DOI: https://doi.org/10.1038/s41598-025-85844-z
PMID: https://pubmed.ncbi.nlm.nih.gov/39805957
تاريخ النشر: 2025-01-13
افتح
تأثيرات التحكم في الديدان الخيطية واختبارات السلامة لتحضير ديدينتونيا فلاجرا في الأغنام
الملخص
Duddingtonia flagrans هو فطر يصطاد الديدان الخيطية ويستخدم على نطاق واسع للسيطرة على الديدان الخيطية الطفيلية في الماشية. بعد الابتلاع عن طريق الفم ومروره عبر الجهاز الهضمي للحيوانات، يقوم هذا الكائن الدقيق باصطياد الديدان الخيطية في البراز. على الرغم من أن العديد من الباحثين قد درسوا سلامة هذا الفطر للبشر والحيوانات والبيئة، إلا أن هناك تقارير قليلة تناولت سلامة المنتجات البيولوجية لـ D. flagrans التي تصطاد الديدان الخيطية للحيوانات. في هذه الدراسة، تم اختبار سلامة D. flagrans، بينما تم فحص الآثار السلبية والسمية في الأغنام. أولاً، تم دراسة تأثيرات قتل الديدان الخيطية في الأغنام المصابة طبيعياً بعد إعطاء المجفف بالتجميد.
حالياً، يتم استخدام الأدوية الطاردة للديدان بشكل واسع للتحكم البيولوجي في الطفيليات في الماشية، لكن سوء استخدام هذه الأدوية أدى إلى مشاكل خطيرة في المقاومة؛ لذلك، يجب البحث وتطوير تدابير تحكم جديدة.
المواد والأساليب
سلالة فطرية
الحيوانات
استخدام D. flagrans في السيطرة على الطفيليات الدودية
تجارب سلامة الحيوانات
طرق
الفعالية في الجسم الحي لـ
اختبارات السلامة التجريبية في الجسم الحي
الملاحظات السريرية
قياسات درجة حرارة الجسم والوزن
فحوصات الدم الروتينية
علم الأنسجة العضوية
التحليل الإحصائي
النتائج
كفاءة افتراس الديدان الخيطية في البراز بعد الإدارة البيولوجية الفموية
الملاحظات السريرية
قياسات درجة حرارة الجسم والوزن
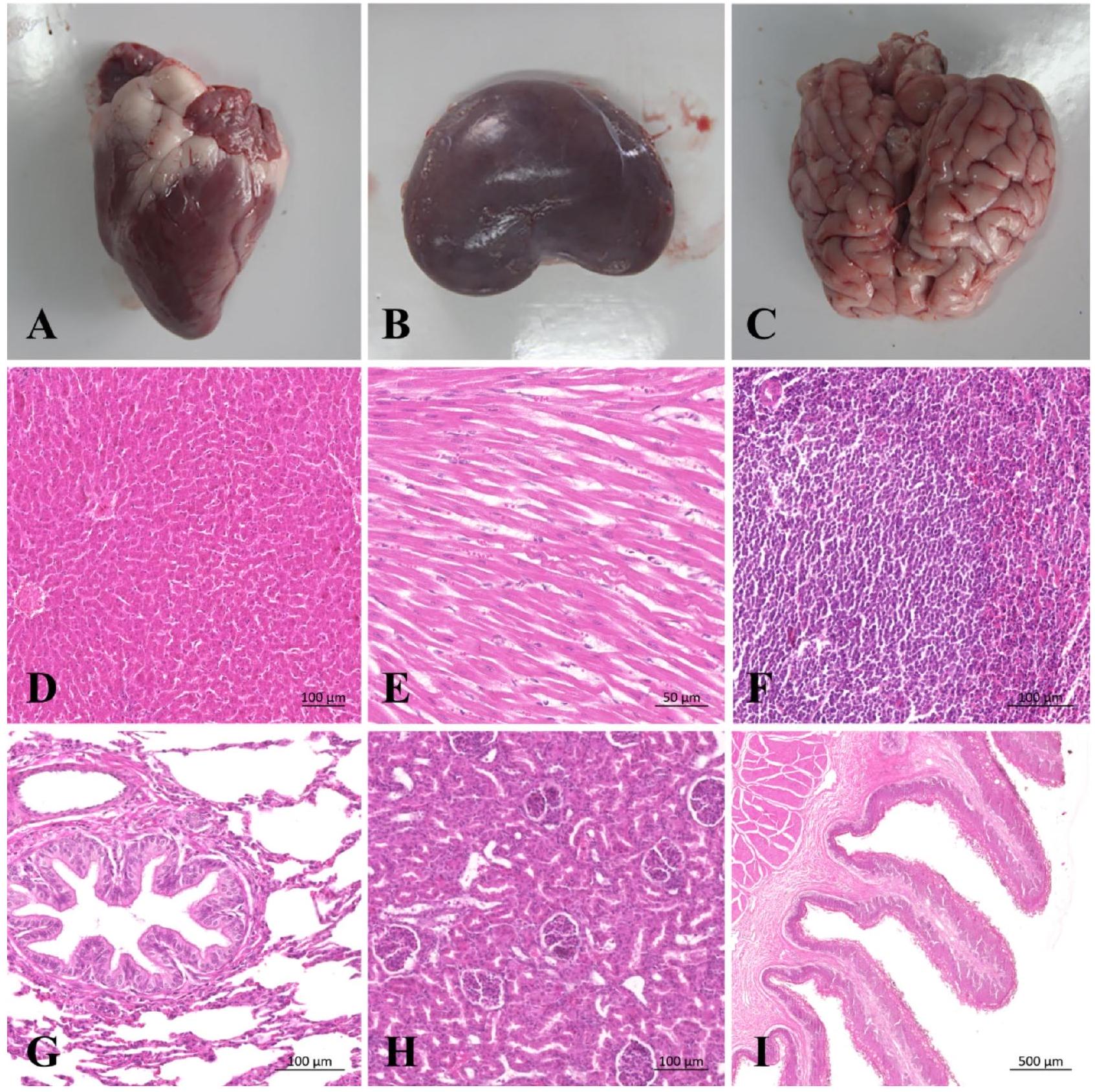
التغيرات المرضية في الأغنام
| المخدرات | دليل البرامج الإلكتروني | غاز البترول المسال | ||||
| تحكم | علاج | فعالية% | تحكم | علاج | نسبة الفعالية% | |
| DF |
|
|
-8.72% |
|
|
92.99% |
| إيفرمكتين |
|
|
٤٩.٤٤٪ |
|
|
85.95% |
| تحكم |
|
|
-24.17% |
|
|
-6.10% |
| جرعة | قبل الجرعة | ثلاثي الأبعاد | 7 د | 15 د | 30 د |
| خمسة أضعاف |
|
|
|
|
|
| عشر مرات |
|
|
|
|
|
| تحكم |
|
|
|
|
|
| جرعة | قبل الجرعة | 3 د | 7 د | 15 د | 30 يوم |
| خمسة أضعاف |
|
|
|
|
|
