive prostate cancer. Oncogene 22, 6005-6012 (2003).
112. Sanchez, V. B., Ali, S., Escobar, A. & Cuajungco, M. P. Transmembrane 163 (TMEM163) protein effluxes zinc. Arch. Biochem. Biophys. 677, 108166 (2019).
113. Styrpejko, D. J. & Cuajungco, M. P. Transmembrane 163 (TMEM163) protein: a new member of the zinc efflux transporter family. Biomedicines 9, 220 (2021).
114. do Rosario, M. C. et al. Variants in the zinc transporter TMEM163 cause a hypomyelinating leukodystrophy. Brain 145, 4202-4209 (2022).
115. Kia, D. A. et al. Identification of candidate Parkinson disease genes by integrating genome-wide association study, expression, and epigenetic data sets. JAMA Neurol. 78, 464-472 (2021).
116. Yuan, Y. et al. A zinc transporter, transmembrane protein 163, is critical for the biogenesis of platelet dense granules. Blood 137, 1804-1817 (2021).
117. Braun, W. et al. Comparison of the NMR solution structure and the x-ray crystal structure of rat metallothionein-2. Proc. Natl Acad. Sci. USA. 89, 10124-10128 (1992).
118. Krężel, A. & Maret, W. The bioinorganic chemistry of mammalian metallothioneins. Chem. Rev. 121, 14594-14648 (2021).
119. Merlos Rodrigo, M. A. et al. Metallothionein isoforms as double agents – their roles in carcinogenesis, cancer progression and chemoresistance. Drug Resist. Updat. 52, 100691 (2020).
120. Go, Y. M., Chandler, J. D. & Jones, D. P. The cysteine proteome. Free Radic. Biol. Med. 84, 227-245 (2015).
121. Marreiro, D. D. et al. Zinc and oxidative stress: current mechanisms. Antioxidants. 6, 24 (2017).
122. Guo, L. et al. STAT5-glucocorticoid receptor interaction and MTF-1 regulate the expression of ZnT2 (Slc30a2) in pancreatic acinar cells. Proc. Natl Acad. Sci. USA. 107, 2818-2823 (2010).
123. Lu, Y. J. et al. Coordinative modulation of human zinc transporter 2 gene expression through active and suppressive regulators. J. Nutr. Biochem. 26, 351-359 (2015).
124. Mocchegiani, E., Giacconi, R. & Malavolta, M. Zinc signalling and subcellular distribution: emerging targets in type 2 diabetes. Trends Mol. Med. 14, 419-428 (2008).
125. O’Donnell, J. S., Teng, M. W. L. & Smyth, M. J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 16, 151-167 (2019).
126. Kim, B., Kim, H. Y. & Lee, W. W. Zap70 regulates TCR-mediated Zip6 activation at the immunological synapse. Front. Immunol. 12, 687367 (2021).
127. Lee, W. W. et al. Age-dependent signature of metallothionein expression in primary CD4 T cell responses is due to sustained zinc signaling. Rejuvenation Res. 11, 1001-1011 (2008).
128. Pommier, A. et al. Inflammatory monocytes are potent antitumor effectors controlled by regulatory CD4+ T cells. Proc. Natl Acad. Sci. USA. 110, 13085-13090 (2013).
129. Aydemir, T. B., Liuzzi, J. P., McClellan, S. & Cousins, R. J. Zinc transporter ZIP8 (SLC39A8) and zinc influence IFN-gamma expression in activated human T cells. J. Leukoc. Biol. 86, 337-348 (2009).
130. Liu, M. J. et al. ZIP8 regulates host defense through zinc-mediated inhibition of NF-kappaB. Cell Rep. 3, 386-400 (2013).
131. Begum, N. A. et al. Mycobacterium bovis BCG cell wall and lipopolysaccharide induce a novel gene, BIGM103, encoding a 7-TM protein: identification of a new protein family having Zn-transporter and Zn-metalloprotease signatures. Genomics 80, 630-645 (2002).
132. Kim, B. et al. Cytoplasmic zinc promotes IL-1 beta production by monocytes and macrophages through mTORC1-induced glycolysis in rheumatoid arthritis. Sci. Signal. 15, eabi7400 (2022).
133. Kang, J. A. et al. ZIP8 exacerbates collagen-induced arthritis by increasing pathogenic T cell responses. Exp. Mol. Med. 53, 560-571 (2021).
134. Abd El-Rehim, D. M. et al. High-throughput protein expression analysis using tissue microarray technology of a large well-characterised series identifies biologically distinct classes of breast cancer confirming recent cDNA expression analyses. Int J. Cancer 116, 340-350 (2005).
135. Lee, D. S. W., Rojas, O. L. & Gommerman, J. L. B cell depletion therapies in autoimmune disease: advances and mechanistic insights. Nat. Rev. Drug Discov. 20, 179-199 (2021).
136. Taniguchi, M. et al. Essential role of the zinc transporter ZIP9/SLC39A9 in regulating the activations of Akt and Erk in B-cell receptor signaling pathway in DT40 cells. PLoS One 8, e58022 (2013).
137. Miyai, T. et al. Zinc transporter SLC39A10/ZIP10 facilitates antiapoptotic signaling during early B-cell development. Proc. Natl Acad. Sci. USA. 111, 11780-11785 (2014).
138. Hojyo, S. et al. Zinc transporter SLC39A10/ZIP10 controls humoral immunity by modulating B-cell receptor signal strength. Proc. Natl Acad. Sci. USA. 111, 11786-11791 (2014).
139. Ma, Z. et al. SLC39A10 upregulation predicts poor prognosis, promotes proliferation and migration, and correlates with immune infiltration in hepatocellular carcinoma. J. Hepatocell. Carcinoma 8, 899-912 (2021).
140. Stafford, S. L. et al. Metal ions in macrophage antimicrobial pathways: emerging roles for zinc and copper. Biosci. Rep. 33, e00049 (2013).
141. Locati, M., Curtale, G. & Mantovani, A. Diversity, mechanisms, and significance of macrophage plasticity. Annu. Rev. Pathol. 15, 123-147 (2020).
142. Gao, H. et al. Metal transporter Slc39a10 regulates susceptibility to inflammatory stimuli by controlling macrophage survival. Proc. Natl Acad. Sci. USA. 114, 12940-12945 (2017).
143. Sriskandan, S. & Altmann, D. M. The immunology of sepsis. J. Pathol. 214, 211-223 (2008).
144. Wong, H. R. et al. Genome-level expression profiles in pediatric septic shock indicate a role for altered zinc homeostasis in poor outcome. Physiol. Genomics. 30, 146-155 (2007).
145. Besecker, B. et al. The human zinc transporter SLC39A8 (Zip8) is critical in zincmediated cytoprotection in lung epithelia. Am. J. Physiol. Lung Cell Mol. Physiol. 294, L1127-L1136 (2008).
146. Besecker, B. Y. et al. A comparison of zinc metabolism, inflammation, and disease severity in critically ill infected and noninfected adults early after intensive care unit admission. Am. J. Clin. Nutr. 93, 1356-1364 (2011).
147. Wessels, I. & Cousins, R. J. Zinc dyshomeostasis during polymicrobial sepsis in mice involves zinc transporter Zip14 and can be overcome by zinc supplementation. Am. J. Physiol. Gastrointest. Liver Physiol. 309, G768-G778 (2015).
148. Hogstrand, C., Kille, P., Nicholson, R. I. & Taylor, K. M. Zinc transporters and cancer: a potential role for ZIP7 as a hub for tyrosine kinase activation. Trends Mol. Med. 15, 101-111 (2009).
149. Adulcikas, J. et al. The zinc transporter SLC39A7 (ZIP7) harbours a highlyconserved histidine-rich N-terminal region that potentially contributes to zinc homeostasis in the endoplasmic reticulum. Comput Biol. Med. 100, 196-202 (2018).
150. Uchida, R. et al. L-type calcium channel-mediated zinc wave is involved in the regulation of IL-6 by stimulating non-IgE with LPS and IL-33 in mast cells and dendritic cells. Biol. Pharm. Bull. 42, 87-93 (2019).
151. Levy, S. et al. Molecular basis for zinc transporter 1 action as an endogenous inhibitor of L-type calcium channels. J. Biol. Chem. 284, 32434-32443 (2009).
152. Maret, W. Zinc in cellular regulation: the nature and significance of “zinc signals”. Int J. Mol. Sci. 18, 2285 (2017).
153. Kim, A. M., Vogt, S., O’Halloran, T. V. & Woodruff, T. K. Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nat. Chem. Biol. 6, 674-681 (2010).
154. Taylor, K. M. et al. Zinc transporter ZIP10 forms a heteromer with ZIP6 which regulates embryonic development and cell migration. Biochem J. 473, 2531-2544 (2016).
155. Kong, B. Y. et al. Maternally-derived zinc transporters ZIP6 and ZIP10 drive the mammalian oocyte-to-egg transition. Mol. Hum. Reprod. 20, 1077-1089 (2014).
156. Nimmanon, T. et al. The ZIP6/ZIP10 heteromer is essential for the zinc-mediated trigger of mitosis. Cell Mol. Life Sci. 78, 1781-1798 (2021).
157. Hogstrand, C. et al. A mechanism for epithelial-mesenchymal transition and anoikis resistance in breast cancer triggered by zinc channel ZIP6 and STAT3 (signal transducer and activator of transcription 3). Biochem. J. 455, 229-237 (2013).
158. Mulay, I. L. et al. Trace-metal analysis of cancerous and noncancerous human tissues. J. Natl Cancer Inst. 47, 1-13 (1971).
159. Chen, P. H. et al. Zinc transporter ZIP7 is a novel determinant of ferroptosis. Cell Death Dis. 12, 198 (2021).
160. Makhov, P. et al. Zinc chelation induces rapid depletion of the X-linked inhibitor of apoptosis and sensitizes prostate cancer cells to TRAIL-mediated apoptosis. Cell Death Differ. 15, 1745-1751 (2008).
161. Zhang, R. et al. Zinc regulates primary ovarian tumor growth and metastasis through the epithelial to mesenchymal transition. Free Radic. Biol. Med. 160, 775-783 (2020).
162. Hernandez-Camacho, J. D., Vicente-Garcia, C., Parsons, D. S. & Navas-Enamorado, I. Zinc at the crossroads of exercise and proteostasis. Redox Biol. 35, 101529 (2020).
163. Ohashi, K. et al. Zinc promotes proliferation and activation of myogenic cells via the PI3K/Akt and ERK signaling cascade. Exp. Cell Res. 333, 228-237 (2015).
164. Lee, H. Y. et al. Deletion of Jazf1 gene causes early growth retardation and insulin resistance in mice. Proc. Natl Acad. Sci. USA. 119, e2213628119 (2022).
165. Jinno, N., Nagata, M. & Takahashi, T. Marginal zinc deficiency negatively affects recovery from muscle injury in mice. Biol. Trace Elem. Res. 158, 65-72 (2014).
166. Lin, P. H. et al. Zinc in wound healing modulation. Nutrients 10, 16 (2017).
167. Postigo, A. A. & Dean, D. C. Differential expression and function of members of the zfh-1 family of zinc finger/homeodomain repressors. Proc. Natl Acad. Sci. USA. 97, 6391-6396 (2000).
168. Taylor, K. M. et al. Protein kinase CK2 triggers cytosolic zinc signaling pathways by phosphorylation of zinc channel ZIP7. Sci. Signal. 5, ra11 (2012).
169. Mnatsakanyan, H., Serra, R. S. I., Rico, P. & Salmeron-Sanchez, M. Zinc uptake promotes myoblast differentiation via Zip7 transporter and activation of Akt signalling transduction pathway. Sci. Rep. 8, 13642 (2018).
170. Nimmanon, T. et al. Phosphorylation of zinc channel ZIP7 drives MAPK, PI3K and mTOR growth and proliferation signalling. Metallomics 9, 471-481 (2017).
171. Mapley, J. I., Wagner, P., Officer, D. L. & Gordon, K. C. Computational and spectroscopic analysis of beta-indandione modified zinc porphyrins. J. Phys. Chem. A. 122, 4448-4456 (2018).
172. Giunta, C. et al. Spondylocheiro dysplastic form of the Ehlers-Danlos syndrome-an autosomal-recessive entity caused by mutations in the zinc transporter gene SLC39A13. Am. J. Hum. Genet. 82, 1290-1305 (2008).
173. Fukada, T. et al. The zinc transporter SLC39A13/ZIP13 is required for connective tissue development; its involvement in BMP/TGF-beta signaling pathways. PLoS One 3, e3642 (2008).
174. Shusterman, E. et al. Zinc transport and the inhibition of the L-type calcium channel are two separable functions of ZnT-1. Metallomics 9, 228-238 (2017).
175. Hennigar, S. R. & McClung, J. P. Zinc transport in the mammalian intestine. Compr. Physiol. 9, 59-74 (2018).
176. Geiser, J., Venken, K. J., De Lisle, R. C. & Andrews, G. K. A mouse model of acrodermatitis enteropathica: loss of intestine zinc transporter ZIP4 (SIc39a4) disrupts the stem cell niche and intestine integrity. PLoS Genet. 8, e1002766 (2012).
177. Dufner-Beattie, J., Kuo, Y. M., Gitschier, J. & Andrews, G. K. The adaptive response to dietary zinc in mice involves the differential cellular localization and zinc regulation of the zinc transporters ZIP4 and ZIP5. J. Biol. Chem. 279, 49082-49090 (2004).
178. Dufner-Beattie, J. et al. The acrodermatitis enteropathica gene ZIP4 encodes a tissue-specific, zinc-regulated zinc transporter in mice. J. Biol. Chem. 278, 33474-33481 (2003).
179. Kury, S. et al. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat. Genet. 31, 239-240 (2002).
180. Wang, K. et al. A novel member of a zinc transporter family is defective in acrodermatitis enteropathica. Am. J. Hum. Genet. 71, 66-73 (2002).
181. Weaver, B. P., Dufner-Beattie, J., Kambe, T. & Andrews, G. K. Novel zincresponsive post-transcriptional mechanisms reciprocally regulate expression of the mouse Slc39a4 and Slc39a5 zinc transporters (Zip4 and Zip5). Biol. Chem. 388, 1301-1312 (2007).
