DOI: https://doi.org/10.1186/s12870-024-04795-1
PMID: https://pubmed.ncbi.nlm.nih.gov/38383286
تاريخ النشر: 2024-02-21
التطبيق الخارجي لثيو يوريا الغنية بالكبريت (STU) للتخفيف من الآثار السلبية لإجهاد الكوبالت في القمح
الملخص
يؤثر إجهاد المعادن الثقيلة على نمو المحاصيل وعوائدها حيث يتأثر نمو القمح (Triticum aestivum L.) سلبًا تحت إجهاد المعادن الثقيلة. درست الدراسة تأثير كلوريد الكوبالت (
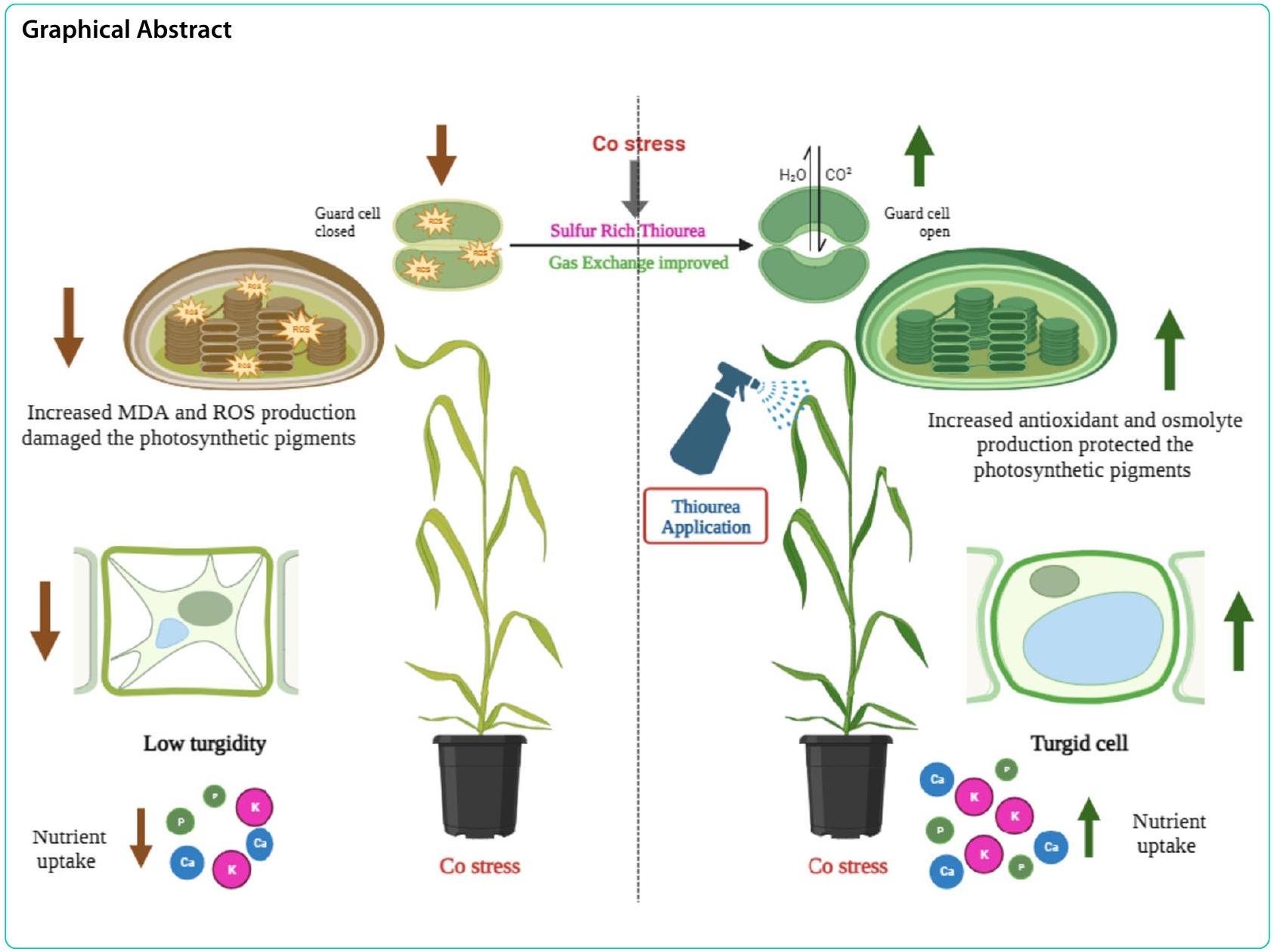
مقدمة
الأراضي الأكثر عرضة لحدوث محتويات عالية من الكوبالت في التربة ذات الأصل البشري. غالبًا ما توجد التربة ذات محتوى الكوبالت العالي بالقرب من عمليات صهر المعادن، وتصنيع الآلات، وعمليات التعدين. مثل المعادن الثقيلة الأخرى، يتسبب الكوبالت في تلف الخلايا ويقلل من نمو النباتات وإنتاجيتها من خلال زيادة نشاط عمليات هابر-وايس وفينتون، مما يؤدي إلى إنتاج أنواع الأكسجين التفاعلية. ت destabilize تركيزات أعلى من الكوبالت مسارات التمثيل الغذائي المتعددة وتسبب ضررًا أكسيديًا للجزيئات الحيوية، مما يؤدي إلى تأكسد الدهون، وتدهور الأغشية، وكربوكسيل البروتينات. تؤدي المستويات المعززة من الكوبالت في النباتات إلى تشويه هيكل البلاستيدات الخضراء، مما يؤدي في النهاية إلى تعطيل امتصاص ثاني أكسيد الكربون بسبب تقليل امتصاص الكربون. تم إزعاج الإنزيمات المستخدمة في المسار الحيوي لتخليق الكلوروفيل بسبب التشويه في هيكل الريبسكو (ريبولوز-1،5-بيسفوسفات كربوكسيلاز/أوكسيجيناز) بسبب استبدال ذرة المغنيسيوم بالكوبالت في الريبسكو، وهو بروتين حاسم لعملية التمثيل الضوئي. أنواع الأكسجين التفاعلية مثل الجذور الحرة لأنيون السوبر أوكسيد.
بيروكسيد (
تطبيق العناصر الغذائية المعدنية أو المنظمات الحيوية، التي تتحكم في آليات فسيولوجية وكيميائية حيوية متعددة على مستوى الأيض والنبات ككل، يحسن الدفاع الطبيعي للنباتات ضد الإجهاد غير الحيوي. الثيويوريا هو محفز لنمو النباتات غني بالكبريت يقوم بتعديل تطور النبات ويمنع بشكل فعال الأضرار التأكسدية التي تفرضها الضغوط غير الحيوية. إنه مادة غير فسيولوجية تعتمد على الثيول وتعمل ككاشف للجذور الحرة، تحتوي على 42% من الكبريت (S) و36% من النيتروجين (N) ويمكن أن تخفض عدم التوازن الأكسدي الناتج عن الإجهاد والإصابات المختلفة للنبات. التطبيق الخارجي للثيويوريا يعزز تحمل المحاصيل للإجهاد. مما يؤدي إلى زيادة في النمو وإنتاجية المحاصيل، واستقرار الأغشية، والقدرة المضادة للأكسدة، وكفاءة التمثيل الضوئي. وقد أفادت عدة دراسات أن تطبيق الثيويوريا يلعب دورًا مهمًا في التكيف مع مجموعة متنوعة من الضغوط غير الحيوية من خلال تحسين المؤشرات المورفوفسيولوجية والكيميائية الحيوية ومساهمات العائد في عدة محاصيل مثل القمح، والذرة، والكانولا، والكميلينا، والشعير.
تم الإبلاغ عن التطبيق الخارجي لـ STU لتقليل الآثار السلبية للإجهاد غير الحيوي في دراسات سابقة. ومع ذلك، فإن دور STU في تخفيف الآثار السامة لإجهاد الكوبالت في أصناف القمح المختلفة محدود ويتطلب مزيدًا من التحقيق. لذلك، افترضت هذه الدراسة أن تطبيقات STU قد تخفف من الآثار السامة لإجهاد الكوبالت في القمح. تم إجراء التحقيق الحالي لتقييم الدور التحسيني لـ STU في أنظمة الدفاع النباتية تحت إجهاد الكوبالت من خلال تحسين الصفات الفسيولوجية للنباتات وأنشطة مضادات الأكسدة في القمح.
المواد والأساليب
نباتات القمح المزروعة تحت ضغط كلوريد الكوبالت في بيت الأسلاك في الحديقة النباتية القديمة، قسم علم النبات، جامعة الزراعة في فيصل آباد
تحديد السمات الشكلية
تحديد أصباغ التمثيل الضوئي
تحديد بيروكسيد الهيدروجين والمالونديالديهايد
تحديد أنشطة مضادات الأكسدة الإنزيمية
تحديد مضادات الأكسدة غير الإنزيمية
تحديد الحماة الأسموزية
. تم قياس البرولين في النباتات من خلال اتباع طريقة بايتس وآخرون [51].
