DOI: https://doi.org/10.1186/s12870-024-04720-6
PMID: https://pubmed.ncbi.nlm.nih.gov/38185637
تاريخ النشر: 2024-01-08
تعديلات نمو القمح بواسطة جزيئات السيلينيوم النانوية تحت ضغط الملوحة
الملخص
يعد ضغط الملوحة عاملاً بيئيًا بارزًا يواجه عقبات في نمو وتطور النباتات. عندما يحتوي التربة على تركيزات عالية من الملح، تواجه الجذور صعوبات في امتصاص الماء، مما يؤدي إلى نقص المياه داخل أنسجة النبات. ونتيجة لذلك، قد تعاني النباتات من نمو مثبط، وتراجع في التطور، وانخفاض في تراكم الكتلة الحيوية. أصبح استخدام الجزيئات النانوية تعديلًا شائعًا في الآونة الأخيرة لتخفيف ضغط الملوحة. درست الدراسة النهج البيولوجي لتحضير جزيئات السيلينيوم النانوية (NP) وتأثيرها على نمو نباتات القمح في ظل ظروف ملوحة. تم استخدام مستخلص أوراق الليمون (Citrus limon L.) للتخليق الأخضر لجزيئات السيلينيوم النانوية (Se-NPs). تم توصيف الجزيئات النانوية التي تم تخليقها بواسطة حيود الأشعة السينية (XRD) وطيف الأشعة تحت الحمراء بتحويل فورييه (FTIR) وتم تطبيقها ورقيًا في نطاق
