DOI: https://doi.org/10.1038/s41598-025-02866-3
PMID: https://pubmed.ncbi.nlm.nih.gov/40410413
تاريخ النشر: 2025-05-23
تقارير علمية
مفتوح
تقييم قابلية الديدان الخيطية للمناعة والمقاومة للأدوية المضادة للديدان باستخدام اختبار حركة WMicrotracker
الملخص
تعاني المجترات الرعوية من العديد من الإصابات الطفيلية، وخاصة تلك التي تسببها الديدان الخيطية المعوية (GIN)، والتي لها تأثير كبير على رفاهيتها وإنتاجيتها. لعلاج هذه الإصابات، أدى الاستخدام المكثف لمضادات الديدان من فئة اللاتونيات الحلقية (ML) إلى ظهور تجمعات من الطفيليات المقاومة للأدوية في جميع أنحاء العالم. الطريقة القياسية للكشف عن المقاومة، اختبار تقليل عدد البيض البرازي (FECRT)، عرضة لسوء التفسير، مما يؤدي إلى قرارات إدارية خاطئة تقوض جهود السيطرة على الطفيليات. وبالتالي، هناك حاجة ملحة لطرق قوية للكشف عن المقاومة في الطفيليات الميدانية. لقد بحثنا في إمكانية اختبار حركة WMicrotracker (WMA)، الذي لم يتم استكشافه سابقًا في تقييم مقاومة ML. قارن الاختبار أولاً قابلية الإيفرمكتين (IVM) بين سلالات بريستول N2 (N2B) من النوع البري، وIVM-selected (IVR10)، و
تشكل الديدان الخيطية الطفيلية تهديدًا كبيرًا لصحة الحيوانات، مع آثار عميقة على رفاهية وإنتاجية الماشية. من بينها، تبرز Haemonchus contortus كطفيلي ديدان خيطية شديد الضراوة، مما يزيد من التحديات التي يواجهها مالكو الماشية ويؤثر على الرفاهية العامة للحيوانات والإنتاج الاقتصادي
