DOI: https://doi.org/10.1038/s41522-024-00488-7
PMID: https://pubmed.ncbi.nlm.nih.gov/38402294
تاريخ النشر: 2024-02-24
كيف تمنع البروبيوتيك، والبريبايوتيك، والسينيبيوتيك، والبروستبيوتيك تسوس الأسنان: منظور ميكروبيوم الفم
الملخص
تسوس الأسنان، وهو مرض فموي شائع للغاية، يؤثر على جزء كبير من السكان العالميين. الطرق التقليدية التي تقضي بشكل عشوائي على الميكروبات تعطل التوازن الطبيعي للميكروبات الفموية. على النقيض من ذلك، تهدف استراتيجيات التدخل الحيوي إلى استعادة هذا التوازن من خلال إدخال ميكروبات مفيدة أو تثبيط تلك المسببة للتسوس. على مدى الثلاثين عامًا الماضية، حظيت التحضيرات الميكروبية باهتمام كبير في أبحاث طب الأسنان للوقاية من تسوس الأسنان وعلاجه. ومع ذلك، على عكس الأمراض ذات الصلة في الجهاز الهضمي، والمهبل، والجهاز التنفسي، يحدث تسوس الأسنان على الأنسجة الصلبة مثل مينا الأسنان ويرتبط ارتباطًا وثيقًا بالإفراط المحلي في إنتاج الأحماض الذي تسهله الأغشية الحيوية المسببة للتسوس. لذلك، فإنه من غير الكافي الاعتماد فقط على الآليات السابقة لتحديد دور التحضيرات الميكروبية في تجويف الفم. يجب أن تتضمن وجهة نظر أكثر شمولاً النظر في مفاهيم الأغشية الحيوية المسببة للتسوس. توضح هذه المراجعة أحدث تقدم في الأبحاث، وآليات العمل، والتحديات، واتجاهات البحث المستقبلية المتعلقة بالبروبيوتيك، والبربيوتيك، والسنبيوتيك، والبوستبيوتيك للوقاية من تسوس الأسنان وعلاجه، مع الأخذ في الاعتبار الآليات المرضية الفريدة لتسوس الأسنان. مع فهم معزز للميكروبات الفموية، ستظهر العلاجات الميكروبية الشخصية كاتجاه بحثي حاسم في المستقبل.
لمنع تسوس الأسنان، يجب أن تهدف الاستراتيجيات الحالية إلى كبح نمو البكتيريا المسببة للتسوس من خلال استهداف عوامل ضراوتها، مع تعزيز ميكروبيوتا سكانية متنوعة وصحية.
خلفية تسوس الأسنان
الميكروبات المرتبطة بتسوس الأسنان
في الفيلم الحيوي أحادي النوع الذي يتكون فقط من S. mutans، هناك 393 جينًا معبرًا عنه بشكل مختلف في S. mutans داخل الفيلم الحيوي ثنائي النوع.
ستربتوكوكوس موتانس
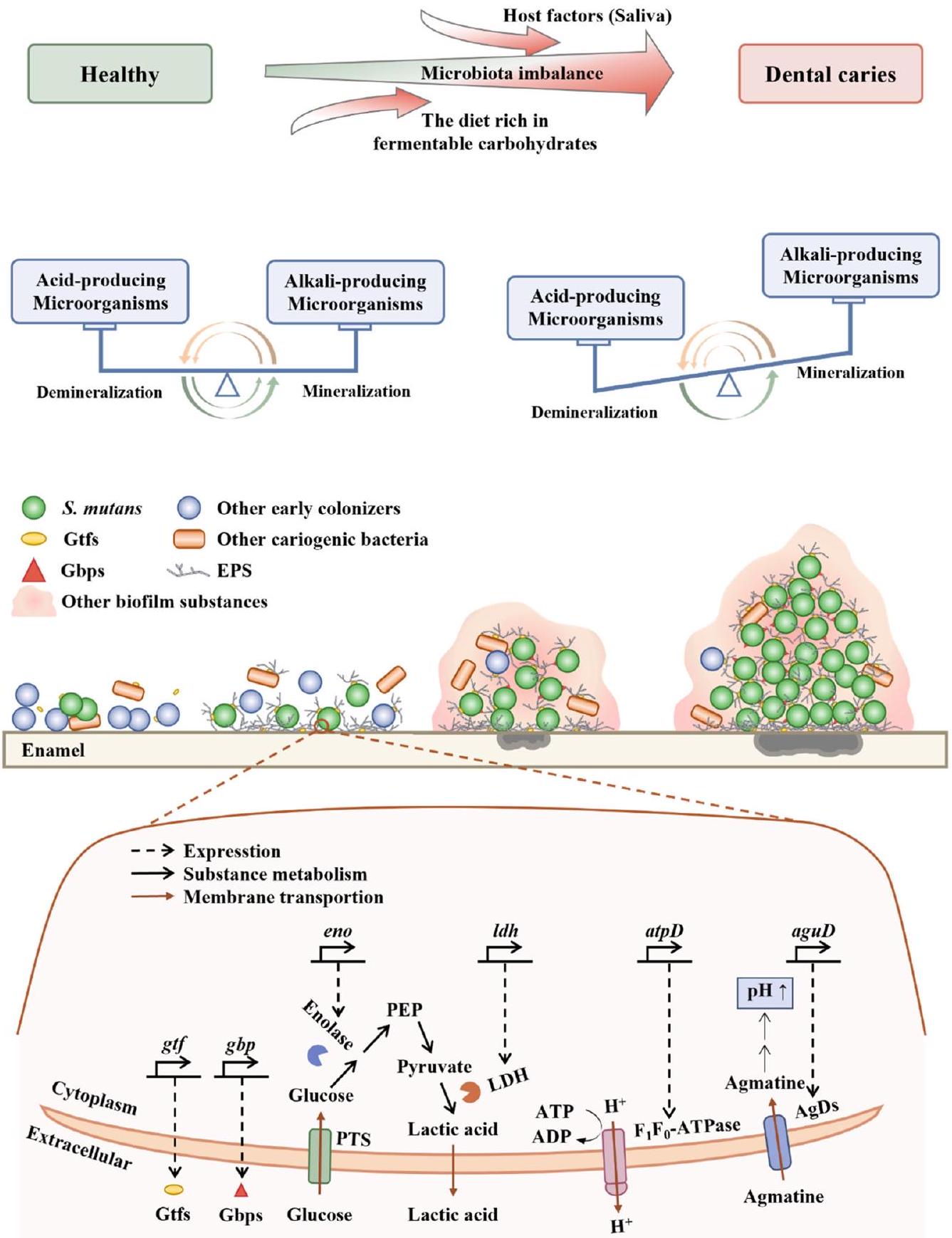
يمكن أن ينتشر بسهولة، بفضل الشحنة السلبية لـ
الميكروبات، التي تساعد في احتباس وتراكم الحمض داخل الغشاء الحيوي
تقلل الجينات بشكل كبير من شدة الضراوة لـ S. mutans، في نماذج تسوس الأسنان لدى القوارض
تدابير الوقاية من تسوس الأسنان – التدخلات البيولوجية
البكتيريا التي هي مسببات الأمراض اللثوية
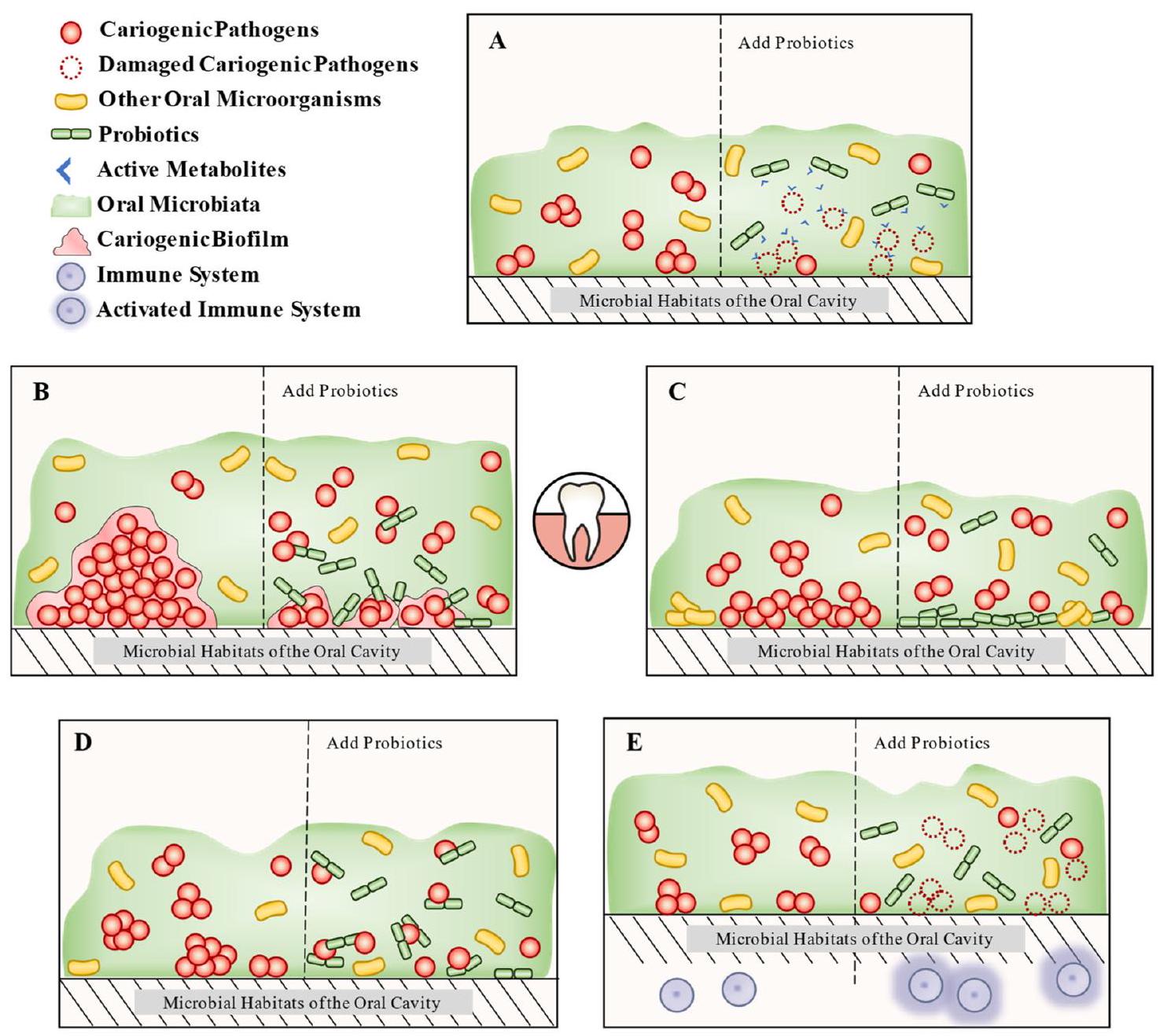
البروبيوتيك
خلفية
آليات للوقاية من تسوس الأسنان
إنتاج المستقلبات النشطة
تكوين الأغشية الحيوية وكثافة الكائنات الدقيقة، كما تم الكشف عنه في دراسة محاكاة
أشكال ممرضة. لقد تم إثبات أن
إنترلوكين-
سيارة التطبيق
جدل
تسيطر عليها الكولاجين من النوع الأول. ومع ذلك، فإن بكتيريا حمض اللبنيك، بما في ذلك
البريبايوتيك
سكر
الكحول السكري
أوليجوسكريدات
| مركبة | سلالة الاختبار | جرعة | تردد | عينة | نتيجة | مرجع |
| حليب بودرة | ل. باراكاسسي |
|
مرة واحدة يومياً لمدة 3 أشهر | 124 طفلًا تتراوح أعمارهم بين 1.5 إلى 5 سنوات | قلل من عدد S. mutans في اللعاب وأخر تطور تسوس الأسنان الجديد | 187 |
| حليب | ل. باراكاسي |
|
مرة واحدة يومياً لمدة 4 أسابيع | 30 مريضًا مصابًا بشق الشفة والحنك غير المتلازمي تم علاجهم تقويمياً بمتوسط عمر 19 عامًا | قلل من العدد
|
188 |
| زبادي | ب. حيواني |
|
مرة واحدة يوميًا لمدة أسبوعين | 49 طفلًا صحيًا تتراوح أعمارهم بين 6-12 عامًا | لم يتمكن من تقليل مستويات سلالات S. mutans و Lactobacillus في اللعاب | 189 |
| زبادي | ب. لاكتيس | غير واضح | مرة واحدة يومياً لمدة أسبوعين | 30 فردًا تتراوح أعمارهم بين 10-30 عامًا يخضعون لعلاج تقويم الأسنان | تقليل العدد الإجمالي للميكروبات في اللويحات السنية | 190 |
| زبادي | ب. لاكتيس BB12 |
|
300 جرام يوميًا لمدة أسبوعين | 66 طالبًا تتراوح أعمارهم بين 18-30 عامًا في المراحل الأولية من تسوس الأسنان | قلل عدد S. mutans و Lactobacillus في مجموعة البروبيوتيك | 191 |
| جبن | L. acidophilus NCFM أو L. rhamnosus Lr-32 (ديبونت
|
|
50 جرام يوميًا لمدة 16 أسبوعًا | 60 من مرتدي أطقم الأسنان المسنين | قلل من استعمار الكانديدا الفموية | 192 |
| جبن | ل. رهمنوسوس GG و ل. رهمنوسوس LC705 |
|
|
74 بالغًا تتراوح أعمارهم بين 18 و35 عامًا | قلل من عدد S. mutans خلال فترة ما بعد العلاج | 193 |
| جبن | اللاكتوباسيلس كاسي LAFTIL26 |
|
50 جرام مرتين يومياً لمدة أسبوعين مع وجبتي الإفطار والعشاء | 60 بالغًا بمتوسط عمر 28 | لم يتمكن من تقليل عدد S. mutans و Lactobacillus في مجموعة البروبيوتيك | 194 |
| آيس كريم | ب. لاكتيس Bb-12 و ل. أسيودوفيلوس La-5 |
|
مرة واحدة يومياً لمدة 7 أيام | 60 طفلًا صحيًا تتراوح أعمارهم بين 6-12 عامًا | قلل عدد بكتيريا S. mutans في اللعاب | 195 |
| حبوب الإفطار | L. paracasei F19 |
|
مرة واحدة يومياً لمدة 9 أشهر | 179 رضيعًا تتراوح أعمارهم بين 4 أشهر | لا تأثير على تكرار تسوس الأسنان أو المكورات العقدية المسببة للتسوس أو اللبنيّات. | 196 |
| مصاصة جديدة بإطلاق بطيء | ب. حيوانات اللبن BB-12 |
|
مرتين يوميًا لمدة عامين | 106 رضيعًا تتراوح أعمارهم بين 1-2 شهر | لا تأثير على الاستعمار الفموي لبكتيريا B. animalis lactis BB-12 والستربتوكوكوس المتحول في الإدارة المبكرة | ١٩٧ |
| علكة | L. reuteri ATCC 55730 و ATCC PTA 1 |
|
ثلاث مرات يومياً بعد الوجبات لمدة 3 أسابيع | 80 بالغًا صحيًا تتراوح أعمارهم بين 21 و 24 عامًا | قلل بشكل كبير من مستويات المكورات العقدية المسببة للتسوس في اللعاب | 198 |
| جبن قريش | ل. أسيدوفيلوس و ب. لاكتيس BB12 (مشتقات حليب الأم ب-أكتيف بلس)
|
غير واضح | مرة واحدة يومياً لمدة 7 أيام قبل الإفطار | 60 بالغًا خاليًا من التسوس تتراوح أعمارهم بين 20-25 عامًا | تحسين كبير في درجة حموضة اللعاب وتقليل عدد بكتيريا S. mutans في اللعاب | 199 |
| جبن قريش | L. acidophilus-SD 5221 (أكتيف بلس؛ نستله، تشيناي، الهند) |
|
مع غداءهم لمدة 30 يومًا | 60 مريضًا تقويم الأسنان تتراوح أعمارهم بين 14 و29 عامًا | خفضت بشكل كبير مستويات
|
٢٠٠ |
| عصير الجزر والأناناس (جيفيلوس) | ل. رهمنوسوس GG |
|
خمسة أيام في الأسبوع لمدة 7 أشهر | 530 طفلًا صحيًا تتراوح أعمارهم بين 3-6 سنوات | قلل من العدد
|
٢٠١ |
| غسول الفم (بروبيورا
|
س. أوراليس KJ3sm، س. أوبرس KJ2sm، وس. راتوس JH145 |
|
مرتين يومياً لمدة 4 أسابيع | 20 بالغين أصحاء تتراوح أعمارهم بين 21 و35 عامًا | خفضت مستويات S. mutans | ٢٠٢ |
أرجينين
اليوريا والنترات
تأثيرات النترات، فإن المزيد من التحقيقات ضرورية لاستكشاف آليات أخرى من خلالها يمكن أن تمنع النترات تسوس الأسنان.