*المراسلة:
زهير حسنين
zuhair@uaar.edu.pk
سبحان دانش
sd96850@gmail.com
¹قسم علم النبات، جامعة الحكومة، فيصل آباد، باكستان
المقدمة
(ROS) داخل خلايا النبات. تسبب ROS، بما في ذلك بيروكسيد الهيدروجين (
المواد والأساليب
النهج الأخضر لتخليق SeNPs
توصيف الجزيئات النانوية
إنبات البذور
| ملمس التربة | طين رملي طيني |
|
صفر |
| ECe
|
0.60 |
|
٢.٤٥ |
| درجة الحموضة | ٧.٦ |
|
1.90 |
| المادة العضوية
|
0.49 | متاح
|
7.3 |
| تشبع | ٣٦ | متاح
|
40 |
تحليل نشاط إنزيمات مضادات الأكسدة
نشاط سوبر أكسيد ديسموتاز (SOD)
نشاط الكاتالاز (CAT)
نشاط البيروكسيداز (POD)
محلول عازل فوسفات بتركيز 50 مللي مولار
محتويات المالونديالديهايد (MDA)
إجمالي الأحماض الأمينية الحرة (TFA)
بيروكسيد الهيدروجين (
خصائص العائد
التحليل الإحصائي
النتائج
توصيف
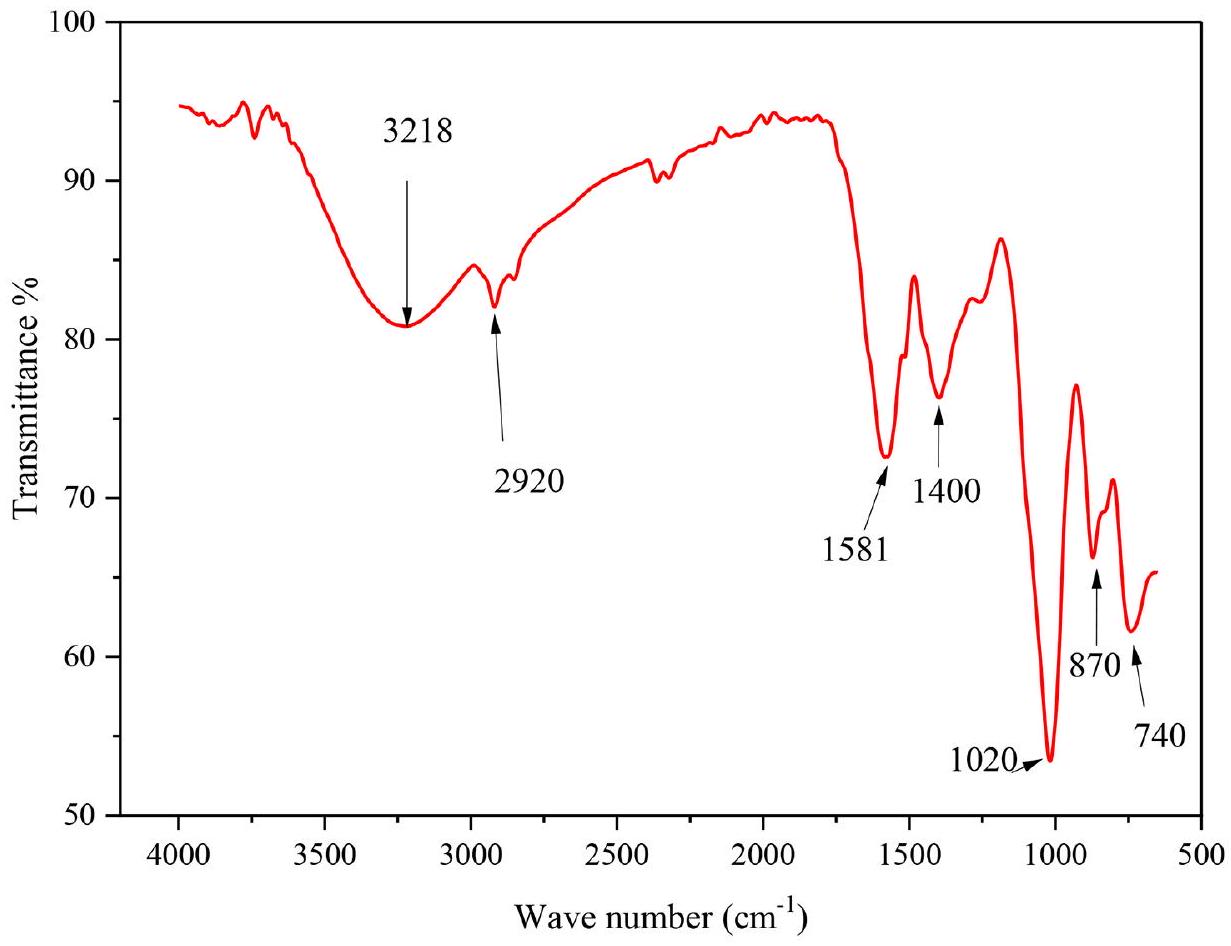
2914.82، و
ارتفاع النبات
الكتلة الحيوية/النبات
