DOI: https://doi.org/10.1038/s41368-023-00271-y
PMID: https://pubmed.ncbi.nlm.nih.gov/38296940
تاريخ النشر: 2024-01-31
المغنيسيوم يعزز تكوين الأوعية الدموية والتكامل العظمي في حالات السكري
الملخص
لقد اعتُبر السكري لفترة طويلة عامل خطر في علاج الزرع وضعف شفاء الجروح في الأنسجة الفموية الرخوة والصلبة. لقد ثبت أن المغنيسيوم يعزز شفاء العظام في الظروف الطبيعية. هنا، نوضح الآلية التي من خلالها
المقدمة
نُشر على الإنترنت: 31 يناير 2024
النتائج
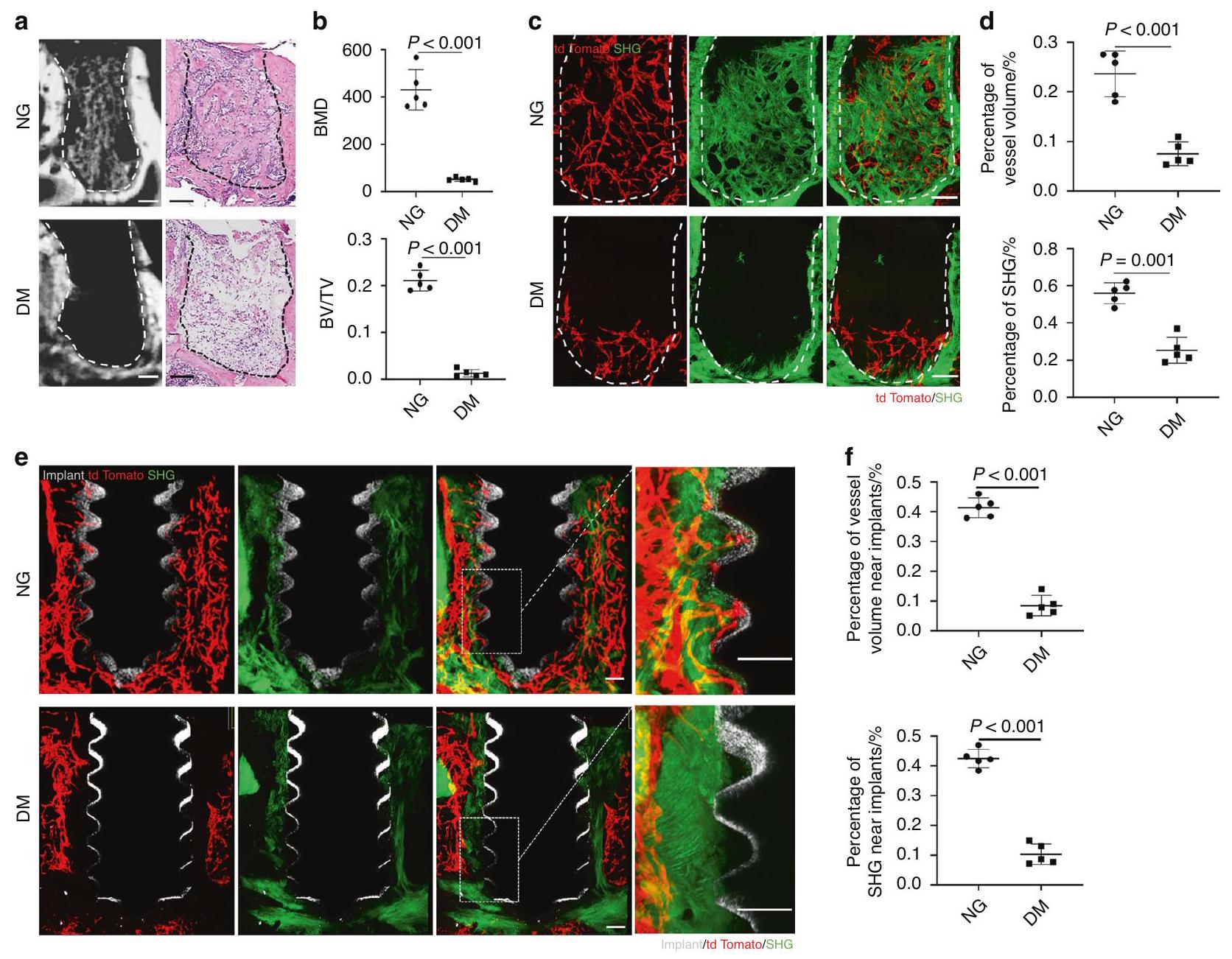
لكشف تأثير السكري على شفاء العظام الفكية، أنشأنا نموذج فئران سكري عن طريق حقن STZ واستخرجنا الأضراس السفلية الأولى. أظهر تحليل Micro-CT أن الفئران DM كانت لديها انخفاض كبير في تكوين العظام مقارنة بالفئران NG. أكدت صبغة H&E للعظام الفكية المنزوعة الكالسيوم الشكل النسيجي للفراغات الفارغة في الفئران DM مقارنة بتكوين العظام الواضح في الفئران NG (الشكل 1أ، ب).
منخفضة بشكل كبير وكانت إشارات الكولاجين شبه غائبة (الشكل 1ج، د).
