DOI: https://doi.org/10.1186/s13195-024-01407-w
PMID: https://pubmed.ncbi.nlm.nih.gov/38378607
تاريخ النشر: 2024-02-20
العلاقة بين مرض الأميلويد، مرض الأوعية الدموية الصغيرة في الدماغ، خلل النظام الغليمفاتي، والإدراك: دراسة قائمة على المشاركين في استمرارية مرض الزهايمر
الملخص
الخلفية: يعتبر خلل الغليمفاتي مسارًا حاسمًا للخرف. تعتبر أمراض الزهايمر (AD) التي تتواجد مع مرض الأوعية الدموية الصغيرة في الدماغ (CSVD) هي الأكثر شيوعًا كسبب للخرف. نفترض أن أمراض الزهايمر وCSVD قد تكون مرتبطة بخلل الغليمفاتي، مما يساهم في ضعف الإدراك. الطريقة: تم تضمين المشاركين الذين أكملوا تصوير PET للأميلويد، وتصوير الألياف الانتشاري (DTI)، وتسلسل الاسترداد المعتمد على السائل (FLAIR) من مبادرة تصوير الأعصاب لمرض الزهايمر (ADNI). تم تقييم كثافات المادة البيضاء (WMH)، وهي العلامة الأكثر شيوعًا لـ CSVD، من صور T2FLAIR وتمثل عبء CSVD. تم استخدام PET للأميلويد لتقييم A
الاستنتاج: قدمت دراستنا دليلًا على أن كل من مرض الزهايمر (A
الخلفية
أثبتت أمراض الزهايمر (AD)، وخاصةً
وCSVD على وظيفة الغليمفاتي بشكل منفصل في نماذج AD وCSVD، على التوالي. القليل من الدراسات بحثت في الارتباط المستقل بين CSVD وأمراض الزهايمر مع وظيفة الغليمفاتي في vivo.
تم استخدامه للتنبؤ بالتحول إلى خرف مرض باركنسون [30].
لذلك، في هذه الدراسة، نهدف إلى التحقيق في (1) ارتباط CSVD و
الطرق والمواد
الموافقة الأخلاقية
المشاركون
[33]. تم ذكر المعلومات عن المشاركين الذين قاموا بتمارين السحب في المادة التكميلية 1.
التقييم العصبي النفسي
اكتساب الرنين المغناطيسي
تصوير PET للأميلويد
المخيخ بالكامل (المادة البيضاء والرمادية) لكل موضوع.
تحليل الصور
تقسيم WMH
تحديد حجم الجمجمة الكلي (TIV)
أخيرًا، تم الحصول على TIV الإجمالي باستخدام وظيفة تقدير TIV في CAT12.
تقييم DTI-ALPS
- معالجة مسبقة لـ DTI
2) تحليل مؤشر DTI-ALPS
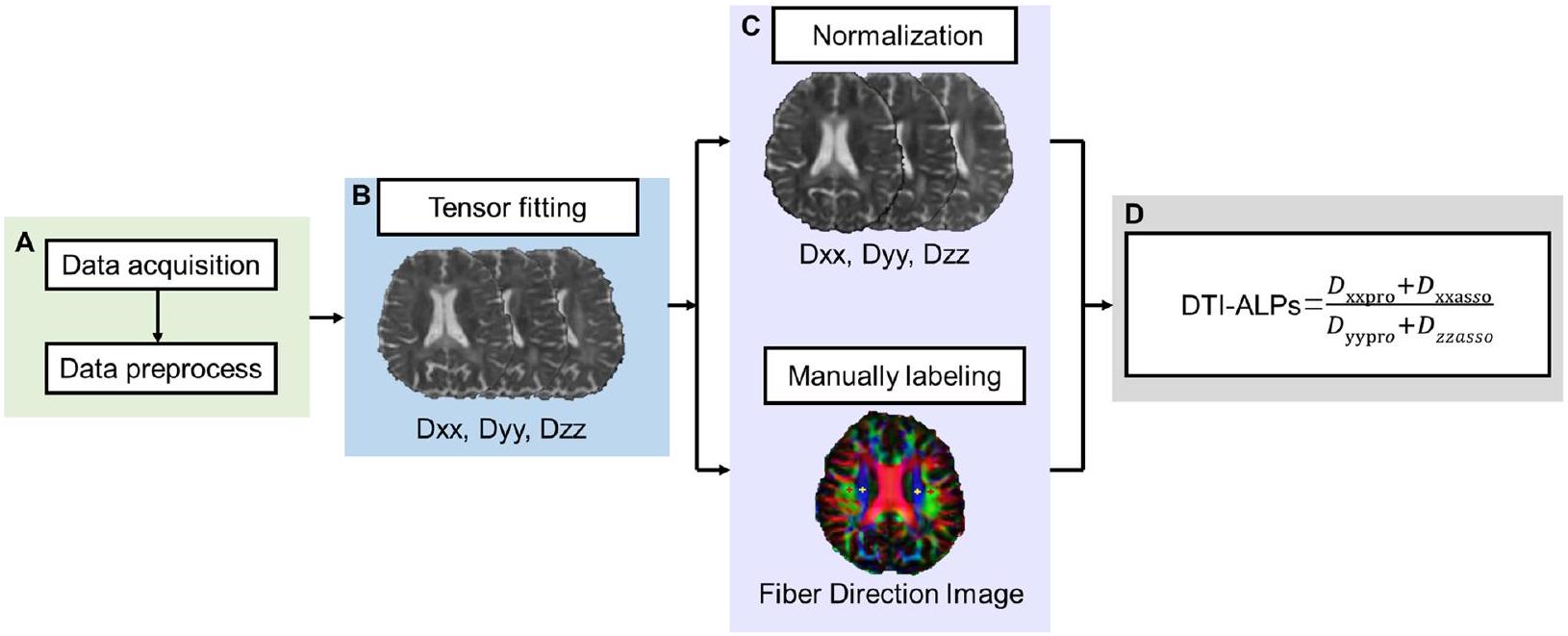
تصنيف PVS
تقسيم حجم المشيمة الوعائية
المجموعات
التحليل الإحصائي
تم إجراء تحليل الارتباط الجزئي لتحليل العلاقة المستقلة العالمية
درجة تقييم PVS في العقدة PVS والمادة البيضاء. في النموذج الأول، قمنا أولاً بتحديد العمر والجنس والحجم الكلي للدماغ كمتغيرات مصاحبة. في النموذج 2، نضيف أيضًا APOE.