في الحالة، لم يتم العثور على اختلافات كبيرة في الكتلة الحيوية/نمو النبات بين مجموعة التحكم SS ومجموعات SS التجريبية المعالجة بـ
من
وزن 1000 حبة
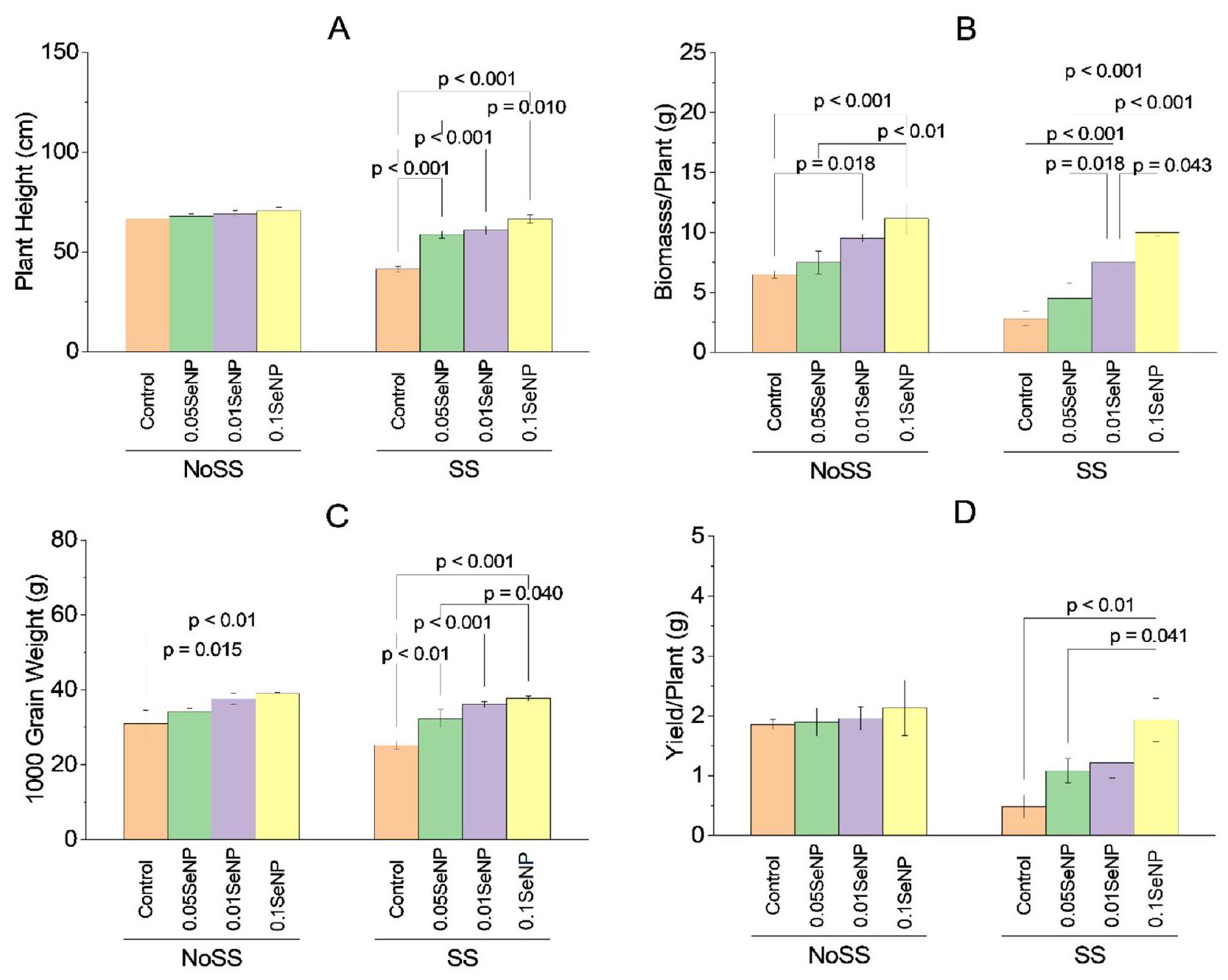
مجموعة التجارب SS المعالجة بـ
العائد/نبات
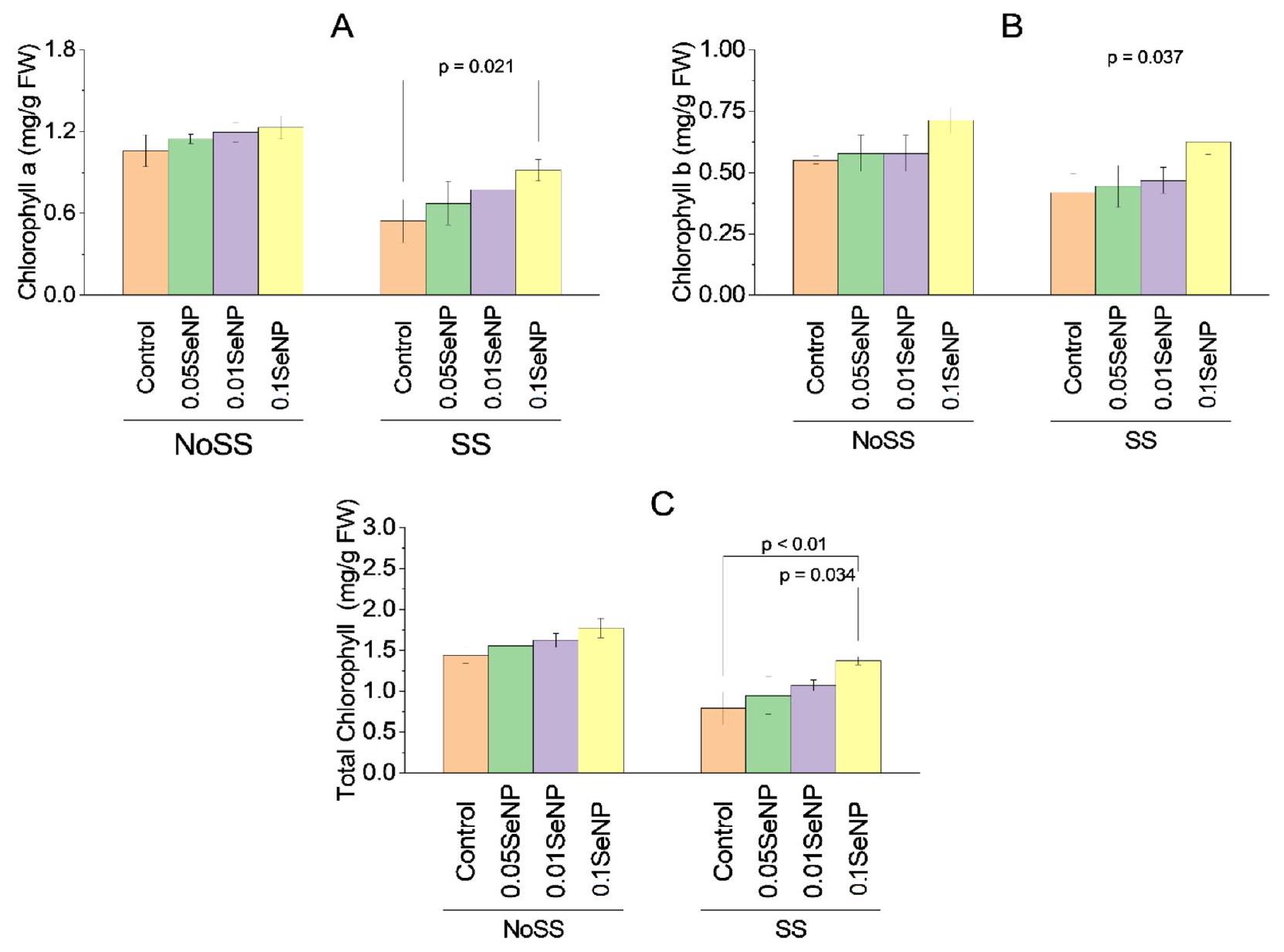
كلوروفيللا
كلوروفيل ب
من
إجمالي الكلوروفيل
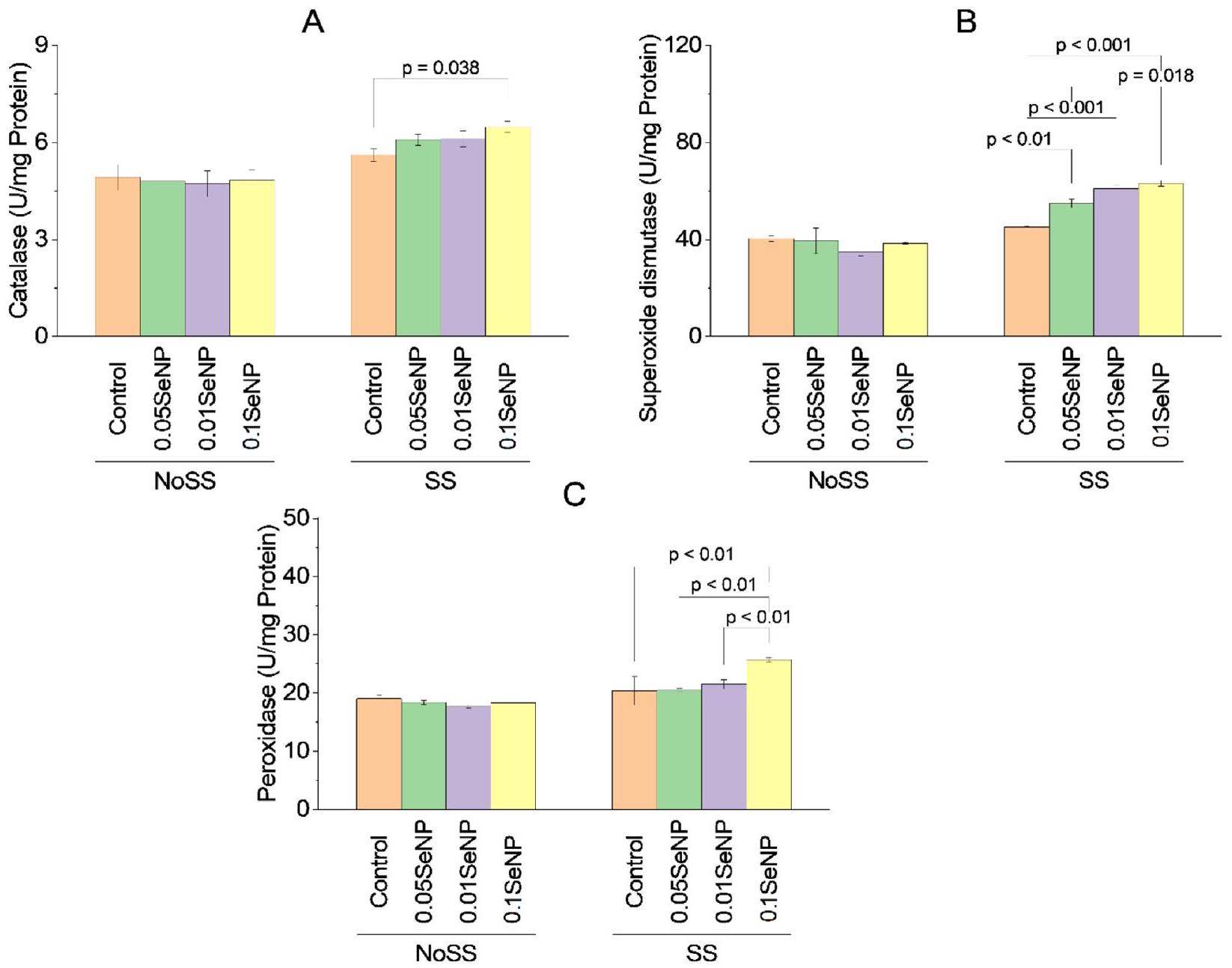
كاتالاز
مجموعة التجارب SS المعالجة بـ
سوبر أكسيد ديسموتاز
بيروكسيداز
إجمالي الأحماض الأمينية الحرة
MDA
في محتوى MDA فوق التحكم NoSS، مع
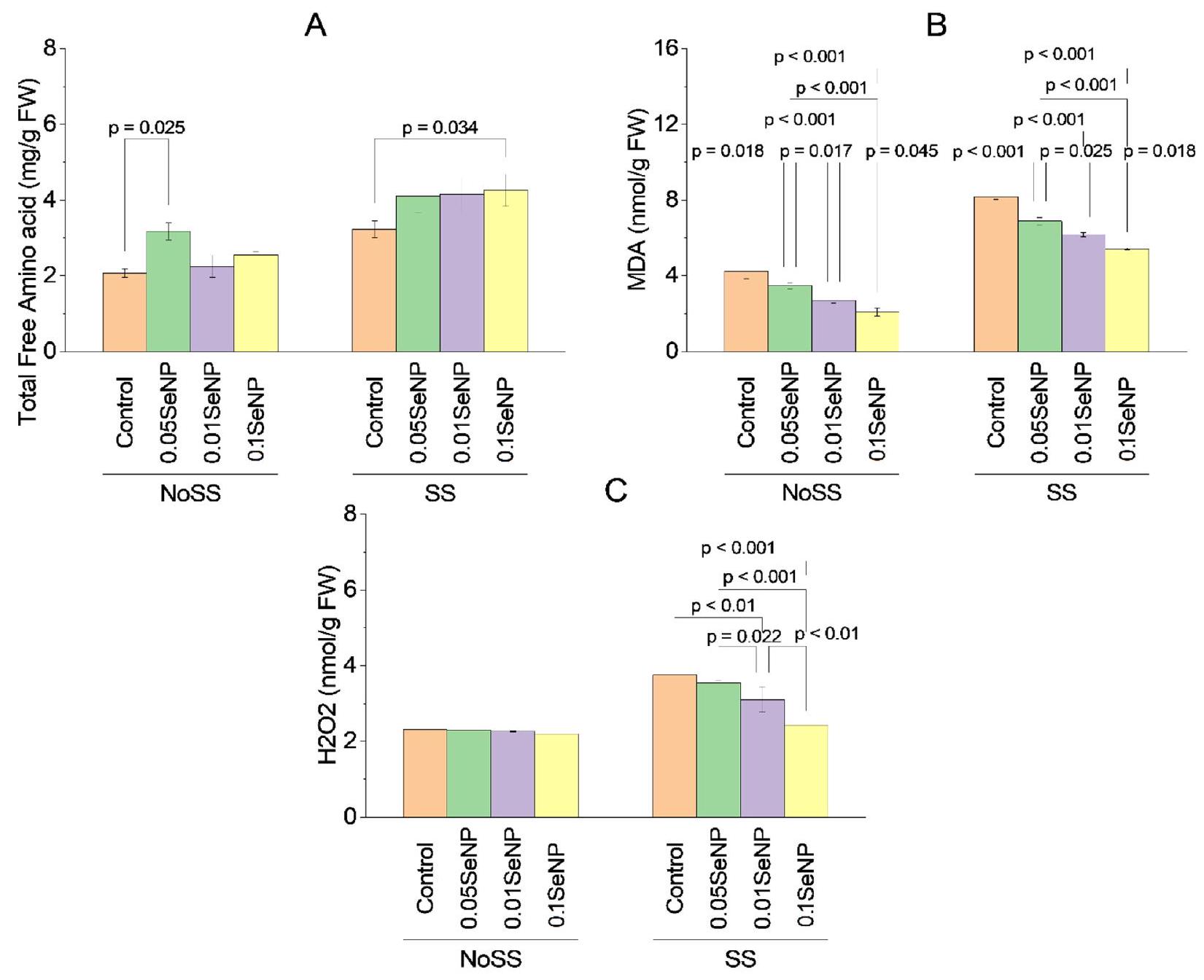
بيروكسيد الهيدروجين
المجموعات المعالجة بـ
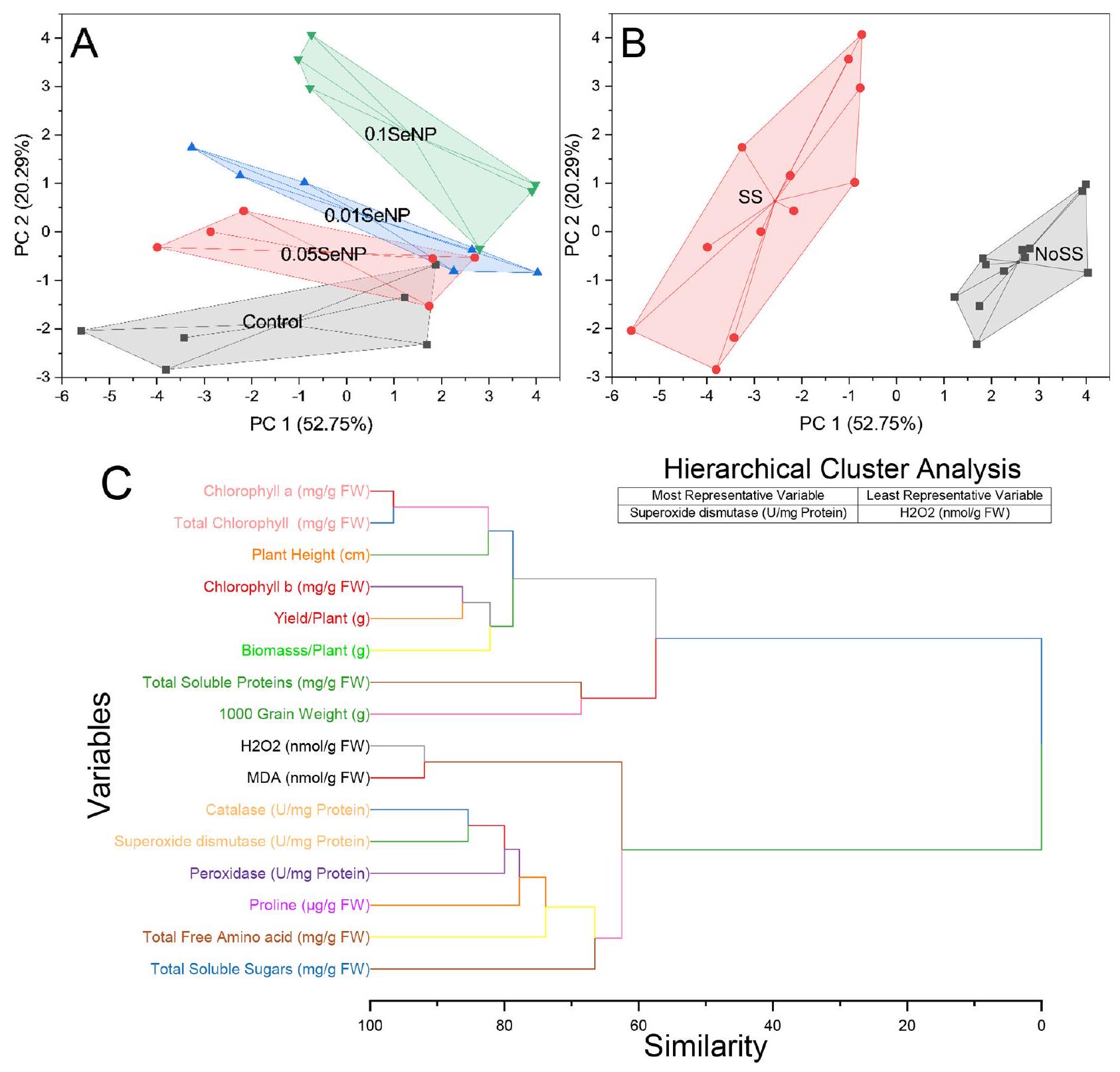
نقاط مثل (-5.59553، -2.04089)، (-3.80546، -2.84626)، (-3.42874، -2.18605)، وغيرها. تقع هذه النقاط بشكل منفصل عن الكتلة 1، ويتم رسم غلاف محدب حولها أيضًا، مما يخلق مضلعًا مميزًا يحيط بالكتلة الثانية (الشكل 7B). تُظهر المتغيرات الكلوروفيل أ (ملغ/غ من الوزن