DOI: https://doi.org/10.2147/jir.s445806
PMID: https://pubmed.ncbi.nlm.nih.gov/38406326
تاريخ النشر: 2024-02-01
فيتامين ك: العدوى، الالتهاب، والمناعة الذاتية
الملخص
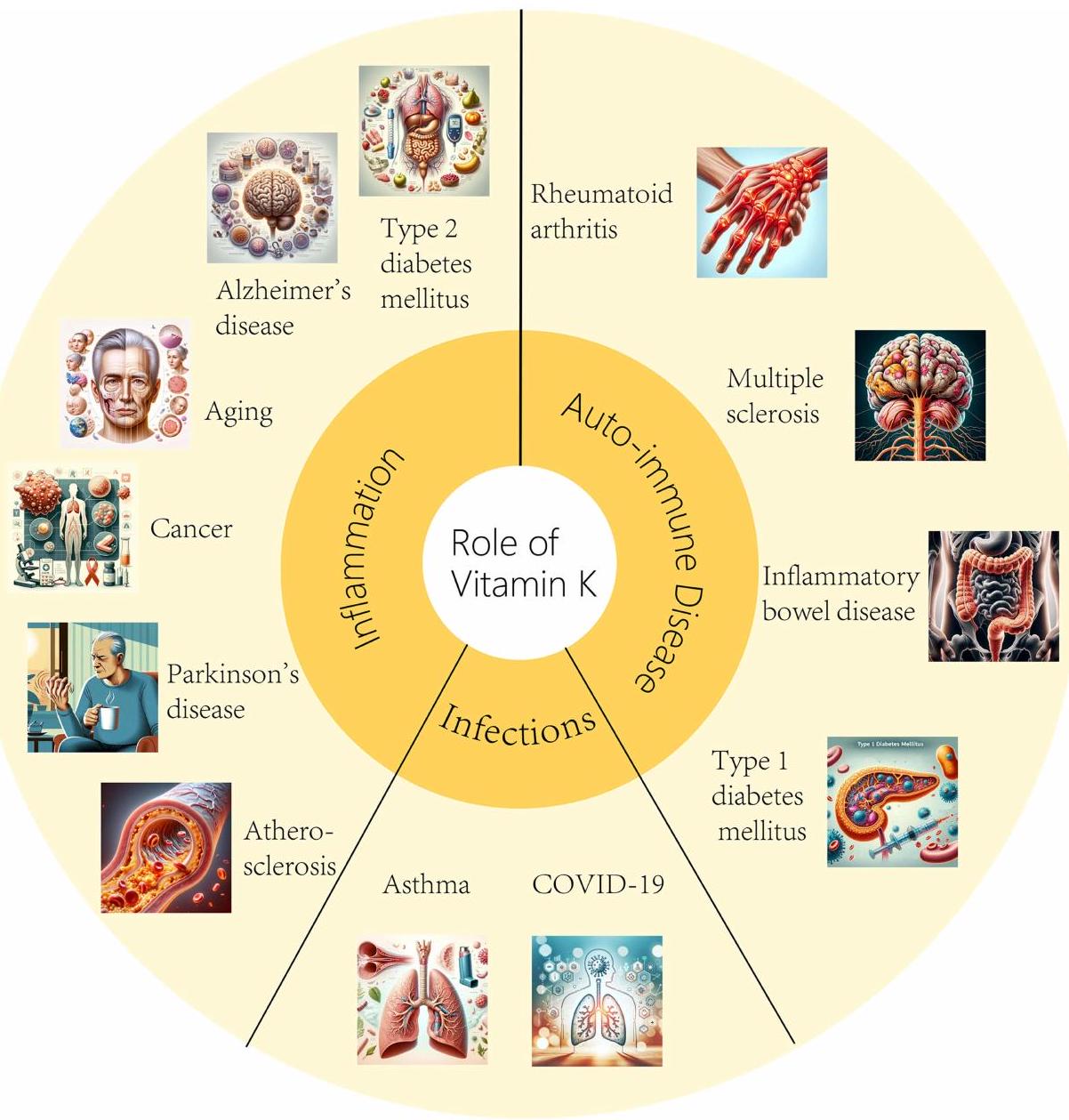
فيتامين ك (VK) يتكون من مجموعة من المواد ذات النشاط الحيوي الكلوروفيل الكينون ويظهر في الطبيعة في شكل VK1 و VK2. نظرًا لأن التعرف الأول عليه نشأ من قدرته على تعزيز تجلط الدم، فإنه يعرف بفيتامين التجلط. ومع ذلك، استنادًا إلى أبحاث واسعة، أظهر VK إمكانات للوقاية والعلاج من أمراض مختلفة. الدراسات التي تظهر التأثيرات المفيدة لـ VK على المناعة، والقدرة المضادة للأكسدة، وتنظيم الميكروبات المعوية، وتطور الظهارة، وحماية العظام قد جذبت اهتمامًا متزايدًا في السنوات الأخيرة. تركز هذه المقالة الاستعراضية على آلية عمل VK وتأثيراته الوقائية والعلاجية المحتملة على العدوى (مثل الربو، COVID-19)، الالتهاب (مثل في مرض السكري من النوع 2، مرض الزهايمر، مرض باركنسون، السرطان، الشيخوخة، تصلب الشرايين) واضطرابات المناعة الذاتية (مثل مرض الأمعاء الالتهابي، مرض السكري من النوع 1، التصلب المتعدد، التهاب المفاصل الروماتويدي). بالإضافة إلى ذلك، تعتبر البروتينات المعتمدة على VK (VKDPs) آلية حاسمة أخرى يمارس من خلالها VK تأثيرات مضادة للالتهابات ومعدلة للمناعة. تستكشف هذه المراجعة الدور المحتمل لـ VK في منع الشيخوخة، ومكافحة الاضطرابات العصبية، وعلاج الأمراض مثل السرطان والسكري. على الرغم من أن الأبحاث الحالية تعين VK كأداة علاجية للتطبيقات السريرية العملية في العدوى، والالتهابات، والأمراض المناعية الذاتية، إلا أن الأبحاث المستقبلية ضرورية لتوضيح آلية العمل بمزيد من التفصيل والتغلب على القيود الحالية.
المقدمة
(AD)، مرض باركنسون (PD)، السرطان، الشيخوخة، تصلب الشرايين) واضطرابات المناعة الذاتية (مثل مرض الأمعاء الالتهابي (IBD)، مرض السكري من النوع 1 (T1DM)، التصلب المتعدد (MS)، والتهاب المفاصل الروماتويدي (RA)). تهدف هذه المراجعة إلى تلخيص الأدبيات الحالية بشأن آثار مكملات VK في العدوى والاستجابة المناعية، بما في ذلك الأدلة من الدراسات قبل السريرية والسريرية.
الخصائص الكيميائية والفسيولوجية لفيتامين ك
فيتامين ك والعدوى
الربو
COVID-19
فيتامين ك والالتهاب
مرض السكري من النوع 2 (T2DM)
تتمثل الوظيفة المعروفة لفيتامين K في التجلط، ولكنه يلعب أيضًا دورًا مهمًا في استقرار مستوى السكر في الدم، وتحسين حساسية الأنسولين، والسيطرة على مرض السكري.
مرض الزهايمر (AD)
| كائن | تناول VK | غرض | استنتاج | مرجع |
| رجل شاب صحي | تناول الغذاء المعتاد | ناقش العلاقة بين استجابة الأنسولين الحادة ومدخول فيتامين K. | تناول كميات عالية من فيتامين ك مفيد لتحسين حساسية الأنسولين. | [23] |
| رجل شاب صحي | VK2 | استكشاف تأثير تناول أقراص VK2 لمدة أسبوع على تحمل الجلوكوز لدى رجل شاب صحي. | VK2 مفيد لتوازن مستوى السكر في الدم. | [24] |
| الرجال والنساء | تناول الغذاء المعتاد | دراسة العلاقة بين تناول فيتامين K وحالة سكر الدم الناتجة عن الأنسولين لدى الرجال والنساء. | VKI و VK2 مفيدان لتحسين اضطراب سكر الدم. | [25] |
| رجال ونساء كبار في السن | تناول الغذاء المعتاد | تحقيق ما إذا كان تناول مكملات فيتامين ك لمدة 36 شهرًا يمكن أن يحسن مقاومة الأنسولين لدى الرجال والنساء المسنين. | تناول VKI مفيد لتحسين الأنسولين لدى الرجال المسنين. | [26] |