المواد والطرق
المواد
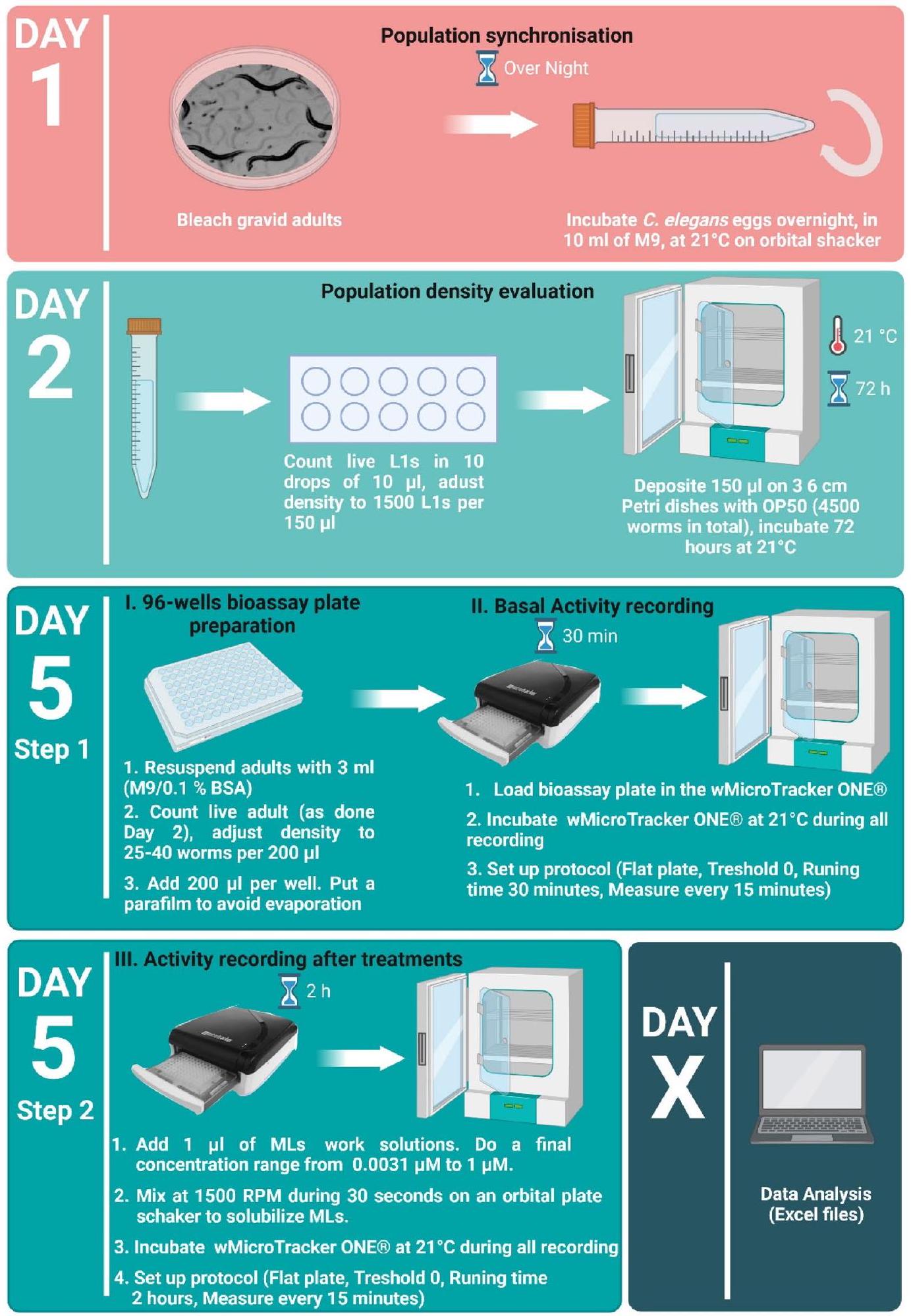
سلالات الديدان الخيطية C. elegans وظروف الزراعة
عزلات H. contortus
اختبار حركة الديدان (WMA)
WMA على سلالات C. elegans
النشاط التقييمي
النشاط القاعدي
تم حساب نسب الحركة لكل بئر معالج كتحفيز مضاعف بالنسبة للديدان المعالجة بـ DMSO والتي تم تعيينها على 100. لتسهيل المقارنة مع اختبارات ظاهرة أخرى، يقدم الجدول 1 ملخصًا لـ
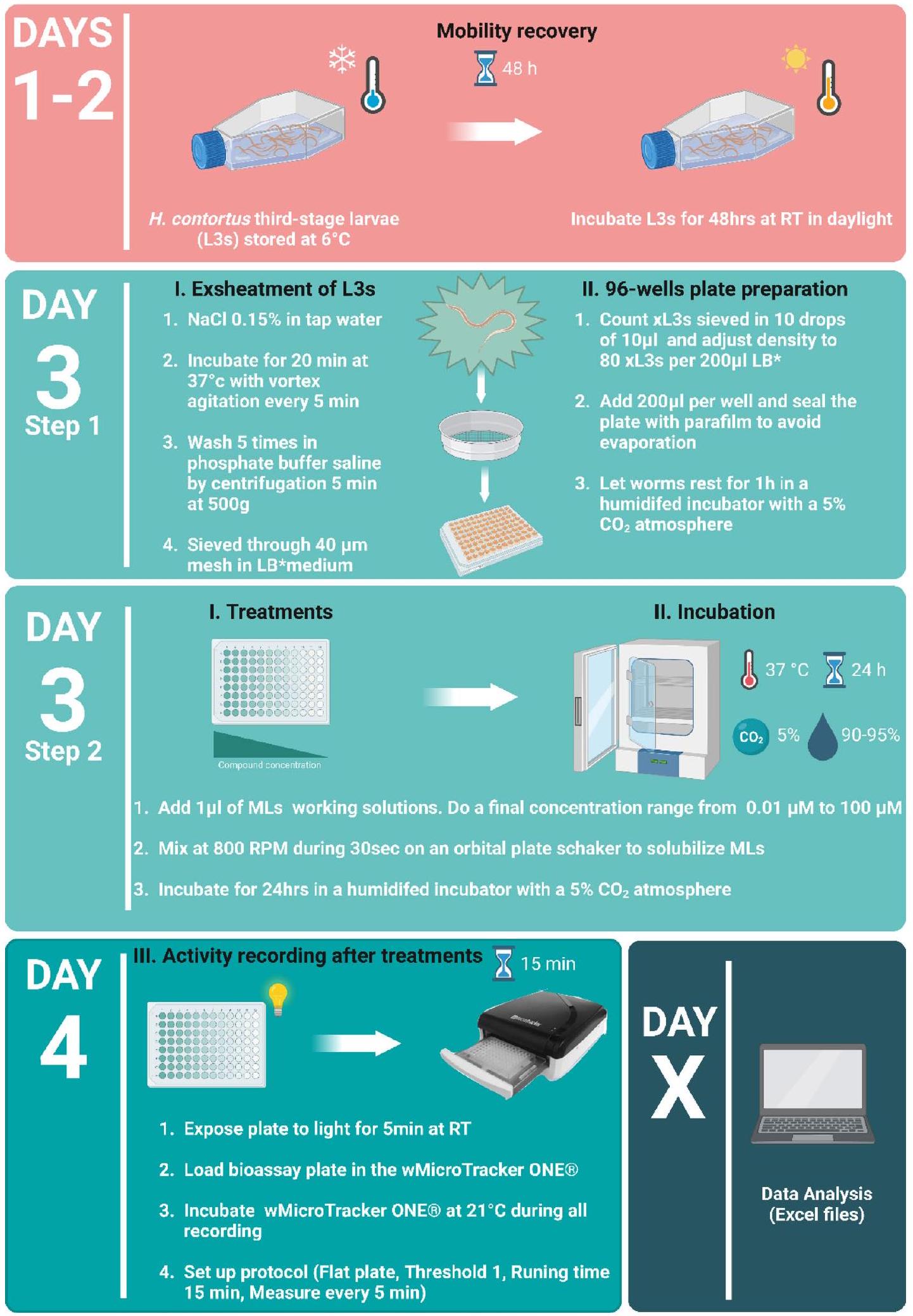
WMA على عزلات H. contortus
تحليل الجرعة والاستجابة وحساب عامل المقاومة (RF)
التحليل الإحصائي
| تحليل | معلومات عن التجارب | سلالات “C. elegans” | ||||||||||
| N2B | NHR-8 | IVR10 | ||||||||||
| اختبار الحركة | IVM | موكس | إي بي آر | IVM | موكس | إي بي آر | IVM | موكس | إي بي آر | المراجع | ||
| البالغون، متتبع ديدان | ٣٣.٥٢ | ٥٩.١٨ | 54.84 | ٢٩.٢٦ | 63.80 | ٤٠.١٥ | ٧١.٢٠ | ٨٨.١٦ | ١٠١.٤١ | الدراسة الحالية | ||
| ل4، متعقب ديدان | 190 | ND | ND | ND | ND | ND | ND | ND | ND | 31 | ||
| البالغون، متعقب الديدان الدقيقة | ٢٩٠ | ١٢٠ | ND | ND | ND | ND | ND | ND | ND | ٣٤ | ||
| ل4، متعقب ديدان | 150-500 | ND | 100-300 | ND | ND | ND | ND | ND | ND | ND | ٢٨ | |
| اختبار تطوير اليرقات | أطباق آجار | 1.69 | 1.77 | 1.19 | ND | ND | ND | 12.43 | 3.06 | 17.82 | ١٣ | |