الرطب) والكلوروفيل الكلي (ملغ/غ من الوزن الرطب) معامل تشابه قدره 3.45938، مما يشير إلى تشابه قوي في قياساتهما. وهذا يشير إلى ارتباط وثيق بين هذه المتغيرات، مما يعكس دورها المترابط في تقييم محتوى الكلوروفيل. وبالمثل، فإن المتغيرات H 2 O 2 (
البحث الإضافي من أجل التعرف والفهم. علاوة على ذلك، تشترك المتغيرات البروتينات القابلة للذوبان الكلية (ملغ/غ وزن رطب) ووزن 1000 حبة (غ) في تشابه قدره 31.41099، مما يشير إلى ارتباط ملحوظ بين هذه المتغيرات. وهذا يقترح وجود علاقة محتملة بين محتوى البروتينات القابلة للذوبان الكلية ووزن 1000 حبة، مما يعكس اعتمادها المتبادل في تطوير الحبوب وتقييم الجودة. أخيرًا، تظهر المتغيرات السكريات القابلة للذوبان الكلية (ملغ/غ وزن رطب) ومتغير غير محدد قيمة تشابه قدرها 33.47375، مما يدل على تشابه كبير. وهذا يقترح وجود علاقة محتملة بين محتوى السكريات القابلة للذوبان الكلية والمتغير غير المحدد، مما يستدعي مزيدًا من التحقيق للتعرف والتوصيف (الشكل 7C).
