DOI: https://doi.org/10.1186/s13054-024-04803-8
PMID: https://pubmed.ncbi.nlm.nih.gov/38302945
تاريخ النشر: 2024-02-01
دمج البروتينات مع الأحماض الدهنية غير المشبعة المتعددة n-3 (EPA + DHA) ووسائطها المساعدة على حل الالتهابات للحفاظ على كتلة العضلات الهيكلية
الملخص
لا يزال النقاش حول الاستراتيجية المثلى للتغذية للمرضى الذين يعانون من حالات حرجة مستمراً، ولكن يجب تكييف التغذية مع احتياجات كل مريض على حدة. المرضى الذين يعانون من حالات حرجة معرضون لخطر انهيار العضلات، مما يؤدي إلى فقدان كتلة العضلات وتأثيراتها السريرية الناتجة. تم استكشاف توقيت بدء التغذية وأهداف البروتين في التجارب الأخيرة. تشير هذه التجارب إلى أن توفير البروتين “المعتدل” (الحد الأقصى
المقدمة
(MPS) وانهيار بروتين العضلات (MPB) [9]. في المرضى الذين يعانون من حالات حرجة، يتعطل هذا التوازن لصالح الانهيار، حيث يعتمد مدى الاضطراب على شدة المرض [10]. تعزز الالتهابات المفرطة أو غير المحلولة هذا الخلل: حيث تمنع الالتهابات مسار إشارة الهدف الثديي من رابيمايسين (mTOR) الذي يعزز MPS، ويساهم ذلك في فقدان بروتين العضلات الصافي الذي لوحظ خلال المرض الحرج والإنتان [11].
الالتهاب شائع جدًا في المرض الحرج [12] ويحفز التحلل البروتيني داخل العضلات الهيكلية [13، 14]. الهدف من انهيار العضلات هو تحريك الأحماض الأمينية لإنتاج الطاقة (أي الأكسدة) أو تكوين الجلوكوز، مع استخدام الأمونيا الناتجة من إزالة الأمين الأولية لتخليق وإخراج اليوريا، ولدعم المناعة، وإصلاح الأنسجة وإنتاج بروتينات المرحلة الحادة [15]. ومع ذلك، بعد المرحلة الحادة الأولية، فإن استمرار الالتهاب ضار، مما يزيد من خطر الضعف المكتسب في وحدة العناية المركزة [16] والمرض الحرج المستمر [17]، مع ارتفاع نسبة اليوريا إلى الكرياتينين المرتبطة به، وهي علامة على انهيار العضلات [18]. يحتوي الالتهاب على مرحلتين متميزتين، البدء والحل: في المرض الحرج المستمر، يبدو أن هذه المرحلة الثانية معطلة.
كيف يمكن مواجهة هذه الظاهرة المرضية الضارة؟ حتى وقت قريب، كانت الاستراتيجيات الرئيسية تتضمن تقديم جرعات كافية من البروتينات، ونسب أعلى من الأحماض الأمينية الأساسية والمتفرعة [19-21]، وفي النهاية النشاط البدني [22]. الاستراتيجية الأحدث، التي تضيف إلى السابقة، هي استخدام الأحماض الدهنية غير المشبعة المتعددة أوميغا-3 طويلة السلسلة (n-3 PUFAs) لدفع حل الالتهاب واستعادة صحة العضلات من خلال تنشيط مسار الحل [9]. يتم مناقشة هذه النتائج الجديدة هنا.
دور البروتينات والأحماض الأمينية
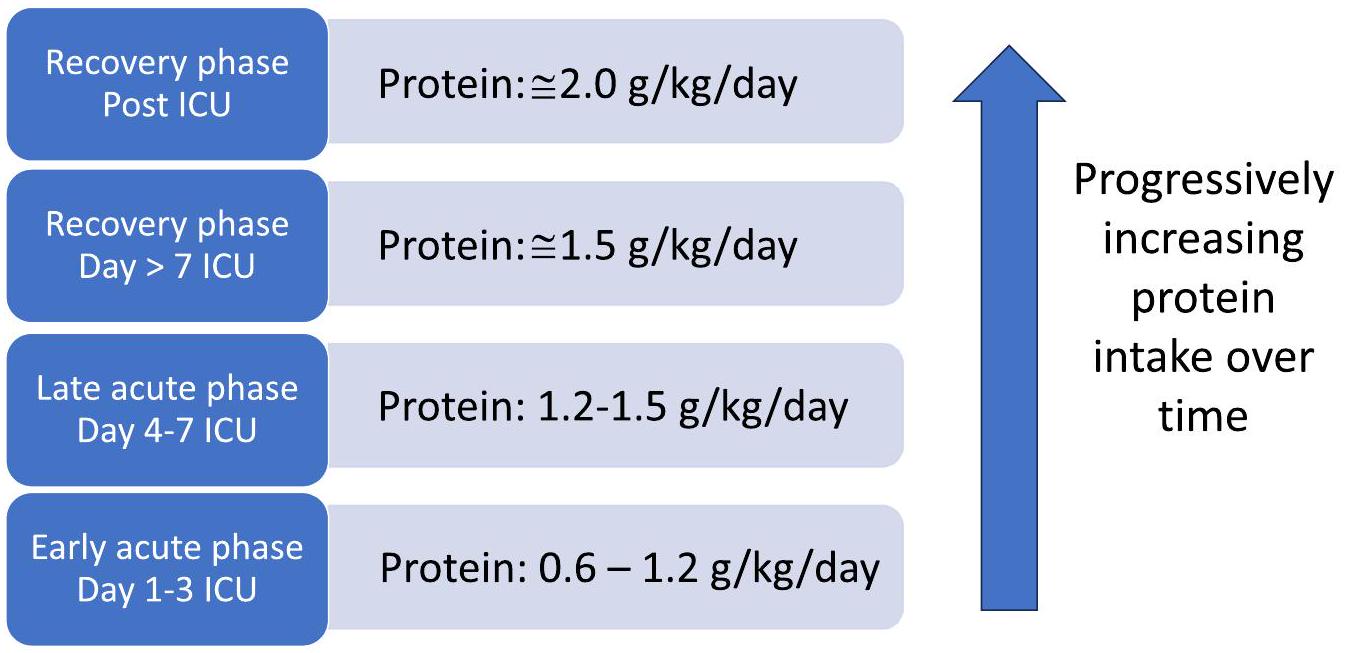
على الرغم من الإرشادات، لا تزال الجرعة المثلى من البروتين والتوقيت محل نقاش، حيث إن التأثير على انهيار بروتين العضلات غير واضح. أفاد وانغ وآخرون بوجود علاقة خطية عكسية بين مدخول البروتين والوفيات خلال 30 يومًا [25]، بينما لاحظ زوسمان وآخرون تقليل الوفيات مع مدخول بروتين يتجاوز