السنبيوتيك
البوستبيوتيك
التقنيات، مثل المجال الكهربائي، والتسونيد بالموجات فوق الصوتية، والضغط العالي، والأشعة السينية، وتفريغ الكهرباء عالي الجهد، وتسخين المجال المغناطيسي، والمجال المغناطيسي المعتدل، وتكنولوجيا البلازما، متاحة أيضًا
الخاتمة وآفاق المستقبل
يمكن تحقيق علاج تسوس الأسنان. علاوة على ذلك، فإن خطط العلاج الشخصية ليست محدودة بالتحضيرات الميكروبية الفردية ويمكن استخدامها جنبًا إلى جنب مع أساليب العلاج الأخرى لتعزيز النتائج العلاجية العامة وتحقيق هدف شفاء تسوس الأسنان. تحسين الفعالية والسلامة هو اتجاه حاسم للبحوث المستقبلية. استنادًا إلى استكشاف أعمق لآليات عمل التحضيرات الميكروبية، يمكن أن يؤدي تحسين وتعديل التركيبات وفقًا لنتائج البحث إلى تعزيز التأثيرات العلاجية وسلامة التحضيرات الميكروبية.
نُشر على الإنترنت: 24 فبراير 2024
References
- Wen, P. Y. F., Chen, M. X., Zhong, Y. J., Dong, Q. Q. & Wong, H. M. Global burden and inequality of dental caries, 1990 to 2019. J. Dent. Res. 101, 392-399 (2022).
- Nomura, R. et al. Potential involvement of Streptococcus mutans possessing collagen binding protein Cnm in infective endocarditis. Sci. Rep. 10, 19118 (2020).
- Philip, N., Suneja, B. & Walsh, L. J. Ecological approaches to dental caries prevention: paradigm shift or shibboleth? Caries Res. 52, 153-165 (2018).
- Yu, O. Y., Lam, W. Y., Wong, A. W., Duangthip, D. & Chu, C. H. Nonrestorative management of dental caries. Dent. J. 9, 121 (2021).
- Marsh, P. D., Head, D. A. & Devine, D. A. Ecological approaches to oral biofilms: control without killing. Caries Res. 49, 46-54 (2015).
- Meurman, J. H., Antila, H. & Salminen, S. Recovery of Lactobacillus strain GG (ATCC 53103) from saliva of healthy volunteers after consumption of yoghurt prepared with the bacterium. Microb. Ecol. Health Dis. 7, 295-298 (1994).
- Chattopadhyay, I. et al. Can metagenomics unravel the impact of oral bacteriome in human diseases? Biotechnol. Genet. Eng. Rev. 39, 85-117 (2022).
- Achtman, M. & Zhou, Z. Metagenomics of the modern and historical human oral microbiome with phylogenetic studies on Streptococcus mutans and Streptococcus sobrinus. Philos. Trans. R. Soc. B Biol. Sci. 375, 20190573 (2020).
- Wade, W. G. The oral microbiome in health and disease. Pharmacol. Res. 69, 137-143 (2013).
- Rosier, B. T., Marsh, P. D. & Mira, A. Resilience of the oral microbiota in health: mechanisms that prevent dysbiosis. J. Dent. Res. 97, 371-380 (2018).
- Kilian, M. The oral microbiome-friend or foe? Eur. J. Oral. Sci. 126, 5-12 (2018).
- Kanasi, E. et al. Clonal analysis of the microbiota of severe early childhood caries. Caries Res. 44, 485-497 (2010).
- Hajishengallis, E., Parsaei, Y., Klein, M. I. & Koo, H. Advances in the microbial etiology and pathogenesis of early childhood caries. Mol. Oral Microbiol. 32, 24-34 (2017).
- Forssten, S. D., Bjorklund, M. & Ouwehand, A. C. Streptococcus mutans, caries and simulation models. Nutrients 2, 290-298 (2010).
- Gong, Y. et al. Global transcriptional analysis of acid-inducible genes in Streptococcus mutans: multiple two-component systems involved in acid adaptation. Microbiology 155, 3322-3332 (2009).
- Kim, D. et al. Spatial mapping of polymicrobial communities reveals a precise biogeography associated with human dental caries. Proc. Natl. Acad. Sci. USA 117, 12375-12386 (2020).
- Peres, M. A. et al. Oral diseases: a global public health challenge. Lancet 394, 249-260 (2019).
- Palmer, R. J. et al. Interbacterial adhesion networks within early oral biofilms of single human hosts. Appl. Environ. Microbiol. 83, e00407-e00417 (2017).
- Baker, J. L. et al. Deep metagenomics examines the oral microbiome during dental caries, revealing novel taxa and co-occurrences with host molecules. Genome Res. 31, 64-74 (2021).
- Liu, G., Wu, C., Abrams, W. R. & Li, Y. Structural and functional characteristics of the microbiome in deep-dentin caries. J. Dent. Res. 99, 713-720 (2020).
- Jenkinson, H. F. & Lamont, R. J. Oral microbial communities in sickness and in health. Trends Microbiol. 13, 589-595 (2005).
- Kazemtabrizi, A., Haddadi, A., Shavandi, M. & Harzandi, N. Metagenomic investigation of bacteria associated with dental lesions: a cross-sectional study. Med. Oral Patol. Oral Cir. Bucal 25, e240-e251 (2020).
- Peterson, S. N., Snesrud, E., Schork, N. J. & Bretz, W. A. Dental caries pathogenicity: a genomic and metagenomic perspective. Int. Dent. J. 61, 11-22 (2011).
- Kluytmans, J., van Belkum, A. & Verbrugh, H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 10, 505-520 (1997).
- Sivamaruthi, B. S., Kesika, P. & Chaiyasut, C. A review of the role of probiotic supplementation in dental caries. Probiotics Antimicrob. Proteins 12, 1300-1309 (2020).
- He, J. et al. RNA-Seq reveals enhanced sugar metabolism in Streptococcus mutans co-cultured with Candida albicans within mixed-species biofilms. Front. Microbiol. 8, 1036 (2017).
- Priya, A., Selvaraj, A., Divya, D., Karthik Raja, R. & Pandian, S. K. In vitro and in vivo anti-infective potential of thymol against early childhood caries causing dual species Candida albicans and Streptococcus mutans. Front. Pharmacol. 12, 760768 (2021).
- Chen, J. et al. Core microbiota promotes the development of dental caries. Appl. Sci. 11, 3638 (2021).
- Belda-Ferre, P. et al. The oral metagenome in health and disease. ISME J. 6, 46-56 (2012).
- Pang, L. et al. Metagenomic analysis of dental plaque on pit and fissure sites with and without caries among adolescents. Front. Cell. Infect. Microbiol. 11, 740981 (2021).
- Loesche, W. J. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 50, 353-380 (1986).
- Legenova, K. & Bujdakova, H. The role of Streptococcus mutans in the oral biofilm. Epidemiol. Mikrobiol. Imunol. 64, 179-187 (2015).
- Gross, E. L. et al. Bacterial 16 S sequence analysis of severe caries in young permanent teeth. J. Clin. Microbiol. 48, 4121-4128 (2010).
- Nicolas, G. G. & Lavoie, M. C. Streptococcus mutans and oral streptococci in dental plaque. Can. J. Microbiol. 57, 1-20 (2011).
- Balakrishnan, M., Simmonds, R. S. & Tagg, J. R. Dental caries is a preventable infectious disease. Aust. Dent. J. 45, 235-245 (2000).
- Bowen, W. H. Rodent model in caries research. Odontology 101, 9-14 (2013).
- Palmer, C. A. et al. Diet and caries-associated bacteria in severe early childhood caries. J. Dent. Res. 89, 1224-1229 (2010).
- Lin, Y., Chen, J., Zhou, X. & Li, Y. Inhibition of Streptococcus mutans biofilm formation by strategies targeting the metabolism of exopolysaccharides. Crit. Rev. Microbiol. 47, 667-677 (2021).
- Klein, M. I., Hwang, G., Santos, P. H. S., Campanella, O. H. & Koo, H. Streptococcus mutans-derived extracellular matrix in cariogenic oral biofilms. Front. Cell. Infect. Microbiol. 5, 10 (2015).
- Pleszczynska, M., Wiater, A., Janczarek, M. & Szczodrak, J. (1->3)-
-D-glucan hydrolases in dental biofilm prevention and control: a review. Int. J. Biol. Macromol. 79, 761-778 (2015). - Poulin, M. B. & Kuperman, L. L. Regulation of biofilm exopolysaccharide production by cyclic di-guanosine monophosphate. Front. Microbiol. 12, 730980 (2021).
- Bowen, W. H., Burne, R. A., Wu, H. & Koo, H. Oral biofilms: pathogens, matrix and polymicrobial interactions in microenvironments. Trends Microbiol. 26, 229-242 (2018).
- Alves, L. A. et al. CovR regulates Streptococcus mutans susceptibility to complement immunity and survival in blood. Infect. Immun. 84, 3206-3219 (2016).
- Goodman, S. D. et al. Biofilms can be dispersed by focusing the immune system on a common family of bacterial nucleoidassociated proteins. Mucosal Immunol. 4, 625-637 (2011).
- Xiao, J. et al. The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixedspecies oral biofilm. PLoS Pathog. 8, e1002623 (2012).
- Guo, L., McLean, J. S., Lux, R., He, X. & Shi, W. The wellcoordinated linkage between acidogenicity and aciduricity via insoluble glucans on the surface of Streptococcus mutans. Sci. Rep. 5, 18015 (2015).
- Banas, J. A. Virulence properties of Streptococcus mutans. Front. Biosci. Landmark 9, 1267-1277 (2004).