النتائج
المشاركون
| CN A – (
|
CN A + (
|
MCI A + (
|
AD A + (N=19) |
|
|
| التركيبة السكانية | |||||
| العمر (بالسنوات) |
|
|
|
|
0.123 |
| ذكر،
|
17 (42.5%) | 17 (35.4%) | 7 (26.9%) | 9 (47.4%) | 0.367 |
| التعليم (السنوات) |
|
|
|
|
0.144 |
| APOE
|
9 (23.1%)
|
22 (45.8%)
|
15 (57.7%) | 11 (57.9%) | 0.763 |
| عوامل الخطر الوعائية | |||||
| ارتفاع ضغط الدم، ن (%) | 14 (35.0%) | 22 (45.8%) | 13 (50.0%) | 8 (44.4%) | 0.630 |
| السكري
|
3 (7.5%) | 6 (12.5%) | 0 | 0 | 0.128 |
| فرط شحميات الدم
|
16 (40%) | ٢٦ (٥٤.٢٪) | 12 (46.2%) | 8 (44.4%) | 0.608 |
| التدخين،
|
6 (15.0%) | 9 (18.8%) | 6 (23.1%) | 1 (5.6%) | 0.458 |
| أمراض القلب،
|
6 (15.0%) | 4 (8.3%) | 4 (15.4%) | 3 (16.7%) | 0.701 |
| خصائص التصوير | |||||
| TIV (
|
|
|
|
|
0.901 |
| عبء WMH |
|
|
|
|
< 0.001 |
| وجود فجوة، ن (%) | 2 (5%) | 5 (10.4%) | 2 (7.7%) | 1 (5.3%) | 0.781 |
| وجود النزيف الدقيق (%) | 4 (10%) | 3 (6.3%) | 3 (11.5%) | 6 (31.6%) | 0.036 |
| العالمية أ
|
|
|
|
|
< 0.001 |
| درجة تصنيف PVS | |||||
| العقد القاعدية PVS | 2.00 (2.00-3.75) | 2.50 (2.00-3.00) | 3.00 (1.00-3.25) | 3.00 (1.00-4.00) | 0.041 |
| المادة البيضاء PVS | 2.00 (2.00-2.75) | 3.00 (2.00-3.00) | 2.00 (1.75-3.00) | 3.00 (2.00-3.00) | 0.987 |
| حجم المشيمية الوعائية |
|
|
|
|
0.029 |
| دي تي آي – ألبس |
|
|
|
|
< 0.001 |
حجم المشيمية
CN الحالة المعرفية الطبيعية، MCI ضعف إدراكي خفيف، AD مرض الزهايمر، TIV الحجم الكلي داخل الجمجمة، WMH فرط كثافة المادة البيضاء، PVS الفضاء المحيط بالأوعية الدموية
ارتباطات علامات الغليمفاتيك مع
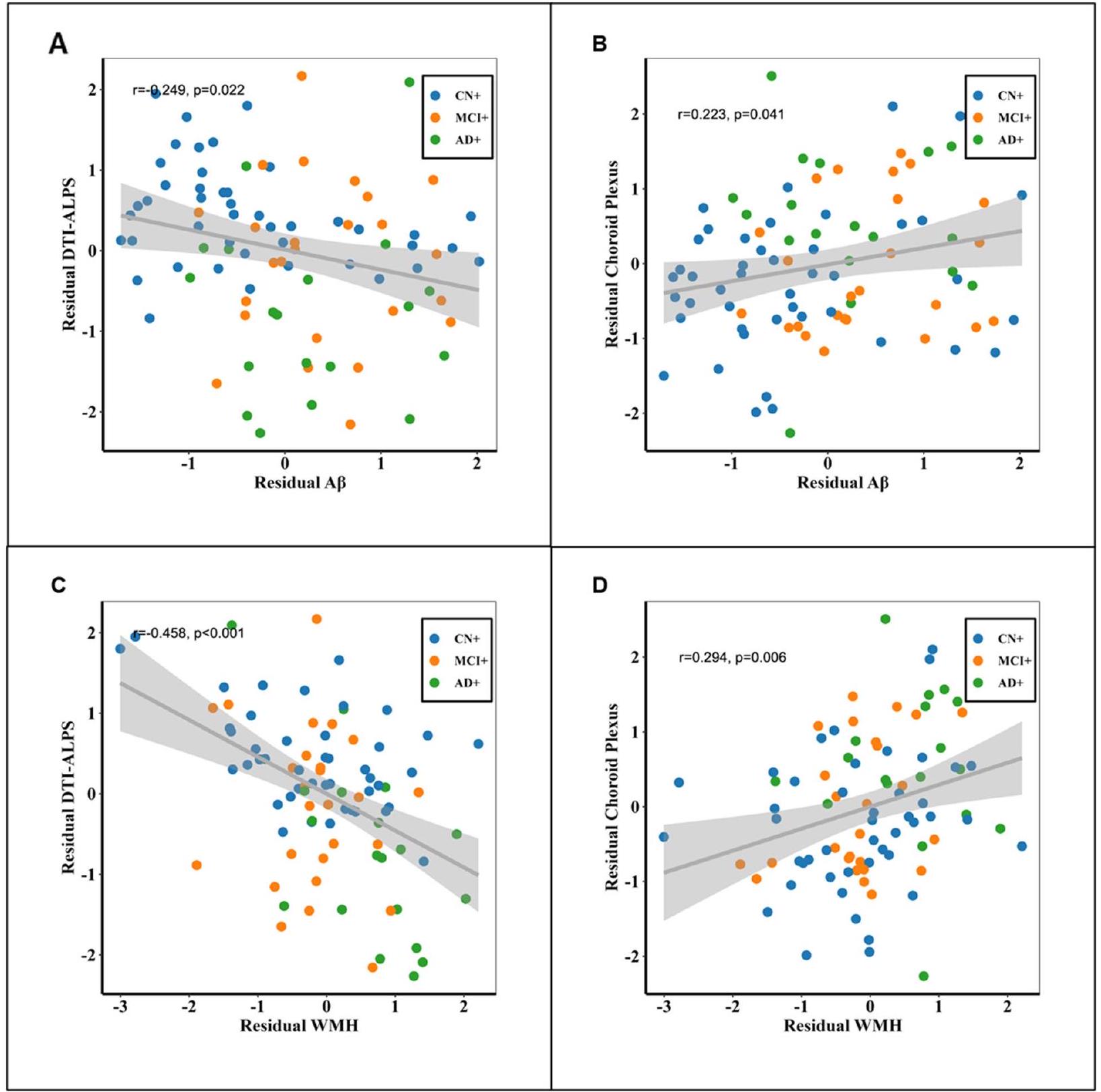
نظرًا للتأثير المحتمل لعوامل الخطر الوعائية على علامات الغليمفاتي، قمنا بتكرار التحليل المذكور أعلاه بشكل خاص في المشاركين الذين لديهم عامل خطر وعائي واحد على الأقل. بالإضافة إلى ذلك، أجرينا ارتباطات
بين علامات الغليمفاتيك والأميلويد/WMH، مع تصحيح إضافي لدرجة عوامل الخطر الوعائية. أظهرت كلا التحليلين أن العلاقة بين الأميلويد/WMH وعلامات الغليمفاتيك (DTI-ALPS وحجم المشيمة الشوكية) ظلت ذات دلالة إحصائية. يمكن العثور على مزيد من التفاصيل في المواد التكميلية 6 و7.
| العقد القاعدية PVS | المادة البيضاء PVS | |||
|
|
|
|
|
|
| النموذج 1 | ||||
|
|
-1.238 | 0.347 | -0.867 | 0.494 |
| عبء WMH | 0.542 | 0.173 | 0.054 | 0.889 |
| النموذج 2 | ||||
|
|
-1.212 | 0.379 | -0.754 | 0.575 |
| عبء WMH | 0.489 | 0.225 | -0.054 | 0.890 |
النموذج 2: تم تضمين العمر، الجنس، حجم الدماغ الكلي، وحالة APOE كمتغيرات مصاحبة
حجم الجمجمة الكلي TIV، فرط شدة المادة البيضاء WMH، الفضاء المحيط بالأوعية PVS
الارتباطات بين علامات الغليمفاتي والأداء المعرفي لدى المشاركين الإيجابيين للأميلويد
ومع ذلك، لم تكن هناك ارتباطات ذات دلالة إحصائية بين عبء PVS في العقد القاعدية وأداء المادة البيضاء PVS
مع الأداء المعرفي. يمكن العثور على مزيد من التفاصيل في الجدول 3.