بزيادة الوفيات مع مدخول بروتين أعلى [27،28]. اختبرت تجربة EFFORT البروتين وصف جرعات مختلفة من البروتين (
يتطلب الأفراد الأكبر سنًا تناول بروتين أعلى للتغلب على مقاومة الأيض التي تحدث مع تقدم العمر: معدل تخليق البروتين العضلي هو أعلى بكثير عند مستويات تناول البروتين المنخفضة في الأفراد الأصغر سنًا [38]. تظهر دراسات توازن النيتروجين نتائج مشابهة: تحسنت الاستجابة لتناول البروتين في مرضى وحدة العناية المركزة الذين يعانون من إصابات بشكل خطي حتى تناول
من خلال قياس معدلات تخليق البروتين العضلي، قارن تشابل وآخرون مرضى وحدة العناية المركزة مع ضوابط صحية وتمكنوا من إظهار أنه لم يكن هناك
فرق في هضم البروتين وتوافر الأحماض الأمينية بعد الوجبة. ومع ذلك، كان غنى البروتين العضلي بالفينيل ألانين (كمقياس لإدماج البروتين الغذائي في بروتين العضلات الهيكلية)
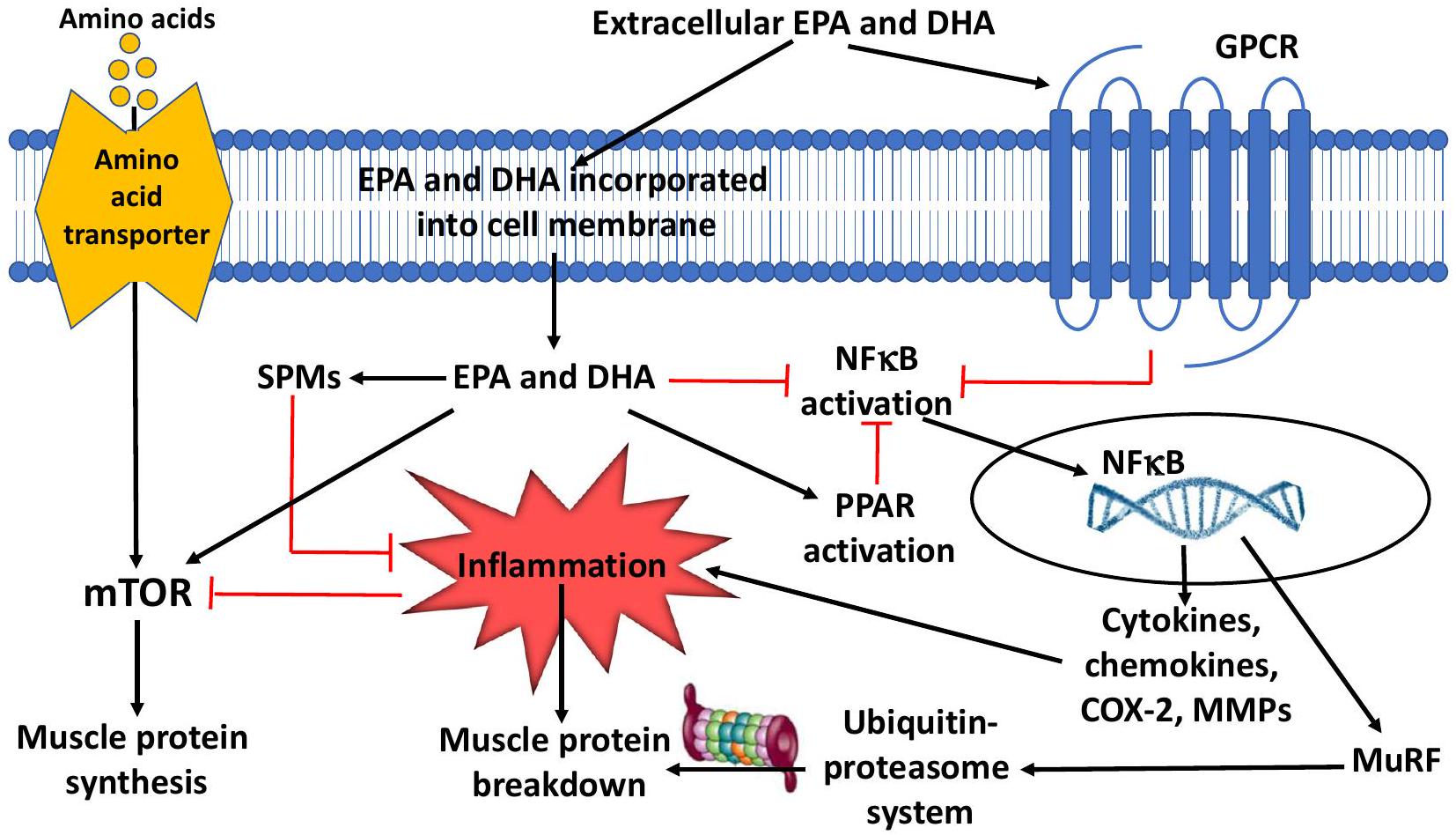
الالتهاب، EPA، DHA، والوسائط المساعدة على الحل
تقليل الالتهاب وتعزيز حله (الشكل 2)، قد يقلل EPA وDHA من تحلل البروتين العضلي وفقدانه [48].
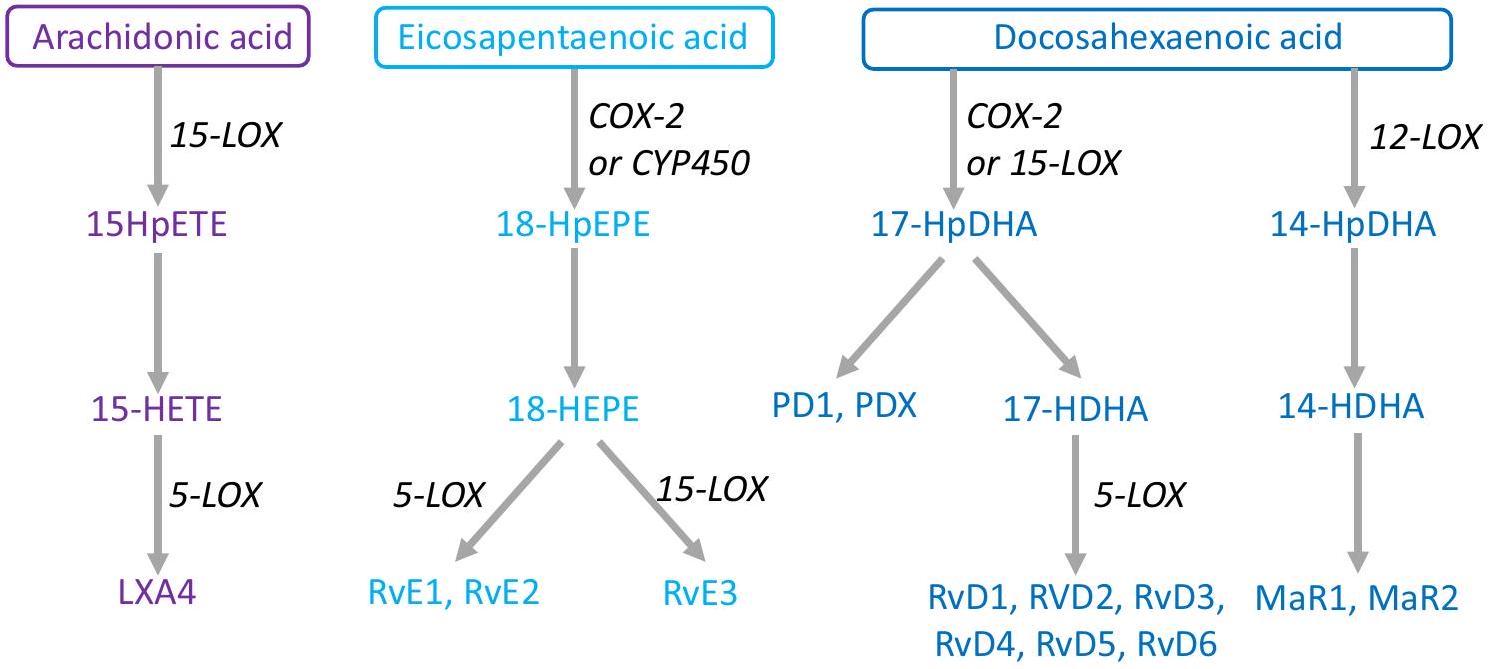
SPMs غير موجودة في النظام الغذائي، بل يتم إنتاجها داخليًا من خلال الأيض الخاص بحمض الأراكيدونيك وEPA وDPA وDHA إلى سلسلة واسعة من مشتقات الأحماض الدهنية؛ ومن الأمثلة الأكثر دراسة تشمل ليبكسين A4 (من حمض الأراكيدونيك) وريزولفين E1 (من EPA) وريزولفين D1 وD2 (من DHA). ينتج ليبكسين A4 تأثيراته المضادة للالتهابات من خلال تحفيز المونوسيتات والماكروفاجات لزيادة البلعمة وتحفيز إنتاج IL-10 مع تقليل إفراز السيتوكينات المؤيدة للالتهابات. يقلل ريزولفين E1 من تنشيط العدلات والتصاق البطانة، بينما يقلل أيضًا من إنتاج أنواع الأكسجين التفاعلية. كما تعزز SPMs قتل الميكروبات وإزالةها. يعمل ريزولفين D1 على خلايا البطانة من خلال زيادة تركيزات أكسيد النيتريك الداخلي والبروستاسيكلين وتقليل مستقبلات الالتصاق وإنتاج ROS والسيتوكينات المؤيدة للالتهابات. يقلل ريزولفين D2 من هجرة الخلايا الشجرية وإنتاج IL-12 [51]. باختصار، تعمل SPMs على تثبيط المزيد من تجنيد العدلات، وتعزيز إفراز السيتوكينات المؤيدة للحل مع تقليل السيتوكينات المؤيدة للالتهابات، وزيادة إزالة الميكروبات والحطام الخلوي. في البشر، تعتبر زيوت السمك مصدرًا مهمًا لـ EPA وDHA. وقد وُجد أن مكملات EPA وDHA تزيد من مستوياتها الدائرة
العديد من SPMs [52]. وقد أظهرت الدراسات أن تخليق SPM محفوظ تطوريًا: الفئران والضفادع والأميبا جميعها تخ синтез SPMs مما يظهر أهمية حل الالتهابات في إصلاح الأنسجة والتعافي عبر الطبيعة [53]. نطاق التركيز لنشاط SPMs هو في مستوى النانومولار/البيكومولار. توجد بمستويات نشطة حيويًا في معظم الأنسجة، بما في ذلك الدماغ والعقد اللمفاوية والأنسجة الدهنية [49-51، 54].