182. Yu, Y. Y., Kirschke, C. P. & Huang, L. Immunohistochemical analysis of ZnT1, 4, 5, 6, and 7 in the mouse gastrointestinal tract. J. Histochem Cytochem. 55, 223-234 (2007).
183. McMahon, R. J. & Cousins, R. J. Regulation of the zinc transporter
184. Wu, J., Ma, N., Johnston, L. J. & Ma, X. Dietary nutrients mediate intestinal host defense peptide expression. Adv. Nutr. 11, 92-102 (2020).
185. Podany, A. B. et al. ZnT2-mediated zinc import into paneth cell granules is necessary for coordinated secretion and paneth cell function in mice. Cell Mol. Gastroenterol. Hepatol. 2, 369-383 (2016).
186. Hennigar, S. R. & Kelleher, S. L. TNFalpha post-translationally targets ZnT2 to accumulate zinc in lysosomes. J. Cell Physiol. 230, 2345-2350 (2015).
187. Ohashi, W. et al. Zinc transporter SLC39A7/ZIP7 promotes intestinal epithelial self-renewal by resolving ER stress. PLoS Genet. 12, e1006349 (2016).
188. Turner, J. R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799-809 (2009).
189. Higashimura, Y. et al. Zinc deficiency activates the IL-23/Th17 axis to aggravate experimental colitis in mice. J. Crohns Colitis 14, 856-866 (2020).
190. Hering, N. A., Fromm, M. & Schulzke, J. D. Determinants of colonic barrier function in inflammatory bowel disease and potential therapeutics. J. Physiol. 590, 1035-1044 (2012).
191. Guthrie, G. J. et al. Influence of ZIP14 (slc39A14) on intestinal zinc processing and barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 308, G171-G178 (2015).
192. Kim, J. et al. Deletion of metal transporter Zip14 (SIc39a14) produces skeletal muscle wasting, endotoxemia, Mef2c activation and induction of miR-675 and Hspb7. Sci. Rep. 10, 4050 (2020).
193. Aydemir, T. B. & Cousins, R. J. The multiple faces of the metal transporter ZIP14 (SLC39A14). J. Nutr. 148, 174-184 (2018).
194. McGourty, K. et al. ZnT2 is critical for TLR4-mediated cytokine expression in colonocytes and modulates mucosal inflammation in mice. Int J. Mol. Sci. 23, 11467 (2022).
195. Hennigar, S. R. et al. ZnT 2 is a critical mediator of lysosomal-mediated cell death during early mammary gland involution. Sci. Rep. 5, 8033 (2015).
196. Liu, M. J. et al. ZIP8 regulates host defense through zinc-mediated inhibition of NF-кВ. Cell Rep. 3, 386-400 (2013).
197. Li, D. et al. A pleiotropic missense variant in SLC39A8 is associated with Crohn’s disease and human gut microbiome composition. Gastroenterology 151, 724-732 (2016).
198. Vergnano, A. M. et al. Zinc dynamics and action at excitatory synapses. Neuron 82, 1101-1114 (2014).
199. Kalappa, B. I. et al. AMPA receptor inhibition by synaptically released zinc. Proc. Natl Acad. Sci. USA. 112, 15749-15754 (2015).
200. Huang, Y. Z., Pan, E., Xiong, Z. Q. & McNamara, J. O. Zinc-mediated transactivation of TrkB potentiates the hippocampal mossy fiber-CA3 pyramid synapse. Neuron 57, 546-558 (2008).
201. Pan, E. et al. Vesicular zinc promotes presynaptic and inhibits postsynaptic longterm potentiation of mossy fiber-CA3 synapse. Neuron 71, 1116-1126 (2011).
202. Eom, K. et al. Intracellular
203. Anderson, C. T., Kumar, M., Xiong, S. & Tzounopoulos, T. Cell-specific gain modulation by synaptically released zinc in cortical circuits of audition. Elife 6, e29893 (2017).
204. Kumar, M., Xiong, S., Tzounopoulos, T. & Anderson, C. T. Fine control of sound frequency tuning and frequency discrimination acuity by synaptic zinc signaling in mouse auditory cortex. J. Neurosci. 39, 854-865 (2019).
205. Besser, L. et al. Synaptically released zinc triggers metabotropic signaling via a zinc-sensing receptor in the hippocampus. J. Neurosci. 29, 2890-2901 (2009).
206. Palmiter, R. D., Cole, T. B., Quaife, C. J. & Findley, S. D. ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc. Natl Acad. Sci. USA. 93, 14934-14939 (1996).
207. Sikora, J., Kieffer, B. L., Paoletti, P. & Ouagazzal, A. M. Synaptic zinc contributes to motor and cognitive deficits in 6-hydroxydopamine mouse models of Parkinson’s disease. Neurobiol. Dis. 134, 104681 (2020).
208. Upmanyu, N. et al. Colocalization of different neurotransmitter transporters on synaptic vesicles is sparse except for VGLUT1 and ZnT3. Neuron 110, 1483-1497.e1487 (2022).
209. McAllister, B. B. & Dyck, R. H. Zinc transporter 3 (ZnT3) and vesicular zinc in central nervous system function. Neurosci. Biobehav. Rev. 80, 329-350 (2017).
210. Perez-Rosello, T. et al. Tonic zinc inhibits spontaneous firing in dorsal cochlear nucleus principal neurons by enhancing glycinergic neurotransmission. Neurobiol. Dis. 81, 14-19 (2015).
211. Sindreu, C., Palmiter, R. D. & Storm, D. R. Zinc transporter ZnT-3 regulates presynaptic Erk1/2 signaling and hippocampus-dependent memory. Proc. Natl Acad. Sci. USA. 108, 3366-3370 (2011).
212. Mellone, M. et al. Zinc transporter-1: a novel NMDA receptor-binding protein at the postsynaptic density. J. Neurochem. 132, 159-168 (2015).
213. Krall, R. F. et al. Synaptic zinc inhibition of NMDA receptors depends on the association of GluN2A with the zinc transporter ZnT1. Sci. Adv. 6, eabb1515 (2020).
214. Chowanadisai, W. et al. Neurulation and neurite extension require the zinc transporter ZIP12 (slc39a12). Proc. Natl Acad. Sci. USA. 110, 9903-9908 (2013).
215. Kambe, T., Yamaguchi-Iwai, Y., Sasaki, R. & Nagao, M. Overview of mammalian zinc transporters. Cell Mol. Life Sci. 61, 49-68 (2004).
216. Scarr, E. et al. Increased cortical expression of the zinc transporter SLC39A12 suggests a breakdown in zinc cellular homeostasis as part of the pathophysiology of schizophrenia. NPJ Schizophr. 2, 16002 (2016).
217. Bogdanovic, M. et al. The ZIP3 zinc transporter is localized to mossy fiber terminals and is required for kainate-induced degeneration of CA3 neurons. J. Neurosci. 42, 2824-2834 (2022).
218. De Benedictis, C. A. et al. Expression analysis of zinc transporters in nervous tissue cells reveals neuronal and synaptic localization of ZIP4. Int J. Mol. Sci. 22, 4511 (2021).
219. Pickrell, J. K. et al. Detection and interpretation of shared genetic influences on 42 human traits. Nat. Genet. 48, 709-717 (2016).
220. Park, J. H. et al. SLC39A8 deficiency: a disorder of manganese transport and glycosylation. Am. J. Hum. Genet. 97, 894-903 (2015).
221. Müller, N. Inflammation and the glutamate system in schizophrenia: implications for therapeutic targets and drug development. Expert Opin. Ther. Targets 12, 1497-1507 (2008).
222. Tseng, W. C. et al. Schizophrenia-associated SLC39A8 polymorphism is a loss-offunction allele altering glutamate receptor and innate immune signaling. Transl. Psychiatry 11, 136 (2021).
223. Derewenda, U. et al. Phenol stabilizes more helix in a new symmetrical zinc insulin hexamer. Nature 338, 594-596 (1989).
224. Barman, S. & Srinivasan, K. Diabetes and zinc dyshomeostasis: can zinc supplementation mitigate diabetic complications? Crit. Rev. Food Sci. Nutr. 62, 1046-1061 (2022).
225. Davidson, H. W., Wenzlau, J. M. & O’Brien, R. M. Zinc transporter 8 (ZnT8) and
226. Rutter, G. A. & Chimienti, F. SLC30A8 mutations in type 2 diabetes. Diabetologia 58, 31-36 (2015).
227. Tamaki, M. et al. The diabetes-susceptible gene SLC30A8/ZnT8 regulates hepatic insulin clearance. J. Clin. Invest. 123, 4513-4524 (2013).
228. Sladek, R. et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445, 881-885 (2007).
229. Fukunaka, A. & Fujitani, Y. Role of zinc homeostasis in the pathogenesis of diabetes and obesity. Int J. Mol. Sci. 19, 476 (2018).
230. Ma, Q. et al. ZnT8 loss-of-function accelerates functional maturation of hESCderived
231. Regnell, S. E. & Lernmark, Å. Early prediction of autoimmune (type 1) diabetes. Diabetologia 60, 1370-1381 (2017).
232. Lemaire, K. et al. Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. Proc. Natl Acad. Sci. USA. 106, 14872-14877 (2009).
233. Wenzlau, J. M. et al. The cation efflux transporter
234. Smidt, K. et al. SLC30A3 responds to glucose- and zinc variations in beta-cells and is critical for insulin production and in vivo glucose-metabolism during beta-cell stress. PLoS One 4, e5684 (2009).
235. Petersen, A. B. et al. siRNA-mediated knock-down of ZnT 3 and ZnT 8 affects production and secretion of insulin and apoptosis in INS-1E cells. Apmis 119, 93-102 (2011).
236. Hardy, A. B. et al. Zip4 mediated zinc influx stimulates insulin secretion in pancreatic beta cells. PLoS One 10, e0119136 (2015).
237. Liu, Y. et al. Characterization of zinc influx transporters (ZIPs) in pancreatic
238. Gyulkhandanyan, A. V. et al. Investigation of transport mechanisms and regulation of intracellular
239. Solomou, A. et al. Over-expression of Slc30a8/ZnT8 selectively in the mouse a cell impairs glucagon release and responses to hypoglycemia. Nutr. Metab. 13, 46 (2016).
240. Balaz, M. et al. Subcutaneous adipose tissue zinc-a2-glycoprotein is associated with adipose tissue and whole-body insulin sensitivity. Obesity 22, 1821-1829 (2014).
241. Wang, W. & Seale, P. Control of brown and beige fat development. Nat. Rev. Mol. Cell Biol. 17, 691-702 (2016).
242. Fukunaka, A. et al. Zinc transporter ZIP13 suppresses beige adipocyte biogenesis and energy expenditure by regulating C/EBP-
243. Hay, N. Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nat. Rev. Cancer 16, 635-649 (2016).
244. Luo, X. et al. Emerging roles of lipid metabolism in cancer metastasis. Mol. Cancer 16, 76 (2017).
245. Gumulec, J. et al. Insight to physiology and pathology of zinc(II) ions and their actions in breast and prostate carcinoma. Curr. Med. Chem. 18, 5041-5051 (2011).
246. Takahashi, Y., Ogra, Y. & Suzuki, K. T. Nuclear trafficking of metallothionein requires oxidation of a cytosolic partner. J. Cell Physiol. 202, 563-569 (2005).
247. Nagel, W. W. & Vallee, B. L. Cell cycle regulation of metallothionein in human colonic cancer cells. Proc. Natl Acad. Sci. USA. 92, 579-583 (1995).
248. Formigari, A., Santon, A. & Irato, P. Efficacy of zinc treatment against ironinduced toxicity in rat hepatoma cell line H4-II-E-C3. Liver Int. 27, 120-127 (2007).
249. Chen, W. Y. et al. Expression of metallothionein gene during embryonic and early larval development in zebrafish. Aquat. Toxicol. 69, 215-227 (2004).
250. Chen, W. Y., John, J. A., Lin, C. H. & Chang, C. Y. Expression pattern of metallothionein, MTF-1 nuclear translocation, and its dna-binding activity in zebrafish (Danio rerio) induced by zinc and cadmium. Environ. Toxicol. Chem. 26, 110-117 (2007).
251. Xia, N., Liu, L., Yi, X. & Wang, J. Studies of interaction of tumor suppressor p53 with apo-MT using surface plasmon resonance. Anal. Bioanal. Chem. 395, 2569-2575 (2009).
252. Rana, U. et al. Zinc binding ligands and cellular zinc trafficking: apo-metallothionein, glutathione, TPEN, proteomic zinc, and Zn-Sp1. J. Inorg. Biochem. 102, 489-499 (2008).
253. Huang, M., Shaw, I. C. & Petering, D. H. Interprotein metal exchange between transcription factor Illa and apo-metallothionein. J. Inorg. Biochem. 98, 639-648 (2004).
254. Parreno, V., Martinez, A. M. & Cavalli, G. Mechanisms of Polycomb group protein function in cancer. Cell Res. 32, 231-253 (2022).
34
255. Di Foggia, V. et al. Bmi1 enhances skeletal muscle regeneration through MT1mediated oxidative stress protection in a mouse model of dystrophinopathy. J. Exp. Med. 211, 2617-2633 (2014).
256. Dünkelberg, S. et al. The interaction of sodium and zinc in the priming of T cell subpopulations regarding Th17 and treg cells. Mol. Nutr. Food Res. 64, e1900245 (2020).
257. Spiering, R. et al. Membrane-bound metallothionein 1 of murine dendritic cells promotes the expansion of regulatory T cells in vitro. Toxicol. Sci. 138, 69-75 (2014).
258. Li, S. et al. Metallothionein 3 promotes osteoblast differentiation in C2C12 cells via reduction of oxidative stress. Int J. Mol. Sci. 22, 4312 (2021).
259. Shin, C. H. et al. Identification of XAF1-MT2A mutual antagonism as a molecular switch in cell-fate decisions under stressful conditions. Proc. Natl Acad. Sci. USA. 114, 5683-5688 (2017).