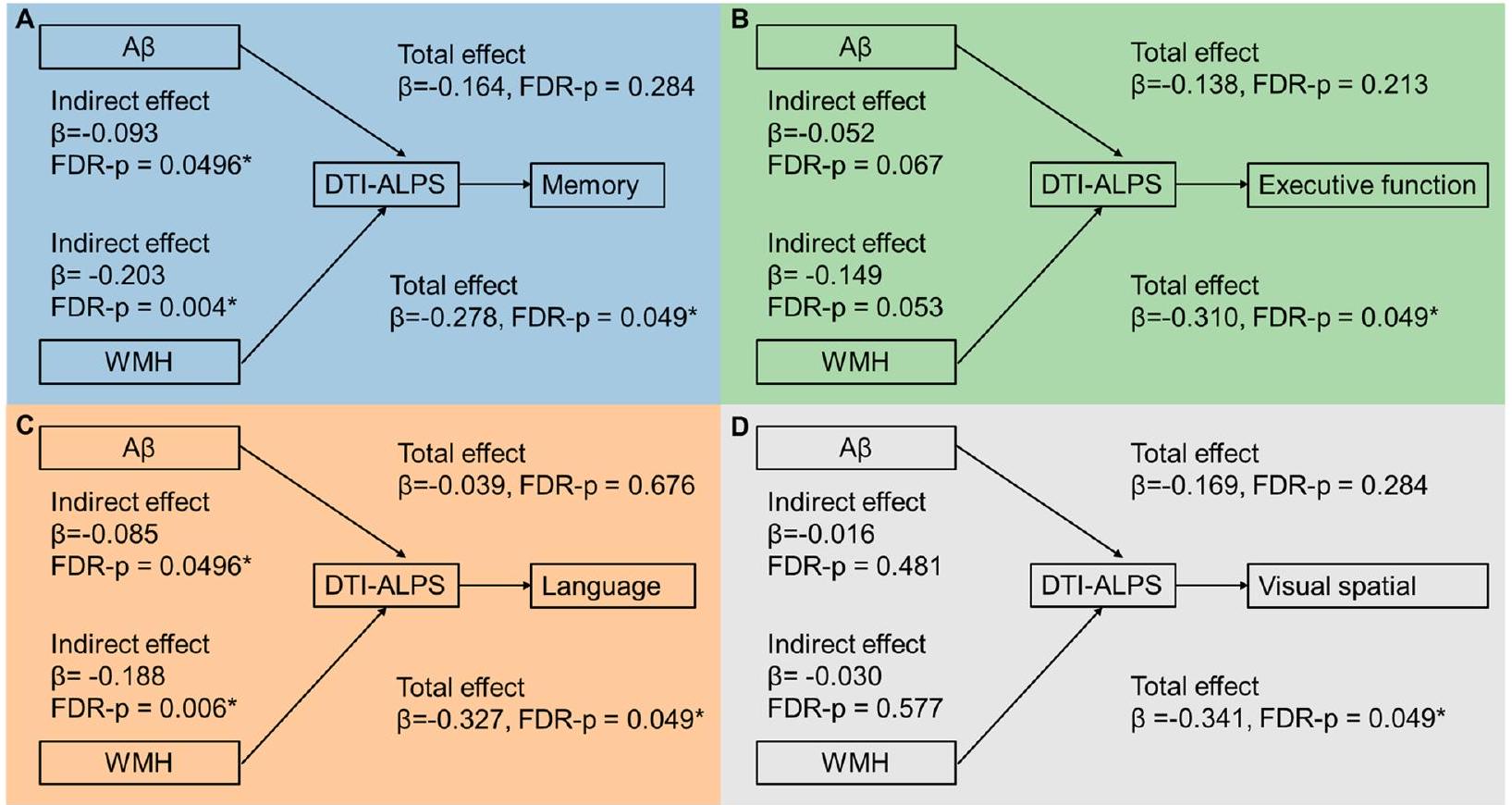
علامات الغليمفاتي كوسيط مهم بين
نقاش
| DTI-ALPS | المشيمة الشوكية | PVS في العقد القاعدية | PVS في المادة البيضاء | |||||
|
|
FDR-p |
|
FDR-p | Rho | FDR-p | Rho | FDR-p | |
| الذاكرة | 0.470 | < 0.001 | -0.315 | 0.007 | 0.060 | 0.704 | 0.054 | 0.788 |
| الوظيفة التنفيذية | 0.358 | 0.001 | -0.321 | 0.007 | -0.042 | 0.704 | 0.114 | 0.601 |
| البصرية المكانية | 0.223 | 0.040 | -0.233 | 0.031 | -0.076 | 0.704 | -0.030 | 0.261 |
| اللغة | 0.419 | < 0.001 | -0.261 | 0.021 | -0.082 | 0.704 | 0.201 | 0.788 |

لم نجد الارتباط بين شدة تضخم PVS و
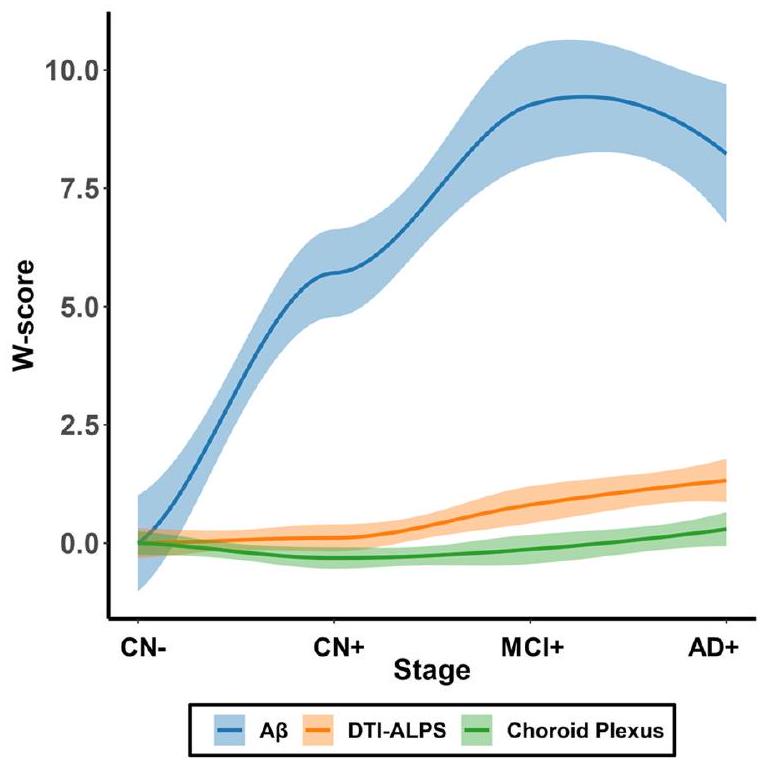
من خلال رسم تغييرات DTI-ALPS وحجم المشيمة الشوكية مقابل تراكم الأميلويد على طول استمرارية AD، وجدنا أن
في المراحل المبكرة من نشوء AD [40]. تؤكد هذه النتائج الجماعية على الطبيعة المعقدة والمتعددة الأوجه لتقدم AD، مما يتطلب استكشافًا أعمق للعلاقات الزمنية بين العوامل المختلفة المعنية في المرض.