| عشر مرات |
|
|
|
|
|
| تحكم |
|
|
|
|
|
نتائج فحص الدم الروتيني
نتائج الكيمياء الحيوية للدم
معاملات الأعضاء
نقاش
| جرعة | الوقت | WBC (
|
ليم (%) | مون (%) | العصبي (%) | إيو (%) | با (%) |
| خمسة أضعاف | قبل الجرعة |
|
|
|
|
|
|
| 15 د |
|
|
|
|
|
|
|
| 30 د |
|
|
|
|
|
|
|
| عشر مرات | قبل الجرعة |
|
|
|
|
|
|
| 15 د |
|
|
|
|
|
|
|
| 30 د |
|
|
|
|
|
|
|
| تحكم | قبل الجرعة |
|
|
|
|
|
|
| 15 د |
|
|
|
|
|
|
|
| 30 د |
|
|
|
|
|
|
|
| جرعة | الوقت |
|
حجم الكريات الحمراء المتوسط (%) | HCT (%) | MCHC (غرام/ديسيلتر) | الهيموغلوبين (غرام/ديسيلتر) | MPV (فل.) |
| خمسة أضعاف | قبل الجرعة |
|
|
|
|
|
|
| 15 د |
|
|
|
|
|
|
|
| 30 يوم |
|
|
|
|
|
|
|
| عشر مرات | قبل الجرعة |
|
|
|
|
|
|
| 15 د |
|
|
|
|
|
|
|
| 30 د |
|
|
|
|
|
|
|
| تحكم | قبل الجرعة |
|
|
|
|
|
|
| 15 د |
|
|
|
|
|
|
|
| 30 د |
|
|
|
|
|
|
| جرعة | الوقت | TG (ملليمول/لتر) | اليوريا (مللي مول/لتر) | ALT (وحدة/لتر) | AST (وحدة/لتر) | TP (غرام/لتر) | ALB (غ/ل) |
| خمسة أضعاف | قبل الجرعة |
|
|
|
|
|
|
| 15 د |
|
|
|
|
|
|
|
| 30 يوم |
|
|
|
|
|
|
|
| عشر مرات | قبل الجرعة |
|
|
|
|
|
|
| 15 د |
|
|
|
|
|
|
|
| 30 د |
|
|
|
|
|
|
|
| تحكم | قبل الجرعة |
|
|
|
|
|
|
| 15 د |
|
|
|
|
|
|
|
| 30 د |
|
|
|
|
|
|
| جرعة
|
الوزن (كجم) | معامل العضو (%) | ||||
| قلب | كبد | الطحال | رئة | كُلى | ||
| خمسة أضعاف |
|
|
|
|
|
|
| عشر مرات |
|
|
|
|
|
|
| تحكم |
|
|
|
|
|
|
توفر البيانات
نُشر على الإنترنت: 13 يناير 2025
References
- Fiałkowska, E., Fiałkowski, W. & Pajdak-Stós, A. The relations between predatory fungus and its rotifer preys as a noteworthy example of intraguild predation (IGP). Microb. Ecol. 79, 73-83 (2020).
- Luns, F. D. et al. Coadministration of nematophagous Fungi for biological control over nematodes in bovine in the South-Eastern Brazil. Biomed Res. Int. 2018, 1-6 (2018).
- Silva, A. R. et al. Biological control of sheep gastrointestinal nematodiasis in a tropical region of the southeast of Brazil with the nematode predatory fungi Duddingtonia flagrans and Monacrosporium thaumasium. Parasitol. Res. 105, 1707-1713 (2009).