أن
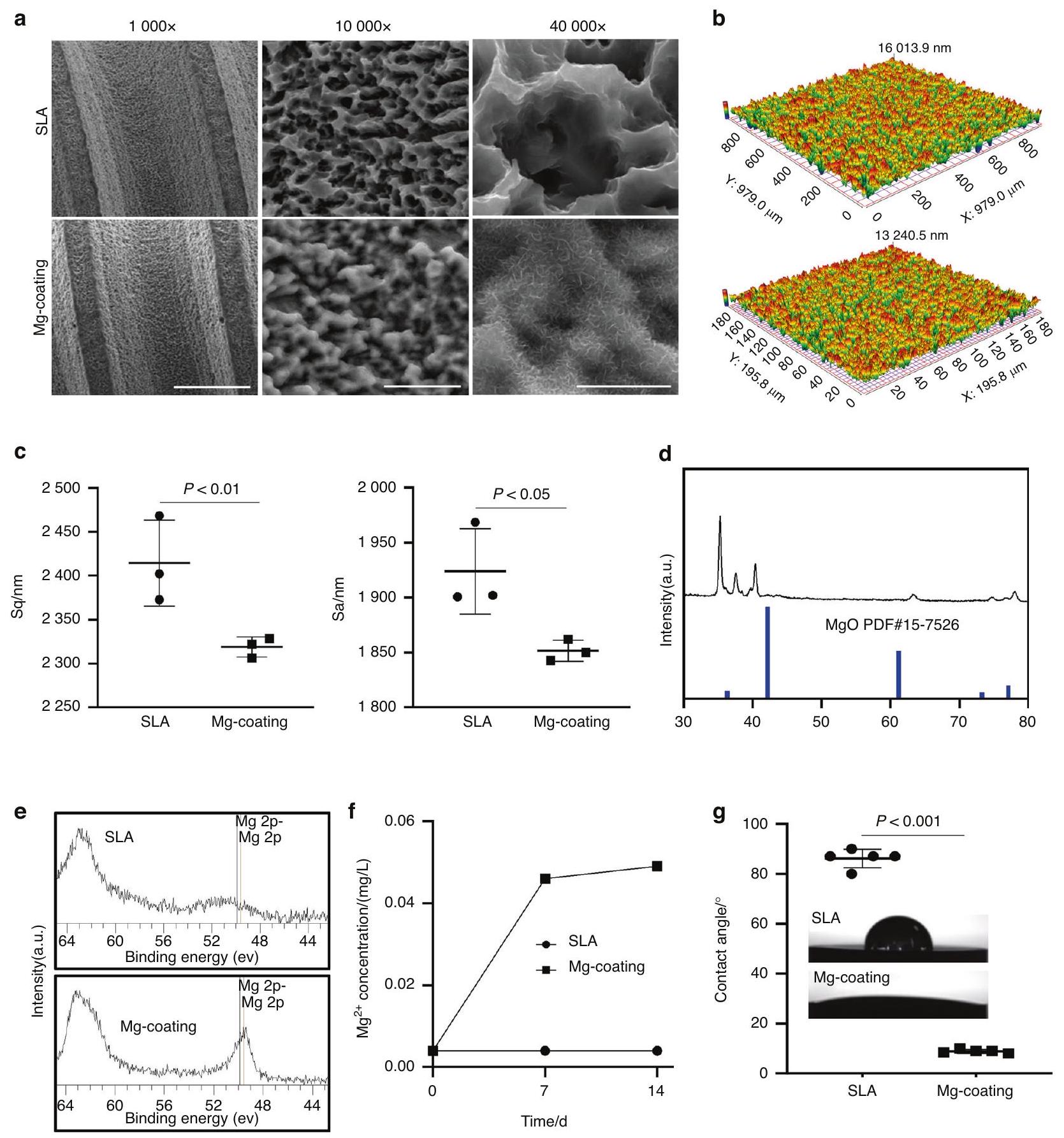
بعد ذلك، قمنا بتطوير زراعة وأوراق مغطاة بمادة المغنيسيوم من خلال التخليق الهيدروحراري. أظهرت مسح SEM أن الطلاء احتفظ بهيكل البناء الملولب للزراعة بينما تم ترسيب أملاح غير عضوية متجانسة على سطح الزراعة في هيكل يشبه النانو، لكنها لم تسد مسام سطح الزراعة SLA تحت تكبير أعلى (الشكل 3أ). السطح
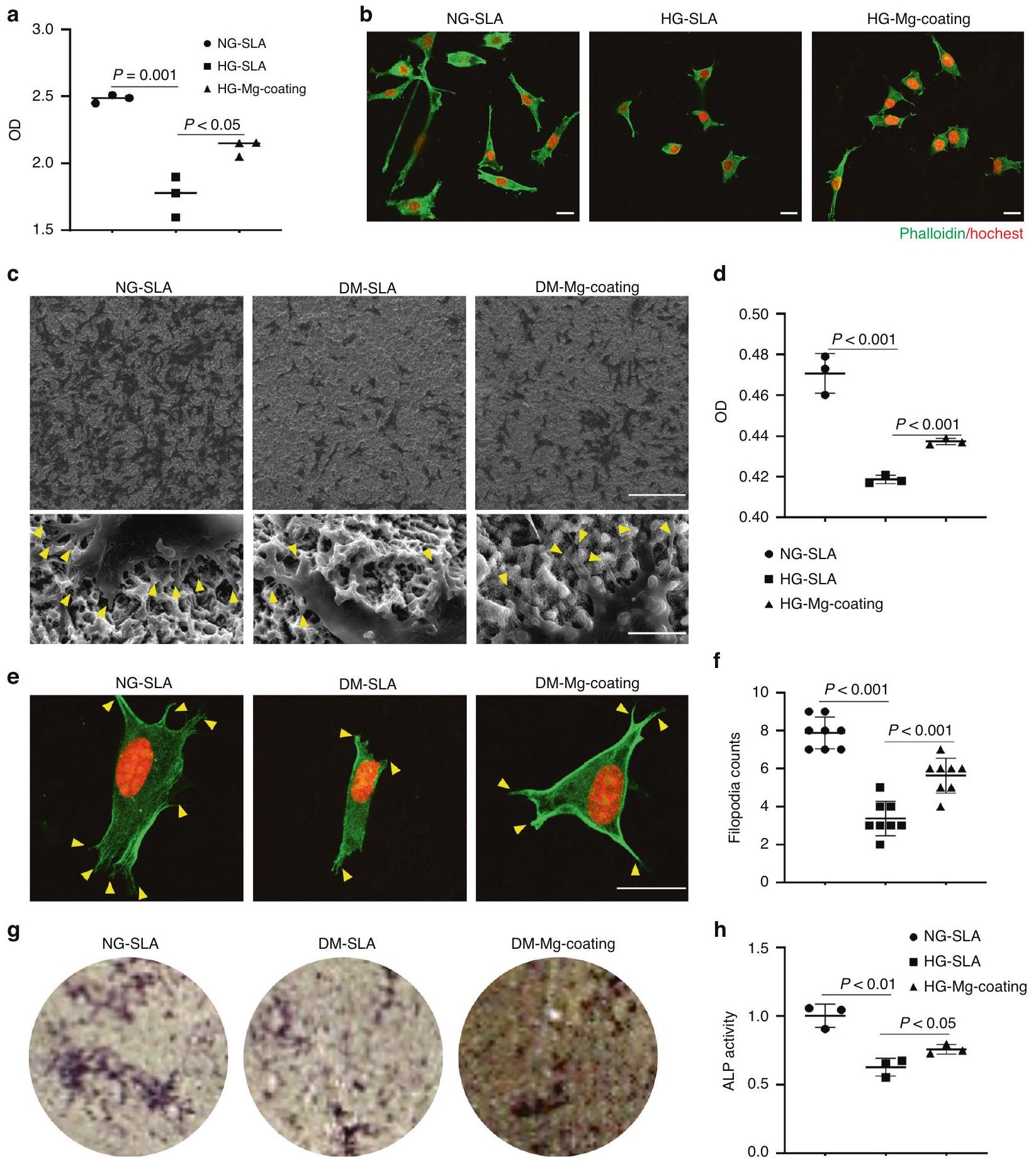
للتحقق من تأثير زراعة الطلاء المغنيسيوم على التوعية الدموية والتكامل العظمي في الفئران المصابة بالسكري، قمنا بإدخال الزرعات في Cdh
تم ملء نسيج العظام في المنطقة المجاورة لسطح الغرسات في مجموعة الطلاء بالمغنيسيوم (الشكل 5 ج، د). بدوره، المزيد من الطماطم
الاندماج العظمي تحت تأثير السكري مقارنة بزراعة الغرسات المطلية بالتيتانيوم، مؤكدًا التأثير العظمي لـ
تم إجراء تحليل تسلسل RNA لاستكشاف الآلية التي من خلالها
تؤدي أنواع الأكسجين التفاعلية إلى اضطرابات استقلابية وحتى موت الخلايا من خلال هجوم الميتوكوندريا. وقد أثبت قياس جودة الميتوكوندريا أن
نقاش
إصلاح الأضرار.