تحسين وظيفة الغليمفاتي بشكل فعال. والأهم من ذلك، أن اكتشافنا من منظور وظيفة الغليمفاتي أضاف أدلة قائمة على الآلية لمرض الزهايمر المختلط (الآفات المرتبطة بمرض الزهايمر مع مرض الأوعية الدموية الصغيرة)، بالإضافة إلى الدراسات السابقة التي تركزت على دورها التراكمي في الاتصال الوظيفي أو الهيكلي للدماغ ككل.
كان لدراستنا عدة قيود. أولاً، كانت تقييماتنا لـ CSVD تعتمد فقط على عبء WMH، دون النظر في علامات CSVD المهمة الأخرى مثل الفجوات والنزيف الدقيق. من المهم الاعتراف بأن WMH يمكن أن يكون له أصول غير وعائية، مما قد يؤدي إلى إدخال بعض التحيز في نتائجنا. ومع ذلك، من الجدير بالذكر أن معظم الدراسات المسببة قد ربطت بشكل أساسي بين WMH و CSVD. علاوة على ذلك، فإن انتشار الفجوات المنخفض
في الختام، قدمت دراستنا دليلاً على أن علم الأمراض المرتبط بمرض الزهايمر (
معلومات إضافية
شكر وتقدير
معلومات اتحاد ADNI
مساهمات المؤلفين
تمويل
توفر البيانات والمواد
الإعلانات
موافقة الأخلاقيات والموافقة على المشاركة
موافقة للنشر
المصالح المتنافسة
تفاصيل المؤلف
تم الاستلام: 2 مايو 2023 تم القبول: 4 فبراير 2024
References
- Rasmussen MK, Mestre H, Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018;17(11):1016-24.
- Nedergaard M, Goldman SA. Glymphatic failure as a final common pathway to dementia. Science. 2020;370(6512):50-6.
- Simon
, Wang , Ismail , Braun , Schindler AG, Reemmer J, et al. Loss of perivascular aquaporin-4 localization impairs glymphatic exchange and promotes amyloid plaque formation in mice. Alzheimer’s Res Ther. 2022;14(1):1-25. - Harrison IF, Siow B, Akilo AB, Evans PG, Ismail O, Ohene Y, et al. Noninvasive imaging of CSF-mediated brain clearance pathways via assessment of perivascular fluid movement with diffusion tensor MRI. Elife. 2018;7:e34028.
- Peng W, Achariyar TM, Li B, Liao Y, Mestre H, Hitomi E, et al. Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2016;93:215-25.
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid
. Sci Transl Med. 2012;4(147):147ra11-ra11. - Da Mesquita S, Louveau A, Vaccari A, Smirnov I, Cornelison RC, Kingsmore KM, et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature. 2018;560(7717):185-91.
- Hsu JL, Wei YC, Toh CH, Hsiao IT, Lin KJ, Yen TC, et al. Magnetic resonance images implicate that glymphatic alterations mediate cognitive dysfunction in Alzheimer disease. Ann Neurol. 2023;93(1):164-74.
- Mortensen KN, Sanggaard S, Mestre H, Lee H, Kostrikov S, Xavier AL, et al. Impaired glymphatic transport in spontaneously hypertensive rats. J Neurosci. 2019;39(32):6365-77.
- Venkat P, Chopp M, Zacharek A, Cui C, Zhang L, Li Q, et al. White matter damage and glymphatic dysfunction in a model of vascular dementia in rats with no prior vascular pathologies. Neurobiol Aging. 2017;50:96-106.
- Wang M, Ding F, Deng S, Guo X, Wang W, Iliff JJ, et al. Focal solute trapping and global glymphatic pathway impairment in a murine model of multiple microinfarcts. J Neurosci. 2017;37(11):2870-7.
- Huang P, Zhang R, Jiaerken Y, Wang S, Yu W, Hong H, et al. Deep white matter hyperintensity is associated with the dilation of perivascular space. J Cereb Blood Flow Metab. 2021;41(9):2370-80.
- Li Y, Zhou Y, Zhong W, Zhu X, Chen Y, Zhang K, et al. Choroid plexus enlargement exacerbates white matter hyperintensity growth through glymphatic impairment. Ann Neurol. 2023;94:182-95.
- Toledo JB, Arnold SE, Raible K, Brettschneider J, Xie SX, Grossman M, et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain. 2013;136(9):2697-706.
- Wardlaw JM, Benveniste H, Nedergaard M, Zlokovic BV, Mestre H, Lee H, et al. Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Rev Neurol. 2020;16(3):137-53.
- Mestre H, Kostrikov S, Mehta RI, Nedergaard M. Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clin Sci. 2017;131(17):2257-74.
- Taoka T, Masutani Y, Kawai H, Nakane T, Matsuoka K, Yasuno F, et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases. Jpn J Radiol. 2017;35:172-8.
- Zhang W, Zhou Y, Wang J, Gong X, Chen Z, Zhang X, et al. Glymphatic clearance function in patients with cerebral small vessel disease. Neuroimage. 2021;238:118257.
- Siow TY, Toh CH, Hsu J-L, Liu G-H, Lee S-H, Chen N-H, et al. Association of sleep, neuropsychological performance, and gray matter volume
with glymphatic function in community-dwelling older adults. Neurology. 2022;98(8):e829-38. - Lee H-J, Lee DA, Shin KJ, Park KM. Glymphatic system dysfunction in obstructive sleep apnea evidenced by DTI-ALPS. Sleep Med. 2022;89:176-81.
- Zhang Y, Zhang R, Ye Y, Wang S, Jiaerken Y, Hong H, et al. The influence of demographics and vascular risk factors on glymphatic function measured by diffusion along perivascular space. Front Aging Neurosci. 2021;13:693787.
- Tian Y, Cai X, Zhou Y, Jin A, Wang S, Yang Y, et al. Impaired glymphatic system as evidenced by low diffusivity along perivascular spaces is associated with cerebral small vessel disease: a population-based study. Stroke Vasc Neurol. 2023;8(5):413-23.
- Mestre H, Tithof J, Du T, Song W, Peng W, Sweeney AM, et al. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat Commun. 2018;9(1):4878.