ربط مكغلوري وآخرون [57] الزيادة التدريجية في n-3 PUFAs في العضلات مع مرور الوقت بتغيرات في مستويات بعض البروتينات المعنية في الإشارات الأيضية في العضلات. أظهروا زيادة تعتمد على الوقت في كيناز التيروزين 2 الذي كان أعلى بعد 4 أسابيع من القاعدة وزيادات متواضعة، على الرغم من عدم كونها ذات دلالة، في كيناز البروتين الريبوسومي S6 كيناز بيتا-1 (P70S6K) وفي بروتين ربط عامل بدء الترجمة 4E. كان mTOR أعلى في الأسبوع 2 مقارنة بالقاعدة. تشير هذه التأثيرات إلى أن أحماض n-3 PUFAs قد تعزز MPS. تم استكشاف ذلك في دراستين من قبل سميث وآخرون [56، 59]. في أولى هذه الدراسات [59]، زادت المكملات اليومية بـ 1.86 غرام من EPA و
مرتبط بزيادة التأثير الأنابولي لإشارات أخرى. أفاد مكغلوري وآخرون [60] أن مكملات الإناث البالغات الشابات بـ 2.97 غرام من EPA و2.03 غرام من DHA يوميًا أدت إلى الحفاظ بشكل أفضل على حجم الكوادريسيبس خلال 2 أسابيع من تثبيت الساق وأن هذا كان مرتبطًا بالاحتفاظ بمعدل أعلى من تخليق البروتين العضلي خلال فترة التثبيت. مؤخرًا، أظهر إنجلين وآخرون [61] تقليلًا يعتمد على الجرعة من EPA بالإضافة إلى DHA لتفكك البروتين في الجسم بالكامل خلال فترة ما بعد الامتصاص وزيادة تعتمد على الجرعة من EPA بالإضافة إلى DHA في تخليق البروتين في الجسم بالكامل خلال فترة التغذية في مرضى داء الانسداد الرئوي المزمن. مجتمعة، تشير هذه الدراسات البشرية إلى أن EPA وDHA تغيران تعبير وتفعيل بروتينات الإشارات الأنابولية في العضلات الهيكلية البشرية، وتقلل من MPB، وتزيد من MPS. هذه التأثيرات ذات صلة بالحالات التي يوجد فيها خطر فقدان كتلة العضلات بسبب الشيخوخة أو عدم الاستخدام أو المرض. في هذا السياق، حددت تحليل ميتا حديث للدراسات البشرية في مجموعات سكانية متغايرة أن الكتلة النحيفة وكتلة العضلات الهيكلية تستفيد من تناول أعلى من EPA وDHA [62].
وآخرون أن ريسولفين D1 قلل بشكل كبير من إصابة الكبد في نموذج الإيثانول/الليبوبوليسكاريد. ومن المثير للاهتمام أن هذه الفائدة تبدو محددة للغاية حيث لم تكن هناك فائدة مع ريسولفين E1 [71].
الخاتمة
الاختصارات
| CRP | بروتينات C-reactive |
| EPA | حمض الإيكوسابنتاينويك |
| DHA | حمض الدوكوساهيكسانويك |
| mTOR | هدف الثدييات لمستقبل الراباميسين |
| n-3 PUFA | حمض دهن أوميغا-3 غير المشبع |
| SPMs | وسائط متخصصة لحل الالتهابات |
مساهمات المؤلفين
التمويل
توفر البيانات والمواد
الإعلانات
موافقة الأخلاقيات والموافقة على المشاركة
المصالح المتنافسة
نشر على الإنترنت: 01 فبراير 2024
References
- Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol. 2000;89(1):81-8.
- Jannas-Vela S, Espinosa A, Candia AA, Flores-Opazo M, Penailillo L, Valenzuela
. The role of omega- 3 polyunsaturated fatty acids and their lipid mediators on skeletal muscle regeneration: a narrative review. Nutrients. 2023;15:4. - Puthucheary Z, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, Hopkinson N, Padhke R, Dew T, Sidhu P, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310(15):1591-600.
- McNelly AS, Bear DE, Connolly BA, Arbane G, Allum L, Tarbhai A, Cooper JA, Hopkins PA, Wise MP, Brealey D, et al. Effect of intermittent or continuous feed on muscle wasting in critical illness: a phase 2 clinical trial. Chest. 2020;158(1):183-94.
- Nakanishi N, Tsutsumi R, Okayama Y, Takashima T, Ueno Y, Itagaki T, Tsutsumi Y, Sakaue H, Oto J. Monitoring of muscle mass in critically ill patients: comparison of ultrasound and two bioelectrical impedance analysis devices. J Intensive Care. 2019;7:61.
- Weijs P, Looijaard W, Beishuizen A, Girbes A, Oudemans-van Straaten H. Early high protein intake is associated with low mortality and energy overfeeding with high mortality in non-septic mechanically ventilated critically ill patients. Crit Care. 2014;18(6):701.
- Lim SL, Ong KC, Chan YH, Loke WC, Ferguson M, Daniels L. Malnutrition and its impact on cost of hospitalization, length of stay, readmission and 3-year mortality. Clin Nutr. 2012;31(3):345-50.
- Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16-31.
- Therdyothin A, Phiphopthatsanee N, Isanejad M. The effect of omega-3 fatty acids on sarcopenia: mechanism of action and potential efficacy. Mar Drugs. 2023;21:7.
- Deutz N, Singer P, Wierzchowska-McNew R, Viana M, Ben-David I, Pantet O, Thaden J, Ten Have G, Engelen M, Berger M. Comprehensive metabolic amino acid flux analysis in critically ill patients. Clin Nutr. 2021;40(5):2876-97.
- Lang CH, Frost RA, Vary TC. Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab. 2007;293(2):E453-459.
- Cox CE. Persistent systemic inflammation in chronic critical illness. Respir Care. 2012, 57(6):859-864; discussion 864-856.
- Haberecht-Muller S, Kruger E, Fielitz J. Out of control: the role of the ubiquitin proteasome system in skeletal muscle during inflammation. Biomolecules. 2021;11:9.
- Ji Y, Li M, Chang M, Liu R, Qiu J, Wang K, Deng C, Shen Y, Zhu J, Wang W, et al. Inflammation: roles in skeletal muscle atrophy. Antioxidants (Basel). 2022;11:9.