قام نت وآخرون (2023) أيضًا بإنشاء هيدروجيل يحتوي على المغنيسيوم لتعزيز تكوين الأوعية الدموية في الجروح السكرية لتعزيز الشفاء.
مستويات الإجهاد التأكسدي، تحسين جودة الميتوكوندريا، زيادة نواة Nrf2 upstream من SLC7A11 وزيادة مستويات ATF4، افترضنا أن
المواد والطرق
فئران
بناء نموذج السكري
الجراحة
تم تثبيتها في المقبس بعد الاستخراج تحت المجهر الاستريو. في اليوم السابع بعد الاستخراج أو الزرع، تم جمع الفكوك.
تم تنفيذ عملية تنظيف الأنسجة PEGASOS كما تم الإبلاغ عنها سابقًا.
تم الحصول على الصور من خلال مجهر متعدد الفوتونات (Lecia SP8 DIVE). تم الحصول على إشارات TdTomato تحت خط ليزر تحفيز بطول موجي 561 نانومتر. تم الحصول على الزرع باستخدام ليزر ياقوتي بطول موجي 950 نانومتر. تم الحصول على تصوير الإشارات التوافقية الثانية (SHG) باستخدام كاشف غير مزال. تم استخدام برنامج IMARIS (الإصدار 9.1.2؛ أكسفورد إنسترومنتس) لتحليل البيانات ومعالجة الصور. منطقة
تم تثبيت الفكوك المحصودة مع الزرعات في 4% PFA عند
الفكوك مع الزرعات كانت مثبتة في
تم زراعة خلايا بطانة الوريد السري البشري (HUVECs) المقدمة من المختبر الرئيسي للدولة للعلاج الحيوي، مستشفى غرب الصين الثاني الجامعي، جامعة سيتشوان، في وسط دلبوكو المعدل (DMEM) (هايكلاون، مختبرات، لوغان، يوتا، الولايات المتحدة الأمريكية) يحتوي على 10% من مصل الجنين البقري (جيبكو، غراند آيلاند، نيويورك، الولايات المتحدة الأمريكية)،
تم زراعة خلايا HUVECs في لوحة 96 بئر بكثافة 5000 خلية لكل بئر. ثم تم إضافة وسط NG أو وسط HG أو وسط HG المحتوي على
يحتوي على
تفاعل البوليميراز المتسلسل العكسي الكمي (qRT-PCR)
اختبار شفاء الجروح الخدش
خفض الجين
تصنيع زرعات بطبقة مغنيسيوم وطبقة تيتانيوم
توصيف المواد للعينات
اختبار حيوية الخلايا
تم تثبيت الخلايا باستخدام 4% PFA لمدة 10 دقائق وتم صبغ الهيكل الخلوي بواسطة الفالودين وصبغ النوى بواسطة DAPI. تم الكشف عن التصوير الفلوري للخلايا بواسطة المجهر الضوئي الماسح بالليزر (FV3000؛ أوليمبوس، اليابان). بعد يوم واحد من الزراعة على الركائز، تم تثبيت الخلايا طوال الليل باستخدام
صبغة الفوسفاتاز القلوية
تحليلات نسيجية وهستومورفومترية
تسلسل RNA وتحليل البيانات
تحليل تدفق الخلايا
البلوت الغربي
اختبار نشاط SOD
التحليلات الإحصائية
توفر البيانات والمواد
شكر وتقدير
مساهمات المؤلفين
معلومات إضافية
REFERENCES
- Buser, D., Sennerby, L. & De Bruyn, H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontology 73, 7-21 (2017).
- De Angelis, F. et al. Implant survival and success rates in patients with risk factors: results from a long-term retrospective study with a 10 to 18 years follow-up. Rev. Med. Pharmacol. Sci. 21, 433-437 (2017).
- de Oliveira, P. et al. Obesity/metabolic syndrome and diabetes mellitus on periimplantitis. Trends Endocrinol. Metab. 31, 596-610 (2020).
- Javed, F. & Romanos, G. E. Chronic hyperglycemia as a risk factor in implant therapy. Periodontology 81, 57-63 (2019).
- Quan, Y. (ed.) Dental Implant Treatment in Medically Compromised Patients. (Springer Press, 2020).
- Ko, K. I., Sculean, A. & Graves, D. T. Diabetic wound healing in soft and hard oral tissues. Transl. Res.: J. Lab. Clin. Med. 236, 72-86 (2021).
- Claes, L., Recknagel, S. & Ignatius, A. Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 8, 133-143 (2012).
- Stegen, S., van Gastel, N. & Carmeliet, G. Bringing new life to damaged bone: the importance of angiogenesis in bone repair and regeneration. Bone 70, 19-27 (2015).
- Tuckermann, J. & Adams, R. H. The endothelium-bone axis in development, homeostasis and bone and joint disease. Nat. Rev. Rheumatol. 17, 608-620 (2021).
- Hu, X. F., Wang, L., Xiang, G., Lei, W. & Feng, Y. F. Angiogenesis impairment by the NADPH oxidase-triggered oxidative stress at the bone-implant interface: Critical mechanisms and therapeutic targets for implant failure under hyperglycemic conditions in diabetes. Acta Biomater. 73, 470-487 (2018).
- Beckman, J. A. & Creager, M. A. Vascular complications of diabetes. Circul. Res. 118, 1771-1785 (2016).
- Xu, Z. et al. Thermosensitive hydrogel incorporating Prussian blue nanoparticles promotes diabetic wound healing via ROS scavenging and mitochondrial function restoration. ACS Appl. Mater. Interfaces 14, 14059-14071 (2022).
- de Baaij, J. H., Hoenderop, J. G. & Bindels, R. J. Magnesium in man: implications for health and disease. Physiol. Rev. 95, 1-46 (2015).