- Zhang, W., Liu, D., Yu, Z., Hou, B. & Wang, R. Comparative genome and transcriptome analysis of the nematode-trapping fungus duddingtonia flagrans reveals high pathogenicity during nematode infection. Biol. Control 143, 104159 (2020).
- Tabata, A. C. et al. Biological control of gastrointestinal nematodes in horses fed with grass in association with nematophagus fungi Duddingtonia flagrans and Pochonia Chlamydosporia. Biol. Control 182, 105219 (2023).
- Mendes, L. Q. et al. In vitro association of Duddingtonia flagrans with ivermectin in the control of gastrointestinal nematodes of water buffaloes. Rev. MVZ Cordoba 27, 3 (2022).
- Voinot, M. et al. Integrating the control of helminths in dairy cattle: deworming, rotational grazing and nutritional pellets with parasiticide fungi. Vet. Parasitol. 278, 109038 (2020).
- Ferreira, S. R., de Araújo, J. V., Braga, F. R., Araujo, J. M. & Fernandes, F. M. In vitro predatory activity of nematophagous fungi Duddingtonia flagrans on infective larvae of Oesophagostomum spp. after passing through gastrointestinal tract of pigs. Trop. Anim. Health Prod. 3, 1589-1593 (2011).
- Araujo, J. M. et al. Control of Strongyloides westeri by nematophagous fungi after passage through the gastrointestinal tract of donkeys. Rev. Bras. Parasitol. Vet. 21, 157-60 (2012).
- Braga, F. R. et al. Destruction of Strongyloides venezuelensis infective larvae by fungi Duddingtonia flagrans, Arthrobotrys robusta and Monacrosporium sinense. Rev. Soc. Bras. Med. Trop. 44, 389-391 (2011).
- Paz-Silva, A. et al. Ability of the fungus Duddingtonia flagrans to adapt to the cyathostomin egg-output by spreading chlamydospores. Vet. Parasitol. 179, 277-282 (2011).
- Hiura, E. et al. Fungi predatory activity on embryonated Toxocara canis eggs inoculated in domestic chickens (Gallus gallus Domesticus) and destruction of second stage larvae. Parasitol. Res. 114, 3301-3308 (2015).