| أطباق أagar | 1.63 | ND | ND | 0.96 | ND | ND | ND | ND | ND | 15 | ||
| ضخ البلعوم | شريحة بثمانية قنوات | 1420 | ٩٠٠ | ND | ND | ND | ND | ND | ND | ND | ٣٤ | |
| شاشة شريحة | 51 | 42 | ND | ND | ND | ND | ND | ND | ND | ٣٤ | ||
النتائج
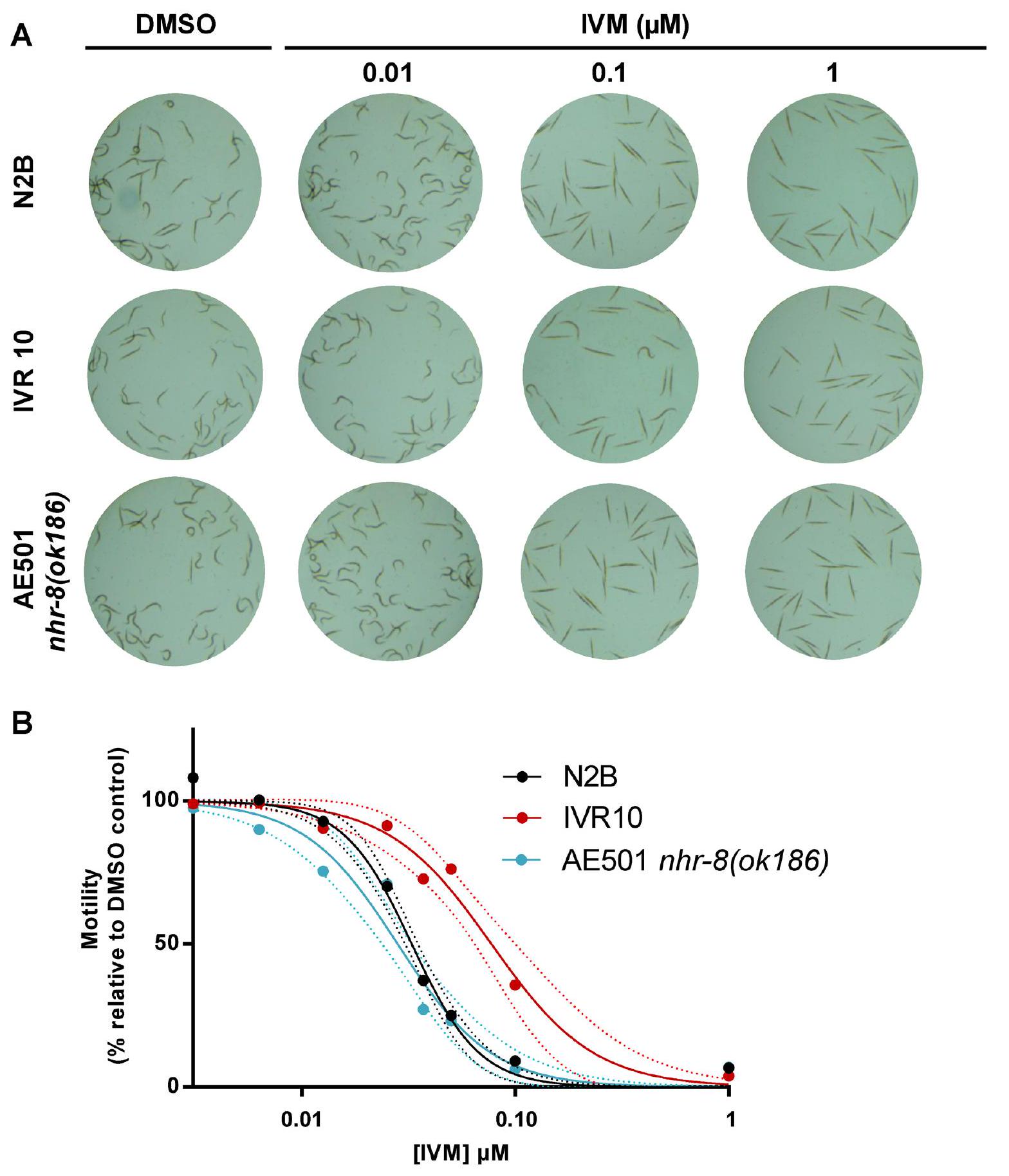
اختبار الحركة لتقييم فعالية IVM في الديدان الخيطية البالغة C. elegans
اختبار الحركة للتمييز بين السلالات القابلة للإصابة والمقاومة
| N2B | IVR10 | AE501 nhr-8(ok186) | |||
| علاج | يعني
|
معدل
|
RF | متوسط
|
RF |
| IVM |
|
|
2.12 |
|
0.87 |
| موكس |
|
|
1.49 |
|
1.08 |
| إي بي آر |
|
|
1.85 |
|
0.73 |
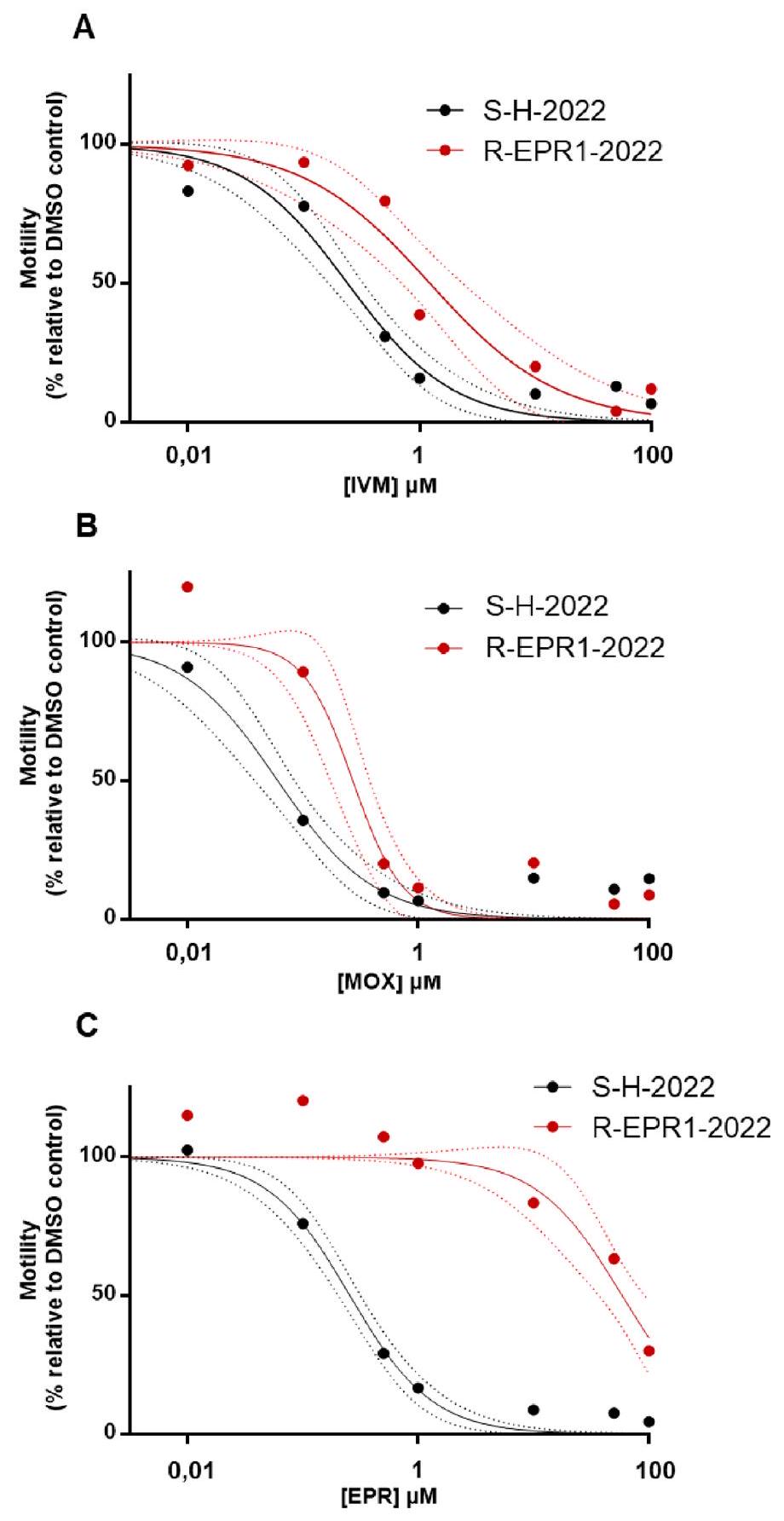
نقاش
| S-H-2022 |
|
||
| علاج | متوسط
|
يعني
|
RF |
| IVM |
|
|
5.24 |
| موكس |
|
|
٥.٤٠ |
| إي بي آر |
|
|
234.19 |
متحمل بشكل كبير لـ EPR، دون مقاومة ناتجة عن المختبر. هذا يعكس بشكل أفضل الظروف الواقعية ويبرز قابلية تكيف هذه الطريقة لتقييم فعالية الأدوية في العزلات الميدانية، لا سيما في اكتشاف المقاومة الناشئة. علاوة على ذلك، يجب أن يحسن فهمنا لكيفية تأثير الفئات الفرعية داخل مجموعة أكبر على المقاومة العامة، وهو أمر أساسي لمراقبة وإدارة ديناميات مقاومة الأدوية بشكل فعال. من خلال قياس الحركة بشكل مباشر، يوفر WMi نهجًا قويًا لتقييم AR عبر تنوع.