| نساء ما قبل السكري | مع أو بدون 500
|
افحص التأثير التنظيمي لمكملات VKI على توازن الجلوكوز في النساء المعرضات للإصابة بالسكري. | يمكن أن يحسن تناول مكملات VKI مستوى سكر الدم لدى النساء المصابات بمرحلة ما قبل السكري. | [27] |
| نساء ما قبل السكري | مع أو بدون
|
دراسة ما إذا كانت مكملات VKI تؤثر على استقلاب الجلوكوز أو حساسية الأنسولين لدى النساء المصابات بمرحلة ما قبل السكري. | مكملات VKI مفيدة لتحسين مستوى السكر في الدم وحساسية الأنسولين لدى النساء المعرضات للإصابة بالسكري. | [28] |
مرض باركنسون (PD)
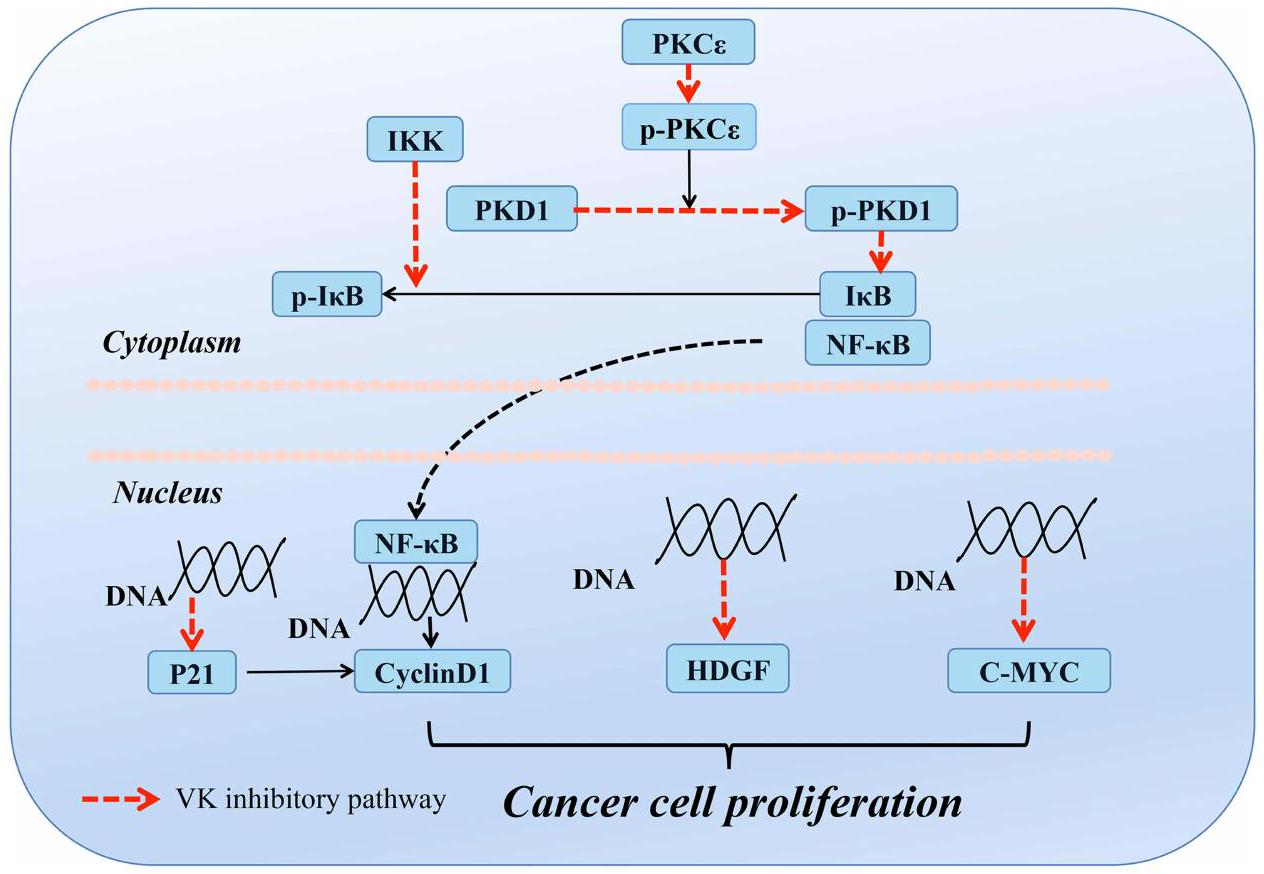
السرطان
الشيخوخة
تصلب الشرايين
فيتامين K والأمراض المناعية الذاتية
مرض الأمعاء الالتهابي (IBD)
داء السكري من النوع الأول (TIDM)
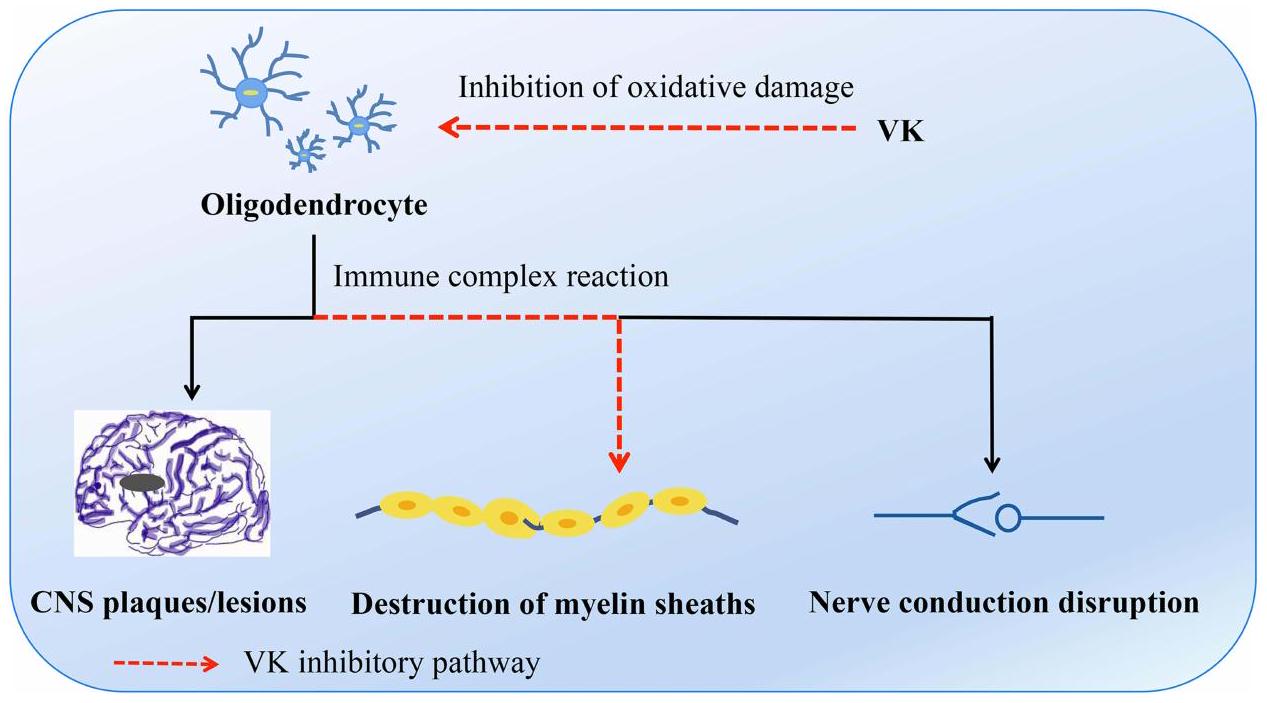
التصلب المتعدد (MS)
التهاب المفاصل الروماتويدي (RA)
المناقشات
| المؤلف، السنة (البلد) (المرجع) | المشاركون، العمر | تصميم (الطول) | تعرض التدخل | أثر التدخل على المرض |
| شيراكِي وآخرون 2000 اليابان
|
241 PMO 67.2 سنة | مرتقب
|
|
|
| إيواموتو وآخرون 2001 اليابان
|
72 PMO 65.3 سنة | محتمل 2 سنة |
|
|
| بوروسونو وآخرون 2006 إندونيسيا
|
63 PMO 60.8 سنة | RCT 48 و |
|
|
| بولتون-سميث وآخرون 2007 المملكة المتحدة
|
244 صحي W 68.2 ي | RCT 2 سنة |
|
|
| كنابين وآخرون 2007 | 325 بي إم دبليو | RCT |
|
|
| تشونغ وآخرون 2008 كندا
|
400 PMOa 59.1 ي | RCT 2-4 سنوات |
|
|
| هيراوا وآخرون 2008 اليابان
|
44 PMW 68.4 ي | من المحتمل |
|
|
| تسوجاوا وآخرون 2008 اليابان
|
379 غرب 63.0 ي | محتمل 3 سنوات | ارتفاع مؤشر كتلة الجسم مقابل انخفاض مؤشر كتلة الجسم |
|
| جي وآخرون 2011 كوريا
|
78 PMW 67.8 ي | RCT 6 أشهر |
|
|
| كنابين وآخرون 2013 | 244 بي إم دبليو | RCT |
|
|
|
|
||||
| جيانغ وآخرون 2014 الصين
|
213 PMW 64.4 ي | RCT لي |
|
|
| رون وآخرون 2016 الدنمارك
|
148 PMOa 67.5 ي | RCT لي |
|
|
| شيا وآخرون 2009 الولايات المتحدة
|
388 (235:153) صحي 68 سنة | RCT 3 سنوات |
|
|
| تشونغ وآخرون 2015 الولايات المتحدة
|
3401 (2245:1156) CKD 61.9 سنة | متابعة 13.3 سنة |
|
|
| كرناتوسكا وآخرون 2015 بولندا
|
42 (20:22) مرضى غير خاضعين للغسيل الكلوي يعانون من مرض الكلى المزمن 58 سنة | RCT 270 يوم |
|
|
| براندنبورغ وآخرون 2017 ألمانيا
|
99 (18:81) AVC 69.1 ي | RCT لي |
|
|
| شيا وآخرون 2020 الولايات المتحدة
|
3891(2154:1737)
|
متابعة 13 سنة |
|
|
| يوشيدا وآخرون 2008 الولايات المتحدة
|
2719 (1472:1247) صحي 68 سنة | RCT 36 شهر |
|
|
| شيا وآخرون 2017 الولايات المتحدة
|
401 (237:164) من البالغين الأكبر سناً الذين يعيشون في المجتمع
|
RCT 3 سنوات |
|
|
|
|
||||
| كنابين وآخرون 2018 هولندا
|
214 PMW 60 ي | RCT 3 سنوات |
|
|
| أغوايو-رويز وآخرون 2020 المكسيك
|
RCT 3 أشهر | |||
| 40 (24:16) T2D 56 سنة | (1)
|
(1):
|
||
| ساكك وآخرون 2020 إيران
|
68 (42:26) T2D 57.6 سنة | RCT 12 أسبوع |
|
|
الاستنتاجات وآفاق المستقبل
يجب تحسين الوقاية والعلاج بشكل أكبر. لذلك، فإن البحث المستقبلي مطلوب لاستكشاف هذه القضية بشكل أعمق، ويأمل أن يتم تطبيق الأدوار الإيجابية لفيتامين ك في الوقاية من الأمراض وعلاجها في العلاج السريري العملي في أقرب وقت ممكن.