- Gillis C, Carli F. Promoting perioperative metabolic and nutritional care. Anesthesiology. 2015;123(6):1455-72.
- Lad H, Saumur TM, Herridge MS, Dos Santos CC, Mathur S, Batt J, Gilbert PM. Intensive care unit-acquired weakness: not just another muscle atrophying condition. Int J Mol Sci. 2020;21:21.
- Zhang Z, Ho KM, Gu H, Hong Y, Yu Y. Defining persistent critical illness based on growth trajectories in patients with sepsis. Crit Care. 2020;24(1):57.
- Haines RW, Zolfaghari P, Wan Y, Pearse RM, Puthucheary Z, Prowle JR. Elevated urea-to-creatinine ratio provides a biochemical signature of muscle catabolism and persistent critical illness after major trauma. Intensive Care Med. 2019;45(12):1718-31.
- Nakamura K, Kihata A, Naraba H, Kanda N, Takahashi Y, Sonoo T, Hashimoto H, Morimura N. Beta-hydroxy-beta-methylbutyrate, arginine, and glutamine complex on muscle volume loss in critically III patients: a randomized control trial. JPEN J Parenter Enteral Nutr. 2020;22(2):205-2112.
- Viana M, Becce F, Pantet O, Schmidt S, Bagnoud G, Thaden J, Ten Have G, Engelen M, Voidey A, Deutz N. MM B: impact of
-hydroxy- methylbutyrate (HMB) on muscle loss and protein metabolism in critically ill patients: a RCT. Clin Nutr. 2021;40:4878-87. - Lv X, Zhou C, Yan Q, Tan Z, Kang J, Tang S. Elucidating the underlying mechanism of amino acids to regulate muscle protein synthesis: effect on human health. Nutrition. 2022;103-104: 111797.
- Singer P, Blaser AR, Berger MM, Calder PC, Casaer M, Hiesmayr M, Mayer K, Montejo-Gonzalez JC, Pichard C, Preiser JC, et al. ESPEN practical and partially revised guideline: clinical nutrition in the intensive care unit. Clin Nutr. 2023;42(9):1671-89.
- McClave S, Taylor B, Martindale R, Warren M, Johnson D, Braunschweig C, McCarthy M, Davanos E, Rice T, Cresci G, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically III patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2016;40(2):159-211.
- Reintam Blaser A, Starkopf J, Alhazzani W, Berger M, Casaer M, Deane A, Fruhwald S, Hiesmayr M, Ichai C, Jakob S, et al. Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med. 2017;43(3):380-98.
- Wang D, Lin Z, Xie L, Huang K, Ji Z, Gu C, Wang S. Impact of early protein provision on the mortality of acute critically ill stroke patients. Nutr Clin Pract. 2022;37(4):861-8.
- Zusman O, Theilla M, Cohen J, Kagan I, Bendavid I, Singer P. Resting energy expenditure, calorie and protein consumption in critically ill patients: a retrospective cohort study. Crit Care. 2016;20(1):367.
- Heyland DK, Patel J, Compher C, Rice TW, Bear DE, Lee ZY, Gonzalez VC, O’Reilly K, Regala R, Wedemire C, et al. The effect of higher protein dosing in critically ill patients with high nutritional risk (EFFORT Protein): an international, multicentre, pragmatic, registry-based randomised trial. Lancet. 2023;401(10376):568-76.
- Pardo E, Lescot T, Preiser JC, Massanet P, Pons A, Jaber S, Fraipont V, Levesque E, Ichai C, Petit L, et al. Association between early nutrition support and 28-day mortality in critically ill patients: the FRANS prospective nutrition cohort study. Crit Care. 2023;27(1):7.
- Lin J, Chen W, Ye X, Lv C, Liu Y, Jiang X, Tong Z, Liu Y, Ke L, Li W, et al. Trajectories of protein intake and 28-day mortality in critically ill patients: a secondary analysis of a cluster-randomized controlled trial. Clin Nutr. 2022;41(8):1644-50.
- Matejovic M, Huet O, Dams K, Elke G, Vaquerizo Alonso C, Csomos A, Krzych LJ, Tetamo R, Puthucheary Z, Rooyackers O, et al. Medical nutrition therapy and clinical outcomes in critically ill adults: a European multinational, prospective observational cohort study (EuroPN). Crit Care. 2022;26(1):143.
- Lee ZY, Yap CSL, Hasan MS, Engkasan JP, Barakatun-Nisak MY, Day AG, Patel JJ, Heyland DK. The effect of higher versus lower protein delivery in critically ill patients: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2021;25(1):260.
- Koekkoek W, van Setten C, Olthof LE, Kars J, van Zanten ARH. Timing of PROTein INtake and clinical outcomes of adult critically ill patients on prolonged mechanical VENTilation: the PROTINVENT retrospective study. Clin Nutr. 2019;38(2):883-90.
- Chapple LS, Kouw IWK, Summers MJ, Weinel LM, Gluck S, Raith E, Slobodian P, Soenen S, Deane AM, van Loon LJC, Chapman MJ. Muscle protein synthesis after protein administration in critical illness. Am J Respir Crit Care Med. 2022;206(6):740-9.
- Puthucheary Z, Rooyackers O. Anabolic resistance: an uncomfortable truth for clinical trials in preventing intensive care-acquired weakness and physical functional impairment. Am J Respir Crit Care Med. 2022;206(6):660-1.
- Schuetz P, Fehr R, Baechli V, Geiser M, Deiss M, Gomes F, Kutz A, Tribolet P, Bregenzer T, Braun N, et al. Individualised nutritional support in medical inpatients at nutritional risk: a randomised clinical trial. Lancet. 2019;393(10188):2312-21.
- Merker M, Felder M, Gueissaz L, Bolliger R, Tribolet P, Kagi-Braun N, Gomes F, Hoess C, Pavlicek V, Bilz S, et al. Association of baseline inflammation with effectiveness of nutritional support among patients with diseaserelated malnutrition: a secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020;3(3):e200663.
- van Ruijven IM, Stapel SN, Girbes ARJ, Weijs PJM. Early high protein provision and mortality in ICU patients including those receiving continuous renal replacement therapy. Eur J Clin Nutr. 2022;76(9):1303-8.
- Phillips SM. Nutritional supplements in support of resistance exercise to counter age-related sarcopenia. Adv Nutr. 2015;6(4):452-60.
- Dickerson RN, Maish GO 3rd, Croce MA, Minard G, Brown RO. Influence of aging on nitrogen accretion during critical illness. JPEN J Parenter Enteral Nutr. 2015;39(3):282-90.
- Hoffer L. How much protein do parenteral amino acid mixtures provide? Am J Clin Nutr. 2011;94(6):1396-8.
- de Azevedo JRA, Lima HCM, Frota P, Nogueira I, de Souza SC, Fernandes EAA, Cruz AM. High-protein intake and early exercise in adult intensive care patients: a prospective, randomized controlled trial to evaluate the impact on functional outcomes. BMC Anesthesiol. 2021;21(1):283.
- Nakamura K, Nakano H, Naraba H, Mochizuki M, Takahashi Y, Sonoo T, Hashimoto H, Morimura N. High protein versus medium protein delivery under equal total energy delivery in critical care: a randomized controlled trial. Clin Nutr. 2021;40(3):796-803.
- Verceles AC, Serra M, Davis D, Alon G, Wells CL, Parker E, Sorkin J, Bhatti W, Terrin ML. Combining exercise, protein supplementation and electric stimulation to mitigate muscle wasting and improve outcomes for survivors of critical illness-the ExPrES study. Heart Lung. 2023;58:229-35.
- Calder PC. n-3 PUFA and inflammation: from membrane to nucleus and from bench to bedside. Proc Nutr Soc. 2020;8:1-13.
- Calder PC. Eicosanoids. Essays Biochem. 2020;64(3):423-41.