- Zhang, Y. et al. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat. Med. 22, 1160-1169 (2016).
- Lin, S. et al. A magnesium-enriched 3D culture system that mimics the bone development microenvironment for vascularized bone regeneration. Adv. Sci. (Weinh.) 6, 1900209 (2019).
- Han, H. S. et al. Biodegradable magnesium alloys promote angio-osteogenesis to enhance bone repair. Adv. Sci. (Weinh.) 7, 2000800 (2020).
- Zhu, D., You, J., Zhao, N. & Xu, H. Magnesium regulates endothelial barrier functions through TRPM7, MagT1, and S1P1. Adv. Sci. (Weinh.) 6, 1901166 (2019).
- Yin, M. et al. Multifunctional magnesium organic framework-based microneedle patch for accelerating diabetic wound healing. ACS Nano 15, 17842-17853 (2021).
- Sales, C. H. & Pedrosa Lde, F. Magnesium and diabetes mellitus: their relation. Clin. Nutrition (Edinburgh, Scotland) 25, 554-562 (2006).
- WA, E. L., Naser, I. A., Taleb, M. H. & Abutair, A. S. The effects of oral magnesium supplementation on glycemic response among type 2 diabetes patients. Nutrients 11, 44 (2018).
- Veronese, N. et al. Oral magnesium supplementation for treating glucose metabolism parameters in people with or at risk of diabetes: a systematic review and metaanalysis of double-blind randomized controlled trials. Nutrients 13, 4074 (2021).
- He, T. et al. A comparison of micro-CT and histomorphometry for evaluation of osseointegration of PEO-coated titanium implants in a rat model. Sci. Rep. 7, 16270 (2017).
- Calvo-Guirado, J. L. et al. Histological and histomorphometric evaluation of immediate implant placement on a dog model with a new implant surface treatment. Clin. Oral Implants Res. 21, 308-315 (2010).
- Jing, D. et al. Tissue clearing and its application to bone and dental tissues. J. Dental Res. 98, 621-631 (2019).
- Jing, D. et al. Tissue clearing of both hard and soft tissue organs with the PEGASOS method. Cell Res. 28, 803-818 (2018).
- Sivaraj, K. K. & Adams, R. H. Blood vessel formation and function in bone. Development (Cambridge, England) 143, 2706-2715 (2016).
- Lin, W. et al. Mapping the immune microenvironment for mandibular alveolar bone homeostasis at single-cell resolution. Bone Res. 9, 17 (2021).
- Kusumbe, A. P., Ramasamy, S. K. & Adams, R. H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 507, 323-328 (2014).
- Peng, Y., Wu, S., Li, Y. & Crane, J. L. Type H blood vessels in bone modeling and remodeling. Theranostics 10, 426-436 (2020).
- Brem, H. & Tomic-Canic, M. Cellular and molecular basis of wound healing in diabetes. J. Clin. Investig. 117, 1219-1222 (2007).
- Orasanu, G. & Plutzky, J. The pathologic continuum of diabetic vascular disease. J. Am. College Cardiol. 53, S35-S42 (2009).
- Devlin, H., Garland, H. & Sloan, P. Healing of tooth extraction sockets in experimental diabetes mellitus. J. Oral Maxillofac. Surg. 54, 1087-1091 (1996).
- Xiong, Y. et al. A whole-course-repair system based on neurogenesisangiogenesis crosstalk and macrophage reprogramming promotes diabetic wound healing. Adv. Mater. (Deerfield Beach, Fla.), e2212300, https://doi.org/ 10.1002/adma. 202212300 (2023).
- Rohlenova, K., Veys, K., Miranda-Santos, I., De Bock, K. & Carmeliet, P. Endothelial cell metabolism in health and disease. Trends Cell Biol. 28, 224-236 (2018).
- Potente, M., Gerhardt, H. & Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 146, 873-887 (2011).
- Sawada, N. et al. Endothelial PGC-1a mediates vascular dysfunction in diabetes. Cell Metab. 19, 246-258 (2014).
- Giacco, F. & Brownlee, M. Oxidative stress and diabetic complications. Circulation Res. 107, 1058-1070 (2010).
- Eelen, G., de Zeeuw, P., Simons, M. & Carmeliet, P. Endothelial cell metabolism in normal and diseased vasculature. Circulation Res. 116, 1231-1244 (2015).
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 414, 813-820 (2001).
- Wang, Y. et al. Integrated regulation of stress responses, autophagy and survival by altered intracellular iron stores. Redox Biol. 55, 102407 (2022).
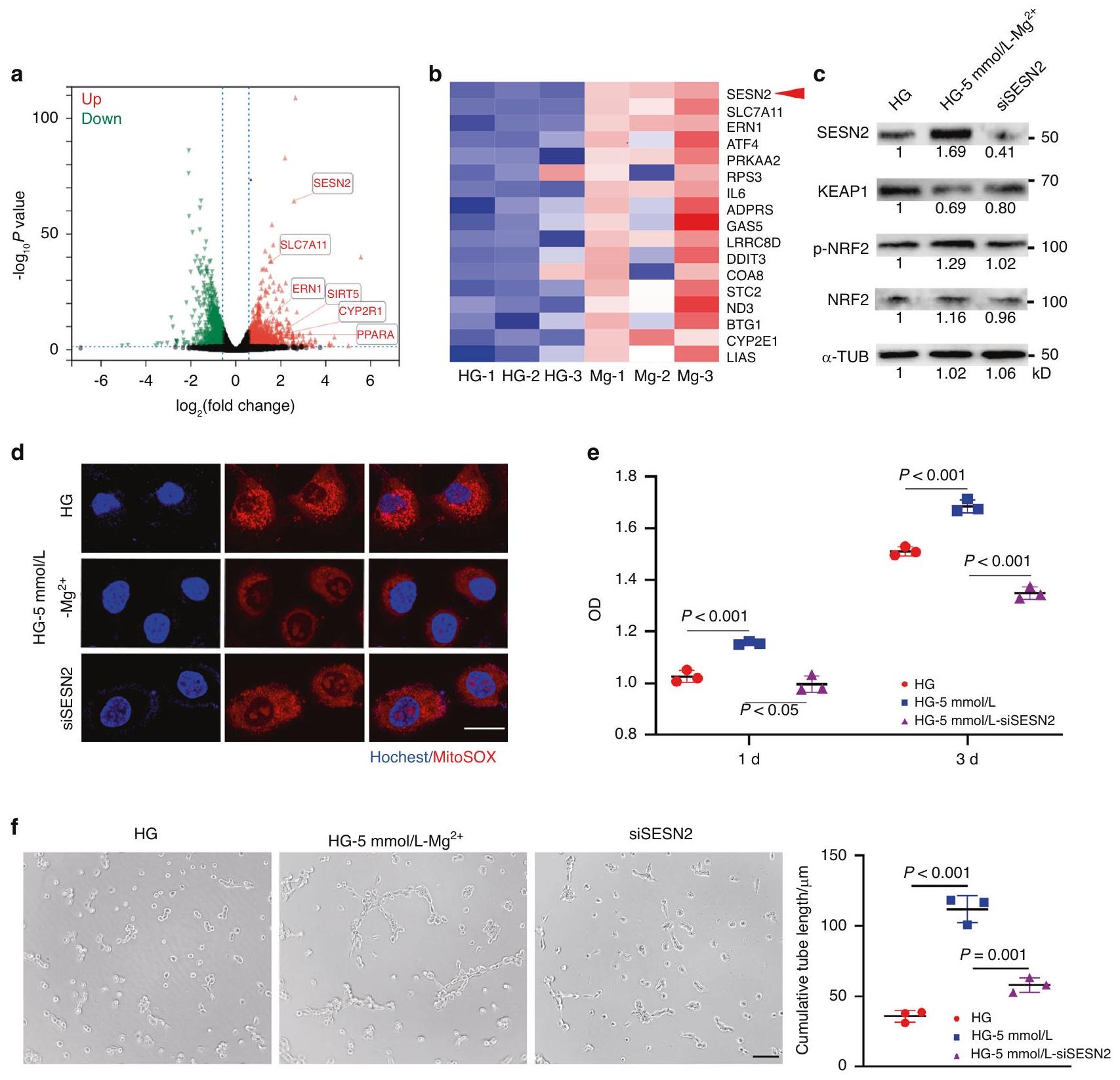
- Ding, S., Ma, N., Liu, H., Tang, M. & Mei, J. Sesn2 attenuates the damage of endothelial progenitor cells induced by angiotensin II through regulating the Keap1/Nrf2 signal pathway. Aging 12, 25505-25527 (2020).