- Koo, H., Allan, R. N., Howlin, R. P., Stoodley, P. & Hall-Stoodley, L. Targeting microbial biofilms: current and prospective therapeutic strategies. Nat. Rev. Microbiol. 15, 740-755 (2017).
- Matsumi, Y. et al. Contribution of glucan-binding protein A to firm and stable biofilm formation by Streptococcus mutans. Mol. Oral Microbiol. 30, 217-226 (2015).
- Abranches, J. et al. Biology of oral streptococci. Microbiol. Spectr. 6, https://doi.org/10.1128/microbiolspec.GPP3-0042-2018 (2018).
- Xu, X., Zhou, X. D. & Wu, C. D. The tea catechin epigallocatechin gallate suppresses cariogenic virulence factors of Streptococcus mutans. Antimicrob. Agents Chemother. 55, 1229-1236 (2011).
- Ma, Q. et al. Acetylation of lactate dehydrogenase negatively regulates the acidogenicity of Streptococcus mutans. mBio 13, e0201322 (2022).
- Cotter, P. D. & Hill, C. Surviving the acid test: responses of grampositive bacteria to low pH. Microbiol. Mol. Biol. Rev. 67, 429-453 (2003).
- Liu, Y.-L., Nascimento, M. & Burne, R. A. Progress toward understanding the contribution of alkali generation in dental biofilms to inhibition of dental caries. Int. J. Oral Sci. 4, 135-140 (2012).
- Li, Y. H. & Tian, X. L. Quorum sensing and bacterial social interactions in biofilms. Sensors 12, 2519-2538 (2012).
- Matsumoto-Nakano, M. Role of Streptococcus mutans surface proteins for biofilm formation. Jpn. Dent. Sci. Rev. 54, 22-29 (2018).
- Lei, L. et al. Modulation of biofilm exopolysaccharides by the Streptococcus mutans vicX gene. Front. Microbiol. 6, 1432 (2015).
- Sadeghinejad, L. et al. Mechanistic, genomic and proteomic study on the effects of BisGMA-derived biodegradation product on cariogenic bacteria. Dent. Mater. 33, 175-190 (2017).
- Woelber, J. P., Al-Ahmad, A. & Alt, K. W. On the pathogenicity of the oral biofilm: a critical review from a biological, evolutionary, and nutritional point of view. Nutrients 14, 2174 (2022).
- Dashiff, A. & Kadouri, D. E. Predation of oral pathogens by Bdellovibrio bacteriovorus 109J. Mol. Oral Microbiol. 26, 19-34 (2011).
- Van Essche, M. et al. Killing of anaerobic pathogens by predatory bacteria. Mol. Oral Microbiol. 26, 52-61 (2011).
- Zarco, M. F., Vess, T. J. & Ginsburg, G. S. The oral microbiome in health and disease and the potential impact on personalized dental medicine. Oral Dis. 18, 109-120 (2012).
- Mercenier, A., Pavan, S. & Pot, B. Probiotics as biotherapeutic agents: present knowledge and future prospects. Curr. Pharm. Des. 9, 175 (2003).
- Hill, C. et al. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506-514 (2014).
- Saiz, P., Taveira, N. & Alves, R. Probiotics in oral health and disease: a systematic review. Appl. Sci. 11, 8070 (2021).
- Simark-Mattsson, C. et al. Lactobacillus-mediated interference of mutans streptococci in caries-free vs. caries-active subjects. Eur. J. Oral Sci. 115, 308-314 (2007).
- Inchingolo, A. D. et al. Oralbiotica/oralbiotics: the impact of oral microbiota on dental health and demineralization: a systematic review of the literature. Children 9, 1014 (2022).
- Teughels, W., Van Essche, M., Sliepen, I. & Quirynen, M. Probiotics and oral healthcare. Periodontology 48, 111-147 (2008). 2000.
- Talarico, T. L., Casas, I. A., Chung, T. C. & Dobrogosz, W. J. Production and isolation of reuterin, a growth inhibitor produced by Lactobacillus reuteri. Antimicrob. Agents Chemother. 32, 1854-1858 (1988).
- Gänzle, M. G., Höltzel, A., Walter, J., Jung, G. & Hammes, W. P. Characterization of reutericyclin produced by Lactobacillus reuteri LTH2584. Appl. Environ. Microbiol. 66, 4325-4333 (2000).
- Caglar, E. et al. Effect of yogurt with Bifidobacterium DN-173 010 on salivary mutans streptococci and lactobacilli in young adults. Acta Odontol. Scand. 63, 317-320 (2005).
- Darbandi, A. et al. Bacteriocins: properties and potential use as antimicrobials. J. Clin. Lab. Anal. 36, e24093 (2022).
- Rogers, L. A. The inhibiting effect of Streptococcus lactis on Lactobacillus bulgaricus. J. Bacteriol. 16, 321-325 (1928).
- Heng, B. C. Reluctance of medical professionals in adopting natural-cycle and minimal ovarian stimulation protocols in human clinical assisted reproduction. Reprod. Biomed. Online 15, 9-11 (2007).
- Wang, Y., Qin, Y., Zhang, Y., Wu, R. & Li, P. Antibacterial mechanism of plantaricin LPL-1, a novel class lla bacteriocin against Listeria monocytogenes. Food Control 97, 87-93 (2019).
- Surachat, K., Sangket, U., Deachamag, P. & Chotigeat, W. In silico analysis of protein toxin and bacteriocins from Lactobacillus paracasei SD1 genome and available online databases. PLoS One 12, e0183548 (2017).
- Nagao, J. et al. Lantibiotics: insight and foresight for new paradigm. J. Biosci. Bioeng. 102, 139-149 (2006).
- Yang, S.-C., Lin, C.-H., Sung, C. T. & Fang, J.-Y. Antibacterial activities of bacteriocins: application in foods and pharmaceuticals. Front. Microbiol. 5, 241 (2014).
- Jin, X., An, S., Kightlinger, W., Zhou, J. & Hong, S. H. Engineering Escherichia coli to produce and secrete colicins for rapid and selective biofilm cell killing. AIChE J. 67, e17466 (2021).
- Dobson, A., Cotter, P. D., Ross, R. P. & Hill, C. Bacteriocin production: a probiotic trait? Appl. Environ. Microbiol. 78, 1-6 (2012).
- Radaic, A. et al. Modulation of pathogenic oral biofilms towards health with nisin probiotic. J. Oral. Microbiol. 12, 1809302 (2020).
- Conrads, G., Westenberger, J., Luerkens, M. & Abdelbary, M. M. H. Isolation and bacteriocin-related typing of Streptococcus dentisani. Front. Cell Infect. Microbiol. 9, 110 (2019).
- Jaffar, N., Ishikawa, Y., Mizuno, K., Okinaga, T. & Maeda, T. Mature biofilm degradation by potential probiotics: Aggregatibacter actinomycetemcomitans versus Lactobacillus spp. PLoS One 11, e0159466 (2016).
- Walker, G. V. et al. Salivaricin E and abundant dextranase activity may contribute to the anti-cariogenic potential of the probiotic candidate Streptococcus salivarius JH. Microbiology 162, 476-486 (2016).
- Huang, X. et al. A highly arginolytic Streptococcus species that potently antagonizes Streptococcus mutans. Appl. Environ. Microbiol. 82, 2187-2201 (2016).
- Di Pierro, F., Zanvit, A., Nobili, P., Risso, P. & Fornaini, C. Cariogram outcome after 90 days of oral treatment with Streptococcus salivarius M18 in children at high risk for dental caries: results of a randomized, controlled study. Clin. Cosmet. Investig. Dent. 7, 107-113 (2015).
- Satpute, S. K. et al. Biosurfactant/s from lactobacilli species: properties, challenges and potential biomedical applications. J. Basic Microbiol. 56, 1140-1158 (2016).
- Sharma, D., & Singh Saharan, B. Simultaneous production of biosurfactants and bacteriocins by probiotic Lactobacillus casei MRTL3. Int. J. Microbiol. 2014, 698713 (2014).
- Rodrigues, L. R., Teixeira, J. A. & Oliveira, R. Low-cost fermentative medium for biosurfactant production by probiotic bacteria. Biochem. Eng. J. 32, 135-142 (2006).
- Saravanakumari, P. & Mani, K. Structural characterization of a novel xylolipid biosurfactant from Lactococcus lactis and analysis of antibacterial activity against multi-drug resistant pathogens. Bioresour. Technol. 101, 8851-8854 (2010).
- Thavasi, R., Jayalakshmi, S. & Banat, I. M. Effect of biosurfactant and fertilizer on biodegradation of crude oil by marine isolates of Bacillus megaterium, Corynebacterium kutscheri and Pseudomonas aeruginosa. Bioresour. Technol. 102, 772-778 (2011).
- Ciandrini, E. et al. Characterization of biosurfactants produced by Lactobacillus spp. and their activity against oral streptococci biofilm. Appl. Microbiol. Biotechnol. 100, 6767-6777 (2016).
- Tahmourespour, A., Salehi, R. & Kasra Kermanshahi, R. Lactobacillus acidophilus-derived biosurfactant effect on gtfB and gtfC expression level in Streptococcus mutans biofilm cells. Braz. J. Microbiol. 42, 330-339 (2011).
- Tan, Y., Leonhard, M., Moser, D. & Schneider-Stickler, B. Inhibition activity of Lactobacilli supernatant against fungal-bacterial multispecies biofilms on silicone. Microb. Pathog. 113, 197-201 (2017).
- Gudina, E. J., Teixeira, J. A. & Rodrigues, L. R. Isolation and functional characterization of a biosurfactant produced by Lactobacillus paracasei. Colloids Surf. B Biointerfaces 76, 298-304 (2010).
- Özcelik, S., Kuley, E. & Özogul, F. Formation of lactic, acetic, succinic, propionic, formic and butyric acid by lactic acid bacteria. LWT Food Sci. Technol. 73, 536-542 (2016).
- Lin, X., Chen, X., Chen, Y., Jiang, W. & Chen, H. The effect of five probiotic lactobacilli strains on the growth and biofilm formation of Streptococcus mutans. Oral Dis. 21, E128-E134 (2015).
- Bustamante, M., Oomah, B. D., Mosi-Roa, Y., Rubilar, M. & BurgosDiaz, C. Probiotics as an adjunct therapy for the treatment of halitosis, dental caries and periodontitis.Probiotics Antimicrob. Proteins 12, 325-334 (2020).
- Redanz, S. et al. Live and let die: hydrogen peroxide production by the commensal flora and its role in maintaining a symbiotic microbiome. Mol. Oral Microbiol. 33, 337-352 (2018).
- Herrero, E. R. et al. Antimicrobial effects of commensal oral species are regulated by environmental factors. J. Dent. 47, 23-33 (2016).
- El Oirdi, S. et al. Isolation and identification of Lactobacillus plantarum 4F, a strain with high antifungal activity, fungicidal effect, and biopreservation properties of food. J. Food Process. Preserv. 45, e15517 (2021).
- Lai, W.-K. et al. Developing lactic acid bacteria as an oral healthy food. Life 11, 268 (2021).
- Barzegari, A. et al. The battle of probiotics and their derivatives against biofilms. Infect. Drug Resist. 13, 659-672 (2020).
- Wasfi, R., Abd El-Rahman, O. A., Zafer, M. M. & Ashour, H. M. Probiotic Lactobacillus sp. inhibit growth, biofilm formation and gene expression of caries-inducing Streptococcus mutans. J. Cell. Mol. Med. 22, 1972-1983 (2018).
- Matsubara, V. H., Wang, Y., Bandara, H. M. H. N., Mayer, M. P. A. & Samaranayake, L. P. Probiotic lactobacilli inhibit early stages of Candida albicans biofilm development by reducing their growth, cell adhesion, and filamentation. Appl. Microbiol. Biotechnol. 100, 6415-6426 (2016).
- James, K. M., MacDonald, K. W., Chanyi, R. M., Cadieux, P. A. & Burton, J. P. Inhibition of Candida albicans biofilm formation and modulation of gene expression by probiotic cells and supernatant. J. Med. Microbiol. 65, 328-336 (2016).
- Cortes-Acha, B. et al. Development and viability of biofilms grown on experimental abutments mimicking dental implants: an in vivo model. Med. Oral Patol. Oral. Cir. Bucal 24, e511-e517 (2019).
- Jung, H.-Y. et al. Collagen peptide in a combinatorial treatment with Lactobacillus rhamnosus inhibits the cariogenic properties of Streptococcus mutans: an in vitro study. Int. J. Mol. Sci. 23, 1860 (2022).
- Lin, T.-H., Lin, C.-H. & Pan, T.-M. The implication of probiotics in the prevention of dental caries. Appl. Microbiol. Biotechnol. 102, 577-586 (2018).
- Singh, T. P., Kaur, G., Kapila, S. & Malik, R. K. Antagonistic activity of Lactobacillus reuteri strains on the adhesion characteristics of selected pathogens. Front. Microbiol. 8, 486 (2017).
- Burton, J. P. et al. Influence of the probiotic Streptococcus salivarius strain M18 on indices of dental health in children: a randomized double-blind, placebo-controlled trial. J. Med. Microbiol. 62, 875-884 (2013).