- Calder PC. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2015;1851(4):469-84.
- Burger B, Kuhl CMC, Candreva T, Cardoso RDS, Silva JR, Castelucci BG, Consonni SR, Fisk HL, Calder PC, Vinolo MAR, Rodrigues HG. Oral administration of EPA-rich oil impairs collagen reorganization due to elevated production of IL-10 during skin wound healing in mice. Sci Rep. 2019;9(1):9119.
- Phillips N, Gray SR, Combet E, Witard OC. Long-chain n-3 polyunsaturated fatty acids for the management of age- and disease-related declines in skeletal muscle mass, strength and physical function. Curr Opin Clin Nutr Metab Care. 2023;10:1097.
- Serhan CN, Dalli J, Colas RA, Winkler JW, Chiang N. Protectins and maresins: new pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim Biophys Acta. 2015;1851(4):397-413.
- Serhan
, Levy . Resolvins in inflammation: emergence of the proresolving superfamily of mediators. J Clin Invest. 2018;128(7):2657-69. - Chiang N, Serhan CN. Specialized pro-resolving mediator network: an update on production and actions. Essays Biochem. 2020;64(3):443-62.
- Calder PC. Eicosapentaenoic and docosahexaenoic acid derived specialised pro-resolving mediators: concentrations in humans and the effects of age, sex, disease and increased omega-3 fatty acid intake. Biochimie. 2020;178:105-23.
- Arroyo Portilla C, Tomas J, Gorvel JP, Lelouard H. From species to regional and local specialization of intestinal macrophages. Front Cell Dev Biol. 2020;8:624213.
- Serhan CN. Discovery of specialized pro-resolving mediators marks the dawn of resolution physiology and pharmacology. Mol Aspects Med. 2017;58:1-11.
- Andersson A, Nalsen C, Tengblad S, Vessby B. Fatty acid composition of skeletal muscle reflects dietary fat composition in humans. Am J Clin Nutr. 2002;76(6):1222-9.
- Smith GI , Atherton P , Reeds DN , Mohammed BS , Rankin D , Rennie MJ , Mittendorfer B. Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia-hyperaminoacidaemia in healthy young and middle-aged men and women. Clin Sci (Lond). 2011;121(6):267-78.
- McGlory C, Galloway SD, Hamilton DL, McClintock C, Breen L, Dick JR, Bell JG, Tipton KD. Temporal changes in human skeletal muscle and blood lipid composition with fish oil supplementation. Prostaglandins Leukot Essent Fatty Acids. 2014;90(6):199-206.
- Browning LM, Walker CG, Mander AP, West AL, Madden J, Gambell JM, Young S, Wang L, Jebb SA, Calder PC. Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. Am J Clin Nutr. 2012;96(4):748-58.
- Smith GI, Atherton P, Reeds DN, Mohammed BS, Rankin D, Rennie MJ, Mittendorfer B. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr. 2011;93(2):402-12.
- McGlory C, Gorissen SHM, Kamal M, Bahniwal R, Hector AJ, Baker SK, Chabowski A, Phillips SM. Omega-3 fatty acid supplementation attenuates skeletal muscle disuse atrophy during two weeks of unilateral leg immobilization in healthy young women. FASEB J. 2019;33(3):4586-97.
- Engelen M, Jonker R, Sulaiman H, Fisk HL, Calder PC, Deutz NEP. omega-3 polyunsaturated fatty acid supplementation improves postabsorptive and prandial protein metabolism in patients with chronic obstructive pulmonary disease: a randomized clinical trial. Am J Clin Nutr. 2022;116(3):686-98.
- Bird JK, Troesch B, Warnke I, Calder PC. The effect of long chain omega-3 polyunsaturated fatty acids on muscle mass and function in sarcopenia: a scoping systematic review and meta-analysis. Clin Nutr ESPEN. 2021;46:73-86.
- Winter M, Heitplatz B, Koppers N, Mohr A, Bungert AD, Juratli MA, Strucker B, Varga G, Pascher A, Becker F. The impact of phase-specific macrophage depletion on intestinal anastomotic healing. Cells. 2023;12:7.
- Xu R, Weber MC, Hu X, Neumann PA, Kamaly N. Annexin A1 based inflammation resolving mediators and nanomedicines for inflammatory bowel disease therapy. Semin Immunol. 2022;61-64:101664.
- Shi J, Wu Z, Li Z, Ji J. Roles of macrophage subtypes in bowel anastomotic healing and anastomotic leakage. J Immunol Res. 2018;2018:6827237.
- Jordan PM, Werz O. Specialized pro-resolving mediators: biosynthesis and biological role in bacterial infections. FEBS J. 2022;289(14):4212-27.
- Quiros M, Feier D, Birkl D, Agarwal R, Zhou DW, Garcia AJ, Parkos CA, Nusrat A. Resolvin E1 is a pro-repair molecule that promotes intestinal epithelial wound healing. Proc Natl Acad Sci U S A. 2020;117(17):9477-82.
- Gallo CG, Fiorino S, Posabella G, Antonacci D, Tropeano A, Pausini E, Pausini C, Guarniero T, Hong W, Giampieri E, et al. The function of specialized pro-resolving endogenous lipid mediators, vitamins, and other micronutrients in the control of the inflammatory processes: Possible role in patients with SARS-CoV-2 related infection. Prostaglandins Lipid Mediat. 2022;159:106619.
- Shimizu T, Saito T, Aoki-Saito H, Okada S, Ikeda H, Nakakura T, Fukuda H, Arai S, Fujiwara K, Nakajima Y, et al. Resolvin E3 ameliorates high-fat diet-induced insulin resistance via the phosphatidylinositol-3-kinase/Akt signaling pathway in adipocytes. FASEB J. 2022;36(3):e22188.
- Castor-Macias J, Larouche J, Wallace E, Spence B, Eames A, Yang B, Davis C, Brooks S, Maddipati K, Markworth J, Aguilar C. Maresin 1 repletion improves muscle regeneration after volumetric muscle loss. bioRxiv 2022.
- Hardesty JE, Warner JB, Song YL, Rouchka EC, McClain CJ, Warner DR, Kirpich IA. Resolvin D1 attenuated liver injury caused by chronic ethanol and acute LPS challenge in mice. FASEB J. 2023;37(1):e22705.
ملاحظة الناشر
- *المراسلة:
ميت م. بيرجر
mette.berger@unil.ch
قسم التغذية البشرية، كلية الطب وعلوم الصحة، جامعة ستيلينبوش، ستيلينبوش، جنوب أفريقيا
كلية الطب، جامعة ساوثهامبتون، ساوثهامبتون، المملكة المتحدة
مركز أبحاث الطب الحيوي في ساوثهامبتون، مستشفى جامعة ساوثهامبتون NHS Foundation Trust، ساوثهامبتون، المملكة المتحدة
قسم الجراحة، جامعة أوريغون للصحة والعلوم، بورتلاند، أوريغون، الولايات المتحدة الأمريكية
كلية البيولوجيا والطب، جامعة لوزان، لوزان، سويسرا
DOI: https://doi.org/10.1186/s13054-024-04803-8
PMID: https://pubmed.ncbi.nlm.nih.gov/38302945
Publication Date: 2024-02-01
Combining proteins with n-3 PUFAs (EPA + DHA) and their inflammation pro-resolution mediators for preservation of skeletal muscle mass
Abstract
The optimal feeding strategy for critically ill patients is still debated, but feeding must be adapted to individual patient needs. Critically ill patients are at risk of muscle catabolism, leading to loss of muscle mass and its consequent clinical impacts. Timing of introduction of feeding and protein targets have been explored in recent trials. These suggest that “moderate” protein provision (maximum
Introduction
(MPS) and muscle protein breakdown (MPB) [9]. In critically ill patients, this balance is disturbed in favour of breakdown, with the extent of disturbance depending upon the severity of illness [10]. Excessive or unresolved inflammation promotes this imbalance: Inflammation inhibits the mammalian target of rapamycin (mTOR) signalling pathway that promotes MPS, and this contributes to the net muscle protein loss observed during critical illness and sepsis [11].