تحديد العناصر الغذائية المعدنية
إرشادات النباتات
التحليل الإحصائي
النتائج
السمات الشكلية
| الأصناف | العلاجات | الوزن الطازج للساق (ملغ/غ FW) | الوزن الطازج للجذر (ملغ/غ FW) | الوزن الجاف للساق (ملغ/غ FW) | الوزن الجاف للجذر (ملغ/غ FW) | طول الساق (سم) | طول الجذر (سم) | مساحة الورقة (سم 2) |
| FSD-2008 | التحكم |
|
|
|
|
|
|
|
| STU |
|
|
|
|
|
|
|
|
| Co |
|
|
|
|
|
|
|
|
| STU+Co |
|
|
|
|
|
|
|
|
| Zincol-2016 | التحكم |
|
|
|
|
|
|
|
| STU |
|
|
|
|
|
|
|
|
| Co |
|
|
|
|
|
|
|
|
| STU+Co |
|
|
|
|
|
|
|
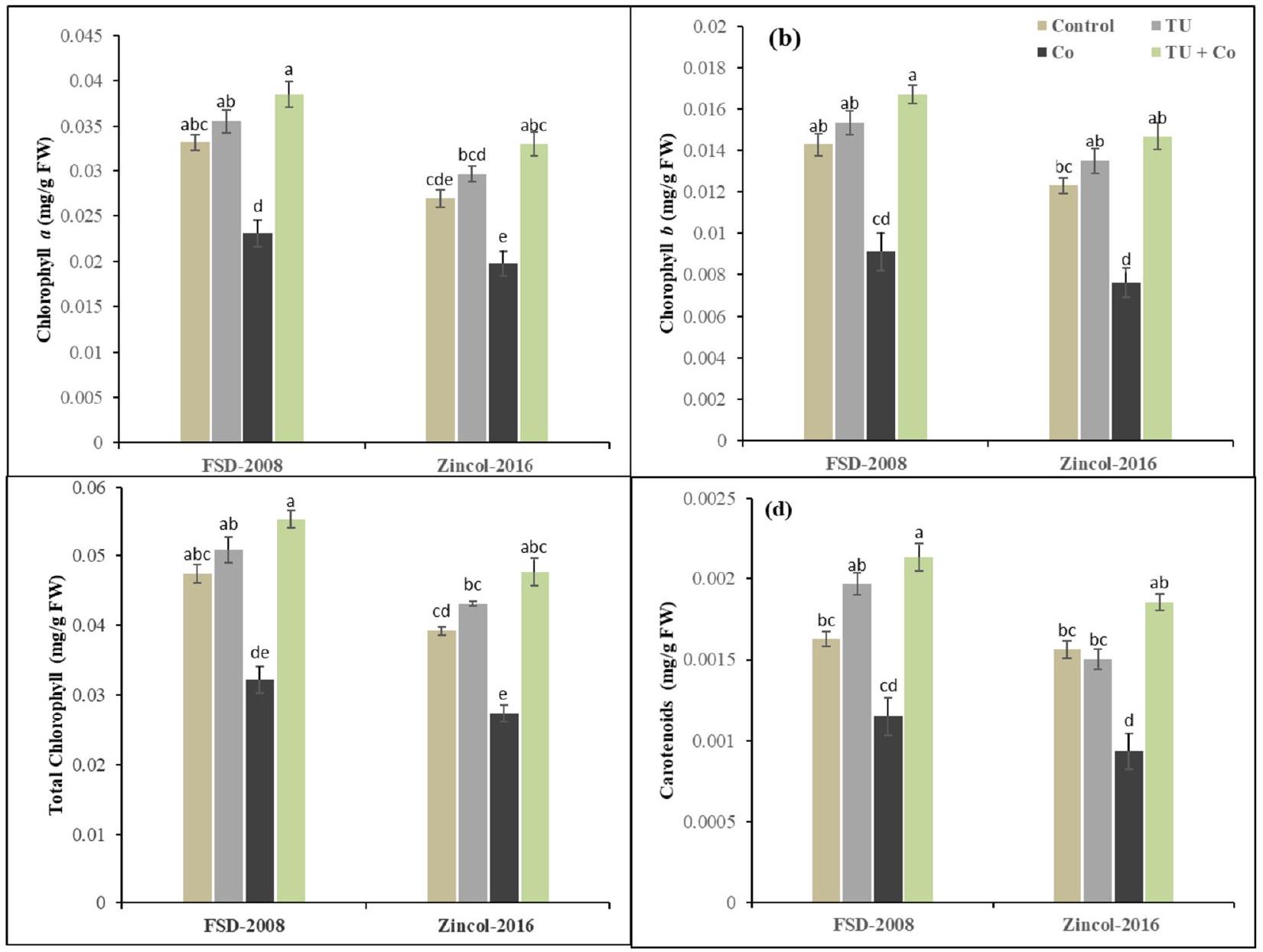
أصباغ التمثيل الضوئي
بالنسبة لأصباغ التمثيل الضوئي. كشفت النتائج أن ضغط الكوبالت قلل من الكلوروفيل
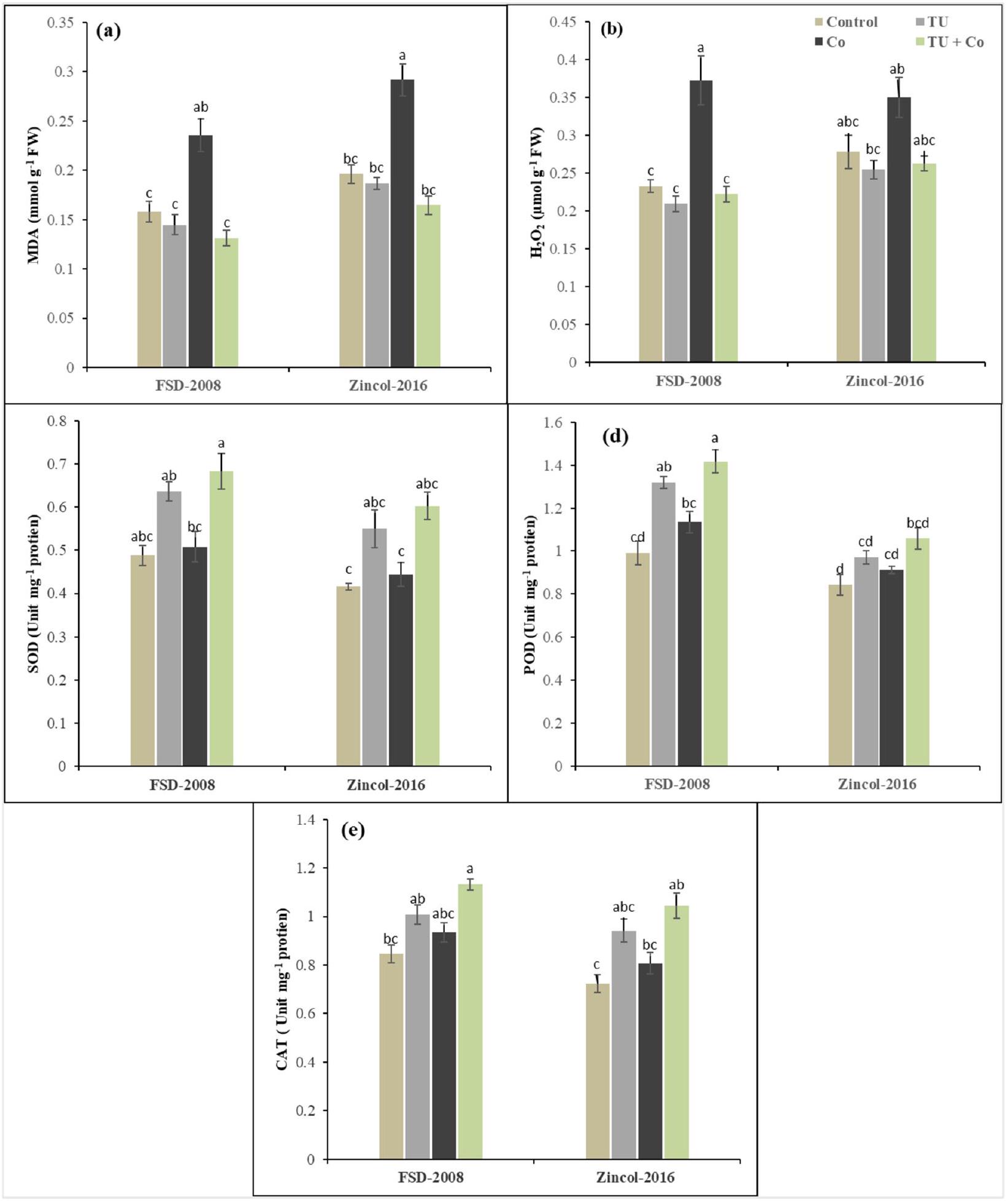
استجابة MDA و
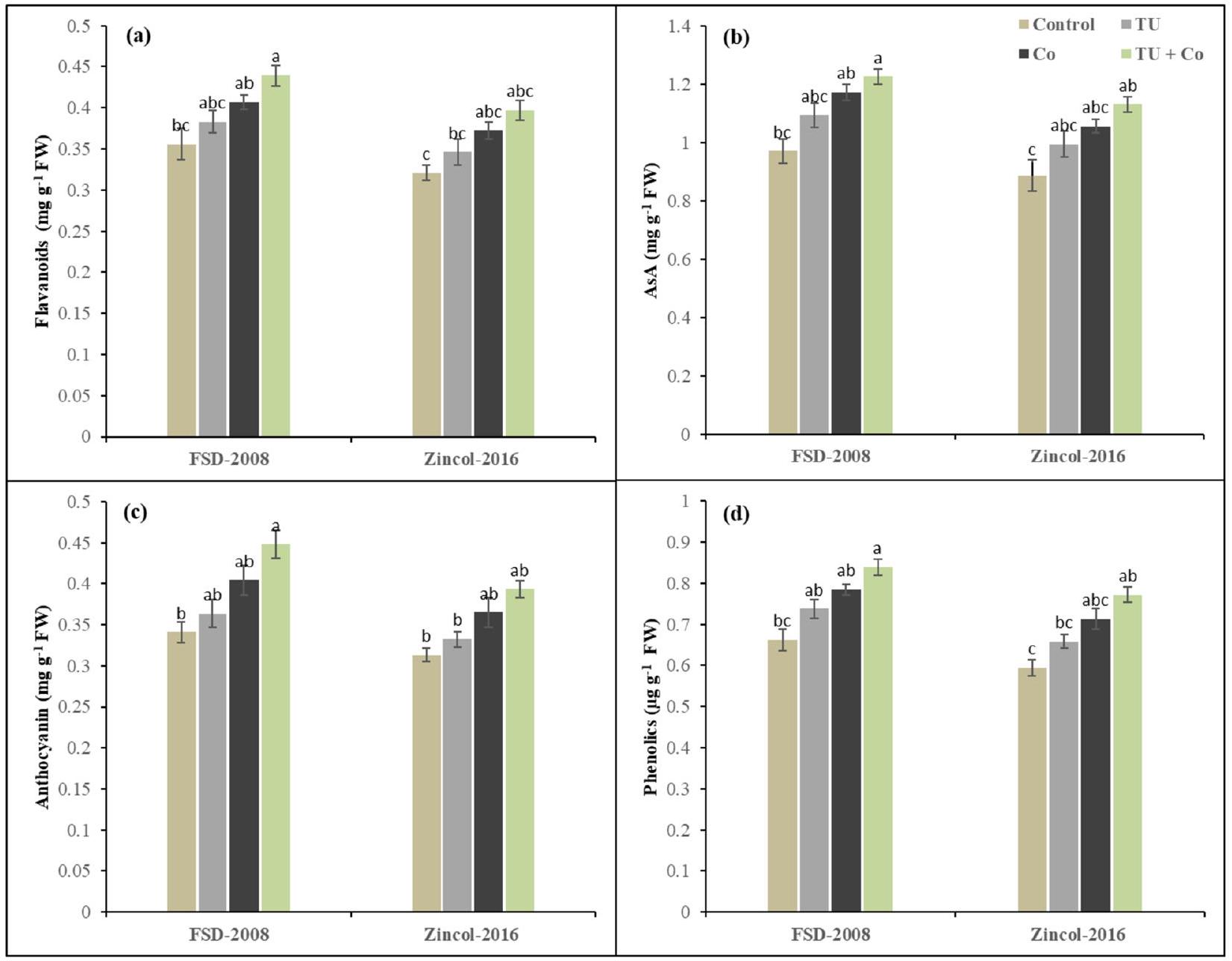
مضادات الأكسدة غير الإنزيمية
حماة الأسموزية
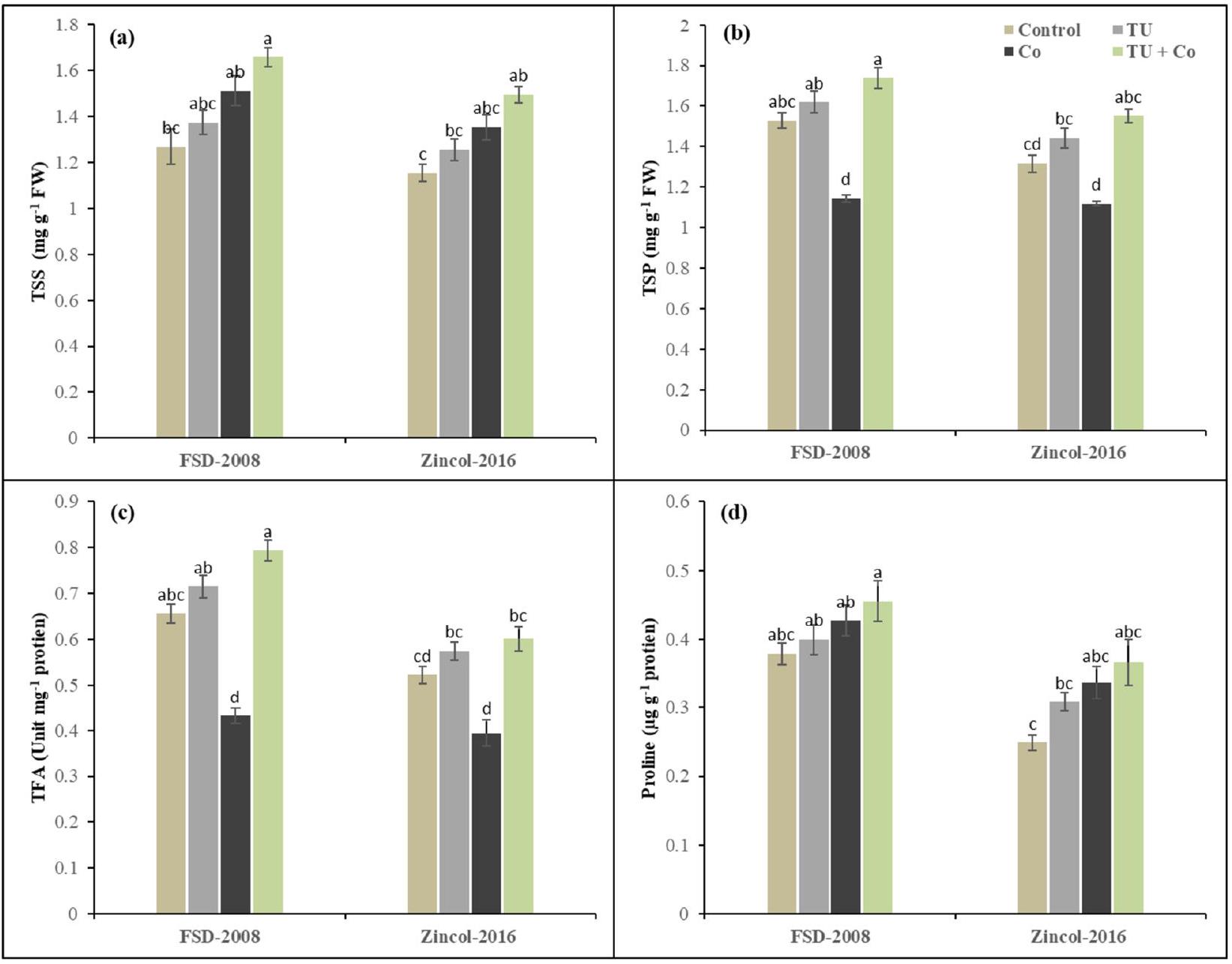
غير ذي دلالة بالنسبة للمواد الحافظة للأسموزة. وقد أظهرت النتائج أن إجهاد الكوبالت زاد من TSS بـ (
المغذيات المعدنية
تحليل الارتباط وخريطة الحرارة
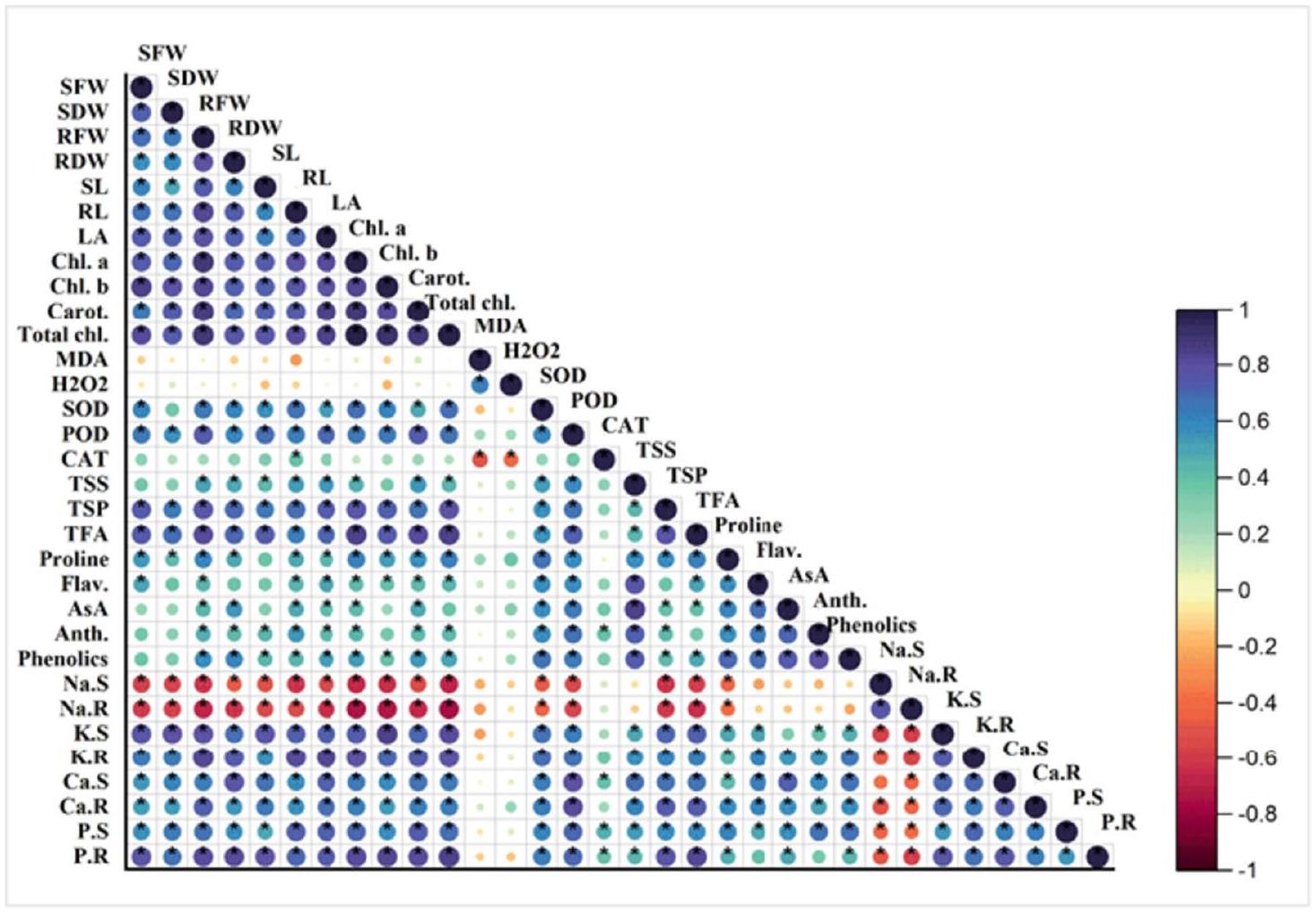
تم إنشاء تحليل خريطة الحرارة عبر المؤشرات الشكلية، والتمثيل الضوئي، والبيوكيميائية، ومحتويات الأيونات في كلا الصنفين من القمح. تُظهر اختلافات الألوان في الصناديق قوة التفاعل بين المؤشرات المذكورة أعلاه والعلاجات. كانت ألوان المقياس تتراوح من الأزرق (إيجابي بشدة) إلى الأحمر الداكن (سلبي بشدة) وكانت مرتبطة ارتباطًا وثيقًا بقوة تدرج اللون المستخدم في صناديق خريطة الحرارة. تم ملاحظة أعلى تعزيز في المؤشرات الشكلية، وأصباغ التمثيل الضوئي، ومضادات الأكسدة الإنزيمية وغير الإنزيمية، والأسموليتات، والفوسفور، والبوتاسيوم، وأيونات الكالسيوم، بينما كان أدنى مستوى من أيونات الصوديوم و
نقاش
| أنواع زراعية | علاجات | أطلق
|
جذر
|
أطلق
|
جذر
|
أطلق
|
جذر
|
إطلاق P (ملغ/غ من الوزن الطازج) | جذر P (ملغ/غ من الوزن الطازج) |
| FSD-2008 | تحكم |
|
|
|
|
|
|
|
|
| STU |
|
|
|
|
|
|
|
|
|
| كو |
|
|
|
|
|
|
|
|
|
| STU+Co |
|
|
|
|
|
|
|
|
|
| زين-كول-2016 | تحكم |
|
|
|
|
|
|
|
|
| STU |
|
|
|
|
|
|
|
|
|
| كو |
|
|
|
|
|
|
|
|
|
| STU+Co |
|
|
|
|
|
|
|
|