- Voinot, M. et al. Control of Strongyles in first-season grazing ewe lambs by integrating deworming and thrice-weekly administration of parasiticidal fungal spores. Pathogens 10, 1338 (2021).
- Salmo, R. et al. Formulating parasiticidal Fungi in dried edible gelatins to reduce the risk of infection by Trichuris Sp. among continuous grazing bison. Pathogens 13, 82 (2024).
- Braga, F. R., Ferraz, C. M., da Silva, E. N. & de Araújo, J. V. Efficiency of the Bioverm
(Duddingtonia flagrans) fungal formulation to control in vivo and in vitro of Haemonchus Contortus and Strongyloides papillosus in sheep. Biotech10, 62(2020). - Voinot, M. et al. Safety of daily administration of pellets containing parasiticidal fungal spores over a long period of time in heifers. Biocontrol Sci. Technol. 32, 1249-1259 (2021).
- Buzatti, A. et al. Duddingtonia flagrans in the control of gastrointestinal nematodes of horses. Exp. Parasitol. 159, 1-4 (2015).
- Mendoza-de Gives, P. Soil-borne nematodes: Impact in agriculture and livestock and sustainable strategies of prevention and control with special reference to the use of nematode natural enemies. Pathogens 11, 640 (2022).
- Souza, D. C. et al. Compatibility study of Duddingtonia flagrans conidia and its crude proteolytic extract. Vet. Parasitol. 322, 110030 (2023).
- Healey, K., Lawlor, C., Knox, M. R., Chambers, M. & Lamb, J. Field evaluation of Duddingtonia flagrans IAH 1297 for the reduction of worm burden in grazing animals: Tracer studies in sheep. Vet. Parasitol. 253, 48-54 (2018).
- Wang, B. B. et al. In vitro and in vivo studies of the native isolates of nematophagous fungi from China against the larvae of trichostrongylides. J. Basic Microbiol. 7, 265-275 (2017).
- Wang, W. R. Study on the clinical application model of nematode-trapping fungus-Duddingtonia flagrans (Master’s thesis of Inner Mongolia Agricultural University, 2018).
- Eysker, M. et al. The impact of daily Duddingtonia flagransapplication to lactating ewes on gastrointestinal nematodes infections in their lambs in the Netherlands. Vet. Parasitol. 41, 91-100 (2006).
- Faessler, H., Torgerson, P. R. & Hertzberg, H. Failure of Duddingtonia flagrans to reducegastrointestinal nematode infections in dairy ewes. Vet. Parasitol. 147(1-2), 96-102 (2007).
- Simon, A., Theodor, P. P. & Wolfgang, H. HTSeq-A Python framework to work with high-throughput sequencing data. Bioinformatics 2, 166-169 (2015).
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) et al. Safety and efficacy of BioWorma
(Duddingtonia flagrans NCIMB 30336) as a feed additive for all grazing animals. EFSA J.18, e06208 (2020). - Braga, F. R. et al. Interaction of the nematophagous fungus Duddingtonia flagrans on Amblyomma cajannense engorged females and enzymatic characterisationof its chitinase. Biocontrol Sci. Technol. 23, 584-594 (2013).
(2025) 15:1843 - Kazda, M., Langer, S. & Bengeelsdorf, F. R. Fungi open new possibilities for anaerobicfermentation of organic residues. Energy Sustain. Soc. 4, 6 (2014).
- Pajdak-Stosa, A., Wazny, R. & Fiałkowska. E. Can a predatory fungus (Zoophagus sp.) endanger the rotifer populations in activated sludge? Fungal Ecol. 23, 75-78 (2016).
- Crook, E. K. et al. Prevalence of anthelmintic resistance on sheep and goat farms in the mid-atlantic region and comparison of in vivo and in vitro detection methods. Small Ruminant Res. 143, 89-96 (2016).
مساهمات المؤلفين
الإعلانات
المصالح المتنافسة
موافقة الأخلاقيات
الموافقة المستنيرة
معلومات إضافية
معلومات إعادة الطباعة والتصاريح متاحة علىwww.nature.com/reprints.
ملاحظة الناشر: تظل شركة سبرينجر ناتشر محايدة فيما يتعلق بالمطالبات القضائية في الخرائط المنشورة والانتماءات المؤسسية.