Inflammation is highly prevalent in critical illness [12] and triggers proteolysis within skeletal muscle [13, 14]. The aim of muscle catabolism is to mobilize amino acids for energy production (i.e. oxidation) or gluconeogenesis, with the ammonia released from the initial deamination being used for urea synthesis and excretion, and to support immunity, tissue repair and the production of acute-phase proteins [15]. However, after the initial acute phase, persistence of inflammation is deleterious, increasing the risk of ICU acquired weakness [16] and persistent critical illness [17], with its associated elevated urea-to-creatinine ratio, a signature of muscle catabolism [18]. Inflammation has two distinct phases, initiation and resolution: In persistent critical illness, this 2nd stage seems to be impaired.
How can this deleterious pathophysiological phenomenon be countered? Until recently, the principal strategies involved the delivery of adequate doses of proteins, higher proportions of essential and branched chain amino acids [19-21], and eventually physical activity [22]. The most recent strategy, which adds to the previous, is the use of very long-chain omega-3 polyunsaturated fatty acids (n-3 PUFAs) to drive resolution of inflammation and restore muscle health by activation of the resolution pathway [9]. These new findings are discussed herein.
Role of proteins and amino acids
Despite the guidelines, the optimal protein dose and timing remain debated, as the impact on muscle protein breakdown is not clear. Wang et al. reported an inverse linear relationship between protein intake and 30-day mortality [25], while Zusman et al. observed mortality reduction with a protein intake exceeding
increased mortality with higher protein intakes [27,28]. The EFFORT protein trial tested the prescription of different protein doses (
Older individuals require higher protein intakes to overcome the anabolic resistance that occurs with ageing: The myofibrillar fractional synthesis rate is significantly higher at lower protein intake levels in younger individuals [38]. Nitrogen balance studies show similar results: Response to protein intake in ICU patients with trauma improved linearly up until an intake of
Through measuring myofibrillar protein synthesis rates, Chapple et al. compared ICU patients to healthy controls and were able to show that there was no
difference in protein digestion and amino acid availability post meal. However, muscle protein phenylalanine enrichment (as a measure of dietary protein incorporation into skeletal muscle protein) was

Inflammation, EPA, DHA, and pro-resolving mediators
reducing inflammation and promoting its resolution (Fig. 2), EPA and DHA might decrease muscle proteolysis and loss [48].


SPMs are not present in the diet, rather they are produced endogenously via the respective metabolism of arachidonic acid, EPA, DPA, and DHA into a wide-ranging series of fatty acid derivatives; examples of the most commonly studied include lipoxin A4 (from arachidonic acid), resolvin E1 (from EPA), and resolvins D1 and D2 (from DHA). Lipoxin A4 produces its anti-inflammatory effects through induction of monocytes and macrophages to increase phagocytosis and stimulation of IL-10 production with reduction of pro-inflammatory cytokine release. Resolvin E1 decreases neutrophil activation and endothelial adhesion, while also decreasing production of reactive oxygen species. The SPMs also enhance microbial killing and clearance. Resolvin D1 acts upon endothelial cells by increasing intracellular nitric oxide and prostacyclin concentrations and decreasing adhesion receptors, ROS generation, and pro-inflammatory cytokines. Resolvin D2 decreases migration of dendritic cells and IL-12 production [51]. In summary, SPMs work to inhibit further neutrophil recruitment, promote secretion of pro-resolution cytokines with attenuation of pro-inflammatory cytokines, and increase clearance of microbes and cellular debris. In humans, fish oils are an important source of EPA and DHA. Supplementation of EPA and DHA has been found to increase circulating levels of
many SPMs [52]. It has been shown that SPM synthesis is evolutionarily conserved: mice, frogs, and amoeba all synthesize SPMs showing the importance of inflammation resolution in tissue repair and recovery across nature [53]. The concentration range for activity of SPMs is in the nanomolar/picomolar level. They are found at bioactive levels throughout most tissues, including the brain, lymph nodes, and adipose tissue [49-51, 54].
McGlory et al. [57] linked the progressive increase in n-3 PUFAs in muscle over time with changes in levels of certain proteins involved in anabolic signalling in the muscle. They demonstrated a time-dependent increase in protein tyrosine kinase 2 which was higher after 4 weeks than at baseline and modest, though not significant, time-dependent increases in ribosomal protein S6 kinase beta-1 (P70S6K) and in eukaryotic translation initiation factor 4E-binding protein 1. mTOR was higher at week 2 than at baseline. These effects suggest that n-3 PUFAs acids could promote MPS. This was explored in two studies by Smith et al. [56, 59]. In the first of these studies [59], daily supplementation with 1.86-g EPA and
to be linked to augmenting the anabolic effect of other signals. McGlory et al. [60] reported that supplementation of young adult females with 2.97-g EPA and 2.03-g DHA daily resulted in better maintenance of quadriceps volume during 2 weeks of leg immobilization and that this was linked to retention of a higher rate of myofibrillar protein synthesis during the immobilization period. Most recently, Engelen et al. [61] showed an EPA plus DHA dose-dependent reduction of whole-body protein breakdown during the postabsorptive period and an EPA plus DHA dose-dependent increase in whole-body protein synthesis during the feeding period in patients with chronic obstructive pulmonary disease. Taken together, these human studies indicate that EPA and DHA alter the expression and activation of anabolic signalling proteins in human skeletal muscle, decrease MPB, and increase MPS. These effects are of relevance to situations where there is risk of loss of muscle mass due to ageing, disuse, or disease. In this context, a recent meta-analysis of human studies in heterogeneous population groups identified that lean mass and skeletal muscle mass are favoured by higher intakes of EPA and DHA [62].
et al. has shown that resolvin D1 significantly decreased hepatic injury in an ethanol/lipopolysaccharide model. Interestingly, this benefit appears very stereospecific as there was no benefit with resolvin E1 [71].
Conclusion
Abbreviations
| CRP | C-reactive proteins |
| EPA | Eicosapentaenoic acid |
| DHA | Docosahexaenoic acid |
| mTOR | Mammalian target of rapamycin receptor |
| n-3 PUFA | Omega-3 polyunsaturated fatty acid |
| SPMs | Specialized pro-resolving mediators |
Author contributions
Funding
Availability of data and materials
Declarations
Ethics approval and consent to participate
Competing interest
Published online: 01 February 2024
References
- Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol. 2000;89(1):81-8.
- Jannas-Vela S, Espinosa A, Candia AA, Flores-Opazo M, Penailillo L, Valenzuela
. The role of omega- 3 polyunsaturated fatty acids and their lipid mediators on skeletal muscle regeneration: a narrative review. Nutrients. 2023;15:4. - Puthucheary Z, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, Hopkinson N, Padhke R, Dew T, Sidhu P, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310(15):1591-600.
- McNelly AS, Bear DE, Connolly BA, Arbane G, Allum L, Tarbhai A, Cooper JA, Hopkins PA, Wise MP, Brealey D, et al. Effect of intermittent or continuous feed on muscle wasting in critical illness: a phase 2 clinical trial. Chest. 2020;158(1):183-94.
- Nakanishi N, Tsutsumi R, Okayama Y, Takashima T, Ueno Y, Itagaki T, Tsutsumi Y, Sakaue H, Oto J. Monitoring of muscle mass in critically ill patients: comparison of ultrasound and two bioelectrical impedance analysis devices. J Intensive Care. 2019;7:61.
- Weijs P, Looijaard W, Beishuizen A, Girbes A, Oudemans-van Straaten H. Early high protein intake is associated with low mortality and energy overfeeding with high mortality in non-septic mechanically ventilated critically ill patients. Crit Care. 2014;18(6):701.
- Lim SL, Ong KC, Chan YH, Loke WC, Ferguson M, Daniels L. Malnutrition and its impact on cost of hospitalization, length of stay, readmission and 3-year mortality. Clin Nutr. 2012;31(3):345-50.
- Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16-31.
- Therdyothin A, Phiphopthatsanee N, Isanejad M. The effect of omega-3 fatty acids on sarcopenia: mechanism of action and potential efficacy. Mar Drugs. 2023;21:7.
- Deutz N, Singer P, Wierzchowska-McNew R, Viana M, Ben-David I, Pantet O, Thaden J, Ten Have G, Engelen M, Berger M. Comprehensive metabolic amino acid flux analysis in critically ill patients. Clin Nutr. 2021;40(5):2876-97.
- Lang CH, Frost RA, Vary TC. Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab. 2007;293(2):E453-459.
- Cox CE. Persistent systemic inflammation in chronic critical illness. Respir Care. 2012, 57(6):859-864; discussion 864-856.
- Haberecht-Muller S, Kruger E, Fielitz J. Out of control: the role of the ubiquitin proteasome system in skeletal muscle during inflammation. Biomolecules. 2021;11:9.
- Ji Y, Li M, Chang M, Liu R, Qiu J, Wang K, Deng C, Shen Y, Zhu J, Wang W, et al. Inflammation: roles in skeletal muscle atrophy. Antioxidants (Basel). 2022;11:9.
- Gillis C, Carli F. Promoting perioperative metabolic and nutritional care. Anesthesiology. 2015;123(6):1455-72.
- Lad H, Saumur TM, Herridge MS, Dos Santos CC, Mathur S, Batt J, Gilbert PM. Intensive care unit-acquired weakness: not just another muscle atrophying condition. Int J Mol Sci. 2020;21:21.
- Zhang Z, Ho KM, Gu H, Hong Y, Yu Y. Defining persistent critical illness based on growth trajectories in patients with sepsis. Crit Care. 2020;24(1):57.
- Haines RW, Zolfaghari P, Wan Y, Pearse RM, Puthucheary Z, Prowle JR. Elevated urea-to-creatinine ratio provides a biochemical signature of muscle catabolism and persistent critical illness after major trauma. Intensive Care Med. 2019;45(12):1718-31.
- Nakamura K, Kihata A, Naraba H, Kanda N, Takahashi Y, Sonoo T, Hashimoto H, Morimura N. Beta-hydroxy-beta-methylbutyrate, arginine, and glutamine complex on muscle volume loss in critically III patients: a randomized control trial. JPEN J Parenter Enteral Nutr. 2020;22(2):205-2112.
- Viana M, Becce F, Pantet O, Schmidt S, Bagnoud G, Thaden J, Ten Have G, Engelen M, Voidey A, Deutz N. MM B: impact of
-hydroxy- methylbutyrate (HMB) on muscle loss and protein metabolism in critically ill patients: a RCT. Clin Nutr. 2021;40:4878-87. - Lv X, Zhou C, Yan Q, Tan Z, Kang J, Tang S. Elucidating the underlying mechanism of amino acids to regulate muscle protein synthesis: effect on human health. Nutrition. 2022;103-104: 111797.
- Singer P, Blaser AR, Berger MM, Calder PC, Casaer M, Hiesmayr M, Mayer K, Montejo-Gonzalez JC, Pichard C, Preiser JC, et al. ESPEN practical and partially revised guideline: clinical nutrition in the intensive care unit. Clin Nutr. 2023;42(9):1671-89.
- McClave S, Taylor B, Martindale R, Warren M, Johnson D, Braunschweig C, McCarthy M, Davanos E, Rice T, Cresci G, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically III patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2016;40(2):159-211.
- Reintam Blaser A, Starkopf J, Alhazzani W, Berger M, Casaer M, Deane A, Fruhwald S, Hiesmayr M, Ichai C, Jakob S, et al. Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med. 2017;43(3):380-98.
- Wang D, Lin Z, Xie L, Huang K, Ji Z, Gu C, Wang S. Impact of early protein provision on the mortality of acute critically ill stroke patients. Nutr Clin Pract. 2022;37(4):861-8.
- Zusman O, Theilla M, Cohen J, Kagan I, Bendavid I, Singer P. Resting energy expenditure, calorie and protein consumption in critically ill patients: a retrospective cohort study. Crit Care. 2016;20(1):367.
- Heyland DK, Patel J, Compher C, Rice TW, Bear DE, Lee ZY, Gonzalez VC, O’Reilly K, Regala R, Wedemire C, et al. The effect of higher protein dosing in critically ill patients with high nutritional risk (EFFORT Protein): an international, multicentre, pragmatic, registry-based randomised trial. Lancet. 2023;401(10376):568-76.
- Pardo E, Lescot T, Preiser JC, Massanet P, Pons A, Jaber S, Fraipont V, Levesque E, Ichai C, Petit L, et al. Association between early nutrition support and 28-day mortality in critically ill patients: the FRANS prospective nutrition cohort study. Crit Care. 2023;27(1):7.
- Lin J, Chen W, Ye X, Lv C, Liu Y, Jiang X, Tong Z, Liu Y, Ke L, Li W, et al. Trajectories of protein intake and 28-day mortality in critically ill patients: a secondary analysis of a cluster-randomized controlled trial. Clin Nutr. 2022;41(8):1644-50.
- Matejovic M, Huet O, Dams K, Elke G, Vaquerizo Alonso C, Csomos A, Krzych LJ, Tetamo R, Puthucheary Z, Rooyackers O, et al. Medical nutrition therapy and clinical outcomes in critically ill adults: a European multinational, prospective observational cohort study (EuroPN). Crit Care. 2022;26(1):143.
- Lee ZY, Yap CSL, Hasan MS, Engkasan JP, Barakatun-Nisak MY, Day AG, Patel JJ, Heyland DK. The effect of higher versus lower protein delivery in critically ill patients: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2021;25(1):260.
- Koekkoek W, van Setten C, Olthof LE, Kars J, van Zanten ARH. Timing of PROTein INtake and clinical outcomes of adult critically ill patients on prolonged mechanical VENTilation: the PROTINVENT retrospective study. Clin Nutr. 2019;38(2):883-90.
- Chapple LS, Kouw IWK, Summers MJ, Weinel LM, Gluck S, Raith E, Slobodian P, Soenen S, Deane AM, van Loon LJC, Chapman MJ. Muscle protein synthesis after protein administration in critical illness. Am J Respir Crit Care Med. 2022;206(6):740-9.
- Puthucheary Z, Rooyackers O. Anabolic resistance: an uncomfortable truth for clinical trials in preventing intensive care-acquired weakness and physical functional impairment. Am J Respir Crit Care Med. 2022;206(6):660-1.
- Schuetz P, Fehr R, Baechli V, Geiser M, Deiss M, Gomes F, Kutz A, Tribolet P, Bregenzer T, Braun N, et al. Individualised nutritional support in medical inpatients at nutritional risk: a randomised clinical trial. Lancet. 2019;393(10188):2312-21.
- Merker M, Felder M, Gueissaz L, Bolliger R, Tribolet P, Kagi-Braun N, Gomes F, Hoess C, Pavlicek V, Bilz S, et al. Association of baseline inflammation with effectiveness of nutritional support among patients with diseaserelated malnutrition: a secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020;3(3):e200663.
- van Ruijven IM, Stapel SN, Girbes ARJ, Weijs PJM. Early high protein provision and mortality in ICU patients including those receiving continuous renal replacement therapy. Eur J Clin Nutr. 2022;76(9):1303-8.
- Phillips SM. Nutritional supplements in support of resistance exercise to counter age-related sarcopenia. Adv Nutr. 2015;6(4):452-60.
- Dickerson RN, Maish GO 3rd, Croce MA, Minard G, Brown RO. Influence of aging on nitrogen accretion during critical illness. JPEN J Parenter Enteral Nutr. 2015;39(3):282-90.
- Hoffer L. How much protein do parenteral amino acid mixtures provide? Am J Clin Nutr. 2011;94(6):1396-8.