نقاش
تم الإبلاغ عن تنظيم توازن الأيونات في النباتات تحت ضغط الملوحة [37]. من ناحية أخرى، غالبًا ما يتسبب ضغط الملوحة العالي في تعطيل امتصاص وتوزيع العناصر الغذائية الأساسية، بما في ذلك المغنيسيوم (Mg) والبوتاسيوم (K)، والتي تعتبر ضرورية لتخليق الكلوروفيل [38]. يمكن لجزيئات السيلينيوم النانوية (SeNPs) تعديل ناقلات الأيونات والقنوات، مما يعزز الامتصاص الفعال والنقل للعناصر الغذائية الأساسية. وهذا يضمن توافر كافٍ من المغنيسيوم والبوتاسيوم لتخليق الكلوروفيل، مما يؤدي إلى زيادة محتوى الكلوروفيل [38].
الخاتمة
شكر وتقدير
مساهمات المؤلفين
التمويل
توفر البيانات
الإعلانات
موافقة الأخلاقيات والموافقة على المشاركة
الموافقة على النشر
المصالح المتنافسة
تم النشر عبر الإنترنت: 08 يناير 2024
References
- Shereen A, Asma A, Shirazi MU, Khan MA, Ali M, Arif M. Physio-biochemical analysis of salinity tolerance in sodium contrasting rice (Oryza sativa L.) genotypes. Pakistan J Bot. 2022;54:787-94.
- Ali F, Bano A, Hassan TU, Nazir M, Khan RT. Plant growth promoting rhizobacteria induced modulation of physiological responses in rice under salt and drought stresses. Pakistan J Bot. 2023;55:447-52.
- Bouabdallah M, Mahmoudi H, Ghnaya T, Hannachi H, Taheri A, Ouerghi Z et al. Spermidine as an elevator of salinity induced stress on two varieties of Triticum durum Desf. (Karim and Razzek). Pakistan J Bot. 2022;54: 771-9.
- Khan A, Shafi M, Bakht J, Anwar S, Khan MO. Effect of salinity (NaCl) and seed priming
on biochemical parameters and biological yield of wheat. Pakistan J Bot. 2021;53:779-89. - NazT, Akhtar J, Mazhar lqbal M, Anwar-Ul-Haq M, Murtaza G, Khan Niazi N, et al. Assessment of gas exchange attributes, chlorophyll contents, ionic composition and antioxidant enzymes of bread wheat genotypes in boron toxic, saline and boron. Researchrepository Murdoch Edu Au. 2019;21:1271-8.
- Omara AE-D, Hafez EM, Osman HS, Rashwan E, El-Said MAA, Alharbi K, et al. Collaborative impact of Compost and beneficial rhizobacteria on soil properties, physiological attributes, and productivity of wheat subjected to deficit irrigation in salt affected soil. Plants. 2022;11:877.
- Hu J, Hu X, Duan H, Zhang H, Yu Q. Na
and homeostasis is important for salinity and drought tolerance of Calligonum mongolicum. Pakistan J Bot. 2021;53:1927-34. - Ait-El-Mokhtar M, Baslam M, Ben-Laouane R, Anli M, Boutasknit A, Mitsui T, et al. Alleviation of detrimental effects of salt stress on date palm (Phoenix dactylifera L .) by the application of arbuscular mycorrhizal fungi and/or compost. Front Sustain Food Syst. 2020;4:131.
- Kravchik M, Bernstein N. Effects of salinity on the transcriptome of growing maize leaf cells point at cell-age specificity in the involvement of the antioxidative response in cell growth restriction. BMC Genomics. 2013;14:24.
- Naz T, Mazhar lqbal M, Tahir M, Hassan MM, Rehmani MIA, Zafar MI, et al. Foliar application of potassium mitigates salinity stress conditions in spinach (Spinacia oleracea L.) through reducing nacl toxicity and enhancing the activity of antioxidant enzymes. Hortic. 2021;7:566.
- Kaya C, Akram NA, Ashraf M, Sonmez O. Exogenous application of humic acid mitigates salinity stress in maize (Zea mays L.) plants by improving some key physico-biochemical attributes. Cereal Res Commun. 2018;46:67-78.
- Huang S, Gill S, Ramzan M, Ahmad MZ, Danish S, Huang P, et al. Uncovering the impact of AM fungi on wheat nutrient uptake, ion homeostasis, oxidative stress, and antioxidant defense under salinity stress. Sci Rep. 2023;13:8249.
- Ahmed N, Khalid S, Grewal AG, Ali MA, Anjum MA, Rahi AA, et al. Performance of mango scion cultivars under various levels of artificially induced salinity stress. Pakistan J Bot. 2020;52:1143-58.
- Zafar-ul-Hye M, Yaseen R, Abid M, Abbas M, Ahmad M, Rahi AA, et al. Rhizobacteria having ACC-deaminase and biogas slurry can mitigate salinity adverse effects in wheat. Pakistan J Bot. 2022;54:297-303.
- Farooq F, Rashid N, Ibrar D, Hasnain Z, Ullah R, Nawaz M, et al. Impact of varying levels of soil salinity on emergence, growth and biochemical attributes of four Moringa oleifera landraces. PLoS ONE. 2022;17:e0263978.
- Hossen MS, Karim MF, Fujita M, Bhuyan MHMB, Nahar K, Masud AAC, et al. Comparative physiology of Indica and Japonica rice under salinity and drought stress: an intrinsic study on osmotic adjustment, oxidative stress, antioxidant defense and methylglyoxal detoxification. Stresses. 2022;2:156-78.
- Hasanuzzaman M, Parvin K, Bardhan K, Nahar K, Anee TI, Masud AAC, et al. Biostimulants for the regulation of reactive oxygen species metabolism in plants under abiotic stress. Cells. 2021;10:2537.
- Shareef HJ, Abdi G, Fahad S. Change in photosynthetic pigments of date palm offshoots under abiotic stress factors. Folia Oecol. 2020;47:45-51.
- Taqdees Z, Khan J, Khan W-D, Kausar S, Afzaal M, Akhtar I. Silicon and zinc nanoparticles-enriched miscanthus biochar enhanced seed germination, antioxidant defense system, and nutrient status of radish under NaCl stress. Crop Pasture Sci. 2022;73:556-72.