260. Korkola, N. C. & Stillman, M. J. Structural role of cadmium and zinc in metallothionein oxidation by hydrogen peroxide: the resilience of metal-thiolate clusters. J. Am. Chem. Soc. 145, 6383-6397 (2023).
261. Ma, H. et al. HMBOX1 interacts with MT2A to regulate autophagy and apoptosis in vascular endothelial cells. Sci. Rep. 5, 15121 (2015).
262. Murphy, M. P. et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 4, 651-662 (2022).
263. Song, Q. X. et al. Potential role of oxidative stress in the pathogenesis of diabetic bladder dysfunction. Nat. Rev. Urol. 19, 581-596 (2022).
264. Vatner, S. F. et al. Healthful aging mediated by inhibition of oxidative stress. Ageing Res. Rev. 64, 101194 (2020).
265. Niu, B. et al. Application of glutathione depletion in cancer therapy: enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials 277, 121110 (2021).
266. Otterbein, L. E., Foresti, R. & Motterlini, R. Heme oxygenase-1 and carbon monoxide in the heart: the balancing act between danger signaling and prosurvival. Circ. Res. 118, 1940-1959 (2016).
267. Maret, W. & Li, Y. Coordination dynamics of zinc in proteins. Chem. Rev. 109, 4682-4707 (2009).
268. Pluth, M. D., Tomat, E. & Lippard, S. J. Biochemistry of mobile zinc and nitric oxide revealed by fluorescent sensors. Annu Rev. Biochem. 80, 333-355 (2011).
269. Rowsell, S. et al. Crystal structure of human MMP9 in complex with a reverse hydroxamate inhibitor. J. Mol. Biol. 319, 173-181 (2002).
270. Choi, S., Liu, X. & Pan, Z. Zinc deficiency and cellular oxidative stress: prognostic implications in cardiovascular diseases. Acta Pharm. Sin. 39, 1120-1132 (2018).
271. D’Amico, E., Factor-Litvak, P., Santella, R. M. & Mitsumoto, H. Clinical perspective on oxidative stress in sporadic amyotrophic lateral sclerosis. Free Radic. Biol. Med. 65, 509-527 (2013).
272. Wu, W., Bromberg, P. A. & Samet, J. M. Zinc ions as effectors of environmental oxidative lung injury. Free Radic. Biol. Med. 65, 57-69 (2013).
273. Roel, M. et al. Crambescin C1 exerts a cytoprotective effect on HepG2 cells through metallothionein induction. Mar. Drugs 13, 4633-4653 (2015).
274. Cavalca, E. et al. Metallothioneins are neuroprotective agents in lysosomal storage disorders. Ann. Neurol. 83, 418-432 (2018).
275. Yang, M. & Chitambar, C. R. Role of oxidative stress in the induction of metallothionein-2A and heme oxygenase-1 gene expression by the antineoplastic agent gallium nitrate in human lymphoma cells. Free Radic. Biol. Med. 45, 763-772 (2008).
276. Qu, W., Pi, J. & Waalkes, M. P. Metallothionein blocks oxidative DNA damage in vitro. Arch. Toxicol. 87, 311-321 (2013).
277. Koh, J. Y. & Lee, S. J. Metallothionein-3 as a multifunctional player in the control of cellular processes and diseases. Mol. Brain. 13, 116 (2020).
278. Álvarez-Barrios, A. et al. Antioxidant defenses in the human eye: a focus on metallothioneins. Antioxidants 10, 89 (2021).
279. Maret, W. The redox biology of redox-inert zinc ions. Free Radic. Biol. Med. 134, 311-326 (2019).
280. Oteiza, P. I. Zinc and the modulation of redox homeostasis. Free Radic. Biol. Med. 53, 1748-1759 (2012).
281. Hübner, C. & Haase, H. Interactions of zinc- and redox-signaling pathways. Redox Biol. 41, 101916 (2021).
282. Kim, H. G. et al. The epigenetic regulator SIRT6 protects the liver from alcoholinduced tissue injury by reducing oxidative stress in mice. J. Hepatol. 71, 960-969 (2019).
283. Hwang, S. et al. Interleukin-22 ameliorates neutrophil-driven nonalcoholic steatohepatitis through multiple targets. Hepatology 72, 412-429 (2020).
284. Wang, B. et al. D609 protects retinal pigmented epithelium as a potential therapy for age-related macular degeneration. Signal Transduct. Target Ther. 5, 20 (2020).
285. Phillippi, J. A. et al. Basal and oxidative stress-induced expression of metallothionein is decreased in ascending aortic aneurysms of bicuspid aortic valve patients. Circulation 119, 2498-2506 (2009).
286. Bahadorani, S., Mukai, S., Egli, D. & Hilliker, A. J. Overexpression of metalresponsive transcription factor (MTF-1) in Drosophila melanogaster ameliorates life-span reductions associated with oxidative stress and metal toxicity. Neurobiol. Aging 31, 1215-1226 (2010).
287. Esposito, K. et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation 106, 2067-2072 (2002).
288. Stankovic, R. K., Chung, R. S. & Penkowa, M. Metallothioneins I and II: neuroprotective significance during CNS pathology. Int J. Biochem. Cell Biol. 39, 484-489 (2007).
289. Inoue, K., Takano, H. & Satoh, M. Protective role of metallothionein in coagulatory disturbance accompanied by acute liver injury induced by LPS/D-GalN. Thromb. Haemost. 99, 980-983 (2008).
290. Inoue, K. et al. Role of metallothionein in coagulatory disturbance and systemic inflammation induced by lipopolysaccharide in mice. Faseb J. 20, 533-535 (2006).
291. Takano, H. et al. Protective role of metallothionein in acute lung injury induced by bacterial endotoxin. Thorax 59, 1057-1062 (2004).
292. Subramanian Vignesh, K. et al. Granulocyte macrophage-colony stimulating factor induced Zn sequestration enhances macrophage superoxide and limits intracellular pathogen survival. Immunity 39, 697-710 (2013).
293. Liu, Y. et al. EOLA1 protects lipopolysaccharide induced IL-6 production and apoptosis by regulation of MT2A in human umbilical vein endothelial cells. Mol. Cell Biochem. 395, 45-51 (2014).
294. Wu, H. et al. Metallothionein deletion exacerbates intermittent hypoxia-induced renal injury in mice. Toxicol. Lett. 232, 340-348 (2015).
295. Vasto, S. et al. Zinc and inflammatory/immune response in aging. Ann. N. Y. Acad. Sci. 1100, 111-122 (2007).
296. Majumder, S. et al. Loss of metallothionein predisposes mice to diethylnitrosamine-induced hepatocarcinogenesis by activating NF-kappaB target genes. Cancer Res. 70, 10265-10276 (2010).
297. Butcher, H. L. et al. Metallothionein mediates the level and activity of nuclear factor kappa B in murine fibroblasts. J. Pharm. Exp. Ther. 310, 589-598 (2004).
298. Pan, Y. et al. Metallothionein 2 A inhibits
299. Toh, P. P. et al. Modulation of metallothionein isoforms is associated with collagen deposition in proliferating keloid fibroblasts in vitro. Exp. Dermatol. 19, 987-993 (2010).
300. Cong, W. et al. Metallothionein prevents age-associated cardiomyopathy via inhibiting NF-kB pathway activation and associated nitrative damage to 2-OGD. Antioxid. Redox Signal. 25, 936-952 (2016).
301. Read, S. A. et al. Zinc is a potent and specific inhibitor of IFN-
302. Chen, Q. Y., DesMarais, T. & Costa, M. Metals and mechanisms of carcinogenesis. Annu. Rev. Pharm. Toxicol. 59, 537-554 (2019).
303. Ganger, R. et al. Protective effects of zinc against acute arsenic toxicity by regulating antioxidant defense system and cumulative metallothionein expression. Biol. Trace Elem. Res. 169, 218-229 (2016).
304. Polykretis, P. et al. Cadmium effects on superoxide dismutase 1 in human cells revealed by NMR. Redox Biol. 21, 101102 (2019).
305. Petering, D. H., Loftsgaarden, J., Schneider, J. & Fowler, B. Metabolism of cadmium, zinc and copper in the rat kidney: the role of metallothionein and other binding sites. Environ. Health Perspect. 54, 73-81 (1984).
306. Chen, X. et al. The association between renal tubular dysfunction and zinc level in a Chinese population environmentally exposed to cadmium. Biol. Trace Elem. Res. 186, 114-121 (2018).
307. Hu, Y. et al. The role of reactive oxygen species in arsenic toxicity. Biomolecules 10, 240 (2020).
308. Rahman, M. T. & De Ley, M. Arsenic induction of metallothionein and metallothionein induction against arsenic cytotoxicity. Rev. Environ. Contam Toxicol. 240, 151-168 (2017).
309. Ho, E. Zinc deficiency, DNA damage and cancer risk. J. Nutr. Biochem. 15, 572-578 (2004).
310. Song, Y. et al. Marginal zinc deficiency increases oxidative DNA damage in the prostate after chronic exercise. Free Radic. Biol. Med. 48, 82-88 (2010).
311. Stepien, M. et al. Circulating copper and zinc levels and risk of hepatobiliary cancers in Europeans. Br. J. Cancer 116, 688-696 (2017).
312. Jayaraman, A. K. & Jayaraman, S. Increased level of exogenous zinc induces cytotoxicity and up-regulates the expression of the
313. Wu, X., Tang, J. & Xie, M. Serum and hair zinc levels in breast cancer: a metaanalysis. Sci. Rep. 5, 12249 (2015).
314. Seeler, J. F. et al. Metal ion fluxes controlling amphibian fertilization. Nat. Chem. 13, 683-691 (2021).
315. Kambe, T., Hashimoto, A. & Fujimoto, S. Current understanding of ZIP and ZnT zinc transporters in human health and diseases. Cell Mol. Life Sci. 71, 3281-3295 (2014).
316. Margalioth, E. J., Schenker, J. G. & Chevion, M. Copper and zinc levels in normal and malignant tissues. Cancer 52, 868-872 (1983).
317. Gammoh, N. Z. & Rink, L. Zinc in infection and inflammation. Nutrients 9, 624 (2017).
318. Cui, Y. et al. Levels of zinc, selenium, calcium, and iron in benign breast tissue and risk of subsequent breast cancer. Cancer Epidemiol. Biomark. Prev. 16, 1682-1685 (2007).
319. Santoliquido, P. M., Southwick, H. W. & Olwin, J. H. Trace metal levels in cancer of the breast. Surg. Gynecol. Obstet. 142, 65-70 (1976).
320. Taylor, K. M. et al. The emerging role of the LIV-1 subfamily of zinc transporters in breast cancer. Mol. Med. 13, 396-406 (2007).
321. Kasper, G. et al. Expression levels of the putative zinc transporter LIV-1 are associated with a better outcome of breast cancer patients. Int J. Cancer 117, 961-973 (2005).
322. Yamashita, S. et al. Zinc transporter LIVI controls epithelial-mesenchymal transition in zebrafish gastrula organizer. Nature 429, 298-302 (2004).
323. Kowalski, P. J., Rubin, M. A. & Kleer, C. G. E-cadherin expression in primary carcinomas of the breast and its distant metastases. Breast Cancer Res. 5, R217-R222 (2003).
324. Oka, H. et al. Expression of E-cadherin cell adhesion molecules in human breast cancer tissues and its relationship to metastasis. Cancer Res. 53, 1696-1701 (1993).
325. Lopez, V. & Kelleher, S. L. Zip6-attenuation promotes epithelial-to-mesenchymal transition in ductal breast tumor (T47D) cells. Exp. Cell Res. 316, 366-375 (2010).
326. Shen, H., Qin, H. & Guo, J. Concordant correlation of LIV-1 and E-cadherin expression in human breast cancer cell MCF-7. Mol. Biol. Rep. 36, 653-659 (2009).
327. Matsui, C. et al. Zinc and its transporter ZIP6 are key mediators of breast cancer cell survival under high glucose conditions. FEBS Lett. 591, 3348-3359 (2017).
328. Gao, T. et al. The mechanism between epithelial mesenchymal transition in breast cancer and hypoxia microenvironment. Biomed. Pharmacother. 80, 393-405 (2016).
329. Dave, B., Mittal, V., Tan, N. M. & Chang, J. C. Epithelial-mesenchymal transition, cancer stem cells and treatment resistance. Breast Cancer Res. 14, 202 (2012).
330. Chung, C. H., Bernard, P. S. & Perou, C. M. Molecular portraits and the family tree of cancer. Nat. Genet. 32, 533-540 (2002).
331. Tozlu, S. et al. Identification of novel genes that co-cluster with estrogen receptor alpha in breast tumor biopsy specimens, using a large-scale real-time reverse transcription-PCR approach. Endocr. Relat. Cancer 13, 1109-1120 (2006).
332. Althobiti, M. et al. Oestrogen-regulated protein SLC39A6: a biomarker of good prognosis in luminal breast cancer. Breast Cancer Res Treat. 189, 621-630 (2021).
333. Kambe, T. [Overview of and update on the physiological functions of mammalian zinc transporters]. Nihon Eiseigaku Zasshi. 68, 92-102 (2013).
334. Kagara, N., Tanaka, N., Noguchi, S. & Hirano, T. Zinc and its transporter ZIP10 are involved in invasive behavior of breast cancer cells. Cancer Sci. 98, 692-697 (2007).
335. Pal, D., Sharma, U., Singh, S. K. & Prasad, R. Association between ZIP10 gene expression and tumor aggressiveness in renal cell carcinoma. Gene 552, 195-198 (2014).
336. Pawlus, M. R., Wang, L. & Hu, C. J. STAT3 and HIF1alpha cooperatively activate HIF1 target genes in MDA-MB-231 and RCC4 cells. Oncogene 33, 1670-1679 (2014).
337. Armanious, H. et al. STAT3 upregulates the protein expression and transcriptional activity of beta-catenin in breast cancer. Int J. Clin. Exp. Pathol. 3, 654-664 (2010).
338. Chung, S. S., Giehl, N., Wu, Y. & Vadgama, J. V. STAT3 activation in HER2overexpressing breast cancer promotes epithelial-mesenchymal transition and cancer stem cell traits. Int J. Oncol. 44, 403-411 (2014).