الاختصارات
مساهمات المؤلفين
تمويل
الإفصاح
References
- Wong F, de la Fuente-Nunez C, Collins JJ. Leveraging artificial intelligence in the fight against infectious diseases. Science. 2023;381 (6654):164-170. doi:10.1126/science.adh1114
- Sangeetha Vijayan P, Xavier J, Valappil MP. A review of immune modulators and immunotherapy in infectious diseases. Mol Cell Biochem. 2023. doi:10.1007/s11010-023-04825-w
- Zheng Y, Liu Q, Goronzy JJ, Weyand CM. Immune aging – A mechanism in autoimmune disease. Semin Immunol. 2023;69:101814. doi:10.1016/j. smim.2023.101814
- Merra G, Dominici F, Gualtieri P, et al. Role of vitamin K2 in bone-vascular crosstalk. Int J Vitam Nutr Res. 2022. doi:10.1024/0300-9831/a000761
- Swanson JC, Suttie JW. Vitamin K dependent in vitro production of prothrombin. Biochemistry. 1982;21(23):6011-6018. doi:10.1021/bi00266a044
- Schurgers LJ, Vermeer C. Determination of phylloquinone and menaquinones in food. Effect of food matrix on circulating vitamin K concentrations. Haemostasis. 2000;30(6):298-307. doi:10.1159/000054147
- Dunlop E, Jakobsen J, Jensen MB, et al. Vitamin K content of cheese, yoghurt and meat products in Australia. Food Chem. 2022;397:133772. doi:10.1016/j.foodchem.2022.133772
- Kamao M, Suhara Y, Tsugawa N, et al. Vitamin K content of foods and dietary vitamin K intake in Japanese young women. J Nutr Sci Vitaminol. 2007;53(6):464-470. doi:10.3177/jnsv.53.464
- Stafford DW. The vitamin K cycle. J Thromb Haemost. 2005;3(8):1873-1878. doi:10.1111/j.1538-7836.2005.01419.x
- Bentley R, Meganathan R. Biosynthesis of vitamin K (menaquinone) in bacteria. Microbiol Rev. 1982;46(3):241-280. doi:10.1128/mr.46.3.241280.1982
- Beulens JWJ, Booth SL, van den Heuvel EG, Stoecklin E, Baka A, Vermeer C. The role of menaquinones (vitamin
) in human health. Br J Nutr. 2013;110(8):1357-1368. doi:10.1017/S0007114513001013 - Shearer MJ, Okano T. Key pathways and regulators of vitamin K function and intermediary metabolism. Annu Rev Nutr. 2018;38:127-151. doi:10.1146/annurev-nutr-082117-051741
- Saglani S, Yates L, Lloyd CM. Immunoregulation of asthma by type 2 cytokine therapies: treatments for all ages? Eur J Immunol. 2023;53(8): e2249919. doi:10.1002/eji. 202249919
- Kimur I, Tanizaki Y, Sato S, Saito K, Takahashi K. Menaquinone (vitamin K2) therapy for bronchial asthma. II. Clinical effect of menaquinone on bronchial asthma. Acta Med Okayama. 1975;29(2):127-135.
- Janssen R, Vermeer C. Vitamin K deficit and elastolysis theory in pulmonary elasto-degenerative diseases. Med Hypotheses. 2017;108:38-41. doi:10.1016/j.mehy.2017.07.029
- Jespersen T, Kampmann FB, Dantoft TM, et al. The association of vitamin K status with lung function and disease in a general population.
Open Res. 2023;9:5. - Aziz M, Fatima R, Assaly R. Elevated interleukin-6 and severe COVID-19: a meta-analysis. J Med Virol. 2020;92(11):2283-2285. doi:10.1002/ jmv. 25948
- McElvaney OJ, Curley GF, Rose-John S, McElvaney NG. Interleukin-6: obstacles to targeting a complex cytokine in critical illness. Lancet Respir Med. 2021;9(6):643-654. doi:10.1016/S2213-2600(21)00103-X
- Liu B, Li M, Zhou Z, Guan X, Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun. 2020;111:102452. doi:10.1016/j.jaut.2020.102452
- Visser MPJ, Dofferhoff ASM, van den Ouweland JMW, et al. Effects of Vitamin D and K on Interleukin-6 in COVID-19. Front Nutr. 2021;8:761191. doi:10.3389/fnut.2021.761191
- Mangge H, Prueller F, Dawczynski C, et al. Dramatic decrease of vitamin K2 subtype menaquinone-7 in COVID-19 patients. Antioxidants. 2022;11:7.
- Su J, Luo Y, Hu S, Tang L, Ouyang S. Advances in research on type 2 diabetes mellitus targets and therapeutic agents. Int J Mol Sci. 2023;24:17. doi:10.3390/ijms241713381
- Sakamoto N, Nishiike T, Iguchi H, Sakamoto K. Relationship between acute insulin response and vitamin K intake in healthy young male volunteers. Diabetes Nutr Metab. 1999;12(1):37-41.
- Sakamoto N, Nishiike T, Iguchi H, Sakamoto K. Possible effects of one week vitamin K (menaquinone-4) tablets intake on glucose tolerance in healthy young male volunteers with different descarboxy prothrombin levels. Clin Nutr. 2000;19(4):259-263. doi:10.1054/clnu.2000.0102
- Yoshida M, Booth SL, Meigs JB, Saltzman E, Jacques PF. Phylloquinone intake, insulin sensitivity, and glycemic status in men and women12. Am J Clin Nutr. 2008;88(1):210-215. doi:10.1093/ajcn/88.1.210
- Yoshida M, Jacques PF, Meigs JB, et al. Effect of vitamin K supplementation on insulin resistance in older men and women. Diabetes Care. 2008;31(11):2092-2096. doi:10.2337/dc08-1204
- Rasekhi H, Karandish M, Jalali MT, et al. Phylloquinone supplementation improves glycemic status independent of the effects of adiponectin levels in premonopause women with prediabetes: a double-blind randomized controlled clinical trial. J Diabetes Metab Disord. 2015;14(1):1. doi:10.1186/s40200-014-0127-9
- Rasekhi H, Karandish M, Jalali MT, et al. The effect of vitamin K1 supplementation on sensitivity and insulin resistance via osteocalcin in prediabetic women: a double-blind randomized controlled clinical trial. Eur J Clin Nutr. 2015;69(8):891-895. doi:10.1038/ejcn.2015.17
- Zhang Q, Yan Y. The role of natural flavonoids on neuroinflammation as a therapeutic target for Alzheimer’s disease: a narrative review. Neural Regen Res. 2023;18(12):2582-2591. doi:10.4103/1673-5374.373680
- Alisi L, Cao R, De Angelis C, et al. The relationships between vitamin K and cognition: a review of current evidence. Front Neurol. 2019;10:239. doi:10.3389/fneur.2019.00239
- Wattenberg BW. Intra- and intercellular trafficking in sphingolipid metabolism in myelination. advances in Biological Regulation. 2019;71:97-103. doi:10.1016/j.jbior.2018.11.002
- Manfioletti G, Brancolini C, Avanzi GC, Schneider C. The protein encoded by a growth arrest-specific gene (gas6) is a new member of the vitamin K-dependent proteins related to protein S, a negative coregulator in the blood coagulation cascade. Mol Cell Biol. 1993;13:4976-4985. doi:10.1128/mcb.13.8.4976-4985.1993
- Bellido-Martín L, de Frutos PG. Vitamin K-dependent actions of Gas6. Vitam Horm. 2008;78:185-209.
- Funakoshi H, Yonemasu T, Nakano T, Matumoto K, Nakamura T. Identification of Gas6, a putative ligand for Sky and Axl receptor tyrosine kinases, as a novel neurotrophic factor for hippocampal neurons. J Neurosci Res. 2002;68(2):150-160. doi:10.1002/jnr. 10211
- Yagami T, Ueda K, Asakura K, et al. Gas6 rescues cortical neurons from amyloid beta protein-induced apoptosis. Neuropharmacology. 2002;43 (8):1289-1296. doi:10.1016/S0028-3908(02)00333-7
- Liu D, Guo H, Griffin JH, Fernández JA, Zlokovic BV. Protein S confers neuronal protection during ischemic/hypoxic injury in mice. Circulation. 2003;107(13):1791-1796. doi:10.1161/01.CIR.0000058460.34453.5A
- Pluta R, Januszewski S, Czuczwar SJ. Brain ischemia as a prelude to alzheimer’s disease. Front Aging Neurosci. 2021;13:636653. doi:10.3389/ fnagi.2021.636653
- Dexter DT, Jenner P. Parkinson disease: from pathology to molecular disease mechanisms. Free Radic Biol Med. 2013;62:132-144. doi:10.1016/j.freeradbiomed.2013.01.018
- Lopez-Fabuel I, Martin-Martin L, Resch-Beusher M, Azkona G, Sanchez-Pernaute R, Bolaños JP. Mitochondrial respiratory chain disorganization in Parkinson’s disease-relevant PINK1 and DJ1 mutants. Neurochem Int. 2017;109:101-105. doi:10.1016/j.neuint.2017.03.023
- Vos M, Esposito G, Edirisinghe JN, et al. Vitamin K2 is a mitochondrial electron carrier that rescues pink1 deficiency. Science. 2012;336 (6086):1306-1310. doi:10.1126/science. 1218632
- Prasuhn J, Kasten M, Vos M, et al. The use of vitamin K2 in patients with parkinson’s disease and mitochondrial dysfunction (PD-K2): a theranostic pilot study in a placebo-controlled parallel group design. Front Neurol. 2020;11:592104. doi:10.3389/fneur.2020.592104
- Yu Y-X, Yu X-D, Cheng Q-Z, Tang L, Shen M-Q. The association of serum vitamin K2 levels with Parkinson’s disease: from basic case-control study to big data mining analysis. Aging. 2020;12(16):16410-16419. doi:10.18632/aging. 103691
- da Silva FL, Coelho Cerqueira E, de Freitas MS, Gonçalves DL, Costa LT, Follmer C. Vitamins K interact with N-terminus
-synuclein and modulate the protein fibrillization in vitro. Exploring the interaction between quinones and -synuclein. Neurochem Int. 2013;62(1):103-112. doi:10.1016/j.neuint.2012.10.001 - Zafar E, Maqbool MF, Iqbal A, et al. A comprehensive review on anticancer mechanism of bazedoxifene. Biotechnol Appl Biochem. 2022;69 (2):767-782. doi:10.1002/bab. 2150
- Fernald K, Kurokawa M. Evading apoptosis in cancer. Trends Cell Biol. 2013;23(12):620-633. doi:10.1016/j.tcb.2013.07.006
- Bouzahzah B, Nishikawa Y, Simon D, Carr BI. Growth control and gene expression in a new hepatocellular carcinoma cell line, Hep40: inhibitory actions of vitamin K. J Cell Physiol. 1995;165(3):459-467. doi:10.1002/jcp.1041650303
- Chlebowski RT, Dietrich M, Akman S, Block JB. Vitamin K3 inhibition of malignant murine cell growth and human tumor colony formation. Cancer Treat Rep. 1985;69(5):527-532.
- Wu FY, Liao WC, Chang HM. Comparison of antitumor activity of vitamins K1, K2 and K3 on human tumor cells by two (MTT and SRB) cell viability assays. Life Sci. 1993;52(22):1797-1804. doi:10.1016/0024-3205(93)90469-J
- Hitomi M, Yokoyama F, Kita Y, et al. Antitumor effects of vitamins K1, K2 and K3 on hepatocellular carcinoma in vitro and in vivo. Int J Oncol. 2005;26(3):713-720.