- Ha Kim, J., Jang, H. J., Lee, N.-K. & Paik, H.-D. Antibacterial and antibiofilm effect of cell-free supernatant of Lactobacillus brevis KCCM 202399 isolated from korean fermented food against Streptococcus mutans KCTC 5458. J. Microbiol. Biotechnol. 32, 56-63 (2022).
- Haukioja, A., Loimaranta, V. & Tenovuo, J. Probiotic bacteria affect the composition of salivary pellicle and streptococcal adhesion in vitro. Oral Microbiol. Immunol. 23, 336-343 (2008).
- Tenovuo, J. Antimicrobial function of human saliva-how important is it for oral health? Acta Odontol. Scand. 56, 250-256 (1998).
- Boris, S., Suárez, J. E. & Barbés, C. Characterization of the aggregation promoting factor from Lactobacillus gasseri, a vaginal isolate. J. Appl. Microbiol. 83, 413-420 (1997).
- Lang, C. et al. Specific Lactobacillus/mutans Streptococcus coaggregation. J. Dent. Res. 89, 175-179 (2010).
- Sliepen, I. et al. Microbial interactions influence inflammatory host cell responses. J. Dent. Res. 88, 1026-1030 (2009).
- Wattanarat, O. et al. Significant elevation of salivary human neutrophil peptides 1-3 levels by probiotic milk in preschool children with severe early childhood caries: a randomized controlled trial. Clin. Oral Investig. 25, 2891-2903 (2021).
- Pahumunto, N., Sophatha, B., Piwat, S. & Teanpaisan, R. Increasing salivary IgA and reducing Streptococcus mutans by probiotic Lactobacillus paracasei SD1: a double-blind, randomized, controlled study. J. Dent. Sci. 14, 178-184 (2019).
- Balzaretti, S. et al. A novel rhamnose-rich hetero-exopolysaccharide isolated from Lactobacillus paracasei DG activates THP-1 human monocytic cells. Appl. Environ. Microbiol. 83, e02702-e02716 (2017).
- Amargianitakis, M., Antoniadou, M., Rahiotis, C. & Varzakas, T. Probiotics, prebiotics, synbiotics and dental caries. new perspectives, suggestions, and patient coaching approach for a cavity-free mouth. Appl. Sci. 11, 5472 (2021).
- Nadelman, P., Magno, M. B., Masterson, D., da Cruz, A. G. & Maia, L. C. Are dairy products containing probiotics beneficial for oral health? a systematic review and meta-analysis. Clin. Oral Investig. 22, 2763-2785 (2018).
- Gedalia, I. et al. Enamel softening with Coca-Cola and rehardening with milk or saliva. Am. J. Dent. 4, 120-122 (1991).
- Kashket, S. & Yaskell, T. Effectiveness of calcium lactate added to food in reducing intraoral demineralization of enamel. Caries Res. 31, 429-433 (1997).
- Schüpbach, P., Neeser, J. R., Golliard, M., Rouvet, M. & Guggenheim, B. Incorporation of caseinoglycomacropeptide and caseinophosphopeptide into the salivary pellicle inhibits adherence of mutans streptococci. J. Dent. Res. 75, 1779-1788 (1996).
- Swarna, S. K. & Nivedhitha, M. S. Probiotics in prevention of dental caries-a literature review. Biosci. Biotechnol. Res. Commun. 13, 517-526 (2020).
- de Alvarenga, J. A. et al. Probiotic effects of lactobacillus paracasei 28.4 to inhibit Streptococcus mutans in a gellan-based formulation. Probiotics Antimicrob. Proteins 13, 506-517 (2021).
- Yelin, I. et al. Genomic and epidemiological evidence of bacterial transmission from probiotic capsule to blood in ICU patients. Nat. Med. 25, 1728-1732 (2019).
- Gruner, D., Paris, S. & Schwendicke, F. Probiotics for managing caries and periodontitis: systematic review and meta-analysis. J. Dent. 48, 16-25 (2016).
- Corby, P. M. et al. Microbial risk indicators of early childhood caries. J. Clin. Microbiol. 43, 5753-5759 (2005).
- Wen, Z. T., Huang, X., Ellepola, K., Liao, S. & Li, Y. Lactobacilli and human dental caries: more than mechanical retention. Microbiology 168, 001196 (2022).
- Henne, K., Rheinberg, A., Melzer-Krick, B. & Conrads, G. Aciduric microbial taxa including Scardovia wiggsiae and Bifidobacterium spp. in caries and caries free subjects. Anaerobe 35, 60-65 (2015).
- Caufield, P. W., Schön, C. N., Saraithong, P., Li, Y. & Argimón, S. Oral lactobacilli and dental caries: a model for niche adaptation in humans. J. Dent. Res. 94, 110S-118S (2015).
- Newhouse, M. T. & Dolovich, M. Spacer devices for asthma. J. Pediatr. 109, 913-914 (1986).
- Gibson, G. R. & Roberfroid, M. B. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 125, 1401-1412 (1995).
- Gibson, G. R. et al. Expert consensus document: the international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 14, 491-502 (2017).
- Guerrero-Wyss, M., Durán Agüero, S. & Angarita Dávila, L. D-tagatose is a promising sweetener to control glycaemia: a new functional food. Biomed. Res. Int. 2018, e8718053 (2018).
- Mayumi, S. et al. Potential of prebiotic D-tagatose for prevention of oral disease. Front. Cell Infect. Microbiol. 11, 767944 (2021).
- Nagamine, Y. et al. D-tagatose effectively reduces the number of Streptococcus mutans and oral bacteria in healthy adult subjects: a chewing gum pilot study and randomized clinical trial. Acta Med. Okayama 74, 307-317 (2020).
- Kojima, Y., Ohshima, T., Seneviratne, C. J. & Maeda, N. Combining prebiotics and probiotics to develop novel synbiotics that suppress oral pathogens. J. Oral Biosci. 58, 27-32 (2016).
- Söderling, E. & Pienihäkkinen, K. Effects of xylitol and erythritol consumption on mutans streptococci and the oral microbiota: a systematic review. Acta Odontol. Scand. 78, 599-608 (2020).
- Gibson, G. R., Probert, H. M., Loo, J. V., Rastall, R. A. & Roberfroid, M. B. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr. Res. Rev. 17, 259-275 (2004).
- Roberfroid, M. et al. Prebiotic effects: metabolic and health benefits. Br. J. Nutr. 104, S1-S63 (2010).
- Cocco, F. et al. The caries preventive effect of 1 -year use of low-dose xylitol chewing gum. a randomized placebo-controlled clinical trial in high-caries-risk adults. Clin. Oral Investig. 21, 2733-2740 (2017).
- Söderling, E., Alaräisänen, L., Scheinin, A. & Mäkinen, K. K. Effect of xylitol and sorbitol on polysaccharide production by and adhesive properties of Streptococcus mutans. Caries Res. 21, 109-116 (1987).
- Watthanasaen, S. et al. Xylitol-containing chewing gum for caries prevention in students with disabilities: a randomised trial. Oral Health Prev. Dent. 15, 519-527 (2017).
- Gauthier, L., Vadeboncoeur, C. & Mayrand, D. Loss of sensitivity to xylitol by Streptococcus mutans LG-1. Caries Res. 18, 289-295 (1984).
- Falony, G. et al. Long-term effect of erythritol on dental caries development during childhood: a posttreatment survival analysis. Caries Res. 50, 579-588 (2016).
- Thabuis, C. et al. Effects of maltitol and xylitol chewing-gums on parameters involved in dental caries development. Eur. J. Paediatr. Dent. 14, 303-308 (2013).
- Salli, K., Söderling, E., Hirvonen, J., Gürsoy, U. K. & Ouwehand, A. C. Influence of
-fucosyllactose and galacto-oligosaccharides on the growth and adhesion of Streptococcus mutans. Br. J. Nutr. 124, 824-831 (2020). - Sharon, N. Carbohydrates as future anti-adhesion drugs for infectious diseases. Biochim. Biophys. Acta 1760, 527-537 (2006).
- Oku, T. & Nakamura, S. Threshold for transitory diarrhea induced by ingestion of xylitol and lactitol in young male and female adults. J. Nutr. Sci. Vitaminol. 53, 13-20 (2007).
- Koopman, J. E. et al. Stability and resilience of oral microcosms toward acidification and Candida outgrowth by arginine supplementation. Microb. Ecol. 69, 422-433 (2015).
- Bacali, C. et al. Oral microbiome: getting to know and befriend neighbors, a biological approach. Biomedicines 10, 671 (2022).
- Zheng, X. et al. Ecological effect of arginine on oral microbiota. Sci. Rep. 7, 7206 (2017).
- He, J. et al. L-arginine modifies the exopolysaccharide matrix and thwarts Streptococcus mutans outgrowth within mixed-species oral biofilms. J. Bacteriol. 198, 2651-2661 (2016).
- Koopman, J. E. et al. Changes in the oral ecosystem induced by the use of 8% arginine toothpaste. Arch. Oral Biol. 73, 79-87 (2017).
- Yin, W. et al. The anti-caries efficacy of a dentifrice containing
arginine and 1450 ppm fluoride as sodium monofluorophosphate assessed using quantitative light-induced fluorescence (QLF). J. Dent. 41, S22-S28 (2013). - Bijle, M. N. A., Ekambaram, M., Lo, E. C. & Yiu, C. K. Y. The combined enamel remineralization potential of arginine and fluoride toothpaste. J. Dent. 76, 75-82 (2018).
- Carda-Diéguez, M., Moazzez, R. & Mira, A. Functional changes in the oral microbiome after use of fluoride and arginine containing dentifrices: a metagenomic and metatranscriptomic study. Microbiome 10, 159 (2022).
- Cheng, X . et al. Magnesium-dependent promotion of
production increases ecological competitiveness of oral commensal streptococci. J. Dent. Res. 99, 847-854 (2020). - Burne, R. A. & Marquis, R. E. Alkali production by oral bacteria and protection against dental caries. FEMS Microbiol. Lett. 193, 1-6 (2000).
- Zaura, E. & Twetman, S. Critical appraisal of oral pre- and probiotics for caries prevention and care. Caries Res. 53, 514-526 (2019).
- Sánchez, G. A., Miozza, V. A., Delgado, A. & Busch, L. Total salivary nitrates and nitrites in oral health and periodontal disease. Nitric Oxide 36, 31-35 (2014).
- Doel, J. J. et al. Protective effect of salivary nitrate and microbial nitrate reductase activity against caries. Eur. J. Oral Sci. 112, 424-428 (2004).
- Green, S. J. Nitric oxide in mucosal immunity. Nat. Med. 1, 515-517 (1995).
- Allaker, R. P., Silva Mendez, L. S., Hardie, J. M. & Benjamin, N. Antimicrobial effect of acidified nitrite on periodontal bacteria. Oral Microbiol. Immunol. 16, 253-256 (2001).
- Rosier, B. T., Buetas, E., Moya-Gonzalvez, E. M., Artacho, A. & Mira, A. Nitrate as a potential prebiotic for the oral microbiome. Sci. Rep. 10, 12895 (2020).
- Li, H. et al. Salivary nitrate-an ecological factor in reducing oral acidity. Oral Microbiol. Immunol. 22, 67-71 (2007).
- Jockel-Schneider, Y. et al. Stimulation of the nitrate-nitrite-NOmetabolism by repeated lettuce juice consumption decreases gingival inflammation in periodontal recall patients: a randomized, double-blinded, placebo-controlled clinical trial. J. Clin. Periodontol. 43, 603-608 (2016).
- Gee, L. C. & Ahluwalia, A. Dietary nitrate lowers blood pressure: epidemiological, pre-clinical experimental and clinical trial evidence. Curr. Hypertens. Rep. 18, 17 (2016).
- Vanhatalo, A. et al. Nitrate-responsive oral microbiome modulates nitric oxide homeostasis and blood pressure in humans. Free Radic. Biol. Med. 124, 21-30 (2018).
- Velmurugan, S. et al. Dietary nitrate improves vascular function in patients with hypercholesterolemia: a randomized, double-blind, placebo-controlled study. Am. J. Clin. Nutr. 103, 25-38 (2015).
- Markowiak, P. & Śliżewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 9, 1021 (2017).
- Swanson, K. S. et al. The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 17, 687-701 (2020).
- Nunpan, S., Suwannachart, C. & Wayakanon, K. Effect of prebioticsenhanced probiotics on the growth of Streptococcus mutans. Int. J. Microbiol. 2019, 4623807 (2019).
- Tester, R. & AI-Ghazzewi, F. A preliminary study of the synbiotic effects of konjac glucomannan hydrolysates (GMH) and lactobacilli on the growth of the oral bacterium Streptococcus mutans. Nutr. Food Sci. 41, 234-237 (2011).
- Bijle, M. N., Neelakantan, P., Ekambaram, M., Lo, E. C. M. & Yiu, C. K. Y. Effect of a novel synbiotic on Streptococcus mutans. Sci. Rep. 10, 7951 (2020).
- Salminen, S. et al. The international scientific association of probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 18, 649-667 (2021).
- Barros, C. P. et al. Paraprobiotics and postbiotics: concepts and potential applications in dairy products. Curr. Opin. Food Sci. 32, 1-8 (2020).
- Moradi, M. et al. Postbiotics produced by lactic acid bacteria: the next frontier in food safety. Compr. Rev. Food Sci. Food Saf. 19, 3390-3415 (2020).