© المؤلفون 2025
كلية الطب البيطري، جامعة الزراعة في منغوليا الداخلية، هولون بوير، منغوليا الداخلية، جمهورية الصين الشعبية. المختبر الرئيسي للتشخيص والعلاج السريري للأمراض الحيوانية، وزارة الزراعة، المركز الوطني لتعليم الطب البيطري التجريبي، بكين، جمهورية الصين الشعبية. كلية الصيدلة، جامعة هيتزه، طريق الجامعة 2269، هيتزه 274015، جمهورية الصين الشعبية. شركة روي بو للتكنولوجيا الزراعية المحدودة، هولون بوير، منغوليا الداخلية، جمهورية الصين الشعبية. البريد الإلكتروني: wr2006@163.com; 865583480@qq.com
DOI: https://doi.org/10.1038/s41598-025-85844-z
PMID: https://pubmed.ncbi.nlm.nih.gov/39805957
Publication Date: 2025-01-13
OPEN
Nematode controlling effects and safety tests of Duddingtonia flagrans biological preparation in sheep
Abstract
Duddingtonia flagrans is a nematode-trapping fungus that is widely used to control parasitic nematodes in livestock. After oral ingestion and passage through the digestive tract of animals, this microorganism captures nematodes in feces. Although many researchers have examined the safety of this fungus for humans, animals, and the environment, few reports have discussed the safety of nematode-trapping D. flagrans biologics for animals. In this study, D. flagrans safety was tested, while adverse effects and toxicities were examined in sheep. First, the nematode killing effects in naturally parasitized sheep after administration of lyophilized
Currently, to biologically control parasites in livestock, anthelmintic drugs are widely applied but the misuse of these drugs has led to serious resistance issues; therefore, new control measures must be researched and developed
Materials and methods
Fungal strain
Animals
D. flagrans use in controlling helminth parasites
Animal safety experiments
Methods
In vivo efficacy of
In vivo safety experimental tests
Clinical observations
Body temperature and weight measurements
Routine blood tests
Organ histomorphology
Statistical analysis
Results
Nematode predation efficiency in feces after oral biologic administration
Clinical observations
Body temperature and weight measurements
Pathological changes in sheep
| Drugs | EPG | LPG | ||||
| Control | Treatment | Efficacy% | Control | Treatment | Efficacy% | |
| DF |
|
|
-8.72% |
|
|
92.99% |
| Ivermectin |
|
|
49.44% |
|
|
85.95% |
| Control |
|
|
-24.17% |
|
|
-6.10% |
| Dosage | Pre-dose | 3d | 7 d | 15 d | 30 d |
| 5-fold |
|
|
|
|
|
| 10-fold |
|
|
|
|
|
| Control |
|
|
|
|
|
| Dosage | Pre-dose | 3 d | 7 d | 15 d | 30d |
| 5-fold |
|
|
|
|
|
| 10-fold |
|
|
|
|
|
| Control |
|
|
|
|
|
Routine blood test results
Blood biochemistry results
Organ coefficients
Discussion

| Dosage | Time | WBC (
|
Lym (%) | Mon (%) | Neu (%) | Eo (%) | Ba (%) |
| 5-fold | Pre-dose |
|
|
|
|
|
|
| 15 d |
|
|
|
|
|
|
|
| 30 d |
|
|
|
|
|
|
|
| 10-fold | Pre-dose |
|
|
|
|
|
|
| 15 d |
|
|
|
|
|
|
|
| 30 d |
|
|
|
|
|
|
|
| Control | Pre-dose |
|
|
|
|
|
|
| 15 d |
|
|
|
|
|
|
|
| 30 d |
|
|
|
|
|
|
|
| Dosage | Time |
|
MCV (%) | HCT (%) | MCHC (g/dl) | Hb (g/dl) | MPV (fl.) |
| 5-fold | Pre-dose |
|
|
|
|
|
|
| 15 d |
|
|
|
|
|
|
|
| 30 d |
|
|
|
|
|
|
|
| 10-fold | Pre-dose |
|
|
|
|
|
|
| 15 d |
|
|
|
|
|
|
|
| 30 d |
|
|
|
|
|
|
|
| Control | Pre-dose |
|
|
|
|
|
|
| 15 d |
|
|
|
|
|
|
|
| 30 d |
|
|
|
|
|
|
| Dosage | Time | TG (mmol/L) | UREA (mmol/L) | ALT (U/L) | AST (U/L) | TP (g/L) | ALB (g/L) |
| 5-fold | Pre-dose |