- Ozaki I, Zhang H, Mizuta T, et al. Menatetrenone, a vitamin K2 analogue, inhibits hepatocellular carcinoma cell growth by suppressing cyclin D1 expression through inhibition of nuclear factor kappaB activation. Clin Cancer Res. 2007;13(7):2236-2245. doi:10.1158/1078-0432.CCR-06-2308
- Xia J, Matsuhashi S, Hamajima H, et al. The role of PKC isoforms in the inhibition of NF-
activation by vitamin K 2 in human hepatocellular carcinoma cells. J Nutr Biochem. 2012;23(12):1668-1675. doi:10.1016/j.jnutbio.2011.11.010 - Maniwa Y, Kasukabe T, Kumakura S. Vitamin K2 and cotylenin A synergistically induce monocytic differentiation and growth arrest along with the suppression of c-MYC expression and induction of cyclin G2 expression in human leukemia HL-60 cells. Int J Oncol. 2015;47(2):473-480. doi:10.3892/ijo.2015.3028
- Otsuka M, Kato N, Shao RX, et al. Vitamin K2 inhibits the growth and invasiveness of hepatocellular carcinoma cells via protein kinase A activation. Hepatology. 2004;40(1):243-251. doi:10.1002/hep. 20260
- Liu W, Nakamura H, Yamamoto T, et al. Vitamin K2 inhibits the proliferation of HepG2 cells by up-regulating the transcription of p21 gene. Hepatol Res. 2007;37(5):360-365. doi:10.1111/j.1872-034X.2007.00058.x
- Maurya PK, Kumar P, Chandra P. Biomarkers of oxidative stress in erythrocytes as a function of human age. World J Methodol. 2015;5 (4):216-222. doi:10.5662/wjm.v5.i4.216
- Harshman SG, Shea MK. The role of vitamin K in chronic aging diseases: inflammation, cardiovascular disease, and osteoarthritis. Curr Nutr Rep. 2016;5(2):90-98. doi:10.1007/s13668-016-0162-x
- Popa DS, Bigman G, Rusu ME. The role of vitamin K in humans: implication in aging and age-associated diseases. Antioxidants. 2021;10:4.
- Tsugawa N, Shiraki M. Vitamin K nutrition and bone health. Nutrients. 2020;12(7). doi:10.3390/nu12071909
- Shea MK, Kritchevsky SB, Hsu FC, et al. The association between vitamin K status and knee osteoarthritis features in older adults: the health, aging and body composition study. Osteoarthritis Cartilage. 2015;23(3):370-378. doi:10.1016/j.joca.2014.12.008
- Binkley NC, Krueger DC, Kawahara TN, Engelke JA, Chappell RJ, Suttie JW. A high phylloquinone intake is required to achieve maximal osteocalcin gamma-carboxylation. Am J Clin Nutr. 2002;76(5):1055-1060. doi:10.1093/ajcn/76.5.1055
- Lin X, Brennan-Speranza TC, Levinger I, Yeap BB. Undercarboxylated osteocalcin: experimental and human evidence for a role in glucose homeostasis and muscle regulation of insulin sensitivity. Nutrients. 2018;10(7):847. doi:10.3390/nu10070847
- Luukinen H, Käkönen SM, Pettersson K, et al. Strong prediction of fractures among older adults by the ratio of carboxylated to total serum osteocalcin. J Bone Miner Res. 2000;15(12):2473-2478. doi:10.1359/jbmr.2000.15.12.2473
- Sim M, Lewis JR, Prince RL, et al. The effects of vitamin K-rich green leafy vegetables on bone metabolism: a 4 -week randomised controlled trial in middle-aged and older individuals. Bone Rep. 2020;12:100274. doi:10.1016/j.bonr.2020.100274
- Bultynck C, Munim N, Harrington DJ, et al. Prevalence of vitamin K deficiency in older people with Hip fracture. Acta Clin Belg. 2020;75 (2):136-140. doi:10.1080/17843286.2018.1564174
- Moore AE, Kim E, Dulnoan D, et al. Serum vitamin K(1) (phylloquinone) is associated with fracture risk and Hip strength in post-menopausal osteoporosis: a cross-sectional study. Bone. 2020;141:115630. doi:10.1016/j.bone.2020.115630
- Hooshmand S, Kern M, Metti D, et al. The effect of two doses of dried plum on bone density and bone biomarkers in osteopenic postmenopausal women: a randomized, controlled trial. Osteoporos Int. 2016;27(7):2271-2279. doi:10.1007/s00198-016-3524-8
- Yamaguchi M, Weitzmann MN. Vitamin K2 stimulates osteoblastogenesis and suppresses osteoclastogenesis by suppressing NF-кB activation. Int J Mol Med. 2011;27(1):3-14. doi:10.3892/ijmm.2010.562
- Capozzi A, Scambia G, Lello S. Calcium, vitamin D, vitamin K2, and magnesium supplementation and skeletal health. Maturitas. 2020;140:55-63. doi:10.1016/j.maturitas.2020.05.020
- Dai L, Schurgers LJ, Shiels PG, Stenvinkel P. Early vascular ageing in chronic kidney disease: impact of inflammation, vitamin K, senescence and genomic damage. Nephrol Dial Transplant. 2020;35(Suppl 2):ii31-ii7. doi:10.1093/ndt/gfaa006
- Mozos I, Stoian D, Luca CT. Crosstalk between vitamins A, B12, D, K, C, and E status and arterial stiffness. Dis Markers. 2017;2017:8784971. doi:10.1155/2017/8784971
- Roumeliotis S, Dounousi E, Salmas M, Eleftheriadis T, Liakopoulos V. vascular calcification in chronic kidney disease: the role of vitamin Kdependent matrix gla protein. Front Med Lausanne. 2020;7:154. doi:10.3389/fmed.2020.00154
- Ueland T, Dahl CP, Gullestad L, et al. Circulating levels of non-phosphorylated undercarboxylated matrix Gla protein are associated with disease severity in patients with chronic heart failure. Clin Sci (Lond). 2011;121(3):119-127. doi:10.1042/CS20100589
- Puzantian H, Akers SR, Oldland G, et al. Circulating dephospho-uncarboxylated matrix gla-protein is associated with kidney dysfunction and arterial stiffness. Am J Hypertens. 2018;31(9):988-994. doi:10.1093/ajh/hpy079
- Caluwé R, Vandecasteele S, Van Vlem B, Vermeer C, De Vriese AS. Vitamin K2 supplementation in haemodialysis patients: a randomized dose-finding study. Nephrol Dial Transplant. 2014;29(7):1385-1390. doi:10.1093/ndt/gft464
- Shioi A, Morioka T, Shoji T, Emoto M. The inhibitory roles of vitamin K in progression of vascular calcification. Nutrients. 2020;12(2). doi:10.3390/nu12020583
- Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology. 2014;146(6):1489-1499. doi:10.1053/j.gastro.2014.02.009
- Nadeem MS, Kumar V, Al-Abbasi FA, Kamal MA, Anwar F. Risk of colorectal cancer in inflammatory bowel diseases. Semin Cancer Biol. 2020;64:51-60. doi:10.1016/j.semcancer.2019.05.001
- Yoon SM. Micronutrient deficiencies in inflammatory bowel disease: trivial or crucial? Intest Res. 2016;14(2):109-110. doi:10.5217/ ir.2016.14.2.109
- Lai Y, Masatoshi H, Ma Y, Guo Y, Zhang B. Role of vitamin K in intestinal health. Front Immunol. 2021;12:791565. doi:10.3389/ fimmu.2021.791565
- Varsha MK, Thiagarajan R, Manikandan R, Dhanasekaran G. Vitamin K1 alleviates streptozotocin-induced type 1 diabetes by mitigating free radical stress, as well as inhibiting NF-
activation and iNOS expression in rat pancreas. Nutrition. 2015;31(1):214-222. doi:10.1016/j. nut.2014.05.012 - Iwamoto J, Seki A, Sato Y, Matsumoto H, Takeda T, Yeh JK. Vitamin
prevents hyperglycemia and cancellous osteopenia in rats with streptozotocin-induced type 1 diabetes. Calcif Tissue Int. 2011;88(2):162-168. doi:10.1007/s00223-010-9441-5 - Dulamea AO. Role of oligodendrocyte dysfunction in demyelination, remyelination and neurodegeneration in multiple sclerosis. Adv Exp Med Biol. 2017;958:91-127. doi:10.1007/978-3-319-47861-6_7
- Lasemi R, Kundi M, Moghadam NB, Moshammer H, Hainfellner JA. Vitamin K2 in multiple sclerosis patients. Wien Klin Wochenschr. 2018;130(9-10):307-313. doi:10.1007/s00508-018-1328-x
- Moriya M, Nakatsuji Y, Okuno T, Hamasaki T, Sawada M, Sakoda S. Vitamin K2 ameliorates experimental autoimmune encephalomyelitis in Lewis rats.