- Holz, C. et al. Lactobacillus paracasei DSMZ16671 reduces mutans Streptococci: a short-term pilot study. Probiotics Antimicrob. Proteins 5, 259-263 (2013).
- Moradi, M., Molaei, R. & Guimarães, J. T. A review on preparation and chemical analysis of postbiotics from lactic acid bacteria. Enzym. Microb. Technol. 143, 109722 (2021).
- el-Nezami, H., Kankaanpää, P., Salminen, S. & Ahokas, J. Physicochemical alterations enhance the ability of dairy strains of lactic acid bacteria to remove aflatoxin from contaminated media. J. Food Prot. 61, 466-468 (1998).
- Schwendicke, F., Horb, K., Kneist, S., Dörfer, C. & Paris, S. Effects of heat-inactivated Bifidobacterium BB12 on cariogenicity of Streptococcus mutans in vitro. Arch. Oral Biol. 59, 1384-1390 (2014).
- Tareb, R., Bernardeau, M., Gueguen, M. & Vernoux, J.-P. In vitro characterization of aggregation and adhesion properties of viable and heat-killed forms of two probiotic Lactobacillus strains and interaction with foodborne zoonotic bacteria, especially Campylobacter jejuni. J. Med. Microbiol. 62, 637-649 (2013).
- Pahumunto, N. et al. Reducing mutans streptococci and caries development by Lactobacillus paracasei SD1 in preschool children: a randomized placebo-controlled trial. Acta Odontol. Scand. 76, 331-337 (2018).
- Ritthagol, W., Saetang, C. & Teanpaisan, R. Effect of probiotics containing Lactobacillus paracasei SD1 on salivary mutans streptococci and lactobacilli in orthodontic cleft patients: a doubleblinded, randomized, placebo-controlled study. Cleft Palate Craniofac. J. 51, 257-263 (2014).
- Nozari, A., Motamedifar, M., Seifi, N., Hatamizargaran, Z. & Ranjbar, M. A. The effect of Iranian customary used probiotic yogurt on the children’s salivary cariogenic microflora. J. Dent. 16, 81-86 (2015).
- Pinto, G. S., Cenci, M. S., Azevedo, M. S., Epifanio, M. & Jones, M. H. Effect of yogurt containing Bifidobacterium animalis subsp. lactis DN-173010 probiotic on dental plaque and saliva in orthodontic patients. Caries Res. 48, 63-68 (2014).
- Zare Javid, A. et al. Effects of the consumption of probiotic yogurt containing Bifidobacterium lactis Bb12 on the levels of Streptococcus mutans and lactobacilli in saliva of students with initial stages of dental caries: a double-blind randomized controlled trial. Caries Res. 54, 68-74 (2020).
- Miyazima, T., Ishikawa, K., Mayer, M., Saad, S. & Nakamae, A. Cheese supplemented with probiotics reduced the Candida levels in denture wearers-RCT. Oral Dis. 23, 919-925 (2017).
- Ahola, A. J. et al. Short-term consumption of probiotic-containing cheese and its effect on dental caries risk factors. Arch. Oral Biol. 47, 799-804 (2002).
- Mortazavi, S. & Akhlaghi, N. Salivary Streptococcus mutans and Lactobacilli levels following probiotic cheese consumption in adults: a double blind randomized clinical trial*. J. Res. Med. Sci. 17, 57-66 (2012).
- Ashwin, D. et al. Effect of probiotic containing ice-cream on salivary mutans streptococci (SMS) levels in children of 6-12 years of age: a randomized controlled double blind study with six-months follow up. J. Clin. Diagn. Res. 9, ZC06-ZC09 (2015).
- Hasslof, P., West, C. E., Videhult, F. K., Brandelius, C. & StecksenBlicks, C. Early intervention with probiotic Lactobacillus paracasei F19 has no long-term effect on caries experience. Caries Res. 47, 559-565 (2013).
- Taipale, T., Pienihakkinen, K., Salminen, S., Jokela, J. & Soderling, E. Bifidobacterium animalis subsp. lactis BB-12 administration in early childhood: a randomized clinical trial of effects on oral colonization by mutans streptococci and the probiotic. Caries Res. 46, 69-77 (2012).
- Caglar, E. et al. Effect of chewing gums containing xylitol or probiotic bacteria on salivary mutans streptococci and lactobacilli. Clin. Oral Investig. 11, 425-429 (2007).
- Srivastava, S., Saha, S., Kumari, M. & Mohd, S. Effect of probiotic curd on salivary pH and Streptococcus mutans: a double blind parallel randomized controlled trial. J. Clin. Diagn. Res. 10, ZC13-ZC16 (2016).
- Jose, J. E., Padmanabhan, S. & Chitharanjan, A. B. Systemic consumption of probiotic curd and use of probiotic toothpaste to reduce Streptococcus mutans in plaque around orthodontic brackets. Am. J. Orthod. Dentofac. Orthop. 144, 67-72 (2013).
- Pohjavuori, S. et al. Effect of consumption of Lactobacillus rhamnosus GG and calcium, in carrot-pineapple juice on dental caries risk in children. Int. J. Probiotics Prebiotics 5, 221-228 (2010).
- Zahradnik, R. T. et al. Preliminary assessment of safety and effectiveness in humans of ProBiora
, a probiotic mouthwash. J. Appl. Microbiol. 107, 682-690 (2009).
شكر وتقدير
مساهمات المؤلفين
المصالح المتنافسة
معلومات إضافية
http://www.nature.com/reprints
© المؤلف(ون) 2024
- ¹مختبر قوانغدونغ الإقليمي الرئيسي لعلم الأحياء البحرية، قسم الأحياء، كلية العلوم، جامعة شانتو، شانتو 515063 قوانغدونغ، جمهورية الصين الشعبية.
معهد الأبحاث للغذاء المستقبلي، قسم علوم الغذاء والتغذية، جامعة بوليتكنك هونغ كونغ، هونغ كونغ، جمهورية الصين الشعبية. كلية علوم الحياة، الجامعة الصينية في هونغ كونغ، شاتين، المناطق الجديدة، هونغ كونغ، جمهورية الصين الشعبية. البريد الإلكتروني: bbzhang@stu.edu.cn
DOI: https://doi.org/10.1038/s41522-024-00488-7
PMID: https://pubmed.ncbi.nlm.nih.gov/38402294
Publication Date: 2024-02-24
How probiotics, prebiotics, synbiotics, and postbiotics prevent dental caries: an oral microbiota perspective
Abstract
Dental caries, a highly prevalent oral disease, impacts a significant portion of the global population. Conventional approaches that indiscriminately eradicate microbes disrupt the natural equilibrium of the oral microbiota. In contrast, biointervention strategies aim to restore this balance by introducing beneficial microorganisms or inhibiting cariogenic ones. Over the past three decades, microbial preparations have garnered considerable attention in dental research for the prevention and treatment of dental caries. However, unlike related pathologies in the gastrointestinal, vaginal, and respiratory tracts, dental caries occurs on hard tissues such as tooth enamel and is closely associated with localized acid overproduction facilitated by cariogenic biofilms. Therefore, it is insufficient to rely solely on previous mechanisms to delineate the role of microbial preparations in the oral cavity. A more comprehensive perspective should involve considering the concepts of cariogenic biofilms. This review elucidates the latest research progress, mechanisms of action, challenges, and future research directions regarding probiotics, prebiotics, synbiotics, and postbiotics for the prevention and treatment of dental caries, taking into account the unique pathogenic mechanisms of dental caries. With an enhanced understanding of oral microbiota, personalized microbial therapy will emerge as a critical future research trend.
prevent dental caries, current strategies should aim to suppress the overgrowth of specific cariogenic bacteria by targeting their virulence factors, while also promoting a diverse and healthy resident microbiota
Dental caries Background
Microorganisms associated with dental caries
to the mono-species biofilm comprising solely S. mutans, there are 393 differentially expressed genes in S. mutans within the dual-species biofilm
Streptococcus mutans

can easily diffuse, facilitated by the negative charge of
microorganisms, aiding in the retention and accumulation of acid within the biofilm
genes significantly reduces the virulence of S. mutans, in rodent caries models
Dental caries prevention measures-biological interventions
bacteria that are periodontal pathogens
Probiotics
Background
Mechanisms to prevent dental caries
Production of active metabolites

formation of biofilm and the density of microorganisms, as revealed in a simulation study
pathogenic forms. It has been demonstrated that
of interleukin-
The application vehicle
Controversy
dominated by type I collagen. However, lactic acid bacteria, including
Prebiotics
Sugar
Sugar alcohol
Oligosaccharides
| Vehicle | Test strain | Dose | Frequency | Sample | Result | Reference |
| Milk powder | L. paracasssei |
|
once daily for 3 months | 124 children aged 1.5-5 | reduced the count of S. mutans in saliva and delayed the development of new dental caries | 187 |
| Milk | L. paracasei |
|
once daily for 4 weeks | 30 orthodontically treated nonsyndromic cleft lip and palate patients with a mean age of 19 | reduced the count of
|
188 |
| Yogurt | B. animalis |
|
once daily for 2 weeks | 49 healthy children aged 6-12 | could not reduce the levels of salivary S. mutans and Lactobacillus | 189 |
| Yogurt | B. lactis | unclear | once daily for 2 weeks | 30 individuals aged 10-30 undergoing orthodontic treatment | reduced total microbial counts in dental plaque | 190 |
| Yogurt | B. lactis BB12 |
|
300 g daily for 2 weeks | 66 students aged 18-30 with initial stages of dental caries | reduced the count of S. mutans and Lactobacillus in the probiotic group | 191 |
| Cheese | L. acidophilus NCFM or L. rhamnosus Lr-32 (DuPont
|
|
50 g daily for 16 weeks | 60 elderly denture wearers | reduced the colonization of oral Candida | 192 |
| Cheese | L. rhamnosus GG and L. rhamnosus LC705 |
|
|
74 adults aged 18-35 | reduced the count of S. mutans during the posttreatment period | 193 |
| Cheese | L. casei LAFTIL26 |
|
50 g twice daily for 2 weeks with breakfast and dinner meals | 60 adults with a mean age of 28 | could not reduce the count of S. mutans and Lactobacillus in the probiotic group | 194 |
| Ice cream | B. lactis Bb-12 and L. acidophilus La-5 |
|
once daily for 7 days | 60 healthy children aged 6-12 | reduced the count of salivary S. mutans | 195 |
| Cereal | L. paracasei F19 |
|
once daily for 9 months | 179 infants aged 4 months | no impact on the frequency of dental caries, mutans streptococci, or lactobacilli | 196 |
| Novel slow-release pacifier | B. animalis lactis BB-12 |
|
twice daily for 2 years | 106 infants aged 1-2 months | no impact on the oral colonization of B. animalis lactis BB-12 and mutans streptococci in the early administration | 197 |
| Chewing gum | L. reuteri ATCC 55730 and ATCC PTA 1 |
|
three times daily after meals for 3 weeks | 80 healthy adults aged 21-24 | significantly reduced the levels of salivary mutans streptococci | 198 |
| Curd | L. acidophilus and B. lactis BB12 (Mother dairy b-activ Plus
|
unclear | once daily for 7 days before breakfast | 60 caries-free adults aged 20-25 | Significantly improved salivary pH and reduced the count of salivary S. mutans | 199 |
| Curd | L. acidophilus-SD 5221 (Active Plus; Nestle, Chennai, India) |
|
with their lunch for 30 days | 60 orthodontic patients aged 14-29 | significantly reduced the levels of
|
200 |
| Carrot-pineapple juice (Gefilus’) | L. rhamnosus GG |
|
five times a week for 7 months | 530 healthy children aged 3-6 | reduced the count of
|
201 |
| Mouthwash (ProBiora
|
S. oralis KJ3sm, S. uberis KJ2sm, and S. rattus JH145 |
|
twice daily for 4 weeks | 20 healthy adults aged 21-35 | reduced the levels of S. mutans | 202 |
Arginine
Urea and nitrates
effects of nitrate, further investigation is warranted to explore other mechanisms through which nitrate can prevent dental caries.
Synbiotics
Postbiotics
technologies, such as electric field, ultrasonication, high pressure, X-rays, high voltage electrical discharge, magnetic field heating, moderate magnetic field, and plasma technology, are also available
Conclusion and future perspectives
treatment of dental caries can be achieved. Furthermore, personalized treatment schemes are not limited to single microbial preparations and can be used alongside other treatment modalities to enhance the overall therapeutic outcomes and achieve the goal of curing dental caries. Improving efficacy and safety is a critical direction for future research. Based on a further exploration of the mechanisms of action of microbial preparations, refining and optimizing formulations according to research findings can enhance the therapeutic effects and safety of microbial preparations.
Published online: 24 February 2024
References
- Wen, P. Y. F., Chen, M. X., Zhong, Y. J., Dong, Q. Q. & Wong, H. M. Global burden and inequality of dental caries, 1990 to 2019. J. Dent. Res. 101, 392-399 (2022).
- Nomura, R. et al. Potential involvement of Streptococcus mutans possessing collagen binding protein Cnm in infective endocarditis. Sci. Rep. 10, 19118 (2020).
- Philip, N., Suneja, B. & Walsh, L. J. Ecological approaches to dental caries prevention: paradigm shift or shibboleth? Caries Res. 52, 153-165 (2018).
- Yu, O. Y., Lam, W. Y., Wong, A. W., Duangthip, D. & Chu, C. H. Nonrestorative management of dental caries. Dent. J. 9, 121 (2021).