أظهرت نتائج هذه الدراسة أنه تحت إجهاد الكوبالت، تلعب تطبيقات STU دورًا إيجابيًا في تحسين الخصائص الشكلية لصنفي القمح (الجدول 1). أدت سمية الكوبالت إلى تقليل المعلمات الشكلية مثل الوزن الطازج والجاف، وطول الجذر والساق لكلا صنفي القمح كما تم الإبلاغ عنه في الدراسات السابقة [55،56] من خلال تقليل امتصاص العناصر الغذائية، حالة الماء في النبات، وموصلية الثغور [14]. في الوقت نفسه، لوحظت أقصى تقليل في صنف القمح Zincol-2016 مقارنة بـ FSD-2008. ومع ذلك، حسنت تطبيقات STU الخارجية مؤشرات نمو النبات بما في ذلك طول الساق والجذر، والوزن الطازج والجاف في كلا صنفي القمح المزروعة تحت إجهاد الكوبالت كما وجدت في الدراسات السابقة [57] بينما أظهر FSD-2008 استجابة قصوى تجاه STU وأظهر أقصى تحسين في معلمات النمو من خلال إزالة سمية الكوبالت
سمية الإجهاد مقارنة بـ Zincol-2016. قد يحسن تطبيق STU مساحة سطح الورقة مما يساعد على التقاط المزيد من الضوء، وبالتالي تعزيز تثبيت الكربون الذي ينظم إنتاج المواد المساعدة ويوجهها نحو تطوير المصارف مما يعزز تطوير المحاصيل ومعلمات العائد. تحت ظروف الإجهاد، عزز الثيويوريا السعة الأسموزية من خلال تحسين انتفاخ الخلايا مما سمح للنبات بالحفاظ على توازن مياهه لزيادة معدل النتح مما أدى بدوره إلى تحسين المؤشرات الشكلية في النباتات. حسنت تطبيقات STU الورقية عند
مما يؤدي إلى إنشاء الكاروتينات والكلوروفيل. بسبب ارتفاع إمكانات أكسدة الكوبالت وتثبيط الآليات الإنزيمية المسؤولة عن إنتاج الكلوروفيل، قد يكون هناك ارتباط بين انخفاض تركيز الكلوروفيل تحت الظروف المجهدة والخطوات الاختزالية المثبطة في مسار تخليق الكلوروفيل. تحت إجهاد الكوبالت، يؤدي إنشاء الجذور الحرة للأكسجين إلى إتلاف غشاء الخلية،
مما يؤدي إلى انهيار البلاستيدات الخضراء أو التخليق الحيوي للمنتجات الوسيطة في العملية مما يؤدي إلى تطوير الكاروتينات والكلوروفيل [60-62]. ومع ذلك، حسنت تطبيقات STU الورقية تكوين صبغات التمثيل الضوئي التي تلعب دورًا إيجابيًا في كفاءة التمثيل الضوئي التي تعتبر ضرورية لتطوير ونمو كلا صنفي القمح بينما أظهر FSD-2008 أقصى تحسين في صبغات التمثيل الضوئي مقارنة بـ Zincol-2016 كما تم الإبلاغ عنه في التحقيقات السابقة [63-65]. تم تقليل الأضرار التأكسدية لصبغات التمثيل الضوئي وخاصة مستويات الكاروتينات والكلوروفيل من خلال تطبيق STU في كلا الصنفين من القمح تحت إجهاد الكوبالت [66]. قد يؤدي استخدام الثيويوريا إلى زيادة في التمثيل الضوئي بسبب دوره في الفيريدوكسين الذي يعزز تخليق والحفاظ على محتويات الكلوروفيل مما يعزز معدل التمثيل الضوئي وكفاءة الامتصاص التي تدعم تطوير ونمو النباتات [67].