توفر البيانات
تاريخ الاستلام: 30 أكتوبر 2024؛ تاريخ القبول: 16 مايو 2025
نُشر على الإنترنت: 23 مايو 2025
References
- Fitzpatrick, J. L. Global food security: the impact of veterinary parasites and parasitologists. Vet. Parasitol. 195, 233-248 (2013).
- Martin, R. J., Robertson, A. P., Choudhary, S. & Ivermectin An anthelmintic, an insecticide, and much more. Trends Parasitol. 37, 48-64 (2021).
- Prichard, R. K. & Geary, T. G. Perspectives on the utility of moxidectin for the control of parasitic nematodes in the face of developing anthelmintic resistance. Int. J. Parasitol. Drugs Drug Resist. 10, 69-83 (2019).
- European Medicines Agency. Reflection paper on anthelmintic resistance (Draft 2). Eur. Med. Agency. 44, 1-16 (2016).
- Bourguinat, C. et al. Macrocyclic lactone resistance in dirofilaria immitis: failure of heartworm preventives and investigation of genetic markers for resistance. Vet. Parasitol. 210, 167-178 (2015).
- Laing, R., Gillan, V. & Devaney, E. Ivermectin – Old drug, new tricks?? Trends Parasitol. 33, 463-472 (2017).
- Doyle, S. R. et al. Genome-wide analysis of Ivermectin response by onchocerca volvulus reveals that genetic drift and soft selective sweeps contribute to loss of drug sensitivity. PLoS Negl. Trop. Dis. 11, e0005816 (2017).
- Morgan, E. R. et al. 100 Questions in livestock helminthology research. Trends Parasitol. 35, 52-71 (2019).
- Osei-Atweneboana, M. Y. et al. Phenotypic evidence of emerging Ivermectin resistance in onchocerca volvulus. PLoS Negl. Trop. Dis. 5, 1-11 (2011).
- Wit, J., Dilks, C. M., Andersen, E. C. & Program, B. S. Nematodes Underst. Anthelmintic Resist. 37, 240-250 (2022).
- Hahnel, S. R., Dilks, C. M., Heisler, I., Andersen, E. C. & Kulke, D. Caenorhabditis elegans in anthelmintic research – Old model, new perspectives. Int. J. Parasitol. Drugs Drug Resist. 14, 237-248 (2020).
- James, C. E. & Davey, M. W. Increased expression of ABC transport proteins is associated with Ivermectin resistance in the model nematode Caenorhabditis elegans. Int. J. Parasitol. 39, 213-220 (2009).
- Ménez, C., Alberich, M., Kansoh, D., Blanchard, A. & Lespine, A. Acquired tolerance to Ivermectin and moxidectin after drug selection pressure in the nematode Caenorhabditis elegans. Antimicrob. Agents Chemother. 60, 4809-4819 (2016).
- Driscoll, M., Dean, E., Reilly, E., Bergholz, E. & Chalfie, M. Genetic and molecular analysis of a Caenorhabditis elegans betatubulin that conveys benzimidazole sensitivity. J. Cell. Biol. 109, 2993-3003 (1989).
- Ménez, C. et al. The transcription factor NHR-8: A new target to increase Ivermectin efficacy in nematodes. PLOS Pathog. 15, e1007598 (2019).
- Kaplan, R. M. et al. World association for the advancement of veterinary parasitology (W.A.A.V.P.) guideline for diagnosing anthelmintic resistance using the faecal egg count reduction test in ruminants, horses and swine. Vet Parasitol 318, (2023).
- Morgan, E. R., Lanusse, C., Rinaldi, L., Charlier, J. & Vercruysse, J. Confounding factors affecting faecal egg count reduction as a measure of anthelmintic efficacy. Parasite 29, 20 (2022).
- Simonetta, S. H. & Golombek, D. A. An automated tracking system for Caenorhabditis elegans locomotor behavior and circadian studies application. J. Neurosci. Methods. 161, 273-280 (2007).
- Simonetta, S. H., Migliori, M. L., Romanowski, A. & Golombek, D. A. Timing of locomotor activity circadian rhythms in Caenorhabditis elegans. PLoS One. 4, e7571 (2009).
- Bianchi, J. I. et al. Reliable screening of dye phototoxicity by using a Caenorhabditis elegans fast bioassay. PLoS One. 10, 1-15 (2015).
- Buckingham, S. D., Partridge, F. A., Sattelle, D. B. & Automated high-throughput, motility analysis in Caenorhabditis elegans and parasitic nematodes: applications in the search for new anthelmintics. Int. J. Parasitol. Drugs Drug Resist. 4, 226-232 (2014).
- Liu, M. et al. Bioassay-guided isolation of three anthelmintic compounds from Warburgia ugandensis Sprague subspecies ugandensis, and the mechanism of action of polygodial. Int. J. Parasitol. 48, 833-844 (2018).
- Liu, M. et al. Bioassay-Guided isolation of anthelmintic components from semen pharbitidis, and the mechanism of action of pharbitin. Int J. Mol. Sci 23, (2022).