- Muri, J. & Kopf, M. Redox regulation of immunometabolism. Nat. Rev. Immunol. 21, 363-381 (2021).
- Rupp, F., Liang, L., Geis-Gerstorfer, J., Scheideler, L. & Hüttig, F. Surface characteristics of dental implants: a review. Dental Mater. 34, 40-57 (2018).
© The Author(s) 2024
State Key Laboratory of Oral Diseases & National Center for Stomatology & National Clinical Research Center for Oral Diseases & West China Hospital of Stomatology, Sichuan University, Chengdu, China and State Key Laboratory of Oral Diseases & National Center for Stomatology & National Clinical Research Center for Oral Diseases & Department of Oral Implantology, West China Hospital of Stomatology, Sichuan University, Chengdu, China
Correspondence: Quan Yuan (yuanquan@scu.edu.cn) or Shiwen Zhang (sw.zhang2018@scu.edu.cn)
These authors contributed equally: Linfeng Liu, Feiyu Wang
DOI: https://doi.org/10.1038/s41368-023-00271-y
PMID: https://pubmed.ncbi.nlm.nih.gov/38296940
Publication Date: 2024-01-31
Magnesium promotes vascularization and osseointegration in diabetic states
Abstract
Diabetes has long been considered a risk factor in implant therapy and impaired wound healing in soft and hard oral tissues. Magnesium has been proved to promote bone healing under normal conditions. Here, we elucidate the mechanism by which
INTRODUCTION
Published online: 31 January 2024

RESULTS
To detect the effect of diabetes on the healing of alveolar bone, we generated a diabetic mice model by STZ injection and extracted the first mandibular molars. Micro-CT analysis showed that DM mice had a significant reduction in bone formation compared to normoglycemic (NG) mice. H&E staining of the decalcified alveolar bone confirmed the empty extraction socket histomorphology in DM mice in contrast to obvious bone formation in NG mice (Fig. 1a, b).