Neuroimmunol. 2005;170(1-2):11-20. doi:10.1016/j.jneuroim.2005.08.001 - Li J, Wang H, Rosenberg PA. Vitamin K prevents oxidative cell death by inhibiting activation of 12-lipoxygenase in developing oligodendrocytes. J Neurosci Res. 2009;87(9):1997-2005. doi:10.1002/jnr. 22029
- Carrié I, Portoukalian J, Vicaretti R, Rochford J, Potvin S, Ferland G. Menaquinone-4 concentration is correlated with sphingolipid concentrations in rat brain. J Nutr. 2004;134(1):167-172. doi:10.1093/jn/134.1.167
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:6937):356-61. doi:10.1038/nature01661
- Smolen JS, Aletaha D, Barton A, et al. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18002. doi:10.1038/nrdp.2018.2
- Bowman SJ. Hematological manifestations of rheumatoid arthritis. Scand
Rheumatol. 2002;31(5):251-259. doi:10.1080/ 030097402760375124 - Pereira L, Monteiro R. Tailoring gut microbiota with a combination of Vitamin K and probiotics as a possible adjuvant in the treatment of rheumatic arthritis: a systematic review. Clin Nutr ESPEN. 2022;51:37-49. doi:10.1016/j.clnesp.2022.08.002
- Huang YJ, Han L, Li J, Chen C. Acquired coagulation dysfunction resulting from vitamin K-dependent coagulation factor deficiency associated with rheumatoid arthritis: a case report. World J Clin Cases. 2022;10(1):236-241. doi:10.12998/wjcc.v10.i1.236
- Xu W, Chen S, Wang X, et al. Suppressive effect of vitamin K2 against mitogen-activated peripheral blood mononuclear cells of rheumatoid arthritis patients. Int J Clin Pharmacol Ther. 2021;59(1):55-62. doi:10.5414/CP203827
- Shiraki M, Shiraki Y, Aoki C, Miura M. Vitamin K2 (menatetrenone) effectively prevents fractures and sustains lumbar bone mineral density in osteoporosis. J Bone Miner Res. 2000;15(3):515-521. doi:10.1359/jbmr.2000.15.3.515
- Iwamoto J, Takeda T, Ichimura S. Effect of menatetrenone on bone mineral density and incidence of vertebral fractures in postmenopausal women with osteoporosis: a comparison with the effect of etidronate. J Orthop Sci. 2001;6(6):487-492. doi:10.1007/s007760100002
- Purwosunu Y, Rachman IA, Reksoprodjo S, Sekizawa A. Vitamin K2 treatment for postmenopausal osteoporosis in Indonesia. J Obstetrics Gynaecol Res. 2006;32(2):230-234. doi:10.1111/j.1447-0756.2006.00386.x
- Bolton-Smith C, McMurdo MET, Paterson CR, et al. Two-year randomized controlled trial of vitamin K1 (Phylloquinone) and Vitamin D3 plus calcium on the bone health of older women. J Bone Miner Res. 2007;22(4):509-519. doi:10.1359/jbmr.070116
- Knapen MH, Schurgers LJ, Vermeer C. Vitamin K2 supplementation improves Hip bone geometry and bone strength indices in postmenopausal women. Osteoporos Int. 2007;18(7):963-972. doi:10.1007/s00198-007-0337-9
- Cheung AM, Tile L, Lee Y, et al. Vitamin K supplementation in postmenopausal women with osteopenia (ECKO Trial): a randomized controlled trial. PLoS Med. 2008;5(1):- 12. doi:10.1371/journal.pmed. 0050196
- Hirao M, Hashimoto J, Ando W, Ono T, Yoshikawa H. Response of serum carboxylated and undercarboxylated osteocalcin to alendronate monotherapy and combined therapy with vitamin K2 in postmenopausal women. J Bone Miner Metab. 2008;26(3):260-264. doi:10.1007/ s00774-007-0823-3
- Tsugawa N, Shiraki M, Suhara Y, et al. Low plasma phylloquinone concentration is associated with high incidence of vertebral fracture in Japanese women. J Bone Miner Metab. 2008;26(1):79-85. doi:10.1007/s00774-007-0790-8
- Je SH, Joo NS, Choi BH, et al. Vitamin K supplement along with vitamin D and calcium reduced serum concentration of undercarboxylated osteocalcin while increasing bone mineral density in Korean postmenopausal women over sixty-years-old. J Korean Med Sci. 2011;26 (8):1093-1098. doi:10.3346/jkms.2011.26.8.1093
- Knapen MH, Drummen NE, Smit E, Vermeer C, Theuwissen E. Three-year low-dose menaquinone-7 supplementation helps decrease bone loss in healthy postmenopausal women. Osteoporos Int. 2013;24(9):2499-2507. doi:10.1007/s00198-013-2325-6
- Jiang Y, Zhang ZL, Zhang ZL, et al. Menatetrenone versus alfacalcidol in the treatment of Chinese postmenopausal women with osteoporosis: a multicenter, randomized, double-blinded, double-dummy, positive drug-controlled clinical trial. Clin Interv Aging. 2014;9:121-127. doi:10.2147/CIA.S54107
- Rønn SH, Harsløf T, Pedersen SB, Langdahl BL. Vitamin K2 (menaquinone-7) prevents age-related deterioration of trabecular bone microarchitecture at the tibia in postmenopausal women. Europ
Endocrinol. 2016;175(6):541-549. doi:10.1530/EJE-16-0498 - Shea MK, O’Donnell CJ, Hoffmann U, et al. Vitamin K supplementation and progression of coronary artery calcium in older men and women23. Am J Clin Nutr. 2009;89(6):1799-1807. doi:10.3945/ajcn.2008.27338
- Cheung CL, Sahni S, Cheung BM, Sing CW, Wong IC. Vitamin K intake and mortality in people with chronic kidney disease from NHANES III. Clin Nutr. 2015;34(2):235-240. doi:10.1016/j.clnu.2014.03.011
- Kurnatowska I, Grzelak P, Masajtis-Zagajewska A, et al. Effect of vitamin K2 on progression of atherosclerosis and vascular calcification in nondialyzed patients with chronic kidney disease stages 3-5. Pol Arch Med Wewn. 2015;125(9):631-640. doi:10.20452/pamw. 3041
- Brandenburg VM, Reinartz S, Kaesler N, et al. Slower progress of aortic valve calcification with vitamin K supplementation. Circulation. 2017;135(21):2081-2083. doi:10.1161/CIRCULATIONAHA.116.027011
- Shea MK, Barger K, Booth SL, et al. Vitamin K status, cardiovascular disease, and all-cause mortality: a participant-level meta-analysis of 3 US cohorts. Am J Clin Nutr. 2020;111(6):1170-1177. doi:10.1093/ajcn/nqaa082
- Shea MK, Dawson-Hughes B, Gundberg CM, Booth SL. Reducing undercarboxylated osteocalcin with vitamin K supplementation does not promote lean tissue loss or fat gain over 3 years in older women and men: a randomized controlled trial. J Bone Miner Res. 2017;32 (2):243-249. doi:10.1002/jbmr. 2989
- Knapen MHJ, Jardon KM, Vermeer C. Vitamin K-induced effects on body fat and weight: results from a 3 -year vitamin K2 intervention study. Eur J Clin Nutr. 2018;72(1):136-141. doi:10.1038/ejcn.2017.146
- Aguayo-Ruiz JI, García-Cobián TA, Pascoe-González S, et al. Effect of supplementation with vitamins D3 and K2 on undercarboxylated osteocalcin and insulin serum levels in patients with type 2 diabetes mellitus: a randomized, double-blind, clinical trial. Diabetol Metab Syndr. 2020;12(1):73. doi:10.1186/s13098-020-00580-w
- Rahimi Sakak F, Moslehi N, Niroomand M, Mirmiran P. Glycemic control improvement in individuals with type 2 diabetes with vitamin K(2) supplementation: a randomized controlled trial. Eur J Nutr. 2021;60(5):2495-2506. doi:10.1007/s00394-020-02419-6
انشر عملك في هذه المجلة
DOI: https://doi.org/10.2147/jir.s445806
PMID: https://pubmed.ncbi.nlm.nih.gov/38406326
Publication Date: 2024-02-01
Vitamin K: Infection, Inflammation, and Auto-Immunity
Abstract
Vitamin K (VK) comprises a group of substances with chlorophyll quinone bioactivity and exists in nature in the form of VK1 and VK2. As its initial recognition originated from the ability to promote blood coagulation, it is known as the coagulation vitamin. However, based on extensive research, VK has shown potential for the prevention and treatment of various diseases. Studies demonstrating the beneficial effects of VK on immunity, antioxidant capacity, intestinal microbiota regulation, epithelial development, and bone protection have drawn growing interest in recent years. This review article focuses on the mechanism of action of VK and its potential preventive and therapeutic effects on infections (eg, asthma, COVID-19), inflammation (eg, in type 2 diabetes mellitus, Alzheimer’s disease, Parkinson’s disease, cancer, aging, atherosclerosis) and autoimmune disorders (eg, inflammatory bowel disease, type 1 diabetes mellitus, multiple sclerosis, rheumatoid arthritis). In addition, VK-dependent proteins (VKDPs) are another crucial mechanism by which VK exerts anti-inflammatory and immunomodulatory effects. This review explores the potential role of VK in preventing aging, combating neurological abnormalities, and treating diseases such as cancer and diabetes. Although current research appoints VK as a therapeutic tool for practical clinical applications in infections, inflammation, and autoimmune diseases, future research is necessary to elucidate the mechanism of action in more detail and overcome current limitations.
Introduction
(AD), Parkinson’s disease (PD), cancer, aging, atherosclerosis) and autoimmune disorders (eg, inflammatory bowel disease (IBD), type 1 diabetes mellitus (T1DM), multiple sclerosis (MS), and rheumatoid arthritis (RA)). This review aims to summarize the current literature regarding the implications of VK supplementation in infections and the immune response, including evidence from both preclinical and clinical studies.
Chemical and Physiological Characteristics of Vitamin K
Vitamin K and Infections
Asthma
COVID-19
Vitamin K and Inflammation
Type 2 Diabetes Mellitus (T2DM)
known function of VK is coagulation, it also plays an important role in stabilizing blood sugar, improving insulin sensitivity and controlling diabetes.