339. Taylor, K. M. et al. ZIP7-mediated intracellular zinc transport contributes to aberrant growth factor signaling in antihormone-resistant breast cancer Cells. Endocrinology 149, 4912-4920 (2008).
340. Ziliotto, S. et al. Activated zinc transporter ZIP7 as an indicator of anti-hormone resistance in breast cancer. Metallomics 11, 1579-1592 (2019).
341. Huang, L., Kirschke, C. P., Zhang, Y. & Yu, Y. Y. The ZIP7 gene (SIc39a7) encodes a zinc transporter involved in zinc homeostasis of the Golgi apparatus. J. Biol. Chem. 280, 15456-15463 (2005).
342. de Nonneville, A. et al. Prognostic and predictive value of LIV1 expression in early breast cancer and by molecular subtype. Pharmaceutics 15, 938 (2023).
343. Vogel-Gonzalez, M., Musa-Afaneh, D., Rivera Gil, P. & Vicente, R. Zinc favors triple-negative breast cancer’s microenvironment modulation and cell plasticity. Int J. Mol. Sci. 22, 9188 (2021).
344. Yap, X. et al. Over-expression of metallothionein predicts chemoresistance in breast cancer. J. Pathol. 217, 563-570 (2009).
345. Jadhav, R. R. et al. Genome-wide DNA methylation analysis reveals estrogenmediated epigenetic repression of metallothionein-1 gene cluster in breast cancer. Clin. Epigenetics. 7, 13 (2015).
346. Lopez, V., Foolad, F. & Kelleher, S. L. ZnT2-overexpression represses the cytotoxic effects of zinc hyper-accumulation in malignant metallothionein-null T47D breast tumor cells. Cancer Lett. 304, 41-51 (2011).
347. Lim, D., Jocelyn, K. M., Yip, G. W. & Bay, B. H. Silencing the Metallothionein-2A gene inhibits cell cycle progression from G1- to S-phase involving ATM and cdc25A signaling in breast cancer cells. Cancer Lett. 276, 109-117 (2009).
348. Sun, L. et al. Zinc regulates the ability of Cdc25C to activate MPF/cdk1. J. Cell Physiol. 213, 98-104 (2007).
349. Banin, S. et al. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 281, 1674-1677 (1998).
350. Deng, C. et al. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 82, 675-684 (1995).
351. Li, D., Stovall, D. B., Wang, W. & Sui, G. Advances of zinc signaling studies in prostate cancer. Int J. Mol. Sci. 21, 667 (2020).
352. Zhao, J. et al. Comparative study of serum zinc concentrations in benign and malignant prostate disease: a systematic review and meta-analysis. Sci. Rep. 6, 25778 (2016).
353. McNeal, J. E. Normal histology of the prostate. Am. J. Surg. Pathol. 12, 619-633 (1988).
354. Costello, L. C. & Franklin, R. B. A comprehensive review of the role of zinc in normal prostate function and metabolism; and its implications in prostate cancer. Arch. Biochem. Biophys. 611, 100-112 (2016).
355. Vartsky, D. et al. Prostatic zinc and prostate specific antigen: an experimental evaluation of their combined diagnostic value. J. Urol. 170, 2258-2262 (2003).
356. Dakubo, G. D. et al. Altered metabolism and mitochondrial genome in prostate cancer. J. Clin. Pathol. 59, 10-16 (2006).
357. Feng, P. et al. The involvement of Bax in zinc-induced mitochondrial apoptogenesis in malignant prostate cells. Mol. Cancer 7, 25 (2008).
358. Nardinocchi, L. et al. Zinc downregulates HIF-1alpha and inhibits its activity in tumor cells in vitro and in vivo. PLoS One 5, e15048 (2010).
359. Uzzo, R. G. et al. Zinc inhibits nuclear factor-kappa B activation and sensitizes prostate cancer cells to cytotoxic agents. Clin. Cancer Res. 8, 3579-3583 (2002).
360. Ishii, K. et al. Evidence that the prostate-specific antigen (PSA)/Zn2+ axis may play a role in human prostate cancer cell invasion. Cancer Lett. 207, 79-87 (2004).
361. Uzzo, R. G. et al. Diverse effects of zinc on NF-kappaB and AP-1 transcription factors: implications for prostate cancer progression. Carcinogenesis 27, 1980-1990 (2006).
362. Ishii, K. et al. Inhibition of aminopeptidase N (AP-N) and urokinase-type plasminogen activator (uPA) by zinc suppresses the invasion activity in human urological cancer cells. Biol. Pharm. Bull. 24, 226-230 (2001).
363. Singh, K. K., Desouki, M. M., Franklin, R. B. & Costello, L. C. Mitochondrial aconitase and citrate metabolism in malignant and nonmalignant human prostate tissues. Mol. Cancer 5, 14 (2006).
364. Fontana, F., Anselmi, M. & Limonta, P. Unraveling the peculiar features of mitochondrial metabolism and dynamics in prostate cancer. Cancers. 15, 1192 (2023).
365. Costello, L. C. et al. Human prostate cancer ZIP1/zinc/citrate genetic/metabolic relationship in the TRAMP prostate cancer animal model. Cancer Biol. Ther. 12, 1078-1084 (2011).
366. Costello, L. C. & Franklin, R. B. The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: connecting the dots. Mol. Cancer 5, 17 (2006).
367. Franklin, R. B. et al. hZIP1 zinc uptake transporter down regulation and zinc depletion in prostate cancer. Mol. Cancer 4, 32 (2005).
368. An, Y. et al. A novel tetrapeptide fluorescence sensor for early diagnosis of prostate cancer based on imaging
369. Fong, L. Y. et al. Human-like hyperplastic prostate with low ZIP1 induced solely by Zn deficiency in rats. Proc. Natl Acad. Sci. USA. 115, E11091-e11100 (2018).
370. Costello, L. C., Franklin, R. B., Zou, J. & Naslund, M. J. Evidence that human prostate cancer is a ZIP1-deficient malignancy that could be effectively treated with a zinc ionophore (Clioquinol) approach. Chemotherapy 4, 152 (2015).
371. Huang, L., Kirschke, C. P. & Zhang, Y. Decreased intracellular zinc in human tumorigenic prostate epithelial cells: a possible role in prostate cancer progression. Cancer Cell Int. 6, 10 (2006).
372. Makhov, P. et al. Transcriptional regulation of the major zinc uptake protein hZip1 in prostate cancer cells. Gene 431, 39-46 (2009).
373. Thiagalingam, A. et al. RREB-1, a novel zinc finger protein, is involved in the differentiation response to Ras in human medullary thyroid carcinomas. Mol. Cell Biol. 16, 5335-5345 (1996).
374. Zhang, S. et al. p16 INK4a gene promoter variation and differential binding of a repressor, the ras-responsive zinc-finger transcription factor, RREB. Oncogene 22, 2285-2295 (2003).
36
375. Gioeli, D. Signal transduction in prostate cancer progression. Clin. Sci. 108, 293-308 (2005).
376. Milon, B. C. et al. Ras responsive element binding protein-1 (RREB-1) downregulates hZIP1 expression in prostate cancer cells. Prostate 70, 288-296 (2010).
377. Aguirre-Portoles, C. et al. ZIP9 is a druggable determinant of sex differences in melanoma. Cancer Res. 81, 5991-6003 (2021).
378. Berg, A. H. et al. Identification and characterization of membrane androgen receptors in the ZIP9 zinc transporter subfamily: I. Discovery in female atlantic croaker and evidence ZIP9 mediates testosterone-induced apoptosis of ovarian follicle cells. Endocrinology 155, 4237-4249 (2014).
379. Thomas, P., Pang, Y., Dong, J. & Berg, A. H. Identification and characterization of membrane androgen receptors in the ZIP9 zinc transporter subfamily: II. Role of human ZIP9 in testosterone-induced prostate and breast cancer cell apoptosis. Endocrinology 155, 4250-4265 (2014).
380. Desouki, M. M. et al. hZip2 and hZip3 zinc transporters are down regulated in human prostate adenocarcinomatous glands. Mol. Cancer 6, 37 (2007).
381. Kelleher, S. L., McCormick, N. H., Velasquez, V. & Lopez, V. Zinc in specialized secretory tissues: roles in the pancreas, prostate, and mammary gland. Adv. Nutr. 2, 101-111 (2011).
382. Franklin, R. B. et al. Human ZIP1 is a major zinc uptake transporter for the accumulation of zinc in prostate cells. J. Inorg. Biochem. 96, 435-442 (2003).
383. Prasad, R. R. et al. Stage-specific differential expression of zinc transporter SLC30A and SLC39A family proteins during prostate tumorigenesis. Mol. Carcinog. 61, 454-471 (2022).
384. Kim, Y. R. et al. HOXB13 downregulates intracellular zinc and increases NFkappaB signaling to promote prostate cancer metastasis. Oncogene 33, 4558-4567 (2014).
385. Beck, F. W. et al. Differential expression of
386. Inoue, K. et al. Osteopenia and male-specific sudden cardiac death in mice lacking a zinc transporter gene, Znt5. Hum. Mol. Genet. 11, 1775-1784 (2002).
387. Wei, H. et al. Differential expression of metallothioneins (MTs) 1,2 , and 3 in response to zinc treatment in human prostate normal and malignant cells and tissues. Mol. Cancer 7, 7 (2008).
388. Han, Y. C. et al. Metallothionein 1 h tumour suppressor activity in prostate cancer is mediated by euchromatin methyltransferase 1. J. Pathol. 230, 184-193 (2013).
389. Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 73, 17-48 (2023).
390. Costello, L. C. et al. Decreased zinc and downregulation of ZIP3 zinc uptake transporter in the development of pancreatic adenocarcinoma. Cancer Biol. Ther. 12, 297-303 (2011).
391. Li, M. et al. Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. Proc. Natl Acad. Sci. USA. 104, 18636-18641 (2007).
392. Shakri, A. R. et al. Upregulation of ZIP14 and altered zinc homeostasis in muscles in pancreatic cancer cachexia. Cancers. 12, 3 (2019).
393. Li, M. et al. Down-regulation of ZIP4 by RNA interference inhibits pancreatic cancer growth and increases the survival of nude mice with pancreatic cancer xenografts. Clin. Cancer Res. 15, 5993-6001 (2009).
394. Liu, M. et al. ZIP4 promotes pancreatic cancer progression by repressing ZO-1 and Claudin-1 through a ZEB1-dependent transcriptional mechanism. Clin. Cancer Res. 24, 3186-3196 (2018).
395. Liu, M. et al. ZIP4 increases expression of transcription factor ZEB1 to promote Integrin
396. Shi, X. et al. Circular RNA ANAPC7 inhibits tumor growth and muscle wasting via PHLPP2-AKT-TGF-
397. Xu, X. et al. ZIP4, a novel determinant of tumor invasion in hepatocellular carcinoma, contributes to tumor recurrence after liver transplantation. Int J. Biol. Sci. 10, 245-256 (2014).
398. Zhang, Y. et al. ZIP4 regulates pancreatic cancer cell growth by activating IL-6/ STAT3 pathway through zinc finger transcription factor CREB. Clin. Cancer Res. 16, 1423-1430 (2010).
399. Zhang, Y. et al. A novel epigenetic CREB-miR-373 axis mediates ZIP4-induced pancreatic cancer growth. EMBO Mol. Med. 5, 1322-1334 (2013).
400. Shi, X. et al. Circular RNA ANAPC7 inhibits tumor growth and muscle wasting via PHLPP2-AKT-TGF-beta signaling axis in pancreatic cancer. Gastroenterology 162, 2004-2017.e2002 (2022).
401. Krebs, A. M. et al. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat. Cell Biol. 19, 518-529 (2017).
402. Franklin, R. B., Zou, J. & Costello, L. C. The cytotoxic role of RREB1, ZIP3 zinc transporter, and zinc in human pancreatic adenocarcinoma. Cancer Biol. Ther. 15, 1431-1437 (2014).
403. Li, K. et al. Metallothionein-1G suppresses pancreatic cancer cell stemness by limiting activin A secretion via NF-KB inhibition. Theranostics 11, 3196-3212 (2021).
404. Li, P. et al. Association between zinc intake and risk of digestive tract cancers: a systematic review and meta-analysis. Clin. Nutr. 33, 415-420 (2014).
405. Jaiswal, A. S. & Narayan, S. Zinc stabilizes adenomatous polyposis coli (APC) protein levels and induces cell cycle arrest in colon cancer cells. J. Cell Biochem. 93, 345-357 (2004).
406. Shangkuan, W. C. et al. Risk analysis of colorectal cancer incidence by gene expression analysis. PeerJ 5, e3003 (2017).
407. Yagi, K. et al. Three DNA methylation epigenotypes in human colorectal cancer. Clin. Cancer Res. 16, 21-33 (2010).
408. Hou, L., Liu, P. & Zhu, T. Long noncoding RNA SLC30A10 promotes colorectal tumor proliferation and migration via miR-21c/APC axis. Eur. Rev. Med Pharm. Sci. 24, 6682-6691 (2020).
409. Yao, H. et al. KCTD9 inhibits the Wnt/
410. Chen, Y. H. et al. Role of GAC63 in transcriptional activation mediated by betacatenin. Nucleic Acids Res. 35, 2084-2092 (2007).
411. Zhao, H. et al. Wnt signaling in colorectal cancer: pathogenic role and therapeutic target. Mol. Cancer 21, 144 (2022).
412. Barresi, V. et al. Transcriptome analysis reveals an altered expression profile of zinc transporters in colorectal cancer. J. Cell Biochem. 119, 9707-9719 (2018).
413. Sheng, N. et al. Knockdown of SLC39A7 inhibits cell growth and induces apoptosis in human colorectal cancer cells. Acta Biochim. Biophys. Sin. (Shanghai). 49, 926-934 (2017).
414. Jbara, A. et al. RBFOX2 modulates a metastatic signature of alternative splicing in pancreatic cancer. Nature 617, 147-153 (2023).