was significantly reduced and the collagen signals was almost absent (Fig. 1c, d).

that
Next, we developed Mg-coating implants and sheets by hydrothermal synthesis. SEM scanning showed that the coating retained threaded construction structure of the implant while homogeneous inorganic salts were deposited on the implant surface in a nanopattern-like structure, but did not block the pores of the SLA implant surface under higher magnification (Fig. 3a). The surface


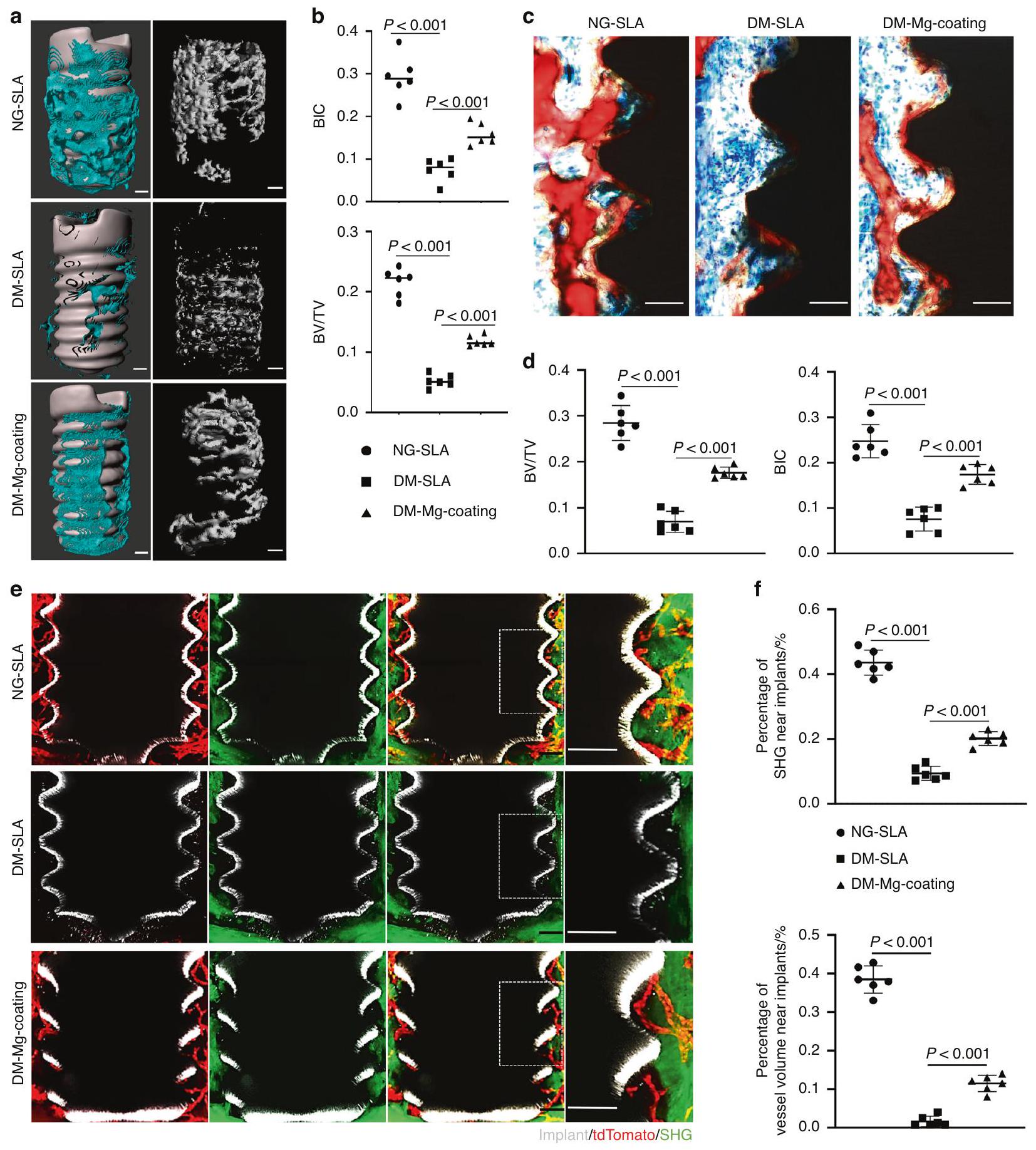
To verify the effect of Mg -coating implants on vascularization and osseointegration in diabetic mice, we inserted implants in Cdh
bone tissue was filled in the neighboring area to the surface of the implants in Mg-coating group (Fig. 5c, d). In turn, more Tomato

osseointegration under diabetes compared to titanium-coatings implants, confirming the osteogenic effect of
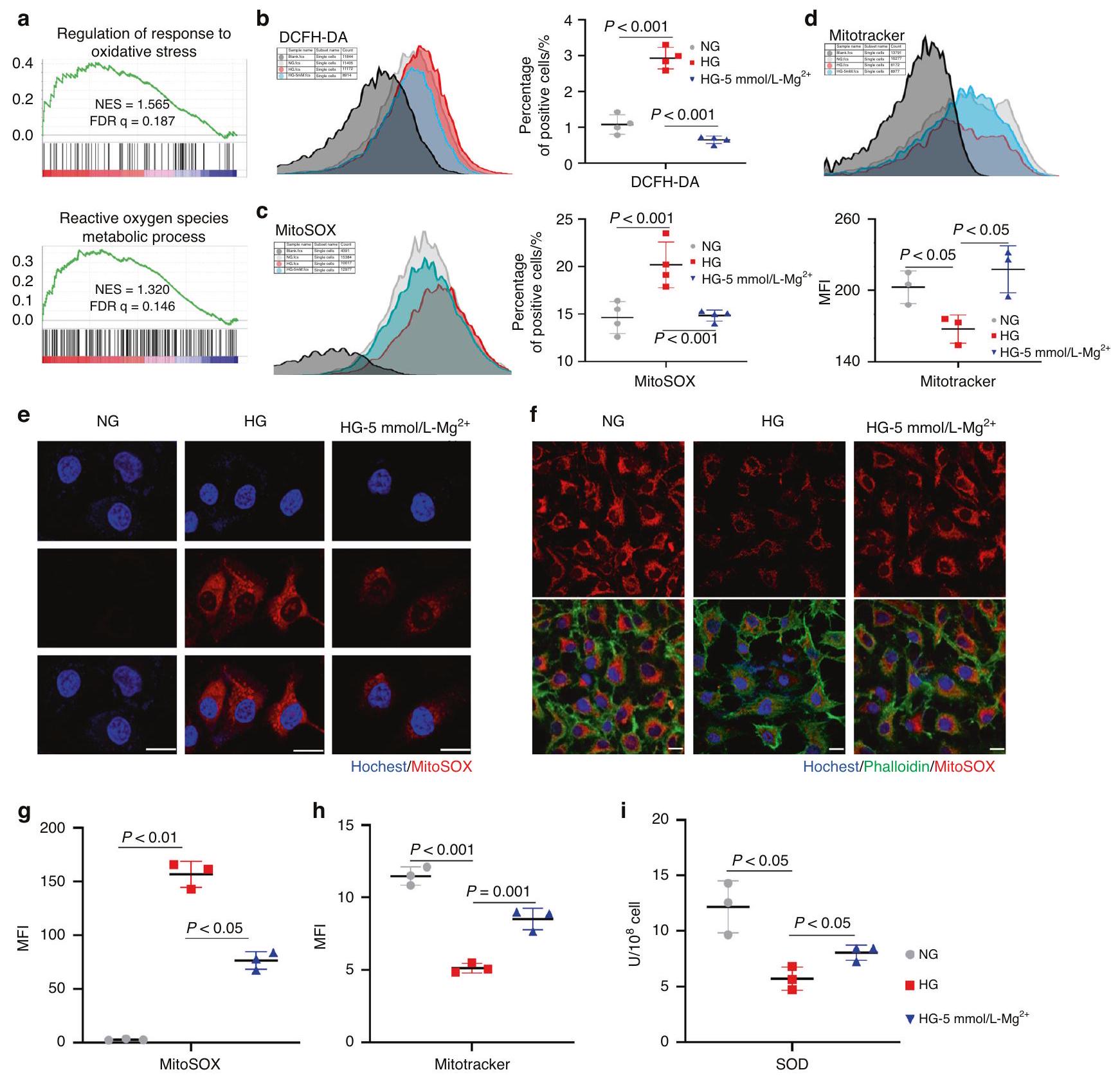
RNA-sequencing analysis was performed to explore the mechanism by which

reactive oxygen species result in metabolic disorders and even death of cells by mitochondrial attack. Mitochondrial quality measuring proved that

DISCUSSION
damage repair.
et al. (2023) also constructed magnesium-containing hydrogels to strengthen diabetic wound angiogenesis to promote healing.
oxidative stress levels, improved mitochondrial quality, increased Nrf2 nucleation upstream of SLC7A11 and upregulate ATF4 levels, we speculated that
MATERIALS AND METHODS
Mice
Construction of the diabetic model
Surgery
screwed in the socket after extraction under the stereomicroscope. On day 7 after extraction or implantation, mandibles were harvested.
The PEGASOS tissue clearing process was performed as reported before.