- Azmat R, Altaf I, Moin S, Ahmed W, Alrefaei AF, Ali S. A study of photo-biological reactions under
nanoparticle accumulation in Spinacia oleracea. Pakistan J Bot. 2023;55:1359-64. - Adhikari A, Khan MA, Imran M, Lee K-E, Kang S-M, Shin JY et al. The combined inoculation of Curvularia lunata AR11 and biochar stimulates synthetic silicon and potassium phosphate use efficiency, and mitigates Salt and drought stresses in rice. Front Plant Sci. 2022;13.
- Kareem HA, Saleem MF, Saleem S, Rather SA, Wani SH, Siddiqui MH, et al. Zinc oxide nanoparticles interplay with physiological and biochemical attributes in terminal heat stress alleviation in Mungbean (Vigna radiata L). Front Plant Sci. 2022;13:101.
- Alagesan
, Venugopal S. Green synthesis of selenium nanoparticle using leaves extract of withania somnifera and its biological applications and photocatalytic activities. Bionanoscience. 2019;9:105-16. - Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1-15.
- Giannopolitis CN, Ries SK. Superoxide dismutases: I. occurrence in higher plants. Plant Physiol. 1977;59:309-14.
- Chance B, Maehly AC. Assay of catalases and peroxidases. Methods Enzymol. 1955;2 C:764-75.
- Cakmak I, Horst WJ. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant. 1991;83:463-8.
- Velikova V, Yordanov I, Edreva A. (2000). Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Science. 2000; 151: 59-66.
- OriginLab Corporation. OriginPro. Northampton. MA, USA.: OriginLab; 2021.
- Chellapa LR, Shanmugam R, Indiran MA, Samuel SR. Biogenic nanoselenium synthesis, its antimicrobial, antioxidant activity and toxicity. Bioinspired, Biomim Nanobiomaterials. 2020;9:184-9.
- Šoln K, Koce JD. Oxidative stress in roots: detection of lipid peroxidation and total antioxidative capacity. Methods Mol Biol. 2022;2447:221-31.
- Qi W-Y, Li Q, Chen H, Liu J, Xing S-F, Xu M, et al. Selenium nanoparticles ameliorate Brassica napus L. cadmium toxicity by inhibiting the respiratory burst and scavenging reactive oxygen species. J Hazard Mater. 2021;417:125900.
- Hussein H-AA, Darwesh OM, Mekki BB. Environmentally friendly nanoselenium to improve antioxidant system and growth of groundnut cultivars under sandy soil conditions. Biocatal Agric Biotechnol. 2019;18:101080.
- Farouk S, Elhindi KM, Alotaibi MA. Silicon supplementation mitigates salinity stress on Ocimum basilicum L. via improving water balance, ion homeostasis, and antioxidant defense system. Ecotoxicol Environ Saf. 2020;206:111396.
- Ikram M, Raja NI, Mashwani Z-U-R, Omar AA, Mohamed AH, Satti SH, et al. Phytogenic selenium nanoparticles elicited the physiological, biochemical, and antioxidant Defense System Amelioration of Huanglongbing-infected ‘Kinnow’ Mandarin plants. Nanomaterials. 2022;12:356.
- Shafi A, Zahoor I, Mushtaq U. Proline accumulation and oxidative stress: diverse roles and mechanism of tolerance and adaptation under salinity stress. Salt stress, microbes, and plant interactions: mechanisms and molecular approaches. Springer; 2019. pp. 269-300.
- Sardar R, Ahmed S, Shah AA, Yasin NA. Selenium nanoparticles reduced cadmium uptake, regulated nutritional homeostasis and antioxidative system in Coriandrum sativum grown in cadmium toxic conditions. Chemosphere. 2022;287:132332.
- Ghassemi-Golezani K, Abdoli S. Alleviation of salt stress in rapeseed (Brassica napus L .) plants by biochar-based rhizobacteria: new insights into the mechanisms regulating nutrient uptake, antioxidant activity, root growth and productivity. Arch Agron Soil Sci. 2023;69:1548-65.
ملاحظة الناشر
DOI: https://doi.org/10.1186/s12870-024-04720-6
PMID: https://pubmed.ncbi.nlm.nih.gov/38185637
Publication Date: 2024-01-08
Modulations of wheat growth by selenium nanoparticles under salinity stress
Abstract
Salinity stress is a prominent environmental factor that presents obstacles to the growth and development of plants. When the soil contains high salt concentrations, the roots face difficulties in absorbing water, resulting in water deficits within the plant tissues. Consequently, plants may experience inhibited growth, decreased development, and a decline in biomass accumulation. The use of nanoparticles has become a popular amendment in recent times for the alleviation of salinity stress. The study investigated the biological approach for the preparation of Se nanoparticles (NP) and their effect on the growth of wheat plants under saline conditions. The leaf extract of lemon (Citrus limon L.) was used for the green synthesis of selenium nanoparticles (Se-NPs). The synthesized NPs were characterized by X-ray diffraction (XRD) and Fourier-transform infrared spectroscopy (FTIR) and were applied foliar in the range of
*Correspondence:
Zuhair Hasnain
zuhair@uaar.edu.pk
Subhan Danish
sd96850@gmail.com
¹Botany Department, Government College University, Faisalabad, Pakistan
Introduction
(ROS) within plant cells. ROS, including hydrogen peroxide (
Materials and methods
Green synthesis approach of SeNPs
Nanoparticles characterization