415. Marasco, L. E. & Kornblihtt, A. R. The physiology of alternative splicing. Nat. Rev. Mol. Cell Biol. 24, 242-254 (2023).
416. Wan, L. et al. Splicing factor SRSF1 promotes pancreatitis and KRASG12Dmediated pancreatic cancer. Cancer Discov. 13, 1678-1695 (2023).
417. Thorsen, K. et al. Alternative splicing of SLC39A14 in colorectal cancer is regulated by the Wnt pathway. Mol. Cell Proteom. 10, M110 002998 (2011).
418. Cao, X. et al. Cadmium induced BEAS-2B cells apoptosis and mitochondria damage via MAPK signaling pathway. Chemosphere 263, 128346 (2021).
419. Jin, Y. H. et al. Cadmium is a mutagen that acts by inhibiting mismatch repair. Nat. Genet. 34, 326-329 (2003).
420. Hung, K. C. et al. The expression profile and prognostic significance of metallothionein genes in colorectal cancer. Int J. Mol. Sci. 20, 3849 (2019).
421. Arriaga, J. M., Greco, A., Mordoh, J. & Bianchini, M. Metallothionein 1 G and zinc sensitize human colorectal cancer cells to chemotherapy. Mol. Cancer Ther. 13, 1369-1381 (2014).
422. Liu, X. et al. Metallothionein 2 A (MT2A) controls cell proliferation and liver metastasis by controlling the MST1/LATS2/YAP1 signaling pathway in colorectal cancer. Cancer Cell Int. 22, 205 (2022).
423. Arriaga, J. M. et al. Metallothionein expression in colorectal cancer: relevance of different isoforms for tumor progression and patient survival. Hum. Pathol. 43, 197-208 (2012).
424. Chen, H. et al. Nutrient intakes and adenocarcinoma of the esophagus and distal stomach. Nutr. Cancer 42, 33-40 (2002).
425. Rogers, M. A. et al. A case-control study of element levels and cancer of the upper aerodigestive tract. Cancer Epidemiol. Biomark. Prev. 2, 305-312 (1993).
426. Pakseresht, M. et al. Dietary habits and gastric cancer risk in north-west Iran. Cancer Causes Control. 22, 725-736 (2011).
427. He, Y. et al. Cancer incidence and mortality in Hebei province, 2013. Medicine 96, e7293 (2017).
428. Li, D. et al. Cancer survival in Cixian of China, 2003-2013: a population-based study. Cancer Med. 7, 1537-1545 (2018).
429. Liang, D. et al. Gastric cancer burden of last 40 years in North China (Hebei Province): a population-based study. Medicine 96, e5887 (2017).
430. Guo, Y. & He, Y. Comprehensive analysis of the expression of SLC30A family genes and prognosis in human gastric cancer. Sci. Rep. 10, 18352 (2020).
431. Guan, X. et al. Dual inhibition of MYC and SLC39A10 by a novel natural product STAT3 inhibitor derived from Chaetomium globosum suppresses tumor growth and metastasis in gastric cancer. Pharm. Res. 189, 106703 (2023).
432. Zhang, Y. et al. SLC39A7, regulated by miR-139-5p, induces cell proliferation, migration and inhibits apoptosis in gastric cancer via Akt/mTOR signaling pathway. Biosci. Rep. 40, BSR20200041 (2020).
433. Janssen, A. M. et al. Metallothionein in human gastrointestinal cancer. J. Pathol. 192, 293-300 (2000).
434. Lin, S. et al. Transcription factor myeloid zinc-finger 1 suppresses human gastric carcinogenesis by interacting with metallothionein 2 A. Clin. Cancer Res. 25, 1050-1062 (2019).
435. Cho, Y. H. et al. A role of metallothionein-3 in radiation-induced autophagy in glioma cells. Sci. Rep. 10, 2015 (2020).
436. Li, K. et al. MT1M regulates gastric cancer progression and stemness by modulating the Hedgehog pathway protein GLI1. Biochem. Biophys. Res. Commun. 670, 63-72 (2023).
437. Fiches, G. N. et al. Profiling of immune related genes silenced in EBV-positive gastric carcinoma identified novel restriction factors of human gammaherpesviruses. PLoS Pathog. 16, e1008778 (2020).
438. Takahashi, S. Molecular functions of metallothionein and its role in hematological malignancies. J. Hematol. Oncol. 5, 41 (2012).
439. Pan, Y. et al. Epigenetic upregulation of metallothionein 2 A by diallyl trisulfide enhances chemosensitivity of human gastric cancer cells to docetaxel through attenuating NF-кB activation. Antioxid. Redox Signal. 24, 839-854 (2016).
440. Habel, N. et al. Zinc chelation: a metallothionein 2 A ‘s mechanism of action involved in osteosarcoma cell death and chemotherapy resistance. Cell Death Dis. 4, e874 (2013).
441. Zalewska, M., Trefon, J. & Milnerowicz, H. The role of metallothionein interactions with other proteins. Proteomics 14, 1343-1356 (2014).
442. Kolenko, V., Teper, E., Kutikov, A. & Uzzo, R. Zinc and zinc transporters in prostate carcinogenesis. Nat. Rev. Urol. 10, 219-226 (2013).
443. Kim, C. H., Kim, J. H., Lee, J. & Ahn, Y. S. Zinc-induced NF-kappaB inhibition can be modulated by changes in the intracellular metallothionein level. Toxicol. Appl Pharmacol. 190, 189-196 (2003).
444. Fong, L. Y. & Magee, P. N. Dietary zinc deficiency enhances esophageal cell proliferation and N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumor incidence in C57BL/6 mouse. Cancer Lett. 143, 63-69 (1999).
445. Abnet, C. C. et al. Zinc concentration in esophageal biopsy specimens measured by x-ray fluorescence and esophageal cancer risk. J. Natl Cancer Inst. 97, 301-306 (2005).
446. Fong, L. Y., Nguyen, V. T. & Farber, J. L. Esophageal cancer prevention in zincdeficient rats: rapid induction of apoptosis by replenishing zinc. J. Natl Cancer Inst. 93, 1525-1533 (2001).
447. Wu, C. et al. Genome-wide association study identifies common variants in SLC39A6 associated with length of survival in esophageal squamous-cell carcinoma. Nat. Genet. 45, 632-638 (2013).
448. Cui, X. B. et al. SLC39A6: a potential target for diagnosis and therapy of esophageal carcinoma. J. Transl. Med. 13, 321 (2015).
449. Cheng, X. et al. Solute carrier family 39 member 6 gene promotes aggressiveness of esophageal carcinoma cells by increasing intracellular levels of zinc, activating phosphatidylinositol 3 -kinase signaling, and up-regulating genes that regulate metastasis. Gastroenterology 152, 1985-1997.e1912 (2017).
450. Jin, J. et al. Knockdown of zinc transporter ZIP5 (SLC39A5) expression significantly inhibits human esophageal cancer progression. Oncol. Rep. 34, 1431-1439 (2015).
451. Kumar, A., Chatopadhyay, T., Raziuddin, M. & Ralhan, R. Discovery of deregulation of zinc homeostasis and its associated genes in esophageal squamous cell carcinoma using cDNA microarray. Int J. Cancer 120, 230-242 (2007).
452. Li, Q. et al. Knockdown of zinc transporter ZIP5 by RNA interference inhibits esophageal cancer growth in vivo. Oncol. Res. 24, 205-214 (2016).
453. Huang, J. X. et al. Relationship between COX-2 and cell cycle-regulatory proteins in patients with esophageal squamous cell carcinoma. World J. Gastroenterol. 16, 5975-5981 (2010).
454. Shimizu, M. et al. Metallothionein 2 A expression in cancer-associated fibroblasts and cancer cells promotes esophageal squamous cell carcinoma progression. Cancers. 13, 4552 (2021).
455. Wong, T. S., Gao, W. & Chan, J. Y. Transcription regulation of E-cadherin by zinc finger E -box binding homeobox proteins in solid tumors. Biomed. Res Int. 2014, 921564 (2014).
456. ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Consortium. Pan-cancer analysis of whole genomes. Nature 578, 82-93 (2020).
457. Agrawal, A. et al. Zinc-binding groups modulate selective inhibition of MMPs. ChemMedChem 3, 812-820 (2008).
458. Puerta, D. T. & Cohen, S. M. Examination of novel zinc-binding groups for use in matrix metalloproteinase inhibitors. Inorg. Chem. 42, 3423-3430 (2003).
459. Lheureux, S., Braunstein, M. & Oza, A. M. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J. Clin. 69, 280-304 (2019).
460. Wei, T. et al. ZnT7 RNAi favors Raf(GOF)scrib(-/-)-induced tumor growth and invasion in Drosophila through JNK signaling pathway. Oncogene 40, 2217-2229 (2021).
461. Aguirre-Portolés, C. et al. ZIP9 is a druggable determinant of sex differences in melanoma. Cancer Res. 81, 5991-6003 (2021).
462. Jaiswal, S. & Libby, P. Clonal haematopoiesis: connecting ageing and inflammation in cardiovascular disease. Nat. Rev. Cardiol. 17, 137-144 (2020).
463. Bekele, T. H. et al. Dietary recommendations for ethiopians on the basis of priority diet-related diseases and causes of death in ethiopia: an umbrella review. Adv. Nutr. 14, 895-913 (2023).
464. Mohammadifard, N. et al. Trace minerals intake: Risks and benefits for cardiovascular health. Crit. Rev. Food Sci. Nutr. 59, 1334-1346 (2019).
465. Libby, P. The changing landscape of atherosclerosis. Nature 592, 524-533 (2021).
466. Förstermann, U., Xia, N. & Li, H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res. 120, 713-735 (2017).
467. Conway, D. E. et al. Endothelial metallothionein expression and intracellular free zinc levels are regulated by shear stress. Am. J. Physiol. Cell Physiol. 299, C1461-C1467 (2010).
468. Hara, T. et al. Role of ScI39a13/ZIP13 in cardiovascular homeostasis. PLoS One 17, e0276452 (2022).
469. Allen-Redpath, K. et al. Marginal dietary zinc deficiency in vivo induces vascular smooth muscle cell apoptosis in large arteries. Cardiovasc Res. 99, 525-534 (2013).
470. Alcantara, E. H. et al. Long-term zinc deprivation accelerates rat vascular smooth muscle cell proliferation involving the down-regulation of JNK1/2 expression in MAPK signaling. Atherosclerosis 228, 46-52 (2013).
471. Patrushev, N., Seidel-Rogol, B. & Salazar, G. Angiotensin II requires zinc and downregulation of the zinc transporters ZnT 3 and ZnT 10 to induce senescence of vascular smooth muscle cells. PLoS One 7, e33211 (2012).
472. min, L. J., Mogi, M., Iwai, M. & Horiuchi, M. Signaling mechanisms of angiotensin II in regulating vascular senescence. Ageing Res Rev. 8, 113-121 (2009).
473. Reed, G. W., Rossi, J. E. & Cannon, C. P. Acute myocardial infarction. Lancet 389, 197-210 (2017).
474. McIntosh, R. et al. The critical role of intracellular zinc in adenosine A(2) receptor activation induced cardioprotection against reperfusion injury. J. Mol. Cell Cardiol. 49, 41-47 (2010).
475. Du, L. et al. The critical role of the zinc transporter Zip2 (SLC39A2) in ischemia/reperfusion injury in mouse hearts. J. Mol. Cell Cardiol. 132, 136-145 (2019).
476. Zhao, H. et al. Endoplasmic reticulum stress/Ca(2+)-calmodulin-dependent protein kinase/signal transducer and activator of transcription 3 pathway plays a role in the regulation of cellular zinc deficiency in myocardial ischemia/reperfusion injury. Front. Physiol. 12, 736920 (2021).
477. Zhang, H. et al. The zinc transporter ZIP7 (SIc39a7) controls myocardial reperfusion injury by regulating mitophagy. Basic Res. Cardiol. 116, 54 (2021).
478. Beharier, O. et al. ZnT-1 protects HL-1 cells from simulated ischemia-reperfusion through activation of Ras-ERK signaling. J. Mol. Med. 90, 127-138 (2012).
479. Bruinsma, J. J., Jirakulaporn, T., Muslin, A. J. & Kornfeld, K. Zinc ions and cation diffusion facilitator proteins regulate Ras-mediated signaling. Dev. Cell. 2, 567-578 (2002).
480. Lazarczyk, M. et al. Regulation of cellular zinc balance as a potential mechanism of EVER-mediated protection against pathogenesis by cutaneous oncogenic human papillomaviruses. J. Exp. Med. 205, 35-42 (2008).
481. Murphy, E. & Steenbergen, C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol. Rev. 88, 581-609 (2008).
482. Smith, M. J. et al. Redox and metal profiles in human coronary endothelial and smooth muscle cells under hyperoxia, physiological normoxia and hypoxia: Effects of NRF2 signaling on intracellular zinc. Redox Biol. 62, 102712 (2023).
483. Cai, L. et al. Attenuation by metallothionein of early cardiac cell death via suppression of mitochondrial oxidative stress results in a prevention of diabetic cardiomyopathy. J. Am. Coll. Cardiol. 48, 1688-1697 (2006).
484. Wang, Y. et al. Inactivation of GSK-3beta by metallothionein prevents diabetesrelated changes in cardiac energy metabolism, inflammation, nitrosative damage, and remodeling. Diabetes 58, 1391-1402 (2009).
485. Dong, F. et al. Metallothionein prevents high-fat diet induced cardiac contractile dysfunction: role of peroxisome proliferator activated receptor gamma coactivator 1alpha and mitochondrial biogenesis. Diabetes 56, 2201-2212 (2007).
486. Wang, J. et al. Cardiac metallothionein induction plays the major role in the prevention of diabetic cardiomyopathy by zinc supplementation. Circulation 113, 544-554 (2006).
487. Hu, N. et al. Cardiac-specific overexpression of metallothionein rescues nicotineinduced cardiac contractile dysfunction and interstitial fibrosis. Toxicol. Lett. 202, 8-14 (2011).
488. Zhou, G. et al. Metallothionein suppresses angiotensin II-induced nicotinamide adenine dinucleotide phosphate oxidase activation, nitrosative stress, apoptosis, and pathological remodeling in the diabetic heart. J. Am. Coll. Cardiol. 52, 655-666 (2008).