Images were acquired through a multiphoton microscope (Lecia SP8 DIVE). TdTomato signals were acquired under a 561 nm excitation laser line. The implant was acquired with a sapphire laser at 950 nm wavelength. Second harmonic signals (SHG) imaging was acquired with a non-descanned detector. IMARIS software (version 9.1.2; Oxford Instruments) was used for data analysis and image processing. A region
The harvested mandibles with implants were fixed in 4% PFA at
The mandibles with implants were fixed in
Human Umbilical Vein Endothelial Cells (HUVECs) provided by State Key Laboratory of Biotherapy, West China Second University Hospital, Sichuan University, were cultured in Dulbecco’s modified Eagle medium (DMEM) (Hyclone, Laboratories, Logan, UT, USA) containing 10% fetal bovine serum (Gibco, Grand Island, NY, USA),
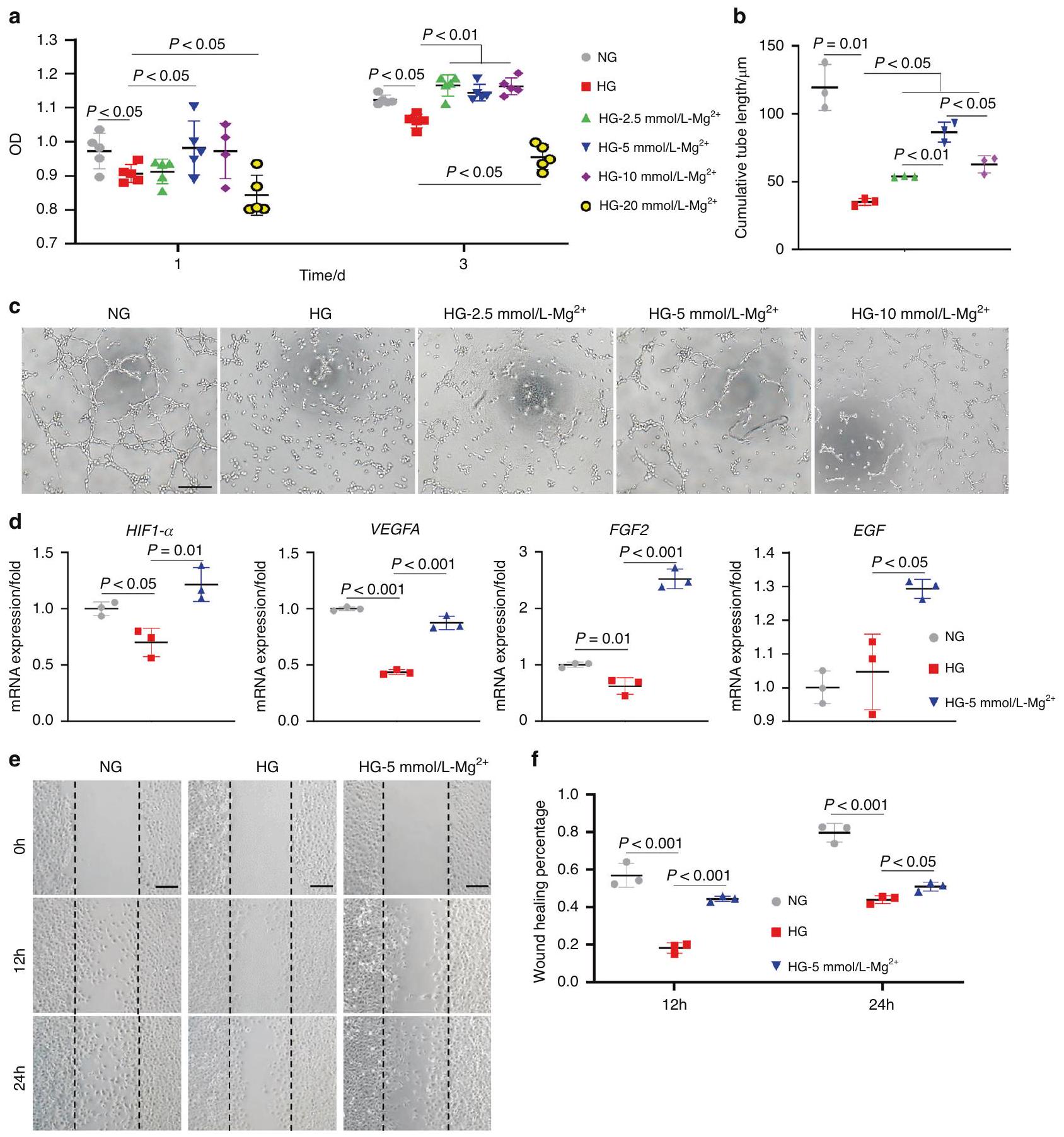
HUVECs were seeded in a 96-well plate at a density of 5000 cells per well. Then NG medium, HG medium, or HG medium containing
containing
Quantitative reverse-transcription PCR (qRT-PCR)
Scratch wound healing assay
Gene knockdown
Fabrication of Mg-coating and Ti-coating implants
Materials characterization of samples
Cell viability assay
fixed with 4% PFA for 10 min and the cell cytoskeleton was stained by phalloidin and the nuclei stained by DAPI. Fluorescent imaging of cells was detected by laser scanning confocal microscopy (FV3000; Olympus, Japan). After 1 day of culture on substrates, cells were fixed overnight with
Alkaline phosphatase staining
Histologic and histomorphometric analyses
RNA-Sequencing and data analysis
Flow cytometry analysis
Western blot
SOD activity assay
Statistical analyses
DATA AND MATERIALS AVAILABILITY
ACKNOWLEDGEMENTS
AUTHOR CONTRIBUTIONS
ADDITIONAL INFORMATION
REFERENCES
- Buser, D., Sennerby, L. & De Bruyn, H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontology 73, 7-21 (2017).
- De Angelis, F. et al. Implant survival and success rates in patients with risk factors: results from a long-term retrospective study with a 10 to 18 years follow-up. Rev. Med. Pharmacol. Sci. 21, 433-437 (2017).
- de Oliveira, P. et al. Obesity/metabolic syndrome and diabetes mellitus on periimplantitis. Trends Endocrinol. Metab. 31, 596-610 (2020).
- Javed, F. & Romanos, G. E. Chronic hyperglycemia as a risk factor in implant therapy. Periodontology 81, 57-63 (2019).
- Quan, Y. (ed.) Dental Implant Treatment in Medically Compromised Patients. (Springer Press, 2020).
- Ko, K. I., Sculean, A. & Graves, D. T. Diabetic wound healing in soft and hard oral tissues. Transl. Res.: J. Lab. Clin. Med. 236, 72-86 (2021).
- Claes, L., Recknagel, S. & Ignatius, A. Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 8, 133-143 (2012).
- Stegen, S., van Gastel, N. & Carmeliet, G. Bringing new life to damaged bone: the importance of angiogenesis in bone repair and regeneration. Bone 70, 19-27 (2015).