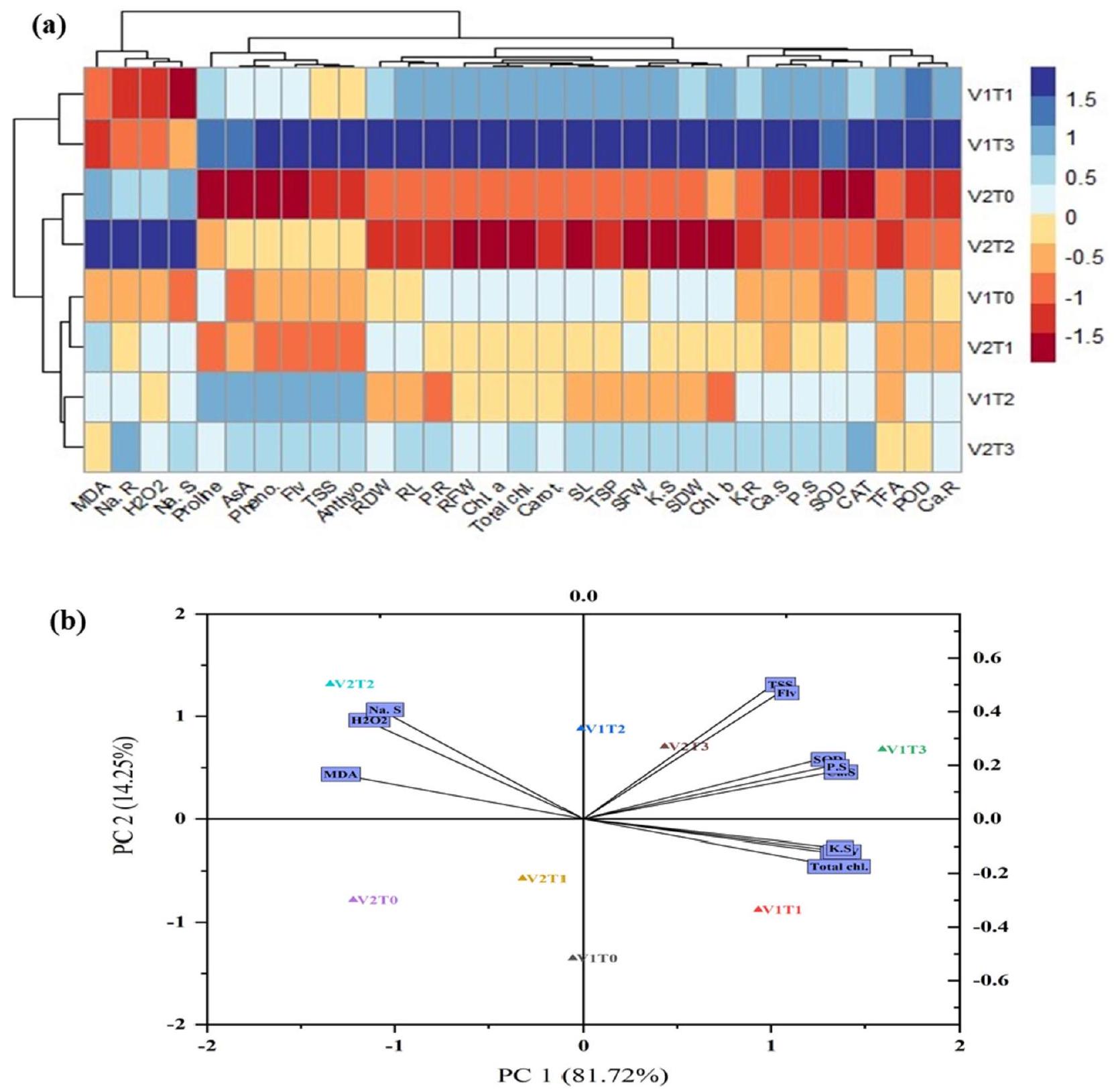
تخليق المواد الكيميائية المزالة للسموم لـ ROS. يكمن الاختلاف في كيفية تراكم النباتات لهذه المركبات وتجنب الأضرار الخلوية للعمل تحت ظروف صعبة (الأشكال 3، 4 و 5). يعزز STU استقرار غشاء الخلية من خلال قمع MDA وكونه كاشف مباشر للجذور الحرة الذي يعادل الجذور الحرة الداخلية
مرتبط ارتباطًا وثيقًا بتراكمها الواسع، خاصة في الجذور، كما يمنع دخول وربط الأيونات الأساسية مثل
الخاتمة
شكر وتقدير
مساهمات المؤلفين
تمويل
توفر البيانات والمواد
الإعلانات
موافقة الأخلاقيات والموافقة على المشاركة
موافقة على النشر
المصالح المتنافسة
تفاصيل المؤلف
نُشر على الإنترنت: 21 فبراير 2024
References
- Zulfiqar U, Ahmad M, Valipour M, Ishfaq M, Maqsood MF, Iqbal R, Ali MF, Roy R, El Sabagh A. Evaluating Optimum Limited Irrigation and Integrated Nutrient Management Strategies for Wheat Growth, Yield and Quality. Hydrology. 2023;10(3):56.
- Victoria O, Idorenyin U, Asana M, Jia L, Shuoshuo L, Yang S, Okoi IM, Ping A, Egrinya EA. Seed treatment with 24-epibrassinolide improves wheat germination under salinity stress. Asian J Agric Biol. 2023;2023(3). https:// doi.org/10.35495/ajab.2022.076.
- Taratima W, Kunpratum N, Maneerattanarungroj P. Effect of salinity stress on physiological aspects of pumpkin (Cucurbita moschata
4. Mukhtar T, Rehman SU, Sultan T, Munis FH, Chaudhary HJ. Induction of heat tolerance in tomato cultivar with heat tolerant bacteria under field condition. Asian J Agric Biol. 2022;2022(2):202103112. https://doi.org/10. 35495/ajab.2021.03.112.
5. Abd El-Fattah DA, Hashem FA, Abd-Elrahman SH. Impact of applying organic fertilizers on nutrient content of soil and lettuce plants, yield quality and benefit-cost ratio under water stress conditions. Asian J Agric Biol. 2022;2022(2):202102086. https://doi.org/10.35495/ajab.2021.02.086.
6. Qasim M, Ahmed W, Safdar U, Maqbool R, Bin Sajid H, Noor H. Effect of drought stress on fertile tillers of wheat genotypes (Triticum aestivum L). Int J Agric Biosci. 2022;11:172-80.
7. Zulfiqar U, Haider FU, Maqsood MF, Mohy-Ud-Din W, Shabaan M, Ahmad M, Kaleem M, Ishfaq M, Aslam Z, Shahzad B. Recent advances in microbial-assisted remediation of cadmium-contaminated soil. Plants. 2023;12(17):3147.
8. Zulfiqar U, Jiang W, Xiukang W, Hussain S, Ahmad M, Maqsood MF, Ali N, Ishfaq M, Kaleem M, Haider FU, Farooq N. Cadmium phytotoxicity, tolerance, and advanced remediation approaches in agricultural soils. Front Plant Sci. 2022;13:773815.
9. Hu X, Wei X, Ling J, Chen J. Cobalt: an essential micronutrient for plant growth? Front Plant Sci. 2021;12:768523.
10. Leyssens L, Vinck B, Van Der Straeten C, Wuyts F, Maes L. Cobalt toxicity in humans-A review of the potential sources and systemic health effects. Toxicology. 2017;387:43-56.
11. Jiang M, Wang K, Wang Y, Zhao Q, Wang W. Technologies for the cobalt-contaminated soil remediation: a review. Sci Total Environ. 2022;813:151908.
12. Eskander SB, Saleh HM. Heavy metal-induced oxidative stress and related cellular process. In: Cellular and molecular phytotoxicity of heavy metals. 2020. p. 99-123.
13. Bano A, Gupta A, Rai S, Fatima T, Sharma S, Pathak N. Mechanistic role of reactive oxygen species and its regulation via the antioxidant system under environmental stress. Plant Stress Physiol Perspec Agric. 2021;23:1-8.
14. Singh A, Roychoudhury A. Protective chemicals and metabolites in stabilizing photosynthesis and respiration machinery during abiotic stresses. In: Photosynthesis and respiratory cycles during environmental stress response in plants. FL, USA: Apple Academic Press Inc.; 2022. p. 351-72.
15. Ozfidan-Konakci C, Yildiztugay E, Elbasan F, Kucukoduk M, Turkan I. Hydrogen sulfide
16. Sachdev S, Ansari SA, Ansari MI, Fujita M, Hasanuzzaman M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants. 2021;10(2):277.
17. Ghous M, Amir Bakhtavar M, Nawaz F. Halophyte quinoa: a potential hyperaccumulator of heavy metals for phytoremediation. Asian J Agric Biol. 2022;4:1-9.
18. Luqman M, Shahbaz M, Maqsood MF, Farhat F, Zulfiqar U, Siddiqui MH, Masood A, Aqeel M, Haider FU. Effect of strigolactone on growth, photosynthetic efficiency, antioxidant activity, and osmolytes accumulation in different maize (Zea mays L.) hybrids grown under drought stress. Plant Signal Behav. 2023;18(1):2262795.
19. Li Y, Mo X, Xiong J, Huang K, Zheng M, Jiang Q, Su G, Ou Q, Pan H, Jiang C. Deciphering the probiotic properties and safety assessment of a novel multi-stress-tolerant aromatic yeast Pichia kudriavzevii HJ2 from marine mangroves. Food Biosci. 2023;56:103248.