489. Zhang, Y. et al. Cardiac overexpression of metallothionein rescues cold exposure-induced myocardial contractile dysfunction through attenuation of cardiac fibrosis despite cardiomyocyte mechanical anomalies. Free Radic. Biol. Med. 53, 194-207 (2012).
38
490. Cai, L. et al. Inhibition of superoxide generation and associated nitrosative damage is involved in metallothionein prevention of diabetic cardiomyopathy. Diabetes 54, 1829-1837 (2005).
491. Gu, J. et al. Metallothionein preserves Akt2 activity and cardiac function via inhibiting TRB3 in diabetic hearts. Diabetes 67, 507-517 (2018).
492. Dabravolski, S. A. et al. Interplay between
493. Woodier, J., Rainbow, R. D., Stewart, A. J. & Pitt, S. J. Intracellular zinc modulates cardiac ryanodine receptor-mediated calcium release. J. Biol. Chem. 290, 17599-17610 (2015).
494. Gaburjakova, J. & Gaburjakova, M. The cardiac ryanodine receptor provides a suitable pathway for the rapid transport of zinc (
495. Mor, M. et al. ZnT-1 enhances the activity and surface expression of T-type calcium channels through activation of Ras-ERK signaling. Am. J. Physiol. Cell Physiol. 303, C192-C203 (2012).
496. Liu, B., Cai, Z. Q. & Zhou, Y. M. Deficient zinc levels and myocardial infarction : association between deficient zinc levels and myocardial infarction: a metaanalysis. Biol. Trace Elem. Res. 165, 41-50 (2015).
497. Wang, J. et al. Downregulation of the zinc transporter SLC39A13 (ZIP13) is responsible for the activation of CaMKII at reperfusion and leads to myocardial ischemia/reperfusion injury in mouse hearts. J. Mol. Cell Cardiol. 152, 69-79 (2021).
498. Chen, Z. et al. Zinc ameliorates human aortic valve calcification through GPR39 mediated ERK1/2 signalling pathway. Cardiovasc. Res. 117, 820-835 (2021).
499. Fang, Y. et al. Slc39a2-mediated zinc homeostasis modulates innate immune signaling in phenylephrine-induced cardiomyocyte hypertrophy. Front. Cardiovasc. Med. 8, 736911 (2021).
500. Jiang, D. S. et al. IRF8 suppresses pathological cardiac remodelling by inhibiting calcineurin signalling. Nat. Commun. 5, 3303 (2014).
501. Jiang, D. S. et al. Interferon regulatory factor 9 protects against cardiac hypertrophy by targeting myocardin. Hypertension 63, 119-127 (2014).
502. Jiang, D. S. et al. Interferon regulatory factor 7 functions as a novel negative regulator of pathological cardiac hypertrophy. Hypertension 63, 713-722 (2014).
503. Lin, W. et al. Zinc transporter Slc39a8 is essential for cardiac ventricular compaction. J. Clin. Invest. 128, 826-833 (2018).
504. Lehuen, A., Diana, J., Zaccone, P. & Cooke, A. Immune cell crosstalk in type 1 diabetes. Nat. Rev. Immunol. 10, 501-513 (2010).
505. Baekkeskov, S. et al. Identification of the 64 K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature 347, 151-156 (1990).
506. Vehik, K. et al. Hierarchical order of distinct autoantibody spreading and progression to type 1 diabetes in the TEDDY study. Diabetes Care. 43, 2066-2073 (2020).
507. Palmer, J. P. et al. Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science 222, 1337-1339 (1983).
508. Achenbach, P. et al. Autoantibodies to zinc transporter 8 and SLC30A8 genotype stratify type 1 diabetes risk. Diabetologia 52, 1881-1888 (2009).
509. Kawasaki, E. et al. Differences in the humoral autoreactivity to zinc transporter 8 between childhood- and adult-onset type 1 diabetes in Japanese patients. Clin. Immunol. 138, 146-153 (2011).
510. Vermeulen, I. et al. Contribution of antibodies against IA-2
511. Wenzlau, J. M. et al. Kinetics of the post-onset decline in zinc transporter 8 autoantibodies in type 1 diabetic human subjects. J. Clin. Endocrinol. Metab. 95, 4712-4719 (2010).
512. Long, A. E. et al. Humoral responses to islet antigen-2 and zinc transporter 8 are attenuated in patients carrying HLA-A24 alleles at the onset of type 1 diabetes. Diabetes 62, 2067-2071 (2013).
513. Ye, J. et al. Attenuated humoral responses in HLA-A24-positive individuals at risk of type 1 diabetes. Diabetologia 58, 2284-2287 (2015).
514. Énée, É. et al.
515. Scotto, M. et al. Zinc transporter (ZnT)8(186-194) is an immunodominant CD8+ T cell epitope in HLA-A2 + type 1 diabetic patients. Diabetologia 55, 2026-2031 (2012).
516. Culina, S. et al. Islet-reactive CD8(+) T cell frequencies in the pancreas, but not in blood, distinguish type 1 diabetic patients from healthy donors. Sci. Immunol. 3, eaao4013 (2018).
517. Lampasona, V. & Liberati, D. Islet autoantibodies. Curr. Diab. Rep. 16, 53 (2016).
518. Wenzlau, J. M. et al. A common nonsynonymous single nucleotide polymorphism in the SLC30A8 gene determines ZnT8 autoantibody specificity in type 1 diabetes. Diabetes 57, 2693-2697 (2008).
519. Kawasaki, E. et al. Association between anti-ZnT8 autoantibody specificities and SLC30A8 Arg325Trp variant in Japanese patients with type 1 diabetes. Diabetologia 51, 2299-2302 (2008).
520. Shruthi, S., Mohan, V., Maradana, M. R. & Aravindhan, V. In silico identification and wet lab validation of novel cryptic
521. Hanna, S. J. et al. Slow progressors to type 1 diabetes lose islet autoantibodies over time, have few islet antigen-specific CD8(+) T cells and exhibit a distinct CD95(hi) B cell phenotype. Diabetologia 63, 1174-1185 (2020).
522. Wenzlau, J. M. et al. Changes in zinc transporter 8 autoantibodies following type 1 diabetes onset: the type 1 diabetes genetics consortium autoantibody workshop. Diabetes Care. 38, S14-S20 (2015).
523. Flannick, J. et al. Loss-of-function mutations in SLC30A8 protect against type 2 diabetes. Nat. Genet. 46, 357-363 (2014).
524. Choi, B. Y. et al. Zinc transporter 3 (ZnT3) gene deletion reduces spinal cord white matter damage and motor deficits in a murine MOG-induced multiple sclerosis model. Neurobiol. Dis. 94, 205-212 (2016).
525. Penkowa, M. & Hidalgo, J. Metallothionein I+II expression and their role in experimental autoimmune encephalomyelitis. Glia 32, 247-263 (2000).
526. Kim, B. et al. Cytoplasmic zinc promotes IL-1
527. Yoon, B. R. et al. Preferential induction of the T cell auxiliary signaling molecule B7-H3 on synovial monocytes in rheumatoid arthritis. J. Biol. Chem. 291, 4048-4057 (2016).
528. Cassat, J. E. & Skaar, E. P. Metal ion acquisition in Staphylococcus aureus: overcoming nutritional immunity. Semin. Immunopathol. 34, 215-235 (2012).
529. Baum, M. K. et al. Randomized, controlled clinical trial of zinc supplementation to prevent immunological failure in HIV-infected adults. Clin. Infect. Dis. 50, 1653-1660 (2010).
530. Kehl-Fie, T. E. & Skaar, E. P. Nutritional immunity beyond iron: a role for manganese and zinc. Curr. Opin. Chem. Biol. 14, 218-224 (2010).
531. Bao, B. et al. Zinc supplementation decreases oxidative stress, incidence of infection, and generation of inflammatory cytokines in sickle cell disease patients. Transl. Res. 152, 67-80 (2008).
532. Laskaris, P. et al. Administration of zinc chelators improves survival of mice infected with aspergillus fumigatus both in monotherapy and in combination with caspofungin. Antimicrob. Agents Chemother. 60, 5631-5639 (2016).
533. Corbin, B. D. et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 319, 962-965 (2008).
534. Hantke, K. Bacterial zinc uptake and regulators. Curr. Opin. Microbiol. 8, 196-202 (2005).
535. Lappann, M. et al. In vitro resistance mechanisms of Neisseria meningitidis against neutrophil extracellular traps. Mol. Microbiol. 89, 433-449 (2013).
536. Botella, H. et al. Metallobiology of host-pathogen interactions: an intoxicating new insight. Trends Microbiol. 20, 106-112 (2012).
537. Branch, A. H., Stoudenmire, J. L., Seib, K. L. & Cornelissen, C. N. Acclimation to nutritional immunity and metal intoxication requires zinc, manganese, and copper homeostasis in the pathogenic neisseriae. Front Cell Infect. Microbiol. 12, 909888 (2022).
538. Ishida, T. J. A. J. B. S. R. Review on the role of
539. Alamir, O. F., Oladele, R. O. & Ibe, C. Nutritional immunity: targeting fungal zinc homeostasis. Heliyon 7, e07805 (2021).
540. Subramanian Vignesh, K. & Deepe, G. S. Jr. Immunological orchestration of zinc homeostasis: the battle between host mechanisms and pathogen defenses. Arch. Biochem. Biophys. 611, 66-78 (2016).
541. Wagner, D. et al. Elemental analysis of Mycobacterium avium-, Mycobacterium tuberculosis-, and Mycobacterium smegmatis-containing phagosomes indicates pathogen-induced microenvironments within the host cell’s endosomal system. J. Immunol. 174, 1491-1500 (2005).
542. Botella, H. et al. Mycobacterial p(1)-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host Microbe 10, 248-259 (2011).
543. Neyrolles, O., Wolschendorf, F., Mitra, A. & Niederweis, M. Mycobacteria, metals, and the macrophage. Immunol. Rev. 264, 249-263 (2015).
544. Neyrolles, O., Mintz, E. & Catty, P. Zinc and copper toxicity in host defense against pathogens: mycobacterium tuberculosis as a model example of an emerging paradigm. Front. Cell Infect. Microbiol. 3, 89 (2013).
545. Sayadi, A., Nguyen, A. T., Bard, F. A. & Bard-Chapeau, E. A. Zip14 expression induced by lipopolysaccharides in macrophages attenuates inflammatory response. Inflamm. Res. 62, 133-143 (2013).
546. Stocks, C. J. et al. Uropathogenic Escherichia coli employs both evasion and resistance to subvert innate immune-mediated zinc toxicity for dissemination. Proc. Natl Acad. Sci. USA. 116, 6341-6350 (2019).
547. Padilla-Benavides, T . et al. A novel
548. Chandrangsu, P., Rensing, C. & Helmann, J. D. Metal homeostasis and resistance in bacteria. Nat. Rev. Microbiol. 15, 338-350 (2017).
549. Sensi, S. L. et al. The neurophysiology and pathology of brain zinc. J. Neurosci. 31, 16076-16085 (2011).
550. Szewczyk, B. Zinc homeostasis and neurodegenerative disorders. Front Aging Neurosci. 5, 33 (2013).
551. Walsh, D. M. et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416, 535-539 (2002).
552. Adlard, P. A. et al. Metal chaperones prevent zinc-mediated cognitive decline. Neurobiol. Dis. 81, 196-202 (2015).
553. Bjorklund, N. L. et al. Absence of amyloid
554. Bush, A. I. The metallobiology of Alzheimer’s disease. Trends Neurosci. 26, 207-214 (2003).
555. Whitfield, D. R. et al. Depression and synaptic zinc regulation in Alzheimer disease, dementia with lewy bodies, and Parkinson disease dementia. Am. J. Geriatr. Psychiatry 23, 141-148 (2015).
556. Adlard, P. A., Parncutt, J. M., Finkelstein, D. I. & Bush, A. I. Cognitive loss in zinc transporter-3 knock-out mice: a phenocopy for the synaptic and memory deficits of Alzheimer’s disease? J. Neurosci. 30, 1631-1636 (2010).
557. Adlard, P. A. et al. A novel approach to rapidly prevent age-related cognitive decline. Aging Cell. 13, 351-359 (2014).
558. Lang, M. et al. Genetic inhibition of solute-linked carrier 39 family transporter 1 ameliorates a
559. Meloni, G. et al. Metal swap between Zn 7 -metallothionein-3 and amyloid-betaCu protects against amyloid-beta toxicity. Nat. Chem. Biol. 4, 366-372 (2008).
560. Lyubartseva, G., Smith, J. L., Markesbery, W. R. & Lovell, M. A. Alterations of zinc transporter proteins
561. Bosomworth, H. J., Adlard, P. A., Ford, D. & Valentine, R. A. Altered expression of ZnT10 in Alzheimer’s disease brain. PLoS One 8, e65475 (2013).
562. Song, L. et al. ZIP9 mediates the effects of DHT on learning, memory and hippocampal synaptic plasticity of male Tfm and APP/PS1 mice. Front Endocrinol. 14, 1139874 (2023).
563. Sikora, J. & Ouagazzal, A. M. Synaptic zinc: an emerging player in Parkinson’s disease. Int J. Mol. Sci. 22, 4724 (2021).
564. Valiente-Gabioud, A. A. et al. Structural basis behind the interaction of
565. Sepers, M. D. & Raymond, L. A. Mechanisms of synaptic dysfunction and excitotoxicity in Huntington’s disease. Drug Discov. Today 19, 990-996 (2014).
566. Fourie, C. et al. Dietary zinc supplementation prevents autism related behaviors and striatal synaptic dysfunction in Shank3 Exon 13-16 mutant mice. Front. Cell Neurosci. 12, 374 (2018).
567. Lee, K. et al. Dietary zinc supplementation rescues fear-based learning and synaptic function in the Tbr1(+/-) mouse model of autism spectrum disorders. Mol. Autism 13, 13 (2022).
568. Squadrone, S., Brizio, P., Abete, M. C. & Brusco, A. Trace elements profile in the blood of Huntington’ disease patients. J. Trace Elem. Med. Biol. 57, 18-20 (2020).