Alzheimer’s Disease (AD)
| Object | VK intake | Purpose | Conclusion | Ref |
| Healthy young man | Usual dietary intake | Discuss Relationship between acute insulin response and VK intake. | High VK intake is beneficial to improve the insulin sensitivity. | [23] |
| Healthy young man | VK2 | Explore the effect of one-week VK2 tablet intake on glucose tolerance of healthy young man. | VK2 is beneficial to blood glucose balance. | [24] |
| Men and women | Usual dietary intake | Study the relationship between VK intake and insulin-induced blood glucose status in men and women. | VKI and VK2 are beneficial to improve blood glucose disorder. | [25] |
| Older men and women | Usual dietary intake | Investigate whether VK supplementation for 36 months can improve insulin resistance in elderly men and women. | VKI intake is beneficial to improve insulin in elderly men. | [26] |
| Prediabetic women | With or without 500
|
Examine the regulatory effect of VKI supplementation on glucose homeostasis in prediabetic women. | Supplementation of VKI can improve blood glucose status of women with pre-diabetes mellitus. | [27] |
| Prediabetic women | With or without
|
Study whether VKI supplementation affects glucose metabolism or insulin sensitivity in women with pre-diabetes mellitus. | VKI supplementation is beneficial to improve blood glucose status and insulin sensitivity for pre-diabetic women. | [28] |
Parkinson’s Disease (PD)
Cancer

Aging
Atherosclerosis
Vitamin K and Auto-Immune Disease
Inflammatory Bowel Disease (IBD)
Type I Diabetes Mellitus (TIDM)
Multiple Sclerosis (MS)
Rheumatoid Arthritis (RA)
Discussions

| Author, Year (Country) (Ref) | Participants, Age | Design (Length) | Intervention Exposure | Effect of Intervention on Illness |
| Shiraki et al 2000 Japan
|
241 PMO 67.2 y | Prospective
|
|
|
| Iwamoto et al 2001 Japan
|
72 PMO 65.3 y | Prospective 2 y |
|
|
| Purwosunu et al 2006 Indonesia
|
63 PMO 60.8 y | RCT 48 w |
|
|
| Bolton-Smith et al 2007 UK
|
244 Healthy W 68.2 y | RCT 2 y |
|
|
| Knapen et al 2007 | 325 PMW | RCT |
|
|
| Cheung et al 2008 Canada
|
400 PMOa 59.1 y | RCT 2-4 y |
|
|
| Hirao et al 2008 Japan
|
44 PMW 68.4 y | Prospective ly |
|
|
| Tsugawa et al 2008 Japan
|
379 W 63.0 y | Prospective 3 y | High VKI vs low VKI |
|
| Je et al 2011 Korea
|
78 PMW 67.8 y | RCT 6 mo |
|
|
| Knapen et al 2013 | 244 PMW | RCT |
|
|
|
|
||||
| Jiang et al 2014 China
|
213 PMW 64.4 y | RCT ly |
|
|
| Rønn et al 2016 Denmark
|
148 PMOa 67.5 y | RCT ly |
|
|
| Shea et al 2009 USA
|
388 (235:153) Healthy 68 y | RCT 3 y |
|
|
| Cheung et al 2015 USA
|
3401 (2245:1156) CKD 61.9 y | Follow-up 13.3 y |
|
|
| Kurnatowska et al 2015 Poland
|
42 (20:22) Nondialyzed patients with CKD 58 y | RCT 270 days |
|
|
| Brandenburg et al 2017 Germany
|
99 (18:81) AVC 69.1 y | RCT ly |
|
|
| Shea et al 2020 USA
|
3891(2154:1737)
|
Follow-up 13 y |
|
|
| Yoshida et al 2008 USA
|
2719 (1472:1247) healthy 68 y | RCT 36 mo |
|
|
| Shea et al 2017 USA
|
401 (237:164) older community-dwelling adults
|
RCT 3 y |
|
|
|
|
||||
| Knapen et al 2018 Netherlands
|
214 PMW 60 y | RCT 3 y |
|
|
| Aguayo-Ruiz et al 2020 Mexico
|
RCT 3 mo | |||
| 40 (24:16) T2D 56 y | (1)
|
(1):
|
||
| Sakak et al 2020 Iran
|
68 (42:26) T2D 57.6 y | RCT 12 w |
|
|

Conclusions and Future Perspective
prevention and treatment needs to be further refined. Future research is therefore warranted to further explore this issue, and it is hoped that the positive roles of VK in disease prevention and treatment could be applied to practical clinical treatment as soon as possible.
Abbreviations
Author Contributions
Funding
Disclosure
References
- Wong F, de la Fuente-Nunez C, Collins JJ. Leveraging artificial intelligence in the fight against infectious diseases. Science. 2023;381 (6654):164-170. doi:10.1126/science.adh1114
- Sangeetha Vijayan P, Xavier J, Valappil MP. A review of immune modulators and immunotherapy in infectious diseases. Mol Cell Biochem. 2023. doi:10.1007/s11010-023-04825-w
- Zheng Y, Liu Q, Goronzy JJ, Weyand CM. Immune aging – A mechanism in autoimmune disease. Semin Immunol. 2023;69:101814. doi:10.1016/j. smim.2023.101814
- Merra G, Dominici F, Gualtieri P, et al. Role of vitamin K2 in bone-vascular crosstalk. Int J Vitam Nutr Res. 2022. doi:10.1024/0300-9831/a000761
- Swanson JC, Suttie JW. Vitamin K dependent in vitro production of prothrombin. Biochemistry. 1982;21(23):6011-6018. doi:10.1021/bi00266a044
- Schurgers LJ, Vermeer C. Determination of phylloquinone and menaquinones in food. Effect of food matrix on circulating vitamin K concentrations. Haemostasis. 2000;30(6):298-307. doi:10.1159/000054147
- Dunlop E, Jakobsen J, Jensen MB, et al. Vitamin K content of cheese, yoghurt and meat products in Australia. Food Chem. 2022;397:133772. doi:10.1016/j.foodchem.2022.133772
- Kamao M, Suhara Y, Tsugawa N, et al. Vitamin K content of foods and dietary vitamin K intake in Japanese young women. J Nutr Sci Vitaminol. 2007;53(6):464-470. doi:10.3177/jnsv.53.464
- Stafford DW. The vitamin K cycle. J Thromb Haemost. 2005;3(8):1873-1878. doi:10.1111/j.1538-7836.2005.01419.x
- Bentley R, Meganathan R. Biosynthesis of vitamin K (menaquinone) in bacteria. Microbiol Rev. 1982;46(3):241-280. doi:10.1128/mr.46.3.241280.1982
- Beulens JWJ, Booth SL, van den Heuvel EG, Stoecklin E, Baka A, Vermeer C. The role of menaquinones (vitamin
) in human health. Br J Nutr. 2013;110(8):1357-1368. doi:10.1017/S0007114513001013 - Shearer MJ, Okano T. Key pathways and regulators of vitamin K function and intermediary metabolism. Annu Rev Nutr. 2018;38:127-151. doi:10.1146/annurev-nutr-082117-051741
- Saglani S, Yates L, Lloyd CM. Immunoregulation of asthma by type 2 cytokine therapies: treatments for all ages? Eur J Immunol. 2023;53(8): e2249919. doi:10.1002/eji. 202249919
- Kimur I, Tanizaki Y, Sato S, Saito K, Takahashi K. Menaquinone (vitamin K2) therapy for bronchial asthma. II. Clinical effect of menaquinone on bronchial asthma. Acta Med Okayama. 1975;29(2):127-135.
- Janssen R, Vermeer C. Vitamin K deficit and elastolysis theory in pulmonary elasto-degenerative diseases. Med Hypotheses. 2017;108:38-41. doi:10.1016/j.mehy.2017.07.029
- Jespersen T, Kampmann FB, Dantoft TM, et al. The association of vitamin K status with lung function and disease in a general population.
Open Res. 2023;9:5. - Aziz M, Fatima R, Assaly R. Elevated interleukin-6 and severe COVID-19: a meta-analysis. J Med Virol. 2020;92(11):2283-2285. doi:10.1002/ jmv. 25948
- McElvaney OJ, Curley GF, Rose-John S, McElvaney NG. Interleukin-6: obstacles to targeting a complex cytokine in critical illness. Lancet Respir Med. 2021;9(6):643-654. doi:10.1016/S2213-2600(21)00103-X
- Liu B, Li M, Zhou Z, Guan X, Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun. 2020;111:102452. doi:10.1016/j.jaut.2020.102452
- Visser MPJ, Dofferhoff ASM, van den Ouweland JMW, et al. Effects of Vitamin D and K on Interleukin-6 in COVID-19. Front Nutr. 2021;8:761191. doi:10.3389/fnut.2021.761191
- Mangge H, Prueller F, Dawczynski C, et al. Dramatic decrease of vitamin K2 subtype menaquinone-7 in COVID-19 patients. Antioxidants. 2022;11:7.
- Su J, Luo Y, Hu S, Tang L, Ouyang S. Advances in research on type 2 diabetes mellitus targets and therapeutic agents. Int J Mol Sci. 2023;24:17. doi:10.3390/ijms241713381
- Sakamoto N, Nishiike T, Iguchi H, Sakamoto K. Relationship between acute insulin response and vitamin K intake in healthy young male volunteers. Diabetes Nutr Metab. 1999;12(1):37-41.
- Sakamoto N, Nishiike T, Iguchi H, Sakamoto K. Possible effects of one week vitamin K (menaquinone-4) tablets intake on glucose tolerance in healthy young male volunteers with different descarboxy prothrombin levels. Clin Nutr. 2000;19(4):259-263. doi:10.1054/clnu.2000.0102
- Yoshida M, Booth SL, Meigs JB, Saltzman E, Jacques PF. Phylloquinone intake, insulin sensitivity, and glycemic status in men and women12. Am J Clin Nutr. 2008;88(1):210-215. doi:10.1093/ajcn/88.1.210
- Yoshida M, Jacques PF, Meigs JB, et al. Effect of vitamin K supplementation on insulin resistance in older men and women. Diabetes Care. 2008;31(11):2092-2096. doi:10.2337/dc08-1204
- Rasekhi H, Karandish M, Jalali MT, et al. Phylloquinone supplementation improves glycemic status independent of the effects of adiponectin levels in premonopause women with prediabetes: a double-blind randomized controlled clinical trial. J Diabetes Metab Disord. 2015;14(1):1. doi:10.1186/s40200-014-0127-9
- Rasekhi H, Karandish M, Jalali MT, et al. The effect of vitamin K1 supplementation on sensitivity and insulin resistance via osteocalcin in prediabetic women: a double-blind randomized controlled clinical trial. Eur J Clin Nutr. 2015;69(8):891-895. doi:10.1038/ejcn.2015.17
- Zhang Q, Yan Y. The role of natural flavonoids on neuroinflammation as a therapeutic target for Alzheimer’s disease: a narrative review. Neural Regen Res. 2023;18(12):2582-2591. doi:10.4103/1673-5374.373680
- Alisi L, Cao R, De Angelis C, et al. The relationships between vitamin K and cognition: a review of current evidence. Front Neurol. 2019;10:239. doi:10.3389/fneur.2019.00239
- Wattenberg BW. Intra- and intercellular trafficking in sphingolipid metabolism in myelination. advances in Biological Regulation. 2019;71:97-103. doi:10.1016/j.jbior.2018.11.002
- Manfioletti G, Brancolini C, Avanzi GC, Schneider C. The protein encoded by a growth arrest-specific gene (gas6) is a new member of the vitamin K-dependent proteins related to protein S, a negative coregulator in the blood coagulation cascade. Mol Cell Biol. 1993;13:4976-4985. doi:10.1128/mcb.13.8.4976-4985.1993
- Bellido-Martín L, de Frutos PG. Vitamin K-dependent actions of Gas6. Vitam Horm. 2008;78:185-209.