- Jiang Q, Zhang L, Ding G, Davoodi-Bojd E, Li Q, Li L, et al. Impairment of the glymphatic system after diabetes. J Cereb Blood Flow Metab. 2017;37(4):1326-37.
- Georgiopoulos C, Tisell A, Holmgren RT, Eleftheriou A, Rydja J, Lundin F, et al. Noninvasive assessment of glymphatic dysfunction in idiopathic normal pressure hydrocephalus with diffusion tensor imaging. J Neurosurg. 2023;1(aop):1-9.
- Si X, Guo T, Wang Z, Fang Y, Gu L, Cao L, et al. Neuroimaging evidence of glymphatic system dysfunction in possible REM sleep behavior disorder and Parkinson’s disease. NPJ Parkinsons Dis. 2022;8(1):54.
- Carotenuto A, Cacciaguerra L, Pagani E, Preziosa P, Filippi M, Rocca MA. Glymphatic system impairment in multiple sclerosis: relation with brain damage and disability. Brain. 2022;145(8):2785-95.
- Jiang D, Liu L, Kong Y, Chen Z, Rosa-Neto P, Chen K, et al. Regional glymphatic abnormality in behavioral variant frontotemporal dementia. Ann Neurol. 2023;94(3):442-56.
- Choi JD, Moon Y, Kim H-J, Yim Y, Lee S, Moon W-J. Choroid plexus volume and permeability at brain MRI within the Alzheimer disease clinical spectrum. Radiology. 2022;304(3):635-45.
- Jeong SH, Jeong HJ, Sunwoo MK, Ahn SS, Lee SK, Lee PH, et al. Association between choroid plexus volume and cognition in Parkinson disease. Eur J Neurol. 2023;30(10):3114-23.
- Chun MY, Jang H, Kim S-J, Park YH, Yun J, Lockhart SN, et al. Emerging role of vascular burden in AT (N) classification in individuals with Alzheimer’s and concomitant cerebrovascular burdens. J Neurol Neurosurg Psychiatry. 2023;95:44-51.
- Keuss SE, Coath W, Nicholas JM, Poole T, Barnes J, Cash DM, et al. Associations of
-amyloid and vascular burden with rates of neurodegeneration in cognitively normal members of the 1946 British birth cohort. Neurology. 2022;99(2):e129-41. - Taoka T, Ito R, Nakamichi R, Kamagata K, Sakai M, Kawai H, et al. Reproducibility of diffusion tensor image analysis along the perivascular space (DTI-ALPS) for evaluating interstitial fluid diffusivity and glymphatic function: CHanges in Alps index on Multiple conditiON acqulsition eXperiment (CHAMONIX) study. Jpn J Radiol. 2022;40(2):147-58.
- Ashburner J, Barnes G, Chen C-C, Daunizeau J, Flandin G, Friston K, et al. SPM12 Manual. 2021.
- Zhu Y-C, Dufouil C, Mazoyer B, Soumaré A, Ricolfi F, Tzourio C, et al. Frequency and location of dilated Virchow-Robin spaces in elderly people: a population-based 3D MR imaging study. Am J Neuroradiol. 2011;32(4):709-13.
- Evans TE, Knol MJ, Schwingenschuh P, Wittfeld K, Hilal S, Ikram MA, et al. Determinants of perivascular spaces in the general population: a pooled cohort analysis of individual participant data. Neurology. 2023;100(2):e107-22.
- Luo X, Jiaerken Y, Yu X, Huang P, Qiu T, Jia Y, et al. Associations between APOE genotype and cerebral small-vessel disease: a longitudinal study. Oncotarget. 2017;8(27):44477.
- Alfons A, Ateş NY, Groenen PJF. Robust Mediation Analysis: The R Package robmed. J Stat Softw. 2022;103(13):1-45.
- Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, et al. Decreased clearance of CNS
-amyloid in Alzheimer’s disease. Science. 2010;330(6012):1774-. - Gertje EC, van Westen D, Panizo C, Mattsson-Carlgren N, Hansson O. Association of enlarged perivascular spaces and measures of small vessel and Alzheimer disease. Neurology. 2021;96(2):e193-202.
- Jeong SH, Cha J, Park M, Jung JH, Ye BS, Sohn YH, et al. Association of enlarged perivascular spaces with amyloid burden and cognitive decline in Alzheimer disease continuum. Neurology. 2022;99(16):e1791-802.
- Bouvy WH, Biessels GJ, Kuijf HJ, Kappelle LJ, Luijten PR, Zwanenburg JJ. Visualization of perivascular spaces and perforating arteries with 7T magnetic resonance imaging. Invest Radiol. 2014;49(5):307-13.
- Xu Z, Xiao N, Chen Y, Huang H, Marshall C, Gao J, et al. Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain A
accumulation and memory deficits. Mol Neurodegener. 2015;10(1):1-16. - Lucey BP.
-amyloid can’t go with the flow. Sci Transl Med. 2016;8(368):368195-368195. - Zhang L, Chopp M, Luo H, Jiang Q, Ding G, Zhang ZG. Abstract WP238: glymphatic system dysfunctions contributes to the progression of cognitive decline in a rat model of Alzheimer’s disease. Stroke. 2023;54(Suppl_1):AWP238-AWP.
- Hawkes CA, Jayakody N, Johnston DA, Bechmann I, Carare RO. Failure of perivascular drainage of
-amyloid in cerebral amyloid angiopathy. Brain Pathol. 2014;24(4):396-403. - Kim SH, Ahn JH, Yang H, Lee P, Koh GY, Jeong Y. Cerebral amyloid angiopathy aggravates perivascular clearance impairment in an Alzheimer’s disease mouse model. Acta Neuropathol Commun. 2020;8(1):1-20.
- Ismail R, Parbo P, Madsen LS, Hansen AK, Hansen KV, Schaldemose JL, et al. The relationships between neuroinflammation, beta-amyloid and tau deposition in Alzheimer’s disease: a longitudinal PET study. J Neuroinflammation. 2020;17:1-11.
- Louveau A, Plog BA, Antila S, Alitalo K, Nedergaard M, Kipnis J. Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J Clin Investig. 2017;127(9):3210-9.
- Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A, Plog BA, Liao Y, et al. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci. 2013;33(46):18190-9.
- Rey J, Sarntinoranont M. Pulsatile flow drivers in brain parenchyma and perivascular spaces: a resistance network model study. Fluids Barriers CNS. 2018;15(1):20.
- Lahna D, Schwartz DL, Woltjer R, Black SE, Roese N, Dodge H, et al. Venous collagenosis as pathogenesis of white matter hyperintensity. Ann Neurol. 2022;92(6):992-1000.
- Fulop GA, Tarantini S, Yabluchanskiy A, Molnar A, Prodan Cl, Kiss T, et al. Role of age-related alterations of the cerebral venous circulation in the pathogenesis of vascular cognitive impairment. Am J Physiol Heart Circ Physiol. 2019;316(5):H1124-40.
- Naessens DMP, Coolen BF, de Vos J, VanBavel E, Strijkers GJ, Bakker E. Altered brain fluid management in a rat model of arterial hypertension. Fluids Barriers CNS. 2020;17(1):41.