- Garbin, V. P. et al. Chemical characterization and in vitro anthelmintic activity of Citrus bergamia Risso and Citrus X paradisii Macfad essential oil against Haemonchus contortus Kirby isolate. Acta Trop. 217, 105869 (2021).
- Liu, M., Landuyt, B., Klaassen, H., Geldhof, P. & Luyten, W. Screening of a drug repurposing library with a nematode motility assay identifies promising anthelmintic hits against Cooperia oncophora and other ruminant parasites. Vet. Parasitol. 265, 15-18 (2019).
- Taki, A. C. et al. High-Throughput phenotypic assay to screen for anthelmintic activity on Haemonchus contortus. Pharmaceuticals 14, 616 (2021).
- Munguía, B. et al. Sensitivity of Haemonchus contortus to anthelmintics using different in vitro screening assays: a comparative study. Parasites Vectors. 15, 1-11 (2022).
- Suárez, G., Alcántara, I. & Salinas, G. Caenorhabditis elegans as a valuable model for the study of anthelmintic pharmacodynamics and drug-drug interactions: the case of Ivermectin and eprinomectin. Front. Pharmacol. 13, 1-9 (2022).
- Charvet, C. L., Guégnard, F., Courtot, E., Cortet, J. & Neveu, C. Nicotine-sensitive acetylcholine receptors are relevant Pharmacological targets for the control of multidrug resistant parasitic nematodes. Int. J. Parasitol. Drugs Drug Resist. 8, 540-549 (2018).
- Jouffroy, S. et al. First report of eprinomectin-resistant isolates of Haemonchus contortus in 5 dairy sheep farms from the Pyrénées atlantiques département in France. Parasitology 150, 365-373 (2023).
- Risi, G. et al. Caenorhabditis elegans Infrared-Based motility assay identified new hits for nematicide drug development. Vet. Sci. 6, 29 (2019).
- Mickiewicz, M. et al. Inhibitory effect of dimethyl sulfoxide on the development of gastrointestinal nematode larvae in the larval development test. Abst. J. Veter. Res. 69(1), 83-90. https://doi.org/10.2478/jvetres-2025-0016 (2025).
- Preez, G. et al. Oxygen consumption rate of Caenorhabditis elegans as a high-throughput endpoint of toxicity testing using the seahorse XFe96 extracellular flux analyzer. Sci. Rep. 10, 1-11 (2020).
- Preston, S. et al. Low cost whole-organism screening of compounds for anthelmintic activity. Int. J. Parasitol. 45, 333-343 (2015).
- Dolinská, M., Königová, A., Letková, V., Molnár, L. & Várady, M. Detection of Ivermectin resistance by a larval development testBack to the past or a step forward? Vet. Parasitol. 198, 154-158 (2013).
- Ardelli, B. F., Stitt, L. E., Tompkins, J. B. & Prichard, R. K. A comparison of the effects of Ivermectin and moxidectin on the nematode Caenorhabditis elegans. Vet. Parasitol. 165, 96-108 (2009).
- Kotze, A. C. & Prichard, R. K. Anthelmintic resistance in Haemonchus contortus. in 397-428 (2016). https://doi.org/10.1016/bs.a par.2016.02.012
- Kaplan, R. M. & Biology Epidemiology, diagnosis, and management of anthelmintic resistance in Gastrointestinal nematodes of livestock. Vet. Clin. North. Am. Food Anim. Pract. 36, 17-30 (2020).
- Hahnel, S. R., Roberts, W. M., Heisler, I., Kulke, D. & Weeks, J. C. Comparison of electrophysiological and motility assays to study anthelmintic effects in Caenorhabditis elegans. Int. J. Parasitol. Drugs Drug Resist. 16, 174-187 (2021).
- Kotze, A. C. & Prichard, R. K. Anthelmintic Resistance in Haemonchus Contortus. History, Mechanisms and Diagnosisvol. 93 (Elsevier Ltd, 2016). Advances in Parasitology.
- MARTIN, P., ANDERSON, N. & JARRETT, R. Detecting benzimidazole resistance with faecal egg count reduction tests and in vitro assays. Aust Vet. J. 66, 236-240 (1989).
- McIntyre, J. et al. Hidden in plain sight – Multiple resistant species within a Strongyle community. Vet. Parasitol. 258, 79-87 (2018).
- Kaplan, R. M. et al. A novel approach for combining the use of in vitro and in vivo data to measure and detect emerging moxidectin resistance in Gastrointestinal nematodes of goats. Int. J. Parasitol. 37, 795-804 (2007).
- Demeler, J., Kleinschmidt, N., Küttler, U., Koopmann, R. & von Samson-Himmelstjerna, G. Evaluation of the egg hatch assay and the larval migration Inhibition assay to detect anthelmintic resistance in cattle parasitic nematodes on farms. Parasitol. Int. 61, 614-618 (2012).
- Dolinská, M. U., Königová, A., Babják, M. & Várady, M. Comparison of two in vitro methods for the detection of Ivermectin resistance in Haemonchus contortus in sheep. Helminthol 53, 120-125 (2016).
- Kotze, A. C., Jambre, L., O’Grady, J. & L. F. & A modified larval migration assay for detection of resistance to macrocyclic lactones in Haemonchus contortus, and drug screening with Trichostrongylidae parasites. Vet. Parasitol. 137, 294-305 (2006).
- Buller, H. et al. Veterinary diagnostic practice and the use of rapid tests in antimicrobial stewardship on UK livestock farms. Front Vet. Sci 7, (2020).
- Vande Velde, F., Charlier, J., Hudders, L., Cauberghe, V. & Claerebout, E. Beliefs, intentions, and beyond: A qualitative study on the adoption of sustainable Gastrointestinal nematode control practices in Flanders’ dairy industry. Prev. Vet. Med. 153, 15-23 (2018).
- Blanchard, A. et al. Deciphering the molecular determinants of cholinergic anthelmintic sensitivity in nematodes: when novel functional validation approaches highlight major differences between the model Caenorhabditis elegans and parasitic species. PLoS Pathog. 14, 1-28 (2018).
- George, M. M., Lopez-Soberal, L., Storey, B. E., Howell, S. B. & Kaplan, R. M. Motility in the L3 stage is a poor phenotype for detecting and measuring resistance to avermectin/milbemycin drugs in Gastrointestinal nematodes of livestock. Int. J. Parasitol. Drugs Drug Resist. 8, 22-30 (2018).
- Page, A. P., Stepek, G., Winter, A. D. & Pertab, D. Enzymology of the nematode cuticle: A potential drug target? Int. J. Parasitol. Drugs Drug Resist. 4, 133-141 (2014).