- Marsh, P. D., Head, D. A. & Devine, D. A. Ecological approaches to oral biofilms: control without killing. Caries Res. 49, 46-54 (2015).
- Meurman, J. H., Antila, H. & Salminen, S. Recovery of Lactobacillus strain GG (ATCC 53103) from saliva of healthy volunteers after consumption of yoghurt prepared with the bacterium. Microb. Ecol. Health Dis. 7, 295-298 (1994).
- Chattopadhyay, I. et al. Can metagenomics unravel the impact of oral bacteriome in human diseases? Biotechnol. Genet. Eng. Rev. 39, 85-117 (2022).
- Achtman, M. & Zhou, Z. Metagenomics of the modern and historical human oral microbiome with phylogenetic studies on Streptococcus mutans and Streptococcus sobrinus. Philos. Trans. R. Soc. B Biol. Sci. 375, 20190573 (2020).
- Wade, W. G. The oral microbiome in health and disease. Pharmacol. Res. 69, 137-143 (2013).
- Rosier, B. T., Marsh, P. D. & Mira, A. Resilience of the oral microbiota in health: mechanisms that prevent dysbiosis. J. Dent. Res. 97, 371-380 (2018).
- Kilian, M. The oral microbiome-friend or foe? Eur. J. Oral. Sci. 126, 5-12 (2018).
- Kanasi, E. et al. Clonal analysis of the microbiota of severe early childhood caries. Caries Res. 44, 485-497 (2010).
- Hajishengallis, E., Parsaei, Y., Klein, M. I. & Koo, H. Advances in the microbial etiology and pathogenesis of early childhood caries. Mol. Oral Microbiol. 32, 24-34 (2017).
- Forssten, S. D., Bjorklund, M. & Ouwehand, A. C. Streptococcus mutans, caries and simulation models. Nutrients 2, 290-298 (2010).
- Gong, Y. et al. Global transcriptional analysis of acid-inducible genes in Streptococcus mutans: multiple two-component systems involved in acid adaptation. Microbiology 155, 3322-3332 (2009).
- Kim, D. et al. Spatial mapping of polymicrobial communities reveals a precise biogeography associated with human dental caries. Proc. Natl. Acad. Sci. USA 117, 12375-12386 (2020).
- Peres, M. A. et al. Oral diseases: a global public health challenge. Lancet 394, 249-260 (2019).
- Palmer, R. J. et al. Interbacterial adhesion networks within early oral biofilms of single human hosts. Appl. Environ. Microbiol. 83, e00407-e00417 (2017).
- Baker, J. L. et al. Deep metagenomics examines the oral microbiome during dental caries, revealing novel taxa and co-occurrences with host molecules. Genome Res. 31, 64-74 (2021).
- Liu, G., Wu, C., Abrams, W. R. & Li, Y. Structural and functional characteristics of the microbiome in deep-dentin caries. J. Dent. Res. 99, 713-720 (2020).
- Jenkinson, H. F. & Lamont, R. J. Oral microbial communities in sickness and in health. Trends Microbiol. 13, 589-595 (2005).
- Kazemtabrizi, A., Haddadi, A., Shavandi, M. & Harzandi, N. Metagenomic investigation of bacteria associated with dental lesions: a cross-sectional study. Med. Oral Patol. Oral Cir. Bucal 25, e240-e251 (2020).
- Peterson, S. N., Snesrud, E., Schork, N. J. & Bretz, W. A. Dental caries pathogenicity: a genomic and metagenomic perspective. Int. Dent. J. 61, 11-22 (2011).
- Kluytmans, J., van Belkum, A. & Verbrugh, H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 10, 505-520 (1997).
- Sivamaruthi, B. S., Kesika, P. & Chaiyasut, C. A review of the role of probiotic supplementation in dental caries. Probiotics Antimicrob. Proteins 12, 1300-1309 (2020).
- He, J. et al. RNA-Seq reveals enhanced sugar metabolism in Streptococcus mutans co-cultured with Candida albicans within mixed-species biofilms. Front. Microbiol. 8, 1036 (2017).
- Priya, A., Selvaraj, A., Divya, D., Karthik Raja, R. & Pandian, S. K. In vitro and in vivo anti-infective potential of thymol against early childhood caries causing dual species Candida albicans and Streptococcus mutans. Front. Pharmacol. 12, 760768 (2021).
- Chen, J. et al. Core microbiota promotes the development of dental caries. Appl. Sci. 11, 3638 (2021).
- Belda-Ferre, P. et al. The oral metagenome in health and disease. ISME J. 6, 46-56 (2012).
- Pang, L. et al. Metagenomic analysis of dental plaque on pit and fissure sites with and without caries among adolescents. Front. Cell. Infect. Microbiol. 11, 740981 (2021).
- Loesche, W. J. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 50, 353-380 (1986).
- Legenova, K. & Bujdakova, H. The role of Streptococcus mutans in the oral biofilm. Epidemiol. Mikrobiol. Imunol. 64, 179-187 (2015).
- Gross, E. L. et al. Bacterial 16 S sequence analysis of severe caries in young permanent teeth. J. Clin. Microbiol. 48, 4121-4128 (2010).
- Nicolas, G. G. & Lavoie, M. C. Streptococcus mutans and oral streptococci in dental plaque. Can. J. Microbiol. 57, 1-20 (2011).
- Balakrishnan, M., Simmonds, R. S. & Tagg, J. R. Dental caries is a preventable infectious disease. Aust. Dent. J. 45, 235-245 (2000).
- Bowen, W. H. Rodent model in caries research. Odontology 101, 9-14 (2013).
- Palmer, C. A. et al. Diet and caries-associated bacteria in severe early childhood caries. J. Dent. Res. 89, 1224-1229 (2010).
- Lin, Y., Chen, J., Zhou, X. & Li, Y. Inhibition of Streptococcus mutans biofilm formation by strategies targeting the metabolism of exopolysaccharides. Crit. Rev. Microbiol. 47, 667-677 (2021).
- Klein, M. I., Hwang, G., Santos, P. H. S., Campanella, O. H. & Koo, H. Streptococcus mutans-derived extracellular matrix in cariogenic oral biofilms. Front. Cell. Infect. Microbiol. 5, 10 (2015).
- Pleszczynska, M., Wiater, A., Janczarek, M. & Szczodrak, J. (1->3)-
-D-glucan hydrolases in dental biofilm prevention and control: a review. Int. J. Biol. Macromol. 79, 761-778 (2015). - Poulin, M. B. & Kuperman, L. L. Regulation of biofilm exopolysaccharide production by cyclic di-guanosine monophosphate. Front. Microbiol. 12, 730980 (2021).
- Bowen, W. H., Burne, R. A., Wu, H. & Koo, H. Oral biofilms: pathogens, matrix and polymicrobial interactions in microenvironments. Trends Microbiol. 26, 229-242 (2018).
- Alves, L. A. et al. CovR regulates Streptococcus mutans susceptibility to complement immunity and survival in blood. Infect. Immun. 84, 3206-3219 (2016).
- Goodman, S. D. et al. Biofilms can be dispersed by focusing the immune system on a common family of bacterial nucleoidassociated proteins. Mucosal Immunol. 4, 625-637 (2011).
- Xiao, J. et al. The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixedspecies oral biofilm. PLoS Pathog. 8, e1002623 (2012).
- Guo, L., McLean, J. S., Lux, R., He, X. & Shi, W. The wellcoordinated linkage between acidogenicity and aciduricity via insoluble glucans on the surface of Streptococcus mutans. Sci. Rep. 5, 18015 (2015).
- Banas, J. A. Virulence properties of Streptococcus mutans. Front. Biosci. Landmark 9, 1267-1277 (2004).
- Koo, H., Allan, R. N., Howlin, R. P., Stoodley, P. & Hall-Stoodley, L. Targeting microbial biofilms: current and prospective therapeutic strategies. Nat. Rev. Microbiol. 15, 740-755 (2017).
- Matsumi, Y. et al. Contribution of glucan-binding protein A to firm and stable biofilm formation by Streptococcus mutans. Mol. Oral Microbiol. 30, 217-226 (2015).
- Abranches, J. et al. Biology of oral streptococci. Microbiol. Spectr. 6, https://doi.org/10.1128/microbiolspec.GPP3-0042-2018 (2018).
- Xu, X., Zhou, X. D. & Wu, C. D. The tea catechin epigallocatechin gallate suppresses cariogenic virulence factors of Streptococcus mutans. Antimicrob. Agents Chemother. 55, 1229-1236 (2011).
- Ma, Q. et al. Acetylation of lactate dehydrogenase negatively regulates the acidogenicity of Streptococcus mutans. mBio 13, e0201322 (2022).
- Cotter, P. D. & Hill, C. Surviving the acid test: responses of grampositive bacteria to low pH. Microbiol. Mol. Biol. Rev. 67, 429-453 (2003).
- Liu, Y.-L., Nascimento, M. & Burne, R. A. Progress toward understanding the contribution of alkali generation in dental biofilms to inhibition of dental caries. Int. J. Oral Sci. 4, 135-140 (2012).
- Li, Y. H. & Tian, X. L. Quorum sensing and bacterial social interactions in biofilms. Sensors 12, 2519-2538 (2012).
- Matsumoto-Nakano, M. Role of Streptococcus mutans surface proteins for biofilm formation. Jpn. Dent. Sci. Rev. 54, 22-29 (2018).
- Lei, L. et al. Modulation of biofilm exopolysaccharides by the Streptococcus mutans vicX gene. Front. Microbiol. 6, 1432 (2015).
- Sadeghinejad, L. et al. Mechanistic, genomic and proteomic study on the effects of BisGMA-derived biodegradation product on cariogenic bacteria. Dent. Mater. 33, 175-190 (2017).
- Woelber, J. P., Al-Ahmad, A. & Alt, K. W. On the pathogenicity of the oral biofilm: a critical review from a biological, evolutionary, and nutritional point of view. Nutrients 14, 2174 (2022).
- Dashiff, A. & Kadouri, D. E. Predation of oral pathogens by Bdellovibrio bacteriovorus 109J. Mol. Oral Microbiol. 26, 19-34 (2011).
- Van Essche, M. et al. Killing of anaerobic pathogens by predatory bacteria. Mol. Oral Microbiol. 26, 52-61 (2011).
- Zarco, M. F., Vess, T. J. & Ginsburg, G. S. The oral microbiome in health and disease and the potential impact on personalized dental medicine. Oral Dis. 18, 109-120 (2012).
- Mercenier, A., Pavan, S. & Pot, B. Probiotics as biotherapeutic agents: present knowledge and future prospects. Curr. Pharm. Des. 9, 175 (2003).
- Hill, C. et al. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506-514 (2014).
- Saiz, P., Taveira, N. & Alves, R. Probiotics in oral health and disease: a systematic review. Appl. Sci. 11, 8070 (2021).
- Simark-Mattsson, C. et al. Lactobacillus-mediated interference of mutans streptococci in caries-free vs. caries-active subjects. Eur. J. Oral Sci. 115, 308-314 (2007).
- Inchingolo, A. D. et al. Oralbiotica/oralbiotics: the impact of oral microbiota on dental health and demineralization: a systematic review of the literature. Children 9, 1014 (2022).
- Teughels, W., Van Essche, M., Sliepen, I. & Quirynen, M. Probiotics and oral healthcare. Periodontology 48, 111-147 (2008). 2000.
- Talarico, T. L., Casas, I. A., Chung, T. C. & Dobrogosz, W. J. Production and isolation of reuterin, a growth inhibitor produced by Lactobacillus reuteri. Antimicrob. Agents Chemother. 32, 1854-1858 (1988).
- Gänzle, M. G., Höltzel, A., Walter, J., Jung, G. & Hammes, W. P. Characterization of reutericyclin produced by Lactobacillus reuteri LTH2584. Appl. Environ. Microbiol. 66, 4325-4333 (2000).
- Caglar, E. et al. Effect of yogurt with Bifidobacterium DN-173 010 on salivary mutans streptococci and lactobacilli in young adults. Acta Odontol. Scand. 63, 317-320 (2005).
- Darbandi, A. et al. Bacteriocins: properties and potential use as antimicrobials. J. Clin. Lab. Anal. 36, e24093 (2022).
- Rogers, L. A. The inhibiting effect of Streptococcus lactis on Lactobacillus bulgaricus. J. Bacteriol. 16, 321-325 (1928).
- Heng, B. C. Reluctance of medical professionals in adopting natural-cycle and minimal ovarian stimulation protocols in human clinical assisted reproduction. Reprod. Biomed. Online 15, 9-11 (2007).
- Wang, Y., Qin, Y., Zhang, Y., Wu, R. & Li, P. Antibacterial mechanism of plantaricin LPL-1, a novel class lla bacteriocin against Listeria monocytogenes. Food Control 97, 87-93 (2019).
- Surachat, K., Sangket, U., Deachamag, P. & Chotigeat, W. In silico analysis of protein toxin and bacteriocins from Lactobacillus paracasei SD1 genome and available online databases. PLoS One 12, e0183548 (2017).
- Nagao, J. et al. Lantibiotics: insight and foresight for new paradigm. J. Biosci. Bioeng. 102, 139-149 (2006).