- Azarpazhooh MR, Avan A, Cipriano LE, Munoz DG, Sposato LA, Hachinski V. Concomitant vascular and neurodegenerative pathologies double the risk of dementia. Alzheimers Dement. 2018;14(2):148-56.
- Sweeney MD, Montagne A, Sagare AP, Nation DA, Schneider LS, Chui HC, et al. Vascular dysfunction-the disregarded partner of Alzheimer’s disease. Alzheimers Dement. 2019;15(1):158-67.
ملاحظة الناشر
ساهم هوي هونغ ولو وي هونغ بالتساوي في هذا العمل.
*المراسلة:
بييو هوانغ
huangpy@zju.edu.cn
مينمينغ زانغ
zhangminming@zju.edu.cn
القائمة الكاملة لمعلومات المؤلف متاحة في نهاية المقالة
DOI: https://doi.org/10.1186/s13195-024-01407-w
PMID: https://pubmed.ncbi.nlm.nih.gov/38378607
Publication Date: 2024-02-20
The relationship between amyloid
Check for updates pathology, cerebral small vessel disease, glymphatic dysfunction, and cognition: a study based on Alzheimer’s disease continuum participants
Abstract
Background Glymphatic dysfunction is a crucial pathway for dementia. Alzheimer’s disease (AD) pathologies co-existing with cerebral small vessel disease (CSVD) is the most common pathogenesis for dementia. We hypothesize that AD pathologies and CSVD could be associated with glymphatic dysfunction, contributing to cognitive impairment. Method Participants completed with amyloid PET, diffusion tensor imaging (DTI), and T2 fluid-attenuated inversionrecovery (FLAIR) sequences were included from the Alzheimer’s Disease Neuroimaging Initiative (ADNI). White matter hyperintensities (WMH), the most common CSVD marker, was evaluated from T2FLAIR images and represented the burden of CSVD. Amyloid PET was used to assess A
Conclusion Our study provided evidence that both AD pathology (A
Background
Alzheimer’s disease (AD) pathologies, especially
pathologies and CSVD on glymphatic function is separately proved in AD and CSVD models, respectively. Few studies have investigated the independent association of CSVD and AD pathologies with glymphatic function in vivo.
been used to predict conversion to Parkinson’s disease dementia [30].
Therefore, in this study, we aim to investigate (1) the association of CSVD and
Methods and materials
Ethical approval
Participants
[33]. The information on participants with chin-ups was mentioned in Supplementary Material 1.
Neuropsychological assessment
MRI acquisition
Amyloid PET
whole cerebellum (white and gray matter) for each subject.
Imaging analysis
WMH segmentation
The quantification of total intracranial volume (TIV)
Finally, the overall TIV was obtained using the CAT12 estimating TIV function.
DTI-ALPS evaluation
- Preprocessing of DTI
2) DTI-ALPS index analysis

PVS rating
Choroid plexus volume segmentation
Groups
Statistical analysis
Partial correlation analysis was carried out to analyze the independent relationship of global
ganglia PVS and white matter PVS rating score. In the first model, we firstly set age, sex, and TIV as covariates. In model 2, we additionally include APOE
Results
Participants
| CN A – (
|
CN A + (
|
MCI A + (
|
AD A + (N=19) |
|
|
| Demographics | |||||
| Age (years) |
|
|
|
|
0.123 |
| Male,
|
17 (42.5%) | 17 (35.4%) | 7 (26.9%) | 9 (47.4%) | 0.367 |
| Education (years) |
|
|
|
|
0.144 |
| APOE
|
9 (23.1%)
|
22 (45.8%)
|
15 (57.7%) | 11 (57.9%) | 0.763 |
| Vascular risk factors | |||||
| Hypertension, N (%) | 14 (35.0%) | 22 (45.8%) | 13 (50.0%) | 8 (44.4%) | 0.630 |
| Diabetes,
|
3 (7.5%) | 6 (12.5%) | 0 | 0 | 0.128 |
| Hyperlipidemia,
|
16 (40%) | 26 (54.2%) | 12 (46.2%) | 8 (44.4%) | 0.608 |
| Smoking,
|
6 (15.0%) | 9 (18.8%) | 6 (23.1%) | 1 (5.6%) | 0.458 |
| Heart disease,
|
6 (15.0%) | 4 (8.3%) | 4 (15.4%) | 3 (16.7%) | 0.701 |
| Imaging characteristics | |||||
| TIV (
|
|
|
|
|
0.901 |
| WMH burden |
|
|
|
|
< 0.001 |
| Presence of lacune, N (%) | 2 (5%) | 5 (10.4%) | 2 (7.7%) | 1 (5.3%) | 0.781 |
| Presence of microbleed (%) | 4 (10%) | 3 (6.3%) | 3 (11.5%) | 6 (31.6%) | 0.036 |
| Global A
|
|
|
|
|
< 0.001 |
| PVS rating score | |||||
| Basal ganglia PVS | 2.00 (2.00-3.75) | 2.50 (2.00-3.00) | 3.00 (1.00-3.25) | 3.00 (1.00-4.00) | 0.041 |
| White matter PVS | 2.00 (2.00-2.75) | 3.00 (2.00-3.00) | 2.00 (1.75-3.00) | 3.00 (2.00-3.00) | 0.987 |
| Choroid plexus volume |
|
|
|
|
0.029 |
| DTI-ALPS |
|
|
|
|
< 0.001 |
The choroid volume
CN Cognitive normal, MCI Mild cognitive impairment, AD Alzheimer’s disease, TIV Total intracranial volume, WMH White matter hyperintensity, PVS Perivascular space

Associations of glymphatic markers with

Considering the potential impact of vascular risk factors on glymphatic markers, we replicated the aforementioned analysis specifically in participants with at least one vascular risk factor. Additionally, we conducted correlations
between glymphatic markers and amyloid/WMH, with an additional correction for the vascular risk factor score. Both analyses showed that the association between amyloid/WMH and glymphatic markers (DTI-ALPS and choroid plexus volume) remained significant. Further details can be found in Supplementary Material 6 and 7.
| Basal ganglia PVS | White matter PVS | |||
|
|
|
|
|
|
| Model 1 | ||||
|
|
-1.238 | 0.347 | -0.867 | 0.494 |
| WMH burden | 0.542 | 0.173 | 0.054 | 0.889 |
| Model 2 | ||||
|
|
-1.212 | 0.379 | -0.754 | 0.575 |
| WMH burden | 0.489 | 0.225 | -0.054 | 0.890 |
Model 2: age, sex, TIV, and APOE status were included as covariates
TIV Total intracranial volume, WMH White matter hyperintensity, PVS Perivascular space
Associations between glymphatic markers and cognitive performance on amyloid positive participants
However, there were no significant correlations between basal ganglia PVS burden and white matter PVS
burden with cognitive performance. Further details can be found in Table 3.