- Maclean, M. J. et al. Does evaluation of in vitro microfilarial motility reflect the resistance status of dirofilaria immitis isolates to macrocyclic lactones? Parasites Vectors. 10, 17-23 (2017).
- Francis, E. K. & Šlapeta, J. A new diagnostic approach to fast-track and increase the accessibility of Gastrointestinal nematode identification from faeces: FECPAKG2 egg nemabiome metabarcoding. Int. J. Parasitol. 52, 331-342 (2022).
- Francis, E. K. et al. A mixed amplicon metabarcoding and sequencing approach for surveillance of drug resistance to levamisole and benzimidazole in Haemonchus spp. Int. J. Parasitol. 54, 55-64 (2024).
- Besier, R. B., Kahn, L. P., Sargison, N. D., Van Wyk, J. A. & Diagnosis Treatment and management of Haemonchus contortus in small ruminants. in 181-238 (2016). https://doi.org/10.1016/bs.apar.2016.02.024
- Arsenopoulos, K. V., Fthenakis, G. C., Katsarou, E. I. & Papadopoulos, E. Haemonchosis: A challenging parasitic infection of sheep and goats. Animals 11, 363 (2021).
- Hodgkinson, J. E. et al. Refugia and anthelmintic resistance: concepts and challenges. Int. J. Parasitol. Drugs Drug Resist. 10, 51-57 (2019).
شكر وتقدير
مساهمات المؤلفين
الإعلانات
المصالح المتنافسة
معلومات إضافية
معلومات إعادة الطبع والتصاريح متاحة علىwww.nature.com/reprints.
ملاحظة الناشر: تظل شركة سبرينغر ناتشر محايدة فيما يتعلق بالمطالبات القضائية في الخرائط المنشورة والانتماءات المؤسسية.
© المؤلف(ون) 2025
INTHERES، جامعة تولوز، INRAE، ENVT، تولوز، فرنسا. IHAP، جامعة تولوز، INRAE، ENVT، تولوز، فرنسا. البريد الإلكتروني:melanie.alberich@inrae.fr; anne.lespine@inrae.fr
DOI: https://doi.org/10.1038/s41598-025-02866-3
PMID: https://pubmed.ncbi.nlm.nih.gov/40410413
Publication Date: 2025-05-23
scientific reports
OPEN
Evaluation of nematode susceptibility and resistance to anthelmintic drugs with a WMicrotracker motility assay
Abstract
Grazing ruminants suffer from various helminth infections particularly those caused by gastrointestinal nematode (GIN) parasites, which have a considerable impact on their welfare and productivity. To treat these infections, the intensive use of macrocyclic lactone (ML) anthelmintics has led to the emergence of drug-resistant parasite populations worldwide. The standard method for detecting resistance, the Faecal Egg Count Reduction Test (FECRT), is susceptible to misinterpretation, leading to flawed management decisions that undermine parasite control efforts. Thus, there is a pressing need for robust resistance detection methods in field parasites. We investigated the potential of the WMicrotracker motility assay (WMA), previously unexplored in ML resistance assessment. The assay first compared ivermectin (IVM) susceptibility among wild-type Bristol N2 (N2B), IVM-selected (IVR10), and
Parasitic nematodes pose a significant threat to animal health, with profound implications for livestock welfare and productivity. Among them, Haemonchus contortus stands out as a highly pathogenic nematode parasite, exacerbating the challenges faced by livestock owners and impacting overall animal well-being and economic output
Materials and methods
Materials
C. elegans nematode strains and cultivation conditions
H. contortus isolates
Worm motility assay (WMA)
WMA on C. elegans strains
(Score Activity
(Basal Activity
Motility percentages were calculated for each treated well as -fold induction relative to DMSO treated worms which was set to 100 . To facilitate comparison with other phenotypic assays, Table 1 provides a summary of
WMA on H. contortus isolates
Dose-response analysis and resistance factor (RF) calculation
Statistical analysis


| Assay | Informations about experiments | C. elegansstrains | ||||||||||
| N2B | NHR-8 | IVR10 | ||||||||||
| Motility assay | IVM | MOX | EPR | IVM | MOX | EPR | IVM | MOX | EPR | References | ||
| Adults, Worm Microtracker | 33.52 | 59.18 | 54.84 | 29.26 | 63.80 | 40.15 | 71.20 | 88.16 | 101.41 | Current study | ||
| L4, Worm Microtracker | 190 | ND | ND | ND | ND | ND | ND | ND | ND | 31 | ||
| Adults, Worm Microtracker | 290 | 120 | ND | ND | ND | ND | ND | ND | ND | 34 | ||
| L4, Worm Microtracker | 150-500 | ND | 100-300 | ND | ND | ND | ND | ND | ND | ND | 28 | |
| Larval development assay | Agar plates | 1.69 | 1.77 | 1.19 | ND | ND | ND | 12.43 | 3.06 | 17.82 | 13 | |
| Agar plates | 1.63 | ND | ND | 0.96 | ND | ND | ND | ND | ND | 15 | ||
| Pharynx pumping | 8-channel chip | 1420 | 900 | ND | ND | ND | ND | ND | ND | ND | 34 | |
| ScreenChip | 51 | 42 | ND | ND | ND | ND | ND | ND | ND | 34 | ||
Results
Motility assay to assess IVM efficacy in adult C. elegans
Motility assay to discriminate susceptible from resistant

| N2B | IVR10 | AE501 nhr-8(ok186) | |||
| Treatment | Mean
|
Mean
|
RF | Mean
|
RF |
| IVM |
|
|
2.12 |
|
0.87 |
| MOX |
|
|
1.49 |
|
1.08 |
| EPR |
|
|
1.85 |
|
0.73 |
Discussion

| S-H-2022 |
|
||
| Treatment | Mean
|
Mean
|
RF |
| IVM |
|
|
5.24 |
| MOX |
|
|
5.40 |
| EPR |
|
|
234.19 |
highly tolerant to EPR, without laboratory-induced resistance. This better reflects real-world conditions and highlights the adaptability of this method for assessing drug efficacy in field isolates, particularly in detecting emerging resistance. Furthermore, it should improve our understanding of how subpopulations within a larger population can influence overall resistance, which is essential for effectively monitoring and managing drug resistance dynamics. By directly quantifying motility, WMi provides a robust approach for evaluating AR across diverse
Data availability
Received: 30 October 2024; Accepted: 16 May 2025
Published online: 23 May 2025
References
- Fitzpatrick, J. L. Global food security: the impact of veterinary parasites and parasitologists. Vet. Parasitol. 195, 233-248 (2013).