20. Patade VY, Nikalje GC, Srivastava S. Role of thiourea in mitigating different environmental stresses in plants. Protective chemical agents in the amelioration of plant abiotic stress: biochemical and molecular perspectives. Jun. 2020;22:467-82.
21. Ahmad M, Waraich EA, Zulfiqar U, Ullah A, Farooq M. Thiourea application increases seed and oil yields in camelina under heat stress by modulating the plant water relations and antioxidant defense system. J Soil Sci Plant Nut. 2023;23(1):290-307.
22. Ahmad S, Aziz AU, Ullah A, Raza MA. Effect of vermicompost and organic matter in enhancing wheat tolerance against drought stress. Int J Agric Biosci. 2022;11(3):165-7.
23. Srivastava AK, Sablok G, Hackenberg M, Deshpande U, Suprasanna P. Thiourea priming enhances salt tolerance through co-ordinated regulation of microRNAs and hormones in Brassica juncea. Sci Rep. 2017;7(01):45490.
24. Ahmad M, Waraich EA, Zulfiqar U, Hussain S, Yasin MU, Farooq M. Thiourea application improves the growth and seed and oil yields in canola by modulating gas exchange, antioxidant defense, and osmoprotection under heat stress. J Soil Sci Plant Nut. 2023;22(03):3655-66.
25. Zhang LJ, Buatois LA, Mángano MG. Potential and problems in evaluating secular changes in the diversity of animal-substrate interactions at ichnospecies rank. Terra Nova. 2022;34(5):433-40.
26. Haroon M, Anas M, Naurin I, Afzal R, Irfan U, Tariq H, Idrees F, Taj MH, Rukh M. Autoimmunity in plants; a powerful weapon in kingdom plantae to combat stresses. Int J Agric Biosci. 2023;12(3):159-64.
27. Singh RP, Singh D. Response of lentil to thiourea application under rain fed conditions of Central India. Int J Curr Microbiol App Sci. 2017;06:2556-60.
28. Wahid A, Basra S, Farooq M. Thiourea: a molecule with Immense Biological significance for plants. Int J Agri Bio. 2017;19(04):23-32.
29. Naz S, Perveen S. Response of wheat (Triticum aestivum L. Var. Gal-axy-2013) to pre-sowing seed treatment with thiourea under drought stress. Pak J Bot. 2021;53:1209-17.
30. Yadav T, Kumar A, Yadav R, Yadav G, Kumar R, Kushwaha M. Salicylic acid and thiourea mitigate the salinity and drought stress on physiological traits governing yield in pearl millet-wheat. Saudi J Biol Sci. 2020;27:2010-7.
31. Baqer RA, AI-Kaaby HK, Adul-Qadir LH. Antioxidant responses in wheat plants (Triticum aestivum L.) treated with thiourea. Plant Arch. 2020;20:717-22.
32. Haider FU, Virk AL, Rehmani MIA, Skalicky M, Ata-ul-Karim ST, Ahmad N, Soufan W, Brestic M, Sabagh AE, Liqun C. Integrated application of thiourea and biochar improves maize growth, antioxidant activity and reduces cadmium bioavailability in cadmium-contaminated soil. Front Plant Sci. 2022;12:809322.
33. Ahmad M, Waraich EA, Zulfiqar U, Ullah A, Farooq M. Thiourea application improves heat tolerance in camelina (Camelina sativa L. Crantz) by modulating gas exchange, antioxidant defense and osmoprotection. Ind Crop Prod. 2021;170:113826.
34. Ahmad M, Waraich EA, Hussain S, Ayyub CM, Ahmad Z, Zulfiqar U. Improving heat stress tolerance in Camelina sativa and Brassica napus through Thiourea seed priming. J Plant Growth Reg. 2021;3:1-7.
35. Ahmad M, Waraich EA, Hussain S, Zulfiqar U, Teshome FT, Gastelbondo M, Imran M, Farooq M. Exogenous application of thiourea improves the growth, seed yield, and seed fatty acid profile in late sown camelina. J Soil Sci Plant Nutr. 2023;23(01):1306-25.
36. Smilanick JL, Hershberger W, Bonde MR, Nester SE. Germinability of teliospores of Tilletia indica after hot water and sodium hypochlorite treatments. Plant Dis. 1997;81(8):932-5.
37. Arnon DI, Hoagland DR. Crop production in artificial culture solutions and in soils with special reference to factors influencing yields and absorption of inorganic nutrients. Soil Sci. 1940;50:463-85.
38. Witzenberger A, Hack H. Erlaeuterungen Zum BBCH-Dezimal-Code fuer die Entwicklungsstadien Des Getreides-Mit Abbildungen. Gesunde Pflanzen. 1989;41:384-8.
39. Lancashire PD, Bleiholder H, Langelüddecke P, Stauss R, Van den Boom T, Weber E, Witzen-berger A. A uniform decimal code for growth stages of crops and weeds. Ann Appl Biol. 1991;119:561-601.
40. Arnon DI. Copper enzyme in isolated chloroplast. Polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949;24:1-15.
41. Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in Acid rain-treated Bean plants: protective role of exogenous polyamines. Plant Sci. 2000;151:59-66.
42. Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: I. kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125(1):189-98.
43. Chance B, Maehly AC. Assay of catalases and peroxidases. 1955. p. 764-75.
44. Giannopolitis CN, Ries SK. Superoxide dismutases: I. occurrence in higher plants. Plant Physiol. 1977;59:309-14.
45. Kim MS, Kim C, Jo DH, Ryu YW. Effect of fungal elicitors and heavy metals on the production of flavonol glycosides in the cell cultures of Ginko Biloba. J Microbiol Biotech. 1999;9:661-7.
46. Mukherjee SP, Choudhuri MA. Implication of water stress-Induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedling. Physiol Plant. 1983;58:166-70.
47. Stark D, Wray V. Anthocyanins. In: Harborne JB, editor. Methods in Plant Biochemistry. Volume 1. London, UK: Acdemic; 1989. pp. 325-56.
48. Noreen Z, Ashraf M. Assessment of variation in antioxidative defense system in salt treated pea (Pisum sativum) cultivars and its putative use as salinity tolerance markers. J Plant Physiol. 2009;166:1764-74.
49. Handle EV. Direct microdetermination of sucrose. Anal Biochem. 1968;22:280-3.
50. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1-2):248-54.
51. Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39:205-7.
52. Allen SE, Grimshaw HM, Rowlan AP. Chemical analysis. In: Chapmman SB, editor. Methods in plant Ecology. Oxford: Blackwell Scientific Publications; 1976. p. 332-3.
53. Jackson ML. Soil chemical analysis: advanced course. UW-Madison Libraries Parallel Press; 2005.
54. Bashir MH, Am NM, Khan AZ, Aziz S, Ullah F, Qasim M. Characterization and advancement of microsatellite (SSR) markers for various stresses in wheat. Int J Agri Biosci. 2022;11(2):8.
55. Ali S, Gill RA, Ulhassan Z, Najeeb U, Kanwar MK, Abid M, Mwamba TM, Huang Q, Zhou W. Insights on the responses of Brassica napus cultivars against the cobalt-stress as revealed by carbon assimilation, anatomical changes and secondary metabolites. EEB. 2018;156:183-96.
56. Nazir A, Wahid A. Spray of stress protective chemicals alleviates cobalt toxicity on growth, water and nutrients status of hybrid maize (Zea mays L). Russ J Plant Physiol. 2023;70(3):36.
57. Liu J, Wang J, Lee S, Wen R. Copper-caused oxidative stress triggers the activation of antioxidant enzymes via ZmMPK3 in maize leaves. PLoS ONE. 2018;13(09):0203612.
58. Ur Rehman H, Iqbal Q, Farooq M, Wahid A, Afzal I, Basra SMA. Sulphur application improves the growth, seed yield and oil quality of canola. Acta Physiol Plant. 2013;35:2999-3006.
59. Salam A, Afridi MS, Khan AR, Azhar W, Shuaiqi Y, Ulhassan Z, Qi J, Xuo N, Chunyan Y, Chen N, Gan Y. Cobalt Induced toxicity and tolerance in plants: insights from Omics approaches. Heavy metal toxicity and tolerance in plants: a Biological. Omics Genetic Eng Approach. 2023;207-29. John Wiley & Sons Ltd
60. Salam A, Khan AR, Liu L, Yang S, Azhar W, Ulhassan Z, Zeeshan M, Wu J, Fan X, Gan Y. Seed priming with zinc oxide nanoparticles downplayed ultrastructural damage and improved photosynthetic apparatus in maize under cobalt stress. J Hazard Mater. 2022;423:127021.
61. Tang J, Li Y, Liu X, Yu G, Zheng F, Guo Z, Zhang Y, Shao W, Wu S, Li H. Cobalt induces neurodegenerative damages through impairing autophagic flux by activating hypoxia-inducible factor-1a triggered ROS overproduction. Sci Total Environ. 2023;857:159432.
62. Chandra R, Kang H. Mixed heavy metal stress on photosynthesis, transpiration rate, and chlorophyll content in poplar hybrids. For Sci Technol. 2016;12(02):55-61.
63. Fonseca MDCD, Bossolani JW, de Oliveira SL, Moretti LG, Portugal JR, Scudeletti D, de Oliveira EF, Crusciol CAC. Bacillus subtilis inoculation improves nutrient uptake and physiological activity in sugarcane under drought stress. Microorganisms. 2022;10(04):809.
64. Perveen A, Wahid A, Mahmood S, Hussain I, Rasheed R. Possible mechanism of medium-supplemented thiourea in improving growth, gas exchange, and photosynthetic pigments in cadmium-stressed maize (Zea mays). Rev Bras Bot. 2015;38:71-9.
65. Suryavanshi P, Buttar GS. Effects of exogenous osmoprotectants on physiological characteristics of wheat. Int J Curr Microbiol App Sci. 2018;7:1077-89.
66. Verma CB. Physiological traits and Productivity of Wheat (Triticum aestivum L.) in relation to Foliar Spray of Zinc, Urea, and Thiourea under Rainfed Condition. Agric Sci Dig. 2019;39(02):124-7.
67. Kumar P, Yadav S, Singh MP. Bioregulators application improved heat tolerance and yield in chickpea (Cicer arietinum L.) by modulating zeaxanthin cycle. Plant Physiol Rep. 2020;25:677-88.
68. Dauphinee AN, Fletcher JI, Denbigh GL, Lacroix CR, Gunawardena AH. Remodelling of lace plant leaves: antioxidants and ROS are key regulators of programmed cell death. Planta. 2017;246:133-47.
69. Ren L, Wang MR, Wang QC. ROS-induced oxidative stress in plant cryopreservation: occurrence and alleviation. Planta. 2021;254:1-18.
70. Wa Lwalaba JL, Zvogbo G, Mulembo M, Mundende M, Zhang G. The effect of cobalt stress on growth and physiological traits and its association with cobalt accumulation in barley genotypes differing in cobalt tolerance. J Plant Nutr. 2017;40(15):2192-9.
71. Pandey N, Pathak GC, Pandey DK, Pandey R. Heavy metals, Co, Ni, Cu, Zn and Cd, produce oxidative damage and evoke differential antioxidant responses in spinach. Braz J Plant Physiol. 2009;21:103-11.