Glymphatic markers as a significant mediator between
Discussion
| DTI-ALPS | Choroid plexus | Basal ganglia PVS | White matter PVS | |||||
|
|
FDR-p |
|
FDR-p | Rho | FDR-p | Rho | FDR-p | |
| Memory | 0.470 | < 0.001 | -0.315 | 0.007 | 0.060 | 0.704 | 0.054 | 0.788 |
| Executive function | 0.358 | 0.001 | -0.321 | 0.007 | -0.042 | 0.704 | 0.114 | 0.601 |
| Visual-spatial | 0.223 | 0.040 | -0.233 | 0.031 | -0.076 | 0.704 | -0.030 | 0.261 |
| Language | 0.419 | < 0.001 | -0.261 | 0.021 | -0.082 | 0.704 | 0.201 | 0.788 |


we did not find the correlation between PVS enlargement severity and
By plotting the changes of DTI-ALPS and choroid plexus volume against amyloid accumulation along AD continuum, we found that
in the early stages of AD pathogenesis [40]. These collective findings emphasize the complex and multifaceted nature of AD progression, requiring a deeper exploration of the temporal relationships among various factors involved in the disease.
effectively improve glymphatic function. More importantly, our finding from the view of glymphatic function added the mechanism-based evidence for mixed AD dementia (AD pathologies combined with CSVD), in addition to prior studies focusing on their addictive role on whole brain functional or structural connectivity.
Our study had several limitations. Firstly, our evaluation of CSVD was solely based on WMH burden, without considering other important CSVD markers such as lacunes and microbleeds. It is important to acknowledge that WMH can have non-vascular origins, which might potentially introduce some bias into our results. However, it is worth noting that most pathogenic studies have primarily associated WMH with CSVD. Furthermore, the low prevalence of lacunes (
In conclusion, our study provided evidence that AD pathology (
Supplementary Information
Acknowledgements
ADNI consortia information
Authors’ contributions
Funding
Availability of data and materials
Declarations
Ethics approval and consent to participate
Consent for publication
Competing interests
Author details
Received: 2 May 2023 Accepted: 4 February 2024
References
- Rasmussen MK, Mestre H, Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018;17(11):1016-24.
- Nedergaard M, Goldman SA. Glymphatic failure as a final common pathway to dementia. Science. 2020;370(6512):50-6.
- Simon
, Wang , Ismail , Braun , Schindler AG, Reemmer J, et al. Loss of perivascular aquaporin-4 localization impairs glymphatic exchange and promotes amyloid plaque formation in mice. Alzheimer’s Res Ther. 2022;14(1):1-25. - Harrison IF, Siow B, Akilo AB, Evans PG, Ismail O, Ohene Y, et al. Noninvasive imaging of CSF-mediated brain clearance pathways via assessment of perivascular fluid movement with diffusion tensor MRI. Elife. 2018;7:e34028.
- Peng W, Achariyar TM, Li B, Liao Y, Mestre H, Hitomi E, et al. Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2016;93:215-25.
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid
. Sci Transl Med. 2012;4(147):147ra11-ra11. - Da Mesquita S, Louveau A, Vaccari A, Smirnov I, Cornelison RC, Kingsmore KM, et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature. 2018;560(7717):185-91.
- Hsu JL, Wei YC, Toh CH, Hsiao IT, Lin KJ, Yen TC, et al. Magnetic resonance images implicate that glymphatic alterations mediate cognitive dysfunction in Alzheimer disease. Ann Neurol. 2023;93(1):164-74.
- Mortensen KN, Sanggaard S, Mestre H, Lee H, Kostrikov S, Xavier AL, et al. Impaired glymphatic transport in spontaneously hypertensive rats. J Neurosci. 2019;39(32):6365-77.
- Venkat P, Chopp M, Zacharek A, Cui C, Zhang L, Li Q, et al. White matter damage and glymphatic dysfunction in a model of vascular dementia in rats with no prior vascular pathologies. Neurobiol Aging. 2017;50:96-106.
- Wang M, Ding F, Deng S, Guo X, Wang W, Iliff JJ, et al. Focal solute trapping and global glymphatic pathway impairment in a murine model of multiple microinfarcts. J Neurosci. 2017;37(11):2870-7.
- Huang P, Zhang R, Jiaerken Y, Wang S, Yu W, Hong H, et al. Deep white matter hyperintensity is associated with the dilation of perivascular space. J Cereb Blood Flow Metab. 2021;41(9):2370-80.
- Li Y, Zhou Y, Zhong W, Zhu X, Chen Y, Zhang K, et al. Choroid plexus enlargement exacerbates white matter hyperintensity growth through glymphatic impairment. Ann Neurol. 2023;94:182-95.
- Toledo JB, Arnold SE, Raible K, Brettschneider J, Xie SX, Grossman M, et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain. 2013;136(9):2697-706.
- Wardlaw JM, Benveniste H, Nedergaard M, Zlokovic BV, Mestre H, Lee H, et al. Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Rev Neurol. 2020;16(3):137-53.
- Mestre H, Kostrikov S, Mehta RI, Nedergaard M. Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clin Sci. 2017;131(17):2257-74.
- Taoka T, Masutani Y, Kawai H, Nakane T, Matsuoka K, Yasuno F, et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases. Jpn J Radiol. 2017;35:172-8.
- Zhang W, Zhou Y, Wang J, Gong X, Chen Z, Zhang X, et al. Glymphatic clearance function in patients with cerebral small vessel disease. Neuroimage. 2021;238:118257.
- Siow TY, Toh CH, Hsu J-L, Liu G-H, Lee S-H, Chen N-H, et al. Association of sleep, neuropsychological performance, and gray matter volume
with glymphatic function in community-dwelling older adults. Neurology. 2022;98(8):e829-38. - Lee H-J, Lee DA, Shin KJ, Park KM. Glymphatic system dysfunction in obstructive sleep apnea evidenced by DTI-ALPS. Sleep Med. 2022;89:176-81.
- Zhang Y, Zhang R, Ye Y, Wang S, Jiaerken Y, Hong H, et al. The influence of demographics and vascular risk factors on glymphatic function measured by diffusion along perivascular space. Front Aging Neurosci. 2021;13:693787.
- Tian Y, Cai X, Zhou Y, Jin A, Wang S, Yang Y, et al. Impaired glymphatic system as evidenced by low diffusivity along perivascular spaces is associated with cerebral small vessel disease: a population-based study. Stroke Vasc Neurol. 2023;8(5):413-23.
- Mestre H, Tithof J, Du T, Song W, Peng W, Sweeney AM, et al. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat Commun. 2018;9(1):4878.
- Jiang Q, Zhang L, Ding G, Davoodi-Bojd E, Li Q, Li L, et al. Impairment of the glymphatic system after diabetes. J Cereb Blood Flow Metab. 2017;37(4):1326-37.
- Georgiopoulos C, Tisell A, Holmgren RT, Eleftheriou A, Rydja J, Lundin F, et al. Noninvasive assessment of glymphatic dysfunction in idiopathic normal pressure hydrocephalus with diffusion tensor imaging. J Neurosurg. 2023;1(aop):1-9.
- Si X, Guo T, Wang Z, Fang Y, Gu L, Cao L, et al. Neuroimaging evidence of glymphatic system dysfunction in possible REM sleep behavior disorder and Parkinson’s disease. NPJ Parkinsons Dis. 2022;8(1):54.