|
|
|
|
|
|
| 15 d |
|
|
|
|
|
|
|
| 30 d |
|
|
|
|
|
|
|
| 10-fold | Pre-dose |
|
|
|
|
|
|
| 15 d |
|
|
|
|
|
|
|
| 30 d |
|
|
|
|
|
|
|
| Control | Pre-dose |
|
|
|
|
|
|
| 15 d |
|
|
|
|
|
|
|
| 30 d |
|
|
|
|
|
|
| Dosage(
|
weight (kg) | Organ Coefficient (%) | ||||
| heart | Liver | spleen | lung | kidney | ||
| 5-fold |
|
|
|
|
|
|
| 10-fold |
|
|
|
|
|
|
| Control |
|
|
|
|
|
|
Data availability
Published online: 13 January 2025
References
- Fiałkowska, E., Fiałkowski, W. & Pajdak-Stós, A. The relations between predatory fungus and its rotifer preys as a noteworthy example of intraguild predation (IGP). Microb. Ecol. 79, 73-83 (2020).
- Luns, F. D. et al. Coadministration of nematophagous Fungi for biological control over nematodes in bovine in the South-Eastern Brazil. Biomed Res. Int. 2018, 1-6 (2018).
- Silva, A. R. et al. Biological control of sheep gastrointestinal nematodiasis in a tropical region of the southeast of Brazil with the nematode predatory fungi Duddingtonia flagrans and Monacrosporium thaumasium. Parasitol. Res. 105, 1707-1713 (2009).
- Zhang, W., Liu, D., Yu, Z., Hou, B. & Wang, R. Comparative genome and transcriptome analysis of the nematode-trapping fungus duddingtonia flagrans reveals high pathogenicity during nematode infection. Biol. Control 143, 104159 (2020).
- Tabata, A. C. et al. Biological control of gastrointestinal nematodes in horses fed with grass in association with nematophagus fungi Duddingtonia flagrans and Pochonia Chlamydosporia. Biol. Control 182, 105219 (2023).
- Mendes, L. Q. et al. In vitro association of Duddingtonia flagrans with ivermectin in the control of gastrointestinal nematodes of water buffaloes. Rev. MVZ Cordoba 27, 3 (2022).
- Voinot, M. et al. Integrating the control of helminths in dairy cattle: deworming, rotational grazing and nutritional pellets with parasiticide fungi. Vet. Parasitol. 278, 109038 (2020).
- Ferreira, S. R., de Araújo, J. V., Braga, F. R., Araujo, J. M. & Fernandes, F. M. In vitro predatory activity of nematophagous fungi Duddingtonia flagrans on infective larvae of Oesophagostomum spp. after passing through gastrointestinal tract of pigs. Trop. Anim. Health Prod. 3, 1589-1593 (2011).
- Araujo, J. M. et al. Control of Strongyloides westeri by nematophagous fungi after passage through the gastrointestinal tract of donkeys. Rev. Bras. Parasitol. Vet. 21, 157-60 (2012).
- Braga, F. R. et al. Destruction of Strongyloides venezuelensis infective larvae by fungi Duddingtonia flagrans, Arthrobotrys robusta and Monacrosporium sinense. Rev. Soc. Bras. Med. Trop. 44, 389-391 (2011).
- Paz-Silva, A. et al. Ability of the fungus Duddingtonia flagrans to adapt to the cyathostomin egg-output by spreading chlamydospores. Vet. Parasitol. 179, 277-282 (2011).
- Hiura, E. et al. Fungi predatory activity on embryonated Toxocara canis eggs inoculated in domestic chickens (Gallus gallus Domesticus) and destruction of second stage larvae. Parasitol. Res. 114, 3301-3308 (2015).
- Voinot, M. et al. Control of Strongyles in first-season grazing ewe lambs by integrating deworming and thrice-weekly administration of parasiticidal fungal spores. Pathogens 10, 1338 (2021).
- Salmo, R. et al. Formulating parasiticidal Fungi in dried edible gelatins to reduce the risk of infection by Trichuris Sp. among continuous grazing bison. Pathogens 13, 82 (2024).