72. Rashid M, Hampton JG, Rolston MP, Khan KM, Saville DJ. Heat stress during seed development affects forage brassica (Brassica napus L.) seed quality. J Agron Crop Sci. 2018;204:147-54.
73. Qamer Z, Chaudhary MT, Du X, Hinze L, Azhar MT. Review of oxidative stress and antioxidative defense mechanisms in Gossypium hirsutum L. in response to extreme abiotic conditions. J Cotton Res. 2021;4:1-9.
74. Faryal S, Ullah R, Khan MN, Ali B, Hafeez A, Jaremko M, Qureshi KA. Thioureacapped nanoapatites amplify osmotic stress tolerance in Zea mays L. by conserving photosynthetic pigments, osmolytes biosynthesis and antioxidant biosystems. Molecules. 2022;27:5744.
75. Kaya C, Ashraf M, Sönmez O. Promotive effect of exogenously applied thiourea on key physiological parametersand oxidative defense mechanism in salt-stressed Zea mays L. plants. Braz J Plant Physiol. 2015;39(5):786-95.
76. Hassanein RA, Amin AA, Rashad ES, Ali H. Effect of thiourea and salicylic acid on antioxidant defense of wheat plants under drought stress. Int J ChemTech Res. 2015;7(01):346-54.
77. Singh T, Sandhu PS, Chahal GK, Walia SS. Foliar thiourea confers moisture stress tolerance in rainfed maize through elevated antioxidative defence system, osmolyte accumulation and starch synthesis grown under different planting methods. J Plant Growth Regul. 2022;42:199-217.
78. Asthir B, Thapar R, Farooq M, Bains NS. Exogenous application of thiourea improves the performance of late sown wheat by inducing terminal heat resistance. Int J Agric Biol. 2013;15:1337-42.
79. Saleem I, Ahmed SR, Lahori AH, Mierzwa-Hersztek M, Bano S, Afzal A, Muhammad MT, Afzal M, Vambol V, Vambol S, Zhang Z. Utilizing thioureamodified biochars to mitigate toxic metal pollution and promote mustard (Brassica campestris) plant growth in contaminated soils. J Geochem Explor. 2024;257:107331.
80. Shukla A, Pathak SK, Singh S, Srivastava S. Application of thiourea ameliorates stress and reduces accumulation of arsenic in wheat (Triticum aestivum L.) plants grown in contaminated field. J Plant Growth Regul. 2023:42(10):6171-82.
81. Yadav T, Yadav RK, Yadav G, Kumar A, Makarana G. Salicylic acid and thiourea ameliorated adverse effects of salinity and drought-induced changes in physiological traits and yield of wheat. Cereal Res Commun. 2023. https:// doi.org/10.1007/s42976-023-00382-6.
82. Nikolaeva MK, Maevskaya SN, Shugaev AG, Bukhov NG. Effect of drought on chlorophyll content and antioxidant enzyme activities in leaves of three wheat cultivars varying in productivity. Russ J Plant Physiol. 2010;57:87-95.
83. Chung WH. Unraveling new functions of superoxide dismutase using yeast model system: beyond its conventional role in superoxide radical scavenging. J Microbiol. 2017;55:409-16.
84. Kaur G, Asthir BJBP. Proline: a key player in plant abiotic stress tolerance. Biol Plant. 2015;2015(59):609-19.
85. Taiz L, Zeiger E, Møller IM, Murphy A. Plant physiology and development, vol. 6. Massachusetts: Sinauer Associates Incorporated; 2015.
86. Zeeshan M, Hu YX, Guo XH, Sun CY, Salam A, Ahmad S, Muhammad I, Nasar J, Jahan MS, Fahad S, Zhou XB. Physiological and transcriptomic study reveal SeNPs-mediated AsIII stress detoxification mechanisms involved modulation of antioxidants, metal transporters, and transcription factors in Glycine max L.(Merr.) Roots. Environ Pollut. 2023;317:120637.
87. Rehman AU, Nazir S, Irshad R, Tahir K, ur Rehman K, Islam RU, Wahab Z. Toxicity of heavy metals in plants and animals and their uptake by magnetic iron oxide nanoparticles. J Mol Liq. 2021;321:114455.
ملاحظة الناشر
- *المراسلات:
عثمان ذوالفقار
usman.zulfiqar@iub.edu.pk
طلحة شودري
chaudhary.talha@stud.uni-mate.hu
قائمة كاملة بمعلومات المؤلف متاحة في نهاية المقال
DOI: https://doi.org/10.1186/s12870-024-04795-1
PMID: https://pubmed.ncbi.nlm.nih.gov/38383286
Publication Date: 2024-02-21
Exogenous application of sulfur-rich thiourea (STU) to alleviate the adverse effects of cobalt stress in wheat
Abstract
Heavy metal stress affects crop growth and yields as wheat (Triticum aestivum L.) growth and development are negatively affected under heavy metal stress. The study examined the effect of cobalt chloride (

Introduction
ones most susceptible to the occurrence of high cobalt contents in soil of anthropogenic origin. High cobaltcontent soils are frequently found close to metal smelting, machinery manufacturing, and mining operations [11]. Like other heavy metals, Co causes cell damage and decreases plant growth and yield by upregulating the Haber-Weiss and Fenton processes, which result in the production of reactive oxygen species. Higher concentrations of Co destabilize multiple metabolic pathways and induce oxidative damage to biomolecules, resulting in lipid peroxidation, membrane degradation, and protein carboxylation [12]. Cobalt-enhanced levels in plants distort chloroplast structure, ultimately leading to disruption in carbon dioxide assimilation due reduction in the uptake of carbon [13]. Enzymes used in the biosynthetic pathway of chlorophyll were disturbed by the distortion in the structure of rubisco (ribulose-1,5-bisphosphatecarboxylase/oxygenase) due to the replacement of Mg atom by Co in rubisco that is a crucial protein for the photosynthetic process [14]. Reactive oxygen species (ROS) like superoxide anion radicals (
peroxide (
Applying mineral nutrients or bio-regulators, which control multiple physiological and biochemical mechanisms at the metabolic and whole plant levels, improves plants’ natural defense against abiotic stress [18]. Thiourea is a sulfur-rich plant growth promoter that modulates plant development and effectively prevents the plants from oxidative damage imposed by abiotic stress [19, 20]. It is a non-physiological thiol-based ROS scavenger that contains sulfur (S) 42% and nitrogen (N) 36% [21] and can lower the stress-prompted redox imbalance and different injuries of the plant [22, 23]. Exogenously applied STU enhances the stress tolerance of crops [24, 25]. Causing an increase in growth and crop productivity, membrane stability, antioxidant potential, and photosynthetic efficiency [26,27]. Several studies have reported that STU application plays a significant role in coping with a variety of abiotic stress by improving the morphophysiological, biochemical, and yield contribution indices in several crops such as wheat [28-30], maize [31], canola [32], camelina [33, 34], and barley [35].
The exogenous application of STU to lower the negative effects of abiotic stress has been reported in previous studies. However, the role of STU in alleviating the toxic effects of Co stress in the different wheat varieties is limited and requires further investigation. Therefore, this study hypothesized that STU applications may alleviate the toxic effects of Co stress in wheat. The current investigation was conducted to evaluate the ameliorative role of STU to plant defense systems under Co stress by improving plant physiological attributes and antioxidant activities in wheat.
Materials and methods
wheat plants grown under Cobalt chloride stress in the Old Botanical Garden wire house, Department of Botany, University of Agriculture Faisalabad (
Determination of morphological attributes
Determination of photosynthetic pigments
Determination of hydrogen peroxide and malondialdehyde
Determination of enzymatic antioxidant activities
Determination of non-enzymatic antioxidants
Determination of osmo-protectants
method. Proline in plants was measured by following the method of Bates et al. [51].
Determination of mineral nutrients
Plant guidelines
Statistical analysis
Results
Morphological attributes
| Cultivars | Treatments | Shoot fresh weight (mg/g FW) | Root fresh weight (mg/g FW) | Shoot dry weight (mg/g FW) | Root dry weight (mg/g FW) | Shoot length (cm) | Root length (cm) | Leaf area (cm 2) |
| FSD-2008 | Control |
|
|
|
|
|
|
|
| STU |
|
|
|
|
|
|
|
|
| Co |
|
|
|
|
|
|
|
|
| STU+Co |
|
|
|
|
|
|
|
|
| Zincol-2016 | Control |
|
|
|
|
|
|
|
| STU |
|
|
|
|
|
|
|
|
| Co |
|
|
|
|
|
|
|
|
| STU+Co |
|
|
|
|
|
|
|
Photosynthetic pigments
for photosynthetic pigments. Results have revealed that cobalt stress decreased chlorophyll

Response of MDA and
Non-enzymatic antioxidants
Osmo-protectants
non-significant for osmo-protectants. The results have revealed that cobalt stress increased TSS by (
Mineral nutrients
Correlation analysis and heat map


Analysis of the heat map was created across the morphological, photosynthetic, biochemical, and ion contents in both cultivars of wheat. The variation of colors in the boxes shows the interaction strength between the recorded above-mentioned indices and treatments. Scale colors from blue (strongly positive) to dark red (strongly negative) were closely correlated to the strength of the color gradient utilized in the heat map boxes. The highest enhancement was observed in the morphological indices, photosynthetic pigments, enzymatic and non-enzymatic antioxidants, osmolytes, phosphorous, potassium, and calcium ions while the lowest level of sodium ions and
Discussion

| Cultivars | Treatments | Shoot
|
Root
|
Shoot
|
Root
|
Shoot
|
Root
|
Shoot P (mg/g FW) | Root P (mg/g FW) |
| FSD-2008 | Control |
|
|
|
|
|
|
|
|
| STU |
|
|
|
|
|
|
|
|
|
| Co |
|
|
|
|
|
|
|
|
|
| STU+Co |
|
|
|
|
|
|
|
|
|
| Zin-col-2016 | Control |
|
|
|
|
|
|
|
|
| STU |
|
|
|
|
|
|
|
|
|
| Co |
|
|
|
|
|
|
|
|
|
| STU+Co |
|
|
|
|
|
|
|
|

The outcome of this study revealed that under cobalt stress, STU applications play a positive role in improving the morphological characteristics of wheat cultivars (Table 1). Cobalt-induced toxicity reduced the morphological parameters such as fresh and dry weight, and root and shoot length of both wheat cultivars as reported in the previous studies [55,56] by reducing the nutrient uptake, plant water status, and stomatal conductance [14]. At the same time, the maximum reduction was observed in the wheat cultivar Zincol-2016 as compared to FSD-2008. However, exogenous applications of STU improved the plant growth indices including shoot and root length, and fresh and dry weight in both wheat cultivars grown under cobalt stress as found in the previous studies [57] while FSD-2008 showed maximum response towards STU and showed maximum improvement in growth parameters by detoxifying the cobalt
stress-toxicity as compared to Zincol-2016. STU application may improve the leaf surface area which helps to capture more light, hence enhancing the carbon fixation that regulates assimilates production and splits them towards developing sinks enhancing crop development and yield parameters. Under stress conditions, thiourea enhanced the osmotic capacity by improving the cell turgidity that allowed the plant to hold its water balance to raise the transpiration rate which in turn improved the morphological indies in the plants. Foliar applications of STU at

leading to the creation of carotenoids and chlorophyll. Because of high Co redox potential and inhibition of the enzymatic mechanisms responsible for chlorophyll production, there may be a correlation between the drop in chlorophyll concentration under stressful circumstances and the reductive steps inhibited in the chlorophyll biosynthetic pathway. Under cobalt stress, the creation of oxygen radicals damages the membrane of the cell,
leading to the breakdown of chloroplasts or the biosynthesis of intermediate products in the process leading to the development of carotenoids and chlorophyll [60-62]. However, STU foliar applications improved the formation of photosynthetic pigments which play a positive role in the photosynthetic efficiency that is essential for the development and growth of both wheat varieties while FSD-2008 showed maximum improvement in the photosynthetic pigments as compared to the Zincol-2016 as reported in the previous investigations [63-65]. The oxidative damage of photosynthetic pigments especially carotenoids and chlorophyll levels lowered by the application of STU in both varieties of wheat under Co-stress [66]. Thiourea usage may result in an upsurge in photosynthesis due to its role in ferredoxin that promotes synthesis and sustaining chlorophyll contents thereby boosting the photosynthetic rate and absorption efficiency that subsidizes the development and growth of plants [67].