- Carotenuto A, Cacciaguerra L, Pagani E, Preziosa P, Filippi M, Rocca MA. Glymphatic system impairment in multiple sclerosis: relation with brain damage and disability. Brain. 2022;145(8):2785-95.
- Jiang D, Liu L, Kong Y, Chen Z, Rosa-Neto P, Chen K, et al. Regional glymphatic abnormality in behavioral variant frontotemporal dementia. Ann Neurol. 2023;94(3):442-56.
- Choi JD, Moon Y, Kim H-J, Yim Y, Lee S, Moon W-J. Choroid plexus volume and permeability at brain MRI within the Alzheimer disease clinical spectrum. Radiology. 2022;304(3):635-45.
- Jeong SH, Jeong HJ, Sunwoo MK, Ahn SS, Lee SK, Lee PH, et al. Association between choroid plexus volume and cognition in Parkinson disease. Eur J Neurol. 2023;30(10):3114-23.
- Chun MY, Jang H, Kim S-J, Park YH, Yun J, Lockhart SN, et al. Emerging role of vascular burden in AT (N) classification in individuals with Alzheimer’s and concomitant cerebrovascular burdens. J Neurol Neurosurg Psychiatry. 2023;95:44-51.
- Keuss SE, Coath W, Nicholas JM, Poole T, Barnes J, Cash DM, et al. Associations of
-amyloid and vascular burden with rates of neurodegeneration in cognitively normal members of the 1946 British birth cohort. Neurology. 2022;99(2):e129-41. - Taoka T, Ito R, Nakamichi R, Kamagata K, Sakai M, Kawai H, et al. Reproducibility of diffusion tensor image analysis along the perivascular space (DTI-ALPS) for evaluating interstitial fluid diffusivity and glymphatic function: CHanges in Alps index on Multiple conditiON acqulsition eXperiment (CHAMONIX) study. Jpn J Radiol. 2022;40(2):147-58.
- Ashburner J, Barnes G, Chen C-C, Daunizeau J, Flandin G, Friston K, et al. SPM12 Manual. 2021.
- Zhu Y-C, Dufouil C, Mazoyer B, Soumaré A, Ricolfi F, Tzourio C, et al. Frequency and location of dilated Virchow-Robin spaces in elderly people: a population-based 3D MR imaging study. Am J Neuroradiol. 2011;32(4):709-13.
- Evans TE, Knol MJ, Schwingenschuh P, Wittfeld K, Hilal S, Ikram MA, et al. Determinants of perivascular spaces in the general population: a pooled cohort analysis of individual participant data. Neurology. 2023;100(2):e107-22.
- Luo X, Jiaerken Y, Yu X, Huang P, Qiu T, Jia Y, et al. Associations between APOE genotype and cerebral small-vessel disease: a longitudinal study. Oncotarget. 2017;8(27):44477.
- Alfons A, Ateş NY, Groenen PJF. Robust Mediation Analysis: The R Package robmed. J Stat Softw. 2022;103(13):1-45.
- Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, et al. Decreased clearance of CNS
-amyloid in Alzheimer’s disease. Science. 2010;330(6012):1774-. - Gertje EC, van Westen D, Panizo C, Mattsson-Carlgren N, Hansson O. Association of enlarged perivascular spaces and measures of small vessel and Alzheimer disease. Neurology. 2021;96(2):e193-202.
- Jeong SH, Cha J, Park M, Jung JH, Ye BS, Sohn YH, et al. Association of enlarged perivascular spaces with amyloid burden and cognitive decline in Alzheimer disease continuum. Neurology. 2022;99(16):e1791-802.
- Bouvy WH, Biessels GJ, Kuijf HJ, Kappelle LJ, Luijten PR, Zwanenburg JJ. Visualization of perivascular spaces and perforating arteries with 7T magnetic resonance imaging. Invest Radiol. 2014;49(5):307-13.
- Xu Z, Xiao N, Chen Y, Huang H, Marshall C, Gao J, et al. Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain A
accumulation and memory deficits. Mol Neurodegener. 2015;10(1):1-16. - Lucey BP.
-amyloid can’t go with the flow. Sci Transl Med. 2016;8(368):368195-368195. - Zhang L, Chopp M, Luo H, Jiang Q, Ding G, Zhang ZG. Abstract WP238: glymphatic system dysfunctions contributes to the progression of cognitive decline in a rat model of Alzheimer’s disease. Stroke. 2023;54(Suppl_1):AWP238-AWP.
- Hawkes CA, Jayakody N, Johnston DA, Bechmann I, Carare RO. Failure of perivascular drainage of
-amyloid in cerebral amyloid angiopathy. Brain Pathol. 2014;24(4):396-403. - Kim SH, Ahn JH, Yang H, Lee P, Koh GY, Jeong Y. Cerebral amyloid angiopathy aggravates perivascular clearance impairment in an Alzheimer’s disease mouse model. Acta Neuropathol Commun. 2020;8(1):1-20.
- Ismail R, Parbo P, Madsen LS, Hansen AK, Hansen KV, Schaldemose JL, et al. The relationships between neuroinflammation, beta-amyloid and tau deposition in Alzheimer’s disease: a longitudinal PET study. J Neuroinflammation. 2020;17:1-11.
- Louveau A, Plog BA, Antila S, Alitalo K, Nedergaard M, Kipnis J. Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J Clin Investig. 2017;127(9):3210-9.
- Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A, Plog BA, Liao Y, et al. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci. 2013;33(46):18190-9.
- Rey J, Sarntinoranont M. Pulsatile flow drivers in brain parenchyma and perivascular spaces: a resistance network model study. Fluids Barriers CNS. 2018;15(1):20.
- Lahna D, Schwartz DL, Woltjer R, Black SE, Roese N, Dodge H, et al. Venous collagenosis as pathogenesis of white matter hyperintensity. Ann Neurol. 2022;92(6):992-1000.
- Fulop GA, Tarantini S, Yabluchanskiy A, Molnar A, Prodan Cl, Kiss T, et al. Role of age-related alterations of the cerebral venous circulation in the pathogenesis of vascular cognitive impairment. Am J Physiol Heart Circ Physiol. 2019;316(5):H1124-40.
- Naessens DMP, Coolen BF, de Vos J, VanBavel E, Strijkers GJ, Bakker E. Altered brain fluid management in a rat model of arterial hypertension. Fluids Barriers CNS. 2020;17(1):41.
- Azarpazhooh MR, Avan A, Cipriano LE, Munoz DG, Sposato LA, Hachinski V. Concomitant vascular and neurodegenerative pathologies double the risk of dementia. Alzheimers Dement. 2018;14(2):148-56.
- Sweeney MD, Montagne A, Sagare AP, Nation DA, Schneider LS, Chui HC, et al. Vascular dysfunction-the disregarded partner of Alzheimer’s disease. Alzheimers Dement. 2019;15(1):158-67.
Publisher’s Note
Hui Hong and Luwei Hong contributed equally to this work.
*Correspondence:
Peiyu Huang
huangpy@zju.edu.cn
Minming Zhang
zhangminming@zju.edu.cn
Full list of author information is available at the end of the article