- de Azevedo JRA, Lima HCM, Frota P, Nogueira I, de Souza SC, Fernandes EAA, Cruz AM. High-protein intake and early exercise in adult intensive care patients: a prospective, randomized controlled trial to evaluate the impact on functional outcomes. BMC Anesthesiol. 2021;21(1):283.
- Nakamura K, Nakano H, Naraba H, Mochizuki M, Takahashi Y, Sonoo T, Hashimoto H, Morimura N. High protein versus medium protein delivery under equal total energy delivery in critical care: a randomized controlled trial. Clin Nutr. 2021;40(3):796-803.
- Verceles AC, Serra M, Davis D, Alon G, Wells CL, Parker E, Sorkin J, Bhatti W, Terrin ML. Combining exercise, protein supplementation and electric stimulation to mitigate muscle wasting and improve outcomes for survivors of critical illness-the ExPrES study. Heart Lung. 2023;58:229-35.
- Calder PC. n-3 PUFA and inflammation: from membrane to nucleus and from bench to bedside. Proc Nutr Soc. 2020;8:1-13.
- Calder PC. Eicosanoids. Essays Biochem. 2020;64(3):423-41.
- Calder PC. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2015;1851(4):469-84.
- Burger B, Kuhl CMC, Candreva T, Cardoso RDS, Silva JR, Castelucci BG, Consonni SR, Fisk HL, Calder PC, Vinolo MAR, Rodrigues HG. Oral administration of EPA-rich oil impairs collagen reorganization due to elevated production of IL-10 during skin wound healing in mice. Sci Rep. 2019;9(1):9119.
- Phillips N, Gray SR, Combet E, Witard OC. Long-chain n-3 polyunsaturated fatty acids for the management of age- and disease-related declines in skeletal muscle mass, strength and physical function. Curr Opin Clin Nutr Metab Care. 2023;10:1097.
- Serhan CN, Dalli J, Colas RA, Winkler JW, Chiang N. Protectins and maresins: new pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim Biophys Acta. 2015;1851(4):397-413.
- Serhan
, Levy . Resolvins in inflammation: emergence of the proresolving superfamily of mediators. J Clin Invest. 2018;128(7):2657-69. - Chiang N, Serhan CN. Specialized pro-resolving mediator network: an update on production and actions. Essays Biochem. 2020;64(3):443-62.
- Calder PC. Eicosapentaenoic and docosahexaenoic acid derived specialised pro-resolving mediators: concentrations in humans and the effects of age, sex, disease and increased omega-3 fatty acid intake. Biochimie. 2020;178:105-23.
- Arroyo Portilla C, Tomas J, Gorvel JP, Lelouard H. From species to regional and local specialization of intestinal macrophages. Front Cell Dev Biol. 2020;8:624213.
- Serhan CN. Discovery of specialized pro-resolving mediators marks the dawn of resolution physiology and pharmacology. Mol Aspects Med. 2017;58:1-11.
- Andersson A, Nalsen C, Tengblad S, Vessby B. Fatty acid composition of skeletal muscle reflects dietary fat composition in humans. Am J Clin Nutr. 2002;76(6):1222-9.
- Smith GI , Atherton P , Reeds DN , Mohammed BS , Rankin D , Rennie MJ , Mittendorfer B. Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia-hyperaminoacidaemia in healthy young and middle-aged men and women. Clin Sci (Lond). 2011;121(6):267-78.
- McGlory C, Galloway SD, Hamilton DL, McClintock C, Breen L, Dick JR, Bell JG, Tipton KD. Temporal changes in human skeletal muscle and blood lipid composition with fish oil supplementation. Prostaglandins Leukot Essent Fatty Acids. 2014;90(6):199-206.
- Browning LM, Walker CG, Mander AP, West AL, Madden J, Gambell JM, Young S, Wang L, Jebb SA, Calder PC. Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. Am J Clin Nutr. 2012;96(4):748-58.
- Smith GI, Atherton P, Reeds DN, Mohammed BS, Rankin D, Rennie MJ, Mittendorfer B. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr. 2011;93(2):402-12.
- McGlory C, Gorissen SHM, Kamal M, Bahniwal R, Hector AJ, Baker SK, Chabowski A, Phillips SM. Omega-3 fatty acid supplementation attenuates skeletal muscle disuse atrophy during two weeks of unilateral leg immobilization in healthy young women. FASEB J. 2019;33(3):4586-97.
- Engelen M, Jonker R, Sulaiman H, Fisk HL, Calder PC, Deutz NEP. omega-3 polyunsaturated fatty acid supplementation improves postabsorptive and prandial protein metabolism in patients with chronic obstructive pulmonary disease: a randomized clinical trial. Am J Clin Nutr. 2022;116(3):686-98.
- Bird JK, Troesch B, Warnke I, Calder PC. The effect of long chain omega-3 polyunsaturated fatty acids on muscle mass and function in sarcopenia: a scoping systematic review and meta-analysis. Clin Nutr ESPEN. 2021;46:73-86.
- Winter M, Heitplatz B, Koppers N, Mohr A, Bungert AD, Juratli MA, Strucker B, Varga G, Pascher A, Becker F. The impact of phase-specific macrophage depletion on intestinal anastomotic healing. Cells. 2023;12:7.
- Xu R, Weber MC, Hu X, Neumann PA, Kamaly N. Annexin A1 based inflammation resolving mediators and nanomedicines for inflammatory bowel disease therapy. Semin Immunol. 2022;61-64:101664.
- Shi J, Wu Z, Li Z, Ji J. Roles of macrophage subtypes in bowel anastomotic healing and anastomotic leakage. J Immunol Res. 2018;2018:6827237.
- Jordan PM, Werz O. Specialized pro-resolving mediators: biosynthesis and biological role in bacterial infections. FEBS J. 2022;289(14):4212-27.
- Quiros M, Feier D, Birkl D, Agarwal R, Zhou DW, Garcia AJ, Parkos CA, Nusrat A. Resolvin E1 is a pro-repair molecule that promotes intestinal epithelial wound healing. Proc Natl Acad Sci U S A. 2020;117(17):9477-82.
- Gallo CG, Fiorino S, Posabella G, Antonacci D, Tropeano A, Pausini E, Pausini C, Guarniero T, Hong W, Giampieri E, et al. The function of specialized pro-resolving endogenous lipid mediators, vitamins, and other micronutrients in the control of the inflammatory processes: Possible role in patients with SARS-CoV-2 related infection. Prostaglandins Lipid Mediat. 2022;159:106619.
- Shimizu T, Saito T, Aoki-Saito H, Okada S, Ikeda H, Nakakura T, Fukuda H, Arai S, Fujiwara K, Nakajima Y, et al. Resolvin E3 ameliorates high-fat diet-induced insulin resistance via the phosphatidylinositol-3-kinase/Akt signaling pathway in adipocytes. FASEB J. 2022;36(3):e22188.
- Castor-Macias J, Larouche J, Wallace E, Spence B, Eames A, Yang B, Davis C, Brooks S, Maddipati K, Markworth J, Aguilar C. Maresin 1 repletion improves muscle regeneration after volumetric muscle loss. bioRxiv 2022.
- Hardesty JE, Warner JB, Song YL, Rouchka EC, McClain CJ, Warner DR, Kirpich IA. Resolvin D1 attenuated liver injury caused by chronic ethanol and acute LPS challenge in mice. FASEB J. 2023;37(1):e22705.
Publisher’s Note
- *Correspondence:
Mette M. Berger
mette.berger@unil.ch
Division of Human Nutrition, Faculty of Medicine and Health Sciences, Stellenbosch University, Stellenbosch, South Africa
Faculty of Medicine, University of Southampton, Southampton, UK
NIHR Southampton Biomedical Research Centre, University Hospital Southampton NHS Foundation Trust, Southampton, UK
Surgery Department, Oregon Health and Science University, Portland, OR, USA
Faculty of Biology and Medicine, Lausanne University, Lausanne, Switzerland