synthesizing detoxifying chemicals for ROS. The difference lies in how plants accumulate these compounds and avoid cellular damage to function under demanding conditions (Figs. 3, 4 and 5). STU enhances cell membrane stability by suppressing MDA and a direct ROS scavenger that neutralizes endogenous
intimately linked with their widespread accumulation, particularly in roots, and also prevent the entry and binding of essential ions like
Conclusion
Acknowledgements
Authors’ contributions
Funding
Availability of data and materials
Declarations
Ethics approval and consent to participate
Consent for publication
Competing interests
Author details
Published online: 21 February 2024
References
- Zulfiqar U, Ahmad M, Valipour M, Ishfaq M, Maqsood MF, Iqbal R, Ali MF, Roy R, El Sabagh A. Evaluating Optimum Limited Irrigation and Integrated Nutrient Management Strategies for Wheat Growth, Yield and Quality. Hydrology. 2023;10(3):56.
- Victoria O, Idorenyin U, Asana M, Jia L, Shuoshuo L, Yang S, Okoi IM, Ping A, Egrinya EA. Seed treatment with 24-epibrassinolide improves wheat germination under salinity stress. Asian J Agric Biol. 2023;2023(3). https:// doi.org/10.35495/ajab.2022.076.
- Taratima W, Kunpratum N, Maneerattanarungroj P. Effect of salinity stress on physiological aspects of pumpkin (Cucurbita moschata
4. Mukhtar T, Rehman SU, Sultan T, Munis FH, Chaudhary HJ. Induction of heat tolerance in tomato cultivar with heat tolerant bacteria under field condition. Asian J Agric Biol. 2022;2022(2):202103112. https://doi.org/10. 35495/ajab.2021.03.112.
5. Abd El-Fattah DA, Hashem FA, Abd-Elrahman SH. Impact of applying organic fertilizers on nutrient content of soil and lettuce plants, yield quality and benefit-cost ratio under water stress conditions. Asian J Agric Biol. 2022;2022(2):202102086. https://doi.org/10.35495/ajab.2021.02.086.
6. Qasim M, Ahmed W, Safdar U, Maqbool R, Bin Sajid H, Noor H. Effect of drought stress on fertile tillers of wheat genotypes (Triticum aestivum L). Int J Agric Biosci. 2022;11:172-80.
7. Zulfiqar U, Haider FU, Maqsood MF, Mohy-Ud-Din W, Shabaan M, Ahmad M, Kaleem M, Ishfaq M, Aslam Z, Shahzad B. Recent advances in microbial-assisted remediation of cadmium-contaminated soil. Plants. 2023;12(17):3147.
8. Zulfiqar U, Jiang W, Xiukang W, Hussain S, Ahmad M, Maqsood MF, Ali N, Ishfaq M, Kaleem M, Haider FU, Farooq N. Cadmium phytotoxicity, tolerance, and advanced remediation approaches in agricultural soils. Front Plant Sci. 2022;13:773815.
9. Hu X, Wei X, Ling J, Chen J. Cobalt: an essential micronutrient for plant growth? Front Plant Sci. 2021;12:768523.
10. Leyssens L, Vinck B, Van Der Straeten C, Wuyts F, Maes L. Cobalt toxicity in humans-A review of the potential sources and systemic health effects. Toxicology. 2017;387:43-56.
11. Jiang M, Wang K, Wang Y, Zhao Q, Wang W. Technologies for the cobalt-contaminated soil remediation: a review. Sci Total Environ. 2022;813:151908.
12. Eskander SB, Saleh HM. Heavy metal-induced oxidative stress and related cellular process. In: Cellular and molecular phytotoxicity of heavy metals. 2020. p. 99-123.
13. Bano A, Gupta A, Rai S, Fatima T, Sharma S, Pathak N. Mechanistic role of reactive oxygen species and its regulation via the antioxidant system under environmental stress. Plant Stress Physiol Perspec Agric. 2021;23:1-8.
14. Singh A, Roychoudhury A. Protective chemicals and metabolites in stabilizing photosynthesis and respiration machinery during abiotic stresses. In: Photosynthesis and respiratory cycles during environmental stress response in plants. FL, USA: Apple Academic Press Inc.; 2022. p. 351-72.
15. Ozfidan-Konakci C, Yildiztugay E, Elbasan F, Kucukoduk M, Turkan I. Hydrogen sulfide
16. Sachdev S, Ansari SA, Ansari MI, Fujita M, Hasanuzzaman M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants. 2021;10(2):277.
17. Ghous M, Amir Bakhtavar M, Nawaz F. Halophyte quinoa: a potential hyperaccumulator of heavy metals for phytoremediation. Asian J Agric Biol. 2022;4:1-9.
18. Luqman M, Shahbaz M, Maqsood MF, Farhat F, Zulfiqar U, Siddiqui MH, Masood A, Aqeel M, Haider FU. Effect of strigolactone on growth, photosynthetic efficiency, antioxidant activity, and osmolytes accumulation in different maize (Zea mays L.) hybrids grown under drought stress. Plant Signal Behav. 2023;18(1):2262795.
19. Li Y, Mo X, Xiong J, Huang K, Zheng M, Jiang Q, Su G, Ou Q, Pan H, Jiang C. Deciphering the probiotic properties and safety assessment of a novel multi-stress-tolerant aromatic yeast Pichia kudriavzevii HJ2 from marine mangroves. Food Biosci. 2023;56:103248.
20. Patade VY, Nikalje GC, Srivastava S. Role of thiourea in mitigating different environmental stresses in plants. Protective chemical agents in the amelioration of plant abiotic stress: biochemical and molecular perspectives. Jun. 2020;22:467-82.
21. Ahmad M, Waraich EA, Zulfiqar U, Ullah A, Farooq M. Thiourea application increases seed and oil yields in camelina under heat stress by modulating the plant water relations and antioxidant defense system. J Soil Sci Plant Nut. 2023;23(1):290-307.
22. Ahmad S, Aziz AU, Ullah A, Raza MA. Effect of vermicompost and organic matter in enhancing wheat tolerance against drought stress. Int J Agric Biosci. 2022;11(3):165-7.
23. Srivastava AK, Sablok G, Hackenberg M, Deshpande U, Suprasanna P. Thiourea priming enhances salt tolerance through co-ordinated regulation of microRNAs and hormones in Brassica juncea. Sci Rep. 2017;7(01):45490.
24. Ahmad M, Waraich EA, Zulfiqar U, Hussain S, Yasin MU, Farooq M. Thiourea application improves the growth and seed and oil yields in canola by modulating gas exchange, antioxidant defense, and osmoprotection under heat stress. J Soil Sci Plant Nut. 2023;22(03):3655-66.
25. Zhang LJ, Buatois LA, Mángano MG. Potential and problems in evaluating secular changes in the diversity of animal-substrate interactions at ichnospecies rank. Terra Nova. 2022;34(5):433-40.
26. Haroon M, Anas M, Naurin I, Afzal R, Irfan U, Tariq H, Idrees F, Taj MH, Rukh M. Autoimmunity in plants; a powerful weapon in kingdom plantae to combat stresses. Int J Agric Biosci. 2023;12(3):159-64.
27. Singh RP, Singh D. Response of lentil to thiourea application under rain fed conditions of Central India. Int J Curr Microbiol App Sci. 2017;06:2556-60.
28. Wahid A, Basra S, Farooq M. Thiourea: a molecule with Immense Biological significance for plants. Int J Agri Bio. 2017;19(04):23-32.
29. Naz S, Perveen S. Response of wheat (Triticum aestivum L. Var. Gal-axy-2013) to pre-sowing seed treatment with thiourea under drought stress. Pak J Bot. 2021;53:1209-17.
30. Yadav T, Kumar A, Yadav R, Yadav G, Kumar R, Kushwaha M. Salicylic acid and thiourea mitigate the salinity and drought stress on physiological traits governing yield in pearl millet-wheat. Saudi J Biol Sci. 2020;27:2010-7.
31. Baqer RA, AI-Kaaby HK, Adul-Qadir LH. Antioxidant responses in wheat plants (Triticum aestivum L.) treated with thiourea. Plant Arch. 2020;20:717-22.
32. Haider FU, Virk AL, Rehmani MIA, Skalicky M, Ata-ul-Karim ST, Ahmad N, Soufan W, Brestic M, Sabagh AE, Liqun C. Integrated application of thiourea and biochar improves maize growth, antioxidant activity and reduces cadmium bioavailability in cadmium-contaminated soil. Front Plant Sci. 2022;12:809322.
33. Ahmad M, Waraich EA, Zulfiqar U, Ullah A, Farooq M. Thiourea application improves heat tolerance in camelina (Camelina sativa L. Crantz) by modulating gas exchange, antioxidant defense and osmoprotection. Ind Crop Prod. 2021;170:113826.
34. Ahmad M, Waraich EA, Hussain S, Ayyub CM, Ahmad Z, Zulfiqar U. Improving heat stress tolerance in Camelina sativa and Brassica napus through Thiourea seed priming. J Plant Growth Reg. 2021;3:1-7.
35. Ahmad M, Waraich EA, Hussain S, Zulfiqar U, Teshome FT, Gastelbondo M, Imran M, Farooq M. Exogenous application of thiourea improves the growth, seed yield, and seed fatty acid profile in late sown camelina. J Soil Sci Plant Nutr. 2023;23(01):1306-25.
36. Smilanick JL, Hershberger W, Bonde MR, Nester SE. Germinability of teliospores of Tilletia indica after hot water and sodium hypochlorite treatments. Plant Dis. 1997;81(8):932-5.
37. Arnon DI, Hoagland DR. Crop production in artificial culture solutions and in soils with special reference to factors influencing yields and absorption of inorganic nutrients. Soil Sci. 1940;50:463-85.