- Martin, R. J., Robertson, A. P., Choudhary, S. & Ivermectin An anthelmintic, an insecticide, and much more. Trends Parasitol. 37, 48-64 (2021).
- Prichard, R. K. & Geary, T. G. Perspectives on the utility of moxidectin for the control of parasitic nematodes in the face of developing anthelmintic resistance. Int. J. Parasitol. Drugs Drug Resist. 10, 69-83 (2019).
- European Medicines Agency. Reflection paper on anthelmintic resistance (Draft 2). Eur. Med. Agency. 44, 1-16 (2016).
- Bourguinat, C. et al. Macrocyclic lactone resistance in dirofilaria immitis: failure of heartworm preventives and investigation of genetic markers for resistance. Vet. Parasitol. 210, 167-178 (2015).
- Laing, R., Gillan, V. & Devaney, E. Ivermectin – Old drug, new tricks?? Trends Parasitol. 33, 463-472 (2017).
- Doyle, S. R. et al. Genome-wide analysis of Ivermectin response by onchocerca volvulus reveals that genetic drift and soft selective sweeps contribute to loss of drug sensitivity. PLoS Negl. Trop. Dis. 11, e0005816 (2017).
- Morgan, E. R. et al. 100 Questions in livestock helminthology research. Trends Parasitol. 35, 52-71 (2019).
- Osei-Atweneboana, M. Y. et al. Phenotypic evidence of emerging Ivermectin resistance in onchocerca volvulus. PLoS Negl. Trop. Dis. 5, 1-11 (2011).
- Wit, J., Dilks, C. M., Andersen, E. C. & Program, B. S. Nematodes Underst. Anthelmintic Resist. 37, 240-250 (2022).
- Hahnel, S. R., Dilks, C. M., Heisler, I., Andersen, E. C. & Kulke, D. Caenorhabditis elegans in anthelmintic research – Old model, new perspectives. Int. J. Parasitol. Drugs Drug Resist. 14, 237-248 (2020).
- James, C. E. & Davey, M. W. Increased expression of ABC transport proteins is associated with Ivermectin resistance in the model nematode Caenorhabditis elegans. Int. J. Parasitol. 39, 213-220 (2009).
- Ménez, C., Alberich, M., Kansoh, D., Blanchard, A. & Lespine, A. Acquired tolerance to Ivermectin and moxidectin after drug selection pressure in the nematode Caenorhabditis elegans. Antimicrob. Agents Chemother. 60, 4809-4819 (2016).
- Driscoll, M., Dean, E., Reilly, E., Bergholz, E. & Chalfie, M. Genetic and molecular analysis of a Caenorhabditis elegans betatubulin that conveys benzimidazole sensitivity. J. Cell. Biol. 109, 2993-3003 (1989).
- Ménez, C. et al. The transcription factor NHR-8: A new target to increase Ivermectin efficacy in nematodes. PLOS Pathog. 15, e1007598 (2019).
- Kaplan, R. M. et al. World association for the advancement of veterinary parasitology (W.A.A.V.P.) guideline for diagnosing anthelmintic resistance using the faecal egg count reduction test in ruminants, horses and swine. Vet Parasitol 318, (2023).
- Morgan, E. R., Lanusse, C., Rinaldi, L., Charlier, J. & Vercruysse, J. Confounding factors affecting faecal egg count reduction as a measure of anthelmintic efficacy. Parasite 29, 20 (2022).
- Simonetta, S. H. & Golombek, D. A. An automated tracking system for Caenorhabditis elegans locomotor behavior and circadian studies application. J. Neurosci. Methods. 161, 273-280 (2007).
- Simonetta, S. H., Migliori, M. L., Romanowski, A. & Golombek, D. A. Timing of locomotor activity circadian rhythms in Caenorhabditis elegans. PLoS One. 4, e7571 (2009).
- Bianchi, J. I. et al. Reliable screening of dye phototoxicity by using a Caenorhabditis elegans fast bioassay. PLoS One. 10, 1-15 (2015).
- Buckingham, S. D., Partridge, F. A., Sattelle, D. B. & Automated high-throughput, motility analysis in Caenorhabditis elegans and parasitic nematodes: applications in the search for new anthelmintics. Int. J. Parasitol. Drugs Drug Resist. 4, 226-232 (2014).
- Liu, M. et al. Bioassay-guided isolation of three anthelmintic compounds from Warburgia ugandensis Sprague subspecies ugandensis, and the mechanism of action of polygodial. Int. J. Parasitol. 48, 833-844 (2018).
- Liu, M. et al. Bioassay-Guided isolation of anthelmintic components from semen pharbitidis, and the mechanism of action of pharbitin. Int J. Mol. Sci 23, (2022).
- Garbin, V. P. et al. Chemical characterization and in vitro anthelmintic activity of Citrus bergamia Risso and Citrus X paradisii Macfad essential oil against Haemonchus contortus Kirby isolate. Acta Trop. 217, 105869 (2021).
- Liu, M., Landuyt, B., Klaassen, H., Geldhof, P. & Luyten, W. Screening of a drug repurposing library with a nematode motility assay identifies promising anthelmintic hits against Cooperia oncophora and other ruminant parasites. Vet. Parasitol. 265, 15-18 (2019).
- Taki, A. C. et al. High-Throughput phenotypic assay to screen for anthelmintic activity on Haemonchus contortus. Pharmaceuticals 14, 616 (2021).
- Munguía, B. et al. Sensitivity of Haemonchus contortus to anthelmintics using different in vitro screening assays: a comparative study. Parasites Vectors. 15, 1-11 (2022).
- Suárez, G., Alcántara, I. & Salinas, G. Caenorhabditis elegans as a valuable model for the study of anthelmintic pharmacodynamics and drug-drug interactions: the case of Ivermectin and eprinomectin. Front. Pharmacol. 13, 1-9 (2022).
- Charvet, C. L., Guégnard, F., Courtot, E., Cortet, J. & Neveu, C. Nicotine-sensitive acetylcholine receptors are relevant Pharmacological targets for the control of multidrug resistant parasitic nematodes. Int. J. Parasitol. Drugs Drug Resist. 8, 540-549 (2018).
- Jouffroy, S. et al. First report of eprinomectin-resistant isolates of Haemonchus contortus in 5 dairy sheep farms from the Pyrénées atlantiques département in France. Parasitology 150, 365-373 (2023).
- Risi, G. et al. Caenorhabditis elegans Infrared-Based motility assay identified new hits for nematicide drug development. Vet. Sci. 6, 29 (2019).