- Yang, S.-C., Lin, C.-H., Sung, C. T. & Fang, J.-Y. Antibacterial activities of bacteriocins: application in foods and pharmaceuticals. Front. Microbiol. 5, 241 (2014).
- Jin, X., An, S., Kightlinger, W., Zhou, J. & Hong, S. H. Engineering Escherichia coli to produce and secrete colicins for rapid and selective biofilm cell killing. AIChE J. 67, e17466 (2021).
- Dobson, A., Cotter, P. D., Ross, R. P. & Hill, C. Bacteriocin production: a probiotic trait? Appl. Environ. Microbiol. 78, 1-6 (2012).
- Radaic, A. et al. Modulation of pathogenic oral biofilms towards health with nisin probiotic. J. Oral. Microbiol. 12, 1809302 (2020).
- Conrads, G., Westenberger, J., Luerkens, M. & Abdelbary, M. M. H. Isolation and bacteriocin-related typing of Streptococcus dentisani. Front. Cell Infect. Microbiol. 9, 110 (2019).
- Jaffar, N., Ishikawa, Y., Mizuno, K., Okinaga, T. & Maeda, T. Mature biofilm degradation by potential probiotics: Aggregatibacter actinomycetemcomitans versus Lactobacillus spp. PLoS One 11, e0159466 (2016).
- Walker, G. V. et al. Salivaricin E and abundant dextranase activity may contribute to the anti-cariogenic potential of the probiotic candidate Streptococcus salivarius JH. Microbiology 162, 476-486 (2016).
- Huang, X. et al. A highly arginolytic Streptococcus species that potently antagonizes Streptococcus mutans. Appl. Environ. Microbiol. 82, 2187-2201 (2016).
- Di Pierro, F., Zanvit, A., Nobili, P., Risso, P. & Fornaini, C. Cariogram outcome after 90 days of oral treatment with Streptococcus salivarius M18 in children at high risk for dental caries: results of a randomized, controlled study. Clin. Cosmet. Investig. Dent. 7, 107-113 (2015).
- Satpute, S. K. et al. Biosurfactant/s from lactobacilli species: properties, challenges and potential biomedical applications. J. Basic Microbiol. 56, 1140-1158 (2016).
- Sharma, D., & Singh Saharan, B. Simultaneous production of biosurfactants and bacteriocins by probiotic Lactobacillus casei MRTL3. Int. J. Microbiol. 2014, 698713 (2014).
- Rodrigues, L. R., Teixeira, J. A. & Oliveira, R. Low-cost fermentative medium for biosurfactant production by probiotic bacteria. Biochem. Eng. J. 32, 135-142 (2006).
- Saravanakumari, P. & Mani, K. Structural characterization of a novel xylolipid biosurfactant from Lactococcus lactis and analysis of antibacterial activity against multi-drug resistant pathogens. Bioresour. Technol. 101, 8851-8854 (2010).
- Thavasi, R., Jayalakshmi, S. & Banat, I. M. Effect of biosurfactant and fertilizer on biodegradation of crude oil by marine isolates of Bacillus megaterium, Corynebacterium kutscheri and Pseudomonas aeruginosa. Bioresour. Technol. 102, 772-778 (2011).
- Ciandrini, E. et al. Characterization of biosurfactants produced by Lactobacillus spp. and their activity against oral streptococci biofilm. Appl. Microbiol. Biotechnol. 100, 6767-6777 (2016).
- Tahmourespour, A., Salehi, R. & Kasra Kermanshahi, R. Lactobacillus acidophilus-derived biosurfactant effect on gtfB and gtfC expression level in Streptococcus mutans biofilm cells. Braz. J. Microbiol. 42, 330-339 (2011).
- Tan, Y., Leonhard, M., Moser, D. & Schneider-Stickler, B. Inhibition activity of Lactobacilli supernatant against fungal-bacterial multispecies biofilms on silicone. Microb. Pathog. 113, 197-201 (2017).
- Gudina, E. J., Teixeira, J. A. & Rodrigues, L. R. Isolation and functional characterization of a biosurfactant produced by Lactobacillus paracasei. Colloids Surf. B Biointerfaces 76, 298-304 (2010).
- Özcelik, S., Kuley, E. & Özogul, F. Formation of lactic, acetic, succinic, propionic, formic and butyric acid by lactic acid bacteria. LWT Food Sci. Technol. 73, 536-542 (2016).
- Lin, X., Chen, X., Chen, Y., Jiang, W. & Chen, H. The effect of five probiotic lactobacilli strains on the growth and biofilm formation of Streptococcus mutans. Oral Dis. 21, E128-E134 (2015).
- Bustamante, M., Oomah, B. D., Mosi-Roa, Y., Rubilar, M. & BurgosDiaz, C. Probiotics as an adjunct therapy for the treatment of halitosis, dental caries and periodontitis.Probiotics Antimicrob. Proteins 12, 325-334 (2020).
- Redanz, S. et al. Live and let die: hydrogen peroxide production by the commensal flora and its role in maintaining a symbiotic microbiome. Mol. Oral Microbiol. 33, 337-352 (2018).
- Herrero, E. R. et al. Antimicrobial effects of commensal oral species are regulated by environmental factors. J. Dent. 47, 23-33 (2016).
- El Oirdi, S. et al. Isolation and identification of Lactobacillus plantarum 4F, a strain with high antifungal activity, fungicidal effect, and biopreservation properties of food. J. Food Process. Preserv. 45, e15517 (2021).
- Lai, W.-K. et al. Developing lactic acid bacteria as an oral healthy food. Life 11, 268 (2021).
- Barzegari, A. et al. The battle of probiotics and their derivatives against biofilms. Infect. Drug Resist. 13, 659-672 (2020).
- Wasfi, R., Abd El-Rahman, O. A., Zafer, M. M. & Ashour, H. M. Probiotic Lactobacillus sp. inhibit growth, biofilm formation and gene expression of caries-inducing Streptococcus mutans. J. Cell. Mol. Med. 22, 1972-1983 (2018).
- Matsubara, V. H., Wang, Y., Bandara, H. M. H. N., Mayer, M. P. A. & Samaranayake, L. P. Probiotic lactobacilli inhibit early stages of Candida albicans biofilm development by reducing their growth, cell adhesion, and filamentation. Appl. Microbiol. Biotechnol. 100, 6415-6426 (2016).
- James, K. M., MacDonald, K. W., Chanyi, R. M., Cadieux, P. A. & Burton, J. P. Inhibition of Candida albicans biofilm formation and modulation of gene expression by probiotic cells and supernatant. J. Med. Microbiol. 65, 328-336 (2016).
- Cortes-Acha, B. et al. Development and viability of biofilms grown on experimental abutments mimicking dental implants: an in vivo model. Med. Oral Patol. Oral. Cir. Bucal 24, e511-e517 (2019).
- Jung, H.-Y. et al. Collagen peptide in a combinatorial treatment with Lactobacillus rhamnosus inhibits the cariogenic properties of Streptococcus mutans: an in vitro study. Int. J. Mol. Sci. 23, 1860 (2022).
- Lin, T.-H., Lin, C.-H. & Pan, T.-M. The implication of probiotics in the prevention of dental caries. Appl. Microbiol. Biotechnol. 102, 577-586 (2018).
- Singh, T. P., Kaur, G., Kapila, S. & Malik, R. K. Antagonistic activity of Lactobacillus reuteri strains on the adhesion characteristics of selected pathogens. Front. Microbiol. 8, 486 (2017).
- Burton, J. P. et al. Influence of the probiotic Streptococcus salivarius strain M18 on indices of dental health in children: a randomized double-blind, placebo-controlled trial. J. Med. Microbiol. 62, 875-884 (2013).
- Ha Kim, J., Jang, H. J., Lee, N.-K. & Paik, H.-D. Antibacterial and antibiofilm effect of cell-free supernatant of Lactobacillus brevis KCCM 202399 isolated from korean fermented food against Streptococcus mutans KCTC 5458. J. Microbiol. Biotechnol. 32, 56-63 (2022).
- Haukioja, A., Loimaranta, V. & Tenovuo, J. Probiotic bacteria affect the composition of salivary pellicle and streptococcal adhesion in vitro. Oral Microbiol. Immunol. 23, 336-343 (2008).
- Tenovuo, J. Antimicrobial function of human saliva-how important is it for oral health? Acta Odontol. Scand. 56, 250-256 (1998).
- Boris, S., Suárez, J. E. & Barbés, C. Characterization of the aggregation promoting factor from Lactobacillus gasseri, a vaginal isolate. J. Appl. Microbiol. 83, 413-420 (1997).
- Lang, C. et al. Specific Lactobacillus/mutans Streptococcus coaggregation. J. Dent. Res. 89, 175-179 (2010).
- Sliepen, I. et al. Microbial interactions influence inflammatory host cell responses. J. Dent. Res. 88, 1026-1030 (2009).
- Wattanarat, O. et al. Significant elevation of salivary human neutrophil peptides 1-3 levels by probiotic milk in preschool children with severe early childhood caries: a randomized controlled trial. Clin. Oral Investig. 25, 2891-2903 (2021).
- Pahumunto, N., Sophatha, B., Piwat, S. & Teanpaisan, R. Increasing salivary IgA and reducing Streptococcus mutans by probiotic Lactobacillus paracasei SD1: a double-blind, randomized, controlled study. J. Dent. Sci. 14, 178-184 (2019).
- Balzaretti, S. et al. A novel rhamnose-rich hetero-exopolysaccharide isolated from Lactobacillus paracasei DG activates THP-1 human monocytic cells. Appl. Environ. Microbiol. 83, e02702-e02716 (2017).
- Amargianitakis, M., Antoniadou, M., Rahiotis, C. & Varzakas, T. Probiotics, prebiotics, synbiotics and dental caries. new perspectives, suggestions, and patient coaching approach for a cavity-free mouth. Appl. Sci. 11, 5472 (2021).
- Nadelman, P., Magno, M. B., Masterson, D., da Cruz, A. G. & Maia, L. C. Are dairy products containing probiotics beneficial for oral health? a systematic review and meta-analysis. Clin. Oral Investig. 22, 2763-2785 (2018).
- Gedalia, I. et al. Enamel softening with Coca-Cola and rehardening with milk or saliva. Am. J. Dent. 4, 120-122 (1991).
- Kashket, S. & Yaskell, T. Effectiveness of calcium lactate added to food in reducing intraoral demineralization of enamel. Caries Res. 31, 429-433 (1997).
- Schüpbach, P., Neeser, J. R., Golliard, M., Rouvet, M. & Guggenheim, B. Incorporation of caseinoglycomacropeptide and caseinophosphopeptide into the salivary pellicle inhibits adherence of mutans streptococci. J. Dent. Res. 75, 1779-1788 (1996).
- Swarna, S. K. & Nivedhitha, M. S. Probiotics in prevention of dental caries-a literature review. Biosci. Biotechnol. Res. Commun. 13, 517-526 (2020).
- de Alvarenga, J. A. et al. Probiotic effects of lactobacillus paracasei 28.4 to inhibit Streptococcus mutans in a gellan-based formulation. Probiotics Antimicrob. Proteins 13, 506-517 (2021).
- Yelin, I. et al. Genomic and epidemiological evidence of bacterial transmission from probiotic capsule to blood in ICU patients. Nat. Med. 25, 1728-1732 (2019).
- Gruner, D., Paris, S. & Schwendicke, F. Probiotics for managing caries and periodontitis: systematic review and meta-analysis. J. Dent. 48, 16-25 (2016).
- Corby, P. M. et al. Microbial risk indicators of early childhood caries. J. Clin. Microbiol. 43, 5753-5759 (2005).
- Wen, Z. T., Huang, X., Ellepola, K., Liao, S. & Li, Y. Lactobacilli and human dental caries: more than mechanical retention. Microbiology 168, 001196 (2022).
- Henne, K., Rheinberg, A., Melzer-Krick, B. & Conrads, G. Aciduric microbial taxa including Scardovia wiggsiae and Bifidobacterium spp. in caries and caries free subjects. Anaerobe 35, 60-65 (2015).
- Caufield, P. W., Schön, C. N., Saraithong, P., Li, Y. & Argimón, S. Oral lactobacilli and dental caries: a model for niche adaptation in humans. J. Dent. Res. 94, 110S-118S (2015).
- Newhouse, M. T. & Dolovich, M. Spacer devices for asthma. J. Pediatr. 109, 913-914 (1986).
- Gibson, G. R. & Roberfroid, M. B. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 125, 1401-1412 (1995).
- Gibson, G. R. et al. Expert consensus document: the international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 14, 491-502 (2017).
- Guerrero-Wyss, M., Durán Agüero, S. & Angarita Dávila, L. D-tagatose is a promising sweetener to control glycaemia: a new functional food. Biomed. Res. Int. 2018, e8718053 (2018).
- Mayumi, S. et al. Potential of prebiotic D-tagatose for prevention of oral disease. Front. Cell Infect. Microbiol. 11, 767944 (2021).
- Nagamine, Y. et al. D-tagatose effectively reduces the number of Streptococcus mutans and oral bacteria in healthy adult subjects: a chewing gum pilot study and randomized clinical trial. Acta Med. Okayama 74, 307-317 (2020).
- Kojima, Y., Ohshima, T., Seneviratne, C. J. & Maeda, N. Combining prebiotics and probiotics to develop novel synbiotics that suppress oral pathogens. J. Oral Biosci. 58, 27-32 (2016).