569. Niu, L. et al. Disruption of zinc transporter ZnT 3 transcriptional activity and synaptic vesicular zinc in the brain of Huntington’s disease transgenic mouse. Cell Biosci. 10, 106 (2020).
570. Ayton, S. et al. Brain zinc deficiency exacerbates cognitive decline in the
571. Kaneko, M. et al. Zinc transporters ZnT3 and ZnT6 are downregulated in the spinal cords of patients with sporadic amyotrophic lateral sclerosis. J. Neurosci. Res. 93, 370-379 (2015).
572. Huang, J. et al. Structural basis of the zinc-induced cytoplasmic aggregation of the RNA-binding protein SFPQ. Nucleic Acids Res. 48, 3356-3365 (2020).
573. Gordon, P. M., Hamid, F., Makeyev, E. V. & Houart, C. A conserved role for the ALS-linked splicing factor SFPQ in repression of pathogenic cryptic last exons. Nat. Commun. 12, 1918 (2021).
574. Younas, N. et al. SFPQ and Tau: critical factors contributing to rapid progression of Alzheimer’s disease. Acta Neuropathol. 140, 317-339 (2020).
575. Bayik, D. & Lathia, J. D. Cancer stem cell-immune cell crosstalk in tumour progression. Nat. Rev. Cancer 21, 526-536 (2021).
576. Polyak, K. & Weinberg, R. A. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat. Rev. Cancer 9, 265-273 (2009).
577. Medema, J. P. Cancer stem cells: the challenges ahead. Nat. Cell Biol. 15, 338-344 (2013).
578. Singh, A. & Settleman, J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 29, 4741-4751 (2010).
579. Holohan, C. et al. Cancer drug resistance: an evolving paradigm. Nat. Rev. Cancer 13, 714-726 (2013).
580. Nabhan, C. et al. Caspase activation is required for gemcitabine activity in multiple myeloma cell lines. Mol. Cancer Ther. 1, 1221-1227 (2002).
581. Cui, X. et al. ZIP4 confers resistance to zinc deficiency-induced apoptosis in pancreatic cancer. Cell Cycle 13, 1180-1186 (2014).
582. Hessmann, E., Johnsen, S. A., Siveke, J. T. & Ellenrieder, V. Epigenetic treatment of pancreatic cancer: is there a therapeutic perspective on the horizon? Gut 66, 168-179 (2017).
583. Jiang, Y. et al. ZIP4 promotes non-small cell lung cancer metastasis by activating snail-N-cadherin signaling axis. Cancer Lett. 521, 71-81 (2021).
584. Wu, D. M. et al. SLC39A4 expression is associated with enhanced cell migration, cisplatin resistance, and poor survival in non-small cell lung cancer. Sci. Rep. 7, 7211 (2017).
585. Fan, Q., Zhang, W., Emerson, R. E. & Xu, Y. ZIP4 is a novel cancer stem cell marker in high-grade serous ovarian cancer. Cancers 12, 3692 (2020).
586. Ivan, C. et al. Epigenetic analysis of the Notch superfamily in high-grade serous ovarian cancer. Gynecol. Oncol. 128, 506-511 (2013).
587. Geles, K. G. et al. NOTCH3-targeted antibody drug conjugates regress tumors by inducing apoptosis in receptor cells and through transendocytosis into ligand cells. Cell Rep. Med. 2, 100279 (2021).
588. Farra, R. et al. Strategies for delivery of siRNAs to ovarian cancer cells. Pharmaceutics 11, 547 (2019).
589. Li, H. et al. ZIP10 drives osteosarcoma proliferation and chemoresistance through ITGA10-mediated activation of the PI3K/AKT pathway. J. Exp. Clin. Cancer Res. 40, 340 (2021).
590. Chen, Q. et al. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature 533, 493-498 (2016).
591. Maynard, A. et al. Therapy-induced evolution of human lung cancer revealed by single-cell RNA sequencing. Cell 182, 1232-1251.e1222 (2020).
592. Ni, C. et al. ZIP1(+) fibroblasts protect lung cancer against chemotherapy via connexin-43 mediated intercellular
593. Jia, C., Guo, Y. & Wu, F. G. Chemodynamic therapy via fenton and fenton-like nanomaterials: strategies and recent advances. Small 18, e2103868 (2022).
594. Ho, E., Wong, C. P. & King, J. C. Impact of zinc on DNA integrity and age-related inflammation. Free Radic. Biol. Med. 178, 391-397 (2022).
595. He, Y. et al. Evaluation of miR-21 and miR-375 as prognostic biomarkers in oesophageal cancer in high-risk areas in China. Clin. Exp. Metastasis. 34, 73-84 (2017).
596. Jin, J. et al. Methylation-associated silencing of miR-193b improves the radiotherapy sensitivity of esophageal cancer cells by targeting cyclin D1 in areas with zinc deficiency. Radiother. Oncol. 150, 104-113 (2020).
597. Kang, Y. et al. Advances in targeted therapy mainly based on signal pathways for nasopharyngeal carcinoma. Signal Transduct. Target Ther. 5, 245 (2020).
598. Criscitiello, C., Morganti, S. & Curigliano, G. Antibody-drug conjugates in solid tumors: a look into novel targets. J. Hematol. Oncol. 14, 20 (2021).
599. Nagayama, A., Vidula, N., Ellisen, L. & Bardia, A. Novel antibody-drug conjugates for triple negative breast cancer. Ther. Adv. Med. Oncol. 12, 1758835920915980 (2020).
600. Trail, P. A., Dubowchik, G. M. & Lowinger, T. B. Antibody drug conjugates for treatment of breast cancer: novel targets and diverse approaches in ADC design. Pharm. Ther. 181, 126-142 (2018).
601. Barroso-Sousa, R. & Tolaney, S. M. Clinical development of new antibody-drug conjugates in breast cancer: to infinity and beyond. BioDrugs 35, 159-174 (2021).
602. Lim, W. F., Mohamad Yusof, M. I., Teh, L. K. & Salleh, M. Z. Significant decreased expressions of CaN, VEGF, SLC39A6 and SFRP1 in MDA-MB-231 xenograft breast tumor mice treated with moringa oleifera leaves and seed residue (MOLSr) extracts. Nutrients 12, 2993 (2020).
603. Nolin, E. et al. Discovery of a ZIP7 inhibitor from a Notch pathway screen. Nat. Chem. Biol. 15, 179-188 (2019).
604. Chen, J. et al. Androgen dihydrotestosterone (DHT) promotes the bladder cancer nuclear AR-negative cell invasion via a newly identified membrane androgen receptor (mAR-SLC39A9)-mediated Gai protein/MAPK/MMP9 intracellular signaling. Oncogene 39, 574-586 (2020).
605. Seok, J. et al. Anti-oncogenic effects of dutasteride, a dual 5-alpha reductase inhibitor and a drug for benign prostate hyperplasia, in bladder cancer. J. Transl. Med. 21, 129 (2023).
606. Ashrafizadeh, M. et al. Noncoding RNAs as regulators of STAT3 pathway in gastrointestinal cancers: Roles in cancer progression and therapeutic response. Med. Res. Rev., 43, 1263-1321 (2023).
607. Yang, J. et al. Gene profile identifies zinc transporters differentially expressed in normal human organs and human pancreatic cancer. Curr. Mol. Med. 13, 401-409 (2013).
608. Ferrari, G., Thrasher, A. J. & Aiuti, A. Gene therapy using haematopoietic stem and progenitor cells. Nat. Rev. Genet. 22, 216-234 (2021).
609. Pramanik, S. K. et al. Nanoparticles for super-resolution microscopy: intracellular delivery and molecular targeting. Chem. Soc. Rev. 51, 9882-9916 (2022).
610. Wandt, V. K. et al. Ageing-associated effects of a long-term dietary modulation of four trace elements in mice. Redox Biol. 46, 102083 (2021).
611. Vrieling, F. & Stienstra, R. Obesity and dysregulated innate immune responses: impact of micronutrient deficiencies. Trends Immunol. 44, 217-230 (2023).
612. Wang, X. et al. The zinc transporter Slc39a5 controls glucose sensing and insulin secretion in pancreatic
613. Wang, G. et al. Metastatic cancers promote cachexia through ZIP14 upregulation in skeletal muscle. Nat. Med. 24, 770-781 (2018).
614. Yu, Y. et al. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood 136, 726-739 (2020).
615. Carvalho, C. S. et al. Blood cell responses and metallothionein in the liver, kidney and muscles of bullfrog tadpoles, Lithobates catesbeianus, following exposure to different metals. Environ. Pollut. 221, 445-452 (2017).
616. Chen, G. H. et al. Functional analysis of MTF-1 and MT promoters and their transcriptional response to zinc ( Zn ) and copper ( Cu ) in yellow catfish Pelteobagrus fulvidraco. Chemosphere 246, 125792 (2020).
617. Santoro, A. et al. The glutathione/metallothionein system challenges the design of efficient
618. Zaręba, N. & Kepinska, M. The function of transthyretin complexes with metallothionein in Alzheimer’s disease. Int J. Mol. Sci. 21, 9003 (2020).
619. Manso, Y. et al. Characterization of the role of metallothionein-3 in an animal model of Alzheimer’s disease. Cell Mol. Life Sci. 69, 3683-3700 (2012).
620. Kang, Y. C. et al. Cell-penetrating artificial mitochondria-targeting peptideconjugated metallothionein 1 A alleviates mitochondrial damage in Parkinson’s disease models. Exp. Mol. Med. 50, 1-13 (2018).
621. Carrasco, J. et al. Metallothionein-I and -III expression in animal models of Alzheimer disease. Neuroscience 143, 911-922 (2006).
622. Manso, Y. et al. Characterization of the role of the antioxidant proteins metallothioneins 1 and 2 in an animal model of Alzheimer’s disease. Cell Mol. Life Sci. 69, 3665-3681 (2012).
623. Nakamura, S. et al. Role of metallothioneins 1 and 2 in ocular neovascularization. Invest Ophthalmol. Vis. Sci. 55, 6851-6860 (2014).
624. Tiwari, R. et al. SPINK1 promotes colorectal cancer progression by downregulating Metallothioneins expression. Oncogenesis 4, e162 (2015).
625. Na, H. et al. Novel roles of DC-SIGNR in colon cancer cell adhesion, migration, invasion, and liver metastasis. J. Hematol. Oncol. 10, 28 (2017).
626. Mendes Garrido Abregú, F., Caniffi, C., Arranz, C. T. & Tomat, A. L. Impact of zinc deficiency during prenatal and/or postnatal life on cardiovascular and metabolic diseases: experimental and clinical evidence. Adv. Nutr. 13, 833-845 (2022).
627. Read, S. A., Obeid, S., Ahlenstiel, C. & Ahlenstiel, G. The role of zinc in antiviral immunity. Adv. Nutr. 10, 696-710 (2019).
628. Gomes, M. J. C., Martino, H. S. D. & Tako, E. Zinc-biofortified staple food crops to improve zinc status in humans: a systematic review. Crit. Rev. Food Sci. Nutr. 63, 4966-4978 (2023).
629. Gibson, R. S., King, J. C. & Lowe, N. A review of dietary zinc recommendations. Food Nutr. Bull. 37, 443-460 (2016).
630. Fairweather-Tait, S. J. & de Sesmaisons, A. Approaches used to estimate bioavailability when deriving dietary reference values for iron and zinc in adults. Proc. Nutr. Soc. 78, 1-7 (2018).
631. Duan, M. et al. Zinc nutrition and dietary zinc supplements. Crit. Rev. Food Sci. Nutr. 63, 1277-1292 (2023).
632. Brown, K. H. et al. International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr. Bull. 25, S99-S203 (2004).
633. Tran, C. D. et al. Zinc absorption as a function of the dose of zinc sulfate in aqueous solution. Am. J. Clin. Nutr. 80, 1570-1573 (2004).
634. Sapota, A. et al. The bioavailability of different zinc compounds used as human dietary supplements in rat prostate: a comparative study. Biometals 27, 495-505 (2014).
635. Chukwuma, C. I. et al. A comprehensive review on zinc(II) complexes as antidiabetic agents: The advances, scientific gaps and prospects. Pharm. Res. 155, 104744 (2020).
636. Jansen, J., Karges, W. & Rink, L. Zinc and diabetes-clinical links and molecular mechanisms. J. Nutr. Biochem. 20, 399-417 (2009).
637. Tang, Y. et al. Zinc supplementation partially prevents renal pathological changes in diabetic rats. J. Nutr. Biochem. 21, 237-246 (2010).
638. Jayawardena, R. et al. Effects of zinc supplementation on diabetes mellitus: a systematic review and meta-analysis. Diabetol. Metab. Syndr. 4, 13 (2012).
639. Ranasinghe, P. et al. Effects of Zinc supplementation on serum lipids: a systematic review and meta-analysis. Nutr. Metab. 12, 26 (2015).
640. Pompano, L. M. & Boy, E. Effects of dose and duration of zinc interventions on risk factors for type 2 diabetes and cardiovascular disease: a systematic review and meta-analysis. Adv. Nutr. 12, 141-160 (2021).
641. Özcelik, D. et al. Zinc supplementation attenuates metallothionein and oxidative stress changes in kidney of streptozotocin-induced diabetic rats. Biol. Trace Elem. Res. 150, 342-349 (2012).
642. Barman, S., Pradeep, S. R. & Srinivasan, K. Zinc supplementation alleviates the progression of diabetic nephropathy by inhibiting the overexpression of oxidative-stress-mediated molecular markers in streptozotocin-induced experimental rats. J. Nutr. Biochem. 54, 113-129 (2018).
643. Liu, F. et al. Zinc supplementation alleviates diabetic peripheral neuropathy by inhibiting oxidative stress and upregulating metallothionein in peripheral nerves of diabetic rats. Biol. Trace Elem. Res. 158, 211-218 (2014).
644. Foster, M., Chu, A., Petocz, P. & Samman, S. Zinc transporter gene expression and glycemic control in post-menopausal women with Type 2 diabetes mellitus. J. Trace Elem. Med Biol. 28, 448-452 (2014).