Seed germination
| Soil texture | Sandy-clay-loam |
|
Nil |
| ECe
|
0.60 |
|
2.45 |
| pH | 7.6 |
|
1.90 |
| Organic matter
|
0.49 | Available
|
7.3 |
| Saturation | 36 | Available
|
40 |
Analysis of activity of antioxidant enzymes
Superoxide dismutase (SOD) activity
Catalase (CAT) activity
Peroxidase (POD) activity
of 50 mM phosphate buffer
Malondialdehyde (MDA) contents
Total free amino acids (TFA)
Hydrogen peroxide (
Yield attributes
Statistical analysis
Results
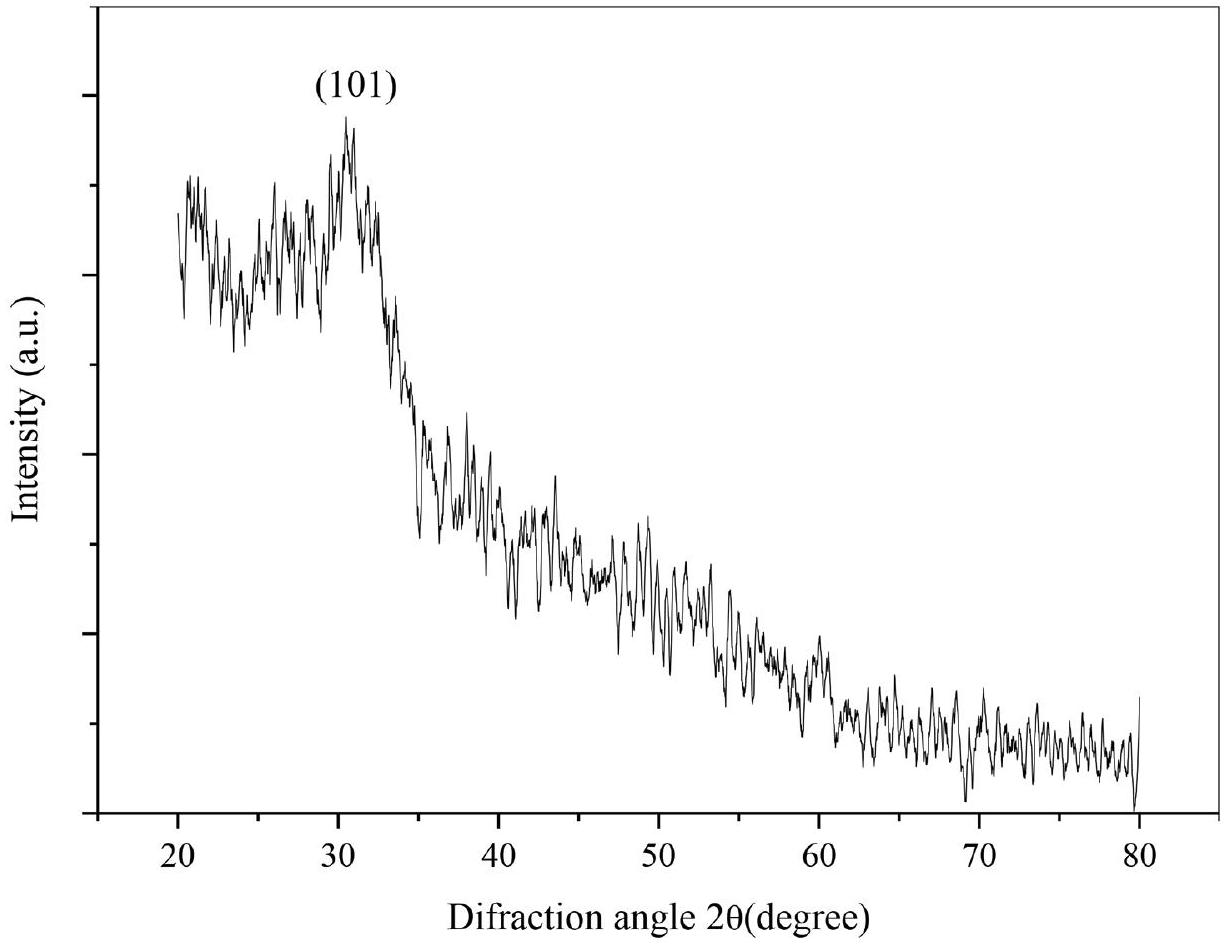
Characterization
2914.82, and
Plant height


Biomass/plant
condition, no significant variations were found in biomass/plant growth between the SS control and the SS experimental groups treated with
of
1000 grains weight

the SS experimental group treated with
Yield/plant

Chlorophylla
Chlorophyll b
of
Total chlorophyll
Catalase
the SS experimental group treated with
Superoxide dismutase
Peroxidase

Total free amino acids
MDA
in MDA content over the NoSS control, with

Hydrogen peroxide
groups treated with

by points like (-5.59553, -2.04089), (-3.80546, -2.84626), (-3.42874, -2.18605), and others. These points are located separately from Cluster 1, and a convex hull is drawn around them as well, creating a distinct polygon that encompasses the second cluster (Fig. 7B). The variables Chlorophyll a (mg/g FW) and Total Chlorophyll (mg/g FW) demonstrate a similarity coefficient of 3.45938, indicating a strong resemblance in their respective measurements. This suggests a close association between these variables, potentially reflecting their interconnected role in chlorophyll content assessment. Similarly, the variables H 2 O 2 (
further research for identification and understanding. Furthermore, the variables Total Soluble Proteins (mg/g FW) and 1000 Grain Weight (g) share a similarity of 31.41099, indicating a notable association between these variables. This suggests a potential relationship between total soluble protein content and the weight of 1000 grains, potentially reflecting their interdependency in grain development and quality assessment. Finally, the variables Total Soluble Sugars (mg/g FW) and an unidentified variable exhibit a similarity value of 33.47375 , signifying a substantial resemblance. This suggests a potential relationship between total soluble sugar content and the unidentified variable, warranting further investigation for identification and characterization (Fig. 7C).
Discussion
reported to regulate ion homeostasis in plants under salinity stress [37]. On the other hand high salinity stress often interrupts the uptake and distribution of essential nutrients, including magnesium ( Mg ) and potassium ( K ), which are crucial for chlorophyll synthesis [38]. SeNPs can modulate ion transporters and channels, promoting the efficient uptake and translocation of essential nutrients. This ensures an adequate supply of Mg and K for chlorophyll biosynthesis, leading to increased chlorophyll content [38].
Conclusion
Acknowledgements
Author contributions
Funding
Data availability
Declarations
Ethics approval and consent to participate
Consent for publication
Competing interests
Published online: 08 January 2024
References
- Shereen A, Asma A, Shirazi MU, Khan MA, Ali M, Arif M. Physio-biochemical analysis of salinity tolerance in sodium contrasting rice (Oryza sativa L.) genotypes. Pakistan J Bot. 2022;54:787-94.
- Ali F, Bano A, Hassan TU, Nazir M, Khan RT. Plant growth promoting rhizobacteria induced modulation of physiological responses in rice under salt and drought stresses. Pakistan J Bot. 2023;55:447-52.
- Bouabdallah M, Mahmoudi H, Ghnaya T, Hannachi H, Taheri A, Ouerghi Z et al. Spermidine as an elevator of salinity induced stress on two varieties of Triticum durum Desf. (Karim and Razzek). Pakistan J Bot. 2022;54: 771-9.
- Khan A, Shafi M, Bakht J, Anwar S, Khan MO. Effect of salinity (NaCl) and seed priming
on biochemical parameters and biological yield of wheat. Pakistan J Bot. 2021;53:779-89. - NazT, Akhtar J, Mazhar lqbal M, Anwar-Ul-Haq M, Murtaza G, Khan Niazi N, et al. Assessment of gas exchange attributes, chlorophyll contents, ionic composition and antioxidant enzymes of bread wheat genotypes in boron toxic, saline and boron. Researchrepository Murdoch Edu Au. 2019;21:1271-8.
- Omara AE-D, Hafez EM, Osman HS, Rashwan E, El-Said MAA, Alharbi K, et al. Collaborative impact of Compost and beneficial rhizobacteria on soil properties, physiological attributes, and productivity of wheat subjected to deficit irrigation in salt affected soil. Plants. 2022;11:877.
- Hu J, Hu X, Duan H, Zhang H, Yu Q. Na
and homeostasis is important for salinity and drought tolerance of Calligonum mongolicum. Pakistan J Bot. 2021;53:1927-34. - Ait-El-Mokhtar M, Baslam M, Ben-Laouane R, Anli M, Boutasknit A, Mitsui T, et al. Alleviation of detrimental effects of salt stress on date palm (Phoenix dactylifera L .) by the application of arbuscular mycorrhizal fungi and/or compost. Front Sustain Food Syst. 2020;4:131.