- Tuckermann, J. & Adams, R. H. The endothelium-bone axis in development, homeostasis and bone and joint disease. Nat. Rev. Rheumatol. 17, 608-620 (2021).
- Hu, X. F., Wang, L., Xiang, G., Lei, W. & Feng, Y. F. Angiogenesis impairment by the NADPH oxidase-triggered oxidative stress at the bone-implant interface: Critical mechanisms and therapeutic targets for implant failure under hyperglycemic conditions in diabetes. Acta Biomater. 73, 470-487 (2018).
- Beckman, J. A. & Creager, M. A. Vascular complications of diabetes. Circul. Res. 118, 1771-1785 (2016).
- Xu, Z. et al. Thermosensitive hydrogel incorporating Prussian blue nanoparticles promotes diabetic wound healing via ROS scavenging and mitochondrial function restoration. ACS Appl. Mater. Interfaces 14, 14059-14071 (2022).
- de Baaij, J. H., Hoenderop, J. G. & Bindels, R. J. Magnesium in man: implications for health and disease. Physiol. Rev. 95, 1-46 (2015).
- Zhang, Y. et al. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat. Med. 22, 1160-1169 (2016).
- Lin, S. et al. A magnesium-enriched 3D culture system that mimics the bone development microenvironment for vascularized bone regeneration. Adv. Sci. (Weinh.) 6, 1900209 (2019).
- Han, H. S. et al. Biodegradable magnesium alloys promote angio-osteogenesis to enhance bone repair. Adv. Sci. (Weinh.) 7, 2000800 (2020).
- Zhu, D., You, J., Zhao, N. & Xu, H. Magnesium regulates endothelial barrier functions through TRPM7, MagT1, and S1P1. Adv. Sci. (Weinh.) 6, 1901166 (2019).
- Yin, M. et al. Multifunctional magnesium organic framework-based microneedle patch for accelerating diabetic wound healing. ACS Nano 15, 17842-17853 (2021).
- Sales, C. H. & Pedrosa Lde, F. Magnesium and diabetes mellitus: their relation. Clin. Nutrition (Edinburgh, Scotland) 25, 554-562 (2006).
- WA, E. L., Naser, I. A., Taleb, M. H. & Abutair, A. S. The effects of oral magnesium supplementation on glycemic response among type 2 diabetes patients. Nutrients 11, 44 (2018).
- Veronese, N. et al. Oral magnesium supplementation for treating glucose metabolism parameters in people with or at risk of diabetes: a systematic review and metaanalysis of double-blind randomized controlled trials. Nutrients 13, 4074 (2021).
- He, T. et al. A comparison of micro-CT and histomorphometry for evaluation of osseointegration of PEO-coated titanium implants in a rat model. Sci. Rep. 7, 16270 (2017).
- Calvo-Guirado, J. L. et al. Histological and histomorphometric evaluation of immediate implant placement on a dog model with a new implant surface treatment. Clin. Oral Implants Res. 21, 308-315 (2010).
- Jing, D. et al. Tissue clearing and its application to bone and dental tissues. J. Dental Res. 98, 621-631 (2019).
- Jing, D. et al. Tissue clearing of both hard and soft tissue organs with the PEGASOS method. Cell Res. 28, 803-818 (2018).
- Sivaraj, K. K. & Adams, R. H. Blood vessel formation and function in bone. Development (Cambridge, England) 143, 2706-2715 (2016).
- Lin, W. et al. Mapping the immune microenvironment for mandibular alveolar bone homeostasis at single-cell resolution. Bone Res. 9, 17 (2021).
- Kusumbe, A. P., Ramasamy, S. K. & Adams, R. H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 507, 323-328 (2014).
- Peng, Y., Wu, S., Li, Y. & Crane, J. L. Type H blood vessels in bone modeling and remodeling. Theranostics 10, 426-436 (2020).
- Brem, H. & Tomic-Canic, M. Cellular and molecular basis of wound healing in diabetes. J. Clin. Investig. 117, 1219-1222 (2007).
- Orasanu, G. & Plutzky, J. The pathologic continuum of diabetic vascular disease. J. Am. College Cardiol. 53, S35-S42 (2009).
- Devlin, H., Garland, H. & Sloan, P. Healing of tooth extraction sockets in experimental diabetes mellitus. J. Oral Maxillofac. Surg. 54, 1087-1091 (1996).
- Xiong, Y. et al. A whole-course-repair system based on neurogenesisangiogenesis crosstalk and macrophage reprogramming promotes diabetic wound healing. Adv. Mater. (Deerfield Beach, Fla.), e2212300, https://doi.org/ 10.1002/adma. 202212300 (2023).
- Rohlenova, K., Veys, K., Miranda-Santos, I., De Bock, K. & Carmeliet, P. Endothelial cell metabolism in health and disease. Trends Cell Biol. 28, 224-236 (2018).
- Potente, M., Gerhardt, H. & Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 146, 873-887 (2011).
- Sawada, N. et al. Endothelial PGC-1a mediates vascular dysfunction in diabetes. Cell Metab. 19, 246-258 (2014).
- Giacco, F. & Brownlee, M. Oxidative stress and diabetic complications. Circulation Res. 107, 1058-1070 (2010).
- Eelen, G., de Zeeuw, P., Simons, M. & Carmeliet, P. Endothelial cell metabolism in normal and diseased vasculature. Circulation Res. 116, 1231-1244 (2015).
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 414, 813-820 (2001).
- Wang, Y. et al. Integrated regulation of stress responses, autophagy and survival by altered intracellular iron stores. Redox Biol. 55, 102407 (2022).
- Ding, S., Ma, N., Liu, H., Tang, M. & Mei, J. Sesn2 attenuates the damage of endothelial progenitor cells induced by angiotensin II through regulating the Keap1/Nrf2 signal pathway. Aging 12, 25505-25527 (2020).
- Muri, J. & Kopf, M. Redox regulation of immunometabolism. Nat. Rev. Immunol. 21, 363-381 (2021).
- Rupp, F., Liang, L., Geis-Gerstorfer, J., Scheideler, L. & Hüttig, F. Surface characteristics of dental implants: a review. Dental Mater. 34, 40-57 (2018).
© The Author(s) 2024
State Key Laboratory of Oral Diseases & National Center for Stomatology & National Clinical Research Center for Oral Diseases & West China Hospital of Stomatology, Sichuan University, Chengdu, China and State Key Laboratory of Oral Diseases & National Center for Stomatology & National Clinical Research Center for Oral Diseases & Department of Oral Implantology, West China Hospital of Stomatology, Sichuan University, Chengdu, China
Correspondence: Quan Yuan (yuanquan@scu.edu.cn) or Shiwen Zhang (sw.zhang2018@scu.edu.cn)
These authors contributed equally: Linfeng Liu, Feiyu Wang