645. Sakurai, H., Yoshikawa, Y. & Yasui, H. Current state for the development of metallopharmaceutics and anti-diabetic metal complexes. Chem. Soc. Rev. 37, 2383-2392 (2008).
646. Tang, K. S. The current and future perspectives of zinc oxide nanoparticles in the treatment of diabetes mellitus. Life Sci. 239, 117011 (2019).
647. Patel, A. et al. Therapeutic value of zinc supplementation in acute and persistent diarrhea: a systematic review. PLoS One 5, e10386 (2010).
648. Chang, M. N. et al. Effects of different types of zinc supplement on the growth, incidence of diarrhea, immune function, and rectal microbiota of newborn dairy calves. J. Dairy Sci. 103, 6100-6113 (2020).
649. Bhandari, N. et al. Substantial reduction in severe diarrheal morbidity by daily zinc supplementation in young north Indian children. Pediatrics 109, e86 (2002).
650. Brooks, W. A. et al. Effect of weekly zinc supplements on incidence of pneumonia and diarrhoea in children younger than 2 years in an urban, low-income population in Bangladesh: randomised controlled trial. Lancet 366, 999-1004 (2005).
651. Dong, J., Li, H. & Min, W. Preparation, characterization and bioactivities of Athelia rolfsii exopolysaccharide-zinc complex (AEPS-zinc). Int J. Biol. Macromol. 113, 20-28 (2018).
652. Martinelli, D. et al. MEDNIK syndrome: a novel defect of copper metabolism treatable by zinc acetate therapy. Brain 136, 872-881 (2013).
653. Camarata, M. A., Ala, A. & Schilsky, M. L. Zinc maintenance therapy for wilson disease: a comparison between zinc acetate and alternative zinc preparations. Hepatol. Commun. 3, 1151-1158 (2019).
654. Duncan, A., Yacoubian, C., Watson, N. & Morrison, I. The risk of copper deficiency in patients prescribed zinc supplements. J. Clin. Pathol. 68, 723-725 (2015).
655. Guo, C. H. & Wang, C. L. Effects of zinc supplementation on plasma copper/zinc ratios, oxidative stress, and immunological status in hemodialysis patients. Int J. Med. Sci. 10, 79-89 (2013).
656. Hemilä, H. Zinc lozenges and the common cold: a meta-analysis comparing zinc acetate and zinc gluconate, and the role of zinc dosage. JRSM Open. 8, 2054270417694291 (2017).
657. Granum, B. Opinion of the Scientific Committee on Consumer safety (SCCS) Final opinion on water-soluble zinc salts used in oral hygiene products. Regul. Toxicol. Pharmacol. 99, 249-250 (2018).
658. Franklin, R. B. & Costello, L. C. The important role of the apoptotic effects of zinc in the development of cancers. J. Cell Biochem. 106, 750-757 (2009).
659. Hashemi, M. et al. Cytotoxic effects of intra and extracellular zinc chelation on human breast cancer cells. Eur. J. Pharmacol. 557, 9-19 (2007).
660. Richter, M. et al. Zinc chelators inhibit eotaxin, RANTES, and MCP-1 production in stimulated human airway epithelium and fibroblasts. Am. J. Physiol. Lung Cell Mol. Physiol. 285, L719-L729 (2003).
661. Albulescu, L. O. et al. Preclinical validation of a repurposed metal chelator as an early-intervention therapeutic for hemotoxic snakebite. Sci. Transl. Med. 12, eaay8314 (2020).
662. Nyborg, J. K. & Peersen, O. B. That zincing feeling: the effects of EDTA on the behaviour of zinc-binding transcriptional regulators. Biochem J. 381, e3-e4 (2004).
663. Hellmich, H. L. et al. Protective effects of zinc chelation in traumatic brain injury correlate with upregulation of neuroprotective genes in rat brain. Neurosci. Lett. 355, 221-225 (2004).
664. Bareggi, S. R. & Cornelli, U. Clioquinol: review of its mechanisms of action and clinical uses in neurodegenerative disorders. CNS Neurosci. Ther. 18, 41-46 (2012).
665. Doraiswamy, P. M. & Finefrock, A. E. Metals in our minds: therapeutic implications for neurodegenerative disorders. Lancet Neurol. 3, 431-434 (2004).
666. Labbé, R. F., Vreman, H. J. & Stevenson, D. K. Zinc protoporphyrin: a metabolite with a mission. Clin. Chem. 45, 2060-2072 (1999).
667. Faller, P. & Hureau, C. Bioinorganic chemistry of copper and zinc ions coordinated to amyloid-beta peptide. Dalton Trans. 7, 1080-1094 (2009).
668. Jackson, K. W. & Mahmood, T. M. Atomic absorption, atomic emission, and flame emission spectrometry. Anal. Chem. 66, 252r-279r (1994).
669. Carter, K. P., Young, A. M. & Palmer, A. E. Fluorescent sensors for measuring metal ions in living systems. Chem. Rev. 114, 4564-4601 (2014).
670. Denk, C. et al. Design, synthesis, and evaluation of a low-molecular-weight (11) C-labeled tetrazine for pretargeted PET imaging applying bioorthogonal in vivo click chemistry. Bioconjug. Chem. 27, 1707-1712 (2016).
671. Aper, S. J., Dierickx, P. & Merkx, M. Dual Readout BRET/FRET Sensors for Measuring Intracellular Zinc. ACS Chem. Biol. 11, 2854-2864 (2016).
672. Wei, T. et al. Directed evolution of the genetically encoded zinc(II) FRET sensor ZapCY1. Biochim Biophys. Acta Gen. Subj. 1866, 130201 (2022).
673. Bacart, J. et al. The BRET technology and its application to screening assays. Biotechnol. J. 3, 311-324 (2008).
674. Qin, Y. et al. Measuring steady-state and dynamic endoplasmic reticulum and Golgi Zn2+ with genetically encoded sensors. Proc. Natl Acad. Sci. Usa. 108, 7351-7356 (2011).
675. Chabosseau, P. et al. Mitochondrial and ER-targeted eCALWY probes reveal high levels of free
676. Hessels, A. M. et al. eZinCh-2: a versatile, genetically encoded FRET sensor for cytosolic and intraorganelle
677. Hessels, A. M., Taylor, K. M. & Merkx, M. Monitoring cytosolic and ER Zn(2+) in stimulated breast cancer cells using genetically encoded FRET sensors. Metallomics 8, 211-217 (2016).
678. Park, J. G., Qin, Y., Galati, D. F. & Palmer, A. E. New sensors for quantitative measurement of mitochondrial
679. Lin, Y. et al. ZIP4 is a novel molecular marker for glioma. Neuro Oncol. 15, 1008-1016 (2013).
680. Saravanan, R. et al. Zinc transporter LIV1: a promising cell surface target for triple negative breast cancer. J. Cell Physiol. 237, 4132-4156 (2022).
681. Gou, Y. et al. The transcription of ZIP9 is associated with the macrophage polarization and the pathogenesis of hepatocellular carcinoma. Front Immunol. 13, 725595 (2022).
682. Changizzadeh, P. N., Mukkamalla, S. K. R. & Armenio, V. A. Combined checkpoint inhibitor therapy causing diabetic ketoacidosis in metastatic melanoma. J. Immunother. Cancer 5, 97 (2017).
683. Sveen, A. et al. The exon-level biomarker SLC39A14 has organ-confined cancerspecificity in colorectal cancer. Int J. Cancer 131, 1479-1485 (2012).
684. Karandish, M. et al. The effect of curcumin and zinc co-supplementation on glycemic parameters in overweight or obese prediabetic subjects: a phase 2 randomized, placebo-controlled trial with a multi-arm, parallel-group design. Phytother. Res. 35, 4377-4387 (2021).
685. Islam, M. R. et al. Zinc supplementation for improving glucose handling in prediabetes: a double blind randomized placebo controlled pilot study. Diabetes Res Clin. Pract. 115, 39-46 (2016).
686. Foster, M., Petocz, P. & Samman, S. Inflammation markers predict zinc transporter gene expression in women with type 2 diabetes mellitus. J. Nutr. Biochem. 24, 1655-1661 (2013).
687. Nazem, M. R. et al. Zinc supplementation ameliorates type 2 diabetes markers through the enhancement of total antioxidant capacity in overweight patients. Postgrad. Med. J. 99, 862-867 (2023).
688. Fung, E. B. et al. Zinc supplementation improves markers of glucose homeostasis in thalassaemia. Br. J. Haematol. 190, e162-e166 (2020).
689. Bao, B. et al. Zinc decreases C-reactive protein, lipid peroxidation, and inflammatory cytokines in elderly subjects: a potential implication of zinc as an atheroprotective agent. Am. J. Clin. Nutr. 91, 1634-1641 (2010).
690. Ben Abdallah, S. et al. Twice-Daily Oral Zinc in the Treatment of Patients With Coronavirus Disease 2019: A Randomized Double-Blind Controlled Trial. Clin. Infect. Dis. 76, 185-191 (2023).
691. Rodriguez, J. A. M. et al. Effect and tolerability of a nutritional supplement based on a synergistic combination of
692. Faghfouri, A. H. et al. Regulation of NLRP3 inflammasome by zinc supplementation in Behçet’s disease patients: a double-blind, randomized placebocontrolled clinical trial. Int Immunopharmacol. 109, 108825 (2022).
693. Faghfouri, A. H. et al. Immunomodulatory and clinical responses to zinc gluconate supplementation in patients with Behçet’s disease: a doubleblind, randomized placebo-controlled clinical trial. Clin. Nutr. 41, 1083-1092 (2022).
694. Bobat, R. et al. Safety and efficacy of zinc supplementation for children with HIV1 infection in South Africa: a randomised double-blind placebo-controlled trial. Lancet 366, 1862-1867 (2005).
695. Roy, S. K. et al. Zinc supplementation in children with cholera in Bangladesh: randomised controlled trial. BMJ 336, 266-268 (2008).
696. Veenemans, J. et al. Effect of supplementation with zinc and other micronutrients on malaria in Tanzanian children: a randomised trial. PLoS Med. 8, e1001125 (2011).
697. Fung, E. B. et al. Zinc supplementation improves bone density in patients with thalassemia: a double-blind, randomized, placebo-controlled trial. Am. J. Clin. Nutr. 98, 960-971 (2013).
698. Guo, C. H., Chen, P. C., Hsu, G. S. & Wang, C. L. Zinc supplementation alters plasma aluminum and selenium status of patients undergoing dialysis: a pilot study. Nutrients 5, 1456-1470 (2013).
699. Kobayashi, H. et al. Oral zinc supplementation reduces the erythropoietin responsiveness index in patients on hemodialysis. Nutrients 7, 3783-3795 (2015).
700. Lin, L. C., Que, J., Lin, L. K. & Lin, F. C. Zinc supplementation to improve mucositis and dermatitis in patients after radiotherapy for head-and-neck cancers: a double-blind, randomized study. Int J. Radiat. Oncol. Biol. Phys. 65, 745-750 (2006).
701. Ribeiro, S. M. et al. Effect of zinc supplementation on antioxidant defenses and oxidative stress markers in patients undergoing chemotherapy for colorectal cancer: a placebo-controlled, prospective randomized trial. Biol. Trace Elem. Res. 169, 8-16 (2016).
702. Qiao, Y. L. et al. Total and cancer mortality after supplementation with vitamins and minerals: follow-up of the Linxian General Population Nutrition Intervention Trial. J. Natl Cancer Inst. 101, 507-518 (2009).
703. Ye, W. et al. A sensitive FRET biosensor based on carbon dots-modified nanoporous membrane for 8-hydroxy-2′-Deoxyguanosine (8-OHdG) detection with Au@ZIF-8 nanoparticles as signal quenchers. Nanomaterials 10, 2044 (2020).
704. Qin, Y. et al. Development of an optical
705. Han, Y., Goldberg, J. M., Lippard, S. J. & Palmer, A. E. Superiority of SpiroZin2 Versus FluoZin-3 for monitoring vesicular
706. Nolan, E. M. & Lippard, S. J. Small-molecule fluorescent sensors for investigating zinc metalloneurochemistry. Acc. Chem. Res. 42, 193-203 (2009).
707. Ueno, S. et al. Mossy fiber Zn2+ spillover modulates heterosynaptic N-methyl-D-aspartate receptor activity in hippocampal CA3 circuits. J. Cell Biol. 158, 215-220 (2002).
708. Kao, Y. Y. et al. Zinc oxide nanoparticles interfere with zinc ion homeostasis to cause cytotoxicity. Toxicol. Sci. 125, 462-472 (2012).
709. Sensi, S. L. et al. A new mitochondrial fluorescent zinc sensor. Cell Calcium 34, 281-284 (2003).
710. You, Y. et al. Phosphorescent sensor for biological mobile zinc. J. Am. Chem. Soc. 133, 18328-18342 (2011).
711. Meeusen, J. W., Tomasiewicz, H., Nowakowski, A. & Petering, D. H. TSQ (6-methoxy-8-p-toluenesulfonamido-quinoline), a common fluorescent sensor for cellular zinc, images zinc proteins. Inorg. Chem. 50, 7563-7573 (2011).
© The Author(s) 2023
Department of Anatomical and Cellular Pathology, State Key Laboratory of Translational Oncology, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong, China; State Key Laboratory of Digestive Disease, Institute of Digestive Disease, The Chinese University of Hong Kong, Hong Kong, China; CUHK-Shenzhen Research Institute, The Chinese University of Hong Kong, Shenzhen, China; Department of Pathology, Nanfang Hospital and Basic Medical College, Southern Medical University, Guangdong Province Key Laboratory of Molecular Tumor Pathology, Guangzhou, China; Institute of Biomedical Research, Taihe Hospital, Hubei University of Medicine, Shiyan, China; Department of Pediatrics, The Chinese University of Hong Kong, Hong Kong, China and Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Hong Kong, China
Correspondence: Wei Kang (weikang@cuhk.edu.hk) or Ka Fai To (kfto@cuhk.edu.hk)
These authors contributed equally: Bonan Chen, Peiyao Yu