- Braga, F. R., Ferraz, C. M., da Silva, E. N. & de Araújo, J. V. Efficiency of the Bioverm
(Duddingtonia flagrans) fungal formulation to control in vivo and in vitro of Haemonchus Contortus and Strongyloides papillosus in sheep. Biotech10, 62(2020). - Voinot, M. et al. Safety of daily administration of pellets containing parasiticidal fungal spores over a long period of time in heifers. Biocontrol Sci. Technol. 32, 1249-1259 (2021).
- Buzatti, A. et al. Duddingtonia flagrans in the control of gastrointestinal nematodes of horses. Exp. Parasitol. 159, 1-4 (2015).
- Mendoza-de Gives, P. Soil-borne nematodes: Impact in agriculture and livestock and sustainable strategies of prevention and control with special reference to the use of nematode natural enemies. Pathogens 11, 640 (2022).
- Souza, D. C. et al. Compatibility study of Duddingtonia flagrans conidia and its crude proteolytic extract. Vet. Parasitol. 322, 110030 (2023).
- Healey, K., Lawlor, C., Knox, M. R., Chambers, M. & Lamb, J. Field evaluation of Duddingtonia flagrans IAH 1297 for the reduction of worm burden in grazing animals: Tracer studies in sheep. Vet. Parasitol. 253, 48-54 (2018).
- Wang, B. B. et al. In vitro and in vivo studies of the native isolates of nematophagous fungi from China against the larvae of trichostrongylides. J. Basic Microbiol. 7, 265-275 (2017).
- Wang, W. R. Study on the clinical application model of nematode-trapping fungus-Duddingtonia flagrans (Master’s thesis of Inner Mongolia Agricultural University, 2018).
- Eysker, M. et al. The impact of daily Duddingtonia flagransapplication to lactating ewes on gastrointestinal nematodes infections in their lambs in the Netherlands. Vet. Parasitol. 41, 91-100 (2006).
- Faessler, H., Torgerson, P. R. & Hertzberg, H. Failure of Duddingtonia flagrans to reducegastrointestinal nematode infections in dairy ewes. Vet. Parasitol. 147(1-2), 96-102 (2007).
- Simon, A., Theodor, P. P. & Wolfgang, H. HTSeq-A Python framework to work with high-throughput sequencing data. Bioinformatics 2, 166-169 (2015).
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) et al. Safety and efficacy of BioWorma
(Duddingtonia flagrans NCIMB 30336) as a feed additive for all grazing animals. EFSA J.18, e06208 (2020). - Braga, F. R. et al. Interaction of the nematophagous fungus Duddingtonia flagrans on Amblyomma cajannense engorged females and enzymatic characterisationof its chitinase. Biocontrol Sci. Technol. 23, 584-594 (2013).
(2025) 15:1843 - Kazda, M., Langer, S. & Bengeelsdorf, F. R. Fungi open new possibilities for anaerobicfermentation of organic residues. Energy Sustain. Soc. 4, 6 (2014).
- Pajdak-Stosa, A., Wazny, R. & Fiałkowska. E. Can a predatory fungus (Zoophagus sp.) endanger the rotifer populations in activated sludge? Fungal Ecol. 23, 75-78 (2016).
- Crook, E. K. et al. Prevalence of anthelmintic resistance on sheep and goat farms in the mid-atlantic region and comparison of in vivo and in vitro detection methods. Small Ruminant Res. 143, 89-96 (2016).
Author contributions
Declarations
Competing interests
Ethics approval
Informed consent
Additional information
Reprints and permissions information is available at www.nature.com/reprints.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
© The Author(s) 2025
College of Veterinary Medicine, Inner Mongolia Agricultural University, Hohhot, Inner Mongolia, People’s Republic of China. Key Laboratory of Clinical Diagnosis and Treatment of Animal Diseases, Ministry of Agriculture, National Animal Medicine Experimental Teaching Center, Beijing, People’s Republic of China. College of Pharmacy Heze University, University Road 2269, Heze 274015, People’s Republic of China. Rui Pu Agricultural Technology Co., Ltd, Hohhot, Inner Mongolia, People’s Republic of China. email: wr2006@163.com; 865583480@qq.com