- Söderling, E. & Pienihäkkinen, K. Effects of xylitol and erythritol consumption on mutans streptococci and the oral microbiota: a systematic review. Acta Odontol. Scand. 78, 599-608 (2020).
- Gibson, G. R., Probert, H. M., Loo, J. V., Rastall, R. A. & Roberfroid, M. B. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr. Res. Rev. 17, 259-275 (2004).
- Roberfroid, M. et al. Prebiotic effects: metabolic and health benefits. Br. J. Nutr. 104, S1-S63 (2010).
- Cocco, F. et al. The caries preventive effect of 1 -year use of low-dose xylitol chewing gum. a randomized placebo-controlled clinical trial in high-caries-risk adults. Clin. Oral Investig. 21, 2733-2740 (2017).
- Söderling, E., Alaräisänen, L., Scheinin, A. & Mäkinen, K. K. Effect of xylitol and sorbitol on polysaccharide production by and adhesive properties of Streptococcus mutans. Caries Res. 21, 109-116 (1987).
- Watthanasaen, S. et al. Xylitol-containing chewing gum for caries prevention in students with disabilities: a randomised trial. Oral Health Prev. Dent. 15, 519-527 (2017).
- Gauthier, L., Vadeboncoeur, C. & Mayrand, D. Loss of sensitivity to xylitol by Streptococcus mutans LG-1. Caries Res. 18, 289-295 (1984).
- Falony, G. et al. Long-term effect of erythritol on dental caries development during childhood: a posttreatment survival analysis. Caries Res. 50, 579-588 (2016).
- Thabuis, C. et al. Effects of maltitol and xylitol chewing-gums on parameters involved in dental caries development. Eur. J. Paediatr. Dent. 14, 303-308 (2013).
- Salli, K., Söderling, E., Hirvonen, J., Gürsoy, U. K. & Ouwehand, A. C. Influence of
-fucosyllactose and galacto-oligosaccharides on the growth and adhesion of Streptococcus mutans. Br. J. Nutr. 124, 824-831 (2020). - Sharon, N. Carbohydrates as future anti-adhesion drugs for infectious diseases. Biochim. Biophys. Acta 1760, 527-537 (2006).
- Oku, T. & Nakamura, S. Threshold for transitory diarrhea induced by ingestion of xylitol and lactitol in young male and female adults. J. Nutr. Sci. Vitaminol. 53, 13-20 (2007).
- Koopman, J. E. et al. Stability and resilience of oral microcosms toward acidification and Candida outgrowth by arginine supplementation. Microb. Ecol. 69, 422-433 (2015).
- Bacali, C. et al. Oral microbiome: getting to know and befriend neighbors, a biological approach. Biomedicines 10, 671 (2022).
- Zheng, X. et al. Ecological effect of arginine on oral microbiota. Sci. Rep. 7, 7206 (2017).
- He, J. et al. L-arginine modifies the exopolysaccharide matrix and thwarts Streptococcus mutans outgrowth within mixed-species oral biofilms. J. Bacteriol. 198, 2651-2661 (2016).
- Koopman, J. E. et al. Changes in the oral ecosystem induced by the use of 8% arginine toothpaste. Arch. Oral Biol. 73, 79-87 (2017).
- Yin, W. et al. The anti-caries efficacy of a dentifrice containing
arginine and 1450 ppm fluoride as sodium monofluorophosphate assessed using quantitative light-induced fluorescence (QLF). J. Dent. 41, S22-S28 (2013). - Bijle, M. N. A., Ekambaram, M., Lo, E. C. & Yiu, C. K. Y. The combined enamel remineralization potential of arginine and fluoride toothpaste. J. Dent. 76, 75-82 (2018).
- Carda-Diéguez, M., Moazzez, R. & Mira, A. Functional changes in the oral microbiome after use of fluoride and arginine containing dentifrices: a metagenomic and metatranscriptomic study. Microbiome 10, 159 (2022).
- Cheng, X . et al. Magnesium-dependent promotion of
production increases ecological competitiveness of oral commensal streptococci. J. Dent. Res. 99, 847-854 (2020). - Burne, R. A. & Marquis, R. E. Alkali production by oral bacteria and protection against dental caries. FEMS Microbiol. Lett. 193, 1-6 (2000).
- Zaura, E. & Twetman, S. Critical appraisal of oral pre- and probiotics for caries prevention and care. Caries Res. 53, 514-526 (2019).
- Sánchez, G. A., Miozza, V. A., Delgado, A. & Busch, L. Total salivary nitrates and nitrites in oral health and periodontal disease. Nitric Oxide 36, 31-35 (2014).
- Doel, J. J. et al. Protective effect of salivary nitrate and microbial nitrate reductase activity against caries. Eur. J. Oral Sci. 112, 424-428 (2004).
- Green, S. J. Nitric oxide in mucosal immunity. Nat. Med. 1, 515-517 (1995).
- Allaker, R. P., Silva Mendez, L. S., Hardie, J. M. & Benjamin, N. Antimicrobial effect of acidified nitrite on periodontal bacteria. Oral Microbiol. Immunol. 16, 253-256 (2001).
- Rosier, B. T., Buetas, E., Moya-Gonzalvez, E. M., Artacho, A. & Mira, A. Nitrate as a potential prebiotic for the oral microbiome. Sci. Rep. 10, 12895 (2020).
- Li, H. et al. Salivary nitrate-an ecological factor in reducing oral acidity. Oral Microbiol. Immunol. 22, 67-71 (2007).
- Jockel-Schneider, Y. et al. Stimulation of the nitrate-nitrite-NOmetabolism by repeated lettuce juice consumption decreases gingival inflammation in periodontal recall patients: a randomized, double-blinded, placebo-controlled clinical trial. J. Clin. Periodontol. 43, 603-608 (2016).
- Gee, L. C. & Ahluwalia, A. Dietary nitrate lowers blood pressure: epidemiological, pre-clinical experimental and clinical trial evidence. Curr. Hypertens. Rep. 18, 17 (2016).
- Vanhatalo, A. et al. Nitrate-responsive oral microbiome modulates nitric oxide homeostasis and blood pressure in humans. Free Radic. Biol. Med. 124, 21-30 (2018).
- Velmurugan, S. et al. Dietary nitrate improves vascular function in patients with hypercholesterolemia: a randomized, double-blind, placebo-controlled study. Am. J. Clin. Nutr. 103, 25-38 (2015).
- Markowiak, P. & Śliżewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 9, 1021 (2017).
- Swanson, K. S. et al. The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 17, 687-701 (2020).
- Nunpan, S., Suwannachart, C. & Wayakanon, K. Effect of prebioticsenhanced probiotics on the growth of Streptococcus mutans. Int. J. Microbiol. 2019, 4623807 (2019).
- Tester, R. & AI-Ghazzewi, F. A preliminary study of the synbiotic effects of konjac glucomannan hydrolysates (GMH) and lactobacilli on the growth of the oral bacterium Streptococcus mutans. Nutr. Food Sci. 41, 234-237 (2011).
- Bijle, M. N., Neelakantan, P., Ekambaram, M., Lo, E. C. M. & Yiu, C. K. Y. Effect of a novel synbiotic on Streptococcus mutans. Sci. Rep. 10, 7951 (2020).
- Salminen, S. et al. The international scientific association of probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 18, 649-667 (2021).
- Barros, C. P. et al. Paraprobiotics and postbiotics: concepts and potential applications in dairy products. Curr. Opin. Food Sci. 32, 1-8 (2020).
- Moradi, M. et al. Postbiotics produced by lactic acid bacteria: the next frontier in food safety. Compr. Rev. Food Sci. Food Saf. 19, 3390-3415 (2020).
- Holz, C. et al. Lactobacillus paracasei DSMZ16671 reduces mutans Streptococci: a short-term pilot study. Probiotics Antimicrob. Proteins 5, 259-263 (2013).
- Moradi, M., Molaei, R. & Guimarães, J. T. A review on preparation and chemical analysis of postbiotics from lactic acid bacteria. Enzym. Microb. Technol. 143, 109722 (2021).
- el-Nezami, H., Kankaanpää, P., Salminen, S. & Ahokas, J. Physicochemical alterations enhance the ability of dairy strains of lactic acid bacteria to remove aflatoxin from contaminated media. J. Food Prot. 61, 466-468 (1998).
- Schwendicke, F., Horb, K., Kneist, S., Dörfer, C. & Paris, S. Effects of heat-inactivated Bifidobacterium BB12 on cariogenicity of Streptococcus mutans in vitro. Arch. Oral Biol. 59, 1384-1390 (2014).
- Tareb, R., Bernardeau, M., Gueguen, M. & Vernoux, J.-P. In vitro characterization of aggregation and adhesion properties of viable and heat-killed forms of two probiotic Lactobacillus strains and interaction with foodborne zoonotic bacteria, especially Campylobacter jejuni. J. Med. Microbiol. 62, 637-649 (2013).
- Pahumunto, N. et al. Reducing mutans streptococci and caries development by Lactobacillus paracasei SD1 in preschool children: a randomized placebo-controlled trial. Acta Odontol. Scand. 76, 331-337 (2018).
- Ritthagol, W., Saetang, C. & Teanpaisan, R. Effect of probiotics containing Lactobacillus paracasei SD1 on salivary mutans streptococci and lactobacilli in orthodontic cleft patients: a doubleblinded, randomized, placebo-controlled study. Cleft Palate Craniofac. J. 51, 257-263 (2014).
- Nozari, A., Motamedifar, M., Seifi, N., Hatamizargaran, Z. & Ranjbar, M. A. The effect of Iranian customary used probiotic yogurt on the children’s salivary cariogenic microflora. J. Dent. 16, 81-86 (2015).
- Pinto, G. S., Cenci, M. S., Azevedo, M. S., Epifanio, M. & Jones, M. H. Effect of yogurt containing Bifidobacterium animalis subsp. lactis DN-173010 probiotic on dental plaque and saliva in orthodontic patients. Caries Res. 48, 63-68 (2014).
- Zare Javid, A. et al. Effects of the consumption of probiotic yogurt containing Bifidobacterium lactis Bb12 on the levels of Streptococcus mutans and lactobacilli in saliva of students with initial stages of dental caries: a double-blind randomized controlled trial. Caries Res. 54, 68-74 (2020).
- Miyazima, T., Ishikawa, K., Mayer, M., Saad, S. & Nakamae, A. Cheese supplemented with probiotics reduced the Candida levels in denture wearers-RCT. Oral Dis. 23, 919-925 (2017).
- Ahola, A. J. et al. Short-term consumption of probiotic-containing cheese and its effect on dental caries risk factors. Arch. Oral Biol. 47, 799-804 (2002).
- Mortazavi, S. & Akhlaghi, N. Salivary Streptococcus mutans and Lactobacilli levels following probiotic cheese consumption in adults: a double blind randomized clinical trial*. J. Res. Med. Sci. 17, 57-66 (2012).
- Ashwin, D. et al. Effect of probiotic containing ice-cream on salivary mutans streptococci (SMS) levels in children of 6-12 years of age: a randomized controlled double blind study with six-months follow up. J. Clin. Diagn. Res. 9, ZC06-ZC09 (2015).
- Hasslof, P., West, C. E., Videhult, F. K., Brandelius, C. & StecksenBlicks, C. Early intervention with probiotic Lactobacillus paracasei F19 has no long-term effect on caries experience. Caries Res. 47, 559-565 (2013).
- Taipale, T., Pienihakkinen, K., Salminen, S., Jokela, J. & Soderling, E. Bifidobacterium animalis subsp. lactis BB-12 administration in early childhood: a randomized clinical trial of effects on oral colonization by mutans streptococci and the probiotic. Caries Res. 46, 69-77 (2012).
- Caglar, E. et al. Effect of chewing gums containing xylitol or probiotic bacteria on salivary mutans streptococci and lactobacilli. Clin. Oral Investig. 11, 425-429 (2007).
- Srivastava, S., Saha, S., Kumari, M. & Mohd, S. Effect of probiotic curd on salivary pH and Streptococcus mutans: a double blind parallel randomized controlled trial. J. Clin. Diagn. Res. 10, ZC13-ZC16 (2016).
- Jose, J. E., Padmanabhan, S. & Chitharanjan, A. B. Systemic consumption of probiotic curd and use of probiotic toothpaste to reduce Streptococcus mutans in plaque around orthodontic brackets. Am. J. Orthod. Dentofac. Orthop. 144, 67-72 (2013).
- Pohjavuori, S. et al. Effect of consumption of Lactobacillus rhamnosus GG and calcium, in carrot-pineapple juice on dental caries risk in children. Int. J. Probiotics Prebiotics 5, 221-228 (2010).
- Zahradnik, R. T. et al. Preliminary assessment of safety and effectiveness in humans of ProBiora
, a probiotic mouthwash. J. Appl. Microbiol. 107, 682-690 (2009).
Acknowledgements
Author contributions
Competing interests
Additional information
http://www.nature.com/reprints
© The Author(s) 2024
- ¹Guangdong Provincial Key Laboratory of Marine Biology, Department of Biology, College of Science, Shantou University, Shantou 515063 Guangdong, PR China.
Research Institute for Future Food, Department of Food Science and Nutrition, The Hong Kong Polytechnic University, Hong Kong, PR China. School of Life Sciences, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong, PR China. e-mail: bbzhang@stu.edu.cn