38. Witzenberger A, Hack H. Erlaeuterungen Zum BBCH-Dezimal-Code fuer die Entwicklungsstadien Des Getreides-Mit Abbildungen. Gesunde Pflanzen. 1989;41:384-8.
39. Lancashire PD, Bleiholder H, Langelüddecke P, Stauss R, Van den Boom T, Weber E, Witzen-berger A. A uniform decimal code for growth stages of crops and weeds. Ann Appl Biol. 1991;119:561-601.
40. Arnon DI. Copper enzyme in isolated chloroplast. Polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949;24:1-15.
41. Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in Acid rain-treated Bean plants: protective role of exogenous polyamines. Plant Sci. 2000;151:59-66.
42. Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: I. kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125(1):189-98.
43. Chance B, Maehly AC. Assay of catalases and peroxidases. 1955. p. 764-75.
44. Giannopolitis CN, Ries SK. Superoxide dismutases: I. occurrence in higher plants. Plant Physiol. 1977;59:309-14.
45. Kim MS, Kim C, Jo DH, Ryu YW. Effect of fungal elicitors and heavy metals on the production of flavonol glycosides in the cell cultures of Ginko Biloba. J Microbiol Biotech. 1999;9:661-7.
46. Mukherjee SP, Choudhuri MA. Implication of water stress-Induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedling. Physiol Plant. 1983;58:166-70.
47. Stark D, Wray V. Anthocyanins. In: Harborne JB, editor. Methods in Plant Biochemistry. Volume 1. London, UK: Acdemic; 1989. pp. 325-56.
48. Noreen Z, Ashraf M. Assessment of variation in antioxidative defense system in salt treated pea (Pisum sativum) cultivars and its putative use as salinity tolerance markers. J Plant Physiol. 2009;166:1764-74.
49. Handle EV. Direct microdetermination of sucrose. Anal Biochem. 1968;22:280-3.
50. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1-2):248-54.
51. Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39:205-7.
52. Allen SE, Grimshaw HM, Rowlan AP. Chemical analysis. In: Chapmman SB, editor. Methods in plant Ecology. Oxford: Blackwell Scientific Publications; 1976. p. 332-3.
53. Jackson ML. Soil chemical analysis: advanced course. UW-Madison Libraries Parallel Press; 2005.
54. Bashir MH, Am NM, Khan AZ, Aziz S, Ullah F, Qasim M. Characterization and advancement of microsatellite (SSR) markers for various stresses in wheat. Int J Agri Biosci. 2022;11(2):8.
55. Ali S, Gill RA, Ulhassan Z, Najeeb U, Kanwar MK, Abid M, Mwamba TM, Huang Q, Zhou W. Insights on the responses of Brassica napus cultivars against the cobalt-stress as revealed by carbon assimilation, anatomical changes and secondary metabolites. EEB. 2018;156:183-96.
56. Nazir A, Wahid A. Spray of stress protective chemicals alleviates cobalt toxicity on growth, water and nutrients status of hybrid maize (Zea mays L). Russ J Plant Physiol. 2023;70(3):36.
57. Liu J, Wang J, Lee S, Wen R. Copper-caused oxidative stress triggers the activation of antioxidant enzymes via ZmMPK3 in maize leaves. PLoS ONE. 2018;13(09):0203612.
58. Ur Rehman H, Iqbal Q, Farooq M, Wahid A, Afzal I, Basra SMA. Sulphur application improves the growth, seed yield and oil quality of canola. Acta Physiol Plant. 2013;35:2999-3006.
59. Salam A, Afridi MS, Khan AR, Azhar W, Shuaiqi Y, Ulhassan Z, Qi J, Xuo N, Chunyan Y, Chen N, Gan Y. Cobalt Induced toxicity and tolerance in plants: insights from Omics approaches. Heavy metal toxicity and tolerance in plants: a Biological. Omics Genetic Eng Approach. 2023;207-29. John Wiley & Sons Ltd
60. Salam A, Khan AR, Liu L, Yang S, Azhar W, Ulhassan Z, Zeeshan M, Wu J, Fan X, Gan Y. Seed priming with zinc oxide nanoparticles downplayed ultrastructural damage and improved photosynthetic apparatus in maize under cobalt stress. J Hazard Mater. 2022;423:127021.
61. Tang J, Li Y, Liu X, Yu G, Zheng F, Guo Z, Zhang Y, Shao W, Wu S, Li H. Cobalt induces neurodegenerative damages through impairing autophagic flux by activating hypoxia-inducible factor-1a triggered ROS overproduction. Sci Total Environ. 2023;857:159432.
62. Chandra R, Kang H. Mixed heavy metal stress on photosynthesis, transpiration rate, and chlorophyll content in poplar hybrids. For Sci Technol. 2016;12(02):55-61.
63. Fonseca MDCD, Bossolani JW, de Oliveira SL, Moretti LG, Portugal JR, Scudeletti D, de Oliveira EF, Crusciol CAC. Bacillus subtilis inoculation improves nutrient uptake and physiological activity in sugarcane under drought stress. Microorganisms. 2022;10(04):809.
64. Perveen A, Wahid A, Mahmood S, Hussain I, Rasheed R. Possible mechanism of medium-supplemented thiourea in improving growth, gas exchange, and photosynthetic pigments in cadmium-stressed maize (Zea mays). Rev Bras Bot. 2015;38:71-9.
65. Suryavanshi P, Buttar GS. Effects of exogenous osmoprotectants on physiological characteristics of wheat. Int J Curr Microbiol App Sci. 2018;7:1077-89.
66. Verma CB. Physiological traits and Productivity of Wheat (Triticum aestivum L.) in relation to Foliar Spray of Zinc, Urea, and Thiourea under Rainfed Condition. Agric Sci Dig. 2019;39(02):124-7.
67. Kumar P, Yadav S, Singh MP. Bioregulators application improved heat tolerance and yield in chickpea (Cicer arietinum L.) by modulating zeaxanthin cycle. Plant Physiol Rep. 2020;25:677-88.
68. Dauphinee AN, Fletcher JI, Denbigh GL, Lacroix CR, Gunawardena AH. Remodelling of lace plant leaves: antioxidants and ROS are key regulators of programmed cell death. Planta. 2017;246:133-47.
69. Ren L, Wang MR, Wang QC. ROS-induced oxidative stress in plant cryopreservation: occurrence and alleviation. Planta. 2021;254:1-18.
70. Wa Lwalaba JL, Zvogbo G, Mulembo M, Mundende M, Zhang G. The effect of cobalt stress on growth and physiological traits and its association with cobalt accumulation in barley genotypes differing in cobalt tolerance. J Plant Nutr. 2017;40(15):2192-9.
71. Pandey N, Pathak GC, Pandey DK, Pandey R. Heavy metals, Co, Ni, Cu, Zn and Cd, produce oxidative damage and evoke differential antioxidant responses in spinach. Braz J Plant Physiol. 2009;21:103-11.
72. Rashid M, Hampton JG, Rolston MP, Khan KM, Saville DJ. Heat stress during seed development affects forage brassica (Brassica napus L.) seed quality. J Agron Crop Sci. 2018;204:147-54.
73. Qamer Z, Chaudhary MT, Du X, Hinze L, Azhar MT. Review of oxidative stress and antioxidative defense mechanisms in Gossypium hirsutum L. in response to extreme abiotic conditions. J Cotton Res. 2021;4:1-9.
74. Faryal S, Ullah R, Khan MN, Ali B, Hafeez A, Jaremko M, Qureshi KA. Thioureacapped nanoapatites amplify osmotic stress tolerance in Zea mays L. by conserving photosynthetic pigments, osmolytes biosynthesis and antioxidant biosystems. Molecules. 2022;27:5744.
75. Kaya C, Ashraf M, Sönmez O. Promotive effect of exogenously applied thiourea on key physiological parametersand oxidative defense mechanism in salt-stressed Zea mays L. plants. Braz J Plant Physiol. 2015;39(5):786-95.
76. Hassanein RA, Amin AA, Rashad ES, Ali H. Effect of thiourea and salicylic acid on antioxidant defense of wheat plants under drought stress. Int J ChemTech Res. 2015;7(01):346-54.
77. Singh T, Sandhu PS, Chahal GK, Walia SS. Foliar thiourea confers moisture stress tolerance in rainfed maize through elevated antioxidative defence system, osmolyte accumulation and starch synthesis grown under different planting methods. J Plant Growth Regul. 2022;42:199-217.
78. Asthir B, Thapar R, Farooq M, Bains NS. Exogenous application of thiourea improves the performance of late sown wheat by inducing terminal heat resistance. Int J Agric Biol. 2013;15:1337-42.
79. Saleem I, Ahmed SR, Lahori AH, Mierzwa-Hersztek M, Bano S, Afzal A, Muhammad MT, Afzal M, Vambol V, Vambol S, Zhang Z. Utilizing thioureamodified biochars to mitigate toxic metal pollution and promote mustard (Brassica campestris) plant growth in contaminated soils. J Geochem Explor. 2024;257:107331.
80. Shukla A, Pathak SK, Singh S, Srivastava S. Application of thiourea ameliorates stress and reduces accumulation of arsenic in wheat (Triticum aestivum L.) plants grown in contaminated field. J Plant Growth Regul. 2023:42(10):6171-82.
81. Yadav T, Yadav RK, Yadav G, Kumar A, Makarana G. Salicylic acid and thiourea ameliorated adverse effects of salinity and drought-induced changes in physiological traits and yield of wheat. Cereal Res Commun. 2023. https:// doi.org/10.1007/s42976-023-00382-6.
82. Nikolaeva MK, Maevskaya SN, Shugaev AG, Bukhov NG. Effect of drought on chlorophyll content and antioxidant enzyme activities in leaves of three wheat cultivars varying in productivity. Russ J Plant Physiol. 2010;57:87-95.
83. Chung WH. Unraveling new functions of superoxide dismutase using yeast model system: beyond its conventional role in superoxide radical scavenging. J Microbiol. 2017;55:409-16.
84. Kaur G, Asthir BJBP. Proline: a key player in plant abiotic stress tolerance. Biol Plant. 2015;2015(59):609-19.
85. Taiz L, Zeiger E, Møller IM, Murphy A. Plant physiology and development, vol. 6. Massachusetts: Sinauer Associates Incorporated; 2015.
86. Zeeshan M, Hu YX, Guo XH, Sun CY, Salam A, Ahmad S, Muhammad I, Nasar J, Jahan MS, Fahad S, Zhou XB. Physiological and transcriptomic study reveal SeNPs-mediated AsIII stress detoxification mechanisms involved modulation of antioxidants, metal transporters, and transcription factors in Glycine max L.(Merr.) Roots. Environ Pollut. 2023;317:120637.
87. Rehman AU, Nazir S, Irshad R, Tahir K, ur Rehman K, Islam RU, Wahab Z. Toxicity of heavy metals in plants and animals and their uptake by magnetic iron oxide nanoparticles. J Mol Liq. 2021;321:114455.
Publisher’s Note
- *Correspondence:
Usman Zulfiqar
usman.zulfiqar@iub.edu.pk
Talha Chaudhary
chaudhary.talha@stud.uni-mate.hu
Full list of author information is available at the end of the article