- Mickiewicz, M. et al. Inhibitory effect of dimethyl sulfoxide on the development of gastrointestinal nematode larvae in the larval development test. Abst. J. Veter. Res. 69(1), 83-90. https://doi.org/10.2478/jvetres-2025-0016 (2025).
- Preez, G. et al. Oxygen consumption rate of Caenorhabditis elegans as a high-throughput endpoint of toxicity testing using the seahorse XFe96 extracellular flux analyzer. Sci. Rep. 10, 1-11 (2020).
- Preston, S. et al. Low cost whole-organism screening of compounds for anthelmintic activity. Int. J. Parasitol. 45, 333-343 (2015).
- Dolinská, M., Königová, A., Letková, V., Molnár, L. & Várady, M. Detection of Ivermectin resistance by a larval development testBack to the past or a step forward? Vet. Parasitol. 198, 154-158 (2013).
- Ardelli, B. F., Stitt, L. E., Tompkins, J. B. & Prichard, R. K. A comparison of the effects of Ivermectin and moxidectin on the nematode Caenorhabditis elegans. Vet. Parasitol. 165, 96-108 (2009).
- Kotze, A. C. & Prichard, R. K. Anthelmintic resistance in Haemonchus contortus. in 397-428 (2016). https://doi.org/10.1016/bs.a par.2016.02.012
- Kaplan, R. M. & Biology Epidemiology, diagnosis, and management of anthelmintic resistance in Gastrointestinal nematodes of livestock. Vet. Clin. North. Am. Food Anim. Pract. 36, 17-30 (2020).
- Hahnel, S. R., Roberts, W. M., Heisler, I., Kulke, D. & Weeks, J. C. Comparison of electrophysiological and motility assays to study anthelmintic effects in Caenorhabditis elegans. Int. J. Parasitol. Drugs Drug Resist. 16, 174-187 (2021).
- Kotze, A. C. & Prichard, R. K. Anthelmintic Resistance in Haemonchus Contortus. History, Mechanisms and Diagnosisvol. 93 (Elsevier Ltd, 2016). Advances in Parasitology.
- MARTIN, P., ANDERSON, N. & JARRETT, R. Detecting benzimidazole resistance with faecal egg count reduction tests and in vitro assays. Aust Vet. J. 66, 236-240 (1989).
- McIntyre, J. et al. Hidden in plain sight – Multiple resistant species within a Strongyle community. Vet. Parasitol. 258, 79-87 (2018).
- Kaplan, R. M. et al. A novel approach for combining the use of in vitro and in vivo data to measure and detect emerging moxidectin resistance in Gastrointestinal nematodes of goats. Int. J. Parasitol. 37, 795-804 (2007).
- Demeler, J., Kleinschmidt, N., Küttler, U., Koopmann, R. & von Samson-Himmelstjerna, G. Evaluation of the egg hatch assay and the larval migration Inhibition assay to detect anthelmintic resistance in cattle parasitic nematodes on farms. Parasitol. Int. 61, 614-618 (2012).
- Dolinská, M. U., Königová, A., Babják, M. & Várady, M. Comparison of two in vitro methods for the detection of Ivermectin resistance in Haemonchus contortus in sheep. Helminthol 53, 120-125 (2016).
- Kotze, A. C., Jambre, L., O’Grady, J. & L. F. & A modified larval migration assay for detection of resistance to macrocyclic lactones in Haemonchus contortus, and drug screening with Trichostrongylidae parasites. Vet. Parasitol. 137, 294-305 (2006).
- Buller, H. et al. Veterinary diagnostic practice and the use of rapid tests in antimicrobial stewardship on UK livestock farms. Front Vet. Sci 7, (2020).
- Vande Velde, F., Charlier, J., Hudders, L., Cauberghe, V. & Claerebout, E. Beliefs, intentions, and beyond: A qualitative study on the adoption of sustainable Gastrointestinal nematode control practices in Flanders’ dairy industry. Prev. Vet. Med. 153, 15-23 (2018).
- Blanchard, A. et al. Deciphering the molecular determinants of cholinergic anthelmintic sensitivity in nematodes: when novel functional validation approaches highlight major differences between the model Caenorhabditis elegans and parasitic species. PLoS Pathog. 14, 1-28 (2018).
- George, M. M., Lopez-Soberal, L., Storey, B. E., Howell, S. B. & Kaplan, R. M. Motility in the L3 stage is a poor phenotype for detecting and measuring resistance to avermectin/milbemycin drugs in Gastrointestinal nematodes of livestock. Int. J. Parasitol. Drugs Drug Resist. 8, 22-30 (2018).
- Page, A. P., Stepek, G., Winter, A. D. & Pertab, D. Enzymology of the nematode cuticle: A potential drug target? Int. J. Parasitol. Drugs Drug Resist. 4, 133-141 (2014).
- Maclean, M. J. et al. Does evaluation of in vitro microfilarial motility reflect the resistance status of dirofilaria immitis isolates to macrocyclic lactones? Parasites Vectors. 10, 17-23 (2017).
- Francis, E. K. & Šlapeta, J. A new diagnostic approach to fast-track and increase the accessibility of Gastrointestinal nematode identification from faeces: FECPAKG2 egg nemabiome metabarcoding. Int. J. Parasitol. 52, 331-342 (2022).
- Francis, E. K. et al. A mixed amplicon metabarcoding and sequencing approach for surveillance of drug resistance to levamisole and benzimidazole in Haemonchus spp. Int. J. Parasitol. 54, 55-64 (2024).
- Besier, R. B., Kahn, L. P., Sargison, N. D., Van Wyk, J. A. & Diagnosis Treatment and management of Haemonchus contortus in small ruminants. in 181-238 (2016). https://doi.org/10.1016/bs.apar.2016.02.024
- Arsenopoulos, K. V., Fthenakis, G. C., Katsarou, E. I. & Papadopoulos, E. Haemonchosis: A challenging parasitic infection of sheep and goats. Animals 11, 363 (2021).
- Hodgkinson, J. E. et al. Refugia and anthelmintic resistance: concepts and challenges. Int. J. Parasitol. Drugs Drug Resist. 10, 51-57 (2019).
Acknowledgements
Author contributions
Declarations
Competing interests
Additional information
Reprints and permissions information is available at www.nature.com/reprints.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
© The Author(s) 2025
INTHERES, Université de Toulouse, INRAE, ENVT, Toulouse, France. IHAP, Université de Toulouse, INRAE, ENVT, Toulouse, France. email: melanie.alberich@inrae.fr; anne.lespine@inrae.fr