- Funakoshi H, Yonemasu T, Nakano T, Matumoto K, Nakamura T. Identification of Gas6, a putative ligand for Sky and Axl receptor tyrosine kinases, as a novel neurotrophic factor for hippocampal neurons. J Neurosci Res. 2002;68(2):150-160. doi:10.1002/jnr. 10211
- Yagami T, Ueda K, Asakura K, et al. Gas6 rescues cortical neurons from amyloid beta protein-induced apoptosis. Neuropharmacology. 2002;43 (8):1289-1296. doi:10.1016/S0028-3908(02)00333-7
- Liu D, Guo H, Griffin JH, Fernández JA, Zlokovic BV. Protein S confers neuronal protection during ischemic/hypoxic injury in mice. Circulation. 2003;107(13):1791-1796. doi:10.1161/01.CIR.0000058460.34453.5A
- Pluta R, Januszewski S, Czuczwar SJ. Brain ischemia as a prelude to alzheimer’s disease. Front Aging Neurosci. 2021;13:636653. doi:10.3389/ fnagi.2021.636653
- Dexter DT, Jenner P. Parkinson disease: from pathology to molecular disease mechanisms. Free Radic Biol Med. 2013;62:132-144. doi:10.1016/j.freeradbiomed.2013.01.018
- Lopez-Fabuel I, Martin-Martin L, Resch-Beusher M, Azkona G, Sanchez-Pernaute R, Bolaños JP. Mitochondrial respiratory chain disorganization in Parkinson’s disease-relevant PINK1 and DJ1 mutants. Neurochem Int. 2017;109:101-105. doi:10.1016/j.neuint.2017.03.023
- Vos M, Esposito G, Edirisinghe JN, et al. Vitamin K2 is a mitochondrial electron carrier that rescues pink1 deficiency. Science. 2012;336 (6086):1306-1310. doi:10.1126/science. 1218632
- Prasuhn J, Kasten M, Vos M, et al. The use of vitamin K2 in patients with parkinson’s disease and mitochondrial dysfunction (PD-K2): a theranostic pilot study in a placebo-controlled parallel group design. Front Neurol. 2020;11:592104. doi:10.3389/fneur.2020.592104
- Yu Y-X, Yu X-D, Cheng Q-Z, Tang L, Shen M-Q. The association of serum vitamin K2 levels with Parkinson’s disease: from basic case-control study to big data mining analysis. Aging. 2020;12(16):16410-16419. doi:10.18632/aging. 103691
- da Silva FL, Coelho Cerqueira E, de Freitas MS, Gonçalves DL, Costa LT, Follmer C. Vitamins K interact with N-terminus
-synuclein and modulate the protein fibrillization in vitro. Exploring the interaction between quinones and -synuclein. Neurochem Int. 2013;62(1):103-112. doi:10.1016/j.neuint.2012.10.001 - Zafar E, Maqbool MF, Iqbal A, et al. A comprehensive review on anticancer mechanism of bazedoxifene. Biotechnol Appl Biochem. 2022;69 (2):767-782. doi:10.1002/bab. 2150
- Fernald K, Kurokawa M. Evading apoptosis in cancer. Trends Cell Biol. 2013;23(12):620-633. doi:10.1016/j.tcb.2013.07.006
- Bouzahzah B, Nishikawa Y, Simon D, Carr BI. Growth control and gene expression in a new hepatocellular carcinoma cell line, Hep40: inhibitory actions of vitamin K. J Cell Physiol. 1995;165(3):459-467. doi:10.1002/jcp.1041650303
- Chlebowski RT, Dietrich M, Akman S, Block JB. Vitamin K3 inhibition of malignant murine cell growth and human tumor colony formation. Cancer Treat Rep. 1985;69(5):527-532.
- Wu FY, Liao WC, Chang HM. Comparison of antitumor activity of vitamins K1, K2 and K3 on human tumor cells by two (MTT and SRB) cell viability assays. Life Sci. 1993;52(22):1797-1804. doi:10.1016/0024-3205(93)90469-J
- Hitomi M, Yokoyama F, Kita Y, et al. Antitumor effects of vitamins K1, K2 and K3 on hepatocellular carcinoma in vitro and in vivo. Int J Oncol. 2005;26(3):713-720.
- Ozaki I, Zhang H, Mizuta T, et al. Menatetrenone, a vitamin K2 analogue, inhibits hepatocellular carcinoma cell growth by suppressing cyclin D1 expression through inhibition of nuclear factor kappaB activation. Clin Cancer Res. 2007;13(7):2236-2245. doi:10.1158/1078-0432.CCR-06-2308
- Xia J, Matsuhashi S, Hamajima H, et al. The role of PKC isoforms in the inhibition of NF-
activation by vitamin K 2 in human hepatocellular carcinoma cells. J Nutr Biochem. 2012;23(12):1668-1675. doi:10.1016/j.jnutbio.2011.11.010 - Maniwa Y, Kasukabe T, Kumakura S. Vitamin K2 and cotylenin A synergistically induce monocytic differentiation and growth arrest along with the suppression of c-MYC expression and induction of cyclin G2 expression in human leukemia HL-60 cells. Int J Oncol. 2015;47(2):473-480. doi:10.3892/ijo.2015.3028
- Otsuka M, Kato N, Shao RX, et al. Vitamin K2 inhibits the growth and invasiveness of hepatocellular carcinoma cells via protein kinase A activation. Hepatology. 2004;40(1):243-251. doi:10.1002/hep. 20260
- Liu W, Nakamura H, Yamamoto T, et al. Vitamin K2 inhibits the proliferation of HepG2 cells by up-regulating the transcription of p21 gene. Hepatol Res. 2007;37(5):360-365. doi:10.1111/j.1872-034X.2007.00058.x
- Maurya PK, Kumar P, Chandra P. Biomarkers of oxidative stress in erythrocytes as a function of human age. World J Methodol. 2015;5 (4):216-222. doi:10.5662/wjm.v5.i4.216
- Harshman SG, Shea MK. The role of vitamin K in chronic aging diseases: inflammation, cardiovascular disease, and osteoarthritis. Curr Nutr Rep. 2016;5(2):90-98. doi:10.1007/s13668-016-0162-x
- Popa DS, Bigman G, Rusu ME. The role of vitamin K in humans: implication in aging and age-associated diseases. Antioxidants. 2021;10:4.
- Tsugawa N, Shiraki M. Vitamin K nutrition and bone health. Nutrients. 2020;12(7). doi:10.3390/nu12071909
- Shea MK, Kritchevsky SB, Hsu FC, et al. The association between vitamin K status and knee osteoarthritis features in older adults: the health, aging and body composition study. Osteoarthritis Cartilage. 2015;23(3):370-378. doi:10.1016/j.joca.2014.12.008
- Binkley NC, Krueger DC, Kawahara TN, Engelke JA, Chappell RJ, Suttie JW. A high phylloquinone intake is required to achieve maximal osteocalcin gamma-carboxylation. Am J Clin Nutr. 2002;76(5):1055-1060. doi:10.1093/ajcn/76.5.1055
- Lin X, Brennan-Speranza TC, Levinger I, Yeap BB. Undercarboxylated osteocalcin: experimental and human evidence for a role in glucose homeostasis and muscle regulation of insulin sensitivity. Nutrients. 2018;10(7):847. doi:10.3390/nu10070847
- Luukinen H, Käkönen SM, Pettersson K, et al. Strong prediction of fractures among older adults by the ratio of carboxylated to total serum osteocalcin. J Bone Miner Res. 2000;15(12):2473-2478. doi:10.1359/jbmr.2000.15.12.2473
- Sim M, Lewis JR, Prince RL, et al. The effects of vitamin K-rich green leafy vegetables on bone metabolism: a 4 -week randomised controlled trial in middle-aged and older individuals. Bone Rep. 2020;12:100274. doi:10.1016/j.bonr.2020.100274
- Bultynck C, Munim N, Harrington DJ, et al. Prevalence of vitamin K deficiency in older people with Hip fracture. Acta Clin Belg. 2020;75 (2):136-140. doi:10.1080/17843286.2018.1564174
- Moore AE, Kim E, Dulnoan D, et al. Serum vitamin K(1) (phylloquinone) is associated with fracture risk and Hip strength in post-menopausal osteoporosis: a cross-sectional study. Bone. 2020;141:115630. doi:10.1016/j.bone.2020.115630
- Hooshmand S, Kern M, Metti D, et al. The effect of two doses of dried plum on bone density and bone biomarkers in osteopenic postmenopausal women: a randomized, controlled trial. Osteoporos Int. 2016;27(7):2271-2279. doi:10.1007/s00198-016-3524-8
- Yamaguchi M, Weitzmann MN. Vitamin K2 stimulates osteoblastogenesis and suppresses osteoclastogenesis by suppressing NF-кB activation. Int J Mol Med. 2011;27(1):3-14. doi:10.3892/ijmm.2010.562
- Capozzi A, Scambia G, Lello S. Calcium, vitamin D, vitamin K2, and magnesium supplementation and skeletal health. Maturitas. 2020;140:55-63. doi:10.1016/j.maturitas.2020.05.020
- Dai L, Schurgers LJ, Shiels PG, Stenvinkel P. Early vascular ageing in chronic kidney disease: impact of inflammation, vitamin K, senescence and genomic damage. Nephrol Dial Transplant. 2020;35(Suppl 2):ii31-ii7. doi:10.1093/ndt/gfaa006
- Mozos I, Stoian D, Luca CT. Crosstalk between vitamins A, B12, D, K, C, and E status and arterial stiffness. Dis Markers. 2017;2017:8784971. doi:10.1155/2017/8784971
- Roumeliotis S, Dounousi E, Salmas M, Eleftheriadis T, Liakopoulos V. vascular calcification in chronic kidney disease: the role of vitamin Kdependent matrix gla protein. Front Med Lausanne. 2020;7:154. doi:10.3389/fmed.2020.00154
- Ueland T, Dahl CP, Gullestad L, et al. Circulating levels of non-phosphorylated undercarboxylated matrix Gla protein are associated with disease severity in patients with chronic heart failure. Clin Sci (Lond). 2011;121(3):119-127. doi:10.1042/CS20100589
- Puzantian H, Akers SR, Oldland G, et al. Circulating dephospho-uncarboxylated matrix gla-protein is associated with kidney dysfunction and arterial stiffness. Am J Hypertens. 2018;31(9):988-994. doi:10.1093/ajh/hpy079
- Caluwé R, Vandecasteele S, Van Vlem B, Vermeer C, De Vriese AS. Vitamin K2 supplementation in haemodialysis patients: a randomized dose-finding study. Nephrol Dial Transplant. 2014;29(7):1385-1390. doi:10.1093/ndt/gft464
- Shioi A, Morioka T, Shoji T, Emoto M. The inhibitory roles of vitamin K in progression of vascular calcification. Nutrients. 2020;12(2). doi:10.3390/nu12020583
- Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology. 2014;146(6):1489-1499. doi:10.1053/j.gastro.2014.02.009
- Nadeem MS, Kumar V, Al-Abbasi FA, Kamal MA, Anwar F. Risk of colorectal cancer in inflammatory bowel diseases. Semin Cancer Biol. 2020;64:51-60. doi:10.1016/j.semcancer.2019.05.001
- Yoon SM. Micronutrient deficiencies in inflammatory bowel disease: trivial or crucial? Intest Res. 2016;14(2):109-110. doi:10.5217/ ir.2016.14.2.109
- Lai Y, Masatoshi H, Ma Y, Guo Y, Zhang B. Role of vitamin K in intestinal health. Front Immunol. 2021;12:791565. doi:10.3389/ fimmu.2021.791565
- Varsha MK, Thiagarajan R, Manikandan R, Dhanasekaran G. Vitamin K1 alleviates streptozotocin-induced type 1 diabetes by mitigating free radical stress, as well as inhibiting NF-
activation and iNOS expression in rat pancreas. Nutrition. 2015;31(1):214-222. doi:10.1016/j. nut.2014.05.012 - Iwamoto J, Seki A, Sato Y, Matsumoto H, Takeda T, Yeh JK. Vitamin
prevents hyperglycemia and cancellous osteopenia in rats with streptozotocin-induced type 1 diabetes. Calcif Tissue Int. 2011;88(2):162-168. doi:10.1007/s00223-010-9441-5 - Dulamea AO. Role of oligodendrocyte dysfunction in demyelination, remyelination and neurodegeneration in multiple sclerosis. Adv Exp Med Biol. 2017;958:91-127. doi:10.1007/978-3-319-47861-6_7
- Lasemi R, Kundi M, Moghadam NB, Moshammer H, Hainfellner JA. Vitamin K2 in multiple sclerosis patients. Wien Klin Wochenschr. 2018;130(9-10):307-313. doi:10.1007/s00508-018-1328-x
- Moriya M, Nakatsuji Y, Okuno T, Hamasaki T, Sawada M, Sakoda S. Vitamin K2 ameliorates experimental autoimmune encephalomyelitis in Lewis rats.