- Kravchik M, Bernstein N. Effects of salinity on the transcriptome of growing maize leaf cells point at cell-age specificity in the involvement of the antioxidative response in cell growth restriction. BMC Genomics. 2013;14:24.
- Naz T, Mazhar lqbal M, Tahir M, Hassan MM, Rehmani MIA, Zafar MI, et al. Foliar application of potassium mitigates salinity stress conditions in spinach (Spinacia oleracea L.) through reducing nacl toxicity and enhancing the activity of antioxidant enzymes. Hortic. 2021;7:566.
- Kaya C, Akram NA, Ashraf M, Sonmez O. Exogenous application of humic acid mitigates salinity stress in maize (Zea mays L.) plants by improving some key physico-biochemical attributes. Cereal Res Commun. 2018;46:67-78.
- Huang S, Gill S, Ramzan M, Ahmad MZ, Danish S, Huang P, et al. Uncovering the impact of AM fungi on wheat nutrient uptake, ion homeostasis, oxidative stress, and antioxidant defense under salinity stress. Sci Rep. 2023;13:8249.
- Ahmed N, Khalid S, Grewal AG, Ali MA, Anjum MA, Rahi AA, et al. Performance of mango scion cultivars under various levels of artificially induced salinity stress. Pakistan J Bot. 2020;52:1143-58.
- Zafar-ul-Hye M, Yaseen R, Abid M, Abbas M, Ahmad M, Rahi AA, et al. Rhizobacteria having ACC-deaminase and biogas slurry can mitigate salinity adverse effects in wheat. Pakistan J Bot. 2022;54:297-303.
- Farooq F, Rashid N, Ibrar D, Hasnain Z, Ullah R, Nawaz M, et al. Impact of varying levels of soil salinity on emergence, growth and biochemical attributes of four Moringa oleifera landraces. PLoS ONE. 2022;17:e0263978.
- Hossen MS, Karim MF, Fujita M, Bhuyan MHMB, Nahar K, Masud AAC, et al. Comparative physiology of Indica and Japonica rice under salinity and drought stress: an intrinsic study on osmotic adjustment, oxidative stress, antioxidant defense and methylglyoxal detoxification. Stresses. 2022;2:156-78.
- Hasanuzzaman M, Parvin K, Bardhan K, Nahar K, Anee TI, Masud AAC, et al. Biostimulants for the regulation of reactive oxygen species metabolism in plants under abiotic stress. Cells. 2021;10:2537.
- Shareef HJ, Abdi G, Fahad S. Change in photosynthetic pigments of date palm offshoots under abiotic stress factors. Folia Oecol. 2020;47:45-51.
- Taqdees Z, Khan J, Khan W-D, Kausar S, Afzaal M, Akhtar I. Silicon and zinc nanoparticles-enriched miscanthus biochar enhanced seed germination, antioxidant defense system, and nutrient status of radish under NaCl stress. Crop Pasture Sci. 2022;73:556-72.
- Azmat R, Altaf I, Moin S, Ahmed W, Alrefaei AF, Ali S. A study of photo-biological reactions under
nanoparticle accumulation in Spinacia oleracea. Pakistan J Bot. 2023;55:1359-64. - Adhikari A, Khan MA, Imran M, Lee K-E, Kang S-M, Shin JY et al. The combined inoculation of Curvularia lunata AR11 and biochar stimulates synthetic silicon and potassium phosphate use efficiency, and mitigates Salt and drought stresses in rice. Front Plant Sci. 2022;13.
- Kareem HA, Saleem MF, Saleem S, Rather SA, Wani SH, Siddiqui MH, et al. Zinc oxide nanoparticles interplay with physiological and biochemical attributes in terminal heat stress alleviation in Mungbean (Vigna radiata L). Front Plant Sci. 2022;13:101.
- Alagesan
, Venugopal S. Green synthesis of selenium nanoparticle using leaves extract of withania somnifera and its biological applications and photocatalytic activities. Bionanoscience. 2019;9:105-16. - Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1-15.
- Giannopolitis CN, Ries SK. Superoxide dismutases: I. occurrence in higher plants. Plant Physiol. 1977;59:309-14.
- Chance B, Maehly AC. Assay of catalases and peroxidases. Methods Enzymol. 1955;2 C:764-75.
- Cakmak I, Horst WJ. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant. 1991;83:463-8.
- Velikova V, Yordanov I, Edreva A. (2000). Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Science. 2000; 151: 59-66.
- OriginLab Corporation. OriginPro. Northampton. MA, USA.: OriginLab; 2021.
- Chellapa LR, Shanmugam R, Indiran MA, Samuel SR. Biogenic nanoselenium synthesis, its antimicrobial, antioxidant activity and toxicity. Bioinspired, Biomim Nanobiomaterials. 2020;9:184-9.
- Šoln K, Koce JD. Oxidative stress in roots: detection of lipid peroxidation and total antioxidative capacity. Methods Mol Biol. 2022;2447:221-31.
- Qi W-Y, Li Q, Chen H, Liu J, Xing S-F, Xu M, et al. Selenium nanoparticles ameliorate Brassica napus L. cadmium toxicity by inhibiting the respiratory burst and scavenging reactive oxygen species. J Hazard Mater. 2021;417:125900.
- Hussein H-AA, Darwesh OM, Mekki BB. Environmentally friendly nanoselenium to improve antioxidant system and growth of groundnut cultivars under sandy soil conditions. Biocatal Agric Biotechnol. 2019;18:101080.
- Farouk S, Elhindi KM, Alotaibi MA. Silicon supplementation mitigates salinity stress on Ocimum basilicum L. via improving water balance, ion homeostasis, and antioxidant defense system. Ecotoxicol Environ Saf. 2020;206:111396.
- Ikram M, Raja NI, Mashwani Z-U-R, Omar AA, Mohamed AH, Satti SH, et al. Phytogenic selenium nanoparticles elicited the physiological, biochemical, and antioxidant Defense System Amelioration of Huanglongbing-infected ‘Kinnow’ Mandarin plants. Nanomaterials. 2022;12:356.
- Shafi A, Zahoor I, Mushtaq U. Proline accumulation and oxidative stress: diverse roles and mechanism of tolerance and adaptation under salinity stress. Salt stress, microbes, and plant interactions: mechanisms and molecular approaches. Springer; 2019. pp. 269-300.
- Sardar R, Ahmed S, Shah AA, Yasin NA. Selenium nanoparticles reduced cadmium uptake, regulated nutritional homeostasis and antioxidative system in Coriandrum sativum grown in cadmium toxic conditions. Chemosphere. 2022;287:132332.
- Ghassemi-Golezani K, Abdoli S. Alleviation of salt stress in rapeseed (Brassica napus L .) plants by biochar-based rhizobacteria: new insights into the mechanisms regulating nutrient uptake, antioxidant activity, root growth and productivity. Arch Agron Soil Sci. 2023;69:1548-65.