Neuroimmunol. 2005;170(1-2):11-20. doi:10.1016/j.jneuroim.2005.08.001 - Li J, Wang H, Rosenberg PA. Vitamin K prevents oxidative cell death by inhibiting activation of 12-lipoxygenase in developing oligodendrocytes. J Neurosci Res. 2009;87(9):1997-2005. doi:10.1002/jnr. 22029
- Carrié I, Portoukalian J, Vicaretti R, Rochford J, Potvin S, Ferland G. Menaquinone-4 concentration is correlated with sphingolipid concentrations in rat brain. J Nutr. 2004;134(1):167-172. doi:10.1093/jn/134.1.167
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:6937):356-61. doi:10.1038/nature01661
- Smolen JS, Aletaha D, Barton A, et al. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18002. doi:10.1038/nrdp.2018.2
- Bowman SJ. Hematological manifestations of rheumatoid arthritis. Scand
Rheumatol. 2002;31(5):251-259. doi:10.1080/ 030097402760375124 - Pereira L, Monteiro R. Tailoring gut microbiota with a combination of Vitamin K and probiotics as a possible adjuvant in the treatment of rheumatic arthritis: a systematic review. Clin Nutr ESPEN. 2022;51:37-49. doi:10.1016/j.clnesp.2022.08.002
- Huang YJ, Han L, Li J, Chen C. Acquired coagulation dysfunction resulting from vitamin K-dependent coagulation factor deficiency associated with rheumatoid arthritis: a case report. World J Clin Cases. 2022;10(1):236-241. doi:10.12998/wjcc.v10.i1.236
- Xu W, Chen S, Wang X, et al. Suppressive effect of vitamin K2 against mitogen-activated peripheral blood mononuclear cells of rheumatoid arthritis patients. Int J Clin Pharmacol Ther. 2021;59(1):55-62. doi:10.5414/CP203827
- Shiraki M, Shiraki Y, Aoki C, Miura M. Vitamin K2 (menatetrenone) effectively prevents fractures and sustains lumbar bone mineral density in osteoporosis. J Bone Miner Res. 2000;15(3):515-521. doi:10.1359/jbmr.2000.15.3.515
- Iwamoto J, Takeda T, Ichimura S. Effect of menatetrenone on bone mineral density and incidence of vertebral fractures in postmenopausal women with osteoporosis: a comparison with the effect of etidronate. J Orthop Sci. 2001;6(6):487-492. doi:10.1007/s007760100002
- Purwosunu Y, Rachman IA, Reksoprodjo S, Sekizawa A. Vitamin K2 treatment for postmenopausal osteoporosis in Indonesia. J Obstetrics Gynaecol Res. 2006;32(2):230-234. doi:10.1111/j.1447-0756.2006.00386.x
- Bolton-Smith C, McMurdo MET, Paterson CR, et al. Two-year randomized controlled trial of vitamin K1 (Phylloquinone) and Vitamin D3 plus calcium on the bone health of older women. J Bone Miner Res. 2007;22(4):509-519. doi:10.1359/jbmr.070116
- Knapen MH, Schurgers LJ, Vermeer C. Vitamin K2 supplementation improves Hip bone geometry and bone strength indices in postmenopausal women. Osteoporos Int. 2007;18(7):963-972. doi:10.1007/s00198-007-0337-9
- Cheung AM, Tile L, Lee Y, et al. Vitamin K supplementation in postmenopausal women with osteopenia (ECKO Trial): a randomized controlled trial. PLoS Med. 2008;5(1):- 12. doi:10.1371/journal.pmed. 0050196
- Hirao M, Hashimoto J, Ando W, Ono T, Yoshikawa H. Response of serum carboxylated and undercarboxylated osteocalcin to alendronate monotherapy and combined therapy with vitamin K2 in postmenopausal women. J Bone Miner Metab. 2008;26(3):260-264. doi:10.1007/ s00774-007-0823-3
- Tsugawa N, Shiraki M, Suhara Y, et al. Low plasma phylloquinone concentration is associated with high incidence of vertebral fracture in Japanese women. J Bone Miner Metab. 2008;26(1):79-85. doi:10.1007/s00774-007-0790-8
- Je SH, Joo NS, Choi BH, et al. Vitamin K supplement along with vitamin D and calcium reduced serum concentration of undercarboxylated osteocalcin while increasing bone mineral density in Korean postmenopausal women over sixty-years-old. J Korean Med Sci. 2011;26 (8):1093-1098. doi:10.3346/jkms.2011.26.8.1093
- Knapen MH, Drummen NE, Smit E, Vermeer C, Theuwissen E. Three-year low-dose menaquinone-7 supplementation helps decrease bone loss in healthy postmenopausal women. Osteoporos Int. 2013;24(9):2499-2507. doi:10.1007/s00198-013-2325-6
- Jiang Y, Zhang ZL, Zhang ZL, et al. Menatetrenone versus alfacalcidol in the treatment of Chinese postmenopausal women with osteoporosis: a multicenter, randomized, double-blinded, double-dummy, positive drug-controlled clinical trial. Clin Interv Aging. 2014;9:121-127. doi:10.2147/CIA.S54107
- Rønn SH, Harsløf T, Pedersen SB, Langdahl BL. Vitamin K2 (menaquinone-7) prevents age-related deterioration of trabecular bone microarchitecture at the tibia in postmenopausal women. Europ
Endocrinol. 2016;175(6):541-549. doi:10.1530/EJE-16-0498 - Shea MK, O’Donnell CJ, Hoffmann U, et al. Vitamin K supplementation and progression of coronary artery calcium in older men and women23. Am J Clin Nutr. 2009;89(6):1799-1807. doi:10.3945/ajcn.2008.27338
- Cheung CL, Sahni S, Cheung BM, Sing CW, Wong IC. Vitamin K intake and mortality in people with chronic kidney disease from NHANES III. Clin Nutr. 2015;34(2):235-240. doi:10.1016/j.clnu.2014.03.011
- Kurnatowska I, Grzelak P, Masajtis-Zagajewska A, et al. Effect of vitamin K2 on progression of atherosclerosis and vascular calcification in nondialyzed patients with chronic kidney disease stages 3-5. Pol Arch Med Wewn. 2015;125(9):631-640. doi:10.20452/pamw. 3041
- Brandenburg VM, Reinartz S, Kaesler N, et al. Slower progress of aortic valve calcification with vitamin K supplementation. Circulation. 2017;135(21):2081-2083. doi:10.1161/CIRCULATIONAHA.116.027011
- Shea MK, Barger K, Booth SL, et al. Vitamin K status, cardiovascular disease, and all-cause mortality: a participant-level meta-analysis of 3 US cohorts. Am J Clin Nutr. 2020;111(6):1170-1177. doi:10.1093/ajcn/nqaa082
- Shea MK, Dawson-Hughes B, Gundberg CM, Booth SL. Reducing undercarboxylated osteocalcin with vitamin K supplementation does not promote lean tissue loss or fat gain over 3 years in older women and men: a randomized controlled trial. J Bone Miner Res. 2017;32 (2):243-249. doi:10.1002/jbmr. 2989
- Knapen MHJ, Jardon KM, Vermeer C. Vitamin K-induced effects on body fat and weight: results from a 3 -year vitamin K2 intervention study. Eur J Clin Nutr. 2018;72(1):136-141. doi:10.1038/ejcn.2017.146
- Aguayo-Ruiz JI, García-Cobián TA, Pascoe-González S, et al. Effect of supplementation with vitamins D3 and K2 on undercarboxylated osteocalcin and insulin serum levels in patients with type 2 diabetes mellitus: a randomized, double-blind, clinical trial. Diabetol Metab Syndr. 2020;12(1):73. doi:10.1186/s13098-020-00580-w
- Rahimi Sakak F, Moslehi N, Niroomand M, Mirmiran P. Glycemic control improvement in individuals with type 2 diabetes with vitamin K(2) supplementation: a randomized controlled trial. Eur J Nutr. 2021;60(5):2495-2506. doi:10.1007/s00394-020-02419-6
